Attached files
| file | filename |
|---|---|
| EX-31 - Benda Pharmaceutical, Inc. | v185527_ex31.htm |
| EX-32 - Benda Pharmaceutical, Inc. | v185527_ex32.htm |
U.S.
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-K
|
x
|
ANNUAL
REPORT UNDER PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE
ACT OF 1934 FOR THE FISCAL YEAR ENDED DECEMBER 31, 2009
|
|
¨
|
TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934 FOR THE TRANSITION PERIOD FROM ______________ TO
______________
|
Commission
File Number: 0-16397
BENDA
PHARMACEUTICAL, INC.
(Exact
name of small business issuer as specified in its charter)
|
Delaware
|
41-2185030
|
|
|
(State
or other jurisdiction of incorporation or
organization)
|
(IRS
Employer Identification No.)
|
Taibei
Mingju, 4th
Floor,
6 Taibei
Road, Wuhan, Hubei Province, 430015, PRC
(Address
of principal executive offices)
+86
(27) 85494916
(Issuer’s
telephone number)
SECURITIES
REGISTERED UNDER SECTION 12(b) OF THE EXCHANGE ACT: NONE
SECURITIES
REGISTERED UNDER SECTION 12(g) OF THE EXCHANGE ACT:
Common
Stock, Par Value $.001 Per Share
(Title of
Class)
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes ¨ No x
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes ¨ No x
Check
whether the issuer: (1) filed all reports required to be filed by Section 13 or
15(d) of the Exchange Act during the past 12 months (or for such shorter period
that the registrant was required to file such reports), and (2) has been subject
to such filing requirements for the past 90 days. YES x NO
¨
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of
this chapter) during the preceding 12 months (or for such shorter period that
the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not
be contained, to the best of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference Part III of this Form 10-K or
any amendment to this Form 10-K. x
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller
reporting company. See the definitions of “large accelerated filer,”
“accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act.
|
Large
accelerated filer
|
¨
|
Accelerated
filer
|
¨
|
|
|
Non-accelerated
filer
(Do
not check if a smaller reporting company)
|
¨
|
Smaller
reporting company
|
x
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act). YES ¨ NO x
As of May
17, 2010, 105,155,355 shares of the registrant's Common Stock were outstanding.
The aggregate market value of the voting common equity held by non-affiliates
(based on the closing bid price of such stock as reported on May 13, 2010 by the
Over-the-Counter Bulletin Board) was approximately $1,525,209.
Documents Incorporated by
Reference:
None.
|
Item
Number and Caption
|
Page
|
|
|
PART
I
|
||
|
Item
1.
|
Description
of Business
|
1
|
|
Item
1A.
|
Risk
Factors
|
|
|
Item
2.
|
Description
of Properties
|
46
|
|
Item
3.
|
Legal
Proceedings
|
48
|
|
Item
4.
|
(Removed
and Reserved)
|
49
|
|
PART
II
|
||
|
Item
5.
|
Market
for Common Equity and Related Stockholder Matters
|
49
|
|
Item
6.
|
Selected
Financial Data
|
|
|
Item
7.
|
Management's
Discussion and Analysis or Plan of Operations
|
51
|
|
Item
7A
|
Quantitative
and Qualitative Disclosures About Market Risk
|
|
|
Item
8.
|
Financial
Statements
|
60
|
|
Item
9.
|
Changes
in and Disagreements With Accountants on Accounting and Financial
Disclosure
|
61
|
|
Item 9A(T).
|
Controls
and Procedures
|
62
|
|
PART
III
|
||
|
Item
10.
|
Directors,
Executive Officers, and Corporate Governance
|
62
|
|
Item
11.
|
Executive
Compensation
|
66
|
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
67
|
|
Item
13.
|
Certain
Relationships and Related Transactions and Director
Independence
|
69
|
|
Item
14.
|
Principal
Accountant Fees and Services
|
74
|
|
PART
IV
|
77
|
|
|
Item
15.
|
Exhibits,
Financial Statement Schedules
|
|
ITEM 1. DESCRIPTION OF
BUSINESS.
Summary
Benda
Pharmaceutical, Inc. (“we”, “us”, “our”, “Benda” or the “Company”), through our
wholly owned subsidiary Ever Leader Holdings Limited (“Ever Leader”), is a
pharmaceutical company that identifies, discovers, develops and manufactures
both conventional medications and Traditional Chinese Medicines (“TCMs”) for the
treatment of some of the largest common ailments and diseases (e.g., common
cold, diabetes, cancer). We are also dedicated to the development, manufacturing
and commercialization of gene therapy products.
Benda
owns all of the capital stock of Ever Leader Holdings Limited, a holding company
incorporated under the laws of Hong Kong SAR on October 29, 2005. Ever Leader
owns 95% of the issued and outstanding capital stock of Hubei Tongji Benda Ebei
Pharmaceutical Co., Ltd. (“Benda Ebei”), a Sino-Foreign Equity Joint Venture
company incorporated under the laws of the PRC. Benda Ebei owns: (i) 95% of the
issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co., Ltd.
(“Jiangling Benda”), a company formed under the laws of the PRC; (ii) 95% of the
issued and outstanding capital stock of Yidu Benda Chemical Co., Ltd. (“Yidu
Benda”), a company incorporated under the laws of the PRC; (iii) 75% of the
issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co.,
Ltd. (“BJ Shusai”), a company incorporated under the laws of the PRC; and (iv)
60.13% of the issued and outstanding capital stock of Shenzhen SiBiono Gene
Technology Co., Ltd. (“SiBiono”), a company incorporated under the laws of the
PRC.
We
distribute our high value, branded medicines, through agents who sell them to
hospitals that administer them to patients. We sell generics to medical
wholesalers for resale to hospitals. The company sells its Over the Counter
(“OTC”) medicines to wholesalers specializing in selling to retail chain drug
stores. Our “Active Pharmaceutical Ingredients” (“APIs”) are typically sold to
large drug manufacturers under long-term supply contracts. The bulk chemicals
are purchased by other Chinese drug companies.
History and Recent
Developments
We were
organized as a Minnesota corporation on February 17, 1982. The technology on
which our original products were based, including Spread Spectrum Technology,
permit data and telemetry to be transmitted simultaneously over telephone wire
without interfering with normal voice service. Our products were known as
data/voice multiplexing (“DVM”) equipment and were aimed at operating telephone
companies in the telecommunications market. Our lack of financial resources
caused us to pursue a plan of dissolution as approved by our Board of Directors
and approved by our shareholders on November 30, 1993.
During
fiscal 1994, we began implementing a plan of voluntary dissolution pursuant to
Minnesota law that was approved by our shareholders at a Special Shareholders’
Meeting held on November 30, 1993. Under our plan of dissolution, most of our
assets were sold during 1994 with some payments deferred into 1995 and beyond.
The recovery period ran through 1997. During fiscal 1995, most of the tangible
asset sales were collected and only technology licenses remained to be
collected. During fiscal 1996, we continued to collect license fees and payments
on one equipment lease. The results of the plan of dissolution were successful
and all liabilities and expenses were either paid or were covered in
reserves.
1
On
November 17, 2000, a Special Meeting of the shareholders of the Company was held
at which time the plan of dissolution was revoked. Pursuant to the proposal for
revocation, a liquidating dividend of approximately $212,000 was paid pro-rata
to our shareholders in August 2001. We have been inactive since
1994.
On
October 7, 2005, we and Applied Spectrum Technologies, Inc., a Delaware
corporation (“Applied - Delaware”) entered into a certain Agreement and Plan of
Merger (“Plan of Merger”). Pursuant to the Plan of Merger, subject to
stockholder approval, we were to merge with and into Applied - Delaware for the
purposes of the redomestication from the State of Minnesota to the State of
Delaware (the “Merger”). On October 24, 2005, we filed a Definitive Proxy
Statement pursuant to Section 14(a) of the Securities Exchange Act of 1934, as
amended, with the Securities and Exchange Commission for the purpose of voting
on the Merger.
On
November 14, 2005, the holders of a majority of our outstanding shares of common
stock approved the Merger. The Merger was completed on November 17, 2005, and
the Articles of Merger were filed with the States of Minnesota and Delaware on
November 17, 2005.
On
December 14, 2005, Norwood Venture Corp. (“Norwood”), a former shareholder of
the Company and KI Equity Partners III, LLC (“KI Equity”) entered into a certain
securities purchase agreement, as amended, under which KI Equity agreed to
purchase and Norwood agreed to sell an aggregate of 2,281,302 shares of common
stock of the Company, representing approximately 77.2% of the Company’s
outstanding shares of common stock, to KI Equity at a price of $175,000. The
closing of the transactions under the Purchase Agreement occurred on December
29, 2005.
Kevin R.
Keating, our former President and sole officer and director who resigned on
November 15, 2006, is the father of Timothy J. Keating, the majority member of
Keating Investments, LLC. Keating Investments, LLC is the managing member of KI
Equity Partners III, LLC, which is the party that acquired the controlling
interest in us pursuant to the Purchase Agreement. Keating Investments, LLC is
also the managing member and 90% owner of Keating Securities, LLC, a registered
broker-dealer. Kevin R. Keating is not affiliated with and has no equity
interest in Keating Investments, LLC, KI Equity Partners III, LLC or Keating
Securities, LLC and disclaims any beneficial interest in the shares of Applied
Spectrum’s common stock to be acquired by KI Equity Partners III,
LLC.
We did
not become engaged in the pharmaceutical business until November of 2006. Before
closing a share exchange transaction in November 2006, we were a shell company
with nominal assets and operations, whose sole business was to identify,
evaluate and investigate various companies with the intent that, if such
investigation warrants, a business combination be negotiated and completed
pursuant to which we (formerly known as Applied Spectrum Technologies, Inc.)
would acquire a target company with an operating business with the intent of
continuing the acquired company's business as a publicly held entity. We entered
in an Exchange Agreement dated September 7, 2006 (the “Exchange Agreement”) with
KI Equity Partners II, LLC (“KI Equity”), Ever Leader, a company incorporated
under the laws of Hong Kong, and the owners of 100% of the capital shares of
Ever Leader. The closing of the Exchange Agreement occurred on November 15,
2006. At the closing of the Exchange Agreement, we acquired all of Ever Leader's
capital shares (the “Ever Leader Shares”) from the Ever Leader Shareholders, and
the Ever Leader Shareholders transferred and contributed all of their Ever
Leader Shares to us. In exchange, we issued 64,942,360 shares of our Common
Stock to the Ever Leader Shareholders.
As a
result of the closing of the Exchange Agreement, Ever Leader became our wholly
owned subsidiary and we adopted Ever Leader’s main operational business. The
Exchange transaction, for accounting and financial reporting purposes, is deemed
to be a reverse acquisition, where we (the legal acquirer) are considered the
accounting acquiree and Ever Leader (the legal acquiree) is considered the
accounting acquirer, and thus the historical financial statements of Ever Leader
are the financial statements of Benda.
2
Financing
The
closing of the Exchange Agreement described above was contingent on a minimum of
$10,000,000 (or such lesser amount as mutually agreed to by Ever Leader and the
placement agent) being subscribed for, and funded into escrow, by certain
accredited and institutional investors ("Investors") in a private placement
offering for the purchase of Units, each Unit consisting of 54,087 shares of our
Common Stock ("Common Stock") and 54,087 common stock purchase warrants promptly
after the closing of the Exchange transaction under terms and conditions
approved by our board of directors immediately following the Exchange (the
“Financing”). The closing of the Financing was contingent on the closing of the
Exchange transaction, and the Exchange transaction was contingent on the closing
of the Financing. On November 15, 2006, we completed this private placement
offering. We received gross proceeds of approximately $12 million in connection
with the Financing from the Investors. Pursuant to Subscription Agreements
entered into with these Investors, we sold 480 Units for a total of 25,961,760
shares of its Common Stock and warrants to purchase an additional 25,961,760
shares of our common stock to the Investors. The price per Unit in the Financing
was $25,000.
Keating
Securities, LLC (“Placement Agent”), an affiliate of Keating Investments, LLC,
acted as placement agent in connection with the Financing. For their services,
the Placement Agents received a commission equal to 7.5% of the gross proceeds
from the offering and a non-accountable expense allowance equal to 1.5% of the
gross proceeds. In addition, the Placement Agents received, for nominal
consideration, warrants to purchase 10% of the number of shares of common stock
sold in connection with the Financing, which in the aggregate totaled 2,596,176
shares of our common stock at an exercise price of $0.555 per share. The
warrants are fully vested and have a term of five years. The Placement Agent
warrants will have registration rights similar to the registration rights
afforded to the holders of Common Stock and Warrants subscribed for in the
Financing. We also paid for the out-of-pocket expenses incurred by the Placement
Agent and all purchasers in the amount of $100,000.
In order
to finance the acquisition of a majority of the shares of Shenzhen SiBiono
GeneTech Co., Ltd. (“SiBiono”), on April 5, 2007, we entered into an Investment
Agreement (“April Financing”) with certain accredited and institutional
investors (“Investors”) who had also participated in the subscription for
$12,000,000 of our common stock pursuant to certain Securities Purchase
Agreements dated November 15, 2006 (“November Financing”). Pursuant to the
Investment Agreement, the Investors purchased a total of 252 Units for
$7,560,000 with each Unit consisting of (i) a convertible promissory note in the
principal amount of Thirty Thousand Dollars ($30,000) which shall be convertible
into 54,087 shares of the Company's common stock, par value $0.001 per share,
and (ii) a warrant to acquire 54,087 shares of Common Stock at an exercise price
of $0.555 per share. The Notes bear an interest rate of four percent per annum
until the Buyer elects to exercise the right to convert, and matured on March
28, 2009. The Notes have reached the Maturity Date. We have not repaid the
principal amounts of the Notes as of the date of this annual
report.
In March
2007 the Company and the Investors entered into a Modification Agreement
amending the November Financing Documents to allow for certain issuances of the
Company's securities, including additional purchases of the Company's equity
securities pursuant to the Investment Agreement; shares issuances required under
the Equity Transfer Agreements; and issuances of options pursuant to an approved
Qualified Employment Stock Option Plan. All of the investors in the November
Financing had the right to participate in the purchase of additional units under
the Investment Agreement and all of such investors either participated in the
Investment Agreement or have waived their right to participate in such. In
addition, those investors that did not participate in the Investment Agreement
also waived their right to object to the changes to the Warrants, Registration
Rights Agreement and Make Good Agreement which were set forth in the
Modification Agreement.
On or
prior to forty five (45) days from the Closing Date of the Investment Agreement,
we were required to deliver to the Buyers our financial statements for the years
ending December 31, 2005 and December 31, 2006, audited by Kempisty &
Company Certified Public Accountants, P.C., prepared in accordance with GAAP,
during each year involved and fairly presenting in all material respects our
financial position as of the dates thereof and the results of our operations and
cash flows for each such year then ended. Such financial statements for the
years ending December 31, 2005 and December 31, 2006 were filed with our Form
10KSB for the year ending December 31, 2006 filed with the Securities and
Exchange Commission on May 4, 2007. In addition, on or prior to seventy five
(75) days from the Closing Date, we are also required to deliver to the Buyers
audited financial statements for SiBiono for the required time periods for the
Form 8-K filing required by the Securities and Exchange Commission. Such
financial statements were filed with our Amendment No. 1 to Form 8K filed June
15, 2007.
Shenzhen
SiBiono GeneTech Co., Ltd.
On April
5, 2007, Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., a Sino-Foreign Equity
Joint Venture company incorporated under the laws of the PRC (“Benda Ebei”), of
which Ever Leader Holdings Limited, a company incorporated under the laws of
Hong Kong SAR ("Ever Leader") and a wholly owned subsidiary of Benda
Pharmaceutical, Inc. (the “Company”), owns 95% of the outstanding common stock,
has entered into Equity Transfer Agreements with certain shareholders of
Shenzhen SiBiono Gene Technology Co., Ltd. (“SiBiono”), a corporation
established and validly existing under the law of the PRC, to purchase a total
of approximately 57.57% of the shares of SiBiono's common stock for total
consideration of RMB60,000,000 due and payable on or before April 30,
2007.
3
In
connection with the Equity Transfer Agreements, we entered into a Financial
Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”)
for financial consultancy services rendered by Super Pioneer. Pursuant to the
Financial Consultancy Agreement, we agreed to issue 2,100,000 shares of our
common stock to Super Pioneer within three months from the date of the
agreement. Super Pioneer agreed to lock up the shares for a period of twelve
months from the date of the issuance of the shares (the “Lock-up Period”).
Within three months from the Lock-up Period, in the event that the public
trading price of our shares did not reach $3.6 per share and we are not listed
in the capital market of NASDAQ or AMEX, Super Pioneer shall have the option to
require us to redeem 1,960,000 shares of the stock owned by Super Pioneer at a
price of $3.6 per share. Such option shall expire within one month from the last
date of the three month period.
On June
11, 2007, Benda Ebei entered into Equity Transfer Agreements with Yaojin Wang
and Huimin Zhang, shareholders of SiBiono, for the purchase of an additional
2.56% of the shares of SiBiono's common stock for total consideration of
RMB2,560,000 due and payable on or before June 30, 2007. Accordingly, Benda Ebei
holds a total of 60.13% of the shares of SiBiono's common stock.
In
connection with the Equity Transfer Agreements, we entered into Technical
Consultancy Agreements with Yaojin Wang and Huimin Zhang for technical
consultancy services rendered by Yaojin Wang and Huimin Zhang. Pursuant to the
Technical Consultancy Agreements, we agreed to issue 33,585 shares of our common
stock to Yaojin Wang and 55,975 shares of our common stock to Huimin Zhang
within three months from the date of the agreement. Yaojin Wang and Huimin Zhang
agreed to lock up their shares for a period of twelve months from the date of
the issuance of the shares (the “Lock-up Period”). Within three months from the
Lock-up Period, in the event that the public trading price of our shares did not
reach $3.6 per share and we are not listed in the capital market of NASDAQ or
AMEX, Yaojin Wang and Huimin Zhang shall have the option to require us to redeem
the shares of the stock owned by Yaojin Wang and Huimin Zhang at a price of $3.6
per share. Such option shall expire within one month from the last date of the
three month period. The redemption requests were made to the Company in January
2008 and the company has already obtained oral consent from Super Pioneer, Wang
and Zhang that the payment would be deferred to the year of 2010.
Business
Our
operations are headquartered in Wuhan, Hubei Province, China. We are a mid-sized
Chinese pharmaceutical company that identifies, discovers, develops and
manufactures both conventional medications, Traditional Chinese Medicines
(“TCMs”) for the treatment of some of the largest common ailments and diseases
(e.g., common cold, diabetes, cancer), gene therapy product for the treatment of
cancer and Herbal TCM Oral Liquid for the treatment of anti-respiratory tract
infections.
We
currently have five core operating companies:
|
·
|
Yidu
Benda develops, manufactures and sells bulk chemicals (or
pharmaceutical intermediated), which are the raw materials used to make
“Active Pharmaceutical Ingredients”
(“APIs”).
|
|
·
|
Jiangling
Benda
develops, manufactures and sells APIs, which are one of the two
components of any capsules, tablets and fluids that are pharmaceutically
active. An API is the substance in a drug that produces the desired
medicinal effect. The “excipient” is the inert material that holds the API
(such as gelatin or water).
|
|
·
|
Benda Ebei
develops, manufactures and sells (a) conventional finished
medicines, which are non-patented, branded, proprietary small volume
injection solutions (vials) used for a variety of treatments including
hepatitis; and (b) Traditional Chinese Medicines (“TCMs”), which are
herb-based and natural medicines used in TCM therapies (via our newly
formed subsidiary Beijing Shusai). Some of the medicines we produce are of
our own origination and protected from competition by certificates issued
by China’s State Food and Drug Administration (“SFDA”).
There are no differences between the regulatory processes for
conventional medicines and Traditional Chinese Medicines. Traditional
Chinese Medicines are ready-make medicines, which are produced according
certain curing principles and prescriptions and can be used immediately,
such as pills, medicinal granules and
capsules.
|
|
·
|
BJ
Shusai
develops, manufactures and sell herbal TCM oral liquid for the treatment
of anti-respiration tract infections. However due to the fact that the
SFDA experienced an overhaul in its policies and regulatory systems in an
effort to fight against corruption in Chinese pharmaceutical industry,
thus BJ Shusai’s operation has been adversely affected by this recent
policy changes which prohibits some state-owned hospitals from forming
alliances with private companies. The management could not estimate that
such situation could be resolved in the coming
future.
|
|
·
|
SiBiono
develops, manufactures and sells gene therapy products. As a pioneer in
gene therapy in China, SiBiono's mission is to develop innovative gene
therapy products for the improvement of human health and life quality. The
Company has developed two core technology platforms: Viral Vector Gene
Delivery System and Non-Viral Vector Gene Delivery System focusing on
development of gene therapy product for cancer and cardiovascular
diseases. SiBiono’s flagship product, Gendicine is the commercialized gene
therapy for the treatment of
cancer.
|
4
Each core
Benda operating company has its own manufacturing facility located near Wuhan,
in Hubei Province and Shenzhen, in Guangdong Province. Good Manufacturing
Practices (“GMP”) certification was first issued to Benda Ebei on November 11,
2003 and renew on December 3, 2009 for the production of injection vials. On
November 26, 2007, Benda Ebei received a GMP certificate for the production of
tablets. Benda Ebei was designated a High and New Technology Enterprise by the
Science and Technology Bureau of Hubei Province on July 6, 2005 for a period of
two years. This designation represents formal recognition by the provincial
government that a company has developed or acquired new technology of
significance, and triggers a number of government support and incentive
policies, including availability of land for expansion, research grants and
discounts on bank loan interest. The designation expired on July 6, 2007;
however, we have passed the re-examination and the new designation was received
on August 2007. Although we have not received any support from the government to
date, such a designation may be useful in obtaining incentives in the
future.
Our Yidu
Benda facility produces bulk chemical intermediates for raw material medicine
and does not require GMP certification. Yidu Benda was temporarily closed mid
January to upgrade its waste water treatment system to comply with new
environmental standards enforced by PRC local government. Yidu Benda has
completed its upgrading of the waste water system and passed the government’s
verification and testing of equipments in October 2007. It is now permitted for
the testing on actual production process. Once the actual products are produced,
then the environmental government bodies will re-test the production results.
The management could not estimate the exact timing for obtaining the final
approval on the actual production process. Furthermore, the management is
searching for new products to be produced in Yidu Benda which with higher profit
margin.
Our
Jiangling Benda facility was closed for renovation in July 2004 to comply with
GMP standards. The Jiangling Benda plant reopened on August 10, 2007 and has
been producing Ribose, the only product that does not require GMP approval.
Jiangling Benda also plans to produce three other types of active pharmaceutical
ingredients and they are Ribavirin, Asarin and Levofloxacin which need to have
GMP Certificate. On April 9, 2008, Jiangling Benda received the approved GMP
Certificate which authorizing the production of Ribavrin. The other two
products, Asarin and Levolfozacin, are still under the stage of GMP certificate
approving process. The management could not estimate the exact timing for
obtaining those certificates.
On
October 16, 2003, SiBiono successfully obtained a New Drug License from the
State Food & Drug Administration of China (SFDA), and then, in April 4,
2004, SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for
Pharmaceutical Product”, so far being fully qualified for the market launch of
Recombinant Human Ad-p53 Injection, trademarked as Gendicine ® in
China. Gendicine ® is the
commercialized gene therapy product approved in the PRC government agency. On
May 19, 2008, SiBiono received an official notice from the PRC State of SFDA in
which it mentioned that during the random inspection performed by the PRC State
of SFDA on April 8 to April 10, 2008, the PRC State of SFDA discovered there
were several production procedures that did not meet the requirement stated in
GMP, thus it required SiBiono to perform necessary improvements in order to
fulfill the GMP requirements and the PRC State of SFDA collected back the
distributed GMP certificate until the necessary improvements being carried out
and passed the examination that conducted by SFDA. On June 10, 2008, SiBiono
received another official notice from Guangdong Province SFDA and they demanded
the same requirements as stated in the official notice which issued by the PRC
State of SFDA dated on May 19, 2008. On November 24, 2008, SiBiono received
another official notice from Guangdong Province SFDA which mentioned that after
the examination conducted by Shenzhen City SFDA, the Guangdong Province SFDA
consent SiBiono to carry out production on a trial basis. It further required
SiBiono strictly to follow the requirements of GMP to organize trail production
and follow the procedures to apply for GMP Certificate verification. On July 14,
2009, SiBiono obtained the final approved GMP Certificate, in order words, the
SFDA allows SiBiono to resume its production and sales.
5
Good
Manufacturing Practices (“GMP”) is an internationally-recognized standard for
pharmaceutical plant design and construction. GMP has been defined as “that part
of quality assurance which ensures that products are consistently produced and
controlled to the quality standards appropriate for their intended use and as
required by the marketing authorization” (World Health Organization). GMP covers
all aspects of the manufacturing process: defined manufacturing process;
validated critical manufacturing steps; suitable premises, storage, transport;
qualified and trained production and quality control personnel; adequate
laboratory facilities; approved written procedures and instructions; records to
show all steps of defined procedures taken; full traceability of a product
through batch processing records and distribution records; and systems for
recall and investigation of complaints. The validity of the GMP approval lasts
for five years upon the issuing date and when the expiration date is
approaching, the manufacturing company needs to make an application for the
renewal.
We
distribute our high value, branded medicines, through agents who sell them to
hospitals that administer them to patients. We sell generics to medical
wholesalers for resale to hospitals. We sell our Over the Counter (“OTC”)
medicines to wholesalers specializing in selling to retail chain drug stores.
Our APIs are typically sold to large drug manufacturers under long-term supply
contracts. Our bulk chemicals are purchased by other Chinese drug
companies.
History
and Corporate Organization
Ever
Leader was incorporated in Hong Kong on October 29, 2005 for the purpose of
functioning as an off-shore holding company to obtain ownership interests in
various entities (collectively “Benda”) that were previously owned, either
directly or indirectly, by Mr. Yiqing Wan (“Wan”) and his wife, Ms. Wei Xu
(“Xu”).
The
following paragraphs summarize the original ownership structure of various
entities owned by Wan and Xu and the subsequent reorganization and transfer of
ownership interests in these entities, either directly or indirectly, to Ever
Leader.
Ownership
Structure Prior to Reorganization
Hubei
Benda Science and Technology Development Co., Ltd. (“Benda Science”) was
incorporated in the Province of Hubei, PRC in October of 2002, primarily
functioning as a holding company with ownership interests in various entities
operated by Wan and Xu. Wan and Xu are the sole owners of Benda Science, with
ownership interests of 10% and 90%, respectively.
Benda
Ebei was incorporated in the Province of Hubei, PRC in April of 2001. Benda Ebei
has registered capital of $2,419,404 which is fully paid up. Prior to the
reorganization of Benda as further described in the paragraphs below, Benda
Science, Wan, and Xu were the sole owners of Benda Ebei, with ownership
interests of 60%, 20%, and 20%, respectively. Benda Ebei develops, manufactures,
and sells small volume injection solutions (vials) and other conventional
medicines.
Jiangling
Benda was incorporated in the Province of Hubei, PRC in October of 2001.
Jiangling Benda has registered capital of $967,738 which is fully paid. Prior to
the reorganization of Benda, Benda Science and Wan were the sole owners of
Jiangling Benda, with ownership interests of 90% and 10%, respectively.
Jiangling Benda develops, manufactures and sells active pharmaceutical
ingredients (“APIs”). Jiangling Benda’s primary production facility was closed
for upgrades and renovations in July 2004 in order to secure a GMP certification
from the Chinese SFDA. The Jiangling Benda plant reopened on August 10, 2007 and
has been producing Ribose, the only product that does not require GMP approval.
Jiangling Benda also plans to produce three other types of active pharmaceutical
ingredients and they are Ribavirin, Asarin and Levofloxacin which need to have
GMP Certificate. On April 9, 2008, Jiangling Benda received the approved GMP
Certificate which authorizes the production of Ribavrin. The other two products, Asarin and Levolfozacin, are
still undergoing the GMP certificate approving process. The management cannot estimate the
exact timing for obtaining those
certificates.
6
Yidu
Benda was incorporated in the Province of Hubei, PRC in March of 2002. Yidu
Benda has registered capital of $4,233,854 which is fully paid. Prior to the
reorganization of Benda, Benda Science and Wan were the sole owners of Yidu
Benda, with ownership interests of 90% and 10%, respectively. Yidu Benda
develops, manufactures and sells bulk chemicals (or pharmaceutical
intermediates) for use in the production of APIs. The organization and ownership
structure of Benda prior to reorganization is as follows:
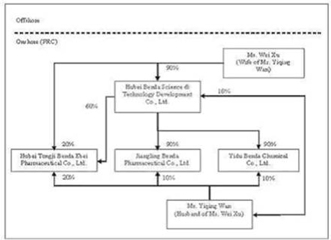
Reorganization
and Revised Ownership Structure
As
previously stated in the paragraphs above, Ever Leader was incorporated in Hong
Kong on October 29, 2005 for the purpose of functioning as an off-shore holding
company to obtain ownership interests in various Benda entities that were
previously owned, either directly or indirectly, by Wan and Xu. Ms. Mo Mo Hon
(“Hon”), a Hong Kong SAR resident, was the sole registered shareholder of Ever
Leader, holding the single issued and outstanding share of Ever Leader in trust
for Xu.
Pursuant
to three separate Equity Transfer Agreements entered into in November of 2005
among Ever Leader, Benda Science, Xu, and Wan, Ever Leader obtained a 95%
ownership interest in Benda Ebei in exchange for a commitment to pay $2,298,434
in aggregate consideration to Benda Science, Wan, and Xu. The $2,298,434
acquisition price represented 95% of the $2,419,404 of registered capital of
Benda Ebei, but was not representative of the fair value of the assets acquired
or liabilities assumed. Specifically, as transfers of ownership interests in PRC
entities to offshore holding companies for zero or nominal consideration is
prohibited by the Chinese Government (regardless of whether these PRC entities
and offshore holding companies are directly or indirectly owned and controlled
by the same individual or individuals), an amount equal to 95% of the value of
the registered capital of Benda Ebei was established for purposes of the
transfer of the 95% ownership interest in Benda Ebei (directly and indirectly
100% owned and controlled by Wan and Xu) to Ever Leader (beneficially 100% owned
and controlled by Xu). As a result of each of these entities being 100% directly
and indirectly controlled by Wan and Xu, this transaction has been accounted for
as a combination of entities under common control (see additional discussion of
accounting treatment in the paragraphs that follow), with Ever Leader’s
commitment to pay $2,298,434 in aggregate consideration to Benda Science, Wan
and Xu being reflected as a current liability at both December 31,2005 and 2004,
with corresponding reductions to paid-in capital.
7
Pursuant
to an Equity Transfer Agreement entered into on December 3, 2005 among Benda
Ebei, Benda Science, and Wan, Benda Science transferred and assigned its 90%
ownership interest in Jiangling Benda to Benda Ebei and Wan transferred and
assigned a 5% ownership interest in Jiangling Benda to Benda Ebei (for zero
consideration as Benda Ebei and Jiangling Benda were both directly and
indirectly 100% owned and controlled by Wan and Xu).
Pursuant
to a second Equity Transfer Agreement entered into on December 4, 2005 among
Benda Ebei, Benda Science, and Wan, Benda Science transferred and assigned its
90% ownership interest in Yidu Benda to Benda Ebei and Wan transferred and
assigned a 5% ownership interest in Yidu Benda to Benda Ebei (for zero
consideration as Benda Ebei and Yidu Benda were both directly and indirectly
100% owned and controlled by Wan and Xu).
The
organization and ownership structure of the Company subsequent to the
consummation of the reorganization as summarized in the paragraphs above is as
follows:
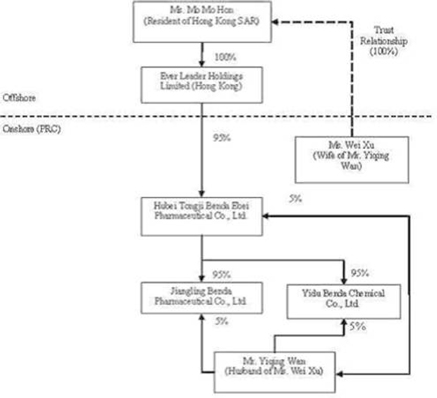
In July
of 2006, Benda Ebei invested approximately $112,500 for a 75% ownership interest
in Beijing Shusai, with the remaining 25% owned by an unrelated PRC individual.
Beijing Shusai, a PRC limited liability company, was incorporated on June 15,
2006 and commenced primary operations in July 2006. Benda Ebei is setting up
self-operated and franchised Pharyngitis Clinics in leading hospitals throughout
major cities in China. It is currently operating two clinics for the Pharyngitis
Killer therapy in Beijing, PRC.
8
On
September 5, 2006, Ever Leader increased its number of authorized shares of
common stock from 10,000 to 1,000,000 and effected a 100 to 1 stock split,
resulting in Hon (the original sole registered shareholder of Ever Leader
holding one share in trust for Xu) receiving 99 additional shares in the
Company.
On
September 5, 2006, Ever Leader transferred and assigned 711,202 shares of common
stock to Xia Pharmaceutical, Inc. (“XIA”), an offshore holding company
incorporated in the British Virgin Islands (“BVI”) that is 100% owned and
controlled by Wan and Xu.
On
September 5, 2006, Ever Leader issued 288,698 shares of common stock to 19
entities (some of whom are considered related parties) at par value.
Additionally, Hon transferred and assigned her ownership interest in her 100
shares of Ever Leader to one of these entities.
The
organization and ownership structure of the Company subsequent to the
consummation of the reorganization as summarized in the paragraphs above is as
follows:
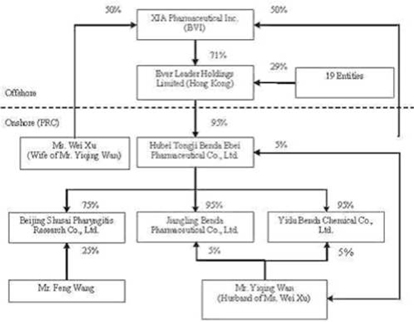
9
The
organizational chart after the acquisition of SiBiono would be stated as
follows:
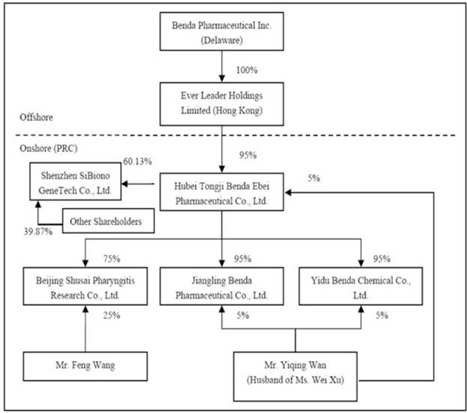
PRINCIPAL
PRODUCTS
In
2009, our
revenues were principally derived from sales of products listed in Figure
1. We have SFDA approval for all medicines and active
pharmaceutical ingredients that we market. Sales of herbal TCM’s and bulk
chemicals do not require SFDA approval. Our medicines have undergone
pharmacological experiments in order to research the medicines’ effect and
mechanism on organisms. On the other side, the experiments also research the
organisms’ effect on the medicine. It includes pharmacodynamics and
pharmacokinetics. Pharmacological experiments and clinical trials have
similarities. Clinical trials are part of pharmacological experiments. However,
pharmacological experiments mainly use animals as research subjects, while
clinical trials generally utilize patients as research subjects. Pharmacological
experiments are carried out by regulations of non-clinical medicine research and
quality control issued by SFDA.
Main
Products
|
Manufacturer
|
Product
|
Type
|
Function
|
|||
|
Benda
Ebei
|
Jixuening
injection vial
|
Generic
|
Haemostatic
(stops bleeding)
|
|||
|
Benda
Ebei
|
Xujing
injection vial
|
Generic
|
Haemostatic
|
|||
|
Benda
Ebei
|
Nokeqing
injection vial
|
Generic
|
Used
to treat hepatitis
|
|||
|
Benda
Ebei
|
Yidingshu
injection vial
|
Generic
|
Vitamin
to treat lack of Riboflavin
|
|||
|
Benda
Ebei
|
Shusai-A
injection vial
|
Generic
|
Anti-inflammatory
analgesic
|
|||
|
Benda
Ebei
|
Suzheng-B
injection vial
|
Generic
|
Vitamin;
complementary medicine used to treat hepatitis
|
|||
|
Benda
Ebei
|
Ribavirin
injection vial利巴韦林
|
Generic
|
Anti-virus,
to treat acute upper respiratory tract infection
|
|||
|
Benda
Ebei
|
Gentamycin
Sulfate Injection vial
|
Generic
|
Broad
spectrum antibiotic
|
|||
|
Benda
Ebei
|
Vitamin
B6 injection vial
|
Generic
|
Vitamin;
complementary medicine used to treat hepatitis
|
|||
|
Benda
Ebei
|
Inosine
injection vial
|
Generic
|
Nutrition,
complementary medicine used to treat hepatitis
|
|||
|
Benda
Ebei
|
Vitamin
C injection vial
|
Generic
|
To
treat deficiency of vitamin C
|
|||
|
Jiangling
Benda
|
Ribavirin
API (1)
|
API
|
Ribavirin
drug manufacture.
|
|||
|
Jiangling
Benda
|
Asarin
API (1)
|
API
|
Asarin
manufacture to treat acute upper respiratory system
infection
|
|||
|
Jiangling
Benda
|
Levofloxacin
Mesylate API (1)
|
API
|
Broad
spectrum antibiotic drug manufacture
|
|||
|
Jiangling
Benda
|
Ribose
|
API
|
Used
to manufacture antibiotic drug
|
|||
|
Yidu
Benda
|
Triazol
carboxylic acid methyl ester (“TCA”)
|
Bulk chemical
|
Ribavirin
manufacture, anti-virus
|
|||
|
SiBiono
|
Gendicine
|
Gene
Therapy
|
Treatment
of
cancer
|
10
|
(1)
|
Jiangling
Benda facility was closed for renovation in July 2004 to comply with GMP
standards. The Jiangling Benda plant reopened on August 10, 2007 and has
been producing Ribose, the only product that does not require GMP
approval. Jiangling Benda also plans to produce three other types of
active pharmaceutical ingredients and they are Ribavirin, Asarin and
Levofloxacin which need to have GMP Certificate. On April 9,
2008, Jiangling Benda received the approved GMP Certificate which
authorizes the production of Ribavrin. The
other two products, Asarin and Levolfozacin, are still undergoing
the GMP
certificate approving process.
The management cannot
estimate the exact timing for obtaining those
certificates.
|
SFDA
Compared to the FDA
The SFDA
approval process is similar to the FDA approval process. They both require three
phases of clinical trials.
|
·
|
Phase
I: Test the safety of drugs. 20-80 cases are required by FDA, while 20-30
cases are required by SFDA.
|
|
·
|
Phase
II: Test the efficacy of drugs. Several hundred cases are required by FDA,
while 100 cases are required by
SFDA.
|
11
|
·
|
Phase
III: Expand the sample group and further test the safety and effectiveness
of drugs. Several hundreds, even thousands are required by FDA, while 300
hundred cases are required by SFDA.
|
Before a
drug can be sold in China, the drug needs to undergo the above three phases,
namely pre-clinical trial, clinical trial and finally, GMP approval. The
approval of a drug by SFDA does not guarantee the approval by FDA. However,
drugs approved by SFDA can be exempted from certain steps in US clinical trials
before applying for FDA.
Benda Ebei
Products
Of our
branded medicines, the Shusai-A Nefopam Hydrochloride solution, sold in
injection vials, is particularly noteworthy. According to a pharmacological
experiment, Nefopam Hydrochloride has an analgesic effect 10.4 times greater
than that of aspirin. Furthermore, it is not addictive and causes no known side
effects.
Pharmacological
experiments research the medicines’ effect and mechanism on organisms. On the
other side, the experiments also research the organisms’ effect on the medicine.
It includes pharmacodynamics and pharmacokinetics. Pharmacological experiments
and clinical trials have similarities. Clinical trials are part of
pharmacological experiments. However, pharmacological experiments mainly use
animals as research subjects, while clinical trials generally utilize patients
as research subjects. Pharmacological experiments are carried out by regulations
of non-clinical medicine research and quality control issued by
SFDA.
The
chemical name of this product is Nefopam hydrochloride. It mainly contains:
5-Methyl-1-Phenyl-3,4,5,6-Tetrahydrocannabinol-1H-2,5-benzoxazocine, fenazoxine
hydrochloride. It is a new type of Non-narcotic analgesics which has the
function of low-grade Antipyretic and muscle relaxants. Its chemical structure
belongs to O-methyl benzene ring of diphenhydramine, so it does not have the
attribute of Non-steroidal anti-inflammatory drugs, nor Opioid receptor agonist.
It is effective for middle-grade and heavy-grade pain. Intramuscular injecting
20mg of Nefopam hydrochloride equals to intramuscular injecting 12mg morphine.
It has light effectiveness on respiration inhibition. It has no inhibition on
circulatory system. It has no tolerance or dependence. It can be rapidly oral
absorbed, Tmax 1-3 hours, and it has obvious effect while first pass. T1/2 4-8
hours, the binding rate of plasma protein is 71%-76%. It is metabolized by liver
to lose its pharmacological activities. Most of it will be excreted through
kidney. The prototype drug will be less than 5% and only a little will be
excreted along with excretion. It is used for pain-killing after operation,
cancer pain, and acute pain. It is also used for visceral smooth muscle cramps
such as acute gastritis, in-biliary ascariasis, ureterolithiasis.
The
clinical trials were conducted in 1993, instructed by Doctor Guozhong, Peng.
Ebei plant paid for all clinical trial expenses. It passed the clinical trials
in 6 clinical institutes including the provincial hospital of Hebei, the second
and the forth hospital attached Hebei School of Medicine, Bethune international
peace hospital and etc. The efficacy rate is around 80% for all 374 cases
examined. There is no follow up results.
Generics
are common, low-cost, medicines used by doctors in hospitals nationwide. Our
generics have been marketed for more than 10 years and are generally used by
low-income patients in rural and country districts. The profit margins for our
generics, which constitute about 3 per cent of Benda Ebei’s current sales
volume, are lower than those of our branded products. However, our generic
products are an effective means for promoting our corporate name, image and
brands nationwide.
Jiangling Benda
Products
Jiangling
Benda produces and plans to produce four types of active pharmaceutical
ingredients and they are Ribavirin, Asarin, Levofloxacin and Ribose whereas the
production of Ribose does not require the GMP certificate, but the production of
the other three products do require the GMP certificate.
12
|
·
|
Ribavirin
has been used to produce antivirus medicine to treat SARS and SARS-like
illnesses. Ribavirin is also used to treat severe virus pneumonia in
infants and young children and a viral liver infection known as hepatitis
C. It can be used in patients who have hepatitis C or human
immunodeficiency virus (“HIV”) infection. Alliance Pharm, Inc. is advising
us on modifications to our production processes in our effort to achieve
U.S. FDA certification. Currently, there is only one other pharmaceutical
company in PRC that has received U.S. FDA certification to produce
Ribavirin API. On April 9, 2008, Jiangling Benda received the
approved GMP Certificate from the SFDA which authorizing the production of
Ribavrin.
|
|
·
|
Asarin
is used to treat infections of the upper respiratory system. Our Asarin
API is synthesized chemically rather than being extracted from natural raw
materials, making it a cost effective and price competitive product.
Benda’s Asarin received SFDA approval as a new API on December 27, 2005.
We plan to extend our reach further down the value chain and manufacture
consumer-ready Asarin medicines, in injection, vial and pill form, from
our Asarin API. We have already filed for SFDA approval for these three
types of finished Asarin
products.
|
|
·
|
Levofloxacin
Mesylate is a synthetic broad spectrum antibacterial agent for oral and
intravenous administration. Benda’s Levofloxacin received SFDA approval as
new drug ingredient on March 5,
2006.
|
|
·
|
Ribose
is a kind of active pharmaceutical ingredient and which is used to
manufacture antibiotic drug and which does not the approval from
SFDA.
|
Yidu Benda
Product
Yidu
plans to produce one type of bulk chemicals:
|
·
|
Triazol
carboxylic acid methyl ester (“TCA”). This is our main bulk chemical
product.
|
Due to a
government order issued by the local government on January 10, 2007, our Yidu
Benda plant has been shut down since the middle of January 2007 for improvement
of our waste water treatment systems. The order requires us to finish the
improvement and be compliant by June 30, 2007. On September 25, and October 1,
2007, Yidu Benda had passed the environmental assessment and safety assessment
by the Yichang Environmental Protection Bureau and Yichang Safety Supervision
Bureau These two bureaus have issued “Environmental Influence Report” and
“Safety Assessment Report” in November and December of 2007, respectively.
Yichang Environmental Protection Bureau issued an approval document, (Document
Number: Yichang Environmental Audit [2007] No. 111) and permitted the trial
production of Yidu Benda on December 28, 2007, at which time Yidu Benda resumed
full production. The related government agencies through trial production
physically inspect the products produced by the newly installed production
facilities. The main purpose for doing so is to ensure that the quality of the
products is such that there will be no harm to the environment. In addition,
Yidu Benda had also passed the examinations conducted by Yichang Public Security
Bureau, Yichang Lightning Protection Institute and Yichang Special Equipment
Inspection and Test Institute, in terms of the fire apparatus and facilities,
lightning protection and static proof facilities etc. It is now permitted for
the testing on actual production process. Once the actual products are produced,
then the environmental government bodies will re-test the production results.
The
management could not estimate the exact timing for obtaining the final
approval on
the actual production process. Furthermore, the management is searching for
new
products to
be produced in
Yidu Benda which with higher profit margin.
SiBiono
Product
SiBiono
is a gene therapy company dedicated to the development, manufacturing and
commercialization of gene therapy products. As a pioneer in gene therapy in
China, SiBiono's mission is to develop innovative gene therapy products for the
improvement of human health and life quality. The Company has developed two core
technology platforms: Viral Vector Gene Delivery System and Non-Viral Vector
Gene Delivery System focusing on development of gene therapy product for cancer
and cardiovascular diseases.
On
October 16, 2003, SiBiono successfully obtained a New Drug License from the
State Food & Drug Administration of China (SFDA), and then, in April 4,
2004, SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for
Pharmaceutical Product”, so far being fully qualified for the market launch of
Recombinant Human Ad-p53 Injection, trademarked as Gendicine
® in China. Gendicine ® is the
first ever commercialized gene therapy product approved in the world by a
government agency. Gendicine is recognized by the world's first class journals
as a major milestone in the field of gene research and biotechnology and is
expect to make important contribution to mankind's endeavor for improving human
health.
13
MARKETING
AND DISTRIBUTION METHODS OF PRODUCTS AND SERVICES
Prescription
Medicines
Two types
of distribution channels exist in the Chinese medicine industry.
For high
value branded medicines: Pharmaceutical Manufacturers à Agents à Sub Agents à Medicine
Representatives à
Hospitals or Pharmacies à Patients
For low
value generics: Pharmaceutical Manufacturers à Wholesalers à Secondary
Wholesalers à
Hospitals or Pharmacies à Patients
The major
difference between an agent and a wholesaler is that the agent has exclusive
product sales rights from each manufacturer in each region, which is generally a
province. Sometimes manufacturers have several wholesalers in a
region.
The table
below illustrates price markups along the distribution channel for a typical
Benda Ebei branded drug, Shusai-A.
Shusai-A
Price Markup Pattern
|
Purchase
Price per
piece in
RMB
|
Price
Markup
|
|||||||
|
Patients
|
40.00 | 54 | % | |||||
|
Retail/Hospitals
|
26.00 | 622 | % | |||||
|
Medicine Reps
|
[3.60 | ] | 29 | % | ||||
|
Sub-agents
|
2.80 | 56 | % | |||||
|
Agents
|
1.80 | |||||||
|
Benda
Ebei
|
n/a | |||||||
The
highest price markup along the distribution channel is on sales by medicine reps
to hospitals because such markups finance kick-backs paid by the reps to
doctors. This unfortunate, but common, practice is condemned by PRC’s patients
and medical industry regulators, but no effective method has been found to stamp
it out. In reality, there are “kick-backs” phenomena which are paid by the reps
to doctors, not by our company to doctors. However, in the normal course of
business, sales commission would be incurred between our company and clients,
such as agents or wholesalers etc., in order to have a sense of incentive to
them. This sales commission scheme is fully disclosed in the sales contracts and
is also allowed by the relevant PRC regulations.
Benda
Ebei sells its products to agents or wholesalers. This method minimizes the need
for a direct sales force and distances Benda Ebei from questionable kick-backs
and potential legal consequences.
Active
Pharmaceutical Ingredients (“APIs”)
Our APIs
are purchased by other Chinese drug companies on an order-by-order basis. The
domestic industry is tight-knit and API marketing still relies on word-of-mouth,
reputation, and personal contacts. Although we have temporarily closed the
Jiangling plant to complete renovation and obtain GMP certification, we have
maintained relationships with all our former clients and expect to bring on
other drug companies as new customers.
Jiangling
Plant is primarily engaged in producing Ribose, a bulk chemical which does not
require GMP approval and Active Pharmaceutical Ingredients (API) which need GMP
approval.
Jiangling
Benda was re-opened in August 2007. The products that are planned to be produced
in Jiangling Benda are as follows:
a)
Ribavirin API (Anti-virus)
b) Asarin
API (Antibiotic)
c)
Levofloxacin (Antibiotic)
d)
Ribose
These
four products are classified as API whereas the production of Ribavirin, Asarin
and Levofloxacin need to obtain GMP approval, however the production of Ribose
does not need the GMP approval. Currently, Jiangling Benda only produces Ribose
and which is a kind of API.
14
Bulk
chemicals
We market
our bulk chemicals by cultivating strong, long-term relationships with loyal
customers. We usually supply customers pursuant to annual renewable contracts.
Customers usually start by buying small quantities and gradually increasing
order sizes. We also enjoy long-standing relationships with a number of
important exporters. These sales contracts are signed annually.
Beijing
Shusai Pharyngitis Research Co., Ltd.
Beijing
Shusai Pharyngitis Research Co., Ltd, a recently established subsidiary of Benda
Ebei, handles our Pharyngitis Killer therapy operation, promotion, and
distribution. Key functional departments are as follows:
|
·
|
Training.
Trains doctors, doctor assistants, and medical
workers.
|
|
·
|
Advisory.
Advises each clinic on how best to apply the Pharyngitis Killer
treatment.
|
|
·
|
Business
Development. Extend the footprint of Pharyngitis clinics and implement
patient outreach programs.
|
|
·
|
Marketing.
Formulate and execute marketing
plans.
|
|
·
|
Finance.
Provide internal financial services to support business
operations.
|
|
·
|
Logistics.
Ensure no bottlenecks or shortages in product supply to the
clinics.
|
Special
Marketing Initiatives
|
(1)
|
Qiweiben Capsule
Initiative. Benda Ebei intends to develop a series of products
based on the Qiweiben Capsule and designed to treat diabetes. They will be
sold through diabetes recovery centers and regional
distributors.
|
|
(2)
|
Jixuening Initiative.
We plan to develop a group of haemostatic medicines based on our core
Jixuening brand. Benda Ebei’s Jixuening has been listed in the Catalog of Basic Medicines Covered
by Social Medical
Security.
|
|
(3)
|
Analgesic Initiative.
The treatment of pain attracts more attention from PRC’s medical community
and hospitals around the country that are setting up pain clinics. Our
Shusai-A and Lappaconitine Hydrobromide products are uniquely powerful
pain killers and are not addictive. We plan to leverage their popularity
to promote our other pain killers and thereby build a series of pain
killer medicines.
|
15
|
(4)
|
Asarin Initiative. We
intend to form a group of medicines, based on Asarin, which will be
designed to cure upper respiratory tract
infection.
|
STATUS
OF PUBLICLY ANNOUNCED NEW
PRODUCTS/SERVICES
We expect
that the following products in our development pipeline will generate growth in
our revenues in the next few years. We expect to begin mass production for
products once we receive SFDA’s approval.
The
registration of a medicine must first be undertaken at the provincial level.
First, you need to make an application in the provincial Food and Drug
administration, then you must pass the on-the-spot examination of the provincial
registration office and the initial inspection of information experts. After
that, you can apply for the experts’ technological evaluation of National
Medicine Analysis and Judgment center. Only after you received the technological
evaluation, your registration can be transferred to SFDA to have administrative
ratification. After the ratification, you will be issued new license and
ratification number. In most situations, after the application and record of
provincial Food and Drug administration, the company’s products will not be
examined again by National Medicine Analysis and Judgment center, which is
similar to the first step of technological check.
Due to
the restructuring of SFDA, the process of approval new drug has been on hold
since the beginning of 2007. We do not know how long it will take to resume the
approval process.
The
Company can only obtain the license for the new medicine and the production
license after the completion of the clinical experiments. The procedures of
obtaining new drug certificate with the SFDA are as follows:
1. Submit
the application to the Province SFDA;
2. Then
the Province SFDA will perform the physical inspection;
3. If we
pass the physical inspection, the related application would be transferred to
the State SFDA;
4. After
ratification, the new drug license and ratification number would be
issued.
Development
Status of Key Products in Our Pipeline
|
Name of Product
|
Type
|
Main Function
|
Status
|
|||
|
Pharyngitis
Killer
|
Herbal
TCM Oral Liquid and Treatment
|
Anti-respiratory
tract infections
|
Market
launch underway; SFDA Certificate not necessary
|
|||
|
Qiweiben
Capsule
|
Branded
TCM
|
Diabetes
treatment
|
New
Medicine Application is accepted by SFDA; awaiting for SFDA approval and
production permit。
|
|||
|
Yan
Long Anti-cancer Oral Liquid
|
Branded
TCM
|
Treatment
of cancers of the digestive tract
|
Awaiting
for SFDA approval (1)
|
|||
|
500mg:5ml
Tranexamic Acid Injection vial
|
Generic
|
Haemostatic
|
SFDA
production approval H20044601 received; Put in production
line.
|
|||
|
200mg:2ml
Ribavirin Injection vial
|
Generic
|
Antibiotic
|
SFDA
production approval H42021048 received; Put in production
line.
|
16
|
1000mg:2.5mlVitamin
C Injection vial
|
Generic
|
Vitamin
|
Achieved
State acceptance and hearing Y0405945; SFDA production approval H20067577
received; Put in production line.
|
|||
|
0.1g:2ml
Lomefloxacin Aspartate Injection vial
|
Generic
|
Antibiotic
|
SFDA
production approval H20056701 received; Put in production
line.
|
|||
|
0.2g:5ml
Lomefloxacin Aspartate Injection vial
|
Generic
|
Antibiotic
|
SFDA
production approval H20056702 received; Put in production
line.
|
|||
|
Lappaconitine
Hydrobromide Injection vial
|
Branded
Medicine
|
Analgesic
|
SFDA
production approval H20055966 received; Put in production
line.
|
|||
|
Asarin
Injection vial
|
Generic
|
Treatment
of upper respiratory infection
|
Filing
completed at provincial bureau level; filed with SFDA in May
2006
|
|||
|
Asarin
pill
|
Generic
|
Treatment
of upper respiratory infection
|
Filing
completed at provincial bureau level; filed with SFDA in August 2006; Need
further bio-clinical trial.
|
|||
|
Asarin
granular medicine
|
Generic
|
Treatment
of upper respiratory infection
|
Filing
completed at provincial bureau level; filing with SFDA in September
2006
|
|||
|
Asarin
oral liquid
|
Generic
|
Treatment
of upper respiratory infection
|
Filing
completed at provincial bureau level; filing with SFDA in September 2006;
Need further bio-clinical trial.
|
|||
|
Lysine
Hydrochloride Injection vial
|
Generic
|
Amino
acid
|
Filing
completed at provincial bureau level; filed with SFDA in July 2006; Need
further bio-clinical trial.
|
|||
|
Arginine
Monohydrochloride Injection vial
|
Generic
|
Amino
acid
|
Filing
completed at provincial bureau level; filed with SFDA in July 2006; Need
further bio-clinical trial.
|
|||
|
100mg:5ml
Levofloxacin Hydrochloride Injection vial
|
Generic
|
Antibiotic
|
Filing
completed at provincial bureau level; filed with SFDA in July 2006; Need
further bio-clinical trial.
|
|||
|
500mg:5ml
Levofloxacin Hydrochloride Injection vial
|
Generic
|
Antibiotic
|
Filing
completed at provincial bureau level; filed with SFDA in July 2006; Need
further bio-clinical trial.
|
|||
|
a-Asarin raw
medicines
|
API
|
Upper
respiratory infection
|
SFDA
production approval H20059540 received; prepare for
production.
|
|||
|
Levofloxacin
mesylate API
|
API
|
Antivirus
|
Filing
completed at provincial bureau level; SFDA approval received; prepare for
production.
|
|||
|
GCLE
|
Bulk
chemical
|
Production
of Antibiotics
|
As
it is a chemical product, it does not need SFDA
approval.
|
17
(1)
Yanlong oral solution has finished its earlier stage of research, and the
Company is in the process of applying for SFDA’s permission of clinical trials.
The new drug certificate will only be issued after the clinical
trials.
New
Branded Medicines
Our
upcoming branded medicines include Qiweiben capsule and Yanlong anti-cancer oral
liquid, which are proprietary traditional Chinese medicines, and Pharyngitis
Killer Therapy, which is a combination of herb-based traditional Chinese
medicine and treatments. We expect these products to have a significant positive
impact on our future operational results.
Our New Branded Traditional Chinese
Medicines
|
Features
|
Pharyngitis
Killer
|
Qiweiben
Capsule
|
Yan
Long Anti-cancer Oral Liquid
|
|||
|
Targeted IP/ Formula Protection Period (1)
|
Not Applicable (2)
|
7+7 years (7)
|
7+7 years (7)
|
|||
|
Our Ownership
|
75% (3)
|
100%
|
100% with
reservation (4)
|
|||
|
Completion Date Of Clinical
Tests
|
Not Applicable
|
Pening (6)
|
Pending
(6)
|
|||
|
New Medicine Certification
Date
|
Not Applicable
|
Pening (6)
|
Pending (6)
|
|||
|
Expected installation of GMP quality production
line (5)
|
Not Applicable
|
Pending (6)
|
Pending (6)
|
|||
|
Expected SFDA Production
Certification
|
Not Applicable
|
Pending (6)
|
Pending (6)
|
|||
|
Expected Commencement of
Production
|
Launched June 2006
|
Pending (6)
|
Pending
(6)
|
(1) The
first protection period will commence when the proprietary TCM protection
application is approved. The second protection period can be applied for when
the first protection period is expired.
(2) TCM
therapies do not require SFDA approval. We chose not to apply for patent
protection for this product’s formula due to our concerns about disclosures
required in the patent application process.
(3) Benda
owns 75% of Beijing Shusai Pharyngitis Research Co., Ltd., a company that owns
all product and market exploitation rights to Pharyngitis Killer
Therapy.
(4) The
inventor of Yanlong Anti-cancer oral liquid, Mr. Yan Li, has reserved the right
to sell the product to one hospital in Hong Kong, one hospital in Taiwan and one
hospital in Shenzhen province.
(5) GMP
certification is expected once the facility installation for the products below
referenced products and examination by the related local government agency is
completed.
18
In order
to produce
Qiwweiben, the following table shows the production facilities are
purchased and installed, and they are all located in Benda Ebei and waiting for
the government agency for inspection:
|
No.
|
|
Equipment
|
|
Model No.
|
|
Quantity
|
|
1
|
Muller
|
300A
|
2
|
|||
|
2
|
powder
shifter
|
ZS-350
|
2
|
|||
|
3
|
powder
shifter
|
XS-650
|
1
|
|||
|
4
|
3D
mixer blender
|
HD-1000
|
1
|
|||
|
5
|
Ultrasonic
spray drier
|
FL-120
|
1
|
|||
|
6
|
Wet
mixer granulator
|
GHL-250
|
1
|
|||
|
7
|
Slot
shape Mixer
|
CHL-150
|
2
|
|||
|
8
|
granule
drier
|
TC-Z-Ⅱ
|
1
|
|||
|
9
|
Capsule
filling machine
|
NJP-1200
|
1
|
|||
|
10
|
Capsule
polishing machine
|
YPJ-Ⅱ
|
1
|
|||
|
11
|
fast
packing machine
|
DPH-250D
|
1
|
In order
to produce Yanlong Anti-cancer Oral Liquid, the following table shows the
production facilities are purchased and installed, and they are all located in
Benda Ebei and waiting for the government agency for inspection:
|
No.
|
Equipment
|
Model No.
|
Category
|
|||
|
1
|
Solution
Preparation Reservoir
|
PLG-1.0
|
||||
|
2
|
Fluid
Storage Reservoir
|
ZYG-1.0
|
||||
|
3
|
High
Level Reservoir
|
N/A,
general machinery
|
Liquefy
|
|||
|
4
|
Micro-filters
|
N/A,
general machinery
|
System
|
|||
|
5
|
Filters
for Sugar Syrup
|
N/A,
general machinery
|
||||
|
6
|
Sugar
Dissolving Reservoir
|
HTG-0.5
|
||||
|
7
|
Bottle
Filling Machine
|
QCX60
|
||||
|
8
|
Sterilization
System
|
AQS1.2
|
Bottling
|
|||
|
9
|
Labeling
Machine
|
TWJA
|
System
|
|||
|
10
|
Light
Examine Workstations
|
N/A
|
(6) Due
to recent restructuring within SFDA, the approval process for new medicines has
been ceased since November 2006, except for the first class new medicines which
are drugs that have never been sold or appeared in the market in China. The
management expects that such situation would still last for an indefinite
period.
(7)
According to the relevant Chinese patent drug policies of SFDA, all national
protective Chinese patent drugs can apply for a seven-year protective period.
During the period, other pharmaceutical enterprises cannot produce imitation
products. After the first seven years, the company can apply for another seven
years’ protective period.
Pharyngitis Killer Therapy
(Anti-Respiratory Tract Infections)
Product Description: This is
an entirely natural Chinese medicine and treatment containing no hormones or
antibiotics, which cures a wide range of upper respiratory tract infections,
ranging from the common cough to more advanced respiratory illnesses, such as
acute and chronic pharyngitis, tracheitis and bronchitis which has already been
proven by clinical trails which are conducted by the company together with the
co-operated hospitals.
The
clinical trials were conducted by the company together with co-operated
hospitals which was based on the sample size 50 chronic respiratory patients who
either had the symptom pharyngoxerosis throat itch, chest distress and feeling
suffocated or oppressed, throat swelling and sore or vocal cord weary and
hoarseness. The medical products used as a comparison were Pharyngitis Killer
Therapy (in form of herb drink) alone and chronic comprehensive
therapy.
We
believe the oral liquid taken alone has a success rate of up to 80% in treating
chronic pharyngitis; however, if accompanied by further treatment, success rates
of up to 98% can be achieved.
19
This
medicine, in the form of herbal drink, was developed and kept secret over many
generations by the Wang family of Beijing. The formula and treatment was
initially invented in 682 A.D. by Wang Zhaojing, a famous doctor in Chinese
medical history. We believe the oral liquid taken alone has a success rate of up
to 80% in treating chronic pharyngitis; however, if accompanied by further
treatment, success rates of up to 98% can be achieved.
However,
the above data was only conducted by the company together with co-operated
hospitals which was based on the sample size 50 chronic respiratory patients. In
order words, such clinical trials were not performed by the formal requirement
of SFDA.
Our
Pharyngitis Killer therapy:
|
·
|
Swiftly
smoothes away throat itching and
coughing
|
|
·
|
Controls
the development of disease in the mucus of the
mouth
|
|
·
|
Rebuilds
the immune function of the throat
area
|
|
·
|
Provides
a natural therapy, which is without side effects and free of
antibiotics
|
Market Outlook: With the
recent urbanization and industrialization of PRC, pollution has become a big
problem. Air pollution in particular causes several diseases of the pharynx.
Researchers believe that approximately 10% of the people who live in cities
suffer from pharynx disease, while in some rural areas, the ratio is a lower,
though still significant, 5.5%. Until now treatments for upper respiratory tract
infections have relied on antibiotics to control acute pharyngitis temporarily.
However, no medicine in the market has thus far been effective at treating
chronic pharyngitis. Pharyngitis Killer therapy overcomes the deficiencies of
other treatments and eradicates the disease.
Purchase: The operating
company for the Pharyngitis Killer therapy is Beijing Shusai. The total
registered capital is 1.2 million RMB. As part of the joint venture, Mr. Wang
contributed the Pharyngitis Killer formula and treatment as intangible assets,
valued at 300,000 RMB, in return for 25% of Benda Shusai’s equity. Benda Ebei
invested 900,000 RMB in cash for 75% equity.
IP protection/ technology
confidentiality: Pharyngitis Killer is a traditional Chinese medicine and
as such does not require SFDA approval. Benda Ebei has chosen not to file for
patent protection on the formula in order to avoid disclosing the product
formula. It believes that the formula and treatment are effectively protected
by: (a) the separation of auxiliary ingredients with a proprietary catalyst
powder called Yao Yin; (b) a proprietary production method for the oral liquid;
and (3) by proprietary treatment techniques. We plan to apply for patent
protection for both the processing method used to make the oral liquid and the
treatment method applied on patients.
Promotion: We plan to market
Pharyngitis Killer as a stand alone oral liquid medicine that can be combined
with surgery-like treatment to achieve results. In August 2006, the PRC State
Administration of Traditional Chinese Medicine (“SATCM”) issued an official
promotional document to provincial SATCM’s, in which Pharyngitis Killer therapy
is categorized as a recommended TCM and treatment. This official promotional
document was issued on August 18, 2006 based on an expert panel review of
Pharyngitis Killer conducted in August 2006.
Marketing and Distribution:
We are setting up self-operated and franchised Pharyngitis Clinics. Benda Ebei
currently has two clinics in full operation in Beijing. Benda Ebei has prepared
advertising and marketing campaigns to support the rapid expansion of the
franchise, which will begin after the closing of this offering. Our
self-operated clinics will be located inside hospitals. A proportion of the
gross revenues will be paid to the host hospitals in lieu of rent and as
compensation for any marketing efforts made on our behalf. Franchised
Pharyngitis Killer clinics will be operated by franchisees, which will buy the
medicine from us and pay us a franchising fee. We plan to open franchising
clinics only at the beginning of the roll-out period in order to accelerate the
revenue growth and minimize start-up costs. At a later stage, we plan to open
self operated clinics only because we expect that they will be comparatively
more profitable and easier to manage.
20
Status of
Planned Pharyngitis Clinics
Due to
the restructuring of SFDA, the related policies of SFDA have changed such that
the local hospitals are not allowed to co-operate with third parties. These
changes have caused us to discontinue our plans of setting of Pharyngitis
Clinics. The
management of the company is seeking and considering other alternatives to
launch this particular drug, however up to now there is no definite
plan yet.
Qiweiben Capsule (alleviates
symptoms of diabetes)
Product Description: Benda
Ebei has the exclusive right to produce a new, effective, herbal medicine
against diabetes, which was developed in cooperation with Shandong Haiyang
Biotech Co., Ltd. (“Haiyang”). Qiweiben capsule is a medicine used to reduce
symptoms of patients suffering from type II diabetes and improve their sexual
ability. It contains 7 active pharmaceutical ingredients (“APIs”) extracted from
animals and plants; its major API is an extract from virgin male silk moths.
This medicine has the following key benefits:
|
(1)
|
Reduces
blood glucose.
|
|
(2)
|
Cures
erectile dysfunction and reduces sexual dysfunction caused by diabetes.
The Qiweiben capsule is unique amongst diabetic medicines in this
regard.
|
|
(3)
|
As
it is a complementary medicine based on natural ingredients, the patient
reduces intake of chemical
medicines.
|
Market Outlook: Diabetes has
become a common and frequently occurring disease that threatens the health of an
increasing portion of the population. According to the 5 th
International Diabetes Union conference held in Beijing in 2002, there are 130
million type II diabetes patients around the world, over 40 million of which are
in PRC. In the 21 st
century, type II diabetes is expected to be epidemic in developing countries
such as PRC and India. Such a large patient group provides a significant market
opportunity for Qiweiben Capsule. Artificial insulin is currently the most
widely used medicine for the treatment of diabetes. Patients using artificial
insulin can become addicted to it and also suffer the discomfort of needle
injections. These disadvantages can be reduced by using our Qiweiben capsule as
a complement to artificial insulin. Pilot studies conducted by Haiyang in 2001
have shown that the Qiweiben capsule significantly reduces one or more symptoms
of diabetes in 88% of cases.
Rights Purchase: Qiweiben is
the brand name of this medicine. The registered drug name is Qiwei Xiaoke
capsule. The SFDA production permit (SFDA Z200110150) was held by Shandong Leaf
Pharmaceutical Co., Ltd. On March 14, 2004, Benda Ebei agreed to pay Haiyang RMB
5 million ($625,000) for the technology required for the extraction of virgin
male silk moth essence, the SFDA production permit (SFDA Z200110150) and the New
Medicine Certificate (SFDA Z20010134). The purchase agreement provided for the
immediate transfer of the production permit and the deferred payment of the RMB
5 million ($625,000) within three years after Benda Ebei begins to sell the
product.
IP protection/ technology
confidentiality: Benda Ebei will apply for TCM protection for Qiweiben
and expects a reply from the SFDA no later than 6 months thereafter. According
to new regulations, protected TCM status is granted for 7 years from the
registration date, during which period no other party may produce it.
Thereafter, a further 7 year protection may be granted to the right holder
depending on factors such as the effectiveness, production standards, quality
and safety of the product. Benda Ebei also has pending patents for the
protection of technological and manufacturing processes used to produce this
medicine.
21
Production: We have been
building Qiweiben production facilities. In order to produce qiwweiben, the
following table shows the production facilities or equipment which have been
purchased and installed. The equipment are all located in Benda Ebei and waiting
for the government agency for inspection:
|
No.
|
|
Equipment
|
|
Model No.
|
|
Quantity
|
|
1
|
Muller
|
300A
|
2
|
|||
|
2
|
powder
shifter
|
ZS-350
|
2
|
|||
|
3
|
powder
shifter
|
XS-650
|
1
|
|||
|
4
|
3D
mixer blender
|
HD-1000
|
1
|
|||
|
5
|
Ultrasonic
spray drier
|
FL-120
|
1
|
|||
|
6
|
Wet
mixer granulator
|
GHL-250
|
1
|
|||
|
7
|
Slot
shape Mixer
|
CHL-150
|
2
|
|||
|
8
|
granule
drier
|
TC-Z-Ⅱ
|
1
|
|||
|
9
|
Capsule
filling machine
|
NJP-1200
|
1
|
|||
|
10
|
Capsule
polishing machine
|
YPJ-Ⅱ
|
1
|
|||
|
11
|
fast
packing machine
|
DPH-250D
|
1
|
We intend
to start manufacturing and marketing the product immediately after receiving GMP
certification. However, due to recent restructuring within SFDA, the approval
process for new medicines has been ceased since November 2006, except for the
first class new medicines. The management expects that such situation would
still last for an indefinite period.
Clinical
Experiments:
Qi Wei
Xiao Ke capsule is a Chinese traditional medicine capsule whose main raw
material is male Sangcan moth of no copulation. Biochemistry engineering
technique is used to extract its useful components, which are produced to
traditional Chinese medicine capsule preparation by adding six additional kinds
of traditional Chinese medicinal materials.
Experiments
with animals have shown that it can lower the blood glucose of streptozotocin
diabetes big mouse and ALX diabetes little mouse and can increase the big
mouse’s glucose tolerance content of ALX and prolong the submerged weight
swimming time of ALX diabetes little mouse.
The male
Sangcan moth is rich in brain hormone, ecdysone, male hormone, etc. and contains
many amino acid, vitamins and microelement that are needed by the human body.
The biological extracts of the male Sangcan moth which are added with milk
veteh, the extracts of root of yellow kudzuvine and sealwort, and the extracts
of medlar, and then mixed with trichosanthes and rhubarb micro mist, are
adequate for traditional medicine’s curing for deficiency of both yin and yang,
II diabetes of Qi deficiency and blood silt. It is tested for 420 clinical
researches in Beijing Guang An Men Hospital-a subordinate hospital of China
Academy of Chinese Medical Sciences and Shandong traditional Chinese medicine
hospital, Liaoning traditional Chinese medicine hospital, and Jiangsu
traditional Chinese medicine hospital which are four clinical experiment base of
new drug. The total effective rate is 88.67% regardless of whether the disease
history is long or short, and it has no poisonous side effect to human
body.
The
medicine was conferred patent of invention by National Patent Bureau in 1996
(patent No. Z96105259·7). Qi Wei Xiao Ke capsule received the new drug license
(SFDA No.: Z20010134) and production approval license (SFDA No.: Z200110150)
issued by SFDA in October, 2001.
Up to
date, we have already submitted the related documents and awaiting for the
approval of SFDA.
22
Yan Long Anti-cancer Oral
Liquid
Product Description: The Yan
Long Anti-Cancer Oral Liquid (“Yan Long”) is a conventional Chinese patent drug
effective against cancers of the digestive tract such as anal, bile duct, colon,
esophageal, gallbladder, liver, pancreatic, rectal, and gastric cancers. It is
especially effective in helping patients withstand chemotherapy treatment. Yan
Long has successfully completed first and second phase clinical trials and Benda
Ebei plans to begin the third phase.
Yanlong
Anti-cancer Oral Liquid is a kind of pure mixture contains Chinese traditional
medicine such as: Baiying, Morel, Salvia, Gentiana, Angelica, etc.
Starting
in 1989, Yanlong anti-cancer oral liquid was given to 30 patients with primary
liver cancer for the clinical trails. The number of the survivals for the year
of 1990, 1991, 1992 and 1993 were 23, 17, 11 and 8 respectively. By March 2004,
among the eight surviving patients, two do not have any signs of recurrence,
three take care of themselves but are still physically weak, three regressed and
returned to the hospital for treatment. I n this clinical research, the survival
rate of the patients observed for over 14 years reached 26.67%.
Inventor: The development of
Yan Long has been the life work of Dr. Yan Li, one of the nation’s most
well-recognized experts in cancer studies. Dr. Li has been an oncology
specialist for over 40 years and has held various prestigious positions in the
cancer research field in PRC. He was born in 1931. In 1956, he went to study in
Beijing College of TCM and graduated in 1962. After that, he worked in the
Beijing TCM Hospital Oncology Department for 12 years. In 1970, he was promoted
to physician in charge. In 1974, he transferred to the institute of oncology of
Beijing Medical University. In 1984, he was appointed as vice president of
Beijing Sino-Japan Friendship Hospital. He developed an anti-cancer oral liquid
based on his decades of research and experience and named it Yan Long
Anti-Cancer Oral Liquid.
Rights Purchase: Benda Ebei
and Dr. Yan Li (“Li”) signed an agreement regarding the production, marketing,
and sales of Yan Long. Benda Ebei signed agreement to buy all rights to Yan Long
from Mr. Li for RMB 5 million ($625,000). Once sales of Yan Long commence a
monthly payment of RMB 150,000 ($18,750) per month for the first six months and
RMB 300,000 ($37,500) thereafter will be paid. Benda Ebei will cease payment
when the accumulated monthly payments reach the RMB 5 million ($625,000) agreed
purchase price. Li and Benda Ebei are obliged to keep the Yan Long formula
secret. Li retains the right to produce and sell Yan Long in his own clinic.
Benda Ebei management believes that this will not significantly impact Benda
Ebei’s revenue from Yan Long.
IP protection/ technology
confidentiality:
Benda Ebei has applied for patent protection for Yan Long and expects to receive
a 10 year patent when the registration process is completed
Production: The production
facilities and equipment for Yan Long were installed and implemented in July
2007. We intend to start manufacturing and marketing the product immediately
after receiving GMP certification. Due to recent restructuring within SFDA, the
approval process for new medicines has been ceased since November 2006, except
for the first class new medicines. The management expects that such situation
would still last for an indefinite period. The process of third phase clinic
trail of Yang Long, application for IP protection and the application of
certificate are pending.
New Bulk
Chemicals
We are in
the process of developing and bringing to market
7-Phenylacetamido-3-chloromethyl cephalosporanic acid P-Metoxy Benzyl Ester
(“GCLE”), which is a medicine intermediate, chemical name which is used for
cephalotin antibiotics. Japan Otsuka Chemical Industry Company originally
developed the process to produce GCLE and held the worldwide patent on the GCLE
production process. The PRC patent protection period expired in 2005. Since then
Benda’s research center and the Wuhan Institute of Chemical Technology have
jointly mastered this process of GCLE production. We have completed small-scale
and mid-scale trial productions, and expect to be able to produce 200 tons of
GCLE in 2007 and 500 tons in 2008. Domestic demand for this product is expected
to increase by 28% in 2006 to 1,200 metric tons 3. This
demand is currently met by imports.
23
This new
product will be produced in our Yidu plant. Due to a government order issued by
the local government on January 10, 2007, our Yidu Benda plant has been
shut down
since the middle of January 2007 for improvement of our waste water treatment
systems. The order requires us to finish the improvement and be compliant by
June 30, 2007.
Yidu
Benda has completed its upgrading of the waster water system and passed
the
government’s
verification and testing of equipments in October 2007. It is now permitted for
the testing on actual production process. Once the actual products are produced,
then the environmental government bodies will re-test the production results.
The
management could not estimate the exact timing for obtaining the final approval
on the actual production process.
|
3
|
Source:
China National Medical Information Center Southern Sub
center
|
INDUSTRY
AND COMPETITIVE FACTORS
There is
certain industry and competitive factors which we believe will be critical to
achieving our growth:
|
·
|
Rapidly
growing Chinese pharmaceutical market. In 2005, China was the
9th
largest and fastest growing pharmaceutical market in the world. The
Chinese currently spend about $12 per capita on pharmaceuticals compared
to $340 per capital in the U.S. As the Chinese population ages and becomes
wealthier, the already large Chinese pharmaceutical market is poised for
continued explosive growth. According to IMS Health, Inc., a research
firm, the Chinese pharmaceutical market grew by over 28% and 20% in 2004
and 2005, respectively. According to Boston Consulting Group, China’s
pharmaceutical market will become the 5 th
largest in the world by 2010. Further, a recent report by McKinsey &
Co. reported that Chinese healthcare spending will grow from $21 billion
in 2000 to approximately $323 billion by 2025, or at a compounded growth
rate of 11.6%. (Farrell D., Gersch U., Stephenson E., (2006) The Value of
China’s Emerging Middle
Class, McKinsey
Quarterly)
|
|
·
|
Benda is
uniquely positioned to capture market share. Our growth potential
will be largely driven by the gene therapy product which is known as
Gendicine and used for the treatment of cancer. On October 16, 2003,
SiBiono successfully obtained a New Drug License from the State Food &
Drug Administration of China (SFDA), and then, in April 4, 2004, SiBiono
obtained “Manufacture Certificate” and “Certificate of GMP for
Pharmaceutical Product”, so far being fully qualified for the market
launch of Recombinant Human Ad-p53 Injection, trademarked as
Gendicine ®
in China. Gendicine ®
is the first ever commercialized gene therapy product approved in the
world by a government agency. Gendicine is recognized by the world's first
class journals as a major milestone in the field of gene research and
biotechnology and is expect to make important contribution to mankind's
endeavor for improving human
health.
|
|
·
|
Low cost
producer.
Our continuing success in optimizing our manufacturing processes
and minimizing our production costs provides us with a competitive
advantage. For example, we have recently developed innovative processes
that achieve Ribavirin (an API in common anti-viral injections) yield
rates 4.5 per cent higher than the competition, while at the same time
reducing Ribavirin manufacturing costs by 9 per
cent.
|
|
·
|
Government
sponsored industry consolidation presents opportunities for
acquisitions. According to Business China
magazine, China’s thousands of domestic companies account for 70 percent
of the pharmaceutical market. Anticipating the effects of WTO entry and in
an effort to compete with foreign firms, the Chinese government has
decided to nurture its own large pharmaceutical companies, by encouraging
the consolidation of its government-owned companies. To this end, the
Chinese State Economic and Trade Commission (SETC) announced plans to
consolidate the industry and support the development of 10 to 15 largest
pharmaceutical firms. According to government statistics China currently
has about 3,500 drug companies, down from over 5,000 in 2004. The number
is expected to drop further. As the industry undergoes further
consolidation, Benda will have the opportunity to grow by
acquisition.
|
24
|
·
|
Benda’s GMP
compliant manufacturing processes provide us with a strong competitive
advantage in the Chinese healthcare market. The government has
formulated an Action Plan for the Modernization of Chinese Medicine to
boost the quality of Chinese medicine and enhance China's ability to
compete in world markets. As such, all domestic producers of APIs were
required to be GMP compliant by the end of 2004.
Since the end of June 2004, the SFDA has been closing down
manufacturers that do not meet the new GMP standards. In contrast to many
of its competitors, Benda has made significant investments in refitting
our manufacturing systems to comply with GMP standards. Our Benda Ebei
plant received GMP certificate from SFDA on November 11, 2003 and renew on
December 3, 2008 for the production of injection vials; while on November
26, 2007, Benda Ebei received a GMP for the production of tablets. Our
Jiangling Benda plants received GMP certificate on April 9, 2008 which
authorizing the production of Ribavrin. Our SiBiono received GMP
certificate on October 16, 2003. Our Yidu Benda facilities, which produce
bulk chemicals, are not subject to GMP certification
requirement.
|
RAW
MATERIALS AND PRINCIPAL SUPPLIERS
Benda
Ebei Suppliers
Benda
Ebei plant manufactures injection vials from APIs. Benda Ebei’s largest supplier
of APIs was Hubei Fourth Ring Pharmaceutical Co., Ltd. In 2008,
Benda Ebei’s largest
supplier of APIs was Nan Yang Re Gong Co. Ltd and Benda Ebei
purchased approximatel $2.9 million worth
of Tranexamic Acid from this supplier, equivalent to 28 per cent of Benda
Ebei’s raw
materials purchases by value。In
2009,
Benda
Ebei’s largest
supplier of APIs was Hubei
Sihuan Pharmaceutical Co.,Ltd and Benda
Ebei purchased approximately $3.4 million,
worth of Tranexamic Acid equivalent to 38
per cent
of Benda Ebei’s raw
materials purchases by value.
Jiangling
Benda Suppliers
Our
Jiangling Benda plant manufactures APIs from bulk chemicals. Jiangling Benda’s
largest supplier of bulk chemicals is Wuhan Zhongnan Supplying Co., Ltd. In
2004, Jiangling Benda purchased $400,000 worth of Acetic Anhydride from this
supplier, equivalent to 26.2 per cent of Jiangling Benda’s raw materials
purchases by value. The Jiangling Benda plant has made no further raw materials
purchases since its closure for renovation.
25
Jiangling
Benda re-opened on August 10, 2007 and started producing Ribose. In year of
2008, the largest
supplier was Hubei
Zhijiang Shanyau Chemical Co. Ltd., and
approximately $74 K
worth of raw materials was purchased from this suppliers, equivalent to
13.16% of
total the raw materials purchases by value. In year
of
2009, the
largest supplier was Xinxiang
Weide
Chemical
Co. Ltd., and approximately $556,000 worth
of raw materials was purchased from this suppliers, equivalent to 29% of
total the raw materials purchases by value
Yidu
Benda Suppliers
Our Yidu
Benda plant manufactures bulk chemicals from other bulk chemicals.
Due to an
government order issued by the local government on January 10, 2007, our Yidu
Benda plant has been shut down since the middle of January 2007 for improvement
of our waste water treatment systems. The order requires us to finish the
improvement and be compliant by June 30, 2007.
Yidu
Benda has completed its upgrading of the waste water system and passed the
government’s verification and testing of equipments in October 2007. It is now
permitted for the testing on actual production process. Therefore, there was no
supplier incurred in the year of 2008 and 2009.
Packaging
Material Suppliers
Our
packaging materials are purchased from two main suppliers. In 2007,
we purchased approximately
$0.9 MM
worth of
plastic packaging material from Anlu ZhongYa Plastics Package Factory,
equivalent to 11 per cent of our packaging supplies by value. In 2008, we
purchased approximately $0.62 MM
worth of
plastic packaging material from Anlu ZhongYa
Plastics Package Factory and Anlu Zhong’Ao
Printing Factory, equivalent to 20 per
cent of our packaging supplies by value.
The
standardized purchasing contract, which is designed by the PRC government
agency, is being adopted. The major terms (or components) of the purchasing
contracts are stated are as follows:
|
1.
|
payment
schedule which is based on the
negotiation;
|
|
2.
|
the
goods are inspected and received according to the PRC regulations for
materials of producing drug;
|
|
3.
|
breach
contract, then parties have the right to sue in the PRC local civil
courts;
|
As there
were many transaction contracts during the years, since the company formed
purchasing contract with the suppliers for lots of material purchased, therefore
we just listed three of those and shown as a sample for reference.
However,
our company does not depend on any sole source suppliers, since the raw
materials market is opened in the sense that we could have plenty of choices of
the suppliers (each category of materials purchased may have three to five
suppliers in the market). We only choose those suppliers who could offer better
terms to us and having a long term co-operation relationship.
26
OUR
INTELLECTUAL PROPERTY
We regard
our service marks, trademarks, trade secrets, patents and similar intellectual
property as critical factors to our success. We rely on patent, trademark and
trade secret law, as well as confidentiality and license agreements with certain
of our employees, customers and others to protect our proprietary
rights.
Pursuant
to the PRC TCM Protection Regulation, certain ready-made TCM products which have
received SFDA approval have automatic protected intellectual property rights for
a seven-year period from the date of grant of such approval. An application can
subsequently be made to extend such protection for up to three consecutive
seven-year periods. Once this protection period has expired, a company may apply
for patent protection.
To a
large extent, we rely on such protection regulation to protect our intellectual
property rights with respect to such products. In addition, as of December 31,
2005, we filed four patents for manufacturing technologies, primarily relating
to our medicine products and manufacturing techniques and the applications are
still in the approval process
Our
Patents
Two types
of medicine-related patents exist in PRC: the medicine production technique
patent and medicine invention formula patent. In PRC, illegal infringements of
production technique patents are widespread. A tiny modification to a filed
medicine production technique might be construed as a new one and argued as not
violating the patent laws. Therefore, Benda Ebei has historically tried to avoid
applying for this kind of patent in order to protect its technological
secrets.
Invention
formula patents are as easily imitated as production technique patents. However,
since only one SFDA production certificate can be issued on new branded medicine
based on the major pharmaceutical ingredients used, a minor modification to the
formula would not warrant a new SFDA production certificate. This means that,
even if another manufacturer gets to know a patented formula, it won’t be able
to produce it, at least in PRC, without the proper SFDA production
certificate.
SiBiono patents
Our
company has already applied for 6 patents and they are shown in the following
table:
27
|
Item
|
Patent name
|
|
Countries
/ Date
|
|
Application
Number (1)
|
|
Publication
Number (2)
|
|
Approved Patent
Number (3)
|
|
Name of
Patent
Inventor
(6)
|
|
Name of
Applicant
(6)
|
|
Patent
Assignees
|
|
|
1
|
A
new method for manufacturing recombinant adenovirus
|
|||||||||||||||
|
A
|
China
|
98123346.5
|
CN1228474A
|
ZL98123346.5
|
Peng
|
Peng
|
SiBiono
|
|||||||||
|
Date
|
12/14/1998
|
9/15/1999
|
7/3/2002
|
|||||||||||||
|
2
|
A
recombinant constructed by a virus vector and a human tumor suppressor
gene and its use
|
|||||||||||||||
|
A
|
China
|
02115228.4
|
CN1401778A
|
ZL02115228.4
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
|||||||||
|
Date
|
5/8/2002
|
3/12/2003
|
11/24/2004
|
|||||||||||||
|
B
|
PCT
(4)
|
WO2004/078987A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
||||||||||
|
Date
|
3/8/2004
|
9/16/2004
|
N/A
|
|||||||||||||
|
3
|
Recombinant
gene medicine of adenovirus vector and and gene p54 for treating
proloferative diseases
|
|||||||||||||||
|
A
|
China
|
03125129.3
|
CN1471977A
|
ZL03125129.3
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
|||||||||
|
Date
|
5/10/2003
|
2/4/2004
|
7/25/2007
|
|||||||||||||
|
B
|
PCT
(4)
|
WO2004/104204A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
||||||||||
|
Date
|
5/9/2004
|
12/2/2004
|
N/A
|
|||||||||||||
|
4
|
The
application of recombinant adenoviral p53 as cancer vaccine (tentative
title)
|
|||||||||||||||
|
A
|
China
|
200510002779.1
|
CN1679641A
|
ZL200510002779.1
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
|||||||||
|
Date
|
1/26/2005
|
10/12/2005
|
8/29/2007
|
|||||||||||||
|
B
|
PCT
(4)
|
WO2006/079244A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
||||||||||
|
Date
|
1/26/2005
|
8/3/2006
|
N/A
|
|||||||||||||
|
C
|
US
(5)
|
11/075035
|
2005/0281785A1
|
Not
Approved
|
Peng
/ Zhang
|
Unidentified
Yet
|
N/A
|
|||||||||
|
Date
|
3/7/2005
|
12/22/2005
|
N/A
|
|||||||||||||
|
5
|
Human
Embryonic Kidney (HEK) sub-clone cell line
|
|||||||||||||||
|
A
|
China
|
03126889.7
|
CN1513985A
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
|||||||||
|
Date
|
6/13/2003
|
7/21/2004
|
N/A
|
|||||||||||||
|
B
|
PCT
(4)
|
WO2004/111239
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
||||||||||
|
Date
|
5/9/2004
|
12/23/2004
|
N/A
|
|||||||||||||
|
6
|
The
complex of polypeptide liposome and human VGEF gene, and its use and human
VGEF gene, and its use
|
|||||||||||||||
|
A
|
China
|
02134321.7
|
CN1389269A
|
Not
Approved
|
Peng
/ Zhang / Zhu
|
Peng
/ Zhang / Zhu
|
N/A
|
|||||||||
|
Date
|
7/4/2002
|
1/8/2003
|
N/A
|
28
Note:
(1)
Application number is obtained when application is submitted;
(2)
Publication number is obtained after the first phase examination;
(3)
Approved patent number is obtained after the final examination;
(4) PCT
is referred to an International Patent Organization in Paris;
(5) US is
referred to the application is made in United States of America
alone;
(6) Peng
is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is referred
to Jinya Zhu.
The
validity of the patent is 20 years since the application date.
On
January 29, 2008, SiBiono entrusted Grandall Legal Group Shenzhen Law Firm to
issue a legal letter to Zhaohui Peng, one of the shareholders of Sibiono and the
inventor of Gendicine, demanding that he transfer all the title of patents to
SiBiono, as SiBiono is the rightful owner of the patents.
On June
18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights
to the patent “A new method for manufacturing recombinant adenovirus” and
related research results to SiBiono as a payment for the registered capital. In
return, Zhaohui Peng was granted 32.03% of the common stock of
SiBiono.
From 1999
to 2007, SiBiono successfully obtained various technology funds from various
government technology agencies to support the further research and development
activities of Gendicine. Due to this significant funding obtained by SiBiono,
Sibiono developed five additional patents which are summarized in the above
table.
As
indicated in the above table, Item 1, the patent “A new method for manufacturing
recombinant adenovirus” had been assigned to SiBiono; however, the other
approved patents (item 2 through item 4) in PRC still have not been assigned to
SiBiono. The Group believes that all the above mentioned patents should be
rightfully transferred to SiBiono, a subsidiary of the Group. Accordingly, the
above mentioned legal letter was issued on this ground.
29
On August
27, 2008, the Group through its subsidiary, SiBiono filed an application to the
Guongdong Province Shenzhen City (Middle) Peoples’ Court to demand Zhaozhu Peng
to transfer back all the above mentioned patents to SiBiono. This case
has been brought to the court twice, and we are still waiting for the
judgment.
Our
Trademarks
Benda
currently owns four trademarks: Jixuening, Benda, Suzheng-B, and Shusai-A. Benda
has filed trademark applications, the approvals of which are pending, for other
13 medicine names or general trademarks and the applications are still in the
approval process.
WORK
SAFETY ISSUES
On
November 10, 2005, there was a small explosion in the Yidu plant resulting in
the deaths of two of our workers. This tragic accident resulted from the
violation of our operating procedures by one of the workers killed in the
explosion. We paid RMB 260,000 ($32,000) to each of the victims’ families in
settlement of any compensation issues. On November 11, 2005, there was another
explosion in the same factory resulting from a chemical reaction triggered by
the prior explosion. Fortunately management had anticipated this incident and
the plant had already been temporally sealed so nobody was killed.
Following
the events of November 10 and 11, 2005 we have put in place very strict work
safety procedures and revised the design of the relevant production process in
order to avoid similar incidents in the future. We believe Benda is not liable
for any further material liabilities arising from these explosions.
RESEARCH
AND DEVELOPMENT ACTIVITIES DURING THE PRIOR TWO FISCAL YEARS
We spent
$349,361 and $1,461,951 on direct research and development (“R&D”) efforts
in 2009 and 2008 respectively. Rather than spend considerable sums internally on
R&D in a market where we cannot easily protect our results of development,
we prefer to leverage our management team’s extensive industrial network and
knowledge in finding new drugs and treatments that may be potentially very
successful but which have not yet been brought to market. We carefully evaluate
each of these drugs before making a purchase decision. We believe each of our
new products will enjoy considerable success in the market.
We also
leverage our network of leading research institutions and universities to
optimize the effectiveness of our investment in production R&D. We allocate
and fund research projects to these institutions. Our in-house scientists
coordinate and supervise the outsourced R&D processes. We have the rights to
all results of the R&D efforts including IP rights. In order to protect our
interests we usually divide each project into several sections and assign each
section to a different research institution. Each research ally therefore only
works on a limited part of each project. Some universities and institutes that
work with us are:
|
|
·
|
Life
Science College of Wuhan
University.
|
|
|
·
|
Biochemical
College of Sanxia University
|
|
|
·
|
Jilin
Medical University
|
|
|
·
|
Shanghai
Institute of Pharmacy of Chinese Academy of
Sciences.
|
|
|
·
|
Wuhan
University of Chemical Technology.
|
The
science and technology cooperation agreement between College of Chemistry and
Life Science of China Three Gorges University and Yidu Benda:
Party A:
China Three Gorges University (Party A for short in the
following)
30
Party B:
Yidu Benda Chemical Co.Ltd. (Party B for short in the following)
To
increase the science and technology progress of Yidu Benda Chemical Engineering,
Inc. (“Yidu Benda”), to improve the quality of its products and its production
efficiency, and to fully make use of scientific intelligence, technologies and
experimental equipments of College of Chemistry and Life Science of China Three
Gorges University (“University”), after negotiation, the two parties decided to
cooperatively build a “drug raw material research and development group”. The
concrete agreements are as follows:
|
1.
|
The
“drug raw material research and development group” is consist of members
of both parties, including one group leader, two assistant group leaders
and five science and technology workers. The group leader is assigned by
University, while the two assistant leaders are assigned by the two
parties, one for each party. The remaining five workers are decided by
negotiation according to the demands of project
research.
|
|
2.
|
The
research and development projects are put forward by Yidu Benda, and are
decided after the demonstration of the two
parties.
|
|
3.
|
The
research funds are supported by Yidu Benda, and University shall supply
all the experimental equipment and the related condition according to
needs, in which including: University provide certain number of chemistry
and chemical engineering apparatus and equipment for common usage and help
Yidu Benda to build a basic laboratory and assist Yidu Benda to build a
pilot laboratory.
|
|
4.
|
The
expense of the research and development projects includes: drug reagent,
low priced and easily worn detecting fees, the transportation fees between
school and company and allowance of research
workers.
|
|
5.
|
University
can directly send relative researchers to Yidu Benda to organize and guide
project task research according to
needs.
|
|
6.
|
The
property rights and interests of the project achievements will be
appointed by Project Contract.
|
COMPLIANCE
WITH ENVIRONMENTAL LAW
We comply
with the Environmental Protection Law of PRC as well as applicable local
regulations. In addition to statutory and regulatory compliance, we actively
ensure the environmental sustainability of our operations. Penalties would be
levied upon us if we fail to adhere to and maintain certain standards. Such
failure has not occurred in the past, and we generally do not anticipate that it
will occur in the future, but no assurance can be given in this
regard.
Our Yidu
Benda facility produces bulk chemical intermediates for raw material medicine
and does not require GMP certification. Yidu Benda was temporarily closed since
mid January to upgrade its waste water treatment system to comply with new
environmental standards enforced by PRC local government.
Due to an
government order issued by the local government on January 10, 2007, our Yidu
Benda plant has been shut down since the mid of January 2007 for improvement of
our waste water treatment systems. The order requires us to finish the
improvement and be compliant by June 30, 2007. Up to date, the company had spent
approximately $0.2 MM to re-design and improve the existing system. Nowadays,
Yidu Benda has completed its upgrading of the waste water system and passed the
government’s verification and testing of equipments in October 2007. It is now
permitted for the testing on actual production process. Once the actual products
are produced, then the environmental government bodies will re-test the
production results. The management could not estimate the exact timing for
obtaining the final approval on the actual production process. Furthermore, the
management is searching for new products to be produced in Yidu Benda which with
higher profit margin.
SHENZHEN
SIBIONO GENE TECHNOLOGY CO., LTD.
On April
5, 2007, Hubei Tongji Benda Ebei Pharmaceutical Co., Ltd., a Sino-Foreign Equity
Joint Venture company incorporated under the laws of the PRC (“Benda Ebei”), of
which Ever Leader Holdings Limited, a company incorporated under the laws of
Hong Kong SAR ("Ever Leader") and a wholly owned subsidiary of Benda
Pharmaceutical, Inc. (the “Company”), owns 95% of the outstanding common stock,
has entered into Equity Transfer Agreements with certain shareholders of
Shenzhen SiBiono Gene Technology Co., Ltd. (“SiBiono”), a corporation
established and validly existing under the law of the PRC, to purchase a total
of approximately 57.57% of the shares of SiBiono’s common stock for total
consideration of RMB60,000,000 due and payable on or before April 30,
2007.
31
In
connection with the Equity Transfer Agreements, we entered into a Financial
Consultancy Agreement with Super Pioneer International Limited (“Super Pioneer”)
for financial consultancy services rendered by Super Pioneer. Pursuant to the
Financial Consultancy Agreement, we agreed to issue 2,100,000 shares of our
common stock to Super Pioneer within three months from the date of the
agreement. Super Pioneer agreed to lock up the shares for a period of twelve
months from the date of the issuance of the shares (the “Lock-up Period”).
Within three months from the Lock-up Period, in the event that the public
trading price of our shares did not reach $3.6 per share and we are not listed
in the capital market of NASDAQ or AMEX, Super Pioneer shall have the option to
require us to redeem 1,960,000 shares of the stock owned by Super Pioneer at a
price of $3.6 per share. Such option expired within one month from the last date
of the three month period. The redemption requests were made to the Company
in January 2008 and the company has already obtained oral consent from Super
Pioneer that the payment would be deferred to the year of 2010.
On June
11, 2007, Benda Ebei entered into Equity Transfer Agreements with Yaojin Wang
and Huimin Zhang, shareholders of SiBiono, for the purchase of an additional
2.56% of the shares of SiBiono’s common stock for total consideration of
RMB2,560,000 due and payable on or before June 30, 2007. Accordingly, Benda Ebei
holds a total of 60.13% of the shares of SiBiono’s common stock.
In
connection with the Equity Transfer Agreements, we entered into Technical
Consultancy Agreements with Yaojin Wang and Huimin Zhang for technical
consultancy services rendered by Yaojin Wang and Huimin Zhang. Pursuant to the
Technical Consultancy Agreements, we agreed to issue 33,585 shares of our common
stock to Yaojin Wang and 55,975 shares of our common stock to Huimin Zhang
within three months from the date of the agreement. Yaojin Wang and Huimin Zhang
agreed to lock up their shares for a period of twelve months from the date of
the issuance of the shares (the “Lock-up Period”). Within three months from the
Lock-up Period, in the event that the public trading price of our shares did not
reach $3.6 per share and we are not listed in the capital market of NASDAQ or
AMEX, Yaojin Wang and Huimin Zhang shall have the option to require us to redeem
the shares of the stock owned by Yaojin Wang and Huimin Zhang at a price of $3.6
per share. Such option expired within one month from the last date of the three
month period. The redemption requests were made to the Company in January
2008 and the company has already obtained oral consent from Wang and Zhang that
the payment would be deferred to the year of 2010.
Business of
SiBiono
Shenzhen
SiBiono GeneTech Co., Ltd. (hereinafter referred to as SiBiono) is a gene
therapy company dedicated to the development, manufacturing and
commercialization of gene therapy products. The Company was founded in early
1998 and is located in Shenzhen Hi-Tech Industrial Park, Shenzhen, China. As a
pioneer in gene therapy in China, SiBiono’s mission is to develop innovative
gene therapy products for the improvement of human health and life quality. The
Company has developed two core technology platforms: Viral Vector Gene Delivery
System and Non-Viral Vector Gene Delivery System focusing on development of gene
therapy product for cancer and cardiovascular diseases.
On
October 16, 2003, SiBiono successfully obtained a New Drug License from the
State Food & Drug Administration of China (SFDA), and then, in April 2004,
SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for
Pharmaceutical Product”, so far being fully qualified for the market launch of
Recombinant Human Ad-p53 Injection, trademarked as Gendicine ® in
China. Gendicine ® is the
first ever commercialized gene therapy product approved in the world by a
government agency. It is only approved and commercialized in PRC alone; however
there is no other gene therapy product like Gendicine has been approved and
commercialized in other countries. One of the factor causing this situation may
be due to the fact that China’s lack of regulatory obstacles in conducting gene
therapy trails.
32
SiBiono
has established the validated GMP manufacturing plant for the production of gene
therapy drugs. A complete set of quality-control assays and large-scale
production processes were implemented in SiBiono in accordance with
international regulations and standards for consistent manufacture of high
quality gene therapy products. Based on SiBiono’s QC procedures and standards,
the SFDA constituted and issued the national technological guideline - “Points
to Consider for Human Gene Therapy and Product Quality Control” in May 2004.
This document was also published in the magazine of Biopharm International for
reference and peer review.
SiBiono
has undertaken a number of national and provincial research and development
projects, including biotechnology projects of “National 863 Plan”, projects of
“National 973 Plan”, key research project of “National Tenth Five-Year-Plan”,
projects funded by “National Innovation Fund”, projects of “National Key
Scientific Development Plan”, National Hi-tech Industrialization Projects, Key
Platform Technology Development Projects in Guangdong Province, as well as
Hi-Tech Industrialization Projects of Shenzhen Municipality. By participating in
those national projects, the reputation of SiBiono could be raised in China and
also it allows us to apply for the funding from the local government agencies to
support our gene therapy research activities.
The
Company has 60.19 million RMB as its registered capital and currently has about
80 full-time employees.
Milestones of
SiBiono
Since its
establishment in March of 1998, SiBiono has evolved from a small start-up
company to an internationally recognized gene therapy company with its
successful launch of the world’s first approved gene therapy product
“Gendicine”. The Company has achieved the following major milestones in the past
(Table 1):
Table 1. Milestones of SiBiono’s
Development
|
Time
|
|
Events
|
|
October
2006
|
SiBiono
was awarded with “Global Entrepolis @ Singapore” Innovation Award. The GES
Award, honoring the “Technopreneur of the Year” in the Asia-Pacific
region, was presented by the President of Singapore, SR Nathan at the
Opening Ceremony of Global Entrepolis@Singapore 2006. A record-breaking
224 entries from applicants in 14 countries and territories in the
Asia-Pacific region submitted the application for
competition.
|
|
|
Wall
Street Journal Asia presented the story of SiBiono in 2 separate
issues.
|
||
|
December
2005
|
Dr.
Zhaohui Peng was awarded a Special Recognition Award by ISCGT
(International Society for Cell and Gene Therapy of Cancer) in recognition
for SiBiono GeneTech, Co., Ltd.'s great contribution to the gene therapy
field.
|
|
|
June
2005
|
Recombinant
Human Ad-p53 Injection (Gendicine) was granted the “State Key-New product
Certificate” issued jointly by the Ministry of Science and Technology, the
Ministry of Commerce, the General Administration of Quality Supervision,
Inspection and Quarantine and the State Environmental Protection
Administration of the People’s Republic of China.
|
|
|
April
2004
|
Gendicine
was launched into market
|
|
|
March
2004
|
SiBiono’s
Gendicine manufacturing facility is granted with “Certificate of GMP for
Pharmaceutical Product” by
SFDA.
|
33
|
January
20, 2004
|
|
SiBiono
was granted “Manufacture Certificate” for Gendicine by the
SFDA
|
|
October
16, 2003
|
Gendicine
was granted “New Drug License” by the SFDA, and became the first gene
therapy product ever approved by a government agency in the
world.
|
|
|
September
2003
|
Mr.
Zeng Qinghong, Vice President of P.R.China, visited SiBiono GeneTech and
gave the Chinese Brand name (今又生) for
Gendicine.
|
|
|
September
2003
|
Completion
of the clinical trials, defense and assessment of “recombinant human p53
adenoviral injection” product
|
|
|
July
2003
|
Corporate
restructuring was finished, the registration capital increased to be 48.19
million RMB
|
|
|
November
2002
|
SiBiono
was granted the Pharmaceutical Manufacture Permission by the Guangdong
Drug Administration.
|
|
|
September
1998
|
SiBiono
obtained SFDA’s permission to initiate Gendicine clinical
trials.
|
|
|
March
1998
|
Shenzhen
SiBiono GeneTech Co., Ltd. was
established.
|
Dr.
Zhaohui Peng is the founder and Chairman of SiBiono. He graduated from a medical
university in China, served as director of a research institute at the South
Medical University in Guangzhou and as a visiting professor at both the
University of Chiba in Japan and the University of California. He also conducted
research at two US biotech companies. Dr. Peng has devoted more than fifteen
years to gene therapy research, development and commercialization. He led
the research, development, industrialization and commercialization of Gendicine.
He is also the main inventor of all patents in SiBiono.
Attribute
to his great contribution to the gene therapy field by developing and launching
the world's first gene medicine product - Gendicine®, Dr. Peng was honored with
the “Gene Therapy Achievement Award” at the 2005 ISCGT Annual Conference in
December 2005, the person of cover story on Forbes (Chinese Edition) entitled
with the “Technology Pioneer in China” in October 2006, the “Certificate of
Recognition” for outstanding achievement and innovative contribution to
bioscience research and development by California State Assembly in November
2006, and the nominee of “CCTV 2006 People of the Year in China’s Economy” in
November 2006.
On
September 9, 2007, Benda achieved a great success in the national forum on "Gene
Therapy for Tumors" sponsored by the Chinese Academy of Medical Sciences and the
China Pharmaceutical Biotechnology Association. The forum is China’s large
annual medical biotechnology conference run from September 7 - 10 in Qing Dao,
Shandong Province. In conjunction with the national conference, SiBiono held a
national forum discussing “Gene Therapy for Tumors”. Approximately 600 leading
Chinese doctors attended SiBiono’s forum, representing 26 different provinces
and over 200 Tier I Chinese hospitals (Tier I hospitals offer the best medical
services in China; there are currently 775 Tier I hospitals in
China).
The main
purpose of the forum was to allow 15 national renowned medical experts to share
their experiences in the effective application of Gendicine ® to
maximize its treatment efficacy.
As of
December 31, 2007, SiBiono shipped 10,652 of the 16,000 vials ordered. From
January 1 to February 25, 2008, another 733 vials out of the 16,000 vials order
had shipped. From February 26 to July 10, 2008, an additional 2,412 vials
shipped. Accordingly, we have shipped and received payment for a total of 13,797
vials of the 16,000 vial order. The 16,000 vial order for Gendicine was ordered
by various selling agents with average selling price RMB2,500 per
vial.
In
January 2008, our quality control department recognized that during the process
of purification, one of the components of Gendicine did not fulfill the
requirements of the internal quality control; therefore further purification was
necessary. Gendicine is a gene therapy product which needs to undergo a series
of internal quality control processes. Once the problem was located, our quality
control and production department solved the issue immediately.
34
On May
19, 2008, SiBiono received an official notice from the PRC State of SFDA in
which it mentioned that during the random inspection performed by the PRC State
of SFDA on April 8 to April 10, 2008, the PRC State of SFDA discovered there
were several production procedures that did not meet the requirement stated in
GMP, thus it required SiBiono to perform necessary improvements in order to
fulfill the GMP requirements and the PRC State of SFDA collected back the
distributed GMP certificate until the necessary improvements being carried out
and passed the examination that conducted by SFDA. On June 10, 2008, SiBiono
received another official notice from Guangdong Province SFDA and they
demanded the same requirements as stated in the official notice which issued by
the PRC State of SFDA dated on May 19, 2008. On November 24, 2008, SiBiono
received another official notice from Guangdong Province SFDA which mentioned
that after the examination conducted by Shenzhen City SFDA, the Guangdong
Province SFDA consent SiBiono to carry out production on a trial basis. It
further required SiBiono to strictly follow the requirements of GMP to organize
trial production and follow the procedures to apply for GMP Certificate
verification. On July 14, 2009, SiBiono obtained the final approved GMP
Certificate, in order words, the SFDA allows SiBiono to resume its production
and sales.
Commercial Bank
Note
On August
14, 2007, Benda Ebei entered into a three year non-interest bearing commercial
bank note issuable agreement with Shanghai Pudong Development Bank. The total
commercial note issuable limit is Rmb 60 million; however 50% of the deposit has
to be made to the bank in order to secure the issuance of the commercial bank
note, thus the net available amount is Rmb 30 million. The repayment period of
each commercial note payable is six months. If the net amount of each commercial
bank note payable is not settled on the due date, the penalty will be the
penalty rate of the PRC bank loan on a daily and compound basis.
The
credit facility was originally guaranteed by SiBiono and secured by the
buildings, machinery and equipment of Benda Ebei. The Sibiono guarantee may have
been a violation of the terms of the convertible promissory notes entered into
with certain accredited and institutional investors on April 5, 2007. However,
on December 15, 2007, Benda Ebei received a consent letter from Pudong Bank that
Pudong Bank agreed to cancel SiBiono’s guarantee toward this credit
facility.
On
January 21, 2008, Benda Ebei entered into a supplementary agreement with
Shanghai Pudong Development Bank, to supplement the commercial bank note
issuance agreement dated on August 14, 2007. According to this supplementary
agreement, the credit facility is further secured by the buildings, machinery
and equipment of Jiangling Benda.
As of
December 31, 2009, Benda Ebei and Jiangling Benda deposited an amount of
$4,409,334 in Shanghai Pudong Development Bank as deposit for the issuance of
commercial bank notes. Such deposits will be released when the commercial bank
notes are cleared. As of December 31, 2009, the balance of the
commercial bank notes payable was $8,160,503. Thus the net commercial bank
notes payable was $3,751,169 as of December 31, 2009.
35
On
January 21, 2008, Benda Ebei entered into a supplementary agreement with
Shanghai Pudong Development Bank, to supplement the commercial bank note
issuance agreement dated on August 14, 2007. According to this supplementary
agreement, the credit facility is further secured by the buildings, machinery
and equipment of Jiangling Benda. As of December 31, 2009, the net book value of
secured property and equipment was approximately Rmb121 million (or $17.7
million) in total.
EMPLOYEES
As of
December 31, 2009, we had
approximately 400 full-time
employees, including 48 sales people, 19 technology and R&D staff, and
333 production
staff and no part-time employees. Among our current
employees are two Ph.D.s and 17 holders of master’s
degrees. All executives have received master-degree level executive training and
all members of our staff have undertaken GMP
training.
We have a
sales office in each of three cities that promote our
branded medicines. As of December 31, 2009,
approximately 18 people in our Central China Sales Office were located in Wuhan,
and 16 people were in our Southern China Sales Office located in
Shenzhen.
RISK
FACTORS
An
investment in our common stock involves investment risks and the possibility of
the loss of an investor’s entire investment. A prospective investor should
evaluate all information about us and the risk factors discussed below in
relation to his financial circumstances before investing in us.
Risks
Relating to Our Business
|
|
·
|
WE
NEED TO MANAGE GROWTH IN OPERATIONS TO MAXIMIZE OUR POTENTIAL GROWTH AND
ACHIEVE OUR EXPECTED REVENUES AND OUR FAILURE TO MANAGE GROWTH WILL CAUSE
A DISRUPTION OF OUR OPERATIONS RESULTING IN THE FAILURE TO GENERATE
REVENUE.
|
In order
to maximize potential growth in our current and potential markets, we believe
that we must expand our manufacturing and marketing operations. This expansion
will place a significant strain on our management and our operational,
accounting, and information systems. We expect that we will need to continue to
improve our financial controls, operating procedures, and management information
systems. We will also need to effectively train, motivate, and manage our
employees. Our failure to manage our growth could disrupt our operations and
ultimately prevent us from generating the revenues we expect.
In order
to achieve the above mentioned targets, the general strategies of our company
are to maintain and search for hard-working employees who have innovative
initiatives; on the other hands, our company will also keep a close eye on
expanding opportunities, for example, acquisition of state-owned
enterprises.
|
|
·
|
WE
CANNOT ASSURE YOU THAT OUR ORGANIC GROWTH STRATEGY WILL BE SUCCESSFUL
WHICH MAY RESULT IN A NEGATIVE IMPACT ON OUR GROWTH, FINANCIAL CONDITION,
RESULTS OF OPERATIONS AND CASH
FLOW.
|
36
One of
our strategies is to grow organically through increasing the distribution and
sales of our products by penetrating existing markets in PRC and entering new
geographic markets in PRC as well as other parts of Asia and the United States.
However, many obstacles to entering such new markets exist, including, but not
limited to, international trade and tariff barriers, shipping and delivery
costs, costs associated with marketing efforts abroad and maintaining attractive
foreign exchange ratios. We cannot, therefore, assure you that we will be able
to successfully overcome such obstacles and establish our products in any
additional markets. Our inability to implement this organic growth strategy
successfully may have a negative impact on our growth, future financial
condition, results of operations or cash flows.
|
|
·
|
WE
CANNOT ASSURE YOU THAT OUR ACQUISITION GROWTH STRATEGY WILL BE SUCCESSFUL
RESULTING IN OUR FAILURE TO MEET GROWTH AND REVENUE
EXPECTATIONS.
|
In
addition to our organic growth strategy, we also expect to grow through
strategic acquisitions. We intend to pursue opportunities to acquire businesses
in PRC that are complementary or related in product lines and business structure
to us. We may not be able to locate suitable acquisition candidates at prices
that we consider appropriate or to finance acquisitions on terms that are
satisfactory to us. If we do identify an appropriate acquisition candidate, we
may not be able to negotiate successfully the terms of an acquisition, or, if
the acquisition occurs, integrate the acquired business into our existing
business. Acquisitions of businesses or other material operations may require
debt financing or additional equity financing, resulting in leverage or dilution
of ownership. Integration of acquired business operations could disrupt our
business by diverting management away from day-to-day operations. The
difficulties of integration may be increased by the necessity of coordinating
geographically dispersed organizations, integrating personnel with disparate
business backgrounds and combining different corporate cultures. We also may not
be able to maintain key employees or customers of an acquired business or
realize cost efficiencies or synergies or other benefits we anticipated when
selecting our acquisition candidates. In addition, we may need to record
write-downs from future impairments of intangible assets, which could reduce our
future reported earnings. At times, acquisition candidates may have liabilities
or adverse operating issues that we fail to discover through due diligence prior
to the acquisition. In addition to the above, acquisitions in PRC, including of
state owned businesses, will be required to comply with laws of the
People's Republic of China ("PRC"), to the extent applicable. There can be no
assurance that any given proposed acquisition will be able to comply with PRC
requirements, rules and/or regulations, or that we will successfully obtain
governmental approvals which are necessary to consummate such acquisitions, to
the extent required. If our acquisition strategy is unsuccessful, we will not
grow our operations and revenues at the rate that we anticipate. If our
acquisition strategy is not successful, our revenues and profit will be solely
dependent upon our organic growth.
|
|
·
|
WE
HAVE PREVIOUSLY HAD AN EXPLOSION AT OUR YIDU PLANT THAT RESULTED IN TWO
DEATHS AND ANY SUCH OCURRENCE IN THE FUTURE CAN EXPOSE US TO LIABILITY FOR
SUCH AN EVENT
|
On
November 10, 2005, there was a small explosion in the Yidu plant resulting in
the deaths of two of our workers. This tragic accident resulted from the
violation of our operating procedures by one of the workers killed in the
explosion. We paid RMB 260,000 (or $32,500) to each of the victims’ families in
settlement of any compensation issues. On November 11, 2005, there was another
explosion in the same factory resulting from a chemical reaction triggered by
the prior explosion. Fortunately management had anticipated this incident and
the plant had already been temporarily sealed so that no further injury
occurred.
Following
the events of November 10 and 11, 2005, Yidu plant was temporarily closed for
approximately one month; during the one month plant shut down, we put in place
very strict work safety procedures and revised the design of the relevant
production process in order to avoid similar incidents in the future. We believe
Benda is not liable for any further material liabilities arising from these
explosions. Notwithstanding this fact, there can be a future explosion that may
result in death or serious injury. If this occurs and in light of the fact that
it previously occurred, may result in exposure to extensive liability to
us.
37
Currently,
we do not have any insurance policy for Yidu Benda due to the fact that it has
been closed since January 2007 for improvement of our waste water treatment
systems. However, once Yidu Benda resumes to its production, we will deploy
appropriate insurance policy for the workers.
|
|
·
|
IF
WE NEED ADDITIONAL CAPITAL TO FUND OUR GROWING OPERATIONS, WE MAY NOT BE
ABLE TO OBTAIN SUFFICIENT CAPITAL AND MAY BE FORCED TO LIMIT THE SCOPE OF
OUR OPERATIONS.
|
If
adequate additional financing is not available on reasonable terms, we may not
be able to undertake plant expansion, purchase additional machinery and purchase
equipment for our operations and we would have to modify our business plans
accordingly. There is no assurance that additional financing will be available
to us.
In
connection with our growth strategies, we may experience increased capital needs
and accordingly, we may not have sufficient capital to fund our future
operations without additional capital investments. Our capital needs will depend
on numerous factors, including (i) our profitability; (ii) the release of
competitive products by our competition; (iii) the level of our investment in
research and development; and (iv) the amount of our capital expenditures,
including acquisitions. We cannot assure you that we will be able to obtain
capital in the future to meet our needs.
In recent
years, the securities markets in the United States have experienced a high level
of price and volume volatility, and the market price of securities of many
companies have experienced wide fluctuations that have not necessarily been
related to the operations, performances, underlying asset values or prospects of
such companies. For these reasons, our shares of common stock can also be
expected to be subject to volatility resulting from purely market forces over
which we will have no control. If we need additional funding, the market
fluctuations affect on our stock price could limit our ability to obtain equity
financing.
If we
cannot obtain additional funding, we may be required to: (i) limit our
investments in research and development; (ii) limit our marketing efforts; and
(iii) decrease or eliminate capital expenditures.
Such
reductions could materially adversely affect our business and our ability to
compete.
Even if
we do find a source of additional capital, we may not be able to negotiate terms
and conditions for receiving the additional capital that are acceptable to us.
Any future capital investments could dilute or otherwise materially and
adversely affect the holdings or rights of our existing shareholders. In
addition, new equity or convertible debt securities issued by us to obtain
financing could have rights, preferences and privileges senior to our common
stock. We cannot give you any assurance that any additional financing will be
available to us, or if available, will be on terms favorable to us.
|
|
·
|
WE
MAY BE REQUIRED TO REPURCHASE SHARES OF OUR COMMON STOCK IN CONNECTION
WITH THE FINANCIAL CONSULTANCY AGREEMENT AND TECHNICAL CONSULTANCY
AGREEMENTS. THE REPURCHASE OF THESE SHARES MAY HAVE A NEGATIVE IMPACT ON
OUR PROFITABILITY.
|
In
connection with the Equity Transfer Agreements, we entered into a Financial
Consultancy Agreement on April 5, 2007 with Super Pioneer for financial
consultancy services rendered by Super Pioneer, and Technical Consultancy
Agreements on June 11, 2007 with Yaojin Wang and Huimin Zhang for technical
consultancy services rendered. Pursuant to these Agreements, we agreed to issue
2,100,000 shares of our common stock to Super Pioneer, 33,585 shares of our
common stock to Yaojin Wang, and 55,975 shares of our common stock to Huimin
Zhang within three months from the date of the agreements. The parties to the
agreements agreed to lock up the shares for a period of twelve months from the
date of the issuance of the shares (the “Lock-up Period”). Within three months
from the Lock-up Period, in the event that the public trading price of our
shares did not reach $3.60 per share and we are not listed in the capital market
of NASDAQ or AMEX, Super Pioneer shall have the option to require us to redeem
1,960,000 shares of the stock owned by Super Pioneer and Yaojin Wang and Huimin
Zhang shall have the option to require us to redeem the shares of the stock
owned by Yaojin Wang and Huimin Zhang at a price of $3.60 per share. Such option
shall expire within one month from the last date of the three month period.
Therefore, as of July 5, 2008, we may be required to redeem 1,960,000 shares of
common stock held by Super Pioneer at a price of $3.60 per share for a total of
$7,560,000. As of September 11, 2008, we may be required to redeem the 33,585
shares of our common stock held by Yaojin Wang at $3.60 per share for a total of
$120,906 and the 55,975 shares held by Huimin Zhang at $3.60 per share for a
total of $201,510. According to the signed agreements, the redemption would have
been required to be performed in September 2008. The redemption requests were
made to the Company in January 2008 and the company has already obtained oral
consent from Super Pioneer, Wang and Zhang that the payment would be deferred to
the year of 2010.
As
of May 17, 2010, the last closing bid price of our common stock was $.04. If we
would be required to make payment for the shares of our common stock a price of
$3.60 per share, we will not have these funds to devote to our operations and
could have a negative impact on our profitability.
38
|
·
|
JIANGLING BENDA AND
YIDU BENDA CURRENTLY ARE ENTITLED TO A BENEFICIAL TAX EXEMPTION FOR A FIVE
YEAR PERIOD; HOWEVER, SUCH TAX EXEMPTION MAY BE INTERPRETED TO BE NOT IN
COMPLIANCE WITH PRC TAX LAWS IN THE FUTURE CAUSING US TO SET ASIDE CERTAIN
CONTINGENCY FUNDS FOR DEALING WITH POTENTIAL RETROSPECTIVE TAX
LIABILITIES.
|
Jiangling
Benda and Yidu Benda are entitled to enjoy the “Two exemption Three half” tax
holiday based on the local government’s policy to encourage outside investment
into the locality. However, the definition of the “Two exemption Three half”
policy defined by Yidu City and Jiangling County’s governments is different from
the usual understanding of the term. According to PRC tax laws, “Two exemption
Three half” policy means foreign investment enterprises including Benda Ebei may
enjoy an exemption from corporate income tax for 2 years starting from its first
profitable year, followed by 3 years at a rate that is one half of the regular
rate for corporate income tax. However, in the documents issued by Yidu City and
Jiangling County’s governments, the term means that an outside enterprise can
enjoy the “two exemption three half” privilege treatment only with respect to the part
allotted to the local government . According to our Chinese legal
counsel, under current PRC tax laws, most enterprises are required to pay
corporate income tax at a rate of 33% of its income before tax, of which, about
90.91% (30% of the corporate income) is paid over to the Central government,
only about 9.09% (3% of the corporate incomes) is reserved for the local
government. Accordingly, there is a risk that the local government may only be
able to dispose of the “9.09% (3%)” reserved for the local government and
Jiangling Benda and Yidu Benda actually would receive much less preferential
treatment than a foreign investment enterprise could enjoy.
The
beneficial tax exemption of Jiangling Benda and Yidu Benda was started in the
year of 2005, and they would subject to corporate income tax rate 18% starting
from the year of 2007 and they would subject to corporate income tax at 25%,
full income tax rate, according to the new PRC corporate income tax with the
effective date January 1, 2008.
If
Jiangling Benda and Yidu Benda could not have any beneficial tax exemption, the
tax contingency, at full rate 25%, would be as follows:
|
|
a)
|
For
the year of 2009: Jiangling Benda: Nil; Yidu Benda:
Nil;
|
|
|
b)
|
For
the year of 2008: Jiangling Benda: Nil; Yidu Benda:
Nil.
|
As to the
tax treatment promised by local governments to purely domestic enterprises,
i.e., Jiangling Benda and Yidu Benda, invested by non-local (but not foreign)
investors under the so called preferential policy announced by local
governments, our consultation with PRC certified public accountants and lawyers,
is that the above policy is not compliant with the PRC laws. Even though
such practice exists in many areas across the country, the policy faces the risk
of being ruled illegal at any time for non-compliance with relevant laws. In
this event, there is a risk that we might be assessed retrospective tax
liabilities.
39
|
·
|
WE MAY HAVE
DIFFICULTY DEFENDING OUR INTELLECTUAL PROPERTY RIGHTS FROM INFRINGEMENT
RESULTING IN LAWSUITS REQUIRING US TO DEVOTE FINANCIAL AND MANAGEMENT
RESOURCES THAT WOULD HAVE A NEGATIVE IMPACT ON OUR OPERATING
RESULTS.
|
We regard
our service marks, trademarks, trade secrets, patents and similar intellectual
property as critical to our success. We rely on trademark, patent and trade
secret law, as well as confidentiality and license agreements with certain of
our employees, customers and others to protect our proprietary rights. We have
received trademark and patent protection for certain of our products in the
People's Republic of China. No assurance can be given that our patents and
licenses will not be challenged, invalidated, infringed or circumvented, or
that our intellectual property rights will provide competitive advantages to us.
There can be no assurance that we will be able to obtain a license from a
third-party technology that we may need to conduct our business or that such
technology can be licensed at a reasonable cost.
Presently,
all of our products are sold to clients in PRC. To date, no trademark or patent
filings have been made other than in PRC. To the extent that we market our
products in other countries, we may have to take additional action to protect
our intellectual property. The measures we take to protect our proprietary
rights may be inadequate and we cannot give you any assurance that our
competitors will not independently develop formulations and processes that are
substantially equivalent or superior to our own or copy our
products.
Currently,
the State Food and Drug Administration (“SFDA”) does not automatically stay drug
registration approval upon initiation of an infringement lawsuit by a third
party. At present, we must wait until a copycat manufacturer has received
marketing approval from SFDA before we can bring an infringement lawsuit.
Furthermore, Chinese courts have been hesitant to issue preliminary injunctions
to suspend sales until a final judgment is issued in the lawsuit. Our sales
could be lowered were a competitor to infringe our intellectual property rights
by marketing one or more versions of SFDA-approved drugs proprietary to us, such
as the Qiweiben capsule, until we can curtail such infringement through legal
action. Pursuing infringement lawsuits would require us to devote financial and
management resources that could impact the results of our
operations.
|
·
|
WE DEPEND ON THE
SUPPLY OF RAW MATERIALS, AND ANY ADVERSE CHANGES IN SUCH INTENSE
COMPETITION FROM EXISTING AND NEW ENTITIES MAY ADVERSELY AFFECT OUR
REVENUES AND
PROFITABILITY.
|
We
compete with other companies, many of whom are developing or can be expected to
develop products similar to ours. Our markets are large with many competitors.
The market for pharmaceutical raw materials manufacturing is particularly
competitive. In this market, our competitors include a number of contract
manufacturers and, from time to time, demand for particular products may be
greatly exceeded by production capacity. Many of our competitors are more
established than we are, and have significantly greater financial, technical,
marketing and other resources than us. Some of our competitors may have greater
name recognition and a larger customer base. These competitors may be able to
respond more quickly to new or changing opportunities and customer requirements
and may be able to undertake more extensive promotional activities, offer more
attractive terms to customers, and adopt more aggressive pricing policies. We
intend to create greater brand awareness for our brand name so that we can
successfully compete with our competitors. We cannot assure you that we will be
able to compete effectively with current or future competitors or that the
competitive pressures we face will not harm our business.
40
Our
company does not depend on any sole source suppliers, since the raw materials
market is opened in the sense that we could have plenty of choices of the
suppliers. We only choose those suppliers who could offer better terms to us and
having a long term co-operation relationship.
|
|
·
|
OUR
PRODUCTS AND THE PROCESSES COULD EXPOSE US TO SUBSTANTIAL PRODUCT
LIABILITY CLAIMS WHICH WILL NEGATIVELY IMPACT OUR
PROFITABILITY.
|
We face
an inherent business risk of exposure to product liability claims in the event
that the use of our products is alleged to have resulted in adverse side
effects. Side effects or marketing or manufacturing problems pertaining to any
of our products could result in product liability claims or adverse publicity.
These risks will exist for those products in clinical development and with
respect to those products that have received regulatory approval for commercial
sale. We do not have insurance to cover any potential product liability claims.
To date, we have not experienced any product liability claims. However, that
does not mean that we will not have any such claims with respect to our products
in the future which will negatively impact our profitability.
|
·
|
WE
DEPEND ON OUR KEY MANAGEMENT PERSONNEL AND THE LOSS OF THEIR SERVICES
COULD ADVERSELY AFFECT OUR
BUSINESS.
|
We place
substantial reliance upon the efforts and abilities of our executive officers,
Yiqing Wan, our Chairman and Chief Executive Officer, Wei Xu, our Vice President
of Operations, Hui Long, our Vice President of Technology. We have entered into
a five year employment contract with Mr. Wan and three year contracts with Ms.
Xu. As per his contract, Mr. Wan is to receive salary of $160,000 per year, Ms.
Xu is to receive $100,000, with the possibility of a discretionary bonus as
determined by the Board of Directors. Each employment contract immediately
terminates upon death or disability, and may be terminated by the Company either
with or without cause after 30 days notice, or terminated by the officer for
good reason with 60 days notice. We are not currently aware of the plans of any
key employees to retire or leave the Company. However, the loss of the services
of any of our executive officers could have a material adverse effect on our
business, operations, revenues or prospects. We do not maintain key man life
insurance on the lives of these individuals.
|
|
·
|
MR.
YIQING WAN AND MS. WEI XU ARE HUSBAND AND WIFE. THE SEPARATION OR DIVORCE
OF THE COUPLE IN THE FUTURE COULD ADVERSELY AFFECT OUR
BUSINESS.
|
Mr.
Yiqing Wan, our Chairman and Chief Executive Officer, and Ms. Wei Xu, Vice
President of Operations, are married. They are two of our principal executives
and are a vital part of our operations. If they were to become separated or
divorced and could not amicably work with each other, one of them may decide to
cease his or her employment with us. Alternatively, their work performance may
not be satisfactory if they become preoccupied with such negative situation. In
both cases, our operations could be negatively affected by our production
resulting in a decrease in revenues.
|
|
·
|
MANAGEMENT
EXERCISES SIGNIFICANT CONTROL OVER MATTERS REQUIRING SHAREHOLDER APPROVAL
WHICH MAY RESULT IN THE DELAY OR PREVENTION OF A CHANGE IN OUR
CONTROL.
|
Mr.
Yiqing Wan, our Chairman and Chief Executive Officer, and Ms. Wei Xu, our Vice
President of Operations, each have a 50% equity ownership in XIA Pharmaceutical
Inc. Through their joint ownership in XIA Pharmaceutical, Inc., Mr. Wan and Ms.
Xu currently share voting power equal to approximately 42.50% of our voting
securities. When combined with the common stock ownership of our other officers
and directors, management has combined voting power in our Company equal to
approximately 42.50% of our voting securities. As a result, management through
such stock ownership exercises significant control over all matters requiring
shareholder approval, including the election of directors and approval of
significant corporate transactions. This concentration of ownership in
management may also have the effect of delaying or preventing a change in
control of us that may be otherwise viewed as beneficial by shareholders other
than management.
41
|
|
·
|
INTERNATIONAL
OPERATIONS REQUIRE US TO COMPLY WITH A NUMBER OF UNITED STATES AND
INTERNATIONAL REGULATIONS WHICH MAY HAVE A NEGATIVE IMPACT ON OUR
GROWTH.
|
We are
required to comply with a number of international regulations in countries
outside of the United States. In addition, we must comply with the Foreign
Corrupt Practices Act, or FCPA, which prohibits U.S. companies or their agents
and employees from providing anything of value to a foreign official for the
purposes of influencing any act or decision of these individuals in their
official capacity to help obtain or retain business, direct business to any
person or corporate entity or obtain any unfair advantage. Any failure by us to
adopt appropriate compliance procedures and ensure that our employees and agents
comply with the FCPA and applicable laws and regulations in foreign
jurisdictions could result in substantial penalties and/or restrictions in our
ability to conduct business in certain foreign jurisdictions. We believe we are
currently in compliance with such regulations. The U.S. Department of The
Treasury's Office of Foreign Asset Control, or OFAC, administers and enforces
economic and trade sanctions against targeted foreign countries, entities and
individuals based on U.S. foreign policy and national security goals. As a
result, we are restricted from entering into transactions with certain
targeted foreign countries, entities and individuals except as permitted by OFAC
which may reduce our future growth.
|
|
·
|
WE
MAY INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES
CORPORATE GOVERNANCE AND ACCOUNTING
REQUIREMENTS.
|
We may
incur significant costs associated with our public company reporting
requirements, costs associated with newly applicable corporate governance
requirements, including requirements under the Sarbanes-Oxley Act of 2002 and
other rules implemented by the Securities and Exchange Commission. We expect all
of these applicable rules and regulations to significantly increase our legal
and financial compliance costs and to make some activities more time consuming
and costly. We also expect that these applicable rules and regulations may make
it more difficult and more expensive for us to obtain director and officer
liability insurance and we may be required to accept reduced policy limits and
coverage or incur substantially higher costs to obtain the same or similar
coverage. As a result, it may be more difficult for us to attract and retain
qualified individuals to serve on our board of directors or as executive
officers. We are currently evaluating and monitoring developments with respect
to these newly applicable rules, and we cannot predict or estimate the amount of
additional costs we may incur or the timing of such costs.
|
|
·
|
WE
MAY NOT BE ABLE TO MEET THE ACCELERATED FILING AND INTERNAL CONTROL
REPORTING REQUIREMENTS IMPOSED BY THE SECURITIES AND EXCHANGE COMMISSION
RESULTING IN A POSSIBLE DECLINE IN THE PRICE OF OUR COMMON STOCK AND OUR
INABILITY TO OBTAIN FUTURE
FINANCING.
|
As
directed by Section 404 of the Sarbanes-Oxley Act, the Securities and Exchange
Commission adopted rules requiring each public company to include a report of
management on the company's internal controls over financial reporting in its
annual reports. In addition, the independent registered public accounting firm
auditing a company's financial statements must also attest to and report on
management's assessment of the effectiveness of the company's internal controls
over financial reporting as well as the operating effectiveness of the company's
internal controls. We will not be subject to these requirements for the fiscal
year ended December 31, 2009.
While we
expect to expend significant resources in developing the necessary documentation
and testing procedures required by Section 404 of the Sarbanes-Oxley Act, there
is a risk that we may not be able to comply timely with all of the requirements
imposed by this rule. In the event that we are unable to receive a positive
attestation from our independent registered public accounting firm with respect
to our internal controls, investors and others may lose confidence in the
reliability of our financial statements and our stock price and ability to
obtain equity or debt financing as needed could suffer.
42
In
addition, in the event that our independent registered public accounting firm is
unable to rely on our internal controls in connection with its audit of our
financial statements, and in the further event that it is unable to devise
alternative procedures in order to satisfy itself as to the material accuracy of
our financial statements and related disclosures, it is possible that we would
be unable to file our Annual Report on Form 10-K with the Securities and
Exchange Commission, which could also adversely affect the market price of our
common stock and our ability to secure additional financing as
needed.
Risks
Relating to the People's Republic of China
|
|
·
|
THE
SALES PRICES OF SOME MEDICINES ARE CURRENTLY CONTROLLED BY THE CHINESE
GOVERNMENT AND THAT MAY ADVERSELY AFFECT OUR
BUSINESS.
|
Prices
paid by end consumers for many of our medicines are regulated by PRC's State
Development and Reform Commission. PRC justifies its need to control the drug
prices on the basis that, at present, only workers at state or private companies
have health insurance. About 900 million rural Chinese people and 35 million
urban unemployed Chinese people lack insurance coverage and cannot afford
expensive drugs. Our future profitability might suffer if a significant portion
of our revenues were to be derived from products whose final selling prices were
state-controlled and if those prices were held at levels close to or below our
cost of sales.
|
|
·
|
SALES
OF OUR PRODUCTS COULD BE HARMED BY THE WIDESPREAD PRESENCE OF COUNTERFEIT
MEDICATION IN PRC NEGATIVELY IMPACTING OUR
PROFITABILITY.
|
Chinese
counterfeiting of pharmaceuticals and other products affecting public health has
grown in tandem with counterfeiting and piracy of goods such as brand-name
clothing, compact discs and computer software. Exact data are impossible to
collect, but the FBI believes that more than half of the pharmaceuticals sold in
PRC are counterfeit. Examples of the seriousness of the problem include: six
months after Viagra was introduced in 2002, state media reported that some 90
percent of little blue pills sold in Shanghai were counterfeit; and 192,000
Chinese patients were reported to have died in 2001 from fake drugs. Counterfeit
products shrink markets for legitimate goods. This situation affects Benda and
other major domestic and foreign drug manufacturers in PRC, especially for
products marketed through the OTC rather than hospital channel. However, we
believe the Chinese authorities are becoming increasingly vigilant against
counterfeiting because in 2001 the authorities closed 1,300 factories while
investigating 480,000 cases of counterfeit drugs. Currently, active
pharmaceutical ingredients are governed only by chemical regulations. We believe
that a major step towards controlling the problem would be taken should the SFDA
be given oversight over PRC’s bulk chemicals producers. However, our ability to
increase sales as rapidly as we would like, and our profitability, could be
affected if this problem persists or worsens.
|
|
·
|
THERE
COULD BE CHANGES IN GOVERNMENT REGULATIONS TOWARDS THE PHARMACEUTICAL AND
HEALTH SUPPLEMENT INDUSTRIES THAT MAY ADVERSELY AFFECT OUR GROWTH AND
PROFITABILITY.
|
The
manufacture and sale of APIs in the PRC is heavily regulated by many state,
provincial and local authorities. These regulations have significantly increased
the difficulty and costs involved in obtaining and maintaining regulatory
approvals for marketing new and existing products. Our future growth and
profitability depend to a large extent on our ability to obtain regulatory
approvals.
The SFDA
of PRC recently implemented new guidelines for licensing of APIs. All existing
manufacturers with licenses were required to apply for GMP certifications by
June 30, 2004, and to receive approvals by December 31, 2004. We have received
certification for our Benda Ebei injection vial production facilities and expect
to receive certifications for the remaining plant that require such
certification in 2008. However, should we fail to receive or maintain the GMP
certifications under the new guidelines in the future; our businesses would be
materially and adversely affected.
43
Moreover,
the laws and regulations regarding acquisitions of the pharmaceutical industry
in the PRC may also change and may significantly impact our ability to grow
through acquisitions.
|
|
·
|
CERTAIN
POLITICAL AND ECONOMIC CONSIDERATIONS RELATING TO THE PRC COULD ADVERSELY
AFFECT OUR COMPANY.
|
The PRC
is transitioning from a planned economy to a market economy. While the PRC
government has pursued economic reforms since its adoption of the open-door
policy in 1978, a large portion of the PRC economy is still operating under
five-year plans and annual state plans. Through these plans and other economic
measures, such as control on foreign exchange, taxation and restrictions on
foreign participation in the domestic market of various industries, the PRC
government exerts considerable direct and indirect influence on the economy.
Many of the economic reforms carried out by the PRC government are unprecedented
or experimental, and are expected to be refined and improved. Other
political, economic and social factors can also lead to further readjustment of
such reforms. This refining and readjustment process may not necessarily have a
positive effect on our operations or future business development. Our
operating results may be adversely affected by changes in the PRC's
economic and social conditions as well as by changes in the policies of the PRC
government, such as changes in laws and regulations (or the official
interpretation thereof), measures which may be introduced to control inflation,
changes in the interest rate or method of taxation, and the imposition of
additional restrictions on currency conversion.
|
|
·
|
THE
RECENT NATURE AND UNCERTAIN APPLICATION OF MANY PRC LAWS APPLICABLE TO US
CREATE AN UNCERTAIN ENVIRONMENT FOR BUSINESS OPERATIONS AND THEY COULD
HAVE A NEGATIVE EFFECT ON US.
|
The PRC
legal system is a civil law system. Unlike the common law system, the civil law
system is based on written statutes in which decided legal cases have little
value as precedents. In 1979, the PRC began to promulgate a comprehensive system
of laws and has since introduced many laws and regulations to provide general
guidance on economic and business practices in the PRC and to regulate foreign
investment. Progress has been made in the promulgation of laws and regulations
dealing with economic matters such as corporate organization and governance,
foreign investment, commerce, taxation and trade. The promulgation of new laws,
changes of existing laws and the abrogation of local regulations by
national laws could have a negative impact on our business and business
prospects.
Since all
the Company’s operation plants are all located in China, therefore we have to
comply with those relevant PRC laws and regulations. The changes of those
relevant PRC laws and regulations do affect our operation, for example, due to
the changes of the relevant regulations of SFDA in PRC, it may take more time to
approve the application of new drug certificate so that the forecasted
operation plan for such particular drug product would be delayed and thus
the potential earning of revenue and net profit would be affectee. In this
aspect, the new drug certificate application progresses of Qiweiben Capsule and
Yan Long Anti-cancer Oral Liquid are delayed. Furthermore, due to the
changes of the requirements of the Environmental PRC agencies, Yidu Benda’s
operation was ceased since January 2007, therefore the company needs to
re-design the production facilities so the process of re-opening is also
delayed. As these laws, regulations and legal requirements are relatively
recent, their interpretation and enforcement involve significant
uncertainty.
|
|
·
|
THE
APPROVAL OF THE CHINESE SECURITIES REGULATORY COMMISSION (“CRSC”) MAY BE
REQUIRED IN CONNECTION WITH THIS OFFERING UNDER A RECENTLY ADOPTED PRC
REGULATION; SINCE THIS OFFERING DID NOT COMMENCE PRIOR TO THE EFFECTIVE
DATE OF THE REGULATION, WE MAY BE REQUIRED TO OBTAIN CRSC APPROVAL FOR
THIS OFFERING AND WE CAN NOT CURRENTLY PREDICT THE CONSEQUENCES OF ANY
FAILURE TO OBTAIN SUCH
APPROVAL.
|
44
On August
8, 2006, six PRC regulatory agencies, including the Chinese Securities
Regulatory Commission, or CSRC, promulgated a regulation that became effective
on September 8, 2006. This regulation, among other things, purports to require
offshore special purpose vehicles, or SPVs, formed for listing purposes through
acquisitions of PRC domestic companies and controlled by PRC individuals, such
as our company, to obtain the approval of the CSRC prior to publicly listing
their securities on an overseas stock exchange. While the application of this
new regulation is not yet clear, we believe, based on the advice of our PRC
counsel, that CSRC approval is not required if trading of our shares of common
stock commenced prior to the effective date of the regulation. Although the CSRC
is expected to promulgate formal implementing rules and/or regulations and
possibly other clarifications, the procedures, criteria and timing for obtaining
any required CSRC approval have not been established and it is unclear when
these will be established. Therefore, since this offering did not commence prior
to the effective date of the regulation and our shares of common stock did not
commence trading prior to the effective date of the regulation, we may be
required to obtain CSRC approval for this offering and we cannot currently
predict the criteria, timing or procedures for obtaining the CSRC approval or
the consequences of any failure to obtain such approval.
|
·
|
RECENT
PRC REGULATIONS RELATING TO THE ESTABLISHMENT OF OFFSHORE SPECIAL PURPOSE
COMPANIES BY PRC RESIDENTS MAY SUBJECT OUR PRC RESIDENT SHAREHOLDERS TO
PERSONAL LIABILITY AND LIMIT OUR ABILITY TO INJECT CAPITAL INTO OUR PRC
SUBSIDIARIES, LIMIT OUR PRC SUBSIDIARIES’ ABILITY TO DISTRIBUTE PROFITS TO
US, OR OTHERWISE ADVERSELY AFFECT
US.
|
The State
Administration of Foreign Exchange (“SAFE”) was one of six PRC regulatory
agencies promulgating a regulation that became effective on September 8, 2006
requiring PRC residents to register with the local SAFE branch before
establishing or controlling any company outside of China for the purpose of
capital financing with assets or equities of PRC companies, referred to in the
notice as an “offshore special purpose company.” PRC residents that are
shareholders of offshore special purpose companies established before November
1, 2005 were required to register with the local SAFE branch before March 31,
2006. Our current beneficial owners who are PRC residents have registered with
the local SAFE branch as required under the SAFE notice. The failure of these
beneficial owners to timely amend their SAFE registrations pursuant to the SAFE
notice or the failure of future beneficial owners of our company who are PRC
residents to comply with the registration procedures set forth in the SAFE
notice may subject such beneficial owners to fines and legal sanctions and may
also limit our ability to contribute additional capital into our PRC
subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to our
company or otherwise adversely affect our business.
Other
Risks
|
|
·
|
CURRENCY
CONVERSION AND EXCHANGE RATE VOLATILITY COULD ADVERSELY AFFECT OUR
FINANCIAL CONDITION.
|
The PRC
government imposes control over the conversion of Renminbi into foreign
currencies. Under the current unified floating exchange rate system, the
People's Bank of China publishes an exchange rate, which we refer to as the PBOC
exchange rate, based on the previous day's dealings in the inter-bank foreign
exchange market. Financial institutions authorized to deal in foreign currency
may enter into foreign exchange transactions at exchange rates within an
authorized range above or below the PBOC exchange rate according to market
conditions.
Pursuant
to the Foreign Exchange Control Regulations of the PRC issued by the State
Council which came into effect on April 1, 1996, and the Regulations on the
Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which
came into effect on July 1, 1996, regarding foreign exchange control, conversion
of Renminbi into foreign exchange by Foreign Investment Enterprises, or FIEs,
for use on current account items, including the distribution of dividends and
profits to foreign investors, is permissible. FIEs are permitted to convert
their after-tax dividends and profits to foreign exchange and remit such foreign
exchange to their foreign exchange bank accounts in the PRC. Conversion of
Renminbi into foreign currencies for capital account items, including direct
investment, loans, and security investment, is still under certain restrictions.
On January 14, 1997, the State Council amended the Foreign Exchange Control
Regulations and added, among other things, an important provision, which
provides that the PRC government shall not impose restrictions on recurring
international payments and transfers under current account
items.
45
Enterprises
in the PRC (including FIEs) which require foreign exchange for transactions
relating to current account items, may, without approval of the State
Administration of Foreign Exchange, or SAFE, effect payment from their foreign
exchange account or convert and pay at the designated foreign exchange banks by
providing valid receipts and proofs.
Convertibility
of foreign exchange in respect of capital account items, such as direct
investment and capital contribution, is still subject to certain restrictions,
and prior approval from the SAFE or its relevant branches must be
sought.
Furthermore,
the Renminbi is not freely convertible into foreign currencies nor can freely
remitted abroad. Under the PRC’s Foreign Exchange Control Regulations and the
Administration of Settlement, Sales and Payment of Foreign Exchange Regulations,
foreign invested enterprises are permitted either to repatriate or distribute
its profits or dividends in foreign currencies out of its foreign exchange
accounts, or exchange Renminbi for foreign currencies through banks authorized
to conduct foreign exchange business. The conversion of Renminbi into foreign
exchange by foreign invested enterprises for recurring items, including the
distribution of dividends to foreign investors, is permissible. The conversion
of Reminbi into foreign currencies for capital items, such as direct investment,
loans and security investment, is subject, however, to more stringent
controls.
Since
1994, the exchange rate for Renminbi against the United States dollar has
remained relatively stable, most of the time in the region of approximately
RMB8.28 to $1.00. However, in 2005, the Chinese government announced that it
would begin pegging the exchange rate of the Chinese Renminbi against a number
of currencies, rather than just the U.S. dollar and, the exchange rate for the
Renminbi against the U.S. dollar became RMB8.02 to $1.00. As our operations are
primarily in PRC, any significant revaluation or devaluation of the Chinese
Renminbi may materially and adversely affect our cash flows, revenues and
financial condition. We may not be able to hedge effectively against in any
such case. For example, to the extent that we need to convert United States
dollars into Chinese Renminbi for our operations, appreciation of this currency
against the United States dollar could have a material adverse effect on our
business, financial condition and results of operations. Conversely, if we
decide to convert Chinese Renminbi into United States dollars for other business
purposes and the United States dollar appreciates against this currency, the
United States dollar equivalent of the Chinese Renminbi we convert would be
reduced. There can be no assurance that future movements in the exchange rate of
Renminbi and other currencies will not have an adverse effect on our financial
condition. Benda’s operating companies are FIEs to which the Foreign Exchange
Control Regulations are applicable. There can be no assurance that we will be
able to obtain sufficient foreign exchange to pay dividends or satisfy other
foreign exchange requirements in the future.
|
|
·
|
IT
MAY BE DIFFICULT TO AFFECT SERVICE OF PROCESS AND ENFORCEMENT OF LEGAL
JUDGMENTS UPON OUR COMPANY AND OUR OFFICERS AND DIRECTORS BECAUSE THEY
RESIDE OUTSIDE THE UNITED STATES.
|
As our
operations are presently based in PRC and a majority of our directors and all of
our officers reside in PRC, service of process on our company and such directors
and officers may be difficult to effect within the United States. Also, our main
assets are located in PRC and any judgment obtained in the United States against
us may not be enforceable outside the United States.
46
|
|
·
|
ANY
FUTURE OUTBREAK OF AVIAN INFLUENZA, OR THE ASIAN BIRD FLU, OR ANY OTHER
EPIDEMIC IN PRC COULD HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS
OPERATIONS, FINANCIAL CONDITION AND RESULTS OF
OPERATIONS.
|
Since
mid-December 2003, a number of Asian countries have reported outbreaks of highly
pathogenic avian influenza in chickens and ducks. Since all of our operations
are in PRC, an outbreak of the Asian Bird Flu in PRC in the future may disrupt
our business operations and have a material adverse effect on our financial
condition and results of operations. For example, a new outbreak of Asian Bird
Flu, or any other epidemic, may reduce the level of economic activity in
affected areas, which may lead to a reduction in our revenue if our clients
cancel existing contracts or defer future expenditures. In addition, health or
other government regulations may require temporary closure of our offices, or
the offices of our customers or partners, which will severely disrupt our
business operations and have a material adverse effect on our financial
condition and results of operations.
|
|
·
|
OUR
BUSINESS MAY BE AFFECTED BY UNEXPECTED CHANGES IN REGULATORY REQUIREMENTS
IN THE JURISDICTIONS IN WHICH WE
OPERATE.
|
We are
subject to many general regulations governing business entities and their
behavior in PRC and in other jurisdictions in which we have, or plan to have,
operations and market our products. In particular, we are subject to laws and
regulations covering food, dietary supplements and APIs. Such regulations
typically deal with licensing, approvals and permits. Any change in product
licensing may make our products more or less available on the market. Such
changes may have a positive or negative impact on the sale of our products and
may directly impact the associated costs in compliance and our operational and
financial viability. Such regulatory environment also covers any existing or
potential trade barriers in the form of import tariff and taxes that may make it
difficult for us to import our products to certain countries and regions,
such as Hong Kong, which would limit our international expansion.
|
|
·
|
WE
MAY EXPERIENCE CURRENCY FLUCTUATION AND LONGER EXCHANGE RATE PAYMENT
CYCLES WHICH WILL NEGATIVELY AFFECT THE COSTS OF OUR PRODUCTS SOLD AND THE
VALUE OF OUR LOCAL CURRENCY
PROFITS.
|
The local
currencies in the countries in which we sell our products may fluctuate in value
in relation to other currencies. Such fluctuations may affect the costs of our
products sold and the value of our local currency profits. While we are not
conducting any meaningful operations in countries other than PRC at the present
time, we may expand to other countries and may then have an increased risk of
exposure of our business to currency fluctuation.
|
|
·
|
SINCE
MOST OF OUR ASSETS ARE LOCATED IN PRC, ANY DIVIDENDS OF PROCEEDS FROM
LIQUIDATION IS SUBJECT TO THE APPROVAL OF THE RELEVANT CHINESE GOVERNMENT
AGENCIES.
|
Our
assets are predominantly located inside PRC. Under the laws governing foreign
invested enterprises in PRC, dividend distribution and liquidation are allowed
but subject to special procedures under the relevant laws and rules. Any
dividend payment will be subject to the decision of the board of directors and
subject to foreign exchange rules governing such repatriation. Any liquidation
is subject to the relevant government agency's approval and supervision as well
as the foreign exchange control. This may generate additional risk for our
investors in case of dividend payment and liquidation.
Risks
Associated with Our Shares of Common Stock
|
|
·
|
OUR
SHARES OF COMMON STOCK ARE VERY THINLY TRADED, AND THE PRICE MAY NOT
REFLECT OUR VALUE AND THERE CAN BE NO ASSURANCE THAT THERE WILL BE AN
ACTIVE MARKET FOR OUR SHARES OF COMMON STOCK EITHER NOW OR IN THE
FUTURE.
|
47
Our
shares of common stock are very thinly traded, and the price if traded may not
reflect our value. There can be no assurance that there will be an active market
for our shares of common stock either now or in the future. The market liquidity
will be dependent on the perception of our operating business and any steps that
our management might take to bring us to the awareness of investors. There can
be no assurance given that there will be any awareness generated. Consequently,
investors may not be able to liquidate their investment or liquidate it at a
price that reflects the value of the business. If a more active market should
develop, the price may be highly volatile. Because there may be a low price for
our shares of common stock, many brokerage firms may not be willing to effect
transactions in the securities. Even if an investor finds a broker willing to
effect a transaction in the shares of our common stock, the combination of
brokerage commissions, transfer fees, taxes, if any, and any other selling costs
may exceed the selling price. Further, many lending institutions will not permit
the use of such shares of common stock as collateral for any loans.
Certain
of our stockholders have been granted registration rights which entitle them to
require us to register their shares of our common stock at the same time that
the shares of our common stock are being registered. The inclusion of this
additional stock in the registration may negatively affect the resale price
that may be obtained for the shares of our common stock when they are
registered.
|
|
·
|
WE
ARE SUBJECT TO THE PENNY STOCK RULES WHICH WILL MAKE THE SHARES OF OUR
COMMON STOCK MORE DIFFICULT TO
SELL.
|
We are
subject to the SEC’s “penny stock” rules as our shares of common stock sell
below $5.00 per share. Penny stocks generally are equity securities
with a price of less than $5.00. The penny stock rules require broker-dealers to
deliver a standardized risk disclosure document prepared by the SEC which
provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also
provide the customer with current bid and offer quotations
for the penny stock, the compensation of the broker-dealer and its salesperson,
and monthly account statements showing the market value of
each penny stock held in the customer's account. The bid and offer quotations,
and the broker-dealer and salesperson compensation information must be given to
the customer orally or in writing prior to completing the transaction and must
be given to the customer in writing before or with the customer's
confirmation.
In
addition, the penny stock rules require that prior to a transaction, the broker
dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the
purchaser's written agreement to the transaction. The penny
stock rules are burdensome and may reduce purchases of any offerings and reduce
the trading activity for shares of our common stock. As long as our shares of
common stock are subject to the penny stock rules, the holders
of such shares of common stock may find it more difficult to sell their
securities.
48
|
|
·
|
THERE
CAN BE NO ASSURANCE THAT THE PRICE OF OUR SHARES OF COMMON STOCK WILL MEET
OR EXCEED THE EXERCISE PRICE OF THE WARRANTS DURING THE EXERCISE PERIOD OR
AT ANY TIME THEREAFTER.
|
Unless
the price of our shares of Common Stock equals or exceeds the exercise price of
the Warrants at the time of such exercise, an Investor may not be able to
exercise his Warrants profitably. There can be no assurance that the price of
our shares of Common Stock will meet or exceed the exercise price of the
Warrants during the exercise period or at any time thereafter. Accordingly,
should an Investor choose to exercise the Warrants, the value of our shares of
Common Stock purchased upon such exercise may be less than the Warrant exercise
price the Investor pays. The Warrant may be worthless and expire unexercised if
the price of our shares of Common Stock does not exceed the Warrant exercise
price. Please see “Description of Securities - Warrants” for additional
information as to the terms of the Warrants.
|
|
·
|
MANAGEMENT
EXERCISES SIGNIFICANT CONTROL OVER MATTERS REQUIRING SHAREHOLDER APPROVAL
AND INVESTORS WILL HAVE CONTROL OVER COMPANY
ACTIONS.
|
As a
practical matter, our officers and directors will have control of us and will be
able to assert significant influence over the election of directors and other
matters presented for a vote of stockholders. Even after the Offering is
complete, a majority of our shares of Common Stock will be owned by Mr. Yiqing
Wan and Ms. Wei Xu, husband and wife. Through their concentration of voting
power, they could delay, deter or prevent a change in our control or other
business combinations that might otherwise be beneficial to the other
stockholders. In deciding how to vote on such matters, Mr. Yiqing Wan and Ms.
Wei Xu may be influenced by interests that conflict with other stockholders’
interests. Investors will not have a voice in management decisions and will
exercise very little control. In the event that the requisite approval of
stockholders is obtained, dissenting or non-participating stockholders generally
would be bound by such vote. Accordingly, Offerees should not subscribe for
Units in this Offering unless they are willing to entrust all aspects of
operational control to our current management team. Further, Investors in the
Offering will rely on our management team to use the proceeds as they determine
to be in our best interest. Although management has indicated its current
intended uses, those may be changed upon their decision.
In
addition, our existing officers, directors, affiliates and other insiders are
permitted to purchase Units in the Offering. If they do so, their interest in us
after the acquisition may be even greater.
|
|
·
|
INVESTORS
MAY EXPERIENCE IMMEDIATE AND SUBSTANTIAL DILUTION IN NET TANGIBLE BOOK
VALUE PER SHARE OF OUR COMMON STOCK IF THEY ELECT TO EXERCISE THE
WARRANTS.
|
Investors
may experience immediate and substantial dilution in net tangible book value per
share of our common stock if they elect to exercise the Warrants. The exercise
price of the Warrants may be substantially higher than the net tangible book
value per share. Accordingly, if Investors exercise the Warrants, they will
likely experience immediate and substantial dilution in the net tangible book
value per share and further dilution if we issue shares of our common stock in
the future. As a result of this dilution, in the event of our subsequent
liquidation, Investors exercising their Warrants may receive significantly less
than the full exercise price that they paid for the shares of our Common
Stock.
|
|
·
|
INVESTORS
MAY EXPERIENCE IMMEDIATE AND SUBSTANTIAL DILUTION IN NET TANGIBLE BOOK
VALUE PER SHARE OF OUR COMMON STOCK IN THE EVENT WE ISSUE SHARES OF OUR
COMMON STOCK IN THE FUTURE.
|
There are
additional authorized but unissued shares of our Common Stock that may be later
issued by our management for any purpose without the consent or vote of the
stockholders. Investors purchasing in this Offering may be further diluted in
their percentage ownership on an as-converted basis in the event additional
shares are issued by us in the future.
49
|
|
·
|
OUR
ARTICLES OF INCORPORATION AUTHORIZE THE ISSUANCE OF SHARES OF PREFERRED
STOCK WHICH, IF ISSUED, THE RIGHTS, PREFERENCES, DESIGNATIONS AND
LIMITATIONS OF SUCH PREFERRED STOCK COULD OPERATE TO THE DISADVANTAGE OF
THE SHARES OF OUR OUTSTANDING COMMON
STOCK.
|
Our
articles of incorporation authorize the issuance of shares of preferred stock,
the rights, preferences, designations and limitations of which may be set by the
board of directors. While no preferred stock is currently outstanding or subject
to be issued, the articles of incorporation have authorized issuance of up to
5,000,000 shares of preferred stock (“Preferred Stock”) in the discretion of the
board of directors. Such Preferred Stock may be issued upon filing of amended
Articles of Incorporation and the payment of required fees; no further
shareholder action is required. If issued, the rights, preferences, designations
and limitations of such Preferred Stock would be set by our board of directors
and could operate to the disadvantage of the shares of our outstanding Common
Stock. Such terms could include, among others, preferences as to dividends and
distributions on liquidation.
ITEM 2. DESCRIPTION OF
PROPERTIES.
Production
Facilities and Equipment
Locations
Our head
office is located in Wuhan, the capital city of Hubei Province. Wuhan is the
biggest hub city in Central China. Divided by the Yangtze River, Wuhan has come
to be known as the Three Towns of Wuhan, with Hankou and Hanyang on the west
bank, and Wuchang on the east. Hubei borders with Henan to the north, Anhui to
the east, Jiangxi to the southeast, Hunan to the south, Chongqing to the west,
and Shanxi to the northwest.
50
Addresses
of Benda Offices and Plants
|
Address
|
Function
|
Size
|
||
|
Hubei
Tongji Benda Ebei Pharmaceutical Co. Ltd., Taibei Mingju, 4th
Floor, 6 Taibei Road, Wuhan, Hubei Province, PRC
|
Headquarters/
administration office
|
800
sq feet (Leased)
|
||
|
Hubei
Tongji Benda Ebei Pharmaceutical Co. Ltd. Shanlihe,Yingshan District,
Guangshui City, Hubei Province, PRC
|
GMP
certified injection vial production plant
|
121,110
sq feet
|
||
|
Jiangling
Benda Pharmaceuticals Co., Ltd., 84 South Street, Tanqiao Town, Jiangling
County, Hubei Province, PRC
|
API
production plant under renovation/ awaiting GMP
certification
|
182,750
sq feet
|
||
|
Yidu
Chemical Industry Co., Ltd., Chayuansi Village, Yidu City, Hubei Province,
PRC
|
Bulk
chemicals production plant
|
172,000
sq feet
|
||
|
Shenzhen
SiBiono Gene Technology Co., Ltd. No.19, Science & Technology
Middle Road, Shenzhen Hi-Tech Industrial Park(Middle), Shenzhen,
PRC
|
Gene
therapy production plant
|
140,000
sq feet
|
||
|
Beijing
Shusai Pharyngitis Research Co., Ltd., 6-3-601, Yangguang Xinganxian,
Yiyuan, Anhuibeili, Chaoyang District, Beijing, PRC
|
Operating,
promoting, and distributing Pharyngitis Killer
|
2,000
sq feet (leased) office space in
Beijing
|
Finished
Medicines - The Benda Ebei Plant
The Benda
Ebei plant produces and packages Benda’s finished medicines. The plant employs
about 200 personnel and has six world-class production lines and 11 stand-alone
machinery for injection vials, with an annual aggregate production capacity of
approximately 900 million units. In PRC, based on management estimate, there are
about 197 factories with similar manufacturing facilities for injection vials.
Benda Ebei’s capacity is sixth in PRC and first in Hubei province. In 2008 and
2007, Benda Ebei produced approximately 420 million and 418 million units of
injection vials respectively, equivalent to 46% capacity. The production
processes and equipment at Benda Ebei meets international quality and control
standards and have been GMP certified by the SFDA since November 2003 and renew
on December 3, 2008 for the production of injection vials; while on November 26,
2008, Benda Ebei received a GMP for the production of tables.
51
Benda
Ebei’s plant will produce lozenges, capsules, granules, oral liquid and pills.
Benda Ebei has invested approximately $5 million on its oral medicine production
facilities. To date, we have already completed the equipment and facilities
installation We expect the production of oral liquid medicines in second quarter
of 2008. The Yanlong Anti-cancer Oral Liquid, Qiweiben Capsule will be produced
in this section of the plant. (Please refer to the section of New Branded
Medicines for the status of progress of these two new drugs.)
Active
Pharmaceutical Ingredients - The Jiangling Benda Plant
We
manufacture APIs at our Jiangling Benda plant. We closed the plant in July 2004
in order to renovate the buildings, equipment and processes and secure GMP
certification by the SFDA. Upon completion, the new plant will be comprised of
three large facilities on a total land area of 68 acres. The total expense of
these renovations will be approximately $3 million. All the equipment has been
ordered.
Our
Jiangling Benda facility was closed for renovation in July 2004 to comply with
GMP standards. The Jiangling Benda plant reopened on August 10, 2007 and has
been producing Ribose, the only product that does not require GMP approval.
Jiangling Benda also plans to produce three other types of active pharmaceutical
ingredients and they are Ribavirin, Asarin and Levofloxacin which need to have
GMP Certificate. On April 9, 2008, Jiangling Benda received the approved
GMP Certificate which authorizing the production of Ribavrin. The other
two products, Asarin and Levolfozacin, are still under the stage of GMP
certificate approving process. The management could not estimate the exact
timing for obtaining those certificates.
Bulk
chemicals - The Yidu Benda Plant
Yidu
Benda was temporarily closed since mid January 2007 to upgrade its waste water
treatment system to comply with new environmental standards enforced by PRC
local government.
Yidu
Benda has completed its upgrading of the waste water system and passed the
government’s verification and testing of equipments in October 2007. It is now
permitted for the testing on actual production process. Once the
actual products are produced, then the environmental government
bodies will re-test the production results. The management could not estimate
the exact timing for obtaining the final approval on the actual production
process
Gene
therapy – SiBiono Plant
On
October 16, 2003, SiBiono successfully obtained a New Drug License from SFDA,
and then, in April 4, 2004, SiBiono obtained “Manufacture Certificate” and
“Certificate of GMP for Pharmaceutical Product”, so far being fully qualified
for the market launch of Recombinant Human Ad-p53 Injection, trademarked as
Gendicine
® in China. Gendicine ® is the
commercialized gene therapy product approved in the PRC government agency.
On May 19, 2008, SiBiono received an official notice from the PRC State of SFDA
in which it mentioned that during the random inspection performed by the PRC
State of SFDA on April 8 to April 10, 2008, the PRC State of SFDA discovered
there were several production procedures that did not meet the requirement
stated in GMP, thus it required SiBiono to perform necessary improvements in
order to fulfill the GMP requirements and the PRC State of SFDA collected back
the distributed GMP certificate until the necessary improvements being carried
out and passed the examination that conducted by SFDA. On June 10, 2008,
SiBiono received another official notice from Guangdong Province SFDA and they
demanded the same requirements as stated in the official notice which issued by
the PRC State of SFDA dated on May 19, 2008. On November 24, 2008, SiBiono
received another official notice from Guangdong Province SFDA which mentioned
that after the examination conducted by Shenzhen City SFDA, the Guangdong
Province SFDA consent SiBiono to carry out production on a trial basis.
It
further required SiBiono strictly to follow the requirements of GMP to organize
trail
production and follow the procedures to apply for GMP Certificate verification.
On July 14, 2009, SiBiono obtained the final approved GMP
Certificate, in order words, the SFDA allows SiBiono to resume its production
and sales.
|
1.
|
On
November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement
with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s
common stock for a total consideration of Rmb12.48 million (Rmb6.24
million in cash and shares of our common stock equal to Rmb6.24 million)
(or $1.71 million) which was due and payable on or before March 31,
2007.
|
Due to
the fact that the signed agreement on November 23, 2006 was not practically
executable according to the PRC regulations, Benda Ebei asked Zhang to terminate
the signed agreement and sign a new agreement that was feasible under PRC
regulations with essentially the same terms.
However,
Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration
Commission (the “Commission”) in April 2007 for enforcement of the original
agreement. Zhang requested the Commission to require Benda Ebei to pay for the
total consideration, penalty for late payment and the related legal and
arbitration expenses.
52
On
November 27, 2007, Shenzhen Arbitration Commission determined that:
|
|
1.
|
Benda
Ebei should pay for the consideration of Rmb 6.24 million, equal to 50% of
the total consideration set forth in the Equity Transfer Agreement. For
the other 50% of the total consideration which was supposed to be settled
in the form of issuing common stock, since Zhang did not make an
arbitration request on how to execute the arrangement, the Arbitration
Commission did not make an award on this particular
part.
|
|
|
2.
|
Benda
Ebei should pay for the penalty of Rmb
46,800;
|
|
|
3.
|
Benda
Eebi should pay for legal and arbitration expenses of Rmb
268,971.
|
On May
22, 2008, Benda Ebei applied to Shenzhen People Court to terminate above
mentioned arbitration. The
termination is based on the ground that Xiaozhi Zhang does not own
all 6.24% of SiBiono’s common
stock. In fact, he only owns 3.28% of SiBiono’s stock.
The application has
been accepted by Shenzhen People Court and is waiting for its further
investigation.
|
2.
|
SiBiono
patents - on January 29, 2008, SiBiono entrusted Grandall Legal Group
Shenzhen Law Firm to issue a legal letter to Zhaohui Peng, one of the
shareholders of Sibiono and the inventor of Gendicine, demanding that he
transfer all the title of patents to
SiBiono.
|
On June
18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights
to the patent “A new method for manufacturing recombinant adenovirus” and
related research results to SiBiono as a payment for the registered capital. In
return, Zhaohui Peng was granted 32.03% of the common stock of
SiBiono.
From 1999
to 2007, SiBiono successfully obtained various technology funds from various
government technology agencies to support the further research and development
activities of Gendicine. Due to this significant funding obtained by SiBiono,
Sibiono developed five additional patents which are summarized as
follows:
|
Countries
/
|
Application
|
Publication
|
Approved
Patent
|
Name
of Patent
|
Name
of
|
Patent
|
|||||||||||
|
Item
|
Patent
name
|
Date
|
Number
(1)
|
Number
(2)
|
Number
(3)
|
Inventor
(6)
|
Applicant
(6)
|
Assignees
|
|||||||||
|
1
|
A
new method for manufacturing recombinant adenovirus
|
||||||||||||||||
| A |
China
|
98123346.5 |
CN1228474A
|
ZL98123346.5
|
Peng
|
Peng
|
SiBiono
|
||||||||||
|
Date
|
1998/12/14
|
1999/9/15
|
2002/7/3
|
||||||||||||||
|
2
|
A
recombinant constructed by a virus vector and a
|
||||||||||||||||
|
human
tumor suppressor gene and its use
|
|||||||||||||||||
| A |
China
|
02115228.4 |
CN1401778A
|
ZL02115228.4
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
||||||||||
|
Date
|
2002/5/8
|
2003/3/12
|
2004/11/24
|
||||||||||||||
| B |
PCT
|
(4)
|
5 |
WO2004/078987A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A | |||||||||
|
Date
|
2004/3/8
|
2004/9/16
|
N/A | ||||||||||||||
|
3
|
Recombinant
gene medicine of adenovirus vector and
|
||||||||||||||||
|
and
gene p54 for treating proloferative diseases
|
|||||||||||||||||
| A |
China
|
03125129.3 |
CN1471977A
|
ZL03125129.3
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
||||||||||
|
Date
|
2003/5/10
|
2004/2/4
|
2007/7/25
|
||||||||||||||
| B |
PCT
|
(4)
|
8 |
WO2004/104204A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A | |||||||||
|
Date
|
2004/5/9
|
2004/12/2
|
N/A | ||||||||||||||
|
4
|
The
application of recombinant adenoviral p53
|
||||||||||||||||
|
as
cancer vaccine (tentative title)
|
|||||||||||||||||
| A |
China
|
200510002779.1 |
CN1679641A
|
ZL200510002779.1
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
||||||||||
|
Date
|
2005/1/26
|
2005/10/12
|
2007/8/29
|
||||||||||||||
| B |
PCT
|
(4)
|
1 |
WO2006/079244A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A | |||||||||
|
Date
|
2005/1/26
|
2006/8/3
|
N/A | ||||||||||||||
| C |
US
|
(5)
|
11/075035 | 2005/0281785A1 |
Not
Approved
|
Peng
/ Zhang
|
Unidentified
Yet
|
N/A | |||||||||
|
Date
|
2005/3/7
|
2005/12/22
|
N/A | ||||||||||||||
|
5
|
Human
Embryonic Kidney (HEK) sub-clone cell line
|
||||||||||||||||
| A |
China
|
03126889.7 |
CN1513985A
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A | ||||||||||
|
Date
|
2003/6/13
|
2004/7/21
|
N/A | ||||||||||||||
| B |
PCT
|
(4)
|
7 |
WO2004/111239
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A | |||||||||
|
Date
|
2004/5/9
|
2004/12/23
|
N/A | ||||||||||||||
|
6
|
The
complex of polypeptide liposome and human VGEF
|
||||||||||||||||
|
gene,
and its use and human VGEF gene, and its use
|
|||||||||||||||||
|
A
|
China
|
02134321.7 |
CN1389269A
|
Not
Approved
|
Peng
/ Zhang / Zhu
|
Peng
/ Zhang / Zhu
|
N/A | ||||||||||
|
Date
|
2002/7/4
|
2003/1/8
|
N/A |
Note:
|
|
(1)
|
Application
number is obtained when application is
submitted;
|
|
|
(2)
|
Publication
number is obtained after the first phase
examination;
|
|
|
(3)
|
Approved
patent number is obtained after the final
examination;
|
|
|
(4)
|
PCT
is referred to an International Patent Organization in
Paris;
|
|
|
(5)
|
US
is referred to the application is made in United States of America
alone;
|
|
|
(6)
|
Peng
is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is
referred to Jinya Zhu.
|
53
As
indicated in the above table, Item 1, the patent “A new method for manufacturing
recombinant adenovirus” had been assigned to SiBiono; however, the other
approved patents (item 2 through item 4) in PRC still have not been
assigned to SiBiono. The Group believes that all the above mentioned patents
should be rightfully transferred to SiBiono, a subsidiary of the Group.
Accordingly, the above mentioned legal letter was issued on this
ground.
On August
27, 2008, the Group through its subsidiary, SiBiono filed an application to the
Guongdong Province Shenzhen City (Middle) Peoples’ Court and demand Zhaozhu Peng
to transfer back all the mentioned patents that mentioned in above to
SiBiono. The case has been
accepted by
the Court and is
waiting for its further investigation.
|
3.
|
The
Company has become aware that Excalibur Limited Partnership and Excalibur
Limited Partnership II (the "Plaintiffs") filed a motion for summary
judgment in lieu of a complaint pursuant to CPLR § 3213 (the "Motion")
with the Supreme Court of the State of New York (the "Court"), alleging
that the Company has been delinquent on the payment of an aggregate sum of
$600,000 and accrued interest and costs arising from the Convertible
Promissory Notes that were issued to the Plaintiffs in April 2007 in
connection with a $7,560,000 private placement. Pursuant to the motion,
the Plaintiffs requested that the Court (1) enter summary judgment in
favor of Excalibur Limited Partnership (“Excalibur Limited”) in the amount
of $390,000 plus all accrued interest and costs, and, (2) enter summary
judgment in favor of Excalibur Small Cap Opportunities LP (“Excalibur
Small Cap”) in the amount of $210,000 and accrued interest and
costs. On July 29, 2009, the Court entered a judgment against the
Company in favor of the Plaintiffs in the amount of $674,251.65 in
connection with the Convertible Promissory Notes issued to the Plaintiffs
in April 2007 in a private
placement.
|
On March
4, 2009, the Company received a Notice and Default and Payment Demand letter
(the "Default Letter") from Pope Investments LLC ("Pope") in connection with its
convertible promissory note in the amount of $5,520,000 (the "Note") purchased
in our April 2007 private placement offering. The Default Letter provided notice
of default based on the Company's failure to make a required interest payment on
the Note by February 20, 2009. The Default Letter further demanded full payment
of all interest, liquidated damages and accrued interest thereon in the amount
of $130,364.37 by March 14, 2009, or Pope will accelerate the maturity date of
the full principal amount of the Note. On April 7, 2009, the Company
received further Default Letters and Payment Demand from Pope, Excalibur Limited
and Excalibur Small Cap demanding payment in full of the balance of the Notes,
which matured on March 28, 2009. The Company was notified on June 15,
2009, that on May 11, 2009 Pope filed a motion for summary judgment in lieu of a
complaint against us pursuant to CPLR § 3213 (the “Motion”) with the Supreme
Court of the State of New York (the “Court”), alleging that the Company has been
delinquent on the payment of an aggregate sum of $5,520,000 and accrued interest
and costs arising from the Note that the Company issued to Pope in April
2007. Pursuant to the motion, the Plaintiffs requested that the Court
enter summary judgment in favor of Plaintiff in the amount of $5,994,617.53
constituting principal and interest, plus costs.
On June
23, 2009, the Company filed an Affidavit in Opposition to Motion for Summary
Judgment in Lieu of Complaint with the Court requesting that Plaintiff’s Motion
be denied. On October 14, 2009, this motion was denied, and the court entered a
judgment in favor of Pope in the amount of $5,520,000 plus
interest.
On July
30, 2009, the Company received a Notice of Default from three additional
investors in the April 2007 private placement offering holding Notes totaling
$90,000.
|
4.
|
On
December 30, 2009, the Company was served with a Summons and Complaint
filed by Pope Investments, LLC (“Pope”) in the Court of Chancery of the
State of Delaware (the “Court”) against the Company and our officers and
directors. Pope filed the Summons and Complaint as a judgment
creditor and as a shareholder of the Company. Pope alleges
that the assets and profits of our subsidiary company have been
wrongfully diverted by our officers and directors and requests the
appointment of a receiver to liquidate and wind down the business affairs
of the Company. In connection with the filing of the Summons and
Complaint, Pope has also filed a motion for a Preliminary Injunction
Motion seeking to enjoin the Company and our officers and directors from
taking any further actions to divert the corporate assets and profits of
our subsidiary company and for expedited discovery proceedings.
Pope further requests the imposition of a constructive trust, an
accounting, damages for an alleged breach of fiduciary duty by the
Company’s officers and directors, and attorney fees.
|
|
A
hearing on the application of Pope Investments, LLC ("Pope") seeking the
appointment of a receiver was held on March 29, 2010. The parties filed
post-trial briefs on April 7, 2010. As of this date (May 12, 2010), the
Court has not yet ruled on Pope's application. Since the hearing, the
remaining defendants, including the Benda directors and officers named as
defendants, have filed a motion to dismiss Pope's complaint against them.
No date has yet been set for Pope's response to the motion or the parties'
arguments to the Court on the
motion.
|
54
Except as
disclosed above, we are not aware of any pending or threatened legal proceedings
in which we are involved.
ITEM 4. (REMOVED AND
RESERVED)
PART
II
ITEM 5. MARKET FOR COMMON EQUITY, RELATED
STOCKHOLDER MATTERS AND SMALL BUSINESS ISSUER PURCHASES OF EQUITY
SECURITIES.
Our
common stock was traded on the national, over-the-counter market under the
symbol ASTI after our initial public offering in January 1988. However, we were
notified by NASD that, due to low trading volume, it would not report
transactions in our common stock after October 13, 1989.
On
September 16, 2005, our common stock commenced quotation on the Over-the-Counter
Bulletin Board (“OTCBB”). Our common stock, having $.001 par value per share
("Common Stock"), is traded on the otcbb under the symbol "BPMA". As of May 13,
2010, there were approximately 807 holders of record of our common
stock.
The
following table sets forth, for the periods indicated, the reported high and low
closing bid quotations for our common stock as reported on the OTCBB. The bid
prices reflect inter-dealer quotations, do not include retail markups, markdowns
or commissions and do not necessarily reflect actual transactions.
|
Quarter Ended
|
High Bid
|
Low Bid
|
||||||
|
December
31, 2009
|
$ | .09 | $ | .03 | ||||
|
September
30, 2009
|
$ | .04 | $ | .01 | ||||
|
June
30, 2009
|
$ | .05 | $ | .01 | ||||
|
March 31, 2009
|
$ | .09 | $ | .02 | ||||
|
December
31, 2008
|
$ | .19 | $ | .04 | ||||
|
September
30, 2008
|
$ | .26 | $ | .13 | ||||
|
June
30, 2008
|
$ | .34 | $ | .25 | ||||
|
March 31, 2008
|
$ | .77 | $ | .30 | ||||
|
December
31, 2007
|
$ | 2.69 | $ | .65 | ||||
|
September
30, 2007
|
$ | 2.60 | $ | 1.90 | ||||
|
June
30, 2007
|
$ | 3.00 | $ | 1.15 | ||||
|
March 31, 2007
|
$ | 1.65 | $ | .85 | ||||
|
December
29, 2006
|
$ | 1.05 | $ | .76 | ||||
|
September
30, 2006
|
$ | .55 | $ | .40 | ||||
|
June
30, 2006
|
$ | .55 | $ | .40 | ||||
|
March
31, 2006
|
$ | .75 | $ | .40 | ||||
Transfer
Agent and Registrar
Computershare
Trust Company, Inc. is currently the transfer agent and registrar for our Common
Stock. Its address is 350 Indiana Street, Suite 800, Golden, Colorado 80401. Its
phone number is (303) 262-0600.
55
Dividend
Policy
Any
future determination as to the declaration and payment of dividends on shares of
our Common Stock will be made at the discretion of our board of directors out of
funds legally available for such purpose. We are under no contractual
obligations or restrictions to declare or pay dividends on our shares of Common
Stock. In addition, we currently have no plans to pay such dividends. However,
even if we wish to pay dividends, because our cash flow is dependent on dividend
distributions from our affiliated entities in PRC, we may be restricted from
distributing dividends to our holders of shares of our common stock in the
future if at the time we are unable to obtain sufficient dividend distributions
from Ebei, Jiangling, Yudi or Shusai. Our board of directors currently intends
to retain all earnings for use in the business for the foreseeable future. See
“Risk Factors.”
Recent
Sales of Unregistered Securities
On August
25, 2008, 3,810,976 shares were issued to the April 2007 PIPE investors as make
good shares pursuant to the Make Good and Escrow Agreement. The above shares of
common stock were issued under an exemption from registration under Section 4(2)
of the Securities Act of 1933, as amended ("Securities Act"). As such, the above
shares of common stock will be restricted shares, and the holder thereof may not
sell, transfer or otherwise dispose of such shares without registration under
the Securities Act or an exemption therefrom.
On July
10, 2008, 540,870 shares were issued to Jayhawk Private Equity Fund upon
conversion of certain promissory notes. The above shares of common stock
were issued under an exemption from registration under Section 4(2) of the
Securities Act of 1933, as amended ("Securities Act"). As such, the above shares
of common stock will be restricted shares, and the holder thereof may not sell,
transfer or otherwise dispose of such shares without registration under the
Securities Act or an exemption therefrom.
On May 1,
2008, 523,438 shares were issued to PIPE investors as penalty for registration
delay expenses. The above shares of common stock were issued under an
exemption from registration under Section 4(2) of the Securities Act of 1933, as
amended ("Securities Act"). As such, the above shares of common stock will be
restricted shares, and the holder thereof may not sell, transfer or otherwise
dispose of such shares without registration under the Securities Act or an
exemption therefrom.
On
January 3, 2008, 110,000 shares were issued to the independent directors in lieu
of their remuneration. The above shares of common stock were issued under
an exemption from registration under Section 4(2) of the Securities Act of 1933,
as amended ("Securities Act"). As such, the above shares of common stock will be
restricted shares, and the holder thereof may not sell, transfer or otherwise
dispose of such shares without registration under the Securities Act or an
exemption therefrom.
56
ITEM 6. SELECTED FINANCIAL
DATA
Not
applicable to a smaller reporting company.
ITEM 7. MANAGEMENT'S DISCUSSION AND
ANALYSIS OR PLAN OF
OPERATION
Forward-Looking
Statements
The
following discussion may contain certain forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933 and Section 21E of the
Securities Exchange Act of 1934. Such statements are intended to be covered by
the safe harbors created by such provisions. These statements include the plans
and objectives of management for future growth of the Company, including plans
and objectives related to the consummation of acquisitions and future private
and public issuances of the Company's equity and debt securities. The
forward-looking statements included herein are based on current expectations
that involve numerous risks and uncertainties. Assumptions relating to the
foregoing involve judgments with respect to, among other things, future
economic, competitive and market conditions and future business decisions, all
of which are difficult or impossible to predict accurately and many of which are
beyond the control of the Company. Although the Company believes that the
assumptions underlying the forward-looking statements are reasonable, any of the
assumptions could be inaccurate and, therefore, there can be no assurance that
the forward-looking statements included in this Form 10-K will prove to be
accurate. In light of the significant uncertainties inherent in the
forward-looking statements included herein, the inclusion of such information
should not be regarded as a representation by the Company or any other person
that the objectives and plans of the Company will be achieved.
The words
“we,” “us” and “our” refer to the Company. The words or phrases “would be,”
“will allow,” “intends to,” “will likely result,” “are expected to,” “will
continue,” “is anticipated,” “estimate,” “project,” or similar expressions are
intended to identify “forward-looking statements.” Actual results could differ
materially from those projected in the forward looking statements as a result of
a number of risks and uncertainties, including but not limited to: (a) limited
amount of resources devoted to achieving our business plan; (b) our failure to
implement our business plan within the time period we originally planned to
accomplish; (c) because we are seeking to merge with an operating business which
has not yet been identified, you will be unable to determine whether we will
ever become profitable; and (d) other risks that are discussed in this Form 10-K
or included in our previous filings with the Securities and Exchange
Commission.
Critical Accounting
Policies
Accounting
policies discussed in this section are those that we consider to be most
critical to an understanding of our financial statements because they inherently
involve significant judgment and uncertainties. For all of these
estimates, we caution that future events rarely develop exactly as forecast, and
the best estimates routinely require adjustment.
Revenue
Recognition
Among the
most important accounting policies affecting the Group’s consolidated financial
statements is its policy of recognizing revenue. Under this policy, all of the
following criteria must be met in order for us to recognize
revenue:
|
|
1.
|
Persuasive
evidence of an arrangement exists;
|
|
|
2.
|
Delivery
has occurred or services have been
rendered;
|
|
|
3.
|
The
seller's price to the buyer is fixed or determinable;
and
|
|
|
4.
|
Collectibility
is reasonably assured.
|
The
majority of the Group's revenue results from sales contracts
with distributors and revenue is recorded upon the shipment of goods.
Management conducts credit background checks for new customers as a means
to reduce the subjectivity of assuring collectibility. Sales are presented
net of value added tax (VAT). No return allowance is made as products returns
are insignificant based on historical experience.
Estimates
Affecting Accounts Receivable and Inventories
The
preparation of our consolidated financial statements requires management to make
estimates and assumptions that affect our reporting of assets and liabilities
(and contingent assets and liabilities). However, it is explicated that the
changes in estimation were not material in the preparation of our consolidation
financial statements.
As of
December 31, 2009 and 2008, the Group provided a $5,502,311 and $3,788,102,
respectively for the allowance of doubtful accounts against trade receivables.
Management's estimate of the appropriate allowance on those accounts receivable
for the reporting periods was based on the aged nature of these accounts. In
making its judgment, management assessed its customers' ability to continue to
pay their outstanding invoices and the collectibility of those accounts on a
timely basis, and whether their financial position might deteriorate
significantly in the future, which would result in their inability to pay their
debts to the Company.
57
Inventories,
which are primarily comprised of raw materials, packaging materials, and
finished goods, are stated at the lower of weighted average cost or net
realizable value. Cost being determined on the basis of a moving average. The
Group evaluates the need for reserves associated with obsolete, slow-moving and
non-salable inventory by reviewing net realizable values on a periodic
basis.
For the
year ended December 31, 2009 and 2008, the Group provided a reserve against its
obsolete, slow-moving or non-salable inventory amounting to $9,262 and $244,915,
respectively.
Management
determination of this allowance was based on potential impairments to the
current carrying value of the inventories due to potential obsolescence of aged
inventories. In making its estimate, management considered the probable demand
for our products in the future and historical trends in the turnover of our
inventories. While the Company currently believes that there is little
likelihood that actual results will differ materially from these current
estimates.
58
Operational
Results
Year ended December 31, 2009
Compared to Year ended December 31, 2008
The
following table provides key components of our operational results for the year
ended December 31, 2009 and 2008 for Benda Pharmaceutical, Inc.
|
YEAR ENDED DECEMBER 31,
|
||||||||
|
(Restated)
|
||||||||
|
2009
|
2008
|
|||||||
|
Revenue
|
$ | 21,990,915 | $ | 25,041,986 | ||||
|
Cost
of goods sold
|
(13,720,676 | ) | (15,740,107 | ) | ||||
|
Gross
profit
|
8,270,239 | 9,301,879 | ||||||
|
Selling
expenses
|
2,378,852 | 2,708,968 | ||||||
|
General
and administrative expenses
|
||||||||
|
Bad
debts
|
1,424,072 | 2,730,434 | ||||||
|
Depreciation
and amortization expense
|
1,273,051 | 837,440 | ||||||
|
Goodwill
impairment
|
- | 4,141,109 | ||||||
|
Inventory
wirtten down to net realizable value
|
- | 392,535 | ||||||
|
Other
general and administrative expenses
|
3,224,561 | 4,189,341 | ||||||
|
Research
and development expenses
|
349,361 | 1,461,951 | ||||||
|
Total
operating expenses
|
8,649,897 | 16,461,778 | ||||||
|
Operating
loss
|
(379,658 | ) | (7,159,899 | ) | ||||
|
Other
income (expenses)
|
||||||||
|
Rental
income
|
241,005 | - | ||||||
|
Government
subsidies / grants (Note 13)
|
454,491 | - | ||||||
|
Interest
income
|
129,513 | 133,251 | ||||||
|
Interest
expense
|
(2,298,411 | ) | (5,359,788 | ) | ||||
|
Other
expenses
|
(14,833 | ) | (1,311,140 | ) | ||||
|
Loss
before income taxes
|
(1,867,893 | ) | (13,697,576 | ) | ||||
|
Income
taxes (Note 18)
|
818,289 | 776,295 | ||||||
|
Net
Loss
|
(2,686,182 | ) | (14,473,871 | ) | ||||
|
Less:
Net loss attributable to the noncontrolling interests
|
(748,712 | ) | (2,993,073 | ) | ||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (1,937,470 | ) | $ | (11,480,798 | ) | ||
|
Other
Comprehensive Loss
|
||||||||
|
Foreign
currency translation (loss) gain
|
(120,157 | ) | 3,460,265 | |||||
|
Comprehensive
Loss
|
(2,806,339 | ) | (11,013,606 | ) | ||||
|
Comprehensive
loss attributable to the noncontrolling interest
|
(764,484 | ) | (2,344,747 | ) | ||||
|
Comprehensive
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (2,041,855 | ) | $ | (8,668,859 | ) | ||
59
Net
Revenue:
The
Company has five core operating segments: Benda Ebei, Jiangling Benda, Yidu
Benda, Beijing Shusai and SiBiono. Benda Ebei manufactures branded/generic
medicines; Jiangling Benda manufactures active pharmaceutical ingredients (API);
Yidu Benda manufactures bulk chemicals; Beijing Shusai operates and distributes
Pharyngitis Killer Therapy; and SiBiono is a gene therapy company dedicated to
the development, manufacturing and commercialization of gene therapy product,
Gedicine.
Net
revenue decreased by $3.05 million (or 12.18%) to $21.99 million for the year
ended December 31, 2009 from $25.04 million for the year ended December 31,
2008. Decrease in revenue is principally attributed by the following
factors:
|
1.
|
One
of the Benda’s subsidiaries, Benda Ebei’s net revenue decreased $4.24
million (or 19.25%) to $17.78 million for the year ended December 31, 2009
from $22.02 million for the year ended December 31, 2008. It was mainly
due to the keen competition in generic medicine and overall economic
downturn.
|
|
2.
|
One
of the Benda’s subsidiaries, Jiangling Benda which resumed its production
in October 2007 and achieved $1.61 million and $0.69 million for the year
ended December 31, 2009 and 2008,
respectively.
|
Jiangling
Benda plans to produce four types of active pharmaceutical ingredients and they
are Ribavirin, Asarin, Levofloxacin and Ribose whereas the production of Ribose
does not require the GMP certificate, but the production of the other three
products do require the GMP certificate.
On April 9, 2008, Jiangling Benda
received the approved GMP Certificate from the Chinese State Food and
Drug Administration ("SFDA") which authorizing the production of Ribavrin.
The other two products, Asarin and Levolfozacin, are still under the stage of
GMP certificate approving process. The management could not estimate the exact
timing for obtaining those certificates.
|
3.
|
One
of the Benda’s subsidiaries, Yidu Benda ceased operation due to the fact
that the plant was closed since mid January of 2007 to upgrade its waste
water treatment system to comply with new environmental standards enforced
by PRC local government.
|
Yidu
Benda has completed its upgrading of the waste water system and passed the
government’s verification and testing of equipments in October 2007. It is now
permitted for the testing on actual production process. Once the actual products
are produced, then the environmental government bodies will re-test the
production results. The management could not estimate the exact timing for
obtaining the final approval on the actual production process. Furthermore, the
management is searching for new products to be produced in Yidu Benda which with
higher profit margin.
|
4.
|
One
of the Benda’s subsidiaries, Beijing Shusai which incorporated on July 15,
2006. China’s State Food and Drug Administration (SFDA) recently
experienced an overhaul in its policies and regulatory systems in an
effort to fight against corruption in Chinese pharmaceutical industry.
Beijing Shusai’s operation has been adversely affected by this recent
policy changes which prohibits some state-owned hospitals from forming
alliances with private companies. The management could not estimate that
such situation could be resolved in the coming
future.
|
|
5.
|
One
of the Benda’s subsidiaries, SiBiono, acquired and effective since April
1, 2007, net revenue increased $0.26 million (or 11%) to $2.59 million for
the year ended December 31, 2009 from $2.33 million for the year ended
December 31, 2008.
|
SiBiono
GMP - On October 16, 2003, SiBiono successfully obtained a New Drug License from
the State Food & Drug Administration of China (SFDA), and then, in April 4,
2004, SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for
Pharmaceutical Product”, so far being fully qualified for the market launch of
Recombinant Human Ad-p53 Injection, trademarked as Gendicine ®
in China. Gendicine ® is the
commercialized gene therapy product approved in the PRC government agency. On
May 19, 2008, SiBiono received an official notice from the PRC State of SFDA in
which it mentioned that during the random inspection performed by the PRC State
of SFDA on April 8 to April 10, 2008, the PRC State of SFDA discovered there
were several production procedures that did not meet the requirement stated in
GMP, thus it required SiBiono to perform necessary improvements in order to
fulfill the GMP requirements and the PRC State of SFDA collected back the
distributed GMP certificate until the necessary improvements being carried out
and passed the examination that conducted by SFDA. On June 10, 2008,
SiBiono received another official notice from Guangdong Province SFDA and they
demanded the same requirements as stated in the official notice which issued by
the PRC State of SFDA dated on May 19, 2008. On November 24, 2008, SiBiono
received another official notice from Guangdong Province SFDA which mentioned
that after the examination conducted by Shenzhen City SFDA, the Guangdong
Province SFDA consent SiBiono to carry out production on a trial basis. It
further required SiBiono strictly to follow the requirements of GMP to organize
trail production and follow the procedures to apply for GMP Certificate
verification.
On July
14, 2009, SiBiono obtained the final approved GMP Certificate, in order words,
the SFDA allows SiBiono to resume its production and sales.
Cost
of Goods Sold
Cost of
goods sold decreased $2.02 million (or 12.83%) to $13.72 million for the year
ended December 31, 2009 from $15.74 million for the year ended December 31, 2008
primarily due to the decreased in the sales volume in Benda
Ebei.
60
Gross
Profit
Gross
profit decreased $1.03 million (or 11.09%) to $8.27 million for the year ended
December 31, 2009 from $9.30 million for the year ended December 31, 2008, which
was mainly because of the decrease in the products market price due to the
overall economic downturn.
Selling
Expenses:
Selling
expenses slightly decreased $0.33 million (or 12.19%) to $2.38 million for the
year ended December 31, 2009 from $2.71 million for the year ended December 31,
2008, primarily due to the management performed cost control in the reporting
period.
General
and Administrative Expenses:
General
and administrative decreased $7.48 million (or 54.40%) to $6.27 million for the
year ended December 31, 2009 from $13.75 million for the year ended December 31,
2008, primarily due to no goodwill impairment, less bad debt expense, inventory
impairment and R&D expense incurred in 2009.
Operating
Loss:
The
Company resulted an operating loss $0.38 million for the year ended December 31,
2009, while the operating loss from comparative period for 2008 was $7.16
million.
Interest
Expenses:
Interest
expense was $2.30 million and $5.36 million for the year ended December 31, 2009
and 2008 respectively. The main component of it was the financing cost
associated with the issuance of convertible promissory note.
Other
Income / (Expenses)
The net
other income (expense) was 0.01 million and 1.31 million for the year ended
December 31, 2009 and 2008 respectively. The expense incurred in 2008 was due to
$1.1 million Registration Delay Penalty and $0.27 million expense by the
issuance of 523,438 shares of common stock for the make good agreement. There
was no such expense incurred for the year ended December 31, 2009.
Income
Taxes:
Benda is
subject to Delaware, United State of America tax, but no provision for income
taxes were made for the year ended December 31, 2009 and 2008 as Benda did not
have reportable taxable income for the period.
Ever
Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no
provisions for income taxes were made for the year ended December 31, 2009 and
2008 as Ever Leader did not have reportable taxable income for the
periods.
Benda
Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and
is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in
the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC enterprise
income tax for two years starting from the first profit-making year, followed by
a 50% reduction in the state income taxes, for the following three years,
commencing from the first profitable year.
Jiangling
Benda and Yidu Benda are cross-municipal investment entities and enjoy the same
tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were
therefore exempt from PRC enterprise income tax for two years starting from the
first profit-making year, followed by a 50% reduction in the state income taxes,
for the following three years, commencing from the first profitable year.
Cross-municipal investments entities refer to entities that are incorporated in
one municipal region but have investments in another municipal
region.
The
exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the
year of 2006, after which they are subject to a 50% reduction in income
taxes, whereas the full income tax rate is 33%. The remaining tax holidays
was expired in 2009.
However,
starting and effective from January 1, 2008, the full income tax rate would be
changed from 33% to 25% according to the new PRC taxation regulations. Therefore
these subsidiaries will be subject to the regular full income tax rate at 25%
after the tax holidays expire.
According
to the new taxation regulations starting and effective from January 1, 2008,
Beijing Shusai is subject to the full income tax rate of 25%.
61
According
to the new taxation regulations starting and effective from January 1, 2008,
SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is
subject to the full income tax rate of 25% gradually in five years as
following:
|
Year
|
Tax
rate
|
|||
|
2008
|
18 | % | ||
|
2009
|
20 | % | ||
|
2010
|
22 | % | ||
|
2011
|
24 | % | ||
|
2012
and thereafter
|
25 | % | ||
Benda
Ebei recorded $872,643 and $1,109,249 income tax for the year ended December 31,
2009 and 2008 respectively.
LIQUIDITY
AND CAPITAL RESOURCES
Net cash
used in operating activities was $0.29 million for the year ended December 31,
2009, while for the year ended December 31, 2008 was $0.83 million.
|
a)
|
Non-cash
operating activities, reconciliation items to the net
income
|
For the
year ended December 31, 2008, an amount about $13.68 million non-cash operating
activities was reconciled back to the net income and which mainly included
amortization of debt discount and debt issue cost, penalty payment made in form
of share issuance, bad debt provision, amortization of intangible assets,
goodwill impairment, and depreciation.
However,
for the year ended December 31, 2009, about $4.87 million of non-cash operating
activities was reconciled back to the net income and summarized as
follows:
|
1.
|
$0.92
million that incurred as interest expenses related to the amortization of
discount on long-term debt and issuance cost associated with the warrants
and the beneficial conversion features;
and
|
|
2.
|
Other
factors: $1.42 million incurred on bad debt provision; $2.19 million
incurred on depreciation; and $0.71 million incurred on amortization of
intangible assets.
|
|
b)
|
Trade
receivables
|
The net
amount of trade receivable increased by $3.08 million for the year ended
December 31, 2009. The management also noticed that the net balance of the trade
receivable, as of December 31, 2009, was a significant asset to the company.
However, management believes that the above situation is temporarily due
to the following reasons:
|
a)
|
Customers
whom have sales relationship with our company are all relatively big
business wholesale enterprises and they have all passed the examination of
GMP Certificate so that the collectibility from those is out of
question;
|
|
b)
|
The
management realized that it did affect the cash flow situation of the
company; therefore the company will put more efforts to reduce the balance
of trade receivables.
|
For the
year ended December 31, 2009 and 2008, the amounts provided by (used in)
investing activities were $0.13 million and ($4.57) million respectively. It was
mainly because higher capital expenditure in 2008 and release of restricted cash
in 2009.
Financing
cash outflow was $0.22 million for the reporting period year ended December 31,
2009, while the financing cash inflow was $3.6 million for the reporting period
year ended December 31, 2008. The major component of it was a net amount
$4.3 million borrowed from the commercial banks during the year ended December
31, 2008.
62
ITEM 8. FINANCIAL STATEMENTS
The
following financial statements required by this item are filed herewith
following the signature page to this report:
63
|
ITEM
9.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
|
On March
1, 2010, Benda Pharmaceutical, Inc. (the “Company”) was notified Kempisty &
Company Certified Public Accountants, P.C., the Company’s independent registered
public accounting firm (“K&Co”), intended to cease auditing services for
public companies and that certain employees of Kempisty would be providing
services for MaloneBailey, LLP (“MB”). On March 1, 2010, K&Co resigned
as the independent registered public accounting firm of the Company and, with
the approval of the Audit Committee of the Company’s Board of Directors, MB was
engaged as the Company’s independent registered public accounting
firm.
K&Co
performed audit of the Company’s consolidated financial statements for the
fiscal year ended December 31, 2008. K&Co’s report did not contain an
adverse opinion or a disclaimer of opinion and were not qualified or modified as
to uncertainty, audit scope, or accounting principles, except for an explanatory
paragraph related to the Company’s ability to continue as a going
concern.
During
the fiscal years ended December 31, 2009 and December 31, 2008 and the
subsequent interim period up through the March 1, 2010, there were no (i)
disagreements between the Company and K&Co on any matter of accounting
principles or practices, financial statement disclosure, or auditing scope or
procedure, which disagreements, if not resolved to its satisfaction, would have
caused K&Co to make reference to the subject matter of such disagreements in
connection with its report, or (ii) “reportable events,” as described in Item
304(a)(1)(v) of Regulation S-K.
On March
1, 2010, the Company furnished K&Co with a copy of this report prior to
filing with the Securities and Exchange Commission (“SEC”) and requested that
K&Co furnish it with a letter addressed to the SEC stating whether or not it
agreed with the statements made by the Company in this report insofar as they
relate to K&Co’s audit services and engagement as the Company’s independent
registered public accounting firm. K& Co has furnished a letter
addressed to the SEC dated March 1, 2010, a copy of which is attached hereto as
Exhibit 16.
As noted
above, on March 1, 2010, the Company engaged the services of MB as the
independent registered public accounting firm of the Company. During the
fiscal years ended December 31, 2009 and 2008 and from December 31, 2009 through
the engagement of MB as the Company’s independent registered public accounting
firm, neither the Company nor anyone on its behalf consulted MB with respect to
any accounting or auditing issues involving the Company. In particular,
there was no discussion with the Company regarding the application of accounting
principles to a specified transaction, the type of audit opinion that might be
rendered on the financial statements, or any matter that was either the subject
of a disagreement, as described in Item 304 of Regulation S-K, with K&Co, or
a “reportable event” as described in Item 304(a)(1)(v) of Regulation
S-K.
64
ITEM 9A(T). CONTROLS AND
PROCEDURES
Evaluation of Disclosure
Controls and Procedures
Management
of the Company is responsible for establishing and maintaining effective
internal control over financial reporting as defined in Rule 13a-15(f) under the
Exchange Act. The Company’s internal control over financial reporting is
designed to provide reasonable assurance to the Company’s management and Board
of Directors regarding the preparation and fair presentation of published
financial statements in accordance with United State’s generally accepted
accounting principles (US GAAP), including those policies and procedures that:
(i) pertain to the maintenance of records that, in reasonable detail,
accurately and fairly reflect the transactions and dispositions of the assets of
the company, (ii) provide reasonable assurance that transactions are
recorded as necessary to permit preparation of financial statements in
accordance with US GAAP and that receipts and expenditures are being made only
in accordance with authorizations of management and directors of the company,
and (iii) provide reasonable assurance regarding prevention or timely
detection of unauthorized acquisition, use, or disposition of the company’s
assets that could have a material effect on the financial
statements.
Management
conducted an evaluation of the effectiveness of internal control over financial
reporting based on the framework in Internal Control — Integrated Framework
issued by the Committee of Sponsoring Organizations of the Treadway
Commission. Management’s assessment included an evaluation of the design
of our internal control over financial reporting and testing of the operational
effectiveness of our internal control over financial reporting. Based on
the evaluation of these disclosure controls and procedures, and in light of the
material weaknesses found in our internal controls, the Chief Executive Officer
concluded that our disclosure controls and procedures were not
effective.
Management Report on
Internal Control Over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting. Under the
supervision of our Chief Executive Officer, the Company conducted an evaluation
of the effectiveness of our internal control
over financial reporting as of December 31, 2009 using the criteria established
in Internal Control—Integrated Framework issued by
the Committee of Sponsoring Organizations of the Treadway Commission
(COSO).
A
material weakness is a deficiency, or combination of deficiencies, in internal
control over financial reporting, such that there is
a reasonable possibility that a material misstatement of our annual or interim
financial statements will not be prevented
or detected on a timely basis. In our assessment of the effectiveness of
internal control over financial reporting as of
December 31, 2009, we determined that control deficiencies existed that
constituted material weaknesses, as described below.
1.There
is a risk of management override given that our officers have a high degree of
involvement in our day to day operations.
2.Significant
errors were found in our prior years accounting treatments that require
restatements of our prior years filed financial statements.
3.There
is personal loan to executives which is a violation of Section 402 of the
Sarbanes-Oxley Act of 2002
Management
is currently evaluating remediation plans for the above control deficiencies.
Accordingly, we concluded that these control deficiencies resulted in a
reasonable possibility that a material misstatement of the annual or interim
financial statements will not be prevented or detected on a timely basis by the
company’s internal controls. As a result of the material weaknesses described
above, management has concluded that the Company did not maintain effective
internal control over financial reporting as of December 31, 2009 based on
criteria established in Internal Control—Integrated Framework issued by
COSO.
This
annual report does not include an attestation report of the Company’s registered
public accounting firm regarding internal control over financial reporting.
Management’s report was not subject to attestation by our registered public
accounting firm pursuant to temporary rules of the Securities and Exchange
Commission that permit the Company to provide only management’s report in this
Annual Report.
Changes in Internal
Controls
During
the quarter ended December 31, 2009 there were no changes in our internal
control over financial reporting that materially affected, or are reasonably
likely to materially affect, our internal control over financial
reporting. Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements. Therefore,
even those systems determined to be effective can provide only reasonable
assurance with respect to financial statement preparation and
presentation.
PART
III
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS OF
THE REGISTRANT
The
following table sets forth the names, positions and ages of our executive
officers and directors. All of our directors serve until the next annual meeting
of stockholders or until their successors are elected and qualify. Officers are
elected by the board of directors and their terms of office are, except to the
extent governed by employment contract, at the discretion of the board of
directors.
|
Name
|
Age
|
Position
|
Date of Appointment
|
|||
|
Yiqing Wan
|
46
|
Chief Executive Officer,
Chief Financial Officer and Chairman
|
November 10, 2006
|
|||
|
Wei Xu
|
45
|
Vice President of Operations
|
November 15, 2006
|
|||
|
Jun Tang
|
42
|
Director
|
April 25, 2008
|
|||
|
Hui Long
|
47
|
Vice President of Technology
|
November 15, 2006
|
|||
|
Jingbo Wu
|
46
|
Vice President
|
November 15, 2006
|
|||
|
Dr. Q.Y. Ma
|
51
|
Chairman of Compensation Committee and Director
|
March 20, 2007
|
65
Business Experience
Descriptions:
Mr.
Yiqing Wan, Chairman, and Chief Executive Officer and Chief Financial
Officer
Mr. Wan
was appointed as Chairman and Chief Executive Officer on November 10, 2006. Mr
Wan was appointed as the Chief Financial Officer in September 2009 upon Eric
Yu’s resignation from the position. Mr. Wan founded Benda Ebei in April 2001and
has served as its president and general manager overseeing the day to day
operations of the Company since such time. Before founding Benda Ebei, Mr. Wan
was Chairman of Zhanjiang Jinhui Pharmaceutical Corp. (from 1995 to 1998),
Chairman of Shandong Jinhai Real Estate Development Co. (from 1992 to 1995),
Manager of Hainan Pharmaceutical Co. Ltd Guangzhou Division (from 1990 to 1992),
and Director of Yichang No.4 Drug Plant (from 1982 to 1990). Mr. Wan has held a
range of operational and executive positions in a number of pharmaceutical
enterprises for more than two decades and has developed significant management
experience in production planning and implementation and in product marketing.
Mr. Wan earned B.S. degree in Biological Engineering from Sanxia University in
1982.
Ms.
Wei Xu, Vice President of Operations
Ms. Xu
was appointed as Vice President of Operations on November 15, 2006. In April,
2007, she was appointed as the General Manger of Shenzhen SiBiono Gene Tech Co.,
Ltd., a subsidiary of the company. From 2002 to present, she serves as
Chairperson of a related company, Hubei Benda Science and Technology Co., Ltd.
Prior to such time, she was the Chairman at Hubei Tongji Benda Pharmaceutical
Co., Ltd. (from 1999 to 2004), General Manager at the Zhanjiang Jinhui
Pharmaceutical Co., Ltd (from 1995 to 1998) and Manager at the Hainan
Pharmaceutical Co., Ltd., Guangzhou Division (from 1991 to 1995).
Jun
Tang, Director
Jun Tang
is now serving as president and Chief Executive Officer in Xin Hua Du Industrial
Group Co., a group company incorporated in the PRC covering retail, real estate,
mining, high-tech and tourism industries with a subsidiary already listed both
in Hong Kong Stock Exchange and in PRC A-shares market and a subsidiary to
be listed in PRC A-shares market in the year of 2008.
Prior to
joining Xin Hua Du Industrial Group Co. this April of 2008, Mr. Tang served as
the president and director of Shanda Interactive Entrainment Limited, a company
listed in NASDAQ, and now remain as a member of Shanda's board of directors and
serve as an advisor to the CEO of Shanda.
Prior to
joining Shanda in 2004, Mr. Tang served as the president of Microsoft China Co.,
Ltd. from March 2002 to January 2004 and the general manager of Microsoft Asia
product support and service and Microsoft Global Technical Engineering Center
from January 1998 to March 2002. He received the Microsoft Chairman Bill Gates
Award in 1998 and the Microsoft Top Honor Award in 2002 from Microsoft
Corporation, and remains the honorary president of Microsoft China Co. Ltd.
Prior to joining Microsoft, Dr. Tang founded Intertex Company, a software and
entertainment company, in Los Angeles, California in 1993.
Mr. Tang
received his doctorate degree, master’s degree and bachelor’s degree in the
U.S., Japan and China, respectively. Mr. Tang was honoured as PRC Top 10
Economic Person, PRC Top 10 IT Person, PRC Top 10 Valuable CEO etc.
Mr.
Hui Long, Vice President of Technology
Mr. Long
was appointed as Vice President of Technology on November 15, 2006. Mr. Long has
held senior technical and production management positions with pharmaceutical
enterprises for approximately 20 years. From 2001 to present, he is the General
Manager of Jiangling Benda Pharmaceutical Co., Ltd. Prior to such time, he was
he deputy general manager of Zhanjiang Jinhui Pharmaceuticals Co. Ltd (from 1995
to 2000), the Chief of Technical Division of Sanxia Pharmaceutical Co., Ltd
(from 1993 to 1995) and t. Mr. Long earned a B.S. in Biological Engineering from
Sanxia University in 1982 and an M.S. in Biological Engineering from Sanxia
University in 1988.
66
Mr.
Jingbo Wu, Vice President
Mr. Wu
was appointed as Vice President on November 15, 2006. Since 2002, Mr. Wu has
been the general manager of North Hubei Tongji Benda Pharmaceutical Company
Ltd., overseeing the company’s daily activities and GMP authentication. Prior to
this position, he was the deputy general manager of Ebei Pharmaceutical Corp.
(from 2000 to 2001) and the deputy general manager of Zhejiang Jinhui
Pharmaceutical Co. Ltd. (from 1999 to 2000). Mr. Wu received his certification
of graduation in Business Administration from Wuhan University in
1995.
Dr.
Q.Y. Ma, Director
Dr. Ma
was appointed as a Director on March 20, 2007. Dr. Ma has over 20 years of
R&D and managerial experience in US. Dr. Ma has been the managing director
of Time Innovation Ventures, a venture capital firm focused on funding
technology start-ups and joint ventures in China, since 2000. He has also been a
director of ComTech, a Chinese semiconductor company, since 2004. He served as
an Associate Professor of electrical engineering at the University of Hong Kong
from 1998 to 2005. Dr. Ma also served as an Associate Professor at the
Department of Electrical Engineering at Columbia University and in the
Department of Radiology at Harvard Medical School from 1994 to 2005. He has
served as a consultant to IBM, General Electric, TRW Inc. and DuPont. Dr. Ma is
a co-founder of and advisor to Semiconductor Manufacturing International Corp.,
and the Chairman of TiMed. He has served as an adviser to the Ministry of
Information Industry, Beijing Government, and as senior advisor to Zhangjiang
Hi-Tech Park in Shanghai. He has published over 160 papers and obtained 7
patents. Dr. Ma received his PhD from Columbia University, and attended the
Executive Program of Stanford University's School of Business.
67
Employment
Agreements
On
November 15, 2006, we executed employment agreements with each of our executive
officers, specifically, Yiqing Wan, our Chief Executive Officer; Wei Xu, our
Vice President of Operations; Hui Long, our Vice President of Technology; Daping
Gu, our Vice President of Marketing; and Jingbo Wu, our
Vice President. The employment agreements are for terms of three years, except
for Yiqing Wan, whose term is for five years. The employment agreements provide
for annual salaries and annual bonuses in amounts as set forth in the table
herein entitled “Executive Compensation Summary.”
Audit Committee and Audit
Committee Financial Expert
The Audit
Committee assists the Board of Directors in its oversight of the quality and
integrity of the accounting, auditing and reporting practices of the Company.
The Audit Committee has the authority and responsibility to hire one or more
independent auditors to audit the Company’s books, records and financial
statements and to review the Company’s systems of accounting (including its
systems of internal control), to discuss with such independent auditors the
results of such audit and review, to conduct periodic independent reviews of the
systems of accounting (including systems of internal control), and to make
reports periodically to the Board of Directors with respect to its findings. The
Board of Directors has determined that each Audit Committee member has
sufficient knowledge in financial and auditing matters to serve on the
Committee. The committee is also responsible for making recommendations to the
Board of Directors or otherwise acting with respect to corporate governance
matters, including board size and membership qualifications, new director
orientation, committee structure and membership, communications with
shareholders, and board and committee self-evaluations. The Audit Committee
operates under a written charter adopted by our Board of Directors.
The
members of the Audit Committee are independent of the Company (as defined under
Rule 4200(a)(15) of the NASDAQ Marketplace Rules). We currently do not have a
member of the Audit Committee that is a “financial expert” as defined under Item
407 of Regulation S-B.
Code of
Ethics
A code of
ethics relates to written standards that are reasonably designed to deter
wrongdoing and to promote:
|
|
·
|
Honest
and ethical conduct, including the ethical handling of actual or apparent
conflicts of interest between personal and professional
relationships;
|
|
|
·
|
Full,
fair, accurate, timely and understandable disclosure in reports and
documents that are filed with, or submitted to, the SEC and in other
public communications made by an
issuer;
|
68
|
|
·
|
Compliance
with applicable governmental laws, rules and
regulations;
|
|
|
·
|
The
prompt internal reporting of violations of the code to an appropriate
person or persons identified in the code;
and
|
|
|
·
|
Accountability
for adherence to the code.
|
Due to
the limited scope of our current operations, we have not adopted a corporate
code of ethics that applies to its principal executive officer, principal
accounting officer, or persons performing similar functions.
Indemnification
Under
Delaware law and pursuant to our articles of incorporation and bylaws, we may
indemnify our officers and directors for various expenses and damages resulting
from their acting in these capacities. Insofar as indemnification for
liabilities arising under the Securities Act of 1933 may be permitted to our
officers or directors pursuant to those provisions, our counsel has informed us
that, in the opinion of the SEC, this indemnification is against public policy
as expressed in the Securities Act, and is therefore unenforceable.
Section 16(a) Beneficial
Ownership Reporting
Section
16(a) of the Securities Exchange Act of 1934, as amended, requires that
directors, executive officers and persons who own more than 10% of the
outstanding common stock of certain reporting companies file initial reports of
ownership and reports of changes in ownership in such common stock with the
Securities and Exchange Commission ("SEC"). Officers, directors and
stockholders who own more than 10% of the outstanding common stock of certain
reporting companies are required by the SEC to furnish such companies with
copies of all Section 16(a) reports they file. We are required to comply with
Section 16(a). Accordingly, stock ownership information contained in this report
is based on what is known to us.
ITEM 11. EXECUTIVE
COMPENSATION
The
following table shows the compensation paid over the past three fiscal years
with respect to: (i) the Company’s President as of the end of the 2009, 2008 and
2007 fiscal year; (ii) the two other most highly compensated executive officers
(in terms of salary and bonus) serving at the end of the 2009, 2008 and 2007
fiscal year whose annual salary and bonus exceeded $100,000; and (iii) up to two
additional individuals who would be in category (ii) but for the fact that the
individual was not serving as an executive officer of the Company at the end of
the last completed fiscal year (the “named executive officers”):
SUMMARY COMPENSATION TABLE
|
Name and
principal position
|
Year
|
Salary ($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings ($)
|
All Other
Compensation
($)
|
Total ($)
|
|||||||||||||||||||||||||
|
Yiqing
Wan, CEO and President
|
2009
|
160,000 | 160,000 | |||||||||||||||||||||||||||||||
|
2008
|
$ | 160,000 | $ | 160,000 | ||||||||||||||||||||||||||||||
|
2007
|
$ | 160,000 | — | — | — | — | — | — | $ | 160,000 | ||||||||||||||||||||||||
|
Wei
Xu, Vice President
|
2009
|
100,000 | 100,000 | |||||||||||||||||||||||||||||||
|
2008
|
$ | 100,000 | $ | 100,000 | ||||||||||||||||||||||||||||||
|
2007
|
$ | 100,000 | — | — | — | — | — | — | $ | 100,000 | ||||||||||||||||||||||||
|
Hui
Long, Vice President
|
2009
|
17,580 | 17,580 | |||||||||||||||||||||||||||||||
|
2008
|
$ | 6,170 | $ | 6,170 | ||||||||||||||||||||||||||||||
|
2007
|
$ | 6,170 | — | — | — | — | — | — | $ | 6,170 | ||||||||||||||||||||||||
|
Daping
Gu, Vice President
|
2009
|
17,580 | 17,580 | |||||||||||||||||||||||||||||||
|
2008
|
$ | 6,000 | $ | 6,000 | ||||||||||||||||||||||||||||||
|
2007
|
$ | 6,000 | — | — | — | — | — | — | $ | 6,000 | ||||||||||||||||||||||||
|
Jingbo
Wu, Vice President
|
2009
|
17,580 | 17,580 | |||||||||||||||||||||||||||||||
|
2008
|
$ | 6,000 | $ | 6,000 | ||||||||||||||||||||||||||||||
|
2007
|
$ | 6,000 | — | — | — | — | — | — | $ | 6,000 | ||||||||||||||||||||||||
|
Eric
Yu, CFO*
|
2009
|
12,631 | 12,631 | |||||||||||||||||||||||||||||||
|
2008
|
$ | 100,000 | $ | 100,000 | ||||||||||||||||||||||||||||||
|
2007
|
$ | 89,000 | — | — | — | — | — | — | $ | 89,000 | ||||||||||||||||||||||||
*Eric Yu resigned from his position
of Director and CFO in September 2009.
69
No stock
options were granted or exercised by any executive officer during the fiscal
year ended December 31, 2009.
For the
year ended December
31,
2009, we paid
$90,000 as the
director remuneration to our independent
directors.
We have
entered into a five year employment contract with Mr. Wan and three year
contracts with Ms. Xu and Mr. Long. Pursuant to the Employment Agreements
entered into with the executive officers, starting from January 1, 2007, the
compensation of CEO, CFO and Vice President, Wei Xu would be as
follows:
|
|
1.
|
CEO,
CFO, Yiqing Wan’s yearly salary would be
$160,000;
|
|
|
2.
|
Vice
President, Wei Xu’s salary would be
$100,000.
|
|
|
3.
|
The
other Vice Presidents’ yearly salary would remain the same as the fiscal
year of 2006.
|
There
would be no specific salary increment and bonus scheme for the above mentioned
key managements for the next three years; it all depends on the profitability of
the company and subject to the Board of Directors’ approval.
Each
employment contract immediately terminates upon death or disability, and may be
terminated by the Company either with or without cause after 30 days notice, or
terminated by the officer for good reason with 60 days notice. We are not
currently aware of the plans of any key employees to retire or leave the
Company.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN
BENEFICIAL OWNERS AND
MANAGEMENT.
The
following table sets forth information as of December 31, 2009 with respect to
the beneficial ownership of the outstanding shares of the Company’s capital
stock by (i) each person known by the Company who will beneficially own five
percent (5%) or more of the outstanding shares; (ii) the officers and directors
of the Company; and (iii) all the aforementioned officers and directors as a
group.
Beneficial
ownership is determined in accordance with the rules of the Securities and
Exchange Commission and generally includes voting or investment power with
respect to securities. Shares of common stock subject to options, warrants or
convertible securities exercisable or convertible within 60 days of May 13, 2010
are deemed outstanding for computing the percentage of the person or entity
holding such options, warrants or convertible securities but are not deemed
outstanding for computing the percentage of any other person.
70
|
Name of Beneficial Owner
|
Amount
of Beneficial
Ownership
|
Percent
of Beneficial
Ownership
(4)
|
|||||
|
XIA Pharmaceutical
Inc. (1)
|
44,687,136
|
(2)
|
42.50
|
%
|
|||
|
Hui
Long (1)
|
0
|
0.00
|
%
|
||||
|
Yiqing
Wan (1)
|
44,687,136
|
(2)
|
42.50
|
%
|
|||
|
Wei
Xu (1)
|
44,687,136
|
(2)
|
42.50
|
%
|
|||
|
Dr.
Q.Y. Ma (1)
|
0
|
0.00
|
%
|
||||
|
Daping
Gu (1)
|
0
|
0.00
|
%
|
||||
|
Ruilu
Song (1)
|
0
|
0.00
|
%
|
||||
|
Jingbo
Wu (1)
|
0
|
0.00
|
%
|
||||
|
Pope
Investments, LLC (3)
5100
Poplar Ave., Suite 805
Memphis,
TN 38137
|
54,108,458
|
39.52
|
%
|
||||
|
All
Executive Officers and Directors as a group (7 persons)
|
44,687,136
|
42.50
|
%
|
||||
|
(1)
|
Address
is c/o Room 13, Floor 25, Sunny New World Tower, No. 231 Xin Hua Road,
Jianghan District, Wuhan, Hubei,
PRC.
|
|
(2)
|
Yiqing
Wan and Wei Xu each have a 50% equity ownership in XIA Pharmaceutical Inc.
They are both our executive officers and Yiqing Wan is a director. In
addition, they are husband and
wife.
|
|
(3)
|
William
P. Wells is the manager of Pope Investments, LLC (“Pope”) and exercises
sole voting and investment control over such shares. In addition to the
22,337,998 shares of common stock, Pope also holds warrants for the right
to purchase a total of 21,818,452 additional shares, and a Convertible
Promissory Note convertible into 9,952,008 additional
shares.
|
|
(4)
|
Applicable
percentage of ownership is based on 105,155,355 shares of common stock
outstanding as of May 13, 2010 together with securities exercisable or
convertible into shares of common stock within sixty (60) days of May 13,
2010 for each stockholder. Beneficial ownership is determined in
accordance with the rules of the SEC and generally includes voting or
investment power with respect to securities. Shares of common stock
subject to securities exercisable or convertible into shares of common
stock that are currently exercisable or exercisable within 60 days of May
13, 20109 are deemed to be beneficially owned by the person holding such
options for the purpose of computing the percentage of ownership of such
person, but are not treated as outstanding for the purpose of computing
the percentage ownership of any other
person.
|
71
ITEM
13. CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS.
Described
below are certain transactions or series of transactions since inception between
Benda and our subsidiaries and our executive officers, directors and the
beneficial owners of 5% or more of our common stock, on an as converted basis,
and certain persons affiliated with or related to these persons, including
family members, in which they had or will have a direct or indirect material
interest in an amount that exceeds $120,000 other than compensation arrangements
that are otherwise required to be described under "Executive
Compensation."
Kevin R.
Keating
Keating Securities, LLC (the
“Placement
Agent”) acted as placement
agent in connection with the Financing. For their services, the
Placement Agent received a commission equal to 7.5% of the gross proceeds or
approximately $900,000 from the offering and a non-accountable expense allowance
equal to 1.5% of the gross proceeds or approximately $180,000. In addition, the Placement Agent received,
for nominal consideration, five-year warrants to purchase 2,596,176 shares of
our common stock, or 10% of the number of shares of Common Stock sold in the
offering, at an exercise price of $0.555 (“Placement Agent Warrants”). We also paid for the out-of-pocket
expenses incurred by the Placement Agent and all purchasers in the amount of
approximately $100,000. As additional compensation for the Placement Agent's
services, we will also pay the Placement Agent the Warrant Solicitation Fee with respect to the
exercise, in whole or in part, of any Warrant equal to 3.0% of the total exercise price of the
Common Stock issued in such exercise of such Warrant. Such cash Warrant
Solicitation Fees shall be paid to the Placement Agent, in immediately available funds,
within three (3) business days following receipt, directly or indirectly, by us,
of any cash or other proceeds from the exercise of such Warrant.
At or prior to the Closing, pursuant to
the terms of the Exchange Agreement, we will enter into a certain
financial advisory agreement with Keating Securities, LLC ("Keating
Securities"), a registered broker-dealer, under which Keating Securities will be
compensated by us for its advisory services rendered to us in connection with the Exchange Agreement. The
transaction advisory fee will be $395,000. This fee shall be paid upon the
Closing of the Exchange Agreement.
Kevin R. Keating, our former President
and sole officer and director who resigned on November 15, 2006, is the father of Timothy J. Keating,
the principal member of Keating Investments, LLC. Keating Investments, LLC is
the managing member of KI Equity, which is our majority stockholder. Keating
Investments, LLC is also the managing member and 100% owner of Keating Securities, LLC, a registered
broker-dealer. Kevin R. Keating is not affiliated with and has no equity
interest in Keating Investments, LLC, KI Equity, or Keating Securities, LLC and
disclaims any beneficial interest in the shares of our common stock owned by KI Equity. Similarly,
Keating Investments, LLC, KI Equity and Keating Securities, LLC disclaim any
beneficial interest in the shares of our common stock currently owned by Kevin
R. Keating.
Effective January 1, 2006, we entered
into a contract with Vero
Management, L.L.C. ("Vero") for managerial and administrative services. Vero has
not been engaged to provide, and Vero does not render, legal, accounting,
auditing, investment banking or capital formation services. Kevin R. Keating,
our former officer and director, is the manager
of Vero. The term of the contract is for one year, but the contract may be
terminated at any time. In consideration of the services provided, Vero is paid
$2,500 for each month in which services are rendered.
Mark
R. Littell
and Norwood
On December 14, 2005, KI Equity entered
into a Purchase Agreement with Norwood Venture Corp. (“Norwood”) for 2,281,302 shares of our common
stock, representing 77.2% of the common shares then outstanding. Mark R. Little,
our former Chairman, Chief
Executive Officer, President and Chief Financial Officer who resigned on
December 30, 2005, is the President and controlling shareholder of Norwood. In
connection with the Purchase Agreement, Mark R. Littell and Norwood entered into
an agreement releasing Applied from any and
all claims they have against us.
During 2006, Norwood paid an accrued
legal expense on behalf of Applied in the amount of $1,568, which was recorded
as additional paid-in capital.
In connection with the
Purchase Agreement, we
paid Norwood approximately $18,936 for consulting services rendered by it to
Applied Spectrum.
72
Related
Party Transactions a nd Long Term Loan Receivable
Due from
related parties at December 31, 2009 and 2008 were comprised as
follows:
|
December 31,
|
December 31,
|
|||||||||
|
Relationship
|
2009
|
2008
|
||||||||
|
Current
|
||||||||||
|
Qin
Yu
|
Vice
president
|
|||||||||
|
Due
to SiBiono
|
$ | 2,024 | $ | 4,314 | ||||||
|
Xiaoji
Zhang
|
Minority
shareholder
|
|||||||||
|
Due
to SiBiono
|
5,423 | 5,439 | ||||||||
|
Hua
Xu
|
General Manager's Sister
|
|||||||||
|
Due
to SiBiono
|
22,726 | - | ||||||||
|
Rong
He
|
Manager
|
|||||||||
|
Due
to SiBiono
|
688 | 1,247 | ||||||||
| $ | 30,861 | $ | 11,000 | |||||||
|
Non
current
|
||||||||||
|
Yiqing
Wan
|
CEO
& Director
|
|||||||||
|
Due
to Ever Leader
|
$ | 646,586 | $ | 650,790 | ||||||
|
Due
to Benda Ebei
|
520,712 | 439,719 | ||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Due
to Yidu Benda
|
1,602,950 | 1,607,173 | ||||||||
|
Due
to Ever Leader
|
230,216 | 231,713 | ||||||||
|
Feng
Wang
|
Minority
shareholder
|
|||||||||
|
Due
to Beijing Shusai
|
32,262 | 32,348 | ||||||||
| $ | 3,032,726 | $ | 2,961,743 | |||||||
The
balance owned by the CEO & Director, and the Company under his control,
totaled $3,000,464 and $2,929,395 as of December 31, 2009 and 2008,
respectively. This is a violation of Section 402 of the Sarbanes-Oxley Act of
2002 which prohibits on personal loans to executives.
73
Due to
related parties at December 31, 2009 and 2008 were comprised as
follows:
|
December 31,
|
December 31,
|
|||||||||
|
|
Relationship
|
2009
|
2008
|
|||||||
|
Current
|
||||||||||
|
Wei
Xu
|
VP,
CEO's Spouse & Director
|
|||||||||
|
Due
to SiBiono
|
$ | 234,569 | $ | 188,065 | ||||||
|
Due
from Ever Leader
|
1,356,172 | 968,000 | ||||||||
|
Due
from Benda
|
36,184 | - | ||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Due
from Benda Ebei
|
28,528 | - | ||||||||
|
Due
from Jiangliang Benda
|
793,864 | - | ||||||||
|
Due
from Beijing Shusai
|
14,111 | - | ||||||||
|
SiBiono
Zhongjia Gene Tech (SZ) Co., Ltd.
|
Associate
company
|
|||||||||
|
Due
from SiBiono
|
103,948 | - | ||||||||
|
Yiqing,
Wan
|
CEO
& Director
|
|||||||||
|
Due
from SiBiono
|
224,071 | 237,780 | ||||||||
|
Hua
Xu
|
General
Manager's Sister
|
|||||||||
|
Due
from SiBiono
|
- | 25,523 | ||||||||
|
Hua
Shen
|
Vice
president
|
|||||||||
|
Due
from SiBiono
|
- | 33,253 | ||||||||
|
Dongyi
Tien
|
Manager
|
|||||||||
|
Due
from SiBiono
|
- | 364 | ||||||||
|
Pong
Tsaiohuei
|
Minority
shareholder
|
|||||||||
|
Due
from SiBiono
|
- | 4,818 | ||||||||
| $ | 2,791,447 | $ | 1,457,803 | |||||||
|
Non
current
|
||||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Due
from Benda Ebei
|
$ | - | $ | 28,602 | ||||||
|
Due
from Jiangliang Benda
|
- | 811,073 | ||||||||
|
Due
from Beijing Shusai
|
- | 14,262 | ||||||||
|
Wei
Xu
|
VP, CEO's Spouse & Director
|
|||||||||
|
Due
from Benda Ebei
|
23,894 | 48,922 | ||||||||
|
Due
from Beijing Shusai
|
65,339 | 65,691 | ||||||||
|
Yiqing,
Wan
|
CEO
& Director
|
|||||||||
|
Due
from Yidu Benda
|
559 | 560 | ||||||||
|
Hui
Xu
|
Manager
|
|||||||||
|
Due
from Benda Ebei
|
28,410 | 28,483 | ||||||||
| $ | 118,202 | $ | 997,593 | |||||||
Except
for the loans from the shareholder Wei Xu by Everleader which bears interest
rate at 12% per annum, unsecure and matures within six months, the above
advances bear no interest and the above loans due to related parties are
unsecured, non-interest bearing and are not convertible into equity. Proceeds
from the above loans were used primarily for general working capital purposes,
among which the current portion does not have definitive terms and for
those portions which are long-term debts in nature, they are expected to be
repaid by the Company in over 12-month period.
Reorganization Related
Transactions
Ever Leader was incorporated in Hong
Kong on October 29, 2005 for the purpose of functioning as an off-shore holding
company to obtain ownership interests in various Benda entities that were
previously owned, either directly or indirectly, by Wan and Xu. Ms. Mo Mo
Hon (“Hon”), a Hong Kong SAR resident, is the
sole registered shareholder of Ever Leader, holding the single issued and
outstanding share of Ever Leader in trust for Xu.
74
Pursuant to three separate Equity
Transfer Agreements
entered into in November of 2005 among Ever Leader, Benda Science, Xu, and Wan,
Ever Leader obtained a 95% ownership interest in Benda Ebei in exchange for a
commitment to pay $2,298,434 in aggregate consideration to Benda Science,
Wan, and Xu. The $2,298,434 acquisition
price represented 95% of the $2,419,404 of registered capital of Benda Ebei, but
was not representative of the fair value of the assets acquired or liabilities
assumed. Specifically, as transfers of ownership interests in PRC entities to offshore holding
companies for zero or nominal consideration is prohibited by the Chinese
Government (regardless of whether these PRC entities and offshore holding
companies are directly or indirectly owned and controlled by the same
individual or individuals), an amount equal
to 95% of the value of the registered capital of Benda Ebei was established for
purposes of the transfer of the 95% ownership interest in Benda Ebei (directly
and indirectly 100% owned and controlled by Wan and Xu) to Ever Leader (beneficially 100% owned
and controlled by Xu).
Pursuant to an Equity Transfer
Agreement entered into on December 3, 2005 among Benda Ebei, Benda Science, and
Wan, Benda Science transferred and assigned its 90% ownership interest in
Jiangling Benda to Benda
Ebei and Wan transferred and assigned a 5% ownership interest in Jiangling Benda
to Benda Ebei (for zero consideration as Benda Ebei and Jiangling Benda were
both directly and indirectly 100% owned and controlled by Wan and
Xu).
Pursuant to a second Equity Transfer
Agreement entered into on December 4, 2005 among Benda Ebei, Benda Science, and
Wan, Benda Science transferred and assigned its 90% ownership interest in Yidu
Benda to Benda Ebei and Wan transferred and assigned a 5% ownership interest in Yidu Benda to Benda
Ebei (for zero consideration as Benda Ebei and Yidu Benda were both directly and
indirectly 100% owned and controlled by Wan and Xu).
75
The organization and ownership
structure of the Company subsequent to the consummation of the reorganization as
summarized in the paragraphs above is as follows:
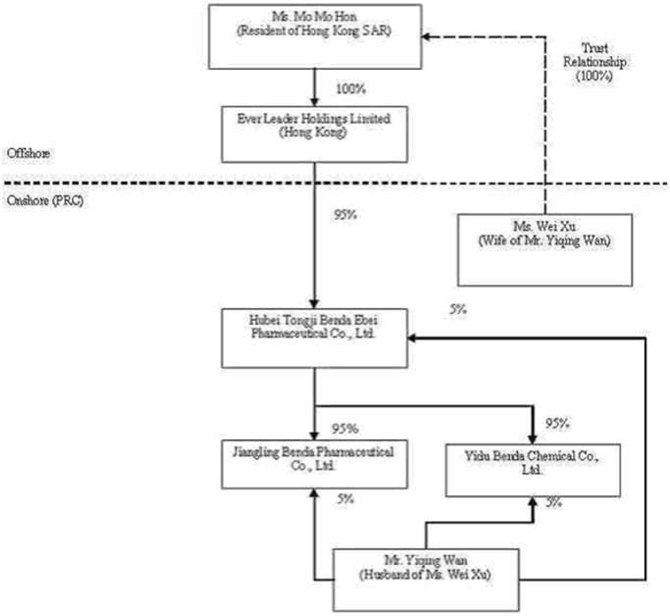
In July
of 2006, Benda Ebei invested approximately $112,500 for a 75% ownership interest
in Beijing Shusai, with the remaining 25% owned by an unrelated PRC individual.
Beijing Shusai, a PRC limited liability company, was incorporated on June 15,
2006 and commenced primary operations in July 2006, operating two clinics in
Beijing, PRC.
On
September 5, 2006, Ever Leader increased its number of authorized shares of
common stock from 10,000 to 1,000,000 and effected a 100 to 1 stock split,
resulting in Hon (the original sole registered shareholder of Ever Leader
holding one share in trust for Xu) receiving 99 additional shares in the
Company.
On
September 5, 2006, Ever Leader transferred and assigned 711,202 shares of common
stock to Xia Pharmaceutical, Inc. (“XIA”), an offshore holding company
incorporated in the British Virgin Islands (“BVI”) that is 100% owned and
controlled by Wan and Xu.
76
On
September 5, 2006, Ever Leader issued 288,698 shares of common stock to 19
entities (some of whom are considered related parties) at par value.
Additionally, Hon transferred and assigned her ownership interest in her 100
shares of Ever Leader to one of these entities.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND
SERVICES
(1)AUDIT
FEES
The
aggregate fees paid for each of the last two fiscal years for professional
services rendered by the principal accountant for the audit of the Company's
annual financial statements and review of financial statements included in the
Company's Form 10-K (17 CFR 249.308a) or 10-Q (17 CFR 249.308b) or services
that are normally provided by the accountant in connection with statutory and
regulatory filings or engagements for those fiscal years was $220,000 to MB and $125,000 to K&Co for the
fiscal year ended December 31, 2009 and $420,734 to K&Co for the fiscal year
ended December 31, 2008.
(2)AUDIT-RELATED
FEES
There
were no fees billed in each of the last two fiscal years for assurance and
related services by the principal accountant that are reasonably related to the
performance of the audit or review of the Company's financial
statements.
(3)TAX
FEES
There
were no fees billed in each of the last two fiscal years for professional
services rendered by the principal accountant for tax compliance, tax advice,
and tax planning.
(4) ALL
OTHER FEES
There
were no other fees billed in each of the last two fiscal years for products and
services provided by the principal accountant, other than the services reported
above.
RE-APPROVAL
POLICIES AND PROCEDURES
Before
the accountant is engaged by the issuer to render audit or non-audit services,
the engagement is approved by the Company's the board of directors acting as the
audit committee.
ITEM 15. EXHIBITS, LIST AND REPORTS ON FORM
8-K.
(a)Exhibits.
77
EXHIBIT
INDEX
The
following exhibits are incorporated by reference or included as part of this
report:
|
Exhibit
Number
|
Description
|
|
|
2.1
|
Articles
of Merger between Applied Spectrum Technologies, Inc., a Minnesota
corporation, and Applied Spectrum Technologies, Inc., a Delaware
corporation, together with the Agreement and Plan of Merger
(1)
|
|
|
2.2
|
Exchange
Agreement by and among Applied Spectrum Technologies, Inc. (“Applied
Spectrum”); KI Equity Partners, III, LLC (“KI Equity”); Ever Leader
Holdings Limited (“Ever Leader”); and each of the equity owners of Ever
Leader Shareholders, dated September 7, 2006 *(2)
|
|
|
2.3
|
Voting
Agreement by and among the Ever Leader Shareholders and KI Equity, dated
November 15, 2006 (4)
|
|
|
2.4
|
Escrow
Agreement by and among Applied Spectrum, Ever Leader, Keating Securities,
LLC and Steele Street State Bank, the escrow agent, dated November 15,
2006 (4)
|
|
|
2.5
|
Make
Good Agreement by and among Keating Securities, LLC, Applied Spectrum,
Ever Leader, Mr. Yiqing Wan and Ms. Wei Xu, and Moveup Investments
Limited, dated November 15, 2006 (4)
|
|
|
2.6
|
Make
Good Escrow Agreement by and among Keating Securities, LLC, Applied
Spectrum, Ever Leader, Mr. Yiqing Wan and Ms. Wei Xu, and Moveup
Investments Limited, dated November 15, 2006 (4)
|
|
|
3.1
|
Certificate
of Incorporation of Applied Spectrum Technologies, Inc., a Delaware
corporation. (1)
|
|
|
3.2
|
Bylaws
of Applied Spectrum Technologies, Inc., a Delaware corporation.
(1)
|
|
|
4.1
|
Lock-Up
Agreement amongst Applied Spectrum, Keating Securities, LLC, Yiqing Wan,
Wei Xu and Moveup Investments Limited (4)
|
|
|
4.2
|
Specimen
Stock Certificate for Shares of Common Stock of the Company
(3)
|
|
|
10.1
|
Placement
Agent Agreement dated October 17, 2006 between Applied Spectrum and
Keating Securities, LLC (4)
|
|
|
10.2
|
Securities
Purchase Agreement by and among Applied Spectrum, Ever Leader and the
Investor listed on the attached Schedule of Buyers (4)
|
|
|
10.3
|
Registration
Rights Agreement (4)
|
|
|
10.4
|
Form
of Common Stock Purchase Warrant (4)
|
|
|
10.5
|
Form
of Placement Agent Stock Purchase Warrant (4)
|
|
|
10.6
|
Employment
Agreement with Yiqing Wan (4)
|
|
|
10.7
|
Employment
Agreement with Wei Xu (4)
|
|
|
10.8
|
Employment
Agreement with Hui Long (4)
|
|
|
10.9
|
Employment
Agreement with Daping Gu (4)
|
|
|
10.10
|
Employment
Agreement with Ruilu Song (4)
|
78
|
10.11
|
Employment
Agreement with Jingbo Wu (4)
|
|
|
10.12
|
Form
Investment Agreement between the Company and Buyers (5)
|
|
|
10.13
|
Equity
Transfer Agreement with Shenzhen Yuanzheng Investment Development Co., Ltd
(5)
|
|
|
10.14
|
Financial
Consultancy Agreement (5)
|
|
|
10.15
|
Equity
Transfer Agreement with Shenzhen Yuanxing Gene City Development Co., Ltd.
(5)
|
|
|
10.16
|
Modification
and Amendment Agreement dated April 5, 2007 (5)
|
|
|
10.17
|
Technical
Consultancy Agreement with Huimin Zhang (6)
|
|
|
10.18
|
Equity
Transfer Agreement with Huimin Zhang (6)
|
|
|
10.19
|
Technical
Consultancy Agreement with Yaojin Wang (6)
|
|
|
10.20
|
Equity
Transfer Agreement with Yaojin Wang (6)
|
|
|
10.21
|
Interview
by Eric Yu for WallSt.net dated July 26, 2007 (7)
|
|
|
10.22
|
Rights
Purchase Agreement with Dr. Yan Li (7)
|
|
|
10.23
|
The
Science and Technology Cooperation Agreement between College of Chemistry
and Life Science of China Three Gorges University and Yidu Benda
(7)
|
|
|
31.1
|
Certification
of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002. Included as Exhibit in this Form 10-K.
|
|
|
31.2
|
Certification
of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002. Included as Exhibit in this Form 10-K.
|
|
|
32.1
|
Certification
of Chief Executive Officer of the Company, pursuant to 18 U.S.C. Section
1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of
2002. Included as Exhibit in this Form 10-K.
|
|
|
32.2
|
Certification
of Chief Financial Officer of the Company, pursuant to 18 U.S.C. Section
1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of
2002. Included as Exhibit in this Form
10-K.
|
|
(1)
|
Incorporated
by reference to the Company's Current Report on Form 8-K dated November
17, 2005 and filed on November 22, 2005 (SEC File No.
000-16397).
|
|
(2)
|
Incorporated
by reference to the Company's Current Report on Form 8-K dated September
7, 2006 and filed on September 7, 2006 (SEC File No.
000-16397).
|
|
(3)
|
Incorporated
by reference to the Company’s Registration Statement on Form S-1 (SEC File
No. 33-17959).
|
|
(4)
|
Incorporated
by reference to the Company's Current Report on Form 8-K dated November
15, 2006 and filed on November 17, 2006 (SEC File No.
000-16397).
|
|
(5)
|
Incorporated
by reference to the Company’s Current Report on Form 8-K dated April 5,
2007 and filed on April 6, 2007 (SEC File No.
000-16397).
|
79
|
(6)
|
Incorporated
by reference to the Company’s Current Report on Amendment No. 1 to Form
8-K dated April 5, 2007 and filed on June 15, 2007 (SEC File No.
000-16397).
|
|
(7)
|
Incorporated by reference to the
Company’s Amendment No. 2 to Registration Statement on Form SB-2 (SEC File
No. 33-17959).
|
|
(b)
|
Reports
on Form 8-K.
|
80
SIGNATURES
In
accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused
this report to be signed on its behalf by the undersigned, thereunto duly
authorized.
|
BENDA
PHARMACEUTICAL, INC.
|
||
|
Date:
May 18, 2010
|
By:
|
/s/
Yiqing Wan
|
|
Yiqing
Wan
Chief
Executive Officer, Chief Financial Officer,
President,
and Director
|
||
In
accordance with the requirements of the Securities Exchange Act of 1934, this
report has been signed by the following persons on behalf of the registrant and
in the capacities and on August 1, 2008.
|
Name
|
Title
|
Date
|
||
|
/s/
Yiqing Wan
|
Chief
Executive Officer and
|
May
18, 2010
|
||
|
Yiqing
Wan
|
President
/ Director
|
|||
|
/s/
Jun Tang
|
Director
|
May
18, 2010
|
||
|
Jun
Tang
|
||||
|
/s/
Dr. Q.Y. Ma
|
Director
|
May
18, 2010
|
||
|
Dr.
Q.Y. Ma
|
81
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors
Benda
Pharmaceutical, Inc.
Wuhan,
Hubei Province, PRC
We have
audited the accompanying consolidated balance sheet of Benda Pharmaceutical,
Inc. (the “Company”) as of December 31, 2009 and the related statements of
operations, changes in shareholders’ equity (deficit) and cash flows for the
year then ended. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these
financial statements based on our audit.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. The Company is not required at
this time, to have, nor were we engaged to perform, an audit of its internal
control over financial reporting. Our audit included consideration of internal
control over financial reporting as a basis for designing audit procedures that
are appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company's internal control over financial
reporting. Accordingly, we express no such opinion. An audit includes examining,
on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall financial statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Benda Pharmaceutical, Inc. at
December 31, 2009 and the results of its operations and its cash flows for the
year then ended in conformity with accounting principles generally accepted in
the in the United States of America.
The
accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. As discussed in Note 1 to the financial
statements, the Company has incurred losses for the year ended
December 31, 2009 and had a working capital deficiency at December 31, 2009.
These factors raise substantial doubt about its ability to continue as a going
concern. Management’s plans concerning this matter are also described in Note 1.
The accompanying financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
/s/MALONEBAILEY,
LLP
www.malonebailey.com
Houston,
Texas
May 17,
2010
Page 1 of
39
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors
Benda
Pharmaceutical, Inc.
Wuhan,
Hubei Province, PRC
We have
audited the condensed Parent Only balance sheet of Benda Pharmaceutical, Inc.
(the “Company”) as of December 31, 2009 and the related condensed Parent Only
statements of income and cash flows for the year then ended included in Footnote
24 to the Consolidated Financial Statements of Benda Pharmaceutical, Inc. These
Parent Only condensed financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these
financial statements based on our audit.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. The Company is not required at
this time, to have, nor were we engaged to perform, an audit of its internal
control over financial reporting. Our audit included consideration of internal
control over financial reporting as a basis for designing audit procedures that
are appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company's internal control over financial
reporting. Accordingly, we express no such opinion. An audit includes examining,
on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall financial statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our
opinion, the condensed Parent Only financial statements referred to above
present fairly, in all material respects, the financial position of Benda
Pharmaceutical, Inc. at December 31, 2009 and the results of its operations and
its cash flows for the year then ended in conformity with accounting principles
generally accepted in the in the United States of America.
The
accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. As discussed in Note 1 to the financial
statements, the Company has incurred losses for the year ended December 31, 2009
and had a working capital deficiency at December 31, 2009. These factors raise
substantial doubt about its ability to continue as a going concern. Management’s
plans concerning this matter are also described in Note 1. The accompanying
financial statements do not include any adjustments that might result from the
outcome of this uncertainty.
/s/MALONEBAILEY,
LLP
www.malone-bailey.com
Houston,
Texas
May 17,
2010
Page 2 of
39
|
KEMPISTY
& COMPANY
|
|
CERTIFIED
PUBLIC ACCOUNTANTS, P.C.
|
|
15
MAIDEN LANE - SUITE 1003 - NEW YORK, NY 10038 - TEL (212) 406-7272 - FAX
(212) 513-1930
|
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of
Directors
Benda
Pharmaceutical, Inc.
We have
audited the accompanying consolidated balance sheets of Benda Pharmaceutical,
Inc. and subsidiaries (the "Company") as of December 31, 2008 and the related
consolidated statements of operations, changes in stockholders’ equity and cash
flows for each of the year then ended. These financial statements are the
responsibility of the Company's management. Our responsibility is to express an
opinion on these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. The Company is not required at
this time, to have, nor were we engaged to perform, an audit of its internal
control over financial reporting. Our audit included consideration of internal
control over financial reporting as a basis for designing audit procedures that
are appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company's internal control over financial
reporting. Accordingly, we express no such opinion. An audit includes examining,
on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall financial statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Benda Pharmaceutical, Inc. and
subsidiaries at December 31, 2008 and the results of its operations and its cash
flows for the year then ended in conformity with accounting principles generally
accepted in the in the United States of America.
As
discussed in Note 25 to the consolidated financial statements, the
accompanying consolidated balance sheet as of December 31, 2008 and the related
statements of operations, cash flows and stockholders' deficit for the year then
ended have been restated.
The
accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. As discussed in Note 1 to the financial
statements, the Company has incurred losses for the year ended December 31, 2008
and had a working capital deficiency at December 31, 2008. These factors raise
substantial doubt about its ability to continue as a going concern. Management’s
plans concerning this matter are also described in Note 1. The accompanying
financial statements do not include any adjustments that might result from the
outcome of this uncertainty.
Kempisty
& Company
Certified
Public Accountants PC
New York,
New York
May 17,
2010
Page 3 of
39
|
KEMPISTY
& COMPANY
|
|
CERTIFIED
PUBLIC ACCOUNTANTS, P.C.
|
|
15
MAIDEN LANE - SUITE 1003 - NEW YORK, NY 10038 - TEL (212) 406-7272 - FAX
(212) 513-1930
|
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of
Directors
Benda
Pharmaceutical, Inc.
We have
audited the condensed Parent Only balance sheet of Benda Pharmaceutical, Inc. as
of December 31, 2008 and the related condensed Parent Only statements of
operations and cash flows for the year then ended included in Footnote 24 to the
Consolidated Financial Statements of Benda Pharmaceutical, Inc. These
Parent Only condensed financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these
financial statements based on our audit.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. The Company is not required at
this time, to have, nor were we engaged to perform, an audit of its internal
control over financial reporting. Our audit included consideration of internal
control over financial reporting as a basis for designing audit procedures that
are appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company's internal control over financial
reporting. Accordingly, we express no such opinion. An audit includes examining,
on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall financial statement presentation. We believe that our audit provides a
reasonable basis for our opinion.
In our
opinion, the condensed Parent Only financial statements referred to above
present fairly, in all material respects, the financial position of Benda
Pharmaceutical, Inc. at December 31, 2008 and the results of its operations and
its cash flows for the year then ended in conformity with accounting principles
generally accepted in the in the United States of America.
The
accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. As discussed in Note 1 to the financial
statements, the Company has incurred losses for the year ended December 31, 2008
and had a working capital deficiency at December 31, 2008. These factors raise
substantial doubt about its ability to continue as a going concern. Management’s
plans concerning this matter are also described in Note 1. The accompanying
financial statements do not include any adjustments that might result from the
outcome of this uncertainty.
Kempisty
& Company
Certified
Public Accountants PC
New York,
New York
May 17,
2010
Page 4 of
39
Benda
Pharmaceutical, Inc.
Consolidated
Balance Sheets
|
(Restated)
|
||||||||
|
December
31
|
December
31
|
|||||||
|
2009
|
2008
|
|||||||
|
Assets
|
||||||||
|
Current
Assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 191,095 | $ | 584,266 | ||||
|
Trade
receivables, net (Note 5)
|
12,405,018 | 9,328,914 | ||||||
|
Advance
for inventory purchase
|
2,110,857 | 230,888 | ||||||
|
Notes
receivable
|
81,426 | 154,691 | ||||||
|
Inventories
(Note 6)
|
2,038,987 | 2,344,561 | ||||||
|
Due
from related parties, short term (Note 14)
|
30,861 | 11,000 | ||||||
|
Prepaid
expenses and other current assets
|
1,720,237 | 508,854 | ||||||
|
Total
current assets
|
18,578,481 | 13,163,174 | ||||||
|
Due
from related parties, long term (Note 14)
|
3,032,726 | 2,961,743 | ||||||
|
Property
and equipment, net (Note 7)
|
28,658,131 | 28,907,035 | ||||||
|
Intangible
assets, net (Note 8)
|
6,629,501 | 7,369,482 | ||||||
|
Debt
issuance costs (Note 19)
|
- | 55,485 | ||||||
|
Restricted
cash (Note 10)
|
4,409,334 | 5,162,184 | ||||||
|
Other
assets (Note 11)
|
2,285,581 | 2,291,602 | ||||||
|
Total
Assets
|
$ | 63,593,754 | $ | 59,910,705 | ||||
|
Liabilities
& Shareholders' Equity
|
||||||||
|
Current
Liabilities
|
||||||||
|
Accounts
payable
|
902,079 | 605,317 | ||||||
|
Customer
deposit
|
1,507,147 | 1,056 | ||||||
|
Other
payable
|
4,569,503 | 2,232,874 | ||||||
|
Accrued
liabilities
|
6,175,538 | 3,940,480 | ||||||
|
Convertible
notes (Note 19)
|
7,260,000 | 6,395,951 | ||||||
|
Short-term
debt (Note 12)
|
11,576,121 | 12,281,729 | ||||||
|
Accrued
VAT and other taxes
|
795,013 | 954,509 | ||||||
|
Acquisition
price payable (Note 1)
|
1,422,743 | 1,426,491 | ||||||
|
Wages
payable
|
1,187,075 | 1,020,474 | ||||||
|
Due
to related parties, short term (Note 14)
|
2,791,447 | 1,457,803 | ||||||
|
Redeemable
common stock, 2,049,560 shares at $3.6 per share (Note 15)
|
7,376,366 | 7,376,366 | ||||||
|
Total
current liabilities
|
45,563,032 | 37,693,050 | ||||||
|
Government
grant payable (Note 13)
|
1,789,439 | 2,234,210 | ||||||
|
Due
to related parties, long-term (Note 14)
|
118,202 | 997,593 | ||||||
|
Deferred income
tax liability
|
778,026 | 834,458 | ||||||
|
Total
liabilities
|
48,248,699 | 41,759,311 | ||||||
|
Shareholders'
Equity
|
||||||||
|
Preferred
stock, $0.001 par value; 5,000,000 shares authorized;
|
||||||||
|
None
issued and outstanding
|
- | - | ||||||
|
Common
stock, $0.001 par value; 150,000,000 shares authorized;
|
||||||||
|
105,155,355
shares issued and outstanding
|
105,155 | 105,155 | ||||||
|
Additional
paid in capital
|
22,108,427 | 22,108,427 | ||||||
|
Statutory
surplus reserve fund (Note 16)
|
2,642,775 | 2,642,775 | ||||||
|
Accumulated
deficit
|
(17,481,559 | ) | (15,544,089 | ) | ||||
|
Accumulative
other comprehensive income
|
6,268,111 | 6,372,496 | ||||||
|
Shares
issuable for services
|
503,860 | 503,860 | ||||||
|
Total
Benda Pharmaceutical, Inc.'s Shareholders' Equity
|
14,146,769 | 16,188,624 | ||||||
|
Noncontrolling
Interest
|
1,198,286 | 1,962,770 | ||||||
|
Total
Shareholders' Equity
|
15,345,055 | 18,151,394 | ||||||
|
Total
Liabilities & Shareholders' Equity
|
$ | 63,593,754 | $ | 59,910,705 | ||||
The
accompanying notes are an integral part of these consolidated financial
statements.
Page 5 of
39
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Operations
|
YEAR
ENDED DECEMBER 31,
|
||||||||
|
(Restated)
|
||||||||
|
2009
|
2008
|
|||||||
|
Revenue
|
$ | 21,990,915 | $ | 25,041,986 | ||||
|
Cost
of goods sold
|
(13,720,676 | ) | (15,740,107 | ) | ||||
|
Gross
profit
|
8,270,239 | 9,301,879 | ||||||
|
Selling
expenses
|
2,378,852 | 2,708,968 | ||||||
|
General
and administrative expenses
|
||||||||
|
Bad
debts
|
1,424,072 | 2,730,434 | ||||||
|
Depreciation
and amortization expense
|
1,273,051 | 837,440 | ||||||
|
Goodwill
impairment
|
- | 4,141,109 | ||||||
|
Inventory
wirtten down to net realizable value
|
- | 392,535 | ||||||
|
Other
general and administrative expenses
|
3,224,561 | 4,189,341 | ||||||
|
Research
and development expenses
|
349,361 | 1,461,951 | ||||||
|
Total
operating expenses
|
8,649,897 | 16,461,778 | ||||||
|
Operating
loss
|
(379,658 | ) | (7,159,899 | ) | ||||
|
Other
income (expenses)
|
||||||||
|
Rental
income
|
241,005 | - | ||||||
|
Government
subsidies / grants (Note 13)
|
454,491 | - | ||||||
|
Interest
income
|
129,513 | 133,251 | ||||||
|
Interest
expense
|
(2,298,411 | ) | (5,359,788 | ) | ||||
|
Other
expenses (Note 17)
|
(14,833 | ) | (1,311,140 | ) | ||||
|
Loss
before income taxes
|
(1,867,893 | ) | (13,697,576 | ) | ||||
|
Income
taxes (Note 18)
|
818,289 | 776,295 | ||||||
|
Net
Loss
|
(2,686,182 | ) | (14,473,871 | ) | ||||
|
Less:
Net loss attributable to the noncontrolling interests
|
(748,712 | ) | (2,993,073 | ) | ||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (1,937,470 | ) | $ | (11,480,798 | ) | ||
|
Other
Comprehensive Loss
|
||||||||
|
Foreign
currency translation (loss) gain
|
(120,157 | ) | 3,460,265 | |||||
|
Comprehensive
Loss
|
(2,806,339 | ) | (11,013,606 | ) | ||||
|
Comprehensive
loss attributable to the noncontrolling interest
|
(764,484 | ) | (2,344,747 | ) | ||||
|
Comprehensive
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (2,041,855 | ) | $ | (8,668,859 | ) | ||
|
Net
loss per share - basic and diluted
|
||||||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (0.03 | ) | $ | (0.14 | ) | ||
|
Weighted
average shares outstanding - basic and diluted
|
105,155,355 | 101,965,464 | ||||||
The
accompanying notes are an integral part of these consolidated financial
statements.
Page 6 of
39
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Changes in Shareholders' Equity
|
Statutory
|
Accumulated
|
|||||||||||||||||||||||||||||||||||
|
Additional
|
Surplus
|
Other
|
Shares Issuable
|
Non-
|
Total
|
|||||||||||||||||||||||||||||||
|
Common
Stock
|
Paid-in
|
Reserve
|
Retained
|
Comprehensive
|
for
|
Controlling
|
Shareholder's
|
|||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Fund
|
Deficit
|
Income
|
Services
|
Interest
|
Equity
|
||||||||||||||||||||||||||||
|
Balance
at December 31, 2007 (Restated)
|
100,170,071 | $ | 100,170 | $ | 21,547,929 | $ | 2,310,681 | $ | (3,731,197 | ) | $ | 3,560,557 | $ | 503,860 | $ | 4,307,517 | $ | 28,599,517 | ||||||||||||||||||
|
Shares
issued in lieu of director renumeration 02/18/08, 110,000 shares @$0.69
each
|
110,000 | 110 | 75,790 | - | - | - | - | - | 75,900 | |||||||||||||||||||||||||||
|
Shares
issued in lieu of penalty for late filing on SB-2 on March 15, 2008.
03/15/08, 523,438 shares @ $0.440 each (Note 17)
|
523,438 | 523 | 229,789 | - | - | - | - | - | 230,312 | |||||||||||||||||||||||||||
|
Shares
issued for conversion of convertible notes on July 10, 2008. 07/10/2008,
540,870 shares @$0.554662 each (Note 19)
|
540,870 | 541 | (8,039 | ) | - | - | - | - | - | (7,498 | ) | |||||||||||||||||||||||||
|
Shares
issued for "Make good agreeement" 08/25/2008 3,810,976 shares @$0.07 (Note
17)
|
3,810,976 | 3,811 | 262,958 | - | - | - | - | - | 266,769 | |||||||||||||||||||||||||||
|
Foreign
currency translation adjustment
|
- | - | - | - | - | 2,811,939 | - | 648,326 | 3,460,265 | |||||||||||||||||||||||||||
|
Net
loss for the year ended 12/31/08
|
- | - | - | - | (11,480,798 | ) | - | - | (2,993,073 | ) | (14,473,871 | ) | ||||||||||||||||||||||||
|
Appropriation
to statutory reserve
|
- | - | - | 332,094 | (332,094 | ) | - | - | - | |||||||||||||||||||||||||||
|
Balance
at December 31, 2008 (Restated)
|
105,155,355 | $ | 105,155 | $ | 22,108,427 | $ | 2,642,775 | $ | (15,544,089 | ) | $ | 6,372,496 | $ | 503,860 | $ | 1,962,770 | 18,151,394 | |||||||||||||||||||
|
Foreign
currency translation adjustment
|
- | - | - | - | (104,385 | ) | - | (15,772 | ) | (120,157 | ) | |||||||||||||||||||||||||
|
Net
loss for the year ended 12/31/09
|
- | - | - | (1,937,470 | ) | - | - | (748,712 | ) | (2,686,182 | ) | |||||||||||||||||||||||||
|
Balance
at December 31, 2009
|
105,155,355 | $ | 105,155 | $ | 22,108,427 | $ | 2,642,775 | $ | (17,481,559 | ) | $ | 6,268,111 | $ | 503,860 | $ | 1,198,286 | $ | 15,345,055 | ||||||||||||||||||
The
accompanying notes are an integral part of these consolidated financial
statements.
Page 7 of
39
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Cash Flows
|
YEAR
ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
(Restated)
|
||||||||
|
Cash
Flows From Operating Activities
|
||||||||
|
Net
loss
|
$ | (2,686,182 | ) | $ | (14,473,871 | ) | ||
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
||||||||
|
Bad
debt provision
|
1,424,072 | 2,730,434 | ||||||
|
Inventory
written down to net realizable value
|
- | 392,535 | ||||||
|
Goodwill
impairment provision
|
- | 3,635,114 | ||||||
|
Loss
on disposals of fixed assets
|
43,044 | - | ||||||
|
Depreciation,
including amounts in cost of sales
|
2,194,394 | 2,086,178 | ||||||
|
Amortization
of intangible assets
|
712,369 | 679,738 | ||||||
|
Amortization
of debt issuance costs
|
55,485 | 264,962 | ||||||
|
Amortization
of convertible notes discount
|
864,049 | 3,520,876 | ||||||
|
Capitalized
interest relates to construction-in-progress
|
(364,164 | ) | - | |||||
|
Income
tax benefit
|
(54,354 | ) | (198,730 | ) | ||||
|
Penalty
to investors settled by issuance of common stock
|
- | 497,081 | ||||||
|
Directors
remuneration settled by issuance of common stock
|
- | 75,900 | ||||||
|
Changes
in operating assets and liabilities:
|
||||||||
|
Trade
receivables
|
(4,521,677 | ) | (1,593,970 | ) | ||||
|
Advance
for inventory purchase
|
(1,880,576 | ) | 676,222 | |||||
|
Inventories
|
299,415 | (784,748 | ) | |||||
|
Prepaid
expenses and other current assets
|
(1,212,712 | ) | (609,700 | ) | ||||
|
Accounts
payable
|
298,354 | 423,265 | ||||||
|
Customer
deposit
|
1,506,095 | (159,762 | ) | |||||
|
Others
payable
|
2,342,495 | 1,949,869 | ||||||
|
Accrued
expense
|
1,117,190 | 23,762 | ||||||
|
Wages
payable
|
168,053 | 355,688 | ||||||
|
Accrued
taxes
|
(156,987 | ) | (324,876 | ) | ||||
|
Government
grant payable
|
(439,830 | ) | - | |||||
|
Net
cash used in operating activities
|
(291,467 | ) | (834,033 | ) | ||||
|
Cash
Flows From Investing Activities
|
||||||||
|
Addition
of notes receivable
|
(1,118,610 | ) | (155,044 | ) | ||||
|
Collection
of notes receivable
|
1,191,469 | 7,208 | ||||||
|
Restricted
cash
|
739,286 | (2,546,930 | ) | |||||
|
Purchases
of property and equipment and construction-in-progress
|
(589,896 | ) | (1,708,213 | ) | ||||
|
Disposal
of intangible assets
|
- | 172,548 | ||||||
|
Advance
to related parties
|
(90,844 | ) | (342,724 | ) | ||||
|
Net
cash provided by (used in) investing activities
|
131,405 | (4,573,155 | ) | |||||
|
Cash
Flows From Financing Actives
|
||||||||
|
Borrowing
(advance) from related party
|
454,253 | (738,222 | ) | |||||
|
Repayments
of borrowings under bank loans, net
|
(673,338 | ) | 4,295,967 | |||||
|
Net
cash provided by (used in) financing activities
|
(219,085 | ) | 3,557,745 | |||||
|
Effect
of exchange rate changes on cash
|
(14,024 | ) | 699,589 | |||||
|
Net
decrease in cash and cash equivalents
|
(393,171 | ) | (1,149,854 | ) | ||||
|
Cash
and cash equivalents, beginning of period
|
584,266 | 1,734,120 | ||||||
|
Cash
and cash equivalents, end of period
|
$ | 191,095 | $ | 584,266 | ||||
|
Supplemental
Disclosure of Cash Flow Information
|
||||||||
|
Cash
paid for interest
|
$ | 536,106 | $ | 984,621 | ||||
|
Cash
paid for income taxes
|
$ | 1,118,912 | $ | 740,717 | ||||
|
Non-Cash
Investing Activities
|
||||||||
|
Transfer
of construction-in-progress to property and equipment
|
$ | 2,412,086 | $ | - | ||||
|
Purchase
of construction-in-progress on credit
|
$ | 1,119,428 | $ | - | ||||
The
accompanying notes are an integral part of these consolidated financial
statements.
Page 8 of
39
Benda
Pharmaceutical, Inc.
Notes
to Consolidated Financial Statements
(Amounts
expressed in U.S. Dollars)
|
1.
|
Organization
|
Benda
Pharmaceutical, Inc. (“Benda”) is a corporation organized under Delaware Law and
headquartered in Hubei Province, the People’s Republic of China
(“PRC”).
Ever
Leader Holdings Limited (“Ever Leader”), a wholly owned subsidiary of Benda, is
a company incorporated under the laws of Hong Kong SAR.
Ever
Leader owns 95% of the issued and outstanding capital of Hubei Tongji Benda Ebei
Pharmaceutical Co. Ltd. (“Benda Ebei”), a Sino-Foreign Equity Joint Venture
company incorporated under the laws of PRC. Mr. Yiqing Wan owns 5% of the issued
and outstanding capital stock of Benda Ebei. Benda Ebei owns: (i) 95% of the
issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co. Ltd.,
(“Jiangling Benda”) a company formed under the laws of PRC; (ii) 95% of the
issued and outstanding capital stock of Yidu Benda Chemical Co. Ltd., (“Yidu
Benda”) a company incorporated under the laws of PRC; and (iii) 75% of the
issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co.
Ltd., (“Beijing Shusai”) a company incorporated under the laws of PRC. Mr.
Yiqing Wan owns: (i) 5% of the issued and outstanding capital stock of Jingling
Benda; and (ii) 5% of the issued and outstanding capital stock of Yidu Benda.
Mr. Feng Wang owns 25% of the issued and outstanding capital stock of Beijing
Shusai.
On April
5, 2007, Benda Ebei entered into an Equity Transfer Agreements with Shenzhen
Yuanzheng Investment Development Co., Ltd. and Shenzhen Yuanxing Gene City
Development Co., Ltd., the shareholders of Shenzhen SiBiono GeneTech Co., Ltd
(“SiBiono”), to purchase 27.57% and 30% respectively of the shares of SiBiono’s
common stock for total consideration of RMB 60 million due and payable on or
before April 30, 2007. On June 11, 2007, Benda Ebei entered into an Equity
Transfer Agreement with Huimin Zhang and Yaojin Wang, the individual
shareholders of SiBiono, to purchase 1.6% and 0.96% respectively of the shares
of SiBiono’s common stock for total consideration of RMB 2.56 million due and
payable on or before June 30, 2007. Altogether, the total consideration for
60.13% shares of SiBiono’s common stock was RMB 62.56 million or $8.58 million.
As of December 31, 2009, an accumulated amount, approximately RMB 52. 83 million
or $7.16 million was paid leaving a balance of RMB 9.73 million or $1.42
million.
Benda,
Ever Leader, Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and SiBiono
shall be referred to herein collectively as the “Company”. The Company is
engaged principally in the business of identifying, discovering, developing, and
manufacturing conventional medicines, active pharmaceuticals, bulk chemicals (or
pharmaceutical immediates), and Traditional Chinese Medicines (“TCM”) for the
treatment of some of the most widespread common ailments and diseases (e.g.
common cold, diabetes, and cancer).
Page 9 of
39
As of
December 31, 2009, the organization and ownership structure of the Company is as
follows:
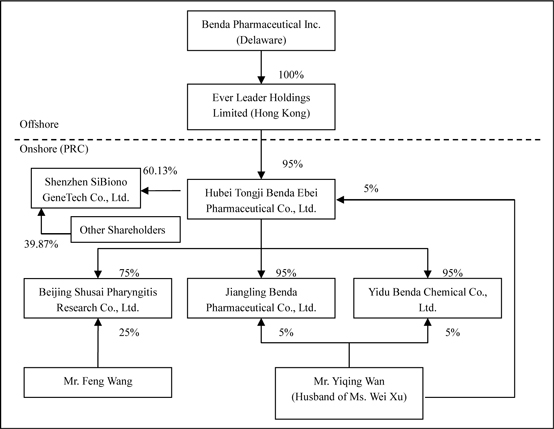
Going
Concern
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As reflected in the accompanying
consolidated financial statements, the Company has recurring losses and has a
working capital deficiency at December 31, 2009. These conditions raise
substantial doubt as to the Company’s ability to continue as a going
concern.
While the
Company is attempting to produce sufficient revenues, the Company’s cash
position may not be enough to support the Company’s daily operations. Management
intends to raise additional funds by way of a public or private offering.
Management believes that the actions presently being taking to further implement
its business plan and generate sufficient revenues provide the opportunity for
the Company to continue as a going concern. While the Company believes in the
viability of its strategy to increase revenues and in its ability to raise
additional funds, there can be no assurance to that effect. The ability of the
Company to continue as a going concern is dependent upon the Company’s ability
to further implement its business plan and generate sufficient revenues. The
financial statements do not include any adjustments that might be necessary if
the Company is unable to continue as a going concern.
|
2.
|
Basis
of Preparation
|
The
accompanying consolidated financial statements of the Company have been prepared
in accordance with the accounting principles generally accepted in the United
States of America (“U.S. GAAP”).
These
consolidated financial statements include the accounts of Benda, Ever Leader,
Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and Sibiono for the full
year. All significant inter-company balances and transactions have been
eliminated in the consolidation.
Page 10
of 39
Certain
amounts in the consolidated financial statements for the prior year have been
reclassified to conform to the presentation of the current year.
|
3.
|
Use
of Estimates
|
The
preparation of financial statements in conformity with US GAAP requires
management to make estimates and assumptions that affect the reported amounts of
assets and liabilities and disclosure of contingent assets and liabilities at
the date of the financial statements, and the reported amounts of revenue and
expenses during the reporting period. Actual results when ultimately realized
could differ from those estimates.
|
4.
|
Significant
Accounting Policies
|
Fair
Value of Financial Instruments
The
Company adopted ASC 820 “Fair Value Measurements,” which defines fair value,
establishes a three-level valuation hierarchy for disclosures of fair value
measurement and enhances disclosures requirements for fair value measures.
Current assets and current liabilities qualified as financial instruments and
management believes their carrying amounts are a reasonable estimate of fair
value because of the short period of time between the origination of such
instruments and their expected realization and if applicable, their current
interest rate is equivalent to interest rates currently available. The three
levels are defined as follow:
• Level 1
— inputs to the valuation methodology are quoted prices (unadjusted) for
identical assets or liabilities in active markets.
• Level 2
— inputs to the valuation methodology include quoted prices for similar assets
and liabilities in active markets, and inputs that are observable for the assets
or liability, either directly or indirectly, for substantially the full term of
the financial instruments.
• Level 3
— inputs to the valuation methodology are unobservable and significant to the
fair value.
As of the
balance sheet date, the estimated fair values of the financial instruments were
not materially different from their carrying values as presented due to the
short maturities of these instruments and that the interest rates on the
borrowings approximate those that would have been available for loans of similar
remaining maturity and risk profile at respective period-ends. Determining
which category an asset or liability falls within the hierarchy requires
significant judgment. The Company evaluates the hierarchy disclosures each
period.
Cash
and Cash Equivalents
Cash and cash equivalents include cash
on hand, cash on deposit with various financial institutions, and all
highly-liquid investments with original maturities of three months or less at
the time of purchase.
Restricted
Cash
The
restricted cash is recorded as asset when the Company deposits cash in the bank
as collateral for note payable, separately from cash and cash
equivalents.
Trade
receivables
Trade
receivables are recognized and carried at original invoiced amount less an
allowance for uncollectible accounts, as needed. The allowance for losses
on trade receivables reflects management’s best estimate of probable losses
determined principally on the basis of historical experience. The
allowance for losses is determined primarily on the basis of management’s best
estimate of probable losses, including specific allowances for known
troubled accounts. All accounts or portions thereof deemed to
be uncollectible or to require an excessive collection cost are written off
to the allowance for losses. When facts subsequently become available to
indicate that the amount provided as the allowance was incorrect, an adjustment
which classified as a change in estimate is made.
Page 11
of 39
Inventories
Inventories
are stated at the lower of cost, as determined on a weighted average basis, or
market. Costs of inventories include purchase and related costs incurred in
bringing the products to their present location and condition. Market value is
determined by reference to selling prices after the balance sheet date or
to management’s estimates based on prevailing market conditions. The
management writes down the inventories to market value if it is below cost.
The management also regularly evaluates the composition of its inventories
to identify slow-moving and obsolete inventories to determine if valuation
allowance is required.
Property
and Equipment
Property
and equipment are recorded at cost and depreciated using the straight-line
method, with an estimated 5% salvage value of original cost, over the estimated
useful lives of the assets as follows:
|
Buildings
|
20-30 years
|
|
|
Machinery
and equipment
|
10-15
years
|
|
|
Motor
Vehicles
|
5
years
|
|
|
Electronics
and office equipment
|
5
years
|
Expenditures
for repairs and maintenance, which do not improve or extend the expected useful
lives of the assets, are expensed as incurred while major replacements and
improvements are capitalized.
When
property or equipment is retired or disposed of, the cost and accumulated
depreciation are removed from the accounts, with any resulting gains or losses
being included in net income or loss in the year of disposition.
Intangible
Assets
The
Company’s intangible assets are stated at cost less accumulated amortization and
are comprised of land-use rights, drug permits and licenses and
patents. Land-use rights are related to land the Company occupies in
Hubei and Guangdong Province, PRC and are being amortized on a straight-line
basis over a period of 40 to 50 years. Other intangible assets are
being amortized on a straight-line basis over a period of 8 to 10
years.
Impairment
of Long-Lived Assets
The
Company accounts for impairment of plant and equipment and amortizable
intangible assets in accordance with ASC 360, “Accounting for Impairment of
Long-Lived Assets and Long-Lived Assets to be Disposed Of”, which requires the
Company to evaluate a long-lived asset for recoverability when there is
event or circumstance that indicate the carrying value of the asset may
not be recoverable. An impairment loss is recognized when the carrying
amount of a long-lived asset or asset Company is not recoverable (when
carrying amount exceeds the gross, undiscounted cash flows from use and
disposition) and is measured as the excess of the carrying amount over the
asset’s (or asset Company’s) fair value.
Revenue
Recognition
The
Company generates revenue from the sales of conventional medicines, active
pharmaceuticals, bulk chemicals (or pharmaceutical immediates), and Traditional
Chinese Medicines (“TCM”) for the treatment of some of the most widespread
common ailments and diseases. Sales are recognized when the following four
revenue criteria are met:
|
|
1.
|
Persuasive
evidence of an arrangement exists;
|
|
|
2.
|
Delivery
has occurred or services have been
rendered;
|
|
|
3.
|
The
seller's price to the buyer is fixed or determinable;
and
|
|
|
4.
|
Collectability
is reasonably assured.
|
Sales are
presented net of Value Added Tax (VAT). No return allowance is made as products
returns are insignificant based on historical experience.
Page 12
of 39
Research
and Development
Research
and development costs are expensed as incurred and consist primarily of salaries
and related expenses of personnel engaged in research and development
activities. The Company spent $349,361 and $1,461,951 on direct research and
development (“R&D”) efforts for the year ended 2009 and 2008,
respectively.
Income
Taxes
The
Company accounts for income taxes in accordance with the accounting standard
which requires an asset and liability approach for financial accounting and
reporting for income taxes and allows recognition and measurement of deferred
tax assets based upon the likelihood of realization of tax benefits in future
years. Under the asset and liability approach, deferred taxes are provided for
the net tax effects of temporary differences between the carrying amounts of
assets and liabilities for financial reporting purposes and the amounts used for
income tax purposes. A valuation allowance is provided for deferred tax assets
if it is more likely than not these items will either expire before the Company
is able to realize their benefits, or that future deductibility is
uncertain.
The
Company adopted the accounting standard for uncertainty in income taxes which
prescribes a comprehensive model for how a company should recognize,
measure, present and disclose in its financial statements uncertain tax
positions that the Company has taken or expects to take on a tax return
(including a decision whether to file or not to file a return in a particular
jurisdiction).
Comprehensive
Income
The
Company reports comprehensive income, its components, and accumulated balances
in its financial statements. Accumulated other comprehensive income
represents the accumulated balance of foreign currency translation
adjustments.
Foreign
Currency Translation
The
functional currency of the Company is the Renminbi (“RMB”), the PRC’s
currency. The Company maintains its financial statements using the
functional currency. Monetary assets and liabilities denominated in
currencies other than the functional currency are translated into the functional
currency at rates of exchange prevailing at the balance sheet dates.
Transactions denominated in currencies other than the functional currency are
translated into the functional currency at the exchange rates prevailing at the
dates of the transaction. Exchange gains or losses arising from foreign currency
transactions are included in the net income (loss) for the respective
periods.
For
financial reporting purposes, the financial statements of the Company, which are
prepared using the RMB, are translated into the Company’s reporting currency,
United States Dollars. Balance sheet accounts are translated using
the closing exchange rate in effect at the balance sheet date and income and
expense accounts are translated using the average exchange rate prevailing
during the reporting period. Adjustments resulting from the translation, if any,
are included in accumulated other comprehensive income (loss) in stockholder’s
equity.
The
exchange rates in effect at December 31, 2009 and 2008 were stated as follows:
(for RMB 1.00):
|
December 31,
2009
|
December 31,
2008
|
|||||||
|
Fixed
rate
|
$ | 0.1463 | $ | 0.1467 | ||||
|
Average
rate
|
$ | 0.1466 | $ | 0.1442 | ||||
Loss
Per Share
Basic
earnings per share amounts are calculated based on the weighted average number
of shares of common stock outstanding during each period. Diluted earnings per
share is based on the weighted average numbers of shares of common stock
outstanding for the periods, including dilutive effects of stock options,
warrants granted and convertible preferred stock. Dilutive options and warrants
that are issued during a period or that expire or are canceled during a period
are reflected in the computations for the time they were outstanding during the
periods being reported. Since the Company has incurred losses for both periods,
the impact of the common stock equivalents would be antidilutive and therefore
are not included in the calculation.
Page 13
of 39
Government
grants
Grants
from the PRC government are recognized at their fair value where there is a
reasonable assurance that the grant will be received and the Company will comply
with all attached conditions. Government grants are recognized as other income
or gains in the period when received and when government confirms that all
attached conditions are met. During the years ended December 31, 2009 and 2008,
the Company recognized other income from PRC government of $454,491 and $0
respectively.
Related
parties
A party
is considered to be related to the Company if the party directly or indirectly
or through one or more intermediaries, controls, is controlled by, or is under
common control with the Company. Related parties also include principal owners
of the Company, its management, members of the immediate families of principal
owners of the Company and its management and other parties with which the
Company may deal if one party controls or can significantly influence the
management or operating policies of the other to an extent that one of the
transacting parties might be prevented from fully pursuing its own separate
interests. A party which can significantly influence the management or operating
policies of the transacting parties or if it has an ownership interest in one of
the transacting parties and can significantly influence the other to an extent
that one or more of the transacting parties might be prevented from fully
pursuing its own separate interests is also a related party.
Recently
Issued Accounting Pronouncements
In
June 2009, the Financial Accounting Standards Board (FASB) issued a
standard that established the FASB Accounting Standards Codification (ASC) and amended the
hierarchy of generally accepted accounting principles (ASC) and amended the
hierarchy of generally accepted accounting principles (GAAP) such that the ASC
became the single source of authoritative nongovernmental U.S. GAAP. The ASC did
not change current U.S. GAAP, but was intended to simplify user access to all
authoritative U.S. GAAP by providing all the authoritative literature related to
a particular topic in one place. All previously existing accounting standard
documents were superseded and all other accounting literature not included in
the ASC is considered non-authoritative. New accounting standards issued
subsequent to June 30, 2009 are communicated by the FASB through Accounting
Standards Updates (ASUs). The Company adopted the ASC on July 1, 2009. This
standard did not have an impact on the Company’s consolidated results of
operations or financial condition. However, throughout the notes to the
consolidated financial statements references that were previously made to
various former authoritative U.S. GAAP pronouncements have been changed to
coincide with the appropriate section of the ASC.
In
December 2007, the FASB issued and, in April 2009, amended a new
business combinations standard codified within ASC 805, which changed the
accounting for business acquisitions. Accounting for business combinations under
this standard requires the acquiring entity in a business combination to
recognize all (and only) the assets acquired and liabilities assumed in the
transaction and establishes the acquisition-date fair value as the measurement
objective for all assets acquired and liabilities assumed in a business
combination. Certain provisions of this standard impact the determination of
acquisition-date fair value of consideration paid in a business combination
(including contingent consideration); exclude transaction costs from acquisition
accounting; and change accounting practices for acquisition-related
restructuring costs, in-process research and development, indemnification
assets, and tax benefits. The Company adopted the standard for business
combinations and adjustments to an acquired entity’s deferred tax asset and
liability balances and it had no immediate impact on the Company’s consolidated
financial position or results of operations.
Page 14
of 39
In
December 2007, the FASB issued a new standard which established the
accounting for and reporting of noncontrolling interests (NCIs) in partially
owned consolidated subsidiaries and the loss of control of subsidiaries. Certain
provisions of this standard indicate, among other things, that NCIs (previously
referred to as minority interests) be treated as a separate component of equity,
not as a liability (as was previously the case); that increases and decreases in
the parent’s ownership interest that leave control intact be treated as equity
transactions, rather than as step acquisitions or dilution gains or losses; and
that losses of a partially owned consolidated subsidiary be allocated to the NCI
even when such allocation might result in a deficit balance. This standard also
required changes to certain presentation and disclosure requirements. The
Company adopted the standard beginning January 1, 2009. The provisions of
the standard were applied to all NCIs prospectively, except for the presentation
and disclosure requirements, which were applied retrospectively to all periods
presented. As a result, upon adoption, the Company retroactively reclassified
the “Minority interest in subsidiaries” balance previously included in the
“Other liabilities” section of the consolidated balance sheet to a new component
of equity with respect to NCIs in consolidated subsidiaries. The adoption also
impacted certain captions previously used on the consolidated statement of
income, largely identifying net income including NCI and net income attributable
to the Company. The adoption of this standard did not have a material
impact on the Company’s consolidated financial position or results of
operations.
In
April 2009, the FASB issued an accounting standard which provides guidance
on (1) estimating the fair value of an asset or liability when the volume
and level of activity for the asset or liability have significantly declined and
(2) identifying transactions that are not orderly. The standard also
amended certain disclosure provisions for fair value measurements and
disclosures in ASC 820 to require, among other things, disclosures in interim
periods of the inputs and valuation techniques used to measure fair value as
well as disclosure of the hierarchy of the source of underlying fair value
information on a disaggregated basis by specific major category of investment.
For the Company, this standard was effective prospectively beginning
April 1, 2009. The adoption of this standard did not have a material impact
on the Company’s consolidated results of operations or financial
condition.
In
June 2009, the FASB issued an accounting standard that revised the
consolidation guidance for variable-interest entities. The modifications include
the elimination of the exemption for qualifying special purpose entities, a new
approach for determining who should consolidate a variable-interest entity, and
changes to when it is necessary to reassess who should consolidate a
variable-interest entity. The standard is effective January 1, 2010. The
Company is currently evaluating the impact of this standard, but would not
expect it to have a material impact on the Company’s consolidated results of
operations or financial condition.
In
August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair
Value, which provides additional guidance on how companies should measure
liabilities at fair value under ASC 820. The ASU clarifies that the quoted price
for an identical liability should be used. However, if such information is not
available, a entity may use, the quoted price of an identical liability when
traded as an asset, quoted prices for similar liabilities or similar liabilities
traded as assets, or another valuation technique (such as the market or income
approach). The ASU also indicates that the fair value of a liability is not
adjusted to reflect the impact of contractual restrictions that prevent its
transfer and indicates circumstances in which quoted prices for an identical
liability or quoted price for an identical liability traded as an asset may be
considered level 1 fair value. This ASU is effective October 1, 2009. The
adoption of this standard did not have a material impact on the Company’s
consolidated results of operations or financial condition.
|
5.
|
Trade
Receivables
|
The
Company’s trade receivables consist of the following:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Trade
receivables, gross
|
$ | 17,607,329 | $ | 13,117,016 | ||||
|
Allowance
for doubtful accounts
|
(5,202,311 | ) | (3,788,102 | ) | ||||
|
Trade
receivables, net
|
$ | 12,405,018 | $ | 9,328,914 | ||||
Page 15
of 39
|
6.
|
Inventories
|
The
Company’s inventories were comprised as follows:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Raw
materials
|
$ | 489,348 | $ | 753,918 | ||||
|
Packing
materials
|
290,601 | 481,724 | ||||||
|
Other
materials / supplies
|
83,247 | 88,412 | ||||||
|
Finished
goods
|
588,604 | 720,575 | ||||||
|
Work-in-process
|
596,449 | 544,847 | ||||||
|
Total
inventories at cost
|
2,048,249 | 2,589,476 | ||||||
|
Less:
Reserves on inventories
|
(9,262 | ) | (244,915 | ) | ||||
|
Total
inventories, net
|
$ | 2,038,987 | $ | 2,344,561 | ||||
|
7.
|
Property
and Equipment
|
The Company’s property and equipment at December 31, 2009 and 2008 was compris ed as
follows:
|
December 31, 2009
|
December 31, 2008
|
|||||||
|
Buildings
|
$ | 14,380,679 | $ | 12,183,053 | ||||
|
Machinery
and equipment
|
13,982,775 | 13,943,173 | ||||||
|
Office
equipment
|
131,930 | 128,729 | ||||||
|
Motor
vehicles
|
348,598 | 349,516 | ||||||
|
Cost
|
28,843,982 | 26,604,471 | ||||||
|
Less:
Accumulated Depreciation
|
||||||||
|
Buildings
|
$ | (2,401,030 | ) | $ | (1,812,814 | ) | ||
|
Machinery
and equipment
|
(5,331,797 | ) | (3,880,979 | ) | ||||
|
Office
equipment
|
(73,966 | ) | (53,840 | ) | ||||
|
Motor
vehicles
|
(142,688 | ) | (93,248 | ) | ||||
|
Accumulated
Depreciation
|
(7,949,481 | ) | (5,840,881 | ) | ||||
|
Construction
in progress
|
$ | 7,763,630 | $ | 8,143,445 | ||||
|
Total
property and equipment, net
|
$ | 28,658,131 | $ | 28,907,035 | ||||
As
mentioned in Note 12, Benda Ebei entered into a commercial bank note issuance
agreement with Shanghai Pudong Development Bank on August 14, 2007 and a
supplementary agreement on January 21, 2008. Under the agreement the
credit facility is secured by the buildings, machinery and equipment of Benda
Ebei and Jiangling Benda. Meanwhile Benda Ebei entered into a
short-term loan agreement with Guang Shui Rural Credit Union on October 9, 2009,
under which the short-term bank loan is secured by the machinery of Benda Ebei.
As of December 31, 2009, the net book value of pledged property and equipment
was approximately RMB 121 million (or $17.7 million) in total.
The total
depreciation expense was $2,194,394 and $2,086,178 for the years ended December
31, 2009 and 2008, respectively, and is broken down as follows:
Page 16
of 39
|
YEAR
ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cost
of sales
|
$ | 1,633,712 | $ | 1,224,199 | ||||
|
Operating
expenses
|
560,682 | 861,979 | ||||||
|
Total
|
$ | 2,194,394 | $ | 2,086,178 | ||||
|
8.
|
Intangible
Assets
|
The
Company’s intangible assets at December 31, 2009 and 2008 were comprised as
follows:
|
December 31, 2009
|
December 31,
2008
|
|||||||
|
Land-use
rights
|
$ | 4,386,064 | $ | 4,415,740 | ||||
|
Drugs
permits and licenses
|
1,821,435 | 1,826,234 | ||||||
|
Technology
formulas
|
43,890 | 44,006 | ||||||
|
Patent
|
3,776,720 | 3,786,670 | ||||||
|
Cost
|
10,028,109 | 10,072,650 | ||||||
|
Less:
Accumulated Amortization
|
||||||||
|
Land-use
rights
|
$ | (222,946 | ) | $ | (179,511 | ) | ||
|
Drugs
permits and licenses
|
(1,784,671 | ) | (1,606,750 | ) | ||||
|
Technology
formulas
|
(15,544 | ) | (11,185 | ) | ||||
|
Patent
|
(1,375,447 | ) | (905,722 | ) | ||||
|
Accumulated
amortization
|
(3,398,608 | ) | (2,703,168 | ) | ||||
|
Total
intangible assets, net
|
$ | 6,629,501 | $ | 7,369,482 | ||||
The total
amortization expense was $712,369 and $679,738 for the years ended December
31, 2009 and 2008, respectively.
|
9.
|
Goodwill
|
The
changes in the carrying amount of goodwill for 2009 and 2008 are summarized
below:
|
December
31,
|
December
31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Balance,
beginning of year
|
$ | - | $ | 3,720,247 | ||||
|
Impairment
|
- | (3,635,114 | ) | |||||
|
Translation
and other
|
- | (85,113 | ) | |||||
|
Balance,
end of year
|
- | - | ||||||
Goodwill
is tested for impairment at the reporting unit level (operating segment or one
level below an operating segment) in the fourth quarter of each year,
unless an event occurs that would cause us to believe the value is impaired at
an interim date.
Page 17
of 39
The
performance of the impairment test involves a two-step process. The first step
involves comparing the fair values of the applicable reporting units with
their aggregate carrying values, including goodwill. We determine the fair value
of our reporting units using the income approach methodology of valuation
that includes the discounted cash flow method. If the carrying amount of a
reporting unit exceeds the reporting unit’s fair value, we perform the
second step of the goodwill impairment test to determine the amount
of impairment loss. The second step involves comparing the implied fair
value of the affected reporting unit’s goodwill with the carrying value of
that goodwill.
We
performed the annual impairment test in the fourth quarter of 2008 and
determined that the goodwill assigned to operating segment, Sibiono, was fully
impaired. The fair value of the reporting unit was estimated using the expected
present value of future cash flows.
|
10.
|
Restricted
Cash
|
The
Company’s restricted cash at December 31, 2009 and December 31, 2008 was
comprised as follows:
|
December
31,
|
December
31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Deposits
for issuance of commercial notes
|
$ | 4,409,334 | $ | 5,162,184 | ||||
The
restricted cash are deposits required by the bank for outstanding notes
payable.
|
11.
|
Other
assets
|
The
Company’s long term other assets were comprised as follows:
|
December
31,
|
December
31,
|
||||||||
|
2009
|
2008
|
||||||||
|
Right
to purchase the 6.24% equity shares in SiBiono
|
$ | 1,825,824 | $ | 1,830,634 |
(a)
|
||||
|
Long
term advance of inventory purchase
|
459,757 | 460,968 | |||||||
|
Total
|
$ | 2,285,581 | $ | 2,291,602 | |||||
|
(a)
|
Right
to purchase the 6.24% equity shares in
SiBiono
|
On
November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement with
Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s common
stock for a total consideration of RMB12.48 million (RMB 6.24 million in cash
and shares of our common stock equal to RMB 6.24 million) which was due and
payable on or before March 31, 2007.
Due to
the fact that the signed agreement on November 23, 2006 was not practically
executable according to the PRC regulations, Benda Ebei asked Zhang to terminate
the signed agreement and sign a new agreement that was feasible under PRC
regulations with essentially the same terms.
However,
Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration
Commission (the “Commission”) in April 2007 for enforcement of the original
agreement. Zhang requested the Commission to require Benda Ebei to pay for the
total consideration, penalty for late payment and the related legal and
arbitration expenses.
On
November 27, 2007, Shenzhen Arbitration Commission determined that Benda Ebei
should pay the consideration of RMB 6.24 million, equal to 50% of the total
consideration set forth in the Equity Transfer Agreement. For the other 50% of
the total consideration which was supposed to be settled in the form of issuing
common stock, since Zhang did not make an arbitration request on how to execute
the arrangement, the Arbitration Commission did not make an award on this
particular part.
Page 18
of 39
Following
this arbitration decision, Benda Ebei recognized the liability as other payable
of RMB 12.48 million, plus the penalty and related legal and arbitration
expenses, totaling approximately RMB 12.80 million or $1,898,355 and $1,903,357
at December 31, 2009 and 2008 respectively. Accordingly Benda Ebei recognized
the right to purchase the 6.24% equity shares in SiBiono and recorded as an
asset at RMB 12.48 million or $1,825,824 and $1,830,634 at December 31, 2009 and
2008 respectively.
On May
22, 2008, Benda Ebei applied to Shenzhen People Court to terminate the above
mentioned arbitration. The termination is based on the grounds that Xiaozhi
Zhang does not own all 6.24% of SiBiono’s common stock. He only owns 3.28% of
SiBiono’s stock. The application has been accepted by Shenzhen People Court and
is waiting for its determination.
12.
Short-term
debt
The
Company’s short term debt was comprised as follows:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Ebei
- one year bank loan due in October 2010, bear interest at 9% per annum,
secured by Ebei Benda’s Machinery
|
$ | 438,900 | $ | - | ||||
|
Ebei-
bank acceptance notes from SHPudong Development Bank with redemption dates
various from one to six months subsequent to year end, secured by
buildings, machinery and equipment of Benda Ebei and Jiangling
Benda.
|
8,160,503 | 9,297,169 | ||||||
|
Sibiono-
three-year bank loan due in April 2008 bearing annual interest at 6.366%.
Loan is currently in default.
|
2,976,718 | 2,984,560 | (a) | |||||
| $ | 11,576,121 | $ | 12,281,729 | |||||
(a)
SiBiono –
Bank Loan in default
As of
December 31, 2009 and 2008, Sibiono, had an outstanding bank loan for the amount
of $2,976,718 and $2,984,560, respectively, which was used primarily to fund
construction in progress projects and for general working capital purposes. The
loan carries annual interest rate of 6.34% and matured in April 2008. The loan
is personally guaranteed by Zhaohui Peng, the former Chairman and a shareholder
of SiBiono and is collateralized by Sibiono’s land use right.
The loan
is in default since the maturity date. During 2008, SiBiono was sued for default
on the bank loan and judgment has been made requiring Sibiono to repay the
loan principle amount and related interest. The loan is collateralized by
Sibiono’s land use right, the judgment agreed that the lender bank can apply for
government permission to sell the land use right owned by Sibiono to repay the
debt. Sibiono’s management is actively seeking ways to refinance this loan,
currently the lender bank has not exercised its rights on the land use
right.
13.
Government
Grant Payable
As of
December 31, 2009 and 2008, long term debt payable is related to various
technology funds obtained from various government technology agencies to support
the Company’s gene therapy research and development activities during the past
years and has been recorded as long term debt payable.
The
obligations will be discharged once the examinations by the various government
technology agencies are conducted and these agencies confirm that the
examinations are carried out and completed.
Page 19
of 39
During
the year ended December 31, 2009, an amount of RMB3,000,000 (or $454,491)
of long term debt payable was discharged and recorded as government
subsidies.
14.
Related
Party Transactions
Due from
related parties at December 31, 2009 and 2008 were comprised as
follows:
|
December 31,
|
December 31,
|
|||||||||
|
Relationship
|
2009
|
2008
|
||||||||
|
Current
|
||||||||||
|
Qin
Yu
|
Vice
president
|
|||||||||
|
Due
to SiBiono
|
$ | 2,024 | $ | 4,314 | ||||||
|
Xiaoji
Zhang
|
Minority
shareholder
|
|||||||||
|
Due
to SiBiono
|
5,423 | 5,439 | ||||||||
|
Hua
Xu
|
General Manager's Sister
|
|||||||||
|
Due
to SiBiono
|
22,726 | - | ||||||||
|
Rong
He
|
Manager
|
|||||||||
|
Due
to SiBiono
|
688 | 1,247 | ||||||||
| $ | 30,861 | $ | 11,000 | |||||||
|
Non
current
|
||||||||||
|
Yiqing
Wan
|
CEO
& Director
|
|||||||||
|
Due
to Ever Leader
|
$ | 646,586 | $ | 650,790 | ||||||
|
Due
to Benda Ebei
|
520,712 | 439,719 | ||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Due
to Yidu Benda
|
1,602,950 | 1,607,173 | ||||||||
|
Due
to Ever Leader
|
230,216 | 231,713 | ||||||||
|
Feng
Wang
|
Minority
shareholder
|
|||||||||
|
Due
to Beijing Shusai
|
32,262 | 32,348 | ||||||||
| $ | 3,032,726 | $ | 2,961,743 | |||||||
The
balance owned by the CEO & Director, and the Company under his control,
totaled $3,000,464 and $2,929,395 as of December 31, 2009 and 2008,
respectively. This is a violation of Section 402 of the Sarbanes-Oxley Act of
2002 which prohibits personal loans to executives.
Page 20
of 39
Due to
related parties at December 31, 2009 and 2008 were comprised as
follows:
|
December
31,
|
December
31,
|
|||||||||
|
Relationship
|
2009
|
2008
|
||||||||
|
Current
|
||||||||||
|
Wei
Xu
|
VP, CEO's Spouse & Director
|
|||||||||
|
Due
to SiBiono
|
$ | 234,569 | $ | 188,065 | ||||||
|
Due
from Ever Leader
|
1,356,172 | 968,000 | ||||||||
|
Due
from Benda
|
36,184 | - | ||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Due
from Benda Ebei
|
28,528 | - | ||||||||
|
Due
from Jiangliang Benda
|
793,864 | - | ||||||||
|
Due
from Beijing Shusai
|
14,111 | - | ||||||||
|
SiBiono
Zhongjia Gene Tech (SZ) Co., Ltd.
|
Associate
company
|
|||||||||
|
Due
from SiBiono
|
103,948 | - | ||||||||
|
Yiqing,
Wan
|
CEO
& Director
|
|||||||||
|
Due
from SiBiono
|
224,071 | 237,780 | ||||||||
|
Hua
Xu
|
General
Manager's Sister
|
|||||||||
|
Due
from SiBiono
|
- | 25,523 | ||||||||
|
Hua
Shen
|
Vice
president
|
|||||||||
|
Due
from SiBiono
|
- | 33,253 | ||||||||
|
Dongyi
Tien
|
Manager
|
|||||||||
|
Due
from SiBiono
|
- | 364 | ||||||||
|
Pong
Tsaiohuei
|
Minority
shareholder
|
|||||||||
|
Due
from SiBiono
|
- | 4,818 | ||||||||
| $ | 2,791,447 | $ | 1,457,803 | |||||||
|
Non
current
|
||||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Due
from Benda Ebei
|
$ | - | $ | 28,602 | ||||||
|
Due
from Jiangliang Benda
|
- | 811,073 | ||||||||
|
Due
from Beijing Shusai
|
- | 14,262 | ||||||||
|
Wei
Xu
|
VP,
CEO's Spouse & Director
|
|||||||||
|
Due
from Benda Ebei
|
23,894 | 48,922 | ||||||||
|
Due
from Beijing Shusai
|
65,339 | 65,691 | ||||||||
|
Yiqing,
Wan
|
CEO
& Director
|
|||||||||
|
Due
from Yidu Benda
|
559 | 560 | ||||||||
|
Hui
Xu
|
Manager
|
|||||||||
|
Due
from Benda Ebei
|
28,410 | 28,483 | ||||||||
| $ | 118,202 | $ | 997,593 | |||||||
Except
for the loans from the shareholder Wei Xu by Everleader which bears interest
rate at 12% per annum, unsecure and matures within six months, the above
advances bear no interest and the above loans due to related parties are
unsecured, non-interest bearing and are not convertible into equity. Proceeds
from the above loans were used primarily for general working capital purposes,
among which the current portion does not have definitive terms and for
those portions which are long-term debts in nature, is expected to be repaid by
the Company in over 12-month period.
15.
Redeemable
Common Stock Issuable For Services
On April
1, 2007, Benda entered into a Financial Consultancy Agreement with Super Pioneer
International Limited (“Super Pioneer”); on June 11, 2007, Benda entered into a
Technical Consultancy Agreement with Yaojin Wang (‘Wang”) and Huiming Zhang
(“Zhang”) for financial and technical consultancy services to be
rendered. Pursuant to the Financial and Technical Consultancy
Agreements (the “Agreements”), Benda agreed to issue an aggregate of 2,189,560
shares of its common stock to Super Pioneer (2.1 million shares, out of which
1.96 million shares is redeemable), Wang (33,585 shares, redeemable) and Zhang
(55,975 share, redeemable) within three months from the date of the Agreements.
Since the issuance of common shares to Super Pioneer, Wang and Zhang was in the
form of financial and technical consultancy services to be rendered, the
corresponding amount $7,882,416 was recorded as consulting and professional
fees.
Page 21
of 39
Super
Pioneer, Wang and Zhang also agreed to refrain from selling shares of Benda’s
common stock for a period of twelve months from the date of the issuance of the
shares (the “Lock-up Period”). Within three months from expiration of
the Lock-up Period, in the event that the public trading price of Benda’s common
stock did not reach $3.60 per share and Benda’s common stock was not listed on
either the NASDAQ or AMEX stock exchanges, Super Pioneer, Wang, and Zhang have
the option to require Benda to redeem an aggregate 2,049,560 shares of Benda’s
common stock owned by Super Pioneer, Wang, and Zhang at a price of $3.60 per
share. Such option expired within one month from the last date of the three
month period. The redemption requests were made to the Company in January 2008
and the company obtained oral consent from Super Pioneer, Wang and Zhang that
the payment would be deferred to the year of 2010.
In
accordance with ASC 480-10-25-5, as the Agreements governing the issuance of the
2,189,560 shares of common stock to Super Pioneer, Wang, and Zhang contain
provisions requiring Benda to repurchase 2,049,560 of these shares at a
redemption price of $3.60 per share at the option of the holders, these
2,049,560 shares have been classified as redeemable common stock, as a liability
at December 31, 2009.
16.
Statutory
Reserves
As
stipulated by the relevant laws and regulations for enterprises operating in the
PRC, the Company is required to make annual appropriations to a statutory
surplus reserve fund for each of its PRC subsidiaries. Specifically,
the Company is required to allocate 15% its profits after taxes at the fiscal
year end, as determined in accordance with the PRC accounting standards
applicable to the Company’s PRC subsidiaries, to a statutory surplus reserve
until such reserve reaches 50% of the registered capital of the Company’s PRC
subsidiaries. As of December 31, 2009 and 2008, the Company’s statutory reserves
total $2,642,775 and the registered capital of the Company’s PRC subsidiaries
was $21,719,896.
17.
Other
Income / (Expenses)
Among the
other income / (expenses), there was an amount incurred for the penalty to
investors. For the years ended December 31, 2009 and 2008, the amount of penalty
to investors consisted of the following events:
|
YEAR
ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Registeration
delay expense
|
$ | - | $ | 1,113,405 | ||||
|
Make
Good Agreement
|
- | 266,768 | ||||||
| $ | - | $ | 1,380,173 | |||||
|
|
a)
|
In
accordance with ASC 815-40 (previously FASB staff position No.
EITF00-19-2), the Company recorded $1,113,405 in Registration Delay
Expense, for the year ended December 31, 2008. Out of the total penalty,
$230,312 was settled by issuance of 523,438 shares of common stock.
According to the signed agreement, the penalty would be capped at 10% of
the total fund raised and such cap was reached in June 2008, thus there
would be no more penalties incurred onwards. Furthermore, the Registration
Statement was effective in August
2008.
|
|
|
b)
|
According
to the Investment Agreements (issuance of convertible promissory notes) in
March 2007, the securities underlying the Notes and Warrants issued to the
Investors are also subject to the terms of a Make Good Agreement entered
into in connection with a financing the Company executed in November of
2006 (the “Make Good Agreement”). The Company further represented to the
Investors that its target net income for fiscal year 2007 (“FY07 Net
Income”) will be greater than or equal to $10.0 million (adjusted for a
variety of non-cash charges) (the “Performance Threshold”). In
the event the Performance Threshold is not attained, the Company is
required to issue to the Investors a pro rata portion of 1,000,000 shares
of the Company’s common stock for every one (1) cent by which the
Company’s earnings per share, determined on a fully diluted basis, is less
than $0.07. On Aug 25, 2008, the Company issued 3,810,976 shares of common
stock in lieu of the non-achievement of the Performance Threshold at $0.07
per share, totaling to $266,768 and booked as
penalty.
|
Page 22
of 39
18.
Income
Taxes
United
States
Benda is
subject to the United States of America tax law, but no provision for income
taxes were made for the years ended December 31, 2009 and 2008 as Benda did not
have reportable taxable income for the periods.
Hong
Kong
Ever
Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax law, but
no provisions for income taxes were made for the years ended December 31, 2009
and 2008 as Ever Leader did not have reportable taxable income for the
periods.
PRC
Benda
Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and
is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in
the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC
enterprise income tax for two years starting from the first profit-making year,
followed by a 50% reduction in income taxes, for the following three
years.
Jiangling
Benda and Yidu Benda are cross-municipal investment entities and enjoy the same
tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were
therefore exempt from PRC enterprise income tax for two years starting from the
first profit-making year, followed by a 50% reduction in income taxes, for the
following three years. Cross-municipal investments entities refer to entities
that are incorporated in one municipal region but have investments in another
municipal region.
The
exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the
year of 2006, after which they are subject to 50% reduction in income taxes;
whereas the full income tax rate is 33%. The remaining tax holidays was expired
in 2009.
However,
starting and effective from January 1, 2008, the full income tax rate was
changed from 33% to 25% according to the new PRC taxation regulations. Therefore
these subsidiaries will be subject to the regular full income tax rate at 25%
after the tax holidays expire.
According
to the new taxation regulations starting and effective from January 1, 2008,
Beijing Shuhai is subject to the full income tax rate of 25%.
According
to the new taxation regulations starting and effective from January 1, 2008,
SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is
subject to the full income tax rate of 25% gradually in five years as
follows:
|
Year
|
Tax rate
|
|||
|
2008
|
18 | % | ||
|
2009
|
20 | % | ||
|
2010
|
22 | % | ||
|
2011
|
24 | % | ||
|
2012 and thereafter
|
25 | % | ||
Page 23
of 39
The
income tax expense in the consolidated statements of operations consists
of:
|
YEAR ENDED DECEMBER 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Current
income taxes expenses:
|
||||||||
|
PRC
Enterprise Income tax expense
|
$ | 872,643 | $ | 1,109,249 | ||||
|
Income
tax benefit
|
(54,354 | ) | (332,954 | ) | ||||
|
Income
tax, net
|
$ | 818,289 | $ | 776,295 | ||||
The
income tax benefit arises from the incremental difference of the intangible
amortization due to difference between intangible assets book value and tax
value from the acquisition of Sibiono at March 31, 2007.
A
reconciliation between the income tax computed at the U.S statutory rate and the
Company’s provision for income tax for the years of 2009 and 2008 are as
follows:
|
YEAR ENDED DECEMBER 31
|
||||||||
|
2009
|
2008
|
|||||||
|
U.S
Statutory rate
|
34.0 | % | 34.0 | % | ||||
|
Foreign
income not recognized in the U.S
|
-34.0 | % | -34.0 | % | ||||
|
PRC
statutory rate
|
25.0 | % | 25.0 | % | ||||
|
Tax
relief and holiday granted to the Subsidiary
|
0.0 | % | -12.5 | % | ||||
|
Tax
rate change due to valuation allowance to deferred tax assets from
NOL
|
(61.7 | )% | (18.2 | )% | ||||
|
Effective
income tax rate
|
(36.7 | )% | (5.7 | )% | ||||
According
to the Chinese tax law, the Group pays Chinese enterprise income taxes at each
individual subsidiary. The income taxes of $872,643 and $1,109,249 for the year
ended December 31, 2009 and 2008 respectively were from Ebei Benda which was the
only subsidiary with pretax income. The other subsidiaries, Jiangling Benda,
Yidu Benda, Beijing Shusai and SiBiono collectively have accumulated NOL of $5.6
million and $3.1 million at December 31, 2009 and 2008 respectively. The
components of the deferred income tax assets consisted of allowance for bad
debt, inventory reserve and NOL. The Company established a valuation allowance
to fully offset the net deferred income tax assets because it is less likely
that the Company will be able to generate future taxable income necessary to
realize those net deferred income tax assets, considering the Company’s history
of significant operating losses.
The tax
effects of temporary differences that have given rise to the deferred tax
liabilities consist of the following:
|
YEAR ENDED DECEMBER 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Deferred
tax liability - intangible amortization
|
$ | 778,026 | $ | 834,458 | ||||
Page 24
of 39
19.
Convertible
Promissory Note
In March
and April of 2007, the Company entered into Investment Agreements (“Agreements”)
with certain accredited and institutional investors (“Investors”) pursuant to
which the Investors purchased from the Company a total of 252 Units (“Units”),
resulting in aggregate gross proceeds to the Company $7,560,000, with each Unit
consisting of: (i) a Convertible Promissory Note (“Note”) in the principal
amount of $30,000 and convertible into 54,087 shares of the Company's common
stock; and (ii) a Common Stock Warrant (“Warrant”) to acquire 54,087 shares
of the Company’s common stock at an exercise price of $0.555 per share and
expiring on November 14, 2011. The Notes are immediately convertible
at the option of each Investor, bear interest at a rate of 4% per annum, and
mature on March 28, 2009.
The
Company has accounted for the Warrants issued in conjunction with the Notes in
accordance with the provisions of ASC 470-20. Accordingly, the Warrants were
valued using a Black-Scholes option pricing model with the following
assumptions: (i) a risk free interest rate of 4.63%, (ii) a contractual life of
4.6 years, (iii) an expected volatility of 25%, and (iv) a dividend yield of
zero. The relative fair value of the Warrants, based on an allocation
of the value of the Notes and the value of the Warrants issued in conjunction
with the Notes, was recorded as a debt discount (with a corresponding increase
to additional paid-in capital) in the amount of $5,174,911, and is being
amortized to interest expense over the expected term of the Notes.
Additionally,
the difference between the effective conversion price of the Notes into shares
of the Company’s common stock, and the fair value of the Company’s common stock
on the date of issuance of the Notes, resulted in a beneficial conversion
feature of $2,385,089 (capped at the $7,560,000 of gross proceeds raised less
the previously calculated $5,174,911 debt discount associated with Warrants
issued in conjunction with the Notes). This beneficial conversion feature was
recorded as an additional debt discount (with a corresponding increase to
additional paid-in capital) and is being amortized to interest expense over the
expected term of the Notes.
On July
10, 2008, 10 units were converted to shares of 540,870. Therefore, as of
December 31, 2009 and 2008, the aggregate $7,260,000 principal balance of the
notes remained outstanding and for the year ended December 31, 2009 and 2008,
the Company recorded $1,089,000 and $695,000 of interest expense related to the
Notes.
During
the year ended December 31, 2009 and 2008, the Company recorded $864,049 and
$3,520,876 in interest expense related to the amortization of the debt discount
associated with the Warrants and the debt discount associated with the
beneficial conversion feature.
The
Company also incurred $529,200 in placement agent commissions related to the
issuance of the Notes and Warrants. This amount was recorded by the
Company as debt issue costs and is being amortized over expected term of the
Notes. For the year ended December 31, 2009, the Company recorded
$55,485 in amortization expense related to debt issue costs.
The
securities underlying the Notes and Warrants issued to the Investors are also
subject to the terms of a Make Good Agreement entered into in connection with a
financing the Company executed in November of 2006 (the “Make Good Agreement”).
See note 17 for details.
In the
accounting for the Convertible Promissory Note, the Company’s analysis of the
Performance Threshold had no effect because of the cap of the debt discounted
and beneficial conversion feature as calculated in accordance with ASC
470.
Page 25
of 39
20.
Warrants
The
following table summarizes the continuity of the Company’s warrants for the year
ended December 31, 2009 and 2008.
|
December 31, 2009
|
December 31, 2008
|
|||||||||||||||
|
Weighted
|
Weighted
|
|||||||||||||||
|
Average
|
Average
|
|||||||||||||||
|
Exercise
|
Exercise
|
|||||||||||||||
|
Warrants
|
Price
|
Warrants
|
Price
|
|||||||||||||
|
Outstanding
at beginning of period
|
43,353,303 | $ | 0.57 | 40,797,983 | $ | 0.56 | ||||||||||
|
Granted
|
- | - | 2,555,320 | 0.77 | ||||||||||||
|
Exercised
|
- | - | - | - | ||||||||||||
|
Expired
|
- | - | - | - | ||||||||||||
|
Cancelled
|
- | - | - | - | ||||||||||||
|
Outstanding
at end of period
|
43,353,303 | $ | 0.57 | 43,353,303 | $ | 0.57 | ||||||||||
|
Exercisable
at end of period
|
43,353,303 | $ | 0.57 | 43,353,303 | $ | 0.57 | ||||||||||
As of
December 31, 2010, the range of warrants prices for shares under warrants, the
weighted average remaining contractual life and aggregate intrinsic value are as
follows:
|
Weighted
|
||||||||||||||||
|
Exercise
|
Number of
|
Contractual Life
|
Aggregate
|
|||||||||||||
|
Expiration Date
|
Price
|
Warrants
|
(in years)
|
Intrinsic Value
|
||||||||||||
|
$
|
#
|
#
|
$
|
|||||||||||||
|
11/15/06
|
$ | 0.555 | 25,961,760 | 1.87 | - | |||||||||||
|
11/15/06
|
$ | 0.555 | 1,747,169 | 1.87 | - | |||||||||||
|
03/28/07
|
$ | 0.555 | 13,089,054 | 2.24 | - | |||||||||||
|
01/02/08
|
$ | 0.770 | 1,597,075 | 3.01 | - | |||||||||||
|
01/02/08
|
$ | 0.770 | 958,245 | 3.01 | - | |||||||||||
| 43,353,303 | 2.05 | - | ||||||||||||||
21.
Commitments
and Contingencies
Legal
Contingencies
|
(a)
|
On
December 30, 2009, the Company was served with a Summons and Complaint
filed by Pope Investments, LLC (“Pope”) in the Court of Chancery of the
state of Delaware (the “Court”) against the Company and its officers and
directors. Pope filed the Summons and Complaint as a judgment creditor and
as a shareholder of the Company. Pope alleges that the assets and profits
of the Company’s subsidiary company have been wrongfully diverted by its
officers and directors and requests the appointment of a receiver to
liquidate and wind down the business affairs of the Company. In connection
with the filing of the Summons and Complaint, Pope has also filed a motion
for a Preliminary Injunction Motion seeking to enjoin the Company and its
officers and directors from taking any further actions to divert the
corporate assets and profits of the subsidiary company and for expedited
discovery proceedings. Pope further requests the imposition of a
constructive trust, an accounting, and damages for an alleged breach of
fiduciary duty by the Company’s officers and directors, and attorney fee.
By the time of this report, a judgment has not been
made.
|
|
(b)
|
As
mentioned in Note 11, following this arbitration decision, Benda Ebei has
the obligation to pay the total acquisition cost payable of RMB12.48
million, plus the penalty and related legal and arbitration expenses,
totaling approximately RMB12.9 million (or $1.89 million), which was
recorded as other payable at December 31,
2009.
|
On May
22, 2008, Benda Ebei applied to Shenzhen People Court to terminate above
mentioned arbitration. The termination is based on the ground that Xiaozhi Zhang
does not own all 6.24% of SiBiono’s common stock. In fact, he only owns 3.28% of
SiBiono’s stock. The application has been accepted by Shenzhen People Court and
is waiting for its determination.
Page 26
of 39
|
(c)
|
The
Company has become aware that Excalibur Limited Partnership and Excalibur
Limited Partnership II (the "Plaintiffs") filed a motion for summary
judgment in lieu of a complaint pursuant to CPLR § 3213 (the "Motion")
with the Supreme Court of the State of New York (the "Court"), alleging
that the Company has been delinquent on the payment of an aggregate sum of
$600,000 and accrued interest and costs arising from the Convertible
Promissory Notes that were issued to the Plaintiffs in April 2007 in
connection with a $7,560,000 private placement. Pursuant to the motion,
the Plaintiffs requested that the Court (1) enter summary judgment in
favor of Excalibur Limited Partnership (“Excalibur Limited”) in the amount
of $390,000 plus all accrued interest and costs, and, (2) enter summary
judgment in favor of Excalibur Small Cap Opportunities LP (“Excalibur
Small Cap”) in the amount of $210,000 and accrued interest and costs. On
July 29, 2009, the Court entered a judgment against the Company in favor
of the Plaintiffs in the amount of $674,251.65 in connection with the
Convertible Promissory Notes issued to the
Plaintiffs.
|
|
(d)
|
On
March 4, 2009, the Company received a Notice and Default and Payment
Demand letter (the "Default Letter") from Pope Investments LLC ("Pope") in
connection with its convertible promissory note in the amount of
$5,520,000 (the "Note") purchased in our April 2007 private placement
offering. The Default Letter provided notice of default based on the
Company's failure to make a required interest payment on the Note by
February 20, 2009. The Default Letter further demanded full payment of all
interest, liquidated damages and accrued interest thereon in the amount of
$130,364.37 by March 14, 2009, or Pope will accelerate the maturity date
of the full principal amount of the Note. On April 7, 2009, the
Company received further Default Letters and Payment Demand from Pope,
Excalibur Limited and Excalibur Small Cap demanding payment in full of the
balance of the Notes, which matured on March 28, 2009. The Company was
notified on June 15, 2009 that on May 11, 2009 Pope filed a motion for
summary judgment in lieu of a complaint against us pursuant to CPLR §3213 (the
“Motion”) with the Supreme Court of the State of New York (the “Court”),
alleging that the Company has been delinquent on the payment of an
aggregate sum of $5,520,000 and accrued interest and costs arising from
the Note that the Company issued to Pope in April 2007. Pursuant to the
Motion, the Plaintiffs requested that the Court enter summary judgment in
favor of Plaintiff in the amount of $5,994,617.53 constituting principal
and interest, plus costs. On June 23, 2009, the Company filed an Affidavit
in Opposition to Motion for Summary judgment in lieu of Complain with the
Court requesting that Plaintiff’s Motion be denied. On October 14, 2009,
this motion was denied, and the Court entered a judgment in favor of Pope
in the amount of $5,520,000 plus
interest.
|
|
(e)
|
On
July 30, 2009, the Company received a Notice of Default from three
additional investors in the April 2007 private placement offering holding
Notes totaling $90,000.
|
For (c)
(d) and (e) discussed above, we recorded the convertible note payable principle
amount and related accrued interest at December 31, 2009.
Page 27
of 39
Operating
Leases
The
Company leases office space in Ebei and plant in Shenzhen. In February 2009,
Sibiono also entered into a two-year sublease agreement with monthly rental
income of $19,106. Approximate future minimum lease payments are as
follows:
|
Year
|
Amount
|
|||
|
2010
|
$ | 144,486 | ||
|
2011
|
35,112 | |||
|
2012
|
35,112 | |||
|
2013
|
35,112 | |||
|
2014
|
8,778 | |||
|
Total
|
258,600 | |||
|
Less
sublease income
|
267,479 | |||
|
Net
|
(8,879 | ) | ||
Concentration
of Credit Risk
A
significant portion of the Company's cash at December 31, 2009 and 2008 is
maintained at various financial institutions in the PRC which do not provide
insurance for amounts on deposit.
The
Company has not experienced any losses in such accounts and believes it is not
exposed to significant credit risk in this area.
The
Company operates principally in the PRC and grants credit to its customers in
this geographic region. Although the PRC is economically stable, it is
always possible that unanticipated events in foreign countries could disrupt the
Company’s operations.
The
following table shows the individual customer’s revenue and account receivable
balance which was higher than 5% of total revenue and total account receivables
for the years ended December 31, 2009 and 2008:
|
YEAR ENDED DECEMBER 31
|
|||||||||||||
|
2009
|
2008
|
||||||||||||
|
Revenue
|
$
|
21,990,915
|
%
|
$
|
25,041,986
|
%
|
|||||||
|
Individual customer's
revenue
|
|||||||||||||
|
1
|
Zhuhai
Gongbei Pharmaceutical Co, Ltd.
|
5,286,243
|
24
|
%
|
4,861,888
|
19
|
%
|
||||||
|
2
|
Shenyang
Pharmaceutical Co. Ltd.
|
2,878,894
|
13
|
%
|
2,620,888
|
10
|
%
|
||||||
|
3
|
Shenzhen
Huihua Pharmaceutical Co. Ltd.
|
2,544,147
|
12
|
%
|
2,788,933
|
11
|
%
|
||||||
|
4
|
Hubei
Hengchuan Health Products Co.,Ltd.
|
1,828,339
|
8
|
%
|
2,746,580
|
11
|
%
|
||||||
|
5
|
Jiangxi
Huiren Pharmaceutical Co. Ltd.
|
1,612,011
|
7
|
%
|
2,073,862
|
8
|
%
|
||||||
|
Account
receivable, gross
|
$
|
17,607,329
|
%
|
$
|
13,117,016
|
%
|
|||||||
|
Individual customer's account receivable gross
balance
|
|||||||||||||
|
1
|
Zhuhai
Gongbei Pharmaceutical Co, Ltd.
|
2,561,356
|
15
|
%
|
1,742,158
|
13
|
%
|
||||||
|
2
|
Shenzhen
Huihua Pharmaceutical Co. Ltd.
|
1,851,956
|
11
|
%
|
1,238,820
|
9
|
%
|
||||||
|
3
|
Hubei
Hengchuan Health Products Co.,Ltd.
|
1,592,476
|
9
|
%
|
1,269,568
|
10
|
%
|
||||||
|
4
|
Shenyang
Pharmaceutical Co. Ltd.
|
1,519,355
|
9
|
%
|
1,087,027
|
8
|
%
|
||||||
|
5
|
Jiangxi
Huiren Pharmaceutical Co. Ltd.
|
1,461,258
|
8
|
%
|
990,665
|
8
|
%
|
||||||
|
22.
|
Segment
Information
|
The
Company states the segment information according to the requirement stated in
ASC 280-10-50. The Company produces five different categories of products and
each category of product is produced in different subsidiaries or operation
plants. The details are stated as follows:
Page 28
of 39
|
1.
|
Benda
Ebei produces conventional medicines which including branded and generic
medicines;
|
|
2.
|
Jiangling
Benda produces active pharmaceutical ingredients,
APIs;
|
|
3.
|
Yidu
Benda produces bulk
chemicals;
|
|
4.
|
Beijing
Shusai produces pharyngitis killer therapy;
and
|
|
5.
|
SiBiono
produces gene therapy medicines,
Gendicine.
|
Since
each subsidiary produces the corresponding products by using the production
facilities of each subsidiary, therefore according to the requirement stated ASC
280-10-50, the Company reports the segment information according to the
un-identical products that produced in each subsidiary.
Selected
financial information for each of these segments for the years ended December
31, 2009 and 2008 were as follows:
Page 29
of 39
|
YEAR ENDED DECEMBER 31,
|
||||||||
|
Branded/Generic medicine segment
|
2009
|
2008
|
||||||
|
Revenue
from external customers
|
$ | 17,783,062 | $ | 22,021,248 | ||||
|
Cost
of sales
|
(11,223,358 | ) | (14,761,544 | ) | ||||
|
Gross
profit
|
6,559,704 | 7,259,704 | ||||||
|
Gross
margin
|
37 | % | 33 | % | ||||
|
Research
and development
|
(225,559 | ) | (19,540 | ) | ||||
|
Selling
expense
|
(850,172 | ) | (1,329,197 | ) | ||||
|
General
and administrative expense
|
(922,279 | ) | (2,206,046 | ) | ||||
|
Segment
contribution
|
$ | 4,561,694 | $ | 3,704,921 | ||||
|
Contribution
margin
|
26 | % | 17 | % | ||||
|
Total
assets, segment
|
$ | 26,431,912 | $ | 21,582,724 | ||||
|
Active pharmaceutical ingredients segment
|
2009
|
2008
|
||||||
|
Revenue
from external customers
|
$ | 1,614,704 | $ | 689,481 | ||||
|
Cost
of sales
|
(2,084,590 | ) | (778,212 | ) | ||||
|
Gross
profit
|
(469,886 | ) | (88,731 | ) | ||||
|
Gross
margin
|
-29 | % | -13 | % | ||||
|
Research
and development
|
(1,169 | ) | (5,776 | ) | ||||
|
Selling
expense
|
(31,211 | ) | (5,581 | ) | ||||
|
General
and administrative expense
|
(522,787 | ) | (1,721,987 | ) | ||||
|
Segment
contribution
|
$ | (1,025,053 | ) | $ | (1,822,075 | ) | ||
|
Contribution
margin
|
-63 | % | -264 | % | ||||
|
Total
assets, segment
|
$ | 12,623,094 | $ | 12,624,190 | ||||
|
Bulk chemicals segment
|
2009
|
2008
|
||||||
|
Revenue
from external customers
|
$ | - | $ | - | ||||
|
Cost
of sales
|
- | - | ||||||
|
Gross
profit
|
- | - | ||||||
|
Gross
margin
|
0 | % | 0 | % | ||||
|
Research
and development
|
- | - | ||||||
|
Selling
expense
|
- | - | ||||||
|
General
and administrative expense
|
(620,229 | ) | (605,118 | ) | ||||
|
Segment
contribution
|
$ | (620,229 | ) | $ | (605,118 | ) | ||
|
Contribution
margin
|
0 | % | 0 | % | ||||
|
Total
assets, segment
|
$ | 8,508,245 | $ | 9,099,810 | ||||
|
Pharynigitis killer therapy segment
|
2009
|
2008
|
||||||
|
Revenue
from external customers
|
$ | - | $ | 4,565 | ||||
|
Cost
of sales
|
- | (251 | ) | |||||
|
Gross
profit
|
- | 4,314 | ||||||
|
Gross
margin
|
0 | % | 95 | % | ||||
|
Research
and development
|
- | - | ||||||
|
Selling
expense
|
(733 | ) | (4,370 | ) | ||||
|
General
and administrative expense
|
(25,242 | ) | (38,049 | ) | ||||
|
Segment
contribution
|
$ | (25,975 | ) | $ | (38,105 | ) | ||
|
Contribution
margin
|
0 | % | -835 | % | ||||
|
Total
assets, segment
|
$ | 87,628 | $ | 110,359 | ||||
|
Gendicine (Ad-p53) segment
|
2009
|
2008
|
||||||
|
Revenue from external customers
|
$ | 2,593,149 | $ | 2,326,692 | ||||
|
Cost
of sales
|
(412,728 | ) | (200,100 | ) | ||||
|
Gross
profit
|
2,180,421 | 2,126,592 | ||||||
|
Gross
margin
|
84 | % | 91 | % | ||||
|
Research
and development
|
(122,633 | ) | (1,436,635 | ) | ||||
|
Selling
expense
|
(1,496,736 | ) | (1,369,820 | ) | ||||
|
General
and administrative expense
|
(2,750,313 | ) | (5,754,878 | ) | ||||
|
Segment
contribution
|
$ | (2,189,261 | ) | $ | (6,434,741 | ) | ||
|
Contribution
margin
|
-84 | % | -277 | % | ||||
|
Total
assets, segment
|
$ | 14,689,633 | $ | 15,521,611 | ||||
|
YEAR
ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Total
revenue from external customers
|
$ | 21,990,915 | $ | 25,041,986 | ||||
|
Cost
of sales
|
(13,720,676 | ) | (15,740,107 | ) | ||||
|
Gross
profit
|
8,270,239 | 9,301,879 | ||||||
|
Gross
margin
|
38 | % | 37 | % | ||||
|
Research
and development
|
(349,361 | ) | (1,461,951 | ) | ||||
|
Selling
expense
|
(2,378,852 | ) | (2,708,968 | ) | ||||
|
General
and administrative expense
|
(4,840,850 | ) | (10,326,078 | ) | ||||
|
Segment
contribution
|
$ | 701,176 | $ | (5,195,118 | ) | |||
|
Contribution
margin
|
3 | % | -21 | % | ||||
|
Total
assets, segment
|
$ | 62,340,512 | $ | 58,938,694 | ||||
Page 30
of 39
The
results of the total consolidated net profit before income taxes for the
reporting periods are as follows:
|
YEAR ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Total
segment contribution
|
$ | 701,176 | $ | (5,195,118 | ) | |||
|
Unallocated
amounts:
|
||||||||
|
Rental
income
|
241,005 | - | ||||||
|
Government
subsidies / grants (Note 22)
|
454,491 | - | ||||||
|
Other
income/(expenses)
|
(421,426 | ) | (1,311,140 | ) | ||||
|
Other
corporate expenses
|
(2,843,139 | ) | (7,191,318 | ) | ||||
|
Total
net loss before noncontrolling interest and income taxes
|
$ | (1,867,893 | ) | $ | (13,697,576 | ) | ||
The other
corporate expenses per the above table for the year ended December 31, 2009 and
2008 composed of the following events:
|
YEAR ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Wages
and salaries
|
$ | 355,243 | $ | 417,276 | ||||
|
Audit
and accounting
|
309,493 | 330,919 | ||||||
|
Consulting
fee
|
16,672 | 747,937 | ||||||
|
Investor
relation, transfer agent and filing fees
|
19,156 | 33,563 | ||||||
|
Director
renumeration
|
90,000 | 185,468 | ||||||
|
Legal
fee
|
201,086 | 137,805 | ||||||
|
Taxes
and levies
|
70,000 | - | ||||||
|
Interest
expense
|
1,762,305 | 4,527,956 | ||||||
|
Miscellaneous
|
19,184 | 810,394 | ||||||
|
Total
|
$ | 2,843,139 | $ | 7,191,318 | ||||
For the
details of information of this particular, it should be read in conjunction
with the management discussion and analysis section.
The
following table shows the reconciliation between the segments assets and the
total assets for the years ended December 31, 2009 and 2008:
|
December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Total
assets, segment
|
$ | 62,340,512 | $ | 58,938,694 | ||||
|
Total
assets of corporate:
|
||||||||
|
Cash
and cash equivalent
|
12,046 | 31,070 | ||||||
|
Prepaid
expesnes and deposit
|
230 | 2,953 | ||||||
|
Due
from related parties
|
876,802 | 882,503 | ||||||
|
Construction-in-progress
capitalized interest
|
364,164 | - | ||||||
|
Debit
issuance costs
|
- | 55,485 | ||||||
|
Total
assets
|
$ | 63,593,754 | $ | 59,910,705 | ||||
Page 31
of 39
The
following table shows how the noncontrolling interest for year ended
December 31, 2009 and 2008 was derived:
|
Year Ended December 31, 2009
|
||||||||||||||||||||||||
|
Benda
|
Jiangling
|
Yidu
|
Beijing
|
Shenzhen
|
||||||||||||||||||||
|
Ebei
|
Benda
|
Benda
|
Shusai
|
SiBiono
|
Total
|
|||||||||||||||||||
|
Segment
operating profit / (loss)
|
$ | 4,561,694 | (1,025,053 | ) | (620,229 | ) | (25,975 | ) | (2,189,261 | ) | $ | 701,176 | ||||||||||||
|
Interest
income/ (expenses)
|
(223,585 | ) | (231 | ) | 5 | - | (182,782 | ) | (406,593 | ) | ||||||||||||||
|
Other
income / (expenses)
|
(15,331 | ) | 56,888 | 3,970 | - | 180,647 | 226,174 | |||||||||||||||||
|
Government
subsidy
|
- | - | - | - | 454,491 | 454,491 | ||||||||||||||||||
|
Income
taxes
|
(872,643 | ) | - | - | - | 54,354 | (818,289 | ) | ||||||||||||||||
|
Income
/ (loss) before noncontrolling interest
|
$ | 3,450,135 | (968,396 | ) | (616,254 | ) | (25,975 | ) | (1,682,551 | ) | $ | 156,959 | ||||||||||||
|
Noncontrolling
interest percentage
|
5.00 | % | 5.00 | % | 5.00 | % | 25.00 | % | 39.87 | % | ||||||||||||||
|
Noncontrolling
interest
|
$ | 7,848 | (48,420 | ) | (30,813 | ) | (6,494 | ) | (670,833 | ) | $ | (748,712 | ) | |||||||||||
|
Year Ended December 31, 2008
|
||||||||||||||||||||||||
|
Benda
|
Jiangling
|
Yidu
|
Beijing
|
Shenzhen
|
||||||||||||||||||||
|
Ebei
|
Benda
|
Benda
|
Shusai
|
SiBiono
|
Total
|
|||||||||||||||||||
|
Segment
operating profit / (loss)
|
$ | 3,704,921 | (1,822,075 | ) | (605,118 | ) | (38,105 | ) | (6,434,741 | ) | $ | (5,195,118 | ) | |||||||||||
|
Interest
income/ (expenses)
|
(382,124 | ) | 1,466 | 52 | - | (317,975 | ) | (698,581 | ) | |||||||||||||||
|
Other
income / (expenses)
|
(6,808 | ) | (4,233 | ) | 3,943 | 6,828 | 64,890 | 64,620 | ||||||||||||||||
|
Income
taxes
|
(1,109,249 | ) | - | - | - | 332,954 | (776,295 | ) | ||||||||||||||||
|
Income
/ (loss) before noncontrolling interest
|
$ | 2,206,740 | (1,824,842 | ) | (601,123 | ) | (31,277 | ) | (6,354,872 | ) | $ | (6,605,374 | ) | |||||||||||
|
Noncontrolling
interest percentage
|
5.00 | % | 5.00 | % | 5.00 | % | 25.00 | % | 39.87 | % | ||||||||||||||
|
Noncontrolling
interest
|
$ | (330,269 | ) | (91,242 | ) | (30,056 | ) | (7,819 | ) | (2,533,687 | ) | $ | (2,993,073 | ) | ||||||||||
|
23.
|
Subsequent
Events
|
Sibiono
and North American Gene Diagnostics and Therapeutics Ltd. (HK) entered into a
business agreement to set up Shenzhen Sibiono Zhongjia Gene Technology Ltd.
during June 2009. The business license of the new joint entity was obtained in
January 2010 and Sibiono made the capital contribution of RMB 800,000 in
February 2010. The new entity's legal representative is Mr. Wan, Yiqing. The
registered capital is RMB 2 million. Sibiono's share of the registered capital
is 40% (RMB 800,000), the other party’s share is 60% (RMB 1.2
million).
In April
2010, Jiangling Benda obtained a one year loan from Hubei Province Rural Credit
with principle amount of RMB 2 million ($292,556) and bears interest at 6% per
annum. The loan is secured by a third party commercial loan guarantee company
for a fee.
|
24.
|
Condensed
Parent Company Financial
Information
|
Basis of
Presentation
The
condensed parent company financial statements have been prepared in accordance
with Rule 12-04, Schedule I of Regulation S-X, as the restricted net assets of
the subsidiaries of Benda Pharmaceutical, Inc. exceed 25% of the consolidated
net assets of Benda Pharmaceutical, Inc. The ability of the Company’s Chinese
operating subsidiaries to pay dividends may be restricted due to the foreign
exchange control policies and availability of cash balances of the Chinese
operating subsidiaries. Because substantially all of the Company’s operations
are conducted in China and a substantial majority of its revenues are generated
in China, a majority of the Company’s revenue being earned and currency received
are denominated in Renminbi (RMB). RMB is subject to the exchange control
regulation in China, and, as a result, the Company may be unable to
distribute any dividends outside of China due to PRC exchange control
regulations that restrict its ability to convert RMB into US
Dollars.
Page 32
of 39
The
condensed parent company financial statements have been prepared using the same
accounting principles and policies described in the notes to the consolidated
financial statements, with the only exception being that the parent company
accounts for its subsidiaries using the equity method. Refer to the consolidated
financial statements and notes presented above for additional information and
disclosures with respect to these financial statements.
Benda
Pharmaceutical, Inc.
Condensed
Parent Company Balance Sheets
|
(Restated)
|
||||||||
|
December 31
|
December 31
|
|||||||
|
2009
|
2008
|
|||||||
|
Assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 5,625 | $ | 24,379 | ||||
|
Prepaid
expenses
|
- | 2,720 | ||||||
|
Debt
issuance cost
|
- | 55,485 | ||||||
|
Investment
in subsidiaries, at equity in net assets
|
33,553,956 | 33,131,876 | ||||||
|
Total
Assets
|
$ | 33,559,581 | $ | 33,214,460 | ||||
|
Liabilities
& Shareholders' Equity
|
||||||||
|
Accrued
liabilities
|
$ | 4,046,912 | $ | 2,820,169 | ||||
|
Convertible
notes
|
7,260,000 | 6,395,951 | ||||||
|
Wages
payable
|
693,350 | 433,350 | ||||||
|
Due
to related parties, short term (Note 14)
|
36,184 | - | ||||||
|
Redeemable
common stock, 2,049,560 shares at $3.6 per share (Note 15)
|
7,376,366 | 7,376,366 | ||||||
|
Total
liabilities
|
19,412,812 | 17,025,836 | ||||||
|
Shareholders'
Equity
|
||||||||
|
Preferred
stock, $0.001 par value; 5,000,000 shares authorized;
|
||||||||
|
None
issued and outstanding
|
- | - | ||||||
|
Common
stock, $0.001 par value; 150,000,000 shares authorized;
|
||||||||
|
105,155,355
shares issued and outstanding
|
105,155 | 105,155 | ||||||
|
Additional
paid in capital
|
22,108,427 | 22,108,427 | ||||||
|
Statutory
surplus reserve fund (Note 16)
|
2,642,775 | 2,642,775 | ||||||
|
Accumulated
deficit
|
(17,481,559 | ) | (15,544,089 | ) | ||||
|
Accumulative
other comprehensive income
|
6,268,111 | 6,372,496 | ||||||
|
Shares
issuable for services
|
503,860 | 503,860 | ||||||
|
Total
Benda Pharmaceutical, Inc.'s Shareholders' Equity
|
14,146,769 | 16,188,624 | ||||||
|
Total
Liabilities & Shareholders' Equity
|
$ | 33,559,581 | $ | 33,214,460 | ||||
Page 33
of 39
Benda
Pharmaceutical, Inc.
Condensed
Parent Company Statements of Income
|
YEAR ENDED DECEMBER 31,
|
||||||||
|
(Restated)
|
||||||||
|
2009
|
2008
|
|||||||
|
General
and administrative expenses
|
$ | (455,401 | ) | $ | (2,628,032 | ) | ||
|
Interest
expense
|
(2,008,534 | ) | (4,481,124 | ) | ||||
|
Total
Expenses
|
(2,463,935 | ) | (7,109,156 | ) | ||||
|
Equity
in undistributed income of subsidiaries
|
526,465 | (4,371,642 | ) | |||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (1,937,470 | ) | $ | (11,480,798 | ) | ||
Benda
Pharmaceutical, Inc.
Condensed
Parent Company Statements of Cash Flows
|
YEAR
ENDED DECEMBER 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
(Restated)
|
||||||||
|
Cash
Flows From Operating Activities
|
||||||||
|
Net
loss
|
$ | (1,937,470 | ) | $ | (11,480,798 | ) | ||
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
||||||||
|
Amortization
of debt issuance costs
|
55,485 | 264,962 | ||||||
|
Amortization
of convertible notes discount
|
864,049 | 3,520,876 | ||||||
|
Penalty
to investors settled by issuance of common stock
|
- | 497,081 | ||||||
|
Directors
remuneration settled by issuance of common stock
|
- | 75,900 | ||||||
|
Changes
in operating assets and liabilities:
|
||||||||
|
Prepaid
expenses
|
2,720 | (2,100 | ) | |||||
|
Equity
in undistributed income of subsidiaries
|
(526,465 | ) | 4,371,642 | |||||
|
Accrued
expense
|
1,226,743 | 1,715,467 | ||||||
|
Wages
payable
|
260,000 | 260,000 | ||||||
|
Net
cash used in operating activities
|
(54,938 | ) | (776,970 | ) | ||||
|
Cash
Flows From Financing Actives
|
||||||||
|
Borrowing
from related party
|
36,184 | - | ||||||
|
Net
cash provided by financing activities
|
36,184 | - | ||||||
|
Effect
of exchange rate changes on cash
|
- | 727,894 | ||||||
|
Net
decrease in cash and cash equivalents
|
(18,754 | ) | (49,076 | ) | ||||
|
Cash
and cash equivalents, beginning of period
|
24,379 | 73,455 | ||||||
|
Cash
and cash equivalents, end of period
|
$ | 5,625 | $ | 24,379 | ||||
Page 34
of 39
25.
Restatement of Previously Issued Financial Statements
On May
12, 2010, the Company discovered that its financial statements for the year
ended December 31, 2008 and 2007 should not be relied upon due to multiple
errors found in the accounting treatment of a business combination transaction
completed in March 2007 resulting in adjustment of assets and liabilities to
fair market value. The Company also adjusted certain other assets and intangible
assets due to errors in the accounting treatment of these assets resulting in
additional expenses for the prior period. The Company also adjusted the income
tax expense resulting in additional income tax expenses.
To
correct the above noted errors, the Company has restated the accompanying
Consolidated Balance Sheets as of December 31, 2008 and 2007, its Consolidated
Statements of Operations and cash flow for the years ended December 31, 2008 and
2007, and the notes to the consolidated financial statements.
Effects
of Restatement
To
correct the above noted errors, the Company has restated the accompanying
Consolidated Balance Sheets as of December 31, 2008, its Consolidated Statements
of Operations and Cash Flows for the year ended December 31, 2008, and the notes
to the consolidated financial statements. The impact of the 2007 restatement is
not presented here.
The
following is a summary items affected by the corrections described
above:
Page 35
of 39
Consolidated Balance
Sheets
Benda
Pharmaceutical, Inc.
Consolidated
Balance Sheets
|
As
of December 31, 2008
|
|||||||||||||
|
As
previously
|
|||||||||||||
|
reported
|
Adjustments
|
As
restated
|
|||||||||||
|
Assets
|
|||||||||||||
|
Current
Assets
|
|||||||||||||
|
Cash and cash
equivalents
|
$ | 569,019 | $ | 15,247 | $ | 584,266 | a | ||||||
|
Trade receivables,
net
|
9,336,915 | -8,001 | 9,328,914 | a | |||||||||
|
Advance for inventory
purchase
|
230,888 | 230,888 | a | ||||||||||
|
Notes
receivable
|
146,685 | 8,006 | 154,691 | a | |||||||||
|
Inventories
|
2,344,562 | -1 | 2,344,561 | a | |||||||||
|
Due from related parties, short
term
|
11,000 | - | 11,000 | ||||||||||
|
Other
receivable
|
504,349 | -504,349 | a | ||||||||||
|
Prepaid expenses and other
current assets
|
1,689,758 | -1,180,904 | 508,854 | a, b | |||||||||
|
Total
current assets
|
14,602,288 | -1,439,114 | 13,163,174 | ||||||||||
|
Due from related parties, long
term
|
2,961,744 | -1 | 2,961,743 | a | |||||||||
|
Property and equipment,
net
|
29,057,225 | -150,190 | 28,907,035 | c | |||||||||
|
Intangible assets,
net
|
6,003,102 | 1,366,380 | 7,369,482 | d | |||||||||
|
Debt issuance
costs
|
55,485 | - | 55,485 | ||||||||||
|
Restricted
cash
|
6,162,849 | -1,000,665 | 5,162,184 | e | |||||||||
|
Refundable purchase price
paid
|
1,200,000 | -1,200,000 | - | f | |||||||||
|
Other
assets
|
1,830,634 | 460,968 | 2,291,602 | a | |||||||||
|
Total
Assets
|
$ | 61,873,327 | -1,962,622 | $ | 59,910,705 | ||||||||
|
Liabilities
& Shareholders' Equity
|
|||||||||||||
|
Current
Liabilities
|
|||||||||||||
|
Accounts
payable
|
605,317 | - | 605,317 | ||||||||||
|
Customer
deposit
|
1,056 | - | 1,056 | ||||||||||
|
Other
payable
|
2,622,874 | -390,000 | 2,232,874 | g | |||||||||
|
Accrued
liabilities
|
3,999,153 | -58,673 | 3,940,480 | g | |||||||||
|
Convertible
notes
|
6,395,951 | - | 6,395,951 | ||||||||||
|
Short-term
debt
|
13,569,986 | -1,288,257 | 12,281,729 | e | |||||||||
|
Accrued VAT and other
taxes
|
516,302 | 438,207 | 954,509 | b | |||||||||
|
Acquisition price
payable
|
1,426,491 | - | 1,426,491 | ||||||||||
|
Wages
payable
|
630,475 | 389,999 | 1,020,474 | g | |||||||||
|
Due to related parties, short
term
|
1,457,803 | - | 1,457,803 | ||||||||||
|
Redeemable common stock,
2,049,560 shares at $3.6 per share
|
7,376,366 | 7,376,366 | h | ||||||||||
|
Total
current liabilities
|
31,225,408 | 6,467,642 | 37,693,050 | ||||||||||
|
Government grant
payable
|
2,234,210 | - | 2,234,210 | ||||||||||
|
Due to related parties,
long-term
|
997,593 | - | 997,593 | ||||||||||
|
Deferred income tax
liability
|
834,458 | 834,458 | c | ||||||||||
|
Total
liabilities
|
34,457,211 | 7,302,100 | 41,759,311 | ||||||||||
|
Redeemable
common stock, 2,049,560 shares at $3.6 per share
|
7,376,366 | -7,376,366 | - | h | |||||||||
|
Shareholders'
Equity
|
|||||||||||||
|
Preferred
stock, $0.001 par value; 5,000,000 shares authorized;
|
|||||||||||||
|
None issued and
outstanding
|
- | - | |||||||||||
|
Common
stock, $0.001 par value; 150,000,000 shares authorized;
|
|||||||||||||
|
105,155,355 shares issued and
outstanding
|
105,155 | - | 105,155 | ||||||||||
|
Additional paid in
capital
|
22,108,427 | - | 22,108,427 | ||||||||||
|
Statutory surplus reserve
fund
|
2,642,775 | - | 2,642,775 | ||||||||||
|
Accumulated
deficit
|
(16,047,561 | ) | 503,472 | (15,544,089 | ) | i | |||||||
|
Accumulative other comprehensive
income
|
6,347,547 | 24,949 | 6,372,496 | i | |||||||||
|
Shares issuable for
services
|
503,860 | - | 503,860 | ||||||||||
|
Total
Benda Pharmaceutical, Inc.'s Shareholders' Equity
|
15,660,203 | 528,421 | 16,188,624 | ||||||||||
|
Noncontrolling
Interest
|
4,379,547 | -2,416,777 | 1,962,770 | i | |||||||||
|
Total
Shareholders' Equity
|
20,039,750 | -1,888,356 | 18,151,394 | ||||||||||
|
Total
Liabilities & Shareholders' Equity
|
61,873,327 | -1,962,622 | $ | 59,910,705 | |||||||||
Page 36
of 39
Consolidated Statement of
Operations
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Operations
|
As
previously
|
(Restated)
|
||||||||||||
|
reported
|
Adjustments
|
2008
|
|||||||||||
|
Revenue
|
$
|
25,041,986
|
$
|
-
|
$
|
25,041,986
|
|||||||
|
Cost
of goods sold
|
(15,740,107
|
)
|
-
|
(15,740,107
|
)
|
||||||||
|
Gross
profit
|
9,301,879
|
-
|
9,301,879
|
||||||||||
|
Selling
expenses
|
2,708,968
|
-
|
2,708,968
|
||||||||||
|
General
and administrative expenses
|
|||||||||||||
|
Bad
debts
|
2,730,434
|
-
|
2,730,434
|
||||||||||
|
Amortization
of intangibles
|
153,303
|
(153,303
|
)
|
-
|
c, d | ||||||||
|
Amortization
of debt issue costs
|
264,962
|
(264,962
|
)
|
-
|
|
j | |||||||
|
Depreciation
and amortization expense
|
861,979
|
(24,539
|
)
|
837,440
|
c | ||||||||
|
Director
remuneration
|
185,468
|
(185,468
|
)
|
-
|
j | ||||||||
|
Goodwill
impairment
|
7,776,223
|
(3,635,114
|
)
|
4,141,109
|
|
c | |||||||
|
Inventory
wirtten down to net realizable value
|
392,535
|
-
|
392,535
|
||||||||||
|
Other
general and administrative expenses
|
3,787,684
|
401,657
|
4,189,341
|
|
j | ||||||||
|
Research
and development expenses
|
1,461,951
|
-
|
1,461,951
|
||||||||||
|
Total
operating expenses
|
20,323,507
|
(3,861,729
|
)
|
16,461,778
|
|||||||||
|
Operating
income / (loss)
|
(11,021,628
|
)
|
3,861,729
|
(7,159,899
|
)
|
||||||||
|
Other
income (expenses)
|
|||||||||||||
|
Interest
income
|
-
|
133,251
|
133,251
|
|
j | ||||||||
|
Interest
expense
|
(4,961,430
|
)
|
(398,358
|
)
|
(5,359,788
|
)
|
j | ||||||
|
Other
income (expenses)
|
(1,311,140
|
)
|
-
|
(1,311,140
|
)
|
||||||||
|
-
|
|||||||||||||
|
Loss
before income taxes
|
(17,294,198
|
)
|
3,596,622
|
(13,697,576
|
)
|
||||||||
|
Income
taxes
|
-
|
776,295
|
776,295
|
|
b | ||||||||
|
Net
Loss
|
(17,294,198
|
)
|
2,820,327
|
(14,473,871
|
)
|
||||||||
|
-
|
|||||||||||||
|
Less:
Net loss attributable to the noncontrolling interests
|
(1,478,278
|
)
|
(1,514,795
|
)
|
(2,993,073
|
)
|
|||||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$
|
(15,815,920
|
)
|
$
|
4,335,122
|
$
|
(11,480,798
|
)
|
|||||
|
Consolidated
Statement of Cash Flows
|
|||||||||||||
Page 37
of 39
Consolidated Statement of
Cash Flows
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Cash Flows
|
For
the year ended December 31, 2008
|
|||||||||||||
|
As
previously
reported
|
Adjustments
|
As
restated
|
|||||||||||
| Cash Flows From Operating Activities | |||||||||||||
|
Net
loss
|
$ | (15,815,920 | ) | $ | 1,342,049 | $ | (14,473,871 | ) |
i
|
||||
|
Adjustments
to reconcile net loss to net cash used in
|
|||||||||||||
|
Bad
debt provision
|
2,730,434 | - | 2,730,434 | ||||||||||
|
Inventory
written down to net realizable value
|
392,535 | - | 392,535 | ||||||||||
|
Goodwill
impairment provision
|
7,776,223 | (4,141,109 | ) | 3,635,114 |
c
|
||||||||
|
Minority
interest
|
(1,478,278 | ) | 1,478,278 | - |
i
|
||||||||
|
Depreciation,
including amounts in cost of sales
|
2,086,178 | - | 2,086,178 |
|
|||||||||
|
Amortization
of intangible assets
|
705,259 | (25,521 | ) | 679,738 |
c,
d
|
||||||||
|
Amortization
of debt issuance costs
|
264,962 | - | 264,962 | ||||||||||
|
Amortization
of convertible notes discount
|
3,520,876 | - | 3,520,876 | ||||||||||
|
Income
tax benefit
|
- | (198,730 | ) | (198,730 | ) |
c
|
|||||||
|
Penalty
to investors settled by issuance of common stock
|
497,081 | - | 497,081 | ||||||||||
|
Directors
remuneration settled by issuance of common stock
|
75,900 | - | 75,900 | ||||||||||
|
Changes
in operating assets and liabilities:
|
|||||||||||||
|
Trade
receivables
|
(2,213,453 | ) | 619,483 | (1,593,970 | ) |
a
|
|||||||
|
Other
receivables
|
195,547 | (195,547 | ) | - |
a
|
||||||||
|
Short
term loan receivable
|
(146,685 | ) | 146,685 | - |
a
|
||||||||
|
Advance
for inventory purchase
|
- | 676,222 | 676,222 |
a
|
|||||||||
|
Inventories
|
(784,749 | ) | 1 | (784,748 | ) |
a
|
|||||||
|
Prepaid
expenses and other current assets
|
359,673 | (969,373 | ) | (609,700 | ) |
a,
b
|
|||||||
|
Accounts
payable
|
2,528,101 | (2,104,836 | ) | 423,265 |
g
|
||||||||
|
Customer
deposit
|
- | (159,762 | ) | (159,762 | ) |
g
|
|||||||
|
Others
payable
|
- | 1,949,869 | 1,949,869 |
g
|
|||||||||
|
Accrued
expense
|
- | 23,762 | 23,762 |
g
|
|||||||||
|
Wages
payable
|
- | 355,688 | 355,688 |
g
|
|||||||||
|
Accrued
taxes
|
(1,503,800 | ) | 1,178,924 | (324,876 | ) |
b
|
|||||||
|
Net
cash used in operating activities
|
(810,116 | ) | (23,917 | ) | (834,033 | ) |
|
||||||
|
Cash Flows From Investing
Activities
|
|
||||||||||||
|
Addition
of notes receivable
|
- | (155,044 | ) | (155,044 | ) |
a
|
|||||||
|
Collection
of notes receivable
|
- | 7,208 | 7,208 |
a
|
|||||||||
|
Restricted
cash
|
- | (2,546,930 | ) | (2,546,930 | ) |
e
|
|||||||
|
Purchases
of property and equipment and construction-in-progress
|
(1,708,213 | ) | - | (1,708,213 | ) |
|
|||||||
|
Disposal
of intangible assets
|
172,548 | - | 172,548 |
|
|||||||||
|
Advance
to related parties
|
- | (342,724 | ) | (342,724 | ) |
a
|
|||||||
|
Net
cash used in investing activities
|
(1,535,665 | ) | (3,037,490 | ) | (4,573,155 | ) | |||||||
|
Cash Flows From Financing
Actives
|
|||||||||||||
|
Borrowing
(advance) from related party
|
(1,080,947 | ) | 342,725 | (738,222 | ) |
a
|
|||||||
|
Proceeds
and repayments of borrowings under government debts payable,
net
|
- | - | |||||||||||
|
Proceeds
and repayments of borrowings under commercial bank notes,
net
|
- | ||||||||||||
|
Proceeds
and repyaments of borrowings under government debts
payable
|
322,884 | (322,884 | ) | - |
a
|
||||||||
|
Repayments
of borrowings under bank loans, net
|
1,852,796 | 2,443,171 | 4,295,967 |
g
|
|||||||||
|
Net
cash provided by (used in) financing activities
|
1,094,733 | 2,463,012 | 3,557,745 | ||||||||||
|
Effect
of exchange rate changes on cash
|
553,827 | 145,762 | 699,589 |
i
|
|||||||||
|
Net
decrease in cash and cash equivalents
|
(697,221 | ) | (452,633 | ) | (1,149,854 | ) | |||||||
|
Cash and cash equivalents,
beginning of period
|
1,266,240 | 467,880 | 1,734,120 |
a
|
|||||||||
|
Cash and cash equivalents, end
of period
|
$ | 569,019 | $ | 15,247 | $ | 584,266 |
a
|
||||||
Page 38
of 39
a – these
are minor reclassifications between current asset items
b – We
reversed Benda Ebei’s income tax expense during the year ended December 31, 2008
because of a book loss. The reversal of the income tax expense resulted in a
decrease in income tax expense, increase in prepaid taxes and decrease of tax
payable. However, Benda Ebei did have taxable income for the year ended December
31, 2008, thus the reversal of the income tax expense should be
cancelled.
c - When
SiBiono was acquired at March 31, 2007, the assets and liabilities of SiBiono
were not fair valued at March 31, 2007. The differences in these items are due
to the difference between the fair value per valuation and book value at March
31, 2007.
d –
Certain R&D expenditures were capitalized as intangible assets in prior
years. We expensed these expenditures in the period in which these expenditures
occur.
e –
Restricted cash reclassification.
f -
Certain R&D expenditures were capitalized as other assets in prior years. We
expensed these expenditures in the period in which these expenditures
occur.
g - these
are minor reclassifications between current liability items
h – The
redeemable common stock was recorded as “temporary equity” at December 31, 2008.
We determined that it should have been classified as liability sine the
redemption request was made by the stock holders in January 2008 and payment was
deferred.
i – The
combination of the above.
j – This
is minor reclassifications between G&A expense and interest
expense.
Page 39
of 39
