Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ev3 Inc. | c57804e8vk.htm |
| EX-99.1 - EX-99.1 - ev3 Inc. | c57804exv99w1.htm |
Exhibit 99.2

| 1Q 2010 Highlights April 29, 2010 |

| 2 Forward-Looking Statements Statements contained in this presentation that relate to future, not past, events are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Forward-looking statements often can be identified by words such as "expect," "anticipate," "intend," "plan," "will," "may," "believe," "could," "continue," "future," "estimate," "outlook," "guidance," or the negative of these words, other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause ev3's actual results to be materially different than those expressed in or implied by ev3's forward-looking statements. For ev3, particular uncertainties and risks include, among others, ev3's future operating results and financial performance, fluctuations in foreign currency exchange rates, the effect of worldwide economic conditions, the timing of regulatory approvals and introduction of new products, market acceptance of new products, success of clinical testing, availability of third party reimbursement, impact of competitive products and pricing, the effect of regulatory actions and the cost and effect of changes in tax and other legislation. More detailed information on these and additional factors that could affect ev3's actual results are described in ev3's filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K. Except as required by law, ev3 undertakes no obligation to publicly update its forward-looking statements. |

| 3 Use of Non-GAAP Financial Measures ev3 uses certain non-GAAP financial measures in this presentation. ev3 uses non-GAAP financial measures as supplemental measures of performance and believes these measures provide useful information to investors in evaluating our operations, period over period. However, non-GAAP financial measures have limitations as analytical tools, and should not be considered in isolation or as a substitute for ev3's financial results prepared in accordance with GAAP. In addition, investors should note that any non- GAAP financial measures ev3 uses may not be the same non-GAAP financial measures, and may not be calculated in the same manner, as that of other companies. We have posted a reconciliation of our non- GAAP financial measures to the most directly comparable GAAP financial measures on our website at www.ev3.net. |

| 4 Q1 '10 Financial Summary Net sales of $123.9 million +23% vs. Q1 2009; +21% constant currency* Peripheral vascular sales +13% vs. Q1 2009; +11% constant currency* Plaque excision +12% vs. Q1 2009, +12% constant currency* Peripheral stents +6% vs. Q1 2009; +4% constant currency* Thrombectomy and embolic protection +17%; +16% constant currency* Procedural support and other (PTA, support catheters, guidewires, etc.) +28%; +26% constant currency* Neurovascular sales +44% vs. Q1 2009; +38% constant currency* Embolic product and stents (Embolic Coils, Onyx, Pipeline, Solitaire) +66% vs. Q1 2009; +60% constant currency* Neuro access and delivery products (Neuro Balloons, Catheters, Alligator) +13% vs. Q1 2009; +9% constant currency* International revenue +31% vs. Q1 2009; +24% constant currency* Gross margin was 76.4%, an improvement of 730 basis points over Q1 2009 GAAP EPS of $0.09 Non-GAAP adjusted EPS* of $0.20; +186% vs. Q1 2009 Cash flow from operating activities totaled $17.3 million in Q1 2010; cash and cash equivalents totaled $116.8M at end of Q1 2010 * These are non-GAAP financial measures. Non-GAAP net sales on a constant currency basis exclude the foreign exchange impact. The foreign exchange impact is the impact from foreign exchange rates on the current period sales compared to prior period sales using the prior period's foreign exchange rates. Non-GAAP adjusted net EPS and non-GAAP adjusted net income excludes amortization and non-cash stock-based compensation. Q1 2009 also excludes a non-cash FoxHollow lease reserve adjustment and a realized gain on divestiture of investments. Q1 2010 also excludes charges relating to the change in fair value of contingent consideration. For reconciliations of ev3's non-GAAP financials, see ev3's website at www.ev3.net. |

| 5 Recent Operational Highlights - Peripheral Vascular Continuing progress in U.S. launch of TurboHawk(tm) Plaque Excision System Incremental opportunity to treat calcified above-the-knee lesions PowerCross(tm) .018 PTA balloon launched globally in 1Q'10 - in addition to EverCross(tm) and NanoCross(tm) balloons, ev3 now offers complete PTA platform Supply agreement with MEDRAD announced in April in preparation for DEFINITIVE AR pilot study DURABILITY II clinical study enrollment completed - 287 patients enrolled Clinical trial enrollment progressing: DEFINITIVE Ca++ U.S. IDE study: 82/133 patients DEFINITIVE LE study: 286/800 patients |
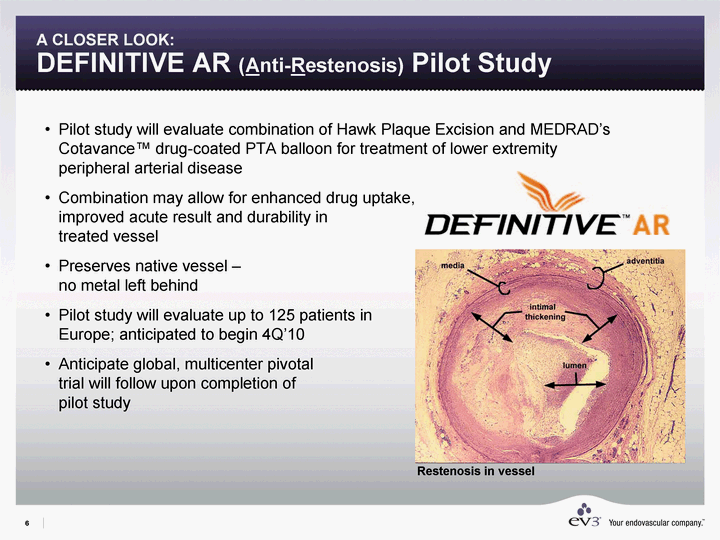
| 6 A CLOSER LOOK: DEFINITIVE AR (Anti-Restenosis) Pilot Study Pilot study will evaluate combination of Hawk Plaque Excision and MEDRAD's Cotavance(tm) drug-coated PTA balloon for treatment of lower extremity peripheral arterial disease Combination may allow for enhanced drug uptake, improved acute result and durability in treated vessel Preserves native vessel - no metal left behind Pilot study will evaluate up to 125 patients in Europe; anticipated to begin 4Q'10 Anticipate global, multicenter pivotal trial will follow upon completion of pilot study Restenosis in vessel |

| 7 Recent Operational Highlights - Neurovascular International launch for Pipeline(tm) Embolization Device (PED) and Solitaire FR Revascularization Device for ischemic stroke progressing very well Now have approx. 113 physicians who are certified or working towards Pipeline certification Early launch of Axium(tm) MicroFX PGLA & Nylon coils in 1Q'10 in U.S. and Europe Patient enrollment initiated in SWIFT (Solitaire With Intention For Thrombectomy) U.S. IDE study in February 2010 - 13/200 patients enrolled Pipeline Embolization Device PMA expected to be submitted to FDA for approval in May 2010 Anticipate PMA approval in 2011 |

| 8 A CLOSER LOOK: SWIFT (Solitaire with Intention for Thrombectomy) Study Investigational Use Only in the United States: Not FDA approved or cleared Flow restoration upon deployment Clot retrieval Multi-center, prospective, randomized controlled trial comparing recanalization rates of Solitaire(tm) FR Flow Restoration device and Concentric Medical's Merci Retriever(r) in treatment of acute ischemic stroke Designed to enroll 200 patients at 20 centers in U.S. and one European center Study will measure arterial recanalization of occluded target vessel without any symptomatic intracranial hemorrhage, time to achieve initial recanalization and assess patient neurological condition at 90 days |

| 9 Actual Results vs. Last Quarter & Year Ago * Constant Currency; For reconciliations, see our website at www.ev3.net. ** All quarters presented are adjusted for amortization and non-cash stock-based compensation. Q1 2009 is also adjusted for a non-cash FoxHollow lease reserve adjustment and a realized gain on divestiture of investments. Q4 2009 and Q1 2010 are also adjusted for charges relating to the change in fair value of contingent consideration. For reconciliations, see our website at www.ev3.net. Q4 2009 Actual Q1 2009 Actual $000's except EPS Net sales Gross profit Gross margin GAAP net income (loss) GAAP earnings (loss) per share Adjusted net income** Adjusted earnings per share** $100,395 $69,407 69.1% $(1,809) $(0.02) $7,070 $0.07 $126,753 $96,214 75.9% $25,716 $0.23 Q1 2010 Actual $123,854 $9,864 $0.09 $22,332 $0.20 $94,627 76.4% Peripheral vascular (PV) Plaque excision Neurovascular (NV) Embolic products and stents International % Chg Seq. % Chg YOY/CC* $74,777 $20,485 $49,077 $32,470 $50,897 $73,705 $24,048 $53,048 $33,482 $53,571 $66,202 $18,308 $34,193 $19,547 $38,741 1% -15% -7% -3% -5% 13%/11% 12%/12% 44%/38% 66%/60% 31%/24% -2% 23%/21% -2% 36% $13,001 $0.12 -24% -25% -13% -13% 216% 186% |

| 10 Total Q1 '10 Revenue Growth of 21%* Total International ($M) +24%* Worldwide Peripheral Vascular ($M) +11%* Worldwide Neurovascular ($M) +38%* *Constant currency compared to Q1 2009; For reconciliations, see our website at www.ev3.net. +21%* Q1 Total Worldwide Sales ($M) Q1 2009 2010 Q1 Q1 |

| 11 Key 2010 Financial Indicators $M Operating Margin Net Sales Adjusted EPS1 Cash Flow from Operations $ SG&A as % of net sales 50% 49% 47% 1. All quarters presented are adjusted for amortization, non-cash stock-based compensation and charges relating to the change in fair value of contingent consideration. Q2 2009 is also adjusted for a non-cash tax benefit resulting from the acquisition of Chestnut. For reconciliations, see our website at www.ev3.net. $ M Q2 % Q3 Q4 Q2 Q3 Q4 GAAP EPS $0.23 $0.06 $0.12 Q1 $0.09 Q1 Q2 Q3 Q4 Q1 50% 2010 2009 |

| 12 Balance Sheet is Strengthening - We are Generating Cash Cash / Cash Equivalents Cash flow from operating activities totaled $17.3M in Q1'10 Cash/cash equivalents increased $18.8M in Q1'10 vs Q4'09 Total debt decreased to $5.8M *Q2 09 included $26.2M payment for Chestnut Medical |

| 13 Improving Cost Structure - Continuing Progress Gross Margin % of sales SG&A % of sales Improved manufacturing efficiencies, volume growth and favorable product mix Continued focus on leveraging cost structure and improving margins |

| 14 Financial Summary: Continuing to Improve Performance Net Sales $123,854 $100,395 $109,086 $112,838 $126,753 Gross Profit $94,627 $69,407 $78,608 $84,230 $96,214 Gross Margin 76.4% 69.1% 72.1% 74.6% 75.9% Net Income (Loss) $9,864 $(1,809) $23,989 $6,736 $13,001 Adjusted EPS per Diluted Share* $0.20 $0.07 $0.14 $0.17 $0.23 GAAP Diluted EPS (Loss) $0.09 $(0.02) $0.23 $0.06 $0.12 Q1 2010 Q1 2009 Q2 2009 Q3 2009 Q4 2009 $000's except EPS Adjusted Net Income* $22,332 $7,070 $14,614 $19,435 $25,716 * All quarters presented are adjusted for amortization and non-cash stock-based compensation. Q1 2009 is also adjusted for a non-cash FoxHollow lease reserve adjustment and a realized gain on divestiture of investments. Q2 2009 is also adjusted for charges relating to the change in fair value of contingent consideration and a non-cash tax benefit resulting from the acquisition of Chestnut. Q3 2009, Q4 2009 and Q1 2010 are also adjusted for charges relating to the change in fair value of contingent consideration. For reconciliations, see our website at www.ev3.net. |

| 15 Guidance - As of April 29, 2010 Full-Year 2010 Guidance Revenues: Q2 2010: $129 to $133 million Full-Year 2010: $520 to $530 million Adjusted EPS1 per diluted share: Q2 2010: $0.18 to $0.211,2 Full-Year 2010: $0.87 to $0.921,2 Adjusted EPS guidance is based on weighted average diluted shares outstanding of approximately 115.1 and 115.4 million for Q2 10 and full-year 2010, respectively, and is adjusted to exclude the impact of amortization expense, non-cash stock-based compensation, and charges relating to the change in fair value of contingent consideration. These are forward-looking non-GAAP financial measures. For reconciliations, see our website at www.ev3.net. Q2 10 GAAP EPS: $0.02 to $0.05; Full-Year 2010 GAAP EPS: $0.36 to $0.41 |

| 16 2008 % of sales 2007 2010 Goal Business Model Structure - Select P&L Information Sales 100% 100% 100% Gross margin 66% 65% 73% Selling, general & administrative* 55% 69% 50% Research & development 12% 17% 11% Stock-based comp, amortization & contingent consideration 11% 11% 10% Adjusted net income (loss)** 2.3% (22.4%) 15% * SG&A includes operating impact of Chestnut from the date of acquisition. ** Adjusted net income (loss) excludes amortization and non-cash stock-based compensation. For the year ended December 31, 2007, Adjusted net loss also excludes $70.7M of Acquired IPR&D. For the year ended December 31, 2008, Adjusted net income also excludes non-cash goodwill and other intangible asset impairment charges of $299.3M. For the year ended December 31, 2009, Adjusted net income also excludes a non-cash FoxHollow lease reserve adjustment of $3.4M, realized gain on investments of $4.1M, accounting charges relating to the change in fair value of contingent consideration of $4.9M and a non-cash tax benefit resulting from the purchase accounting for the acquisition of Chestnut of $19.0M. For the year ended December 31, 2010, Adjusted net income also excludes charges relating to the change in fair value of contingent consideration of $17.2M. These are non-GAAP financial measures. For reconciliations, see our website at www.ev3.net. 100% 77% 47% 11% 11% 20% 2009 |

| Growth Drivers / Milestones Next 3-9 Months Procedure penetration, improving margins and leveraging existing cost structure Peripheral Vascular business increased sales force productivity growth in plaque excision business - TurboHawk(tm) above-the-knee new product launches TurboHawk(tm) below-the-knee 4Q'10 EverFlex+ 4Q'10 ongoing progress in clinical studies initial data from DEFINITIVE LE clinical study start of DEFINITIVE AR pilot study in Europe 4Q'10 Neurovascular business new product launches OUS launch of Pipeline(tm) Embolization Device to treat large/giant aneurysms OUS launch of Solitaire(tm) FR device for ischemic stroke Axium(tm) MicroFX PGLA and Nylon coils ongoing progress in clinical studies submit U.S. PMA for Pipeline PUFS study continuing enrollment in U.S. SWIFT and COCOA clinical trials International market penetration, expansion to new geographies, direct distribution |


| NASDAQ: EVVV www.ev3.net Contact Info: Julie Tracy - Sr. VP, Chief Communications Officer (949) 680-1375 jtracy@ev3.net |
