Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ORTHOVITA INC | d8k.htm |
 2010
Corporate Presentation
: VITA
Exhibit 99.1 |
 2
Safe Harbor Statement
This presentation contains forward-looking statements by Orthovita regarding our
current expectations of future events that involve risks and uncertainties,
including without limitation, our VITOSS
™
, VITAGEL
™
, VITASURE
™
and CORTOSS
™
products and other aspects of our business.
Such statements are based on our current expectations and are subject to a number of
substantial risks and uncertainties that could cause actual results or
timeliness to differ materially from those addressed in the forward-looking
statements. Factors that may cause such a difference include, but are not
limited to, our dependence on the commercial success of our approved products, the need
to maintain regulatory approvals to sell our products, our ability to manage commercial
scale manufacturing capability and capacity, our success in expanding our
commercial product portfolio and distribution
channel,
competition
and
other
risk
factors
listed
from
time
to
time
in
reports
filed
by
Orthovita
with
the
Securities
and
Exchange
Commission,
including
but
not
limited
to
risks
described
in
our
most
recently
filed
Form
10-K
under
the
caption
“Risk
Factors.”
Further
information
about
these
and
other
relevant
risks
and
uncertainties
may
be
found
in
Orthovita’s
filings
with
the
Commission, all of which are available from the Commission as well as from Orthovita
upon request. Orthovita undertakes no obligation to publicly update any
forward-looking statements. |
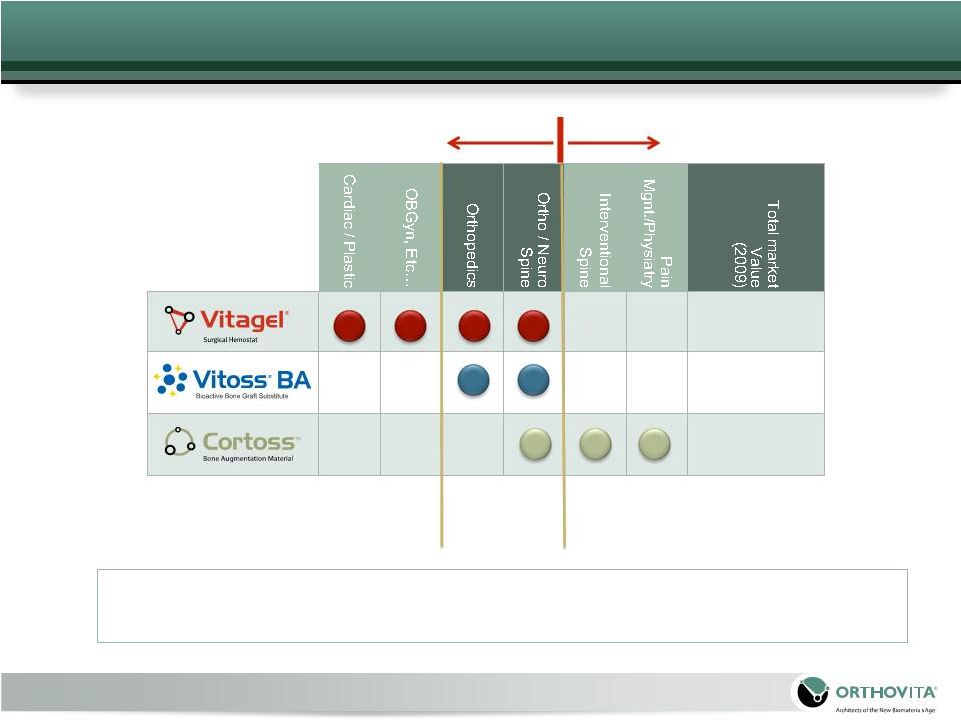
 Positioned to be a
leading provider of novel, well researched Orthobiologics and Biosurgery
technologies for patients, surgeons and hospitals.
|
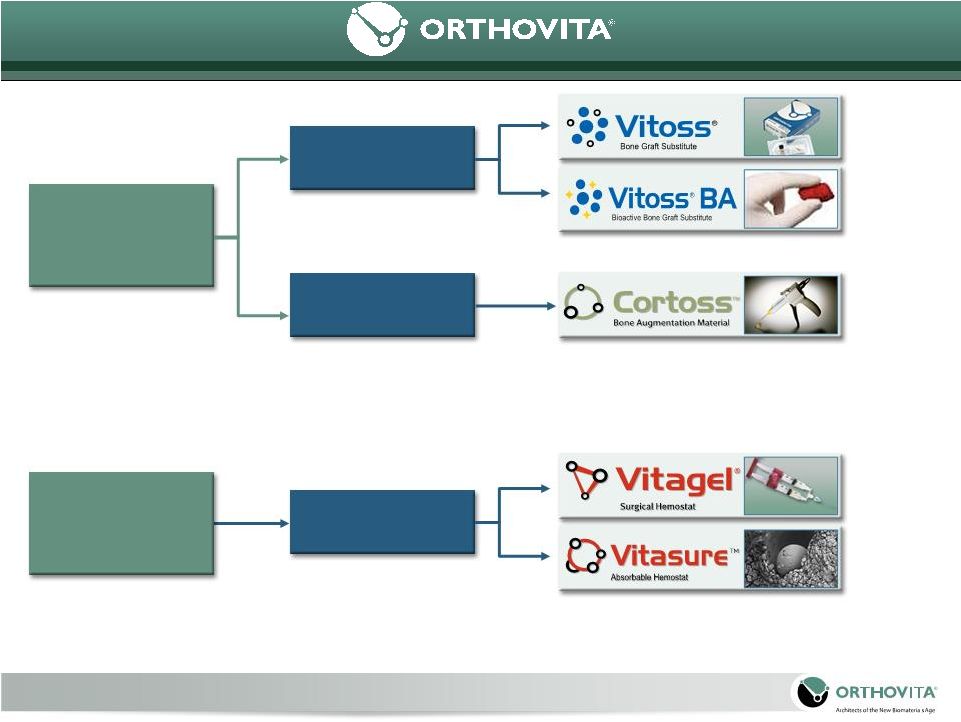 Biosurgery
Hemostats
Fixation
Fusion
Orthobiologics
4
Products and technologies
related to the management
of inter-operative bleeding
and soft tissue healing
Products and
technologies related
to the fusion, fixation
and regeneration of
the human skeleton |
 5
|
 US
Bone
Grafting
Market
2008
–
1,450,900
procedures
Autograft
DBX
–
Synthes
Grafton
–
Osteotech
Allomatrix
–
Wright Medical
Etc…
Allograft Chips
Stem Cells
DBMs
InFuse
–
Medtronic
Op-1
–
Stryker Spine
Trinity Evolution
–
Orthofix
Osteocel
–
Nuvasive
Mastergraft
–
Medtronic
Healos
–
J&J Depuy
Spine
Etc…
*Millennium Research Group: US Markets for Orthopedic Biomaterials
2009
BMPs
Other Synthetics
®
®
®
®
®
®
®
®
® |
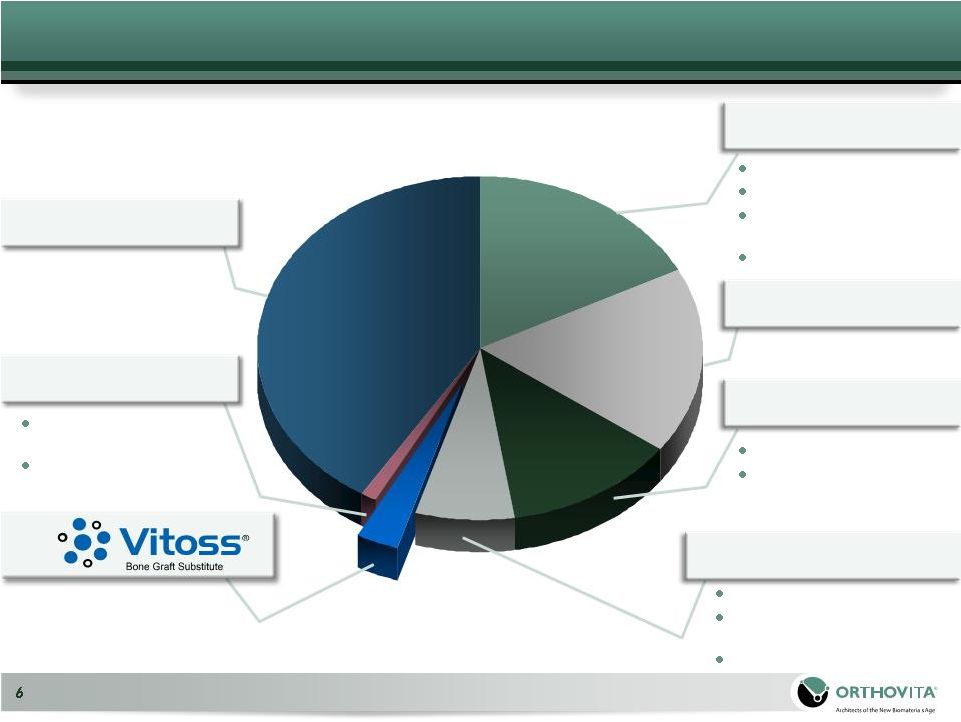
 7
JJ
Depuy
Spine
Healos
Medtronic Spine
*Millennium Research Group: US Markets for Orthopedic Biomaterials
2009 Mastergraft
All Other
Synthetic BGSs
33%
16%
16%
35%
US Synthetic Bone Graft Substitute Market 2008 –
~$150mm |
 8
Eliminates risks associated with autograft, allograft and BMPs
Broadest product range in category
*
When used as directed with BMA
Vitoss Bone Graft Substitute – Value
Proposition
Provides all three key components for bone regeneration
*
:
Scaffold – Cells - Signals
Vitoss Bioactive speeds bone regeneration vs standard
Vitoss
Equivalent efficacy to autograft, the gold standard
* |
 9
The most pre-clinical and clinical research of any product in its class
Vitoss Comparative Effectiveness |
  10
Vitoss Franchise Market Leadership
Early
Market
Entry
–
January
2002
Over 300,000 implantations
Most extensive bibliography in
its class
Covers majority of non-
structural bone grafting
indications
20 studies in spine with 745
patients
Extend Reach into BMP Market
Demonstrated effectiveness
with lower procedural cost
Vitoss BA enhances
conversion rationale
Leader in technology innovation |
 11 |
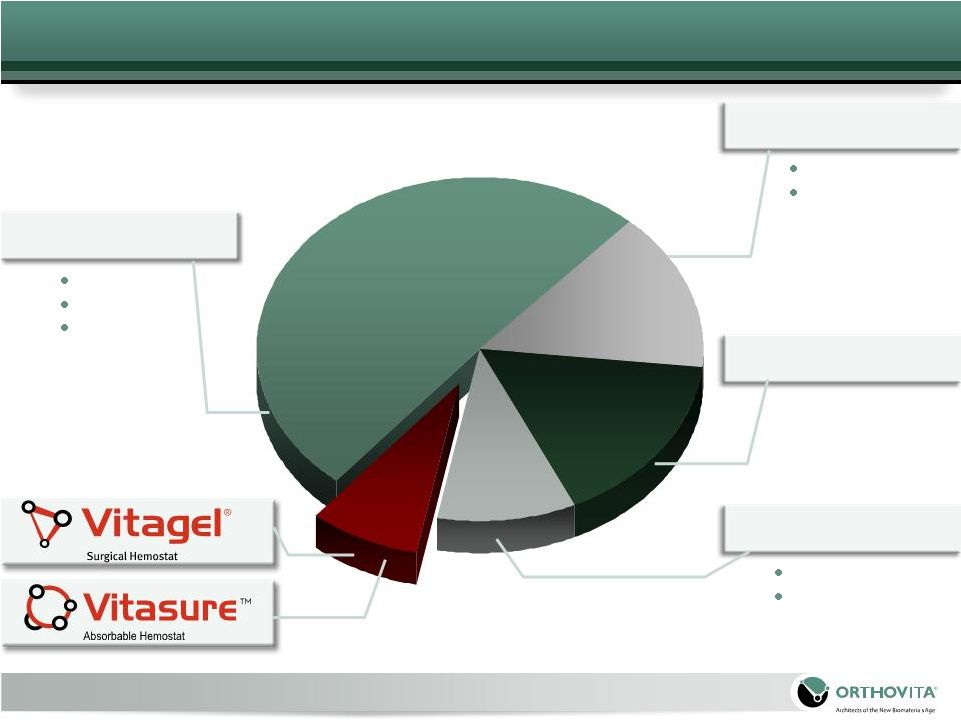 Floseal
®
Tisseel
®
Coseal
®
Surgiflo™
Evicel™
Bioglue
®
Hemostase™
Orthovita
Hemostat
Market
2008
–
$460
Million
Baxter
Cryolife
Ethicon
Platelet Gels
12
*2008, Millennium Research Group
“US Market for Surgical Hemostats, Internal Tissue Sealants and Adhesion Barriers, 2008"; Cryolife 2008 Year End Earnings;
*2008, Millennium Research Group
“US Market for Surgical Hemostats, Internal Tissue Sealants and Adhesion Barriers, 2008"; Cryolife 2008 Year End Earnings; |
 13
Safety:
Plant based technology eliminates many of the safety issues
associated with thrombin based competitors (FloSeal, Baxter)
Ease-of-Use:
Always ready, easy-to-use, and inexpensive
Controls Bleeding, Facilitates Healing:
Only
hemostat
with
the
combination
of
microfibrillar
collagen,
thrombin and the patient’s own plasma; supports hemostasis
and fibroblast attachment
Knee
Arthroplasty
Research:
Less drainage, reduced transfusion
Animal Research:
Less adhesions, faster healing
Vitagel Surgical Hemostat
Vitasure
Absorbable Hemostat
Hemostasis Franchise – Value Proposition
|
 14
Strategic
Role
of
Biosurgery
2010
Synergistic and complementary
soft-tissue product offering
to ORTHOBIOLOGICS product
platform
Opportunistic surgical sale
Supports account penetration in
new and developing geographies
Wide and diverse call pattern
allows business development in
major ORTHOBIOLOGICS
accounts |
 15 |
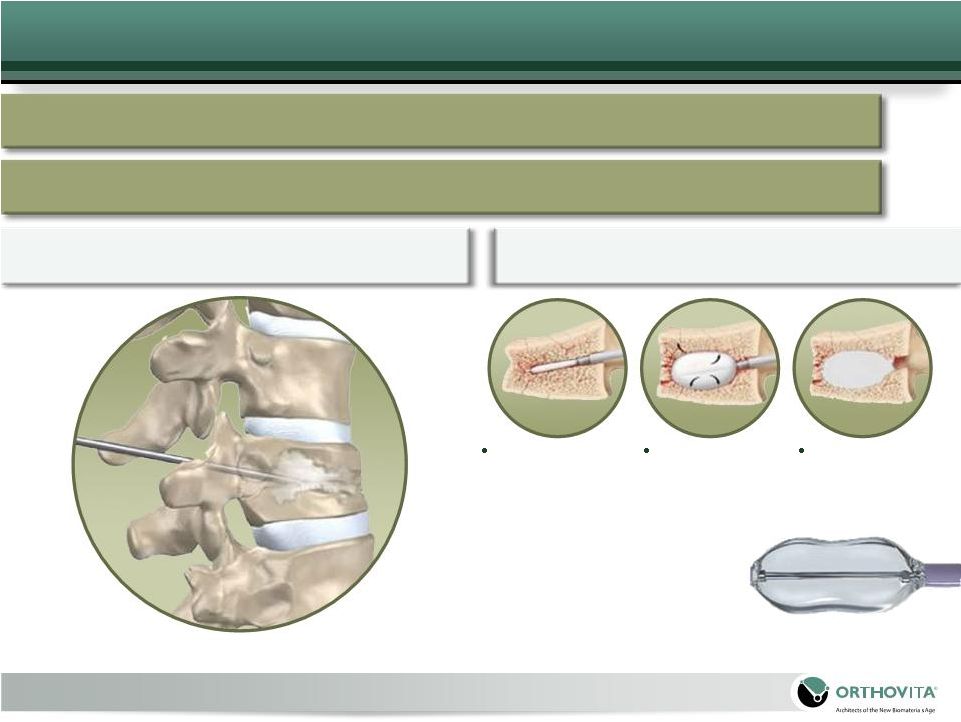 16
Treatment Options
Surgical Intervention
Vertebroplasty (VP)
Kyphoplasty (KP)
Catheter inserted
percutaneously
through pedicle
into body of
vertebra
using AP and
lateral fluroscopic
images
to guide placement
Balloon inflated
to provide some
restoration of
vertebral body
height
PMMA injected
to help maintain
correction
Conservative Care – Analgesics, Bed Rest,
Bracing |
 17
•
An injectable, bioactive composite
that mimics the mechanical
properties of human cortical bone
•
First FDA-cleared alternative to
polymethylmethacrylate
(PMMA)
cement for use in vertebral
augmentation procedure
•
Indicated for the fixation of
pathological fractures of the
vertebral body using vertebral
augmentation
•
Painful vertebral compression
fractures can result from
osteoporosis, benign lesions
(hemangioma), and malignant
lesions (metastatic cancers,
myeloma).
•
Patent protected to 2022
Cortoss Bone Augmentation Material – VCF
System
|
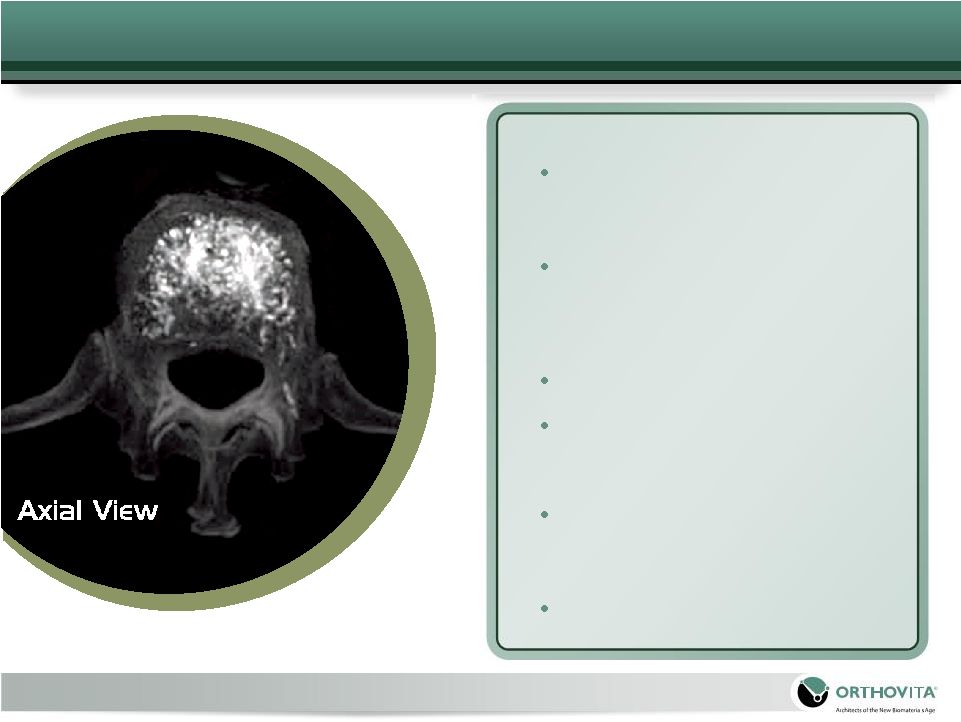  18
Therapeutic Flow and Fill
Material flow promotes an non-
intrusive diffuse, symmetric fill
pattern
Physiological load transfer
through treated vertebra(e)
Safety
No MMA monomer release
Lower injection volumes
Unparalleled Control
Material preparation is an on-
demand process that takes
seconds to complete
Easy
‘start
&
stop’
capability
as
needed to control leaks
Cortoss Bone Augmentation Material – Value
Propositions
|
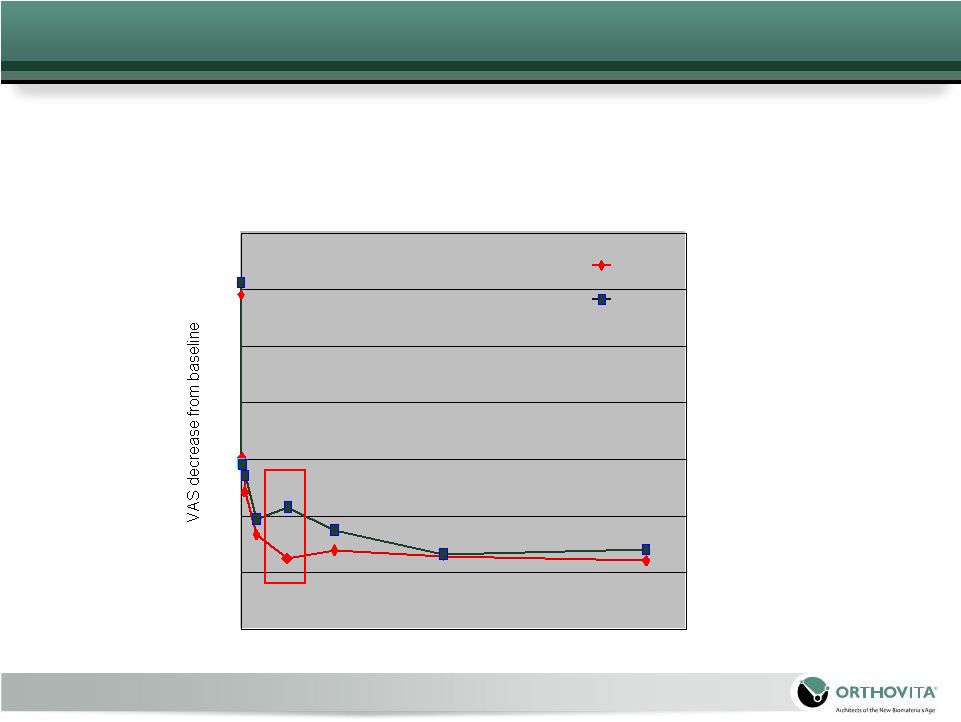 19
0
10
20
30
40
50
60
70
0
100
200
300
400
500
600
700
800Time
point (days) Cortoss
PMMA
FDA IDE Clinical Trial – Pain Results
At 3-months: Cortoss patients had a statistically significant
reduction in pain versus PMMA patients
|
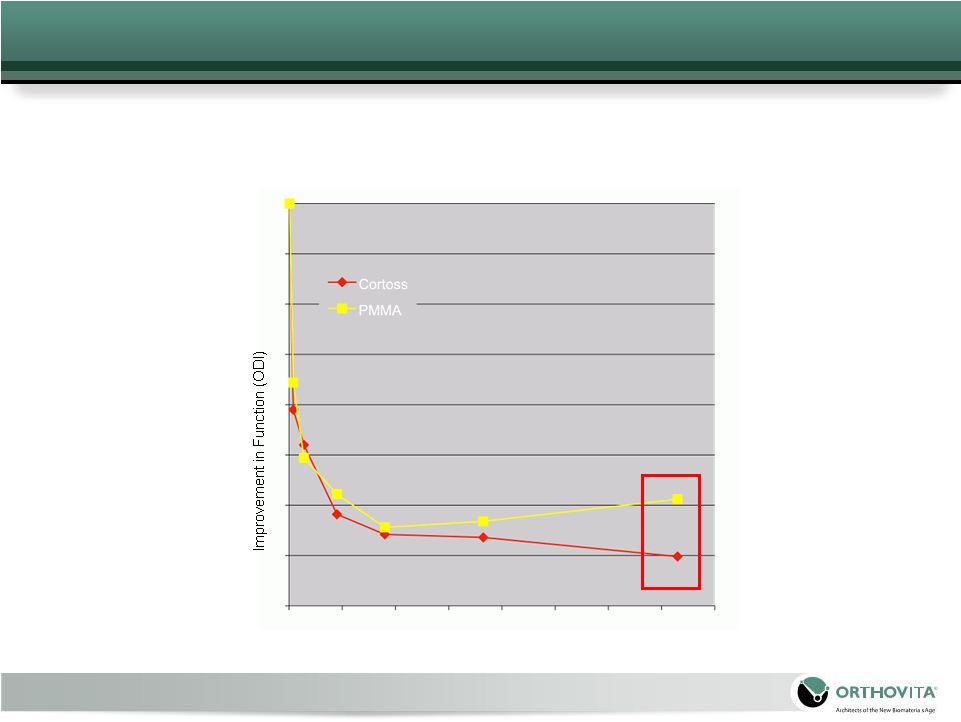 20
At
24-months:
A
statistically
significant,
greater
proportion of
Cortoss
patients
maintained
or
improved
function
(97%
vs
88%)
compared to the PMMA patients
FDA IDE Clinical Trial – Function Results
Time (Days) |
 21
The CORTOSS population experienced measurable clinical and
cost benefits over the PMMA cohort in the following outcomes:
43.4%
fewer
adjacent
fractures
in
‘first-fracture’
Cortoss
patients
Fracture related re-hospitalizations:
2.9%
Cortoss
vs.
11.4%
PMMA
(4x)
Subsequent PVPs
or KPs:
7.4%
Cortoss
vs.
22.7%
PMMA
(3x)
The
Cortoss
patients
required
an
average
of
30% less material
to achieve desired fill
FDA IDE Clinical Trial – Additional Benefits
|
 22
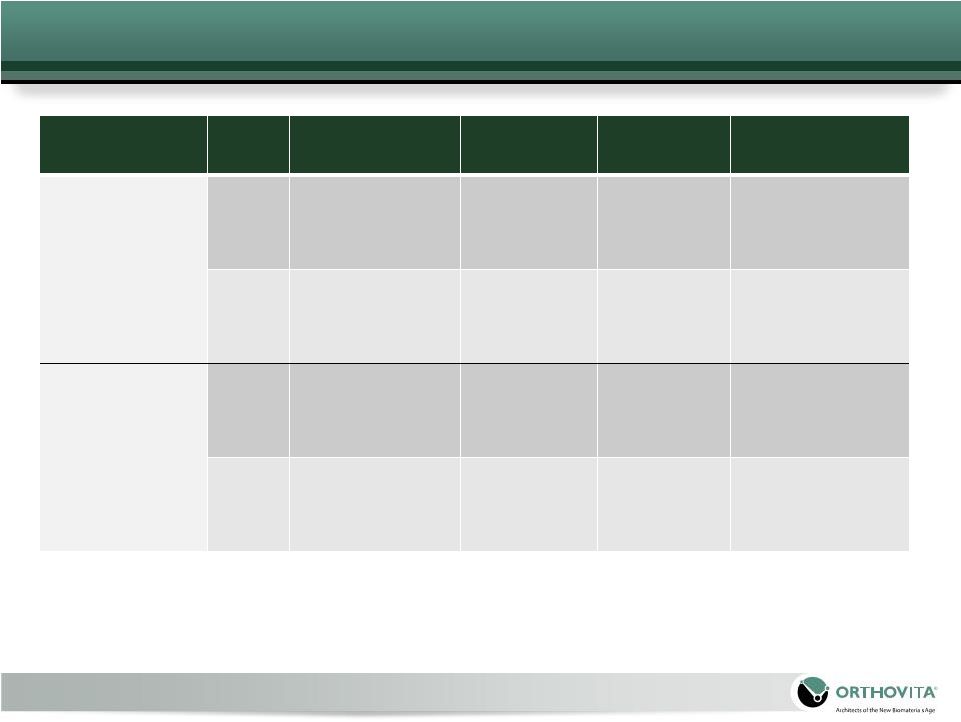
Medicare 2010 Facility Reimbursement
Procedure
Levels
Treated
In-patient
Out-patient
ASC
Physician
Vertebroplasty
1
$7,028 –
16,219
1
$2,141
$1,275
$489 -
518
3
2
2
$7,028 –
16,219
$3,211
$1,913
$718 -
747
Kyphoplasty
1
$7,028 –
16,219
$5,979
$3,551
$546 -
568
2
$7,028 –
16,219
$8,964
$5,327
$803 -
825
1
Reimbursement range dependent on patient co-morbidities
2
Additional levels are reimbursed at 50% of the first level
3
Variance is related to the levels treated. Thoracic is reimbursed at a higher rate
than lumbar Note:
These
are
national
averages,
specific
geographies
may
differ |
 23
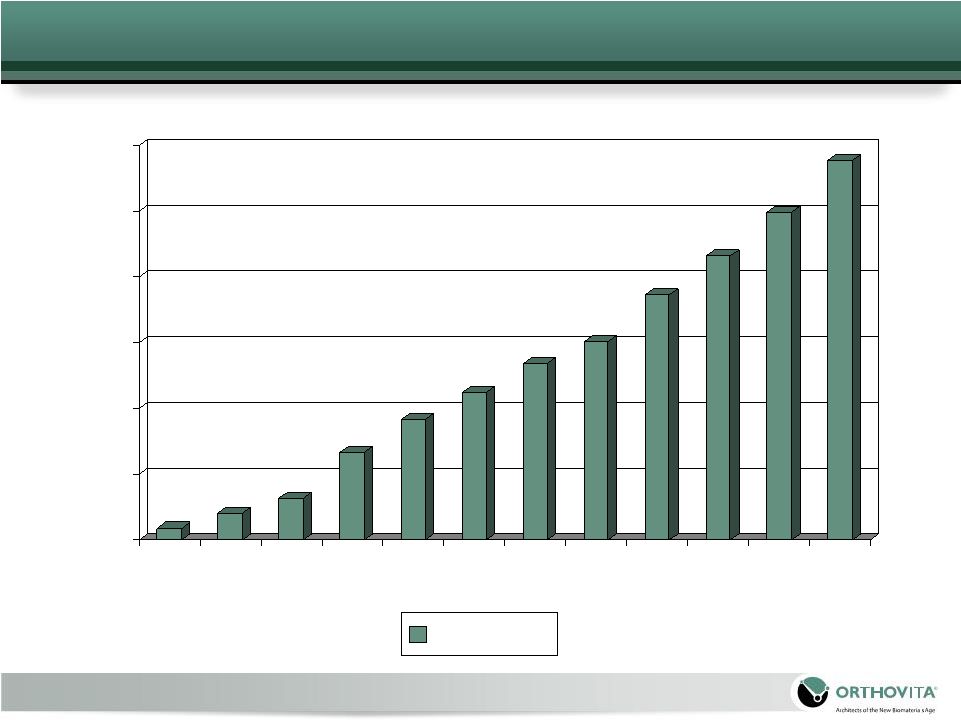
Cortoss Account Growth
0
50
100
150
200
250
300
July
Sep
Nov
Jan
Mar
May
Accounts |
 External Challenges 2010
Recent controversies surrounding vertebroplasty have led to
a reduction in procedures, a movement from inpatient to
outpatient treatment, greater scrutiny by insurance providers
and, subsequently, a very challenging environment for
CORTOSS. |
 Cortoss
Challenges 2010 Publication of NEJM articles
Temporary(?) reduction in referrals
Market size smaller than projected
Payment of fines for inappropriate overnight stays
Accelerated shift from hospital to outpatient procedures
Distraction sales force focus from Vitoss/Vitagel business
Passing of Health Care Reform Bill
Increased scrutiny of new technology by hosp. admins.
Increased lag-time from introduction to sale
Delayed sales ramp with CORTOSS
External events that affected VCF market: |
 Impact
to Sales Force The environment is increasingly requiring our reps maintain
a perpetual, physical presence within the hospitals to:
-
Gain product access
-
Develop key relationships
-
Secure case continuity
-
Defend current business
The sales process is increasingly more time and labor
intensive to generate the same revenue. |
 27
$460MM
$1.2BN
$500MM
~ $1.75B Market
Orthopedics/Spine
Our
unique
portfolio
of
biomaterials
provides
for
sizeable
value
creation
in
the
current
spine
and orthopedic markets. We are adjusting our distribution and call patterns to
address the new challenges and optimize in-
and outpatient procedural access.
Inpatients
Outpatients / ASC
Orthovita
and
the
target
audience
–
Integrating
Cortoss |
 28
Recent
Events:
Noridian
LCD
Review
Noridian
is a CMS contractor that administers Medicare
reimbursement for 8 western states
On May 11 , Noridian
announced it was considering the non-
coverage of reimbursement for vertebroplasty and kyphoplasty
Prompted by two small studies published in New England Journal
of Medicine that questioned effectiveness of vertebroplasty
Concerned that these procedures have been used inappropriately
Without first trying conservative care
Treatment of more levels than necessary
“Disturbing
incidence of complications” Noridian
has established a comment period from May 11 to
September 6 , 2010
Political ramifications of eliminating Medicare coverage to
elderly osteoporotic patients in severe pain
Outcome may be better definition/tracking of appropriate use
Facet
injections
a
recent
example;
Noridian
mandated
creation
of
a
registry
`
th
th
th |
 29
Industry
Develop advocacy by major industry groups (MDMA/ADVAMED)
Work collectively with key players to develop a concerted industry
response
Physicians and surgeons
Offer
to
coordinate
efforts
with
medical
societies
and
KOLs
to
respond to Noridian
Develop patient testimonials to submit to Noridian
Patients
Direct mailing to National Osteoporosis Foundation advocacy team
Awareness mailing to patients in affected geography
Government
Assess how and where to involve local representatives for
advocacy and response
Orthovita Response to Noridian LCD
Review |
 30
Financial Overview |
 31
Strong Growth, Improving Margins
1
2007
Net
loss
includes
a
$16.6mm
charge
for
the
repurchase
of
a
royalty
obligation
Dollars in Millions
2007
2008
2009
Q1 2010
Sales
$58.0
$76.9
$92.9
$24.1
Gross Margin
65%
66%
68%
69%
SG&A Expense
$44.6
$53.5
$57.2
$15.8
R&D Expense
$6.4
$6.7
$6.8
$1.3
Operating Loss
$(13.7)
$(9.2)
$(1.1)
$(0.4)
Net Loss
$(29.9)
$(10.8)
$(3.9)
$(1.2)
Cash and
Investments
$19.5
1 |
 4
Point
Plan
–
Manage
to
the
Environment
Expense reductions implemented in Q2 2010
Develop specialized distribution channels
Hospital Inpatient Market – Spine/Neurosurgeon
Outpatient Market – Interventional Radiologist
Continue to develop product pipeline to leverage each
specialty
Manage reimbursement issues in VCF
|
 33
Going Forward -
Core Strategies and Tactics
Maximize
Growth in
Existing Products
Pipeline
Development Goals
Potential to Fill Gaps
via Licenses &
Acquisitions
Tight focus on
profitable sales growth
–
manage to
environment
Maximize cross-sell of
all products to each
hospital customer
Develop IR/INR
specialty sales focus
Expand Cortoss label
to other indications
2011
–
Cortoss cavity
creation device
2011
–
Next
Generation of Vitoss
2011
–
Vitagel
Cellpaker
update in
conjunction with bone
marrow concentration
device
Bioactive Structural
Spine Implants
–
Regulatory clarity
Utilize collagen facility
capacity for new
product development
Acquire portfolio of
cavity creation devices
to meet all clinical
needs
Build-out Biosurgery
portfolio
Maximize proprietary
Bioactive implant
market opportunity |
 THANK
YOU |
