Attached files
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended March 31, 2010
|
|
|
or
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from to
|
Commission file number: 000-51323
Micrus Endovascular Corporation
(Exact name of registrant as specified in its charter)
|
Delaware
|
23-2853441
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
821 Fox Lane
|
95131
|
|
San Jose, California
(Address of principal executive offices)
|
(Zip Code)
|
(408) 433-1400
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $0.01 par value per share
|
The NASDAQ Stock Market, LLC
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o
|
Accelerated filer x
|
Non-accelerated filer o
|
Smaller reporting company o
|
|
(Do not check if a smaller reporting company)
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
As of September 30, 2009, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $126.1 million based on the closing sale price of such stock as reported on the NASDAQ Global Market. Shares of common stock held by each officer and director as of that date and by each person who owned 5% or more of the registrant’s outstanding common stock as of September 30, 2009 have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of May 28, 2010 registrant had outstanding 16,571,131 shares of common stock, $0.01 par value per share.
DOCUMENTS INCORPORATED BY REFERENCE
The Registrant has incorporated by reference portions of its Proxy Statement for its 2010 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this annual report.
MICRUS ENDOVASCULAR CORPORATION
|
Page
|
|||||
|
3
|
|||||
|
23
|
|||||
|
36
|
|||||
|
36
|
|||||
|
37
|
|||||
|
37
|
|||||
|
37
|
|||||
|
40
|
|||||
|
41
|
|||||
|
54
|
|||||
|
55
|
|||||
|
86
|
|||||
|
86
|
|||||
|
86
|
|||||
|
87
|
|||||
|
87
|
|||||
|
87
|
|||||
|
87
|
|||||
|
87
|
|||||
|
87
|
|||||
|
|
88
|
||||
2
FORWARD-LOOKING STATEMENTS
Certain information contained in or incorporated by reference in this Report contains forward-looking statements that involve risks and uncertainties. The statements contained in this Report that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), including statements regarding our expectations, beliefs, intentions or strategies regarding the future. All forward-looking statements included in this Report are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. Our actual results could differ materially from those discussed herein. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in Item 1A, Risk Factors, and elsewhere in this Report. References herein to “Micrus,” “the Company,” “we,” “our,” “us” and similar words or phrases are references to Micrus Endovascular Corporation and its subsidiaries, unless the context otherwise requires. Unless otherwise provided in this Report, trademarks identified by ® and ™ are registered trademarks or trademarks, respectively, of Micrus Endovascular Corporation or its subsidiaries. All other trademarks are the properties of their respective owners.
The Company
We develop, manufacture and market implantable and disposable medical devices used in the treatment of cerebral vascular diseases. Our products are used by interventional neuroradiologists, interventional neurologists and endovascularly trained neurosurgeons to treat both cerebral aneurysms responsible for hemorrhagic stroke and intracranial atherosclerosis which may lead to ischemic stroke. Hemorrhagic and ischemic stroke are both significant causes of death and disability worldwide.
Our product lines consist of endovascular systems that enable a physician to gain access to the brain in a minimally invasive manner through the vessels of the arterial system. We believe that our products provide a safe and reliable alternative to more invasive neurosurgical procedures for treating aneurysms. Our proprietary family of embolic coils anatomically conforms to a wide diversity of aneurysm shapes and sizes and rapidly deploys. We also supply products such as guide catheters, microcatheters, occlusion balloon catheters, guidewires and stents for the treatment of both hemorrhagic and ischemic stroke. In addition, our Cerecyte® microcoil product line incorporates bioactive filaments of an absorbable material called polyglycolic acid (“PGA”), which reside within the central lumen of our microcoils. We believe, based on interim data from the Cerecyte Coil Trial (“CCT”) and single center peer review journal publications, that Micrus platinum and Cerecyte coils result in superior clinical outcomes when compared to data from the International Subarachnoid Aneurysm Trial (“ISAT”).
We continue to expand our product line beyond microcoils and access systems. In January 2006, we entered into a license, development and distribution agreement with Biotronik AG (“Biotronik”) which provides us with exclusive access to certain stent technologies for neurovascular applications. In February 2006, Biotronik received CE Mark authorization for the PHAROS® stent for both the treatment of cerebral aneurysms and the treatment of ischemic disease (atherosclerosis). In March 2006, we launched our PHAROS® stent in certain countries that recognize the CE Mark, providing us with our first commercial product for the treatment of ischemic disease. The PHAROS® Vitesse® is our second generation balloon-expandable stent for intracranial ischemic stenosis and the treatment of wide-neck aneurysms. In June 2008, we launched the PHAROS® Vitesse® intracranial stent for commercial distribution in the European Union and all other countries that recognize the CE Mark. In August 2009, we received U.S. Food and Drug Administration (“FDA”) approval of our investigational device exemption for the PHAROS® Vitesse® Intracranial Stent Study for Ischemic Therapy (“VISSIT”). The VISSIT study is the first industry-sponsored, randomized, prospective clinical trial designed to compare the clinical outcomes between patients who are stented for intracranial ischemic stenosis versus treated with medical therapy. We are currently enrolling patients in our VISSIT Investigational Device Exemption (“IDE”) trial in the United States, Europe and China.
On November 30, 2006, we completed the acquisition of VasCon, LLC (“VasCon”), a privately held company engaged in the development and manufacture of vascular access and delivery devices. The acquisition of VasCon adds expertise in developing clinically advanced access and catheter systems to our core competencies and provides us with manufacturing capabilities that will facilitate cost reductions for a wide range of our products. In connection with the acquisition, we formed Micrus Design Technology, Inc. (“MDT”) to develop and manufacture neurovascular access and delivery products for us, including our family of Courier® microcatheters, Neuropath® guide catheters and our Ascent® Occlusion Balloon Catheter. On January 1, 2009, we merged MDT into Micrus Endovascular Corporation.
3
In October 2007, we entered into a Stock Purchase Agreement with The Cleveland Clinic Foundation (“The Cleveland Clinic”) and acquired ReVasc Technologies, Inc. (“ReVasc”), a wholly-owned subsidiary of The Cleveland Clinic. This acquisition provides us with an exclusive license to revascularization technology for the treatment of intra-cranial thrombus or clot which can also cause ischemic stroke. In January 2008, we entered into a license, development and commercialization agreement with Genesis Medical Interventional, Inc. (“Genesis”). Under the terms of the agreement, we licensed the rights to Genesis’ F.A.S.T. Funnel Catheter and clot retrieval system for the treatment of ischemic stroke.
In December 2007, we received regulatory approval to sell our stretch-resistant microcoils in Japan and in July 2008, we received approval from Ministry of Health, Labor and Welfare (“MHLW”) to sell our Cerecyte® microcoils in Japan. We currently market our products through a direct sales force in the United States, Canada, the United Kingdom, Germany, Austria and France. We market our products through a network of exclusive distributors in the rest of Europe, Latin America, Asia and the Middle East.
In fiscal 2008, we launched our Cashmere® microcoil systems and our Courier® ENZO® deflectable microcatheter. The Cashmere® is a conformable and stretch-resistant bare platinum or Cerecyte® microcoil designed to provide stable framing or filling of aneurysms that may require a softer microcoil, such as aneurysms with irregular shapes or ruptured aneurysms. The Courier® ENZO® deflectable microcatheter is designed to offer improved maneuverability through the brain’s tortuous vasculature and to enable in vivo repositioning of the microcatheter in the aneurysm, allowing physicians to more efficiently fill aneurysms, which may lead to improved outcomes. We believe that ENZO® is the only deflectable microcatheter available for use in the neurointerventional market.
In fiscal 2009, we launched the Neuropath® guide catheter, which combines robust proximal support with a highly flexible and visible tip designed to facilitate atraumatic vascular access. The Neuropath® guide catheter is used as a conduit for delivery of the microcatheter or other devices, such as coils, stents and balloons, to the aneurysm. We intend to continue to pursue this non-embolic product line expansion with the goal of increasing our revenue opportunity per procedure. We also launched the DeltaPaq™ microcoil system for the treatment of cerebral aneurysms in both bare platinum and Cerecyte® versions. Our DeltaPaq™ microcoil is based on the proprietary Delta Wind™ technology which is designed to enable physicians to achieve greater coil packing density within the aneurysm which may reduce the rate of recanalization and the need for re-treatment. The DeltaPaq™ microcoil system supplements our framing and finishing coils in the filling segment of the coil market. We also introduced a new detachment control box and remote control cable. The Enpower® Detachment Control Box features updated electronic controls, a new ergonomic interface as well as a single long life lithium ion battery system which obviates the need to replace batteries for each case. The remote cable enables physicians to detach the coil using an in-the-sterile-field control system. In fiscal 2009, we received both 510(k) clearances and CE Mark authorizations for our DeltaPlush microcoils, EnPower® Detachment Control System, Ascent® Occlusion Balloon Catheter and One2One™ family of guidewires.
In fiscal 2009, we also completed enrollment in the CCT, a trial which was designed to assess the differences in clinical outcomes between our Cerecyte microcoils and bare platinum microcoils. In October 2008, we also initiated the Presidio® And Cerecyte® (“PAC”) large and giant aneurysm study, which is a prospective registry designed to evaluate the differences in clinical outcomes between our Cerecyte® microcoils and bare platinum microcoils specifically in large and giant aneurysms.
In fiscal 2010, we launched our DeltaPlush™ microcoil, which has been designed to be our softest finishing coil. The DeltaPlush™ microcoil incorporates our exclusive Delta Wind™ technology resulting in a microcoil with the softness and flexibility to find and fill gaps, helping to provide superior finishing at the aneurysm neck.
In fiscal 2010, we also launched our Ascent® Occlusion Balloon Catheter which is intended for use in the blood vessels of the peripheral and neurovasculature where temporary occlusion is desired. The Ascent® balloon catheter enables physicians to use a vessel selective technique of temporary arterial occlusion which is useful in selectively stopping or controlling blood flow and assisting in the placement of coils in a wide neck aneurysm.
In fiscal 2010, we received regulatory approval from the Republic of Korea to sell our MicruSphere® and HeliPaq® microcoils.
On September 30, 2009, we entered into a Distribution Agreement (the “IDS Distribution Agreement”) with IDS (Hong Kong) Ltd. (“IDS”) pursuant to which IDS serves as our exclusive distributor of implantable and disposable medical devices used in the treatment of neurovascular diseases in Hong Kong and China. IDS will promote and market our products in Hong Kong and China and is required to purchase a certain amount of the products, in escalating quantities, over a five year period commencing upon receiving regulatory approval in order to maintain its exclusive distributor status in such territories. In connection with the IDS Distribution Agreement, we also entered into an agreement with IDS to provide marketing and sales support to IDS.
4
We plan to begin selling our products in China upon receiving regulatory and reimbursement approvals. The timing of these approvals is uncertain. We did not have any sales in China during fiscal 2010, but we currently anticipate selling our products in China in fiscal 2011.
We have executive offices in San Jose, California and sales offices in Switzerland and the United Kingdom as well as a development and manufacturing facility in Miramar, Florida. We were incorporated under the laws of the State of Delaware in 1996.
Information on revenues, gross profits and total assets for our business segments and by geographic area appears in Note 14 of the “Notes to Consolidated Financial Statements” for the year ended March 31, 2010, which are included in “Item 8 – Financial Statements and Supplementary Data” of this report and are incorporated herein by reference.
Industry Overview
Strokes can be either hemorrhagic stroke, most often caused by ruptured cerebral aneurysms, or ischemic stroke, caused by the narrowing or blockage of vessels within or leading to the brain. Both hemorrhagic and ischemic stroke often result in irreversible neurological impairment or death. According to the American Heart Association, stroke is the third leading cause of death in the United States. Patients who survive a stroke are often left with disabilities, including paralysis, coma, impaired cognition, decreased coordination, loss of visual acuity, loss of speech, loss of sensation or some combination of these conditions. A significant need for effective prevention and treatment of stroke exists because of the severity of the disorder, its prevalence in society, the shortcomings of current therapies and the high cost of treatment and care.
As noted above, hemorrhagic stroke is most often caused by the bulging or rupture of cerebral aneurysms. A cerebral aneurysm is an outward bulging of an artery in the brain that can develop at weak points in the arterial wall. In some cases, the patient will experience symptoms such as headache, blurred vision or dizziness as the aneurysm grows, but in many cases patients will have no symptoms. The most devastating complication of a cerebral aneurysm occurs when the aneurysm ruptures, decreasing blood flow to brain tissue and leading to increased pressure on the brain. Rupture of a cerebral aneurysm typically occurs suddenly and without warning, often leading to catastrophic brain injury or death.
Historically, patients diagnosed with a cerebral aneurysm that has ruptured or is determined to be at risk of rupturing underwent a craniotomy and aneurysmal clipping, a highly-invasive surgical procedure in which a neurosurgeon creates an opening in the skull, dissects or retracts brain tissue to gain access to the aneurysm, and places a metal clip at the base of the aneurysm to stop further blood flow into the aneurysm, halting its growth and preventing future rupture. This procedure is typically performed by a neurosurgeon at a specialized hospital or medical center. Aneurysmal clipping requires a lengthy recovery time and has the significant expense, morbidity and complication risks associated with a major neurosurgical procedure.
In the 1990s, interventional neuroradiologists and to a lesser extent endovascularly trained neurosurgeons, who collectively are referred to in the industry as “neurointerventionalists,” started using an alternative procedure to clipping, known as embolic coiling, to treat cerebral aneurysms. Rather than reaching the aneurysm by opening the skull to gain access to the aneurysm in an embolic coiling procedure, access is obtained through a catheterization procedure in which the physician inserts a guidewire followed by a catheter into the femoral artery of the upper leg and threads them under fluoroscopy through the arterial system to the brain and ultimately into the opening of the aneurysm. The neurointerventionalist then typically advances a number of embolic coils through the microcatheter to fill the aneurysm. Embolic coils are small platinum coils that typically range in size from approximately 1.5 mm to 20 mm and once advanced through the microcatheter and into the aneurysm, they successively fill the aneurysm. The multiple strands of microcoils effectively form a barrier at the neck of the aneurysm which decreases, stagnates or stops blood flow into the aneurysm, enabling formation of a clot and scar tissue which prevent further growth or rupture of the aneurysm. Since the mid-1990s, embolic coiling has become a widely accepted treatment for cerebral aneurysms because it is a less invasive procedure than surgical clipping and results in lower overall treatment cost, shorter recovery times, and less trauma to the patient.
In 2002, The Lancet, a leading medical journal, published the results of the ISAT, an independent, randomized clinical trial involving 2,143 patients in Europe, North America and Australia that compared surgical clipping with embolic coiling as a method of treating cerebral aneurysms. The ISAT concluded, based on a survey of patients published in The Lancet in October 2002, that among the patients participating in the trial, endovascular intervention with detachable platinum coils resulted in a 23% relative and 7% absolute reduction in the risk of major brain injury or death compared with neurosurgical clipping of the aneurysm at one year follow up. The seven-year follow up data published in The Lancet in September 2005 indicated a continued clinical advantage for patients who underwent coiling versus clipping procedures.
5
Market Opportunity
According to the American Heart Association, approximately 720,000 strokes occur annually in the United States. Ischemic stroke affects approximately 620,000 patients annually while hemorrhagic stroke affects approximately 100,000 patients. We believe that a majority of the hemorrhagic strokes are caused by cerebral aneurysms. We believe that embolic coiling is being used to treat approximately 45% of the patients diagnosed with cerebral aneurysms in the United States. Industry sources also indicate that approximately 65-70% of patients diagnosed with cerebral aneurysms in certain European countries are treated using embolic coiling procedures. We believe that embolic coiling procedures can be used to treat a similar percentage of patients with cerebral aneurysms in the United States as awareness grows among patients and physicians of the advantages of embolic coiling. Industry sources estimate that in Japan embolic coiling is growing at an annual rate of approximately 15% and in China coiling procedures appear to be growing at greater than 20% year over year. The latest industry sources further estimate that the worldwide sale of endovascular devices for the treatment of hemorrhagic stroke was between $600 million to $700 million in 2008.
We believe that growth drivers in the market for embolic coiling products include the overall trend towards less invasive procedures, an increasing number of neurointerventionalists trained to perform embolic coiling procedures, and the aging population in which aneurysms occur with greater frequency.
The key challenges of embolic coiling procedures are the following:
|
•
|
Access to the Aneurysm Site. Specialized products are required to access the complex vasculature of the brain, properly access the aneurysm site and perform a coiling procedure. These access products include microcatheters and guidewires. In order to navigate the complex vascular anatomy of the brain, access products must have enough column strength to be pushed significant distances through this vasculature, yet be flexible enough to travel to distal portions of the brain without injuring blood vessels.
|
|
•
|
Framing and Filling the Aneurysm. In order to effectively treat an aneurysm, the neurointerventionalist must fill the aneurysm with a sufficient volume of coils to disrupt and stagnate blood flow, which is required for aneurysm to ultimately heal. Aneurysms vary in shape and size and, consequently, neurointerventionalists seek an embolic coiling solution that enables coils to conform to the aneurysm’s shape and most effectively fill the aneurysm without requiring extensive manipulation of the coil. Coils that frame, or conform to, the aneurysm wall reduce the risk of rupture, thus decreasing the opportunity for procedural complications. Additionally, microcoils that safely permit the interventionalist to increase the packing density of the aneurysm, will likely lead to improved clinical outcomes.
|
|
•
|
Coverage of the Neck of the Aneurysm. It is important to effectively cover the neck of the aneurysm with coils to help reduce recanalization and improve the chances of a better clinical outcome.
|
|
•
|
Deployment. Once embolic coils are placed in the aneurysm, the neurointerventionalist must be able to quickly and reliably detach the coils from the device positioning unit (“DPU”) within the aneurysm. Unreliable detachment mechanisms can lead to inadvertent retraction of the embolic coil as the neurointerventionalist withdraws the positioning unit only to discover that the coil is still attached. Further, any delay in deployment may increase procedure time and its attendant risks.
|
|
•
|
Recanalization. Industry sources estimate that recanalization, or the continued or renewed growth of the aneurysm, occurs in approximately 15% to 25% of aneurysms treated with embolic coiling. Experts believe that one of the reasons for recanalization is due to inadequate filling of the aneurysm with the embolic coils. Studies have shown that while the recanalization rate is higher for patients treated with embolic coiling procedures compared to aneurysm clipping, embolic coiling has been demonstrated to be a safer treatment approach for aneurysms. Therefore, embolic coiling solutions that decrease recanalization rates and reduce the need for retreatment are highly desirable.
|
|
•
|
Risk of Rupture. Embolic coiling solutions that enhance safety and limit the risk of rupture or re-rupture in the treatment of aneurysms are also essential. Successful framing and filling of the aneurysm requires precise placement of the embolic coil. Neurointerventionalists seek embolic coiling solutions that minimize stress on the aneurysm wall in the course of placing or repositioning the coil in order to reduce the risk of rupture.
|
The Micrus Solution
We are focused on a broad range of cerebral vascular treatments and have developed a proprietary embolic coiling solution, stents and access products that are designed to effectively access and treat cerebral aneurysms and ischemic disease. Complementing our bare platinum microcoil product line, we have also developed a line of microcoils that incorporate bioactive filaments. In addition, we have recently introduced the proprietary DeltaPaq™ and DeltaPlush™ microcoil systems which are designed to enable physicians to achieve greater coil packing density and uniformity of coils at the aneurysm neck.
6
Our solutions have the following key features:
Anatomically Conforming Coils. Our proprietary spherical MicruSphere®, Cashmere® and Presidio® microcoils deploy into a three-dimensional configuration that assumes an aneurysm’s shape upon deployment and are designed to provide effective framing of the aneurysm. Our microcoils are designed to require limited manipulation during placement, thereby reducing the need for significant microcoil repositioning thus reducing potential for procedural complications.
Enhanced Coverage of the Neck of the Aneurysm. We believe that effective neck coverage reduces the rate of recanalization. Our microcoils are designed to facilitate coverage of the neck of the aneurysm in two ways. First, the three dimensional configuration of our spherical MicruSphere®, Cashmere® and Presidio® microcoils provide the framework to stabilize the neck of the aneurysm. Second, our DeltaPaq™ filling coils and UltiPaq® and DeltaPlush™ finishing coils are designed to be soft and flexible, permitting coverage across the neck of the aneurysm.
Unique Framing and Filling Technology Designed to Increase Packing Density. Our Presidio® microcoils are stretch resistant microcoils designed to deliver stable, predictable aneurysm framing and filling to increase coverage of the aneurysm wall and neck with a single coil deployment. In addition, we have introduced a completely new coil technology to the market, called “Delta Wind.” This unique triangular shaped primary wind allows the DeltaPaq™ and DeltaPlush™ to change directions every quarter of a millimeter, far surpassing the filling and finishing performance capabilities of traditional round primary wind coils. The Delta Wind™ technology is designed to increase packing density which previous published studies have suggested lower the risk of coil compaction and recanalization rates.
Proprietary Finishing Coils. Our UltiPaq® and DeltaPlush™ microcoils are extra-soft, stretch-resistant finishing coils used to provide additional aneurysm neck coverage and to more thoroughly fill in the aneurysm after placement of one or more MicruSphere®, Presidio®, Cashmere® and/or DeltaPaq™ microcoils. We launched our DeltaPlush™ microcoil in January 2010. DeltaPlush™ has been designed to be our softest finishing coil.
Deployment Technology. Our proprietary electronic microcoil deployment system employs a resistive heating fiber deployment mechanism that enables neurointerventionalists to quickly and reliably deploy the microcoil. Our electronic microcoil deployment system has been designed so that microcoil deployment time remains consistent regardless of the number of coils used in the procedure. We believe that our electronic microcoil deployment system enables neurointerventionalists to more rapidly deploy microcoils and generally reduce procedure time. We have also recently introduced a change to our DPU which we believe enhances clinical performance while reducing our manufacturing costs.
Bioactive Technology. Cerecyte® is our proprietary microcoil product line that incorporates filaments comprised of PGA within the lumen of the microcoils. MicruSphere®, Cashmere®, Presidio®, HeliPaq® and UltiPaq®, DeltaPaq™ and DeltaPlush™ are all available in Cerecyte® versions. We are conducting two post market studies, a prospective randomized trial and a registry, which could provide additional data regarding the potential benefits of Cerecyte®. Both the CCT and Cerecyte® Registry completed enrollment in fiscal 2009 and the data is being assembled for presentation and submission to peer review journals in fiscal 2011.
Balloon Assisted Coiling. Our Ascent® Occlusion Balloon Catheter is a coaxial dual lumen balloon catheter designed for use over any 0.014 inch guidewire. The 0.014 dual lumen catheter is designed to improve the stability of the balloon potentially resulting in a more effective and safer procedure. It is intended to be used for temporary vascular occlusion and to assist in delivery of microcoils into the aneurysm.
Stent Platform. Our PHAROS® stent is a rapid exchange balloon-delivered device which enables the neurointerventionalist to deliver and deploy a stent in one step, eliminating the need for pre-dilation of the constricted vasculature. We believe this feature may reduce overall procedural time and cost. We believe that the balloon catheter marker bands combined with the radiopacity of the stainless steel stent provide for excellent visibility resulting in improved placement accuracy of deployment. We also believe that the PHAROS® stent’s combination of Rapid Exchange Technology™ and a trackable tip will enable physicians to effectively access the tortuous and distal anatomy of the brain. In March 2006, we launched the PHAROS® stent in certain countries outside of the United States that recognize the CE Mark. In March 2008, we filed an IDE with the FDA for our next generation stent, the cobalt chromium steel PHAROS® Vitesse®, for the treatment of neurovascular stenosis. In July 2008, we received FDA conditional approval of our IDE for the PHAROS® VISSIT study which allowed us to initiate the study. In August 2009, the FDA granted approval of the VISSIT study. The VISSIT study is the first industry sponsored, randomized, prospective clinical trial designed to compare the clinical outcomes between patients who receive a stent for the treatment of their intracranial ischemic stenosis versus being treated with medical therapy. We are currently enrolling patients in our VISSIT IDE trial in the United States, Europe and China.
7
Improved Access Products. We launched an access product line which includes the Courier® line of microcatheters, the Ascent® Occlusion Balloon Catheter, the Neuropath® guide catheter and the Watusi® line of guidewires. We believe that our Courier® microcatheters provide neurointerventionalists with more predictable and secure access to the complex and distal anatomy of the cerebral vasculature. The Neuropath® guide catheter combines robust proximal support with a highly flexible and visible tip designed to facilitate atraumatic vascular access. The Neuropath® guide catheter is used as a conduit for delivery of the microcatheter or other devices such as coils to the aneurysm. We intend to continue to pursue this non-embolic product line expansion with the goal of increasing our revenue opportunity per procedure.
Micrus Strategy
Our objective is to develop and commercialize innovative, minimally invasive medical devices that provide a comprehensive solution to physicians for the treatment of hemorrhagic and ischemic stroke. The key elements of our strategy to achieve our objective include:
Expand Our Hemorrhagic Market Share through Continued Product Innovation. We believe that our microcoils, catheters, balloons, wires, and stents offer safer, more effective and less technically demanding treatment options for neurointerventionalists which have resulted in the rapid growth of our revenues. We believe that continued product innovations such as the introductions of our proprietary DeltaPaq™ and DeltaPlush™ microcoils, Cerecyte® line of bioactive microcoils, our Cashmere® microcoils, our Presidio® microcoils, our Ascent® Occlusion Balloon Catheter, our Courier® microcatheters and our Neuropath® guide catheters will allow us to further grow our market share. We are continuing to develop new technologies which we believe may further enhance aneurysm occlusion and reduce the rate of recanalization.
Increase Our Per-Procedure Revenues. 6% of our revenue for fiscal 2010 has come from the sale of non-embolic products. This product line expansion includes stents, microcatheters, balloons, guidewires and accessories.
Enter the Ischemic Stroke Market. In October 2007, we acquired an exclusive license to revascularization and clot retrieval technology for the treatment of ischemic stroke through the acquisition of ReVasc now known as ReVive™ SE, from The Cleveland Clinic. In January 2008, we entered into a license, development and commercialization agreement with Genesis. Under the terms of the agreement, we licensed the rights to Genesis’ F.A.S.T. Funnel Catheter and clot retrieval and revascularization system for the treatment of ischemic stroke. The Genesis technology is now known as ReVive™ CX. Through our agreement with Biotronik, we launched our first product addressing the ischemic stroke market, our PHAROS® stent. We intend to continue developing and acquiring additional stent platforms for this market. In March 2008, we filed an IDE with the FDA for our next generation stent, the PHAROS® Vitesse®, for the treatment of intracranial stenosis. We are currently enrolling patients in our VISSIT IDE trial in the United States, Europe and China.
Leverage Our Sales and Marketing Expansion. We intend to continue to expand our direct sales force in North America, Europe and Asia as necessary and further increase our presence in the Asian markets through distributors. This expansion should provide us access to more hospitals, garner more per-procedure revenues and expand our market share.
Continue to Penetrate Asian Markets. We believe that Japan and China represent significant potential markets for our products and, in March 2006, we launched our sales and marketing efforts in Japan through our distribution partner, Goodman. In December 2007, we received regulatory approval to sell our stretch-resistant microcoils in Japan and we received regulatory approval for our Cerecyte® microcoils in July 2008. We will begin selling our products in China with the assistance of our Chinese distributor IDS upon receiving regulatory and reimbursement approvals.
License or Acquire Complementary Products and Technologies. In addition to growing our business through internal product development efforts, we will continue to look for opportunities to license and/or acquire technologies to provide solutions for the treatment of a variety of cerebral vascular conditions. In July 2005, we acquired certain deflectable catheter technologies from Vascular FX. In January 2006, we entered into a license, development and distribution agreement with Biotronik, a company with stent design and manufacturing expertise, pursuant to which we collaborate with Biotronik to develop certain neurovascular stent products. This agreement provides us with the exclusive worldwide right to market stent products developed jointly by Biotronik and us. In November 2006, we acquired certain neurovascular catheter patent and process technology through the acquisition of VasCon. In October 2007, we acquired an exclusive license to revascularization technology for the treatment of ischemic stroke through the acquisition of ReVasc from The Cleveland Clinic. In January 2008, we entered into a license, development and commercialization agreement with Genesis. Under the terms of the agreement, we licensed the rights to Genesis’ F.A.S.T. Funnel Catheter and clot retrieval system for the treatment of ischemic stroke. In April 2008, we entered into a co-development agreement with Chemence Medical Products, Inc. (“Chemence”) to jointly develop a liquid embolic product for the treatment of cerebral aneurysms using Chemence’s cyanoacrylate technology, development capabilities and intellectual property. In August 2009, we entered into a license, development and commercialization agreement with Flexible Stenting Solutions, Inc (“FSS”) to jointly develop a flow diversion product for neurovascular indications using both Micrus and FSS technology, development capabilities and intellectual property.
8
In January 2010, we entered into an exclusive license and option agreement with Bay Street Medical, Inc. (“Bay Street”), which granted us a worldwide exclusive license and option to purchase intellectual property assets held by Bay Street related to its proprietary stent delivery and locking technology. By continuing to acquire complementary products, we believe that we can address a broader range of physician and patient needs.
Products
The following table shows our principal products and indicates significant applications for these products. Most of our products are intended for single use and are either disposed of or, in the case of microcoils, remain in the patient after the procedure. All of our products set forth in the following table have received CE Mark authorization and, except for our PHAROS® stent, are covered by the FDA 510(k) process.
|
Product Line
|
Sizes
|
Product Description
|
||
 MicruSphere® Microcoil
|
2-18 mm diameter
|
Three-dimensional framing microcoil; stabilizes the aneurysm. Available in bare platinum and Cerecyte®.
|
||
 Presidio® Microcoil
|
4-20 mm diameter
|
Framing and filling coil to deliver more neck and wall coverage in a single deployment. Available in Cerecyte®.
|
||
 Cashmere® Complex Microcoil
|
2-12 mm diameter
|
Three-dimensional framing and filling complex coil for aneurysms which require a softer coil. Available in Cerecyte® and stretch resistant platinum.
|
||
 DeltaPaq™ Microcoil
|
1.5-10 mm diameter, up to 25cm in length
|
Filling microcoil featuring the new Delta Wind triangular primary wind featuring natural deflection points allowing the coil to change direction more easily, facilitating greater packing density. Available in bare platinum and Cerecyte®.
|
9
|
Product Line
|
Sizes
|
Product Description
|
 DeltaPlush™ Microcoil
|
1.5mm-4mm, up to 8cm in length
|
Designed for finishing, DeltaPlush™ microcoil incorporates our exclusive Delta Wind™ technology resulting in a 10 system microcoil with the softness and flexibility to find and fill empty gaps and open spaces, to help provide superior finishing at the aneurysm neck. Available in bare platinum and Cerecyte®.
|
 UltiPaq® Microcoil
|
2-4 mm diameter
|
Finishing microcoil; soft, stretch resistant, pliable microcoil designed to complete filling of the aneurysm. Available in bare platinum and Cerecyte®.
|
||
 HeliPaq® HeliPaq SR® Microcoil
|
HeliPaq® 2-20 mm diameter; HeliPaq SR® 2-10 mm diameter
|
Filling microcoil; occludes aneurysm following framing. Available in bare platinum and Cerecyte®.
|
||
 InterPaq® Microcoil
|
4 and 6 mm diameter
|
Filling microcoil; occludes aneurysm following framing. Available in bare platinum.
|
||
 Cerecyte® Microcoil
|
2-20 mm diameter
|
Available in MicruSphere®, Cashmere®, Presidio®, Ultipaq®, DeltaPaq™, DeltaPlush™ and HeliPaq SR®. Includes filaments comprised of PGA, a bioactive material.
|
||
 Courier® ENZO® Microcatheter
|
.0170" and 0190” inner diameter and 150 cm length
|
Unique deflectable device used to deliver embolics into the aneurysm.
|
10
|
Product Line
|
Sizes
|
Product Description
|
 Courier® Microcatheter
|
.0170” and .0190” inner diameter and 150 cm length
|
Device used to deliver embolics into the aneurysm.
|
||
 Neuropath® Guide Catheter
|
5 and 6 french internal diameter and a 90 and 100cm length
|
The Neuropath® guide catheter uses Progressive Shaft Technology™ to facilitate the smooth delivery of multiple devices including microcatheters, balloons, guidewires and microcoils. It comes in a variety of tip shapes and sizes.
|
||
 Watusi® Guidewire
|
.014” diameter and 205 cm length
|
Device used to guide catheters and other devices to the aneurysm or stenosis site.
|
||
 PHAROS® Vitesse® Stent System
|
2.5 mm - 4.0 mm outer diameter and 8 mm – 20 mm length
|
For use as scaffolding of wide-neck aneurysms to ensure that the microcoil is not dislodged and for use in opening intracranial arteries that have narrowed.
|
||
 Ascent® Balloon Catheter
|
4 mm diameter and higher. Multiple lengths
|
A coaxial dual lumen balloon catheter designed for use over any 0.014-inch guidewire. It is intended for use for temporary vascular occlusion and to assist in delivery of diagnostic and therapeutic agents.
|
11
|
Product Line
|
Sizes
|
Product Description
|
||
 EnPower® Detachment Control Box and Connecting Cable
|
|
Next-generation control box provides electronic control via a lithium ion battery and initiates detachment of our proprietary microcoil system. The EnPower® also features a remote cable which enables physicians to detach the coils from the patient field.
|
Microcoil Products
We offer a range of microcoils designed to enable neurointerventionalists to treat a wide variety of aneurysms. These include our MicruSphere®, Cashmere®, DeltaPaq™, DeltaPlush™ and Ultipaq® microcoil systems which are available in bare platinum and Cerecyte® versions, and the Presidio® line of microcoils which also incorporates our proprietary Cerecyte® technology. All of our microcoils utilize our rapid deployment system and perform certain specific functions:
|
•
|
Frame. Our MicruSphere®, Presidio® and Cashmere® microcoils are typically the first microcoils used by neurointerventionalists to frame the aneurysm. The MicruSphere® microcoil folds automatically into a spherical three-dimensional shape that conforms to the shape of the aneurysm. This conforming shape reduces the need for the clinician to manipulate and reposition the coil multiple times, shortens procedure time, and reduces the potential for complications. Additional microcoils may then be placed within the first microcoil in smaller sizes in an approach known as the “Russian doll technique,” sequentially filling the aneurysm.
|
|
•
|
Frame and Fill. Our Presidio® and Cashmere® microcoils feature longer lengths and their own unique shapes required to frame and fill the aneurysm. This can allow for more platinum to be deployed at the neck of the aneurysm, as well as greater packing density.
|
|
•
|
Fill. The DeltaPaq™ features a completely new coil technology, called “Delta Wind.” This unique triangular shaped primary wind allows the DeltaPaq™ to change directions rapidly, far surpassing traditional round primary winds. The Delta Wind technology is designed to increase packing density, which previous studies have suggested results in a reduction in recanalization. The DeltaPaq™ is available in both bare platinum and with Cerecyte. The Cashmere® microcoil is a stretch resistant complex coil which may also be used as a filling coil. The Cashmere® combines extra coil softness with a 3 dimensional secondary shape in a 14 system coil. Attributes of a 14 system coil help deliver more packing density per cm of coil compared with smaller system microcoils. Our proprietary DeltaPaq™, HeliPaq® and HeliPaq SR® products are filling microcoils used to fill gaps which may remain in the center of the aneurysm after placement of one or more of our MicruSphere® or Presidio® framing microcoils. Both the HeliPaq® and the HeliPaq SR® automatically form a helical shape upon deployment, which allows filling of complex gaps in the aneurysm. The HeliPaq SR® employs a stretch-resistant system designed to prevent the microcoil from stretching in an unwanted manner while being positioned in the aneurysm. InterPaq® microcoils are filling coils used in larger size aneurysms requiring a greater volume of coil mass in order to be adequately filled.
|
|
•
|
Finish. The DeltaPlush™ microcoil with “Delta Wind” technology is designed to be our softest finishing coil. The DeltaPlush™ is available in both bare platinum and with Cerecyte. Our UltiPaq® microcoil is an extra-soft, stretch-resistant finishing coil, used to provide additional aneurysm neck coverage and to more thoroughly fill in the aneurysm after placement of one or more MicruSphere®, Presidio®, Cashmere® and/or HeliPaq® microcoils.
|
|
•
|
Cerecyte®. Our proprietary Cerecyte® microcoil product line incorporates filaments comprised of PGA into most of our current line of microcoils — MicruSphere®, Presidio®, Cashmere®, DeltaPaq™, DeltaPlush™, HeliPaq®, HeliPaq SR® and UltiPaq®. Because the PGA filaments run through the center of our microcoils, our Cerecyte microcoils possess the same handling characteristics as our standard platinum microcoils.
|
12
In January 2009, we completed the enrollment of 500 patients in our CCT. The CCT is a prospective randomized multi-center trial which directly compares Micrus Cerecyte® bioactive microcoils to Micrus bare platinum microcoils for the treatment of intracranial aneurysms. We also completed the enrollment of 250 patients in our Cerecyte® Registry in January 2009. The Cerecyte® Registry is a prospective non-randomized United States multi-center registry designed to document the clinical and angiographic outcomes of intracranial aneurysms treated with our Cerecyte® bioactive coils. Data from these studies are being assembled for presentation and submission to peer review journals in fiscal 2011. Interim data from the CCT presented at the American Society of Neuroradiology in May 2010 suggest that our Cerecyte and bare platinum microcoils outperform the ISAT study published results for bare platinum coils in both investigator assessed angiographic occlusion rate and safety. The ISAT results are the industry’s benchmark.
PHAROS® and PHAROS® Vitesse® Balloon-Expandable Stent
Our PHAROS® stent is a balloon-delivered device which can be used both to treat ischemic disease and for scaffolding of wide-neck aneurysms to ensure that the microcoil is not dislodged. For the treatment of ischemic disease, our PHAROS® stent dilates intracranial arteries that have narrowed and allows the neurointerventionalist to deliver and deploy a stent in one step. We believe this feature will help reduce overall procedural time and cost. We believe that the balloon catheter marker bands combined with the radiopacity of the stent provide for excellent visibility resulting in improved accuracy of deployment.
We believe that PHAROS® Rapid Exchange Technology and trackable tip will enable a physician to access the tortuous and distal anatomy of the brain. In March 2006, we launched the PHAROS® stent in certain countries outside the United States that recognize the CE Mark. In March 2008, we filed an IDE with FDA for our next generation stent, the cobalt chromium steel PHAROS® Vitesse®, for the treatment of neurovascular stenosis. In July 2008, we received FDA conditional approval of our IDE for the VISSIT study of our next generation PHAROS® Vitesse® stent. In August 2009, we received approval for the study. The VISSIT study is the first industry sponsored, randomized, prospective clinical trial designed to compare the clinical outcomes between patients who are stented for intracranial ischemic stenosis versus treated with medical therapy. We are currently enrolling patients in our VISSIT IDE trial in the United States, Europe and China.
Access Products
We offer the following products:
|
•
|
Ascent® Balloon Catheter. The Ascent® Occlusion Balloon Catheter is a coaxial dual lumen balloon catheter designed for use over any 0.014 inch guidewire. The 0.014 dual lumen catheter is designed to improve the stability of the balloon, potentially resulting in a more effective and safer procedure. It is intended to be used for temporary vascular occlusion and to assist in delivery of microcoils and other therapeutic and diagnostic agents into the aneurysm.
|
|
•
|
Courier® Microcatheter. Our Courier® microcatheter is a device used to deliver microcoils to the aneurysm. Our Courier® microcatheter features our proprietary Endurance™ technology designed to enhance both tip shaping and tip shape retention, both of which are vital to optimal coil delivery. It is available in straight and pre-shaped configurations. Our Courier® Microcatheter has been designed to provide the neurointerventionalist with the ability to navigate the tortuous vasculature of the brain. The microcatheter’s design and hydrophilic coating enable a high level of stability, tip shape retention and overall tracking.
|
|
•
|
Neuropath® Guide catheter. The Neuropath® guide catheter uses Progressive Shaft Technology and facilitates the smooth delivery of multiple devices including microcatheters, balloons, guidewires and micro coils. It comes in a variety of tip shapes as well as varying sizes. It is currently available in a 5 and 6 french internal diameter and a 90 and 100cm length.
|
|
•
|
Courier® ENZO® Microcatheter. Introduced to the United States in September 2007 and to Europe in January 2008, the ENZO® deflectable tip microcatheter is designed to offer improved maneuverability through the brain’s tortuous vasculature and to enable in vivo repositioning of the microcatheter in the aneurysm, allowing physicians to more efficiently fill aneurysms, which may lead to improved outcomes. We believe that ENZO® is the only deflectable tip microcatheter available for use in the neurovascular market.
|
Microcoil Delivery System and Deployment Mechanism
In addition to the detachable microcoil, our microcoil delivery system is comprised of a DPU, a connecting cable and the EnPower® Detachment Control Box. Our DPU is a flexible catheter to which a Micrus microcoil is attached. Deployment of the microcoil occurs when the neurointerventionalist activates a resistive heater at the tip of the DPU, shearing the polyethylene fiber that holds the microcoil onto the DPU. Our deployment technology results in fast and reliable deployment of the microcoil from the DPU.
13
Sales and Marketing
We market our products to interventional neuroradiologists and neurosurgeons who generally practice at centers located in major metropolitan areas. There are currently approximately 300 - 400 neurointerventionalists in the United States who perform embolic coiling procedures. We believe that less than one-third of these physicians perform a substantial majority of the total number of embolic coiling procedures performed in the United States each year. We believe that a similar number of neurointerventionalists are practicing in Europe and Asia.
We have developed relationships with a number of these neurointerventionalists who perform a large number of cerebral vascular procedures. In fiscal 2010, a substantial portion of our product sales in the United States was to an estimated 118 hospitals. In order to encourage the continued adoption of our products, we believe that we need to continue to build and maintain relationships with these neurointerventionalists. We believe that these relationships are enhanced by the presence of our direct sales organization. Sales of embolic coiling products involve a long-term relationship between the sales representative and neurointerventionalist where the sales representative must initially be present for product demonstrations and to monitor procedures. We recruit our sales representatives based on their experience with minimally invasive devices and prior success in the medical device industry. We provide ongoing sales and product training to our employees and distributors and continually monitor their performance. We also market our products at various industry trade shows and conferences.
In the United States and Canada, we market our products through our direct sales force to neurointerventionalists, while in Europe, we rely on both a direct sales force and a distribution network. In Asia Pacific and Latin America, we market our products through a distribution network. We currently have a North American direct sales force of 34, a European direct sales force of 18, an Asia Pacific direct sales force of four and two direct sales people in Latin America. We may add clinical and sales support personnel at both the direct and distributor level in Asia Pacific and Europe to ensure a high level of global physician support for all our products. Our marketing group currently consists of 10 employees in North America and 4 employees in Europe.
We have entered into agreements with distributors in several European countries, as well as portions of the Middle East, Asia and Latin America. Our distributors are experienced in the interventional device markets and have relationships with leading neurointerventionalists and institutions in those countries. Our standard distribution agreement generally (i) provides our distributors with an exclusive right to distribute our products in a certain territory; (ii) restricts them from selling products that are competitive with our products for the limited duration of our agreement with them; (iii) obligates them to obtain the necessary authorizations, licenses and approvals to import, market and distribute our products within the applicable territory; and (iv) obligates them to promote and distribute our products within the applicable territory.
We believe that Japan represents a significant market for our products. On September 30, 2005, we entered into a five-year, exclusive distribution agreement with Goodman to promote and market our products in Japan. In February 2006, we received the requisite local regulatory approvals to sell certain of our products in Japan through Goodman, and the sale of such products in Japan commenced in March 2006. On September 20, 2007, we amended the distribution agreement with Goodman to, among other things, extend the duration of the distribution agreement to six years from the original date of the distribution agreement. In December 2007, we received regulatory approval to sell our stretch-resistant microcoils in Japan, and we received regulatory approval for our Cerecyte® microcoils in July 2008.
Information about our revenues from sales to unaffiliated customers is included in Note 14 of the “Notes to Consolidated Financial Statements,” which are included in “Item 8 – Financial Statements and Supplementary Data” of this report.
We generate revenues from sales to hospitals and third-party distributors. Once a sale has occurred, the customer has no right of return. We provide the customers with limited warranty privileges.
Goodman, our distributor in Japan, accounted for 12% of revenues for both years ended March 31, 2010 and 2009. No customer accounted for 10% or more of our revenues for the year ended March 31, 2008.
Research and Development
Our product development efforts are focused on designing microcoils, guidewires, microcatheters, balloons, thrombectomy devices and stents for the treatment of hemorrhagic and ischemic stroke. We are working to develop next generation microcoils to frame and fill the aneurysm more efficiently and thoroughly. Also under development are next-generation balloon expandable stents for the treatment of intracranial atherosclerosis, self-expanding stents and covered stents, as well as occlusion balloons to augment the treatment of aneurysms with microcoils.
14
Additionally, we are developing technologies to treat acute ischemic stroke. In October 2007, we acquired an exclusive license to revascularization and clot retrieval technology for the treatment of ischemic stroke, known as ReVive™ SE, through the acquisition of ReVasc from The Cleveland Clinic. In January 2008, we entered into a license, development and commercialization agreement with Genesis. Under the terms of the agreement, we licensed the rights to Genesis’ F.A.S.T. Funnel Catheter and clot retrieval and revascularization system, known as ReVive™ CX, for the treatment of ischemic stroke. In April 2008, we entered into a co-development agreement with Chemence to jointly develop a liquid embolic product for the treatment of cerebral aneurysms using Chemence’s cyanoacrylate technology, development capabilities and intellectual property. In August 2009, we entered into a license, development and commercialization agreement with FSS to jointly develop a flow diversion product for neurovascular indications using both Micrus and FSS technology, development capabilities and intellectual property. In January 2010, we entered into a license, development and commercialization agreement with Bay Street regarding their stent delivery system.
As of March 31, 2010, we had 37 full-time employees engaged in research and development activities. Research and development expenses for the fiscal years ended March 31, 2010, 2009 and 2008 were $10.4 million, $10.2 million and $13.7 million, respectively.
Biotronik Collaboration
In January 2006, we entered into a license, development and distribution agreement with Biotronik, pursuant to which we will collaborate with Biotronik to develop certain neurovascular products and we will be the exclusive worldwide distributor for jointly developed neurovascular products. Biotronik granted us an exclusive license to certain patents, know-how and other proprietary technology in the neurovascular field.
Under the terms of our agreement, we paid an up-front licensing fee of approximately $0.6 million to Biotronik and were required to make milestone payments to Biotronik upon receipt of approvals to market stent products we jointly developed for the treatment of neurovascular disease and royalty payments on the products sold. We are also required to pay Biotronik royalties equal to 15% of the actual net sales of our PHAROS® stent on a quarterly basis. In February 2006, Biotronik received CE Mark authorization for the PHAROS® stent intended for both the treatment of aneurysms and the treatment of ischemic diseases. As a consequence, we made milestone payments to Biotronik of approximately $0.7 million in both March and April 2006. We paid an additional cost associated with the PHAROS® stent development of $102,000 in the first quarter of fiscal 2007. Additionally, we have incurred royalties to Biotronik of approximately $318,000, $169,000 and $150,000 for the products sold in fiscal 2010, 2009 and 2008, respectively. There are no future milestone payments to Biotronik related to the PHAROS® stent. Additionally, we will continue to fund ongoing project development based on the terms of this agreement.
ReVasc Acquisition
In October 2007, we entered into a Stock Purchase Agreement (the “ReVasc Agreement”) with The Cleveland Clinic and acquired ReVasc, a wholly-owned subsidiary of The Cleveland Clinic for an aggregate up-front purchase price of $1.0 million. Pursuant to the ReVasc Agreement, we also agreed to pay The Cleveland Clinic up to $5.0 million in additional payments upon the achievement of certain milestones set forth in the ReVasc Agreement. This includes minimum milestone payments of at least $2.0 million due to The Cleveland Clinic by October 2010. The first milestone payment in the amount of $500,000 was paid in March 2008. In January 2010, we paid The Cleveland Clinic an additional milestone payment of $2.0 million, which did not count towards the minimum milestone payments.
ReVasc was a party to a license agreement with The Cleveland Clinic (the “ReVasc License Agreement”) pursuant to which The Cleveland Clinic granted ReVasc an exclusive license to its revascularization technology for the treatment of ischemic stroke. In connection with the acquisition, the parties amended the ReVasc License Agreement to provide, among other matters, for the payment to The Cleveland Clinic of certain royalties for sales of products based on the technology subject to the ReVasc License Agreement.
On December 7, 2007, we merged ReVasc into Micrus. Following the merger, Micrus became the direct recipient of the license of the revascularization technology from The Cleveland Clinic under the ReVasc License Agreement. The ReVasc technology is now known as ReVive™ SE.
Genesis Collaboration
In January 2008, we entered into a license, development and commercialization agreement with Genesis. Under the terms of the agreement, we licensed the rights to Genesis’ F.A.S.T. Funnel Catheter, a revascularization platform and clot retrieval system for the treatment of ischemic stroke. In fiscal 2011, we plan to finalize our development and validation work on these Ischemic products and intend to perform safety studies outside the United States. The transaction with Genesis includes an initial up-front payment of $0.8 million, a development milestone payment of $150,000, which was paid in October 2008, and royalties on potential future product sales. Additionally, we paid $277,000 and $351,000 in consulting fees to Genesis in fiscal 2010 and 2009, respectively, for the funnel catheter product development. The Genesis technology is now known as ReVive™ CX.
15
Disposition of Cardiac and Peripheral Catheter Platform Assets
In January 2008, we entered into an Asset Purchase and Supply Agreement (the “Merit Agreement”) with Merit Medical Systems, Inc. (“Merit”) pursuant to which we sold certain cardiac and peripheral catheter platform assets and technology (the “Merit Transaction”). The majority of the assets sold were originally acquired by us in November 2006 in connection with our purchase of VasCon. Pursuant to the Merit Agreement, we received an up-front payment of $1.5 million and received an additional $1.5 million in December 2008 upon the completion of our obligation to help Merit build a production line for coronary guide catheters. Under the terms of the Merit Agreement, we also agreed to manufacture and supply certain guide catheters to Merit for a period of up to one year following the closing. This obligation expired in January 2009, and we no longer supply guide catheters to Merit.
In connection with the Merit Transaction, we also entered into a license agreement granting Merit the right to use certain non-patented intellectual property in the cardiology and peripheral radiology fields and a non-competition agreement, whereby we agreed not to engage in certain competitive business activities in the fields of cardiology and peripheral radiology for a period of five years.
Our remaining obligations to Merit terminated on September 30, 2009 and therefore, the deferred gain of $1.9 million was recognized as other income in the quarter ended September 30, 2009.
Chemence Collaboration
In April 2008, we entered into a co-development agreement with Chemence to jointly develop a liquid embolic product for the treatment of cerebral aneurysms using Chemence’s cyanoacrylate technology, development capabilities and intellectual property. We will be responsible for overseeing the regulatory and clinical process and will be the exclusive worldwide distributor for the neurovascular product developed based on this collaborative agreement. Under the terms of the agreement, we have made an up-front payment of $100,000 to Chemence and will make additional payments of up to $200,000 upon achieving certain development milestones.
FSS Collaboration
In August 2009, we entered into a License, Development and Commercialization Agreement (the “FSS Agreement”) with FSS. Under the terms of the FSS Agreement, Micrus and FSS will jointly develop a flow diversion product for neurovascular indications using both Micrus and FSS technology, development capabilities and intellectual property. We will be responsible for overseeing the regulatory and clinical process and will manufacture neurovascular products developed under the terms of this collaborative agreement. The transaction included an initial up-front payment of $0.5 million, future development and regulatory milestone payments and royalties on potential future product sales. In March 2010, we amended the original agreement with FSS to expand the projects focus to include a non-flow diversion variation of the FSS stent platform for use as a neurovascular stent.
Bay Street Agreement
In January 2010, we entered into an Exclusive License and Option Agreement (the “Bay Street Agreement”) with Bay Street. Pursuant to the terms of the Bay Street Agreement, Bay Street granted us a worldwide exclusive license and option to purchase intellectual property assets held by Bay Street related to its proprietary stent delivery and locking technology (the “Bay Street Assets”). The transaction included an initial up-front payment of $0.5 million for a six month evaluation of the exclusive license. We have the option to extend the exclusive license for an additional six months upon paying to Bay Street an option payment of $0.5 million. We also have the option to purchase the Bay Street Assets before the option period ends pursuant to an Asset Purchase Agreement that will be an addendum to the Bay Street Agreement. Under the terms of the Asset Purchase Agreement, if we elect to purchase the Bay Street Assets, we would be required to pay an initial purchase payment of $1.0 million, a future sales-based milestone payment and royalties on future product sales.
Physician Advisors
We rely extensively on our physician advisors to advise us on our research and development efforts and to provide feedback on the clinical use of our products. Our advisors are experts in interventional neuroradiology and cerebral vascular diseases. We regularly consult with our physician advisors regarding our research and development efforts, preclinical trials and clinical trials.
16
Some of our physician advisors receive a consulting fee for providing services to Micrus. All of our physician advisors are reimbursed for reasonable expenses. In addition, our medical advisors receive compensation for clinical studies they conduct for us. All of our medical advisors are employed by other organizations and may have commitments to or have consulting arrangements with other companies, including our competitors, which may limit their availability to consult with us. Although these advisors may contribute significantly to our business, we generally do not expect them to devote more than a small portion of their time to us. We also routinely seek advice, input and feedback on our products and business from a larger broader group of physicians and advisors, some of whom may also receive appropriate compensation for their time and expert opinions.
Manufacturing
We manufacture and/or assemble, inspect, test and package all our proprietary microcoils and microcatheters at our headquarters in San Jose, California, or in Miramar, Florida. Most of our non-embolic products are manufactured in Miramar, Florida. As of March 31, 2010, we had 164 employees in manufacturing, quality control, manufacturing engineering and materials and logistics.
We have substantial design, manufacturing and applications engineering expertise in the development of small vessel access and delivery systems and intend to continue to leverage this expertise to develop new products. By designing and manufacturing most of the components of our products, we have been able to maintain greater control of quality and manufacturing process changes. Our microcoils are very small in size, ranging from 1.0 mm to 20.0 mm in diameter and are manufactured using microfabrication techniques. We have developed proprietary manufacturing technologies and processes in the areas of platinum memory shaping, metal fabrication, balloon fabrication and microcatheter and stent fabrication.
Trained product personnel assemble and test each of our components and products in controlled environment rooms. At various assembly stages each lot of product undergoes thorough testing to ensure compliance with applicable regulations, including Quality System Regulations (“QSR”) requirements in the United States and ISO 13485 certification standards in Europe. These standards specify the requirements necessary for a quality management system to consistently provide products that meet customer requirements and to include processes for achieving the outputs of the quality management system that are required in order to obtain a CE Mark to sell medical devices within the European Union. Our quality assurance group verifies that product fabrication and inspection process steps meet our stringent quality specifications and applicable regulatory requirements. Upon successful completion of these steps, the products are packaged, sterilized and prepared for shipment. We typically ship products as orders are received.
We have implemented quality control systems as part of our manufacturing processes, which we believe are in substantial compliance with United States Good Manufacturing Practices (“GMP”) or QSR requirements. Our San Jose facility has also been inspected by the California Department of Health Services on behalf of the State of California and under contract with the FDA, and is registered with the State of California to manufacture our products. We believe that we are in compliance with the FDA GMP for medical devices, and our facilities are subject to inspection by the FDA. The most recent of such inspections occurred in April 2010 in San Jose, California and in September 2007 in our Doral, Florida facility, prior to the relocation of that plant to Miramar, Florida. However, we cannot assure that we will remain in compliance with GMP and our failure to do so could have a material adverse effect on our business, operating results and financial condition.
We purchase the raw materials required for production from various qualified outside vendors. In addition, the deployment control box is manufactured by an outside supplier. We rely on single sources for some of our critical components, including the deployment control box, the platinum used to manufacture the microcoils and certain custom hypodermic tubing material. In addition, we have a sole source subcontract arrangement for sterilization services. We believe that we have alternative sources for most of the components purchased from single sources currently and generally maintain an adequate supply of products to avoid production interruptions. Where we do not have a qualified second source vendor for a product component and depending on the exact component, we believe that it would take us from two days to a month to either manufacture the product component ourselves or have a readily available new supply of the product component. Any unanticipated interruption in the supply of these components and services could have a material adverse effect on us.
Patents and Proprietary Rights
Our success depends in part on our ability to obtain and maintain proprietary protection for our products, technology and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek protection of our proprietary position by filing United States and foreign patent applications to protect technology, inventions and improvements that are important to our business. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position. We hold 76 issued United States patents and 121 issued foreign patents expiring between 2015 and 2027. In addition, we have 37 United States and 66 foreign patent applications pending covering various aspects of our products and technology.
17
The issued patents relate, among other things, to subject matter in the following areas:
|
•
|
vaso-occlusive microcoils and devices, and methods for manufacturing such coils and devices;
|
|
•
|
microcoil deployment systems;
|
|
•
|
bioactive microcoils;
|
|
•
|
intracranial vascular stents;
|
|
•
|
catheters for neurovascular intervention;
|
|
•
|
embolic clot retrieval devices; and
|
|
•
|
bioactive material placement systems and methods.
|
In addition to developing our own technology, we have obtained licenses to certain patents and other intellectual property, including for materials used as coating on our guidewires and for certain types of coils. These licenses grant us the right to use the licensed patents to make, use and sell products that contain the licensed technology. We pay for these licenses through a combination of fixed payments and royalties on sales of covered products. Each of these licenses continues until expiration of the licensed patents. Payments under these license arrangements currently do not account for a material portion of our expenses.
Although we work aggressively to protect our technology, there is no assurance that any patents will be issued from current pending patent applications or from future patent applications. We also cannot assure that the scope of any patent protection will exclude competitors or provide competitive advantages to us, that any of our patents will be held valid if subsequently challenged, or others will not claim rights in or ownership of our patents and proprietary rights. Furthermore, there can be no assurance that others have not developed or will develop similar products, duplicate any of our products or design around our patents.
The medical device industry has been characterized by extensive litigation regarding patents and other intellectual property rights, and many companies in the industry have employed intellectual property litigation to gain a competitive advantage.
In addition to patents, we rely on trademark, trade secret and other intellectual property laws, nondisclosure agreements and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must develop and maintain the proprietary aspects of our technologies. We require our employees, consultants and advisors to execute confidentiality agreements in connection with their employment, consulting or advisory relationships with us. We also require our employees, consultants and advisors who we expect to work on our products to agree to disclose and assign to us all inventions conceived while working for us, using our property or which relate to our business. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary.
Competition
We compete primarily with the neurovascular division of Boston Scientific; Codman Neurovascular, a division of Johnson & Johnson; ev3 Inc and MicroVention, a division of Terumo. Boston Scientific, Codman Neurovascular and ev3 offer broad product lines consisting of embolic microcoils, microcatheters, stents, balloons and guidewires. Boston Scientific, Codman Neurovascular, ev3 and MicroVention currently market a variety of microcatheters which are compatible with our coil systems.
Currently, Boston Scientific is the only company we are aware of other than Micrus that sells bioactive microcoils. Boston Scientific markets the Matrix coil which features a platinum core coated with an absorbable suture material intended to cause a tissue reaction. MicroVention markets the HydroCoil® which is an embolic microcoil that swells in the presence of fluid to provide greater volumetric occlusion to an aneurysm. Codman Neurovascular markets a bare platinum line of microcoils but does not market bioactive or stretch resistant microcoils. Through its acquisition by Terumo, MicroVention now markets microcatheters, guide catheters and guidewires as well, joining Boston Scientific, Codman Neurovascular, ev3 and Micrus in this market segment. Boston Scientific has received from the FDA a Humanitarian Device Exemption (“HDE”) to market stents for the treatment of hemorrhagic and ischemic stoke. Codman Neurovascular has received an HDE to market a stent indicated for the treatment of hemorrhagic stroke.
18
Boston Scientific, MicroVention, ev3 and Codman Neurovascular are all large publicly traded companies or divisions of large publicly traded companies and enjoy several competitive advantages over us, including: greater financial and personnel resources; significantly greater name recognition; established relationships with neurointerventionalists; established distribution networks; greater resources for product research and development; greater experience in, and resources for, launching, marketing, distributing and selling products; and more broad-based and deeper product lines. On June 1, 2010, Covidien plc, a large publicly traded company, announced that it had entered into a definitive merger agreement with ev3. Upon completion of the merger, ev3 will become a division of Covidien plc.
We believe that the principal competitive factors in the market for medical devices used in the treatment of cerebral vascular diseases include:
|
•
|
improved patient outcomes as a result of physician use of the device;
|
|
•
|
access to and acceptance by leading physicians;
|
|
•
|
depth of product line;
|
|
•
|
product quality and reliability;
|
|
•
|
ease of use for physicians;
|
|
•
|
sales and marketing capability; and
|
|
•
|
brand recognition and reputation.
|
Our current or potential competitors may succeed in developing technologies and products that are more effective than those developed by us or that would render our products obsolete or noncompetitive. Additionally, there can be no assurance that we will be able to effectively compete with such competitors in the manufacturing, marketing and sale of our products. At any time, other companies may develop alternative treatments, products or procedures for the treatment of cerebral aneurysms that compete directly or indirectly with our products. If alternative treatments prove to be superior to our products, adoption of our products could be negatively affected and our future revenues could suffer.
Our ability to develop safe, effective and reliable products in a timely manner is the key to our competitive position. Consequently, our success will depend on how quickly we are able to respond to medical and technological changes through the development, clinical evaluation and commercialization of new products. Product development involves a high degree of risk and there can be no assurance that our research and development efforts will result in commercially successful products.
Government Regulation
|
|
United States
|
The research, development, manufacture, labeling, distribution and marketing of our products are subject to extensive regulation by the FDA and other regulatory bodies. Our current products are regulated by the FDA as medical devices, and we are required to obtain review and clearance or approval from the FDA prior to commercializing our devices.
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either prior 510(k) clearance or prior premarket approval (“PMA”) from the FDA. The FDA classifies medical devices into one of three classes. Class I devices are subject to general controls (e.g. establishment registration and device listing, labeling, medical devices reporting (“MDR”), 510(k) premarket notification and prohibitions against adulteration and misbranding). Many class I devices are exempt from 510(k) premarket notification. Class II medical devices are subject to both general controls and special controls and typically require prior 510(k) clearance before they may be commercially marketed. The FDA will clear marketing of a medical device through the 510(k) process if it is demonstrated that the new product is substantially equivalent to another legally marketed device, including a 510(k)-cleared product, and otherwise meets the FDA’s requirements. Devices deemed by the FDA to pose a great risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a legally marketed device are placed in class III, most of which require premarket approval. Both premarket clearance and premarket approval applications are subject to the payment of user fees, paid at the time of submission for FDA review. The FDA officially reclassified neurovascular embolization devices such as our microcoils products to class II medical devices effective January 28, 2005. For our microcoil products, catheter products and guidewire product, we have obtained multiple 510(k) clearances.
19
510(k) Clearance
To obtain 510(k) clearance, we must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications. The FDA’s 510(k) clearance pathway usually takes from three to twelve months from the date the application is submitted, but it can take significantly longer.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a significant change in its intended use, will require a new 510(k) clearance or could require premarket approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket approval is obtained. If the FDA requires us to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
Premarket Approval
A PMA application must be submitted if the device cannot be cleared through the 510(k) process. A PMA application must be supported by extensive data including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use.
After a PMA application is accepted for filing, the FDA begins an in-depth review of the submitted information, which generally takes between one and three years, but may take significantly longer. During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a pre-approval inspection of the manufacturing facility to ensure compliance with quality system regulations. New PMA applications or PMA supplements are required for significant modifications to the manufacturing process, labeling or design of an approved device. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application, and may not require such extensive clinical data or the convening of an advisory panel.
Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
|
•
|
quality system regulation (QSR), which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures during the manufacturing process (otherwise known as Good Manufacturing Practices or GMPs);
|
|
•
|
labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and
|
|
•
|
medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur.
|
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
|
•
|
fines, injunctions, and civil penalties;
|
|
•
|
recall or seizure of our products;
|
|
•
|
operating restrictions, partial suspension or total shutdown of production;
|
|
•
|
refusing our request for 510(k) clearance or premarket approval of new products;
|
|
•
|
withdrawing 510(k) clearance or premarket approvals that are already granted; and
|
20
|
•
|
criminal prosecution.
|
We are also subject to unannounced inspections by the FDA and the Food and Drug Branch of the California Department of Health Services, and these inspections may include the manufacturing facilities of our subcontractors.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain market authorization by a foreign country may be longer or shorter than that required for FDA market authorization, and the requirements may differ. For example, in China, we have submitted market applications for our microcoils, microcatheters and the PHAROS® intracranial stent. The regulatory process in China includes administrative review of technical documentation, test reports and labeling and also physical testing of products to test standards before a license for commercial distribution is granted. The regulations in Central and South American countries vary substantially from country to country. We have received market authorizations for our microcoils and certain other products in the following Central and South American countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Peru and Venezuela.
The primary regulatory environment in Europe is that of the European Union, which consists of countries encompassing most of the major countries in Europe. The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout Europe. The method of assessing conformity varies depending on the class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body.” This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s product. An assessment by a Notified Body in one country within the European Union is required in order for a manufacturer to commercially distribute the product throughout the European Union.
In Japan, medical devices must be approved by the MHLW prior to importation and commercial sale. Manufacturers of medical devices outside of Japan must utilize a contractually bound Japanese entity to submit an application for device approval to the MHLW. The MHLW evaluates each device for safety and efficacy. As part of its approval process, the MHLW may require that the product be tested in Japanese laboratories. The approval process for products such as our existing microcoil products is typically 12-15 months once an application has been accepted for review. Other medical devices may require a longer review period for approval. Once approved, the manufacturer may import the device into Japan for sale by the manufacturer’s contractually bound importer or distributor.
After a device is approved for importation and commercial sale in Japan, the MHLW continues to monitor sales of approved products for compliance with labeling regulations, which prohibit the promotion of devices for unapproved uses and reporting regulations, which require reporting of product malfunctions, including serious injury or death caused by any approved device. Failure to comply with applicable regulatory requirements can result in enforcement action by the MHLW, which may include fines, injunctions, and civil penalties, recall or seizure of our products, operating restrictions, partial suspension or total shutdown of sales in Japan, or criminal prosecution.
In addition to MHLW oversight, the regulation of medical devices in Japan is also governed by the Japanese Pharmaceutical Affairs Law (“PAL”). PAL was substantially revised in July 2002, and the new provisions were implemented in stages through April 2005. Revised provisions of the approval and licensing system of medical devices in Japan, which constitutes the core of import regulations, came into effect on April 1, 2005. The revised law changes class categorizations of medical devices in relation to risk, introduces a third party certification system, strengthens safety countermeasures for biologically derived products, and reinforces safety countermeasures at the time of resale or rental. The revised law also abolishes the ICC system and replaces it with the “primary distributor” system. Under the revised PAL, manufacturers outside of Japan must now appoint a “primary distributor” located in Japan that holds a primary distributor license for medical devices to provide primary distribution services including conducting quality assurance and safety control tasks for each product at the time an application for the approval of such product is submitted to the MHLW. We are unable at this time to determine the impact of such changes on our approved products, products for which we have already applied for approval in Japan or future products. We do not anticipate that these changes will have a material impact on our existing level of third-party reimbursement for sales of our products in Japan.
21
Third-Party Reimbursement
We believe that the procedures performed using our products are generally already reimbursable under government programs and most private health insurance plans. Accordingly, we believe that providers in the United States will generally not be required to obtain new billing authorizations or codes in order to be compensated for performing medically necessary procedures using our products on insured patients or patients covered under government programs such as Medicare and Medicaid. We also believe that our procedures will be generally reimbursable under governmental programs and private health insurance plans in Japan. In Japan, we are required to obtain regulatory clearance for our products to be eligible for reimbursements by third party payors, even though reimbursement for embolic coiling procedure is already in place. In China, there is only limited healthcare reimbursement available for the treatment of stroke.
We cannot assure that reimbursement polices of third party payors will not change in the future with respect to some or all of the procedures using our products and systems. See “Item 1A - Risk Factors” — If neurointerventionalists are unable to obtain sufficient reimbursement for procedures performed with our products, it is unlikely that our products will be widely used” for a discussion of various risks associated with reimbursement from third party payors.
Product Liability and Insurance
We maintain general liability insurance, product liability insurance, directors and officers’ liability insurance, workers compensation insurance and other insurance coverage that we believe are customary in types and amounts for the type of business that we operate. Medical device companies are subject to an inherent risk of product liability and other liability claims in the event that the use of their products results in personal injury claims. Any such claims could have an adverse impact on us. There can be no assurance that product liability or other claims will not exceed such insurance coverage limits or that such insurance will continue to be available on commercially acceptable terms, if at all.
Environmental
Our company is subject to a variety of federal, state and local environmental protection measures. We believe that our operations comply in all material respects with applicable environmental laws and regulations. Our compliance with these requirements did not have a material effect upon our capital expenditures, cash flows, earnings or competitive position during the past fiscal year, and is not expected to in the future. Given the scope and nature of these laws, however, there can be no assurance that our compliance with environmental laws and regulations will not have a material impact on our results of operations.
Employees
As of March 31, 2010, in the United States we had 307 full time employees, including 50 in sales and marketing, 164 in operations and manufacturing, 37 in research and development, 20 in quality assurance and regulatory compliance, and 36 in general and administrative functions. As of March 31, 2010, we had 34 employees in Europe, including 22 in sales and marketing and 12 in general and administrative functions. None of our employees are represented by a collective bargaining agreement and we have never experienced a work stoppage.
Seasonality
Our worldwide sales frequently reflect a degree of seasonality. In both the Americas and Europe we typically experience somewhat lower demand in the second fiscal quarter than throughout the rest of the fiscal year as a result of the summer vacation schedule.
Available Information
We are required to file reports under the Exchange Act with the Securities and Exchange Commission (the “SEC”). You may read and copy our materials on file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information regarding the SEC’s Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website at http://www.sec.gov that contains reports, proxy and information statements and other information.
You may also obtain copies of our annual report on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K and amendments to these reports as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC free of charge by visiting the investor relations page on our website, www.micrusendovascular.com. Information contained on our website is not part of this annual report on Form 10-K.
22
Certain Factors that May Affect Our Business and Future Results
Some of the information included herein contains forward-looking statements as defined in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such forward-looking statements are based on the beliefs of, estimates made by and information currently available to our management and are subject to certain risks, uncertainties and assumptions. Any statements contained herein (including, without limitation, statements to the effect that the Company, we, or management “may,” “will,” “expects,” “anticipates,” “estimates,” “predicts,” “continues,” “plans,” “believes,” or “projects,” “should,” “could,” “would,” “intends” or statements concerning “potential” or “opportunity,” and any variations thereof, comparable terminology or the negative thereof) that are not statements of historical fact should be construed as forward-looking statements. Our actual results may vary materially from those expected in these forward-looking statements. The realization of such forward-looking statements may be impaired by risks including, but not limited to the following:
Current worldwide economic conditions may adversely affect our business, operating results and financial condition, as well as further decrease our stock price.
General worldwide economic conditions have experienced a downturn due to the effects of the subprime lending crisis, European sovereign debt crisis, general credit market crisis, collateral effects on the finance and banking industries, concerns about inflation, slower economic activity, decreased consumer confidence, reduced corporate profits and capital spending, adverse business conditions and liquidity concerns. Our business is not immune. Some of the procedures that use our products are elective and therefore can be deferred by patients. In light of the current economic conditions, patients who do not have insurance covering the total cost of elective procedures may choose to defer or forego them. In addition, in the U.S. and other countries where healthcare coverage is heavily dependent on employment status, increasing numbers of patients may have no or reduced healthcare coverage. Furthermore, government instability may cause certain governments to delay, withhold or reduce reimbursement for our products which could adversely affect our revenues.
The worldwide economic downturn also may have other adverse implications for our business. For example, our customers’ and distributors’ ability to borrow money from their existing lenders, or to obtain credit from other sources to purchase our products may be impaired. Though we maintain allowances for doubtful accounts that are sufficient to cover estimated losses which may occur when customers cannot make their required payments and though such estimated losses have historically been within our expectations and the provisions established, we cannot guarantee that we will continue to experience the same loss rates that we have in the past, especially given the current turmoil in the worldwide economy. A significant change in the liquidity or financial condition of our customers or those governments that reimburse our customers for our products could cause unfavorable trends in our receivable collections and additional allowances may be required, thus adversely affecting our operating results. In addition, the worldwide economic crisis may adversely impact our suppliers’ ability to provide us with materials and components, which could adversely affect our business and operating results. Like the stock prices of many other companies, our stock price has recently decreased substantially. If investors have concerns that our business, operating results and financial condition will be negatively impacted by a continued worldwide economic downturn, our stock price could further decrease.
Our future success is dependent on the continued growth in embolic coiling procedures and our ability to convince a concentrated customer base of neurointerventionalists to use our products as an alternative to other available products.
Our future success and revenue growth are significantly dependent upon an increase in the use of embolic coiling as a procedure to treat cerebral aneurysms. If the number of embolic coiling procedures does not increase or if a new procedure that does not employ our products becomes a more acceptable alternative among neurointerventionalists, our business would be seriously harmed.
The number of interventional neuroradiologists and neurosurgeons trained to conduct embolic coiling procedures is relatively small, both in the United States and abroad. There are currently approximately 300-400 neurointerventionalists in the United States who perform embolic coiling procedures. We believe that less than one-third of these physicians perform a substantial majority of the total number of embolic coiling procedures per year. For the year ended March 31, 2010, a substantial portion of our product sales in the United States were to an estimated 118 hospitals. The growth in the number of interventional neuroradiologists and neurosurgeons in the United States is constrained by the lengthy training programs required to educate these physicians. Accordingly, our revenue growth will be primarily dependent on our ability to increase sales of our products to our existing customers and to increase sales of products to trained neurointerventionalists that currently use products offered by our competitors. We believe that neurointerventionalists who do not currently use our products will not widely adopt our products unless they determine, based on experience, clinical data and published peer reviewed journal articles, that our products provide benefits or an attractive alternative to the clipping of aneurysms or the use of competitors’ products. We believe that neurointerventionalists base their decision to use an alternative procedure or product on the following criteria, among others:
23
|
•
|
extent of clinical and multi-center trial evidence supporting patient benefits;
|
|
•
|
their level of experience with the alternative product;
|
|
•
|
perceived liability risks generally associated with the use of new products and procedures;
|
|
•
|
availability of reimbursement within healthcare payment systems; and
|
|
•
|
costs associated with the purchase of new products and equipment.
|
In addition, we believe that recommendations and support of our products by influential physicians are essential for market acceptance and adoption. That support may be influenced by the results of clinical studies of our products or those of our competitors. If we do not receive continued support from such influential physicians, neurointerventionalists and hospitals may not use our products. In such circumstances, we may not achieve expected revenue levels and our business will suffer.
The results of clinical trials could negatively impact our business.
We believe that neurointerventionalists base their decision to use an alternative procedure or product in part on the result of clinical evidence supporting patient benefits. Consequently, results of clinical research and trials on our existing products and new products in development, as well as those of our competitors, will influence demand for our products. To the extent that clinical trials report negative news about our products or positive news about our competitors products we may lose sales.
For example, we recently announced some interim data from an ongoing clinical study comparing results of coiling with our Cerecyte® microcoils against Micrus bare platinum microcoils. Based on our preliminary discussions with Dr. Molyneux, the principal investigator of the trial, we believe that he will present interim data demonstrating that, based on the core lab assessment of angiographic success, the Cerecyte® and Micrus bare platinum groups are superior to those of the ISAT study and that the safety outcomes for Micrus Cerecyte® and Micrus bare platinum coils are also superior to the coiling results reported in the ISAT study, as well as all other published randomized data. In addition, Dr. Molyneux has advised us that due to the strong performance of Micrus bare platinum coils, it is unlikely that the data will demonstrate a statistically significant difference in angiographic outcome between the two groups. Our Cerecyte® coils are more expensive than our bare platinum coils, although we believe that our bare platinum coils are priced lower than many of our large competitors. It is unclear whether the impact of this study will result in a decline in our sales of Cerecyte® coils or an increase in our overall sales as a result of the superior performance of our coils generally. If we experience a decline in sales of our Cerecyte® coils which is not offset by increases in revenue generated from our bare platinum coils our operating results will be harmed.
Our industry is experiencing increased scrutiny by governmental authorities, which has led to increased compliance costs and potentially more rigorous regulation
The medical device industry is subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. These authorities have been increasing their scrutiny of certain activities of medical device companies, including their conduct of clinical trials, their handling of conflicts of interests and financial arrangements with health care providers (“HCPs”) and their product promotional practices. We are about to commence our first human clinical trials which will require us to comply with patient safety and enrollment regulations. We anticipate that government authorities will continue to scrutinize our industry closely and we may be subject to more rigorous regulation by governmental authorities in the future. This increased government scrutiny has led us to incur increased costs on compliance, human resources costs and the diversion of management and employee focus and we anticipate that such costs will continue to increase. We have adopted a number of compliance procedures and provide regular compliance training to our employees who interact with HCPs but we cannot assure that our activities will not be subject to inquiry or greater action or oversight by governmental authorities or that we will be able to comply with any new regulations. Any failure by us to adopt appropriate compliance procedures and ensure that our employees and agents comply with applicable laws and regulations could result in substantial penalties and/or restrictions in our business activities and the sales of our products.
We have been profitable for each of the last five quarters, but there is no assurance that we will continue to be profitable in the future.
We were incorporated in the State of Delaware in 1996, and began commercial sales of our microcoil products in 2000. We have incurred annual net losses since our inception, including net losses of $11.1 million, $16.3 million, and $5.5 million in fiscal 2009, 2008, and 2007, respectively. In the fourth quarter of fiscal 2009 we achieved our first profitable quarter with $0.6 million of net income and in fiscal 2010 we reported our first profitable fiscal year with $11.5 million of net income. At March 31, 2010, we reduced our accumulated deficit to approximately $70.9 million. There is no assurance that we will continue to be profitable in the future. We expect our operating expenses to increase as we, among other things:
24
|
•
|
grow our internal and third-party sales and marketing forces to expand the sales of our products in the United States and internationally;
|
|
•
|
increase our research and development efforts to improve upon our existing products and develop new products;
|
|
•
|
perform clinical research and trials on our existing products and product candidates;
|
|
•
|
expand our regulatory resources in order to obtain governmental approvals for our existing product enhancements and new products;
|
|
•
|
acquire and/or license new technologies; and
|
|
•
|
expand manufacturing.
|
As a result of these activities, we may not be able to sustain or increase profitability on an ongoing basis.
Our quarterly operating and financial results and our gross margins are likely to fluctuate significantly in future periods.
Our quarterly operating and financial results are difficult to predict and may fluctuate significantly from period to period. The level of our revenues, gross margins and results of operations at any given time will be based primarily on the following factors:
|
•
|
neurointerventionalist and patient acceptance of our products;
|
|
•
|
changes in the number of embolic coiling procedures performed to treat cerebral aneurysms;
|
|
•
|
the seasonality of our product sales;
|
• the mix of our products sold;
|
•
|
stocking patterns for distributors;
|
|
•
|
the development of new procedures to prevent and treat hemorrhagic and ischemic stroke;
|
|
•
|
results of clinical research and trials on our existing products and products in development or those of our competitors;
|
|
•
|
demand for, and pricing of, our products;
|
|
•
|
levels of third-party reimbursement for our products;
|
|
•
|
timing of new product offerings, acquisitions, licenses or other significant events involving us or our competitors;
|
|
•
|
increases in the costs of manufacturing and selling our products;
|
|
•
|
the amount and timing of our operating expenses;
|
|
•
|
potential litigation expenses;
|
|
•
|
fluctuations in foreign currency exchange rates;
|
|
•
|
regulatory approvals and legislative changes affecting the products we may offer or those of our competitors;
|
|
•
|
the effect of competing technological and market developments;
|
|
•
|
changes in our ability to obtain and maintain FDA and other domestic and foreign regulatory approval or clearance for our products;
|
|
•
|
inventory adjustments we may have to make in any quarter;
|
|
•
|
interruption in the manufacturing or distribution of our products;
|
|
•
|
our ability to maintain and expand our sales force and operational personnel;
|
25
|
•
|
the ability of our suppliers to timely provide us with an adequate supply of materials and components; and
|
|
•
|
amount and timing of capital expenditures and other costs relating to any potential expansion of our operations.
|
Many of the products we may seek to develop and introduce in the future will require FDA approval or clearance and will be required to meet similar regulatory requirements in other countries where we seek to market our products, without which we cannot begin to commercialize them. Forecasting the timing of sales of our products is difficult due to the delay inherent in seeking FDA and other clearance or approval, or the failure to obtain such clearance or approval. In addition, we will be increasing our operating expenses as we build our commercial capabilities. Accordingly, we may experience significant, unanticipated quarterly losses. Because of these factors, our operating results in one or more future quarters may fail to meet the expectations of securities analysts or investors, which could cause our stock price to decline significantly.
We may not be able to develop new products or product enhancements that will be accepted by the market.
Our success will depend in part on our ability to develop and introduce new products and enhancements to our existing products. We cannot assure that we will be able to successfully develop or market new products or that any of our future products will be accepted by the neurointerventionalists who use our products or the payors who reimburse for many of the procedures performed with our products. The success of any new product offering or enhancement to an existing product will depend on several factors, including:
|
•
|
our ability to properly identify and anticipate neurointerventionalist and patient needs;
|
|
•
|
our ability to develop new products or enhancements in a timely manner;
|
|
•
|
our ability to leverage the results of clinical and multi-center trial evidence supporting patient benefits;
|
|
•
|
our ability to obtain the necessary regulatory approvals for new products or product enhancements;
|
|
•
|
our ability to provide adequate training to potential users of our products;
|
|
•
|
our ability to receive adequate reimbursement for our procedures;
|
|
•
|
results of clinical research and trials on our existing products and products in development and those of our competitors;
|
|
•
|
demand for, and pricing of, our products;
|
|
•
|
levels of third-party reimbursement for our products; and
|
|
•
|
our ability to develop an effective marketing and distribution network.
|
If we do not develop new products or product enhancements in time to meet market demand or if there is insufficient demand for our products or enhancements, we may not achieve expected revenue levels and our business will suffer.
Our relationships with physicians and other consultants require us to comply with a number of United States and international regulations.
We are required to comply with a number of United States and international laws and regulations related to our financial relationships with physicians and other healthcare providers. In addition, we must comply with the Foreign Corrupt Practices Act (“FCPA”) which prohibits United States companies or their agents and employees from providing anything of value to a foreign official for the purposes of influencing him or her to help obtain or retain business, direct business to any person or corporate entity, or obtain any unfair advantage. While we have taken numerous steps to ensure compliance with these laws and regulations, they are subject to evolving interpretations, making it difficult to ensure compliance. If we are found to be in violation of any of these laws or regulations, we may face serious consequences, including civil and criminal penalties for us and our officers and directors, exclusion of our products from government-funded healthcare programs, termination of customer contracts, and reputational harm.
26
We operate in a highly competitive market segment, face competition from large, well-established medical device manufacturers with significant resources, and may not be able to increase penetration in our markets or otherwise compete effectively.
The market for medical devices for treatment of cerebral vascular diseases is intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. We compete primarily with the neurovascular division of Boston Scientific, the market leader, as well as Codman Neurovascular, ev3 and MicroVention. At any time, other companies may develop alternative treatments, products or procedures for the treatment of cerebral aneurysms that compete directly or indirectly with our products. If alternative treatments prove to be superior to our microcoil or other products, continued use or adoption of our products could be negatively affected and our future revenues could suffer.
In addition, most of our current and potential competitors are either large publicly traded companies or divisions or subsidiaries of large publicly traded companies and enjoy several competitive advantages over us, including:
|
•
|
greater financial and personnel resources;
|
|
•
|
greater name recognition;
|
|
•
|
established relationships with neurointerventionalists;
|
|
•
|
established distribution networks;
|
|
•
|
greater experience in obtaining and maintaining FDA, and other regulatory approvals for products and product enhancements, and greater experience in developing compliance programs for compliance with numerous federal, state, local and similar laws in non-United States jurisdictions;
|
|
•
|
greater resources for product research and development;
|
|
•
|
greater experience in, and resources for, launching, marketing, distributing and selling products; and
|
|
•
|
broader product lines.
|
Except for our agreements with our distributors, we have no material long-term purchase agreements with our customers, who may at any time switch to the use of our competitors’ products.
For these reasons, we may not be able to compete successfully against our current or potential future competitors and sales of our products and our revenues may decline.
|
|
Our sales in international markets subject us to foreign currency exchange and other risks and costs that could harm our business.
|
A substantial portion of our revenues are derived from outside the United States. For the fiscal years ended March 31, 2010, 2009 and 2008, revenues from customers outside the United States represented approximately 51%, 50% and 51%, respectively, of our revenues. We anticipate that revenues from international customers will continue to represent a substantial portion of our revenues as we continue to expand in new international markets including China and Japan. Because we generate revenues in foreign currencies, we are subject to the effects of exchange rate fluctuations. For the fiscal year ended March 31, 2010, approximately 33% of our revenues were denominated in currencies other than the U.S. dollar. The functional currency of our Swiss subsidiary is the Swiss franc. The functional currency of our UK subsidiary is the British pound.
In Europe, our revenues are denominated in Swiss francs, euros, British pounds and U.S. dollars. Accordingly, we are exposed to market risk related to changes between those currencies in which we conduct business. For the preparation of our consolidated financial statements, the financial results of our Swiss and UK subsidiaries are translated into U.S. dollars based on average exchange rates during the applicable period. If the U.S. dollar appreciates against the Swiss franc and British pound, the revenues we recognize from sales by our European subsidiaries will be adversely impacted.
27
Historically, we have also been exposed to risks from fluctuations in currency exchange rates due to intercompany loans made to Micrus Endovascular SA (“Micrus SA”), our Swiss subsidiary, in 2001 in connection with its incorporation. These loans are denominated in Swiss francs and will fluctuate in value against the U.S. dollar, causing us to recognize foreign exchange gains and losses. Foreign exchange gains or losses as a result of exchange rate fluctuations in any given period could harm our operating results and negatively impact our revenues. Additionally, if the effective price of our products were to increase as a result of fluctuations in foreign currency exchange rates, demand for our products could decline and adversely affect our results of operations and financial condition.
We are subject to various additional risks as a consequence of doing business internationally which could harm our business, including the following:
|
•
|
unexpected delays or changes in regulatory requirements;
|
|
•
|
local economic and political instability or other potentially adverse conditions;
|
|
•
|
lack of experience in certain geographical markets;
|
|
•
|
increased difficulty in collecting accounts receivables in certain foreign countries;
|
|
•
|
delays and expenses associated with tariffs and other trade barriers;
|
|
•
|
difficulties and costs associated with attracting and maintaining third party distributors;
|
|
•
|
compliance with foreign laws and regulations; and
|
|
•
|
adverse tax consequences or overlapping tax structures.
|
If we fail to increase our direct sales force in a timely manner, our business could suffer.
We have a limited domestic and international direct sales force. We also have a distribution network for sales in the major markets in Europe, Latin America, Asia Pacific and the Middle East. As we launch new products and increase our marketing efforts with respect to existing products, we will need to expand the number of our direct sales personnel on a worldwide basis. The establishment and development of a more extensive sales force will be expensive and time consuming. There is significant competition for sales personnel experienced in interventional medical device sales. If we are unable to attract, motivate and retain qualified sales personnel and thereby increase our sales force, we may not be able to increase our revenues.
If we fail to properly manage our anticipated growth, our business could suffer.
We have experienced, and may continue to experience, periods of rapid growth and expansion, which have placed, and will likely continue to place, a significant strain on our limited personnel and other resources. In particular, the expansion of our fabrication facility and the continuing expansion of our direct sales force will require significant management, technical and administrative resources. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.
To achieve our revenue goals, we must successfully increase production in our fabrication facility as required by customer demand. We may in the future experience difficulties in increasing production, including problems with production yields and quality control and assurance and in satisfying and maintaining compliance with regulatory requirements. These problems could result in delays in product availability and increases in expenses. Any such delay or increased expense could adversely affect our ability to generate revenues.
Future growth will also impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. In addition, rapid and significant growth will place a strain on our administrative and operational infrastructure. In order to manage our operations and growth we will need to continue to improve our operational, financial and management controls, reporting systems and procedures. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our operating results and business could suffer.
28
We are required to evaluate our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act and are exposed to future risks of non compliance.
Pursuant to Section 404 of the Sarbanes-Oxley Act (“Section 404”), we are required to furnish a report by our management on our internal control over financial reporting. The report contains, among other matters, an assessment of the effectiveness of our internal control over financial reporting as of the end of our fiscal year, including a statement as to whether our internal control over financial reporting is effective. This assessment must include disclosure of any material weaknesses in our internal control over financial reporting identified by management. The report must also contain a statement that our independent registered public accounting firm has issued an attestation report on the effectiveness of internal control over financial reporting.
We completed our assessment of our internal control over financial reporting as required by Section 404 for the fiscal year ended March 31, 2010. Our assessment, testing and evaluation resulted in our conclusion that as of March 31, 2010, our internal control over financial reporting was effective. Our independent registered accounting firm has also expressed the opinion that our internal controls over financial reporting were effective during that period. However, our controls may not prove to be adequate for future periods, and we cannot predict the outcome of our testing in future periods. If our internal controls are deemed to be ineffective in future periods, our financial results and the market price of our stock could be adversely affected. In any event, we will incur additional expenses and commitment of management’s time in connection with further evaluations, which may adversely affect our future operating results and financial condition.
Our future capital needs are uncertain and we may need to raise additional funds in the future, and such funds may not be available on acceptable terms or at all.
We believe that our current cash position, together with the cash to be generated from expected product sales and the funds available under our credit facility (subject to compliance with conditions and covenants of the credit agreement) will be sufficient to meet our projected operating requirements for at least the next 12 months. However, after such period we may be required to seek additional funds from public and private stock or debt offerings, borrowings under lease lines or other sources. Our capital requirements will depend on many factors, including:
|
•
|
the revenues generated by sales of our products;
|
|
•
|
the costs associated with expanding our sales and marketing efforts;
|
|
•
|
the expenses we incur in manufacturing and selling our products;
|
|
•
|
the costs of developing and acquiring new products or technologies;
|
|
•
|
the cost of obtaining and maintaining FDA and other domestic and foreign approval or clearance of our products and products in development;
|
|
•
|
the expenses we incur related to compliance with the United States FCPA and laws and regulations in non-United States jurisdictions;
|
|
•
|
costs associated with compliance with the Sarbanes-Oxley Act and rules and regulations affecting public companies promulgated by the SEC and The NASDAQ Stock Market;
|
|
•
|
the costs associated with our facilities expansion, if any; and
|
|
•
|
the costs associated with increased capital expenditures.
|
As a result of these factors, we may need to raise additional funds, and such funds may not be available on favorable terms, or at all. Furthermore, if we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our potential products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to develop or enhance our products, execute our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated customer requirements. In these events, our ability to achieve our development and commercialization goals would be adversely affected.
29
If we choose to acquire new and complementary businesses, products or technologies instead of developing them ourselves, we may be unable to complete these acquisitions or to successfully integrate them in a cost- effective and non-disruptive manner.
Our success depends on our ability to continually enhance and broaden our product offerings in response to changing customer demands, competitive pressures and technologies. We may in the future pursue the acquisition of additional complementary businesses, products or technologies instead of developing them ourselves. We do not know if we will be able to successfully complete any such acquisitions, or whether we will be able to successfully integrate any acquired business, product or technology or retain any key employees. Integrating any business, product or technology we acquire could be expensive and time consuming, disrupt our ongoing business, distract our management and expose us to unanticipated liabilities. If we are unable to integrate any acquired businesses, products or technologies effectively, our business will suffer. In addition, any amortization or charges resulting from the costs of acquisitions could harm our business and operating results.
Our operations are subject to environmental, health and safety, and other laws and regulations, with which compliance is costly and which expose us to penalties for non-compliance.
Our business, properties and products are subject to foreign, federal, state and local laws and regulations relating to the protection of the environment, natural resources and worker health and safety and the use, management, storage, and disposal of hazardous substances, wastes, and other regulated materials. Because we operate real property, various environmental laws may also impose liability on us for the costs of cleaning up and responding to hazardous substances that may have been released on our property, including releases unknown to us. These environmental laws and regulations also could require us to pay for environmental remediation and response costs at third-party locations where we disposed of or recycled hazardous substances. The costs of complying with these various environmental requirements, as they now exist or may be altered in the future, could adversely affect our financial condition and operating results.
We are dependent on a single source supplier for components and materials used in our devices, and the loss of any of these suppliers, or their inability to supply us with an adequate supply of materials, could harm our business.
We rely on third-party suppliers for components and materials used in our products and rely on a single source for many of the microcoil and delivery system components, including tubing and connectors. Our dependence on third-party suppliers involves several risks, including limited control over pricing, availability, quality, delivery schedules and supplier compliance with regulatory requirements. Any delays in delivery of such components or provision of such services or shortages of such components could cause delays in the shipment of our products, which could significantly harm our business. We generally acquire our single source components pursuant to purchase orders placed in the ordinary course of business, and we have no guaranteed supply arrangements with any of our single source suppliers. Because of our reliance on these vendors, we may also be subject to increases in component costs. These increases could significantly harm our business. For us to be successful, our third-party suppliers must also be able to provide us with the materials and components of our products in substantial quantities, in compliance with regulatory requirements, in accordance with agreed upon specifications, at acceptable cost and on a timely basis. Our anticipated growth may strain the ability of suppliers to deliver an increasingly large supply of materials and components.
If we are unable to obtain sufficient quantities of high quality components and materials to meet customer demand on a timely basis, we could lose customers, our reputation may be harmed and our business could suffer. If any one or more of our third-party suppliers cease to provide us with sufficient quantities of our materials or components in a timely manner or on terms acceptable to us, we would have to seek alternative sources of supply. We could incur delays while we locate and engage alternative qualified suppliers and we might be unable to engage alternative suppliers on favorable terms. Any such disruption or increased expenses could harm our commercialization efforts and adversely affect our ability to generate revenues.
We rely on independent contract manufacturers for the manufacture and assembly of certain of our products and components. Reliance on independent contract manufacturers involves several risks, including the potential inadequacy of capacity, the unavailability of or interruptions in access to certain process technologies and reduced control over product quality, compliance with regulatory requirements, delivery schedules, manufacturing yields and costs. Such manufacturers have possession of and at times title to molds for certain manufactured components of our products. Shortages of raw materials, production capacity constraints or delays by our contract manufacturers could negatively affect our ability to meet our production obligations and result in increased prices for affected parts. Any such reduction, constraint or delay may result in delays in shipments of our products or increases in the prices of components, either of which could have a material adverse effect on our business, operating results and financial condition. We have no supply agreements with our current contract manufacturers and utilize purchase orders which are subject to supplier acceptance. The unanticipated loss of any of our contract manufacturers could cause delays in our ability to deliver product while we identify and qualify a replacement manufacturer. If our current or future independent contract manufacturers are unable to meet our requirements for manufactured components, our business could suffer.
30
Our operations are currently conducted at several locations that may be at risk from earthquakes or other natural disasters.
We currently conduct our manufacturing, development and management activities in San Jose, California, near known earthquake fault zones, and in Miramar, Florida, where there is a risk of hurricanes. We have taken precautions to safeguard our facilities, including insurance, health and safety protocols, and off-site storage of computer data. However, any future natural disaster, such as an earthquake or hurricane, could cause substantial delays in our operations, damage or destroy our equipment or inventory, and cause us to incur additional expenses. A disaster could seriously harm our business and results of operations.
If we are unable to effectively manage our inventory held on consignment by our intended customers, we will not achieve our expected results.
A significant portion of our inventory is held on consignment by hospitals that purchase the inventory as they use it. In these consignment locations, we do not have physical possession of the consigned inventory. We therefore have to rely on information from our customers as well as periodic inspections by our sales personnel to determine when our products have been used. We have in the past experienced problems managing appropriate consigned inventory levels and as a result we recorded an impairment of inventory for anticipated obsolescence in fiscal 2004 and an impairment of excess inventory in both fiscal 2004 and 2005. If we are not able to effectively manage appropriate consigned inventory levels, we may suffer inventory losses that will reduce our gross profit levels. There can be no assurance that any efforts to strengthen our monitoring and management of consigned inventory will be adequate to meaningfully reduce the risk of inventory loss.
We are dependent on our senior management team, key clinical advisors and scientific personnel, and the loss of any of them could harm our business.
Our continued success depends in part upon the continued availability and contributions of our senior management team and the continued participation of our key clinical advisors. We have entered into agreements with certain members of our senior management team, but none of these agreements guarantee the services of the individual for a specified period of time. We also rely on the skills and talents of our scientific personnel because of the complexity of our products. The loss of members of our senior management, key clinical advisors or scientific personnel, or our inability to attract or retain other qualified personnel or advisors could have a material adverse effect on our results of operations and financial condition.
We face certain litigation risks that could harm our business.
At any given time we are likely to have one or more lawsuits filed against us asserting various claims. The results of complex legal proceedings are difficult to predict. Moreover, many of the complaints filed against us do not specify the amount of damages that plaintiffs seek, and we therefore are unable to estimate the possible range of damages that might be incurred should these lawsuits be resolved against us. An unfavorable outcome or settlement of one or more of these lawsuits could have a material adverse effect on our financial condition, liquidity and results of operations. Even if these lawsuits are not resolved against us, the uncertainty and expense associated with unresolved lawsuits could seriously harm our business, financial condition and reputation. Litigation is costly, time-consuming and disruptive to normal business operations. The costs of defending lawsuits has been significant, will continue to be costly and may not be covered by our insurance policies. The defense of these lawsuits could also result in continued diversion of our management’s time and attention away from business operations, which could harm our business.
The medical device industry is characterized by patent litigation, which could be costly, result in the diversion of management’s time and efforts and require us to pay damages.
The medical device industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights. Accordingly, we may in the future be subject to further litigation and administrative proceedings over such rights with other companies in our industry. Our competitors may assert that at least one of our products, its components, or the methods we employ in the use or manufacture of our products are covered by and infringe the competitors’ United States or foreign patents held by them. In addition, should our patents or applications have claims that encompass the same scope as claims pending or issued to a third party competitor, that third party may claim that its claims have priority over ours because they invented the claimed subject matter first. Because patent applications generally take many years to issue, there may be third party applications presently pending of which we are unaware, that may in the future result in issued patents that at least one of our products, its components, or the methods we employ in the use or manufacture of our product(s) may infringe. There could also be issued patents that one or more components of our products may inadvertently be infringing, of which we are unaware. As the number of participants in the market for cerebral vascular treatments and the number of issued patents in this technology area grows, the possibility of being charged with patent infringement increases.
31
Any infringement claims against us may cause us to incur substantial costs, could place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation. If the relevant patent claims are upheld as valid and enforceable and we are found to infringe, we could be required to pay substantial damages and/or royalties and could be prevented from selling our products unless we could obtain a license or were able to redesign our products to avoid infringement. Any such license may not be available on reasonable terms, if at all. If we fail to obtain any required licenses or make any necessary changes to our products or technologies, we may be unable to commercialize one or more of our products or practice the methods we employ in the use or manufacture of our products.
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
Our success depends significantly on our ability to procure proprietary rights to the technologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not be sufficient to adequately protect our intellectual property or permit us to gain or keep any competitive advantage. For example, any of our pending United States or foreign patent applications may ultimately not issue as a patent or, alternatively, may issue with claims that are of little or no value to us. In addition, once issued, a valuable patent may be challenged successfully by third parties and invalidated. In addition, our patent protection for material aspects of our products and methods is presently being pursued with applications that have been filed but not issued, such that these material aspects are not presently protected by patents. Competitors may further be able to get around having to license our technology in order to avoid infringement by designing around our issued and published patent claims, thereby staying clear of our proprietary rights. Similarly, competitors may develop products and methods that are equivalent or superior to ours. Our confidentiality agreements and intellectual property assignment agreements with our employees, consultants and advisors may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. Furthermore, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States. Both the process of procuring patent rights and the process of managing patent disputes can be time consuming and expensive.
In the event a competitor infringes upon our patent or other intellectual property rights, enforcing those rights may be difficult and time consuming. Even if successful, litigation to enforce our intellectual property rights or to defend our patents against challenge could be prolonged, costly and could divert our management’s attention. We may not have sufficient resources to enforce our intellectual property rights or to defend our patents against a challenge.
If we fail to obtain, or experience significant delays in obtaining, FDA clearances or approvals for our future products or product enhancements, or to comply with similar regulatory requirements in other countries where we market our products, our ability to commercially distribute and market our products could suffer.
Our medical devices are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. Our failure to comply with such regulations could lead to the imposition of injunctions, suspensions or loss of regulatory clearances or approvals, product recalls, termination of distribution or product seizures or the need to invest substantial resources to comply with various existing or new requirements. In the more egregious cases, criminal sanctions, civil penalties, disgorgement of profits or closure of our manufacturing facilities are possible. The process of obtaining regulatory clearances or approvals to market a medical device, particularly from the FDA, can be costly and time consuming, and there can be no assurance that such clearances or approvals will be granted on a timely basis, if at all. In particular, the FDA permits commercial distribution of most new medical devices only after the device has received 510(k) clearance or is the subject of an approved pre-market approval application, or PMA. The FDA will clear the marketing of a medical device through the 510(k) process if it is demonstrated that the new product has the same intended use, is substantially equivalent to another legally marketed device, including a 510(k)-cleared product, and otherwise meets the FDA’s requirements. The PMA approval process is more costly, lengthy and uncertain than the 510(k) clearance process and requires the development and submission of clinical studies supporting the safety and effectiveness of the device. Product modifications may also require the submission of a new 510(k) clearance, or the approval of a PMA before the modified product can be marketed. Changes in labeling and manufacturing site for a PMA approved device may require the submission and approval of a PMA supplement. Any products we develop that require regulatory clearance or approval may be delayed, if approved at all. In addition, we believe that some of our new products will require an approved PMA before we can commercially distribute the device and we cannot assure that any new products or any product enhancements we develop will be subject to the shorter 510(k) clearance process instead of the more lengthy PMA requirements. Additionally, certain of our products under development may involve both device and drug or biologic regulation and we will need to comply with drug and biologic regulations in addition to medical device requirements. Accordingly, we anticipate that the regulatory review and approval process for some of our future products or product enhancements may take significantly longer than anticipated or what we have experienced in the past. We will also be required to pay a medical device user fee and may also be required to pay a drug or biologic user fee. There is no assurance that the FDA will not require that a certain new product or product enhancement go through the lengthy and expensive PMA approval process.
32
We have no experience in obtaining PMA approval. We also have no experience in obtaining drug or biologic approval, and will need to rely on third party assistance in navigating the regulatory approval pathway for future combination products.
Further, pursuant to FDA regulations, we can only market our products for cleared or approved uses. Certain of our products may be used by physicians for indications other than those cleared or approved by the FDA, but we cannot promote the products for such off-label uses.
Modifications to our marketed products may require new 510(k) clearances or pre-market approvals, or may require us to cease marketing or recall the modified products until clearances are obtained.
Any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a change in its intended use, requires a new 510(k) clearance or, possibly, PMA approval. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review a manufacturer’s decision. The FDA may not agree with any of our past or future decisions regarding whether new clearances or approvals are necessary. If the FDA requires us to seek 510(k) clearance or PMA approval for any modification to a previously cleared product, we may be required to cease marketing and/or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Further, our products could be subject to recall if the FDA determines that our products are not safe for any reason including but not limited to new safety data from use of the product, or manufacturing defects. Any recall or FDA requirement that we seek additional approvals or clearances could result in delays, fines, costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
If we or our suppliers fail to comply with the FDA’s quality system regulations, the manufacture of our products could be delayed.
We and our suppliers are required to comply with the FDA’s quality system regulations, which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of our products. The FDA enforces these quality system regulations through unannounced inspections. If we or one of our suppliers fail a quality system regulations inspection or if any corrective action plan is not sufficient, or is very expensive or time consuming to implement, the manufacture of our products could be delayed until satisfactory corrections are made, or in the event we are unable to correct the problems we may not be able to continue manufacturing and distributing the particular device or devices. Such a delay could potentially disrupt our business, harm our reputation and adversely affect our sales and revenues.
If hospitals are unable to obtain sufficient reimbursement for procedures performed with our products, it is unlikely that our products will be widely used.
Successful sales of our products will depend on the availability of adequate reimbursement from third-party payors. Healthcare institutions that purchase medical devices for treatment of their patients generally rely on third-party payors to cover the use of the product for the particular procedure and reimburse all or part of the costs and fees associated with the procedures performed with these devices. Currently, the costs of our products distributed domestically are being reimbursed by third party payors. There is no guarantee that coverage and adequate reimbursement will be available in the future for our existing and/or new products. Both public and private insurance reimbursement plans are central to new product acceptance. Hospitals are unlikely to use our products if they do not receive reimbursement adequate to cover the cost of our products and related procedures.
In international markets, market acceptance may depend, in part, upon the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country, and include both government sponsored healthcare and private insurance. Currently, the costs of our products distributed internationally, other than in some Latin American countries, are being reimbursed by public and private healthcare insurers. We may not obtain international reimbursement approvals in a timely manner, if at all, and our failure to receive international reimbursement approvals would negatively impact market acceptance of our products in the international markets in which those approvals are sought.
In addition, in certain countries, such as France, Germany, China and Japan, we are required to obtain regulatory clearance for our products to be eligible for reimbursements by third party payors, even though reimbursement for embolic coiling procedures is already in place.
Healthcare costs have risen significantly over the past decade. There have been and continue to be proposals by legislators, regulators and third-party payors to keep these costs down. Certain proposals, if passed, may impose limitations on the amounts of reimbursement available for our products from governmental agencies or third-party payors, or additional taxes on medical devices. These proposals could have a negative impact on the demand for our products and services, and therefore on our financial position and results of operations.
33
In March 2010, President Obama signed into law major health care reform legislation under the Patient Protection and Affordable Health Care Act of 2010 (the "PPACA"), which was modified by the Health Care and Education Reconciliation Act. The PPACA imposes a 2.3% excise tax on domestic sales of medical devices beginning on January 1, 2013. PPACA also implements provisions that could affect the way in which physicians and hospitals are compensated for performing medical procedures involving our products in the future. The PPACA also expands the scope of the Federal False Claims act and increases fraud and abuse penalties and other government enforcement tools, which could adversely impact healthcare companies, including us. Various healthcare reform proposals also have emerged at the state level. We cannot predict what healthcare initiatives, if any, will be implemented at the state level or the exact effect newly enacted laws or any future legislation or regulation will have on us. However, an expansion in government’s role in the U.S. healthcare industry may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business and results of operations, possibly materially. In addition, the enacted excise tax on medical devices could materially and adversely affect our operating results.
Future reimbursement may be subject to increased restrictions both in the United States and in international markets. Third-party reimbursement and coverage may not be available or adequate in either the United States or international markets. Future legislation, regulation or reimbursement policies of third-party payors may adversely affect, possibly materially, the demand for our existing products or our products currently under development and limit our ability to sell our products on a profitable basis.
Changes to existing accounting pronouncements or taxation rules or practices may affect how we conduct our business and affect our reported results of operations.
New accounting pronouncements or tax rules and varying interpretations of accounting pronouncements or taxation practice have occurred and may occur in the future. A change in accounting pronouncements or interpretations or taxation rules or practices can have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. Changes to existing rules and pronouncements, future changes, if any, or the questioning of current practices or interpretations may adversely affect our reported financial results or the way we conduct our business.
We may become subject to product liability claims which could require us to pay damages that exceed our insurance coverage.
Our business exposes us to potential product liability claims that are inherent in the testing, manufacture and sale of medical devices for neurointerventional procedures. These procedures involve significant risk of serious complications, including intracranial bleeding, brain injury, paralysis and even death. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or the inability to secure coverage in the future. In addition, we could have to pay an amount in excess of policy limits, which would have to be paid out of cash reserves. If longer-term patient results and experience indicate that our products or any component cause tissue damage, motor impairment or other adverse effects, we could be subject to significant liability. Finally, even a meritless or unsuccessful product liability claim could harm our reputation in the industry, lead to significant legal fees and could result in the diversion of management’s attention from managing our business.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at other medical device companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees have wrongfully used or disclosed alleged trade secrets of their former employers or that we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize product candidates, which could severely harm our business.
The price of our common stock has fluctuated and we expect will continue to fluctuate substantially and you may not be able to sell your shares at or above your purchase price.
The market price of our common stock has been and we expect will continue to be highly volatile and may fluctuate substantially due to many factors, including:
|
•
|
volume and timing of orders for our products;
|
|
•
|
the outcome of clinical and multi-center trial evidence supporting patient benefits;
|
|
•
|
the introduction of new products or product enhancements by us or our competitors;
|
34
|
•
|
disputes or other developments with respect to intellectual property rights;
|
|
•
|
our ability to develop, obtain regulatory clearance for, and market, new and enhanced products on a timely basis;
|
|
•
|
product liability claims or other litigation;
|
|
•
|
quarterly variations in our or our competitors’ results of operations;
|
|
•
|
sales of large blocks of our common stock, including sales by our executive officers and directors;
|
|
•
|
changes in governmental regulations or in the status of our regulatory approvals or applications;
|
|
•
|
changes in the availability of third-party reimbursement in the United States or other countries;
|
|
•
|
the results of clinical research and trials on our existing products and products in development or those of our competitors;
|
|
•
|
changes in revenues or earnings estimates or recommendations by securities analysts; and
|
|
•
|
general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
Furthermore, to the extent there is an inactive market for our common stock, the value of your shares and your ability to sell your shares at the time you wish to sell them may be impaired. An inactive market may also impair our ability to raise capital by selling shares and may impair our ability to acquire other companies, products or technologies by using our shares as consideration.
Because of their significant stock ownership, our executive officers, directors and principal stockholders may be able to exert control over us and our significant corporate decisions.
Based on shares outstanding at March 31, 2010, our executive officers, directors, and stockholders holding more than 5% of our outstanding common stock and their affiliates, in the aggregate, beneficially owned approximately 41% of our outstanding common stock. As a result, these stockholders, acting together, may have the ability to determine the outcome of matters submitted to our stockholders for approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets. This concentration of ownership may harm the market price of our common stock by, among other things:
|
•
|
delaying, deferring or preventing a change in control of our company;
|
|
•
|
impeding a merger, consolidation, takeover or other business combination involving our company; or
|
|
•
|
causing us to enter into transactions or agreements that are not in the best interests of all stockholders.
|
Future sales of our common stock may depress our stock price.
Our current stockholders hold a substantial number of shares of our common stock that they are able to sell in the public market. A significant portion of these shares are held by a small number of stockholders. Sales by our current stockholders of a substantial number of shares could significantly reduce the market price of our common stock. Moreover certain holders of our common stock have the right to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders.
We have registered 8,527,739 shares of common stock that we may issue under our 1998 Stock Plan (“1998 Plan”), 2005 Equity Incentive Plan (“2005 Plan”) and 2005 Employee Stock Purchase Plan (“Purchase Plan”). These shares can be freely sold in the public market upon issuance. The sale by any of these holders of a large number of securities in the public market could reduce the trading price of our common stock and impede our ability to raise future capital.
We do not intend to pay cash dividends.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings for us in the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. In addition, the terms of any future debtor credit facility may preclude us from paying any dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of potential gain for the foreseeable future.
35
We may become involved in securities class action litigation that could divert management’s attention and harm our business.
The stock market in general, The NASDAQ Stock Market and the market for medical device companies in particular, continues to experience extreme price and volume fluctuations that are unrelated or disproportionate to companies’ operating performance. Further, the market prices of securities of medical device companies have been particularly volatile. These broad market and industry factors may materially harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a particular company’s securities, securities class action litigation has often been brought against that company. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could materially harm our financial condition and results of operations.
|
|
Anti-takeover provisions in our organizational documents and Delaware law may discourage or prevent a change of control, even an acquisition which would be beneficial to our stockholders, and thereby affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.
|
Our amended and restated certificate of incorporation and amended bylaws contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions:
|
•
|
authorize the issuance of preferred stock which can be created and issued by the board of directors without prior stockholder approval, with rights senior to those of the common stock;
|
|
•
|
provide for a classified board of directors, with each director serving a staggered three-year term;
|
|
•
|
prohibit our stockholders from filling board vacancies, calling special stockholder meetings, or taking action by written consent;
|
|
•
|
prohibit our stockholders from making certain changes to our amended and restated certificate of incorporation or bylaws except with 66 2/3% stockholder approval; and
|
|
•
|
require advance written notice of stockholder proposals and director nominations.
|
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These and other provisions in our amended and restated certificate of incorporation, restated bylaws and Delaware law could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including delaying or impeding a merger, tender offer, or proxy contest involving our company. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
None.
Our worldwide headquarters are located in San Jose, California. On June 6, 2005, we entered into a non-cancelable seven-year lease pursuant to which we lease approximately 42,000 square feet of building space, which we currently use for administrative, sales, marketing, research and development, manufacturing and distribution facilities.
On March 11, 2008, we entered into a non-cancelable ten-year lease in Miramar, Florida. The facility comprises a total of approximately 27,000 square feet, which we currently use for administrative, manufacturing, research and development and distribution facilities.
On December 4, 2007, our wholly owned subsidiary, Micrus SA, entered into a non-cancelable eight-year lease for office space in Switzerland. The office space comprises a total of approximately 5,500 square feet and provides space for sales, marketing and administrative functions.
36
Additionally, we lease office space in the United Kingdom for our wholly-owned subsidiary, Micrus Endovascular UK Limited (“Micrus UK”), under a non-cancelable lease agreement with a term through December 2010. We use this space for our sales, marketing and administrative functions.
We otherwise believe that our existing facilities are adequate to meet our current and near term future needs.
None.
None.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock is traded on The NASDAQ Stock Market under the symbol “MEND.” The following table sets forth the high and low daily bid prices per share of our common stock, as reported by The NASDAQ Stock Market.
|
Fiscal Year Ended March 31, 2010
|
High
|
Low
|
||||||
|
First Quarter
|
$
|
9.54
|
$
|
5.77
|
||||
|
Second Quarter
|
$
|
13.71
|
$
|
7.80
|
||||
|
Third Quarter
|
$
|
15.98
|
$
|
11.16
|
||||
|
Fourth Quarter
|
$
|
22.24
|
$
|
14.92
|
||||
|
Fiscal Year Ended March 31, 2009
|
High
|
Low
|
||||||
|
First Quarter
|
$
|
14.99
|
$
|
9.92
|
||||
|
Second Quarter
|
$
|
16.00
|
$
|
10.97
|
||||
|
Third Quarter
|
$
|
14.12
|
$
|
8.70
|
||||
|
Fourth Quarter
|
$
|
11.95
|
$
|
4.13
|
||||
The last reported sale price of our common stock on The NASDAQ Stock Market on May 28, 2010 was $17.28 per share. As of May 28, 2010, there were approximately 52 holders of record of our common stock.
Dividend Policy
We have never declared a divided or paid any cash dividends on our common stock. Because we currently intend to retain any future earnings to fund the development and growth of our business, we do not anticipate paying any cash dividends in the near future.
Unregistered Securities Sold in Fiscal 2010
None
Issuer Purchases of Equity Securities
We do not have a stock repurchase program and did not repurchase any of our equity securities during the year ended March 31, 2010.
37
Securities Authorized for Issuance Under Equity Compensation Plans
|
Plan Category
|
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights
|
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights
|
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Colum (a))
|
||||||||||
|
Equity compensation plans approved by security holders
|
4,073,882
|
(1
|
)
|
$
|
12.50
|
2,079,326
|
(2
|
)
|
|||||
|
Equity compensation plans not approved by security holders
|
-
|
-
|
-
|
||||||||||
|
Total
|
4,073,882
|
(1
|
)
|
$
|
12.50
|
2,079,326
|
(2
|
)
|
|||||
______
|
(1)
|
Includes 865,700 shares subject to options outstanding under our 1998 Plan and 3,208,182 shares subject to options and restricted stock units outstanding under our 2005 Plan.
|
|
(2)
|
Includes 1,437,859 shares of common stock reserved for future issuance under our 2005 Plan and 641,467 shares of common stock reserved for future issuance under our Purchase Plan. As of April 1, 2010, the number of shares available for issuance under the foregoing plans automatically increased to 2,104,525 shares available for issuance under the 2005 Plan and 863,689 shares available for issuance under the Purchase Plan.
|
38
Stock Performance Graph
Notwithstanding any statement to the contrary in any of our previous or future filings with the Securities and Exchange Commission, the following information relating to the price performance of our common stock shall not be deemed “filed” with the Commission or “soliciting material” under the Securities Exchange Act of 1934 and shall not be incorporated by reference into any such filings.
The following graph shows a comparison of cumulative total return for our common stock, The NASDAQ Composite Index, The NASDAQ Medical Equipment Index and The Russell 2000 Index. Such returns are based on historical results and are not intended to suggest future performance. The graph assumes $100 was invested in our common stock and in each of the indexes on June 16, 2005 (the date our common stock commenced trading on The NASDAQ Stock Market). Data for The NASDAQ Composite Index, The NASDAQ Medical Equipment Index and The Russell 2000 Index assume reinvestment of dividends. We have never paid dividends on our common stock and have no present plans to do so.
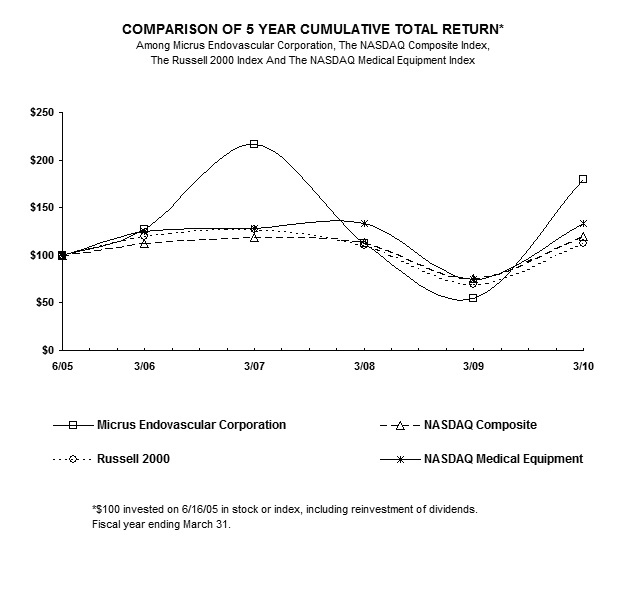
39
The following selected consolidated financial data should be read in conjunction with “Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the notes thereto included in this report. The selected consolidated statements of operations data for the fiscal years ended March 31, 2010, 2009, and 2008 and the selected consolidated balance sheet data as of March 31, 2010 and 2009 are derived from the audited consolidated financial statements that are included elsewhere in this report. The selected consolidated statements of operations data for the fiscal years ended March 31, 2007 and 2006 and the selected consolidated balance sheet data as of March 31, 2008, 2007 and 2006 are derived from our audited consolidated financial statements, which are not included in this report. The historical results are not necessarily indicative of the results of operations to be expected in any future periods.
Consolidated Statements of Operations
|
Years Ended March 31,
|
||||||||||||||||||||
|
2010 (2)(3)
|
2009 (2)
|
2008 (2)
|
2007 (2)(4)
|
2006 (5)
|
||||||||||||||||
|
(In thousands, except per share amounts)
|
||||||||||||||||||||
|
Revenues
|
$
|
91,090
|
$
|
78,196
|
$
|
69,213
|
$
|
58,795
|
$
|
32,781
|
||||||||||
|
Cost of goods sold (1)
|
22,406
|
20,847
|
17,301
|
15,361
|
9,710
|
|||||||||||||||
|
Gross profit
|
68,684
|
57,349
|
51,912
|
43,434
|
23,071
|
|||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development (1)
|
10,449
|
10,243
|
13,718
|
7,904
|
6,589
|
|||||||||||||||
|
Sales and marketing (1)
|
26,816
|
29,312
|
29,385
|
24,121
|
15,171
|
|||||||||||||||
|
General and administrative (1)
|
21,266
|
26,983
|
26,971
|
19,308
|
10,307
|
|||||||||||||||
|
Impairment of intangible assets
|
-
|
462
|
-
|
-
|
-
|
|||||||||||||||
|
Total operating expenses
|
58,531
|
67,000
|
70,074
|
51,333
|
32,067
|
|||||||||||||||
|
Income (loss) from operations
|
10,153
|
(9,651
|
)
|
(18,162
|
)
|
(7,899
|
)
|
(8,996
|
)
|
|||||||||||
|
Interest and investment income
|
45
|
258
|
1,223
|
1,618
|
1,295
|
|||||||||||||||
|
Interest expense
|
(127
|
)
|
(52
|
)
|
(3
|
)
|
(14
|
)
|
(12
|
)
|
||||||||||
|
Other income (expense), net
|
2,096
|
(2,111
|
)
|
488
|
565
|
(632
|
)
|
|||||||||||||
|
Income (loss) before income taxes
|
12,167
|
(11,556
|
)
|
(16,454
|
)
|
(5,730
|
)
|
(8,345
|
)
|
|||||||||||
|
Income tax benefit (expense)
|
(637
|
)
|
502
|
194
|
247
|
84
|
||||||||||||||
|
Net income (loss)
|
11,530
|
(11,054
|
)
|
(16,260
|
)
|
(5,483
|
)
|
(8,261
|
)
|
|||||||||||
|
Accretion of redeemable convertible
|
||||||||||||||||||||
|
preferred stock to redemption value
|
||||||||||||||||||||
|
including beneficial conversion feature
|
-
|
-
|
-
|
-
|
(659
|
)
|
||||||||||||||
|
Net income (loss) attributable to common stockholders
|
$
|
11,530
|
$
|
(11,054
|
)
|
$
|
(16,260
|
)
|
$
|
(5,483
|
)
|
$
|
(8,920
|
)
|
||||||
|
Net income (loss) per share attributable to common stockholders:
|
||||||||||||||||||||
|
Basic
|
$
|
0.72
|
$
|
(0.70
|
)
|
$
|
(1.05
|
)
|
$
|
(0.38
|
)
|
$
|
(0.79
|
)
|
||||||
|
Diluted
|
$
|
0.69
|
$
|
(0.70
|
)
|
$
|
(1.05
|
)
|
$
|
(0.38
|
)
|
$
|
(0.79
|
)
|
||||||
|
Weighted-average number of shares used in per share calculation:
|
||||||||||||||||||||
|
Basic
|
16,004
|
15,692
|
15,438
|
14,621
|
11,240
|
|||||||||||||||
|
Diluted
|
16,666
|
15,692
|
15,438
|
14,621
|
11,240
|
|||||||||||||||
40
|
March 31,
|
||||||||||||||||||||
|
2010
|
2009
|
2008
|
2007 (4)
|
2006 (5)
|
||||||||||||||||
|
(In thousands)
|
||||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
||||||||||||||||||||
|
Total assets
|
$
|
80,236
|
$
|
61,506
|
$
|
72,332
|
$
|
73,097
|
$
|
62,114
|
||||||||||
|
Total stockholders' equity
|
$
|
65,336
|
$
|
42,574
|
$
|
48,180
|
$
|
56,294
|
$
|
51,316
|
||||||||||
|
Accumulated deficit
|
$
|
(70,886
|
)
|
$
|
(82,416
|
)
|
$
|
(71,362
|
)
|
$
|
(55,102
|
)
|
$
|
(49,619
|
)
|
|||||
|
(1) Non-cash stock-based compensation expense included in results of operations is as follows:
|
||||||||||||||||||||
|
Years Ended March 31,
|
||||||||||||||||||||
|
2010(3)
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
(In thousands)
|
||||||||||||||||||||
|
Cost of goods sold
|
$
|
603
|
$
|
560
|
$
|
471
|
$
|
218
|
$
|
26
|
||||||||||
|
Research and development
|
$
|
685
|
$
|
592
|
$
|
533
|
$
|
222
|
$
|
22
|
||||||||||
|
Sales and marketing
|
$
|
1,541
|
$
|
1,498
|
$
|
1,325
|
$
|
802
|
$
|
169
|
||||||||||
|
General and administrative
|
$
|
3,158
|
$
|
2,995
|
$
|
2,629
|
$
|
1,332
|
$
|
172
|
||||||||||
|
(2)
|
In fiscal 2010, 2009, 2008 and 2007, loss from operations, net loss and basic and diluted net loss per share include the impact of Accounting Standards Codification (“ASC”) 718, Compensation - Stock Compensation, which was not present in prior years. Refer to Notes 2 and 12 of the “Notes to Consolidated Financial Statements” which are included in Item 8 “Financial Statements and Supplementary Data.”
|
|
(3)
|
In fiscal 2010, we recorded adjustments to cost of goods sold and operating expenses to record additional expenses primarily related to stock-based compensation expense that were not correctly recorded in prior periods. Refer to Note 2 of the “Notes to Consolidated Financial Statements” which are included in Item 8 “Financial Statements and Supplementary Data.”
|
|
(4)
|
On November 30, 2006, we completed our acquisition of VasCon. In connection with the acquisition, we formed MDT. The results of operations of MDT are included in the accompanying consolidated statements of operations for all periods or partial periods subsequent to the acquisition date. Additionally, the acquired assets and liabilities assumed in the acquisition are included in the consolidated balance sheets subsequent to the acquisition date. See Note 3 of the “Notes to Consolidated Financial Statements,” which are included in “Item 8 – Financial Statements and Supplementary Data” of this report for further details regarding the transaction. On January 1, 2009, we merged MDT into Micrus Endovascular Corporation.
|
|
(5)
|
On September 20, 2005, we completed our acquisition of Neurologic UK Limited (“Neurologic”). In connection with the acquisition, we formed Micrus UK, our subsidiary in the United Kingdom. The results of operations of Micrus UK are included in the accompanying consolidated statements of operations for all periods or partial periods subsequent to the acquisition date. Additionally, the acquired assets and liabilities assumed in the acquisition are included in the consolidated balance sheets subsequent to the acquisition date. See Note 3 of the “Notes to Consolidated Financial Statements,” which are included in “Item 8 – Financial Statements and Supplementary Data” of this report for further details regarding the transaction.
|
The following discussion and analysis of the financial condition and results of operations of the Company should be read in conjunction with the consolidated financial statements and the related notes included elsewhere in this report, and with other factors described from time to time in our other filings with the Securities and Exchange Commission. This Annual Report on Form 10-K contains forward-looking statements that involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in the forward-looking statements due to a number of factors, including those discussed in Part I, Item 1A “Risk Factors” above and elsewhere in this Annual Report on Form 10-K.
41
Overview
We develop, manufacture and market implantable and disposable medical devices used in the treatment of cerebral vascular diseases. Our products are used by interventional neuroradiologists, interventional neurologists and endovascularly trained neurosurgeons to treat both cerebral aneurysms responsible for hemorrhagic stroke and intracranial atherosclerosis which may lead to ischemic stroke. Hemorrhagic and ischemic stroke are both significant causes of death and disability worldwide. Our product lines consist of endovascular systems that enable a physician to gain access to the brain in a minimally invasive manner through the vessels of the arterial system. We believe that our products provide a safe and reliable alternative to more invasive neurosurgical procedures for treating aneurysms. Our proprietary three-dimensional, embolic coils anatomically conform and rapidly deploy within an aneurysm, forming a scaffold that conforms to a wide diversity of aneurysm shapes and sizes. We also supply accessories for use with our microcoils and other products for the treatment of neurovascular disease including microcatheters, balloon catheters, guidewires and stents. We plan on growing our business by continuing to penetrate our existing hemorrhagic and ischemic stroke markets, bringing new products and technologies to interventional neuroradiologists, interventional neurologists and neurosurgeons, and by entering new geographic territories such as Asia where we commenced selling our products in Japan through our distribution partner, Goodman, in March 2006. We also plan to market our products in China upon receiving regulatory approvals.
Our revenues are derived primarily from sales of our microcoils. We also sell stents, access products and accessories for use with our microcoils, which accounted for approximately 6%, 6% and 5% of our revenues in fiscal 2010, 2009 and 2008, respectively. Geographically, our revenues are generally from sales to customers in the Americas, Europe and Asia Pacific. Our products are shipped from our facilities in the United States and a logistics facility in the Netherlands, to either hospitals or distributors. In select hospitals, our products are held on consignment, and remain on site, free of charge until used. Revenue is generally recognized upon shipment after the receipt of a purchase order. In arrangements which specify the title transfer upon delivery, revenue is not recognized until the product is delivered. In the case of consigned goods, revenue is recognized when a replenishment order is made.
We anticipate that our cost of goods sold will generally increase in absolute dollars during those quarters in which our sales increase or we incur additional manufacturing costs in anticipation of the commercial introduction of new products. Furthermore, our gross margin percentage may decrease in those quarters in which we initiate sales of new products or product lines, or enter new geographic territories. Our gross margin percentage may also decrease in those quarters in which we have a higher proportion of sales to distributors with lower average selling prices.
Our product development efforts are primarily focused on expanding our product offerings for the hemorrhagic and ischemic stroke markets. In August 2004, we introduced our Cerecyte® microcoil product line and we have launched ten new products in the last 24 months, including microcoils, stents, microcatheters, guidewires and balloon catheters. During fiscal 2009, we launched the Neuropath® guide catheter, which combines robust proximal support with a highly flexible and visible tip designed to facilitate atraumatic vascular access. The Neuropath® guide catheter is used as a conduit for delivery of the microcatheter or other devices, such as coils, stents and balloons, to the aneurysm. We intend to continue to pursue this non-embolic product line expansion with the goal of increasing our revenue opportunity per procedure. We also launched Cerecyte and stretch resistant versions of our DeltaPaq™ microcoil system for the treatment of cerebral aneurysms. Our DeltaPaq™ microcoil system is designed to enable physicians to achieve greater coil packing density within the aneurysm which may reduce the rate of recanalization and the need for re-treatment. The DeltaPaq™ microcoil system supplements our framing and finishing coils in the filling segment of the coil market. Additionally, we launched the PHAROS® Vitesse® intracranial stent for commercial distribution in the European Union and all other countries recognizing the CE Mark. The PHAROS® Vitesse® is our second generation balloon-expandable stent for intracranial ischemic stenosis and wide-neck aneurysm treatment. We have received FDA conditional approval of our investigational device exemption for the PHAROS® VISSIT study. The VISSIT study is the first industry sponsored, randomized, prospective clinical trial designed to compare the clinical outcomes between patients who are stented for intracranial ischemic stenosis versus treated with medical therapy. We are in the process of initiating study sites in the United States, Europe and China.
In fiscal 2010, we launched our DeltaPlush™ microcoil which has been designed to be our softest finishing coil, enabling more efficient and safer aneurysm treatment. The DeltaPlush™ microcoil incorporates our exclusive Delta Wind™ technology resulting in a microcoil with the softness and flexibility to find and fill gaps, helping to provide superior finishing at the aneurysm neck. It is available in bare platinum and Cerecyte®. In January of 2010, we launched our Ascent® Occlusion Balloon Catheter which is intended for use in the blood vessels of the peripheral and neurovasculature where temporary occlusion is desired. The Ascent® balloon catheter offers a vessel selective technique of temporary arterial occlusion which is useful in selectively stopping or controlling blood flow.
42
We intend to continue to expand our direct sales force in North America, Europe and Asia Pacific as necessary and further increase our presence in the Asia Pacific markets through distributors. In March 2006, we launched our sales and marketing efforts in Japan through our distribution partner, Goodman. In December 2007, we received regulatory approval to sell our stretch-resistant microcoils in Japan, and in July 2008, we received regulatory approval to sell our Cerecyte microcoils in Japan. We recorded product sales to Goodman of $11.0 million, $9.3 million and $6.3 million in fiscal 2010, 2009 and 2008, respectively. We plan to begin selling our products in China upon receiving regulatory and reimbursement approvals. The timing of these approvals is uncertain. We did not have any sales in China during fiscal 2010. We currently anticipate selling our products in China in fiscal 2011.
We currently anticipate that the broadening of our product line and our further expansion into the Asian market will be primarily funded with our currently available cash and cash expected to be generated from operations. We introduced our first proprietary, three-dimensional microcoil in May 2000. Our revenues have grown from $1.8 million in fiscal 2001 to $91.1 million in fiscal 2010.
We achieved our first profitable year in fiscal 2010, with a profit of $11.5 million as a result of our revenue growth and effective cost management, and we expect to achieve another profitable year in fiscal 2011. As of March 31, 2010, we had cash and cash equivalents of $30.1 million. We believe that our current cash position and the cash expected to be generated from operations will be sufficient to meet our working capital and capital expenditure requirements for at least the next twelve months. As of March 31, 2010, we had an accumulated deficit of $70.9 million.
Recent Developments
On August 21, 2009, we entered into the FSS Agreement. Under the terms of the FSS Agreement, Micrus and FSS will jointly develop a flow diversion product for neurovascular indications using both Micrus and FSS technology, development capabilities and intellectual property. We will be responsible for overseeing the regulatory and clinical process and will manufacture neurovascular products developed under the terms of this collaborative agreement. The transaction included an initial up-front payment by the Company of $0.5 million, future development and regulatory milestone payments and royalties on potential future product sales.
On September 30, 2009, we terminated our distribution agreement with Beijing Tian Xian Fu Medical Appliance Co. LTD (“TXF”) and entered into a distribution agreement with IDS. Pursuant to the IDS Distribution Agreement, IDS will serve as our exclusive distributor of implantable and disposable medical devices used in the treatment of neurovascular diseases in China.
Pursuant to the IDS Distribution Agreement, IDS will promote and market our products in China and is required to purchase a certain amount of our products over a five year period commencing upon receiving regulatory approval in order to maintain its exclusive distributor status in such territories, escalating over the five year term. The minimum purchase requirement commences on the date when our products are included in the National Reimbursement List of Interventional Medical Devices issued by the Minister of Health of the People’s Republic of China. We will supply IDS with its requirements for our products and have granted IDS a license to use certain of our trademarks in connection with IDS’ sale and distribution of our products in China. The term of the IDS Distribution Agreement is five years, subject to the right of the parties to terminate earlier based upon the occurrence of certain events. In connection with the IDS Distribution Agreement, we have also entered into an agreement with IDS whereby we have agreed to provide marketing and sales support to IDS.
In January 2010, we entered into an Exclusive License and Option Agreement (the “Bay Street Agreement”) with Bay Street. Pursuant to the terms of the Bay Street Agreement, Bay Street granted us a worldwide exclusive license and option to purchase intellectual property assets held by Bay Street related to its proprietary stent delivery and locking technology (the “Bay Street Assets”). The transaction included an initial up-front payment of $0.5 million for a six month evaluation of the exclusive license. We have the option to extend the exclusive license for an additional six months upon paying to Bay Street an option payment of $0.5 million. We also have the option to purchase the Bay Street Assets before the option period ends pursuant to an Asset Purchase Agreement that will be an addendum to the Bay Street Agreement. Under the terms of the Asset Purchase Agreement, if we elect to purchase the Bay Street Assets, we would be required to pay an initial purchase payment of $1.0 million, a future sales-based milestone payment and royalties on future product sales.
In January 2010, we paid The Cleveland Clinic a milestone payment of $2.0 million pursuant to the ReVasc Agreement.
43
Results of Operations
The following table sets forth the results of our operations, expressed as percentages of revenues, for the fiscal years ended March 31, 2010, 2009 and 2008:
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
%
|
%
|
%
|
||||||||||
|
Consolidated Statements of Operations Data:
|
||||||||||||
|
Revenues
|
100
|
%
|
100
|
%
|
100
|
%
|
||||||
|
Cost of goods sold
|
25
|
%
|
27
|
%
|
25
|
%
|
||||||
|
Gross profit
|
75
|
%
|
73
|
%
|
75
|
%
|
||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
12
|
%
|
13
|
%
|
20
|
%
|
||||||
|
Sales and marketing
|
29
|
%
|
37
|
%
|
42
|
%
|
||||||
|
General and administrative
|
23
|
%
|
35
|
%
|
39
|
%
|
||||||
|
Impairment of intangible assets
|
0
|
%
|
1
|
%
|
0
|
%
|
||||||
|
Total operating expenses
|
64
|
%
|
86
|
%
|
101
|
%
|
||||||
|
Income (loss) from operations
|
11
|
%
|
(13
|
)%
|
(26
|
)%
|
||||||
|
Interest and investment income
|
0
|
%
|
0
|
%
|
2
|
%
|
||||||
|
Interest expense
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Other income (expense), net
|
2
|
%
|
(2)
|
%
|
1
|
%
|
||||||
|
Income (loss) before income taxes
|
13
|
%
|
(15
|
)%
|
(23
|
)%
|
||||||
|
Income tax benefit (expense)
|
(0
|
)%
|
1
|
%
|
0
|
%
|
||||||
|
Net income (loss)
|
13
|
%
|
(14
|
)%
|
(23
|
)%
|
||||||
Fiscal Years Ended March 31, 2010 and 2009
Revenues
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2010
|
2009
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Americas
|
$
|
50,133
|
$
|
44,067
|
$
|
6,066
|
14
|
%
|
||||||||
|
Europe
|
28,486
|
23,461
|
5,025
|
21
|
%
|
|||||||||||
|
Asia Pacific
|
12,471
|
10,668
|
1,803
|
17
|
%
|
|||||||||||
|
$
|
91,090
|
$
|
78,196
|
$
|
12,894
|
16
|
%
|
|||||||||
Our revenues are derived primarily from sales of our microcoils used in the treatment of cerebral vascular diseases. The overall increase in revenues in fiscal 2010 compared to fiscal 2009 was primarily due to an increase in the number of microcoil products sold. Factors driving the increase included growth in the overall market for embolic coils, increase market share in all operating segments, continued market penetration of the DeltaPaq™ microcoil and the introduction of new embolic coil products such as the DeltaPlush™ microcoil along with the revenues generated from sales of new non-embolic products such as the PHAROS® Vitesse® intracranial stent.
Revenues from the Americas increased 14% to $50.1 million for fiscal 2010 and revenues from Europe increased 21% to $28.5 million for fiscal 2010, both compared to fiscal 2009. Our revenues increased in the Americas and Europe primarily due to continued market penetration of the DeltaPaq™ microcoil and the introduction of the DeltaPlush™ microcoil. Revenues from Asia Pacific increased 17% to $12.5 million in fiscal 2010 and included sales to our distributor in Japan of $11.0 million, compared with revenues of $10.7 million for fiscal 2009 which included sales to our distributor in Japan of $9.3 million. We plan to begin selling our products in China upon receiving regulatory approvals. We did not have any sales in China during fiscal 2010.
44
Revenues from embolic coils increased 17% to $85.4 million for fiscal 2010 as compared to fiscal 2009 primarily due to increased market penetration of the DeltaPaq™ and the introduction of the DeltaPlush™ microcoil system, and partially offset by the decline in the overall number of procedures in the United Kingdom. Revenues from our non-embolic and accessories products increased to $5.7 million in fiscal 2010 compared with revenues of $4.9 million in fiscal 2009 primarily due to continued market penetration of our PHAROS® Vitesse® stent and the launch of our Ascent® Occlusion Balloon Catheter. We expect our embolic and non-embolic sales to increase in the future as a result of market growth, continued market penetration of products released during the past two years, including the launch of our next-generation DeltaPaq™ and our DeltaPlush™ microcoil systems, and the launch of our Ascent® Occlusion Balloon Catheter family.
New products continue to represent an important component of our growth strategy, with 27% of our revenues in fiscal 2010 coming from products introduced in the past 24 months. Among these, our DeltaPaq™ microcoil line represented 17% of the total revenue for fiscal 2010. We are also pleased with the strong reception of our newly launched DeltaPlush™ microcoil system, which comprised 5% of fiscal 2010 revenues. With the launch of the Neuropath® guide catheter, the PHAROS® Vitesse® stent and the Ascent® Occlusion Balloon Catheter, we are now in a position to capture a significantly greater portion of hemorrhagic procedure revenues.
Gross Profit
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2010
|
2009
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Cost of goods sold
|
$
|
22,406
|
$
|
20,847
|
$
|
1,559
|
7
|
%
|
||||||||
|
Gross profit
|
$
|
68,684
|
$
|
57,349
|
$
|
11,335
|
20
|
%
|
||||||||
Cost of goods sold consists primarily of materials, direct labor, depreciation, overhead costs associated with manufacturing, impairments of inventory, warranty expenses, amortization of intangible assets that were acquired by us as part of the acquisition of VasCon, amortization of capitalized license technology associated with our PHAROS® stent product and royalties related to certain access device products. The increase in cost of goods sold during fiscal 2010 as compared to fiscal 2009 was primarily related to the increase in sales of our embolic products as well as an increase of $460,000 in inventory provisions primarily related to excess inventories for certain slow moving products and an increase of $102,000 in royalties.
Our gross margin was 75% in fiscal 2010 as compared to 73% in fiscal 2009. The increase was primarily due to an increase in revenues from sales of higher margin products. We expect our gross margin to fluctuate in future periods based on the mix of our product sales.
Operating Expenses
Research and Development
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2010
|
2009
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Research and development
|
$
|
10,449
|
$
|
10,243
|
$
|
206
|
|
2
|
%
|
|||||||
Research and development expenses consist primarily of costs associated with the design, development, and testing of new products. Such costs are expensed as they are incurred and include salaries and related personnel costs, fees paid to outside consultants, and other direct and indirect costs related to research and product development. Research and development expenses increased in fiscal 2010 compared to fiscal 2009 primarily due to an increase of $1.3 million in technology acquisition costs primarily consisting of a $0.5 million upfront payment to FSS to jointly develop a flow diversion product and a $0.5 million upfront payment to Bay Street to obtain a license for its proprietary stent delivery and locking technology. The increase was partially offset by a decrease of $1.0 million in personnel costs due to a change in our employee bonus program and lower headcount and a decrease of $232,000 in material and supplies expenses.
Sales and Marketing
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2010
|
2009
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Sales and marketing
|
$
|
26,816
|
$
|
29,312
|
$
|
(2,496
|
)
|
(9)
|
%
|
|||||||
45
Sales and marketing expenses consist primarily of compensation costs of our direct sales force and marketing personnel, as well as overhead costs related to these activities. Also included are costs associated with promotional literature and videos, trade show participation, and education and training of physicians. Sales and marketing expenses decreased in fiscal 2010 compared to fiscal 2009 primarily as a result of our expense management efforts, including a decrease of $1.7 million in travel and personnel costs, a decrease of $0.8 million in meeting and conference costs and a decrease of $0.6 million in consulting costs. These decreases were partially offset by higher sales incentives compensation of $1.0 million resulting from a higher level of sales.
General and Administrative
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2010
|
2009
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
General and administrative
|
$
|
21,266
|
$
|
26,983
|
$
|
(5,717)
|
(21)
|
%
|
||||||||
General and administrative expenses consist primarily of compensation and related costs for finance, human resources, facilities, information technology, insurance, and professional services. Professional services are principally comprised of outside legal, audit, Sarbanes Oxley compliance and information technology consulting. General and administrative expenses decreased in fiscal 2010 compared to fiscal 2009 due to a $3.6 million reduction in legal and professional service fees primarily related to the settlement cost of the patent litigation with Boston Scientific and the conclusion of the United States Department of Justice monitorship, as well as a decrease of $0.8 million attributable primarily to the reversal of estimated sales tax previously recorded, a decrease of $0.7 million in SOX compliance and consulting costs, a decrease of $383,000 in personnel costs primarily due to a change in our employee bonus program and a decrease of $300,000 in travel costs.
Out of Period Adjustments
In fiscal 2010, we recorded adjustments to cost of goods sold, operating expenses and certain balance sheet accounts to record additional expenses primarily related to stock-based compensation expense that were not correctly recorded in prior periods. The net adjustments resulted in our reporting $281,000 in additional pre-tax expenses. These adjustments both individually and in the aggregate were not material to any of the fiscal 2009 and 2010 interim or fiscal 2009 and 2010 full year consolidated financial statements.
Other Income (Expense), Net
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2010
|
2009
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Interest and investment income
|
$
|
45
|
$
|
258
|
$
|
(213
|
)
|
(83
|
)%
|
|||||||
|
Interest expense
|
(127
|
)
|
(52
|
)
|
(75
|
)
|
144
|
%
|
||||||||
|
Other income (expense), net
|
2,096
|
(2,111
|
)
|
4,207
|
199
|
%
|
||||||||||
|
Total other income (expense), net
|
$
|
2,014
|
$
|
(1,905
|
)
|
$
|
3,919
|
206
|
%
|
|||||||
Interest and investment income consists of interest earned on interest bearing accounts. Interest and investment income decreased in fiscal 2010 compared to fiscal 2009 due to lower interest rates.
Other income (expense), net consists primarily of foreign exchange gains and losses resulting from differences in exchange rates between the time of recording of the transaction and the settlement of foreign currency denominated receivables and payables. Other income (expense), net increased in fiscal 2010 compared to fiscal 2009 primarily due to the recognition of the deferred gain of $1.9 million in connection with the sale of non-neurological, cardiac and peripheral catheter assets and technology assets to Merit and gains resulting from foreign currency translations most notably the impact of the strengthening of the British pound against the U.S. dollar.
Income Taxes
|
Years Ended March 31,
|
|||||||
|
2010
|
2009
|
||||||
|
(In thousands, except percentages)
|
|||||||
|
Income tax provision (benefit)
|
$
|
637
|
$
|
(502
|
)
|
||
|
Effective tax rate
|
5
|
%
|
(4
|
)%
|
|||
46
We recorded an income tax expense of approximately $0.6 million during fiscal 2010. The income tax expense consists primarily of state and Swiss income tax on operating profits. Prior to fiscal 2010, we incurred net operating losses for both federal and state purposes and, as a result, paid no federal or state income taxes. We had an income tax benefit of approximately $0.5 million in fiscal 2009. The net income tax benefit in fiscal 2009 consists primarily of deferred tax benefit for the tax effect of the amortization related to the intangible assets acquired in the Neurologic transaction which is not deductible, the reduction in deferred tax liability due to the impairment of such intangible assets, and losses from our United Kingdom subsidiary.
As of March 31, 2010, we had federal, state and foreign net operating loss carryforwards (“NOLs”) of approximately $36.9 million, $28.8 million and $2.1 million, respectively. The federal NOLs will expire at various dates beginning in 2022, the state NOLs expire beginning in 2015 and the foreign NOLs will expire beginning in 2016. We also had federal and state research and development tax credit carryforwards of approximately $1.9 million and $1.7 million, respectively, as of March 31, 2010. The federal credits will expire beginning in 2019 and the state credits can be carried forward indefinitely. Due to the uncertainty of our ability to generate sufficient taxable income to realize the carryforwards prior to their expiration, we have recorded a valuation allowance at March 31, 2010 to offset our federal and state deferred tax assets.
Fiscal Years Ended March 31, 2009 and 2008
Revenues
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2009
|
2008
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Americas
|
$
|
44,067
|
$
|
37,565
|
$
|
6,502
|
17
|
%
|
||||||||
|
Europe
|
23,461
|
24,195
|
(734
|
)
|
(3
|
)%
|
||||||||||
|
Asia Pacific
|
10,668
|
7,453
|
3,215
|
43
|
%
|
|||||||||||
|
$
|
78,196
|
$
|
69,213
|
$
|
8,983
|
13
|
%
|
|||||||||
Our revenues are derived primarily from sales of our microcoils used in the treatment of cerebral vascular diseases. The overall increase in revenues in fiscal 2009 compared to fiscal 2008 was primarily due to an increase in the number of microcoil products sold. Factors driving the increase included growth in the overall market for embolic coils, an increase in market share in both the Americas and Asia Pacific, and the introduction of new products.
Revenues from the Americas increased 17% to $44.1 million primarily due to continued market penetration of the Cerecyte® Cashmere® microcoil and the introduction of the DeltaPaq™ microcoil. Revenues from Europe decreased 3% to $23.5 million, compared to fiscal 2008 primarily due to the unfavorable impact on revenues from the weakening of the British pound against the U.S. dollar and a decline in overall number of procedures in the United Kingdom. Revenues from Asia Pacific increased 43% to $10.7 million and included product sales to our distributor in Japan of $9.3 million, compared with revenues of $7.5 million for fiscal 2008 which included sales to our distributor in Japan of $6.3 million. We plan to begin selling our products in China upon receiving regulatory approvals. We did not have any sales in China during fiscal 2009.
Revenues from Latin America increased to $3.1 million in fiscal 2009 compared with revenues of $1.6 million in fiscal 2008 due to an overall increase in product sales to our distributors in the region and a change in our revenue recognition policy for sales made to Latin American distributors from a cash collection basis to upon shipment basis (see Note 2 of the “Notes to Consolidated Financial Statements,” which are included in “Item 8 – Financial Statements and Supplementary Data”). As a result of the change in our revenue recognition policy for sales made to Latin American distributors, we recognized approximately $0.7 million of Latin American deferred revenue in the first quarter of fiscal 2009.
Revenues from embolic coils increased 12% to $73.1 million for fiscal 2009 as compared to fiscal 2008 primarily due to the launch of the DeltaPaq™ microcoil system and increased market penetration of both the Cashmere® and the Presidio® microcoil systems, partially offset by the unfavorable impact of foreign currency exchange rates, most notably the weakening of the British pound against the U.S. dollar. Revenues from our non-embolic and accessories products increased to $4.9 million in fiscal 2009 compared with revenues of $3.7 million in fiscal 2008 primarily due to the launch of the Neuropath® guide catheter and PHAROS® Vitesse® stent, and volume increases across multiple product lines. We expect our embolic and non-embolic sales to increase in the future as a result of market growth, continued market penetration of products released during the past two years, including our launch of the next-generation DeltaPaq™ microcoil system and an Ascent Occlusion Balloon Catheter family.
47
New products continue to represent an important component of our growth strategy, with 16% of our revenues in fiscal 2009 coming from products introduced in the past 24 months. Among these, our Cashmere® microcoil line represented 8% of the total revenue for fiscal 2009. We are also pleased with the strong reception for our newly launched DeltaPaq™ microcoil system, which comprised 6% of fiscal 2009 revenues. With the launch of the Neuropath® guide catheter and the Ascent® Occlusion Balloon Catheter, we are now in a position to capture a significantly greater portion of hemorrhagic procedure revenues.
Gross Profit
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2009
|
2008
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Cost of goods sold
|
$
|
20,847
|
$
|
17,301
|
$
|
3,546
|
20
|
%
|
||||||||
|
Gross profit
|
$
|
57,349
|
$
|
51,912
|
$
|
5,437
|
10
|
%
|
||||||||
Cost of goods sold consists primarily of materials, direct labor, depreciation, overhead costs associated with manufacturing, impairments of inventory, warranty expenses, amortization of intangible assets that were acquired by us as part of the acquisition of VasCon, amortization of capitalized license technology associated with our PHAROS® stent product and royalties related to certain access device products. The increase in cost of goods sold during fiscal 2009 as compared to fiscal 2008 was primarily related to the increase in sales of our products as well as an increase of $235,000 in royalties.
Gross margin was 73% in fiscal 2009 and 75% in fiscal 2008. The decrease was primarily due to higher levels of distributor sales of lower margin products primarily in Japan and certain European markets. We expect our gross margin to fluctuate in future periods based on the mix of our product sales.
Operating Expenses
Research and Development
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2009
|
2008
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Research and development
|
$
|
10,243
|
$
|
13,718
|
$
|
(3,475
|
)
|
(25
|
)%
|
|||||||
Research and development expenses consist primarily of costs associated with the design, development, and testing of new products. Such costs are expensed as they are incurred and include salaries and related personnel costs, fees paid to outside consultants, and other direct and indirect costs related to research and product development. Research and development expenses decreased in fiscal 2009 compared to fiscal 2008 primarily due to a decrease of $3.9 million in technology acquisition costs mainly due to a $3.0 million charge in fiscal 2008 for in-process research and development in connection with the acquisition of ReVasc Technologies, Inc. to obtain the rights to pre-regulatory approved revascularization technology and a $0.9 million charge in fiscal 2008 associated with the acquisition of thrombectomy technologies from Genesis. In addition, there was a decrease in outside services of $0.9 million primarily associated with the development of our stent product. This decrease was partially offset by an increase of $0.9 million in personnel cost due to increased headcount at our Florida operations as we ramp up research and development efforts for neurovascular access and delivery products, and an increase of $303,000 in consulting fees.
Sales and Marketing
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2009
|
2008
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Sales and marketing
|
$
|
29,312
|
$
|
29,385
|
$
|
(73
|
)
|
0
|
%
|
|||||||
Sales and marketing expenses consist primarily of compensation costs of our direct sales force and marketing personnel, as well as overhead costs related to these activities. Also included are costs associated with promotional literature and videos, trade show participation, and education and training of physicians. Sales and marketing expenses decreased slightly in fiscal 2009 compared to fiscal 2008 primarily due to termination costs of $0.7 million related to sales personnel in Europe in fiscal 2008, as well as a decrease of $232,000 in recruiting expense and a decrease of $154,000 in education and training expense. These decreases were partially offset by higher sales incentives compensation of $0.6 million resulting from higher level of sales and changes in the sales compensation structure, an increase of $213,000 in product samples and an increase of $174,000 in stock-based compensation expense.
48
General and Administrative
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2009
|
2008
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
General and administrative
|
$
|
26,983
|
$
|
26,971
|
$
|
12
|
0
|
%
|
||||||||
General and administrative expenses consist primarily of compensation and related costs for finance, human resources, facilities, information technology, insurance, and professional services. Professional services are principally comprised of outside legal, audit, Sarbanes Oxley compliance and information technology consulting. General and administrative expenses remained essentially unchanged in fiscal 2009 compared to fiscal 2008. General and administrative expenses included increases of $1.7 million in patent litigation settlement cost in connection with the patent litigation with Boston Scientific, higher finance and administrative personnel costs of $1.2 million due to increased headcount and salary increases for current employees, additional expense of $0.5 million for estimated sales tax in certain states and an increase of $367,000 in stock-based compensation expense.
These increases were partially offset by a decrease of $3.7 million in legal fees primarily resulting from the settlement of the patent litigation with Boston Scientific in September 2008 and the conclusion of the United States Department of Justice monitorship in July 2008.
Impairment of Intangible Assets
In the fourth quarter of fiscal 2009, we performed an assessment of our intangible assets acquired from Neurologic. Management concluded from the assessment that an impairment existed for the intangible asset group (distribution agreement, customer relationships and non-compete agreements) as the undiscounted value was less than its carrying value. As a result, we recorded an impairment charge of $462,000 for this asset group based on the amount by which the carrying amount of these assets exceeded their fair value. There was no impairment charge related to intangible assets in fiscal 2008.
Other Income (Expense), Net
|
Years Ended March 31,
|
Change
|
|||||||||||||||
|
2009
|
2008
|
$
|
%
|
|||||||||||||
|
(In thousands, except percentages)
|
||||||||||||||||
|
Interest and investment income
|
$
|
258
|
$
|
1,223
|
$
|
(965
|
)
|
(79
|
)%
|
|||||||
|
Interest expense
|
(52
|
)
|
(3
|
)
|
(49
|
)
|
1633
|
%
|
||||||||
|
Other income (expense), net
|
(2,111
|
)
|
488
|
(2,599
|
)
|
(533
|
)%
|
|||||||||
|
Total other income (expense), net
|
$
|
(1,905
|
)
|
$
|
1,708
|
$
|
(3,613
|
)
|
(212
|
)%
|
||||||
Interest and investment income consists of interest earned on interest bearing accounts. Interest and investment income decreased in fiscal 2009 compared to fiscal 2008 due to lower cash and investment balances earning interest.
Other income (expense), net consists primarily of foreign exchange gains and losses resulting from differences in exchange rates between the time of recording of the transaction and the settlement of foreign currency denominated receivables and payables. Other income (expense), net decreased in fiscal 2009 compared to fiscal 2008 primarily due to foreign exchange losses resulting from the re-measurement of foreign currency transactions, most notably the impact of the weakening of the British pound against the U.S. dollar.
Income Taxes
|
Years Ended March 31,
|
|||||||
|
2009
|
2008
|
||||||
|
(In thousands, except percentages)
|
|||||||
|
Income tax benefit
|
$
|
(502
|
)
|
$
|
(194
|
)
|
|
|
Effective tax rate
|
(4
|
)%
|
(1
|
)%
|
|||
49
We have incurred net operating losses for both federal and state purposes since inception and, as a result, we have paid no federal or state income taxes. We had an income tax benefit of approximately $502,000 and $194,000 in fiscal 2009 and 2008, respectively. The net income tax benefit in fiscal 2009 consists primarily of deferred tax benefit for the tax effect of the amortization related to the intangible assets acquired in the Neurologic transaction which is not deductible, the reduction in deferred tax liability due to the impairment of such intangible assets, and losses from our United Kingdom subsidiary. The net income tax benefit in fiscal 2008 includes a deferred income tax expense of approximately $72,000 for the Swiss subsidiary’s operating profits and a deferred tax benefit of approximately $266,000 for the tax effect of the amortization related to the intangible assets acquired in the Neurologic transaction which is not deductible and the tax benefit of operating losses for our United Kingdom subsidiary.
Liquidity and Capital Resources
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
(In thousands)
|
||||||||||||
|
Cash flow activities:
|
||||||||||||
|
Net cash provided by (used in) operating activities
|
$
|
13,864
|
$
|
(7,048
|
)
|
$
|
(8,820
|
)
|
||||
|
Net cash used in investing activities
|
$
|
(3,548
|
)
|
$
|
(5,007
|
)
|
$
|
(3,437
|
)
|
|||
|
Net cash provided by financing activities
|
$
|
2,405
|
$
|
4,128
|
$
|
3,165
|
||||||
As of March 31, 2010, we had cash and cash equivalents of $30.1 million, compared to $17.1 million at March 31, 2009. We believe that our current cash position and the cash expected to be generated from operations will be sufficient to meet our working capital and capital expenditure requirements for at least the next twelve months.
Net cash provided by operating activities during fiscal 2010 was $13.9 million as compared to net cash used in operating activities of $7.1 million and $8.8 million during fiscal 2009 and 2008, respectively. Net cash provided by operating activities during fiscal 2010 resulted primarily from net income adjusted by non-cash items such as stock-based compensation expense, depreciation and amortization, provision for excess and obsolete inventories, deferred income taxes, the recognition of the deferred gain in connection with the sale of non-neurological, cardiac and peripheral catheter assets and technology assets to Merit, the effect of foreign exchange rate changes on intercompany balances; an increase in accrued payroll which was attributable to increased headcount and the timing of the payroll payments and an increase in accrued liabilities due to an increase in income taxes on profits arising in the United States and Switzerland. These factors were partially offset by an increase in accounts receivable resulting from higher level of sales; an increase in inventory due to an increase in the number of consignment locations and an increase in the number of units in existing consignment locations primarily due to new products released and a decrease in accounts payable due to the timing of payments made to our vendors.
Net cash used in operating activities during fiscal 2009 resulted primarily from operating losses; an increase in accounts receivable which resulted from the sale of a greater number of microcoil products and timing of collections for those sales; an increase in inventory resulting from an increase in the number of consignment locations due to the addition of new customers and the launch of new products; a decrease in accounts payable due to the timing of payments to our vendors; a decrease in accrued payroll and other related expenses primarily due to lower accrued bonuses and accrued commissions; a decrease in accrued liabilities and other non-current liabilities primarily due to lower accrued legal fees primarily resulting from the settlement of the patent litigation with Boston Scientific and the conclusion of the United States Department of Justice monitorship. These factors were partially offset by a decrease in prepaid expense and non-cash items such as stock-based compensation expense, depreciation and amortization, deferred income taxes, impairment of intangible assets and the effect of foreign exchange rate changes on intercompany balances.
Net cash used in operating activities during fiscal 2008 resulted primarily from: operating losses; an increase in accounts receivable which resulted from the sale of a greater number of microcoil products and timing of collections for those sales; an increase in inventory due to an increase in the number of consignment locations and the buildup of finished goods in anticipation of future sales; an increase in prepaid expenses and other current assets, primarily due to deposits paid in advance of our global sales meeting; and an increase in other non-current assets, primarily due to payments of a broker’s commission and a security deposit in connection with the lease at our new Florida facility. These factors were partially offset by an increase in accounts payable due to the timing of our payments to our vendors; an increase in accrued payroll and other related expenses which was attributable to increased headcount and the timing of payroll payments; an increase in accrued liabilities and other non-current liabilities primarily due to accrued milestone payments to ReVasc and Genesis and higher accrued professional fees associated with legal fees; and non-cash items such as stock-based compensation expense, depreciation and amortization, our provision for excess and obsolete inventories and the effect of foreign exchange rate changes on intercompany balances.
50
Net cash used in investing activities during fiscal 2010 was $3.5 million as compared to $5.0 million and $3.4 million during fiscal 2009 and 2008, respectively. Net cash used in investing activities during fiscal 2010 was related to the purchase of capital equipment, the earn-out payment associated with the purchase of VasCon and a milestone payment to The Cleveland Clinic pursuant to the ReVasc agreement.
Net cash used in investing activities during fiscal 2009 was related to the purchase of capital equipment, the earn-out payments associated with the purchase of VasCon and Neurologic, partially offset by proceeds from the sale of property and equipment to third parties and the sale of certain assets and technologies to Merit.
Net cash used in investing activities during fiscal 2008 was related to the purchase of capital equipment, prepayments made related to leasehold improvements in connection with the lease at our new Florida facility, the earn-out payment associated with the purchase of Neurologic, partially offset by proceeds from the sale of property and equipment to third parties and the sale of certain assets and technologies to Merit.
Net cash provided by financing activities during fiscal 2010 was $2.4 million as compared to $4.1 million and $3.2 million during fiscal 2009 and 2008, respectively. Net cash provided by financing activities during fiscal 2010 was related to excess tax benefit from stock-based compensation, proceeds from the exercise of stock options and the purchase of common stock under our employee stock purchase plan, partially offset by repayment of the line of credit to Wells Fargo Bank.
Net cash provided by financing activities during fiscal 2009 consisted of borrowings under the line of credit with Wells Fargo Bank, exercise of stock options and the purchase of common stock under our employee stock purchase plan.
Net cash provided by financing activities during fiscal 2008 consisted of proceeds from the exercise of stock options and the purchase of common stock under our employee stock purchase plan.
To the extent that existing cash and cash generated from operations are insufficient to fund our future activities, we would seek to borrow funds under our credit facility. We have a revolving line of credit with Wells Fargo Bank with a maximum borrowing in the amount up to $15.0 million. However, given that certain financial and other covenants of the credit agreement are required to be met, these funds may not be available to us. Accordingly, we may need to reduce discretionary spending and raise additional funds through public or private equity or debt financing. Although we are currently not a party to any definitive agreement with respect to potential investments in, or acquisitions of, complementary businesses, services or technologies, we may enter into such agreements in the future. Additional funds may not be available on terms favorable to us, or at all. Failure to manage the discretionary spending or raise additional capital as required may adversely impact our ability to achieve our intended business objectives.
Critical Accounting Policies and Estimates
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States (“GAAP”). In doing so, we have to make estimates and assumptions that affect our reported amounts of assets, liabilities, revenues and expenses, as well as related disclosure of contingent assets and liabilities. In many cases, we could reasonably have used different accounting policies and estimates. In some cases, changes in the accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ materially from our estimates. To the extent that there are material differences between these estimates and actual results, our financial condition or results of operations will be affected. We base our estimates on past experience and other assumptions that we believe are reasonable under the circumstances, and we evaluate these estimates on an ongoing basis. We refer to accounting estimates of this type as critical accounting policies and estimates, which we discuss below. Our management has reviewed our critical accounting policies and estimates with our accounting advisors, audit committee and board of directors.
Although our significant policies are more fully described in Note 2 of the “Notes to Consolidated Financial Statements,” which are included in “Item 8 – Financial Statements and Supplementary Data,” we believe that the following accounting policies to be critical to the judgment and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition. Our revenues are derived primarily from the sale of our microcoil product line to hospitals and third-party distributors. We also sell access products and accessories for use with our microcoils. Revenues are recognized when evidence of an arrangement exists, delivery to the customer has occurred, the selling price is fixed or determinable and collectibility is reasonably assured. Revenue is generally recognized upon shipment after the receipt of a purchase order. In arrangements which specify the title transfer upon delivery, revenue is not recognized until the product is delivered. In the case of consigned goods, revenue is recognized when a replenishment order is made.
51
Allowance for Doubtful Accounts. In estimating the collectibility of our accounts receivable, we analyze historical bad debts, customer concentrations, customer credit-worthiness, current economic trends, and changes in customer payment terms. We regularly review the adequacy of our accounts receivable allowance after considering changes in customers’ financial condition and the aging of account receivable balances. If there are unanticipated future events, this allowance may need to be adjusted.
Excess and Obsolete Inventory. We calculate an inventory provision for estimated obsolescence or excess inventories based upon historical scrap rates and assumptions about future demand for our products and market conditions. Our microcoil products have a five-year shelf life and our access products have a shelf life between one and three years. Our products are subject to demand fluctuations based on the availability and demand for alternative products. Our inventory, which consists primarily of microcoils, is at risk of obsolescence following the introduction and development of new or enhanced products. Our estimates and assumptions for excess and obsolete inventory are reviewed and updated on a quarterly basis. The estimates we use for demand are also used for near-term capacity planning and inventory purchasing and are consistent with our revenue forecasts. Future product introductions and related inventories may require additional provision based upon changes in market demand or introduction of competing technologies. Provision for excess and obsolete inventories result in a corresponding expense to cost of goods sold.
Valuation of Goodwill and Intangibles. When we acquire another company, the purchase price is allocated, as applicable, between acquired in-process research and development, other identifiable intangible assets, tangible net assets and goodwill as required by GAAP. In-process research and development is the value assigned to those projects for which the related products have not received regulatory approval and have no alternative future use. In-process research and development is recorded as a charge on the date of acquisition. Through March 31, 2010, there has been no in-process research and development acquired by us in a business combination. Goodwill is capitalized, subject to periodic review for impairment. Under our accounting policy, we perform an annual review of goodwill in the fourth quarter of each fiscal year, or more often if indicators of impairment exist. We evaluate our identifiable intangible assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be recoverable. Evaluations of possible impairment and, if applicable, adjustments to carrying values require us to estimate, among other factors, future cash flows over the life of the assets being evaluated, useful lives, and fair market values of our reporting units and assets. When we conduct our evaluation of goodwill, the fair value of goodwill is assessed using valuation techniques that require management judgment and actual results may differ from assumed or estimated amounts. Should conditions be different from management’s last assessment, significant write-downs of goodwill may be required. Through March 31, 2010, we determined that there has been no impairment related to goodwill. Our impairment testing conducted in fiscal 2010 and 2008 indicated no such impairment on intangible assets. However, in fiscal 2009 we determined that $462,000 of intangible assets related to the acquisition of Neurologic in September 2005 were impaired and recorded an impairment charge.
Accounting for Income Taxes. Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We have recorded a valuation allowance on our federal and state net deferred tax assets as of March 31, 2010 and our federal, state and Swiss net deferred tax assets as of March 31, 2009 due to the uncertainties related to our ability to utilize our deferred tax assets in the foreseeable future. These deferred tax assets primarily consist of certain net operating loss carry forwards and accruals deductible in different periods.
Stock-based Compensation. We currently use the Black-Scholes option-pricing model to determine the fair value of stock options and employee stock purchase plan shares on the date of grant. The Black-Scholes model requires that we make certain assumptions that are factored into the valuation analysis, including estimating the length of time employees will retain their vested stock options before exercising them (“expected term”) and the estimated volatility of our common stock price over the expected term and the number of options that will ultimately not complete their vesting requirements (“forfeitures”). Changes in the subjective assumptions can materially affect the estimate of fair value of stock-based compensation and consequently, the related amount recognized in the consolidated statements of income.
Prior to fiscal 2010, we determined expected volatility based on median results of a peer group analysis of companies similar in size and financial leverage to us. Beginning in fiscal 2010, we used combined volatility including that of the peer group and the historical volatility of our stock. We have elected to use the simplified method for estimating our expected term as allowed by relevant accounting standards under certain conditions including our inability to rely on historical exercise data. We will continue to use the simplified method until we have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term of our options. The risk-free rate is indexed to the five-year Treasury note interest at the date of grant and expected forfeiture rate is based on our historical forfeiture information.
All stock-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods.
52
Some exercises result in tax deductions in excess of previously recorded benefits based on the option value at the time of grant (“windfall” tax benefits). We recognize windfall tax benefits associated with the exercise of stock options directly to stockholders’ equity only when realized. Accordingly, deferred tax assets are not recognized for net operating loss carryforwards resulting from windfall tax benefits occurring from April 1, 2006 onward. A windfall tax benefit occurs when the actual tax benefit realized by the company upon an employee’s disposition of a share-based award exceeds the deferred tax asset, if any, associated with the award that the company had recorded. When assessing whether a tax benefit relating to share-based compensation has been realized, we have elected to follow the with-and-without approach, under which net operating loss carryforwards and other tax attributes from continuing operations are utilized before utilizing share-based compensation deductions. Also, we have elected to ignore the indirect tax effects of share-based compensation deductions in computing our research and development tax credit. We will recognize the full effect of these deductions in the statements of operations when the valuation allowance is released. See Note 12 of the “Notes to Consolidated Financial Statements,” which are included in “Item 8 – Financial Statements and Supplementary Data” for further information regarding our stock-based compensation disclosures.
Impact of Inflation and Changing Prices
The Company does not believe that the impact of inflation and changing prices has had a material effect on its operations or financial results at any time in the last three fiscal years.
Recent Accounting Pronouncement
In December 2008, the Financial Accounting Standards Board (“FASB”) issued authoritative guidance for employers’ disclosures about postretirement benefit plan assets, which provides guidance on an employer’s disclosures about plan assets of a defined benefit pension or other postretirement plan, including disclosures about investment policies and strategies, categories of plan assets, fair value measurements of plan assets and significant concentrations of risk. The guidance is effective for fiscal years ending after December 2009. The adoption of the guidance did not have a material impact on our consolidated financial statements.
In January 2010, the FASB issued updated guidance related to fair value measurements and disclosures, which requires a reporting entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and to describe the reasons for the transfers. In addition, in the reconciliation for fair value measurements using significant unobservable inputs, or Level 3, a reporting entity should disclose separately information about purchases, sales, issuances and settlements (that is, on a gross basis rather than one net number). The updated guidance also requires that an entity should provide fair value measurement disclosures for each class of assets and liabilities and disclosures about the valuation techniques and inputs used to measure fair value for both recurring and non-recurring fair value measurements for Level 2 and Level 3 fair value measurements. The updated guidance is effective for interim or annual financial reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances and settlements in the roll forward activity in Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010 and for interim periods within those fiscal years. We adopted this guidance in the fourth quarter of fiscal 2010.
In February 2010, the FASB issued amended guidance on subsequent events. Under this amended guidance, SEC filers are no longer required to disclose the date through which subsequent events have been evaluated in originally issued and revised financial statements. We adopted this guidance in the fourth quarter of fiscal 2010 and the adoption did not have a material impact on our consolidated financial statements.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as of March 31, 2010.
Contractual Obligations
We have obligations under non-cancelable operating leases with various expiration dates through 2019 and purchase commitments for inventory, capital equipment and operating expenses, such as materials for research and development and consulting.
53
As of March 31, 2010, our contractual obligations were as follows (in thousands):
|
Payments Due by Period
|
||||||||||||||||||||
|
Less than
|
1-3
|
3-5
|
More than
|
|||||||||||||||||
|
Contractual obligations:
|
Total
|
1 year
|
years
|
years
|
5 years
|
|||||||||||||||
|
Non-cancelable operating lease obligations
|
$
|
5,489
|
$
|
1,054
|
$
|
2,460
|
$
|
1,026
|
$
|
949
|
||||||||||
|
Purchase obligations
|
3,458
|
3,458
|
-
|
-
|
-
|
|||||||||||||||
|
Minimum milestone payments to The Cleveland Clinic
|
1,500
|
1,500
|
-
|
-
|
-
|
|||||||||||||||
|
Royalty payments to Vascular FX, LLC
|
896
|
250
|
646
|
-
|
-
|
|||||||||||||||
|
Earn out payment to VasCon
|
309
|
309
|
-
|
-
|
-
|
|||||||||||||||
|
Total
|
$
|
11,652
|
$
|
6,571
|
$
|
3,106
|
$
|
1,026
|
$
|
949
|
||||||||||
We are required to pay The Cleveland Clinic up to $5.0 million in payments upon the achievement of certain milestones set forth in the ReVasc Agreement. We paid a $0.5 million milestone payment in March 2008 and an additional $2.0 million milestone payment in January 2010. We have accrued an additional $1.5 million as of March 31, 2010 for milestone payments that will become due by October 2010, regardless of whether the related milestones are achieved.
We are required to pay Vascular FX, LLC a royalty equal to 25% of the greater of (i) the applicable aggregate mandatory minimum sales of $1.0 million or (ii) the actual net sales of our deflectable catheter product, both on an annual basis. The royalty period is six years beginning in November 2007. As of March 31, 2010, we accrued royalty payments of approximately $102,000 to Vascular FX.
The future earn-out payments to the former VasCon shareholders will be an amount not to exceed $10.0 million based on the sales and manufacturing performance as set forth in the asset purchase agreement. These future earn-out payments will be paid over three years. We paid the first and second year earn-out amounts of $378,000 and $0.9 million in April 2008 and June 2009, respectively. As of March 31, 2010, we have accrued for the final earn-out payment of $309,000 which will be paid in the first quarter of fiscal 2011.
We are required to pay Biotronik royalties equal to 15% of the actual net sales of our PHAROS® stent on a quarterly basis.
We are required to pay FSS up to $2.0 million in payments upon the achievement of certain milestones set forth in the FSS Agreement and royalties on potential future product sales. The additional milestone payments are contingent on certain events.
Foreign Currency Exchange Risks. We are exposed to risks from fluctuations in currency exchange rates due to intercompany loans made to Micrus SA, our Swiss subsidiary, in 2001 in connection with its incorporation. These loans are denominated in Swiss francs and will fluctuate in value against the U.S. dollar, causing us to recognize foreign exchange gains and losses. The functional currency of our Swiss subsidiary is the Swiss franc. The functional currency of our UK subsidiary is the British pound. In Europe, our revenues are denominated in the Swiss franc, euro, British pound and U.S. dollar. Accordingly, we are exposed to market risk related to changes between these currencies. For example, if the Swiss franc appreciates against the currencies in which our receivables are denominated, we will recognize foreign currency losses. For the preparation of our consolidated financial statements, the financial results of our Swiss subsidiary are translated into U.S. dollars based on average exchange rates during the applicable period. A hypothetical 10% decline in the value of the Swiss franc versus the U.S. dollar would cause us to recognize a loss of $191,000 related to our loan with Micrus SA and a $7,000 increase in our comprehensive loss from our investment in Micrus SA as of March 31, 2010. A hypothetical 10% decline in the value of the British pound versus the U.S. dollar would cause us to recognize a $470,000 increase in our comprehensive loss from our investment in Micrus UK as of March 31, 2010. A hypothetical 10% decline in the value of the euro versus the Swiss franc would cause us to recognize a loss of $445,000 based on our foreign denominated receivables as of March 31, 2010.
In fiscal 2010, approximately 33% of our revenues were denominated in currencies other than the U.S. dollar. In future periods, we believe that a greater portion of our revenues could be denominated in currencies other than the U.S. dollar, thereby increasing our exposure to exchange rate gains and losses on non-U.S. currency transactions.
Interest Rate Risk. Our cash is invested in bank deposits and money market funds denominated in U.S. dollars. The carrying value of these cash equivalents approximates fair market value. Our investments in marketable securities are subject to interest rate risk, which is the risk that our financial condition and results of operations could be adversely affected due to movements in interest rates.
At March 31, 2010, our cash and cash equivalent balance was $30.1 million. Based on our annualized average interest rate, a 10% decrease in the interest rate on such balances would result in a reduction in interest income of approximately $2,500 on an annual basis.
As of March 31, 2010, we do not invest in or trade market risk sensitive instrument or have any debt subject to interest rate fluctuations.
54
Index to Consolidated Financial Statements
|
Page
|
||||
|
56
|
||||
|
57
|
||||
|
58
|
||||
|
59
|
||||
|
60
|
||||
|
61
|
||||
|
Supplementary Data
|
||||
|
89
|
||||
55
To the Board of Directors and Stockholders
of Micrus Endovascular Corporation
In our opinion, the accompanying consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of Micrus Endovascular Corporation and its subsidiaries at March 31, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended March 31, 2010 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2010, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
San Jose, California
June 8, 2010
56
MICRUS ENDOVASCULAR CORPORATION
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
(In thousands, except share
|
||||||||
|
and per share amounts)
|
||||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
30,072
|
$
|
17,050
|
||||
|
Accounts receivable, net of allowance for doubtful accounts
|
||||||||
|
of $217 and $119 at March 31, 2010 and 2009, respectively
|
15,454
|
12,205
|
||||||
|
Inventories
|
13,769
|
11,857
|
||||||
|
Prepaid expenses and other current assets
|
1,760
|
1,237
|
||||||
|
Total current assets
|
61,055
|
42,349
|
||||||
|
Property and equipment, net
|
5,841
|
6,982
|
||||||
|
Goodwill
|
7,169
|
6,762
|
||||||
|
Intangible assets, net
|
5,394
|
4,684
|
||||||
|
Deferred tax assets
|
312
|
260
|
||||||
|
Other assets
|
465
|
469
|
||||||
|
Total assets
|
$
|
80,236
|
$
|
61,506
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable
|
$
|
1,902
|
$
|
2,138
|
||||
|
Accrued payroll and other related expenses
|
6,080
|
5,515
|
||||||
|
Deferred tax liabilities
|
138
|
-
|
||||||
|
Short-term borrowings
|
-
|
2,500
|
||||||
|
Accrued liabilities
|
6,334
|
7,877
|
||||||
|
Total current liabilities
|
14,454
|
18,030
|
||||||
|
Other non-current liabilities
|
446
|
902
|
||||||
|
Total liabilities
|
14,900
|
18,932
|
||||||
|
Commitments and contingencies (Note 9)
|
||||||||
|
Stockholders' equity:
|
||||||||
|
Preferred stock, $0.01 par value;
|
||||||||
|
Authorized: 1,000,000 shares; none issued and outstanding
|
-
|
-
|
||||||
|
Common stock, $0.01 par value;
|
||||||||
|
Authorized: 50,000,000 shares
|
||||||||
|
Issued and outstanding:16,423,452 shares and 15,820,369 shares
|
||||||||
|
at March 31, 2010 and 2009, respectively
|
164
|
158
|
||||||
|
Additional paid-in capital
|
138,019
|
127,121
|
||||||
|
Accumulated other comprehensive loss
|
(1,961
|
)
|
(2,289
|
)
|
||||
|
Accumulated deficit
|
(70,886
|
)
|
(82,416
|
)
|
||||
|
Total stockholders' equity
|
65,336
|
42,574
|
||||||
|
Total liabilities and stockholders' equity
|
$
|
80,236
|
$
|
61,506
|
||||
The accompanying notes are an integral part of these consolidated financial statements.
57
MICRUS ENDOVASCULAR CORPORATION
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
(In thousands, except per share amounts)
|
||||||||||||
|
Revenues
|
$
|
91,090
|
$
|
78,196
|
$
|
69,213
|
||||||
|
Cost of goods sold
|
22,406
|
20,847
|
17,301
|
|||||||||
|
Gross profit
|
68,684
|
57,349
|
51,912
|
|||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
10,449
|
10,243
|
13,718
|
|||||||||
|
Sales and marketing
|
26,816
|
29,312
|
29,385
|
|||||||||
|
General and administrative
|
21,266
|
26,983
|
26,971
|
|||||||||
|
Impairment of intangible assets
|
-
|
462
|
-
|
|||||||||
|
Total operating expenses
|
58,531
|
67,000
|
70,074
|
|||||||||
|
Income (loss) from operations
|
10,153
|
(9,651
|
)
|
(18,162
|
)
|
|||||||
|
Interest and investment income
|
45
|
258
|
1,223
|
|||||||||
|
Interest expense
|
(127
|
)
|
(52
|
)
|
(3
|
)
|
||||||
|
Other income (expense), net
|
2,096
|
(2,111
|
)
|
488
|
||||||||
|
Income (loss) before income taxes
|
12,167
|
(11,556
|
)
|
(16,454
|
)
|
|||||||
|
Income tax benefit (expense)
|
(637
|
)
|
502
|
194
|
||||||||
|
Net income (loss)
|
$
|
11,530
|
$
|
(11,054
|
)
|
$
|
(16,260
|
)
|
||||
|
Net income (loss) per share:
|
||||||||||||
|
Basic
Diluted
|
$
|
0.72
|
$
|
(0.70
|
)
|
$
|
(1.05
|
)
|
||||
|
$
|
0.69
|
$
|
(0.70
|
)
|
$
|
(1.05
|
)
|
|||||
|
Weighted-average number of shares used in per share calculations:
Basic
|
16,004
|
15,692
|
15,438
|
|||||||||
|
Diluted
|
16,666
|
15,692
|
15,438
|
|||||||||
The accompanying notes are an integral part of these consolidated financial statements.
58
MICRUS ENDOVASCULAR CORPORATION
|
Accumulated
|
||||||||||||||||||||||||||||
|
Additional
|
Deferred
|
Other
|
Total
|
|||||||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Stock-based
|
Comprehensive
|
Accumulated
|
Stockholders'
|
|||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Compensation
|
Loss
|
Deficit
|
Equity
|
||||||||||||||||||||||
|
(In thousands)
|
||||||||||||||||||||||||||||
|
Balance at March 31, 2007
|
15,249 | 152 | 111,920 | (164 | ) | (512 | ) | (55,102 | ) | 56,294 | ||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (16,260 | ) | (16,260 | ) | |||||||||||||||||||
|
Translation adjustments
|
- | - | - | - | 1 | - | 1 | |||||||||||||||||||||
|
Total comprehensive loss
|
(16,259 | ) | ||||||||||||||||||||||||||
|
Exercise of stock options
|
270 | 3 | 2,152 | - | - | - | 2,155 | |||||||||||||||||||||
|
Issuance of common stock under employee stock
purchase plan
|
93 | 1 | 1,009 | - | - | - | 1,010 | |||||||||||||||||||||
|
Restricted stock awards issued
|
1 | - | 21 | - | - | - | 21 | |||||||||||||||||||||
|
Restricted stock units vested
|
3 | - | - | - | - | - | - | |||||||||||||||||||||
|
Restricted stock withheld
for minimum taxes
|
(1 | ) | - | (29 | ) | - | - | - | (29 | ) | ||||||||||||||||||
|
Stock-based compensation
(including amount capitalized in inventory of $51)
|
- | - | 4,821 | - | - | - | 4,821 | |||||||||||||||||||||
|
Amortization of deferred stock-based compensation
|
- | - | - | 163 | - | - | 163 | |||||||||||||||||||||
|
Deferred stock-based compensation associated with stock options forfeited
|
- | - | (1 | ) | 1 | - | - | - | ||||||||||||||||||||
|
Non-employee stock-based
compensation
|
- | - | 4 | - | - | - | 4 | |||||||||||||||||||||
|
Balance at March 31, 2008
|
15,615 | 156 | 119,897 | - | (511 | ) | (71,362 | ) | 48,180 | |||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (11,054 | ) | (11,054 | ) | |||||||||||||||||||
|
Translation adjustments
|
- | - | - | - | (1,705 | ) | - | (1,705 | ) | |||||||||||||||||||
|
Swiss pension plan
adjustment
|
- | - | - | - | (73 | ) | - | (73 | ) | |||||||||||||||||||
|
Total comprehensive loss
|
(12,832 | ) | ||||||||||||||||||||||||||
|
Exercise of stock options
|
83 | 1 | 747 | - | - | - | 748 | |||||||||||||||||||||
|
Issuance of common stock under employee stock purchase plan
|
120 | 1 | 879 | - | - | - | 880 | |||||||||||||||||||||
|
Restricted stock awards issued
|
1 | - | 9 | - | - | - | 9 | |||||||||||||||||||||
|
Restricted stock units vested
|
3 | - | - | - | - | - | - | |||||||||||||||||||||
|
Restricted stock withheld for
minimum taxes
|
(2 | ) | - | (17 | ) | - | - | - | (17 | ) | ||||||||||||||||||
|
Stock-based compensation (including amount released from inventory of $8)
|
- | - | 5,606 | - | - | - | 5,606 | |||||||||||||||||||||
|
Balance at March 31, 2009
|
15,820 | $ | 158 | $ | 127,121 | $ | - | $ | (2,289 | ) | $ | (82,416 | ) | $ | 42,574 | |||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||
|
Net income
|
- | - | - | - | - | 11,530 | 11,530 | |||||||||||||||||||||
|
Translation adjustments
|
- | - | - | - | 315 | - | 315 | |||||||||||||||||||||
|
Swiss pension plan
adjustment
|
- | - | - | - | 13 | - | 13 | |||||||||||||||||||||
|
Total comprehensive income
|
11,858 | |||||||||||||||||||||||||||
|
Exercise of stock options
|
461 | 5 | 4,084 | - | - | - | 4,089 | |||||||||||||||||||||
|
Issuance of common stock under employee stock purchase plan
|
140 | 1 | 726 | - | - | - | 727 | |||||||||||||||||||||
|
Restricted stock awards issued
|
1 | - | 9 | - | - | - | 9 | |||||||||||||||||||||
|
Restricted stock units vested
|
3 | - | - | - | - | - | - | |||||||||||||||||||||
|
Restricted stock withheld for
minimum taxes
|
(1 | ) | - | (11 | ) | - | - | - | (11 | ) | ||||||||||||||||||
|
Stock-based compensation (including amount released from inventory of $6)
|
- | - | 6,001 | - | - | - | 6,001 | |||||||||||||||||||||
|
Tax benefit from stock-based
compensation
|
- | - | 89 | - | - | - | 89 | |||||||||||||||||||||
|
Balance at March 31, 2010
|
16,424 | $ | 164 | $ | 138,019 | $ | - | $ | (1,961 | ) | $ | (70,886 | ) | $ | 65,336 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
59
MICRUS ENDOVASCULAR CORPORATION
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
(In thousands)
|
||||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net income (loss)
|
$
|
11,530
|
$
|
(11,054
|
)
|
$
|
(16,260
|
)
|
||||
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating
activities:
|
||||||||||||
|
Depreciation and amortization
|
3,119
|
3,383
|
3,178
|
|||||||||
|
Provision for doubtful accounts
|
96
|
49
|
(145
|
)
|
||||||||
|
Loss on disposal of equipment
|
56
|
28
|
35
|
|||||||||
|
Gain from sale of assets and technologies in connection with Merit transaction
|
(1,866
|
)
|
-
|
-
|
||||||||
|
Provision for excess and obsolete inventories
|
542
|
74
|
240
|
|||||||||
|
Impairment of intangible assets
|
-
|
462
|
-
|
|||||||||
|
Stock-based compensation
|
5,987
|
5,645
|
4,958
|
|||||||||
|
Effect of foreign exchange rate changes on intercompany balances
|
(1,093
|
)
|
2,231
|
(1,296
|
)
|
|||||||
|
Deferred income taxes
|
102
|
(608
|
)
|
(209
|
)
|
|||||||
|
Changes in operating assets and liabilities, net of effect of acquisitions:
|
||||||||||||
|
Accounts receivable
|
(2,891
|
)
|
(1,962
|
)
|
(2,115
|
)
|
||||||
|
Inventories
|
(2,130
|
)
|
(1,041
|
)
|
(2,786
|
)
|
||||||
|
Prepaid expenses and other current assets
|
(500
|
)
|
270
|
(156
|
)
|
|||||||
|
Other assets
|
17
|
(21
|
)
|
(148
|
)
|
|||||||
|
Accounts payable
|
(292
|
)
|
(1,425
|
)
|
1,920
|
|||||||
|
Accrued payroll and other related expenses
|
512
|
(2,077
|
)
|
1,582
|
||||||||
|
Accrued liabilities
|
832
|
(147
|
)
|
1,403
|
||||||||
|
Other non-current liabilities
|
(157
|
)
|
(855
|
)
|
979
|
|||||||
|
Net cash provided by (used in) operating activities
|
13,864
|
(7,048
|
)
|
(8,820
|
)
|
|||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Acquisition of property and equipment
|
(669
|
)
|
(2,688
|
)
|
(2,013
|
)
|
||||||
|
Advance payments for leasehold improvements at new Florida facility
|
-
|
-
|
(802
|
)
|
||||||||
|
Proceeds from sale of property and equipment
|
3
|
13
|
110
|
|||||||||
|
Proceeds from sale of assets and technologies
|
-
|
1,500
|
1,500
|
|||||||||
|
Earn-out payment in connection with acquisition of VasCon, LLC
|
(882
|
)
|
(378
|
)
|
-
|
|||||||
|
Earn-out payment in connection with acquisition of Neurologic UK Ltd.
|
-
|
(3,454
|
)
|
(2,232
|
)
|
|||||||
|
Payment to The Cleveland Clinic for licensed technology
|
(2,000
|
)
|
-
|
-
|
||||||||
|
Net cash used in investing activities
|
(3,548
|
)
|
(5,007
|
)
|
(3,437
|
)
|
||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Borrowings under bank line of credit
|
-
|
2,500
|
-
|
|||||||||
|
Payment on bank line of credit
|
(2,500
|
)
|
-
|
-
|
||||||||
|
Excess tax benefit from stock-based compensation
|
89
|
-
|
-
|
|||||||||
|
Proceeds from exercise of stock options
|
4,089
|
748
|
2,155
|
|||||||||
|
Proceeds from employee stock purchase plan
|
727
|
880
|
1.010
|
|||||||||
|
Net cash provided by financing activities
|
2,405
|
4,128
|
3,165
|
|||||||||
|
Effect of foreign exchange rate changes on cash
|
301
|
(549
|
)
|
82
|
||||||||
|
Net increase (decrease) in cash and cash equivalents
|
12,721
|
(7,927
|
)
|
(9,092
|
)
|
|||||||
|
Cash and cash equivalents at beginning of year
|
17,050
|
25,526
|
34,536
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
30,072
|
$
|
17,050
|
$
|
25,526
|
||||||
|
Supplemental disclosure of cash flow information:
|
||||||||||||
|
Interest paid
|
$
|
126
|
$
|
38
|
$
|
3
|
||||||
|
Supplemental schedule of non-cash investing and financing activities:
|
||||||||||||
|
Accrued earn-out payment in connection with the acquisition of VasCon, LLC
|
$
|
309
|
$
|
886
|
$
|
378
|
||||||
|
Accrued earn-out payment in connection with the acquisition of Neurologic UK Ltd.
|
$
|
-
|
$
|
-
|
$
|
2,997
|
||||||
The accompanying notes are an integral part of these consolidated financial statements.
60
MICRUS ENDOVASCULAR CORPORATION
Note 1 — Formation and Business of the Company
Micrus Endovascular Corporation (the “Company”) was incorporated under the laws of the state of Delaware in June 1996. The Company develops, manufactures and markets both implantable and disposable medical devices used in the treatment of cerebral vascular diseases. The Company’s products are used by interventional neuroradiologists, interventional neurologists, and neurosurgeons to treat both cerebral aneurysms responsible for hemorrhagic stroke and intracranial atherosclerosis, which may lead to ischemic stroke. Hemorrhagic and ischemic stroke are both significant causes of death and disability worldwide.
Note 2 — Summary of Significant Accounting Policies
Principles of consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
The Company’s international subsidiaries use their local currency as the functional currency. Assets and liabilities are translated at exchange rates prevailing at the balance sheet dates. Revenue, expense, gain and loss accounts are translated at average exchange rates during the period. Resulting translation adjustments are recorded directly to accumulated other comprehensive income (loss).
Reclassifications
Certain amounts in the prior year consolidated financial statements have been reclassified to conform to the current year presentation. These reclassifications had no impact on previously reported stockholders’ equity or net loss.
Revision of prior periods' cash flows presentations
The Company previously presented the remeasurement of foreign exchange differences on intercompany balances in “Effect of foreign exchange rate changes on cash and cash equivalents” instead of as an “Adjustment to reconcile net income/(loss) to net cash provided by/(used in) operating activities.” Consequently, for periods presented or prior periods to be presented, the Company is revising the impact by reclassifying the resulting amounts from “Effect of foreign exchange rate changes on cash and cash equivalents” to “Cash provided by/(used in) operating activities.” This revision does not impact the Company's previously reported overall net change in cash and cash equivalents in its consolidated statements of cash flows for any period presented. The Company does not believe these adjustments are material to any of the periods impacted. This item has been adjusted in the consolidated statements of cash flows for the fiscal years ended March 31, 2009 and 2008 in this Form 10-K and will also be adjusted in three months period ended June 30, 2009. The impact on the Company's consolidated statements of cash flows data is as follows (in thousands):
|
As previously
|
||||||||
|
reported
|
As revised
|
|||||||
|
Fiscal year ended March 31, 2008
|
||||||||
|
Net cash used in operating activities
|
$ | (7,524 | ) | $ | (8,820 | ) | ||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
$ | (1,214 | ) | $ | 82 | |||
|
Net decrease in cash and cash equivalents
|
$ | (7,796 | ) | $ | (9,092 | ) | ||
|
Fiscal year ended March 31, 2009
|
||||||||
|
Net cash used in operating activities
|
$ | (9,279 | ) | $ | (7,048 | ) | ||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
$ | 1,682 | $ | (549 | ) | |||
|
Net decrease in cash and cash equivalents
|
$ | (10,158 | ) | $ | (7,927 | ) | ||
|
Three months ended June 30, 2009
|
||||||||
|
Net cash provided by operating activities
|
$ | 4,268 | $ | 3,152 | ||||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
$ | (912 | ) | $ | 204 | |||
|
Net increase in cash and cash equivalents
|
$ | 3,231 | $ | 2,115 | ||||
61
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Out of Period Adjustments
In fiscal 2010, the Company recorded adjustments to cost of goods sold, operating expenses and certain balance sheet accounts to record additional expenses primarily related to stock-based compensation expense that were not properly recorded in prior periods. The net adjustments resulted in the reporting of $281,000 in additional pre-tax expenses. These adjustments both individually and in the aggregate were not material to any of the fiscal 2009 and 2010 interim or fiscal 2009 and 2010 full year consolidated financial statements.
Use of estimates
Preparing consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions. These estimates and assumptions affect the reported amounts of assets and liabilities, and the revenues and expenses which are reported during the Company's reporting periods. In addition, they affect the disclosure of contingent assets and liabilities, as reported on the dates of the Company's consolidated financial statements. Actual results could differ from these estimates. These estimates and assumptions include reserves and write-downs related to accounts receivable and inventories, the recoverability and fair value of long-term assets, and deferred tax assets and related valuation allowances.
Revenue recognition
The Company generates revenue primarily from the sale of its microcoil product line. The Company also sells stents, access products and accessories for use with its microcoils. Revenue is generated from sales to hospitals and third-party distributors.
Revenue is recognized when evidence of an arrangement exists, delivery to the customer has occurred, the selling price is fixed or determinable and collectibility is reasonably assured. Revenue is generally recognized upon shipment after the receipt of a purchase order. In arrangements which specify the title transfer upon delivery, revenue is not recognized until the product is delivered. In the case of consigned goods, revenue is recognized when a replenishment order is made.
The evidence of an arrangement generally consists of a contract or a purchase order approved by the customer.
Delivery to the customer occurs when the customer takes title to the product. Generally title passes upon shipment, but may occur when the product is received by the customer based on the terms of the agreement with the customer.
The selling price for all sales are fixed and agreed with the customer prior to shipment and are generally based on established list prices.
The Company performs credit checks on new customers and periodic credit checks on existing customers. Accordingly, collectibility is generally assured prior to shipment. In the event a sale is made to a customer for whom collectibility is not reasonably assured, the Company either requires prepayment of the order or revenue is deferred and recognized upon collection. The Company maintains a reserve for amounts which may not be collectible.
The Company maintains inventory at various hospital locations under the custody of hospital personnel for use in procedures. The Company recognizes revenue on sales to these customers when the revenue criteria have been met, which occurs when the hospital informs the Company that product has been removed from inventory and used in a procedure.
Once a sale has occurred, the customer has no right of return.
Sales to distributors are recognized at the time of shipment, provided that the Company has received an order, the price is fixed or determinable and collectibility is reasonably assured. Non-refundable fees received from distributors upon entering into multi-year distribution agreements, where there is no culmination of a separate earnings process, are deferred and amortized over the term of the distribution agreement or the expected period of performance, whichever is longer.
62
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Sales made to the Company’s Latin American distributors are made according to similar contractual terms as sales made to other distributors. However, due to historically longer delays in receiving payments and a higher level of receivable write-offs relating to its Latin American distributors, the Company had concluded that through March 31, 2008 collectibility was not reasonably assured at the time that the distributor took title to the inventory. Accordingly, the Company had recognized revenues from sales to Latin American distributors when cash was collected. The Company evaluated its experience with Latin American distributors and concluded that collectibility was now reasonably assured upon shipment, and began recognizing revenue upon shipment to these distributors beginning in the first quarter of fiscal 2009. Revenue recognized upon shipment to its Latin American distributors was $2.8 million and $2.5 million for fiscal 2010 and 2009, respectively. Additionally, the deferred revenue balance at March 31, 2008 of $0.7 million for these distributors and the related cost of goods sold of $273,000 that had been deferred was recognized as revenue and cost of goods sold, respectively, in the first quarter of fiscal 2009. Revenue recognized on a cash basis on sales made to its Latin American distributors was $1.6 million for fiscal 2008.
Product warranty
The Company provides its customers with limited warranty privileges. To date, product returns under warranty have not been significant. The warranty accrual as of March 31, 2010 and 2009 was immaterial to the financial position of the Company and the change in the accrual balance for the current and two prior fiscal years was immaterial to the Company’s results of operations and cash flows.
Cost of goods sold
The Company’s cost of goods sold includes the cost of products sold to customers including materials, direct labor, depreciation, overhead costs associated with manufacturing, impairments of inventory and warranty expenses. Cost of goods sold also includes amortization of capitalized license technology and acquired intangible assets resulting from transactions with Biotronik AG (“Biotronik”) and VasCon, LLC (“VasCon”), respectively.
Concentration of credit risk
Financial instruments which potentially subject the Company to concentrations of credit risk include cash and cash equivalents and accounts receivable. The Company maintains cash and cash equivalents with various major financial institutions. Deposits in these banks may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
The Company grants credit to its customers, which are primarily located in the United States, Europe, Asia Pacific and Latin America, and performs ongoing credit evaluations on its customers and collateral is generally not required for trade receivables. The Company maintains an allowance for potential credit losses and such losses have been within the Company’s expectations.
The Company had one customer which accounted for 12% of revenues for the year ended March 31, 2010 and 2009 and no customer accounted for more than 10% of revenues for the year ended March 31, 2008. The same customer also accounted for 15% and 20% of accounts receivable at March 31, 2010 and 2009, respectively.
Certain significant risks and uncertainties
Most of the Company’s products require approval from the U.S. Food and Drug Administration and foreign regulatory agencies prior to commercialized sale and are subject to continued regulations once approved. There can be no assurance that the Company’s new products or new versions of previous products will receive these required approvals. If the Company was denied such approvals or such approvals were delayed, it could have a materially adverse impact on the Company.
A portion of the Company’s sales operations are based outside of the United States, principally in Europe, Asia Pacific and Latin America. As a result, the Company must comply with a wide variety of foreign laws and regulations. In particular, the Company may be materially adversely affected by changes in the political, social and economic conditions in these countries, and by changes in government policies with respect to such matters as laws and regulations, methods to address inflation, currency conversion and restrictions, and rates and methods of taxation.
63
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Certain of the components and materials used in the Company’s devices are provided by single source suppliers. The loss of any of these suppliers, or their inability to supply the Company with an adequate supply of materials could have a materially adverse impact on the Company.
Cash and cash equivalents
The Company considers all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. Cash equivalents are generally invested in money market funds. Cash equivalents are carried at cost, which approximates fair value.
Fair value of financial instruments
The carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and other liabilities, approximate fair value due to their short maturities.
Allowance for doubtful accounts
The Company maintains an allowance for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments. The Company provides an allowance for specific customer accounts where collection is doubtful and also provides an allowance for other accounts based on historical collection and write-off experience. If the financial condition of the Company's customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
Inventories
Inventories of raw materials, work-in-progress and finished goods are stated at the lower of cost or market, cost being determined under a standard cost method, which approximates actual cost on a first-in, first-out basis.
The Company calculates an inventory provision for estimated obsolescence or excess inventories based upon historical scrap rates and assumptions about future demand for its products and market conditions. The Company’s microcoil products have a five-year shelf life and its stents and access products have a shelf life between one and three years. The Company’s products are subject to demand fluctuations based on the availability and demand for alternative products. The Company’s inventory, which consists primarily of microcoils, is at risk of obsolescence following the introduction and development of new or enhanced products. The Company’s estimates and assumptions for excess and obsolete inventory are reviewed and updated on a quarterly basis. The estimates that the Company uses for demand are also used for near-term capacity planning and inventory purchasing and are consistent with its revenue forecasts. Future product introductions and related inventories may require additional provision based upon changes in market demand or introduction of competing technologies. Provision for excess and obsolete inventories result in a corresponding expense to cost of goods sold.
Property and equipment
Property and equipment are stated at cost and depreciated on a straight-line basis over the estimated useful lives of the related assets, which is generally three to ten years. Computer hardware and software are depreciated over three to five years, machinery and other equipment are depreciated over five to seven years, furniture and fixtures are depreciated over five to ten years, office equipment is depreciated over three to five years. Amortization of leasehold improvements is computed using the straight-line method over the shorter of the remaining lease term or the estimated useful life of the related assets. Construction in progress is not depreciated until the related asset is placed in service. Upon sale or retirement of assets, the costs and related accumulated depreciation and amortization are removed from the consolidated balance sheets and the resulting gain or loss is reflected in the statements of operations. Maintenance and repairs are expensed as incurred.
Goodwill and intangible assets
Goodwill represents the excess of the purchase price over the fair value of in-process research and development, and the net tangible and identifiable intangible assets acquired in a business combination. Negative goodwill represents the excess fair value of the net assets acquired in a business combination over the purchase price. If the acquisition involves contingent consideration, the negative goodwill is recorded as a deferred credit in the consolidated balance sheet and is reduced by the contingent consideration that is paid, with any additional contingent consideration being recorded as goodwill.
64
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
In-process research and development ("IPR&D") is the value assigned to those projects for which the related products have not received regulatory approval and have no alternative future use. For acquisitions subsequent to December 31, 2008, acquired in-process research and development assets are capitalized as indefinite-lived intangible assets until the completion or abandonment of the associated research and development efforts. During the development period, these assets are assessed for impairment at least annually. Upon completion of the development process for the acquired IPR&D, the associated asset is considered definite-lived and amortized over the estimated useful life of the asset. Development costs incurred after the acquisition date are charged to research and development.
Intangible assets resulting from acquisitions are estimated by management based on the fair value of the assets received. Intangible assets from acquisitions are comprised of existing process technology, distribution agreements, non-compete agreements and customer relationships, and are carried at cost less accumulated amortization. Amortization is computed using the straight-line method over their estimated useful lives ranging from five to seven years.
Intangible assets not resulting from acquisitions are comprised of patents and licensed technology, and are carried at cost less accumulated amortization. Amortization of patents is computed using the straight-line method over their estimated useful lives of ten years. Patent application costs, maintenance costs and costs incurred in obtaining the license rights to technology in the research phase are expensed as incurred. Amortization of licensed technology is computed using the straight-line method over its estimated useful life of seven years, starting when the Company began selling the product and generating revenue, which was essentially when it became available for its intended use.
Impairment of goodwill and long-lived assets
The Company evaluates its goodwill for possible impairment on an annual basis or more frequently when events or changes in circumstances indicate that the carrying amounts may not be fully recoverable. The Company performs the impairment test annually in the fourth quarter of its fiscal year. Any impairment charges resulting from the application of this test are immediately recorded as a charge to earnings in the Company’s consolidated statements of operations. Through March 31, 2010, there have been no impairment charges related to goodwill.
The Company reviews long-lived assets, excluding goodwill, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment loss is recognized when the estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount. Impairment, if any, is measured as the amount by which the carrying amount of a long-lived asset exceeds its fair value. Any impairment charges are immediately recorded as a charge to earnings in the Company’s consolidated statements of operations. The Company recorded an impairment charge of $462,000 in fiscal 2009 related to intangible assets acquired from Neurologic UK Limited (“Neurologic”).
Comprehensive income (loss)
Comprehensive income (loss) generally represents all changes in stockholders’ equity except those resulting from investments or contributions by stockholders. The Company’s foreign currency translation adjustments and the Swiss pension plan benefit adjustment represent the only components of comprehensive income (loss) excluded from reported net income (loss). These components of comprehensive income (loss) are presented in the statements of stockholders’ equity.
The balance of accumulated other comprehensive loss consisted of the following components:
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Foreign currency translation adjustments
|
$
|
(1,901
|
)
|
$
|
(2,216
|
)
|
||
|
Swiss pension plan benefit adjustment (Note 13)
|
(60
|
)
|
(73
|
)
|
||||
|
$
|
(1,961
|
)
|
$
|
(2,289
|
)
|
|||
Research and development
Research and development costs are charged to operations as incurred and consist primarily of costs associated with evaluating in-process technology, purchases of intellectual property, personnel costs and supplies.
65
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Advertising costs
Advertising costs are expensed as incurred and are included in sales and marketing expenses.
Shipping and handling of products
Amounts billed to customers for shipping and handling of products are included in revenues. Costs incurred related to shipping and handling of products are included in cost of goods sold.
Foreign currency transactions
Other income (expense), net, includes foreign currency gains or losses related to a loan with the Company’s Swiss subsidiary, and currency gains or losses resulting from differences in exchange rates between the time of recording of the transaction and the cash settlement of foreign currency denominated receivables and payables. The Company recorded currency gains (losses) for the years ended March 31, 2010, 2009 and 2008 of $262,000, $(2.1) million and $0.5 million, respectively.
Income taxes
The Company accounts for income taxes using the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
Net income (loss) per share
Basic net income (loss) per share is computed by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net income (loss) per share is computed by giving effect to all potential dilutive common shares, including stock options and restricted stock units. There is no difference between basic and diluted net loss per share for periods presented prior to fiscal 2010 due to the Company’s net losses.
Anti-dilutive securities
The following outstanding stock options and restricted stock units were excluded from the computation of diluted net loss per common share for the periods presented because their impact would have been anti-dilutive (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Shares issuable upon exercise of common stock options
|
983
|
4,018
|
3,656
|
|||||||||
|
Shares issuable upon settlement of restricted stock units
|
-
|
4
|
7
|
|||||||||
|
983
|
4,022
|
3,663
|
||||||||||
Stock-based compensation
The Company has adopted various stock plans that provide for the grant of stock awards to employees and directors. The Company also has an employee stock purchase plan which enables employees to purchase the Company’s common stock.
Stock-based compensation cost is measured at the grant date based on the fair value of the award. The fair value of the option is estimated at the date of grant using the Black-Scholes option pricing model and is recognized as expense on a straight-line basis over the requisite service period, which is generally the vesting period. The Black-Scholes model requires the Company to make certain assumptions that are factored into the valuation analysis, including risk free interest rate, expected volatility, forfeiture rates, dividend yield and expected term. Risk free interest rate is based on U.S. Treasury rates appropriate for the expected term. Prior to fiscal 2010, expected volatility was determined based on median results of a peer group analysis of companies similar in size and financial leverage to the Company. Beginning in fiscal 2010, the Company used combined volatility including that of the peer group and the historical volatility of its own stock. Forfeiture rates are based on historical factors related to the Company common stock. The assumed dividend yield is zero as the Company does not expect to declare any dividends in the foreseeable future.
66
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
The Company has elected to use the simplified method for estimating its expected term as allowed by relevant accounting standards under certain conditions, including the Company’s inability to rely on historical exercise data. The Company will continue to use the simplified method until it has sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term of its options.
Some exercises result in tax deductions in excess of previously recorded benefits based on the option value at the time of grant (“windfall” tax benefits). The Company recognizes windfall tax benefits associated with the exercise of stock options directly to stockholders’ equity only when realized. Accordingly, deferred tax assets are not recognized for net operating loss carryforwards resulting from windfall tax benefits occurring from April 1, 2006 onward. A windfall tax benefit occurs when the actual tax benefit realized by the company upon an employee’s disposition of a share-based award exceeds the deferred tax asset, if any, associated with the award that the company had recorded. When assessing whether a tax benefit relating to share-based compensation has been realized, the Company has elected to follow the with-and-without approach, under which net operating loss carryforwards and other tax attributes from continuing operations are utilized before utilizing share-based compensation deductions. Also, the Company has elected to ignore the indirect tax effects of share-based compensation deductions in computing our research and development tax credit. The Company will recognize the full effect of these deductions in the statements of operations when the valuation allowance is released.
Recent accounting pronouncements
In December 2008, the Financial Accounting Standards Board (“FASB”) issued authoritative guidance for employers’ disclosures about postretirement benefit plan assets, which provides guidance on an employer’s disclosures about plan assets of a defined benefit pension or other postretirement plan, including disclosures about investment policies and strategies, categories of plan assets, fair value measurements of plan assets and significant concentrations of risk. The guidance is effective for fiscal years ending after December 2009. The adoption of the guidance did not have a material impact on the Company's consolidated financial statements.
In January 2010, the FASB issued updated guidance related to fair value measurements and disclosures, which requires a reporting entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and to describe the reasons for the transfers. In addition, in the reconciliation for fair value measurements using significant unobservable inputs, or Level 3, a reporting entity should disclose separately information about purchases, sales, issuances and settlements (that is, on a gross basis rather than one net number). The updated guidance also requires that an entity should provide fair value measurement disclosures for each class of assets and liabilities and disclosures about the valuation techniques and inputs used to measure fair value for both recurring and non-recurring fair value measurements for Level 2 and Level 3 fair value measurements. The updated guidance is effective for interim or annual financial reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances and settlements in the roll forward activity in Level 3 fair value measurements, which are effective for fiscal years beginning after December 15, 2010 and for interim periods within those fiscal years. The Company adopted this guidance in the fourth quarter of fiscal 2010.
In February 2010, the FASB issued amended guidance on subsequent events. Under this amended guidance, SEC filers are no longer required to disclose the date through which subsequent events have been evaluated in originally issued and revised financial statements. The Company adopted this guidance in the fourth quarter of fiscal 2010 and the adoption did not have a material impact on the Company’s consolidated financial statements.
Note 3 — Business Combinations, Assets Disposition and Technology Acquisition and License Agreements
Fiscal 2007 Business Combination
VasCon
On November 30, 2006, the Company completed the acquisition of VasCon, a privately held company engaged in the development and manufacture of vascular access and delivery devices located in Florida. At the acquisition date, the fair value of the net assets acquired exceeded the purchase consideration resulting in negative goodwill in the amount of $1.6 million. Because the acquisition involved contingent consideration that may exceed the negative goodwill amount, the negative goodwill was recorded as a deferred credit in the consolidated balance sheet and is being reduced by any earned contingent consideration of up to $10.0 million based on sales and manufacturing performance that will be paid over three years, with any additional contingent consideration being recorded as goodwill. At March 31, 2010, 2009 and 2008, the Company accrued for the third, second and first year earn-out payments to the former VasCon shareholders of approximately $309,000, $0.9 million and $378,000, respectively, all of which were recorded as a reduction of the contingent purchase price balance. The Company paid the first and the second year earn-out payments in April 2008 and in June 2009, respectively. The third year earn-out will be paid in the first quarter of fiscal 2011.
67
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Fiscal 2006 Business Combination
Neurologic
On September 20, 2005, the Company entered into a Share Purchase Agreement (“Neurologic Purchase Agreement”) acquiring all of the outstanding capital stock of Neurologic, a privately held distributor of the Company’s products in the United Kingdom (“UK”). The acquisition of Neurologic included future multi-year revenue based earn-out payments. All earn-out payments shall be equal to one-third of Neurologic’s product sales during specified periods. At March 31, 2008 and 2007, the Company accrued for additional considerations under the earn-out provisions of approximately $3.0 million and $2.2 million for the third and second year earn-out payments, respectively, all of which were recorded as additions to goodwill (See Note 6). The Company paid the second and third year earn-out payments in April 2007 and April 2008, respectively. The final earn-out payment of $457,000 was made in June 2008.
Assets Disposition
Merit
On January 31, 2008, the Company entered into an Asset Purchase and Supply Agreement (the “Merit Agreement”) with Merit Medical Systems, Inc. (“Merit”), pursuant to which the Company sold its non-neurological, cardiac and peripheral catheter assets and technology (the “Merit Transaction”). The majority of the assets sold were originally acquired by the Company in November 2006 in connection with its purchase of VasCon. Pursuant to the Merit Agreement, the Company received an up-front payment of $1.5 million and received an additional payment of $1.5 million in December 2008 upon the completion of its obligation to help Merit build a production line for coronary guide catheters and get it fully operational. Though certain elements of this transaction (namely the acquired assets and licensing rights, and the production line assistance for coronary guide catheters) had been delivered as of March 31, 2009, the Company was still obligated to deliver the regulatory documentation and production line assistance for the peripheral guiding sheaths and/or cardiovascular microcatheters. As the Company lacked the ability to separate the multiple obligations of this transaction, the up-front payment of $1.5 million and the additional payment of $1.5 million, net of direct and incremental costs incurred and the net book value of assets transferred to Merit, had been deferred until such time as all obligations of the transaction were delivered. The Company’s remaining obligations to Merit terminated on September 30, 2009 and therefore, the deferred gain of $1.9 million was recognized as other income in the quarter ended September 30, 2009.
Technology Acquisition and License Agreements
Bay Street Medical
In January 2010, the Company entered into an Exclusive License and Option Agreement (the “Bay Street Agreement”) with Bay Street. Pursuant to the terms of the Bay Street Agreement, Bay Street granted the Company a worldwide exclusive license and option to purchase intellectual property assets held by Bay Street related to its proprietary stent delivery and locking technology (the “Bay Street Assets”). The transaction included an initial up-front payment of $0.5 million for a six month evaluation of the exclusive license. The Company has the option to extend the exclusive license for an additional six months upon paying to Bay Street an option payment of $0.5 million. The Company also has the option to purchase the Bay Street Assets before the option period ends pursuant to an Asset Purchase Agreement that will be an addendum to the Bay Street Agreement. Under the terms of the Asset Purchase Agreement, if the Company elects to purchase the Bay Street Assets, the Company would be required to pay an initial purchase payment of $1.0 million, a future sales-based milestone payment, and royalties on future product sales.
68
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
The initial up-front payment of $0.5 million was recorded as research and development expense upon the effective date of the Bay Street Agreement as the technology has yet to receive regulatory approvals and at the present time there is no commercial future use for the technology. Any additional payments will be recorded when triggered and the appropriate accounting treatment, whether to capitalize these costs or recognize them as expense, will be determined at that time.
Flexible Stenting Solutions
On August 21, 2009, the Company entered into a License, Development and Commercialization Agreement (the “FSS Agreement”) with Flexible Stenting Solutions, Inc. (“FSS”). Under the terms of the FSS Agreement, Micrus and FSS will jointly develop a flow diversion product for neurovascular indications using both Micrus and FSS technology, development capabilities and intellectual property. Micrus will be responsible for overseeing the regulatory and clinical process.
The transaction included an initial up-front payment by the Company of $0.5 million, future development and regulatory milestone payments of $2.0 million upon the achievement of certain milestones set forth in the FSS Agreement and royalties on potential future product sales. The initial up-front payment of $0.5 million was recorded as research and development expense upon the effective date of the FSS Agreement as the technology has yet to receive regulatory approvals and at the present time there is no commercial future use for the technology. The additional milestone payments will be recorded when triggered and the appropriate accounting treatment, whether to capitalize these costs or recognize them as expense, will be determined at that time.
Genesis
On January 16, 2008, the Company entered into a license, development and commercialization agreement (the “Genesis Agreement”) with Genesis Medical Interventional, Inc. (“Genesis”). Under the terms of the Genesis Agreement, the Company licensed the rights to Genesis’s F.A.S.T. Funnel Catheter and clot retrieval system for the treatment of ischemic stroke. The transaction included an initial up-front payment of $0.8 million, a future development milestone payment of $150,000 payable upon the earlier of the date of first commercial sale or September 30, 2008 and royalties on potential future product sales. Both the initial up-front payment of $0.8 million and future milestone payment of $150,000 were recorded as research and development expense upon the effective date of the Genesis Agreement. The Company paid the milestone payment of $150,000 in October 2008.
ReVasc
On October 26, 2007, the Company entered into a Stock Purchase Agreement (the “ReVasc Agreement”) with The Cleveland Clinic Foundation (“The Cleveland Clinic”) and ReVasc Technologies, Inc. (“ReVasc”), a wholly-owned subsidiary of The Cleveland Clinic, pursuant to which the Company acquired all of the outstanding stock of ReVasc from The Cleveland Clinic for an aggregate up-front purchase price of $1.0 million. ReVasc did not have the necessary set of activities to be considered as a business and as such this transaction was classified as a technology acquisition. Pursuant to the ReVasc Agreement, the Company also agreed to pay The Cleveland Clinic up to $5.0 million in additional payments upon the achievement of certain milestones set forth in the ReVasc Agreement, with minimum milestone payments of at least $2.0 million due to The Cleveland Clinic by October 2010.
The Company acquired only pre-regulatory approved technology and did not assume any other assets or liabilities in connection with the acquisition of ReVasc. Accordingly, the ReVasc Agreement was accounted for as a purchase of in-process research and development. The Company recorded $3.0 million as research and development expense during fiscal 2008, representing the up-front purchase price of $1,000,000 and future minimum milestone payments of $2.0 million that will become due by October 2010, regardless whether the related milestones are achieved. In March 2008, the Company paid $500,000 of the minimum milestone payment to The Cleveland Clinic. The remaining minimum milestone payments of $1.5 million are included in accrued liabilities at March 31, 2010 (see Note 5).
On January 19, 2010, the Company paid The Cleveland Clinic an additional milestone payment of $2.0 million pursuant to the ReVasc Agreement, which did not count towards the minimum milestone payments. The amount has been capitalized into intangible assets and amortization will begin upon the commencement of sales of the product.
69
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Note 4 — Fair Value Measurements
Effective April 1, 2008, the Company adopted FASB’s authoritative guidance for fair value measurement and disclosures for financial assets and liabilities, as well as for any other assets and liabilities that are carried at fair value on a recurring basis.
The guidance defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The guidance also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The guidance describes three levels of inputs that may be used to measure fair value:
|
Level 1
|
Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities.
|
|
Level 2
|
Quoted prices in markets that are not active; or other inputs that are observable, either directly or indirectly, for substantially the full term of the asset or liability.
|
|
Level 3
|
Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable.
|
The Company’s cash equivalents are classified within Level 1 of the fair value hierarchy because they are invested in money market funds and valued using quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency.
The Swiss pension plan unfunded benefit obligation is classified within Level 3 since there is no observable market for the asset or liability and requires the Company to develop its relevant assumptions.
The following table presents assets measured at fair value on a recurring basis at March 31, 2010 (in thousands):
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market funds
|
$
|
21,378
|
$
|
-
|
$
|
-
|
$
|
21,378
|
||||||||
|
Liabilities:
|
||||||||||||||||
|
Swiss pension plan unfunded benefit obligation (Note 13)
|
$
|
-
|
$
|
-
|
$
|
155
|
$
|
155
|
||||||||
The following table summarizes the change in fair value of Level 3 financial liabilities (in thousands):
|
Level 3
|
||||
|
Fair value beginning of year
|
$
|
148
|
||
|
Change in benefit obligation
|
59
|
|||
|
Change in plan assets
|
(52
|
)
|
||
|
Fair value end of year
|
$
|
155
|
||
70
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Note 5 — Balance Sheet Components
Inventories
Inventories consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Raw materials
|
$
|
2,058
|
$
|
1,918
|
||||
|
Work-in-progress
|
1,894
|
1,968
|
||||||
|
Finished goods
|
3,290
|
2,741
|
||||||
|
Consigned inventory
|
6,527
|
5,230
|
||||||
|
$
|
13,769
|
$
|
11,857
|
|||||
Property and equipment
Property and equipment consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Computer equipment and software
|
$
|
1,814
|
$
|
1,955
|
||||
|
Furniture, fixtures and equipment
|
7,707
|
7,542
|
||||||
|
Leasehold improvements
|
2,168
|
2,132
|
||||||
|
Construction in progress
|
218
|
108
|
||||||
|
Total cost
|
11,907
|
11,737
|
||||||
|
Less accumulated depreciation and amortization
|
(6,066
|
)
|
(4,755
|
)
|
||||
|
$
|
5,841
|
$
|
6,982
|
|||||
Depreciation and amortization expense related to property and equipment was $1.8 million, $1.7 million and $1.3 million for the years ended March 31, 2010, 2009 and 2008, respectively.
Accruals
Accrued payroll and other related expenses consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Accrued bonuses
|
$
|
1,226
|
$
|
1,437
|
||||
|
Accrued salaries
|
1,444
|
1,106
|
||||||
|
Accrued vacation
|
2,169
|
2,000
|
||||||
|
Accrued commissions
|
767
|
544
|
||||||
|
Accrued payroll taxes
|
474
|
428
|
||||||
|
$
|
6,080
|
$
|
5,515
|
|||||
71
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Accrued liabilities consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Milestone payments to The Cleveland Clinic
|
$
|
1,500
|
$
|
1,500
|
||||
|
Professional fees
|
936
|
277
|
||||||
|
Sales tax and VAT payable
|
506
|
876
|
||||||
|
Income taxes payable
|
417
|
-
|
||||||
|
Raw material inventory receipts not invoiced
|
345
|
363
|
||||||
|
Earn-out payment in connection with acquisition of VasCon
|
309
|
886
|
||||||
|
Travel and entertainment
|
305
|
270
|
||||||
|
Deferred gain from Merit transaction
|
-
|
1,866
|
||||||
|
Other
|
2,016
|
1,839
|
||||||
|
$
|
6,334
|
$
|
7,877
|
|||||
Other non-current liabilities
Other non-current liabilities consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Swiss pension plan unfunded benefit obligation
|
$
|
155
|
$
|
148
|
||||
|
Income taxes payable
|
95
|
74
|
||||||
|
Deferred revenue from Goodman Co., Ltd. distribution agreement
|
56
|
169
|
||||||
|
Contingent purchase price in connection with acquisition of VasCon
|
-
|
332
|
||||||
|
Other non-current liabilities
|
140
|
179
|
||||||
|
$
|
446
|
$
|
902
|
|||||
On September 30, 2005, the Company entered into a five-year, exclusive Distribution Agreement with Goodman Co., Ltd. (“Goodman”). Under the terms of the Distribution Agreement, Goodman will promote and market the Company’s full line of products, as such products are approved, in Japan and will purchase a minimum of $27.3 million of such products over the five year term of the agreement, ranging from $2.0 million during the fiscal year ended March 31, 2006 to $9.0 million during the fiscal year ended March 31, 2010. On September 20, 2007, the Company amended the distribution agreement with Goodman to, among other things, extend the duration of the distribution agreement to six years from the original date of the distribution agreement. In connection with the Distribution Agreement, Goodman paid the Company an up-front cash payment of $0.8 million which has been recorded as deferred revenue. The Company is recognizing the deferred revenue on a straight-line basis over the six year term of the agreement.
Note 6 — Goodwill and Intangible Assets
Goodwill
Activity related to goodwill consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Balance beginning of year
|
$
|
6,762
|
$
|
8,549
|
||||
|
Addition related to earn-out payment in connection with acquisition of Neurologic
|
-
|
457
|
||||||
|
Foreign currency translation
|
407
|
(2,244
|
)
|
|||||
|
Balance end of year
|
$
|
7,169
|
$
|
6,762
|
||||
All of the Company’s goodwill has been allocated to the United Kingdom business segment.
The Company performs its annual impairment test of goodwill in the fourth quarter of its fiscal year. Through March 31, 2010, there has been no impairment charges related to goodwill.
72
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Intangible assets
Intangible assets consisted of the following (in thousands):
|
Gross Carrying Amount
|
Accumulated Amortization
|
Net
|
|||||||||||||||||||||||||||||||||||||||
|
Useful
|
|
Foreign
|
|
|
Foreign
|
|
|
|
|||||||||||||||||||||||||||||||||
|
Life
|
March 31,
|
Addition
|
currency
|
March 31,
|
March 31,
|
(Additions)
|
currency
|
March 31,
|
March 31,
|
March 31,
|
|||||||||||||||||||||||||||||||
|
(Years)
|
2009
|
translation
|
2010
|
2009
|
translation
|
2010
|
2010
|
2009
|
|||||||||||||||||||||||||||||||||
|
Existing process
technology
|
7
|
$
|
4,590
|
$
|
-
|
$
|
-
|
$
|
4,590
|
$
|
(1,530
|
)
|
$
|
(656
|
)
|
$
|
-
|
$
|
(2,186
|
)
|
$
|
2,404
|
$
|
3,060
|
|||||||||||||||||
|
Distribution agreements
|
5
|
1,558
|
-
|
93
|
1,651
|
(1,278
|
)
|
(212
|
)
|
(66
|
)
|
(1,556
|
)
|
95
|
280
|
||||||||||||||||||||||||||
|
Capitalized license
technology
|
7
|
1,565
|
2,000
|
-
|
3,565
|
(559
|
)
|
(224
|
)
|
-
|
(783
|
)
|
2,782
|
1,006
|
|||||||||||||||||||||||||||
|
Patents – microcoil
|
10
|
1,100
|
-
|
-
|
1,100
|
(990
|
)
|
(110
|
)
|
-
|
(1,100
|
)
|
-
|
110
|
|||||||||||||||||||||||||||
|
Non-compete
agreements
|
6
|
444
|
-
|
26
|
470
|
(325
|
)
|
(54
|
)
|
(16
|
)
|
(395
|
)
|
75
|
119
|
||||||||||||||||||||||||||
|
Customer relationships
|
5
|
609
|
-
|
38
|
647
|
(500
|
)
|
(83
|
)
|
(26
|
)
|
(609
|
)
|
38
|
109
|
||||||||||||||||||||||||||
|
$
|
9,866
|
$
|
2,000
|
$
|
157
|
$
|
12,023
|
$
|
(5,182
|
)
|
$
|
(1,339
|
)
|
$
|
(108
|
)
|
$
|
(6,629
|
)
|
$
|
5,394
|
$
|
4,684
|
||||||||||||||||||
Amortization of intangible assets included in the results of operations is as follows (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Cost of goods sold
|
$
|
879
|
$
|
879
|
$
|
974
|
||||||
|
Operating expenses
|
460
|
832
|
867
|
|||||||||
|
$
|
1,339
|
$
|
1,711
|
$
|
1,841
|
|||||||
The expected future amortization of intangible assets is as follows (in thousands):
|
For Years Ending March 31,
|
Amortization
|
|||
|
2011
|
$
|
1,063
|
||
|
2012
|
1,189
|
|||
|
2013
|
1,165
|
|||
|
2014
|
835
|
|||
|
2015 and thereafter
|
1,142
|
|||
|
$
|
5,394
|
|||
Note 7— Line of Credit
On November 5, 2008, the Company entered into a credit agreement with Wells Fargo Bank to provide the Company with a revolving line of credit (the “Credit Agreement”). The Credit Agreement provides for maximum borrowings in an amount up to $15.0 million. If borrowings under the Credit Agreement exceed $7.5 million, all borrowings are subject to a borrowing base which is based on eligible accounts receivable. Borrowings are collateralized by a first priority security interest in all of the Company’s assets (except for certain permitted liens that are senior to Wells Fargo Bank’s security interest).
At the Company’s option, borrowings bear interest at either 2.25% over the bank’s prime rate or 3.50% over the one-month, two-month or three-month LIBOR. The interest rate on the borrowings as of March 31, 2010 was 4.0%. The Credit Agreement requires that the Company comply with certain financial and other covenants for borrowings to be permitted. The more significant financial covenants include (i) a minimum modified quick ratio and (ii) a minimum profitability, excluding certain non-cash items. On May 20, 2009, the Company amended the Credit Agreement with Wells Fargo Bank extending the maturity date to August 1, 2010 and adjusting the minimum limits for the financial covenants based on the Company’s financial forecast for fiscal 2010.
73
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
The Company paid off its borrowings of $2.5 million in March 2010. At March 31, 2010, the Company had no outstanding borrowings under the line of credit and was in compliance with all covenants of the Credit Agreement.
Note 8— Income Taxes
As of March 31, 2010, the Company had federal, state and foreign net operating loss carryforwards (“NOLs”) of approximately $36.9 million, $28.8 million and $2.1 million, respectively. The federal NOLs will expire at various dates beginning in 2022, the state NOLs expire beginning in 2015 and the foreign NOLs will expire beginning in 2016. The federal and state loss carryforwards that are attributable to excess tax deductions from stock option exercises are not included in the deferred tax assets shown below. The benefit of approximately $12.4 million and $8.4 million of federal and state loss carryforwards, respectively, will be credited to equity when realized.
The Company also had federal and state research and development tax credit carryforwards of approximately $1.9 million and $1.7 million, respectively, as of March 31, 2010. The federal credits will expire beginning in 2019 and the state credits can be carried forward indefinitely.
The Tax Reform Act of 1986 limits the use of net operating loss and tax credit carryforwards in the case of an “ownership change” of a corporation. An ownership change, as defined, may restrict utilization of tax attribute carryforwards. The Company experienced ownership changes, as defined in Section 382 of the Internal Revenue Code, in May 2002 and June 2005, but the previously limited net operating loss and tax credit carryovers have now become available to offset taxable income in future periods. If an ownership change has occurred subsequent to June 2005, all net operating loss carryovers and all tax credit carryovers arising prior to the ownership change would be subject to limitation in the post change period for US tax purposes.
The related benefit from income taxes consisted of the following (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
State
|
485
|
-
|
-
|
|||||||||
|
Foreign
|
36
|
73
|
-
|
|||||||||
|
Total current income tax provision
|
521
|
73
|
-
|
|||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
-
|
-
|
-
|
|||||||||
|
State
|
-
|
-
|
-
|
|||||||||
|
Foreign
|
116
|
(575
|
)
|
(194
|
)
|
|||||||
|
Total deferred income tax expense (benefit)
|
116
|
(575
|
)
|
(194
|
)
|
|||||||
|
Total income tax expense (benefit)
|
$
|
637
|
$
|
(502
|
)
|
$
|
(194
|
)
|
||||
In fiscal 2010, the Company recorded an income tax expense of approximately $637,000. The income tax expense consists primarily of state and Swiss income tax on operating profits. Prior to fiscal 2010, the Company incurred net operating losses for both federal and state purposes and, as a result, paid no federal or state income taxes. In fiscal 2009, the Company recorded an income tax benefit of approximately $502,000. The net income tax benefit consists primarily of a deferred tax benefit for the tax effect of the amortization related to the intangible assets acquired in the Neurologic transaction which is not deductible, the reduction in deferred tax liability due to the impairment of such intangible assets, and the United Kingdom subsidiary’s operating losses. In fiscal 2008, the Company recorded an income tax benefit of approximately $194,000. The net income tax benefit includes a deferred income tax expense of approximately $72,000 for the Swiss subsidiary’s operating profits and a deferred tax benefit of approximately $266,000 for the tax effect of the amortization related to the intangible assets acquired in the Neurologic transaction which is not deductible and the tax benefit of operating losses for its United Kingdom subsidiary.
74
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Net deferred tax assets (liabilities) consisted of the following (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Net operating loss carryforwards
|
$
|
11,140
|
$
|
14,048
|
||||
|
Basis difference in fixed assets
|
3,412
|
3,143
|
||||||
|
Accruals deductible in different periods
|
5,673
|
4,960
|
||||||
|
Credit carryforwards
|
2,489
|
2,136
|
||||||
|
Total deferred tax assets
|
22,714
|
24,287
|
||||||
|
Less valuation allowance
|
(21,487
|
)
|
(23,696
|
)
|
||||
|
Net deferred tax assets
|
1,227
|
591
|
||||||
|
Deferred tax liabilities:
|
||||||||
|
Accruals deductible in different periods
|
(200
|
)
|
(188
|
)
|
||||
|
Basis difference in fixed and intangible assets
|
(854
|
)
|
(143
|
)
|
||||
|
Total deferred tax liabilities
|
(1,054
|
)
|
(331
|
)
|
||||
|
Net deferred tax assets
|
$
|
173
|
$
|
260
|
||||
The Company has recorded a valuation allowance against its federal and state deferred tax assets as of March 31, 2010 and its federal, state and Swiss deferred tax assets as of March 31, 2009, due to the uncertainty surrounding the realization of such assets. Management evaluates on a periodic basis the recoverability of deferred tax assets. At such time as it is determined that it is more likely than not that deferred tax assets are realizable, the valuation allowance will be reduced.
The effective tax rate differs from the United States federal statutory rate as a result of the following:
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Income tax expense (benefit) at statutory rate
|
35
|
%
|
(35
|
)%
|
(35
|
)%
|
||||||
|
State taxes, net of federal benefit
|
5
|
%
|
(5
|
)%
|
(4
|
)%
|
||||||
|
In process research and development write-off
|
0
|
%
|
0
|
%
|
7
|
%
|
||||||
|
Technology acquisition
|
7
|
%
|
0
|
%
|
2
|
%
|
||||||
|
Non-US income taxed at different rates
|
(5
|
)%
|
9
|
%
|
1
|
%
|
||||||
|
Change in valuation allowance
|
(43
|
)%
|
17
|
%
|
24
|
%
|
||||||
|
Nondeductible deferred compensation
|
7
|
%
|
10
|
%
|
6
|
%
|
||||||
|
Other
|
(1
|
)%
|
0
|
%
|
(2
|
)%
|
||||||
|
Effective income tax rate
|
5
|
%
|
(4
|
)%
|
(1
|
)%
|
||||||
The domestic and foreign components of income (loss) before income taxes were as follows (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Domestic
|
$
|
9,995
|
$
|
(7,093
|
)
|
$
|
(15,474
|
)
|
||||
|
Foreign
|
2,172
|
(4,463
|
)
|
(980
|
)
|
|||||||
|
Income (loss) before income taxes
|
$
|
12,167
|
$
|
(11,556
|
)
|
$
|
(16,454
|
)
|
||||
75
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Effective April 1, 2007, the Company adopted the provision of ASC 740-10-5-6 (formerly known as “FIN 48”), which requires that the Company recognize the financial statement effects of a tax position when it becomes more likely than not, based on the technical merits, that the position will be sustained upon examination. As a result of the implementation of ASC 740-10-5-6, the Company recognized a $192,000 increase in its unrecognized tax benefits. None was accounted for as an increase in the April 1, 2007 balance of accumulated deficit since the benefit relates to attribute carryovers for which the related deferred tax asset was subject to a full valuation allowance. At the adoption date of April 1, 2007 and at March 31, 2010, the Company had zero and $22,000 of accrued interest and penalties related to tax contingencies, respectively. Prior to the fiscal year ended March 31, 2009, since the unrecognized tax benefit relates to attribute carryovers for which the related deferred tax asset was subject to a full valuation allowance, the recognition of the unrecognized tax benefits will not affect the Company’s effective tax rate. For the fiscal year ended March 31, 2010, $22,000 of the $84,000 increase in unrecognized tax benefits will affect the Company’s effective tax rate if recognized. The Company has elected to include interest and penalties as a component of tax expense. The Company does not anticipate that the amount of unrecognized tax benefits relating to tax positions existing at March 31, 2010 will significantly increase or decrease within the next 12 months. Because of net operating loss and credit carryforwards, substantially all of the Company’s tax years, dating to inception in 1996, remain open to federal tax examination. Most state and foreign jurisdictions have 3 to 10 open tax years at any point in time.
Tax filings are based on tax laws which are subject to significant and varied interpretation. It is often unclear whether a particular position taken in a tax return will ultimately be sustained. The Company has reviewed its filing positions and believes that it has adequately accrued for such uncertainties.
The following table summarizes the activity related to the Company’s unrecognized tax benefit for the year ended March 31, 2010, 2009 and 2008 (in thousands):
|
March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Unrecognized tax benefits at beginning of year
|
$ | 360 | $ | 232 | $ | 192 | ||||||
|
Gross increases - prior year tax positions
|
- | - | - | |||||||||
|
Gross increases - current year tax positions
|
84 | 128 | 40 | |||||||||
|
Settlements with taxing authorities
|
- | - | - | |||||||||
|
Expiration of statute of limitations
|
- | - | - | |||||||||
|
Unrecognized tax benefits at end of year
|
$ | 444 | $ | 360 | $ | 232 | ||||||
Note 9 — Commitments and Contingencies
Lease commitments
On June 6, 2005, the Company entered into a non-cancelable seven-year operating lease agreement (“Lease”). Pursuant to the Lease, the Company has leased approximately 42,000 square feet of building space which is being used as the Company’s headquarters in the United States. The Lease commenced in January 2006. The Lease provides a right to extend the term for one period of sixty months that may be exercised by the Company.
The Lease provides for a base rent that increases periodically and averages approximately $41,445 monthly over the lease period and is accounted for on a straight-line basis. The Lease also provides for certain additional payments including the Company’s share of landlord’s operating expenses, including project costs, property taxes and overhead management fees.
On March 11, 2008, the Company entered into a non-cancelable ten-year lease in Miramar, Florida, which commenced in September 2008. The facility comprises a total of approximately 27,000 square feet. The operating lease provides for a base rent that increases periodically and averages approximately $29,047 monthly over the lease period and is accounted for on a straight-line basis. The operating lease also provides for certain additional payments including the Company’s share of landlord’s operating expenses and applicable sales tax.
On December 4, 2007, the Company’s wholly owned subsidiary, Micrus Endovascular SA (“Micrus SA”), entered into a non-cancelable eight-year lease for office space in Switzerland. The office space comprises a total of approximately 5,500 square feet.
76
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Additionally, the Company leases office space for its wholly-owned subsidiary, Micrus UK, under a non-cancelable lease agreement with a term through December 2010.
The combined annual rent for Micrus SA and Micrus UK operating leases is approximately $189,000. The leases also provide for certain additional payments including the Company’s share of the landlord’s operating expenses.
Future minimum lease payments are as follows (in thousands):
|
Minimum
|
||||
|
For Years Ending March 31,
|
Lease Payments
|
|||
|
2011
|
$
|
1,054
|
||
|
2012
|
1,026
|
|||
|
2013
|
937
|
|||
|
2014
|
497
|
|||
|
2015
|
508
|
|||
|
Thereafter
|
1,467
|
|||
|
$
|
5,489
|
|||
Rent expense for the years ended March 31, 2010, 2009 and 2008 was $0.9 million, $1.0 million and $0.7 million, respectively.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnification. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations, and accordingly, the Company has not accrued any amounts for such indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
Litigation
The Company is from time to time subject to various lawsuits. The Company does not believe that it is probable that the resolution of pending litigation will have a material adverse effect on the Company’s consolidated financial statements; however, the outcome of litigation is inherently uncertain.
Note 10 — Preferred Stock
The Company’s Certificate of Incorporation, as amended, authorizes the Company to issue 1,000,000 shares of $0.01 par value preferred stock. As of March 31, 2010, there are no shares of preferred stock issued or outstanding.
Note 11 — Common Stock
The Company’s Certificate of Incorporation, as amended, authorizes the Company to issue 50,000,000 shares of $0.01 par value common stock. Each holder of common stock has the right to one vote and is also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding having priority rights as to dividends. No dividends have been declared or paid as of March 31, 2010.
77
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Note 12 — Stock Option Plans and Other Employee Benefits
Stock Option Plans
The Company’s stock option program is a long-term retention program that is intended to attract, retain and provide incentives for talented employees, officers and directors, and to align stockholder and employee interests. The Company considers the stock option program critical to its operations and productivity. As of March 31, 2010, the Company has two stock option plans: the 1998 Stock Plan (the “1998 Plan”), and the 2005 Equity Incentive Plan as amended (the “2005 Plan”). Currently, the Company grants options from the 2005 Plan, which permits the Company to grant options to all employees, including executive officers, and outside consultants, and directors. Effective June 16, 2005, no new options may be granted under the 1998 Plan. Stock options issued under the Company’s stock option plans generally vest based on 4 years of continuous service and have ten-year contractual terms.
1998 Stock Plan
As of June 16, 2005, no new stock option grants were permitted under the 1998 Plan. However, all options previously granted under the 1998 Plan continue to be administered under the 1998 Plan. As of March 31, 2010, options to purchase 865,700 shares of common stock were outstanding under the 1998 Plan.
2005 Equity Incentive Plan
The 2005 Plan became effective upon the Company’s IPO. The 2005 Plan provides for the issuance of stock options, stock appreciation rights, stock awards (stock and stock units) and cash awards. The Company initially reserved a total of 2,395,020 shares of its common stock for issuance under the 2005 Plan. In addition, the 2005 Plan provides for an automatic annual increase in the number of shares reserved for issuance thereunder on each April 1st by an amount equal to the lesser of (i) 5% of the Company’s total number of outstanding shares on the immediately preceding March 31st; (ii) 666,666 shares, or (iii) a number of shares determined by the Company’s Board of Directors. The shares reserved under the 2005 Plan will also be increased as a result of the forfeiture or repurchase of shares issued under the 1998 Plan and the cancellation of unexercised options under the 1998 Plan. As of March 31, 2010, there were 4,646,041 remaining shares reserved for issuance under the 2005 Plan, of which 1,437,859 were available for grant and 3,208,182 shares were subject to outstanding options.
Acceleration Agreements
On January 29, 2008, the Company entered into agreements with certain executive officers (the “Accelerated Employees”) to fully accelerate the vesting of options to purchase its common stock issued under the Company’s 2005 Equity Incentive Plan and/or 1998 Stock Plan and held by such Accelerated Employees if, within the period 3 months prior to or 12 months following a change of control of the Company or sale of substantially all of the Company’s assets, an Accelerated Employee ceases being employed by the Company because either such Accelerated Employee is involuntary terminated by the Company (or any subsidiary) without “cause” or such Accelerated Employee voluntarily quits within 60 days of an event which constitutes “good reason.”
2005 Employee Stock Purchase Plan
The 2005 Employee Stock Purchase Plan (the “Purchase Plan”) became effective upon the Company’s IPO. The Purchase Plan provides employees with an opportunity to purchase the Company’s common stock through accumulated payroll deductions.
The Company initially reserved a total of 222,222 shares of common stock for issuance under the Purchase Plan. The Purchase Plan provides for annual increases in the total number of shares available for issuance under this plan on April 1st of each year beginning on April 1, 2006, by a number of shares that is equal to the lesser of: (1) 2% of the outstanding shares of the Company’s common stock on the immediately preceding March 31st; (2) 222,222 shares; or (3) a lesser number determined by the Company’s Board of Directors. As of March 31, 2010, there were 641,467 shares reserved for issuance under the Purchase Plan.
The Purchase Plan permits participants to purchase the Company’s common stock through payroll deductions of up to 15% of the participant’s compensation, provided that no participant may purchase shares with a value that exceeds $25,000 per year, or more than 1,111 shares per purchase period. Amounts deducted and accumulated for the participant’s account are used to purchase shares of the Company’s common stock on the last trading day of each purchase period at a price of at least 85% of the lesser of the fair market values of the common stock at the beginning of the offering period or at the end of the purchase period.
78
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
The Purchase Plan provides for offering periods of 12 months and purchase periods of 6 months or such shorter period as may be established by the Company’s Board of Directors. The offering periods start on April 1st and October 1st of each year. During the year ended March 31, 2010, there were 139,595 shares issued at a purchase price ranging from $5.07 to $10.73 per share and during the year ended March 31, 2009, there were 119,732 shares issued at a purchase price ranging from $5.07 to $10.51 per share under the Purchase Plan.
Stock-Based Compensation
The Company currently uses the Black-Scholes option pricing model to determine the fair value of employee stock options and employee stock purchase plan shares.
The determination of the fair value of employee stock options and employee stock purchase plan shares has been estimated using the following weighted-average valuation assumptions:
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Employee Stock Option Plans:
|
||||||||||||
|
Expected term (in years)
|
6
|
6
|
6
|
|||||||||
|
Volatility
|
44
|
%
|
35
|
%
|
42
|
%
|
||||||
|
Risk-free interest rate
|
2.6
|
%
|
3.2
|
%
|
4.0
|
%
|
||||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Weighted average fair value at date of grant
|
$
|
5.11
|
$
|
4.62
|
$
|
9.13
|
||||||
|
Employee Stock Purchase Plan:
|
||||||||||||
|
Expected term (in years)
|
0.5
|
0.5
|
0.5
|
|||||||||
|
Volatility
|
72
|
%
|
45
|
%
|
43
|
%
|
||||||
|
Risk-free interest rate
|
0.5
|
%
|
2.5
|
%
|
3.9
|
%
|
||||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
The fair value of each purchase right granted under the Company’s Purchase Plan during the year ended March 31, 2010, 2009 and 2008 was estimated at the date of grant using the Black-Scholes option pricing model, and is not subject to revaluation as a result of subsequent stock price fluctuations.
Stock-based compensation expense included in results of operation is as follows (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Cost of goods sold
|
$
|
603
|
$
|
560
|
$
|
471
|
||||||
|
Research and development
|
685
|
592
|
533
|
|||||||||
|
Sales and marketing
|
1,541
|
1,498
|
1,325
|
|||||||||
|
General and administrative
|
3,158
|
2,995
|
2,629
|
|||||||||
|
$
|
5,987
|
$
|
5,645
|
$
|
4,958
|
|||||||
Additionally, approximately $6,000 and $51,000 in stock-based compensation expense has been capitalized in inventory at March 31, 2010 and 2008, respectively. At March 31, 2009, approximately $8,000 in stock-based compensation expense has been released from inventory.
As of March 31, 2010, there was approximately $7.3 million of total stock-based compensation expense, after estimated forfeitures, related to unvested employee stock options and restricted stock units, which is expected to be recognized over an estimated weighted average amortization period of 2.4 years.
79
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Stock-based compensation expense recognized for the year ended March 31, 2008 related to the amortization of deferred stock-based compensation was $163,000. The Company had fully recognized amortization of deferred stock-based compensation in the fourth quarter ended March 31, 2008.
Stock-based compensation expense recognized for the years ended March 31, 2008 relating to non-employee options was $4,000. All non-employee options were fully-vested as of March 31, 2008.
General stock option information
The following table sets forth the summary of option activity for the year ended March 31, 2010:
|
|
Average
|
Remaining
|
Aggregate
|
|||||||||||||
|
Exercise
|
Contractual
|
|
Intrinsic
|
|||||||||||||
|
Shares
|
Price
|
Term
|
Value
|
|||||||||||||
|
(in thousands)
|
(in years)
|
(in thousands)
|
||||||||||||||
|
Options outstanding at March 31, 2009
|
4,018
|
$
|
12.69
|
|||||||||||||
|
Options granted
|
865
|
$
|
11.10
|
|||||||||||||
|
Options exercised
|
(461
|
)
|
$
|
8.88
|
||||||||||||
|
Options forfeited
|
(231
|
)
|
$
|
14.86
|
||||||||||||
|
Options expired
|
(117
|
)
|
$
|
18.56
|
||||||||||||
|
Options outstanding at March 31, 2010
|
4,074
|
$
|
12.50
|
6.8
|
$
|
30,607
|
||||||||||
|
Options exercisable at March 31, 2010
|
2,674
|
$
|
12.26
|
5.8
|
$
|
20,800
|
||||||||||
The total aggregate intrinsic value of options exercised during the years ended March 31, 2010, 2009 and 2008 was $3.0 million, $322,000 and $3.5 million, respectively. The Company realized a tax benefit of $89,000 for the year ended March 31, 2010 in connection with the options exercised during the year ended March 31, 2010. The closed market value per share of the Company’s common stock as of March 31, 2010 was $19.72 as reported by The NASDAQ Stock Market.
The following table summarizes information related to options outstanding and exercisable by exercise price at March 31, 2010 (in thousands, except per share data):
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||||
|
Weighted
|
||||||||||||||||||||||
|
Average
|
Weighted
|
Weighted
|
||||||||||||||||||||
|
Remaining
|
Average
|
Average
|
||||||||||||||||||||
|
Range of
|
Number
|
Contractual Life
|
Exercise
|
Number
|
Exercise
|
|||||||||||||||||
|
Exercise Prices
|
Outstanding
|
(years)
|
Price
|
Exercisable
|
Price
|
|||||||||||||||||
|
$
|
0.68 - $ 1.01
|
35
|
2.6
|
$
|
0.83
|
35
|
$
|
0.83
|
||||||||||||||
|
$
|
1.15 - $ 1.15
|
78
|
3.9
|
$
|
1.15
|
78
|
$
|
1.15
|
||||||||||||||
|
$
|
4.93 - $ 7.40
|
631
|
4.8
|
$
|
5.68
|
616
|
$
|
5.66
|
||||||||||||||
|
$
|
7.48 - $10.62
|
1,037
|
7.7
|
$
|
9.14
|
428
|
$
|
9.40
|
||||||||||||||
|
$
|
10.75 - $15.22
|
1,091
|
6.9
|
$
|
12.78
|
711
|
$
|
12.85
|
||||||||||||||
|
$
|
15.30 - $20.14
|
768
|
7.5
|
$
|
18.33
|
512
|
$
|
18.33
|
||||||||||||||
|
$
|
20.24 - $24.75
|
434
|
7.0
|
$
|
22.39
|
294
|
$
|
22.56
|
||||||||||||||
|
$
|
0.68 - $24.75
|
4,074
|
6.8
|
$
|
12.50
|
2,674
|
$
|
12.26
|
||||||||||||||
80
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
The following table sets forth the summary of restricted stock units activity for the year ended March 31, 2010:
|
Weighted-
|
||||||||||||||||
|
Weighted-
|
Average
|
|||||||||||||||
|
Average
|
Remaining
|
Aggregate
|
||||||||||||||
|
Exercise
|
Contractual
|
Intrinsic
|
||||||||||||||
|
Shares
|
Price
|
Term
|
Value
|
|||||||||||||
|
(in thousands)
|
(in years)
|
(in thousands)
|
||||||||||||||
|
Non-vested restricted stock units at March 31, 2009
|
3
|
$
|
-
|
|||||||||||||
|
Awarded
|
-
|
$
|
-
|
|||||||||||||
|
Vested
|
(3
|
)
|
$
|
-
|
||||||||||||
|
Forfeited
|
-
|
$
|
-
|
|||||||||||||
|
Non-vested restricted stock units at March 31, 2010
|
-
|
$
|
-
|
-
|
$
|
-
|
||||||||||
401(k) Savings Plan
The Company has a 401(k) income deferral plan (the “401(k) Plan”). Eligible participants may contribute up to 75% of their pretax salary up to the maximum allowed under Internal Revenue Service regulations. According to the terms of the 401(k) Plan, the Company may make discretionary matching contributions to the 401(k) Plan each year, allocable to all plan participants. The Company made no discretionary contributions during the years ended March 31, 2010, 2009 and 2008.
Note 13 — Defined Benefit Plan
The Company has a qualified defined benefit pension plan for all eligible Swiss employees of its wholly-owned subsidiary in Switzerland, Micrus SA. Retirement benefits are provided based on employees’ years of service and earnings, or in accordance with applicable employee benefit regulations. The Company’s practice is to fund amounts sufficient to meet the requirements set forth in the applicable employee benefit and tax regulations.
Consistent with typical Swiss practice, the pension plan is funded through a guaranteed insurance contract with an insurance company, in this case Swiss Life. Swiss Life is responsible for the investment strategy of the insurance premiums that the Company submits. Swiss Life does not hold individual assets per participating employer. Instead, Swiss Life pools assets from all participating employers, including Micrus SA and invests them in accordance with its own strategies and risk assessments. The goal of these investment practices is for Swiss Life to be able to pay pension benefits on behalf of the participating employers when they come due, while maintaining sufficient equity to absorb fluctuations in the value of its assets and liabilities. Under the term of contract between the Company and Swiss Life, the interest rate as well as the surrender value is guaranteed for each participant, with Swiss Life assuming any risk to the value of the underlying assets. Together with other Swiss life insurance companies, Swiss Life is a member of a security fund, whose purpose is to cover any shortfall in the event that Swiss Life is not able to fulfill its contractual agreements. Conversely, when the market performance of the aggregated assets exceeds the pension plan guaranteed interest income, a maximum of 10% of the market gains will be allocated to Swiss Life.
The Company may make contributions in excess of the legally required minimum contribution level. Any voluntary contributions by the Company are not expected to exceed deductible limits in accordance with Switzerland tax regulations.
81
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
The following table sets forth summarized information on the defined benefit pension plan (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Change in benefit obligation:
|
||||||||
|
Benefit obligation at beginning of year
|
$
|
827
|
$
|
599
|
||||
|
Service cost
|
81
|
85
|
||||||
|
Interest cost
|
29
|
17
|
||||||
|
Participant contribution
|
77
|
62
|
||||||
|
Actuarial gain
|
(8
|
)
|
(14
|
)
|
||||
|
Benefit payments
|
(280
|
)
|
(176
|
)
|
||||
|
Transfers in and additional contributions
|
94
|
226
|
||||||
|
Plan amendments
|
-
|
109
|
||||||
|
Currency exchange rate changes
|
66
|
(81
|
)
|
|||||
|
Benefit obligation at end of year
|
886
|
827
|
||||||
|
Change in plan assets:
|
||||||||
|
Fair value of plan assets at beginning of year
|
679
|
517
|
||||||
|
Actual return on plan assets
|
29
|
36
|
||||||
|
Employer contributions
|
78
|
84
|
||||||
|
Participant contributions
|
77
|
62
|
||||||
|
Benefit payments
|
(280
|
)
|
(176
|
)
|
||||
|
Transfers in and additional contributions
|
94
|
226
|
||||||
|
Currency exchange rate changes
|
54
|
(70
|
)
|
|||||
|
Fair value of plan assets at end of year
|
731
|
679
|
||||||
|
Unfunded status at end of year
|
$
|
155
|
$
|
148
|
||||
The net periodic pension cost for the Swiss pension plan for fiscal 2010 and 2009 was based on long-term asset rates of return of 3.4% and 3.2%, respectively. In developing these expected long-term rate of return assumptions, consideration was given to expected returns based on the current investment policy and historical return for the asset classes.
The following table presents selected pension plan information (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Projected benefit obligation
|
$
|
886
|
$
|
827
|
||||
|
Accumulated benefit obligation
|
$
|
814
|
$
|
756
|
||||
|
Fair value of plan assets
|
$
|
731
|
$
|
679
|
||||
82
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
A summary of the components of net periodic benefit cost for the pension plan is as follows (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Service cost
|
$ | 81 | $ | 85 | $ | 81 | ||||||
|
Interest cost
|
29 | 17 | 13 | |||||||||
|
Expected return on plan assets
|
(25 | ) | (14 | ) | (11 | ) | ||||||
|
Net periodic benefit cost
|
$ | 85 | $ | 88 | $ | 83 | ||||||
Amounts included in accumulated other comprehensive loss are as follows (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Actuarial gain
|
$ | (13 | ) | $ | (36 | ) | ||
|
Plan amendments
|
- | 109 | ||||||
|
Total recognized in other comprehensive loss
|
$ | (13 | ) | $ | 73 | |||
At March 31, 2010 and 2009, the assets of the plans are held in trust and allocated as follows:
|
2010
|
2009
|
|||||||
|
Equity securities
|
3
|
%
|
5
|
%
|
||||
|
Debt securities
|
10
|
%
|
9
|
%
|
||||
|
Cash and cash equivalents
|
3
|
%
|
9
|
%
|
||||
|
Fixed interests
|
71
|
%
|
63
|
%
|
||||
|
Investment in participations and associated companies
|
1
|
%
|
1
|
%
|
||||
|
Real estate
|
11
|
%
|
11
|
%
|
||||
|
Other investments
|
1
|
%
|
2
|
%
|
||||
The following table presents a rollforward of pension plan assets measured at fair value on a recurring basis using unobservable inputs (Level 3) at March 31, 2010 (in thousands)
|
Level 3
|
||||
|
Fair value beginning of year
|
$
|
679
|
||
|
Actual return on plan assets
|
29
|
|||
|
Purchases, sales and settlements
|
23
|
|||
|
Fair value end of year
|
$
|
731
|
||
The benefits expected to be paid out are as follows (in thousands):
|
For Years Ending March 31,
|
Pension Plan
|
|||
|
Benefit
|
||||
|
2011
|
$
|
66
|
||
|
2012
|
$
|
66
|
||
|
2013
|
$
|
66
|
||
|
2014
|
$
|
66
|
||
|
2015
|
$
|
66
|
||
|
Thereafter
|
$
|
300
|
||
83
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Note 14 — Segments
Revenues from unaffiliated customers by geographic area, based on the customer’s shipment locations, were as follows (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
United States
|
$
|
44,731
|
$
|
39,007
|
$
|
33,753
|
||||||
|
Japan
|
11,041
|
9,319
|
6,250
|
|||||||||
|
United Kingdom
|
6,649
|
7,531
|
9,100
|
|||||||||
|
Rest of the world
|
28,669
|
22,339
|
20,110
|
|||||||||
|
$
|
91,090
|
$
|
78,196
|
$
|
69,213
|
|||||||
The Company’s long-lived assets, excluding goodwill and intangible assets, by geographic area were as follows (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
United States
|
$
|
5,602
|
$
|
6,691
|
||||
|
United Kingdom
|
57
|
69
|
||||||
|
Rest of the world
|
182
|
222
|
||||||
|
$
|
5,841
|
$
|
6,982
|
|||||
The Company identifies its operating segments based on how management views and evaluates the Company’s operations, which is primarily based on geographic location. The Company has determined it operates in three business segments, the Americas, Europe and Asia Pacific. The products and services sold by each segment are substantially the same and the Company evaluates performance and allocates resources primarily based on revenues and gross profit. In previous years, the Company’s Europe (excluding the United Kingdom) and the United Kingdom segments were segregated as two business segments. Previous year information in the tables that follow has been restated to conform to the current year classification.
Revenues and gross profit for these segments were as follows (in thousands):
|
Years Ended March 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Revenues:
|
||||||||||||
|
Americas
|
$
|
50,133
|
$
|
44,067
|
$
|
37,565
|
||||||
|
Europe
|
28,486
|
23,461
|
24,195
|
|||||||||
|
Asia Pacific
|
12,471
|
10,668
|
7,453
|
|||||||||
|
$
|
91,090
|
$
|
78,196
|
$
|
69,213
|
|||||||
|
Gross Profit:
|
||||||||||||
|
Americas
|
$
|
39,872
|
$
|
32,952
|
$
|
29,621
|
||||||
|
Europe
|
20,449
|
17,004
|
17,564
|
|||||||||
|
Asia Pacific
|
8,363
|
7,393
|
4,727
|
|||||||||
|
$
|
68,684
|
$
|
57,349
|
$
|
51,912
|
|||||||
Total assets by operating segments at March 31, 2010 and 2009 were as follows (in thousands):
|
March 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Americas
|
$
|
60,525
|
$
|
43,537
|
||||
|
Europe
|
19,711
|
17,969
|
||||||
|
$
|
80,236
|
$
|
61,506
|
|||||
84
MICRUS ENDOVASCULAR CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (Continued)
Note 15 — Subsequent Events
The Company has evaluated subsequent events through the filing date of this Form 10-K and has determined that there were no subsequent events to recognize or disclose in these financial statements.
Note 16 — Quarterly Financial Information (unaudited)
The following table represents certain unaudited quarterly information for the eight quarters ended March 31, 2010. In management’s opinion, this information has been prepared on the same basis as the audited financial statements and includes all the adjustments necessary to fairly state the unaudited quarterly results of operations set forth herein.
|
First
|
Second
|
Third
|
Fourth
|
|||||||||||||
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
|||||||||||||
|
(in thousands, except per share amounts)
|
||||||||||||||||
|
2010:
|
||||||||||||||||
|
Revenues
|
$
|
21,223
|
$
|
21,510
|
$
|
22,793
|
$
|
25,564
|
||||||||
|
Gross profit
|
$
|
15,659
|
$
|
16,605
|
$
|
17,294
|
$
|
19,126
|
||||||||
|
Net income attributable to common stockholders
|
$
|
2,264
|
$
|
3,606
|
$
|
3,320
|
$
|
2,340
|
||||||||
|
Net income per share attributable to common stockholders:
|
||||||||||||||||
|
Basic
|
$
|
0.14
|
$
|
0.23
|
$
|
0.21
|
$
|
0.14
|
||||||||
|
Diluted
|
$
|
0.14
|
$
|
0.22
|
$
|
0.20
|
$
|
0.13
|
||||||||
|
2009:
|
||||||||||||||||
|
Revenues
|
$
|
18,324
|
$
|
20,792
|
$
|
18,322
|
$
|
20,758
|
||||||||
|
Gross profit
|
$
|
13,731
|
$
|
15,178
|
$
|
13,545
|
$
|
14,895
|
||||||||
|
Net income (loss) attributable to common stockholders
|
$
|
(6,602
|
)
|
$
|
(2,736
|
)
|
$
|
(2,292
|
)
|
$
|
576
|
|||||
|
Net income (loss) per share attributable to common stockholders:
Basic and Diluted
|
$
|
(0.42
|
)
|
$
|
(0.17
|
)
|
$
|
(0.15
|
)
|
$
|
0.04
|
|||||
85
None
(a) Evaluation of disclosure controls and procedures.
With the participation of our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), management has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) as of the end of the period covered by this report. Based on such evaluation, our CEO and CFO have concluded that, as of the end of such period, our disclosure controls and procedures are effective.
(b) Management’s report on internal control over financial reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f), to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with United States generally accepted accounting principles. Under the supervision and with the participation of our management, including our CEO and CFO, we conducted an evaluation of the design and operational effectiveness of our internal control over financial reporting as of March 31, 2010 based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Our management, including the CEO and CFO, does not expect our disclosure controls or our internal control over financial reporting will prevent or detect all errors or all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Based on our evaluation utilizing the criteria set forth in Internal Control — Integrated Framework issued by COSO, our management (including our CEO and CFO) concluded that our internal control over financial reporting was effective as of March 31, 2010. Our assessment of the effectiveness of internal control over financial reporting as of March 31, 2010 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report included at “Item 8 – Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
(c) Changes in internal control over financial reporting.
There have not been any changes in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934) during the fourth quarter of our fiscal year ended March 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
86
Information required by this item is incorporated by reference to the Micrus Proxy Statement for the 2010 Annual Meeting of Stockholders.
Information required by this item is incorporated by reference to the Micrus Proxy Statement for the 2010 Annual Meeting of Stockholders.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information required by this item is incorporated by reference to the Micrus Proxy Statement for the 2010 Annual Meeting of Stockholders
Information required by this item is incorporated by reference to the Micrus Proxy Statement for the 2010 Annual Meeting of Stockholders.
Information required by this item is incorporated by reference to the Micrus Proxy Statement for the 2010 Annual Meeting of Stockholders.
(a) The following documents are filed as part of this Annual Report on Form 10-K:
(1) Financial Statements. The following statements of Micrus Endovascular Corporation and the report of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm, are included in Part II, Item 8.
(2) Financial Statement Schedules. The following schedule is required to be filed by Item 15(b).
Schedule II — Valuation and Qualifying Accounts for each of the three years in the period ended March 31, 2010
All other schedules have been omitted because they are either inapplicable or the required information has been provided in the consolidated financial statements or the notes thereto.
(b) Exhibits. The list of exhibits on the Index to Exhibits on pages 90 through 92 of this report is incorporated herein by reference.
87
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, Micrus Endovascular Corporation has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
MICRUS ENDOVASCULAR CORPORATION
|
||||
|
By:
|
/s/ JOHN T. KILCOYNE
|
|||
|
John T. Kilcoyne
|
||||
|
Chairman and Chief Executive Officer
|
||||
Date: June 8, 2010
POWERS OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints John T. Kilcoyne and Gordon T. Sangster, and each of them, his true and lawful attorneys-in-fact and agents with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any amendments to this Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Form 10-K has been signed by the following persons in the capacities and on the dates indicated:
|
Signatures
|
Title
|
Date
|
||
|
/s/ JOHN T. KILCOYNE
|
Chairman and Chief Executive Officer
|
June 8, 2010
|
||
|
John T. Kilcoyne
|
(Principal Executive Officer)
|
|||
|
/s/ GORDON T. SANGSTER
|
Chief Financial Officer
|
June 8, 2010
|
||
|
Gordon T. Sangster
|
(Principal Financial and Accounting Officer)
|
|||
|
/s/ MICHAEL EAGLE
|
Director
|
June 8, 2010
|
||
|
Michael Eagle
|
||||
|
/s/ FRED HOLUBOW
|
Director
|
June 8, 2010
|
||
|
Fred Holubow
|
||||
|
/s/ L. NELSON HOPKINS
|
Director
|
June 8, 2010
|
||
|
L. Nelson Hopkins, M.D.
|
||||
|
/s/ FRANCIS J. SHAMMO
|
Director
|
June 8, 2010
|
||
|
Francis J. Shammo
|
||||
|
/s/ JEFFREY H. THIEL
|
Director
|
June 8, 2010
|
||
|
Jeffrey H. Thiel
|
||||
|
/s/ GREGORY H. WOLF
|
Director
|
June 8, 2010
|
||
|
Gregory H. Wolf
|
88
|
Balance At
|
Additions/
|
|||||||||||||||
|
Beginning
|
Charged to
|
Balance at
|
||||||||||||||
|
of Year
|
Expenses
|
Deductions
|
End of Year
|
|||||||||||||
|
Allowance for Doubtful Accounts
|
(In thousands)
|
|||||||||||||||
|
Year Ended March 31, 2010
|
$ | 119 | $ | 96 | $ | 2 | $ | 217 | ||||||||
|
Year Ended March 31, 2009
|
$ | 95 | $ | 49 | $ | (25 | ) | $ | 119 | |||||||
|
Year Ended March 31, 2008
|
$ | 234 | $ | (145 | ) | $ | 6 | $ | 95 | |||||||
|
Valuation Allowance - Deferred Tax Assets
|
||||||||||||||||
|
Year Ended March 31, 2010
|
$ | 23,696 | $ | - | $ | (2,209 | ) | $ | 21,487 | |||||||
|
Year Ended March 31, 2009
|
$ | 21,419 | $ | 2,277 | $ | - | $ | 23,696 | ||||||||
|
Year Ended March 31, 2008
|
$ | 17,750 | $ | 3,669 | $ | - | $ | 21,419 | ||||||||
89
INDEX TO EXHIBITS
|
Exhibit
Number
|
Description
|
|
3.1
|
Certificate of Incorporation (Filed as Exhibit 3.2 of Amendment No. 3 to the Company’s Registration Statement on Form S-1 filed on May 17, 2005 (Registration No. 333-123154) (“Amendment No. 3”), and incorporated herein by reference)
|
|
3.2
|
Bylaws (Filed as Exhibit 3.4 of Amendment No. 3, and incorporated herein by reference)
|
|
4.1
|
Specimen Stock Certificate (Filed as Exhibit 4.1 of the Company’s Registration Statement on Form S-1 filed on March 4, 2005 (Registration No. 333-123154) (“Form S-1”), and incorporated herein by reference)
|
|
4.2
|
Warrant dated as of December 11, 2000 among the Registrant and Roberts Mitani Capital, LLC (Filed as Exhibit 4.2 of Form S-1, and incorporated herein by reference)
|
|
4.3
|
Amended and Restated Stockholders’ Rights Agreement dated as of February 21, 2005 among the Registrant and the parties listed therein (Filed as Exhibit 4.3 of Form S-1, and incorporated herein by reference)
|
|
4.4
|
Form of Common Stock Warrant issued in connection with the Series E Preferred Stock and Warrant Purchase Agreement dated February 21, 2005, among the Company and the purchasers of the Company’s Series E Preferred Stock (Filed as Exhibit 4.4 to the Company’s Quarterly Report on Form 10-Q filed on February 14, 2006, and incorporated herein by reference)
|
|
10.1*
|
1996 Stock Option Plan (Filed as Exhibit 10.1 of Form S-1, and incorporated herein by reference)
|
|
10.2*
|
1998 Stock Plan (Filed as Exhibit 10.2 of Form S-1, and incorporated herein by reference)
|
|
10.3*
|
2005 Equity Incentive Plan (Filed as Exhibit 10.3 of Amendment No. 4 to the Company’s Registration Statement on Form S-1 filed on May 23, 2005 (Registration No. 333-123154) (“Amendment No. 4”), and incorporated herein by reference)
|
|
10.4*
|
2005 Equity Incentive Plan — Form of Incentive Stock Option Agreement for Executive Officers and Directors (Filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on August 15, 2005, and incorporated herein by reference)
|
|
10.5*
|
2005 Equity Incentive Plan — Form of Nonstatutory Stock Option Agreement for Executive Officers and Directors (Filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on August 15, 2005, and incorporated herein by reference)
|
|
10.6*
|
2005 Employee Stock Purchase Plan, as amended (Filed as Exhibit 10.4 of Amendment No. 6 to the Company’s Registration Statement on Form S-1 filed on June 13, 2005 (Registration No. 333-123154) (“Amendment No. 6”), and incorporated herein by reference)
|
|
10.7*
|
Letter Agreement dated November 15, 2004 with John R. Kilcoyne (Filed as Exhibit 10.7 of Form S-1, and incorporated herein by reference)
|
|
10.8*
|
Letter Agreement dated November 5, 2003 with Robert A. Stern (Filed as Exhibit 10.8 of Form S-1, and incorporated herein by reference)
|
|
10.9*
|
Letter Agreement dated June 12, 1998 with Tom M. Holdych (Filed as Exhibit 10.9 of Form S-1, and incorporated herein by reference)
|
|
10.10*
|
Letter Agreement dated May 23, 2003 with Edward F. Ruppel, Jr. (Filed as Exhibit 10.10 of Form S-1, and incorporated herein by reference)
|
|
10.11*
|
Letter Agreement dated October 25, 2004 with Eckhard H. Reitz (Filed as Exhibit 10.13 of Form S-1, and incorporated herein by reference)
|
|
10.12*
|
Letter Agreement dated February 16, 2005 with Robert C. Colloton (Filed as Exhibit 10.23 of Amendment No. 6, and incorporated herein by reference)
|
|
10.13
|
Office Lease dated June 6, 2005 between the Registrant and WW/LJ GATEWAYS LTD., a California limited partnership, for office space located at 821 Fox Lane in San Jose, California (Filed as Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on July 5, 2005, and incorporated herein by reference)
|
|
10.14†
|
Distribution Agreement, dated September 30, 2005, between Micrus Endovascular Corporation and Goodman Co., Ltd. (Filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2005, and incorporated herein by reference)
|
|
10.15
|
Share Purchase Agreement, dated September 20, 2005, between Mark Ellis and James Mackenzie and Micrus Endovascular Corporation (Filed as Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on September 26, 2005, and incorporated herein by reference)
|
|
10.16†
|
License Agreement, effective July 1, 2005, between Micrus Endovascular Corporation and Micrus Endovascular SA (Filed as Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2005, and incorporated herein by reference)
|
90
|
Exhibit
Number
|
Description
|
|
10.17†
|
Contract Manufacturing Agreement, effective July 1, 2005, between Micrus Endovascular Corporation and Micrus Endovascular SA (Filed as Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2005, and incorporated herein by reference)
|
|
10.18†
|
Agreement for Sharing Development Costs, effective July 1, 2005, between Micrus Endovascular Corporation and Micrus Endovascular SA (Filed as Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2005, and incorporated herein by reference)
|
|
10.19†
|
Support Services Agreement, effective July 1, 2005, between Micrus Endovascular Corporation and Micrus Endovascular SA (Filed as Exhibit 10.8 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2005, and incorporated herein by reference)
|
|
10.20†
|
Technology Transfer Agreement, effective July 28, 2005, between Micrus Endovascular Corporation and Vascular FX (Filed as Exhibit 10.9 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2005, and incorporated herein by reference)
|
|
10.21†
|
License, Development and Distribution Agreement, effective January 6, 2006, between Micrus Endovascular Corporation and Biotronik AG (Filed as Exhibit 10.23 to the Company’s Annual Report on Form 10-K filed on June 16, 2006 and incorporated herein by reference)
|
|
10.22
|
Form of Director and Executive Officer Indemnification Agreement (Filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on February 14, 2007)
|
|
10.23*
|
Amended and Restated Employee Cash Bonus Plan with respect to Executive Officers (Filed as Exhibit 10.26 to the Company’s Quarterly Report on Form 10-Q filed on August 8, 2007)
|
|
10.24†
|
Amendment No. 1 to Distribution Agreement, dated September 20, 2007, between Micrus Endovascular Corporation and Goodman Co., Ltd. (Filed as Exhibit 10.27 to the Company’s Quarterly Report on Form 10-Q filed on November 9, 2007)
|
|
10.25†
|
Distribution Agreement, dated July 31, 2007, between Micrus Endovascular Corporation and Beijing Tianxinfu Medical Appliance Co. Ltd. (Filed as Exhibit 10.28 to the Company’s Quarterly Report on Form 10-Q filed on November 9, 2007)
|
|
10.26†
|
Stock Purchase Agreement, dated October 26, 2007, between Micrus Endovascular Corporation, ReVasc Technologies, Inc. and The Cleveland Clinic Foundation which includes as an exhibit thereto the Amended and Restated License Agreement, dated October 26, 2007, between ReVasc Technologies, Inc. and The Cleveland Clinic Foundation (Filed as Exhibit 10.29 to the Company’s Quarterly Report on Form 10-Q filed on February 11, 2008)
|
|
10.27*
|
Letter Agreement dated November 12, 2007 with Gordon Sangster (Filed as Exhibit 10.30 to the Company’s Quarterly Report on Form 10-Q filed on February 11, 2008)
|
|
10.28*
|
Form of Acceleration Agreement (Filed as Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on January 29, 2008, and incorporated herein by reference)
|
|
10.29*
|
New Product Bonus Incentive Program (Filed as Exhibit 10.29 to the Company’s Quarterly Report on Form 10-Q filed on June 12, 2008)
|
|
10.30†
|
Settlement and License Agreement, dated September 22, 2008, between Micrus Endovascular Corporation, Boston Scientific Corp. and Target Therapeutics, Inc. (Filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2008)
|
|
10.31†
|
Settlement and Release Agreement, dated September 22, 2008, between Micrus Endovascular Corporation and the Regents of the University of California (Filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2008)
|
|
10.32
|
Credit Agreement, dated November 5, 2008, between Micrus Endovascular Corporation and Wells Fargo Bank, National Association (Filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2008)
|
|
10.33
|
First Amendment to Credit Agreement, dated February 3, 2009, between Micrus Endovascular Corporation and Wells Fargo Bank, National Association (Filed as Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on February 9, 2009)
|
|
10.34
|
Amendment to Offer Letter, dated December 15, 2008, between Micrus Endovascular Corporation and John R. Kilcoyne (Filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on February 2, 2009)
|
|
10.35
|
Amendment to Offer Letter, dated December 15, 2008, between Micrus Endovascular Corporation and Robert A. Stern (Filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on February 2, 2009)
|
|
10.36
|
Amendment to Offer Letter, dated December 15, 2008, between Micrus Endovascular Corporation and Carolyn M. Bruguera (Filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on February 2, 2009)
|
|
10.37
|
Amendment to Offer Letter, dated December 12, 2008, between Micrus Endovascular Corporation and Robert C. Colloton (Filed as Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed on February 2, 2009)
|
|
10.38*
|
Bonus Program with respect to Executive Officers (Filed as Exhibit 10.38 to the Company’s Annual Report on Form 10-K filed on June 11, 2009)
|
|
10.39*
|
Amended and Restated 2005 Equity Incentive Plan (Filed as Exhibit 10.39 to the Company’s Annual Report on Form 10-K filed on June 11, 2009)
|
91
|
Exhibit
Number
|
Description
|
|
10.40*
|
First Amendment to Technology Transfer Agreement dated January 28, 2009, between Micrus Endovascular Corporation and Vascular FX, LLC, a Delaware limited liability company (Filed as Exhibit 10.40 to the Company’s Annual Report on Form 10-K filed on June 11, 2009)
|
|
10.41
|
Second Amendment to the Credit Agreement dated May 20, 2009, between Micrus Endovascular Corporation and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 10.1 of Form 8-K filed on May 27, 2009)
|
|
10.42†
|
Distribution Agreement, dated September 30, 2009, between Micrus Endovascular Corporation and IDS (Hong Kong) Ltd. (Filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on November 9, 2009)
|
|
21.1#
|
List of Subsidiaries
|
|
23.1#
|
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm
|
|
24.1#
|
Powers of Attorney (appears on the signature page of this form)
|
|
31.1#
|
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
31.2#
|
Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
32.1#
|
Certifications Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
__________
|
#
|
Filed herewith.
|
|
*
|
Indicates a management contract or compensatory plan or arrangement, as required by Item 15(a)3.
|
|
†
|
Confidential treatment requested for certain portions of this Exhibit pursuant to Rule 24b-2 promulgated under the Securities Exchange Act, which portions are omitted and filed separately with the Securities and Exchange Commission.
|
92
