Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HeartWare International, Inc. | c99612e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - HeartWare International, Inc. | c99612exv99w1.htm |
Exhibit 99.2

| HeartWare(r) Ventricular Assist System Technology & Surgical Overview Martin Strueber, MD Hannover Medical School |

| EVALUATION OF THE MINIMALLY INVASIVE HEARTWARE VENTRICULAR ASSIST SYSTEM AS A LONG TERM SUPPORT DEVICE Martin Strueber, Hannover, Germany; Paul Jansz, Sydney, Australia; George Wieselthaler, Vienna, Austria; Gerry O^Driscoll, Perth, Australia; Michiel Morshuis, Bad Oeynhausen, Germany; Asghar Khaghani, Harefield, Great Britain; Stephan Schueler, Newcastle upon Tyne, Great Britain; Roland Hetzer, Berlin, Germany; Andreas Hoffmeier, Munster, Germany; and Daniel Duveau, Nantes, France. The following data is based on 100 patients unless otherwise noted. Data from the post CE Mark cohort is from registry data and has not been monitored or adjudicated. |

| Global Clinical Experience Overview The HeartWare Ventricular Assist System is the newest circulatory support device to be commercially launched in Europe More than 300 implants to date worldwide Initial clinical trial enrolled 50 patients in Europe and Australia - enrollment completed Dec 2008 CE Mark approval granted and commercial experience began in March 2009 European Registry includes data on 50 patients in commercial period |

| 100 Implants Across Five Countries Australia Paul Jansz, St. Vincent's, Sydney Gerry O^Driscoll, Royal Perth Hospital Austria Georg Wieselthaler, University of Vienna France Daniel Duveau, Hopital Guillaume et Rene Laennec, Nantes Germany Roland Hetzer, Deutsches Herzzentrum Berlin Andreas Hoffmeier, Muenster Michiel Morshuis, Herz und Diabeteszenstrum, Bad Oeynhausen Martin Strueber, Medizinische Hochschule Hannover United Kingdom Asghar Khaghani, Harefield Stephan Schueler, Freeman Hospital |

| The HeartWare Ventricular Assist System HVAD miniaturized implantable blood pump 10 liters of flow Implanted in the pericardial space Via median sternotomy or left thoracotomy No surgical pump pocket Centrifugal flow with Hydromagnetic suspension Wear-less, wide channel impeller design Design optimizes flow, pump surface washing, and hemocompatibility |
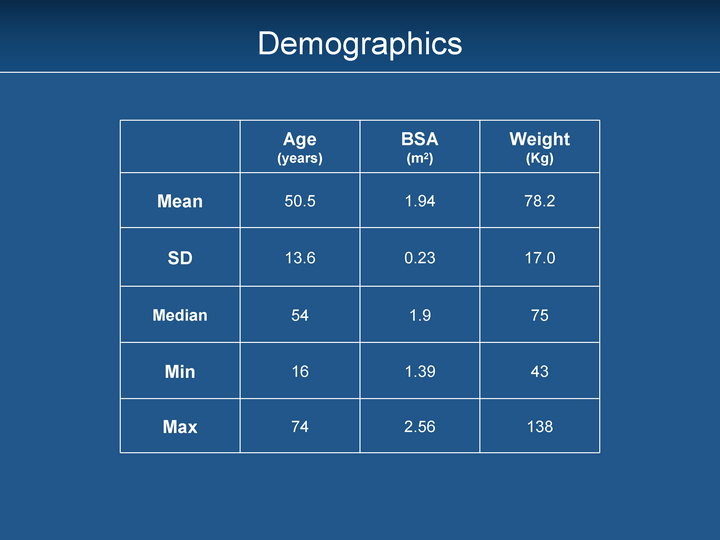
| Age (years) BSA (m2) Weight (Kg) Mean 50.5 1.94 78.2 SD 13.6 0.23 17.0 Median 54 1.9 75 Min 16 1.39 43 Max 74 2.56 138 Demographics |

| Gender |

| Operative |

| Operative Approach at Implant |

| Etiology of Heart Failure |
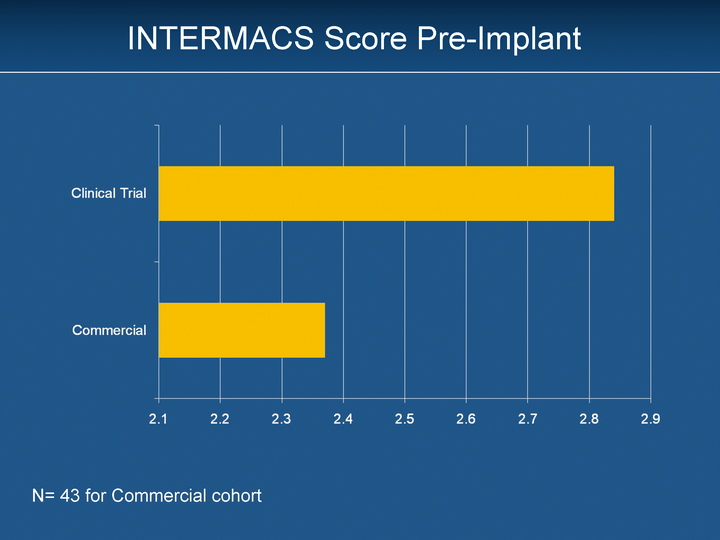
| INTERMACS Score Pre-Implant N= 43 for Commercial cohort |

| Clinical Trial (n =50) Commercial (n = 50) Mean 444.4 203.8 SD 287 90 Median 449 198 Min 12 3 Max 1108 392 Duration of Support (days) |

| % Patients Affected Event Rate Respiratory Failure 15% 0.17 Renal Failure 9% 0.10 Infection - Sepsis 9% 0.10 Infection - Driveline 15% 0.17 Stroke Ischemic 4% 0.05 Hemorrhagic 7% 0.08 * Pagani et al. Extended Mechanical Circulatory Support With a Continuous-Flow Rotary Left Ventricular Assist Device. JACC 2009;54:312-321. Adverse Events and Rates (n = 100) |

| Infections Clinical Trial (n = 50) Commercial (n = 50) Sepsis 4 5 Driveline 9 6 |

| Neurologic Events Post Implant (All) Clinical Trial (n = 50) Commercial (n = 50) CVA Ischemic 2 2 Hemorrhagic 5 2 TIA 2 2 |

| RVAD Post Implant Clinical Trial (n = 50) Commercial (n = 50) Need for RVAD 3 2 |
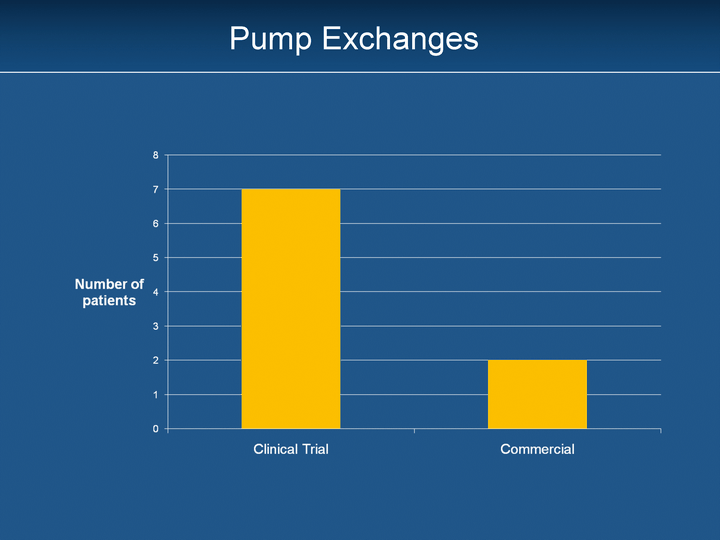
| Pump Exchanges |

| Current Status Clinical Trial (n = 50) Commercial (n = 50) Alive at 180 days 45 (90%) 45 (90%) Transplanted at 180 Days 7 4 Mean Time to Transplant 331 Days 133 Days Median Duration (All) 449 Days 199 Days Average Duration (All) 444 Days 204 Days |

| Kaplan-Meier Survival Curve 90% 180 days 85% 77% Clinical Trial Patients (n =50) Commercial Patients (n = 50) 180 days 90% 87% |

| Kaplan-Meier Survival Curve 90% 86% 78% 180 days Clinical & Commercial (n = 100) |

| Long term circulatory support with the miniature HeartWare Ventricular Assist System is feasible The device performs well for greater than one year with low adverse event rates The data also demonstrate versatility of the HeartWare System to support a wide range of patients Minimally invasive implant techniques were possible Outcomes have continued to improve after initial clinical trial experience Conclusions |
