Attached files
| file | filename |
|---|---|
| 8-K - DELCATH SYSTEMS, INC. | form8-k.htm |
Exhibit 99.1

Investor Presentation
June 2010
NASDAQ: DCTH

2
Forward-looking Statements
This presentation contains forward-looking statements, within the meaning
of federal securities laws, related to future events and future financial
performance. Many of these statements involve known and unknown risks
and uncertainties, which could cause actual results to differ materially from
expected results, performance or achievements expressed or implied by
statements made herein. These risks are described in the prospectus
supplement, the accompanying prospectus, and the other documents
incorporated by reference therein that Delcath files with the Securities and
Exchange Commission including the 2009 Annual Report on Form 10-K and
in Delcath’s Quarterly Reports on Form 10-Q. All of Delcath’s plans and
objectives made in this presentation are based upon management’s current
expectations, but many such expectations are based upon economic,
clinical and regulatory uncertainties, and thus, may differ materially from
actual results.
of federal securities laws, related to future events and future financial
performance. Many of these statements involve known and unknown risks
and uncertainties, which could cause actual results to differ materially from
expected results, performance or achievements expressed or implied by
statements made herein. These risks are described in the prospectus
supplement, the accompanying prospectus, and the other documents
incorporated by reference therein that Delcath files with the Securities and
Exchange Commission including the 2009 Annual Report on Form 10-K and
in Delcath’s Quarterly Reports on Form 10-Q. All of Delcath’s plans and
objectives made in this presentation are based upon management’s current
expectations, but many such expectations are based upon economic,
clinical and regulatory uncertainties, and thus, may differ materially from
actual results.
3
Company Highlights
§ The Delcath Chemosaturation System (PHP) is a proprietary system delivering
ultra-high chemotherapy doses to the liver with manageable systemic toxicities
ultra-high chemotherapy doses to the liver with manageable systemic toxicities
§ Minimally invasive approach to targeted, regional cancer therapy that combines
a existing drug and straightforward delivery system
a existing drug and straightforward delivery system
§ Successful Phase III trial results reported
§ FDA submission process underway
§ Platform technology with potential use in a range of organs for both cancer
and infectious disease, including HCV and HBV
and infectious disease, including HCV and HBV
§ Large, unmet market opportunity: 2.6 million liver cancer patients worldwide
§ Issued patents, orphan drug designations present competitive advantages
§ Deep and experienced management team
Establishing a New Paradigm in Cancer Therapy

4
Significant Combination Product Approval and Commercialization Experience
Deep and Experienced Management Team

5
§ Built new leadership team with in-depth drug and device product and
commercialization experience
commercialization experience
§ Established manufacturing facility
§ Reported successful Phase III Trial results
§ Increased enrollment momentum in separate Phase II clinical trial
§ Improved visibility in medical community
§ Raised over $35 million in equity capital
§ Positioned for 2011 commercialization
The New Delcath: Executing a Sustainable Growth Strategy
Recent Accomplishments

6
Spectrum of Liver Cancer Treatments
Existing Treatments Involves Significant Limitations

7
IHP: Proof of Concept, but High Morbidity and Non-Repeatable
Open Surgical IHP

8
Advantages of Chemosaturation
§ ISOLATION
§ Treats entire liver
§ SATURATION
§ Allows for ~ 100x effective
dose escalation of drug
agents at tumor site
dose escalation of drug
agents at tumor site
§ FILTRATION
§ Controls systemic
toxicities
toxicities
The Delcath Chemosaturation System™
Note: Image not to scale.
Converts Traumatic Open Surgery to Minimally Invasive, Repeatable Procedure

9
Melphalan
§ Well understood, dose dependant, tumor preferential, alkylating cytotoxic
agent that demonstrates no hepatic toxicity
agent that demonstrates no hepatic toxicity
§ Manageable systemic toxicities associated with Neutropenia and Cytopenia
§ Drug dosing over 10x higher than FDA-approved dose via systemic IV
chemotherapy
chemotherapy
§ Dose delivered to tumor is approx. 100x higher than that of systemic IV
chemotherapy
chemotherapy
The Drug of Choice in Liver Cancer Therapy

10
Clinical Development Program Phase I Phase II Phase III
Phase III
Melanoma Metastases
(CS melphalan vs. BAC)
Primary Liver Cancer (OS)
(CS doxorubicin vs. Nexavar™)*
Phase II
Neuroendocrine Metastases
(melphalan)
Primary Liver Cancer
(melphalan)
Adenocarcinoma Metastases
(melphalan)
Melanoma Metastases
(melphalan)
Currently Approved Clinical Trials
* Approved under prior IDE, but inactive.

11
Phase I Trial - Proof of Concept (2005)
Phase I Ocular Melanoma Patients
§ 11 evaluable patients
Safety data - Phase I Trial (all patients)
§ Maximum Dose - 3.5 mg/kg
• Grade IV toxicities observed
§ Optimal Dose - 3.0 mg/kg
• Manageable hematological toxicities - Neutropenia & Cytopenia
Tolerability Data
§ Side effect profile approximates standard melphalan (0.25 mg/kg)
Encouraging Early Clinical Data

12
Phase III Clinical Trial Highlights
§ NCI-led pivotal study
§ Granted Fast Track review designation
§ Special Protocol Assessment (SPA) with FDA to support NDA (505(b)(2))
regulatory pathway (SPA reviewed and validated by outside counsel)
regulatory pathway (SPA reviewed and validated by outside counsel)
§ Randomized, 92 patient multi-center trial (93 enrolled)
§ Top line data released April 21, 2010
§ Complete trial investigators data set presented at ASCO on June 5, 2010
(blinded independent core lab top line data released April 21, 2010)
(blinded independent core lab top line data released April 21, 2010)
93-Patient Randomized, Non-blinded, Multi-center, NCI-Led Study

13
Phase III Clinical Trial Design
Randomized to CS
92 patients: ocular
or cutaneous melanoma
CS/Melphalan
Treat every 4 weeks x 4 rounds
(responders can receive up to 6 rounds)
Cross-over
Primary Trial Endpoint
§ Statistically significant differene in Hepatic
Progression Free Survival (“hPFS”): p < 0.05
Progression Free Survival (“hPFS”): p < 0.05
§ Over 80% of Oncologic drugs approved by FDA
2005 - 2007 on endpoints other than overall
survival*
2005 - 2007 on endpoints other than overall
survival*
Modeled hPFS for Trial Success:
7.73 months (CS)
vs.
4 months (BAC)
Secondary Trial Endpoints
§ Hepatic response and duration of hepatic response
§ Overall response and duration of overall response
§ Overall Survival - Diluted by Cross Over
§ SAP calls for analysis of various patient cohorts
Fully Powered with Well-defined and FDA Accepted Endpoints
Pre-CS (Baseline)
Post-CS (22+ Months)
Hepatic Response - Metastatic Melanoma

14
Phase III Clinical Trial Results (ASCO)
§ Trial results exceed primary endpoint expectations; p value = 0.001
§ Treatment arm shows 5x median hPFS compared to control arm
§ CS/PHP median hPFS of 245 days compared to 49 days for BAC
§ Hazard Ratio = .301
§ Patients failed prior therapies (radiation, chemo, immuno, image guided
local)
local)
§ 90% Ocular, 10% Cutaneous - No difference in response
§ Overall PFS 186 vs. 46 days for BAC
§ 34% response rate for CS/PHP compared to 2% for BAC
§ 52% stable disease for CS/PHP compared to 27% for BAC
§ 86% overall clinical benefit (CR + PR + SD)
We are pleased with the Trial Results

15
Phase III Clinical Trial Results (ASCO)
§ Majority of BAC patients crossed over and obtained similar response from treatment
§ OS Secondary endpoint - No difference (due to cross over treatment response)
§ OS cohort analysis - all positive trends
§ Median survival of 298 days for treatment arm compared to 124 in non-crossover BAC patients
§ Median survival of 398 days for BAC Cross Over patients vs. 124 non-crossover BAC patients
§ Safety profile as expected - in line with current FDA approved labeling for IV administration of
Melphalan and Phase I CS/PHP study results
Melphalan and Phase I CS/PHP study results
§ Treatment related Deaths:
§ 3/40 patients (7.5%) 3/116 procedures (2.6%)
§ Neutropenic Sepsis (n=2) 5%, Hepatic Failure (n=1) 2.5% (95% tumor burden)
§ Current approved labeling for Melphalan - 3% to 10% mortality rate.
§ Instituting REMS (Risk Evaluation & Mitigation Strategy) to address proper management
associated with safe use.
associated with safe use.
We are very pleased with the Trial Results

16
Phase III Trial Review
§ Conducted under valid SPA
§ PFS primary endpoint often used for FDA
approval* (OS used < 20%)
approval* (OS used < 20%)
§ Primary endpoint exceeded
§ Secondary endpoints supports results
§ OS cohort analysis - favorable
§ Safety profile - expected and in accordance with
currently approved labeling for melphalan
currently approved labeling for melphalan
Very Successful Trial
*“Review of Oncology and Hematology Drug Product Approvals at the US Food and Drug Administration Between July 2005 and December 2007”,
Sridhara, Johnson, Justice, Keegan, Chakravarty, Pazdur; Journal of the National Cancer Institute; January 29, 2010
Sridhara, Johnson, Justice, Keegan, Chakravarty, Pazdur; Journal of the National Cancer Institute; January 29, 2010
17
Phase III Trial “Questions”
§ Do we have a valid SPA?
§ FDA letter dated February 9, 2006 - SPA deemed acceptable
§ SPA reviewed and deemed valid in written opinion of outside,
independent counsel
independent counsel
§ “No statistically significant overall survival benefit demonstrated”?
§ Trial design required by FDA provided for OS secondary endpoint
with a cross over provision which yielded expected results
with a cross over provision which yielded expected results
§ SAP calls for analysis of OS trends in patient cohorts - all positive
§ Is hPFS as a primary endpoint enough for potential FDA approval?
§ YES - PFS primary endpoint very often used for FDA approval*
§ SPA requires hPFS as the primary endpoint
§ YES
Phase III trial achieved all of its pre-determined goals

18
Phase III Trial “Questions”
§ Primary endpoint exceeded?
§ YES
§ Secondary endpoints supportive?
§ YES
§ Safety profile?
§ Expected and in accordance with currently approved melphalan
§ What indication will FDA potentially approve?
§ March 9th Pre-NDA meeting with FDA - Phase III and other data
presented and deemed acceptable by FDA for NDA application for
the indication “Treatment of metastatic melanoma to the liver,
ocular or cutaneous”
presented and deemed acceptable by FDA for NDA application for
the indication “Treatment of metastatic melanoma to the liver,
ocular or cutaneous”
§ No treatment result differences between ocular and cutaneous.
§ No requirement under SPA for enrollment minimum of either
ocular or cutaneous melanoma.
ocular or cutaneous melanoma.
Phase III trial achieved all of its pre-determined goals

19
Leading Clinical Sites & Investigators
10-Center Trial Run by Leading Cancer Surgeons and Medical Oncologists
National Cancer Institute (Bethesda, MD) — Marybeth S. Hughes, M.D., FACS
University of Pittsburgh Medical Center (Pittsburgh, PA) — James F. Pingpank, Jr., M.D., FACS
University of Maryland Medical Center (Baltimore, MD) — H. Richard Alexander, Jr., M.D., FACS
Moffitt Cancer Center (Tampa, FL) — Jonathan S. Zager, M.D., FACS
§ John Wayne Cancer Institute (Santa Monica, CA) — Mark Faries, M.D., FACS
§ University of Texas Medical Branch (Galveston, TX) — Orhan S. Ozkan, M.D.
§ Swedish Medical Center (Denver, CO) — Charles Nutting, D.O., FSIR
§ St. Luke’s Cancer Center (Bethlehem, PA) — Sanjiv S. Agarwala, M.D.
§ Albany Medical Center (Albany, NY) — Gary P. Siskin, M.D., FSIR
§ Morristown Memorial Hospital (Morristown, NJ) — Eric D. Whitman, M.D., FACS

20
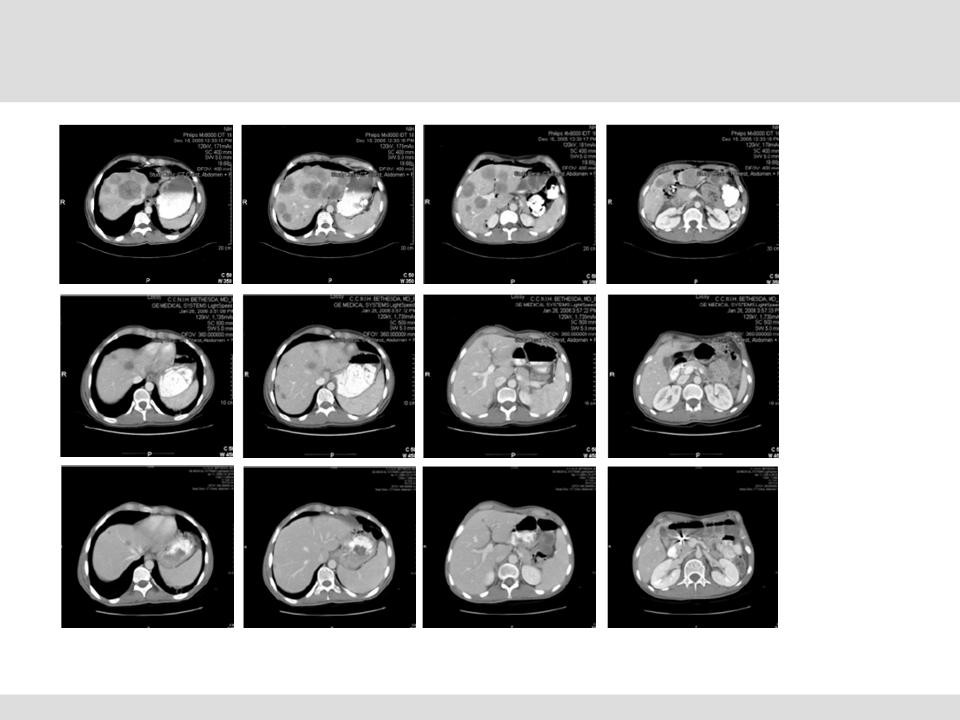
Phase I/II NCI Trials - Neuroendocrine
Neuroendocrine Tumor Trial Results (n=23)*
Promising Response Rate in Potentially Large Market
Pre-CS
(Baseline)
Post-CS #2
(+4 Months)
Post-CS #1
(+6 Weeks)
*Presentation at American Hepato-Pancreo-Biliary Association 2008 annual meeting
21
Phase I/II NCI Trials - Neuroendocrine
Pre-CS
(Baseline)
Post-CS
Treatment #2
(+4 Months)
Post-CS
Treatment #1
(+6 Weeks)

22
Intellectual Property
Patent Protection
§ 7 issued U.S. patents, 9 foreign counterparts granted and 5 pending
§ Primary device patent set to expire August 2016
§ Portfolio protection extends through 2023
§ Post FDA approval up to 5 years of patent extension available
FDA Protection
§ Orphan Drug Designation granted for melphalan in the treatment of ocular
melanoma, cutaneous melanoma and metastatic neuroendocrine tumors, as well as
for doxorubicin in the treatment of HCC
melanoma, cutaneous melanoma and metastatic neuroendocrine tumors, as well as
for doxorubicin in the treatment of HCC
§ Additional Orphan Drug applications to be filed for other drugs and indications,
including HCC and CRC
including HCC and CRC
Multiple Levels of Protection

23
Initial U.S. Liver Cancer Market Potential
$6.8 Billion Maximum Potential U.S. Market for Above Five Diseases
* Assumes 2.5 PHP treatments per patient at an ASP of $20,000.
§ On label annual market estimated over $775 Million in U.S. alone
§ Less than 10% of global liver cancer cases in U.S.
§ Less than 10% of liver cancer patients qualify for surgery, currently
the “gold standard” treatment option,
the “gold standard” treatment option,
§ 90% of liver cancer patients have few, if any viable treatment options
§ Majority of all end stage cancer patients die of liver failure

24
Commercialization “Questions”
§ Not an IV or pill…will clinicians embrace CS procedure?
§ Liver cancer patients have typically failed all Medical Oncology
treatments and are sent to the Surgeon for management.
Surgeons can only resect 10%, so patients currently have few, if
any options. Surgeons, Oncologists and Interventional
Radiologists have expressed excitement at the prospects of CS as
a new procedure for their patients with unresectable liver mets.
treatments and are sent to the Surgeon for management.
Surgeons can only resect 10%, so patients currently have few, if
any options. Surgeons, Oncologists and Interventional
Radiologists have expressed excitement at the prospects of CS as
a new procedure for their patients with unresectable liver mets.
§ Will clinicians use system “off label”?
§ Although we would never promote off label use, clinicians have
stated that they will utilize CS for CR, HCC, NET, patients once it is
approved for Melanoma Liver Mets as there are few, if any viable
options for those patients
stated that they will utilize CS for CR, HCC, NET, patients once it is
approved for Melanoma Liver Mets as there are few, if any viable
options for those patients
§ How will the procedure be reimbursed?
§ Still a work in progress
§ Coverage has been approved by a private payer for compassionate
use of CS for sarcoma mets to the liver
use of CS for sarcoma mets to the liver

25
Three-Pronged Business Strategy
Commercialization
§ Gain regulatory approval
• Goal: receive CE approval by mid 2011
• Goal: receive FDA approval by mid 2011
§ Build out direct specialty sales force for U.S.
§ Direct and Distribution partners OUS
Pursue Asian Strategic Alliances
§ Invest and develop markets for China, Korea and Japan
§ Chi-Fu Trading Company Ltd. signed 2/9/2010 for Taiwan
Establish U.S. and EU Pharma Alliances
§ Co-develop and fund additional indications for Delcath Chemosaturation
System™
System™
Invest In and Develop a World Class Oncology Company
26
Partnering Opportunities
§ HCC — survival trial - CS doxorubicin vs. sorafenib
§ HCC — survival trial - CS melphalan vs. sorafenib
§ Neuroendocrine Liver Mets — hPFS trial with melphalan
§ Colorectal Liver Mets — hPFS trial CS melphalan vs. BAC
§ Colorectal Liver Mets — hPFS trial CS irinotecan/oxaliplatin vs.
BAC
BAC
§ HCV and HBV — initiate testing of high dose interferon/anti-virals
in Asia
in Asia
§ Develop Systems for Other Organs — lung, brain, pelvis, limbs,
others
others
Strategic Alliances Will Help Drive Development in Additional Indications

27
Financials

28
Financial Summary
Financial & Operating Overview
§ Follow On Offering: Raised ~ $35 million in 2009
§ Burn Rate: Approximately $1.8 million/month
§ Cash: ~$29.6 million at May 31, 2010
§ Debt: None
§ Shares Out: 37.3 million (43.9 million fully diluted*)
§ Institutional Ownership: ~ 28% at December 31, 2009
§ Market Capitalization: ~ $580 million as of May 31, 2010
* Fully diluted includes an additional 3.55 million options at $4.40, 2.79 million warrants at $3.53, and 257,910 unvested restricted shares.
Capital Structure Strengthened Significantly in 2009

29
Company Highlights
§ The Delcath Chemosaturation System is a proprietary system delivering ultra-
high chemotherapy doses to the liver with manageable systemic toxicities
high chemotherapy doses to the liver with manageable systemic toxicities
§ Minimally invasive approach to targeted, regional cancer therapy that combines
a existing drug and straightforward delivery system
a existing drug and straightforward delivery system
§ Successful Phase III trial results reported
§ FDA submission process underway
§ Platform technology with potential use in a range of organs for both cancer and
infectious disease, including HCV and HBV
infectious disease, including HCV and HBV
§ Large, unmet market opportunity: 2.6 million liver cancer patients worldwide
§ Issued patents, orphan drug designations present competitive advantages
§ Deep and experienced management team
Establishing a New Paradigm in Cancer Therapy

30
Investor Presentation
June 2010
NASDAQ: DCTH
