Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | a56535e8vk.htm |
| EX-99.1 - EX-99.1 - Ardea Biosciences, Inc./DE | a56535exv99w1.htm |
Exhibit 99.2

| EFFICACY AND SAFETY OF A RANGE OF DOSES OF RDEA594, A NOVEL URICOSURIC AGENT, AS MONOTHERAPY IN GOUT PATIENTS: RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, PHASE 2 EXPERIENCE F. Perez-Ruiz, V. Hingorani, J. Welp, B. Sheedy, K. Manhard, Z. Shen, J. Miner, M. Nguyen, D. Wilson, L. Yeh, B. Quart Annual European Congress of Rheumatology EULAR 2010 16-19 June 2010 Rome |

| Disclosures F. Perez-Ruiz: Consultant for Ardea Biosciences, Novartis Pharmaceuticals, and Menarini. V Hingorani: Consultant for Ardea Biosciences, Inc. J. Welp, B. Sheedy, K. Manhard, Z. Shen, J. Miner, M. Nguyen, D. Wilson, L. Yeh, and B. Quart: Employees, Ardea Biosciences, Inc. 2 |

| >99% Reabsorption Excretion ~100% of uric acid is initially filtered through glomerular filtration URAT1 Inhibitors Increase the Urinary Excretion of Uric Acid URAT1 Enomoto; Urat1 identification in Nature May2002 D Levinson & L Sorensen; Renal Handling of Uric Acid Proximal Tubule Benzbromarone RDEA594 3 |

| Overview of RDEA594 Main metabolite of RDEA806 (HIV NNRTI) Attributes of RDEA594: Inhibitor of URAT1 No activity in purine catabolism pathways No activity against HIV Linear PK with high bioavailability No mitochondrial toxicity, little oxidative metabolism and no activity against multiple nuclear receptor targets seen with other uricosuric drugs 4 |

| Summary of Results From Phase 2a Phase 2a results for reduction of serum urate (sUA) with RDEA594: 200 mg QD: 40% response (sUA< 6 mg/dL) after one week 400 mg QD: 60% response after one week 83% response for patients with mild to moderate renal impairment 100% response with combination allopurinol 300 mg 5 5 |

| Phase 2a - Consistent Response in Mild to Moderate Renal Impairment with RDEA594 400 mg QD 300 mg QD 6 *estimated by Cockcroft-Gault (C-G) * |
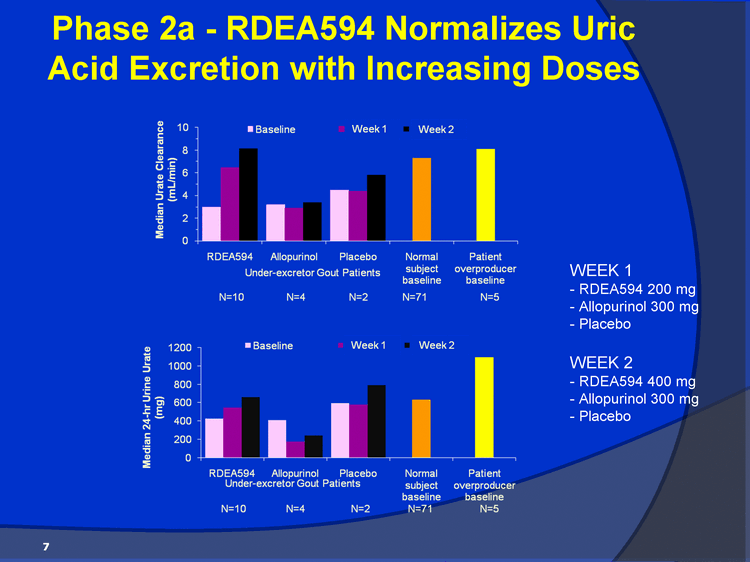
| Phase 2a - RDEA594 Normalizes Uric Acid Excretion with Increasing Doses 7 WEEK 1 - RDEA594 200 mg - Allopurinol 300 mg - Placebo WEEK 2 - RDEA594 400 mg - Allopurinol 300 mg - Placebo |

| RDEA594-202 Phase 2b, Randomized, Double-Blind, Multicenter, Placebo-Controlled, Safety and Efficacy Study of RDEA594 Versus Placebo in the Treatment of Hyperuricemia in Patients with Gout 8 |

| Study Design Study Population 123 patients with sUA ^ 8 mg/dL (480 µmol/L) at screening Diagnosis of gout: ARA criteria (1977) Underexcreters of uric acid (UaCl < 6.0 mL/min) Estimated CrCl by C-G method ^ 60 mL/min Endpoints: Primary - proportion of subjects whose urate level is < 6.0 mg/dL (360 µmol/L) following 4 weeks of dosing by randomized treatment group Secondary - absolute and percent reduction from baseline in sUA levels at each weekly visit, and incidence of gout flares Methods - direct LC-MS/MS method in plasma and indirect UV method in serum for analysis of urate levels in blood 9 |

| Study Timeline and Dose Titration Schema ^14 days 1-2 weeks 4 weeks 2 weeks Screening Period Colchicine Treatment ULT Washout Randomize if no gout flare during run-in period 200 mg Off d r u g Off d r u g Off d r u g 200 mg Placebo 400 mg 200 mg 400 mg 600 mg Screening & Run-in Period - 2-4 weeks: urate lowering therapy (ULT) washout initiate colchicine Treatment Period - 4 weeks: weekly titration up to randomized dose for higher doses Off d r u g 10 |

| Patient Disposition 11 |

| Subject Characteristics Baseline Parameter 200 mg Group 400 mg Group 600 mg Group Placebo Baseline Parameter N=31 N=33 N=32 N=27 Mean age (years) 51 53 53 50 Male (%) 100 97 97 100 Caucasian (%) 97 97 94 96 BMI (kg/m2) 31 30 31 31 Median sUA 9.6 9.6 10.0 9.2 Mean calculated CrCL from 24-hr urine (mL/min) 99 96 87 108* Presence of tophus (%) 16 27 16 19 Prior ULT (%) 26 45 31 37 Mean gout flares in prior year (n, range) 3.0 (0-8) 3.8 (0-30) 3.6 (0-11) 3.6 (0-11) 12 *p<0.05 compared to pooled RDEA594 |

| Primary Endpoint - Percent of Patients < 6 mg/dL (360 µmol/L) at Week 4 13 ITT population n = 27 31 33 32 P= NS P=0.0001 P<0.0001 Plasma urate assay using direct method of analysis. |

| Proportion of Patients Achieving Target U t R d ti L l t W k Urate Reduction Levels at Week 4 70% evel P 0.0001 50% 60% arget Urate Le P=0.0001 30% 40% s Reaching Ta 4 mg/dL (240 mol/L) 5 /dL( l/L) Plasma urate P=0.0044 10% 20% ent of Patients mg/dL (300 mol/6 mg/dL (360 mol/L) P=0.02 0% Placebo 200 400 600 Perce RDEA594 Dose (mg) ITT population 14 Plasma urate assay using direct method of analysis. |
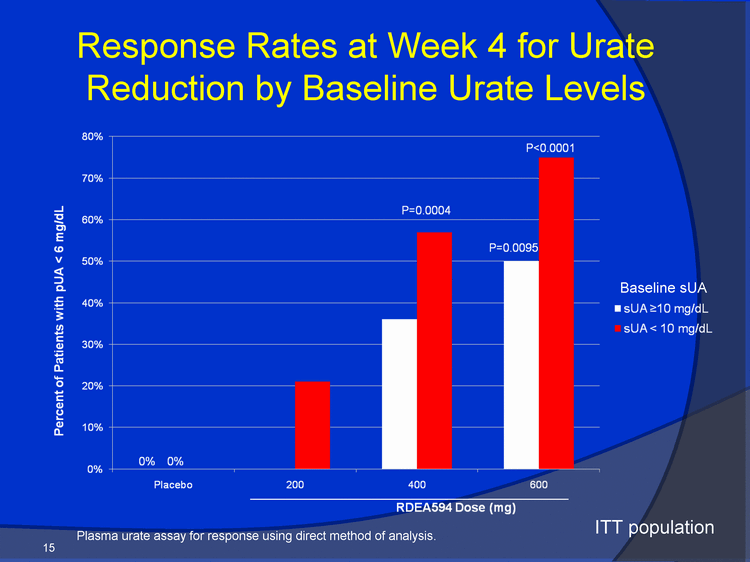
| Response Rates at Week 4 for Urate R d ti b B li U t L l 80% P0.0001 Reduction by Baseline Urate Levels 60% 70% mg/dL P=0.0004 40% 50% with pUA 6 m sUA 10 mg/dL P=0.0095 Baseline sUA 20% 30% nt of Patients sUA 10 mg/dL 0% 10% Pl b 200 400 600 Percen 0% 0% Placebo RDEA594 Dose (mg) 15 ITT population Plasma urate assay for response using direct method of analysis. |

| Secondary Endpoint - Median Change in Serum Urate ** ** ** ** ** ** ** ** ** ** ** * *p=0.004; **p<0.0001 versus placebo Serum urate assay using indirect method of analysis. 16 |

| Consistent sUA Reduction with Reduced Creatinine Clearance* 200 mg 400 mg 600 mg N= 1 7 16 1 9 16 3 10 16 6 10 13 Placebo 17 * CrCl calculated from 24-hr urine clearance. Serum urate assay using indirect method of analysis. Baseline CrCL in mL/min: (Mean = 46) |

| Number of Patients with Flares by Administered Dose Administered Dose Mean Duration of Flares 600 mg 1.7 days 400 mg 2 days 200 mg 4 days Placebo 1 day 18 |

| Patients with Grade 1-2 Elevations in Serum Creatinine (SCr) and Normal Baselines Randomized Dose Randomized Dose Randomized Dose Randomized Dose RDEA594 200 mg (n=31) RDEA594 400 mg (n=33) RDEA594 600 mg (n=32) Placebo (n=27) Patients with Grade 1-2 Elevations in Serum Creatinine at Day 28 2 (6%) 2 (6%) 2 (6%) 1 (4%) Same rate of transient sCR elevations during the screening period No grade 3 or 4 elevations SCr elevations often associated with flares Four patients randomized to RDEA594 had elevated SCr at baseline Two normalized on drug and two remained elevated 19 |

| Incidence of the Most Frequent Adverse Events* *Adverse events reported by at least 2 subjects that were considered at least possibly related to treatment RDEA594 Dose Group 20 No Serious Adverse Events Two discontinuations due to adverse events, both on 400 mg dose: one patient with vertigo, and one patient with elevated sCR that returned to normal range while receiving RDEA594 |

| Conclusions RDEA594 produced rapid and sustained reductions in urate levels, with statistically and clinically significant increases in response rates with the 400 mg and 600 mg doses No safety concerns and no dose-related side effects were observed Profile of RDEA594 has been consistent across multiple clinical studies Results of this trial demonstrate that RDEA594 is a promising new drug for the treatment of hyperuricemia in gout patients and support the initiation of Phase 3 clinical trials 21 |

| Acknowledgements 22 Patients for their participation in the studies Investigators and their staff members: Bulgaria V. Kadinov D. Yaneva K. Yablanski S. Kuzmanova A. Toncheva A. Batalov Canada B. Lasko G. Tellier I. Wilderman R. Hart W. Arkinstal Czech Republic Z. Stejfova D. Galatikova J. Nohejl Bulgaria V. Kadinov D. Yaneva K. Yablanski S. Kuzmanova A. Toncheva A. Batalov Germany K-H. Krause C. Grigat E. Schell Poland M. Nowicki J. Ilkowski J. Brzezicki J. Rozciecha E. Wielosz Georgia L. Kilsonia L. Shalamberidze Germany K-H. Krause C. Grigat E. Schell Poland M. Nowicki J. Ilkowski J. Brzezicki J. Rozciecha E. Wielosz US M. Guice J. Wilson B. Rankin Poland M. Nowicki J. Ilkowski J. Brzezicki J. Rozciecha E. Wielosz |
