Attached files
| file | filename |
|---|---|
| EX-23.2 - EXHIBIT 23.2 - JUNIPER PHARMACEUTICALS INC | exhibit232.htm |
| EX-32.1 - EXHIBIT 32.1 - JUNIPER PHARMACEUTICALS INC | exhibit321.htm |
| EX-23.1 - EXHIBIT 23.1 - JUNIPER PHARMACEUTICALS INC | exhibit231.htm |
| EX-31.2 - EXHIBIT 31(I).2 - JUNIPER PHARMACEUTICALS INC | exhibit31i2.htm |
| EX-31.1 - EXHIBIT 31(I).1 - JUNIPER PHARMACEUTICALS INC | exhibit31i1.htm |
| EX-32.2 - EXHIBIT 32.2 - JUNIPER PHARMACEUTICALS INC | exhibit322.htm |
|
UNITED STATES
|
||
|
SECURITIES AND EXCHANGE COMMISSION
|
||
|
Washington, D.C.
|
||
|
FORM 10-K/A
Amendment No. 1
|
||
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE
|
|
|
ACT OF 1934
|
||
|
For the fiscal year ended December 31, 2009
|
||
|
OR
|
||
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
|
|
|
EXCHANGE ACT OF 1934
|
||
|
For the transition period from ____________ to ____________
|
||
|
Commission File number 1-10352
|
||
|
(Exact name of registrant as specified in its charter)
|
||
|
Delaware
|
59-2758596
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
|
incorporation or organization)
|
Identification No.)
|
|
|
354 Eisenhower Parkway
|
||
|
Livingston, New Jersey
|
07039
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Registrant’s telephone number, including area code:
(973) 994-3999
|
||
|
Securities registered pursuant to Section 12(b) of the Act: None
|
||
|
Securities registered pursuant to Section 12(g) of the Act:
|
||
|
Common Stock, $.01 par value
|
NASDAQ Global Market
|
|
|
(Title of each class)
|
(Name of exchange on which
|
|
|
registered)
|
||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
o
|
Accelerated filer
|
x
|
||||
|
Non-accelerated filer
|
o
|
Smaller reporting company
|
o
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
o Yes x No
The aggregate market value of Common Stock held by non-affiliates of the registrant on June 30, 2009, the last business day of the registrant’s most recently completed second fiscal quarter, based on the closing price on that date of $1.14 was $57,554,824.
Number of shares of Common Stock of Columbia Laboratories, Inc. issued and outstanding as of April 27, 2010 are 65,605,886.
Explanatory Note
The purpose of this Annual Report on Form 10-K/A, Amendment No. 1 (“Form 10-K/A”) is to amend and restate the Annual Report on Form 10-K for the fiscal year ended December 31, 2009 of Columbia Laboratories, Inc., a Delaware corporation (together with its subsidiaries, “Columbia,” the “Company,” “we,” “our,” and “us”), that was filed with the Securities and Exchange Commission (the “SEC”) on March 12, 2010 (the “2009 10-K”), to include Part III, Items 10 through 14 that were previously omitted from the 2009 10-K in reliance on General Instruction G(3) to Form 10-K. General Instruction G(3) to Form 10-K provides that registrants may incorporate by reference certain information from a definitive proxy statement which involves the election of directors if such definitive proxy statement is filed with the SEC within 120 days after the end of the fiscal year. We do not now anticipate that the Company’s definitive proxy statement involving the election of directors will be filed by April 30, 2010 (i.e., within 120 days after the end of the Company’s 2009 fiscal year). The reference on the cover of the 2009 10-K to the incorporation by reference of the registrant’s definitive proxy statement into Part III of the Annual Report is hereby deleted.
In addition, as required by Rule 12b-15 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), new certifications (the “Certifications”) by our principal executive officer and principal financial officer are filed as exhibits to this Annual Report on Form 10-K/A under Item 15 of Part IV hereof.
For purposes of this Annual Report on Form 10-K/A, and in accordance with Rule 12b-15 under the Exchange Act, Items 10 through 14 of our 2009 10-K have been amended and restated in their entirety. In addition, Item 15 of Part IV is being refiled and has been amended to reflect the filing of the Certifications. Except as stated herein, this Form 10-K/A does not reflect events occurring after the filing of the Form 10-K on March 12, 2010 and no attempt has been made in this Form 10-K/A to modify or update other disclosures as presented in the 2009 10-K and included herein. Accordingly, this Form 10-K/A should be read in conjunction with our other filings with the SEC subsequent to the filing of our 2009 10-K, as information in such filings may update or supersede certain information contained in this Form 10-K/A.
Index to Annual Report on Form 10-K/A, Amendment No. 1
Fiscal Year Ended December 31, 2009
Part I 1
|
Item 1.
|
Business
|
|
|
Item 1A.
|
Risk Factors
|
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
|
Item 2.
|
Properties
|
|
|
Item 3.
|
Legal Proceedings
|
|
|
Item 4.
|
(Removed and Reserved)
|
|
Part II
|
Item 5.
|
Market for the Registrant’s Common Equity and Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
Item 6.
|
Selected Financial Data
|
|
|
Item 7.
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risks
|
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|
|
Item 9A.
|
Controls and Procedures
|
|
|
Item 9B.
|
Other Information
|
|
Part III
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
|
Item 11.
|
Executive Compensation
|
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
|
Item 13.
|
Certain Relationships, Related Transactions, and Director Independence
|
|
|
Item 14.
|
Principal Accountant Fees and Services
|
|
Part IV
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
|
“CRINONE®,” “PROCHIEVE®” and “STRIANT®” are registered trademarks of Columbia Laboratories, Inc. RepHresh®, Replens® and Advantage-S® are registered trademarks of Lil’ Drug Store Products, Inc. Other brands, names and trademarks contained in this Annual Report are the property of their respective owners.
Item 1. Business
General
We are in the business of developing, manufacturing and selling pharmaceutical products that utilize our proprietary bioadhesive drug delivery technologies. We are focused predominantly on the women’s reproductive healthcare market. Our bioadhesive vaginal gel products provide patient-friendly solutions for infertility, pregnancy support, amenorrhea, and other obstetric, gynecologic and medical conditions.
Our sales organization in the U.S. currently promotes our natural progesterone gel product, CRINONE 8%. We also continue to sell PROCHIEVE 8% in the U.S. CRINONE and PROCHIEVE are two brands of the same product that is approved in the U.S. for supplementation or replacement of progesterone as part of an Assisted Reproductive Technology (“ART”) treatment for infertile women with progesterone deficiency and for treatment of secondary amenorrhea. Outside the U.S., CRINONE has been approved for marketing for one or more medical indications including supplementation or replacement as part of an ART treatment for infertile women, treatment of secondary amenorrhea, the prevention of hyperplasia in postmenopausal women receiving hormone replacement therapy (“HRT”), the reduction of symptoms of premenstrual syndrome (“PMS”), menstrual irregularities, dysmenorrhea, and dysfunctional uterine bleeding. We promote CRINONE in the U.S. to a full range of reproductive endocrinologists, obstetricians and gynecologists who treat infertility. We also promote STRIANT testosterone buccal system for the treatment of hypogonadism in men; however, our continuing focus in fiscal 2010 is to increase prescriptions of CRINONE.
We derive additional revenues from our established marketing partnership with Merck Serono S.A. (“Merck Serono”), through which CRINONE 8% is commercialized in territories outside the U.S
All of our products and product candidates utilize our Bioadhesive Delivery System (“BDS”), which consists principally of a polymer (polycarbophil) and an active ingredient. The BDS is based upon the principle of bioadhesion, a process by which the polymer adheres to epithelial surfaces or mucosa. Our vaginal products adhere to the vaginal epithelium and the buccal products adhere to the mucosal membrane of the gum and cheek. The polymer remains attached to epithelial surfaces or mucosa and is discharged upon normal cell turnover, a physiological process that, depending upon the area of the body, occurs every 12 to 72 hours, or longer. Both vaginal and buccal BDS products provide sustained and controlled delivery of active drug ingredients. Its extended period of attachment permits use of BDS in products when extended duration of effectiveness is desirable or required.
Recent Events
On March, 3, 2010, we entered into a definitive agreement to sell , subject to shareholder approval, substantially all of our progesterone related assets and 11.2 million shares of common stock to Watson Pharmaceuticals, Inc. (the “Watson Transaction”) for a $47 million upfront payment plus royalties of 10 to 20 percent of annual net sales of certain progesterone products. Additional payments up to $45.5 million can be earned by the successful completion of clinical development milestones in the ongoing PREGNANT Study, regulatory filings, receipt of regulatory approvals and product launches. Watson will fund the development of a second-generation vaginal progesterone product as part of a comprehensive life-cycle management strategy. Watson will also have the right to designate a member of Columbia's Board of Directors. The closing of the transaction is subject to customary conditions, including approval by Columbia’s stockholders. It is expected to close during the second quarter of 2010.See “Item 7 - MD&A - Watson Transaction and Debt Restructuring.”
On March 3, 2010 we also entered into contingent agreements with the holders of our senior debt and our convertible subordinated notes under which, if the Watson transaction closes and the other conditions to consummation of such agreements are satisfied, we will repay our outstanding debt on an accelerated and discounted basis and issue the holders of convertible subordinated notes shares of common stock and warrants to purchase common stock. See “Item 1. Business - Watson Transactions and Debt Restructuring.”
The Company is currently engaged solely in one business segment -- the development, licensing and sale of pharmaceutical products. In certain foreign countries, these products may be classified as medical devices or cosmetics by those countries’ regulatory agencies. See Note 10 to the consolidated financial statements for information on foreign operations.
Operations
Our sales and marketing organization operates solely in the U. S., and is specifically focused on a select group of REIs and OB/GYNs. We also market STRIANT to general endocrinologists, urologists and a select number of primary care physicians. Our marketing and sales efforts for STRIANT are primarily focused on maintaining the current prescription levels. We have entered into partnerships to commercialize our products outside the U.S. and within certain markets in the U.S., and seek to enter into additional partnerships to commercialize our products in new countries and with additional audiences in the U.S. that we do not currently address.
We are substantially dependent on three manufacturers for the products that we sell to marketing partners around the world and commercialize ourselves in the U.S. Our CRINONE and PROCHIEVE vaginal gel products are each manufactured in bulk by Fleet Laboratories Limited, Watford, Herts, United Kingdom (“Fleet”) and filled into overwrapped single-use disposable applicators by Maropack AG, Zell, Switzerland (“Maropack”). Our single buccal product is manufactured for us by Mipharm S.p.A., Milan, Italy (“Mipharm”). Lubrizol, Inc. (“Lubrizol”) is the only supplier of medical grade, cross-linked polycarbophil, the polymer used in our BDS-based products.
Our wholly owned subsidiary, Columbia Laboratories (Bermuda) Ltd., entered into a new agreement dated December 8, 2009, with Fleet, our long-standing manufacturer of our progesterone vaginal gel, for delivery in bulk containers. Pursuant to the agreement, Fleet exclusively manufactures and supplies, and we exclusively purchase from Fleet, our requirements of bulk progesterone gel. We granted Fleet a non-exclusive, royalty-free license to use and practice our intellectual property solely and exclusively in connection with the manufacture and supply of bulk progesterone gel to us. Fleet will manufacture and supply us with bulk progesterone gel at a fixed price per batch in pounds sterling pursuant to rolling monthly forecasts and firm quarterly forecasts. The price may be adjusted annually on the anniversary date of the agreement to take into account any documented decrease or increase in the cost of raw materials or any other decrease or increase in the cost of manufacturing. The initial term of the agreement is five years and the agreement automatically renews for additional periods of two years unless either party gives to the other party, not less than six months prior to expiration of the agreement, written notice of its intention not to extend the agreement; provided, however, that upon termination of the agreement, Fleet agrees to perform its obligations under the agreement until the earlier of one year and Columbia’s engagement and qualification of an alternative manufacturer.
Columbia Laboratories (Bermuda) Ltd. has an agreement with Maropack to fill our bulk progesterone gel into overwrapped single-use disposable applicators. The current term of the agreement is one (1) year with automatic one (1) year renewals. Either party may terminate the agreement on six (6) months prior written notice before the end of any renewal term. Prices are renegotiated annually based on forecasted production volumes. Payments under the agreement are made in Swiss francs.
Columbia Laboratories (Bermuda) Ltd. entered into an agreement dated May 7, 2002 with Mipharm to manufacture at least eighty-five percent (85%) of our requirements for our STRIANT testosterone buccal product for sale in the U. S., Europe and Latin America. Pursuant to the agreement Mipharm built and operates a dedicated suite for the manufacture of hormone products, one-half the cost of which was paid by us. The original term of the agreement is twelve (12) years with automatic three (3) year renewals. Either party may terminate the agreement on twelve (12) months prior written notice before the end of any term. The price of the product may increase based on increases in labor costs in Italy or raw materials. Payments under the agreement are made in Euros.
|
Products
|
||
|
Progesterone Products
|
· CRINONE® 8% (progesterone gel) marketed and sold by the Company in the U.S.
· CRINONE® 8% sold to Merck Serono for resale outside the U.S.
· PROCHIEVE® 8% (progesterone gel) sold by the Company in the U.S.
· PROCHIEVE® 4% sold to Ascend Therapeutics, Inc., for resale in the U.S., pursuant to an agreement that will terminate on July 23, 2010.
|
|
|
Other Products
|
· STRIANT® (testosterone buccal system) marketed and sold by the Company in the U.S.
· STRIANT® sold to Mipharm, S.p.A. for resale in Italy.
· Royalty and licensing revenues.
|
In 2009, we also sold Replens® Vaginal Moisturizer and RepHresh® Vaginal Gel to Lil’ Drug Store Products, Inc. (“Lil’ Drug Store”) for resale pursuant to a supply agreement that expired on October 31, 2009.
Progesterone Products: CRINONE and PROCHIEVE
Progesterone is a hormone manufactured by a woman’s ovaries in the second half of the menstrual cycle and by the placenta during pregnancy. Progesterone is responsible for preparing the uterus for pregnancy and, if pregnancy occurs, maintaining it until birth, or, if pregnancy does not occur, inducing menstruation.
Our principal product is a sustained release gel that delivers natural progesterone vaginally. Our vaginal progesterone gel product is marketed under the two brand names CRINONE and PROCHIEVE. CRINONE/PROCHIEVE utilizes the Company’s patented BDS, which enables the progesterone to achieve a preferential uptake of drug from the vagina to the uterus, or a “First Uterine Pass Effect™.” The product is available in two strengths, an 8% progesterone gel and a 4% progesterone gel. It is the first product designed and FDA approved to deliver progesterone directly to the uterus, thereby providing a therapeutic benefit and avoiding high blood levels of metabolites seen with orally-delivered synthetic progestins.
The Company sells CRINONE and PROCHIEVE brand progesterone gels in the U.S. CRINONE brand progesterone gel is sold outside the U.S. by Merck Serono under a worldwide (excluding the U.S.) license from the Company. Upon closing of the Watson Transaction, Watson will be responsible for marketing and selling CRINONE AND PROCHIEVE in the U.S.
CRINONE/PROCHIEVE in the 8% progesterone gel is approved in the U.S. for progesterone supplementation or replacement as part of an Assisted Reproductive Technology (“ART”) treatment for infertile women with progesterone deficiency. CRINONE/PROCHIEVE in both the 8% and 4% progesterone gels is approved in the U.S. for the treatment of secondary amenorrhea (loss of menstrual period). Outside the U.S., CRINONE has been approved for marketing for one or more medical indications in 60 countries. The medical indications include: progesterone supplementation or replacement as part of an ART treatment for infertile women; the treatment of secondary amenorrhea; the prevention of hyperplasia in post-menopausal women receiving hormone replacement therapy (“HRT”); the reduction of symptoms of premenstrual syndrome (“PMS”); menstrual irregularities; dysmenorrhea; and dysfunctional uterine bleeding. PROCHIEVE is not marketed outside the U.S.
CRINONE 8% is principally marketed to REIs who generally perform the more technical procedures to assist women who are infertile to become pregnant. Our 2010 focus for CRINONE commercialization will be to continue to seek to convert sales of pharmacy compounded intramuscular progesterone injections, progesterone suppositories, and progesterone tablets to sales of CRINONE. Marketing materials showing our compilation of 20 clinical trials that have been conducted to compare CRINONE to other forms of progesterone provide us with a compelling case for the efficacy of CRINONE. These data show that CRINONE is as effective as, and in some cases numerically more effective than, all the other delivery systems for progesterone. Importantly, the completion of our large prospective, randomized trial conducted at Brigham and Women’s hospital which demonstrated equivalent efficacy of CRINONE compared to intramuscular (IM) injections adds significant weight to this already large database. In the seven clinical trials that included an arm evaluating patient preference, patients preferred CRINONE over the competing product in all seven clinical trials. Our sales force uses these materials to persuade physicians to prescribe CRINONE over the competing progesterone injections and suppositories that are pharmacy compounded, as well as over Endometrin®, a vaginal progesterone tablet that requires three doses per day to achieve results comparable to once-daily CRINONE.
PROCHIEVE 8% continues to be available to obstetricians and gynecologists who may use progesterone in conjunction with clomiphene citrate to assist women who are infertile become pregnant. We expect that in 2010 we will continue to invest significant resources in the development program for PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix as measured by transvaginal ultrasound at mid-pregnancy. This program includes a clinical trial in pregnant women which we have named the PREGNANT (PR OCHIEVE® E xtending G estatio N A N ew T herapy) study. In 2007, we reported data from our completed clinical trial of PROCHIEVE 8% in pregnant women with a history of a prior preterm birth. In that clinical trial, the study endpoints were not met, and the trial demonstrated that there was no benefit from administering vaginal progesterone to that patient population. However, a secondary analysis of the data from that earlier study demonstrated a statistically significant improvement in the rate of preterm birth and infant outcomes in trial participants who had a short cervix at mid-pregnancy. This secondary analysis was conducted on a subset of patients with a short cervix at mid-pregnancy from the previous trial; the PREGNANT clinical trial is designed to confirm these data in a larger trial. The PREGNANT study was designed based in part on discussions with the FDA and the data published in the following article Vaginal Progesterone is Associated with a Decrease in Risk for Early Preterm Birth and Improved Neonatal Outcome in Women with a Short Cervix: a Secondary Analysis from a Randomized, Double-blind, Placebo-controlled Trial. DeFranco EA, O’Brien JM, Adair CD, et al., Ultrasound Obstet Gynecol 2007;30:697-705. Based on those positive data and our discussions with the U.S. Food and Drug Administration (“FDA”), we designed the Phase III PREGNANT study. The PREGNANT study is a randomized, double-blind, placebo-controlled Phase III clinical trial designed to evaluate the ability of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix of between 1.0 and 2.0 centimeters as measured by transvaginal ultrasound at mid-pregnancy. The primary endpoint of this clinical trial is a reduction in preterm births at less than or equal to 32 weeks versus placebo.
In October 2008, we announced a collaboration with the Perinatology Research Branch (PRB) of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development, part of the National Institutes of Health (NIH), under which we amended the study protocol to reflect the addition of nine NIH sponsored sites and an increase in the number of patients from 300 to 450. With the increase in patients, the power of the study to show statistically significant improvements in both the obstetrical endpoints and infant outcomes becomes even stronger. All clinical data, whether generated by NIH sites or our sites, will be collected centrally and, assuming success in the study, the results will be available to us for regulatory filings. We believe that, if the study is successful, the participation of the NIH will have a positive impact on physicians’ adoption of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix at mid-pregnancy as measured by transvaginal ultrasound, which will lead to improved patient care and a more rapid reduction in the incidence of preterm birth. If the results of the PREGNANT trial confirm the results seen in the earlier clinical trial, we expect to file a NDA supplement seeking approval of PROCHIEVE 8% for this indication and utilize the name PROCHIEVE solely in connection with the preterm birth indication. We expect to enroll at least 450 patients in the PREGNANT study and we expect to complete enrollment in the second quarter of 2010, with the last baby born and study results available in the fourth quarter of 2010.
In September 2007, we licensed PROCHIEVE 4% (which is marketed only in the U.S.) to Ascend Therapeutics, Inc. (“Ascend”) to market this product effective January 1, 2008 under a five year license and supply agreement. Merck Serono suspended any promotional support for the 4% progesterone vaginal gel outside the U.S. but maintains the marketing rights. On January 21, 2010, Ascend notified us that it was terminating the license and supply agreement as of July 23, 2010, pursuant to the terms of the agreement.
The most common side effects of CRINONE/PROCHIEVE 8% are breast enlargement, constipation, somnolence, nausea, headache, and perineal pain. The most common side effects of PROCHIEVE 4% when used in combination with estrogen include cramps, fatigue, depression, emotional lability, sleep disorder, and headache. CRINONE /PROCHIEVE is contraindicated in the U.S. in patients with active thrombophlebitis or thromboembolic disorders, or a history of hormone-associated thrombophlebitis or thromboembolic disorders, missed abortion, undiagnosed vaginal bleeding, liver dysfunction or disease, and known or suspected malignancy of the breast or genital organs.
Other Vaginal Gel Women’s Products
Replens® Vaginal Moisturizer. Replens Vaginal Moisturizer. Replens is a vaginal gel product indicated for replenishment of vaginal moisture on a sustained basis and to relieve the discomfort associated with vaginal dryness. Replens was the first product developed utilizing the BDS. In May 2000, the Company sold the U.S. rights for Replens to Lil’ Drug Store, pursuant to an agreement under which the Company received royalties of 10% of sales of Replens in the U.S. until October 2005. On June 29, 2004, the Company sold the remaining worldwide marketing rights for Replens to Lil’ Drug Store and executed two related agreements with Lil’ Drug Store: a promotion agreement that expired at the end of 2006 and a five year supply agreement for Lil’ Drug Store’s requirements for Replens in non-U.S. markets that on expired October 31, 2009. See “Item 1. Business - Licensing and Development Agreements.”
RepHresh® Vaginal Gel. RepHresh Vaginal Gel is a feminine hygiene product that can eliminate vaginal odor. On June 29, 2004, the Company sold the worldwide marketing rights to the product to Lil’ Drug Store and executed two related agreements with Lil’ Drug Store: a promotion agreement that expired at the end of 2006 and a five year supply agreement that expired on October 31, 2009. See “Item 1. Business - Licensing and Development Agreements.”
Products Outside of the Women’s Reproductive Healthcare Market
STRIANT (testosterone buccal system). STRIANT is approved in the U.S., and several European countries for treatment of hypogonadism in men, but is currently marketed only in the U.S. and Italy. Hypogonadism is characterized by a deficiency or absence of endogenous testosterone production. Signs and symptoms of hypogonadism can include decreased libido (sexual desire), erectile dysfunction (ED), fatigue, depression, reduced muscle mass, and osteoporosis. The purpose of testosterone replacement therapy is to provide and maintain normal levels of testosterone. It is estimated that hypogonadism affects 38.7% of men aged 45 years or older in the U.S., approximately one million of whom currently receive treatment. The treatment for hypogonadism is to replace testosterone through one of many available delivery systems including transdermal patches, topical gels, injectable formulations of testosterone and the STRIANT buccal system.
STRIANT utilizes the BDS to achieve controlled and sustained delivery of testosterone via the buccal cavity - the small depression in the mouth where the gum meets the upper lip above the incisor teeth. The product, which has the appearance of a small monoconvex tablet, rapidly adheres to the buccal mucosa. STRIANT is absorbed into the bloodstream and delivered directly into the superior vena cava (major blood vessel), bypassing the gastrointestinal system and liver. In clinical trials, STRIANT produced circulating testosterone concentrations in hypogonadal males approximating physiologic levels seen in healthy young men.
The clinical data supporting the approval of STRIANT by the FDA were generated from a 12-week U.S. multi-center, open-label, single arm trial that evaluated the efficacy, safety and tolerability of STRIANT in 98 men with hypogonadism. The most frequent adverse events that occurred with STRIANT in that trial at an incidence of 1% or greater which were possibly, probably or definitely related to the use of STRIANT were: gum or mouth irritation (9.2%), bitter taste (4.1%), gum pain (3.1%), gum tenderness (3.1%), headache (3.1%), gum edema (2.0%), and taste perversion (2.0%). A total of 16 patients reported 19 gum-related adverse events. Of these, ten patients (10.2%) reported 12 events of mild intensity, four patients (4.1%) reported five events of moderate intensity, and two patients (2.0%) reported two events of severe intensity. Four patients (4.1%) discontinued treatment with STRIANT due to gum- or mouth-related adverse events, including two with severe gum irritation, one with mouth irritation and one with "bad taste in mouth." The majority of the gum-related adverse events were transient and resolved within one to 14 days. Patients on STRIANT should be advised to regularly inspect the gum region where they apply STRIANT and report any abnormality to their health care professional.
STRIANT is not indicated for women and must not be used in women. Testosterone supplements may cause fetal harm. STRIANT should not be used in patients with known hypersensitivity to any of its ingredients, including testosterone U.S.P that is chemically synthesized from soy. Androgens are contraindicated in men with carcinoma of the breast or known carcinoma of the prostate. Edema with or without congestive heart failure may be a serious complication in patients with preexisting cardiac, renal or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required. Gynecomastia frequently develops and occasionally persists in patients being treated with androgens for hypogonadism. The treatment of hypogonadal men with testosterone esters may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung diseases. Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia and prostatic carcinoma. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirements.
We market and sell STRIANT in the U.S. STRIANT sales constituted less than 1% of the market for testosterone replacement products in 2009. Due to our focus on increasing prescriptions for our progesterone gel products and increasing our overall business in products for women’s reproductive health, our marketing and sales organization is not undertaking activities beyond those that we believe are required to maintain current U.S. sales of STRIANT.
In October 2002, the Company and Ardana plc (then Ardana Biosciences, Ltd., “Ardana”) entered into a license and supply agreement under which Ardana licensed and sold STRIANT in several European countries (excluding Italy). See “Item 1. Business - Licensing and Development Agreements.” In July 2008, we terminated the license and supply agreement with Ardana. Prior to termination, Ardana had marketed and sold STRIANT in the United Kingdom itself, and sold STRIANT in Ireland, Germany, Sweden, Finland, Norway, Denmark, and the Netherlands through other distributors. Distribution in countries other than Italy and the United Kingdom has been discontinued; the marketing authorization in the United Kingdom has been transferred to our subsidiary, Columbia Laboratories (UK) Ltd. Revenues are not expected to be material.
In May 2003, the Company and Mipharm entered into a license and supply agreement under which Mipharm will market, distribute and sell STRIANT in Italy. See “Item 1. Business - Licensing and Development Agreements.” Mipharm launched STRIANT into the Italian market in November 2007. In 2009, Mipharm assigned its rights to market STRIANT in Italy to Sandoz, S.p.A.
Advanced Formula Legatrin PM. In May 2000, the Company licensed Advanced Formula Legatrin PM®, a product for the relief of occasional pain and sleeplessness associated with minor muscle aches to Lil’ Drug Store, who pays the Company a royalty of 20% of the net sales of the product. The license agreement had an initial five-year term with provisions for automatic renewal. The license for Advanced Formula Legatrin PM was renewed to May 2015. See “Item 1. Business - Licensing and Development Agreements."
Research and Development
The Company spent $8.6 million in 2009, $6.2 million in 2008 and $5.8 million in 2007 on research and development activities. The expenditures in 2009, 2008 and 2007 were primarily costs associated with the Company’s clinical study of PROCHIEVE 8% (progesterone gel) for the prevention of recurrent preterm birth, discussed below. The expenditures in 2008 also included some small residual costs associated with the development of a vaginally-administered lidocaine candidate to prevent and relieve dysmenorrhea. The Company cannot predict whether it will be successful in the development of the products listed below or any other product candidates.
Generally the Company’s drug development activities take the following steps in the U.S. (and comparable steps in foreign countries). After the Company formulates an active drug ingredient into the BDS, it files an Investigational New Drug Application (“IND”) with the FDA to begin to test the product in humans. The IND becomes effective and the studies may begin if the FDA does not disapprove the IND within 30 days of its submission. The IND describes how, where, and by whom the studies will be conducted; information about the safety of the active drug ingredient; how it is thought to work in the body; any toxic effects it may have; and how it is manufactured. All clinical studies must also be reviewed and approved by an Institutional Review Board (“IRB”) that is responsible for the study site. Progress reports on clinical studies must be submitted at least annually to the FDA and the IRB.
Clinical studies are divided into three phases. Phase I studies typically involve small numbers of normal, healthy volunteers. Phase I studies are intended to assess a drug’s safety profile, including the safe dosage range. Phase I studies also determine how the drug is absorbed, distributed, metabolized, and excreted, as well as the duration of its action. Columbia has historically developed products using already approved active ingredients and developed them in our BDS technology. This has typically meant that Phase I studies are not required. Phase II studies involve volunteer patients (people with the disease intended to be treated) to assess the drug’s effectiveness and to further evaluate its safety. Phase III studies usually involve larger numbers of patients in clinics and hospitals to confirm the product’s efficacy and identify possible adverse events. Phase III studies are the “pivotal” studies that regulatory agencies require to show both safety and efficacy on a statistically representative population of people intended to be treated.
Following the completion of all three phases of clinical trials, the Company analyzes all of the data and files a New Drug Application (“NDA”) with the FDA if the data successfully demonstrate both safety and effectiveness. The NDA contains all of the scientific information that the Company has gathered. NDAs typically run thousands of pages. If the FDA approves the NDA, the new product becomes available for physicians to prescribe. The Company must continue to submit periodic reports to the FDA, including any cases of adverse reactions and appropriate quality-control records. For some medicines, the FDA requires additional studies after approval (Phase IV studies) to evaluate long-term effects of the drug. The FDA also has the authority to require a Risk Evaluation and Mitigation Strategy (REMS) from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks. The development, clinical testing and filing of an application to the respective regulatory agencies of those countries where the drug is intended to be approved for marketing and sales can cost millions of dollars.
PROCHIEVE 8% in Preventing Preterm Birth. In February 2007, we reported the results of our Phase III multi-center, randomized, double-blind, placebo-controlled, clinical trial designed to assess the efficacy, safety and tolerability of PROCHIEVE 8% in reducing the risk of preterm birth in pregnant women with a previous preterm birth before 35 weeks gestation. The study did not achieve a statistically significant reduction in the incidence of preterm birth at week 32, the primary endpoint in the study population. The incidence and profile of adverse events in patients receiving PROCHIEVE 8% was similar to placebo.
In April 2007, we reported that evaluation of a secondary endpoint of the study revealed a possible effect of PROCHIEVE 8% in delaying cervical shortening. Although an effect on cervical length was not the primary focus of this trial, pursuant to the study protocol, cervical length measurements were performed on all women at baseline (at approximately 20 weeks gestation) and at 28 weeks gestation. Data from the study show a statistically significant delay in cervical shortening in patients treated with PROCHIEVE 8%, and suggest a correlation between the cervical length data, PROCHIEVE 8% administration, and both a reduction in the likelihood of preterm birth and an improvement in infant outcomes.
Evaluation of treatment by cervical length at baseline revealed that the “responders” to progesterone were patients with a short cervix at baseline. Further evaluation of all randomized patients, including patients randomized with a short cervix only, show that patients with a cervical length less than 3.0 cm had a significant treatment effect to reduce the incidence of preterm birth at less than 37 weeks gestation. A further evaluation of the patients with baseline short cervical length revealed that in women with a baseline cervical length less than 2.8 cm, there was a statistically significant reduction in preterm birth at less than or equal to 32 weeks gestation. These delays in delivery were associated with significant improvements in infant outcomes.
On the basis of these analyses and discussions with the FDA, we designed the PREGNANT study which is underway. This randomized, double-blind, placebo-controlled Phase III clinical trial is designed to evaluate the ability of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix of between 1.0 and 2.0 centimeters as measured by transvaginal ultrasound at mid-pregnancy. The primary endpoint of the PREGNANT clinical trial is a reduction in preterm births at less than or equal to 32 weeks gestation versus placebo.
In 2008, we recruited 19 study sites, filed the protocol with each site’s IRB and trained their staff on the protocol for the PREGNANT study. In October 2008, we announced a collaboration with the Perinatology Research Branch (PRB) of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development, part of the National Institutes of Health (NIH) under which we amended the study protocol to reflect the addition of nine NIH sponsored sites and an increase in the number of patients from 300 to 450. With the increase in patients, the power of the study to show statistically significant improvements in both the obstetrical endpoints and infant outcomes becomes even stronger. All clinical data, whether generated by NIH sites or our sites, will be collected centrally and, assuming success in the study, the results will be available to us for regulatory filings. We believe that, if the study is successful, the participation of the NIH will have a positive impact on physicians’ adoption of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix at mid-pregnancy as measured by transvaginal ultrasound, which will lead to improved patient care and a more rapid reduction in the incidence of preterm birth. We expect to enroll at least 450 patients in the PREGNANT study and we expect to complete enrollment in the second quarter of 2010, with the last baby born and study results available in the fourth quarter of 2010.
Watson Development Collaboration. Pursuant to our agreement with Watson, we will collaborate with respect to the development of progesterone products. In connection therewith, the parties agreed to establish a joint development committee to oversee and supervise all development activities. Columbia will be responsible for completion of the PREGNANT study and such other activities as determined by the joint development committee. We will be responsible for the costs of conducting the PREGNANT study and the preparation, filing and approval process of the related new drug application (or supplemental new drug application) up to a maximum amount of $7 million from January 1, 2010. All other development costs incurred in connection with the development collaboration will be paid by Watson.
Progesterone Progressive Hydration Vaginal Product. We are developing a vaginal progesterone product using our progressive hydration patented technology that would extend patent protection for our progesterone franchise for an additional 6 years. The strategy would be to transition from the current product to the new product, which initially would be protected by both the progesterone delivery and progressive hydration patents.
PROCHIEVE 4% for the Prevention of Endometrial Hyperplasia. In 2004, a third party study was initiated to evaluate the long-term effects of Hormone Replacement Therapy in menopausal women. The study investigators selected PROCHIEVE 4% as the active progesterone to be administered to all menopausal women with an intact uterus who receive estrogen to prevent them from developing endometrial hyperplasia. We are supplying PROCHIEVE 4% for this trial, therefore, our costs are minimal, but we will have access to the data and could possibly utilize the data for publication purposes in a peer reviewed medical journal. The study is now expected to be completed in 2011.
Terbutaline Vaginal Gel. In December 2002, the Company entered into a development and license agreement with Ardana to develop the Company’s terbutaline vaginal gel product candidate for the treatment of infertility, dysmenorrhea and endometriosis. In 2007, Ardana elected to suspend development of the product as a result of slow recruitment in a proof of concept clinical trial. In July 2008, we terminated the development and license agreement pursuant to our rights under the agreement to terminate it in the event of the insolvency of Ardana. Ardana announced in June 2008 that it suspended trading in its shares, was no longer in a position to continue its operations, and had appointed administrators of the company. We may consider further investment, if resources allow, at a later date.
Testosterone Progressive Hydration Vaginal Tablet. In October 2000, the Company completed a Phase I trial of its testosterone progressive hydration vaginal tablet for women. The study demonstrated that testosterone could be delivered vaginally over a period of days. A preliminary clinical plan, with a focus on reducing the size of systemic uterine fibroids is under review. We are not currently investing further in this drug candidate due to our investment in the PREGNANT trial. We may consider further investment, if resources allow, at a later date.
Vaginally Administered Carbamide Peroxide. The Company is conducting pre-clinical development activities for a vaginally-administered carbamide peroxide product for treating or preventing vaginal infections. The product candidate is being investigated to determine the benefit of releasing and maintaining a very low concentration of peroxide over an extended period of time, in order to provide the benefits of oxygen release without adversely affecting normally-desired local vaginal flora. We do not plan to invest in development of this drug candidate at this time, but may consider further investment as resources become available at a later date.
Peptide Delivery System. The Company has completed a program that demonstrates that the BDS can deliver therapeutic doses of small chain peptides for extended periods of time using the Company’s progressive hydration buccal technology.
Licensing and Development Agreements
Merck Serono S.A.
In May 1995, the Company entered into a license and supply agreement with American Home Products Corporation, now Wyeth (“Wyeth”), for its Wyeth-Ayerst Laboratories division to market CRINONE worldwide. The Company agreed to supply CRINONE at a price equal to 30% of Wyeth’s net selling price. In July 1999, Wyeth assigned the license and supply agreement to Serono (now Merck Serono).
In December 2006, the Company purchased from Merck Serono the full U.S. marketing rights for CRINONE. As a result, the Company holds the U.S. marketing rights to both CRINONE and PROCHIEVE brand progesterone vaginal gel products, and Merck Serono retains the marketing rights to CRINONE for the rest of the world. Upon closing of the Watson Transaction, U.S. marketing rights to CRINONE and PROCHIEVE will be sold to Watson.
CRINONE is sold outside the U.S. by Merck Serono, Our license and supply agreement with Merck Serono expires on May 19, 2010, and is renewable upon mutual agreement of the parties for five (5) year periods at commercial terms to be agreed upon. Merck Serono has informed us that they wish to renew the agreement for an additional 5 years, and we are in negotiations with them to renew the agreement on terms that are not materially different from the current agreement. There can be no assurance, however, that we will reach a renewal agreement or that any renewal agreement will not differ materially from the current agreement.
Mipharm S.p.A.
In May 2003, the Company and Mipharm entered into an agreement under which Mipharm will market, distribute and sell STRIANT in Italy. In exchange for these rights, Mipharm is obligated to pay the Company an aggregate of $1.4 million upon achievement of certain milestone events, including $0.4 million that was paid in 2003. We received a payment of $0.1 million, less VAT withholding, in 2004 on account of the UK approval of STRIANT and a payment of $0.2 million, less VAT withholding, in 2007 on marketing authorization received by Mipharm in Italy. Mipharm will provide additional performance payments upon the achievement of certain levels of sales in Italy and the Company will receive a percentage markup on the cost of goods for each unit sold. Mipharm is a manufacturer of STRIANT under a May 2002 agreement. In 2007, Mipharm launched sales of STRIANT in Italy. In 2009, Mipharm assigned its rights to market STRIANT in Italy to Sandoz, S.p.A.
Ardana plc
In October 2002, the Company and Ardana entered into a license and supply agreement under which Ardana would market, distribute and sell STRIANT in 18 European countries (excluding Italy). Under the agreement, the Company received $6.0 million. In December 2002, the Company and Ardana executed a development and license agreement (described above) to develop the Company’s terbutaline vaginal gel product. In 2007, Ardana elected to suspend development of the product as a result of slow recruitment in a proof of concept clinical trial. In July 2008, we terminated the license and supply agreement and the development and license agreement pursuant to our rights under the agreements to terminate it in the event of the insolvency of Ardana. Ardana announced in June 2008 that it suspended trading in its shares, was no longer in a position to continue its operations, and had appointed administrators of the company. In the quarter ended September 30, 2008, the Company recognized $2.9 million of deferred revenue from the cancellation of the agreement.
Lil’ Drug Store Products, Inc.
In June 2004, the Company and Lil’ Drug Store entered into an asset purchase agreement, a five year supply agreement, and a 2½ year promotion agreement. Under the agreements, Lil’ Drug Store acquired the Company’s over-the-counter women’s healthcare products, RepHresh® Vaginal Gel and Advantage-S® Bioadhesive Contraceptive Gel, and foreign marketing rights for Replens® Vaginal Moisturizer. The Company sold the U.S. marketing rights for Replens to Lil’ Drug Store in May 2000. Under the terms of the asset purchase agreement, Lil’ Drug Store also purchased the U.S. inventory of RepHresh and Advantage-S from the Company. The production and sale of Advantage-S was discontinued during 2006. The promotion agreement expired at the end of 2006. The Company supplied RepHresh and foreign requirements for Replens under the supply agreement until it expired on October 31, 2009.
In May 2000, the Company licensed Advanced Formula Legatrin PM®, a product for the relief of occasional pain and sleeplessness associated with minor muscle aches, to Lil’ Drug Store. Lil’ Drug Store pays the Company a royalty of 20% of the net sales of the product. The license agreement had an initial five-year term with provisions for automatic renewal. The license for Advanced Formula Legatrin PM was renewed to May 2015.
Ascend Therapeutics, Inc.
In September 2007, the Company and Ascend entered into a five year license and supply agreement for the Company’s PROCHIEVE 4% progesterone gel, pursuant to which Ascend is responsible for marketing and sales of PROCHIEVE 4% in the U.S. Ascend will purchase product from the Company at a transfer price equal to 35% of Ascend’s net selling price with minimum annual purchase obligations that increase over the life of the agreement. On January 21, 2010, Ascend notified us that it was terminating the license and supply agreement, as of July 23, 2010, pursuant to the terms of the agreement.
Financing Agreements
On July 31, 2002, PharmaBio Development (“PharmaBio”), an affiliate of Quintiles Transnational Corp., agreed to pay $4.5 million in four equal quarterly installments commencing third quarter 2002 for the right to receive a 5% royalty on net sales of the Company’s women’s healthcare products in the U.S. for five years, beginning in the first quarter of 2003. The royalty payments were subject to an aggregate minimum ($8 million) and maximum ($12 million) amounts. Because the minimum amount exceeded $4.5 million, the Company recorded the amounts received as liabilities. The excess of the minimum ($8 million) paid by the Company over the $4.5 million received by the Company was recognized as interest expense over the five-year term of the agreement, assuming an interest rate of 17%. The final payment under this agreement was made in February 2008.
On March 5, 2003, the Company and PharmaBio entered into a second agreement under which PharmaBio paid $15 million to the Company over a 15-month period that commenced with the signing of the agreement. In return, PharmaBio receives a 9% royalty on net sales of STRIANT in the U.S. up to agreed annual sales levels, and a 4.5% royalty of net sales above those levels. The royalty term is seven years. Royalty payments commenced in the third quarter of 2003 and are subject to aggregate minimum ($30 million) and maximum ($55 million) amounts. Because the minimum amount exceeds $15 million, the Company has recorded the amounts received as liabilities. The excess of the minimum ($30 million) to be paid by the Company over the $15 million received by the Company is being recognized as interest expense over the seven-year term of the agreement, assuming an interest rate of 15%. As of December 31, 2009, the Company has paid $13.5 million in royalties (including the true-up payment for the difference between royalties paid through November 2006 and $13.0 million) to PharmaBio under this agreement. The balance of the minimum royalty payments, estimated to be approximately $16.5 million is due in November, 2010.
On July 22, 2009, the Company and PharmaBio entered into an amendment to their agreement pursuant to which they agreed that, when the minimum royalty payment is due, the Company may, in its sole discretion, either pay the balance due or issue to PharmaBio a secured promissory note for that balance. The note would bear interest quarterly in arrears at the rate of 10% per annum and be due on November 30, 2011. In consideration for the right to issue the secured promissory note, the Company (a) agreed that during the period from July 22, 2009 through November 30, 2010, the Company will escrow any proceeds from sales of assets outside the ordinary course of business in excess of $15.0 million but not exceeding the difference between the amount of royalties actually received by PharmaBio under the agreement and $30.0 million, and (b) granted PharmaBio a warrant to purchase 900,000 shares of the Company’s common stock. In further consideration for the right to issue the secured promissory note, the Company has agreed that if it issues the secured promissory note on November 30, 2010, the Company will also on that date grant PharmaBio a warrant to purchase 900,000 shares of the Company’s common stock.
On March 3, 2010 we entered into contingent agreements with PharmaBio and the holders of our convertible subordinated notes under which, if the Watson transaction closes and the other conditions to consummation of such agreements are satisfied, we will repay our outstanding debt on an accelerated and discounted basis and issue the holders of convertible subordinated notes shares of common stock and warrants to purchase common stock. See “Item 1. Business - Watson Transaction and Debt Restructuring.”
Patents, Trademarks and Proprietary Information
We actively seek protection for our products and technology by means of U.S. and foreign patents, trademarks, and copyrights, as appropriate. The following table sets forth U.S. patents granted to the Company since 2002.
|
Year Granted
|
Nature of Patent
|
|
|
2009
|
Low concentration of peroxide for treating or preventing vaginal infections.
|
|
|
2006
|
Bioadhesive progressive hydration tablets using desmopressin or prostaglandin E2 as the active ingredient.
|
|
|
2004
|
Compositions and methods for safely preventing or treating premature labor using a beta-adrenergic agonist, such as terbutaline.
|
|
|
2004
|
Methods of safely treating endometriosis or infertility, and for improving fertility, using a beta-adrenergic agonist.
|
|
|
2003
|
Use of progestin therapy for maintaining amenorrhea.
|
|
|
2003
|
Bioadhesive progressive hydration tablet.
|
|
|
2002
|
Use of certain polycarboxylic acid polymers for vaginal pH buffering to prevent miscarriage and premature labor associated with bacterial vaginosis.
|
The Company continues to develop the core BDS and has filed additional patent applications in the U.S. and other countries around the world. In 2008, we also filed patent applications in the U.S. and around the world for use of progesterone to prevent or treat preterm birth in women with a short cervix. Our patent applications, if allowed, would strengthen our intellectual property position, providing patent protection until the year 2028 for PROCHIEVE 8% in women with a short cervix at mid-pregnancy. We believe our patents are important to our business and we intend to continue to protect them, including through legal action, when appropriate. While patent applications do not ensure the ultimate issuance of a patent, and having patent protection cannot ensure that competitors will not emerge, this is a fundamental step in protecting the Company’s technologies.
The following table sets forth the expiration dates of the principal U.S. patents for the Company’s marketed products and current development projects.
|
Subject of patent
|
Year of Expiration
|
Product or Project
|
||
|
Progressive hydration tablets
|
2019
|
STRIANT
—
testosterone progressive hydration vaginal tablet
—
peptide delivery system
—
progesterone progressive hydration vaginal product
|
||
|
First Uterine Pass Effect™
|
2018
|
vaginally administered lidocaine
—
terbutaline vaginal gel
—
testosterone vaginal gel
|
||
|
Progesterone delivery
|
2013
|
CRINONE /PROCHIEVE
|
||
The Company owns registrations of “CRINONE”, “STRIANT”, and “STRIANT SR” as trademarks in countries throughout the world and “PROCHIEVE” in the U.S. Applications for the registration of trademarks do not ensure the ultimate registration of these marks; however, the Company believes marks with pending applications will be registered. In addition, there can be no assurance that such trademarks will afford the Company adequate protection or that the Company will have the financial resources to enforce its rights under such trademarks.
The Company also relies on confidentiality and nondisclosure agreements to protect its intellectual property. There can be no assurance that other companies will not acquire information that the Company considers to be proprietary. Moreover, there can be no assurance that other companies will not independently develop know-how comparable, or superior, to that of the Company.
Sales of Products
Our products consist of our “Progesterone Products” that we promote through our own sales force to reproductive endocrinologists and obstetricians and gynecologists, sell to wholesalers and specialty pharmacies, and sell to licensees for resale. We supplement our Progesterone Products revenues by selling other products that use our BDS, which we refer to as “Other Products.” As of December 31, 2009:
|
Products
|
||
|
Progesterone Products
|
· CRINONE® 8% (progesterone gel) marketed and sold by the Company in the U.S.
· CRINONE® 8% sold to Merck Serono for resale outside the U.S.
· PROCHIEVE® 8% (progesterone gel) sold by the Company in the U.S.
· PROCHIEVE® 4% sold to Ascend Therapeutics, Inc. for resale in the U.S pursuant to an agreement that will terminate on July 23, 2010.
|
|
|
Other Products
|
· STRIANT® (testosterone buccal system) marketed and sold by the Company in the U.S.
· STRIANT® sold to Mipharm, S.p.A. for resale in Italy.
· Royalty and licensing revenues.
|
In 2009, we also sold Replens® Vaginal Moisturizer and RepHresh® Vaginal Gel to Lil’ Drug Store for resale pursuant to a supply agreement that expired on October 31, 2009.
Prior to establishing our own sales force in 2002, we generally out-licensed marketing rights to our products. In October 2002, our sales force began to call on obstetricians and gynecologists to encourage prescriptions for PROCHIEVE 8%. The sales force began sales efforts for PROCHIEVE 4% in April 2003, and in September 2003 began to call on endocrinologists, urologists and certain primary healthcare doctors to encourage prescriptions for STRIANT.
On December 22, 2006, the Company acquired the U.S. marketing rights to CRINONE and added reproductive endocrinologists to its infertility physician targets. In addition to these specialists, who typically handle the more sophisticated infertility treatments, the Company’s sales force calls on obstetricians and gynecologists, general endocrinologists, urologists and certain primary healthcare physicians. Our 35 person sales force is predominantly focused on promoting CRINONE to women’s reproductive healthcare providers with the aim of building the Company’s infertility business.
Upon closing of the Watson Transaction, U.S. marketing rights to CRINONE and PROCHIEVE will be sold to Watson.
Success of Marketing Efforts
Our business is dependent on market acceptance of our products by physicians, healthcare payors, patients, and the medical community. Medical doctors’ willingness to prescribe our products depends on many factors, including:
|
·
|
Perceived efficacy of our products;
|
|
|
·
|
Convenience and ease of administration;
|
|
|
·
|
Prevalence and severity of adverse side effects in both clinical trials and commercial use;
|
|
|
·
|
Availability of alternative treatments;
|
|
|
·
|
Cost effectiveness;
|
|
|
·
|
The pricing of our products; and
|
|
|
·
|
Our ability to obtain third party coverage or reimbursement for our products.
|
Even though we have received regulatory approval for CRINONE/PROCHIEVE and STRIANT, and even if we receive regulatory approval and satisfy the above criteria for any of our other investigational indications and product candidates, physicians may not prescribe our products. We promote CRINONE and STRIANT on our own behalf in the U.S. We have entered into agreements with other companies for the distribution and marketing of PROCHIEVE 4% in the U.S., CRINONE, and STRIANT in certain countries outside the U.S. Factors that could affect our success in marketing our products include:
|
·
|
The effectiveness of our production, distribution and marketing capabilities;
|
|
|
·
|
The successful marketing of our products by our distribution and marketing partners;
|
|
|
·
|
The success of competing products; and
|
|
|
·
|
The availability and extent of reimbursement from third party payors.
|
If any of our products or product candidates fail to achieve market acceptance, we or our marketing partners may be unable to sell the products successfully, which would limit our ability to generate revenue and could harm our business.
Competition
We and our marketing partners compete against established pharmaceutical and consumer product companies which market products addressing similar needs. Further, numerous companies are developing, or may develop, enhanced delivery systems and products that compete with our present and proposed products. It is possible that we may not have the resources to withstand these and other competitive forces. Some of these competitors possess greater financial, research and technical resources than our Company or our partners. Moreover, these companies may possess greater marketing capabilities than our Company or our partners, including the resources to implement extensive advertising campaigns.
The pharmaceutical industry is subject to change as new delivery technologies are developed, new products enter the market, generic versions of available drugs become available and treatment paradigms evolve to reflect these and other medical research discoveries. We face significant competition in all areas of our business. The rapid pace of change in the pharmaceutical industry continually creates new opportunities for existing competitors and start-ups and can quickly render existing products less valuable. Customer requirements and physician and patient preferences continually change as new treatment options emerge, are more or less heavily promoted and become less expensive. As a result, we may not gain, and may lose, market share.
CRINONE/PROCHIEVE, a natural progesterone product, competes in markets with other progestins, both synthetic and natural, that may be delivered by pharmacy-compounded injections, by pharmacy-compounded vaginal suppositories, with Prometrium® (oral micronized progesterone) marketed by Solvay Pharmaceuticals, Inc. (“Solvay”), and Endometrin® (progesterone vaginal insert) marketed by Ferring Pharmaceuticals, Inc. (“Ferring”). CRINONE/PROCHIEVE and Endometrin are the only progestin products approved by FDA for use in infertility or for use in pregnant women. Endometrin was approved by the FDA in June 2007.
STRIANT competes against other testosterone products that can be delivered by injection, transdermal patch and transdermal gel. Some of the more successful testosterone products include AndroGel® (testosterone gel) marketed by Solvay, Testim® (testosterone gel) marketed by Auxilium Pharmaceuticals, Inc. (“Auxilium”), and Androderm® (testosterone transdermal system) marketed by Watson Pharma, Inc. . Competition is based primarily on delivery method. Transdermal testosterone gels currently have the largest market share and transdermal testosterone patches have the next largest market share, followed by injectable products. STRIANT is priced comparably to the gels and patches.
Customers
Our customers include trade customers, such as drug wholesalers, specialty pharmacies, and chain drug stores, and our marketing partners. We make calls on the Company’s trade customers and doctors to promote CRINONE, PROCHIEVE and STRIANT. Our practice, in the case of our trade customers, is to ship our products promptly upon receipt of purchase orders from customers; consequently, backlog orders are not significant. In the case of our marketing partners, firm purchase orders are received by the Company ninety to one hundred twenty days in advance of the expected shipping date.
Revenue by Product
The following table sets forth the percentage of the Company's consolidated net revenues, consisting of sales, licensing fees, sales force promotional fees, and royalty revenues, by revenue source for each product accounting for 3% or more of consolidated revenues in any of the three years ended December 31:
|
2009
|
2008
|
2007
|
||||
|
CRINONE®
|
69%
|
59%
|
64%
|
|||
|
RepHresh®
|
10%
|
9%
|
11%
|
|||
|
Replens®
|
10%
|
8%
|
8%
|
|||
|
PROCHIEVE®
|
5%
|
7%
|
5%
|
|||
|
STRIANT®
|
5%
|
6%
|
7%
|
|||
|
Royalty income
|
1%
|
9%
|
2%
|
|||
|
Licensing fees
|
0%
|
2%
|
3%
|
|||
|
Total
|
100%
|
100%
|
100%
|
The following table presents information about Columbia’s net revenues, including royalty and license revenue, by customer for each of the three years ended December 31:
|
(in millions)
|
2009
|
2008
|
2007
|
|||
|
Merck-Serono (formerly Serono)
|
$ 8.6
|
$ 9.2
|
$ 8.2
|
|||
|
Lil' Drug Store Products, Inc.
|
6.6
|
6.2
|
6.0
|
|||
|
Cardinal Healthcare
|
5.7
|
5.6
|
6.0
|
|||
|
McKesson
|
4.6
|
5.0
|
3.9
|
|||
|
All others (none over 5%)
|
6.7
|
10.2
|
5.5
|
|||
|
Total
|
$ 32.2
|
$ 36.2
|
$ 29.6
|
The following table sets forth the Company's consolidated net revenues, based on sales by geographic area, for each area accounting for 5% or more of consolidated revenues in any of the three years ended December 31:
Sales by Geographic Area
|
(in millions)
|
2009
|
2008
|
2007
|
|||
|
United States
|
$ 20.0
|
$ 18.0
|
$ 15.2
|
|||
|
Switzerland
|
8.6
|
9.2
|
8.1
|
|||
|
Other European Countries
|
3.6
|
9.0
|
6.3
|
|||
|
Subtotal International
|
12.2
|
18.2
|
14.4
|
|||
|
Total
|
$ 32.2
|
$ 36.2
|
$ 29.6
|
Employees
As of February 23, 2010, the Company had 62 employees: 4 in management, 4 in production, 39 in sales and marketing, 5 in research and development and 12 in support functions. Our success is highly dependent on our ability to attract and retain qualified employees. Competition for employees is intense in the pharmaceutical industry. We believe we have been successful in our efforts to recruit qualified employees, but we cannot guarantee that we will continue to be as successful in the future. None of the Company's employees are represented by a labor union or are subject to collective bargaining agreements. We believe that our relationship with our employees is good.
The Company has employment agreements with four employees: Mr. Condella, interim chief executive officer, Mr. Mills, president and chief operating officer, Mr. McGrane, senior vice president, general counsel and secretary, and Mr. Gyenes, senior vice president, chief financial officer and treasurer. The Board of Directors of the Company has adopted an amended and restated Incentive Plan and an Indemnification Agreement for Officers and Directors and an Executive Change of Control Severance Agreement for Officers. On March 11, 2009, the Company entered into amended and restated employment agreements and new Executive Change in Control Severance Agreements with Messrs. Mills and McGrane, in order to effect certain technical changes required in order to comply with Internal Revenue Code 409A and the regulations thereunder. On July 15, 2009 and December 11, 2009, the Company entered into agreements with Messrs. Gyenes and Condella, respectively.
Watson Transaction and Debt Restructuring
On March 3, 2010, the Company, Watson Pharmaceuticals, Inc., as a guarantor of the Buyer’s obligations (“Watson”), and Coventry Acquisition, Inc., a subsidiary of Watson (the “Buyer”), entered into a Purchase and Collaboration Agreement (the “Purchase Agreement”). Pursuant to the Purchase Agreement, the Company agreed to sell, subject to shareholder approval, to the Buyer (i) substantially all of its assets primarily relating to the research, development, regulatory approval, manufacture, distribution, marketing, sale and promotion of pharmaceutical products containing progesterone as an active ingredient, including CRINONE 8% progesterone gel, PROCHIEVE 4% progesterone gel and PROCHIEVE 8% progesterone gel, each sold by the Company in the U.S. (collectively, the “Progesterone Products”), including certain intellectual property, promotional materials, contracts, product data and regulatory approvals and regulatory filings (the “Purchased Assets”), and (ii) 11,200,000 shares (the “Shares”) of the Company’s Common Stock. After the closing, the Company will retain certain assets and rights relating to its progesterone business, including all rights necessary to perform its obligations under its agreement with Merck Serono. The transactions pursuant to the Purchase Agreement and the ancillary agreements thereto are referred to collectively herein as the “Watson Transaction.”
At the closing of the Watson Transaction, in consideration for the sale of the Purchased Assets and the Shares, the Buyer will pay the Company $47 million in cash and assume certain liabilities associated with the Purchased Assets. In addition, the Buyer agreed to pay the Company up to $45.5 million in cash upon the achievement of several contingent milestones. The Buyer also agreed to make royalty payments to the Company of 10 to 20 percent of annual net sales of certain progesterone products; provided, however that royalty rates would be reduced by 50% in a particular country if a generic entry by a third party occurs in such country and certain other circumstances are fulfilled. In addition, if the Buyer commercializes a product through a third party outside of the U.S., in lieu of royalties, the Company will be entitled to 20% of gross profits associated with such commercialization. If the Buyer or its affiliates effects a generic entry with respect to a progesterone product in a country in the circumstances permitted by the Purchase Agreement, in lieu of royalties payable in respect of net sales for such generic product, the Company will be entitled to 20% of the gross profits associated with the commercialization of such generic product in such country.
Pursuant to the Purchase Agreement, the Company and the Buyer have also agreed to collaborate with respect to the development of progesterone products. In connection therewith, the parties agreed to establish a joint development committee to oversee and supervise all development activities. The Company will be responsible for completion of the PREGNANT Study and such other activities as determined by the joint development committee. The Company will be responsible for the costs of conducting the PREGNANT Study and the preparation, filing and approval process of the related new drug application (or the supplemental new drug application) up to a maximum of $7 million incurred after January 1, 2010. All other development costs incurred in connection with the development collaboration will be paid by the Buyer.
The parties also agreed to enter into various ancillary agreements, including an Investor’s Rights Agreement (pursuant to which the Buyer will have the right to designate a member of the Company’s board of directors for the period set forth therein, the Buyer will obtain certain registration rights pertaining to the Shares and the Buyer will agree to certain transfer restrictions pertaining to the Shares), a Supply Agreement pursuant to which the Company will supply PROCHIEVE 4%, PROCHIEVE 8% and CRINONE 8% to the Buyer for sale in the U.S. at a price equal to 110% of cost of goods sold, and a License Agreement relating to the grant of certain intellectual property licenses.
The closing of the Watson Transaction is subject to customary closing conditions, including Company stockholder approval.
As part of the Purchase Agreement, from the date of the closing of the Watson Transaction until the second anniversary of the date on which the Company and the Buyer terminate their relationship with respect to the joint development of progesterone products, the Company agreed not to manufacture, develop or commercialize products containing progesterone or any other products for the preterm birth indication, subject to certain exceptions. The joint development collaboration is terminable by either party five years after the closing of the Watson Transaction.
The Shares are being offered and sold to the Buyer under the Purchase Agreement in reliance on exemptions from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), pursuant to Section 4(2) under the Securities Act and Rule 506 promulgated thereunder, based on the nature of the Buyer and certain representations made by the Buyer to the Company.
PharmaBio Amendment
On March 3, 2010, the Company entered into an amendment (the “PharmaBio Amendment”) with PharmaBio to the Investment and Royalty Agreement dated March 5, 2003, between the Company and PharmaBio, as previously amended and supplemented (the “PharmaBio Agreement”). The PharmaBio Amendment provides for the early termination of the PharmaBio Agreement by permitting the Company to make certain payments required thereunder on an accelerated and discounted basis on the date the Company consummates (and contingent upon the Company consummating) a transfer of assets, sale of stock, licensing agreement and/or similar transaction yielding gross cash proceeds to the Company of $40 million or more.
Note Purchase and Amendment Agreements
On March 3, 2010, the Company entered into Note Purchase and Amendment Agreements (the “Note Purchase Agreements”) with all of the holders (the “Holders”) of the Company’s Convertible Subordinated Notes due December 31, 2011 (the “Notes”). Under the Note Purchase Agreements, the Company agreed to purchase, subject to the satisfaction of certain conditions, the approximately $40 million in aggregate principal amount of Notes held by the Holders. The aggregate purchase price for the Notes is $26 million in cash (plus accrued and unpaid interest through but excluding the date of the closing of the Note purchases), warrants to purchase 7,750,000 shares of Common Stock at an exercise price of $1.35 per share (the “Warrants”) and 7,407,407 shares of Common Stock. The closings of the transactions contemplated by the Note Purchase Agreements are subject to various conditions, including the consummation of the Watson Transaction. Pursuant to the Note Purchase Agreements, the Holders consented, effective on March 3, 2010, to an amendment to the Notes (the “Amendment”) that eliminates the right of any holder of the Notes to cause the Company to redeem the Notes by virtue of the Watson Transaction. The Amendment terminates if the note purchase closings do not occur on or prior to August 31, 2010 and in certain other circumstances. Each Note Purchase Agreement may be terminated in certain circumstances, including, among others, by any party thereto, if the closings thereunder do not occur on or prior to August 31, 2010.
The warrants to be issued under the Note Purchase Agreements will be exercisable, subject to the limitations set forth therein, during the period commencing 180 days after, and ending on the fifth anniversary of their issuance, unless earlier exercised or terminated as provided in such warrants.
Under the terms of the Note Purchase Agreements, the Company has granted the Holders who are “Affiliates” (as defined under Rule 405 of the Securities Act) of the Company certain registration rights with respect to the resale of the shares of the Company’s common stock to be issued under the Note Purchase Agreements and the shares of Company common stock issuable upon the exercise of the warrants to be issued under the Note Purchase Agreements.
Under the Note Purchase Agreements, until 45 days after the Company’s announcement of the results of the PREGNANT Study, if the Company issues any shares of Company common stock (or common stock equivalents) for a price that is less than $2.00 per share, the Company must offer the Holders, subject to certain exceptions, the right to exchange their warrants for cash payments of up to an aggregate of $3,999,996.
The shares and warrants to be issued under the Note Purchase Agreements are being offered and sold in reliance on exemptions from the registration requirements of the Securities Act pursuant to Section 4(2) under the Securities Act and Rule 506 promulgated thereunder, based on the nature of the Holders and certain representations made by them to the Company.
None of the Shares or the shares and warrants to be issued under the Note Purchase Agreements have been registered under the Securities Act (or the laws of any state or other jurisdiction) and may not be offered or sold in the U.S. absent registration or an applicable exemption from the registration requirements thereof. This Form 10-K does not constitute an offer for the sale of any securities of the Company or a solicitation of any offer to buy any securities of the Company.
The foregoing is a summary of the terms of the Purchase Agreement (and the related ancillary agreements), the Note Purchase Agreements, the Warrants, the Amendment and the PharmaBio Amendment, and does not purport to be complete and is qualified in its entirety by reference to the full text of the Purchase Agreement (and the related ancillary agreements), the Note Purchase Agreements, the Warrants, the Amendment and the PharmaBio Amendment, which were more fully summarized and filed as exhibits to the Company’s current report on Form 8-K filed with the SEC on March 4, 2010.
Additional Information about the Proposed Transactions and Where to Find It:
This communication is not a solicitation of a proxy from any security holder of the Company. In connection with stockholder approval of certain transactions contemplated by the Purchase Agreement, the Company intends to file with the SEC a preliminary proxy statement and a definitive proxy statement and it intends to mail to its security holders a definitive proxy statement and other materials. THE PROXY STATEMENT WILL BE SENT TO COMPANY SECURITY HOLDERS AND WILL CONTAIN IMPORTANT INFORMATION ABOUT THE COMPANY, WATSON, THE BUYER, THE TRANSACTIONS CONTEMPLATED BY THE PURCHASE AGREEMENT AND RELATED MATTERS. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PROXY STATEMENT AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC CAREFULLY WHEN THEY ARE AVAILABLE BEFORE MAKING ANY VOTING OR INVESTMENT DECISION WITH RESPECT TO THE TRANSACTIONS CONTEMPLATED BY THE PURCHASE AGREEMENT. Free copies of the proxy statement and other documents filed with the SEC by the Company, when they become available, can be obtained through the website maintained by the SEC at www.sec.gov. In addition, free copies of the proxy statement will be available from the Company by contacting Lawrence A. Gyenes at (973) 486-8860 or lgyenes@columbialabs.com or on the Company’s investor relations website at www.cbrxir.com.
Participation in the Solicitation:
The Company and its directors and executive officers and certain other members of management may be deemed to be participants in the solicitation of proxies from the Company’s stockholders in connection with the transactions contemplated by the Purchase Agreement. Information regarding the special interests of these directors, executive officers and members of management in the transactions contemplated by the Purchase Agreement and certain related transactions will be included in the proxy statement and other relevant documents filed with the SEC. Additional informational regarding the Company’s directors and executive officers is also included in this Annual Report on Form 10-K for the fiscal year ended December 31, 2009, and the Company’s proxy statement, dated April 9, 2009, filed with the SEC on April 17, 2009. The Company’s Form 10-K and proxy statement are available free of charge at the SEC’s website at www.sec.gov and from the Company by contacting it as described above.
Available Information
The Company's Internet address is www.columbialabs.com. Through a link on the “Investor” section of this website, which is also accessible at www.cbrxir.com, we make available, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with or furnish it to the SEC. In addition, we will provide electronic or paper copies of our filings free of charge upon request. Information contained on our corporate website or any other website is not incorporated into this Annual Report and does not constitute a part of this Annual Report.
In addition, the public may read and copy any materials filed by the Company with the SEC at the SEC’s Reference Room, which is located at 100 F Street NE, Washington, D.C., 20549. Interested parties may call (800) SEC-0330 for further information on the Reference Room. The SEC also maintains a website containing reports, proxy materials and information statements, among other information, at http://www.sec.gov.
Corporate Information
Columbia was incorporated as a Delaware corporation in 1986. Our principal executive offices are located at 354 Eisenhower Parkway, Livingston, New Jersey 07039, and our telephone number is (973) 994-3999. The Company's wholly-owned subsidiaries are Columbia Laboratories (Bermuda) Ltd. ("Columbia Bermuda"), Columbia Laboratories (France) SA ("Columbia France") and Columbia Laboratories (UK) Limited ("Columbia UK”).
We have a history of losses and we may not have sufficient funds to continue operations unless we are able to raise additional funds which may not be available to us.
We have had a history of losses since our founding. For the fiscal year ended December 31, 2009, we had a net loss of $21.9 million. If we and our partners are unable to successfully develop and market our products, and otherwise increase sales of our products, and contain our operating expenses, we may not have sufficient funds to continue operations unless we are able to raise additional funds from sales of securities or otherwise. Additional financing may not be available to us on acceptable terms, if at all.
We have a substantial amount of debt.
As of December 31, 2009, we had remaining future minimum payments due to PharmaBio pursuant to certain financing agreements of approximately $16.5 million, initially payable in November 2010. On July 22, 2009, we entered into an agreement with PharmaBio pursuant to which we can, in our sole discretion, extend the due date of the balance of our obligation to PharmaBio to November 2011. In addition, as of December 31, 2009, we had outstanding approximately $40 million principal amount of our convertible subordinated notes due December 31, 2011. Our annual interest expense is more than $8 million of which approximately $3.2 million is the annual cash portion of the expense relating to the convertible subordinated notes due December 31, 2011. Unless we generate substantial additional sales from our products or raise substantial additional capital, we may not be able to pay the interest on our debt or repay our debt at maturity. Our substantial amount of debt may make it difficult for us to access debt and equity markets to raise additional capital. On March 3, 2010 we entered into contingent agreements with PharmaBio and the holders of our convertible subordinated notes under which, if the Watson transaction closes and the other conditions to consummation of such agreements are satisfied, we will repay our outstanding debt on an accelerated and discounted basis and issue the holders of convertible subordinated notes shares of common stock and warrants to purchase common stock. See “Item 1. Business - Watson Transaction and Debt Restructuring.”
Our Phase III PREGNANT Study to reduce the incidence of preterm birth may be further delayed and may not be successful.
Our lead Research & Development opportunity is the PREGNANT study of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix as measured by transvaginal ultrasound in mid-pregnancy. This randomized, double-blind, placebo-controlled clinical trial will evaluate the effect of PROCHIEVE 8% on reducing the risk of preterm birth in women with a cervical length between 1.0 and 2.0 centimeters as measured by transvaginal ultrasound at mid-pregnancy. The primary endpoint is a reduction in the incidence of preterm birth at less than or equal to 32 weeks gestation vs. placebo. The study which we expect to complete by the end of 2010 may be further delayed and may not demonstrate the safety or efficacy of the product for this indication. In addition, the study may provide insufficient safety or efficacy data to meet FDA requirements for approval of the indication. In such event, a substantial portion of the contingent payments under the Watson Transaction would not be paid. Even if the study is successful, we may not have sufficient funds to complete the FDA approval process or to commercialize the product.
Our business is heavily dependent on the continued sale of CRINONE 8% by Merck Serono.
Our operating results are heavily dependent on the revenues and royalties derived from the sale of CRINONE 8% to Merck Serono for sale outside the U.S. Revenues from these sales of in 2009 constituted approximately 27% of our total revenues. We do not control the amount and timing of marketing resources that Merck Serono devotes to our product. Failure of Merck Serono to effectively market CRINONE 8% in its territories outside the U.S. could have a material adverse effect on our business, financial condition and results of operations. Our license and supply agreement with Merck Serono expires on May 19, 2010, and is renewable upon mutual agreement of the parties for five (5) year periods at commercial terms to be agreed upon. Merck Serono has informed us that they wish to renew the agreement for an additional 5 years, and we are in negotiations with them to renew the agreement on terms that are not materially different from the current agreement. There can be no assurance, however, that we will reach a renewal agreement or that any renewal agreement will not differ materially from the current agreement.
The price of our Common Stock has been and may continue to be volatile.
Historically, the market price of our Common Stock has fluctuated over a wide range. Between 2007 and 2008, our Common Stock traded in a range from $.92 to $5.25 per share. In 2009, our Common Stock traded in a range from $.65 to $1.74 per share. It is likely that the price of our Common Stock will fluctuate in the future. The market prices of securities of small specialty pharmaceutical companies, including ours, from time to time experience significant price and volume fluctuations. In particular, the market price of our Common Stock may fluctuate significantly due to a variety of factors, including: the results of clinical trials for our product candidates; FDA’s determination with respect to new drug applications for new products and new indications; and our ability to develop additional products. In addition, the occurrence of any of the risks described in these “Risk Factors” could have a material and adverse impact on the market price of our Common Stock.
The current stock market and credit market conditions are extremely volatile and may restrict our ability to raise additional funds to meet our capital needs.
The current stock market and credit market conditions are extremely volatile. It is difficult to predict whether these conditions will continue or worsen and, if so, whether the conditions would impact the Company and whether the impact would be material. In particular, constriction and volatility in the equity and debt markets may restrict our future ability to access these markets to meet our future capital or liquidity needs.
A decline in the price of our common stock could affect our ability to raise further working capital and adversely impact our ability to continue operations.
A prolonged decline in the price of our common stock could result in a reduction in the liquidity of our common stock and a reduction in our ability to raise capital. Because a significant portion of our operations has been and will continue to be financed through the sale of equity securities and equity linked securities (warrants and convertible debt), a decline in the price of our common stock could be especially detrimental to our liquidity and our operations. Such reductions may force us to reallocate funds from other planned uses and may have a significant negative effect on our business plans and operations, including our ability to develop our product candidates and continue our current operations. If we are unable to raise sufficient capital in the future, and we are unable to generate funds from operations sufficient to meet our obligations, we will not be able to have the resources to continue our normal operations.
If we do not meet the continued listing requirements of the NASDAQ Global Market, our common stock may be delisted.
Our common stock is listed on the NASDAQ Global Market. The NASDAQ Global Market requires us to continue to meet certain listing standards. During 2009, the closing price of our common stock on the NASDAQ Global Market ranged from $0.65 to $1.74. On December 9, 2009, we received a letter from the NASDAQ Global Market indicating that for 30 consecutive business days the Company’s common stock did not maintain a minimum closing bid price of $1.00 (“Minimum Bid Price Requirement”) per share as required by NASDAQ Listing Rule 5450(a)(1). On January 13, 2010, we received a notice from the NASDAQ Global Market indicating that the Company had regained compliance with the Minimum Bid Price Requirement for continued listing on the NASDAQ Global Market as set forth in Marketplace Rule 5450(a)(1). The notice stated that the closing bid price of the Company's common stock had been at or above the required minimum $1.00 per share for the previous 10 consecutive business days. While we are currently in compliance with the NASDAQ Global Market continued listing requirements, we cannot assure you that we will remain in compliance. If we do not meet the NASDAQ Global Market’s continued listing standards, we will be notified by the NASDAQ Global Market and we will be required to take corrective action to meet the continued listing standards; otherwise our common stock will be delisted from the NASDAQ Global Market. A delisting of our common stock on the NASDAQ Global Market would reduce the liquidity and market price of our common stock and the number of investors willing to hold or acquire our common stock, which could negatively impact our ability to access the public capital markets. A delisting would also reduce the value of our equity compensation plans, which could negatively impact our ability to retain key employees.
The development of our pharmaceutical products is uncertain and subject to a number of significant risks.
Some of our pharmaceutical products are in various stages of development. In the U.S. and most foreign countries, we must complete extensive human clinical trials that demonstrate the safety and efficacy of a product in order to apply for regulatory approval to market the product.
The process of developing product candidates involves a degree of risk and may take several years. Product candidates that appear promising in the early phases of development may fail to reach the market for several reasons, including:
|
·
|
Clinical trials may show our product candidates to be ineffective for the indications studied or to have harmful side effects;
|
|
|
·
|
Product candidates may fail to receive regulatory approvals required to bring the products to market;
|
|
|
·
|
Manufacturing costs or other factors may make our product candidates uneconomical; and
|
|
|
·
|
The proprietary rights of others and their competing products and technologies may prevent our product candidates from being effectively commercialized.
|
Success in early clinical trials does not ensure that large-scale clinical trials will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals.
The length of time necessary to complete clinical trials and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly and may be difficult to predict. The speed with which we can complete clinical trials and applications for marketing approval will depend on several factors, including the following:
|
·
|
The rate of patient enrollment which is a function of factors including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the study, and the nature of the study protocol;
|
|
|
·
|
Institutional review board, or IRB, approval of the study protocol and the informed consent form;
|
|
|
·
|
Prior regulatory agency review and approval;
|
|
|
·
|
Analysis of data obtained from clinical activities, which are susceptible to varying interpretations and which interpretations could delay, limit or prevent regulatory approval;
|
|
|
·
|
Changes in the policies of regulatory authorities for drug approval during the period of product development; and
|
|
|
·
|
The availability of skilled and experienced staff to conduct and monitor clinical studies and to prepare the appropriate regulatory applications.
|
In addition, developing product candidates is very expensive and will continue to have a significant impact on our ability to generate profits. Factors affecting our product development expenses include:
|
·
|
Our ability to raise any additional funds that we need to complete our trials;
|
|
|
·
|
The number and outcome of clinical trials conducted by us and/or our collaborators;
|
|
|
·
|
The number of products we may have in clinical development;
|
|
|
·
|
In licensing or other partnership activities, including the timing and amount of related development funding, license fees or milestone payments; and
|
|
|
·
|
Future levels of our revenue.
|
Clinical trials are expensive and can take years to complete, and there is no guarantee that the clinical trials will demonstrate sufficient safety and/or efficacy of the products to meet FDA requirements, or those of foreign regulatory authorities.
We may experience adverse events in clinical trials, which could delay or halt our product development.
Our product candidates may produce serious adverse events. These adverse events could interrupt, delay or halt clinical trials of our product candidates and could result in FDA or other regulatory authorities denying approval of our product candidates for any or all targeted indications. An Institutional Review Board or independent data safety monitoring board, the FDA, other regulatory authorities, or we ourselves may suspend or terminate clinical trials at any time. Our product candidates may prove not to be safe for human use.
Delays or failures in obtaining regulatory approvals may delay or prevent marketing of the products that we are developing.
Other than PROCHIEVE 8% (progesterone gel) which is being evaluated for the reduction of the risk of preterm birth in women with a short cervix at mid-pregnancy, and PROCHIEVE 4% (progesterone gel), which is being evaluated for the prevention of endometrial hyperplasia in women with an intact uterus undergoing estrogen replacement therapy, none of our product candidates have received regulatory approval from the FDA or any foreign regulatory authority. The regulatory approval process typically is extremely expensive, takes many years, and the timing or likelihood of any approval cannot be accurately predicted. Delays in obtaining regulatory approval can be extremely costly in terms of lost sales opportunities and increased clinical trial costs. If we fail to obtain regulatory approval for our current or future product candidates or expanded indications for currently marketed products, we will be unable to market and sell such products and indications and therefore may never be profitable.
As part of the regulatory approval process, we must conduct clinical trials for each product candidate to demonstrate safety and efficacy. The number of clinical trials that will be required varies depending on the product candidate, the indication being evaluated, the trial results, and the regulations applicable to any particular product candidate.
The results of initial clinical trials of our product candidates do not necessarily predict the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials. The data collected from the clinical trials of our product candidates may not be sufficient to support FDA or other regulatory approval. In addition, the continuation of a particular study after review by an IRB or independent data safety monitoring board does not necessarily indicate that our product candidate will achieve the clinical endpoint.
The FDA and other regulatory agencies can delay, limit or deny approval for many reasons, including:
|
·
|
A product candidate may not be deemed to be safe or effective;
|
|
|
·
|
The manufacturing processes or facilities we have selected may not meet the applicable requirements; and
|
|
|
·
|
Changes in their approval policies or adoption of new regulations may require additional clinical trials or other data.
|
Any delay in, or failure to receive, approval for any of our product candidates could prevent us from growing our revenues or achieving profitability.
Healthcare insurers and other payors may not pay for our products or may impose limits on reimbursement.
Our ability to commercialize our prescription products will depend, in part, on the extent to which reimbursement for our products is available from third-party payors, such as health maintenance organizations, health insurers and other public and private payors. If we succeed in bringing new prescription products to market or expand the approved label for existing products, we cannot be assured that third-party payors will pay for such products, or establish and maintain price levels sufficient for realization of an appropriate return on our investment in product development.
Many health maintenance organizations and other third-party payors use formularies, or lists of drugs for which coverage is provided under a healthcare benefit plan, to control the costs of prescription drugs. Each payor that maintains a drug formulary makes its own determination as to whether a new drug will be added to the formulary and whether particular drugs in a therapeutic class will have preferred status over other drugs in the same class. This determination often involves an assessment of the clinical appropriateness of the drug and, in some cases, the cost of the drug in comparison to alternative products. Our current or our future products may not be added to payors’ formularies, our products may not have preferred status to alternative therapies, and formulary decisions may not be conducted in a timely manner. Once reimbursement at an agreed level is approved by a third-party payor, we may lose that reimbursement entirely or we may lose the similar or better reimbursement we receive compared to competitive products. As reimbursement is often approved for a period of time, this risk is greater at the end of the time period, if any, for which the reimbursement was approved. We may also decide to enter into discount or formulary fee arrangements with payors, which could result in us receiving lower or discounted prices for CRINONE, PROCHIEVE and STRIANT or future products.
We face significant competition from pharmaceutical companies, which may adversely impact our market share.
We and our marketing partners compete against established pharmaceutical companies that market products addressing similar needs. Further, numerous companies are developing, or may develop, enhanced delivery systems and products that compete with our present and proposed products. It is possible that we may not have the resources to withstand these and other competitive forces. Some of these competitors may possess greater financial, research and technical resources than our company or our partners. Moreover, these companies may possess greater marketing capabilities than our company or our partners, including the resources to implement extensive advertising campaigns.
The pharmaceutical industry is subject to change as new delivery technologies are developed, new products enter the market, generic versions of available drugs become available, and treatment paradigms evolve to reflect these and other medical research discoveries. We face significant competition in all areas of our business. The rapid pace of change in the pharmaceutical industry continually creates new opportunities for existing competitors and start-ups, and can quickly render existing products less valuable. Customer requirements and physician and patient preferences continually change as new treatment options emerge, are more or less heavily promoted, and become less expensive. As a result, we may not gain, and may lose, market share.
CRINONE/PROCHIEVE, a natural progesterone product, competes in markets with other progestins, both synthetic and natural, including Endometrin® (progesterone vaginal insert) marketed by Ferring, Prometrium® (oral micronized progesterone) marketed by Solvay, pharmacy-compounded injections and pharmacy-compounded vaginal suppositories. In June 2007, Ferring obtained FDA approval for and launched Endometrin®, a competing product for use in infertility. Ferring is one of the leading companies in the infertility market and, in addition to Endometrin, offers gonadotropin hormones generally used for the treatment of infertility. Ferring may have greater awareness among key reproductive endocrinology opinion leaders than Columbia.
STRIANT competes against other testosterone products that can be delivered by injection, transdermal patch and transdermal gel. Some of the more successful testosterone products include AndroGel® (testosterone gel) marketed by Solvay, Testim® (testosterone gel) marketed by Auxilium, and Androderm® (testosterone transdermal system) marketed by Watson. Competition is based primarily on delivery method. Transdermal testosterone gels currently have the largest market share and transdermal testosterone patches have the next largest market share, followed by injectable products. STRIANT is priced comparably to the gels and patches.
Our products could demonstrate hormone replacement risks.
In the past, certain studies of female hormone replacement therapy products, such as estrogen, have reported an increase in health risks. Progesterone is a natural female hormone present at normal levels in most women through their lifetimes. However, some women require progesterone supplementation due to a natural or chemical-related progesterone deficiency. It is possible that data suggesting risks or problems may come to light in the future which could demonstrate a health risk associated with progesterone or progestin supplementation or our 8% and 4% progesterone gels. It is also possible that future study results for hormone replacement therapy could be negative and could result in negative publicity about the risks and benefits of hormone replacement therapy. As a result, physicians and patients may not wish to prescribe or use progestins, including our progesterone gels.
Similarly, while testosterone is a natural male hormone, present at normal levels in most men through their lifetimes, some men require testosterone replacement therapy, or TRT, to normalize their testosterone levels. It is possible that data suggesting risks or problems may come to light in the future that could demonstrate a health risk associated with TRT or STRIANT. It is also possible that future study results for hormone replacement therapy could be negative and could result in negative publicity about the risks and benefits of TRT. As a result, physicians and patients may not wish to prescribe or use TRT products, including STRIANT.
In addition, investors may become concerned about these issues and decide to sell our Common Stock. These factors could adversely affect our business and the price of our Common Stock.
We may be exposed to product liability claims.
We could be exposed to future product liability claims by consumers. Although we presently maintain product liability insurance coverage at what we believe is a commercially reasonable level, such insurance may not be sufficient to cover all possible liabilities. An award against us in an amount greater than our insurance coverage could have a material adverse effect on our operations. Some customers require us to have a minimum level of product liability insurance coverage before they will purchase or accept our products for distribution. If we fail to satisfy insurance requirements, our ability to achieve broad distribution of our products could be limited. This could have a material adverse effect upon our business and financial condition.
Steps taken by us to protect our proprietary rights might not be adequate; in which case, competitors may infringe on our rights or develop similar products. The U.S. and foreign patents upon which our original Bioadhesive Delivery System was based have expired.
Our success and competitive position are partially dependent on our ability to protect our proprietary position for our technology, products and product candidates. We rely primarily on a combination of patents, trademarks, copyrights, trade secret laws, third-party confidentiality and nondisclosure agreements, and other methods to protect our proprietary rights. The steps we take to protect our proprietary rights, however, may not be adequate. Third parties may infringe or misappropriate our patents, copyrights, trademarks, and similar proprietary rights. Moreover, we may not be able or willing, for financial, legal or other reasons, to enforce our rights.
Bio-Mimetics, Inc. held the patent upon which our original Bioadhesive Delivery System, or BDS, was based and granted us a license under that patent. Bio-Mimetics’ patent contained broad claims covering controlled release products that include a bioadhesive. However, this U.S. patent and its corresponding foreign patents expired in November 2003. Based upon the expiration of the original Bio-Mimetics patent, other parties will be permitted to make, use or sell products covered by the claims of the Bio-Mimetics patent, subject to other patents, including those which we hold. We have obtained numerous patents with claims covering improved methods of formulating and delivering therapeutic compounds using the BDS. We cannot assure you that any of these patents will enable us to prevent infringement, or that our competitors will not develop alternative methods of delivering compounds, potentially resulting in competitive products outside the protection that may be afforded by our patents. Other companies may independently develop or obtain patent or similar rights to equivalent or superior technologies or processes. Additionally, although we believe that our patented technology has been independently developed and does not infringe on the proprietary rights of others, we cannot assure you that our products do not and will not infringe on the proprietary rights of others. In the event of infringement, we may be required to modify our technology or products, obtain licenses or pay license fees. We may not be able to do so in a timely manner or upon acceptable terms and conditions. This may have a material adverse effect on our operations.
The standards that the U.S. Patent and Trademark Office and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Limitations on patent protection in some countries outside the U.S., and the differences in what constitutes patentable subject matter in these countries, may limit the protection we seek outside of the U.S. For example, methods of treating humans are not patentable subject matter in many countries outside of the U.S. In addition, laws of foreign countries may not protect our intellectual property to the same extent as would laws of the U.S. In determining whether or not to seek a patent or to license any patent in a particular foreign country, we weigh the relevant costs and benefits, and consider, among other things, the market potential of our product candidates in the jurisdiction and the scope and enforceability of patent protection afforded by the law of the jurisdiction.
We own registrations of the following as trademarks in countries throughout the world: CRINONE, STRIANT, and STRIANT SR, and PROCHIEVE in the U.S. These trademarks, however, may not afford us adequate protection or we may not have the financial resources to enforce our rights under these trademarks.
We are subject to government regulation, which could affect our ability to sell products.
Nearly every aspect of the development, manufacture and commercialization of our pharmaceutical products is subject to time-consuming and costly regulation by various governmental entities, including the Food and Drug Administration, or FDA, the Drug Enforcement Administration and state agencies, as well as regulatory agencies in those foreign countries in which our products are manufactured or distributed. The FDA has the power to seize adulterated or misbranded products and unapproved new drugs, to require their recall from the market, to enjoin further manufacture or sale, and to publicize certain facts concerning a product.
We employ various quality control measures in our efforts to ensure that our products conform to their intended specifications and meet the standards required under applicable governmental regulations, including FDA’s current Good Manufacturing Practices regulations. Notwithstanding our efforts, our products or the ingredients we purchase from our suppliers for inclusion in our products may contain undetected defects or non-conformities with specifications. Such defects or non-conformities could compel us to recall the affected product, make changes to or restrict distribution of the product, or take other remedial actions. The occurrence of such events may harm our relations with or result in the loss of customers, injure our reputation, impair market acceptance of our products, harm our financial results, and, in certain circumstances, expose us to product liability or other claims.
We are dependent on third-party suppliers of raw materials for our products, the loss of whom could impair our ability to manufacture and sell our products.
Medical grade, cross-linked polycarbophil, the polymer used in our BDS-based products is currently available from only one supplier, Lubrizol, Inc., or Lubrizol. We believe that Lubrizol will supply as much of the material as we require because our products rank among the highest value-added uses of the polymer. In the event that Lubrizol cannot or will not supply enough of the product to satisfy our needs, we will be required to seek alternative sources of polycarbophil. An alternative source of polycarbophil may not be available on satisfactory terms or at all, which would impair our ability to manufacture and sell our products.
We currently purchase testosterone from only one supplier and progesterone from two suppliers. If our suppliers are unable or unwilling to satisfy our needs, we will be required to seek alternative sources of supply. While several alternative sources of progesterone and testosterone exist, the time needed to obtain regulatory approvals for new suppliers may impair our ability to manufacture and sell our products.
We are dependent upon third-party developers and manufacturers, the loss of which could result in a loss of revenues.
We rely on third parties to develop and manufacture our products, including Fleet, which manufacturers our vaginal gel products in bulk, Maropack, which fills our vaginal gel products into applicators and Mipharm which manufacturers STRIANT. These third parties may not be able to satisfy our needs in the future, and we may not be able to find or obtain FDA approval of alternate developers and manufacturers. Delays in the development and manufacture of our products could have a material adverse effect on our business. This reliance on third parties could have an adverse effect on our profit margins. Any interruption in the manufacture of our products would impair our ability to deliver our products to customers on a timely and competitive basis, and could result in the loss of revenues.
The loss of our key executives could have a significant impact on our company.
Our success depends in large part upon the abilities and continued service of our executive officers and other key employees. Our employment agreements with our executive officers are terminable by them on short notice. The loss of key employees may result in a significant loss in the knowledge and experience that we, as an organization, possess, and could cause significant delays in, or outright failure of, the development and commercialization of our products and product candidates. If we are unable to attract and retain qualified and talented senior management personnel, our business may suffer.
We may be limited in our use of our net operating loss carryforwards.
As of December 31, 2009, we had certain net operating loss carryforwards of approximately $162.0 million that may be used to reduce our future U.S. federal income tax liabilities. Our ability to use these loss carryforwards to reduce our future U.S. federal income tax liabilities could be lost if we were to experience more than a 50% change in ownership within the meaning of Section 382(g) of the Internal Revenue Code. If we were to lose the benefits of these loss carryforwards, our future earnings and cash resources would be materially and adversely affected.
Sales of large amounts of Common Stock may adversely affect our market price. The issuance of preferred stock or convertible debt may adversely affect rights of common stockholders.
As of February 23, 2010, we had 65,630,051 shares of Common Stock outstanding, of which 61,324,589 shares were freely tradable by non-affiliates. As of that date, approximately 4,305,462 shares of Common Stock were restricted or held by affiliates. We also have the following securities outstanding: series B convertible preferred stock, series C convertible preferred stock, series E convertible preferred stock, convertible subordinated notes, warrants, and options. If all of these securities are exercised or converted, an additional 27,931,558 shares of Common Stock will be outstanding, all of which will have been registered for resale under the Securities Act. The exercise and conversion of these securities is likely to dilute the book value per share of our Common Stock. In addition, the existence of these securities may adversely affect the terms on which we can obtain additional equity financing.
Pursuant to our agreement with Watson, we have agreed to sell Watson 11.2 million shares of Common Stock. In addition, pursuant to our contingent agreements with the holders of our convertible subordinated notes we have agreed to issue to the holders of convertible subordinated notes warrants to purchase 7,750,000 shares of Common Stock at an exercise price of $1.35 per share and 7,407,407 shares of Common Stock.
In March 2002, our Board of Directors authorized shares of series D junior participating preferred stock in connection with its adoption of a stockholder rights plan, under which we issued rights to purchase series D convertible preferred stock to holders of our Common Stock. Upon certain triggering events, such rights become exercisable to purchase shares of Common Stock (or, in the discretion of our Board of Directors, series D convertible preferred stock) at a price substantially discounted from the then current market price of our Common Stock.
Under our certificate of incorporation, our Board of Directors has the authority to issue up to 1.0 million shares of preferred stock and to determine the price, rights, preferences and privileges of those shares without any further vote or action by our stockholders. In addition, we may issue convertible debt without shareholder approval. The rights of the holders of Common Stock are subject to, and may be adversely affected by, the rights of the holders of any shares of preferred stock or convertible debt that may be issued in the future. While we have no present intention to authorize or issue any additional series of preferred stock or convertible debt, such preferred stock or convertible debt, if authorized and issued, may have other rights, including economic rights senior to the Common Stock, and, as a result, their issuance could have a material adverse effect on the market value of our Common Stock.
We acquired marketing rights to CRINONE in the U.S. in December 2006, and we may never realize the anticipated benefits of the acquisition.
In December 2006, we purchased the marketing rights in the U.S. to CRINONE from Merck Serono, and we began in 2007 to call on reproductive endocrinologists, a medical specialty in infertility. Our goal is to grow CRINONE prescriptions with these specialists. We believe the reproductive endocrinologists are particularly important because of their influence on prescribing practices of obstetricians and gynecologists who also treat infertility. Our efforts to grow the CRINONE business may not be successful and we may fail to realize the anticipated benefits of the acquisition.
Our corporate compliance program cannot guarantee that we are in compliance with all potentially applicable regulations.
We are a relatively small company and we rely heavily on third parties to conduct many important functions. As a pharmaceutical company, we are subject to a large body of legal and regulatory requirements. In addition, as a publicly traded company we are subject to significant regulations, including the Sarbanes-Oxley Act of 2002, some of which have either only recently been adopted or are currently proposals subject to change. We cannot assure you that we are or will be in compliance with all potentially applicable laws and regulations. Failure to comply with all potentially applicable laws and regulations could lead to the imposition of fines, cause the value of our common stock to decline, impede our ability to raise capital or lead to the de-listing of our stock.
We could be negatively impacted by future interpretation or implementation of federal and state fraud and abuse laws, including anti-kickback laws, false claims laws and federal and state anti-referral laws.
Various federal and state laws pertain to health care fraud and abuse, including anti-kickback laws, false claims laws and physician self-referral laws. Violations of these laws are punishable by criminal and/or civil sanctions, including, in some instances, imprisonment and exclusion from participation in federal and state health care programs, including Medicare, Medicaid, and veterans’ health programs. We do not currently participate in government programs; including Medicare (except Medicare Part D), Medicaid and veteran’s health programs and we have not been challenged by a governmental authority under any of these laws and believe that our operations are in compliance with such laws.
However, because of the far-reaching nature of these laws, we may be required to alter one or more of our practices to be in compliance with these laws. Health care fraud and abuse regulations are complex, and even minor, inadvertent irregularities can potentially give rise to claims that the law has been violated. Any violations of these laws could result in a material adverse effect on our business, financial condition and results of operations. If there is a change in law, regulation or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which could have a material adverse effect on our business, financial condition and results of operations.
We could become subject to false claims litigation under federal or state statutes, which can lead to civil money penalties, criminal fines and imprisonment, and/or exclusion from participation in federal health care programs. These false claims statutes include the federal False Claims Act, which allows any person to bring suit alleging the false or fraudulent submission of claims for payment under federal programs or other violations of the statute and to share in any amounts paid by us to the government in fines or settlement. Such suits, known as qui tam actions, have increased significantly in recent years and have increased the risk that companies like us may have to defend a false claim action. We could also become subject to similar false claims litigation under state statutes. If we are unsuccessful in defending any such action, such action may have a material adverse effect on our business, financial condition and results of operations.
Anti-takeover provisions could impede or discourage a third-party acquisition of our company. This could prevent stockholders from receiving a premium over market price for their stock.
We are a Delaware corporation. Anti-takeover provisions of Delaware law impose various obstacles to the ability of a third party to acquire control of our company, even if a change in control would be beneficial to our existing stockholders. In addition, our Board of Directors has adopted a stockholder rights plan and has designated a series of preferred stock that could be used defensively if a takeover is threatened. Our incorporation under Delaware law, our stockholder rights plan, and our ability to issue additional series of preferred stock, could impede a merger, takeover or other business combination involving our company or discourage a potential acquirer from making a tender offer for our Common Stock. This could reduce the market value of our Common Stock if investors view these factors as preventing stockholders from receiving a premium for their shares.
We are exposed to market risk from foreign currency exchange rates.
With two operating subsidiaries and third party manufacturers in Europe, economic and political developments in the European Union can have a significant impact on our business. All of our products are currently manufactured in Europe. We are exposed to currency fluctuations related to payment for the manufacture of our products in Euros and other currencies and selling them in U.S. dollars and other currencies.
None.
Item 2. Properties
As of December 31, 2009, the Company leased the following properties:
|
Location
|
Use
|
Square feet
|
Expiration
|
Annual Rent
|
|||||||||
|
Livingston, NJ
|
Corporate office
|
9,450
|
October, 2013
|
$
|
217,350
|
||||||||
|
Paris, France
|
European logistics office
|
150
|
3 months notice
|
$
|
19,529
|
||||||||
Item 3. Legal Proceedings
Claims and lawsuits have been filed against the Company and its subsidiaries from time to time. Although the results of pending claims are always uncertain, the Company does not believe the results of any such actions, individually or in the aggregate, will have a material adverse effect on the Company’s financial position or results of operation. Additionally, the Company believes that it has adequate reserves or insurance coverage in respect of these claims, but no assurance can be given as to the sufficiency of such reserves or insurance in the event of any unfavorable outcome resulting from these actions.
In connection with the 1989 purchase of the assets of Bio-Mimetics, Inc., which assets consisted of the patents underlying the Company's BDS, other patent applications, and related technology, the Company agreed to pay Bio-Mimetics a royalty equal to two percent of the net sales of products based on the assets up to an aggregate of $7.5 million or until the last of the relevant patents expired. The Company determined that the obligation to pay royalties on STRIANT, PROCHIEVE, and CRINONE terminated in September of 2006, with the expiration of a certain Canadian patent, but continues on Replens® and RepHresh®. On December 28, 2007, Bio-Mimetics filed a complaint in the U.S. District Court for Massachusetts (Bio-Mimetics, Inc. v. Columbia Laboratories, Inc.) alleging breach of contract and unfair or deceptive trade practices, for the Company’s failure to continue royalty payments on STRIANT, PROCHIEVE, and CRINONE. To date, the Company has paid approximately $3.9 million in royalty payments and Bio-Mimetics seeks a judgment that we are obligated to pay the remaining $3.6 million in full. The Company has denied all such allegations and believes it has no contractual liability to Bio-Mimetics for the disputed royalty payments and intends to defend this action vigorously.
Item 4. (Removed and Reserved)
None.
Item 5. Market for the Registrant’s Common Equity and Related Stockholder Matters and
Issuer Purchases of Equity Securities
The Company’s Common Stock is traded on the NASDAQ Global Market under the symbol CBRX. The following table sets forth for the periods indicated the high and low sales prices of the Common Stock on the NASDAQ Global Market.
|
High
|
Low
|
||||||
|
Fiscal Year Ended December 31, 2008
|
|||||||
|
First Quarter
|
$ | 2.51 | $ | 1.89 | |||
|
Second Quarter
|
4.29 | 2.13 | |||||
|
Third Quarter
|
4.47 | 2.15 | |||||
|
Fourth Quarter
|
2.76 | 0.92 | |||||
|
Fiscal Year Ended December 31, 2009
|
|||||||
|
First Quarter
|
$ | 1.74 | $ | 1.09 | |||
|
Second Quarter
|
1.70 | 1.06 | |||||
|
Third Quarter
|
1.59 | 1.08 | |||||
|
Fourth Quarter
|
1.51 | 0.65 | |||||
At March 3, 2010, there were approximately 300 shareholders of record of the Company’s Common Stock, one shareholder of record of the Company’s Series B convertible preferred stock (“Series B Preferred Stock”), 3 shareholders of record of the Company’s contingently redeemable Series C Convertible Preferred Stock (“Series C Preferred Stock”) and 7 shareholders of record of the Company’s Series E convertible preferred stock (“Series E Preferred Stock”). The Company estimates that there were approximately 4,600 beneficial owners of its Common Stock on such date.
The Series C Preferred Stock was issued and sold by the Company in January 1999 to 24 accredited investors, through which the Company raised approximately $6.4 million, net of expenses. The Series C Preferred Stock has a stated value of $1,000 per share, and is convertible into Common Stock at the lower of: (i) $3.50 per share of Common Stock and (ii) 100% of the average of the closing prices during the three trading days immediately preceding the conversion notice, not to exceed 2,352,361 shares as of December 31, 2009. The Series C Preferred Stock pays a 5% dividend, payable quarterly in arrears on the last day of the quarter. The security holders of Series C Preferred Stock have certain redemption rights due to events beyond the control of the Company such as delisting, dividend defaults and certain other defaults. The terms of the Series C Preferred Stock have remained the same since inception.
During 2005, the Company raised $6.9 million from the issuance and sale of 69,000 shares of Series E Preferred Stock. The Series E Preferred Stock has a stated value of $100 per share. Each share of the Series E Preferred Stock may be converted by the holder into 50 shares of Common Stock, subject to adjustment, and will automatically be converted into Common Stock at that rate upon the date that the average of the daily market prices of the Company’s Common Stock for the 20 consecutive trading days preceding such date exceeds $6.00 per share. The Series E Preferred Stock pays no dividends and contains voting rights equal to the number of shares of Common Stock into which each share of Series E Preferred Stock is convertible. Upon liquidation of the Company, the holders of the Series E Preferred Stock are entitled to $100 per share.
On March 10, 2006, the Company raised $30 million in gross proceeds to the Company from the issuance and sale of 7,428,220 shares of its Common Stock at a price of $4.04 per share and warrants to purchase 1,857,041 shares of Common Stock with an exercise price of $5.39 per share. The warrants became exercisable on September 9, 2006, and expire on March 13, 2011, unless earlier exercised or terminated. Proceeds were used for general corporate purposes.
On December 22, 2006, the Company raised approximately $40 million in gross proceeds to the Company from the issuance and sale of convertible subordinated notes. The notes bear interest at a rate of 8% per annum and mature on December 31, 2011. They are convertible into shares of Common Stock at a conversion price of $5.25. Investors also received warrants to purchase 2,285,714 shares of Common Stock at an exercise price of $5.50 per share. The warrants became exercisable on June 20, 2007, and expire on December 22, 2011, unless earlier exercised or terminated. The Company used the proceeds of this offering to acquire from Merck Serono the U.S. marketing rights to CRINONE for $33 million, purchase Serono’s current inventory of that product, and pay other costs related to the transaction. On April 1, 2007, the Company recorded a liability from the contract with Merck Serono for certain sales returns associated with sales made by Merck Serono. The Company recorded the estimated liability of $1,000,000 as an increase in the purchase price that is being amortized over the remaining term of the license. The balance of approximately $3.7 million was used for general corporate purposes.
All of such securities were issued in unregistered offerings pursuant to Section 4(2) of the Securities Act of 1933, as amended or Regulation D thereunder.
On August 26, 2008, the Company raised approximately $4.7 million in gross proceeds to the Company from the issuance and sale of 1,333,000 shares of its common stock at a price of $3.50 per share in a registered offering. During 2008, outstanding options were exercised resulting in the issuance of 318,149 shares of Common Stock and the receipt of $0.6 million by the Company. Proceeds were used for general corporate purposes. In addition, 350 shares of Series C Preferred Stock were converted into 235,426 shares of Common Stock, and 4,547 shares of Series E Preferred Stock were converted into 227,350 shares of Common Stock.
On January 6, 2009, the Company raised approximately $0.75 million in gross proceeds to the Company from the issuance and sale of 451,807 shares of its common stock at a price of $1.66 per share in a registered offering. On October 22, 2009, the Company raised approximately $11.8 million in gross proceeds to the Company from the issuance and sale of 10,900,000 shares of its common stock at a price of $1.08 per share and warrants to purchase 5,450,000 shares of Common Stock with an exercise price of $1.52 per share in a registered offering. The warrants became exercisable on April 30, 2010, and expire on April 30, 2015, unless earlier exercised or terminated. Proceeds will be used for general corporate purposes including the completion of the preterm birth clinical trial. In addition, on March 25, 2009, 175 shares of Series C Preferred Stock were converted into 117,449 shares of Common Stock. During 2009, no options were exercised
Equity Compensation Plan Information
The following table sets forth aggregate information for the fiscal year ended December 31, 2009, regarding the Company’s compensation plans, including individual compensation agreements, under which equity securities of the Company are authorized for issuance:
|
Number of securities
|
||||||||||||
|
remaining available for
|
||||||||||||
|
Number of securities to be
|
Weighted average
|
future issuance under
|
||||||||||
|
issued upon exercise of
|
exercise price of
|
equity compensation plans
|
||||||||||
|
outstanding options,
|
outstanding options,
|
[excluding securities reflected
|
||||||||||
|
Plan Category
|
warrants and rights
|
warrants and rights
|
in column (a)]
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by security holders
|
5,960,304 | $ | 2.20 | 6,071,254 | ||||||||
|
Equity compensation plans not approved by security holders
|
450,000 | $ | 7.79 | - | ||||||||
|
Total
|
6,410,304 | $ | 2.59 | 6,071,254 | ||||||||
The Company has two shareholder-approved equity compensation plans. The 1996 Long-term Performance Plan (the “1996 Plan”), adopted in October 1996, provided for the grant of stock options, stock appreciation rights and restricted stock to certain designated employees of the Company, non-employee directors of the Company and certain other persons performing significant services for the Company as designated by the Compensation Committee of the Board of Directors. On May 13, 2008, shareholders approved the 2008 Long-term Incentive Plan (the “2008 Plan”), which provides for the grant of stock options, stock appreciation rights and restricted stock to certain designated employees of the Company, non-employee directors of the Company and certain other persons performing significant services for the Company as designated by the Compensation Committee of the Board of Directors. Upon approval of the 2008 Plan, the Company stopped making grants under the 1996 Plan.
The equity compensation plan not approved by the security holders represents 10 year warrant grants to key executives of the Company in 1999 and 2001.
Stockholder Rights Plan
On March 12, 2002, the Company adopted a Stockholder Rights Plan (the “Rights Plan”) designed to protect company stockholders in the event of takeover activity that would deny them the full value of their investment. The Rights Plan was not adopted in response to any specific takeover threat. In adopting the Rights Plan, the Board declared a dividend distribution of one preferred stock purchase right for each outstanding share of Common Stock of the Company, payable to stockholders of record at the close of business on March 22, 2002. The rights will become exercisable only in the event, with certain exceptions, a person or group of affiliated or associated persons acquires 15% or more of the Company’s voting stock, or a person or group of affiliated or associated persons commences a tender or exchange offer which, if successfully consummated, would result in such person or group owning 15% or more of the Company’s voting stock. The rights will expire on March 12, 2012. Each right, once exercisable, will entitle the holder (other than rights owned by an acquiring person or group) to buy one one-thousandth of a share of a series of the Company’s Series D Junior Participating Preferred Stock at a price of $30 per one-thousandth of a share, subject to adjustments. In addition, upon the occurrence of certain events, holders of the rights (other than rights owned by an acquiring person or group) would be entitled to purchase either the Company’s preferred stock or shares in an “acquiring entity” at approximately half of market value. Further, at any time after a person or group acquires 15% or more (but less than 50%) of the Company’s outstanding voting stock, subject to certain exceptions, the Board of Directors may, at its option, exchange part or all of the rights (other than rights held by an acquiring person or group) for shares of the Company's Common Stock having a fair market value on the date of such acquisition equal to the excess of (i) the fair market value of preferred stock issuable upon exercise of the rights over (ii) the exercise price of the rights. The Company generally will be entitled to redeem the rights at $0.01 per right at any time prior to the close of business on the tenth day after there has been a public announcement of the beneficial ownership by any person or group of 15% or more of the Company’s voting stock, subject to certain exceptions.
Dividend Policy
The Company has never paid a cash dividend on its Common Stock and does not anticipate the payment of cash dividends in the foreseeable future. The Company intends to retain any earnings for use in the development and expansion of its business. The Company is required to pay a 5% dividend on its Series C Preferred Stock on the last day of each quarter.
Applicable provisions of the Delaware General Corporation Law may affect the ability of the Company to declare and pay dividends on its Common Stock as well as on its Series C Preferred Stock. In particular, pursuant to the Delaware General Corporation Law, a company may pay dividends out of its surplus, as defined, or out of its net profits, for the fiscal year in which the dividend is declared and/or the preceding year. Surplus is defined in the Delaware General Corporation Law to be the excess of net assets of the company over capital. Capital is defined to be the aggregate par value of shares issued unless otherwise established by the Board of Directors.
|
Comparison of Five-Year Cumulative Total Return*
|
|
Columbia Laboratories, Inc., Russell 2000 Index, Value Line Drug, And Peer Group
|
|
|
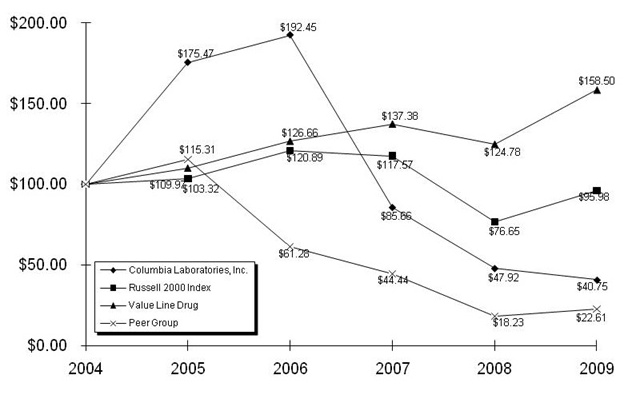
|
* Assumes $100 invested at the close of trading on December 31, 2004 in Columbia Laboratories, Inc. Common Stock, Russell 2000 Index, Value Line Drug, and Peer Group.
|
||||||
|
Source: Value Line, Inc.
|
||||||
|
Factual material is obtained from sources believed to be reliable, but the publisher is not responsible for any errors or omissions contained herein.
|
||||||
Peer Group Companies are Acadia Pharmaceuticals, Inc., Adolor Corp., Ariad Pharmaceuticals, Inc., ArQule, Inc., BioSante Pharmaceuticals, Inc., Cytokinetics, DepoMed, Inc., GenVec Inc., MiddleBrook Pharmaceuticals, Inc., Neurocrine Biosciences, Inc., SuperGen, Inc., Unigene, and Vical, Inc.
Item 6. Selected Financial Data
The following selected financial data are derived from the Company’s audited financial statements and are qualified in their entirety by reference to, and should be read in conjunction with, such consolidated financial statements and Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Annual Report. The historical results are not necessarily indicative of the results we expect for future periods.
|
|
December 31 | |||||||||
|
2009
|
2008
|
2007
|
2006
|
2005
|
||||||
|
Statement of Operations Data:
|
||||||||||
|
(000's except per share data)
|
||||||||||
|
Net revenues
|
$ 32,196
|
$ 36,229
|
$ 29,627
|
$ 17,393
|
$ 22,041
|
|||||
|
Gross profit
|
23,002
|
25,294
|
20,613
|
9,573
|
13,929
|
|||||
|
Operating expenses
|
36,165
|
32,552
|
28,721
|
20,733
|
21,160
|
|||||
|
Interest expense
|
8,851
|
7,882
|
7,946
|
2,670
|
3,491
|
|||||
|
Net loss
|
(21,870)
|
(14,077)
|
(14,292)
|
(12,485)
|
(10,104)
|
|||||
|
Loss per common share
|
(0.39)
|
(0.27)
|
(0.28)
|
(0.26)
|
(0.25)
|
|||||
|
Weighted average number of common shares
|
||||||||||
|
outstanding-basic and diluted
|
56,359
|
52,439
|
51,124
|
48,089
|
41,752
|
|||||
|
Balance Sheet Data:
|
||||||||||
|
(000's)
|
||||||||||
|
Working capital (deficiency)
|
$ 14,256
|
$ 12,305
|
$ 14,461
|
$ 23,410
|
$ (3,471)
|
|||||
|
Total assets
|
43,757
|
45,622
|
56,589
|
65,839
|
14,732
|
|||||
|
Notes payable
|
32,966
|
30,075
|
27,536
|
25,299
|
-
|
|||||
|
Long-term portion of financing agreements
|
15,234
|
13,126
|
11,426
|
13,277
|
10,921
|
|||||
|
Redeemable preferred series C stock
|
600
|
775
|
1,125
|
3,200
|
3,250
|
|||||
|
Shareholders' equity (deficiency)
|
(13,766)
|
(5,893)
|
2,015
|
12,616
|
(20,573)
|
|||||
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Overview
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is intended to help the reader understand the Company’s financial condition and results of operations. The MD&A is provided as a supplement to, and should be read in conjunction with, our financial statements and the accompanying notes (“Notes”).
We are in the business of developing, manufacturing and selling pharmaceutical products that utilize our proprietary bioadhesive drug delivery technologies. Our bioadhesive vaginal gel products provide patient-friendly solutions for infertility, pregnancy support, amenorrhea, and other obstetric, gynecologic and medical conditions.
We have a 35 person sales organization that promotes our natural progesterone gel product, CRINONE 8% in the U.S. We acquired the U.S. marketing rights to CRINONE in December 2006, and promote it to a full range of reproductive endocrinologists, obstetricians and gynecologists who treat infertility. We also promote STRIANT testosterone buccal system for the treatment of hypogonadism in men; however, our focus is on increasing prescriptions of CRINONE.
We derive additional revenues from our established marketing partnership with Merck Serono through which CRINONE 8% is commercialized in territories outside the U.S
On March 3, 2010, we entered into a definitive agreement to sell substantially all of our progesterone related assets and 11.2 million shares of common stock to Watson Pharmaceuticals, Inc. for a $47 million upfront payment plus royalties of 10 to 20 percent of annual net sales of certain progesterone products. Additional payments up to $45.5 million can be earned by the successful completion of clinical development milestones in the ongoing PREGNANT Study, regulatory filings, receipt of regulatory approvals and product launches. Watson will fund the development of a second-generation vaginal progesterone product as part of a comprehensive life-cycle management strategy. See “Item 1. Business - Watson Transaction and Debt Restructuring.”
On March 3, 2010 we also entered into contingent agreements with the holders of our senior debt and our convertible subordinated notes under which, if the Watson transaction closes and the other conditions to consummation of such agreements are satisfied, we will repay our outstanding debt on an accelerated and discounted basis and issue the holders of convertible subordinated notes shares of common stock and warrants to purchase common stock. See “Item 1. Business - Watson Transaction and Debt Restructuring.”
Our net loss for 2009 was $21.9 million, or $0.39 per basic and diluted common share. We expect to continue to incur operating losses in the near future because of the significant non-cash items related to the CRINONE acquisition that our future financial statements will reflect, significant sales, distribution and research and development costs and increased payments on our consolidated debt. Our sales and distribution expenses are expected to be higher in 2010. In 2010, we expect that our research and development expenses will be higher than those in 2009, primarily as a result of our investment in our PREGNANT clinical trial of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix at mid-pregnancy including the preparation for filing an NDA early in 2011.
Our 2009 revenues reflect the third full year of our marketing both brands of 8% progesterone gel in the U.S., and in particular, our attention to CRINONE 8%. In 2009, we recognized the commercial benefit of the medical/marketing foundations laid in previous years. Prescriptions for CRINONE climbed 12% from December 2008 to December 2009. This increase is in spite of a reduction in total ART procedures in 2009 due, most likely, to the cash out-of-pocket nature of infertility therapy, and the economy. Our CRINONE 8% sales and marketing activities in 2009 included:
|
·
|
Continued very strong presence at the annual meeting of the American Society of Reproductive Medicine in 2009. After three years of our strong presence at ASRM, we are receiving recognition as a major player in the market for infertility treatments.
|
|
·
|
Emphasis in our marketing materials to four new clinical trials added to our previous 16 clinical trials that have been conducted to compare CRINONE to other forms of progesterone, which provide us with a compelling case for the efficacy of CRINONE. We believe that this data shows that CRINONE is as effective as, and in some cases numerically more effective, than other delivery systems for progesterone. Of particular note is the completion of the well powered, prospective randomized, Harvard-affiliated, Brigham & Women’s study which concluded that CRINONE’s efficacy is statistically equivalent to painful IM therapy, with significant patient preference. This is consistent with the other six clinical trials that included an arm evaluating patient preference. Patients statistically preferred CRINONE over the competing product in all seven clinical trials.
|
|
·
|
Meetings of our Infertility Advisory Committee of respected reproductive endocrinologists from around the U.S. who provide us with insight on how to best communicate to physicians and patients all the clinical information that is available for CRINONE.
|
|
·
|
Numerous Speaker Programs where key opinion leaders and a number of reproductive endocrinologists speak on behalf of CRINONE.
|
Our partner Merck Serono has exclusive rights to market CRINONE in all countries outside of the U.S. Increased unit volume of CRINONE in non-U.S. markets by Merck Serono, who pays us a transfer price on CRINONE sales, contributed to growth for CRINONE in 2009. Non-U.S. progesterone units were up 8% over 2008 while sales dollars were down 6% primarily due to lower selling prices related to foreign exchange rates relative to the dollar, and price adjustments for government tenders. We expect that CRINONE sales will continue to increase outside the U.S., especially in China where the product was approved in December of 2008 and launched in mid 2009. Our license and supply agreement with Merck Serono expires on May 19, 2010, and is renewable upon mutual agreement of the parties for five (5) year periods at commercial terms to be agreed upon. Merck Serono has informed us that they wish to renew the agreement for an additional 5 years, and we are in negotiations with them to renew the agreement on terms that are not materially different from the current agreement. There can be no assurance, however, that we will reach a renewal agreement or that any renewal agreement will not differ materially from the current agreement.
Clinical development of PROCHIEVE 8% for Prevention of Preterm Birth in Women with Mid-pregnancy Short Cervix.
We expect that in 2010 we will invest significant resources in the development program for PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix as measured by transvaginal ultrasound at mid-pregnancy. This program includes a clinical trial in pregnant women that we have named the PREGNANT study. In 2007, we reported data from our completed clinical trial of PROCHIEVE 8% in pregnant women with a history of prior preterm birth. In that clinical trial, the study endpoints were not met, and the trial demonstrated that there was no benefit of administering vaginal progesterone to this patient population. However, secondary analyses of the data from this earlier study demonstrated a statistically significant improvement in the rate of preterm birth and infant outcomes in trial participants who had a short cervix in mid-pregnancy. The PREGNANT clinical trial is designed to confirm these data in a larger trial. If the results of the PREGNANT trial confirm the results seen in the earlier clinical trial, we expect to file a NDA supplement seeking approval of PROCHIEVE 8% for this indication.
The clinical trial data were published in October 2007 in the peer-reviewed journal Ultrasound in Obstetrics & Gynecology (also known as the “White Journal”), which, was followed by the publication of an abstract entitled “ Progesterone Reduces the Rate of Cervical Shortening in Women at Risk for Preterm Birth ” in the December 2007 supplement of the American Journal of Obstetrics and Gynecology . Because we were able to publish data in advance of the 2008 Annual Meeting of the Society for Maternal-Fetal Medicine in late January 2008, the data underlying this abstract were discussed in an oral presentation at that meeting.
The PREGNANT study was designed based in part on the data set forth in the White Journal and discussions with the FDA. This randomized, double-blind, placebo-controlled Phase III clinical trial is designed to evaluate the ability of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix of between 1.0 and 2.0 centimeters as measured by transvaginal ultrasound at mid-pregnancy. The primary endpoint of this clinical trial is a reduction in preterm births at less than or equal to 32 weeks versus placebo.
In October 2008 we announced a collaboration with the Perinatology Research Branch (PRB) of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development, part of the National Institutes of Health (NIH) under which we amended the study protocol to reflect the addition of nine NIH sponsored sites and an increase in the number of patients from 300 to 450. With the increase in patients, the power of the study to show statistically significant improvements in both the obstetrical endpoints and infant outcomes becomes even stronger. All clinical data, whether generated by NIH sites or our sites, will be collected centrally and, assuming success in the study, the results will be available to us for regulatory filings. We believe that, if the study is successful, the participation of the NIH will have a positive impact on physicians’ adoption of PROCHIEVE 8% to reduce the risk of preterm birth in women with a short cervix at mid-pregnancy as measured by transvaginal ultrasound, which will lead to improved patient care and a more rapid reduction in the incidence of preterm birth. We expect to enroll at least 450 patients in the PREGNANT study and we expect to complete enrollment in the second quarter of 2010, with the last baby born and study results in the fourth quarter of 2010.
In 2008, we continued to invest in our vaginal lidocaine drug candidate which we were evaluating to treat the severe uterine cramps that result in the debilitating pain of dysmenorrhea. In the U.S. alone, this common, painful condition seriously affects about 5.6 million women in the age range of 20 to 45 to the point where they frequently miss work. This figure exclude very young women between the onset of menstruation and age 20 who can suffer dysmenorrhea at a higher percentage than the more mature female population between the ages of 20 and 45.
In September 2008, we announced results from a 70-patient Phase II cross-over study in patients with dysmenorrhea. The primary endpoint of the study was to show a difference between lidocaine and placebo in terms of the time-weighted average patient-assessed pain intensity over four treatment days. Data from the clinical trial did not show a significant difference between the pain scores for the lidocaine and placebo treatment cycles. Patients in the clinical trial were also asked to make a subjective assessment of the treatment at the end of each cycle, and to compare the first and second cycles to one another. The data suggest a trend for patients to favor their lidocaine treatment cycle. The clinical trial did not reveal any significant adverse events and those adverse events that occurred were similar in both kind and frequency for lidocaine and placebo.
Results of Operations
Summary
Our products consist of Progesterone Products that we promote through our own sales force to REIs and OB/GYNs and sell to wholesalers and specialty pharmacies and from sales to licensees. We supplement our Progesterone Product revenue by selling other products that use our BDS which we refer to as “Other Products”. Most of the Other Product revenue is based on sales of products to licensees.
Products
|
Progesterone Products
|
·
|
CRINONE 8% progesterone gel marketed and sold by us in the U.S.
|
||
|
·
|
CRINONE 8% sold by us to Merck Serono for resale outside the U.S.
|
|||
|
·
|
PROCHIEVE 8% progesterone gel sold by us in the U.S.
|
|||
|
·
|
PROCHIEVE 4% progesterone gel sold to Ascend for sale in the U.S
pursuant to an agreement that will terminate on July 23, 2010.
|
|||
| · | STRIANT testosterone buccal system marketed and sold by us in the U.S. | |||
| · | STRIANT sold to MiPharm for resale in Italy. | |||
| · | Replens® Vaginal Moisturizer sold to Lil’ Drug Store for resale outside the U.S. | |||
| · | RepHresh® Vaginal Gel sold to Lil’ Drug Store for resale on a worldwide basis. | |||
| · | Royalty and licensing revenues. | |||
All of our products are manufactured in Europe by third parties on behalf of our foreign subsidiaries who sell the products to our worldwide licensees, and to the Company in the case of the products we commercialize ourselves in the U.S. Because our European revenues reflect these sales and are reduced only by our product manufacturing costs, we have historically shown a profit from our European operations.
Revenues from our U.S. operations principally relate to the Company’s products that we promote to physicians through our sales representatives, as well as income from products that we have licensed. The Company charges our U.S. operations all Selling and Distribution expenses that support our marketing, sales and distribution efforts. Research and Development expenses are charged to our U.S. operations for product development which principally supports new products and new label indications for products to be sold in this country. In addition, the majority of our General and Administrative expenses represent the Company’s management activities as a public company and are charged to our U.S. operations. The amortization of the repurchase of the U.S. rights to CRINONE is also charged to our U.S. operations. As a result, we have historically shown a loss from our U.S. operations that has been significantly greater than, and offsets, the profits from our European operations.
|
(In thousands, except
|
Percentage
|
Percentage
|
|||
|
percentages)
|
from prior
|
from prior
|
|||
|
2009
|
year
|
2008
|
year
|
2007
|
|
|
Net revenues
|
$32,196
|
(11%)
|
$36,229
|
22%
|
$29,628
|
Net revenues decreased 11% in 2009 to $32.2 million as compared to $36.2 million in 2008 and $29.6 million in 2007. Net revenues from Progesterone Products decreased 1% to $23.8 million from $24.1 million in 2008 and compared with $20.5 million in 2007. The decrease from 2008 levels was primarily as a result of decreased sales of CRINONE outside the U.S and PROCHIEVE in the U.S. Net revenues from domestic CRINONE in 2009 increased 10% over 2008, with unit volume accounting for most of the increase. Total prescriptions for CRINONE in 2009 were 14% higher than 2008 and 27% higher than 2007. These increases were achieved despite a major economic downturn during 2009 impacting patients’ decisions to postpone or forego elective infertility procedures that are not reimbursed by health insurers in many major markets, including California. CRINONE net revenues from sales outside the U.S. in 2009 were 6% lower than in 2008 and can be attributed to a number of factors including lower selling prices due to foreign exchange rates relative to the dollar and price adjustments for government tenders. Sales volumes for CRINONE sold outside the U.S. were 8% higher than in 2008. PROCHIEVE net revenues in 2009 were $1.6 million or $0.5 million below net revenues of $2.1 million for 2008.
Net revenues increased 23% in 2008 to $36.3 million as compared to $29.6 million in 2007 and $17.4 million in 2006. Net revenues from Progesterone Products increased 18% to $24.1 million from $20.5 million in 2007 and $11.2 million in 2006. Unit sales increased for Progesterone Products from 2007 to 2008 by 30% worldwide. This volume increase was offset by price and other sales adjustments due Merck Serono. The increase in net revenues from Progesterone Products in 2008 is as a result of expanding the sales force across the U.S. in late 2007 and realizing the growth from converting doctors over to CRINONE. U.S. sales increased by 21%. International CRINONE marketed by Merck Serono grew by 13%. The growth in 2007 in Progesterone Products was primarily as a result of the acquisition of U.S. CRINONE marketing rights purchased from Merck Serono in December 2006 and the cancellation of the sale of a semi-annual batch of CRINONE to Merck Serono in anticipation of the U.S. rights acquisition.
Net revenues from Other Products in 2009 decreased by 31% or $3.8 million, to $8.4 million from $12.2 million in 2008. The principal driver of the decrease was the recognition of the deferred revenue from Ardana in 2008 as a result of its bankruptcy coupled with lower STRIANT sales both domestically and internationally.
Net revenues from Other Products in 2008 increased by 33% to $12.2 million from $9.2 million in 2007. The principal driver of the increase is the recognition of the deferred revenue from Ardana as a result of their bankruptcy. The deferred income that accelerated as a result of the bankruptcy was $2.9 million. International sales of Replens were up 16%. Net revenues in 2007 were up 48% over 2006 from $6.2 million to $9.2 million. The principal drivers for the increase during this period were additional batch orders of RepHresh from Lil’ Drug Stores and increased STRIANT sales.
Gross profit as a percentage of net revenues was 71% in 2009 as compared with 70% in 2008 and 2007. Although the gross profit percentage did not materially change, there were a number of offsetting events including the recognition of the deferred income from the Ardana bankruptcy with no underlying costs, increases in CRINONE and STRIANT profitability primarily through the effects of higher volume and, to a lesser extent, price increases offset by international CRINONE price adjustments recognized for government tenders during the period which lowered international CRINONE margins.
Gross profit as a percentage of net revenues was 70% in 2008 as compared with 70% in 2007 and 55% in 2006. Although the gross profit percentage did not change, there were a number of offsetting events including the recognition of the deferred income from the Ardana bankruptcy with no underlying costs, increases in CRINONE and STRIANT profitability primarily through the effects of higher volume and, to a lesser extent, price increases offset by international CRINONE price adjustments recognized for government tenders during the period, lowering international CRINONE margins. For 2007, the gross profit percentage for Progesterone Products improved 26% based on the shift from the previous Merck Serono license arrangement to the current full U.S. ownership of CRINONE. Gross profit percentage on Other Products decreased to 63% in 2007 from 73% in 2006 principally due to the loss of promotion fee income from Lil’ Drug Store.
Selling and Distribution
|
(In thousands, except
|
Percentage
|
Percentage
|
|||
|
percentages)
|
from prior
|
from prior
|
|||
|
2009
|
year
|
2008
|
year
|
2007
|
|
|
Selling and distribution
|
$11,982
|
(6%)
|
$12,685
|
25%
|
$10,112
|
|
As a percentage of revenue
|
37%
|
2pp
|
35%
|
1pp
|
34%
|
Note: pp = percentage points
Selling and distribution expenses include payroll, employee benefits, equity compensation and other personnel-related costs associated with sales and marketing personnel, and advertising, promotions, tradeshows, seminars, and other marketing-related programs. Selling and distribution expenses were approximately $12.0 million, $12.7 million and $10.1 million in 2009, 2008 and 2007, respectively. Selling and distribution expenses decreased by approximately 6% in 2009 compared to 2008 and increased by approximately 25% in 2008 compared to 2007. The primary reason for the 2009 decrease was the leveling off of marketing expenditures from 2008 levels when the sales organization was increased by more than 30% over 2007 levels. The increase in 2008 from 2007 reflected increases in sales force and marketing expenses.
Included in the 2009 expenses were sales force and management costs of approximately $6.7 million, product marketing expenses of approximately $4.3 million and $1.0 million in sales information and distribution costs. Expenses in 2008 included approximately $6.2 million in sales force and management costs, approximately $5.4 million in product marketing expenses and approximately $1.1 million in distribution costs. Expenses in 2007 included approximately $4.7 million in sales force and management costs, approximately $4.3 million in product marketing expenses and approximately $1.1 million in distribution costs.
General and Administrative
|
(In thousands, except
|
Percentage
|
Percentage
|
|||
|
percentages)
|
from prior
|
from prior
|
|||
|
2009
|
year
|
2008
|
year
|
2007
|
|
|
General and administrative
|
$10,559
|
23%
|
$8,615
|
10%
|
$7,825
|
|
As a percentage of revenue
|
33%
|
8pp
|
25%
|
(1)pp
|
26%
|
General and administrative costs include payroll, employee benefits, equity compensation, and other personnel-related costs associated with finance, legal, regulatory affairs, information technology, facilities and certain human resources, and other administrative personnel, as well as legal costs and other administrative fees. General and Administrative expenses increased by approximately $1.9 million, or 23%, to approximately $10.6 million in 2009 from approximately $8.6 million in 2008, which was an increase of $0.8 million from $7.8 million in 2007. The increase in 2009 reflected additional legal fees for patent applications for preterm birth ($0.3 million), the Bio-Mimetics litigation ($0.6 million), business development activities ($0.3 million) and severance costs ($0.5 million). The increase in 2008 cost over 2007 reflected additional legal fees for patent applications for preterm birth ($0.3 million), the Bio-Mimetics litigation ($0.4 million), and additional accounting expenses from the change in auditors and the restatement of 2006 financial statements ($0.3 million).
|
(In thousands, except
|
Percentage
|
Percentage
|
|||
|
percentages)
|
from prior
|
from prior
|
|||
|
2009
|
year
|
2008
|
year
|
2007
|
|
|
Research and development
|
$8,579
|
38%
|
$6,206
|
7%
|
$5,779
|
|
As a percentage of revenue
|
27%
|
10pp
|
17%
|
(3)pp
|
20%
|
Research and development costs include payroll, employee benefits, equity compensation and other personnel-related costs associated with product development, external contractors and consultants as well as the cost of conducting and administering clinical studies and the cost of regulatory activities and fees for our products. Research and development expenses increased $2.4 million, or 38%, to approximately $8.6 million in 2009 from $6.2 million 2008, which was an increase of $0.4 million from $5.8 million in 2007. The increase in 2009 was primarily due to higher costs for the PREGNANT study, including the enrollment of a greater number of patients at more study centers in 2009 versus 2008. The clinical PREGNANT trial expenses in 2008 were lower than in 2007, when most of the trial start up costs were incurred. This was partly offset by the absence of expenses related to the development of vaginal lidocaine in 2009. We completed a Phase II study of this candidate in the third quarter of 2008; associated costs were $0.8 million in 2008 and $1.4 million in 2007.
Amortization of CRINONE® Acquisition
We purchased the marketing rights for U.S. sales of CRINONE® 8% from Merck Serono in December 2006 for $33.0 million. In the second quarter of 2007, we recognized a $1.0 million adjustment to the purchase price to reflect contingent liabilities for Merck Serono sales returns. The $33.0 million charge is being amortized over 6.75 years, and the $1.0 million charge is being amortized over 6.5 years. Amortization expense of the acquisition cost for CRINONE® U.S. marketing rights for 2009, 2008 and 2007 was $5.0 million.
Other Income and Expense
Interest expense was $8.9 million, $7.9 million and $7.9 million in 2009, 2008 and 2007, respectively. Interest expense in 2009 included cash interest of $3.2 million and $3.1 million in charges associated with amortization of the beneficial conversion feature, amortization of the warrant costs and issuance costs, related to the Company's convertible subordinated notes.
Interest expense in 2009, 2008, and 2007 included approximately $2.2 million, $1.9 million and $2.3 million, respectively, as a result of amortizing the difference between the minimum amounts to be paid to PharmaBio and the amounts received as interest expense under the PharmaBio agreements.
Other income in 2009 reflected foreign exchange losses of approximately $0.2 million. In 2008 and 2007, other income included interest income from marketable securities of $0.3 million and $1.0 million, respectively.
State Income Tax Benefit
In each of the years 2009, 2008 and 2007, we realized proceeds from the sale of its New Jersey state net operating losses of $0.4 million, $0.9 million, and $0.8 million, respectively.
Net Loss
The net loss for 2009 was $21.9 million or $0.39 per share as compared to a net loss of $14.1 million, or $0.27 per share, in 2008 and a net loss of $14.3 million or $0.28 per share in 2007.
Contractual Obligations
As previously disclosed, in July 2002 and March 2003, the Company entered into agreements with PharmaBio, under which we received upfront money in exchange for royalty payments on our women’s healthcare products and STRIANT, respectively. We owe royalty payments to PharmaBio for a fixed period of time. These royalty payments are subject to minimum and maximum amounts. On February 29, 2008, the Company made the final payment under the STRIANT agreement. As discussed below, the remaining minimum payment on the STRIANT agreement must be made by November 2011. In addition, the Company enters into operating leases for many of our facility and equipment needs. These leases allow us to conserve cash by paying a monthly lease rental fee for the use of, rather than purchasing, facilities and equipment. At the end of the lease, we have no further obligation to the lessor.
On December 22, 2006, the Company issued (i) subordinated convertible notes in aggregate principal amount of $40 million (the “Convertible Notes”) and (ii) warrants to purchase 2,285,714 shares of Common Stock (the “Warrants”) with an exercise price of $5.50 per share. A portion of the proceeds were used to acquire the U.S. marketing rights to CRINONE® and the balance was used to pay related expenses and for working capital. The Convertible Notes bear interest at the rate of 8% per annum, payable quarterly in arrears commencing April 1, 2007. The Convertible Notes are convertible into shares of Common Stock at a conversion price of $5.25 per share. The conversion price is subject to adjustment if the Company subdivides or combines the outstanding Common Stock and under certain other circumstances. The maturity date of the Convertible Notes is December 31, 2011. In the event of a “change of control”, as defined in the Convertible Notes, the holder of each note is entitled to a “make-whole premium” if the holder exercises its rights to convert the Convertible Note, in whole or in part, during the “change of control redemption/conversion period,” as defined in the Convertible Notes. The make-whole premium is calculated in accordance with paragraph 5(e)(iii)(B) of the notes and decreases as our Common Stock price increases and the date of the change of control extends from the closing. No make-whole premium is due if the stock price is $3.50 or less or $10.50 or greater. In the event of a change of control, the holder of each note may also require the Company to redeem all or any portion of the note. The Convertible Notes contain customary events of default. The Convertible Notes are subordinated to the Company’s obligations to PharmaBio. The Warrants were exercisable beginning on June 20, 2007, and ending on December 22, 2011, at an exercise price of $5.50 per share, subject to adjustment in certain circumstances. The Company will be required to make certain cash payments to the holders of the Convertible Notes and Warrants if it does not meet its registration obligations under the agreement relating to the transaction.
On July 22, 2009, the Company and PharmaBio entered into an amendment (the “Second Amendment”) to the STRIANT Agreement, in which it was agreed that when the minimum royalty payment is due the Company may, in its sole discretion, either pay the balance due under the STRIANT Agreement or issue to PharmaBio a secured promissory note for that balance. In consideration for the right to issue the secured promissory note, the Company has (a) agreed that during the period from July 22, 2009 through November 30, 2010, the Company will escrow any proceeds from sales of assets outside the ordinary course of business in excess of $15.0 million but not exceeding the difference between the amount of royalties actually received by PharmaBio under the STRIANT Agreement and $30.0 million, and (b) granted PharmaBio a warrant to purchase 900,000 shares of the Company’s Common Stock. In further consideration for the right to issue the secured promissory note, the Company has agreed that if it issues the secured promissory note on November 30, 2010, the Company will on that date grant PharmaBio a second warrant to purchase 900,000 shares of the Company’s Common Stock. Each warrant is exercisable beginning November 30, 2010 and expires on the date five years from its issue date. The warrants are exercisable at $1.15 per share, permit cashless exercise, and provide piggyback registration rights. If the Company issues the secured promissory note, it would bear interest quarterly in arrears at the rate of 10% per annum, be due on November 30, 2011, be secured by Columbia’s assets, and contain customary representations, warranties, and events of default. Using the Black Scholes valuation model, the Company determined the value of the initial warrant to purchase 900,000 shares of the Company’s Common Stock to be $719,904, or $0.80 per share, which is being amortized over the 16 months through November 2010. The amortization expense recorded in the quarter ended December 31, 2009 was approximately $225,000.
On March 3, 2010 we entered into contingent agreements with PharmaBio and the holders of our Convertible Notes under which, if the Watson transaction closes and the other conditions to consummation of such agreements are satisfied, we will repay our outstanding debt on an accelerated and discounted basis and issue the holders of Convertible Notes shares of common stock and warrants to purchase common stock. See “Item 1. Business - Watson Transaction and Debt Restructuring.”
Our future contractual obligations without giving effect to the Watson Transaction or the contingent agreements with PharmaBio and the holders of our Convertible Notes, include the following:
|
For the Fiscal Years Ended December 31,
|
||||||||||||||||||||||||
|
Total
|
2010
|
2011
|
2012
|
2013
|
Beyond
|
|||||||||||||||||||
|
$40 million convertible notes
|
$ | 40,000 | $ | - | $ | 40,000 | $ | - | $ | - | $ | - | ||||||||||||
|
Interest on $40 million convertible notes
|
7,200 | 3,200 | 4,000 | - | - | - | ||||||||||||||||||
|
PharmaBio Striant®
|
||||||||||||||||||||||||
|
finance agreement
|
16,526 | 145 | 16,381 | - | - | - | ||||||||||||||||||
|
Operating lease obligations
|
936 | 271 | 245 | 233 | 187 | - | ||||||||||||||||||
|
Executive agreements
|
1,961 | 1,961 | - | - | - | - | ||||||||||||||||||
|
Total
|
$ | 66,623 | $ | 5,577 | $ | 60,626 | $ | 233 | $ | 187 | $ | - | ||||||||||||
Recent Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements (a consensus of the FASB Emerging Issues Task Force), which amends ASC 605-25, Revenue Recognition: Multiple-Element Arrangements. ASU No. 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how to allocate consideration to each unit of accounting in the arrangement. This ASU replaces all references to fair value as the measurement criteria with the term selling price and establishes a hierarchy for determining the selling price of a deliverable. ASU No. 2009-13 also eliminates the use of the residual value method for determining the allocation of arrangement consideration. Additionally, ASU No. 2009-13 requires expanded disclosures. This ASU will become effective for revenue arrangements entered into or materially modified after the fiscal year 2010. Earlier application is permitted with required transition disclosures based on the period of adoption. We are currently evaluating the application date and the impact of this standard on our consolidated financial statements.
In June 2009, the FASB issued Statement of Financial Accounting Standards (“SFAS”) ASC 105 (formerly No. 168). The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles (the “Codification”). This standard replaces SFAS No. 162, The Hierarchy of Generally Accepted Accounting Principles, and establishes only two levels of U.S. generally accepted accounting principles (“GAAP”), authoritative and nonauthoritative. The FASB Accounting Standards Codification (“ASC”) will become the source of authoritative, nongovernmental GAAP, except for rules and interpretive releases of the SEC, which are sources of authoritative GAAP for SEC registrants. All other nongrandfathered, non-SEC accounting literature not included in the Codification will become nonauthoritative. This standard is effective for financial statements for interim or annual reporting periods ending after September 15, 2009. The adoption of the Codification changed the Company’s references to GAAP accounting standards but did not impact the Company’s results of operations, financial position or liquidity.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 805, Business Combinations (formerly SFAS No.141(R), Business Combinations). The new standard applies to all transactions or other events in which an entity obtains control of one or more businesses. Additionally, the new standard requires the acquiring entity in a business combination to recognize all (and only) the assets acquired and liabilities assumed in the transaction; establishes the acquisition-date fair value as the measurement date for all assets acquired and liabilities assumed; and requires the acquirer to disclose additional information needed to evaluate and understand the nature and financial effect of the business combination. ASC 805 will impact the Company in the event of any future acquisition.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 260, Earnings Per Share (formerly FASB Staff Position (“FSP”) Emerging Issues Task Force (“EITF”) 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities). The new guidance clarifies that non-vested share-based payment awards that entitle their holders to receive nonforfeitable dividends or dividend equivalents before vesting should be considered participating securities and included in basic earnings per share. The Company’s adoption of the new accounting standard did not have a material effect on previously reported or current earnings per share.
Effective January 1, 2009, the Company adopted a new accounting standard included in FASB ASC 470-20, “Debt with Conversion and other Options (Including Partial Cash Settlement) ,” which applies to all convertible debt instruments that have a net settlement feature; which means that such convertible debt instruments, by their terms, may be settled either wholly or partially in cash upon conversion. FASB ASC 470-20 requires issuers of convertible debt instruments that may be settled wholly or partially in cash upon conversion to separately account for the liability and equity components in a manner reflective of the issuer’s nonconvertible debt borrowing rate. Previous guidance provided for accounting for this type of convertible debt instrument entirely as debt. FASB ASC 470-20 was effective for financial statements issued for fiscal years beginning after December 15, 2008 and interim periods within those fiscal years. The adoption of the new accounting standard did not affect the Company’s financial statements.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 815-40 “Determining Whether an Instrument (or an Embedded Feature) is Indexed to an Entity’s Own Stock” (“EITF 07-5”). The new standard provides that an entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. It also clarifies the impact of foreign currency denominated strike prices and market-based employee stock option valuation instruments on the evaluation. EITF 07-5 is effective for fiscal years beginning after December 15, 2008. The adoption of ASC 815-40 did not affect the Company’s financial statements.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 470 “Transition Guidance for Conforming to Issue No. 98-5 (“EITF no. 08-4”)”. The objective of the new standard to provide transition guidance for conforming changes made to EITF No. 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Rations,” that result from EITF No. 00-27 “Application of Issue No. 98-5 to Certain Convertible Instruments,” and SFAS No. 150, “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity.” This standard is effective for financial statements issued for fiscal years ending after December 15, 2008. The adoption of this new standard did not affect the Company’s accounting for the convertible notes and related warrants transactions.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future material effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources.
Impact of Inflation
Sales revenues, manufacturing costs, selling and distribution expenses, general and administrative expenses, and research and development costs tend to reflect the general inflationary trends.
Liquidity and Capital Resources
Cash and cash equivalents were $14.8 million and $12.5 million at December 31, 2009 and December 31, 2008, respectively.
The Company believes that the approximately $14.8 million of cash on hand at December 31, 2009 will allow it to sustain its operations for the next twelve months.
|
2009
|
2008
|
2007
|
|||
|
Cash flows:
|
|||||
|
Operating activities
|
$(9,055,667)
|
$ (5,136,488)
|
$ (7,914,299)
|
||
|
Investing activities
|
(49,853)
|
(375,926)
|
(102,021)
|
||
|
Financing activities
|
11,332,531
|
959,191
|
(40,803)
|
Operating Activities:
The net loss in 2009 of $21.9 million is reduced by non-cash items totaling $13.8 million resulting in cash losses of $8.1 million. Changes to assets and liabilities increased the amount by $1.0 million.
The net loss in 2008 of $14.1 million is reduced by non-cash items totaling $10.1 million leaving cash losses of $4.0 million. Changes to assets and liabilities increased the amount by $1.4 million.
Investing Activities:
In 2009 the Company invested $0.1 million in processing equipment and computer equipment and software upgrades.
In 2008 the Company invested $0.4 million in processing equipment and computer equipment and software upgrades.
Net cash raised in financing activities in 2009 was $11.3 million. The Company raised $0.75 million from the issuance of 451,807 shares in January, and $11.5 million from the issuance of 10,900,000 shares in October 2009 in a public offering. The Company acquired $0.1 million of treasury stock.
Net cash raised in financing activities in 2008 was $1.0 million. The Company raised $4.1 million in equity placement of 1,333,000 shares in August. Stock option exercises raised an additional $0.6 million through the issuance of 318,149 shares. Other financing activities included the final payment made to PharmaBio under the 2002 Women’s Healthcare products arrangement of $3.6 million. The Company bought $0.1 million of treasury stock and paid $0.1 million in Series C Preferred Stock dividends.
As previously discussed, on July 31, 2002, we entered into an investment and royalty agreement with PharmaBio under which we received $4.5 million in return for a 5% royalty to PharmaBio on net sales of the Company’s women’s healthcare products in the U.S. for five years, beginning in the first quarter of 2003. The royalty payments were subject to aggregate minimum ($8 million) and maximum ($12 million) amounts. Because the minimum amount exceeded $4.5 million, the Company recorded the amounts received as liabilities. The excess of the minimum ($8 million) paid by the Company over the $4.5 million received by the Company was recognized as interest expense over the five-year term of the agreement, assuming an interest rate of 17%. The final payment under this agreement was made in February 2008.
Also, as previously discussed, on March 5, 2003, we entered into a second investment and royalty agreement with PharmaBio under which we received $15.0 million in return for a 9% royalty to PharmaBio on net sales of STRIANT in the U.S. up to agreed annual sales revenues, and a 4.5% royalty of net sales above those levels. The royalty term is seven years. Royalty payments commenced in the 2003 third quarter and are subject to aggregate minimum ($30.0 million) and maximum ($55.0 million) amounts, including a true-up payment due on November 14, 2006 for the difference between royalties paid to that period and $13.0 million. On April 14, 2006, the Company made an advance payment of $11.6 million on the contractually required true-up payment. This amount represented the present value of a $12.0 million true-up payment due November 14, 2006, calculated using a six percent annual discount factor. Because the minimum amount exceeds $15.0 million, the Company has recorded the amounts received as liabilities. The excess of the minimum ($30.0 million) to be paid by the Company over the $15.0 million received by the Company is being recognized as interest expense over the seven-year term of the agreement, assuming an interest rate of 15%. As of December 31, 2009, the Company has paid $13.5 million in royalties (including the true-up payment) to PharmaBio under this agreement. The balance of the minimum royalty payments due November, 2010 is estimated to be approximately $16.5 million.
On July 22, 2009, the Company and PharmaBio entered into an amendment to this agreement in which they agreed that, when the minimum royalty payment is due, the Company may, in its sole discretion, either pay the balance due or issue to PharmaBio a secured promissory note for that balance. The note would bear interest quarterly in arrears at the rate of 10% per annum and be due on November 30, 2011. In consideration for the right to issue the secured promissory note, the Company (a) agreed that during the period from July 22, 2009 through November 30, 2010, the Company will escrow any proceeds from sales of assets outside the ordinary course of business in excess of $15.0 million but not exceeding the difference between the amount of royalties actually received by PharmaBio under the agreement and $30.0 million, and (b) granted PharmaBio a warrant to purchase 900,000 shares of the Company’s common stock. In further consideration for the right to issue the secured promissory note, the Company has agreed that if it issues the secured promissory note on November 30, 2010, the Company will also on that date grant PharmaBio a warrant to purchase 900,000 shares of the Company’s common stock. Each warrant is exercisable beginning November 30, 2010 and expires on the date five years from its issue date. The warrants are exercisable at $1.15 per share, permit cashless exercise, and provide piggyback registration rights. The second promissory note would be secured by Columbia’s assets, and contain customary representations, warranties, and events of default. Using the Black Scholes valuation model, the Company determined the value of the initial warrant to purchase 900,000 shares of the Company’s Common Stock to be $719,904, or $0.80 per share, which is being amortized over the 16 month period from July 2009 through November 2010. The amortization expense recorded for 2009 was approximately $225,000.
The Company has an effective registration statement that we filed with the Securities and Exchange Commission (the “SEC”) using a shelf registration process. Under the shelf registration process, we may offer from time to time Common Stock, preferred stock, debt securities and warrants up to an aggregate amount of $50 million. To date the Company has sold approximately $12.5 million in Common Stock under the registration statement. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct our business. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the marketing of one or more of our products and the development and/or commercialization of one or more of our product candidates.
As of December 31, 2009, the Company had outstanding exercisable options and warrants that, if exercised, would result in approximately $51.5 million of additional capital and would cause the number of shares of Common Stock outstanding to increase by 16,903,059 shares. However, there can be no assurance that any such options or warrants will be exercised. The aggregate intrinsic value of exercisable options and warrants at December 31, 2009 were $0.0 and $0.0, respectively.
In connection with the 1989 purchase of the assets of Bio-Mimetics, which assets consisted of the patents underlying the Company's BDS, other patent applications and related technology, the Company pays Bio-Mimetics a royalty equal to two percent of the net sales of products based on the BDS up to an aggregate of $7.5 million or until the last of the relevant patents expires. The Company is required to prepay 25% of the remaining royalty obligation, in cash or stock at the option of the Company, if the closing price of the Company’s Common Stock is $20.00 or more on March 2, or within 30 days after that date, of any year. Royalty payments on STRIANT, PROCHIEVE®, and CRINONE® expired in September of 2006, but continue on Replens® and RepHresh®. On December 28, 2007, Bio-Mimetics filed a complaint in the U.S. District Court for Massachusetts (Bio-Mimetics, Inc. v Columbia Laboratories, Inc.) alleging breach of contract, and unfair or deceptive trade practices for the Company’s failure to continue royalty payments on STRIANT®, PROCHIEVE®, and CRINONE®. To date, the Company has paid approximately $3.9 million in royalty payments and Bio-Mimetics seeks a judgment that we are obligated to pay the remaining $3.6 million in full. The Company denies the allegations and intends to defend this action vigorously.
The Company anticipates it will spend approximately $0.1 million on equipment in 2010.
As of December 31, 2009, the Company had available net operating loss carryforwards of approximately $162.0 million to offset its potential future U.S. taxable income. There can be no assurance that the Company will have sufficient income to utilize the net operating loss carryforwards or that the net operating loss carryforwards will be available at that time.
In accordance with Statement of Financial Accounting Standards ASC 740, as of December 31, 2009 and 2008, other assets in the accompanying consolidated balance sheet include deferred tax assets of approximately $62.9 million, $56.1 million, respectively, (comprised primarily of a net operating loss carryforward) for which a valuation allowance has been recorded because the probability of realizing the deferred tax assets are not determinable.
Watson Transaction and Debt Restructuring
On March 3, 2010, the Company, Watson Pharmaceuticals, Inc., as a guarantor of the Buyer’s obligations (“Watson”), and Coventry Acquisition, Inc., a subsidiary of Watson (the “Buyer”), entered into a Purchase and Collaboration Agreement (the “Purchase Agreement”) which is subject to stockholder approval. Pursuant to the Purchase Agreement, the Company agreed to sell, subject to shareholder approval, to the Buyer (i) substantially all of its assets primarily relating to the research, development, regulatory approval, manufacture, distribution, marketing, sale and promotion of pharmaceutical products containing progesterone as an active ingredient, including CRINONE 8% progesterone gel, PROCHIEVE 4% progesterone gel and PROCHIEVE 8% progesterone gel, each sold by the Company in the U.S. (collectively, the “Progesterone Products”), including certain intellectual property, promotional materials, contracts, product data and regulatory approvals and regulatory filings (the “Purchased Assets”), and (ii) 11,200,000 shares (the “Shares”) of the Company’s Common Stock. The Company will retain certain assets and rights relating to its progesterone business, including all rights necessary to perform its obligations under its agreement with Merck Serono. The transactions pursuant to the Purchase Agreement and the ancillary agreements thereto are referred to collectively herein as the “Watson Transaction.”
At the closing of the Watson Transaction, in consideration for the sale of the Purchased Assets and the Shares, the Buyer agreed to pay the Company $47 million in cash and assume certain liabilities associated with the Purchased Assets. In addition, the Buyer agreed to pay the Company up to $45.5 million in cash upon the achievement of several contingent milestones. The Buyer also agreed to make royalty payments to the Company of 10 to 20 percent of annual net sales of certain progesterone products; provided, however that royalty rates would be reduced by 50% in a particular country if a generic entry by a third party occurs in such country and certain other circumstances are fulfilled and, if the Buyer commercializes a product through a third party outside of the U.S., in lieu of royalties, the Company will be entitled to 20% of gross profits associated with such commercialization. In addition, if the Buyer or its affiliates effects a generic entry with respect to a progesterone product in a country in the circumstances permitted by the Purchase Agreement, in lieu of royalties payable in respect of net sales for such generic product, the Company will be entitled to 20% of the gross profits associated with the commercialization of such generic product in such country.
Pursuant to the Purchase Agreement, the Company and the Buyer have also agreed to collaborate with respect to the development of progesterone products. In connection therewith, the parties agreed to establish a joint development committee to oversee and supervise all development activities. The Company will be responsible for completion of the PREGNANT Study and such other activities as determined by the joint development committee. The Company will be responsible for the costs of conducting the PREGNANT Study and the preparation, filing and approval process of the related new drug application (or the supplemental new drug application) up to a maximum of $7 million from January 1, 2010. All other development costs incurred in connection with the development collaboration will be paid by the Buyer.
Upon the closing of the Watson Transaction which is subject to customary closing conditions including Company stockholder approval, the operations of the Company will be materially changed as our revenue streams will be dependent primarily on royalty streams from our partners, Watson and Merck Serono (if our agreement with Merck Serono is renewed), supplemented by payments for the achievement of significant development and approval milestones. In addition, the Company will receive revenues under its Supply Agreement with Watson. On the operating expense side, the Company intends to eliminate all selling and distribution expenses, reduce certain of its general and administrative expenses in support of the sales and distribution efforts and be limited to $7 million in third-party out-of-pocket costs to complete the PREGNANT study and, if successful, prepare the FDA filings and pay the related filing fees. The Company will have no financial obligations to fund additional development initiatives on the Progesterone Products. The Company will place into a restricted account the required operating cash for financing the completion of the PREGNANT study and preparation for and filing of the related new drug application (or the supplemental new drug application) up to the amount to fulfill our $7 million obligation.
On March 3, 2010, the Company entered into an amendment (the “PharmaBio Amendment”) with PharmaBio to the Investment and Royalty Agreement dated March 5, 2003, between the Company and PharmaBio, as previously amended and supplemented (the “PharmaBio Agreement”). The PharmaBio Amendment provides for the early termination of the PharmaBio Agreement by permitting the Company to make certain payments required thereunder on an accelerated and discounted basis on the date the Company consummates (and contingent upon the Company consummating) a transfer of assets, sale of stock, licensing agreement and/or similar transaction yielding gross cash proceeds to the Company of $40 million or more.
Lastly, on March 3, 2010, the Company entered into Note Purchase and Amendment Agreements (the “Note Purchase Agreements”) with all of the holders (the “Holders”) of the Company’s Convertible Subordinated Notes due December 31, 2011 (the “Notes”). Under the Note Purchase Agreements, the Company agreed to purchase, subject to the satisfaction of certain conditions, the approximately $40 million in aggregate principal amount of Notes held by the Holders. The aggregate purchase price for the Notes will be $26 million in cash (plus accrued and unpaid interest through but excluding the date of the closing of the Note purchases), warrants to purchase 7,750,000 shares of Common Stock at an exercise price of $1.35 per share (the “Warrants”) and 7,407,407 shares of Common Stock. The closings of the transactions contemplated by the Note Purchase Agreements are subject to various conditions, including the consummation of the Watson Transaction. Pursuant to the Note Purchase Agreements, the Holders consented, effective on March 3, 2010, to an amendment to the Notes (the “Amendment”) that eliminates the right of any holder of the Notes to cause the Company to redeem the Notes by virtue of the Watson Transaction. The Amendment terminates if the note purchase closings do not occur on or prior to August 31, 2010 and in certain other circumstances. Each Note Purchase Agreement may be terminated in certain circumstances, including, among others, by any party thereto, if the closings thereunder do not occur on or prior to August 31, 2010.
Critical Accounting Policies and Estimates
Our financial statements and accompanying notes are prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). The preparation of financial statements requires management to make estimates and assumptions that affect the reported amount of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. There can be no assurance that actual results will not differ from those estimates. These estimates and assumptions are affected by management’s application of accounting policies. Critical accounting policies for us include revenue recognition, impairment of intangible assets, and accounting for the agreements with PharmaBio. For a detailed discussion on the application of these and other accounting policies, see Note 1 of the consolidated financial statements included in Item 8 of this Annual Report on Form 10-K.
Revenue recognition. The Company’s revenue recognition is significant because revenue is a key component of our results of operations. In addition, revenue recognition determines the timing of certain expenses, such as commissions and royalties. Revenue results are difficult to predict, and any shortfall in revenue or delay in recognizing revenue could cause operating results to vary significantly from quarter to quarter. Revenues on sales of products by Columbia are discussed in detail below. License fees are recorded over the life of the license. Royalty revenues, based on sales by licensees, are recorded as revenues as those sales are made by licensees.
Sales Returns. Revenues from the sale of products are recorded at the time goods are shipped to customers. The Company believes that it has not made any shipments in excess of its customers' ordinary course of business inventory levels. Our return policy allows product to be returned for a period beginning three months prior to the product expiration date and ending twelve months after the product expiration date. Provisions for returns on sales to wholesalers, distributors and retail chain stores are estimated based on a percentage of sales, using such factors as historical sales information, distributor inventory levels and product prescription data, and are recorded as a reduction to sales in the same period as the related sales are recognized. We also continually analyze the reserve for future sales returns and increase such reserve if deemed appropriate. The Company purchases prescription data on all its products from IMS Health, a leading provider of market intelligence to the pharmaceutical and healthcare industries. The Company also purchases certain information regarding inventory levels from its larger wholesale customers. This information includes for each of the Company’ products, the quantity on hand, the number of days of inventory on hand, and a 28 day forecast of sales by units. Using this information and historical information, the Company estimates potential returns by taking the number of product units sold by the Company by expiration date and then subtracting actual units and potential units that may be sold to end users (consumers) based on prescription data up to five months prior to the product’s expiration date. The Company records a provision for returns on a quarterly basis using an estimated rate and adjusts the provision if its analysis indicates that the potential for product non-salability exists. Sales adjustments for international sales are estimated to recognize changes in foreign exchange rates and government tenders that may fluctuate within a year.
Accounting For PharmaBio Agreements. In July 2002 and March 2003, the Company entered into agreements with PharmaBio under which the Company received upfront money paid in quarterly installments in exchange for royalty payments on certain of the Company’s products to be paid to PharmaBio for a fixed period of time. The royalty payments are subject to minimum and maximum amounts. Because the minimum amounts exceed the amount received by the Company, the Company has recorded the monies received as liabilities. We are recording the excess of the minimum to be paid by the Company over the amount received by the Company as interest expense over the terms of the agreements.
Forward-Looking Statements
This Annual Report on Form 10-K contains statements that are forward-looking. These statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements include, without limitation, the Company’s expectations regarding sales, earnings or other future financial performance and liquidity, completion or outcome of clinical studies, product introductions, entry into new geographic regions, and general optimism about future operations or operating results. Some of these statements can be identified by the use of forward-looking terminology such as "prospects," "outlook," "believes," "estimates," "intends," "may," "will," "should," "anticipates," "expects, or "plans," or the negative or other variation of these or similar words, or by discussion of trends and conditions, strategy or risks and uncertainties.
These forward looking expectations are based on current assumptions within the bounds of management’s knowledge of our business and operations and which management believes are reasonable. These assumptions are subject to risks and uncertainties, and actual results could differ materially from expectations because of issues and uncertainties such as those listed in “Risk Factors” and elsewhere in this Annual Report, which, among others, should be considered in evaluating our future financial performance. All subsequent written and oral forward-looking statements attributable to the Company or persons acting on behalf of the Company are expressly qualified in their entirety by the cautionary statements in this Annual Report. Readers are advised to consult any further disclosures the Company may make on related subjects in subsequent reports filed with the SEC.
The Company does not believe that it has material exposure to market rate risk. The Company may, however, require additional financing to fund future obligations and no assurance can be given that the terms of future sources of financing will not expose the Company to material market risk. Expenditures primarily related to manufacturing in 2009 were approximately $0.6 million less than they would have been if the average 2008 exchange rates had been in effect in 2009.
Item 8. Financial Statements and Supplementary Data
The financial statements and supplementary data required by this item are set forth at the pages indicated in Item 15, set forth in this annual report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None
Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures designed to ensure that the information the Company must disclose in its filings with the SEC is recorded, processed, summarized and reported on a timely basis. The Company ’s management, under the supervision and with the participation of the Chief Executive Officer and the Chief Financial Officer, evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of December 31, 2009. Based on this evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2009, the Company’s disclosure controls and procedures were effective.
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a – 15 or 15d – 15 that occurred during the last fiscal quarter that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Management’s Annual Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining an adequate system of internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Further, because of changes in conditions, effectiveness of internal control over financial reporting may vary over time.
Management of the Company conducted an evaluation of the effectiveness, as of December 31, 2009, of the Company’s internal control over financial reporting based on the Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO Framework”). Based on its evaluation under the COSO Framework, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2009.
BDO Seidman, LLP, an independent registered public accounting firm, has issued an attestation report on the Company’s internal control over financial reporting (see Report of Independent Registered Public Accounting Firm).
Board of Directors and Shareholders
Columbia Laboratories, Inc.
Livingston, NJ
We have audited Columbia Laboratories, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Item 9A. Management’s Annual Report on Internal Control over Financial Reporting”. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Columbia Laboratories, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Columbia Laboratories, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations and comprehensive operations, shareholders’ deficiency, and cash flows for the years then ended and our report dated March 12, 2010, expressed an unqualified opinion thereon.
/s/ BDO Seidman, LLP
BDO Seidman, LLP
Woodbridge, NJ
March 12, 2010
Item 9B. Other Information
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Our Executive Officers and Directors as of April 27, 2010, were as follows:
|
Name
|
Age
|
Position with the Company
|
||
|
Frank C. Condella
|
55
|
Interim Chief Executive Officer, Director
|
||
| Robert S. Mills | 57 | President & Chief Operating Officer, Director | ||
|
Lawrence A. Gyenes
|
59
|
Senior Vice President, Chief Financial Officer and Treasurer
|
||
|
Michael McGrane
|
60
|
Senior Vice President, General Counsel and Secretary
|
||
|
Stephen G. Kasnet
|
64
|
Chairman of the Board
|
||
|
Edward A. Blechschmidt
|
57
|
Vice Chairman of the Board
|
||
|
Valerie L. Andrews
|
50
|
Director
|
||
|
Anthony R. Campbell
|
62
|
Director
|
||
|
James S. Crofton
|
58
|
Director
|
||
|
Selwyn P. Oskowitz, M.D.
|
64
|
Director
|
Officers serve at the discretion of the Board of Directors. There is no family relationship between any of the executive officers or between any of the executive officers and the Company’s directors. There is no arrangement or understanding between any executive officer, director and any other person pursuant to which the executive officer was selected or director was elected, except with respect to Messrs. Condella’s, Mills’, McGrane’s and Gyenes’ employment agreements.
Mr. Condella has served as Interim Chief Executive Officer since December 2009 and has been a Director of Columbia since March 2009. Mr. Condella has over 30 years of experience in both privately and publicly held companies, all of which were in the life sciences industry. He was Chief Executive Officer of Skyepharma plc from March 2006 to September 2008, President of European Operations and Managing Director, UK, at IVAX Corporation from 2002 to February 2006, and President and Chief Executive Officer of Faulding Pharmaceutical Co., from 2000 to 2001. Previously, he was Vice-President of Specialty Care Products at Hoffman-La Roche and Vice-President and General Manager of the Lederle unit of American Home Products. Mr. Condella is the non-executive Chairman of Skyepharma plc and a director of Fulcrum Pharma plc. Mr. Condella holds a MBA degree and a B.S. degree in pharmacy from Northeastern University.
Mr. Condella has a wide ranging business background, including senior leadership roles in the pharmaceutical and healthcare industry, with particular experience in marketing and sales.
Mr. Mills has served as President and has been a member of the Company’s Board of Directors since January 2006. He was Chief Executive Officer from March 2006 to December 2009. Mr. Mills joined Columbia in May 2001 as Senior Vice President, Operations and was named Chief Operating Officer in September 2003. Prior to joining the Company, Mr. Mills served five years as Senior Vice President, Manufacturing Operations, at Watson Pharmaceuticals, Inc. from 1996 to 2001. During his 34-year career in the pharmaceutical industry he also served as Vice President, Operations, at Alpharma, Inc. from 1993 to 1996 and held various positions with Aventis SA, including Director-Plant Operations. Mr. Mills holds a B.S. degree from Grove City College.
Mr. Mills has a wide ranging business background, including senior leadership roles in the pharmaceutical and healthcare industry, with particular experience in manufacturing operations. As previously disclosed, on April 15, 2010, Mr. Mills resigned from his position as President and Chief Operating Officer of the Company and resigned from the Company’s Board of Directors, both effective May 14, 2010, pursuant to his Amended and Restated Employment Agreement with the Company, dated March 11, 2009 (the “Employment Agreement”), as amended by an Addendum to the Employment Agreement on December 11, 2009 (the “Addendum”), for Good Reason (as defined in the Employment Agreement and Addendum) pursuant to Section 6(d) of the Employment Agreement. The Employment Agreement was filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008. The Addendum was filed as an Exhibit to the Company’s Current Report on Form 8-K dated December 11, 2009.
Mr. Gyenes has served as Senior Vice President, Chief Financial Officer and Treasurer since July 2009. Mr. Gyenes has over 35 years of financial experience in both privately and publicly held companies, most all of which were in the life sciences industry. He most recently served as Senior Vice President and Chief Financial Officer of Acusphere, Inc., a former NASDAQ-listed specialty pharmaceutical company. Prior to joining Acusphere, Mr. Gyenes was Chief Financial Officer of Zila, Inc., a former NASDAQ-listed oral cancer screening company, a consultant to investment management firms, and Senior Vice President and Chief Financial Officer of Savient Pharmaceuticals, Inc., a NASDAQ-listed specialty pharmaceutical company. He also held senior financial positions with Reliant Pharmaceuticals, Inc., Rand McNally & Co., CompuServe Corporation, Helene Curtis, Inc., and G.D. Searle & Co., and was a consultant to DuPont Pharmaceuticals Company in its sale to Bristol-Meyers Squibb. Mr. Gyenes holds an MBA degree from the University of Chicago and a BS degree in Accounting from the University of Illinois.
Mr. McGrane has served as Senior Vice President since January 2006, and our General Counsel and Secretary since January 2002. He joined the Company from The Liposome Company, Inc., a biotechnology company, where he served as Vice President, General Counsel and Secretary from 1999 to 2001, prior to which he was Vice President, General Counsel and Secretary to Novartis Consumer Health, Inc. from 1997 to 1998. Previously, Mr. McGrane held various positions, including Associate General Counsel, with Novartis Pharmaceuticals Corporation from 1984 to 1996, and was Regulatory Counsel to the U.S. Food and Drug Administration from 1975 to 1984. Mr. McGrane received his J.D. degree from Georgetown University and his B.A. degree from Cornell College. He is a member of the New Jersey bar.
Mr. Kasnet has been a director of the Company since August 2004 and Chairman of the Board since November 2004. He is a director and chairman of the audit committee of Two Harbor Investment Corp (real estate) and a director of First Ipswich Bancorp. (banking). He was President and Chief Executive Officer of Dartmouth Street Capital LLC (real estate) from April 2007 through September 2009. He was President and Chief Executive Officer of Harbor Global Company, Ltd. (investment management and real estate), from June 2000 through 2006. He previously held senior management positions with various financial organizations, including Pioneer Group, Inc.; First Winthrop Corporation and Winthrop Financial Associates; and Cabot and Forbes. He serves as Chairman of the Board of Rubicon Ltd. (forestry) and is a director of Tenon Ltd. (wood products). He was Chairman of Warren Bank from 1990 to 2003. He is also a trustee and vice president of the board of The Governor’s Academy, Byfield, MA.
Mr. Kasnet has extensive experience in matters of finance, corporate strategy and senior leadership relevant to public companies.
Mr. Blechschmidt has been a director of Columbia since August 2004 and Vice Chairman of the Board since November 2004. He was Chief Executive Officer of Novelis, Inc. (aluminum rolled products) from December 2006 to May 2007. He was Chairman, Chief Executive Officer and President of Gentiva Health Services (home healthcare) from March 2000 until his retirement in July 2002. He previously served as Chief Executive Officer and a Director of Olsten Corporation (“Olsten”) (staffing services), the conglomerate from which Gentiva Health Services was split off and taken public. Before joining Olsten, Mr. Blechschmidt was President and Chief Executive Officer of both Siemens' Nixdorf Americas (information technology) and Siemens' Pyramid Technology (information technology), prior to which he served more than 20 years with Unisys Corporation (information technology), ultimately as Chief Financial Officer. He is currently a director of Healthsouth Corp. (healthcare), Lionbridge Technologies, Inc. (business services), Diamond Foods, Inc (snack foods) and, VWR International, LLC, (laboratory supplies).
Mr. Blechschmidt has extensive experience in matters of finance, corporate strategy and senior leadership relevant to public companies, including in the healthcare field.
Ms. Andrews has been a director of Columbia since October 2005 and is Vice President and Deputy General Counsel of Vertex Pharmaceuticals Incorporated. Before joining Vertex in 2002, Ms. Andrews was Executive Director of Licensing for Massachusetts General and The Brigham and Women’s Hospitals, and prior to that a partner in the law firm of Hill & Barlow. She served as a law clerk to Chief Judge Levin H. Campbell of the U.S. Court of Appeals for the First Circuit from 1988 to 1989, and earlier rose to the rank of Captain in the U.S. Air Force.
Ms. Andrews has extensive experience in business and legal matters, including senior leadership roles in the pharmaceutical and healthcare industry, with particular experience in corporate governance, strategic transactions, risk management, and compliance matters.
Mr. Campbell has been a director of Columbia since December 2008 and is a Portfolio Manager and Senior Analyst for Dorset Management Corporation since January 2000 and a Director of Knott Partners Management, LLC (investment advisors) since 2004. Mr. Campbell founded Windsor Partners, L.P. (investment advisors) in 1986. He was Principal and Managing Director of Berg Capital Corporation, a registered investment advisor, from 1984 through 1985, and also served as General Partner of Chelsea Partners, a private investment partnership, during that time. Mr. Campbell was a Vice President at the First Boston Corporation from 1975 through 1984, and from 1969 until 1975 was at McLeod, Young, Weir, Ltd. (investment advisors) in Canada and was appointed a Vice President in 1973.
Mr. Campbell has extensive experience in matters of finance and corporate strategy relevant to public companies.
Mr. Crofton has been a director of Columbia since October 2005. He has been Senior Vice President and Chief Financial Officer of Sarnoff Corporation (technology) since 1999. Previously, Mr. Crofton was Chief Financial Officer of EA Industries, Inc. (electronics manufacturing), and prior to that served in various positions, including Vice President of Finance, with Unisys Corporation (information technology). Mr. Crofton was previously a director of American Mold Guard, Inc. (construction materials).
Mr. Crofton has extensive experience in matters of finance, corporate strategy and senior leadership relevant to public companies. He qualifies as an “audit committee financial expert” within the meaning of the SEC regulations.
Dr. Oskowitz has been a director of the Company since January 1999. Dr. Oskowitz has been an assistant professor of obstetrics, gynecology and reproductive biology at Harvard Medical School since 1993. He is a reproductive endocrinologist at, and Director of, Boston IVF, a fertility clinic with which he has been associated since 1986. Dr. Oskowitz is a past President of the Boston Fertility Society.
Dr. Oskowitz has extensive experience as a practicing reproductive endocrinologist and in academic medicine with particular experience in understanding the scientific nature of our business and assisting us in drug development.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the rules issued thereunder, require our directors and executive officers and beneficial owners of more than 10% of the outstanding shares of our Common Stock to file reports of ownership and changes in ownership of our Common Stock with the SEC. Copies of these reports are furnished to Columbia. The Company is required to identify any of those individuals who failed to file such reports on a timely basis. Based solely on our review of the copies of such reports furnished to us and representations from the persons subject to Section 16(a) with respect to our company, we believe that during 2009 all of our executive officers, directors and 10% stockholders complied with the Section 16(a) requirements.
BOARD OF DIRECTORS AND CORPORATE GOVERNANCE
The Board in General
Directors are required by Columbia to be less than age 72 when elected or appointed unless the Board waives that provision with respect to an individual director whose continued service is deemed uniquely important to the Company. The Board can fill vacancies and newly created directorships, as well as amend the bylaws to provide for a greater or lesser number of directors.
The Board is currently comprised of eight members. The members of the Board are Valerie L. Andrews, Edward A. Blechschmidt, Anthony R. Campbell, Frank C. Condella, Jr., James S. Crofton, Stephen G. Kasnet, Robert S. Mills, and Selwyn P. Oskowitz, M.D.
The Role of the Board in Corporate Governance
Pursuant to the Company’s bylaws and the General Corporation Law of the State of Delaware, Columbia’s business and affairs are managed under the direction of the Board. The Board plays an important role in the governance of the Company and in directing management’s overriding objective, the pursuit of long-term growth and increasing stockholder value. The responsibilities of the Board include:
|
•
|
Establishing the Company’s strategic plan;
|
||
|
•
|
Establishing broad corporate policies and reviewing overall performance;
|
||
|
•
|
Overseeing Company management;
|
||
|
•
|
Management succession;
|
||
|
•
|
Review and approval of the annual operating plan prepared by management;
|
||
|
•
|
Monitoring performance in comparison to the operating plan;
|
||
|
•
|
Consideration of topics relevant to the Company’s ability to carry out its strategic plan;
|
||
|
•
|
Review of the Company’s investor relations program; and
|
||
|
•
|
Review and approval of proposed major commitments of corporate resources.
|
Company Management Changes in 2009
In exercising its responsibilities for overseeing Company management and management succession, in July 2009 the Board recruited Lawrence A. Gyenes to be the Company’s Senior Vice President, Chief Financial Officer, and Treasurer, following the June 11, 2009 resignation of James A. Meer. Mr. Gyenes brings to the Company 35 years of financial experience in both privately and publicly held companies and a strong financial and industry transaction background, as well as a demonstrated ability to communicate effectively with the investment community.
In December 2009 the Board announced the appointment, effective December 15, of Frank C. Condella, Jr. as Interim Chief Executive Officer. Mr. Condella has served on the Company’s Board since March 2009. He brings to the Company a strong focus on strategic partnership opportunities, as well as many years of management experience at both small and large specialty pharmaceutical companies - particularly ones with drug delivery and formulation expertise. At that time, Robert S. Mills, who had previously served as Chief Executive Officer agreed to continue to serve on the management team as President and Chief Operating Officer and refocus his talents on the Company’s operations.
Board Leadership Structure and Communication with Independent Directors
Since November 2004, Mr. Kasnet, an independent director, has served as Chairman of the Board and presides at regular meetings of the Board. The Board believes that this structure is appropriate because it results in a balanced leadership, combining an independent Chair with members of management involved in the day-to-day operation of the Company’s business. Although, no single leadership model is right for all companies and at all times, the Board recognizes that depending on the circumstances, other leadership models, such as a combination of the Chairman and Chief Executive Officer with a strong, independent, clearly-defined lead director role, might be appropriate. Accordingly, the Board will periodically review its leadership structure.
Members of the Board are kept informed of the Company’s business through discussions with the Company’s Chief Executive Officer (the “CEO”) and other senior officers, by reviewing materials provided to them, and by participating in meetings of the Board and its committees. The Board’s independent directors meet regularly in executive session without management participation. This encourages open discussion.
Share Ownership Guidelines for Independent Directors
On November 17, 2009, the Board adopted guidelines for independent directors to own and hold, as a minimum, that number of shares of the Company’s Common Stock having a market value of at least two times the director’s annual retainer upon the later of (a) three years after the date of original adoption of the guidelines or (b) three years of becoming a director, Each director should make incremental progress toward the ownership goal over the course of the applicable period. The Board believes that these ownership expectations are an important tool in aligning the interests of the Company’s independent directors with the long-term interests of shareholders.
Communications with the Board
The Board has implemented a process by which the Company’s stockholders can communicate directly with independent directors of the Board. The Company’s stockholders who want to communicate with the Board or any individual director may write to:
Columbia Laboratories, Inc.
354 Eisenhower Parkway
Plaza I, Second Floor
Livingston, NJ 07039
Attn: Board of Directors
- or -
directors@columbialabs.com
The letter should include a statement indicating that the sender is a stockholder of the Company. The Company’s General Counsel will review all stockholder letters to the Board and depending on the subject matter will:
|
|
Promptly forward any letter that deals with the function of the Board or committees of the Board (or is otherwise appropriate for Board attention) to the director or directors to whom it is addressed;
|
|
|
The General Counsel or another member of management will, at each meeting of the Board, present a summary of all letters received since the last meeting that were not forwarded to the Board and will make those letters available to the Board upon request.
Meetings and Attendance During 2009
The Board held 17 meetings in 2009. The Board had, and continues to have, four standing committees, as described below. Each director who served as a director during 2009 participated in 75% or more of the meetings of the Board and the committees on which he or she served during the year ended December 31, 2009. At each regular meeting of the Board, the independent directors meet in private without members of management.
We typically schedule a Board meeting in conjunction with our Annual Meeting of Stockholders and expect that our directors will attend, absent a valid reason such as a schedule conflict. Eight of the nine individuals then serving as directors attended our 2009 Annual Meeting of Stockholders.
Committees of the Board
The Board has the following four committees: (1) Audit Committee, (2) Compensation Committee, (3) Nominating and Corporate Governance Committee, and (4) Scientific Committee. The Board has adopted a written charter for each of the first three committees. The committee charters are posted on our “Investor Relations” website, www.cbrxir.com, which may also be accessed through our corporate website, www.columbialabs.com.
Below is a description of the duties and composition of each standing committee of the Board. Directors hold committee memberships for a term of one year.
Audit Committee
The primary function of the Audit Committee is to oversee Columbia’s reporting processes on behalf of the Board and to report the results of its activities to the Board. The Audit Committee’s primary duties and responsibilities are to:
|
·
|
Serve as an independent and objective party to monitor the Company’s financial reporting process, including the review of the financial reports and other financial information provided by the Company to governmental or regulatory bodies, the public or other users, and internal control systems (including any material weaknesses, significant deficiencies and significant changes in internal controls reported to the Audit Committee by the outside auditor or management);
|
|
·
|
Approve the engagement of the Company’s independent registered public accounting firm;
|
|
·
|
Review and appraise the audit efforts of the Company’s independent registered public accounting firm;
|
|
·
|
Provide an open avenue of communication among the Company’s independent registered public accounting firm and financial and senior management of the Company;
|
|
·
|
Review financial press releases;
|
|
·
|
Review and address conflicts of interests of the Company’s directors and executive officers; and
|
|
·
|
Monitor, review, and recommend actions relating to transactions and dealings with related parties.
|
The Audit Committee acts pursuant to the Audit Committee Charter adopted by the Board on May 12, 2004.
While the Audit Committee has the powers and responsibilities set forth in its charter, it is not the responsibility of the Audit Committee to plan or conduct audits or to determine that Columbia’s financial statements are complete and accurate or are in compliance with generally accepted accounting principles. This is the responsibility of management and the Company’s independent registered public accounting firm.
All of the members of the Audit Committee have been determined by the Board to be independent within the meaning of the applicable NASDAQ Marketplace Rules and Section 10A(m)(3) of the Exchange Act. The Company has identified James S. Crofton as an “audit committee financial expert” as that term is defined in applicable regulations of the SEC.
Members: Mr. Crofton (chair), Mr. Campbell, and Mr. Kasnet Meetings last year: five
Compensation Committee
Information about the Compensation Committee can be found in Item 11 below under the heading “Compensation Discussion and Analysis.”
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee provides assistance to the Board in identifying, screening, and recommending qualified candidates to serve as directors of Columbia. The Committee also oversees matters of corporate governance and provides counsel to the Board with respect to Board organization, membership, and function. The Nominating and Corporate Governance Committee acts pursuant to the Nominating and Corporate Governance Committee Charter adopted by the Board on August 16, 2004.
The Nominating and Corporate Governance Committee is responsible for proposing to the Board nominees for election or reelection to the Board based upon recommendations from the Chairman, the CEO, other Board members, and Columbia stockholders.
Board candidates are considered by the Nominating and Corporate Governance Committee on a case-by-case basis. A candidate for election to the Board must possess the ability to apply good business judgment and must be in a position to properly exercise his or her duties of loyalty and care in his or her representation of the interests of stockholders. Candidates should also exhibit proven leadership capabilities, high integrity and experience with a high level of responsibilities within their chosen fields, and have the ability to quickly grasp complex principles of business, finance, and transactions regarding the Company’s industry. In general, preferred candidates will currently hold, or have recently held, an established executive level position and have extensive experience in business, finance, law, science, research, or government. The Nominating and Corporate Governance Committee will consider these criteria for nominees identified by the Committee, by stockholders, or through other sources. When current Board members are considered for nomination for reelection, the Nominating and Corporate Governance Committee will take into consideration their prior Board contributions and performance as well as the composition of the Board as a whole, including whether the Board reflects the appropriate balance of independence, sound judgment, business specialization, technical skills, diversity, and other desired qualities. The Nominating and Corporate Governance Committee will make a preliminary assessment of each proposed nominee based upon the résumé and biographical information, an indication of the individual’s willingness to serve, and other relevant information. This information will be evaluated against the criteria set forth above and the specific needs of the Company at that time. Based upon a preliminary assessment of the candidate(s), those who appear best suited to meet the needs of the Company may be invited to participate in a series of interviews, which are used as a further means of evaluating potential candidates. On the basis of information learned during this process, the Nominating and Corporate Governance Committee will determine which nominee(s) to submit for election. The Nominating Committee will use the same process for evaluating all nominees, regardless of the original source of the nomination.
The Nominating and Corporate Governance Committee and the Board believe that diversity along multiple dimensions, including opinions, skills, perspectives, personal and professional experiences and other differentiating characteristics, is an important element of its nomination recommendations. The Board considers each nominee in the context of the Board as a whole, with the objective of assembling a Board that can best maintain the success of the Company’s business. Although the Board does not have a formal diversity policy, the Nominating and Corporate Governance Committee and the Board periodically review the Board’s membership in light of the Company’s business and strategic objectives, consider whether the directors possess the requisite skills, experience and perspectives to oversee the Company in achieving those goals, and may seek additional directors from time to time as a result of its considerations.
The Nominating and Corporate Governance Committee has adopted a policy that does not permit a non-employee director to be nominated for election as a director at the next Annual Meeting of Stockholders if the director will attain the age of 72 during the term for which he or she would be nominated.
All of the members of the Nominating and Corporate Governance Committee have been determined by the Board to be independent within the meaning of the applicable NASDAQ Marketplace Rules.
Members: Mr. Kasnet (chair), Ms. Andrews, Mr. Blechschmidt, and Dr. Oskowitz. Meetings last year: two
Scientific Committee
The Scientific Committee is responsible for reviewing and advising Company management on the Company’s research and development programs.
All of the members of the Scientific Committee have been determined by the Board to be independent within the meaning of the applicable NASDAQ Marketplace Rules.
Members: Dr. Oskowitz (chair), Mr. Blechschmidt, and Mr. Campbell Meetings last year: three
Code of Business Conduct and Ethics
The Board has adopted a Code of Business Conduct and Ethics that applies to all of our directors, executive officers (including our CEO and our Chief Financial Officer and principal accounting officer), and employees of the Company. The Code was filed as Exhibit 14 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. Our Code of Business Conduct and Ethics is posted on our “Investor Relations” website, www.cbrxir.com, which may also be accessed through our corporate website: www.columbialabs.com. We will provide an electronic or paper copy of this document free of charge upon request. If, in the future, we should amend our Code of Business Conduct and Ethics or grant a waiver to our CEO or our Chief Financial Officer and principal accounting officer or controller with respect to our Code of Business Conduct and Ethics, then we will post the amendment or a description of the waiver on our “Investor Relations” website, www.cbrxir.com, which may also be accessed through our corporate website: www.columbialabs.com or in a report on Form 8-K.
Item 11. Executive Compensation
COMPENSATION DISCUSSION AND ANALYSIS
Introduction
Our Compensation Committee (which is referred to in this Form 10-K/A as the “Committee” or as the “Compensation Committee”) provides assistance to the Board in fulfilling its responsibility to oversee and participate in the creation and administration of executive compensation programs and practices. Responsibilities of the Committee include, among other things:
|
·
|
Review and determination of the annual salary of the Company’s CEO and other officers;
|
|
·
|
Review and approval of the Company’s management incentive compensation policies and programs; and
|
|
·
|
Review and approval of equity compensation programs for the Company’s employees, directors and consultants, including grants of options, restricted stock and other awards thereunder.
|
The Committee acts pursuant to the Compensation Committee Charter adopted by the Board of Directors on March 11, 2009. This Charter can be found on our “Investor Relations” website, www.cbrxir.com, which may also be accessed through our corporate website, www.columbialabs.com. Two Directors served on the Compensation Committee for the full year 2009: Valerie L. Andrews (Chair) and Edward A. Blechschmidt. In addition, Denis M. O’Donnell, M.D. served on the Committee until the 2009 Annual Meeting of Stockholders when he did not stand for reelection. James S Crofton was elected to the Committee at the Board’s meeting on May 21, 2009, and served for the remainder of the year, and Frank C. Condella, Jr. served on the Committee from his election to the Board on March 10, 2009, until his appointment, effective December 15, 2009, as the Interim Chief Executive Officer of the Company. Each of these individuals has been determined by the Board to be independent within the meaning of the applicable NASDAQ Marketplace Rules.
The Committee meets at regularly scheduled times during the year and on an ad hoc basis as business needs necessitate. In 2009, the Committee held six meetings. In addition to the assistance provided by the Company’s management, the Committee has the authority under its charter to retain independent consultants and legal counsel to provide guidance on matters related to executive compensation and other related matters as directed by the Committee. In 2009, the Committee retained counsel to advise it on Mr. Condella’s employment agreement as Interim CEO, but did not retain consultants.
Risk Assessment
The Compensation Committee regularly undertakes a qualitative assessment of the extent to which the Company’s compensation program may aggravate or mitigate risk-taking behavior by executive officers. The Compensation Committee concluded that the Company’s executive compensation program maintains an appropriate balance between risks and rewards, particularly in light of the actions taken by the Compensation Committee to help mitigate risk through the establishment of stock ownership guidelines.
Stock Ownership Guidelines for Named Executive Officers
On November 17, 2009, in order to preserve the linkage between the interests of the Company’s executive officers and those of shareholders, the Compensation Committee recommended and the Board adopted guidelines for executive officer share ownership that are consistent with competitive practice and responsible corporate governance. Executive officers will be expected to establish a significant level of direct ownership. The CEO is expected to own and hold that number of shares having a value of at least two times base salary, and the other executive officers are expected to own and hold that number of shares having a value of at least the amount of the base salary. The CEO and each named executive officer have five years to comply with the guideline. The Compensation Committee believes that these ownership expectations are an important tool in aligning the interests of the Company’s executives with the long-term interests of shareholders.
Executive Compensation Philosophy and Objectives
Our compensation program for the individuals named in the Summary Compensation Table (the “named executive officers”) is designed and implemented based on our pay-for-performance compensation philosophy. Our named executive officers for 2009 were Frank C. Condella Jr., Interim CEO (“CEO”), Robert S. Mills, President and COO (“COO”), Lawrence A. Gyenes, Senior Vice President, Chief Financial Officer and Treasurer (“CFO”), Michael McGrane, Senior Vice President, General Counsel and Secretary (“GC”), and James A. Meer, who resigned effective June 11, 2009. Mr. Condella became Interim CEO on December 15, 2009. Mr. Gyenes joined the Company as CFO on July 15, 2009.
We strive to adhere to this philosophy by differentiating the pay and rewards of our executive officers based on their demonstrated performance and potential to contribute to the long-term success of the Company. Competing for talent in the rapidly changing and increasingly competitive pharmaceutical industry is both challenging and critical to our success. We need and want the best people to be excited and motivated to work at Columbia and to understand that their rewards are driven by the Company’s performance and by their individual contributions to the Company’s performance. The quality of the Company’s talent is a key component of long-term stockholder value.
The Company has entered into employment agreements with its executive officers because we believe that they are a fair and effective way to maintain focus on our business in the face of market and other volatility in our industry. The agreements with Messrs. Mills, Gyenes, and McGrane provide for severance and change of control payments. As Interim CEO, Mr.Condella’s employment agreement does not have severance and change of control provisions.
We have established a total rewards framework that supports our compensation philosophy through the following objectives:
|
·
|
to afford our executives a competitive total rewards opportunity comparable to organizations with which we compete for executive talent;
|
|
·
|
to allow us to attract and retain executives who can perform and succeed in our fast-paced and challenging environment; and
|
|
·
|
to deliver pay in a cost efficient manner that aligns employees’ rewards with stockholders’ long-term interests.
|
Compensation Program Elements and Pay Level Determination
The Committee undertakes discussions and assessments of compensation related programs and the performance of management throughout the year. Early in the Company’s fiscal year, the Committee reviews and recommends base salaries, annual cash incentive bonus payments, and equity incentives for all executive officers based on the prior year’s performance, which are then approved by all independent (non-employee) directors.
As part of the review process, the CEO provides to the Committee an individual assessment of the major accomplishments of each executive officer over the prior year and recommends compensation actions for each executive officer. The Committee evaluates the performance of our CEO and recommends to the Board for its approval all compensation elements and amounts to be awarded to our CEO. Our CEO, who is a member of the Board of Directors, does not participate in Board decisions relating to his own compensation. The key metrics we use to measure the performance of our executive officers can be grouped in the following categories:
|
·
|
Financial – We evaluate measures of our financial performance, including revenue growth and other matters such as expense management.
|
|
·
|
Strategic – We monitor the performance of our executive officers in furthering the strategic success of the Company. This includes achieving targeted revenues, completing recruitment in a clinical study, ensuring talent is effectively managed, and evaluating and establishing new partnership opportunities.
|
|
·
|
Operational – We include operational measures in our determination of success, including the quality of our leadership development and teamwork, and effective recruitment and retention of talented employees.
|
The Committee considers the recommendation of the CEO and other information (including each executive’s significant accomplishments, external competitiveness, Company performance, progress towards strategic objectives, and internal equity among executive officers) and applies its knowledge and discretion to determine the compensation for each executive officer.
To understand external competitiveness, the Committee compares each element of total compensation against a peer group of publicly traded pharmaceutical and biotechnology companies. Company management recommends a list of companies as the peer group, factoring stage of development, types of products sold or developed, market capitalization, revenues, and number of employees. The Committee reviews the list of companies and determines the peer group composition as it deems appropriate and reasonable. For 2009, the Committee determined the final peer group to consist of 13 publicly-traded companies. The Committee considers both the mean and median base salaries, annual incentive bonuses, and equity awards to executives of the peer group of companies in relation to the Company’s executive compensation, as well as the background, education, and experience of each named executive officer.
Public Company Peer Group
The following companies comprise our public company peer group.
Acadia Pharmaceuticals, Inc.
Adolor Corp.
Ariad Pharmaceuticals, Inc.
ArQule, Inc.
Biosante Pharmaceuticals, Inc.
Cytokinetics, Incorporated
DepoMed, Inc.
GenVec Inc.
MiddleBrook Pharmaceuticals, Inc.
Neurocrine Biosciences, Inc.
SuperGen, Inc.
Unigene Laboratories Inc.
Vical, Inc.
Components of our Executive Compensation Program
Total compensation for our named executive officers is a mix of cash and equity awards. Base salaries and discretionary annual incentive bonuses are paid in cash. Long term incentives consist of equity awards, including stock options and restricted stock awards. Indirect compensation consists of standard employee benefits.
Each component of compensation and selected benefits is summarized in the following table.
|
Component
|
Purpose/Description
|
|
Base salary
|
Competitive fixed income for performance of day-to-day responsibilities, paid semi-monthly.
|
|
Annual incentive bonus
|
Rewards achievement of annual goals that support short-term (annual) business objectives, paid in cash after the relevant fiscal year.
|
|
Equity compensation
|
Fosters a culture of ownership, aligns compensation with stockholder interests, and promotes long-term retention with the Company. Consists primarily of the following equity-based awards.
|
|
· Stock options
|
Provides compensation tied to the price of our Common Stock. The awards generally vest in increments of 25% on each of the first four anniversaries of the grant date and have no value if our Common Stock price is below the exercise price.
|
|
· Restricted stock
|
Provides compensation tied to the price of our Common Stock. Supports an ownership mentality, encouraging our executives to act in a manner consistent with the long-term interests of the Company and its stockholders. Restricted stock grants vest over time.
|
|
Benefits
|
Standard employee benefits, such as health, dental, vision, disability, and life insurance.
|
|
Retirement Benefits
|
Standard employee 401(k) plan. The Company matches 50% of the first 4% of contributions. The Company suspended the matching contribution in February 2009 and reinstated it on January 1, 2010.
|
|
Perquisites
|
None
|
While the general mix of each component is considered in the design of our total compensation program, the Committee does not target a specific mix of pay either in its program design or in its compensation determinations. By design, our executive officers have more variability in their compensation than non-executives, to more closely tie their compensation to the Company’s overall performance. Company management also provides the Committee a tally sheet for each executive officer that sets forth all components of the executive’s compensation, including salary, cash bonus, value of equity compensation, the dollar value to the executive and cost to the Company of all benefits, and the actual projected payout obligation under potential severance and change-in-control scenarios. The tally sheets show the impact of the proposed award or payment on each compensation component and on aggregate compensation. The Committee makes all executive compensation recommendations and decisions with reference to the provided tally sheets, with a goal of establishing and administering an overall executive compensation program that is fair and reasonable both to our executives and our stockholders.
Base Salary
We pay our executive officers base salaries to provide a baseline level of compensation that is both competitive with the external market and commensurate with each executive officer’s past performance, experience, responsibilities, and skills. The base salary levels of our executive officers may be increased from time to time to recognize external competitive compensation levels, internal pay equity, and individual contributions and performance.
Changes in Base Salaries for 2010
Generally, the Committee compares our executive officers with base salaries for comparable positions in the peer group companies. In connection with determining the base salary levels for the Company’s executive officers, the Committee compares their total cash compensation (i.e. base salary plus target annual bonus) to total cash compensation for comparable positions in the peer group companies. As a result of the December 2009 appointment of Mr. Condella as the Interim Chief Executive Officer and Mr. Mills as President and Chief Operating Officer, Mr. Mills’ base salary for 2010 was decreased by 10% to reflect the change in his position. Mr. Gyenes was hired on July 15, 2009, and therefore the Committee did not recommend an increase in his base salary for 2010. The Committee recommended a 1.5% cost of living increase in Mr. McGrane’s base salary from the previous year to $300,000.
The table below shows annual 2009 and 2010 salaries for each named executive officer who was employed by the Company as of the end of the 2009 fiscal year.
|
Name
|
Position
|
Ending 2009 Salary
|
2010 Salary
|
Percentage (%) Increase
(Decrease)
|
|
Frank C. Condella Jr.
|
Interim CEO
|
$375,000
|
$375,000
|
none
|
|
Robert S. Mills
|
President & Chief Operating Officer
|
$390,000
|
$350,000
|
(10%)
|
|
Lawrence Gyenes
|
Senior Vice President, Chief Financial Officer & Treasurer
|
$325,000
|
$325,000
|
none
|
|
Michael McGrane
|
Senior Vice President, General Counsel & Secretary
|
$295,700
|
$300,000
|
1.5%
|
2009 Annual Cash Incentive Bonus
We maintain an annual cash incentive program (the “Incentive Plan”), the purpose of which is to motivate and reward the attainment of annual Company and individual performance. For all participants, annual incentive opportunities, which are expressed as a percentage of base salary, can range from 0% to 150% of targeted levels, depending on the degree of attainment of pre-established Company goals for that particular year. Bonus targets for Messrs. Mills, Gyenes, and McGrane are 50%, 40%, and 40% of salary, respectively, pursuant to their individual employment agreements. As Interim CEO, Mr.Condella does not participate in the Incentive Plan.
Actual payouts under the Incentive Plan are recommended by the Committee to the Board based on achievement of corporate goals, overall individual performance, and the broad discretion of the Committee and Board. Our corporate goals are jointly established at the beginning of each year by management and the Committee and are approved by the Committee and the Board. Once the corporate goals are finalized and approved by the Board, they are clearly communicated to executives. Executives are aware of the overall bonus program, targets, annual goals, and performance measures that impact the annual bonus payout.
The extent to which corporate goals are achieved is assessed by the Committee with input from the CEO and other members of management. The Committee considers the following in assessing cash bonuses:
|
·
|
The extent to which corporate goals are achieved or exceeded;
|
|
·
|
The overall success of the Company throughout the year as determined by factors such as progress in key programs, execution of the strategic plan, and share price; and
|
|
·
|
Positive or negative events occurring throughout the year unrelated to pre-established corporate goals.
|
For the fiscal year ended December 31, 2009, the Compensation Committee recommended a 45% achievement award for 2009 under the Incentive Plan. Actual. The following table summarizes the Company’s goals and results for 2009 and the Committee’s assessments of goal achievements.
|
Company Goal
|
Weight
|
Results
|
Achievement
|
|
Achieve 2009 targeted revenue and earnings before interest, taxes, depreciation, amortization, and stock compensation expense.
|
30%
|
Goal partially met by earnings before interest, taxes, depreciation, amortization, and stock compensation expense
|
20%
|
|
Complete enrollment in the PREGNANT Study by
December 31, 2009.
|
30%
|
Goal not met
|
0%
|
|
Ensure Company’s capital structure is sufficient for ongoing activities.
|
20%
|
Goal partially met through financing activities
|
5%
|
|
Advance business development initiatives.
|
10%
|
Goal met
|
10%
|
|
Strengthen Financial Operations and Investor Relations function.
|
10%
|
Goal met
|
10%
|
|
Total
|
100%
|
45%
|
The cash bonus awards to the named executive officers for 2009 performance, other than Mr. Condella, who was not eligible, were determined as follows:
|
Name
|
Position
|
2009 Target Bonus
|
Company Performance Factor
|
Individual Performance Factor
|
2009 Bonus
|
|
Robert S. Mills
|
President & Chief Operating Officer
|
$195,000
|
45%
|
90%
|
$78,000
|
|
Lawrence Gyenes
|
Senior Vice President, Chief Financial Officer & Treasurer
|
$130,000
|
45%
|
140%
|
$35,7501
|
|
Michael McGrane
|
Senior Vice President, General Counsel & Secretary
|
$118,280
|
45%
|
100%
|
$53,226
|
1 Mr. Gyenes’ 2009 bonus was prorated from his July 15, 2009 employment date.
Equity Compensation
An equity compensation program is provided to all employees to foster a culture of ownership, align compensation with stockholder interests, and promote long-term retention with the organization. Each year the Committee determines the types of awards to be used for equity compensation. In doing so, the Committee considers the ability of each type of award to achieve key compensation objectives (such as employee retention, motivation, and attraction), the needs of the business, competitive market practices, dilution, and expense constraints, as well as tax and accounting implications.
The exercise price for each stock option awarded under the Columbia Laboratories, Inc. 1996 Long-Term Performance Plan (the “1996 Plan”) and the Columbia Laboratories, Inc. 2008 Long-Term Incentive Plan (the “2008 Plan”) is equal to or greater than the fair market value (i.e. the average of the high and low prices for the 1996 Plan and the closing price for the 2008 Plan) for the Company’s Common Stock on the NASDAQ Global Market on the date of grant. The 2008 Plan was adopted by the Stockholders at the 2008 Annual Meeting and supplants the 1996 Plan for all grants following the adoption of the 2008 Plan. We refer to the 2008 Plan and the 1996 Plan, collectively, as our “Long-term Performance Plans.” Stock option grants and restricted stock awards are made at Board and Committee meetings that are generally scheduled a year in advance and scheduling decisions are made without regard to anticipated financial reporting dates or other major announcements by the Company.
In general, newly hired employees, including executive officers, are granted options and/or restricted stock effective on the first day of employment, with the options having an exercise price set at the fair market value (i.e. the closing price) for our Common Stock on the NASDAQ Global Market on the employment start date. The employees’ start dates are scheduled without regard to anticipated financial reporting dates or other major announcements by the Company. Mr. Condella was granted 100,000 options on December 11, 2009 as part of his employment agreement when he joined Columbia as Interim Chief Executive Officer, effective December 15, 2010. Mr. Gyenes was granted 125,000 options on July 15, 2009 as part of his employment agreement when he joined the Company as CFO on July 15, 2009.
We have historically made an annual grant of employee stock options at the time of the annual review of each executive’s performance, usually in late February or early March. In 2010 the Committee decided to defer the recommendation of an annual grant of stock options for all employees during the first six months of the year due to the pending Watson Transaction. Generally, option grants vest 25% on each of the first four anniversaries of the grant date to provide an incentive for employees to remain with the Company and to increase shareholder value. Pursuant to our stock option plans, the closing of the Watson Transaction would vest all stock options and restricted shares. As a result, the objective for granting equity compensation as a long term incentive for increasing shareholder value and retaining talent would not be achieved for awards made prior to the closing of the Watson Transaction. The Committee will reconsider an annual grant of stock options and restricted stock following the Special Meeting of Stockholders at which the stockholders will vote on the Watson Transaction.
Benefits and Perquisites
All named executive officers are offered the standard benefit plans that are offered to other full-time employees of the Company. These standard benefits include health, dental, vision, life insurance, and both short- and long-term disability. In addition, the Company has provided a 401(k) plan and generally a 50% match of contributions up to 4% of salary. The Company suspended the matching contributions to the plan in February 2009, and reinstated it on January 1, 2010. Mr. Condella receives a payment of $2,000 per month in lieu of receiving group medical, dental and vision benefits that are available to the other executive officers. The Company does not provide perquisites for our executive officers.
Termination or Change in Control
The Company has entered into employment agreements with each of our named executive officers in which each executive officer has agreed to certain confidentiality and non-competition provisions. The employment agreements for Messrs. Mills, Gyenes and McGrane contain severance arrangements that provide for payments and other benefits if the officer’s employment is involuntarily terminated or not renewed by the Company. Mr. Condella’s agreement as Interim CEO does not provide a severance arrangement.
The employment agreements for Messrs. Mills, Gyenes and McGrane also incorporate an executive change in control severance agreement that provides payments to the executive under certain circumstances. Payments under the agreements are subject to a “double trigger,” meaning payments require both a change in control and a termination by the Company without cause or by the executive for “good reason.” We believe agreements of this type can be important components of our effort to recruit and retain senior executives, particularly for companies at our stage of development and in our relatively high-risk industry. Mr. Condella’s employment agreement does not contain an executive change in control severance provision.
A further discussion of the terms and payouts under each of these agreements is set forth below under the heading Potential Payments upon Termination or Change in Control.
Tax Considerations
Section 162(m) of the Internal Revenue Code limits to $1 million the deductibility for federal income tax purposes of annual compensation paid by a publicly held company to its chief executive officer and its other named executive officers (other than its chief financial officer), unless certain conditions are met. To the extent feasible, we structure executive compensation to preserve deductibility for federal income tax purposes. In this regard, our equity incentive plans are designed to preserve, to the extent otherwise available, the deductibility of income realized pursuant to these plans. Nevertheless, we retain the flexibility to authorize compensation that may not be deductible if we believe it is in the best interest of the Company.
Compensation Committee Report
The Compensation Committee evaluates and establishes compensation for executive officers and oversees the Company’s compensation policies, the Long-term Performance Plans, Incentive Plan, and other benefit programs. Management has the primary responsibility for the Company’s financial statements and reporting process, including the disclosure of executive compensation. We have reviewed and discussed with management this Compensation Discussion and Analysis on Form 10-K/A. The Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Form 10-K/A for filing with the SEC.
Valerie L. Andrews, Chair
Edward A. Blechschmidt
James S. Crofton
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
|
Name and Principal Position
|
Year
|
Salary
|
Stock
Awards1
|
Option
Awards2
|
Non-Equity Incentive Plan Compensation3
|
Bonus4
|
Change in Pension Value and Nonqualified Deferred Compensation Earnings5
|
All other compen-sation6
|
Total
|
|||||||||||||||||||
|
Frank C. Condella Jr.,6
Interim Chief Executive Officer
|
2009
|
$
|
17,067
|
$
|
-
|
$
|
61,425
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
1,092
|
$
|
79,584
|
|||||||||||
|
Robert S. Mills,
President and Chief Operating Officer
|
2009
|
$
|
390,000
|
$
|
31,920
|
$
|
130,806
|
$
|
78,000
|
$
|
-
|
$
|
-
|
$
|
1,300
|
$
|
632,026
|
|||||||||||
|
2008
|
$
|
383,933
|
$
|
55,920
|
$
|
263,837
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
4,600
|
$
|
708,290
|
||||||||||||
|
2007
|
$
|
351,332
|
$
|
28,000
|
$
|
281,220
|
$
|
123,760
|
$
|
-
|
$
|
-
|
$
|
4,500
|
$
|
788,812
|
||||||||||||
|
Lawrence A. Gyenes,7
Senior Vice President, Chief Financial Officer, and Treasurer
|
2009
|
$
|
150,208
|
$
|
-
|
$
|
102,584
|
$
|
35,750
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
288,542
|
|||||||||||
|
James A. Meer,8
Senior Vice President, Chief Financial Officer, and Treasurer
|
2009
|
$
|
138,022
|
$
|
23,275
|
$
|
100,907
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
372,167
|
$
|
634,371
|
|||||||||||
|
2008
|
$
|
272,500
|
$
|
40,775
|
$
|
181,090
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
4,600
|
$
|
498,965
|
||||||||||||
|
2007
|
$
|
260,000
|
$
|
19,600
|
$
|
196,854
|
$
|
70,000
|
$
|
-
|
$
|
-
|
$
|
3,444
|
$
|
549,898
|
||||||||||||
|
Michael McGrane,
Senior Vice President, General Counsel, and Secretary
|
2009
|
$
|
295,700
|
$
|
23,275
|
$
|
100,907
|
$
|
53,226
|
$
|
-
|
$
|
-
|
$
|
986
|
$
|
474,094
|
|||||||||||
|
2008
|
$
|
294,867
|
$
|
40,775
|
$
|
182,305
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
4,600
|
$
|
522,547
|
||||||||||||
|
2007
|
$
|
288,883
|
$
|
22,400
|
$
|
224,976
|
$
|
76,000
|
$
|
-
|
$
|
-
|
$
|
3,911
|
$
|
616,170
|
||||||||||||
|
1
|
This column represents the grant date fair values for restricted stock granted in 2007, 2008 and 2009 to the named executive officers. The 2007 and 2008 stock award values were recalculated from amounts shown in prior annual reports to reflect their grant date fair values, as required by SEC rules effective for 2010. Pursuant to SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. The Company estimates that no forfeitures will occur. During the year ended December 31 2009, 37,625 restricted shares were forfeited for executive officers. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report on Form 10-K (Note 9, Share-Based Payments). These amounts reflect the Company’s accounting expense for these awards, and do not correspond to the actual value that will be recognized by the named executive officers.
|
|
2
|
This column represents the grant date fair values of the stock options awarded in 2009, 2008 and 2007, respectively, The 2007 and 2008 option award values were recalculated from the amounts shown in prior annual reports to reflect the grant date fair value, as required by SEC rules effective for 2010. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2009 Annual Report (Note 9, Share-Based Payments). The Company estimates a 10% rate for forfeitures; during the year ended December 31 2009, 300,500 options were forfeited for executive officers.
|
|
3
|
The Company does not pay a bonus separate from the awards under the Company’s Incentive Plan.
|
|
4
|
The Company does not have a pension plan and does not offer nonqualified deferred compensation.
|
|
5
|
This column represents the Company’s matching contributions to Messrs. Mills, McGrane and Meer's 401(k) savings account of 50% of the first 4% of pay. Mr. Condella receives an addition payment of $2,000 per month in lieu of his receiving group medical, dental and vision benefits which are available to other executive employees. The Company does not provide any additional perquisites for our executive officers. The Company does not pay other compensation and does not offer other perquisites. Mr. Meer received a severance payment from the Company of $371,250, due to his resignation from the Company effective June 11, 2009.
|
|
6
|
Mr. Condella joined the Company on December 15, 2009.
|
|
7
|
Mr. Gyenes joined the Company on July 15, 2009.
|
|
8
|
Mr. Meer left the Company on June 11, 2009.
|
2009 Grants of Plan-Based Awards Table
The following table provides information about equity and non-equity awards granted to the named executive officers in 2009.
|
Estimated Possible Payouts Under Non-Equity Incentive Plan Awards1
|
All Other Stock Awards: Number of Shares of Stock or Units (#)
|
All Other Option Awards: Number of Securities Underlying Options (#)
|
Exercise or Base Price of Option Awards ($/share)2
|
Grant Date Fair Value of Stock and Option Awards3
|
||||
|
Name of Executive
|
Grant Date
|
Threshold
|
Target
|
Maximum
|
||||
|
Mr. Condella,
|
||||||||
|
Interim Chief Executive Officer6
|
12/11/2009
|
0
|
0
|
0
|
0
|
100,0005
|
$0.87
|
$61,420
|
|
Mr. Mills,
President &
Chief Operating
Officer
|
0
|
$195,000
|
$292,500
|
|||||
|
3/11/2009
|
24,0004
|
$31,920
|
||||||
|
3/11/2009
|
105,0005
|
$1.33
|
$98,104
|
|||||
|
3/3/2009
|
35,0005
|
$1.33
|
$32,701
|
|||||
|
Mr. Meer,
Senior Vice President &
Chief
Financial
Officer
|
0
|
$96,250
|
$144,375
|
|||||
|
3/11/2009
|
17,5004
|
$23,275
|
||||||
|
3/11/2009
|
108,0005
|
$1.33
|
$100,907
|
|||||
|
3/11/2009
|
||||||||
|
Mr. Gyenes,
Senior Vice President &
Chief Financial Officer
|
0
|
$130,000
|
$195,000
|
|||||
|
7/15/2009
|
125,0005
|
$1.17
|
$102,585
|
|||||
|
Mr. McGrane,
Senior Vice President &
General Counsel
|
0
|
$118,280
|
$177,420
|
|||||
|
3/11/2009
|
17,5004
|
$23,275
|
||||||
|
3/11/2009
|
81,0005
|
$1.33
|
$75,680
|
|||||
|
3/11/2009
|
27,0005
|
$1.33
|
$25,227
|
|||||
1These columns show the range of possible payouts for 2009 under the Incentive Plan as described in the section titled “2009 Annual Cash Incentive Bonus” in the “Compensation Discussion and Analysis” section above. Messrs. Mills, Gyenes and McGrane received payouts for 2009 under the Incentive Plan. The actual payouts for these named executive officers are reported in the “Non-Equity Incentive Plan Compensation” column of the “Summary Compensation Table” above.
2Exercise price is the closing price on the NASDAQ Global Market on the date of grant.
3 These amounts represent the grant date fair value of awards for fiscal years 2009, 2008 and 2007, computed in accordance with the Financial Accounting Standards Board Accounting Standards Codification Topic 718. See Note 8 to the consolidated financial statements in our Form 10-K for the fiscal year ended December 31, 2009 regarding assumptions underlying the valuation of equity awards. To see the value actually received by the named executive officers in fiscal year 2009, see the Option Exercises and Stock Vested in 2009 table.
4Represents restricted stock awards granted under the 2008 Plan, of which 25% vests on each of the first, second, third, and fourth anniversaries of the grant date. The shares are held by the Company in escrow until vested. The shares may not be sold, transferred, assigned, hypothecated, pledged, encumbered or otherwise disposed of, whether voluntarily or by operation of law, at any time before they vest. The shares vest immediately if the grantee dies or there is a change in control of the Company, and are canceled upon any other termination of service by the grantee to the Company. The grantee is responsible for any income or other taxes due with respect to the shares, including on account of the vesting of the shares. The grantee may elect to have shares withheld by the Company upon vesting for taxes.
5Represents stock option awards granted under the 2008 Plan that have a seven year term, of which 25% vests on each of the first, second, third, and fourth anniversaries of the grant date.
6 Pursuant to his employment agreement, Mr. Condella does not participate in the Company’s Incentive Plan.
Outstanding Equity Awards at 2009 Fiscal Year-End
The following table provides information on the holdings of stock options and stock awards by the named executive officers as of December 31, 2009. The table includes unexercised and unvested option awards and unvested stock awards. Each equity grant is shown separately for each named executive officer. The vesting schedule for each grant is shown following this table, based on the option or stock award grant date. The market value of the stock awards is based on the closing market price of Columbia Common Stock as of December 31, 2009, which was $1.08.
|
Option Awards1
|
Stock Awards2
|
|||||||||||
|
Name of Executive
|
Option Grant Date
|
Number of Securities Underlying Unexercised Options
Exercisable
|
Number of Securities Underlying Unexercised Options
Un-Exercisable
|
Option Exercise Price
|
Option Expiration Date
|
Stock Award Grant Date
|
Number of Shares or Units of Stock That Have Not Vested
|
Market Value of Shares or Units of Stock That Have Not Vested
|
Equity Incentive Plan Awards: Number of Unearned Shares or Units That Have Not Vested
|
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares or Units That Have Not Vested
|
||
|
Mr. Condella Interim CEO
|
12/11/2009
|
100,000
|
$0.87
|
12/11/2016
|
||||||||
|
Mr. Mills
President &
Chief
Operating
Officer
|
05/30/2001
|
50,000
|
-
|
$5.900
|
05/30/2011
|
|||||||
|
10/18/2001
|
18,750
|
-
|
$4.140
|
10/18/2011
|
||||||||
|
01/18/2002
|
18,750
|
-
|
$3.675
|
01/18/2012
|
||||||||
|
03/14/2003
|
39,375
|
-
|
$2.975
|
03/14/2013
|
||||||||
|
10/28/2003
|
30,000
|
-
|
$10.675
|
10/28/2013
|
||||||||
|
05/12/2004
|
60,000
|
-
|
$4.050
|
05/12/2014
|
||||||||
|
02/25/2005
|
206,350
|
-
|
$2.050
|
02/25/2015
|
||||||||
|
05/17/2005
|
65,000
|
-
|
$2.750
|
05/17/2012
|
||||||||
|
05/15/2006
|
2,5000
|
$2,700
|
||||||||||
|
05/15/2006
|
37,500
|
12,500
|
$4.335
|
05/15/2013
|
||||||||
|
|
02/28/2007
|
10,000
|
$10,800
|
|||||||||
|
02/28/2007
|
225,000
|
75,000
|
$1.420
|
02/28/2014
|
||||||||
|
03/03/2008
|
32,500
|
97,500
|
$2.400
|
03/03/2015
|
||||||||
|
03/03/2008
|
11,250
|
33,750
|
$3.000
|
03/03/2015
|
||||||||
|
03/11/2009
|
140,000
|
$1.330
|
03/11/2016
|
|||||||||
|
03/11/2009
|
24,000
|
$25,920
|
||||||||||
|
Mr. Gyenes
Senior Vice President & Chief
Financial
Officer
|
07/15/2009
|
125,000
|
$1.170
|
07/15/2016
|
||||||||
|
Mr. McGrane
Senior Vice President &
General Counsel
|
01/02/2002
|
125,000
|
$3.435
|
01/02/2012
|
||||||||
|
01/02/2002
|
50,000
|
$5.935
|
01/02/2012
|
|||||||||
|
03/14/2003
|
43,875
|
$2.975
|
03/14/2013
|
|||||||||
|
05/12/2004
|
50,000
|
$4.050
|
05/12/2014
|
|||||||||
|
02/25/2005
|
12,435
|
$2.050
|
02/25/2015
|
|||||||||
|
05/17/2005
|
55,000
|
$2.750
|
05/17/2012
|
|||||||||
|
05/15/2006
|
1,719
|
$1,857
|
||||||||||
|
05/15/2006
|
20,625
|
6,875
|
$4.335
|
05/15/2013
|
||||||||
|
02/28/2007
|
8,000
|
$8,640
|
||||||||||
|
02/28/2007
|
180,000
|
60,000
|
$1.420
|
02/28/2014
|
||||||||
|
03/03/2008
|
26,000
|
78,000
|
$2.400
|
03/03/2015
|
||||||||
|
03/03/2008
|
4,000
|
12,000
|
$3.000
|
03/03/2015
|
||||||||
|
03/03/2008
|
13,125
|
$14,175
|
||||||||||
|
03/11/2009
|
108,000
|
$1.330
|
03/11/2016
|
|||||||||
|
03/11/2009
|
17,500
|
$18,900
|
||||||||||
1Option Awards Vesting Schedule:
|
Grant Date
|
Vesting Schedule
|
|
05/30/2001
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
10/18/2001
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
01/02/2002
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
01/02/2002
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
01/18/2002
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
03/14/2003
|
50% vested on each of the first and second anniversaries from date of grant.
|
|
10/28/2003
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
05/12/2004
|
25%vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
02/25/2005
|
100% vested on the date of grant, except in the case of Mr. Mills for whom 43,750 options vested on each of the first, second, third, and fourth anniversaries from date of grant for 175,000 shares.
|
|
05/17/2005
|
25% vested on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
05/15/2006
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
02/28/2007
|
25% vested at date of grant and 25% vests on each of the first, second, and third anniversaries from date of grant.
|
|
03/03/2008
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
03/11/2009
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
07/15/2009
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
12/11/2009
|
100% vests on the first anniversary of the grant date; provided, however, that if a significant corporate transaction is consummated prior to the first anniversary, 50% of such options shall vest on the date such significant corporate transaction is consummated. The determination of whether a significant corporate transaction has been consummated shall be made by the Board in a timely fashion and upon a written request by the executive to the Company’s Chairman of the Board of Directors.
|
2Stock Awards Vesting Schedule:
|
Grant Date
|
Vesting Schedule
|
|
05/15/2006
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
02/28/2007
|
25% vests on each of the first , second, third, and fourth anniversaries from date of grant.
|
|
03/03/2008
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
|
03/11/2009
|
25% vests on each of the first, second, third, and fourth anniversaries from date of grant.
|
Option Exercises and Stock Vested in 2009
The following table provides information for each of the named executive officers on stock options exercised and restricted stock vested during 2009, including the number of shares acquired upon exercise or vesting and the value realized before any withholding tax and broker commission.
|
Name
|
Option Awards
|
Stock Awards
|
||
|
Number of Shares Acquired on Exercise (#)
|
Value Realized On Exercise ($)
|
Number of Shares Acquired on Vesting (#)
|
Value Realized on
Vesting ($)
|
|
|
Frank C. Condella Jr.
|
-
|
-
|
36,000(1)
12,000(2)
|
$46,440
$12,960
|
|
Robert S. Mills
|
-
|
-
|
2,500(3)
5,000(4)
6,000(5)
|
$3,750
$6,850
$9,180
|
|
Lawrence Gyenes
|
-
|
-
|
-
|
-
|
|
James A. Meer
|
-
|
-
|
3,500(4)
4,375(5)
|
$4,795
$6,694
|
|
Michael McGrane
|
-
|
-
|
1,719(3)
4,000(4)
4,375(5)
|
$2,579
$5,480
$6,694
|
|
(1)
|
36,000 shares of Common Stock vested on September 30, 2009, with a closing market price of $1.29.
|
|
(2)
|
12,000 shares of Common Stock vested on December 31, 2009, with a closing market price of $1.08.
|
|
(3)
|
2,500 and 1,719 shares of Common Stock vested for Mr. Mills and Mr. McGrane, respectively, on May 15, 2009, with a closing market price of $1.50 per share.
|
|
(4)
|
5,000, 3,500, and 4,000 shares of Common Stock vested for Mr. Mills, Mr. Meer and Mr. McGrane, respectively, on February 28, 2009 with a closing market price of $1.37 per share.
|
|
(5)
|
6,000, 4,375, and 4,375 shares of Common Stock vested for Mr. Mills, Mr. Meer and Mr. McGrane, respectively, on March 3, 2009, with a closing market price of $1.53 per share.
|
Potential Payments upon Termination or Change in Control
We have entered into employment agreements with our named executive officers. The employment agreements of Messrs. Mills, McGrane and Gyenes require us to provide to them under specified circumstances cash compensation, benefits, and/or acceleration of the vesting of equity awards in the event of termination of employment. These agreements and plans are described below. The employment agreement for Mr. Condella as Interim CEO does not contain comparable obligations.
In the event of termination of employment of Mr. Mills, McGrane or Gyenes by the Company without cause1, or resignation by the executive with good reason2, the named executive officer will be entitled to the following:
|
Severance Payment
|
Base salary plus the greater of such person’s cash bonus paid in the preceding year or his target bonus.
|
|
Benefits
|
(i) For a period of twelve months following the termination date, continuation of medical, dental and vision coverage in effect on the termination date and (ii) payment for accrued and unused vacation days.
|
|
Salary
|
Base salary through the date of termination.
|
|
Expenses
|
Reimbursement for any previously unreimbursed business expenses.
|
In the event of his termination as a result of change in control3, the named executive officer will be entitled to the following:
|
Severance Payment
|
Base salary plus the greater of such person’s cash bonus paid in the preceding year or his target bonus.
|
|
Benefits
|
(i) A lump sum payment equal to the value of the fringe benefits provided to him for the year prior to the change in control and (ii) payment for accrued and unused vacation days.
|
|
Options
|
Full vesting of outstanding options.
|
|
Restricted Stock
|
Full vesting of all outstanding restricted stock grants.
|
|
Salary
|
Base salary through the date of termination.
|
|
Expenses
|
Reimbursement for any previously unreimbursed business expenses.
|
|
1
|
The executive employment agreements define “cause” as (i) the failure or refusal to perform, in any material respect, duties faithfully and diligently; (ii) gross negligence, recklessness or malfeasance; (iii) any criminal act; (iv) any act of fraud or other material misconduct resulting or intending to result directly or indirectly in gain or personal enrichment at the expense of Company; (v) any conduct relating to the business of Company that could reasonably be expected to have a materially detrimental effect on the business or financial condition of the Company; (vi) misconduct which materially discredits or damages Company, or violates Company’s policies or procedures, after Company has notified the executive of the actions Company deems to constitute non-compliance; and (vii) a material breach of obligations relating to confidential information, non-solicitation and non-competition..
|
|
2
|
The executive employment agreements define “good reason” as (i) a material diminution of responsibilities, or working conditions, or duties; (ii) a material diminution in base salary; (iii) a material negative change in the terms or status of an employment agreement; or (iv) an office relocation of more than 100 miles.
|
|
3
|
The change-in-control provisions in the employment agreements for Messrs Mills and McGrane require the Company to pay them an excise tax gross up payment if an excise tax is imposed following a change-in-control. Mr. Gyenes’ employment agreement does not contain a similar provision. The Company currently estimates that no excise tax would be due. The executive employment agreements define “change in control” as a consolidation or merger of Company in which Company is not the continuing or surviving entity or pursuant to which shares of Company’s common stock would be converted to cash, securities or other property, other than a merger of Company in which the holders of Company’s common stock immediately prior to the merger have the same proportionate ownership of common stock of the surviving entity immediately after the merger, or (ii) a sale, lease, exchange or transfer of all, or substantially all, of the assets of the company; (b) a stockholder approval of a plan or proposal for the liquidation or dissolution of the Company; (c) a person shall become a beneficial owner of 40% or more of Company’s outstanding common stock; or (d) during any period of two consecutive years, individuals who at the beginning of such period constitute the entire Board shall cease for any reason to constitute a majority thereof unless the election, or the nomination for election by Company’s stockholders, of each new director was approved by a vote of at least 50% of the directors eligible to vote who were directors at the beginning of the period.
|
The following table describes the potential payments and benefits under the Company’s agreements and plans to which the named executive officers would be entitled upon termination of their employment had such termination occurred on December 31, 2009.
|
Cash Severance
Payment1
|
Vacation Pay2
|
Continuation of Medical/Welfare Benefits
(present value)
|
Acceleration and Continuation of Equity Awards (unamortized expense as of December 31, 2009)5,6
|
Excise Tax Gross-up
|
Total Termination Benefits
|
||
|
Mr. Mills
|
|||||||
|
· Voluntary resignation by employee without good reason
|
N/A
|
$30,000
|
N/A
|
$0
|
N/A
|
$30,000
|
|
|
· Termination by the Company without cause or resignation by employee with good reason
|
$585,000
|
$30,000
|
$1,176(3)
|
$0
|
N/A
|
$616,176
|
|
|
· Termination by the Company without cause or resignation by employee for good reason after change in control (CIC)
|
$585,000
|
$30,000
|
$5,330(4)
|
$58,860
|
7
|
$679,190
|
|
|
Mr. Gyenes
|
|||||||
|
· Voluntary resignation by employee without good reason
|
N/A
|
$25,000
|
N/A
|
$0
|
N/A
|
$25,000
|
|
|
· Termination by the Company without cause or resignation by employee with good reason
|
$455,000
|
$25,000
|
$15,674(3)
|
$0
|
N/A
|
$495,674
|
|
|
· Termination by the Company without cause or resignation by employee for good reason after CIC
|
$455,000
|
$25,000
|
$18,476(4)
|
$0
|
7
|
$498,476
|
|
|
Mr. McGrane
|
|||||||
|
· Voluntary resignation by employee without good reason
|
N/A
|
$22,746
|
N/A
|
$0
|
N/A
|
$22,746
|
|
|
· Termination by the Company without cause or resignation by employee with good reason
|
$413,980
|
$22,746
|
N/A
|
$0
|
N/A
|
$436,726
|
|
|
· Termination by the Company without cause or resignation by employee for good reason after CIC
|
$413,980
|
$22,746
|
$3,305(4)
|
$43,572
|
7
|
$483,603
|
|
N/A – Not Applicable
|
1
|
Payment of the amount of base salary and the greater of preceding year or target bonus based on such person’s 2009 base salary and target bonus.
|
|
2
|
Assumes no vacation taken in year of termination, and a termination date of December 31, 2009. Unused and accrued vacation benefits are paid in a lump sum.
|
|
3
|
Represents Company paid costs in 2009 for medical, dental and vision insurance. This benefit is for twelve months.
|
|
4
|
Represents Company paid costs in 2009 for life, medical, dental, vision, and short- and long-term disability insurance, and 401(k) match. This benefit is paid in a lump sum.
|
|
5
|
All stock options vest upon a change-in-control pursuant to the terms of the 1996 Plan and the 2008 Plan. Represents the intrinsic value of both vested and unvested stock options on December 31, 2009, based on the difference between the closing market price of the Company’s Common Stock on December 31, 2009 ($1.08) and the applicable exercise price of all stock options.
|
|
6
|
All restricted stock shares vest upon a change-in-control pursuant to the terms of the 1996 Plan and the 2008 Plan. Assumes the value of all vesting shares of restricted stock at $1.08 a share, the closing market price of the Company’s Common Stock on December 31, 2009.
|
|
7
|
Mr. Mills’ and Mr. McGrane’s change-in-control employment agreements provide for the Company to pay them an excise tax gross up payment if an excise tax is imposed following a change-in-control. Mr. Gyenes’ employment agreement does not contain a similar provision. The Company currently estimates that no excise tax would be due.
|
2009 Director Compensation
Directors who are employees receive no additional compensation for serving on the Board. In 2009, we provided the following annual compensation to directors who were not employees.
|
Name of Director
|
Fees Earned and
Paid in Cash1
|
Stock
Awards2
|
Option
Awards3
|
Total ($)
|
|
Stephen Kasnet
|
$67,500
|
$25,000
|
–
|
$92,500
|
|
Edward Blechschmidt
|
$52,000
|
$25,000
|
–
|
$77,000
|
|
Denis O’Donnell4
|
$16,417
|
–
|
–
|
$16,417
|
|
Valerie Andrews
|
$45,000
|
$25,000
|
–
|
$70,000
|
|
James Crofton
|
$52,500
|
$25,000
|
–
|
$77,500
|
|
Selwyn Oskowitz
|
$38,583
|
$25,000
|
–
|
$63,583
|
|
Anthony Campbell
|
$44,917
|
$25,000
|
$16,984
|
$86,901
|
|
Frank C. Condella, Jr.5
|
$32,833
|
$107,599
|
–
|
$140,432
|
|
1
|
This column reports the amount of cash compensation earned in 2009 for Board and Committee service. The Company currently provides to the non-employee directors reimbursement for expenses and the following compensation. The Board has reduced retainers paid in calendar year 2010 by 20%.
|
|
Annual Retainer, Chairman
|
$
|
45,000
|
||
|
Annual Retainer, Vice Chairman
|
$
|
30,000
|
||
|
Annual Director Retainer (except Chairman and Vice Chairman)
|
$
|
20,000
|
||
|
Annual Committee Retainer (except Audit Committee)
|
$
|
1,000
|
||
|
Annual Committee Retainer (Audit Committee)
|
$
|
2,000
|
||
|
Additional Annual Retainer: Committee Chair (except Audit and Compensation Committees)
|
$
|
1,000
|
||
|
Additional Annual Retainer: Audit Committee Chair
|
$
|
15,000
|
||
|
Additional Annual Retainer: Compensation Committee Chair
|
$
|
6,000
|
||
|
Meeting Attendance Fees (per day)
|
$
|
1,500
|
(in person)
|
|
|
$
|
500
|
(by telephone)
|
||
|
Value of restricted stock granted upon election at annual meeting consists of a grant of the number of shares of restricted stock under the Company’s Long-term Performance Plans determined by dividing $25,000 by the fair market value of the Company’s Common Stock on the NASDAQ Global Market on the date of grant.
|
$
|
25,000
|
|
|
2
|
This column represents the aggregate grant date fair value of awards of restricted stock granted during the 2009 fiscal year, computed in accordance with ASC 718. The aggregate grant date fair value for the restricted stock awards to each director upon his or her reelection to the Board at the 2009 Annual Meeting of Stockholders was $25,000. (See note 6 below for additional awards to Mr.Condella). Each director had an aggregate of 8,620 shares of unvested restricted Common Stock outstanding at 2009 fiscal year end.
|
|
3
|
This column represents the aggregate grant date fair value of option awards made to during the 2009 fiscal year, computed in accordance with ASC 718. Mr. Campbell was granted 25,000 stock options in 2009.
|
|
4
|
Dr. Denis O’Donnell did not seek reelection to the Board at the 2009 Annual Meeting of Stockholders.
|
|
5
|
The Board elected Frank C. Condella, Jr., as a director of the Company on March 10, 2009. Compensation in this table does not reflect compensation paid to him beginning December 15, 2009, as Interim Chief Executive Officer, which is reflected in the Summary Compensation Table.
|
|
6
|
In addition to Mr. Condella’s restricted stock award described in Note 2 above, he received the following restricted stock awards in 2009 (i) 16,556 shares on March 10, 2009, valued at $25,000, upon his initial election to the Board, and (ii) 12,000 shares on July 20, 2009, valued at $13,320; 12,000 shares on August 3, 2009, valued at $14,040; 12,000 shares on September 1, 2009, valued at $14,760; and 12,000 shares on October 1, 2009, valued at $15,480; each pursuant to his consulting agreement dated July 18, 2009, relating to partnering activities for the Company’s progesterone products.
|
Aggregate total number of shares underlying stock option awards outstanding at 2009 fiscal year end for the independent directors are shown below:
|
Name
|
Number of Shares Underlying Options
|
|
Valerie Andrews
|
12,000
|
|
Edward Blechschmidt
|
15,000
|
|
James Crofton
|
12,000
|
|
Stephen Kasnet
|
12,000
|
|
Selwyn Oskowitz
|
83,000
|
|
Anthony Campbell
|
25,000
|
The members of the Compensation Committee serving at any time during 2009 were Valerie L. Andrews, Edward A. Blechschmidt, Frank C. Condella, Jr., James S. Crofton, and Denis M. O’Donnell, M.D. None of the Company’s executive officers served during fiscal year 2009 or currently serve and the Company anticipates that none will serve, as a member of the board of directors or compensation committee of any entity (other than the Company) that has one or more executive officers that serves on the Company’s Board or the Compensation Committee.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Common Stock. The following table sets forth, as of April 27, 2010, information with respect to the beneficial ownership of shares of Columbia’s Common Stock by:
each person known to us to be the beneficial owner of more than 5% of the shares of Columbia’s Common Stock;
|
|
each of Columbia’s directors and director nominees;
|
|
|
each of Columbia’s named executive officers; and
|
|
|
all of Columbia’s current directors and named executive officers as a group.
|
The number of shares beneficially owned by each person, director, director nominee, or named executive officer is determined under rules of the SEC; the information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares for which the individual has sole or shared voting power or investment power and also any shares with respect to which the person has the right to acquire sole or shared voting or investment power on or before June 26, 2010 (60 days after April 27, 2010) through the conversion of shares of convertible preferred stock or convertible debt or the exercise of any stock option, warrant or other right. Unless we indicate otherwise, each person has sole investment and/or voting power (or shares such powers with his or her spouse) with respect to the class of shares set forth in the following tables.
|
Common Stock
|
||
|
Name and Address of
Beneficial Owner
|
Number of Shares
Beneficially
Owned (1)
|
Percentage
of Total (2)
|
|
David M. Knott/Dorset Management
Corporation (3)
485 Underhill Boulevard, Suite 205
Syosset, New York 11791
|
6,107,803
|
8.9%
|
|
John P. Curran (4)
230 Park Avenue
New York, New York 10017
|
6,600,272
|
9.99%
|
|
Goldman Capital Management, Inc. (5)
320 Park Avenue
New York, New York 10022
|
4,585,350
|
7.0%
|
|
Perry Corp. and Richard C. Perry (6)
767 Fifth Avenue, 19th Floor
New York, NY 10153
|
4,457,142
|
6.8%
|
|
Directors and Named Executive Officers:
|
||
|
Valerie L. Andrews (7)(8)
|
48,981
|
*
|
|
Edward A. Blechschmidt (7)(8)
|
84,731
|
*
|
|
Anthony R. Campbell (8)(9)
|
6,381,642
|
9.3%
|
|
Frank C. Condella, Jr.
|
162,764
|
*
|
|
James S. Crofton (7)(8)
|
48,981
|
*
|
|
Lawrence A. Gyenes
|
50,000
|
*
|
|
Stephen G. Kasnet (7)(8)
|
136,981
|
*
|
|
Michael McGrane (7)(8)
|
754,259
|
1.1%
|
|
Robert S. Mills (7)(8)
|
1,079,474
|
1.6%
|
|
Selwyn P. Oskowitz, M.D. (7)(8)
|
123,681
|
*
|
|
All Directors and Executive Officers as a Group (10 persons) (7) (8) (9)
|
8,871,494
|
13.2%
|
|
|
_________________________
|
|
*
|
Signifies less than 1%
|
|
(1)
|
Includes shares that may be acquired through the conversion of shares of convertible preferred stock or convertible debt or the exercise of warrants, stock options, or other rights, in each case, that are convertible or exercisable on or before June 26, 2010.
|
|
(2)
|
Based on 65,605,886 shares outstanding at April 27, 2010. In calculating the percentage of ownership, all shares of Common Stock of which the identified person or group has the right to acquire beneficial ownership on or before June 26, 2010 are deemed to be outstanding for the purpose of computing the percentage of the shares of Common Stock owned by that person or group. These shares are not, however, deemed to be outstanding for the purpose of computing the percentage of the shares of Common Stock owned by any other person or group.
|
|
(3)
|
Based on Schedule 13D/A, filed on March 18, 2010 with the SEC by Dorset Management Corporation and David M. Knott in which (a) Dorset Management Corporation reported beneficial ownership of 6,012,473 shares of Common Stock, which includes 2,152,381 shares of Common Stock issuable upon the conversion of the Notes and the conversion of shares of Series E Preferred Stock, and 657,002 shares of Common Stock that are issuable upon the exercise of warrants or options to purchase shares of Common Stock, and (b) David M. Knott reported beneficial ownership of the shares reported as beneficially owned by Dorset Management Corporation plus 95,330 additional shares of Common Stock.
|
|
(4)
|
Based on Schedule 13G/A filed on April 27, 2010 with the SEC by John P. Curran, which reported beneficial ownership of 6,600,272 shares of Common Stock. The Schedule 13G reported that Mr. Curran does not have sole voting and dispositive power over any shares of Columbia’s Common Stock and has shared voting and dispositive power over 6,600,272 of Columbia’s Common Stock. Mr. Curran disclaimed beneficial ownership in the securities reported in the Schedule 13G/A except to the extent of his pecuniary interest therein.
|
|
(5)
|
Based on a telephone conversation on March 17, 2010, between Frank C. Condella, Jr., Interim Chief Executive Officer of the Company, and Neil Goldman, President of Goldman Capital Management, Inc., in which Mr. Goldman reported beneficial ownership of 4,585,350 shares of Common Stock.
|
|
(6)
|
Based on a Schedule 13D filed on March 12, 2010 with the SEC by Perry Corp. and Richard C. Perry, which reported beneficial ownership of 4,457,142 shares of Common Stock issuable upon conversion of Notes or exercise of warrants to purchase Common Stock.
|
|
(7)
|
Includes shares which may be acquired upon the exercise of options exercisable within 60 days after April 27, 2010, as follows: Ms. Andrews, 12,000 shares; Mr. Blechschmidt, 15,000 shares; Mr. Campbell, 25,000 shares; Mr. Crofton, 12,000 shares; Mr. Kasnet, 12,000 shares; Mr. McGrane, 663,810 shares; Mr. Mills, 977,674 shares; and, Dr. Oskowitz, 83,000 shares.
|
|
(8)
|
Includes restricted shares that were unvested on April 27, 2010, as follows: Ms. Andrews, 19,230 shares; Mr. Blechschmidt, 19,230 shares; Mr. Campbell, 19,230 shares; Mr. Condella, 19,230 shares; Mr. Crofton, 19,230 shares; Mr. Kasnet, 19,230 shares; Mr. McGrane, 25,875 shares; Mr. Mills, 35,000 shares; and, Dr. Oskowitz, 19,230 shares.
|
|
(9)
|
Includes direct ownership of 122,265 shares of Common Stock and indirect ownership of 126,574 shares of Common Stock (including 100,000 shares that have been pledged as security). Also includes beneficial ownership of the shares described in footnote 3 above reported as beneficially owned by Dorset Management Corporation and David M. Knott.
|
Series B Preferred Stock. Michael Nissan and Marla S. Nissan of 876 Park Avenue, New York, New York 10021, hold all 130 outstanding shares of the Series B Preferred Stock as of April 27, 2010.
Series E Preferred Stock. The following table sets forth, as of April 27, 2010, information with respect to each person known to us to be the beneficial owner of more than 5% of Columbia’s Series E Preferred Stock. No director, director nominee, or named executive officer owns any shares of Series E Preferred Stock.
|
Series E Preferred Stock
|
||
|
Name and Address of Beneficial Owner
|
Number of Shares Beneficially Owned
|
Percentage
of Total (1)
|
|
Perry Corp. and Richard C. Perry (2)
767 Fifth Avenue, 19th Floor
New York, NY 10153
|
35,000
|
59.3%
|
|
Knott Partners Offshore Master Fund, L.P.
485 Underhill Boulevard, Suite 205
Syosset, New York 11791
|
9,580
|
16.2%
|
|
Knott Partners, L.P.
485 Underhill Boulevard, Suite 205
Syosset, New York 11791
|
7,980
|
13.5%
|
|
Shoshone Partners, L.P.
485 Underhill Boulevard, Suite 205
Syosset, New York 11791
|
5,180
|
8.8%
|
_______________
|
(1)
|
Based on 59,000 shares of Series E Preferred Stock outstanding at April 27, 2010.
|
|
(2)
|
Based on a Schedule 13D filed on March 12, 2010 with the SEC by Perry Corp. and Richard C. Perry, which reported beneficial ownership of 1,750,000 shares of Common Stock issuable upon conversion of Preferred Stock.
|
As of April 27, 2010, we know of no persons, other than those listed above, who beneficially own, as determined under rules of the SEC, more than 5% of our outstanding shares of Common Stock, Series B Preferred Stock or Series E Preferred Stock.
Item 13. Certain Relationships, Related Transactions, and Director Independence
The Board has analyzed the independence of each director and nominee and has determined that, with the exception of Messrs. Condella and Mills, Interim CEO and President, respectively, each director qualifies as an “independent” director under the applicable NASDAQ Marketplace Rules, including that each such director or nominee is free of any relationship that would interfere with his individual exercise of independent judgment. In analyzing the independence of Mr. Campbell, the Board considered his position as a Portfolio Manager and Senior Analyst for Dorset Management Corporation, the beneficial owner of more than 5% of the Company’s Common Stock.
All of the Company’s committees are comprised solely of independent directors.
Certain Relationships and Related Party Transactions
We have a policy against our directors, officers, employees, and consultants entering into transactions that present actual or potential conflicts of interests. A conflict of interest can arise when a director, officer, employee, or consultant takes an action or has an interest that may make it difficult for him or her to perform his or her work objectively and effectively. Conflicts of interest may also arise when a director, officer, employee, or consultant (or his or her family members) receives improper personal benefits as a result of the director’s, officer’s, employee’s, or consultant’s relationship to us. This policy is reflected in our Code of Business Conduct and Ethics. In addition, the Audit Committee of the Board, pursuant to its charter, is responsible for reviewing and addressing conflicts of interest of directors and executive officers; as well as monitoring and reviewing (including discussing with management and the independent auditor) and, if appropriate, recommending to the full Board the approval or ratification of any transactions or courses of dealing with related parties that are required to be disclosed pursuant to SEC Regulation S-K, Item 404.
Item 14. Principal Accountant Fees and Services
BDO Seidman, LLP (“BDO Seidman”), serves as the Company’s independent registered public accounting firm and has served in that capacity since July 2008. The decision to engage BDO Seidman as the Company’s independent registered public accounting firm was approved by the Audit Committee of the Board.
The Audit Committee considered the independence of BDO Seidman and whether the audit and non-audit services BDO Seidman provide to the Company are compatible with maintaining that independence. The Audit Committee has adopted a set of policies governing the provision of non-audit services by BDO Seidman; those policies are included in the Audit Committee’s report. See “Board of Directors and Corporate Governance – Audit Committee.” The Audit Committee has adopted procedures by which the Audit Committee must approve in advance all services provided by and fees paid to the Company’s independent registered public accounting firm. The advance approval requirement was not waived in any instance during the past fiscal year.
Fees and Services of BDO Seidman, LLP
The following table sets forth the aggregate fees billed to the Company by BDO Seidman, LLP for the fiscal year ended December 31, 2009 and 2008:
|
2009
|
2008
|
|||||
|
Audit Fees (1)
|
$
|
420,700
|
$
|
348,000
|
||
|
Audit-Related Fees (2)
|
16,000
|
2,500
|
||||
|
Tax Fees (3)
|
53,500
|
-
|
||||
|
All Other Fees
|
-
|
-
|
||||
|
Total
|
$
|
490,200
|
350,500
|
|||
|
|
(1)
|
Audit fees consisted of fees for audit work performed in the audit of financial statements, including the audit of our internal controls over financial reporting required by Section 404 of the Sarbanes-Oxley Act, as well as fees for quarterly reviews and registration statements.
|
|
|
(2)
|
Audit-related fees consisted principally of fees for consulting on financial accounting and reporting standards for certain transactions and related matters.
|
|
|
(3)
|
Tax fees consisted principally of fees for work performed with respect to tax compliance and tax planning.
|
The Audit Committee has adopted a formal policy on auditor independence requiring the advance approval by the Audit Committee of all audit and non-audit services provided by our independent registered public accounting firm. In determining whether to approve any services by our independent registered public accounting firm, the Audit Committee reviews the services and the estimated fees and considers whether approval of the proposed services will have a detrimental impact on the auditor’s independence. On an annual basis, our management reports to the Audit Committee all audit and non-audit services performed during the previous twelve months and all fees billed by our independent registered public accounting firm for such services.
In fiscal 2008 and 2009, all audit and non-audit services and the corresponding fees were approved by the Audit Committee.
Item 15. Exhibits and Financial Statement Schedules
(a)(1)(2) Financial Statements and Financial Statement Schedules
Indexes to financial statements and financial statement schedules appear on F-1 and F-26, respectively.
(b) Exhibits
|
Exhibit
No
|
Description
|
|
|
3.1
|
Restated Certificate of Incorporation of the Company, as amended (14)
|
|
|
3.2
|
Amended and Restated By-laws of Company (3)
|
|
|
4.1
|
Certificate of Designations, Preferences and Rights of Series C Convertible Preferred Stock of the Company, dated as of January 7, 1999 (3)
|
|
|
4.2
|
Securities Purchase Agreement, dated as of January 7, 1999, between the Company and each of the purchasers named on the signature pages thereto (3)
|
|
|
4.3
|
Securities Purchase Agreement, dated as of January 19, 1999, among the Company, David M. Knott and Knott Partners, L.P. (3)
|
|
|
4.4
|
Form of Warrant to Purchase Common Stock (3)
|
|
|
4.5
|
Warrant to Purchase Common Stock granted to James J. Apostolakis on September 23, 1999 (5)
|
|
|
4.6
|
Certificate of Designations of Series E Convertible Preferred Stock, filed May 10, 2005 with the Delaware Secretary of State (13)
|
|
|
4.7
|
Preferred Stock Purchase Agreement, dated as of May 10, 2005, among Columbia Laboratories, Inc., Perry Partners L.P. and Perry Partners International, Inc. (13)
|
|
|
4.8
|
Securities Purchase Agreement, dated March 10, 2006, by and between Columbia Laboratories, Inc. and the Purchasers listed on Exhibit A thereto (15)
|
|
|
4.9*
|
Form of Restricted Stock Agreement (17)
|
|
|
4.10*
|
Form of Option Agreement (24)
|
|
|
4.11
|
Securities Purchase Agreement, dated December 21, 2006, by and between Columbia Laboratories, Inc. and the Purchasers listed on Exhibit A thereto (19)
|
|
|
10.1
|
1996 Long-term Performance Plan, as amended, of the Company (2)
|
|
|
10.2
|
Asset Purchase, License and Option Agreement between Bio-Mimetics, Inc. and Columbia Laboratories, Inc., dated November 22, 1989 (1)
|
|
|
10.4
|
Settlement Agreement and Release dated as of March 16, 2000 between Columbia Laboratories (Bermuda) Ltd. and Lake Consumer Products, Inc. (5)
|
|
|
10.5
|
License Agreement dated April 18, 2000, between the Company and Lil’ Drug Store Products, Inc. (6)
|
|
|
10.6
|
Rights Agreement dated as of March 13, 2002, by and between Columbia Laboratories, Inc. and First Union National Bank, as Rights Agent (7)
|
|
|
10.7†
|
Semi-Exclusive Supply Agreement dated May 7, 2002 between the Company and Mipharm S.p.A. (8)
|
|
|
10.8†
|
Amended and Restated License and Supply Agreement dated June 4, 2002 between the Company and Ares Trading S.A.(8)
|
|
|
10.9†
|
Investment and Royalty Agreement dated March 5, 2003 between the Company and PharmaBio Development Inc. (9)
|
|
|
10.10†
|
License and Supply Agreement Dated May 27, 2003 between the Company and Mipharm S.p.A. (10)
|
|
|
10.11*
|
Form of Indemnification Agreement for Officers and Directors (11)
|
|
|
10.12†
|
Asset Purchase Agreement Dated June 29, 2004, between the Company and Lil’ Drug Store Products, Inc. (12)
|
|
|
10.13†
|
Supply Agreement dated June 29, 2004, between the Company and Lil’ Drug Store Products, Inc.(12)
|
|
|
10.14
|
Letter Agreement Supplement to STRIANT Investment and Royalty Agreement dated April 14, 2006 (16)
|
|
|
10.15*
|
Separation Agreement by and between Columbia Laboratories, Inc. and David L. Weinberg effective as of December 12, 2006 (18)
|
|
|
10.16†
|
Agreement, dated December 21, 2006, by and among Ares Trading S.A., Serono, Inc., the Company and its wholly-owned subsidiary, Columbia Laboratories (Bermuda), Ltd (19)
|
|
|
10.17
|
Amendment No. 1 to the Amended and Restated License and Supply Agreement, entered into December 21, 2006, by and between Ares Trading S.A and Columbia Laboratories (Bermuda), Ltd. (19)
|
|
|
10.18
|
Description of the Registrant’s Compensation and Reimbursement Practices for Non-employee Directors. (20)
|
|
|
10.19
|
Lease Agreement between Allwood Associates I and Columbia Laboratories, Inc., dated July 6, 2007 (20)
|
|
|
10.20†
|
License and Supply Agreement between Columbia Laboratories, Inc. and Ascend Therapeutics, Inc., dated September 27, 2007 (21)
|
|
|
10.21
|
Supply Agreement between Columbia Laboratories (Bermuda) Limited and Fleet Laboratories Limited, dated July 12, 1996 (22)
|
|
|
10.22
|
Packaging Agreement between Columbia Laboratories (Ireland) Ltd. and Maropack AG, dated October 28, 1993 (22)
|
|
|
10.23*
|
Columbia Laboratories, Inc., 2008 Long-Term Incentive Plan (23)
|
|
|
10.24*
|
Columbia Laboratories, Inc., Amended and Restated Incentive Plan (24)
|
|
|
10.25*
|
Form of Executive Change of Control Severance Agreement (24)
|
|
|
10.26
|
Stock Purchase Agreement, by and between the Company and Numoda Corporation, dated January 6, 2009 (25)
|
|
|
10.27*
|
Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and Robert S. Mills dated March 11, 2009 (24)
|
|
|
10.28*
|
Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and Michael McGrane dated March 11, 2009 (29)
|
|
|
10.29*
|
Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and James A Meer dated March 11, 2009 (26)
|
|
|
10.30*
|
Separation and Release Agreement by and between Columbia Laboratories, Inc. and James A. Meer effective as of May 18, 2009 (26)
|
|
|
10.31*
|
Employment Agreement by and between Columbia Laboratories, Inc. and Lawrence Gyenes dated July 15, 2009 (27).
|
|
|
10.32
|
Second Amendment to Investment and Royalty Agreement, by and between the Company and PharmaBio Development, Inc., July 22, 2009 (28)
|
|
|
10.33
|
Placement Agent Agreement, by and among the Company, Oppenheimer & Co., Inc. and The Benchmark Company, LLC, dated October 22, 2009 (30)
|
|
|
10.34
|
Form of Subscription Agreement (30)
|
|
|
10.35
|
Form of Warrant to Purchase Common Stock (30)
|
|
|
10.36*
|
Columbia Laboratories Stock Ownership Guidelines for Officers and Directors (31)
|
|
|
10.37
|
Manufacturing and Supply Agreement between Fleet Laboratories and Columbia Laboratories (Bermuda), Ltd., dated December 8, 2009 (32)
|
|
|
10.38*
|
Employment Agreement by and between Columbia Laboratories, Inc. and Frank C. Condella, Jr., dated December 11, 2009 (33)
|
|
|
10.39*
|
Addendum to Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and Robert S. Mills dated December 11, 2009 (33)
|
|
|
10.40*
|
Addendum to Executive Change in Control Severance Agreement by and between Columbia Laboratories, Inc. and Robert S. Mills dated December 11, 2009 (33).
|
|
|
14
|
Code of Ethics of the Company (11)
|
|
|
21
|
Subsidiaries of the Company (34)
|
|
|
23.1
|
Consent of BDO Seidman, LLP (35)
|
|
|
23.2
|
Consent of McGladrey & Pullen, LLP (35)
|
|
|
31(i).1
|
Certification of Chief Executive Officer of the Company (35)
|
|
|
31(i).2
|
Certification of Chief Financial Officer of the Company (35)
|
|
|
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (35)
|
|
|
32.2
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. (35)
|
|
|
†
|
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
|
*
|
Management contract or compensatory plans or arrangements
|
|
|
1/
|
Incorporated by reference to the Registrant's Registration Statement on Form S-1 (File No. 33-31962) declared effective on May 14, 1990
|
|
2/
|
Incorporated by reference to the Registrant's Proxy Statement dated May 10, 2000
|
|
3/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1998
|
|
4/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 1999
|
|
5/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1999
|
|
6/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2000
|
|
7/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated March 12, 2002
|
|
8/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 14, 2002
|
|
9/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002
|
|
10/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 14, 2003
|
|
11/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2003
|
|
12/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 4, 2004
|
|
13/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated May 12, 2005
|
|
14/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2005
|
|
15/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated March 16, 2006
|
|
16/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated April 17, 2006
|
|
17/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated May 17, 2006
|
|
18/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 15, 2006
|
|
19/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 26, 2006
|
|
20/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, dated August 8, 2007
|
|
21/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, dated November 8, 2007
|
|
22/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007
|
|
23/
|
Incorporated by reference to the Registrant’s Proxy Statement dated April 8, 2008
|
|
24/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008
|
|
25/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated January 8, 2009
|
|
26/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated May 12, 2009
|
|
27/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated July 15, 2009
|
|
28/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated July 24, 2009
|
|
29/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 6, 2009
|
|
30/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated October 23, 2009
|
|
31/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated November 17, 2009
|
|
32/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 8, 2009
|
|
33/
|
Incorporated by reference to the Registrant’s Current Report Form 8-K, dated December 14, 2009
|
|
34/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2009
|
|
35/
|
Filed herewith
|
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
COLUMBIA LABORATORIES, INC.
|
||
|
Date: April 30, 2010
|
By:
|
/s/ Mr. Lawrence A. Gyenes
|
|
Lawrence A. Gyenes, Senior Vice President
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
/s/
|
Frank C. Condella, Jr.
|
April 30, 2010
|
|||
|
Frank C. Condella, Jr.
|
Interim Chief Executive Officer
(Principal Executive Officer)
|
||||
|
/s/
|
Robert S. Mills
|
April 30, 2010
|
|||
|
Robert S. Mills
|
President and Chief Operating Officer
|
||||
|
/s/
|
Mr. Lawrence A. Gyenes
|
April 30, 2010
|
|||
|
Mr. Lawrence A. Gyenes
|
Senior Vice President, Chief Financial Officer and Treasurer
(Principal Financial and Accounting Officer)
|
||||
|
/s/
|
Valerie L. Andrews
|
April 30, 2010
|
|||
|
Valerie L. Andrews
|
Director
|
||||
|
/s/
|
Edward A. Blechschmidt
|
April 30, 2010
|
|||
|
Edward A. Blechschmidt
|
Vice Chairman of the Board of Directors
|
||||
|
/s/
|
Anthony R. Campbell
|
April 30, 2010
|
|||
|
Anthony R. Campbell
|
Director
|
||||
|
/s/
|
Stephen G. Kasnet
|
April 30, 2010
|
|||
|
Stephen G. Kasnet
|
Chairman of the Board of Directors
|
||||
|
/s/
|
Selwyn P. Oskowitz
|
April 30, 2010
|
|||
|
Selwyn P. Oskowitz
|
Director
|
COLUMBIA LABORATORIES, INC. AND SUBSIDIARIES
INDEX TO FINANCIAL STATEMENTS
|
Page
|
|
|
Reports of Independent Registered Public Accounting Firms
|
F-2– F-3
|
|
Consolidated Balance Sheets as of December 31, 2009 and 2008
|
F-4 – F-5
|
|
Consolidated Statements of Operations for the Three Years Ended December 31, 2009, 2008 and 2007
|
F-6
|
|
Consolidated Statements of Comprehensive Operations for the Three Years Ended December 31, 2009, 2008 and 2007
|
F-7
|
|
Consolidated Statements of Shareholders' Equity (Deficiency) for the Three Years Ended December 31, 2009, 2008 and 2007
|
F-8
|
|
Consolidated Statements of Cash Flows for the Three Years Ended December 31, 2009, 2008 and 2007
|
F-9 – F-10
|
|
Notes to Consolidated Financial Statements
|
F-11
|
F - 1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
Columbia Laboratories, Inc.
Livingston, NJ
We have audited the accompanying consolidated balance sheets of Columbia Laboratories, Inc. as of December 31, 2009 and 2008 and the related consolidated statements of operations and comprehensive operations, shareholders’ deficiency, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Columbia Laboratories, Inc. at December 31, 2009 and 2008, and the results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Columbia Laboratories, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 12, 2010, expressed an unqualified opinion thereon.
/s/ BDO Seidman, LLP
BDO Seidman, LLP
Woodbridge, NJ
March 12, 2010
F - 2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
Of Columbia Laboratories, Inc.
We have audited the accompanying consolidated statements of operations, comprehensive operations, shareholders’ equity (deficiency) and cash flows for the year ended December 31, 2007 of Columbia Laboratories, Inc. and Subsidiaries. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the results of operations of Columbia Laboratories, Inc. and Subsidiaries and their cash flows for the year ended December 31, 2007, in conformity with U.S. generally accepted accounting principles.
As disclosed in Note 2 to the consolidated financial statements, effective January 1, 2007, the Company adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes - an Interpretation of FASB Statement No. 109”.
/s/ McGladrey & Pullen, LLP
McGLADREY & PULLEN, LLP
New York, New York
March 25, 2008
F - 3
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2009 AND 2008
ASSETS
|
2009
|
2008
|
|||||||
|
Cash and cash equivalents of which $12,225,732 and
|
$ | 14,757,615 | $ | 12,497,382 | ||||
|
$12,099,318 is interest bearing
|
||||||||
|
Accounts receivable, net of allowances for
|
4,262,851 | 3,562,277 | ||||||
|
doubtful accounts of $100,000 and $100,000
|
||||||||
|
Inventories
|
2,532,722 | 2,377,139 | ||||||
|
Prepaid expenses and other current assets
|
1,097,525 | 1,102,525 | ||||||
|
Total current assets
|
22,650,713 | 19,539,323 | ||||||
|
Machinery and equipment
|
2,547,460 | 2,479,602 | ||||||
|
Computer software
|
545,616 | 534,302 | ||||||
|
Office equipment and furniture and fixtures
|
669,599 | 698,920 | ||||||
| 3,762,675 | 3,712,824 | |||||||
|
Less-accumulated depreciation and amortization
|
(3,071,196 | ) | (2,890,967 | ) | ||||
| 691,479 | 821,857 | |||||||
| 18,770,332 | 23,815,060 | |||||||
| 1,644,695 | 1,446,249 | |||||||
| $ | 43,757,219 | $ | 45,622,489 | |||||
(Continued)
F - 4
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2009 AND 2008
LIABILITIES AND SHAREHOLDERS’ DEFICIENCY
|
2009
|
2008
|
|||||||||
|
CURRENT LIABILITIES:
|
||||||||||
|
Current portion of financing agreements
|
$ | 144,897 | $ | 168,034 | ||||||
|
Accounts payable
|
3,662,091 | 2,085,463 | ||||||||
|
Accrued expenses
|
4,588,088 | 4,980,643 | ||||||||
|
Total current liabilities
|
8,395,076 | 7,234,140 | ||||||||
|
NOTES PAYABLE
|
32,965,863 | 30,074,966 | ||||||||
|
DEFERRED REVENUE
|
328,367 | 305,433 | ||||||||
|
LONG-TERM PORTION OF FINANCING AGREEMENTS
|
15,234,406 | 13,126,210 | ||||||||
|
TOTAL LIABILITIES
|
56,923,712 | 50,740,749 | ||||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||||
|
Contingently redeemable series C preferred stock
|
600,000 | 775,000 | ||||||||
|
600 and 775 shares issued and outstanding in 2009 and
|
||||||||||
|
2008, respectively (liquidation preference of $600,000 and
|
||||||||||
| $775,000) | ||||||||||
|
SHAREHOLDERS' DEFICIENCY:
|
||||||||||
|
Preferred stock, $.01 par value; 1,000,000 shares authorized
|
||||||||||
|
Series B convertible preferred stock, 130 shares issued
|
||||||||||
|
and outstanding (liquidation preference of $13,000)
|
1 | 1 | ||||||||
|
Series E convertible preferred stock 59,000 shares
|
||||||||||
|
issued and outstanding (liquidation preference of $5,900,000)
|
590 | 590 | ||||||||
|
Common Stock $.01 par value; 100,000,000 shares
|
||||||||||
|
authorized; 65,761,986 and 54,007,579 shares issued
|
657,619 | 540,076 | ||||||||
|
Capital in excess of par value
|
242,637,646 | 228,686,942 | ||||||||
|
Less cost of 131,935 and 63,644 treasury shares
|
(280,813 | ) | (189,229 | ) | ||||||
|
Accumulated deficit
|
(256,979,263 | ) | (235,109,705 | ) | ||||||
|
Accumulated other comprehensive income
|
197,727 | 178,065 | ||||||||
|
Shareholders' deficiency
|
(13,766,493 | ) | (5,893,260 | ) | ||||||
|
TOTAL LIABILITIES AND SHAREHOLDERS' DEFICIENCY
|
$ | 43,757,219 | $ | 45,622,489 | ||||||
The accompanying notes to consolidated financial statements
are an integral part of these statements
F - 5
COLUMBIA LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE YEARS ENDED DECEMBER 31, 2009
|
2009
|
2008
|
2007
|
||||||||||
|
NET REVENUES
|
$ | 32,196,381 | $ | 36,229,114 | $ | 29,627,638 | ||||||
|
COST OF REVENUES
|
9,194,538 | 10,934,615 | 9,014,540 | |||||||||
|
Gross profit
|
23,001,843 | 25,294,499 | 20,613,098 | |||||||||
|
OPERATING EXPENSES:
|
||||||||||||
|
Selling and distribution
|
11,982,229 | 12,685,618 | 10,111,796 | |||||||||
|
General and administrative
|
10,559,298 | 8,615,381 | 7,824,741 | |||||||||
|
Research and development
|
8,579,035 | 6,206,157 | 5,778,641 | |||||||||
|
Amortization of licensing right
|
5,044,728 | 5,044,728 | 5,005,768 | |||||||||
|
Total operating expenses
|
36,165,290 | 32,551,884 | 28,720,946 | |||||||||
|
Loss from operations
|
(13,163,447 | ) | (7,257,385 | ) | (8,107,848 | ) | ||||||
|
OTHER INCOME (EXPENSE):
|
||||||||||||
|
Interest income
|
33,830 | 299,805 | 979,953 | |||||||||
|
Interest expense
|
(8,851,253 | ) | (7,882,183 | ) | (7,946,048 | ) | ||||||
|
Other, net
|
(243,720 | ) | (100,516 | ) | (5,440 | ) | ||||||
| (9,061,143 | ) | (7,682,894 | ) | (6,971,535 | ) | |||||||
|
Net loss before taxes
|
(22,224,590 | ) | (14,940,279 | ) | (15,079,383 | ) | ||||||
|
State income tax benefits
|
355,032 | 863,770 | 787,593 | |||||||||
|
Net loss
|
$ | (21,869,558 | ) | $ | (14,076,509 | ) | $ | (14,291,790 | ) | |||
|
LOSS PER COMMON
|
||||||||||||
|
SHARE - BASIC AND DILUTED
|
$ | (0.39 | ) | $ | (0.27 | ) | $ | (0.28 | ) | |||
|
WEIGHTED - AVERAGE NUMBER OF
|
||||||||||||
|
COMMON SHARES OUTSTANDING
|
||||||||||||
|
BASIC AND DILUTED
|
56,358,843 | 52,439,327 | 51,124,266 | |||||||||
The accompanying notes to consolidated financial statements
are an integral part of these statements.
F - 6
CONSOLIDATED STATEMENTS OF COMPREHENSIVE OPERATIONS
FOR THE THREE YEARS ENDED DECEMBER 31, 2009
|
2009
|
2008
|
2007
|
|||||
|
NET LOSS
|
$ (21,869,558)
|
$ (14,076,509)
|
$(14,291,790)
|
||||
|
Other comprehensive income (loss):
|
|||||||
|
Foreign currency translation
|
19,662
|
(29,539)
|
8,560
|
||||
|
Comprehensive loss
|
$ (21,849,896)
|
$ (14,106,048)
|
$(14,283,230)
|
||||
The accompanying notes to consolidated financial statements
are an integral part of these statements.
F - 7
COLUMBIA LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIENCY)
FOR THE THREE YEARS ENDED DECEMBER 31, 2009
|
Series B Convertible
Preferred Stock
|
Series E Convertible
Preferred Stock
|
Common Stock
|
Capital in
Excess of
Par Value
|
Treasury
Stock
|
Accumulated
Deficit
|
Accumulated Other
Comprehensive
Income (Loss)
|
Total
|
|||||||||||||||
|
Number of
Shares
|
Amount
|
Number of
Shares
|
Amount
|
Number of
Shares
|
Amount
|
|||||||||||||||||
|
Balance, December 31, 2006
|
130
|
$ 1
|
69,000
|
$ 690
|
49,694,213
|
$ 496,942
|
$ 218,687,977
|
$ (26,880)
|
$ (206,741,406)
|
$ 199,044
|
$ 12,616,368
|
|||||||||||
|
Options exercised
|
43,050
|
431
|
62,810
|
63,241
|
||||||||||||||||||
|
Share based compensation expense
|
155,690
|
1,557
|
1,646,365
|
1,647,922
|
||||||||||||||||||
|
Conversion of Series C Preferred Stock
|
1,564,548
|
15,645
|
2,059,355
|
2,075,000
|
||||||||||||||||||
|
Conversion of Series E Preferred Stock
|
(5,453)
|
(55)
|
272,650
|
2,727
|
(2,672)
|
-
|
||||||||||||||||
|
Purchase of treasury stock
|
(27,150)
|
(27,150)
|
||||||||||||||||||||
|
Dividends on preferred stock
|
(76,894)
|
(76,894)
|
||||||||||||||||||||
|
Translation adjustment
|
8,560
|
8,560
|
||||||||||||||||||||
|
Net loss
|
(14,291,790)
|
(14,291,790)
|
||||||||||||||||||||
|
Balance, December 31, 2007
|
130
|
$ 1
|
63,547
|
$ 635
|
51,730,151
|
$ 517,302
|
$ 222,376,941
|
$ (54,030)
|
$ (221,033,196)
|
$ 207,604
|
$ 2,015,257
|
|||||||||||
|
Issuance of common stock
|
1,333,000
|
13,330
|
4,082,298
|
4,095,628
|
||||||||||||||||||
|
Options exercised
|
318,149
|
3,182
|
591,673
|
594,855
|
||||||||||||||||||
|
Conversion of Series C Preferred Stock
|
235,426
|
2,354
|
347,646
|
350,000
|
||||||||||||||||||
|
Conversion of Series E Preferred Stock
|
(4,547)
|
(45)
|
227,350
|
2,273
|
(2,228)
|
-
|
||||||||||||||||
|
Share based compensation expense
|
163,503
|
1,635
|
1,345,756
|
1,347,391
|
||||||||||||||||||
|
Purchase of treasury stock
|
(135,199)
|
(135,199)
|
||||||||||||||||||||
|
Dividends on preferred stock
|
(55,144)
|
(55,144)
|
||||||||||||||||||||
|
Translation adjustment
|
(29,539)
|
(29,539)
|
||||||||||||||||||||
|
Net loss
|
(14,076,509)
|
(14,076,509)
|
||||||||||||||||||||
|
Balance, December 31, 2008
|
130
|
$ 1
|
59,000
|
$ 590
|
54,007,579
|
$ 540,076
|
$ 228,686,942
|
$ (189,229)
|
$ (235,109,705)
|
$ 178,065
|
$ (5,893,260)
|
|||||||||||
|
Issuance of common stock
|
11,351,807
|
113,517
|
11,342,786
|
11,456,303
|
||||||||||||||||||
|
Issuance of warrants
|
719,904
|
719,904
|
||||||||||||||||||||
|
Conversion of Series C Preferred Stock
|
117,449
|
1,174
|
173,826
|
175,000
|
||||||||||||||||||
|
Share based compensation expense
|
285,151
|
2,852
|
1,746,376
|
1,749,228
|
||||||||||||||||||
|
Purchase of treasury stock
|
(91,584)
|
(91,584)
|
||||||||||||||||||||
|
Dividends on preferred stock
|
(32,188)
|
(32,188)
|
||||||||||||||||||||
|
Translation adjustment
|
19,662
|
19,662
|
||||||||||||||||||||
|
Net loss
|
(21,869,558)
|
(21,869,558)
|
||||||||||||||||||||
|
Balance, December 31, 2009
|
130
|
$ 1
|
59,000
|
$ 590
|
65,761,986
|
$ 657,619
|
$ 242,637,646
|
$ (280,813)
|
$ (256,979,263)
|
$ 197,727
|
$ (13,766,493)
|
|||||||||||
F - 8
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE THREE YEARS ENDED DECEMBER 31, 2009
|
2009
|
2008
|
2007
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss
|
$ | (21,869,558 | ) | $ | (14,076,509 | ) | $ | (14,291,790 | ) | |||
|
Adjustments to reconcile net loss to net
|
||||||||||||
|
cash used in operating activities -
|
||||||||||||
|
Depreciation and amortization
|
5,737,242 | 5,510,802 | 5,462,888 | |||||||||
|
Amortization of beneficial conversion features
|
1,658,981 | 1,466,591 | 1,265,496 | |||||||||
|
Amortization of warrants
|
1,231,916 | 1,092,197 | 971,548 | |||||||||
|
Provision for doubtful accounts
|
- | 4,267 | 15,000 | |||||||||
|
Provision for sales returns
|
1,226,357 | 1,399,991 | 992,502 | |||||||||
|
Write-down of inventories
|
81,718 | 746,905 | 80,961 | |||||||||
|
Share based compensation
|
1,749,228 | 1,347,391 | 1,647,922 | |||||||||
|
Non-cash interest expense on financing agreements
|
2,085,059 | 1,702,842 | 1,381,407 | |||||||||
|
Recognition of deferred income (Ardana)
|
- | (2,891,188 | ) | - | ||||||||
|
Loss on disposal of fixed assets
|
- | 3,048 | - | |||||||||
|
Changes in assets and liabilities -
|
||||||||||||
|
(Increase) decrease in:
|
||||||||||||
|
Accounts receivable
|
(700,574 | ) | 244,449 | (1,520,937 | ) | |||||||
|
Inventories
|
(237,301 | ) | (76,915 | ) | (1,023,052 | ) | ||||||
|
Prepaid expenses and other current assets
|
5,000 | 184,775 | (293,534 | ) | ||||||||
|
Other assets
|
9,175 | 943 | (418,405 | ) | ||||||||
|
Increase (decrease) in:
|
||||||||||||
|
Accounts payable
|
1,576,628 | (130,480 | ) | (1,370,828 | ) | |||||||
|
Accrued expenses
|
(1,632,472 | ) | (1,281,338 | ) | (211,709 | ) | ||||||
|
Deferred revenue
|
22,934 | (384,259 | ) | (601,768 | ) | |||||||
|
Net cash used in operating activities
|
(9,055,667 | ) | (5,136,488 | ) | (7,914,299 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||
|
Purchase of property and equipment
|
(49,853 | ) | (375,926 | ) | (102,021 | ) | ||||||
|
Net cash used in investing activities
|
(49,853 | ) | (375,926 | ) | (102,021 | ) | ||||||
(Continued)
F - 9
COLUMBIA LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE THREE YEARS ENDED DECEMBER 31, 2009
|
2009
|
2008
|
2007
|
|||||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|||||||||||||
|
Net proceeds from issuance of common stock
|
11,456,303 | 4,095,628 | - | ||||||||||
|
Proceeds from exercise of options
|
- | 594,855 | 63,241 | ||||||||||
|
Payment for purchase of treasury stock
|
(91,584 | ) | (135,199 | ) | (27,150 | ) | |||||||
|
Payment pursuant to financing agreements
|
- | (3,540,949 | ) | - | |||||||||
|
Dividends paid
|
(32,188 | ) | (55,144 | ) | (76,894 | ) | |||||||
|
Net cash or provided by or (used in) financing activities
|
11,332,531 | 959,191 | (40,803 | ) | |||||||||
|
EFFECT OF EXCHANGE RATE CHANGES ON
|
|||||||||||||
|
CASH
|
33,222 | (171,206 | ) | 8,557 | |||||||||
|
NET INCREASE/(DECREASE) IN CASH AND
|
|||||||||||||
|
CASH EQUIVALENTS
|
2,260,233 | (4,724,429 | ) | (8,048,566 | ) | ||||||||
|
CASH AND CASH EQUIVALENTS,
|
|||||||||||||
|
Beginning of year
|
12,497,382 | 17,221,811 | 25,270,377 | ||||||||||
|
CASH AND CASH EQUIVALENTS,
|
|||||||||||||
|
End of year
|
$ | 14,757,615 | $ | 12,497,382 | $ | 17,221,811 | |||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH
|
|||||||||||||
|
FLOW INFORMATION
|
|||||||||||||
|
Interest paid
|
$ | 3,200,000 | $ | 3,200,000 | $ | 2,488,889 | |||||||
|
Taxes paid
|
$ | 52,000 | $ | 27,403 | $ | 34,759 | |||||||
|
Accrual of financing costs
|
$ | - | $ | - | $ | 25,000 | |||||||
|
Increase of US Crinone License Right cost
|
$ | - | $ | - | $ | 1,000,000 | |||||||
|
Conversion of Series C preference shares to
|
|||||||||||||
|
common stock
|
|
$ | 175,000 | $ | 350,000 | $ | 2,075,000 | ||||||
|
Conversion of Series E preference shares to
|
|||||||||||||
|
common stock
|
|
$ | - | $ | 454,700 | $ | 545,300 | ||||||
|
Issuance of Warrants for Option to Extend
|
|||||||||||||
|
Pharmabio Debt
|
|
$ | 719,904 | $ | - | $ | - | ||||||
The accompanying notes to consolidated financial statements
are an integral part of these statements
F - 10
COLUMBIA LABORATORIES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES :
Organization -
Columbia Laboratories, Inc. (the "Company" or “Columbia”) was incorporated as a Delaware corporation in December 1986. The Company is primarily dedicated to research, development, and commercialization of women’s healthcare and endocrinology products, including those that treat or are intended to treat infertility, endometriosis, dysmenorrhea, preterm birth for women with a short cervix at mid-pregnancy and hormonal deficiencies. The Company has also developed a buccal delivery system for peptides. The Company’s products primarily utilize its patented Bioadhesive Delivery System technology.
Principles of Consolidation -
The consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Accounting Estimates -
The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates are used for, but are not limited to sales return reserves, license fees, payments to distributors, intangible assets, and share based compensation. Actual results could differ from those estimates in the near term.
Foreign Currency -
The assets and liabilities of the Company's foreign subsidiaries are translated into U.S. dollars at current exchange rates and revenue and expense items are translated at average rates of exchange prevailing during the period. Resulting translation adjustments are accumulated as a separate component of shareholders' equity. Transaction gains and losses are reflected in the Statements of Operations.
Accounts Receivable -
Accounts receivable are reported at their outstanding unpaid principal balances reduced by allowances for doubtful accounts. The Company estimates doubtful accounts based on historical bad debts, factors related to specific customers’ ability to pay and current economic trends. The Company writes off accounts receivable against the allowance when a balance is determined to be uncollectible.
The estimated fair value of the convertible subordinated notes payable and beneficial conversion feature amounted to $34,507,598 and $33,009,841 at December 31, 2009 and 2008, respectively. This value is the aggregate of the estimated future cash flows associated with the settlement of the notes payable, determined through consideration of market conditions, including available interest rates, credit spreads and the Company’s liquidity, and the intrinsic value of the beneficial conversion feature. The carrying value of the convertible notes payable and beneficial conversion feature amounted to $32,965,863 and $30,074,966 at December 31, 2009 and 2008, respectively. The fair value of accounts receivable, accounts payable and the financing agreements described in Note 5 approximate their carrying amount.
F - 11
Inventories are stated at the lower of cost (first-in, first-out) or market. Components of inventory cost include materials, labor and manufacturing overhead. Inventories consist of the following:
|
December 31,
|
|||
|
2009
|
2008
|
||
|
Finished goods
|
$ 1,343,742
|
$ 1,745,222
|
|
|
Raw materials
|
1,188,980
|
631,917
|
|
|
Total
|
$ 2,532,722
|
$ 2,377,139
|
|
Shipping costs are included in selling and distribution expenses and amounted to approximately $169,000, $152,000 and $102,000, in 2009, 2008 and 2007 respectively.
Property and Equipment -
Property and equipment is stated at cost less accumulated depreciation. Leasehold improvements are amortized over the lesser of the useful life or the term of the respective leases. Depreciation is computed on the straight-line basis over the estimated useful lives of the respective assets, as follows:
|
Years
|
||||
|
Software
|
3
|
|||
|
Machinery and equipment
|
5-10
|
|||
|
Furniture and fixtures
|
5
|
|||
Costs of major additions and improvements are capitalized and expenditures for maintenance and repairs that do not extend the term of the assets are expensed. Upon sale or disposition of property and equipment, the cost and related accumulated depreciation are eliminated from the accounts and any resultant gain or loss is credited or charged to operations.
Depreciation expense amounted to approximately $180,000, $203,000 and $215,000 in 2009, 2008 and 2007, respectively.
Concentration of Risk -
The Company sells its products to customers worldwide. The Company performs ongoing credit evaluations of its customers and generally does not require collateral. See Note 10 for customer concentrations.
The Company depends on one supplier for a key excipient (ingredient) used in its products and one supplier for one of the active pharmaceutical ingredients.
F - 12
On December 22, 2006, the Company acquired the U.S. rights to CRINONE (progesterone gel). The cost of the acquisition was $33,000,000 in cash and is being amortized over a 6.75-year period. On April 1, 2007, the Company recorded a liability from the contract with Merck Serono for certain sales returns associated with sales made by Merck Serono. The Company recorded the estimated liability of $1,000,000 as an increase in the purchase price that is being amortized over the remaining term of the license.
|
2009
|
2008
|
||
|
Balance at January 1
|
$ 34,000,000
|
$ 34,000,000
|
|
|
Accumulated amortization
|
(15,229,668)
|
(10,184,940)
|
|
|
Balance at December 31
|
$ 18,770,332
|
$ 23,815,060
|
Amortization expense amounted to $5,044,728, $5,044,728, and $5,005,768 in 2009, 2008, and 2007, respectively.
Amortization expenses for future periods are expected to be:
|
Year
|
Amortization
|
|
2010
|
$ 5,044,728
|
|
2011
|
5,044,728
|
|
2012
|
5,044,728
|
|
2013
|
3,636,148
|
|
Total
|
$ 18,770,332
|
Income Taxes -
Deferred tax assets or liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities, as measured by enacted tax rates. A valuation allowance is provided against deferred income tax assets in circumstances where management believes the recoverability of a portion of the assets is more likely than not. The Company has provided a full valuation allowance against its net deferred tax assets as of December 31, 2009 and 2008.
Long-lived Assets -
Following the acquisition of any long-lived assets, the Company continually evaluates whether later events and circumstances have occurred that indicate the remaining estimated useful life of the long-lived asset may warrant revision or that the remaining balance of the long-lived asset may not be recoverable. When factors indicate that a long-lived asset may be impaired, the Company uses an estimate of the underlying product's future cash flows, including amounts to be received over the remaining life of the long-lived asset from license fees, royalty income, and related revenue in measuring whether the long-lived asset is recoverable. Unrecoverable amounts are charged to operations.
Accrued Expenses -
Accrued expenses consist of the following:
|
2009
|
2008
|
||
|
Sales returns & price adjustments
|
$ 1,910,292
|
$ 2,789,316
|
|
|
Salaries
|
939,861
|
332,657
|
|
|
Interest
|
800,000
|
800,000
|
|
|
Professional fees
|
317,801
|
483,193
|
|
|
Inventory management fees
|
265,012
|
359,376
|
|
|
Marketing expenses
|
5,419
|
34,652
|
|
|
Royalties/Other
|
349,703
|
181,449
|
|
|
Total
|
$ 4,588,088
|
$ 4,980,643
|
F - 13
Revenue Recognition -
Revenues on sales of products by Columbia are discussed in detail below. License fees are recorded over the life of the license. Royalty revenues, based on sales by licensees, are recorded as revenue as those sales are made by the licensees.
Sales Return Reserves -
Revenues from the sale of products are recorded at the time goods are shipped to customers. The Company believes that it has not made any shipments in excess of its customers' ordinary course of business inventory levels. The Company’s return policy allows product to be returned for a period beginning three months prior to the product expiration date and ending twelve months after the product expiration date. Provisions for returns on sales to wholesalers, distributors and retail chain stores are estimated based on a percentage of sales, using such factors as historical sales information, distributor inventory levels and product prescription data, and are recorded as a reduction to sales in the same period as the related sales are recognized. The Company assumes that its customers are using the first-in, first-out method in filling orders so that the oldest saleable product is used first. The Company records a provision for returns on a quarterly basis using an estimated rate and adjusts the provision if its analysis indicates that the potential for product non-saleability exists.
An analysis of the reserve for sales returns is as follows:
|
2009
|
2008
|
2007
|
|||
|
Balance at beginning of year
|
$ 1,864,316
|
$ 1,923,765
|
$ 1,240,234
|
||
|
Addition related to Crinone®
|
-
|
-
|
1,000,000
|
||
|
Adjusted balance at beginning of year
|
1,864,316
|
1,923,765
|
2,240,234
|
||
|
Provision:
|
|||||
|
Related to current period sales
|
671,259
|
674,020
|
527,819
|
||
|
Related to prior period sales
|
555,098
|
725,971
|
500,000
|
||
|
1,226,357
|
1,399,991
|
1,027,819
|
|||
|
Returns:
|
|||||
|
Related to current period sales
|
(28,092)
|
(130,551)
|
(61,125)
|
||
|
Related to 2006 Crinone® purchase
|
(31,793)
|
(300,152)
|
(328,896)
|
||
|
Related to prior period sales
|
(1,147,165)
|
(1,028,737)
|
(954,267)
|
||
|
(1,207,050)
|
(1,459,440)
|
(1,344,288)
|
|||
|
Balance at end of period
|
$ 1,883,623
|
$ 1,864,316
|
$ 1,923,765
|
The Company believes that the greatest potential for uncertainty in estimating sales returns is the estimation of future prescriptions. They are wholly dependent on the Company’s ability to sell and market the products. If prescriptions are lower in future periods, then the current reserve will be inadequate.
In the fourth quarter of 2006, the Company purchased the U.S. rights to CRINONE for $33 million. In 2007, the Company recorded an estimated liability of $1.0 million for certain sales returns associated with sales made by Merck Serono.
Sales returns provisions for 2009 were $1.2 million of which $0.7 million was based on 2009 sales and the balance was related to sales from prior periods. Sales returns provisions for the year 2008 were $1.4 million of which $0.7 million was based on 2008 sales and the balance was based on previous year sales. In 2007, sales return provisions were $2.0 million including $1.0 million in the second quarter as an increase in the purchase price of the U.S. rights to CRINONE for future returns as a result of product sold by Merck Serono that was still in the channel at the time of purchase of such rights.
F - 14
License Fees -
License revenue consists of up-front, milestone and similar payments under license agreements and is recognized when earned under the terms of the applicable agreements. Milestone payments represent payments for the occurrence of contract-specified events and coincide with the achievement of a substantive element in a multi-element arrangement. License revenue, including milestone payments, is deferred and recognized in revenues over the estimated product life cycle or the length of relevant patents, whichever is shorter.
Payments to Distributors -
The Company estimates fees it pays its distributors and specialty pharmacies for customer services that include supplemental sales calling, providing information about their customers and the processing of sales returns. The fees for these services have historically been charged to selling and distribution expenses. In 2009 and 2008, these charges were split between selling and marketing expenses and as reduction to sales; the costs charged to selling and distribution expense in 2009 and 2008 were $0.2 million and $0.5 million, respectively. The costs charged as a reduction to sales in 2009 and 2008 were $0.6 million and $.07 million, respectively. In 2007 these fees were $0.6 million and were charged to selling and distribution expense.
Advertising Expense -
All costs associated with advertising and promoting products are expensed in the year incurred. Advertising and promotion expense was approximately $1.2 million in 2009, $1.9 million in 2008 and $0.9 million in 2007 and is included in selling and distribution expense.
Research and Development Costs -
Company-sponsored research and development costs related to future products are expensed as incurred.
Share-based compensation -
The Company recognizes compensation expense [ASC 718, “Share Based Payment”, formerly SFAS 123(R)], for all stock-based awards made to employees and directors including employee stock options based on estimated fair values.
ASC 718, “Share Based Payment”, requires companies to estimate the fair value of stock-based awards on the date of grant using an option pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s Consolidated Statements of Operations. Employee stock-based compensation expenses for the years ended December 31, 2009, 2008 and 2007, were $1,749,228, $ 1,314,571 and $1,490,059 respectively, which consisted primarily of stock-based compensation expense related to employee stock options.
Stock-based compensation expense recognized in the years ended December 31, 2009, 2008 and 2007 included compensation expense for share-based awards granted prior to, but not yet vested as of December 31, 2005, based on the fair value on the grant date estimated in accordance with the pro forma provisions of ASC 718,“Share Based Payment”, and compensation expense for the stock-based awards granted or modified subsequent to December 31, 2005, based on the fair value on the grant date estimated in accordance with the provisions of ASC 718. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Loss per Share -
Basic loss per share is computed by dividing the net loss plus preferred dividends by the weighted-average number of shares of Common Stock outstanding during the period. Diluted earnings per share gives effect to dilutive options, warrants and other potential Common Stock outstanding during the year. Shares to be issued upon the exercise of the outstanding options and warrants or the conversion of the preferred stock are not included in the computation of diluted loss per share as their effect is anti-dilutive. Outstanding options and warrants excluded from the calculation amounted to 16,903,059; 9,731,213; and 9,704,058 at December 31, 2009, 2008 and 2007, respectively.
F - 15
Cash Equivalents -
The Company considers all investments purchased with an original maturity of three months or less to be cash equivalents.
Reclassifications -
For comparability purposes, certain prior year amounts in the Consolidated Financial Statements have been reclassified, where appropriate, to conform to the financial statement presentation used in 2009.
Recent Accounting Pronouncements -
In October 2009, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements (a consensus of the FASB Emerging Issues Task Force), which amends ASC 605-25, Revenue Recognition: Multiple-Element Arrangements. ASU No. 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how to allocate consideration to each unit of accounting in the arrangement. This ASU replaces all references to fair value as the measurement criteria with the term selling price and establishes a hierarchy for determining the selling price of a deliverable. ASU No. 2009-13 also eliminates the use of the residual value method for determining the allocation of arrangement consideration. Additionally, ASU No. 2009-13 requires expanded disclosures. This ASU will become effective for revenue arrangements entered into or materially modified after the fiscal year 2010. Earlier application is permitted with required transition disclosures based on the period of adoption. We are currently evaluating the application date and the impact of this standard on our consolidated financial statements.
In June 2009, the FASB issued Statement of Financial Accounting Standards (“SFAS”) ASC 105 (formerly No. 168). The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles (the “Codification”). This standard replaces SFAS No. 162, The Hierarchy of Generally Accepted Accounting Principles, and establishes only two levels of U.S. generally accepted accounting principles (“GAAP”), authoritative and nonauthoritative. The FASB Accounting Standards Codification (“ASC”) will become the source of authoritative, nongovernmental GAAP, except for rules and interpretive releases of the SEC, which are sources of authoritative GAAP for SEC registrants. All other nongrandfathered, non-SEC accounting literature not included in the Codification will become nonauthoritative. This standard is effective for financial statements for interim or annual reporting periods ending after September 15, 2009. The adoption of the Codification changed the Company’s references to GAAP accounting standards but did not impact the Company’s results of operations, financial position or liquidity.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 805, Business Combinations (formerly SFAS No.141(R), Business Combinations). The new standard applies to all transactions or other events in which an entity obtains control of one or more businesses. Additionally, the new standard requires the acquiring entity in a business combination to recognize all (and only) the assets acquired and liabilities assumed in the transaction; establishes the acquisition-date fair value as the measurement date for all assets acquired and liabilities assumed; and requires the acquirer to disclose additional information needed to evaluate and understand the nature and financial effect of the business combination. ASC 805 will impact the Company in the event of any future acquisition.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 260, Earnings Per Share (formerly FASB Staff Position (“FSP”) Emerging Issues Task Force (“EITF”) 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities). The new guidance clarifies that non-vested share-based payment awards that entitle their holders to receive nonforfeitable dividends or dividend equivalents before vesting should be considered participating securities and included in basic earnings per share. The Company’s adoption of the new accounting standard did not have a material effect on previously reported or current earnings per share.
Effective January 1, 2009, the Company adopted a new accounting standard included in FASB ASC 470-20, “Debt with Conversion and other Options (Including Partial Cash Settlement) ,” which applies to all convertible debt instruments that have a net settlement feature; which means that such convertible debt instruments, by their terms, may be settled either wholly or partially in cash upon conversion. FASB ASC 470-20 requires issuers of convertible debt instruments that may be settled wholly or partially in cash upon conversion to separately account for the liability and equity components in a manner reflective of the issuer’s nonconvertible debt borrowing rate. Previous guidance provided for accounting for this type of convertible debt instrument entirely as debt. FASB ASC 470-20 was effective for financial statements issued for fiscal years beginning after December 15, 2008 and interim periods within those fiscal years. The adoption of the new accounting standard did not affect the Company’s financial statements.
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 815-40 “Determining Whether an Instrument (or an Embedded Feature) is Indexed to an Entity’s Own Stock” (“EITF 07-5”). The new standard provides that an entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. It also clarifies the impact of foreign currency denominated strike prices and market-based employee stock option valuation instruments on the evaluation. EITF 07-5 is effective for fiscal years beginning after December 15, 2008. The adoption of ASC 815-40 did not affect the Company’s financial statements.
F - 16
Effective January 1, 2009, the Company adopted a new accounting standard included in ASC 470 “Transition Guidance for Conforming to Issue No. 98-5 (“EITF no. 08-4”)”. The objective of the new standard to provide transition guidance for conforming changes made to EITF No. 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Rations,” that result from EITF No. 00-27 “Application of Issue No. 98-5 to Certain Convertible Instruments,” and SFAS No. 150, “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity.” This standard is effective for financial statements issued for fiscal years ending after December 15, 2008. The adoption of this new standard did not affect the Company’s accounting for the convertible notes and related warrants transactions.
(2) INCOME TAXES
Effective January 1, 2007, the Company adopted the provisions of FASB ASC 740, “Accounting for Uncertainty in Income Taxes - An Interpretation of FASB No 109.” FASB ASC 740 provides detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in the financial statements in accordance with SFAS No. 109. Tax positions must meet a “more-likely-than-not” recognition threshold at the effective date to be recognized upon the adoption of FASB ASC 740 and in subsequent periods. Upon the adoption of FASB ASC 740, the Company had no unrecognized tax benefits. During the year ended December 31, 2009, the Company recognized the expiration through December 31, 2009 of certain tax losses and had no adjustments for uncertain tax benefits.
The reconciliation of the effective income tax rate to the federal statutory rate is as follows:
|
2009
|
2008
|
2007
|
|||
|
Federal income tax rate
|
(34.0%)
|
(34.0%)
|
(35.0%)
|
||
|
Statutory rate over expected future
|
|||||
|
federal benefit
|
0.0%
|
0.0%
|
1.0%
|
||
|
Foreign income tax benefit/loss
|
(7.7%)
|
(18.2%)
|
(12.6%)
|
||
|
State tax net of federal benefit
|
(5.2%)
|
(11.0%)
|
(9.9%)
|
||
|
Permanent Items:
|
|||||
|
Incentive Stock Options
|
1.7%
|
1.0%
|
0.0%
|
||
|
R&D Credit
|
0.0%
|
0.0%
|
0.0%
|
||
|
Other
|
0.1%
|
1.0%
|
1.6%
|
||
|
Effect of permanent differences
|
1.8%
|
2.0%
|
1.6%
|
||
|
Effective income tax rate
|
(45.1%)
|
(61.2%)
|
(54.9%)
|
||
|
Increase in valuation allowance
|
43.5%
|
55.4%
|
49.7%
|
||
|
Effective income tax rate
|
(1.6%)
|
(5.8%)
|
(5.2%)
|
As of December 31, 2009, the Company has U.S. tax net operating loss carryforwards of approximately $162 million which expire through 2029. The Company also has unused tax credits of approximately $1.4 million which expire at various dates through 2028. Utilization of net operating loss carryforwards may be limited in any year due to limitations in the Internal Revenue Code.
F - 17
The Company recognizes interest and penalties, if any, related to uncertain tax positions in general and administrative expenses. No interest and penalties related to uncertain tax positions were accrued at December 31, 2009.
|
Deferred Tax Assets (Liabilities)
|
|||
|
2009
|
2008
|
||
|
Share Based awards compensation
|
$ 671,444
|
$ 533,937
|
|
|
Allowance for doubtful accounts
|
28,125
|
28,125
|
|
|
Allowance for returns
|
706,359
|
591,241
|
|
|
Inventory reserve
|
30,083
|
163
|
|
|
Book accumulated depreciation net of tax
|
(21,484)
|
(19,096)
|
|
|
Accum amortization - CRINONE license
|
3,138,209
|
2,096,436
|
|
|
Vacation & bonus accrual
|
24,652
|
15,000
|
|
|
Inventory capitalization
|
40,253
|
25,604
|
|
|
Patents
|
487,688
|
112,500
|
|
|
Long term debt (book amortization beneficial conversion)
|
(1,530,254)
|
(2,152,372)
|
|
|
Federal net operating loss
|
55,055,721
|
50,962,090
|
|
|
State net operating loss
|
2,948,608
|
2,573,071
|
|
|
Unused R&D credit
|
1,383,187
|
1,323,385
|
|
|
Contributions
|
488
|
-
|
|
|
Net Deferred Tax Assets
|
62,963,079
|
56,090,084
|
|
|
Less Valuation Allowance:
|
|||
|
Federal
|
(62,963,079)
|
(56,090,084)
|
|
|
Deferred Taxes Assets
|
$ -
|
$ -
|
In 2009, the Company reduced net operating loss amounts and valuation allowances by $7.5 million due to the expiration of tax losses.
The Company files federal income tax returns as well as multiple state, local and foreign jurisdiction tax returns. Tax years ended December 31, 2005 or later remain subject to examination by the IRS. State and local jurisdiction tax returns remain subject to examination for tax years ended December 31, 2005 or later.
(3) STRATEGIC ALLIANCE AGREEMENTS:
In May 1995, the Company entered into a worldwide license and supply agreement with American Home Products Corporation (“Wyeth”) under which its Wyeth-Ayerst Laboratories division marketed CRINONE. The Company supplied CRINONE to Wyeth at a price equal to 30% of Wyeth’s net selling price. On July 2, 1999, Wyeth assigned the license and supply agreement to Ares-Serono (now “Merck Serono”). In June 2002 the Company acquired the right to market a second brand of its 8% and 4% progesterone gel products under the trade name “PROCHIEVE® ” to obstetricians, gynecologists and all other physicians in the U.S. that were not on Merck Serono’s target list of fertility specialists. Under this agreement the Company paid a 30% royalty to Merck Serono based on net sales of the product and an additional royalty of 40% of PROCHIEVE’s net sales to the infertility specialist market. The Company paid approximately $1,365,000 to Merck Serono in accordance with this agreement in 2006. In December 2006, the Company acquired the U.S. marketing rights to CRINONE from Merck Serono and eliminated further PROCHIEVE royalty payments. The Company continues to supply CRINONE to Merck Serono for all non-U.S. requirements. During the year ended December 31, 2009, the Company recorded an adjustment to revenues of $320,000, primarily related to estimated price adjustments for CRINONE sold to Merck Serono in the fourth quarter of 2008. During the year ended December 31, 2008, the Company recorded an adjustment to revenues of $350,000 related to estimated price adjustments for CRINONE sold to Merck Serono in 2007. Also in 2008, the Company recorded a $575,000 charge for estimated 2008 price adjustments under the agreement. These adjustments are for the effects of government tenders awarded and foreign exchange differences from established rates at the beginning of each year. Our license and supply agreement with Merck Serono expires on May 19, 2010, and is renewable upon mutual agreement of the parties for five (5) year periods at commercial terms to be agreed upon. Merck Serono has informed us that they wish to renew the agreement for an additional 5 years, and we are in negotiations with them to renew the agreement on terms that are not materially different from the current agreement. There can be no assurance, however, that we will reach a renewal agreement or that any renewal agreement will not differ materially from the current agreement.
In March 1999, the Company entered into a license and supply agreement with Mipharm SpA under which Mipharm SpA will be the exclusive marketer of the Company’s previously unlicensed women’s healthcare products in Italy, Portugal, Greece and Ireland, with a right of first refusal for Spain. Under the terms of the agreement, the Company has received $0.5 million, net of expenses, and expects to receive future milestone payments as products are made available by the Company.
F - 18
Effective May 5, 2000, the Company licensed its Legatrin® PM brand to Lil’ Drug Store. Under the terms of this agreement, the Company receives license fees equal to 20% of the licensee’s net sales of Legatrin PM. This agreement had a five-year term with provisions for renewal and contains an option that allows the licensee to acquire this brand from the Company. The license for Advanced Formula Legatrin PM renewed automatically to May 2010.
On October 16, 2002, the Company and Ardana entered into a license and supply agreement for STRIANT in 18 European countries (excluding Italy). Under the agreement the Company received $6.0 million. In July 2008, the Company terminated the development and license agreement pursuant to its rights under the agreement to terminate it in the event of the insolvency of Ardana. Ardana announced in June 2008 that it suspended trading in its shares, was no longer in a position to continue its operations, and had appointed administrators of the company. In the quarter ended September 30, 2008, the Company recognized $2.9 million of deferred revenue from the cancellation of the agreement. The Company recognized license revenue under this agreement of$0.0, $3.2 and $0.7 million in 2009, 2008, and 2007, respectively.
In May 2003, the Company and Mipharm entered into an agreement under which Mipharm will market, distribute and sell STRIANT in Italy. In exchange for these rights, Mipharm is obligated to pay the Company an aggregate of $1.4 million upon achievement of certain milestone events, including $350,000 that was paid in 2003. The Company received a payment of $100,000, less VAT withholding, in 2004 on account of the UK approval of STRIANT and a payment of $150,000, less VAT withholding, in 2007 on marketing authorization in Italy in late 2006. Mipharm will provide additional performance payments upon the achievement of certain levels of sales in Italy, and the Company will receive a percentage markup on the cost of goods for each unit sold. Mipharm is a manufacturer of STRIANT under a May 2002 agreement. The Company is recognizing the license revenue on this agreement over a 132 month period and accordingly has recognized revenue of $53,199 in 2009 and 2008, and $41,574 in 2007. The remaining $254,636 as of December 31, 2009 is shown as part of deferred revenue in the accompanying consolidated balance sheets.
On September 27, 2007, the Company entered into a License and Supply Agreement with Ascend Therapeutics, Inc. (“Ascend”), pursuant to which the Company granted Ascend an exclusive, five year license to market and sell the Company’s PROCHIEVE 4% (progesterone gel) product in the U.S. effective January 1, 2008. Ascend will purchase product from Columbia at a transfer price equal to 35% of Ascend’s net selling price with minimum annual purchase obligations that increase over the life of the agreement. On January 21, 2010, Ascend notified the Company that it was terminating the license and supply agreement as of July 23, 2010, pursuant to the terms of the agreement.
(4) NOTES PAYABLE:
On December 22, 2006, the Company raised approximately $40 million in gross proceeds to the Company from the sale of convertible subordinated notes to a group of existing institutional investors. The notes bear interest at a rate of 8% per annum and are subordinated to the PharmaBio financing agreements (see Note 5) and mature on December 31, 2011. They are convertible into a total of approximately 7.6 million shares of Common Stock at a conversion price of $5.25. Investors also received warrants to purchase 2,285,714 shares of Common Stock at an exercise price of $5.50 per share. The warrants became exercisable on June 20, 2007, and expire on December 22, 2011, unless earlier exercised or terminated. The Company used the proceeds of this offering to acquire from Merck Serono the U.S. marketing rights to CRINONE for $33.0 million and purchased Merck Serono’s existing inventory of that product. The balance of the proceeds was used to pay other costs related to the transaction and for general corporate purposes.
The Company recorded original issue discounts of $6,272,566 to the notes based upon the fair value of warrants granted. In addition, beneficial conversion features totaling $8,482,090 have been recorded as a discount to the notes. These discounts are being amortized at an imputed rate over the five year term of the related notes. For the years ended December 31, 2009, 2008 and 2007, $2,890,897, $2,535,788 and $2,237,043, respectively, of amortization related to these discounts is classified as interest expense in the consolidated statements of operations. Unamortized discounts of $7,034,137 and $9,925,034 have been reflected as a reduction to the face value of the convertible notes in the consolidated balance sheets as of December 31, 2009 and 2008, respectively.
F - 19
(5) FINANCING AGREEMENTS:
In an agreement dated July 31, 2002, PharmaBio Development (“PharmaBio”), agreed to pay $4.5 million, to be paid in four equal quarterly installments commencing third quarter 2002, for the right to receive a 5% royalty on the net sales of the Company’s women’s healthcare products in the U.S. for five years beginning in the first quarter of 2003. The royalty payments were subject to minimum ($8 million) and maximum ($12 million) amounts, and because the minimum amount exceeds $4.5 million, the Company has recorded the amounts received as liabilities. The excess of the minimum ($8 million) to be paid by the Company over the $4.5 million received by the Company is being recognized as interest expense over the five-year term of the agreement, assuming an interest rate of 17%. The Company recorded $0, $0 and $617,016 as interest expense for the years 2009, 2008 and 2007, respectively. The agreement called for a true-up payment, if by February 28, 2005, the Company had not made $2,750,000 in royalty payments to PharmaBio. The amounts paid to PharmaBio were $0 for 2009, $0 for 2008 and $647,884 for 2007. The final payment of $3.6 million was made on February 29, 2008.
In an agreement dated March 5, 2003 (the “STRIANT Agreement”), PharmaBio agreed to pay the Company $15 million in five quarterly installments commencing with the signing of the STRIANT Agreement. In return, PharmaBio will receive a 9% royalty on net sales of STRIANT in the U.S. up to agreed annual sales revenues, and a 4.5% royalty of net sales above those levels. The royalty term is seven years. Royalty payments commenced in the 2003 third quarter and are subject to minimum ($30 million) and maximum ($55 million) amounts. Because the minimum amount exceeds the $15 million received by the Company, the Company has recorded the amounts received as liabilities. The excess of the minimum ($30 million) to be paid by the Company over the $15 million received by the Company is being recognized as interest expense over the seven-year term of the STRIANT Agreement, assuming an interest rate of 15%. The Company recorded $2,245,647, $1,878,364 and $1,648,756 as interest expense in 2009, 2008 and 2007, respectively. The STRIANT Agreement called for a true-up payment on November 14, 2006 equal to the difference between royalties paid through and for the third quarter of 2006 and $13,000,000. On April 14, 2006, the Company entered into a letter agreement (the “Letter Agreement”) with PharmaBio pursuant to which the Company agreed to pay approximately $12 million of this true-up payment seven months early. Accordingly, on April 14, 2006, the Company paid PharmaBio $11,585,235 (the “Early Payment”), which was the present value of a November 14, 2006 $12 million true-up payment using a six percent (6%) annual discount factor. In consideration of such payment, PharmaBio agreed that PharmaBio would be deemed (solely for purposes of the STRIANT Agreement) to have received on account of that payment $12 million for purposes of the true-up payment. In the event that, as of the payment date for the true-up payment, the aggregate amount of royalties paid under the STRIANT Agreement, including the Early Payment, exceeded $13 million, the Company would have been entitled to have such excess reimbursed. Including the Early Payment, the Company has paid PharmaBio approximately $13.5 million through 2009.The balance of the minimum royalty payments, estimated to be $16.5 million, is due November 2010.
Liabilities from financing agreements consist of the following:
|
December 31
|
|||
|
2009
|
2008
|
||
|
STRIANT Agreement
|
$ 15,379,303
|
$ 13,294,244
|
|
|
Less: current portion
|
144,897
|
168,034
|
|
|
Total
|
$ 15,234,406
|
$ 13,126,210
|
|
On July 22, 2009, the Company and PharmaBio entered into an amendment (the “Second Amendment”) to the STRIANT Agreement, in which they agreed that when the minimum royalty payment is due the Company may, in its sole discretion, either pay the balance due under the STRIANT Agreement or issue to PharmaBio a secured promissory note for that balance. In consideration for the right to issue the secured promissory note, the Company has (a) agreed that during the period from July 22, 2009 through November 30, 2010, the Company will escrow any proceeds from sales of assets outside the ordinary course of business in excess of $15.0 million but not exceeding the difference between the amount of royalties actually received by PharmaBio under the STRIANT Agreement and $30.0 million, and (b) granted PharmaBio a warrant to purchase 900,000 shares of the Company’s Common Stock. In further consideration for the right to issue the secured promissory note, the Company has agreed that if it issues the secured promissory note on November 30, 2010, the Company will on that date grant PharmaBio a second warrant to purchase 900,000 shares of the Company’s Common Stock. Each warrant is exercisable beginning November 30, 2010 and expires on the date five years from its issue date. The warrants are exercisable at $1.15 per share, permit cashless exercise, and provide piggyback registration rights. If the Company issues the secured promissory note, it would bear interest quarterly in arrears at the rate of 10% per annum, be due on November 30, 2011, be secured by Columbia’s assets, and contain customary representations, warranties, and events of default. Using the Black Scholes valuation model, the Company determined the value of the initial warrant to purchase 900,000 shares of the Company’s Common Stock to be $719,904, or $0.80 per share, which is being amortized over the 16 months through November 2010. The amortization expense recorded in the quarter ended December 31, 2009 was approximately $225,000.
F - 20
(6) CONTINGENTLY REDEEMABLE SERIES C CONVERTIBLE PREFERRED STOCK
In January 1999, the Company raised approximately $6.4 million, net of expenses, from the issuance and sale of Series C Convertible Preferred Stock (“Series C Preferred Stock”). The Series C Preferred Stock has a stated redemption value of $1,000 per share. The Series C Preferred Stock is convertible into Common Stock at the lower of: (i) $3.50 per common share or (ii) 100% of the average of the closing prices during the three trading days immediately preceding the conversion notice (not to exceed 2,705,236 shares). The Series C Preferred Stock pays a 5% dividend, payable quarterly in arrears on the last day of the quarter. In 2003, 500 shares of Series C Preferred Stock were converted into 142,857 Common Shares; in 2006, 50 shares of Series C Preferred Stock were converted into 14,285 Common Shares, in 2007; 2,075 shares of Series C Preferred Stock were converted into 1,564,548 Common Shares; in 2008, 350 shares of Series C Preferred Stock were converted into 235,426 Common Shares and in 2009, 175 shares of Series C Preferred Stock were converted into 117,449 Common Shares. Each holder of Series C Preferred Stock has the right to redeem all or a portion of their shares in cash and upon the occurrence of certain events under the Series C Preferred Stock certificate of designations.
(7) SHAREHOLDERS’ EQUITY
Preferred Stock - Authorized Preferred Stock is 1,000,000 shares at a par value of $0.01 per share.
In August 1991, the Company completed a private placement of 150,000 shares of Series B Convertible Preferred Stock (“Series B Preferred Stock”). Each share of Series B Preferred Stock is convertible into 20 shares of Common Stock. At December 31, 2009 only 130 shares remain outstanding.
Upon liquidation of the Company, the holders of the Series B Preferred Stock are entitled to $100 per share. The Series B Preferred Stock will be automatically converted into Common Stock upon the occurrence of certain events. Holders of the Series B Preferred Stock are entitled to one vote for each share of Common Stock into which the preferred stock is convertible.
On March 12, 2002, the Company adopted a Shareholder Rights Plan (“Rights Plan”) designed to protect company shareholders in the event of takeover activity that would deny them the full value of their investment. The Rights Plan was not adopted in response to any specific takeover threat. In adopting the Rights Plan, the Board declared a dividend distribution of one preferred stock purchase right for each outstanding share of Common Stock of the Company, payable to shareholders of record at the close of business on March 22, 2002. The rights will become exercisable only in the event, with certain exceptions, a person or group of affiliated or associated persons acquires 15% or more of the Company’s voting stock, or a person or group of affiliated or associated persons commences a tender or exchange offer, which if successfully consummated, would result in such person or group owning 15% or more of the Company’s voting stock. The rights will expire on March 12, 2012. Each right, once exercisable, will entitle the holder (other than rights owned by an acquiring person or group) to buy one one-thousandth of a share of a series of the Company’s Series D Junior Participating Preferred Stock at a price of $30 per one-thousandth of a share, subject to adjustments. In addition, upon the occurrence of certain events, holders of the rights (other than rights owned by an acquiring person or group) would be entitled to purchase either the Company’s preferred stock or shares in an “acquiring entity” at approximately half of market value. Further, at any time after a person or group acquires 15% or more (but less than 50%) of the Company’s outstanding voting stock, subject to certain exceptions, the Board of Directors may, at its option, exchange part or all of the rights (other than rights held by an acquiring person or group) for shares of the Company’s Common Stock having a fair market value on the date of such acquisition equal to the excess of (i) the fair market value of preferred stock issuable upon exercise of the rights over (ii) the exercise price of the rights. The Company generally will be entitled to redeem the rights at $0.01 per right at any time prior to the close of business on the tenth day after there has been a public announcement of the beneficial ownership by any person or group of 15% or more of the Company’s voting stock, subject to certain exceptions. These rights are deemed to have no value and accordingly have not been recorded in the accompanying financial statements.
On May 10, 2005, the Company raised $6.9 million from the issuance and sale of 69,000 shares of Series E Convertible Preferred Stock (“Series E Preferred Stock”). The Series E Preferred Stock has a stated value of $100 per share. Each share of the Series E Preferred Stock may be converted by the holder into 50 shares of Common Stock, subject to adjustment, and will automatically be converted into Common Stock at that rate upon the date that the average of the daily market prices of the Company’s Common Stock for the 20 consecutive trading days preceding such date exceeds $6.00 per share. The Series E Preferred Stock pays no dividends and contains voting rights equal to the number of shares of Common Stock into which each share of Series E Preferred Stock is convertible. Upon liquidation of the Company, the holders of the Series E Preferred Stock are entitled to $100 per share. In 2007, 5,453 shares of Series E Preferred Stock were converted into 272,650 shares of Common Stock. In 2008, 4,547 shares of Series E Preferred Stock were converted into 227,350 shares of Common Stock.
F - 21
Common Stock -
During the first quarter of 2009, the Company issued 451,807 shares of Common Stock in a registered offering with gross proceeds of $750,000. During the fourth quarter of 2009, the Company issued 10,900,000 shares of Common Stock in a registered offering with proceeds net of offering costs of $10,706,305. Also, in 2009, 175 shares of Series C Preferred Stock were converted into 117,449 shares of Common Stock. During 2009, no options were exercised. The Company granted 326,776 shares of restricted stock to its key employees and to members of the Board of Directors.
During 2008, the Company issued 1,330,000 shares of Common Stock in an offering with proceeds net of offering costs of $4,095,628. The Company issued 318,149 shares of Common Stock for the exercise of stock options for proceeds of $594,855. Also, in 2008, 350 shares of Contingently Redeemable Series C Preferred Stock were converted into 235,426 Shares of Common Stock and 4,547 shares of Series E Preferred Stock were converted into 227,350 Shares of Common Stock. The Company granted 163,503 shares of restricted stock to its key employees and to members of the Board of Directors.
During 2007, the Company issued 43,050 shares of Common Stock for the exercise of stock options with proceeds of $63,241, and 155,690 shares of restricted Common Stock were granted to its key employees and to members of the Board of Directors. Also, in 2007, 2,075 shares of Contingently Redeemable Series C Preferred Stock were converted into 1,564,548 Shares of Common Stock.
As of December 31, 2009, the Company had warrants outstanding for the purchase shares of Common Stock. Information on outstanding warrants is as follows:
|
Weighted Average Exercise Price
|
Warrants
|
|
|
$ 1.15
|
900,000
|
|
|
1.52
|
5,450,000
|
|
|
5.39
|
1,857,041
|
|
|
5.50
|
2,285,714
|
|
|
5.85
|
100,000
|
|
|
8.35
|
350,000
|
|
|
$ 3.24
|
10,942,755
|
|
During 2009, warrants to purchase 900,000 shares of the Company’s Common Stock at an exercise price of $1.15 per share were issued in conjunction with the right to issue the secured promissory note to PharmaBio which by their terms expire November 30, 2015. Also in 2009, warrants to purchase 5,450,000 shares of the Company’s Common Stock at an exercise price of $1.52 per share were issued to investors in the October 2009 financing which by their terms expire on April 30, 2015.
During 2006, warrants to purchase 1,857,041 shares of the Company’s Common Stock at an exercise price of $5.39 per share were issued to investors in the March 2006 financing which by their terms expire March 11, 2011. Also in 2006, warrants to purchase 2,285,714 shares of the Company’s Common Stock at an exercise price of $5.50 per share were issued to investors in the December 2006 financing which by their terms expire on December 22, 2011.
No warrants were issued in 2008 and 2007. No warrants were exercised in 2009, 2008, or 2007.
F - 22
(8) STOCK-BASED COMPENSATION
The following table summarizes stock-based compensation costs for the years ended December 31, 2009, 2008 and 2007:
|
2009
|
2008
|
2007
|
|||
|
Employee stock-based compensation in:
|
|||||
|
Cost of revenue
|
$ 73,630
|
$ 68,046
|
$ 148,589
|
||
|
Selling and distribution
|
450,226
|
175,375
|
160,573
|
||
|
General and administrative
|
1,085,785
|
973,148
|
1,014,702
|
||
|
Research and development
|
139,587
|
98,002
|
166,195
|
||
|
Total employee stock-based compensation
|
|||||
|
in operating expenses
|
1,675,598
|
1,246,525
|
1,341,470
|
||
|
Total employee stock-based compensation
|
$ 1,749,228
|
$ 1,314,571
|
$ 1,490,059
|
Stock based compensation for consultants amounted to $222,554, $32,820 and $157,863 for 2009, 2008 and 2007, respectively. No tax benefit has been recognized due to net losses during the periods presented.
As of December 31, 2009, total unamortized share-based compensation cost related to non-vested stock options was $900,525 which is expected to be recognized over the remaining vesting period of the outstanding options, up to the next 47 months. The Company selected the Black-Scholes option pricing model as the most appropriate model for determining the estimated fair value for share-based awards. The use of the Black-Scholes model requires the use of extensive actual employee exercise behavior data and the use of a number of complex assumptions including expected volatility, risk-free interest rate, and expected dividends.
The assumptions used to value options granted are as follows:
|
2009
|
2008
|
2007
|
|||
|
Risk free interest rate
|
1.71%
|
2.25%
|
4.53%
|
||
|
Expected term
|
4.2 years
|
4.75 years
|
4.52 years
|
||
|
Dividend yield
|
0.0
|
0.0
|
0.0
|
||
|
Expected volatility
|
93.04%
|
85.49%
|
85.68%
|
The Company estimated the volatility of its stock based on expected volatility of the Company’s stock which includes consideration of historical volatility in accordance with guidance in ASC 718 and SAB 110 (Expensing Employee Stock Options). The Company did not consider implied volatility because there are no comparable options traded on its stock. The risk-free interest rate assumption is based upon observed interest rates appropriate for the estimated term of the employee stock options. The dividend yield assumption is based on the Company’s history and expectation of dividend payouts on Common Stock.
The expected term of employee stock options represents the weighted-average period that employees are expected to hold the options before exercise. The Company derived the expected term assumption based on the Company’s historical settlement experience, while giving consideration to options that have life cycles less than the contractual terms and vesting schedules in accordance with guidance in ASC 718,“Share Based Payment”, formerly SFAS 123(R) and SAB 110.
F - 23
Stock Option Plans -
In May of 2008, the Company adopted the 2008 Long-term Incentive Plan (“2008 Plan”) which provides for the grant of stock options, stock appreciation rights and restricted stock to certain designated employees of the Company, Non-Employee directors of the Company and certain other persons performing significant services for the Company as designated by the Compensation Committee of the Board of Directors. Six million shares of Common Stock have been reserved for issuance under the 2008 Plan.
In October 1996, the Company adopted the 1996 Long-term Performance Plan (“1996 Plan”) which provides for the grant of stock options, stock appreciation rights and restricted stock to certain designated employees of the Company, non-employee directors of the Company and certain other persons performing significant services for the Company as designated by the Compensation/Stock Option Committee of the Board of Directors. Upon approval of the 2008 Plan, the Company stopped granting options under the 1996 Plan.
The Company’s stock options have a maximum term of ten years from the date of grant. Options granted prior to 2006 have a ten year term. Since 2006, the Company has been granting stock options with a seven year term. Options generally vest over a four-year period, with 25% vesting on each of the first four anniversaries of the date of grant. The 2007 annual option grant to employees vested 25% of the grant upon the grant date with the balance to vest equally over the next three years. The 2008 annual grant vests over 4 years. The Company’s general policy is to issue new shares upon the exercise of stock options
A summary of the status of the Company’s two stock option plans as of December 31, 2009, 2008, and 2007 is presented below:
|
2009
|
2008
|
2007
|
||||||
|
Weighted-
|
Weighted-
|
Weighted-
|
||||||
|
Average
|
Average
|
Average
|
||||||
|
Exercise
|
Exercise
|
Exercise
|
||||||
|
Price
|
Price
|
Shares
|
Price
|
|||||
|
Outstanding at beginning of year
|
4,863,488
|
$3.47
|
4,936,335
|
$4.64
|
4,686,552
|
$8.57
|
||
|
Granted
|
1,842,525
|
1.40
|
1,275,700
|
2.58
|
1,710,850
|
1.50
|
||
|
Exercised
|
-
|
-
|
(318,149)
|
1.87
|
(43,050)
|
1.47
|
||
|
Forfeited
|
(745,709)
|
4.05
|
(1,030,398)
|
9.10
|
(1,418,017)
|
12.84
|
||
|
Outstanding at end of year
|
5,960,304
|
2.70
|
4,863,488
|
3.47
|
4,936,335
|
4.64
|
||
|
Options exercisable at year end
|
3,168,023
|
2,688,841
|
3,196,121
|
|||||
The weighted average grant date fair values of options granted in 2009, 2008 and 2007 was $1.40, $2.58 and $1.50 per share respectively.
The weighted average exercise price and the weighted average remaining contractual life of the outstanding options expected to vest at December 31, 2009 amounted to $3.41 and 3.7 years, respectively.
The aggregate intrinsic value of options outstanding, options expected to vest and options exercisable at December 31, 2009 were $0, $0 and $0, respectively. The intrinsic value of options exercised in 2008, and 2007, respectively, were $1,121,000 and $97,000.
During 2009, there was no cash received from the exercise of options.
Restricted stock grants consist of grants of the Company’s Common Stock that may vest in the future. The Board has set a one, two, or four year vesting period for most of the issued restricted shares. The fair value of each restricted share grant is equal to the market price of the Company’s Common Stock at the date of grant. Expense relating to restricted shares is at the closing price amortized ratably over the vesting period.
F - 24
A summary of the Company’s restricted stock activity and related information for 2009 is as follows:
|
2009
|
2008
|
2007
|
||||||||||
|
Weighted-
|
Weighted-
|
Weighted-
|
||||||||||
|
Average Grant
|
Average Grant
|
Average Grant
|
||||||||||
|
Shares
|
Date Fair Value
|
Shares
|
Date Fair Value
|
Shares
|
Date Fair Value
|
|||||||
|
Unvested at beginning of period
|
238,115
|
$ 2.23
|
197,096
|
$ 2.36
|
146,875
|
$ 4.49
|
||||||
|
Granted
|
326,776
|
1.31
|
172,553
|
2.36
|
159,390
|
1.79
|
||||||
|
Vested
|
(186,227)
|
3.70
|
(122,484)
|
2.94
|
(105,469)
|
1.69
|
||||||
|
Forfeited
|
(41,625)
|
1.66
|
(9,050)
|
1.94
|
(3,700)
|
1.40
|
||||||
|
Unvested at December 31,
|
337,039
|
$ 1.55
|
238,115
|
$ 2.23
|
197,096
|
$ 2.36
|
||||||
As of December 31, 2009, there was $0.3 million of total unrecognized compensation costs related to non-vested restricted share-based compensation. The remaining cost is expected to be recognized over a weighted average period of 0.7 years. The total fair value of shares vested during the years ended December 31, 2009, 2008 and 2007 was $0.7 million, $0.4 million and $0.2 million respectively.
(9) COMMITMENTS AND CONTINGENCIES:
Cash and cash equivalents -
The Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. The Company believes that there is no credit risk with respect to these accounts.
Leases -
The Company leases office space and office equipment under noncancelable operating leases. Lease expense for each of the three years ended December 31, 2009, 2008 and 2007 totaled $308,079, $330,772 and $209,478, respectively. Future minimum lease payments as of December 31, 2009 are as follows:
|
2010
|
$ 271,226
|
|
|
2011
|
244,532
|
|
|
2012
|
233,066
|
|
|
2013
|
187,031
|
|
|
Beyond
|
-
|
|
|
Total
|
$ 935,855
|
F - 25
Royalties -
In 1989, the Company purchased the assets of Bio-Mimetics Inc., which assets consisted of the patents underlying the Company’s Bioadhesive Delivery System (BDS), other patent applications and related technology, for $2,600,000, in the form of 9% convertible debentures which were converted into 500,000 shares of Common Stock during 1991, and $100,000 in cash. In addition, Bio-Mimetics, Inc. is entitled to a royalty equal to 2% of the net sales of products based on the BDS up to an aggregate amount of $7,500,000. The royalty payments are payable over the life of the patent(s) which are specific to each product or fifteen years, whichever is longer. The Company is required to prepay 25% of the remaining royalty obligation, in cash or stock at the option of the Company, if the closing price of the Company’s Common Stock is $20 or more on March 2, or within 30 days after the date, of any year. The Company may not assign the patents underlying the BDS without the prior written consent of Bio-Mimetics, Inc. until the aggregate royalties have been paid. Royalty expense under this agreement amounted to $104,967, $132,023, and $114,466 in 2009, 2008 and 2007, respectively. See “Legal Proceedings”
Legal Proceedings -
Claims and lawsuits have been filed against the Company from time to time. Although the results of pending claims are always uncertain, the Company does not believe the results of any such actions, individually or in the aggregate, will have a material adverse effect on the Company’s financial position or results of operation. Additionally, the Company believes that it has adequate reserves or adequate insurance coverage in respect of these claims, but no assurance can be given as to the sufficiency of such reserves or insurance in the event of any unfavorable outcome resulting from these actions.
In connection with the 1989 purchase of the assets of Bio-Mimetics, Inc., which assets consisted of the patents underlying the Company's BDS, other patent applications, and related technology, the Company agreed to pay Bio-Mimetics a royalty equal to two percent of the net sales of products based on the assets up to an aggregate of $7.5 million or until the last of the relevant patents expired. The Company determined that the obligation to pay royalties on STRIANT, PROCHIEVE, and CRINONE terminated in September of 2006, with the expiration of a certain Canadian patent, but continues on Replens® and RepHresh®. On December 28, 2007, Bio-Mimetics filed a complaint in the U.S. District Court for Massachusetts (Bio-Mimetics, Inc. v. Columbia Laboratories, Inc.) alleging breach of contract, violation of the covenant of good faith and fair dealing, and unjust enrichment for the Company’s failure to continue royalty payments on STRIANT, PROCHIEVE, and CRINONE. To date, the Company has paid approximately $3.9 million in royalty payments and Bio-Mimetics seeks a judgment that we are obligated to pay the remaining $3.6 million in full. The Company has denied all such allegations and believes it has no contractual liability to Bio-Mimetics for the disputed royalty payments and intends to defend this action vigorously.
F - 26
(10) GEOGRAPHIC INFORMATION AND CUSTOMER CONCENTRATION:
Geographic Information -
The Company and its subsidiaries are engaged in one line of business, the development, licensing and sale of pharmaceutical products. The Company conducts its international business through its Bermuda subsidiary which contracts with various manufacturers located in the United Kingdom, Switzerland and Italy, to make product for both its international and domestic operations. Most arrangements with licensees are made by the Bermuda company. These customers sell their products into several countries. The Company’s two largest international customers are Merck Serono and Lil’ Drug Store.
The following table shows selected information by geographic area:
|
(Loss) profit from
|
Identifiable
|
|||||
|
Revenues
|
Operations
|
Assets
|
||||
|
As of and for the year
|
||||||
|
ended December 31, 2009-
|
||||||
|
United States
|
$ 19,997,178
|
$ (18,771,745)
|
$ 37,263,748
|
|||
|
Switzerland
|
8,610,429
|
5,608,298
|
6,493,471
|
|||
|
Other
|
3,588,774
|
- |
-
|
|||
|
Subtotal International
|
12,199,203
|
5,608,298
|
6,493,471
|
|||
|
Total
|
$ 32,196,381
|
$ (13,163,447)
|
$ 43,757,219
|
|||
|
As of and for the year
|
||||||
|
ended December 31, 2008-
|
||||||
|
United States
|
$ 17,950,995
|
$ (14,978,075)
|
$ 38,806,638
|
|||
|
Switzerland
|
9,168,230
|
7,720,690
|
6,815,851
|
|||
|
Other
|
9,109,889
|
-
|
-
|
|||
|
Subtotal International
|
18,278,119
|
7,720,690
|
6,815,851
|
|||
|
Total
|
$ 36,229,114
|
$ (7,257,385)
|
$ 45,622,489
|
|||
|
As of and for the year
|
||||||
|
ended December 31, 2007-
|
||||||
|
United States
|
$ 15,257,884
|
$ (14,339,632)
|
$ 49,253,642
|
|||
|
Switzerland
|
8,101,831
|
6,231,784
|
7,335,635
|
|||
|
Other
|
6,267,923
|
-
|
-
|
|||
|
Subtotal International
|
14,369,754
|
6,231,784
|
7,335,635
|
|||
|
Total
|
$ 29,627,638
|
$ (8,107,848)
|
$ 56,589,277
|
|||
F - 27
Customer Concentration -
The following table presents information about Columbia’s revenues by customer, including royalty and license revenue for the three years ended December 31:
|
2009
|
2008
|
2007
|
% Chg 2009
|
|||||
|
MerckSerono
|
$ 8,627,333
|
$ 9,168,230
|
$ 8,151,292
|
-5.90%
|
||||
|
Lil' Drug Store Products, Inc.
|
6,592,945
|
6,218,949
|
5,958,925
|
6.01%
|
||||
|
Cardinal Healthcare
|
5,662,507
|
5,612,748
|
6,098,510
|
0.89%
|
||||
|
McKesson
|
4,574,128
|
4,990,960
|
3,888,354
|
-8.35%
|
||||
|
All others (none over 5%)
|
6,739,468
|
10,238,227
|
5,530,557
|
-34.17%
|
||||
|
Total
|
$ 32,196,381
|
$ 36,229,114
|
$ 29,627,638
|
-11.13%
|
||||
Revenue by Product
The following table sets forth the breakdown of the Company's consolidated net revenues, consisting of sales, royalty and licensing income, by revenue source for each product accounting for 10% or more of consolidated revenues in any of the three years ended December 31:
|
2009
|
2008
|
2007
|
||||
|
CRINONE®
|
$ 22,166,867
|
$ 21,439,847
|
$ 18,904,512
|
|||
|
RepHresh®
|
3,021,944
|
3,316,193
|
3,326,927
|
|||
|
PROCHIEVE®
|
1,642,161
|
2,697,255
|
1,527,727
|
|||
|
Other
|
5,365,409
|
8,775,819
|
5,868,472
|
|||
|
Total
|
$ 32,196,381
|
$ 36,229,114
|
$ 29,627,638
|
F - 28
The following table summarizes selected quarterly data for the years ended December 31, 2009 and 2008:
|
2009
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Full
Year
|
|||||
|
Net sales
|
$ 7,224,370
|
$ 8,286,623
|
$ 7,807,944
|
$ 8,443,644
|
$ 31,762,581
|
|||||
|
Fee income and other
|
||||||||||
|
income
|
96,817
|
137,081
|
95,013
|
104,889
|
433,800
|
|||||
|
Gross profit
|
5,541,169
|
6,084,353
|
5,341,397
|
6,034,924
|
23,001,843
|
|||||
|
Loss from operations
|
(3,247,187)
|
(3,060,393)
|
(3,422,766)
|
(3,433,101)
|
(13,163,447)
|
|||||
|
Net loss
|
(5,333,567)
|
(5,240,661)
|
(5,853,262)
|
(5,442,068)
|
(21,869,558)
|
|||||
|
Basic and diluted loss
|
||||||||||
|
per common share*
|
$ (0.10)
|
$ (0.10)
|
$ (0.11)
|
$ (0.09)
|
$ (0.39)
|
|||||
|
2008
|
||||||||||
|
Net sales
|
$ 8,606,298
|
$ 8,827,369
|
$ 8,141,662
|
$ 6,893,915
|
$ 32,469,244
|
|||||
|
Fee income and other
|
||||||||||
|
income
|
306,637
|
320,252
|
2,975,696
|
157,285
|
3,759,870
|
|||||
|
Gross profit
|
5,952,838
|
6,197,812
|
8,257,768
|
4,886,081
|
25,294,499
|
|||||
|
Loss from operations
|
(2,378,635)
|
(2,399,902)
|
(86,884)
|
(2,391,964)
|
(7,257,385)
|
|||||
|
Net loss
|
(4,248,795)
|
(4,326,172)
|
(2,050,136)
|
(3,451,406)
|
(14,076,509)
|
|||||
|
Basic and diluted loss
|
||||||||||
|
per common share*
|
$ (0.08)
|
$ (0.08)
|
$ (0.04)
|
$ (0.06)
|
$ (0.27)
|
|||||
* The addition of earnings (loss) per share by quarter may not equal total earnings (loss) per share for the year.
F - 29
12) SUBSEQUENT EVENT
In connection with the preparation of the Company’s financial statements at December 31, 2009, subsequent events have been evaluated through the date of the filing of this Form 10-K.
Watson Transaction and Debt Restructuring
On March 3, 2010, the Company, Watson Pharmaceuticals, Inc., as a guarantor of the Buyer’s obligations (“Watson”), and Coventry Acquisition, Inc., a subsidiary of Watson (the “Buyer”), entered into a Purchase and Collaboration Agreement (the “Purchase Agreement”). Pursuant to the Purchase Agreement, the Company agreed to sell, subject to shareholder approval, to the Buyer (i) substantially all of its assets primarily relating to the research, development, regulatory approval, manufacture, distribution, marketing, sale and promotion of pharmaceutical products containing progesterone as an active ingredient, including CRINONE 8% progesterone gel, PROCHIEVE 4% progesterone gel and PROCHIEVE 8% progesterone gel, each sold by the Company in the U.S. (collectively, the “Progesterone Products”), including certain intellectual property, promotional materials, contracts, product data and regulatory approvals and regulatory filings (the “Purchased Assets”), and (ii) 11,200,000 shares (the “Shares”) of the Company’s Common Stock. After the closing, the Company will retain certain assets and rights relating to its progesterone business, including all rights necessary to perform its obligations under its agreement with Merck Serono. The transactions pursuant to the Purchase Agreement and the ancillary agreements thereto are referred to collectively herein as the “Watson Transaction.”
At the closing of the Watson Transaction, in consideration for the sale of the Purchased Assets and the Shares, the Buyer will pay the Company $47 million in cash and assume certain liabilities associated with the Purchased Assets. In addition, the Buyer agreed to pay the Company up to $45.5 million in cash upon the achievement of several contingent milestones. The Buyer also agreed to make royalty payments to the Company of 10 to 20 percent of annual net sales of certain progesterone products; provided, however that royalty rates would be reduced by 50% in a particular country if a generic entry by a third party occurs in such country and certain other circumstances are fulfilled. In addition, if the Buyer commercializes a product through a third party outside of the U.S., in lieu of royalties, the Company will be entitled to 20% of gross profits associated with such commercialization. If the Buyer or its affiliates effects a generic entry with respect to a progesterone product in a country in the circumstances permitted by the Purchase Agreement, in lieu of royalties payable in respect of net sales for such generic product, the Company will be entitled to 20% of the gross profits associated with the commercialization of such generic product in such country.
Pursuant to the Purchase Agreement, the Company and the Buyer have also agreed to collaborate with respect to the development of progesterone products. In connection therewith, the parties agreed to establish a joint development committee to oversee and supervise all development activities. The Company will be responsible for completion of the PREGNANT Study and such other activities as determined by the joint development committee. The Company will be responsible for the costs of conducting the PREGNANT Study and the preparation, filing and approval process of the related new drug application (or the supplemental new drug application) up to a maximum of $7 million incurred after January 1, 2010. All other development costs incurred in connection with the development collaboration will be paid by the Buyer.
The parties also agreed to enter into various ancillary agreements, including an Investor’s Rights Agreement (pursuant to which the Buyer will have the right to designate a member of the Company’s board of directors for the period set forth therein, the Buyer will obtain certain registration rights pertaining to the Shares and the Buyer will agree to certain transfer restrictions pertaining to the Shares), a Supply Agreement pursuant to which the Company will supply PROCHIEVE 4%, PROCHIEVE 8% and CRINONE 8% to the Buyer for sale in the U.S. at a price equal to 110% of cost of goods sold, and a License Agreement relating to the grant of certain intellectual property licenses.
The closing of the Watson Transaction is subject to customary closing conditions, including Company stockholder approval.
As part of the Purchase Agreement, from the date of the closing of the Watson Transaction until the second anniversary of the date on which the Company and the Buyer terminate their relationship with respect to the joint development of progesterone products, the Company agreed not to manufacture, develop or commercialize products containing progesterone or any other products for the preterm birth indication, subject to certain exceptions. The joint development collaboration is terminable by either party five years after the closing of the Watson Transaction.
The Shares are being offered and sold to the Buyer under the Purchase Agreement in reliance on exemptions from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), pursuant to Section 4(2) under the Securities Act and Rule 506 promulgated thereunder, based on the nature of the Buyer and certain representations made by the Buyer to the Company.
F - 30
PharmaBio Amendment
On March 3, 2010, the Company entered into an amendment (the “PharmaBio Amendment”) with PharmaBio to the Investment and Royalty Agreement dated March 5, 2003, between the Company and PharmaBio, as previously amended and supplemented (the “PharmaBio Agreement”). The PharmaBio Amendment provides for the early termination of the PharmaBio Agreement by permitting the Company to make certain payments required thereunder on an accelerated and discounted basis on the date the Company consummates (and contingent upon the Company consummating) a transfer of assets, sale of stock, licensing agreement and/or similar transaction yielding gross cash proceeds to the Company of $40 million or more.
Note Purchase and Amendment Agreements
On March 3, 2010, the Company entered into Note Purchase and Amendment Agreements (the “Note Purchase Agreements”) with all of the holders (the “Holders”) of the Company’s Convertible Subordinated Notes due December 31, 2011 (the “Notes”). Under the Note Purchase Agreements, the Company agreed to purchase, subject to the satisfaction of certain conditions, the approximately $40 million in aggregate principal amount of Notes held by the Holders. The aggregate purchase price for the Notes is $26 million in cash (plus accrued and unpaid interest through but excluding the date of the closing of the Note purchases), warrants to purchase 7,750,000 shares of Common Stock at an exercise price of $1.35 per share (the “Warrants”) and 7,407,407 shares of Common Stock. The closings of the transactions contemplated by the Note Purchase Agreements are subject to various conditions, including the consummation of the Watson Transaction. Pursuant to the Note Purchase Agreements, the Holders consented, effective on March 3, 2010, to an amendment to the Notes (the “Amendment”) that eliminates the right of any holder of the Notes to cause the Company to redeem the Notes by virtue of the Watson Transaction. The Amendment terminates if the note purchase closings do not occur on or prior to August 31, 2010 and in certain other circumstances. Each Note Purchase Agreement may be terminated in certain circumstances, including, among others, by any party thereto, if the closings thereunder do not occur on or prior to August 31, 2010.
The warrants to be issued under the Note Purchase Agreements will be exercisable, subject to the limitations set forth therein, during the period commencing 180 days after, and ending on the fifth anniversary of their issuance, unless earlier exercised or terminated as provided in such warrants.
Under the terms of the Note Purchase Agreements, the Company has granted the Holders who are “Affiliates” (as defined under Rule 405 of the Securities Act) of the Company certain registration rights with respect to the resale of the shares of the Company’s common stock to be issued under the Note Purchase Agreements and the shares of Company common stock issuable upon the exercise of the warrants to be issued under the Note Purchase Agreements.
Under the Note Purchase Agreements, until 45 days after the Company’s announcement of the results of the PREGNANT Study, if the Company issues any shares of Company common stock (or common stock equivalents) for a price that is less than $2.00 per share, the Company must offer the Holders, subject to certain exceptions, the right to exchange their warrants for cash payments of up to an aggregate of $3,999,996.
The shares and warrants to be issued under the Note Purchase Agreements are being offered and sold in reliance on exemptions from the registration requirements of the Securities Act pursuant to Section 4(2) under the Securities Act and Rule 506 promulgated thereunder, based on the nature of the Holders and certain representations made by them to the Company.
None of the Shares or the shares and warrants to be issued under the Note Purchase Agreements have been registered under the Securities Act (or the laws of any state or other jurisdiction) and may not be offered or sold in the U.S. absent registration or an applicable exemption from the registration requirements thereof. This Form 10-K does not constitute an offer for the sale of any securities of the Company or a solicitation of any offer to buy any securities of the Company.
The foregoing is a summary of the terms of the Purchase Agreement (and the related ancillary agreements), the Note Purchase Agreements, the Warrants, the Amendment and the PharmaBio Amendment, and does not purport to be complete and is qualified in its entirety by reference to the full text of the Purchase Agreement (and the related ancillary agreements), the Note Purchase Agreements, the Warrants, the Amendment and the PharmaBio Amendment, which were more fully summarized and filed as exhibits to the Company’s current report on Form 8-K filed with the SEC on March 4, 2010.
F - 31
Accounting Treatment of the Transaction
The Company will allocate the $47 million initial proceeds, net of transaction expenses to 1) the then fair value of the 11.2 million common shares; and 2) the elimination of the remaining book value of the CRINONE intangible assets (which was $18.8 million as of December 31, 2009). The excess, if any, will be recorded as revenue over the remaining research & development period for the PREGNANT study through the Company's filing and FDA acceptance of the new drug application which is expected to occur by mid-2011. The accounting treatment for the debt extinguishments will need to be evaluated at a later date, subject to the timing of the Watson Transaction and the Company's share price at the transaction date.
F - 32
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Columbia Laboratories, Inc.
Livingston, NJ
The audits referred to in our report dated March 12, 2010 relating to the consolidated financial statements of Columbia Laboratories, Inc., which is contained in Item 8 of this Form 10-K also included the audits of the financial statement schedule listed in the accompanying index. These financial statement schedules are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statement schedules based on our audits.
In our opinion such financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly, in all material respects, the information set forth therein.
(Signed BDO Seidman, LLP)
Woodbridge, NJ
March 12, 2010
F - 33
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
of Columbia Laboratories, Inc.
Our audit of the consolidated financial statements referred to in our report dated March 25, 2008 included elsewhere in this Annual Report on Form 10-K also included the 2007 information in the financial statement schedule of Columbia Laboratories, Inc. listed in Item 15(a) of this Form 10-K. This schedule is the responsibility of Columbia Laboratories, Inc.’s management. Our responsibility is to express an opinion based on our audits of the consolidated financial statements.
In our opinion, the 2007 information in the financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly in all material respects the information set forth therein.
/s/ McGladrey & Pullen, LLP
McGLADREY & PULLEN, LLP
New York, New York
March 25, 2008
F - 34
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS
FOR THE THREE YEARS ENDED DECEMBER 31, 2009
|
Charged to
|
|||||||||
|
Balance at
|
(credited to)
|
Balance
|
|||||||
|
beginning
|
costs and
|
at end
|
|||||||
|
Description
|
of year
|
expenses
|
Deductions (A)
|
of year
|
|||||
| YEAR ENDED DECEMBER 31, 2009: | |||||||||
|
Allowance for doubtful accounts
|
$ 100,000
|
$ -
|
$ -
|
$ 100,000
|
|||||
| YEAR ENDED DECEMBER 31, 2008: | |||||||||
|
Allowance for doubtful accounts
|
$ 95,733
|
$ 4,267
|
$ -
|
$ 100,000
|
|||||
| YEAR ENDED DECEMBER 31, 2007: | |||||||||
|
Allowance for doubtful accounts
|
$ 100,000
|
$ 15,000
|
$ 19,267
|
$ 95,733
|
|||||
(A) Deductions represent the write-off of uncollectible accounts.
F - 35
EXHIBIT INDEX
|
Exhibit
No
|
Description
|
|
3.1
|
Restated Certificate of Incorporation of the Company, as amended (14)
|
|
3.2
|
Amended and Restated By-laws of Company (3)
|
|
4.1
|
Certificate of Designations, Preferences and Rights of Series C Convertible Preferred Stock of the Company, dated as of January 7, 1999 (3)
|
|
4.2
|
Securities Purchase Agreement, dated as of January 7, 1999, between the Company and each of the purchasers named on the signature pages thereto (3)
|
|
4.3
|
Securities Purchase Agreement, dated as of January 19, 1999, among the Company, David M. Knott and Knott Partners, L.P. (3)
|
|
4.4
|
Form of Warrant to Purchase Common Stock (3)
|
|
4.5
|
Warrant to Purchase Common Stock granted to James J. Apostolakis on September 23, 1999 (5)
|
|
4.6
|
Certificate of Designations of Series E Convertible Preferred Stock, filed May 10, 2005 with the Delaware Secretary of State (13)
|
|
4.7
|
Preferred Stock Purchase Agreement, dated as of May 10, 2005, among Columbia Laboratories, Inc., Perry Partners L.P. and Perry Partners International, Inc. (13)
|
|
4.8
|
Securities Purchase Agreement, dated March 10, 2006, by and between Columbia Laboratories, Inc. and the Purchasers listed on Exhibit A thereto (15)
|
|
4.9*
|
Form of Restricted Stock Agreement (17)
|
|
4.10*
|
Form of Option Agreement (24)
|
|
4.11
|
Securities Purchase Agreement, dated December 21, 2006, by and between Columbia Laboratories, Inc. and the Purchasers listed on Exhibit A thereto (19)
|
|
10.1
|
1996 Long-term Performance Plan, as amended, of the Company (2)
|
|
10.2
|
Asset Purchase, License and Option Agreement between Bio-Mimetics, Inc. and Columbia Laboratories, Inc., dated November 22, 1989 (1)
|
|
10.4
|
Settlement Agreement and Release dated as of March 16, 2000 between Columbia Laboratories (Bermuda) Ltd. and Lake Consumer Products, Inc. (5)
|
|
10.5
|
License Agreement dated April 18, 2000, between the Company and Lil’ Drug Store Products, Inc. (6)
|
|
10.6
|
Rights Agreement dated as of March 13, 2002, by and between Columbia Laboratories, Inc. and First Union National Bank, as Rights Agent (7)
|
|
10.7†
|
Semi-Exclusive Supply Agreement dated May 7, 2002 between the Company and Mipharm S.p.A. (8)
|
|
10.8†
|
Amended and Restated License and Supply Agreement dated June 4, 2002 between the Company and Ares Trading S.A.(8)
|
|
10.9†
|
Investment and Royalty Agreement dated March 5, 2003 between the Company and PharmaBio Development Inc. (9)
|
|
10.10†
|
License and Supply Agreement Dated May 27, 2003 between the Company and Mipharm S.p.A. (10)
|
|
10.11*
|
Form of Indemnification Agreement for Officers and Directors (11)
|
|
10.12†
|
Asset Purchase Agreement Dated June 29, 2004, between the Company and Lil’ Drug Store Products, Inc. (12)
|
|
10.13†
|
Supply Agreement dated June 29, 2004, between the Company and Lil’ Drug Store Products, Inc.(12)
|
|
10.14
|
Letter Agreement Supplement to STRIANT Investment and Royalty Agreement dated April 14, 2006 (16)
|
|
10.15*
|
Separation Agreement by and between Columbia Laboratories, Inc. and David L. Weinberg effective as of December 12, 2006 (18)
|
|
10.16†
|
Agreement, dated December 21, 2006, by and among Ares Trading S.A., Serono, Inc., the Company and its wholly-owned subsidiary, Columbia Laboratories (Bermuda), Ltd (19)
|
|
10.17
|
Amendment No. 1 to the Amended and Restated License and Supply Agreement, entered into December 21, 2006, by and between Ares Trading S.A and Columbia Laboratories (Bermuda), Ltd. (19)
|
|
10.18
|
Description of the Registrant’s Compensation and Reimbursement Practices for Non-employee Directors. (20)
|
|
10.19
|
Lease Agreement between Allwood Associates I and Columbia Laboratories, Inc., dated July 6, 2007 (20)
|
|
10.20†
|
License and Supply Agreement between Columbia Laboratories, Inc. and Ascend Therapeutics, Inc., dated September 27, 2007 (21)
|
|
10.21
|
Supply Agreement between Columbia Laboratories (Bermuda) Limited and Fleet Laboratories Limited, dated July 12, 1996 (22)
|
|
10.22
|
Packaging Agreement between Columbia Laboratories (Ireland) Ltd. and Maropack AG, dated October 28, 1993 (22)
|
|
10.23*
|
Columbia Laboratories, Inc., 2008 Long-Term Incentive Plan (23)
|
|
10.24*
|
Columbia Laboratories, Inc., Amended and Restated Incentive Plan (24)
|
|
10.25*
|
Form of Executive Change of Control Severance Agreement (24)
|
|
10.26
|
Stock Purchase Agreement, by and between the Company and Numoda Corporation, dated January 6, 2009 (25)
|
|
10.27*
|
Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and Robert S. Mills dated March 11, 2009 (24)
|
|
10.28*
|
Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and Michael McGrane dated March 11, 2009 (29)
|
|
10.29*
|
Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and James A. Meer dated March 11, 2009 (26)
|
|
10.30*
|
Separation and Release Agreement by and between Columbia Laboratories, Inc. and James A. Meer effective as of May 18, 2009 (26)
|
|
10.31*
|
Employment Agreement by and between Columbia Laboratories, Inc. and Lawrence Gyenes dated July 15, 2009 (27).
|
|
10.32
|
Second Amendment to Investment and Royalty Agreement, by and between the Company and PharmaBio Development, Inc., July 22, 2009 (28)
|
|
10.33
|
Placement Agent Agreement, by and among the Company, Oppenheimer & Co., Inc. and The Benchmark Company, LLC, dated October 22, 2009 (30)
|
|
10.34
|
Form of Subscription Agreement (30)
|
|
10.35
|
Form of Warrant to Purchase Common Stock (30)
|
|
10.36*
|
Columbia Laboratories Stock Ownership Guidelines for Officers and Directors (31)
|
|
10.37
|
Manufacturing and Supply Agreement between Fleet Laboratories and Columbia Laboratories (Bermuda), Ltd., dated December 8, 2009 (32)
|
|
10.38*
|
Employment Agreement by and between Columbia Laboratories, Inc. and Frank C. Condella, Jr., dated December 11, 2009 (33)
|
|
10.39*
|
Addendum to Amended and Restated Employment Agreement by and between Columbia Laboratories, Inc. and Robert S. Mills dated December 11, 2009 (33)
|
|
10.40*
|
Addendum to Executive Change in Control Severance Agreement by and between Columbia Laboratories, Inc. and Robert S. Mills dated December 11, 2009 (33).
|
|
14
|
Code of Ethics of the Company (11)
|
|
21
|
Subsidiaries of the Company (34)
|
|
23.1
|
Consent of BDO Seidman, LLP (35)
|
|
23.2
|
Consent of McGladrey & Pullen, LLP (35)
|
|
31(i).1
|
Certification of Chief Executive Officer of the Company (35)
|
|
31(i).2
|
Certification of Chief Financial Officer of the Company (35)
|
|
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (35)
|
|
32.2
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. (35)
|
|
†
|
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
*
|
Management contract or compensatory plans or arrangements
|
|
1/
|
Incorporated by reference to the Registrant's Registration Statement on Form S-1 (File No. 33-31962) declared effective on May 14, 1990
|
|
2/
|
Incorporated by reference to the Registrant's Proxy Statement dated May 10, 2000
|
|
3/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1998
|
|
4/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 1999
|
|
5/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1999
|
|
6/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2000
|
|
7/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated March 12, 2002
|
|
8/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 14, 2002
|
|
9/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002
|
|
10/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 14, 2003
|
|
11/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2003
|
|
12/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 4, 2004
|
|
13/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated May 12, 2005
|
|
14/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2005
|
|
15/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated March 16, 2006
|
|
16/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated April 17, 2006
|
|
17/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated May 17, 2006
|
|
18/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 15, 2006
|
|
19/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 26, 2006
|
|
20/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, dated August 8, 2007
|
|
21/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, dated November 8, 2007
|
|
22/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007
|
|
23/
|
Incorporated by reference to the Registrant’s Proxy Statement dated April 8, 2008
|
|
24/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008
|
|
25/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated January 8, 2009
|
|
26/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated May 12, 2009
|
|
27/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated July 15, 2009
|
|
28/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated July 24, 2009
|
|
29/
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q dated August 6, 2009
|
|
30/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated October 23, 2009
|
|
31/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated November 17, 2009
|
|
32/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 8, 2009
|
|
33/
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, dated December 14, 2009
|
|
34/
|
Incorporated by reference to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2009
|
|
35/
|
Filed herewith
|
