Attached files
| file | filename |
|---|---|
| 8-K - Inspyr Therapeutics, Inc. | v190398_8k.htm |
EXECUTIVE
INFORMATIONAL OVERVIEW
 |
GenSpera,
Inc.
2511
N Loop 1604 W, Suite 204
San
Antonio, TX 78258
Phone:
(210) 479-8112
Fax:
(210) 479-8113
www.genspera.com
|
Novel,
Targeted Approach to Chemotherapy Using Prodrugs
|
Snapshot
|
July
6, 2010
|
GenSpera,
Inc. (“GenSpera” or “the Company”) is a biotechnology company developing
targeted therapies to treat cancerous tumors. The Company’s novel approach uses
a prodrug†—an
inactive precursor of a drug that converts into its active form at a targeted
site—to deliver a potent, cell-killing agent directly to tumors. GenSpera’s
prodrugs employ one of two techniques: (1) targeting tumor-associated blood
vessels; or (2) targeting tumors directly. In contrast to existing anti-angiogenic drugs, which
may only block new blood vessel formation, the Company’s lead prodrug candidate,
G-202, attacks existing tumor vasculature, potentially
debilitating the tumor’s nutrient supply and causing cancer regression without
toxicity to other areas of the body. G-202 has caused tumor regression in animal
models of breast, prostate, bladder, and kidney cancer. A Phase I clinical trial
with G-202 is ongoing at two major cancer centers. GenSpera’s technology can
also be used to attack cancer cells directly by targeting the prodrug to enzymes found solely at tumor
sites. Using this approach, GenSpera is developing G-115, which targets prostate
cancer. The Company owns and controls all rights to G-202 and G-115 and seeks a
strategic partnership to maximize the value of these prodrugs. GenSpera has
headquarters in San Antonio, Texas, and trades on the Over-the-Counter Bulletin
Board (OTC.BB) as “GNSZ.”
Recent
Financial Data
|
Ticker (Exchange)
|
GNSZ (OTC.BB)
|
|
|
Recent
Price (07/06/2010)
|
$2.21
|
|
|
52-week
Range*
|
$1.55
- $3.60
|
|
|
Shares
Outstanding
|
17.5
million
|
|
|
Market
Capitalization
|
~$38.7
million
|
|
|
Average
3-month Volume
|
21,323
|
|
|
Insider
Owners +5%
|
34%
|
|
|
Institutional
Owners
|
N/A
|
|
|
EPS
(Year ended 03/31/2010)
|
($0.14)
|
|
|
Employees
|
2
|
*Note:
GenSpera’s shares began trading on the OTC.BB on November 2, 2009.
Key
Points
|
n
|
GenSpera’s
prodrug candidates combine 12ADT, a plant-derived cytotoxin, with a
prodrug delivery system that activates only within a tumor. Unlike
standard chemotherapies, which
mainly target rapidly dividing cells, 12ADT kills cells independently of
their division rate.
|
|
n
|
To
date, four patients have been enrolled in the Phase I study with G-202.
GenSpera seeks to enroll up to 30 patients and expects to complete the
trial in the second quarter 2011.
|
|
n
|
GenSpera’s
technology was developed over 15 years at Johns Hopkins and other global
research centers and funded by over $15 million in grants from the U.S.
National Institutes of Health, the National Cancer Institute, and the U.S.
Department of Defense, among others.
|
|
n
|
The
Company holds seven patents and four pending patent applications, which
were acquired without any milestone or royalty payments due to third
parties.
|
|
n
|
GenSpera’s
management team has extensive experience identifying oncology treatments
and bringing them to the clinic. The Company’s Scientific Advisory Board
is composed of individuals who are both inventors of GenSpera’s technology
and major shareholders.
|
|
n
|
At March 31, 2010, the Company’s
cash and cash equivalent position was ~$2.7 million. Subsequently,
GenSpera raised roughly $2.7 million in gross proceeds in May
2010.
|
†BOLD WORDS ARE REFERENCED IN
THE GLOSSARY ON PAGES 53-55.
 |
Table of Contents
|
Snapshot
|
1
|
|
Recent
Financial Data
|
1
|
|
Key
Points
|
1
|
|
Executive
Overview
|
3
|
|
Growth
Strategy
|
7
|
|
Intellectual
Property
|
9
|
|
Company
Leadership
|
10
|
|
Core
Story
|
13
|
|
Cancer
|
13
|
|
GenSpera’s
Prodrug Technology
|
18
|
|
G-202:
GenSpera’s Lead Prodrug Candidate
|
23
|
|
Developing
Prodrugs to Target Tumors Directly
|
31
|
|
Competition
|
33
|
|
Milestones
|
38
|
|
Key
Points to Consider
|
39
|
|
Historical
Financial Results
|
40
|
|
Risks
|
43
|
|
Recent
Events
|
51
|
|
Glossary
|
53
|
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 2
|
 |
Executive
Overview
GenSpera,
Inc. (“GenSpera” or “the Company”) is a development-stage company focused on
discovering and developing targeted cancer therapeutics to treat a wide range of
solid tumors, including breast, prostate, bladder, and kidney cancers. The
Company’s novel prodrug technology combines a potent cytotoxin with a prodrug
delivery system that activates the drug only within the tumor. The Company’s
business strategy entails developing a series of therapies based on its
target-activated prodrug technology platform, identifying potentially attractive
drug candidates with solid intellectual property (IP) protection, and then
developing these compounds through Phase I/II clinical trials. Once a candidate
reaches this stage, the Company intends to license the rights for further
development to more established pharmaceutical companies, which could then
finalize drug development and market the resulting therapeutic.
GenSpera
initiated a Phase I clinical trial with its lead prodrug, G-202, in early 2010.
Upon completion of its Phase I trial, the Company expects to initiate multiple
Phase II trials for G-202 in several different cancer types. GenSpera’s second
drug, G-115, is designed to target prostate cancer. The Company owns and
controls all rights to both G-202 and G-115. GenSpera aims to establish a
strategic partnership to maximize the value of its drugs as they advance in the
clinic.
Cancer
The human
body is composed of trillions of cells that constantly grow, divide, and die.
For the most part, the cells in the body are healthy and perform their vital
functions. However, when healthy cells do not perform properly, they typically
self-destruct and are replaced. Cancer cells reproduce uncontrollably,
regardless of their abnormalities. While the exact mechanism of transformation
that causes normal cells to become cancerous remains unknown, cancer cells are
believed to develop due to mutations caused by changed or damaged DNA. Instead
of dying when they should, the abnormal cells constantly grow and divide,
producing new cells that are not needed by the body.
Once
cancerous cells are established, they may rapidly invade surrounding tissues. As
cancer cells grow, they require sufficient nutrition, which is initially
acquired by feeding off of the body’s systems in a parasitic manner. In order
for a tumor to continue to grow beyond the size of roughly a pinhead, its cancer
cells recruit blood vessels through a process called tumor angiogenesis. This development
enables cancer cells to achieve self-sustainable growth by acquiring their own
blood supply, which further fuels the spread of tumors.
Cancer is
the second most common cause of death in the U.S. In 2009, the American Cancer
Society (ACS) estimated that roughly 1,500 individuals succumb to cancer daily,
totaling more than 560,000 cancer-related deaths annually (Source: the ACS’s
Cancer Facts & Figures
2009). The disease also impacts countries on an economic level. In 2009,
the U.S. National Institutes of Health approximated the overall cost of cancer
in 2008 to be $228.1 billion, encompassing $93.2 billion for direct medical
costs and $134.9 billion for lost productivity due to illness or premature
death.
Traditional
chemotherapy involves treating patients with cytotoxins, which are compounds or
agents that are toxic to cells. In the early stages of cancer, chemotherapy may
be combined with surgery or radiation to improve the efficacy of a treatment
regimen. In the later stages of the disease—after the cancer has spread to
another region of the body—chemotherapy is often the only treatment option for
many forms of cancer. However, traditional chemotherapies have several prominent
disadvantages:
|
(1)
|
they
affect both healthy and cancer cells indiscriminately, causing negative
side effects;
|
|
(2)
|
they
exert their toxic effect when cells divide, which can be ineffective in
tumors with cells that divide at a slower rate than cells in normal
tissues; and
|
|
(3)
|
cancer
cells may develop a drug
resistance after repeated exposure to current chemotherapies, thus
limiting the number of times a therapy can be used
effectively.
|
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
3
|
 |
GenSpera’s
Prodrug Chemotherapy
Researchers
are investigating prodrug chemotherapy as a technique to deliver higher
concentrations of cytotoxic agents to tumors while avoiding the toxicity to
healthy tissues of the body. Prodrug technology entails administering an
inactive form of a cytotoxin, called a prodrug, to a patient. The prodrug is
designed to be activated only through a conversion that occurs exclusively at a
tumor site. If successfully developed, GenSpera believes that prodrug therapies
could provide an effective therapeutic approach for a broad range of solid
tumors caused by breast, prostate, lung, colon, and other cancers. The Company’s
prodrug technology involves attaching a targeting/masking agent to an
active drug, temporarily making the drug both soluble for intravenous
administration and inactive in the bloodstream. Once the compound reaches its
target, the masking agent is removed by an enzyme found at the tumor location.
With the agent detached, the drug is reactivated and becomes insoluble,
precipitating directly into nearby cancer cells. The cancer is killed due to the
toxic effects of the activated drug.
GenSpera
uses a two-tiered strategy to target its medicine specifically to tumor sites.
The Company identifies select enzymes (proteases) that are found at
higher levels in tumors relative to other tissues in the body. Upon identifying
these enzymes, GenSpera creates peptides that are recognized
predominantly by those enzymes in the tumor and not by enzymes in normal
tissues. Because enzyme recognition is required to remove the selected peptide
(the targeting/masking agent) and activate the cytotoxin, this aspect seeks to
ensure that the compound does not cause toxicity in areas of the body other than
the targeted site.
GenSpera
uses 12ADT—a chemically modified form of the cytotoxin thapsigargin that kills fast-,
slow-, and non-dividing cells—as the therapeutic element of its prodrugs,
including in its lead candidate, G-202. Thapsigargin is a potent and novel
cytotoxin extracted from the plant Thapsia garganica (T.
garganica), which is 10- to 100-fold more potent than the National Cancer
Institute’s reference chemotherapeutic agents. 12ADT functions by dramatically
raising the level of calcium inside cells, which leads to cell death. The
Company believes that 12ADT addresses several issues prevalent with current
chemotherapies as it does not appear to trigger the development of resistance to
its effects, and it is able to target cells regardless of their rate of
division. GenSpera believes that targeting cell death independently of cell
division is important for three reasons: (1) it allows the drug to be effective
against tumor cells that divide more slowly than normal cells in the body (e.g.,
prostate cancer); (2) the drug can be effective against the very slowly dividing
blood vessel cells within solid tumors; and (3) the drug may also kill cancer stem cells, which in
general are also very slow dividing.
Presently,
the Company is engaged in the development of two prodrug candidates, G-202 and
G-115, as overviewed in Figure 1. GenSpera uses two approaches in its prodrugs:
(1) targeting the blood supply that supports tumor growth; and (2) targeting the
tumor directly. Each of these approaches is briefly summarized following Figure
1 and more fully detailed on page 19.
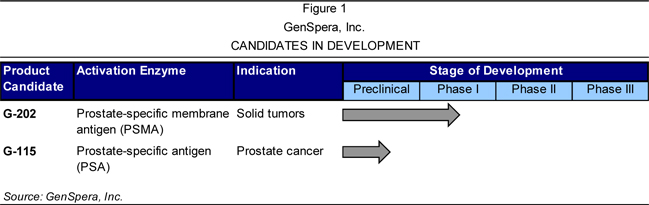
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
4
|
 |
Targeting
the Blood Supply that Supports Tumor Growth
GenSpera
is currently developing its lead prodrug candidate, G-202, which targets the
blood vessels of solid tumors. G-202 is selectively activated within solid
tumors by an enzyme present on the tumor blood vessels, thus destroying the
existing tumor by ceasing its blood supply. The Company believes that this
technique is a dramatic improvement to existing anti-angiogenic drugs, which primarily
stop the growth of new blood vessels.
G-202
combines the cytotoxic activity of 12ADT with a specific peptide that masks its
activity until it is delivered to the target site. The peptide can only be
removed by prostate-specific
membrane antigen (PSMA)—a process referred to as the targeted delivery of
the active drug. PSMA is expressed in non-cancerous and cancerous prostate
tissues, at lower levels in some non-prostate tissues (e.g., kidney), and in the
endothelial cells of
vasculature associated with non-prostate cancers (Source: Journal of Carcinogenesis
2006, 15[5]:21). Because PSMA is expressed in tumor-associated blood vessels,
G-202 and any other PSMA-targeted prodrugs that GenSpera may develop could be
able to attack the blood supplies of many different tumor types.
In
preclinical testing, G-202 was shown to cause tumor regression in animal models
with solid tumors of breast, prostate, bladder, and kidney cancer. G-202’s
demonstrated anti-tumor effects in these cancer types further the belief that
G-202 may have broad application as a therapy for a variety of human solid
tumors due to its ability to selectively target PSMA-producing endothelial cells
within tumors. In these studies, the administration of G-202 caused noticeable
regression of the tumor—in some cases with no visible re-growth for
approximately one month following the last treatment. G-202 was well tolerated
at dose levels that caused regression of tumor growth, with no signs of
toxicity. At the highest doses, transient weight loss was documented, which
quickly recovered after each course of the therapy. The data also indicated that
even after repeated dosing cycles, G-202 did not activate drug resistance in
tumor cells.
GenSpera
believes that its prodrug technology may be able to eliminate cancerous tumors
with dosing that is effective for a significant length of time. Further, the
Company believes that this is more likely to be achieved in humans than in mice
due to the ability to infuse the drug into human
patients and an anticipated longer half-life of G-202 in the
human bloodstream versus that of laboratory animals. Given its efficacy profile,
G-202 could also be effective as a monotherapy, thus reducing the
costs and time required to conduct clinical trials.
Current
Status of G-202
In early
2010, GenSpera initiated a Phase I study with G-202 to evaluate its safety and
its pharmacokinetics in
cancer patients. The open-label, dose-escalation Phase I study could include up
to 30 refractory cancer patients—individuals who have relapsed following
treatment with other chemotherapies—with any type of solid tumor. GenSpera
expects this approach to enhance patient accrual rates and provide the Company
with safety data across a range of cancer types. While the primary endpoints of
the study are to evaluate the safety, tolerability, and pharmacokinetics of the
drug in humans, the design of the trial also allows the collection of efficacy
data.
The trial
is ongoing at two major cancer centers: (1) the Sidney Kimmel Comprehensive
Cancer Center at Johns Hopkins in Baltimore, Maryland; and (2) the University of
Wisconsin Carbone Cancer Center in Madison, Wisconsin. As of June 2010, four
patients had been enrolled in the study. Dependent upon the successful
completion of the Phase I study, GenSpera plans to conduct up to four Phase II
clinical trials over 18 months to determine the therapeutic efficacy of G-202 in
different tumor types. Moreover, GenSpera may seek to establish a strategic
partnership to maximize the value of G-202 as the compound progresses through
future clinical trials.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
5
|
 |
Targeting
the Tumor Directly
Compared
to other cancers, prostate cancer has a large proportion of slowly proliferating
cells that are resistant to treatment with conventional cytotoxic agents.
Current therapies generally attack and destroy rapidly dividing cells, and thus
spare cancer cells that divide slowly—a feature that is characteristic of
prostate cancers. GenSpera’s technology has a broad range of potential
applications, including techniques that pair cytotoxic derivatives of
thapsigargin to peptides in order to target prostate tumors directly. As such,
GenSpera sought to develop a prodrug candidate using this approach that avoided
the negative aspects of current prostate cancer therapies.
GenSpera’s
first approach to this strategy couples thapsigargin to peptides that are
selectively cleaved by a prostate cancer-specific protease called prostate-specific antigen
(PSA), which led to GenSpera’s lead candidate for this technique, G-115.
PSA is active within tumor sites and in normal prostate tissue but is inactive
within the bloodstream—characteristics that form the basis for tumor-specific
delivery of cytotoxic agents. G-115 was selected as the lead development
candidate in the PSA-targeted prodrug program due to its ability to dramatically
inhibit the growth of tumors in animal models of human prostate cancer. GenSpera
aims to obtain Investigational New Drug (IND) approval for G-115 in the third
quarter 2011. Additionally, the Company seeks to establish a strategic
partnership to maximize the value of G-115 as it progresses in the
clinic.
It is
important to note that G-115 and G-202 are non-competing product candidates; the
Company intends to market G-115 to urologists and market G-202 to medical
oncologists.
Corporate
Information
Incorporated
in Delaware in 2003, GenSpera was founded as part of a business development
initiative based on technology and IP owned by Johns Hopkins. In early 2004, the
IP underlying the Company’s technologies was assigned from Johns Hopkins to its
co-inventors—Drs. John T. Isaacs, Samuel R. Denmeade, Soren Brogger Christensen,
and Hans Lilja—who in turn awarded an option to license the IP to the Company in
return for continued protection of the patent portfolio. GenSpera exercised this
option in early 2008 by reimbursing Johns Hopkins for previous patent
prosecution costs. The co-inventors, who now comprise the Company’s Scientific
Advisory Board, assigned the IP to the Company in April 2008. GenSpera’s
activities between 2004 and 2007 were limited to continued prosecution of the
relevant patent portfolio.
After
filing its Form 10-K in March 2010 without a “going concern” statement and
raising $2.7 million in gross proceeds in May 2010, GenSpera believes that its
ability to continue supporting its development initiatives financially
differentiates the Company from many other biotechnology entities.
Presently,
GenSpera’s Common Stock trades on the Over-the-Counter Bulletin Board (OTC.BB)
under the ticker symbol “GNSZ.”
Headquarters
and Employees
GenSpera’s
headquarters are located in San Antonio, Texas, where the Company leases a
roughly 850-square foot facility. GenSpera employs two full-time individuals who
serve as the Company’s executive officers.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
6
|
 |
Growth
Strategy
Leveraging
the management team’s expertise for identifying promising treatments and
bringing them into the clinic, GenSpera’s business strategy entails developing
therapeutics based on the Company’s target-activated prodrug technology
platform. The Company plans to advance these candidates through Phase I/II
clinical trials and subsequently license the rights for further development to
major pharmaceutical companies. While the Company has identified four prodrug
candidates—G-202, G-114, G-115, and Ac-GKAFRR-L12ADT (as overviewed on pages
23-32)—GenSpera is presently focused on advancing G-202 and G-115. The Company
will likely commence the development of the remaining candidates when sufficient
resources are available. GenSpera is focused on minimizing its infrastructure in
order to keep its burn rate as low as possible as it develops its lead
molecules.
Manufacturing
and Development
Under the
direction of key personnel, GenSpera plans to outsource its preclinical
development (e.g., toxicology), manufacturing, and clinical development
initiatives to contract research organizations (CROs) and contract manufacturing
organizations (CMOs). Commonly engaged in the pharmaceutical and biotechnology
industries, CROs and CMOs are third parties that act on behalf of their clients
and specialize in the execution of project-oriented research activities and
processes. The Company intends to maintain Good Laboratory Practice (GLP)
and Good Manufacturing Practice
(GMP) standards by outsourcing its activities to organizations with
approved facilities and manufacturing practices.
Commercialization
After
developing candidates through Phase I/II clinical trials, the Company intends to
license its therapies to third parties for further clinical development and
commercialization. Table 1 highlights several recent examples of agreements for
the license or sale of various clinical-stage cancer therapies. GenSpera
believes that G-202 offers an even greater value for potential partners because
it may address a broad range of solid tumors.
Table
1
VALUE
CREATION: PRIOR EXAMPLES OF LICENSES OR SALES OF CLINICAL-STAGE CANCER DRUGS
|
Acquirer/Licenser
|
Pharmaceutical Application
|
|||||||
|
Year
|
from drug developer
|
Clinical Status
|
Benefits to Drug Developer
|
|||||
|
2007
|
GlaxoSmithKline plc
|
·
|
Metastatic melanoma drug
|
·
|
Upfront cash payment of $80M
|
|||
|
|
from Synta Pharmaceuticals Corp.
|
·
|
Phase II
|
·
|
Up to $300M at commercial milestones and
|
|||
|
up to $585M at other identified milestones
|
||||||||
|
Astellas Pharma Inc.
|
·
|
Prostate cancer drug
|
·
|
Upfront payment of $110M
|
||||
|
from Medivation, Inc.
|
·
|
Phase III
|
·
|
Up to $655M in milestone payments
|
||||
|
sanofi-aventis SA
|
·
|
Two inhibitors of cancer proteins
|
·
|
Upfront cash payment and potential
|
||||
|
from Exelixis Inc.
|
·
|
Phase I
|
development and regulatory milestone
|
|||||
|
2009
|
payments that could exceed $1B
|
|||||||
|
sanofi-aventis SA
|
·
|
An inhibitor of cancer protein
|
·
|
Acquisition of company for up to $500M,
|
||||
|
from BiPar Sciences, Inc.
|
·
|
Phase II
|
divided into varying milestone payments
|
|||||
|
Johnson & Johnson
|
·
|
Prostate cancer drug
|
·
|
Acquisition of company for nearly $1B
|
||||
|
from Cougar Biotechnology, Inc.
|
·
|
Phase III
|
||||||
|
2010
|
Novartis AG
|
·
|
Several cancer protein inhibitors
|
·
|
Upfront payment of $45M and up to $422M
|
|||
|
|
from Array BioPharma Inc.
|
·
|
Phase I
|
for development and regulatory milestones
|
||||
Sources:
GenSpera, Inc. and Crystal Research Associates, LLC.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
7
|
 |
Research
Programs and Grants
In
addition to developing second-generation approaches to its current programs,
GenSpera intends to continue the characterization of its lead
molecules in the laboratories of Drs. Isaacs and Denmeade at the Sidney Kimmel
Comprehensive Cancer Center at Johns Hopkins. To date, the development of
GenSpera’s technology platform has been supported by approximately $10 million
in scientific grants for research performed in Dr. Isaacs’ and Dr. Denmeade’s
laboratories at the Kimmel Cancer Center. The funding for research at the Kimmel
Cancer Center has been provided by the U.S. National Institutes of Health, the
National Cancer Institute’s Rapid Access to Intervention Development (RAID)
program, the NCI’s Specialized Programs of Research Excellence (SPORE), and the
Prostate Cancer Foundation (formerly CaPCURE), among others. Additional grants
totaling roughly $5 million have supported research in Europe and elsewhere by
Drs. Christensen and Lilja. GenSpera believes that the more than 15 years of
support of its technologies by peer-reviewed funding agencies offers evidence of
the technology platforms’ acceptance and recognized potential by academic and
medical communities.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
8
|
 |
Intellectual
Property
The
intellectual property (IP) supporting GenSpera’s technology was developed over
15 years at Johns Hopkins University and the University of Copenhagen with over
$15 million in scientific grants from the National Cancer Institute, the U.S.
Department of Defense, and the U.S. National Institutes of Health, among others.
GenSpera’s IP portfolio contains seven issued patents and four pending patent
applications (as listed in Table 2), which protect the Company’s core prodrug
platform and ancillary technologies. While GenSpera plans to continue to
prosecute claims included in all four of its U.S. patent applications, the
Company is placing a particular emphasis on protecting the outstanding claims
covered in its core PSMA prodrug patent application. PSMA is a protein that is
over-expressed in prostate cancer and in the tumor-associated vasculature of
several solid tumor types, which is targeted by GenSpera’s lead prodrug
candidate, G-202. The Company also protects its proprietary information through
confidentiality agreements with employees, consultants, sponsored researchers,
and significant scientific collaborators.
GenSpera,
Inc.
INTELLECTUAL
PROPERTY
|
Patents
|
||||||||||
|
Number
|
Country
|
Filing Date
|
Issue Date
|
Exp. Date
|
Title
|
|||||
|
6,265,540
|
U.S.
|
05/19/1998
|
07/24/2001
|
05/18/2018
|
Tissue specific prodrug (PSA)
|
|||||
|
6,410,514
|
U.S.
|
06/07/2000
|
06/25/2002
|
06/06/2020
|
Tissue specific prodrug (PSA)
|
|||||
|
6,504,014
|
U.S.
|
06/07/2000
|
01/07/2003
|
06/06/2020
|
Tissue specific prodrug (thapsigargin)
|
|||||
|
6,545,131
|
U.S.
|
07/28/2000
|
04/08/2003
|
07/27/2020
|
Tissue specific prodrug (thapsigargin)
|
|||||
|
7,053,042
|
U.S.
|
07/28/2000
|
05/30/2006
|
07/27/2020
|
Activation of peptide prodrugs by hK2
|
|||||
|
7,468,354
|
U.S.
|
11/30/2001
|
12/23/2008
|
11/29/2021
|
Tissue specific prodrugs (G-202, PSMA)
|
|||||
|
7,635,682
|
U.S.
|
01/06/2006
|
12/22/2009
|
01/05/2026
|
Tumor activated prodrugs (G-115)
|
|||||
|
Pending Patent Applications
|
||||||||||
|
Number
|
Country
|
Filing Date
|
Issue Date
|
Exp. Date
|
Title
|
|||||
|
US 2007/0160536
|
U.S.
|
01/06/2006
|
Pending
|
N/A
|
Tumor activated prodrugs (PSA, G-115)
|
|||||
|
US 2008/0247950
|
U.S.
|
03/15/2007
|
Pending
|
N/A
|
Activation of peptide prodrugs by hK2
|
|||||
|
US 2009/0163426
|
U.S.
|
11/25/2008
|
Pending
|
N/A
|
Tissue specific prodrugs (PSMA)
|
|||||
|
US 2010/0120697
|
U.S.
|
11/05/2009
|
Pending
|
N/A
|
Tumor activated prodrugs (G-115)
|
|||||
Sources:
the U.S. Patent and Trademark Office (USPTO), the World Intellectual Property
Organization (WIPO), and GenSpera, Inc.
GenSpera
owns and controls all rights to both G-202 and G-115, the Company’s second
anti-cancer drug in development. In December 2009, the Company was issued its
most recent U.S. patent, entitled “Tumor activated prodrugs” (No. 7,635,682),
which strengthens GenSpera’s IP position for G-115 and its use in prostate
cancer and other prostate conditions (e.g., enlarged prostate).
The
patents and patent applications supporting GenSpera’s technology were initially
owned by Johns Hopkins University and assigned to the Company in April 2008. The
co-inventors of the IP—Drs. Isaacs, Denmeade, Christensen, and Lilja—remain
affiliated with GenSpera as members of its Scientific Advisory Board. While
GenSpera has no further financial obligations to the inventors of the IP or to
Johns Hopkins, the institution retains a fully paid, royalty-free, non-exclusive
license to use the IP for nonprofit purposes.
To
maintain a competitive advantage, GenSpera plans to continue to pursue
protection for its core and ancillary technologies, either independently or with
scientific collaborators or strategic partners. Primarily, GenSpera is focused
on filing patent applications in the U.S. The Company also intends to obtain
licenses or options to acquire licenses to IP that may be useful in furthering
GenSpera’s research, development, and commercialization
initiatives.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
9
|
 |
Company
Leadership
GenSpera’s
leadership possesses biotechnology and pharmaceutical experience, including
identifying and bringing oncology treatments to clinical development. Dr. Craig
A. Dionne, the Company’s chief executive officer, chief financial officer,
president, and chairman, is also a co-founder of the Company, and brings roughly
21 years of experience identifying and investigating clinical oncology
treatments. Supporting Dr. Dionne is Dr. Russell Richerson, who has over 25
years of experience in the biotechnology and diagnostics industries. GenSpera’s
Board of Directors oversees the conduct of and supervises the Company’s key
management. The Company’s Scientific Advisory Board includes Drs. Isaacs and
Denmeade, who are also co-founders of GenSpera. GenSpera’s executive management
and Board of Directors are overviewed in Table 3 followed by detailed
biographies; the Scientific Advisory Board is summarized on pages
11-12.
Table 3
GenSpera,
Inc.
LEADERSHIP
|
Craig
A. Dionne, Ph.D.
|
Chief
Executive Officer, Chief Financial Officer, President, and Chairman of the
Board
|
|
Russell
Richerson, Ph.D.
|
Chief
Operating Officer and Secretary
|
|
John
M. Farah, Jr., Ph.D.
|
Director
|
|
Scott
Ogilvie, J.D.
|
Director
|
Source:
GenSpera, Inc.
Craig
A. Dionne, Ph.D., Chief Executive Officer, Chief Financial Officer, President,
and Chairman of the Board
Dr.
Dionne has roughly 21 years of experience in the pharmaceutical industry, with
increasing levels of responsibility throughout his career. He recently served as
executive vice president, research and therapeutics with the Prostate Cancer
Foundation, a nonprofit foundation that seeks improved treatments for advanced
prostate cancer. This position gave Dr. Dionne a global perspective of potential
advances in this therapeutic arena. Prior to the Prostate Cancer Foundation, he
served five years as vice president in discovery research at Cephalon, Inc.
(CEPH-NASDAQ), where he was responsible for drug discovery programs in the areas
of oncology and neurobiology. His efforts at Cephalon were key in the
identification of four drugs that were brought into clinical evaluation over a
span of six years, including two targeted primarily toward prostate cancer and
one anti-angiogenic agent for solid tumors. Dr. Dionne has extensive experience
in corporate collaborations, having served on joint management teams in various
capacities with eight different corporate partners while with Cephalon. Prior to
joining Cephalon in 1992, he received a doctorate from the University of Texas
in 1984, was trained as a postdoctoral fellow at Dana-Farber Cancer Institute,
and worked as a research fellow at Rhone-Poulenc Rorer Pharmaceuticals, Inc. Dr.
Dionne has had an extensive scientific career as demonstrated by co-inventorship
on six issued U.S. patents as well as co-authorship of over 50 scientific
publications.
Russell
Richerson, Ph.D., Chief Operations Officer and Secretary
Dr.
Richerson has over 25 years of experience in the biotechnology and diagnostic
industries. Dr. Richerson most recently served as vice president of operations
with the Molecular Profiling Institute (a for-profit spinoff of the
Translational Genomics Research Institute) and Ameripath, Inc., and as the chief
operating officer at the International Genomics Consortium (a nonprofit medical
research organization). During Dr. Richerson’s career, he has served as the vice
president of diagnostic research and development with Ventana Medical Systems
(acquired in 2008 by Roche Diagnostics Division, a subsidiary of F. Hoffmann-La
Roche Ltd [ROG-SWX]), Prometheus Laboratories Inc., and CellzDirect, Inc. In
addition, Dr. Richerson spent 11 years at Abbott Laboratories (ABT-NYSE) in
numerous management roles, most recently as director of molecular probes and
director of the AxSym® program.
His career also includes positions at Pandex Laboratories (acquired by Baxter
International Inc. [BAX-NYSE]), E. I. du Pont de Nemours and Company (DD-NYSE),
Coulter Diagnostics, and Boehringer Mannheim Diagnostics. Dr. Richerson holds a
B.S. in medical technology from Louisiana State University and a Ph.D. in
biochemistry from the University of Texas at Austin.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
10
|
 |
John
M. Farah, Jr., Ph.D., Director on the Board of Directors
Dr. Farah
is vice president, intercontinental operations at Cephalon, which he joined in
1992. He is responsible for ensuring corporate support and managing sales
performance of international partners in the Americas and Asia Pacific with
specific growth initiatives for Cephalon in China and Japan. Dr. Farah’s prior
roles included the responsibility to promote and negotiate research and
development and commercial alliances with multinational and regional
pharmaceutical firms, as well as responsibilities in scientific affairs, product
licensing, and academic collaborations. He currently serves on the Board of
Directors of Aeolus Pharmaceuticals Inc. (AOLS-OTC).
Scott
Ogilvie, J.D., Director on the Board of Directors
Mr.
Ogilvie is president of AFIN International, Inc., a private equity/business
advisory firm that he founded in 2006. Prior to December 31, 2009, he was chief
executive officer of Gulf Enterprises International, Ltd, a company that brings
strategic partners, expertise, and investment capital to the Middle East and
North Africa. Mr. Ogilvie previously served as chief operating officer of CIC
Group, Inc., an investment manager. He began his career as a corporate and
securities lawyer with Hill, Farrer & Burrill LLP and has extensive public
and private corporate board experience in finance, real estate, and technology
companies. Mr. Ogilvie currently serves on the Board of Directors of Neuralstem,
Inc. (CUR-NYSE Amex), Innovative Card Technologies, Inc. (INVC-OTC), and
Preferred Voice Inc, (PRFV-OTC).
Scientific
Advisory Board
GenSpera’s
Scientific Advisory Board, listed in Table 4, encompasses leading researchers
who are inventors of the Company’s technology as well as shareholders. The
members of the Company’s Scientific Advisory Board are active in terms of
creating new intellectual property that GenSpera can use as well as in helping
the Company develop its drug candidates through the preclinical
process.
Table 4
GenSpera,
Inc.
SCIENTIFIC
ADVISORY BOARD
|
John
T. Isaacs, Ph.D.
|
Chief
Scientific Advisor, Chairman of the Scientific Advisory Board,
and
|
|
Co-founder
|
|
|
Samuel
R. Denmeade, M.D.
|
Chief
Clinical Advisor and Co-founder
|
|
Soren
Brogger Christensen, Ph.D.
|
Member
|
|
Hans
Lilja, M.D., Ph.D.
|
Member
|
Source:
GenSpera, Inc.
John
T. Isaacs, Ph.D., Chief Scientific Advisor, Chairman of the Scientific Advisory
Board, and Co-founder
Dr.
Isaacs is a professor of oncology at the Kimmel Cancer Center as well as a
professor in the urology department of the James Buchanan Brady Urological
Institute, part of Johns Hopkins School of Medicine in Baltimore, Maryland. He
has had a longstanding interest in the regulation of the growth of both the
normal and abnormal prostate, with a particular interest in developing new
approaches for the treatment of prostate cancer. Dr. Isaacs is the
editor-in-chief of the journal The Prostate and is on the
Editorial Board of Clinical
Cancer Research, Cancer
Research, Endocrine Related Cancer, and Cancer and Metastases Reviews.
From 1990 to 1991, he was president of the Society for Basic Urological
Research. Dr. Isaacs has also been the director of the Cellular and Molecular
Medicine Graduate Program in the Johns Hopkins School of Medicine and has served
on the Experimental Therapeutics Study Section with the U.S. National Institutes
of Health. He has been a director of the Experimental Therapeutics Division and
a co-director of both the Prostate Cancer Program and the Chemical Therapeutics
Program in the Kimmel Cancer Center. He also served on the Scientific Advisory
Board of Cephalon, and has published over 300 scientific
publications.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
11
|
 |
Samuel
R. Denmeade, M.D., Chief Clinical Advisor and Co-founder
Dr.
Denmeade is an associate professor of oncology and pharmacology at the Kimmel
Cancer Center and an associate professor in the Chemical and Biomolecular
Engineering Department at Johns Hopkins. He is also a member of the Anti-Cancer
Drug Development Training Program at Johns Hopkins. Dr. Denmeade’s laboratory
focus is on the development of targeted therapies for the treatment of prostate
cancer. In particular, his laboratory has developed prodrug therapies that are
activated by prostate cancer-specific proteases. Dr. Denmeade has published more
than 40 papers in this area. He receives funding through the National Cancer
Institute’s Prostate Cancer SPORE award and through awards from both the
prostate and breast cancer research programs of the U.S. Department of Defense.
Dr. Denmeade is a Board-certified medical oncologist and a member of the
clinical Genitourinary Oncology Group at Johns Hopkins. He has been the
principal investigator on a number of prostate cancer clinical trials. Dr.
Denmeade is on the Editorial Board of The Prostate and is serving
on the Physical Imaging Study Section for the Department of Defense’s Prostate
Cancer Research Program. He is a consultant for Cephalon and Protox Therapeutics
Inc. (PRX-TSX), and also serves on Protox’s Scientific Advisory
Board.
Soren
Brogger Christensen, Ph.D.
Dr.
Christensen is professor, Department of Medicinal Chemistry, the Faculty of
Pharmaceutical Sciences, University of Copenhagen, Denmark, and is
internationally recognized for his work in the isolation and identification of
natural products with significant biological activities. He has also served as
chairman of the Department of Medicinal Chemistry. He discovered the
antiparasitic effects of licochalcone A and is
co-inventor on several patents in the IP portfolio of LICA Pharmaceuticals A/S,
a closely held Danish biopharmaceutical research and development company. Dr.
Christensen co-founded LICA Pharmaceuticals and formerly served as a member of
its Board of Directors. He was the first to elucidate the chemical structure of
thapsigargin, and his laboratory is actively engaged in exploring further
derivatives of the bioactive compound. Dr. Christensen has co-authored over 130
scientific publications and is co-inventor on several key patents in GenSpera’s
IP portfolio.
Hans
Lilja, M.D., Ph.D.
Dr. Lilja
is attending research clinical chemist at the Department of Clinical
Laboratories with joint appointments at the Departments of Urology and Medicine
(Genitourinary Oncology Services) at Memorial Sloan-Kettering Cancer Center
(MSKCC) in New York. He is also professor (visiting) at the Department of
Laboratory Medicine, Lund University, University Hospital (UMAS), Malmö, Sweden,
where he previously was trained and most recently held a tenured full-time
position as professor and chief physician until he was recruited to MSKCC. Dr.
Lilja is an internationally recognized authority on the biology of PSA and hK2.
He holds several diagnostic patents and is co-inventor for one of the most
widely used commercial PSA assays. Dr. Lilja has won
numerous international awards for his work on PSA assay methods and has
co-authored more than 130 peer-reviewed publications. His continuing research on
prostate cancer biomarkers has expanded to also include additional human
glandular kallikreins, which hold an important place in GenSpera’s pipeline of
prostate cancer therapeutics.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
12
|
 |
Core
Story
GenSpera,
Inc. (“GenSpera” or “the Company”) is a development-stage biotechnology company
focused on the discovery and development of prodrug cancer therapeutics. A
prodrug is an inactive precursor of a drug that is converted into its active
form at a targeted site. The Company believes that, if successfully developed,
prodrug therapies have the potential to provide an effective therapeutic
approach for a broad range of solid tumors.
GenSpera
has proprietary technologies that appear to meet the requirements for an
effective prodrug in animal models. In addition, the cytotoxic agent used in the
Company’s prodrug candidates may address several limitations of current cancer
drugs due to the following key characteristics of GenSpera’s cytotoxic agent:
(1) it kills slow- and non-dividing cancer cells as well as rapidly
proliferating (dividing) cancer cells; (2) it does not appear to trigger tumor
resistance to its effects; and (3) it limits cytotoxicity to healthy cells by
remaining inactive until it reaches the tumor site. GenSpera plans to develop
its prodrug candidates through Phase I/II clinical trials and then license the
experimental drugs to third parties under the assumption that such entities
would continue to develop, market, sell, and distribute the resulting
products.
CANCER
The human
body is composed of trillions of cells that constantly grow, divide, and die.
For the most part, the cells in the body are healthy and perform their vital
functions. When healthy cells do not behave properly, they self-destruct and are
replaced. However, cancer cells differ in this aspect because they reproduce
regardless of their abnormalities. There are over 200 types of cancers, all of
which entail the uncontrolled division and growth of abnormal cells in the body.
Cancers are most often named for the organ or type of cell in which they
originate, despite the fact that cancer cells can eventually spread into other
parts of the body through the bloodstream or lymphatic system.
While the
exact mechanism of transformation that causes normal cells to become cancerous
remains unknown, cancer cells are believed to originally develop as a result of
mutations due to changed or damaged DNA. The abnormal cells continuously grow
and divide, producing new cells that are not needed by the body. Once cancer
cells develop, they are able to rapidly invade surrounding tissues to the extent
that normal processes in the surrounding tissue and organs may be inhibited or
completely stopped. Another difference between cancer cells and healthy cells is
that when touching another cell healthy cells undergo contact inhibition—a natural
process that prevents the cell from multiplying. In contrast, cancer cells
continue to multiply even when in contact with other cells, enabling the disease
to form a mass of tissue called a tumor or growth.
The
Growth and Spread of Cancer
As cancer
cells grow, they require sufficient nutrition, which is initially acquired by
feeding off of the body’s systems in a parasitic manner. However, in order to
grow beyond the size of roughly a pinhead, cancer cells also recruit blood
vessels through a process called tumor angiogenesis. This activity enables
cancer cells to achieve self-sustainable growth by acquiring their own blood
supply, which further promotes the growth and spread of tumors. Figure 2 (page
14) illustrates the lifecycle of cancerous cells, from their origin, through the
recruitment of blood vessels for growth, and to their spread to other parts of
the body.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
13
|
 |
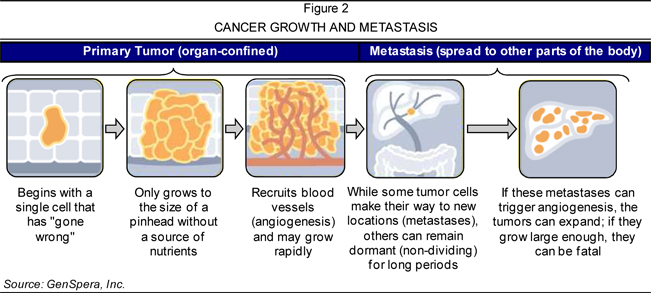
Cancer
spreads, or metastasizes, when cancer
cells break away from the primary tumor site and move to other regions of the
body via the blood or the lymphatic system. When the cells reach a new
destination, they may once again begin to divide and invade the surrounding
area. Ultimately, these cells form a new tumor, called a metastatic tumor, in a
different part of the body. The cancer cells located in the metastatic tumor are
similar to the cells from the original tumor. For example, if the cancer
originated in the breast and subsequently spread to the lungs, the cells located
in the lungs would be considered metastatic breast cancer cells.
While
cancer can be fatal even if it does not metastasize, a significant portion of
cancer-related deaths are caused by tumor metastases. Most common forms of
cancer (e.g., prostate, breast, colon, and lung cancer) develop in organs that
can be completely or partially removed by surgery. Although removing such organs
has negative effects, these procedures could cure patients if the cancer does
not spread. The metastasis of cancer causes many serious consequences,
particularly if it spreads to an essential part of the body (e.g., the brain) or
if excessive cell division in an organ disrupts the body’s natural metabolism
(Source: American Cancer Society [ACS]).
Current
Cancer Treatments
Once
cancer has metastasized, it can either be treated with a single therapy or a
combination of therapies. The type of treatment generally depends on the type of
primary cancer, the patient’s age and health, the size and location of the
metastasis, and the treatments the patient has had in the past. The following
therapies can be used for various purposes, including to control or stop the
growth of cancer or to relieve the symptoms or side effects caused by other
treatment methods.
|
n
|
Chemotherapy. This
technique uses a drug or a combination of drugs to slow or reverse the
spread of cancer. Chemotherapeutics work by targeting rapidly dividing
cells, such as cancer cells. However, fast-growing healthy cells—including
blood cells forming in the bone marrow as well as cells in the digestive
tract, reproductive system (sexual organs), and hair follicles—may also be
affected. Side effects include fatigue, nausea, vomiting, pain, hair loss,
and anemia, among others.
|
|
n
|
Surgery. The Mayo
Foundation for Medical Education and Research considers cancer surgery to
be the foundation of cancer treatment. An operation to repair or remove
part of the body is used for a variety of purposes, including for cancer
prevention, diagnosis, determining the cancer’s progression (stage), to
enhance the efficacy of an alternate therapy, or to relieve symptoms or
side effects. As such, cancer surgery may be employed alone or may be
supplemented with other treatments, such as chemotherapy, radiation,
biological therapy, or hormone
therapy.
|
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
14
|
 |
|
n
|
Radiation Therapy. This
treatment uses high-energy radiation that kills cancer cells and reduces
the size of tumors. Like surgery, radiation therapy is a localized
treatment that only affects cancer cells in the treated area. Radiation
most often comes from a machine (external radiation), but the therapy can
also be administered from a small container of radioactive material
implanted directly into or near the tumor (internal radiation). The
National Cancer Institute estimates that 50% of cancer patients are
treated with radiation therapy, either alone or in combination with other
cancer treatments. Side effects include skin changes, fatigue, diarrhea,
hair loss at the treated area, nausea, vomiting, and swelling, among
others.
|
|
n
|
Biological Therapy.
This type of treatment works with a patient’s immune system to help fight
cancer or control side effects caused by other cancer treatments. While
the exact mechanism that causes biological therapy to be effective is
unknown, researchers speculate that the technique may prevent cancer from
spreading, stop or slow the growth of cancer cells, or enhance the immune
system’s ability to destroy cancer cells. Side effects include fatigue,
fever, chills, gastrointestinal upset, and body aches, among others. While
this type of therapy can come in the form of pills or at-home injections,
other biological treatments must be given intravenously at the hospital or
a clinic.
|
|
n
|
Hormone Therapy.
Depending on the type of cancer, hormones can either help cancer cells to
grow (e.g., prostate cancer and breast cancer), or hormones can kill
cancer cells or slow or stop their growth. As such, hormone therapy may
entail taking medications that interfere with natural hormone activity or
stop the production of hormones. In some cases, surgical removal of the
gland that produces the hormones may be necessary. Side effects of various
hormone treatments include hot flashes, impotence, a loss of desire for
sexual relations, male breast enlargement, nausea, vomiting, vaginal
spotting, irregular menstrual periods, fatigue, skin rash, loss of
appetite or weight gain, and headaches, among
others.
|
|
n
|
Cryosurgery. This is a
non-surgical technique that entails freezing and killing abnormal cells on
the skin or inside the body to treat several conditions, including
non-metastatic liver cancer, cancer that has spread to the liver from
another site, non-metastatic prostate cancer, cancerous and non-cancerous
bone tumors, early-stage skin cancers, and some precancerous conditions.
While still under study to determine its long-term efficacy, cryosurgery
is believed to be less costly than other treatments with fewer side
effects.
|
Traditional,
localized approaches—such as surgery or radiation therapy—can be largely
ineffective for metastatic cancers because the effects of the treatment are
primarily exhibited at the treatment site. In addition, while chemotherapy is
widely used for metastatic disease, this type of therapy causes significant
repercussions. Many traditional chemotherapies target cancer cells by killing
cells during the act of cell division, as cancer cells divide more rapidly than
normal cells of the body. However, this strategy also harms healthy cells of the
body that also rapidly divide, causing hair loss, gastrointestinal distress, and
bone marrow suppression. Additionally, these therapies have little activity
against slow-growing tumors (e.g., prostate cancer) and have virtually no
activity on cancer stem cells—which drive the growth of tumors yet have a slow
proliferation rate. Cancer stem cells that are not eradicated during treatment
can lead to cancer recurrence. As well, many cancers can also become resistant
to conventional chemotherapies. Numerous approaches have attempted to resolve
these drawbacks by targeting the delivery of cancer drugs directly to the tumor
site. Nevertheless, GenSpera believes that these approaches have generally had
limited success due to the factors summarized below.
|
n
|
Conventional
targeted delivery systems may still allow the cancer therapeutic to enter
into the bloodstream, making it difficult to deliver sufficient
concentrations to the tumor and increasing the chances of side effects in
normal tissues.
|
|
n
|
Angiogenesis
inhibitors, which aim to cut off the blood supply to tumors, primarily
prevent new blood vessels from growing. Therefore tumor growth can be
slowed, but not reversed.
|
|
n
|
The
development of resistance to the drug as well as a medicine’s
ineffectiveness against slowly dividing tumor cells continue to be major
obstacles facing drug therapies for
cancer.
|
Roche
Holding AG’s Avastin®, a
widely used angiogenesis inhibitor, primarily stops the growth of new
tumor-related blood vessels. Avastin® is often
used in combination with traditional chemotherapeutic agents, which shrink the
tumors while Avastin® slows
the growth. In lung cancer patients, using Avastin® in
combination with paclitaxel and carboplatin (PC) increased median survival by
roughly two months versus PC therapy alone. Similar results were reported for
Avastin® in
combination with IFL
chemotherapy in colorectal cancer. Despite the limited survival advantage
and its high costs at roughly $4,400 per month—global sales of Avastin® in 2009
were 6.2 billion Swiss francs (approximately $5.8 billion) (Source: BIOWORLD
Today and Roche Holdings AG [www.roche.com]).
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
15
|
 |
While
this may validate the approach of targeting cancer blood vessels, GenSpera
believes that significant improvements can be made to Avastin® and
other existing therapies. For example, when Avastin® is used
in combination with other chemotherapeutic agents, the patient retains all of
the side effects of the original chemotherapy as well as the new side effects of
Avastin®. The
Company’s product candidate, G-202, is being developed as a monotherapy.
Additionally, while Avastin® blocks a
protein to prevent the formation of new tumor vasculature, GenSpera’s prodrug
technology differs in that it kills new and existing tumor vasculature.
Furthermore, G-202 attacks cancer cells independently of cell division and may
therefore be useful in slow- as well as fast-growing tumors. G-202 may also kill
cancer stem cells—cells that have a very low proliferation rate but are largely
responsible for tumor growth. Because of their slow-dividing nature, cancer stem
cells may evade treatment by standard chemotherapeutic agents and are often the
cause of cancer relapse.
Market
Size for Cancer Therapies
Although
significant improvements in cancer diagnosis have been made in recent years and
various therapies have been developed to treat the disease, cancer continues to
be a leading healthcare challenge worldwide. In the U.S., nearly one out of two
men and roughly one out of three women develop cancer in their lifetime (Source:
the ACS’s Cancer Facts &
Figures 2009). In 2009 alone, the ACS estimated that nearly 1.5 million
new cases of cancer would be diagnosed in the U.S. and over 560,000 patients
would die as a result of cancer—over 1,500 people per day—making cancer the
second most common cause of death in the U.S. behind cardiovascular
disease.
With
global sales of $47.7 billion, cancer is considered to be one of the largest,
fastest growing markets in the pharmaceutical sector (Source: Business Insights’
The Cancer Market Outlook to
2014, December 2009). The cancer therapy market encompasses four key
segments: (1) chemotherapy; (2) hormone therapy; (3) target therapy; and (4)
immunotherapy. BBC Research indicated that target therapy presently captures the
largest share of the market with estimated 2008 sales of $22.9 billion. Further,
this segment is expected to triple by 2013, reaching roughly $69.1 billion in
sales (Source: Cancer
Therapies: Technologies and Global Markets). In contrast, the second
largest category, chemotherapy, was expected to reach approximately $14.3
billion in 2008. Figure 3 portrays the estimated values of each the four key
segments in the global cancer therapy market from 2006 to 2013.
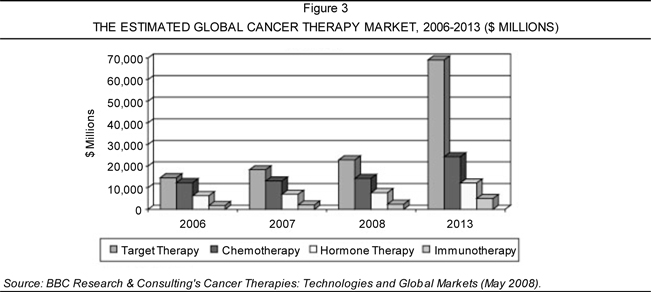
Table 5
(page 17) from the ACS estimates the new cases and related deaths in 2010 for a
variety of cancers common in the U.S. GenSpera believes that its prodrug
therapy, if successfully developed, has the potential to treat a broad range of
solid tumors. The highlighted sections in Table 5 summarize the potential U.S.
patient populations that may be amenable to the Company’s prodrug therapies,
representing potential target markets.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
16
|
 |
Table 5
ESTIMATED
NEW CANCER CASES AND DEATHS BY SEX, U.S., 2010*
|
Estimated New Cases
|
Estimated Deaths
|
|||||||||||||||||||||||
|
Both Sexes
|
Male
|
Female
|
Both Sexes
|
Male
|
Female
|
|||||||||||||||||||
|
All
Sites
|
1,529,560 | 789,620 | 739,940 | 569,490 | 299,200 | 270,290 | ||||||||||||||||||
|
Oral
cavity & pharynx
|
36,540 | 25,420 | 11,120 | 7,880 | 5,430 | 2,450 | ||||||||||||||||||
|
Tongue
|
10,990 | 7,690 | 3,300 | 1,990 | 1,300 | 690 | ||||||||||||||||||
|
Mouth
|
10,840 | 6,430 | 4,410 | 1,830 | 1,140 | 690 | ||||||||||||||||||
|
Pharynx
|
12,660 | 9,880 | 2,780 | 2,410 | 1,730 | 680 | ||||||||||||||||||
|
Other
oral cavity
|
2,050 | 1,420 | 630 | 1,650 | 1,260 | 390 | ||||||||||||||||||
|
Digestive
system
|
274,330 | 148,540 | 125,790 | 139,580 | 79,010 | 60,570 | ||||||||||||||||||
|
Esophagus
|
16,640 | 13,130 | 3,510 | 14,500 | 11,650 | 2,850 | ||||||||||||||||||
|
Stomach
|
21,000 | 12,730 | 8,270 | 10,570 | 6,350 | 4,220 | ||||||||||||||||||
|
Small
intestine
|
6,960 | 3,680 | 3,280 | 1,100 | 610 | 490 | ||||||||||||||||||
|
Colon†
|
102,900 | 49,470 | 53,430 | 51,370 | 26,580 | 24,790 | ||||||||||||||||||
|
Rectum
|
39,670 | 22,620 | 17,050 | |||||||||||||||||||||
|
Anus,
anal canal, & anorectum
|
5,260 | 2,000 | 3,260 | 720 | 280 | 440 | ||||||||||||||||||
|
Liver
& intrahepatic bile duct
|
24,120 | 17,430 | 6,690 | 18,910 | 12,720 | 6,190 | ||||||||||||||||||
|
Gallbladder
& other biliary
|
9,760 | 4,450 | 5,310 | 3,320 | 1,240 | 2,080 | ||||||||||||||||||
|
Pancreas
|
43,140 | 21,370 | 21,770 | 36,800 | 18,770 | 18,030 | ||||||||||||||||||
|
Other
digestive organs
|
4,880 | 1,660 | 3,220 | 2,290 | 810 | 1,480 | ||||||||||||||||||
|
Respiratory
system
|
240,610 | 130,600 | 110,010 | 161,670 | 89,550 | 72,120 | ||||||||||||||||||
|
Larynx
|
12,720 | 10,110 | 2,610 | 3,600 | 2,870 | 730 | ||||||||||||||||||
|
Lung
& bronchus
|
222,520 | 116,750 | 105,770 | 157,300 | 86,220 | 71,080 | ||||||||||||||||||
|
Other
respiratory organs
|
5,370 | 3,740 | 1,630 | 770 | 460 | 310 | ||||||||||||||||||
|
Bones
& joints
|
2,650 | 1,530 | 1,120 | 1,460 | 830 | 630 | ||||||||||||||||||
|
Soft
tissue (including heart)
|
10,520 | 5,680 | 4,840 | 3,920 | 2,020 | 1,900 | ||||||||||||||||||
|
Skin
(excluding basal & squamous)
|
74,010 | 42,610 | 31,400 | 11,790 | 7,910 | 3,880 | ||||||||||||||||||
|
Melanoma-skin
|
68,130 | 38,870 | 29,260 | 8,700 | 5,670 | 3,030 | ||||||||||||||||||
|
Other
nonepithelial skin
|
5,880 | 3,740 | 2,140 | 3,090 | 2,240 | 850 | ||||||||||||||||||
|
Breast
|
209,060 | 1,970 | 207,090 | 40,230 | 390 | 39,840 | ||||||||||||||||||
|
Genital
system
|
311,210 | 227,460 | 83,750 | 60,420 | 32,710 | 27,710 | ||||||||||||||||||
|
Uterine
cervix
|
12,200 | 12,200 | 4,210 | 4,210 | ||||||||||||||||||||
|
Uterine
corpus
|
43,470 | 43,470 | 7,950 | 7,950 | ||||||||||||||||||||
|
Ovary
|
21,880 | 21,880 | 13,850 | 13,850 | ||||||||||||||||||||
|
Vulva
|
3,900 | 3,900 | 920 | 920 | ||||||||||||||||||||
|
Vagina
& other genital, female
|
2,300 | 2,300 | 780 | 780 | ||||||||||||||||||||
|
Prostate
|
217,730 | 217,730 | 32,050 | 32,050 | ||||||||||||||||||||
|
Testis
|
8,480 | 8,480 | 350 | 350 | ||||||||||||||||||||
|
Penis
& other genital, male
|
1,250 | 1,250 | 310 | 310 | ||||||||||||||||||||
|
Urinary
system
|
131,260 | 89,620 | 41,640 | 28,550 | 19,110 | 9,440 | ||||||||||||||||||
|
Urinary
bladder
|
70,530 | 52,760 | 17,770 | 14,680 | 10,410 | 4,270 | ||||||||||||||||||
|
Kidney
& renal pelvis
|
58,240 | 35,370 | 22,870 | 13,040 | 8,210 | 4,830 | ||||||||||||||||||
|
Ureter
& other urinary organs
|
2,490 | 1,490 | 1,000 | 830 | 490 | 340 | ||||||||||||||||||
|
Eye
& orbit
|
2,480 | 1,240 | 1,240 | 230 | 120 | 110 | ||||||||||||||||||
|
Brain
& other nervous system
|
22,020 | 11,980 | 10,040 | 13,140 | 7,420 | 5,720 | ||||||||||||||||||
|
Endocrine
system
|
46,930 | 11,890 | 35,040 | 2,570 | 1,140 | 1,430 | ||||||||||||||||||
|
Thyroid
|
44,670 | 10,740 | 33,930 | 1,690 | 730 | 960 | ||||||||||||||||||
|
Other
endocrine
|
2,260 | 1,150 | 1,110 | 880 | 410 | 470 | ||||||||||||||||||
|
Lymphoma
|
74,030 | 40,050 | 33,980 | 21,530 | 11,450 | 10,080 | ||||||||||||||||||
|
Hodgkin
lymphoma
|
8,490 | 4,670 | 3,820 | 1,320 | 740 | 580 | ||||||||||||||||||
|
Non-Hodgkin
lymphoma
|
65,540 | 35,380 | 30,160 | 20,210 | 10,710 | 9,500 | ||||||||||||||||||
|
Myeloma
|
20,180 | 11,170 | 9,010 | 10,650 | 5,760 | 4,890 | ||||||||||||||||||
|
Leukemia
|
43,050 | 24,690 | 18,360 | 21,840 | 12,660 | 9,180 | ||||||||||||||||||
|
Acute
lymphocytic leukemia
|
5,330 | 3,150 | 2,180 | 1,420 | 790 | 630 | ||||||||||||||||||
|
Chronic
lymphocytic leukemia
|
14,990 | 8,870 | 6,120 | 4,390 | 2,650 | 1,740 | ||||||||||||||||||
|
Acute
myeloid leukemia
|
12,330 | 6,590 | 5,740 | 8,950 | 5,280 | 3,670 | ||||||||||||||||||
|
Chronic
myeloid leukemia
|
4,870 | 2,800 | 2,070 | 440 | 190 | 250 | ||||||||||||||||||
|
Other
leukemia‡
|
5,530 | 3,280 | 2,250 | 6,640 | 3,750 | 2,890 | ||||||||||||||||||
|
Other
& unspecified primary sites‡
|
30,680 | 15,170 | 15,510 | 44,030 | 23,690 | 20,340 | ||||||||||||||||||
*
Rounded to the nearest 10; estimated new cases exclude basal and squamous cell
skin cancers and in situ carcinomas except urinary bladder. About 54,010 female
carcinoma in situ of the breast and 46,770 melanoma in situ will be newly
diagnosed in 2010.
†
Estimated deaths for colon and rectum cancers are combined.
‡ More
deaths than cases may reflect lack of specificity in recording underlying cause
of death on death certificates or an undercount in the case
estimate.
Note:
Estimated new cases are based on 1995-2006 incidence rates from 44 states and
the District of Columbia as reported by the North American Association of
Central Cancer Registries, representing about 89% of the U.S. population.
Estimated deaths are based on data from U.S. Mortality Data, 1969 to 2007,
National Center for Health Statistics, CDC, 2010.
|
Source: American Cancer Society, Inc., Surveillance and Health Policy Research, 2010.
|
Potential Target Markets for GenSpera
|
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
17
|
 |
GENSPERA’S
PRODRUG TECHNOLOGY
Prodrug
chemotherapy is a relatively new approach to treat cancer that is being
investigated as a means to deliver higher concentrations of cytotoxic agents to
tumors while avoiding the toxicity of these high doses in other areas of the
body. In prodrug chemotherapy, the patient is administered the inactive form of
a cytotoxin—called a “prodrug”—which converts into its active form only at the
targeted tumor site. GenSpera aims to develop a prodrug chemotherapy that
improves upon the weaknesses of current therapies and evades the obstacles
listed on page 15, particularly for metastatic cancers. As overviewed on page
19, GenSpera’s prodrugs target cancer cells in one of two ways: (1) by targeting
tumor-associated blood vessels; or (2) by targeting tumors
directly.
The
Company’s lead prodrug candidate, G-202, is designed to attack existing tumor
vasculature, potentially debilitating the tumor’s nutrient supply and causing
cancer regression without affecting healthy tissues within the body. GenSpera
believes that it has validated G-202 as a drug candidate to treat various forms
of solid tumors—including breast, bladder, kidney, and prostate cancers—based on
its ability to cause tumor regression in animal models of these diseases.
GenSpera is conducting Phase I clinical testing of G-202 at two major cancer
centers: (1) the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; and
(2) the University of Wisconsin Carbone Cancer Center.
GenSpera’s
technology can also be used to attack cancer cells directly by targeting the
prodrug to enzymes believed to be found solely at a tumor location. The
Company’s primary candidate using this approach, G-115, targets
prostate-specific antigen (PSA), a protein produced by the cells of the prostate
gland that is used by physicians as a “tumor marker” to detect the presence of
prostate cancer.
GenSpera
believes that its prodrugs may minimize toxicity to other areas of the body
because it is converted into its active cytotoxic form by specific enzymes that
are over-expressed at cancer sites. In addition to minimizing side effects in
healthy tissues, the prodrugs are designed to have greater anti-tumor efficacy
based upon the novel mode of action of the Company’s proprietary cytotoxic
agent. GenSpera’s prodrug technology is fully detailed below and on pages 19-23
of the Core Story. The Company believes that its prodrug therapy, if
successfully developed, has the potential to treat a broad range of solid
tumors.
Prodrugs
are often developed by adding an appendage to the original parent drug molecule
in order to alter its physicochemical properties and
enhance its ability to be delivered. Thus, prodrugs are created to overcome the
barrier(s) to utility found in the parent drug molecule. There are several
common barriers to drug delivery, including lack of site specificity, which
often causes negative side effects in patients. The process of transforming
GenSpera’s prodrugs—from the original cytotoxin thapsigargin to its derivative
12ADT to its final prodrug format—is depicted in Figure 4.
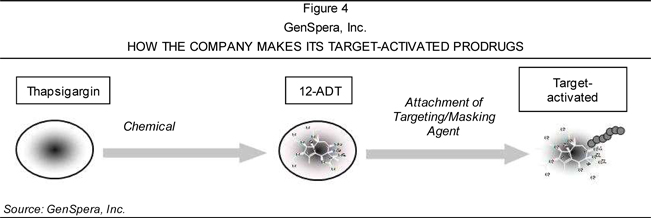
GenSpera
is working to design prodrugs that deliver the parent drug through the systemic
circulation to the desired site of action in a safe, effective, and efficient
manner. The Company seeks to identify specific enzymes that are found at high
levels in tumors relative to other tissues in the body. Upon identifying these
enzymes, GenSpera creates peptides that are recognized predominantly by those
enzymes in the tumor and not by enzymes in normal tissues. This double layer of
recognition adds to the tumor-targeting benefits found in the Company’s
prodrugs. GenSpera leverages two separate approaches as platforms to develop its
prodrug candidates, each of which overviewed below.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
18
|
 |
|
(1)
|
Targeting the Tumor’s Blood
Supply. For this technique, the Company has designed a prodrug
candidate that includes a peptide to temporarily mask the activity of the
cytotoxin while also helping to target the cytotoxin to the site of the
tumor. The peptide, which also facilitates intravenous delivery of the
prodrug, can only be removed by an enzyme that is commonly found in blood
vessels supporting solid tumors. Once the peptide is removed, the
cytotoxin is released into the cells of the tumor’s blood vessels, causing
the network to collapse. Once the tumor’s nutrient source has been
destroyed, the tumor cells begin to starve, ultimately leading to their
death. This platform is applied to G-202, GenSpera’s lead prodrug
candidate.
|
|
(2)
|
Targeting the Tumor Cells
Directly. This method involves selecting peptides for prodrugs that
can be removed by enzymes found in tumors. Once the peptide is removed,
the active drug precipitates directly into nearby cancer cells. The drug’s
cytotoxicity causes the death of cancer cells within the tumor. Using this
approach, GenSpera has developed two prodrug candidates for prostate
cancer—including G-115, its lead development candidate for this
platform—with encouraging results in animal studies thus far. The Company
also intends to target other forms of cancer via this platform. GenSpera
chose G-115 as its lead development candidate in the PSA-targeted prodrug
program due to its enhanced PSA-substrate and in vivo anti-tumor
activity, as well as its broader intellectual property (IP)
coverage.
|
GenSpera’s
target-activated prodrug technology platforms may enable the Company to address
a wide range of solid tumors, as shown in Figure 5. GenSpera has also proposed
several alternate applications for its technology, including serving as an aid
in diagnostic imaging or alleviating symptoms associated with benign prostatic hypertrophy
(BPH).
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
19
|
 |
The
Potential of GenSpera’s Prodrug Therapies
Numerous
cancer treatments (e.g., chemotherapy) use cytotoxins—compounds or agents that
are toxic to cells—to kill cancer cells. Cytotoxins can have direct destructive
effects on specific cells in the body. However, current chemotherapies for
cancer patients entail the use of compounds that are toxic to both cancer and
healthy cells. In the early stages of cancer, chemotherapy can be combined with
surgery or radiation, but in the later stages of the disease, it is the
preferred or only treatment option for many patients. However, the Company
believes that there are several major drawbacks of chemotherapy, as listed
below.
|
n
|
Side Effects. Currently
available cytotoxic agents cause toxicity to both cancer cells and
non-cancer cells in the body, often leading to serious side effects. In
particular, rapidly dividing cells—such as those from hair follicles or
bone marrow—often suffer significant damage from chemotherapy, potentially
leading to hair loss and impaired immune systems, among other side
effects.
|
|
n
|
Incomplete Tumor Kill.
Many leading chemotherapies act by preventing further division of cancer
cells. Once a cytotoxin is administered to a cancer patient, the
fast-dividing cancer cells are often destroyed at a more rapid rate than
slower-dividing, non-cancerous cells. As such, efficacy is generally lower
for tumors containing cells that divide
slowly.
|
|
n
|
Resistance. After
repeated exposure to chemotherapy drugs, cancer cells often develop a
resistance to the agent, thus limiting the number of times that a
treatment can be administered
effectively.
|
Conversely,
prodrug chemotherapy is being investigated as a means to deliver higher
concentrations of the cytotoxic agents to the tumor location, while avoiding the
toxicity of these higher doses in the rest of the body. Prodrug therapy entails
administering the cytotoxin to the patient in an inactive form, which is
converted into the active cytotoxin at the tumor site. GenSpera believes that,
if successfully developed, prodrug therapies have the potential to be effective
treatments for a broad range of solid tumors. In animal models, the Company’s
proprietary technologies appear to meet the requirements for an effective
prodrug, as further detailed on pages 25-29 under “Anti-tumor Efficacy Studies
of G-202.” GenSpera anticipates that its cytotoxin addresses two additional
issues prevalent with current cancer therapeutics: (1) it kills both slowly and
non-dividing cancer cells as well as rapidly dividing cancer cells; and (2) it
does not appear to prompt drug resistance in cancer cells.
How
GenSpera’s Prodrugs Work
GenSpera’s
prodrugs employ a novel process to prevent its cytotoxin from harming healthy,
non-cancerous cells in the body, whether rapidly or slowly dividing. The
Company’s technology supports the creation of prodrugs by attaching
targeting/masking agents to a cytotoxin in a manner that allows conversion of
the prodrug to its active form only at a tumor site. GenSpera has chemically
modified a potent cytotoxin called thapsigargin to develop 12ADT, a derivative
of thapsigargin that is also capable of killing fast-, slow-, and non-dividing
cells indiscriminately.
The
Company believes that targeting cell death independently of cell division is
important for three reasons:
|
(1)
|
it
allows the drug to be effective against tumor cells that divide more
slowly than normal cells in the body (e.g., prostate
cancer);
|
|
(2)
|
the
drug can be effective against the slow-dividing blood vessel cells within
solid tumors; and
|
|
(3)
|
the
drug may also kill cancer stem cells, which in general also divide
slowly.
|
A
simplified depiction of GenSpera’s therapeutic process is provided in Figure 6
(page 21), followed by details of thapsigargin, 12ADT, and the targeting/masking
agents.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
20
|
 |
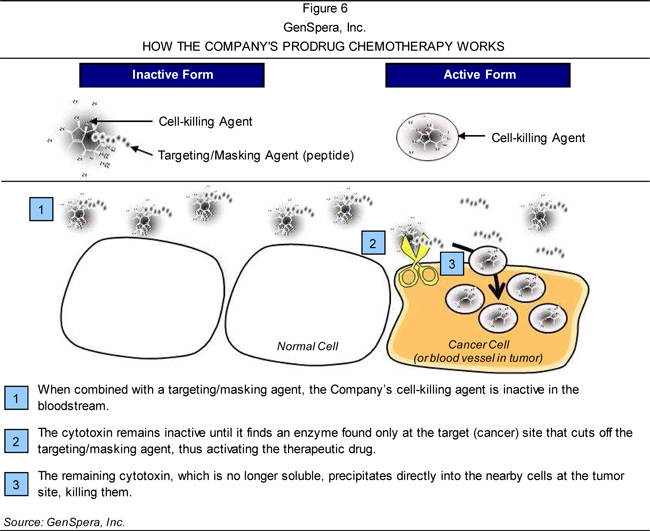
Thapsigargin
Thapsigargin
is a highly potent compound extracted from the Mediterranean plant Thapsia garganica (T.
garganica). Thapsigargin is a well-characterized and studied compound
that, unlike other cytotoxic agents, has the ability to kill slow and
non-dividing cells in addition to the rapidly dividing cells that are typically
associated with cancer. It is important to note that thapsigargin induces apoptosis, or programmed cell
death, which is a type of non-inflammatory cell death that also occurs during
normal tissue development. Thapsigargin elicits cell death by blocking the SERCA pump, which works to
maintain a low level of calcium within cells. Once the SERCA pump is blocked,
the concentration of cytosolic calcium increases
rapidly, leading to the death of the treated cells via apoptosis. Because the
SERCA pump is expressed in the majority of tissues in the body, it is critical
for the delivery mechanism to only release the active drug at the cancer sites
or tumor vasculature within the patient.
While
T. garganica is
relatively common in the wild, to the Company’s knowledge, Thapsibiza S.L. is
the only commercial supplier of T. garganica seeds, which are
harvested from the coastal regions of the Mediterranean Sea. In April 2007,
GenSpera obtained the proper permits from the U.S. Department of Agriculture
(USDA) for the import of T.
garganica seeds into the U.S. and, in January 2008, entered into a
five-year sole-source agreement with Thapsibiza. Per the material terms of the
agreement, the Company is permitted to seek additional suppliers to supplement
its supply from Thapsibiza. However, Thapsibiza is expected to exclusively
provide T. garganica
seeds to GenSpera at the current price of €300 per kilogram (kg). Thapsibiza can
increase the price without notice to compensate for increased governmental
taxes. As long as GenSpera continues to develop drugs derived from thapsigargin,
the Company is required to purchase a minimum of 50 kg of T. garganica seeds per harvest period
year.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
21
|
 |
Thapsigargin Production
Technology Research
GenSpera’s
research for G-202 is covered by patents and supported by laboratories at Johns
Hopkins and the University of Copenhagen via academic grants awarded to the
Company’s collaborators. The University of Copenhagen has received a $4.4
million grant from the Danish Research Council for Strategic Research to develop
a metabolically engineered moss strain as a sustainable production platform for
plant products. GenSpera is contributing a total of $100,000 to the grant.
Production of thapsigargin has been selected as a pilot project called
Sustainable Production of Thapsigargin using Light (SPOTLight). Dr. Craig A.
Dionne, GenSpera’s chief executive officer, chief financial officer, president,
and chairman, serves on the Advisory Board for SPOTLight. A positive outcome of
GenSpera’s trials with a prodrug of thapsigargin (e.g., G-202) could create
significant demand for thapsigargin. SPOTLight is focused on meeting this demand
by developing a sustainable platform thapsigargin supply using only carbon
dioxide, water, and sunlight with easily cultivated moss cells.
The National Cancer
Institute’s Evaluation of Thapsigargin’s Effects
Thapsigargin
has been included on the National Cancer Institute’s Developmental Therapeutics
Program, an anticancer compound screening program designed to identify novel
chemical leads and biological mechanisms. The National Cancer Institute’s
experiments demonstrated that thapsigargin possesses broad growth inhibitory
activity and is 10 to 100 times more potent than Bristol-Myers Squibb Co.’s
(BMY-NYSE) Taxol® (paclitaxel) or doxorubicin, which served as
the reference cytotoxic agents. A similar experiment published in the Journal of the National Cancer
Institute in 2003 evaluated a broader panel of prostate cancer cell lines
and other non-cancer cell types than the previous screening. Equivalent results
were achieved, indicating that thapsigargin killed cells non-selectively,
regardless of whether the test cells were normal, malignant, or of prostatic
origin. Further, the data also showed that thapsigargin effectively killed both
rapidly and very slowly dividing cells in contrast to Taxol® or
doxorubicin, which diminished in efficacy when tested against these slower
dividing cells. Figure 7 illustrates the ability of each of the different drugs
to achieve apoptosis in high- or low-proliferation cell cultures that were
exposed to the product for 48 hours.
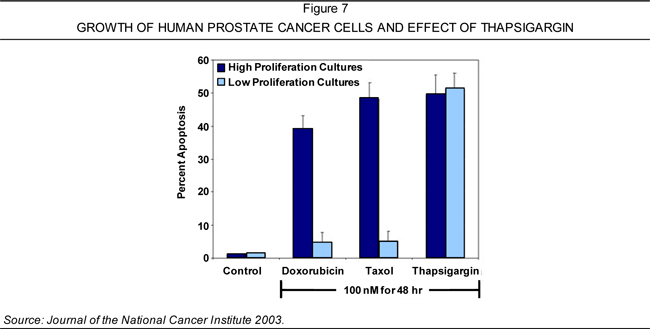
Creating
Prodrugs from Thapsigargin
In the
past, thapsigargin has not been used as a cancer drug because its natural form
is toxic to healthy body tissues in addition to cancer cells and tumors. Over
the past 15 years, the members of GenSpera’s Scientific Advisory Board have
focused on targeting the active drug specifically to the tumor in order to
reduce or eliminate the damage to healthy cells and tissues. More specifically,
the Company has investigated ways to attach masking agents that render
thapsigargin and its analogs temporarily nontoxic, while maintaining the ability
to selectively remove the masking agent at the tumor site, thus activating the
compound exactly where its toxic effects are more effective.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
22
|
 |
Important
factors in the development of GenSpera’s prodrug were determining how to attach
the peptide to thapsigargin and identifying the best location for attachment.
The laboratory of Dr. Samuel R. Denmeade (biography on page 12), one of
GenSpera’s co-founders and currently the chief clinical advisor of the Company’s
Scientific Advisory Board, discovered that GenSpera’s target enzymes—enzymes
found primarily at tumor sites—were unable to remove the final amino acid of peptides
attached directly to thapsigargin. Therefore, the development of a prodrug
required the identification of a chemical derivative of thapsigargin that met
several key requirements:
|
n
|
was
equally as effective as thapsigargin in killing cancer
cells;
|
|
n
|
could
be chemically coupled to the desired peptide;
and
|
|
n
|
was
able to regain its activity once it was exposed to the target enzyme at
the tumor site.
|
12ADT
As
GenSpera’s targeting/masking agents cannot be directly attached to thapsigargin,
the Company created 12ADT, a therapeutically active analog of thapsigargin that
fulfills the key requirements listed above. 12ADT enables GenSpera to attach its
targeting/masking agents to its proprietary compounds and is currently the chief
therapeutic component of GenSpera’s products, including its lead prodrug
candidate G-202. 12ADT functions by dramatically raising the level of calcium
inside cells, which leads to cell death. It is the subject of the Company’s
patent protection, as are the specific peptide sequences that GenSpera attaches
to 12ADT (detailed below under “Targeting/Masking Agents”). GenSpera’s two core
patents—both of which are entitled “Tissue Specific Prodrug”—contain claims that
cover the composition of 12ADT. In addition, while the creation of 12ADT is
based on numerous years of research by members of GenSpera’s Scientific Advisory
Board, the Company does not owe any milestone or royalty payments to third
parties.
Targeting/Masking
Agents
GenSpera
uses peptides to target/mask its agents as they are delivered to the target site
(tumors) within the body. Peptides are short strings of amino acids—the building
blocks of many components found in cells. The Company selects peptides that
specifically target 12ADT to tumors. When attached to 12ADT, GenSpera’s
targeting/masking agent is able to temporarily mask the activity of 12ADT,
essentially making it inactive. The agent also makes the prodrug highly soluble
in blood, making intravenous administration possible. The Company’s technology
capitalizes on the ability of the masking peptides to be removed by chemical
reactors in the body called enzymes as well as the highly specific recognition
of particular peptides by particular enzymes. As such, the peptide can only be
removed by a specific enzyme found at the target (tumor) site. Once the peptide
is removed, 12ADT returns to its naturally active, insoluble state and
precipitates directly into the nearby cells. A set of GenSpera’s patents and
patent applications cover the process by which the Company’s refined and
specific targeting/masking peptides combine with 12ADT.
G-202:
GENSPERA’S LEAD PRODRUG CANDIDATE
G-202 is
a prodrug chemotherapy designed to treat cancer by selectively destroying the
blood supply that supports solid tumors. GenSpera creates G-202 by chemically
modifying thapsigargin to its equally active derivative, 12ADT, which is then
coupled with a specific peptide that masks the activity of the drug until it is
delivered to its target site. G-202’s protective peptide is preferentially
removed by the prostate-specific membrane antigen (PSMA).
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
23
|
 |
PSMA
Expression
PSMA is a
prostate differentiation antigen that is also expressed at minimal levels in a
few specific cell types beyond the prostate. The antigen was first identified as
a surface protein in normal (non-cancerous) prostate epithelium and in a large
percentage of primary and metastatic prostate cancers. For this reason, PSMA is
currently used as a marker for prostate cancer. ProstaScint®, a
product marketed by EUSA Pharma (USA), Inc., is an approved agent that uses
labeled antibodies to seek and bind to PSMA in order to identify both primary
prostate cancer and its metastases.
While
PSMA was originally thought to be specific to the prostate, it is now known to
be expressed in the vasculature of many solid tumors (e.g., breast, colon, lung,
prostate, etc.). As such, PSMA-targeted prodrugs may be able to attack many
different tumor types. As such, GenSpera has chosen PSMA as the activating
enzyme in G-202. To the Company’s knowledge, data to date has suggested that the
expression of PSMA by endothelial cells is confined to tumor vasculature. Based
on this information, the Company does not expect PSMA-targeted therapeutics to
harm blood vessels in any non-cancerous tissues. Therefore, prodrugs that are
PSMA-targeted may have a broader application because they are designed to
specifically attack the blood supply of a large number of different tumor types,
and thus have a clinical profile of a potent angiotoxic agent.
The
Clinical Opportunity: Disrupting Tumor Blood Supply
Angiogenesis—the
physiological process involving the formation of new blood vessels from
pre-existing vessels—is a standard process in growth and development as well as
in wound healing. However, angiogenesis is also the process by which a tumor may
grow beyond a clinically insignificant size. Without the nutrition and
oxygenation from a local blood supply, tumors cannot grow to be more than
several millimeters in size.
Interrupting
angiogenesis to tumors has been employed as a method to slow or potentially
reverse tumor growth. For example, Roche Holding AG’s Avastin®, an
FDA-approved monoclonal
antibody, inhibits the activity of vascular endothelial growth factor
(VEGF), a substance that is important for the growth and survival of endothelial
cells. Other approved drugs may also work in part via anti-angiogenesis,
including Bayer HealthCare’s and Onyx Pharmaceuticals, Inc.’s (ONXX-NASDAQ)
Nexavar®, Pfizer
Inc.’s (PFE-NYSE) Sutent®, and
Celgene Corporation’s Thalomid®—all of
which are further detailed in the Competition section on pages 33-37. While
these medicines may have limited therapeutic effects—with median patient
survival times increasing only by several months—GenSpera anticipates that the
aforementioned products may help clinically validate the therapeutic approach of
disrupting blood supply to tumors in addition to confirming the market
potential.
The sales
of several currently marketed anti-angiogenic agents are shown in Table 6.
Although these medicines are approved and have hundreds of millions to billion
dollar sales—achievements that validate both the cellular target of the products
as well as the market potential—the Company believes that there remains a vast
window for improvement in therapeutic response. GenSpera’s approach, called
tumor angiotoxicity, is designed to destroy both the existing and newly growing
tumor vasculature versus solely blocking new blood vessel formation (as found in
commercially available products). The Company anticipates that its technique may
lead to a more immediate collapse of nutrient supply to the tumors, improving
the rate of destruction at the tumor site.
ANNUAL
SALES OF MARKETED ANTI-ANGIOGENIC AGENTS
|
Company
Name
|
Drug
|
Sales
(2009)
|
||
|
Roche
Holdings AG
|
Avastin®
|
$5.9
billion
|
||
|
Pfizer
Inc.
|
Sutent®
|
$964
million
|
||
|
Celgene
Corporation
|
Thalomid®
|
$437
million
|
||
|
Bayer
HealthCare and Onyx Pharmaceuticals, Inc.
|
Nexavar®
|
$235
million
|
Sources:
Crystal Research Associates, LLC and company web sites.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
24
|
 |
Based on
preclinical studies, the Company believes that G-202 has the characteristics of
a favorable prodrug candidate. To be clinically successful, GenSpera believes
that the preclinical candidate should possess certain attributes, as listed on
the left side of Table 7. The right side of Table 7 lists the pharmacokinetics
of G-202 in relation to the Company’s desired prodrug
characteristics.
Table
7
GenSpera,
Inc.
AN
EVALUATION OF SEVERAL FAVORABLE CHARACTERISTICS OF PRODRUGS VERSUS G-202
|
Favorable Prodrug Characteristic
|
G-202 Pharmacokinetic Results
|
||
|
The prodrug is relatively stable in its
|
·
|
The half-life (time for the initial concentration to be reduced by 50%) of
|
|
|
inactive form in the bloodstream.
|
the inactive prodrug in the bloodstream is 5.5 hours in mice and 10
|
||
|
hours in monkeys.
|
|||
|
There is a high accumulation of the active
|
·
|
Tumor tissue accumulates significant levels of the active form (12ADT)
|
|
|
drug in the tumor tissue, with very low
|
at 24 hours after the last dose, with less than a 50% decrease over
|
||
|
concentrations in other tissues.
|
the subsequent two days, and with relatively high levels still found
|
||
|
after five days.
|
|||
|
·
|
Only very low concentrations of 12ADT were found in other tissues,
|
||
|
such as the kidney, skeletal muscle, or brain.*
|
|||
|
There are low concentrations of the active
|
·
|
The concentration of 12ADT in the bloodstream never exceeded 1% of
|
|
|
form of the drug in the bloodstream relative
|
the concentration of G-202 (the inactive prodrug) over a 16-hour period.
|
||
|
to the inactive prodrug.
|
|||
|
The difference in toxicity between the
|
·
|
12ADT is toxic to mice at over 1,000-fold lower doses than the
|
|
|
inactive prodrug and its corresponding
|
therapeutic doses of G-202.
|
||
|
active drug should be as high as possible.
|
|||
|
The activated drug is actively taken up by
|
·
|
Target enzymes were selected for their activity on the outer surface of
|
|
|
adjacent cancer cells for a "bystander"
|
cells. Hence, the activated drug is free to kill any cells within its
|
||
|
killing effect.
|
immediate vicinity.
|
||
*
Higher concentrations were found in the liver without apparent toxicity, which
the Company believes is most likely a part of the liver’s normal
function in clearing cytotoxic drugs from the bloodstream.
Source:
GenSpera, Inc.
In
summary, the Company believes that G-202 exhibits favorable characteristics as a
prodrug candidate. G-202 is stable in its inactive form in the bloodstream and,
therefore, dosing levels and the frequency of infusions are easily controlled.
In addition, G-202 has demonstrated the ability to target tumors with minimal
leakage of the active form into the bloodstream or other tissues. As such, the
dosing levels of G-202 could be increased, if necessary. G-202 may also not
require combination with other therapies, which could permit faster and less
costly clinical trials.
Anti-tumor
Efficacy Studies of G-202
GenSpera
has conducted several studies using G-202 in a variety of animal models with
solid tumors, including prostate, bladder, renal (kidney), and breast cancers.
In these studies, the administration of G-202 stopped tumor growth—and in most
cases caused noticeable regression of the tumor—with no visible re-growth for
roughly one month following the last treatment. G-202 was well tolerated at dose
levels that caused cessation of tumor growth with no signs of toxicity. At the
highest doses, transient weight loss was documented, which quickly recovered
after each course of therapy. In addition, the data indicated that even after
several dosing cycles, G-202 did not activate drug resistance in tumor cells.
The Company has also completed definitive toxicology studies of G-202 in rats
and monkeys.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
25
|
 |
G-202’s Anti-tumor Activity
Against Prostate Cancer
To
initially determine whether G-202 could serve as an anti-tumor agent in live
animals, GenSpera evaluated the agent for activity in the LNCaP mouse xenograft model. LNCaP cells
express PSMA at roughly the same level as found in a human prostate. When
administered via intravenous injection at a dose of 17 mg/kg daily for four
days, G-202 nearly ceased tumor growth. G-202 was also well tolerated with no
signs of morbidity or toxicity during the study.
The
Company subsequently evaluated G-202 for anti-tumor efficacy against the CWR22R-H human prostate tumor
xenograft, which expresses higher levels of PSMA than LNCaP tumors. Mice with
CWR22R-H tumors were treated with G-202 at either 17 mg/kg for 10 consecutive
days or 56 mg/kg for three consecutive days. Both treatment regimens inhibited
tumor growth for approximately 32 to 40 days before re-growth was detected. In
addition, between the two different regimens, no significant difference in
anti-tumor response was observed. The anti-tumor effect of the more intense
dosing regimen, which is shown in Figure 8, was accompanied by weight loss
(maximum of roughly 17%), which resolved and reached control values by day 27.
The lower-intensity dosing regimen of G-202, which was equivalent to the total
dose of the 56 mg/kg regimen, was also accompanied by a marginal (<10%) but
rapidly reversible weight loss. The results of these two studies demonstrated
that G-202 could produce significant anti-tumor effects at doses that were well
tolerated by mice.
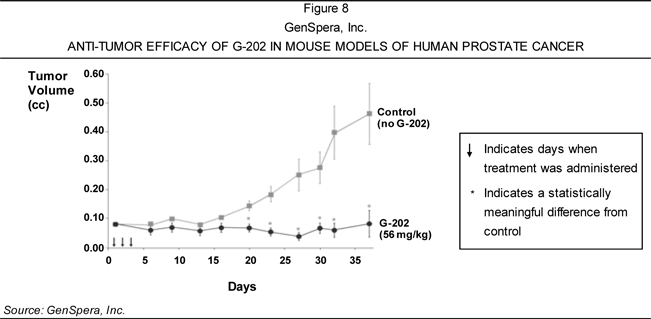
GenSpera’s
next study was designed to evaluate G-202 for its ability to exert extended
anti-tumor activity after several administration cycles at high dose levels.
Mice with CWR22R-H were treated with four courses of G-202 intravenously at a
dose of 56 mg/kg for three consecutive days. Each treatment course occurred at
10-day intervals. The data from the study demonstrated that the dosing regimen
significantly inhibited tumor growth over the course of therapy from day 7 until
the experiment was terminated on day 69. Similar to the previous experiment with
56 mg/kg dosing levels, tumor growth became apparent roughly 20 days after the
last treatment. In addition, the mice also temporarily lost weight (maximum of
approximately 12%), which recovered quickly after each course of
therapy.
Based on
the preliminary findings, which evaluated established but small tumors, GenSpera
could not determine whether G-202 caused tumor regression or merely extended a
stagnant growth period. As such, the Company also evaluated the ability of G-202
to cause tumor regression in animals with relatively large CWR22R-H tumors that
had re-grown after the discontinuation of treatment with GenSpera’s related
PSMA-targeted prodrug, 12ADT-Asp-Glu (also called G-201). In this study, G-202
was administered intravenously at a dose of 56 mg/kg for three separate cycles
of dosing: days 1-3, days 16-18, and days 26-28. Data indicated that each dosing
cycle caused noticeable regression of the large tumors, suggesting that G-202
exerts cytotoxic and tumor-reducing effects in vivo. In addition, the
tumors did not manifest a resistance to the drug, even after multiple dosing
cycles.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
26
|
 |
G-202’s Anti-tumor Effect
Against Human Bladder, Kidney, and Breast Cancers
Some
researchers have demonstrated PSMA expression in the new vasculature of a
variety of tumor types, including renal, bladder, colon, neuroendocrine,
pancreatic, lung cancers, and the majority of breast cancers and sarcomas.
Prostate cancer is not included because research has shown that endothelial
cells within prostate cancers do not appear to make PSMA (Source: Cancer Research 1999; 59:
3192-3198). A report published in 2006 demonstrated that PSMA may serve as an
interface on the molecular level, coordinating both extracellular and
intracellular signals during angiogenesis (Source: Molecular and Cellular
Biology 26(14):5310-5324). In this study, PSMA knockout mice had marked
impairment of new blood vessel formation, indicating that G-202 may also be
effective against non-prostate cancer tumors due to its ability to selectively
destroy PSMA-producing endothelial cells present within the tumor.
Based on
these findings, the Company evaluated the anti-tumor effect of G-202 in a mouse
model of human bladder cancer by administering three consecutive daily 56 mg/kg
doses of G-202 to nude
mice bearing TSU-Pr1 xenografts for four
cycles. The treated mice exhibited significant inhibition of tumor growth versus
vehicle-treated controls, which received vehicle alone—15% solutol/15% propylene
glycol in phosphate-buffered saline. The treated versus control (T/C) ratio of
the tumor volume on day 25 was 0.33. By day 33, the tumor volume in six of the
eight treated control animals was greater than 0.5 cc, while all but one of the
animals treated with G-202 was less than 0.5 cc. Calculated from the ratios of
measured tumor volume at day 33 to starting tumor volume for each individual
animal, the relative volume of control tumors was 13.2 versus 3.2 for
G-202-treated tumors, as shown in Figure 9.
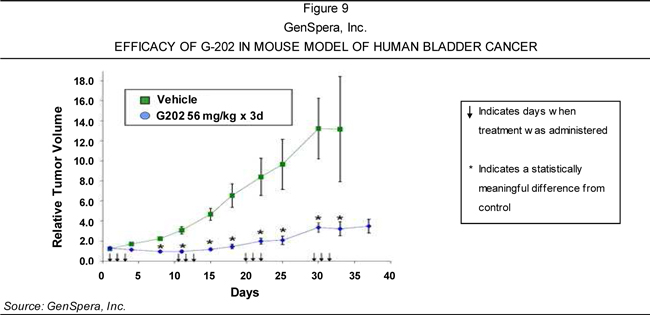
In
addition, GenSpera evaluated G-202 for anti-tumor efficacy in a mouse model of
human renal (kidney) cancer. Five consecutive daily doses of 56 mg/kg were given
to nude mice for nearly three courses of treatment. In total, 14 doses were
administered, as indicated by the downward facing arrows shown in Figure 10
(page 28). As displayed in Figure 10, the data from this study demonstrated
tumor regression when contrasted with the size of the tumor at the beginning of
the experiment. After the third course of G-202 treatment, the treated versus
control (T/C) ratio on day 30 was 0.12 versus vehicle controls. By day 30, the
group treated with G-202 experienced a 75% reduction in overall tumor size. The
experiment was also repeated at a dosing level of 5.6 mg/kg (one-tenth of the
original dose) on the same dosing schedule. These results further validated the
anti-tumor effects of G-202—even at a significantly lower dose—relative to
vehicle controls, with a T/C ratio of 0.57 on day 30.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
27
|
 |
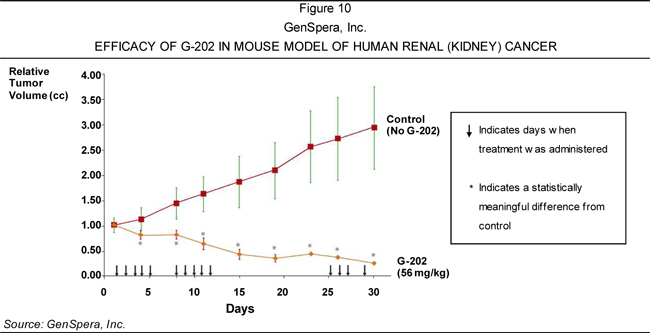
G-202 was
also tested using a human breast tumor model in nude mice to evaluate its
anti-tumor effects. Only two consecutive daily doses of 56 mg/kg G-202 were
administered to the mice. This therapy resulted in complete regression in six
out of eight treated animals, indicating that 75% of animals may have been cured
with only one cycle of dosing. Tumors that re-grow can be treated with repeated
dosings resulting in permanent tumor regressions. The data from this study is
shown in Figure 11.
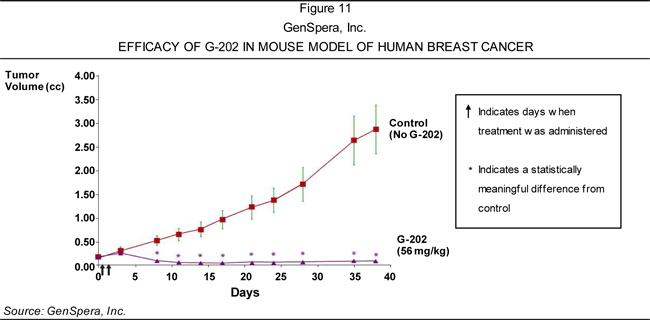
It is
important to note the broad therapeutic safety window for G-202. In the human
renal cancer model, 14 daily doses of drug were administered to mice, as
illustrated by downward facing arrows in Figure 10. Nevertheless, the Company
anticipates that only two doses may be necessary as demonstrated in the human
breast cancer model, potentially allotting a safety window that the Company
believes is significant for cancer therapeutics.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
28
|
 |
Summary of
Data
The
anti-tumor effects of G-202 on the growth of breast, renal, bladder, and
prostate cancer xenografts indicate that G-202 may have broad application as a
therapy for a variety of human solid tumors due to its ability to selectively
target PSMA-producing endothelial cells within these tumors. Further, the
effects are achievable at doses of G-202 that are well tolerated in mice.
GenSpera believes that it may be able to eliminate cancerous tumors with dosing
that is effective for an extended length of time. Further, the Company also
anticipates that this is more likely to be achieved in humans than in mice due
to the ability to infuse the drug and a potentially longer half-life of G-202 in
the human bloodstream versus that of laboratory animals. Given its efficacy
profile, GenSpera also expects that G-202 will likely be effective as a
monotherapy, thus reducing the costs and time required to conduct clinical
trials.
Development
Status
In
September 2009, GenSpera announced that the FDA approved its IND application to
initiate a Phase I study with G-202 in cancer patients. The ongoing study
(overviewed below) is designed to evaluate its safety and pharmacokinetics in
humans, and determine an appropriate dosing regimen for subsequent clinical
studies. Assuming successful completion of the Phase I clinical trial, GenSpera
expects to conduct up to four Phase II clinical trials to determine the
therapeutic efficacy of G-202 in various cancer types.
Phase
I Clinical Study
The Phase
I trial is ongoing at two locations: (1) the University of Wisconsin Carbone
Cancer Center in Madison, Wisconsin, with Dr. George Wilding as principal
investigator; and (2) the Sidney Kimmel Comprehensive Cancer Center at Johns
Hopkins, Baltimore, Maryland, for which Dr. Michael Carducci is serving as
principal investigator. GenSpera is conducting this study in cancer patients
with any type of solid tumor who have relapsed after treatment with other
anticancer agents—a strategy that is expected to facilitate enrollment and
provide safety data across a variety of cancer patients. While the primary
endpoints of the open-label, dose-escalation study are to evaluate the safety,
tolerability, and pharmacokinetics of the drug in humans, the design of the
trial also allows the collection of efficacy data.
In
January 2010, GenSpera treated the first patient at the University of Wisconsin
Carbone Cancer Center. The study is progressing according to a predetermined
schedule (in terms of dose escalation) agreed to by the FDA. In the first
cohort, participants enroll one after another, with each patient requiring
roughly six weeks. GenSpera seeks to enroll up to 30 patients in the Phase I
study. As of June 2010, four patients had been enrolled in the study. The
Company expects to complete the trial in the second quarter 2011.
Planned
Phase II Clinical Program
Dependent
upon the successful completion of the Phase I study, GenSpera plans to conduct
up to four Phase II clinical trials over 18 months to determine the therapeutic
efficacy of G-202 in different tumor types. Each trial is expected to focus on a
single tumor type—a strategy that the Company believes can maximize efficiency.
The Phase II program requires additional G-202 product, which could take
approximately one year and $1.5 million to produce.
GenSpera
plans to license G-202 during the Phase II program. In the event that the
Company is unable to do so on acceptable terms, it will likely proceed with
Phase III clinical trials.
Clinical
Strategy
Unlike
the first generation of angiogenic drugs, which only blocked new vessel
formulation, GenSpera believes that its approach destroys the existing tumor
vasculature, collapsing the tumor’s nutrient supply and destroying the tumor at
a more rapid rate. Because G-202 is capable of attacking the blood supply to a
variety of solid tumors, the Company believes that there is a wide range of
clinical applications for G-202, including those overviewed in Table 8 (page
30). The Company expects to continue to evaluate G-202 in other preclinical
animal models of various cancers in addition to evaluating other potential
clinical opportunities as human patient data is collected in early clinical
trials.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
29
|
 |
Table 8
GenSpera,
Inc.
POTENTIAL
MARKETS FOR G-202
|
Cancer Type
|
Estimated Number of
New Cases (2006)
|
Probability of Developing
(birth to death)
|
||||
|
Male
|
Female
|
|||||
|
Prostate
|
192,280
|
1
in 6
|
—
|
|||
|
Breast
|
194,280
|
n/a
|
1
in 8
|
|||
|
Urinary
Bladder
|
70,980
|
1
in 27
|
1
in 84
|
|||
|
Kidney
|
57,760
|
n/a
|
n/a
|
|||
Source:
CA: A Cancer Journal for Clinicians 2009; 59:225-249.
GenSpera
believes that G-202 could have activity across a wide variety of solid tumors.
The Company plans to evaluate G-202 in multiple cancer types, creating value for
potential corporate partners by demonstrating breadth of activity.
Manufacturing
and Development
To
leverage GenSpera’s experience and available financial resources, the Company
does not intend to develop its own manufacturing facilities. Rather, GenSpera
plans to outsource all drug manufacturing to a Good Manufacturing Practice
(GMP)-compliant contract manufacturer. GenSpera entered into an Alliance
Agreement with Cedarburg Hauser Pharmaceutical Services to perform most of the
Company’s contract manufacturing efforts. Under the terms of this agreement,
independent work orders have been and will likely continue to be constructed for
various tasks, including manufacture of chemical intermediates and reference
standards, manufacture of G-202 in compliance with GMP standards, and
development of analytical methods in support of GenSpera’s development programs.
The Company plans to continually improve its efficiency by refining its current
manufacturing process as well as the final drug formulation to simplify storage
and related procedures. GenSpera has identified a viable formulation of G-202
for the clinic and for commercial purposes. The Company has sufficient G-202 to
supply the Phase I clinical trial.
GenSpera
believes that its current stockpile of T. garganica seeds can
generate 2.5 kg of drug product, and as such, the Company is in possession of
sufficient material to take G-202 through late-stage clinical
testing.
Other
Potential Business Applications for G-202
In
addition to chemotherapy, GenSpera believes that G-202 has several
characteristics that may be beneficial in other business applications, including
prostate cancer and benign prostatic hypertrophy (BPH). BPH is a condition in
older men that is characterized by an enlarged prostate in addition to urinary
problems. GenSpera anticipates that the size of the prostate gland may be
reduced when G-202 is administered to treat tumors because PSMA is found within
both the healthy and enlarged prostate. This potential size reduction may have a
positive effect by relieving symptoms associated with prostate cancer and BPH.
Approximately one in four men over the age of 40 experience BPH symptoms
(Source: Boehringer Ingelheim Limited). The American Urological Association
(AUA) estimates that BPH is prevalent in over 50% of men above age 60, a figure
that increases to 90% by age 85 (Source: the AUA’s Clinical Guidelines on the
Management of BPH 2003). The potential utility of G-202 in the treatment
of BPH is closely related to its toxicity profile in humans. While not currently
a primary business focus, this potential effect is expected to be closely
monitored in early clinical trials.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE
30
|
 |
DEVELOPING
PRODRUGS TO TARGET TUMORS DIRECTLY
GenSpera’s
technology has a broad range of potential applications, including the pairing of
cytotoxic derivatives of thapsigargin to peptides that are only removable by
enzymes expressed in a particular tumor type. The laboratories of Dr. John T.
Isaacs and Dr. Denmeade are working to develop other lead compounds that
specifically release thapsigargin within prostate tumors. Biographies for Dr.
Isaacs and Dr.
Denmeade, who are co-founders of GenSpera presently serving as Scientific
Advisory Board members, are provided on page 11 and page 12, respectively. The
Company’s first approach coupled thapsigargin to peptides that were selectively
cleaved by a prostate cancer-specific protease called prostate-specific antigen
(PSA), which led to GenSpera’s development candidate, G-115. The Company’s
second program entails the delivery of thapsigargin selectively to prostate
tumors via human glandular
kallikrein 2 (hK2), a distinct prostate cancer-specific protease. The hK2
program has resulted in several molecules with anticancer properties in animal
models, and the Company is currently using peptide optimization to identify a
legitimate development candidate.
PSA-targeted
Prodrugs for Prostate Cancer
Compared
to other cancers, prostate cancer has a large proportion of slowly proliferating
cells that are resistant to treatment with conventional cytotoxic agents.
Currently available therapies generally attack and destroy rapidly dividing
cells, as opposed to cells that divide slowly. The side effects associated with
the present therapies are severe because cell division is also a frequent and
normal process of healthy non-malignant tissues. Therefore, to develop an
efficacious drug, it is important to use an agent that is capable of killing
slowly proliferating cancer cells and can be delivered in a way that minimizes
the toxicity to other parts of the body. GenSpera believes that its core
technologies accomplish these two primary objectives.
PSA is
active within tumor sites and in the normal prostate tissue but is inactive in
the bloodstream—characteristics that form the basis for tumor-specific delivery
of cytotoxic agents. While PSA is not active in the bloodstream, its level is
measurable and is used as a clinical test to detect prostate cancer and follow
response to therapy.
To
develop a PSA-activated prodrug, GenSpera focused on the identification of a
derivative of thapsigargin that could be chemically coupled to a PSA-substrate
peptide, yet retain all the activity of the parent agent after it was released
from the prodrug by PSA. The Company focused on the peptide’s synthetic
feasibility, optimization of its activity as a PSA substrate, and its relative
stability to cleavage by other proteases that are common in the body. Adhering
to these conditions, GenSpera identified an initial PSA-activated prodrug,
G-114. However, continued screening and optimization of PSA-cleavable peptides
led to the discovery of G-115, an improved prodrug that the Company believes
exhibits 10-fold greater activity as a PSA substrate versus G-114. G-114 has
been designated as the back-up drug for G-115 due to its significant activity as
an anti-tumor agent in animal models of prostate cancer.
G-115
G-115 is
a prodrug that is selectively activated within prostate tumors by PSA. GenSpera
chose G-115 as its lead development candidate in the PSA-targeted prodrug
program due to its enhanced PSA-substrate and in vivo anti-tumor activity,
as well as its broader intellectual property (IP) coverage. To evaluate G-115’s
anti-tumor response, mice bearing a high PSA-producing human prostate cancer
xenograft were treated with 7 mg/kg for 10 consecutive days. The data from the
study, shown in Figure 12, demonstrated that G-115 inhibited tumor growth and
exhibited well-behaved pharmacokinetics in mice.
GenSpera
plans to obtain an IND for G-115 in the third quarter 2011. The Company seeks to
establish a strategic partnership to maximize the value of G-115 as it
progresses in the clinic. It is important to note that G-115 and G-202 are
non-competing product candidates: the Company intends to market G-115 to
urologists, while marketing G-202 to medical oncologists. In the U.S. and many
counties in Europe, urologists are the first medical professionals to treat
prostate cancer patients. When their methods and tools fail, the patient is
often recommended to a medical oncologist.
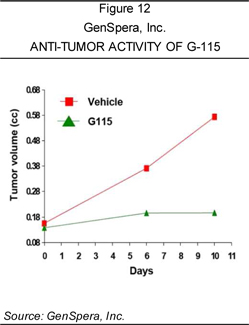
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE
31
|
 |
hK2-targeted
Prodrugs for Prostate Cancer
Prostate
cancer cells also secrete the protease hK2, which may be used to activate
thapsigargin prodrugs. Through the laboratories of Dr. Isaacs and Dr. Denmeade,
GenSpera has identified peptides that are selectively cleaved by hK2. The
Company has coupled them to 12ADT to generate a family of hK2-activated
thapsigargin prodrugs, including Ac-GKAFRR-L12ADT, which GenSpera considers to
be the most fully characterized prodrug of this species. In 2006, the data
obtained from Ac-GKAFRR-L12ADT were published in The Prostate.
Ac-GKAFRR-L12ADT demonstrated an anti-tumor effect in animal models of prostate
cancer while it was being administered. However, the prodrug was rapidly cleared
from the body and there was a moderately low range of therapeutic doses relative
to toxicity. Presently, the Company is focused on developing second-generation
hK2-activated thapsigargin prodrugs with improved formulations and increased
half-lives.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE
32
|
 |
Competition
The
pharmaceutical, biopharmaceutical, and biotechnology industries are highly
competitive. While GenSpera is not aware of any medicine in development that is
designed to destroy both existing and newly grown tumor vasculature in a manner
similar to G-202, the Company may face competition from other companies that are
marketing or developing agents that function, at least in part, by attacking
tumor-associated blood vessels (e.g., OXiGENE, Inc.’s ZYBRESTAT™ or Roche’s
Avastin®, which
are overviewed on page 36 and page 37, respectively). GenSpera believes that the
already-approved anti-angiogenic products validate the concept of targeting
tumor-associated endothelial cells as a commercially viable cancer
therapy.
Nevertheless,
it is important to note that the molecular mechanism of G-202’s cytotoxicity is
novel among anticancer agents as it inhibits the activity of the SERCA pump to
cause cell death (as detailed on page 21). Further, G-202 as a monotherapy has
led to tumor regression in animal models of cancer versus existing
anti-angiogenic agents that may only be able to prevent further tumor growth.
Currently marketed drugs have been approved based upon anti-tumor effects
demonstrated in the clinic. However, a significant unmet need persists for more
effective anticancer agents that significantly extend patient survival in
addition to having anti-tumor effects. As well, to the Company’s knowledge, it
is the only entity with a targeting mechanism based upon the enzymatic activity
of PSMA. GenSpera believes that this is, in part, due to the substrate
specificity of the enzyme as well as the Company’s solid patent position in this
area.
GenSpera
may also compete with large, well-funded companies that are developing or have
developed drug candidates of a different type that address the same patient
populations. While not an exhaustive collection of GenSpera’s competitors, Table
9 lists the companies that are believed to be representative of the type of
competition that the Company may face.
|
Table
9
|
||||||||||||||||||
|
GenSpera,
Inc.
|
||||||||||||||||||
|
COMPETITION
|
||||||||||||||||||
|
Symbol
|
Last
Trade
|
52-week
|
Avg
Vol
|
Market
|
||||||||||||||
|
Company
|
(Exchange)
|
(07/06/10)
|
Range
|
(3
month)
|
Cap
|
|||||||||||||
|
Access
Pharmaceuticals, Inc.
|
ACCP
(OTC.BB)
|
$ | 2.00 | $ | 1.52 - $5.00 | 30,444 | $ | 30.91M | ||||||||||
|
ACT
Biotech, Inc.
|
Closely
held
|
— | — | — | — | |||||||||||||
|
Bayer
HealthCare (part of Bayer AG)*
|
BAYN
(XETRA)
|
€ | 45.12 | € | 35.36 - €56.71 | 5,190,030 | € | 37.3B | ||||||||||
|
Biogen
Idec Inc.
|
BIIB
(NASDAQ)
|
$ | 48.38 | $ | 41.75 - $60.28 | 3,336,450 | $ | 12.92B | ||||||||||
|
Biotest
AG*
|
BIO
(XETRA)
|
€ | 35.50 | € | 31.09 - €47.49 | 4,245 | 234.13 | M | ||||||||||
|
Celgene
Corporation
|
CELG
(NASDAQ)
|
$ | 49.02 | $ | 45.27 - $65.79 | 4,652,230 | $ | 22.59B | ||||||||||
|
F.
Hoffmann-La Roche Ltd**
|
ROG
(SWX)
|
CHF
146.80
|
CHF
143.70 -
|
2,127,600 |
CHF
103.1B
|
|||||||||||||
|
CHF
187.40
|
||||||||||||||||||
|
ImmunoGen,
Inc.
|
IMGN
(NASDAQ)
|
$ | 8.44 | $ | 6.25 - $10.90 | 998,439 | $ | 484.97M | ||||||||||
|
Ipsen
S.A.
|
IPN
(Euronext Paris)
|
€ | 24.79 | € | 24.06 - €42.24 | 263,341 | € | 2.1B | ||||||||||
|
Merck
& Co., Inc.
|
MRK
(NYSE)
|
$ | 34.65 | $ | 25.05 - $41.56 | 19,763,000 | $ | 108.05B | ||||||||||
|
Onyx
Pharmaceuticals, Inc.
|
ONXX
(NASDAQ)
|
$ | 19.90 | $ | 19.54 - $36.75 | 1,179,840 | $ | 1.25B | ||||||||||
|
OXiGENE,
Inc.
|
OXGN
(NASDAQ)
|
$ | 0.29 | $ | 0.29 - $2.37 | 549,842 | $ | 20.06M | ||||||||||
|
Pfizer
Inc.
|
PFE
(NYSE)
|
$ | 14.29 | $ | 14.00 - $20.36 | 69,620,200 | $ | 115.27B | ||||||||||
|
sanofi-aventis
SA
|
SNY
(NYSE)
|
$ | 29.47 | $ | 28.01 - $41.59 | 3,369,520 | $ | 77.17B | ||||||||||
|
*
Amounts in euros; €1 ˜ US$1.26 at
07/06/10.
|
**
Amounts in Swiss francs; CHF 1 ˜ US$0.94 at
07/06/10.
|
|||||||||||||||||
|
Sources:
Yahoo! Finance, Google Finance, and Crystal Research Associates,
LLC.
|
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE
33
|
 |
Access Pharmaceuticals, Inc.
(www.accesspharma.com)
Access
Pharmaceuticals is a Texas-based biopharmaceutical company that develops and
commercializes products for the treatment and supportive care of cancer
patients. Access Pharmaceuticals’ pipeline includes ProLindac™, an oncology drug
that is currently in Phase II clinical testing in Europe with ovarian cancer
patients. ProLindac™ is a novel DACH platinum prodrug that has
been shown in both preclinical models and in human trials to be active in a wide
variety of solid tumors, and may eliminate some of the toxic side effects seen
in currently marketed DACH platinum medicines. ProLindac™ has completed a Phase
II monotherapy study in ovarian cancer patients. Also in development is
thiarabine (4-thio Ara-C), a small molecule therapy licensed from the Southern
Research Institute in Birmingham, Alabama, that has been evaluated in two Phase
I studies in patients with solid tumors. Based on preclinical and clinical data
to date, Access Pharmaceuticals plans to further investigate thiarabine in
leukemia and lymphoma patients.
ACT Biotech, Inc. (www.actbiotech.com)
ACT
Biotech is a closely held, California-based pharmaceutical company focused on
the development and commercialization of targeted cancer drugs. Its lead program
is the development of Telatinib, a small molecule angiogenesis inhibitor. Data
from over 250 patients involved in two Phase I monotherapy trials and four
combination therapy trials showed signs of efficacy in various cancer types and
improved safety versus other VEGF or VEGF receptor-directed anti-angiogenic
drugs. In June 2010, Telatinib received Orphan Drug designation from
the FDA for the treatment of gastric cancer. Telatinib is currently in Phase II
clinical testing in the U.S. and Europe for the first-line treatment of advanced
gastric cancer patients in combination with standard-of-care chemotherapy for
which results are expected in late 2010. A second ACT Biotech program entails
the development of ACTB1003, an oral kinase inhibitor with multiple modes of
action, which targets cancer by inhibiting angiogenesis, inducing tumor cell
apoptosis, and targeting specific cancer mutations. The company’s IND
application for ACTB1003 was accepted by the FDA in early 2010, and ACT Biotech
is preparing for Phase I clinical trials in multiple cancer
indications.
Bayer HealthCare (www.bayer.com)
Bayer
HealthCare, the U.S.-based pharmaceuticals unit of Bayer HealthCare LLC (a
division of Bayer AG), has collaborated with Onyx (detailed on page 36) to
develop and market Nexavar®
(sorafenib) in tablet form. Nexavar® is a
multi-kinase inhibitor
indicated for the treatment of advanced renal cell carcinoma, the most common
type of kidney cancer, and for unresectable hepatocellular carcinoma, the
most common form of liver cancer. In advanced renal cell carcinoma patients,
Nexavar®
demonstrated improved progression-free survival from 12 weeks to 24 weeks. The
active ingredient in Nexavar®,
sorafenib, targets both the tumor cell and tumor vasculature. Nexavar® is
approved in over 80 countries for liver cancer and in more than 90 countries for
advanced kidney cancer. Nexavar® is also
being evaluated as an individual or combination therapy in lung, thyroid,
breast, and colorectal cancer, among others.
Biogen Idec Inc. (www.biogenidec.com)
Headquartered
in Massachusetts, Biogen Idec is a biotechnology company engaged in the
development, manufacturing, and commercialization of novel therapies. Under a
collaboration agreement, Biogen Idec and Genentech, Inc. (a wholly owned member
of the Roche Group) are developing and marketing Rituxan®
(rituximab), a targeted B-cell therapy that is indicated for the treatment of
non-Hodgkin’s lymphoma.
Rituxan® is
approved as a single agent or a combination therapy for treatment of select
stages and forms of B-cell non-Hodgkin’s lymphoma and as part of a combination
therapy to treat chronic
lymphocytic leukemia. The company’s lead development candidate is a
humanized anti-CD20 monoclonal antibody called GA101, which is being developed
under a collaboration agreement with Genentech. GA101 is in Phase III clinical
trials for chronic lymphocytic leukemia and Phase II testing for non-Hodgkin’s
B-cell lymphoma. Biogen Idec is also developing a candidate with technology
licensed from ImmunoGen, called BIIB015, for the treatment of solid tumors.
BIIB015 has ImmunoGen’s cell-killing agent, DM4, attached. Phase I clinical
testing with BIIB015 is ongoing.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE
34
|
 |
Biotest AG (www.biotest.com)
Biotest
is a German pharmaceutical company that specializes in hematology and immunology
products. Through the licensing of technology from ImmunoGen, Biotest is
currently developing BT-062, a Tumor-Activated Prodrug (TAP) compound for the
treatment of multiple myeloma. BT-062 comprises a monoclonal antibody and
ImmunoGen’s cell-killing agent, DM4. In early 2008, BT-062 was granted Orphan
Drug status by the FDA, and in December 2008, the European Commission also
granted BT-062 Orphan Drug designation for multiple myeloma after the compound
was assessed by the European Medicines Agency (EMEA). BT-062 is currently
undergoing Phase I clinical trials at four cancer clinics in the U.S. to test
the safety and tolerability of the compound for use in humans, as well as to
provide initial information on its efficacy. Biotest is also making preparations
to extend the clinical development program to Europe.
Celgene Corporation (www.celgene.com)
New
Jersey-based Celgene is a multinational biopharmaceutical company that aims to
improve the lives of patients worldwide. Celgene currently markets Thalomid®
(thalidomide), an orally administered immunomodulatory agent. Thalomid® has been
shown to have anti-angiogenic activity. Thalomid® is
indicated for the treatment of patients with newly diagnosed multiple myeloma
(cancer of the plasma cell) when used in combination with dexamethasone, a
steroid that reduces inflammation and swelling. As well, Celgene is developing
Revlimid®
(lenalidomide), which is approved in combination with dexamethasone to treat
multiple myeloma patients who have received at least one prior therapy.
Revlimid® is also
in clinical testing for chronic lymphocytic leukemia, non-Hodgkin’s lymphoma,
and solid tumors, among other indications. Celgene plans to complete a Phase III
trial with its lead compound for small cell lung cancer, Amrubicin, in
2010.
Genentech, Inc. (www.gene.com)
Headquartered
in San Francisco, California, Genentech is a biotechnology company that focuses
on the discovery, development, manufacture, and commercialization of medicines
for patients with significant unmet medical needs. Genentech is a wholly owned
member of the Roche Group and serves as the headquarters for all Roche
pharmaceutical operations in the U.S., including marketing products such as
Avastin®
(bevacizumab), a bioengineered antibody for treating various types of colon,
breast, lung, and other cancers (described in greater detail on page 37).
Genentech’s pipeline includes two Phase II oncology candidates: (1) ABT-263, a
small molecule in Phase I clinical trials in combination with other medicines to
treat solid tumors and hematologic malignancies and in Phase I/II clinical
trials as a single agent for various forms of cancer; and (2) MetMAb, a
humanized monoclonal antibody in Phase II clinical testing in combination with
Tarceva®
(erlotinib) for second- and third-line metastatic non-small cell lung
cancer.
ImmunoGen, Inc. (www.immunogen.com)
Headquartered
in Massachusetts, ImmunoGen is focused on the development of targeted anticancer
therapeutics. ImmunoGen’s pipeline is primarily created from the company’s
proprietary TAP technology, which was designed to facilitate the development of
anticancer drugs that are more effective and better tolerated than currently
available therapies. A TAP compound consists of a monoclonal antibody that binds
specifically to an antigen found on cancer cells that is attached to a potent
cell-killing agent. ImmunoGen has several TAP compounds in human clinical
testing: (1) BT-062, which is in Phase I for the treatment of multiple myeloma;
and (2) IMGN388, a TAP compound that uses an integrin-targeting antibody
developed by Centocor, Inc. (part of Johnson & Johnson [JNJ-NYSE]), which is
in Phase I clinical testing for the treatment of solid tumors. ImmunoGen has
also licensed rights to develop products using its TAP technology to several
major biotechnology and pharmaceutical companies, including Genentech,
sanofi-aventis, Biogen Idec, Biotest, Bayer HealthCare, and Amgen, Inc.
(AMGN-NASDAQ).
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE
35
|
 |
Ipsen S.A. (www.ipsen.com)
Headquartered
in Paris, France, Ipsen is a global biopharmaceutical group focused on specialty
and primary care drugs in oncology, endocrinology, neurology, and hematology,
among others. The company is supporting over 20 research and development
programs, including several in the oncology arena: (1) Decapeptyl®, a Phase
III hormone therapy for breast cancer; (2) Toremifene citrate, a Phase III
candidate being developed to treat and prevent prostate cancer; and (3) BN 83495
(STX 64), a Phase I therapy for post-menopausal breast cancer expressing
estrogenic receptors, among others. Ipsen’s pipeline also includes several
preclinical anticancer candidates, such as Angiomates (STX 140), a family of
small molecules that exhibit both anti-angiogenic and anti-proliferative
(killing cancer cells) properties.
Merck & Co., Inc. (www.merck.com)
With
headquarters in Whitehouse Station, New Jersey, Merck is a global pharmaceutical
company developing medicines, vaccines, biologic therapies, and consumer and
animal products. Its oncology program entails products for the prevention,
treatment, and supportive care of cancer. The company aims to be the leader in
the discovery, development, and delivery of targeted anticancer therapies and is
also pursuing cancer vaccines. Merck’s targeted oncology candidates focus on key
pathways and processes involved in cancer growth and progression. The company
has two Phase III cancer candidates: (1) Ridaforolimus (MK-8669, AP23573), an
oral inhibitor of mTOR—a protein involved in regulating normal cell growth,
division, and survival as well as new blood vessel formation—to treat soft
tissue sarcoma; and (2) V503, a vaccine to prevent nine types of human
papillomavirus (HPV).
Onyx Pharmaceuticals, Inc.
(www.onyx-pharm.com)
Onyx is a
California-based biopharmaceutical company that seeks to improve the lives of
cancer patients. In addition to the collaboration with Bayer HealthCare for the
development and marketing of Nexavar®, Onyx is
also developing carfilzomib, a proteasome inhibitor that is being evaluated in
multiple clinical settings, including an ongoing Phase IIb trial in patients
with relapsed and refractory multiple myeloma, a Phase II trial in patients with
relapsed and/or refractory multiple myeloma who have relapsed after one to three
prior therapies, and a Phase Ib/II study in patients with solid tumors. Onyx’s
pipeline also includes PD 0332991, a cell cycle kinase inhibitor in Phase II
clinical development with Pfizer, Inc., and ONX 0801, an inhibitor of
thymidylate synthase (TS), a crucial enzyme for cell growth and division. ONX
0801 functions through a combination of two approaches: (1) receptor-mediated
targeting of tumor cells; and (2) inhibiting TS. ONX 0801 is currently in Phase
I clinical testing. Onyx has an exclusive worldwide license to ONX 0801, which
was originally discovered at the Institute of Cancer Research in London, called
BGC 945. Onyx has also acquired an option to license rights to ONX 0803, an
orally available, potent, and selective inhibitor of Janus kinase 2 (JAK2), and
ONX 0805, also a JAK2 inhibitor, from Singapore-based S*BIO Pte Ltd. JAK2 has
been implicated across a broad range of conditions, including cancer and
autoimmune diseases, and may be used as a potential target in next-generation
cancer therapies. The agreement grants Onyx option rights to exclusively develop
and commercialize the compounds for any potential indication in the U.S.,
Canada, and Europe.
OXiGENE, Inc. (www.oxigene.com)
OXiGENE
is a biopharmaceutical company based in Massachusetts that aims to develop
therapeutics for patients with cancer and sight-threatening eye diseases and
conditions. OXiGENE is developing several pipeline candidates based on vascular
disruption technology, which aims to deprive solid tumors of their blood supply
via vascular-disrupting agents (VDAs). In contrast to anti-angiogenic therapies,
which prevent the formation of new blood vessels, VDAs disrupt newly formed
blood vessels by rapidly reducing blood flow to the tumor, therefore depriving
it of oxygen and nutrients that are necessary for survival. OXiGENE’s VDAs
include ZYBRESTAT™, which is in clinical trials for anaplastic thyroid cancer
(Phase II/III), non-small cell lung cancer (Phase II), and ovarian cancer (Phase
II), as well as OXi4503, which is currently being evaluated as a monotherapy in
patients with advanced solid tumors in a Phase I dose-escalation study.
Nevertheless, data from preclinical studies executed by OXiGENE and its
scientific collaborators have shown that OXiGENE’s VDAs may work synergistically
with select anti-angiogenic drugs to produce anti-tumor
effects.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 36
|
 |
Pfizer Inc. (www.pfizer.com)
Headquartered
in New York, Pfizer is a research-based biomedical and pharmaceutical company.
While Pfizer markets treatments for a variety of cancers, one of its more recent
developments is its angiogenesis inhibitor, Sutent®, which
is administered orally via tablet. By blocking the actions of VEGF, Sutent® is able
to cut off the blood supply that feeds tumors and destroys cellular
reproduction. Sutent® is
indicated for the treatment of advanced gastrointestinal tumors and advanced
renal cell carcinomas. In addition, Sutent® is
currently involved in Phase II testing for gastric and colorectal cancer and
Phase III clinical trials for various other cancers with unmet medical needs,
including breast, lung, liver, and prostate cancers.
Roche Holdings AG (www.roche.com)
Headquartered
in Basel, Switzerland, Roche is a global healthcare company with products
addressing oncology, virology, inflammation, metabolism, the central nervous
system, in vitro
diagnostics, tissue-based cancer diagnostics, and diabetes, among others. Roche
acquired Genentech as well as its pipeline in 2009, including Avastin®.
Approved by the FDA in February 2004, Avastin® is
considered to be the first FDA-approved therapy for an angiogenesis inhibitor.
Avastin® is
designed to specifically restrain vascular endothelial growth factor (VEGF), a
potent protein that is believed to be a critical element of a tumor’s ability to
grow and spread in the body. While Avastin® has not
demonstrated activity as a monotherapy, it has been indicated for colon and lung
cancer when administered intravenously following chemotherapy. Avastin® has been
shown to increase progression-free survival by roughly four months for carcinoma
of the colon or rectum, and by approximately two months for unresectable,
locally advanced, recurrent or metastatic non-squamous, non-small cell lung
cancer. Moreover, Avastin® is also
FDA-approved as a breast cancer treatment, although there is currently no data
supporting a reduction in disease-related symptoms or an increased survival rate
for this indication. Several serious side effects are associated with
Avastin®,
including gastrointestinal perforation, slow wound healing, and severe bleeding,
among others.
sanofi-aventis SA (www.sanofi-aventis.com)
Headquartered
in Paris, France, sanofi-aventis is a pharmaceutical group engaged in the
research, development, manufacture, and marketing of medicines, vaccines, and
integrated healthcare solutions. The company is developing product candidates in
various therapeutic areas, including oncology, cardiovascular disease, metabolic
disorders, thrombosis, central nervous system, internal medicine, ophthalmology,
and vaccines, among others. In the oncology arena, sanofi-aventis has six
candidates in late phases of development. Of these, two have Fast Track status
from the FDA: (1) cabazitaxel, a tubulin inhibitor, for the second-line
treatment of prostate cancer; and (2) BSI-201, a PARP-1 inhibitor, to treat
triple negative metastatic breast cancer. Cabazitaxel is a new molecular entity
in the registration phase. Additionally, using ImmunoGen’s TAP technology,
sanofi-aventis is developing SAR3419, which is in Phase I clinical testing for
the treatment of non-Hodgkin’s lymphoma. Currently in Phase I studies, SAR3419
is a compound that combines a CD19-targeting monoclonal antibody and a toxic
substance called maytansinoid DM4.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 37
|
 |
Milestones
Recent
Milestones
GenSpera
has achieved several significant milestones in the past 12 months, as listed
below.
|
n
|
Received
FDA approval for its IND application for G-202 in September
2009
|
|
n
|
Began
trading on the Over-the-Counter Bulletin Board (OTC.BB) under the symbol
“GNSZ” in November 2009
|
|
n
|
Commenced
the Phase I clinical study with G-202 at both the Sidney Kimmel
Comprehensive Cancer Center at Johns Hopkins and the University of
Wisconsin Carbone Cancer Center
|
|
n
|
Treated
the first patient in the Phase I study with G-202 in January
2010
|
|
n
|
Raised
roughly $2.7 million in gross proceeds in May
2010
|
Potential
Milestones
The
Company has identified the following key milestones that it aims to achieve in
the next several years relating to its product candidates, as summarized
below.
|
n
|
Complete
the Phase I clinical trial with G-202 in the second quarter
2011
|
|
n
|
Complete
Phase II clinical studies with G-202 in the fourth quarter
2012
|
|
n
|
License
G-202 to a third party during Phase
I/II
|
Note: If GenSpera is unable to
license G-202 to a third party during Phase I/II on acceptable terms, the
Company intends to proceed with Phase III Clinical
trials.
|
n
|
Finalize
formulation and toxicology work for G-115, potentially filing an IND in
the second half of 2011
|
Figure 13
depicts the Company’s intended development timelines for G-202 and
G-115.
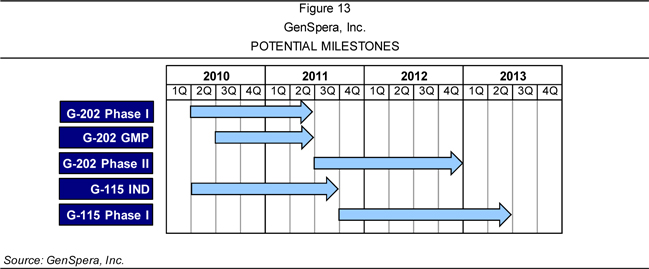
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 38
|
 |
Key
Points to Consider
|
n
|
GenSpera’s
prodrug technology entails the attachment of a targeting/masking agent to
an active drug, making the drug inactive and soluble in the bloodstream.
The Company uses specific peptides as its targeting/masking agents, and an
analog of the cytotoxin thapsigargin, called 12ADT, as the active drug.
The Company’s prodrugs are designed to either target the blood supply that
is supporting tumor growth or target the tumor
directly.
|
|
|
o
|
The
prodrug can only be activated once the targeting/masking agent is removed
by an enzyme that is expressed at the site of a tumor. With the agent
detached, the drug becomes insoluble and precipitates directly into nearby
cells.
|
|
|
o
|
The
Company’s patent-protected peptides, which are based on years of research
by GenSpera’s Scientific Advisory Board members, were developed to attack
multiple targets.
|
|
n
|
Traditional
cancer treatments (e.g., surgery or radiation therapy) are localized
therapies that lose efficacy once the cancer has spread. Chemotherapeutics
are also widely used, but as many are not targeted, their toxic effects
harm both healthy tissues and cancer
cells.
|
|
|
o
|
In
contrast, GenSpera’s prodrug technology may be more effective for cancer
that has spread due to its ability to deliver higher concentrations of
cytotoxic agents to tumors while avoiding the toxicity of these higher
doses in the rest of the body.
|
|
n
|
The
Company’s lead prodrug candidate, G-202, is composed of 12ADT and a
targeting/masking agent. Once G-202 is administered intravenously into the
bloodstream, it can only be activated at a target site by
prostate-specific membrane antigen (PSMA), which is expressed in
cancer-supporting vasculature but not in normal blood
vessels.
|
|
|
o
|
G-202
may be able to inhibit further tumor angiogenesis and attack existing
tumor vasculature—stopping growth by depleting the cancer’s nutrient
supply and potentially causing tumor regression—versus the current
generation of anti-angiogenesis drugs that GenSpera believes only slow or
prevent further growth of tumors, but do not cause tumor
regression.
|
|
|
o
|
Based
on G-202’s preclinical data in breast, prostate, bladder, and kidney
cancer, as well as its specific, robust mechanism of action, the Company
believes that G-202 can demonstrate increased efficacy and less toxicity
versus currently available chemotherapies, which cause toxicity to all
rapidly dividing cells in the body, including healthy, non-cancerous
cells.
|
|
|
o
|
A
Phase I clinical trial with G-202 is ongoing at two major cancer centers.
The trial is advancing according to a schedule determined in dialog with
the U.S. Food and Drug Administration, with four patients enrolled to
date. The study’s primary endpoints include determining the safety,
tolerability, and pharmacokinetics of G-202. Efficacy data may be
collected in parallel. If successful, the Company plans to initiate up to
four Phase II clinical trials in various cancer
types.
|
|
n
|
GenSpera’s
second prodrug technology approach attacks cancer cells directly by
targeting enzymes found primarily in tumors. G-115, the primary
development candidate in this area, targets a protein used by physicians
to detect the presence of prostate cancer, called prostate-specific
antigen (PSA). The Company plans to obtain an Investigational New Drug
(IND) for G-115 in the third quarter
2011.
|
|
n
|
GenSpera
is supported by a management team with extensive experience in identifying
oncology treatments and bringing them to the clinic as well as its
Scientific Advisory Board, which is composed of members who are all
inventors of the technology as well as
shareholders.
|
|
n
|
As
of March 31, 2010, the Company’s cash and cash equivalent position was
~$2.7 million. In May 2010, GenSpera raised an additional $2.7 million in
gross proceeds.
|
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 39
|
 |
Historical
Financial Results
Table 10,
Table 11 (page 41), and Table 12 (page 42) provide a summary of GenSpera’s key
historical financial statements: its Condensed Statements of Losses, Condensed
Balance Sheets, and Condensed Statements of Cash Flows.
|
Table
10
|
|
GenSpera,
Inc.
|
|
(A
Development Stage Company)
|
|
CONDENSED
STATEMENTS OF LOSSES
|
|
FOR
THE THREE MONTHS ENDED MARCH 31, 2010 AND 2009
|
|
AND FOR THE PERIOD FROM INCEPTION (NOVEMBER 21,
2003) TO MARCH 31, 2010
|
|
(Unaudited)
|
|
Cumulative Period
|
||||||||||||
|
from
|
||||||||||||
|
November 21, 2003
|
||||||||||||
|
(date of
|
||||||||||||
|
inception) to
|
||||||||||||
|
Three Months ended March 31,
|
March 31,
|
|||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
Operating
expenses:
|
||||||||||||
|
General
and administrative expenses
|
$ | 395,880 | $ | 199,717 | $ | 3,110,269 | ||||||
|
Research
and development
|
354,065 | 309,502 | 5,865,606 | |||||||||
|
Total
operating expenses
|
749,945 | 509,219 | 8,975,875 | |||||||||
|
Loss
from operations
|
(749,945 | ) | (509,219 | ) | (8,975,875 | ) | ||||||
|
Finance
cost
|
— | (472,938 | ) | (518,675 | ) | |||||||
|
Change
in fair value of derivative liability
|
(1,423,492 | ) | (572,785 | ) | (2,854,042 | ) | ||||||
|
Interest
income (expense), net
|
3,373 | (2,608 | ) | (13,001 | ) | |||||||
|
Loss
before provision for income taxes
|
(2,170,064 | ) | (1,557,550 | ) | (12,361,593 | ) | ||||||
|
Provision
for income taxes
|
— | — | — | |||||||||
|
Net
loss
|
$ | (2,170,064 | ) | $ | (1,557,550 | ) | $ | (12,361,593 | ) | |||
|
Net
loss per Common Share, basic and diluted
|
$ | (0.14 | ) | $ | (0.12 | ) | ||||||
|
Weighted
average shares outstanding
|
15,649,956 | 12,699,314 | ||||||||||
|
Source:
GenSpera, Inc.
|
||||||||||||
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 40
|
 |
|
Table
11
|
||||||||
|
GenSpera,
Inc.
|
||||||||
|
(A
Development Stage Company)
|
||||||||
|
CONDENSED
BALANCE SHEETS
|
||||||||
|
March
31,
|
December
31,
|
|||||||
|
2010
|
2009
|
|||||||
|
(Unaudited)
|
||||||||
|
Assets
|
||||||||
|
Current
assets:
|
||||||||
|
Cash
|
$ | 2,711,281 | $ | 2,255,311 | ||||
|
Total
current assets
|
2,711,281 | 2,255,311 | ||||||
|
Fixed
assets, net of accumulated depreciation of $1,500 and $708
|
14,333 | 15,125 | ||||||
|
Intangible
assets, net of accumulated amortization of $30,695 and
$26,858
|
153,473 | 157,310 | ||||||
|
Total
assets
|
$ | 2,879,087 | $ | 2,427,746 | ||||
|
Liabilities
and stockholders' deficit
|
||||||||
|
Current
liabilities:
|
||||||||
|
Accounts
payable and accrued expenses
|
$ | 232,070 | $ | 78,198 | ||||
|
Accrued
interest - stockholder
|
9,195 | 8,107 | ||||||
|
Convertible
Note payable - stockholder, current portion
|
35,000 | 35,000 | ||||||
|
Total
current liabilities
|
276,265 | 121,305 | ||||||
|
Convertible
Note payable, net of discount of $0 and $11,046
|
— | — | ||||||
|
Convertible
Notes payable - stockholder, long-term portion
|
70,000 | 70,000 | ||||||
|
Derivative
liabilities
|
3,655,387 | 2,290,686 | ||||||
|
Total
liabilities
|
4,001,652 | 2,481,991 | ||||||
|
Commitments
and contingencies
|
||||||||
|
Stockholders'
equity deficit:
|
||||||||
|
Preferred
Stock, par value $.0001 per share; 10,000,000 shares authorized,
none issued and outstanding
|
— | — | ||||||
|
Common
Stock, par value $.0001 per share; 80,000,000 shares authorized,
16,033,187 and 15,466,446 shares issued and
outstanding
|
1,603 | 1,547 | ||||||
|
Additional
paid-in capital
|
11,237,425 | 10,135,737 | ||||||
|
Deficit
accumulated during the development stage
|
(12,361,593 | ) | (10,191,529 | ) | ||||
|
Total
stockholders' equity deficit
|
(1,122,565 | ) | (54,245 | ) | ||||
|
Total
liabilities and stockholders' equity deficit
|
$ | 2,879,087 | $ | 2,427,746 | ||||
|
Source:
GenSpera, Inc.
|
||||||||
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 41
|
 |
|
Table
12
|
||||||||||||
|
GenSpera,
Inc.
|
||||||||||||
|
(A
Development Stage Company)
|
||||||||||||
|
CONDENSED
STATEMENTS OF CASH FLOWS
|
||||||||||||
|
FOR
THE THREE MONTHS ENDED MARCH 31, 2010 AND 2009
|
||||||||||||
|
AND
FOR THE PERIOD FROM INCEPTION (NOVEMBER 21, 2003) TO MARCH 31,
2010
|
||||||||||||
|
(Unaudited)
|
||||||||||||
|
Cumulative
Period
|
||||||||||||
|
from
|
||||||||||||
|
November
21, 2003
|
||||||||||||
|
(date
of inception) to
|
||||||||||||
|
Three
months ended March 31,
|
March
31,
|
|||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
Cash
flows from operating activities:
|
||||||||||||
|
Net
loss
|
$ | (2,170,064 | ) | $ | (1,557,550 | ) | $ | (12,361,593 | ) | |||
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
||||||||||||
|
Depreciation
and amortization
|
4,629 | 3,837 | 32,195 | |||||||||
|
Stock-based
compensation
|
186,742 | 29,554 | 2,667,086 | |||||||||
|
Warrants
issued for financing costs
|
— | 467,840 | 467,840 | |||||||||
|
Change
in fair value of derivative liability
|
1,423,492 | 572,785 | 2,854,042 | |||||||||
|
Contributed
services
|
— | — | 774,000 | |||||||||
|
Amortization
of debt discount
|
— | 5,098 | 20,675 | |||||||||
|
Changes
in assets and liabilities:
|
||||||||||||
|
Increase
(decrease) in accounts payable and accrued expenses
|
154,960 | (94,479 | ) | 267,689 | ||||||||
|
Cash
used in operating activities
|
(400,241 | ) | (572,915 | ) | (5,278,066 | ) | ||||||
|
Cash
flows from investing activities:
|
||||||||||||
|
Acquisition
of property and equipment
|
— | — | (15,833 | ) | ||||||||
|
Acquisition
of intangibles
|
— | — | (184,168 | ) | ||||||||
|
Cash
used in investing activities
|
— | — | (200,001 | ) | ||||||||
|
Cash
flows from financing activities:
|
||||||||||||
|
Proceeds
from sale of Common Stock and Warrants
|
806,210 | 699,985 | 8,034,347 | |||||||||
|
Proceeds
from exercise of Warrants
|
50,001 | — | 50,001 | |||||||||
|
Proceeds
from Convertible Notes - stockholder
|
— | — | 155,000 | |||||||||
|
Repayments
of Convertible Notes - stockholder
|
— | — | (50,000 | ) | ||||||||
|
Cash
provided by financing activities
|
856,211 | 699,985 | 8,189,348 | |||||||||
|
Net
increase in cash
|
455,970 | 127,070 | 2,711,281 | |||||||||
|
Cash,
beginning of period
|
2,255,311 | 534,290 | — | |||||||||
|
Cash,
end of period
|
$ | 2,711,281 | $ | 661,360 | $ | 2,711,281 | ||||||
|
Supplemental
cash flow information:
|
||||||||||||
|
Cash
paid for interest
|
$ | — | $ | 79 | ||||||||
|
Cash
paid for income taxes
|
$ | — | $ | — | ||||||||
|
Non-cash
financial activities:
|
||||||||||||
|
Derivative
liability reclassified to equity upon exercise of Warrants
|
$ | 58,791 | $ | — | ||||||||
|
Source:
GenSpera, Inc.
|
||||||||||||
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 42
|
 |
Risks
Some of
the information in this Executive Informational Overview®
(EIO®) relates
to future events or future business and financial performance. Such statements
can only be predictions and the actual events or results may differ from those
described due to the risks presented in GenSpera’s statements on Forms 10-K,
10-Q, as well as other forms filed from time to time. The content of this report
with respect to GenSpera has been compiled primarily from information available
to the public released by the Company through U.S. Securities and Exchange
Commission (SEC) filings. GenSpera is solely responsible for the accuracy of
this information. Information as to other companies has been prepared from
publicly available information and has not been independently verified by the
Company. Certain summaries of activities have been condensed to aid the reader
in gaining a general understanding. For more information about GenSpera, please
refer to the Company’s website at www.genspera.com.
Investors
should carefully consider the risks and information about GenSpera’s business
described below. Investors should not interpret the order in which these
considerations are presented as an indication of their relative importance. The
risks and uncertainties described below are not the only risks that the Company
faces. Additional risks and uncertainties not presently known to GenSpera or
that the Company currently believes to be immaterial may also adversely affect
its business. If any of the following risks and uncertainties develops into
actual events, GenSpera’s business, financial condition, and results of
operations could be materially and adversely affected.
RISKS
RELATING TO THE COMPANY’S STAGE OF DEVELOPMENT
As
a result of GenSpera’s limited operating history, investors cannot rely upon its
historical performance to make an investment decision.
Since
inception in 2003 and through March 31, 2010, the Company has raised
approximately $8,084,000 in capital. During this same period, GenSpera has
recorded accumulated losses totaling $12,361,593. As of March 31, 2010, the
Company had working capital of $2,435,016 and a stockholders’ deficit of
$1,122,565. GenSpera’s net losses for the two most recent fiscal years ended
December 31, 2008 and 2009 were $3,326,261 and $5,132,827, respectively. Since
inception, the Company has generated no revenue.
GenSpera’s
limited operating history means that there is a high degree of uncertainty in
its ability to achieve the following: (1) develop and commercialize its
technologies and proposed products; (2) obtain approval from the FDA; (3)
achieve market acceptance of its proposed product, if developed; (4) respond to
competition; or (5) operate the business, as management has not previously
undertaken such actions as a company. No assurances can be given as to exactly
when, if at all, GenSpera will be able to fully develop, license, commercialize,
market, sell, and derive material revenues from its proposed products in
development.
The
Company will need to raise additional capital to continue
operations.
GenSpera
currently generates no cash. The Company has relied entirely on external
financing to fund operations. Such financing has come primarily from the sale of
Common Stock to third parties and the exercise of Warrants/Options. GenSpera has
expended and will continue to expend substantial amounts of cash in the
development, preclinical, and clinical testing of its proposed products. The
Company will require additional cash to conduct drug development, to establish
and conduct preclinical and clinical trials, and for general working capital
needs. GenSpera anticipates that it will require an additional $13 million to
take its lead drug through Phase II clinical evaluations, which is currently
anticipated to occur in the fourth quarter 2012.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 43
|
 |
As of
March 31, 2010, GenSpera had cash on hand of approximately $2,711,000, and an
additional $2,695,000 was raised in a financing completed in May 2010.
Collectively, the Company anticipates that these funds can support its
operations through June 2011. Presently, the Company has an average monthly cash
burn rate of roughly $185,000, which GenSpera expects to increase to
approximately $550,000
per month in the second half of 2010 as the Company funds development of G-115
and manufactures more G-202 in preparation for the Phase II clinical program. It
is expected that the monthly burn rate will decrease considerably in the first
half of 2011 and then increase again with the advent of Phase II clinical
studies. These projections are based upon the assumption that the Company does
not engage in an extraordinary transaction or otherwise face unexpected events
or contingencies. Accordingly, GenSpera will need to raise additional capital to
fund anticipated operating expenses after June 2011. In the event the Company is
not able to secure financing, GenSpera may have to delay, reduce the scope of,
or eliminate one or more of its research, development, or commercialization
programs. Any such change may materially harm its business, financial condition,
and operations.
GenSpera’s
long-term capital requirements are expected to depend on many
factors:
|
n
|
its
development programs;
|
|
n
|
the
progress and costs of preclinical studies and clinical
trials;
|
|
n
|
the
time and costs involved in obtaining regulatory
clearance;
|
|
n
|
the
costs involved in preparing, filing, prosecuting, maintaining, and
enforcing patent claims;
|
|
n
|
the
costs and the ability of the Company to license its
products;
|
|
n
|
competing
technological and market
developments;
|
|
n
|
market
acceptance of its proposed products, if developed;
and
|
|
n
|
the
costs for recruiting and retaining employees, consultants, and
professionals.
|
GenSpera
cannot assure investors that financing, whether from external sources or related
parties, will be available if needed or on favorable terms. If additional
financing is not available when required or is not available on acceptable
terms, the Company may be unable to fund operations and planned growth, develop
or enhance its technologies, take advantage of business opportunities. or
respond to competitive market pressures.
Raising
needed capital may be difficult as a result of GenSpera’s limited operating
history.
When
making investment decisions, investors typically look at a company’s historical
performance in evaluating the risks and operations of the business and the
business’s future prospects. GenSpera’s limited operating history makes such
evaluation and an estimation of its future performance substantially more
difficult. As a result, investors may be unwilling to invest in the Company or
such investment may be on terms or conditions that are not acceptable. If
GenSpera is unable to secure such additional finance, it may need to cease
operations.
The
Company may not be able to commercially develop its technologies.
GenSpera
has concentrated its research and development on its prodrug technologies. The
Company’s ability to generate revenue and operate profitably will depend on its
ability to develop these technologies for human applications. GenSpera’s
technologies are primarily directed toward developing therapeutic cancer agents.
The Company cannot guarantee that the results obtained in the preclinical and
clinical evaluation of its therapeutic agents will be sufficient to warrant FDA
approval. Even if GenSpera’s therapeutic agents are approved by the FDA, there
is no guarantee that they will exhibit an enhanced efficacy relative to
competing therapeutic modalities such that they will be adopted by the medical
community. Without significant adoption by the medical community, the Company’s
agents will have limited commercial potential, which could harm GenSpera’s
ability to generate revenues, operate profitably, or remain a viable
business.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 44
|
 |
Inability
to complete preclinical and clinical testing and trials will impair GenSpera’s
viability.
In the
first quarter 2010, the Company commenced its first clinical trials of G-202 at
the University of Wisconsin Carbone Cancer Center in Madison, Wisconsin, and at
the Sydney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
Although GenSpera has commenced its clinical trials, the outcome of the trials
is uncertain and, if the Company is unable to satisfactorily complete such
trials or if such trials yield unsatisfactory results, GenSpera will be unable
to commercialize its proposed products. No assurances can be given that the
Company’s clinical trials will be successful. The failure of such trials could
delay or prevent regulatory approval and could harm GenSpera’s ability to
generate revenues, operate profitably, or remain a viable business.
Future
financing will result in dilution to existing stockholders.
GenSpera
will require additional financing in the future. The Company is authorized to
issue 80 million shares of Common Stock and 10 million shares of Preferred
Stock. Such securities may be issued without the approval or consent of
GenSpera’s stockholders. The issuance of equity securities in connection with a
future financing will result in a decrease of the Company’s current
stockholders’ percentage ownership.
RISKS
RELATING TO INTELLECTUAL PROPERTY AND GOVERNMENT REGULATION
GenSpera
may not be able to withstand challenges to its intellectual property (IP)
rights.
The
Company relies on IP, including issued and pending patents, as the foundation of
its business. GenSpera’s IP rights may come under challenge. No assurances can
be given that, even if issued, the Company’s patents will survive claims
alleging invalidity or infringement on other patents. The viability of
GenSpera’s business will suffer if such patent protection becomes limited or is
eliminated.
The
Company may not be able to adequately protect its IP.
Considerable
research with regard to GenSpera’s technologies has been performed in countries
outside the U.S. The laws protecting IP in some of those countries may not
provide protection for the Company’s trade secrets and IP. If GenSpera’s trade
secrets or IP are misappropriated in those countries, the Company may be without
adequate remedies to address the issue. At present, GenSpera is not aware of any
infringement of its IP. In addition to patents, the Company relies on
confidentiality and assignment of invention agreements to protect its IP. These
agreements provide for contractual remedies in the event of misappropriation.
GenSpera does not know to what extent, if any, these agreements and any remedies
for their breach will be enforced by a court. In the event the Company’s IP is
misappropriated or infringed upon and an adequate remedy is not available, its
future prospects will greatly diminish.
GenSpera’s
proposed products may not receive FDA approval.
The FDA
and comparable government agencies in foreign countries impose substantial
regulations on the manufacture and marketing of pharmaceutical products through
lengthy and detailed laboratory, preclinical, and clinical testing procedures,
sampling activities, and other costly and time-consuming procedures.
Satisfaction of these regulations typically takes several years or more and
varies largely based upon the type, complexity, and novelty of the proposed
product. On September 4, 2009, GenSpera received approval from the FDA for its
first IND to commence clinical trials with G-202. Although the Company began the
G-202 Phase I clinical trial on January 19, 2010, GenSpera cannot assure
investors that it will successfully complete the trial. Further, the Company
cannot yet accurately predict when it might first submit any product license
application for FDA approval or whether any such product license application
would be granted on a timely basis, if at all. Any delay in obtaining, or
failure to obtain, such approvals could have a materially adverse effect on the
commercialization of GenSpera’s products and the viability of the
Company.
|
CRYSTAL RESEARCH ASSOCIATES, LLC
|
EXECUTIVE INFORMATIONAL OVERVIEW®
|
PAGE 45
|
 |
GENERAL
RISKS RELATING TO GENSPERA’S BUSINESS AND BUSINESS MODEL
The
Company depends on Dr. Craig A. Dionne, its chief executive officer, chief
financial officer, president, and chairman, and Dr. Russell Richerson, the
Company’s chief operating officer and secretary, for its continued
operations.
GenSpera
only has two full-time employees. The loss of Dr. Dionne or Dr. Richerson would
be detrimental to the business. Although the Company has entered into employment
agreements with Dr. Dionne and Dr. Richerson, there can be no assurance that
these individuals will continue to provide services to GenSpera. A voluntary or
involuntary termination of employment by Dr. Dionne or Dr. Richerson could have
a materially adverse effect on the business. Further, as part of their
employment agreements, Dr. Dionne and Dr. Richerson agreed to not compete with
the Company for a certain amount of time following the termination of their
employment. Once the applicable time of these provisions expires, Dr. Dionne and
Dr. Richerson may be employed by a competitor of GenSpera in the
future.
GenSpera may be required to make
significant payments to members of its management team if the Company terminates
their employment or
institutes a change of control.
GenSpera
is a party to employment agreements with Dr. Dionne and Dr. Richerson. In the
event that the Company terminates the employment of either of these executives
or experiences a change in control or, in certain cases, if such executives
terminate their employment with GenSpera, such executives will be entitled to
receive certain severance and related payments. Additionally, in such instance,
certain securities held by Dr. Dionne and Dr. Richerson will become immediately
vested and exercisable. Upon the occurrence of any such event, the Company’s
obligation to make such payments could significantly impact its working capital
and, accordingly, GenSpera’s ability to execute its business plan, which could
have a materially adverse effect on the business. Also, these provisions may
discourage potential takeover attempts.
The
Company will require additional personnel to execute its business
plan.
GenSpera’s
anticipated growth and expansion into areas and activities requiring additional
expertise—such as clinical testing, regulatory compliance, manufacturing, and
marketing—may require the addition of new management personnel and the
development of additional expertise by existing management. There is intense
competition for qualified personnel in such areas. There can be no assurance
that the Company will be able to continue to attract and retain the qualified
personnel necessary for the development of its business.
GenSpera’s
competitors have significantly greater experience and financial
resources.
The
Company competes against numerous companies, many of which have substantially
greater financial and other resources than GenSpera. Several such enterprises
have research programs and efforts to treat the same diseases that GenSpera
targets. Companies such as Merck and Ipsen, among others, have substantially
greater resources and experience than the Company does and are situated to
compete with GenSpera effectively. As a result, the Company’s competitors may
bring rival products to market that would result in a decrease in demand for
GenSpera’s product, if developed, which could have a materially adverse effect
on the viability of the Company.
GenSpera intends to rely exclusively
upon the third-party FDA-approved manufacturers and suppliers for its
products.
The
Company currently has no internal manufacturing capability and will rely
exclusively on FDA-approved licensees, strategic partners, or third-party
contract manufacturers or suppliers. Should GenSpera be forced to manufacture
its products, the Company cannot give investors any assurance that it will be
able to develop internal manufacturing capabilities or procure third-party
suppliers. In the event that GenSpera seeks third-party suppliers, they may
require the Company to purchase a minimum amount of materials or could require
other unfavorable terms. Any such event would materially impact GenSpera’s
prospects and could delay the development and sale of its products. Moreover,
the Company cannot give investors any assurance that any contract manufacturers
or suppliers that it selects will be able to supply products in a timely or
cost-effective manner or in accordance with applicable regulatory requirements
or GenSpera’s specifications.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 46
|
 |
The Company’s business is dependent
upon securing sufficient quantities of a natural product that currently grows
in very specific
locations outside of the U.S.
The
therapeutic component of GenSpera’s products, including G-202, is referred to as
12ADT. 12ADT functions by dramatically raising the levels of calcium inside
cells, leading to cell death. 12ADT is produced from a material called
thapsigargin. Thapsigargin is derived from the seeds of a plant referred to as
Thapsia garganica
(T. garganica), which
grows along the coastal regions of the Mediterranean Sea. GenSpera currently
secures the seeds from Thapsibiza S.L., a third-party supplier. There can be no
assurances that the countries from which the Company can secure T. garganica will continue to
allow Thapsibiza to collect such seeds or to export the seeds to the U.S. In the
event that GenSpera is no longer able to import these seeds, the Company will
not be able to produce its proposed drug and its business will be adversely
affected.
The
current manufacturing process of G-202 requires acetonitrile.
The
current manufacturing process for G-202 requires the common solvent
acetonitrile. Beginning in late 2008, there was a temporary worldwide shortage
of acetonitrile for a number of reasons. During that period of time, GenSpera
observed that the available supply of acetonitrile was of variable quality, some
of which is not suitable for the Company’s purposes. If GenSpera is unable to
successfully change its manufacturing methods to avoid the reliance upon
acetonitrile, the Company may incur prolonged production timelines and increased
production costs if an acetonitrile shortage reoccurs. In an extreme case, this
situation could adversely affect GenSpera’s ability to manufacture G-202
altogether, thus significantly impacting the Company’s future
operations.
In order to secure market share and
generate revenues, GenSpera’s proposed products must be accepted by the
healthcare
community.
The
Company’s proposed products, if approved for marketing, may not achieve market
acceptance as hospitals, physicians, patients, or the medical community in
general may decide not to accept and use them. GenSpera is attempting to develop
products that will likely be first approved for marketing in late-stage cancer
where there is no truly effective standard of care. If approved for use in the
late stages of disease progression, the drugs will then be evaluated in earlier
stages where they would represent substantial departures from established
treatment methods and will compete with a number of conventional drugs and
therapies manufactured and marketed by major pharmaceutical companies. It is too
early in the development cycle for GenSpera to accurately predict its major
competitors. Nonetheless, the degree of market acceptance of any of GenSpera’s
developed products will depend on the following factors:
|
n
|
its
demonstration to the medical community of the clinical efficacy and safety
of its proposed products;
|
|
n
|
its
ability to create products that are superior to alternatives currently on
the market;
|
|
n
|
its
ability to establish in the medical community the potential advantage of
GenSpera’s treatments over alternative treatment methods;
and
|
|
n
|
the
reimbursement policies of government and third-party
payors.
|
If the
healthcare community does not accept the Company’s products for any of the
foregoing reasons or for any other reason, GenSpera’s business will be
materially harmed.
The
Company may be required to secure land for cultivation and harvesting of T. garganica.
GenSpera
believes that it can satisfy its needs for clinical development of G-202 through
completion of Phase III clinical studies from T. garganica that grows
naturally in the wild. In the event that G-202 is approved for commercial
marketing, the Company’s current supply of T. garganica may not be
sufficient for the anticipated demand. GenSpera estimates that in order to
secure sufficient quantities of T. garganica to commercialize
a product comprising G-202, the Company will need to secure roughly 1,000 acres
of land to cultivate and grow T. garganica. GenSpera
anticipates that the cost to lease such land would be
$400,000 per year but has not yet fully assessed what other costs would be
associated with a full-scale farming operation. There can be no assurances that
the Company can secure such acreage or, even if GenSpera is able to do so, that
it could adequately grow sufficient quantities of T. garganica to satisfy any
commercial objectives that involve G-202. The Company’s inability to secure
adequate seeds will result in GenSpera not being able to develop and manufacture
its proposed drug and will adversely impact its business.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 47
|
 |
T. garganica and thapsigargin
can cause severe skin irritation.
It has
been known for centuries that the plant T. garganica can cause severe
skin irritation when contact is made between the plant and the skin. In 1978,
thapsigargin was determined to be the skin-irritating component of the plant
T. garganica. The
therapeutic component of GenSpera’s products, including G-202, is derived from
thapsigargin. The Company obtains thapsigargin from the above-ground seeds of
T. garganica, which are
harvested by hand. Those who harvest the seeds must wear protective clothing and
gloves to avoid skin contact. Although GenSpera obtains the seeds from a
third-party contractor located in Spain who has contractually waived any and all
liability associated with collecting the seeds, it is possible that the
contractor or those employed by the contractor may suffer medical issues related
to the harvesting and subsequently seek compensation from the Company via, for
example, litigation. Despite GenSpera’s contractual relationship with the
third-party contractor, no assurances can be given that the Company will not be
the subject of litigation related to harvesting.
The
synthesis of 12ADT must be conducted in special facilities.
There are
a limited number of manufacturing facilities qualified to handle and manufacture
therapeutic toxic agents and compounds. This limits the potential number of
possible manufacturing sites for GenSpera’s therapeutic compounds derived from
T. garganica. No
assurances can be provided that these facilities will be available for the
manufacture of the Company’s compounds under its time schedules or within the
parameters of its manufacturing budget. In the event facilities are not
available for manufacturing GenSpera’s compounds, its business and future
prospects will be adversely affected.
G-202
has not been subjected to large-scale manufacturing procedures.
To date,
G-202 has only been manufactured at a scale adequate to supply early-stage
clinical trials. There can be no assurances that the current procedure for
manufacturing G-202 will work at a larger scale adequate for commercial needs.
In the event that G-202 cannot be manufactured in sufficient quantities,
GenSpera’s future prospects could be significantly impacted.
The
Company may not have adequate insurance coverage.
The
testing, manufacturing, marketing, and sale of human therapeutic products entail
an inherent risk of product liability claims. GenSpera cannot assure investors
that substantial claims will not be asserted against the Company. In the event
that GenSpera is forced to expend significant funds on defending such claims
beyond its current coverage, and in the event those funds come from operating
capital, the Company will be required to reduce its business activities, which
could lead to significant losses.
Provisions in Delaware law and
executive employment agreements may prevent or delay a change of control.
GenSpera
is subject to the Delaware anti-takeover laws regulating corporate takeovers.
These anti-takeover laws prevent Delaware corporations from engaging in a merger
or sale of more than 10% of its assets with any stockholder, including all
affiliates and associates of the stockholder, who owns 15% or more of the
corporation’s outstanding voting stock for three years following the date that
the stockholder acquired 15% or more of the corporation’s assets unless one of
the following exemptions applies:
|
n
|
the
Board of Directors approved the transaction in which the stockholder
acquired 15% or more of the corporation’s
assets;
|
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 48
|
 |
|
n
|
after
the transaction in which the stockholder acquired 15% or more of the
corporation’s assets, the stockholder owned at least 85% of the
corporation’s outstanding voting stock, excluding shares owned by
directors, officers, and employee stock plans in which employee
participants do not have the right to determine confidentially whether
shares held under the plan will be tendered in a tender or exchange offer;
or
|
|
n
|
on
or after this date, the merger or sale is approved by the Board of
Directors and the holders of at least two-thirds of the outstanding voting
stock that is not owned by the
stockholder.
|
A
Delaware corporation may opt out of the Delaware anti-takeover laws if its
certificate of incorporation or bylaws so provide. GenSpera has not opted out of
the provisions of the anti-takeover laws. As such, these laws could prohibit or
delay mergers or other takeover or change of control of GenSpera and may
discourage attempts by other companies to acquire the Company.
In
addition, employment agreements with certain executive officers provide for the
payment of severance and acceleration of the vesting of Options and Restricted
Stock in the event of termination of the executive officer following a change of
control of GenSpera. These provisions could have the effect of discouraging
potential takeover attempts.
RISKS
RELATING TO GENSPERA’S COMMON STOCK
There
is no established public market for the Company’s securities.
On
September 18, 2009, GenSpera’s Common Shares began trading on the OTC.BB.
Notwithstanding, there has been sporadic trading in the Company’s Common Shares.
Accordingly, there is no established public market for GenSpera’s securities. An
investment in the Company’s Common Stock should be considered totally illiquid.
No assurances can be given that a public market for GenSpera’s securities will
ever materialize. Additionally, even if a public market for the Company’s
securities develops and its securities become traded, the trading volume may be
limited, making it difficult for an investor to sell shares.
GenSpera
faces risks related to compliance with corporate governance laws and financial
reporting standards.
The
Sarbanes-Oxley Act of 2002 as well as related new rules and regulations
implemented by the SEC and the Public Company Accounting Oversight Board require
changes in the corporate governance practices and financial reporting standards
for public companies. These new laws, rules, and regulations, including
compliance with Section 404 of the Sarbanes-Oxley Act of 2002 relating to
internal control over financial reporting, will materially increase the
Company’s legal and financial compliance costs and make some activities more
time-consuming and burdensome. As a result, management may be required to devote
more time to compliance, which could result in a reduced focus on development,
thereby adversely affecting the Company’s development activities. Also, the
increased costs will require the Company to seek financing sooner that may
otherwise have been required.
Starting
in 2007, Section 404 of the Sarbanes-Oxley Act of 2002 requires a company’s
management to assess the company’s internal control over financial reporting
annually and include a report on such assessment in its Annual Report filed with
the SEC. For small reporting companies with fiscal years ending on or after June
15, 2010, independent registered public accounting firms will be required to
audit both the design and operating effectiveness of internal controls and
management’s assessment of the design and the operating effectiveness of such
internal controls. If this deadline is not extended, GenSpera will be required
to expand substantial capital in connection with compliance.
Because
of GenSpera’s limited resources, the Company’s management has concluded that its
internal control over financial reporting may not be effective in providing
reasonable assurance regarding the reliability of financial reporting and the
preparation of financial statements for external purposes in accordance with
U.S. generally accepted accounting principles. To mitigate the current limited
resources and limited employees, GenSpera relies heavily on direct management
oversight of transactions, along with the use of legal and accounting
professionals. As it grows, GenSpera plans to increase its employee count, which
will enable the Company to implement adequate segregation of duties within the
Committee of Sponsoring Organizations of the Treadway Commission internal
control framework.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 49
|
 |
GenSpera
does not intend to pay cash dividends.
The
Company does not anticipate paying cash dividends in the foreseeable future.
Accordingly, any gains on an investment in GenSpera will need to come through an
increase in the price of the Company’s Common Stock. The lack of a market for
GenSpera’s Common Stock makes such gains highly unlikely.
The
Company’s Board of Directors has broad discretion to issue additional
securities.
GenSpera
is entitled under its Certificate of Incorporation to issue up to 80,000,000
Common and 10,000,000 “blank check” Preferred Shares. Blank check Preferred
Shares provide the Board of Directors broad authority to determine voting,
dividend, conversion, and other rights. As of March 31, 2010, the Company had
issued and outstanding 16,033,187 Common Shares and 8,003,903 Common Shares
reserved for issuance upon the exercise of current outstanding Options,
Warrants, and Convertible Securities. Accordingly, GenSpera will be entitled to
issue up to 55,962,910 additional Common Shares and 10,000,000 additional
Preferred Shares. The Company’s Board may generally issue those Common and
Preferred Shares, or Options or Warrants to purchase those shares, without
further approval by GenSpera’s shareholders. Any Preferred Shares that GenSpera
may issue will have such rights, preferences, privileges, and restrictions as
may be designated from time-to-time by its Board, including preferential
dividend rights, voting rights, conversion rights, redemption rights, and
liquidation provisions. It is likely that the Company will be required to issue
a large amount of additional securities to raise capital to further its
development and marketing plans. It is also likely that GenSpera will be
required to issue a large amount of additional securities to directors,
officers, employees, and consultants as compensatory grants in connection with
their services, both in the form of stand-alone grants or under the Company’s
various stock plans. The issuance of additional securities may cause substantial
dilution to GenSpera’s shareholders.
The Company’s officers and scientific
advisors beneficially own roughly 41% of GenSpera’s outstanding Common
Shares.
GenSpera’s
officers and scientific advisors own approximately 41% of its issued and
outstanding Common Shares. As a consequence of their level of stock ownership,
the group will substantially retain the ability to elect or remove members of
GenSpera’s Board of Directors and thereby control management. This group of
shareholders has the ability to significantly control the outcome of corporate
actions requiring shareholder approval, including mergers and other changes of
corporate control, going private transactions, and other extraordinary
transactions any of which may be in opposition to the best interest of the other
shareholders and may negatively impact the value of an investment in the
Company.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 50
|
 |
Recent
Events
Recently,
GenSpera’s technology and its founders have been featured in the San Antonio Business Journal,
MSN Money (www.moneycentral.msn.com),
and PCRP Perspectives,
a newsletter issued as part of the U.S. Department of Defense’s Prostate Cancer
Research Program (PCRP). An overview of GenSpera’s recent announcements is
provided below, referring the reader to the Company’s website for complete press
releases (www.genspera.com).
05/25/2010—GenSpera, Inc.
announced that on May 18, 2010, the Company entered into a Securities Purchase
Agreement with a number of institutional and accredited investors. GenSpera
offered and sold the investors 1,347,500 units at $2.00 per unit resulting in
gross proceeds of approximately $2,695,000.
05/18/2010—Announced that Dr.
Craig A. Dionne, the Company’s chief executive officer, chief financial officer,
president, and chairman, was expected to present a corporate overview, including
a company update and future outlook, at the Source Capital Group Small Cap
Virtual Conference on Tuesday, May 18, 2010. The virtual conference showcased
emerging small- and micro-cap companies in a live forum.
02/02/2010—Announced that the
U.S. Patent and Trademark Office (USPTO) issued its patent application, entitled
“Tumor Activated Prodrugs,” as U.S. Patent No. 7,635,682. The patent covers the
composition and potential uses of G-115, the Company’s second anticancer drug in
development, further strengthening GenSpera’s intellectual property (IP)
position for G-115 and its use in prostate cancer and other prostate pathologies
including enlarged prostate. The term of the patent extends to
2026.
01/27/2010—Announced that the
first patient was treated in the Phase I clinical study of GenSpera’s lead
prodrug candidate, G-202, at the University of Wisconsin Carbone Cancer Center
in Madison, Wisconsin. The trial is also being conducted at the Sidney Kimmel
Comprehensive Cancer Center at Johns Hopkins in Baltimore,
Maryland.
11/03/2009—Announced that its
Common Stock began trading on November 2, 2009, on the Over-the-Counter Bulletin
Board (OTC.BB) under the symbol “GNSZ.”
09/11/2009—Announced that the
U.S. Food and Drug Administration (FDA) approved its Investigational New Drug
(IND) application for G-202.
08/14/2009—Received a Notice
of Effectiveness from the U.S. Securities and Exchange Commission (SEC)
accepting the Company’s Form S-1 (filed in July 2009), the general form for
registration of securities under the Securities Act of 1933, and thereby
registering GenSpera’s shares for public trading.
08/12/2009—Filed an amendment
to the previously submitted Form S-1.
07/31/2009—Filed Form S-1 with
the SEC relating to the resale of 4,516,120 shares of the Company’s Common
Stock. GenSpera’s Common Stock was not then traded on any market or exchange,
and the Company had not yet applied for listing or quotation on any public
market. GenSpera anticipated seeking sponsorship to trade its Common Stock on
the OTC.BB once the registration became effective.
07/29/2009—Entered into a
Securities Purchase Agreement with a number of accredited investors. Per the
terms of the agreement, GenSpera sold units aggregating approximately $907,000
to the investors. The price per unit was $1.50. Each unit consisted of the
following: (1) one share of the Company’s Common Stock; and (2) one half Common
Stock Purchase Warrant. The Warrants had a five-year term and allowed investors
to purchase GenSpera’s Common Shares at $3.00 per share. The Warrants contained
anti-dilution protection against stock splits, stock dividends, and similar
transactions. GenSpera incurred $79,583 in fees and expenses for the
transaction. The Company also issued 40,001 additional Common Stock Purchase
Warrants under the same terms as the investor Warrants to compensate certain
finders.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 51
|
 |
07/24/2009—Received
notification from the FDA that its IND for G-202 was on clinical hold pending
the Company’s response to certain questions provided by the FDA. The questions
concerned the design of GenSpera’s proposed Phase I clinical trial.
07/10/2009—Issued a Common
Stock Purchase Warrant to purchase 150,000 Common Shares as reimbursement for
due diligence expenses. The Warrants had a five-year term and entitled the
holder to purchase GenSpera’s Common Stock at $3.00 per share.
06/29/2009—Entered into a
Securities Purchase Agreement with a number of accredited investors. Per the
terms of the agreement, GenSpera sold the investors units aggregating roughly
$2,131,000 at a price of $1.50 per unit. Each unit consisted of the following:
(1) one share of the Company’s Common Stock; and (2) one half Common Stock
Purchase Warrant. The Warrants had a five-year term and allowed investors to
purchase GenSpera’s Common Shares at $3.00 per share. The Warrants also
contained anti-dilution protection in the event of stock splits, stock
dividends, or other similar transactions. GenSpera incurred $142,467 in fees and
expenses for the transaction, $50,000 of which had been paid through the
issuance of 33,334 units. The Company also issued 43,894 additional Common Stock
Purchase Warrants under the same terms as the investor Warrants to compensate
certain finders.
06/23/2009—Submitted an IND
application to the FDA for G-202.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 52
|
 |
Glossary
Amino Acid—Organic compounds
that are the building blocks of many components found in cells. The sequence of
amino acids affects how these components react biologically.
Angiogenesis—The physiological
process involving the growth of new blood vessels from pre-existing vessels. It
is a normal process in growth and development as well as in wound
healing.
Angiotoxic—Toxic to blood
vessels.
Anti-angiogenic Drugs—Drugs
that attempt to interrupt tumor-associated angiogenesis. Current anti-angiogenic
drugs, such as Genentech’s Avastin®, act by
blocking the formation of new blood vessels supporting the tumor.
Apoptosis—Programmed cell
death. This physiological process is necessary for the elimination of
superfluous, diseased, or damaged cells.
Assays—Biological tests,
measurements, or analyses to determine whether compounds have the desired effect
either in a living organism, outside an organism, or in an artificial
environment.
Benign Prostatic Hypertrophy
(BPH)—Enlargement of the prostate gland, which surrounds the male
urethra, causing frequent urination. This condition is common in older
men.
Cancer Stem
Cells—Self-renewing cells responsible for sustaining a cancer and for
producing differentiated progeny that form the bulk of the cancer. Cancer stem
cells identified in leukemia and certain solid tumors are believed to be
critical therapeutic targets.
Characterization—Analysis that
may include chemistry data, purity, potency, quality, stability, strength,
pharmacokinetics, dose response, and efficacy.
Chemotherapies—The treatment
of cancers with cytotoxic agents.
Chronic Lymphocytic
Leukemia—The most common form of childhood leukemia, also known as
lymphoblastic leukemia. In this disease, the bone marrow produces large
quantities of immature lymphocytes (white blood cells).
Contact Inhibition—Cessation
of cellular movement, growth, and division upon contact with other
cells.
CWR22R-H—A cell line derived
from human prostatic carcinoma.
Cytosolic—Of or pertaining to
the cytosol, the aqueous part of the cytoplasm within which various particles
and organelles are suspended.
Cytotoxin—Any drug that has a
toxic effect on cells. Cytotoxic drugs are commonly used in chemotherapy to
inhibit the proliferation of cancerous cells.
DACH Platinum—A form of
platinum that has a similar mechanism of action to other derivatives of
platinum. However, it is the only form of platinum shown to be clinically
effective in the treatment of colorectal cancer.
Doxorubicin—A chemotherapeutic
administered via injection to treat a variety of cancers, including bladder,
breast, head and neck, some types of leukemia, ovary, and prostate cancers,
among others.
Drug Resistance—The ability of
bacteria and other disease-causing microorganisms to withstand a drug to which
they were once sensitive (and were once stalled or killed
outright).
Endothelial Cells—Cells that
line the blood vessels of the body.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 53
|
 |
Enzymes—Any of several complex
proteins that are produced by cells and act as catalysts in specific biochemical
reactions. For GenSpera’s purpose, select enzymes found only at tumor sites
remove the Company’s targeting/masking agent from 12ADT.
Good Laboratory Practice
(GLP)—An international standard that provides a framework to plan,
perform, monitor, record, report, and archive laboratory studies. These studies
are undertaken to generate data by which the hazards and risks to users,
consumers, and third parties can be assessed for pharmaceuticals (only
preclinical studies), agrochemicals, cosmetics, and food additives, among
others. These practices help to assure regulatory authorities that the data
submitted are a true reflection of the results obtained during the study and can
therefore be relied upon when making risk/safety assessments.
Good Manufacturing Practice
(GMP)—The quality system regulation overseen by the FDA, which includes
requirements related to the methods used in, and the facilities and controls
used for, designing, manufacturing, packaging, labeling, storing, installing,
and servicing medical devices intended for human use.
Half-Life—The time required
for half the amount of a drug to be eliminated from the body.
Harvest Period Year—The period
from May 1 until April 30 of the following year.
Hepatocellular Carcinoma—A
carcinoma derived from parenchymal cells of the liver.
Human Glandular Kallikrein 2
(hK2)—A serine protease in the human kallikrein gene family that is 80%
identical to PSA at the protein level. Similar to PSA, hK2 is expressed
primarily in the prostate, thus making hK2 an attractive biomarker for prostate
cancer development. HK2 is also the activating enzyme for GenSpera’s prodrug
candidate Ac-GKAFRR-L12ADT.
IFL Chemotherapy—Concurrent
treatment with irinotecan, leucovorin (folinic acid), and
fluorouracil.
Infuse—To administer or inject
by slowly but continuously introducing a solution into a vein.
Licochalcone A—Extract from
Chinese licorice roots.
LNCaP—A widely studied
metastatic prostate cancer cell line that is androgen responsive.
Lymphatic System—A network of
vessels in the body, separate from blood circulation, that transports immune
system cells to fight off germs, infections, and diseases. The lymphatic system
includes the lymph nodes, which act as immune system command
centers.
Maytansinoid—An agent derived
from maytansine. Maytansine is isolated from the East African shrubs Maytenus serrata and M. buchananii. It
demonstrates activity related to inhibiting or preventing the proliferation of
tumor cells, most likely due to its inhibition of DNA synthesis.
Metastasizes—Cancer spreading
from one part of the body to another.
Monoclonal Antibody—Any of a
class of highly specific antibodies produced by the clones of a single hybrid
cell formed in the laboratory by the fusion of a B-cell with a tumor cell. It is
widely used in medical and biological research.
Monotherapy—A therapy that is
effective on its own, rather than needing to be combined with other
treatments.
Multi-Kinase Inhibitor—A
therapy that targets several cancer pathways at once.
Non-Hodgkin’s
Lymphoma—Malignant tumors of the lymphatic system consisting of several
subtypes of lymphatic cancer.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 54
|
 |
Nude Mice—Immunologically
deficient mice used to permit growth of tumor cells from mice or other species,
such as humans.
Orphan Drug—A medication in
development that seeks to treat an Orphan Disease, which is a rare illness
affecting fewer than 200,000 people, or a common disease that has been ignored
because it is less prominent in the U.S. compared with developing nations.
According to the U.S. National Institutes of Health, there are approximately
7,000 of these diseases.
Peptides—Any compound
consisting of two or more amino acids, the building blocks of proteins. For
GenSpera’s purposes, when a specific peptide is attached to thapsigargin or
12ADT, it masks the activity of the cytotoxin. Once removed, the cytotoxin
becomes active again.
Pharmacokinetics—The study of
how and where drug levels are affected by absorption, distribution, metabolism,
and elimination processes in the body.
Physicochemical—Relating to
both physical and chemical properties.
Prodrug—A pharmacologic
compound that is administered in an inactive form. Once absorbed by the body, it
is metabolized and converted to the active form of the drug.
Prostate-Specific Antigen
(PSA)—A protein in the blood produced by prostate tissue that serves as a
tumor marker. PSA is also the activating enzyme for GenSpera’s prodrug
candidates G-114 and G-115.
Prostate-Specific Membrane Antigen
(PSMA)—An enzyme that is found in the blood vessels supporting tumors but
not in normal blood vessels. It is also found in normal prostate cells and in
prostate cancers. PSMA is the activating enzyme for GenSpera’s lead prodrug
candidate, G-202.
Proteases—Enzymes that aid in
the breakdown of proteins in the body.
PSMA Knockout Mice—Mice that
lack the PSMA gene.
SERCA Pump—An intracellular
protein that keeps cytosolic calcium low, allowing calcium to regulate important
cellular processes such as cell growth, division, differentiation, cell death,
and apoptosis (programmed cell death).
Substrate—The substance that
is acted upon by an enzyme.
Targeting/Masking Agent—An
agent that masks the toxicity of a cytotoxin while simultaneously helping target
the cytotoxin to a tumor site.
Taxol® (Paclitaxel)—A chemotherapy
drug administered via injection to treat several types of cancer, most commonly
ovarian, breast, and non-small cell lung cancer.
Thapsigargin—A potent and
novel cytotoxin extracted from the plant, T. garganica. It is 10- to
100-fold more potent than the National Cancer Institute’s reference
chemotherapeutic agents and is capable of killing fast-, slow-, and non-dividing
cancer cells.
TSU-Pr1—A cell line that is
derived from the human bladder carcinoma.
Unresectable—Unable to
surgically remove part or all of an organ or other structure.
Vasculature—The blood vessels
or arrangement of blood vessels in an organ or part.
Xenograft—A surgical graft of
tissue from one species to an unlike species.
|
CRYSTAL
RESEARCH ASSOCIATES, LLC
|
EXECUTIVE
INFORMATIONAL OVERVIEW®
|
PAGE 55
|
 |

|
Jeffrey
J. Kraws and Karen B. Goldfarb
Phone:
(609) 306-2274
Fax:
(609) 395-9339
Email: eio@crystalra.com
Web:
www.crystalra.com
|
Legal Notes and Disclosures:
This report has been prepared by GenSpera, Inc. (“GenSpera” or “the
Company”) with the assistance of Crystal Research Associates, LLC (“CRA”) based
upon information provided by the Company. CRA has not independently verified
such information. In addition, CRA has been compensated by the Company in cash
of fifty-eight thousand five hundred U.S. dollars and fifty thousand
Options/Warrants for its services in creating and updating the base report, for
updates, and for printing costs.
Some of
the information in this report relates to future events or future business and
financial performance. Such statements constitute forward-looking information
within the meaning of the Private Securities Litigation Act of 1995. Such
statements can be only predictions and the actual events or results may differ
from those discussed due to, among other things, the risks described in
GenSpera’s reports on its 10-K, 10-Q, and other forms filed from time to time.
The content of this report with respect to GenSpera has been compiled primarily
from information available to the public released by GenSpera. The Company is
solely responsible for the accuracy of that information. Information as to other
companies has been prepared from publicly available information and has not been
independently verified by GenSpera or CRA. Certain summaries of scientific
activities and outcomes have been condensed to aid the reader in gaining a
general understanding. For more complete information about GenSpera, the reader
is directed to the Company’s website at www.genspera.com. This report is
published solely for information purposes and is not to be construed as an offer
to sell or the solicitation of an offer to buy any security in any state. Past
performance does not guarantee future performance. Additional information about
GenSpera and its public filings, as well as copies of this report, can be
obtained in either a paper or electronic format by calling (210)
479-8112.
