Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - BIOCANCELL THERAPEUTICS INC. | ex23-1.htm |
As filed with the Securities and Exchange Commission on June 11, 2010
Registration No. 333-162088
___________________________________________________________________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No . 3 to
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
BIOCANCELL THERAPEUTICS INC.
(Exact Name of Registrant As Specified in Its Charter)
|
Delaware
|
2834
|
20-4630076
|
||
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(Primary Standard Industrial
Classification Code Number)
|
(I.R.S. Employer
Identification Number)
|
Beck Science Center
8 Hartom St, Har Hotzvim
Jerusalem 97775 Israel
972-2-548-6555
(Address and Telephone Number of Registrant’s Principal Executive Offices)
Corporation Service Company
2711 Centerville Road
Suite 400
Wilmington, Delaware 19808
(302) 636-5400
(Name, Address, Including Zip Code, and Telephone Number,
Including Area Code, of Agent for Service)
Copy to:
Dr. Shachar Hadar, Adv.
Gross, Kleinhendler, Hodak, Halevy, Greenberg & Co., Law Offices
1 Azrieli Center, Round Tower, 40th floor
Tel Aviv 67021, Israel
Tel: 972-3- 6074444
Fax: 972-3- 6074422
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o
|
Accelerated filer o
|
|
|
Non-accelerated filer o (Do not check if a smaller reporting company)
|
Smaller reporting company þ
|
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. The company may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION, DATED JUNE 11, 2010
|
BIOCANCELL THERAPEUTICS INC.
Shares of Common Stock
Series 3 Warrants
Series 4 Warrants
We are offering on a best-efforts basis up to units , with each unit consisting of shares of our common stock, par value $0.01 per share , Series 3 warrants and Series 4 warrants . The offering is not underwritten on a firm basis.
Each Series 3 warrant shall be exercisable into one share of our common stock at an exercise price of NIS (approximately $ ). Series 3 warrants will be exercisable for two years from the date on which they are issued. Each Series 4 Warrant shall be exercisable into one share of our common stock at an exercise price of NIS (approximately $ ). Series 4 warrants will be exercisable for four years from the date on which they are issued. The units will separate immediately and the shares of our common stock and the Series 3 warrants and Series 4 warrants comprising the units will be issued and will trade separately.
We anticipate that the offering price will be between NIS and NIS per unit. The offering price will be determined in an auction process and shall not be lower than the minimum price for the purpose of this offering. For further details regarding the auction process, see under the heading “Plan of Distribution” beginning on page 73 of this prospectus.
Currently, there is no public market for our common stock in the United States, and no assurances can be given that a public market will develop or, if developed, that it will be sustained. Our common stock is listed on the Tel Aviv Stock Exchange, or TASE, and trades under the symbol “BICL”. On June 10, 2010 , the closing price of one share of our common stock was NIS 2.36 . The shares of our common stock and warrants which are being offered under this prospectus will be listed for trading on the TASE.
|
|
Per Unit
|
|
Total
|
||
|
Public offering price
|
NIS
|
|
NIS
|
|
|
|
Offering proceeds, before expenses
|
NIS
|
|
NIS
|
|
|
Investing in our common stock involves a high degree of risk. Before buying any shares, you should review the discussion of risk factors with respect to our common stock under the heading “Risk Factors” beginning on page 5 of this prospectus.
You should read this prospectus and any prospectus supplement carefully before you decide to invest. You should not assume that the information in this prospectus is accurate as of any date other than the date on the front of this document.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is , 2010 .
TABLE OF CONTENTS
|
|
||||
|
PROSPECTUS SUMMARY
|
1 | |||
|
RISK FACTORS
|
5 | |||
|
USE OF PROCEEDS
|
20 | |||
|
DIVIDEND POLICY
|
20 | |||
|
DETERMINATION OF OFFERING PRICE
|
21 | |||
|
DILUTION
|
21 | |||
|
OUR BUSINESS
|
22 | |||
|
MANAGEMENT
|
35 | |||
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
38 | |||
|
EXECUTIVE COMPENSATION
|
40 | |||
|
CORPORATE GOVERNANCE
|
47 | |||
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
54 | |||
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
|
66 | |||
|
MARKET PRICE OF COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
|
68 | |||
|
DESCRIPTION OF SECURITIES
|
68 | |||
|
PLAN OF DISTRIBUTION
|
73 | |||
|
EXPERTS
|
75 | |||
|
LEGAL MATTERS
|
75 | |||
|
COMMISSION’S POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
75 | |||
|
ADDITIONAL INFORMATION
|
75 | |||
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties. These forward-looking statements, which are usually accompanied by words such as “may,” “might,” “will,” “should,” “could,” “would,” “intends,” “estimates,” “predicts,” “potential,” “continue,” “believes,” “anticipates,” “plans,” “expects,” “prospective” and similar expressions, relate to, without limitation, statements about our market opportunities, our strategy, our competition, our projected revenue and expense levels and the adequacy of our available cash resources. This prospectus also may contain forward-looking statements attributed to third parties relating to their estimates regarding our industry. You should not place undue reliance on any of the forward-looking statements contained in this prospectus.
The cautionary statements contained in “Risk Factors” and other similar statements contained elsewhere in this prospectus identify important factors with respect to such forward-looking statements, including certain risks and uncertainties that could cause our actual results, performance or achievements to differ materially from those expressed or forecasted in, or implied by, such forward-looking statements.
Although we believe that the expectations reflected in these forward-looking statements are based upon reasonable assumptions, no assurance can be given that such expectations will be attained or that any deviations will not be material. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. We disclaim any obligation or undertaking to disseminate any updates or revision to any forward-looking statement contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based.
PROSPECTUS SUMMARY
The following summary highlights selected information contained in this prospectus. This summary does not contain all the information you should consider before investing in the Units. Before making any investment decision, you should read the entire prospectus carefully, including the “Risk Factors” section, the financial statements and the notes to the financial statements.
Our Business
We are a development-stage biopharmaceutical company focused on the discovery, development and commercialization of novel therapies for treating cancer-related diseases. Our research and development activities build upon the research of Professor Abraham Hochberg of the Hebrew University of Jerusalem, who isolated the human H19 gene and determined that it is expressed in over forty different forms of cancer, including superficial bladder carcinoma, and pancreatic and ovarian cancer, while laying dormant and non-expressed in non-cancerous cells. Professor Hochberg’s research discovered that the H19 gene is significantly expressed in cancerous cells of between 70% and 84% of adult cancer patients, varying between cancer types. Research has also demonstrated that the H19 gene plays a significant role in the tumor development process by enabling tumor cells to survive under stress conditions, such as low serum and low oxygen levels, that are typical conditions of the environment in which cancerous cells develop. This survival supports the growth of the tumor and the development of metastases.
We completed a Phase I/IIa trial of our drug -candidate , BC-819, designed for use in patients suffering from bladder carcinoma that have failed standard treatment . The Phase I/IIa trial resulted in no severe adverse side effects directly attributable to the tested therapy. In March 2008, we began FDA-approved Phase IIb trials for this therapy. In addition, pursuant to an IND application to the FDA from December 2008, we commenced treating patients in Israel in May 2009 for a Phase I/IIa trial, also using BC-819, designed to treat ovarian cancer. In addition, pursuant to an IND application to the FDA from December 2008, we commenced a Phase I/IIa trial for this therapy, also using BC-819, designed to treat pancreatic cancer, in Israel in August 2009 and in the United States in March 2010 .
Our Goal
Our goal is to become a leader in the development and commercialization of research and therapeutic product -candidates and other applications utilizing target genes such as H19 and IGF2. Our key strategies to achieve this goal are as follows:
|
§
|
Build and Maintain a Versatile and Effective Intellectual Property Portfolio. We intend to develop an effective intellectual property portfolio that will support our efforts in developing and commercializing gene-based therapeutic product-candidates and in taking advantage of new opportunities that may materialize in this developing field. Our patent strategy is to continue to seek intellectual property rights coverage for our prototype drug BC-819 and for all of our molecules and to file patent applications claiming composition-of-matter and method-of-use on individual molecules of commercial interest.
|
|
§
|
Pursue Therapeutic Product Opportunities. We intend to utilize our target gene-based discoveries, know-how and expertise to develop drugs that selectively compromise and kill forms of cancer that express target genes.
|
|
§
|
Leverage Our Intellectual Property Position, Expertise and Knowledge of Target Genes to Continue to Establish Strategic Collaborations. We intend to enter into strategic collaborations for the funding, development and commercialization of therapeutic products utilizing the H19 and IGF2 target genes.
|
Risks Related to Our Business
We are a development stage company and, as such, our success is subject to the performance and efficacy of the experimental product -candidates we are developing and our ability to secure adequate financing on favorable terms. There are several risk factors to consider when deciding whether to make an investment in our common stock, including risk factors related to our operations in Israel and the complexity of the regulatory process to which the approval of our therapies are subject, that are explained in detail in this prospectus under the heading “Risk Factors”.
Some of the most significant risks related to our business and to owning our common stock include the following:
1
|
§
|
The approach that we have adopted to discover and develop prospective therapeutic products is new and may never lead to marketable products.
|
|
§
|
We will require substantial additional funds to complete our research and development activities and, if additional funds are not available, we may need to significantly scale back or cease our operations.
|
|
§
|
Any prospective therapeutic products that we may develop will be required to undergo a time-consuming, costly and burdensome pre-market approval process, and we may be unable to obtain regulatory approval for any of our prospective therapeutic products.
|
|
§
|
Even if we receive regulatory approval to market our prospective therapeutic products, the market may not be receptive to our prospective therapeutic products upon their commercial introduction which will prevent us from becoming profitable.
|
|
§
|
If we fail to comply with our obligations under our license with Yissum or other licenses or related agreements that we are a party to and that we may enter into in the future, we could lose license rights that may be necessary for developing our target gene-based therapeutic product-candidates.
|
|
§
|
Risks related to our operations in Israel, including due to political and economic conditions as well as certain Israeli legislation and regulation to which we are subject.
|
|
§
|
If our common stock is accepted for quotation on the OTC Bulletin Board, it may be thinly traded, so you may be unable to sell at or near ask prices or at all if you need to sell your shares to raise money or otherwise desire to liquidate your shares.
|
|
§
|
The application of the “penny stock” rules to our common stock could limit the trading and liquidity of the common stock, adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
|
The Auction Process
We plan to conduct this offering using an auction process for all investors, both individual and institutional. To participate in the auction, investors will submit binding bids to purchase units that specify the number of units the investor would be interested in purchasing and the price the investor would be willing to pay. We intend to use the auction to determine the offering price per unit for the offering. All valid bids to purchase units at or above the determined offering price per unit will receive an allocation of units at the offering price. If the number of units represented by successful bids exceeds the number of units we are offering, then all bidders making bids above the offering price per unit will receive all the units for which they bid, all bidders making bids at the offering price per unit will receive some of the units for which they bid, on a pro-rata basis, and all bidders making bids below the offering price per unit will receive no units. See the section entitled “Plan of Distribution – The Auction Process” for a description of how this process will work.
Corporate Information
We were incorporated as DBT Biopharmaceuticals Inc. in Delaware on July 26, 2004 and changed our name to BioCancell Therapeutics Inc. on February 25, 2005. We conduct our business and operations through our wholly owned subsidiary, BioCancell Therapeutics Israel Ltd., incorporated in Israel on October 18, 2004. Our principal executive office is located at Beck Science Center, 8 Hartom St, Har Hotzvim, Jerusalem 97775 Israel. Our telephone number is 972-2-548-6555. On August 17, 2006, we listed our common stock on the TASE as a part of our initial public offering. Shares of our common stock trade on the TASE under the symbol “BICL”. Our website is located at www.biocancell.com . The information found on, or accessible through, our website is not part of, or incorporated by reference into, this prospectus.
In this prospectus, the terms “BioCancell,” “Company,” “we,” “us” and “our” each refer to BioCancell Therapeutics, Inc. and its wholly owned subsidiary, BioCancell Therapeutics Israel Ltd.
2
THE OFFERING
|
Securities Offered
|
|
shares of common stock
Series 3 warrants
Series 4 warrants
The securities will be sold in up to units, each comprising shares of our common stock, Series 3 warrants and 100 Series 4 warrants.
|
|
|
|
|
|
TASE symbol
|
Common stock – BICL
|
|
|
Proposed TASE symbols
|
Series 3 warrants - BICL.W3
|
|
Series 4 warrants - BICL.W4
|
||
|
Common stock:
|
||
|
Number outstanding before this offering:
|
20,621,113
|
|
|
Number to be outstanding after this offering:
|
|
|
|
Series 3 warrants:
|
||
|
Number outstanding before this offering:
|
--
|
|
|
Number to be outstanding after this offering:
|
|
|
|
Terms of Series 3 Warrant
|
Each Series 3 warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Series 3 warrants will be exercisable for two years from the date on which they are issued.
|
|
|
Series 4 warrants:
|
||
|
Number outstanding before this offering:
|
--
|
|
|
Number to be outstanding after this offering:
|
|
|
|
Terms of Series 4 Warrant
|
Each Series 4 warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Series 4 warrants will be exercisable for four years from the date on which they are issued.
|
|
|
Risk Factors
|
Investing in our common stock involves a high degree of risk. Please review the discussion of risk factors with respect to an investment in our common stock set forth in “Risk Factors” beginning on Page 6 .
|
|
|
Use of Proceeds
|
We intend to use the net proceeds from this offering to fund our research and development activities and for working capital and general corporate purposes.
|
|
|
Where you can find more information:
|
If you have any questions relating to this prospectus, you should contact:
|
|
|
Mr. Uri Danon
BioCancell Therapeutics Inc.
Beck Science Center, 8 Hartom St
Har Hotzvim, Jerusalem 97775 Israel
Phone: 972-2-548-6555
|
Currently, there is no public market for our common stock in the United States and no assurances can be given that a public market will develop or, if developed, that it will be sustained. Our common stock is listed on the TASE under the symbol “BICL”. On June 10, 2010, the reported closing price of our common stock on the TASE was NIS 2.36. The shares of our common stock and Warrants which are being offered under this prospectus will be listed for trading on TASE promptly after the registration statement filed with the SEC of which this prospectus is a part is declared effective.
SUMMARY OF CONSOLIDATED FINANCIAL DATA
The summary consolidated financial data set forth below is derived from our consolidated financial statements. The consolidated results of operations for the years ended, and consolidated balance sheet data as of, December 31, 2009 and 2008 are derived from our audited consolidated financial statements included elsewhere in this prospectus. The consolidated balance sheet data as of March 31, 2010, the statements of operations data for the three months ended March 31, 2010 and 2009 and for the period from inception through March 31, 2010, are derived from our unaudited consolidated financial statements included elsewhere in this prospectus.
It is important that you read this information together with “Management’s Discussion and Analysis of Financial Conditions and Results of Operations,” “Risk Factors” and the financial statements and the notes to the financial statements beginning on page F-1 of this prospectus. The historical results presented below are not necessarily indicative of results to be expected in any future periods.
3
Consolidated Statements of Operations Data:
|
Three month period ended
March 31,
|
Year ended
December 31,
|
Cumulative from
inception to
March 31,
|
|||
|
2010
|
2009
|
2009
|
2008
|
2010
|
|
|
U.S. dollars in thousands
|
|||||
|
(except share and per share data)
|
|||||
|
Research and development expenses
|
$ | 534 | $ | 585 | $ | 2,617 | $ | 3,155 | $ | 11,803 | ||||||||||
|
Less: Chief Scientist grants and BIRD Foundation grants
|
(29 | ) | (171 | ) | (604 | ) | (461 | ) | (2,046 | ) | ||||||||||
|
Research and development expenses, net
|
505 | 414 | 2,013 | 2,694 | 9,757 | |||||||||||||||
|
General and administrative expenses
|
428 | 324 | 1,457 | 1,913 | 6,721 | |||||||||||||||
|
Operating loss
|
933 | 738 | 3,470 | 4,607 | 16,478 | |||||||||||||||
|
Interest expense (income), net
|
(2 | ) | 1 | (3 | ) | (61 | ) | (15 | ) | |||||||||||
|
Loss (gain) from marketable securities, net
|
- | - | - | 89 | (6 | ) | ||||||||||||||
|
Interest on convertible notes and discount amortization
|
93 | 79 | 341 | 147 | 582 | |||||||||||||||
|
Revaluation of warrants
|
1,186 | 1,037 | 3,053 | (1,653 | ) | 2,586 | ||||||||||||||
|
Other financing expenses (income), net
|
6 | (110 | ) | (58 | ) | (187 | ) | (279 | ) | |||||||||||
|
Net loss
|
$ | 2,216 | $ | 1,745 | $ | 6,803 | $ | 2,942 | $ | 19,346 | ||||||||||
|
Basic and diluted net loss per share
|
$ | 0.13 | $ | 0.12 | $ | 0.44 | $ | 0.22 | $ | 1.85 | ||||||||||
|
Weighted-average common shares used in computing basic and diluted net loss per share
|
17,213,498 | 14,479,859 | 15,453,126 | 13,413,452 | 10,473,498 |
|
Consolidated Balance Sheet Data:
|
March 31,
2010
|
December 31,
2009
|
December 31,
2008
|
|||||||||
|
Total current assets
|
$
|
3,100
|
$
|
985
|
$
|
2,994
|
||||||
|
Total long-term assets
|
272
|
296
|
185
|
|||||||||
|
Property and equipment, net
|
101
|
106
|
|
131
|
||||||||
|
Total assets
|
3,688
|
1,565
|
3,310
|
|||||||||
|
Total current liabilities
|
550
|
683
|
834
|
|||||||||
|
Total long-term liabilities
|
5,572
|
4,248
|
|
526
|
||||||||
|
Total stockholders’ equity (deficit)
|
(2,434
|
)
|
(3,366
|
)
|
1,950
|
|||||||
|
Total liabilities and stockholders’ equity (deficit)
|
|
$
|
3,688
|
$
|
1,565
|
$
|
3,310
|
|||||
4
RISK FACTORS
An investment in our Units offered by this prospectus involves a substantial risk of loss. Before you invest, you should carefully consider the risks and uncertainties described below and the other information in this prospectus. If any of the following risks materialize, our business, operating results and financial condition could be harmed and the value of our common stock could decline. This means that you could lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our operations.
Risks Related to Our Business and Industry, Our Financial Results and Our Need for Financing
The approach that we have adopted to discover and develop prospective therapeutic products is new and may never lead to marketable products.
We have concentrated our efforts on developing therapeutic product-candidates utilizing target genes, such as the H19 and IGF2 genes, which is a new field of drug research and development. To the best of our knowledge, no person has developed any therapeutic product utilizing these target genes that has been approved for marketing. The scientific discoveries that form the basis for our efforts to develop drugs utilizing the target genes are relatively new. The scientific evidence to support the feasibility of developing such drugs based on these discoveries is both preliminary and limited. Further, our focus solely on developing therapeutic products utilizing target genes as opposed to more proven technologies for the development of therapeutic products increases the risks associated with the ownership of our common stock. If we are unsuccessful in developing drugs utilizing the target genes, we may be required to change the scope and direction of our product development activities. In that case, if we are not able to identify and successfully implement an alternative product development strategy, our business may fail.
We do not have a history of commercial sales and do not anticipate earning operating income over the coming years, and our failure to receive marketing approval for any of our prospective therapeutic products or our failure to otherwise achieve and sustain profitability would negatively impact our ability to continue our business operations.
We were formed on July 26, 2004 and continue to be a development-stage company that is in the early stage of drug development. As of the date of this prospectus, we have not received marketing approval for any of our prospective therapeutic products and as a result have not recorded any sales. We expect that we will operate at a loss over the coming years as we do not expect to generate any revenue from operations in the near term. We may or may not be able to develop or receive marketing approval for drugs from our research and development efforts. In addition, even if we obtain all necessary approvals to market any of our prospective therapeutic products, there is no certainty that there will be a sufficient demand to justify the production and marketing of any such product. The market for any drug based on target genes may be small or may not even develop, and the creation and growth of a market for any such drug depends on a number of factors including:
|
§
|
regulatory approval;
|
|
§
|
physicians accepting the benefits of the use of drugs utilizing the target genes;
|
|
§
|
perceived risks generally associated with the use of new medical products;
|
|
§
|
the availability of alternative treatments or procedures that are perceived to be or are more effective, safer, easier to use or less costly than drugs that utilize the target genes;
|
|
§
|
availability of adequate reimbursement by patients’ third party insurers for drugs that utilize the target genes; and
|
|
§
|
marketing efforts and publicity regarding any drug that we may develop utilizing the target genes.
|
If our prospective therapeutic products utilizing the target genes do not gain wide market acceptance, we may not be able to achieve our anticipated growth, revenues or profitability and we may not be able to continue our business operations.
Scientific or technological difficulties may impede our research and development activities.
Our research and development activities to develop drugs utilizing the target genes may be impeded due to scientific or technological difficulties or our lack of complete understanding of the target genes. There is no certainty that research and development of the target genes will give rise to a marketable drug or that we will succeed in developing a marketable drug in a timely manner or in accordance with our estimated budgets. Even if we are successful in developing such drugs, there is no certainty that the drugs, when developed, will be found to be sufficiently effective and safe for use to receive regulatory approval for marketing, which would adversely impact our potential revenues, results of operations and financial condition. Several other companies, in addition to us, are currently in stages of development of such drugs. The creation of a market for such drugs may take several years and may require significant resources to create, and there is a risk that the market for these drugs may never come into existence or may be very limited in size. Our inability to create a market for drugs utilizing the target genes would adversely impact our potential revenues, results of operations and financial condition.
5
We will require substantial additional funds to complete our research and development activities and, if additional funds are not available, we may need to significantly scale back or cease our operations.
We will require substantial funds to discover, develop, protect and conduct research and development for our target genes-based prospective therapies, including pre-clinical testing and clinical trials of any of our prospective therapeutic products, and to manufacture and market any such product that may be approved for commercial sale. From our inception, we have raised a cumulative amount of $16,840,000 including income generated by the exercise of options by employees, directors and consultants. For details, see “Management's Discussion and Analysis of Financial Condition and Results of Operations – Overview” below. However, they may prove to be insufficient for these activities. Our financing needs may change substantially because of the results of our research and development, competition, clinical trials and costs arising from additional regulatory approvals. We may not succeed in raising additional funds if needed. The timing of our need for additional funds will depend on a number of factors, which factors are difficult to predict or may be outside of our control, including:
|
§
|
progress in our research and development programs;
|
|
§
|
the resources, time and costs required to initiate and complete our research and development and to initiate and complete pre-clinical and clinical studies and to obtain regulatory approvals for our prospective therapeutic products;
|
|
§
|
the timing, receipt and amount of milestone, royalty and other payments from present and future collaborators, if any; and
|
|
§
|
costs necessary to protect our intellectual property.
|
If our estimates and predictions relating to any of these factors are incorrect, we may need to modify our operating plan. Additional funds may not be available to us when needed on acceptable terms, or at all. Debt financing, if available, may involve restrictive covenants that could limit our flexibility in conducting future business activities. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some or all of our technologies or our prospective therapeutic products. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail one or more of our research or development programs, which would adversely impact our potential revenues, results of operations and financial condition.
Our auditors have expressed substantial doubt as to whether we can continue as a going concern.
The audit report covering the December 31, 2009 consolidated financial statements contains an explanatory paragraph that states that our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. Our financial statements do not include any adjustments to the value of our assets or the classification of our liabilities that might result if we would be unable to continue as a going concern. We have incurred operating losses since inception, have not generated any product sales revenues and have not achieved profitable operations. Our deficit, accumulated during the development stage through March 31, 2010 , aggregated $ 19,346 ,000 and we expect to continue to incur substantial losses in future periods while we continue to test and prepare our product candidates for the market. We have not generated any revenues from operations since our inception and have incurred substantial losses. Following the closing of the private placements of our securities in March 2010 , we believe that we have sufficient cash to meet our planned operating needs until November 2010 . We will require substantial additional funds to complete our research and development activities and there can be no assurance that we will be able to raise any or all of the capital required. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Future Operations,” for a more detailed description of our financial condition.
We have limited sources for funding our research and development activities and a lack of adequate funding may cause a cessation of all or part of our research and development activities.
A significant portion of our research and development activities has been financed by the research grants that we have received and continue to receive from the Israeli government through the Office of the Chief Scientist of the Ministry of Industry, Trade and Labor and the bi-national BIRD Foundation. There is no certainty that we will be able to obtain additional sources of funding for our research and development activities should there be a termination of funding from the Office of the Chief Scientist. A lack of adequate funding may cause a cessation of all or part of our research and development activities and business operations. Under the terms of the exclusive license granted to us by Yissum, Yissum has the right to terminate the license in the event that we become bankrupt or insolvent, or if our business is placed in the hands of a receiver, assignee or trustee. For more information, see “Material Operating Arrangements — Exclusive License Agreement with Yissum” below.
We will need additional capital in the future. If additional capital is not available, we may not be able to continue to operate our business pursuant to our business plan or we may have to discontinue our operations entirely.
Based on our proposed use of proceeds, we will likely need significant additional financing, which we may seek to raise through, among other things, public and private equity offerings and debt financing. Any equity financings will be dilutive to existing stockholders, and any debt financings will likely involve covenants restricting our business activities. Additional financing may not be available on acceptable terms, or at all.
6
Risks Related to Development, Clinical Testing and
Regulatory Approval of Our Prospective Therapeutic Products
Any prospective therapeutic products that we may develop will be required to undergo a time-consuming, costly and burdensome pre-market approval process, and we may be unable to obtain regulatory approval for any of our prospective therapeutic products.
Any prospective therapeutic products that we may develop will be subject to extensive governmental regulations relating to development, clinical trials, manufacturing and commercialization. Rigorous pre-clinical testing and clinical trials and an extensive regulatory approval processes are required to be successfully completed in the United States and in many foreign jurisdictions such as the European Union and Japan before a new therapeutic product may be offered and sold in any of these countries or regions. Satisfaction of these and other regulatory requirements is costly, time-consuming, uncertain and subject to unanticipated delays.
In the United States, the prospective therapeutic products that we intend to develop and market are regulated by the United States Food and Drug Administration, or FDA, under the FDA’s new drug development and review process. The time required to obtain FDA and other approvals for our prospective therapeutic products is unpredictable. Before such prospective therapeutic products can be marketed, we must obtain clearance from the FDA first through submission of an investigational new drug application (IND), then through successful completion of human testing under three phases of clinical trials and finally through submission of a new drug application (NDA). Even after successful completion of clinical testing, there is a risk that the FDA may request further information from us, disagree with our findings or otherwise undertake a lengthy review of our NDA submission. There can be no assurance that the FDA will approve of any NDA that we may submit. It is possible that none of the prospective therapeutic products that we may develop will obtain the appropriate regulatory approvals necessary for us to commence the offer and sale of such products. Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from a particular prospective therapeutic product.
Because we intend to market any drug that we may develop in jurisdictions in addition to the United States, such as the European Union and Japan, we will likely incur the same costs or more in satisfying foreign regulatory requirements governing the conduct of clinical trials, manufacturing and marketing and commercialization of our prospective therapeutic products. Approval by the FDA by itself does not assure approval by regulatory authorities outside the United States. Each of these foreign regulatory approval processes includes all of the risks associated with the FDA approval process, as well as risks attributable to having to satisfy local regulations within each of these foreign jurisdictions. Our inability to obtain regulatory approval outside the U .S. may adversely compromise our business prospects.
If the pre-clinical and clinical studies that are required to be conducted by us to gain regulatory approval are delayed or unsuccessful, we may not be able to market our prospective therapeutic products.
We may experience delays in any phase of the development of our prospective therapeutic products and their commercial launch, including during research and development and clinical trials. Implementing a clinical study is time-consuming and expensive, and the outcome of any clinical study is uncertain. The completion of any of these studies may be delayed or halted for numerous reasons, including, but not limited to, the following:
|
§
|
the FDA, institutional review boards, the European Union Notified Bodies, the Israeli Ministry of Health, or other regulatory authorities do not approve a clinical study protocol or place a clinical study on hold;
|
|
§
|
patients do not enroll in a clinical study or results from patient participants are not received at the expected rate;
|
|
§
|
patients experience adverse events from our drug candidate;
|
|
§
|
patients die during a clinical study for a variety of reasons that may or may not be related to our prospective therapeutic products, including the advanced stage of their disease and other medical problems;
|
|
§
|
third party clinical investigators do not perform the clinical studies in accordance with the anticipated schedule or consistent with the clinical study protocol and good clinical practices or other third party organizations do not perform data collection and analysis in a timely or accurate manner;
|
|
§
|
regulatory inspections of manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend the clinical studies;
|
|
§
|
changes in governmental regulations or administrative actions;
|
|
§
|
the interim results of the clinical study are inconclusive or negative; and
|
|
§
|
the study design, although approved and completed, is inadequate to demonstrate effectiveness and safety.
|
Our dependence upon clinical trials in developing our prospective therapeutic products may impede us from reaching advanced stages of development, and might cause termination of all or part of our commercial operations. To date, the above described situations regarding potential delays in research and development activities and clinical trials have yet to occur in a manner which adversely affects our research and development activities. Notwithstanding the foregoing, tough FDA criteria have made patient recruitment for the superficial bladder cancer Phase IIb clinical trial, a more difficult and time-consuming issue than was initially expected.
7
We have limited experience in conducting and managing clinical trials.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals for our prospective therapeutic products. We may rely on third parties for clinical development activities and our reliance on third parties will reduce our control over these activities. Accordingly, third party contractors may not complete activities on schedule, or may not conduct clinical trials in accordance with regulatory requirements or our trial design. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be required to replace them which may have the effect of delaying the affected trial.
The difficulties of identifying and recruiting suitable patients for clinical studies may be exacerbated in the case of patients diagnosed with superficial bladder carcinoma, and further, the clinical results and data that we obtain from any clinical testing that we may conduct in the future may not be reliable, any of which factor may significantly compromise our ability to develop a drug utilizing a target gene for the treatment of superficial bladder carcinoma.
The difficulties of identifying and recruiting suitable patients for clinical studies may be exacerbated in the case of patients diagnosed with superficial bladder carcinoma because of the high demand for such patients’ involvement in current and future clinical trials for potential drugs for the treatment of superficial bladder carcinoma. Further, the supply of suitable patients diagnosed with superficial bladder carcinoma is low because of the strict inclusion criteria requirements . The results and data obtained from any clinical testing that we may conduct in the future may not be reliable because our recruitment of suitable patients diagnosed with superficial bladder carcinoma may require that we administer our clinical studies in the developing world. Third party clinical investigators in the developing world may not perform clinical studies in accordance with the anticipated schedule or consistent with the clinical study protocol and good clinical practices or may not perform data collection and analysis in a timely or accurate manner. The realization of any of the foregoing risks may significantly compromise our ability to develop a drug utilizing a target gene for the treatment of superficial bladder carcinoma, which would adversely impact our potential revenues, results of operations and financial condition. We performed a Phase I/IIa superficial bladder carcinoma clinical trial at Wolfson and Meir Medical Centers in Israel, without difficulties in enrolling subjects or adhering to study protocols. We are performing a Phase IIb clinical trial for this application at Asaf Harofe, Hadassah, Hillel Yafe, Wolfson, Meir, Sheba and Bnei Tzion Medical Centers in Israel, and BCG Oncology in Arizona. The tough FDA criteria have made patient recruitment in this trial a more difficult and time-consuming issue than was initially expected. Our researchers have not reported experiencing any difficulties adhering to study protocols.
We may not commence additional clinical testing for any of our prospective therapeutic products and the commercial value of any clinical study that we may commence and conduct in the future will significantly depend upon our choice of indication and our selection of a patient population for our clinical study of an indication, and our inability to commence clinical testing or our choice of clinical strategy may significantly compromise our business prospects.
Our ability to commence clinical studies depends upon successful completion of and positive scientific results from pre-clinical studies. In the event that we successfully complete a clinical study, the commercial value of any such study will significantly depend upon our choice of indication and our selection of a patient population for that indication. Because the target genes are expressed in various types of cancer, our prototype molecules may have the ability to treat many different kinds of cancer. Thus, we may incorrectly assess the market opportunities of an indication or may incorrectly estimate or fail to fully appreciate the scientific and technological difficulties associated with treating an indication. Furthermore, the quality and robustness of the results and data of any clinical study that we may conduct in the future will depend upon our selection of a patient population for clinical testing. Our inability to commence clinical testing or our choice of clinical strategy may significantly compromise our business prospects.
Our inability to address the chemistry, manufacturing and control concerns of regulatory bodies would significantly compromise our business prospects.
In the event that we commence clinical studies for any of our prospective therapeutic products, our ability to successfully complete clinical studies and to apply for and obtain regulatory approval for marketing will depend upon our ability to develop an established manufacturing process assuring consistent production of a therapeutic product of a defined quality for all phases of clinical testing and for commercial production. Our inability to satisfy the chemistry, manufacturing and control concerns of regulatory bodies such as the FDA would either prevent us from completing clinical studies or prevent us from obtaining regulatory approval for marketing, either of which would significantly compromise our business prospects.
If we, or if our service providers or any third party manufacturers, fail to comply with regulatory requirements , we or they could be subject to enforcement actions, which could adversely affect our ability to market and sell our prospective therapeutic products.
If we, or if our service providers or any third party manufacturers fail to comply with applicable federal, state or foreign laws or regulations, we could be subject to enforcement actions, which could adversely affect our ability to develop, market and sell our prospective therapeutic products successfully and could harm our reputation and lead to reduced acceptance of our prospective therapeutic products. These enforcement actions may include:
8
|
§
|
restrictions on, or prohibitions against, marketing our prospective therapeutic products;
|
|
§
|
restrictions on importation of our prospective therapeutic products;
|
|
§
|
suspension of review or refusal to approve new or pending applications;
|
|
§
|
suspension or withdrawal of product approvals;
|
|
§
|
product seizures;
|
|
§
|
injunctions; and
|
|
§
|
civil and criminal penalties and fines.
|
If we do not comply with laws regulating the protection of the environment and health and human safety, our business prospects could be adversely affected.
Our research and development activities may involve the use of hazardous materials, chemicals and various radioactive compounds, and we may maintain quantities of various flammable and toxic chemicals in our facilities in Israel. We may not have the requisite safety procedures in place to handle and dispose of these materials and any such procedures that we have or may have in place to comply with applicable regulations cannot eliminate the risk of accidental contamination or injury. If an accident occurs, we may be held liable for resulting damages which could be substantial. We also may be subject to numerous environmental, health and workplace safety laws and regulations including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of bio hazardous materials. We may incur substantial costs to comply with, and substantial fines or penalties if we violate any of these laws or regulations, which could significantly compromise our business prospects.
Risks Related to Competition and Commercialization of Our Prospective Therapeutic Products
The pharmaceutical and biotechnology market is highly competitive. If we are unable to compete effectively with existing products, new treatment methods and new technologies, we may be unable to commercialize any therapeutic products that we may develop in the future.
The biotechnology market is highly competitive, is subject to rapid technological change and is significantly affected by existing rival drugs and medical procedures, new product introductions and the market activities of other participants. Pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations may pursue the research and development of technologies, drugs or other therapeutic products for the treatment of some or all of the diseases that we are targeting or that we expect to target including superficial bladder carcinoma , and pancreatic and ovarian cancer. We also may face competition from products which have already been approved and accepted by the medical community for the treatment of these same indications. We believe a significant number of products are currently under development, and may become commercially available in the future, for the treatment of some or all of the diseases that we are targeting or that we expect to target including superficial bladder carcinoma , and pancreatic and ovarian cancer. We know of two products undergoing Phase III clinical testing for bladder cancer, namely Apaziquone (a bioreductive prodrug and a chemical analog of the chemotherapeutic agent mitomycin C with antineoplastic and cytotoxic activities) and Urocidin (which is designed to inhibit cancer cell division, induce apoptosis and stimulate anticancer cytokine synthesis).
Our competitors may develop products more rapidly or more effectively than us. Many of our competitors have:
|
§
|
much greater experience and much greater financial, technical and human resources than we have at every stage of the discovery, development, manufacture and commercialization process;
|
|
§
|
more extensive experience in pre-clinical testing, conducting clinical trials, obtaining and maintaining regulatory approvals and in manufacturing and marketing therapeutic products;
|
|
§
|
products that have been approved or are in late stages of development;
|
|
§
|
established distribution networks;
|
|
§
|
collaborative arrangements in our target markets with leading companies and research institutions; and
|
|
§
|
entrenched and established relationships with healthcare providers and payors.
|
As a result of any of the foregoing factors, our competitors may develop or commercialize products with significant advantages over any therapeutic products that we may develop. If our competitors are more successful in commercializing their products than us, their success could adversely affect our competitive position and harm our business prospects.
Even if we receive regulatory approval to market our prospective therapeutic products, the market may not be receptive to our prospective therapeutic products upon their commercial introduction which will prevent us from becoming profitable.
We may have difficulties convincing the medical community and third party payors to accept and use any of our prospective therapeutic products that may be approved for commercialization in the future. Key participants in pharmaceutical marketplaces, such as physicians, third party payors and consumers may not accept therapies utilizing the target genes. Even if such therapies are accepted by these participants, the medical community may not consider effectiveness and safety alone as a sufficient basis for prescribing our prospective therapeutic products in lieu of other alternative treatment methods and medications that are available. Other factors that we believe will affect market acceptance of our prospective therapeutic products include:
9
|
§
|
the timing of our receipt of any marketing approvals, the terms of any approvals and the countries in which approvals are obtained;
|
|
§
|
the safety, efficacy and ease of administration;
|
|
§
|
the success of physician education programs;
|
|
§
|
the availability of government and third party payor reimbursement;
|
|
§
|
the pricing of our prospective therapeutic products, particularly as compared to alternative treatment methods and medications; and
|
|
§
|
the extent to which alternative treatment methods and medications are more readily available as compared to the availability of any prospective therapeutic products that we may develop in the future.
|
Any therapeutic products that we may develop may become subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, thereby adversely affecting the profitability of our business.
The regulations that govern pricing for new medical products vary widely from country to country. As a result, we might obtain regulatory approval for a product in a particular country, but then be subjected to pricing regulations in that country that delay our commercial launch of the product and negatively impact the revenues we are able to generate from the sale of the product in that country. In addition, our ability to commercialize any approved products successfully will depend in part on the extent to which reimbursement for these products will be available from government health administration authorities, private health insurers and other organizations. Even if we succeed in bringing one or more therapeutic products to the market, these products may not be considered cost-effective, and the amount reimbursed for any products may be insufficient to allow us to sell our therapeutic products on a competitive basis. If the price we are able to charge for any therapeutic products that we develop and for which we obtain regulatory approval is inadequate in light of our development and other costs, our profitability could be adversely affected.
Risks Related to Our Dependence on Third Parties
We may not be able to execute our business strategy if we are unable to enter into or maintain our collaborations with other companies that can provide capabilities and funds for the development and commercialization of our prospective therapeutic products.
We do not have any capability for sales, marketing or distribution and have limited capabilities for product development including obtaining regulatory approval of any therapeutic product that we may develop. Accordingly, our current business strategy is to enter into collaborations with major pharmaceutical, biotechnology and other companies to jointly develop specific product candidates and to jointly commercialize them if they are approved. In such collaborations, we would expect our collaborators to provide substantial capabilities in clinical development, regulatory affairs, marketing and sales. We cannot guarantee whether and to what extent we will be able to enter into collaborative arrangements to develop and commercialize our prospective therapeutic products and to fund such development and commercialization, and any such collaborative arrangement that we may enter into may include terms and conditions that are unfavorable to us and we may be unsuccessful in maintaining any such collaborations that we enter into, which could significantly compromise our business prospects.
We rely on a limited number of third parties for the supply of the component polyethylenimine and other raw materials required for our research and development activities and if we are unable to reach agreements with these third parties or if we are unable to maintain our contractual relationships with these third parties, our research and development activities would be delayed.
We rely on third parties for the provision of materials required for our research and development activities. Obtaining these materials requires various approvals as well as reaching a commercial agreement on acceptable terms with the provider of the materials. We may not be able to reach agreements with a sufficient number of suppliers or do so on terms acceptable to us. If we are unable to reach acceptable agreements with a sufficient number of suppliers of research materials, our research and development activities will be delayed and our ability to implement our business plan will be compromised.
If any collaborator terminates or fails to perform its obligations under agreements with us, the development and commercialization of our prospective therapeutic products could be delayed or terminated.
Our expected dependence on collaborators for capabilities and funding means that our business would be adversely affected if any collaborator terminates its collaboration agreement with us or fails to perform its obligations under any such agreement. Our current or future collaborations, if any, may not be scientifically or commercially successful. Disputes may arise in the future with respect to the ownership of rights to technology or therapeutic products developed with collaborators, which could have an adverse effect on our ability to develop and commercialize our prospective therapeutic products. If any collaborator terminates its collaboration with us, for breach or otherwise, it would be difficult for us to attract new collaborators and it could adversely affect how we are perceived in the business and financial communities. If any of these occur, the research, development and commercialization of one or more of our prospective therapeutic products could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
10
We have no manufacturing experience or resources and we must incur significant costs to develop this expertise or rely on third parties to manufacture our prospective therapeutic products.
We have no manufacturing experience. In order to develop products, apply for regulatory approvals and commercialize our prospective therapeutic products, we will need to develop, contract for or otherwise arrange for the necessary manufacturing capabilities. Manufacturing of our prospective therapeutic products must comply with the current Good Manufacturing Practice requirements set forth in the Quality System Regulation. The manufacturing process for our prospective therapeutic products is an element of the FDA approval process and we will need to contract with manufacturers who can satisfy the FDA requirements on an ongoing basis before we seek to obtain FDA approval. In addition, if we receive the necessary regulatory approval for any of our prospective therapeutic products, we also expect to rely on third parties, including our collaborators, to manufacture our therapeutic products in quantities sufficient for clinical trials and commercial marketing. We may experience difficulty in obtaining adequate manufacturing capacity for our commercial needs. If we are unable to obtain or maintain contract manufacturing for our prospective therapeutic products, or are unable to do so on commercially reasonable terms, we may not be able to successfully develop and commercialize our prospective therapeutic products.
Risks Related to Our Operations
If we are unable to retain Professor Abraham Hochberg, Dr. Patricia Ohana and other qualified key management and scientists, staff consultants and advisors, our ability to implement our business plan may be adversely affected.
Our success largely depends on the skills, experience and efforts of certain of our senior management. The loss of the service of any of these persons, including Professor Abraham Hochberg and Dr. Patricia Ohana, would likely significantly delay or prevent our achievement of product development and our other business objectives. Our employment agreements with our key personnel, including Professor Abraham Hochberg and Dr. Patricia Ohana, are terminable by the employee upon the provision of 90 days or three months advance written notice to us. We do not carry key man life insurance on any of our executive officers or other key employees. Should either of Professor Hochberg or Dr. Ohana cease his or her employment with us or should we otherwise lose his or her services, our ability to conduct our research and development activities would be significantly compromised.
We are exposed to a risk of substantial loss due to claims that may be filed against us in the future because our insurance policies may not fully cover the risk of loss associated with our operations.
We are exposed to the risk of having claims seeking monetary damages being filed against us for loss or harm suffered by participants of our clinical studies or for loss or harm suffered by users of any drug that may receive approval for commercialization in the future. In either event, the FDA or the regulatory authorities of other countries or regions may commence investigations of the safety and effectiveness of any such clinical trial or commercialized drug, the manufacturing processes and facilities or marketing programs utilized in respect of any such trial or drug, and may result in mandatory or voluntary recalls of any commercialized drug or other significant enforcement action such as limiting the indications for which any such drug may be used, or suspension or withdrawal of approval for any such drug. Investigations by the FDA or any other regulatory authority in other countries or regions also could delay or prevent the completion of any of our other clinical development programs. In the event that we are required to pay damages for any such claim, we may be forced to seek bankruptcy or to liquidate because our asset and revenue base may be insufficient to satisfy the payment of damages and any insurance that we have obtained or may obtain for product or clinical trial liability may not provide sufficient coverage against potential liabilities. Our current clinical trials insurance policy provides coverage in the amount of up to $3,000,000 in the aggregate .
Risks Related to Government Regulation
We may be subject to U.S. federal and state and also foreign healthcare fraud and abuse laws and regulations and other regulatory reforms, and a finding of our failure to comply with such laws, regulations and reforms could have a material adverse effect on our business.
Our operations may be directly or indirectly affected by various broad U.S. federal and state healthcare fraud and abuse laws. These include the U.S. federal anti-kickback statute, which prohibits any person from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in return for or to induce the referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of an item or service, for which payment may be made under U.S. federal healthcare programs, such as the Medicare and Medicaid programs. The U.S. federal anti-kickback statute is very broad in scope, and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, many states have adopted laws similar to the U.S. federal anti-kickback statute, and some of these laws are broader than that statute in that their prohibitions are not limited to items or services paid for by a U.S. federal healthcare program but, instead, apply regardless of the source of payment. Violations of these laws could result in fines, imprisonment or exclusion from government-sponsored programs.
11
Risks Related to Our Intellectual Property and Potential Litigation
Our exclusive license to certain patents will expire in 2017 and we may face increased competition from third parties who will be able to license the patents that we have used in our research and development activities.
Some of the patents underlying the exclusive license that Yissum Research Development Company of the Hebrew University of Jerusalem (Yissum) has granted to us, will expire beginning in 2017, after which time, any one or more of our competitors could develop generic alternatives to our prospective drugs , to the extent that these are not subject to additional protections, such as orphan drug status .
We are required, and may be required in the future, to license patent rights from third party owners in order to develop our prospective therapeutic products in the future. If we cannot obtain such licenses or if such owners do not properly maintain or enforce the patents underlying such licenses, our competitive position and business prospects will be harmed.
We currently license technologies and other patents in conducting our research and development activities and we may be required to obtain additional licenses in the future in the event that we believe it is necessary or useful for our business and our research and development efforts to use third party intellectual property or if our efforts would infringe upon the intellectual property rights of third parties.
Additionally, should we succeed in obtaining any such license, our business prospects will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed intellectual property, in particular, those patents for which we secured exclusive rights such as the exclusive license granted to us by Yissum in connection with our research on the H19 gene. Our licensors may terminate our license, may not successfully prosecute or may fail to maintain their patent applications to which we are licensed, may determine not to pursue litigation against other persons that are infringing these patents or may pursue such litigation less aggressively than we would. Without protection for the intellectual property that we have been licensed and that we may obtain licenses in the future, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive position and harm our business prospects.
If we fail to comply with our obligations under our license with Yissum or other licenses or related agreements that we are a party to and that we may enter into in the future, we could lose license rights that may be necessary for developing our target gene-based therapeutic products.
The exclusive license granted to us by Yissum and any license that we may enter into in the future in connection with our efforts to develop drugs utilizing the target genes may impose various development, commercialization, funding, royalty, diligence, sublicensing, insurance and other obligations on us. Our obligations under any of these license agreements could include, without limitation:
|
§
|
royalty payments;
|
|
§
|
annual maintenance fees;
|
|
§
|
providing progress reports;
|
|
§
|
maintaining insurance coverage;
|
|
§
|
paying fees related to prosecution, maintenance and enforcement of patent rights;
|
|
§
|
minimum annual payments; and
|
|
§
|
performing commercially reasonable diligent efforts to develop and to introduce therapeutic products into the commercial market as soon as practicable.
|
If we were to breach any of our material obligations as described below, the licensor may have the right to terminate the license which could result in our being unable to develop, manufacture and sell products that are covered by the licensed technology or a competitor’s gaining access to the licensed technology. Under the terms of the exclusive license granted to us by Yissum, Yissum has the right to terminate the license in the event that we become bankrupt or insolvent, or if our business is placed in the hands of a receiver, assignee or trustee. In addition, Yissum has the right to terminate the license for any material breach of the license by us in the event that we fail to remedy such material breach within ninety days of Yissum’s notice of our material breach and its intent to terminate, provided that the material breach is curable within ninety days. In the event that the material breach cannot be remedied within ninety days, Yissum may not terminate the license if we take reasonable commercial action to cure such breach as promptly as practicable. The Israeli Contract Law (Remedies for Breach of Contract) — 1970, defines the term “material breach” as a breach, with regards to which, it may be assumed that a reasonable person would not have entered into the specific agreement had that person foreseen the breach and the outcome thereof, or a breach which is specifically defined as material in the agreement. Acts which may constitute a material breach of the license agreement by us may include, for example: the granting of sublicenses not in compliance with the provisions of the license agreement, a breach of our obligations to pay royalties and provide the necessary reports with respect thereto, a breach of our obligations not to disclose or misuse certain confidential information of Yissum, and a breach of our obligations to develop and commercialize the licensed technology (including our obligation to fund certain research and development activities) and to conduct patent prosecution and maintenance, as further described below.
12
We have agreed to provide research and development funding to Yissum in connection with the license, which we may terminate upon 90 days prior written notice to Yissum. In such event, we are required to compensate Yissum for all expenses incurred by it prior to the notification date in connection with its research efforts and all additional expenses that Yissum had assumed the obligation to cover prior to the notification date. The research and development funding was initially set for a period of two years with the possibility to extend the term by mutual agreement. We have been extending the funding period on a yearly basis, and it is currently valid until December 2010 .
In addition, we have agreed to prepare, register and maintain any patent application or patent that may arise out of our research and development efforts pursuant to our license with Yissum and to bear all expenses of preparation, registration and maintenance. We agreed to keep Yissum informed of filing and prosecutions pursuant to the agreement, including submission of copies of all official actions, relevant correspondence, applications, continuations or like proceedings, and responses thereto. We agreed to consult Yissum regarding any abandonment of the prosecution of patent applications arising out of the license. In the event that we decide not to commence or continue the process of patent registration in a certain country, we must notify Yissum of this decision. Yissum may then individually prepare, register and maintain any such patent. We must inform Yissum of our desire to assume the expenses incurred by Yissum in connection with its patent registration within 90 days from the date in which Yissum notifies us of its decision to prepare, register and maintain such patent. In the event that we decide not to assume these expenses, or in the absence of our reply within the above 90 day period, the exclusive, worldwide license granted to us by Yissum will no longer be applicable in such countries in which we elected not to file or to abandon the filing, prosecution or maintenance of patents pursuant to the license.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
We currently rely and intend to rely in the future on trade secrets, know-how and technology that are not protected by patents to maintain our competitive position. In order to protect our proprietary technology and processes, we also rely in part on confidentiality agreements with our collaborators, employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such party. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive position and harm our business prospects.
If we are not able to obtain and enforce patent protection for our inventions, our ability to develop and commercialize our prospective therapeutic products will be harmed.
Our success depends, to a considerable extent, on our ability to protect proprietary methods and technologies that we develop under the patent and other intellectual property laws of the United States and other countries, so that we may prevent others from unlawfully using our inventions and proprietary information. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the U.S. Patent and Trademark Office, or PTO, and its foreign counterparts use to grant patents are not always applied predictably or uniformly and may change. There also is no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. Even if our rights are not directly challenged, disputes among third parties could lead to the weakening or invalidation of our intellectual property rights. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed with respect to any patents issued to us or to others. Additionally, the mere issuance of a patent does not guarantee that it is valid or enforceable against third parties.
A third party may sue us for infringing its patent rights. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of third party proprietary rights. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be significant, and the litigation would divert our management’s efforts. From a financial perspective, there is a risk that we would not be able to sustain the costs of any such litigation and would be forced to seek bankruptcy or to liquidate because of our limited asset and revenue base.
13
Risks Related to Our Operations in Israel
If there are significant shifts in the political, economic and military conditions in Israel, it could have a material adverse effect on our operations.
Our operations will be directly influenced by the political, economic and military conditions affecting Israel at any given time. A change in the security and political situation in Israel and in the economy could impede the raising of the funds required to finance our research and development plans, to create joint ventures with third parties and could otherwise have a material adverse effect on our business, operating results and financial condition. Although Israel has entered into various agreements with Egypt, Jordan and the Palestinian Authority, there have been times since October 2000 when Israel has experienced an increase in unrest and terrorist activity. The establishment in 2006 of a government in the Palestinian Authority by representatives of the Hamas militant group has created additional unrest and uncertainty in the region. In mid-2006, there was a war between Israel and the Hezbollah in Lebanon, resulting in thousands of rockets being fired from Lebanon up to 50 miles into Israel. In January 2009, Israel attacked, during three weeks, Hamas strongholds in the Gaza strip, in reaction to rockets that were fired from Gaza up to 25 miles into Israel. Major hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners could result in damage to our facilities and likewise have a material adverse effect on our business, operating results and financial condition. Furthermore, several countries restrict business with Israeli companies. This may impair our ability to sell our prospective therapeutic products in certain countries.
Our operations could be disrupted as a result of the obligation of our personnel to perform military service.
All of our executive officers and key employees of our business reside in Israel and some are required to perform annual military reserve duty and may be called for active duty under emergency circumstances at any time. Our operations could be disrupted by the absence for a significant period of time of one or more of these officers or key employees due to military service. Any such disruption could adversely affect our business, results of operations and financial condition.
Because a substantial portion of our revenues is expected to be generated in U.S. Dollars and Euros, our potential revenue may be reduced due to currency exchange rate fluctuations.
In the future, we expect that a substantial portion of our revenues will be generated in U.S. Dollars and Euros. Our financial records are maintained in New Israel Shekels, which is the functional currency of the Company. Our financial statements are prepared using the U.S. dollar as the reporting currency. As a result, our financial results might be affected by fluctuations in the exchange rates of currencies in the countries in which our prospective therapeutic products may be sold. Furthermore, we will likely be exposed to the risk that the rate of inflation in Israel will exceed the rate of devaluation of the Israeli currency in relation to the U.S. Dollar or the Euro, or that the timing of this devaluation will lag behind inflation in Israel. Because inflation has the effect of increasing the U.S. Dollar and Euro costs of an Israeli company’s operations, it would have an adverse effect on our U.S. Dollar-measured results of operations.
Currency exchange controls may restrict our ability to utilize our cash flows.
We intend to receive proceeds from sales of any prospective therapeutic product we may develop and also to pay our operational costs and expenses in U.S. Dollars, Euros and other foreign currencies. However, we may be subject to existing or future rules and regulations on currency conversion. In 1998, the Israeli currency control regulations were liberalized significantly, and there are currently no currency controls in place. Legislation remains in effect, however, pursuant to which such currency controls could be imposed in Israel by administrative action at any time. We cannot assure you that such controls will not be reinstated, and if reinstated, would not have an adverse effect on our operations.
The ability of any Israeli company , such as our wholly-owned subsidiary, to pay dividends is subject to Israeli law and the amount of cash dividends payable may be subject to devaluation in the Israeli currency.
The ability of an Israeli company , such as our wholly-owned subsidiary, to pay dividends is governed by Israeli law, which provides that cash dividends may be paid only out of retained earnings as determined for statutory purposes in Israeli currency. In the event of a devaluation of the Israeli currency against the U.S. Dollar, the amount in U.S. Dollars available for payment of cash dividends out of prior years’ earnings will decrease. Cash dividends paid by an Israeli corporation to a United States resident corporate parent are subject to the Convention for the Avoidance of Double Taxation between Israel and the United States. Under the terms of the Convention, such dividends are subject to taxation by both Israel and the United States. Any change in these laws or regulations could adversely affect our ability to declare and pay dividends.
14
The termination or reduction of tax and other incentives that the Israeli Government provides to domestic companies, such as our wholly-owned subsidiary BioCancell Therapeutics Israel Ltd., may increase the costs involved in operating a company in Israel.
The Israeli Government currently provides tax and capital investment incentives to domestic companies, as well as grant and loan programs relating to research and development and marketing and export activities. In recent years, the Israeli Government has reduced the benefits available under these programs and the Israeli Governmental authorities have indicated that the government may in the future further reduce or eliminate the benefits of those programs. Our wholly-owned Israeli subsidiary, BioCancell Therapeutics Israel Ltd., currently takes advantage of these programs. We cannot assure you that such benefits and programs would continue to be available in the future to BioCancell Therapeutics Israel Ltd. If such benefits and programs were terminated or further reduced, it could have an adverse affect on our business, operating results and financial condition.
Any Israeli government grants that we receive for research and development expenditures limit or prohibit our ability to manufacture products and transfer know-how outside of Israel and require us to satisfy specified conditions.
We receive grants from the Israeli government through the Office of the Chief Scientist of the Ministry of Industry, Trade and Labor , for the financing of a significant portion of our research and development expenditures. Israeli law requires that the manufacture of products developed with government grants be carried out in Israel , unless the OCS approves otherwise. Such approval, if given, is generally conditioned on an increase in the total amount to be repaid to the OCS, to an amount of up to 300% of the funds received , plus interest (the exact amount would depend on the extent of the manufacturing to be conducted outside of Israel ) . The restriction on manufacturing outside of Israel does not apply to the extent that plans to manufacture were disclosed when application for funding was filed. Transfer of OCS-funded technologies and know-how outside of Israel is restricted , unless conducted in accordance with the restrictions set forth under Israeli law. Israeli law further specifies that both the transfer of know-how as well as the transfer of intellectual property rights in such know-how are subject to the same restrictions , namely the approval of the OCS. Such approval, if given, might be subject to special reimbursement which may be required to be paid to the Government of Israel.
If we fail to comply with the conditions imposed by the Office of the Chief Scientist, including the payment of royalties with respect to grants received, we may be subject to certain sanctions which are set forth under Israeli law, including the possible refund of any payments previously received, together with interest and penalties. The difficulties in obtaining the approval of the OCS for the transfer of manufacturing rights , technology, know-how and intellectual property rights in such know-how, outside of Israel , could prevent us from entering into strategic alliances or other transactions that provide for such a transfer which in turn could adversely affect our business, results of operations and financial condition.
Our stockholders generally may have difficulties enforcing a U.S. judgment against us, our executive officers and directors and some of the experts named in this prospectus, or asserting U.S. securities law claims in Israel.
It may be difficult for our stockholders to effect service of process on some or all of our executive officers, directors and the experts named in this prospectus. Furthermore, most of our assets and most of the assets of our executive officers and directors and some of the experts named in this prospectus are located outside the United States. Therefore, a judgment obtained against us or any of them in the United States, including one based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to assert U.S. securities law claims in original actions instituted in Israel.
Even if an Israeli court agrees to hear such a claim, it may determine that Israeli, and not U.S., law is applicable to the claim. Under Israeli law, if U.S. law is found to be applicable to such a claim, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would be governed by Israeli law. There is little binding case law in Israel addressing these matters.
Furthermore, Israeli courts might not enforce judgments rendered outside Israel, which may make it difficult to collect on judgments rendered against us. Subject to certain time limitations, an Israeli court may declare a foreign civil judgment enforceable only if it finds that:
|
§
|
the judgment was rendered by a court which was, according to the laws of the state of the court, competent to render the judgment;
|
|
§
|
the judgment may no longer be appealed;
|
|
§
|
the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and
|
|
§
|
the judgment is executory in the state in which it was given.
|
Even if these conditions are satisfied and subject to exceptional cases, an Israeli court will not enforce a foreign judgment if it was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts or if its enforcement is likely to prejudice the sovereignty or security of the State of Israel. An Israeli court also will not declare a foreign judgment enforceable if:
15
|
§
|
the judgment was obtained by fraud;
|
|
§
|
there is a finding of lack of due process;
|
|
§
|
the judgment was rendered by a court not competent to render it according to the laws of private international law in Israel;
|
|
§
|
the judgment is at variance with another judgment that was given in the same matter between the same parties and that is still valid; or
|
|
§
|
at the time the action was brought in the foreign court, a suit in the same matter and between the same parties was pending before a court or tribunal in Israel.
|
Risks Related to Our Units and this Offering
We are subject to certain provisions of the Israeli Securities Law — 1968 and the Israeli Companies Law — 1999 that differ from the default provisions generally applicable to Delaware corporations, which provisions we have also adopted in our Amended and Restated Certificate of Incorporation; certain of these provisions may be unenforceable and stockholder rights may be adversely affected.
As a result of the listing of our securities on the TASE, we are subject to certain provisions of the Israeli Securities Law — 1968. Pursuant to Section 39A of the Israeli Securities Law — 1968, rules and regulations of the Israeli Companies Law — 1999 listed in the Fourth Schedule apply to issuers incorporated in jurisdictions outside Israel which offer securities to the public in Israel. Our Amended and Restated Certificate of Incorporation contains certain provisions addressing these matters of Israeli law that materially differ from the default provisions included in the Delaware General Corporation Law. While Delaware permits companies to adopt provisions that differ from the default Delaware provisions, certain of the provisions which apply to us pursuant to Israeli law and which are contained in our Amended and Restated Certificate of Incorporation may be inconsistent with Delaware public policy and may not be upheld by Delaware courts in the event of a dispute.
In addition, pursuant to Section 39A of the Israeli Securities Law, we are subject to a number of other provisions of the Israeli Companies Law which are also addressed in our Amended and Restated Certificate of Incorporation, including provisions applicable to:
|
§
|
our Chairman of the Board of Directors and our Chief Executive Officer (Sections 95 and 121(c) of the Israeli Companies Law)
|
|
§
|
proxy statements required under the Israeli Companies Law (Sections 87 and 89 of the Israeli Companies Law);
|
|
§
|
our Audit Committee (Sections 114 to 117 of the Israeli Companies Law), our Internal Auditor (Sections 146 to 153 of the Israeli Companies Law);
|
|
§
|
derivative claims and class action lawsuits (Sections 194 to 218 of the Israeli Companies Law), independent directors (Sections 239 to 249(a) of the Israeli Companies Law);
|
|
§
|
duties of officers (Sections 252 to 256 of the Israeli Companies Law); and
|
|
§
|
transactions with controlling stockholders (Sections 270(d) and 275 to 282 of the Israeli Companies Law and all regulations promulgated thereunder).
|
The provisions of Israeli law addressed in our Amended and Restated Certificate of Incorporation are only applicable to the extent permitted by Delaware law. In the event of a conflict between the provisions in our Amended and Restated Certificate of Incorporation and Delaware law, Delaware law will prevail. Therefore, stockholders may possess fewer rights than are enumerated in our Amended and Restated Certificate of Incorporation.
The obligations associated with being an independent public company require significant resources and management attention.
As a reporting company, we are subject to the reporting requirements of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Sarbanes-Oxley Act of 2002. We, like all other public and reporting companies, incur additional expenses and, to a lesser extent, application of our management’s time, in our efforts to comply with our reporting obligations under the Exchange Act to file annual, quarterly and current reports and to comply with Section 404 of the Sarbanes-Oxley Act of 2002 regarding internal controls over financial reporting. Our failure to prepare and disclose this information in a timely manner could subject us to penalties under federal securities laws, expose us to lawsuits and restrict our ability to access financing.
Pursuant to an exemption afforded to new registrants , we have not yet evaluated our internal controls over financial reporting in order to allow management to report on, and our independent auditors to attest to, our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act of 2002 and the rules and regulations of the SEC, which we collectively refer to as “Section 404”. We will be required to include our Section 404 management’s assessment of internal control over financial reporting beginning with our annual report for the year ending December 31, 2010. Pursuant to current SEC rules, we will be required to include our independent auditor’s attestation on management’s report on internal control over financial reporting beginning with our first annual report for the fiscal year ending on or after December 15, 2010.
We intend to comply with the Section 404 management assessment of internal control over financial reporting beginning with our annual report for the year ending December 31, 2010. However, our lack of familiarity with Section 404 may divert management’s time and resources away from executing our business plan. If, in the future, management identifies one or more material weaknesses, or our external auditors are unable to attest that our management’s report is fairly stated or to express an opinion on the effectiveness of our internal controls, this could result in a loss of investor confidence in our financial reports, have an adverse effect on our stock price and/or subject us to sanctions or investigation by regulatory authorities.
16
If our common stock is accepted for quotation on the OTC Bulletin Board, it may be thinly traded, so you may be unable to sell at or near ask prices or at all if you need to sell your shares to raise money or otherwise desire to liquidate your shares.
If our common stock is accepted for quotation on the OTC Bulletin Board, it may be thinly traded on the OTC Bulletin Board, meaning there would be a low volume of buyers and sellers of the shares. Thus, we will be required to undertake efforts to develop market recognition for us and support for our shares of common stock in the public market. The price and volume for our common stock that will develop cannot be assured. The number of persons interested in purchasing our common stock at or near ask prices at any given time, may be relatively small or non-existent. This situation may be attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be periods of several days, weeks or months when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price.
If our common stock is accepted for quotation on the OTC Bulletin Board, while we are trading on the OTC Bulletin Board, the trading volume we develop may be limited by the fact that many major institutional investment funds, including mutual funds, as well as individual investors follow a policy of not investing in OTC Bulletin Board stocks and certain major brokerage firms restrict their brokers from recommending OTC Bulletin Board stocks because they are considered speculative, volatile and thinly traded. We cannot give you any assurance that an active public trading market for our common stock will develop or be sustained in the U.S.
The application of the “penny stock” rules to our common stock could limit the trading and liquidity of the common stock, adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
If our common stock is accepted for quotation on the OTC Bulletin Board, as long as the trading price of our common stock is below $5 per share, the open-market trading of our common stock will be subject to the “penny stock” rules, unless we otherwise qualify for an exemption from the “penny stock” definition. The “penny stock” rules impose additional sales practice requirements on certain broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). These regulations, if they apply, require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the associated risks. Under these regulations, certain brokers who recommend such securities to persons other than established customers or certain accredited investors must make a special written suitability determination regarding such a purchaser and receive such purchaser’s written agreement to a transaction prior to sale. These regulations may have the effect of limiting the trading activity of our common stock, reducing the liquidity of an investment in our common stock and increasing the transaction costs for sales and purchases of our common stock as compared to other securities.
The OTC Bulletin Board is a quotation system, not an issuer listing service, market or exchange. Therefore, buying and selling stock on the OTC Bulletin Board is not as efficient as buying and selling stock through an exchange.
The OTC Bulletin Board is a regulated quotation service that displays real-time quotes, last sale prices and volume limitations in over-the-counter securities. Because trades and quotations on the OTC Bulletin Board involve a manual process, the market information for such securities cannot be guaranteed. In addition, quote information, or even firm quotes, may not be available. The manual execution process may delay order processing and intervening price fluctuations may result in the failure of a limit order to execute or the execution of a market order at a significantly different price. Execution of trades, execution reporting and the delivery of legal trade confirmation may be delayed significantly. Consequently, one may not be able to sell shares of our common stock at the optimum trading prices.
When fewer shares of a security are being traded on the OTC Bulletin Board, volatility of prices may increase and price movement may outpace the ability to deliver accurate quote information. Lower trading volumes in a security may result in a lower likelihood of an individual’s orders being executed, and current prices may differ significantly from the price one was quoted by the OTC Bulletin Board at the time of the order entry.
Orders for OTC Bulletin Board securities may be canceled or edited like orders for other securities. All requests to change or cancel an order must be submitted to, received and processed by the OTC Bulletin Board. Due to the manual order processing involved in handling OTC Bulletin Board trades, order processing and reporting may be delayed, and an individual may not be able to cancel or edit his order. Consequently, one may not able to sell shares of common stock at the optimum trading prices.
17
The dealer’s spread (the difference between the bid and ask prices) may be large and may result in substantial losses to the seller of securities on the OTC Bulletin Board if the common stock or other security must be sold immediately. Further, purchasers of securities may incur an immediate “paper” loss due to the price spread. Moreover, dealers trading on the OTC Bulletin Board may not have a bid price for securities bought and sold through the OTC Bulletin Board. Due to the foregoing, demand for securities that are traded through the OTC Bulletin Board may be decreased or eliminated.
We expect volatility in the price of our common stock, which may subject us to securities litigation.
If established, the market for our common stock may be characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may in the future be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
As a new investor, you will experience immediate and substantial dilution.
Purchasers in this offering will immediately experience substantial dilution in net tangible book value. Because our common stock has in the past been sold at prices substantially lower than the initial public offering price that you will pay, you will suffer immediate dilution of $ per share in net tangible book value, based on an initial offering price of $ per share of common stock , the mid point of the range on the cover of the prospectus, assuming allocation of the entire purchase price to the common share component of the units, and after deducting the estimated offering expenses payable by us . The above calculation assumes no exercise of the warrants offered in this offering . The exercise of outstanding options and conversion of convertible notes may result in further dilution.
18
Risks Related to the Auction Process for Our Offering
The auction process may result in a phenomenon known as the “winner’s curse,” and, as a result, investors may experience significant losses.
The auction process may result in a phenomenon known as the “winner’s curse.” At the conclusion of the auction, bidders that receive allocations of units in this offering (successful bidders) may infer that there is little incremental demand for our shares above or equal to the public trading price. As a result, successful bidders may conclude that they paid too much for our units and could seek to immediately sell their shares to limit their losses should our stock price decline. In this situation, other investors that did not submit successful bids may wait for this selling to be completed, resulting in reduced demand for our common stock in the public market and a significant decline in our stock price. Therefore, we caution investors that submitting successful bids and receiving allocations may be followed by a significant decline in the value of their investment in our common stock shortly after our offering. Furthermore, although our shares of common stock are currently listed on TASE, there is limited trading in the shares of our common stock and therefore a limited public market in which an investor may seek to resell our shares.
The auction process may result in a situation in which less price sensitive investors play a larger role in the determination of our clearing price and constitute a larger portion of the investors in our offering, and, therefore, the offering price may not be sustainable.
In a typical public offering, a majority of the securities sold to the public are purchased by professional investors that have significant experience in determining valuations for companies. These professional investors typically have access to, or conduct their own, independent research and analysis regarding investments. Other investors typically have less access to this level of research and analysis, and as a result, may be less sensitive to price when participating in our auction process. Because of our auction process, these less price sensitive investors may have a greater influence in setting our offering price and may have a higher level of participation in our offering than is normal. This, in turn, could cause our auction process to result in an offering price that is higher than the price professional investors are willing to pay for our units. As a result, our stock price may decrease once trading of our common stock begins. Also, because professional investors may have a substantial degree of influence on the trading price of our shares over time, the price of our common stock may decline and not recover after our offering. Furthermore, if our public offering price is above the level that investors determine is reasonable for our shares, some investors may attempt to short sell the stock after trading begins, which would create additional downward pressure on the trading price of our common stock.
Successful bidders may receive the full number of units subject to their bids, so potential investors should not make bids for more units than they are prepared to purchase.
Successful bidders may be allocated all or almost all of the units that they bid for in the auction. Therefore, we caution investors against submitting a bid that does not accurately represent the number of units that they are willing and prepared to purchase.
If research analysts publish or establish target prices for our common stock that are below the offering price for our units or the then current trading market price of our shares, the price of our shares of common stock may fall.
Although the offering price of our units may have little or no relationship to the price determined using traditional valuation methods, we believe that research analysts will rely upon these methods to establish target prices for our common stock. If research analysts publish target prices for our common stock that are below the offering price of our units or the then current trading market price of our shares, our stock price may decline.
The mechanics of our bid process make it difficult for persons not having an account with an authorized entity at the time of the bid process to place a bid for our units.
We are conducting our bid process in compliance with the rules of the TASE and the Israeli Securities Authority. As a result of the manner in which we are conducting the offering and while the bidding process is open to everyone, bids must be submitted through authorized entities. For description of the offering mechanism, see “Plan of Distribution.” The need to submit a bid through an authorized entity makes it difficult and costly for persons not having an account with an authorized entity to bid on our units.
The amount of proceeds from this offering is dependent upon the outcome of the auction process.
The amounts set forth in the “Use of Proceeds” section indicate our proposed use of proceeds from this offering. Due to the nature of the auction process, the outcome of the auction will determine the proceeds of the offering and there is no assurance that we will be able to sell any units in this offering.
19
USE OF PROCEEDS
We are offering a maximum of units of our securities on a best efforts basis. The offering price per unit will be determined by an auction process as detailed in “Plan of Distribution – The Auction Process”. There is no assurance that we will sell the maximum number of units . The following table sets forth the uses of proceeds, assuming the sale of 25%, 50%, 75% and 100% of the securities offered for sale in this offering and assuming an offering price per unit of NIS (approximately $ ), which is the mid point of the range listed on the cover page of this prospectus. On June 10, 2010, the closing price of one share of our common stock was NIS 2.36. The proceeds included herein are the immediate proceeds of the offering, and do not include any proceeds that may be received from exercise of warrants in the future . Such exercise is not guaranteed, and we will not receive any warrant proceeds unless, and to the extent that, warrants are exercised. For further discussion see Management’s Discussion and Plan of Operation below.
| If 25% of Units are sold | If 50% of Units are sold | If 75% of Units are sold | If 100% of Units are sold | |||||||||||||
| U.S. dollars in thousands | ||||||||||||||||
|
Gross Proceeds
|
$ | $ | $ | $ | ||||||||||||
|
Less: Offering expenses
|
( | ) | ( | ) | ( | ) | ( | ) | ||||||||
|
Net proceeds from offering
|
||||||||||||||||
|
Use of Proceeds
|
||||||||||||||||
|
Research and Development expenses
|
||||||||||||||||
|
Funding clinical trials for superficial bladder carcinoma cancer
|
||||||||||||||||
|
Funding clinical trials for ovarian cancer
|
||||||||||||||||
|
Funding clinical trials for pancreatic cancer
|
||||||||||||||||
|
Funding expansion of our BC-819 drug candidate
|
||||||||||||||||
|
Further development of BC-821
|
||||||||||||||||
|
Sub-total
|
||||||||||||||||
|
Administrative expenses
|
||||||||||||||||
|
Funding for working capital and for general corporate purposes
|
||||||||||||||||
|
Sub-total
|
||||||||||||||||
|
TOTALS
|
$ | $ | $ | $ | ||||||||||||
The above figures represent only estimated costs and expenses.
Working capital and general corporate purposes include amounts required to pay salaries, professional fees, public reporting costs, office-related expenses and other corporate expenses, including interest and overhead. Pending these uses, we intend to invest most of the net proceeds from this offering in short-term, investment-grade, interest-bearing securities or deposits.
The timing and amounts of our actual expenditures will depend on several factors, including the timing of our entry into collaboration agreements, the progress of our clinical trials, the progress of our research and development programs, the results of other pre-clinical and clinical studies and the timing and costs of regulatory approvals. As a result, our management will have broad discretion in the allocation of the net proceeds of this offering for any purpose, and investors will be relying on the judgment of our management with regard to the use of these net proceeds.
Because there is no minimum offering amount required as a condition to closing this offering, we may sell less than all of the units offered hereby, which may significantly reduce the amount of proceeds received by us. In the case that the offering does not reach the maximum and the total proceeds are less than those indicated in the table, we will have the discretion to apply the available net proceeds to various indicated uses within the dollar limits established in the table above.
DIVIDEND POLICY
We do not anticipate paying any dividends on our common stock in the foreseeable future. We expect to retain future earnings, if any, for use in our development activities and the operation of our business. The payment of any future dividends will be subject to the discretion of our board of directors and will depend, among other things, upon our results of operations, financial condition, cash requirements, prospects and other factors that our board of directors may deem relevant. Additionally, our ability to pay future dividends may be restricted by the terms of any debt financing, tax considerations and Israeli law, which provides that dividends may be paid only out of retained earnings as determined for statutory purposes in Israeli currency.
20
DETERMINATION OF OFFERING PRICE
The price at which the units covered by this prospectus will be determined by an auction process is detailed in “Plan of Distribution – The Auction Process”. The minimum price in the offering and the exercise prices of the warrants will be announced by us at least five (5) TASE trading hours before the auction by filing a report with the Israeli Securities Authority and a current report on Form 8-K with the SEC. These parameters will be determined by our management based on management's assessment of, among others, prevailing market conditions, the historical performance of our stock on TASE and early bids received by institutional investors. See “Plan of Distribution – The Auction Process”.
Each Series 3 Warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Each Series 4 Warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ).
We cannot be sure that the public offering price will correspond to the price at which the common stock will trade in the public market following this offering or that an active trading market for the common stock will develop and continue after this offering.
For further details, please see “Plan of Distribution – The Auction Process”.
DILUTION
If you purchase shares of our common stock in this offering, your interest will be diluted to the extent of the difference between the public offering price per share and the net tangible book value per share of our common stock after this offering. Our net tangible book value as of March 31, 2010, was approximately ($2,434,000), or approximately ($0.12) per share. Net tangible book value per share is equal to total assets minus the sum of total liabilities and intangible assets divided by the total number of shares outstanding.
Dilution in net tangible book value per share to new investors represents the difference between the amount per share paid by purchasers of shares of our common stock in this offering and the net tangible book value per share immediately after completion of this offering. After giving effect to the sale of shares of our common stock in this offering at an assumed public offering price of $ per share, the mid point of the range listed on the cover page of this prospectus (assuming allocation of the entire purchase price to the common share component of the units), and after deducting estimated offering expenses, our net tangible book value as of March 31, 2010, would have been $ , or $ per share. This amount represents an immediate increase in net tangible book value to existing shareholders of $ per share and an immediate dilution in net tangible book value of $ per share to purchasers of shares of our common stock in this offering, as illustrated in the following table:
|
Assumed public offering price per share
|
$ | |||||||
|
Net tangible book value per share as of March 31, 2010
|
$ | (2,434,000 | ) | |||||
|
Increase in net tangible book value per share after giving effect to this offering
|
$ | |||||||
|
Pro forma net tangible book value per share as of March 31, 2010
|
$ | |||||||
|
Dilution in net tangible book value per share to new investors
|
$ | |||||||
This table assumes no exercise of outstanding options or warrants, conversion of outstanding convertible debentures and the exercise of the warrants offered in this offering. To the extent that options or warrants are exercised, or convertible debentures are converted, there may be further dilution to new investors.
The as adjusted information discussed above is illustrative only. Our net tangible book value following the completion of the offering is subject to adjustment based on the actual offering price and terms of this offering.
21
OUR BUSINESS
We are a development-stage biopharmaceutical company focused on the discovery, development and commercialization of novel therapies for treating cancer-related diseases. Our research and development activities build upon the research of Professor Abraham Hochberg of the Hebrew University of Jerusalem, who isolated the human H19 gene and determined that it is expressed in over forty different forms of cancer, including superficial bladder carcinoma , and pancreatic and ovarian cancer, while laying dormant and non-expressed in non-cancerous cells. Professor Hochberg’s research discovered that the H19 gene is significantly expressed in cancerous cells of adults. Research has also demonstrated that the H19 gene plays a significant role in the tumor development process by enabling tumor cells to survive under stress conditions, such as low serum and low oxygen levels, that are typical conditions of the environment in which cancerous cells develop. This survival supports the growth of the tumor and the development of metastases.
Recently-conducted research has led to significant scientific breakthroughs in the understanding of cancer, in particular with respect to the contribution of stem cells to the development of the disease. Stem cells possess the ability to regenerate themselves through mitotic cell division and differentiate into a diverse range of specialized cell types. Pluripotent stem cells have the potential to differentiate into any of the three germ layers: endoderm (interior stomach lining, gastrointestinal tract, the lungs), mesoderm (muscle, bone, blood, urogenital), or ectoderm (epidermal tissues and nervous system); thus, Pluripotent stem cells can give rise to any fetal or adult cell type. Pluripotent cells soon undergo further specialization into multipotent cells, which can give rise to several other limited cell types. A hematopoietic cell, for example, can develop into several types of blood cells, but cannot develop into muscle cells or other types of cells. Leading researchers have opined that adult multipotent stem cells are also the source of adult cancer stem cells, which are present in many, and are possibly in all, cancerous tumors. This theory is consistent with a number of other theories which are currently accepted by cancer researchers.
A significant problem with each of the current methods for treatment of cancer is the return of tumors. According to the new theory described above, even the most aggressive anti-cancer drugs, such as those used in chemotherapy, destroy the tumor cells, but in fact do not treat the source of the tumor — adult cancer stem cells. As a result, the theory postulates that anti-cancer drugs should treat adult cancer stem cells which are the source of returning tumors. In addition, according to the accepted theory among cancer researchers, the disease is created as a result of a long process of accumulation of genetic mutations. The fact that cells in most body tissues continuously undergo self-renewal, however, undermines the theory regarding the accumulation of genetic mutations. Only adult cancer stem cells, which are present in each tissue for many years, accumulate genetic mutations throughout the years, thus supporting the creation and return of tumors.
The research and understanding of the origin of cancer and metastases has progressed significantly in recent years. It is currently understood that embryogenesis, which is the process by which the embryo is formed and develops, and the process of the development of cancer, possess similar characteristics. The H19 gene is expressed and has a significant role in both processes. The process of formation of metastases is similar in its characteristics to epithelial mesenchymal transition, or EMT, which is a program of development of biological cells characterized by loss of cell adhesion, repression of E-cadherin expression, and increased cell mobility. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation. Similar to EMT, the process of formation of metastasis is also characterized by loss of cell adhesion, repression of E-cadherin expression, and increased cell mobility. Studies have demonstrated the involvement of the H19 gene in EMT.
In light of the recently achieved scientific breakthroughs in cancer research, and the role of the H19 gene in such processes, we believe that an anti-cancer drug based on the H19 gene has the potential to provide benefits that are competitive with existing treatment methods.
Our Prospective Drugs
We are currently focused on developing our prospective drug, BC-819. The detection of expression of the H19 gene is the biotechnological foundation of our potential therapy. Our development of BC-819 builds upon the research of Professor Hochberg who discovered that the H19 gene is a diagnostic marker for cancerous growths, through the identification of the expression of the H19 gene. Based upon these discoveries, we have developed BC-819, which is a double stranded DNA plasmid construct that incorporates the gene for diphtheria toxin (DTA) under the regulation of the H19 promoter sequence. The DTA gene is transcribed into a cell-killing DTA peptide only in cells that produce H19 RNA. The H19 regulatory gene, which is crucial to the growth of tumor cells, is utilized instead as the activator of the intra-cellular synthesis of DTA. The synthesis of this toxin peptide inhibits cellular protein synthesis, thereby causing the cancer cells to commit suicide. Once the BC-819 DNA enters an H19-positive cancer cell, the cell can offer no resistance and is marked for death. The net result of this mechanism is highly selective tumor cell destruction. The strong safety profile of the plasmid is due, in part, to the fact that it produces the “A” portion only of diphtheria toxin inside cancerous cells. This toxin lacks the ability to penetrate other cells provided by the “B” portion, and consequently only acts to destroy the cell in which it is produced. Therefore, this new therapeutic modality is specific for the H19-positive cancer cell and thus far has not been detected with known toxic effects on normal cells — a safety feature that is unique when compared to currently available cancer treatments.
22
BC-819 has also demonstrated potential with respect to combination therapy. Even in cells that produce small amounts of H19 regulatory RNA, it appears that BC-819 acts to reverse the resistance of the cancer cells to chemotherapeutic agents. Moreover, as a result of our therapeutic approach, the plasmid being used to deliver the cytotoxic DTA gene does not incorporate into the genome of the host. We believe that this has significant advantages over gene therapy platforms for cancer that depend on a “genetic correction” strategy.
We are developing two BC-819-based strategies. The first therapy is for bladder cancer wherein DTA-H19 is mixed with a transfection agent (to facilitate entry of BC-819 molecules into the cancer cells) for bladder instillation. The second BC-819 approach is for advanced stage cancers such as pancreatic and ovarian carcinomas in which plasmid DNA is injected directly into the tumor or instilled into the peritoneal cavity. Different routes of administration are employed depending upon the type of tumor (intravesical administration for bladder cancer, intratumoral injection for pancreatic and hepatocellular carcinoma and intraperitoneal administration for ovarian cancer with ascites). In addition, we are developing formulations for systemic administration for use in combination with intratumoral or hepatic artery infusions and other oncotherapeutic agents.
Pursuant to an agreement with Yissum Research Development Company of the Hebrew University of Jerusalem, which we refer to in this prospectus as “Yissum”, Yissum has granted to us an exclusive, worldwide license for the use, development and commercialization of the H19 gene in consideration of which we have agreed to pay certain royalties to Yissum described elsewhere in this prospectus.
Our primary strategic objective is to continue development of our prospective drug BC-819 for the treatment of superficial bladder carcinoma while broadening the scope of development to include additional applications.
We completed Phase I/IIa trials of BC-819 , designed for use in patients suffering from bladder carcinoma. The Phase I/IIa trials resulted in no severe adverse side effects directly attributable to the tested therapy. In March 2008, we began the FDA-approved Phase IIb trials for this therapy. In addition, we had a Pre-IND meeting with the FDA in April 2008, to discuss proposed clinical trial protocols for two additional uses of this therapy — ovarian and pancreatic cancer — which were approved and the proposed pre-clinical protocol designs (animal species and mode of administration) were accepted, subject to the completion of additional toxicology studies (pharmacokinetics and histopathology studies). These studies were successfully completed. We filed IND applications for final FDA approval of the clinical trials during December 2008. On January 8, 2009, we were entitled to commence a Phase I/IIa clinical trial for treatment of pancreatic cancer, as the FDA had not contacted us regarding potential concerns or objections to our IND application within 30 days of its submission. On July 21, 2009 , we received the approval of the Israeli Ministry of Health to commence the Phase I/IIa clinical trial for treatment of pancreatic cancer. We commenced such trial in Israel in August 2009. On January 12, 2009, we were entitled to commence Phase I/IIa clinical trials for treatment of ovarian cancer as the FDA had not contacted us regarding potential concerns or objections to our IND application within 30 days of its submission. We commenced the trial and began screening and treating patients in Israel in May 2009 and in the United States in June 2009. On August 20, 2009, the United States Department of Health and Human Services informed us that it has granted orphan drug status to BC-819 for its use in treating ovarian cancer. An “ orphan drug ” is a drug for a disease that affects a relatively small number of people in a population. In the U .S. , an orphan drug is defined as one that treats a disease affecting less than 200,000 people each year. In order to encourage the development of drugs for such diseases, benefits and incentives can be granted to the drug developers. The main standard benefit for orphan drugs in the U .S. is the right to market the drug exclusively for 7 years from the date it is approved. Additional benefits include tax benefits on R&D expenses, and waived FDA fees.
In addition to pursuing clinical trials of BC-819 for the treatment of superficial bladder carcinoma, pancreatic and ovarian cancer, we intend to continue our pre-clinical research related to the use of BC-819 for the treatment of various other forms of cancer as we seek to develop additional drugs based on various implementations of the biotechnology developed by Professor Hochberg. The following is a summary of some of our other research and development activities that are in the preliminary stage of laboratory research:
|
§
|
BC-820. BC-820 is a potential therapeutic product that activates both the synthesis of DTA and the protein Tumor Necrosis Factor (TNF) of the cytokine family. Like BC-819, BC-820 penetrates cancerous cells expressing the H19 gene and activates the synthesis of DTA and TNF in a targeted manner.
|
|
§
|
BC-821. BC-821 is a potential therapeutic product that penetrates cancerous cells and activates the synthesis of DTA only in cells expressing the P4 promoter of the target gene IGF2, which like the H19 target gene is expressed only in cancerous cells and not in non-cancerous cells. It thus has the potential to serve as a complementary product to BC-819, allowing the targeted destruction of cancer cells in a larger number of patients.
|
|
§
|
BC-822. This potential therapeutic product seeks to suppress the growth of cancerous cells by re-sequencing a cancer cell’s RNA by way of inserting a small interfering Ribonucleic Acid (siRNA) sequence that prevents the expression of the H19 gene. Like BC-819, the potential therapy would first penetrate cells expressing the H19 gene and then covalently attach the new siRNA sequence.
|
In pursuing our objectives, we may enter into strategic collaborations with third parties who have the expertise and the resources necessary for the performance of large scale clinical trials, who have well established marketing, distribution and manufacturing infrastructures or who have experience and expertise in preparing registrations with the FDA and other regulatory authorities for the receipt of marketing approval.
Potentially Treatable Diseases
Superficial Bladder Carcinoma. We believe that BC-819 may be effective in treating superficial bladder carcinoma. Superficial bladder carcinoma is a specific form of bladder cancer resulting from the development and progression of cancerous tumors within the urothelium layer. The H19 gene is expressed at high levels in areas afflicted by superficial bladder carcinoma.
Pancreatic Cancer. Cancerous cells may develop in either the exocrine cells or the endocrine cells of the pancreas and the H19 gene is expressed at high levels in these cancerous regions.
Ovarian Cancer. Ovarian cancer is a disease in which normal ovarian cells begin to grow in an uncontrolled, abnormal manner and produce tumors in one or both ovaries. The H19 gene is expressed at high levels in areas afflicted by ovarian cancer.
23
Our Process of Research and Development — Target Identification and Validation.
Target validation involves proving that DNA, RNA or a protein molecule is directly involved in a disease process and can be a suitable target for development of a new therapeutic drug. Drug target validation is among the most critical challenges facing pharmaceutical companies today. Our efforts have been conducted by a research team headed by our Chief Scientific Officer, Professor Hochberg at the Hebrew University in Jerusalem, and include the following milestones:
|
§
|
Diagnostics — Use of H19 as a Diagnostic and Prognostic Tool. Professor Hochberg’s research team has discovered that the H19 gene serves as a diagnostic marker for cancerous growths, through the identification of the expression of the H19 gene. A sensitive method called In-Situ Hybridization analysis (ISH) can detect even a single malignant cell expressing the H19 gene. ISH enables the detection of the expression of H19 in the examined tissue, while providing precise anatomic information regarding the location of the H19 presence in the tissue and cell. The detection of expression of the H19 gene is the biotechnological foundation of our potential therapy. The diagnostic marker enables the diagnosis of cancerous tumors in early stages. Such diagnosis supports the prognosis of the tumor.
|
|
§
|
Pre-Clinical Studies. Between the years 2000 – 2005, Professor Hochberg’s research team conducted extensive animal studies in BC-819. Plasmid was introduced into the bladders of bladder-carcinoma carrying rats (orthotropic model) and mice (heterotopic model). Significant tumor growth inhibition was observed after treatment. Non-good laboratory practices (GLP) toxicology studies were performed in mice and in rats (syngeneic and nude models). Between the years 2006 – 2007, Harlen Biotech Israel Ltd. conducted toxicology studies in rats and mice, in accordance with Good Laboratory Practices Regulations. The studies included repeated injections at increasing dosages into the abdominal cavity of mice and intravesical administration into the urinary bladder of rats. The equivalent dosage given to the animals was higher than the expected human dosage. No gross pathological findings were evident in the intravesical administration study, and mild to moderate side effects were evident in the intraperitoneal administration study. In recent experiments which were conducted in the laboratories of the University of Munich, lung cancer carrying mice were treated with BC-819. More tumor growth inhibition was observed in mice which were treated with BC-819 than in mice which did not receive treatment. We are currently reviewing the results of the study and will consider further development of this application subject to our available resources.
|
|
§
|
Compassionate Use Patients. BC-819 has been administered, under “compassionate use” provisions in Israel, to ovarian and liver cancer patients who had failed chemotherapy and were in the ultimate stages of cancer, and bladder cancer patients who had failed chemotherapy and were in the ultimate stages of cancer, and urinary bladder and renal pelvis transitional cell carcinoma (TCC) patients who had failed chemotherapy and were candidates for radical cystectomy and nephrectomy, respectively. In 2003, two patients with resistant bladder cancer were treated with BC-819. Prior to the BC-819 treatment, the patients underwent a transurethral resection, but the tumors returned. Both patients were treated by direct introduction of BC-819 into the bladder using a catheter. The BC-819 treatment resulted in a significant decrease of the superficial bladder tumor, no unwanted toxicity was demonstrated in healthy cells, no severe adverse side effects which can be related to the drug were diagnosed, and no plasmid was detected in the patients’ blood.
|
Two additional patients with very large metastases in their livers showed shrinkage of these tumors following treatment with BC-819 in 2004 and 2006. No unwanted toxicity was demonstrated in healthy cells and no severe adverse side effects which can be related to the drug were diagnosed.
A patient suffering from ovarian cancer characterized by intra-peritoneal distribution of metastases and ascites (liquid containing cancerous cells that builds up in the peritoneum as a result of the cancer) was treated with BC-819 from 2007, after the failure of conventional chemotherapy treatment. In the framework of the treatments, the patient received a number of different doses of BC-819 administered by intra-peritoneal infusions via catheter. The results indicated that the drug caused no serious adverse events at any dosage, and a decrease of 50% in the ovarian cancer marker protein CA-125 in the patient’s blood was measured, as well as a significant decrease in the number of cancerous cells in the ascites. The patient survived for 18 months after the commencement of treatment.
A patient suffering from TCC in his renal pelvis, who had previously undergone nephrectomy of his right kidney for this disease, and was a candidate for nephrectomy of his left kidney, was treated with BC-819 from the end of 2008. The results showed that no new growths were formed in the renal pelvis, and the treatment did not cause any serious adverse effects.
|
§
|
Phase I/IIa Clinical Trials. In 2006 – 2007, we conducted a Phase I/IIa, Dose-Escalation, Safety and Proof of Concept Study of BC-819 in Refractory Superficial Bladder Cancer. This study was designed to assess the safety and preliminary efficacy of BC-819 given by intravesical infusions into the bladder of 18 patients with superficial bladder cancer who had failed previous treatment. Escalating doses of 2 mg, 4 mg, 6 mg, 12mg and 20mg of BC-819 were utilized. No severe adverse side effects which can be related to the drug were diagnosed, other than in one case for which the reason was uncertain. This patient was hospitalized following complaint of urination urgency. The patient was released after two days and did not suffer additional adverse side effects during the treatment. We did not discover any dose limiting toxicity. As a result of the Phase I/IIa study, we concluded that the optimal dose to be used in Phase II trials would be 20mg.
|
At the beginning of the BC-819 treatment of patients in this study, all of the bladder tumors were removed, except for one (the diameter of which was 0.5 cm to 1 cm), which was left as a marker to gauge the influence of the treatment, despite the fact that the standard of care for bladder cancer patients involves removing all tumors. The parameters for examination of the initial efficacy include the reappearance of tumors, elimination or decrease in the size of the marker, and the aggravation of the disease. We examined efficacy in all patients participating in the trial, including patients who did not receive the optimal dose, although no control group was used in the trial as it is not the accepted practice to use a control group in a Phase I trial, whose primary purpose relates to the safety of the drug. Due to the small size of the trial and the absence of a control group, no p-values were calculated. Approximately 72% of the patients presented response to the treatment. The initial estimation of the drug’s efficacy indicates that it has the ability to eliminate or decrease the size of tumors, and to prevent the reappearance of new tumors. Approximately 56% of the patients finished the study without tumor recurrence. We detected reappearance of tumors mainly in patients who received doses which were substantially lower than the optimal dose. Intravesical administration of BC-819 resulted in complete ablation of the marker tumor without any new tumors in 4 of the 18 patients for a 22% overall complete response rate. The marker was eliminated, or reduced by at least 50%, in approximately 44% of the patients in the study. We detected only one patient with aggravation of the disease, meaning aggravation of the stage or the appearance of high grade tumors.
24
On March 6, 2008, we filed with the FDA a pre-IND application regarding future potential Phase I/IIa clinical trials for testing BC-819 for the treatment of pancreatic, ovarian and liver cancer. The purpose of the trials, as presented to the FDA, is to examine the safety and preliminary efficacy of BC-819, in a series of increased doses and various indications. At the Pre-IND meeting in April 2008, the proposed clinical trial protocols were approved, the proposed pre-clinical protocol design (animal species and mode of administration) was accepted but further toxicology studies were requested (more pharmacokinetics and histopathology studies), which studies were successfully completed. On January 8, 2009, we were entitled to commence a Phase I/IIa clinical trial for treatment of pancreatic cancer, as the FDA had not contacted us regarding potential concerns or objections to our IND application within 30 days of its submission. On July 21, 2009, we received the approval of the Israeli Ministry of Health to commence the Phase I/IIa clinical trial for treatment of pancreatic cancer. We commenced such trial in Israel in August 2009. The clinical trial is also being performed at the University of Maryland, Baltimore and will be partly funded by a $950,000 grant from the Israel-U.S. Binational Industrial Research and Development Fund. On January 12, 2009, we were entitled to commence Phase I/IIa clinical trials for treatment of ovarian cancer as the FDA had not contacted us regarding potential concerns or objections to our IND application within 30 days of its submission. We commenced the trial and began screening and treating patients in Israel in May 2009 and the United States in June 2009.
|
§
|
Phase IIb Clinical Trial. We filed an investigational new drug (IND) application with the FDA for the performance of a Phase IIb clinical trial with patients suffering from superficial bladder cancer who had failed previous treatment. The application was approved in January 2008, and we immediately began recruiting patients. The purpose of this trial is to measure the efficacy and safety of BC-819 at a dose of 20mg, as tested in the previous Phase I/IIa clinical trial. The trial is being conducted in a U.S.-based medical center in Arizona by BCG Oncology, PC and at seven medical centers in Israel. This two-stage trial includes 33 patients, divided into groups of 18 and 15. Each participant will receive six weekly treatments of BC-819. Patients responding to the treatment will be offered nine additional maintenance treatments. The tough FDA criteria have made patient recruitment a more difficult and time-consuming issue than was initially expected. Notwithstanding the foregoing, as clinical studies are currently ongoing, we are expected to continue gathering safety and efficacy data on larger subject populations. Subsequent studies, and additional data which we expect to obtain from additional research and development activities may not corroborate previous findings with respect to safety and efficacy, which were obtained during the studies previously conducted. The FDA alone will determine whether our BC-819 product is both safe and effective for commercial use in the United States after substantial additional clinical studies.
|
The two efficacy criteria that need to be met in order to determine a complete response in a patient, are non-recurrence and ablation of a single tumor left in each patient’s bladder, solely for the purpose of the clinical trial. Of these, we believe that non-recurrence is the major problem facing refractory superficial bladder cancer, and that this criterion is therefore of greater importance in determining what to expect from the treatment in market conditions.
In April 2010, we received interim results for this clinical trial. These showed that 15 patients out of 18 (83%) responded to treatment (either non-recurrence, tumor ablation or both), 10 patients (56%) showed non-recurrence, 9 patients (50%) showed tumor ablation, and 4 patients (22%) had a complete response (both non-recurrence and tumor ablation). No Serious Adverse Events (SAEs) related to the drug candidate were recorded in this (or any other) clinical trial of BC-819.
In total, six patients showed a complete response, of whom two were discounted from the final statistics – one that was excluded from the patient count because she discontinued treatment before it was completed, and one that was included in the patient count, but was not listed as a complete response on account of a change in the criteria during the course of the trial. The FDA had required five patients to show a complete response, and, when presented with the complete interim results, approved the continuation of the trial and the commencement of enrollment of the second and last treatment group (comprised of 15 patients).
The following chart illustrates the stages to which each of our potential therapies has been developed, reflecting the relative development status of each of our potential therapies (but does not represent the relative amounts invested by us in each initiative):
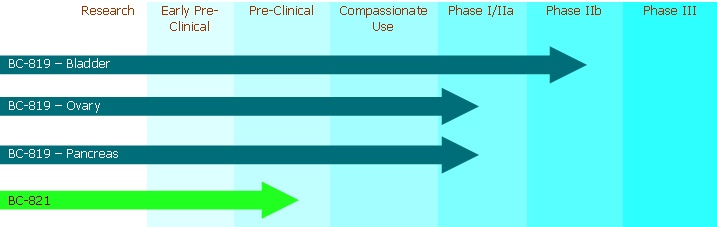
Competition for Our Prospective Therapeutic Products
Our principal competitors in the field of researching and developing drugs for the treatment of cancer types including superficial bladder carcinoma, pancreatic cancer and ovarian cancer, include biotechnology and multinational pharmaceutical companies. We also compete with research and academic institutions around the world in the race to discover genes, techniques and other patentable assets central to the research and development of drugs for the treatment of such diseases.
25
Of the cancer applications for which we are testing BC-819, the only application for which there is an efficacious drug on the market is bladder cancer. This market is thought to be relatively conservative. The current drugs of choice are BCG, a non-specific immunotherapeutic agent, with FDA's black box warning for side effects as well as the chemotherapy agent, Mitomycin C (approved in Europe). With current competition in all of our applications being chemotherapy and immunotherapy agents, both projected by mechanism to be toxic with a safety profile inferior to that of BC-819, there is a clear medical and market need for new agents such as BC-819, demonstrating improved safety profile.
To the best of our knowledge, there are a number of treatment methods for treating these cancer types that would compete with any drug that we may develop and commercialize, including our prospective drug BC-819, which include:
|
§
|
Surgery. Surgery is the most common treatment method for invasive cancerous growths related to bladder cancer. Transurethral resection is the surgical method that is most often utilized for the removal of superficial bladder cancer tumors.
|
For patients with pancreatic cancer, surgery offers the only known possibility of cure, but even this is effective in only a small number of patients (10 – 20%). Even when resection is possible, the median survival times of 13 – 25 months and five-year survival rates of 10 – 20% have been reported. Prognosis is poor because of a high rate of local recurrence and metastases despite resection.
Initial surgery is almost always necessary in the management of suspected ovarian cancer. A total abdominal hysterectomy and bilateral salpingo-oophorectomyare typically performed.
|
§
|
Radiation Therapy. Radiation therapy involves the use of X-rays to destroy cancerous cells.
|
|
§
|
Chemotherapy. Chemotherapy uses special anti-cancer drugs that destroy cancerous cells. For bladder cancer patients, chemotherapy is administered directly into the bladder using a catheter for patients who are in the early stages of superficial bladder carcinoma. Chemotherapy is otherwise administered intravenously when superficial bladder carcinoma has become invasive.
|
To the best of our knowledge, some of the more commonly prescribed drugs used in intravesical chemotherapy are: Mitomycin-C, which has not been approved by the FDA for the treatment of bladder cancer and is marketed by the Bristol-Myers Squibb Company and Supergene; Epirubicin, which is marketed by Pfizer, Inc.; and Doxorubicin, which is marketed by Ortho Biotech, Thiotepa (marketed by Bedford Laboratories) and Valrubicin (supplied by SYNCHEM OHG), which are approved by the FDA for the treatment of superficial TCC. To the best of our knowledge, some of the more commonly prescribed drugs used in intravenous chemotherapy are Taxol, Carboplatin, Cisplatin and Gemcitabine.
The chemotherapy treatment plan in pancreatic cancer includes the downstaging of the tumor by shrinking the tumor volume to the extent that vascular involvement is lessened and resection is then rendered possible. Recently, many investigators have reported the utility of chemotherapy using gemcitabine, in which early studies showed that patients experienced an improvement in disease-related symptoms. However, the median survival time of 5.65 months and the 12-month survival rate of 18% for gemcitabine-treated patients is considered by most experts to be disappointing. The combination of gemcitabine and irradiation cause both acute and late toxicity of the gastrointestinal tract.
The majority of patients with epithelial ovarian cancer will require chemotherapy following the operation in an attempt to eradicate residual disease. Platinum-based adjuvant treatment, or immune system stimulator, can reduce the risk of relapse in patients with early-stage ovarian cancer, resulting in disease-free survival of approximately 80% of patients. Intravenous administration of taxane- and platinum- based chemotherapy is the current standard of postoperative care for patients with advanced ovarian cancer. Platinum analogues, such as carboplatin and cisplatin, are the most active agents in this disease. In contrast, taxanes such as paclitaxel and docetaxel exert their cytotoxic effects through a unique mechanism of action involving binding to and stabilization of the tubulin polymer. Despite the improved median overall survival in patients with regimens such as paclitaxel and carboplatin, relapse still occurs in the majority of those with advanced disease, and only 10 to 30% of such patients have long-term survival.
|
§
|
Immunotherapy. Immunotherapy is a method that employs a patient’s immune system to fight cancerous cells. Although the precise biological mechanism of activation is unknown, the administration of Bacille Calmette Guerin (BCG) is used to treat a number of superficial bladder cancer types because BCG is believed to stimulate a patient’s immune system to thwart the growth of cancerous cells.
|
|
§
|
Interferon. Interferons are human proteins that are introduced into the human body in order to stimulate the host’s immune system to thwart the growth of cancerous cells. Although the precise biological mechanism of activation is unknown, it is believed that interferons impede or suspend the growth of cancerous cells, compromise the ability of cancerous cells to defend against the host’s immune system and strengthen the host’s immune system. Interferons have been administered in combination with BCG. The University of Iowa has conducted Phase III clinical trials to examine the relative effectiveness of the interferon/BCG combined therapy as compared to each of the stand alone treatments.
|
In addition to the foregoing, we are aware of two products undergoing Phase III clinical testing for bladder cancer, namely Apaziquone (a bioreductive prodrug and a chemical analog of the chemotherapeutic agent mitomycin C with antineoplastic and cytotoxic activities) and Urocidin (which is designed to inhibit cancer cell division, induce apoptosis and stimulate anticancer cytokine synthesis). We are also aware of several new treatment methods for superficial bladder carcinoma , including photodynamic treatment, which kills cancerous cells using laser, and synergo technology, which combines hypothermia with chemotherapeutical substances, that have not been approved by the FDA.
26
We are also aware of several drugs for the treatment of ovarian cancer in various stages of development, including: (i) Avastin, recombinant humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF), developed by Genentech; (ii) Tarceva, small molecule human epidermal growth factor type 1 or epidermal growth factor receptor (HER1/EGFR) inhibitor, developed by Roche Holdings Ltd; and (iii) epothilone B, a cytotoxic, novel tubulin polymerizing compound known as an epothilone which inhibits cancer cells with a similar mechanism as paclitaxel, developed by Novartis.
The results of our Phase I/IIa bladder carcinoma clinical study showed that the safety profile of BC-819 is excellent. The incidence and severity of adverse events in this small study population were lower than published results for BCG and chemotherapy. For instance in a typical Phase III study of BCG and epirubicin (de Reijke et al. 2005), adverse events included hematuria in 41% and 28% of patients treated with BCG or epirubicin, respectively, while only 11% of patients treated with BC-819 suffered hematuria. Severe dysuria was reported in 26% and 10% of patients treated with BCG and epirubicin, respectively, whereas there were no cases of severe dysuria reported for BC-819. No local reactions caused cessation of treatment with BC-819, whereas 26% and 9% of patients treated with BCG and epirubicin, respectively, had to have treatment stopped due to local toxicities. Using a plasmid with an expression cassette of the diphtheria toxin, no immune response will be encountered as happens when using an adenovirus; moreover, people born in Western countries are routinely immunized against this toxin. Further, our treatments are not affected by multi-drug resistance effects, a major problem in chemotherapy. Notwithstanding the foregoing, the FDA alone will determine whether our BC-819 product is both safe and effective for commercial use in the United States after substantial additional clinical studies.
Expenditures on Research and Development
From the time of our inception on July 26, 2004 through December 31, 2009 , we invested approximately $ 11,269 ,000 in our research and development activities. We have funded our research and development expenses from our own resources and from various non-diluting grants, including grants from the Office of the Chief Scientist of the Israeli Ministry of Industry, Trade and Labor (OCS).
Our aggregate research and development budgetary expenditures for our first year of OCS funding for superficial bladder carcinoma (July 2005 to June 2006) were NIS 2.734 million (approximately $850,000), of which NIS 1.225 million (approximately $380,000) was funded by the OCS. Our aggregate research and development budgetary expenditures for the second year (August 2006 to July 2007) and third year (August 2007 to July 2008) were NIS 4.127 million (approximately $1,280,000) and NIS 5.473 million (approximately $1,700,000), respectively. In the second and third years, the OCS funded 60% of the approved research and development expenditures paid in Israel and 30% of the research and development costs paid to sub-contractors and consultants outside Israel. During July 2009 the OCS approved a fourth year of funding, in an aggregate amount of NIS 2.815 million (approximately $718,000).
In March 2009, the OCS approved a grant for us to perform a Phase I/IIa clinical trial of BC-819 for ovarian cancer. The OCS will fund up to 60% of NIS 1.7 million (approximately $447,000) in local expenses and up to 30% of NIS 1.8 million (approximately $484,000) in foreign expenses.
According to the Israeli Regulations for the Promotion of Research and Development in Industry 1996, we must pay the OCS royalties at a rate of 3% of the sum of sales of products during the first three years from the date of commencement, 4% of the sum of sales during the following three years and 5% of the sum of sales from the seventh year onward until the full repayment of the grants received by us, which are linked to the U.S. Dollar and bear annual interest at LIBOR rates. As of the date of this prospectus, we have not began repayments.
In December 2007, the Israel-U.S. Binational Industrial Research and Development Foundation (BIRD) approved a grant in the amount of $950,000 for our collaboration with the Virginia Bioscience Commercialization Center. The grant will partially fund our Phase I /IIa clinical trial for the use of BC-819 as a treatment for pancreatic cancer, which will be conducted at the University of Maryland Medical Center in Baltimore .
Under the terms of the grant agreement between BIRD, the Virginia Bioscience Commercialization Center and us, we will have to repay the grant within twelve months of the successful completion of the project. We are entitled to extend the repayment period to 2 years in return for total repayment of 113% of the grant amount, to 3 years in return for total repayment of 125%, to 4 years in return for total repayment of 138%, or to 5 years or more in return for total repayment of 150% of the grant amount.
Intellectual Property
As of the date of this prospectus, our patent portfolio includes 65 patent applications in seven families in various stages of examination, 25 of which have been granted in the United States, Europe, Israel, China, Australia, South Korea, Russia, Singapore, Mexico, Japan, Canada and the Czech Republic. All of our patents and patent applications were licensed to us from Yissum and are subject to the Yissum license agreement. The table below details which patent groups are related to which of our product candidates.
27
|
Patent Group
|
Product
|
Expiration Dates
|
|||
|
Use of the H19 gene as a tumor marker
|
BC-819
|
Between
03/07/2014
and
03/06/2015
|
|||
|
Methods and compositions for inducing tumor-specific cytotoxicity
|
BC-819
BC-821
|
Between
10/03/2017
and
10/04/2018
|
|||
|
Nucleic acid agents for down regulating H19, and methods of using same
|
BC-822
|
—
|
|||
|
Nucleic acid constructs, pharmaceutical compositions and methods of using same for treating cancer
|
BC- 820
|
—
|
|||
|
Nucleic acid constructs and methods for specific silencing of H19
|
BC- 822
|
—
|
|||
|
H19 silencing nucleic acid agents for treating rheumatoid arthritis (siRNA + RA)
|
BC-822
|
—
|
|||
|
Constructs containing multiple diphtheria toxin expression cassettes
|
N/A
|
—
|
|||
Pursuant to an exclusive license agreement with Yissum, which is described in more detail below, we have an exclusive, worldwide license for the development, use, manufacturing and commercialization of products arising out of patents owned by, and patent applications filed by, Yissum in connection with the H19 gene. All of our patents and patent applications were licensed to us from Yissum and are subject to the Yissum license agreement.
Government Regulation
Our operations are subject to many governmental regulations. In the event that we complete Phase III clinical trials and are in a position to manufacture and market our prospective therapeutic products, the marketing of our prospective therapeutic products would be conditioned upon obtaining the consent of health authorities in each of the countries in which our prospective therapeutic products would be marketed, including the FDA and the European Agency for the Evaluation of Medicinal Products. In order to market our products outside of the United States, we must comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in countries outside of the United States might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed below regarding FDA approval as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others.
United States Food and Drug Administration. We must obtain the approval of the FDA to market any drugs that we may develop in the United States, as well as adhere to other U.S. and state regulations. If we seek to market new drugs, we will be required to file a new drug application and obtain FDA approval. FDA regulations govern the following activities that we may perform, or that we may have performed on our behalf, to ensure that any drugs that we may develop are safe and effective for their intended uses:
28
|
§
|
pre-clinical (animal) testing including toxicology studies;
|
|
§
|
submission of an investigational new drug application (IND);
|
|
§
|
human testing in clinical trials, Phases I, II and III;
|
|
§
|
recordkeeping and retention;
|
|
§
|
pre-marketing review through submission of a new drug application (NDA);
|
|
§
|
drug labeling and manufacturing, the latter of which must comply with current good manufacturing practice regulations;
|
|
§
|
drug marketing, sales and distribution; and
|
|
§
|
post-marketing study commitments (Phase IV), post-marketing surveillance, complaint handling, reporting of deaths or serious injuries and repair or recall of drugs.
|
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
|
§
|
warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;
|
|
§
|
disqualification of clinical investigator and/or sponsor from current and future studies;
|
|
§
|
clinical hold on clinical trials;
|
|
§
|
operating restrictions, partial suspension or total shutdown of production;
|
|
§
|
refusal to approve an NDA;
|
|
§
|
post-marketing withdrawal of approval; and
|
|
§
|
criminal prosecution.
|
The FDA’s Pre-clinical and IND Requirements. The first step to obtaining FDA approval of a new drug involves development, purification and pre-clinical testing of a pharmaceutically active agent in laboratory animals. Once sufficient pre-clinical data has been collected to demonstrate that the drug is reasonably safe for initial use in humans, an IND can be prepared and submitted to the FDA for review. In the IND review process, FDA physicians and scientists evaluate the proposed clinical trial protocol, chemistry and manufacturing control, pharmacologic mechanisms of action of the drug and toxicologic effects of the drug in animals and in vitro. Within 30 days of the IND’s submission, the drug review division of the FDA may contact us regarding potential concerns and, if necessary, implement a clinical hold until certain issues are resolved satisfactorily. If it does not take any action, we may proceed with clinical trials on the 31st day.
Clinical Trials. Clinical trials represent the ultimate pre-market testing ground for unapproved drugs, generally taking several years to complete. Before testing can begin, an institutional review board (IRB) must have reviewed and approved the use of human subjects in the clinical trial. During clinical trials, an investigational compound is administered to humans and evaluated for its safety and effectiveness in treating, preventing or diagnosing a specific disease or condition. The clinical trials consist of Phase I, Phase II, and Phase III testing. During clinical trials, the FDA and IRB closely monitor the studies and may suspend or terminate trials at any time for a number of reasons, such as finding that patients are being exposed to an unacceptable health risk. The results of clinical trials comprise the single most important factor in the approval or disapproval of a new drug.
NDA Review. An NDA, requesting approval to market the drug for one or more indications, may be submitted to the FDA once sufficient data has been gathered through pre-clinical and clinical testing. An NDA includes all animal and human testing data and analyses of the data, as well as information about how the drug behaves in the human body and how it is manufactured. The NDA is reviewed by a team of FDA physicians, chemists, statisticians, microbiologists, pharmacologists and other experts, who evaluate whether the studies submitted show that the drug is safe and effective for its proposed use. The FDA reviewers may request further information from us, consult with outside experts or disagree with our findings or interpretation of the data. Each reviewer prepares a written evaluation, and the reviewing team discusses the evaluations. Accelerated approval may be given to some new drugs for serious and life-threatening illnesses that lack satisfactory treatments. At the end of its review, the FDA may approve the new drug to be marketed or decide that a new drug is “approvable” or “not approvable.” In either of the latter cases, we may meet with FDA officials to discuss and correct deficiencies.
Pervasive and Continuing Regulation in the United States. After a drug is approved for marketing and enters the marketplace, numerous regulatory requirements continue to apply. These include, but are not limited to:
29
|
§
|
The FDA’s current good manufacturing practice regulations require manufacturers, including third party manufacturers, to follow stringent requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product;
|
|
§
|
Labeling regulations and the FDA prohibitions against the promotion of drug for uncleared or unapproved uses (known as off-label uses), as well as requirements to provide adequate information on both risks and benefits during promotion of the drug;
|
|
§
|
Clearance or approval of product modifications or use of the drug for an indication other than approved in the NDA;
|
|
§
|
Adverse drug experience regulations, which require us to report information on rare, latent or long-term drug effects not identified during pre-market testing;
|
|
§
|
Post-market testing and surveillance requirements, including Phase IV trials, when necessary to protect the public health or to provide additional safety and effectiveness data for the drug; and
|
|
§
|
The FDA’s recall authority, whereby it can ask, or under certain conditions order, drug manufacturers to recall from the market a product that is in violation of governing laws and regulations.
|
After a drug receives approval, any modification in conditions of use, active ingredient(s), route of administration, dosage form, strength or bioavailability, will require a new clearance or approval. We may be able to submit a 505(b)(2) NDA referring to pre-clinical and certain clinical studies presented in the drug’s original NDA, accompanied by additional clinical data necessary to demonstrate the safety and effectiveness of the product with the proposed changes. Additional clinical studies may be required for proposed changes.
Fraud and Abuse Laws in the United States. A variety of U.S. federal and state laws apply to the sale, marketing and promotion of drugs that are paid for, directly or indirectly, by U.S. federal or state healthcare programs such as Medicare and Medicaid. The restrictions imposed by these laws are in addition to those imposed by the FDA, the United States Federal Trade Commission and corresponding state agencies. Some of these laws significantly restrict or prohibit certain types of sales, marketing and promotional activities by drug manufacturers. Violation of these laws may result in significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties and exclusion or debarment from U.S. federal and state healthcare and other programs. Many private health insurance companies also prohibit payment to entities that have been sanctioned, excluded or debarred by U.S. federal agencies.
Anti-Kickback Statutes in the United States. The U.S. federal anti-kickback statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending of a good or service, for which payment may be made in whole or in part under a U.S. federal healthcare program such as the Medicare and Medicaid programs. The definition of “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, the furnishing of supplies or equipment, payments of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that, if any one purpose of an arrangement involving remuneration is to induce referrals or otherwise generate business involving goods or services reimbursed in whole or in part under U.S. federal healthcare programs, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other U.S. federal healthcare programs. In addition, some kickback allegations have been claimed to violate the United States False Claims Act (as discussed below).
The U.S. federal anti-kickback statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the statute is broad and may technically prohibit many innocuous or beneficial arrangements, the Office of Inspector General of the Department of Health and Human Services, or OIG, has issued a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the anti-kickback statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG or the U.S. Department of Justice.
Many states have adopted laws similar to the U.S. federal anti-kickback statute. Some of these state prohibitions are broader than the U.S. federal statute, and apply to the referral of patients and recommendations for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs. Government officials have focused certain enforcement efforts on marketing of healthcare items and services, among other activities, and have brought cases against individuals or entities with sales personnel who allegedly offered unlawful inducements to potential or existing physician customers in an attempt to procure their business.
United States False Claims Act. The United States False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the U.S. federal government or knowingly making, or causing to be made, a false statement in order to have a false claim paid. The U.S. federal government’s interpretation of the scope of the law has in recent years grown increasingly broad. Most states also have statutes or regulations similar to the United States False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these U.S. federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment. Several drug manufacturers have been prosecuted under the false claims laws for allegedly providing free drugs to physician customers with the expectation that the physician customers would bill U.S. federal programs for the product. In addition, several recent cases against drug manufacturers have alleged that the manufacturers improperly promoted their products for “off-label” use, outside of the scope of the FDA-approved labeling.
United States Health Insurance Portability and Accountability Act of 1996. The United States Health Insurance Portability and Accountability Act of 1996, or HIPAA, created a new U.S. federal healthcare fraud statute that prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government-sponsored programs. Among other things, HIPAA also imposes new criminal penalties for knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services, along with theft or embezzlement in connection with a healthcare benefits program and willful obstruction of a criminal investigation involving a U.S. federal healthcare offense.
30
Regulations in Europe. In Europe, we must obtain authorization from the European Agency for the Evaluation of Medicinal Products, commonly known as the European Medicines Evaluation Agency (EMEA) before marketing medicinal products. Authorization can be obtained through either the (i) “centralized” procedure, with applications made directly to the EMEA leading to the grant of a European marketing authorization by the European Commission, or (ii) “mutual recognition” procedure, in which applications are made to one or more Member States leading to national marketing authorizations mutually recognized by other Member States. If and when we receive marketing authorization, EU law regulates our distribution, classification for supply, labeling and packaging, and advertising of medicinal products for human use. The EU also regulates the manufacture of medicinal products, requiring us to meet the Good Manufacturing Practice requirements set forth in the Quality System regulation (cGMP). EU pharmacovigilance directives and regulations require us to establish post-market surveillance systems that include individual adverse reaction case reports, periodic safety update reports, and company-sponsored post-authorization safety studies. If a medicinal product’s overall risk and benefit profile is found to have changed significantly for any reason, we may be required to vary, withdraw or suspend the use of any such EMEA-approved drug.
Regulations in Israel. Our operations in Israel also are subject to approval by Israel’s Ministry of Health and the Helsinki Committee. All phases of clinical studies conducted in Israel must be conducted in accordance with the Public Health Regulations (Medical Experiments Involving Human Subjects, 1980, including amendments and addenda thereto) and the International Conference for Harmonization Good Clinical Practice Guidelines. The regulations stipulate that a medical trial on humans will only be approved after the Helsinki Committee at the hospital intending to perform the trial has approved the medical trial and notified the medical director at the hospital in writing. The Helsinki Committee will not approve the performance of the medical trial unless it is fully satisfied that it has advantages to the trial participants and society at large that justify the risk and inconvenience for the participants and that the medical and scientific information justifies the performance of the requested medical trial. The medical director also must be satisfied that the trial is not contrary to the Helsinki Declaration or to other regulations. The Ministry of Health also licenses and inspects pharmaceutical manufacturers, requiring manufacturers to meet internationally recognized cGMP standards.
Under the Israeli Law for the Encouragement of Industrial Research and Development, 1984 and related regulations, which we refer to as the “Research Law”, recipients of grants from the OCS are prohibited from manufacturing products developed using these grants outside of the State of Israel without special approvals, although the Research Law does enable companies to seek prior approval for conducting certain manufacturing activities outside of Israel without being subject to increased royalties. If we receive approval to manufacture the products developed with government grants outside of Israel, we will be required to pay an increased total amount of royalties to OCS, up to 300% of the grant amounts plus interest, depending on the manufacturing volume that is performed outside of Israel, as well as at a possibly increased royalty rate.
Additionally, under the Research Law, we are prohibited from transferring OCS-financed technologies and related intellectual property rights outside of the State of Israel except under limited circumstances, and only with the approval of the Research Committee of the OCS.
We may not receive the required approvals for any proposed transfer and, if received, we may be required to pay the OCS a portion of the consideration that we receive upon any sale of such technology to a non-Israeli entity. The scope of the support received, the royalties that we have already paid to the OCS, the amount of time that has elapsed between the date on which the know-how was transferred and the date on which the OCS grants were received and the sale price and the form of transaction will be taken into account in order to calculate the amount of the payment to the OCS. Approval of the transfer of technology to non-residents of the State of Israel is required, and may be granted in specific circumstances only if the recipient abides by the provisions of applicable laws, including the restrictions on the transfer of know-how and the obligation to pay royalties. No assurances can be made that approval to any such transfer, if requested, will be granted.
In March 2005, an amendment to the Research Law was enacted. One of the main modifications included in the amendment was an authorization of the Research Committee to allow the transfer outside of Israel of know-how derived from an approved program and the related manufacturing rights. In general, the Research Committee may approve transfer of know-how in limited circumstances as follows:
31
|
§
|
in the event of a sale of the know-how itself to a non affiliated third party, provided that upon such sale the owner of the know-how pays to the OCS an amount, in cash, as set forth in the Research Law. In addition, the amendment provides that if the purchaser of the know-how gives the selling Israeli company the right to exploit the know-how by way of an exclusive, irrevocable and unlimited license, the research committee may approve such transfer in special cases without requiring a cash payment.
|
|
§
|
in the event of a sale of the company which is the owner of know-how, pursuant to which the company ceases to be an Israeli company, provided that upon such sale, the owner of the know-how makes a cash payment to the OCS as set forth in the Research Law.
|
|
§
|
in the event of an exchange of know-how such that in exchange for the transfer of know-how outside of Israel, the recipient of the know-how transfers other know-how to the company in Israel in a manner in which the OCS is convinced that the Israeli economy realizes a greater, overall benefit from the exchange of know-how.
|
Another provision in the amendment concerns the transfer of manufacturing rights. The Research Committee of the OCS may, in special cases, approve the transfer of manufacture or of manufacturing rights of a product developed within the framework of the approved program or which results therefrom, outside of Israel.
The State of Israel does not own intellectual property rights in technology developed with OCS funding and there is no restriction on the export of products manufactured using technology developed with OCS funding. The technology is, however, subject to transfer of technology and manufacturing rights restrictions as described above. OCS approval is not required for the export of any products resulting from the research or development or for the licensing of any technology in the ordinary course of business.
Material Operating Arrangements
Exclusive License Agreement with Yissum. On November 14, 2005, we entered into a license agreement with Yissum, which was subsequently amended on November 22, 2005 and September 11, 2007, pursuant to which Yissum has granted to us an exclusive, worldwide license for the development, use, manufacturing and commercialization of products arising out of patents owned by, and patent applications filed by, Yissum in connection with the H19 gene.
Under the terms of the Yissum license, Yissum retains right, title and interest in the products, technologies or other inventions arising out of our research and development of these patents and patent applications, except for intellectual property developed with funding from the OCS, which will be owned by us and transferred to Yissum only upon our dissolution or upon decision by the OCS that it no longer requires us to own the intellectual property developed with its funding. We have the right to grant sub-licenses to third parties in accordance with the terms set forth in the Yissum license.
We have agreed to provide research and development funding to Yissum in connection with the license, which we may terminate upon 90 days prior written notice to Yissum. In such event, we are required to compensate Yissum for all expenses incurred by it prior to the notification date in connection with its research efforts and all additional expenses that Yissum had assumed the obligation to cover prior to the notification date. The research and development funding was initially set for a period of two years with the possibility to extend the term by mutual agreement. We have been extending the funding period on a yearly basis, and it is currently valid until December 2010 .
In addition, we have agreed to prepare, register and maintain any patent application or patent that may arise out of our research and development efforts pursuant to our license with Yissum and to bear all expenses of preparation, registration and maintenance. We agreed to keep Yissum informed of filing and prosecutions pursuant to the agreement, including submission of copies of all official actions, relevant correspondence, applications, continuations or like proceedings, and responses thereto. We agreed to consult Yissum regarding any abandonment of the prosecution of patent applications arising out of the license. In the event that we decide not to commence or continue the process of patent registration in a certain country, we must notify Yissum of this decision. Yissum may then individually prepare, register and maintain any such patent. We must inform Yissum of our desire to assume the expenses incurred by Yissum in connection with its patent registration within 90 days from the date in which Yissum notifies us of its decision to prepare, register and maintain such patent. In the event that we decide not to assume these expenses, or in the absence of our reply within the above 90 day period, the exclusive, worldwide license granted to us by Yissum will no longer be applicable in such countries in which we elected not to file or to abandon the filing, prosecution or maintenance of patents pursuant to the license. We undertook to use commercially reasonable efforts at our own expense to protect against third party’s infringement of the patents arising out of the license and to advise Yissum upon learning of such infringement. We also undertook to use commercially reasonable efforts at our own expense to defend any action, claim or demand made by any entity in connection with rights in the patents, and to notify Yissum immediately upon learning of any such action or claim.
We have agreed to pay Yissum 5% of all “net sales” as royalties and to pay Yissum 10% of the income that we receive from granting sub-licenses to third parties up to revenues of $30 million each year, and 6.5% of all additional income that we receive from granting sub-licenses to third parties.
We are required to indemnify Yissum, the Hebrew University of Jerusalem, their employees, their executive officers, delegates and any other persons acting on their behalf under the license against any liability, including product liability, damages, losses, expenses, fees and reasonable legal expenses arising out of our actions or omissions in performing the Yissum license, including the use, development and manufacturing of patents arising out of it and the granting of sub-licenses thereunder, provided that any such loss was not caused by the intentional misconduct or gross negligence of the indemnitees.
We have agreed to maintain, and to add Yissum as an additional insured party with respect to, product liability insurance as well as an insurance policy with respect to the foregoing indemnification prior to the time when we commence clinical trials and close our first commercial sale. We also have agreed to obtain liability insurance with respect to clinical trials prior to the time when we commence clinical trials.
32
We have the right to terminate the Yissum license upon three months prior written notice provided that we have paid all amounts owing to Yissum under the license. Yissum has the right to terminate the license in the event that we become bankrupt or insolvent, or if our business is placed in the hands of a receiver, assignee or trustee. In addition, Yissum has the right to terminate the license for any material breach of it by us in the event that we fail to remedy such material breach within ninety days of Yissum’s notice of our material breach and its intent to terminate, provided that the material breach is curable within ninety days. In the event that the material breach cannot be remedied within ninety days , Yissum may not terminate the license if we take reasonable commercial action to cure such breach as promptly as practicable. The Israeli Contract Law (Remedies for Breach of Contract) — 1970, defines the term “material breach” as a breach, with regards to which, it may be assumed that a reasonable person would not have entered into the specific agreement had that person foreseen the breach and the outcome thereof, or a breach which is specifically defined as material in the agreement. Acts which may constitute a material breach of the license agreement by us may include, for example: the granting of sublicenses not in compliance with the provisions of the license agreement, a breach of our obligations to pay royalties and provide the necessary reports with respect thereto, a breach of our obligation to develop and commercialize the licensed technology including our obligation to fund certain research and development activities, a breach of our obligations to conduct patent prosecution and maintenance, and a breach of our obligations not to disclose or misuse certain confidential information of Yissum. The termination of the license also entails termination of all licenses granted thereunder. Any termination of the license shall not terminate any of our obligations, including our obligation to pay royalties that matured prior to the effective date of termination.
We may assign the Yissum license only in connection with the sale of all or substantially all of our assets. Otherwise, we must receive the prior written consent of Yissum to assign the license which consent shall not be unreasonably withheld.
Each license granted to us under the Yissum agreement expires upon the expiration of the underlying patent, or, if no patent has been registered, then after nine years from the date of the first commercial sale of the product of such license, provided that we may extend the license for an additional one year period in any such circumstance by continuing to pay royalties for such license.
Polyplus-transfection SA. Polyplus-transfection SA is currently our sole supplier of the component polyethylenimine (PEI) which is used to enhance our prospective drug BC-819’s ability to penetrate cancerous cells in the bladder. Polyplus-transfection SA has agreed to comply with the Good Manufacturing Practice requirements set forth in the Quality System Regulation in manufacturing PEI for us.
Supply Agreement with Althea Technologies Inc. In January 2007, our subsidiary, BioCancell Therapeutics Israel Ltd. entered into a continuous production agreement with Althea Technologies Inc., an American manufacturer for the production of our plasmids. To date, the manufacturer has executed three production campaigns of the DTA-H19 plasmid, one for the production of non-GMP material, the second for GMP material that is used for the Phase IIb clinical study for treatment of superficial bladder cancer, and the third for the production of GMP material for Phase I/IIa clinical trials of BC-819 in the treatment of ovarian and pancreatic cancer. All campaigns were successful, resulting with sufficient material, all of it within the specifications.
Supply Agreement with VGXI USA. In September 2008, our subsidiary, BioCancell Therapeutics Israel Ltd. entered into a continuous production agreement with VGXI USA (“VGXI”), a business entity registered in the state of Texas, a DBA of VGX International Inc. of Korea. Pursuant to the agreement, we may purchase from, and have plasmids produced by VGXI under cGMP conditions. We have no commitment to purchase any minimum quantity of plasmids nor shall VGXI have any commitment to produce and sell any minimum quantity of plasmids. We shall become obligated to purchase, and VGX shall be obligated to produce plasmids only upon execution and delivery of a purchase order for such plasmids.
Agreements for the Performance of Clinical Trials. Between November 2007 and October 2009, we entered into agreements with the Wolfson Medical Center, Meir Medical Center, Hillel Yaffeh Medical Center, Sheba Medical Center, Assaf Harofe Medical Center, Hadassah Hospital, Bnai Zion Medical Center and BCG Oncology PC, pursuant to which Phase II clinical trials will be administered by each for the purposes of assessing the efficacy and safety of our prospective drug BC-819 on patients suffering from superficial bladder carcinoma. Between April 2009 and October 2009, we entered into agreements with the Wolfson Medical Center, Meir Medical Center and Hadassah Hospital, pursuant to which Phase I/IIa clinical trials will be administered by each for the purposes of assessing the safety and efficacy of our prospective drug BC-819 on patients suffering from ovarian cancer. Between May 2009 and March 2010 , we entered into agreements with the Hadassah Hospital, Sheba Medical Center , Meir Medical Center and University of Maryland Medical Center , pursuant to which Phase I/IIa clinical trials will be administered by each for the purposes of assessing the safety and efficacy of our prospective drug BC-819 on patients suffering from pancreatic cancer. Each of these agreements was approved by the appropriate hospital authority.
33
Pursuant to the terms of each of these agreements, we will provide funding for the clinical trials and the hospitals will provide us with the medical personnel required by the trials, including researchers and facilities, and will supervise the trials in order to verify the trials’ compliance with trial protocols, the directives of the regulatory authorities, the terms and conditions set forth by the Helsinki Committee or applicable institutional review board for the performance of the trials and any other relevant law and regulatory directives. We have agreed to compensate each of the hospitals based upon numerical factors including the number of participants in the trials and the number of patient visits.
Each of the hospitals have ceded to us all right, title and interest in any and all intellectual property rights of any kind that may be created or developed from the trials.
We have agreed to indemnify each of the hospitals and their medical personnel who participate in the trials against any loss or damage arising out of the trials, as long as the trials are administered in accordance with the terms set forth in such hospitals’ agreement with us, and as long as such loss or damage was not caused by the negligence of the indemnitees.
Employees and Advisory Board
As of the date of this prospectus, we have 16 employees who are employed by us or our wholly - owned subsidiary, BioCancell Therapeutics Israel Ltd . Nine of these employees conduct clinical development or research and development for us; the other seven are managers or administrators. The majority of our research and development work is completed by Professor Hochberg’s laboratory team at the Hebrew University of Jerusalem. The team members are qualified in the life sciences and in medicine: three of these members have doctorates in the natural sciences and one specializes in pathology.
Our Scientific Advisory Board includes world-renowned experts in the field of cancer therapy including Professor Mark L. Tykocinski, who was President of the American Society for Investigative Pathology and is currently President of the Association of Pathology Chairs, Professor Aaron Ciechanover, who was awarded the Nobel Prize in Chemistry in 2004, Professor Roger D. Kornberg, who was awarded the Nobel Prize in Chemistry in 2006, Professor Hermona Soreq, Dean of the Faculty of Mathematics and Science at the Hebrew University of Jerusalem, Professor Yechezkel Barenholz, a professor of Biochemistry at the Hebrew University-Hadassah Medical School, and Professor Yaakov Naparstek, Chairman of Medicine at Hadassah University Hospital. The members of our Scientific Advisory Board provide us with general and strategic consultation and development services and assistance with respect to our research and development activities. As compensation for their work, the members of our Scientific Advisory Board receive an advisor’s fee of $1,000 for each meeting of the Scientific Advisory Board in which they participate, plus expenses. In addition, our Board of Directors has committed to grant to each member of the Scientific Advisory Board options to purchase 30,000 shares of our common stock pursuant to our 2007 Stock Option Plan. No member of our Scientific Advisory Board is an officer, employee or director of either BioCancell Therapeutics Inc. or BioCancell Therapeutics Israel Ltd.
All of our current employees have signed personal employment agreements for monthly salaries. Under these employment agreements, our employees have promised to cede to us all right, title and interest to any and all intellectual property created during their course of employment to us and they have undertaken not to make use of it, and not to compete with us for a period of 12 months after termination of their employment with us.
None of our employees are represented by a collective bargaining agreement, nor have we experienced any work stoppages. We believe that our relations with our employees are good. We have adopted a Code of Ethics that applies to all of our employees, officers and members of the Board of Directors. The Code of Ethics is available on our website at www.biocancell.com, and in print to any interested party that requests it.
Legal Proceedings
We are not a party to any legal proceeding.
Facilities
Our offices are located at Har Hotzvim, Jerusalem, in buildings in which we lease a total space of 213 square meters for a term expiring in September 2010. Our annual rent under the lease is approximately $48,000 plus VAT. Most of our research and development activities are conducted in the research laboratories of the Hebrew University of Jerusalem.
34
MANAGEMENT
As of the date of this prospectus, our directors and executive officers, their ages and positions held are as follows:
|
Name
|
Age
|
Position
|
|||||||||
|
Uri Danon
|
44
|
Chief Executive Officer
|
|||||||||
|
Ira Weinstein
|
54
|
Chief Financial and Operating Officer
|
|||||||||
|
Abraham Hochberg (2)
|
72
|
Chief Scientific Officer and Director
|
|||||||||
|
Patricia Ohana
|
54
|
Vice President of Clinical Development
|
|||||||||
|
Moshe Landsberg
|
58
|
Vice President of Technology
|
|||||||||
|
Doron Amit
|
35
|
Vice President of Business Development
|
|||||||||
|
Doron Nevo (3)
|
54
|
Chariman of the Board of Directors
|
|||||||||
|
Aviv Boim (2)
|
42
|
Director
|
|||||||||
| Hanoch Rappaport (1) | 43 |
Director
|
|||||||||
| David Schlachet (1)(2) | 64 | Director | |||||||||
|
Orly Yarkoni (1)(3)
|
54 | Director | |||||||||
|
(1)
|
Member of the Audit Committee
|
|
(2)
|
Member of the Executive Committee
|
|
(3)
|
Member of the Compensation Committee
|
The following is a brief account of the education and business experience during the past five years of each director and executive officer, indicating the principal occupation during that period, and the name and principal business of the organization in which such occupation and employment were carried out.
Uri Danon, Chief Executive Officer
On October 18, 2009, we entered into an employment agreement with Mr. Uri Danon, pursuant to which he has served as our Chief Executive Officer since November 1, 2009. Mr. Danon served as our President between 2005 and 2006, and was Chief Executive Officer of Atox Bio, Inc . (which develops treatments for autoimmune diseases and sepsis), between 2003 and 2009 . He previously managed international projects for Teva Pharmaceutical Industries, Ltd., including the development of the drug Copaxone in a solution in pre-filled syringes (a drug with sales of over $2 .8 billion per year), and was CEO of Epigenesis, Ltd., a cell therapy development company. He holds an MBA in Marketing from Bar Ilan University, and a B.Sc. in Industrial Engineering and Management from Tel Aviv University.
Ira Weinstein , Chief Financial and Operating Officer
Mr. Weinstein has served as our Chief Financial Officer and as our Chief Operating Officer since May 2007. Prior to taking his position with us, Mr. Weinstein was Chief Executive Officer of Hapto Biotech (Israel) Ltd. from April 2004 to April 2007, and was Chief Executive Officer of Incure Ltd. from January 2004 to February 2005. Prior to these positions, Mr. Weinstein served as Chief Financial Officer and Chief Operating Officer of Keryx Biopharmaceuticals, Inc., a NASDAQ-listed public company. Mr. Weinstein also has served as a consultant to the New York State Department of Health and has held teaching positions at Baruch College, Touro College, St. John’s University and Coventry Polytechnic University. Mr. Weinstein holds an M.B.A. in Management and a Bachelor of Business Administration degree in Accounting from Baruch College, City University of New York.
Abraham Hochberg , Chief Scientific Officer and Director
Professor Hochberg has served as our Chief Scientific Officer since December 2005 and as a member of our Board of Directors since March 2006. He served as our Chairman of the Board of Directors between July 2006 and October 2009. Professor Hochberg has been a biochemist and molecular biologist in the Department of Biological Chemistry at the Hebrew University of Jerusalem for the past 48 years. Professor Hochberg is recognized as a world-leading expert on the H19 gene and is considered to have made many seminal contributions in the fields of imprinted genes, the H19 gene, the IGF2 gene and oncology. Professor Hochberg earned a Ph.D. in Molecular Biology, summa cum laude, from the Hebrew University of Jerusalem. He also holds a B.A. in Archeology from the Hebrew University of Jerusalem. As our technology is based on over 15 years of Prof. Hochberg’s research, he is qualified to serve on our Board by virtue of his unparalleled understanding of our business.
35
Patricia Ohana , Vice President of Clinical Development
Dr. Ohana has served as our Vice President of Clinical Development since September 2006. Dr. Ohana also has been a biochemist and molecular biologist in the Department of Biological Chemistry at the Hebrew University of Jerusalem for the past 26 years. Dr. Ohana holds a Ph.D. in Biological Chemistry, a M.Sc. in Biochemistry and a B.Sc. in Chemistry, each from the Hebrew University of Jerusalem.
Moshe Landsberg , Vice President of Technology
Mr. Landsberg has served as our Vice President of Technology since April 2007. Prior to taking his position with us, Mr. Landsberg was the Chief Operating Officer of BioPreventative Ltd. (which later became Analyte Works Ltd.) and was Quality Assurance director at Common Sense Ltd., and Chief Operating Officer and Quality Assurance director at Lithotech Medical Ltd. Mr. Landsberg also served various executive positions at Biotechnology General (Israel) Ltd. for 17 years. Mr. Landsberg currently holds a teaching position at the Agriculture Faculty of the Hebrew University of Jerusalem and is a consultant to several leading pharmaceutical, biopharmaceutical and biotechnology companies in Israeli including Teva Pharmaceutical Industries Ltd., Taro Pharmaceutical Industries Ltd., Perrigo Israel, MediWound Ltd., the Israeli Institute for Biological Research and the Weizmann Institute of Science. Mr. Landsberg holds a M.Sc. in Applied Microbiology from the Hebrew University of Jerusalem and a B.S. degree in Life Sciences from Tel Aviv University.
As part of our continued efforts to improve budgetary efficiency, on April 15, 2010, we terminated the services of Mr. Landsberg, effective July 15, 2010. Mr. Landsberg's current duties, including responsibility for manufacturing, logistics and quality assurance, will thereafter be performed by other members of our management.
Doron Amit, Vice President of Business Development
Dr. Amit oversees our business development activity, and is in charge of the research and development of BC-821, our 2 nd generation drug candidate, since co-inventing it together with Prof. Abraham Hochberg. He is a former director of the US Funds Department and the chief life science analyst at FreeMind Group LLC, an international consultancy firm, where he managed a team of bio-med consultants and was responsible for raising funds from non-dilutive sources such as government agencies and private foundations for biotechnology companies and research institutions. He has provided business consultancy services for various biotechnology and medical device companies in Israel and in the U.S. Dr. Amit holds a Ph.D. in Molecular Biology and an MBA, magna cum laude, both from the Hebrew University of Jerusalem. He also holds an M.Sc., summa cum laude, in Molecular Biology, and a Bachelor of Medical Sciences, both from the Hebrew University of Jerusalem.
Doron Nevo , Chairman of the Board of Directors
Mr. Nevo has served as a director since November 2004 , and Chairman of the Board since March 2010 . Mr. Nevo has founded and served as President and Chief Executive Officer of a number of companies, including KiloLambda Technologies, Ltd., an optical components and subsystems company, NKO, Inc., a company that designed and developed carrier-grade IP telephony system platforms and established its own IP network ; and Clalcom Ltd. and Barak, Ltd., leading international telecommunications carriers in Israel. Prior to founding Clalcom, Mr. Nevo held various executive positions with Sprint International, Inc ., a global telecom carrier. Mr. Nevo currently serves on a number of boards of directors of companies in various fields, including Audiocodes, Ltd., Elcom Technologies, KiloLambda, Inc., Pro4Tech , Ltd . and Etgar Investment Portfolios Corp. Ltd. Mr. Nevo holds a B.Sc. in Electrical Engineering from the Technion and a M.Sc. in Telecommunications Management from Brooklyn Polytechnic , NY. Mr. Nevo is qualified to serve on our Board of Directors by virtue of his vast business knowledge and experience.
Aviv Boim, Director
Mr. Boim has served as a director since August 2008. Mr. Boim previously served as Chief Executive Officer of Tikcro Technologies, Ltd., and Orckit Communications Ltd., where he held the position of Chief Financial Officer for nearly ten years. Prior to joining Orckit, Mr. Boim was a banker with BT Alex. Brown Incorporated, an investment banking firm. Mr. Boim holds a B.A. and a M.A. in economics and management from Tel Aviv University and an L.L.B. from Tel Aviv University Law School. Mr. Boim currently serves on our Board of Directors as a representative, and is an affiliate, of Tikcro Technologies, Ltd. See “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Certain Relationships and Related Transactions” below. Mr. Boim is qualified to serve on our Board of Directors by virtue of his investment banking and finance knowledge and experience which are relevant to a company in our position.
Hanoch Rappaport , Director
Mr. Rappaport has served as a director since November 2004. Mr. Rappaport serves as the Chief Financial Officer of the Provident Fund of the Employees of the Hebrew University of Jerusalem Ltd. Mr. Rappaport also served as corporate credit officer at Bank Hapoalim. Mr. Rappaport currently serves as Investment Manager at A. Heifetz & Co. Mr. Rappaport holds a B.A. in Economics and Political Science from Bar-Ilan University and an M.B.A. from the Tel Aviv International School of Management. Mr. Rappaport currently serves as a member of our Board of Directors and is an affiliate of the Provident Fund of the Employees of the Hebrew University of Jerusalem Ltd. See “Security Ownership of Certain Beneficial Owners and Management” and “Certain Relationships and Related Transactions” below. Mr. Rappaport is qualified to serve on our Board of Directors by virtue of his finance knowledge and experience.
36
Orly Yarkoni, Director
Orly Yarkoni has served as a director since January 2010, and is deemed independent under Israeli law and in accordance with Section 6.2 of our Amended and Restated Certificate of Incorporation. She has amassed over 20 years in the insurance industry, most recently as Chief Executive Officer of Yashir - I.D.I. Insurance Co., Ltd. She has served as a member of the Board of Governors of the Israeli Securities Authority, and currently serves as a director of Peninsula Finance, Ltd., Menorah Mivtachim Insurance, Ltd., Ma'ayanot Eden, Ltd. Plasto-Sac, Ltd. and Amot Investments, Ltd. Ms. Yarkoni holds a B.Sc. (cum laude) in Mathematics from the Hebrew University of Jerusalem, Israel, and is a member of the Israeli Association of Actuaries. Ms. Yarkoni is qualified to serve on our Board of Directors by virtue of her vast business knowledge and experience, especially with publicly-traded companies.
David Schlachet, Director
David Schlachet has served as a director since January 2010, and is deemed independent under Israeli law and in accordance with Section 6.2 of our Amended and Restated Certificate of Incorporation. He was previously Chief Executive Officer of Syneron Medical Ltd., Managing Partner of Biocom (a venture capital fund specializing in the life sciences area), senior VP and Chief Financial Officer of Strauss Elite Holdings, VP Finance & Administration of the Weizmann Institute of Science, and Chief Executive Officer of the Weizmann Institute's technology transfer company, Yeda R&D Co. Ltd.
Mr. Schlachet serves as an independent director on the Board of the Tel Aviv Stock Exchange (TASE) and as a director and audit committee member of the TASE Clearing House. He is a director of Nasdaq-listed EzChip Semiconductor Ltd. and Syneron Medical Ltd., and of TASE-listed Taya Investments Ltd., Mazor Surgical Technologies Ltd. and Adgar Investments and Developments Ltd., as well as several privately-owned companies. He was previously active chairman of both Harel Capital Markets Ltd., and Elite Industries, Ltd. Mr. Schlachet holds an M.B.A in Finance from Tel-Aviv University, and a B.Sc. in Chemical Engineering from the Technion. Mr. Schlachet is qualified to serve on our Board of Directors by virtue of his vast business, scientific and finance knowledge and experience, particularly with biotechnology start-up companies.
37
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of the date of this prospectus, information regarding the beneficial ownership of our common stock by (i) each person who is known to us to be the owner of more than five percent of our common stock, (ii) each of our directors, (iii) each of the named executive officers and (iv) all directors and executive officers as a group. For purposes of this table, a person or group of persons is deemed to have beneficial ownership of any shares that such person has the right to acquire within 60 days of the date of this prospectus. Unless otherwise indicated, the address of each of the persons listed in this table is as follows: Beck Science Center, 8 Hartom St, Jerusalem 97775 Israel. Information in this Section is based on information provided to us by the individuals and entities listed below.
|
Name and Address of Beneficial Owner
|
Total Number of
Shares of Common
Stock Beneficially
Owned (1)
|
Percentage
Ownership of
Common Stock (1)
|
|||||||||
|
Five percent or more beneficial owners:
|
|
|
|||||||||
|
Clal Biotechnology Industries, Ltd.
45th Floor, 3 Azrieli Center, Tel Aviv 67023 Israel (2)
|
6,895,368
|
29.13
|
%
|
||||||||
|
Tikcro Technologies, Ltd.
126 Yigal Allon St, Tel Aviv 67443 Israel (3)
|
8,417,966
|
29.81
|
%
|
||||||||
|
Provident Fund of the Employees of the Hebrew
University of Jerusalem Ltd.
2/2 Hi-Tech Village
Givat Ram, Jerusalem 91904 Israel (4)
|
1,227,471
|
5.82
|
%
|
||||||||
|
Directors and named executive officers:
|
|||||||||||
|
Uri Danon (8)
|
220,750
|
1.06
|
%
|
||||||||
|
Abraham Hochberg (5)
|
2,211,254
|
10.69
|
%
|
||||||||
|
Moshe Landsberg (6)
|
121,893
|
*
|
|||||||||
|
Aviv Boim (3)
|
8,417,966
|
29.81
|
%
|
||||||||
|
Doron Nevo (9)
|
168,126
|
*
|
|||||||||
|
Hanoch Rappaport (7)
|
1,247,471
|
5.91
|
%
|
||||||||
|
David Schlachet (10)
|
5,000
|
*
|
|
||||||||
|
Orly Yarkoni (10)
|
5,000
|
*
|
|||||||||
|
All directors and officers as a group (8 persons) (11)
|
12,397,461
|
42.55
|
%
|
||||||||
|
*
|
Less than 1%.
|
||||||||||
|
(1)
|
Assumes the full exercise of all options and warrants held by the holder that are exercisable within 60 days of the date of this prospectus. Percent of class based on 20,621,113 shares of our common stock outstanding as of the date of this prospectus.
|
|
(2)
|
Consists of 6, 895,368 shares of our common stock owned by Clal Biotechnology Industries, Ltd. (“CBI”) of which (i) 3,047,001 shares of our common stock underlie convertible debentures and warrants which are convertible or exercisable within 60 days of the date of this prospectus; and (ii) 13, 921 shares of our common stock are owned by Epsilon Mutual Funds (1991 ) Ltd., an affiliate of CBI. The table does not reflect 150,000 shares of our common stock issuable upon the exercise of warrants issued to Clal Finance Ltd., an affiliate of CBI, in August 2006 in connection with our initial public offering on the TASE; these warrants are exercisable at any time after the date on which shares of our common stock have traded on the TASE for more than an average price of NIS 10 for 30 consecutive days. The amount of shares underlying the convertible debenture referred to above, assumes full conversion of the convertible debenture at the end of the 60 day period commencing on the date of this prospectus. The amount of shares underlying the convertible debenture may vary following the date of this prospectus upon the consummation of certain M&A events as set forth in the convertible debenture, or as a result of certain anti-dilution adjustments provided for under the convertible debenture. For more information on such rights, see “Description of Securities — Anti-Dilution Adjustments” below.
|
|
|
(3)
|
Includes 7, 617,503 shares of our common stock underlying convertible debentures and warrants which are convertible or exercisable within 60 days of the date of this prospectus. Mr. Boim is an employee of Tikcro Technologies, Ltd. The amount of shares underlying the convertible debenture referred to above, assumes full conversion of the convertible debenture at the end of the 60 day period commencing on the date of this prospectus. The amount of shares underlying the convertible debenture may vary following the date of this prospectus upon the consummation of certain M&A events as set forth in the convertible debenture, or as a result of certain anti-dilution adjustments provided for under the convertible debenture. For more information on such rights, see “Description of Securities — Anti Dilution Adjustments” below.
|
|
(4)
|
Includes 457,050 shares of our common stock underlying convertible debentures and warrants which are convertible or exercisable within 60 days of the date of this prospectus. The amount of shares underlying the convertible debenture referred to above, assumes full conversion of the convertible debenture at the end of the 60 day period commencing on the date of this prospectus. The amount of shares underlying the convertible debenture may vary following the date of this prospectus upon the consummation of certain M&A events as set forth in the convertible debenture, or as a result of certain anti-dilution adjustments provided for under the convertible debenture. For more information on such rights, see “Description of Securities — Anti-Dilution Adjustments” below.
|
|
(5)
|
Consists of 2,211,254 shares of our common stock owned by Abraham Hochberg, of which 70,000 shares of our common stock underlie options that are exercisable within 60 days of the date of this prospectus.
|
38
|
(6)
|
Consists of 121,893 shares of our common stock owned by Moshe Landsberg, of which 113,750 shares of our common stock underlie options that are exercisable within 60 days of the date of this prospectus.
|
|
(7)
|
Includes 457,050 shares of our common stock underlying convertible debentures and warrants which are convertible or exercisable within 60 days of the date of this prospectus (as detailed in Note 4 above), and 20,000 shares of our common stock owned by Mr. Rappaport, underlying options that are exercisable within 60 days of the date of this prospectus. Mr. Rappaport is the Chief Financial Officer of the Provident Fund of the Employees of the Hebrew University of Jerusalem Ltd. The amount of shares underlying the convertible debenture referred to above, assumes full conversion of the convertible debenture at the end of the 60 day period commencing on the date of this prospectus. The amount of shares underlying the convertible debenture may vary following the date of this prospectus upon the consummation of certain M&A events as set forth in the convertible debenture, or as a result of certain anti-dilution adjustments provided for under the convertible debenture. For more information on such rights, see “Description of Securities — Anti-Dilution Adjustments” below.
|
|
(8)
|
Consists of 220,750 shares of our common stock owned by Uri Danon, of which 149,375 shares of our common stock underlie options that are exercisable within 60 days of the date of this prospectus.
|
|
(9)
|
Consists of 168,126 shares of our common stock owned by Doron Nevo, of which 78,126 shares of our common stock underlie options that are exercisable within 60 days of the date of this prospectus.
|
|
|
(10)
|
Consists of 5,000 shares of our common stock owned by the director, underlying options that are exercisable within 60 days of the date of this prospectus.
|
|
|
(11)
|
Includes 8,515,805 shares of our common stock underlying convertible debentures, warrants and options which are convertible or exercisable within 60 days of the date of this prospectus. The amount of shares underlying the convertible debenture referred to above, assumes full conversion of the convertible debenture at the end of the 60 day period commencing on the date of this prospectus. The amount of shares underlying the convertible debenture may vary following the date of this prospectus upon the consummation of certain M&A events as set forth in the convertible debenture, or as a result of certain anti-dilution adjustments provided for under the convertible debenture. For more information on such rights, see “Description of Securities — Anti Dilution Adjustments” below.
|
Voting Agreements
On July 30, 2008, in connection with the closing of the private placement of our securities pursuant to Subscription and Registration Rights Agreements with Clal Biotechnology Industries Ltd. (CBI), Tikcro Technologies Ltd. (Tikcro) and the Provident Fund of the Employees of the Hebrew University of Jerusalem Ltd., CBI, Tikcro, Professor Abraham Hochberg, and Mr. Avi Barak, entered into an Irrevocable Voting Agreement. For more information regarding these transactions, see “Certain Relationships and Related Transactions — Private Placements” below.
Pursuant to the Irrevocable Voting Agreement, the parties agreed, subject to applicable law, to vote or cause to be voted all shares of our common stock or other voting securities directly or indirectly owned by it or him at any general meeting of our stockholders at which members of our Board of Directors are to be elected in favor of the election of one nominee recommended by each of CBI, Tikcro and Professor Hochberg for as long as CBI, Professor Hochberg and Tikcro, respectively, hold at least seven percent of our capital stock on an as converted basis, including any type of debt securities convertible into shares of our equity but excluding warrants or options to purchase shares of our common stock. The parties further agreed not to vote to terminate the membership of any such nominee on our Board of Directors without the prior written consent of the applicable nominating party. As of the date of this prospectus, there is no CBI-nominated director on our Board, and CBI has not nominated a candidate.
The Israel Securities Authority regards the parties to the Irrevocable Voting Agreement as mutual holders of a “control block” pursuant to the Israeli Companies Law because these parties collectively hold more than 45% of the voting rights of our stockholders. As a result, a transaction between us and any of the parties to the Irrevocable Voting Agreement would be regarded as an interested transaction under the Israeli Companies Law. For more information on the treatment of interested transactions under the Israeli Companies Law, see “Corporate Governance — Business Combinations; Interested Transactions” below.
On November 22, 2009, CBI, Professor Abraham Hochberg and Mr. Avi Barak notified the Company that they had signed a voting agreement (the “ New Voting Agreement ”), according to which each party (Professor Hochberg and Mr. Barak are together considered one party) will be required to vote at general meetings for the election of one director designated by the other party. This agreement is in addition to (and does not amend) the existing voting agreement of July 30, 2008, such that, in total, the parties to the New Voting Agreement will vote together regarding two nominees of each party on the Board of Directors. The parties further agreed not to vote to terminate the membership of any such nominee on our Board of Directors without the prior written consent of the applicable nominating party. In the event that the service of a nominee as director terminates, the parties undertook to act to convene a general meeting, and to vote for the appointment of a candidate for the position of director nominated by the party that previously nominated the director whose service terminated.
The right to nominate directors under the New Voting Agreement will be in effect as long as a party holds at least 7% of the outstanding shares of the Company (including convertible bonds, on an as-converted basis, but excluding warrants), but in any case not beyond July 30, 2012. The parties to the New Voting Agreement also agreed to vote against any resolution increasing the number of directors on the Board beyond nine. The Company is not a party to either voting agreement.
39
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth the aggregate cash compensation paid during the 2008 and 2009 fiscal years to our Chief Executive Officer and to our executive officers other than our CEO whose annual salary and bonuses exceeded $100,000 for the applicable years.
|
Name and Principal Position
|
Year
|
Salary
($) (1)
|
Bonus
($) (1)
|
Stock
Awards
($) (1)
|
Option
Awards
($) (1)
|
Non-Equity
Incentive
Plan
Compensation
($) (1)
|
Nonqualified
Deferred
Compensation
Earnings
($) (1)
|
All Other
Compensation
($) (1)
|
Total
($) (1)
|
||||||||||||||||||||||||||||||||
|
Avi Barak (2)
Chief Executive Officer and Director until 11/09
|
2009
|
$
|
133,253
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
74,284
|
(5
|
)
|
$
|
207,537
|
||||||||||||||||||||||
|
2008
|
143,100
|
100,000
|
—
|
7,473
|
(4
|
)
|
—
|
—
|
74,659
|
(5
|
)
|
325,232
|
|||||||||||||||||||||||||||||
|
Uri Danon
Chief Executive Officer from 11/09
|
2009
|
21,209
|
—
|
—
|
229,713
|
(3
|
)
|
—
|
—
|
8,508
|
(6
|
)
|
259,431
|
||||||||||||||||||||||||||||
|
Abraham Hochberg (6)
Chief Scientific Officer and Director
|
2009
|
101,866
|
—
|
—
|
—
|
—
|
—
|
48,004
|
(7
|
)
|
149,870
|
||||||||||||||||||||||||||||||
|
2008
|
102,099
|
3,654
|
—
|
4,982
|
(4
|
)
|
—
|
—
|
52,904
|
(7
|
)
|
163,639
|
|||||||||||||||||||||||||||||
|
Moshe Landsberg
Vice President,
Technology
|
2009
|
126,136
|
26,121
|
—
|
—
|
—
|
—
|
23,256
|
(8
|
)
|
175,514
|
||||||||||||||||||||||||||||||
|
2008
|
$
|
134,492
|
$
|
16,478
|
—
|
$
|
13,708
|
—
|
—
|
$
|
22,614
|
(8
|
)
|
$
|
187,292
|
||||||||||||||||||||||||||
|
(1)
|
All compensation received by our executive officers is paid in NIS. For the purposes of completing this table, we converted each NIS denominated amount into U.S. dollars by multiplying the NIS amount by the representative exchange rate as it was published by the Bank of Israel on each date on which the compensation was calculated, so that no single conversion rate has been used.
|
|
(2)
|
Mr. Barak received compensation from us solely for his services as our Chief Executive Officer, and not in his capacity as a member of our Board of Directors.
|
|
(3)
|
On September 21, 2009, our Board of Directors approved the conditions of Mr. Danon’s employment agreement which was entered into on October 18, 2009. Pursuant to the terms of Mr. Danon’s employment agreement, Mr. Danon was granted options to purchase 450,000 shares of our common stock at an exercise price of NIS 3.18 (approximately $0.86) per share, pursuant to the 2007 Stock Option Plan, of which options to purchase 80,000 shares will vest immediately, and the remainder will vest over the course of four years, with partial acceleration in return for the achievement of pre-determined milestones by pre-set dates.
|
|
(4)
|
On October 22, 2008, our Board of Directors approved the grants to Mr. Barak and Professor Hochberg of options to purchase 180,000 and 120,000 shares of our common stock, respectively, under our 2007 Stock Option Plan. On December 3, 2008, the grant of these options was approved by our stockholders as an interested transaction under the Israeli Companies Law with a controlling shareholder due to Mr. Barak’s and Professor Hochberg’s execution of an Irreversible Voting Agreement with two of our stockholders. See “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Corporate Governance — Business Combinations; Interested Transactions.”
|
|
(5)
|
The items described as All Other Compensation to Mr. Barak for the year ended December 31, 2009 include (i) $29,355 in expenses related to the use of a company car by Mr. Barak, (ii) $3,312 in expenses related to the use of a company cell phone by Mr. Barak, and (iii) $41,616 in contributions by our Israeli subsidiary to executive insurance for Mr. Barak’s benefit. The items described as All Other Compensation to Mr. Barak for the year ended December 31, 2008 include (i) $26,549 in expenses related to the use of a company car by Mr. Barak, (ii) $6,084 in expenses related to the use of a company cell phone by Mr. Barak, and (iii) $42,026 in contributions by our Israeli subsidiary to executive insurance for Mr. Barak’s benefit.
|
|
(6)
|
Prof. Hochberg receives compensation from us solely for his services as our Chief Scientific Officer, and not in his capacity as a member of our Board of Directors.
|
|
(7)
|
The items described as All Other Compensation to Mr. Danon for the year ended December 31, 2009 include (i) $2,165 in expenses related to the use of a company car by Mr. Danon, and (ii) $6,343 in contributions by our Israeli subsidiary to executive insurance for Mr. Danon’s benefit.
|
40
|
(8)
|
The items described as All Other Compensation to Professor Hochberg for the year ended December 31, 2009 include (i) $20,557 in expenses related to the use of a company car by Professor Hochberg, (ii) $3,029 in expenses related to the use of a company cell phone by Professor Hochberg, and (iii) $24,419 in contributions by our Israeli subsidiary to senior employees insurance for Professor Hochberg’s benefit. The items described as All Other Compensation to Professor Hochberg for the year December 31, 2008 include (i) $22,581 in expenses related to the use of a company car by Professor Hochberg, (ii) $7,493 in expenses related to the use of a company cell phone by Professor Hochberg, and (iii) $22,830 in contributions by our Israeli subsidiary to senior employees insurance for Professor Hochberg’s benefit.
|
|
(9)
|
The items described as All Other Compensation to Mr. Landsberg for the year ended December 31, 2009 include (i) $21,922 in expenses related to the use of a company car by Mr. Landsberg, and (ii) $1,334 in expenses related to the use of a company cell phone by Mr. Landsberg. The items described as All Other Compensation to Mr. Landsberg for the year ended December 31, 2008 include (i) $21,041 in expenses related to the use of a company car by Mr. Landsberg, and (ii) $1,573 in expenses related to the use of a company cell phone by Mr. Landsberg.
|
Narrative to Summary Compensation Table
Our compensation committee evaluates and sets the compensation policies and procedures for our executive officers. Except as provided for in the employment agreements described below, annual reviews generally determine future salary and bonus amounts for our executive officers, as a part of the Company’s compensation procedures.
Employment Agreements
Avi Barak
Generally. On April 4, 2006, we entered into an employment agreement with Mr. Avi Barak pursuant to which he served as our Chief Executive Officer on a full-time basis. On August 11, 2009, we received a letter from Mr. Barak, announcing his intention to resign his position as CEO, effective January 30, 2010. We appointed Mr. Uri Danon as CEO in Mr. Barak’s place, as of November 1, 2009. Mr. Barak must maintain the confidentiality of all proprietary information of ours that he received through his employment with us.
Salary and Other Social Benefits. The agreement provided Mr. Barak with a monthly salary of $12,000. His salary was subject to adjustment throughout the term of the agreement due to increases in the Israeli Consumer Price Index. We also provided Mr. Barak with other social benefits such as a company car, executive insurance, a laptop computer and a cellular telephone. Mr. Barak was entitled to participate in our advanced studies fund and senior employees insurance. We reimbursed Mr. Barak for reasonable expenses incurred by him in the course of his employment with us. On December 1, 2008, Mr. Barak announced that in view of the global economic crisis, he agreed to reduce his monthly salary by 7.5%.
Bonuses. We committed to pay Mr. Barak a one-time bonus of one percent of funds received between March 1, 2009 and March 1, 2012 (in any form, except for loans from banks and grants under the auspices of the Ministry of Industry, Trade and Labor, such as Chief Scientist grants and bi-national funds), provided that at least $10 million is raised and that Mr. Barak continued to hold a position with us . For the purposes of calculating the bonus, amounts provided by known pre-existing investors (i.e. entities from which we have received funds or assets prior to March 1, 2009 ) would be deducted from the total amount received. In any case, the bonus was not to exceed $100,000. The criteria for the award of this bonus were not reached before Mr. Barak's resignation took effect .
Non-Competition and Non-Solicitation. Under the terms of his employment agreement, Mr. Barak must refrain from competing with us for one year from the date of termination of his employment with us. Further, for one year after his employment terminates, Mr. Barak may not offer or solicit any of our or our subsidiary’s employees, consultants, customers, suppliers, distributors, agents or contractors away from their dealings with us or our subsidiary. He has also granted all rights in any products that he developed during the course of his employment with us to our Israeli subsidiary, BioCancell Therapeutics Israel Ltd.
Stock Option Grant. Pursuant to the terms of Mr. Barak’s employment agreement, we granted Mr. Barak options to purchase 500,000 shares of our common stock at an exercise price of $0.01 per share. Mr. Barak exercised these options on March 28, 2007. On October 22, 2008, our Board of Directors approved the grant to Mr. Barak of options to purchase 180,000 shares of common stock pursuant to our 2007 Stock Option Plan, at an exercise price of $0.597 per share. On December 3, 2008, the grant of these options to Mr. Barak was approved by our stockholders as an interested transaction under the Israeli Companies Law with a controlling shareholder due to Mr. Barak’s execution of an Irreversible Voting Agreement with several of our stockholders and Professor Hochberg, our Chief Scientific Officer. See “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Corporate Governance — Business Combinations; Interested Transactions.” Upon his resignation, the unvested option to purchase 105,000 shares of our common stock previously granted to Mr. Barak was forfeited.
41
Mr. Uri Danon
Generally. On October 18, 2009, we entered into an employment agreement with Mr. Uri Danon, pursuant to which he has served as our Chief Executive Officer since November 1, 2009. Either we or Mr. Danon may terminate this agreement upon the provision of ninety days advance written notice to the other party expressing an intention to terminate the agreement. We also may terminate this agreement for cause, defined in the agreement as including the following on the part of Mr. Danon: (i) a material breach of any term of the agreement; (ii) any breach of Mr. Danon's fiduciary duties to us, including, without limitation, any material conflict of interest for the promotion of his benefit; (iii) fraud, felonious conduct or dishonesty; (iv) embezzlement of our funds; (v) any conduct which is materially injurious to us, monetary or otherwise; (vi) conviction of any felony; (vii) misconduct, gross negligence or willful misconduct in performance of duties and/or responsibilities assigned in the agreement; or (viii) refusal to perform the duties and/or responsibilities assigned in the agreement for any reason other than illness or incapacity, or disregard or insubordination of any lawful resolution and/or instruction of the Board of Directors with respect to Mr. Danon's duties and/or responsibilities towards us.
Salary and Other Social Benefits. The agreement provides Mr. Danon with a monthly salary of 42,000 NIS (approximately $11,350). His salary is subject to adjustment throughout the term of the agreement due to cost-of-living increases. We will also provide Mr. Danon with other social benefits such as a company car, a laptop computer, a cellular telephone, and pension and similar payments. We will reimburse Mr. Danon for reasonable expenses incurred by him in the course of his employment with us.
Bonuses. Mr. Danon is entitled to receive a bonus at such time as we shall raise an aggregate amount of $10 million between January 30, 2010 and the termination of his employment with us, in any form, except for loans from financial institutions and grants under the auspices of the Israeli Ministry of Industry, Trade and Labor, such as Chief Scientist grants and bi-national funds. The amount of the bonus will be $100,000, subject to a deduction of a pro-rata portion based on the funds raised provided by investors from which we have received funds or assets prior to January 30, 2010. Payment will be made in cash if more than $5 million in aggregate has been raised in equity offerings, or in stock options otherwise.
Non-Competition, Non-Solicitation and Confidentiality. Under the terms of his employment agreement, Mr. Danon must refrain from competing with us during the term of his employment and for one year from the date of termination of his employment with us. Further, during his employment and for one year after his employment terminates, Mr. Danon may not offer or solicit any of our or our subsidiary’s employees away from their dealings with us or our subsidiary. He also must grant us all rights in any products that he develops during the course of his employment with us. In addition, Mr. Danon must maintain the confidentiality of all proprietary information of ours that he receives through his employment with us.
Stock Option Grant. Pursuant to the terms of Mr. Danon's employment agreement, we granted Mr. Danon options to purchase 450,000 shares of our common stock at an exercise price of NIS 3.18 (approximately $0.86) per share, pursuant to our 2007 Stock Option Plan, of which options to purchase 80,000 shares vested immediately, and the remainder are vesting over the course of four years, with partial acceleration in return for the achievement of pre-determined milestones by pre-set dates.
Professor Abraham Hochberg
Generally. On December 1, 2005, we entered into an employment agreement with Professor Abraham Hochberg pursuant to which he serves as our Chief Scientific Officer . Under the terms of this agreement, Professor Hochberg manages our research and development activities and reports these activities to our Board of Directors. We may terminate this agreement upon the provision of six months advance written notice. Professor Hochberg may terminate this agreement upon the provision of three months notice. We also may terminate this agreement for cause, meaning any of the following: (a) a material breach of Professor Hochberg’s obligations regarding confidentiality and non-competition, as set out in the agreement; (b) conviction of any felony involving moral turpitude affecting us; (c) any material breach of his employment agreement which has not been cured by him within 15 days after his receipt of notice from us, containing a description of the breach or breaches alleged to have occurred; (d) the habitual neglect or gross failure by Professor Hochberg to adequately perform the duties of his position; (e) any act of moral turpitude or criminal action connected to his employment with us; or (f) Professor Hochberg’s refusal to comply with or his violation of lawful instructions of our CEO or Board of Directors. In addition, we may terminate this agreement in the event that Professor Hochberg is prevented from continuing his employment with us due to medical reasons for 90 consecutive days or for an aggregate of 120 days per fiscal year, but in the event of such termination, Professor Hochberg will be entitled to receive three months additional salary from us and also severance payments in accordance with the Israeli Severance Pay Law. Professor Hochberg must maintain the confidentiality of all of our proprietary information that he receives through his employment with us. On October 22, 2008 our Board of Directors approved the extension of Professor Hochberg's employment agreement for a period of three years , following the termination of the initial term of three years .
Salary and Other Social Benefits. The agreement provides Professor Hochberg with a monthly salary of $6,000, which was raised to $8,500 in July 2007 upon the approval of our stockholders. In October 2008, our Board of Directors recommended that our stockholders restate Professor Hochberg’s salary at a fixed monthly rate of NIS 36,000. His salary is subject to adjustment throughout the terms of the agreement due to increases in the Israeli Consumer Price Index. We also provide Professor Hochberg with other social benefits such as a company car. Professor Hochberg is entitled to participate in our advanced studies fund and senior employees insurance as well as annual leave and convalescence pay and sick leave. We reimburse Professor Hochberg for reasonable expenses incurred by him in the course of his employment with us. On December 1, 2008, Professor Hochberg announced that in view of the global economic crisis, he agrees to reduce his monthly salary by 7.5% until further notice. On December 3, 2008, our stockholders approved the extension of the employment agreement and restatement of Professor Hochberg’s salary as an interested transaction under the Israeli Companies Law with a controlling shareholder, due to Professor Hochberg’s execution of an Irreversible Voting Agreement with several of our other stockholders. See “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Corporate Governance — Business Combinations; Interested Transactions”.
42
Bonuses. Under the terms of his employment agreement, Professor Hochberg is entitled to an annual bonus, determined at the discretion of our Chief Executive Officer, in consultation with our Board of Directors, and subject to applicable law. Because Professor Hochberg is one of our significant stockholders, payment of this bonus is subject to the approval of our audit committee, our Board of Directors and our stockholders.
In addition, Professor Hochberg receives a bonus of 7.5% of the amount of grants that we receive in which he is listed as the leading researcher in the research to be funded by such grants, and that are approved for our use by our Board of Directors, other than grants provided by the Office of the Chief Scientist of the Ministry of Industry, Trade and Labor of Israel.
Non-Competition and Non-Solicitation. Under the terms of the employment agreement, Professor Hochberg may not offer or solicit any of our or our subsidiary’s employees, consultants, customers, suppliers, distributors, agents or contractors away from their dealings with us or our subsidiary during his employment and for 12 months after his employment terminates. Professor Hochberg has promised to cede to us all right, title and interest to any and all intellectual property created during his course of employment with us and has undertaken not to make use of it and not to compete with us for a period of twelve months after termination of his employment with us.
Agreement Regarding Allocation of Royalties With Yissum. In accordance with the directives of the management of the Hebrew University of Jerusalem, any royalties that we pay pursuant to our exclusive license agreement with Yissum are allocated as follows: 40% to Professor Hochberg; 20% to Professor Hochberg's research laboratory; and 40% to Yissum and the Hebrew University of Jerusalem. For more information regarding this license agreement, see “Our Business — Material Operating Agreements”.
Stock Option Grant. On October 22, 2008, our Board of Directors approved the grant of options to purchase 120,000 shares of our common stock at an exercise price of $0.597 per share. These options will vest in twelve equal quarterly portions. On December 3, 2008, the grant of these options to Professor Hochberg was approved by our stockholders as an interested transaction under the Israeli Company Law with a controlling shareholder due to Professor Hochberg’s execution of an Irrevocable Voting Agreement with several of our stockholders and Mr. Barak, our Chief Executive Officer and a member of our Board of Directors. See “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Corporate Governance — Business Combinations, Interested Transactions.”
Mr. Moshe Landsberg
Generally. On February 15, 2007, we entered into a consulting agreement with Mr. Moshe Landsberg pursuant to which he serves as our Vice President of Technology on a part-time basis commencing on April 1, 2007, and later, upon mutual agreement, on a full-time basis. We may terminate this agreement upon the provision of four months advance written notice expressing our intention to terminate the agreement. Mr. Landsberg may terminate this agreement upon the provision of two months advance written notice to us expressing his intention to terminate the agreement. In addition, we may terminate this agreement in the event that Mr. Landsberg is prevented from continuing his consultancy with us due to medical reasons for two consecutive months or for an aggregate of 120 days per fiscal year. Mr. Landsberg must maintain the confidentiality of all of our proprietary information that he receives through his consulting with us.
As part of our continued efforts to improve budgetary efficiency, on April 15, 2010, we terminated the services of Mr. Landsberg, effective July 15, 2010. Mr. Landsberg's current duties, including responsibility for manufacturing, logistics and quality assurance, will thereafter be performed by other members of our management.
Salary and Other Social Benefits. The agreement provides Mr. Landsberg with a monthly salary of NIS 40,000 and NIS 50,000 as compensation for his part-time and full-time consultancy with us, respectively (approximately $10,000 and $12,500, respectively). His payment terms may be adjusted in the future according to the Israeli Consumer Price Index to account for the cost of living. We also provide Mr. Landsberg with other social benefits such as a company car.
Bonuses. We have agreed to pay Mr. Landsberg an annual bonus of two monthly salaries in the event that Mr. Landsberg satisfies certain performance criteria as agreed upon in advance and in writing with us.
Non-Competition and Non-Solicitation. Under the terms of the consulting agreement, Mr. Landsberg must refrain from competing with us during the term of his consultancy and for 12 months from the date of termination of his consulting with us. Further, during his consultancy and for 12 months thereafter, Mr. Landsberg may not offer or solicit any of our or our subsidiary’s employees, consultants, customers, suppliers, distributors, agents or contractors away from their dealings with us or our subsidiary. He also must grant all rights in any products that he develops during the course of his consulting with us to our subsidiary, BioCancell Therapeutics Israel Ltd.
Stock Option Grant. Pursuant to the terms of Mr. Landsberg’s consulting agreement, on October 22, 2008, our Board of Directors approved the grant of options to purchase 260,000 shares of our common stock at an exercise price of NIS 1.27 (approximately 31 cents) per share. These options will vest according to the following schedule: one sixteenth of the options will vest at the end of every calendar quarter, from the time of grant and until all are vested. As his termination is not for Cause (as defined in his option agreement), the unvested portion of his option will vest upon his termination becoming effective.
43
Outstanding Equity Awards at December 31, 2009
The following are all unexercised options, unvested shares of common stock and any other awards granted under our 2004 Stock Option Plan and 2007 Stock Option Plan held by any of our named executive officers as of December 31, 2009 :
|
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
|
||||||||||||
|
OPTION AWARDS
|
STOCK AWARDS
|
|||||||||||
|
Name
|
Number of Securities
Underlying Unexercised Options
|
Equity Incentive
Plan Awards:
Number of Securities Underlying Unexercised Unearned Options
|
Equity Incentive
Plan Awards:
Number of Securities Underlying Unexercised Unearned Options
|
Option Exercise
Price
|
Option Expiration Date
|
Number of Shares or
Units of Stock that
have not Vested
|
Market Value of
Shares of Units of
Stock that Have Not Vested
|
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other
Rights that have not
Vested
|
Equity Incentive Plan Awards:
Market or Payout Value of Unearned
Shares, Units or other Rights that
have not Vested
|
|||
|
Avi Barak
|
75,000
|
105,000
|
$0.597
|
12/3/2018
|
-
|
-
|
-
|
-
|
||||
|
Uri Danon
|
103,125
|
346,875
|
$0.84
|
12/31/2019
|
-
|
-
|
-
|
-
|
||||
|
Abraham Hochberg
|
50,000
|
70,000
|
$0.597
|
12/3/2018
|
-
|
-
|
-
|
-
|
||||
|
Moshe Landsberg
|
81,250
|
178,750
|
$0.34
|
12/3/2018
|
-
|
-
|
-
|
-
|
||||
Director Compensation
The following table sets forth information regarding the compensation paid to each of our directors and past directors who were not our employees during the year ended December 31, 2009 :
| Name |
Fees
Earned or
Paid
in Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive
Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||
|
Jacob Ben Gur
|
$ | 12,567 | — | — | — | — | — | $ | 12,567 | |||||||||||||||||||
|
\
|
12,437 | — | — | — | — | — | 12,437 | |||||||||||||||||||||
|
Doron Nevo
|
6,989 | — | 10,213 | — | — | — | 17,202 | |||||||||||||||||||||
|
Hanoch Rappaport
|
2,861 | — | 10,213 | — | — | — | 13,074 | |||||||||||||||||||||
|
Alexander Levitzki
|
3,937 | — | 1,246 | — | — | — | $ | 3,408 | ||||||||||||||||||||
|
Ofer Goldberg
|
— | — | — | — | — | — | — | |||||||||||||||||||||
|
Aviv Boim
|
$ | — | — | — | — | — | — | — | ||||||||||||||||||||
Narrative to Director Compensation Table
Members of our Board of Directors who are independent directors (as defined in our Amended and Restated Certificate of Incorporation) receive the fixed compensation established under Israeli regulations, NIS 31,700 (approximately $ 8,455 ) per annum, plus NIS 2,120 (approximately $ 565 ) per meeting (60% of such amount for participation via teleconference, and 50% of such amount for approving a written resolution).
Non-employee Members of our Board of Directors who are not independent directors receive compensation of NIS 1,800 (approximately $ 480 ) per meeting. No other directors receive any compensation for their service on our Board of Directors.
44
Director and Officer Indemnification
Our Second Amended and Restated Bylaws provides that each person who was or is a party or is threatened to be made a party to or is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he, or a person of whom he is the legal representative, is or was a director or officer of our company or is or was serving at the request of our company as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans, whether the basis of such proceeding is alleged action or inaction in an official capacity or in any other capacity while serving as director, officer, employee or agent, shall be indemnified and held harmless by us to the fullest extent permitted by the Delaware General Corporation Law, as amended from time to time, against all costs, charges, expenses, liabilities and losses (including attorneys’ fees, judgments, fines, ERISA excise taxes or penalties and amounts paid or to be paid in settlement) reasonably incurred or suffered by such person in connection therewith.
In addition, our Second Amended and Restated Bylaws provides that the right to indemnification conferred in the our Second Amended and Restated Bylaws shall be a contract right and shall include the right to be paid by us the expenses incurred in defending any such proceeding in advance of its final disposition .
We currently maintain directors’ and officers’ liability insurance for the purpose of paying these types of claims. The insurance policy provides coverage in an amount of $5,000,000 per claim or per the aggregate loss arising from all claims for each insurance period, and an additional coverage of up to $1,000,000 for legal expenses.
BioCancell Therapeutics, Inc. 2004 Stock Option Plan and 2007 Stock Option Plan
General Provisions. Our Board of Directors adopted our 2004 Stock Option Plan to allocate up to 2,024,003 shares of our common stock to our directors, employees and consultants. Our Board of Directors adopted our 2007 Stock Option Plan to allocate up to an additional 2,000,000 shares of our common stock to our directors, employees and consultants. Each plan is administered by our Board of Directors and any committee that our Board of Directors may appoint for such purpose. Our Board of Directors or its designated committee may grant options and restricted stock in addition to other compensation instruments under each of the plans. With respect to options, they may grant four types of options under either plan: Approved 102 Capital Gains Options, which are granted only to our directors and employees and qualify for capital gains tax treatments; Approved 102 Ordinary Income Options, which qualify for ordinary income tax treatment; Unapproved 102 Options; and 3( i ) Options, which are non-qualified stock options which are granted mostly to our consultants. The number of shares authorized to be issued under each of the plans will be proportionately adjusted for any increase or decrease in the number of issued shares of common stock resulting from a stock split, reverse stock split, combination or reclassification of the stock or the payment of a stock dividend with respect to the common stock or any other increase or decrease in the number of issued shares of common stock effected without receipt of consideration.
The exercise price of an option granted under our 2004 Stock Option Plan or 2007 Stock Option Plan will be determined by the Board of Directors or its designated committee at the time of the option grant.
The vesting schedule of each grant is detailed in the corresponding options allocation agreement . The committee, though, may, in its absolute discretion and on such terms and conditions as it deems appropriate, accelerate or otherwise change the time at which options granted under either plan or any portion of any such option will vest. Option grants under either plan also may contain performance goals and measures and the provisions in one option grant need not be identical to any other option grant. All options granted under either plan will expire ten years from the date of grant unless terminated earlier, provided that options granted under Section 422 of the United States Internal Revenue Code of 1986 to a stockholder that holds ten percent or more of our common stock will expire five years from the date of grant unless terminated earlier. With respect to each of the plans, our Board of Directors may reallocate to other employees, directors or consultants the unvested portion of an option that expires prior to its expiration date and the vested but unexercised and unvested portions of an option that was either cancelled or repurchased by us and any such reallocation of shares must be recycled within the plan pursuant to which such option was granted. Our Board of Directors may not reallocate the vested but unexercised portions of an option that expires upon its expiration date.
In the event of the disability or death of a grantee and, in the case of our 2004 Stock Option Plan, the retirement of a grantee, and, in the case of our 2007 Stock Option Plan, the termination of the grantee’s employment by us without cause (as that term is defined in our 2007 Stock Option Plan), the grantee or his legal delegates or successors, as the case may be, may only exercise that portion of the option that had vested as of the date of any such event and may exercise the vested portion within one year from the date of such event. In cases of retirement for options granted under our 2004 Stock Option Plan, if the grantee dies within one year from the date of retirement, the vested portion may be exercised by such deceased grantee’s successor within one year from the date of death so long as the option has not otherwise expired. Furthermore, for options granted under our 2004 Stock Option Plan, in the event that the grantee’s employment was terminated by us without cause (as that term is defined in our 2004 Stock Option Plan), the grantee may exercise that portion of the option that had vested as of such date of termination and may exercise such vested portion within 90 days from such date. In the event that the grantee’s employment was terminated by us for cause or was terminated by the grantee, the grantee shall no longer have the right to exercise any option granted under our 2004 Stock Option Plan held by him irrespective of whether and to what extent his options have vested. For options granted under our 2007 Stock Option Plan, in the event that the grantee’s employment was terminated by us for cause, the grantee may exercise that portion of the option that had vested as of such date of termination and may exercise the vested portion within 30 days from such date.
Our Board of Directors or its appointed committee is entitled, at any time and from time to time, to modify the terms of either plan or to suspend or completely cancel either of the plans, and any such modification, suspension or cancellation may have retroactive effect, provided that the modification, suspension or cancellation does not adversely affect the rights of the grantees in a material way, and any such material and adverse modification, suspension or cancellation will be invalid unless it is approved by the affected grantee.
45
Termination of an Option. Subject to our Board of Director’s approval, the committee administering either plan may, from time to time, cancel all or any portion of an option granted under such plan, and our obligation with respect to options of such plan will be discharged through (i) payment to the grantee of an amount in cash equal to the excess, if any, of the fair market value of the cancelled option at the date of such cancellation over the aggregate exercise price of the option, (ii) the issuance or transfer to the grantee of common stock with a fair market value at the date of such transfer equal to any such excess, or (iii) a combination of cash and shares with a combined value equal to any such excess, as determined by the committee, in its sole discretion.
Also, in the event of a merger or consolidation in which we are not the surviving entity, an acquisition of all of our capital stock or the sale of all or substantially all of our assets, we, in our sole and absolute discretion, may cancel all outstanding options granted under our 2004 Stock Option Plan that remain unexercised prior to the consummation of any such transaction, provided that we send a cancellation notice to each grantee of our 2004 Stock Option Plan at least twenty days prior to the consummation of any such transaction during which notification period each grantee may exercise his options. In cases of merger, our Board of Directors may exchange all outstanding options granted under our 2004 Stock Option Plan that remain unexercised prior to the consummation of the merger for the securities of the surviving corporation or to pay the fair market value of any such option. In the event that we do not send a cancellation notice, or in the event of any changes in our capital structure by reason of stock split, stock dividends, reorganization, issue of rights or convertible or capital stock or any corporate transaction or other events with an similar impact, we may make an equitable adjustment in the number of shares resulting from the exercise of options granted under our 2004 Stock Option Plan and/or the exercise price of such options in order to prevent significant dilution.
With respect to our 2007 Stock Option Plan, in the event of a merger or consolidation in which we are not the surviving entity, an acquisition of all of our capital stock or the sale of all or substantially all of our assets, the person acquiring us, in its sole and absolute discretion, either may convert or exchange options granted under our 2007 Stock Option Plan to options to purchase securities of such person or may cancel all outstanding options granted under our 2007 Stock Option Plan that remain unexercised prior to the consummation of any such transaction, provided that it sends a cancellation notice to each grantee of our 2007 Stock Option Plan at least twenty days prior to the consummation of any such transaction during which notification period each grantee may exercise his options. Other than transactions in which we are acquired, in the event of any changes in our capital structure by reason of stock split, stock dividends, reorganization, issue of rights or convertible or capital stock or any corporate transaction or other events with an similar impact, we may make an equitable adjustment in the number of shares resulting from the exercise of options granted under our 2007 Stock Option Plan and/or the exercise price of such options in order to prevent significant dilution.
Outstanding Grants. As of the date of this prospectus, we have 478,426 options outstanding under our 2004 Stock Option Plan. The options under our 2004 Stock Option Plan are not listed for trading on the TASE.
As of the date of this prospectus, we have 1, 897,750 options outstanding under our 2007 Stock Option Plan. We have not issued any shares of our common stock upon exercise of options granted under our 2007 Stock Option Plan. The options under our 2007 Stock Option Plan are not listed for trading on the TASE.
Equity Compensation Plans
The following table sets forth information as of December 31, 2009, regarding our equity compensation plans, our 2004 Stock Option Plan and our 2007 Stock Option Plan, under which we grant securities exercisable for shares of our common stock to employees, directors and consultants of our company and employees, directors and consultants of our present and future subsidiaries.
|
Plan Category
|
Number of Shares
to Be Issued upon
Exercise of
Outstanding Options
|
Weighted Average
Exercise Price of
Outstanding Options
|
Number of Shares
Remaining Available
for Future Issuance
under Equity
Compensation Plans
(excluding shares
in Column A)
|
|||||||||
|
Equity Compensation Plans approved by securityholders
|
585,489
|
$
|
0. 16
|
-
|
||||||||
|
Equity Compensation Plans not approved by securityholders
|
1, 786 ,500
|
$
|
0. 61
|
213 ,500
|
||||||||
|
Total
|
2,371 ,989
|
$
|
0. 50
|
213,500
|
||||||||
46
CORPORATE GOVERNANCE
As a result of the listing of our securities on the TASE, we are subject to certain provisions of the Israeli Securities Law — 1968. Pursuant to Section 39A of the Israeli Securities Law, rules and regulations of the Israeli Companies Law — 1999 listed in the Fourth Schedule apply to issuers incorporated in jurisdictions outside Israel which offer securities to the public in Israel. Under Articles V, VI, VII, VIII, IX, XI and XII of our Amended and Restated Certificate of Incorporation, we are subject to such provisions of the Israeli Companies Law — 1999 and the Israeli Securities Regulations to the extent permitted by Delaware law. The following describes the applicable provisions of the Israeli Companies Law that apply to us in addition to the laws of the State of Delaware.
Number of Directors; Election; Removal; Filling Vacancies; Independent Directors
Our Second Amended and Restated Bylaws provide that our Board of Directors shall consist of at least two, and no more than nine, members. Pursuant to an Irrevocable Voting Agreement, certain of our stockholders have agreed to vote their shares of our common stock in favor of nominees for election to our Board of Directors appointed by such stockholders. See “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Certain Relationships and Related Transactions — Private Placements.”
Pursuant to Sections 95 and 121(c) of the Israeli Companies Law and Article VI of our Amended and Restated Certificate of Incorporation, we are subject to certain procedural and board composition requirements. A director that holds the office of Chairman of the Board of Directors may not also hold the office, or be granted the powers of, the Chief Executive Officer, unless authorized in accordance with Section 121(c) of the Israeli Companies Law. Section 121(c) provides that the stockholders at a general meeting of a public company may resolve that for a period of no more than three years the Chairman of the Board of Directors may be authorized to fulfill the duties of the Chief Executive Officer, provided that , in counting the votes at the meeting at least one of the following is true: (i) the majority must include at least two-thirds of the stockholders who are not holders of control in the corporation present at the vote and abstaining votes must not be taken into account in counting the votes of such stockholders; or (ii) the total of opposing votes from among the stockholders referenced in subsection (i) does not exceed 1% of all of the voting rights in the corporation.
Pursuant to Sections 239 through 249A of the Israeli Companies Law, our Board of Directors must at all times include at least two directors who are elected by stockholders where at least one of the following is true: (i) the majority of the votes represented at the stockholders’ meeting includes at least one-third of all of the votes of the stockholders who are not holders of control in the corporation and who participate in the voting (with abstentions not being included in the total votes of the foregoing stockholders); or (ii) the total of opposing votes from among the stockholders referenced in subsection (i) does not exceed 1% of all of the voting rights in the corporation. Directors elected in accordance with this provision are deemed to be “independent directors.”
For purposes of this provision, “control” is defined in the Israeli Companies Law as the ability to direct the activity of the corporation, and a person shall be presumed to control a corporation if he holds 50% or more of the means of control of the corporation such as the right to vote at a general meeting or a corresponding body of the corporation or the right to appoint directors or the general manager of the corporation. If at the time of the appointment of an independent director, all of the members of the board of directors are of one gender, the second independent director shall be of the other gender.
Any vacancy in the office of an independent director may be filled only by the stockholders and may not be filled by the remaining directors. If at any time there are fewer than two independent directors, Board of Directors must call a special stockholders’ meeting for the election of independent directors. An independent director may not be removed from office without cause and no person may serve as an independent director for more than six one-year terms. An independent director is required to be a resident of the State of Israel, provided that a public company, the shares of which have been offered to the public outside of Israel, or are listed on a stock exchange outside of Israel, may appoint an independent director who is not an Israeli resident.
An independent director must have professional qualifications or accounting and financial expertise, and at least one of the independent directors must have accounting and financial expertise.
No individual may be appointed as an independent director if, at the time of appointment or at any time during the preceding two years, he, his relative, partner, employer or a corporate body of which he is a controlling party had an interest in the corporation, in a person who was a controlling party of the corporation at the time of the appointment, or in another corporate body. For the purposes of this provision, (i) “interest” means an employment relationship, commercial or professional ties in general or control, as well as service as an officer, other than service as a director who was appointed as an independent director in a corporation that is due to offer shares to the public for the first time, and (ii) “other corporate body” means a corporate body in which the corporation or controlling member of it is a controlling member at the time of the appointment or during the two years before the time of appointment.
47
A director in a corporation cannot be appointed as an independent director in another corporation if at that time a director of the other corporation serves as an independent director of the first corporation. An independent director may not receive any compensation from the corporation, except as determined by applicable law and except for insurance coverage and indemnification by the corporation’s bylaws or as determined by other applicable law. An individual cannot be appointed as an independent director if he holds any other position or owns any business which might give rise to a conflict of interest with his role as a director, or if such circumstances might harm his ability to act as a director. An individual cannot be appointed as an independent director if he is a member of the Israel Securities Authority or an employee thereof, or if he is a member of the board of directors or an employee of an Israeli stock exchange.
A general meeting of our stockholders at which the appointment of an Independent Director is on the agenda may only be convened if the nominee has declared that he fulfils the conditions required for being appointed as an Independent Director.
Each committee of our Board of Directors authorized to exercise any of the powers of our Board of Directors must include at least one independent director.
The term of office of an independent director is three years, and we may appoint an incumbent independent director for one additional term of three years. If our Board of Directors learns of a suspicion that an independent director no longer meets the condition required under the applicable law for his appointment, or that he has violated his fiduciary duty of loyalty, the board of directors shall discuss the matter at the meeting first convened after it learned of the matter. If our Board of Directors determines that the independent director no longer meets a condition required under applicable law for his appointment or that he has violated his fiduciary duty of loyalty, than the board of directors must call a special meeting of our stockholders for the removal of that independent director. The reasons of our Board of Directors must be presented at the meeting of our stockholders, and the independent director must be given a reasonable opportunity to present his position; the decision of our stockholders to terminate an independent directors’ term of office must be adopted with the same majority that was necessary for his appointment.
An Israeli court may, pursuant to the application of a director or stockholder, order the removal of an independent director if it determines that the director has ceased to fulfill one of the conditions required under the Israeli Companies Law for his appointment, or that he has committed a breach of a fiduciary duty to the corporation.
For a period of two years following the termination of an independent director, a corporation may not directly or indirectly enter into any employment, consulting or other arrangement for fees with such person, and such person may not serve as a director or officer of the corporation, including a corporation controlled by such person.
Director Liability
We are subject to Sections 252 through 256 of the Israeli Companies Law, which are addressed in Article XII of our Amended and Restated Certificate of Incorporation. These sections provide that: (i) an officer owes a duty of care to the corporation as provided in Sections 35 and 36 of the Civil Wrongs Ordinance, (ii) an officer shall act with the standard of proficiency which a reasonable officer, in the same position and in the same circumstances, would act; and (iii) an officer shall owe a fiduciary duty to the corporation, and shall act in good faith and for the benefit of the corporation. We may approve, subject to the applicable provisions of the Israeli Companies Law with respect to transaction approval procedures, an act that would otherwise be deemed a violation of an officer’s fiduciary duty to the corporation if (1) the officer acted in good faith and neither the act nor the approval of the act prejudices the corporation, and (2) the officer disclosed the essence of his personal interest in the act, including any substantial fact or document, a reasonable time before the date of the discussion of the approval.
Business Combinations; Interested Transactions
Pursuant to Sections 270(4) and 275 through 282 of the Israeli Companies Law, and in accordance with Section 6.4 of our Amended and Restated Certificate of Incorporation, we may not enter into an “interested transaction” unless the transaction is approved by the following in the following order: (i) the audit committee of our Board of Directors, (ii) our Board of Directors; and (iii) the meeting of stockholders, on condition that one of the following applies: (A) the majority of the votes at the meeting of stockholders includes at least one-third of all of the votes of stockholders who do not have a personal interest in the approval of the transaction and who are participating in the vote (abstentions shall not be included in the total of the votes of such stockholders), or (B) the total of opposing votes from among such stockholders does not exceed 1% of all the voting rights in the corporation. For purposes of this provision, “personal interest” means a personal interest of a person in an action or a transaction of a corporation including a personal interest of his relative of and of another corporation in which he or his relative are interested parties, excluding personal interests deriving only from the holding of stock.
The term “interested transaction” means an extraordinary transaction of a public company with its controlling stockholder (as defined in Sections 1 and 268 of the Israeli Companies Law), or an extraordinary transaction of a public company with another person in whom the holder of control has a personal interest, including a private offering in which the holder of control has a personal interest and also a contract of a public company with its holder of control or such holder’s relative, if he also is an officer of the corporation, with respect to his service and employment conditions, and if he is an employee of the corporation but is not an officer, then with respect to his employment by the corporation.
48
In addition, certain procedures must be followed by any stockholder voting on an interested transaction, or who has a personal interest in the interested transaction, and by any director who has a personal interest in the interested transaction.
We are governed by the Israeli Securities Regulations (Transactions between a Company and its Controlling Shareholder) — 2001 to the full extent permitted by Delaware law. The Israeli Securities Regulations generally govern transactions between a company and a controlling stockholder and the stockholders’ meetings convened for the purpose of approving a transaction with a controlling stockholder. For purposes of these provisions, the term “controlling stockholder” is defined in the Israeli Companies Law. The regulations require that the company give notice to the stockholders of a transaction with a controlling stockholder and that the company provide a transaction report containing all relevant details of the transaction. The regulations govern the contents of the notice and the transaction report and provide that the Israel Securities Authority may deliver a directive to the company requesting that the company amend the transaction report.
In connection with the closing of a private placement of our securities in July 2008, certain of our stockholders entered into an Irrevocable Voting Agreement regarding the election of certain of our directors. Because these parties hold in the aggregate more than 45% of the voting rights of our stockholders, the Israeli Securities Authority regards these parties to have formed a control block under the Israeli Securities Regulations, and as a result an exceptional transaction between us and any of these parties would be regarded as an interested transaction. For more information regarding the Irrevocable Voting Agreement, see “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” and “Certain Relationships and Related Transactions.”
Meetings of Stockholders
Pursuant to Sections 87 and 89 of the Israeli Companies Law and Article V of our Amended and Restated Certificate of Incorporation, we must comply with certain requirements regarding meetings of our stockholders. A notice of any annual or special meeting of our stockholders must be accompanied by a form of proxy card setting forth the resolutions to be presented by our Board of Directors for a vote at such meeting. In addition, the Israeli Companies Law provides that stockholders in a public company may vote at a stockholders’ meeting by means of a voting paper in which the stockholder indicates how he votes on resolutions relating to: (i) the appointment and removal of directors; (ii) approval of acts or transactions requiring the approval of the general meeting pursuant to Sections 255 and 268 through 275 of the Israeli Companies Law; (iii) approval of a merger pursuant to Section 320 of the Israeli Companies Law; (iv) any other matter with respect to which the company’s charter provides that decisions of the general meeting also may be passed by means of a voting paper; and (v) other matters prescribed by the Israeli Minister of Justice pursuant to Section 89 of the Israeli Companies Law.
Pursuant to Section 88 of the Israeli Companies Law which we have adopted in Article V of our Amended and Restated Certificate of Incorporation, the Board of Directors and any person at the request of whom the Board of Directors convenes a stockholders meeting, may approach the stockholders in writing through the Company, in order to convince them to vote in a certain manner on the resolutions set forth in clauses (i) to (v) above. The Company is then required to send the position notification to its stockholders. If the agenda of a stockholders meeting includes any of the resolutions set forth in clauses (i) to (v) above, a stockholder may approach the Company and request to send a position notification on his behalf to the other stockholders. The distribution of such position notifications is subject to certain technical requirements set forth in regulations enacted by virtue of Section 89 of the Companies Law.
Pursuant to Section 2.4 of our Second Amended and Restated Bylaws, each notice of a stockholders meeting shall be given, personally or by mail, not fewer than the minimum number of days required for public companies under the Israeli Companies Law and all regulations provided thereunder, provided that such number of days is not less than the minimum number of days required under Delaware law.
Each beneficial stockholder whose shares are held by a bank or a member of the TASE and are registered on the books of the company, or in the name of any company acting for The Depository Trust Company, shall be permitted to participate at the meeting and to vote such stock by proxy.
Committees of the Board of Directors; Audit Committee, Compensation Committee and Executive Committee
Our Board of Directors may designate committees of the Board, determine the obligations of any such committee and identify such powers as our Board of Directors may delegate to a committee.
Pursuant to Section 114 through 117 of the Israeli Companies Law and Article VII of our Amended and Restated Certificate of Incorporation, our Board of Directors must appoint an audit committee comprised of at least three (3) directors. The members of our audit committee must include the independent directors, and may not include (i) the Chairman of our Board of Directors, (ii) any director who is either employed by, or provides services to the company on a regular basis (other than in the capacity as a director), or (iii) any director who is either a controlling stockholder of us or any relative of a controlling stockholder.
Under the Israeli Companies Law, the audit committee must have the following duties: (i) to identify deficiencies in the management of the company in consultation with its Internal Auditor and auditing auditors, and recommending corrective measures if needed; and (ii) to consider the approval of actions as set forth in Sections 255 and 268 through 275 of the Israeli Companies Law, including an interested transaction.
The charter of our audit committee provides that our audit committee will be appointed by our Board of Directors and will oversee the following subjects: internal controls and risk management; internal audit; auditors, compliance, reporting responsibilities; and approval of transactions. Our audit committee must consist of no less than three members of the Board of Directors, two of which are considered independent directors under the applicable standards of Israeli law. Our audit committee is currently composed of three members: Orly Yarkoni, David Schlachet and Hanoch Rappaport .
49
Our Board of Directors also maintains a compensation committee whose duty is to make recommendations to our Board of Directors regarding employee and director compensation issues, including the compensation of new executives and directors, material changes to the compensation of current executives and directors, and the awarding of employee stock options. Our compensation committee is currently composed of four members , Doron Nevo , Aviv Boim, Hanoch Rappaport and Orly Yarkoni .
Upon and as a condition to the closing of the private placement of our securities to Clal Biotechnology Industries Ltd., Tikcro Technologies Ltd. and Provident Fund of the Hebrew University Ltd. in July 2008, our Board of Directors appointed an executive committee initially consisting of four members of the Board, Professor Hochberg, an external director (currently David Schlachet ), and the members of our Board of Directors nominated by each of Clal Biotechnology Industries Ltd. and Tikcro Technologies Ltd. As of the date of this prospectus, CBI does not have a nominee on the Board, so the Executive Committee currently consists of three members of the Board: Professor Hochberg , Mr. David Schlachet and Tikcro’s nominee, Mr. Aviv Boim. As required under the July 2008 private placement agreements, approval of our Board of Directors of the consummation of any material transaction involving us, the adoption of our annual budgets and any matters relating to our investment policies and working plan, are subject to the approval of our executive committee. See “Certain Relationships and Related Transactions — Private Placements” regarding our obligations with respect to the formation of the executive committee.
We do not maintain a standing nominating committee of our Board of Directors.
Internal Auditor
We are subject to certain Israeli laws regarding our internal auditor. We must have an internal auditor appointed by our Board of Directors based upon the proposal of our audit committee. The internal auditor must be a natural person who is not (i) an interested party (as interested under applicable law), or a relative of such interested party, (ii) an officer of the company or a relative of any officer of the company, or (iii) the company’s auditing accountant or its designee.
The internal auditor must perform his duties consistent with Sections 3(a), 4(b), 8 through 10, 14(b) and 14(c) of the Israeli Internal Audit Law, 1992, subject to the provisions of the Israeli Companies Law. The Israeli Internal Auditing Law governs internal auditing in public entities such as governmental offices, governmental companies and statutory institutes. The Israeli Companies Law applies seven general sections from the Israeli Internal Auditing Law on the internal auditor of a public company, which sections relate to the Internal Auditor’s residence, experience, education and criminal record, the internal auditor’s professional standards, restrictions over the internal auditor’s additional positions and conflicts of interest, the internal auditor’s authority, the internal auditor’s report as evidence and permission to expand and/or preserve the internal auditor’s authority by the company, subject to Sections 146 through 153 of the Israeli Companies Law.
The internal auditor must submit periodic or annual audit plans to our audit committee or to our Board of Directors, as determined by our Board of Directors, for its advance approval. The Chairman of the Board of Directors or the chair of our audit committee may delegate to the internal auditor additional audit duties for urgent examination in addition to the audit plan, in which case the findings will be reported to the appointing officer. The internal auditor must, among other duties, audit our compliance with applicable laws and proper business practices. The internal auditor must report his findings to the Chairman of the Board of Directors, the chairman of our audit committee or our general manager.
The internal auditor’s term in office may not be terminated without his agreement, and he shall not be suspended from office, unless our Board of Directors determines to terminate the internal auditor based upon the recommendation of our audit committee and after the internal auditor was given a reasonable opportunity to state his case before our Board of directors and before our audit committee. Notwithstanding any other provision set forth in our Amended and Restated Certificate of Incorporation or Second Amended and Restated bylaws, the majority of our entire Board of Directors shall constitute a quorum for the termination of office of the internal auditor by our Board of Directors.
Stockholders’ Rights to Examine Books and Records
Pursuant to Sections 184 and 185 of the Israeli Companies Law, our stockholders have the right, upon written request, to receive copies of the minutes of meetings of stockholders, our stock ledger, copies of our governing documents, and a list of our stockholders. Stockholders also have the right, upon written request setting forth the purpose thereof, to receive copies of any document relating to any act or transaction requiring the consent of stockholders pursuant to Sections 255 and 268 through 275 of the Israeli Companies Law. We may refuse a stockholder’s request if we, acting through our Board of Directors, believe that such demand was not made in good faith or that the requested documents include a commercial secret or patent or that the disclosure would otherwise have an adverse effect on the corporation.
50
Compromises and Arrangements with Creditors and Stockholders
Pursuant to Article XI of our Amended and Restated Certificate of Incorporation, whenever a compromise or arrangement is proposed between a company and its creditors or any class of them and/or between the company and its stockholders or any class of them, any court of equitable jurisdiction within the State of Delaware may, on the application of the company or of any creditor or stockholder, or on the application of any receiver or receivers appointed for the company under the provisions of Section 291 of Title 8 of the Delaware Code, or on the application of trustees in dissolution or of any receiver or receivers appointed for the company under the provision of Section 279 of Title 8 of the Delaware Code, order a meeting of the creditors or class of creditors, or of the stockholders or class of stockholders, as the case may be, to be summoned in such manner as the court directs. If a majority in number representing three-fourths in value of the creditors or class of creditors, and/or of the stockholders or class of stockholders, as the case may be, agree to any compromise or arrangement and to any reorganization of the company as a consequence of such compromise or arrangement, such compromise or arrangement and the reorganization shall, if sanctioned by the court to which the application has been made, be binding on all creditors or class of creditors, or on all of the stockholders or class of stockholders and on the company.
Corporate Action without a Stockholder Meeting
Under Section 4.2 of our Amended and Restated Certificate of Incorporation, if our stockholders take action by written consent, we will be required to give prompt notice of such action to any stockholder who did not execute such written consent.
Special Tender Offers; Forced Sale of Shares
Sections 328 through 340 and Section 342A of the Israeli Companies Law are applicable to us and are addressed in Article IX of our Amended and Restated Certificate of Incorporation. These provisions relate to special tender offers and forced sales of shares. Such sections provide that in a public company, no purchase shall be affected as a result of which a person shall become a holder of a control block (25% of the voting rights of the company), if there is no such holder in the company, and no purchase shall be effected as a result of which the purchaser’s holding shall increase above 45% of the voting rights of the company, if there is no other person holding more than 45% of the voting rights in the company, other than by way of a tender offer in accordance with the Israeli Companies Law.
Notwithstanding the foregoing, Section 328(b) of the Israeli Companies Law provides that such a transaction, in which a control block is acquired, may be effected if approved by the general meeting as a transaction, the purpose of which is to create a control block of 25% or 45% of our voting rights, as applicable.
Where a special tender offer has been made, our Board of Directors must give its opinion to the offerees regarding the advisability of the special tender offer and must disclose all personal interests of each of the directors in such tender offer. Our Board of Directors need not give such an opinion, though, if it is unable to do so, provided that it gives notice of its reasons for not so doing. A special tender offer must be made to all offerees, and the offer may only be accepted by a majority of the votes of those offerees who gave notice of their position with respect to the offer. The votes of a holder of a controlling interest in the offeror, a holder of a control block in us or any person acting on their or on the offeror’s behalf, shall not be taken into account. Where a special purchase offer has been accepted, offerees who have not given notice of their position in respect of the tender offer, or who have objected to it, may consent to the offer, no more than four days after the last day for accepting the tender offer, and they shall be considered to have consented to the offer from the outset.
An officer in a target company may negotiate with an offeror for the improvement of the conditions of his offer, and may negotiate with other in order to obtain a counter offer. An officer in a target company who commits an act, other than negotiations as aforesaid, the purpose of which is to forestall an existing or anticipated special tender offer, or to harm the chances of its being accepted, shall be liable to the offeror and the offerees for any damage resulting from his acts, unless he acted in good faith and had reasonable grounds for presuming that the act done by him was for the good of the corporation.
A special tender offer may not be accepted unless shares conferring at least 5% of the voting rights in the company have been purchased. Shares purchased in contravention of these requirements of the Israeli Companies Law will not confer any right and will be “dormant” shares as defined in the Israeli Companies Law as long as the shares are owned by the purchaser.
Where a special tender offer has been accepted, the offeror, or any person controlling the offeror at the time of the offering, and any company controlled by them, shall not for a period of one year following the date of the tender offer, make another tender offer for the purchase of shares, and they shall not effect a merger of the company unless they undertook to do so in the special tender offer.
A person may not purchase shares of a public company such that after the purchase he holds more than 90% of the shares other than by way of a tender offer of all of the shares. When a stockholder owns more than 90% of all of the shares, he shall not purchase any additional shares for so long as he continues to hold at least 90% of the shares.
51
Where a complete tender offer is accepted by the offerees in such a way that the rate of holding of the offerees who did not accept the offer is less than 5% of the issued shares, all of the shares that the offeror sought to purchase shall be transferred to him. Where a complete tender offer is not accepted, the offeror shall not purchase shares from any offerees who have accepted the offer that will confer on him a holding of more than 90% of all of the outstanding shares.
Upon application of an offeree submitted no later than three months after the date of receipt of the complete tender offer, a court may rule that the consideration paid for shares in a tender offer was less than their fair value and that fair value should be paid.
Class and Derivative Action
We are subject to Sections 194 through 218 of the Israeli Companies Law. These provisions relate to class and derivative actions. Any of our stockholders or directors, which we referred to as a “Plaintiff”, may file a derivative action if the conditions set forth in the above provisions have been met. Any person wishing to file a derivative action shall address the corporation in writing, demanding that it exhaust its right by instituting an action, which we referred to as a “Demand.” The Demand must be addressed to the Chairman of the Board of Directors and must set out in detail the facts giving rise to the cause of action and the reasons for its submission. We may proceed in one of the following ways upon receipt of a Demand: (i) take any action or pass any resolution resulting in the dropping of the cause of action; (ii) reject the Demand for reasons specified in its resolution; (iii) resolve to file a suit. We must inform the Plaintiff of the way in which we elect to proceed. A Plaintiff may file a derivative action with the approval of the court, if one of the following applies: (i) the action taken or the resolution made, did not, in the Plaintiff’s opinion, bring about the dropping of the cause of action; (ii) we rejected the Demand; (iii) we gave notice to the Plaintiff that it has resolved to file a suit, but no suit was filed within seventy five days of the date of such notice; or (iv) we did not respond to the Demand in accordance with Section 196 of the Israeli Companies Law. A derivative action requires the approval of the court, which shall approve it if convinced that the claim, and the conduct thereof, are prima facie in the best interests of the corporation, and that the Plaintiff is not acting with lack of good faith. A Plaintiff may not withdraw a derivative action, and may not enter into an arrangement or settlement with the defendant, other than with the consent of the court. The application for such consent shall specify all details of the arrangement or settlement, including any payment offered to the Plaintiff.
The Israeli Class Action Law provides that a person having a cause of action with respect to a security may, subject to the terms and conditions set forth in the foregoing act, and with the consent of the relevant court, sue on behalf of a group all of whose members have a cause of action that has materially similar factual and legal ground.
Director Independence Requirements
Director Independence under Israeli Law
Our common stock is listed on the TASE and trades under the symbol “BICL”. As a requirement of listing on the TASE, we must comply with certain director independence requirements. These requirements are incorporated in Article VI of our Amended and Restated Certificate of Incorporation, which is filed as an exhibit to this registration statement. Pursuant to these requirements, two independent directors, Orly Yarkoni and David Schlachet, serve on our Board of Directors. Other than through their current positions as independent directors on our Board of Directors, neither such director has had any employment relationship with us, has had any commercial or professional ties in general or through control or has served as one of our executive officers. We have no knowledge of the ownership by either director of any securities issued by us or owned by our wholly owned subsidiary, except for options to each of the directors to purchase 30,000 shares of our common stock, as approved by our Board of Directors and a general meeting of our stockholders .
In addition, our Amended & Restated Certificate of Incorporation requires that at least one independent director sit on each committee of our Board of Directors . David Schlachet is a member of our Audit and Executive Committees. Orly Yarkoni is a member of our Audit and Compensation Committees. Our other directors are not considered to be independent directors under Israeli law.
Director Independence Using an Independence Definition of a National Securities Exchange
We are not listed as an issuer, nor have we applied to be listed as an issuer, on any U.S. national securities exchange or inter-dealer quotation system. For purposes of compliance with applicable securities rules, the following describes the independence standards required by the NASDAQ Stock Market, Inc., and assesses whether our directors would be considered to be independent directors under NASDAQ’s independence standards were they so required.
An independent director is “a person other than an executive officer or employee of the company or any other individual having a relationship which, in the opinion of the company’s board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.” Under NASDAQ Rule 5605(a)(2) , an independent director may not: (i) have been employed by the company or its subsidiaries or parent company during the preceding three years; (ii) have received, or had a family member that received, compensation in excess of $ 120 ,000 from the company during any twelve month period during the preceding three years (other than for board or committee services, benefits under a retirement plan or payments to a family member who is a non-executive employee of the company); (iii) have had a family member that was an executive officer of the company during the preceding three years;
52
(iv) have received from or given to the company during the current and three preceding years, either directly or through family members or entities under his control, any property or services totaling the greater of $200,000 or 5% of the recipient’s gross revenues for that year, unless the payments arose from investments in the company’s securities or a non-discretionary charitable contribution matching program; (v) be employed or have been employed during the last three years, or have a family member who is employed or was employed, as an executive officer of another company where any of the issuer’s executive officers sat on the compensation committee; or (vi) be or have a family member who is a partner at the company’s outside auditor or was a partner or employee of the company’s outside auditor who worked on the company’s audit at any time during the preceding three years. Share ownership in a company will not by itself disqualify a director from being independent.
Under NASDAQ rules, at least a majority of the members of a listed company’s board of directors must be independent. We have determined that each of Hanoch Rappaport, Doron Nevo , David Schlachet, Orly Yarkoni and Aviv Boim are independent under NASDAQ rules and, as a result, we currently comply with the NASDAQ independence standards for board of directors composition.
Each company listed on NASDAQ must appoint an audit committee of at least three members and adopt an audit committee charter describing the committee’s responsibilities for oversight of accounting and financial issues. All directors on the audit committee must be independent according to the definition described above. As of the date of this prospectus, our audit committee would meet these standards, since all three members of our audit committee, Orly Yarkoni, David Schlachet and Hanoch Rappaport, would be deemed to satisfy the NASDAQ independence requirements for audit committee purposes .
Companies listed on NASDAQ are not required to maintain standing compensation or nomination committees. They may instead rely upon a majority of independent directors sitting on the board of directors to discharge the duties of such committees, such as setting the compensation of executive officers and the nomination of directors. If the board chooses to appoint a compensation or nomination committee, however, then the committee must be comprised of entirely independent directors. The compensation committee of our Board of Directors would meet NASDAQ’s standards for independence, since all four members of our compensation committee , Doron Nevo, Aviv Boim, Hanoch Rappaport and Orly Yarkoni, would be deemed to satisfy the NASDAQ independence requirements.
53
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements, which involve risk and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of certain factors including, but not limited to, those discussed in “Risk Factors” and elsewhere in this prospectus.
Overview
We were incorporated in the United States under the laws of the State of Delaware on July 26, 2004 and commenced operations on October 1, 2004. We filed a prospectus for an initial public offering on the Tel Aviv Stock Exchange, or TASE, and, since August 17, 2006, our securities have been publicly traded in Israel on the TASE. For TASE purposes, we file Hebrew financial statements in New Israeli Shekels in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board. For our filings in the United States, we prepare the U.S. financial statements in accordance with accounting principles generally accepted in the United States of America (GAAP).
We and our wholly owned subsidiary in Israel, BioCancell Therapeutics Israel Ltd., focus our activities on the research and development of drugs for the treatment of superficial bladder cancer, pancreatic cancer and ovarian cancer. The leading drug developed by us has been tested for a number of cancer types in pre-clinical lab animal trials, compassionate use human trials and a Phase I/IIa clinical trial. We are now performing an advanced Phase IIb clinical trial on bladder cancer patients , a Phase I/IIa clinical trials on pancreatic and ovarian cancer patients. Additionally, we are presently evaluating indications for the possible use of this drug, and others under development, to treat other types of cancer.
We are a development stage company. Therefore, there is no certainty regarding our ability to complete the development of any of our product -candidates , receive regulatory permits and succeed in our marketing efforts. Our operations since inception have been directed primarily toward developing research and development activities, conducting pre-clinical and clinical testing of our product - candidates, business strategies, raising capital, exploring marketing channels and recruiting personnel.
From our inception, we have raised a cumulative amount of $ 16,840 ,000 including as a result of the exercise of options by our employees, directors and consultants. During 2005 and the first half of 2006, we raised in private placements an amount of $2,951,000 from private investors and from Yissum Research Development Company of the Hebrew University of Jerusalem Ltd., a founder of our company. We raised an additional $4,976,000 in connection with our initial public offering in August 2006 on the TASE. On May 15, 2008 , we executed a private placement to Clal Biotechnology Industries Ltd ., or CBI , from which we received aggregate gross proceeds of $669,000 (net proceeds of $653,000). On July 30, 2008, we carried out private placements with Tikcro Technologies Ltd. and the Provident Fund of the Employees of the Hebrew University of Jerusalem Ltd., institutional investors, whereby three investors received an aggregate amount of 1,222,780 shares of our common stock at a price of about $0.60 per share, non-registered convertible notes payable , convertible into an aggregate of 5,058,002 shares of our common stock at a conversion price of about $0.72 per share and three non-registered warrants to purchase an aggregate of up to 6,280,783 shares of our common stock at a price of about $0.72 per share exercisable for five years. The aggregate gross proceeds from the private placements were approximately $3.65 million (net proceeds of $3,609,000). During May and June 2009, we sold 1,099,756 shares of treasury stock, at an average price of $0.92 per share, and total proceeds of $1,017,000. In August 2009, we sold 713,000 shares of treasury stock, at an average price of $0.79 per share, and total proceeds of $552,000. Following the sale, we did not hold any shares of treasury stock. In March 2010, we executed private placements to institutional and individual investors of 4,157,500 shares of common stock at a price of approximately $0.79 per share, and warrants to purchase an additional 4,157,500 shares of our common stock, exercisable immediately upon their issuance with a life of four years and an exercise price of approximately $1.15. The aggregate gross proceeds were $3,285,000 at an approximate price of $0.79 per share (net proceeds of $3,072,000).
We have incurred operating losses since inception, have not generated any product sales revenues and have not achieved profitable operations. Our deficit, accumulated during the development stage through March 31, 2010 , aggregated $19, 346 ,000 and we expect to continue to incur substantial losses in future periods while we continue to test and prepare our product candidates for the market. We believe we have sufficient cash to meet our planned operating needs until November 2010 , based on our current cash levels.
We are highly dependent on the success of our research, development and licensing efforts and, ultimately, upon regulatory approval and market acceptance of our products under development. Our short and long-term capital requirements depend upon a variety of factors, including market acceptance for our technologies and product - candidates and various other factors. The continuation of the stages of development and the realization of assets related to the planned activities depend on future events, including the receipt of interim financing and achieving operational profitability in the future. It is not possible to forecast accurately the results of these activities.
The biotechnology industry is characterized by strong competition, resulting from the risk of frequent technological changes. Entry into this market requires the investment of enormous resources and continuous development. Our future success is dependent on several factors, including the quality of the product's technology, the product's price, and the creation of an advantage over the competition.
54
Our research and development activities are carried out by our Israeli subsidiary primarily through a laboratory research team at the Hebrew University in Jerusalem. The laboratory is managed by our Chief Scientific Officer . All of our assets are presently situated in Israel.
Costs and Expenses
Research and Development Expenses
Research and development costs are expensed and recorded in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) subtopic 73-10. Research and development costs comprise costs incurred in performing research and development activities, including salaries and related costs, consultants and sub-contractors costs, clinical trials costs, patent fees, materials and depreciation costs. We are running three main research and development projects: clinical trials for each of bladder, ovarian and pancreatic cancer. Completion of the projects is subject to a number of factors unknown and/or not under our control, including, but not limited to, clinical trial expectations of the FDA, the participation of sufficient volunteers that meet inclusion criteria in clinical trials, and the required granting of final market approval by the FDA. Therefore, the nature and scope of costs needed to bring each of these projects to conclusion is not estimable. If the bladder cancer trials conclude successfully, we expect to receive final FDA approval and commence sales in 2014 – 2015 . On account of anticipated FDA fast-track development for life-saving drugs, we expect the ovarian and pancreatic cancer trial projects to conclude by 2014-2015 , and if successful, for sales to commence shortly thereafter. Delays in completing a project on schedule would entail additional operating costs for the period of delay, and could adversely affect our liquidity in the pre-sales period.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation, travel and overhead costs for financial, legal and administrative personnel, insurance fees, fees for professional services, including investor relations, legal, accounting and other consulting fees and other general corporate expenses. Overhead costs consist primarily of rent, telecommunications, utilities and depreciation expenses.
Stock-Based Compensation
New employees typically receive stock option awards. We also grant additional stock option awards to existing employees and directors, usually once a year. The Company records stock-based compensation as an expense in the statement of operations in accordance with ASC subtopic 505-50 and 718-10. The cost of stock-based compensation awards is measured at their fair value at the date of the award. Fair value is determined using the Black-Scholes -Merton option pricing model. We have accounted for stock-based compensation in this way from our inception.
Non-operating expenses (income), net
Non-operating expenses (income), net consists primarily of interest income, net which primarily consists of interest income earned on cash, cash equivalent and investment securities balances, loss (income) from marketable securities, net, interest on convertible notes and discount amortization, fair value adjustments of our warrants and foreign currency exchange gains and losses.
Income Tax Expense
We account for income taxes under the asset and liability method in accordance with the ASC subtopic 740-10. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided for the amount of deferred tax assets that, based on available evidence, are more likely than not to be realized.
ASC subtopic 740-10 clarifies the accounting for uncertainty in income taxes recognized in a company's financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. Interest and penalties related to unrecognized tax benefits are recognized as a component of income tax expense.
55
Results of Operation
Three Months Ended March 31, 2010 and March 31 , 2009 , Years Ended December 31, 2009 and December 31 , 2008 , and the Development Stage Period (cumulative from inception to March 31, 2010 )
Research and Development Expenses
|
Three Months Ended
March 31, 2010
|
Three Months Ended
March 31, 2009
|
Year Ended
December 31, 2009
|
Year Ended
December 31, 2008
|
Cumulative From
Inception to
March 31, 2010
|
||||||||||||||||
|
U.S. Dollars in Thousands
|
||||||||||||||||||||
|
Research and Development Expenses, Net
|
$ | 505 | $ | 414 | $ | 2,013 | $ | 2,694 | $ | 9,757 | ||||||||||
Research and development expenses increased by approximately $ 91 ,000, or 22.0 %, to $ 505 ,000 for the three months ended March 31, 2010 , from $ 414 ,000 for the three months ended March 31, 2009 . Research and development expenses increased due to increased clinical trial compensation, patent expenses and decreased funding from OCS, BIRD Foundation and other grants. This was partially offset by less than anticipated clinical trials expenses in bladder, pancreatic and ovarian cancer due to slower than anticipated enrollment rate of patients. R&D compensation expenses increased to $264 ,000 for the three months ended March 31, 2010, from $192 ,000 for the three months ended March 31 , 2009. Research and development expenses for the three month period ending March 31, 2010 were comprised mainly of compensation to personnel, as disclosed above, and other clinical trial expenses of $ 128 ,000. By comparison, research and development expenses for the three month period ending March 31, 2009 , were comprised mainly of compensation to personnel of $ 192 ,000 and other clinical trial expenses of $ 340 ,000.
It is anticipated that our level research and development expenses will increase as our clinical trials move forward, dependent upon the enrollment of patients and the availability of funding .
56
The following table summarizes information about our research and development expenses for the three months ended March 31, 2010 and March 31, 2009 and the cumulative period from inception to March 31, 2010:
|
Three Months Ended
March 31, 2010
|
Three Months Ended
March 31, 2009
|
Cumulative From
Inception to
March 31, 2010
|
||||||||
|
|
U.S. Dollars in Thousands
|
|||||||||
|
Clinical Trials:
|
||||||||||||
|
Bladder cancer
Phase I/IIa
|
$
|
-
|
$
|
-
|
$
|
897
|
||||||
|
Bladder cancer
Phase IIb
|
20
|
36
|
2,461
|
|||||||||
|
Pancreatic cancer Phase I/IIa
|
33
|
27
|
488
|
|||||||||
|
Ovarian cancer Phase I/IIa
|
70
|
273
|
1,797
|
|||||||||
|
Liver cancer
|
-
|
-
|
20
|
|||||||||
|
Clinical trial compensation
|
155
|
78
|
1,324
|
|||||||||
|
General expenses
|
5
|
4
|
64
|
|||||||||
|
283
|
418
|
7,051
|
||||||||||
|
Pre-clinical expenses:
|
||||||||||||
|
Compensation
|
109
|
114
|
3,654
|
|||||||||
|
Material
|
33
|
5
|
300
|
|||||||||
|
Patents
|
86
|
15
|
459
|
|||||||||
|
Depreciation
|
8
|
11
|
126
|
|||||||||
|
General expenses
|
15
|
22
|
213
|
|||||||||
|
251
|
167
|
4,752
|
||||||||||
|
534
|
585
|
11,803
|
||||||||||
|
Less: Chief Scientist and BIRD Foundation grants
|
(29
|
)
|
(171
|
)
|
(2,046
|
)
|
||||||
|
Total Research and Development Expenses, Net
|
$
|
505
|
$
|
414
|
$
|
9,757
|
||||||
Research and development expenses, net decreased by $681 thousand, or 25.3%, to $2,013,000 for the year ended December 31 , 2009 from $2,694,000 for the year ended December 31, 2008. Research and development expenses decreased due to less than anticipated clinical trials expenses in bladder and pancreatic cancer due to slower than anticipated enrollment of patients, reduced pre-clinical compensation and patent expenses which were offset by increased clinical trial expenses in ovarian cancer and increased OCS, BIRD Foundation and other grants. Research and development expenses for the period ending December 31, 2009 amounted to approximately $2,617,000, comprised mainly of compensation to personnel of $986,000 as compared to $1,271,000, due to the reduction in personnel, and clinical trial expenses of $1,334,000 as compared to $1,482,000 as of December 31, 2009 and 2008, respectively. It is anticipated that our level research and development expenses will remain relatively the same as our clinical trials move forward, dependent upon the enrollment of patients and the availability of funding.
The following table summarizes information about our research and development expenses for the years ended December 31, 2009 and December 31, 2008:
|
Year Ended
December 31, 2009
|
Year Ended
December 31, 2008
|
||||||
|
|
U.S. Dollars in Thousands
|
||||||
|
Clinical Trials:
|
|||||||
|
Bladder cancer
Phase I/IIa
|
$
|
-
|
$
|
25
|
|||
|
Bladder cancer
Phase IIb
|
208
|
404
|
|||||
|
Pancreatic cancer Phase I/IIa
|
165
|
287
|
|||||
|
Ovarian cancer Phase I/IIa
|
961
|
763
|
|||||
|
Liver cancer
|
-
|
3
|
|||||
|
Clinical trial compensation
|
434
|
399
|
|||||
|
General expenses
|
23
|
15
|
|||||
|
Pre-clinical expenses:
|
|||||||
|
Compensation
|
552
|
872
|
|||||
|
Material
|
40
|
133
|
|||||
|
Patents
|
107
|
141
|
|||||
|
Depreciation
|
41
|
48
|
|||||
|
General expenses
|
86
|
65
|
|||||
|
2,617
|
3,155
|
||||||
|
Chief Scientist and BIRD Foundation grants
|
(604
|
)
|
(461
|
)
|
|||
|
Total Research and Development Expenses, Net
|
$
|
2,013
|
$
|
2,694
|
|||
57
General and Administrative Expenses
|
Three Months Ended
March 31, 2010
|
Three Months Ended
March 31, 2009
|
Year Ended
December 31, 2009
|
Year Ended
December 31, 2008
|
Cumulative From
Inception to
March 31, 2010
|
||||||||||||||||
|
U.S. Dollars in Thousands
|
||||||||||||||||||||
|
General and Administrative Expenses
|
$ | 428 | $ | 324 | $ | 1,457 | $ | 1,913 | $ | 6,721 | ||||||||||
General and administrative expenses increased by $104,000, or 32.1%, to $428,000 for the three months ended March 31, 2010 from $324,000 for the three months ended March 31, 2009. General and administrative expenses were higher in 2010 than in 2009 due primarily to stock-based compensation to employees and directors which were offset by reduced consulting fees and professional service fees (primarily legal and accounting). The consulting expenses incurred due to the S-1 filing and filing with the TASE in 2009 in anticipation of the upcoming round of financing were recorded as deferred stock issuance costs in the balance sheet, to be offset against expected proceeds from the intended fundraising. The main components of general and administrative expenses were compensation expenses of $302,000 as compared to $146,000, professional service and consulting fees of $74,000 as compared to $120,000 for the periods ended March 31, 2010 and 2009, respectively. It is anticipated that our level general and administrative expenses will remain relatively the same in the upcoming quarters.
The following table summarizes information about our general and administrative expenses for the three months ended March 31, 2010 and March 31, 2009 and the cumulative period from inception to March 31, 2010:
|
Three Months Ended
March 31, 2010
|
Three Months Ended
March 31, 2009
|
Cumulative From
Inception to
March 31, 2010
|
||||||||||
|
|
U.S. Dollars in Thousands
|
|||||||||||
|
Compensation
|
$
|
302
|
$
|
146
|
$
|
3,498
|
||||||
|
Professional services and consulting fees
|
74
|
120
|
2,206
|
|||||||||
|
Rent & office related expenses
|
29
|
30
|
510
|
|||||||||
|
Travel
|
-
|
7
|
122
|
|||||||||
|
Insurance
|
8
|
4
|
67
|
|||||||||
|
Corporate and filing fees
|
6
|
2
|
102
|
|||||||||
|
Other general expenses
|
9
|
15
|
216
|
|||||||||
|
Total General and Administrative Expenses
|
$
|
428
|
$
|
324
|
$
|
6,721
|
||||||
General and administrative expenses decreased $456,000, or 24%, to $1,457,000 for the year ended December 31, 2009 from $1,913,000 for the year ended December 31, 2008. General and administrative expenses were lower in 2009 than in 2008 due primarily to decreased professional fees (primarily legal and accounting) incurred in 2009, on account of our private placement transactions and the filing of an S-1 registration statement in 2008. The main components of general and administrative expense were compensation costs of $715,000 as compared to $712,000, and professional services and consulting fees of $451,000 as compared to $917,000, for the periods ending December 31, 2009 and 2008, respectively.
The following table summarizes information about our general and administrative expenses for the years ended December 31, 2009 and December 31, 2008:
|
|
For the Year Ended December 31, 2009
|
For the Year Ended
December 31, 2008
|
||||||
|
U.S. Dollars in Thousands
|
||||||||
|
Compensation
|
$
|
715
|
$
|
712
|
||||
|
Professional services & consulting fees
|
451
|
917
|
||||||
|
Rent & office related expenses
|
129
|
137
|
||||||
|
Travel
|
11
|
50
|
||||||
|
Insurance
|
24
|
19
|
||||||
|
Corporate fees
|
64
|
15
|
||||||
|
Other general expenses
|
63
|
63
|
||||||
|
Total General and Administrative Expenses
|
$
|
1,457
|
$
|
1,913
|
||||
58
Non-operating expenses (income), net
Non-operating expenses (income), net, increased $ 276 ,000 to $ 1,283 ,000 for the three months ended March 31, 2010 from $ 1,007 ,000 for the period ended March 31, 2009 . The increase in non-operating expenses (income), net, resulted primarily from the fair value adjustment of our warrants which are being accounted for as a derivative financial instrument, as described below. The primary drivers of the adjustment of our warrants were the increases in our stock price and the implied volatility of the underlying stock. An increase in the price of our common stock increases the value of the warrants and thus results in a loss in our income statement. Conversely, a decline in the price of our common stock would decrease the value of the warrants and thus result in a gain in our statement of operations. We recorded a charge of approximately $ 1,186 thousand as compared to $1, 037 thousand for the three months ended March 31, 2010 and 2009 , respectively, resulting from revaluation of warrants to shareholders.
The following table summarizes information about our non-operating expenses (income), net , for the three months ended March 31, 2010 and March 31, 2009 and the cumulative period from inception to March 31, 2010 :
|
|
Three Months Ended
March 31, 2010
|
Three Months Ended
March 31, 2009
|
Cumulative From
Inception to
March 31, 2010
|
|||||||||
|
U.S. Dollars in Thousands
|
||||||||||||
|
Interest expense (income), net
|
$
|
(2
|
)
|
$
|
1
|
$
|
(15
|
)
|
||||
|
Loss (income) from marketable securities, net
|
-
|
-
|
(6
|
)
|
||||||||
|
Interest on convertible notes and discount amortization
|
93
|
79
|
582
|
|||||||||
|
Revaluation of warrants
|
1,186
|
1,037
|
2,586
|
|||||||||
|
Other financing expense (income), net
|
6
|
(110
|
)
|
(279
|
)
|
|||||||
|
|
$
|
1,283
|
$
|
1,007
|
$
|
2,868
|
||||||
Non-operating expenses (income), net, increased $ 4,998 ,000, to $ 3,333,000 for the year ended December 31, 2009 from income of $ 1,665,000 for the year ended December 31, 2008. The increase in non-operating expenses (income), net, resulted primarily from the fair value adjustment which results from our outstanding warrants which are being accounted for as a derivative financial instrument, as described below. The primary drivers of the adjustment of our warrants were the increases in our stock price and the implied volatility of the underlying stock. An increase in the price of our common stock increases the value of the warrants and thus results in a loss in our statement of operations. Conversely, a decline in the price of our common stock would decrease the value of the warrants and thus result in a gain in our statement of operations. We recorded a charge of approximately $3,053,000 as compared to income of ($1,653,000) for the periods ending December 31, 2009 and 2008, respectively, resulting from revaluation of warrants to shareholders.
59
|
Year Ended
December 31,
2009
|
Year Ended
December 31,
2008
|
|||||||
|
U.S. Dollars in Thousands
|
||||||||
|
Interest expense, net
|
$
|
(3
|
)
|
$
|
(61
|
)
|
||
|
Loss from marketable securities, net
|
-
|
89
|
||||||
|
Interest on convertible notes and discount amortization
|
341
|
147
|
||||||
|
Revaluation of warrants
|
3,053
|
(1,653
|
)
|
|||||
|
Other financing income, net
|
(58
|
)
|
(187
|
)
|
||||
|
|
$
|
3,333
|
$
|
(1,665
|
)
|
|||
Our warrants to noteholders are valued using the Binomial model. Prior to the second quarter of 2009, our warrants to noteholders were valued using the Black-Scholes -Merton model. These models use the variables of the price of the underlying stock, the strike price, the continuously compounded risk free interest rate, the continuously compounded annual dividend yield, the time in years until the expiration of the option, the implied volatility of the underlying stock and the standard deviation. Given the recent foreign currency fluctuation’s effect on the exercise price of the warrants throughout the life of the warrant, our assumptions have changed regarding the possible exercise patterns, and these possibilities can be addressed through the Binomial model in contrast to the Black-Scholes -Merton model which only allows for one exercise date. The change in the model did not have a material impact on our consolidated financial position, results of our operations or cash flows.
The following table shows the changes in the underlying parameters used in the valuation for valuing the warrants:
|
As of
March 31, 2010
|
As of
March 31, 2009
|
As of
December 31, 2009
|
As of
December 31, 2008
|
|||||||||||||
|
Price of underlying stock
|
3.311 NIS
|
1.316 NIS
|
2.435 NIS
|
0.405 NIS
|
||||||||||||
|
Strike price
|
$ | 0.716 | $ | 0.716 | $ | 0.716 | $ | 0.716 | ||||||||
|
Continuously compounded risk free interest rate for warrants
|
2.41 | % | 1.24 | % | 2.68 | % | 4.00 | % | ||||||||
|
Continuously compounded annual dividend rate
|
0 | % | 0 | % | 0 | % | 0 | % | ||||||||
|
Time in years until the expiration of the warrant*
|
3.33 years
|
4.33 years
|
3.58 years
|
4.58 years
|
||||||||||||
|
Implied volatility for the underlying stock
|
142.28-146.53 | % | 93.6 | % | 145.48-148.52 | % | 61.3 | % | ||||||||
|
|
* the Binomial model addresses this parameter differently than the Black-Scholes-Merton model as explained above.
|
Income Tax (Expense) Benefit
The federal tax rates applicable to us, as an entity incorporated in Delaware in the United States, are progressive corporate tax rates of up to 34%.
At December 31, 2009 , we had net operating loss (NOL) carryforwards in the United States amounting to $ 1,794 ,000, which will expire beginning in 2024 through 2029 . The Tax Reform Act of 1986 imposed substantial restrictions on the utilization of NOL and tax credits in the event of an ownership change of a corporation. Thus, in accordance with Internal Revenue Code, Section 382, our initial public offering, or IPO, and other ownership changes that have transpired, may limit our ability to utilize its NOL and credit carryforwards although we have not yet determined to what extent.
Pursuant to the tax laws applicable to Israeli residents, dividends received from a company that is not an Israeli resident are subject to tax in Israel, at the rate of 20% or 25%, depending on the identity of the stockholder (individual or company) and the ownership percentage. According to the tax laws in the United States, such a dividend is subject to withholding tax at the rate of 30%, which could be reduced to the rate of 25% or 12.5% (depending on the identity of the stockholder and the ownership percentage), in accordance with the Treaty to Prevent Double Taxation between Israel and the United States. In order to enjoy the tax treaty's benefits, several procedural requirements must be met. As of December 31, 2009 , we believe that we are currently a dual tax resident in both Delaware and Israel.
60
The tax rates applicable to our Israeli subsidiary and to us as a dual resident for tax purposes, are as follows: 2009 – 26%, 2010 – 25%, 2011 – 24%, 2012 – 23%, 2013 – 22%, 2014 – 21 %, 2015 – 20% and 2016 thereafter – 18 %. At December 31, 2009 , our wholly owned Israeli subsidiary had NOL carryforwards in Israel amounting to NIS 51,461 ,000 (approximately $ 13,000 ,000), which under current tax law can be carried forward indefinitely.
Liquidity and Capital Resources
We are a development stage company and have not experienced significant revenue generating activities since our formation. We have incurred operating losses for each year since our inception in 2004. To achieve operating profits, we, alone or with others, must successfully identify, develop and market product candidates. Our principal activities, from the beginning of our development stage, have been organizational matters, issuance of stock, product research and development, fund raising and market research. We have financed our operations from inception primarily through public offerings of our common stock, various private placement transactions, and option exercises.
We believe that we have sufficient cash to meet our planned operating needs until the end of November 2010, based on our current cash levels. We therefore will need to raise additional capital through future equity or debt financing to finance such initiatives.
In the near-term, we expect to continue to incur significant and increasing operating losses as a result of the research and development expenses we expect to incur in developing our product candidates and the general and administrative expenses we expect to incur as a reporting company under the Securities Exchange Act of 1934, as amended . We do not anticipate earning operating income over the coming years as the development process will be subject to extensive governmental regulations relating to development , clinical trials, manufacturing and commercialization and we may be unable to obtain regulatory approval for any of our prospective therapeutic products .
As of March 31, 2010 , we had $ 2,801 ,000 in cash, cash equivalents, and marketable securities, an increase of $2,177 ,000 from December 31, 2009 .
|
Three Months Ended
March 31, 2010
|
Three Months Ended
March 31, 2009
|
For the Year Ended
December 31, 2009
|
For the Year Ended
December 31, 2008
|
Cumulative From
Inception to
March 31, 2010
|
||||||||||||||||
|
|
U.S. Dollars in Thousands
|
|||||||||||||||||||
|
Net cash used in operating activities
|
$
|
( 861
|
)
|
$
|
( 805
|
)
|
$
|
(3, 654
|
)
|
$
|
(3,757
|
)
|
$
|
(15,871
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
$
|
( 1
|
)
|
$
|
( 1
|
)
|
(19
|
)
|
2,231
|
|
$
|
2, 039
|
||||||||
|
Net cash provided by financing activities
|
$
|
3,038
|
$
|
-
|
$
|
1,568
|
$
|
4,257
|
$
|
16,629
|
||||||||||
Operating Activities
Net cash used in operations was $ 861 ,000 for the three months ended March 31, 2010 as compared to net cash used in operating activities of $ 805 ,000 for the three months ended March 31, 2009. The net cash used in operations was mainly used for operating expenses. The difference between our net loss of $2,216,000 and our net cash used in operations was attributable mostly to $124 ,000 of operating expenses that were the result of non-cash, stock-based compensation to employees and directors, and depreciation and amortization of $ 9, 000, while recognizing income of $1,186 ,000 of fair value adjustment of the derivative instruments. Net cash used in operations was $3, 654 ,000 for the year ended December 31, 2009 .
Currently all of our funds are held as cash and cash equivalents. As of March 31, 2010, we have no material commitments for capital expenditures. It is anticipated that our level of net cash used in operations will remain the same as our clinical trials move forward. Our cash reserves are currently the sole resource of funding for our current operations. We will require substantial additional funds to complete our research and development activities and, if additional funds are not available, we may need to significantly scale back or cease our operations, and our level of activity may change based on ability to secure future funding.
Net cash used in operations from our inception is attributable mostly to our net loss offset by non-cash items, primarily change in fair value of our warrants and stock-based compensation, as delineated in our Consolidated Statement of Cash Flows. It is anticipated that our level of net cash used in operations will remain the same as our clinical trials move forward. Our cash reserves are currently the sole resource of funding for our current operations. We will require substantial additional funds to complete our research and development activities and, if additional funds are not available, we may need to significantly scale back or cease our operations, and our level of activity may change based on ability to secure future funding.
61
Investing Activities
Net cash used in investing activities in the three months ended March 31, 2010 and 2009, is attributable primarily to the acquisition of equipment, as delineated in our Consolidated Statement of Cash Flows. Net cash provided by (used in) investing activities in the years ended December 31, 2009 and 2008 , and from our inception is attributable mostly to the proceeds from marketable securities less the investments in marketable securities and acquisition of equipment, as delineated in our Consolidated Statement of Cash Flows . We redeemed our marketable securities for our ongoing activities and we do not expect this to continue because currently all of our funds are held as cash and cash equivalents.
Financing Activities
Net cash flow provided by financing activities was $ 3,038 ,000 for the three months ended March 31, 2010 as compared to net cash provided by financing activities of $0 for the three months ended March 31, 2009. This was mainly attributable to the proceeds from the private placements to institutional and individual investors of 4,157,500 shares of our common stock of our stock at a price of about $0.79, and warrants to purchase an additional 4,157,500 shares of our common stock, exercisable immediately upon their issuance with a life of four years and an exercise price of at a price of about $1.15. The aggregate gross proceeds were $3,287,000 at an approximately price of $0.79 per share (net proceeds of $3,072,000), as described above . Cash flow provided by financing activities in the year ended December 31, 2009 was $1,568,000 as compared to net cash provided by financing activities of $4,257,000 in the year ended December 31, 2008 . Cash flow used in financing activities for the development stage period (cumulative from inception to March 31, 2010 ) stems from the net proceeds from our initial public offering in Israel and the conversion of our series A convertible preferred stock in 2006, net proceeds from the proceeds from a private placement allocation of shares of our common stock and warrants to Tikcro Technologies, Ltd., CBI and The Provident Fund of the Hebrew University of Jerusalem in 2008 , net proceeds from the proceeds from a private placement allocation of shares of our common stock and warrants in March 2010 and the exercise of stock options less the purchase of treasury stock. During May and June 2009, our subsidiary sold 1,099,756 shares of treasury stock, at an average price of $0.92 per share, and total proceeds of $1,017,000. In August 2009, we sold 713,000 shares of treasury stock, at an average price of $0.79 per share, and total proceeds of $552,000. Following the sale, we did not hold any shares of treasury stock.
Our financing needs may change substantially because of the results of our research and development, competition, clinical trials and costs arising from additional regulatory approvals. We may not succeed in raising additional funds if needed. The timing of our need for additional funds will depend on a number of factors as described in the “ Risk Factor s” Section under “We will require substantial additional funds to complete our research and development activities and, if additional funds are not available, we may need to significantly scale back or cease our operations”.
Future Operations
We believe we have sufficient cash to meet our planned operating needs until November 2010 , based on our current cash levels. Furthermore, our business strategy includes growth through additional business combinations and licensing which could require use of a significant amount of our available cash and raising additional capital. We therefore will be required to raise additional capital through future debt or equity financing to finance such initiatives. However, we cannot be certain that additional financing will be available on acceptable terms, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Also see discussion in the “ Risk Factor s” Section under “Our auditors have expressed substantial doubt as to whether we can continue as a going concern”.
62
Our short and long-term capital requirements depend upon a variety of factors, including market acceptance for our technologies and product candidates and various other factors, many of which we cannot control, including:
|
·
|
continued progress of and increased spending related to our research and development activities, including our plan to hire additional research and development employees;
|
|
·
|
progress with pre-clinical experiments and clinical trials;
|
|
·
|
increased general and administrative expenses related to our being a reporting company; prosecuting and enforcing patent claims;
|
|
·
|
technological and market developments;
|
|
·
|
the ability to establish product development arrangements;
|
|
·
|
the cost of manufacturing development;
|
|
·
|
effective marketing activities and arrangements; and
|
|
·
|
licensing activity.
|
Off-Balance Sheet Arrangements
We have not entered into any transactions with unconsolidated entities in which we have financial guarantees, subordinated retained interests, derivative instruments or other contingent arrangements that expose us to material continuing risks, contingent liabilities or any other obligations under a variable interest in an unconsolidated entity that provides us with financing, liquidity, market risk or credit risk support.
Critical Accounting Policies and Significant Estimates
While our significant accounting policies are more fully described in the notes to our audited consolidated financial statements for the years ended December 31, 2009 and 2008 , we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements.
Accounting for Stock-based Compensation
We record stock-based compensation as an expense in the statement of operations in accordance with the ASC subtopic 505-50 and 718-10, which requires measurement of compensation cost for all stock-based awards at fair value on date of grant and recognition of compensation over the service period for awards expected to vest . Compensation cost of all stock-based compensation awards are recorded at their fair value at the date of the award over the service period for awards expected to vest . Fair value is determined using the Black-Scholes -Merton option pricing model which considers the exercise price relative to the market value of the underlying stock, the expected stock price volatility, the risk-free interest rate and the dividend yield, and the estimated period of time option grants will be outstanding before they are ultimately exercised. We also determine the fair value of stock options and warrants granted to non-employees, for accounting purposes, using the Black-Scholes -Merton valuation model. We have accounted for stock-based compensation under ASC subtopic 505-50 and 718-10 from our inception. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results differ from our estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. We consider various factors when estimating expected forfeitures, including historical experience. Actual results may differ substantially from these estimates.
63
With the exception of stock options granted to employees prior to August 17, 2006, the date of our first filing with the TASE in connection with our IPO, we determine the fair value of stock options granted to employees and directors using the Black-Scholes -Merton valuation model, which considers the exercise price relative to the market value of the underlying stock, the expected stock price volatility, the risk-free interest rate and the dividend yield, and the estimated period of time option grants will be outstanding before they are ultimately exercised. We also determine the fair value of stock options granted to non-employees, for accounting purposes, using the Black-Scholes -Merton valuation model. Prior to our IPO, the market value of the underlying stock was based on estimates, including volatility estimates that are inherently highly uncertain and subjective, since prior to our IPO there had been no public market for our stock. Subsequent to our IPO, we did not have sufficient history to actually predict our volatility, therefore, our assumptions about stock price volatility were based on the volatility rates of comparable publicly held companies. These rates may or may not reflect our actual stock price volatility. Our assumptions about our stock price volatility are based on a rate that we derived by taking into consideration the volatility rates of comparable publicly held companies as well as our own historical volatility rates. In future periods and as we accumulate our own volatility history over longer periods of time, we will increase the weighting of our own volatility history in calculating expected volatility. Had we made different assumptions about the market value of our stock, stock price volatility or the estimated time option and warrant grants will be outstanding before they are ultimately exercised, the related stock based compensation expense and our net loss and net loss per share amounts could have been significantly different.
The pre-IPO options were granted with a par value exercise price. Due to the par value amount of $0.01, the fair value of these options was estimated to be equal to our share price at the grant date, based on stock issuances that took place surrounding the grant date. The expenses recorded in the statement of operations on account of stock-based transactions were $ 124 ,000 and $ 22 ,000 for the three months ending March 31, 2010 and 2009 and $1, 765 ,000 for the development stage period (cumulative from inception to March 31, 2010 ).
The parameter used in the valuation of options granted to employees in 2004-2005 was the price of the share on grant date as described above. The parameters used in the valuation of grants in 2006 – 2010 were based on the Black-Scholes -Merton model for valuing options for employees:
|
Year
|
Volatility
|
Expected Average
Term of the Option
|
Risk-free Rate
|
Estimate Value of
the Share on
the Grant Date
|
|||||||||
|
2006
|
0.6
|
3 years
|
4.07% – 5.00
|
%
|
$
|
0.83 – $1.45
|
|||||||
|
2008
|
0.6
|
10 years
|
6.37
|
%
|
$
|
0.10
|
|||||||
|
2009
|
0.8
|
10 years
|
4.96
|
%
|
$
|
0.63
|
|||||||
|
2010
|
0.9
|
10 years
|
4.97
|
%
|
$
|
0.88
|
The fair value of options granted to non-employees has been computed and accounted for in accordance with ASC subtopic 505-50 and 718-10 and was measured according to the Black-Scholes -Merton option-pricing model. To the extent that there are non-employee options for which a measurement date was not yet reached , the stock option compensation is revalued at the end of each reporting period.
Share-based payment expense has not been recorded in the statement of operations with respect to the award of an additional 150 ,000 contingent options that are milestone based, and at this point are not expected to vest .
64
Accounting for Income Taxes
As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process involves management estimating our actual current tax exposure together with assessing temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. We must then assess the likelihood that our deferred tax assets will be recovered from future taxable income and, to the extent we believe that recovery is not more likely than not, we must establish a valuation allowance. Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We have almost fully offset our U.S. net deferred tax asset with a valuation allowance. Our lack of earnings history and the uncertainty surrounding our ability to generate US taxable income prior to the expiration of such deferred tax assets were the primary factors considered by our management in establishing the valuation allowance.
We recognize tax benefits from tax positions only if they have at least a “more likely than not” chance of being sustained upon a challenge by the respective taxing authorities. Recognition and measurement of uncertain tax positions are also subject to management’s estimates and judgment of whether they are more likely than not to be sustained, and the amount to be recognized . Interest and penalties related to unrecognized tax benefits are recognized as a component of income tax expense.
Valuation of Financial Instruments Issued in Private Placement Financing
On July 30, 2008 , we completed private placements with institutional investors, for the purchase of: (i) shares of our common stock, (ii) debentures convertible into shares of our common stock and (iii) warrants to purchase shares of our common stock. The terms of the convertible debentures and warrants are described under “Description of Securities — Convertible Debentures” and “Description of Securities — Warrants”.
To account for these private placements, we estimated the fair value of the three components embodied in the agreements. We used various valuation models and techniques to determine the individual values of the three components. These models use the variables of the price of the underlying stock, the strike price, the continuously compounded risk free interest rate, the continuously compounded annual dividend yield, the time in years until the expiration of the option, the implied volatility for the underlying stock and the standard normal cumulative distribution function. While we believe we have applied appropriate judgment in the assumptions and estimates, variations in judgment in applying assumptions and estimates used in the valuations could have a material effect upon the valuation results, and thus, on our financial statements. The $3.650 million of proceeds from the private placements were first allocated to the warrants, which were classified as derivative instruments in accordance with the guidance in ASC subtopic 815-15. The warrants are considered derivatives since they are not indexed solely to our own stock as they must be settled in a currency other than our functional currency, and the warrants meet all of the characteristics of a derivative instrument as described in ASC subtopic 815-15. The convertible notes payable are classified as long-term liabilities and have been recorded at their initial relative fair value. The warrants have been recorded as a liability, with a corresponding discount to the Convertible Notes Payable, based on their fair values, and are revalued at each reporting date. Changes in the fair value are included in the statement of operations. Our warrants to noteholders are valued using the Binomial model. Prior to this period, our warrants to noteholders were valued using the Black-Scholes -Merton model. These models use the variables of the price of the underlying stock, the strike price, the continuously compounded risk free interest rate, the continuously compounded annual dividend yield, the time in years until the expiration of the option, the implied volatility of the underlying stock and the standard deviation. Given the recent foreign currency fluctuation’s effect on the exercise price of the warrants throughout the life of the warrant, our assumptions have changed regarding the possible exercise patterns, and these possibilities can be addressed through the Binomial model in contrast to the Black-Scholes -Merton model which only allows for exercise date. While we believe we have applied appropriate judgment in the assumptions and estimates, variations in judgment in applying assumptions and estimates used in the valuations could have a material effect upon the valuation results, and thus, on our financial statements. For further details regarding these estimates, see the discussion on non-operating expenses (income), net above.
65
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Except as set forth below, since January 1, 200 8 , there has not been, nor is there currently proposed, any transaction or series of similar transactions to which we were a party or are a party in which:
|
§
|
the amounts involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years; and
|
|
§
|
a director, executive officer, holder of more than 5% of our common stock or any member of their immediate family had or will have a direct or indirect material interest.
|
License Agreements
Yissum has granted to us an exclusive, worldwide license for the use, development and commercialization of the H19 gene in consideration of which we have agreed to pay certain royalties to Yissum described elsewhere in this prospectus. See “Our Business — Material Operating Agreements” for a more detailed description of this agreement between Yissum and us.
Private Placements
Clal Biotechnology Industries Ltd.
On March 12, 2008, we entered into a Subscription and Registration Rights Agreement with Clal Biotechnology Industries Ltd. (CBI), pursuant to which we agreed to issue 650,000 shares of our common stock to CBI for a purchase price of NIS 3.52 (approximately $1. 03 ) per share, for an aggregate purchase price of NIS 2,288,000 (approximately $ 669,000 ). The transaction was subject to the satisfaction of several conditions, including the approval of the transaction by our stockholders, the approval of the listing of the shares of common stock issuable to CBI in the transaction by TASE, and approval of the transaction by the Israeli Anti-trust Authority.
On May 1, 2008, our stockholders approved the terms of the transaction as a transaction the result of which would be the sale to CBI of a control block of 25% of our issued capital and voting rights, in accordance with Section 328(b)(1) of the Israeli Companies Law.
On May 15, 2008, we completed the sale of the shares of our common stock to CBI contemplated by the March 12, 2008 Subscription and Registration Rights Agreement with CBI.
Pursuant to the terms of the Subscription and Registration Rights Agreement with CBI dated March 12, 2008, we granted CBI certain rights with respect to the registration under the Securities Act of the securities issued to it under that agreement. For more information, see “Description of Capital Stock — Registration Rights.”
CBI, Tikcro Technologies Ltd. and Provident Fund of Hebrew University Ltd.
On June 22, 2008, we entered into Subscription and Registration Rights Agreements with each of CBI, which at that time was deemed to be a “controlling shareholder” under the Israeli Companies Law, Tikcro Technologies Ltd., and Provident Fund of the Hebrew University Ltd. (Provident), for the purchase of an aggregate of (i) 1,222,780 shares of our common stock at a price per share equal to $0.597, for an aggregate purchase price equal to $730,000, (ii) debentures in an aggregate principal amount of $2,920,000 convertible in the aggregate into up to 5,058,002 shares of our common stock at a price per share of $0.716, and (iii) warrants to purchase up to 6,280,783 shares of our common stock at an exercise price of $0.716 per share. The terms of the convertible debentures and warrants are described below under “Description of Securities — Convertible Debentures” and “Description of Securities — Warrants” below.
66
On July 30, 2008 we completed the sale and issuance of the securities to Tikcro, CBI and Provident contemplated by the Subscription and Registration Rights Agreements dated June 22, 2008.
In connection with the Subscription and Registration Rights Agreements dated June 22, 2008, we granted to CBI, Tikcro and Provident certain anti-dilution rights and adjustments with respect to the shares of our common stock, convertible debentures and warrants issued to them under those agreements. See “Description of Securities — Anti-Dilution Adjustments” below.
We granted CBI, Tikcro and Provident certain rights with respect to the registration of the securities issued to those parties under such agreements under the Securities Act. For more information, see “Description of Capital Stock — Registration Rights.”
As a condition to the closing of the transactions contemplated by the Subscription and Registration Rights Agreement with Tikcro, we also agreed to undertake the following actions:
|
§
|
Executive Committee. We agreed to designate an executive committee of our Board of Directors consisting of four members of the board, Professor Hochberg, an external director (currently Mr. David Schlachet), and the members of our Board of Directors nominated by each of Tikcro and CBI. The functions of the executive committee are to include the consummation of any material transaction involving us, the adoption of our annual budgets and any matters relating to our investment policies and working plan.
|
|
§
|
Consulting Fees. We agreed to pay Tikcro a consulting fee, for as long as a director designated by Tikcro is a member of our board of directors, for consulting services to be provided to us by a representative of Tikcro. The consulting fee consists of an annual payment of $30,000 and an annual issuance of 63,939 shares of our common stock.
|
|
§
|
Information Rights. We undertook to furnish CBI and Tikcro certain financial information about us in order to enable the timely reporting of their financial statements, as required under applicable law.
|
In connection with, and as a condition to, the closing of the transactions contemplated by the Subscription and Registration Rights Agreements dated June 22, 2008, CBI, Tikcro, Avi Barak and Professor Hochberg entered into an Irrevocable Voting Agreement regarding voting of shares of our common stock held by such parties in elections of members of our Board of Directors. For more information about the Irrevocable Voting Agreement, see “Security Ownership of Certain Beneficial Owners and Management — Voting Agreement” above.
Purchase and Exercise of Series 1 Warrants
Between August 14 and 17, 2008, our wholly owned Israeli subsidiary purchased on the TASE 1,812,756 of our Series 1 Warrants, at a purchase price of NIS 0.02 each. On August 18, 2008, our subsidiary exercised all of these warrants for 1,812,756 shares of our common shares at an exercise price of NIS 9.73 each. All remaining outstanding and unexercised Series 1 Warrants expired on August 18, 2008. We funded the exercise of these warrants through an equity investment by us in our subsidiary. By directing our wholly-owned subsidiary to purchase and exercise warrants to purchase our shares, using funding provided by us, we were able to create a pool of treasury stock, at an insignificant cost. This can be used beneficially as directed by our Board of Directors, by taking advantage of market conditions to issue stock in the future without expensive and lengthy fundraising procedures, such as the publishing of a prospectus. During May and June 2009, we sold 1,099,756 shares of treasury stock, at an average price of $0.92 per share, and total proceeds of approximately $1,017,000. In August 2009, we sold 713,000 shares of treasury stock, at an average price of $0.79 per share, and total proceeds of approximately $552,000. Following the August sale, we did not hold any shares of treasury stock.
Executive Compensation
For more information regarding transactions in which our directors and executive officers had or will have a direct or indirect material interest, see “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation” above.
67
MARKET PRICE OF COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our shares of common stock are listed for trading on the TASE under the symbol “BICL”. Public trading of shares of our common stock commenced on the TASE on August 17, 2006. We have never declared any dividends on any shares of our common stock, and we do not have any present intention to do so in the future.
Currently, there is no public market for our common stock in the United States and no assurances can be given that a public market will develop or, if developed, that it will be sustained.
The closing price of our common stock as reported on the TASE on June 10, 2010 was NIS 2.36 .
DESCRIPTION OF SECURITIES
The following information describes our common stock, other security convertible into or exercisable for shares of our common stock and provisions of our Amended and Restated Certificate of Incorporation and our Second Amended and Restated Bylaws. This description is only a summary. You also should refer to our Amended and Restated Certificate of Incorporation and our Second Amended and Restated Bylaws, which have been filed as exhibits to the registration statement of which this prospectus is a part.
Our authorized capital stock consists of 50,000,000 shares of common stock, par value $0.01 per share. Our Amended and Restated Certificate of Incorporation does not currently authorize the issuance of preferred stock.
Units
Each unit consists of shares of our common stock, Series 3 and Series 4 warrants . Each Series 3 Warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Series 3 warrants will be exercisable for two years from the date on which they are issued. Each Series 4 Warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Series 4 warrants will be exercisable for four years from the date on which they are issued.
Common Stock
As of the date of this prospectus, there are 20,621,113 shares of common stock outstanding. There are 41 record stockholders of our common stock. Our common stock is listed on the TASE, and trades under the symbol “BICL”.
The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders. Our stockholders do not have cumulative voting rights in the election of directors. Accordingly, holders of a majority of the voting shares are able to elect all of the directors. The remaining directors may fill a vacancy on our Board of Directors, if the vacancy occurred by reason of death, resignation or the creation of a new directorship. Our independent directors , for the purpose of the Israeli requirements we are subject to, must be elected through satisfaction of one of the following two conditions: either (i) the majority of the votes at the stockholder meeting includes at least one third of all of the votes by stockholders who are not controlling stockholders or their representatives, not counting abstentions, or (ii) the total number of opposing votes by the non-controlling stockholders does not exceed 1% of all the voting rights. Certain of our stockholders have entered into an Irrevocable Voting Agreement regarding the voting of their shares of our common stock in the election of members of our Board of Directors. See “Security Ownership of Certain of Certain Beneficial Owners and Management — Voting Agreement.”
Dividends may be declared by our Board of Directors at any regular or special meeting and may be paid in cash or property or in shares of capital stock. The directors may set apart a reserve available for dividends for any proper purpose. We have not distributed a dividend since the time of our incorporation and we have not established a policy of dividend distribution.
Convertible Debentures
In connection with the Subscription and Registration Rights Agreements dated June 22, 2008 that we entered into with CBI, Tikcro and Provident, we issued on July 30, 2008 to each of CBI, Tikcro and Provident convertible debentures in a principal amount of $800,000, $2,000,000 and $120,0000, respectively. These convertible debentures bear interest at the rate of 10% per annum, compounded annually, of the outstanding principal amount from July 31, 2008, the date on which we issued the convertible debentures, until the date of actual repayment. Interest accrued on the convertible debentures between their issuance date and July 31, 2010 will be capitalized as additional principal amount on the convertible debentures. We are obligated to pay on a quarterly basis in cash interest accrued on the convertible debentures following July 31, 2010. The convertible debentures are convertible, in whole or in part at the option of the holder at any time prior to July 31, 2012, into a number of shares of our common stock equal to the principal amount of the debentures, including capitalized interest accrued between July 31, 2008 and July 31, 2010, at a price of $0.716 per share.
68
The conversion price and terms of the convertible debentures are subject to certain adjustments; see “Description of Securities — Anti-Dilution Adjustments” below. The convertible debentures mature, and any principal amount thereunder not converted into shares of common stock becomes due and payable, on July 31, 2012.
In the event that (i) we consolidate or merge with another entity and we are not the surviving entity in the transaction, (ii) we sell, lease or exchange substantially all of our assets, (iii) a person or entity acquires more than 50% of our then outstanding stock, or (iv) a person or entity controls more than a majority of our Board of Directors, then in each such case, in addition to the right to convert the convertible debentures into shares of our common stock, at the option of the holder, each convertible debenture will:
|
§
|
become immediately due and payable (including accrued but unpaid interest); or
|
|
§
|
upon conversion and at any time after such event shall be redeemable for the shares or other securities or property to which the holder would have been entitled had the holder converted the convertible debenture immediately prior to such event.
|
We are obligated under the convertible debentures to give the holders thereof at least 14 days’ notices of such a change of control event.
Upon the occurrence of any of the following events of default on payment, the holders may, by notice in writing sent to us, declare the convertible debenture due and payable: (i) a default in the payment of interest on the principal amount when due and such default has continued for more than ten days; (ii) a default in the payment of the principal at the expressed or any accelerated due date and such default has continued for more than ten days, or, solely with respect to the convertible debentures held by CBI and Tikcro; (iii) a representative of CBI or Tikcro is not a member of each of our Board of Directors and our executive committee, other than by the resignation of such person and the refusal of CBI or Tikcro, respectively, to appoint someone in his stead (as long as CBI or Tikcro, respectively, holds securities (on an as-converted basis) that represent at least seven percent of our issued and outstanding share capital; provided that in such case, CBI or Tikcro, respectively, shall have the right to demand the repayment of the loan amount plus an amount equal to the interest accruable over a period of four years from the closing date less any amount of interest actually paid to CBI or Tikcro, respectively.
In addition to the events of financial default described above, when any of the following non-financial defaults or cross defaults has occurred, then the convertible debenture shall immediately become due and payable:
69
|
·
|
a default in the payment when due of the principal of or interest on any debt of ours to a bank or another financial institution or pursuant to which the creditor has a registered security interest over our assets or any of our subsidiaries’ assets, having an unpaid principal amount in excess of $100,000 (whether by lapse of time, by declaration, by call for redemption or otherwise), and such default or event shall continue beyond the period of grace, if any, allowed with respect thereto;
|
|
·
|
a default in the observance or performance of any of the negative covenants set forth in the convertible debenture and listed below;
|
|
·
|
a default in the observance or performance of any of the covenants set forth in the Subscription and Registration Rights Agreement, including: (i) failure to use best efforts to register the securities underlying the convertible debentures with the SEC; (ii) failure to comply with a holder’s demand that we file a registration statement; (iii) failure to file a short-form registration statement upon the request of the holder when we are eligible to do so; and (iv) failure to give the holder adequate notice of filing of short-form registrations in certain circumstances);
|
|
·
|
final non-appealable judgment(s) for the payment of money aggregating in excess of $100,000 (and $500,000 with respect to any major supplier of ours with annual payments by us exceeding 20% of our annual expenditures) is or are outstanding against us or against any of our property or assets and any such judgment or judgments aggregating in excess of $100,000 has or have remained unpaid, unvacated, unbonded or unstayed by appeal or otherwise for a period of 30 days from the date of its or their entry;
|
|
·
|
if we are delisted from the TASE and our shares of common stock are not listed within 60 days of such delisting on NASDAQ or in an alternate exchange reasonably satisfactory to the lender;
|
|
·
|
if there shall be commenced against us (other than by CBI, Tikcro or Provident) any proceedings related to us under any bankruptcy, reorganization, arrangement, insolvency, readjustment of debt, receivership, dissolution or liquidation law or statute of any jurisdiction, whether now or hereinafter in effect, and any such proceedings shall remain undismissed for a period of 60 days, or if a receiver or trustee shall be appointed for us or for all or a substantial part of our assets, and any such receivership or trusteeship shall remain undischarged for a period of 60 days;
|
|
·
|
if we apply for an arrangement with our creditors or participants, or if we shall make an assignment of our assets for the benefit of our creditors (other than in the ordinary course of business); or
|
|
·
|
upon the levy of an attachment or the institution of execution proceedings (other than by the lender) against the whole or a substantial portion of our assets, where such attachment or execution proceeding is not discharged within 60 days.
|
We undertook that for as long as the loan amount has not been repaid or converted pursuant to the terms convertible debentures, we would not, without the prior written consent of (i) the lender with respect to subsections (a), (c), (e) or (f) below; or (ii) of the executive committee with respect to subsections (b) or (d) below:
|
(a)
|
materially change the general nature of our business;
|
|
(b)
|
transfer, license, encumber, pledge or dispose of more than 20% of our assets; provided that if the Executive Committee approves such transaction the lender shall have the right to demand the repayment of the loan amount plus an amount equal to the interest accruable over a period of 4 years from the closing date less any amount of interest actually paid to the lender;
|
|
(c)
|
distribute any cash or share dividends to our stockholders;
|
|
(d)
|
a consummation of certain merger and acquisition events in which all or part of the consideration payable by the acquirer is in a cash; provided that if the Executive Committee approves such transaction the lender shall have the right to demand the repayment of the loan amount plus an amount equal to the interest accruable over a period of 4 years from the closing date less any amount of interest actually paid to the lender;
|
|
(e)
|
make any loan or other extension of credit to our distributors, customers, or employees other than loans and advances granted in the ordinary course of business and consistent with past practices; or
|
|
(f)
|
(i) receive any loan or advance from a third party or incur any debt, other than (a) inter-company loans granted by us to our subsidiaries or credit from our suppliers incurred in the ordinary course of business, consistent with past practices; and (b) a loan of up to $2 million with the same terms as set forth in the convertible note, or (ii) issue a guarantee in an aggregate amount in excess of $50,000.
|
We undertook to maintain and reserve, free from pre-emptive or similar rights, such number of authorized but unissued shares of common stock so that the convertible debentures may be converted without additional authorization of common stock, for so long as the convertible debentures have not been repaid or converted in full. We further agreed not to enter into, or be a party to, any transaction, arrangement or agreement with any affiliate (an entity which controls or is under common control with us) without each respective lender’s prior written consent. We undertook to use our best efforts and allocate the budget required to achieve and satisfy certain milestones. Any change in excess of 10% to the budget devoted to the attainment of such milestones and to the schedule for its deployment shall require the prior written approval of the executive committee.
Warrants
Series 1 Warrants
Between August 14 and 17, 2008, our wholly owned Israeli subsidiary purchased on the TASE 1,812,756 of our Series 1 warrants, at a purchase price of 0.02 NIS each. On August 18, 2008, the subsidiary exercised all of these warrants for 1,812,756 shares of our common stock at an exercise price of 9.73 NIS each. All remaining outstanding and unexercised Series 1 warrants expired on August 18, 2008. We funded the exercise of these warrants through an equity investment by us in our subsidiary.
Series 2 Warrants
On August 14, 2006, we issued to institutional and individual investors 2,500,000 Series 2 warrants exercisable in the aggregate to 2,500,000 shares of our common stock. The Series 2 warrants, with an exercise price per warrant of NIS 11.55 (approximately $3.10) linked to the consumer price index in Israel that published in July 2006, are listed on TASE and can be exercised until August 11, 2010.
70
Series 3 and Series 4 Warrants
Each unit offered in this offering consists of shares of our common stock, Series 3 warrants and Series 4 warrants. Each Series 3 warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Series 3 warrants will be exercisable for a period of two years commencing on the date on which they are issued. Each Series 4 warrant shall be exercisable into one share of our common stock at an exercise price equal to NIS (approximately $ ). Series 4 warrants will be exercisable for a period of four years commencing on the date on which they are issued. The exercise price of the Series 3 warrants and the Series 4 warrants will not be linked to the Israeli Consumer Price Index .
The exercise prices of the warrants will be announced by us at least five (5) TASE trading hours before the auction by filing a report with the Israeli Securities Authority and a current report on Form 8-K with the SEC. These parameters will be determined by our management based on management's assessment of, among others, prevailing market conditions, the historical performance of our stock on TASE and early bids by institutional investors.
Shares of our common stock resulting from the exercise of the warrants will award their holder identical rights to those of the existing stockholders of the Company in effect on the date of exercise, including any dividend or other rights declared by our Board of Directors, in effect on the exercise date or thereafter.
Until the expiration date of the Series 3 warrants and the Series 4 warrants, we shall reserve a sufficient amount of common stock to accommodate for the exercise of the warrants and, if required, increase in the registered stock capital of the Company. In case of a modification of the par-value of our shares of common stock, the number of shares of common stock to be issued upon the exercise of a warrant will be adjusted accordingly.
In the event of a voluntary liquidation of our company, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our shares of common stock in proportion to the nominal value of their respective holdings. Any existing warrant holders will be deemed as if they had exercised their entire amount of warrants in full, and will also be eligible to partake in the distribution, after taking into account a deduction of the exercise price of the warrants (unless a written notice is provided by a warrant holder regarding the waver of such right). We shall not, without the consent of the warrant holders, alter the applicable rights of the holder of shares of common stock and shall not issue a new type of stock (not including preferred shares) which is eligible to participate in the distribution in case of liquidation of the Company.
A warrant which is not exercised by its expiration date, i.e., the exercise notice and the exercise price is not delivered to us (in the case of registered warrant holders) or to a member of the TASE Clearing House (in the case of warrants held by a member of the TASE Clearing House) by the expiration date, shall not award its holder any right or claims against us, and shall expire on the aforementioned date. We will also need to receive an allocation letter in order to issue the shares of common stock underlying the warrant.
We will publish a notification regarding the upcoming expiration date of a series of warrants.
In any case of distribution of bonus stock, in respect to the holders of warrants not exercised at such date, the aggregate exercise price of each warrant will remain unchanged, however, the exercise price per share of common stock will decrease accordingly.
If we offer to our stockholders securities of any kind by way of rights issuance, the exercise price of the warrants shall not be adjusted, however, the number of shares of common stock to be issued pursuant the exercise of the warrant that have not been exercised on the record date that establishes the right to acquire rights in rights issuance, shall be adjusted according to the benefit component of the rights.
In case of a declaration of a cash dividend, there will be no modification of the exercise price and/or the number of shares of common stock to be issued pursuant the exercise of the warrant. Notwithstanding the foregoing, the Company shall be permitted, but not obligated, to determine that, with respect to a certain series of unexercised warrants, the exercise price shall be adjusted by multiplying the ratio between the “X dividend” price and the closing price of the shares of common stock as quoted on the TASE on the record date determined for the right to receive the dividend.
Additional Warrants
In connection with the Subscription and Registration Rights Agreements that we entered into with each of Clal Biotechnology Industries Ltd. (CBI), Tikcro Technologies Ltd. (Tikcro) and Provident Fund of the Hebrew University Ltd. (Provident) on June 22, 2008, we issued warrants to each of CBI, Tikcro and Provident on July 31, 2008 to acquire up to 1,720,763, 4,301,906 and 258,114 shares of our common stock, respectively. These warrants are exercisable at any time prior to July 29, 2013 for a per share exercise price of $0.716. The exercise price of the warrants is subject to certain adjustments; see “Anti-Dilution Adjustments” below. For more information regarding the private placement of our securities that we completed in July 2008, see “Certain Relationships and Related Transactions — Private Placements.”
In connection with our initial public offering on the TASE, each of Clal Finance Ltd. and Altshuler Shaham, who served as underwriters at the public offering, received 150,000 warrants, exercisable for 4 years from the time that the average 30-day closing price of the share is greater than 130% of the IPO price (which was NIS 7.70, hence the average must be greater than NIS 10.01). The exercise price is 30% of the IPO price (i.e. NIS 2.31). In the event that the average 30-day closing price of the share does not reach the NIS 10.01 threshold within the four year period commencing on the date of the initial public offering, August 14 , 2006, the warrants will expire on such fourth anniversary.
71
Options Granted Pursuant to Our Stock Option Plans
For information on options granted to our directors, employees and consultants pursuant to our 2004 and 2007 Stock Option Plans, see “BioCancell Therapeutics Inc. 2004 Stock Option Plan and 2007 Stock Option Plan” above.
Anti-Dilution Rights
Pursuant to the terms of the Subscription and Registrations Rights Agreements dated June 22, 2008 that we entered into with, and the convertible debenture and warrants that we issued to, CBI, Tikcro and Provident, until such time as we have raised equity financing in excess of $15,000,000 (excluding any amounts invested by CBI, Tikcro and Provident and $5,000,000 raised in equity financings consummated within three months of the closing of the transactions contemplated by these agreements), if we issue any securities to any party for a per share purchase price, conversion price or exercise price, as applicable, less than any of the per share purchase price, conversion price or exercise price paid or payable for the shares of our common stock, convertible debentures or warrants, respectively, issued to CBI, Tikcro and Provident in the transaction, we will be (i) obligated to issue an additional number of shares of our common stock such that the number of shares originally issued to CBI, Tikcro and Provident at the closing plus such number of additional of shares of our common stock equal the number of shares of our common stock that would have been issued at the price at which we sold our securities such subsequent issuance for the purchase price paid at the closing for shares of our common stock, and (ii) the conversion price of convertible debentures and the exercise price of the warrants shall be reduced to equal the price at which our securities were sold in such subsequent issuance. These adjustment provisions do not apply to securities issued to our directors, officers, employees or consultants pursuant to awards under our stock option plans approved by our Board of Directors, securities issued upon a stock split, recapitalization, reclassification or dividend with respect to our capital stock, securities issued upon the exercise of options and warrants outstanding at the closing, or up to 15% of our capital stock issued by us in connection with an acquisition approved by our Board of Directors.
These agreements with CBI, Tikcro and Provident also provide that, until such time as we have raised equity financing in excess of $15,000,000 (excluding any amounts invested by CBI, Tikcro and Provident and $5,000,000 raised in equity financings consummated within three months of the closing of the transactions contemplated by these agreements), in the event that we issued any type of security including, but not limited to, any type of debt, warrant or equity securities the terms more favorable than those applicable to the securities issued to CBI, Tikcro and Provident, then, at the option of the CBI, Tikcro and Provident, the securities issued to those parties shall be construed as containing the more favorable terms afforded to such third party, as though such terms were previously provided herein retroactively from the closing date. Furthermore, if we issue convertible debentures which are publicly traded, the CBI, Tikcro and Provident have the right to have their convertible debenture exchanged for such publicly traded convertible debentures in an amount equal to the outstanding principal amount of their respective convertible debenture at that time.
Registration Rights
Pursuant to the terms of the Subscription and Registration Rights Agreements that we entered into in March and June 2008 with CBI, Tikcro and Provident, we agreed to use our best efforts to prepare and file, within nine months of the closing of those transactions on May 15 and July 30, 2008, and to cause to be declared effective, a registration statement with the SEC for the registration under the Securities Act of the shares of our common stock that we issued to CBI, Tickro and Provident, and the shares of our common stock that are issuable to those parties upon the conversion of the convertible debentures and exercise of the warrants that we issued to those parties, under those agreements. We are required under these agreements to qualify our shares of common stock for listing on NASDAQ (or, if we do not meet NASDAQ qualifications for listing, to qualify the shares for quotation on the OTCBB) immediately after the registration statement is declared effective.
Furthermore, these agreements require us to register, on two occasions after the later of (i) six months after the effectiveness of our initial registration statement under the Securities Act and (ii) 12 months after the closing of the transactions contemplated by those agreements if no such registration has been declared on such date, all of the shares of our common stock that held by CBI, Tickro and Provident that such party requests to be registered under the Securities Act, provided that such requested are for at least 25% of all of the shares of our common stock then held by such party for which such party is entitled to registration rights.
We also agreed at any such party’s request to (i) register such party’s shares of our common stock on Form S-3 whenever we are eligible to use Form S-3, and (ii) to include such party’s shares in any registration statement that we file for a primary offering of our securities under the Securities Act.
All of these registrations are subject to certain conditions and limitations, including the right of underwriters to limit the number of shares included in an offering.
We are required to bear certain expenses incurred in connection with such registration. These agreements include mutual indemnification provisions in connection with the registration.
If we grant registration right to any person or entity that are more favorable than those to which CBI, Tikcro and Provident are then entitled (including, but not limited to, preferences in underwriter’s cutbacks), such more favorable rights shall be deemed to have been granted to these parties as well.
72
PLAN OF DISTRIBUTION
We are offering up to shares of our common stock, Series 3 warrants, and Series 4 warrants on a best-efforts basis.
There is currently no public or other trading market in the U.S. for our shares of common stock, and we cannot give any assurance to you that the shares offered by this prospectus will have a market value, or that they can be resold for at least the offered price if and when an active secondary market might develop, or that a public market for our shares will be sustained even if one is ultimately developed. Our shares of common stock are currently listed for trade on TASE.
Our shares of common stock will be sold on our behalf by our officers and directors. Potential investors include, but are not limited to, friends, family members and business acquaintances of our officers and directors. The intended methods of communication include, without limitation, telephone calls and personal contacts. There can be no assurance that all, or any, of the offered securities will be sold.
Our officers and directors will not register as broker/dealers under Section 15 of the Exchange Act in reliance upon Rule 3a4-1. Rule 3a4-1 sets forth those conditions under which a person associated with an issuer may participate in the offering of the issuer’s securities and not be deemed to be a broker/dealer. The conditions are that:
|
·
|
The person is not statutorily disqualified, as that term is defined in Section 3(a)(39) of the Exchange Act, at the time of his participation,
|
|
·
|
The person is not compensated in connection with his participation by the payment of commissions or other remuneration based either directly or indirectly on transactions in securities,
|
|
·
|
The person is not at the time of their participation, an associated person of a broker/dealer, and
|
|
·
|
The person meets the conditions of paragraph (a)(4)(ii) of Rule 3a4-1 of the Exchange Act, in that he (a) primarily performs, or is intended primarily to perform at the end of the offering, substantial duties for or on behalf of the issuer otherwise than in connection with transactions in securities, (b) is not a broker or dealer, or an associated person of a broker or dealer, within the preceding 12 months, and (c) does not participate in selling and offering of securities for any issuer more than once every 12 months other than in reliance on paragraphs (a)(4)(i) or (a)(4)(iii).
|
The Auction Process
The following describes the auction process being used to determine the price and the number of the units to be issued. We believe allowing open participation in this offering through an auction process aligns with our corporate culture and business mission. The auction process will be conducted pursuant to regulations of the TASE and the Israeli Securities Authority. This offering method differs from methods that have been traditionally used in most other public offerings in the U.S.
We will appoint a member of the TASE to act as our offering coordinator to administrate the offering.
Prior to the auction, we may obtain early bids of Israeli institutional investors. Such process is regulated by applicable rules of TASE and the Israeli Securities Authority. Summary date with respect to these bids, if obtained, will be announced by us prior to the commencement of the auction.
The bidding process will take place during one business day in Israel. Each bidder may submit up to three irrevocable bids, at different prices, to the offering coordinator or to other entities in Israel (which we referred to as the authorized entities) that are authorized to receive bids (which will be members of the TASE as well). Bids received by the authorized entities will be transferred, in sealed envelops, to the offering coordinator which will keep them in a sealed box until one hour after the closing of the auction. Each bid will specify the number of units the investor proposes to purchase and the price the investor is willing to pay for the units, which will be no less than the minimum price announced by us prior to the bidding process. Any bid lower than the minimum price will be void. By submitting the bid, each investor irrevocably undertakes to purchase and pay for the units that will be issued to such investor, if any. Under regulations promulgate by the Israeli Securities Authority, each authorized entity and the offering coordinator guarantees the bids submitted in the auction through such authorized entity or offering coordinator, as the case may be.
One hour after the close of the bidding process, the offering coordinator, in the presence of our independent accountants, will review all bids to determine the offering price per unit and the number of the units to be issued. Only investors who made bids during the bid process will be permitted to purchase units. See the section entitled “Risk Factors - Risks Related to the Auction Process for Our Offering.”
73
Determination of Unit Price
The principal factor in establishing the offering price to be paid for the units offered in this offering will be the “offering price per unit” resulting from the highest price at which bids were placed for purchase of all units offered in this offering, or, if the amount of the units for which bids were placed is lower than the number of units offered in this offering, the minimum price shall be offering price per unit. As indicated, the bid can be at the minimum price or at any price above that minimum and no bids lower than the minimum price will be accepted. The “offering price per unit” will also determine the allocation of shares to successful bidders.
First, we will arrange all bids in descending order of price. Then we will count the bids in descending order, until we have counted bids for 100% of the units. The price offered in the last bid so counted will be the offering price per unit. For example, if the number of units included in the bids is less than 100% of the number of units offered, the offering price per unit will be the minimum price. If the number of units included in the bids is equal or greater than 100% of the number of units offered, the offering price per unit will be the highest bid price at or which bids have been made for 100% of the offering. All units will be sold at the same price.
The following table illustrates a hypothetical bid for an offering of 40,000 units, with the minimum price per unit set at NIS 900. The table indicates we would receive aggregate offers to purchase 50,000 units at NIS 900 to 1,050. Since the 50,000 Units bid exceed the 40,000 units available, these units will be allocated among all the highest bidders. All bidders making bids above the offering price per unit will receive all the units for which they bid, all bidders making bids at the offering price per unit will receive some of the units for which they bid, on a pro-rata basis, and all bidders making bids below the offering price per unit will receive no units. Thus, 22,000 units would be sold to bidders who bid above the offering price per unit. Since 20,000 units were bid for at the offering price per unit, but only 18,000 were available, bidders at the offering price per unit would receive 90% of the units for which they bid. With NIS 950 as the offering price per unit, this is the price that all successful bidders from NIS 950 to 1,050 would pay for each unit bid and allocated, and the gross proceeds to us would be 38,000,000 NIS (40,000 units x 950 NIS/unit).
All bids below 950 NIS, in this case all 8,000 units bid at 900 NIS per unit, which was the stated minimum public offering price, would be rejected.
|
Number of Units Requested by Bidders
|
Bid Price
|
Aggregate Number of Units at Bid Price and Greater
|
Number of Units Successfully Bid For
|
Percent of Successful Bids
|
||||
|
12,000
|
1,050 NIS
|
12,000
|
12,000
|
100%
|
||||
|
10,000
|
1,000 NIS
|
22,000
|
10,000
|
100%
|
||||
|
20,000
|
950 NIS
|
42,000
|
18,000
|
90%
|
||||
|
8,000
|
900 NIS
|
50,000
|
0
|
0%
|
As the above hypothetical bid process illustrates, this auction process will determine the proceeds to us, the only variable being the number of valid firm bids received (and associated price). The “offering price per unit” is determined by the bids, and thus we do not have the ability to arbitrarily choose. Of course, we do not know how many offers to purchase will be submitted or what the prices will be for any offers to purchase. The above hypothetical table is included merely to explain our auction process. The final allocation of the units will be conducted in accordance with the applicable regulations of the TASE and the Israeli Securities Authority.
The minimum price in the offering and the exercise prices of the warrants will be announced by us at least five (5) TASE trading hours before the commencement of the auction by filing a report with the Israeli Securities Authority and a current report on Form 8-K with the SEC. These parameters will be determined by our management based on management's assessment of, among others, prevailing market conditions, the historical performance of our stock on TASE and early bids received by institutional investors.
Acceptance of Bids
The offering coordinator will open a bank account on our behalf to which all authorized entities be instructed to deposit the consideration for the units to be issued. The escrow account will be managed exclusively by the offering coordinator on behalf of the company. Until 10:00 am (Israel time) on a TASE trading day following the closing of the auction, the offering coordinator will provide the authorized entities the results of the auction. Pursuant to Israeli regulation, until 12:00 am (Israel time) on that day, the consideration will be wired by the authorized entities to the offering coordinator which will transfer the consideration to us upon and subject to the issuance of the securities offered in this offering.
74
EXPERTS
The consolidated financial statements of BioCancell Therapeutics, Inc. (a Development Stage Company), as of December 31, 2009 and 2008 , and for each of the years in the two-year period ended December 31, 2009 , and the cumulative period from October 1, 2004 (inception of operations) through December 31, 2009 , have been included herein, in reliance upon the report of Somekh Chaikin, a member firm of KPMG International, an independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
The audit report covering the December 31, 2009 consolidated financial statements contains an explanatory paragraph that states that the Company's recurring losses from operations raise substantial doubt about the entity's ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of that uncertainty. Also, this same report refers to the adoption by the Company, effective January 1, 2008, of the new accounting and disclosure requirements with respect to its assets and liabilities measured at fair value on a recurring basis, and effective January 1, 2009, of the new requirements with respect to nonfinancial assets and liabilities measured at fair value on a non-recurring basis .
LEGAL MATTERS
The validity of the securities offered in this prospectus is being passed upon for us by our counsel, Gross, Kleinhendler, Hodak, Halevy, Greenberg & Co ., Tel Aviv, Israel .
COMMISSION’S POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
We are a Delaware corporation. Article XII of our Amended and Restated Certificate of Incorporation provides to the fullest extent permitted under the Delaware General Corporation Law (“DGCL”), that our directors or officers shall not be personally liable to us or our stockholders for damages for breach of such director’s or officer’s fiduciary duty. Section 145 of the DGCL provides that a corporation may indemnify directors, officers, employees and agents against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement in connection with specified actions, suits or proceedings whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation — a “derivative action”), if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe their conduct was unlawful. A similar standard is applicable in the case of derivative actions, except that indemnification is permitted only for expenses (including attorneys’ fees) incurred in connection with the defense or settlement of such action, and the statute requires court approval before there can be any indemnification for expenses where the person seeking indemnification has been found liable to the corporation. The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s charter, bylaws, disinterested director vote, stockholder vote, agreement or otherwise.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable.
ADDITIONAL INFORMATION
We have filed a registration statement on Form S-1 with the SEC, to register the units being offered by this prospectus. In addition, we file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy any reports, statements or other information that we file at the SEC’s public reference facilities at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information regarding the public reference facilities. The SEC maintains a website, http://www.sec.gov, that contains reports, proxy statements and information statements and other information regarding registrants that file electronically with the SEC, including us. Our SEC filings are also available to the public from commercial document retrieval services. Information contained on our website should not be considered part of this prospectus.
You may also request a copy of our filings at no cost by writing or telephoning us at:
BioCancell Therapeutics Inc.
Beck Science Center
8 Hartom St, Har Hotzvim
Jerusalem 97775 Israel
972-2-548-6555
75
BioCancell Therapeutics
Inc. and Subsidiary
(Development Stage Company)
Consolidated Financial Statements
As of March 31, 2010
(Unaudited)
F-1
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Financial Statements as of March 31, 2010
| Table of Contents | Page |
| Consolidated Balance Sheets | F-3 |
| Consolidated Statements of Operations | F-5 |
| Consolidated Statements of Cash Flows | F-6 |
| Notes to the Consolidated Financial Statements | F-8 |
F-2
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Balance Sheets (Unaudited)
|
March 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
U.S. dollars in thousands
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 2,801 | $ | 624 | ||||
|
Receivable from Chief Scientist and BIRD Foundation
|
74 | 45 | ||||||
|
Prepaid expenses 1
|
169 | 228 | ||||||
|
Other current assets
|
56 | 88 | ||||||
|
Total current assets
|
3,100 | 985 | ||||||
|
Deferred stock issuance costs
|
215 | 178 | ||||||
|
Long-term assets
|
||||||||
|
Deposit in respect of employee severance benefits
|
212 | 237 | ||||||
|
Long-term prepaid expenses
|
60 | 59 | ||||||
|
Total long-term assets
|
272 | 296 | ||||||
|
Property and equipment at cost, net of $139 thousand
|
||||||||
|
and $130 thousand accumulated depreciation as of
|
||||||||
|
March 31, 2010 and December 31, 2009, respectively
|
101 | 106 | ||||||
|
Total assets
|
$ | 3,688 | $ | 1,565 | ||||
1 The amounts recorded as of March 31, 2010 and December 31, 2009 include $14 and $28 thousand to a related party, respectively.
The accompanying notes form an integral part of the financial statements.
F-3
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Balance Sheets (Unaudited)
|
March 31,
|
December 31,
|
|||||||||||
|
2010
|
2009
|
|||||||||||
|
Note
|
U.S. dollars in thousands
|
|||||||||||
|
Current liabilities
|
||||||||||||
|
Accounts payable 1
|
$ | 197 | $ | 239 | ||||||||
|
Accrued expenses and others 2
|
236 | 298 | ||||||||||
|
Accrued vacation pay
|
64 | 80 | ||||||||||
|
Employees and related liabilities
|
53 | 66 | ||||||||||
|
Total current liabilities
|
550 | 683 | ||||||||||
|
Long-term liabilities
|
||||||||||||
|
Liability for employee severance benefits
|
175 | 204 | ||||||||||
|
Convertible notes payable
|
3,4 | 748 | 644 | |||||||||
|
Warrants to noteholders
|
3 | 4,649 | 3,400 | |||||||||
|
Total long-term liabilities
|
5,572 | 4,248 | ||||||||||
|
Contingent liabilities and commitments
|
2 | |||||||||||
|
Stockholders' equity (deficit)
|
||||||||||||
|
Common stock, $0.01 par value per share (50,000,000 shares
|
||||||||||||
|
authorized, 20,584,050 and 16,356,550 shares issued and
|
||||||||||||
|
outstanding, as of March 31, 2010, and December 31, 2009,
|
||||||||||||
|
respectively)
|
206 | 164 | ||||||||||
|
Additional paid-in capital
|
19,846 | 16,692 | ||||||||||
|
Accumulated other comprehensive income
|
287 | 335 | ||||||||||
|
Accumulated deficit
|
(22,773 | ) | (20,557 | ) | ||||||||
|
Total stockholders' equity (deficit)
|
(2,434 | ) | (3,366 | ) | ||||||||
|
Total liabilities and stockholders' equity (deficit)
|
$ | 3,688 | $ | 1,565 | ||||||||
1 The amount recorded as of March 31, 2010 includes $17 to a related party.
2 The amount recorded as of December 31, 2009 includes $8 thousand to a related party.
The accompanying notes form an integral part of the financial statements.
F-4
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Operations (Unaudited)
|
Cumulative
|
||||||||||||
|
from inception
|
||||||||||||
|
Three month period ended
|
through
|
|||||||||||
|
March 31
|
March 31
|
March 31,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
(except share and per share data)
|
||||||||||||
|
Research and development expenses
|
$ | 534 | $ | 585 | $ | 11,803 | ||||||
|
Less: Chief Scientist and BIRD Foundation grants
|
(29 | ) | (171 | ) | (2,046 | ) | ||||||
|
Research and development expenses, net
|
505 | 414 | 9,757 | |||||||||
|
General and administrative expenses 1
|
428 | 324 | 6,721 | |||||||||
|
Operating loss
|
933 | 738 | 16,478 | |||||||||
|
Interest expense (income), net
|
(2 | ) | 1 | (15 | ) | |||||||
|
Gain from marketable securities, net
|
- | - | (6 | ) | ||||||||
|
Interest on convertible notes and discount amortization
|
93 | 79 | 582 | |||||||||
|
Revaluation of warrants
|
1,186 | 1,037 | 2,586 | |||||||||
|
Other financing expense (income), net
|
6 | (110 | ) | (279 | ) | |||||||
|
Net loss
|
$ | 2,216 | $ | 1,745 | $ | 19,346 | ||||||
|
Basic and diluted net loss per share
|
$ | 0.13 | $ | 0.12 | $ | 1.85 | ||||||
|
Weighted-average common shares used in computing
|
||||||||||||
|
basic and diluted net loss per share
|
17,213,498 | 14,479,859 | 10,473,498 | |||||||||
|
|
1 The amounts recorded for the three month period ending March 31, 2010 and for the cumulative period include $21 thousand $95 thousand to a related party, respectively.
|
The accompanying notes form an integral part of the financial statements.
F-5
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Cash Flows (Unaudited)
|
Cumulative
|
||||||||||||
|
from inception
|
||||||||||||
|
Three month period ended
|
through
|
|||||||||||
|
March 31
|
March 31
|
March 31,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$ | (2,216 | ) | $ | (1,745 | ) | $ | (19,346 | ) | |||
|
Adjustments to reconcile net loss to net cash flows from
|
||||||||||||
|
operating activities:
|
||||||||||||
|
Income and expenses not involving cash flows:
|
||||||||||||
|
Increase (decrease) in liability for employee severance
|
||||||||||||
|
benefits
|
(32 | ) | 12 | 162 | ||||||||
|
Fair value adjustment of marketable securities
|
- | - | 133 | |||||||||
|
Depreciation and amortization
|
9 | 11 | 134 | |||||||||
|
Stock-based compensation
|
124 | 22 | 1,765 | |||||||||
|
Revaluation of warrants
|
1,186 | 1,037 | 2,586 | |||||||||
|
Accrued interest and amortization of discount to notes
|
||||||||||||
|
payable, and exchange difference thereon
|
115 | 107 | 590 | |||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Decrease (increase) in other current assets
|
33 | (2 | ) | (57 | ) | |||||||
|
Decrease (increase) in prepaid expenses
|
63 | (38 | ) | (144 | ) | |||||||
|
Increase in Chief Scientist and BIRD Foundation receivable
|
(28 | ) | (83 | ) | (7 | ) | ||||||
|
Investment in marketable securities (trading)
|
- | - | (7,883 | ) | ||||||||
|
Proceeds from marketable securities (trading)
|
- | - | 5,970 | |||||||||
|
Decrease (increase) in severance pay deposits
|
29 | (15 | ) | (190 | ) | |||||||
|
Decrease (increase) in long-term receivables
|
- | 1 | (53 | ) | ||||||||
|
Increase (decrease) in accounts payable
|
(46 | ) | (183 | ) | 181 | |||||||
|
Increase(decrease) in employees and related liabilities
|
(14 | ) | 32 | 72 | ||||||||
|
Increase (decrease) in accrued vacation pay
|
(17 | ) | 15 | 30 | ||||||||
|
Increase (decrease) in accrued expenses
|
(67 | ) | 24 | 186 | ||||||||
|
Net cash used in operating activities
|
(861 | ) | (805 | ) | (15,871 | ) | ||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Investment in marketable securities (trading)
|
- | - | (921 | ) | ||||||||
|
Proceeds from marketable securities (trading)
|
- | - | 3,173 | |||||||||
|
Sale of property and equipment
|
2 | - | 2 | |||||||||
|
Acquisition of property and equipment
|
(3 | ) | (1 | ) | (215 | ) | ||||||
|
Net cash provided by (used in) investing activities
|
$ | (1 | ) | $ | (1 | ) | $ | 2,039 | ||||
The accompanying notes form an integral part of the financial statements.
F-6
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Cash Flows (Unaudited) (cont’d)
|
Cumulative
|
||||||||||||
|
from inception
|
||||||||||||
|
Three month period ended
|
through
|
|||||||||||
|
March 31
|
March 31
|
March 31,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Issuance of common stock, net of issuance costs of $215
|
||||||||||||
|
thousand
|
$ | 3,072 | $ | - | $ | 13,465 | ||||||
|
Increase in deferred stock issuance stocks
|
(34 | ) | - | (211 | ) | |||||||
|
Issuance of Series A convertible preferred stock
|
- | - | 2,118 | |||||||||
|
Payments of debtors for shares
|
- | - | 473 | |||||||||
|
Issuance of Series A options warrant
|
- | - | 772 | |||||||||
|
Issuance of Series B options warrant
|
- | - | 1,028 | |||||||||
|
Receipt of grant from Chief Scientist
|
- | - | 2 | |||||||||
|
Repayment of stockholder loans
|
- | - | 360 | |||||||||
|
Treasury stock purchase
|
- | - | (4,951 | ) | ||||||||
|
Treasury stock sale
|
- | - | 1,568 | |||||||||
|
Convertible notes payable
|
- | - | 176 | |||||||||
|
Warrants to noteholders
|
- | - | 1,829 | |||||||||
|
Net cash provided by financing activities
|
3,038 | - | 16,629 | |||||||||
|
Effect of exchange rate on cash
|
1 | (240 | ) | 4 | ||||||||
|
Increase (decrease) in cash and cash equivalents
|
2,177 | (1,046 | ) | 2,801 | ||||||||
|
Cash and cash equivalents at beginning of period
|
624 | 2,779 | - | |||||||||
|
Cash and cash equivalents at end of period
|
$ | 2,801 | $ | 1,733 | $ | 2,801 | ||||||
|
Non-cash transactions
|
||||||||||||
|
Conversion of stockholder loans
|
$ | - | $ | - | $ | 360 | ||||||
|
Issuance of common stock to founders
|
- | - | 43 | |||||||||
|
Issuance of option warrants to underwriters
|
- | - | 358 | |||||||||
|
Exercise of stock options by Company consultants
|
- | - | 1 | |||||||||
|
|
||||||||||||
|
Conversion of series A convertible preferred stock to common stock
|
$ | - | $ | - | $ | 33 | ||||||
The accompanying notes form an integral part of the financial statements.
F-7
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 1 – Business and Summary of Significant Accounting Policies
|
A.
|
BioCancell Therapeutics, Inc. (hereafter "the Parent") was incorporated in the United States as a private company under the laws of the State of Delaware on July 26, 2004 and commenced operations on October 1, 2004.
|
|
B.
|
The principal activities of the Parent and its subsidiary in Israel, BioCancell Therapeutics Israel Ltd. (the "Subsidiary"), (hereafter collectively referred to as “the Company”) are research and development of drugs for the treatment of superficial bladder cancer, pancreatic cancer and ovarian cancer. The leading drug developed by the Company has been successfully tested for a number of cancer types in pre-clinical lab animal trials, compassionate use human trials and a Phase I/IIa clinical trial. The Company is now performing an advanced Phase IIb clinical trial on bladder cancer patients, a Phase I/IIa clinical trial on ovarian cancer patients and a Phase I/IIa clinical trials of BC-819 for treatment of pancreatic cancer. The Company is evaluating indications for the possible use of this drug, and others under development, to treat other types of cancer.
|
The Company is in the development stage. Therefore, there is no certainty regarding the Company’s ability to complete the product’s development, receipt of regulatory permits, alternative treatments or procedures that may be developed, and success of its marketing. The continuation of the stages of development and the realization of assets related to the planned activities depend on future events, including the receipt of interim financing and achieving operational profitability in the future. The Company has generated only limited income since its inception and has incurred substantial losses and expects that it will operate at a loss over the coming years, as it does not expect to generate any revenue from operations in the near term. The Company is initiating activities to raise capital for ensuring future operations although there are still significant doubts as to the ability of the Company to continue operating as a “going concern”. The Company believes that it has sufficient cash to meet its planned operating needs until the end of November 2010, based on its current cash level. It is not possible to estimate the final outcome of these activities. These financial statements do not include any adjustments to the value of assets and liabilities and their classification, which may be required if the Company cannot continue operating as a “going concern”.
The biotechnology industry is characterized by strong competition, resulting from the risk of frequent technological changes. Entry into this market requires the investment of considerable resources and continuous development. The Company's future success is dependent on several factors, including the quality of the Company's technology, the product's price, and the creation of an advantage over the competition.
|
C.
|
The Company's research and development activities are carried out by its Subsidiary primarily through a laboratory research team at the Hebrew University in Jerusalem. The Hebrew University laboratory is managed by the Chief Scientist of the Company, who is a related party. All of the Company’s net assets are situated in Israel.
|
|
D.
|
The Company filed a prospectus for an initial public offering on the Tel Aviv Stock Exchange (“TASE”) and beginning August 17, 2006 has been publicly traded on the TASE. On June 23, 2009 the Company’s Registration Statement on Form S-1 was deemed effective by the United States Securities and Exchange Commission (SEC) and as of that date it is a reporting company to the SEC.
|
|
E.
|
In October 2009, the Company published a shelf prospectus with the TASE (which is valid for two years) and a Form S-1/A with the U.S. Securities and Exchange Commission in anticipation of its upcoming fundraising. As of March 31, 2010, the Company has not received final comments from the SEC regarding its filing and has recorded $215 thousand as deferred stock issuance costs in anticipation of this planned fundraising.
|
|
F.
|
Basis of Presentation
|
The accompanying consolidated financial statements include the accounts of BioCancell Therapeutics, Inc. and its subsidiary and are presented in accordance with accounting principles generally accepted in the United States of America. All significant intercompany balances and transactions have been eliminated in consolidation.
The accompanying unaudited consolidated financial statements were prepared in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X of the SEC for interim financial information and, therefore, do not include all disclosures necessary for a complete presentation of financial condition, results of operations, and cash flows in conformity with generally accepted accounting principles. All adjustments which are, in the opinion of management, of a normal recurring nature and are necessary for a fair presentation of the interim financial statements have been included. Nevertheless, these financial statements should be read in conjunction with the consolidated financial statements and related notes included in the Company’s Consolidated Financial Statements for the year ended December 31, 2009. The results of operations for the three months ended March 31, 2009 and 2010 are not necessarily indicative of the results that may be expected for the entire fiscal year or any other interim period. Certain amounts have been reclassified in order to conform to the current year presentation.
The Company also files Hebrew language, New Israel Shekel based financial statements, prepared in accordance with International Financial Reporting Standards (IFRS), with the TASE.
F-8
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 1 – Business and Summary of Significant Accounting Policies (cont’d)
|
|
G.
|
Development Stage Enterprise
The Company’s principal activities to date have been the research and development of its products and the Company has not generated revenues from its planned, principal operation. Accordingly, the Company’s financial statements are presented as those of a development stage enterprise.
|
|
|
H.
|
Reporting Currency
|
|
|
The financial records of the Parent and its Subsidiary are maintained in New Israel Shekels, which is the functional currency of the Company. These financial statements have been prepared using the U.S. dollar as the reporting currency.
All assets and liabilities are translated at period-end exchange rates. Items in the statement of stockholders’ equity (deficit) are at actual historical exchange rates. Statement of operations items are translated at the average exchange rate for the period. The adjustment resulting from translating the financial statements is included in accumulated other comprehensive income, which is reflected as a separate component of stockholders’ equity.
The following table summarizes the representative rate of exchage of the U.S. dollar to the New Israel Shekel (NIS):
|
|
Rate at March 31,
|
Rate at December 31,
|
Average rate of exchange
for the three month period ended March 31,
|
||||||||||
|
NIS
|
||||||||||||
|
2010
|
3.713 | n/a | 3.734 | |||||||||
|
2009
|
4.188 | 3.775 | 4.059 | |||||||||
|
|
The average rate of exchange during the development stage period (from inception through March 31, 2010) was NIS 4.096 to $1.
The translated figures should not be construed to represent actual amounts in, or convertible into, U.S. dollars.
|
|
|
I.
|
Transactions in Foreign Currency
Transactions in foreign currency are recorded at the exchange rate as of the transaction date. Exchange gains or losses from the disposition of monetary assets or liabilities or from the reporting of monetary assets or liabilities according to exchange rates other than those originally recorded, or from exchange rates recorded in prior financial statements, are recognized in the Statement of Operations.
|
|
|
J.
|
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported results of operations during the reporting periods. Actual results may differ from such estimates.
Significant items subject to such estimates and assumptions include the valuation of derivatives, deferred tax assets and stock-based compensation. The current economic environment has increased the degree of uncertainty inherent in those estimates and assumptions.
|
F-9
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 1 – Business and Summary of Significant Accounting Policies (cont’d)
K. Derivative Instruments
|
|
The Company recognizes all derivative instruments as either assets or liabilities in the balance sheet at their respective fair values. The Company carries its derivatives at fair value on the balance sheet and recognizes any subsequent changes to fair value in earnings.
|
The Company issued derivative instruments in the form of warrants to purchase an aggregate of up to 6,280,783 shares of common stock (at a price of 71.6 cents per share) as part of the financing described in Note 4 below. The warrants have been recorded as a liability, at fair value, and changes in the fair value of the instruments are included in the Statement of Operations under the caption "revaluation of warrants.
L. Segment and Geographic Information
|
|
The Company's business is currently comprised of one geographic and operating segment, focused on the discovery, development and commercialization of novel therapies for treating cancer-related diseases. The Company's development and administration operations are maintained in Israel. The Company’s chief operating decision maker uses measurements aggregated at the entity-wide level to manage the business.
|
|
|
M.
|
Net Loss Per Share
|
Basic net loss per share is computed by dividing the net loss for the period by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing the net loss for the period by the weighted-average number of common shares plus dilutive potential common stock considered outstanding during the period. However, as the Company generated net losses in all periods presented, potentially dilutive securities, comprised of incremental common shares issuable upon the conversion of Series A convertible preferred stock and the exercise of warrants and stock options, are not reflected in diluted net loss per share because such shares are antidilutive.
The following table summarizes the securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic EPS in the future and were not included in the computation of basic and diluted EPS because doing so would have been antidilutive for the periods presented:
|
Cumulative
|
||||||||||||
|
from October
|
||||||||||||
| 1, 2004 | ||||||||||||
|
For the three month period ended
|
(inception) to
|
|||||||||||
|
March 31
|
March 31
|
March 31
|
||||||||||
|
2010
|
2009
|
2010 | ||||||||||
|
Series 2 Warrants
|
2,500,000 | 2,500,000 | 2,500,000 | |||||||||
|
Warrants to underwriter
|
300,000 | 300,000 | 300,000 | |||||||||
|
Convertible notes payable
|
4,078,212 | 4,078,212 | 4,078,212 | |||||||||
|
Warrants for interest on convertible notes payable
|
707,709 | 272,626 | 707,709 | |||||||||
|
Warrants to investors
|
10,166,202 | 5,573,619 | 10,166,202 | |||||||||
|
Stock options to employees, directors and consultants under Stock Option Plans
|
2,569,489 | 1,667,989 | 2,569,489 | |||||||||
| 20,321,612 | 14,392,446 | 20,321,612 | ||||||||||
F-10
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 2 – Contingent Liabilities and Commitments
|
|
A.
|
Operating leases
|
1. Vehicles
The Company has lease agreements for 9 vehicles for 36 months each. As required by the agreements, the Company deposited a security deposit to ensure the fulfillment of its contractual commitment. At March 31, 2010, the balance of the deposit was $23 thousand and represents the last three months of the lease. The deposit is linked to the Israeli CPI and does not bear interest.
The minimum annual payments in connection with the agreement are as follows:
|
U.S. dollars in thousands
|
||||
|
2010
|
$ | 90 | ||
|
2011
|
$ | 73 | ||
|
2012
|
$ | 25 | ||
2. Rental Agreement
|
|
In November 2006 the Company entered into a rental agreement for its offices for a 24 month period with an option to extend. During 2008 and 2009 the Company extended the rental agreement for additional periods of 12 months until September 16, 2010. The monthly rental (including maintenance fee and parking) is $4 thousand + VAT. The Company presented the lessor with a bank guarantee in the amount of $16 thousand to ensure fulfillment of its contractual obligations.
|
|
|
The following table summarizes the Company’s rent expense for the periods presented:
|
|
Three month period ending March 31, 2010
|
Three month period ending March 31, 2009
|
Cumulative from inception through
March 31, 2010
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Rent expense
|
$ | 14 | $ | 12 | $ | 195 | ||||||
The minimum annual payment in connection with the agreement is as follows:
|
U.S. dollars in thousands
|
||||
|
2010
|
$ | 25 | ||
F-11
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 2 – Contingent Liabilities and Commitments (cont’d)
|
|
B.
|
Consulting Agreement
|
As of July 2008, the Company entered into an agreement with Tikcro Technologies Ltd., whereby in exchange for consultancy services provided by it, the Company will pay Tikcro Technologies Ltd. an annual amount of $30 thousand, 63,939 common shares and reimbursement of expenses. The Company recorded in the first year of the agreement a provision in the amount of $40 thousand in connection with these shares. The Company believes that it did not receive the consultancy services as provided for in the agreement for that year. Therefore, the Board of Directors of the Company, with the advice of its legal advisor, decided to cancel the provision. As of the date of these financials statements, the Company has not paid these fees and the companies are in discussions regarding the consulting fee. In July 2009, the Company began to receive consultancy services. As of March 31, 2010, the Company has recorded as general and administrative expenses approximately $8 thousand for these services and an additional $14 thousand in regard to the 63,939 shares issued for the second year of the agreement. Additionally, the Company has recorded as a prepaid expense approximately $14 thousand in connection with these shares.
|
|
C.
|
Clinical Trial Agreements between the Company and Medical Centers in the U.S. and Israel (collectively, “Hospitals”)
|
|
|
The Company has entered into indemnification agreements with several medical centers which are more fully described in the Company’s annual financial statements and that at present the Company is not aware of any need for such indemnification.
|
Note 3 – Fair Value Measurements
Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering such assumptions, ASC subtopic 820-35 establishes a three-tier value hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
Level 1 - Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 - Include other inputs that are directly or indirectly observable in the marketplace.
Level 3 - Unobservable inputs which are supported by little or no market activity.
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
F-12
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 3 – Fair Value Measurements (cont'd)
The Company’s warrants to noteholders are valued using the Binomial model. Prior to the second quarter of 2009, the Company’s warrants to noteholders were valued using the Black-Scholes model. Given the recent foreign currency fluctuation’s effect on the exercise price of the Warrants throughout the life of the Warrant, the Company’s assumptions have changed regarding the possible exercise patterns, and these possibilities can be addressed through the Binomial model in contrast to the Black & Scholes model which only allows for one exercise date. The change in the model did not have a material impact on our consolidated financial position, results of our operations or cash flows. These models use the variables of the price of the underlying stock, the strike price, the continuously compounded risk free interest rate, the continuously compounded annual dividend yield, the time in years until the expiration of the option and the implied volatility of the underlying stock. Some of the inputs to this valuation are unobservable in the market and are significant, requiring significant judgment using the best information available.
The Company recorded a charge of approximately $1,186 thousand and $1,037 thousand for the three months ended March 31, 2010 and March 31, 2009, respectively, and $2,586 thousand for the development stage period, resulting from revaluation of warrants to shareholders, which has been recorded in the statement of operations.
Assets and liabilities measured at fair value are summarized below:
|
Fair value measurement at reporting date using
|
||||||||||||||||
|
Significant
|
||||||||||||||||
|
Quoted Prices in
|
Other
|
Significant
|
||||||||||||||
|
Active Markets
|
Observable
|
Unobservable
|
||||||||||||||
|
for Identical
|
Inputs
|
Inputs
|
||||||||||||||
|
March 31, 2010
|
Assets (Level 1)
|
(Level 2)
|
(Level 3)
|
|||||||||||||
|
Description
|
U.S. dollars in thousands
|
|||||||||||||||
|
Warrants to noteholders
|
$ | 4,649 | - | - | $ | 4,649 | ||||||||||
|
Total Liabilities
|
$ | 4,649 | - | - | $ | 4,649 | ||||||||||
|
Fair value measurement at reporting date using
|
||||||||||||||||
|
Significant
|
||||||||||||||||
|
Quoted Prices in
|
Other
|
Significant
|
||||||||||||||
|
Active Markets
|
Observable
|
Unobservable
|
||||||||||||||
|
for Identical
|
Inputs
|
Inputs
|
||||||||||||||
|
March 31, 2009
|
Assets (Level 1)
|
(Level 2)
|
(Level 3)
|
|||||||||||||
|
Description
|
U.S. dollars in thousands
|
|||||||||||||||
|
Warrants to noteholders
|
$ | 3,400 | - | - | $ | 3,400 | ||||||||||
|
Total Liabilities
|
$ | 3,400 | - | - | $ | 3,400 | ||||||||||
F-13
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 3 – Fair Value Measurements (cont'd)
The following table presents the Company’s activity for liabilities measured at fair value using significant unobservable inputs (Level 3) as defined in SFAS 157, as of March 31, 2010:
|
Level 3
|
||||
|
U.S. dollars in
|
||||
|
thousands
|
||||
|
Balance at December 31, 2009
|
$ | 3,400 | ||
|
Loss resulting from revaluation of warrants to
|
1,186 | |||
|
shareholders, included in the statement of
|
||||
|
operations for the three months ended
|
||||
|
March 31, 2010
|
||||
|
Adjustment for translation differences
|
63 | |||
|
Balance at March 31, 2010
|
$ | 4,649 | ||
Other financial instruments include cash and cash equivalents, accounts receivable, other current assets, accounts payable, accrued expenses and others, and convertible notes payable. Except for the convertible notes payable, the fair value of these instruments at March 31, 2010 and December 31, 2009 approximate the carrying value amounts of all the aforementioned financial instruments. Estimated fair values for the convertible notes payable have been determined using the Binomial model. Carrying amounts and the related estimated fair value of the convertible notes payable are as follows:
|
March 31, 2010
|
March 31, 2009
|
|||||||||||||||
|
U.S. dollars in thousands
|
||||||||||||||||
|
Carrying
|
Carrying
|
|||||||||||||||
|
Amount
|
Fair Value
|
Amount
|
Fair Value
|
|||||||||||||
|
Convertible notes payable
|
$ | 748 | $ | 5,719 | $ | 644 | $ | 3,483 | ||||||||
The difference between the carrying amount as compared to the fair value of the convertible notes payable represents the unamortized portion of the beneficial conversion feature of the convertible notes payable.
F-14
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 4 - Convertible Notes Payable
In July 2008, the Company carried out private placements with institutional investors (the “Investors”), whereby 3 investors received an aggregate amount of 1) 1,222,780 shares of common stock (at a price of 59.7 cents per share) (the “Common Shares”), 2) non-registered convertible notes payable (the “Convertible Notes Payable”) convertible into an aggregate of up to 5,058,002 common shares (at a conversion price of 71.6 cents per share) and 3) non-registered warrants (the “Warrants”) to purchase an aggregate of up to 6,280,783 shares of common stock (at a price of 71.6 cents per share) exercisable for 5 years. The Company's gross proceeds from the private placements were approximately $3.650 million (NIS 12.662 million). As long as they are not converted, the Convertible Notes Payable bear dollar - linked interest of 10% per annum, to be added to the principal (and considered as part of the principal for the purposes of conversion) for the first nine quarters and paid quarterly thereafter, for the remaining 7 quarters.
The Convertible Notes Payable were classified as long-term liabilities and were recorded at their initial relative fair value. The Convertible Notes Payable are presented net of unamortized discounts for the portions allocated to the Warrants, Common Shares and the beneficial conversion feature inherent in the instrument. The interest due on the Convertible Notes Payable is accrued to the value of the instrument, as the period for cash payment of interest, as described above, has not yet commenced.
|
U.S. dollars in
|
||||
|
Movement during the period:
|
thousands
|
|||
|
Balance at December 31, 2009
|
$ | 644 | ||
|
Accrued interest
|
70 | |||
|
Amortization of discount for the three month period
|
34 | |||
|
ended March 31, 2010
|
||||
|
Balance of Convertible Notes Payable at March
|
||||
|
31, 2010
|
$ | 748 | ||
Note 5 - Stockholders' Equity
During March 2010, the Company received an aggregate investment of NIS 12,264,000 (approximately $3,287 thousand) from a number of investors. In return the Company allocated an aggregate amount of 4,157,500 shares, at a price per share of NIS 2.95 (approximately $0.78) and 4,157,500 non-registered warrants (the “Warrants”) to purchase 4,157,500 shares of common stock. The Warrants are exercisable at a price per share of NIS 4.25 (approximately $1.12) for four years. The net gross proceeds, after deducting the fundraising expenses of $215 thousand, was recorded in the additional paid in capital in exchange for the shares issued to the investors.
The following table summarizes information about the changes regarding the common stock outstanding and additional paid-in-capital for the period ending March 31, 2010:
|
Number of
|
Common stock |
Additional
|
||||||||||
|
shares
|
Amount
|
Paid-in capital
|
||||||||||
|
U.S. dollars in thousands (except share data)
|
||||||||||||
|
Balance as of December 31, 2009
|
16,356,550 | $ | 164 | $ | 16,692 | |||||||
|
Issuance of common stock to private
|
||||||||||||
|
investors (net of approximately $215,000 of issuance costs)
|
4,157,500 | 42 | 3,030 | |||||||||
|
Stock- based compensation
|
- | - | 124 | |||||||||
|
Exercise of stock options and warrants
|
70,000 | * | - | |||||||||
|
Balance as at March 31, 2010
|
20,584,050 | $ | 206 | $ | 19,846 | |||||||
* less than $1 thousand
F-15
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Notes to the Consolidated Financial Statements as of March 31, 2010 (Unaudited)
Note 6 - Financial Instruments and Risk Management
|
A.
|
Concentration of credit risk
|
Financial instruments that may subject the Company to significant concentrations of credit risk consist mainly of cash and cash equivalents and deposits in respect of employee severance benefits.
Cash and cash equivalents are maintained with major financial institutions in Israel. Deposits in respect of employee severance benefits are maintained with major insurance companies and financial institutions in Israel.
|
B.
|
Concentration of business risk
|
The Company uses materials required for its research and development activities that are currently available from a limited number of sources. The Company believes that it will not experience delays in the supply of critical components in the future. If the Company experiences such delays and there is an insufficient inventory of critical components at that time, the Company's operations and financial results would be adversely affected.
Note 7– Significant Events During the Period
|
|
A.
|
At a General Meeting of the shareholders held on January 21, 2010, the shareholders authorized the increase of the authorized capital of the Company to 65,000,000 common shares at a par value of $0.01, subject to a future resolution of the Board.
|
|
|
B.
|
In the interests of expediting patient recruitment and to reduce expenses relating to the clinical development of the Company’s developing drug therapy in patients with ovarian cancer, in March 2010 the Company ceased recruiting patients at two university hospitals in the U.S. in order to commence patient recruitment in the near future at alternate sites in Israel.
|
In addition, the company has requested from the FDA to change the enrollment to inclusion criteria to include ovarian cancer patients who do not have ascites.
|
C.
|
On March 7, 2010, following the recommendations of the Compensation and Audit Committees, the Board of Directors appointed current director Mr. Doron Nevo as Chairman of the Board, and approved a compensation package including an allocation of 168,750 options at an exercise price of NIS 3.51 (the 22-day average closing price of the Company's shares on the TASE) (approximately $0.94) which shall vest over twelve calendar quarters, with an acceleration such that if he is removed from the position of Chairman (except for cause) during the first year, a total of 56,250 options will be vested, and if he is removed (except for cause) thereafter, whatever is already vested shall remain so, and an additional 14,063 options shall vest.
Additionally, the Board of Directors approved an allocation of 80,000 options at an exercise price of NIS 3.17 (approximately $0.84) to three directors.
|
Note 8 – Subsequent events
In April 2010, the Company announced that it had received the interim results of its Phase IIb clinical trial of the drug candidate BC-819 for use in superficial bladder cancer in patients who had not responded to conventional treatment (BCG or chemotherapy), and that the Food and Drug Administration (FDA) had approved the continuation of the trial, and the commencement of enrollment of the second and last treatment group (comprised of 15 patients).
F-16
BIOCANCELL THERAPEUTICS INC. AND SUBSIDIARY
(Development Stage Company)
CONSOLIDATED FINANCIAL STATEMENTS
As of and for the Years Ended
December 31, 2009 and 2008
and for the Development Stage Period
F-17
BIOCANCELL THERAPEUTICS INC. AND SUBSIDIARY
(Development Stage Company)
CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2008
|
Table of Contents
|
Page
|
|||
|
Report of Independent Registered Public Accounting Firm
|
F-19
|
|||
|
Consolidated Balance Sheets
|
F-20
|
|||
|
Consolidated Statements of Operations
|
F-22
|
|||
|
Statement of Changes in Stockholders’ Equity and Comprehensive Loss
|
F-23
|
|||
|
Consolidated Statements of Cash Flows
|
F-26
|
|||
|
Notes to the Consolidated Financial Statements
|
F-29
|
|||
F-18
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
BioCancell Therapeutics Inc. (Development Stage Company):
We have audited the accompanying consolidated balance sheets of BioCancell Therapeutics, Inc. and subsidiary (collectively, "the Company") (Development Stage Company) as of December 31, 2009, and 2008, and the related consolidated statements of operations, changes in stockholders’ equity (deficit) and comprehensive loss, and cash flows for each of the years in the two-year period ended December 31, 2009, and for the cumulative period from October 1, 2004 (inception of operations) through December 31, 2009. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of BioCancell Therapeutics, Inc. and subsidiary (Development Stage Company) as of December 31, 2009 and 2008, and the results of their operations, and their cash flows for each of the years in the two-year period ended December 31, 2009, and for the cumulative period from October 1, 2004 (inception of operations) through December 31, 2009, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1B to the consolidated financial statements, the Company has suffered recurring losses from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1B. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in Note 2 to the consolidated financials statements, the Company has applied the new accounting and disclosure requirements issued by the FASB as of January 1, 2008, with respect to its assets and liabilities measured at fair value on a recurring basis. With respect to nonfinancial assets and liabilities measured at fair value on a non-recurring basis, the Company has adopted the new requirements as of January 1, 2009.
Somekh Chaikin
Certified Public Accountants (Israel)
A Member Firm of KPMG International
Jerusalem, Israel
March 17, 2010
F-19
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Balance Sheets
|
December 31,
|
December 31,
|
|||||||||||
|
2009
|
2008
|
|||||||||||
|
Note
|
U.S. dollars in thousands
|
|||||||||||
|
Current assets
|
||||||||||||
|
Cash and cash equivalents
|
3 | $ | 624 | $ | 2,779 | |||||||
|
Receivable from Chief Scientist and BIRD Foundation
|
8A,B | 45 | 72 | |||||||||
|
Deferred stock issuance costs
|
1 | E | 178 | - | ||||||||
|
Prepaid expenses 1
|
228 | 109 | ||||||||||
|
Other current assets
|
88 | 34 | ||||||||||
|
Total current assets
|
1,163 | 2,994 | ||||||||||
|
Long-term assets
|
||||||||||||
|
Deposits in respect of employee severance benefits
|
6 | 237 | 141 | |||||||||
|
Long-term prepaid expenses
|
8 | H | 59 | 44 | ||||||||
|
Total long-term assets
|
296 | 185 | ||||||||||
|
Property and equipment, net
|
4 | 106 | 131 | |||||||||
|
Total assets
|
$ | 1,565 | $ | 3,310 | ||||||||
1 The amounts recorded as of December 31, 2009 include $28 thousand to a related party.
The accompanying notes form an integral part of the financial statements.
F-20
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Balance Sheets
|
December 31,
|
December 31,
|
|||||||||||
|
2009
|
2008
|
|||||||||||
|
Note
|
U.S. dollars in thousands
|
|||||||||||
|
Current liabilities
|
||||||||||||
|
Accounts payable 1
|
5 | $ | 239 | $ | 493 | |||||||
|
Accrued expenses and others 2
|
298 | 239 | ||||||||||
|
Accrued vacation pay
|
80 | 63 | ||||||||||
|
Employees and related liabilities
|
66 | 39 | ||||||||||
|
Total current liabilities
|
683 | 834 | ||||||||||
|
Long-term liabilities
|
||||||||||||
|
Liability for employee severance benefits
|
6 | 204 | 144 | |||||||||
|
Convertible notes payable
|
7,9 | C | 644 | 303 | ||||||||
|
Warrants to noteholders
|
7,9 | C | 3,400 | 79 | ||||||||
|
Total long-term liabilities
|
4,248 | 526 | ||||||||||
|
Contingent liabilities and commitments
|
8 | |||||||||||
|
Stockholders' equity (deficit)
|
9 | |||||||||||
|
Common stock, $0.01 par value per share (50,000,000
|
||||||||||||
|
shares authorized, 16,356,550 and
|
||||||||||||
|
16,292,611 shares issued and outstanding as of
|
||||||||||||
|
December 31, 2009, and 2008, respectively)
|
164 | 163 | ||||||||||
|
Additional paid-in capital
|
16,692 | 16,425 | ||||||||||
|
Accumulated other comprehensive income
|
335 | 684 | ||||||||||
|
Treasury stock
|
- | (4,951 | ) | |||||||||
|
Accumulated deficit
|
(20,557 | ) | (10,371 | ) | ||||||||
|
Total stockholders' equity (deficit)
|
(3,366 | ) | 1,950 | |||||||||
|
Total liabilities and stockholders' equity (deficit)
|
$ | 1,565 | $ | 3,310 | ||||||||
|
1
|
The amounts recorded as of December 31, 2009 and December 31, 2008 include $0 thousand and $23 thousand to a related party, respectively.
|
|
2
|
The amounts recorded as of December 31, 2009 and December 31, 2008 include $8 thousand and $8 thousand to a related party, respectively.
|
The accompanying notes form an integral part of the financial statements.
F-21
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Operations
|
Cumulative
|
||||||||||||||||
|
from October 1,
|
||||||||||||||||
|
2004 (inception)
|
||||||||||||||||
|
For the year ended December 31,
|
to December 31,
|
|||||||||||||||
|
2009
|
2008
|
2009
|
||||||||||||||
|
U.S. dollars in thousands
|
||||||||||||||||
|
Note
|
(except share and per share data)
|
|||||||||||||||
|
Research and development expenses
|
$ | 2,617 | $ | 3,155 | $ | 11,269 | ||||||||||
|
Less: Chief Scientist, BIRD Foundation and
|
||||||||||||||||
|
other grants
|
8A,B | (604 | ) | (461 | ) | (2,017 | ) | |||||||||
|
Research and development expenses, net
|
2,013 | 2,694 | 9,252 | |||||||||||||
|
General and administrative expenses1
|
1,457 | 1,913 | 6,293 | |||||||||||||
|
Operating loss
|
3,470 | 4,607 | 15,545 | |||||||||||||
|
Interest income, net
|
(3 | ) | (61 | ) | (13 | ) | ||||||||||
|
Loss (gain) from marketable securities, net
|
- | 89 | (6 | ) | ||||||||||||
|
Interest on convertible notes and discount
|
||||||||||||||||
|
amortization
|
7,9 | C | 341 | 147 | 489 | |||||||||||
|
Revaluation of warrants
|
7,9 | C | 3,053 | (1,653 | ) | 1,400 | ||||||||||
|
Other financing income, net
|
(58 | ) | (187 | ) | (285 | ) | ||||||||||
|
Net loss
|
6,803 | 2,942 | 17,130 | |||||||||||||
|
Basic and diluted net loss per share
|
$ | 0.44 | $ | 0.22 | $ | 1.69 | ||||||||||
|
Weighted-average common shares used
|
||||||||||||||||
|
in computing basic and diluted net loss
|
||||||||||||||||
|
per share
|
15,453,126 | 13,413,452 | 10,152,880 | |||||||||||||
|
1
|
The amounts recorded for the year ending December 31, 2009 and December 31, 2008 and for the cumulative period include $3 thousand, $71 thousand and $74 thousand to related parties.
|
The accompanying notes form an integral part of the financial statements.
F-22
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Changes in Stockholders' Equity (Deficit) and Comprehensive Loss
Amounts accumulated during development stage (October 1, 2004 to December 31, 2009):
|
|
Series A convertible
|
|||||||||||||
|
preferred stock
|
Common stock
|
|||||||||||||
|
Accumulated
|
||||||||||||||
|
Additional
|
other
|
Total
|
||||||||||||
|
Number of
|
Number of
|
paid-in
|
Treasury
|
comprehensive
|
Accumulated
|
Comprehensive
|
stockholders'
|
|||||||
|
shares
|
Amount
|
shares
|
Amount
|
capital
|
stock
|
income
|
deficit
|
loss
|
equity
|
|||||
|
U.S. dollars in thousands (except share data)
|
||||||||||||||
|
Issuance of common stock to founders
|
-
|
-
|
4,282,500
|
43
|
-
|
-
|
-
|
(43)
|
-
|
-
|
||||
|
Stock-based compensation to employees
|
-
|
-
|
-
|
-
|
44
|
-
|
-
|
-
|
-
|
44
|
||||
|
Stock-based compensation to non-employees
|
-
|
-
|
-
|
-
|
41
|
-
|
-
|
-
|
-
|
41
|
||||
|
Comprehensive loss:
|
||||||||||||||
|
Foreign currency translation reserve
|
-
|
-
|
-
|
-
|
-
|
-
|
(5)
|
-
|
(5)
|
(5)
|
||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(300)
|
(300)
|
(300)
|
||||
|
Comprehensive loss
|
(305)
|
|||||||||||||
|
Balance as of December 31, 2004
|
-
|
-
|
4,282,500
|
43
|
85
|
-
|
(5)
|
(343)
|
(220)
|
|||||
|
Issuance of Series A convertible preferred stock (at $0.83 per
|
||||||||||||||
|
share)
|
2,854,532
|
28
|
-
|
-
|
1,795
|
-
|
-
|
-
|
-
|
1,823
|
||||
|
Conversion of stockholder loans
|
-
|
-
|
360,955
|
4
|
296
|
-
|
-
|
-
|
-
|
300
|
||||
|
Stock-based compensation to employees
|
-
|
-
|
-
|
-
|
193
|
-
|
-
|
-
|
-
|
193
|
||||
|
Stock-based compensation to non-employees
|
-
|
-
|
-
|
-
|
148
|
-
|
-
|
-
|
-
|
148
|
||||
|
Comprehensive loss:
|
||||||||||||||
|
Foreign currency translation reserve
|
-
|
-
|
-
|
-
|
-
|
-
|
21
|
-
|
21
|
21
|
||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(834)
|
(834)
|
(834)
|
||||
|
Comprehensive loss
|
(1,118)
|
|||||||||||||
|
Balance as of December 31, 2005
|
2,854,532
|
28
|
4,643,455
|
47
|
2,517
|
-
|
16
|
(1,177)
|
1,431
|
|||||
The accompanying notes form an integral part of the financial statements.
F-23
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Changes in Stockholders' Equity (Deficit) and Comprehensive Loss (cont'd)
Amounts accumulated during development stage (October 1, 2004 to December 31, 2009) (cont’d):
|
Series A convertible
|
||||||||||||
|
preferred stock
|
Common stock
|
|||||||||||
|
Accumulated
|
||||||||||||
|
Additional
|
other
|
Total
|
||||||||||
|
Number of
|
Number of
|
paid-in
|
Treasury
|
comprehensive
|
Accumulated
|
Comprehensive
|
stockholders'
|
|||||
|
shares
|
Amount
|
shares
|
Amount
|
capital
|
stock
|
income
|
deficit
|
loss
|
equity
|
|||
|
U.S. dollars in thousands (except share data)
|
||||||||||||
|
Issuance of Series A convertible preferred stock (at $0.83 per
|
||||||||||||
|
share)
|
397,057
|
4
|
-
|
-
|
291
|
-
|
- | - | - |
295
|
||
|
Payments of debtors for shares
|
-
|
-
|
-
|
-
|
473
|
-
|
-
|
-
|
-
|
473
|
||
|
Conversion of stockholder loans
|
72,192
|
1
|
-
|
-
|
59
|
- | - | - | - |
60
|
||
|
Conversion of series A convertible preferred stock to common
|
||||||||||||
|
stock
|
(3,323,781)
|
(33)
|
3,323,781
|
33
|
-
|
-
|
-
|
-
|
-
|
-
|
||
|
Issuance of common stock to public (net of approximately
|
||||||||||||
|
$1,035,000 of issuance costs) (at $1.23 per share)
|
-
|
-
|
3,200,000
|
32
|
2,786
|
-
|
-
|
-
|
-
|
2,818
|
||
|
Issuance of series 1 option warrants
|
-
|
-
|
-
|
-
|
772
|
-
|
-
|
-
|
-
|
772
|
||
|
Issuance of series 2 option warrants
|
-
|
-
|
-
|
-
|
1,028
|
-
|
-
|
-
|
-
|
1,028
|
||
|
Stock-based compensation to employees
|
-
|
-
|
-
|
-
|
698
|
-
|
-
|
-
|
-
|
698
|
||
|
Stock-based compensation to non-employees
|
-
|
-
|
-
|
-
|
473
|
-
|
-
|
-
|
-
|
473
|
||
|
Exercise of stock options and warrants
|
-
|
-
|
50,000
|
*
|
-
|
-
|
-
|
-
|
-
|
-
|
||
|
Comprehensive loss:
|
||||||||||||
|
Foreign currency translation reserve
|
-
|
-
|
-
|
-
|
-
|
-
|
287
|
-
|
287
|
287
|
||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(2,316)
|
(2,316)
|
(2,316)
|
||
|
Comprehensive loss
|
(3,147)
|
|||||||||||
|
Balance as of December 31, 2006
|
-
|
-
|
11,217,236
|
112
|
9,097
|
-
|
303
|
(3,493)
|
- |
6,019
|
||
|
Stock-based compensation to employees
|
-
|
-
|
-
|
-
|
44
|
-
|
-
|
-
|
-
|
44
|
||
|
Stock-based compensation to non-employees
|
-
|
-
|
-
|
-
|
78
|
-
|
-
|
-
|
-
|
78
|
||
|
Exercise of stock options and warrants
|
-
|
-
|
1,180,790
|
12
|
2
|
-
|
-
|
-
|
-
|
14
|
||
|
Comprehensive loss:
|
||||||||||||
|
Foreign currency translation reserve
|
-
|
-
|
-
|
-
|
-
|
-
|
334
|
-
|
334
|
334
|
||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(3,936)
|
(3,936)
|
(3,936)
|
||
|
Comprehensive loss
|
(6,749)
|
|||||||||||
|
Balance as of December 31, 2007
|
-
|
-
|
12,398,026
|
124
|
9,221
|
-
|
637
|
(7,429)
|
2,553
|
|||
* less than $1 thousand
The accompanying notes form an integral part of the financial statements.
F-24
|
|
BioCancell Therapeutics Inc. and Subsidiary
|
(Development Stage Company)
Consolidated Statements of Changes in Stockholders' Equity (Deficit) and Comprehensive Loss (cont'd)
Amounts accumulated during development stage (October 1, 2004 to December 31, 2009) (cont’d):
|
Series A convertible
|
|||||||||||||||||
|
preferred stock
|
Common stock
|
||||||||||||||||
|
Accumulated
|
|||||||||||||||||
|
Additional
|
other
|
Total
|
|||||||||||||||
|
Number of
|
Number of
|
paid-in
|
Treasury
|
comprehensive
|
Accumulated
|
Comprehensive
|
stockholders'
|
||||||||||
|
shares
|
Amount
|
shares
|
Amount
|
capital
|
stock
|
income
|
deficit
|
loss
|
equity
|
||||||||
|
U.S. dollars in thousands (except share data)
|
|||||||||||||||||
|
Issuance of common stock to private investors (net of
|
|||||||||||||||||
|
approximately $57,000 of issuance costs)
|
-
|
-
|
1,872,780
|
19
|
2,237
|
-
|
-
|
-
|
-
|
2,256
|
|||||||
|
Repurchase and exercise of series 1 option warrants
|
-
|
-
|
1,812,756
|
18
|
4,923
|
(4,951)
|
-
|
-
|
-
|
(10)
|
|||||||
|
Stock-based compensation to employees
|
-
|
-
|
-
|
-
|
29
|
-
|
-
|
-
|
-
|
29
|
|||||||
|
Stock-based compensation to non-employees
|
-
|
-
|
-
|
-
|
11
|
-
|
-
|
-
|
-
|
11
|
|||||||
|
Exercise of stock options and warrants
|
-
|
-
|
209,049
|
2
|
4
|
-
|
-
|
-
|
-
|
6
|
|||||||
|
Comprehensive loss:
|
|||||||||||||||||
|
Foreign currency translation reserve
|
-
|
-
|
-
|
-
|
-
|
-
|
47
|
-
|
47
|
47
|
|||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(2,942)
|
(2,942)
|
(2,942)
|
|||||||
|
Comprehensive loss
|
(9,644)
|
||||||||||||||||
|
Balance as at December 31, 2008
|
-
|
-
|
16,292,611
|
163
|
16,425
|
(4,951)
|
684
|
(10,371)
|
- |
1,950
|
|||||||
|
Issuance of common stock to consultant
|
-
|
-
|
63,939
|
1
|
55
|
-
|
-
|
-
|
-
|
56
|
|||||||
|
Stock-based compensation to employees
|
-
|
-
|
-
|
-
|
139
|
-
|
-
|
-
|
-
|
139
|
|||||||
|
Stock-based compensation to non-employees
|
-
|
-
|
-
|
-
|
73
|
-
|
-
|
-
|
-
|
73
|
|||||||
|
Sale of treasury stock
|
-
|
-
|
-
|
-
|
-
|
4,951
|
(3,383)
|
-
|
1,568
|
||||||||
|
Comprehensive loss:
|
|||||||||||||||||
|
Foreign currency translation reserve
|
-
|
-
|
(349)
|
-
|
(349)
|
(349)
|
|||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(6,803)
|
(6,803)
|
(6,803)
|
|||||||
|
Comprehensive loss
|
(16,796)
|
||||||||||||||||
|
Balance as at December 31, 2009
|
-
|
-
|
16,356,550
|
164
|
16,692
|
-
|
335
|
(20,557)
|
(3,366)
|
||||||||
The accompanying notes form an integral part of the financial statements.
F-25
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Cash Flows
|
Cumulative
|
||||||||||||
|
For the year
|
For the year
|
from October 1,
|
||||||||||
|
ended
|
ended
|
2004 (inception)
|
||||||||||
|
December 31,
|
December 31,
|
to December 31,
|
||||||||||
|
2009
|
2008
|
2009
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$ | (6,803 | ) | $ | (2,942 | ) | $ | (17,130 | ) | |||
|
Adjustments to reconcile net loss to net cash flows from
|
||||||||||||
|
operating activities:
|
||||||||||||
|
Income and expenses not involving cash flows:
|
||||||||||||
|
Increase in liability for employee severance benefits, net
|
57 | 63 | 194 | |||||||||
|
Fair value adjustment of marketable securities
|
- | 89 | 133 | |||||||||
|
Depreciation and amortization
|
44 | 47 | 125 | |||||||||
|
Stock-based payment compensation
|
240 | 40 | 1,641 | |||||||||
|
Revaluation of warrants
|
3,053 | (1,653 | ) | 1,400 | ||||||||
|
Accrued interest and amortization of discount to notes
|
||||||||||||
|
payable, and exchange difference thereon
|
328 | 147 | 475 | |||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Increase in other current assets
|
(52 | ) | (33 | ) | (90 | ) | ||||||
|
Increase in deferred stock issuance costs
|
(177 | ) | - | (177 | ) | |||||||
|
Increase in prepaid expenses
|
(114 | ) | - | (207 | ) | |||||||
|
Decrease in Chief Scientist and BIRD foundation
|
||||||||||||
|
receivable
|
26 | 321 | 21 | |||||||||
|
Investment in marketable securities (trading)
|
- | - | (7,883 | ) | ||||||||
|
Proceeds from marketable securities (trading)
|
- | - | 5,970 | |||||||||
|
Increase in severance pay deposits
|
(91 | ) | (26 | ) | (219 | ) | ||||||
|
Increase in long-term receivables
|
(14 | ) | (2 | ) | (53 | ) | ||||||
|
Increase (decrease) in accounts payable
|
(248 | ) | 247 | 227 | ||||||||
|
Increase in employees and related liabilities
|
26 | 26 | 86 | |||||||||
|
Increase (decrease) in accrued vacation pay
|
16 | (18 | ) | 47 | ||||||||
|
Increase (decrease) in accrued expenses
|
55 | (63 | ) | 253 | ||||||||
|
Net cash used in operating activities
|
(3,654 | ) | (3,757 | ) | (15,187 | ) | ||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Investment in marketable securities (trading)
|
- | (921 | ) | (921 | ) | |||||||
|
Proceeds from marketable securities (trading)
|
- | 3,173 | 3,173 | |||||||||
|
Acquisition of property and equipment
|
(19 | ) | (21 | ) | (212 | ) | ||||||
|
Net cash provided by (used in) investing activities
|
$ | (19 | ) | $ | 2,231 | $ | 2,040 | |||||
The accompanying notes form an integral part of the financial statements.
F-26
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Cash Flows (Cont’d)
|
For the year ended December 31, 2009
|
For the year ended December 31, 2008
|
Cumulative from October 1, 2004 (inception) to December 31, 2009
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Issuance of common stock and exercise of options
|
$ | - | $ | 7,203 | $ | 10,393 | ||||||
|
Issuance of Series A convertible preferred stock
|
- | - | 2,118 | |||||||||
|
Payments of debtors for shares
|
- | - | 473 | |||||||||
|
Issuance of Series A options warrant
|
- | - | 772 | |||||||||
|
Issuance of Series B options warrant
|
- | - | 1,028 | |||||||||
|
Receipt of grant from Chief Scientist
|
- | - | 2 | |||||||||
|
Repayment of stockholder loans
|
- | - | 360 | |||||||||
|
Treasury stock
|
- | (4,951 | ) | (4,951 | ) | |||||||
|
Sale of Treasury stock
|
1,568 | - | 1,568 | |||||||||
|
Convertible notes payable
|
- | 176 | 176 | |||||||||
|
Warrants to noteholders (long-term derivatives)
|
- | 1,829 | 1,829 | |||||||||
|
Net cash provided by financing activities
|
1,568 | 4,257 | 13,768 | |||||||||
|
Effect of exchange rate on cash
|
(50 | ) | (328 | ) | 3 | |||||||
|
Increase (decrease) in cash and cash equivalents
|
(2,155 | ) | 2,403 | 624 | ||||||||
|
Cash and cash equivalents at beginning of period
|
2,779 | 376 | - | |||||||||
|
Cash and cash equivalents at end of period
|
$ | 624 | $ | 2,779 | $ | 624 | ||||||
The accompanying notes form an integral part of the financial statements.
F-27
BioCancell Therapeutics Inc. and Subsidiary
(Development Stage Company)
Consolidated Statements of Cash Flows (cont’d)
|
For the year ended December 31, 2009
|
For the year ended December 31, 2008
|
Cumulative from October 1, 2004 (inception) to December 31, 2009
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Conversion of stockholder loans
|
$ | - | $ | - | $ | 360 | ||||||
|
Issuance of common stock to founders
|
- | - | 43 | |||||||||
|
Issuance of option warrants to underwriters
|
- | - | 358 | |||||||||
|
Exercise of stock options by Company consultants
|
- | - | 1 | |||||||||
|
Conversion of series A convertible preferred stock to common stock
|
$ | - | $ | - | $ | 33 | ||||||
The accompanying notes form an integral part of the financial statements.
F-28
Note 1 - Organization and Description of Business
A. BioCancell Therapeutics, Inc. (hereafter "the Parent") was incorporated in the United States as a private company under the laws of the State of Delaware on July 26, 2004 and commenced operations October 1, 2004.
B. The principal activities of the Parent and its subsidiary in Israel, BioCancell Therapeutics Israel Ltd. (the "Subsidiary"), (hereafter collectively referred to as “the Company”) are research and development of drugs for the treatment of superficial bladder cancer, pancreatic cancer and ovarian cancer. The leading drug developed by the Company has been successfully tested for a number of cancer types in pre-clinical lab animal trials, compassionate use human trials and a Phase I/IIa clinical trial. The Company is now performing an advanced Phase IIb clinical trial on bladder cancer patients, a Phase I/IIa clinical trial on ovarian cancer patients, and a Phase I/IIa clinical trial for treatment of pancreatic cancer. The Company is evaluating indications for the possible use of this drug, and others under development, to treat other types of cancer.
The Company is in the development stage. Therefore, there is no certainty regarding the Company’s ability to complete the product’s development, receipt of regulatory permits, alternative treatments or procedures that may be developed, and success of its marketing. The continuation of the stages of development and the realization of assets related to the planned activities depend on future events, including the receipt of interim financing and achieving operational profitability in the future. The Company has generated only limited revenues since its inception and has incurred substantial losses and expects that it will operate at a loss over the coming years, as it does not expect to generate any revenue from operations in the near term. The Company is initiating activities to raise capital for ensuring future operations although there are still significant doubts as to the ability of the Company to continue operating as a “going concern”. The Company believes that it has sufficient cash to meet its planned operating needs until the end of September 2010, based on its current cash level. It is not possible to estimate the final outcome of these activities. These financial statements do not include any adjustments to the value of assets and liabilities and their classification, which may be required if the Company cannot continue operating as a “going concern”.
The biotechnology industry is characterized by strong competition, resulting from the risk of frequent technological changes. Entry into this market requires the investment of considerable resources and continuous development. The Company's future success is dependent on several factors, including the quality of the Company's technology, the product's price, and the creation of an advantage over the competition.
C. The Company's research and development activities are carried out by its Subsidiary primarily through a laboratory research team in the Hebrew University of Jerusalem. The Hebrew University laboratory is managed by the Chief Scientist of the Company, who is a related party. All of the Company’s net assets are situated in Israel.
D. The Company filed a prospectus for an initial public offering on the Tel Aviv Stock Exchange (“TASE”) and beginning August 17, 2006 has been publicly traded on the TASE. On June 23, 2009 the Company’s Registration Statement on Form S-1 was deemed effective by the United States Securities and Exchange Commission (SEC) and as of that date it is a reporting company to the SEC.
E. In October 2009, the Company published a shelf prospectus with the TASE (which is valid for two years) and a Form S-1/A with the U.S. Securities and Exchange Commission in anticipation of its upcoming fundraising. As of December 31, 2009, the Company has not received final comments from the SEC regarding its filing and has recorded $178 thousand as deferred stock issuance costs in anticipation of this planned fundraising.
Note 2 - Reporting Principles and Accounting Policies
|
|
A.
|
Basis of presentation
|
The accompanying consolidated financial statements include the accounts of BioCancell Therapeutics, Inc. and its subsidiary and are presented in accordance with accounting principles generally accepted in the United States of America. All significant intercompany balances and transactions have been eliminated in consolidation. All references herein to accounting pronouncements have been changed to conform with ASC section 105-10 (FASB Codification).
|
|
The Company also files Hebrew language New Israel Shekel based financial statements, prepared in accordance with International Financial Reporting Standards (IFRS), with the TASE.
|
|
|
B.
|
Development stage enterprise
|
The Company’s principal activities to date have been the research and development of its products and the Company has not generated revenues from its planned, principal operation. Accordingly, the Company’s financial statements are presented as those of a development stage enterprise.
F-29
Note 2 - Reporting Principles and Accounting Policies (cont'd)
|
|
C.
|
Translation into U.S. Dollars
|
|
|
The financial records of the Parent and its Subsidiary are maintained in New Israel Shekels, which is the functional currency of the Company. These financial statements have been prepared using the U.S. dollar as the reporting currency.
|
All assets and liabilities are translated at year-end exchange rates. Items in the statement of stockholders’ equity (deficit) are at year-end exchange rates. Statement of operations items are translated at the annual average exchange rate. The adjustment resulting from translating the financial statements is included in accumulated other comprehensive income, which is reflected as a separate component of stockholders’ equity (deficit).
|
|
The following table summarizes the representative rate of exchange of the U.S. dollar to the New Israel Shekel (NIS):
|
|
Average rate of
|
||||
|
Rate at
|
exchange
|
|||
|
December 31
|
during the year
|
|||
|
NIS
|
NIS
|
|
2009
|
3.775
|
3.932
|
|
|
2008
|
3.802
|
3.586
|
|
|
The average rate of exchange during the development stage period (from inception through December 31, 2009) was NIS 4.13 to $1.
|
The translated figures should not be construed to represent actual amounts in, or convertible into, U.S. dollars.
|
|
D.
|
Transactions in foreign currency
|
Transactions in foreign currency are recorded at the exchange rate as of the transaction date. Exchange gains or losses from the disposition of monetary assets or liabilities or from the reporting of monetary assets or liabilities according to exchange rates other than those originally recorded, or from exchange rates recorded in prior financial statements, are recognized in the statement of operations.
|
|
E.
|
Use of estimates
|
The preparation of consolidated financial statements in conformity with generally accepted accounting principles in the United States requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported results of operations during the reporting periods. Actual results may differ from such estimates.
Significant items subject to such estimates and assumptions include the valuation of derivatives, deferred tax assets and share-based compensation. The current economic environment has increased the degree of uncertainty inherent in those estimates and assumptions.
|
|
F.
|
Cash and cash equivalents
|
The Company considers all highly liquid investments, which include short-term bank deposits with an original maturity of up to three months, to be cash equivalents.
|
|
G.
|
Marketable securities
|
All of the Company's investment securities, primarily consisting of investments in government and corporate bond, mutual funds and convertible bonds, have been classified as trading securities, and are reported at fair market value, with unrealized gains and losses, net of tax, recorded in the statement of operations. As of December 31, 2009, all funds of the Company are held in cash and cash equivalents.
The cash flows from investments in marketable securities for the year ended December 31, 2008 are classified according to their nature and purpose, as provided for by ASC subtopic 825-10 (previously referred to as SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities).
|
|
H.
|
Derivative instruments
|
|
|
The Company recognizes all derivative instruments as either assets or liabilities in the balance sheet at their respective fair values. The Company carries its derivative instruments at fair value on the balance sheet and recognizes any subsequent changes to fair value in the statement of operations. Beginning January 1, 2009, the Company adopted ASC subtopic 815-10 (previously referred to as SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities (SFAS 161)).
|
The Company's only derivative instruments were issued in the form of warrants to purchase an aggregate of up to 6,280,783 shares of common stock (at a price of 71.6 cents per share) as part of the financing described in Note 9C below. The warrants have been recorded as a liability, at fair value, and is revalued at each reporting date and changes in the fair value of the instruments are included in the statement of operations.
F-30
Note 2 - Reporting Principles and Accounting Policies (cont'd)
|
|
I.
|
Property and equipment, net
|
Property and equipment are stated at cost, net of accumulated depreciation.
Improvements and enhancements are added to the cost of the assets whereas maintenance and repairs are charged to expense as incurred.
Depreciation is calculated by the straight-line method on the basis of the estimated useful lives of the assets. Annual depreciation rates are as follows:
|
%
|
|
Electronic equipment
|
15 | |||
|
Computers
|
33 | |||
|
Furniture
|
6 |
|
|
J.
|
Research and development expenses, net
|
Research and development costs are expensed as incurred. Research and development costs comprise costs incurred in performing research and development activities, including salaries and related costs, consultants and sub-contractors costs, clinical trials costs, patent fees, materials and depreciation costs.
The grant revenue consists of grants received from the OCS in Israel, the Israel-U.S. Binational Industrial Research and Development Foundation (BIRD) and the Jerusalem Development Authority in support of the Company’s research and development activities. These grants are recognized as a reduction of expense as the related costs are incurred (see Note 8A,B and J).
K. Stock-based compensation
The Company records stock-based compensation as an expense in the statement of operations. The cost of stock-based compensation awards is measured at their grant date fair value. Fair value is determined using the Black-Scholes-Merton option pricing model. Expense is recognized over the vesting period, generally 10 years. The Company issues new shares upon share option exercises.
L. Segment and geographic information
|
|
The Company's business is currently comprised of one geographic and operating segment, focused on the discovery, development and commercialization of novel therapies for treating cancer-related diseases. The Company follows ASC subtopic 280-10 (previously referred to as SFAS No. 131, Disclosures about Segments of an Enterprise and Related Information). The Company's development and administration operations are maintained in Israel. The Company’s chief operating decision maker uses measurements aggregated at the entity-wide level to manage the business. All of the Company’s net assets are situated in Israel.
|
|
|
M.
|
Income taxes
|
The Company accounts for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided for the amount of deferred tax assets that, based on available evidence, are not more likely than not to be realized.
The Company recognizes the effect of an income tax position only if that position is more likely than not of being sustained upon examination, based on the technical merits of the position. A recognized income tax position is then measured at the largest amount that is greater than 50% likely of being realized. Interest and penalties related to unrecognized tax benefits are recognized as a component of income tax expense.
|
|
N.
|
Net loss per share
|
Basic net loss per share is computed by dividing the net loss for the period by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing the net loss for the period by the weighted-average number of common shares plus dilutive potential common stock considered outstanding during the period. However, as the Company generated net losses in all periods presented, potentially dilutive securities, comprised of incremental common shares issuable upon the conversion of Series A convertible preferred stock and the exercise of warrants and stock options, are not reflected in diluted net loss per share because such shares are antidilutive.
In the calculation of the net loss per share, the share splits of November 2005 (1:30) and of July 2006 (1:2.855) were accounted for retroactively as if they occurred at the beginning of the first period presented.
F-31
Note 2 - Reporting Principles and Accounting Policies (cont'd)
|
|
N.
|
Net loss per share (cont'd)
|
The following table summarizes the securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic EPS in the future and were not included in the computation of basic and diluted EPS because doing so would have been antidilutive for the periods presented:
|
Cumulative
|
||||
|
from October 1,
|
||||
|
2004 (inception)
|
||||
|
For the year ended December 31
|
to December 31,
|
|||
|
2009
|
2008
|
2009
|
||
|
Series 2 Warrants
|
2,500,000
|
2,500,000
|
2,500,000
|
|
|
Warrants to underwriter
|
300,000
|
300,000
|
300,000
|
|
|
Convertible notes payable
|
4,078,212
|
4,078,212
|
4,078,212
|
|
|
Warrants for interest on convertible
|
||||
|
notes payable
|
597,095
|
172,067
|
597,095
|
|
|
Warrants to investors
|
5,898,088
|
5,473,060
|
5,898,088
|
|
|
Stock options to employees,
|
||||
|
Directors and consultants under
|
||||
|
Stock Option Plans
|
2,521,989
|
1,667,989
|
2,521,989
|
|
|
15,895,384
|
14,191,328
|
15,895,384
|
||
|
|
O.
|
Impairment of long-lived assets
|
The Company evaluates whenever events or changes in circumstances indicate carrying amount may not be recoverable. The Company evaluates the realizability of its long-lived assets based on cash flow expectations for the related assets. If circumstances require a long-lived asset or asset group be tested for possible impairment, the Company first compares undiscounted cash flows expected to be generated by that asset or asset group to its carrying value. If the carrying value of the long-lived asset or asset group is not recoverable on an undiscounted cash flow basis, an impairment is recognized to the extent that the carrying value exceed its fair value. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. Any write-downs are treated as permanent reductions in the carrying amount of the assets.
|
|
P.
|
Fair value measurements
|
On January 1, 2008, the Company adopted the provisions of FASB Statement No. 157, Fair Value Measurements (SFAS 157)), included in ASC Topic 820, Fair Value Measurements and Disclosures, for fair value measurements of financial assets and financial liabilities and for fair value measurements of nonfinancial items that are recognized or disclosed at fair value in the financial statements on a recurring basis. ASC subtopic 820 (Statement 157) defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC subtopic 820 (Statement 157) also establishes a framework for measuring fair value and expands disclosures about fair value measurements (see Note 7).
On January 1, 2009, the Company adopted the provisions of ASC subtopic 820 (Statement 157) for fair value measurements of nonfinancial assets and nonfinancial liabilities that are recognized or disclosed at fair value in the financial statements on a nonrecurring basis. The adoption of ASC subtopic 820 did not have a material impact on the Company’s consolidated financial position and results of operations.
Note 3 - Cash and Cash Equivalents
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
U.S. dollars in thousands
|
||||||||
|
In US dollars:
|
||||||||
|
Cash
|
$ | 142 | $ | 36 | ||||
|
Cash equivalents
|
100 | 1,949 | ||||||
|
In New Israeli Shekels:
|
||||||||
|
Cash
|
278 | 182 | ||||||
|
Cash equivalents
|
104 | 612 | ||||||
|
In Euro*
|
- | - | ||||||
| $ | 624 | $ | 2,779 | |||||
F-32
Note 4 - Property and Equipment, Net
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
U.S. dollars in thousands
|
||||||||
|
Cost
|
||||||||
|
Furniture
|
$ | 22 | $ | 22 | ||||
|
Electronic equipment
|
118 | 111 | ||||||
|
Computers
|
96 | 84 | ||||||
|
Total
|
236 | 217 | ||||||
|
Accumulated depreciation
|
130 | 86 | ||||||
|
Net book value
|
$ | 106 | $ | 131 | ||||
The Company recorded depreciation of $25 thousand and $23 thousand for the years ended December 31, 2009 and December 31, 2008, respectively and depreciation of $130 thousand for the period from inception through December 31, 2009.
Note 5 - Accounts Payable
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
U.S. dollars in thousands
|
||||||||
|
Suppliers
|
$ | 232 | $ | 482 | ||||
|
Credit card payable
|
7 | 11 | ||||||
| $ | 239 | $ | 493 | |||||
Note 6 - Deposits and Liability for Employee Severance Benefits
Under Israeli law, employers are required to make severance payments to dismissed employees and employees leaving employment in certain other circumstances, based on the latest monthly salary multiplied by the number of years of employment as of the balance sheet date. This liability of the Company is partially covered by payments of premiums to insurance companies under approved plans and by a provision in these financial statements. The deposits in respect of employee severance benefits as stated in the balance sheets as of December 31, 2009 and 2008 represent contributions to manager’s insurance policies and are stated at current cash redemption values.
Note 7 - Fair Value Measurements
The Company measures fair value representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering such assumptions, the Company utilizes a three-tier value hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
Level 1 - Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 - Include other inputs that are directly or indirectly observable in the marketplace.
Level 3 - Unobservable inputs which are supported by little or no market activity.
The fair value hierarchy also requires the Company to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The Company’s warrants to noteholders are valued using the Binomial model. Prior to the second quarter of 2009, the Company’s warrants to noteholders were valued using the Black-Scholes-Merton model. Given the recent foreign currency fluctuation’s effect on the exercise price of the Warrants throughout the life of the Warrant, the Company’s assumptions have changed regarding the possible exercise patterns, and these possibilities can be addressed through the Binomial model in contrast to the Black–Scholes-Merton model which only allows for one exercise date. The change in the model did not have a material impact on our consolidated financial position, results of our operations or cash flows. These models use the variables of the price of the underlying stock, the strike price, the continuously compounded risk-free interest rate, the continuously compounded annual dividend yield, the time in years until the expiration of the option and the weighted average implied volatility of the underlying stock, the implied volatility of the Warrant and the implied volatility of the Series 2 Warrants. Some of the inputs to this valuation are unobservable in the market and are significant, requiring significant judgment using the best information available (see Note 9C).
The Company recorded a financing expense (income) charge of approximately $3,053 thousand and $(1,653) thousand for the year ended December 31, 2009 and 2008, respectively, and $1,400 thousand for the development stage period, resulting from revaluation of warrants to noteholders, which has been recorded in the statement of operations.
F-33
Note 7 - Fair Value Measurements (cont’d)
|
|
1.
|
Assets and liabilities measured at fair value on a recurring basis are summarized below:
|
|
Fair value measurement at reporting date using
|
||||||||||||||||
|
Quoted Prices
|
||||||||||||||||
|
in Active
|
Significant
|
|||||||||||||||
|
Markets for
|
Other
|
Significant
|
||||||||||||||
|
December 31,
|
Identical Assets
|
Observable
|
Unobservable
|
|||||||||||||
|
2009
|
(Level 1)
|
Inputs (Level 2)
|
Inputs (Level 3)
|
|||||||||||||
|
Description
|
U.S. dollars in thousands
|
|||||||||||||||
|
Warrants to noteholders
|
$ | 2,640 | $ | - | $ | - | $ | 2,640 | ||||||||
|
Total Liabilities
|
$ | 2,640 | $ | - | $ | - | $ | 2,640 | ||||||||
|
Fair value measurement at reporting date using
|
||||||||||||||||
|
Quoted Prices
|
||||||||||||||||
|
in Active
|
Significant
|
|||||||||||||||
|
Markets for
|
Other
|
Significant
|
||||||||||||||
|
December 31,
|
Identical Assets
|
Observable
|
Unobservable
|
|||||||||||||
|
2008
|
(Level 1)
|
Inputs (Level 2)
|
Inputs (Level 3)
|
|||||||||||||
|
Description
|
U.S. dollars in thousands
|
|||||||||||||||
|
Warrants to noteholders
|
$ | 79 | $ | - | $ | - | $ | 79 | ||||||||
|
Total Liabilities
|
$ | 79 | $ | - | $ | - | $ | 79 | ||||||||
|
|
The Company currently has no other assets measured at fair value.
|
|
|
2.
|
The parameters used in the fair value calculation for the Warrants were as follows:
|
|
As of
|
As of
|
|||||||||||
|
As of July 30,
|
December 31,
|
December 31,
|
||||||||||
|
2008
|
2008
|
2009
|
||||||||||
|
Market price of underlying stock
|
2.08 NIS
|
0.405 NIS
|
2.435 NIS
|
|||||||||
|
Strike price
|
$ | 0.716 | $ | 0.716 | $ | 0.716 | ||||||
|
Continuously compounded risk-free interest rate for
|
||||||||||||
|
the debt feature of the Convertible Note Payable
|
5.04 | % | - | - | ||||||||
|
Continuously compounded risk-free interest rate for
|
||||||||||||
|
Warrants
|
5.33 | % | 4.00 | % | 2.68 | % | ||||||
|
Continuously compounded annual dividend rate
|
0 | % | 0 | % | 0 | % | ||||||
|
Time in years until the expiration of the Convertible
|
||||||||||||
|
Note Payable
|
4 years
|
- | - | |||||||||
|
Time in years until the expiration of the Warrant
|
5 years
|
4.58 years
|
3.58 years
|
|||||||||
|
Implied volatility for the underlying stock
|
55 | % | 61.3 | % | 145.48-148.52 | % | ||||||
F-34
Note 7- Fair Value Measurements (cont’d)
|
|
3.
|
The following table presents the Company’s activity for liabilities measured at fair value using significant unobservable inputs (Level 3), as of December 31, 2009:
|
|
Level 3
|
||||
|
U.S. dollars
|
||||
|
in thousands
|
||||
|
Balance at December 31, 2007
|
- | |||
|
Initial valuation of warrants to shareholders
|
$ | 1,828 | ||
|
Gain from revaluation of warrants to shareholders, included in the statement of operations for the year ended December 31, 2008
|
(1,653 | ) | ||
| 175 | ||||
|
Adjustment for foreign currency translation
|
||||
|
differences
|
(96 | ) | ||
|
Balance at December 31, 2008
|
79 | |||
|
Loss from revaluation of warrants to shareholders,
|
||||
|
included in the statement of operations for the
|
||||
|
year ended December 31, 2009
|
3,053 | |||
| 3,132 | ||||
|
Adjustment for foreign currency translation
|
||||
|
differences
|
268 | |||
|
Balance at December 31, 2009
|
$ | 3,400 | ||
The Company has outstanding convertible notes payable, of which the fair values have been determined using the Binomial model. Carrying amounts and the related estimated fair value of the convertible notes payable are as follows:
|
December 31, 2009
|
December 31, 2008
|
|||||||||||||||
|
Carrying
|
Carrying
|
|||||||||||||||
|
Amount
|
Fair Value
|
Amount
|
Fair Value
|
|||||||||||||
|
U.S. dollars in thousands
|
||||||||||||||||
|
Convertible notes payable
|
$ | 644 | $ | 3,483 | $ | 303 | $ | 3,370 | ||||||||
The difference between the carrying amount as compared to the fair value of the convertible notes payable represents the unamortized portion of the beneficial conversion feature of the convertible notes payable.
Note 8 - Contingent Liabilities and Commitments
|
|
A.
|
Liabilities for the payment of royalties to the Chief Scientist
|
The Company financed part of its research and development activities through grants received from the Office of the Chief Scientist (OCS) in Israel. In consideration for the OCS grants, the Company is obligated to pay royalties at a rate between 3% and 5% of revenues from sales of the product developed with the aid of the OCS, up to repayment of the full amount of the obligation, whichever is earlier. The grants are linked to the exchange rate of the U.S. dollar and bear an agreed-upon interest rate.
Through December 31, 2009 the Subsidiary received from the OCS $1,725,000 ($491,000 and $282,000 for the years 2009 and 2008, respectively).
In March 2009, the Subsidiary received from the OCS approval of the grants applied for in November 2008 for an additional research and development program with a budget of $931 thousand (NIS 3,514 thousand) (covering 60% of expenses in Israel and 30% of expenses overseas) and totaling an anticipated grant of $413 thousand (NIS 1,561 thousand). For the period ended December 31, 2009, the Subsidiary received from the Chief Scientist a total of $324 thousand (NIS 1,301 thousand).
In July 2009, the Subsidiary received from the OCS approval for two additional research and development program grants with a budget of $679 thousand (NIS 2,565,000) (covering 60% of expenses in Israel and 30% of expenses overseas) and totaling an anticipated grant of $354 thousand (NIS 1,336,000). For the period ended December 31, 2009, the Subsidiary received from the Chief Scientist a total of $167 thousand (NIS 642 thousand).
F-35
|
|
B.
|
Liabilities for the payment of grant to the Israel-US Binational Industrial Research and Development Fund (BIRD)
|
In December 2007, the Subsidiary and the Virginia Biosciences Commercialization Center (VBCC), received approval for a grant from the Israel-US Binational Industrial Research and Development Fund (BIRD). The grant will partially fund a Phase I/IIa clinical trial in pancreatic cancer at the National Cancer Institute-designated Massey Cancer Center of Virginia Commonwealth University. The grant is up to an amount of $950,000 or 50% of the predicted expenses of the project.
In accordance with the terms of the agreements between the two companies and the BIRD Foundation, the Company will have to repay the grant within twelve months of the successful completion of the project. The terms of the agreements call for the evaluation of the status of the project by May 1, 2010.
The Company is entitled to extend the repayment period to 2 years in return for total repayment of 113% of the grant amount, to 3 years in return for total repayment of 125%, to 4 years in return for total repayment of 138%, or to 5 years or more in return for total repayment of 150% of the grant amount.
As of December 31, 2009, the Company recognized approximately $78 thousand, which has been recorded as a reduction to research and development expenses. Of this amount, $43 thousand (NIS 161 thousand) has been recorded as a receivable at December 31, 2009.
From inception through December 31, 2009 the Company has received a total of $214 thousand from the BIRD Foundation.
|
|
C.
|
Licensing agreement
|
The Company's research and development activities are based on an exclusive license given to the Company to use technology protected by patent and/or applications filed for registration of patents. These patents were developed by one of the Company's founders, and were originally the property of Yissum, which was a related party of the Company. According to the amended licensing agreement signed between Yissum, the Company and the Subsidiary in November 2005 ("the licensing agreement"), the Company was granted rights for exclusive use of the said patents. Under this amended agreement, the Company undertook to pay royalties to Yissum, at the following rates:
|
|
1.
|
5% of net sales which is defined as the gross amount invoiced by the Company to third parties, which are not sub-licensees, from sales of Licensed Products in an arm’s length transaction, less customary discounts as further detailed in the licensing agreement;
|
|
|
2.
|
10% of total proceeds that the Company will receive from any third party who will receive a sub-license to use the Company's technology for royalties of up to $30 million per year;
|
|
|
3.
|
6.5% of the total proceeds that the Company receives from any third party who will receive a sub-license to use the Company's technology for royalties above $30 million per year.
|
Note 8 - Contingent Liabilities and Commitments (cont’d)
|
|
C.
|
Licensing agreement (cont'd)
|
The licensing agreement will expire on a country-by-country basis, when the patent expires in that country, or if no patent is registered in that country, in the 9th year from the date of the first commercial sale (as defined in the licensing agreement) in that country. However, the Company is permitted to extend the licensing agreement for an additional period in each and every country, by continuing to pay royalties on the revenues from that country. It was also agreed that if the agreement covering each country expires, as aforesaid, the Company will have a license for that country, which is not subject to its obligations pursuant to the licensing agreement, and which has no time limit.
In November 2006, the Board of Directors resolved that the rights of the licensing agreement shall be held exclusively by the Subsidiary and informed Yissum as such on November 29, 2006. The latter acknowledged receipt.
In September 2007, the Company and Yissum modified the licensing agreement, such that all intellectual property developed using OCS funding will be owned by the Subsidiary.
|
|
D.
|
Research and development agreement
|
The Company's research and development activities are carried out by the Subsidiary primarily through a laboratory research team in the Hebrew University of Jerusalem (the “University”) through its technology transfer company, Yissum. The laboratory is managed by a related party of the Company. The research is conducted under an agreement whereby the Company is obligated to pay $35 thousand plus VAT per quarter on a quarterly basis, for this research service. The original agreement was for a two - year period and as of the date of these financial statements, the agreement has been extended until June 30, 2010.
The Company can terminate the agreement upon 90 days prior notice. If the agreement is terminated prior to the end of the research period, the Company shall immediately pay Yissum for the research actually performed and for personnel obligations of Yissum made before the receipt of such written notice or which arise as a result of the termination.
As of December 31, 2009, the Company has recorded a prepaid expense of approximately $71 thousand in respect of this agreement.
F-36
Note 8 - Contingent Liabilities and Commitments (cont’d)
|
|
E.
|
Indemnification agreements
|
The Company has undertaken to indemnify Yissum and the Hebrew University of Jerusalem and any person acting on its behalf and any of their directors, officers, employees and representatives (herein referred to as “Indemnitees”) from and against any liability including, without limitation, product liability, damages, losses or expenses including reasonable legal fees and litigation expenses incurred by or imposed upon the Indemnitees (collectively, “Losses”) only by reason of Company’s acts and/or omissions and/or which derive from Company’s use, development, manufacture, marketing, sale and/or sublicensing of the licensed technology except to the extent such Losses are determined to have resulted from the gross negligence or willful misconduct of Indemnitees.
Additionally, upon the commencement of any clinical trial, the Company is obligated to procure and maintain comprehensive clinical trial liability insurance in amounts commensurate with accepted commercial practice. All required insurance will be at the Company’s sole cost and expense. The Company acquired a clinical trial insurance policy providing Yissum as an additional named insuree. The Company is obligated to obtain comprehensive general liability insurance which shall provide contractual liability coverage for the Company’s indemnification under its agreement with Yissum (see D above). As of December 31, 2009, the Company is in compliance with the terms of the agreement.
|
|
In July 2006, the Company approved the granting of letters of indemnification to directors and officers serving at that time and in the future. In this context, the Company committed that in addition to the terms of indemnification established in its Articles of Incorporation, it would indemnify each of the Directors and Officers for debts or expenses, as detailed in the letter of indemnification, borne on account of activities performed by virtue of their position as Directors and Officers of the Company or of another company, partnership, joint venture, etc. connected directly or indirectly with one or more of the events detailed in the letter of indemnification. See also indemnification in 8F and 8G below.
|
|
|
F.
|
Clinical Trial Agreements between the Company and Medical Centers in the U.S. and Israel (collectively, “Hospitals”)
|
|
|
1.
|
Between November 2007 and December 2009, the Company signed clinical trial agreements with eight medical centers in Israel and BCG Oncology in the U.S.
|
|
|
Under the Clinical Trial Agreements, the Hospitals will carry out a clinical trial study relating to the clinical development of the Company’s developing drug therapy in patients with superficial bladder cancer and subject to compliance with the requirements set forth in the Agreement. In consideration of the Hospitals’ undertaking to conduct the study and for the performance of its obligations, the Company agrees to pay the Hospitals based upon the number of patients, time enrolled and number of treatments, and shall provide, at the Company’s sole expense, the study drug required for the performance of the study as set forth in the agreement. As of December 31, 2009, the Company is not aware of any need for such indemnification.
|
|
|
2.
|
Between April 2009 and December 2009, the Company signed clinical trial agreements with three medical centers in Israel and two university hospitals in the U.S.
|
|
|
Under the Clinical Trial Agreements, the Hospitals will carry out a clinical trial study relating to the clinical development of the Company’s developing drug therapy in patients with ovarian cancer and subject to compliance with the requirements set forth in the Agreement. In consideration of the Hospitals’ undertaking to conduct the study and for the performance of its obligations, the Company agrees to pay the Hospitals based upon the number of patients, time enrolled and number of treatments, and shall provide, at the Company’s sole expense, the study drug required for the performance of the study as set forth in the agreement. As of December 31, 2009, the Company is not aware of any need for such indemnification.
|
F-37
Note 8 - Contingent Liabilities and Commitments (cont’d)
|
|
F.
|
Clinical Trial Agreements between the Company and Medical Centers in the U.S. and Israel (collectively, “Hospitals”) (cont’d)
|
|
|
3.
|
Between May 2009 and December 2009, the Company signed clinical trial agreements with three medical centers in Israel and a medical center in the USA.
|
|
|
Under the Clinical Trial Agreements, the Hospitals will carry out a clinical trial study relating to the clinical development of the Company’s developing drug therapy in patients with pancreatic cancer and subject to compliance with the requirements set forth in the Agreement. In consideration of the Hospitals’ undertaking to conduct the study and for the performance of its obligations, the Company agrees to pay the Hospitals based upon the number of patients, time enrolled and number of treatments, and shall provide, at the Company’s sole expense, the study drug required for the performance of the study as set forth in the agreement. As of December 31, 2009, the Company is not aware of any need for such indemnification.
|
|
|
G.
|
Clinical Trial Agreements between Hebrew University of Jerusalem and the Company and Medical Centers in the U.S. and Israel (collectively, “Hospitals”)
|
The University and the Company committed to indemnify the Hospitals, the study staff, and the employees and/or officers and/or representatives of any of the parties listed above (“Beneficiaries”) from and against all claims, demands, causes of action and suits of whatsoever kind or nature based on damages claimed to have been caused as a result of the performance of the Study and/or any procedures prescribed in the Protocol and/or pertaining to the study (“Loss”) conditional to the following conditions: (i) the Loss was not caused as a result of negligence or misconduct of the Beneficiaries; and (ii) the Beneficiaries performed the study in accordance with the Protocol and the additional requirements set in the clinical trial agreement. The Company shall obtain and maintain at all times during the term of clinical trial agreement and thereafter for a period not less than the applicable statute of limitations, a comprehensive general liability insurance policy for reasonably cognizable claims arising from the activities to be undertaken pursuant to clinical trial agreement in the minimum amount of $3 million dollars per incident. As of December 31, 2009, the Company is in compliance with the terms of the agreement.
F-38
Note 8 - Contingent Liabilities and Commitments (cont’d)
|
|
H.
|
Operating leases
|
1. Vehicles
The Company has lease agreements for 9 vehicles for 36 months each. As required by the agreements, the Company deposited a security deposit to ensure the fulfillment of its contractual commitment. At December 31, 2009, the balance of the deposit was $26 thousand and represents the last three months of the lease. The deposit is linked to the Israeli CPI and does not bear interest.
The minimum annual payments in connection with the agreements are as follows:
|
U.S. dollars
|
||||
|
in thousands
|
||||
|
2010
|
$ | 76 | ||
|
2011
|
59 | |||
|
2012
|
$ | 15 | ||
2. Rental Agreement
|
|
In November 2006 the Company entered into a rental agreement for its offices for a 24 month period with optional extension. During 2008 and 2009 the Company extended the rental agreement for additional periods of 12 months until September 16, 2010. The monthly rental (including maintenance fee and parking) is $4 thousand + VAT. The Company presented the lessor with a bank guarantee in the amount of $12 thousand which was increased to $15 thousand in 2009, to ensure fulfillment of its contractual obligations.
|
|
|
The following table summarizes the Company’s rent expense for the periods presented:
|
|
Cumulative from inception to December 31,
|
||||||||||||
|
2009
|
2008
|
2009
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Rent expense
|
$ | 48 | $ | 48 | $ | 181 | ||||||
The minimum annual payment in connection with the agreement is as follows:
|
U.S. dollars
|
||||
|
in thousands
|
||||
|
2010
|
$ | 36 | ||
F-39
Note 8 - Contingent Liabilities and Commitments (cont’d)
|
|
I.
|
Consulting Agreements
|
|
|
1.
|
As of July 2008, the Company entered into an agreement with Tikcro Technologies Ltd., whereby in exchange for consultancy services provided by it, the Company will pay Tikcro Technologies Ltd. an annual amount of $30 thousand, 63,939 common shares and reimbursement of expenses. The Company recorded in the first year of the agreement a provision in the amount of $40 thousand in connection with these shares. The Company believes that it did not receive the consultancy services as provided for in the agreement for that year. Therefore, the Board of Directors of the Company, with the advice of its legal advisor, decided to cancel the provision. As of the date of these financials statements, the Company has not paid these fees and the companies are in discussions regarding the consulting fee. In July 2009, the Company began to receive consultancy services and as such, recorded as general and administrative expenses approximately $15 thousand for these services and an additional $28 thousand in regard to the 63,939 shares issued for the second year of the agreement. Additionally, the Company has recorded as a prepaid expense approximately $28 thousand in connection with these shares.
|
|
|
2.
|
In October 2009 the Company signed an agreement with a financial advisor with regard to raising capital until May 10, 2010. As part of the agreement, the Company committed to pay a fee to the advisor, to be derived from amounts raised.
|
J. Liabilities for the payment of grant to the Jerusalem Development Authority (JDA)
In November, 2009, the Company received from the JDA a grant of $35 thousand and anticipates an additional grant in 2010. In accordance with the terms of the agreement between the Company and the JDA, the Company is obligated to pay royalties at a rate of 4% of revenues from sales of the product developed, up to an amount equal to the grants received. The grant is linked to the Israeli Consumer Price Index.
Note 9 - Stockholders' Equity
|
|
A.
|
Stock splits
|
|
|
1.
|
In November 2005, the Company's board of directors approved a 1:30 split of the Company's stock. Therefore, the number of authorized and issued shares of the Company increased from 100,000 to 3,000,000. At the same time, the Company's board of directors approved a further increase in the number of authorized shares to 6,137,609, comprised of 5,000,000 shares of common stock, $0.01 par value and 1,137,609 shares of Series A convertible preferred stock par value of $0.01. The stock split was recorded retroactively from inception in accordance with ASC subtopic 505-10 (previously referred to as Accounting Research Bulletin (“ARB”) No. 43).
|
|
|
2.
|
In July 2006, the Company’s board of directors approved a 1:2.855 split of the Company’s stock. The stock split was recorded retroactively from inception in accordance with ASC subtopic 505-10.
|
F-40
Note 9 - Stockholders' Equity (cont’d)
|
|
B.
|
Composition
|
|
As of December 31, 2009
|
As of December 31, 2008
|
|||
|
Number of shares
|
||||
|
Issued and
|
Issued and
|
|||
|
Authorized
|
outstanding
|
Authorized
|
outstanding
|
|
|
Common stock, $0.01 par value
|
||||
|
Each
|
50,000,000
|
16,356,550
|
50,000,000
|
16,292,611
|
On October 1, 2009, the Board of Directors of the Company resolved to increase the authorized capital of the Company to 65,000,000 common shares, subject to a future resolution of the general meeting of the stockholders and future resolution of the Board, which was not received as of balance sheet date (See Note 13).
|
|
C.
|
Common Stock
|
|
|
1.
|
In October 2004, the Company issued 4,282,500 shares of common stock to two of the Company's founders, for no consideration, as founders’ shares.
|
|
|
2.
|
In November 2005, pursuant to an agreement signed in October 2005, the Company issued 360,955 shares of common stock to Yissum, resulting from the conversion of stockholder loans totaling $300 thousand.
|
|
3.
|
In December 2005, the Company issued 2,854,532 shares of Series A convertible preferred stock for consideration of $2,296,000, net of expenses incurred in connection with the fund raising of $76 thousand, including $473 thousand which was in transit at the balance sheet date and is recorded in 2006.
|
During the first quarter of 2006, the Company issued 397,057 shares of Series A convertible preferred stock for consideration of $295 thousand, net of expenses incurred in connection with the fund raising of $35 thousand. In March 2006, the Company issued to Yissum 72,192 shares of Series A convertible preferred stock in consideration for expenses paid by Yissum on the Company’s behalf of $60 thousand.
The Series A convertible preferred stock conferred, inter alia, voting rights, preferential rights in all that relates to the distribution of the Company's assets upon liquidation and income from a merger or acquisition of all or most of the Company's shares or assets and anti-dilution rights if the Company allots shares or rights to shares at a price below that paid by the Series A convertible preferred stockholders.
|
|
4.
|
In conjunction with the Company’s initial public offering on the TASE, the 3,323,781 of Series A convertible preferred shares were converted into shares of common stock, $0.01 par value, on a 1:1 basis. The conversion was completed on August 14, 2006.
|
F-41
Note 9 - Stockholders' Equity (cont’d)
|
|
C.
|
Common Stock (cont’d)
|
|
|
5.
|
In August 2006, the Company filed a prospectus for an initial public offering on the Tel Aviv Stock Exchange, offering to the public the following securities:
|
a. Common stock - 3,200,000 quoted shares, $0.01 par value each.
|
|
b.
|
Series (1) Warrants – 2,500,000 quoted option warrants that are exercisable into 2,500,000 common shares until August 18, 2008, $0.01 par value, at an exercise price of $2.12 each.
|
|
|
c.
|
Series (2) Warrants – 2,500,000 quoted option warrants that are exercisable into 2,500,000 common shares until August 11, 2010, $0.01 par value, at an exercise price of $2.65 each.
|
|
|
d.
|
The marketable securities were offered to the public in 100,000 units that were composed of 32 ordinary shares, 25 Series (1) Warrants and 25 Series (2) Warrants, at a price of $55.89 per unit.
|
The offering was consummated on August 14, 2006 upon the issuance of 3,200,000 shares of Common Stock, 2,500,000 Series 1 Warrants and 2,500,000 Series 2 Warrants. The gross proceeds received by the Company totaled $5,653,000. The expenses incurred in connection with the public offering totaled $1,035,000, of which $358 thousand was due to warrants granted to underwriters as discussed in section G below. The net proceeds of the offering was allocated between stock and warrants based on their relative fair values.
|
|
6.
|
In February 2008, the Company increased its authorized share capital to 50 million shares.
|
|
|
7.
|
In May 2008, the Company executed a private placement allocation of 650,000 common shares to Clal Biotechnology Industries Ltd. (“CBI”) at a price of NIS 3.52 per share, the average closing price of the Company’s shares on the Tel Aviv Stock Exchange over the thirty trading days preceding he Company’s Finance Committee recommendation to approve the transaction. The gross proceeds of the allocation of $653 thousand, less $16 thousand in allocated expenses, were credited to additional paid-in capital upon the allocation of Company shares to CBI.
|
F-42
Note 9 - Stockholders' Equity (cont’d)
|
|
C.
|
Common Stock (cont’d)
|
|
|
8.
|
In July 2008, the Company carried out private placements with institutional investors (the “Investors”), whereby 3 investors received an aggregate amount of 1) 1,222,780 shares of common stock (at a price of 59.7 cents per share) (the “Common Shares”), 2) non-registered convertible notes payable (the “Convertible Notes Payable”) convertible into an aggregate of up to 5,058,002 common shares (at a conversion price of 71.6 cents per share) and 3) non-registered warrants (the “Warrants”) to purchase an aggregate of up to 6,280,783 shares of common stock (at a price of 71.6 cents per share) exercisable for 5 years. The Company's gross proceeds from the private placements were $3,650,000 (NIS 12,662,000). As long as they are not converted, the Convertible Notes Payable bear dollar - linked interest of 10% per annum, to be added to the principal (and considered as part of the principal for the purposes of conversion) for the first nine quarters and paid quarterly thereafter, for the remaining 7 quarters.
|
The Convertible Notes Payable are convertible, in whole or in part at the option of the holder at any time prior to July 31, 2012, into a number of shares of the Company’s common stock equal to the principal amount of the notes payable, including capitalized interest accrued between July 31, 2008 and July 31, 2010, at a price of 71.6 cents per share. The conversion price and terms of the Convertible Notes Payable are subject to certain adjustments. The Convertible Notes Payable mature, and any principal amount thereunder not converted into shares of common stock becomes due and payable, on July 31, 2012. The Warrants are exercisable at any time prior to July 29, 2013 for a per share exercise price of 71.6 cents.
Pursuant to the terms of the agreements entered into by the Company in connection with the private placement, until such time as the Company has raised equity financing in excess of $15,000,000 (excluding any amounts invested by the Investors and $5,000,000 raised in equity financings consummated within three months of the closing of the transactions contemplated by these agreements), if the Company issues any securities to any party for a per share purchase price, conversion price or exercise price, as applicable, less than any of the per share purchase price, conversion price or exercise price paid or payable for the shares of our common stock, convertible debentures or warrants, respectively, issued to the Investors in the transaction, the Company will be (i) obligated to issue an additional number of shares of its common stock such that the number of shares originally issued to the Investors at the closing plus such number of additional of shares of its common stock equal the number of shares of its common stock that would have been issued at the price at which it sold its securities such subsequent issuance for the purchase price paid at the closing for shares of its common stock, and (ii) the conversion price of Convertible Notes Payable and the exercise price of the Warrants shall be reduced to equal the price at which its securities were sold in such subsequent issuance.
F-43
Note 9 - Stockholders' Equity (cont’d)
|
|
C.
|
Common Stock (cont’d)
|
The $3,650,000 of proceeds from the private placements were first allocated to the Warrants, which were classified as derivative instruments. The Warrants are considered derivatives since they are not indexed solely to the Company’s own stock as they must be settled in a currency other than the Company’s functional currency, and the Warrants meet all of the characteristics of a derivative instrument. The allocation is based on their fair value, and the residual amount was allocated among the Common Stock and the Convertible Notes Payable based on their relative fair values (see Note 7).
The Convertible Notes Payable were classified as long-term liabilities and were recorded at their initial relative fair value. The Convertible Notes Payable are presented net of unamortized discounts for the portions allocated to the Warrants, Common Shares and the beneficial conversion feature inherent in the instrument. The interest due on the Convertible Notes Payable is accrued to the value of the instrument. During the current period, interest expense of approximately $282 thousand was recorded.
The Warrants have been recorded as a liability, as mentioned above, with a corresponding discount to the Convertible Notes Payable, based on their fair values, and are revalued at each reporting date. Changes in the fair value are included in the Statement of Operations. During the current period the fair value of the Warrants increased by $2,561,000, of which $2,293,000 is recorded in the statement of operations in revaluation of warrants income (the primary drivers of this change are described in a table further on in this note) and $268 thousand is included in other comprehensive loss representing foreign currency translation differences.
The difference between the effective conversion rate of the Convertible Notes Payable as compared to the fair market value of the Company's common stock on the date of issuance represents a beneficial conversion feature. Thus, the Company recorded an additional discount to the Convertible Notes Payable with a corresponding increase in additional paid-in capital of $1,372,000. The aforesaid discount relating to the beneficial conversion feature is amortized to interest expense, using the effective interest method, over the four year life of the Convertible Notes Payable from the date of issuance. As of December 31, 2009, approximately $68 thousand of such discount has been amortized since the issuance in July 2008.
F-44
|
|
Note 9 – Stockholders’ equity (cont’d)
|
|
|
C.
|
Common Stock (cont'd)
|
|
U.S. dollars
|
||||
|
in thousands
|
||||
|
Initial recording:
|
||||
|
Gross proceeds
|
$ | 3,650 | ||
|
Issuance of Common Stock
|
(12 | ) | ||
|
APIC resulting from issuance of Common stock
|
(262 | ) | ||
|
Discount resulting from the issuance of Warrants
|
(1,828 | ) | ||
|
Discount resulting from the Beneficial Conversion Feature
|
(1,372 | ) | ||
|
Balance of Convertible Notes Payable at July 30, 2008
|
$ | 176 | ||
|
U.S. dollars
|
||||
|
in thousands
|
||||
|
Movement subsequent to issuance:
|
||||
|
Initial valuation at July 30, 2008
|
$ | 176 | ||
|
Accrued interest
|
118 | |||
|
Amortizations of discounts from closing through December 31, 2008
|
9 | |||
|
Balance of Convertible Notes Payable at December 31, 2008
|
303 | |||
|
Accrued interest
|
282 | |||
|
Amortizations of discounts for the year ended December 31, 2009
|
59 | |||
|
Balance of Convertible Notes Payable at December 31, 2009
|
$ | 644 | ||
If the Convertible Notes Payable are not converted before July 31, 2012, the Company will need to pay a total of $3,621,000.
|
|
9.
|
With respect to the 63,939 common shares issued to Tikcro Technologies Ltd. for consultancy services see Note 8I.
|
|
|
D.
|
Treasury Stock
|
During August, 2008, the Subsidiary purchased 1,812,756 Series 1 Warrants of the Company from a third party at a price of NIS 0.02 each. The Subsidiary exercised these warrants to common stock at a strike price of NIS 9.73 per share, paid to the Company by an equity investment by the Company in the Subsidiary. During the year ending December 31, 2009, the Company sold 1,812,756 shares of treasury stock, at an average price of NIS 3.38 (approximately $0.865) per share, and total proceeds of $1,568,000. Following the sale, the Company did not hold any shares of treasury stock. The difference between the total proceeds and the initial relative cost of the treasury stock in the amount of $3,383,000 was recorded as an increase to the accumulated deficit.
F-45
Note 9 - Stockholders' Equity (cont'd)
|
|
E.
|
Stock Option Plans
|
|
|
1.
|
The Company provides for direct grants of common stock, and common stock options to employees and non-employees through the 2004 Stock Option Plan (the 2004 Plan) and the 2007 Stock Option Plan (the 2007 Plan).
|
The Company’s stockholders approved the 2004 Plan in November 2004, following its adoption by the Company’s board of directors. Under the 2004 Plan, the Company may grant stock options, stock appreciation rights, restricted stock, deferred stock, other stock-related awards and performance awards to officers, directors, employees, consultants and other persons who provide services to the Company. As of December 31, 2009, there are no options available under the 2004 Plan. The total number of Company shares of common stock reserved and available for grant under the 2004 Plan was set at 2,024,003 upon its adoption. As of December 31, 2009, no options are available under the 2004 Plan.
In January 2007, the Company’s Board of Directors approved the 2007 Plan. Under the 2007 Plan, the Company may grant stock options, stock appreciation rights, restricted stock, deferred stock, other stock-related awards and performance awards to officers, directors, employees, consultants and other persons who provide services to the Company. The total number of Company shares of Common stock reserved and available for grant under the 2007 Plan was set at 2,000,000 upon its adoption. As of December 31, 2009, there are 213,500 options available under the 2007 Plan.
All options expire ten years after grant date and may be exercised following the employee’s termination by the Company for any reason other than cause, for a period of between 1-24 months. Vesting terms are not defined in the Plans but are determined by the Company’s board of directors at the time of the grant.
|
|
2.
|
The stock option plan was approved in accordance with Section 102 of the Israeli Income Tax Ordinance (for the Israeli employees) as a "capital track" plan, whereby the options will be deposited with a trustee for a period of at least two years when the options were granted. The option recipients will pay tax deriving from the benefit at the time the shares are realized, but no expense related to them will be recognized for tax purposes by the Company. The options to the consultants, which could not be recorded under Section 102 of the Israeli Income Tax Ordinance, will be taxed to their recipients as ordinary income, when the options are exercised, under Section 3(I) of the Israeli Income Tax Ordinance.
|
F-46
Note 9 – Stockholders' Equity (cont'd)
F. Allotment of stock options to employees, directors and consultants of the Company and the Subsidiary as of December 31, 2009
The expenses recorded in the statement of operations on account of stock-based transactions were $212 thousand and $40 thousand for the years ending December 31, 2009 and 2008, respectively, and $1,613 for the development stage period (cumulative from inception).
The options that were granted in 2004 were with a par value exercise price. Due to the par value amount of $0.01, the fair value of these options was estimated to be equal to the Company’s share price at the grant date, based on stock issuances that took place surrounding the grant date.
The parameters used in the valuation of the option granted in 2006, 2008 and 2009, based on the Black–Scholes-Merton model, are as follows:
|
Estimate value
|
|||||||||||||
|
Expected
|
of the stock
|
||||||||||||
|
average term of
|
price on the
|
||||||||||||
|
Year
|
Volatility
|
the option
|
Risk-free rate
|
grant date*
|
|||||||||
|
2006
|
0.6 |
3 years
|
4.07%-5.00 | % | $ | 0.83-$1.45 | |||||||
|
2008
|
0.6 |
10 years
|
6.3705 | % | $ | 0.10 | |||||||
|
2009
|
0.8 |
10 years
|
4.9553 | % | $ | 0.63 | |||||||
|
|
*
|
The market price is fixed in New Israeli Shekel are translated at exchange rate on date of grant.
|
The fair value of options granted to non-employees has been computed and accounted for in accordance with ASC subtopic 505-50 and 718-10 (previously referred to as SFAS 123(R) and Emerging Issues Task Force (EITF) 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services), and was measured according to the Black-Scholes-Merton option-pricing model with the parameters discussed in the table below, and unvested options are revalued at the end of each reporting period.
|
Contractual term
|
Share price as of
|
|||||||||
|
Year
|
Volatility
|
of the option
|
December 31,
|
Risk-free rate
|
||||||
|
2008
|
0.6 |
10 years
|
NIS 0.405
|
6.3705 | % | |||||
|
2009
|
0.8 |
10 years
|
NIS 2.435
|
5.1278 | % | |||||
|
|
G.
|
Options outstanding
|
|
|
1.
|
The following table summarizes information about stock options outstanding as of December 31, 2009, except for milestone based options:
|
|
Options outstanding
|
Options exercisable
|
|||||||||||||||||||||||
|
Weighted
|
Weighted
|
|||||||||||||||||||||||
|
average
|
average
|
|||||||||||||||||||||||
|
remaining
|
Weighted
|
remaining
|
Weighted
|
|||||||||||||||||||||
|
contractual
|
average
|
Aggregate
|
contractual
|
average
|
Aggregate
|
|||||||||||||||||||
|
Number
|
term
|
exercise
|
intrinsic
|
Number
|
term
|
exercise
|
intrinsic
|
|||||||||||||||||
|
outstanding
|
(years)
|
price
|
value
|
outstanding
|
(years)
|
price
|
value
|
|||||||||||||||||
| 256,951 | 5.00 | $ | 0.01 | 256,951 | 5.00 | $ | 0.01 | |||||||||||||||||
| 78,426 | 6.55 | $ | 0.08 | 78,426 | 6.55 | $ | 0.08 | |||||||||||||||||
| 902,612 | 8.92 | $ | 0.33 | 300,814 | 8.92 | $ | 0.33 | |||||||||||||||||
| 390,000 | 8.92 | $ | 0.60 | 215,000 | 8.92 | $ | 0.60 | |||||||||||||||||
| 744,000 | 9.92 | $ | 0.84 | 119,000 | 9.92 | $ | 0.84 | |||||||||||||||||
| 2,371,989 | 8.73 | $ | 0.49 |
$505
|
970,191 | 7.81 | $ | 0.35 |
$288
|
|||||||||||||||
Intrinsic value for stock options is calculated based on the exercise price of the underlying awards and the quoted price of the Company’s common stock as of the reporting date.
F-47
Note 9 – Stockholders' Equity (cont'd)
|
|
G.
|
Options outstanding (cont'd)
|
|
|
Remaining
|
||||||||||||
|
Number of
|
contractual
|
||||||||||||
|
options granted
|
Exercise price
|
term
|
Fair value
|
||||||||||
|
Consultants
|
543,711 | $ | 0.01-$0.33 |
5-8.92 years
|
$ | 0.06-$1.18 | |||||||
|
Directors
|
430,000 | $ | 0.597-$0.84 |
8.92-9.92 years
|
$ | 0.04-$0.51 | |||||||
|
Employees
|
1,398,278 | $ | 0.08-$0.84 |
6.42-9.92 years
|
$ | 0.06-$1.60 | |||||||
|
Total
|
2,371,989 | ||||||||||||
|
|
The stock options with exercise prices fixed in New Israeli Shekel are translated at year-end exchange rates.
|
2. The following table summarizes the Company’s stock option activity during 2009:
|
Weighted
|
||||||||
|
Number of
|
average exercise
|
|||||||
|
stock options
|
price
|
|||||||
|
Balance at January 1, 2009
|
1,667,989 | $ | 0.33 | |||||
|
Granted:
|
||||||||
|
CEO
|
450,000 | $ | 0.84 | |||||
|
Directors
|
40,000 | $ | 0.84 | |||||
|
Employees
|
254,000 | $ | 0.84 | |||||
|
Forfeited
|
(35,938 | ) | $ | 0.33 | ||||
|
Expired
|
(4,062 | ) | $ | 0.33 | ||||
|
Exercised
|
- | - | ||||||
|
Balance at December 31, 2009
|
2,371,989 | $ | 0.49 | |||||
During 2008, a director and consultants exercised 208 thousand options at an exercise price of $0.01 per share. For the years ending December 31, 2009 and 2008, the Company received from the exercise of options $0 thousand and $2 thousand, respectively, and additional paid-in capital increased accordingly. The total intrinsic value of options exercised during the years ended December 31, 2009 and 2008 was $0 thousand and $110 thousand.
The weighted average fair value of options granted for the years 2009, 2008 and cumulatively was $0.51, $0.05 and $0.43 per option, respectively.
|
|
3.
|
Performance based options
|
An additional grant of 150,000 options is subject to market and performance based conditions. No share-based payment expense has been recorded in the statement of operations with respect to this award of 150,000 contingent options.
F-48
Note 9 – Stockholders' Equity (cont'd)
G. Options outstanding (cont’d):
As of December 31, 2009, there was $289 thousand of unrecognized compensation cost, related to nonvested stock options granted under the Company’s various stock option plans. That cost is expected to be recognized as follows (in thousands):
|
Year ending December 31
|
$ | |||
|
2010
|
$ | 172 | ||
|
2011
|
75 | |||
|
2012
|
34 | |||
|
2013
|
8 | |||
| $ | 289 |
|
|
H.
|
On August 1, 2006, the Company signed an agreement with underwriters in connection with its IPO on the Tel Aviv Stock Exchange. Upon the signing of the agreement, the Company issued 300,000 warrants to the underwriters, par value $0.01. The warrants can be exercised, either in part or in whole, for a period of four years from the time that the closing price of the Company’s shares on the stock market for a period of 30 consecutive trading days shall be at least 130% of the price of the shares at the time of the offering, such that the exercise price of the warrants shall be 30% of the share price at the offering.
|
Based on the Up & Call model, the fair value of each warrant that was granted is NIS 5.20 (approximately $1.19) and the total fair value of the warrants issued to the underwriters is $358 thousand. The parameters used in the valuation were the weekly volatility of 5.15% and a risk-free interest rate of 4%.
I. Dividend Distribution Policy
In the Company’s prospectus published on August 3, 2006, the Company stated that in light of the fact that it had incorporated in the U.S., an agreement had not yet been reached with the clearing-house of the Tel Aviv Stock Exchange (“TASE”) concerning procedures to apply when the Company distributes dividends – specifically as it regards the withholding of tax under U.S. law and required reporting. Therefore, the Company committed not to distribute dividends or any other payment to stockholders until an agreement is formalized with the TASE on this matter. The Company further committed to act to finalize such an agreement as soon as possible. As of the balance sheet date, an agreement has not been finalized.
F-49
Note 10- Taxes on Income
A. Tax laws applicable to the Company
The Parent is taxed under the laws of the United States. The Parent was registered as a foreign tax reporting company in Israel and will be taxed in accordance with the Treaty to Prevent Double Taxation between Israel and the US.
The Subsidiary is taxed under the Israeli Income Tax Ordinance and Income Tax (Inflationary Adjustments) Law - 1985 (the " Inflationary Adjustments Law"). Under the Inflationary Adjustments Law, the Subsidiary's results for tax purposes are measured after adjustment to changes in the Israeli CPI. On February 26, 2008, the Israeli Income Tax Law (Inflationary Adjustments) (Amendment No. 20) (Restriction of Period of Application) – 2008 (“the Amendment”) was passed by the Knesset. According to the Amendment, the Inflationary Adjustments Law will no longer be applicable subsequent to the 2007 tax year, except for certain transitional provisions.
Further, according to the Amendment, commencing with the 2008 tax year, the adjustment of income for the effects of inflation for tax purposes will no longer be calculated. Additionally, depreciation on fixed assets and tax loss carryforwards will no longer be linked to future changes in the CPI subsequent to the 2007 tax year, and the balances that have been linked to the CPI through the end of the 2007 tax year will be used going forward.
B. Tax rates applicable to the Company
1. The Parent
The federal tax rates applicable to the Parent, which is incorporated in the United States, are the (progressive) corporate tax rates of up to 34%.
At December 31, 2009, the Parent had net operating loss (“NOL”) carryforwards in the US amounting to $1,794 thousand, which will expire beginning in 2024 to 2029. The Tax Reform Act of 1986 imposed substantial restrictions on the utilization of NOL and tax credits in the event of an ownership change of a corporation. Thus, in accordance with Internal Revenue Code, Section 382, the Company’s IPO and recent financing activities, may limit the Parent’s ability to utilize its NOL and credit carryforwards although the Parent has not yet determined to what extent.
2. The Subsidiary
The Subsidiary is taxed under the Israeli Income Tax Ordinance and Income Tax (Inflationary Adjustments) Law - 1985 (the " Inflationary Adjustments Law"). Under the Inflationary Adjustments Law, the Subsidiary's results for tax purposes are measured after adjustment to changes in the Israeli CPI. On July 14, 2009, the Israeli Parliament (the Knesset) enacted an amendment to the Income Tax Ordinance (No. 147), the Law for Economic Efficiency, which stipulates, inter alia, that the corporate tax rate will be gradually reduced to the following tax rates: 2009 – 26%; 2010 – 25%; 2011 – 24%; 2012 – 23%, 2013 – 22%, 2014 – 21%, 2015– 20% and 2016 and thereafter – 18%. This amendment did not have a material effect on the financial position of the Company. At December 31, 2009, the Subsidiary had NOL carryforwards in Israel amounting to NIS 51 million (approximately $13 million), which under the current tax law can be carried forward indefinitely.
F-50
Note 10– Taxes on Income (cont'd)
|
|
C.
|
The components of loss (income) before income taxes were:
|
|
Amounts
|
||||||||||||
|
accumulated
|
||||||||||||
|
during
|
||||||||||||
|
development
|
||||||||||||
|
2009
|
2008
|
stage
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
US
|
$ | 4,202 | $ | (1,082 | ) | $ | 4,294 | |||||
|
Non-US
|
2,601 | 4,024 | 12,836 | |||||||||
| $ | 6,803 | $ | 2,942 | $ | 17,130 | |||||||
|
|
D.
|
Income tax expense (benefit)
|
Income tax benefit attributable to income from continuing operations consists of the following:
|
Current
|
Deferred
|
Total
|
||||||||||
|
U.S. dollars in thousands
|
||||||||||||
|
Year ended December 31, 2009:
|
$ | - | $ | - | $ | - | ||||||
|
Year ended December 31, 2008:
|
- | - | - | |||||||||
|
Amounts accumulated during development stage
|
$ | - | $ | - | $ | - | ||||||
Income tax expense (benefit) for the years ended December 31, 2009 and 2008 differed from the amounts computed by applying the U.S. federal income tax rate of 34% to pretax income as a result of the following:
|
Years ended December 31
|
||||
|
Amounts
|
||||
|
accumulated
|
||||
|
during
|
||||
|
development
|
||||
|
2009
|
2008
|
stage
|
||
|
U.S. dollars in thousands
|
||||
|
Computed “expected” tax benefit
|
$(2,313)
|
$(1,000)
|
$(5,825)
|
|
|
Non-deductible stock-based compensation
|
86
|
77
|
509
|
|
|
Fair valuation adjustment of Warrants
|
1,053
|
(552)
|
502
|
|
|
Foreign tax rate differential
|
208
|
282
|
731
|
|
|
Other
|
2
|
6
|
15
|
|
|
Change in valuation allowance
|
964
|
1,187
|
4,068
|
|
|
Actual income tax expense (benefit)
|
$ -
|
$ -
|
$ -
|
|
F-51
Note 10 – Taxes on Income (cont'd)
|
|
E. Deferred tax assets and liabilities
|
The types of temporary differences that give rise to significant portions of the Company’s deferred tax assets and liability are set out as follows:
|
Years ended December 31
|
||||
|
2009
|
2008
|
|||
|
U.S. dollars in thousands
|
||||
|
Deferred tax assets:
|
||||
|
Net operating loss carryforwards
|
$3,042
|
$ 2,113
|
||
|
Deferred research & development charge
|
537
|
612
|
||
|
Stock-based compensation expense
|
-
|
-
|
||
|
Patent costs
|
-
|
-
|
||
|
Liability for accrued vacation pay
|
15
|
17
|
||
|
Total gross deferred tax assets
|
3,594
|
2,742
|
||
|
Valuation allowance
|
(3,584)
|
(2,737)
|
||
|
Total net deferred tax assets
|
10
|
5
|
||
|
Deferred tax liabilities:
|
||||
|
Asset for employee severance benefits, net
|
(10)
|
(5)
|
||
|
Securities income
|
-
|
-
|
||
|
Total gross deferred tax liabilities
|
(10)
|
(5)
|
||
|
Net deferred tax asset
|
$ -
|
$ -
|
||
Because of the Company’s lack of earnings history, management believes that it is more likely than not that the Company will not realize the benefits of these deductible temporary differences. Accordingly, its deferred tax assets have been offset by valuation allowance at December 31, 2009 and 2008. The net change in the total valuation allowance for the years ended December 31, 2009 and 2008, was an decrease of $847 thousand, $956 thousand, respectively, and $3,584 thousand for the development stage period.
F-52
Note 10 - Taxes on Income (cont'd)
|
|
F
|
Accounting for uncertainty in income taxes
|
The Company adopted the provisions of ASC subtopic 740-10 (previously referred to as FIN 48) on January 1, 2007. Adoption of ASC subtopic 740-10 had no material impact on the Company’s consolidated results of operations and financial position. As a result, the Company did not record any cumulative-effect adjustment related to adopting ASC subtopic 740-10.
As of January 1, 2007 (adoption date) and for the 12-month periods ended December 31, 2008 and 2009, the Company did not have any unrecognized tax benefits and thus, no interest and penalties related to unrecognized tax benefits were recognized. The Company records interest and penalties on unrecognized tax benefits, if any, as a component of income tax expense. In addition, the Company does not expect that the amount of unrecognized tax benefits will change substantially within the next 12 months.
The Parent and its Subsidiary file income tax returns in the U.S. federal jurisdictions and non-U.S. jurisdictions. The Parent’s U.S. federal income tax returns are open to examination by the Internal Revenue Service for the tax years beginning in 2004. The Subsidiary's Israeli income tax returns remain subject to examination by the Israeli income tax authorities for the tax years beginning in 2004.
Note 11 – Related Party Transactions
|
December 31,
|
December 31,
|
||
|
2009
|
2008
|
||
|
U.S. dollars in thousands
|
|||
|
Business consulting fees paid to related
|
|||
|
companies
|
$3
|
$71
|
|
As of December 31, 2009, half of the business consulting fees was paid to related companies, $28 thousand is recorded in prepaid expenses (See Note 6).
|
|
B.
|
In May 2006, the Company purchased a Directors and Officers insurance policy for the Company, limited to a liability of five million dollars ($5,000,000) per claim and in aggregate during the term of the policy. The term of the policy is until the May 21, 2010. The annual cost of the premium for the insurance policy is $16 thousand. Officers are not required to pay a deductible, while the deductible for the Company is $10 thousand throughout the world except for the USA and Canada, where the deductible for the Company is $50 thousand ($75 thousand for work-related claims).
|
F-53
Note 11 – Related Party Transactions (cont’d)
|
|
C.
|
In July 2006, the Company approved the granting of letters of indemnification to directors and officers serving at that time and in the future (see Note 8 E).
|
|
|
D.
|
As described in Note 8I, the Company entered into an agreement with Tikcro Technologies Ltd., an investor in the July 2008 private placement transaction, whereby it would receive consultancy services.
|
|
|
E.
|
On May 15, 2008, the Company executed a private placement allocation of 650,000 common shares to Clal Biotechnology Industries Ltd. (“CBI”) as described in Note 9C.
|
|
|
F.
|
On July 30, 2008, the Company carried out private placements with institutional investors (the “Investors”), whereby 3 investors received common stock, non-registered Convertible Notes Payable and Warrants as described in Note 9C.
|
Note 12 - Financial Instruments and Risk Management
A. Concentration of credit risk
Financial instruments that may subject the Company to significant concentrations of credit risk consist mainly of cash and cash equivalents and deposits in respect of employee severance benefits.
Cash and cash equivalents are maintained with major financial institutions in Israel. Deposits in respect of employee severance benefits are maintained with major insurance companies and financial institutions in Israel.
B. Concentrations of business risk
The Company uses materials required for our research and development activities that are currently available from a limited number of sources. The Company believes that it will not experience delays in the supply of critical components in the future. If the Company experiences such delays and there is an insufficient inventory of critical components at that time, the Company's operations and financial results would be adversely affected.
Note 13 – Subsequent Events
|
|
A.
|
At a General Meeting of the shareholders held on January 21, 2010, the shareholders authorized the increase of the authorized capital of the Company to 65,000,000 common shares at a par value of $0.01.
|
|
|
B.
|
On March 7, 2010, following the recommendations of the Compensation and Audit Committees, the Board of Directors appointed current director Mr. Doron Nevo as Chairman of the Board, and approved a compensation package including an allocation of 168,750 options (subject to approval of the TASE) at an exercise price of NIS 3.51 (the 22-day average closing price of the Company's shares on the TASE) (approximately $0.94) which shall vest over twelve calendar quarters, with an acceleration such that if he is removed from the position of Chairman (except for cause) in the first year, a total of 56,250 options will be vested, and if he is removed (except for cause) thereafter, whatever is already vested shall remain so, and an additional 14,063 options shall vest. Additionally, he shall receive a cash payment of NIS 31,700 (approximately $8,458) per year paid quarterly, plus NIS 2,120 (approximately $566) per day in which he participates in meetings of the Board or any committee of which he is a member. He shall also receive a per diem of $750 plus expenses for overseas travel, up to 10 days per year without coordination with the Board.
|
|
|
C.
|
On March 10 and March 11, 2010, the Company received an aggregate investment of NIS 10,590,000 from a number of investors. In return the Company allocated an aggregate amount of 3,590,000 shares, at a price per share of NIS 2.95 (approximately $0.78) and 3,590,000 non-registered warrants (the “Warrants”) to purchase 3,590,000 shares of common stock. The Warrants are exercisable at a price per share of NIS 4.25 (approximately $1.12) for four years. The securities allocated in this private placement form approximately 17.97% of the Company’s common stock after the allocation (approximately 17.92% on a fully-diluted basis). The private placement was approved by the Board of Directors on February 25, 2010. The Company has not yet determined the accounting impact of the investment, which is expected to be material.
|
F-54
PROSPECTUS
BIOCANCELL THERAPEUTICS INC.
Shares of Common Stock
Series 3 warrants
Series 4 warrants
, 2010
No dealer, salesperson or other individual has been authorized to give any information or to make any representations not contained in this prospectus in connection with the offering covered by this prospectus. If given or made, such information or representations must not be relied upon as having been authorized by us. This prospectus does not constitute an offer to sell, or a solicitation of an offer to buy the offered securities in any jurisdiction where, or to any person to whom, it is unlawful to make any such offer or solicitation. Neither the delivery of this prospectus nor any offer or sale made hereunder shall, under any circumstances, create an implication that there has not been any change in the facts set forth in this prospectus or in our affairs since the date hereof.
II-1
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the various expenses payable by us in connection with the sale and distribution of the securities offered in this offering. All of the amounts shown are estimated except for the Securities and Exchange Commission (the “SEC”) registration fee.
|
Securities and Exchange Commission registration fee
|
$
|
729.73
|
||
|
Federal taxes
|
*
|
|||
|
States taxes and fees
|
*
|
|||
|
Trustees’ and transfer agents’ fees
|
*
|
|||
|
Printing and engraving expenses
|
*
|
|||
|
Legal fees and expenses
|
*
|
|||
|
Accounting fees and expenses
|
*
|
|||
|
Miscellaneous expenses
|
*
|
|||
|
Total
|
$
|
729.73
|
||
|
* Information to be provided in an amendment to this registration statement.
|
Item 14. Indemnification of Directors and Officers.
Section 145(a) of the Delaware General Corporation Law provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation), because he or she is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding, if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section 145(b) of the Delaware General Corporation Law provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification shall be made with respect to any claim, issue or matter as to which he or she shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, he or she is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or other adjudicating court shall deem proper.
Section 145(g) of the Delaware General Corporation Law provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation would have the power to indemnify the person against such liability under Section 145 of the Delaware General Corporation Law.
Article XII of our Amended and Restated Certificate of Incorporation (the “Charter”), provides that no director of our company shall be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director, except for liability (1) for any breach of the director’s duty of loyalty to us or our stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) in respect of unlawful dividend payments or stock redemptions or repurchases, or (4) for any transaction from which the director derived an improper personal benefit. In addition, our Charter provides that if the Delaware General Corporation Law is amended to authorize the further elimination or limitation of the liability of directors, then the liability of a director of our company shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
Article XII of the Charter further provides that any repeal or modification of such article by our stockholders will not adversely affect any limitation on the personal liability of a director of our company existing at the time of such repeal or modification.
Section 23 of our Second Amended and Restated By-Laws (the “By-Laws”), provides that each person who was or is a party or is threatened to be made a party to or is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he, or a person of whom he is the legal representative, is or was a director or officer of our company or is or was serving at the request of our company as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans, whether the basis of such proceeding is alleged action or inaction in an official capacity or in any other capacity while serving as director, officer, employee or agent, shall be indemnified and held harmless by us to the fullest extent permitted by the Delaware General Corporation Law, as amended from time to time, against all costs, charges, expenses, liabilities and losses (including attorneys’ fees, judgments, fines, ERISA excise taxes or penalties and amounts paid or to be paid in settlement) reasonably incurred or suffered by such person in connection therewith.
II-2
In addition, Section 23 of the By-Laws provides that the right to indemnification conferred in the By-Laws shall be a contract right and shall include the right to be paid by us the expenses incurred in defending any such proceeding in advance of its final disposition .
We also maintain a general liability insurance policy which covers certain liabilities of directors and officers of our company arising out of claims based on acts or omissions in their capacities as directors or officers.
Item 15. Recent Sales of Unregistered Securities.
Sales of BioCancell Therapeutics Inc. Securities
During the three year period prior to the date of this prospectus, we have issued securities in the transactions described below without registration under the Securities Act:
On May 15, 2008, we issued 650,000 shares of our common stock to Clal Biotechnology Industries Ltd. (“CBI”) at a price of NIS 3.52 (approximately $1.03 at the time of issue) per share, for an aggregate purchase price of NIS 2,288,000 (approximately $669,000 at the time of issue). CBI was an accredited investor at the time of the sale, and the sale was exempt from registration pursuant to Section 4(2) of the Securities Act. CBI beneficially owns more than five percent of our outstanding shares of common stock.
On July 30, 2008, we issued to CBI, Tikcro Technologies Ltd. (“Tikcro”) and Provident Fund of the Hebrew University Ltd. (“Provident”) (i) an aggregate of 1,222,780 shares of our common stock at a purchase price equal to $0.597 per share, for an aggregate purchase price of $730,000, (ii) debentures in an aggregate principal amount of $2,920,000 which are convertible in the aggregate up to 5,058,002 shares of our common stock at a price per share of $0.716, and (iii) warrants to purchase up to 6,280,783 shares of our common stock at an exercise price of $0.716 per share. These securities are described in the section of this prospectus entitled “Description of Securities.” Each of CBI, Tikcro and Provident were accredited investors at the time of sale and each of these sales were exempt from registration pursuant to Section 4(2) of the Securities Act. CBI and Tikcro each beneficially owns more than five percent of our outstanding shares of common stock, and a representative of each of CBI, Tikcro and Provident nominated by such party is a member of the Company’s Board of Directors. The table below details the number or principal amount of securities issued to each of CBI, Tikcro and Provident in these sales.
|
Name of Investor
|
Shares of
Common
Stock Issued
|
Principal
Amount of
Convertible
Debentures
Issued
|
Shares of
Common Stock
Issuable Upon
Exercise of
Warrants
Issued
|
|||||||||
|
CBI
|
335,008
|
$
|
800,000
|
1,720,763
|
||||||||
|
Tikcro
|
837,521
|
$
|
2,000,000
|
4,301,906
|
||||||||
|
Provident
|
50,251
|
$
|
120,000
|
258,114
|
||||||||
An aggregate amount of 1,872,780 shares of our common stock, comprised of the 650,000 and 1,222,780 shares of common stock issued at the above mentioned private placements, was registered for resale under the securities Act in our previously filed Registration Statement on Form S-1 (File No. 333-156252).
II-3
In March 2010, we executed private placements to institutional and individual investors of 4,157,500 shares of our common stock at a price per share of NIS 2.95, and warrants to purchase an additional 4,157,500 common shares, exercisable immediately upon their issuance with a life of four years and an exercise price of NIS 4.25. The aggregate gross proceeds were NIS 12,264,625. These sales were exempt from registration pursuant to Section 4(2) of the Securities Act or Regulation S promulgated thereunder as offshore sales to non-United States persons.
Options Exercised Under Grants Issued Pursuant to BioCancell Equity Incentive Plans
During the three year period prior to the date of this prospectus, unregistered options for 745,577 shares of our common stock granted under the 2004 Stock Option Plan have been exercised. The following list describes information regarding these option exercises:
|
·
|
On June 7, 2007, we issued 178,581 shares of our common stock to Ira Weinstein, who is an employee of ours, 80,000 shares of our common stock to Doron Nevo, who is the Chairman of our Board of Directors, and 71,375 shares of our common stock to Uri Danon, who was an officer of ours, upon exercise of options granted under the 2004 Stock Option Plan at an exercise a price of $0.01 per share. This sale was exempt from registration pursuant to Section 4(2) of the Securities Act or Regulation S promulgated thereunder as offshore sales to non-United States persons.
|
|
·
|
On October 17, 2007, we issued 80,000 shares of our common stock to Gil Bianco, who was a director of ours, and 20,834 shares of our common stock to Doron Amit, who is an employee of ours, upon exercise of options granted under the 2004 Stock Option Plan at an exercise a price of $0.01 and $0.08 per share, respectively. This sale was exempt from registration pursuant to Section 4(2) of the Securities Act or Regulation S promulgated thereunder as offshore sales to non-United States persons.
|
|
·
|
On June 22, 2008, we issued 121,300 shares of our common stock to Abraham Czerniak, who is a consultant of ours, and 6,424 shares of our common stock to Talia Grossman, who was a consultant of ours, upon exercise of options granted under the 2004 Stock Option Plan at an exercise a price of $0.01 per share. This sale was exempt from registration pursuant to Section 4(2) of the Securities Act or Regulation S promulgated thereunder as offshore sales to non-United States persons.
|
|
·
|
On August 19, 2008, we issued 80,000 shares of our common stock to Tuvia Gilat, who was a director of ours, upon exercise of an option granted under the 2004 Stock Option Plan at an exercise a price of $0.01 per share. This sale was exempt from registration pursuant to Section 4(2) of the Securities Act or Regulation S promulgated thereunder as offshore sales to non-United States persons.
|
|
·
|
Between February 22 and April 26, 2010, we issued 107,063 shares of our common stock to Ami Sidi, who is a consultant of ours, upon exercise of an option granted under the 2004 Stock Option Plan at an exercise a price of $0.01 per share. This sale was exempt from registration pursuant to Section 4(2) of the Securities Act or Regulation S promulgated thereunder as offshore sales to non-United States persons.
|
Exercises of Unregistered Warrants
Since January 1, 2004, no unregistered warrants for shares of our common stock have been exercised.
II-4
Item 16. Exhibits and Financial Statement Schedules.
The list of exhibits filed as part of this registration statement is included herein in the Exhibit Index below.
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Jerusalem, Israel, on the 10th day of June, 2010 .
|
|
BIOCANCELL THERAPEUTICS INC.
|
|
|
|
/s/ Uri Danon
Name: Uri Danon
Title: Chief Executive Officer
|
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Uri Danon and Ira Weinstein and each of them, his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities (including his capacity as a director and/or officer of BioCancell Therapeutics, Inc.) to sign any or all amendments (including post-effective amendments) to this Registration Statement and any and all additional registration statements pursuant to rule 462(b) of the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the SEC, granting unto each said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
/s/ Uri Danon
Uri Danon
|
Chief Executive Officer
(Principal Executive Officer)
|
June 10, 2010
|
|
/s/ Ira Weinstein
Ira Weinstein
|
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
June 10, 2010
|
|
/s/ Doron Nevo
Doron Nevo
|
Chairman of the Board of Directors
|
June 11, 2010
|
|
/s/ Abraham Hochberg
Abraham Hochberg
|
Director
|
June 11, 2010
|
|
/s/ Aviv Boim
Aviv Boim
|
Director
|
June 11, 2010
|
|
/s/ Hanoch Rappaport
Hanoch Rappaport
|
Director
|
June 11, 2010
|
|
/s/ David Schlachet
David Schlachet
|
Director
|
June 11, 2010
|
|
/s/ Orly Yarkoni
Orly Yarkoni
|
Director
|
June 11, 2010
|
II-6
EXHIBIT INDEX
|
Exhibit No.
|
Description
|
|
|
3 .1
|
Amended and Restated Certificate of Incorporation of BioCancell Therapeutics Inc.†
|
|
|
3.2
|
Second Amended and Restated Bylaws of BioCancell Therapeutics Inc.†
|
|
|
4.1
|
Specimen Common Stock Certificate†
|
|
|
4.2
|
Form of Series 2 Warrant certificate†
|
|
|
4 .3
|
Form of Convertible Debentures issued July 30, 2008†
|
|
|
4.4
|
Form of Warrants issued July 30, 2008†
|
|
|
4.5
|
Form of Series 3 Warrant !
|
|
|
4.6
|
Form of Series 4 Warrant !
|
|
|
5.1
|
Opinion of Gross, Kleinhendler, Hodak, Halevy, Greenberg & Co.~
|
|
|
9 .1
|
Irrevocable Voting Agreement among Clal Biotechnology Industries Ltd., Tikcro Technologies Ltd., Abraham Hochberg and Avi Barak dated July 30, 2008†
|
|
|
9.2
|
Irrevocable Voting Agreement among Clal Biotechnology Industries Ltd., Abraham Hochberg and Avi Barak dated November 22, 2009 &
|
|
|
10.1 (i)
|
Yissum License†
|
|
|
10.1 (ii)
|
Amendment 1 to Yissum License†
|
|
|
10.1 (ii i)
|
Amendment 2 to Yissum License†
|
|
|
10. 2
|
Supply Agreement with Althea Technologies #%
|
|
|
10. 3
|
Supply Agreement with VGXI USA†
|
|
|
10. 4
|
The BIRD Foundation Grant Agreement†
|
|
|
10.5
|
Agreement for the Performance of Clinical Trials with Wolfson Medical Center†
|
|
|
10.6
|
Agreement for the Performance of Clinical Trials with Meir Medical Center†
|
|
|
10.7
|
Agreement for the Performance of Clinical Trials with BCG Oncology PC†
|
|
|
10.8
|
Employment Agreement with Avi Barak†
|
|
|
10.9
|
Employment Agreement with Ira Weinstein†
|
|
|
10.10
|
|
Employment Agreement with Professor Abraham Hochberg†
|
|
10.11
|
Employment Agreement with Dr. Patricia Ohana†
|
|
|
10.12
|
Consulting Agreement with Moshe Landsberg†
|
|
|
10.13(i)
|
|
BioCancell Therapeutics Inc. 2004 Stock Option Plan†
|
|
10.13(ii)
|
|
Form of Award Agreement†
|
|
10.14(i)
|
BioCancell Therapeutics Inc. 2006 Stock Option Plan (adopted in January 2007 and referred to herein as the BioCancell Therapeutics 2007 Stock Option Plan)†
|
|
|
10.14(ii)
|
Form of Award Agreement†
|
|
|
10.15
|
Translation of Lease Agreement with Beck Tech (Jerusalem) Ltd. and Appendices !
|
|
|
10.16
|
Subscription and Registration Rights Agreement with Clal Biotechnology Industries Ltd. dated March 12, 2008†
|
|
|
10.17
|
Subscription and Registration Rights Agreement with Clal Biotechnology Industries Ltd. dated June 22, 2008†
|
|
|
10.18
|
Subscription and Registration Rights Agreement with Tikcro Technologies Ltd. dated June 22, 2008†
|
|
|
10.19
|
Subscription and Registration Rights Agreement with Provident Fund of the Hebrew University Ltd. dated June 22, 2008†
|
|
|
10.20
|
Agreement for the Performance of Clinical Trials with Medical Research Infrastructure and Health Services Fund by Chaim Sheba Fund*
|
|
|
10.21
|
Agreement for the Performance of Clinical Trials with the Fund of Medical Research Development of Infrastructure and Health, Hillel Yafe Medical Center*
|
|
|
10.22
|
Agreement for the Performance of Clinical Trials with Hadasit Medical Research Services and Development Ltd.*
|
|
|
10.23
|
Agreement for the Performance of Clinical Trials with the Fund for Medical Research Development of Infrastructure and Health Services, Assaf Harofe Fund*
|
|
|
10.24
|
Agreement for the Performance of Clinical Trials with Keren Mechkarim of Bnai Zion Medical Center*
|
|
|
10.25
|
Employment Agreement with Uri Danon^
|
|
|
21.1
|
Subsidiaries of BioCancell Therapeutics Inc.†
|
|
|
23.1
|
Consent of Somekh Chaikin, a member firm of KPMG International
|
|
|
23.2
|
Consent of Gross, Kleinhendler, Hodak, Halevy, Greenberg & Co. (contained in Exhibit 5.1) ~
|
|
|
24.1
|
Power of Attorney (included on signature page hereto)@
|
II-7
|
†
|
Incorporated by reference to the Form S-1, filed by the Company with the Securities and Exchange Commission on December 17, 2008.
|
|
*
|
Incorporated by reference to the Form S-1/A, filed by the Company with the Securities and Exchange Commission on February 2, 2009.
|
|
#
|
Incorporated by reference to the Form S-1/A, filed by the Company with the Securities and Exchange Commission on April 7, 2009.
|
|
|
@
|
Incorporated by reference to the Form S-1, filed by the Company with the Securities and Exchange Commission on September 24, 2009.
|
|
|
^
|
Incorporated by reference to the Form 8-K, filed by the Company with the Securities and Exchange Commission on October 21, 2009.
|
|
|
!
|
Incorporated by reference to the Form S-1/A, filed by the Company with the Securities and Exchange Commission on October 21, 2009.
|
|
&
|
Incorporated by reference to Post-Effective Amendment to Form S-1, filed by the Company with the Securities and Exchange Commission on April 26, 2010.
|
|
|
~
|
To be included in a future amendment to this filing.
|
|
|
%
|
Certain confidential portions of this exhibit were omitted and provided separately to the Securities and Exchange Commission pursuant to a request for confidential treatment.
|
II-8
