Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CAMBRIDGE HEART INC | d8k.htm |
 Investor
Presentation June 7, 2010
Exhibit 99.1 |
 Forward Looking
Statements 2
Statements in this presentation that are not purely historical are forward-looking statements within
the meaning of the Private Securities Litigation Reform Act of 1995 including, but not
limited to, statements about existing and potential partnerships and distribution
arrangements that Cambridge Heart, Inc. (the “Company”) is, or may become party to,
statements about the Company’s expected launch of the MTWA Module by Cardiac Sciences
Corporation, future operating results, the Company’s belief that the Company’s existing
cash resources are sufficient to fund operations through March 31, 2011, the Company’s
expectations and estimates regarding MTWA Module metrics (i.e., MTWA Module and sensor sales
prices, gross margins and sensor utilization), and product development plans. These
forward-looking statements are based on current expectations and assumptions that are
subject to risks and uncertainties. Actual results could differ materially from the
results expressed or implied in these forward-looking statements. Material deviations
from our current operating plan, including a delay in launching the MTWA Module, lower than
expected sales of the MTWA Module, lower than expected sales of the Heartwave II System, lower than
expected sales prices or gross margins for the MTWA Module and lower than expected sensor utilization by
purchasers of the MTWA Module, may cause or contribute to such differences. Other factors
that may cause or contributed to such differences include failure to achieve broad market
acceptance of the Company’s MTWA technology, failure of our sales and marketing
organization or partners to market our products effectively, inability to hire and retain
qualified clinical applications specialists in the Company's target markets, failure to
obtain or maintain adequate levels of first-party reimbursement for use of the Company's MTWA
test, customer delays in making final buying decisions, decreased demand for the Company's
products, failure to obtain funding necessary to develop or enhance our technology, adverse results in
future clinical studies of our technology, failure to obtain or maintain patent protection for
our technology and overall economic and market conditions. Many of these factors are
more fully discussed, as are other factors, in Part I, Item 1A. “Risk Factors” of
the Company’s Annual Report on Form 10 K for the fiscal year ended December 31, 2009,
which is on file with the SEC and available at www.EDGAR.com. In addition, any
forward-looking statements represent our estimates only as of today and should not be relied upon as
representing our estimates as of any subsequent date. While the Company may elect to update
forward- looking statements at some point in the future, the Company specifically disclaims
any obligation to do so except as may be legally necessary, even if the Company’s estimates
should change. |
 Corporate
Mandates Impact the number of deaths from sudden
cardiac arrest (SCA) from today’s 300,000 –
400,000 annual incidents
Make MTWA testing a standard annual
diagnostic assessment of ~12 million high-risk
cardiac patients
Leverage MTWA core technology into new,
broader-based diagnostic cardiac applications
3 |
 The
Opportunity New business model, major risks eliminated, attractive
valuation
Key components for success in place
–
Large addressable market of ~ 12 million high risk cardiac patients
–
FDA Clearance
–
CMS and private reimbursement in place
–
Significant IP and clinical validation
–
New marketing partnership set to commence
Recurring revenue stream builds shareholder value
–
Razor/razor-blade model
–
High-margin disposables
4 |
 Overview &
Milestones 1980’s: Early research at MIT
1990: CHI was founded
1994: Landmark publication in NEJM
1996: Initial public offering (CAMH)
1996: First 510(k) & CH2000 launch
1999: Regulatory study completed & received FDA labeling claims
2000: HearTwave
I launch
2005: HearTwave
II launch
2006:
NCD
by
CMS,
Class
II-a
Guideline,
Coverage
by
many
private
payers
2007 –
2008: Co-marketing agreement with SJM
2009:
Agreement
with
Cardiac
Science
Corporation
to
develop
and
market
an Alternans module for integration into existing stress platform
2010: MTWA OEM Module 510(k)
5 |
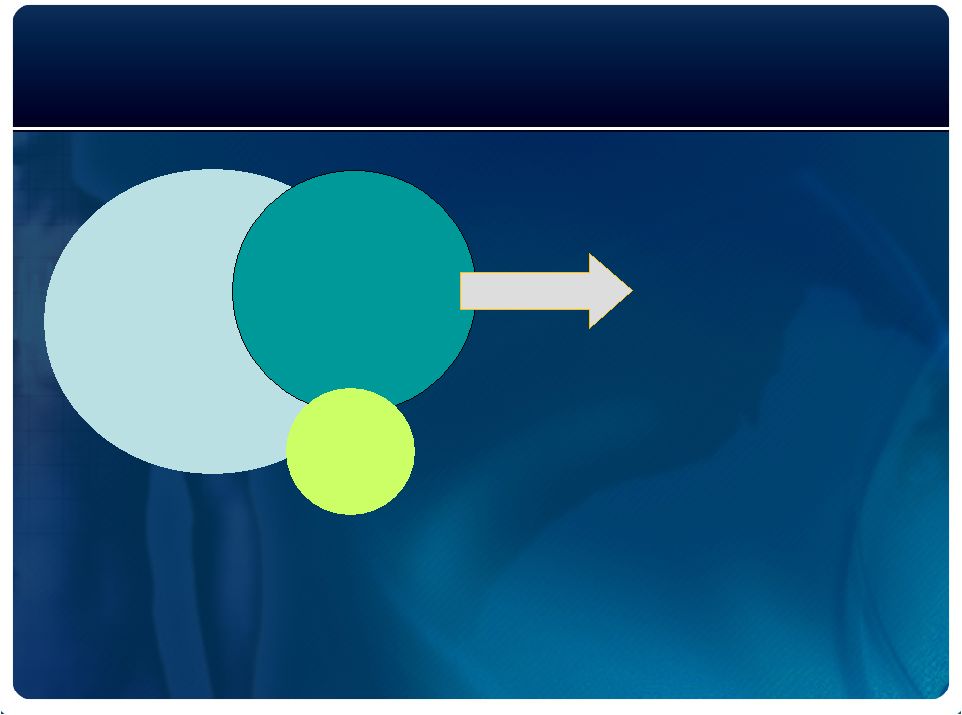 High SCA Risk
Population: Large US Market Opportunity
5 million
Heart Failure
1-2 million
other
8 million
Post MI
~ 12 million
high-risk
individuals
Circulation 2/10/2006 Heart Disease and Stroke Statistics
300,000 –
400,000 deaths per year
6 |
 Microvolt
T-Wave Alternans (MTWA) More effective at
identifying at-risk SCA
patients than invasive
electrophysiology (EP) test
Extraordinary negative
predictive value (NPV)
Ensure delivery of
appropriate therapy
HearTwave®
II
7 |
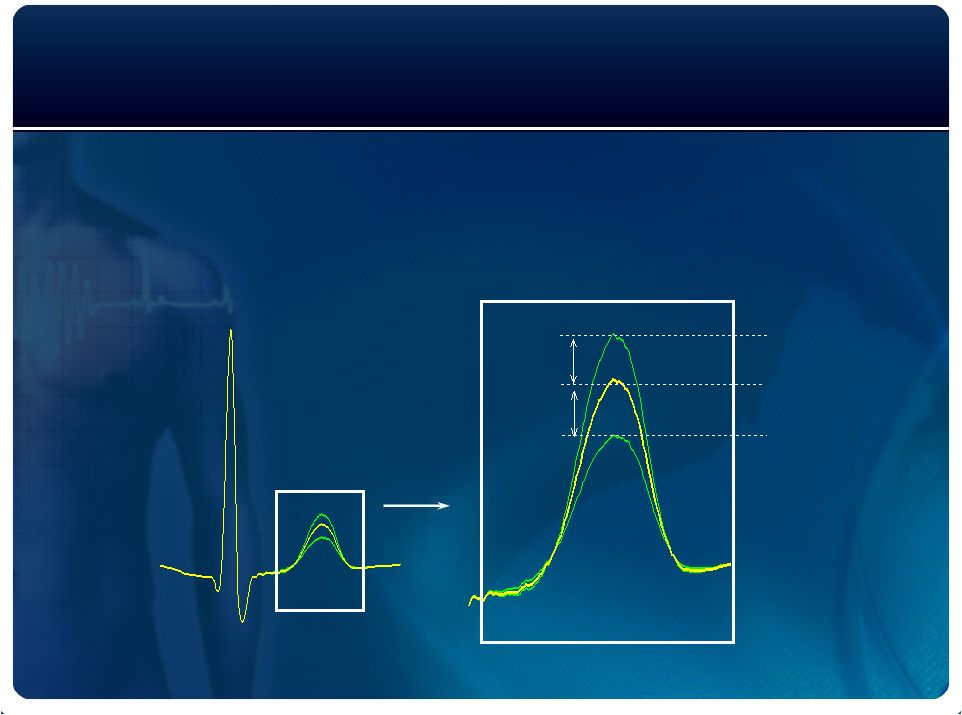 The
Technology MTWA
Analytic Spectral Method
13 patents protect all components of the technology
Even Beats
Odd Beats
Mean
V
alt
V
alt
Q
R
S
T
8 |
 Clinical
Validation 200+ publications in peer-reviewed journals
Large prospective clinical trials
–
ALPHA Study -
Boston Scientific
–
ABCD Study –
St. Jude Medical
–
MASTER Study --
Medtronic
–
Ikeda Study
–
Meta
Analysis
–
Hahnloser
(March
2009
HRS
Supplement)
Applicable patient populations
–
Primary
prevention
(ejection
fraction
35%)
–
Post MI
–
Congestive heart failure
–
Syncope
–
Dilated cardiomyopathy
9 |
 Class IIa
Guideline
American College of Cardiology/American Heart Assoc./European Society of
Cardiology 2006 Guideline for Management of Patients With Ventricular
Arrhythmias and the Prevention of Sudden Cardiac Death (Developed in
collaboration with the HRS and EHRA)
–
Class IIa
It
is
reasonable
to
use
T-Wave
Alternans
for
improving
the
diagnosis
and
risk
stratification
of
patients with ventricular arrhythmias or who are
at risk for developing life-threatening ventricular
arrhythmias. (Level of Evidence: A)
10 |
 CMS National
Coverage “CMS (Medicare) has determined that there is
sufficient evidence to conclude that Microvolt T-
wave Alternans (MTWA) diagnostic testing is
reasonable and necessary
for the evaluation of
patients at risk of sudden cardiac death, only
when the spectral analytic method*
is
used, and CMS is issuing the following national
coverage determination (NCD) for this
indication.”
93025 (3/21/2006)
* Cambridge Heart US Patent # 6,735,466 granted May 2004
11 |
 Medicare
Reimbursement National Average: $196
–
Reimbursement is limited if MTWA is performed at
the same time as standard stress, stress echo or
nuclear stress testing
–
Negatively impacts physician’s ability to integrate
MTWA testing for high-risk cardiac
patients
CHI has requested that CMS remove the current
limitations
–
A positive outcome would provide full reimbursement
for both stress and MTWA tests during the same
patient visit
12 |
 Private
Coverage Private payers followed CMS:
–
Aetna, Cigna, Humana
–
HCSC, BC/BS of NY, Horizon (NJ), Care First, MI, IA, SD,
MN, KS, Wellmark, Premera, BCBS of AZ
–
Harvard Pilgirim
–
Regionals: GHI, Tufts, Med. Mutual of Ohio, Health Net
Coverage typically for ICD indicated population
Other private payers in progress
13 |
 Initial Market
Positioning 14
Historical clinical positioning and marketing
linked MTWA testing to ICD therapy
That positioning limited the market opportunity
and perception of MTWA testing
–
300,000 to 1 million primary prevention patients
–
Focus on EP community and testing of border line patients
–
Entanglement in clinical disagreements on ICD therapy
Additional Challenges
–
Relatively expensive, stand-alone MTWA equipment platform
–
Single product offering and inability to offer a complete
“solution”;
–
Practice integration issues
–
Lack of distribution
–
Economic environment for medical capital equipment |
 New Strategic
Direction Position MTWA as a tool to
“assess” and “manage
” an individual’s risk of SCA in the high risk
population
Make MTWA more accessible to the high risk
population
–
Develop multiple product embodiments and industry partnerships
–
Create an attractive physician ROI
–
Simplify practice integration
Re-organize resources on the primary corporate
value driver (i.e. sensor utilization)
15 |
 SCA Risk
Management in Preserved EF Population
Non-device interventions for reducing SCA risk
Adapted from Myerburg, NEJM 2009; 360(9):938. |
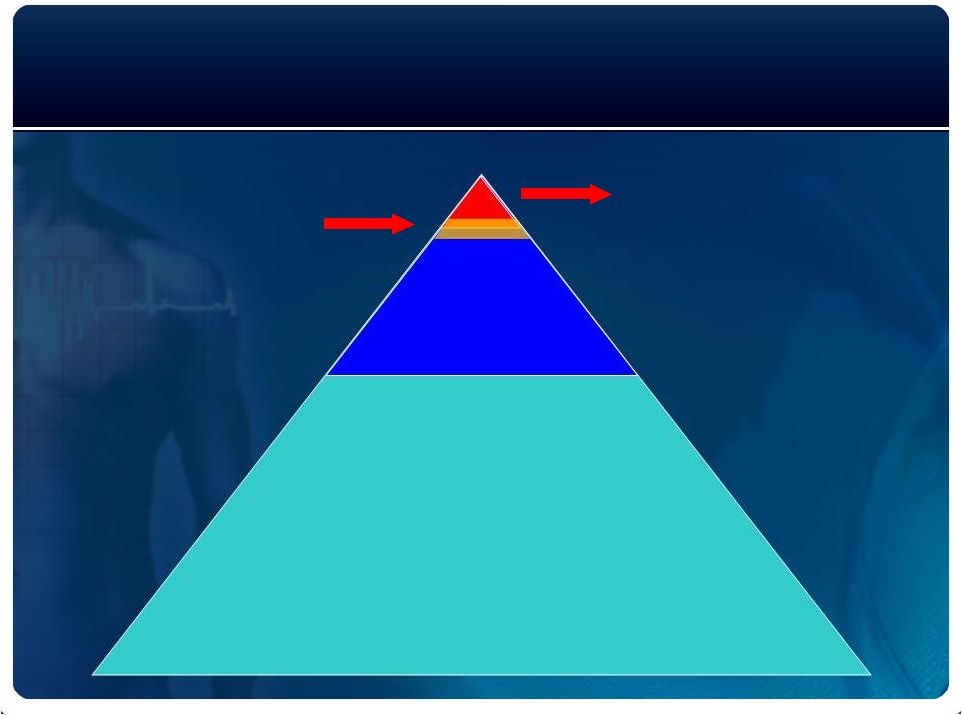 300,000 –
1,000,000 primary
prevention population
Reluctant / borderline
Patients
New Strategic Direction
~ 12 million
post MI, HF
and other high
risk patients
~ 40,000,000
Cardiac stress tests per year |
 OEM Alternans
Module Taps into existing market for stress systems
–
Approximately 4,000 units sold per year (1)
with
an estimated
installed base of 35,000. 40 million tests performed per year.
–
Key players: CSC (39%), GE (30%), Welch Allyn (6%) Schiller
(5%), Mortara
& Philips (4% combined)
(1)
Materially improves physician ROI
–
Reduces technology acquisition costs
–
Maximizes reimbursement
Significantly simplifies practice integration
–
Simplified training
–
Simplified EMR connectivity
(1) Source: Frost & Sullivan, 2009 Report. US market data only.
18 |
 First
Partnership Signed Cardiac Science: US stress market leader
–
5 year, non-exclusive, worldwide
–
Partner will market and sell modules and sensors (CHI to drive
utilization)
•
Module end-user pricing in-line with other stress upgrades/options ($5-$8k).
•
End-user sensor pricing in-line with current CHI of $64 to $84 per sensor.
–
Partner will sell directly to the end-user and retain a portion of the
revenue.
Module will be offered as an upgrade to:
–
Partner’s existing installed base of stress systems
–
Partner’s new system sales
Product Launch –
Q3 2010
–
Module development (CHI & CSC) –
Completed in February 2010
–
Regulatory requirements –
510(k) received in April 2010
–
Marketing & sales training –
On-going
19 |
 2010 Strategic
Initiatives MTWA Module –
Other Stress Platforms
–
CHI’s
current development work is portable to other manufacturer’s cardiac
stress platforms
MTWA Module –
Other embodiments
–
Ambulatory Holter
platform
–
Event recording services
–
Pacemaker programmer
Heartwave II System
–
Independent/corporate distribution networks
•
Geography not covered by CHI’s
existing direct sales force.
20 |
 2010 Strategic
Initiatives Ischemia Application
–
Data suggest MTWA can identify ischemia missed by current tests
–
Pilot study to begin in July 2010
•
PI identified
•
Protocol completed
•
IRB approval in progress
MTWA and Pharmacological Agents
–
Drug development phase
–
Resuscitation of failed drugs
–
Individualized medicine
21 |
 The Market
Opportunity Disposable sensor gross margin in excess of 70%
COGS reductions opportunity based on volume
10% Penetration of ~12 million high risk patients:
$60 Million in annual recurring revenue from the sale of
proprietary sensors
25% Penetration of ~12 million high risk patients:
$150 Million in annual recurring revenue from the sale of
proprietary sensors
22 |
 MTWA Module
Metrics Upfront Module Revenue
–
$1,000 -
$5,000 (platform/channel dependant)
–
70% -
90% gross margin
Recurring Sensor Revenue
–
$35 -
$70 per MTWA test (platform/channel dependant)
–
+70% gross margin
Possible Range of Sensor Utilization/System
–
70 to 140 sensors/system/year
23 |
 Cash Flow
Summary Current Quarterly Revenue -
$800,000 +
20%
–
U.S. HearTwave
Systems
–
U.S. Sensor revenue
–
All other (includes service and ROW sales)
Quarterly Cash Expenditures -
$1,900,000
–
Largely fixed cash expenditures except commissions and minimal COGS
Quarterly Cash Burn -
$1,100,000 plus CAPEX
Cash at March 31, 2010 –
$2.2 million
–
Coupled with proceeds of $2 mm from exercise of Series D Warrants, the
Company believes is sufficient to fund operations through Q1 2011
24 |
 Capital
Structure Series C-1 Convertible Preferred (SJM Mar 2007)
–
$12.5 million junior liquidation preference
–
Converts into 4.2 million common shares at $2.99
Series D Convertible Preferred (Dec 2009)
–
Purchased by directors and existing individual and institutional
shareholders
–
$1.8 million senior liquidation preference
–
Converts into 22.6 million common shares at $0.082
–
Short-term warrants to purchase 11 million common shares at
$0.107 (expires 12/31/10); and long-term warrants to purchase 7
million common shares at $0.142 (exercised as of 6/4/10)
Capital Structure (on as converted and exercised basis)
–
111 million common shares issued and outstanding
–
9 million employee/director options (7 mm at $0.16 exercise price)
25 |
 The
Opportunity New business model, major risks eliminated, low valuation
Key components for success in place
–
Large addressable market of ~ 12 million high risk cardiac patients
–
FDA Clearance
–
CMS and private reimbursement in place
–
Significant IP and clinical validation
–
New marketing partnership set to commence
Recurring revenue stream builds shareholder value
–
Razor/razor-blade model
–
High-margin disposables
26 |
 Investor
Presentation June 7, 2010 |
