Attached files
| file | filename |
|---|---|
| 8-K - 8-K - BIOCANCELL THERAPEUTICS INC. | zk1008447.htm |
Exhibit 99.1

BioCancell Therapeutics, Inc.
BioCancell Therapeutics Inc.
An Innovative Targeted Cancer Therapy Approach
An Innovative Targeted Cancer Therapy Approach
Uri Danon, CEO
June 2010

2
This presentation contains forward-looking statements within the meaning of the Federal Securities Laws and the
Israeli Securities Laws that involves risks and uncertainties . These forward-looking statements, relate to, without
limitation, statements about our market opportunities, our strategy, our competition, the further development and
potential safety and efficacy of our products, our projected revenue and expense levels and the adequacy of our
available cash resources.
Israeli Securities Laws that involves risks and uncertainties . These forward-looking statements, relate to, without
limitation, statements about our market opportunities, our strategy, our competition, the further development and
potential safety and efficacy of our products, our projected revenue and expense levels and the adequacy of our
available cash resources.
Drug discovery and development involve a high degree of risk. Factors that might cause material differences
include, among others, risks relating to: the successful preclinical development of our product candidates; the
completion of clinical trials; the successful completion of the process with the FDA, foreign regulatory bodies and
other governmental regulation, including the FDA`s review of any filings we make in connection with the treatment
protocol; uncertainties related to the ability to attract and retain partners for our technologies and products under
development; and other factors described in our public filings.
include, among others, risks relating to: the successful preclinical development of our product candidates; the
completion of clinical trials; the successful completion of the process with the FDA, foreign regulatory bodies and
other governmental regulation, including the FDA`s review of any filings we make in connection with the treatment
protocol; uncertainties related to the ability to attract and retain partners for our technologies and products under
development; and other factors described in our public filings.
This presentation does not constitute or form part of, and should not be construed as constituting or forming part
of, any offer or invitation to sell or issue, or any solicitation of any offer to purchase or subscribe for, any shares in
the Company, nor shall any part of this presentation nor the fact of its distribution form part of or be relied on in
connection with any contract or investment decision relating thereto, nor does it constitute a recommendation
regarding the securities of the Company.
of, any offer or invitation to sell or issue, or any solicitation of any offer to purchase or subscribe for, any shares in
the Company, nor shall any part of this presentation nor the fact of its distribution form part of or be relied on in
connection with any contract or investment decision relating thereto, nor does it constitute a recommendation
regarding the securities of the Company.
Although we believe that the expectations reflected in these forward-looking statements are based upon
reasonable assumptions, no assurance can be given that such expectations will be attained or that any deviations
will not be material. No reliance may be placed for any purposes whatsoever on the information contained in this
presentation or on its completeness. No representation or warranty, express or implied, is given by or on behalf of
the Company and/or its subsidiaries or any of their directors, officers or employees or any other person as to the
accuracy or completeness of the information or opinions contained in this presentation and no liability whatsoever
is accepted by the Company and/or its subsidiaries, or any of their members, directors, officers or employees or
any other person for any loss howsoever arising, directly or indirectly, from any use of such information or
opinions or other wise arising in connection therewith.
reasonable assumptions, no assurance can be given that such expectations will be attained or that any deviations
will not be material. No reliance may be placed for any purposes whatsoever on the information contained in this
presentation or on its completeness. No representation or warranty, express or implied, is given by or on behalf of
the Company and/or its subsidiaries or any of their directors, officers or employees or any other person as to the
accuracy or completeness of the information or opinions contained in this presentation and no liability whatsoever
is accepted by the Company and/or its subsidiaries, or any of their members, directors, officers or employees or
any other person for any loss howsoever arising, directly or indirectly, from any use of such information or
opinions or other wise arising in connection therewith.
Safe Harbor

3
BioCancell Overview
● Developing innovative Targeted Cancer Therapy for the treatment of
cancer, based on Professor Avraham Hochberg’s research
cancer, based on Professor Avraham Hochberg’s research
● Lead product candidate BC-819 in clinical trials: phase IIb for bladder
cancer, phase I/IIa for pancreatic and ovarian cancer
cancer, phase I/IIa for pancreatic and ovarian cancer
● Intellectual Property: 7 patent families
● Incorporated in Delaware (July 2004), 16 employees
● Listed on TASE (BICL) in Aug. 2006, SEC filing since June 2009
● Fundraising: $17.8M, in private placements and IPO on TASE
● Major Stockholders: Clal Biotechnology Industries Ltd. (18.6%),
Professor Avraham Hochberg (10.4%), Tikcro (3.9%)
Professor Avraham Hochberg (10.4%), Tikcro (3.9%)

4
Technology Platform
● Targeted Cancer Therapy platform based on H19 gene
● H19 is expressed uniquely within cancer cells, while not expressed in
normal cells
normal cells
● BC-819 drug candidate uses the H19 gene to produce diphtheria toxin
in cancer cells, destroying the cancer cells without affecting normal
cells
in cancer cells, destroying the cancer cells without affecting normal
cells
● Diagnosis of H19 gene is prerequisite for treatment
● It is a targeted treatment without side effects*
● Platform potential: H19 expressed in more than 40 different cancer
indications
indications
*Detected to date

After birth, H19 is expressed only in cancer cells, therefore a
significant marker of cancer cells
significant marker of cancer cells
The expression of H19 in cancer cells promote tumor development
Direct mechanistic connection exists between H19 and p53, a
central protein involved in cancer cell proliferation
central protein involved in cancer cell proliferation
H19 - Oncofetal gene
(Fetus)

6
The Drug - BC-819 Plasmid
H19 Promoter
A DNA plasmid containing the H19 gene regulatory sequences
that drive the expression of the Diphtheria Toxin A gene (DTA-
H19)
that drive the expression of the Diphtheria Toxin A gene (DTA-
H19)
Diphtheria Toxin sequence

7
Cancer cell
Normal rapid-dividing cell
Trigger activated
H19’s transcription factors just in cancer cell
nucleus, activate plasmid to produce
diphtheria toxin (DTA)
nucleus, activate plasmid to produce
diphtheria toxin (DTA)
Cancer cell killed
Mechanism of Action

8
Cancer cell
Normal rapid-dividing cell
Trigger activated
Trigger not activated
H19’s transcription factors just in cancer cell
nucleus, activate plasmid to produce
diphtheria toxin (DTA)
nucleus, activate plasmid to produce
diphtheria toxin (DTA)
No H19 transcription factors for activation of
plasmid to produce diphtheria toxin (DTA)
plasmid to produce diphtheria toxin (DTA)
Cancer cell killed
No change
Mechanism of Action

9
The Advantages of BC-819
● Good safety profile and lack of side effects* - prevents patient withdrawal
in chemotherapy treatment
in chemotherapy treatment
● Reduces Multi-Drug Resistance (MDR) - a major disadvantage of
chemotherapy
chemotherapy
● Targeted Cancer Therapy - yields superior success rate
● Same drug for 40 different cancer types expressing H19
● Low cost manufacturing vs. other biological products
*Detected to date

10
2010
2012
2013
2011
Ovarian
cancer
cancer
Phase IIb
Phase I/IIa
All indications have
FDA Fast Track
potential
FDA Fast Track
potential
Bladder
cancer
Phase IIb
FDA Meeting
Phase III
Phase I/IIa (was completed in 2007)
Pancreatic
cancer
cancer
Phase IIb
Phase I/IIa
Phase III
FDA Meeting
FDA Meeting
* Provided clinical success, regulatory approvals, availability of financial resources / strategic collaboration/s

11
Market Size - Treatment of Bladder Cancer
|
New Cases*
|
Prevalence
|
Deaths / Year
|
H19 Expression
in patients |
|
175,000
|
1,500,000
|
37,000
|
84%
|
* Data: Globocan; World Population Prospects and American Cancer Society, estimated 2009. Data
refers to the population in the 7 major pharmaceutical markets
refers to the population in the 7 major pharmaceutical markets
Competitors’ Drugs - Annual Sales Volume:
BCG* (TheraCys/ TICE) - Sanofi-Aventis/ Schering-Plough Corp.
(Organon Pharmaceuticals), $200M
(Organon Pharmaceuticals), $200M
* Adverse events: dysuria, urinary frequency, hematuria, cystitis, nocturia,
* Drug label has black box warning
The cost per patient of bladder cancer from diagnosis to death is the highest of
all cancers, ranging from $96K-$187K
all cancers, ranging from $96K-$187K

12
Market Size - Treatment of Ovarian Cancer
|
New Cases*
|
Prevalence
|
Deaths / Year
|
H19 Expression
in patients |
|
60,000
|
400,000
|
40,000
|
75%
|
* Data: Globocan; World Population Prospects and American Cancer Society, estimated 2009. Data
refers to the population in the 7 major pharmaceutical markets
refers to the population in the 7 major pharmaceutical markets
Competitors’ Drugs - Annual Sales Volume:
Doxil/ Caelyx* - Johnson & Johnson/ Schering-Plough Corp, $650M
Taxotere* (Sanofi-Aventis), $3B
Hycamtin* (GlaxoSmithKline), $325M
Gemzar (Eli Lilly), $1.72B
* Adverse events: Immunosupression, anemia, diarrhea,nausea, hair loss
* For all indications, drug label has black box warning

13
Market Size - Treatment of Pancreatic Cancer
|
New Cases*
|
Prevalence
|
# of deaths per year
|
H19 Expression
in patients
|
|
100,000
|
90,000
|
90,000
|
70%
|
Competitors’ Drugs - Annual Sales Volume:
Gemzar* - Eli Lilly & Co, $1.72B
Tarceva* (in combination with Gemzar) - OSI
Pharmaceuticals/Genentech/Roche, $1.66B
Pharmaceuticals/Genentech/Roche, $1.66B
* Data: Globocan; World Population Prospects and American Cancer Society, estimated 2009. Data
refers to the population in the 7 major pharmaceutical markets
refers to the population in the 7 major pharmaceutical markets
* Adverse events: Immunosupression, anemia, diarrhea,nausea, hair loss
* For all indications

14
● Lung Cancer (NSCLC):
Tarceva (Erlotinib) - $1.66B
● Liver Cancer:
5-Fluorouracil (Adrucil, Efudex, Fluoroplex) - Blockbuster
● Kidney Cancer (RCC):
Sorafenib (Nexavar) - $900M
BC-819 - Additional Indications
BC-819 has been successfully tested in animals for the
treatment of lung cancer, liver cancer and kidney cancer
treatment of lung cancer, liver cancer and kidney cancer
Market potential (annual sales) for other drugs in those
indications:
* Source: Data Monitor

15
Marketed Blockbuster Drugs - Sales
Avastin - originally approved for colorectal cancer
|
2004
|
2005
|
2006
|
2007
|
2008
|
|
$651M
|
$1,571M
|
$2,795M
|
$3,875M
|
$4,914M
|
Herceptin - breast cancer, for “only” 40,000 potential patients
|
2004
|
2005
|
2006
|
2007
|
2008
|
|
$3,188M
|
$3,920M
|
$4,567M
|
$5,206M
|
$5,590M
|
(new approved indications,
contributed to an increase in sales)
contributed to an increase in sales)
Lung (NSCLC) RCC/EMEA HER2- negative
breast cancer
breast cancer
* Source: Data Monitor
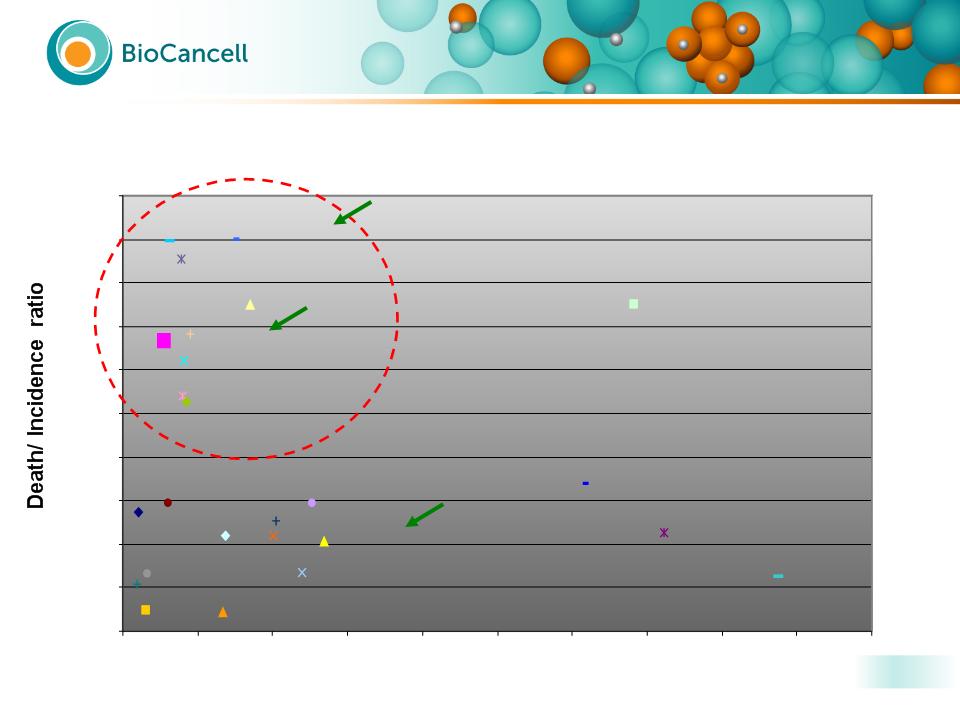
16
ALL
AML
Brain
Breast
CLL
Colorectal
NSCLC
SCLC
Melanoma
NHL
Prostate
Testis
Thyroid
Uterine
Liver
Hodgkin's
Kidney
Bladder
CML
Esophagus
Head&Neck
Myeloma
Ovary
Pancreas
Stomach
0.00
0.10
0.20
0.30
0.40
0.50
0.60
0.70
0.80
0.90
1.00
0
25,000
50,000
75,000
100,000
125,000
150,000
175,000
200,000
225,000
250,000
New Cases - US, 2007
Lethal Cancers: High Unmet Medical Need
BioCancell’s Target Indications
Create High Value

17
Eisai acquired Morphotek (US) for $325M (3/2007). Morphotek's
advanced programs then were MORAb-003 in phase I/II for ovarian
cancer and MORAb-009 in phase I for pancreatic cancer.
advanced programs then were MORAb-003 in phase I/II for ovarian
cancer and MORAb-009 in phase I for pancreatic cancer.
AstraZeneca acquired KuDOS (UK) for $210M (1/2006). KuDOS’
advanced program then was KU 59436, an oral poly-ADP-ribose
polymerase (PARP) enzyme inhibitor, in phase I trial for cancer.
advanced program then was KU 59436, an oral poly-ADP-ribose
polymerase (PARP) enzyme inhibitor, in phase I trial for cancer.
OncoGenex (Nasdaq: OGXI) and Teva (12/2009), entered
co-development & co-promotion agreement for OGX-011, that has
completed phase II in prostate and lung cancer. Upfront = $60M, up to
$370M on future milestones and 10%-25% royalties on sales.
completed phase II in prostate and lung cancer. Upfront = $60M, up to
$370M on future milestones and 10%-25% royalties on sales.
Clavis Pharma (Oslo: CLAVIS.OL) and Clovis Oncology, entered co-
development & co-promotion agreement for CP-4126 (11/2009), in phase
II for pancreatic cancer. Upfront = $15M, up to $365M on future
milestones and tiered double-digit royalties on sales.
development & co-promotion agreement for CP-4126 (11/2009), in phase
II for pancreatic cancer. Upfront = $15M, up to $365M on future
milestones and tiered double-digit royalties on sales.
Value creation by M&A / Licensing

18
● Purpose - assess the safety and preliminary efficacy of BC-819
● 18 patients who failed treatment with BCG or chemotherapy
● Successfully concluded in Aug, 2007 (Journal of Urology; Sidi et al. 2008)
Results:
- No Serious Adverse Events related to BC-819 were detected
- 56% (10/18 patients) did not experience recurrence of tumor
Phase I/IIa in Bladder Cancer

19
Phase IIb in Bladder Cancer
● Purpose - assessing efficacy and safety of BC-819 in 33 patients
● Seven sites in Israel and one in the US (BCG Oncology, Arizona)
● Refractory patients with TCC that failed treatment with BCG or
chemotherapy and whose alternative treatment is bladder removal
chemotherapy and whose alternative treatment is bladder removal
● Interim result of first stage of Phase IIb (18 patients):
- No Serious Adverse Events related to BC-819 were detected
- 84% responders*
- 56% showed non-recurrence of tumor
- 50% showed complete tumor ablation
- 22% showed a complete response**
* Either non-recurrence, tumor ablation or both
** Both non-recurrence and tumor ablation
The major problem
in refractory bladder
cancer;
cancer;
predicts what to expect in
post-marketing approval
treatment
post-marketing approval
treatment

Ovarian Cancer Compassionate Use
Background:
● A 69 year-old female patient, failed all
available treatments, chemotherapy resistance
● IP treated with BC-819 (80mg to 140mg)
After treatment with BC-819:
● Extension of 18 months beyond patient’s
original 3 month life-expectancy
● No SAEs were reported
● Tumor growth was arrested
● Cancer marker CA-125 dropped by ~50%
● Reduction of number of cancerous cells in
patient’s ascitic fluid
● Ascitic fluid level was significantly reduced
● The patient and her physicians reported
significant clinical improvement
31.10.07- before treatments
02.01.08- After treatment #8
(Ascites)

21
Phase I/IIa in Ovarian Cancer
● Purpose - determine safety and the optimal dose for intraperitoneal
delivery of BC-819
delivery of BC-819
● Phase I/IIa, Dose-Escalation, Safety, Pharmacokinetic and Preliminary
Efficacy Study
Efficacy Study
● Performed in Israel
● 11 patients with ovarian cancer to be treated
● FDA granted ‘Orphan Drug’ status for BC-819 in the US, for treatment
of ovarian cancer
of ovarian cancer

22
Animal Model for Pancreatic Cancer
Primary pancreatic tumor ex vivo volume in hamsters treated with BC-
819 was significantly reduced (50%) compared to the control group
819 was significantly reduced (50%) compared to the control group
Only one third of the treated group showed metastatic growths, while
all of the animals in the control group developed metastases
all of the animals in the control group developed metastases

23
Phase I/IIa in Pancreatic Cancer
● Purpose - determine safety and the optimal dose of BC-819
● Phase I/IIa, Dose-Escalation, Safety, Pharmacokinetic and Preliminary
Efficacy Study
Efficacy Study
● Performed in Israel and in the US (at the NCI-designated University of
Maryland, Baltimore Medical Center)
Maryland, Baltimore Medical Center)
● 9 patients with unresectable pancreatic cancer to be treated
intratumorally 4 times with BC-819, twice a week for 2 weeks
intratumorally 4 times with BC-819, twice a week for 2 weeks

24
The 2nd Generation Drug - BC-821
● Use of both the H19 and IGF2-P4
genes (double promoter plasmid)
as a treatment platform for
targeted treatment
genes (double promoter plasmid)
as a treatment platform for
targeted treatment
● Status: pre-clinical results in
animals
animals
● The drug covers 100% of the
eligible cancer patients (30%-50%
more than BC-819)
eligible cancer patients (30%-50%
more than BC-819)

25
Patent (granted worldwide) for BC-819 was submitted
on October 1997
Expiration (without extension): 10/2017 - in US, 10/2018 - Worldwide
Extension Strategies:
Orphan drugs (7 years) - already approved for ovarian cancer
Extension due to drug development process (up to 5 years)
Database protection (5-10 years)
New US legislation (up to 12 years)
New patent application for BC-821 was submitted in 2008
Intellectual Property

26
Management
Uri Danon, CEO
– Former CEO of Atox Bio and Epigenesis
– Managed large-scale international projects, at Teva Pharmaceuticals Industries Ltd., including
Copaxone in a pre-filled syringe
Copaxone in a pre-filled syringe
Ira Weinstein, CFO & COO
– Former CEO of Hapto Biotech (Israel) Ltd. and Incure Ltd.
– Former CFO & COO of Keryx Biopharmaceuticals, Inc. (NASDAQ:KERX; and AIM:KRX)
Prof. Avraham Hochberg, CSO, Founder
– Professor of Biochemistry, Hebrew University, Jerusalem, Israel
– The world-leading expert on H19, with over 140 publications
Dr. Doron Amit, VP Business Development
– Former director of US Funds Department and chief life science analyst at FreeMind Consulting
Group
Group
– A molecular biologist at the Hebrew University of Jerusalem where he leads the research and
development of BioCancell’s 2nd generation drug - BC-821
development of BioCancell’s 2nd generation drug - BC-821
Dr. Patricia Ohana, VP Clinical Development
– A biochemist and molecular biologist at the Hebrew University of Jerusalem where she holds a
position as research scientist in the Department of Biological Chemistry
position as research scientist in the Department of Biological Chemistry
– Involved in many aspects of ground-breaking research on H19 gene led by Professor Hochberg

27
● Prof. Avraham Hochberg, Ph.D., SAB Chairman
– Founder & CSO
– Professor of Biochemistry at the Hebrew University of Jerusalem
– A recipient of the Kaye Award for innovation
● Aaron Ciechanover, M.D., Ph.D.
– Nobel Prize Laureate in Chemistry, 2004
– Distinguished Professor in the Faculty of Medicine and Research Institute at the Technion
● Roger D. Kornberg, Ph.D.
– Nobel Prize Laureate in Chemistry, 2006
– Professor of Structural Biology at the Stanford University School of Medicine
● Yechezkel Barenholz, Ph.D.
– Professor of Biochemistry at the Hebrew University of Jerusalem
– Inventor of Doxil, an anticancer drug with annual sales of over $650 million
● Yaakov Naparstek, M.D.
– Chairman of Medicine at Hadassah University Hospital
● Hermona Soreq, Ph.D.
– Former Dean of the Faculty of Science at the Hebrew University of Jerusalem, Israel
● Mark L. Tykocinski, M.D.
– Dean of Jefferson Medical College and Senior Vice President of Thomas Jefferson University
– Former chairman of Department of Pathology & Laboratory Medicine at University of Pennsylvania
– President / Chair of two leading pathology associations in the U.S.
SAB Members

28
Short-Term Anticipated Events
● Recruitment of the second group of patients in the ovarian cancer
Phase I/IIa clinical trial
Phase I/IIa clinical trial
● Completion of Phase I/IIa in pancreatic cancer
● ‘Orphan Drug’ designation for the treatment of pancreatic cancer
● ‘Fast-Track’ designation for the treatment of ovarian and
pancreatic cancers
pancreatic cancers
● Approval of the lead patent in additional countries

29
● Correct target: H19 gene, which has major role in cancer development,
expressed in over 40 types of cancer. Recently, mechanistically linked to
p53 protein
expressed in over 40 types of cancer. Recently, mechanistically linked to
p53 protein
● Ground-breaking Targeted Cancer Therapy, targeted treatment of
cancer cells, without side effects*
cancer cells, without side effects*
● Interim Phase II results show efficacy
● Blockbuster potential
● Leading interdisciplinary team (with 7 Ph.D.’s)
Summary
*Detected to date

30
Thank You
Please visit us at
www.biocancell.com
www.biocancell.com
