Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Vicor Technologies, Inc. | y03602e8vk.htm |
Exhibit 99.1
| 1 Vicor Technologies, Inc. A Medical Diagnostic Company Dedicated to the Commercialization Of Breakthrough Risk Stratification Technology |

| 2 Safe Harbor Statement This presentation contains forward-looking statements. Forward-looking statements are often signaled by forms of words such as should, could, will, might, plan, projection, forecast, expect, guidance, potential and developing. Actual results could vary materially from those expected due to a variety of risk factors, including our ability to generate revenue from the sale of the PD2i Analyzer; our ability to obtain FDA marketing clearance on the PD2i VS for military and civilian applications and the PD2i-CA for total and cardiac mortality; our ability to continue to receive financing sufficient to continue operations and complete the critical clinical trials; our ability to continue as a going concern; our ability to successfully develop products based on our technologies; our ability to obtain and maintain adequate levels of third-party reimbursement for our products; the impact of competitive products and pricing; the ability to receive regulatory approval for our products; the ability of third-party contract research organizations to perform preclinical testing and clinical trial for our technologies; our ability of third-party manufacturers to manufacture our products; our ability to retain the services of our key personnel; our ability to market and sell our products successfully; our ability to protect our intellectual property; product liability; federal income tax laws and regulations; general market conditions in the medical device and pharmaceutical industries; and other factors identified in our Annual Report on Form 10-K for the fiscal year ended December 31, 2009 and subsequent filings with the Securities and Exchange Commission. The Company undertakes no obligation to update the results of these forward-looking statements to reflect events or circumstances after today or to reflect the occurrence of unanticipated events. |

| 3 Vicor Technologies, Inc. The PD2i Analyzer(tm) |

| 4 Overview PD2i Analyzer(tm) is a Revolutionary Diagnostic Technology Platform technology Risk stratification of large target patient populations Potential new vital sign Rapid, accurate and economical for new healthcare system PD2i Analyzer 510(k) Marketing Clearance 12/29/08 PD2i Analyzer(tm) Product Sales Launch Q1 2010 Substantial Revenue Generation Capability with Existing 510(k) |
| 5 PD2i(r) Advantages User Friendly Data Acquisition Capable of Handling Dirty Data Incentive for Physician Use: Low up-front investment Per test revenue (Vicor and Physician) Huge Market in U.S. and Internationally |
| 6 Autonomic Nervous System Testing Autonomic Nervous System (ANS/DAN) Dysfunction Large untapped market (24+ million patients) Autonomic Neuropathic Complications (Silent Killer) Early ANS Dysfunction Diagnosis Permits: Physicians to adjust medications appropriately Reduces/eliminates effects of ANS Dysfunction |
| 7 PD2i CA(tm) 81,100,000 People Affected Ischemia-Post MI Congestive Heart Failure Total Cardiac Morbidity Sudden Cardiac Death - swift and unexpected Pump failure Cardiovascular Disease |

| 8 INTERNAL MEDICINE 278.01 Morbid Obesity 279.3 Unspecified Immunity Deficiency 296.80 Bipolar Disease 311 Depression 300.00 Anxiety 307.4** Sleep Disorders 530.11 GERD 536.3 Gastroparesis 564.1 Irritable Bowel Syndrome 729.1 Fibromyalgia 309.81 Post-Traumatic Stress Syndrome 782.3 Edema AVAILABLE DIAGNOSTIC CODES (ICD 9 for test authorization) CARDIOLOGY 401.0 - 405.99 Hypertension 412 Post - MI 413.9 Angina 440.9 Atherosclerosis 424.0 Mitral Valve Prolapse Syndrome 425.4 Cardiomyopathy 427.9 Cardiac Dysrhythmias 428.0 Congestive Heart Failure ENDOCRINOLOGY 244.8 Acquired Hypothyroidism 246.9 Thyroid Disorders 256.31 Premature Menopausal Symptoms 627.2 Menopausal Syndromes NEPHROLOGY 402.90 Hypertensive Renal Disease 586 Renal Failure DIABETES 250 - 250.8** NEUROLOGY 307.01 Tension Headache 314.01 ADD/ADHD 332.0 Parkinsonism 333.0 Shy-Drager Syndrom (Orthostatic Hypotension with Multisystem Degeneration) 337.00 Idiopathic Peripheral Autonomic Neuropathy 358.1 Myasthenic Syndrome (Eaton-Lambert) 337.2 Chronic Regional Pain Syndrome or RSD 458.0 Orthostatic Hypotension 340 Multiple Sclerosis 458.1 Chronic Hypotension 346.0 - 346.9** Migraine 742.8 Other Specified Congenital Anomalies of the Nervous System 356.4 Idiopathic Progressive Polyneuropathy 780.2 Syncope and Collapse 356.8 Other Specified Idiopathic Peripheral Neuropathy 780.4 Dizziness, Light-headedness and Vertigo 356.9 Unspecified Peripheral Idiopathic Neuropathy 780.71 Chronic Fatigue Syndrome 357.2 Polyneuropathy in Diiabetes 780.0 Headache 357.4 Polyneuropathy in Other Disease Classified Elsewhere 785.0 Tachycardia (Postural) PULMONOLOGY 780.5 - 780.59 Sleep Disturbances 493.9 - 493.92** Asthma 493.2** COPD (Asthma with Chronic Obstructive Pulmonary Disease) This example was put together to help aid you on proper reimbursement procedures. If you have any questions regarding reimbursement, please feel free to contact David Fater toll-free at 800-998-9964 |

| 9 ATTN: BILLING DEPARTMENT ICD-9 CODING FOR VICOR MONITORING CPT CODING EXAMPLE: STEP 1: Use proper diagnosis code(s) for patient. See reverse side for ICD-9 Coding list.*** (i.e., 250.6 Diabetes with neurological manifestations) STEP 2: CPT CODING for VICOR TESTING CPT CODE DESCRIPTION 2009 NATIONAL REIMBURSEMENT AVERAGE SUBMITTAL REQUEST 95921 Parasympathetic Nervous System Testing $72.49 $100 PT CODING for 3-Lead Rhythm ECG: During the Vicor test a 3-lead ECG is also performed. You may bill for this procedure in addition to Sympathetic and Parasympathetic testing, but you may not be paid separately if it's included in Vicor's testing. ** This code is not specific to our technology. It is not reimbursed by every insurance provider; those that are reimbursing may require a 59 Modifier. (Please refer to your states policies.) CPT CODE DESCRIPTION 2009 NATIONAL REIMBURSEMENT AVERAGE SUBMITTAL REQUEST 93040** Rhythm, ECG, one to three leads; with interpretation and report $13.34 $25 |
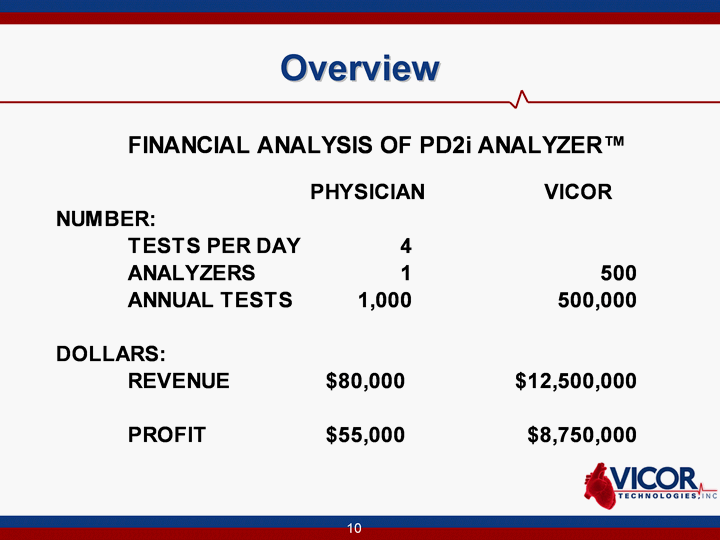
| 10 Overview |
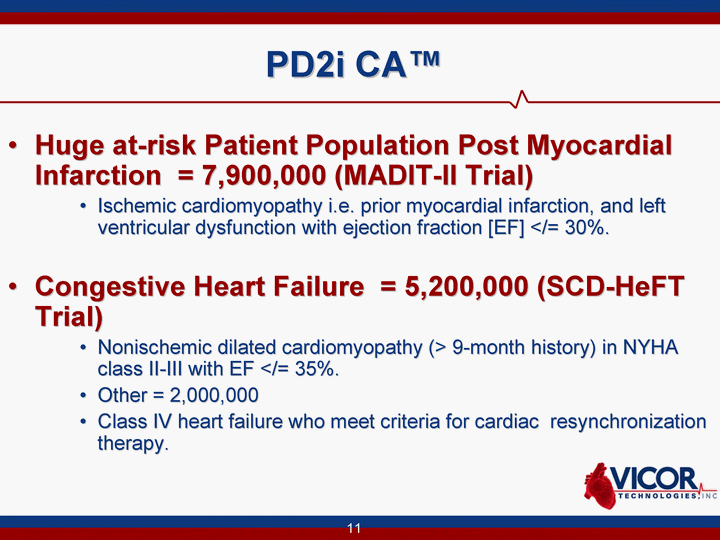
| 11 PD2i CA(tm) Huge at-risk Patient Population Post Myocardial Infarction = 7,900,000 (MADIT-II Trial) Ischemic cardiomyopathy i.e. prior myocardial infarction, and left ventricular dysfunction with ejection fraction [EF] </= 30%. Congestive Heart Failure = 5,200,000 (SCD-HeFT Trial) Nonischemic dilated cardiomyopathy (> 9-month history) in NYHA class II-III with EF </= 35%. Other = 2,000,000 Class IV heart failure who meet criteria for cardiac resynchronization therapy. |
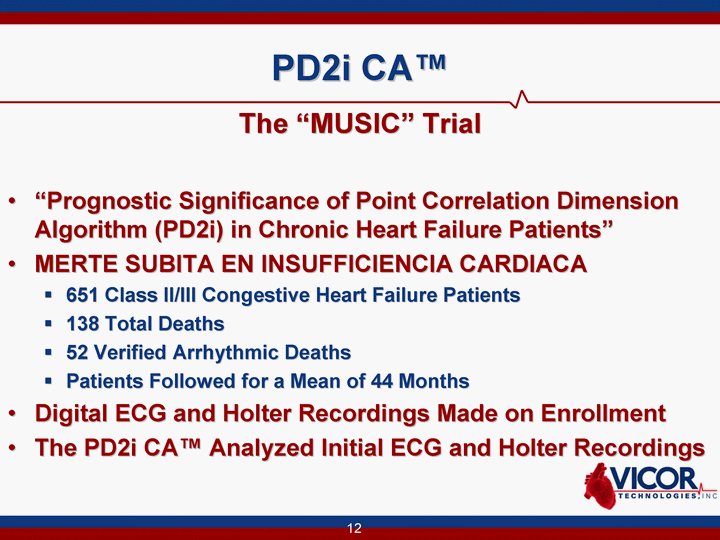
| 12 PD2i CA(tm) The "MUSIC" Trial "Prognostic Significance of Point Correlation Dimension Algorithm (PD2i) in Chronic Heart Failure Patients" MERTE SUBITA EN INSUFFICIENCIA CARDIACA 651 Class II/III Congestive Heart Failure Patients 138 Total Deaths 52 Verified Arrhythmic Deaths Patients Followed for a Mean of 44 Months Digital ECG and Holter Recordings Made on Enrollment The PD2i CA(tm) Analyzed Initial ECG and Holter Recordings |
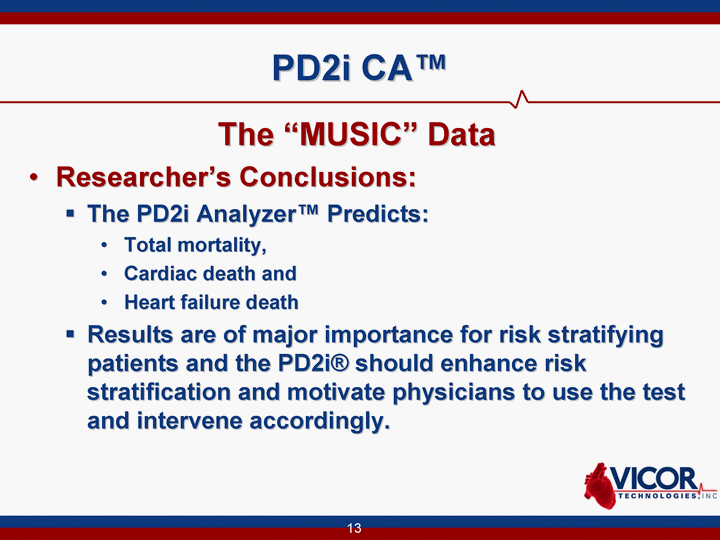
| 13 PD2i CA(tm) The "MUSIC" Data Researcher's Conclusions: The PD2i Analyzer(tm) Predicts: Total mortality, Cardiac death and Heart failure death Results are of major importance for risk stratifying patients and the PD2i(r) should enhance risk stratification and motivate physicians to use the test and intervene accordingly. |
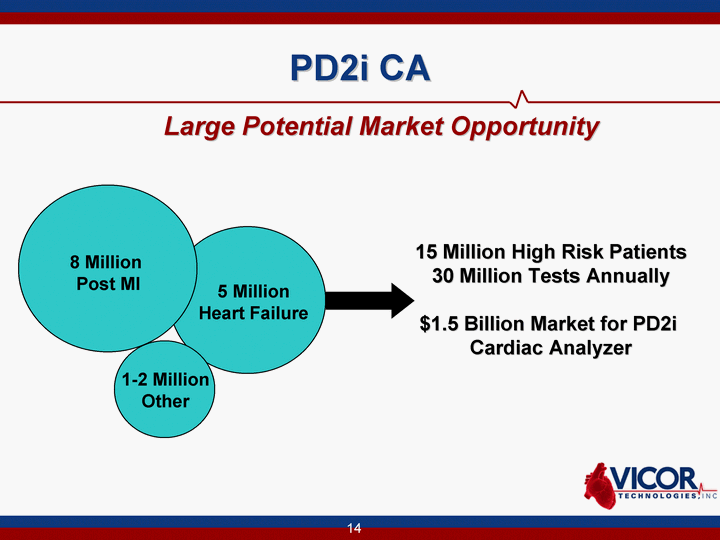
| 14 PD2i CA Large Potential Market Opportunity 5 Million Heart Failure 1-2 Million Other 15 Million High Risk Patients 30 Million Tests Annually $1.5 Billion Market for PD2i Cardiac Analyzer 8 Million Post MI |

| 15 PD2i VS(tm) Collaboration with the U.S. Army (USAISR) Assess the severity of injury and imminent death Civilian sector opportunity: 38,000,000 annual events). Anticipated FDA 510(k) Marketing Clearance in 2010 |

| 16 PD2i VS(tm) USAISR Study #1 USE OF HEART-RATE COMPLEXITY TO DETERMINE THE NEED FOR LIFE SAVING INTERVENTIONS IN COMBAT CASUALTIES Abnormal HRC values exhibited in all patients Those requiring LSI's had Min PD2i values < 1 Findings suggest utility of PD2i for diagnosis of need for LSI |
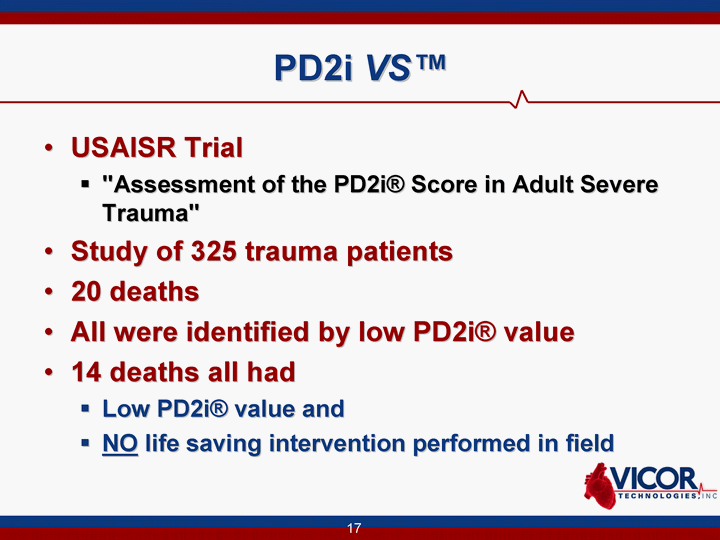
| 17 PD2i VS(tm) USAISR Trial "Assessment of the PD2i(r) Score in Adult Severe Trauma" Study of 325 trauma patients 20 deaths All were identified by low PD2i(r) value 14 deaths all had Low PD2i(r) value and NO life saving intervention performed in field |

| 18 PD2i(r) Marketing Plan PD2i Analyzer(tm) (ANS Testing) Product launch Q1 2010 Initial 25 Independent sales reps in NC and SC in January Targeted marketing of shareholder practices- Q2-2010 Roll out in Q2 and Q3 2010 in other states Sales personnel Other Distributors Cardiac Morbidity Beta tests in several practices- Cedars Sinai Medical Group-Los Angeles Jackson Memorial Hospital North-Miami 510(k) submission 1H 2010 and 510(k) clearance thereafter Use of 25 sales reps in NC and SC initially Thought Leader seminars- 2H 2010 Roll out in 2H 2010 of other states |

| 19 PD2i(r) International Marketing Plan Active Discussions With Distribution Partners: Canada South America Middle East Europe Far East |
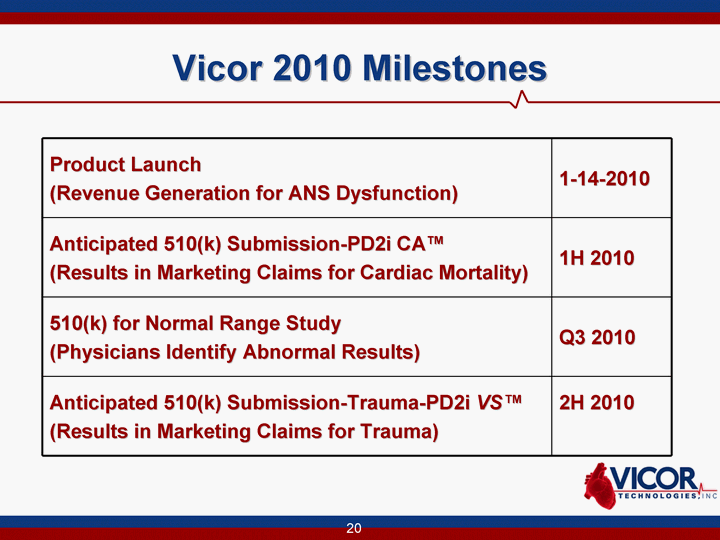
| 20 Vicor 2010 Milestones Product Launch (Revenue Generation for ANS Dysfunction) 1-14-2010 Anticipated 510(k) Submission-PD2i CA(tm) (Results in Marketing Claims for Cardiac Mortality) 1H 2010 510(k) for Normal Range Study (Physicians Identify Abnormal Results) Q3 2010 Anticipated 510(k) Submission-Trauma-PD2i VS(tm) (Results in Marketing Claims for Trauma) 2H 2010 |
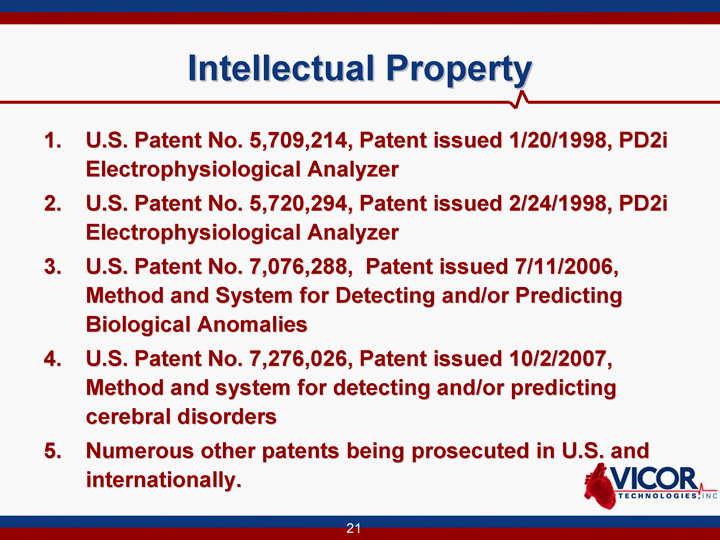
| 21 Intellectual Property U.S. Patent No. 5,709,214, Patent issued 1/20/1998, PD2i Electrophysiological Analyzer U.S. Patent No. 5,720,294, Patent issued 2/24/1998, PD2i Electrophysiological Analyzer U.S. Patent No. 7,076,288, Patent issued 7/11/2006, Method and System for Detecting and/or Predicting Biological Anomalies U.S. Patent No. 7,276,026, Patent issued 10/2/2007, Method and system for detecting and/or predicting cerebral disorders Numerous other patents being prosecuted in U.S. and internationally. |

| 22 Vicor Management Employees and Consultants David Fater - President and CEO Over 13 years experience as a senior executive with 3 public healthcare companies. Spent 24 years as a senior international partner with Ernst & Young. Dr. James Skinner, Ph.D. - Director of Grant R&D Extensive experience as a scientist and manager of large research and development projects. Professor of Medicine at Baylor College of Medicine. Recipient of many research grants. Principal investigator of a program that operated 5 laboratories and 3 core facilities. Dr. Jerry Anchin, Ph.D. - Director of R&D 25 year's accumulated experience in the biotechnology diagnostic and medical device sector. Actively involved in immunology, molecular biology, and drug discovery. |

| 23 Vicor Management Employees and Consultants Dr. Daniel N. Weiss, M.D.,F.A.C.C. - CMO Extensive experience as a practicing cardiologist and electrophysiologist. Partner of Florida Arrhythmia Consultants. Director of the Jim Moran Heart and Vascular Center since 1994. Consultant to Medtronics, St. Jude Medical, and Guidant. Dr. Richard M. Cohen, Ph.D. - Business Development 30 year's experience in worldwide operations and sourcing. COO of four health care companies, including two medical device companies. Thomas J. Bohannon-CPA-Chief Accounting Officer 40 year's experience in accounting and financial reporting. Lloyd Chesney-Chief Technology Officer Michael Harwitz-Senior Systems Architect 25+ year's experience in developing web-based enterprise systems for health care |
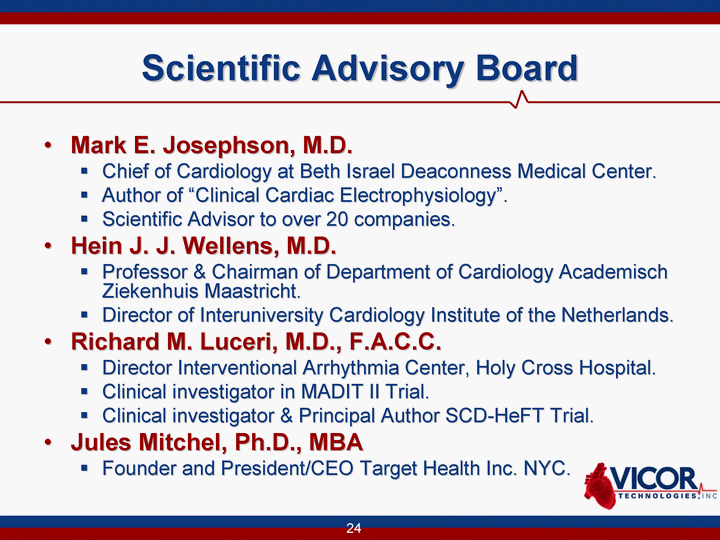
| 24 Scientific Advisory Board Mark E. Josephson, M.D. Chief of Cardiology at Beth Israel Deaconness Medical Center. Author of "Clinical Cardiac Electrophysiology". Scientific Advisor to over 20 companies. Hein J. J. Wellens, M.D. Professor & Chairman of Department of Cardiology Academisch Ziekenhuis Maastricht. Director of Interuniversity Cardiology Institute of the Netherlands. Richard M. Luceri, M.D., F.A.C.C. Director Interventional Arrhythmia Center, Holy Cross Hospital. Clinical investigator in MADIT II Trial. Clinical investigator & Principal Author SCD-HeFT Trial. Jules Mitchel, Ph.D., MBA Founder and President/CEO Target Health Inc. NYC. |
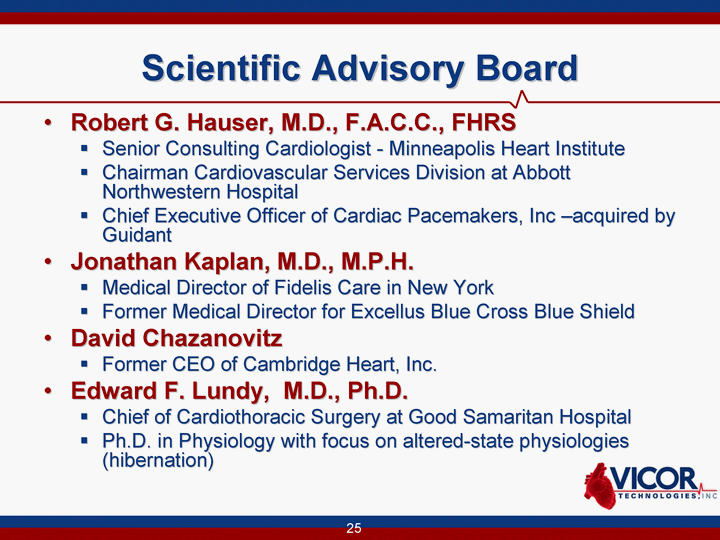
| 25 Scientific Advisory Board Robert G. Hauser, M.D., F.A.C.C., FHRS Senior Consulting Cardiologist - Minneapolis Heart Institute Chairman Cardiovascular Services Division at Abbott Northwestern Hospital Chief Executive Officer of Cardiac Pacemakers, Inc -acquired by Guidant Jonathan Kaplan, M.D., M.P.H. Medical Director of Fidelis Care in New York Former Medical Director for Excellus Blue Cross Blue Shield David Chazanovitz Former CEO of Cambridge Heart, Inc. Edward F. Lundy, M.D., Ph.D. Chief of Cardiothoracic Surgery at Good Samaritan Hospital Ph.D. in Physiology with focus on altered-state physiologies (hibernation) |
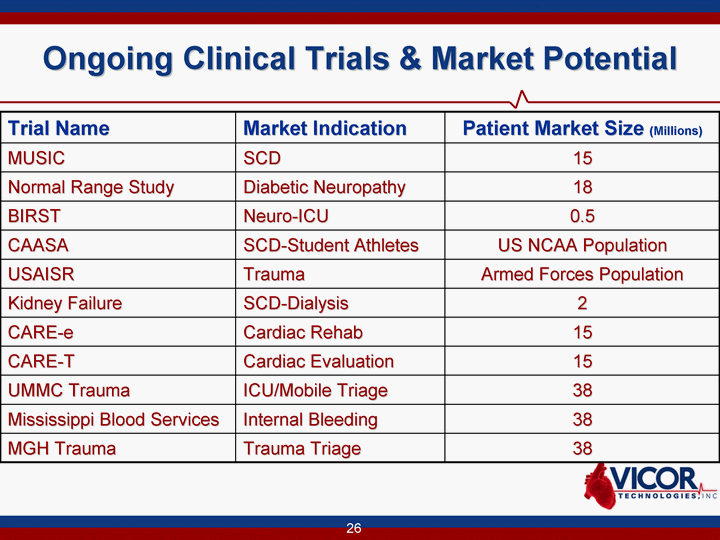
| 26 Ongoing Clinical Trials & Market Potential Trial Name Market Indication Patient Market Size (Millions) MUSIC SCD 15 Normal Range Study Diabetic Neuropathy 18 BIRST Neuro-ICU 0.5 CAASA SCD-Student Athletes US NCAA Population USAISR Trauma Armed Forces Population Kidney Failure SCD-Dialysis 2 CARE-e Cardiac Rehab 15 CARE-T Cardiac Evaluation 15 UMMC Trauma ICU/Mobile Triage 38 Mississippi Blood Services Internal Bleeding 38 MGH Trauma Trauma Triage 38 |
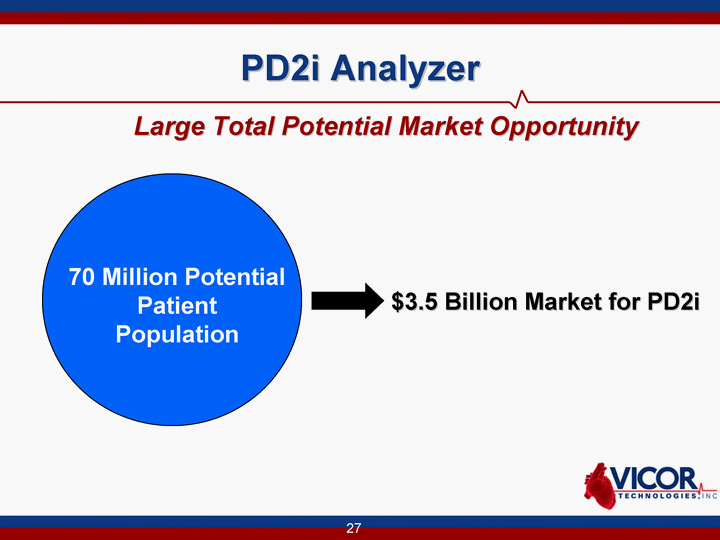
| 27 PD2i Analyzer Large Total Potential Market Opportunity 70 Million Potential Patient Population $3.5 Billion Market for PD2i |

| 28 Investment Highlights Life Saving Technology Substantial Unmet Medical Need in Risk Stratification Substantial Cost Savings to Public and Private Insurers High Margin, High Operating Profit Products Attractive Business Model Highly Experienced Management Team World-class Scientific Advisory Board |

| Thank You. Vicor Technologies, Inc. 2300 NW Corporate Blvd.,Suite 123 Boca Raton, FL 33431 Phone: (877) 528-PD2i (7324) Fax: (561) 995-2449 www.vicortech.com |
