Attached files
| file | filename |
|---|---|
| 8-K - 8K INVESTOR PRESENTATION - JUNIPER PHARMACEUTICALS INC | form8k.htm |

1
Corporate Presentation April 2010
Frank Condella, Interim CEO
Larry Gyenes, CFO

2
This presentation contains forward-looking statements, which statements are indicated by the words “may,” “will,”
“plans,” “believes,” “expects,” “anticipates,” “potential,” and similar expressions. Such forward-looking statements
involve known and unknown risks, uncertainties, and other factors that may cause actual results to differ materially
from those projected in the forward-looking statements.
“plans,” “believes,” “expects,” “anticipates,” “potential,” and similar expressions. Such forward-looking statements
involve known and unknown risks, uncertainties, and other factors that may cause actual results to differ materially
from those projected in the forward-looking statements.
Factors that might cause future results to differ include, but are not limited to, the following: approval of the sale of the
assets and other matters contemplated by the Purchase and Collaboration Agreement with Watson Pharmaceuticals,
Inc. by Columbia's stockholders; the successful marketing of CRINONE® (progesterone gel) and STRIANT®
(testosterone buccal system) in the United States; the successful marketing of CRINONE by Merck Serono; the timely
and successful completion of the ongoing Phase III PREGNANT (PROCHIEVE Extending GestatioN A New Therapy)
Study of PROCHIEVE® 8% (progesterone gel) to reduce the risk of preterm birth in women with a short cervix in mid-
pregnancy; successful development of a next-generation vaginal progesterone product; success in obtaining
acceptance and approval of new products and new indications for current products by the United States Food and
Drug Administration and international regulatory agencies; the impact of competitive products and pricing; our ability
to obtain financing in order to fund our operations and repay our debt as it becomes due; the timely and successful
negotiation of partnerships or other transactions; the strength of the United States dollar relative to international
currencies, particularly the euro; competitive economic and regulatory factors in the pharmaceutical and healthcare
industry; general economic conditions; and other risks and uncertainties that may be detailed, from time-to-time, in
Columbia’s reports filed with the SEC.
assets and other matters contemplated by the Purchase and Collaboration Agreement with Watson Pharmaceuticals,
Inc. by Columbia's stockholders; the successful marketing of CRINONE® (progesterone gel) and STRIANT®
(testosterone buccal system) in the United States; the successful marketing of CRINONE by Merck Serono; the timely
and successful completion of the ongoing Phase III PREGNANT (PROCHIEVE Extending GestatioN A New Therapy)
Study of PROCHIEVE® 8% (progesterone gel) to reduce the risk of preterm birth in women with a short cervix in mid-
pregnancy; successful development of a next-generation vaginal progesterone product; success in obtaining
acceptance and approval of new products and new indications for current products by the United States Food and
Drug Administration and international regulatory agencies; the impact of competitive products and pricing; our ability
to obtain financing in order to fund our operations and repay our debt as it becomes due; the timely and successful
negotiation of partnerships or other transactions; the strength of the United States dollar relative to international
currencies, particularly the euro; competitive economic and regulatory factors in the pharmaceutical and healthcare
industry; general economic conditions; and other risks and uncertainties that may be detailed, from time-to-time, in
Columbia’s reports filed with the SEC.
Completion of the sale of the assets under the Purchase and Collaboration Agreement with Watson Pharmaceuticals,
Inc. is subject to various conditions to closing, and there can be no assurance those conditions will be satisfied or that
such sale or other transactions will be completed on the terms described in the Purchase and Collaboration
Agreement or other agreements related thereto or at all. All forward-looking statements contained herein are neither
promises nor guarantees. Columbia does not undertake any responsibility to revise or update any forward-looking
statements contained herein.
Inc. is subject to various conditions to closing, and there can be no assurance those conditions will be satisfied or that
such sale or other transactions will be completed on the terms described in the Purchase and Collaboration
Agreement or other agreements related thereto or at all. All forward-looking statements contained herein are neither
promises nor guarantees. Columbia does not undertake any responsibility to revise or update any forward-looking
statements contained herein.

3
} Specialty pharmaceutical company leveraging our
bioadhesive drug delivery system and clinical expertise
to develop proprietary products in women’s healthcare
bioadhesive drug delivery system and clinical expertise
to develop proprietary products in women’s healthcare
} CRINONE® 8% progesterone vaginal gel
◦ Sold worldwide for use in infertility treatments
} CRINONE/PROCHIEVE® 8% gel
◦ Currently in pivotal Phase III trial to reduce the risk of preterm
birth in women with short cervix
birth in women with short cervix
◦ Enrollment to complete in Q2 2010
◦ Data available around end of 2010

4
Frank C. Condella, Jr. -- Interim Chief Executive Officer /Board Member
• Chairman of SkyePharma ; Director of Fulcrum Pharma plc.
• Formerly CEO of SkyePharma; IVAX Corporation; Faulding Pharmaceutical Co.;
Hoffman-La Roche.
Hoffman-La Roche.
Robert S. Mills -- President and Chief Operating Officer
• Formerly SVP, manufacturing Operations, Watson Pharmaceuticals;
Alphapharma, Inc.; Aventis, SA
Alphapharma, Inc.; Aventis, SA
Lawrence A. Gyenes -- Senior Vice President, Chief Financial Officer and Treasurer
• Formerly CFO at Acusphere; Zila; Savient & Reliant; 15 years at Searle
Michael McGrane -- Senior Vice President, General Counsel and Secretary
• Formerly General Counsel, The Liposome Co.; Novartis Consumer Health
•George W. Creasy, MD -- Vice President, Clinical Research and Development
• Formerly spent 16 years Johnson & Johnson; Fellow of the American College of
Obstetricians and Gynecologists.
Obstetricians and Gynecologists.
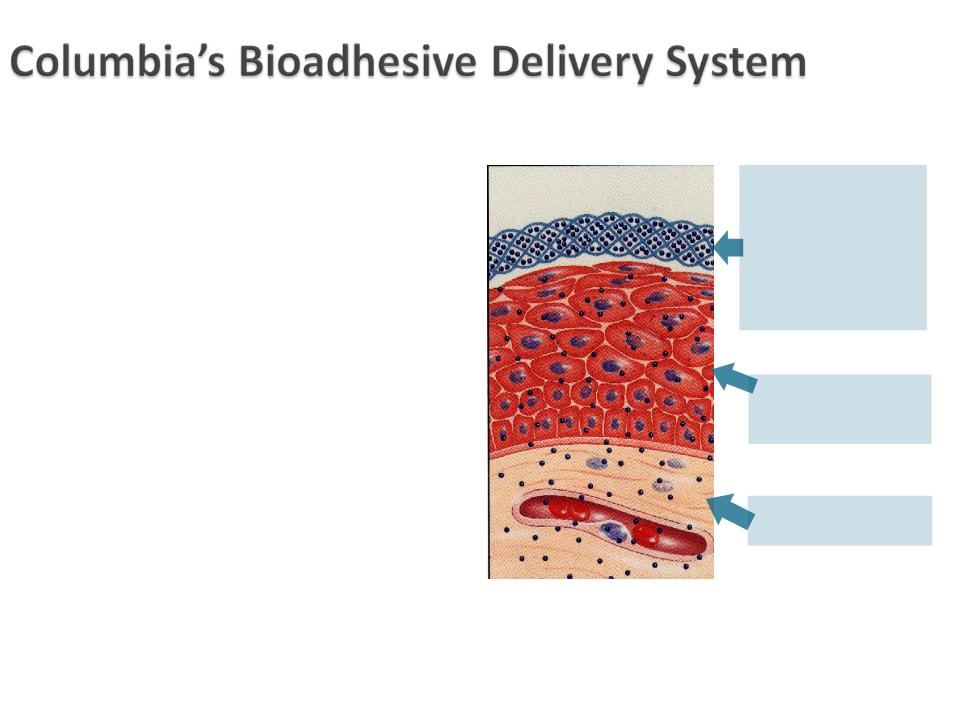
} Technology that uses bioadhesion to
administer medication to patients in a
targeted fashion
administer medication to patients in a
targeted fashion
} Polycarbophil-based, non-immunogenic
(hypo-allergenic) polymer
(hypo-allergenic) polymer
} Adheres to mucosal surface and discharged
upon normal cell turnover
upon normal cell turnover
◦ Oral mucosa for up to 24 hours
◦ Vaginal epithelium for 72+ hours
} Enables controlled and sustained drug
release
release
} Able to deliver broad range of compounds
to address numerous therapeutic areas
to address numerous therapeutic areas
Polycarbophil: a water-
swellable, water-
insoluble polymer with
numerous negative
charges that
swellable, water-
insoluble polymer with
numerous negative
charges that
permit hydrogen
bonding with the cell
surface
bonding with the cell
surface
Active entrapped in the
cross-linked polymer
polycarbophil
cross-linked polymer
polycarbophil
Sustained release of
active to tissue
active to tissue

6
*2008 net revenues include $2.9 million in previously deferred revenue for
STRIANT licensing fees from Ardana as a results of its bankruptcy in Q2 2008.
STRIANT licensing fees from Ardana as a results of its bankruptcy in Q2 2008.
*

7
|
Millions
|
2007
|
2008
|
2009
|
|
Net revenues
|
$29.6
|
$36.2*
|
$32.2
|
|
Cost of revenues
|
(9.0)
|
(10.9)
|
(9.2)
|
|
Gross Profit
|
20.6
|
25.3
|
23.0
|
|
Selling & distribution
|
10.1
|
12.7
|
12.0
|
|
General & administrative
|
7.8
|
8.6
|
10.6
|
|
Research & development
|
5.8
|
6.2
|
8.6
|
|
Amortization of licensing right
|
5.0
|
5.0
|
5.0
|
|
Loss from operations
|
(8.1)
|
(7.3)
|
(13.2)
|
|
Interest expense
|
(7.9)
|
(7.9)
|
(8.9)
|
|
Net loss
|
(14.3)
|
(14.1)
|
(21.9)
|
|
Earnings (loss) per share
|
$(0.28)
|
$(0.27)
|
$(0.39)
|
*2008 net revenues include $2.9 million in previously deferred revenue for
STRIANT licensing fees from Ardana as a results of its bankruptcy in Q2 2008.
STRIANT licensing fees from Ardana as a results of its bankruptcy in Q2 2008.

8
|
Nasdaq: CBRX
|
|
|
Recent market price (4/6/2010)
|
$1.04
|
|
Market capitalization
|
$68.2 million
|
|
Cash and equivalents (12/31/2009)
|
$14.8 million
|
|
Debt obligations
|
|
|
PharmaBio royalty loan due 11/30/2010 (extendable one year)
|
$16.4 million
|
|
Subordinated 8% convertible notes due 12/31/2011
(convertible @ $5.25) |
$40.0 million
|
9

10
$300+ Million Total Market Opportunity

11
$0
$2,000,000
$4,000,000
$6,000,000
$8,000,000
$10,000,000
$12,000,000
$14,000,000
$16,000,000
$18,000,000
2006
2007
2008
2009
CRINONE 8%

12
} Brigham and Women’s study compared CRINONE vs. IM
progesterone in 440 women
progesterone in 440 women
◦ Similar pregnancy rates and outcomes
◦ CRINONE was significantly better tolerated than IM progesterone
◦ Added CRINONE to protocol in preferred position
} Berger analysis compared the efficacy of luteal
supplementation with 3 different progesterone preparations
in women aged 35-40 undergoing IVF-FET
supplementation with 3 different progesterone preparations
in women aged 35-40 undergoing IVF-FET
◦ Clinical pregnancy rates were comparable
◦ Once daily CRINONE may improve compliance
} Toner study found bleeding is rare in IVF patients who
become pregnant when treated with CRINONE + estrogen
become pregnant when treated with CRINONE + estrogen

13

14
} Studies have shown that “short cervix” is the most
powerful predictor of preterm birth
powerful predictor of preterm birth
} Cervical length should be measured in pregnant women
at mid-pregnancy
at mid-pregnancy
} A cervix of <3.0cm at 18-22 weeks is considered “short”
} Progesterone is the most promising treatment to reduce
risk of PTB and improve infant outcomes in women with
a short cervix
risk of PTB and improve infant outcomes in women with
a short cervix
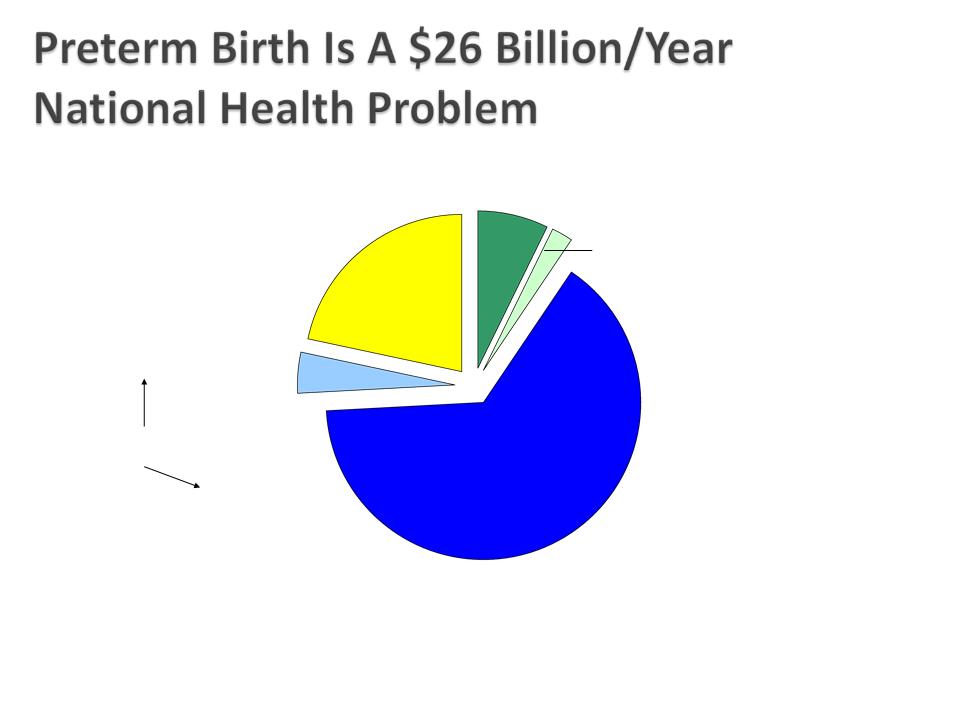
Behrman RE et al. In: Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention.
Washington, DC: The National Academies Press; 2006:329-354.
Washington, DC: The National Academies Press; 2006:329-354.
Lost household and
market productivity
market productivity
$5.7 billion
Maternal delivery costs
$1.9 billion
Children’s early intervention
services
services
$611 million
Infant Costs
Special education services
$1.1 billion
Medical care services
$16.9 billion
~$51,600 Per Preterm Infant
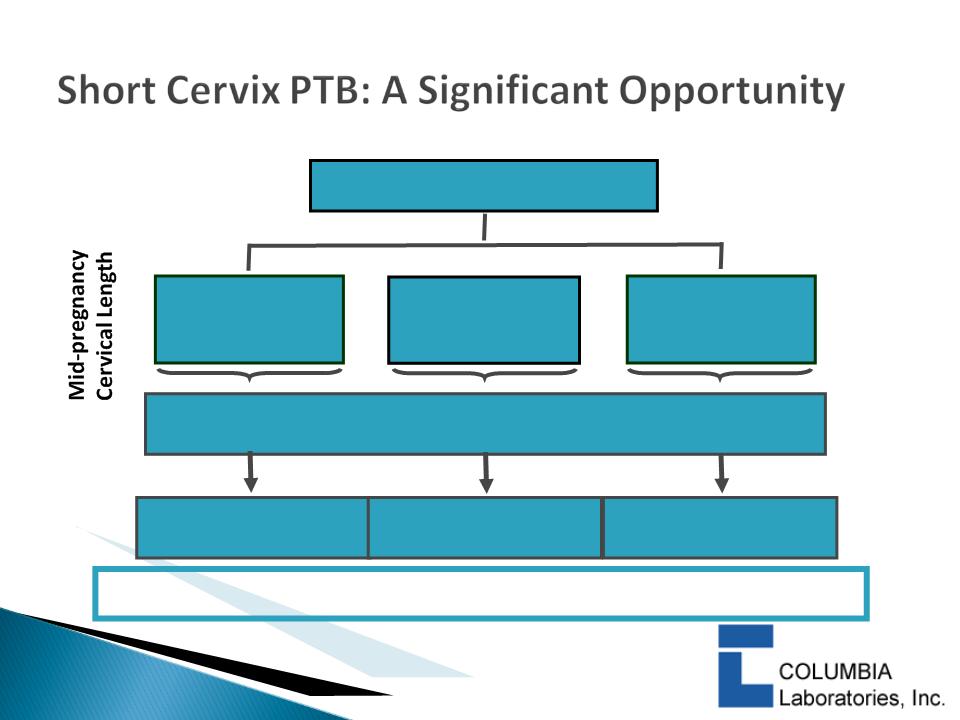
16
$1.7+ Billion Total U.S. Market Opportunity
$225+ million market
potential
potential
4.3 Million Births Annually
>2.5 - 3.0 cm
(20%)
(20%)
$1.1+ billion
market potential
> 2.0 - 2.5 cm
(6%)
(6%)
1.0 - 2.0 cm
(4%)
(4%)
$340+ million market
potential
potential
16 weeks X $83.31 week =
$1,333 per patient

17
} Phase III clinical trial
◦ Double-blind, placebo controlled
◦ 450 pregnant women with cervical length 1.0-2.0cm
◦ ~40 centers (U.S. and abroad)
◦ Primary endpoint: a reduction in preterm births at ≤32 6/7
weeks vs. placebo
weeks vs. placebo
◦ Improved infant outcomes important secondary endpoint
} NIH participation
◦ Validates science and design of trial

18
} Currently enrolled 426 patients as of 4/7/10
} Complete patient enrollment Q2 2010
} Last infant is born Q4 2010
} Report study outcomes Y/E 2010
} File with FDA* 2011
} FDA decision* 2011/2012**
*Assuming positive outcome
**PDUFA limits review time to 10 months; could shorten
to 6 months if granted priority review
to 6 months if granted priority review

19
progesterone
placebo
33 investigators
n=116 (58 Prochieve, 58 Placebo)
As Cervical Length Decreases, The Benefit Of
Treatment Increases
Treatment Increases
Baseline Cervical Length ≤ 3.0 cm
Baseline Cervical Length < 2.8 cm
progesterone
placebo
22 Investigators
N=46 (19 Prochieve, 27 Placebo)
Fishers Exact Test
at <32 weeks*:
at <32 weeks*:
(p = 0.014)
At ≤ 32 Weeks No Preterm Births Were Seen With Prochieve® Vs. 29.6% On Placebo
The Kaplan-Meier method was
used for calculation; (Wilcoxon
P = 0.043).
used for calculation; (Wilcoxon
P = 0.043).
DeFranco et al, Ultrasound Obstet
Gynecol 2007; 30: 697-705
Gynecol 2007; 30: 697-705

20
p=0.016
p=0.077
p=0.16
DeFranco et al, Ultrasound Obstet
Gynecol 2007; 30: 697-705
Gynecol 2007; 30: 697-705

21
DeFranco et al,
Ultrasound Obstet
Gynecol 2007; 30:
697-705
Ultrasound Obstet
Gynecol 2007; 30:
697-705
p=0.013
p=0.026
p=0.05

22
} Progesterone delivery patent à 2013
◦ U.S. Patent No. 5,543,150
◦ Development of a generic would be challenging due to:
– Clinical endpoints (not blood levels) are required to demonstrate bio-
equivalence for a generic gel
equivalence for a generic gel
– A clinical endpoint requires a non-inferiority study in hard-to-treat
infertility subjects
infertility subjects
} Second generation formulation à 2019
◦ U.S. Patent No. 6,248,358
◦ Designed to replace current gel product
◦ Would have certain user benefits over current formulation
◦ Clinical study necessary in approved indications for FDA approval

23
} Progesterone delivery patent à 2013
} Next generation formulation à 2019
} PTB/Short Cervix (patent pending) à 2028
◦ Patent Application No. US-2008/0188829-A1
◦ Claims a method to:
– Identify pregnant women with a short cervix
– Prevent PTB by administering progesterone to minimize the shortening or
effacing of the cervix
effacing of the cervix
– Administer progesterone by any route
– Deliver in a broad range of doses
◦ Includes both natural progesterone & synthetic progestins
◦ Patent applications filed worldwide

24

25
} Watson Pharmaceuticals
◦ Global specialty pharmaceutical company
◦ Branded business focused on Urology & Women’s Health
◦ ~$500 million in branded revenues
◦ $4 billion market capitalization
◦ Field force of 350 calling on OB/GYNs and urologists, plus
specialists focusing on infertility clinics
specialists focusing on infertility clinics

26
} Watson to acquire:
◦ Substantially all of CBRX progesterone assets
◦ 11.2 million shares CBRX common stock
} Watson assumes responsibility for all U.S. sales,
marketing & distribution activities for CRINONE
in infertility
marketing & distribution activities for CRINONE
in infertility
} CBRX continues manufacturing & supply chain
management
management

27
} Watson & CBRX will collaborate on:
◦ Completion of the PREGNANT Study
– CBRX out-of pocket costs capped at $7 million
spent beginning 1/1/2010
spent beginning 1/1/2010
◦ Development of next-generation vaginal
progesterone products for infertility and preterm
birth
progesterone products for infertility and preterm
birth
– Watson will fund development once the
transaction closes
transaction closes

28
} Watson will pay CBRX:
◦ $47 million upfront
◦ Escalating royalties from 10 to 20%
◦ Up to $45.5 million upon CBRX successful completion
of several milestones
of several milestones
} With initial proceeds, CBRX to:
◦ Pre-pay $16 million PharmaBio debt
◦ Retire 100% of the $40 million of 8% convertible notes

29
} Transaction subject to stockholder approval
◦ Needs >50% of voting power in favor
} Preliminary Proxy to SEC on March 19; to stockholders in
April/May
April/May
} Special meeting will be held - likely in May/June
} Columbia’s Board of Directors unanimously recommends
a vote in favor of this transaction
a vote in favor of this transaction

30
} Use new data to drive sales growth of CRINONE in
infertility
infertility
} Close Watson transaction by June
◦ Retire debt in associated transaction
◦ Transition U.S. sales and marketing
◦ Reduce infrastructure costs
} Complete PREGNANT Study
◦ If data is positive, pursue FDA approval of CRINONE/PROCHIEVE for
reducing the risk of preterm birth in women with short cervix
reducing the risk of preterm birth in women with short cervix
} Collaborate with Watson to develop second generation
product to protect long-term royalty stream
product to protect long-term royalty stream
} Establish alternate promotion arrangement for STRIANT

31
• A modest 12% increase in product sales to $17 million
would match the 2009 product contribution margin
would match the 2009 product contribution margin
• The above excludes Merck Serono revenues which
contributed another $9 million in revenues and $6 million in
contribution margin in 2009
contributed another $9 million in revenues and $6 million in
contribution margin in 2009

32
} Collaborate with WPI to maximize preterm birth
opportunity and value for CBRX shareholders
opportunity and value for CBRX shareholders
} Apply proven proprietary drug delivery systems to
develop new products for women’s health needs
develop new products for women’s health needs
} Selectively pursue development opportunities in other
therapeutic areas utilizing platform technologies
therapeutic areas utilizing platform technologies
◦ Expand use of progressive hydration technology for extended
release products
release products
◦ Pursue label expansion for Striant

33
|
Product
|
Indication
|
Clinical Stage
|
|
Crinone/Prochieve 8%
Vaginal Gel
|
Reduce risk of preterm birth in women
with a short cervix in mid-pregnancy |
Pivotal Phase III
|
|
Crinone/Prochieve 8%
Next generation
|
ART (Infertility)
|
Phase I
|
|
Crinone/Prochieve 8%
Next generation
|
Preterm Birth
|
Phase I
|
|
Carbamide Peroxide
Vaginal Tablet
|
Bacterial vaginosis
|
Pre-clinical
|
|
Infertility Product
Subcutaneous
|
Non-progesterone ART
|
Phase I/II
|
|
Testosterone
Vaginal delivery
|
Fibroid reduction
|
Phase I
|
|
Anesthetic
Vaginal Gel
|
Chronic Pelvic Pain (CPP)
|
Phase II
|

34
} Carbamide peroxide for bacterial vaginosis
} In-house development program
◦ Formulation & pre-clinical: approx. 9 months
◦ IND filing
◦ Phase I safety study: approx. 9 months
◦ Phase II proof of concept study: approx. 9 months
◦ Total expected cost: $2.5 million
} Partner for Phase III

35
} Debt reduced; balance sheet strengthened
} Ongoing revenue: potential milestones, royalties from Watson &
Merck Serono, sales of Striant
Merck Serono, sales of Striant
} Reduced operating expenses
} Sufficient cash for operations, completing PREGNANT Study and FDA
filing
filing
} Positive cash flow from preterm birth trial success, filing, and launch
} Lifecycle management program increases likelihood of long term
revenue stream
revenue stream
} CBRX funds new development projects through proof of concept,
then partnered
then partnered

36
} Focused today on:
◦ Building CRINONE sales to infertility market
◦ Developing CRINONE/PROCHIEVE for preterm birth indication
} With stockholder approval of WPI transaction:
◦ CBRX emerges debt free, with a stronger balance sheet
◦ Ongoing royalty revenues from WPI & Merck-Serono, potential milestone
payments, STRIANT sales
payments, STRIANT sales
◦ Significantly lower operating expenses
◦ Burn rate decreases to ~ $1 million per quarter through the completion
of the PREGNANT Study
of the PREGNANT Study
◦ If PREGNANT Study is successful, CBRX will be cash flow & earnings
positive on an annual basis from that point forward
positive on an annual basis from that point forward

37
Corporate Presentation April 2010
Frank Condella, Interim CEO
Larry Gyenes, CFO
