Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - SCOLR Pharma, Inc. | d8k.htm |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
1
SCOLR Pharma
Corporate Presentation
Welcome to a Revolution…
April 2010
Exhibit 99.1 |
 SCOLR Pharma, Inc.
Forward-Looking Statements
This presentation contains forward-looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995,
including without limitation statements about our strategies, product
development programs, revenue opportunities, 2010 revenue
projections, product approval requirements and timelines, that
involve significant risks and uncertainties. These statements
represent management’s present expectations of future events and
are subject to a number of important factors that could cause actual
results to differ materially from those described in the forward-
looking statements.
Please refer to the risk factors and other cautionary language
included in our reports and other filings with the Securities and
Exchange Commission and available on our website.
We assume no obligation to update these forward-looking
statements.
Copyright©2010, SCOLR Pharma, Inc.
2
April 2010 |
 SCOLR Pharma, Inc.
New Strategy:
Redefining our business:
Emphasis on direct sales of our products
License/Partner where we do not have the
resources/capabilities
Alignment of operating costs to “match”
business strategy
Lower operating costs in line with business
strategy
Copyright©2010, SCOLR Pharma, Inc.
3
April 2010 |
 SCOLR Pharma, Inc.
Strategy:
Strong financial performance:
Sales/Licensing revenues
Strong cost control
Direct Sales with higher contribution
Nutritional Supplements
Nuprin®
ibuprofen based tablets
Pseudoephedrine 12 hour tablets (pending
approval)
Advance 12 hour ibuprofen towards
commercialization
Copyright©2010, SCOLR Pharma, Inc.
4
April 2010 |
 SCOLR Pharma, Inc.
5
Nutritional Supplements
–
revenue opportunities
Copyright©2010, SCOLR Pharma, Inc.
April 2010
*Statements not evaluated by the FDA. Not meant to treat, diagnose or cure
any condition or disease |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
6
Nutritional Supplements
–
Label examples
April 2010 |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
7
Unique/Differentiated products
Limited or no competition
National distribution to retail consumers
Niche vitamin/mineral “depletion”
category in the
pharmacy aisle
Focus on products that utilize proven and
recognized ingredients, supported by strong
science with tangible consumer benefits
Expected 2010 revenues between $5-10 million
Nutritional Supplements
–
revenue opportunities
April 2010 |
 SCOLR Pharma, Inc.
Company Background:
Public Company (NYSE Amex: DDD); based in Bothell, WA
High quality, precision, oral drug delivery technologies at prices
comparable to immediate release tablets/capsules
Stephen J. Turner, President & Chief Executive Officer
Joined SCOLR in 1999. Served as Chief Technology Officer in charge of
R&D and Operations since 2003 until being appointed President and
CEO
in
August
2009.
Built
SCOLR’s
R&D,
Intellectual
Property
and
Information Technology Functions.
Richard M. Levy, CPA, Chief Financial Officer
Joined SCOLR in 2005. Strong financial skills with extensive experience
in acquisitions and divestures. Held several executive level positions
with
domestic
and
international
companies,
including
CFO
of
Bank
of
America’s International Private Banking Division and Bank of
America Australia.
Most
recently,
CFO
at
Community
Trust
Bank,
Kentucky,
and
Corporate Controller for Washington Mutual Bank.
Copyright©2010, SCOLR Pharma, Inc.
8
April 2010 |
 SCOLR Pharma, Inc.
Balance Sheet and Capital Structure
December 31, 2009
(In Thousands)
Cash and cash equivalents
$ 1,176
Working Capital
961
Total Assets
$ 3,111
Total Debt
-
(In Millions)
Common Shares Outstanding
41.1
Fully -
Diluted Shares
48.5
Note
-
Excluded
from
above
Completed equity offering, March 2010 with a net proceeds of $3.7 million.
Copyright©2010, SCOLR Pharma, Inc.
April 2010
9
Copyright©2010, SCOLR Pharma, Inc. |
 SCOLR Pharma, Inc.
10
CDT®
–
A
Better
Oral
Drug
Delivery
Alternative
Applicable to broad range of drugs and consumer products
Technologies have been demonstrated across all classes of drugs
(BCS 1-4)
19 validating clinical studies across 7 drugs, n > 800
Standard GRAS ingredients and equipment
Less complex, cost-effective controlled release tablet/capsule
formulations
5 issued controlled-release patents:
Greater release flexibility with less formulation complexity
Robust, predictable and programmable controlled release
Ability to formulate independent of API solubility
Proprietary formulation expertise expands capabilities:
Potential for enhanced solubility in developmental formulations
Potential for enhanced oral bioavailability for select drugs
Copyright©2010, SCOLR Pharma, Inc. |
 SCOLR Pharma, Inc.
11
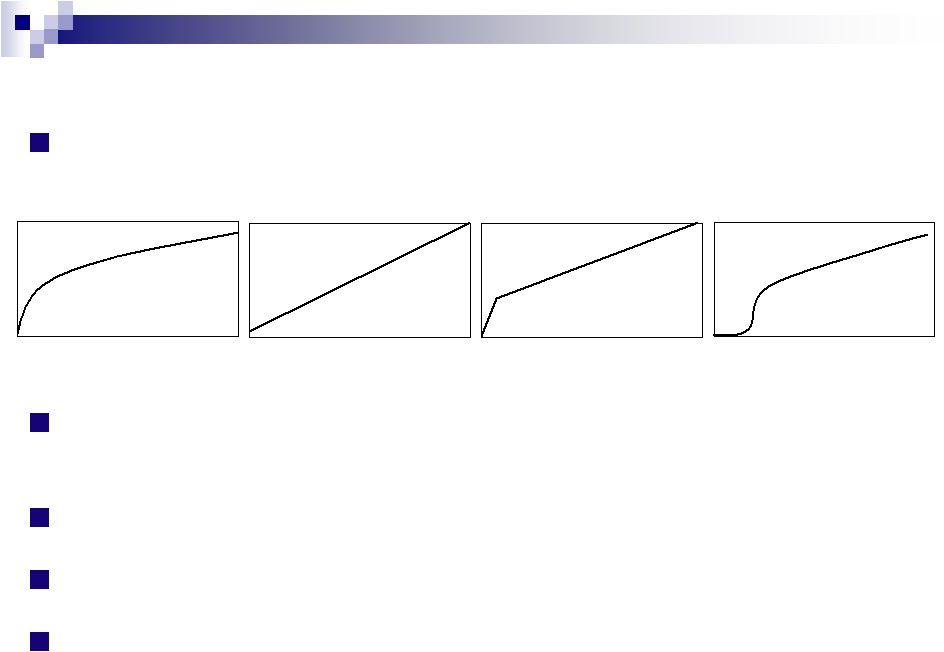
Key Benefits of SCOLR’s
Technologies:
Extremely
flexible
release
profiles:
Precision
near-linear,
first-order and delayed release up to 24 hours
Easy
to
manufacture:
2-3
step
manufacture
reduces
manufacturing time and decreases risk of batch failure
Cost
effective:
Can
use
existing
manufacturing
equipment
High
payload:
Fewer
excipients,
higher
active
loading
Strong
patent
protection:
Significant
patent
life
remaining
Copyright©2010, SCOLR Pharma, Inc.
First Order
Zero Order
Bimodal
Delayed Onset |
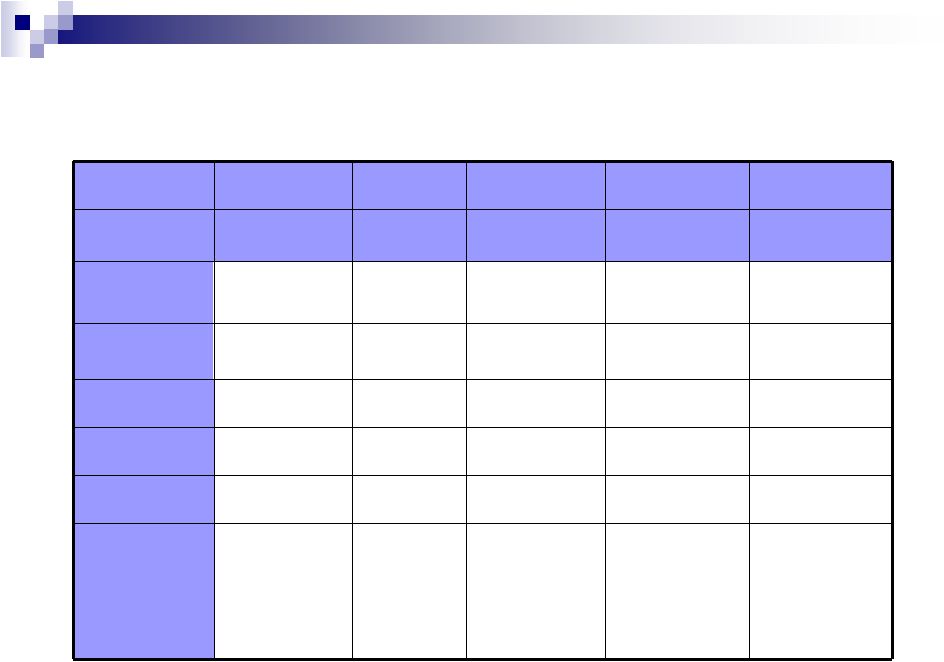 SCOLR Pharma, Inc.
CDT®
Difference:
Readily
Manufacturable
and
Cost-
Effective Extended Release
CDT®
Osmotic
Pump
Multiparticulate
Multi-Layered
Tablet
Proprietary
polymer
SCOLR
Alza
(Oros®)
Flamel
(Micropump®)
Skyepharma
(GeoMatrix®)
Labopharm
(Contramid®)
Process
Description
Granulation or
direct compression
matrix
Laser-drilled
orifice
Fluid bed,
extrusion bead
formation
Granulation or
direct compression
matrix
Granulation or
direct compression
matrix
Mode of Action
Diffusion/erosion
matrix
Reservoir
device
Diffusion/erosion
Diffusion/erosion
matrix
Hydrophilic
polymer matrix
Low
High
Medium
High
High
Yes
Yes
No
Yes
No
2 or 3
18+
36+
12+
Polymer synthesis
Complexity
Payload
Flexibility
Manufacturing
Steps Required
Limitations
(vs. CDT®)
Simple
granulation, can
be incorporated
into tablets or
capsules
Payload
restrictions,
fragile, short
exclusivity
Multiple complex
manufacturing
steps
Specialized
manufacturing
equipment
Limited source,
availability and
application of
polymer
Copyright©2010, SCOLR Pharma, Inc.
April 2010
12
Copyright©2010, SCOLR Pharma, Inc. |
 SCOLR Pharma, Inc.
13
SCOLR CDT®
Ibuprofen tablet compared to existing OTC and
Rx products
Rx products for size reference
only
6 tabs/24 hr
6 tabs/24 hr
2 tabs/24 hr
Not Available in US
Copyright©2010, SCOLR Pharma, Inc.
Commercial/Late-Stage Programs: Ibuprofen |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
14
ER Ibuprofen Program Objectives:
Develop a novel, cost-competitive 12-hour extended-
release ibuprofen tablet based on SCOLR’s
proprietary
and patented CDT®
delivery technology
Tablet Approval Requirements:
Bioequivalent to three 200 mg immediate release (IR)
RLD tablets (Motrin®)*
Stay below Cmax
or safety studies will be required*
Provide onset of pain relief in less than 60 minutes*
Demonstrate efficacy for the full 12 hours, especially
over the last four hours (8-12)**
*As discussed at type “b”
meetings with FDA in 2004, 2006
**Responses during special protocol assessment (SPA) with FDA
|
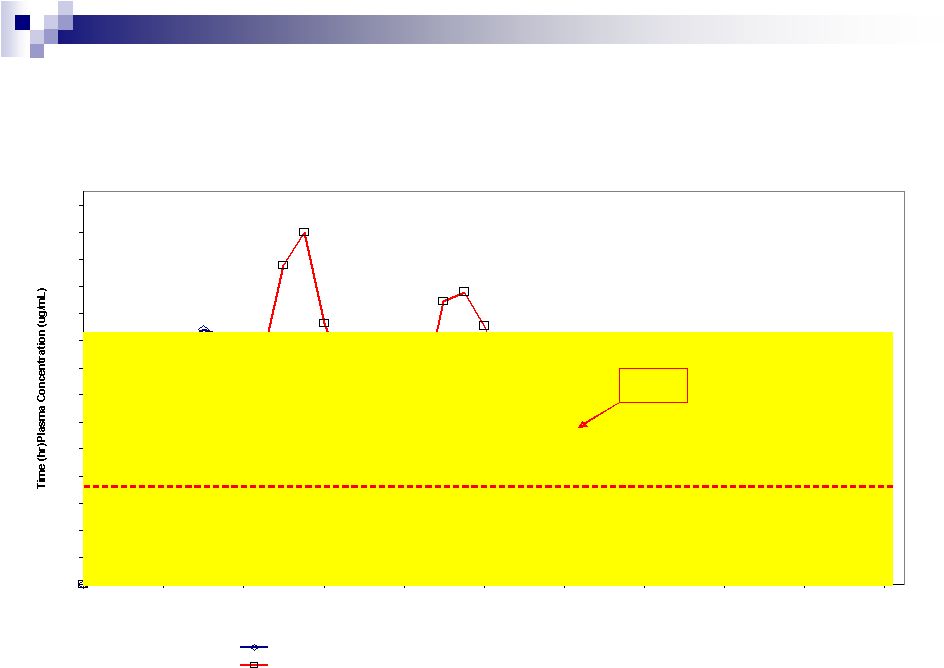 SCOLR Pharma, Inc.
15
as reported by Biovail
Contract Research
Copyright©2010, SCOLR Pharma, Inc.
Ibuprofen –
Reproducible PK response
Figure 11.1 -
Mean plasma ibuprofen concentration versus time (n=34)
0
2
4
6
8
10
12
14
16
18
20
22
24
26
28
0
2
4
6
8
10
12
14
16
18
20
Time (hr)
Treatment A: CDT®
Ibuprofen Tablet Extended Release, 1 x 600 mg, Lot#: 0601318
Treatment B: Motrin IB Ibuprofen Tablet USP, 1 x 200 mg (t.i.d.), Lot#:
MDA132 Min. Effective Concentration
12 Hour
Level
Biovail
Contract Research
A Division of Biovail
Corporation
Study No.: 3313
Ibuprofen Tablets Extended Release 600 mg
Study Reported: November 20, 2006 |
 SCOLR Pharma, Inc.
16
Multiple dose, Dental Pain Trial, Safety & Efficacy trial
256 subject randomized (169 active : 87 placebo)
Extraction of 1-2 impacted molars with confirmed moderate pain
(>5) Subjects given study medication every 12 hours for 48 hours
total Co-Primary Endpoints Achieved:
Effective pain relief over a full 12 hours
Durability of effect with repeat doses over 48 hours
Demonstrated
at
least
a
20%
improvement
over
baseline
(starting)
pain
Key Secondary Endpoint:
“Confirmed”
onset of effect in less than 1 hour
Copyright©2010, SCOLR Pharma, Inc.
Ibuprofen –
Successful pivotal phase III clinical |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
17
April 2010
Addressable Global OTC analgesic market
Ibuprofen (No extended release competitor)
Acetaminophen
(Tylenol®
8 hour, Tylenol Arthritis®)
Naproxen (approved for 8-12 hours)
Significant
upside
potential
if
SCOLR
CDT®
Ibuprofen
demonstrates superiority to other OTC NSAIDs
Cough-Cold combinations based on ibuprofen
Rx ibuprofen and potential combinations for severe
pain (i.e. Vicoprofen®)
Potential Market for Ibuprofen based products:
17
Copyright©2010, SCOLR Pharma, Inc.
Tylenol®, Tylenol-Arthritis are trademarks of Ortho-McNeil,
a Johnson and Johnson company. Vicoprofen® is a trademark of Abbott |
 SCOLR Pharma, Inc.
18
Copyright©2010, SCOLR Pharma, Inc.
Commercial/Late-Stage Programs –
Pseudoephedrine
CDT®
National Brand |
 SCOLR Pharma, Inc.
19
Copyright©2010, SCOLR Pharma, Inc.
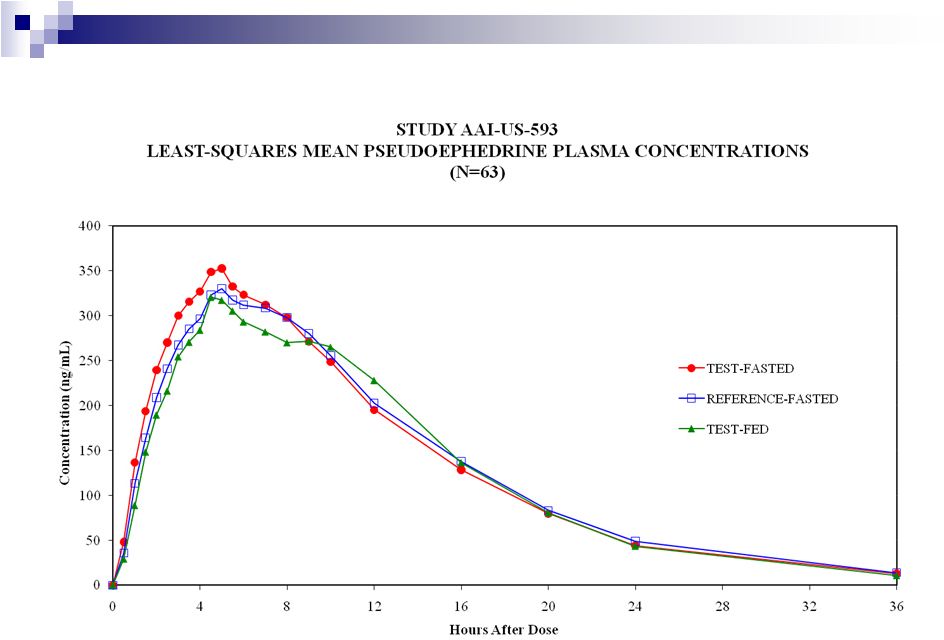
Pseudoephedrine –
Bioequivalence to NBE established |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
20 |
 SCOLR Pharma, Inc.
Copyright©2010, SCOLR Pharma, Inc.
21 |
 SCOLR Pharma, Inc.
22
Summary of Key Benefits
Broadly applicable oral technologies
High and low solubility drugs
High and low dose drugs
Release capability from 0-24 hours
Combination products
Controlled release at costs comparable to immediate release
Common manufacturing processes, with only 2-3 steps
GRAS excipients
Proven commercialized technologies
Demonstrated clinical performance
Patent Protected
Copyright©2010, SCOLR Pharma, Inc. |
