Attached files
Table of Contents
As filed with the Securities and Exchange Commission on May 14, 2010
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Shangri-La Tibetan Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
| British Virgin Islands | 2834 | Not applicable | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
| 53 Niwang Rd Shangri-La County, Diqing, Yunnan Province, China 674400 |
CT Corporation System 111 Eighth Avenue New York, New York 10011 | |
| (+86) 887 823 2158 | (800) 624-0909 | |
| (Address, including zip code, and telephone number, including area code, of principal executive offices) |
(Name, address, including zip code, and telephone number, including area code, of agent for service) |
Copies to:
Bradley A. Haneberg, Esq.
Anthony W. Basch, Esq.
Christopher J. Mugel, Esq.
Kaufman & Canoles, P.C.
Three James Center, 1051 East Cary Street, 12th Floor
Richmond, Virginia 23219
(804) 771-5700 – telephone
(804) 771-5777 – facsimile
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee | ||
| Ordinary Shares(2) |
$15,000,000 | $1,069.50 | ||
| Ordinary Shares(3) |
$2,835,000 | $202.14 | ||
| Placement Agent’s Warrants(3) |
$187 | $0.01 | ||
| Ordinary Shares Underlying Placement Agent’s Warrants(4) |
$1,875,000 | $133.69 | ||
| Total |
$19,710,187 | $1,405.34(5) | ||
| (1) | The registration fee for securities is based on an estimate of the aggregate offering price of the securities, assuming the sale of the securities at the midpoint of the high and low anticipated offering prices set forth in the prospectus, and such estimate is solely for the purpose of calculating the registration fee pursuant to Rule 457(a). |
| (2) | In accordance with Rule 416(a), the Registrant is also registering an indeterminate number of additional ordinary shares that shall be issuable pursuant to Rule 416 to prevent dilution resulting from share splits, share dividends or similar transactions. |
| (3) | This registration statement also covers the resale under a separate resale prospectus by selling shareholders of up to 354,375 ordinary shares previously issued to such selling shareholders named in the resale prospectus. The registrant will not receive any proceeds from the sale of these shares. For purposes of this calculation, the Registrant has assumed the sale of these securities at the midpoint of the high and low anticipated offering prices set forth in the prospectus. |
| (4) | We have agreed to issue, on the closing date of this offering, warrants to our Placement Agent, Anderson & Strudwick, Incorporated (the “Placement Agent”), to purchase up to 10 percent of the aggregate number of ordinary shares sold by the Registrant (the “Placement Agent’s Warrants”). The price to be paid by the Placement Agent for the Placement Agent’s Warrants is $0.001 per warrant. Each Placement Agent’s Warrant may be exercised to purchase one of our ordinary shares. The closing date will be a date mutually acceptable to the Placement Agent and the Registrant after the minimum offering has been sold; provided, however, that the closing date will be on or before October 31, 2010. Assuming a maximum placement, on the closing date the Placement Agent would receive 187,500 Placement Agent’s Warrants at an aggregate purchase price of $187. The exercise price of the Placement Agent’s Warrants is equal to 125% of the price of the ordinary shares offered hereby. Assuming a maximum placement and an exercise price of $10.00 per share, we would receive, in the aggregate, $1,875,000 upon exercise of the Placement Agent’s Warrants. The ordinary shares underlying the Placement Agent’s Warrants are exercisable within one year of the date of this registration statement and are deemed to commence simultaneously with the Placement Agent’s Warrants. |
| (5) | Paid herewith. |
Table of Contents
EXPLANATORY NOTE
This registration statement contains a prospectus to be used in connection with the initial public offering of up to 1,875,000 of the registrant’s ordinary shares on a best-efforts, minimum/maximum basis through the Placement Agent named on the cover page of that prospectus (the “IPO Prospectus”). In addition, the registrant is registering on this registration statement the resale of up to 354,375 ordinary shares (the “Registrable Securities”) held by selling shareholders. Consequently, this registration statement contains a second prospectus to cover these possible resales (the “Resale Prospectus”) by certain of the registrant’s shareholders named under the Resale Prospectus (the “selling shareholders”). The IPO Prospectus and the Resale Prospectus are substantively identical, except for the following principal points:
| • | they contain different front and rear covers (including table of contents); |
| • | they contain different Offering sections in the Prospectus Summary section beginning on page 1; |
| • | they contain different Use of Proceeds sections on page 41; |
| • | the Dilution section is deleted from the Resale Prospectus on page 46; |
| • | a Selling Shareholders section is included in the Resale Prospectus beginning on page 46; |
| • | references in the IPO Prospectus to the Resale Prospectus will be deleted from the Resale Prospectus; and |
| • | the Placement section from the IPO Prospectus on page 104 is deleted from the Resale Prospectus and a Plan of Distribution is inserted in its place. |
The registrant has included in this Registration Statement, after the financial statements, alternate pages to reflect the foregoing differences.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 14, 2010

SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
Minimum Offering: 1,500,000 Ordinary Shares
Maximum Offering: 1,875,000 Ordinary Shares
This is the initial public offering of Shangri-La Tibetan Pharmaceutical, Inc., a British Virgin Islands company. We are offering a minimum of 1,500,000 and a maximum of 1,875,000 our ordinary shares. None of our officers, directors or affiliates may purchase shares in this offering.
We expect that the offering price will be between $7.50 and $8.50 per ordinary share. No public market currently exists for our ordinary shares. The offering price will be determined by the Placement Agent and the Company taking into account apparent demand for the ordinary shares, financial market conditions, market conditions for the Company, and other considerations as deemed to be relevant. We have applied for approval for quotation on the NASDAQ Global Market under the symbol “TBET” for the ordinary shares we are offering. We believe that upon the completion of the offering contemplated by this prospectus, we will meet the standards for listing on the NASDAQ Global Market.
Investing in these ordinary shares involves significant risks. See “Risk Factors” beginning on page 11 of this prospectus.
| Per Ordinary Share | Minimum Offering | Maximum Offering | |||||||
| Assumed public offering price |
$ | 8.00 | $ | 12,000,000 | $ | 15,000,000 | |||
| Placement discount |
$ | 0.56 | $ | 840,000 | $ | 1,056,000 | |||
| Proceeds to us, before expenses |
$ | 7.44 | $ | 11,160,000 | $ | 13,950,000 | |||
We expect our total cash expenses for this offering to be approximately $600,000, exclusive of the above commissions. In addition, we will pay the Placement Agent a non-accountable expense allowance of 1% of the amount of the offering, or $187,500 (maximum offering, exclusive of shares registered under Rule 462(b)) or $150,000 (minimum offering). The Placement Agent must sell the minimum number of securities offered (1,500,000 ordinary shares) if any are sold. The Placement Agent is required to use only its best efforts to sell the securities offered. The offering will terminate upon the earlier of: (i) a date mutually acceptable to us and our Placement Agent after which the minimum offering is sold or (ii) October 31, 2010. Until we sell at least 1,500,000 ordinary shares, all investor funds will be held in an escrow account at SunTrust Bank, Richmond, Virginia. If we do not sell at least 1,500,000 ordinary shares by October 31, 2010, all funds will be promptly returned to investors (within one business day) without interest or deduction. If we complete this offering, net proceeds will be delivered to our company on the closing date. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. If we complete this offering, then on the closing date, we will issue ordinary shares to investors in the offering and Placement Agent Warrants to our Placement Agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of Ordinary Shares sold in this offering.
Table of Contents
These securities have not been approved or disapproved by the Securities and Exchange Commission or any state securities commission nor has the Securities and Exchange Commission or any state securities commission passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
Anderson & Strudwick,
Incorporated
Prospectus dated , 2010
Table of Contents
Conventions That Apply to This Prospectus
Unless otherwise indicated, references in this prospectus to:
| • | “$,” “US$,” “USD” and “U.S. dollars” are to the legal currency of the United States; |
| • | “China” and the “PRC” are to the People’s Republic of China, excluding, for the purposes of this prospectus only, Taiwan and the special administrative regions of Hong Kong and Macau; |
| • | “GMP” means Good Manufacturing Practices, a certification program applicable to Chinese pharmaceutical producers; |
| • | “ordinary shares” or “shares” are to our ordinary shares, par value USD$0.001 per share; |
| • | “NMIP” means the National Medical Insurance Program, China’s national medical insurance program; |
| • | “NDRC” means the National Development and Reform Commission, which imposes price controls on some pharmaceutical products; |
| • | “provinces” are the 31 provincial level governments in China, including 22 provinces, four municipalities directly administered under the PRC central government (for example, Beijing and Shanghai) and five autonomous regions (for example, Guangxi and Tibet); |
| • | “RMB”, “Renminbi” and ¥ are to the legal currency of China; |
| • | “SAFE” means China’s State Administration of Foreign Exchange; |
| • | “SFDA” means the State Food and Drug Administration of the PRC; |
| • | “TCM” means traditional Chinese medicine; and |
| • | “we,” “us,” “our company” and “our” are to Shangri-La Tibetan Pharmaceuticals Inc., a British Virgin Islands company (“TBET”), its predecessor entities and its consolidated subsidiaries, including Chinese Tibetan Pharmaceuticals Limited, a Hong Kong company (“CTP”); Yibo Information Consulting (Shenzhen) Company Ltd., a PRC company (“WFOE”), and Yunnan Shangri-La Tibetan Pharmaceutical Group Limited (“YSTP”) a PRC company, which WFOE controls by contractual arrangements. |
This prospectus contains translations of certain RMB amounts into U.S. dollar amounts at a specified rate solely for the convenience of the reader. Unless otherwise noted, all exchange conversions relating to our financial performance made in this prospectus are based upon a rate of RMB 6.8172 to US$1.00, which was the exchange rate on December 31, 2009. Conversions relating to the health care market and Chinese economy that are based on third-party sources may be based upon exchange rates at different dates.
Unless otherwise stated, we have translated balance sheet amounts with the exception of equity at December 31, 2009 at RMB 6.8172 to US$1.00 as compared to RMB 6.8420 to US$1.00 at December 31, 20082009. We have stated equity accounts at their historical rate. The average translation rates applied to income statement accounts for the year ended December 31, 2009 and the year ended December 31, 2008 were RMB 6.8296 and RMB 7.0685, respectively. We make no representation that the RMB or U.S. dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars or RMB, as the case may be, at any particular rate or at all. On May 13, 2010, the exchange rate was $1.00 to RMB6.82792. See “Risk Factors – Fluctuation of the Renminbi could materially affect our financial condition and results of operations” for discussions of the effects of fluctuating exchange rates on the value of our ordinary shares. Any discrepancies in any table between the amounts identified as total amounts and the sum of the amounts listed therein are due to rounding.
For the sake of clarity, this prospectus follows English naming convention of first name followed by last name, regardless of whether an individual’s name is Chinese or English. For example, the name of our chief executive officer will be presented as “Taylor Guo” or “Taylor Z. Guo”, even though, in Chinese, his name would be presented as “Ziqiang Guo.”
Table of Contents
Unless otherwise indicated, all information in this prospectus assumes:
| • | no person will exercise any outstanding options; |
| • | the sale of 1,875,000 ordinary shares, the maximum number of shares offered in this offering; and |
| • | an assumed initial public offering price of $8.00 per unit, the midpoint of the range set forth on the cover page of this prospectus. |
We have relied on statistics provided by a variety of publicly-available sources regarding China’s expectations of growth, China’s demand for pharmaceutical products and traditional Tibetan medicine. We did not, directly or indirectly, sponsor or participate in the publication of such materials.
Table of Contents
This summary highlights key aspects of the information contained elsewhere in this prospectus. Because it is a summary, it does not contain all of the information that you should consider before making an investment decision. You should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and the accompanying notes to those statements. This summary contains forward-looking statements that involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “should,” “will,” “could,” and similar expressions denoting uncertainty or an action that may, will or is expected to occur in the future. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements. You should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and the notes to those statements.
Overview
Our company is a specialty pharmaceutical company focusing on the research, development, manufacturing and marketing of modernized traditional Tibetan medicines. We develop products in China for promoting health in human respiratory, digestive, urinary and reproductive systems. Our five SFDA-approved modernized traditional Tibetan medicines are designed to address large market opportunities. Our product development pipeline includes potential expanded uses of our existing products for additional medical indications and a number of new product candidates that are intended to address significant medical health needs in China.
We currently sell both prescription and over-the-counter traditional Tibetan medicines. We have developed and presently are selling five pharmaceutical products, all of which have received Chinese-government approval. In addition, we are in the process of developing several additional products for which we hope to secure government approval and to commercialize in the future. We sell our products principally to distributors in China, who in turn sell them chiefly to hospitals, hospital pharmacies and retail pharmacies.
Corporate Information
Our principal executive office is located at 53 Niwang Road, Shangri-La County, Diqing, Yunnan Province, China 674400. Our telephone number is (+86) 887 823 2158. Fax (+86) 887 823 2156. We do not maintain a corporate website at this time.
Geographic Market
Our products are sold throughout China. A majority of our sales are concentrated in the southern provinces of China, most notably Yunnan Province, Guangdong Province, and Zhejiang Province.
1
Table of Contents
Our headquarters and manufacturing facilities are located in Shangri-La County, in Yunnan Province and close to the source of the raw materials we use to formulate our medicines. Yunnan Province is part of the Tibetan Plateau, a massive area at generally high altitude that is the habitat for a wide variety of plant species not readily found elsewhere. The Province has been said to have the greatest biodiversity of any area in China, and to host 60 percent of the plants used in traditional Chinese medicine.
Tibetan Medicine
Tibetan medicine has developed over the past seventeen centuries into a significant system to promote health and treat maladies. While traditional Tibetan medicine is generally considered alternative medicine in the West, it is accepted as mainstream medical practice in China and is regulated as such by the SFDA.
As currently formulated, traditional Tibetan medicine focuses on maintenance of healthy bodily systems and the study and treatment of causes of diseases. By contrast, Western medicine is sometimes said to focus on treatment of diseases rather than the causes of diseases. These different orientations led Yeshi Donden, who served as the current Dalai Lama’s personal physician for twenty years, to remark that, “Western medicine acts quickly and is helpful in cutting acute symptoms. On the other hand, Tibetan medicine acts gradually, over a long period of time.”
Tibetan medicine relies on treatments involving diet, behavior modification, herbal medicines and physical therapies. While changes in diet (types and amount of food and number and times of daily meals) and behavior (meditation, exercise, sleep and eating patterns) are generally considered important first treatments in Tibetan medicine, herbal medicines are typically the most common and important element of treatment. If necessary, Tibetan medicine also incorporates physical therapies such as acupuncture, massage, cupping, moxibustion (heating/burning herbs on the body) baths and inhalation therapy.
Tibetan herbal medications, the type of products we develop and sell, are composed of a wide variety of medicinal herbs, minerals and, to a lesser extent, animal substances. Medications always consist of several ingredients, generally consisting of one major group of ingredients and two minor groups. The major group is addressed to the intended effect. One minor group supports the major group, and the other minor group is intended to help suppress unwanted side effects.
While Tibetan medications have traditionally been used for all types of medical concerns, in the modern context, they have tended to be used to maintain general wellbeing and to treat or ameliorate chronic conditions that conventional Western medications either do not improve or improve only minimally. In such cases, individuals who use Tibetan medications tend to rely on them in part due to their relatively low level of side effects. Because traditional Tibetan medicine is oriented toward prevention, Tibetan medicines are often taken over a long period of time, even in the absence of any symptoms.
2
Table of Contents
Industry and Market Background – Generally
We operate in China’s healthcare industry, which is large and rapidly growing as a result of population growth, and aging of the population, the country’s growing wealth, and substantial government support. A recent OECD study indicates that spending hikes and regulatory changes in China have contributed to significant growth in health-care spending in the country, and that such spending in 2010 is likely to increase another 8.7 percent, with about 4.5 percent of gross domestic product (GDP) allocated to healthcare. The consulting firm Scientia Advisor projects that healthcare spending in China will reach $600 billion by 2015, a threefold increase over 2000 expenditures.
In April 2009, the Chinese government implemented large-scale healthcare reforms in an effort to significantly improve health care facilities and infrastructure and to extent health insurance company to ever larger segments of the population. The State Council allocated $123 billion as part of its New Medical Reform Plan. The Chinese government plans to improve the urban healthcare system by rebuilding and restructuring approximately 3,700 existing urban community health centers and 11,000 community health clinics. The plan will also accommodate the development of approximately 2,400 new urban health centers. In effect, the plan de-emphasizes the prevalence of large, magnet facilities in favor of smaller, more accessible clinics.
As part of its Eleventh Five-Year Plan (2006-2010), the Chinese government has actively supported the Chinese healthcare industry by providing a number of incentives and enacting several programs, including increased funding for building additional hospitals, research centers and other healthcare facilities, enacting healthcare reforms and standards and subsidizing healthcare services for its citizens. The Chinese government has announced it will spend an additional RMB850 billion on healthcare programs from 2009 to 2011, which is designed significantly bolster the Chinese healthcare market.
In addition, the China’s government is working to improve dramatically medical services available for the 800 million rural poor in China. Through the plan, the Chinese government contemplates the development of clinics in every village and a hospital in every county in China by the end of 2011. If successfully implemented, the plan would result in at least 2,000 new county hospitals and 29,000 village clinics.
Pharmaceutical sales and usage play a larger role in the Chinese healthcare market than in the healthcare sectors of many other countries. With a small share of the population presently enjoying insurance coverage, a still-developing state health-care system, and a massive rural and relatively poor population, individuals (who generally must pay for care out of pocket) and health-care providers rely heavily on medicines, both traditional and modern. One report states that the share of healthcare spending devoted to pharmaceuticals in China is three times that of the average for countries in the developed word.
The total sales of medicines in China, including both prescription and over-the-counter medicines, was US$33.9 billion (approximately RMB257 billion) in 2007, representing an increase of 25.6% from 2006 and a 2003-2007 compound average growth rate (“CAGR”) of 17.8%. It has been estimated that the Chinese market became the eighth largest pharmaceutical market in the world in 2007 from ninth in 2006.
Traditional Chinese Medicine (“TCM”) is a major and accepted segment of the health-care industry in China. The market for TCMs in China, including both prescription and over-the-counter medicines, was approximately $5.8 billion in 2005, accounting for approximately 20.9% of all expenditures on medicine in China. TCM products have been widely used in China for thousands of years and are deeply ingrained in the Chinese culture.
Industry and Market Background – Tibetan Medicine
Traditional Tibetan medicine is a sector of the TCM market segment. The market for Tibetan pharmaceuticals in China is a small but growing in relation to the overall pharmaceutical market in China. There are over one hundred producers of traditional Tibetan medicines, of which about 40 (including our company) have received some GMP certification. Many producers are relatively small, engage in little or no research and development, and employ traditional as opposed to modern formulation and manufacturing techniques.
3
Table of Contents
According to China Medicine Source Net (“CMSN”), in 2006, the 17 GMP-certified Tibetan medicine manufacturers in the Tibet Autonomous Region had total annual industrial output of RMB623 million or about $91,018,871. Overall, China’s Tibetan medicine industry in 2006 is estimated to have been about RMB1 billion in size, or about 0.5% of China’s total pharmaceutical industry. Yet, the Tibetan medicine industry as a whole is growing rapidly, CMSN projected that the Tibetan medicine industry is growing at an annual rate of 50 percent.
Our Opportunities
Generally, we believe that a number of demographic, social, economic and policy trends point to continued growth in the Chinese pharmaceutical market generally and the traditional Tibetan pharmaceutical product market in particular. These include:
| • | China’s longstanding preference for TCM and Tibetan medicine remedies; |
| • | the rapid growth of the Chinese economy; |
| • | the aging population in China; |
| • | increases in government spending on public health care, as well as in providing medical care in rural areas; |
| • | government support for modernized TCM and Tibetan medicine as a key component of increasing quality of healthcare; |
| • | the rapidly growing over-the-counter market, of which TCM (including Tibetan medicine) makes up more than half; and |
| • | the relative low price of traditional Chinese medicines compared to Western medicine. |
In addition, we believe that interest in traditional Tibetan medicines outside of China is both significant and growing.
Our Competitive Strengths
We believe that we have developed a strong position in the traditional Tibetan medicine market as a result of access to raw materials, research and development efforts, modern production techniques, an emphasis on quality control, high-quality, effective products and effective marketing. We believe these strengths position us to take advantage of the growth of the Chinese pharmaceutical market generally and the expansion of the traditional Tibetan medicine market in particular. More specifically, we have:
| • | an established portfolio of five SFDA-approved, market-leading pharmaceuticals already in distribution; |
| • | worked to develop promising additional products that are in various stages of clinical testing and review for approval in anticipation of commercialization; |
| • | growing diversity in our product offerings, with four major categories of products for maintenance of health in the respiratory, digestive, urinary and reproductive systems; |
| • | strong research and development capabilities; |
| • | modern, sophisticated formulation and manufacturing techniques; |
| • | strict quality control procedures; |
| • | an experienced management team; |
| • | access to abundant raw materials used in traditional Tibetan medicine, but not generally available outside our geographical region; |
| • | effective marketing capabilities; |
| • | an established distribution network throughout China; and |
| • | received significant governmental support to protect and enhance traditional Tibetan medicine. |
4
Table of Contents
Our Strategies
Our objectives are to maintain and strengthen the position of our products in the Tibetan medicine healthcare segment in China, develop new products and to increase the sales of our products. We will continue to integrate our marketing, sales, management, technology, research and development and capital resources, to continue build our brand awareness, and to become the market leader for the development, manufacture and commercialization of Tibetan pharmaceutical products throughout the world. We intend to achieve these objectives by:
| • | promoting our existing brands to maintain national recognition; |
| • | developing and introducing additional products to broaden or strengthen our existing product pipeline; |
| • | expanding our distribution network for further market penetration; |
| • | building brand awareness; |
| • | expanding beyond the China healthcare market; and |
| • | pursuing strategic acquisition and licensing opportunities. |
Our Challenges and Risks
Our ability to successfully execute our strategies is subject to certain risks and uncertainties, including those relating to:
| • | competition from other traditional Tibetan medicine producers that, while generally smaller than our company, may also benefit from government support; |
| • | competition from other TCM and modern and Western medicine, which also is making inroads into the Chinese pharmaceutical market; |
| • | possible changes in perception of the efficacy of traditional Chinese and Tibetan medicines; |
| • | possible disruptions in access to needed quality raw materials; |
| • | the inability to forecast with certainty our ability to develop, prove the efficacy of and secure government approval for new pharmaceutical products; |
| • | possible changes in national or regional government policies relating to health care generally and the promotion and approval of traditional Tibetan medicines in particular; |
| • | our abilities to implement successfully our growth strategy; and |
| • | our ability to protect and safeguard our brands and product formulations. |
In addition, we face risks and uncertainties that may materially affect our business, financial condition, results of operations and prospects. Thus, you should consider the risks discussed in “Risk Factors” and elsewhere in this prospectus before investing in our ordinary shares.
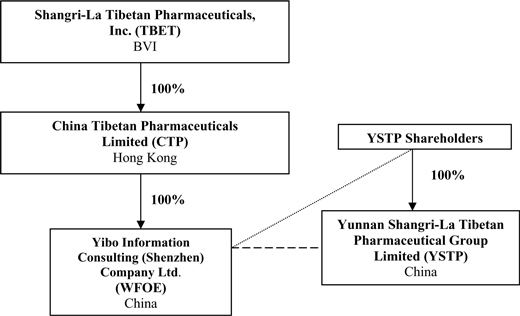
Corporate Structure
Overview
TBET is a holding company incorporated in the British Virgin Islands. TBET owns all of the outstanding capital stock of CTP, our wholly-owned subsidiary in Hong Kong. CTP in turn owns all of the outstanding capital stock of WFOE, our operating subsidiary based in Shenzhen, Guandong Province, China. WFOE has entered into control agreements with all of the owners of YSTP, which agreements allow WFOE to control YSTP. Through our ownership of CTP, CTP’s ownership of WFOE and WFOE’s contractual agreements with the owners of YSTP as well as YSTP itself, we control YSTP.
Corporate History – YSTP
Yunnan Diqing Shangri-La Tibetan Medicine Co., Ltd was incorporated on April 19, 2000 as a domestic Chinese corporation. On December 24, 2002, it changed its name to Yunan Shangri-La Tibetan Pharmaceutical Group Limited (“YSTP”). As YSTP has continued to grow, it has increased its registered capital, which presently stands at RMB 60,000,000 (approximately $8,801,267).
5
Table of Contents
Corporate History – TBET, CPT and WFOE
We formed TBET, CPT and WFOE in 2009, 2010 and 2010, respectively, in anticipation of registering the common shares of TBET in an initial public offering. In connection with the formation of TBET, CPT and WFOE, WFOE entered into certain control agreements with YSTP and its shareholders, pursuant to which we, by virtue of our ownership of CTP and CTP’s ownership of WFOE, control YSTP.
Control Agreements
We conduct our business in China through our subsidiary, WFOE. WFOE, in turn, conducts it business through YSTP, which we consolidate as a variable interest entity. WFOE and YSTP operate in connection with a series of control agreements, rather than through an equity ownership relationship.
Chinese laws and regulations currently do not prohibit or restrict foreign ownership in pharmaceutical businesses. However, Chinese laws and regulations do prevent direct foreign investment in certain industries. On March 26, 2010, to protect the Company’s shareholders from possible future foreign ownership restrictions, YSTP and all of the shareholders of YSTP entered into an Entrusted Management Agreement, Exclusive Option Agreement, Shareholders’ Voting Proxy Agreement and Pledge of Equity Interest Agreement (collectively, the “Control Agreements”) with WFOE in return for ownership interests in TBET. Through the formation of TBET as a holding company, YSTP investors now own 93% of the common shares of TBET. The remaining 7% of TBET’s common shares belong to other investors. TBET, in turn owns 100% of the equity of WFOE.
WFOE, YSTP and each of the shareholders of YSTP entered into the Control Agreements. Through the Control Agreements, we can substantially influence YSTP’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval. As a result of these Control Agreements, which enable us to control YSTP and cause WFOE to absorb 100% of the expected losses and gains of YSTP, we are considered the primary beneficiary of YSTP. Accordingly, we consolidate YSTP’s operating results, assets and liabilities in our financial statements. For a description of these contractual arrangements, see “Our Corporate Structure—Contractual Arrangements with YSTP and YSTP’s Shareholders.”
6
Table of Contents
Our corporate structure is as follows:
|
|
Equity interest | |
|
|
Contractual arrangements including Entrusted Management Agreement and Exclusive Option Agreement. For a description of these agreements, see “Corporate Structure— Contractual Arrangements with YSTP and YSTP’s Shareholders.” | |
|
|
Contractual arrangements including Exclusive Option Agreement, Shareholders’ Voting Proxy Agreement and Pledge of Equity Interest Agreement. For a description of these agreements, see “Corporate Structure— Contractual Arrangements with YSTP and YSTP’s Shareholders.” | |
7
Table of Contents
The Offering
| Ordinary Shares Offered: | Minimum: 1,500,000 ordinary shares(1) Maximum: 1,875,000 ordinary shares(1) | |
| Shares Outstanding Prior to Completion of Offering: | 11,812,500 ordinary shares | |
| Shares to be Outstanding after Offering: | Minimum: 13,312,500 ordinary shares Maximum: 13,687,500 ordinary shares | |
| Assumed Offering Price per Ordinary Share: | $8.00 | |
| Gross Proceeds: | Minimum: $12,000,000 Maximum: $15,000,000 | |
| Proposed NASDAQ Global Market Symbol: | “TBET” (CUSIP No. G80649 109) | |
| Transfer Agent: | Pacific Stock Transfer Company, 4045 S. Spencer Street, Suite 403, Las Vegas, NV 89119 | |
| Risk Factors: | Investing in these securities involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section of this prospectus before deciding to invest in our ordinary shares. | |
| Closing of Offering: | The offering contemplated by this prospectus will terminate upon the earlier of: (i) a date mutually acceptable to us and our Placement Agent after the minimum offering is sold or (ii) October 31, 2010. If we complete this offering, net proceeds will be delivered to our company on the closing date (such closing date being the above mutually acceptable date on or before October 31, 2010, provided the minimum offering has been sold). We will not complete this offering unless our application to list on the NASDAQ Global Market is approved. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. If we complete this offering, then on the closing date, we will issue shares to investors and Placement Agent Warrants to our Placement Agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of ordinary shares sold in this offering. | |
| (1) | We are also concurrently registering for resale under a separate prospectus up to 354,375 ordinary shares held by the selling shareholders named under the prospectus. None of the shares is being offered by us and we will not receive any proceeds from the sale of the ordinary shares. In addition, none of the selling shareholders is an officer or director of our company, CTP, WFOE or YSTP. |
8
Table of Contents
Placement
We have engaged Anderson & Strudwick, Incorporated as our Placement Agent to conduct this offering on a “best efforts, minimum/maximum” basis. The offering is being made without a firm commitment by the Placement Agent, which has no obligation or commitment to purchase any of our ordinary shares. Our Placement Agent is required to use only its best efforts to sell the securities offered. The offering will terminate upon the earlier of: (i) a date mutually acceptable to us and our Placement Agent after which at least 1,500,000 ordinary shares are sold or (ii) October 31, 2010. Until we sell at least 1,500,000 ordinary shares, all investor funds will be held in an escrow account at SunTrust Bank, Richmond, Virginia. If we do not sell at least 1,500,000 ordinary shares by October 31, 2010, all funds will be promptly returned to investors (within one business day) without interest or deduction. If we complete this offering, net proceeds will be delivered to our company on the closing date. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. None of our officers, directors or affiliates may purchase shares in this offering. If we complete this offering, then on the closing date, we will issue shares to investors and Placement Agent Warrants to our Placement Agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of ordinary shares sold in this offering.
We have agreed with our Placement Agent to value our company based on a multiple of approximately seven times our targeted after-tax earnings for the year ending December 31, 2010, subject to the terms of a Make-Good Escrow Agreement to be executed before effectiveness of this registration statement. Although we do not currently pay any taxes on our income, we refer to this amount as our targeted 2010 audited net after-tax income to reflect our and our Placement Agent’s intention that the targeted amount will be net of any taxes that may apply to our company during 2010. If we are unable to achieve these targeted after-tax earnings, then there is a risk that our company would be considered overvalued based on this multiple. In order to mitigate some of this risk, certain shareholders of our company, Hong Yu and Taylor Z. Guo, have agreed to place 720,000 and 30,000 ordinary shares, respectively, that each owns beneficially into escrow that is equal to 40% of the maximum number of ordinary shares to be sold in this offering. Upon closing of this offering, the escrow agent will return any shares in excess of 40% of the actual number of ordinary shares sold in the offering. Such escrowed shares are referred to as the “Make-Good Shares.” The Make-Good Shares will remain in escrow with SunTrust Bank or another bank acceptable to our Placement Agent pending the filing of our company’s Form 10-K for the year ending December 31, 2010.
To the extent our audited after-tax earnings per share (for purposes of this calculation, earnings per share are to be calculated based solely on the number of ordinary shares issued and outstanding immediately after this offering, and not on any subsequently-issued shares) for the year ending December 31, 2010 are less than $0.9863, excluding any expenses associated with releasing the Make-Good Shares back to the original owners as described below, our company will redeem and cancel, pro rata, the Make-Good Shares without any additional consideration to the extent necessary to cause our audited after-tax earnings per share to be equal to $0.9863. We cannot guarantee that we will be able to redeem a sufficient number of Make-Good Shares to increase audited after-tax earnings per share to $0.9863 if our company either has low net income or any net losses in 2010.
Any remaining Make-Good Shares will be released from escrow to our initial shareholders upon the earlier of (i) one (1) business day after the termination of this offering without closing or (ii) thirty (30) calendar days after the filing of the Form 10-K for the year ending December 31, 2010 after redeeming any Make-Good Shares. Additionally, notwithstanding any other terms of the Make-Good Escrow, if our shares trade at or above 2.5 times the price of this offering for a period of five trading days within a ten day trading period, the Make-Good Escrow will terminate and the Make-Good Shares will be released to the initial shareholders. Any delay in redeeming the Make-Good Shares will delay the release of such remaining Make-Good Shares from escrow.
We believe the Make-Good Escrow arrangement benefits the shareholders of our company (other than those who may forfeit shares without consideration) because it is designed to increase the likelihood that our company will achieve the after-tax earnings per share upon which our valuation is based. To the extent Make-Good Shares are redeemed without cost, the after-tax per-share earnings will increase for all remaining outstanding shares. While we believe the Make-Good Escrow arrangement is a benefit to our shareholders, we may be unable to redeem enough Make-Good Shares to reach our targeted 2010 after-tax earnings per share. This could occur if we either have net losses or substantially lower than anticipated earnings. If this were to happen, our audited after-tax earnings
9
Table of Contents
after redemption of the Make-Good Shares could be less than $0.9863 per share. See “Risk Factors – Redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company.”
Placement Agent’s Warrants
In connection with this offering, we will, for a nominal amount, sell to our Placement Agent Warrants, exercisable at a rate of one warrant per share, to purchase up to ten percent of the shares sold in the offering. These warrants are exercisable for a period of five years from the date of issuance at a price equal to 125% of the price of the shares in this offering. If we complete the maximum offering, then on the closing date we will issue 187,500 warrants to the Placement Agent to purchase one ordinary share each. During the term of the warrants, the holders thereof will be given the opportunity to profit from a rise in the market price of our ordinary shares, with a resulting dilution in the interest of our other shareholders. The terms on which we could obtain additional capital during the life of these warrants may be adversely affected because the holders of these warrants might be expected to exercise them when we are able to obtain any needed additional capital in a new offering of securities at a price greater than the exercise price of the warrants. If the Placement Agent exercises all of its warrants, we would have between 1.27% (minimum offering) and 1.37% (maximum offering) more shares outstanding after the Placement Agent’s Warrant exercise than at the conclusion of the offering, assuming no other issuances (including any issuances under the share incentive plan). See “Placement.”
Summary Financial Information
In the table below, we provide you with summary financial data of our company. This information is derived from our consolidated financial statements included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected for any future period. When you read this historical selected financial data, it is important that you read it along with the historical statements and notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| For the Fiscal
Year ended December 31, |
|||||||
| 2009 | 2008 | ||||||
| Total Sales |
$ | 23,008,031 | 15,580,269 | ||||
| Income from Operations |
9,415,877 | 6,091,960 | |||||
| Net Other Income (Expense) |
(170,899 | ) | (158,477 | ) | |||
| Net Income attributable to TBET |
9,244,978 | 5,933,483 | |||||
| Other Comprehensive Income attributable to TBET |
12,709 | 99,172 | |||||
| Comprehensive Income attributable to TBET |
9,257,687 | 6,032,655 | |||||
| Basic and Diluted Earnings per Share (based on 11,782,500 and 11,632,500 TBET shares outstanding, on December 31, 2009 and 2008, respectively) (1) |
0.78 | 0.51 | |||||
| Pro forma Basic and Diluted Earnings per Share (based on 11,062,500 TBET shares outstanding, on each of December 31, 2009 and 2008) (2) |
0.84 | 0.54 | |||||
| December 31, | |||||||
| 2009 | 2008 | ||||||
| Total Assets |
$ | 18,920,333 | 16,787,522 | ||||
| Total Current Liabilities |
3,833,643 | 2,343,715 | |||||
| TBET Shareholders’ Equity |
11,504,574 | 10,874,675 | |||||
| Total Liabilities and Shareholders’ Equity |
18,920,333 | 16,787,522 | |||||
| (1) | We have presented earnings per share in TBET after giving retroactive effect to the reorganization of our company that was completed on March 18, 2010, upon WFOE incorporation approval by Shenzhen Industry and Commerce Bureau. |
10
Table of Contents
| (2) | We have presented these pro forma earnings per share after (a) giving retroactive effect to the WFOE reorganization that was completed on March 18, 2010, and (b) assuming the redemption of all shares placed into escrow as described in the section entitled “Related Party Transactions – Make-Good Shares Subject to Redemption.” Based on 11,812,500 shares issued and outstanding as of May 14, 2010, the number of escrowed shares is based on 40% of an assumed maximum of 1,875,000 common shares. We provide this pro forma earnings for share information to allow potential investors to evaluate our earnings under alternative assumptions that the Make-Good Shares would or would not be redeemed. |
Investment in our securities involves a high degree of risk. You should carefully consider the risks described below together with all of the other information included in this prospectus before making an investment decision. The risks and uncertainties described below are not the only ones we face, but represent the material risks to our business. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, you may lose all or part of your investment. You should not invest in this offering unless you can afford to lose your entire investment.
Risks Related to Our Company
Our recent operating history makes it difficult to evaluate our future prospects and results of operations.
While YSTP was incorporated in 2000, it operated as a very small business until recently, when business began to develop rapidly. We first became profitable in 2006. Accordingly, you should consider our future prospects in light of the risks and uncertainties experienced in evolving markets such as the growing market for traditional Tibetan medicine products in China. Some of these risks and uncertainties relate to our ability to:
| • | develop additional products to attract and retain a larger customer base; |
| • | secure required governmental approvals; |
| • | attract additional customers and increased spending per customer; |
| • | increase awareness of our brand and continue to develop customer loyalty; |
| • | respond to competitive market conditions; |
| • | respond to changes in our regulatory environment; |
| • | manage continuous growth; |
| • | manage risks associated with intellectual property rights; |
| • | maintain effective control of our costs and expenses; |
| • | raise sufficient capital to sustain and expand our business; |
| • | attract, retain and motivate qualified personnel; and |
| • | upgrade our technology to support additional research and development of new pharmaceutical products. |
If we are unsuccessful in addressing any of these risks and uncertainties, our business may be materially and adversely affected.
Potential disruptions in the capital and credit markets may adversely affect our business, including the availability and cost of short-term funds for liquidity requirements, which could adversely affect our results of operations, cash flows and financial condition.
In the last two years, the global economy has experienced a significant contraction, which has affected the availability of business and consumer credit. We may need to rely on the credit markets, particularly for short-term borrowings from banks in China, as well as the capital markets, to meet our financial commitments and short-term liquidity needs if internal funds are not available from our operations. Disruptions in the credit and capital markets, as have been experienced since mid-2008, could adversely affect our ability to draw on such short-term bank facilities. Our access to funds under such credit facilities is dependent on the ability of the banks that are parties to those facilities to meet their funding commitments, which may be dependent on governmental economic policies in China. Those banks may not be able to meet their funding commitments to us if they experience shortages of capital and liquidity or if they experience excessive volumes of borrowing requests from us and other borrowers within a short period of time.
11
Table of Contents
Long-term disruptions in the credit and capital markets, similar to those that have been experienced since mid-2008, could result from uncertainty, changing or increased regulation, reduced alternatives or failures of significant financial institutions and could adversely affect our access to liquidity needed for our business. Any disruption could require us to take measures to conserve cash until the markets stabilize or until alternative credit arrangements or other funding for our business needs can be arranged. Such measures could include deferring capital expenditures, and reducing or eliminating discretionary uses of cash.
Continued market disruptions could cause broader economic downturns, which may lead to lower demand for our products and increased likelihood that our customers will be unable to pay for our products. Further, bankruptcies or similar events by customers may cause us to incur bad debt expense at levels higher than historically experienced. These events would adversely impact our results of operations, cash flows and financial position.
Our ability to develop new Tibetan pharmaceutical products is uncertain.
We expend a significant amount of time and effort to develop and prove new products. Our products require long lead times to develop and test to secure regulatory approval and gain market acceptance. The nature of pharmaceutical development is that some products in which substantial investments are made ultimately prove not to be safe or effective, or otherwise prove not to be commercially viable. In addition to the long lead-time needed to develop, test and secure approval for traditional Tibetan medicine, the products often take a relatively long time to demonstrate their efficacy in use. As a result, the commercial success of any product may develop, if it develops at all, only over a long period of time.
Our operations are capital-intensive, and our business could be adversely affected if we fail to maintain sufficient levels of working capital.
Developing and testing these pharmaceutical products requires substantial investments of capital and effort over extended periods of time. Substantial time is needed to develop formulations suitable for use in products, to refine those formulations, and then to conduct clinical trials to determine the efficacy and safety of the products. At times formulations must be modified based on the results of laboratory or clinical testing. Changes in the availability of capital and credit may impair our ability to sustain development and testing efforts.
Our future capital needs are uncertain and we may need to raise additional funds in the future.
We may require additional cash resources in the future due to many factors, including:
| • | changed business conditions or other future developments; |
| • | the time and expenses required to obtain, regulatory clearances and approvals; |
| • | the resources we devote to developing, manufacturing, marketing and selling our products; |
| • | our ability to identify and our desire or need to pursue acquisitions or other investments; and |
| • | the extent to which our products generate market demand. |
If we need to obtain external financing, we cannot assure you that financing will be available in the amounts or on the terms acceptable to us, if at all. Our future capital needs and other business reasons could require us to sell additional equity or debt securities or obtain credit facilities. The sale of additional equity or equity-linked securities could result in additional dilution to our then existing shareholders. Additional debt financing may include conditions that would restrict our freedom to operate our business, such as conditions that:
| • | limit our ability to pay dividends or require us to seek consent for the payment of dividends; |
| • | increase our vulnerability to general adverse economic and industry conditions; |
| • | require us to dedicate a portion of our cash flow from operations to payments on our debt, thereby reducing the availability of our cash flow to fund capital expenditures, working capital and other general corporate purposes; and |
12
Table of Contents
| • | limit our flexibility in planning for, or reacting to, changes in our business and our industry. |
We cannot guarantee that we will be able to obtain any additional financing on terms that are acceptable to us, or at all.
We presently have a limited number of commercialized products, and are dependent on two products for the vast majority of our sales revenue.
At present, we have five products in commercial distribution. Two of these products account for the vast majority of our sales. Our Gentiana product (for certain respiratory conditions) and our Mandrake product (for certain gynecological conditions) together accounted for 70% of our sales revenue in both 2009 and 2008. While we are developing and working to better distribute other products, we expect these products to continue to generate the majority of our sales in the near future. Should we encounter problems in producing these products, or should competition for these types of products intensify, our revenues and operating results could be adversely affected.
We are dependent on access to raw materials, the availability and quality of which may vary.
Our products are each comprised of multiple ingredients, many of which are plants and herbs grown principally, if not exclusively, in the Tibetan highlands. While we have relatively good access to the raw materials we use, changes in weather, disease and insect infestations, supplier business relations and other circumstances could disrupt or interrupt our access to ingredients we require to manufacture our products. To the extent we do not have access to the raw materials necessary for the production of our products, our operating results would suffer.
We may be unable to consistently purchase needed raw materials of high quality.
We require high quality raw materials raw materials to manufacture our products, and as we purchase such raw materials from third parties, we may not consistently have access to raw materials that meet our standards.
We purchase all medicinal raw materials used for production from third parties. Raw medicinal materials for traditional Tibetan medicines are agricultural products difficult to standardize. During planting and processing, there is no uniform quality standard due to differences in natural climate, soil conditions, picking, drying and processing methods. We have established strict internal quality control standards according to medicinal materials quality standards of Chinese Pharmacopoeia, and we have set up strict operation procedures according to GMP requirements. Our purchased medicinal materials are inspected by our Department of Quality before warehousing. Nonetheless, circumstances may lead to shortages of supply of high-quality raw materials. If we cannot obtain needed quantities of quality ingredients, our ability to maintain production, satisfy customers, and obtain desired operational results will be adversely effected.
We depend on the skill and expertise of our research and development executive personnel.
We believe our strong research and development capabilities contribute to our success and prospects for future growth. There is no assurance, however, that we will be able to retain our personnel and add additional, sufficiently-skilled personnel in the future. If we fail to do so, it may adversely affect our ability to develop, manufacture and market our products, and thus adversely affect our financial condition and results of operations. In addition, competition for these individuals could cause us to offer higher compensation and other benefits in order to attract and retain them, which could also materially and adversely affect our financial condition and results of operations.
If we are unable to attract, train, retain and motivate our salespeople, sales of our products may be materially and adversely affected.
We rely on our salespeople, who are dispersed across China, to market our products to distributors. We believe that our leading position in the traditional Tibetan medicine market has resulted, to a significant extent, from the dedication, efforts and performance of our salespeople. We believe that our future success will depend on those same factors. If we are unable to attract, train, retain and motivate our salespeople, sales of our products may be materially and adversely affected.
13
Table of Contents
We operate in a highly competitive marketplace, which could adversely affect our sales and financial condition.
We compete on the basis of quality, price, product availability and security of supply, product development and customer service. Some competitors are larger than us in certain markets and may have greater financial resources that allow them to be in a better position to withstand changes in the industry. Our competitors may introduce new products based on more competitive alternative technologies that may cause us to lose customers which would result in a decline in our sales volume and earnings. Our customers demand high quality and low cost products and services. The costs of research and development and marketing expansion may continue to increase and thus adversely affect the competitiveness of our products. Competition could cause us to lose market share and certain lines of business, or increase expenditures or reduce pricing, each of which would have an adverse effect on our results of operations and cash flows.
As Tibetan Medicine industry has good development prospects and large market potential, more enterprises will enter into Tibetan Medicine industry in the future; current Tibetan Medicine enterprises will increase investment in research and development to become more competitive. Biological medicine and chemical medicine, as an alternative to traditional Tibetan medicine, will continuously be produced and could become threats to sales of our traditional Tibetan products. In addition, foreign pharmaceutical companies have accelerated their speed of entering China market with their new innovative medicines and special treatment medicine. Through localization, they can reduce the cost of production and gain more market share. All these competition will impact our sales and profitability.
We may not be able to manage the expansion of our operations effectively.
We were incorporated in 2000, became profitable in 2006, and have grown significantly in recent years. Our sales have increased 48% and 41% in 2009 and 2008, respectively. We anticipate that we will continue to grow through organic growth and potentially some strategic acquisitions. To manage the potential growth of our operations, we will be required to improve our operational and financial systems, procedures and controls, to increase manufacturing capacity and output, and to expand, train and manage our growing employee base. Furthermore, we will need to maintain and expand our relationships with suppliers, distributors, hospitals, retail pharmacies and other third parties. Our current and planned operations, personnel, systems, internal procedures and controls may not be adequate to support our future growth. If we are unable to manage our growth effectively, we may not be able to take advantage of market opportunities, execute our business strategies or respond to competitive pressures.
If we do not keep pace with rapid technological change, we will be unable to capture and sustain a strong market position.
The pharmaceutical industry in China is characterized by rapid changes in technology, constant enhancement of industrial know-how and the frequent emergence of new products. Future technological improvements and continued product developments in the pharmaceutical market may render our existing products obsolete or affect their viability and competitiveness. Therefore, our future success will largely depend on our ability to improve our existing products, diversify our product range and develop new and competitively priced products that can meet the requirements of the changing market. Should we fail to respond to these frequent technological advances by improving our existing products or developing new products in a timely manner, or should these products not achieve a desirable level of market acceptance, this may adversely affect our business and profitability.
Our products may prove to have side effects. If side effects associated with our current or future products, or with traditional Tibetan medicines generally, are significant, we may face regulatory, legal and commercial difficulties that could materially adversely affect our revenues and operating results.
Pharmaceutical products sometimes have side effects, which can be minor or life threatening. At present, we know of no material adverse drug reaction among any of our presently-commercialized products. The Chinese National Center for Adverse Drug Reaction Monitoring has not found any adverse reactions among our products. Nonetheless, adverse effects may be discovered over time, or be found in newly-developed products.
14
Table of Contents
If significant side effects of our medicines are identified after they are marketed and sold,
| • | those medicines listed in the national and provincial medicine catalogs may be removed from the catalogs or downgraded to a lower tier; |
| • | regulatory authorities may withdraw or modify their approvals of such medicines; |
| • | we may be required to reformulate these medicines, change the ways in which they are marketed, conduct additional clinical trials, change the labeling of these medicines or implement changes to obtain new approvals for our manufacturing facilities; |
| • | the products may be viewed as less attractive to hospitals, pharmacies and physicians, and as a result we may have less success in marketing and selling those products. |
| • | we may have to recall these medicines from the market and may not be able to re-launch them; |
| • | we may experience a significant decline in sales of the affected products; |
| • | our reputation may suffer; and |
| • | we may become a target of lawsuits. |
The occurrence of any of these events would harm our sales of these products and substantially increase the costs and expenses of marketing these products, which in turn could cause our revenues and net income to decline. In addition, if any severe side effects are discovered to be associated with another manufacturer’s traditional Tibetan medicine products used to treat medical conditions similar to those that our medicines are used to treat, the reputation and, consequently, sales of our medicines could be adversely affected.
If WFOE is required to make a payment under its agreement to bear the losses of YSTP, our liquidity may be adversely affected, which could harm our financial condition and results of operations.
On March 26, 2010, WFOE entered into an Entrusted Management Agreement with YSTP. Pursuant to the Entrusted Management Agreement, WFOE agreed to bear the losses of YSTP. If YSTP suffers losses and WFOE is required to absorb all or a portion of such losses, WFOE will be required to seek reimbursement from YSTP. In such event, it is unlikely that YSTP will be able to make such reimbursement, and WFOE may be unable to recoup the loss WFOE absorbed at such time, if ever. Further, under the Entrusted Management Agreement, WFOE may absorb the losses at a time when WFOE does not have sufficient cash to make such payment and at a time when we or WFOE may be unable to borrow such funds on terms that are acceptable, if at all. As a result, any losses absorbed under the Entrusted Management Agreement may have an adverse effect on our liquidity, financial condition and results of operations.
WFOE’s contractual arrangements with YSTP may result in adverse tax consequences to us.
We could face material and adverse tax consequences if the Chinese tax authorities determine that WFOE’s contractual arrangements with YSTP were not made on an arm’s length basis and adjust our income and expenses for Chinese tax purposes in the form of a transfer pricing adjustment. A transfer pricing adjustment could result in a reduction, for Chinese tax purposes, of adjustments recorded by YSTP, which could adversely affect us by increasing YSTP’s tax liability without reducing WFOE’s tax liability, which could further result in late payment fees and other penalties to YSTP for underpaid taxes.
WFOE’s contractual arrangements with YSTP may not be as effective in providing control over YSTP as direct ownership.
We conduct substantially all of our operations, and generate substantially all of our revenues, through contractual arrangements with YSTP that provide us, through our ownership of WFOE, with effective control over YSTP. We depend on YSTP to hold and maintain contracts with our customers. YSTP also owns substantially all of our intellectual property, facilities and other assets relating to the operation of our business, and employs the personnel for substantially all of our business. Neither our company nor WFOE has any ownership interest in YSTP. Although we have been advised by DeHeng Law Offices, our Chinese legal counsel, that each contract under WFOE’s contractual arrangements with YSTP is valid, binding and enforceable under current Chinese laws and regulations, these contractual arrangements may not be as effective in providing us with control over YSTP as direct ownership of YSTP would be. In addition, YSTP may breach the contractual arrangements. For example, YSTP
15
Table of Contents
may decide not to make contractual payments to WFOE, and consequently to our company, in accordance with the existing contractual arrangements. In the event of any such breach, we would have to rely on legal remedies under Chinese law. These remedies may not always be effective, particularly in light of uncertainties in the Chinese legal system.
The shareholders of YSTP have potential conflicts of interest with us, which may adversely affect our business.
Neither we nor WFOE owns any portion of the equity interests of YSTP. Instead, we rely on WFOE’s contractual obligations to enforce our interest in receiving payments from YSTP. Conflicts of interests may arise between YSTP’s shareholders and our company if, for example, their interests in receiving dividends from YSTP were to conflict with our interest requiring these companies to make contractually-obligated payments to WFOE. As a result, we have required YSTP and each of its shareholders to execute irrevocable powers of attorney to appoint the individual designated by us to be his attorney-in-fact to vote on their behalf on all matters requiring shareholder approval by YSTP and to require YSTP’s compliance with the terms of its contractual obligations. We cannot assure you, however, that when conflicts of interest arise, these companies’ shareholders will act completely in our interests or that conflicts of interests will be resolved in our favor. In addition, these shareholders could violate their agreements with us by diverting business opportunities from us to others. If we cannot resolve any conflicts of interest between us and YSTP’s shareholders, we would have to rely on legal proceedings, which could result in the disruption of our business.
We rely on dividends paid by WFOE for our cash needs.
We rely primarily on dividends paid by WFOE for our cash needs, including the funds necessary to pay dividends and other cash distributions, if any, to our shareholders, to service any debt we may incur and to pay our operating expenses. The payment of dividends by entities organized in China is subject to limitations. Regulations in China currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. Under British Virgin Islands law, we may only pay dividends from surplus (the excess, if any, at the time of the determination of the total assets of our company over the sum of our liabilities, as shown in our books of account, plus our capital), and we must be solvent before and after the dividend payment in the sense that we will be able to satisfy our liabilities as they become due in the ordinary course of business; and the realizable value of assets of our company will not be less than the sum of our total liabilities, other than deferred taxes as shown on our books of account, and our capital. If we determine to pay dividends on any of our ordinary shares in the future, as a holding company, we will be dependent on receipt of funds from WFOE. See “Dividend Policy.”
Pursuant to the Implementation Rules for the new Chinese enterprise income tax law, effective on January 1, 2008, dividends payable by a foreign investment entity to its foreign investors are subject to a withholding tax of up to 10 percent. Pursuant to Article 10 of the Arrangement Between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes on Income effective on December 8, 2006, dividends payable by a foreign investment entity to its Hong Kong investor who owns 25% or above equity are subject to a withholding tax of up to 5 percent.
The payment of dividends by entities organized in China is subject to limitations, procedures and formalities. Regulations in China currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. WFOE is also required to set aside at least 10% of its after-tax profit based on Chinese accounting standards each year to its compulsory reserves fund until the accumulative amount of such reserves reaches 50% of its registered capital.
The transfer to this reserve must be made before distribution of any dividend to shareholders. The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 25% of the registered capital. For the years ended December 31, 2009, we made reserves of RMB9,532,480 ($1,395,579.64); in 2008, we made reserves of RMB 6,191,330 ($875,904.36).
16
Table of Contents
WFOE is also required to allocate a portion of its after-tax profits, as determined by its board of directors, to the general reserve, the staff welfare and bonus funds, and the enterprise expansion reserve, which may not be distributed to equity owners.
Pursuant to the Implementation Rules of the Law on Foreign-Invested Enterprises, effective on December 12, 1990, Foreign-Invested Enterprises are required to allocate a portion of their after-tax profits in accordance with their Articles of Association, to the general reserve, the staff welfare and bonus funds, and the enterprise expansion reserve. According to the Articles of Association of WFOE, the amount of each reserve is determined by WFOE’s board of directors. The general reserve is used to offset future extraordinary losses. The subsidiaries may, upon a resolution passed by the shareholders, convert the general reserve into capital. The employee welfare and bonus reserve is used for the collective welfare of the employees of the subsidiaries. The enterprise expansion reserve is used for the expansion of the subsidiaries’ operations and can be converted to capital subject to approval by the relevant authorities. These reserves represent appropriations of retained earnings determined according to Chinese law.
As of the date of this prospectus, the amounts of these reserves have not yet been determined, and we have not committed to establishing such amounts at this time. Under current Chinese laws, WFOE is required to set aside reserve amounts, but has not yet done so. WFOE has not done so because Chinese authorities grant companies flexibility in making a determination. Chinese law requires such a determination to be made in accordance with the companies’ organizational documents and WFOE’s organizational documents do not require the determination to be made within a particular timeframe. Although we have not yet been required by Chinese authorities to make such determinations or set aside such reserves, Chinese authorities may require WFOE to rectify its noncompliance and we may be fined if we fail to do so after warning within the time period set in the warning.
Additionally, Chinese law requires that the after-tax profits of foreign invested companies be distributed after a portion of after-tax profits is allocated to the reserve, therefore if for any reason, the dividends from WFOE cannot be repatriated to us or not in time, then it may detrimentally affect our cash flow and even cause us to become insolvent.
The retail prices of our principal products could be subject to government price controls.
The retail prices of some prescription and OTC medicines in China are subject to price controls administered by the Price Control Office under the National Development and Reform Commission, or the NDRC, and provincial price control authorities, either in the form of fixed prices or price ceilings. From time to time, the NDRC publishes a list of medicines subject to price controls. The NDRC directly regulates retail prices of certain medicines on the list and authorizes provincial price control authorities to regulate retail prices of the remainder on that list. Price controls apply to retail prices, but where they apply they necessarily also affect a producer’s and distributor’s ability to freely set wholesale prices. At present, none of our products are subject to price controls. Yet, should NDRC or provincial authorities decide to subject our products to price controls, it would impair our ability to set and raise prices and it could adversely affect our operating results.
We may not be able to obtain manufacturing or marketing approval from SFDA for our future products, and failure to obtain approvals for our future products could materially harm our business prospects.
All medicines must be approved by the SFDA before they can be manufactured, marketed or sold in China. The SFDA requires a pharmaceutical manufacturer to have successfully completed clinical trials of a new medicine and demonstrated its manufacturing capability before approval to manufacture that new medicine is granted. Clinical trials are expensive and their results are uncertain. In addition, the SFDA and other regulatory authorities may apply new standards for safety, manufacturing, labeling, marketing and distribution of future products. Complying with these standards may be time-consuming and expensive. Furthermore, our future products may not be efficacious or may have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining approval or may prevent or limit their commercial use. As a result, we may not be able to obtain SFDA or other governmental approvals for our future products on a timely basis or at all. Even if we do obtain approvals, such approvals may be subject to limitations on the indicated uses for which we may market a product, which may limit the size of the market for such a product.
17
Table of Contents
Adverse publicity associated with our company or our products could have a material adverse effect on our results of operations.
We are highly dependent upon consumer perceptions of our company, the “Yunnan Shangri-La Tibetan Pharmaceuticals” brand, the “Xiangbala Jidan” trademark, our other trademarks and the safety and quality of our products. We could be adversely affected if the “Yunnan Shangri-La Tibetan Pharmaceuticals” brand, the “Xiangbala Jidan” trademark or any of our other trademarks is subject to negative publicity. We could also be adversely affected if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to consumers. Because of our dependence upon consumer perceptions, any negative publicity associated with illness or other adverse effects resulting from consumers’ use or misuse of our products, or any similar products distributed by other companies, could also have a material adverse impact on our results of operations.
We may not be able to sufficiently and promptly respond to rapid changes in government regulation, treatment of diseases and customer preferences in the Chinese pharmaceutical industry, which may adversely affect our business, financial condition and results of operations.
The pharmaceutical industry in China is subject to extensive government regulation and supervision. In recent years, the Chinese government has introduced and implemented certain regulatory measures, and announced plans to implement additional rules and regulations with respect to the pharmaceutical industry, including those relating to: (i) changes in legislation or regulations governing the distribution, manufacturing or pricing of pharmaceutical products; (ii) additional quality control, licensing and certification requirements; (iii) changes in legislation or regulations governing the pricing, procurement, prescription and dispensing of essential and other medicines by public hospitals and other healthcare institutions; and (iv) changes in governmental funding for individual healthcare and pharmaceutical services. These measures may lead to significant changes in the Chinese pharmaceutical industry, and could result in increased costs and lowered profit margins for pharmaceutical distributors. The measures could also lead to decreases in the amount of our products and services purchased by our customers or the price they are willing to pay for our products and services. In addition, we cannot assure you that the Chinese government will continue to adopt policies supporting the pharmaceutical industry. The Chinese government may adopt laws and regulations that reduce support or the level of healthcare services and benefits provided in China. We cannot assure you that we will be able to adapt to such changes, and further cannot assure you that our business, financial condition and results of operations will not be adversely affected.
The Chinese pharmaceutical industry is also characterized by rapid advances in science and technology and the emergence or mutation of viruses and bacteria that together lead pharmaceutical manufacturers to discover and develop innovative new medicines and other pharmaceutical products and treatments. Our future success depends on our ability to improve and diversify our product portfolio by identifying such trends. We cannot assure you that we will be able to respond to emerging trends by improving our product portfolio and services or distributing new products in a timely fashion.
In addition to regulatory and industry changes, the preferences and purchasing trends of our customers with regard to pharmaceutical products can rapidly change. Our success depends on our ability to anticipate product lead-time and demand, identify customer preferences and adapt our product selection to these preferences. We must adjust our product availability, selection and inventory levels based on certain business requirements, sales trends and other market data. In addition, our product selection may not accurately reflect product life cycles, seasonality, backorders or customer preferences at any given time. We cannot assure you that we will be able to accurately respond to such changes in customer preferences and purchasing trends, and such failure may have an adverse effect on our business, financial condition, results of operations and profitability.
We have not determined a specific use for a significant portion of the proceeds from this offering, and we may use the proceeds in ways with which you may not agree.
Our management will have considerable discretion in the application of the net proceeds received by us. In addition, we have reserved the right to re-allocate funds currently allocated to that purpose to our general working capital. If that were to happen, then our management would have significant discretion over even more of the net proceeds to be received by our company in this offering. You will not have the opportunity, as part of your
18
Table of Contents
investment decision, to assess whether the proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate purposes that do not improve our efforts to achieve profitability or increase our stock price. The net proceeds from this offering may be placed in investments that do not produce income or that lose value. See “Use of Proceeds.”
We may incur additional environmental protection costs.
Even though the Company has implemented effective control on pollutant discharge according to environmental protection regulations and relative standards, with the increasing of people’s living standard and continuously enhancing of social and environmental protection consciousness, the state and local government may issue new laws and regulations in the future and increase environmental protection standards so as to make the Company pay higher environmental protection expenses. Additionally, as our capacity increases, we may incur more pollutant discharge expenses, which will lead to total increase of our environmental protection spending and impact our profitability.
The Chinese government could cancel its preferential tax policy, and subject us to income tax.
Pursuant to a tax preferential policy of the Yunnan Diqing Tibet Autonomous State (on March 17, 2008, the tax bureau of the Yunnan Diqing Tibet Autonomous State agreed to exempt the Company from national and provincial taxes through 2012. This measure implemented a policy to exempt from income tax enterprises in national autonomous areas that are deemed worthy of encouragement. In 2007, the Fifth Session of the Tenth National People’s Congress passed the Law of Enterprise Income Tax of the People’s Republic of China, which took effect on January 1, 2008 and provides further authority to autonomous area government agencies to decide to reduce or exempt local enterprises from income taxes. While we presently benefit from an exemption, and believe there is reason to expect a renewal or extension of some exemption, there is no assurance that our exemption will be renewed after 2012. If we do not enjoy such an exemption or reduction in the future, our operational results may be adversely affected.
The loss of any of our significant customers could reduce our revenues and our profitability.
Our key customers are principally pharmaceutical distributors in China. A few distributors account for a majority of our sales. We have not entered into long-term supply contracts with any of these major customers. Therefore, there can be no assurance that we will maintain or improve the relationships with these customers, or that we will be able to continue to supply these customers at current levels or at all.
As of December 31, 2009 and 2008, we had two customers that accounted for a significant portion of our revenues:)
| Purchaser Name |
Percentage of Revenues in Year ended December 31, 2009 |
Percentage of Revenues in Year ended December 31, 2008 |
||||
| Kunming Shangri-La Medicine Co., Ltd. |
23.0 | % | 27.7 | % | ||
| Hangzhou Hesheng Medicine Co., Ltd. |
12.6 | % | 10.2 | % |
If we cannot maintain long-term relationships with our major distributors, the loss of a significant portion of our sales to them could have an adverse effect on our business, financial condition and results of operations.
Our products are not included in national and provincial medicine catalogs of the NMIP.
The Ministry of Labor and Social Security, together with other government authorities, determines which medicines are to be included in or removed from the national medicine catalog of the NMIP, thus entitling purchasers of those medicines to coverage and reimbursement under the Chinese health insurance program. Our products are not covered by the NMIP catalog. We have not sought to have any of our commercialized products in the NMIP program. We believe that our medicines can and do compete successfully without NMIP coverage because they are sold a modest prices and in large volume. Yet, if purchasers and end-users may perceive NMIP coverage for a medicine to be a competitive advantage; the lack of NMIP coverage for our products may be a competitive disadvantage.
19
Table of Contents
Failure to obtain approval from the SFDA to convert a provisional national production standard of our principal products to a national final production standard would require us to suspend or cease the production of these products.
After the SFDA approves a new medicine, it normally directs the manufacturer to produce that medicine according to a provisional national production standard, or a provisional standard. A provisional standard is valid for two years, during which the SFDA closely monitors the production process and quality consistency of the medicine in order to develop a national final production standard, or a final standard. Three months before a medicine’s provisional standard expires, the manufacturer of that medicine is required to apply to the SFDA to convert the provisional standard to a final standard. In practice, the SFDA’s approval process is time-consuming and could take a few years. However, during the SFDA’s review period (including after the expiration of the two-year provisional standard period), the manufacturer may continue to produce the medicine according to the provisional standard.
We have six products that have received SFDA approval (five of which have been commercialized), and other products in development that we hope to submit for SFDA approval. We have received final approval for our production processes for the five commercialized products that have received SFDA approval. We are now seeking approval for a seventh product. If our manufacturing processes do not received needed SFDA approval, our ability to grow and our operational results may be adversely affected.
We do not have business interruption, litigation, product liability or other insurance.
The insurance industry in China is still at an early state of development. In particular PRC insurance companies offer limited business products. As a result, we do not have any business liability, product liability, commercial, disruption or key man insurance coverage for our operations in China. Any business interruption, litigation or natural disaster may result in our business incurring substantial costs and the diversion of resources. Furthermore, our business exposes us to the risk of product liability claims, which are inherent in the manufacturing, testing and marketing of medicines. If a product liability lawsuit were to be brought against us, it is likely to divert the attention of our management from pursuing our business strategies and might be costly to defend. The successful assertion of product liability claims against us could result in potentially significant monetary damages and require us to make significant payments or require us to limit or forgo commercialization of those products. Should any natural catastrophes such as earthquakes, fire, floods or any acts of terrorism occur where our production facilities and our primary sources of raw materials are concentrated, or elsewhere in China, which constitutes our sole market,
we might suffer not only significant property damages but also loss of revenues due to interruptions in our business operations.
We may not pay dividends.
We do not anticipate paying any dividends on our ordinary shares. Although we achieved net profitability in 2006, we cannot assure you that our operations will continue to result in sufficient revenues to enable us to operate at profitable levels or to generate positive cash flows. Furthermore, there is no assurance our Board of Directors will declare dividends even if we are profitable. Dividend policy is subject to the discretion of our Board of Directors and will depend on, among other things, our earnings, financial condition, capital requirements and other factors. If we determine to pay dividends on any of our ordinary shares in the future, we will be dependent, in large part, on receipt of funds from YSTP. See “Dividend Policy.”
Our failure to protect our intellectual property rights may undermine our competitive position, and litigation to protect our intellectual property rights or defend against third-party allegations of infringement may be costly and ineffective.
We rely primarily on trademarks, trade secrets, unpatented proprietary technologies, processes and know-how and other contractual restrictions to protect our intellectual property. These afford only limited protection and the actions we take to protect our intellectual property rights may not be adequate. Our trade secrets may otherwise become known or be independently discovered by our competitors. Third parties may infringe upon or
20
Table of Contents
misappropriate our proprietary technologies or other intellectual property rights, which could have a material adverse effect on our business, financial condition or operating results. Policing the unauthorized use of proprietary technology can be difficult and expensive. Also, litigation may be necessary to enforce our intellectual property rights, protect our trade secrets or determine the validity and scope of the proprietary rights of others. The outcome of such potential litigation may not be in our favor and any success in litigation may not be able to adequately protect our rights. Such litigation may be costly and divert management attention away from our business. An adverse determination in any such litigation would impair our intellectual property rights and may harm our business, prospects and reputation. Enforcement of judgments in China is uncertain and even if we are successful in such litigation it may not provide us with an effective remedy. In addition, we have no insurance coverage against litigation costs and would have to bear all costs arising from such litigation to the extent we are unable to recover them from other parties. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
We may be exposed to infringement or misappropriation claims by third parties, which, if adversely determined against us, could cause us to pay significant damage awards.
Our success depends largely on our ability to use and develop our technology and know-how without infringing on the intellectual property rights of third parties. The validity and scope of claims relating to patents, proprietary technologies or other intellectual property rights in the pharmaceutical industry involve complex scientific, legal and factual questions and analysis, and therefore may be highly uncertain. We may be subject to litigation involving claims of patent infringement or violation of intellectual property rights of third parties. The defense and prosecution of intellectual property suits, patent opposition proceedings and related legal and administrative proceedings can be both costly and time consuming and may significantly divert the attention and resources of our technical and management personnel. An adverse determination in any such litigation or proceedings to which we may become a party could subject us to significant liability to third parties, require us to seek licenses from third parties, to pay ongoing royalties or to redesign our products, or subject us to injunctions prohibiting the manufacture and sale of our products or the use of our technologies. Prolonged litigation could also result in our customers or potential customers deferring or limiting their purchase or use of our products until resolution of such litigation.
Customers may confuse our products with products of other traditional Tibetan or Chinese medicine companies that use the Chinese characters of “Tibet” or “Shangri-La” in their company names even where such use is not intended to infringe our trademarks.
We market and sell all of our products under a variety of trademarks. We believe that the public’s recognition of and familiarity with our brand is important to our business and that the growth of the market share of our products will depend to a significant extent upon the goodwill associated with this brand. Under the existing Chinese legal regime for intellectual property rights, limited legal remedies are available for a company to prevent the unauthorized use of its trademarks by other companies as part of their company names, unless these trademarks have been legally recognized as “well-known trademarks.” An unauthorized use of a well-known trademark as part of another company’s name can be restricted if this use is regarded as confusing consumers or misleading the public. Consumers may confuse our products with the traditional Chinese medicine products from other companies using “Tibet” and/or “Shangri-La” in or as part of their company names, and those products may be of inferior quality. This confusion could harm the reputation of our brand and cause consumers to refrain from purchasing our products, which in turn would adversely and materially affect our business, financial condition and results of operations.
Our future success depends in part on our ability to make strategic acquisitions and investments. Our failure to consummate or handle the risks associated with these acquisitions and investments could have a material adverse effect on our market penetration and revenues growth.
As part of our plan to expand our manufacturing capacity and product offerings, we hope to make strategic acquisitions in the highly-fragmented traditional Tibetan medicine sector. Strategic acquisitions could subject us to uncertainties and risks, including:
| • | high acquisition and financing costs; |
| • | potential ongoing financial obligations and unforeseen or hidden liabilities; |
21
Table of Contents
| • | failure to achieve the intended objectives, benefits or revenue-enhancing opportunities; and |
| • | cost of and difficulties in integrating acquired businesses and managing a larger business. |
Our failure to address these risks successfully may have a material adverse effect on our financial condition and results of operations.
Risks Related to Our Industry
The Chinese healthcare industry is highly regulated, and the regulatory framework, requirements and enforcement trends may change frequently.
The healthcare industry in China is highly regulated. We are governed by various local, regional and national regulatory regimes in all aspects of our operations, including licensing and certification requirements and procedures for manufacturers of pharmaceuticals, operating and security standards and environmental protection. We cannot assure you that the legal framework, licensing and certification requirements and enforcement trends in the healthcare industry will not change, or that we will be successful in responding to such changes. Such changes may result in increased costs of compliance, which would adversely affect our business, financial condition and results of operations.
All pharmaceutical manufacturing companies in China are required to obtain certain permits and licenses from various Chinese governmental authorities, including Good Manufacturing Practice, or GMP, certifications for manufacturing operations. We have obtained permits, licenses and GMP certifications required for the manufacture of our pharmaceutical products. These permits and licenses held by us are generally valid for a maximum period of five years and subject to periodic renewal and/or reassessment by the relevant Chinese government authorities and the standards of such renewal or reassessment may change from time to time. We intend to apply for the renewal of these permits, licenses and certifications when required by applicable laws and regulations. Any failure by us to obtain and maintain all licenses, permits and certifications necessary to carry on our business at any time could have a material adverse effect on our business, financial condition and results of operations. In addition, any inability to renew these permits, licenses and certifications could severely disrupt our business, and prevent us from continuing to carry on our business. Any changes in the standards used by governmental authorities in considering whether to renew or reassess our business licenses, permits and certifications, as well as any enactment of new regulations that may restrict the conduct of our business, may also decrease our revenues and/or increase our costs, and materially reduce our profitability and prospects. Further, if the interpretation or implementation of existing laws and regulations changes or new regulations come into effect requiring us to obtain any additional permits, licenses or certifications that were previously not required to operate our existing businesses, we cannot assure you that we may successfully obtain such permits, licenses or certifications.
We are subject to regular inspections, examinations, inquiries or audits by the regulatory departments as part of the process of maintaining or renewing the various permits, licenses and certificates required for the manufacture and distribution of pharmaceutical products and the provision of related logistics services. In the event that any of our products or facilities fails such inspections, our business, profitability and reputation in the industry may be adversely affected.
We may be adversely affected by changes in environmental, employee health and safety and other laws and regulations.
Our operations are subject to a number of national, provincial and local laws and regulations. These include industry laws and regulations relating to environmental protection, employee health and safety, and tax. While we believe that we are and have been in compliance with applicable laws and regulations, we cannot guarantee that changes in laws or regulations, including environmental, employee health and safety, and tax laws and regulations, will not cause us to incur substantial additional expenditures to upgrade or supplement our existing facilities or subject us to an increased rate of taxation or fines and penalties. Any such changes in laws and regulations could have a material adverse effect on our business, financial condition and results of operations.
22
Table of Contents
China’s WTO accession may intensify competition in our businesses within China.
As a consequence of its joining the World Trade Organization (“WTO”), China has lowered tariffs on certain imported pharmaceutical products and opened its pharmaceutical distribution market to foreign participation. As a result, an increasing number of foreign investors, many of which have substantially greater financial and marketing resources than we do, may establish entities or ties with existing domestic pharmaceutical distributors in China to engage in the manufacture or distribution of pharmaceutical products in the domestic market. Such new entities or alliances between foreign investors and our competitors may erode our competitiveness and weaken our financial performance.
The existence of counterfeit pharmaceutical products in China’s pharmaceutical retail market may damage our brand and reputation and have a material adverse effect on our business, financial condition, results of operations and prospects.
Certain Tibetan medicine products distributed or sold in China’s pharmaceutical retail market, including our products, may be counterfeit. Counterfeit products are products sold under the same or very similar brand names and/or having a similar appearance to genuine products. Counterfeit products, including counterfeit pharmaceutical products, are a significant problem in China. Such products divert sales from genuine products, often are of lower cost, often are or lower quality (having different ingredients or formulations, for example), and have the potential to damage the reputation for quality and effectiveness of the genuine product. The counterfeit pharmaceutical product regulation control and enforcement system in China is not able to completely eliminate production and sale of counterfeit pharmaceutical products. Any sale of counterfeit Tibetan products resulting in adverse side effects to consumers, may subject us to negative publicity and other administrative headaches and expenses. It could have a material adverse effect on our business, financial condition, results of operations and prospects.
Our competitors may develop or commercialize products ahead of us.
The pharmaceutical market in China is intensely competitive, rapidly evolving and highly fragmented. Our competitors may develop products that are superior to or more affordable than ours or they may more effectively market products that compete with ours. We face direct competition from manufacturers of other traditional Tibetan
and Chinese medicines that are similar to our products. We also face competition from manufacturers of Western medicines, including multinational companies, that manufacture Western medicines with similar curative effects and that can be used as substitutes for our products. Many of our existing and potential competitors have substantially greater financial, technical, manufacturing and other resources than we do. Our competitors’ greater size in some cases provides them with a competitive advantage with respect to manufacturing costs because of their economies of scale and their ability to purchase raw materials at lower prices. Many of our competitors also have better brand name recognition, more established distribution networks and larger customer bases. In addition, many of our competitors have extensive knowledge of our target markets. As a result, they may be able to devote greater resources to the research, development, promotion and sale of their products or respond more quickly to evolving industry standards and changes in market conditions than we can. Our failure to adapt to changing market conditions and to compete successfully with existing or new competitors may materially and adversely affect our financial condition and results of operations.
The ongoing anti-corruption campaign initiated by the Chinese government targeting state-owned hospitals could adversely affect our sales designated for hospitals.
The Chinese government has recently launched a nationwide campaign against corrupt practices that have been frequently engaged by state-owned hospitals in China, including their acceptance of kickbacks or other illegal gains and benefits in connection with their providing medical services and purchasing medical equipment and medicines. In mid-2006, the Chinese Ministry of Health ordered all state-owned hospitals to review, among other things, their procurement policies and procedures and self-correct problems and deficiencies, if any, by the end of 2006. As a result of this campaign, many state-owned hospitals have since diverted a significant portion of their attention and resources to their self-inspection and self-correction activities and are reviewing their procurement policies. If the anti-corruption campaign becomes more intensified, causing a significant change to the hospitals’ procurement policies and procedures or otherwise resulting in a further delay for state-owned hospitals to resume their normal procurement of our products, our sales designated for hospitals which we indirectly sold to through our distributors
23
Table of Contents
Risks Related to Doing Business in China
Adverse changes in political and economic policies of the Chinese government could have a material adverse effect on the overall economic growth of China, which could reduce the demand for our products and materially adversely affect our competitive position.
We conduct substantially all of our operations and generate all of our revenues in China. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in China. The Chinese economy differs from the economies of most developed countries in many respects, including:
| • | the higher level of government involvement; |
| • | the early stage of development of the market-oriented sector of the economy; |
| • | the rapid growth rate; |
| • | the higher level of control over foreign exchange; and |
| • | the allocation of resources. |
While the Chinese economy has grown significantly since the late 1970s, the growth has been uneven, both geographically and among various sectors of the economy. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on our business. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that apply to us.
The Chinese economy has been transitioning from a planned economy to a more market-oriented economy. Although the Chinese government has in recent years implemented measures emphasizing the utilization of market forces for economic reform, the Chinese government continues to exercise significant control over economic growth in China through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and imposing policies that impact particular industries or companies in different ways.
Changes in China’s political and economic policies could harm our business.
China’s economy has historically been a planned economy subject to governmental plans and quotas and has, in certain aspects, been transitioning to a more market-oriented economy. Although we believe that the economic reform and the macroeconomic measures adopted by the Chinese government have had a positive effect on the economic development of China, we cannot predict the future direction of these economic reforms or the effects these measures may have on our business, financial position or results of operations. In addition, the Chinese economy differs from the economies of most countries belonging to the Organization for Economic Cooperation and Development, or OECD. These differences include:
| • | economic structure; |
| • | level of government involvement in the economy; |
| • | level of development; |
| • | level of capital reinvestment; |
| • | control of foreign exchange; |
| • | methods of allocating resources; and |
| • | balance of payments position. |
As a result of these differences, our business may not develop in the same way or at the same rate as might be expected if the Chinese economy were similar to those of the OECD member countries. See “Our Business”.
24
Table of Contents
Since 1979, the Chinese government has promulgated many new laws and regulations covering general economic matters. Despite these efforts to develop a legal system, China’s system of laws is not yet complete. Even where adequate law exists in China, enforcement of existing laws or contracts based on existing law may be uncertain or sporadic, and it may be difficult to obtain swift and equitable enforcement or to obtain enforcement of a judgment by a court of another jurisdiction. The relative inexperience of China’s judiciary, in many cases, creates additional uncertainty as to the outcome of any litigation. In addition, interpretation of statutes and regulations may be subject to government policies reflecting domestic political changes. Our activities in China will also be subject to administration review and approval by various national and local agencies of China’s government. Because of the changes occurring in China’s legal and regulatory structure, we may not be able to secure the requisite governmental approval for our activities. Although we have obtained all required governmental approval to operate our business as currently conducted, to the extent we are unable to obtain or maintain required governmental approvals, the Chinese government may, in its sole discretion, prohibit us from conducting our business. See “Our Business”
Uncertainties with respect to the Chinese legal system could limit the legal protections available to you and us.
We conduct substantially all of our business through our operating subsidiary in China, WFOE, which is a wholly foreign owned enterprise in China. WFOE is generally subject to laws and regulations applicable to foreign investment in China and, in particular, laws applicable to foreign-invested enterprises. The Chinese legal system is based on written statutes, and prior court decisions may be cited for reference but have limited precedential value. Since 1979, a series of new Chinese laws and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, since these laws and regulations are relatively new and the Chinese legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involve uncertainties, which may limit legal protections available to you and us. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
A slowdown in the Chinese economy may slow down our growth and profitability.
The Chinese economy has grown at an approximately 9 percent rate for more than 25 years, making it the fastest growing major economy in recorded history. We cannot assure you that growth of the Chinese economy will
be steady or that any slowdown will not have a negative effect on our business. Several years ago, the Chinese economy experienced deflation, which may recur in the foreseeable future. More recently, the Chinese government announced its intention to use macroeconomic tools and regulations to slow the rate of growth of the Chinese economy, the results of which are difficult to predict. Adverse changes in the Chinese economy could impact the financial performance of the pharmaceutical industry in China. If such adverse changes were to occur in these industries, commercial shipping could decrease, which could, in turn, reduce the demand for our shipping agency services. See “Our Business”
Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.
Most of our revenues and expenses are denominated in Renminbi. Under Chinese law, the Renminbi is currently convertible under the “current account,” which includes dividends and trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans. Currently, WFOE may purchase foreign currencies for settlement of current account transactions, including payments of dividends to us, without the approval of the State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. However, the relevant Chinese government authorities may limit or eliminate our ability to purchase foreign currencies in the future. Since a significant amount of our future revenues will be denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability to utilize revenues generated in Renminbi to fund our business activities outside China that are denominated in foreign currencies.
Foreign exchange transactions by WFOE under the capital account continue to be subject to significant foreign exchange controls and require the approval of or need to register with Chinese government authorities, including SAFE. In particular, if WFOE borrows foreign currency through loans from us or other foreign lenders, these loans must be registered with SAFE, and if we finance WFOE by means of additional capital contributions, these capital contributions must be approved by certain government authorities, including the National Development
25
Table of Contents
and Reform Commission, or the NDRC, the Ministry of Commerce, or MOFCOM, or their respective local counterparts. These limitations could affect WFOE’s ability to obtain foreign exchange through debt or equity financing.
Recent Chinese regulations relating to the establishment of offshore special purpose vehicles by Chinese residents, if applied to us, may subject the Chinese resident shareholders of us or our parent company to personal liability and limit our ability to acquire Chinese companies or to inject capital into our Chinese subsidiary, limit our Chinese subsidiaries’ ability to distribute profits to us or otherwise materially adversely affect us.
In October 2005, SAFE issued a public notice, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice, which requires Chinese residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of China, referred to as an “offshore special purpose company,” for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any Chinese resident that is the shareholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in China. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any Chinese shareholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the Chinese subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under Chinese laws for evasion of applicable foreign exchange restrictions.
Due to lack of official interpretation, some of the terms and provisions in the SAFE notice remain unclear and implementation by central SAFE and local SAFE branches of the SAFE notice has been inconsistent since its adoption. Because of uncertainty over how the SAFE notice will be interpreted and implemented, we cannot predict how it will affect our business operations or future strategies. For example, our present and prospective Chinese subsidiaries’ ability to conduct foreign exchange activities, such as the remittance of dividends and foreign currency-denominated borrowings, may be subject to compliance with the SAFE notice by our or our parent company’s Chinese resident beneficial holders. In addition, such Chinese residents may not always be able to complete the necessary registration procedures required by the SAFE notice. We also have little control over either our present or prospective direct or indirect shareholders or the outcome of such registration procedures. A failure by our Chinese resident beneficial holders or future Chinese resident shareholders to comply with the SAFE notice, if SAFE requires it, could subject us to fines or legal sanctions, restrict our overseas or cross-border investment activities, limit our subsidiary’s ability to make distributions or pay dividends or affect our ownership structure, which could adversely affect our business and prospects.
The Chinese government could change its policies toward private enterprise or even nationalize or expropriate private enterprises, which could result in the total loss of our investment in that country.
Our business is subject to significant political and economic uncertainties and may be adversely affected by political, economic and social developments in China. Over the past several years, the Chinese government has pursued economic reform policies including the encouragement of private economic activity and greater economic decentralization. The Chinese government may not continue to pursue these policies or may significantly alter them to our detriment from time to time with little, if any, prior notice.
Changes in policies, laws and regulations or in their interpretation or the imposition of confiscatory taxation, restrictions on currency conversion, restrictions or prohibitions on dividend payments to stockholders, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on our business. Nationalization or expropriation could even result in the total loss of our investment in China and in the total loss of your investment in us.
26
Table of Contents
We must remit the offering proceeds to China before they may be used to benefit our business in China, and this process may take a number of months.
The proceeds of this offering must be sent back to the China, and the process for sending such proceeds back to the China may take several months after the closing of this offering. We may be unable to use these proceeds to grow our business until we receive such proceeds in China. In order to remit the offering proceeds to China, we will take the following actions:
| • | First, we will open a special foreign exchange account for capital account transactions. To open this account, we must submit to SAFE certain application forms, identity documents, transaction documents, form of foreign exchange registration of overseas investments of the domestic residents, and foreign exchange registration certificate of the invested company. |
| • | Second, we will remit the offering proceeds into this special foreign exchange account. |
| • | Third, we will apply for settlement of the foreign exchange. In order to do so, we must submit to SAFE certain application forms, identity documents, payment order to a designated person, and a tax certificate. |
The timing of the process is difficult to estimate because the efficiencies of different SAFE branches can vary significantly. Ordinarily the process takes several months but is required to be accomplished within 180 days of application by law.
We face risks related to health epidemics and other outbreaks.
Adverse public health epidemics or pandemics could disrupt business and the economies of China and other countries where we do business. From December 2002 to June 2003, China and other countries experienced an outbreak of a highly contagious form of atypical pneumonia now known as severe acute respiratory syndrome, or SARS. On July 5, 2003, the World Health Organization declared that the SARS outbreak had been contained. However, a number of isolated new cases of SARS were subsequently reported, most recently in central China in April 2004. During May and June of 2003, many businesses in China were closed by the Chinese government to prevent transmission of SARS. Moreover, some Asian countries, including China, have recently encountered
incidents of the H5N1 strain of avian influenza. We are unable to predict the effect, if any, that avian influenza may have on our business. In particular, any future outbreak of SARS, avian influenza or other similar adverse public developments may, among other things, significantly disrupt our business and force us to temporarily close our offices. Furthermore, an outbreak may severely restrict the level of economic activity in affected areas, which may in turn materially adversely affect our financial condition and results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of avian influenza, SARS or any other epidemic.
Fluctuation in the value of the Renminbi may have a material adverse effect on your investment.
Changes in the value of the Renminbi against the U.S. dollar, Euro and other foreign currencies are affected by, among other things, changes in China’s political and economic conditions. Any significant revaluation of the Renminbi may have a material adverse effect on our revenues and financial condition, and the value of, and any dividends payable on our shares in U.S. dollar terms. For example, to the extent that we need to convert U.S. dollars we receive from our initial public offering into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on Renminbi amount we would receive from the conversion. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of paying dividends on our ordinary shares or for other business purposes, appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount available to us.
Recent changes in the Chinese labor law restrict our ability to reduce our workforce in China in the event of an economic downturn and may increase our production costs.
In June 2007, the National People’s Congress of China enacted new labor law legislation called the Labor Contract Law, which became effective on January 1, 2008. To clarify certain details in connection with the implementation of the Labor Contract Law, the Chinese State Council promulgated the Implementing Rules for the
27
Table of Contents
Labor Contract Law on September 18, 2008, which came into effect immediately. The new legislation formalized workers’ rights concerning overtime hours, pensions, layoffs, employment contracts and the role of trade unions. Considered one of the strictest labor laws in the world, among other things, this new law provides for specific standards and procedures for the termination of an employment contract and places the burden of proof on the employer. In addition, the law requires the payment of a statutory severance pay upon the termination of an employment contract in most cases, including the case of the expiration of a fixed-term employment contract. Further, the law requires an employer to conclude an “employment contract without a fixed-term” with any employee who either has worked for the same employer for 10 consecutive years or more or has had two consecutive fixed-term contracts with the same employer. An “employment contract without a fixed term” can no longer be terminated on the ground of the expiration of the contract, although it can still be terminated pursuant to the standards and procedures set forth under the new law. Because of the lack of precedents for the enforcement of such a law, the standards and procedures set forth under the law in relation to the termination of an employment contract have raised concerns among foreign investment enterprises in China that such “employment contract without a fixed term” might in fact become a “lifetime, permanent employment contract.” Finally, under the new law, downsizing of either more than 20 people or more than 10% of the workforce may occur only under specified circumstances, such as a restructuring undertaken pursuant to China’s Enterprise Bankruptcy Law, or where a company suffers serious difficulties in production and/or business operations, or where there has been a material change in the objective economic circumstances relied upon by the parties at the time of the conclusion of the employment contract, thereby making the performance of such employment contract not possible. To date, there has been very little guidance and precedents as to how such specified circumstances for downsizing will be interpreted and enforced by the relevant Chinese authorities. All of our employees working for us exclusively within China are covered by the new law and thus, our ability to adjust the size of our operations when necessary in periods of recession or less severe economic downturns may be curtailed. Accordingly, if we face future periods of decline in business activity generally or adverse economic periods specific to our business, this new law can be expected to exacerbate the adverse effect of the economic environment on our results of operations and financial condition.
If any dividend is declared in the future and paid in a foreign currency, you may be taxed on a larger amount in U.S. dollars than the U.S. dollar amount that you will actually ultimately receive.
If you are a United States holder, you will be taxed on the U.S. dollar value of your dividends, if any, at the time you receive them, even if you actually receive a smaller amount of U.S. dollars when the payment is in fact converted into U.S. dollars. Specifically, if a dividend is declared and paid in a foreign currency, the amount of the dividend distribution that you must include in your income as a U.S. holder will be the U.S. dollar value of the payments made in the foreign currency, determined at the spot rate of the foreign currency to the U.S. dollar on the date the dividend distribution is includible in your income, regardless of whether the payment is in fact converted into U.S. dollars. Thus, if the value of the foreign currency decreases before you actually convert the currency into U.S. dollars, you will be taxed on a larger amount in U.S. dollars than the U.S. dollar amount that you will actually ultimately receive.
We may become a passive foreign investment company, which could result in adverse U.S. tax consequences to U.S. investors.
Based upon the nature of our business activities, we may be classified as a passive foreign investment company (“PFIC”), by the U.S. Internal Revenue Service (“IRS”), for U.S. federal income tax purposes. Such characterization could result in adverse U.S. tax consequences to you if you are a U.S. investor. For example, if we are a PFIC, a U.S. investor will become subject to burdensome reporting requirements. The determination of whether or not we are a PFIC is made on an annual basis and will depend on the composition of our income and assets from time to time. Specifically, we will be classified as a PFIC for U.S. tax purposes if either:
| • | 75% or more of our gross income in a taxable year is passive income; or |
| • | the average percentage of our assets by value in a taxable year which produce or are held for the production of passive income (which includes cash) is at least 50%. |
The calculation of the value of our assets is based, in part, on the then market value of our ordinary shares, which is subject to change. In addition, the composition of our income and assets will be affected by how, and how quickly, we spend the cash we raise in this offering. We cannot assure you that we will not be a PFIC for any taxable year. See “Taxation – United States Federal Income Taxation—Passive Foreign Investment Company.”
28
Table of Contents
If relations between the United States and China worsen, our share price may decrease and we may have difficulty accessing U.S. capital markets.
At various times during recent years, the United States and China have had disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China could adversely affect the market price of our ordinary shares and our ability to access U.S. capital markets.
Because our operations are located in China, information about our operations are not readily available from independent third-party sources.
Because YSTP and WFOE are based in China, our shareholders may have greater difficulty in obtaining information about them on a timely basis than would shareholders of a U.S.-based company. Their operations will continue to be conducted in China and shareholders may have difficulty in obtaining information about them from sources other than the companies themselves. Information available from newspapers, trade journals, or local, regional or national regulatory agencies such as issuance of construction permits and contract awards for development projects will not be readily available to shareholders and, where available, will likely be available only in Chinese. Shareholders will be dependent upon management for reports of their progress, development, activities and expenditure of proceeds.
Our failure to obtain prior approval of the China Securities Regulatory Commission (“CSRC”) of the listing and trading of our ordinary shares on a foreign stock exchange could significantly delay this offering or could have a material adverse effect upon our business, operating results, reputation and trading price of our ordinary shares.
On August 8, 2006, six Chinese regulatory agencies, including the Ministry of Commerce, the State Assets Supervision and Administration Commission, the State Administration of Taxation, the State Administration for Industry and Commerce, the CSRC and SAFE, jointly issued the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors (the “New M&A Rule”). The New M&A Rule became effective on September 8, 2006. This regulation contains provisions that purport to require that an offshore special purpose vehicle (“SPV”) formed for listing purposes and controlled directly or indirectly by Chinese companies or individuals shall obtain the approval of the CSRC prior to the listing and trading of such SPV’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published procedures specifying documents and materials required to be submitted to it by SPVs seeking CSRC approval of overseas listings.
However, the application of the New M&A Rule remains unclear with no consensus currently existing among leading Chinese law firms regarding the scope and applicability of the CSRC approval requirement. Our Chinese counsel, DeHeng Law Offices, has advised us that, based upon their understanding of current Chinese laws and regulations:
| • | We currently control our Chinese affiliate, YSTP, by virtue of WFOE’s VIE agreements with YSTP, but not through equity interest or asset acquisition which are stipulated in the New M&A Rule; and |
| • | In spite of the lack of clarity on this issue, the CSRC has not issued any definitive rule or interpretation regarding whether offerings like the one contemplated by this Prospectus are subject to the New M&A Rule. |
The CSRC has not issued any such definitive rule or interpretation, and we have not chosen to voluntarily request approval under the New M&A Rule. If the CSRC requires that we obtain its approval prior to the completion of this offering, the offering will be delayed until we obtain CSRC approval, which may take several months. There is also the possibility that we may not be able to obtain such approval. If prior CSRC approval was required, we may face regulatory actions or other sanctions from the CSRC or other Chinese regulatory authorities. These authorities may impose fines and penalties upon our operations in China, limit our operating privileges in China, delay or restrict the repatriation of the proceeds from this offering into China, or take other actions that could have a material
29
Table of Contents
adverse effect upon our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our ordinary shares. The CSRC or other Chinese regulatory agencies may also take actions requiring us, or making it advisable for us, to terminate this offering prior to closing.
Chinese regulation of loans and direct investment by offshore holding companies to Chinese entities may delay or prevent us from using the proceeds of this offering to make loans or additional capital contributions to our Chinese operating subsidiaries.
In utilizing the proceeds of this offering in the manner described in “Use of Proceeds,” as an offshore holding company of our Chinese operating subsidiaries, we may make loans or additional capital contributions to our Chinese subsidiaries.
Loans extended by foreign entities such as our company to either an FIE or a domestic Chinese company are subject to Chinese regulatory approvals and registration with the SAFE. For an FIE, such as WFOE, the Chinese regulators approve particular amounts for the FIE’s registered capital, which represents shareholders’ equity investments over a defined period of time, and the FIE’s total investment, which represents the total of the company’s registered capital plus permitted loans. The excess of the total investment over the registered capital represents the maximum amount of borrowings that an FIE is permitted to have under Chinese law. An FIE’s loans must be registered with the SAFE. A domestic Chinese company may borrow medium- and long-term loans from a foreign lender, such as our company, but must obtain NDRC approval for those loans. A domestic Chinese company may also borrow short-term foreign loans, subject, however, to a quota set by the SAFE from time to time.
In addition to loans, we may also decide to finance our subsidiaries by means of capital contributions. These capital contributions, whether to an FIE or a domestic Chinese company, must be approved by the Ministry of Commerce or its local counterparts. We cannot assure you that we will be able to obtain these government approvals on a timely basis, if at all, with respect to future capital contributions by us to our subsidiaries. If we fail to receive such approvals, our ability to use the proceeds of this offering and to capitalize our Chinese operations may be negatively affected, which could adversely affect our liquidity and our ability to fund and expand our business.
Risks Related to This Offering
There may not be an active, liquid trading market for our ordinary shares.
Prior to this offering, there has been no public market for our ordinary shares. An active trading market for our ordinary shares may not develop or be sustained following this offering. You may not be able to sell your shares at the market price, if at all, if trading in our ordinary shares is not active. The initial public offering price was determined by negotiations between us and the Placement Agent based upon a number of factors. The initial public offering price may not be indicative of prices that will prevail in the trading market.
Investors risk loss of use of funds subscribed, with no right of return, during the offering period.
We cannot assure you that all or any shares will be sold. Anderson & Strudwick, our Placement Agent, is offering our shares on a best efforts minimum/maximum basis.” We have no firm commitment from anyone, including our affiliates, to purchase all or any of the shares offered. If subscriptions for a minimum of 1,500,000 shares are not received on or before October 31, 2010, escrow provisions require that all funds received be promptly refunded. If refunded, investors will receive no interest on their funds. During the offering period, investors will not have any use or right to return of the funds.
The market price for our ordinary shares may be volatile, which could result in substantial losses to investors.
The market price for our ordinary shares is likely to be volatile and subject to wide fluctuations in response to factors including the following:
| • | actual or anticipated fluctuations in our quarterly operating results; |
| • | changes in the Chinese pharmaceutical industry; |
30
Table of Contents
| • | changes in the Chinese economy; |
| • | announcements by our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | additions or departures of key personnel; or |
| • | potential litigation. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. As a result, to the extent shareholders sell our ordinary shares in negative market fluctuation, they may not receive a price per share that is based solely upon our business performance. We cannot guarantee that shareholders will not lose some of their entire investment in our ordinary shares.
If our financial condition deteriorates, we may not meet initial objective listing standards related to net income on the NASDAQ Global Market (or, if we are listed at such time, continued listing standards) and our shareholders could find it difficult to sell our shares.
We have applied to list our common shares for trading on the NASDAQ Global Market. We have not yet been informed that our common shares will trade on the NASDAQ Global Market and can provide no assurance that our NASDAQ Global Market listing application will be approved. Additionally, we will not complete this offering unless our application to list on the NASDAQ Global Market is approved. In order to qualify for initial listing on the NASDAQ Global Market upon the completion of this offering, we must meet the following criteria:
| • | (i) Equity Standard: we must have been in operation for at least two years, must have shareholder equity of at least $30,000,000 and must have a market value for our publicly held securities of at least $18,000,000; OR (ii) Market Value Standard: we must have a market value for our publicly held securities of at least $20,000,000 and must have a market value of our listed securities of at least $75,000,000; OR (iii) Income Standard: we must have net income from continuing operations in our last fiscal year (or two of the last three fiscal years) of at least $1,000,000, must have shareholder equity of at least $15,000,000 and must have a market value for our publicly held securities of at least $8,000,000; OR (iv) Total Assets/Total Revenue Standard: we must have total assets and total revenues in our last fiscal year (or two of the last three fiscal years) of at least $75,000,000 each and we must have a market value for our publicly held securities of at least $20,000,000; and |
| • | We must have at least 1.1 million publicly held shares; |
| • | The minimum bid price for our shares must be at least $4.00 per share; |
| • | We must have at least 400 round-lot shareholders; |
| • | We must have at least 3 (in the case of the Income Standard or Equity Standard) or 4 (in the case of the Market Value Standard or Total Assets/Total Revenue Standard) market makers; and |
| • | We must have adopted NASDAQ-mandated corporate governance measures, including a Board of Directors comprised of a majority of independent directors, an Audit Committee comprised solely of independent directors and the adoption of a code of ethics among other items. |
As to the first objective listing requirement, we have applied for listing on the NASDAQ Global Market in reliance on the third test (“net income from continuing operations in our last fiscal year (or two of the last three fiscal years) of at least $1,000,000, must have shareholder equity of at least $15,000,000 and must have a market value for our publicly held securities of at least $8,000,000”). While our net income for 2009 and 2008 satisfied this objective requirement, a deterioration in our financial status combined with a protracted registration and offering period could cause us to fail to meet this requirement.
31
Table of Contents
The NASDAQ Global Market also requires companies to fulfill specific requirements in order for their shares to continue to be listed. In order to qualify for continued listing on the NASDAQ Global Market, we must meet the following criteria:
| • | Equity Standard: our shareholders’ equity must be at least $10,000,000, we must have 750,000 publicly held shares and we must have a market value of publicly held shares of at least $5,000,000; OR Market Value Standard: the market value of our listed securities must be at least $50,000,000, we must have 1,100,000 publicly held shares, and we must have a market value of publicly held shares of at least $15,000,000; OR Total Assets/Total Revenue Standard: our total assets and total revenue in our last fiscal year (or two of the last three fiscal years) must have been at least $50,000,000 each, we must have 1,100,000 publicly held shares, and we must have a market value of publicly held shares of at least $15,000,000; |
| • | The minimum bid price for our shares must be at least $1.00 per share; |
| • | We must have at least 400 round lot shareholders or at least 2,200 total shareholders; |
| • | A market value of publicly held shares (excluding shares held by officers, directors and 10% or greater shareholders) of at least $70 million; |
| • | We must have at least 2 (Equity Standard) or 4 (Market Value Standard or Total Assets/Total Revenue Standard) market makers; and |
| • | We must have adopted NASDAQ-mandated corporate governance measures, including a Board of Directors comprised of a majority of independent directors, an Audit Committee comprised solely of independent directors and the adoption of a code of ethics among other items. |
Although we have applied to have our common shares trade on the NASDAQ Global Market upon closing of this offering, investors should be aware that they will be required to commit their investment funds prior to the approval or disapproval of our listing application by the NASDAQ Global Market. We will not close this offering unless our listing application is approved. If our shares are delisted from the NASDAQ Global Market at some later date, our shareholders could find it difficult to sell our shares.
In addition, we have relied on an exemption to the blue sky registration requirements afforded to “covered securities”. Securities listed on the NASDAQ Global Market are “covered securities.” If we were unable to meet the NASDAQ Global Market’s listing standards, then we would be unable to rely on the covered securities exemption to blue sky registration requirements and we would need to register the offering in each state in which we planned to sell shares. Consequently, we will not complete this offering unless we meet the NASDAQ Global Market’s listing requirements.
In addition, if our common shares are delisted from the NASDAQ Global Market at some later date, we may apply to have our common shares quoted on the Bulletin Board or in the “pink sheets” maintained by the National Quotation Bureau, Inc. The Bulletin Board and the “pink sheets” are generally considered to be less efficient markets than the NASDAQ Global Market. In addition, if our common shares are not so listed or is delisted at some later date, our common shares may be subject to the “penny stock” regulations. These rules impose additional sales practice requirements on broker-dealers that sell low-priced securities to persons other than established customers and institutional accredited investors and require the delivery of a disclosure schedule explaining the nature and risks of the penny stock market. As a result, the ability or willingness of broker-dealers to sell or make a market in our common shares might decline. If our common shares are not so listed or are delisted from the NASDAQ Global Market at some later date or were to become subject to the penny stock regulations, it is likely that the price of our shares would decline and that our shareholders would find it difficult to sell their shares.
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, as well as new rules subsequently implemented by the SEC and NASDAQ, have required changes in corporate governance practices of public companies. We expect these new rules and regulations to significantly increase our legal, accounting and financial compliance costs and to make certain corporate activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements. We estimate that our costs for SEC reporting and continued listing on the NASDAQ Global Market to be between $500,000 and $1,000,000 per year that we did not experience prior to commencement of this offering.
32
Table of Contents
Our classified board structure may prevent a change in our control.
Our board of directors is divided into three classes of directors. Directors of each class are chosen for staggered three-year terms upon the expiration of their current terms, and each year one class of directors is elected by the shareholders. The staggered terms of our directors may reduce the possibility of a tender offer or an attempt at a change in control, even though a tender offer or change in control might be in the best interest of our shareholders. See “Management – Board of Directors and Board Committees.”
Future sales of our ordinary shares may depress our share price.
The market price of our ordinary shares could decline as a result of sales of substantial amounts of our ordinary shares in the public market, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of ordinary shares. There will be an aggregate of 11,812,500 ordinary shares outstanding before the consummation of this offering and 13,687,500 ordinary shares outstanding immediately after this offering, if the maximum offering is raised. All of the ordinary shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act, except for any shares purchased by our “affiliates,” as defined in Rule 144 of the Securities Act. The remaining ordinary shares will be “restricted securities” as defined in Rule 144. These shares may be sold in the future without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act. See “Shares Eligible for Future Sale.”
You will experience immediate and substantial dilution.
The initial public offering price of our ordinary shares is expected to be substantially higher than the pro forma net tangible book value per share of our ordinary shares. Therefore, assuming the completion of the maximum offering, if you purchase ordinary shares in this offering, you will incur immediate dilution of approximately $6.25 or approximately 78% in the pro forma net tangible book value per ordinary share from the price per share that you pay for the ordinary shares. Assuming the completion of the minimum offering, if you purchase ordinary shares in this offering, you will incur immediate dilution of approximately $6.4 or approximately 80% in the pro forma net tangible book value per ordinary share from the price per share that you pay for the ordinary shares. Accordingly, if you purchase ordinary shares in this offering, you will incur immediate and substantial dilution of your investment. See “Dilution.”
Entities controlled by our employees, officers and/or directors will control a majority of our ordinary shares, decreasing your influence on shareholder decisions.
Assuming the sale of the maximum offering, entities controlled by our employees, officers and/or directors will, in the aggregate, beneficially own approximately 80% of our outstanding shares. Assuming the sale of the minimum offering, entities controlled by our employees, officers and/or directors will, in the aggregate, beneficially own approximately 82% of our outstanding ordinary shares. As a result, our employees, officers and directors will possess substantial ability to impact our management and affairs and the outcome of matters submitted to shareholders for approval. These shareholders, acting individually or as a group, could exert control and substantial influence over matters such as electing directors and approving mergers or other business combination transactions. This concentration of ownership and voting power may also discourage, delay or prevent a change in control of our company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and might reduce the price of our ordinary shares. These actions may be taken even if they are opposed by our other shareholders, including those who purchase shares in this offering. See “Principal Shareholders.”
33
Table of Contents
A redemption of shares held by our founders may be insufficient to cause our company to achieve targeted earnings and may reduce our founders’ involvement and stake in our company.
As described in greater detail in the sections entitled “Related Party Transactions – Make-Good Shares Subject to Redemption” and “Placement—Market and Pricing Considerations,” our founders have agreed to place, on a prorated basis, that number of ordinary shares into escrow that is equal to 40% of the number of shares sold in this offering (such escrowed shares, the “Make-Good Shares”) pending determination of our audited net after-tax income for the year ending December 31, 2010. The Make-Good Shares will consist of 720,000 shares beneficially owned by Hong Yu and 30,000 shares beneficially owned by Taylor Z. Guo. Our company will redeem and cancel these Make-Good Shares pro rata without consideration to the extent necessary to cause our earnings per share to be at least $0.9863, excluding any expenses associated with releasing the Make-Good Shares back to the original owners.
We cannot guarantee that we will be able to redeem a sufficient number of Make-Good Shares to increase audited after-tax earnings per share to $0.9863 if our company either has lower net income or any net losses in 2010. To the extent there are an insufficient number of Make-Good Shares available for such redemption, our per-share after tax earnings may be less than $0.9863 for 2010.
As noted above, the holders of the Make-Good Shares are integral to our company’s success. Prior to the commencement of this offering, they collectively own 93% of our issued and outstanding shares. Assuming a maximum offering, they would collectively hold approximately 80%% of our shares upon completion of the offering. In the event all of the Make-Good Shares are redeemed, the founders of YSTP would collectively hold approximately 75% of our shares, assuming a maximum offering.
We will have an ongoing relationship with our Placement Agent that may impact our ability to obtain additional capital.
In connection with this offering, we have sold our Placement Agent Warrants to purchase up to 187,500 shares (assuming the maximum offering) for a nominal amount. These warrants are exercisable for a period of five years from the date of issuance at a price equal to 125% of the price of the ordinary shares in this offering. During the term of the warrants, the holders thereof will be given the opportunity to profit from a rise in the market price of our ordinary shares, with a resulting dilution in the interest of our other shareholders. The term on which we could obtain additional capital during the life of these warrants may be adversely affected because the holders of these warrants might be expected to exercise them when we are able to obtain any needed additional capital in a new offering of securities at a price greater than the exercise price of the warrants. See “Placement.”
We will have an ongoing relationship with our Placement Agent that may impact our shareholders’ ability to impact decisions related to our operations.
In connection with this offering, we have agreed to allow our Placement Agent to designate two non-voting observers to our Board of Directors until the earlier of the date that:
| (i) | the investors that purchase shares in this offering beneficially own less than five percent (5%) of our outstanding shares; or |
| (ii) | the trading price per share is at least four (4) times the offering price for any consecutive 15 trading day period. |
Although our Placement Agent’s observers will not be able to vote, they may nevertheless significantly influence the outcome of matters submitted to the Board of Directors for approval. We have agreed to reimburse the observers for their expenses for attending our Board meetings, subject to a maximum reimbursement of $6,000 per meeting and $12,000 annually, which amount is not more than the reimbursement payable to our directors. The observer will be required to certify that such travel expenses are not reimbursed by any other party. We will also pay observers the same amount as our independent directors receive. As of the date of this prospectus, Mr. L. McCarthy Downs III and Mr. Hayden Zou are serving as our Placement Agent’s observers to our Board of Directors. See “Management – Board of Directors Observer.”
34
Table of Contents
As the rights of shareholders under British Virgin Islands law differ from those under U.S. law, you may have fewer protections as a shareholder.
Our corporate affairs will be governed by our amended and restated memorandum and articles of association (as they may be amended and modified from time to time), the British Virgin Islands Business Companies Act, 2004 (the “BVI Act”), and the ordinary law of the British Virgin Islands. The rights of shareholders to take legal action against our directors, actions by minority shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law are to a large extent governed by the ordinary law of the British Virgin Islands and by the BVI Act. The ordinary law of the British Virgin Islands is derived in part from comparatively limited judicial precedent in the British Virgin Islands as well as from English ordinary law, which has persuasive, but not binding, authority on a court in the British Virgin Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law are not as clearly established as they would be under statutes or judicial precedents in some jurisdictions in the United States. In particular, the British Virgin Islands has a less developed body of securities laws as compared to the United States, and some states (such as Delaware) have more fully developed and judicially interpreted bodies of corporate law.
As a result of all of the above, holders of our shares may have more difficulty in protecting their interests through actions against our management, directors or major shareholders than they would as shareholders of a U.S. company. For a discussion of significant differences between the provisions of the BVI Act and the laws applicable to companies incorporated in the United States and their shareholders, see “Description of Share Capital – Differences in Corporate Law.”
British Virgin Islands companies may not be able to initiate shareholder derivative actions, thereby depriving shareholders of the ability to protect their interests.
British Virgin Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States. The circumstances in which any such action may be brought, and the procedures and defenses that may be available in respect to any such action, may result in the rights of shareholders of a British Virgin Islands company being more limited than those of shareholders of a company organized in the United States. Accordingly, shareholders may have fewer alternatives available to them if they believe that corporate wrongdoing has occurred. The British Virgin Islands courts are also unlikely to recognize or enforce against us judgments of courts in the United States based on certain liability provisions of U.S. securities law; and to impose liabilities against us, in original actions brought in the British Virgin Islands, based on certain liability provisions of U.S. securities laws that are penal in nature. There is no statutory recognition in the British Virgin Islands of judgments obtained in the United States, although the courts of the British Virgin Islands will generally recognize and enforce the non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits. This means that even if shareholders were to sue us successfully, they may not be able to recover anything to make up for the losses suffered.
The laws of the British Virgin Islands provide little protection for minority shareholders, so minority shareholders will have little or no recourse if the shareholders are dissatisfied with the conduct of our affairs.
Under the law of the British Virgin Islands, there is little statutory law for the protection of minority shareholders other than the provisions of the BVI Act dealing with shareholder remedies. The principal protection under statutory law is that shareholders may bring an action to enforce the constituent documents of the corporation, our fourth amended and restated memorandum and articles of association. Shareholders are entitled to have the affairs of the company conducted in accordance with the general law and the articles and memorandum.
There are ordinary law rights for the protection of shareholders that may be invoked, largely dependent on English company law, since the ordinary law of the British Virgin Islands for business companies is limited. Under the general rule pursuant to English company law known as the rule in Foss v. Harbottle, a court will generally refuse to interfere with the management of a company at the insistence of a minority of its shareholders who express dissatisfaction with the conduct of the company’s affairs by the majority or the board of directors. However, every shareholder is entitled to have the affairs of the company conducted properly according to law and the constituent documents of the corporation. As such, if those who control the company have persistently disregarded the
35
Table of Contents
requirements of company law or the provisions of the company’s memorandum and articles of association, then the courts will grant relief. Generally, the areas in which the courts will intervene are the following: (1) an act complained of which is outside the scope of the authorized business or is illegal or not capable of ratification by the majority; (2) acts that constitute fraud on the minority where the wrongdoers control the company; (3) acts that infringe on the personal rights of the shareholders, such as the right to vote; and (4) where the company has not complied with provisions requiring approval of a special or extraordinary majority of shareholders, which are more limited than the rights afforded minority shareholders under the laws of many states in the United States.
We may use the net proceeds in ways with which you may not agree.
While we do have some plans for the use of proceeds from this offering, we have not finally or firmly allocated specific portions of the net proceeds to us from this offering to particular purposes and have not yet determined all of our anticipated uses. Rather, our management will have significant flexibility in applying the net proceeds received by us. See “Use of Proceeds.” You will not have the opportunity, as part of your investment decision, to assess whether such proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the proceeds we receive from this offering. The proceeds we receive may be used for corporate purposes that do not improve our profitability or increase our share price. The proceeds we receive from this offering may also be placed in investments that do not produce income or that may lose value.
36
Table of Contents
We have made statements in this prospectus, including under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Our Business” and elsewhere that constitute forward-looking statements. Forward-looking statements involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “will,” “should,” “could” and similar expressions. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements.
Examples of forward-looking statements include:
| • | projections of revenue, earnings, capital structure and other financial items; |
| • | statements of our plans and objectives; |
| • | statements regarding the capabilities and capacities of our business operations; |
| • | statements of expected future economic performance; |
| • | our expectations regarding governmental support for the development of the traditional Chinese pharmaceutical industry; |
| • | our expectations with respect to our ability to secure high-quality raw materials in the future; |
| • | competition from other manufacturers of pharmaceutical products, including manufacturers of traditional Chinese medicines; |
| • | our ability to effectively protect our existing and future intellectual property and not to infringe on the intellectual property of others; and |
| • | fluctuations in general economic and business conditions in China; and |
| • | assumptions underlying statements regarding us or our business. |
The ultimate correctness of these forward-looking statements depends upon a number of known and unknown risks and events. We discuss many of these risks under the heading “Risk Factors” above. Many factors could cause our actual results to differ materially from those expressed or implied in our forward-looking statements. Consequently, you should not place undue reliance on these forward-looking statements.
The forward-looking statements speak only as of the date on which they are made, and, except as required by law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events.
In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
37
Table of Contents
Overview.
TBET is a holding company incorporated in the British Virgin Islands. TBET owns all of the outstanding capital stock of Chinese Tibetan Pharmaceuticals Limited, a Hong Kong company (“CTP”). CTP in turn owns all of the outstanding capital stock of Yibo Information Consulting (Shenzhen) Company Ltd., a company of the People’s Republic of China (“WFOE”), our operating subsidiary based in Shenzhen, Guangdong Province, China. WFOE has entered into control agreements with all of the owners of Yunnan Shangri-La Tibetan Pharmaceutical Group Limited (“YSTP”), which agreements allow WFOE to control YSTP. Through our ownership of CTP, CTP’s ownership of WFOE and WFOE’s contractual agreements with the owners of YSTP, we control YSTP.
Our Corporate Structure
Our corporate structure is as follows:


|
Equity interest | |

|
Contractual arrangements including Entrusted Management Agreement and Exclusive Option Agreement. For a description of these agreements, see “Corporate Structure— Contractual Arrangements with YSTP and YSTP’s Shareholders.” | |

|
Contractual arrangements including Exclusive Option Agreement, Shareholders’ Voting Proxy Agreement and Pledge of Equity Interest Agreement. For a description of these agreements, see “Corporate Structure— Contractual Arrangements with YSTP and YSTP’s Shareholders.” | |
Corporate History – YSTP
Yunnan Diqing Shangri-La Tibetan Medicine Co., Ltd was incorporated on April 19, 2000 as a domestic Chinese corporation. On December 24, 2002, it changed its name to Yunan Shangri-La Tibetan Pharmaceutical Group Limited (“YSTP”). As YSTP has continued to grow, it has increased its registered capital, which presently stands at RMB 60,000,000 (approximately $8,801,267).
38
Table of Contents
Corporate History – TBET, CPT and WFOE
We formed TBET, CPT and WFOE in 2009, 2010 and 2010, respectively, in anticipation of registering the common shares of TBET in an initial public offering. In connection with the formation of TBET, CPT and WFOE, WFOE entered into certain control agreements with YSTP and its shareholders, pursuant to which we, by virtue of our ownership of CTP and CTP’s ownership of WFOE, control YSTP.
TBET was incorporated in the British Virgin Islands on December 22, 2009 as a limited liability company. TBET is engaged in the research, development, manufacturing, marketing and selling of modernized traditional Tibetan medicines in China. Its wholly owned subsidiary, CTP was incorporated in Hong Kong on January 6, 2010 as a limited liability company. Other than the equity interest in CTP, the Company does not own any assets or conduct any operations.
WFOE was incorporated on March 18, 2010 as a wholly foreign owned enterprise, 100% owned by CTP.
On March 26, 2010, to protect the Company’s shareholders from possible future foreign ownership restrictions, YSTP and all of the shareholders of YSTP entered into an entrusted management agreement with WFOE, which provides that WFOE will be entitled to the full guarantee for the performance of such contracts, agreements or transactions entered into by YSTP. WFOE is also entitled to receive the residual return of YSTP. As a result of the agreement, WFOE will absorb 100% of the expected losses and gains of YSTP, which results in WFOE being the primary beneficiary of YSTP.
Control Agreements
Our relationships with YSTP and each of its shareholders are governed by a series of contractual arrangements. Under Chinese laws, each of WFOE and YSTP is an independent legal entity and neither of them is exposed to liabilities incurred by the other party. Other than pursuant to the contractual arrangements between WFOE and YSTP, YSTP does not transfer any other funds generated from its operations to WFOE.
The shareholders of YSTP entered into and caused YSTP to enter into an Entrusted Management Agreement, Exclusive Option Agreement, Shareholders’ Voting Proxy Agreement and Pledge of Equity Interest Agreement (collectively, the “Control Agreements”) with WFOE in return for ownership interests in MOE. Through the formation of TBET as a holding company, YSTP investors now own 93% of the ordinary shares of TBET. The remaining 7% of TBET’s ordinary shares belong to other investors. TBET, in turn owns 100% of the equity of YSTP.
Through the Control Agreements, we can substantially influence YSTP’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval. As a result of these Control Agreements, which enable us to control YSTP, we are considered the primary beneficiary of YSTP. Accordingly, we consolidate YSTP’s operating results, assets and liabilities in our financial statements.
Contractual Arrangements with YSTP and Its Shareholders
We conduct our business in China through our subsidiary, WFOE. WFOE, in turn, conducts it business through YSTP, which we consolidate as a variable interest entity. WFOE and YSTP operate in connection with a series of control agreements, rather than through an equity ownership relationship.
Chinese laws and regulations currently do not prohibit or restrict foreign ownership in pharmaceutical businesses. However, Chinese laws and regulations do prevent direct foreign investment in certain industries. To protect the Company’s shareholders from possible future foreign ownership restrictions, YSTP and all of the shareholders of YSTP entered into a series of contractual arrangements, or Control Agreements. Under PRC laws, each of WFOE and YSTP is an independent legal entity and neither of them is exposed to liabilities incurred by the other party. Other than pursuant to the contractual arrangements between WFOE and YSTP, YSTP does not transfer any other funds generated from its operations to WFOE. Effective March 26, 2010, WFOE entered into a functionally identical set of Control Agreements with YSTP, which agreements provide as follows.
39
Table of Contents
As of May 6, 2010, YSTP investors own 93% of the ordinary shares of TBET. The remaining 7% of TBET’s ordinary shares belongs to other investors. TBET, in turn owns 100% of the equity of YSTP.
Entrusted Management Agreement. YSTP, WFOE, and the all of the shareholders of YSTP entered into an Entrusted Management Agreement, dated March 26, 2010 that provides that WFOE will be fully and exclusively responsible for the management of YSTP. As consideration for such services, YSTP has agreed to pay the entrusted management fee during the term of this agreement. The entrusted management fee will be equal to YSTP’s estimated earnings. Also, WFOE will assume all operation risks related to the entrusted management of YSTP and bear all losses of YSTP. The term of this agreement will be from the effective date thereof to the earliest of the following: (1) the winding up of YSTP; (2) the termination date of the Entrusted Management Agreement, as agreed by the parties thereto; or (3) the date on which WFOE completes the acquisition of YSTP.
Exclusive Option Agreement. YSTP and all of YSTP’s shareholders entered into an Exclusive Option Agreement with WFOE dated March 26, 2010, which option agreement provides that WFOE will be entitled to acquire such shares from the current shareholders upon certain terms and conditions. In addition, WFOE is entitled to an irrevocable exclusive purchase option to purchase all or part of the assets and business of YSTP, if such a purchase is or becomes allowable under PRC laws and regulations and WFOE so elects. The Exclusive Option Agreement also prohibits YSTP and its shareholders from transferring any portion of the equity interests, business or assets of YSTP to anyone other than WFOE. WFOE has not yet taken any corporate action to exercise this right of purchase, and there is no guarantee that it will do so or will be permitted to do so by applicable law at such times as it may wish to do so.
Shareholders’ Voting Proxy Agreement. All of the shareholders of YSTP have executed a Shareholders’ Voting Proxy Agreement with WFOE effective March 26, 2010 to irrevocably appoint the persons designated by WFOE with the exclusive right to exercise, on their behalf, all of their Voting Rights in accordance with applicable law and YSTP’s Articles of Association, including but not limited to the rights to sell or transfer all or any of their equity interests in YSTP and to appoint and elect the directors and Chair as the authorized legal representative of YSTP. This agreement will be only terminated prior to the completion of acquisition of all of the equity interests in, or all assets or business of YSTP.
Pledge of Equity Agreement. WFOE and all of the shareholders of YSTP have entered in Pledge of Equity Agreement, effective March 26, 2010, pursuant to which all shareholder pledges all of their shares (100%) of YSTP, as appropriate, to WFOE. If YSTP or any of its respective shareholders breaches its respective contractual obligations in “Entrusted Management Agreement”, “Exclusive Option Agreement” and “Shareholders’ Voting Proxy Agreement”, WFOE as Pledgee, will be entitled to certain right to foreclose on the pledged equity interests. Such YSTP shareholders cannot dispose of the pledged equity interests or take any actions that would prejudice WFOE’s interest. This pledge has been recorded with applicable authorities in China to perfect WFOE’s security interest.
40
Table of Contents
After deducting the estimated placement discount and offering expenses payable by us, we expect to receive net proceeds of approximately $10,440,000 from this offering if the minimum offering is sold and $13,200,000 if the maximum offering is sold.
We do not have, at this time, a definitive plan to use the proceeds of the offering in particular ways. Generally, we intend to use the net proceeds of this offering to support and expand operations, with particular priority to efforts to expand our production and marketing capabilities as well as to strengthen and expand our distribution network. The precise amounts and percentage of proceeds we would devote to particular categories of activity will depend not only on the total amount of proceeds we generate in the offering, but also on prevailing market and business conditions as well as on the nature of particular opportunities that may arise from time to time. Similarly, the priority of our prospective uses of proceeds will depend on business and market conditions are they develop. Accordingly, we reserve the right to change the use of proceeds that we presently anticipate and describe herein. Nonetheless, based on our preliminary planning, we anticipate that the proceeds of the offering would be allocated to major categories of expense in approximately the following amounts:
| Use of Net Proceeds |
Percentage of Net Proceeds | ||
| Build Distribution Channel |
42 | % | |
| Brand Awareness, Marketing and Advertising |
20 | % | |
| Production Equipment Upgrade |
16 | % | |
| Working Capital, Advance to Distributors |
15 | % | |
| Sarbanes-Oxley Complaince-Related Professional Fees |
7 | % | |
| Total |
100 | % |
Our management will have considerable discretion in the application of the net proceeds received by us. We reserve the right to re-allocate funds tentatively allocated to that purpose to our general working capital. If that were to happen, then our management would have significant discretion over even more of the net proceeds to be received by our company in this offering. You will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate purposes that do not improve our efforts to achieve profitability or increase our stock price. The net proceeds from this offering may be placed in investments that do not produce income or that lose value.
Pending use of the net proceeds, we intend to invest our net proceeds in short-term, interest bearing, investment-grade obligations. These investments may have a material adverse effect on the U.S. federal income tax consequences of an investment in our common shares. It is possible that we may become a passive foreign investment company for U.S. federal income taxpayers, which could result in negative tax consequences to you. These consequences are described in more detail in “Taxation.”
We anticipate that we will retain any earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future. . Any future determination relating to our dividend policy will be made at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions and future prospects and other factors the Board of Directors may deem relevant.
If we determine to pay dividends on any of our ordinary shares in the future, as a holding company, we will be dependent on receipt of funds from WFOE. Payments of dividends by WFOE to our company are subject to the requirement that foreign invested enterprises may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business. Further, such remittances would require WFOE to provide an application for remittance that includes, in addition to the application form, a foreign registration certificate, board resolution, capital verification report, audit report on profit and stock bonuses, and a tax certificate. There are no such similar foreign exchange restrictions in the British Virgin Islands.
41
Table of Contents
Our business is conducted in China, and the financial records of WFOE and YSTP are maintained in RMB, their functional currency. However, we use the U.S. dollar as our reporting currency; therefore, periodic reports made to shareholders will include current period amounts translated into U.S. dollars using the then-current exchange rates, for the convenience of the readers. Our financial statements have been translated into U.S. dollars in accordance with Accounting Standards Codification (“ASC”) 830-10, “Foreign Currency Matters.” We have translated our asset and liability accounts using the exchange rate in effect at the balance sheet date. We translated our statements of operations using the average exchange rate for the period. We reported the resulting translation adjustments under other comprehensive income. Unless otherwise noted, we have translated balance sheet amounts with the exception of equity at December 31, 2009 at ¥6.8172 to $1.00 as compared to ¥6.842 to $1.00 at December 31, 2008. The average translation rates applied to income statement accounts for the year ended December 31, 2009 and the year ended December 31, 2008 were ¥6.8296 and ¥7.0685, respectively.
We make no representation that any RMB or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, or at all. The Chinese government imposes control over its foreign currency reserves in part through direct regulation of the conversion of RMB into foreign exchange and through restrictions on foreign trade. On May 13, 2010, the Forex rate was RMB6.82792 to $1.00. The Company does not currently engage in currency hedging transactions.
The following table sets forth information concerning exchange rates between the RMB and the U.S. dollar for the periods indicated.
Forex Exchange Rate
| (RMB per U.S. Dollar) | ||||
| Period End | Average | |||
| 2004 |
8.2765 | 8.2767 | ||
| 2005 |
8.0702 | 8.17335 | ||
| 2006 |
7.8041 | 7.93715 | ||
| 2007 |
7.295 | 7.551 | ||
| 2008 |
6.842 | 7.0685 | ||
| 2009 |
6.8172 | 6.8296 | ||
| 2010 |
||||
| January |
6.82679 | 6.82705 | ||
| February |
6.82601 | 6.8292 | ||
| March |
6.82588 | 6.8263 | ||
| April |
6.82523 | 6.82579 | ||
| May (through May 13, 2010) |
6.82792 | 6.82678 | ||
42
Table of Contents
The following table sets forth our capitalization as of December 31, 2009 on a pro forma as adjusted basis giving effect to the sale of the minimum and maximum offering at an assumed public offering price of $8.00 per share and to reflect the application of the proceeds after deducting the estimated 7% placement fees, 1% non-accountable expense allowance and $600,000 in other offering expenses.
You should read this table in conjunction with our financial statements and related notes appearing elsewhere in this prospectus and “Use of Proceeds” and “Description of Capital Stock.”
Minimum Offering (1,500,000 Ordinary shares)
U.S. Dollars
December 31, 2009
| As Reported(1) | Pro Forma Adjusted for IPO(2) |
||||||
| Common shares |
|||||||
|
Shares(3) |
11,782,500 | 13,312,500 | |||||
| Par Value Amount |
$ | 11,782 | $ | 13,312.5 | |||
| Additional Paid-In Capital |
$ | 8,375,795 | $ | 18,814,295 | (4) | ||
| Statutory Reserves |
$ | 2,811,323 | $ | 2,811,323 | |||
| Retained Earnings |
$ | 193,793 | $ | 193,793 | |||
| Accumulated Other Comprehensive Income |
$ | 111,881 | $ | 111,881 | |||
| Total |
$ | 11,504,574 | $ | 21,944,604 | |||
Maximum Offering (1,875,000 Common shares)
U.S. Dollars
December 31, 2009
| As Reported(1) | Pro Forma Adjusted for IPO(2) |
||||||
| Common shares |
|||||||
|
Shares(3) |
11,782,500 | 13,687,500 | |||||
| Par Value Amount |
$ | 11,782 | $ | 13,687.5 | |||
| Additional Paid-In Capital |
$ | 8,325,795 | $ | 21,573,920 | (4) | ||
| Statutory Reserves |
$ | 2,811,323 | $ | 2,811,323 | |||
| Retained Earnings |
$ | 193,793 | $ | 193,793 | |||
| Accumulated Other Comprehensive Income |
$ | 111,881 | $ | 111,881 | |||
| Total |
$ | 11,504,574 | $ | 24,704,604 | |||
| (1) | This column gives effect to the reorganization of our company that was completed on March 18, 2010, upon WFOE incorporation approval by Shenzhen Industry and Commerce Bureau. |
| (2) | Gives effect to the sale of the minimum offering and the maximum offering, as applicable, at an assumed public offering price of $8.00 per share and to reflect the application of the proceeds after deducting a 7% underwriting discounts,1% non-accountable expense allowance and our estimated offering expenses of $600,000. |
| (3) | Upon the closing of this Offering, we will cause Hong Yu and Taylor Z. Guo to place into escrow Make-Good Shares equal to 40% of the number of Shares we sell in this Offering. That amount will be between 600,000 and 750,000 Make-Good Shares, depending on whether we complete a minimum offering, a maximum offering or an offering between the minimum and maximum offering. These escrowed shares, which will be placed into escrow upon closing of this Offering, have not been removed from the Shares presented in the above Capitalization Tables. For further discussion of the escrow arrangement, please see “Summary – Placement”, “Risk Factors – A redemption of shares held by our founders may be insufficient to cause our company to achieve targeted earnings and may reduce our founders’ involvement and stake in our company”, “Related Party Transactions – Make-Good Shares Subject to Redemption.” |
43
Table of Contents
| (4) | Pro forma adjusted for IPO additional paid in capital reflects the net proceeds we expect to receive, after deducting a 7% underwriting discount, a 1% non-accountable expense allowance and approximately $600,000 in expenses, less the par value of the shares offered. In a minimum offering, we expect to receive net proceeds of $10,440,000 ($12,000,000 offering, less underwriting discount of $840,000, non-accountable expense allowance of $120,000 and offering expenses of $600,000), of which $10,438,500 would be attributable to additional paid in capital after deducting $1,500 in aggregate par value amount. In a maximum offering, we expect to receive net proceeds of $13,200,000 ($15,000,000 offering, less underwriting discount of $1,050,000, non-accountable expense allowance of $150,000 and offering expenses of $600,000), of which $13,198,125 would be attributable to additional paid in capital after deducting $1,875 in aggregate par value amount. |
44
Table of Contents
If you invest in our ordinary shares, your interest will be diluted to the extent of the difference between the initial public offering price per ordinary share and the pro forma net tangible book value per ordinary share after the offering. Dilution results from the fact that the per ordinary share offering price is substantially in excess of the book value per ordinary share attributable to the existing shareholders for our presently outstanding ordinary shares. Our net tangible book value attributable to shareholders at December 31, 2009 was $10,807,416 or approximately $0.91 per ordinary share. Net tangible book value per ordinary share as of December 31, 2009 represents the amount of total tangible assets less goodwill, acquired intangible assets and total liabilities, divided by the number of ordinary shares outstanding.
If the minimum offering is sold, we will have 13,312,500 ordinary shares outstanding upon completion of the offering. Our post offering pro forma net tangible book value, which gives effect to receipt of the net proceeds from the offering and issuance of additional shares in the offering, but does not take into consideration any other changes in our net tangible book value after December 31, 2009, will be approximately $21,247,416 or $1.60 per ordinary share. This would result in dilution to investors in this offering of approximately $6.40 per ordinary share or approximately 80% from the assumed offering price of $8.00 per ordinary share. Net tangible book value per ordinary share would increase to the benefit of present stockholders by $0.69 per share attributable to the purchase of the ordinary shares by investors in this offering.
If the maximum offering is sold, we will have 13,687,500 ordinary shares outstanding upon completion of the offering. Our post offering pro forma net tangible book value, which gives effect to receipt of the net proceeds from the offering and issuance of additional shares in the offering, but does not take into consideration any other changes in our net tangible book value after December 31, 2009, will be approximately $24,007,416 or $1.75 per ordinary share. This would result in dilution to investors in this offering of approximately $6.25 per ordinary share or approximately 78% from the assumed offering price of $8.00 per ordinary share. Net tangible book value per ordinary share would increase to the benefit of present shareholders by $0.84 per share attributable to the purchase of the ordinary shares by investors in this offering.
The following table sets forth the estimated net tangible book value per ordinary share after the offering and the dilution to persons purchasing ordinary shares based on the foregoing minimum and maximum offering assumptions.
| Minimum Offering(1) |
Maximum Offering(2) | |||||
| Assumed offering price per ordinary share |
$ | 8.00 | $ | 8.00 | ||
| Net tangible book value per ordinary share before the offering |
$ | 0.91 | $ | 0.91 | ||
| Increase per ordinary share attributable to payments by new investors |
$ | 0.69 | $ | 0.84 | ||
| Pro forma net tangible book value per ordinary share after the offering |
$ | 1.60 | $ | 1.75 | ||
| Dilution per ordinary share to new investors |
$ | 6.40 | $ | 6.25 | ||
| (1) | Assumes net proceeds of $10,440,000 from offering of 1,500,000 common shares, calculated as follows: $12,000,000 offering, less underwriting discount of $840,000, non-accountable expense allowance of $120,000 and offering expenses of $600,000. |
| (2) | Assumes net proceeds of $13,200,000 from offering of 1,875,000 ordinary shares, calculated as follows: $15,000,000 offering, less underwriting discount of $1,050,000, non-accountable expense allowance of $150,000 and offering expenses of $600,000. |
45
Table of Contents
The following charts illustrate our pro forma proportionate ownership, upon completion of the offering under alternative minimum and maximum offering assumptions, by present shareholders and investors in this offering, compared to the relative amounts paid by each. The charts reflect payment by present shareholders as of the date the consideration was received and by investors in this offering at the assumed offering price without deduction of commissions or expenses. The charts further assume no changes in net tangible book value other than those resulting from the offering and provide alternative scenarios depending on whether the Make-Good Shares are redeemed. See “Risk Factors – A redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company,” “Related Party Transactions – Make-Good Shares Subject to Redemption” and “Placement – Market and Pricing Considerations.”
Scenario 1: Pro forma presentation assuming no redemption of any Make-Good Shares
| Shares Purchased | Total Consideration | Average Price | ||||||||||
| Amount (#) | Percent (%) | Amount ($) | Percent (%) | Per Share ($) | ||||||||
| MINIMUM OFFERING |
||||||||||||
| Existing shareholders |
11,812,500 | 88.7 | % | 10,807,416 | 47.4 | % | 0.91 | |||||
| New investors |
1,500,000 | 11.3 | % | 12,000,000 | 52.6 | % | 8.00 | |||||
| Total |
13,312,500 | 100 | % | 22,807,416 | 100 | % | 1.71 | |||||
| Shares Purchased | Total Consideration | Average Price | ||||||||||
| Amount (#) | Percent (%) | Amount ($) | Percent (%) | Per Share ($) | ||||||||
| MAXIMUM OFFERING |
||||||||||||
| Existing shareholders |
11,812,500 | 86.3 | % | 10,807,416 | 41.9 | % | 0.91 | |||||
| New investors |
1,875,000 | 13.7 | % | 15,000,000 | 58.1 | % | 8.00 | |||||
| Total |
13,687,500 | 100 | % | 25,807,416 | 100 | % | 1.89 | |||||
| Scenario 2: Pro forma presentation assuming redemption of all Make-Good Shares | ||||||||||||
| Shares Purchased | Total Consideration | Average Price | ||||||||||
| Amount (#) | Percent (%) | Amount ($) | Percent (%) | Per Share ($) | ||||||||
| MINIMUM OFFERING |
||||||||||||
| Existing shareholders |
11,062,500 | 88.1 | % | 10,807,416 | 47.4 | % | 0.98 | |||||
| New investors |
1,500,000 | 11.9 | % | 12,000,000 | 52.6 | % | 8.00 | |||||
| Total |
12,562,500 | 100 | % | 22,807,416 | 100 | % | 1.82 | |||||
| Shares Purchased | Total Consideration | Average Price | ||||||||||
| Amount (#) | Percent (%) | Amount ($) | Percent (%) | Per Share ($) | ||||||||
| MAXIMUM OFFERING |
||||||||||||
| Existing shareholders |
11,062,500 | 85.5 | % | 10,807,416 | 41.9 | % | 0.98 | |||||
| New investors |
1,875,000 | 14.5 | % | 15,000,000 | 58.1 | % | 8.00 | |||||
| Total |
12,937,500 | 100 | % | 25,807,416 | 100 | % | 1.99 | |||||
46
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our audited historical consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We focus on the research, development, manufacturing, marketing and selling of modernized traditional Tibetan medicines in China. All of our current products are offered and derived from Tibetan based traditional medicines and are manufactured using plant based natural materials, particularly the herbs and minerals found in the high-latitude, low-temperature, and pollution-free environment of Qinghai-Tibet Plateau. As of December 31, 2009, we sell 5 major prescription and over-the-counter Tibetan medicine products, each has certain medicinal functions and has demonstrated safety and efficacy in accordance with the China SFDA requirements for the treatment of at least one or more therapeutic indications.
Our manufacturing facility in China is GMP certified, fully integrated with manufacturing support systems including quality assurance, quality control and regulatory compliance. We have developed our own independent quality control systems in accordance with SFDA regulations. Our quality assurance team devotes significant attention to quality control for designing, manufacturing and testing our products, and is also responsible for ensuring that we are in compliance with all applicable national and local regulations and standards, as well as our internal policies. Our senior management team is also actively involved in setting quality assurance policies and managing internal and external quality performance. These support systems enable us to maintain high standards of quality for our products and deliver reliable products to our customers on a timely basis.
In the last two years, our business has grown rapidly as a result of China’s strengthening economy, our combining promotion of Tibetan culture with educational physician conferences and seminars and the strong demand for our medicine products resulted from the increase in the number of elderly people in China.
Year Ended December 31, 2009 Compared to Year Ended December 31, 2008
Liquidity
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis. At December 31, 2009 our working capital was $7,912,642 as compared to $ 6,238,872 at December 31, 2008.
Our cash balance at December 31, 2009 totaled $4,081,752, an increase of $1,370,200 over our balance at December 31 2008. During the fiscal year of 2009, we received cash from operating activities of $10,143,153.
We have financed our operations over the two years ended December 31, 2009 primarily through cash from operating activities. Net cash provided by operating activities was approximately $10 million and 6 million in fiscal 2009 and 2008, respectively.
Other than the continued strength of China economy and the increased demand of our medicine products from the increase in the number of elderly people in China (all of which we believe may increase our liquidity if they continue), we are not aware of any trends or any demands, commitments, events or uncertainties that will result in or that are reasonably likely to result in our liquidity increasing or decreasing in any material way.
For 2010, we expect our main growth will be organic from existing operations. The demand for our products appears to be getting stronger, which we expect to generate more positive cash flow.
47
Table of Contents
Assuming we generate more positive cash flow from operation, and to the extent demand for our product increases, we may need to consider upgrading our existing production facility and purchasing additional machinery to expand our capacity. We anticipate spending approximately $2.2 million to do the upgrade and purchase. If we were to make such upgrade and purchases prior to the completion of this offering or if our cash flow were insufficient, such purchases could put pressure on our liquidity.
We are also investigating a plan to build our distributor network to a national presence. We expect that the total cost for building and recruiting distributors would be approximately $5.8 million. If this were to happen before we receive proceeds from IPO or if our cash flow were insufficient, we could face liquidity pressure.
Capital Resources
The following table provides certain selected balance sheets comparisons between years ended December 31, 2009 and 2008:
| December 31, | Increase/ |
|||||||||
| 2009 | 2008 | (Decrease) | ||||||||
| Cash |
$ | 4,081,752 | $ | 2,711,552 | $ | 1,370,200 | ||||
| Inventories |
$ | 1,305,537 | $ | 1,419,099 | $ | (113,562 | ) | |||
| Accounts receivable |
$ | 4,608,075 | $ | 2,980,698 | $ | 1,627,377 | ||||
| Other current assets |
$ | 200,836 | $ | 113,517 | $ | 87,319 | ||||
| Due from shareholder |
$ | 1,550,085 | $ | 1,357,721 | $ | 192,364 | ||||
| Total current assets |
$ | 11,746,285 | $ | 8,582,587 | $ | 3,163,698 | ||||
| Plant and equipment |
$ | 6,079,984 | $ | 6,783,108 | $ | (703,124 | ) | |||
| Intangible assets |
$ | 697,158 | $ | 878,720 | $ | (181,562 | ) | |||
| Prepaid expense |
$ | 396,906 | $ | 543,107 | $ | (146,201 | ) | |||
| Total assets |
$ | 18,920,333 | $ | 16,787,522 | $ | 2,132,811 | ||||
| Accounts payable |
$ | 2,830,032 | $ | 1,515,977 | $ | 1,314,055 | ||||
| Other payables |
$ | 1,003,611 | $ | 827,738 | $ | 175,873 | ||||
| Total current liabilities |
3,833,643 | 2,343,715 | $ | 1,489,928 | ||||||
48
Table of Contents
We maintain cash and cash equivalents in China. At December 31, 2009 and 2008, cash and cash equivalents were as follows:
| December 31, | ||||||
| Country |
2009 | 2008 | ||||
| China |
$ | 4,081,752 | $ | 2,711,552 | ||
All of our cash in deposit balances at December 31, 2009 are in the form of RMB held in bank accounts at financial institutions located in the PRC. Cash held in banks in the PRC is not insured. The value of cash on deposit in China of approximately $4.08 million at December 31, 2009 has been converted based on the exchange rate as of December 31, 2009. In 1996, the Chinese government introduced regulations, which relaxed restrictions on the conversion of the RMB; however restrictions still remain, including but not limited to restrictions on foreign invested entities. Foreign invested entities may only buy, sell or remit foreign currencies after providing valid commercial documents at only those banks authorized to conduct foreign exchanges. Furthermore, the conversion of RMB for capital account items, including direct investments and loans, is subject to PRC government approval. Chinese entities are required to establish and maintain separate foreign exchange accounts for capital account items. We cannot be certain Chinese regulatory authorities will not impose more stringent restrictions on the convertibility of the RMB, especially with respect to foreign exchange transactions. Accordingly, cash on deposit in banks in the PRC is not readily deployable by us for purposes outside of China.
Current assets as of December 31, 2009 totaled $11,746,285, an increase of 37% compared to December 31, 2008. Current liabilities as of December 31, 2009 totaled $ 3,833,643, reflecting an increase of 64% from our December 31, 2008 balance.
Inventories as of December 31, 2009 were $1,305,537, a decrease of $113,562 compared to December 31, 2008. This decrease is due primarily to the better control of inventories management.
At December 31, 2009, we have no commitments for capital expenditures. While we do not have any agreements, understandings or commitments at this time for such activities, we may upgrade and purchase machinery for approximately $2.2 million, and build or recruit distributors for approximately $5.8 million. If we undertake any of these activities, we expect to do so without debt financing. Instead, we plan either to use our cash flow resources or proceeds of this offering to make such purchase. As such, any such expenditure would affect our available cash resources but would not affect any debt arrangements.
Principal Factors Affecting our Results of Operations
Revenues
We generate revenue mainly from the sales of our products. Our product revenues represent our total revenues from the sales of our products, less value-added taxes, or VAT.
Our principle products include 25 Ingredients Mandrake Pill and 15 Ingredients Gentiana Pill.
25 Ingredients Mandrake Pill is a product approved for the treatment of various women’s health indications including irregular menstruation, anemia, gynecological rheumatism, endometritis and pelvic inflammation.
15 Ingredients Gentiana Pill, is a product indicated to regulate lung, alleviate coughing and remove phlegm, and for the treatment of bronchus related coughing, asthma, and hoarseness.
49
Table of Contents
The following table sets out a breakdown of our revenues for the two products, and each item expressed as a percentage of our product revenues, for the periods indicated:
| For the year ended December 31, | ||||||||||||
| 2009 | 2008 | |||||||||||
| Sales amount |
%
of product revenues |
Sales amount |
%
of product revenues |
|||||||||
| 25 Ingredients Mandrake Pill |
$ | 11,185,718 | 49 | % | $ | 7,789,246 | 50 | % | ||||
| 15 Ingredients Gentiana Pill |
$ | 4,941,706 | 21 | % | $ | 3,120,079 | 20 | % | ||||
| $ | 16,127,424 | 70 | % | $ | 10,909,325 | 70 | % | |||||
Factors Affecting Revenues
The following factors affect the revenues we derive from our operations. For other factors affecting our revenues, see “Risk Factors—Risks Related to Our Business.”
Industry-wide Factors. We have benefited significantly from the overall economic development in China in recent years and the increase in the number of elderly people in China, which together have resulted in increased expenditures on medicine in China, including traditional Tibetan medicine. The Chinese have long perceived and accepted traditional Tibetan medicine as a safe and effective solution to diseases, having the advantage of causing fewer side effects than western medicine, due to the natural ingredients used. With the improvement of living standards in China, the health care industry has grown substantially in recent years, which also stimulated the domestic demands for Tibetan medicine. As China’s elder population grows, as well as the increased awareness and perception of the safety of traditional Tibetan medicine products, the market demand for traditional Tibetan medicine products will keep growing.
Government accreditation in our industry. The market for Tibetan pharmaceutical products in China is only a very small portion of the overall pharmaceutical market in China. The annual production of Tibetan medicine amounts to approximately 1500 tons with 293 products marketed. However, the growth rate of the market for Tibetan pharmaceuticals is substantial, estimated at 50% a year by China Medicine Source Net (CMSN). One factor spurring growth has been the willingness of Chinese regulators to accredit Tibetan medicine. As of December 31, 2009, 14 Tibetan medicine products have been listed on the Protected Chinese Traditional Medicine List; 24 Tibetan medicine products have been included in the National Medicine Index; and 218 Tibetan medicine products have been certified by the SFDA.
Consolidation and expansion. In recent years, the Tibetan Medicine industry in China has made certain achievements in raw material cultivation, extraction of active ingredients and productions of Tibetan Medicine. The modernized extraction technology is widely applied. However, the equipments and production technology of most Tibetan medicine manufacturers are out dated and many of them are small on scale. Consolidation in the Tibetan medicine industry is likely to increase as inefficient and smaller operators are likely to be acquired or consolidated into larger manufacturer’s operations. As market demand grows stronger for our products, we believe that we must continue to expand our production capacity to seize additional market share. If we fail to make acquisitions or expand our production capacity, our revenue growth could slow.
Competition. We operate in a highly competitive marketplace. We compete our products on the basis of brand recognition, quality, price, product availability, and security of supply, product development and customer service. If any of our competitors come out with a product, not only has strong marketing campaign and lowered price, but also has better perceived quality and effectiveness, it could put great pressure on our products and result in a decline in our sales volume and earnings. Competition could cause us to lose market share and certain lines of business, or increase expenditures or reduce pricing, each of which would have an adverse effect on our results of operations, cash flows and financial condition.
Negative Publicity. Adverse publicity associated with our company or our products could have a material adverse effect on our results of operations. We are highly dependent upon consumer perceptions of our company
50
Table of Contents
and the safety and quality of our products. We could be adversely affected if the “Shangri-La Tibetan Pharmaceuticals” brand, the “Xiangbala” trademark or any of our other trademarks is subject to negative publicity. We could also be adversely affected if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to consumers. Because of our dependence upon consumer perceptions, any negative publicity associated with illness or other adverse effects resulting from consumers’ use or misuse of our products, or any similar products distributed by other companies, could also have a material adverse impact on our results of operations. In addition, the existence of counterfeit pharmaceutical products in the PRC pharmaceutical retail market may damage our brand and reputation and subject us to negative publicity and other administrative headaches and expenses. It could have a material adverse effect on our business, financial condition, results of operations and prospects.
Costs and Expenses
We primarily incur the following costs and expenses:
Costs of goods sold. We incur a number of costs that factor in to costs of goods sold.
| • | raw materials, which are primarily comprised of costs of the necessary active ingredients and supporting ingredients of pharmaceuticals we manufacture; |
| • | labor, including salaries and benefits for employees directly involved in manufacturing activities; |
| • | packaging costs, including cost of packaging materials for our products; |
| • | depreciation and amortization of manufacturing equipment and facilities. Due to our capacity expansion, depreciation and amortization expenses, measured in absolute terms, have increased significantly. We expect that depreciation and amortization expenses in absolute terms will continue to increase in the near future as we continue to grow; and |
| • | overhead, including utility, parts for and maintenance of production equipment, testing, and other expenses related to the manufacturing of our products. |
Selling, general and administrative expenses. Selling, general and administrative expenses consist primarily of compensation expense for our corporate staff and personnel supporting our corporate, professional fees (including consulting, audit and legal fees), travel and entertainment expenses, contractual performance obligations and office administrative and related expenses.
Factors Affecting Cost and Expenses
Supplies and commodity prices. The largest component of our cost relates to the price of raw materials. To the extent the prices of these materials vary, our cost of goods will fluctuate. For this reason, we may be affected by droughts, floods, crop diseases and the like, which tend to make raw material scarcer and thus expensive.
Transition to public company. Once we complete this offering, we expect that our administrative costs will increase significantly, as we need to comply with detailed reporting requirements.
Number of customers. The more customers we have, the greater we expect our selling expenses, travel expenses and the like will be. At present, we are able to sell substantially all of our products to a relatively small number of customers. We believe this concentration of customers has allowed us to focus our marketing and selling efforts in a relatively narrow area. As we expand and build our national distribution network, we expect to see a rise in the selling expense.
51
Table of Contents
Advertising expense. As we started rolling out our advertising campaign to build more brand awareness and consumers recognition, we’ll see increases in our advertising expenses related to our brand image promotion and recognition.
Results of Operations
| For the year ended December 31, | ||||||||||||
| 2009 | 2008 | |||||||||||
| Amount | % | Amount | % | |||||||||
| Revenues |
$ | 23,008,031 | — | $ | 15,580,269 | — | ||||||
| Cost of goods sold |
$ | 11,303,118 | 49 | % | $ | 7,857,884 | 50 | % | ||||
| Gross profit |
$ | 11,704,913 | 51 | % | $ | 7,722,385 | 50 | % | ||||
| Total operating expenses |
$ | 2,289,036 | 10 | % | $ | 1,630,425 | 11 | % | ||||
| Operating income |
$ | 9,415,877 | 41 | % | $ | 6,091,960 | 39 | % | ||||
Our revenues increased from $15.58 million in 2008 to $23.01 million in 2009, or 48% increase. This is primarily due to continuous growth from the Company’s existing five principle products. As of December 31, 2009, our biggest product revenue contributor, 25 Ingredients Mandrake Pill, has increased sales 44% from $7.79 million in 2008 to $11.19 million in 2009. Expanding our existing distributor network also drove our revenue performance during 2009.
Our gross profit margins stayed relatively the same, from 50% in fiscal year 2008 to 51% in the same period of 2009. In 2009 we were able to expand our sales and controlled the cost, and successfully maintained the gross profit margins.
Selling expense increased by $206,026, or 26%, from $782,359 in 2008 to $ 988,385 in 2009. The increase in selling expense resulted from an increase in promotional efforts and media advertisement in 2009 to promote the Company’s 25 Ingredients Mandrake Pill and 15 Ingredients Gentiana Pill.
Research and development costs increased by $115,736, or 23%, from $502,827 in 2008 to $618,562 in 2009. This reflected our increased spending on research and development of new products.
The other general and administrative expenses increased by $336,850 in the year ended December 31, 2009 compared to the year ended December 31, 2008 primarily as a result of business expansion. Payroll expense was majority of the increase in general and administrative expenses.
Net interest expense was $170,900 in 2009, compared to net interest expense of $158,477 in 2008.
Our net income for the years ended December 31, 2009 and 2008 were $9,244,978 and $5,933,483, respectively. The increase in net income is primarily a result of higher sales, efficient control, management of production costs and administrative expenses.
We realized significant growth in our business due to the strengthening of our marketing efforts. We host in-person product presentations, conference and seminars for physicians, other healthcare professionals and research scholars to promote and generate awareness of our pharmaceutical products. For our OTC pharmaceutical products, we also carry out consumer advertising and educational campaigns. As the pharmaceutical market in China continues to grow, we plan on hiring additional personnel for the sale, marketing and distribution of our pharmaceutical products to increase the market recognition of our products and awareness of our brand name.
52
Table of Contents
Consolidated Statement of Cash Flows
In the year ended December 31, 2009, our net increase in cash from operations totaled $1,370,200 and was comprised of $10,143,153 provided by operating activities, $8,785,288 used in financing activities, and the effect of prevailing exchange rates provided on our cash position of $12,334.
In the year ended December 31, 2008, our net increase in cash from operations totaled $1,295,797 and consisted of $6,049,836 provided by operating activities, $ 642,112 used in investing activities, $4,244,182 used in financing activities, and the effect of prevailing exchange rates on our cash position of $132,254.
Cash Provided by Operating Activities
Net cash provided by operating activities in the year ended December 31, 2009 totaled $10,143,153. The activities were mainly comprised of our net income of $9,244,978, an increase in accounts payable of $1,306,164, an increase in accrued expenses and other payables of $208,525, a decrease in inventories of $ 118,509 and prepaid expenses of $ 146,200, These increases were partially offset by an increase in accounts receivable of $ 1,613,598, and amount due from shareholder of $187,085. Non-cash transactions comprised of the followings: (i) $703,124 in depreciation; and (ii) $181,563 in amortization, and (iii) $157,500 in stock-based compensation.
In the year ended December 31, 2008, net cash provided by operations totaled $6,049,836. The activities mainly consisted of our net income of $ 5,933,483, an increase in accounts payable of $573,340, a decrease in advances to suppliers of $199,821 and prepaid expenses of $141,259. These increases were partially offset by an increase in accounts receivable of $ 961,302, inventories of $ 304,580 and amount due from shareholder of $ 137,839, and a decrease in accrued expenses and other payables of $199,798. Non-cash transactions comprised of (i) $611,935 in depreciation and (ii) $175,426 in amortization.
Cash Used in Investing Activities
No cash used in investing activities for the year ended December 31, 2009.
In the year ended December 31, 2008, our cash used in investing activities totaled $642,112. The activities were solely represented our cash payment in connection with the acquisition of plant and equipment.
Cash Used in Financing Activities
For the year ended December 31, 2009, net cash used in financing activities was $8,785,288. The activities were solely represented dividends paid to the shareholders made by YSTP.
For the year ended December 31, 2008, net cash used in financing activities was $4,244,182. The activities were solely represented dividends paid to the shareholders made by YSTP.
Off Balance Sheet Items
Under SEC regulations, we are required to disclose our off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, such as changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors. An off-balance sheet arrangement means a transaction, agreement or contractual arrangement to which any entity that is not consolidated with us is a party, under which we have:
| • | Any obligation under certain guarantee contracts, |
| • | Any retained or contingent interest in assets transferred to an unconsolidated entity or similar arrangement that serves as credit, liquidity or market risk support to that entity for such assets, |
| • | Any obligation under a contract that would be accounted for as a derivative instrument, except that it is both indexed to our stock and classified in stockholder’s equity in our statement of financial position, and |
53
Table of Contents
| • | Any obligation arising out of a material variable interest held by us in an unconsolidated entity that provides financing, liquidity, market risk or credit risk support to us, or engages in leasing, hedging or research and development services with us. |
We do not have any off-balance sheet arrangements that we are required to disclose pursuant to these regulations. In the ordinary course of business, we enter into operating lease commitments, purchase commitments and other contractual obligations. These transactions are recognized in our financial statements in accordance with generally accepted accounting principles in the United States.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations are based upon our audited consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these audited consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. On an on-going basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
A summary of significant accounting policies is included in this item are discussed in further detail in the notes to the audited consolidated financial statements appearing elsewhere in this prospectus. Management believes that the application of these policies on a consistent basis enables us to provide useful and reliable financial information about our operating results and financial condition.
Revenue Recognition
We follow the guidance of ASC 605, “Revenue Recognition,” and the Securities and Exchange Commission’s Staff Accounting Bulletin (“SAB”) No. 104 and SAB Topic 13 for revenue recognition. In general, revenue from the sale of pharmaceutical products is recognized when the products are delivered to and received by the customers, collectability is reasonably assured and the prices are fixed and determinable.
We have distributor arrangements with certain parties for sale of its pharmaceutical products. The distributor agreements do not provide chargeback, price protection, or stock rotation rights. Accordingly, we record the revenue when products are delivered to and received by the distributors.
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results when ultimately realized could differ from those estimates. Significant estimates in the years ended December 31, 2009 and 2008 include the useful life of plant and equipment, intangible assets
Fair Value of Financial Instruments
We follow ASC 820, “Fair Value Measurements and Disclosures,” (SFAS 157), as amended by Financial Accounting Standards Board (FASB) Financial Staff Position (FSP) No. 157-2, on the effective date of FASB Statement No. 157. Those provisions relate to our financial assets and liabilities carried at fair value and our fair value disclosures related to financial assets and liabilities. ASC 820 (SFAS 157) defines fair value, expands related disclosure requirements and specifies a hierarchy of valuation techniques based on the nature of the inputs used to
54
Table of Contents
develop the fair value measures. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. There are three levels of inputs to fair value measurements - Level 1, meaning the use of quoted prices for identical instruments in active markets; Level 2, meaning the use of quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active or are directly or indirectly observable; and Level 3, meaning the use of unobservable inputs. Observable market data should be used when available. We did not have financial instruments during the years ended December 31, 2009 and 2008.
Comprehensive Income
We follow ASC 205, “Presentation of Financial Statements,” and ASC 220 (SFAS 130), “Reporting Comprehensive Income,” to recognize the elements of comprehensive income. Comprehensive income is comprised of net income and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. Comprehensive income for 2009 and 2008 included net income.
Impairment of Long-lived Assets
In accordance with ASC 360-10 (SFAS 144), “Impairment or Disposal of Long-Lived Assets”, we periodically review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. We recognize an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the estimated fair value and the book value of the underlying asset. We did not record any impairment charges during the years ended December 31, 2009 and 2008, respectively.
Newly Adopted Accounting Pronouncements
Effective January 1, 2009, the Group adopted an authoritative guidance issued by the Financial Accounting Standards Board (“FASB”) on business combinations. The guidance retains the fundamental requirements that the acquisition method of accounting (previously referred to as the purchase method of accounting) be used for all business combinations, but requires a number of changes, including changes in the way assets and liabilities are recognized and measured as a result of business combinations. It also requires the capitalization of in-process research and development at fair value and requires the expensing of acquisition-related costs as incurred. The Group has applied this guidance to business combinations completed since January 1, 2009.
Effective January 1, 2009, the Group adopted an authoritative pronouncement issued by the FASB regarding interim disclosures about fair value of financial instruments. The pronouncement requires disclosures about fair value of financial instruments for interim reporting periods as well as in annual financial statements of publicly traded companies. The pronouncement also requires those disclosures in summarized financial information at interim reporting periods. The adoption of this pronouncement did not have any significant impact on the Group’s financial condition or results of operations.
Effective January 1, 2009, the Group adopted an authoritative pronouncement issued by the FASB regarding recognition and presentation of other-than-temporary impairments. The pronouncement amends the other- than-temporary impairment pronouncement in US GAAP for debt securities to make the pronouncement more operational, and improves the presentation and disclosure of other-than-temporary impairments on debt and equity securities in financial statements. The adoption of this pronouncement did not have any significant impact on the Group’s financial condition or results of operations. Effective April 1, 2009, the Company adopted an authoritative pronouncement issued by the FASB regarding determining fair value when the volume and level of activity for the asset or liability have significantly decreased and identifying transactions that are not orderly. The pronouncement provides clarification on estimating fair value when the volume and level of activity for the asset or liability have significantly decreased and on identifying circumstances that indicate a transaction that is not orderly. The pronouncement emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date under the then current market conditions. The adoption of this pronouncement did not have any significant impact on the Group’s financial condition or results of operations.
55
Table of Contents
Effective July 1, 2009, the Group adopted a new Accounting Standards Codification (the “ASC”) as issued by the FASB. The ASC has become the source of the authoritative US GAAP recognized by the FASB to be applied by nongovernmental entities and provides that all such pronouncement carries an equal level of authority. The ASC is not intended to change or alter existing GAAP. The ASC is effective for interim and annual periods ending after September 15, 2009. The adoption of the ASC did not have any significant impact on the Company’s financial condition or results of operations.
56
Table of Contents
Our Company
Our company is a specialty pharmaceutical company focusing on the research, development, manufacturing and marketing of modernized traditional Tibetan medicines. We develop products in China for promoting health in human respiratory, digestive, urinary and reproductive systems. Our five SFDA-approved modernized traditional Tibetan medicines in distribution are designed to address large market opportunities. Our product development pipeline includes potential expanded uses of our existing products for additional medical indications and a number of new product candidates that are intended to address significant medical health needs in China.
We currently sell both prescription and over-the-counter products. We presently sell five products. We also have developed one additional pharmaceutical product that has received Chinese-government approval but that has not yet been introduced into the market. Still other products are in various stages of development, clinical trials, and review for government approval. We sell our products principally to distributors in China, who in turn resell the products principally to hospitals, hospital pharmacies, and retail pharmacies.
Our products are sold throughout China. A majority of sales are concentrated in southern provinces (most notably Yunnan Province, Guangdong Province, and Zhejiang Province. To date, we have not sold our products outside of China.
China’s Economic Development
China’s economy continues the rapid growth it has shown over recent decades. China’s gross domestic product grew from RMB9921.5 billion in 2000 to RMB33535.3 billion in 2009. Real GDP annual growth rates during that period ranged between 8.3 percent and 13 percent. While Chine suffered some consequences of the recent worldwide economic downturn, it has suffered less and recovered more quickly than most countries. Real GDP growth fell from an annual rate of 13 percent in 2007 to 9.0 percent in 2008 and 8.7 percent in 2009. A recent United Nations report estimates that the China economy will grow by about 8.8 percent in 2010. Another report stated that China’s real GDP grew at the end of the first calendar quarter of 2010 rose 11.9 percent from a year earlier. It also has been forecast that China’s economy may surpass Japan’s as the second largest in the world in 2010.
The rapid and sustained growth of the Chinese economy has resulted in steady growth in consumer wealth and spending. Consumer wealth is also broadening throughout the population: a recent Credit Suisse survey found that, between 2004 to 2009, the average household income of the 40-60 percent income group grew by 98 percent, and the income of the bottom 20 percent households also rose by 50 percent (the latter being attributed in part to the indirect benefits of improved farm profitability). The report anticipates that China’s share of global consumption will grow from 5.2 percent (US$1.72 trillion) in 2009 to 23.1 percent (US$15.94 trillion) in 2020, overtaking the US as the largest consumer market in the world.
China’s Healthcare Industry – Generally
From 1940 through the 1950s, China’s government developed a healthcare system that would address the main health considerations of the day – improved sanitation, improved diet and disease prevention. The healthcare system provided universal access to all Chinese citizens. In China’s rural areas local governments generally financed the healthcare system. The results of the government’s focus upon healthcare resulted in a dramatic long-term improvement of the health of China’s citizens. For example, in 1949, the life expectancy of a Chinese citizen was 35 years; by 2009, the life expectancy of a Chinese citizen had risen to over 73 years, more than six years greater than the worldwide average life expectancy.
In the 1980s, the Chinese government, led by Deng Xiaoping, introduced socialist market reforms designed to increase China’s presence in the international community while increasing the standard of living in China. However, these reforms also pushed an ever-increasing portion of healthcare costs upon individual citizens.
57
Table of Contents
Consequently, an ever-widening gap arose between the provision of healthcare services to citizens in wealthy, urban areas and those in poor, rural areas. In 1975, over 85% of the rural population was covered by government-provided healthcare; in 1997, that proportion had decreased to 10% of the rural population.
In April 2009, the Chinese government implemented large-scale healthcare reform. The State Council allocated $123 billion as part of its New Medical Reform Plan. In addition, the plan includes significant improvements to health care facilities and expansion of China’s health related infrastructure. A recent OECD study indicates that spending hikes and regulatory changes in China have contributed to significant growth in health-care spending in the country, and that such spending in 2010 is likely to increase another 8.7 percent, with about 4.5 percent of gross domestic product (GDP) allocated to healthcare, half of which comes from the private sector. The consulting firm Scientia Advisor projects that healthcare spending in China will reach $600B by 2015, a threefold increase over 2000 expenditures.
The Chinese government aims to improve the urban healthcare system by rebuilding and restructuring approximately 3,700 existing urban community health centers and 11,000 community health clinics. The plan will also accommodate the development of approximately 2,400 new urban health centers. In effect, the plan de-emphasizes the prevalence of large, magnet facilities in favor of smaller, more accessible clinics.
In addition, the plan is designed to improve dramatically medical services available for the 800 million rural poor in China. Through the plan, the Chinese government contemplates the development of clinics in every village and a hospital in every county in China by the end of 2011. If successfully implemented, the plan would result in at least 2,000 new county hospitals and 29,000 village clinics.
The rapid increase in the per capita disposable income of Chinese urban residents and the increase in the number of elderly people in China in recent years have resulted in increasing spending on prescription and OTC medicines, including traditional Chinese medicine.
China’s Market for Pharmaceutical Products
The pharmaceutical market in China has grown rapidly in recent years. According to Business Monitor International, the total sales of medicines in China, including prescription and over-the-counter medicines, was US$33.9 billion (approximately RMB257 billion) in 2007, representing an increase of 25.6% from 2006 and a 2003-2007 CAGR of 17.8%. Business Monitor International estimates that the Chinese market became the eighth largest pharmaceutical market in the world in 2007 from ninth in 2006. The following chart sets forth the trend of PRC expenditures for prescription and over-the-counter medicines in the periods indicated:
Sales of prescription medicines are the principal component of pharmaceutical expenditures in China and are mostly made in hospitals. According to Business Monitor International, sales of prescription medicines grew from US$14.1 billion in 2003 to US$26.4 billion in 2007, representing a CAGR of 17.0%. In 2007, total sales of prescription medicines represented 77.9% of China’s total expenditures on medicine sales. The remaining expenditures were spent on over-the-counter medicines. In 2007, sales of over-the-counter medicines amounted to US$7.5 billion, representing a CAGR of 21.0% from 2003 to 2007. In addition to the primary growth drivers of healthcare spending in China described above, another factor expected to increase the amount of healthcare spending in China is the growing trend of PRC consumers to purchase non-prescription, over-the-counter medicines in non-hospital retail pharmacies.
58
Table of Contents
Data source: Business Monitor International
The Chinese pharmaceuticals market is fragmented but rapidly growing. According to Business Monitor International, the total sales of medicine in China grew at a CAGR of approximately 17.8% from 2003 to 2007, which we believe was driven by favorable socio-economic factors and strong government support. Furthermore, the Chinese government has recently announced a reform plan to spend RMB850 billion on health care, which is in addition to the regular healthcare budget, from 2009 to 2011 in order to increase the availability of healthcare, basic medicines and health insurance coverage in China. As a comparison, in 2007, the total healthcare expenditure in China was approximately RMB1.1 trillion, of which approximately RMB230 billion was government spending, according to the Ministry of Health. The healthcare reform plan is expected to accelerate growth in the Chinese pharmaceutical industry.
Pharmaceutical sales and usage play a larger role in the Chinese health-care market than in the health-care sectors of many other countries. With a small share of the population presently enjoying insurance coverage, a still-developing state health-care system, and a massive rural and relatively poor population, individuals (who generally must pay for care out of pocket) and health-care providers rely heavily on medicines, both traditional and modern. One report states that the share of health-care spending devoted to pharmaceuticals in China is three times that of the average for countries in the developed word.
In addition to the country’s general economic growth and the government’s emphasis on improving health care, we believe that China’s aging population is a key contributor to the increased expenditures on medicines because elderly people on average spend more on healthcare than younger people. In China, each urban resident over age 60 on average spent approximately RMB984.0 (US$126.1) on medicines in 2000, five times the average spending by an urban resident below age 60 in the same year, according to the China Industry Development Report for the Pharmaceutical Industry 2004 published by the China National Information Center. The portion of the Chinese population aged 60 and above has increased in both absolute numbers and as a percentage of the total population, and this trend is likely to continue in the next few decades. According to two surveys conducted by the National Bureau of Statistics in 2000 and 2005, the number of people in China aged 60 and above was approximately 130.0 million in 2000, or 10.5% of China’s entire population, and this number increased to approximately 144.1 million by 2005, representing 11.0% of the population.
The significant growth of China’s population aged 60 or above is expected to drive demand for healthcare in China. According to the PRC National Bureau of Statistics, the proportion of the population aged 60 or above in
59
Table of Contents
China has increased from 11.9% in 2003, or approximately 150.0 million people to 13.6%, or approximately 162.2 million people in 2007. Rising life expectancy is also expected to contribute to the growth of China’s aging population, both as an absolute number and as a percentage of the total population. We believe that the aging population in China, which historically spends the most on healthcare, will drive the growth of the PRC healthcare industry. The prevalence of chronic health problems, such as arthritis, cardiovascular diseases and cancer, is expected to increase with the growth of China’s population aged 60 or above. In addition, as living standards continue to improve and health consciousness grows in China, many lifestyle-related diseases are also increasing and becoming more widespread. For example, Business Monitor International estimates that sales of prescription cardiovascular medicines increased by 87% from US$2,765 million in 2003 to US$5,177 million in 2007, primarily as a result of the rising prevalence of heart disease in an aging population and increasingly unhealthy lifestyles in the population at large.
National Medical Insurance Program
The National Medical Insurance Program (“NMIP”), introduced in 1999, is the largest medical insurance program in China. As of the end of 2006, the number of participants enrolled in this program was 157.4 million, according to a statement made by the MLSS on January 18, 2007. According to a February 21, 2006 statement by the MLSS, the Chinese government intends to expand the program enrollment to 300 million of China’s urban population by the end of 2010. We believe that only a small percentage of the Chinese population can afford commercial insurance plans and that a majority of the Chinese population has no medical insurance coverage.
The national medicine catalog of the NMIP provides guidance on which prescription and OTC medicines are included in the program and to what extent the purchases of these medicines are reimbursable. At present, none of our commercialized products is eligible for NMIP reimbursement, and we do not anticipate seeking inclusion in the NMIP program for our medicines. We believe our medicines are better suited to sale at modest prices and in large volume, and that an ample market exists for them among both the insured and uninsured.
In September 2008, the State Council published a draft plan to ease the difficulties and minimize the costs for PRC citizens to obtain proper healthcare treatment. On 17 March 2009, the PRC Government issued the Opinion on Deepening the Healthcare System Reform (the “Opinion”). The State Council subsequently released the Notice on Important Implementing Plans for the Healthcare System Reform 2009-2011 (the “Implementing Plan”). The goal of the healthcare reform plan is to establish a basic, universal healthcare framework to provide Chinese citizens with safe, efficient, convenient and affordable healthcare. The Opinion calls for healthcare reform to be carried out in two steps:
| • | Step One, which will be completed by 2011, aims to increase the accessibility while reducing the cost of healthcare. During this phase, the PRC Government will build up a network of basic healthcare facilities, expand the coverage of the public medical insurance system to cover 90% or more the population, as well as reform the drug supply and public hospital system. |
| • | Step Two, which will take place between 2011 and 2020, envisions the establishment of a universal healthcare system. The entire population should be covered by public medical insurance; drugs and medical services should be accessible and affordable to citizens in all public healthcare facilities. |
While the PRC Government has neither provided a concrete timetable nor steps to implement certain tasks, such as the public hospital reform, it has released execution guidance for other tasks. Most notably, the PRC Government has announced it will spend an additional RMB850 billion from 2009 to 2011 on the healthcare industry. A significant portion will be expended to establish a basic healthcare medical insurance regime, which aims to cover over 90% of the national population by 2011, mainly through the Urban Worker Program, Urban Resident Program and the New Rural Insurance Scheme. The PRC Government further announced that the annual subsidy for each participant will be increased from RMB40 to RMB120 for Urban Resident Program participants, and from RMB80 to RMB120 for New Rural Insurance Scheme participants, starting from 2010. The reform plan will also raise the cap on claim payments from four times the local average annual income to six times such income. Another significant part of the spending plan focuses on healthcare facilities. The PRC Government plans to build 29,000 rural clinics in 2009. In the next three years, it plans to build an additional 5,000 rural clinics, 2,000 county-level hospitals and 2,400 urban community clinics in under-developed areas. This substantial increase in healthcare spending is expected to expedite the growth of the healthcare industry in China.
60
Table of Contents
In addition, the PRC Government has actively promoted the implementation of the New Rural Cooperative Medical Insurance Scheme (“New Rural Insurance Scheme”), which seeks to provide healthcare services to the vast rural areas of China. The program extends to cover approximately 2,729 counties in the PRC, which account for 95.4% of the total number of counties in the PRC. In addition, the program covers approximately 814 million rural residents, which accounts for approximately 91.5% of the total population engaged in the agricultural industry in China as of December 31, 2008.
Prescription Medicines and Hospitals
Most people in China seek both in-patient and out-patient medical treatments at state-owned hospitals, where doctors may only prescribe medicines that are listed on the hospital’s formulary. Hospital administrators generally decide whether to include a particular medicine on their formulary based upon a number of factors, including doctors’ interest in prescribing the medicine, the cost of the medicine, the perceived efficacy of the medicine and the hospital’s budget. Unlike in the United States, where patients typically fill their prescriptions at pharmacies unaffiliated with hospitals, out-patients in China typically fill their prescriptions at hospital pharmacies.
Substantially all hospitals in China are owned and operated by the government. State-owned hospitals generally have effective monopolies in their respective geographic areas, enabling them to use their market power to obtain prescription medicines from pharmaceutical companies at lower prices.
OTC Medicines and Retail Pharmacies
While out-patients in China generally fill their prescriptions at hospital pharmacies, they primarily purchase OTC medicines from retail pharmacies. To the extent that a medical condition can be treated with an OTC medicine, many Chinese people choose to purchase an OTC medicine instead of seeing a doctor in a hospital for a prescription medicine.
The retail pharmacy sector in China is highly fragmented. Retail pharmacies in China include pharmacy chain stores, individual stores, retail chain stores with OTC counters, and OTC counters in supermarkets. While they are expanding quickly, neither pharmacy chain stores nor retail chain stores with OTC counters have developed a nationwide presence in China. As a result, retail pharmacies tend to have less bargaining power than hospitals in procuring medicines from pharmaceutical companies.
A small portion of retail pharmacies in China is authorized for participation in the NMIP. A program participant may be reimbursed for the cost of a medicine included in the provincial medicine catalog only if he or she purchases that medicine from an authorized retail pharmacy. We refer to these pharmacies as authorized pharmacies.
In 2004, the Chinese government authorities began to enforce the regulation prohibiting advertisement of prescription medicines through mass media. However, OTC medicine can be advertised in the mass media.
We believe that Chinese consumers purchase OTC medicines based upon brand name recognition and price. Consumers gain familiarity with an OTC medicine through advertising, word-of-mouth and recommendations by pharmacy salespeople.
Traditional Chinese Medicine
We believe that TCM is and will remain mainstream medicine in China. We also believe that a majority of Chinese consumers gives equal consideration to Western medicine and traditional Chinese medicine in choosing a medicine. According to the China Statistical Yearbook 2006-2008, traditional Chinese medicine accounted for approximately 21% of all medicines sold in China, based upon the sales revenues for each year between 2005 and 2007.
61
Table of Contents
Traditional Chinese medicine has been widely used in China for thousands of years and is therefore deeply ingrained in Chinese culture. The theory underlying traditional Chinese medicine is that the human body is a dynamic energy system, or Qi, that needs to harmonize and balance the different energies within it. Under this theory, a person becomes ill because his or her body experiences disharmony or an imbalance of energy. Traditional Chinese medicine seeks to restore harmony and the balance of energy within the patient’s body, thereby preventing, mitigating or curing diseases.
Historically, traditional Chinese medicine consisted primarily of mixtures of dried herbs and, in some cases, animal parts and minerals. In recent decades, pharmaceutical companies in China have applied modern production technologies to extract active ingredients out of the mixtures and formulate the extracts into a variety of dosage forms such as tablets, capsules and granules, which we refer to as modernized traditional Chinese medicines. These modernized formulations offer patients convenient forms of traditional Chinese medicine and also substantially improve their quality, consistency and dosage precision.
In general, traditional Chinese medicine has long been perceived by many Chinese to be safe and efficacious, while causing fewer side effects than Western medicine. Regulations regarding the safety and efficacy of traditional Chinese medicine in China are administered by the SFDA, the same regulatory agency responsible for oversight of Western medicine.
In 2008, 2,688 out of a total of 19,712 Chinese hospitals were designated as traditional Chinese medicine hospitals, according to the Chinese Ministry of Health Statistical Yearbook 2009. In addition to these traditional Chinese medicine hospitals, a significant majority of hospitals in China, including Western medicine hospitals, has a department dedicated to traditional Chinese medicine, and doctors with Western medical training in other departments of the hospital can also prescribe traditional Chinese medicine to their patients. By the end of 2008, China had 23 government medical schools specializing in traditional Chinese medicine education and practice. Western medical schools in China also offer traditional Chinese medicine curricula to their students.
The Chinese government is committed to supporting and promoting the development of traditional Chinese medicine. In the Chinese pharmaceutical industry five-year plan released in June 2006 by the National Development and Reform Commission, or the NDRC, the NDRC identified traditional Chinese medicine, particularly traditional Chinese medicines used for the treatment of diseases prevalent among middle-aged and elderly people, as a priority area that will receive governmental support. The State Administration of Traditional Chinese Medicine, a national government agency, formulates traditional Chinese medicine industry policies for the development of traditional Chinese medicine and provides research grants for traditional Chinese medicine research and development.
Traditional Tibetan Medicine
Tibetan medicine is classified as one type of Chinese traditional medicine. The Chinese have long perceived and accepted traditional Tibetan medicine as a safe and effective solution to diseases, having the advantage of causing fewer side effects than Western medicine, due to the natural ingredients used.
Tibetan medicine is a centuries-old medical system that employs a complex approach to diagnosis, incorporating techniques such as pulse analysis and urinalysis, and utilizes behavior and dietary modification, medicines composed of natural materials (e.g., herbs and minerals) and physical therapies (e.g. Tibetan acupuncture, moxabustion, etc.) to treat illness.
Traditional Tibetan medicine has a long history. It is a body of medicine that has developed over thousands years, founded on theory. Tibetan medicine is rooted in the traditional Tibetan culture, and is closely bounded with the local religion, philosophy, astronomy, climate, folk-custom, etc. Its theory, thinking mode (way of thinking), technical means, and medical ethic reflects Tibetan traditional culture. Tibetan Medicine is formed through years of clinical practice and summarization on the basis of assimilating medicine of other nationalities around. It bases on the “theory of three categories of disease cause” and forms versatile diagnosis and treatment systems with especially rich clinical experience of treating ordinary and frequent occurring illness. The main documentations of Tibetan medicine are “Four Volume Medicine Tantra”, “King Moon Diagnosis”, “Crystal Beads Herbs”, etc. The knowledge system of Tibetan Medicine and the splendid traditional culture of the Tibetan are the principal resources of the exploitation of Tibetan medicine and afford the theoretical base and cultural support for the development of Tibetan medicine.
62
Table of Contents
The vast Qinghai-Tibet Plateau area and the complicated and varied nature conditions make it possible for versatile plant, animal resources and the unique resource advantage for cultivation and development of Tibetan Medicine.
The market for Tibetan pharmaceuticals in China is a small portion of the overall medical market in China. The annual production of Tibetan medicine amounts to approximately 1500 tons with more than 293 products marketed.
One factor spurring growth has been the willingness of Chinese regulators to accredit products based on Tibetan medicine. At the present time, this accreditation includes:
| • | 14 products that have been listed on the Protected Chinese Traditional Medicine List; |
| • | 24 products that have been included in the National Medicine Index; |
| • | 218 products that have been certified by the SFDA; and |
| • | 20+ products that have registered trademarks in China. |
There are over one hundred producers of traditional Tibetan medicine in China. Many are quite small, employ only traditional manufacturing techniques, and engage in little or no research and development. Roughly 40 companies have received some level of GMP certification. According to China Medicine Source Net (“CMSN”), in 2006, the 17 GMP-certified Tibetan medicine manufacturers in Tibet Autonomous Region had a combined annual industrial output of RMB623 million or about $91,018,871. Overall, China’s Tibetan medicine industry was about RMB1 billion in size in 2006, about 0.5% of China’s total pharmaceutical industry at that time. The Tibetan medicine industry as a whole is growing rapidly, CMSN projected that the Tibetan medicine industry is growing at an annual rate of 50 percent.
Owing to a unique environment featuring large temperature difference between day and night, and alpine-cold and low pressure, we believe the active ingredients and bioactivity of the medicinal plants found in the Qinghai-Tibet Plateau, which lies in part in the area where we reside, are particularly well suited to use in medicine. Tibetan medicinal material growing here are widely accepted and perceived in China to have special non-substitutability and higher pharmaceutical value and curative effects.
63
Table of Contents
OTC Tibetan Medicines
Since the implementation of Drug Classified Management, the OTC medicine market developed rapidly in China. In 1990, the sale of OTC medicines in China was only RMB1.9 billion. In 2007, the total was about RMB51 billion.(about $7.5 billion. reflecting a compound annual growth rate of 28%. The OTC pharmaceutical market is expected to increase at a rate of about 15% per year in the future. TCM and Tibetan medicines comprise the principal part of the OTC pharmaceutical market. The rapid development of OTC market therefore promises substantial opportunities for future growth for TCM and Tibetan medicine suppliers. Currently, the OTC species have reached 4,488, of which 3,511 or about 78% are traditional Chinese or Tibetan products.
Government Support of Tibetan Medicine
The Chinese government gives strong support to the pharmaceutical industry, especially support to the traditional Chinese and Tibetan medicine industry. China has set the goal to change from a large medicine producing country to a stronger, world first class medicine producing country. China plans to build this solid foundation during its Eleventh Five-year Plan period (2006-2010) by promoting the modernization of traditional Chinese and Tibetan medicine, developing modern biotechnology and creating more effective Chinese and Tibetan drugs made from herbs and other natural ingredients
The Eleventh Five-year Plan, Mid-Term and Long-Term Science and Technology Development Program, Healthcare Industry and Traditional Chinese Medicine Industry Development Plan all have listed the development of folk medicines of ethnic minorities as important issue.
The Yunnan Province government has enacted a series of preferential policies targeting Tibetan medicine for the purpose of enabling Yunnan province to become a “Biological Resources Power Province”. These efforts comprise part of an effort by the Province to build a brand awareness for the province as the principal source of resources for biological resources, such as plants and herbs used in medical production These measures include providing funds to scientific research and technology improvement, to simplify the medicine applying procedures, and to relax the requirements to get listed in the medical insurance program.
We believe the Chinese government is committed to supporting and promoting the development of modernized TCM and Tibetan medicine, as evidenced by the government formulating an industry development plan for the modernized TCM and Tibetan medicine sector. In addition, in the Chinese pharmaceutical industry five-year plan released in June 2006, the NDRC identified TCM and Tibetan medicines (particularly TCM and Tibetan medicines used for the treatment of diseases prevalent among middle-aged and elderly people) as a priority area for receiving governmental support.
We believe that TCM and Tibetan medicine will remain mainstream medicines in China and will continue to grow as a result of:
| • | China’s longstanding preference for TCM and Tibetan medicine remedies; |
| • | the growing economy; |
| • | the aging population; |
| • | the increasing participation in the State Basic Medical Insurance System; |
| • | Increase in government spending on public health care; |
| • | government support for modernized TCM and Tibetan medicine as a key component of increasing quality of healthcare; |
| • | existence of well-recognized brands supported by a long history; |
| • | the rapidly growing over-the-counter market, in which TCM and Tibetan medicine makes up more than half; and |
| • | the relative low price compared to Western medicine. |
64
Table of Contents
Our Strengths
We believe that we have developed a strong position in the traditional Tibetan medicine market as a result of access to raw materials, research and development efforts, a focus on quality control, modern production techniques and effective marketing. We believe these assets position us to take advantage of the growth of the Chinese pharmaceutical market generally and the expansion of the traditional Tibetan medicine market in particular. More specifically:
| • | We have an established portfolio of SFDA-approved pharmaceuticals. We presently market five products, both prescription and OTC, and believe these products to be the market leaders among modernized traditional Tibetan medicines for the treatment of diseases of the human respiratory system, digestive system, urinary system and gynecological inflammation. We believe our products are unique in their formulas and composition, and we believe they are well perceived by our product users to have high efficacy and low cost compared to our competitors. |
| • | We have diversity in our product offerings. The company has developed four major categories of products with the treatment of respiratory, digestive and urinary systems, and reproductive diseases. |
| • | We have developed promising additional products. We have received government approval for our Wupeng Pill, a traditional Tibetan medicines that we plan to introduce in the market for treating insect bite, pestilence, diphtheria, anthrax, echinococcosis and leprosy. We’re also applying for government approval on Xuezang Guben Pill, a product for boosting energy and immune system, treating neurasthenia, insomnia and women’s menopausal symptoms. We have a number of other formulations in development, some of which are the currently in clinical trials. |
| • | We have strong research and development capabilities. The company has employed a large number of senior scientists in Tibetan Medicine. We have established a close cooperation with the China Academy of Medical Sciences and the Second Military Medical University of China People’s Liberation Army (PLA) to ensure research and innovation capacity strength in traditional Tibetan medicines. |
| • | We employ modern, sophisticated formulation and manufacturing techniques. Our medicine products are processed with modern equipment and advanced technology, unlike many traditional Tibetan pharmaceutical products. At the same time, the production system of the company consistently adheres to traditional Tibetan medicine principles and concepts, that is, of employing combinations of authentic herbs and traditional processing methods. |
| • | We employ rigorous quality control procedures throughout our operations. We devote substantial effort and attention to quality control, not only in manufacturing, but also in sourcing and selecting raw materials, testing and verifying raw material quality and purity, inspection of product in manufacture and after manufacture, management, and distribution and sales. All of our commercialized products have GMP certification, and our production equipment and facilities also are GMP certified. |
| • | We have an experienced management team. Our management team has extensive experience in the traditional Tibetan medicine sector, including spending the last 10 years exclusively in the Tibetan medicine industry. They have a proven track record of identifying, acquiring and developing modernized traditional Tibetan medicinal products with good sales potential as well as successfully manufacturing, marketing and distributing modernized traditional Tibetan medicine. Both our management team and staff are experienced in operating in the highly-regulated and rapidly-developing Tibetan pharmaceutical sector. |
| • | We have access to abundant raw materials used in traditional Tibetan medicine, but not generally available outside our region. Traditional Tibetan medicines are formulated from a variety of herbs and other ingredients that are available primarily or exclusively in Qinghai-Tibet Plateau. |
| • | We have an established distribution network throughout China. We currently contract with 19 distributors in China and plan to add to these relationships to target new markets. |
65
Table of Contents
Our Strategies
Our objectives are to maintain and strengthen the market leadership of our products in Tibetan medicine segment in China, to develop additional products and to increase the sales of our other products. We will continue to integrate our marketing, sales, management, technology, research and development and capital resources, to continue build our brand awareness, and to become the market leader for the development, manufacture and commercialization of Tibetan pharmaceutical products. We intend to achieve these objectives by taking a variety of steps:
| • | We plan to further promote our existing brands to achieve greater market penetration. We intend to support and grow the existing recognition and reputation of our brands and to maintain our branded pricing strategy through continued sales and marketing efforts. To achieve this goal, we plan to detail the efficacy and highlight the quality and benefits of our fast growing Tibetan pharmaceutical products to physicians at hospitals and clinics in all provinces in China, through combining promotion of Tibetan culture with educational physician conferences and seminars. |
| • | We are pursuing a vigorous research and development program to maintain a pipeline of products in development, and to introduce additional products to diversity our product offerings. We plan to focus our research and development capabilities towards expanding our existing portfolio of approved products. We have five prescription and over-the-counter pharmaceutical products in our portfolio that have been successfully commercialized, a sixth that has received SFDA approval and that we hope to commercialize in the future, and a seventh product that currently is under SFDA review for approval. In addition, we intend to conduct clinical trials for new modernized products and product line extensions for our existing products. We plan to introduce new modernized products to leverage our branded market leadership position, particularly in the therapeutic areas we already have an establishment to develop product line extensions for our existing products. |
| • | We are expanding our distribution network to diversify and broaden our market penetration. We intend to expand our reach by building or acquiring national or regional distribution points to drive additional growth of our existing and future products. We plan to build and improve our information system to better respond market demand, improve business process reorganization and optimization, enhance the collaboration among production, supply and sales, achieve good business cooperation on supply chain system, efficiently meet and exceed customer satisfaction, and better promote our products. |
| • | We will work to further develop procedures and systems to support our plans for expansion in research and development, manufacturing and marketing. Our research and development efforts are focused on new and innovative products with substantial market potential, as well as enhancement on existing products. We have forged research and development collaboration partnerships with some of the most prestigious research and development institutions in China, including China Academy of Medical Sciences and the Second Military Medical University of China PLA. In addition, we have plans to expand our manufacturing capacity and strengthen our distribution network both from the proceeds of this offering and from revenues from our operations. |
| • | We will work to build brand awareness. Our ultimate goal is to have our company name “Shangri-La Tibetan Pharmaceuticals” and our brands and trademarks recognized and trusted by our target consumers. In addition, we plan to continue to broaden our marketing efforts outside of major cities in China and increase our market penetration in cities and rural areas where we already have a presence. |
| • | We hope to expand beyond the Chinese market. We also hope to expand our presence beyond China to international markets. We plan to work with other international pharmaceutical companies in cross selling of our products. |
| • | We will pursue strategic acquisition and licensing opportunities. We intend to selectively pursue strategic acquisition opportunities that we believe would grow our customer base, expand our product lines and distribution network, enhance our manufacturing and technical expertise or otherwise complement our business or further our strategic goals. Pursuing additional acquisitions is a significant component of our growth strategy. Our acquisition targets are Tibetan pharmaceutical companies who have very competitive products, with strong efficacy. |
66
Table of Contents
Our Products
The five Tibetan medicines that Shangri-la currently produces are among those Tibetan medicines made with modern drug manufacturing techniques. The manufacturing equipments and techniques are certified under China’s national standards of Good Manufacturing Practice (GMP), and are in compliance with applicable regulations of the SFDA, which regulates both the pharmaceutical and the nutraceutical industries in China. The automatic production and testing processes facilitate the accurate execution of the formulae and the quality and quantity of the products. Moreover, the strict quality control and inspection procedures similarly enhance the consistent quality of our products.
Approved and Commercialized Products
The following table identifies the five products we have commercialized to date, and summarizes the manner in which they are distributed (whether by prescription or over the counter), and the indications or conditions for which they have received SFDA approval:
| No. |
Product Name |
Form of |
Indication |
Usage, Function | ||||
| 1 | 25 Ingredients Mandrake Pill | RX | Gynecology Disease | Regulate menses, treating endometritis, pelvic inflammations and women anemia | ||||
| 2 | 28 Ingredients Pinang Pill | RX | Urinary Tract | Improvement of kidney and lymph function | ||||
| 3 | 18 Ingredients Chebulic (Myrobalan) Frusemide Pill | RX | Diabetes, Nephropathy |
Improvement of kidney function, treating diabetes | ||||
| 4 | 15 Ingredients Gentiana Pill | OTC | Respiratory System | Regulating lung function, suppressing coughing and removing phlegm. Bronchusbronchus, hoarseness | ||||
| 5 | Pomegranate Nichirin Pill | OTC | Digestive System | Improvement of kidney function and digestive system | ||||
A sixth product, our Wupeng Pill, has received SFDA approval but has not yet been commercialized. The Wupeng Pill is a traditional Tibetan medicine for use in treating insect bites, pestilence, diphtheria, anthrax, echinococcosis and leprosy. We hope to commercialize this product after we assess more fully its market potential.
Two of our products account for the vast majority of our sales. The Gentiana pill (comprised of 15 ingredients) and our Mandrake pill (comprised of 25 ingredients) together accounted for 70 percent of our sales in both 2009 and 2008 Our other current products combine for a much smaller share of our sales, namely our Pinang Pill (28 ingredients), Chebulic (Myrobalan) Frusemide Pill (18 ingredients) and our Pomegranate Nichirin Pill.
Our Mandrake pill is sold both as a prescription and as an OTC medicine. While there is no substantive difference between the two versions, as a prescription medicine, the Mandrake Pill is approved by the SFDA for the treatment of anemia in women, gynecological rheumatism and endometritis and pelvic inflammations. As an OTC medicine, it is approved for the treatment of irregular menstruation. The Mandrake pill is a formulation comprised of extracts of 25 different plants, including mandrake. It was developed based on a traditional recipe from the Tibet medicine mantra. In 2008 and 2009, sales of the Mandrake pill totaled RMB55.1 million and RMB76.4 million (US$11.2 million), representing 50% and 48% of our net revenues, respectively.
Our Gentiana pill is an OTC medicine used to treat coughing, asthma and improve the function of the lung. The Gentiana pill is a formulation comprised of 15 plant extracts and contain active ingredients in traditional Tibetan medicine literature as having phlegm removal and coughing relieving properties. In 2008 and 2009, sales of our Gentiana pill was RMB22.1 million and RMB33.8 million (US$4.9 million), representing 19% and 22 % of our net revenues, respectively.
67
Table of Contents
Product Pipeline
We have our own research, development and laboratory facilities and retain our own professional research and development team. We are actively developing a number of new medicines and formulations that were primarily acquired from third parties, as well as through joint research and development with universities and research institutions. As of March 31, 2010, we were at different stages of developing over 60 product candidates.
We have many formulations in different stages of development. Our Xuezang Guben Pill, a product for boosting energy and immune system, treating neurasthenia, insomnia and women’s menopausal symptoms, is now under review for SFDA approval, and we hope to receive approval sometime in 2011. A few other products are currently in clinical trials and are, we believe, particularly promising. The table below identifies the most promising products in development as well as the functions we believe they perform and the indications or uses to which we believe they can be put, subject to the successful completion of clinical trials and securing SFDA approval:
| Medicines |
Function |
Indication |
Status | |||
| Xuezang Guben pill | Improve kidney function, blood balance, sexual function; regulate endocrine disorder | Treatment of neurasthenia, insomnia, frequent urination, nocturnal emission and women’s menopausal symptoms | Applying for certificate of manufacturing and marketing | |||
| Shengke I | Improve kidney function, guard sperms, help urine, improve secretion function is islet cell. Increase the volume of blood flow in heart and brain | Treatment of diabetes | Phase III clinical testing | |||
| Shengke II | Reduction of sexual dysfunction, impotence; regulation of endocrine disorders. | Treatment of impotence and premature ejaculation, prostrate disease, memory loss | Phase II clinical testing | |||
| Jiuzan pill | Relief from stomach ailments | Treatment of chronic gastroenteritis and peptic ulcers | Phase II clinical testing | |||
| Antai pill | Improves liver function | Treatment of hepatitis B | Pre-clinical | |||
Research and Development
Unlike many producers of traditional Tibetan medicines, we devote considerable effort to product research and development, which efforts we believe contribute greatly to our success and opportunities for growth. Our research and development department is responsible for new medicine initiation and approval, research and development, new medicine trial production, technology project application, and communication with universities and research institutes. Our R&D department is also responsible for analyzing potential drug projects, starting the establishment, intermediate evaluation, consultation, acceptance and other works of research and development projects.
68
Table of Contents
As of May 14, 2010, our research and development team consisted of 11 experienced researchers, engineers and developers. Of these, one holds a doctorate, two have masters degrees, four have bachelor degrees and four have associate degrees. In addition, some of our support employees regularly participate in our research and development efforts. We have developed R&D cooperative relationships with universities and medical institutes, including with China Academy of Medical Science Institute, and the Second Military Medical University of China PLA. In addition, we receive outside help from recognized industry experts. For example, Dr. Keji Chen, who’s China’s only fellow in the prestigious Institute of China Academy of Science coming from pharmaceutical industry, worked as a consultant to the company. Dr. Chen is, was a pioneer in the treatment of joint disease and cardiovascular disease through a combination of Western and traditional Chinese medicine . He is also widely recognized for his work in the treatment of coronary, spleen, and kidney conditions, as well as anti-aging.
Our research and development expenditures were RMB3.5 million and RMB4.2 million (US$0.6 million) in 2008 and 2009, respectively, representing 3.2% and 2.7% of our net revenues, respectively. Our research and development expenditures were primarily spent on the acquisition of product candidates and our research partnerships and, to a lesser degree, on remunerations for our research and development personnel.
Manufacturing
Our production facilities are located in Shangri-La County, Yunnan Province, a top region in China in terms of production of raw materials used in the manufacturing of traditional Tibetan medicines. Our unique location allows us to maintain close relationships with suppliers of medicinal herbs, to have lower transportation costs and to give us the option of farming our own medicinal herbs, which we believe is a competitive advantage. We have a policy of maintaining an inventory that allows us to have at least a three-month supply of raw materials. The company own the facility.
Most of our products are manufactured from an array of ingredients. Compared to most other producers of traditional Tibetan medicines, we employ relatively sophisticated techniques to formulate and produce our products, as well as to maintain quality control of our products and production processes. The ingredients used in our products have passed the drug standard issued by the PRC Ministry of Health, and are included in the “Chinese Pharmacopoeia”. We trace the source of raw materials, implement quality control procedures. We also have a clear inspection standard in place.
Our annual manufacturing capacity is 360 million pills. We have the capacity to increase production with our existing facilities and equipment, we believe that our continuing growth may soon necessitate additional equipment purchases and upgrades to plant facilities. We anticipate using portions of the proceeds to upgrade our production capacity, so we can reach full production limit as we grow our business. Our goal is to eventually increase the annual manufacturing capacity to 500 million pills, or 38% increase over existing manufacturing capacity. This utilization rate is calculated on the basis of one shift per day. Our facilities can be operated using two shifts per day.
Quality Control
We believe that our close attention to quality control, in all aspects of our operations, is an important contributor to our success and potential for growth. While many producers of traditional Tibetan medicines are small and may have fewer resources to devote to quality control and modern production techniques, ensuring quality is a major focus of our business.
We implement quality control procedures in compliance with the GMP standards and other SFDA regulations to ensure consistent quality in our products. The ingredients used in our products have passed the drug standard issued by the PRC Ministry of Health, and are included in the “Chinese Pharmacopoeia”. We trace the source of raw materials, implement quality control procedures that includes the inspection of all incoming raw materials. We inspect and test incoming ingredients, products in the course of production, and after production. All of our products meet or exceed GMP and relevant national drug quality standards. Our production equipment and facilities also are GMP-certified.
69
Table of Contents
We have set up a dedicated quality control department, which is responsible for product quality and raw materials management, internal quality control standards and the development of operating procedures and modifications, improving the quality standards of the test methods, and implementing tests of raw materials and finished products. We have implemented a series of corresponding quality control responsibilities among its personnel governing purchasing, production process control, product sales and after-sales service. In addition, we have set up a drug safety warning rapid response team that is directed by our CEO.
Distributors and Customers
We sell our products to independent distributors who resell these products to hospitals, clinics, retail pharmacies and other healthcare institutions throughout China. These distributors also handle distribution logistics, warehousing and transportation. We do not sell our products directly to hospitals or retail pharmacies.
We have appointed nineteen regional distributors across China, most of which are large pharmaceutical distribution companies affiliated with provincial or municipal governments in the provinces or cities in which they operate. We select our distributors based on their reputation, market coverage and sales experience, as well as financial and economic capabilities. We typically enter into annual supply contracts with our regional distributors, renewable for one year or more upon their expiration. These supply contracts set forth the target sales revenues and product prices and contain guidelines for the sale, order, delivery and payment arrangement of our products, including restrictions on the territories in which the products may be sold.
In 2009 and 2008, the following two distributors each accounted for more than 10% of our revenues: (from
| Purchaser Name |
Percentage of Revenues in Year ended December 31, 2009 |
Percentage of Revenues in Year ended December 31, 2008 |
||||
| Kunming Shangri-La Medicine Co., Ltd. |
23.0 | % | 27.7. | % | ||
| Hangzhou Hesheng Medicine Co., Ltd. |
12.6 | % | 10.2 | % |
In 2008 and 2009, sales to our top five distributors collectively accounted for 69% and 62% of our net revenues, respectively.
Our agreements with our distributors at present operate on annual terms but generally have been renewed. Distributors have been granted exclusive sales territories, but are not required to sell our products exclusively. Distributors are not subject to minimum or annual sales requirements, but we believe our ability to monitory distributor performance closely and terminate or not renew agreements provides us with the ability to incentivize and control distributors. Our terms of sale with distributors call for payment within 60 days.
In the future, we plan to expand significantly the number of distributors with which we work, both to lessen our dependence on existing distributors and to increase our market penetration. We presently plan to expend approximately $5.8 million over a two-year period to recruit additional distributors, achieve broader geographic coverage throughout China, and generally to strengthen our distribution network.
The ultimate customers of our prescription products are principally hospitals and hospital pharmacies. The ultimate customers of our OTC products are primarily retail pharmacies
Suppliers
We purchase raw materials from local farmers who either locally grow the plants or harvest them. We also purchase raw materials from local open markets where there exist many pharmaceutical stores who are specialized in supplying raw materials for production of Tibetan medicine.
We use the following principal suppliers for our operations. Our supplies consist primarily of raw materials for drug production. We believe the materials provided by our suppliers are widely available and do not anticipate that we will be unable to obtain these materials from other suppliers in the event our principal suppliers are unable or unwilling to supply us. One supplier, Chun Sheng, accounted for 18.7 percent of our purchases of ingredients in 2009 and 15.9 percent of our purchases of ingredients in 2009. Kunming Moringstar Pringing Company accounted for 18.3 percent of our purchases of packaging material in 2009 and 17.6% of such purchases in 2008.
70
Table of Contents
Marketing and Sales
We market and sell our products principally through our distribution network. We do not finance distributor advertising and promotion directly, but we do provide information and support to our distributors, We do expect distributor promotion of our products, and look for evidence of this as we monitor our distributor network In addition to promoting our products to distributors and others, as we work to expand our distribution network, we also directly promote our products to end users through advertising on radio and a variety of print media. Our efforts to strengthen our distribution network and to market directly to end users are both parts of our general effort to build brand awareness and name recognition.
Intellectual Property
We rely primarily on a combination of trademark and trade secret protections, as well as employee and third-party confidentiality agreements to safeguard our intellectual property.
Trademarks
The Chinese have domestic laws for the protection of rights in copyrights, patents, trademarks and trade secrets. The Chinese are also a signatory to all of the world’s major intellectual property conventions, including:
| • | Convention establishing the World Intellectual Property Organization (WIPO Convention) (June 4, 1980); |
| • | Paris Convention for the Protection of Industrial Property (March 19, 1985); |
| • | Patent Cooperation Treaty (January 1, 1994); and |
| • | The Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPs) (November 11, 2001). |
The Chinese Trademark Law, adopted in 1982 and revised in 2001, with its implementation rules adopted in 2002, protects registered trademarks. The Trademark Office of the State Administration of Industry and
Commerce (“SAIC”), handles trademark registrations and grants trademark registrations for a term of ten years.
We market Xiangbala Jidan under a trademark that combines graphic designs and Tibetan characters of “Shangri-La.” We maintain eight registrations of our trademarks in the PRC, including registrations of combinations of the Chinese characters and graphic designs for “Zangyu”, “Shengke” and “Xiangbala Jidan”, and “Baofuwan.” A listing of our trademarks registered in China follows:
| No |
Trademark Name |
Registration Number |
Valid Through |
Category | ||||
| 1 | Zangyu | 3273849 | 5/20/2014 | Approved Use, Product Category 5 | ||||
| 2 | Shengke | 3273850 | 1/6/2014 | Approved Use, Product Category 5 | ||||
| 3 | (Design) | 1573695 | 5/20/2011 | Approved Use, Product Category 5 | ||||
| 4 | Xiangbala Jidan | 1785280 | 6/13/2012. | Approved Use, Product Category 5 | ||||
| 5 | (Design) | 1974689 | 12/6/2012 | Approved Use, Product Category 5 | ||||
| 6 | (Design) | 2013260 | 1/20/2013 | Approved Use, Product Category 30 | ||||
| 7 | Baofuwan | 3545820 | 9/13/2015 | Approved Use, Product Category 5 | ||||
| 8 | (Design) | 3545841 | 4/13/2015 | Approved Use, Product Category 5 |
71
Table of Contents
We believe that our trademarks Xiangbala Jidan and Zangyu in particular are well recognized in China among physicians and hospital administrators, pharmacists and osteoporosis patients. We are working to strengthen, expand, and enforce our rights associated with these trademarks and brand names, the protection of which is important to our reputation and branding.
Trade Secrets
Many elements of our pharmaceutical composition, formulation, delivery and manufacturing methods and processes involve proprietary technologies, processes, know-how or data that are non-patentable. Other methods and formulations may be patentable, but we have determined that our business interests are better served by trying to protect them as trade secrets or confidential information. We rely heavily on trade secret protection and confidentiality agreements rather than patent laws to protect our rights in these proprietary technologies, processes, know-how and data. We have taken security measures to protect our rights in this regard. For example, all of our research and development personnel have entered into confidentiality, non-competition and proprietary information agreements with us. In addition, we maintain a segregation of duties among personnel involved in different stages of our production process. This segregation reduces the risk that a breach of these protections by any single staff member would result in a leakage of the entire production process of our products. We also implement other precautions, such as internal document controls and network assurance procedures and the use of a separate dedicated computer server, for our proprietary information and technical data.
Competition
The traditional Chinese medicine and Tibetan medicine sector in China is highly fragmented and intensely competitive. According to the 2006 China Pharmaceutical Market Research Report prepared by Compass International, a Beijing-based research company, in 2005 there were over 1,200 traditional Chinese medicine manufacturers in China (not including manufacturers of traditional sliced herbs). Of these manufacturers, in 2005, large-sized manufacturers contributed to 27.4% of the total sales of this sector, compared to 30.3% and 42.3% of sales by small- and medium-sized manufacturers, respectively, according to the same research report. According to the company size classification standards used by Compass International in the research report and which are set by the National Bureau of Statistics, a company with a headcount of over 2,000, sales of over RMB300 million and assets of over RMB400 million is classified as a large-sized company. On the other hand, for companies that do not meet all three criteria of a large-sized company, if the company has an employee headcount of at least 300, sales of at least RMB30 million and assets of at least RMB40 million, it is designated as a medium-sized company. Otherwise, it is considered to be a small-sized company. Under these standards, we believe that we would be designated as a medium-sized company.
Different categories of traditional Tibetan medicine companies.
The Tibetan medicine industry is only a segment of the TCM sector. It is now in a transition stage, evolving from a sector dominated by small, backyard enterprises to one increasingly characterized by larger-scale, industrial operations. Our competitors can be divided into two categories:
| • | State-run pharmaceutical companies, such as Jingqiu Tibetan Medicine, Jinhe Tibetan Medicine and Tibetan Pharmaceutical Factory; and |
| • | Private or joint-venture enterprises, such as the Cheezheng Tibetan Medical Group and Niemula Tibet Medicine. |
The state-owned enterprises have access to state financial and political support. They also entered into the market earlier than we did, and thus have accumulated management experiences and personnel in the Tibetan medicine business. Meanwhile, the joint-venture enterprises, like us, entered into market later, but, at least in our case, have grown quickly.
Currently the Tibetan drug manufacturers can be divided into two other categories. One category is comprised of many traditional Tibetan medicine companies that produce a variety of different products relies principally on medicinal formulations that have been “inherited” and used for many, many years. The other category, comprised of far fewer companies, employs more modern manufacturing techniques and research and development capabilities to formulate new products. Typically companies in this second
72
Table of Contents
category, in which we count ourselves as a member, focus on fewer products but higher quality and modern techniques. Other examples include such as Tibet Medicine Industry Company and Cheezheng Tibetan Medicine Company. The former one has developed emergency life saving medicine— rh-BNP on acute heart failure, which has changed people’s mind where Tibetan drug manufacturers can only make a living by relying on selling biological resources; the latter’s leading product Cheezheng Pain Relieving Plaster, annual sales in 2009 amounted $59.7 million, which tells people how big potential is for Tibetan medicine for just coming out one successful product.
Growth and increasing approval of traditional Tibetan medicine producers
According to China Medicine Source Net (CMSN), there exist more than 100 Tibetan medicine enterprises throughout China, of which about 40 have secured GMP authentication. In 2006, 17 GMP-certified Tibetan medicine manufacturers in Tibet Autonomous Region have total annual industrial output of RMB 623 million. China’s Tibetan medicine industry was about RMB1 billion in size in 2006, about 0.5% of China’s total pharmaceutical industry. The Tibetan medicine industry is believed to be growing rapidly: CMSN projected the Tibetan medicine industry as a whole to be growing at an average annual rate of about 50%.
Principal Bases of Competition
We believe that traditional Chinese medicine manufacturers primarily compete on the basis of:
| • | brand name and reputation; |
| • | price; |
| • | perceived efficacy; |
| • | side effects; |
| • | marketing ability; |
| • | economies of scale; |
| • | customer service and customer support capabilities; and |
| • | customer base and customer loyalty. |
We believe that our experience, relatively large size, attention to R&D and quality control, product quality and modern manufacturing and marketing techniques all position us to compete effectively in the areas listed above.
Principal competitors in the traditional Tibetan medicine sector
We face direct competition from other China-based manufacturers of traditional Tibetan medicines. We believe that the primary traditional Tibetan medicines manufacturers competing with our 25 Ingredients Mandrake Pill are Qinghai Jinke Tibetan Medicine Co., Ltd. and Qinghai Crystal Beads Tibetan Medicine High-tech Industry Co., Ltd. Both companies are PRC domestic pharmaceutical companies.
The following chart sets forth the primary competitors that compete with our other principal products:
| Our Product |
Primary Competitors | |
| 28 Ingredients Pinang Pill |
Donggeer Pharmaceuticals Co., Ltd. Xiongbalaqu Shenshui Tibetan Medicine Factory | |
| 18 Ingredients Chebulic (Myrobalan) Frusemide Pill |
Tibet Shenhou Pharmaceutical Co., Ltd. Qinghai Caidamu High-tech Medicine Co., Ltd. | |
| 15 Ingredients Gentiana Pill |
Ningxia Duowei Pharmaceutical Co., Ltd. Qinghai Dimaer Tibetan Pharmaceutical Co., Ltd. | |
| Pomegranate Nichirin Pill |
Linzhiyutuo Tibetan Pharmaceutical Co., Ltd., Qinghai Tongtianhe Tibetan Medicine Co., Ltd. |
73
Table of Contents
Environmental Matters
Medicine manufacturers in China must comply with environmental laws and regulations stipulated by the state, provincial and local environment protection authorities. Relevant laws and regulations include provisions relating to the treatment of sewage and exhaust fumes and the limitation of industrial pollution. Pharmaceutical companies are required to carry out an environmental impact assessment before commencing construction of their main production facilities as well as to construct accompanying pollution treatment facilities.
The major waste products of our manufacturing processes include:
| • | organic waste from the extraction process – we have set aside a specific area within our facility to gather this organic waste for local farmers to remove and use as fertilizer; |
| • | waste water – we have our own waste water treatment facilities to treat the waste water from our production processes; and |
| • | alcohol – we have our own facilities for recycling alcohol. |
We comply with the Environmental Protection Law of China as well as the applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
Employees
As of May 14, 2010, we have 173 full-time employees, and no part-time employees. We anticipate that the number of our employees will remain about the same in the near future.
Insurance
We do not carry any building, commercial, liability, product liability or key man insurance. This is not unusual in China, where the business insurance market is relatively undeveloped.
Facilities
YSTP’s manufacturing and office facilities are located in Shangri-La County of Yunnan Province, China. The office facility is located in a single building having 17,014 square feet ; the manufacturing facility is in a single building of 52,374 square feet.
Assuming that we generate positive cash flow from operations and continued growth in demand, we may expand our production facility and purchase additional machines to expand our production capacity. We tentatively plan to expend approximately $2.2 million to upgrade production capabilities.
Legal and Administrative Proceedings
We are currently not a party to any material legal or administrative proceedings, and we are not aware of any threatened material legal or administrative proceedings against us. We may from time to time become a party to various legal or administrative proceedings arising in the ordinary course of our business.
74
Table of Contents
Restriction on Foreign Ownership
The principal regulation in China governing foreign investors’ entry possibility and mode of investment vehicle in the industries in which they intend to invest is the Catalogue of Industrial Guidance for Foreign Investment, effective as of December 11, 2007 (the “Catalogue”). The Catalogue classifies the various industries into four categories: encouraged, permitted, restricted and prohibited. Pursuant to the Catalogue, YSTP is engaged in a “permitted” industry. Such a designation offers businesses distinct advantages. For example, businesses engaged in encouraged industries:
| • | are not subject to restrictions on foreign investment, and, as such, foreigners can own a majority in Sino-foreign joint ventures or establish wholly-owned foreign enterprises in China; and |
| • | if a company has total investment of less than $100 million, the company is subject to regional (not central) government examination and approval which are generally more efficient and less time-consuming. |
The National Development and Reform Commission and the Ministry of Commerce periodically jointly revise the Catalogue of Industrial Guidance for Foreign Investment. As such, there is a possibility that our company’s business in the future may fall into the scope of the definition of a restricted or prohibited industry. Should this occur, we would no longer benefit from the above designation.
Regulation of Foreign Currency Exchange and Dividend Distribution
Foreign Currency Exchange. The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations (1996), as amended, and the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996). Under these regulations, Renminbi are freely convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions, but not for most capital account items, such as direct investment, loan, repatriation of investment and investment in securities outside China, unless the prior approval of SAFE or its local counterparts is obtained. In addition, any loans to an operating subsidiary in China that is a foreign invested enterprise, cannot, in the aggregate, exceed the difference between its respective approved total investment amount and its respective approved registered capital amount. Furthermore, any foreign loan must be registered with SAFE or its local counterparts for the loan to be effective. Any increase in the amount of the total investment and registered capital must be approved by the Chinese Ministry of Commerce or its local counterpart. We may not be able to obtain these government approvals or registrations on a timely basis, if at all, which could result in a delay in the process of making these loans.
The dividends paid by the subsidiary to its shareholder are deemed shareholder income and are taxable in China. Pursuant to the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), foreign-invested enterprises in China may purchase or remit foreign exchange, subject to a cap approved by SAFE, for settlement of current account transactions without the approval of SAFE. Foreign exchange transactions under the capital account are still subject to limitations and require approvals from, or registration with, SAFE and other relevant Chinese governmental authorities.
Dividend Distribution. The principal regulations governing the distribution of dividends by foreign holding companies include the Foreign Investment Enterprise Law (1986), as amended, and the Administrative Rules under the Foreign Investment Enterprise Law (2001).
Under these regulations, foreign investment enterprises in China may pay dividends only out of their retained profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, foreign investment enterprises in China are required to allocate at least 10% of their respective retained profits each year, if any, to fund certain reserve funds unless these reserves have reached 50% of the registered capital of the enterprises. These reserves are not distributable as cash dividends.
75
Table of Contents
Notice 75. On October 21, 2005, SAFE issued Notice 75, which became effective as of November 1, 2005. According to Notice 75, prior registration with the local SAFE branch is required for Chinese residents to establish or to control an offshore company for the purposes of financing that offshore company with assets or equity interests in an onshore enterprise located in China. An amendment to registration or filing with the local SAFE branch by such Chinese resident is also required for the injection of equity interests or assets of an onshore enterprise in the offshore company or overseas funds raised by such offshore company, or any other material change involving a change in the capital of the offshore company.
Moreover, Notice 75 applies retroactively. As a result, Chinese residents who have established or acquired control of offshore companies that have made onshore investments in China in the past are required to complete the relevant registration procedures with the local SAFE branch. Under the relevant rules, failure to comply with the registration procedures set forth in Notice 75 may result in restrictions being imposed on the foreign exchange activities of the relevant onshore company, including the increase of its registered capital, the payment of dividends and other distributions to its offshore parent or affiliate and capital inflow from the offshore entity, and may also subject relevant Chinese residents to penalties under Chinese foreign exchange administration regulations.
Chinese residents who control our company are required to register with SAFE in connection with their investments in us. Such individuals began this registration process on May 14, 2010. If we use our equity interest to purchase the assets or equity interest of a Chinese company owned by Chinese residents in the future, such Chinese residents will be subject to the registration procedures described in Notice 75.
New M&A Regulations and Overseas Listings
On August 8, 2006, six Chinese regulatory agencies, including the Ministry of Commerce, the State Assets Supervision and Administration Commission, the State Administration for Taxation, the State Administration for Industry and Commerce, CSRC and SAFE, jointly issued the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the New M&A Rule, which became effective on September 8, 2006. This New M&A Rule, among other things, includes provisions that purport to require that an offshore special purpose vehicle formed for purposes of overseas listing of equity interests in Chinese companies and controlled directly or indirectly by Chinese companies or individuals obtain the approval of CSRC prior to the listing and trading of such special purpose vehicle’s securities on an overseas stock exchange.
On September 21, 2006, CSRC published on its official website procedures regarding its approval of overseas listings by special purpose vehicles. The CSRC approval procedures require the filing of a number of documents with the CSRC and it would take several months to complete the approval process. The application of this new Chinese regulation remains unclear with no consensus currently existing among leading Chinese law firms regarding the scope of the applicability of the CSRC approval requirement.
Our Chinese counsel, DeHeng Law Offices, has advised us that, based on their understanding of the current Chinese laws and regulations:
| • | We currently control our Chinese affiliate, YSTP, by virtue of WFOE’s VIE agreements with YSTP but not through equity interest acquisition nor asset acquisition which are stipulated in the New M&A Rule; and |
| • | In spite of the above, CSRC currently has not issued any definitive rule or interpretation concerning whether offerings like ours under this prospectus are subject to this new procedure. |
Registration and Approval of Medicine
The pharmaceutical industry in China, including the traditional Chinese medicine sector, is highly regulated. The primary regulatory authority is the SFDA, including its provincial and local branches. As a developer, producer and distributor of medicinal products, we are subject to regulation and oversight by the SFDA and its provincial and local branches. The Law of China on the Administration of Pharmaceuticals provides the basic legal framework for the administration of the production and sale of pharmaceuticals in China and covers the manufacturing, distributing, packaging, pricing and advertising of pharmaceutical products. Its implementing regulations set forth detailed rules with respect to the administration of pharmaceuticals in China. We are also
subject to other Chinese laws and regulations that are applicable to business operators, manufacturers and distributors in general.
76
Table of Contents
A medicine must be registered and approved by the SFDA before it can be manufactured. The registration and approval process requires the manufacturer to submit to the SFDA a registration application containing detailed information concerning the efficacy and quality of the medicine and the manufacturing process and the production facilities the manufacturer expects to use. This process generally takes at least a few months and could be longer, depending on the nature of the medicine under review, the quality of the data provided and the workload of the SFDA. To obtain the SFDA registration and approval necessary for commencing production, the manufacturer is also required to conduct pre-clinical trials, apply to the SFDA for permission to conduct clinical trials, and, after clinical trials are completed, file clinical data with the SFDA for approval.
New Medicine. If a medicine is approved by the SFDA as a new medicine, the SFDA will issue a new medicine certificate to the manufacturer and impose a monitoring period of as long as five years. The length of the monitoring period is specified in the new medicine certificate. During the monitoring period, the SFDA will monitor the safety of the new medicine, and will neither accept new medicine certificate applications for an identical medicine by another pharmaceutical company, nor approve the production or import of an identical medicine by other pharmaceutical companies. For new medicines approved prior to 2002, the monitoring period could be longer than five years. As a result of these regulations, the holder of a new medicine certificate effectively has the exclusive right to manufacture the new medicine during the monitoring period.
Provisional National Production Standard. In connection with the SFDA’s approval of a new medicine, the SFDA will normally direct the manufacturer to produce the medicine according to a provisional national production standard, or a provisional standard. A provisional standard is valid for two years, during which the SFDA closely monitors the production process and quality consistency of the medicine to develop a national final production standard for the medicine, or a final standard. Three months before the expiration of the two-year period, the manufacturer is required to apply to the SFDA to convert the provisional standard to a final standard. Upon approval, the SFDA will publish the final standard for the production of this medicine. There is no statutory timeline for the SFDA to complete its review and grant approval for the conversion. In practice, the approval for conversion to a final standard is time-consuming and could take a few years. However, during the SFDA’s review period, the manufacturer may continue to produce the medicine according to the provisional standard.
Transitional Period. Prior to the latter of (1) the expiration of a new medicine’s monitoring period or (2) the date when the SFDA grants a final standard for a new medicine after the expiration of the provisional standard, the SFDA will not accept applications for an identical medicine nor will it approve the production of an identical medicine by other pharmaceutical companies. Accordingly, the manufacturer will continue to have an exclusive production right for the new medicine during this transitional period.
Continuing SFDA Regulation
Pharmaceutical manufacturers in China are subject to continuing regulation by the SFDA. If an approved medicine, its labeling or its manufacturing process is significantly modified, a new pre-market approval or pre-market approval supplement will be required by the SFDA. A pharmaceutical manufacturer is subject to periodic inspection and safety monitoring by the SFDA to determine compliance with regulatory requirements. The SFDA has a variety of enforcement actions available to enforce its regulations and rules, including fines and injunctions, recall or seizure of products, the imposition of operating restrictions, partial suspension or complete shutdown of production and criminal prosecution.
Pharmaceutical Product Manufacturing
Permits and Licenses for Pharmaceutical Manufacturers. A pharmaceutical manufacturer must obtain a pharmaceutical manufacturing permit from the SFDA’s relevant provincial branch. This permit is valid for five years and is renewable upon its expiration.
Good Manufacturing Practice. A pharmaceutical manufacturer must meet the Good Manufacturing Practice standards, or GMP standards, for each of its production facilities in China in respect of each form of
77
Table of Contents
pharmaceutical products it produces. GMP standards include staff qualifications, production premises and facilities, equipment, raw materials, environmental hygiene, production management, quality control and customer complaint administration. If a manufacturer meets the GMP standards, the SFDA will issue to the manufacturer a Good Manufacturing Practice certificate, or a GMP certificate, with a five-year validity period. However, for a newly established pharmaceutical manufacturer that meets the GMP standards, the SFDA will issue a GMP certificate with only a one-year validity period.
Pharmaceutical Distribution
A distributor of pharmaceutical products in China must obtain a pharmaceutical distribution permit from the relevant provincial or local SFDA branches. The distribution permit is granted if the relevant SFDA provincial branch receives satisfactory inspection results of the distributor’s facilities, warehouse, hygiene environment, quality control systems, personnel and equipment. A pharmaceutical distribution permit is valid for five years.
Restrictions on Foreign Ownership of Pharmaceutical Wholesale and Retail Businesses in China. Chinese regulations on foreign investment currently permit foreign companies to establish or invest in wholly foreign-owned companies or joint ventures that engage in wholesale or retail sales of pharmaceuticals in China. For retail sales, these regulations restrict the number and size of retail pharmacy outlets that a foreign investor may establish. Retail pharmacy chains with more than 30 outlets that sell a variety of branded pharmaceutical products sourced from different suppliers are limited to 49.0% foreign ownership unless the outlets are owned by a third party and operated under a foreign franchise.
Good Supply Practice Standards. The SFDA applies Good Supply Practice standards, or GSP standards, to all pharmaceutical wholesale and retail distributors to ensure the quality of distribution in China. The currently applicable GSP standards require pharmaceutical distributors to implement controls on the distribution of medicine, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. A certificate for GSP standards, or GSP certificate, is valid for five years, except for a newly established pharmaceutical distribution company, for which the GSP certificate is valid for only one year.
Price Controls
The retail prices of prescription and OTC medicines that are included in the national medicine catalog are subject to price controls administered by the Price Control Office under the National Development and Reform Commission, or the NDRC, and provincial price control authorities, either in the form of fixed prices or price ceilings. The controls over the retail price of a medicine effectively set the limits for the wholesale price of that medicine. From time to time, the NDRC publishes and updates a national list of medicines that are subject to price control. Fixed prices and price ceilings on medicines are determined based on profit margins that the NDRC deems reasonable, the type and quality of the medicine, its production costs, the prices of substitute medicines and the extent of the manufacturer’s compliance with the applicable GMP standards. The NDRC directly regulates the price of some of the medicines on the list, and delegates the power to provincial price control authorities to regulate the remainder on the list. For those medicines under the authority of provincial price control authorities, each provincial price control authority regulates medicines manufactured by manufacturers registered in that province. Provincial price control authorities have the discretion to authorize price adjustments based on the local conditions and the level of local economic development.
Only the manufacturer of a medicine may apply for an increase in the retail price of the medicine and it must apply either to the NDRC, if the price of the medicine is nationally regulated, or to the provincial price control authorities in the province where it is registered, if the price of the medicine is provincially regulated. For a provincially regulated medicine, when provincial price control authorities approve an application, they will file the new approved price with the NDRC for confirmation and thereafter the newly approved price will become binding and enforceable across China.
At present, none of our products are subject to price controls.
78
Table of Contents
Tendering Requirement for Hospital Purchases of Medicines
Provincial and municipal government agencies such as provincial or municipal health departments also operate a mandatory tendering process for purchases by state-owned hospitals of a medicine included in provincial medicine catalogs. These government agencies organize a tendering process once every year in their province or city and typically invite manufacturers of provincial catalog medicines that are on the hospitals’ formularies and are in demand by these hospitals to participate in the tendering process. A government-approved committee consisting of physicians, experts and officials is delegated by these government agencies the power to review bids and select one or more medicines for the treatment of a particular medical condition. The selection is based on a number of factors, including bid price, quality and manufacturer’s reputation and service. The bidding price of a wining medicine will become the price required for purchases of that medicine by all state-owned hospitals in that province or city. This price, however, is effective for only one year before the following year’s tendering process, where the manufacturer of the winning medicine must submit a new bid.
The tendering requirement was first introduced in 2004 and has since been implemented across China. We understand that the level of present implementation of the tendering requirement varies among different provinces in China.
79
Table of Contents
Directors and Executive Officers
The following table sets forth our executive officers and directors, their ages and the positions held by them:
| Name |
Age | Position |
Appointed | |||
| Mr. Hong Yu(1) |
50 | Chairman of the Board and Director | 2010 | |||
| Mr. Taylor Z. Guo(1) |
38 | Chief Executive Officer, Director | 2010 | |||
| Ms. Sabrina Y. Ren(1) |
38 | Chief Financial Officer | 2010 | |||
| Dr. Wenbo Chen(1) |
74 | Independent Director | 2010 | |||
| Mr. Youhang Peng( 1) |
48 | Independent Director | 2010 | |||
| Mr. Solomon Chen( 1) |
33 | Independent Director | 2010 |
| (1) | The business address for each of Mr. Yu, Mr. Guo, Ms. Ren, Dr. Chen, Mr. Peng and Mr. Chen is: 53 Niwang Road, Shangri-La County, Yunnan Province, China |
Mr. Hong Yu. Mr. Yu has served as our Chair since April 2010. Mr. Yu is the founder of YSTP, and has served as its Chairman of the Board and General Manager (roughly equivalent to Chief Executive Officer) from 2000 until mid-2009. Mr. Yu holds a MBA from Yunnan Finance and Trade Institute. Mr. Yu was nominated as a director for his knowledge with Tibetan culture and medicine and his leadership of our company.
Mr. Taylor Z. Guo. Mr. Guo has served as a director and our CEO since 2010; he also has served as the General Manager (or CEO) of YSTP since mid-2009. From 2004 to 2009, Mr. Guo was director of Bao-Wei Electronics Limited. Mr. Guo holds a bachelor degree from Dalian University of Technology and a MBA from University of Minnesota. Mr. Guo was nominated as a director because of his operating and management experience.
Ms. Sabrina Y. Ren has served as our Chief Financial Officer since 2010; she serves in the same position for YSTP. From 2000 through 2009, she worked for Yunnan Kangle Pharmaceuticals Co., Ltd. Ms. Ren holds a bachelor degree of Accounting from Henan Financial Institute. Ms. Ren has been retained because of her significant finance and accounting experience, especially in pharmaceutical companies.
Dr. Wenbo Chen. Dr. Chen has served as an independent Director since April 2010. Dr. Chen has been chairman and chief physician of Beijing Medical Center of Famous Physicians since 1993, professor of Beijing University of Chinese Medicine since 1990. Dr. Chen also serves as director of China Geriatric Association and director of Beijing Association of Chinese Medicine. From 1981 through 1998, Dr. Chen was dean and president of Beijing Gulou (Drum Tower) Hospital of Chinese Medicine. Dr. Chen is recognized as a national level expert in traditional Chinese medicine. He was awarded “China’s 100 Top Doctors in Traditional Chinese Medicine”, and enjoys government special allowance issued to him by China Department of State. Dr. Chen has published many books, research papers, and articles. He was the editor for the Journal of Traditional Chinese Medicine. Dr. Chen was nominated as a director because of his outstanding industry knowledge and reputation.
Mr. Youhang Peng. Mr. Peng has served as an independent Director since April 2010. Since 2004, Mr. Peng has been the Senior Managing Director of Caybridge International, Inc., whose principal address is in Coppell, Texas. Mr. Peng holds a bachelor degree from Tsinghua University and a master degree from University of California at Davis. Mr. Peng was nominated as an independent director because of his experience in capital markets.
Mr. Solomon Chen. Mr. Chen has served as an independent Director since April 2010. Since 2004, Mr. Chen has been audit manager of Deloitte Touche Tohmatsu CPA (Shenzhen) . Mr. Chen holds a bachelor degree from Xiamen University. Mr. Chen is a Certified Public Accountant (China) and a Certified Tax Accountant (China). Mr. Chen serves as our Audit Committee financial expert. Mr. Chen was nominated as an independent director because of his experience in audit, accounting, internal control review, and transactional advisory experience in merger and acquisitions.
80
Table of Contents
Summary Compensation Table
The following table shows the annual compensation paid by us for the years ended December 31, 2008 and 2009 to our principal executive officers.
| Name and principal position |
Year | Salary | Bonus | Stock Awards |
All Other Compensation |
Total Paid | ||||||||||
| Mr. Taylor Z. Guo, |
2009 | $ | 30,749 | $ | 0 | $ | 157,500 | 0 | $ | 188,249 | ||||||
| Mr. Hong Yu, |
2009 | $ | 96,638 | 0 | 0 | 0 | $ | 96,638 | ||||||||
| Mr. Hong Yu, |
2008 | $ | 93,372 | 0 | 0 | 0 | $ | 96,372 | ||||||||
| (1) | Mr. Guo did not become CEO of TBET until 2010. In July 2009, he became General Manager (the equivalent of CEO) of YSTP, and he continues to hold that position. Under his employment agreement with TBET effective April 30, 2010, going forward he will receive annual compensation of $61,500. His employment does not provide for bonuses, options or other compensation. For his services as General Manager of YSTP for one-half of 2009, and for those services he was paid a salary of $30,749.00. In addition, Mr. Guo received non-cash compensation for services rendered in 2009 in the form of a non-plan stock award of 150,000 shares of TBET, the compensation value of which has been valued by our auditors are $157,500. As a result, Mr. Guo’s total compensation for 2009 was $188,249. |
| (2) | Mr. Yu served as General Manager of YSTP prior to Mr. Guo’s arrival in 2009. The salary number reported reflects his total compensation by YSTP, including compensation for his services as General Manager. |
Employment Agreements
Generally
Under Chinese law, we may only terminate employment agreements without cause and without penalty by providing notice of non-renewal one month prior to the date on which the employment agreement is scheduled to expire. If we fail to provide this notice or if we wish to terminate an employment agreement in the absence of cause, then we are obligated to pay the employee one month’s salary for each year we have employed the employee. We are, however, permitted to terminate an employee for cause without penalty to our company, where the employee has committed a crime or the employee’s actions or inactions have resulted in a material adverse effect to us.
Our employment agreements with our executive officers generally provide for a term of three years, extendable for an additional two years, and a salary to be paid monthly. The agreements also provide that executive officers are to work an average of forty hours per week and are entitled to all legal holidays as well as other paid leave in accordance with Chinese laws and regulations and our internal work policies. Under such agreements, our executive officers can be terminated for cause without further compensation. The employment agreements also provide that we will pay for all mandatory social security programs for our executive officers in accordance with Chinese regulations. During the agreement and for three (3) years afterward, our executive officers are subject to keep trade secrets confidential. In addition, our employment agreements with our executive officers prevent them from rendering services for our competitors for a period of two (2) years after termination of employment.
To date, we have entered into “retainer agreements” with our officers and the chairman of our board of directors. These agreements went into effect on April 30, 2010; prior to April 30, 2010, YSTP’s officers served without formal, written agreements. The written agreements presently in place require full-time service for us, and provide for the payment of annual salaries plus the reimbursement of reasonably-incurred expenses. They do not provide for bonuses or other forms of compensation. Generally, our written retainer agreements have terms of three years, and automatic renewal terms of two years. They require that the individuals work exclusively for the company, and further provide that during the terms of the agreements and for twenty-four months thereafter the individuals may not render services for our direct or indirect competitors. The agreements further bind the individuals to confidentiality obligations throughout the terms of the agreements and for thirty-six months thereafter.
81
Table of Contents
Hong Yu
Mr. Yu entered into a retainer agreement with YSTP, effective April 30, 2010, providing for Mr. Yu to serve as our chairman of the board of directors. The agreement operates, unless sooner terminated as provided for in the agreement, for a term of the agreement is three years, and it is renewable for an additional two-year term. The agreement provides for payment to Mr. Yu of an annual salary of $96,650, payable in equal monthly installments, as well as reimbursement of expenses she reasonably incurs.
Taylor Z. Guo
Mr. Guo entered into a retainer agreement with YSTP effective April 30, 2010. It provides for Mr. Guo to serve as our CEO. The agreement operates for a term of three years, which term is renewable for a subsequent term of two years. The retainer agreements provides for an annual salary payable to Mr. Guo of $61,500, payable in equal monthly installments, as well as for the reimbursement of reasonable expenses incurred by Mr. Guo. The retainer agreement is terminable by either party upon sixty days prior written notice.
Sabrina Y. Ren
Ms. Ren entered into a retainer agreement with YSTP, effective April 30, 2010, providing for Ms Ren to serve as our CFO. The agreement operates, unless sooner terminated as provided for in the agreement, for a term of the agreement is three years, and it is renewable for an additional two-year term. The agreement provides for payment to Ms. Ren of an annual salary of $44,000, payable in equal monthly installments, as well as reimbursement of expenses she reasonably incurs.
Duties of Directors
Under British Virgin Islands law, our directors have a duty to act honestly, in good faith and with a view to our best interests. Our directors also have a duty to exercise the care, diligence and skills that a reasonably prudent person would exercise in comparable circumstances. See “Description of Share Capital—Differences in Corporate Law” for additional information on our directors’ fiduciary duties under British Virgin Islands law. In fulfilling their duty of care to us, our directors must ensure compliance with our fourth amended and restated memorandum and articles of association. We have the right to seek damages if a duty owed by our directors is breached.
The functions and powers of our board of directors include, among others:
| • | appointing officers and determining the term of office of the officers; |
| • | authorizing the payment of donations to religious, charitable, public or other bodies, clubs, funds or associations as deemed advisable; |
| • | exercising the borrowing powers of the company and mortgaging the property of the company; |
| • | executing checks, promissory notes and other negotiable instruments on behalf of the company; and |
| • | maintaining or registering a register of mortgages, charges or other encumbrances of the company. |
Board of Directors
Our board of directors currently consists of five (5) directors, including the chairman of the board. We expect that all current directors will continue to serve after this offering. There are no family relationships between any of our executive officers and directors.
The directors will be divided into three classes, as nearly equal in number as the then total number of directors permits. Class I directors shall face re-election at our annual general meeting of shareholders in 2010 and every three years thereafter. Class II directors shall face re-election at our annual general meeting of shareholders in 2011 and every three years thereafter. Class III directors shall face re-election at our annual general meeting of shareholders in 2012 and every three years thereafter.
82
Table of Contents
If the number of directors changes, any increase or decrease will be apportioned among the classes so as to maintain the number of directors in each class as nearly as possible. Any additional directors of a class elected to fill a vacancy resulting from an increase in such class will hold office for a term that coincides with the remaining term of that class. Decreases in the number of directors will not shorten the term of any incumbent director. These board provisions could make it more difficult for third parties to gain control of our company by making it difficult to replace members of the Board of Directors.
A director may vote in respect of any contract or transaction in which he is interested, provided, however that the nature of the interest of any director in any such contract or transaction shall be disclosed by him at or prior to its consideration and any vote on that matter. A general notice or disclosure to the directors or otherwise contained in the minutes of a meeting or a written resolution of the directors or any committee thereof of the nature of a director’s interest shall be sufficient disclosure and after such general notice it shall not be necessary to give special notice relating to any particular transaction. A director may be counted for a quorum upon a motion in respect of any contract or arrangement which he shall make with our company, or in which he is so interested and may vote on such motion.
There are no membership qualifications for directors. Further, there are no share ownership qualifications for directors unless so fixed by us in a general meeting.
The Board of Directors maintains a majority of independent directors who are deemed to be independent under the definition of independence provided by NASDAQ Listing Rule 5605(a)(15). Solomon Chen, Dr. Wenbo Chen and Youhang Peng are our independent directors.
Mr. Hong Yu serves as the chairman of the board of directors. He has entered into a retainer agreement for his services as chairman of the board of directors, which agreement carries an initial term of 36 months and a renewal term of 24 months. The agreement provides for an annual salary of $96,650, payable in monthly installments, as well as reimbursement of his reasonably-incurred expenses, for his services as chairman. Mr. Taylor Guo serves as a director, and is also our CEO. He has a retainer agreement compensating him for his services as CEO, but he receives no additional compensation for serving as a director.
Our Board of Directors plays a key role in our risk oversight. The Board of Directors makes all relevant Company decisions. As such, it is important for us to have both our Chief Executive Officer and Chief Financial Officer serve on the Board as they play key roles in the risk oversight or the Company. As a smaller reporting company with a small board of directors, we believe it is appropriate to have the involvement and input of all of our directors in risk oversight matters.
There are no other arrangements or understandings pursuant to which our directors are selected or nominated.
83
Table of Contents
Committees of the Board of Directors
The board of directors may determine by resolution the existence, powers and composition of committees. Board committees that have been established, and their respective compositions, are as follows: an Audit Committee, presently chaired by Solomon Chen and also comprised of Wenbo Chen and Youhang Peng; a Compensation Committee, presently chaired by Youhang Peng and also composed of Solomon Chen and Wenbo Chen; and a Nominating Committee, now chaired by Solomon Chen and with both Wenbo Chen and Youhang Peng serving as members. The Audit Committee is responsible for overseeing the accounting and financial reporting processes of our company and audits of the financial statements of our company, including the appointment, compensation and oversight of the work of our independent auditors. The Compensation Committee of the board of directors reviews and makes recommendations to the board regarding our compensation policies for our officers and all forms of compensation, and also will administer our incentive compensation plans and equity-based plans (but our board retains the authority to interpret those plans). The Nominating Committee of the board of directors is responsible for the assessment of the performance of the board, considering and making recommendations to the board with respect to the nominations or elections of directors and other governance issues. The nominating committee considers diversity of opinion and experience when nominating directors.
Director Compensation
All directors hold office until the next annual meeting of shareholders at which their respective class of directors is re-elected and until their successors have been duly elected and qualified. There are no family relationships among our directors or executive officers. Officers are elected by and serve at the discretion of the Board of Directors. Employee directors do not receive any compensation for their services. Non-employee directors will be entitled to receive $10,000 per year for serving as directors and may receive option grants from our company. In addition, non-employee directors are entitled to receive compensation for their actual travel expenses for each Board of Directors meeting attended.
Summary Compensation Table FY 2009 – Directors
| Name |
Director Fees earned or paid in cash |
Total(1) | ||||||
| Hong Yu (2)( 3) |
$ | 0 | $ | 0 | ||||
| Taylor Z. Guo(2) |
$ | 0 | $ | 0 | ||||
| Dr. Wenbo Chen(2) |
$ | 0 | $ | 0 | ||||
| Mr. Youhang Peng(2) |
$ | 0 | $ | 0 | ||||
| Mr. Solomon Chen(2) |
$ | 0 | $ | 0 | ||||
| (1) | None of the directors received any common share awards, option awards, nonqualified deferred compensation earnings or non-equity incentive plan compensation in fiscal year 2009. |
| (2) | As TBET was not formed until 2010, none of the directors received any compensation in fiscal year 2009. |
| (3) | Mr. Yu has entered into a retainer agreement effective April 30, 2010 that contemplates payment to him of an annual salary of $96,650 for his services as chairman of the board, as well as for reimbursement of his reasonably incurred expenses. The agreement does not provide for separate compensation for his service on the board. He also serves as the chairman of YSTP’s board and, until mid-2009, served as its General Manager. In 1999, he received total compensation of $96,638 for his services as General Manager. |
Contractual Arrangements with Domestic Companies and their Shareholders
We operate our business in China through a series of contractual arrangements with the Domestic Companies and their shareholders. For a description of these contractual arrangements, see “Our Corporate Structure – Contractual Arrangements with Domestic Companies and their Shareholders.”
84
Table of Contents
Relationship with our Placement Agent
In connection with this offering, we have agreed to allow our Placement Agent to designate two non-voting observers to our Board of Directors until the earlier of the date that:
| • | the investors that purchase shares in this offering beneficially own less than 5% of our outstanding shares; or |
| • | the trading price per share is at least $24 per share for any consecutive 15 trading day period. |
It is anticipated that Mr. Downs, our Placement Agent’s Senior Vice President, and Mr. Hayden Zou will serve as the Placement Agent’s observers to our Board of Directors.
Although our placement agent’s observers will not be able to vote, they may nevertheless influence the outcome of matters submitted to the Board of Directors for approval. We have agreed to reimburse the observers for their expenses for attending our Board meetings, subject to a maximum reimbursement of $6,000 per meeting and $12,000 annually, which amount is not more than the reimbursement payable to our directors. The observer will be required to certify that such travel expenses are not reimbursed by any other party. We will also pay observers the same amount as our independent directors receive.
Future Related Party Transactions
The Corporate Governance Committee of our Board of Directors must approve all related party transactions. All material related party transactions will be made or entered into on terms that are no less favorable to us than can be obtained from unaffiliated third parties. Related party transactions that we have previously entered into were not approved by independent directors, as we had no independent directors at that time.
Interested Transactions
A director may vote, attend a board meeting or sign a document on our behalf with respect to any contract or transaction in which he or she is interested. A director must promptly disclose the interest to all other directors after becoming aware of the fact that he or she is interested in a transaction we have entered into or are to enter into. A general notice or disclosure to the board or otherwise contained in the minutes of a meeting or a written resolution of the board or any committee of the board that a director is a shareholder, director, officer or trustee of any specified firm or company and is to be regarded as interested in any transaction with such firm or company will be sufficient disclosure, and, after such general notice, it will not be necessary to give special notice relating to any particular transaction.
Remuneration and Borrowing
The directors may receive such remuneration as our board of directors may determine from time to time. Each director is entitled to be repaid or prepaid all traveling, hotel and incidental expenses reasonably incurred or expected to be incurred in attending meetings of our board of directors or committees of our board of directors or shareholder meetings or otherwise in connection with the discharge of his or her duties as a director. The compensation committee will assist the directors in reviewing and approving the compensation structure for the directors. Our board of directors may exercise all the powers of the company to borrow money and to mortgage or charge our undertakings and property or any part thereof, to issue debentures, debenture stock and other securities whenever money is borrowed or as security for any debt, liability or obligation of the company or of any third party.
Qualification
A director is not required to hold shares as a qualification to office.
Share Incentive Plan
We intend to establish a pool for share options for our employees following the completion of this offering. This pool will contain options to purchase our common shares equal to up to five percent (5%) of the number of common shares outstanding at the conclusion of this offering, not including any shares underlying placement agent’s warrants. This pool will contain options to purchase up to 684,375 of our common shares subject to outstanding
85
Table of Contents
share options or reserved for issuance under our share incentive plan, assuming a maximum offering. The options will vest at a rate of 20% per year for five years. Options granted to employees will have a per share exercise price equal to the market price of the ordinary shares on the date of the option grant, except that options granted to employees on the date of the offering will have an exercise price equal to the offering price.
Limitation of Director and Officer Liability
Under British Virgin Islands law, each of our directors and officers, in performing his or her functions, is required to act honestly and in good faith with a view to our best interests and exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances. Our memorandum and articles of association provide that, to the fullest extent permitted by British Virgin Islands law or any other applicable laws, our directors will not be personally liable to us or our shareholders for any acts or omissions in the performance of their duties. Such limitation of liability does not affect the availability of equitable remedies such as injunctive relief or rescission. These provisions will not limit the liability of directors under United States federal securities laws.
Our articles of association provide that the Company shall, subject to certain limitations, indemnify against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with legal, administrative or investigative proceedings any person. among others, is or was, at the request of the Company, serving as a director of, or in any other capacity or is acting for the Company. We may only indemnify a director if he or she acted honestly and in good faith with the view to our best interests and, in the case of criminal proceedings, the director had no reasonable cause to believe that his or her conduct was unlawful. The decision of our board of directors as to whether the director acted honestly and in good faith with a view to our best interests and as to whether the director had no reasonable cause to believe that his or her conduct was unlawful, is in the absence of fraud sufficient for the purposes of indemnification, unless a question of law is involved. The termination of any proceedings by any judgment, order, settlement, conviction or the entry of no plea does not, by itself, create a presumption that a director did not act honestly and in good faith and with a view to our best interests or that the director had reasonable cause to believe that his or her conduct was unlawful. If a director to be indemnified has been successful in defense of any proceedings referred to above, the director is entitled to be indemnified against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred by the director or officer in connection with the proceedings.
We may purchase and maintain insurance in relation to any of our directors or officers against any liability asserted against the directors or officers and incurred by the directors or officers in that capacity, whether or not we have or would have had the power to indemnify the directors or officers against the liability as provided in our fourth amended and restated memorandum and articles of association.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for our directors, officers or persons controlling our company under the foregoing provisions, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Code of Ethics
We have put in place a code of ethics, entitled the “Code of Business Conduct and Ethics,” that applies to all of our employees, including but not limited to our chief executive officer and chief financial officer.
Transactions among Subsidiaries
We operate our business in China through a series of contractual arrangements between WFOE and YSTP and its shareholders, who are related parties. For a description of these contractual arrangements, see “Our Corporate Structure – Contractual Arrangements with Domestic Companies and their Shareholders.”
86
Table of Contents
Transactions among Certain Directors, Shareholders and Affiliates
Kunming Shangri-La Medicine Co., Ltd. is a distributor of YSTP. Our chairman, Mr. Yu, owns 31% of it. At December 31, 2009 and 2008, YSTP had account receivables from Kunming Shangri-La Medicine of approximately $342,000 and $1,277,550, respectively. Such amounts were normal business transactions, payable in 60 days.
At December 31, 2009, YSTP had account receivables to related parties of approximately $1,550,085. Such amounts were payable pursuant to a loan to Mr. Hong Yu, our founder and chairman. As of May 1, 2010, Mr. Hong Yu has paid off such loan balance in full.
Make-Good Shares Subject to Redemption
As described in more detail in the section entitled “Placement – Market and Pricing Consideration” our company had been valued on a forward-looking basis for purposes of this offering. We and our Placement Agent agreed to value our company at a multiple of approximately seven (7) times our targeted 2010 audited net after-tax income. Based on a target 2010 audited net after-tax income of $13,500,000, we have been valued at approximately $94,500,000. Our targeted 2010 net after-tax income is not a projection but instead represents a negotiated target between our company and our Placement Agent. Although we do not currently pay any taxes on our income, we refer to this amount as our targeted 2010 audited net after-tax income to reflect our and our Placement Agent’s intention that the targeted amount will be net of any taxes that may apply to our company during 2010. Based on the above valuation of our company, our earnings per share would be approximately $0.9863. This amount is calculated by dividing $13,500,000 by 13,687,500 shares outstanding upon completion of a maximum offering, not including any shares in the incentive plan or the exercise of any Placement Agent Warrants.
Valuing a company on a forward-looking basis is subject to a number of risks, including the possibility that the company will not achieve the targeted income levels and that world markets may not maintain the same valuation for companies in general in the future. In order to mitigate some of this risk, certain key members of the management of our company have agreed to place that number of ordinary shares into escrow that is equal to 40% of the maximum number of shares to be sold in this offering. Upon closing of this offering, the escrow agent will return any shares in excess of 40% of the actual number of shares sold in the offering. Such escrowed shares are referred to as the “Make-Good Shares”). The Make-Good Shares will remain in escrow with SunTrust Bank or another bank acceptable to our Placement Agent pending the filing of our company’s Form 10-K for the year ending December 31, 2010.
To the extent our audited after-tax earnings per share issued and outstanding immediately after the offering for the year ending December 31, 2010 are less than $0.9863, excluding any expenses associated with releasing the Make-Good Shares back to the original owners, our company will redeem and cancel, pro rata, the Make-Good Shares without any consideration to the extent necessary to cause our audited after-tax earnings to be equal to $0.9863. We cannot guarantee that we will be able to redeem a sufficient number of Make-Good Shares to increase audited after-tax earnings per share to $0.9863 if our company either has low net income or any net losses in 2010. Any remaining Make-Good Shares will be released from escrow to Hong Yu and Taylor Z. Guo upon the earlier of (i) one (1) business day after the termination of this offering without closing or (ii) thirty (30) calendar days after the filing of the Form 10-K for the year ending December 31, 2010 after redeeming any Make-Good Shares. See “Risk Factors – A redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company.”
Because the Make-Good Shares have been escrowed as a condition of completing the initial public offering and will be released to the holders thereof without regard to such holders’ continued employment if YSTP meets the foregoing criteria, we have determined that no compensatory arrangement exists. Accordingly, we account for the Make-Good Shares as an element of the overall transaction and we do not recognize any compensation expense upon the return of such Make-Good Shares to the holders. If our company does not meet the criteria for releasing the Make-Good Shares, then we will redeem the Make-Good Shares without payment, resulting in the reduction of YSTP ordinary shares outstanding.
87
Table of Contents
Our authorized capital stock consists of 50,000,000 ordinary shares, par value $0.001 per share. As of the date of this prospectus, 11,812,500 ordinary shares are issued and outstanding. An additional 684,500 shares of ordinary shares have been reserved for issuance upon exercise of outstanding options. The following summary description relating to our capital stock does not purport to be complete and is qualified in its entirety by our Memorandum and Articles of Association.
Ordinary Shares
Holders of ordinary shares are entitled to cast one vote for each share on all matters submitted to a vote of shareholders, including the election of directors. The holders of ordinary shares are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds legally available therefor and subject to any preference of any then authorized and issued preferred stock. See “Dividend Policy.” Such holders do not have any preemptive or other rights to subscribe for additional shares. All holders of ordinary shares are entitled to share ratably in any assets for distribution to shareholders upon the liquidation, dissolution or winding up of the Company, subject to any preference of any then authorized and issued preferred stock. The Memorandum of Association authorizes the Board of Directors may by resolution authorize the redemption, purchase or other acquisition of the ordinary shares, and in our Articles of Association the Board did authorize the redemption or purchase, subject to limitations and conditions stated therein. All outstanding ordinary shares are fully paid and nonassessable.
Limitations on the Right to Own Shares
There are no limitations on the right to own our ordinary shares.
Limitations on Transfer of Shares
Our Articles of Association gives our directors, at their discretion, the right to decline to register any transfer of shares.
Disclosure of Shareholder Ownership
There are no provisions in our Memorandum of Association or Articles of Association governing the ownership threshold above which shareholder ownership must be disclosed.
Changes in Capital
We may from time to time by ordinary resolution increase the share capital by such sum, to be divided into shares of such amount, as the resolution shall prescribe. The new shares shall be subject to the same provisions with reference to the payment of calls, lien, transfer, transmission, forfeiture and otherwise as the shares in the original share capital. We may by ordinary resolution:
| • | consolidate and divide all or any of our share capital into shares of larger amount than our existing shares; |
| • | convert all or any of our paid up shares into stock and reconvert that stock into paid up shares of any denomination; |
| • | in many circumstances, sub-divide our existing shares, or any of them, into shares of smaller amount provided that in the subdivision the proportion between the amount paid and the amount, if any, unpaid on each reduced share shall be the same as it was in the case of the share form which the reduced share is derived; and |
| • | cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of its share capital by the amount of the shares so cancelled. |
We may by special resolution reduce our share capital and any capital redemption reserve fund in any manner authorized by law. In addition, if earnings per share at the end of 2010 do not reach the percentage
prescribed in the Make Good Shares escrow agreement, some or all shares in escrow may be cancelled, in which case our total share capital would be reduced.
88
Table of Contents
The BVI Act and the laws of the British Virgin Islands affecting British Virgin Islands companies like us and our shareholders differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the laws of the British Virgin Islands applicable to us and the laws applicable to companies incorporated in the United States and their shareholders.
Mergers and similar arrangements
Under the laws of the British Virgin Islands, two or more companies may merge or consolidate in accordance with Section 170 of the BVI Act. A merger means the merging of two or more constituent companies into one of the constituent companies and a consolidation means the uniting of two or more constituent companies into a new company. In order to merge or consolidate, the directors of each constituent company must approve a written plan of merger or consolidation, which must be authorized by a resolution of shareholders.
While a director may vote on the plan of merger or consolidation even if he has a financial interest in the plan, the interested director must disclose the interest to all other directors of the company promptly upon becoming aware of the fact that he is interested in a transaction entered into or to be entered into by the company.
A transaction entered into by our company in respect of which a director is interested (including a merger or consolidation) is voidable by us unless the director’s interest was (a) disclosed to the board prior to the transaction or (b) the transaction is (i) between the director and the company and (ii) the transaction is in the ordinary course of the company’s business and on usual terms and conditions.
Notwithstanding the above, a transaction entered into by the company is not voidable if the material facts of the interest are known to the shareholders and they approve or ratify it or the company received fair value for the transaction.
Shareholders not otherwise entitled to vote on the merger or consolidation may still acquire the right to vote if the plan of merger or consolidation contains any provision which, if proposed as an amendment to the memorandum or articles of association, would entitle them to vote as a class or series on the proposed amendment. In any event, all shareholders must be given a copy of the plan of merger or consolidation irrespective of whether they are entitled to vote at the meeting to approve the plan of merger or consolidation.
The shareholders of the constituent companies are not required to receive shares of the surviving or consolidated company but may receive debt obligations or other securities of the surviving or consolidated company, other assets, or a combination thereof. Further, some or all of the shares of a class or series may be converted into a kind of asset while the other shares of the same class or series may receive a different kind of asset. As such, not all the shares of a class or series must receive the same kind of consideration.
After the plan of merger or consolidation has been approved by the directors and authorized by a resolution of the shareholders, articles of merger or consolidation are executed by each company and filed with the Registrar of Corporate Affairs in the British Virgin Islands.
A shareholder may dissent from a mandatory redemption of his shares, an arrangement (if permitted by the court), a merger (unless the shareholder was a shareholder of the surviving company prior to the merger and continues to hold the same or similar shares after the merger) or a consolidation. A shareholder properly exercising his dissent rights is entitled to a cash payment equal to the fair value of his shares.
A shareholder dissenting from a merger or consolidation must object in writing to the merger or consolidation before the vote by the shareholders on the merger or consolidation, unless notice of the meeting was not given to the shareholder. If the merger or consolidation is approved by the shareholders, the company must give
89
Table of Contents
notice of this fact to each shareholder within 20 days who gave written objection. These shareholders then have 20 days to give to the company their written election in the form specified by the BVI Act to dissent from the merger or consolidation, provided that in the case of a merger, the 20 days starts when the plan of merger is delivered to the shareholder.
Upon giving notice of his election to dissent, a shareholder ceases to have any shareholder rights except the right to be paid the fair value of his shares. As such, the merger or consolidation may proceed in the ordinary course notwithstanding his dissent.
Within seven days of the later of the delivery of the notice of election to dissent and the effective date of the merger or consolidation, the company must make a written offer to each dissenting shareholder to purchase his shares at a specified price per share that the company determines to be the fair value of the shares. The company and the shareholder then have 30 days to agree upon the price. If the company and a shareholder fail to agree on the price within the 30 days, then the company and the shareholder shall, within 20 days immediately following the expiration of the 30-day period, each designate an appraiser and these two appraisers shall designate a third appraiser. These three appraisers shall fix the fair value of the shares as of the close of business on the day prior to the shareholders’ approval of the transaction without taking into account any change in value as a result of the transaction.
Shareholders’ suits
There are both statutory and ordinary law remedies available to our shareholders as a matter of British Virgin Islands law. These are summarized below:
Prejudiced members
A shareholder who considers that the affairs of the company have been, are being, or are likely to be, conducted in a manner that is, or any act or acts of the company have been, or are, likely to be oppressive, unfairly discriminatory or unfairly prejudicial to him in that capacity, can apply to the court under Section 184I of the BVI Act, among other things, for an order that his shares be acquired, that he be provided compensation, that the Court regulate the future conduct of the company, or that any decision of the company which contravenes the BVI Act or our memorandum and articles of association be set aside.
Derivative actions
Section 184C of the BVI Act provides that a shareholder of a company may, with the leave of the Court, bring an action in the name of the company to redress any wrong done to it.
Just and equitable winding up
In addition to the statutory remedies outlined above, shareholders can also petition for the winding up of a company on the grounds that it is just and equitable for the court to so order. Save in exceptional circumstances, this remedy is only available where the company has been operated as a quasi partnership and trust and confidence between the partners has broken down.
Indemnification of directors and executive officers and limitation of liability
British Virgin Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any provision providing indemnification may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime.
90
Table of Contents
Under our memorandum and articles of association, we indemnify against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with legal, administrative or investigative proceedings for any person who:
| • | is or was a party or is threatened to be made a party to any threatened, pending or completed proceedings, whether civil, criminal, administrative or investigative, by reason of the fact that the person is or was our director; or |
| • | is or was, at our request, serving as a director or officer of, or in any other capacity is or was acting for, another body corporate or a partnership, joint venture, trust or other enterprise. |
These indemnities only apply if the person acted honestly and in good faith with a view to our best interests and, in the case of criminal proceedings, the person had no reasonable cause to believe that his conduct was unlawful.
This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Anti-takeover provisions in our memorandum and articles of association
Some provisions of our memorandum and articles of association may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable, including provisions that provide for a staggered board of directors and prevent shareholders from taking an action by written consent in lieu of a meeting. However, under British Virgin Islands law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association, as amended and restated from time to time, as they believe in good faith to be in the best interests of our company.
Directors’ fiduciary duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction and that the transaction was of fair value to the corporation.
Under British Virgin Islands law, our directors owe the company certain statutory and fiduciary duties including, among others, a duty to act honestly, in good faith, for a proper purpose and with a view to what the directors believe to be in the best interests of the company. Our directors are also required, when exercising powers or performing duties as a director, to exercise the care, diligence and skill that a reasonable director would exercise in comparable circumstances, taking into account without limitation, the nature of the company, the nature of the decision and the position of the director and the nature of the responsibilities undertaken. In the exercise of their powers, our directors must ensure neither they nor the company acts in a manner which contravenes the BVI Act or our memorandum and articles of association, as amended and re-stated from time to time. A shareholder has the right to seek damages for breaches of duties owed to us by our directors.
Shareholder action by written consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. British Virgin Islands law provides that shareholders may approve corporate matters by way of a written resolution without a meeting signed by or on behalf
91
Table of Contents
of shareholders sufficient to constitute the requisite majority of shareholders who would have been entitled to vote on such matter at a general meeting; provided that if the consent is less than unanimous, notice must be given to all non-consenting shareholders. However, our memorandum and articles of association do not permit shareholders to act by written consent.
Shareholder proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings. British Virgin Islands law and our memorandum and articles of association allow our shareholders holding not less than 30% of the votes of the outstanding voting shares to requisition a shareholders’ meeting. We are not obliged by law to call shareholders’ annual general meetings, but our memorandum and articles of association do permit the directors to call such a meeting. The location of any shareholders’ meeting can be determined by the board of directors and can be held anywhere in the world.
Cumulative voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. As permitted under British Virgin Islands law, our memorandum and articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our memorandum and articles of association, directors can be removed from office, with cause, by a resolution of shareholders or by a resolution of directors passed at a meeting of directors called for the purpose of removing the director or for purposes including the removal of the director.
Transactions with interested shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or group who or which owns or owned 15% or more of the target’s outstanding voting shares within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware public corporation to negotiate the terms of any acquisition transaction with the target’s board of directors. British Virgin Islands law has no comparable statute. However, our memorandum and articles of association expressly provide for the same protection afforded by the Delaware business combination statute.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the
92
Table of Contents
corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board. Under the BVI Act and our memorandum and articles of association, we may appoint a voluntary liquidator by a resolution of the shareholders or by resolution of directors.
Variation of rights of shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. Under our memorandum and articles of association, if at any time our shares are divided into different classes of shares, the rights attached to any class may only be varied, whether or not our company is in liquidation, by a resolution passed at a meeting by a majority of the votes cast by those entitled to vote at a meeting of the holders of the issued shares in that class.
Amendment of governing documents
Under the Delaware General Corporation Law, a corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. As permitted by British Virgin Islands law, our memorandum and articles of association may be amended by a resolution of shareholders and, subject to certain exceptions, by a resolution of directors. Any amendment is effective from the date it is registered at the Registry of Corporate Affairs in the British Virgin Islands.
93
Table of Contents
PRINCIPAL SHAREHOLDERS
The following table sets forth information with respect to beneficial ownership of our common shares as of May 14, 2010 by:
| • | Each person who is known by us to beneficially own more than 5% of our outstanding common shares; |
| • | Each of our directors and named executive officers; and |
| • | All directors and named executive officers as a group. |
The number and percentage of common shares beneficially owned before the offering are based on 11,812,500 common shares outstanding as of May 14, 2010. Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common shares. Beneficial ownership is determined in accordance with the rules of the SEC and generally requires that such person have voting or investment power with respect to securities. In computing the number of common shares beneficially owned by a person listed below and the percentage ownership of such person, common shares underlying options, warrants or convertible securities held by each such person that are exercisable or convertible within 60 days of May 14, 2010 are deemed outstanding, but are not deemed outstanding for computing the percentage ownership of any other person. Except as otherwise indicated in the footnotes to this table, or as required by applicable community property laws, all persons listed have sole voting and investment power for all common shares shown as beneficially owned by them. Unless otherwise indicated in the footnotes, the address for each principal shareholder is in the care of: Shangri-La Tibetan Pharmaceutical Group, Inc., 53 Niwang Road, Shangri-La County, Yunnan Province, China. As of the date of the Prospectus, we had 27 shareholders of record.
| Named Executive Officers and Directors |
Amount of Beneficial Ownership(1) |
Percentage Ownership(2) |
|||
| Hong Yu, Chairman of the Board |
3,280,000 | 27.8 | % | ||
| Taylor Z. Guo, CEO and Director(3) |
4,484,500 | 38.0 | % | ||
| Sabrina Y. Ren, CFO |
0 | 0 | % | ||
| Dr. Wenbo Chen, Director |
0 | 0 | % | ||
| Mr. Youhang Peng, Director |
0 | 0 | % | ||
| Mr. Solomon Chen, Director |
0 | 0 | % |
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the common shares. |
| (2) | The number of our common shares outstanding used in calculating the percentage for each listed person excludes the common shares underlying options held by such person. |
| (3) | Mr. Guo owns 150,000 shares directly and is the beneficial owner of another 4,334,500 shares, owned in fact by others, by reason of trust agreements giving him the right to vote those shares. |
94
Table of Contents
Shares Eligible for Future Sale
Prior to this offering, there has been no market for our ordinary shares, and a liquid trading market for our ordinary shares may not develop or be sustained after this offering. Future sales of substantial amounts of ordinary shares, including ordinary shares issued upon exercise of outstanding options and exercise of the warrants offered in this prospectus in the public market after this offering or the anticipation of those sales could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of our equity securities.
Upon the completion of the offering, we will have outstanding 13,687,500 ordinary shares, assuming no the closing of the maximum offering and not including any shares underlying the underwriter warrants. Of these ordinary shares, the 1,875,000 ordinary shares sold in this offering and the up to 354,375 shares sold by the selling shareholders described above will be freely tradable without restriction under the Securities Act, except that any shares purchased by our “affiliates,” as that term is defined in Rule 144 of the Securities Act, may generally only be sold in compliance with the limitations of Rule 144 described below. The remaining approximately 11,458,125 ordinary shares outstanding will be restricted shares held by existing shareholders that could be sold pursuant to Rule 144. We have not agreed to register these restricted shares. We have not issued any warrants to purchase our ordinary shares or other securities convertible into our ordinary shares.
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person (or persons whose shares are aggregated) who is deemed to be an affiliate of our company at the time of sale, or at any time during the preceding three months, and who has beneficially owned restricted shares for at least six months, would be entitled to sell within any three-month period a number of our ordinary shares that does not exceed the greater of 1% of the then outstanding ordinary shares or the average weekly trading volume of ordinary shares during the four calendar weeks preceding such sale. Sales under Rule 144 are subject to certain manner of sale provisions, notice requirements and the availability of current public information about our company. In addition, sales by our affiliates may be subject to the terms of lock-up agreements and Make-Good Escrow agreements. See “Shares Eligible for Future Sale – Lock-Up Agreements” and “Related Party Transactions –Make-Good Shares Subject to Redemption.”
A person who has not been our affiliate at any time during the three months preceding a sale, and who has beneficially owned his or her ordinary shares for at least six months, would be entitled under Rule 144 to sell such shares without regard to any manner of sale, notice provisions or volume limitations described above. Any such sales must comply with the public information provision of Rule 144 until our ordinary shares have been held for one year.
Rule 701
Securities issued in reliance on Rule 701 are also restricted and may be sold by shareholders other than affiliates of our company subject only to manner of sale provisions of Rule 144 and by affiliates under Rule 144 without compliance with its six-month holding period requirement.
Registration on Form S-8
We intend to file a registration statement on Form S-8 under the Securities Act as soon as practicable after the closing of this offering to register up to 1,368,750 of our ordinary shares subject to outstanding stock options or reserved for issuance under our stock incentive plan, such amount being equal to ten percent (10%) of the number of ordinary shares issued and outstanding after the closing of the offering, assuming a maximum offering. This registration will permit the resale of these ordinary shares by nonaffiliates in the public market without restriction under the Securities Act, upon the completion of the lock-up period described below. Ordinary shares registered pursuant to the Form S-8 held by affiliates will be subject to Rule 144 volume limitations. As of the date of this Prospectus, we have not issued any options to purchase our ordinary shares.
95
Table of Contents
Lock-Up Agreements
Each of our executive officers, directors and individuals who on the effective date of the registration statement of which this prospectus is a part are the beneficial owners of more than 5% of our ordinary shares, has agreed not to register, offer, sell, contract to sell or grant any of our ordinary shares or any securities convertible into or exercisable or exchangeable for our ordinary shares or any warrants to purchase our ordinary shares (including, without limitation, securities of our company which may be deemed to be beneficially owned by such individuals in accordance with the rules and regulations of the Securities and Exchange Commission and securities which may be issued upon the exercise of a stock option or warrant) for a period of (a) as to one-half (1/2) of the ordinary shares now or in the future beneficially owned by such individual, ninety (90) days after the date of effectiveness or commencement of sales of this public offering and (b) as to the other one-half of such ordinary shares now or in the future beneficially owned by such individual, one hundred ninety (190) days after the date of effectiveness or commencement of sales of this public offering. Upon the expiration of these lock-up agreements, additional ordinary shares will be available for sale in the public market.
Registration
We are concurrently registering for resale under a separate prospectus up to 354,375 shares of our ordinary stock held by the selling shareholders named in the prospectus. We are not offering any of these shares, and we will not receive any proceeds from the sale of the shares.
96
Table of Contents
Summary of Shares Available for Future Sale
The following table summarizes the total shares potentially available for future sale. To the extent we sell a number of ordinary shares between the minimum and maximum offering, the below tables will be adjusted proportionately as to numbers of shares available for sale (as to option pool and Placement Agent shares) and dates on which such shares may be sold (as to currently outstanding shares).
Minimum Offering
| Shares |
Date Available for Sale | |
| Currently Outstanding Shares: 11,812,500 | ||
| Up to 354,375 |
After the date of this prospectus, the shares will have been registered upon a separate resale prospectus and will be tradable by certain selling shareholders listed in the resale prospectus pursuant to that prospectus. | |
| 9,443,125 |
After 90 days from the date of effectiveness or commencement of sales of the public offering | |
| 1,415,000 |
After 190 days from the date of effectiveness or commencement of sales of the public offering | |
| 600,000 |
After 30 days after filing of Form 10-K for the year ending December 31, 2010, assuming no redemption of Make-Good Shares; any delay in redemption will also delay the release of these shares | |
| Ordinary Shares in Option Pool: Up to 665,625 | From vesting dates through expiration of grants | |
| Ordinary Shares Underlying Placement Agent’s Warrants: 150,000 | After 180 days from the date of effectiveness or commencement of sales of the public offering | |
| Shares Offered in this Offering: 1,500,000 | After the date of this prospectus, these shares will be freely tradable. | |
Maximum Offering
| Shares |
Date Available for Sale | |
| Currently Outstanding Shares: 11,812,500 | ||
| Up to 354,375 |
After the date of this prospectus, the shares will have been registered upon a separate resale prospectus and will be tradable by certain selling shareholders listed in the resale prospectus pursuant to that prospectus. | |
| 9,368,125 |
After 90 days from the date of effectiveness or commencement of sales of the public offering | |
| 1,340,000 |
After 190 days from the date of effectiveness or commencement of sales of the public offering | |
| 750,000 |
After 30 days after filing of Form 10-K for the year ending December 31, 2010, assuming no redemption of Make-Good Shares; any delay in redemption will also delay the release of these shares | |
| Ordinary Shares in Option Pool: Up to 684,375 | From vesting dates through expiration of grants | |
| Ordinary Shares Underlying Placement Agent’s Warrants: 1,875,500 | After 180 days from the date of effectiveness or commencement of sales of the public offering | |
| Shares Offered in this Offering: 1,875,500 | After the date of this prospectus, these shares will be freely tradable. | |
97
Table of Contents
Tax Matters Applicable to U.S. Holders of Our Ordinary Shares
The following sets forth the material British Virgin Islands, Chinese and U.S. federal income tax matters related to an investment in our ordinary shares. It is directed to U.S. Holders (as defined below) of our ordinary shares and is based upon laws and relevant interpretations thereof in effect as of the date of this prospectus, all of which are subject to change. This description does not deal with all possible tax consequences relating to an investment in our ordinary shares, such as the tax consequences under state, local and other tax laws.
The following brief description applies only to U.S. Holders (defined below) that hold ordinary shares as capital assets and that have the U.S. dollar as their functional currency. This brief description is based on the tax laws of the United States in effect as of the date of this prospectus and on U.S. Treasury regulations in effect or, in some cases, proposed, as of the date of this prospectus, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing authorities are subject to change, which change could apply retroactively and could affect the tax consequences described below.
The brief description below of the U.S. federal income tax consequences to “U.S. Holders” will apply to you if you are a beneficial owner of shares and you are, for U.S. federal income tax purposes,
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
WE URGE POTENTIAL PURCHASERS OF OUR SHARES TO CONSULT THEIR OWN TAX
ADVISORS CONCERNING THE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX
CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR SHARES.
People’s Republic of China Enterprise Taxation
The following brief description of Chinese enterprise laws is designed to highlight the enterprise-level taxation on our earnings, which will affect the amount of dividends, if any, we are ultimately able to pay to our shareholders. See “Dividend Policy.” Our company is not subject to any enterprise income tax. Our exemption from enterprise income tax derives from the Implementation Rules for the Law on Enterprise Income Tax, which exempts from enterprise income tax for enterprises located in minority ethnic autonomous areas and engaged in encouraged or permitted industries. If this exemption was to be terminated or we were to fail to qualify to receive these exemptions, we would be subject to taxation at the standard 25% for enterprise income taxes, unless we were otherwise to qualify for a decreased tax rate.
British Virgin Islands Taxation
Under the BVI Act as currently in effect, a holder of ordinary shares who is not a resident of the British Virgin Islands is exempt from British Virgin Islands income tax on dividends paid with respect to the ordinary shares and all holders of ordinary shares are not liable to the British Virgin Islands for income tax on gains realized during that year on sale or disposal of such shares. The British Virgin Islands does not impose a withholding tax on dividends paid by a company incorporated or re-registered under the BVI Act.
There are no capital gains, gift or inheritance taxes levied by the British Virgin Islands on companies incorporated or re-registered under the BVI Act. In addition, shares of companies incorporated or re-registered under the BVI Act are not subject to transfer taxes, stamp duties or similar charges.
There is no income tax treaty or convention currently in effect between the United States and the British Virgin Islands or between China and the British Virgin Islands.
98
Table of Contents
United States Federal Income Taxation
The following does not address the tax consequences to any particular investor or to persons in special tax situations such as:
| • | banks; |
| • | financial institutions; |
| • | insurance companies; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | broker-dealers; |
| • | traders that elect to mark-to-market; |
| • | U.S. expatriates; |
| • | tax-exempt entities; |
| • | persons liable for alternative minimum tax; |
| • | persons holding our ordinary shares as part of a straddle, hedging, conversion or integrated transaction; |
| • | persons that actually or constructively own 10% or more of our voting shares; |
| • | persons who acquired our ordinary shares pursuant to the exercise of any employee share option or otherwise as consideration; or |
| • | persons holding our ordinary shares through partnerships or other pass-through entities. |
Prospective purchasers are urged to consult their tax advisors about the application of the U.S. Federal tax rules to their particular circumstances as well as the state, local, foreign and other tax consequences to them of the purchase, ownership and disposition of our ordinary shares.
Taxation of dividends and other distributions on our ordinary shares
Subject to the passive foreign investment company rules discussed below, the gross amount of distributions made by us to you with respect to the ordinary shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). The dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, for taxable years beginning before January 1, 2011, dividends will be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the ordinary shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a passive foreign investment company (as discussed below) for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Under U.S. Internal Revenue Service authority, ordinary shares are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on the NASDAQ Global Market. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our ordinary shares, including the effects of any change in law after the date of this prospectus.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our ordinary shares will constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.”
99
Table of Contents
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your ordinary shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
Taxation of Dispositions of Ordinary Shares
Subject to the passive foreign investment company rules discussed below, you will recognize taxable gain or loss on any sale, exchange or other taxable disposition of a share equal to the difference between the amount realized (in U.S. dollars) for the share and your tax basis (in U.S. dollars) in the ordinary shares. The gain or loss will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the ordinary shares for more than one year, you will be eligible for reduced tax rates of 0% (for individuals in the 10% or 15% tax brackets) or 15% for all other individuals, assuming the renewal of current capital gains rates prior to their scheduled expiration at the end of 2010. If capital gains preferential rates are not renewed, such gains would be taxable at the personal income rates then in place. The deductibility of capital losses is subject to limitations. Any such gain or loss that you recognize will generally be treated as United States source income or loss for foreign tax credit limitation purposes.
Passive Foreign Investment Company
Based on our current and anticipated operations and the composition of our assets, we do not expect to be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for our current taxable year ending December 31, 2010. Our actual PFIC status for the current taxable year ending December 31, 2010 will not be determinable until the close of such taxable year and, accordingly, there is no guarantee that we will not be a PFIC for the current taxable year. Because PFIC status is a factual determination for each taxable year which cannot be made until the close of the taxable year. A non-U.S. corporation is considered a PFIC for any taxable year if either:
| • | at least 75% of its gross income is passive income; or |
| • | at least 50% of the value of its assets (based on an average of the quarterly values of the assets during a taxable year) is attributable to assets that produce or are held for the production of passive income (the “asset test”). |
We will be treated as owning our proportionate share of the assets and earning our proportionate share of the income of any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock.
We must make a separate determination each year as to whether we are a PFIC. As a result, our PFIC status may change. In particular, because the value of our assets for purposes of the asset test will generally be determined based on the market price of our ordinary shares, our PFIC status will depend in large part on the market price of our ordinary shares. Accordingly, fluctuations in the market price of the ordinary shares may cause us to become a PFIC. In addition, the application of the PFIC rules is subject to uncertainty in several respects and the composition of our income and assets will be affected by how, and how quickly, we spend the cash we raise in this offering. If we are a PFIC for any year during which you hold ordinary shares, we will continue to be treated as a PFIC for all succeeding years during which you hold ordinary shares. However, if we cease to be a PFIC, you may avoid some of the adverse effects of the PFIC regime by making a “deemed sale” election with respect to the ordinary shares.
If we are a PFIC for any taxable year during which you hold ordinary shares, you will be subject to special tax rules with respect to any “excess distribution” that you receive and any gain you realize from a sale or other disposition (including a pledge) of the ordinary shares, unless you make a “mark-to-market” election as discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the ordinary shares will be treated as an excess distribution. Under these special tax rules:
| • | the excess distribution or gain will be allocated ratably over your holding period for the ordinary shares; |
100
Table of Contents
| • | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we were a PFIC, will be treated as ordinary income, and |
| • | the amount allocated to each other year will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to years prior to the year of disposition or “excess distribution” cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the sale of the ordinary shares cannot be treated as capital, even if you hold the ordinary shares as capital assets.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election for such stock to elect out of the tax treatment discussed above. If you make a mark-to-market election for the ordinary shares, you will include in income each year an amount equal to the excess, if any, of the fair market value of the ordinary shares as of the close of your taxable year over your adjusted basis in such ordinary shares. You are allowed a deduction for the excess, if any, of the adjusted basis of the ordinary shares over their fair market value as of the close of the taxable year. However, deductions are allowable only to the extent of any net mark-to-market gains on the ordinary shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as gain on the actual sale or other disposition of the ordinary shares, are treated as ordinary income. Ordinary loss treatment also applies to the deductible portion of any mark-to-market loss on the ordinary shares, as well as to any loss realized on the actual sale or disposition of the ordinary shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such ordinary shares. Your basis in the ordinary shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, the tax rules that apply to distributions by corporations which are not PFICs would apply to distributions by us, except that the lower applicable capital gains rate for qualified dividend income discussed above under “—Taxation of Dividends and Other Distributions on our Ordinary shares” generally would not apply.
The mark-to-market election is available only for “marketable stock”, which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a qualified exchange or other market (as defined in applicable U.S. Treasury regulations), including the NASDAQ Global Market. If the ordinary shares are regularly traded on the NASDAQ Global Market and if you are a holder of ordinary shares, the mark-to-market election would be available to you were we to be or become a PFIC.
Alternatively, a U.S. Holder of stock in a PFIC may make a “qualified electing fund” election with respect to such PFIC to elect out of the tax treatment discussed above. A U.S. Holder who makes a valid qualified electing fund election with respect to a PFIC will generally include in gross income for a taxable year such holder’s pro rata share of the corporation’s earnings and profits for the taxable year. However, the qualified electing fund election is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. We do not currently intend to prepare or provide the information that would enable you to make a qualified electing fund election. If you hold ordinary shares in any year in which we are a PFIC, you will be required to file U.S. Internal Revenue Service Form 8621 regarding distributions received on the ordinary shares and any gain realized on the disposition of the ordinary shares.
You are urged to consult your tax advisors regarding the application of the PFIC rules to your investment in our ordinary shares and the elections discussed above.
Information Reporting and Backup Withholding
Dividend payments with respect to our ordinary shares and proceeds from the sale, exchange or redemption of our ordinary shares may be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding at a current rate of 28%. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or who is otherwise exempt from backup withholding. U.S. Holders who are required to
101
Table of Contents
establish their exempt status generally must provide such certification on U.S. Internal Revenue Service Form W-9. U.S. Holders are urged to consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information.
102
Table of Contents
Enforceability of Civil Liabilities
We are incorporated under the laws of the British Virgin Islands with limited liability. We are incorporated in the British Virgin Islands because of certain benefits associated with being a British Virgin Islands corporation, such as political and economic stability, an effective judicial system, a favorable tax system, the absence of exchange control or currency restrictions and the availability of professional and support services. However, the British Virgin Islands has a less developed body of securities laws as compared to the United States and provides protections for investors to a significantly lesser extent. In addition, British Virgin Islands companies may not have standing to sue before the federal courts of the United States.
Substantially all of our assets are located outside the United States. In addition, a majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or such persons or to enforce against them or against us, judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof.
We have appointed CT Corporation System as our agent to receive service of process with respect to any action brought against us in the United States District Court for the Southern District of New York under the federal securities laws of the United States or of any State of the United States or any action brought against us in the Supreme Court of the State of New York in the County of New York under the securities laws of the State of New York.
DeHeng Law Offices, our counsel as to Chinese law, has advised us that there is uncertainty as to whether the courts of China would (1) recognize or enforce judgments of United States courts obtained against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or (2) be competent to hear original actions brought in each respective jurisdiction, against us or such persons predicated upon the securities laws of the United States or any state thereof.
DeHeng Law Offices has advised us that the recognition and enforcement of foreign judgments are provided for under the Chinese Civil Procedure Law. Chinese courts may recognize and enforce foreign judgments in accordance with the requirements of the Chinese Civil Procedure Law based either on treaties between China and the country where the judgment is made or in reciprocity between jurisdictions. China does not have any treaties or other agreements with the British Virgin Islands or the United States that provide for the reciprocal recognition and enforcement of foreign judgments. As a result, it is uncertain whether a Chinese court would enforce a judgment rendered by a court in either of these two jurisdictions.
We have been advised by Kaufman & Canoles, our counsel as to British Virgin Islands law, that the United States and the British Virgin Islands do not have a treaty providing for reciprocal recognition and enforcement of judgments of courts of the United States in civil and commercial matters and that a final judgment for the payment of money rendered by any general or state court in the United States based on civil liability, whether or not predicated solely upon the U.S. federal securities laws, is unlikely to be enforceable in the British Virgin Islands. We have also been advised by Kaufman & Canoles that a final and conclusive judgment obtained in U.S. federal or state courts under which a sum of money is payable as compensatory damages (i.e., not being a sum claimed by a revenue authority for taxes or other charges of a similar nature by a governmental authority, or in respect of a fine or penalty or multiple or punitive damages) may be the subject of an action on a debt in the court of the British Virgin Islands under the ordinary law doctrine of obligation.
103
Table of Contents
We have engaged Anderson & Strudwick, Incorporated to conduct this offering on a “best efforts, minimum/maximum” basis. The offering is being made without a firm commitment by the Placement Agent, which has no obligation or commitment to purchase any of our shares. None of our officers, directors or affiliates may purchase shares in this offering.
Unless sooner withdrawn or canceled by either us or the Placement Agent, the offering will continue until the earlier of (i) a date mutually acceptable to us and our Placement Agent after which the minimum offering is sold or (ii) October 31, 2010 (the “Offering Termination Date”). The Placement Agent has agreed in accordance with the provisions of SEC Rule 15c2-4 to cause all funds received by the Placement Agent for the sale of the ordinary shares to be promptly deposited in an escrow account maintained by SunTrust Bank, N.A. (the “Escrow Agent”) as escrow agent for the investors in the offering. The Escrow Agent will exercise signature control on the escrow account and will act based on joint instructions from our company and the Placement Agent. On the closing date for the offering, net proceeds in the escrow account maintained by the Escrow Agent will be delivered to our company. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China, which may take as long as six months in the ordinary course. If we do not complete this offering before the Offering Termination Date, all amounts will be promptly returned as described below.
If we complete this offering, then on the closing date, we will issue shares to investors and Placement Agent Warrants to our Placement Agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of ordinary shares sold in this offering. In the event of any dispute between our company and the Placement Agent, including about whether the minimum offering has been sold and whether and how funds are to be reimbursed, the escrow agent is entitled to petition a court of competent jurisdiction to resolve any such dispute.
Investors must pay in full for all ordinary shares at the time of investment. Payment for the units may be made (i) by check, bank draft or money order made payable to “SunTrust Bank” and delivered to the Placement Agent no less than four business days before the date of closing, or (ii) by authorization of withdrawal from securities accounts maintained with the Placement Agent. If payment is made by authorization of withdrawal from securities accounts, the funds authorized to be withdrawn from a securities account will continue to accrue interest, if any interest is to accrue on such amounts, at the contractual rates until closing or termination of the offering, but a hold will be placed on such funds, thereby making them unavailable to the purchaser until closing or termination of the offering. If a purchaser authorizes the Placement Agent to withdraw the amount of the purchase price from a securities account, such Placement Agent will do so as of the date of closing. The Placement Agents will inform prospective purchasers of the anticipated date of closing. If payment is made by check, investors should make all checks payable to the Escrow Agent.
Proceeds deposited in escrow with the Escrow Agent may not be withdrawn by investors prior to the earlier of the closing of the offering or the Offering Termination Date. If the offering is withdrawn or canceled or if the 1,500,000 share minimum offering is not reached and proceeds therefrom are not received by us on or prior to the Offering Termination Date, all proceeds will be promptly returned by the Escrow Agent without interest or deduction to the persons from which they are received (within one business day) in accordance with applicable securities laws. All such proceeds will be placed in a non-interest bearing account pending such time.
Pursuant to that certain placement agreement by and between the Placement Agent and us, the obligations of the Placement Agent to solicit offers to purchase the shares and of investors solicited by the Placement Agent to purchase our ordinary shares are subject to approval of certain legal matters by counsel to the Placement Agent. The Placement Agent’s ability to complete this “best efforts minimum/maximum” transaction is dependent upon the existence of stable U.S. trading markets. As such, the Placement Agent’s obligations under the placement agreement are also subject to various conditions which are customary in transactions of this type, including that, as of the closing of the offering, there shall not have occurred (i) a suspension or material limitation in trading in securities generally on the New York Stock Exchange or the publication of quotations on the NASDAQ Global Market; (ii) a general moratorium on commercial banking activities in the State of New York or China; (iii) the engagement by the United States or China in hostilities which have resulted in the declaration of a national emergency or war if any such event would have a material adverse effect, in the Placement Agent’s reasonable judgment, as to make it impracticable or inadvisable to proceed with the solicitation of offers to consummate the offering with respect to investors solicited by the Placement Agent on the terms and conditions contemplated herein.
104
Table of Contents
We have agreed to indemnify the Placement Agent against certain liabilities, including liabilities under the Securities Act of 1933, or to contribute to payments the Placement Agent may be required to make in respect of those liabilities.
The Placement Agent is offering the ordinary shares, subject to prior sale, when, as and if issued to and accepted by it, subject to conditions contained in the placement agreement, such as the receipt by the Placement Agent of officers’ certificates and legal opinions. The Placement Agent reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part in the event (i) our representations or warranties are incorrect or misleading or we fail to fulfill our agreements with the Placement Agent; (ii) a material adverse change occurs affecting our business, management, property, assets, results of operations, condition or prospects; (iii) trading is suspended on any national securities exchange; (iv) war is declared; (v) a banking moratorium is declared in Virginia, New York or the U.S.; or (vi) any laws, regulations, court or administrative order or other governmental or agency act causes the Placement Agent to believe that our business or the U.S. securities markets will be materially adversely affected. The Placement Agent’s discretion in this regard is broad.
The Placement Agent intends to offer our ordinary shares to its retail customers only in states in which we are permitted to offer our ordinary shares. We have relied on an exemption to the blue sky registration requirements afforded to “covered securities”. Securities listed on the NASDAQ Global Market are “covered securities.” If we were unable to meet the NASDAQ Global Market’s listing standards, then we would be unable to rely on the covered securities exemption to blue sky registration requirements and we would need to register the offering in each state in which we planned to sell shares. Consequently, we will not complete this offering unless we meet the NASDAQ Global Market’s listing requirements.
In connection with this offering, the Placement Agent or certain of the securities dealers may distribute prospectuses electronically. No forms of prospectus other than printed prospectuses and electronically distributed prospectuses that are printable in Adobe PDF format will be used in connection with this offering.
Foreign Regulatory Restrictions on Purchase of our Shares
We have not taken any action to permit a public offering of our shares outside the United States or to permit the possession or distribution of this prospectus outside the United States. People outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering of our shares and the distribution of this prospectus outside the United States.
Commissions and Discounts
The Placement Agent has advised us that it proposes to offer the ordinary shares to the public at the initial public offering price on the cover page of this prospectus. The following table shows the public offering price, Placement Agent fee to be paid by us to the Placement Agent and the proceeds, before expenses, to us.
| Per Ordinary Share | Minimum Offering | Maximum Offering | |||||||
| Assumed public offering price |
$ | 8.00 | $ | 12,000,000 | $ | 15,000,000 | |||
| Placement discount |
$ | 0.56 | $ | 840,000 | $ | 1,056,000 | |||
| Proceeds to us, before expenses |
$ | 7.44 | $ | 11,160,000 | $ | 13,950,000 | |||
We expect our total cash expenses for this offering to be approximately $600,000, exclusive of the above commissions. In addition, we will pay the Placement Agent a non-accountable expense allowance of 1% of the amount of the offering, or $187,500 (maximum offering, exclusive of shares registered under Rule 462(b)) or $150,000 (minimum offering). The Placement Agent must sell the minimum number of securities offered (1,500,000 ordinary shares) if any are sold. The Placement Agent is required to use only its best efforts to sell the securities offered. The offering will terminate upon the earlier of: (i) a date mutually acceptable to us and our Placement Agent after which the minimum offering is sold or (ii) October 31, 2010. Until we sell at least 1,500,000 shares, all investor funds will be held in an escrow account at SunTrust Bank, Richmond, Virginia. If we do not sell at least
105
Table of Contents
1,500,000 shares by October 31, 2010, all funds will be promptly returned to investors (within one business day) without interest or deduction. If we complete this offering, net proceeds will be delivered to our company on the closing date. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. If we complete this offering, then on the closing date, we will issue ordinary shares to investors in the offering and Placement Agent Warrants to our Placement Agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of Ordinary shares sold in this offering.
Placement Agent’s Warrants
We have agreed to sell to the Placement Agent, on the closing date of this offering, at a price of $0.001 per warrant, Placement Agent’s Warrants exercisable at a rate of one warrant per share to purchase 10% of the number of ordinary shares issued by us in connection with the offering. We will issue between 150,000 and 187,500 Placement Agent’s Warrants in connection with this offering, depending on the number of ordinary shares sold in this offering. Each Placement Agent’s Warrant will be exercisable to purchase one ordinary share. The Placement Agent’s Warrants will be exercisable at 125% the offering price per ordinary share for a period of five years after issuance on the closing date of this offering. The Placement Agent’s Warrants may not be exercised, sold, transferred, pledged, assigned or hypothecated for a period of 180 days after the date of effectiveness or commencement of sales of the public offering, except to officers or partners and shareholders of the Placement Agent. This restriction is imposed pursuant to the requirements of FINRA Rule 5110(g)(1). If we do not complete this offering by selling at least the minimum number of ordinary shares, we will not issue any Placement Agent’s Warrants to our Placement Agent.
For the life of the Placement Agent’s Warrants, the holders thereof are given, at nominal costs, the opportunity to profit from a rise in the market price of our ordinary shares with a resulting dilution in the interest of other shareholders. Further, the holders may be expected to exercise the Placement Agent’s Warrant at a time when we would, in all likelihood, be able to obtain equity capital on terms more favorable than those provided in the Placement Agent’s Warrants.
We are required for the life of the Placement Agent’s Warrants to reserve sufficient ordinary shares to deliver upon exercise of the warrants and to take all necessary actions to ensure that we may validly and legally issue fully paid and non-assessable shares on exercise of the warrants.
The Placement Agent has a right to demand registration of the ordinary shares in the event registered ordinary shares are not available at the time of exercise and an exemption from such registration is not otherwise available. If this happens, we will be required to file the registration statement within ninety (90) days after demand and to pay the costs associated with the registration other than the Placement Agent’s counsel fees and any underwriting or selling commissions. We are required to seek the listing of the shares on the same exchange on which our ordinary shares trade, if any.
To the extent we are unable to register the shares, the Placement Agent may exercise the warrant with a cashless exercise, which is designed to give the Placement Agent the economic benefit of exercising the Placement Agent’s Warrants. A cashless exercise, however, would not result in the payment of any exercise price to us.
The Placement Agent’s Warrants also contain anti-dilution provisions, consistent with applicable FINRA rules, to adjust the terms of the Placement Agent’s Warrants as necessary to protect against dilution in the event we reorganize, consolidate, merge or subdivide our shares.
Market and Pricing Considerations
There is not an established market for our ordinary shares. We negotiated with our Placement Agent to determine the offering price of our ordinary shares in this offering using a multiple of approximately seven (7) times our targeted after-tax net income for the fiscal year ending December 31, 2010. Noting past offerings completed by our Placement Agent, we believe that this multiple approximates the valuation multiples utilized in similar offerings for similarly-sized companies.
106
Table of Contents
In addition to prevailing market conditions, the factors considered in determining the applicable multiples were:
| • | The history of, and the prospects for, our company and the industry in which we compete; |
| • | An assessment of our management, its past and present operation, and the prospects for, and timing of, our future revenues; |
| • | The present state of our development; and |
| • | The factors listed above in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
Based on a target 2010 audited net after-tax income of $13,500,000, we have been valued at approximately $94,500,000. Our targeted 2010 net after-tax income is not a projection but instead represents a negotiated target between our company and our Placement Agent. Although we do not currently pay any taxes on our income, we refer to this amount as our targeted 2010 audited net after-tax income to reflect our and our Placement Agent’s intention that the targeted amount will be net of any taxes that may apply to our company during 2010. Because we have 11,812,500 shares outstanding prior to the commencement of this offering, each such share has been valued at $8.00 per share, the assumed offering price in this offering.
If we are unable to achieve the targeted after-tax earnings upon which our valuation is based, then there is a risk that our company would be considered overvalued based on this multiple. In order to mitigate some of this risk, Messrs. Hong Yu and Taylor Z. Guo has agreed to place a number of beneficially owned ordinary shares into escrow that is equal to 40% of the maximum number of shares to be sold in this offering. See “Related Party Transactions—Make-Good Shares Subject to Redemption.”
An active trading market for our ordinary shares may not develop. It is possible that after this offering the ordinary shares will not trade in the public market at or above the initial offering price.
The exercise price for the Placement Agent’s Warrants issued to our Placement Agent in connection with, and conditional on the closing of, this offering has been negotiated between our company and the Placement Agent. The exercise price (125% of the offering price of ordinary shares in this offering), along with the length of time the Placement Agent must wait before exercise (at least 180 days after the closing of this offering) are influenced by the valuation attributed by FINRA in its calculation of the acceptability of aggregate placement consideration.
Discretionary Shares
The Placement Agent will not sell any shares in this offering to accounts over which it exercises discretionary authority, without first receiving written consent from those accounts.
Application for Listing on the NASDAQ Global Market
We have applied to list our ordinary shares on the NASDAQ Global Market under the symbol “TBET.” As this offering is a best-efforts offering, the NASDAQ Global Market has indicated that it is unable to admit our ordinary shares for listing until the completion of the offering and, consequently, the satisfaction of NASDAQ Global Market listing standards. If so admitted, we expect our ordinary shares to begin trading on the NASDAQ Global Market on the day following the closing of this offering. If our ordinary shares are eventually listed on the NASDAQ Global Market, we will be subject to continued listing requirements and corporate governance standards. We expect these new rules and regulations to significantly increase our legal, accounting and financial compliance costs.
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of the ordinary shares, the Placement Agent may engage in transactions that stabilize, maintain or otherwise affect the price of the ordinary shares. In order to facilitate the offering, the Placement Agent may, but is not required to, bid for, and purchase, ordinary shares in the open market to stabilize the price of the ordinary shares. These activities may raise or maintain the market price of the ordinary shares above independent market levels or prevent or retard a decline in the market price of the ordinary shares. The Placement
107
Table of Contents
Agent is not required to engage in these activities, and may end any of these activities at any time. We and the Placement Agent have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
We and the Placement Agent have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
Certain matters as to Virginia law and U.S. federal law in connection with this offering will be passed upon for us and for the Placement Agent by Kaufman & Canoles, P.C. The validity of the shares and certain legal matters relating to the offering as to British Virgin Islands law will be passed upon for us by Kaufman & Canoles, P.C. Certain legal matters relating to the offering as to Chinese law will be passed upon for us by DeHeng Law Offices, People’s Republic of China. Kaufman & Canoles, P.C. may rely upon DeHeng Law Offices with respect to matters governed by Chinese law.
Financial statements as of December 31, 2009 and 2008, and for the years then ended appearing in this prospectus, have been included herein and in the registration statement in reliance upon the report of Acquavella, Chiarelli, Shuster, Berkower & Co., LLP, an independent registered public accounting firm, appearing elsewhere herein, and upon the authority of that firm as experts in accounting and auditing.
Interests of Experts and Counsel
Attorneys with Kaufman and Canoles, P.C., representing our company with respect to this offering beneficially own 177,187 ordinary shares of our company as of the date of this prospectus.
Where You Can Find More Information
We have filed with the SEC under the Securities Act a registration statement on Form S-1 relating to the ordinary shares we and the selling stockholders are offering by this prospectus. This prospectus, which constitutes part of the registration statement filed with the SEC, does not contain all the information included in the registration statement and the exhibits and schedules thereto. For further information with respect to us and our ordinary shares, you should refer to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract, agreement or other document are not necessarily complete, and, where the contract, agreement or other document is an exhibit to the registration statement, any statement with respect to such contract, agreement or document is qualified by the provisions of such exhibit. You may examine and copy the registration statement, including the exhibits, at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can obtain a copy of all or a portion of the registration statement by mail from the Public Reference Section of the SEC at the same address, upon payment of prescribed fees. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains periodic reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the website is http://www.sec.gov.
As a result of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, will file periodic reports, proxy statements and other information with the SEC.
108
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Table of Contents
| F-1 | ||
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 -F-16 | ||
Table of Contents
ACSB Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
| 517 Route one | 1 Penn Plaza | |||
| Iselin, New Jersey, 08830 | 36the Floor | |||
| 732.855.9600 | New York, NY 10119 |
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
Shangri-la Tibetan Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheet of Shangri-la Tibetan Pharmaceuticals, Inc. as of December 31, 2009 and December 31, 2008, and the related consolidated statement of income, stockholders’ equity and comprehensive income, and cash flow for the years ended December 31, 2009 and December 31, 2008. Shangri-la Tibetan Pharmaceuticals, Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that out audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Shangri-la Tibetan Pharmaceuticals, Inc. as of December 31, 2009 and December 31, 2008, and the consolidated results of its operation and its cash flow for the years ended December 31, 2009 and 2008 in conformity with accounting principles generally accepted in the United States of America.
/s/ Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
Certified Public Accountants
New York, N.Y.
May 14, 2010
F-1
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICAS, INC.
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2009 AND 2008
| 2009 | 2008 | |||||
| ASSETS | ||||||
| Current Assets |
||||||
| Cash and cash equivalents |
$ | 4,081,752 | $ | 2,711,552 | ||
| Accounts receivable- related party |
342,000 | 1,277,550 | ||||
| Accounts receivable- non-related party |
4,266,075 | 1,703,148 | ||||
| Other receivables |
101,948 | 101,578 | ||||
| Due from shareholder |
1,550,085 | 1,357,721 | ||||
| Trade deposits paid in advance |
98,888 | 11,939 | ||||
| Inventories |
1,305,537 | 1,419,099 | ||||
| Total current assets |
11,746,285 | 8,582,587 | ||||
| Property, plant and equipment, net |
6,079,984 | 6,783,108 | ||||
| Intangible assets |
697,158 | 878,720 | ||||
| Prepaid expenses |
396,906 | 543,107 | ||||
| Total Assets |
$ | 18,920,333 | $ | 16,787,522 | ||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||
| Current Liabilities |
||||||
| Accounts payable |
$ | 2,830,032 | $ | 1,515,977 | ||
| Accrued expenses and other payables |
933,099 | 721,570 | ||||
| Value-added tax payable |
70,512 | 106,168 | ||||
| Total current liabilities |
3,833,643 | 2,343,715 | ||||
| Long-term Liabilities |
||||||
| Long-term loan |
3,582,116 | 3,569,132 | ||||
| Total liabilities |
7,415,759 | 5,912,847 | ||||
| Stockholders’ Equity: |
||||||
| Common stock, $0.001 par value, 50,000,000 shares authorized, 11,782,500 and 11,632,500 issued and outstanding as of 12/31/09 and 12/31/08. |
11,782 | 11,632 | ||||
| Additional paid in capital |
8,375,795 | 8,218,445 | ||||
| Retained earnings |
193,793 | 1,129,863 | ||||
| Cum. Transl. Adjustment |
111,881 | 99,172 | ||||
| Statutory reserve |
2,811,323 | 1,415,563 | ||||
| Total stockholders’ equity |
11,504,574 | 10,874,675 | ||||
| Total Liabilities and Stockholders’ Equity |
$ | 18,920,333 | $ | 16,787,522 | ||
The accompanying notes are an integral part of these financial statements.
F-2
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF INCOME
FOR THE YEARS ENDED DECEMBER 31, 2009 AND 2008
| 2009 | 2008 | |||||||
| Sales, net |
$ | 23,008,031 | $ | 15,580,269 | ||||
| Cost of sales |
(11,303,118 | ) | (7,857,884 | ) | ||||
| Gross profit |
11,704,913 | 7,722,385 | ||||||
| Selling, general and administrative expense |
(2,289,036 | ) | (1,630,425 | ) | ||||
| Income from operations |
9,415,877 | 6,091,960 | ||||||
| Interest income |
14,062 | 19,172 | ||||||
| Interest expenses and bank charges |
(184,961 | ) | (177,649 | ) | ||||
| Total Other Income (Expense) |
(170,899 | ) | (158,477 | ) | ||||
| Income before income taxes |
9,244,978 | 5,933,483 | ||||||
| Income taxes |
— | — | ||||||
| Net income |
$ | 9,244,978 | $ | 5,933,483 | ||||
| Other comprehensive income (loss) |
12,709 | 99,172 | ||||||
| Comprehensive income (loss) |
9,257,687 | 6,032,655 | ||||||
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2009 AND 2008
| Years Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
||||||||
| Net income |
9,244,978 | $ | 5,933,483 | |||||
| Adjustments to reconcile net income to net cash provided by |
||||||||
| Operating activities: |
||||||||
| Depreciation |
703,124 | 611,935 | ||||||
| Amortization |
181,563 | 175,426 | ||||||
| Stock based compensation |
157,500 | — | ||||||
| Changes in assets and liabilities provided/(used) cash : |
||||||||
| Accounts receivable |
(1,613,598 | ) | (961,302 | ) | ||||
| Other receivables |
0 | (24,758 | ) | |||||
| Due from shareholder |
(187,085 | ) | (137,839 | ) | ||||
| Trade deposit paid |
(86,749 | ) | 199,821 | |||||
| Inventories |
118,509 | (304,580 | ) | |||||
| Intangible assets |
0 | — | ||||||
| Prepaid expenses |
146,200 | 141,259 | ||||||
| Accounts payable |
1,306,164 | 573,340 | ||||||
| Accrued expenses and other payables |
208,525 | (199,798 | ) | |||||
| Value-added tax payable |
(35,977 | ) | 42,848 | |||||
| Net cash provided by operating activities |
10,143,153 | 6,049,836 | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||
| Purchase of property & equipment |
— | (642,112 | ) | |||||
| Purchase of Intangible assets |
— | — | ||||||
| Net cash used in investing activities |
0 | (642,112 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||
| Dividend Paid |
(8,785,288 | ) | (4,244,182 | ) | ||||
| Net cash provided by financing activities |
(8,785,288 | ) | (4,244,182 | ) | ||||
| Effect of exchange rate changes on cash and cash equivalents |
12,334 | 132,253 | ||||||
| NET CHANGE IN CASH AND CASH EQUIVALENTS |
1,370,200 | 1,295,796 | ||||||
| CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR |
2,711,552 | 1,415,756 | ||||||
| CASH AND CASH EQUIVALENTS, END OF YEAR |
$ | 4,081,752 | $ | 2,711,552 | ||||
| SUPPLEMENTAL DISCLOSURES: |
||||||||
| Interest paid |
$ | 180,568 | $ | 174,466 | ||||
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2009 AND 2008
| Shares of Common Stock Outstanding In thousand |
Common Stock |
Additional Paid-in Capital |
Accumulated Retained |
Statuary Reserve |
Accumulated Other Comprehensive Income/(Loss) |
Total | ||||||||||||||||
| Balance, January 1, 2008 |
11,632,500 | $ | 11,632 | $ | 8,218,445 | $ | 316,466 | $ | 539,659 | $ | 0 | $ | 9,086,202 | |||||||||
| Net income/(loss) |
5,933,483 | 5,933,483 | ||||||||||||||||||||
| Distribution- Net income |
(4,244,182 | ) | (4,244,182 | ) | ||||||||||||||||||
| Transfer to statutory reserve |
(875,904 | ) | 875,904 | 0 | ||||||||||||||||||
| Foreign currency translation adjustments |
99,172 | 99,172 | ||||||||||||||||||||
| Balance, December 31, 2008 |
11,632,500 | $ | 11,632 | $ | 8,218,445 | $ | 1,129,863 | 1,415,563 | $ | 99,172 | $ | 10,874,675 | ||||||||||
| Balance, January 1, 2009 |
11,632,500 | $ | 11,632 | $ | 8,218,445 | $ | 1,129,863 | 1,415,563 | $ | 99,172 | $ | 10,874,675 | ||||||||||
| Stock based compensation |
150,000 | 150 | 157,350 | 157,500 | ||||||||||||||||||
| Net (income) loss |
9,244,978 | 9,244,978 | ||||||||||||||||||||
| Distribution- Net income |
(8,785,288 | ) | (8,785,288 | ) | ||||||||||||||||||
| Transfer to statutory reserve |
(1,395,760 | ) | 1,395,760 | 0 | ||||||||||||||||||
| Foreign currency translation adjustments |
12,709 | 12,709 | ||||||||||||||||||||
| Balance, December 31, 2009 |
11,782,500 | $ | 11,782 | $ | 8,375,795 | $ | 193,793 | 2,811,323 | $ | 111,881 | $ | 11,504,574 | ||||||||||
The accompanying notes are an integral part of these financial statements
F-5
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 1 – ORGANIZATION
Shangri-la Tibetan Pharmaceuticals, Inc. (“the Company”) was incorporated on December 22, 2009 under the laws of British Virgin Islands. China Tibetan Pharmaceuticals Limited (“CTP”), the Company’s 100% owned subsidiary, was established in Hong Kong on January 6, 2010 as a limited liability company. Other than the equity interest in CTP, the Company does not own any assets or conduct any operations. CTP holds all of the outstanding equity interest in Yibo Information Consulting (Shenzhen) Company Ltd., a company established on March 18, 2010 in the PRC as a wholly foreign owned enterprise (“WFOE”). Other than the equity interest in WFOE, CTP does not own any assets or conduct any operations. Yunnan Diqing Shangri-La Tibetan Medicine Co., Ltd was incorporated on April 19, 2000 as a domestic Chinese corporation. On December 24, 2002, it changed its name to Yunnan Shangri-La Tibetan Pharmaceuticals Group Limited (“YSTP”). YSTP is engaged in manufacturing, marketing, selling, researching and developing modernized traditional Tibetan medicines in China
WFOE conducts its business through YSTP via a series of contractual arrangements. YSTP is consolidated as a variable interest entity (“VIE”). The Company does not conduct any substantive operations of its own, but conducts its primary business operations through WFOE’s VIE. The Company holds its interest in the VIE through WFOE.
Effective control over the VIE was transferred to the Company through the series of contractual arrangements without transferring legal ownership in the VIE (“reorganization”). As a result of these contractual arrangements, the Company maintained the ability to approve decisions made by the VIE and was entitled to substantially all of the economic benefits of the VIE, and therefore the Company consolidates the VIE. Immediately before and after the reorganization, the ultimate shareholder controlled the VIE; therefore, the reorganization is accounted for as a transaction between entities under common control. Accordingly, the accompanying consolidated financial statements have been prepared as if the current corporate structure had been in existence throughout the periods presented.
Chinese laws and regulations currently do not prohibit or restrict foreign ownership in pharmaceutical manufacturing businesses. However, Chinese laws and regulations do prevent direct foreign investment in certain industries. On March 26, 2010, to protect the Company’s shareholders from possible future foreign ownership restrictions, YSTP and all of the shareholders of YSTP entered into an entrusted management agreement with WFOE, which provides that WFOE will be entitled to the full guarantee for the performance of such contracts, agreements or transactions entered into by YSTP. WFOE is also entitled to receive the residual return of YSTP. As a result of the agreement, WFOE will absorb 100% of the expected losses and gains of YSTP, which results in WFOE being the primary beneficiary of YSTP.
WFOE also entered into a pledge of equity agreement with the principal shareholders, who pledged all their equity interest in these entities to WFOE. The pledge of equity agreement, which were entered into by each principal shareholder, pledged each of the principal shareholders’ equity interest in WFOE as a guarantee for the entrustment payment under the Entrusted Management Agreement. Administration for Industry and Commerce approved and registered such pledge of equity by which WFOE owns the right of pledge legally.
In addition, WFOE entered into an option agreement to acquire the principal shareholders’ equity interest in these entities at such times as it may wish to do so.
F-6
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 1 – ORGANIZATION (Continued)
The followings are brief description of contracts entered between WFOE and YSTP:
(1) Entrusted Management Agreement. The domestic companies, YSTP and WFOE, have entered into an Entrusted Management Agreement, which provides that WFOE will be fully and exclusively responsible for the management of YSTP. As consideration for such services, YSTP has agreed to pay WFOE the management fee during the term of this agreement and the management fee shall equal to YSTP’s estimated earnings before tax. Also, WFOE will assume all operating risks related to this entrusted management service to YSTP and bear all losses of YSTP. The term of this agreement will be from the effective date thereof to the earlier of the following: (1) the winding up of YSTP, or (2) the termination date of this Agreement to be determined by the parties hereto, or (3) the date on which WFOE completes the acquisition of YSTP.
(2) Exclusive Option Agreement. All the shareholders of YSTP as well as YSTP has entered into an Exclusive Option Agreement with WFOE, which provides that WFOE will be entitled to acquire such shares form the current shareholders upon certain terms and conditions, meanwhile WFOE will be entitled an irrevocable exclusive purchase option to purchase all or part of the assets and business of YSTP, if such a purchase is or becomes allowable under PRC laws and regulations. The Exclusive Option Agreement also prohibits the current shareholders of YSTP as well as YSTP from transferring any portion of their equity interests, business or assets to anyone other than WFOE. WFOE has not yet taken any corporate action to exercise this right of purchase, and there is no guarantee that it will do so or will be permitted to do so by applicable law at such times as it may wish to do so.
(3) Shareholders’ Voting Proxy Agreement. All the shareholders of YSTP has executed a Shareholders’ Voting Proxy Agreement to irrevocably appoint the persons designated by WFOE with the exclusive right to exercise, on their behalf, all of their Voting Rights in accordance with the laws and YSTP’s Articles of Association, including but not limited to the rights to sell or transfer all or any of their equity interests of YSTP, and to appoint and elect the directors and Chairman as the authorized legal representative of YSTP. This agreement will be only terminated prior to the completion of acquisition of all of the equity interests in, or all assets or business of YSTP.
(4) Pledge of Equity Agreement. WFOE and the shareholders of YSTP have entered into a Pledge of Equity Agreement, pursuant to which all shareholder pledges all of their shares (100%) of YSTP, as appropriate, to WFOE. If YSTP or any of its respective shareholders breaches its respective contractual obligations in the “Entrusted Management Agreement”, “Exclusive Option Agreement” and “Shareholders’ Voting Proxy Agreement”, WFOE as Pledge, will be entitled to certain rights to foreclose on the pledged equity interests. Such YSTP shareholders cannot dispose of the pledged equity interests or take any actions that would prejudice WFOE’s interest
Except for the disclosed above, there are no arrangements that could require the Company to provide financial support to the variable interest entities, including events or circumstances that could expose the Company to a loss. As stated in the disclosure of various agreements between the Company and its VIE, the Company has rights to acquire any portion of the equity interests of the VIE. Also the Company may allocate its available funds to its VIE for business purpose. There are no fixed terms of such arrangements.
F-7
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The Company’s functional currency is the Chinese Renminbi; however the accompanying consolidated financial statements have been translated and presented in United States Dollars. These financial statements are prepared on a historical pro forma basis.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiary and variable interest entity (“VIE”) for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted the Consolidation Topic of the FASB Accounting Standards Codification (“ASC 810”) which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
Translation Adjustment
As of December 31, 2009 and 2008, the accounts of the Company were maintained, and its financial statements were expressed, in Chinese Yuan Renminbi (“CNY”). Such financial statements were translated into U.S. Dollars (“USD”) in accordance with the Foreign Currency Matters Topic of the FASB Accounting Standards Codification (“ASC 830”), with the CNY as the functional currency. According to ASC 830, all assets and liabilities were translated at the current exchange rate, stockholders’ equity is translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”), as a component of shareholders’ equity. Transaction gains and losses are reflected in the income statement.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”). Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
Risks and Uncertainties
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC’s economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
F-8
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
The Company is subject to substantial risks from, among other things, intense competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history, foreign currency exchange rates and the volatility of public markets.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed.
Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserves are recorded primarily on a specific identification basis. These are no allowances for doubtful accounts as of December 31, 2009 and 2008.
Inventories
Inventories are valued at the lower of cost (determined on a weighted average basis) or market,, if lower. As of December 31, 2009 and 2008, inventories consist of the following:
| December 31, | ||||||
| 2009 | 2008 | |||||
| Raw materials |
$ | 579,118 | $ | 748,648 | ||
| Package materials |
137,902 | 137,136 | ||||
| Finished goods |
$ | 588,517 | $ | 533,315 | ||
| Total |
$ | 1,305,537 | $ | 1,419,099 | ||
F-9
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Property, Plant & Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
| Buildings |
20 years | |
| Machinery and equipment |
10 years | |
| Motor vehicles |
5 years |
As of December 31, 2009 and 2008, Property, Plant & Equipment consist of the following:
| 2009 | 2008 | |||||||
| Buildings |
$ | 5,051,487 | $ | 5,051,487 | ||||
| Plant and machinery |
$ | 3,108,107 | $ | 3,108,107 | ||||
| Motor vehicles |
$ | 658,653 | $ | 658,653 | ||||
| Sub-total |
$ | 8,818,247 | $ | 8,818,247 | ||||
| Less: Accumulated depreciation |
$ | (2,738,263 | ) | $ | (2,035,139 | ) | ||
| Property, plant and equipment, net |
$ | 6,079,984 | $ | 6,783,108 | ||||
Depreciation expenses totaled $703,124 and $611,935 for the years ended December 31, 2009 and 2008, respectively.
Intangible Assets
Intangible assets are amortized using the straight-line method over their estimated period of benefit, ranging from ten to fifty years. Management evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. No impairments of intangible assets have been identified during any of the periods presented. The land use right will expire in 2050. All of the Company’s intangible assets are subject to amortization with estimated lives of:
| Land use right |
50 years | |
| Proprietary technologies |
10 years |
F-10
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Intangible Assets (Continued)
The components of finite-lived intangible assets are as follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Proprietary technologies |
$ | 1,644,962 | $ | 1,644,962 | ||||
| Land use right |
274,160 | 274,160 | ||||||
| 1,919,122 | 1,919,122 | |||||||
| Less: Accumulated amortization: |
(1,221,965 | ) | (1,040,402 | ) | ||||
| $ | 697,158 | $ | 878,720 | |||||
Amortization expense for the years ended December 31, 2009 and 2008 were $181,563, and $175,426 respectively.
The estimated future amortization expenses related to intangible asset as of December 31, 2009 are as follows:
| 2010 |
181,563 | ||
| 2011 |
181,563 | ||
| 2012 |
181,563 | ||
| 2013 |
5,857 | ||
| 2014 |
5,857 | ||
| Thereafter |
$ | 140,755 |
Long-Lived Assets
Effective January 1, 2002, the Company adopted the Property, Plant and Equipment Topic of the FASB Accounting Standard Codification (“ASC 360”), which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of,” and the accounting and reporting provisions of Accounting Principles Board Opinion No. 30, “Reporting the Results of Operations for a Disposal of a Segment of a Business.” The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with ASC 360, which requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. Based on its review, the Company believes that, as of December 31, 2009 and 2008, there were no significant impairments of its long-lived assets.
Revenue Recognition
The Company’s revenue recognition policies are in compliance with the Revenue Recognition Topic of the FASB Accounting Standards Codification (“ASC 605”). Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist and collectibility is reasonably assured.
F-11
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue.
The Company have distributor arrangements with certain parties for sale of its pharmaceutical products. The distributor agreements do not provide chargeback, price protection, or stock rotation rights. Accordingly, revenue is recognized when products are delivered to and received by the distributors.
Prepaid Expenses
Advertising expenses consist primarily of costs of promotion for corporate image and product marketing and costs of direct advertising. The Company records payments made to advertising companies for the purpose of reserving prime-time advertising space as prepaid expenses in the consolidated balance sheet. The Company expenses the prepaid advertising amount when the advertisement is published or aired. All other advertising costs are expensed as incurred.
Also, included in prepaid expenses is GMP authentication, which is being amortized during the period of the certificate of authentication.
Research and development costs
Research and development costs are incurred in the development of the new products and processes, including significant improvements and refinements to existing products. All research and development costs are expensed as incurred.
Income Taxes
The Company utilizes SFAS No. 109, “Accounting for Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The Company is entitled to exemption from PRC income tax between 2008 and 2012. According to tax preferential policy of Yunnan Diqing Tibet Autonomous State and approval of governmental tax agency, after approval of document by the tax bureau of the People’s Government Office of Yunnan Diqing Autonomous State, the tax bureau of the Yunnan Diqing Tibet Autonomous State agreed to exempt the Company from income tax for 5 years between 2008 and 2012.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), “Accounting for uncertainty in Income Taxes.” FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, “Accounting for Contingencies.” FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company’s financial statements. SFAS No. 109 and FIN 48 were superseded by the Income Taxes Topic of the FASB Accounting Standards Codification (“ ASC 740”) in September 2009. However, this portion of the codification has no application to the Company until 2010 as stated above.
F-12
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Statement of Cash Flows
In accordance with the Statement of Cash Flows Topic of the FASB Accounting Standards Codification (“ASC 230”), cash flows from the Company’s operations are based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, accounts receivable and other receivables arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base, most of which are in China. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited. As of December 31, 2009, there was no allowance for uncollectible accounts as previously discussed.
Recent Accounting Pronouncements
In April 2009, the Financial Accounting Standards Board (“FASB”) issued the following new accounting standards:
| • | FASB Staff Position FAS No. 157-4, Determining Whether a Market Is Not Active and a Transaction Is Not Distressed, (“FSP FAS No. 157-4”) provides guidelines for making fair value measurements more consistent with the principles presented in SFAS No. 157. FSP FAS No. 157-4 provides additional authoritative guidance in determining whether a market is active or inactive and whether a transaction is distressed. It is applicable to all assets and liabilities (i.e., financial and non-financial) and will require enhanced disclosures. FSP FAS No. 157-4 was superseded by the Fair Value Measurements and Disclosures Topic of the FASB Accounting Standards Codification (“ASC 820”). |
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal
F-13
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair Value, which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
Note 3 – INDEBTEDNESS
Long-term debts were as follows:
| December 31, | ||||||
| 2009 | 2008 | |||||
| Secured long-term loans |
$ | 3,582,116 | $ | 3,569,132 | ||
The interest expense was $180,568 and $174,466 for the years ended December 31, 2009 and 2008, respectively.
The secured long-term bank loans are secured by a pledge of certain of the Company’s machinery equipment and buildings. The pledged machinery equipment and buildings as of December 31, 2009 and 2008 had a net book value of $ 6,079,984 and $ 6,783,108, respectively.
As of December 31, 2009 and December 31, 2008, the secured long-term loans consisted of 2 bank loans. Both are due in November 2011 with annual interest rate of 5.05%
Note 4 – COMPENSATED ABSENCES
Regulation 45 of the local labor law of the People’s Republic of China (“PRC”) entitles employees to annual vacation leave after 1 year of service. In general, all leave must be utilized annually, with proper notification. Any unutilized leave is cancelled.
F-14
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 5 – TRADE DEPOSITS PAID IN ADVANCE
The Company advances to certain vendors for the purchase of materials. As of December 31, 2009 and 2008, the advances to suppliers amounted to $98,888 and $11,939, respectively.
Note 6 – RELATED PARTY TRANSACTIONS
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. Parties are also considered to be related if they are subject to common control or common significant influence. The Revenue derived from related party transactions for the years ended December 31, 2009 and 2008, were $5,301,094, and $ 4,313,685, respectively. As of December 31, 2009 and December 31, 2008, the balance of accounts receivable from related party were $ 342,000 and $1,277,550. As of December 31, 2009 and December 31, 2008, the balance of due from shareholders were $1,550,085 and $1,357,721, respectively. Such amount is non-interest bearing and due upon demand.
Note 7 – STATUTORY RESERVE
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of December 31, 2009 and 2008, the Company had allocated $ 2,811,323 and $1,415,563, respectively, to these non-distributable reserve funds.
Note 8 – OTHER COMPREHENSIVE INCOME
Balances of related after-tax components comprising accumulated other comprehensive income, included in stockholders’ equity, as of December 31, 2009 and 2008, are as follows:
| Foreign Currency Translation Adjustment |
Accumulated Other Comprehensive Income | |||||
| Balance at December 31, 2007 |
— | — | ||||
| Change for 2008 |
99,172 | 99,172 | ||||
| Balance at December 31, 2008 |
$ | 99,172 | $ | 99,172 | ||
| Change for 2009 |
12,709 | 12,709 | ||||
| Balance at December 31, 2009 |
$ | 111,881 | $ | 111,881 | ||
F-15
Table of Contents
SHANGRI-LA TIBETAN PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
Note 9 – MAJOR CUSTOMERS AND CREDIT RISK
Details of customers accounting for 10% or more of total net sales of the Company are as follows:
| December 31, | |||||||||
| 2009 | 2008 | ||||||||
| Kunming Shangri-La Medicine Co., Ltd |
$ | 5,301,094 | 23.0 | % | $ | 4,313,685 | |||
| Hangzhou Hesheng Medicine Co., Ltd |
$ | 2,907,817 | 12.6 | % | $ | 1,583,471 | |||
| Total |
$ | 8,208,911 | 35.6 | % | $ | 5,897,156 | |||
Note 10 – SEGMENT REPORTING
The Company operates and manages its business as a single segment that includes primarily the development, manufacture and sale of modernized traditional Chinese medicine. The Company’s chief operating decision maker has been identified as Mr. Guo, the Chief Executive Officer, who reviews the consolidated results when making decisions about allocating resources and assessing performance of the Company.
The revenues attributable to our product groups are as follows:
| December 31, | ||||||
| 2009 | 2008 | |||||
| 25 Ingredients Mandrake Pill |
$ | 11,185,718 | $ | 7,789,246 | ||
| 15 Ingredients Gentiana Pill |
$ | 4,941,706 | $ | 3,120,079 | ||
| 18 Ingredients Chebulic ( Myrobalan) Frusemide Pill |
$ | 2,451,670 | $ | 1,567,877 | ||
| Pomegranate Nichirin Pill |
$ | 2,265,810 | $ | 1,535,484 | ||
| 28 Ingredients Pinang Pill |
$ | 2,163,127 | $ | 1,567,583 | ||
| Total |
$ | 23,008,031 | $ | 15,580,269 | ||
Note 11 – COMMON STOCK
In 2009, the Company issued 150,000 shares of Company stock, valued at $157,500, for services rendered through December 31, 2009.
Note 12 – SUBSEQUENT EVENT
For the year ended December 31, 2009, the Company has evaluated subsequent events for potential recognition and disclosure through May 14, 2010, the date of the financial statement issuance.
F-16
Table of Contents
Table of Contents
[Alternate Page]
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 14, 2010

SHANGRI-LA TIBETAN
PHARMACEUTICALS, INC.
354,375 Ordinary Shares
This prospectus relates to the resale by the selling shareholders of up to 354,375 of our ordinary shares. The selling shareholders may sell ordinary shares, from time to time and in the principal market on which our shares is traded, at the prevailing market price or in negotiated transactions. We will not receive any proceeds from the sales by the selling shareholders.
No public market currently exists for our shares. We have applied for approval for quotation on the NASDAQ Global Market under the symbol “TBET” for the ordinary shares we are offering.
The selling shareholders holding 354,375 shares offered through this prospectus may sell their shares once our ordinary shares have been registered and listed on the NASDAQ Global Market or another national exchange. Once, and if, our ordinary shares begin to be traded or quoted on any stock exchange, market, or trading facility, the selling shareholders may sell their shares, from time to time at the market price prevailing on the exchange, market, or trading facility, or at prices related to such prevailing market prices, or in negotiated transactions or a combination of such methods of sale.
Investing in our ordinary shares involves significant risks. See “Risk Factors” beginning on page 11 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of the prospectus. Any representation to the contrary is a criminal offense.
Prospectus dated , 2010
Table of Contents
[ALTERNATE PAGE]
Table of Contents
[ALTERNATE PAGE]
| The Offering |
||
| Ordinary shares Offered by Selling Shareholders | 354,375 shares | |
| Ordinary shares Outstanding | 11,812,500 shares(1) | |
| Use of proceeds | We will not receive any proceeds from the sale of our ordinary shares by the selling shareholders. | |
| NASDAQ Market Symbol | We have applied to use the symbol “TBET” for our ordinary shares. (CUSIP No. G80649 109) | |
| (1) | Based on 11,812,500 of our ordinary shares outstanding as of the date of this prospectus. The number of ordinary shares outstanding excludes up to 1,875,000 ordinary shares to be offered on a best efforts, minimum/maximum offering concurrently herewith. |
8-A
Table of Contents
[ALTERNATE PAGE]
USE OF PROCEEDS
The selling shareholders are selling all of the shares covered by this prospectus for their own accounts. We will not receive any proceeds from the sale of the shares.
41-A
Table of Contents
[ALTERNATE PAGE]
SELLING SHAREHOLDERS
The following table provides, as of the date of this prospectus, information regarding the beneficial ownership of our ordinary shares held by each of the selling shareholders, including:
| • | the number of shares owned by each shareholder prior to this offering; |
| • | the percentage owned by each shareholder prior to completion of the offering; |
| • | the total number of shares that are to be offered for each shareholder; |
| • | the total number of shares that will be owned by each shareholder upon completion of the offering; and |
| • | the percentage owned by each shareholder upon completion of the offering. |
We have agreed to register a total of 354,375 of our ordinary shares held by the selling shareholders. We are registering the shares under this prospectus.
| Name of Selling Shareholder |
Number
of Ordinary shares Beneficially Owned Prior to the Offering |
Percentage
of Common Shares Beneficially Owned Prior to the Offering(1) |
Number
of Ordinary shares Registered for Sale Hereby |
Number
of Ordinary shares Beneficially Owned after Completion of the Offering(2) |
Percentage Common Shares Beneficially Owned after Completion of the Offering(2) |
|||||||
| Xiumei Lan |
200,000 | 1.69 | % | 200,000 | 0 | 0.00 | % | |||||
| Hayden Zou |
200,000 | 1.69 | % | 70,000 | 130,000 | 1.10 | % | |||||
| Peizhen Jin |
30,000 | 0.25 | % | 10,000 | 20,000 | 0.17 | % | |||||
| Fulcan Investments LLC |
24,375 | 0.21 | % | 24,375 | 0 | 0.00 | % | |||||
| Richard Ng |
20,000 | 0.17 | % | 20,000 | 0 | 0.00 | % | |||||
| Daybreak Special Situations Master Fund, Ltd. |
20,000 | 0.17 | % | 20,000 | 0 | 0.00 | % | |||||
| Weixin Li |
5,000 | 0.04 | % | 5,000 | 0 | 0.00 | % | |||||
| Jianhua Zhou |
5,000 | 0.04 | % | 5,000 | 0 | 0.00 | % | |||||
| Total |
504,375 | 4.27 | % | 354,375 | 150,000 | 1.27 | % |
| (1) | Based on 11,812,500 ordinary shares outstanding as of the date of this prospectus. The number of ordinary shares outstanding excludes up to 1,875,000 ordinary shares to be offered on a best efforts, minimum/maximum offering concurrently herewith. |
| (2) | Represents the amount of shares that will be held by the selling shareholders after completion of this offering based on the assumption that all shares registered for sale hereby will be sold. However, the selling shareholders may offer all, some or none of the shares pursuant to this prospectus, and to our knowledge there are currently no agreements, arrangements or understandings with respect to the sale of any of the shares that may be held by the selling shareholders after completion of this offering. |
The selling shareholders acquired the shares for their own accounts in the ordinary course of business, and at the time they acquired the shares, they had no agreements, plans or understandings, directly or indirectly, to distribute the shares. None of the selling shareholders, to our knowledge, has had a material relationship with our company other than as a shareholder at any time within the past three years.
46-A
Table of Contents
[ALTERNATE PAGE]
PLAN OF DISTRIBUTION
Once, and if, our ordinary shares begin to be traded or quoted on any stock exchange, market, or trading facility, the selling shareholders, who hold an aggregate of 354,375 ordinary shares offered through this prospectus, may sell their shares from time to time at the market price prevailing on the exchange, market, or trading facility, or at prices relating to the prevailing market prices, or in negotiated transactions or a combination of such methods of sale. The selling shareholders may use any one or more of the following methods when selling shares:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | settlement of short sales entered into after the date of this prospectus; |
| • | broker-dealers may agree with the selling shareholders to sell a specified number of such shares at a stipulated price per share; |
| • | a combination of any such methods of sale; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; or |
| • | any other method permitted pursuant to applicable law. |
In connection with the sale of our ordinary shares or interest therein, the selling shareholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of our ordinary shares in the course of hedging the positions they assume. The selling shareholders may also sell ordinary shares short and deliver these securities to close out their short positions, or loan or pledge the ordinary shares to broker-dealers, which in turn may sell the securities. The selling shareholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling shareholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. Because the selling shareholders may be deemed to be “underwriters” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements of the Securities Act. We will make copies of this prospectus available to the selling shareholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale. Each selling shareholder has informed us that it does not have any agreement or understanding, directly or indirectly, with any person to distribute the ordinary shares.
We are required to pay certain fees and expenses incurred by us incident to the registration of the shares. We have agreed to indemnify the selling shareholders against certain losses, claims, damages and liabilities.
The resale shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with. The maximum commission or discount to be received by any FINRA member or independent broker/dealer will not be greater than 8% for the sale of any securities being registered pursuant to SEC Rule 415.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale shares may not simultaneously engage in market making activities with respect to our ordinary shares for a period of two business days prior to the commencement of the distribution. In addition, the selling shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of our ordinary shares by the selling shareholders or any other person.
Our placement agent in the public offering, Anderson & Strudwick, may assist the Selling Shareholders with the sale of their ordinary shares. To the extent the placement agent assists with any resale of such ordinary shares, the maximum commission or discount to be received by it in such capacity will not be greater than 8% for the sale of any securities being registered pursuant to SEC Rule 415.
104-A
Table of Contents
Shares Eligible For Future Sale
Prior to the initial public offering being conducted contemporaneously, there has been no market for our ordinary shares, and a liquid trading market for our ordinary shares may not develop or be sustained after this offering. Future sales of substantial amounts of ordinary shares, including ordinary shares issued upon exercise of outstanding options and exercise of the warrants offered in this prospectus in the public market after this offering or the anticipation of those sales could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of our equity securities.
Upon the completion of the initial public offering, we will have outstanding 13,687,500 ordinary shares, the closing of the maximum offering and not including any shares underlying the underwriter warrants. Of these ordinary shares, the 1,875,000 ordinary shares sold in the initial public offering and the 354,375 registered herewith will be freely tradable without restriction under the Securities Act, except that any shares purchased by our “affiliates,” as that term is defined in Rule 144 of the Securities Act, may generally only be sold in compliance with the limitations of Rule 144 described below. The remaining approximately 11,458,500 ordinary shares outstanding will be restricted shares held by existing shareholders that could be sold pursuant to Rule 144. We have not agreed to register these restricted shares. We have not issued any warrants to purchase our ordinary shares or other securities convertible into our ordinary shares.
Registration on Form S-1
We are concurrently registering the initial public offering of a minimum of 1,500,000 and a maximum of 1,875,000 of our ordinary shares.
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the effective date of the initial public registration statement, a person (or persons whose shares are aggregated) who is deemed to be an affiliate of our company at the time of sale, or at any time during the preceding three months, and who has beneficially owned restricted shares for at least six months, would be entitled to sell within any three-month period a number of our ordinary shares that does not exceed the greater of 1% of the then outstanding ordinary shares or the average weekly trading volume of ordinary shares during the four calendar weeks preceding such sale. Sales under Rule 144 are subject to certain manner of sale provisions, notice requirements and the availability of current public information about our company. In addition, sales by our affiliates may be subject to the terms of lock-up agreements and Make-Good Escrow agreements. See “Shares Eligible for Future Sale – Lock-Up Agreements” and “Related Party Transactions – Make-Good Shares Subject to Redemption.”
A person who has not been our affiliate at any time during the three months preceding a sale, and who has beneficially owned his or her ordinary shares for at least six months, would be entitled under Rule 144 to sell such shares without regard to any manner of sale, notice provisions or volume limitations described above. Any such sales must comply with the public information provision of Rule 144 until our ordinary shares have been held for one year.
Rule 701
Securities issued in reliance on Rule 701 are also restricted and may be sold by shareholders other than affiliates of our company subject only to manner of sale provisions of Rule 144 and by affiliates under Rule 144 without compliance with its six-month holding period requirement.
Registration on Form S-8
We intend to file a registration statement on Form S-8 under the Securities Act as soon as practicable after the closing of the initial public offering to register up to 684,375 of our ordinary shares subject to outstanding stock options or reserved for issuance under our stock incentive plan, such amount being equal to five percent (5%) of the number of ordinary shares issued and outstanding after the closing of the initial public offering, assuming a
95-A
Table of Contents
maximum offering. This registration will permit the resale of these ordinary shares by nonaffiliates in the public market without restriction under the Securities Act, upon the completion of the lock-up period described below. Ordinary shares registered pursuant to the Form S-8 held by affiliates will be subject to Rule 144 volume limitations. As of the date of initial public offering effective date, we have not issued any options to purchase our ordinary shares.
Lock-Up Agreements
Each of our executive officers, directors and individuals who on the effective date of the initial public registration statement are the beneficial owners of more than 5% of our ordinary shares, has agreed not to register, offer, sell, contract to sell or grant any of our ordinary shares or any securities convertible into or exercisable or exchangeable for our ordinary shares or any warrants to purchase our ordinary shares (including, without limitation, securities of our company which may be deemed to be beneficially owned by such individuals in accordance with the rules and regulations of the Securities and Exchange Commission and securities which may be issued upon the exercise of a stock option or warrant) for a period of (a) as to one-half (1/2) of the ordinary shares now or in the future beneficially owned by such individual, ninety (90) days after the date of effectiveness or commencement of sales of our initial public offering and (b) as to the other one-half of such ordinary shares now or in the future beneficially owned by such individual, one hundred ninety (190) days after the date of effectiveness or commencement of sales of our initial public offering. Upon the expiration of these lock-up agreements, additional ordinary shares will be available for sale in the public market.
Summary of Shares Available for Future Sale
The following table summarizes the total shares potentially available for future sale. To the extent we sell a number of ordinary shares between the minimum and maximum offering in our initial public offering, the below tables will be adjusted proportionately as to numbers of shares available for sale (as to option pool and placement agent shares) and dates on which such shares may be sold (as to currently outstanding shares).
Minimum Offering
| Shares |
Date Available for Sale | |
| Currently Outstanding Ordinary shares: 11,812,500 |
||
| 354,375 |
After the date of this prospectus, the shares will have been registered and will be tradable by the selling shareholders listed in this resale prospectus. | |
| 9,443,125 |
After 90 days from the date of effectiveness or commencement of sales of the public offering conducted concurrently herewith | |
| 1,415,000 |
After 190 days from the date of effectiveness or commencement of sales of the public offering conducted concurrently herewith | |
| 600,000 |
After 30 days after filing of Form 10-K for the year ending December 31, 2010, assuming no redemption of Make-Good Shares; any delay in redemption will also delay the release of these shares | |
| Ordinary shares in Option Pool: 665,625 |
From vesting dates through expiration of grants | |
| Ordinary shares Underlying Placement Agent’s Warrants: 150,000 |
After 180 days from the date of effectiveness or commencement of sales of the public offering conducted concurrently herewith | |
| Shares Offered in this Offering: 1,500,000 |
After the date of this prospectus, these shares will be freely tradable. | |
96-A
Table of Contents
Maximum Offering
| Shares |
Date Available for Sale | |
| Currently Outstanding Ordinary shares: 11,812,500 |
||
| 354,375 |
After the date of this prospectus, the shares will have been registered and will be tradable by the selling shareholders listed in this resale prospectus. | |
| 9,368,125 |
After 90 days from the date of effectiveness or commencement of sales of the public offering conducted concurrently herewith | |
| 1,340,000 |
After 190 days from the date of effectiveness or commencement of sales of the public offering conducted concurrently herewith | |
| 750,000 |
After 30 days after filing of Form 10-K for the year ending December 31, 2010, assuming no redemption of Make-Good Shares; any delay in redemption will also delay the release of these shares | |
| Ordinary shares in Option Pool: 684,375 |
From vesting dates through expiration of grants | |
| Ordinary shares Underlying Placement Agent’s Warrants: 187,500 |
After 180 days from the date of effectiveness or commencement of sales of the public offering conducted concurrently herewith | |
| Shares Offered in this Offering: 1,875,000 |
After the date of this prospectus, these shares will be freely tradable. | |
97-A
Table of Contents
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution. |
The estimated expenses payable by us in connection with the offering described in this registration statement (other than the placement discounts and commissions) will be as follows. With the exception of the filing fees for the U.S. Securities Exchange Commission, FINRA and NASDAQ, all amounts are estimates.
| U.S. Securities Exchange Commission registration fee |
$ | 1,405.34 | |
| FINRA filing fee |
$ | 2,471.00 | |
| NASDAQ listing fee |
$ | 125,000 | |
| Legal fees and expenses for Chinese counsel |
$ | 70,000 | |
| Legal fees and expenses for British Virgin Islands counsel |
$ | 10,000 | |
| Legal fees and expenses for U.S. counsel |
$ | 230,000 | |
| Accounting fees and expenses |
$ | 130,000 | |
| Printing fees |
$ | 30,000 | |
| Misc. |
$ | 1,123.66 | |
| Total |
$ | 600,000 |
| Item 14. | Indemnification of Directors and Officers |
British Virgin Islands law and our articles of association provide that we may indemnify our directors, officers, advisors and trustee acting in relation to any of our affairs against actions, proceedings, costs, charges, losses, damages and expenses incurred by reason of any act done or omitted in the execution of their duty in their capacities as such. Under our articles of association, indemnification is not available, however, if those events were incurred or sustained by or through their own willful neglect or default.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
| Item 15. | Recent Sales of Unregistered Securities |
We have not issued any unregistered securities in the last three years.
| Item 16. | Exhibits and Financial Statement Schedules |
| (a) | Exhibits |
The following exhibits are filed herewith or incorporated by reference in this prospectus:
| Exhibit |
Document | |
| 1.1 |
Form of Placement Agreement (1) | |
| 3(i).1 |
Articles of Association of the Registrant (1) | |
| 3(i).2 |
Form of Amended and Restated Articles of Association of the Registrant (1) | |
| 3(ii).1 |
Memorandum of Association of the Registrant (1) | |
| 3(ii).2 |
Form of Amended and Restated Memorandum of Association of Registrant (1) | |
II - 1
Table of Contents
| 4.1 |
Specimen Share Certificate (2) | |
| 4.2 |
Form of Placement Agent Warrant (included in Ex. 10.1) (1) | |
| 5.1 |
Form of Opinion of Kaufman & Canoles, P.C., Virginia counsel (1) | |
| 5.2 |
Form of Opinion of Kaufman & Canoles, P.C., British Virgin Islands counsel (1) | |
| 10.1 |
Form of Placement Agent Warrant Agreement (1) | |
| 10.2 |
Translation of Entrusted Management Agreement for YSTP (1) | |
| 10.3 |
Translation of Shareholder Voting Proxy Agreement for WFOE (1) | |
| 10.4 |
Translation of Pledge of Equity Interest Agreement for WFOE (1) | |
| 10.5 |
Translation of Exclusive Option Agreement for YSTP (1) | |
| 10.6 |
Form of Lock-Up Agreement (1) | |
| 10.7 |
Form of Make-Good Escrow Agreement (1) | |
| 10.8 |
Translation of Medicinal Materials Procurement Contract (Ba Sang) (1) | |
| 10.9 |
Translation of Medicinal Materials Procurement Contract (Chun Sheng) (1) | |
| 10.10 |
Translation of Medicinal Materials Procurement Contract (Cili Peichu) (1) | |
| 10.11 |
Translation of Medicinal Materials Procurement Contract (Kunming Morningstar Printing Co.) (1) | |
| 10.12 |
Translation of Medicinal Materials Procurement Contract (Xiong Ba) (1) | |
| 10.13 |
Translation of Sales Contract (Hangzhou Hesheng Medicine Co. Ltd) (1) | |
| 10.14 |
Translation of Sales Contract (Kunming Shangri-La Medicine Co. Ltd.) (1) | |
| 10.15 |
Translation of Agreement on Prescription and Industrialization Development of Tibetan Medicine (Kunming Institute of Botany of Chinese Academy Of Sciences) (1) | |
| 10.16 |
Translation of Agreement on Research into Tibetan Medicine Pharmacology and Effect (Second Military Medical University of Chinese People’s Liberation Army) (1) | |
| 10.17 |
Retainer Contract (Taylor Z. Guo) (1) | |
| 10.18 |
Retainer Contract (Sabrina Ren) (1) | |
| 10.19 |
Retainer Contract (Hong Yu) (1) | |
| 21.1 |
Subsidiaries and Affiliate of the Registrant (1) | |
| 23.1 |
Consent of Acquavella, Chiarelli, Shuster, Berkower & Co., LLP (1) | |
II - 2
Table of Contents
| 23.2 |
Consent of Kaufman & Canoles, Virginia counsel (included in Exhibit 5.1) (1) | |
| 23.3 |
Consent of Kaufman & Canoles, British Virgin Islands counsel (included in Exhibit 5.2) (1) | |
| 23.4 |
Consent of DeHeng Law Offices, Chinese counsel (included in Exhibit 99.1) (1) | |
| 24.1 |
Power of Attorney (included at page II-6) (1) | |
| 99.1 |
Form of Opinion of DeHeng Law Offices, Chinese counsel (1) | |
| 99.2 |
Code of Business Conduct and Ethics (1) | |
| (1) | Filed herewith. |
| (2) | To be filed by amendment. |
| (b) | Financial Statement Schedules |
None.
| Item 17. | Undertakings |
The Registrant hereby undertakes:
| (a) | to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to: |
| (i) | include any prospectus required by section 10(a)(3) of the Securities Act; |
| (ii) | reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (b) | that, for the purpose of determining any liability under the Securities Act, each post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (c) | to file a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering. |
| (d) | to file a post-effective amendment to include any financial statements required by Form 10-K at the start of any delayed offering or throughout a continuous offering. |
| (e) | that insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant, the Registrant has been advised that in |
II - 3
Table of Contents
| the opinion of the SEC, such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registration of expenses incurred or paid by a director, officer or controlling person to the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| (f) | that, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (g) | that, for the purpose of determining liability of the Registrant under the Securities Act to any purchaser in the initial distribution of the securities, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | any preliminary prospectus or prospectus of the Registrant relating to the offering filed pursuant to Rule 424; |
| (ii) | any free writing prospectus relating to the offering prepared by or on behalf of the Registrant or used or referred to by the Registrant; |
| (iii) | the portion of any other free writing prospectus relating to the offering containing material information about the Registrant or its securities provided by or on behalf of the Registrant; and |
| (iv) | any other communication that is an offer in the offering made by the Registrant to the purchaser. |
| (h) | to provide to the Placement Agent at the closing specified in the Placement Agent agreements, certificates in such denominations and registered in such names as required by the Placement Agent to permit prompt delivery to each purchaser. |
II - 4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the People’s Republic of China, on the 14th day of May, 2010.
| SHANGRI-LA TIBETAN PHARMACEUTICALS, INC. | ||
| /s/ Taylor Z. Guo | ||
| Taylor Z. Guo | ||
| Chief Executive Officer (Principal Executive Officer) | ||
| Date: | May 14, 2010 | |
II - 5
Table of Contents
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below does hereby constitute and appoint Taylor Z. Guo his true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement and sign any registration statement for the same offering covered by this Registration Statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933, as amended and all post-effective amendments thereto and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitutes or substitutes, may lawfully do or cause to be done by virtue hereof.
| Date: | May 14, 2010 |
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| Signature |
Title |
Date | ||
| /s/ Hong Yu |
Chairman of the Board of Directors | May 14, 2010 | ||
| Hong Yu | ||||
| /s/ Taylor Z. Guo |
Chief Executive Officer, Director | May 14, 2010 | ||
| Taylor Z. Guo | (Principal Executive Officer) | |||
| /s/ Sabrina Y. Ren |
Chief Financial Officer | May 14, 2010 | ||
| Sabrina Y. Ren | (Principal Accounting and Financial Officer) | |||
| /s/ Wenbo Chen |
Director | May 14, 2010 | ||
| Dr. Wenbo Chen | ||||
| /s/ Youhang Peng |
Director | May 14, 2010 | ||
| Youhang Peng | ||||
| /s/ Solomon Chen |
Director | May 14, 2010 | ||
| Solomon Chen | ||||
II - 6
