Attached files
| file | filename |
|---|---|
| EX-31.2 - Tongli Pharmaceuticals (USA), Inc. | v189393_ex31-2.htm |
| EX-32.2 - Tongli Pharmaceuticals (USA), Inc. | v189393_ex32-2.htm |
| EX-23.1 - Tongli Pharmaceuticals (USA), Inc. | v189393_ex23-1.htm |
| EX-32.1 - Tongli Pharmaceuticals (USA), Inc. | v189393_ex32-1.htm |
| EX-31.1 - Tongli Pharmaceuticals (USA), Inc. | v189393_ex31-1.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
|
x
|
Annual report pursuant to
section 13 or 15(d) of the Securities Exchange Act of 1934.
|
For
the fiscal year ending March 31, 2010
OR
|
¨
|
Transition report pursuant to
Section 13 or 15(d) of the Securities Exchange Act of
1934.
|
For
the transition period from
________ to ________.
Commission
file number 000-52954
Tongli
Pharmaceuticals (USA), Inc.
(Exact
name of registrant as specified in its charter)
|
Delaware
|
84-1090791
|
|
|
(State
or other jurisdiction
of
incorporation or organization)
|
(IRS
Employer
Identification
number)
|
|
|
136-17 Maple Avenue, 11H
Flushing, NY
|
11354
|
|
|
(Address
of Principal Executive Offices)
|
(Zip
Code)
|
718-321-8380
(Registrant’s
Telephone Number, Including Area Code)
Securities
registered pursuant to Section 12(b) of the Act:
None
(Title of
Class)
Name of
each exchange on which registered
None
Securities
registered pursuant to Section 12(g) of the Act:
Common
Stock, $.001 par value per share
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes ¨ No x.
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Exchange
Act. Yes ¨ No x.
Indicate
by check mark whether the registrant (1) has filed all reports required to
be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934
during the preceding 12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been subject to such filing
requirements for the past 90 days. Yes x No ¨.
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company. See
definition of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act.
|
Large
accelerated filer ¨
|
Accelerated
filer ¨
|
|
|
Non-accelerated
filer ¨
|
Smaller
reporting company x
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act). Yes ¨ No x.
The
aggregate market value of the voting and non-voting common stock, other than
shares held by persons who may be deemed affiliates of the registrant, computed
by reference to the closing sales price for the registrant’s Common Stock on
September 30, 2009, as reported on the OTC Bulletin Board, was approximately
$3,672,475.
As of
June 25, 2010, there were 11,395,036 outstanding shares of common stock of the
registrant, par value $.001 per share.
DOCUMENTS
INCORPORATED BY REFERENCE
None
TABLE
OF CONTENTS
| Page | |||
|
Cautionary
Note On Forward Looking Statements
|
-i-
|
||
|
Part
I
|
|||
|
Item
1.
|
Business
|
1
|
|
|
Item
1A.
|
Risk
Factors
|
9
|
|
|
Item
1B.
|
Unresolved
Staff Comments
|
22
|
|
|
Item
2.
|
Description
of Properties
|
22
|
|
|
Item
3.
|
Legal
Proceedings
|
22
|
|
|
Item
4.
|
(Removed
and Reserved)
|
22
|
|
|
Part
II
|
|||
|
Item
5.
|
Market
For Common Equity, Related Stockholder Matters and Issuer Purchases of
Equity Securities
|
23
|
|
|
Item
6.
|
Selected
Financial Data
|
24
|
|
|
Item
7.
|
Management’s
Discussion and Analysis or Plan of Operation
|
24
|
|
|
Item
7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
36
|
|
|
Item
8.
|
Financial
Statements and Supplementary Data
|
36
|
|
|
Item
9.
|
Changes
In and Disagreements With Accountants On Accounting and Financial
Disclosure
|
36
|
|
|
Item
9A(T).
|
Controls
and Procedures
|
36
|
|
|
Item
9B.
|
Other
Information
|
38
|
|
|
Part
III
|
|||
|
Item
10.
|
Directors,
Executive Officers, Promoters and Control Persons; Compliance With Section
16(A) of the Exchange Act
|
39
|
|
|
Item
11.
|
Executive
Compensation.
|
41
|
|
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
43
|
|
|
Item
13.
|
Certain
Relationships and Related Party Transactions
|
44
|
|
|
Item
14.
|
Principal
Accountant Fees and Services
|
45
|
|
|
Part
IV
|
|||
|
Item
15.
|
Exhibits
|
45
|
|
|
Index
to Financial Statements
|
F-1
|
||
Unless
otherwise provided in this Annual Report on Form 10-K, references to “Tongli,”
“we,” “us,” “our” and similar terminology refer to Tongli Pharmaceuticals (USA),
Inc. and its subsidiaries.
CAUTIONARY
NOTE ON FORWARD LOOKING STATEMENTS
In
addition to historical information, this Annual Report on Form 10-K contains
forward looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. The forward-looking statements are subject
to significant known and unknown risks and uncertainties that could
cause actual results to differ materially from those reflected in such
forward-looking statements. Factors that might cause such a difference
include, but are not limited to, those discussed in the sections entitled
“Business”, “Risk Factors”, and “Management’s Discussion and Analysis or Plan of
Operation.” Readers are cautioned not to place undue reliance on these
forward-looking statements, which reflect management’s opinions only as of the
date thereof. We undertake no obligation to revise or publicly release the
results of any revision of these forward-looking statements. Readers
should carefully review the risk factors described in this Report and in other
documents that we file from time to time with the Securities and Exchange
Commission.
In some
cases, you can identify forward-looking statements by terminology such as “may,”
“will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,”
“predicts,” “projects,” “potential,” “proposed,” “intended,” or “continue” or
the negative of these terms or other comparable terminology. You should read
statements that contain these words carefully, because they discuss our
expectations about our future operating results or our future financial
condition or state other “forward-looking” information. There may be events in
the future that we are not able to accurately predict or control. You should be
aware that the occurrence of any of the events described in our risk factors and
other disclosures included in this Report could substantially harm our business,
results of operations and financial condition, and that upon the occurrence of
any of these events, the trading price of our securities could
decline. Although we believe that the expectations reflected in the
forward-looking statements are reasonable, we cannot guarantee future results,
growth rates, and levels of activity, performance or
achievements. Factors that may cause actual results, our performance
or achievements, or industry results, to differ materially from those
contemplated by such forward-looking statements include without
limitation:
|
|
·
|
obtain
sufficient working capital to support our business
plans;
|
|
|
·
|
maintain
or protect our intellectual
property;
|
|
|
·
|
maintain
our proprietary technology;
|
|
|
·
|
expand
our product offerings and maintain the quality of our
products;
|
|
|
·
|
manage
our expanding operations and continue to fill customers’ orders on
time;
|
|
|
·
|
maintain
adequate control of our expenses allowing us to realize anticipated
revenue growth;
|
|
|
·
|
the
impact of government regulation in China and
elsewhere;
|
|
|
·
|
implement
our product development, marketing, sales and acquisition strategies and
adapt and modify them as needed;
|
|
|
·
|
integrate
any future acquisitions;
|
|
|
·
|
our
implementation of required financial, accounting and disclosure controls
and procedures and related corporate governance policies;
and
|
|
|
·
|
anticipate
and adapt to changing conditions in the Chinese herbal medicines industry
resulting from changes in government regulations, mergers and acquisitions
involving our competitors, technological developments and other
significant competitive and market
dynamics.
|
Except as
required by applicable law, including the securities laws of the United States,
we do not intend to update any of the forward-looking statements to conform
these statements to actual results. The following discussion should be read in
conjunction with our financial statements and the related notes that appear
elsewhere in this report.
We cannot
give any guarantee that these plans, intentions or expectations will be
achieved. All forward-looking statements involve risks and
uncertainties, and actual results may differ materially from those discussed in
the forward-looking statements as a result of various factors, including those
factors listed above and described in the “Risk Factors” section of this
Report.
-i-
PART
I
Item
1. Business
Organization
and Business Description
Tongli
Pharmaceuticals (USA), Inc., through a wholly-owned subsidiary, Harbin Tianmu
Pharmaceuticals Co., Ltd. (“HTP” or “Tianmu Pharmaceuticals”), develops,
produces and sells a wide variety of pharmaceuticals and healthcare products in
the People’s Republic of China (“PRC” or “China”) that are based on traditional
Chinese medicine, or TCM. We were formerly known as American Tony
Pharmaceutical, Inc. (“American Tony”). The name change became effective
on October 30, 2008 and was done to better represent the origin and ongoing
business of our company.
On August
12, 2008, American Tony completed a reverse merger with Aim Smart Corporation
(“Aim Smart”), a dormant public shell, which was originally incorporated on
April 27, 1988 in the State of Colorado under the name “Gatwick, Ltd” for
the purpose of seeking out and completing a merger or acquisition with one or
more companies or businesses, and was reorganized as a Delaware corporation in
September 2007. American Tony was a holding company which was
incorporated on November 17, 2006 in the state of Delaware and has had no
significant operations since its inception. The acquisition was
effected by the merger of American Tony into a wholly-owned subsidiary of Aim
Smart.
Under the
terms of the merger agreement, the former American Tony stockholders exchanged
their shares for Aim Smart shares so that, upon the closing of the merger, the
former American Tony stockholders owned 96.7% of the outstanding shares of Aim
Smart. America Tony acquired its controlling interest in Aim Smart for a cost of
$525,000. This interest was acquired solely to effectuate the reverse merger and
was paid for with $276,000 of its own funds and a $249,000 loan from our
Chairman, Mingli Yao. Aim Smart changed its name to American Tony
upon the closing of the reverse merger. Tianmu Pharmaceuticals was
formed under laws of the PRC on November 26, 1999. In February 2007,
American Tony acquired Tianmu Pharmaceuticals through a recapitalization
transaction which was accomplished through the exchange of shares with
Heilongjiang Tongli Technology Co., Ltd. (“TT” or “Tongli Technology”), a
wholly-owned subsidiary of American Tony located in the PRC. TT owns 100% of
Tianmu Pharmaceuticals and doesn’t have any other operations since its
inception.
Our
corporate structure as of the date of this Report is as follows:
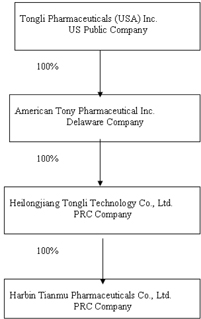
1
Industry
Background
Overview
of Traditional Chinese Medicine
In China,
Traditional Chinese Medicine is not an alternative form of therapy but is used
in the state-run hospitals alongside modern medicine. For its practitioners and
advocates, TCM is a complete medical system that is used to treat disease in all
its forms. TCM is also believed to promote long term wellness and vigor. Many
modern-day drugs have been developed from herbal sources. These include drugs
designed to treat asthma and hay fever such as ephedrine; hepatitis remedies
from fruits and licorice roots and a number of anticancer agents from trees and
shrubs.
The roots
of TCM date back thousands of years and include a number of therapeutic
approaches. These include herbal medications, acupuncture, dietary manipulation,
massage and others. Very early works of Chinese medical literature date back as
much as 2,500 years while other classics appeared approximately 2,000 years ago
during the Han Dynasty. Medicine in China continued to develop throughout the
Middle Ages when emperors commissioned the creation of various scholarly works
that compiled and documented hundreds of medicines derived from herbs, animal
sources and minerals. In addition, these works described their therapeutic uses.
In the 1950s, TCM was further modernized and reformed by the PRC
government.
The
emphasis on wellness and the avoidance of disease is considered by some to be a
key distinction between TCM and western medical practice which has been seen as
more heavily oriented toward the treatment of disease and less toward
prevention. While TCM has remained a substantial part of medical treatment in
China and throughout East Asia, recent decades have seen increasing acceptance
throughout the United States, Europe and elsewhere. This growth is, in part,
driven by increasingly educated and empowered consumers of medical care who seek
organic, natural and alternative approaches to western medical treatments and
prescription drugs. Medical doctors are also accelerating the process of
acceptance, as doctors trained in the western tradition in Europe, the United
States and elsewhere are integrating TCM and alternative treatments in their
everyday practice. Additionally, a growing number of physicians specifically
trained in TCM, acupuncture and other modalities are opening offices in
communities in the U.S. and around the world.
We
believe that the sales of TCM in China reflect the central and still growing
role these therapies play in medical care in that nation. According to Helmut
Kaiser Consultancy, in 2005, total sales revenue for Chinese herbal medicine
manufactured in China was $13.6 billion which accounted for 25.8% of all
medicine manufactured in China. This segment had total profit of $1.76 billion
which accounted for 29% of the total profit of the Chinese drug industry. In
2006, there were approximately 1,400 Chinese herbal medicine manufacturers with
an annual growth rate of 15%, much higher than the comparable period GDP growth.
According to Helmut Kaiser Consultancy, as a result of the increasing wealth of
China and an aging population, it is estimated that by 2010, China will be the
fifth largest market for herbal medicines in the world exceeding more than $24
billion in sales.
Overview
of the Chinese Market
The
People’s Republic of China is undergoing the world’s most important and powerful
economic transformation. This transformation includes the confluence of its
ancient culture with modern trends in business, technology and finance. As a
result, Chinese operating companies are capitalizing on unmatched growth
opportunities in this evolving and growing marketplace. Although average income
is approximately one-tenth that of developed western nations, business growth
and market reform-driven policies have given the country’s 1.3 billion citizens
more purchasing power than ever.
According
to a report published in Newsweek, total consumer spending in China reached $1.7
trillion in 2007, compared with $12 trillion in the U.S. In its China Consumer
Survey published in January 2010, Credit Suisse found that household income in
China of the bottom 20% of those surveyed rose by 50% since 2004, while the top
10% had grown 255% to around RMB34,000 per month. Credit Suisse expects China’s
share of global consumption to increase from 5.2% at US$1.72 trillion in 2009 to
23.1% at US$15.94 trillion in 2020, overtaking the U.S. as the largest consumer
market in the world. Further, research on
Chinese consumers by management consulting firm McKinsey classifies two million
households out of a population of 1.3 billion as “wealthy,” based on fairly
modest annual earnings of more than $30,000. An enormous middle class is rising,
however, numbering some 70 million urban households, but these still earn
$5,000-$10,000 a year. China’s National Bureau of Statistics, based on a random
survey of 65,000 urban households in China, found that the average (annual)
disposable income of urban residents in the first half of 2009 was U.S. $1,300,
an increase of 9.8% compared to the same period last year. When price factors
are deducted, this is equivalent to a real increase of 11.2%. The average
consumption expenditure amount of urban residents in the first half of 2009 was
U.S.$876, an increase of 8.9% compared to the same period last year. When price
factors are deducted, this is equal to a real increase of
10.3%.
2
TCM
Industry Drivers
We
believe that demographic, governmental and related factors in the China will be
favorable to growth and expansion of our business.
Growing Prosperity of the Chinese
People. The increased spending power of China’s population continues to
be reflected in the increased consumption of health products and medical
services between 2007 and 2010. According to Euromonitor data, spending by
Chinese people on these goods and services will increase from $100 billion in
2007 to $145 billion in 2010.
Population
and Aging
|
|
·
|
The
total population of China was 1.32 billion at the end of 2007, according
to official government estimates.
|
|
|
·
|
Due
to improved healthcare, the elderly population of China is
growing.
|
|
|
·
|
The
health/medical costs associated with care for elderly in China are
approximately five (5x) times that of younger
people.
|
|
|
·
|
China
had 170 million elderly people in 2007 but will have an expected 230
million elderly by 2015 according to “Consumer Lifestyles in China:
Consumer Trends, China’s Grey Population,” by Euromonitor,
2009.
|
|
|
·
|
The
proportion of the China’s population aged 65 and over will rise from just
10% of the overall population in 1995 to 22% by 2030, according to the
World Bank.
|
|
|
·
|
From
1995 to 2030 it is estimated that the ratio of working-age people to
pensioners will decrease from 9.7:1 to 4.2:1. China’s national estimates
vary slightly from World Bank figures, but still show in increase in the
proportion of the population over 65 years from 7% in 2000 to 9.4% in
2007, according to China Country Profile 2009, The Economist Intelligence
Unit Ltd.
|
Government Policies in Health Care
and TCM. In April of 2009 the PRC government implemented a new national
medical and health plan. Among other features, this new plan extended national
medical insurance coverage to China’s rural areas, where the bulk of the
population resides. This expanded coverage will eventually encompass virtually
all of China’s 1.3 billion citizens, greatly expanding the market for TCM
pharmaceuticals, as well as other health care products and services. This has
led to massive potential for increased sales growth for Tongli and other
providers of TCM pharmaceutical products.
According
to Espicom Business Intelligence, in the next three years, the PRC government’s
health care investment will rise to $125 billion, compared with $96 billion for
2008. Direct health care subsidies of urban and rural residents will amount to
$57 billion. China’s health care investment is expected to witness a growth of
19.7% and the overall growth rate will reach more than 25%.
Government Support of Traditional
Chinese Medicine. Among its public health initiatives, the Chinese
government officially supports use of TCM to enhance wellness and to treat
chronic and acute diseases. The government has also commenced a program to
evaluate TCM and herbal-based pharmaceuticals for coverage and reimbursement
under national medical insurance. In 2002, TCM was declared a “national
strategic industry” in the government’s “Development Outline of Traditional
Chinese Medicine Modernization (2002 – 2010).”
3
Decreased Competition.
According to the Information Office of the State Council of the PRC, prior to
2009, there were approximately 6,000 Chinese pharmaceutical manufacturers. That
number is being significantly reduced through both marketplace attrition and
direct government involvement, decreasing competition and increasing potential
sales opportunities for the surviving companies. Other companies are expected to
fail through lack of size and innovative and aggressive management. According to
a 2009 report published by KMPG, of the approximately 4,500 pharmaceutical
companies in China, the majority are small players with limited local market
reach, and rapid consolidation between medium and large players in the sector is
anticipated since the Chinese government has been encouraging industry
consolidation with an effort to improve the Good Manufacturing Practice (GMP)
standard, enforce GMP certification and to better control the pricing of
drugs.
Our
Products
We have
been developing pharmaceuticals and health care products that incorporate
elements of Chinese traditional medicine with elements of western
medicine. Tianmu Pharmaceuticals now offers drugs and health care
products in several distinct categories, including:
|
●
|
Antihyperlipidemics. These
tablets, based on principles of Chinese traditional medicine, are used to
reduce cholesterol levels and soften blood vessels in order to improve
circulation. Our antihyperlipidemics are offered as an
affordable alternative to the statins commonly used for this purpose in
western medicine. For the year ended March 31, 2010, sales of
Antihyperlipidemics accounted for approximately 26.30% of our total sales
revenue during the year.
|
|
●
|
Yuxiang Anti-Bacterial
Mouthwash. Comprised of a mixture of medicinal
ingredients that counter disease and odor in the oral cavity and throat,
Yuxiang Mouthwash is designed to purge bad breath caused by gum disease,
abnormal sleep, nervousness, food, alcohol and smoking. We package
Yuxiang Mouthwash in bottles that are small enough to be carried
conveniently, and we target customers who are travelling or away from
home. Our primary points of distribution for Yuxiang Mouthwash are
restaurants and transport carriers. For the year ended March 31,
2010, sales of Yufang Anti-Bacterial Mouthwash accounted for approximately
21.47% of our total sales revenue during the
year.
|
|
●
|
Calcium Gluconate Oral
Liquid. This is a calcium supplement used for the prevention
and treatment of diseases caused by calcium deficiency, such as
osteoporosis, bone hypoplasis, and rickets. The liquid is
particularly recommended for women during menopause or lactation. We
believe that our product has a competitive advantage over other similar
products provided by our competitors, because we have obtained a
pharmaceutical license for this product, which is considerably more
authoritative than the health license under which most of our competitors
market their calcium supplements. For the year ended March 31, 2010, sales
of Calcium Gluconate Oral Liquid accounted for approximately 17.56% of our
total sales revenue during the
year.
|
|
●
|
Yan Li Xiao Capsules.
This product is designed to detoxify the body, clear heat, relieve
inflammatory symptoms and can be used to treat acute tonsillitis,
bacillary dysentery, acute and chronic bronchitis, acute gastroenteritis,
acute mastitis and other infectious diseases. During the year ended
March 31, 2010, sales of Yan Li Xiao Capsule accounted for approximately
27.15% of our total sales revenue during the
year.
|
|
●
|
Fuke Zhidai Tablets.
This product is used to treat abnormal leucorrhea which caused by
chronic cervicitis, endometritis and endocolpitis. During the year
ended March 31, 2010, sales of Fuke Zhidai Tablets accounted
for approximately 7.51% of our total sales during the
year.
|
We have
obtained Drug Register License and Drug Production Certificate for each of
the products listed above from China State Food & Drug
Administration (“SFDA”). Please refer to the “Government Regulation”
section for more discussion.
4
On
December 24, 2008, we signed a patent transfer agreement with a third party
Harbin Lanhai Biochemical Company Limited and paid RMB 7,030,000 (approximate to
USD 1 million) to purchase a nutraceutical product from Lanhai Biochemical
Company Limited. The Company’s ability to conclude this purchase and ultimately
commercialize this product requires, among other things, additional assistance
from the seller and obtaining government approvals. Due to the recent strict
regulation regarding the examination and approval procedure, we are waiting
for suspended governmental approval for the formula to be used in production of
Calcium supplements and expect to obtain such approval from the China SFDA by
2011.
In August
2009, we signed a contract with a third party Harbin Sanmu Pharmaceuticals to
purchase the exclusive rights to manufacture and sell a new product named Yan Li
Xiao Capsule nationwide for the next seven years. We paid Harbin Sanmu
Pharmaceuticals RMB 1,200,000 (approximate to USD 0.18 million) for this new
product namely Yan Li Xiao Capsule in October 2009 and started to manufacture
and distribute it in late 2009.
On March
21, 2010, we signed a patent purchase agreement with a third party, Tonghua
Yisheng Pharmaceuticals Company Limited, to purchase a new product which we
believe has significant potential for us. Total purchase price for this patent
amounted to RMB 33,000,000 (approximate to USD 4.85 million) to be paid in three
installments. We paid the first installment of RMB 11,000,000 (equivalent to USD
1.6 million) to Tonghua Yisheng Pharmaceuticals Company Limited in March 2010
upon execution of the purchase agreement. Our ability to conclude this purchase
and ultimately commercialize this product requires additional assistance from
the seller and obtaining government approvals. We believe this new drug formula
will help to expand our product offering as well as market coverage in the
future.
Manufacturing
Our
manufacturing and warehouse facilities are located in the
Limin Pharmaceutical Technology Park in the City of Harbin.
Our entire site was constructed in compliance with Chinese State Drug
Administration GMP (Good Manufacturing Practices) standards at a total
construction cost of 50 million RMB (approximately $7.3 million), with a
goal of achieving world class standards. In recognition of our
accomplishment, our manufacturing facility has received the National Drug GMP
(Good Manufacturing Practices) Certificate, which is required by laws in order
to carry on pharmaceutical manufacturing in the PRC. We have also received
certificates from the International Organization for Standardization:
specifically, ISO9001:2000 International Quality Management System
Certificate and ISO14001 Environmental Management System
Certificate.
At the
present time, our manufacturing facility has the capacity to produce an annual
output of products with a sales value over 100 million RMB (approximately
$14,650,000). We believe that our current capacity is adequate for at
least the next two years. In the meantime, we have budgeted $3.5
million for capital investment to expand our capacity, and we will need to raise
capital or obtain other funding to finance such expansion.
Marketing
We
currently market exclusively within the PRC. Our distribution network is
comprised of our own direct sales personnel as well as a network of authorized
distribution agents. Currently our sales network includes:
|
●
|
15
regional distribution agents;
|
|
●
|
over
200 city and county level distribution agents;
and
|
|
●
|
four
national distributors, each of whom has the exclusive right to market one
or more of our products if certain designated sales targets are achieved.
For example, we have given Jilin Province San Yuan Medical Ltd. the
exclusive right to market our Calcium Gluconate Oral Solution and our
Clindamycin Hydrochloride Capsule nationwide through March 2011 if it
purchases certain designated minimum quantities of each
product。
|
We
have entered into agreements with four distributors to provide agreed upon
amounts of products at pre-agreed price. In the event a distributor
does not purchase a fixed percentage of the agreed upon amounts for three
consecutive months, we may terminate the agreement. In addition to
that, one agreement provides, among other things, that the distributor can
become the exclusive distributor for a geographical area if certain sales
targets are met.
5
We also
market online through the “China Flagship Medicine Net”, a consortium website
that offers subscribers medical information services and an online purchasing
platform.
Major
Customers
During
the year ended March 31, 2010, our largest distributor contributed 27.2% of our
total sales. No other distributor accounted for more than 10% of our
sales. In addition, our four major
products (Antihyperlipidemics, Yuxiang Anti-Bacterial Mouthwash, Calcium
Gluconate Oral Liquid and Yan Li Xiao Capsule) represented
approximately 65% of the total sales for the year ended March 31,
2010.
Research
and Development
We
currently have limited resources to devote to and limited capabilities to
conduct the development of new products, and as such research and development
activities are not presently material to our business. We, like other TCM
manufacturers, enjoy relatively low research and development expenses as most
TCM medicines are based on standardized formula. In 2008, SFDA promulgated a
notice of registration of Chinese traditional medicine providing that TCM
composed of classic prescriptions shall be exempted from pharmacological and
toxicological tests and studies. The notice defined classic prescription and
classic TCM formulas as those herbal remedies recorded in ancient Chinese
medicine books from Qing Dynasty or earlier which are currently widely used.
According to such notice, the production and manufacturing of TCM products are
subject to non-clinical safety studies only and exempted from pharmacological
and toxicological tests and studies. Thus, TCM products are entitled to obtain
faster SFDA approval. As such, we enjoy relatively low research and development
expenses because most of our products are based on classic TCM formulas that are
covered by this notice.
We have
decreased research and development expenses incurred in the fiscal year
ended March 31, 2010 because our current products are still under normal product
cycle and our patents have provided us sufficient capabilities to meet our
current production and marketing demand, accordingly, we cut off
our investment in our own research and development activities. In
addition, we have switched our research and development strategy to
acquiring new products with significant market potential from third parties
instead of relying on our own efforts which we believe will be more
efficient.
Raw
Materials
We have
developed purchasing relationships with a considerable number of suppliers, and
have multiple sources for most of the raw materials that we require. Our
business would not be significantly affected by the loss of any one
supplier.
A
considerable portion of the raw materials that we require are volatile herbs,
which have a brief shelf life. This situation imposes a risk on our
suppliers, who will often grow the herbs to order in order to insure an
immediate market for their herbs. The situation also necessitates that we
assure ourselves that our raw material requirements are available precisely when
needed. To satisfy these conditions, it is our practice to make
substantial cash advances to our suppliers in order to lock-in our raw material
requirements. As of March 31, 2010, our advances to suppliers totaled
about $1,465,713, which was equal to approximately 17.1% of our revenue for the
year ended March 31, 2010. As our business expands, we expect that the
ratio will decrease. However, unless we develop proprietary sources of raw
material, the payment schedule for our raw materials supply will continue to
have a negative effect on our cash flow.
Competition
According
to the Information Office of the State Council of the PRC, prior to 2009, there
were approximately 6,000 Chinese pharmaceutical manufacturers. That number is
being significantly reduced through both marketplace attrition and direct
government involvement, decreasing competition and increasing potential sales
opportunities for the surviving companies. Other companies are expected to fail
through lack of size and innovative and aggressive management. According to a
2009 report published by KMPG, of the approximately 4,500 pharmaceutical
companies in China, the majority are small players with limited local market
reach, and rapid consolidation between medium and large players in the sector is
anticipated since the Chinese government has been encouraging industry
consolidation with an effort to improve the Good Manufacturing Practice (GMP)
standard, enforce GMP certification and to better control the pricing of
drugs. The market continues to attract new entrants because the per capita
medicine consumption in China is still low, compared to developed countries, and
that shows promise for substantial growth.
6
We
compete with other companies, many of whom are developing, or can be expected to
develop, products similar to ours. Some of our competitors are better
established than we are, have better brand recognition of products that compete
with ours, and have more financial, technical, marketing and other resources
than we presently possess and a larger customer base. These competitors may be
able to respond more quickly to new or changing opportunities and customer
requirements and may be able to undertake more extensive promotional activities,
offer more attractive terms to customers or adopt more aggressive pricing
policies. We cannot assure you that we will be able to compete effectively with
current or future competitors or that the competitive pressures we face will not
harm our business.
We intend
to establish a significant market share by advertising the demonstrated efficacy
of Tianmu Pharmaceutical’s products. We have extensively tested our
products and can cite studies that demonstrate the efficacy of many of them.
This contrasts with a large portion of the over-the-counter pharmaceutical
market in China, which is characterized by unproven products.
Growth
Strategies
In our
fiscal year ended at March 31, 2010, we continued the execution of our product
channel expansion strategy that resulted in increased market penetration of our
products and expanded revenue growth. Management plans to continue the
emphasis on expanded and enhanced marketing and sales in our 2011 fiscal year
and beyond. Part of this strategy involves increasing and improving
our marketing and sales activities to enhance the market leadership of our key
leading products and to increase the sales of other products by expanding our
sales force, solidifying our distribution network and expanding our market
segment coverage, and increasing our marketing and promotional
activities.
Management
also plans to pursue strategic acquisitions of new products with significant
market potential as part of our growth strategy in 2010 and
beyond. We plan to selectively pursue strategic acquisition
opportunities to further consolidate our resources and expand our market
coverage. We believe that such an initiative will provide efficient
means to broaden our product lines, increase our market coverage and complement
our research and development capabilities.
Management
believes that our emphasis on further commercializing and broadening our product
lines, enhanced sales and marketing efforts has the potential to yield
significant increases in revenue in 2010 and beyond.
Government
Regulation
The
pharmaceutical industry in China, including the TCM sector, is highly
regulated. The primary regulatory authority is the SFDA, including
its provincial and local branches. As a developer, producer and distributor of
medicinal products, we are subject to regulation and oversight by the SFDA and
its provincial and local branches. The Law of the PRC on the Administration of
Pharmaceuticals provides the basic legal framework for the administration of the
production and sale of pharmaceuticals in China and covers the manufacturing,
distributing, packaging, pricing and advertising of pharmaceutical products. Its
implementing regulations set forth detailed rules with respect to the
administration of pharmaceuticals in China. We are also subject to other PRC
laws and regulations that are applicable to business operators, manufacturers
and distributors in general.
Under the
SFDA guidelines for licensing of pharmaceutical products, all pharmaceutical
manufacturers must obtain and maintain Good Manufacturing Practices (“GMP”)
Certificate.
Because
our manufacturing facility has obtained the National GMP Certificate, we are
authorized to produce products in four modes: tablets, capsules, granules,
and oral suspensions. In addition to that, in order to market our products
as pharmaceuticals, we are required to obtain Drug Register License and Drug
Production Certificate specific to each product from the provincial branch of
China SFDA. The process of application for such licenses is rigorous, requiring
considerable testing. On average, it costs us approximately 1 million RMB
(approximately $150,000) to get the approval for each product by the SFDA. To
date we have obtained Drug Register License and Drug Production Certificate for
the our products listed under “Our Products” above.
7
The more
readily available license is for “health care products”, which are governed by
the Heilongjiang Province Public Health Bureau. Tianmu Pharmaceuticals has
registered its Yuxiang Anti-Bacterial Mouthwash with this Bureau.
Currently
we have not developed a market in U.S. so we believe we are not subject to any
of regulations by the U.S. Food and Drug Administration.
Environmental
Matters
Our
manufacturing and warehouse facilities are located in the Limin Pharmaceutical
Technology Park in the City of Harbin. We believe that the industrial
zone where we have located our manufacturing facilities is equipped with all
necessary equipment that will enable us to comply with the applicable national,
provincial and local environmental laws related to our operation. We
maintain all the permits and licenses required by the PRC environment
regulations through Limin Pharmaceutical Technology Park, to whom we pay certain
amount of management fees every year.
Intellectual
Property
We have
registered “Tianmu” as our trademark in China. In addition, we have
obtained a patent in China that covers the method of applying blue polyethylene
packaging to the bottles of our Calcium Gluconate Oral Solution. All of our
employees are bound by our policy to not disclose our proprietary information,
although we have no written confidentiality agreements with our
employees.
Employees
We
currently have approximately 86 employees, all of whom are employed on a
full-time basis. 12 employees are in executive management and human
resource & administration, 11 in sales, 6 in accounting, 8 in
technical and 49 in manufacturing.
Executive
Offices in China
Our
executive offices in China are located at 1 Beijing Road, Limin Development
Zone, Harbin, China. We maintain a website at www.tmyy.com.cn. Information
contained on or accessed through our website is not intended to constitute and
shall not be deemed to constitute part of this Report.
8
Item
1A. Risk Factors
Our
business, operations and financial condition are subject to various risks. Some
of these risks are described below and you should take these risks into account
in making a decision to invest in our common stock. If any of the following
risks actually occurs, we may not be able to conduct our business as currently
planned and our financial condition and operating results could be seriously
harmed. In that case, the market price of our common stock could decline and you
could lose all or part of your investment in our common stock.
Risks
Related to Our Business
We
may need additional financing, which may not be available on satisfactory
terms or at all.
The
revenues from the production and sale may not be adequate to support our
expansion and product development programs. We may need substantial additional
funds to build new production facilities, acquire new
products, pursue further research and development, obtain regulatory
approvals, market our products, and file, prosecute, defend and enforce our
intellectual property rights.
At
present we have no commitment from any source for those funds. We cannot
determine, therefore, the terms on which we will be able to raise the necessary
funds. To the extent we raise additional capital by issuing equity
securities, our stockholders may experience dilution. To the extent that we
raise additional capital by issuing debt securities, we may incur substantial
interest obligations, may be required to pledge assets as security for the debt
and may be constrained by restrictive financial and/or operational covenants.
Debt financing would also be superior to our stockholders’ interest in
bankruptcy or liquidation.
There are
no assurances that future funding will be available to us on favorable
terms or at all. If additional funding is not obtained, we will need to reduce,
defer or cancel development programs, planned initiatives or overhead
expenditures, to the extent necessary. The failure to fund our capital
requirements would have a material adverse effect on our business, financial
condition and results of operations.
We
have been heavily dependent on key products.
Our three
major products Antihyperlipidemics, Yuxiang Anti-Bacterial Mouthwash and Calcium
Gluconate Oral Liquid represented approximately 92% of the total sales for the
year ended March 31, 2010. We expect that a significant portion of
our future revenue will continue to be derived from sales of these three
products. If any of these three products were to become subject to a problem
such as loss of patent protection, unexpected side effects, regulatory
proceedings, publicity adversely affecting user confidence or pressure from
competing products, or if a new, more effective treatment should be introduced,
the impact on our revenues could be significant.
We
face competition in the pharmaceutical market in the PRC and such competition
could cause our sales revenue and profits to decline.
According
to SFDA in China, there were approximately 5,071 pharmaceutical manufacturing
companies in the PRC as of the end of June 2004, of which approximately 3,237
manufacturers obtained certificates of Good Manufacturing Practices
Certification (“GMP”). After GMP certification became a mandatory
requirement on July 1, 2004, approximately 1,834 pharmaceutical manufacturers
were forced to cease production. Only the 3,237 pharmaceutical
manufacturers with GMP certifications may continue their manufacturing
operations. As of the end of 2006, there were 4,682 enterprises
manufacturing medicines and formulation in China. The certificates,
permits, and licenses required for pharmaceutical operation in the PRC create a
potentially significant barrier for new competitors seeking entrance into the
market. Despite these obstacles, we face competitors that will
attempt to create, or are already marketing, products in the PRC that are
similar to ours. Many of our current and potential competitors have
significantly longer operating histories and significantly greater managerial,
financial, marketing, technical and other competitive resources, as well as
greater name recognition, than we do. These competitors may be able
to respond more quickly to new or changing opportunities and customer
requirements and may be able to undertake more extensive promotional activities,
offer more attractive terms to customers or adopt more aggressive pricing
policies. We cannot assure you that we will be able to compete
effectively with current or future competitors or that the competitive pressures
we face will not harm our business.
9
Our
business and growth will suffer if we are unable to hire and retain key
personnel that are in high demand.
Our
future success depends on our ability to attract and retain highly skilled
chemists, pharmaceutical engineers, technical, and marketingl, especially
qualified personnel for our operations in China. Qualified individuals are in
high demand in China, and there are insufficient experienced personnel to fill
the demand. Therefore we may not be able to successfully attract or retain
the personnel we need to succeed.
Our
business development would be hindered if we lost the services of some key
personnel. Yao Mingli is the Chief Executive Officer of our company and of its
operating subsidiary, Tianmu Pharmaceuticals. Mr. Yao is responsible for
strategizing not only our business plan but also the means of financing it.
If Mr. Yao were to leave Tianmu Pharmaceuticals or become unable to
fulfill his responsibilities, our business would be imperiled. At the very
least, there would be a delay in the development of Tianmu Pharmaceuticals until
a suitable replacement for Mr. Yao could be retained.
Our
results of operations are dependent on continually developing or acquiring new
and advanced products, technologies, and processes and failure to do so may
cause us to lose our competitiveness in the pharmaceutical industry and may
cause our profits to decline.
To remain
competitive in the pharmaceutical industry, it is important to continually
develop new and advanced products, technologies and processes. There
is no assurance that our competitors’ new products, technologies and processes
will not render our company’s existing products obsolete or
non-competitive. Our company’s competitiveness in the pharmaceutical
market therefore relies upon our ability to enhance our current products,
introduce new products, and develop and implement new technologies and
processes. Our company’s failure to technologically evolve and/or
develop new or enhanced products may cause us to lose our competitiveness in the
pharmaceutical industry and may cause our profits to decline. It is
likely that our efforts to grow our products lines will be focused on
acquisitions of such products from third parties. There are many
risks attendant to the acquisition of assets or companies, including
availability, pricing, competition and, if acquisitions are consummate,
integration. If we are unable to so acquire and integrate new
products, our revenue and profitability may suffer.
The
commercial success of our products depends upon the degree of market acceptance
among the medical community and failure to attain market acceptance among the
medical community may have an adverse impact on our operations and
profitability.
The
commercial performance of our products depends upon the degree of market
acceptance among the medical community, such as hospitals and
physicians. Even if our products are approved by SFDA, and even if
our products are authorized to be eligible for reimbursement under Chinese
national medical insurance programs, there is no assurance that physicians will
prescribe or recommend our products to patients. Furthermore, a
product’s prevalence and use at hospitals may be contingent upon our
relationship with the medical community. The acceptance of our
products among the medical community may depend upon several factors, including
but not limited to, the product’s acceptance by physicians and patients as a
safe and effective treatment, cost effectiveness, potential advantages over
alternative treatments, and the prevalence and severity of side
effects. Failure to attain market acceptance among the medical
community may have an adverse impact on our operations and
profitability.
We
may not be able to obtain the regulatory approvals or clearances that are
necessary to commercialize our products.
The PRC
and other countries impose significant statutory and regulatory obligations upon
the manufacture and sale of pharmaceutical products. Each regulatory
authority typically has a lengthy approval process in which it examines
pre-clinical and clinical data and the facilities in which the product is
manufactured. Regulatory submissions must meet complex criteria to
demonstrate the safety and efficacy of the ultimate products. Addressing these
criteria requires considerable data collection, verification and analysis. We
may spend time and money preparing regulatory submissions or applications
without assurances as to whether they will be approved on a timely basis or at
all.
10
Our
product candidates, some of which are currently in the early stages of
development, will require significant additional development and pre-clinical
and clinical testing prior to their commercialization. These steps and the
process of obtaining required approvals and clearances can be costly and
time-consuming. If our potential products are not successfully developed, cannot
be proven to be safe and effective through clinical trials, or do not receive
applicable regulatory approvals and clearances, or if there are delays in the
process:
|
●
|
the
commercialization of our products could be adversely
affected;
|
|
●
|
any
competitive advantages of the products could be diminished;
and
|
|
●
|
revenues
or collaborative milestones from the products could be reduced or
delayed.
|
Governmental
and regulatory authorities may approve a product candidate for fewer indications
or narrower circumstances than requested or may condition approval on the
performance of post-marketing studies for a product candidate. Even if a product
receives regulatory approval and clearance, it may later exhibit adverse side
effects that limit or prevent its widespread use or that would force us to
withdraw the product from the market.
Any
marketed product and its manufacturer will continue to be subject to strict
regulation after approval. Results of post-marketing programs may limit or
expand the further marketing of products. Unforeseen problems with an approved
product or any violation of regulations could result in restrictions on the
product, including its withdrawal from the market and possible civil
actions.
In
manufacturing our products we will be required to comply with applicable good
manufacturing practices regulations, which include requirements relating to
quality control and quality assurance, as well as the maintenance of records and
documentation. If we cannot comply with regulatory requirements, including
applicable good manufacturing practice requirements, we may not be allowed to
develop or market the product candidates. If we or our manufacturers fail to
comply with applicable regulatory requirements at any stage during the
regulatory process, we may be subject to sanctions, including fines, product
recalls or seizures, injunctions, refusal of regulatory agencies to review
pending market approval applications or supplements to approve applications,
total or partial suspension of production, civil penalties, withdrawals of
previously approved marketing applications and criminal
prosecution.
Our
current and future products may have inadvertent and/or harmful side effects
which would expose us to the risks of litigation and a loss of
revenue.
All
medicines have certain side effects. Although all of our medicines
sold on market have passed proper testing and are approved by SFDA, the products
may still inadvertently adverse effects on the health of the consumers. If such
side effect is identified after marketing and sale of the products, the products
may be required to be withdrawn from the market, or have a change in labeling.
If a product liability claim is brought against us, it may, regardless of merit
or eventual outcome, result in damage to our reputation, breach of contracts
with consumers, decreased demand for our products, costly litigation and loss of
revenue.
Natural
disasters, weather conditions and other environmental factors affect our raw
material supply, and a reduction in the quality or quantity of our herb supplies
may have material adverse consequences on our financial results.
Our
business may be adversely affected by weather and environmental factors beyond
our control, such as natural disasters and adverse weather
conditions. The production of our products depends on the
availability of raw materials, a significant portion of which are herbs.
These herbs tend to be very sensitive crops, which can be readily damaged
by harsh weather, by disease, and by pests. If our suppliers’ crops are
destroyed by drought, flood, storm, blight, or the other woes of farming, we
will not be able to meet the demands of our customers, which will have a
material adverse effect on our business and financial condition and results.
11
If
we lost control of our distribution network, our business would
fail.
We depend
on our distribution network for the success of our business. During the
year ended March 31, 2010, approximately 43.5% of sales were generated from four
major distributors. Competitors may seek to pull our distribution network away
from us. In addition, if dominant members of our distribution network
become dissatisfied with their relationship with Tianmu Pharmaceuticals, a
concerted effort by the distribution network could force us to accept less
favorable financial terms from the distribution network. Either of these
possibilities, if realized, would have an adverse effect on our
business.
We
may not be able to adequately protect our intellectual property, which could
cause us to be less competitive.
We regard
our trademarks, trade secrets, patents and similar intellectual property as
material to our success. We rely on trademark, patent and trade secret law,
as well as
confidentiality and license agreements with certain of our customers and
others to protect our proprietary rights. We have received trademark and patent
protection for
certain of our products in the PRC. No assurance can be given that our patents
and licenses will not be challenged, invalidated, infringed or
circumvented, or that our intellectual property rights will provide competitive
advantages to us. There can be no assurance that we will be able to obtain a
license from a third-party technology that we may need to conduct our business
or that such technology can be licensed at a reasonable cost.
If
our products fail to receive regulatory approval or are severely limited in the
products scope of use, then we may be unable to recoup our research and
development expenditures and we may not be able to adequately sell such
products.
Our
products that are approved to be manufactured as of March 31, 2010 include four
medicines. The production of our pharmaceutical products is subject to the
regulatory approval of the SFDA. The regulatory approval procedure
for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon
the discretion of the SFDA, the approval process may be significantly delayed by
additional clinical testing and require the expenditure of currently unavailable
resources; in such an event, it may be necessary for us to abandon our
application. Even where approval of the product is granted, it may
contain significant limitations in the form of narrow indications, warnings,
precautions, or contra-indications with respect to conditions of use. If
approval of our product is denied, abandoned, or severely limited in terms of
the scope of products use, it may result in the inability to recoup considerable
research and development expenditures already incurred.
Our
certificates, permits, and license are subject to governmental control and
renewal, and the failure to obtain renewal would cause all or part of our
operation to be suspended and have a material adverse effect on our financial
condition.
We are subject to various PRC
laws and regulations pertaining to the pharmaceutical industry. We have has
attained certain certificates, permits, and licenses required for
the operation of a pharmaceutical enterprise and the manufacturing of
pharmaceutical products in the PRC. We obtained the Medicine Production Permit
in 2003 and 2004, which are subject to annual checks by the SFDA. We also
have GMP certificates which are
subject to annual checks by the SFDA. The pharmaceutical production
permits and GMP certificates are each valid for a term of five years and must be
renewed before their expiration. During the renewal process, we will be
re-evaluated by the appropriate governmental authorities and must comply with
the prevailing standards and regulations, which may change from time to time. In
the event that we are not able to renew the certificates, permits and
licenses, all or part of our operations may be suspended by the government,
which would have a material adverse effect on our financial condition.
Furthermore, if escalating compliance costs associated with governmental
standards and regulations restrict or prohibit any part of our operations,
it may adversely affect our results of operations and
profitability.
We may be subject
to the People’s Republic of China’s price control of drugs which may limit our
profitability and even cause us to stop manufacturing certain
products.
The State
Development and Reform Commission of the PRC (“SDRC”) and the price
administration bureaus of the relevant provinces of the PRC in which the
pharmaceutical products are manufactured are responsible for the retail price
control over our pharmaceutical products. The SDRC sets the price
ceilings for certain pharmaceutical products in the PRC. All of our products
except those under the protection periods are subject to such price controls as
of the date of this Memorandum and we prices our medicines well under
government-mandated caps. There is no assurance that whether our other products
will remain unaffected by the price control. Where our products are
subject to a price ceiling, we will need to adjust the product price to meet the
requirement and to accommodate for the pricing of competitors in the competition
for market shares. The price ceilings set by the SDRC may limit our
profitability, and in some instances, such as where the price ceiling is below
production costs, may cause us to stop manufacturing certain products which may
adversely affect our results of operations.
12
Because
we may not be able to obtain business insurance in the PRC, we may not be
protected from risks that are customarily covered by insurance in the United
States.
Business
insurance is not readily available in the PRC. To the extent that we
suffer a loss of a type which would normally be covered by insurance in the
United States, such as product liability and general liability insurance, we
would incur significant expenses in both defending any action and in paying any
claims that result from a settlement or judgment. We have not
obtained fire, casualty and theft insurance, and there is no insurance coverage
for our raw materials, goods and merchandise, furniture and buildings in
China. Any losses incurred by us will have to be borne by us without
any assistance, and we may not have sufficient capital to cover material damage
to, or the loss of, our production facility due to fire, severe weather, flood
or other cause, and such damage or loss would have a material adverse effect on
our financial condition, business and prospects.
We
may be subject to product liability claims, for which we have no
insurance.
We may
produce products which inadvertently have an adverse pharmaceutical effect on
the health of individuals. Existing laws and regulations in China do not
require us to maintain third party liability insurance to cover product
liability claims. However, if a product liability claim is brought against
us, it may, regardless of merit or eventual outcome, result in damage to our
reputation, breach of contracts with our customers, decreased demand for our
products, costly litigations, product recalls, loss of revenue, and our
inability to commercialize some products.
Our
indemnification obligations could adversely affect our business, financial
condition and results of operations.
Our
governing documents require us to indemnify our current and former directors,
officers, employees and agents against most actions of a civil, criminal,
administrative or investigative nature. Generally, we are required to
advance indemnification expenses prior to any final adjudication of an
individual’s culpability. The expense of indemnifying our current and
former directors, officers and employees and agents in their defense or related
expenses as a result of any actions related to the internal investigation and
financial restatement may be significant and in excess of any insurance coverage
we may have. As such, there is a risk that our indemnification
obligations could divert needed financial resources and may adversely affect our
business, financial condition and results of operations.
A
large portion of our common stock is controlled by a small number of
stockholders and as a result, these stockholders are able to influence and
ultimately control the outcome of stockholder votes on various
matters.
Mr.
Mingli Yao, our Chairman and CEO, together with his wife and daughter owns
2,698,333, or 23.7% of our outstanding shares as of the date of this Form
10-K. As a result, these stockholders are able to influence and
potentially control the outcome of stockholder votes on various matters,
including the election of directors and other corporate transactions including
business combinations. In addition, the occurrence of sales of a large
number of shares of our common stock, or the perception that these sales could
occur, may affect our stock price and could impair our ability to obtain capital
through an offering of equity securities. Furthermore, the current ratios of
ownership of our common stock reduce the public float and liquidity of our
common stock which can in turn affect the market price of our common
stock.
13
If
we are unable to maintain appropriate internal financial reporting controls and
procedures, it could cause us to fail to meet our reporting obligations, result
in the restatement of our financial statements, harm our operating results,
subject us to regulatory scrutiny and sanction, and cause investors to lose
confidence in our reported financial information.
Effective
internal controls are necessary for us to provide reliable financial reports and
effectively prevent fraud. As a public company, we have significant
additional requirements for enhanced financial reporting and internal
controls. We will be required to document and test our internal
control procedures in order to satisfy the requirements of Section 404 of the
Sarbanes-Oxley Act of 2002, which requires annual management assessments of the
effectiveness of our internal controls over financial reporting and a report by
our independent registered public accounting firm addressing these assessments.
The process of designing and implementing effective internal controls is a
continuous effort that requires us to anticipate and react to changes in our
business and the economic and regulatory environments and to expend significant
resources to maintain a system of internal controls that is adequate to satisfy
our reporting obligations as a public company.
We cannot
assure you that we will not, in the future, identify areas requiring improvement
in our internal control over financial reporting. We cannot assure you that the
measures we will take to remediate any areas in need of improvement will be
successful or that we will implement and maintain adequate controls over our
financial processes and reporting in the future as we continue our growth. If we
are unable to establish appropriate internal financial reporting controls and
procedures, it could cause us to fail to comply with Sarbanes-Oxley and meet our
reporting obligations, result in the restatement of our financial statements,
harm our operating results, subject us to regulatory scrutiny and sanction, and
cause investors to lose confidence in our reported financial
information.
We
have not yet fully developed independent corporate governance.
As of the
date of this Report, we only have one director that is “independent” (as defined
under Nasdaq Marketplace Rules). Additionally, we have no audit,
compensation, or nominating committees of our board of
directors. This lack of independence and independent controls over
our corporate affairs may result in potential or actual conflicts of interest
between our management and our stockholders. We presently have no
policy to resolve such conflicts. The absence of such standards of
corporate governance may leave our stockholders without protections against
interested director or executive transactions, conflicts of interest and similar
matters, which could negatively impact an investment in our
company.
We
incur increased costs as a result of being a public company.
As a
public company, we incur significant legal, accounting and other expenses that
we did not incur as a private company. In addition, the Sarbanes-Oxley Act of
2002, as well as new rules subsequently implemented by the SEC, have required
changes in corporate governance practices of public companies. We expect these
new rules and regulations to increase our legal, accounting and financial
compliance costs and to make certain corporate activities more time-consuming
and costly. In addition, we will incur additional costs associated with our
public company reporting requirements. We are currently evaluating and
monitoring developments with respect to these new rules, and we cannot predict
or estimate the amount of additional costs we may incur or the timing of such
costs.
Our directors
and officers liability insurance may lapse or be invalid or may fail to cover
any expenses and losses due to lawsuits related to financial reporting errors,
and our indemnification obligations could adversely affect our business,
financial condition and results of operations.
Our
director and officer liability insurance may lapse or otherwise be
unable to cover lawsuit expenses and losses related to financial reporting
errors. Our bylaws require us to indemnify our current and former directors,
officers, employees and agents against most actions of a civil, criminal,
administrative or investigative nature. Generally, we are required to advance
indemnification expenses prior to any final adjudication of an individual’s
culpability. The expense of indemnifying our current and former directors,
officers and employees and agents in their defense or related expenses as a
result of any actions related to the internal investigation and financial
restatement may be significant. Therefore, our indemnification obligations could
result in the diversion of our financial resources and may adversely affect our
business, financial condition and results of operations.
14
We
are not likely to hold annual stockholder meetings in the next few
years.
Management
does not expect to hold annual meetings of stockholders in the next few years,
due to the expense involved. The current members of the Board of Directors
were appointed to that position by the previous directors. If other
directors are added to the Board in the future, it is likely that
the current directors will appoint them. As a result, our
stockholders will have no effective means of exercising control over the
operations of our company.
Potential
environmental liability could have a material adverse effect on our operations
and financial condition.
As a
manufacturer, we are subject to various Chinese environmental laws and
regulations on air emission, waste water discharge, solid wastes and noise.
Although we believe that our operations are in substantial compliance with
current environmental laws and regulations, we may not be able to comply with
these regulations at all times as the Chinese environmental legal regime is
evolving and becoming more stringent. Therefore, if the Chinese government
imposes more stringent regulations in the future, we may have to incur
additional and potentially substantial costs and expenses in order to comply
with new regulations, which may negatively affect our results of operations.
Further, no assurance can be given that all potential environmental liabilities
have been identified or properly quantified or that any prior owner, operator,
or tenant has not created an environmental condition unknown to us. If we fail
to comply with any of the present or future environmental regulations in any
material aspects, we may suffer from negative publicity and be subject to
claims for damages that may require us to pay substantial fines or have our
operations suspended or even be forced to cease operations.
Risks
Associated With Doing Business In China
There are
substantial risks associated with doing business in China, as set forth in the
following risk factors.
The
Chinese government exerts substantial influence over the manner in which we must
conduct our business activities.
We are
dependent on our relationship with the local government in the Chinese province
in which we operate our business. The Chinese government has
exercised and continues to exercise substantial control over virtually every
sector of the Chinese economy through regulation and state
ownership. Our ability to operate in China may be harmed by changes
in its laws and regulations, including those relating to taxation, environmental
regulations, land use rights, property and other matters. The central
or local governments of in the PRC jurisdictions may impose new, stricter
regulations or interpretations of existing regulations that would require
additional expenditures and efforts on our part to ensure our compliance with
such regulations or interpretations. Accordingly, government actions
in the future, including any decision not to continue to support recent economic
reforms and to return to a more centrally planned economy or regional or local
variations in the implementation of economic policies, could have a significant
effect on economic conditions in China or particular regions thereof, and could
require us to divest ourselves of any interest we then hold in Chinese
properties.
Future
inflation in China may inhibit our ability to conduct business in China. In
recent years, the Chinese economy has experienced periods of rapid expansion and
high rates of inflation. Rapid economic growth can lead to growth in
the money supply and rising inflation. If prices for our products
rise at a rate that is insufficient to compensate for the rise in the costs of
supplies, it may have an adverse effect on profitability. These
factors have led to the adoption by Chinese government, from time to time, of
various corrective measures designed to restrict the availability of credit or
regulate growth and contain inflation. High inflation may in the
future cause Chinese government to impose controls on credit and/or prices, or
to take other action, which could inhibit economic activity in China, and
thereby harm the market for our products.
Our
operations and assets in China are subject to significant political and economic
uncertainties and the company may lose all of its assets and operations if the
Chinese government alters its policies to further restrict foreign participation
in business operating in the PRC.
Changes
in PRC laws and regulations, or their interpretation, or the imposition of
confiscatory taxation, restrictions on currency conversion, imports and sources
of supply, devaluations of currency or the nationalization or other
expropriation of private enterprises could have a material adverse effect on our
business, results of operations and financial condition. Under its
current leadership, the Chinese government has been pursuing economic reform
policies that encourage private economic activities and greater economic
decentralization. There is no assurance, however, that the Chinese
government will continue to pursue these policies, or that it will not
significantly alter these policies from time to time without notice. We may lose all of our
assets and operations if the Chinese government alters its policies to further
restrict foreign participation in business operating in the
PRC.
15
We derive all of our sales in China
and a slowdown or other adverse developments in the PRC economy may materially
and adversely affect our customers, demand for our products and our
business.
All of
our sales are generated in China. We anticipate that sales of our
products in China will continue to represent all of our total sales in the near
future. Although the PRC economy has grown significantly in recent
years, we cannot assure you that such growth will continue. The
industry which we are involved in the PRC is relatively new and growing, but we
do not know how sensitive we are to a slowdown in economic growth or other
adverse changes in the PRC economy which may affect demand for our
products. In addition, the Chinese government also exercises
significant control over Chinese economic growth through the allocation of
resources, controlling payment of foreign currency-denominated obligations,
setting monetary policy and providing preferential treatment to particular
industries or companies. Efforts by the Chinese government to slow
the pace of growth of the Chinese economy could result in reduced demand for our
products. A slowdown in overall economic growth, an economic downturn
or recession or other adverse economic developments in the PRC may materially
reduce the demand for our products and materially and adversely affect our
business.
Currency fluctuations and
restrictions on currency exchange may adversely affect our business, including
limiting our ability to convert Chinese Renminbi into foreign currencies and, if
Chinese Renminbi were to decline in value, reducing our revenue in U.S. dollar
terms.
Our
reporting currency is the U.S. dollar and our operations in China use their
local currency as their functional currencies. Substantially all of our revenue
and expenses are in the Chinese currency, the Renminbi. We are subject to the
effects of exchange rate fluctuations with respect to any of these currencies.
For example, the value of the Renminbi depends to a large extent on Chinese
government policies and China’s domestic and international economic and
political developments, as well as supply and demand in the local market. Since
1994, the official exchange rate for the conversion of the Renminbi to the U.S.
dollar had generally been stable and the Renminbi had appreciated slightly
against the U.S. dollar. In July 2005, the Chinese government changed its policy
of pegging the value of the Renminbi to the U.S. dollar. Under this policy,
which was halted in 2008 due to the worldwide financial crisis, the Renminbi was
permitted to fluctuate within a narrow and managed band against a basket of
certain foreign currencies. In June 2010, the Chinese government announced its
intention to again allow the Renminbi to fluctuate within the 2005 parameters.
It is possible that the Chinese government could adopt an even more flexible
currency policy, which could result in more significant fluctuation of Renminbi
against the U.S. dollar, or it could adopt a more restrictive policy. We can
offer no assurance that the Renminbi will be stable against the U.S. dollar or
any other foreign currency.
Our
financial statements are translated into U.S. dollars at the average exchange
rates in each applicable period. To the extent the U.S. dollar strengthens
against foreign currencies, the translation of these foreign currencies
denominated transactions results in reduced revenue, operating expenses and net
income for our international operations. Similarly, to the extent the U.S.
dollar weakens against foreign currencies, the translation of these foreign
currency denominated transactions results in increased revenue, operating
expenses and net income for our international operations. We are also exposed to
foreign exchange rate fluctuations as we convert the financial statements of our
foreign consolidated subsidiaries into U.S. dollars in consolidation. If there
is a change in foreign currency exchange rates, the conversion of the foreign
consolidated subsidiaries’ financial statements into U.S. dollars will lead to a
translation gain or loss which is recorded as a component of other comprehensive
income. In addition, we have certain assets and liabilities that are denominated
in currencies other than the relevant entity’s functional currency. Changes in
the functional currency value of these assets and liabilities create
fluctuations that will lead to a transaction gain or loss. We have not entered
into agreements or purchased instruments to hedge our exchange rate risks,
although we may do so in the future. The availability and effectiveness of any
hedging transaction may be limited and we may not be able to hedge our exchange
rate risks.
16
Changes
in foreign exchange regulations in the PRC may affect our ability to pay
dividends in foreign currency or conduct other foreign exchange
business.
We
receive substantially all of our revenues in Renminbi, the Chinese currency,
which is currently not a freely convertible currency. The
restrictions on currency exchanges may limit our ability to use revenues
generated in RMB to make dividends or other payments in United States
dollars. The PRC government strictly regulates conversion of RMB into
foreign currencies. Over the years, foreign exchange regulations in
the PRC have significantly reduced the government’s control over routine foreign
exchange transactions under current accounts. In the PRC, SAFE
regulates the conversion of the RMB into foreign currencies. Pursuant
to applicable PRC laws and regulations, foreign invested enterprises
incorporated in the PRC are required to apply for “Foreign Exchange Registration
Certificates.” Currently, conversion within the scope of the “current
account” (e.g. remittance of foreign currencies for payment of dividends, etc.)
can be effected without requiring the approval of SAFE. However, conversion of
currency in the “capital account” (e.g. for capital items such as direct
investments, loans, securities, etc.) still requires the approval of
SAFE. In addition, failure to obtain approval from SAFE for currency
conversion on the capital account may adversely impact our capital expenditure
plans and our ability to expand in accordance with our desired
objectives.
The PRC
government also may at its discretion restrict access in the future to foreign
currencies for current account transactions. If the foreign exchange
control system prevents us from obtaining foreign currency, we may be unable to
pay dividends or meet obligations that may be incurred in the future that
require payment in foreign currency.
The application
of PRC regulations relating to the overseas listing of PRC domestic companies is
uncertain, and we may be subject to penalties for failing to request approval of
the PRC authorities prior to listing our shares in the
U.S.
In recent
years several PRC regulatory agencies have adopted merger and acquisition
regulations pertaining to the overseas listing of PRC domestic companies which
require the approval of the China Securities Regulatory Commission
(“CSRC”). Because we have been advised by our PRC legal counsel that
we are not subject to these regulations, we do not intend to request approval
from the CSRC prior to listing our shares on the Over the Counter Bulletin Board
or a national exchange.
However,
there are substantial uncertainties regarding the interpretation, application
and enforcement of these rules, and CSRC has yet to promulgate any written
provisions or formally to declare or state whether the overseas listing of a
PRC-related company structured similar to ours is subject to the approval
of CSRC. Any violation of these rules could result in fines and other
penalties on our operations in China, restrictions or limitations on remitting
dividends outside of China, and other forms of sanctions that may cause a
material and adverse effect to our business, operations and financial
conditions.
The new
mergers and acquisitions regulations also established additional procedures and
requirements that are expected to make merger and acquisition activities by
foreign investors more time-consuming and complex, including requirements in
some instances that the Ministry of Commerce be notified in advance of any
change-of-control transaction in which a foreign investor takes control of a PRC
domestic enterprise that owns well-known trademarks or China’s traditional
brands. We may grow our business in part by acquiring other businesses.
Complying with the requirements of the new mergers and acquisitions regulations
in completing this type of transactions could be time-consuming, and any
required approval processes, including CSRC approval, may delay or inhibit our
ability to complete such transactions, which could affect our ability to expand
our business or maintain our market share.
We
may face regulatory uncertainties that could restrict our ability to issue
equity compensation to our directors and employees and other parties who are PRC
citizens or residents under PRC law.
On April
6, 2007, SAFE issued the “Operating Procedures for Administration of Domestic
Individuals Participating in the Employee Stock Ownership Plan or Stock Option
Plan of An Overseas Listed Company, also know as “Circular 78.” It is not clear
whether Circular 78 covers all forms of equity compensation plans or only those
which provide for the granting of stock options. For any equity compensation
plan which is so covered and is adopted by a non-PRC listed company after April
6, 2007, Circular 78 requires all participants who are PRC citizens to register
with and obtain approvals from SAFE prior to their participation in the plan. In
addition, Circular 78 also requires PRC citizens to register with SAFE and make
the necessary applications and filings if they participated in an overseas
listed company’s covered equity compensation plan prior to April 6,
2007. We have begun to make option and stock grants to some of our
directors and employees, who are PRC citizens and have adopted an equity
compensation plan of 2 million shares. Circular 78 may require PRC citizens who
receive option grants to register with SAFE. We believe that the registration
and approval requirements contemplated in Circular 78 will be burdensome and
time consuming. Failure to comply with such provisions may subject us and
recipients of such options to fines and legal sanctions and prevent us from
being able to grant equity compensation to our PRC employees. In that case, our
ability to compensate our employees and directors through equity compensation
would be hindered and our business operations may be adversely
affected.
17
Because our
principal assets are located outside of the United States and with the exception
of one director, our directors and all our officers reside outside of
the United States, it may be difficult for you to enforce your rights based on
the United States Federal securities laws against us and our officers and
directors in the United States or to enforce judgments of United States courts
against us or them in the PRC.
All of
our officers and directors reside outside of the United States. In addition, our
operating subsidiaries are located in the PRC and all of their assets are
located outside of the United States. China does not have a treaty with United
States providing for the reciprocal recognition and enforcement of judgments of
courts. It may therefore be difficult for investors in the United States to
enforce their legal rights based on the civil liability provisions of the United
States Federal securities laws against us in the courts of either the United
States or the PRC and, even if civil judgments are obtained in courts of the
United States, to enforce such judgments in PRC courts. Further, it is unclear
if extradition treaties now in effect between the United States and the PRC
would permit effective enforcement against us or our officers and directors of
criminal penalties, under the United States Federal securities laws or
otherwise.
We may have
limited legal recourse under PRC law if disputes arise under our contracts with
third parties.
The
Chinese government has enacted some laws and regulations dealing with matters
such as corporate organization and governance, foreign investment, commerce,
taxation and trade. However, their experience in implementing,
interpreting and enforcing these laws and regulations is limited, and our
ability to enforce commercial claims or to resolve commercial disputes is
unpredictable. If our new business ventures are unsuccessful, or other
adverse circumstances arise from these transactions, we face the risk that the
parties to these ventures may seek ways to terminate the transactions, or, may
hinder or prevent us from accessing important information regarding the
financial and business operations of these acquired companies. The
resolution of these matters may be subject to the exercise of considerable
discretion by agencies of the Chinese government, and forces unrelated to the
legal merits of a particular matter or dispute may influence their
determination. Any rights we may have to specific performance, or to seek an
injunction under PRC laws, in either of these cases, are severely limited, and
without a means of recourse by virtue of the Chinese legal system, we may be
unable to prevent these situations from occurring. The occurrence of any
such events could have a material adverse effect on our business, financial
condition and results of operations. Although legislation in China over
the past 30 years has significantly improved the protection afforded to various
forms of foreign investment and contractual arrangements in China, these laws,
regulations and legal requirements are relatively new and their interpretation
and enforcement involve uncertainties, which could limit the legal protection
available to us, and foreign investors, including you. The inability to
enforce or obtain a remedy under any of our future agreements could result in a
significant loss of business, business opportunities or capital and could have a
material adverse impact on our operations.
We must comply with the Foreign
Corrupt Practices Act.
We are
required to comply with the United States Foreign Corrupt Practices Act, which
prohibits U.S. companies from engaging in bribery or other prohibited payments
to foreign officials for the purpose of obtaining or retaining business.
Foreign companies, including some of our competitors, are not subject to these
prohibitions. Corruption, extortion, bribery, pay-offs, theft and other
fraudulent practices occur from time-to-time in mainland China. If our
competitors engage in these practices, they may receive preferential treatment
from personnel of some companies, giving our competitors an advantage in
securing business or from government officials who might give them priority in
obtaining new licenses, which would put us at a disadvantage. Although we
inform our personnel that such practices are illegal, we have not established
formal policies or procedures for prohibiting or monitoring this conduct, and we
can not assure you that our employees or other agents will not engage in such
conduct for which we might be held responsible. If our employees or other
agents are found to have engaged in such practices, we could suffer severe
penalties.
18
Due
to various restrictions under PRC laws on the distribution of dividends by our
PRC operating companies, we may not be able to pay dividends to our
stockholders.
The
Wholly-Foreign Owned Enterprise Law (1986), as amended and the Wholly-Foreign
Owned Enterprise Law Implementing Rules (1990), as amended and the Company Law
of the PRC (2006) contain the principal regulations governing dividend
distributions by wholly foreign owned enterprises. Under these regulations,
wholly foreign owned enterprises, such as WFOE, may pay dividends only out of
their accumulated profits, if any, determined in accordance with PRC accounting
standards and regulations. Additionally, WFOE is required to set aside a certain
amount of their accumulated profits each year, if any, to fund certain reserve
funds. These reserves are not distributable as cash dividends except in
the event of liquidation and cannot be used for working capital purposes.
Furthermore,
if our consolidated subsidiaries in China incur debt on their own in the future,
the instruments governing the debt may restrict its ability to pay dividends or
make other payments. If we or our consolidated subsidiaries are unable to
receive all of the revenues from our operations due to these contractual or
dividend arrangements, we may be unable to pay dividends on our common
stock.
We
may have difficulty establishing adequate management, governance, legal and
financial controls in the PRC.
The PRC
historically has been deficient in western style management, governance and
financial reporting concepts and practices, as well as in modern banking, and
other control systems. Our current management has little experience with
western style management, governance and financial reporting concepts and
practices, and we may have difficulty in hiring and retaining a sufficient
number of qualified employees to work in the PRC. As a result of these
factors, and especially given that we expect to be a publicly listed company in
U.S. and subject to regulation as such, we may experience difficulty in
establishing management, governance legal and financial controls, collecting
financial data and preparing financial statements, books of account and
corporate records and instituting business practices that meet western
standards. We may have difficulty establishing adequate management,
governance, legal and financial controls in the PRC. Therefore, we may, in
turn, experience difficulties in implementing and maintaining adequate internal
controls as required under Section 404 of the Sarbanes-Oxley Act of 2002 and
other applicable laws, rules and regulations. This may result in
significant deficiencies or material weaknesses in our internal controls which
could impact the reliability of our financial statements and prevent us from
complying with SEC rules and regulations and the requirements of the
Sarbanes-Oxley Act of 2002. Any such deficiencies, weaknesses or lack of
compliance could have a materially adverse effect on our business and the public
announcement of such deficiencies could adversely impact our stock
price.
It
may be difficult to protect and enforce our intellectual property rights under
PRC laws.
Intellectual
property rights in China are still developing, and there are uncertainties
involved in the protection and the enforcement of such rights. We will
need to pay special attention to protecting our intellectual property and trade
secrets. Failure to do so could lead to the loss of a competitive
advantage that could not be compensated by our damages award.
If
our land use rights are revoked, we would have no operational
capabilities.
Under
Chinese law land is owned by the state or rural collective economic
organizations. The state issues to tenants the rights to use property. Use
rights can be revoked and the tenants forced to vacate at any time when
redevelopment of the land is in the public interest. The public interest
rationale is interpreted quite broadly and the process of land appropriation may
be less than transparent. Through our operating subsidiary, we rely on
these land use rights as the cornerstone of our operations for both our
manufacturing facility and our corporate headquarters. The loss of such
rights would have a material adverse effect on our company as we would be
required to relocate our facilities and obtain new land use rights, and there is
a risk that we would not be able to accomplish such a relocation with reasonable
cost or at all.
19
Our
failure to fully comply with PRC labor laws, including laws relating to social
insurance, may expose us to potential liability and increased
costs.
Companies
operating in China must comply with a variety of labor laws, including certain
pension, health insurance, unemployment insurance and other welfare-oriented
payment obligations. Our failure to comply with these laws, including
those relating to the payment of social insurance, could have a material adverse
effect on our business in the form of payment obligations as well as paying
administrative fines.
In
addition, the new PRC Labor Contract Law took effect January 1, 2008 and governs
standard terms and conditions for employment, including termination and lay-off
rights, contract requirements, compensation levels and consultation with labor
unions, among other topics. In addition, the law limits
non-competition agreements with senior management and other employees who have
access to confidential information to two years and imposes restrictions or
geographical limits. This new labor contract law could impact our
labor costs, which could adversely impact our results of
operations.
Any
recurrence of severe acute respiratory syndrome, or SARS, or another widespread
public health problem, could adversely affect our operations.
A renewed
outbreak of SARS or another widespread public health problem in the PRC, where
all of our revenue is derived, could have an adverse effect on our operations.
Our operations may be impacted by a number of health-related factors, including
quarantines or closures of some of our offices that could leave us without many
employees to conduct our business which would materially and adversely affect
our operations and financial condition.
Risks
Related to Our Common Stock
There
is currently a limited trading market for our common shares, and you may be
unable to sell at or near ask prices or at all if you need to sell your shares
to raise money or otherwise desire to liquidate your shares.
Our
common stock is currently traded in the over-the-counter market through the OTC
Bulletin Board. The quotation of our shares on the OTC Bulletin Board may result
in a less liquid market available for our existing and potential stockholders to
trade shares of our common stock, could depress the trading price of our common
stock and could have a long-term adverse impact on our ability to raise capital
in the future. While there is an active trading market for our common stock, it
is small. We cannot give you any assurance that a broader or more active public
trading market for our common stock will develop or be sustained, or that
current trading levels will be sustained.
An
active and visible trading market for our common stock may not
develop.
We cannot
predict whether an active market for our common stock will develop in the
future. In the absence of an active trading market:
|
·
|
Investors
may have difficulty buying and selling or obtaining market
quotations;
|
|
·
|
Market
visibility for our common stock may be limited;
and
|
|
|
·
|
A
lack of visibility for our common stock may have a depressive effect on
the market price for our common
stock.
|
20
The OTCBB
is an unorganized, inter-dealer, over-the-counter market that provides
significantly less liquidity than NASDAQ or the NYSE AMEX. The
trading price of the common stock is expected to be subject to significant
fluctuations in response to variations in quarterly operating results, changes
in analysts’ earnings estimates, announcements of innovations by us or our
competitors, general conditions in the industry in which we operate and other
factors. These fluctuations, as well as general economic and market
conditions, may have a material or adverse effect on the market price of our
common stock.
The
market price for our stock may be volatile.
The
market price for our stock may be volatile and subject to wide fluctuations in
response to factors including the following:
|
●
|
actual
or anticipated fluctuations in our quarterly operating
results;
|
|
●
|
changes
in financial estimates by securities research
analysts;
|
|
●
|
conditions
in pharmaceutical markets;
|
|
●
|
changes
in the economic performance or market valuations of other pharmaceutical
companies;
|
|
●
|
announcements
by us or our competitors of new products, acquisitions, strategic
partnerships, joint ventures or capital
commitments;
|
|
●
|
addition
or departure of key personnel;
|
|
●
|
fluctuations
of exchange rates between RMB and the U.S.
dollar;
|
|
●
|
intellectual
property litigation; and
|
|
●
|
general
economic or political conditions in
China.
|
In
addition, the securities market has from time to time experienced significant
price and volume fluctuations that are not related to the operating performance
of particular companies. These market fluctuations may also materially and
adversely affect the market price of our stock.
We
are likely to remain subject to “penny stock” regulation and as a consequence
there are additional sales practice requirements and additional warning issued
by the SEC.
As long
as the trading price of our common stock is below $5.00 per share, the
open-market trading of our common stock will be subject to the “penny stock”
rules of the SEC. The “penny stock” rules impose additional sales practice
requirements on broker-dealers who sell securities to persons other than
established customers and accredited investors (generally those with assets in
excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together
with their spouse). For transactions covered by these rules, the broker-dealer
must make a special suitability determination for the purchase of securities and
have received the purchaser’s written consent to the transaction before the
purchase. Additionally, for any transaction involving a penny stock, unless
exempt, the broker-dealer must deliver, before the transaction, a disclosure
schedule prescribed by the SEC relating to the penny stock market. The
broker-dealer also must disclose the commissions payable to both the
broker-dealer and the registered representative and current quotations for the
securities. Finally, monthly statements must be sent disclosing recent price
information on the limited market in penny stocks. These additional burdens
imposed on broker-dealers may restrict the ability of broker-dealers to sell the
common stock and may affect a stockholder’s ability to resell the common
stock.
There can
be no assurance that our common stock will qualify for exemption from the “penny
stock” rules. In any event, even if our common stock is exempt from such rules,
we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the
SEC the authority to restrict any person from participating in a distribution of
a “penny stock” if the SEC finds that such a restriction would be in the public
interest.
Stockholders
should be aware that, according to SEC Release No. 34-29093, the market for
penny stocks has suffered in recent years from patterns of fraud and abuse. Such
patterns include (i) control of the market for the security by one or a few
broker-dealers that are often related to the promoter or issuer; (ii)
manipulation of prices through prearranged matching of purchases and sales and
false and misleading press releases; (iii) boiler room practices involving
high-pressure sales tactics and unrealistic price projections by inexperienced
sales persons; (iv) excessive and undisclosed bid-ask differential and markups
by selling broker-dealers; and (v) the wholesale dumping of the same securities
by promoters and broker-dealers after prices have been manipulated to a desired
level, along with the resulting inevitable collapse of those prices and with
consequent investor losses. Our management is aware of the abuses that have
occurred historically in the penny stock market.
21
We
do not foresee paying cash dividends in the foreseeable future.
We
currently intend to retain any future earnings for funding growth. We do not
anticipate paying any dividends in the foreseeable future. As a result, you
should not rely on an investment in our securities if you require dividend
income. Capital appreciation, if any, of our shares may be your sole source of
gain for the foreseeable future. Moreover, you may not be able to resell your
shares in our company at or above the price you paid for them.
Our
Article of Incorporation authorize the issuance of shares of preferred stock
which, if issues, the rights, preference, designations and limitations of such
preferred stock could operate to the disadvantage of the shares of our
outstanding common stock.
Our
articles of incorporation authorize the issuance of shares of preferred stock,
the rights, preferences, designations and limitations of which may be set by the
board of directors. While no preferred stock is currently outstanding or subject
to be issued, the articles of incorporation have authorized issuance of up
to 1,000,000 shares of preferred stock (“Preferred Stock”) in the discretion of
the board of directors. Such Preferred Stock may be issued upon filing of
amended Articles of Incorporation and the payment of required fees; no further
shareholder action is required. If issued, the rights, preferences, designations
and limitations of such Preferred Stock would be set by our board of directors
and could operate to the disadvantage of the shares of our outstanding Common
Stock. Such terms could include, among others, preferences as to dividends and
distributions on liquidation.
Item
1B. Unresolved Staff Comments
None.
Item 2. Description of
Properties
The
executive offices and manufacturing facilities of our wholly-owned operating
subsidiary, Tianmu Pharmaceuticals, are located in the
Limin Pharmaceutical Technology Park in the City of Harbin, which
is the capital of Heilongjiang Province.
Under the
PRC law, land is owned by the state or rural collective economic organizations.
The state issues to tenants the rights to use land. Tianmu Pharmaceuticals has
been granted by the government of China, a 50-year land use right certificate,
which will expire in April 2046, to use 50,000 square meters of land for
manufacturing purposes. Currently our offices and manufacturing facility
occupy 15,000 square meters, and our warehouse occupies 1,800 square meters.
Item 3. Legal Proceedings
We are
not a party to any current or pending legal proceedings that, if decided
adversely to us, would have a material adverse effect upon our business, results
of operations, or financial condition, and we are not aware of any threatened or
contemplated proceeding by any governmental authority against us. To
our knowledge, we are not a party to any threatened civil or criminal
action or investigation. However, from time to time, we may become involved
in various lawsuits and legal proceedings that arise in the ordinary course of
business. Litigation is subject to inherent uncertainties, and an adverse result
in these or other matters may arise from time to time that may harm our
business.
Item 4. (REMOVED AND
RESERVED)
22
PART
II
Item 5. Market For
Common Equity, Related Stockholder Matters and Issuer Purchases of Equity
Securities
Market
Information
Our
Common Stock is traded on the OTC Bulletin Board under the symbols
“TGLPE.OB”. The following tables set forth, for the calendar quarter
indicated, the quarterly high and low sales price for our common stock as
reported on the OTC Bulletin Board. Trading in the common stock in
the over-the-counter market has been limited and sporadic and the quotations set
forth below are not necessarily indicative of actual market
conditions. Further, the quotations merely reflect the prices at
which transactions were proposed, and do not necessarily represent actual
transactions.
|
|
High
|
Low
|
|||||
|
2010 by
Quarter
|
|||||||
|
April
1, 2009 - June 30 , 2009
|
$
|
0.51
|
0.51
|
||||
|
July
1, 2009 - September 30, 2009
|
$
|
0.51
|
0.51
|
||||
|
October
1, 2009 - December 31, 2009
|
$
|
0.51
|
0.05
|
||||
|
January
1, 2010 - March 31 , 2010
|
$
|
1.20
|
0.06
|
||||
|
|
|
||||||
|
2009 by
Quarter
|
|||||||
|
April
1, 2008 - June 30, 2008
|
$
|
1.30
|
0.50
|
||||
|
July
1, 2008 - September 30, 2008
|
$
|
0.6
|
0.51
|
||||
|
October
1, 2008 - December 31, 2008
|
$
|
0.51
|
0.51
|
||||
|
January,
2009 - March 31, 2009
|
$
|
0.51
|
0.51
|
||||
On June
25, 2010, the closing price for shares of our common stock, as reported by the
Over-the-Counter Bulletin Board, was $0.55.
Record Holders.
As of
June 25, 2010, there were 667 registered holders of our common stock. As of June
25, 2010 there were 11,395,036 shares of common stock issued and
outstanding.
Dividends
We have
not paid dividends on our common stock in the past and do not anticipate doing
so in the foreseeable future. We currently intend to retain future earnings, if
any, to fund the development and growth of our business. The decision whether to
pay cash dividends on our common stock will be made by our board of directors,
at their discretion, and will depend on our financial condition, operating
results, capital requirements and other factors that the board of directors
considers significant.
Recent
Sales of Unregistered Securities
On April
1, 2009, we issued 39,216 shares of common stock to a law firm as consideration
for services. This issuance was made in reliance upon the exemption from
registration under Section 4(a) of the Securities Act.
Securities
Authorized for Issuance Under Equity Compensation Plans
The
following table indicates shares of common stock authorized for issuance under
our 2009 Incentive Plan as of March 31, 2010:
23
|
Plan
category
|
Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
|
Number of securities
remaining available
for future issuance
|
||||||
|
Equity
compensation plans approved by security holders (a)
|
0 | 830,907 | ||||||
|
Equity
compensation plans not approved by security holders
|
— | — | ||||||
|
Total
|
0 | 830,907 | ||||||
|
(a)
|
There a no
securities to be issued in the future under the Company’s
2009 Incentive Plan as only common shares (1,139,093 shares) have
been issued under such plan to
date.
|
Item
6. Selected Financial Data
We are a
“smaller reporting company” as defined by Regulation S-K and as such, are not
required to provide the information contained in this item pursuant to
Regulation S-K.
Item 7. Management’s Discussion
and Analysis or Plan of Operation.
The
following discussion and analysis of financial condition and results of
operations relates to the operations and financial condition reported in the
financial statements of Tongli Pharmaceuticals(USA) Inc. for the fiscal years
ended March 31, 2010 and 2009 and should be read in conjunction with such
financial statements and related notes included in this report. Those
statements in the following discussion that are not historical in nature
should be considered to be forward-looking statements that are inherently
uncertain. Actual results and the timing of events may differ materially
from those contained in these forward-looking statements due to a number of
factors, including those discussed in the “Cautionary Note on
Forward-Looking Statements” set forth above.
Company
Overview
Tongli
Pharmaceuticals (USA), Inc. (“TP” or the “Company”), formally known as Aim Smart
Corporation (“Aim Smart”), was originally formed in the State of Colorado in
April 1998 and reorganized as a Delaware corporation in September 2007.
Subsequent to the merger, Aim Smart changed its name to American Tony
Pharmaceuticals, Inc. on September 23, 2008, and then changed its name to Tongli
Pharmaceuticals (USA), Inc. on October 30, 2008.
On July
29, 2008, Aim Smart acquired all of the outstanding capital stock of American
Tony Pharmaceuticals, Inc., a Delaware corporation (“American Tony”), by issuing
9,700,000 shares of Aim Smart’s common stock to the shareholders of American
Tony, representing 96.7% of the outstanding shares of Aim Smart. American Tony
paid $525,000 for its controlling interest in Aim Smart and this interest was
acquired solely to effectuate the reverse merger and was paid for with $276,000
of its own funds and a $249,000 loan from its Chairman.
The
acquisition has been accounted for as a reverse merger under the purchase method
of accounting since there was a change of control. Accordingly, American Tony is
treated as the continuing entity for accounting purposes, whereas the entity
formally known as Aim Smart is the legal surviving entity.
American
Tony is a holding company incorporated in the State of Delaware. In February
2007, American Tony acquired, through a wholly-owned subsidiary, Heilongjiang
Tongli Technology Co., Ltd. (“TT” or “Tongli Technology”), all of the registered
capital of Tianmu Pharmaceuticals, a corporation organized under the laws of the
PRC on November 26, 1999. Almost all of our operation is conducted through
Tianmu Pharmaceuticals in PRC. Tianmu Pharmaceuticals is engaged in the business
of manufacturing and marketing pharmaceuticals and health care products in the
PRC, and has been developing pharmaceutical and health care products that
incorporate elements of Chinese traditional medicine with elements of western
medicine. Our research and development activities have been carried out at
relatively low cost because they have been carried out by our in house
R&D team and, in the past, in concert with a number of research
institutes and universities, including the Jilin Research Institute of Chinese
Traditional Medicine, the Sichuan Research Institute of Chinese Medicine, the
Heilongjiang Institute of Chinese Traditional Medicine, the Chemistry Department
of Tsinghua University, and the R&D Center of Harbin Medical University.
24
In 2005,
Tianmu Pharmaceuticals obtained the National Drug GMP (Good Manufacturing
Practices) Certification, and Drug Register License and Drug Production
Certificate for nine products from the China State Food and Drug
Administration (formerly, China State Drug Administration). The
Company’s main products include cholesterol reduction pill, mouthwash,
anti-inflammatory tablet and calcium supplement. These products are sold through
distributors or directly to customers; no service is required after sales are
made. The Company’s primary customers are drug stores and hospitals located in
China.
Development
and Strategy
In
our fiscal year ended at March 31, 2010, we continued the execution
of our product channel expansion strategy that resulted in increased market
penetration of our products and expanded revenue growth. Management
plans to continue the emphasis on expanded and enhanced marketing and sales in
our 2011 fiscal year and beyond. Part of this strategy involves
increasing and improving our marketing and sales activities to enhance the
market leadership of our key leading products and to increase the sales of other
products by expanding our sales force, solidifying our distribution network and
expanding our market segment coverage, and increasing our marketing and
promotional activities.
Management
also plans to pursue strategic acquisitions as part of our growth strategy in
2010 and beyond. We plan to selectively pursue strategic
acquisition opportunities to further consolidate our resources and expand our
market coverage. We believe that such an initiative will provide
effective means to broaden our product lines, increase our market coverage and
complement our research and development capabilities.
Management
believes that our emphasis on further commercializing and broadening our product
lines, enhanced sales and marketing efforts shall continue to yield significant
increases in revenue in 2010 and beyond.
Critical
Accounting Policies and Estimates
Our
consolidated financial information has been prepared in accordance with
generally accepted accounting principles in the United States, which requires us
to make judgments, estimates and assumptions that affect (1) the reported
amounts of our assets and liabilities, (2) the disclosure of our contingent
assets and liabilities at the end of each fiscal period and (3) the reported
amounts of revenues and expenses during each fiscal period. We
continually evaluate these estimates based on our own historical experience,
knowledge and assessment of current business and other conditions, our
expectations regarding the future based on available information and reasonable
assumptions, which together form our basis for making judgments about matters
that are not readily apparent from other sources. Since the use of
estimates is an integral component of the financial reporting process, our
actual results could differ from those estimates. Some of our
accounting policies require a higher degree of judgment than others in their
application.
When
reviewing our financial statements, you should consider (1) our selection of
critical accounting policies, (2) the judgment and other uncertainties affecting
the application of those policies, and (3) the sensitivity of reported results
to changes in conditions and assumptions. We believe the following
accounting policies involve the most significant judgment and estimates used in
the preparation of our financial statements.
Basis
of presentation
The
financial statements have been prepared in conformity with accounting principles
generally accepted in the United States of America. The functional currency of
TT and HTP is the Chinese Renminbi (“RMB”). The accompanying financial
statements include the financial statements of foreign subsidiaries that have
been translated and presented in United States dollars (“USD”).
25
Principles
of consolidation
The
consolidated financial statements include the accounts of the Company, American
Tony, TT and HTP. All significant inter-company accounts and
transactions have been eliminated.
Principles
of consolidation
The
consolidated financial statements include the accounts of the Company, American
Tony, TT and HTP. All significant inter-company accounts and
transactions have been eliminated upon consolidation.
Uses
of estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of
the financial statements and the reported amounts of net revenue and expenses
during each reporting period. Actual results could differ from those
estimates.
Fair
Value of Financial Instruments
The
Company adopted ASC 820, Fair Value Measurements and Disclosures. ASC 820
clarifies the definition of fair value, prescribes methods for measuring fair
value, and establishes a fair value hierarchy to classify the inputs used in
measuring fair value as follows:
Level
1-Inputs are unadjusted quoted prices in active markets for identical assets or
liabilities available at the measurement date.
Level
2-Inputs are unadjusted quoted prices for similar assets and liabilities in
active markets, quoted prices for identical or similar assets and liabilities in
markets that are not active, inputs other then quoted prices that are
observable, and inputs derived from or corroborated by observable market
data.
Level
3-Inputs are unobservable inputs which reflect the reporting entity’s own
assumptions on what assumptions the market participants would use in pricing the
asset or liability based on the best available information.
The
carrying amounts reported in the balance sheets for cash, accounts receivable,
inventory, advance to suppliers, accounts payable and other accrued expenses
approximate their fair market value based on the short-term maturity of these
instruments. The Company did not identify any assets or liabilities that are
required to be presented on the consolidated balance sheets at fair value in
accordance with ASC 820.
Cash
and Cash Equivalents
The
Company maintains cash with financial institutions in the PRC, which are not
insured or otherwise protected. Should any of these institutions
holding the Company’s cash become insolvent, or if the Company is unable to
withdraw funds for any reason, the Company could lose the cash on deposit with
that institution.
Cash and
cash equivalents include cash in hand and cash in time deposits, certificates of
deposit and all highly liquid debt instruments with original maturities of three
months or less.
Accounts
Receivable
Accounts receivables consist primarily
of receivables resulting from sales of products, and are stated at net
realizable value. This value includes an appropriate allowance for estimated
uncollectible accounts. The allowance is calculated based upon the evaluation
and the level of past due accounts and the relationship with and the economic
status of the customers. Management believes that no allowance is
necessary for the period indicated.
26
Inventory
Inventory
is stated at the lower of cost, determined using the weighted average cost
method, and net realizable value. Costs include materials, labor and
manufacturing overhead. Net realizable value is the estimated selling
price, in the ordinary course of business, less estimated costs to complete and
dispose. Management periodically compares the cost of inventory with the market
value and an allowance is made for writing down the inventory to its market
value, if lower than cost. No allowance for inventory markdowns is considered
necessary for the years ended March 31, 2010 and 2009.
Advances
to Suppliers
Advance
to suppliers represent the payments made and recorded in advance for goods and
services to be received. The Company’s management has developed purchasing
relationships with a considerable number of suppliers to ensure multiple sources
for most of the raw materials required. In the opinion of management, the
Company’s business would not be significantly damaged by the loss of any one
supplier.
A
considerable portion of the raw materials that production requires are volatile
herbs, which have a brief shelf life. This situation imposes a risk on
suppliers, who often grow such herbs to order to insure an immediate market for
the herbs. The situation also necessitates that management is assured that
raw material requirements are available precisely when needed. To satisfy
these conditions, it is the practice to make substantial cash advances to
suppliers in order to lock-in raw material requirements. However, unless the
Company develops proprietary sources of raw material supplies, the advance
payment schedule for supplies will continue to have a negative effect on working
capital.
Property
and equipment
Property
and equipment are recorded at cost. The cost of an asset comprises
its purchase price and any directly attributable costs of bringing the asset to
its present working condition and locations for its intended use. Depreciation
is provided in amounts sufficient to amortize the cost of the related assets
over their useful lives using the straight line method for financial reporting
purposes. HTP obtained the right to use a parcel of land on which its office and
production facilities are situated, pursuant to the contract from the local
government of the PRC which expires in April 2046.
Depreciation
is calculated using the straight-line method over the following useful
lives:
|
Buildings
and improvements
|
40
years
|
|
|
Right
to use land
|
Life
of lease
|
|
|
Machinery
and equipment
|
10
years
|
|
|
Office
equipment
|
5
years
|
|
|
Vehicle
|
|
5
years
|
Maintenance, repairs and minor renewals
are charged to expense when incurred. Replacements and major renewals
are capitalized.
Impairment
of Long Lived Assets
Long-lived
assets, which include property, plant and equipment and intangible assets, are
reviewed for impairment whenever events or changes in circumstances indicate
that the carrying amount of an asset may not be recoverable. Recoverability of
long-lived assets to be held and used is measured by a comparison of the
carrying amount of an asset to the estimated undiscounted future cash flows
expected to be generated by the assets.
27
The
Company accounts for the impairment of long-lived assets in accordance with the
guidance of FASB ASC 360-10-20 (formerly referred as Statement of Financial
Accounting Standards No. 144. “Accounting for the Impairment or
Disposal of Long-Lived Assets”). Long-lived assets are
reviewed for impairment when circumstances indicate the carrying value of an
asset may not be recoverable. For assets that are to be held and
used, impairment is recognized when the estimated undiscounted cash flows
associated with the asset or group of assets is less than their carrying
value. If impairment exists, an adjustment is made to write the asset
down to its fair value, and a loss is recorded as the difference between the
carrying value and fair value. Fair values are determined based on
quoted market values, discounted cash flows or internal and external appraisals,
as applicable. Assets to be disposed of are carried at the lower of
carrying value or estimated net realizable value. Based on its review, the
Company believes that, as of March 31, 2010, there were no significant
impairments of its long-lived assets used in operations.
Deferred
income taxes
The
Company accounts for income taxes in accordance with FASB ASC 740 “Income Taxes” which requires
that deferred tax assets and liabilities be recognized for future tax
consequences attributable to differences between financial statement carrying
amounts of existing assets and liabilities and their respective tax
bases. In addition, ASC 740 requires recognition of future tax
benefits, such as carry-forwards, to the extent that realization of such
benefits is more likely than not and that a valuation allowance be provided when
it is more likely than not that some portion of the deferred tax asset will not
be realized. Management reviews this valuation allowance periodically and makes
adjustments as warranted.
Foreign
currency translation
Since the
Company operates primarily in the PRC, the Company’s functional currency is the
Chinese Yuan (“RMB”). For financial reporting purposes, RMB has been
translated into United States dollars (“USD”) as the reporting currency. Equity
accounts are translated at historical rates. Assets and liabilities are
translated at the exchange rate in effect at the balance sheet date. Revenues
and expenses are translated at the average rate of exchange prevailing during
the reporting period. Translation adjustments arising from the use of different
exchange rates from period to period are included as a component of
stockholders’ equity as “Accumulated other comprehensive income”. Gains and
losses resulting from foreign currency translations are included in accumulated
other comprehensive income.
Revenue
Recognition
Revenue
is recognized at the date of shipment to customers when a formal arrangement
exists, the price is fixed or determinable, the delivery is completed, and no
other significant obligations of the Company exist and collectability is
reasonably assured. Payments received before all of the relevant criteria
for revenue recognition are satisfied are recorded as advances from
customers.
Earnings
per share
The
Company computes earnings per share (“EPS’) in accordance with FASB ASC 260
“Earnings per Share”
which requires companies with complex capital structures to present basic and
diluted EPS. Basic EPS is measured as net income divided by the weighted
average common shares outstanding for the period. Diluted EPS is similar
to basic EPS but presents the dilutive effect on a per share basis of potential
common shares (e.g., convertible securities, options and warrants) as if they
had been converted at the beginning of the periods presented, or issuance date,
if later. Potential common shares that have an anti-dilutive effect (i.e.,
those that increase income per share or decrease loss per share) are excluded
from the calculation of diluted EPS.
Comprehensive
income
In
accordance with FASB ASC 220, comprehensive income is defined to include all
changes in equity except those resulting from investments by shareholders and
distributions to shareholders. Among other disclosures, all items
that are required to be recognized under current accounting standards as
components of comprehensive income are required to be reported in a financial
statement that is presented with the same prominence as other financial
statements. Comprehensive income includes net income and the foreign
currency translation gain, net of tax.
28
Statement
of Cash Flows
In
accordance with FASB ASC 230 “Statement of cash Flows,” cash flows from the
Company’s operations are calculated based upon the local currencies. As a
result, amounts related to assets and liabilities reported on the statement of
cash flows will not necessarily agree with changes in the corresponding balances
on the balance sheet.
Segment
reporting
FASB ASC
280-10-50 “Segment Reporting” requires the use of the “management approach”
model for segment reporting. The management approach model is based
on the way a company’s management organized segments within the company for
making operating decisions and assessing performance. Reportable
segments are based on products and services, geography, legal structure,
management structure, or any other manner in which management disaggregates a
company. ASC 280-10-50 has an immaterial effect on the Company’s
financial statements, as the Company consists of only one reportable business
segment. All revenues are from sales to customers in the
PRC. Substantially all of the Company’s assets are located in the
PRC.
Concentration
of risks
During
the year ended March 31, 2010, approximately 43.5% of sales were generated from
four major distributors with the largest distributor representing 27.2% of
sales. In addition, two products manufactured by the Company
represented approximately 47.8% of the total sales for the year ended March 31,
2010, versus one product represented 63.3% of total sales for the year ended
March 31, 2009.
Discussion
of Operating Results
The
results of our operation for the twelve months ended March 31, 2010 compared to
the prior comparative periods are as follows:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Revenues
|
$ | 8,591,858 | $ | 7,442,445 | ||||
|
Cost
of Sales
|
4,807,168 | 4,103,669 | ||||||
|
Gross
Profit
|
3,784,690 | 3,338,776 | ||||||
|
Selling,
general & administrative expeses
|
1,883,142 | 1,238,603 | ||||||
|
Other
income (expenses)
|
(73,247 | ) | 59,423 | |||||
|
Provision
of income taxes
|
855,489 | 254,757.00 | ||||||
|
Net
income
|
972,812 | 1,904,839 | ||||||
|
Foreign
currency translation adjustments
|
11,848 | 174,997 | ||||||
|
Comprehensive
income
|
$ | 984,660 | $ | 2,079,836 | ||||
29
Revenues,
cost of sales and gross profit
Revenues
increased 15.44% to approximately $8.59 million for the twelve months ended
March 31, 2010 from approximately $7.44 million for the twelve months ended
March 31, 2009. The $ 1.15 million increase was attributable to the
increase in sales of our product Antihyperlipidemics, supported by our marketing
efforts, increasing brand recognition and effective pricing strategy and our
distribution to previously unaddressed market. Part of our revenue was also
generated by our recently purchased new product Yan Li Xiao Capsule which
broadened our product offerings and helped to increase our sales. We have the
exclusive right to use this new product’s trademark, manufacture and sell this
product nationwide for the next seven years.
Management
expects that our emphasis on broadening our product pipeline coupled with our
continued sales channel expansions, along with our enhanced sales and marketing
efforts will continue to yield increases in revenue for fiscal year 2011 and
beyond.
Cost of
sales was approximately $4.8 million for the twelve months ended March 31, 2010
compared to $4.1 million for the twelve months ended March 31, 2009. The $0.7
million increase in cost of sales was mainly attributable to the increased sales
of our products, especially due to increased cost of sales incurred associated
with the manufacturing and distribution of our recently purchased new product
Yan Li Xiao Capsule. Cost of sales as a percentage of revenue increased from
55.14% to 55.95% as compared to the prior comparative period.
Gross
profit was approximately $3.78 million for the twelve months ended March 31,
2010 compared to $3.34 million for the twelve months ended March 31, 2009, an
increase of $0.44 million due to increased sales of our products. The gross
profit as a percent of revenues for the twelve months ended March 31, 2010
decreased to 44.05% compared to 44.86% in the prior year mainly due to
increasing of raw material costs as well as relatively higher costs incurred for
our newly acquired Yan Li Xiao Capsule and business tax for Yan Li Xiao sales
These factors led to the increased cost of sales. We expect our gross profit
margin will remain in its current level with slight growth in the
future.
Operating
expenses
Total
operating expenses increased to approximately $1.88 million for the twelve
months ended March 31, 2010 from $1.23 million for the twelve months ended March
31, 2009.
As a
percentage of revenues, operating expenses increased to 21.92% for the
twelve months ended March 31, 2010 compared to 16.64% for the twelve months
ended March 31, 2009. The $0.64 million increase in total operating
expenses was primarily
due to the increase in general and administrative expenses, because, on March
29, 2010, We issued 1,169,093 common shares without restrictive legend to 46
employees under our 2009 stock incentive plan and accordingly we recorded
related general and administrative expenses in the amount of $1,180,784. This
increase was offset by decreased R&D expenses incurred because we our
current products are still under normal product cycle and our current patents
have provided us sufficient capabilities to meet our current production and
marketing demand, accordingly, we cut off our investment in our own
R&D activities. In addition, we have switched our R&D strategy to buy
new products with great market potentials from outside parties instead of
relying on our own efforts which we believe will be more effective. The decrease
in our total operating expense was also attributable to our decreased selling
expense for the twelve months ended March 31, 2010 because we have established
relatively mature marketing network to market our products which reduced our
sales efforts and advertising expenses. Our increased brand awareness
among our targeted customers also helped to reduce our selling expense. These
changes are summarized below:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
General
& administrative expenses
|
$ | 490,323 | $ | 834,194 | ||||
| Non-cash compensation | 1,180,784 | |||||||
|
Research
& development
|
3,462 | 70,670 | ||||||
|
Depreciation
and amortization expenses
|
153,214 | 177,575 | ||||||
|
Selling
expenses
|
55,359 | 156,164 | ||||||
|
Total
Operating expenses
|
$ | 1,883,142 | $ | 1,238,603 | ||||
30
Interest
expense
Net
interest expense was $73,247 for the twelve months ended March 31, 2010
(including interest expense of $73,340 and interest income of $93) compared to
$182,285 for the twelve months ended March 31, 2009. The decrease of net
interest expense for the twelve months ended March 31, 2010 was due to our
repayment of all the bank loans as of March 31, 2009. The only interest expense
relates to the loans from related parties.
Rental
income
Since
June 2007, HTP has leased a portion of its facility to a pharmaceutical college
at a monthly rental of approximately $13,400 (RMB100,000) through September 30,
2008 and $23,300 (RMB160,000) from October 1, 2008 through March 31,
2009. For the year ended March 31, 2009, we reported rental income of
$241,708. As of March 31, 2009, the facility became vacant and
available to the Company for future operation. No rental income was
generated from the facility for the year ended March 31, 2010.
Income
taxes
The
majority of our net income for the period ended March 31, 2010 was from Harbin
Tianmu Pharmaceuticals Co., Ltd. (“HTP”), which conducts substantially all of
our operation in the PRC. Our Chinese subsidiaries are governed by the Income
Tax Law of the PRC concerning the private-run enterprises, which are generally
subject to tax at a statutory rate of 25% on income reported in the statutory
financial statements after appropriate tax adjustments.
The
income tax expense of $855,489 for the twelve months ended March 31, 2010, an
increase of $600,732 or 235.8% than prior comparative period was attributed to
the increased taxable income primarily derived from HTP for the year ended March
31, 2010.
A
reconciliation of tax at United States Federal statutory rate to provision for
income tax recorded in the financial statements is as follows:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
U.S.statutory
income tax rate
|
35.0 | % | 35.0 | % | ||||
|
Foreign
tax rate difference between US and China
|
(10.0 | )% | (10.0 | )% | ||||
|
Effect
of tax deduction due to NOL from China
|
0.0 | % | (20.2 | )% | ||||
|
NOL
from U.S with 100% valuation allowance
|
21.8 | % | 6.70 | % | ||||
|
Actual
consolidated income tax rate
|
46.8 | % | 11.5 | % | ||||
The
components of deferred taxes as of March 31, 2010 and 2009 consist of the
following:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Net
operating loss carry-forwards
|
773,165 | 208,732 | ||||||
|
Valuation
allowance
|
(773,165 | ) | (208,732 | ) | ||||
|
Net
deferred tax asset
|
- | - | ||||||
Management
believes the deferred tax assets as of March 31, 2010 and 2009 do not satisfy
the realization criteria set forth in FASB ASC 740 and has recorded a valuation
allowance for the entire net tax asset.
Net
income
As a
result of the above factors, we reported a net income of $972,812 for the year
ended March 31, 2010, an decrease of $0.93 million or 48.9%, as compared to
the net income of approximately $1.9 million for the year ended March 31, 2009,
For
the year ended March 31, 2010, the decrease in our net income was primarily due
to our increased operating expenses primarily affected by stock based
compensation, especially the $1,180,784 general and administrative expenses
incurred for the 1,169,093 common shares issued to 46 employees under the 2009
incentive plan as discussed above. The decrease in our net income was also
affected by our increased cost of sales associated with the manufacturing and
distribution of our recently purchased new product Yan Li Xiao
Capsule.
Other
comprehensive income
We
operate primarily in the PRC and the functional currency of our operating
subsidiary is the Chinese Renminbi (“RMB”). The RMB is not freely
convertible into foreign currency and all foreign exchange transactions must
take place through authorized institutions. No representation is
intended to imply that the Renminbi amounts could have been, or could be,
converted, realized or settled into USD at the rate on March 31, 2010 or at any
other rate.
31
Translation
adjustments resulting from this process amounted to $11,848 and $174,997 as of
March 31, 2010 and 2009, respectively. The balance sheet amounts with
the exception of equity at March 31, 2010 were translated at 6.8259 RMB to 1.00
USD as compared to 6.8336 RMB to 1.00 USD at March 31, 2009. The equity accounts
were stated at their historical rate. The average translation rates applied to
the income statements accounts for the periods ended March 31, 2010 and 2009
were 6.8289 RMB and 6.8678 RMB, respectively.
Liquidity
and Capital Resources
Liquidity
is the ability of a company to generate funds to support its current and future
operations, satisfy its obligations and otherwise operate on an ongoing basis.
We usually finance our operation and capital expenditures through cash from
operations, short-term bank loans as well as loans from the members of our
management group and an entity owned by our Chairman.
The
report of our independent registered public accounting firm on the financial
statements for the year ended March 31, 2009 includes an explanatory paragraph
indicating substantial doubt as to the our ability to continue as a going
concern. We have taken certain actions and continue to implement changes
designed to improve our financial results and operating cash flows. The actions
include certain cost-saving initiatives and continuous development of new and
existing clients. As of March 31, 2010, our working capital has improved to
$515,312 and the operating results for the twelve months ended March 31, 2010
reflect profitability. We believe that these actions will enable us to move
towards profitability and improve cash flows in our operations through the
coming year.
Total
current assets increased to approximately $1.96 million for the twelve months
ended March 31, 2010 from $1.45 million for the twelve months ended March 31,
2009. The primary changes in our current assets during this period
were from changes in cash, inventory and advance to suppliers. The decrease in
cash from $50,247 at March 31, 2009 to $30,066 at March 31, 2010 was because our
increased business activities required more cash to be used in our operation,
the increase of inventory from $19,016 in March 31, 2009 to the amount of
$109,387 as of March 31, 2010 was due to the consideration of stocking raw
materials to avoid the potential material shortage in the near future, and
stocking finished goods in support of future sales. The increase of advance to
suppliers from $986,281 at March 31, 2009 to $1,465,713 as of March 31, 2010 was
attributed to our financial support to strengthen the relationship with our raw
material suppliers. These advances are short-term in nature and we believe the
risk of uncollectibility is small based our past experience.
Our total
current liabilities as of March 31, 2010 totaled in the amount of $1.44 million
as compared to $1.73 million in prior fiscal year. The decrease in current
liabilities was primarily due to our repayment of related party loans in the
amount of $0.28 million. Our balance sheet at March 31, 2009, also reflects a
balance due to related parties of $1,063,629 which was a working capital
advances made to us by our Chairman of the Company. The decrease of related
party loans by the amount of $0.28 million as compared to that of the previous
year was primarily due to our repayment of such loans when cash generated from
operating became available.
We
believe that we have sufficient funds to operate our existing business for the
next twelve months. However, in addition to funds available from our operating
and loans from related parties, we may need external sources of capital for our
expansion. Currently
we have budgeted $3.5 million for capital improvements. We may pursue
additional debt financing which could be secured by our property and equipment
and approach international equity markets for additional debt and/or equity
financing. There can be no assurance that we will be able to obtain such
additional financing at acceptable terms to us, or at all. To date we have no
commitment from any source for the funds we require.
Discussion
of Cash Flow
Comparison
of cash flows results for the fiscal year ended March 31, 2010 to the fiscal
year ended March 31, 2009, is summarized as follows:
32
|
As of March 31
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash
flow from operating activities
|
$ | 2,114,395 | $ | 1,306,918 | ||||
|
Cash
flow from investing activities
|
$ | (1,786,511 | ) | $ | (885,728 | ) | ||
|
Cash
flow from financing activities
|
$ | (348,096 | ) | $ | (511,566 | ) | ||
Operating
activities
Cash
flows provided by operating activities in the twelve months ended March 31, 2010
amounted to $2,114,395, which consist of our net income of $972,812, adds back
noncash adjustments of $1,675,790 and offset by net changes in operating assets
and liabilities, primarily including increase of advance to suppliers of 478,098
to maintain good relationship with the suppliers and increase in inventory of
$90,309 to stock raw materials to avoid the potential material
shortage in the near future, and stock finished goods in support of future
sales. For the
year ended March 31, 2010, we incurred stock-based compensation expense of
$1,200,784 due to stock issued to outside consultant for services rendered and
stock issued to employees under the 2009 incentive plan. Our cash provided by
operating activities was greatly affected by such expense
incurred.
Cash
flows provided by operating activities during the twelve months ended March 31,
2009 amounted to $1,306,916, which consists of our net income of $1,904,839
,adds back noncash adjustments of $809,854 and offset by net changes in
operating assets and liabilities due to expanded operating activities, including
increase in accounts receivables in the amount of $390,679, increase in advance
to suppliers in the amount of $287,464 to stimulate sales and maintain good
relationship with the suppliers, as well as an increase in accounts payable of
$803,136 in support of expanded operating activities.
Cash
flows from operations during the twelve months ended March 31, 2010 increased by
$807,449 or 61.8% compared with to the same period of 2009. The
increase in our cash flows provided by operations as of March 31, 2010 was
mainly becuase cash from sales were increased and rapid
collection generated more cash to be used in our
operation.
Investing
activities
Cash
flows used in investing activities amounted to $1,786,511 in the twelve months
ended March 31, 2010, which consisted of acquisition of intangible asset of
$175,722. In August 2009, we signed a contract with a third party Harbin Sanmu
Pharmaceuticals to purchase the exclusive rights to manufacture and sell a new
product nationwide for the next seven years. We paid Harbin Sanmu
Pharmaceuticals RMB 1,200,000 (approximate to USD 0.18 million) for this new
product namely Yan Li Xiao Capsule in October 2009 and started to manufacture
and distribute it in late 2009. The increase in our cash flow used in
investing activities also attributable to our payment made on contract deposit
of RMB 1,610,789 related to purchasing a new drug formula from a third
party. On March 21, 2010, we signed a patent purchase agreement with a third
party Tonghua Yisheng Pharmaceuticals Company Limited to purchase a new drug
with great market potentials. Total consideration for this patent transaction
amounted to RMB 33,000,000 (approximate to USD 4.83 million) to be paid in three
installments. The Company paid the first installment of RMB 11,000,000
(equivalent to USD 1.6 million) to Tonghua Yisheng Pharmaceuticals Company
Limited in March 2010 upon signing the purchase agreement. Our ability to
conclude this purchase and ultimately commercialize this product requires
additional assistance from the seller and obtaining government approvals. We
believe this new drug formula will help to expand our product offering as well
as market coverage in the future.
Our cash
flows used in investing activities amounted to $885,728 in the twelve months
ended March 31, 2009, which consist of a contract deposit amounted to
$1,023,619 to acquire a patent pertaining to a formula for a nutraceutical
product, receipt of a refundable deposit in the amount of $414,762, payment for
a portion of cost of Aim Smart of $276,000 and purchase of property and
equipment of $871.
Cash
flows used in investing activities in the twelve months ended March 31, 2009
increased by $900,783 or 101.7% compared to the same period in
2009.
33
Financing
activities
Cash
flows used in financing activities amounted to $348,096 in the twelve months
ended March 31, 2010, which consists of repayment of related party
loans of $348,096.
Our cash
flows used in financing activities amounted to $511,566 in the twelve months
ended March 31, 2009, which consist of proceeds from related party loans of
$1,553,604, offset by repayment to related party of $1,027,627 and repayment of
our bank loan of $1,017,543.
Cash
flows used in financing activities decreased by $163,470 or 31.95% compared to
the same period in 2009.
Off-Balance
Sheet Arrangements
As of the
date of this Report, we do not have any off-balance sheet arrangements that have
or are reasonably likely to have a current or future effect on our financial
condition, changes in financial condition, revenues or expenses, results of
operations, liquidity, capital expenditures or capital resources that are
material to investors. The term “off-balance sheet arrangement” generally means
any transaction, agreement or other contractual arrangement to which an entity
unconsolidated with us is a party, under which we have: (i) any obligation
arising under a guarantee contract, derivative instrument or variable interest;
or (ii) a retained or contingent interest in assets transferred to such entity
or similar arrangement that serves as credit, liquidity or market risk support
for such assets.
Inflation
Inflation
has not had a material impact on our business and we do not expect inflation to
have a material impact on our business in the near future.
Recent
Accounting Pronouncements
In May
2009, the FASB issued guidance now codified as ASC Topic 855, Subsequent Events (“ASC 855”)
which provides authoritative accounting literature related to evaluating
subsequent events. ASC 855 is similar to the current guidance with some
exceptions that are not intended to result in significant change to current
practice. ASC 855 defines subsequent events and also requires the
disclosure of the date through which an entity has evaluated subsequent events
and the basis for that date. The Company’s disclosures have been updated to
reflect the adoption of this standard.
In June
2009, FASB issued guidance now codified as ASC Topic 105, Generally Accepted Accounting
Principles (“ASC 105”). ASC 105 establishes only two levels of GAAP,
authoritative and non-authoritative. The FASB Accounting Standards
Codification (the “Codification”) is the source of authoritative,
nongovernmental GAAP, except for rules and interpretive releases of the
Securities and Exchange Commission (“SEC”), which are sources of authoritative
GAAP for SEC registrants. All other non-grandfathered, non-SEC accounting
literature not included in the Codification will become
non-authoritative. The standard is effective for financial statements for
interim or annual reporting periods ending after September 15,
2009. As the Codification was not intended to change or alter existing
GAAP, it will not have any impact on the Company’s financial position and
results of operations. References made to FASB guidance throughout this
document have been updated for the Codification.
In
June 2009, the FASB issued SFAS No. 168, “The FASB Accounting
Standards Codification and the Hierarchy of Generally Accepted Accounting
Principles, a replacement of FASB Statement No. 162, now included in FASB
ASC 105, “Generally Accepted Accounting Principles”. This guidance establishes
the FASB Accounting Standards Codification as the source of authoritative
accounting principles recognized by the FASB to be applied in the preparation of
financial statements in conformity with generally accepted accounting
principles. This guidance explicitly recognizes rules and interpretive releases
of the SEC under federal securities laws as authoritative GAAP for SEC
registrants and is effective for the Company’s current reporting period. The
Company has updated its financial statement disclosures to reflect the adoption
of this standard.
34
In June
2009, the FASB issued SFAS No. 167, “Amendments to FASB Interpretation
No. 46(R)”, now included in FASB ASC 810-10, “Consolidation”, which amends
FASB Interpretation No. 46 (revised December 2003) to address the
elimination of the concept of a QSPE. This guidance replaces the
quantitative-based risks and rewards calculation for determining which
enterprise has a controlling financial interest in a variable interest entity
with an approach focused on identifying which enterprise has the power to direct
the activities of a variable interest entity and the obligation to absorb losses
of the entity or the right to receive benefits from the entity. Additionally,
this guidance provides more timely and useful information about an enterprise’s
involvement with a variable interest entity and will become effective
January 1, 2010. We are currently evaluating the impact, if any, of these
guidelines.
In August
2009, the FASB issued ASU 2009-5, “Fair Value Measurements and Disclosures
(Topic 820) – Measuring Liabilities at Fair Value” (“FASB ASU 2009-5”), which
provides a single definition of fair value, a framework for measuring fair
value, and requires additional disclosure about the use of fair value to measure
assets and liabilities. ASU 2009-05 provides clarification for
circumstances in which a quoted price in an active market for the identical
liability is not available. In such circumstances, a reporting entity is
required to measure fair value using one or more of the following techniques:
(1) a valuation technique that uses: (a) the quoted price of the identical
liability when traded as an asset; or (b) quoted prices for similar liabilities
or similar liabilities when traded as assets; or (2) another valuation technique
that is consistent with the principles of Topic 820 such as an income approach
or a market approach. The guidance provided in ASU 2009-05 is
effective for the first reporting period (including interim periods) beginning
after issuance.
In
October 2009, the FASB issued Accounting Standards Update (ASU) No. 2009-13
Revenue Recognition (ASC 605):
Multiple-Deliverable Revenue Arrangement, which changes the requirements
for establishing separate units of accounting in a multiple element arrangement
and requires the allocation of arrangement consideration to each deliverable
based on the relative selling price. The selling price for each deliverable is
based on vendor-specific objective evidence (VSOE) if available, third-party
evidence if VSOE is not available, or estimated selling price if neither VSOE or
third-party evidence is available. ASU 2009-13 is effective for revenue
arrangements entered into in fiscal years beginning on or after June 15, 2010.
The Company is currently assessing the impact to its financial condition,
results of operations or cash flows.
In
December 2009, FASB issued ASU 2009-17, Consolidations (Topic 810)
Improvements to Financial Reporting by Enterprises Involved with Variable
Interest Entities , which replaces the quantitative-based risks and
rewards calculation for determining which enterprise, if any, has a controlling
financial interest in a variable interest entity with an approach focused on
identifying which enterprise has the power to direct the activities of a
variable interest entity that most significantly impact the entity’s economic
performance and (1) the obligation to absorb losses of the entity or
(2) the right to receive benefits from the entity. ASU 2009-17 also
requires additional disclosures about an enterprise’s involvement in variable
interest entities. ASU 2009-17 is effective as of the beginning of each
reporting entity’s first annual reporting period that begins after
November 15, 2009. The Company does not expect the adoption of ASU 2009-17
to have a material impact on its consolidated financial statements.
In
January 2010, the FASB issued ASU 2010-04, Accounting for Various Topics –
Technical Corrections to SEC Paragraphs. ASU 2010-04 makes technical
corrections to existing SEC guidance, including the following topics: accounting
for subsequent investments, termination of an interest rate swap, issuance of
financial statements - subsequent events, use of residential method to value
acquired assets other than goodwill, adjustments in assets and liabilities for
holding gains and losses, and selections of discount rate used for measuring
defined benefit obligation. The Company does not expect the adoption of ASU
2010-04 to have a material impact on its consolidated financial
statements.
In
January 2010, the FASB issued new standards in ASC 820, Fair Value Measurements and
Disclosures: Improving Disclosures About Fair Value Measurements. ASU
2010-06 amends Subtopic 820-10 to clarify existing disclosures, require new
disclosures, and include conforming amendments to guidance on employers’
disclosures about postretirement benefit plan assets. ASU 2010-06 is effective
for interim and annual periods beginning after December 15, 2009, except for
disclosures about purchases, sales, issuances, and settlements in the roll
forward of activity in Level 3 fair value measurements. Those disclosures are
effective for fiscal years beginning after December 15, 2010, and for interim
periods within those fiscal years. The Company is currently
evaluating the impact these standards will have on its financial condition,
results of operations, or cash flows and does not expect the adoption of ASU
2010-06 to have a material impact on its consolidated financial
statements.
35
In
January 2010, the FASB issued ASU 2010-05, Compensation – Stock Compensation
(Topic 718): Escrowed
Share Arrangements and the Presumption of Compensation. ASU 2010-05
updates existing guidance to address the SEC staff’s views on overcoming the
presumption that for certain shareholders escrowed share arrangements represent
compensation. The Company does not expect the adoption of ASU 2010-05
to have a material impact on its consolidated financial statements.
In
January 2010, the FASB issued ASU No. 2010-01, Equity (ASC 505): Accounting
for distributions to
Shareholders with Components of Stock and Cash (A Consensus of the FASB
Emerging Issues Task Force). This amendment to ASC 505 clarifies the stock
portion of a distribution to shareholders that allow them to elect to receive
cash or stock with a limit on the amount of cash that will be distributed is not
a stock dividend for purposes of applying ASC 505 and 260. Effective for interim
and annual periods ending on or after December 15, 2009, and would be applied on
a retrospective basis. The Company does not expect the provisions of ASU No.
2010-01 to have a material effect on the financial position, results of
operations or cash flows of the Company.
In
February 2010, the FASB issued ASU 2010-08, Technical Corrections to Various
Topics. ASU 2010-08 clarifies guidance on embedded derivatives and
hedging. ASU 2010-08 is effective for interim and annual periods beginning after
December 15, 2009. The Company does not expect the adoption of ASU 2010-08 to
have a material impact on its consolidated financial statements.
In
February 2010, FASB issued ASU 2010-09 Subsequent Event (Topic 855) Amendments
to Certain Recognition and Disclosure Requirements. ASU 2010-09 removes the
requirement for an SEC filer to disclose a date through which subsequent events
have been evaluated in both issued and revised financial statements. Revised
financial statements include financial statements revised as a result of either
correction of an error or retrospective application of GAAP. All of the
amendments in ASU 2010-09 are effective upon issuance of the final ASU, except
for the use of the issued date for conduit debt obligors. That amendment is
effective for interim or annual periods ending after June 15, 2010. The Company
adopted ASU 2010-09 in February 2010 and did not disclose the date through which
subsequent events have been evaluated.
Item
7A. Quantitative and Qualitative Disclosures About Market
Risk
We are a
“smaller reporting company” as defined by Regulation S-K and as such, are not
required to provide the information contained in this item pursuant to
Regulation S-K.
Item
8. Financial Statements and Supplementary Data
Our
Consolidated Financial Statements and Notes thereto and the report of Paritz
& Company, P.A., our independent registered public accounting firm, are set
forth on pages F-1 through 17 of this Report.
Item
9. Changes In and Disagreements With Accountants On Accounting and
Financial Disclosure.
None.
Item
9A(T). Controls and Procedures.
Disclosure Controls and
Procedures.
Under the
supervision and with the participation of our management, including our Chief
Executive Officer and Chief Financial Officer, we conducted an evaluation of the
effectiveness of the design and operation of our disclosure controls and
procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities
Exchange Act of 1934, as amended, as of the end of the period covered by this
Annual Report on Form 10- K (the “Evaluation Date”). The evaluation of our
disclosure controls and procedures included a review of our processes and the
effect on the information generated for use in this Annul Report on Form 10-K.
In the course of this evaluation, we sought to identify any material weaknesses
in our disclosure controls and procedures and to confirm that any necessary
corrective action, including process improvements, was taken. The purpose of
this evaluation is to determine if, as of the Evaluation Date, our disclosure
controls and procedures were operating effectively such that the information,
required to be disclosed in our SEC reports (i) was recorded, processed,
summarized and reported within the time periods specified in SEC rules and
forms, and (ii) was accumulated and communicated to our management, including
our Chief Executive Officer and Chief Financial Officer, as appropriate to allow
timely decisions regarding required disclosure. Based on that evaluation, our
Chief Executive Officer and Chief Financial Officer have concluded that our
disclosure controls and procedures were not effective as of March 31, 2010 based
on the significant deficiencies described below.
36
Management’s
Report on Internal Control over Financial Reporting
Management
of the Company is responsible for establishing and maintaining adequate internal
control over financial reporting (ICFR) as defined in Rule 13a-15(f) under the
Securities Exchange Act of 1934. Our internal controls over financial reporting
are designed by, or under the supervision of, our Chief Executive Officer and
Chief Financial Officer, or persons performing similar functions, and effected
by our board of directors, management and other personnel, to provide reasonable
assurance regarding the reliability of financial reporting and the preparation
of financial statements for external purposes in accordance with generally
accepted accounting principles.
Our
internal control over financial reporting, and includes those policies and
procedures that:
|
1.
|
Pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of our
assets;
|
|
2.
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted
accounting principles, and that our receipts and expenditures are being
made only in accordance with authorizations of our management and
directors; and
|
|
3.
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of our assets that could have
a material effect on the financial
statements.
|
Management
has evaluated the effectiveness of our internal control over financial reporting
as of March 31, 2010 based on the control criteria established in a
report entitled Internal
Control—Integrated Framework, issued by the Committee of Sponsoring
Organizations of the Treadway Commission. Based on our assessment and
those criteria, our management has concluded that our internal control over
financial reporting was not effective due to the following significant
deficiencies (as defined in Public Company Accounting Oversight Board
Standard No. 2) in the Company’s internal controls over financial reporting.
This is due to the fact that the Company lacks sufficient personnel with the
appropriate level of knowledge, experience and training in the application of US
generally accepted accounting principles (“GAAP”) standards, especially related
to complicated accounting issues. This could cause the Company to be unable to
fully identify and resolve certain accounting and disclosure issues that could
lead to a failure to maintain effective controls over preparation, review and
approval of certain significant account reconciliation from Chinese GAAP to US
GAAP and necessary journal entries.
The
Company has relatively small number of professionals employed by the Company in
bookkeeping and accounting functions, which prevents the Company from
appropriately segregating duties within its internal control systems. The
inadequate segregation of duties is a weakness because it could lead to the
untimely identification and resolution of accounting and disclosure matters or
could lead to a failure to perform timely and effective reviews.
Remediation
of Significant Deficiencies
Based on
the control deficiency identified above, we have designed and plan to implement,
or in some cases have already implemented, the specific remediation initiatives
described below:
|
|
·
|
We
are evaluating the roles of our existing accounting personnel in an effort
to realign the reporting structure of our internal auditing staff in China
that will test and monitor the implementation of our accounting and
internal control procedures.
|
37
|
|
·
|
We
are in the process of completing a review and revision of the
documentation of the Company’s internal control procedures and
policies.
|
|
|
·
|
We
will begin implementation an initiative and training in China to ensure
the importance of internal controls and compliance with established
policies and procedures are fully understood throughout the organization
and will provide additional U.S. GAAP training to all employees involved
with the performance of or compliance with those procedures and
policies.
|
|
|
·
|
We
will implement a formal financial reporting process that includes review
by our Chief Executive Officer and the full Board of Directors of
financial statements prior to filing with the
SEC.
|
|
|
·
|
We
will increase our accounting and financing personnel resources, by
retaining more U.S. GAAP knowledgeable financial
professionals.
|
The
remedial measures being undertaken may not be fully effectuated or may be
insufficient to address the significant deficiencies we identified, and there
can be no assurance that significant deficiencies or material weaknesses in our
internal control over financial reporting will not be identified or occur in the
future. If additional significant deficiencies (or if material weaknesses) in
our internal controls are discovered or occur in the future, among other similar
or related effects: (i) the Company may fail to meet future reporting
obligations on a timely basis, (ii) the Company’s consolidated financial
statements may contain material misstatements, (iii) the Company’s business and
operating results may be harmed.
Based on
this evaluation, our Chief Executive Officer and Chief Financial Officer
concluded that, as of the Evaluation Date, our disclosure controls and
procedures were not operating effectively.
Inherent
Limitations Over Internal Controls
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect all errors or misstatements and all fraud. Therefore, even
those systems determined to be effective can provide only reasonable, not
absolute, assurance that the objectives of the policies and procedures are met.
Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in
conditions, or that the degree of compliance with the policies or procedures may
deteriorate.
This
annual report does not include an attestation report of the company’s registered
public accounting firm regarding internal control over financial reporting.
Management’s report was not subject to attestation by the company’s registered
public accounting firm pursuant to temporary rules of the SEC that permit the
company to provide only management’s report in this annual report.
Changes
in Internal Control over Financial Reporting
There
were no changes in our internal controls over financial reporting identified in
connection with the evaluation that occurred during the quarter ended March 31,
2010 that have materially affected, or are reasonably likely to materially
affect, our internal controls over financial reporting. Our
management has initiated the steps outlined above under “Remediation of
Significant Deficiencies.”
Item 9B. Other Information
None.
38
PART
III
Item 10. Directors, Executive Officers,
Promoters and Control Persons; Compliance With Section 16(A) of the Exchange
Act.
The
following table sets forth the names and ages of our current directors and
executive officers, and positions with us held by each person.
|
Name
|
Age
|
Title
|
||
|
Mingli
Yao
|
60
|
Chief
Executive Officer, Chairman
|
||
|
Ailing
Zhao
|
53
|
Corporate
Secretary, Director
|
||
|
Yuan
Yao
|
30
|
Director
|
||
|
Hui
Shao
|
43
|
Independent
Director
|
||
|
Li
Li
|
43
|
Chief
Financial Officer
|
The term
of office of each director expires at our annual meeting of stockholders or
until their successors are duly elected and qualified. Our officers serve at the
discretion of our Board of Directors.
Mingli Yao. Mr. Yao has
been the Chief Executive Officer and a director of our company since March 2008.
Since 1999, Mr. Yao has been employed as Chairman of Tianmu Pharmaceuticals.
From 1998 to 1999, Mr. Yao was employed as a manager for a
construction company owned by the People’s Government of Heilongjiang
Province. In 1983, Mr. Yao earned an Associate Degree in Electronic
Engineering from the China Haerbin College of Light Industry. Mr. Yao is
the husband of Ailing Zhao and the father of Yuan Yao.
Yuan Yao. Ms. Yao has
been a director of our company since March 2008. Since 2005, Ms. Yao has been
employed as General Manager in charge of business operations of Tianmu
Pharmaceuticals. In 2005 Ms. Yao earned a Bachelor of Arts Degree with a
concentration in Law from the China University of Political Science and Law.
Ms. Yao is the daughter of Mingli Yao and Ailing Zhao.
Ailing Zhao. Mr. Yao has been
a director of our company since March 2008. Since 1999, Ms. Zhao has been
employed as Deputy General Manager in charge of administration of Tianmu
Pharmaceuticals. In 1980 Ms. Zhao earned a Vocational Degree in Business
from the Haerbin Business Vocational School. Ms. Zhao is the
wife of Mingli Yao and the mother of Yuan Yao.
Hui Shao. On January 8, 2009,
Mr. Hui was appointed as an independent director of our company. Mr. Hui is
currently a Senior Vice President of Finance of China Aoxing Pharmaceutical
Company, Inc. (“China Aoxing”), a vertically integrated pharmaceutical company
specializing in research, development, manufacturing and marketing of a broad
range of narcotics and pain management pharmaceutical products in China. Dr.
Shao joined China Aoxing in January 2007 after ten years working in various
aspects of the pharmaceutical industry. From 2003 to 2006, Dr. Shao served as a
buy-side healthcare analyst for investment firms, initially Mehta Partners LLC
and then Kamunting Street Asset Management. From 1997 to 2003 Dr. Shao was
employed as Principal Scientist by Roche Pharmaceuticals in Nutley, New
Jersey. Dr. Shao was awarded a B.S. in Polymer Chemistry at the University of
Science and Technology in China. He then earned a Ph.D. in Organic Chemistry at
the University of California, San Diego, and an M.B.A. in Finance and
Accounting from the Stern School of Business at New
York University.
Li Li. Mr. Li was appointed as
Chief Financial Officer on March 16, 2009. Mr. Li joined Harbin Tianmu
Pharmaceutical Inc., a wholly owned operating subsidiary of our company, as
Chief Financial Officer in 2009. Prior to that, he had served as
Chief Financial Officer of Sanpu Pharmaceutical Co., Ltd. from 2006 to 2008, and
Chief Financial Officer of Harbin Qiulin Co., Ltd. from 1999 to
2005. Mr. Li earned an M.B.A. in Finance from
Peking University.
39
Family
Relationships
Mr.
Mingli Yao and Ms. Ailing Zhao are husband and wife. Ms. Yuan Yao is
their daughter. No other family relationships exist among our directors or
executive officers.
Involvement
in Certain Legal Proceedings
Our
directors, executive officers and control persons have not been involved in any
of the following events during the past five years:
|
|
1.
|
any
bankruptcy petition filed by or against any business of which such person
was a general partner or executive officer either at the time of the
bankruptcy or within two years prior to that
time;
|
|
|
2.
|
any
conviction in a criminal proceeding or being subject to a pending criminal
proceeding (excluding traffic violations and other minor
offenses);
|
|
|
3.
|
being
subject to any order, judgment, or decree, not subsequently reversed,
suspended or vacated, of any court of competent jurisdiction, permanently
or temporarily enjoining, barring, suspending or otherwise limiting his
involvement in any type of business, securities or banking activities;
or
|
|
|
|
|
|
4.
|
being
found by a court of competent jurisdiction (in a civil action), the SEC or
the Commodity Futures Trading Commission to have violated a federal or
state securities or commodities law, and the judgment has not been
reversed, suspended, or vacated.
|
Code
of Conduct and Ethics
We have
not adopted a code of ethics or a code of conduct that applies to its principal
executive officer, principal financial officer, principal accounting officer,
controller, or to persons performing similar functions.
Director
Independence
We
currently have only one independent director on our Board. As of the date
of this 10-K, our common stock is traded on the OTC Bulletin Board. The OTC
Bulletin Board does not impose on us standards relating to director independence
or the makeup of committees with independent directors, or provide definitions
of independence.
Committees
of the Board of Directors
We
currently do not have an audit committee, compensation committee, nominating
committee, executive committee, or any other committees.
Audit
Committee
We do not
presently have a separately-designated standing audit committee. The entire
Board of Directors performs the functions of an audit committee, but no written
charter governs the actions of the Board when performing the functions of what
would generally be performed by an audit committee.
The
primary functions of an audit committee carried out by the entire Board of
Directors include:
|
●
|
appointing,
approving the compensation of, and assessing the independence of our
independent auditors;
|
|
●
|
assisting
in the oversight of the integrity of our financial statements, our
company’s compliance with legal and regulatory requirements, its
independent auditors’ qualifications, and independence and the performance
of the independent auditors;
|
|
●
|
overseeing
the work of our independent auditors, including through the receipt and
consideration of certain reports from the independent
auditors;
|
40
|
●
|
reviewing
and discussing with management and the independent auditors our annual and
quarterly financial statements and related
disclosures;
|
|
●
|
coordinating
the oversight of our internal control over financial reporting, disclosure
controls and procedures and code of conduct and ethics;
and
|
|
●
|
establishing
procedures for the receipt and retention of accounting related complaints
and concerns; meeting independently with our internal auditing staff,
independent auditors and
management.
|
The Board
of Directors has determined that no one on our Board qualified as an “audit
committee financial expert” as defined in Item 401(e) of
Regulation S-B. We believe that our directors are collectively capable of
analyzing and evaluating our financial statements and understanding internal
controls and procedures for financial reporting.
Section 16(a) Beneficial Ownership
Reporting Compliance
Section
16(a) of the Securities Exchange Act of 1934, as amended, requires our officers,
directors and persons who beneficially own more than 10% of a registered class
of our equity securities (“ten percent stockholders”) to file reports of
ownership and changes in ownership with the SEC. Officers, directors and ten
percent stockholders are charged by the SEC regulations to furnish us with
copies of all Section 16(a) forms they file.
Based
solely upon a review of copies of Section 16(a) reports and representations
received by us from reporting persons, and without conducting any independent
investigation of our own, in fiscal year ended on March 31, 2010, our officers,
directors and ten percent stockholders are in compliance with Section 16(a)
except one late filing for each of Ailing Zhao and Mingli Yao in connection with
Ms. Zhao’s transfer of 1,998,333 shares to a third party.
Item 11. Executive
Compensation.
The
following table sets forth all compensation received during the last two fiscal
years by our Chief Executive Officer, Chief Financial Officer and each of the
other most highly compensated executive officers whose total compensation
exceeds $ 100,000 in such fiscal years. These officers are referred
to as the Named Executive Officers in this Report.
All the
executive officers were paid in RMB and the amounts reported in this table have
been converted from Renminbi to U.S. dollars based on the March 31, 2010
conversion rate of RMB 6.8263 to $1.00.
|
SUMMARY COMPENSATION TABLE
|
||||||||||||||||||||||||||||||||||
|
Name and Principal
Position
|
Fiscal
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Change in
Pension Value
and
Nonqualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||||||
|
Yaoming
Li
|
2010
|
$ | 8,777 | - | - | - | - | - | - | $ | 8,777 | |||||||||||||||||||||||
|
Chief
Executive Officer
|
2009
|
$ | 8,777 | - | - | - | - | - | - | $ | 8,777 | |||||||||||||||||||||||
|
Ailing
Zhao,
|
2010
|
$ | 8,777 | - | - | - | - | - | - | $ | 8,777 | |||||||||||||||||||||||
|
Corporate
Secretary
|
2009
|
$ | 8,777 | - | - | - | - | - | - | $ | 8,777 | |||||||||||||||||||||||
|
Li
Li
|
2010
|
$ | 8,777 | - | - | - | - | - | - | $ | 8,777 | |||||||||||||||||||||||
|
Chief
Financial Officer
|
2009
|
$ | 8,777 | - | - | - | - | - | - | $ | 8,777 | |||||||||||||||||||||||
41
Director
Compensation
Mr.
Mingli Yao, Ms. Ailing Zhao and Mr. Yuan Yao are paid in their capacity as
executive officers of our company and they do not receive any additional
compensation for their service as directors.
DIRECTOR
COMPENSATION
|
Name (a)
|
Fees
Earned
or Paid
in Cash
($)(1)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Non-Qualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total ($)
|
|||||||||||||||||||||
|
Mingli
Yao
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
AilingZhao
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Yuan
Yao
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Hui
Shao
|
$
|
4,395
|
$
|
-
|
-
|
-
|
-
|
$
|
4,395
|
|||||||||||||||||||
(1) All
the Directors were paid in RMB and the amounts reported in this table have been
converted from Renminbi to U.S. dollars based on the March 31, 2010 conversion
rate of RMB 6.8263 to $1.00.
We have
historically entered into written agreements with our non-employee
directors. Mr. Hui Shao is a party to such an agreement, pursuant to
which he is entitled to receive an annual cash fee of a $30,000 and 40,000
shares of our common stock. In addition, Mr. Shao is entitled to
reimbursement for reasonable and substantiated expenses incurred by him in
furtherance of his performance of duties to our company. As of the date of this
Annual Report, the 40,000 shares have not been issued yet.
Outstanding
Equity Awards Narrative Disclosure
As of
March 31, 2010, 1,169,093 shares have been issued to employees and consultants
of the Company under the 2009 Incentive Plan.
2009
Incentive Plan
On August
13, 2009, our Board of Directors and the stockholders holding a majority of the
Company’s outstanding voting stock approved the 2009 Incentive Plan (the
“Plan”). The purpose of the Plan is to provide additional incentive to
selected employees, officers, directors and consultants of our company or its
affiliates, to attract and retain the best available personnel for positions of
substantial responsibility, and to promote the long-term success of our
business.
The Plan
currently provides that a maximum of 2,000,000 shares of Common Stock may be
issued under the Plan (subject to adjustment in the event of stock split or
other changes in the Common Stock as provided in the
Plan).
Administration
The Plan
is administered by our Board of Directors. The Board has, by
resolution, delegated authority to our executive officers to issue up to 200,000
shares or options under the Plan to any single recipient without prior Board of
Directors approval (the “Issuance Cap”), with the proviso that: (i) any single
or series of related issuances in excess of the Issuance Cap shall require the
approval of the Board of Directors or a designated committee thereof and (ii)
such officers are required to review with the Board of Directors or a designated
committee thereof no less than quarterly all issuances made under the
Plan.
42
Type
of Awards
We may
grant the following types of awards under the Plan:
|
·
|
Incentive
Stock Option
|
|
·
|
Non-Qualified
Stock Option
|
|
·
|
Restricted
Shares
|
Option
Terms
The
options granted under the Plan will be evidenced by an award agreement. The
Administrator shall specify the grant date, exercise price, terms, conditions,
and restriction for the exercise of the options. No option shall in any event be
exercisable after ten years from the date of grant, and no Incentive Stock
Option granted to a ten percent shareholder shall become exercisable five years
from the date of grant.
The
exercise price shall be no less than the Fair Market Value per Share on the date
of grant and the exercise price of an Incentive Stock Option granted to an
employee who owns more than 10% of the our stock shall be no less than one
hundred and ten percent of the fair market value per Share on the date of
grant.
Eligibility
Employees,
directors, officers and consultants in the service of our company or its
affiliated are eligible to participate in the Plan. Determinations as to
eligibility shall be made by the Administrator. Options issued in the form
of Incentive Stock Options may only be granted to our
employees.
Transferability
No
unexercised or restricted award shall be assignable or transferable by a
recipient other than by will or the laws of descent and distribution; provided,
unless that the Administrator determines otherwise.
Restrictions
on Resale
Certain
of our officers and directors may be deemed to be “affiliates” of the Company as
that term is defined under the Securities Act. The Common Stock acquired
under the Plan by an affiliate may be reoffered or resold only pursuant to an
effective registration statement or pursuant to Rule 144 under the Securities
Act or other exemption from the registration requirements of the Securities
Act.
Item 12. Security Ownership of Certain
Beneficial Owners and Management and Related Stockholder
Matters
Security
Ownership of Certain Beneficial Owners and Management
The table
sets forth below certain information regarding the beneficial ownership of our
common stock as of June 25, 2010, based on 11,395,036 aggregate shares of common
stock outstanding as of such date, by: (i) each person who is known by us to own
beneficially more than 5% of our outstanding common stock with the address of
each such person, (ii) each of our present directors and officers, and (iii) all
officers and directors as a group.
Beneficial
ownership is determined in accordance with SEC rules and generally includes
voting or investment power with respect to securities. Unless otherwise
indicated, the stockholders listed in the table have sole voting and investment
power with respect to the shares indicated. Unless otherwise noted, the
principal address of each of the stockholders, directors and officers listed
below is c/o 1 Beijing Road, Limin Development Zone, Harbin,
China.
43
All share
ownership figures include shares of our Common Stock issuable upon securities
convertible or exchangeable into shares of our Common Stock within sixty (60)
days of June 25, 2010, which are deemed outstanding and beneficially owned by
such person for purposes of computing his or her percentage ownership, but not
for purposes of computing the percentage ownership of any other
person.
|
Name of Beneficial Owner
|
Amount and Nature of
Beneficial Ownership
|
Percentage of Class
|
||||||
|
Mingli
Yao
|
1,998,333
|
17.5
|
%
|
|||||
|
Yuan
Yao
|
700,000
|
6.1
|
%
|
|||||
|
Ailing
Zhao
|
1,998,333
|
17.5
|
%
|
|||||
|
Hui
Shao
|
40,000
|
0.35
|
%
|
|||||
|
Li
Li
|
0
|
0
|
||||||
|
All
officers and directors as a group (5 persons)
|
2,738,333
|
23.95
|
%
|
|||||
Item 13. Certain Relationships and Related
Party Transactions
A note
from Harbin Commercial Bank is secured by certain real estate assets owned by
the principal shareholder of the Company and guaranteed by Heilongjiang Tongli
Technology Co., Ltd. The Company has repaid the loan in full as of March 10,
2009.
The
Company has a month to month sub-lease arrangement for its New York office with
a company owned by its Chairman. This arrangement began on March 31, 2008 and
the monthly rental was $3,950 which was the same as the amounts incurred by the
related entity. This arrangement has ended on March 31, 2009. Started on April
1, 2009, a new lease agreement with a monthly rental of $1,950 under the name of
the chairman has been arranged for the Company’s New York office.
As of
March 31, 2010, the Company has a balance of $ 788,166 due to related parties.
This includes amounts payable to members of our management who have loaned funds
to us to help us fund working capital deficits.
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Harbin
Tianmu Real Estate Development Co., Ltd. (a)
|
$ | (308,705 | ) | $ | (688,808 | ) | ||
|
Chairman
of the Company (a)
|
(458,874 | ) | (354,234 | ) | ||||
|
US
Hua Sky International Investment LLC. (b)
|
(20,587 | ) | (20,587 | ) | ||||
|
Total
|
$ | (788,166 | ) | $ | (1,063,629 | ) | ||
Review,
Approval or Ratification of Related Party Transactions
Our Board
of Director is responsible for reviewing all “Related Person Transactions” as
defined by Item 404 of Regulation S-K of the rules promulgated by the SEC.
Directors and executive officers are responsible for bringing a potential
Related Person Transaction to the attention of our Board.
In
reviewing a related person transaction, the Board will, after reviewing all
material information regarding the transaction, take into account, among other
factors it deems appropriate, whether the transaction is on terms no less
favorable than terms generally available to an unaffiliated third party under
the same or similar circumstances and the extent of the related person’s
interest in the transaction.
44
Item
14. Principal Accountant Fees and Services.
The
following table sets forth fees billed to us by our independent registered
public accounting firms Paritz & Company, P.A. during the fiscal years ended
March 31, 2010 and March 31, 2009 for: (i) services rendered for the audit of
our annual financial statements and the review of our quarterly financial
statements; (ii) services by our independent registered public accounting firms
that are reasonably related to the performance of the audit or review of our
financial statements and that are not reported as audit fees; (iii) services
rendered in connection with tax compliance, tax advice and tax planning; and
(iv) all other fees for services rendered.
|
|
March 31, 2010
|
March 31, 2009
|
||||||
|
Audit
Fees
|
$
|
41,000
|
30,000
|
|||||
|
Audit
Related Fees
|
$
|
-
|
-
|
|||||
|
Tax
Fees
|
$
|
1,250
|
||||||
|
All
Other Fees
|
$
|
-
|
-
|
|||||
|
TOTAL
|
$
|
42,250
|
30,000
|
|||||
Part
IV
Item 15. Exhibits
|
Item
Number
|
Description
|
|
|
23.1*
|
Consent of Paritz & Company, PA
|
|
|
(31)
|
Section
302 Certification
|
|
|
31.1*
|
Certification
of Registrant’s Chief Executive Officer pursuant to Rule
13a-14(a)/15d-14(a) of the Securities Exchange Act of
1934.
|
|
|
31.2*
|
Certification
of Registrant’s Chief Financial Officer pursuant to Rule
13a-14(a)/15d-14(a) of the Securities Exchange Act of
1934.
|
|
|
(32)
|
Section
906 Certification
|
|
|
32.1*
|
Certification
of Registrant’s Chief Executive Officer pursuant to Rule
13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C.
Section 1350.
|
|
|
32.2*
|
Certification
of Registrant’s Chief Financial Officer pursuant to Rule
13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C.
Section 1350
|
* filed
herewith
45
INDEX
TO FINANCIAL STATEMENTS
TONGLI
PHARMACEUTICALS (USA) INC. AND SUBSIDIARIES
|
Report
of Independent Registered Public Accounting Firm
|
F-1
|
|
|
Consolidated
Balance Sheet at March 31, 2010 and 2009
|
F-3
|
|
|
Consolidated
Statements of Income For The Years Ended March 31, 2010 and
2009
|
F-4
|
|
|
Consolidated
Statements of Cash Flows for The Years Ended March 31, 2010 and
2009
|
F-5
|
|
|
Consolidated
Statements of Changes In Stockholders’ Equity for the Years Ended March
31, 2010 and 2009
|
F-6
|
|
|
Notes
To Consolidated Financial Statements
|
F-7
|
F-1
REPORT OF INDEPENDENT
REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors of
Tongli
Pharmaceuticals (USA), Inc. and subsidiaries
We have
audited the accompanying consolidated balance sheets of Tongli Pharmaceuticals
(USA), Inc. and Subsidiaries as of March 31, 2010 and 2009 and the related
consolidated statements of income and other comprehensive income, changes in
stockholders’ equity and cash flows for the years then ended. These
financial statements are the responsibility of the Company's
management. Our responsibility is to express an opinion on these
consolidated financial statements based on our audit.
We
conducted our audit in accordance with standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. The
Company is not required to have, nor were we engaged to perform an audit of is
internal control over financial reporting. Our audit included
consideration of internal control over financial reporting as a basis for
designing audit procedures that are appropriate in the circumstances, but not
for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such
opinion. An audit includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements, assessing
the accounting principles used and significant estimates made by management, as
well as evaluating the overall financial statement presentation. We
believe that our audit provides a reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of Tongli Pharmaceuticals
(USA), Inc. and Subsidiaries as of March 31, 2010 and 2009, and the results of
its operations and its cash flows for the years then ended in conformity with
accounting principles generally accepted in the United States of
America.
/S/
Paritz & Company, P.A.
Hackensack,
New Jersey
June 28,
2010
F-2
TONGLI
PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
CONSOLIDATED
BALANCE SHEETS
|
As
of March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets:
|
||||||||
|
Cash
|
30,066 | 50,247 | ||||||
|
Accounts
Receivable
|
351,991 | 395,170 | ||||||
|
Inventory
|
109,387 | 19,016 | ||||||
|
Prepaid
expense
|
3,900 | - | ||||||
|
Advance
to suppliers
|
1,465,713 | 986,281 | ||||||
|
Total
current assets
|
1,961,057 | 1,450,714 | ||||||
|
Property
and equipment, net
|
6,744,376 | 7,076,746 | ||||||
|
Contract
deposits
|
2,641,418 | 1,028,736 | ||||||
|
Intangible
assets, net
|
163,244 | - | ||||||
|
Total
assets
|
11,510,095 | 9,556,196 | ||||||
|
LIABILITIES
AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current
liabilities:
|
||||||||
|
Accounts
payable
|
101,611 | 119,643 | ||||||
|
Due
to related parties
|
788,166 | 1,063,629 | ||||||
|
Accrued
expenses and other current liabilities
|
555,969 | 542,892 | ||||||
|
Total
current liabilities
|
1,445,746 | 1,726,164 | ||||||
|
Stockholders'
Equity
|
||||||||
|
Preferred
stock, $0.001 par value,
|
||||||||
|
Authorized
1,000,000 shares; none issued
|
- | - | ||||||
|
Common
stock, $0.001 par value
|
||||||||
|
Issued
and outstanding- 11,395,025 and 10,186,716 shares,
respectively
|
11,395 | 10,187 | ||||||
|
Additional
paid-in-capital
|
7,913,799 | 6,665,349 | ||||||
|
Accumulated
other comprehensive income
|
1,142,133 | 1,130,285 | ||||||
|
Retained
earnings
|
997,022 | 24,210 | ||||||
|
Total
Stockholders' equity
|
10,064,349 | 7,830,031 | ||||||
|
Total
liabilities and Stockholders' equity
|
$ | 11,510,095 | $ | 9,556,196 | ||||
See notes
to consolidated financial statements
F-3
TONGLI
PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENT OF INCOME AND OTHER COMPREHENSIVE INCOME
|
For the years ended March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Revenue
|
$ | 8,591,858 | $ | 7,442,445 | ||||
|
Cost
of sales
|
4,807,168 | 4,103,669 | ||||||
|
Gross
Profit
|
3,784,690 | 3,338,776 | ||||||
|
Operating
expenses:
|
||||||||
|
General
& administrative expenses
|
490,323 | 834,194 | ||||||
|
Non
cash Compensation
|
1,180,784 | |||||||
|
Research
& development expenses
|
3,462 | 70,670 | ||||||
|
Depreciation
expenses
|
153,214 | 177,575 | ||||||
|
Selling
expenses
|
55,359 | 156,164 | ||||||
|
Total
operating expenses
|
1,883,142 | 1,238,603 | ||||||
|
Operating
income
|
1,901,548 | 2,100,173 | ||||||
|
Other
Income (expenses):
|
||||||||
|
Interest
expense-related party
|
(73,340 | ) | (182,285 | ) | ||||
|
Other
income
|
93 | - | ||||||
|
Rental
income
|
- | 241,708 | ||||||
|
Total
other income (expenses)
|
(73,247 | ) | 59,423 | |||||
|
Income
before income taxes
|
1,828,301 | 2,159,596 | ||||||
|
Income
Taxes
|
855,489 | 254,757 | ||||||
|
Net
Income
|
972,812 | 1,904,839 | ||||||
|
Other
Comprehensive item:
|
||||||||
|
Foreign
Currency Translation adjustment
|
11,848 | 174,997 | ||||||
|
Comprehensive
income
|
984,660 | 2,079,836 | ||||||
|
Basic
and diluted income per share
|
$ | 0.10 | $ | 0.19 | ||||
|
Basic
and diluted weighted average shares outstanding
|
10,232,231 | 9,968,824 | ||||||
See notes
to consolidated financial statements
F-4
|
TONGLI
PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
|
|
CONSOLIDATED
STATEMENTS OF CASH FLOWS
|
|
For the years ended March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash
flows from operating activities
|
||||||||
|
Net
income
|
$ | 972,812 | $ | 1,904,839 | ||||
|
Adjustments
to reconcile net income to net cash provided
|
||||||||
|
operating
activities:
|
||||||||
|
Depreciation
and amortization
|
352,791 | 346,357 | ||||||
|
Bad
debt
|
||||||||
|
Amortization
of stock compensation
|
48,875 | 97,909 | ||||||
|
Accrued
interest- related party
|
73,340 | 57,141 | ||||||
|
Stock
issued for services
|
19,999 | - | ||||||
|
Non
cash compensation
|
1,180,784 | - | ||||||
|
Changes
in operating assets and liabilities:
|
- | |||||||
|
Accounts
receivable
|
43,607 | (390,679 | ) | |||||
|
Inventory
|
(90,309 | ) | (7,498 | ) | ||||
|
Advances
to suppliers
|
(478,098 | ) | (287,464 | ) | ||||
|
Prepaid
expenses
|
(3,900 | ) | - | |||||
|
Accounts
payable
|
(18,158 | ) | (803,136 | ) | ||||
|
Accrued
expenses and other current liabilities
|
12,651 | 389,447 | ||||||
|
Net
cash provided by operating activities
|
2,114,395 | 1,306,918 | ||||||
|
Cash
flows from investing activities
|
||||||||
|
Acquisition
of property and equipment
|
- | (871 | ) | |||||
|
Refundable
deposit related to terminated acquisitions
|
- | 414,762 | ||||||
|
Payment
of contract deposit
|
(1,610,789 | ) | (1,023,619 | ) | ||||
|
Acquisition
of intangible assets
|
(175,722 | ) | ||||||
|
Payment
made on Aim Smart acquisition
|
- | (276,000 | ) | |||||
|
Net
cash used in investing activities
|
(1,786,511 | ) | (885,728 | ) | ||||
|
Cash
flows from financing activities
|
||||||||
|
Payment
of bank loans, net
|
- | (1,017,543 | ) | |||||
|
Repayment
of related party loans
|
(348,096 | ) | (1,027,627 | ) | ||||
|
Proceeds
from related party loans
|
1,533,604 | |||||||
|
Net
cash (used in) financing activities
|
(348,096 | ) | (511,566 | ) | ||||
|
Effect
of exchange rate changes on cash
|
30 | 9,993 | ||||||
|
Decrease
in cash
|
(20,181 | ) | (80,383 | ) | ||||
|
Cash,
beginning of the period
|
50,247 | 130,630 | ||||||
|
Cash,
end of the period
|
$ | 30,066 | $ | 50,247 | ||||
|
Supplemental
cash flow information
|
||||||||
|
During
the period, cash was paid for the following:
|
||||||||
|
Income
taxes
|
$ | 833,241 | $ | - | ||||
|
Interest
paid
|
$ | - | $ | 158,222 | ||||
|
Noncash
investing and financing activities
|
||||||||
|
Reduction
of related debt in connection with terminated construction
project
|
$ | - | $ | 588,886 | ||||
|
Payment
made by the officer on Aim Smart acquisition
|
$ | - | $ | 249,000 | ||||
See notes
to consolidated financial statements
F-5
CONSOLIDATED
STATEMENT OF CHANGES IN STOCKHOLDERS' EQUITY
|
Accumulated
|
Retained
|
|||||||||||||||||||||||||||||||
|
Other
|
Earnings
|
|||||||||||||||||||||||||||||||
|
Preferred Stock
|
Common Stock
|
Additional
|
Comrehensive
|
(Accumulated
|
||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Paid in capital
|
Income
|
Deficits)
|
Total
|
|||||||||||||||||||||||||
|
Balance
- March 31, 2008
|
- | $ | - | 9,963,216 | $ | 9,963 | $ | 7,092,663 | $ | 955,288 | $ | (1,880,629 | ) | $ | 6,177,285 | |||||||||||||||||
|
Effects
of reverse merger
|
(100,000 | ) | (100 | ) | (524,900 | ) | - | - | (525,000 | ) | ||||||||||||||||||||||
|
Stock
issued for services
|
323,500 | 324 | 146,461 | - | - | 146,785 | ||||||||||||||||||||||||||
|
Unearned
stock compensation
|
- | - | (48,875 | ) | - | - | (48,875 | ) | ||||||||||||||||||||||||
|
Foreign
currency translation adjustment
|
- | - | - | 174,997 | - | 174,997 | ||||||||||||||||||||||||||
|
Net
income
|
- | - | - | 1,904,839 | 1,904,839 | |||||||||||||||||||||||||||
|
Balance
- March 31, 2009
|
- | $ | - | 10,186,716 | $ | 10,187 | $ | 6,665,349 | $ | 1,130,285 | $ | 24,210 | $ | 7,830,031 | ||||||||||||||||||
|
Stock
issued for services
|
- | - | 39,216 | 39 | 19,960 | - | - | 19,999 | ||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||
|
Stock
issued to employees under 2009 stock incentive plan
|
1,169,093 | 1,169 | 1,179,615 | 1,180,784 | ||||||||||||||||||||||||||||
|
Earned
stock compensation
|
- | - | - | 48,875 | - | 48,875 | ||||||||||||||||||||||||||
|
Foreign
currency translation adjustment
|
- | - | - | - | 11,848 | - | 11,848 | |||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||
|
Net
Inocme
|
- | - | - | - | 972,812 | 972,812 | ||||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||
|
Balance
- March 31, 2010
|
- | $ | - | 11,395,025 | 11,395 | 7,913,799 | 1,142,133 | 997,022 | $ | 10,064,349 | ||||||||||||||||||||||
See notes
to consolidated financial statements
F-6
TONGLI
PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS
ENDED MARCH 31, 2010 AND 2009
1 ORGANIZATION
AND BASIS OF PRESENTATION
Organization
Tongli
Pharmaceuticals (USA), Inc. (“TP” or the “Company”), formally known as Aim Smart
Corporation (“Aim Smart”), was originally formed in the State of Colorado in
April 1998 and reorganized as a Delaware corporation in September
2007.
On July
29, 2008, Aim Smart acquired all of the outstanding capital stock of American
Tony Pharmaceutical, Inc., a Delaware corporation (“American Tony”), by issuing
9,700,000 shares of its common stock to the shareholders of American Tony,
representing 96.7% of the outstanding shares of Aim Smart. Immediately after the
Merger, Aim Smart assigned ownership of the merged company to Hua Sky
Investment Ltd. (“Hua Sky”), a wholly-owned subsidiary of Aim Smart, which was
organized under the Business Company Act of the British Virgin Islands.
Subsequent to the Merger, Aim Smart changed its name to Tongli Pharmaceuticals
(USA), Inc. (“TP”). American Tony also paid $525,000 for its controlling
interest in Aim Smart and this interest was acquired solely to effectuate the
reverse merger and was paid for with $276,000 of its own funds and a $249,000
loan from the officer. This cost was charged to additional paid-in capital at
the time of the merger.
The
acquisition has been accounted for as a reverse merger under the purchase method
of accounting since there has been a change of control. Accordingly, American
Tony and its subsidiaries are treated as the continuing entities for accounting
purposes. Whereas the entity formally known as Aim Smart is the legal surviving
entity.
American
Tony is a holding company incorporated in the State of Delaware. In February
2007, American Tony acquired, through a wholly-owned subsidiary, Heilongjiang
Tongli Technology Co., Ltd. (“TT”), all of the registered capital of Harbin
Tianmu Pharmaceuticals Co., Ltd. (“HTP”), a corporation organized under the laws
of the People’s Republic of China (“PRC”) on November 26, 1999. HTP is
engaged in the business of manufacturing and marketing pharmaceutical and health
care products in the PRC.
The
Company’s main products include cholesterol reduction pill, mouthwash,
anti-inflammatory tablet and calcium supplement. These products are sold through
distributors and directly to customers; no service is provided after sales are
made. The Company’s primary customers are drug stores and hospitals located in
China.
Basis
of presentation
The
financial statements have been prepared in conformity with accounting principles
generally accepted in the United States of America. The functional currency of
TT and HTP is the Chinese Renminbi (“RMB”). The accompanying financial
statements include the financial statements of foreign subsidiaries that have
been translated and presented in United States dollars (“USD”).
2 SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES
Principles
of consolidation
The
consolidated financial statements include the accounts of the Company, American
Tony, TT and HTP. All significant inter-company accounts and
transactions have been eliminated upon consolidation.
F-7
Uses
of estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of
the financial statements and the reported amounts of net revenue and expenses
during each reporting period. Actual results could differ from those
estimates.
Fair
Value of Financial Instruments
The
Company adopted ASC 820, Fair Value Measurements and Disclosures. ASC 820
clarifies the definition of fair value, prescribes methods for measuring fair
value, and establishes a fair value hierarchy to classify the inputs used in
measuring fair value as follows:
Level
1-Inputs are unadjusted quoted prices in active markets for identical assets or
liabilities available at the measurement date.
Level
2-Inputs are unadjusted quoted prices for similar assets and liabilities in
active markets, quoted prices for identical or similar assets and liabilities in
markets that are not active, inputs other then quoted prices that are
observable, and inputs derived from or corroborated by observable market
data.
Level
3-Inputs are unobservable inputs which reflect the reporting entity’s own
assumptions on what assumptions the market participants would use in pricing the
asset or liability based on the best available information.
The
carrying amounts reported in the balance sheets for cash, accounts receivable,
inventory, advance to suppliers, accounts payable and other accrued expenses
approximate their fair market value based on the short-term maturity of these
instruments. The Company did not identify any assets or liabilities that are
required to be presented on the consolidated balance sheets at fair value in
accordance with ASC 820.
Cash
and Cash Equivalents
The
Company maintains cash with financial institutions in the PRC, which are not
insured or otherwise protected. Should any of these institutions
holding the Company’s cash become insolvent, or if the Company is unable to
withdraw funds for any reason, the Company could lose the cash on deposit with
that institution.
Cash and
cash equivalents include cash in hand and cash in time deposits, certificates of
deposit and all highly liquid debt instruments with original maturities of three
months or less.
Accounts
Receivable
Accounts
receivables consist primarily of receivables resulting from sales of products,
and are stated at net realizable value. This value includes an appropriate
allowance for estimated uncollectible accounts. The allowance is calculated
based upon the evaluation and the level of past due accounts and the
relationship with and the economic status of the
customers. Management believes that no allowance is necessary for the
period indicated.
Inventory
Inventory
is stated at the lower of cost, determined using the weighted average cost
method, and net realizable value. Costs include materials, labor and
manufacturing overhead. Net realizable value is the estimated selling
price, in the ordinary course of business, less estimated costs to complete and
dispose. Management periodically compares the cost of inventory with the market
value and an allowance is made for writing down the inventory to its market
value, if lower than cost. No allowance for inventory markdown is considered
necessary for the years ended March 31, 2010 and 2009.
F-8
Advances
to Suppliers
Advance
to suppliers represent the payments made and recorded in advance for goods and
services to be received. The Company’s management has developed purchasing
relationships with a considerable number of suppliers to ensure multiple sources
for most of the raw materials required. In the opinion of management, the
Company’s business would not be significantly damaged by the loss of any one
supplier.
A
considerable portion of the raw materials that production requires are volatile
herbs, which have a brief shelf life. This situation imposes a risk on
suppliers, who often grow such herbs to order to insure an immediate market for
the herbs. The situation also necessitates that management is assured that
raw material requirements are available precisely when needed. To satisfy
these conditions, it is the practice to make substantial cash advances to
suppliers in order to lock-in raw material requirements. However, unless the
Company develops proprietary sources of raw material supplies, the advance
payment schedule for supplies will continue to have a negative effect on working
capital.
Property
and equipment
Property
and equipment are recorded at cost. The cost of an asset comprises
its purchase price and any directly attributable costs of bringing the asset to
its present working condition and locations for its intended use. Depreciation
is provided in amounts sufficient to amortize the cost of the related assets
over their useful lives using the straight line method for financial reporting
purposes. HTP obtained the right to use a parcel of land on which its office and
production facilities are situated, pursuant to the contract from the local
government of the PRC which expires in April 2046.
Depreciation
is calculated using the straight-line method over the following useful
lives:
|
Buildings and improvements
|
40 years
|
|
Right to use land
|
Life of lease
|
|
Machinery and equipment
|
10 years
|
|
Office equipment
|
5 years
|
|
Vehicle
|
5 years
|
Maintenance,
repairs and minor renewals are charged to expense when
incurred. Replacements and major renewals are
capitalized.
Impairment
of Long Lived Assets
Long-lived
assets, which include property, plant and equipment and intangible assets, are
reviewed for impairment whenever events or changes in circumstances indicate
that the carrying amount of an asset may not be recoverable. Recoverability of
long-lived assets to be held and used is measured by a comparison of the
carrying amount of an asset to the estimated undiscounted future cash flows
expected to be generated by the assets.
The
Company accounts for the impairment of long-lived assets in accordance with the
guidance of FASB ASC 360-10-20. Long-lived assets are reviewed for
impairment when circumstances indicate the carrying value of an asset may not be
recoverable. For assets that are to be held and used, impairment is
recognized when the estimated undiscounted cash flows associated with the asset
or group of assets is less than their carrying value. If impairment
exists, an adjustment is made to write the asset down to its fair value, and a
loss is recorded as the difference between the carrying value and fair
value. Fair values are determined based on quoted market values,
discounted cash flows or internal and external appraisals, as
applicable. Assets to be disposed of are carried at the lower of
carrying value or estimated net realizable value. Based on its review, the
Company believes that, as of March 31, 2010, there were no significant
impairments of its long-lived assets used in operations.
F-9
Deferred
income taxes
The
Company accounts for income taxes in accordance with FASB ASC 740 “Income Taxes” which requires
that deferred tax assets and liabilities be recognized for future tax
consequences attributable to differences between financial statement carrying
amounts of existing assets and liabilities and their respective tax
bases. In addition, ASC 740 requires recognition of future tax
benefits, such as carry-forwards, to the extent that realization of such
benefits is more likely than not and that a valuation allowance be provided when
it is more likely than not that some portion of the deferred tax asset will not
be realized. Management reviews this valuation allowance periodically and makes
adjustments as warranted.
Foreign
currency translation
Since the
Company operates primarily in the PRC, the Company’s functional currency is the
Chinese Yuan (”RMB”). For financial reporting purposes, RMB has been
translated into United States dollars ("USD") as the reporting currency. Equity
accounts are translated at historical rates. Assets and liabilities are
translated at the exchange rate in effect at the balance sheet date. Revenues
and expenses are translated at the average rate of exchange prevailing during
the reporting period. Translation adjustments arising from the use of different
exchange rates from period to period are included as a component of
stockholders' equity as "Accumulated other comprehensive income". Gains and
losses resulting from foreign currency translations are included in accumulated
other comprehensive income.
Revenue
Recognition
Revenue
is recognized at the date of shipment to customers when a formal arrangement
exists, the price is fixed or determinable, the delivery is completed, and no
other significant obligations of the Company exist and collectability is
reasonably assured. Payments received before all of the relevant criteria
for revenue recognition are satisfied are recorded as advances from
customers.
Earnings
per share
The
Company computes earnings per share (“EPS’) in accordance with FASB ASC 260
“Earnings per Share”
which requires companies with complex capital structures to present basic and
diluted EPS. Basic EPS is measured as net income divided by the weighted
average common shares outstanding for the period. Diluted EPS is similar
to basic EPS but presents the dilutive effect on a per share basis of potential
common shares (e.g., convertible securities, options and warrants) as if they
had been converted at the beginning of the periods presented, or issuance date,
if later. Potential common shares that have an anti-dilutive effect (i.e.,
those that increase income per share or decrease loss per share) are excluded
from the calculation of diluted EPS.
Comprehensive
income
In
accordance with FASB ASC 220, comprehensive income is defined to include all
changes in equity except those resulting from investments by shareholders and
distributions to shareholders. Among other disclosures, all items
that are required to be recognized under current accounting standards as
components of comprehensive income are required to be reported in a financial
statement that is presented with the same prominence as other financial
statements. Comprehensive income includes net income and the foreign
currency translation gain, net of tax.
F-10
Statement
of Cash Flows
In
accordance with FASB ASC 230 “Statement of cash Flows,” cash flows from the
Company’s operations are calculated based upon the local currencies. As a
result, amounts related to assets and liabilities reported on the statement of
cash flows will not necessarily agree with changes in the corresponding balances
on the balance sheet.
Segment
reporting
FASB ASC
280-10-50 “Segment Reporting" requires the use of the “management approach”
model for segment reporting. The management approach model is based
on the way a company’s management organized segments within the company for
making operating decisions and assessing performance. Reportable
segments are based on products and services, geography, legal structure,
management structure, or any other manner in which management disaggregates a
company. ASC 280-10-50 has an immaterial effect on the Company’s
financial statements, as the Company consists of only one reportable business
segment. All revenues are from sales to customers in the
PRC. Substantially all of the Company’s assets are located in the
PRC.
Concentration
of risks
During
the year ended March 31, 2010, approximately 43.5% of sales were generated from
four major distributors with the largest distributor representing 27.2% of
sales. In addition, four products manufactured by the Company
(including Antihyperlipidemics, Anti-bacterial Mouthwash, Calcium Gluconate Oral
Liquid and Yan Li Xiao Capsule) represented approximately 92% of the total sales
for the year ended March 31, 2010, as compared to one product which
represented 63.3% of total sales for the year ended March 31, 2009.
F-11
New
Accounting Pronouncements
In August
2009, the FASB issued ASU 2009-5, “Fair Value Measurements and Disclosures
(Topic 820) – Measuring Liabilities at Fair Value” (“FASB ASU 2009-5”), which
provides a single definition of fair value, a framework for measuring fair
value, and requires additional disclosure about the use of fair value to measure
assets and liabilities. ASU 2009-05 provides clarification for
circumstances in which a quoted price in an active market for the identical
liability is not available. In such circumstances, a reporting entity is
required to measure fair value using one or more of the following techniques:
(1) a valuation technique that uses: (a) the quoted price of the identical
liability when traded as an asset; or (b) quoted prices for similar liabilities
or similar liabilities when traded as assets; or (2) another valuation technique
that is consistent with the principles of Topic 820 such as an income approach
or a market approach. The guidance provided in ASU 2009-05 is
effective for the first reporting period (including interim periods) beginning
after issuance.
In
October 2009, the FASB issued Accounting Standards Update (ASU) No. 2009-13
Revenue Recognition (ASC 605):
Multiple-Deliverable Revenue Arrangement, which changes the requirements
for establishing separate units of accounting in a multiple element arrangement
and requires the allocation of arrangement consideration to each deliverable
based on the relative selling price. The selling price for each deliverable is
based on vendor-specific objective evidence (VSOE) if available, third-party
evidence if VSOE is not available, or estimated selling price if neither VSOE or
third-party evidence is available. ASU 2009-13 is effective for revenue
arrangements entered into in fiscal years beginning on or after June 15, 2010.
The Company is currently assessing the impact to its financial condition,
results of operations or cash flows.
In
December 2009, FASB issued ASU 2009-17, Consolidations (Topic 810)
Improvements to Financial Reporting by Enterprises Involved with Variable
Interest Entities , which replaces the quantitative-based risks and
rewards calculation for determining which enterprise, if any, has a controlling
financial interest in a variable interest entity with an approach focused on
identifying which enterprise has the power to direct the activities of a
variable interest entity that most significantly impact the entity's
economic performance and (1) the obligation to absorb losses of the entity
or (2) the right to receive benefits from the entity. ASU 2009-17 also
requires additional disclosures about an enterprise's involvement in variable
interest entities. ASU 2009-17 is effective as of the beginning of each
reporting entity's first annual reporting period that begins after
November 15, 2009. The Company does not expect the adoption of ASU 2009-17
to have a material impact on its consolidated financial statements.
In
January 2010, the FASB issued ASU 2010-04, Accounting for Various Topics –
Technical Corrections to SEC Paragraphs. ASU 2010-04 makes technical
corrections to existing SEC guidance, including the following topics: accounting
for subsequent investments, termination of an interest rate swap, issuance of
financial statements - subsequent events, use of residential method to value
acquired assets other than goodwill, adjustments in assets and liabilities for
holding gains and losses, and selections of discount rate used for measuring
defined benefit obligation. The Company does not expect the adoption of ASU
2010-04 to have a material impact on its consolidated financial
statements.
F-12
In
January 2010, the FASB issued new standards in ASC 820, Fair Value Measurements and
Disclosures: Improving Disclosures About Fair Value Measurements. ASU
2010-06 amends Subtopic 820-10 to clarify existing disclosures, require new
disclosures, and include conforming amendments to guidance on employers’
disclosures about postretirement benefit plan assets. ASU 2010-06 is effective
for interim and annual periods beginning after December 15, 2009, except for
disclosures about purchases, sales, issuances, and settlements in the roll
forward of activity in Level 3 fair value measurements. Those disclosures are
effective for fiscal years beginning after December 15, 2010, and for interim
periods within those fiscal years. The Company is currently
evaluating the impact these standards will have on its financial condition,
results of operations, or cash flows and does not expect the adoption of ASU
2010-06 to have a material impact on its consolidated financial
statements.
In
January 2010, the FASB issued ASU 2010-05, Compensation – Stock Compensation
(Topic 718): Escrowed
Share Arrangements and the Presumption of Compensation. ASU 2010-05
updates existing guidance to address the SEC staff’s views on overcoming the
presumption that for certain shareholders escrowed share arrangements represent
compensation. The Company does not expect the adoption of ASU 2010-05
to have a material impact on its consolidated financial statements.
In
January 2010, the FASB issued ASU No. 2010-01, Equity (ASC 505): Accounting
for distributions to
Shareholders with Components of Stock and Cash (A Consensus of the FASB
Emerging Issues Task Force). This amendment to ASC 505 clarifies the stock
portion of a distribution to shareholders that allow them to elect to receive
cash or stock with a limit on the amount of cash that will be distributed is not
a stock dividend for purposes of applying ASC 505 and 260. Effective for interim
and annual periods ending on or after December 15, 2009, and would be applied on
a retrospective basis. The Company does not expect the provisions of ASU No.
2010-01 to have a material effect on the financial position, results of
operations or cash flows of the Company.
In
February 2010, FASB issued ASU 2010-09 Subsequent Event (Topic 855) Amendments
to Certain Recognition and Disclosure Requirements. ASU 2010-09 removes the
requirement for an SEC filer to disclose a date through which subsequent events
have been evaluated in both issued and revised financial statements. Revised
financial statements include financial statements revised as a result of either
correction of an error or retrospective application of GAAP. All of the
amendments in ASU 2010-09 are effective upon issuance of the final ASU, except
for the use of the issued date for conduit debt obligors. That amendment is
effective for interim or annual periods ending after June 15, 2010. The Company
adopted ASU 2010-09 in February 2010 and did not disclose the date through which
subsequent events have been evaluated.
3 INVENTORY
As of
March 31, 2010 and 2009, inventory consists the following:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Raw
materials
|
$ | 32,313 | $ | 15,100 | ||||
|
Finished
goods
|
77,074 | 3,916 | ||||||
|
Total
|
$ | 109,387 | $ | 19,016 | ||||
F-13
4 PROPERTY
AND EQUIPMENT
A summary
of property and equipment is as follows:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Building
|
$ | 7,113,343 | $ | 7,105,308 | ||||
|
Right
to use land
|
395,552 | 395,107 | ||||||
|
Machinery
and equipment
|
1,022,933 | 1,021,778 | ||||||
|
Office
equipment
|
16,066 | 16,057 | ||||||
|
Vehicle
|
99,418 | 99,305 | ||||||
|
Total
|
$ | 8,647,311 | $ | 8,637,556 | ||||
|
Less:
accumulated depreciation and amortization
|
1,902,935 | 1,560,809 | ||||||
|
Total
|
$ | 6,744,376 | $ | 7,076,746 | ||||
Depreciation
and amortization expenses were $352,791 and $346,357 for the years ended March
31, 2010 and 2009, respectively.
5 CONTRACT
DEPOSIT
Contract
deposits represents payments under material contracts by which the Company
intends to purchase drug formula to be used in the manufacturing and increasing
its product lines. On December 24, 2008, the Company signed a patent transfer
agreement with a third party Harbin Lanhai Biochemical Company Limited
and paid RMB 7,030,000 (approximately $1.03 million) for the purchase of a
nutraceutical product from Lanhai Biochemical Company Limited. The Company’s
ability to conclude this purchase and ultimately commercialize this product
requires, among other things, additional assistance from the seller and
obtaining government approvals. Due to the recent strict regulation regarding
the examination and approval procedure, the Company is now still waiting for
suspended governmental approval for the formula to be used in production of
Calcium supplements and is expected to obtain such approval from the China State
Food & Drug Administration (“SFDA”) by 2011.
On March
21, 2010, the Company signed a patent purchase agreement
with a third party Tonghua Yisheng Pharmaceuticals Company
Limited for purchase of a new drug. Total contract price for this patent
transaction amounted to RMB 33,000,000 (approximate to USD 4.85 million) to be
paid in three installments. The Company paid the first installment of RMB
11,000,000 (equivalent to USD 1,611,514) to Tonghua Yisheng Pharmaceuticals
Company Limited in March 2010 upon signing the purchase agreement. The Company’s
ability to conclude this purchase and ultimately commercialize this product
requires additional assistance from the seller and obtaining government
approvals.
F-14
6
INTANGIBLE ASSETS
Intangible
assets primarily represent a new product’s sales right specified by a contract
between the Company and an outside party Harbin Sanmu Pharmaceuticals. The
Company paid RMB 1,200,000 (equivalent to USD 175,772) to Harbin Sanmu
Pharmaceuticals in October 2009 for the exclusive rights to use the product’s
trademark, manufacture and sell the product nationwide for seven years.
Management believes that this new product has great market potentials and will
generate additional revenue for fiscal year 2010 and beyond. The Company
amortizes such product sales right using straight-line method for seven years.
As of March 31, 2010, the amortization expense totaled $12,557.
7 DUE
TO RELATED PARTIES
Due to
related parties consist of the following:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Harbin
Tianmu Real Estate Development Co., Ltd. (a)
|
$ | (308,705 | ) | $ | (688,808 | ) | ||
|
Chairman
of the Company (a)
|
(458,874 | ) | (354,234 | ) | ||||
|
US
Hua Sky International Investment LLC. (b)
|
(20,587 | ) | (20,587 | ) | ||||
|
Total
|
$ | (788,166 | ) | $ | (1,063,629 | ) | ||
|
(a)
|
These
loans bear interest at 7% per annum and are due on
demand..
|
|
(b)
|
The
Company has a month to month sub-lease arrangement for its New York office
with a company
owned by its Chairman. This arrangement began on March 31, 2008 and the
monthly rental was $3,950 which was the same as the amounts incurred by
the related entity. This arrangement has ended on March 31, 2009. A
new lease agreement commenced on April 1, 2009 with a monthly rental of
$1,950 under the name of the chairman has been arranged for the Company’s
New York office.
|
8 STOCKHOLDERS’
EQUITY
On March
16, 2009, the Company issued 100,000 shares of common stock to a consultant. The
Company’s contract with this consultant has a term of one year, and the unearned
stock compensation will be amortized as expense over one year from the date of
the grant. As of March 31, 2010, the above one-year stock based
compensation has been fully amortized. The amortization of this stock
compensation for the twelve months ended March 31, 2010 was
$48,875.
On April
1, 2009, the Company issued 39,216 shares of common stock to a law firm for
legal services rendered and recorded at its fair value of $20,000.
In
December 2009 the Company established the 2009 Incentive Plan (“the Plan”) to
provide additional incentive to selected employees, officers, directors and
consultants of the Company and to retain the best available personnel for
positions of substantial responsibility and to promote the long-term success of
the Company. 2,000,000 shares were initially reserved for issuance pursuant to
awards granted under the Plan. Awards may be in the form of incentive stock
options, non-qualified stock options, restricted shares or other arrangements or
programs. On March 29, 2010, The Company issued 1,169,093 common shares to 46
employees without restrictive legend. The Company recorded the fair value of
$1,180,784 for these issued shares.
F-15
9 EARNINGS
(LOSS) PER SHARE
Earnings
(loss) per share are computed by dividing income available to stockholders of
our common stock by the weighted-average number of shares of common stock
outstanding for the twelve months ended March 31, 2010 and 2009. Diluted
earnings per share is computed similar to basic earnings per share except that
the denominator is increased to include the number of additional shares of
common stock that would have been outstanding if the potential shares of common
stock have been issued and if the additional shares of common stock were
dilutive. There are no common stock equivalents available for dilution purposes
as of March 31, 2010 and 2009, respectively. The following demonstrates the
calculation for earnings per share for the twelve months ended March 31, 2010
and 2009:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Net
income
|
$ | 972,812 | $ | 1,904,839 | ||||
|
Weighted
shares outstanding- Basic and diluted
|
10,232,231 | 9,968,824 | ||||||
|
Earning
per share- Basic
|
$ | 0.10 | $ | 0.19 | ||||
|
Earnings
per share- Diluted
|
$ | 0.10 | $ | 0.19 | ||||
10 TAXES
(a)
Corporation
income tax (“CIT”)
The
Company has not recorded a provision for U.S federal income tax for the year
ended March 31, 2010 and 2009 due to the net operating losses in the United
States which the Company has set up 100% valuation allowance.
The
Company’s Chinese subsidiaries are governed by the Income Tax Law of the
People’s Republic of China concerning the private-run enterprises, which are
generally subject to tax at a new statutory rate of 25% on income reported in
the statutory financial statements after appropriate tax
adjustments.
A
reconciliation of tax at United States Federal statutory rate to provision for
income tax recorded in the financial statements is as follows:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
U.S.statutory
income tax rate
|
35.0 | % | 35.0 | % | ||||
|
Foreign
tax rate difference between US and China
|
(10.0 | )% | (10.0 | )% | ||||
|
Effect
of tax deduction due to NOL from China
|
0.0 | % | (20.2 | )% | ||||
|
NOL
from U.S with 100% valuation allowance
|
21.8 | % | 6.70 | % | ||||
|
Actual
consolidated income tax rate
|
46.8 | % | 11.5 | % | ||||
F-16
The
components of deferred taxes as of March 31, 2010 and 2009 consist of the
following:
|
As of
|
||||||||
|
March 31, 2010
|
March 31, 2009
|
|||||||
|
Net
operating loss carry-forwards
|
773,165 | 208,732 | ||||||
|
Valuation
allowance
|
(773,165 | ) | (208,732 | ) | ||||
|
Net
deferred tax asset
|
- | - | ||||||
Management
believes the deferred tax assets as of March 31, 2010 and 2009 do not satisfy
the realization criteria set forth in FASB ASC 740 and has recorded a valuation
allowance for the entire net tax asset.
(b)
Value added tax (“VAT”)
Enterprises
or individuals who sell commodities, engage in repair and maintenance or import
or export goods in the PRC are subject to a value added tax in accordance with
the PRC laws. The value added tax standard rate is 17% of the gross sales price.
A credit is available whereby VAT paid on the purchases of semi-finished
products or raw materials used in the production of the Company’s finished
products can be used to offset the VAT due on the sales of the finished
products.
(3)
Other taxes
The
Company is also subject to 5% of business tax, 7% of City Construction Tax and
4% of Education Fees based on VAT.
11 COMMITMENTS
AND CONTINGENCIES
The
Company entered into agreements with three distributors to provide agreed upon
amounts of products at pre-agreed pricing. These agreements expire in March
2010. In the event a distributor does not purchase a fixed percentage of the
agreed upon amounts for three consecutive months, the Company may terminate the
agreement. In addition, one agreement provides, among other things, that the
distributor can become the exclusive distributor for a geographical area if
certain sales targets are met. Revenues for the year ended March 31, 2010 and
2009 were immaterial from these agreements.
On March
21, 2010, the Company signed a patent purchase agreement with a
third party Tonghua Yisheng Pharmaceuticals Company Limited for purchase of
a new drug. Total
contract price for this patent transaction amounted to RMB 33,000,000
(approximate to USD 4.85 million) to be paid in three installments. The Company
paid the first installment of RMB 11,000,000 (equivalent to USD 1,611,514) to
Tonghua Yisheng Pharmaceuticals Company Limited in March 2010 upon signing the
purchase agreement. The Company’s ability to conclude this purchase and
ultimately commercialize this product requires additional assistance from the
seller and obtaining government approvals.
F-17
12 VULNERABILITY
DUE TO OPERATIONS IN PRC
The
Company’s operations may be adversely affected by significant political,
economic and social uncertainties in the PRC. Although the PRC government has
been pursuing economic reform policies for more than thirty years, no assurance
can be given that the PRC government will continue to pursue such policies or
that such policies may not be significantly altered, especially in the event of
a change in leadership, social or political disruption or unforeseen
circumstances affecting the PRC’s political, economic and social conditions.
There is also no guarantee that the PRC government’s pursuit of economic reforms
will be consistent or effective.
Substantially
all of the Company’s businesses are transacted in RMB, which is not freely
convertible. The Peoples Bank of China or other banks are authorized to buy and
sell foreign currencies at the exchange rates quoted by the Peoples Bank of
China. Approval of foreign currency payments by the Peoples Bank of China or
other institutions requires submitting a payment application form together with
suppliers’ invoices, shipping documents and signed contracts.
Since the
Company has its primary operations in the PRC, the majority of its revenues will
be settled in RMB, not U.S. Dollars. Due to certain restrictions on currency
exchanges that exist in the PRC, the Company’s ability to use revenue generated
in RMB to pay any dividend payments to its shareholders outside of China may be
limited.
The
Company’s business depends on maintaining licenses of its current products from
SFDA. Obtaining licenses for additional products can be expensive and is usually
time consuming. Failure to obtain the required licenses can cause the Company’s
business plan to be delayed. If the delays prevent the Company from generating
positive cash flows or introducing a significant number of products, there will
be a material adverse effect on the Company.
F-18
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned thereunto duly authorized.
Dated:
June 29,
2010
|
|
/s/ Mingli Yao
|
|
Mingli
Yao
|
|
|
Chief
Executive Officer and
Chairman
|
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the Registrant and in the
capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Mingli Yao
|
Chief
Executive Officer and Chairman
|
June
29, 2010
|
||
|
Mingli
Yao
|
||||
|
/s/ Li Li
|
Chief
Financial Officer
|
June
29, 2010
|
||
|
Li
Li
|
||||
|
/s/ Ailing Zhao
|
Corporate
Secretary and Director
|
June
29, 2010
|
||
|
Ailing
Zhao
|
||||
|
/s/ Yuan Yao
|
Director
|
June
29, 2010
|
||
|
Yuan
Yao
|
||||
|
/s/ Hui Shao
|
Director
|
June
29, 2010
|
||
|
Hui
Shao
|
