Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-34584
HARBOR BIOSCIENCES, INC.
(Exact name of Registrant as specified in its charter)
| Delaware | 13-3697002 | |
| (State or other jurisdiction | (I.R.S. Employer | |
| of incorporation or organization) | Identification No.) | |
| 4435 Eastgate Mall, Suite 400 | ||
| San Diego, CA | 92121 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (858) 587-9333
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Each Exchange on Which Registered | |
| Common Stock, par value $0.01 per share | The Nasdaq Stock Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ¨ NO x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ¨ NO x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirement for the past 90 days. YES x NO ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ¨ NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. YES ¨ NO x
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2. (Check One).
| Large accelerated filer ¨ |
Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller Reporting Company x |
Indicate by check mark whether the Registrant is a shell company (as defined in Exchange Act Rule 12b-2). YES ¨ NO x
The aggregate market value of the voting stock held by nonaffiliates of the Registrant as of June 30, 2009, the end of the Company’s most recently completed second fiscal quarter, was approximately $14,497,860 based on the closing stock price of $0.50 for the Registrant’s Common Stock as reported by the Nasdaq Global Market*.
As of March 27, 2010, there were outstanding 29,433,939 shares of the Registrant’s Common Stock, $.01 par value per share.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of Registrant's definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after Registrant’s fiscal year end December 31, 2009, are incorporated by reference into Part III of this Annual Report on Form 10-K.
* Excludes the common stock held by executive officers, directors and stockholders whose ownership exceeded 10% of the Registrant’s common stock outstanding at June 30, 2009. This calculation does not reflect a determination that such persons are affiliates for any other purposes.
Table of Contents
Harbor BioSciences, Inc.
Form 10-K
For the Fiscal Year Ended December 31, 2009
2
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, and the information incorporated herein, contains forward-looking statements that involve and are subject to risks and uncertainties. In particular, statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are contained or incorporated by reference in this Annual Report on Form 10-K. We have based these forward-looking statements on our current expectations about future events. While we believe these expectations reflected in this Annual Report on Form 10-K are reasonable, the inclusion of any forward-looking statements should not be regarded as a representation that any of our plans will be achieved and such forward-looking statements are inherently subject to risks and uncertainties, many of which are beyond our control. Such forward-looking statements include, but are not limited to, statements preceded by, followed by or that otherwise include the words, “believe,” “may,” “might,” “can,” “could,” “will,” “would”, “should,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “project,” “expect,” or similar expressions. The actual future results for Harbor BioSciences, Inc. may differ materially from those discussed here for various reasons, including those discussed in this Annual Report in Part 1, Item 1A under the heading “Risk Factors,” Part II, Item 7 entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere throughout this Annual Report on Form 10-K. Given these risks and uncertainties, you are cautioned not to place undue reliance on such forward-looking statements. The forward-looking statements included in this Annual Report are made only as of the date hereof. We do not undertake any obligation to update any such statements or to publicly announce the results of any revisions to any of such statements as a result of new information, to reflect future events or developments. When used in this Annual Report, unless otherwise indicated, “we,” “our” and “us” refers to Harbor BioSciences, Inc.
PART I
| Item 1. | Business |
GENERAL OVERVIEW
Harbor BioSciences, Inc. (“Harbor BioSciences”), a clinical-stage pharmaceutical company, is engaged in the discovery and development of products for the treatment of diseases related to aging. Our current development efforts are primarily focused on a series of steroid hormone analogs that are derived from the human adrenal metabolome.
We are currently focused on the development of two clinical drug development candidates—APOPTONE® (HE3235), a compound in a Phase I/IIa clinical trial for late-stage prostate cancer and TRIOLEX® (HE3286), a compound currently in early Phase II clinical trials for the treatment of type 2 diabetes and staged for Phase II clinical trials in ulcerative colitis (UC) and rheumatoid arthritis (RA).
Drawn from our unique and proprietary platform, our research program has identified additional lead candidates active in preclinical models of cancer, metabolic conditions, autoimmune conditions, lung inflammation, bone degeneration and organ regeneration.
Our principal executive offices are located at 4435 Eastgate Mall, Suite 400, San Diego, California 92121, and our telephone number is (858) 587-9333. We incorporated in Delaware in 1992.
On March 26, 1997, Hollis-Eden, Inc., a Delaware corporation, was merged with and into us, then known as Initial Acquisition Corp. (IAC), a Delaware corporation. Upon consummation of the merger of Hollis-Eden, Inc. with IAC, Hollis-Eden, Inc. ceased to exist, and IAC changed its name to Hollis-Eden Pharmaceuticals, Inc.
Effective February 9, 2010, we changed our name from Hollis-Eden Pharmaceuticals, Inc. to Harbor BioSciences, Inc. The name change was effected pursuant to Section 253 of the General Corporation Law of the
3
Table of Contents
State of Delaware by the merger of our wholly owned subsidiary into us. We were the surviving corporation and, in connection with the merger, we amended our Amended and Restated Certificate of Incorporation to change our name to Harbor BioSciences, Inc.
Effective February 18, 2010, our common stock began trading under a new Nasdaq symbol, “HRBR” and CUSIP number “41150V 103”.
Harbor BioSciences, TRIOLEX, APOPTONE, and the Harbor BioSciences stylized logo are trademarks of Harbor BioSciences, Inc. This filing also includes trademarks owned by other parties. All other trademarks mentioned are the property of their respective owners. Use or display by us of other parties’ trademarks or products is not intended to and does not imply a relationship with, or endorsements or sponsorship of, us by the trademark or product owners.
Our periodic and current reports that we file with the Securities and Exchange Commission, or SEC, are available free of charge, on our website, as soon as reasonably practicable after we have electronically filed them with, or furnished them to, the SEC. Our Internet address is www.harborbiosciences.com. The reference to our website does not constitute incorporation by reference of the information contained on our website.
Harbor BioSciences’ Approach
Over the last several decades, scientists have developed novel tools to study biological function at the molecular level. These tools enabled an intellectual approach to drug development largely centered on the selection of agents that interacted with validated molecular targets for specific diseases. While this approach has resulted in a number of successful drugs, frequently their use is limited by serious side effects.
At Harbor BioSciences, we embrace a “systems biology” approach to drug development, one that accounts for and leverages the complexity of tissue specific reactions integral to a variety of molecular pathways. Rather than simply blocking or stimulating an isolated target, we are attempting to integrate a number of different scientific disciplines, including molecular biology, high speed computing and engineering, to understand interactions and identify signals that restore homeostasis. There is evidence that dysregulation at the metabolome level can lead to a diverse set of diseases and conditions. Chronic inflammatory processes may be a common link to tissue destruction in diverse diseases such as arthritis, diabetes, HIV, Alzheimer’s disease and cancer. All of these conditions may benefit by a blunting of the inflammatory response and the restoration of homeostasis.
Our development strategy is based on the hypothesis that adrenal products are critical to the regulation of the body’s complex defense system. We believe that in young, healthy adults, adrenal products such as cortisol, dehydroepiandrosterone (DHEA) and its metabolome, provide important signals that determine whether appropriate cytokines are produced at appropriate times to properly regulate immune responses. Under conditions of stress, chronic infections or systemic inflammation, changes to adrenal products themselves, their metabolism, and perturbations of signaling pathways in peripheral tissues, may drive the growth of certain tumors and be causative to diseases of advancing age, including metabolic syndrome, autoimmune diseases, immune mediated inflammatory disease and an impaired ability to fight infections.
Most drug developers are taking a ground up approach, first striving to understand and identify critical components in these intricate cascades, and then trying to design drugs that can successfully block or stimulate specific pathways. In contrast, ours is a top down approach, beginning with the discovery of new members of the adrenal steroid metabolome. Then, by applying pharmaceutical development methodology, our goal is to design compounds that modify critical adrenocorticoid endocrine pathways. We believe this approach has the potential to identify product candidate compounds to treat a myriad of diseases associated with advancing age, including certain cancers, metabolic and autoimmune diseases as well as immune-mediated inflammatory diseases. We believe that successfully applying these principles may have the potential to develop pharmaceuticals to address a number of large and important markets, including many unmet medical needs.
4
Table of Contents
TECHNOLOGY
Platform
Our primary technology development efforts are focused on a series of adrenal steroid hormones and synthetic analogs that may be useful in treating a wide variety of medical conditions, if successfully developed. These adrenal hormones are depleted during advancing age, a process accelerated by infectious diseases and chronic immune system disorders. High plasma concentrations of these hormones are positively correlated with attenuated disease, in certain indications, and their maintenance is often associated with healthy aging.
The chemistry and biochemistry of steroids have been extensively studied and utilized in the development of various drugs, especially for hormonal imbalance, for the treatment of infections and cancer as well as inflammation. Harbor BioSciences’ inventory of greater than 700 steroid compounds is a targeted library synthesized under industry standards of medicinal chemistry. The compound library takes existing drug leads and generates neighbors (analogues) of the leads in the chemical space. Many of the compounds are previously undiscovered metabolic products of (DHEA) as well as structurally novel analogues stabilized against additional metabolism. This library is the largest sample of the DHEA metabolome reported and contains many unique chemical structures with diverse biological properties.
The unifying theme of the targeted library is to make drug-like molecules that have unique target recognition characteristics used to derive a structure-activity relationship (SAR) that imparts affinity and selectivity for the selected target. In addition, design features include bioavailability, usually so that the compound can be given orally, metabolic and chemical stability, and with the necessary novelty for patent purposes. Synthesis of active compounds is optimized to be facile and cost-effective with attention to the need for commercialization.
OUR DRUG CANDIDATES IN DEVELOPMENT
We are currently focused on the development of two proprietary synthetic steroid derivatives derived from the human adrenal steroid metabolome. Our lead clinical drug development candidates are APOPTONE (HE3235), currently in clinical trials for late-stage prostate cancer and TRIOLEX (HE3286), with clinical trials conducted for the treatment of obese type 2 diabetes, metabolic and autoimmune disorders. Each of these compounds is described in more detail below. In addition, our research program, focused on the identification and characterization of new adrenal hormones, has identified additional potential, new human hormones that may become future pharmaceutical candidates or nutraceutical products.
APOPTONE (HE3235)
Prostate Cancer
APOPTONE is a second-generation compound we have selected for clinical development in the area of hormone driven cancers, such as prostate cancer. APOPTONE was discovered by screening our proprietary steroid chemical library against a prostate cancer LNCaP cell line. It was selected based on a combination of its potency against cancer and desirable pharmaceutical properties. It has been tested in a number of preclinical cancer models and has shown indications of activity in controlling the incidence, growth and development of new tumors in these models. We believe that APOPTONE is a disease-modifying agent that may directly induce apoptosis, or cell death, in tumor cells, a result that differs from traditional hormone blockade therapies that interrupt the tumor cell growth signal through direct androgen or estrogen receptor mediated mechanisms. While hormone blockade therapy can effectively control prostate cancer for a period of time, it often fails and the cancer grows again and spreads to other organs, usually the bone.
In 2008, we initiated a Phase I/IIa clinical trial with APOPTONE in late-stage castrate resistant prostate cancer (CRPC) patients who have failed hormone therapy and at least one round of chemotherapy. In December
5
Table of Contents
2009, the trial was amended to include a group of CRPC patients with progressive disease that have not been previously treated with chemotherapy. The open-label dose ranging clinical trial is being conducted in various clinical sites including some sites within the Prostate Cancer Clinical Trial Consortium (PCCTC). It is evaluating the safety, tolerance, pharmacokinetics and potential activity of APOPTONE when administered twice daily in late-stage prostate cancer patients. Potential activity of the compound is measured by the effect on time to disease progression, as determined by prostate-specific antigen (PSA) blood tests, computerized tomography (CT), magnetic resonance imaging (MRI), or bone scintigraphy, and its effect on circulating tumor cells (CTC). Approximately 234,000 patients are diagnosed each year with prostate cancer, and global sales for leading prostate cancer drugs range approximately from $500 million to $1 billion annually.
Breast Cancer
We are also exploring the potential of APOPTONE in breast cancer. In pre-clinical models of MNU-induced breast cancer, APOPTONE successfully treated established tumors and prevented the formation of new tumors. It appeared to be synergistic when given in combination with concurrent taxane chemotherapy.
APOPTONE Development Status
APOPTONE is manufactured using several organic synthesis steps from the starting material androsterone. The active pharmaceutical ingredient is formulated to an oral dosage form using commonly used excipients in approved (oral dosage) products. Non-clinical toxicology studies have been done that enable the use of APOPTONE in clinical studies in late-stage prostate cancer and breast cancer patients using 28-day cycles of therapy. Encouraging data were reported from an ongoing Phase I/IIa clinical trial for castration resistant prostate cancer (CRPC)—also referred to as hormone resistant prostate cancer—at the ASCO Genitourinary Cancers Symposium in San Francisco, March 6, 2010. Preliminary results from the study, conducted which included participating member sites of the Prostate Cancer Clinical Trial Consortium (PCCTC), were first reported on November 16, 2009. The phase I/IIa trial is an open-label study with the primary objectives of assessing safety, tolerability, pharmacokinetics and activity of APOPTONE in men with CRPC and an ECOG performance status score of less than or equal to 2 (ambulatory and capable of at least self-care). Patient cohorts are defined by oral daily doses of 10 mg, 20 mg, 30 mg, 50 mg, 100 mg, 200 mg and 350 mg. Subjects are treated on 28-day cycles until toxicity or disease progression; CT and bone scans are obtained every two cycles to assess progression. Based on encouraging signs of activity, the PCCTC recommended an extension of the current trial into patients that have not been treated with chemotherapy. Accordingly, the subject eligibility criteria were amended to include earlier-stage, chemotherapy-naïve patients in a 100 mg expansion cohort and 10 patients have been enrolled to date. As of February 17, 2010, 42 taxane-resistant prostate cancer patients have been entered into the clinical trial at 7 dose levels. Of these 28 (67%) reached their first reassessment (two 28-day cycles), 15 (54%) of these had stable disease on scans or imaging and have received 1-8 additional treatment cycles before disease progression. Six patients continue to receive treatment. The Kaplan-Meier estimate for the median time to progression is 15.3 weeks (range 4-40) for this ongoing trial. The data are essentially complete for the low dose groups. Due to early signs of activity, the 20 mg dose group was expanded to include 14 patients. Eleven of these were evaluable with an actual median time to progression of 20 weeks (range 8-28). Changes in PSA levels were consistent with the properties of this class of agent. The drug has been well tolerated and dose escalation has proceeded to 350 mg per day with no overt dose-limiting toxicities reported. The 350 mg dose group was expanded to include 10 additional pre-chemotherapy patients in order to gain additional information on the tolerability of APOPTONE at this dose level. Several patent applications have been filed for the pharmaceutical formulation of APOPTONE and its use for the treatment of prostate cancer, breast cancer and benign prostate hypertrophy.
Competition
Taxotere chemotherapy is presently the only approved therapy to treat castrate resistant prostate cancer. Despite current treatments, there is an ongoing need for novel oral agents that can control the growth of prostate
6
Table of Contents
cancer that is progressing on conventional therapies or hormone treatments. Accordingly, there are a number of companies with drug candidates in development targeting late-stage castration resistant prostate cancer, including compounds already in Phase III clinical trials. Abiraterone produced by Cougar Biotechnology, Inc. is an agent that impedes the synthesis of androgens by inhibition of an enzyme that transforms precursor molecules into the hormone testosterone. Many forms of prostate cancer are dependent on the presence of androgens in order to grow. MDV-3100, produced by Medivation, Inc., is an agent that blocks the action of androgens on prostate cancer cells through interference at the androgen receptor and stopping their growth. Provenge, produced by Dendreon, Inc., is an autologous immune cell therapy that primes the patients cells against prostate cancer cells. All three of these therapies are in late stage clinical and regulatory development. Apoptone is believed to be a disease modification agent with a mechanism of action that distinguishes it from these competitive drug candidates.
TRIOLEX (HE3286)
Inflammatory Processes in Chronic Diseases
One of our primary focus areas is diseases of chronic inflammation. Properly regulated, inflammation is a protective, life saving response to invading pathogens. However, when inflammation goes awry and becomes chronic, it can cause devastating tissue damage and loss of organ function. Chronic inflammation is associated with an over-stimulation of the immune system, often resulting in the release of destructive products such as reactive oxygen species, destructive enzymes and other pro-inflammatory mediators. The over-production of these dangerous products is often due to persistent low-grade infections, aging and the body’s inability to differentiate between itself and foreign invaders. Chronic inflammation has been implicated in the pathogenesis of many diseases ranging from autoimmune conditions, such as arthritis and psoriasis, to infectious diseases, including human immunodeficiency virus (HIV), malaria and tuberculosis, and to metabolic diseases, including diabetes and cardiovascular diseases as well as a number of different cancer types.
Current Treatments for Chronic Inflammation
Some of the most widely used drugs for reducing inflammation belong to the corticosteroid class of compounds, which are also derived from the adrenal metabolom. Market research indicates that U.S. physicians issue tens of millions of new prescriptions for corticosteroids each year for a wide range of conditions. While these drugs are highly effective, chronic use leads to immune suppression and other serious side effects including bone loss.
Over the last decade, a number of new drugs have been introduced that are focused on inhibiting a specific component of the pro-inflammatory cascade, including agents that block specific inflammatory cytokines, such as TNF-alpha and IL-1 beta, as well as drugs that inhibit specific enzymes, such as COX-2, that produce pro-inflammatory mediators. While these drugs have demonstrated significant activity in a number of clinical trials involving chronic inflammatory diseases, such as arthritis, inflammatory bowel disease and psoriasis, most have also demonstrated significant limitations. Many cause dangerous immune suppression and other serious side effects that limit usage. Most focus on one specific mediator of inflammation, which means they may not remain effective and are vulnerable to redundancies in biological pathways. Our goal is to develop compounds that provide appropriate regulatory signals at multiple levels to regain control of the inflammatory process and restore homeostasis.
Diabetes, Insulin Resistance, and Chronic Inflammation
Diabetes is a disease of insulin signaling that is comprised of a constellation of syndromes. Insulin is a hormone needed to move glucose from the blood into cells, where it can be stored, or converted to the energy needed to perform properly. When insulin is not present in sufficient quantity or when signaling pathways do not function correctly, the result is high levels of glucose in the blood. Over time, chronically-elevated blood glucose can lead to a host of severe medical conditions including nerve disease, blindness, limb amputation, heart attack,
7
Table of Contents
stroke and death. There are two forms of diabetes: type 1, a chronic condition in which little or no insulin is produced, and type 2, a condition in which the body becomes resistant to the effects of insulin or the body produces some, but not enough, insulin to maintain a normal blood sugar level.
Epidemiological studies have clearly defined risk factors for the development or progression of type 2 diabetes, including genetics, and prenatal and postnatal environmental factors, including low birth weight, obesity, nutrient excess, inactivity, gestational diabetes and advancing age. Each of these risk factors can, via largely undefined mechanisms, lead to insulin resistance, beta-cell dysfunction and overt diabetes. In turn, diabetes-related hyperglycemia and associated metabolic abnormalities can further alter signal transduction and gene-expression thus contributing to a vicious cycle. It is likely that each of these risk factors alter gene expression, possibly in a unique but partly overlapping way. Therefore, superimposition of multiple risk-related and tissue-specific changes in gene expression are required to produce the phenotype of type 2 diabetes.
The need for new classes of agents to treat type 2 diabetes is significant. There are over 22 million Americans with type 2 diabetes and over 170 million type 2 diabetics worldwide. Obese diabetes is a syndrome that is increasing rapidly as a result of advancing age and the rising incidence of obesity. Clinical data indicates only 36% of type 2 diabetics are currently able to maintain the American Diabetes Association maximum recommended HbA1c, (a form of hemoglobin that is primarily used to identify the average plasma glucose concentration over a prolonged period of time), glucose level of less than 7.0 %. Large clinical studies have shown that failure to achieve these glucose targets, especially in obese patients, can progressively lead to severe health consequences including neuropathy, blindness, amputation, heart attack, stroke and death. Patients in large clinical trials consistently have a median BMI of 32 indicating that over half the population of T2DM is obese.
Academic researchers have increasingly linked obesity-induced chronic inflammation with type 2 diabetes and elucidated its potential role in leading to insulin resistance in type 2 diabetes. In the setting of type 2 diabetes, evidence suggests that the pathology may arise through perturbations in NF-kappaB signaling, particularly via the TLR4 receptor. TLR4 is a receptor expressed on the surface of macrophages and other cells and is stimulated by certain pathogens such as bacteria and viruses or certain substances such as dietary fatty acids. Stimulation of the TLR4 receptor induces a cascade of pro-inflammatory signals, which in turn, results in activation events that set a complex network of signaling pathways in motion, which culminates with the activation of NF-kappaB and a number of genes under its control that are involved in the inflammatory and cellular stress response. Persistent stimulation can lead to the chronic inflammatory state and associated pathologies.
The development of widely effective agents to treat type 2 diabetes has been difficult because of heterogeneity in the underlying causes of the disease. Advances in genomic, proteomic and metabolomic sciences over the last decade, however, have led to the development of “targeted” diagnostics and therapeutics. These leverage knowledge of an individual’s genetic makeup to create a more personalized approach to healthcare. Genomic testing enables identification of an individual’s susceptibility to disease, predict how a given patient will respond to a particular drug, and match patients with the right therapeutics. This new science of personalized medicine has the potential to improve the design of clinical trials, eliminate unnecessary treatments, reduce the incidence of adverse reactions to drugs, increase the efficacy of treatments and through identifying the right drug for the right patient, ultimately improves health outcomes.
Current Treatments for Type 2 Diabetes
There are several pharmaceutical approaches to treating type 2 diabetes. These include drugs designed to increase insulin production by the pancreas, reduce glucose production by the liver, and drugs, referred to as insulin sensitizers, designed to increase the body’s sensitivity to insulin, thereby improving glucose disposal from the bloodstream. Metformin is usually the first intervention prescribed by physicians when an individual is diagnosed with type 2 diabetes. Frequently clinicians will combine drugs that have different mechanistic approaches to controlling the diabetes in an effort to achieve appropriate glucose control.
8
Table of Contents
TRIOLEX to Treat Chronic Inflammation in Type 2 Diabetes
TRIOLEX is a next-generation compound that we are developing for the treatment of individuals diagnosed with certain chronic inflammatory processes.
In the setting of type 2 diabetes, evidence suggests that the mechanism of action for TRIOLEX may be through the regulation of the NF-kappaB pathway, particularly when it’s stimulated through the TLR4 receptor. TRIOLEX may be the first in a new class of insulin sensitizers to target obesity-mediated dysregulated metabolism. This is a major component of the type 2 diabetes syndrome that is characterized by the presence of a chronic inflammatory state. Through regulation of the NF-kappaB pathway, our scientists believe potential mechanisms of action for TRIOLEX involve control of genes whose products are involved in the inflammatory signaling pathway including TNF-alpha and IL-6. These cytokines are also thought to be critically involved in the pathogenesis of certain autoimmune diseases such as ulcerative colitis and rheumatoid arthritis, and are also implicated in the pathogenesis of metabolic diseases such as non-alcoholic steatohepatitis, cardiovascular disorders, cancer and in general, diseases associated with advancing age.
Based on biochemical experiments, we believe TRIOLEX action on the NF-kappaB pathway is independent of the PPAR-gamma pathway targeted by other insulin sensitizers. Instead, the action of TRIOLEX is associated with a downregulation of pro-inflammatory JNK, IKK and p38 kinase pathways. Chronic activation of these kinase pathways lead to impairment of the insulin receptor substrate-1 protein (IRS-1) function, an important cellular mediator of insulin signaling.
A single-dose Phase I clinical trial conducted during 2007 demonstrated that TRIOLEX is orally bioavailable in humans, with significant drug concentrations detected in the blood at the lowest dose tested. The findings also showed that all doses of TRIOLEX tested appear to be safe and well tolerated in healthy volunteers with no reported drug related serious adverse side effects to date.
A Phase I/II double-blind, placebo-controlled, multi-dose ranging clinical trial with TRIOLEX in obese insulin-resistant subjects was initiated in 2007 and evaluated the safety, tolerance and pharmacokinetics of TRIOLEX when administered for 28 days to obese adult subjects. The potential activity of TRIOLEX to decrease insulin resistance was assessed. In addition, an open-label cohort of six patients with type 2 diabetes mellitus was studied. TRIOLEX was found to be safe and increased insulin sensitivity in insulin resistant subjects.
During 2008, a Phase IIa clinical trial was initiated with TRIOLEX in type 2 diabetes patients that proceeded in two stages. Stage 1 of this double-blinded placebo controlled 12-week dosing trial was exploratory in nature and enrolled 96 patients who were on a stable dose of metformin with hemoglobin A1c (HbA1c) level in excess of 7.5 percent. The primary objectives of this trial were to evaluate the change in HbA1c from baseline to week 12 and to evaluate the safety and tolerance of TRIOLEX given 10 mg per day (5 mg BID) as compared to placebo. A final analysis of activity (HbA1c) in the clinical study of unaudited data was performed on all subjects that completed dosing on day 84 of the study (72 patients). There was no statistical difference between treatment and placebo for HbA1c in the overall patient population.
A retrospective analysis of unaudited data was performed on the subpopulation of patients that represent the inflamed, obese, insulin-resistant, diabetic population. This group is reflective of the impaired glucose tolerance subjects that responded to treatment in the company’s Phase I study. This analysis included patients who met the following criteria at baseline: BMI greater than or equal to 28; fasting plasma insulin levels greater than or equal to 4 µU/mL; fasting plasma C-peptide levels greater than or equal to 2 ng/mL; and serum monocyte chemotactic protein-1 (MCP-1) levels greater than or equal to 400 pg/mL. This phenotype represented 35% of all subjects (89 patients) with values for these parameters at baseline. Twenty-two individuals with this phenotype completed the 84 days of dosing. Those treated with TRIOLEX (10 patients) were found to show improvements in clinical parameters compared to placebo patients (12 patients). These included statistical trends for a decrease in HbA1c (-0.53%, p = 0.06) and a decrease in fasting plasma glucose (-28.75 mg/dL, p = 0.09), as well as non-significant
9
Table of Contents
decreases in fasting plasma C-peptide (-0.43 ng/mL), fasting plasma insulin (–0.48 µU/mL), fructosamine (–25.75 µmol/L), HOMA2 insulin resistance (–0.65 IR), and increases in HOMA2 insulin sensitivity (11.3 %S) and HOMA2 beta cell function (17.95 %B). The observed changes in these secondary indicators of activity are all consistent with the observed decreases in HbA1c and glucose in this diabetic subpopulation.
Sensitivity analysis using last observation carried forward indicated that individuals in this subpopulation who completed at least 29 days of dosing showed improvement. A biostatistician who is an expert in analyzing data from type 2 diabetes clinical trials independently confirmed these results.
Stage 2 of the Phase IIa clinical trial was designed to be confirmatory of the HbA1c activity and is presently in progress. Overall Triolex has a good safety profile with no consistent pattern of adverse events associated with its use. The side effects associated with the use of currently approved thiazolidenedione insulin sensitizers, have not been observed with TRIOLEX.
Competition in Diabetes
Given the large market opportunities for products that treat the indications for which we are currently developing our compounds, most major pharmaceutical companies and many biotechnology companies have programs directed toward finding drugs to treat the indications that we are exploring and the competition in these markets is intense. In metabolism and type 2 diabetes, there are a number of drugs, such as Actos® from Takeda Pharmaceuticals and Avandia® from GlaxoSmithKline (already approved for improving insulin sensitivity), glucagon-like peptide-1 (such as Victoza by Novo Nordisk), dipeptidyl peptidase-4 inhibitors (such as Januvia by Merck and Onglyza by Bristol Myers Squibb) and numerous other drugs in various stages of development. While Actos® and Avandia® currently account for a significant share of the market for insulin sensitizers to treat type 2 diabetes, they are known to cause the unwanted side effects of weight gain and edema. In addition, both have been given black box warnings by the FDA because of increased risk of treatment-related heart failure.
Autoimmune Disease and Chronic Inflammation
Current Treatments for Autoimmune Diseases
Immune modulators that correct immune dysregulation and chronic inflammatory conditions by inhibition or enhancement of single cytokine targets such as TNF alpha and IL-1 beta or their receptors have been developed by a number of companies. For example, Amgen’s Enbrel® targets TNF-alpha, as does Johnson & Johnson’s Remicade®. Other immune-modulating drugs such as Celebrex from Pfizer target COX-2. While these targeted approaches have shown clinical benefit and have generated significant sales volumes, redundancy in the immune system can limit their effectiveness. In addition, side effects, health care costs and reimbursement issues may limit their long-term global utility. In contrast, we believe our compounds may affect cytokine cascades through direct interactions in the endocrine system. This may make them more attractive drug candidates than those currently available, assuming they are successfully developed and commercialized.
Rheumatoid Arthritis
Rheumatoid arthritis is a type of chronic arthritis that occurs in joints on both sides of the body (such as hands, wrists or knees). In rheumatoid arthritis, the immune system attacks the joints and sometimes other organs. According to the Centers for Disease Control and Prevention, or (CDCP), an estimated 46 million people were treated for some form of arthritis and other rheumatic conditions in 2003, the latest year for which data is available, and an estimated 8 million more people will suffer from arthritis between 2005 and 2015.
Based upon the published studies in rodent models of collagen-induced and collagen antibody-induced arthritis where TRIOLEX demonstrated activity, a Phase I clinical trial was initiated with TRIOLEX in 2008 in rheumatoid arthritis patients. A 28-day oral dose ranging study assessed the safety, pharmacokinetics and
10
Table of Contents
potential for drug-drug interactions in stable rheumatoid arthritis patients also receiving methotrexate. TRIOLEX was found to be safe and well tolerated. No drug-drug interaction was found. TRIOLEX is now positioned to enter clinical studies in patients with active rheumatoid arthritis.
Ulcerative Colitis
Inflammatory bowel disease is comprised of ulcerative colitis, a chronic inflammation of the large intestine, or colon, and Crohn’s disease, a condition of inflammation of the small intestines. Ulcerative colitis and Crohn’s disease together affect approximately 500,000 to 2 million people in the United States.
Based upon published observations with TRIOLEX in preclinical models widely used by the pharmaceutical industry and academia to test agents as potential treatments for ulcerative colitis, in 2008, we commenced a Phase I/II clinical trial with TRIOLEX in ulcerative colitis patients. This Phase I/II dose ranging study evaluated the safety, tolerance, pharmacokinetics and activity of TRIOLEX when administered orally for 28 days to patients with active, mild-to-moderate ulcerative colitis. TRIOLEX at the doses studied was found to be safe and well tolerated but offered no indication of a treatment advantage in this acute inflammatory setting when compared to placebo. Triolex is staged for long-term clinical trials directed towards the control of the chronic inflammatory processes associated with this disease, a clinical setting believed to be consistent with the pharmacological properties of the compound.
Pulmonary Diseases and other Autoimmune Diseases
The Company is also interested in exploring the potential for TRIOLEX and other new compounds from our technology platform in a variety of pulmonary diseases including, cystic fibrosis, chronic pulmonary disease and asthma. In addition TRIOLEX has shown utility in pre-clinical models of multiple sclerosis and lupus erythematosus.
Triolex Development Status
TRIOLEX is manufactured economically using a multi-step organic synthesis from the widely abundant and inexpensive starting material, DHEA. It is formulated for oral administration with commonly used excipients in approved (oral dosage) products. Long term toxicology studies have been completed that qualify TRIOLEX for use in clinical studies of 6 months duration or longer. Diseases associated with chronic inflammation are thought to require drug exposures of extended duration to observe definitive treatment effects. Patent applications have been filed for the composition, pharmaceutical formulations and methods of use to treat a variety of inflammatory diseases including type 2 diabetes and autoimmune conditions such as rheumatoid arthritis and ulcerative colitis.
Government Regulation
General
The manufacturing and marketing of our proposed drug candidates and our research and development activities are, and will continue to be, subject to regulation by federal, state and local governmental authorities in the U.S. and other countries. In the U.S., pharmaceuticals are subject to rigorous regulation by the Food and Drug Administration, (“FDA”), which reviews and approves the marketing of drugs. The Federal Food, Drug and Cosmetic Act, the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the testing, manufacturing, labeling, storage, record keeping, advertising and promotion of our potential products.
11
Table of Contents
Approval Process
The process of obtaining FDA approval for a new drug may take many years and generally involves the expenditure of substantial resources. The steps required before a new drug can be produced and marketed for human use include clinical trials and the approval of a New Drug Application.
Preclinical Testing. In the preclinical phase of development, the promising compound is subjected to extensive laboratory and animal testing to determine if the compound is biologically active and safe.
Investigational New Drug, or IND. Before human tests can start, the drug sponsor must file an IND application with the FDA, showing how the drug is made and the results of animal testing. An IND becomes effective 30 days following receipt by the FDA.
Human Clinical Testing. The human clinical testing program usually involves three phases which generally are conducted sequentially, but which, particularly in the case of anti-cancer and other life-saving drugs, may overlap or be combined. Clinical trials are conducted in accordance with protocols that detail the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Each protocol is submitted to the FDA as part of the IND filing. Each clinical study is conducted under the auspices of an independent Institutional Review Board, or IRB, for each institution at which the study will be conducted. The IRB will consider, among other things, the design of the clinical trial, ethical factors, the risk to human subjects and the potential benefits of therapy relative to risk.
In Phase I clinical trials, studies usually are conducted on healthy volunteers or, in the case of certain terminal illnesses such as AIDS or cancer, patients with disease that has failed to respond to other treatment, to determine the maximum tolerated dose, side effects and pharmacokinetics of a product. Phase II studies are conducted on a small number of patients having a specific disease to determine initial efficacy in humans for that specific disease, the most effective doses and schedules of administration, and possible adverse effects and safety risks. Phase II/III differs from Phase II in that the trials involved may include more patients and, at the sole discretion of the FDA, be considered the “pivotal” trials, or trials that will form the basis for FDA approval. Phase III normally involves the pivotal trials of a drug, consisting of wide-scale studies on patients with the same disease, in order to evaluate the overall benefits and risks of the drug for the treated disease compared with other available therapies. The FDA continually reviews the clinical trial plans and results and may suggest design changes or may discontinue the trials at any time if significant safety or other issues arise.
New Drug Application, or NDA. Upon successful completion of Phase III clinical trials, the drug sponsor files an NDA with the FDA for approval containing all information that has been gathered. The NDA must include the chemical composition of the drug, manufacturing and production details, nonclinical data, results of human tests, and proposed labeling.
The testing and approval process is likely to require substantial time, from several months to years, and there can be no assurance that any FDA approval will be granted on a timely basis, if at all. The approval process is affected by a number of factors, primarily the side effects of the drug (safety) and its therapeutic benefits (efficacy). Additional preclinical or clinical trials may be required during the FDA review period and may delay marketing approval. The FDA may also deny an NDA if applicable regulatory criteria are not met.
Outside the U.S., we will be subject to foreign regulatory requirements governing human clinical trials and marketing approval for our products. The “requirements” governing human clinical trials ex-US usually follow ICH cGCP or country-specific cGCPs which are based on the ICH cGCPs. Regulatory approval outside the U.S. typically includes the risks and costs associated with obtaining FDA approval but may also include additional risks and costs.
Post Approval. If the FDA approves an NDA, the drug sponsor is required to submit reports periodically to the FDA containing adverse reactions, production, quality control and distribution records. The FDA may also
12
Table of Contents
require post-marketing testing to support the conclusion of efficacy and safety of the product, which can involve significant expense. After FDA approval is obtained for initial indications, further clinical trials may be necessary to gain approval for the use of the product for additional indications.
Manufacturing
We do not have, and do not intend to establish, manufacturing facilities to produce our drug candidates or any future products. We plan to control our capital expenditures by using contract manufacturers to make our products. We believe that there are a sufficient number of high-quality FDA-approved contract manufacturers available, and we have had discussions, and in some cases established relationships, to fulfill our near-term production needs for both clinical and commercial use.
The manufacture of our drug candidates or any future products, whether done by outside contractors as planned or internally, will be subject to rigorous regulations, including the need to comply with the FDA’s current Good Manufacturing Practice regulations. As part of obtaining FDA approval for each product, each of the manufacturing facilities must be inspected, approved by and registered with the FDA. In addition to obtaining FDA approval of the prospective manufacturer’s quality control and manufacturing procedures, domestic and foreign manufacturing facilities are subject to periodic inspection by the FDA and/or foreign regulatory authorities.
Patents
We currently own or have obtained licenses to a number of U.S. and foreign patents and patent applications. We consider the protection of our technology, whether owned or licensed, to the exclusion of use by others, to be vital to our business. While we intend to focus primarily on patented or patentable technology, we may also rely on trade secrets, unpatented property, know-how, regulatory exclusivity, patent extensions and continuing technological innovation to develop our competitive position. In the U.S. and certain foreign countries, the exclusivity period provided by patents covering pharmaceutical products may be extended by a portion of the time required to obtain regulatory approval for a product.
In certain countries, some pharmaceutical-related technology such as disease treatment methods are not patentable or only recently have become patentable, and enforcement of intellectual property rights in many countries has been limited or non-existent. Future enforcement of patents and proprietary rights in many countries can be expected to be problematic or unpredictable. We cannot guarantee that any patents issued or licensed to us will provide us with competitive advantages or will not be challenged by others. Furthermore, we cannot be certain that others will not independently develop similar products or will not design around patents issued or licensed to us.
In most cases, patent applications in the U.S. are maintained in secrecy until 18 months after the earliest filing or priority date. Publication of discoveries in the scientific or patent literature, if made, tends to lag behind actual discoveries by at least several months. U.S. patent applicants can elect to prevent publication until the application issues by agreeing not to file the application outside the U.S. This option is rarely used for pharmaceutical patent applications. Consequently, we cannot be certain that a patent owner or licensor of its intellectual property was the first to invent the technology or compounds covered by pending patent applications or issued patents or that it was the first to file patent applications for such inventions. In addition, the patent positions of biotechnology and pharmaceutical companies, including our own, are generally uncertain, partly because they involve complex legal and factual questions.
In addition to the considerations discussed above, companies that obtain patents claiming products, uses or processes that are necessary for, or useful to, the development of our drug candidates or future products could bring legal actions against us claiming infringement. Patent litigation is typically costly and time consuming, and if such an action were brought against us, it could result in significant cost and diversion of our attention. We
13
Table of Contents
may be required to obtain licenses to other patents or proprietary rights, and we cannot guarantee that licenses would be made available on terms acceptable to us. If we do not obtain such licenses, we could encounter delays in product market introductions while we attempt to develop or license technology designed around such patents, or we could find that the development, manufacture or sale of products requiring such licenses is foreclosed.
Further, we cannot guarantee that patents that are issued will not be challenged, invalidated or infringed upon or designed around by others, or that the claims contained in such patents will not interfere with the patent claims of others, or provide us with significant protection against competitive products, or otherwise be commercially valuable. To the extent that we are unable to obtain patent protection for our products or technology, our business may be materially adversely affected by competitors who develop substantially equivalent technology.
For a discussion of the risks associated with patent and proprietary rights, see below under Item 1A “Risk Factors”.
Technology Agreements
In August 2002, we entered into a Sublicense Agreement with Pharmadigm, Inc. (currently known as Inflabloc Pharmaceuticals, Inc.). Under the agreement, we obtained exclusive worldwide rights to certain intellectual property of Pharmadigm and the University of Utah and we agreed to make aggregate payments of $0.9 million in cash or in shares of our common stock, at our option, over the next year. We elected to make such payments in equity and have issued a total of 168,921 shares of our common stock in complete satisfaction of this requirement. We will also make additional milestone and royalty payments to Pharmadigm if we meet specified development and commercialization milestones for products covered by the patents. No such milestones have been met to date. The principal patents licensed under the agreement, originally licensed to Pharmadigm from the University of Utah, relate to inventions by Dr. Raymond Daynes and Dr. Barbara A. Areneo. Dr. Daynes served as a scientific consultant for us from 1999 to mid-2003.
In October 2000, we acquired a 21% equity stake in Aeson Therapeutics Inc. (“Aeson”) and an exclusive worldwide sublicense to three issued patents in the area of adrenal steroids in exchange for $2.0 million in cash and 208,672 shares of our common stock valued at $2 million. As part of the transaction, Aeson and its stockholders granted us an exclusive option to acquire the remainder of Aeson at a predetermined price. In March 2002, we amended certain aspects of our agreements with Aeson. Under the amendments, we paid Aeson $1.2 million, which extended the initial date by which we could exercise our option to acquire the remainder of Aeson to September 30, 2002. We also received additional equity securities of Aeson as a result of this payment. We elected not to exercise the option to acquire the remainder of Aeson by September 30, 2002. On June 7, 2006, we acquired substantially all of the assets of Aeson. As consideration for Aeson’s assets, we agreed (i) to issue a total of 35,000 shares of our common stock to Aeson at the closing of the acquisition and (ii) to issue to Aeson’s stockholders up to a total of 165,000 additional shares of our common stock if certain development milestones are achieved. We have not achieved any of the development milestones to date.
Employees
As of March 27, 2010, we had 19 full-time equivalent, non-union employees. We believe that our relations with our employees are good.
14
Table of Contents
Executive Officers and Senior Management
Our executive officers and senior management and their ages as of March 27, 2010 are as follows:
| Name |
Age | Position | ||
| James M. Frincke, Ph.D. |
59 | Chief Executive Officer | ||
| Christopher L. Reading, Ph.D. |
62 | Chief Scientific Officer | ||
| Dwight R. Stickney, M.D. |
67 | Chief Medical Officer | ||
| Robert W. Weber |
59 | Chief Financial Officer and Secretary |
James M. Frincke, Ph.D. joined Harbor BioSciences, Inc., as Vice President, Research and Development in November 1997, was promoted to Executive Vice President in March 1999, to Chief Scientific Officer in December 2001, to Chief Operating Officer in February 2008 and to Chief Executive Officer in 2009. Dr. Frincke joined Harbor BioSciences, Inc. from Prolinx, Inc., where he served as Vice President, Therapeutics Research and Development from 1995 to 1997. During his 29 years in the biotechnology industry, Dr. Frincke has managed major development programs including drugs, biologicals, and cellular and gene therapy products aimed at the treatment of cancer, infectious diseases, organ transplantation, autoimmune disease and type 2 diabetes. Since joining the biotechnology industry, Dr. Frincke has held vice president, research and development positions in top tier biotechnology companies including Hybritech/Eli Lilly and SyStemix Inc. (acquired by Novartis). In various capacities, he has been responsible for all aspects of pharmaceutical development including early stage research programs, product evaluation, pharmacology, manufacturing, and the management of regulatory and clinical matters for lead product opportunities. Dr. Frincke has authored or co-authored more than 100 scientific articles, abstracts and regulatory filings. Dr. Frincke received his B.S. in Chemistry and his Ph.D. in Chemistry from the University of California, Davis. Dr. Frincke performed his postdoctoral work at the University of California, San Diego.
Christopher L. Reading, Ph.D. became Vice President of Scientific Development in January 1999, was promoted to Executive Vice President, Scientific Development in March 2002 and to Chief Scientific Officer in February 2008. Before Harbor BioSciences, Inc., Dr. Reading was Vice President of Product and Process Development at Novartis Inc.-owned SyStemix Inc. During this time, he successfully filed three investigational new drug applications (INDs) in the areas of stem cell therapy technology and stem cell gene therapy for HIV/AIDS. Prior to joining SyStemix, Dr. Reading served on the faculty of the M.D. Anderson Cancer Center in Houston for nearly 13 years. His positions there included Associate and Assistant Professor of Medicine in the Departments of Hematology and Tumor Biology. During his career, Dr. Reading has given more than 25 national and international scientific presentations, published more than 100 peer-reviewed journal articles and 15 invited journal articles as well as written nearly 20 book chapters, and received numerous grants and contracts which supported his research activities. Dr. Reading has served on the National Science Foundation Advisory Committee for Small Business Innovative Research Grants (SBIR) as well as on the editorial boards of Journal of Biological Response Modifiers and Molecular Biotherapy. He holds a number of patents for his work with monoclonal antibodies and devices. Dr. Reading received his Ph.D. in Biochemistry at the University of California at Berkeley and completed postdoctoral study in tumor biology at The University of California at Irvine. He earned his B.A. in cell biology at the University of California at San Diego.
Dwight R. Stickney, M.D. joined Harbor BioSciences, Inc., as Medical Director, Oncology in May 2000, was appointed Vice President, Medical Affairs in March 2003 and was promoted to Chief Medical Officer in February 2008. Dr. Stickney joined Harbor BioSciences, Inc. from the Radiation Oncology Division of Radiological Associates of Sacramento Medical Group, Inc., in Sacramento, California, where he served as a Radiation Oncologist since 1993. While at Radiological Associates, he served as Chairman of the Radiation Oncology Division from 1997 to 1999 and was a member of the Radiation Study section of the National Institute of Health’s Division of Research Grants from 1993 to 1997. He also served as the Director of Radiation Research for Scripps Clinic and Research Foundation in La Jolla, California. Dr. Stickney has taught in medical academia as Associate Professor of Radiation Medicine at Loma Linda University School of Medicine and has served as
15
Table of Contents
Director of the International Order of Forresters Cancer Research Laboratory and on the Board of Directors of the California Division of the American Cancer Society. Earlier in his career, Dr. Stickney held positions with Burroughs Wellcome and the Centers for Disease Control, and academic teaching appointments at The University of California at Los Angeles and The University of California at Riverside. He has also served as a consultant for a number of biotechnology companies on the design and conduct of clinical trials. Dr. Stickney has authored or co-authored over 80 scientific articles, abstracts and book chapters. He is named inventor on numerous issued patents and patent applications. Dr. Stickney holds a Bachelor of Science in Microbiology, a Masters of Science in Immunology, and a M.D. from Ohio State University. In addition, he is certified as a Diplomat of the American Board of Internal Medicine and Hematology and a Diplomat of the American Board of Radiology, Therapeutic Radiology.
Robert W. Weber joined Harbor BioSciences, Inc., in March 1996 and currently serves as the Chief Financial Officer and Secretary. Mr. Weber has over thirty years of experience in financial management. He has been employed at executive levels by multiple start-up companies and contributed to the success of several turnaround situations. He previously served as Vice President of Finance at Prometheus Products, a subsidiary of Sierra Semiconductor (now PMC Sierra), from 1994 to 1996, and Vice President Finance and Chief Financial Officer for Amercom, a personal computer telecommunications software publishing company, from 1993 to 1994. From February 1988 to August 1993, Mr. Weber served as Vice President Finance and Chief Financial Officer of Instromedix, a company that develops and markets medical devices and software. Mr. Weber brings a broad and expert knowledge of many aspects of financial management. In various capacities, he has been responsible for all aspects of finance and accounting including cost accounting, cash management, SEC filings, treasury, investor relations, private and venture financing, corporate legal matters, acquisitions/divestitures as well as information technology, human resources and facilities. Mr. Weber received a B.S. from GMI Institute of Technology (now Kettering University) and a MBA from the Stanford Graduate School of Business.
| Item 1A. | Risk Factors |
In evaluating our business, you should consider the following discussion of risks, in addition to other information contained in this Annual Report on Form 10-K, as well as our other public filings with the Securities and Exchange Commission. Any of the following risks could materially adversely affect our business, financial condition, results of operations and prospects and, as a result, the market price of our common stock could decline, and you may lose all or part of the money you paid to buy our common stock.
We are still a development stage company.
We have never had any revenues from sales of products. None of our drug candidates has been approved for commercial sale and we do not expect that any of our present or future drug candidates will be commercially available for a number of years, if at all. We have incurred losses since our inception and we expect to continue to incur significant additional operating losses for the foreseeable future as we fund clinical trials and other expenses in support of regulatory approval of our drug candidates.
If we do not obtain government regulatory approval for our products, we cannot sell our products and we will not generate revenues.
Our principal development efforts are currently centered around a proprietary class of small compounds that we believe shows promise for the treatment of several diseases and disorders. However, all drug candidates require approval by the U.S. Food and Drug Administration (“FDA”) before they can be commercialized in the United States as well as approval by various foreign government agencies before they can be commercialized in other countries. These regulations change from time to time and new regulations may be adopted. While limited clinical trials of our drug candidates have been conducted to date, significant additional trials are required, and we may not be able to demonstrate that our drug candidates are safe or effective. In addition, success in early development does not mean that later development will be successful because, for example, drug candidates in
16
Table of Contents
later-stage clinical trials may fail to demonstrate sufficient safety and efficacy despite having progressed through initial clinical testing. Our clinical experience with our drug candidates is limited, and to date our drug candidates have been tested in less than the number of patients that will likely need to be studied to gain regulatory approval. The data collected from clinical trials with larger patient populations may not demonstrate sufficient safety and efficacy to support regulatory approval of these drug candidates. In addition, we do not know whether early results from any of our ongoing clinical trials will be predictive of final results of any such trial. If we are unable to demonstrate the safety and effectiveness of a particular drug candidate to the satisfaction of regulatory authorities, the drug candidate will not obtain required government approval and we will experience potentially significant delays in, or be required to abandon, development of the drug candidate. If we do not receive FDA or foreign approvals for our drug candidates, we will not be able to sell products and will not generate revenues. If we receive regulatory approval of one of our drug candidates, such approval may impose limitations on the indicated uses for which we may market the resulting product, which may limit our ability to generate significant revenues. Further, U.S. or foreign regulatory agencies could change existing, or promulgate new, regulations at any time, which may affect our ability to obtain approval of our drug candidates or require significant additional costs to obtain such approvals. In addition, if regulatory authorities determine that we or a partner conducting research and development activities on our behalf have not complied with regulations in the research and development of one of our drug candidates, then they may not approve the drug candidate and we will not be able to market and sell it. If we were unable to market and sell our drug candidates, our business and results of operations would be materially and adversely affected.
Recent publicity concerning the safety of certain drug products has resulted in heightened scrutiny by the FDA in the process of approving new drugs, which could delay or limit any regulatory approvals we may obtain for our drug candidates.
In light of widely publicized events concerning the safety risk of certain drug products, the FDA, members of Congress, the Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and establishment of risk management programs that may, for instance, restrict distribution of drug products after approval. In addition, the Food and Drug Administration Amendments Act of 2007, or FDAAA, grants significant expanded authority to the FDA, much of which is aimed at improving the safety of drug products before and after approval. In particular, the new law authorizes the FDA to, among other things, require post-approval studies and clinical trials, mandate changes to drug labeling to reflect new safety information and require risk evaluation and mitigation strategies for certain drugs, including certain currently approved drugs. It also significantly expands the federal government’s clinical trial registry and results databank, which we expect will result in significantly increased government oversight of clinical trials. Under the FDAAA, companies that violate these and other provisions of the new law are subject to substantial civil monetary penalties, among other regulatory, civil and criminal penalties. The increased attention to drug safety issues may result in a more cautious approach by the FDA in its review of data from our clinical trials. Data from clinical trials may receive greater scrutiny, particularly with respect to safety, which may make the FDA or other regulatory authorities more likely to require additional preclinical studies or clinical trials by drug development companies. As a result, the FDA may require us to conduct additional preclinical studies or clinical trials during the clinical development of one or more of our drug candidates as a condition precedent to approval which could potentially delay our development plans, limit the indications for which our drug candidates are ultimately approved, and otherwise adversely impact us.
If we do not successfully commercialize our products, we may never achieve profitability.
We have experienced significant operating losses to date because of the substantial expenses we have incurred to acquire and fund development of our drug candidates. We have never had significant operating revenues and have never commercially introduced a product. Our accumulated deficit was approximately $251.8 million as of December 31, 2009. Our net losses for fiscal years 2009, 2008 and 2007 were approximately $15.6 million, $21.6 million and $23.1 million, respectively. Many of our research and development programs are at an
17
Table of Contents
early stage. Potential drug candidates are subject to inherent risks of failure. These risks include the possibilities that no drug candidate will be found safe or effective, meet applicable regulatory standards or receive the necessary regulatory clearances. Even if we were ultimately to receive regulatory approval for one or more of our drug candidates, we may be unable to commercialize them successfully for a variety of reasons. These include, for example, the availability of alternative treatments, lack of cost effectiveness, the cost of manufacturing the product on a commercial scale, the effect of competition with other drugs, or because we may have inadequate financial or other resources to pursue one or more of our drug candidates through commercialization. If we are unable to develop safe, commercially viable drugs, we may never achieve profitability. If we become profitable, we may not remain profitable.
As a result of our intensely competitive industry, we may not gain enough market share to be profitable.
The biotechnology and pharmaceutical industries are intensely competitive. We have numerous competitors in the U.S. and elsewhere. Because we are pursuing potentially large markets, our competitors include major multinational pharmaceutical companies, specialized biotechnology firms as well as academic institutions, government agencies and private and public research institutions. Several of these entities have already successfully marketed and commercialized products that will compete with our drug candidates, assuming that our drug candidates gain regulatory approval. A large number of companies including Merck & Company, Inc., GlaxoSmithKline, Takeda Pharmaceuticals, Amylin Pharmaceuticals, Inc., AstraZeneca, Novartis, Novo Nordisk, Pfizer Inc., Sanofi-Aventis and Eli Lilly and Co. are developing and marketing new drugs for the treatment of type 2 diabetes. Similarly, a large number of companies, including Merck & Company, Inc., Pfizer Inc., Johnson & Johnson Inc. and Amgen Inc., are developing and marketing new drugs for the treatment of chronic inflammatory conditions. In addition, there are also a number of other companies with drug candidates in development targeting late-stage prostate cancer, including compounds already in Phase 3 clinical trials. One or more such compounds may be approved before any of our drug candidates could potentially be approved. Many, if not all, of these competing drug development programs are being conducted by pharmaceutical and biotechnology companies with considerably greater financial resources, human resources and experience than ours.
Many of these competitors have greater financial and other resources, larger research and development staffs and more effective marketing and manufacturing organizations than we do. In addition, academic and government institutions have become increasingly aware of the commercial value of their research findings. These institutions are now more likely to enter into exclusive licensing agreements with commercial enterprises, including our competitors, to develop and market commercial products.
Our competitors may succeed in developing or licensing technologies and drugs that are more effective or less costly than any we are developing. Our competitors may succeed in obtaining FDA or other regulatory approvals for drug candidates before we do. If competing drug candidates prove to be more effective or less costly or better-marketed than our drug candidates, our drug candidates, even if approved for sale, may not be able to compete successfully with our competitors’ existing products or new products under development. Similarly, we cannot predict whether any of our drug candidates, if approved, will have sufficient advantages to cause healthcare professionals to adopt our products over competing products. If we are unable to compete successfully, we may never be able to sell enough products at a price sufficient to permit us to generate profits.
We need to raise additional money before we achieve profitability; if we fail to raise additional money, it could be difficult or impossible to continue our business.
As of December 31, 2009, our cash and cash equivalents totaled approximately $9.7 million. Based on our current plans, we believe these financial resources, and interest earned thereon, will be sufficient to meet our operating expenses and capital requirements into late 2010. However, changes in our research and development plans or other events affecting our operating expenses may result in the expenditure of such cash before that time. We will require substantial additional funds in order to finance our drug discovery and development
18
Table of Contents
programs, fund operating expenses, pursue regulatory clearances, develop manufacturing, marketing and sales capabilities, and prosecute and defend our intellectual property rights. We may seek additional funding through public or private financing or through collaborative arrangements with strategic partners.
You should be aware that in the future:
| • | we may not obtain additional financial resources when necessary or on terms favorable to us, if at all; and |
| • | any available additional financing may not be adequate. |
If we cannot raise additional funds when needed, or on acceptable terms, we will not be able to continue to develop our drug candidates.
We may need to liquidate the Company in a voluntary dissolution under Delaware law or seek protection under the provisions of the U.S. Bankruptcy Code, and in either event, it is unlikely that stockholders would receive any value for their shares.
We have not generated any revenues from product sales, and have incurred losses in each year since our inception in 1994. We expect that it will be very difficult to raise capital to continue our operations and our independent registered public accounting firm has issued an opinion with an explanatory paragraph to the effect that there is substantial doubt about our ability to continue as a going concern. We do not believe that we could succeed in raising additional capital needed to sustain our operations without some strategic transaction, such as a partnership or merger. If we are unable to consummate such a transaction, we expect that we would need to cease all operations and wind down. Although we are currently evaluating our strategic alternatives with respect to all aspects of our business, we cannot assure you that any actions that we take would raise or generate sufficient capital to fully address the uncertainties of our financial position. As a result, we may be unable to realize value from our assets and discharge our liabilities in the normal course of business. If we are unable to settle our obligations to our creditors or if we are unable to consummate a strategic transaction, we would likely need to liquidate the Company in a voluntary dissolution under Delaware law or seek protection under the provisions of the U.S. Bankruptcy Code. In that event, we, or a trustee appointed by the court, may be required to liquidate our assets. In either of these events, we might realize significantly less from our assets than the values at which they are carried on our financial statements. The funds resulting from the liquidation of our assets would be used first to satisfy obligations to creditors before any funds would be available to pay our stockholders, and any shortfall in the proceeds would directly reduce the amounts available for distribution, if any, to our creditors and to our stockholders. In the event we are required to liquidate under Delaware law or the federal bankruptcy laws, it is highly unlikely that stockholders would receive any value for their shares. See “Liquidity and Capital Resources” in Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Note 1 to our financial statements included elsewhere in this Annual Report on Form 10-K.
Our results of operations and liquidity needs could be materially negatively affected by market fluctuations and economic downturn.
Our results of operations could be materially negatively affected by economic conditions generally, both in the U.S. and elsewhere around the world. Continuing concerns over inflation, energy costs, geopolitical issues, the availability and cost of credit, the U.S. mortgage market and a declining residential real estate market in the U.S. have contributed to increased volatility and diminished expectations for the economy and the markets going forward. These factors, combined with volatile oil prices, declining business and consumer confidence and increased unemployment, precipitated an economic recession from which the global economy is in stages of recovery. Domestic and international equity markets continue to experience heightened volatility and turmoil. These events and the continuing market upheavals may have an adverse effect on us. In the event of a market downturn, our results of operations could be adversely affected by those factors in many ways, including making it more difficult for us to raise funds if necessary, and our stock price may further decline.
19
Table of Contents
Failure to protect our proprietary technology could impair our competitive position.
We own or have obtained a license to a number of U.S. and foreign patents and patent applications. Our success depends in part on our ability to obtain and defend patent rights and other intellectual property rights that are important to our ability to commercialize our drug candidates, if approved and our ability to operate our business without infringing the proprietary rights of third parties. We place considerable importance on obtaining patent protection for significant new technologies, products and processes. Legal standards relating to the validity of patents covering pharmaceutical and biotechnology inventions and the scope of claims made under such patents are still developing. In some of the countries in which we intend to market our drug candidates, if approved, pharmaceuticals are either not patentable or have only recently become patentable. Past enforcement of intellectual property rights in many of these countries has been limited or non-existent. Future enforcement of patents and proprietary rights in many other countries may be problematic or unpredictable. Moreover, the issuance of a patent in one country does not assure the issuance of a similar patent in another country. Claim interpretation and infringement laws vary by nation, so the extent of any patent protection is uncertain and may vary in different jurisdictions. Our domestic patent position is also highly uncertain and involves complex legal and factual questions. The applicant or inventors of subject matter covered by patent applications or patents owned by or licensed to us may not have been the first to invent or the first to file patent applications for such inventions. Due to uncertainties regarding patent law and the circumstances surrounding our patent applications, the pending or future patent applications we own or have licensed may not result in the issuance of any patents. Existing or future patents owned by or licensed to us may be challenged, infringed upon, invalidated, found to be unenforceable or circumvented by others. Further, any rights we may have under any issued patents may not provide us with sufficient protection against similar competitive products or technologies that do not infringe our patents or otherwise cover commercially valuable products or processes.
Litigation or other disputes regarding patents and other proprietary rights may be expensive, cause delays in bringing products to market and harm our ability to operate.
The manufacture, use or sale of our drug candidates may infringe on the patent rights of others. If we are unable to avoid infringement of the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming and can preclude, delay or suspend commercialization of our drug candidates. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, or fail to successfully defend an infringement action or have the patents we are alleged to infringe declared invalid, we may:
| • | incur substantial money damages; |
| • | encounter significant delays in bringing our drug candidates to market; |
| • | be precluded from participating in the manufacture, use or sale of our drug candidates or methods of treatment without first obtaining licenses to do so; and/or |
| • | not be able to obtain any required license on favorable terms, if at all. |
In addition, if another party claims the same subject matter or subject matter overlapping with the subject matter that we have claimed in a U.S. patent application or patent, we may decide or be required to participate in interference proceedings in the U.S. Patent and Trademark Office in order to determine the priority of invention. Loss of such an interference proceeding would deprive us of patent protection sought or previously obtained and could prevent us from commercializing our products. Participation in such proceedings could result in substantial costs, whether or not the eventual outcome is favorable. These additional costs could adversely affect our financial results.
20
Table of Contents
Litigation may be expensive and time consuming and may adversely affect our operations.
From time to time, we may be involved in litigation relating to claims arising out of our operations in the normal course of business. Participation in such proceedings is time consuming and could result in substantial costs, whether or not the eventual outcome is favorable. These additional costs could adversely affect our financial results.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we also rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Existing and/or future pricing regulations and reimbursement limitations may limit our potential profits from the sale of our products.
The requirements governing product licensing, pricing and reimbursement vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after product-licensing approval is granted. As a result, we may obtain regulatory approval for a drug candidate in a particular country, but then be subject to price regulations that reduce our profits from the sale of the product. In some foreign markets pricing of prescription pharmaceuticals is subject to continuing government control even after initial marketing approval. In addition, certain governments may grant third parties a license to manufacture our product without our permission. Such compulsory licenses may be on terms that are less favorable to us and would likely have the effect of reducing our revenues.
Varying price regulation between countries can lead to inconsistent prices and some re-selling by third parties of products from markets where products are sold at lower prices to markets where those products are sold at higher prices. Any practice of exploiting price differences between countries could undermine our sales in markets with higher prices and reduce the sales of our future products, if any.
While we do not have any applications for regulatory approval of our drug candidates currently pending, any decline in the size of the markets in which we may in the future sell commercial products, assuming our receipt of the requisite regulatory approvals, could cause the perceived market value of our business and the price of our common stock to decline.
Our ability to commercialize our drug candidates successfully also will depend in part on the extent to which reimbursement for the cost of our drug candidates and related treatments will be available from government health administration authorities, private health insurers and other organizations. Third-party payers are increasingly challenging the prices charged for medical products and services. If we succeed in bringing any of our drug candidates to the market, such drug candidates may not be considered cost effective and reimbursement may not be available or sufficient to allow us to sell such drug candidates on a profitable or competitive basis.
The United States Congress is considering various proposals for fundamental reform of the health care and health insurance systems, including proposals championed by President Obama. Although it is premature to assess the exact effect on us of whatever proposals are to be adopted, it is unknown what the overall effect of such legislation would be on us.
21
Table of Contents
Delays in the conduct or completion of our preclinical or clinical studies or the analysis of the data from our preclinical or clinical studies may result in delays in our planned filings for regulatory approvals, or adversely affect our ability to enter into collaborative arrangements.
The current status of our drug candidates is set forth below. We have either completed or are in the midst of:
| • | Phase I clinical trial with TRIOLEX, in the United States under an IND, for the treatment of metabolic disorders; |
| • | Phase I/II clinical trial with TRIOLEX, in the United States under an IND for the treatment of metabolic disorders; |
| • | Phase I clinical trial with TRIOLEX, in the United States under an IND, for the treatment of metabolic disorders; |
| • | Phase II clinical trial with TRIOLEX, in the United States in type 2 diabetes patients under an IND for the treatment of metabolic disorders; |
| • | Phase I/II clinical trial with TRIOLEX, in the United States under an IND for the treatment of gastrointestinal inflammatory conditions; |
| • | Phase I clinical trial with TRIOLEX, in the United States in rheumatoid arthritis patients under an IND for the treatment of inflammatory conditions; and |
| • | Phase I/IIa clinical trial with APOPTONE, in the United States in late-stage prostate cancer patients who have failed hormone therapy and at least one round of chemotherapy treatment or have not received chemotherapy under an IND for the treatment of hormone-sensitive cancers including prostate cancer. |
We may encounter problems with some or all of our completed or ongoing studies that may cause us or regulatory authorities to delay or suspend our ongoing studies or delay the analysis of data from our completed or ongoing studies. We rely, in part, on third parties to assist us in managing and monitoring our preclinical and clinical studies. We generally do not have control over the amount and timing of resources that our business partners devote to our drug candidates. Our reliance on these third parties may result in delays in completing or failure to complete studies if third parties fail to perform their obligations to us. If the results of our ongoing and planned studies for our drug candidates are not available when we expect or if we encounter any delay in the analysis of the results of studies of our drug candidates:
| • | we may not have the financial resources to continue research and development of any of our drug candidates; |
| • | we may not be able to enter into collaborative arrangements relating to any drug candidate subject to delay in regulatory filing; |
| • | we may lose any competitive advantage associated with early market entry; and |
| • | our ability to generate revenues may be delayed. |
Any of the following reasons, among others, could delay or suspend the completion of our ongoing and future studies:
| • | delays in enrolling volunteers; |
| • | interruptions in the manufacturing of our drug candidates or other delays in the delivery of materials required for the conduct of our studies; |
| • | lower than anticipated retention rate of volunteers in a clinical trial; |
| • | unfavorable efficacy results; |
| • | serious side effects experienced by study participants relating to the drug candidate; |
22
Table of Contents
| • | reaching agreement on acceptable terms with prospective contract research organizations and clinical trial sites; |
| • | failure to conduct a clinical trial in accordance with regulatory requirements or clinical protocols; |
| • | inspection of a clinical trial operations or clinical trial site by regulatory authorities resulting in the imposition of a clinical hold; |
| • | new communications from regulatory agencies about how to conduct these studies; or |
| • | failure to raise additional funds resulting in lack of adequate funding to continue a clinical trial or study. |
If the manufacturers of our drug candidates do not comply with current Good Manufacturing Practices regulations, or cannot produce sufficient quantities of our drug candidates to enable us to continue our development, we will fall behind on our business objectives.
Manufacturers producing our drug candidates must follow current Good Manufacturing Practices regulations enforced by the FDA and foreign equivalents. If a manufacturer of our drug candidates does not conform to current Good Manufacturing Practices regulations and cannot be brought up to such a standard, we will be required to find alternative manufacturers that do conform. This may be a long and difficult process, and may delay our ability to receive FDA or foreign regulatory approval of our drug candidates.
We also rely on our manufacturers to supply us with a sufficient quantity of our drug candidates to conduct clinical trials. If we have difficulty in the future with obtaining our required quantity and quality of supply, we could experience significant delays in our development programs and regulatory process.
Our ability to achieve any significant revenue may depend on our ability to establish effective sales and marketing capabilities.
Our efforts to date have focused on the development and evaluation of our drug candidates. As we continue preclinical and clinical studies and seek to commercialize our drug candidates, we may need to build a sales and marketing infrastructure. As a company, we have no experience in the sales and marketing of pharmaceutical products. If we fail to establish a sufficient marketing and sales force or to make alternative arrangements to have our drug candidates marketed and sold by others on attractive terms, it will impair our ability to commercialize our drug candidates and to enter new or existing markets. Our inability to effectively enter these markets would materially and adversely affect our ability to generate significant revenues.
If we were to lose the services of members of our management team, or fail to attract or retain qualified personnel in the future, our business objectives would be more difficult to implement, adversely affecting our operations.
Our ability to successfully implement our business strategy depends upon the continued services of our management team. If we lose the services of one or more of these individuals, replacement could be difficult and may take an extended period of time and could impede significantly the achievement of our business objectives.
We may face product liability claims related to the use or misuse of our drug candidates, which may cause us to incur significant losses.
We are currently exposed to the risk of product liability claims due to administration of our drug candidates in clinical trials, since the use or misuse of our drug candidates during a clinical trial could potentially result in injury or death. If we are able to commercialize our products, we will also be subject to the risk of losses in the future due to product liability claims in the event that the use or misuse of our commercial products results in injury or death. We currently maintain liability insurance on a claims-made basis. Because we cannot predict the magnitude or the number of claims that may be brought against us in the future, we do not know whether the
23
Table of Contents
insurance policies’ coverage limits are adequate. The insurance is expensive, difficult to obtain and may not be available in the future on acceptable terms, or at all. Any claims against us, regardless of their merit, could substantially increase our costs and cause us to incur significant losses.
Our securities could be subject to extreme price fluctuations that could adversely affect your investment.
The market prices for securities of life sciences companies, particularly those that are not profitable, are highly volatile. Publicized events and announcements, most of which we cannot control, may have a significant impact on the market price of our common stock, which has been, and is likely to continue to be, volatile. For example:
| • | biological or medical discoveries by competitors; |
| • | public concern about the safety of our drug candidates; |
| • | delays in the conduct or analysis of our preclinical or clinical studies; |
| • | unfavorable results from preclinical or clinical studies; |
| • | delays in obtaining or failure to obtain purchase orders of our drug candidates; |
| • | announcements in the scientific and research community; |
| • | changes in the potential commercial markets for our drug candidates; |
| • | unfavorable developments concerning patents or other proprietary rights; |
| • | unfavorable domestic or foreign regulatory or governmental developments or actions; |
| • | broader economic, industry and market trends unrelated to our performance; |
| • | issuances of new equity securities by us, pursuant to our effective shelf registration statement or otherwise; |
| • | discussion of us or our stock price by the financial and scientific press and in online investor communities; or |
| • | additions or departures of key personnel |
may have the effect of temporarily or permanently driving down the price of our common stock. In addition, the stock market from time to time experiences extreme price and volume fluctuations which particularly affect the market prices for emerging and life sciences companies, such as ours, and which are often unrelated to the operating performance of the affected companies. For example, our stock price has ranged from $0.29 to $0.85 between January 1, 2009 and March 27, 2010.
These broad market fluctuations may adversely affect the ability of a stockholder to dispose of his shares at a price equal to or above the price at which the shares were purchased. In addition, in the past, following periods of volatility in the market price of a company’s securities, securities class-action litigation has often been instituted against that company. Any litigation against our company, including this type of litigation, could result in substantial costs and a diversion of management’s attention and resources, which could materially adversely affect our business, financial condition and results of operations.
We may be delisted from The Nasdaq Exchange, which could materially limit the trading market for our common stock.
We received a letter from The Nasdaq Stock Market on September 15, 2009. The letter stated that we were not in compliance with Nasdaq Marketplace Rule 4450(a)(5) because our common stock had closed below $1.00 per share for 30 consecutive business days. The letter also stated that in accordance with Nasdaq Marketplace Rules, we had 180 days, or until March 15, 2010, to regain compliance with the minimum bid price rule.
24
Table of Contents
On March 16, 2010, we received notice from The Nasdaq Stock Market that our application had been approved to transfer the listing of our common stock to The Nasdaq Capital Market from The Nasdaq Global Market. The transfer was effective at the opening of the market on March 18, 2010. Our common stock continues to trade under the symbol “HRBR.”
The Nasdaq Capital Market is a continuous trading market that operates in substantially the same manner as the Nasdaq Global Market. All companies listed on the Nasdaq Capital Market must meet certain financial requirements and adhere to Nasdaq's corporate governance standards. We elected to transfer the listing of our common stock to the Nasdaq Capital Market because we no longer met the minimum bid price rule of The Nasdaq Global Market.
We believe we are in compliance with all applicable criteria for continued listing on The Nasdaq Capital Market, but for the $1.00 per share minimum bid price requirement set forth in Listing Rule 5550(a)(2). We will continue to monitor the bid price of our common stock and will consider available options if its common stock does not trade at a price level likely to result in our gaining compliance with Listing Rule 5550(a)(2) prior to the September 13, 2010 grace period deadline.
We will regain compliance with the minimum bid price rule of The Nasdaq Capital Market if the bid price of our common stock closes at $1.00 per share or more for a minimum of 10 consecutive business days before September 13, 2010. However, if we do not regain compliance with the minimum bid price rule by September 13, 2010, the Nasdaq staff will provide us with a written notification that our common stock will be delisted. At that time, we may appeal this determination to a Hearings Panel (the “Panel”). If we appeal, we will be asked to provide a plan to regain compliance to the Panel. We have been informed by Nasdaq that Panels have historically generally viewed a near-term reverse stock split as the only definitive plan acceptable to resolve a bid price deficiency.
Because stock ownership is concentrated, you and other investors will have minimal influence on stockholders’ decisions.
Richard B. Hollis, our former Chief Executive Officer and a former member of our board of directors, owned approximately 7.8% of our outstanding common stock as of March 27, 2010. As a result, Mr. Hollis may be able to significantly influence all matters requiring stockholder approval, including the election of directors. Such concentration of ownership may also have the effect of delaying or preventing a change in control of our company.
Substantial sales of our stock may impact the market price of our common stock.
Future sales of substantial amounts of our common stock, including shares that we may issue upon exercise of options and warrants or conversion of convertible securities, could adversely affect the market price of our common stock. Further, if we raise additional funds through the issuance of common stock or securities convertible into or exercisable for common stock, the percentage ownership of our stockholders will be reduced and the price of our common stock may fall.
Issuing preferred stock with rights senior to those of our common stock could adversely affect holders of common stock.
Our charter documents give our board of directors the authority to issue shares of preferred stock without a vote or action by our stockholders. The board also has the authority to determine the terms of preferred stock, including price, preferences and voting rights. The rights granted to holders of preferred stock may adversely affect the rights of holders of our common stock. For example, a series of preferred stock may be granted the right to receive a liquidation preference—a pre-set distribution in the event of a liquidation—that would reduce
25
Table of Contents
the amount available for distribution to holders of common stock. In addition, the issuance of preferred stock could make it more difficult for a third party to acquire a majority of our outstanding voting stock. As a result, common stockholders could be prevented from participating in transactions that would offer an optimal price for their shares.
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
Our corporate headquarters are currently located at 4435 Eastgate Mall, Suite 400, San Diego, CA 92121, where we have leased approximately 7,000 square feet of office space through December 2010. We believe that our facilities are adequate for our current operations.
| Item 3. | Legal Proceedings |
From time to time, we may be involved in litigation relating to claims arising out of our operations in the normal course of business. While it is impossible to predict accurately or to determine the eventual outcome of these matters, as of the date of this report, we do not believe that we are engaged in any legal proceedings that are expected, individually or in the aggregate, to have a material adverse effect on our business, financial condition or operating results.
| Item 4. | Removed and Reserved. |
26
Table of Contents
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock is traded on the Nasdaq Capital Market under the symbol HRBR.
The following table sets forth the quarterly high and low sales prices for our common stock from January 1, 2008 through March 27, 2010.
| 2008 |
||||||
| First Quarter |
$ | 2.09 | $ | 1.50 | ||
| Second Quarter |
2.39 | 1.40 | ||||
| Third Quarter |
2.00 | 0.90 | ||||
| Fourth Quarter |
1.37 | 0.28 | ||||
| 2009 |
||||||
| First Quarter |
$ | 0.85 | $ | 0.37 | ||
| Second Quarter |
0.76 | 0.29 | ||||
| Third Quarter |
0.70 | 0.43 | ||||
| Fourth Quarter |
0.67 | 0.43 | ||||
| 2010 |
||||||
| January 1 – March 26 |
$ | 0.75 | $ | 0.45 |
27
Table of Contents
Performance Measurement Comparison(1)
The following graph compares changes through December 31, 2009, in the cumulative total return on our common stock, a broad market index, namely the Nasdaq Composite Index (the “Nasdaq Index”), and an industry index, namely the Nasdaq Biotechnology Index (the “Industry Index”). The Industry Index comprises all companies listed on the Nasdaq Stock Market under SIC 283. All values assume reinvestment of the full amount of all dividends as of December 31 of each year.
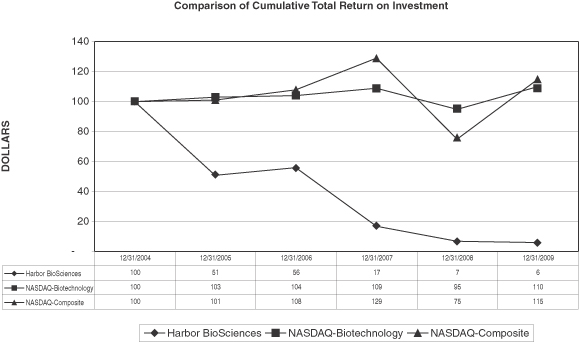
| (1) | “The material in this section is not “soliciting material” is not deemed “filed” with the SEC, and is not to be incorporated by reference into any filing of the Company under the 1933 or 1934 Act.” |
On March 19, 2010, the closing price of our common stock as reported by the Nasdaq Capital Market was $0.59 share. There were approximately 9,000 shareholders of record and beneficial stockholders of our common stock as of such date. We have not paid cash dividends on our common stock and do not intend to do so in the foreseeable future.
There were no unregistered sales of equity securities in 2009.
We made no repurchases of our securities during the year ended December 31, 2009.
28
Table of Contents
| Item 6. | Selected Financial Data |
The following data summarizes certain selected financial data for each of the five years ended December 31, 2009 through 2005 and the period from inception (August 15, 1994) to December 31, 2009. The information presented should be read in conjunction with the financial statements and related notes included elsewhere in this report (in thousands, except per share amounts).
| 2009 | 2008 | 2007 | 2006 | 2005 | Period from Inception (Aug. 15, 1994) to December 31, 2009 |
|||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||
| Contract revenues |
$ | — | $ | — | $ | 645 | $ | 444 | $ | 56 | $ | 1,208 | ||||||||||||
| Research and development |
10,555 | 16,070 | 18,319 | 23,764 | 18,342 | (1) | 171,254 | |||||||||||||||||
| General and administrative |
5,140 | 6,537 | 8,150 | 9,644 | 9,777 | (1) | 87,917 | |||||||||||||||||
| Settlement of Dispute |
— | — | — | — | 3,000 | 3,000 | ||||||||||||||||||
| Total operating expenses |
15,695 | 22,607 | 26,469 | 33,408 | 31,119 | 262,171 | ||||||||||||||||||
| Interest income (expense) |
138 | 1,048 | 2,781 | 2,741 | 1,622 | 17,363 | ||||||||||||||||||
| Other income (expense) |
(69 | ) | (6 | ) | (78 | ) | (8 | ) | — | (8,232 | ) | |||||||||||||
| Net loss |
$ | (15,626 | ) | $ | (21,565 | ) | $ | (23,121 | ) | $ | (30,231 | ) | $ | (29,441 | ) | $ | (251,832 | ) | ||||||
| Net loss per share, basic and diluted |
$ | (0.53 | ) | $ | (0.74 | ) | $ | (0.80 | ) | $ | (1.20 | ) | $ | (1.46 | ) | |||||||||
| Weighted average number of common Shares outstanding, basic and diluted |
29,319 | 29,060 | 28,955 | 25,131 | 20,125 | |||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash and equivalents |
$ | 9,738 | $ | 24,152 | $ | 43,215 | $ | 67,135 | $ | 45,130 | ||||||||||||||
| Total assets |
10,286 | 25,157 | 45,123 | 68,512 | 46,582 | |||||||||||||||||||
| Total current liabilities. |
1,286 | 1,952 | 3,018 | 6,734 | 7,708 | |||||||||||||||||||
| Stockholders’ equity |
$ | 9,000 | $ | 23,205 | $ | 42,105 | $ | 61,778 | $ | 38,874 | ||||||||||||||
| (1) | 2005 Research and development and general and administrative expenses do not include the expense for ASC 718, Share-Based Payment. Share-Based Payment (ASC 718), expense was not included in financial results for any of the previous years prior to 2005. (See—“ ASC 718, Share-Based Payments” in the Notes to Financial Statements). |
29
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion contains forward-looking statements that involve risks and uncertainties. See “Forward-Looking Statements” elsewhere in this Annual Report on Form 10-K. This discussion and analysis should be read in conjunction with the financial statements and notes included elsewhere in this Annual Report.
General
We are a development-stage pharmaceutical company engaged in the discovery and development of drug candidates for the treatment of diseases and disorders in which the body is unable to mount an appropriate immune or metabolic response due to disease or the process of aging. Our initial technology development efforts are primarily focused on a series of adrenal steroid hormones and hormone analogs that are derived from our Hormonal Signaling Technology Platform.
We have been unprofitable since our inception. As of December 31, 2009, we had an accumulated deficit of approximately $251.8 million. We expect to incur substantial additional operating losses and capital expenditures for the foreseeable future as we increase expenditures on research and development and begin to allocate significant and increasing resources to clinical testing and other activities in support of the development of our drug candidates. In addition, in the future, we may have to meet the substantial new challenge of developing the capability to market products if we are successful in obtaining regulatory approval for any of our current or future drug candidates. Accordingly, our activities to date are not as broad in depth or scope as the activities we may undertake in the future, and our historical operations and financial information are not indicative of the future operating results or financial condition or ability to operate profitably as a commercial enterprise when and if we succeed in bringing any drug candidates to market.
On March 26, 1997, Hollis-Eden, Inc., a Delaware corporation, was merged with and into us, then known as Initial Acquisition Corp. (IAC), a Delaware corporation. Upon consummation of the merger of Hollis-Eden, Inc. with IAC, Hollis-Eden, Inc. ceased to exist, and IAC changed its name to Hollis-Eden Pharmaceuticals, Inc.
Effective February 9, 2010, we changed our name from Hollis-Eden Pharmaceuticals, Inc. to Harbor BioSciences, Inc. The name change was effected pursuant to Section 253 of the General Corporation Law of the State of Delaware by the merger of our wholly owned subsidiary into us. We were the surviving corporation and, in connection with the merger, we amended our Amended and Restated Certificate of Incorporation to change our name to Harbor BioSciences, Inc.
Effective February 18, 2010, our common stock began trading under a new Nasdaq symbol, “HRBR” and CUSIP number “41150V 103”.
Results of Operations
We have devoted substantially all of our resources to the payment of research and development expenses and general and administrative expenses. From inception through December 31, 2009, we have incurred approximately $171.2 million in research and development expenses, $87.9 million in general and administrative expenses, and $3.0 million in a settlement of dispute. From inception, (August 15, 1994), through December 31, 2009 we have generated approximately $1.2 million in revenues (which resulted from providing research and development services under our Study Funding Agreement with Cystic Fibrosis Foundation Therapeutics, Inc., (CFFT). We have earned $9.1 million in other income. The other income and expense is comprised of $7.6 million in deemed discount expense, $0.4 million in interest expense and a $0.2 million loss on disposal of assets. These expenses have been offset by $17.3 million in interest income. The combination of these resulted in a net loss of $251.8 million for the period from inception, (August 15, 1994), until December 31, 2009.
30
Table of Contents
Research and development and general and administrative expenses include the expense for ASC 718 share-based payments for all fiscal years starting with 2006, (See —“ASC 718 Share-Based Payments” in the Notes to Financial Statements).
Research and development expenses were $10.6 million, $16.1 million and $18.3 million in 2009, 2008 and 2007, respectively. The research and development expenses relate primarily to the ongoing development, preclinical testing and clinical trials for our drug candidates. Research and development expenses decreased $5.5 million in 2009 compared to 2008 and $2.2 million in 2008 compared to 2007, due primarily to a decrease in general and preclinical research and development projects resulting from reduced personnel and a decline in stock option compensation expense. These decreases were partly offset by an increase in clinical trial expenditures.
General and administrative expenses were $5.1 million, $6.5 million and $8.1 million in 2009, 2008 and 2007, respectively. General and administrative expenses relate to salaries and benefits, facilities, patent fees, legal, accounting/auditing, investor relations, consultants, insurance and travel. General and administrative expenses decreased $1.4 million in 2009 compared to 2008 due mainly to a decrease in salaries expense resulting from reduced personnel, investor communications and stock option compensation expense, offset by an increase in legal fees and Directors and Officers insurance. General and administrative expenses decreased $1.6 million in 2008 compared to 2007 due primarily to a decrease in executive head count, bonuses, Directors and Officers insurance, and in accounting and consulting fees.
Other income and expenses were $0.07 million, $1.0 million and $2.7 million in 2009, 2008 and 2007, respectively. The $0.9 million and $1.7 million decrease in other income and expense for 2009 compared to 2008 and 2008 compared to 2007 was due mainly to lower interest rates and lower cash balances. Included in the 2009 loss was $0.07 million related to the closing of our laboratory.
Liquidity and Capital Resources
Our operations to date have consumed substantial capital without generating any revenues other than the amount received under our collaboration with Cystic Fibrosis Foundation Therapeutics, Inc. (CFFT). We have financed our operations since inception primarily through the sale of our equity securities raising a total of $205 million, net of expenses. In addition, we have received a total of $12 million from the exercise of warrants and stock options from inception. As of December 31, 2009, our cash and cash equivalents totaled approximately $9.7 million. During 2009, we significantly reduced staffing, terminated leases, consolidated office space and other measures to reduce our planned 2010 quarterly cash usage to the range of $2.0 to $2.5 million. Based upon our current plans, we believe that our existing capital resources, together with interest thereon, will be sufficient to meet our operating expenses and capital requirements into late 2010. However, changes in our research and development plans or other events affecting our operating expenses may result in the expenditure of such cash before that time.
Our future capital requirements will depend upon many factors, including progress with preclinical testing and clinical trials, the number and breadth of our programs, the time and costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims and other proprietary rights, the time and costs involved in obtaining regulatory approvals, competing technological and market developments, and our ability to establish collaborative arrangements, effective commercialization, marketing activities and other arrangements. We may incur increasing negative cash flows and net losses for the foreseeable future.
We expect that it will be very difficult to raise capital to continue our operations and our independent registered public accounting firm has issued an opinion with an explanatory paragraph to the effect that there is substantial doubt about our ability to continue as a going concern. We do not believe that we could succeed in raising additional capital needed to sustain our operations without some strategic transaction, such as a partnership or merger. If we are unable to consummate such a transaction, we expect that we would need to cease
31
Table of Contents
all operations and wind down. Although we are currently evaluating our strategic alternatives with respect to all aspects of our business, we cannot assure you that any actions that we take would raise or generate sufficient capital to fully address the uncertainties of our financial position. As a result, we may be unable to realize value from our assets and discharge our liabilities in the normal course of business. If we are unable to settle our obligations to our creditors or if we are unable to consummate a strategic transaction, we would likely need to liquidate the Company in a voluntary dissolution under Delaware law or seek protection under the provisions of the U.S. Bankruptcy Code. In that event, we, or a trustee appointed by the court, may be required to liquidate our assets. In either of these events, we might realize significantly less from our assets than the values at which they are carried on our financial statements. The funds resulting from the liquidation of our assets would be used first to satisfy obligations to creditors before any funds would be available to pay our stockholders, and any shortfall in the proceeds would directly reduce the amounts available for distribution, if any, to our creditors and to our stockholders. In the event we are required to liquidate under Delaware law or the federal bankruptcy laws, it is highly unlikely that stockholders would receive any value for their shares.
Off-Balance Sheet Arrangements
Harbor BioSciences, Inc. currently does not have any off-balance sheet arrangements.
Contractual Obligations:
As of December 31, 2009, we had the following contractual obligations
| Payments Due by Period | |||||||||||||||
| Contractual Obligations |
Total | Less than one year |
One to three years |
Three to five years |
More than Five years | ||||||||||
| Operating Leases |
$ | 205 | $ | 202 | $ | 3 | $ | — | $ | — | |||||
We may also be required to make substantial milestone or royalty payments in cash based on the terms of some of our agreements (See Note 6 to the Financial Statements).
Our operations to date have consumed substantial capital without generating any revenues other than the amount received under our collaboration with Cystic Fibrosis Foundation Therapeutics, Inc., (CFFT). We will continue to require substantial and increasing amounts of funds to conduct necessary research and development and preclinical and clinical testing of our drug candidates, and to market any drug candidates that receive regulatory approval. We do not expect to generate revenue from operations for the foreseeable future, and our ability to meet our cash obligations as they become due and payable may depend for at least the next several years on our ability to sell securities, borrow funds or some combination thereof. Based upon our current plans, we believe that our existing capital resources, together with interest thereon, will be sufficient to meet our operating expenses and capital requirements into late 2010. However, changes in our research and development plans or other events affecting our operating expenses may result in the expenditure of such cash before that time. We may not be successful in raising necessary funds. As of December 31, 2009, our cash and cash equivalents totaled approximately $9.7 million.
Our future capital requirements will depend upon many factors, including progress with preclinical testing and clinical trials, the number and breadth of our programs, the time and costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims and other proprietary rights, the time and costs involved in obtaining regulatory approvals, competing technological and market developments, and our ability to establish collaborative arrangements, effective commercialization, marketing activities and other arrangements. We may incur increasing negative cash flows and net losses for the foreseeable future. We are seeking additional funding through public or private financing or through collaborative arrangements with strategic partners. Our auditor has stated in the opinion that there is substantial doubt about the Company’s ability to continue as a going concern.
32
Table of Contents
Critical Accounting Policies
Certain of our accounting policies require the application of judgment and estimates by management, which may be affected by different assumptions and conditions. These estimates are typically based on historical experience, terms of existing contracts, trends in the industry and information available from other outside sources, as appropriate. We believe the estimates and judgments associated with our reported amounts are appropriate in the circumstances. Actual results could materially vary from those estimates under different assumptions or conditions.
All research and development costs are expensed as incurred. The value of acquired in-process research and development is charged to expense on the date of acquisition. Research and development expenses include, but are not limited to, acquired in-process technology deemed to have no alternative future use, license fees related to license agreements, preclinical and clinical trial studies, payroll and personnel expense, and lab supplies, consulting and research-related overhead. Research and development expenses paid in the form of cash and our stock to related parties aggregated $11.5 million for the period from inception (August 15, 1994) to December 31, 2003 (see Note 6, “Colthurst, Edenland and Mr. Prendergest” and “Aeson Therapeutics”). No such related party expenses were incurred in 2009, 2008 or 2007.
As of January 1, 2006, we account for share-based payments in accordance with ASC 718. Under the fair value recognition provisions of this statement, share-based payments cost is measured at the grant date based on the value of the award and is recognized as expense over the vesting period. Determining the fair value of share-based awards at the grant date requires judgment, including estimating our stock price volatility and employee stock option exercise behavior. If actual results differ significantly from these estimates, stock-based compensation expense and our results of operations could be materially impacted.
Our expected volatility is based upon the historical volatility of our stock. Our expected life for our options is based on historical stock option activity. Because share-based payments expense is recognized in our statement of operations based on awards ultimately expected to vest, the amount of expense has been reduced for estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience. If factors change and we employ different assumptions in the application of ASC 718, the compensation expense that we record in future periods may differ significantly from what we have recorded in the current period.
On July 13, 2006, ASC 740-10, Accounting for Uncertainty in Income Taxes, which is effective for fiscal years beginning after December 15, 2006, which establishes recognition and measurement thresholds that must be met before a tax benefit can be recognized in the financial statements. The Company has adopted ASC 740-10 on January 1, 2007, and it has had no material impact on its financial statements.
Impact of Recently Issued Accounting Pronouncements
Accounting for Collaborative Arrangements Related to the Development and Commercialization of Intellectual Property, ASC 808-10, concluded that a collaborative arrangement is one in which the participants are actively involved and are exposed to significant risks and rewards that depend on the ultimate commercial success of the endeavor. Revenues and costs incurred with third parties in connection with collaborative arrangements would be presented gross or net based on the criteria in ASC 605-45, “Reporting Revenue Gross as a Principal versus Net as an Agent,” and other accounting literature. Payments to or from collaborators would be evaluated and presented based on the nature of the arrangement and its terms, the nature of the entity’s business and whether those payments are within the scope of other accounting literature. The nature and purpose of collaborative arrangements are to be disclosed along with the accounting policies and the classification and amounts of significant financial statement amounts related to the arrangements. Activities in the arrangement conducted in a separate legal entity should be accounted for under other accounting literature; however, required
33
Table of Contents
disclosure under ASC 808-10 applies to the entire collaborative agreement. ASC 808-10 is effective for fiscal years beginning after December 15, 2008, and is to be applied retrospectively to all periods presented for all collaborative arrangements existing as of the effective date. The adoption of this standard did not have a material impact on our financial statements.
In December 2007, the FASB issued ASC 805, Business Combinations, requires an acquirer to recognize the assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions. ASC 805 also requires the acquirer in a business combination achieved in stages to recognize the identifiable assets and liabilities, as well as the non-controlling interest in the acquiree, at the full amounts of their fair values. ASC 805 makes various other amendments to authoritative literature intended to provide additional guidance or to confirm the guidance in that literature to that provided in this Statement. ASC 805 applies to business combinations for which the acquisition date is in fiscal years beginning after December 15, 2008. The adoption of this standard did not have a material impact on our financial statements.
Effective July 1, 2009, the Company adopted a replacement of FASB Statement No. 162, The “FASB Accounting Standards Codification” and the Hierarchy of Generally Accepted Accounting Principles (ASC Topic 105). This standard establishes only two levels of U.S. generally accepted accounting principles (“GAAP”), authoritative and nonauthoritative. The FASB Accounting Standards Codification (the “Codification”) became the source of authoritative, nongovernmental GAAP, except for rules and interpretive releases of the SEC, which are sources of authoritative GAAP for SEC registrants. All other non-grandfathered, non-SEC accounting literature not included in the Codification became nonauthoritative. The Company began using the new guidelines and numbering system prescribed by the Codification when referring to GAAP in the third quarter of fiscal 2009. As the Codification was not intended to change or alter existing GAAP, it did not have any impact on our financial statements.
Effective April 1, 2009, the Company adopted three accounting standard updates that were intended to provide additional application guidance and enhanced disclosures regarding fair value measurements and impairments of securities. They also provide additional guidelines for estimating fair value in accordance with fair value accounting. The first update, as codified in ASC 820-10-65, provides additional guidelines for estimating fair value in accordance with fair value accounting. The second accounting update, as codified in ASC 820-10-65, changes accounting requirements for other-than-temporary-impairment (OTTI) for debt securities by replacing the current requirement that a holder have the positive intent and ability to hold an impaired security to recovery in order to conclude an impairment was temporary with a requirement that an entity conclude it does not intend to sell an impaired security and it will not be required to sell the security before the recovery of its amortized cost basis. The third accounting update, as codified in ASC 825-10-65, increases the frequency of fair value disclosures. These updates were effective for fiscal years and interim periods ended after June 15, 2009. The adoption of these accounting updates did not have any impact on our financial statements.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC 820-10 establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). These tiers include:
| • | Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; |
| • | Level 2, defined as inputs, other than quoted prices in active markets that are directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are no active; and |
| • | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant value drivers are observable. |
34
Table of Contents
Our level 1 assets primarily include our cash and cash equivalents (mainly money market accounts). Valuations are obtained from readily available pricing sources. We do not currently have Level 2 or 3 assets.
We do not have any debt instruments as of December 31, 2009 and 2008
Effective April 1, 2009, the Company adopted a new accounting standard for subsequent events, as codified in ASC 855-10. The update modifies the names of the two types of subsequent events either as recognized subsequent events (previously referred to in practice as Type I subsequent events) or non-recognized subsequent events (previously referred to in practice as Type II subsequent events). In addition, the standard modifies the definition of subsequent events to refer to events or transactions that occur after the balance sheet date, but before the financial statements are issued (for public entities) or available to be issued (for nonpublic entities). The update did not result in significant changes in the practice of subsequent event disclosures, and therefore the adoption did not have any impact on our financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
At December 31, 2009, our investment portfolio included only cash and money market accounts and did not contain fixed-income securities, with the exception of a small amount held in a restricted CD. There would be no material impact to our investment portfolio, in the short term, associated with any change in interest rates, and any decline in interest rates over time will reduce our interest income, while increases in interest rates over time will increase our interest income.
35
Table of Contents
| Item 8. | Financial Statements and Supplementary Data |
| Page | ||
| 37 | ||
38 | ||
39 | ||
44 | ||
| 45 | ||
| 74 | ||
36
Table of Contents
(A Development Stage Company)
Balance Sheets
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (In thousands, except par value) |
||||||||
| ASSETS: |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 9,738 | $ | 24,152 | ||||
| Prepaid expenses |
209 | 262 | ||||||
| Deposits |
48 | 7 | ||||||
| Other receivables |
81 | — | ||||||
| Total current assets |
10,076 | 24,421 | ||||||
| Property and equipment, net of accumulated depreciation of $906 and $1,496 |
176 | 641 | ||||||
| Deposits |
— | 61 | ||||||
| Restricted cash |
34 | 34 | ||||||
| Total assets |
$ | 10,286 | $ | 25,157 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY: |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 136 | $ | 323 | ||||
| Accrued expenses |
1,150 | 1,629 | ||||||
| Total current liabilities |
1,286 | 1,952 | ||||||
| Commitments and contingencies (Notes 6, 11, 12) |
||||||||
| Stockholders’ equity: (Notes 3, 7, 8, 9, 10) |
||||||||
| Preferred stock, $.01, 10,000 shares authorized; no shares issued or outstanding |
— | — | ||||||
| Common stock, $.01 par value, 50,000 shares authorized; 29,493 and 29,228 shares issued and 29,434 and 29,169 outstanding respectively |
294 | 292 | ||||||
| Paid-in capital |
260,884 | 259,465 | ||||||
| Cost of treasury stock (59 shares) |
(346 | ) | (346 | ) | ||||
| Deficit accumulated during development stage |
(251,832 | ) | (236,206 | ) | ||||
| Total stockholders’ equity |
9,000 | 23,205 | ||||||
| Total liabilities and stockholders’ equity |
$ | 10,286 | $ | 25,157 | ||||
The accompanying notes are an integral part of these financial statements.
37
Table of Contents
(A Development Stage Company)
Statements of Operations
| For the year ended December 31, | Inception (Aug.15, 1994) to December 31, 2009 |
|||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||
| ( In thousands, except per share amounts) | ||||||||||||||||
| Revenue: |
||||||||||||||||
| Contract R&D revenue |
$ | — | $ | — | $ | 645 | $ | 1,208 | ||||||||
| Total revenue |
— | — | 645 | 1,208 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
||||||||||||||||
| R & D operating expenses |
9,923 | 15,092 | 17,074 | 161,145 | ||||||||||||
| R & D costs related to common stock and stock option grants for collaborations and technology purchases |
632 | 978 | 1,245 | 10,109 | ||||||||||||
| Total research and development |
10,555 | 16,070 | 18,319 | 171,254 | ||||||||||||
| General and administrative |
||||||||||||||||
| G & A operating expenses |
4,450 | 5,025 | 6,160 | 69,074 | ||||||||||||
| G & A costs related to options / warrants granted |
690 | 1,512 | 1,990 | 18,843 | ||||||||||||
| Total general and administrative |
5,140 | 6,537 | 8,150 | 87,917 | ||||||||||||
| Settlement of dispute |
— | — | — | 3,000 | ||||||||||||
| Total operating expenses |
15,695 | 22,607 | 26,469 | 262,171 | ||||||||||||
| Other income (expense): |
||||||||||||||||
| Loss on disposition of assets |
(69 | ) | (6 | ) | (78 | ) | (217 | ) | ||||||||
| Non-cash amortization of deemed discount and deferred issuance costs on convertible debentures |
— | — | — | (7,627 | ) | |||||||||||
| Interest income |
138 | 1,048 | 2,781 | 17,363 | ||||||||||||
| Interest expense |
— | — | — | (388 | ) | |||||||||||
| Total other income, net |
69 | 1,042 | 2,703 | 9,131 | ||||||||||||
| Net loss |
$ | (15,626 | ) | $ | (21,565 | ) | $ | (23,121 | ) | $ | (251,832 | ) | ||||
| Net loss per share, basic and diluted |
$ | (0.53 | ) | $ | (0.74 | ) | $ | (0.80 | ) | |||||||
| Weighted average number of common shares outstanding, basic and diluted |
29,319 | 29,060 | 28,955 | |||||||||||||
The accompanying notes are an integral part of these financial statements.
38
Table of Contents
Harbor BioSciences, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity
| Preferred stock at par value |
Common stock at par value |
Capital in excess of par value |
Cost of Repurchased Common Stock |
Deficit accumulated during development stage |
Total | ||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||
| ( In thousands ) | |||||||||||||||||||||||||
| Contribution by stockholder |
— | $ | — | — | $ | — | $ | 103 | — | — | $ | — | $ | 103 | |||||||||||
| Common stock issued for cash |
— | — | 2,853 | — | 25 | — | — | — | 25 | ||||||||||||||||
| Common stock issued as consideration for the license agreements (Note 6) |
— | — | 543 | — | 5 | — | — | — | 5 | ||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (1,277 | ) | (1,277 | ) | ||||||||||||||
| Balance at December 31, 1994 |
— | — | 3,396 | — | 133 | — | — | (1,277 | ) | (1,144 | ) | ||||||||||||||
| Common stock issued for cash |
— | — | 679 | — | 250 | — | — | — | 250 | ||||||||||||||||
| Common stock issued as consideration for amendments to the license agreements (Note 6) |
— | — | 76 | — | 28 | — | — | — | 28 | ||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (672 | ) | (672 | ) | ||||||||||||||
| Balance at December 31, 1995 |
— | — | 4,151 | — | 411 | — | — | (1,949 | ) | (1,538 | ) | ||||||||||||||
| Common stock issued in conversion of debt (Note 7) |
— | — | 165 | — | 371 | — | — | — | 371 | ||||||||||||||||
| Common stock issued for cash, net of expenses (Note 7) |
— | — | 580 | — | 1,234 | — | — | — | 1,234 | ||||||||||||||||
| Common stock issued as consideration for termination of a finance agreement |
— | — | 15 | — | 34 | — | — | — | 34 | ||||||||||||||||
| Warrants issued to consultants for services rendered |
— | — | — | — | 24 | — | — | — | 24 | ||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (692 | ) | (692 | ) | ||||||||||||||
| Balance at December 31, 1996 |
— | — | 4,911 | — | 2,074 | — | — | (2,641 | ) | (567 | ) | ||||||||||||||
| Recapitalization of Company upon the merger with Initial Acquisition Corp. (Note 3) |
— | — | 883 | 58 | 6,213 | — | — | — | 6,271 | ||||||||||||||||
| Warrants issued to a certain director upon the successful closure of the merger (Note 3) |
— | — | — | — | 570 | — | — | — | 570 | ||||||||||||||||
| Exercise of warrants, net of expenses |
— | — | 978 | 10 | 5,619 | — | — | — | 5,629 | ||||||||||||||||
| Amortization of deferred compensation |
— | — | — | — | 282 | — | — | — | 282 | ||||||||||||||||
| Exercise of stock options |
— | — | — | — | 1 | — | — | — | 1 | ||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (5,253 | ) | (5,253 | ) | ||||||||||||||
| Balance at December 31, 1997 |
— | — | 6,772 | 68 | 14,759 | — | — | (7,894 | ) | 6,933 | |||||||||||||||
| Exercise of warrants |
— | — | 399 | 4 | 1,196 | — | — | — | 1,200 | ||||||||||||||||
| Exercise of stock options |
— | — | 53 | 1 | 155 | — | — | — | 156 | ||||||||||||||||
39
Table of Contents
Harbor BioSciences, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity—(Continued)
| Preferred stock at par value |
Common stock at par value |
Capital in excess of par value |
Cost of Repurchased Common Stock |
Deficit accumulated during development stage |
Total | |||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||
| ( In thousands ) | ||||||||||||||||||||||
| Private Placement, net of expenses (Note 7) |
4 | — | 1,329 | 13 | 19,877 | — | — | — | 19,890 | |||||||||||||
| Warrants issued for services in lieu of cash (Note 10) |
— | — | — | — | 408 | — | — | — | 408 | |||||||||||||
| Stock issued for license fee (Note 6) |
— | — | 33 | — | 500 | — | — | — | 500 | |||||||||||||
| Stock issued for services in lieu of cash |
— | — | 6 | — | 95 | — | — | — | 95 | |||||||||||||
| Options issued for services in lieu of cash (Note 9) |
— | — | — | — | 240 | — | — | — | 240 | |||||||||||||
| Amortization of deferred compensation |
— | — | — | — | 308 | — | — | — | 308 | |||||||||||||
| Net loss |
— | — | — | — | — | — | — | (5,427 | ) | (5,427 | ) | |||||||||||
| Balance at December 31, 1998 |
4 | — | 8,592 | 86 | 37,538 | — | — | (13,321 | ) | 24,303 | ||||||||||||
| Exercise of warrants |
— | — | 755 | 8 | 5,136 | — | — | — | 5,144 | |||||||||||||
| Exercise of stock options |
— | — | 10 | — | 75 | — | — | — | 75 | |||||||||||||
| Private Placement, net of expenses (Note 7) |
— | — | 1,368 | 14 | 24,759 | — | — | — | 24,773 | |||||||||||||
| Preferred Stock Conversion (Note 7,8) |
(4 | ) | — | 346 | 3 | (3 | ) | — | — | — | — | |||||||||||
| Deferred compensation-Options forfeited (Note 9) |
— | — | — | — | 51 | — | — | — | 51 | |||||||||||||
| Amortization of non-employee options |
— | — | — | — | 559 | — | — | — | 559 | |||||||||||||
| Warrants issued for services in lieu of cash (Note 10) |
— | — | — | — | 2,140 | — | — | — | 2,140 | |||||||||||||
| Options accelerated vesting (Note 9) |
— | — | — | — | 4,900 | — | — | — | 4,900 | |||||||||||||
| Net loss |
— | — | — | — | — | — | — | (15,320 | ) | (15,320 | ) | |||||||||||
| Balance at December 31, 1999 |
— | — | 11,071 | 111 | 75,155 | — | — | (28,641 | ) | 46,625 | ||||||||||||
| Exercise of warrants |
— | — | 133 | 2 | 758 | — | — | — | 760 | |||||||||||||
| Exercise of stock options |
— | — | 1 | — | 5 | — | — | — | 5 | |||||||||||||
| Common Stock issued for 401(k)/401(m) plan |
— | — | 6 | — | 63 | — | — | — | 63 | |||||||||||||
| Common Stock issued for In-Process R&D (Note 6) |
— | — | 209 | 2 | 1,998 | — | — | — | 2,000 | |||||||||||||
| Options granted for license fee |
— | — | 38 | — | 598 | — | — | — | 598 | |||||||||||||
| Amortization of non-employee options |
— | — | — | — | 79 | — | — | — | 79 | |||||||||||||
| Common Stock issued for purchase of technology |
— | — | 132 | 1 | 1,847 | — | — | — | 1,848 | |||||||||||||
| Net loss |
— | — | — | — | — | — | — | (19,515 | ) | (19,515 | ) | |||||||||||
| Balance at December 31, 2000 |
— | — | 11,590 | 116 | 80,503 | — | — | (48,156 | ) | 32,463 | ||||||||||||
40
Table of Contents
Harbor BioSciences, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity—(Continued)
| Preferred stock at par value |
Common stock at par value |
Capital in excess of par value |
Cost of Repurchased Common Stock |
Deficit accumulated during development stage |
Total | |||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||
| ( In thousands ) | ||||||||||||||||||||||
| Exercise of stock options |
— | — | 10 | — | 22 | — | — | — | 22 | |||||||||||||
| Common Stock issued for 401(k)/401(m) plan |
— | — | 16 | — | 96 | — | — | — | 96 | |||||||||||||
| Private Placement, net of expenses (Note 7) |
— | — | 1,280 | 13 | 10,644 | — | — | — | 10,657 | |||||||||||||
| Warrants issued for services in lieu of cash (Note 10) |
— | — | — | — | 80 | — | — | — | 80 | |||||||||||||
| Amortization of non-employee options |
— | — | — | — | 96 | — | — | — | 96 | |||||||||||||
| Warrants issued for services |
— | — | — | — | 208 | — | — | — | 208 | |||||||||||||
| Net loss |
— | — | — | — | — | — | — | (15,762 | ) | (15,762 | ) | |||||||||||
| Balance at December 31, 2001 |
— | — | 12,896 | 129 | 91,649 | — | — | (63,918 | ) | 27,860 | ||||||||||||
| Exercise of stock options |
— | — | — | — | 2 | — | — | — | 2 | |||||||||||||
| Common Stock issued for 401(k)/401(m) plan |
— | — | 26 | — | 137 | — | — | — | 137 | |||||||||||||
| Common Stock issued for sublicense agreement (Note 6) |
— | — | 50 | 1 | 204 | — | — | — | 205 | |||||||||||||
| Common Stock issued to consultants |
— | — | — | — | 17 | — | — | — | 17 | |||||||||||||
| Amortization of non-employee options |
— | — | — | — | 66 | — | — | — | 66 | |||||||||||||
| Warrants issued for services |
— | — | — | — | 247 | — | — | — | 247 | |||||||||||||
| Net loss |
— | — | — | — | — | — | — | (17,502 | ) | (17,502 | ) | |||||||||||
| Balance at December 31, 2002 |
— | — | 12,972 | 130 | 92,322 | — | — | (81,420 | ) | 11,032 | ||||||||||||
| Common Stock issued for 401(k)/401(m) plan |
— | — | 32 | — | 223 | — | — | — | 223 | |||||||||||||
| Exercise of warrants |
— | — | 467 | 5 | 3,323 | — | — | — | 3,328 | |||||||||||||
| Exercise of stock options |
— | — | 85 | 1 | 955 | — | — | — | 956 | |||||||||||||
| Stock options issued |
— | — | — | — | 561 | — | — | — | 561 | |||||||||||||
| Private Placement, net of expenses |
— | — | 1,283 | 13 | 14,290 | — | — | — | 14,303 | |||||||||||||
| Common Stock issued for sublicense agreement (Note 6) |
— | — | 119 | 1 | 644 | — | — | — | 645 | |||||||||||||
| Common Stock issued for milestone payment |
— | — | 50 | 1 | 281 | — | — | — | 282 | |||||||||||||
| Debt Conversion |
— | — | 1,755 | 17 | 9,983 | — | — | — | 10,000 | |||||||||||||
| Common Stock issued in lieu of cash / interest |
— | — | 9 | — | 142 | — | — | — | 142 | |||||||||||||
| Public Offering, net of expenses |
— | — | 2,500 | 25 | 58,576 | — | — | — | 58,601 | |||||||||||||
| Deemed discount on convertible debentures |
— | — | — | — | 6,470 | — | — | — | 6,470 | |||||||||||||
| Warrants issued for services |
— | — | — | — | 1,398 | — | — | — | 1,398 | |||||||||||||
| Amortization of non-employee options |
— | — | — | — | 128 | — | — | — | 128 | |||||||||||||
| Purchase of treasury stock |
— | — | — | — | — | (59 | ) | (346 | ) | — | (346 | ) | ||||||||||
| Net loss |
— | — | — | — | — | — | — | (25,671 | ) | (25,671 | ) | |||||||||||
| Balance at December 31, 2003 |
— | — | 19,272 | 193 | 189,296 | (59 | ) | (346 | ) | (107,091 | ) | 82,052 | ||||||||||
41
Table of Contents
Harbor BioSciences, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity—(Continued)
| Preferred stock at par value |
Common stock at par value |
Capital in excess of par value |
Cost of Repurchased Common Stock |
Deficit accumulated during development stage |
Total | ||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||
| ( In thousands ) | |||||||||||||||||||||||
| Common Stock issued for 401(k) plan |
— | — | 17 | — | 147 | — | — | — | 147 | ||||||||||||||
| Exercise of warrants |
— | — | 6 | — | 11 | — | — | — | 11 | ||||||||||||||
| Exercise of stock options |
— | — | 4 | — | 16 | — | — | — | 16 | ||||||||||||||
| Common Stock issued for In-Process R&D (Note 6) |
— | — | 48 | — | 629 | — | — | — | 629 | ||||||||||||||
| Amortization of non-employee options |
— | — | — | — | 136 | — | — | — | 136 | ||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (24,757 | ) | (24,757 | ) | ||||||||||||
| Balance at December 31, 2004 |
— | — | 19,347 | 193 | 190,235 | (59 | ) | (346 | ) | (131,848 | ) | 58,234 | |||||||||||
| Common Stock issued for 401(k) plan |
— | — | 25 | — | 151 | — | — | — | 151 | ||||||||||||||
| Exercise of warrants |
— | — | 42 | 1 | 260 | — | — | — | 261 | ||||||||||||||
| Exercise of stock options |
— | — | 35 | 1 | 123 | — | — | — | 124 | ||||||||||||||
| Public Offering, net of expenses (Note 7) |
— | — | 1,333 | 13 | 9,502 | — | — | — | 9,515 | ||||||||||||||
| Amortization of non-employee options |
— | — | — | — | 30 | — | — | — | 30 | ||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (29,441 | ) | (29,441 | ) | ||||||||||||
| Balance at December 31, 2005 |
— | — | 20,782 | 208 | 200,301 | (59 | ) | (346 | ) | (161,289 | ) | 38,874 | |||||||||||
| Common Stock issued for 401(k) plan |
— | — | 45 | 1 | 224 | — | — | — | 225 | ||||||||||||||
| Exercise of warrants |
— | — | 10 | — | 1 | — | — | — | 1 | ||||||||||||||
| Warrants issued to consultants |
— | — | — | — | 226 | — | — | — | 226 | ||||||||||||||
| Exercise of stock options |
— | — | 34 | — | 86 | — | — | — | 86 | ||||||||||||||
| Private Placements, net of expenses |
— | — | 8,000 | 80 | 48,697 | — | — | — | 48,777 | ||||||||||||||
| Stock-Based Compensation Expense |
— | — | — | — | 3,534 | — | — | — | 3,534 | ||||||||||||||
| Amortization of non-employee warrants |
— | — | — | — | 13 | — | — | — | 13 | ||||||||||||||
| Restricted stock grant, net of forfeitures |
— | — | 65 | 1 | 401 | — | — | — | 402 | ||||||||||||||
| Common Stock issued for In-Process R&D |
— | — | 35 | — | 180 | — | — | — | 180 | ||||||||||||||
| Deferred Compensation |
— | — | — | — | (309 | ) | — | — | — | (309 | ) | ||||||||||||
| Net loss |
— | — | — | — | — | — | — | (30,231 | ) | (30,231 | ) | ||||||||||||
| Balance at December 31, 2006 |
— | — | 28,971 | 290 | 253,354 | (59 | ) | (346 | ) | (191,520 | ) | 61,778 | |||||||||||
42
Table of Contents
Harbor BioSciences, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity—(Continued)
| Preferred stock at par value |
Common stock at par value |
Capital in excess of par value |
Cost of Repurchased Common Stock |
Deficit accumulated during development stage |
Total | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||
| Common Stock issued for 401(k) plan |
— | — | 96 | 1 | 192 | — | — | — | 193 | ||||||||||||||||||||
| Exercise of stock options |
— | — | 9 | — | 20 | — | — | — | 20 | ||||||||||||||||||||
| Stock-Based Compensation Expense |
— | — | — | — | 3,128 | — | — | — | 3,128 | ||||||||||||||||||||
| Restricted Stock Forfeitures |
— | — | (12 | ) | — | (33 | ) | — | — | — | (33 | ) | |||||||||||||||||
| Amortization of non-employee warrants |
— | — | — | — | 17 | — | — | — | 17 | ||||||||||||||||||||
| Deferred Compensation |
— | — | — | — | 123 | — | — | — | 123 | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (23,121 | ) | (23,121 | ) | ||||||||||||||||||
| Balance at December 31, 2007 |
— | — | 29,064 | 291 | 256,801 | (59 | ) | (346 | ) | (214,641 | ) | 42,105 | |||||||||||||||||
| Common Stock issued for 401(k) plan |
— | — | 164 | 1 | 174 | — | — | — | 175 | ||||||||||||||||||||
| Stock-Based Compensation Expense |
— | — | — | — | 2,404 | — | — | — | 2,404 | ||||||||||||||||||||
| Deferred Compensation |
— | — | — | — | 86 | — | — | — | 86 | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (21,565 | ) | (21,565 | ) | ||||||||||||||||||
| Balance at December 31, 2008 |
— | — | 29,228 | 292 | 259,465 | (59 | ) | (346 | ) | (236,206 | ) | 23,205 | |||||||||||||||||
| Common Stock issued for 401(k) plan |
— | — | 271 | 2 | 96 | — | — | — | 98 | ||||||||||||||||||||
| Restricted Stock Forfeitures |
— | — | (6 | ) | — | (3 | ) | (3 | ) | ||||||||||||||||||||
| Stock-Based Compensation Expense |
— | — | — | — | 1,240 | — | — | — | 1,240 | ||||||||||||||||||||
| Deferred Compensation |
— | — | — | — | 86 | — | — | — | 86 | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (15,626 | ) | (15,626 | ) | ||||||||||||||||||
| Balance at December 31, 2009 |
— | — | 29,493 | $ | 294 | $ | 260,884 | (59 | ) | $ | (346 | ) | $ | (251,832 | ) | $ | 9,000 | ||||||||||||
The accompanying notes are an integral part of these financial statements.
43
Table of Contents
(A Development Stage Company)
Statements of Cash Flows
| 2009 | 2008 | 2007 | Period from Inception (Aug. 15, 1994) to December 31, 2009 |
|||||||||||||
| ( In thousands ) | ||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||
| Net loss |
$ | (15,626 | ) | $ | (21,565 | ) | $ | (23,121 | ) | $ | (251,832 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||
| Depreciation |
208 | 315 | 309 | 2,222 | ||||||||||||
| Disposal of assets |
69 | 6 | 85 | 231 | ||||||||||||
| Compensation expense related to equity awards |
1,323 | 2,490 | 3,218 | 10,658 | ||||||||||||
| Amortization of deemed discount on convertible debentures |
— | — | — | 6,470 | ||||||||||||
| Amortization of deferred issuance cost |
— | — | — | 1,157 | ||||||||||||
| Common stock issued for 401k/401m plan |
98 | 174 | 192 | 1,507 | ||||||||||||
| Common stock issued as consideration for amendments to the license / finance agreements |
— | — | — | 67 | ||||||||||||
| Common stock and options issued as consideration for license fees, milestone payment, interest, note repayment and services |
— | — | — | 2,859 | ||||||||||||
| Expense related to warrants issued as consideration to consultants |
— | — | 17 | 4,369 | ||||||||||||
| Expense related to warrants issued to a director for successful closure of merger |
— | — | — | 570 | ||||||||||||
| Expense related to stock options issued |
— | — | — | 5,718 | ||||||||||||
| Expense related to common stock issued for the purchase of technology |
— | — | — | 1,848 | ||||||||||||
| Common stock issued as consideration for In-Process R&D |
— | — | — | 2,809 | ||||||||||||
| Deferred compensation expense related to options issued |
— | — | — | 1,210 | ||||||||||||
| Changes in assets and liabilities: |
||||||||||||||||
| Prepaid expenses |
53 | 7 | (81 | ) | (209 | ) | ||||||||||
| Deposits |
20 | — | 32 | (48 | ) | |||||||||||
| Other receivable |
(81 | ) | 645 | (645 | ) | (81 | ) | |||||||||
| Other Receivable from related party |
— | — | 4 | — | ||||||||||||
| Accounts payable |
(187 | ) | (132 | ) | (170 | ) | 827 | |||||||||
| Accrued expenses |
(479 | ) | (933 | ) | (3,546 | ) | 1,104 | |||||||||
| Net cash used in operating activities |
(14,602 | ) | (18,993 | ) | (23,706 | ) | (208,544 | ) | ||||||||
| Cash flows provided by investing activities: |
||||||||||||||||
| Proceeds from sale of property and equipment |
197 | — | — | 197 | ||||||||||||
| Purchase of property and equipment |
(9 | ) | (70 | ) | (234 | ) | (2,825 | ) | ||||||||
| Net cash provided by (used in) investing activities |
188 | (70 | ) | (234 | ) | (2,628 | ) | |||||||||
| Cash flows from financing activities: |
||||||||||||||||
| Restricted Cash |
— | — | — | (34 | ) | |||||||||||
| Contributions from stockholder |
— | — | — | 104 | ||||||||||||
| Net proceeds from sale of preferred stock |
— | — | — | 4,000 | ||||||||||||
| Net proceeds from sale of common stock |
— | — | — | 183,534 | ||||||||||||
| Net proceeds from issuance of convertible debentures and warrants |
— | — | — | 9,214 | ||||||||||||
| Purchase of treasury stock |
— | — | — | (346 | ) | |||||||||||
| Proceeds from issuance of debt |
— | — | — | 371 | ||||||||||||
| Net proceeds from recapitalization |
— | — | — | 6,271 | ||||||||||||
| Net proceeds from warrants/options exercised |
— | — | 20 | 17,796 | ||||||||||||
| Net cash provided by financing activities |
— | — | 20 | 220,910 | ||||||||||||
| Net increase (decrease) in cash and equivalents |
(14,414 | ) | (19,063 | ) | (23,920 | ) | 9,738 | |||||||||
| Cash and equivalents at beginning of period |
24,152 | 43,215 | 67,135 | — | ||||||||||||
| Cash and equivalents at end of period |
$ | 9,738 | $ | 24,152 | $ | 43,215 | $ | 9,738 | ||||||||
| Supplemental disclosure of cash flow information: |
||||||||||||||||
| Interest paid |
$ | — | $ | — | $ | — | $ | 388 | ||||||||
| Conversion of debt to equity |
— | — | — | 10,371 | ||||||||||||
| Warrants issued to consultants in lieu of cash, no vesting |
— | — | — | 559 | ||||||||||||
| Warrants issued in lieu of cash, commissions on private placement |
— | — | — | 733 | ||||||||||||
| Warrants issued in connection with convertible debentures |
— | — | — | 371 | ||||||||||||
The accompanying notes are an integral part of these financial statements.
44
Table of Contents
(A Development Stage Company)
Notes to Financial Statements
| 1. | The Company |
Harbor BioSciences, Inc., (“Harbor BioSciences” or the “Company”), a development stage pharmaceutical company, is engaged in the discovery, development and commercialization of products for the treatment of diseases related to aging. From inception (August 15, 1994) through March 1997, the Company's efforts were directed toward organizing, licensing technology and preparing for offerings of shares of its common stock. Since 1997, the Company has been expanding its intellectual property, developing its lead drug candidates, performing preclinical tests and has entered into multiple clinical studies. Our primary technology development efforts are focused on a series of adrenal steroid hormones and synthetic analogs that may be useful in treating a wide variety of medical conditions, if successfully developed. These adrenal hormones are depleted during advancing age, a process accelerated by infectious diseases and chronic immune system disorders. High plasma concentrations of these hormones are positively correlated with attenuated disease, in certain indications, and their maintenance is often associated with healthy aging.
During the past three years, the Company has devoted substantially all of its research, development and clinical efforts and financial resources toward the development of Apoptone and Triolex. The Company has incurred a net loss of $15.6 million in 2009, has had cumulative net losses of $251.8 million from inception to date and has limited financial resources at December 31, 2009.
These events raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. This basis of accounting contemplates the recovery of the Company’s assets and the satisfaction of liabilities in the normal course of business and does not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern. In an effort to preserve cash, the Company initiated steps during 2009 to significantly reduce its operating costs including a substantial reduction in personnel, closure of its laboratory, sale of equipment and reduction of leased space.
The Company is seeking to maximize the value of its remaining assets. The Company is currently evaluating its strategic alternatives, which include the following:
| • | Pursue potential strategic transactions, which could include mergers, license agreements or other collaborations, with third parties; |
| • | Sell or out-license the Company’s remaining assets, including the Company’s library of compounds; or |
| • | Implement an orderly wind down of the Company if other alternatives are not deemed viable and in the best interests of the Company. |
If we do not raise additional cash, the Company will be out of cash to fund further operations by late 2010.
| 2. | Summary of Accounting Policies |
Cash Equivalents
The Company considers any liquid investments with maturity of three months or less when purchased to be cash equivalents. At December 31, 2009 the Company's cash equivalents are approximately $9.7 million and are deposited primarily in a money market account with a large financial institution.
45
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Property and Equipment
Property and equipment are stated at cost. The Company provides for depreciation using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized over the lesser of the lease term or the useful life. The cost of major additions and improvements is capitalized, while maintenance and repair costs that do not improve or extend the lives of the respective assets are charged to operations as incurred.
Property and equipment balances and corresponding lives were as follows:
| December 31 | Lives | |||||||||
| 2009 | 2008 | |||||||||
| (in thousands) | ||||||||||
| Leasehold improvements |
$ | — | $ | 23 | 3 years | |||||
| Machinery, equipment and information systems |
864 | 1,896 | 5-7 years | |||||||
| Furniture and fixtures |
218 | 218 | 5-7 years | |||||||
| Total |
1,082 | 2,137 | ||||||||
| Less: Accumulated depreciation and amortization |
(906 | ) | (1,496 | ) | ||||||
| $ | 176 | $ | 641 | |||||||
Depreciation expense associated with property and equipment was approximately $208,000, $315,000 and $309,000 in 2009, 2008 and 2007, respectively.
In accordance with ASC Topic 360, Property, Plant and Equipment, the Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. Recoverability measurement and estimating of undiscounted cash flows is done at the lowest possible level for which there is identifiable assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. The Company had no impairments in 2009, 2008 and 2007.
Accrued Expenses
Accrued expenses include approximately $0.3 million and $0.5 million in accrued vacation expense, $0.9 million and $1.1 million in other research and development / general and administrative expenses as of December 31, 2009 and 2008, respectively.
Revenue Recognition
In December 2003, the Securities and Exchange Commission (“SEC”) issued ASC Topic 605, Revenue Recognition, which updates and summarizes the Commission’s views on the application of generally accepted accounting principles to revenue recognition in financial statements. The Company believes that its revenue recognition policies conform to the requirements of ASC Topic 605.
Contract revenue is recognized as the services are performed on a cost reimbursement basis. Revenue associated with development milestones, if any, is recognized based upon the achievement of the milestones, as
46
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
defined in the respective agreements. Overall, revenue is considered to be realized or realizable and earned when there is persuasive evidence of a revenue arrangement in the form of a contract or purchase order, the services have been performed, the price is fixed or determinable and collectability is reasonably assured.
Research and Development
All research and development costs are expensed as incurred. The value of acquired in-process research and development is charged to expense on the date of acquisition. Research and development expenses include, but are not limited to, acquired in-process technology deemed to have no alternative future use, license fees related to license agreements, preclinical and clinical trial studies, payroll and personnel expense, lab supplies, consulting and research-related overhead. Research and development expenses paid in the form of cash and Company stock to related parties aggregated $11.5 million for the period from inception (August 15, 1994) to December 31, 2003 (see Note 6, “Colthurst, Edenland and Mr. Prendergest” and “Aeson Therapeutics”). No such related party expenses were incurred in 2009, 2008 or 2007.
Accounting for Share-Based Payments
The Company has an equity-based incentive compensation plan known as “The 2005 Equity Incentive Plan” (the “Plan”). The Plan allows us to grant stock options and other stock or stock-based awards, including stock appreciation rights, stock purchase awards, restricted stock awards and restricted stock units awards. The Plan also allows us to provide equity compensation to non-employee directors and consultants. The exercise price for an option granted under the Plan is typically not less than the fair market value of the common stock subject to such option. The term of any options granted under the Plan may not exceed 10 years from the date of the grant. Options issued to employees generally vest over a four-year period, with 25% vesting on the first anniversary date and the balance vesting monthly during years two, three and four.
Prior to (ASC Topic 718), Compensation-Stock Compensation, all stock options for employees (with the exception of three grants) have been granted at or above the market price where the exercise price of the option equaled or exceeded the market price of the stock on the date of the grant. As a result, under previous rules, there was no stock-based compensation expense for those grants. Compensation expense was taken for the three options granted at below market value (see “2005 Annual Report on Form 10-K, Notes to Financial Statements – No. 9 Stock Options” for more detail). As of December 31, 2009 the Plan has 8,235,608 shares of common stock reserved for issuance.
Effective January 1, 2006, we adopted ASC Topic 718, requiring us to recognize expense related to the fair value of our stock-based compensation awards. We elected the modified prospective transition method as permitted by ASC Topic 718; accordingly, results from prior periods have not been restated. Under this transition method, stock-based compensation expense for the fiscal year ended December 31, 2009, 2008 and 2007 includes:
| a) | compensation expense for all stock-based compensation awards granted prior to January 1, 2006 but not yet vested, based on the grant date fair value estimated in accordance with the original provisions of ASC Topic 718, and |
| b) | compensation expense for all stock-based compensation awards granted subsequent to December 31, 2005 based on the grant-date fair value estimated in accordance with the provisions of ASC Topic 718. |
The fair value of each stock option granted is estimated on the date of grant using the Black-Scholes option-pricing model. The assumptions used to calculate the fair value of options granted are evaluated and revised, as
47
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
necessary, to reflect the Company’s experience. Compensation expense is recognized using the straight-line method for all stock-based awards issued after January 1, 2006. Compensation expense is recognized only for those options expected to vest, with forfeitures estimated at the date of grant based on the Company’s historical experience and future expectations. Prior to the adoption of ASC Topic 718, the effect of forfeitures on the pro forma expense amounts was not recognized. ASC Topic 718, requires forfeitures to be estimated at the time of the grant and revised as necessary in subsequent periods if actual forfeitures differ from those estimates.
Black-Scholes Option Valuation Assumptions (1)
| Fiscal Years Ended | |||||||||
| December 31, 2009 |
December 31, 2008 |
December 31, 2007 |
|||||||
| Risk-free interest rate |
4.5 | % | 3.75 | % | 4.75 | % | |||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | |||
| Expected life(2) |
7.04 years | 6.60 years | 6.25 years | ||||||
| Expected volatility(3) |
85 | % | 75 | % | 76 | % | |||
| (1) | Forfeitures are estimated as 7.88% for 2009, 6.20% for 2008 and 5.05% for 2007 because of the Company’s restructuring in 2009, there were greater than normal forfeitures of stock options. |
| (2) | The 2009 and 2008 expected life is based on historical experience; the 2007 expected life is based on the safe-harbor method. |
| (3) | The expected stock price volatility is estimated based on historical experience. |
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected stock price volatility. Because the Company’s employee stock options have characteristics significantly different from those of traded options, and because changes in subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models do not necessarily provide a reliable single measure of the fair value of the Company’s options.
401(k) Matching Contributions
Our Company sponsors a 401(k) savings plan, to which eligible domestic employees may voluntarily contribute a portion of their income, subject to statutory limitations. In addition to the participant’s own contributions to these 401(k) savings plans, we match such contributions up to a designated level. Total matching contributions related to employee savings plans were approximately $120,000, $175,000 and $192,000 in 2009, 2008 and 2007, respectively.
Income Taxes
The Company provides for income taxes under the principles of ASC Topic 740, Income Taxes, which requires that provision be made for taxes currently due and for the expected future tax effects of temporary differences between book and tax bases of assets and liabilities.
On July 13, 2006 ASC Topic 740 was issued, Accounting for Uncertainty in Income Taxes, which establishes recognition and measurement thresholds that must be met before a tax benefit can be recognized in the financial statements. The Company adopted ASC Topic 740 on January 1, 2007, and it has had no material impact on its financial statements.
48
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Financial Instruments
The Company’s financial instruments consist primarily of cash and accounts payable. These financial instruments are stated at their respective carrying values, which approximate their fair values, due to their short-term nature.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could materially differ from those estimates.
Concentrations of Risk
Financial instruments, which potentially expose the Company to concentrations of credit risk, consist primarily of cash and cash equivalents. The Company places its cash with high quality financial institutions. Cash balances are generally substantially in excess of the amounts insured by the Federal Deposit Insurance Corporation.
Net Loss per Share
Basic net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per share is computed in a manner consistent with basic net loss per share after giving effect to potentially dilutive securities. Potential common shares of 5,010,334, 9,466,150, and 9,702,428 related to the Company’s outstanding stock option and warrants were excluded from the computation of diluted net loss per share for the years ended December 31, 2009, 2008 and 2007 because their effect on net loss per share is anti-dilutive.
Recent Accounting Pronouncements
Accounting for Collaborative Arrangements Related to the Development and Commercialization of Intellectual Property, ASC Subtopic 808-10, concluded that a collaborative arrangement is one in which the participants are actively involved and are exposed to significant risks and rewards that depend on the ultimate commercial success of the endeavor. Revenues and costs incurred with third parties in connection with collaborative arrangements would be presented gross or net based on the criteria in ASC Subtopic 605-45, Reporting Revenue Gross as a Principal versus Net as an Agent, and other accounting literature. Payments to or from collaborators would be evaluated and presented based on the nature of the arrangement and its terms, the nature of the entity’s business and whether those payments are within the scope of other accounting literature. The nature and purpose of collaborative arrangements are to be disclosed along with the accounting policies and the classification and amounts of significant financial statement amounts related to the arrangements. Activities in the arrangement conducted in a separate legal entity should be accounted for under other accounting literature; however, required disclosure under ASC Subtopic 808-10 applies to the entire collaborative agreement. ASC Subtopic 808-10 is effective for fiscal years beginning after December 15, 2008, and is to be applied retrospectively to all periods presented for all collaborative arrangements existing as of the effective date. The adoption of this standard did not have a material impact on our financial statements.
49
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
In December 2007, ASC Topic 805, Business Combinations, was issued which requires an acquirer to recognize the assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions. ASC Topic 805 also requires the acquirer in a business combination achieved in stages to recognize the identifiable assets and liabilities, as well as the non-controlling interest in the acquiree, at the full amounts of their fair values. ASC 805 Topic makes various other amendments to authoritative literature intended to provide additional guidance or to confirm the guidance in that literature to that provided in this Statement. ASC Topic 805 applies to business combinations for which the acquisition date is in fiscal years beginning after December 15, 2008. The adoption of this standard did not have a material impact on our financial statements.
Effective July 1, 2009, the Company adopted a replacement of FASB Statement No. 162, The “FASB Accounting Standards Codification”, of Generally Accepted Accounting Principles (ASC Topic 105). This standard establishes only two levels of U.S. generally accepted accounting principles (“GAAP”), authoritative and nonauthoritative. The FASB Accounting Standards Codification (the “Codification”) became the source of authoritative, nongovernmental GAAP, except for rules and interpretive releases of the SEC, which are sources of authoritative GAAP for SEC registrants. All other non-grandfathered, non-SEC accounting literature not included in the Codification became nonauthoritative. The Company began using the new guidelines and numbering system prescribed by the Codification when referring to GAAP in the third quarter of fiscal 2009. As the Codification was not intended to change or alter existing GAAP, it did not have any impact on our financial statements.
Effective April 1, 2009, the Company adopted three accounting standard updates which were intended to provide additional application guidance and enhanced disclosures regarding fair value measurements and impairments of securities. They also provide additional guidelines for estimating fair value in accordance with fair value accounting. The first update, as codified in ASC Subtopic 820-10-65, Financial Instruments, provides additional guidelines for estimating fair value in accordance with fair value accounting. The second accounting update, as codified in ASC Subtopic 820-10-65, changes accounting requirements for other-than-temporary-impairment (OTTI) for debt securities by replacing the current requirement that a holder have the positive intent and ability to hold an impaired security to recovery in order to conclude an impairment was temporary with a requirement that an entity conclude it does not intend to sell an impaired security and it will not be required to sell the security before the recovery of its amortized cost basis. The third accounting update, as codified in ASC Subtopic 825-10-65, increases the frequency of fair value disclosures. These updates were effective for fiscal years and interim periods ended after June 15, 2009. The adoption of these accounting updates did not have any impact on our financial statements.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC Subtopic 820-10 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements. Our level 1 assets primarily include our cash and cash equivalents (mainly money market accounts). Valuations are obtained from readily available pricing sources. We do not currently have Level 2 or 3 assets.
Effective April 1, 2009, the Company adopted a new accounting standard for subsequent events, as codified in ASC Subtopic 855-10, Subsequent Events. The update modifies the names of the two types of subsequent events either as recognized subsequent events (previously referred to in practice as Type I subsequent events) or non-recognized subsequent events (previously referred to in practice as Type II subsequent events). In addition,
50
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
the standard modifies the definition of subsequent events to refer to events or transactions that occur after the balance sheet date, but before the financial statements are issued (for public entities) or available to be issued (for nonpublic entities). The update did not result in significant changes in the practice of subsequent event disclosures, and therefore the adoption did not have any impact on our financial statements.
| 3. | Recapitalization |
During March 1997, Hollis-Eden Inc. was merged (the “Merger”) with and into the Company (then known as Initial Acquisition Corp. (IAC)). Upon consummation of the Merger, Hollis-Eden Inc. ceased to exist, and IAC changed its name to Hollis-Eden Pharmaceuticals, Inc. IAC (now called Hollis-Eden Pharmaceuticals, Inc.) was the continuing legal entity and registrant for SEC reporting purposes. The IAC Merger was accounted for as a recapitalization of Hollis-Eden Inc. by an exchange of Common Stock of Hollis-Eden Inc., for the net assets of IAC, consisting primarily of $6.5 million in cash and other receivables.
Under the terms of the merger agreement, each share of Hollis-Eden Inc. Common Stock outstanding converted into one share of Common Stock of Hollis-Eden Pharmaceuticals, Inc. Common Stock (“Company Common Stock”), and all warrants and options to purchase Hollis-Eden Inc. Common Stock outstanding converted into the right to receive the same number of shares of Company Common Stock.
Upon the consummation of the Merger, pursuant to an agreement, the Company issued warrants to purchase an aggregate of 50,000 shares of Company Common Stock at an exercise price of $0.10 per share to a director and former officer. Additional paid-in capital was increased by $570,000 with an offsetting $570,000 charge recorded to operations during the three months ended March 31, 1997.
Effective February 9, 2010, we changed our name from Hollis-Eden Pharmaceuticals, Inc. to Harbor BioSciences, Inc. The name change was effected pursuant to Section 253 of the General Corporation Law of the State of Delaware by the merger of our wholly owned subsidiary into us. We were the surviving corporation and, in connection with the merger, we amended our Amended and Restated Certificate of Incorporation to change our name to Harbor BioSciences, Inc.
Effective February 18, 2010, our common stock began trading under a new Nasdaq symbol, "HRBR" and CUSIP number “41150V 103”.
| 4. | Other Receivable from Related Party |
On April 23, 2001, the Company entered into a promissory note with a stockholder/officer in the amount of $16,875. Interest was at 4.5% per annum. The promissory note was paid in full prior to the due date of April 23, 2004.
On May 22, 1998, the Company entered into a promissory note with a stockholder/officer in the amount of $200,000. Interest was at 5.5% per annum. The note was repaid in full in May 2003.
On March 21, 2005, the Company entered into a promissory note with an employee with a maximum loan amount of $20,000. Interest was at 6% per annum. The first installment of $10,000 was made on the commencement date. A second installment of $10,000 was made on April 20, 2005. The loan was repaid with a balance of approximately $2,000 forgiven on May 10, 2007.
51
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
| 5. | Income Taxes |
The Company has available a federal and state net operating loss carryforward of approximately $207.1 million and $163.5 million at December 31, 2009, respectively, which may be carried forward as an offset to taxable income, if any, in future years through its expiration for California in 2019 and for Federal in 2029. The Company has a net federal and state deferred tax asset of approximately $81.0 million and $17.8 million at December 31, 2009, respectively, comprised of research and development credits and the net operating loss carryforwards. The net deferred tax assets have been fully reserved due to the uncertainty of the Company being able to generate taxable income under the more likely than not criteria of ASC Topic 740. The Federal and state net operating loss carryforwards begin expiring in 2017 and 2010, respectively.
The difference between the Company’s expected Federal tax benefit calculated using a 34% tax rate and the Company’s zero tax benefit for all years is primarily related to a full valuation allowance established against the Company’s net operating loss carryforwards for 2009 and the tax effects of stock compensation under ASC Topic 718. The net increase in valuation allowance at December 31, 2009 was approximately $5.4 million.
If certain substantial changes in the Company’s ownership should occur, there would potentially be an annual limitation on the amount of the carryforwards, which could be utilized in a tax year. The Company has not performed a “Section 382” change in control test to date. Until this test is performed, the Company cannot be certain of the use of the loss carryforwards.
All operations are in California and the Company believes it has no tax positions which could more-likely-than not be challenged by tax authorities. We have no unrecognized tax benefits and thus no interest or penalties included in the financial statements. The Company is subject to examination for tax years after 2006 for federal purposes and after 2005 for California state tax purposes.
| 6. | Related Party Licenses and other Agreements and Contingencies |
Colthurst, Edenland and Mr. Prendergast
During 1994, the Company entered into two license agreements and one research, development and option agreement as discussed in the following paragraphs.
Pursuant to a license agreement dated May 18, 1994 (“Colthurst License Agreement”) with related parties, Patrick T. Prendergast, a significant stockholder at the time, and with Colthurst Limited, a company controlled by Mr. Prendergast, the Company acquired the exclusive worldwide rights to Mr. Prendergast's patent rights, know-how and background technology relating to the treatment of human/animal immunodeficiency. The agreement was amended on August 11, 1995 to change the license fee payment terms as discussed below in paragraph four of this Note. Per the license agreement, the Company agreed to pay royalties on product revenues.
On August 25, 1994, the Company entered into a license agreement (“Edenland License Agreement”) with a related party, Edenland Inc., a company controlled by Mr. Prendergast, for the exclusive worldwide rights to Mr. Prendergast's patent rights, know-how and background technology related to the substance tradenamed HE317 and to any other pharmaceutical product that became subject to the license agreement under the research, development and option agreement discussed below. The agreement was amended on August 11, 1995 to change the license fee payment terms as discussed in the following paragraph. Per the Edenland License Agreement, the Company agreed to pay royalties on product revenues.
52
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Effective August 11, 1995, Edenland, Inc., Colthurst Limited and the Company entered into amendments concerning the license fee payment terms to the two agreements described above. Under this amendment, the Company agreed to pay a license fee by April 28, 1996 plus additional license fees within 24 months of April 1996. The balances of these fees were paid in full by May 1997. As consideration for entering into certain amendments, the Company issued 75,472 shares of the Company's common stock to Edenland, Inc. and Colthurst Limited.
Per the amended Colthurst License Agreement, a renewal annual license fee was payable commencing May 1998. The Company paid this fee in 1998 by issuing shares of its common stock and, in 1999, paid in cash.
In August 1994, the Company entered into a Research, Development and Option Agreement, with Edenland, Inc. and Mr. Prendergast. The agreement provided for the development of HE317 to a certain stage of development and granted the Company the right of first option on new products developed by Edenland, Inc. The agreement committed the Company to pay for certain development costs up to the amount of $3.0 million with certain contingencies for funding. In October 1996, the Company and Edenland, Inc. entered into an amendment, which accelerated the date that the $3.0 million payment for HE317 or other product development costs was to be made. The Company paid $2.7 million during 1997 and the remaining $300,000 in April 1998.
On January 20, 2000, the Company reached a settlement regarding various disputes with Mr. Prendergast, Colthurst and Edenland. The parties entered into two new technology agreements, the Technology Assignment Agreement and the Sponsored Research and License Agreement.
The Technology Assignment Agreement (Assignment Agreement) replaced the Colthurst License Agreement. Pursuant to the Assignment Agreement, Mr. Prendergast and Colthurst assigned to the Company ownership of all patents, patent applications and current or future improvements of the technology under the Colthurst License Agreement, including IMMUNITIN, the Company’s lead clinical compound at the time. The annual license fee of $500,000 and the royalty obligations under the Colthurst License Agreement were eliminated. In consideration for the foregoing, the Company agreed to issue to Colthurst 660,000 shares of Common Stock and a warrant to purchase an aggregate of 400,000 shares of Common Stock at $25 per share. Only 132,000 of such shares of Common Stock were issued in 2000, with the remaining 528,000 shares to be issued over the next four years conditioned on continued compliance with the agreement and, in particular, satisfaction of the Conditions (as defined below). In addition, all of the shares under the warrant vest over four years conditioned on continued compliance with the agreement and, in particular, satisfaction of the Conditions (as defined below). The Sponsored Research and License Agreement replaced the Edenland License Agreement and the Research, Development and Option Agreement. Pursuant to the Sponsored Research and License Agreement, Edenland exclusively licensed to the Company a number of compounds, together with all related patents and patent applications, and the Company funded additional preclinical research projects conducted by Edenland. The Company would also have exclusive license rights to all results of such research and would have royalty obligations to Edenland on sales of new products, if any, resulting from such research. None of the compounds licensed under the Sponsored Research and License Agreement have been developed by the Company and, as described below, this agreement is now terminated.
As stated above, the issuance of the additional shares of Common Stock and the vesting of the warrant was dependent upon the satisfaction of certain conditions (the “Conditions”). In accordance with ASC Subtopic 505-50 these future events could not be determined at the date of the agreements (January 2000). Accordingly, the shares and warrants are accounted for as they vest or are issued. During 2000, the Company recorded a research and development charge for $1.9 million representing the fair value of the 132,000 shares issued under the Assignment Agreement.
53
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Because all of the Conditions were not satisfied, the Company did not issue any additional shares to Colthurst and believed it had no obligation to issue any additional shares and that the warrant would not vest as to any shares of Common Stock.
After arbitration proceedings during 2004 and 2005, pursuant to which Colthurst sought more than $25 million in damages for the non-issuance of the 528,000 shares of common stock and the warrant to purchase up to 400,000 shares of common stock, in February 2006 the parties agreed to a settlement and release of all issues in dispute between the parties. Under the settlement agreement, (1) the Company agreed to make a payment of $3 million in cash and (2) the parties agreed to terminate the Sponsored Research and License Agreement between the Company and Edenland Inc. The $3.0 million was accrued as an expense as of December 31, 2005. Under the settlement agreement, the Colthurst parties remain prohibited from conducting any further research, development or commercialization activities of any kind relating in any way to the technology (including IMMUNITIN) that was assigned to the Company under the Assignment Agreement.
Aeson Therapeutics
In October 2000, the Company acquired a 21% equity stake in Aeson Therapeutics Inc. (“Aeson”) and an exclusive worldwide sublicense to three issued patents in the area of adrenal steroids in exchange for $2.0 million in cash and 208,672 shares of Common Stock valued at $2 million. The cash and shares were expensed as in-process R&D during the fourth quarter of 2000. As part of the transaction, Aeson and its shareholders granted the Company an exclusive option to acquire the remainder of Aeson at a predetermined price.
In March 2002, the Company amended certain of its agreements with Aeson. Under the amendments, the Company paid Aeson $1.2 million, which extended the initial date by which the Company could exercise its option to acquire the remainder of Aeson to September 30, 2002. The Company also received additional equity securities as a result of its $1.2 million payment. The $1.2 million payment was expensed as in-process R&D. The Company elected not to exercise the option to acquire the remainder of Aeson by September 30, 2002.
On June 7, 2006, the Company acquired substantially all of the assets of Aeson. As consideration for Aeson’s assets, the Company agreed (i) to issue a total of 35,000 shares of common stock to Aeson at the closing of the acquisition and (ii) to issue to Aeson’s stockholders up to a total of 165,000 additional shares of common stock if certain development milestones are achieved. The acquisition was expensed as in-process research and development. The Company has not achieved any of the development milestones.
Pharmadigm
In August 2002, we entered into a Sublicense Agreement with Pharmadigm, Inc. (currently known as Inflabloc Pharmaceuticals, Inc.). Under the agreement, we obtained exclusive worldwide rights to certain intellectual property of Pharmadigm and the University of Utah and we agreed to make aggregate payments of $0.9 million in cash or in shares of our common stock, at our option, over the next year. This cost was expensed in the third quarter of 2002. We elected to make such payments in equity and have issued a total of 168,921 shares of our common stock in complete satisfaction of this requirement (of which 118,921 shares were issued the quarter ended March 31, 2003). We may also make substantial additional milestone and royalty payments in cash to Pharmadigm if we meet specified development and commercialization milestones for products covered by the patents. To date, no such milestones have been met. The principal patents licensed under the agreement, originally licensed to Pharmadigm from the University of Utah, relate to inventions by Dr. Raymond Daynes and Dr. Barbara A. Areneo. Dr. Daynes served as a scientific consultant to the Company from 1999 to mid-2003.
54
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Congressional Pharmaceutical
In February 2004, the Company acquired Congressional Pharmaceutical Corporation (“CPC”) and replaced CPC as the exclusive licensee to certain intellectual property rights held by the University of Chicago. These intellectual property rights consist of a series of patents and patent applications that relate to discoveries made by David J. Grdina, Ph.D., Professor of Radiation and Cellular Oncology at the University of Chicago. The patented technology covers a series of compounds that have the potential to protect against DNA mutations that can occur as a result of radiation injury or chemotherapy. In the acquisition the Company issued approximately 50,000 shares of common stock to the former stockholders of CPC valued at approximately $650,000, in accordance with Emerging Issues Task Force No. 99-12. In addition, if the Company achieves certain development milestones, it will be required to issue additional shares of our common stock to the former stockholders of CPC. In the event all of the milestones are achieved, the total number of additional shares that the Company would be required to issue to the former stockholders of CPC is 275,000, more than half of which would be issued only upon FDA approval of CPC’s product. No such milestone has been met to date. Furthermore, upon regulatory approval and commercialization of products covered by the licensed intellectual property, the Company may be required to pay royalties to the former stockholders of CPC and the University of Chicago. Following the acquisition, Dr. Grdina agreed to an exclusive consulting arrangement with the Company in the fields of hematopoiesis and radiation and chemotherapy exposure. In March 2007, the Company terminated its consulting agreement with Dr. Grdina. In May 2007, the University of Chicago terminated the license agreement with the Company.
AFRRI Collaboration
The Company performed work on two task orders that were issued under a collaboration with the Armed Forces Radiobiology Research Institute (“AFRRI”). Under these task orders, the Company conducted radiation studies with a subcontractor. The task orders committed AFRRI to reimburse the Company for $2.0 million in subcontractor fees. The reimbursement amounts from AFRRI were recorded in the same timeline as the subcontractor fees, resulting in no impact on the statement of operations. There was no activity during 2007 under the AFRRI collaboration. The Company terminated its collaborative research and development agreement with AFRRI effective August 12, 2007.
Study Funding Agreement
The Company has a Study Funding Agreement with Cystic Fibrosis Foundation Therapeutics, Inc. (“CFFT”). The agreement commits CFFT to provide a total of $1.7 million to be paid in seven tranches based on the Company’s completion of certain agreed-upon events. The agreement also contains a provision indicating that upon termination of this agreement by either party, CFFT shall pay the Company for all work performed through the date of termination, plus reasonable costs of bringing the study to an orderly close.
In return for this funding, the Company has agreed to pay CFFT a minimum royalty over a specified period following regulatory approval in the United States of America. Additional compensation is due to CFFT if net sales of this compound exceed a specified amount over a period of time.
Revenue is recognized under this agreement on a percentage of completion method for each distinct agreed-upon event. No revenue was recorded in 2009.
This agreement expired December 31, 2009.
55
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
| 7. | Common Stock |
Reverse Stock Splits
During February 1995, there was a 3-for-5 reverse stock split of the Company's common stock and in March 1996, a 1-for-2.65 reverse stock split of the Company’s common stock. Both reverse stock splits have been retroactively reflected for all periods presented.
Common Stock Financings
In January 1996, the Company completed a $367,522 round of debt financing with a group of private investors. These notes, with an 8% interest rate, were due on or before the earlier of (i) January 21, 1997 or (ii) the closing of a private or public offering of securities. During April 1996, the debt financing, plus accrued interest, was converted into 164,962 shares of common stock at a price of $2.25 per share. In April 1996, the Company privately issued 580,005 shares of the Company’s common stock at an offering price of $2.25 per share. Total proceeds from this offering aggregated $1,234,499.
During May 1998, the Company completed a private financing totaling $20.6 million in gross proceeds. The Company issued 1,329,201 shares of common stock, (of which 192,061 shares were subject to adjustment based on future average stock price (“Adjustable Common Stock”)), 4,000 shares of 5% Series A Convertible Preferred Stock and warrants to purchase 1,437,475 shares of common stock in the financing. The warrants entitled the holders to purchase up to a total of 1,437,475 shares of common stock at a price of $17.00 per share.
The Convertible Preferred Stock had an initial conversion price of $20.30 for the first seven months, after which it could be adjusted, either up or down, based on the future stock prices of the Company’s common stock. The Convertible Preferred Stock was converted to common stock in January 1999 (See Note 8).
In January 1999, the Company completed two private placements of an aggregate of 1,367,868 shares of common stock at prices ranging from $18.00 to $18.50 per share. In connection with the private placements, the Company issued warrants to purchase an aggregate of 90,000 shares of the Company’s common stock, with an exercise price of $18.25 per share, as a finder’s fee. The Company raised approximately $25.0 million in gross proceeds.
During December 2001, the Company raised $11.5 million in gross proceeds from the sale of 1.28 million shares of newly issued common stock in a private placement at a price of $9.00 per share. The investors were a group of qualified institutional buyers and institutional accredited investors. The Company also issued warrants to purchase up to 128,000 shares of common stock having an exercise price of $12.00 per share to investors. As a finders fee, the Company issued to its placement agent two warrants for a total of 112,640 shares of common stock, one warrant with an exercise price of $9.00 and the other with an exercise price of $12.00.
On February 25, 2003, we completed a private placement in which we issued $10.0 million aggregate principal amount of three-year convertible debentures (“debentures”), bearing interest at 7.5% per year, and warrants to purchase up to 701,760 shares of common stock. The debentures were convertible into common stock at a price of $5.70 per share, which represented a discount from the price of our common stock on the commencement date. Also issued in connection with this private placement were warrants to purchase up to 350,880 shares of common stock which are exercisable at a price per share of $6.17, subject to adjustment, and warrants to purchase up to 350,880 shares of common stock which are exercisable at a price per share of $6.71, subject to adjustment. The warrants were exercisable until February 25, 2007.
56
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
In connection with the issuance of the debentures and warrants, we recorded approximately $3.5 million related to the beneficial conversion feature and approximately $3.0 million for the detachable warrants on the debentures. The total amount of the deemed discount on the debentures as a result of the warrant issuance and the beneficial conversion feature amounts to $6.5 million. The beneficial conversion feature and warrant value (deemed discount) were amortized over the term of the debentures and as conversion of the debentures occurred.
On June 20, 2003, convertible debentures with a face value of $0.5 million were converted into 87,720 shares of our common stock leaving a $9.5 million aggregate principle amount of convertible debentures outstanding. On August 11, 2003, the remaining aggregate principal amount of convertible debentures with a face value of $9.5 million were converted into 1,666,680 shares of our common stock with a value of $5.70 per share.
During June 2003, the Company completed a private placement of common stock and warrants, from which it received gross proceeds of $14.7 million. In October 2003 the Company completed a public offering of an aggregate of 2,500,000 shares of common stock at a price of $25.00 per share and received $62.5 million in gross proceeds from this offering.
On June 1, 2005 the Company raised approximately $10.0 million in gross proceeds from the sale of 1,333,333 shares of the Company’s common stock at an exercise price of $6.17 per share. Additionally, the Company issued a four-year warrant to purchase up to an additional 266,667 shares of common stock at an exercise price of $10.00 per share. In connection with this transaction, the Company incurred approximately $0.5 million in direct costs and recorded net proceeds of approximately $9.5 million.
On February 6, 2006 the Company raised approximately $26.0 million in gross proceeds from the sale of 4,000,000 shares of the Company’s common stock at a price of $6.50 per share. The direct costs related to this financing were $1.7 million, resulting in net proceeds of $24.3 million. Additionally, the Company issued four-year warrants to purchase up to an additional 800,000 shares of common stock at an exercise price of $8.75 per share. The warrants were not exercisable until six months following issuance.
On June 7, 2006 the Company issued 35,000 shares of the Company’s common stock to Aeson Therapeutics, Inc. (Aeson) in connection with the purchase of substantially all of Aeson’s assets, resulting in an expense of $180,000. Upon certain events, the Company may be obligated to issue an additional 165,000 shares. The acquisition was expensed as in-process research and development. To date, the Company has not achieved any of the development milestones.
On November 13, 2006 the Company raised approximately $26.0 million in gross proceeds from the sale of 4,000,000 shares of the Company’s common stock at a price of $6.50 per share. The direct costs related to this financing were $1.6 million, resulting in net proceeds of $24.4 million. Additionally, the Company issued four-year warrants to purchase up to an additional 800,000 shares of common stock at an exercise price of $8.75 per share. The warrants were not exercisable until six months following issuance.
| 8. | Preferred Stock |
During May 1998, as part of a private placement, the Company issued 4,000 shares of Convertible Preferred Stock for proceeds of $4.0 million. The proceeds of the offering are included in the proceeds to the May 1998 financing described in Note 7, above.
57
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
During January 1999, the Company issued 346,127 shares of common stock in connection with the conversion of the Series A Convertible Preferred Stock and additional shares relating to the Adjustable Common Stock. The Adjustable Common Stock was issued during the private placement of May 1998 and was subject to adjustment based on the future average stock price of the Company’s common stock as described in Note 7. Upon conversion, all outstanding shares of Preferred stock and Adjustable Common stock were eliminated.
In November 1999, the Company adopted a Shareholders Rights Plan in which Preferred Stock purchase rights (“Rights”) were distributed as a dividend at the rate of one Right for each share of common stock held as of the close of business on November 29, 1999. Each right entitled stockholders to buy, upon certain events, one one-hundredth of a share of a new Series B junior participating preferred stock of the Company at an exercise price of $100.00. The Rights are designed to guard against partial tender offers and other abusive tactics that might be used in an attempt to gain control of the Company or to deprive stockholders of their interest in the long-term value of the Company. The Rights were exercisable only if a person or group acquires 15% or more of the Company’s common stock or announces a tender offer of which the consummation would result in ownership by a person or group of 15% or more of the Company’s common stock. The Rights were redeemable for one cent per Right at the option of the Board of Directors prior to this event occurring. The Rights expired on November 14, 2009.
Effective October 19, 2009, the Company executed an Amended and Restated Rights Agreement (the “Rights Agreement”) between the Company and American Stock Transfer and Trust Company, LLC, as Rights Agent, amending and restating the Rights Agreement dated as of November 15, 1999 (the “Original Rights Agreement”). The purposes of this amendment of the Original Agreement include, among other things: to extend the expiration date of the Preferred Stock purchase rights issued from November 14, 2009 to November 14, 2019; to change how many new shares of common stock the Rights holders can purchase at a price of $100 per Right (the “Purchase Right”) after the 15% threshold is crossed from two times the number of the Company’s common stock that the Purchase Price is worth to five times the number of Company’s common stock that the Purchase Price is worth; to decrease the redemption price for Company-initiated redemption of the Rights from $0.01 to $0.0001.
| 9. | Stock Options and Restricted Stock |
Stock Options
1997 Stock Option Plan
The 1997 Stock Option Plan (the “1997 Option Plan”) was approved by the Company’s stockholders in 1997. Under the 1997 Option Plan, shares of common stock have been reserved for issuance to employees, officers, directors, and consultants of the Company and provides for the grant of incentive and nonstatutory stock options. The Board of Directors determines terms of the stock option agreements, including vesting requirements. The exercise price of incentive stock options must equal at least the fair market value on the date of grant. The options expire not later than ten years from the date of the grant and generally are exercisable ratably over a three-year or four-year period beginning one-year from the date of the grant.
2005 Equity Incentive Plan
In June 2005, the Company’s stockholders approved an amendment and restatement of the 1997 Option Plan to become the 2005 Equity Incentive Plan (the “2005 Equity Plan”). Options granted under the 1997 Option Plan prior to its amendment and restatement will continue to be subject to the terms and conditions set forth in the agreements evidencing such options and the terms of the 1997 Option Plan except that the Board may elect to
58
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
extend one or more of the features of the 2005 Equity Plan to stock awards granted under the 1997 Option Plan. The approval of the 2005 Equity Plan in June 2005 increased the number of shares reserved for issuance beyond those reserved for issuance under the 1997 Option Plan by 350,000 shares for a total of 5,500,000 reserved shares. The 2005 Equity Plan will allow the Company greater flexibility in designing equity incentives, including stock appreciation rights, stock purchase awards, restricted stock awards and restricted stock unit awards. In December 2005, the Board of Directors amended the 2005 Equity Plan to reserve an additional 100,000 shares to be used only for the grant of stock awards to persons not previously employed by the Company, or following a bona fide period of non-employment, as an inducement material to those persons entering into employment with the Company with the meeting of the Rule 4350(i)(1)(A)(iv) of the Nasdaq Marketplace Rules, and to provide that any such “inducement grants” must be granted either by a majority of the Company’s independent directors or a committee comprised of a majority of independent directors.
On March 18, 2006, the Board of Directors amended and restated the 2005 Equity Plan to reserve an additional 500,000 shares for issuance under the 2005 Equity Plan, which was subsequently approved by the Company’s stockholders in June 2006.
On March 30, 2007 the Board of Directors amended and restated the 2005 Equity Plan to reserve an additional 1,500,000 shares for issuance, for a total of 7,500,000 reserved shares and 100,000 inducement shares. The amendment was approved by the Company’s stockholders in June 2007. The approval of the amendment allows the Company to continue to grant stock options and other awards at levels determined appropriate by our Board of Directors.
On March 28, 2008, the Board of Directors amended and restated the 2005 Equity Plan to reserve an additional 800,000 shares for issuance under the 2005 Equity Plan, which was subsequently approved by the Company’s stockholders in June 2008.
59
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
The following table summarizes stock option activity under the Plan and the 2005 Equity Plan for 1997 through 2009 (in thousands, except per share amounts):
| Price Per Share | ||||||||
| Shares | Range | Weighted Average | ||||||
| 1997 |
||||||||
| Granted |
518 | $ | 6.75-8.70 | $ | 7.13 | |||
| Outstanding, December 31, 1997 |
518 | $ | 6.75-8.70 | $ | 7.13 | |||
| 1998 |
||||||||
| Granted |
341 | 13.25-16.75 | 14.52 | |||||
| Forfeited |
100 | 8.70 | 8.70 | |||||
| Outstanding, December 31, 1998 |
759 | $ | 6.75-16.75 | $ | 10.24 | |||
| 1999 |
||||||||
| Granted |
776 | 10.56-16.63 | 12.70 | |||||
| Forfeited |
61 | 14.06-14.63 | 14.63 | |||||
| Outstanding, December 31, 1999 |
1,474 | $ | 6.75-16.75 | $ | 11.36 | |||
| 2000 |
||||||||
| Granted |
774 | 6.50-15.06 | 8.18 | |||||
| Exercised |
1 | 6.75 | 6.75 | |||||
| Forfeited |
24 | 6.75-15.13 | 14.22 | |||||
| Outstanding, December 31, 2000 |
2,223 | $ | 6.50-16.75 | $ | 10.22 | |||
| 2001 |
||||||||
| Granted |
170 | 3.53-11.84 | 6.13 | |||||
| Forfeited |
65 | 5.09-16.63 | 13.31 | |||||
| Outstanding, December 31, 2001 |
2,328 | $ | 3.53-16.75 | $ | 9.80 | |||
| 2002 |
||||||||
| Granted |
696 | 5.15-10.10 | 9.48 | |||||
| Forfeited |
55 | 5.13-13.13 | 8.17 | |||||
| Outstanding, December 31, 2002 |
2,969 | $ | 3.53-16.75 | $ | 10.98 | |||
| 2003 |
||||||||
| Granted |
943 | 2.25-17.83 | 6.59 | |||||
| Exercised |
85 | 4.50-13.13 | 11.25 | |||||
| Forfeited |
66 | 4.00-16.75 | 12.17 | |||||
| Outstanding, December 31, 2003 |
3,761 | $ | 2.25-17.83 | $ | 8.88 | |||
| 2004 |
||||||||
| Granted |
596 | 8.54-15.20 | 13.69 | |||||
| Exercised |
4 | 3.53-5.29 | 3.75 | |||||
| Forfeited |
46 | 10.56-17.83 | 13.66 | |||||
| Outstanding, December 31, 2004 |
4,307 | $ | 2.25-17.83 | $ | 9.50 | |||
60
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
| Price Per Share | ||||||||
| Shares | Range | Weighted Average | ||||||
| 2005 |
||||||||
| Granted |
408 | 5.22-10.75 | 9.94 | |||||
| Exercised |
13 | 3.53-6.68 | 5.67 | |||||
| Forfeited |
56 | 5.29-10.47 | 8.06 | |||||
| Outstanding, December 31, 2005 |
4,646 | $ | 2.25-17.83 | $ | 9.57 | |||
| 2006 |
||||||||
| Granted |
965 | 4.43-7.08 | 5.67 | |||||
| Exercised |
6 | 2.25-5.29 | 3.86 | |||||
| Forfeited |
67 | 4.60-10.69 | 6.98 | |||||
| Outstanding, December 31, 2006 |
5,538 | $ | 2.25-17.83 | $ | 8.93 | |||
| 2007 |
||||||||
| Granted |
1,740 | 1.64-5.00 | 2.13 | |||||
| Exercised |
9 | 2.25 | 2.25 | |||||
| Forfeited |
1,607 | 2.25-16.75 | 8.29 | |||||
| Outstanding, December 31, 2007 |
5,662 | $ | 1.64-17.83 | $ | 7.02 | |||
| 2008 |
||||||||
| Granted |
1,067 | 0.65-16.63 | 2.07 | |||||
| Forfeited |
442 | 1.26-16.75 | 10.58 | |||||
| Outstanding, December 31, 2008 |
6,287 | $ | 0.65-17.83 | $ | 5.93 | |||
| 2009 |
||||||||
| Granted |
378 | 0.75-14.97 | 4.85 | |||||
| Forfeited |
3,645 | 0.75-16.63 | 6.16 | |||||
| Outstanding, December 31, 2009 |
3,020 | $ | 0.65-17.83 | $ | 5.52 | |||
| Shares (in thousands) |
Weighted- Average Exercise Price per Share |
Weighted- Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value (in thousands) | |||||||
| Outstanding, December 31, 2009 |
3,020 | $ | 5.52 | 4.74 | $ | 0 | ||||
| Exercisable on December 31, 2009 |
2,693 | $ | 5.92 | 4.39 | $ | 0 | ||||
As of December 31, 2009, the total remaining shares of common stock available for grant under the 2005 Equity Plan is 5,209,013 (which includes 66,000 shares under the inducement pool).
61
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
2005 Non-Employee Directors’ Equity Incentive Plan
The 2005 Non-Employee Directors’ Equity Incentive Plan (the “Non-Employee Directors Plan”) was approved by the Company’s stockholders in June 2006. Under the Non-Employee Directors Plan, 150,000 shares of common stock have been reserved for issuance to non-employee directors and provides for the grant of nonstatutory stock options, stock appreciation rights, stock purchase awards, restricted stock awards, restricted stock unit awards, and other forms of equity compensation. The Board of Directors determines terms of the stock awards, including vesting requirements. The exercise price of all options granted under the Non-Employee Directors Plan must equal at least the fair market value on the date of grant. The options expire not later than ten years from the date of the grant and generally are exercisable ratably during the option holder’s continued service period.
On March 18, 2006, the Board of Directors amended and restated the 2005 Non-Employee Director’s Equity Incentive Plan to reserve an additional 150,000 shares for issuance under the 2005 Non-Employee Director’s Equity Incentive Plan which was subsequently approved by the Company’s stockholders in June 2006.
On March 30, 2007 the Board of Directors amended and restated the 2005 Directors’ Plan to reserve an additional 150,000 shares for issuance for a total of 450,000 shares reserved. The Company’s stockholders approved the amendment in June 2007.
On March 28, 2008, the Board of Directors amended and restated the 2005 Non-Employee Director’s Equity Incentive Plan to reserve an additional 150,000 shares for issuance under the 2005 Non-Employee Director’s Equity Incentive Plan, which was subsequently approved by the Company’s stockholders in June 2008.
The following table summarizes stock option activity under the Non-Employee Directors Plan for 2005 – 2009 (in thousands, except per share amounts):
| Shares | Price Per Share | |||||||
| Range | Weighted Average | |||||||
| 2005 |
||||||||
| Granted |
30 | $ | 10.75 | $ | 10.75 | |||
| Outstanding, December 31, 2005 |
30 | $ | 10.75 | $ | 10.75 | |||
| 2006 |
||||||||
| Granted |
253 | $ | 5.43-11.75 | $ | 7.55 | |||
| Outstanding, December 31, 2006 |
283 | $ | 5.43-11.75 | $ | 7.89 | |||
| 2007 |
||||||||
| Granted |
75 | $ | 2.14 | $ | 2.14 | |||
| Forfeited |
30 | $ | 5.43-6.19 | $ | 5.81 | |||
| Outstanding, December 31, 2007 |
328 | $ | 2.14-11.75 | $ | 6.76 | |||
| 2008 |
||||||||
| Granted |
190 | $ | 1.62-10.75 | $ | 2.88 | |||
| Forfeited |
70 | $ | 1.62-10.75 | $ | 5.05 | |||
| Outstanding, December 31, 2008 |
448 | $ | 1.62-11.75 | $ | 5.39 | |||
| 2009 |
||||||||
| Forfeited |
190 | $ | 1.62-10.75 | $ | 4.01 | |||
| Outstanding, December 31, 2009 |
258 | $ | 1.62-11.75 | $ | 6.40 | |||
62
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
| Shares (in thousands) |
Weighted- Average Exercise Price per Share |
Weighted- Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value | |||||||
| Outstanding, December 31, 2009 |
258 | $ | 6.40 | 6.37 | $ | -0- | ||||
| Exercisable on December 31, 2009 |
239 | $ | 6.76 | 6.24 | $ | -0- | ||||
As of December 31, 2009, the total remaining shares of common stock available for grant under the 2005 Non-Employee Directors’ Equity Incentive Plan is 342,000 shares.
Non-Plan Options
During 1995 and 1996, the Company granted non-statutory stock options to purchase a total of 608,000 shares to directors, officers and consultants.
In February 1997, as part of an employment agreement, the Company granted a non-statutory stock option to an executive to purchase 2,400,000 shares of the Company’s common stock at a price of $5.00 per share, which vested ratably over a six-year period. The intrinsic value of the options was $1,848,000. As a result, the Company recorded as deferred compensation a non-cash charge of $1,848,000, which was being amortized ratably over the six-year vesting period. Through February 1999, the Company had amortized a total of $641,333. In March 1999, the Company announced the resignation of this executive, at which time the Company and the executive agreed that the option would remain outstanding for a total of 1,200,000 shares, including the acceleration of vesting of 400,000 shares. This acceleration is considered to be a new grant of options and, as such, the Company took a one-time non-cash charge of $4.9 million during the first quarter of 1999. No change was made to the terms of the option for the remaining 800,000 shares. In February 2008, 400,000 of the options were forfeited and in February 2009, the remaining 800,000 were forfeited.
In March 1999, the Company granted a non-statutory stock option to purchase 300,000 shares to an officer at an exercise price of $16.63. The options were forfeited on December 31, 2008.
On June 17, 2004, the Company granted stock options to purchase a total of 80,000 shares of common stock of the Company, at an exercise price of $11.75 per share, the fair market value of the date of grant, to two new directors. Options to purchase one-third of the total number of shares vest upon the first anniversary of the grant, and options to purchase the remaining shares vest in equal monthly installments over the following two years. At the direction of Nasdaq, with the agreement of the directors, these options were rescinded and cancelled in February 2006 and new options with the same terms were granted under the 2005 Non-Employee Directors’ Equity Incentive Plan. No compensation was recognized upon issuance of new options as the exercise price exceeded the stock price at the date of the new grant. The options were forfeited in May 2007.
On June 24, 2004, the Company granted stock options to purchase 50,000 shares of common stock of the Company, at an exercise price of $11.70 per share, the fair market value at the date of grant, to a new executive officer. Options to purchase one-fourth of the total number of shares vest upon the first anniversary of the grant, and options to purchase the remaining shares vest in equal monthly installments over the following three years. The options were forfeited in November 2006.
On September 20, 2004, the Company granted stock options to purchase 40,000 shares of common stock of the Company, at an exercise price of $10.79 per share, the fair market value at the date of grant, to a new executive officer. Options to purchase one-fourth of the total number of shares vest upon the first anniversary of
63
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
the grant, and options to purchase the remaining shares vest in equal monthly installments over the following three years. The options were forfeited in May 2007.
On August 1, 2007, the Company granted stock options to purchase 150,000 shares of common stock of the Company, at an exercise price of $1.66 per share, the fair market value at the date of grant, to a new executive officer. The options were forfeited in February 2008.
The following table summarizes stock option activity not pursuant to the Plan for 1995 through 2009 (in thousands, except per share amounts):
| Shares | Price Per Share | |||||||
| Range | Weighted Average | |||||||
| 1995 |
||||||||
| Granted |
38 | $ | 2.65-7.95 | $ | 4.64 | |||
| Outstanding, December 31, 1995 |
38 | $ | 2.65-7.95 | $ | 4.64 | |||
| 1996 |
||||||||
| Granted |
570 | 2.25 | 2.25 | |||||
| Outstanding, December 31, 1996 |
608 | $ | 2.25-7.95 | $ | 2.40 | |||
| 1997 |
||||||||
| Granted |
2,400 | 5.00 | 5.00 | |||||
| Forfeited |
50 | 2.25 | 2.25 | |||||
| Outstanding, December 31, 1997 |
2,958 | $ | 2.25-7.95 | $ | 4.51 | |||
| 1998 |
||||||||
| Exercised |
53 | 2.25-5.30 | 2.93 | |||||
| Forfeited |
50 | 2.25 | 2.25 | |||||
| Outstanding, December 31, 1998 |
2,855 | $ | 2.25-7.95 | $ | 4.58 | |||
| 1999 |
||||||||
| Granted |
300 | 16.63 | 16.63 | |||||
| Exercised |
10 | 7.95 | 7.95 | |||||
| Forfeited |
1,220 | 2.25-5.00 | 4.95 | |||||
| Outstanding, December 31, 1999 |
1,925 | $ | 2.25-16.63 | $ | 6.16 | |||
| Outstanding, December 31, 2000 |
1,925 | $ | 2.25-16.63 | $ | 6.16 | |||
| 2001 |
||||||||
| Exercised |
10 | 2.25 | 2.25 | |||||
| Outstanding, December 31, 2001 |
1,915 | $ | 2.25-16.63 | $ | 6.23 | |||
| Outstanding, December 31, 2002 |
1,915 | $ | 2.25-16.63 | $ | 6.23 | |||
| 2003 |
||||||||
| Forfeited |
165 | 2.25 | 2.25 | |||||
| Outstanding, December 31, 2003 |
1,750 | $ | 2.25-16.63 | $ | 6.60 | |||
64
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
| Shares | Price Per Share | |||||||
| Range | Weighted Average | |||||||
| 2004 |
||||||||
| Granted |
90 | $ | 10.79-11.70 | $ | 11.30 | |||
| Outstanding, December 31, 2004 |
1,840 | $ | 2.25-16.63 | $ | 6.83 | |||
| 2005 |
||||||||
| Granted |
28 | $ | 6.39-7.59 | $ | 7.00 | |||
| Exercised |
22 | 2.25 | 2.25 | |||||
| Outstanding, December 31, 2005 |
1,846 | $ | 2.25-16.63 | $ | 6.89 | |||
| 2006 |
||||||||
| Exercised |
28 | $ | 2.25 | $ | 2.25 | |||
| Forfeited |
220 | 2.25-11.70 | 3.10 | |||||
| Outstanding, December 31, 2006 |
1,598 | $ | 5.00-16.63 | $ | 7.49 | |||
| 2007 |
||||||||
| Granted |
150 | $ | 1.66 | $ | 1.66 | |||
| Forfeited |
84 | 7.59-11.70 | 10.58 | |||||
| Outstanding, December 31, 2007 |
1,664 | $ | 1.66-16.63 | $ | 6.81 | |||
| 2008 |
||||||||
| Forfeited |
850 | $ | 1.66-16.63 | $ | 8.52 | |||
| Outstanding, December 31, 2008 |
814 | $ | 5.00-6.39 | $ | 5.02 | |||
| 2009 |
||||||||
| Forfeited |
814 | $ | 5.00-6.39 | $ | 5.02 | |||
| Outstanding, December 31, 2009 |
— | — | — | |||||
| Shares (in thousands) |
Weighted- Average Exercise Price per Share |
Weighted- Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value (in thousands) | |||||
| Outstanding, December 31, 2009 |
— | — | — | — | ||||
| Exercisable on December 31, 2009 |
— | — | — | — |
65
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
For various price ranges, weighted average characteristics of outstanding stock options at December 31, 2009 were as follows:
| Outstanding options | Exercisable options | |||||||||||
| Range of |
Shares | Remaining life (years) |
Weighted average price |
Shares | Weighted average price | |||||||
| $ 0.65-$ 1.64 | 426,614 | 7.21 | $ | 1.60 | 303,103 | $ | 1.60 | |||||
| $ 1.66-$ 1.66 | 893,833 | 6.66 | $ | 1.66 | 725,391 | $ | 1.66 | |||||
| $ 1.68-$ 5.76 | 669,059 | 4.70 | $ | 5.21 | 619,518 | $ | 5.19 | |||||
| $ 5.88-$ 9.91 | 732,788 | 2.45 | $ | 7.66 | 728,239 | $ | 7.67 | |||||
| $10.15-$17.83 | 556,300 | 3.60 | $ | 12.70 | 556,300 | $ | 12.70 | |||||
| Balance as of 12/31/2009 |
3,278,594 | 4.87 | $ | 5.59 | 2,932,551 | $ | 5.99 | |||||
Options exercisable at December 31, 2009, 2008 and 2007 were 2,932,551, 5,411,313 and 5,138,358 at weighted average exercise prices of $5.99, $7.10 and $8.78, respectively.
The weighted average, estimated fair values of employee stock options granted during the fiscal years ended December 31, 2009, 2008 and 2007 were $0.15, $1.02 and $1.14 per share, respectively.
The total stock-based compensation expense included in our statement of operations for the fiscal years ended December 31, 2009, 2008 and 2007 was $1.3 million, $2.5 million and $3.2 million, respectively. Of the $1.3 million stock-based compensation expense in 2009, $1.2 million relates to awards granted prior to January 1, 2009.
As of December 31, 2009, the unrecognized stock-based compensation expense related to non-vested options and restricted shares was approximately $1.7 million which is expected to be recognized over a weighted average period of approximately 1.11 years. During the fiscal year ended December 31, 2009 since no options were exercised, the total intrinsic value of the stock options exercised was $0. The Company issues new shares of common stock upon the exercise of stock options.
Cash proceeds and the intrinsic value related to stock options exercised during the fiscal years 2009, 2008 and 2007 to date, are provided in the following table (in thousands):
| Fiscal year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Proceeds from stock options exercised |
$ | 0 | $ | 0 | $ | 20 | |||
| Tax benefit related to stock options exercised (1) |
NA | NA | NA | ||||||
| Intrinsic value of stock options exercised (2) |
$ | 0 | $ | 0 | $ | 28 | |||
| (1) | ASC Topic 718 requires that the excess tax benefits received related to stock option exercises be presented as financing cash inflows. The Company currently does not receive a tax benefit related to the exercise of stock options due to the Company’s net operating losses. |
| (2) | The intrinsic value of stock options exercised is the amount by which the market price of the stock on the date of exercise exceeded the market price of the stock on the date of grant. |
66
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Restricted Stock
The fair value of restricted stock is based on the trading price of the Company’s common stock on the date of grant. We issued restricted stock for the first time during 2006 to certain employees. Restricted stock activity is as follows:
| (Shares in thousands) |
Shares | Weighted- Average Grant Date Fair Value | |||
| Outstanding at beginning of year |
— | — | |||
| Granted |
68 | $ | 6.20 | ||
| Forfeited |
4 | $ | 6.20 | ||
| Outstanding December 31, 2006 |
64 | $ | 6.20 | ||
| Vested |
22 | $ | 6.20 | ||
| Forfeited |
12 | $ | 6.20 | ||
| Outstanding December 31, 2007 |
30 | $ | 6.20 | ||
| Vested |
12 | $ | 6.20 | ||
| Outstanding December 31, 2008 |
18 | $ | 6.20 | ||
| Vested |
9 | $ | 6.20 | ||
| Forfeited |
6 | $ | 6.20 | ||
| Outstanding December 31, 2009 |
3 | $ | 6.20 | ||
The market price of the common stock on the date of the grant was initially recorded as deferred compensation within the stockholders’ equity section of the Company’s balance sheet and subsequently is being amortized over the 4-year vesting period. During the fiscal years ended December 31, 2009, 2008 and 2007 there was approximately $82,900, $85,700 and $90,000 of compensation expense, respectively, was amortized and is included in general and administrative and research and development expense in the statement of operations.
| 10. | Common Stock Purchase Warrants |
Series A Warrants
During April 1996, in accordance with anti-dilution privileges triggered by an offering in March 1995, the Company issued 1,018,866 Series A Warrants to all stockholders of record as of March 1995 to purchase the same number of shares of common stock at a price of $11.02 per share. The warrants expired January 2002, except for one warrant for 393,250 shares, which expired January 7, 2006.
IAC Management Warrants
During April 1994, the Company issued warrants, to existing shareholders and management, to purchase 160,000 units (the “Units”) at $10.00 per Unit, each unit to be identical to the Units issued as part of its initial public offering. Each Unit consists of (i) one share of common stock, $.01 par value per share and (ii) one Class A Warrant entitling the holder to purchase one share of common stock at a price of $9.00 per share. All the warrants have expired.
67
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Representatives Warrants
In connection with the Company’s initial public offering, the Company issued warrants to the underwriters for 60,000 Units at an exercise price of $11.00 per Unit and 24,000 Class B Warrants at an exercise price of $5.775 per warrant and were exercisable until May 2000. Each Class B Warrant entitled the holder to purchase one Unit (i.e. one share of common stock and one Class A Warrant). The unexercised warrants have expired.
Investor Relations Warrant
During February 1998, as part of payment for services relating to investor relations, the Company issued a warrant to purchase 150,000 shares with an exercise price of $14.75 per share and an expiration date of February 1999. The warrant was estimated to have a value of $408,000, which was expensed in 1998. This warrant was exercised.
1998 Private Placement Warrants
In connection with the May 1998 private placement, the Company issued warrants to purchase 1,437,475 shares of common stock at an exercise price of $17.00 per share. The warrants were exercisable until May 2001. Of the warrants issued, 157,000 were issued as finders fees, and 1,280,475 were issued to the private placement investors. These warrants have expired.
1999 Agent Warrants
In connection with the January 1999, private placement, the Company issued warrants as a finders fee to purchase 90,000 shares of common stock at an exercise price of $18.25 per share. The warrants expired in January 2002.
1999 Consulting Warrant
During March 1999, the Company entered into a three-year agreement with a financial consulting organization affiliated with a director of the Company. The Company agreed to issue as compensation for services, a warrant to purchase 500,000 shares of common stock with an exercise price of $20.50 per share and an expiration date of March 2002. The warrant was not subject to any vesting provisions. The warrant was estimated to have a value of approximately $2.1 million, which was expensed as a non-cash charge during the first quarter of 1999. During 2001, the expiration date for this warrant was extended to March 2003.
During March 2003, the Company amended the consulting arrangement with the same financial organization affiliated with a director. The Company amended the warrant so that the warrant is now exercisable into an aggregate of 250,000 shares of common stock with an exercise price of $10.00 per share and an expiration date of the earlier of March 12, 2006 or thirty days after the consulting agreement is terminated. For accounting purposes, the original warrant was considered cancelled and a new warrant issued as a replacement resulting in a non-cash charge of approximately $0.8 million, which was expensed. The warrant expired without being exercised.
2001 Consulting Warrants
During April 2001, the Company issued warrants to purchase 25,000 shares of common stock at an exercise price of $3.09 per share. The warrants expired April 30, 2006. During July 2001, the Company issued warrants to purchase 25,000 shares of common stock at an exercise price of $6.225 per share. These warrants were
68
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
exercisable until July 31, 2006. These warrants, collectively, were issued for compensation for services and were estimated to have a combined value of approximately $208,000, which was expensed as a non-cash charge. Approximately 15% of these warrants were exercised and the remaining warrants have expired.
During the fourth quarter of 2001, the Company issued three-year warrants to purchase 16,870 shares of common stock with exercise prices ranging from $4.72 to $10.10 per share. The warrants have no vesting period and were issued in lieu of cash for services. An estimated value for these warrants of approximately $80,000 was expensed. The majority of these warrants have not been exercised. The unexercised warrants have expired.
2001 Private Placement Warrants
In connection with the December 2001 private placement, the Company issued warrants to purchase 128,000 shares of common stock to investors with an exercise price of $12.00. Warrants to purchase 68,329 shares of our common stock were exercised and the remaining warrants expired December 11, 2003.
As a finders fee, the Company issued two warrants to the placement agent for a total of 112,640 shares of common stock. One warrant has an exercise price of $9.00 per share and the other an exercise price of $12.00 per share. The value ascribed to these warrants based on the Black-Scholes pricing model was $1.5 million and was included as a charge to equity. These warrants to purchase 68,329 shares of our common stock were previously exercised and the remaining unexercised warrants expired in December 2006.
2002 Consulting Warrants
In March 2002, the Company agreed to issue a three-year warrant to a consultant, Dr. Joseph Hollis, to purchase up to 60,000 shares of common stock at an exercise price of $11.00 per share for services rendered in 2002. Dr. Hollis is the brother of Richard B. Hollis. This warrant expired with 50,000 shares unexercised.
During the fourth quarter of 2002, the Company issued a three-year warrant to purchase up to 10,000 shares of common stock at an exercise price of $4.54 per share. The warrants were issued in lieu of cash for consulting services performed for the Company during 2002. The unexercised warrants have expired.
All of the 2002 warrants were valued at a total of $247,000 using the Black-Scholes pricing model. The value of the warrants was expensed and is included in the 2002 operating expenses.
2003 Convertible Note and Warrants
On February 25, 2003, the Company completed a private placement in which the Company issued $10.0 million aggregate principal amount of three-year convertible debentures (“debentures”), bearing interest at 7.5% per year, and warrants to purchase up to 701,760 shares of common stock. The debentures are convertible into common stock at a price of $5.70 per share, which represented a discount from the price of common stock on the commencement date. Also issued in connection with this private placement were warrants to purchase up to 350,880 shares of common stock which are exercisable at a price per share of $6.17, subject to adjustment, and warrants to purchase up to 350,880 shares of common stock which are exercisable at a price per share of $6.71, subject to adjustment. Approximately half of these warrants have been exercised. The balance of these warrants expired February 2007.
In connection with the issuance of the debentures and warrants, the Company recorded approximately $3.5 million related to the beneficial conversion feature and approximately $3.0 million for the detachable warrants on
69
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
the debentures. The total amount of the deemed discount on the debentures as a result of the warrant issuance and the beneficial conversion feature amounts to $6.5 million. The beneficial conversion feature and warrant value (deemed discount) were amortized over the term of the debentures and as conversion of the debentures occurred.
The placement agent received a warrant to purchase 73,684 shares of common stock having an exercise price of $5.99 per share. The value ascribed to this warrant based on the Black-Scholes pricing model was $0.4 million and was expensed as a non-cash charge. This warrant expired in February 2008.
2003 Private Placement Warrants
In connection with the June 2003 private placement, the Company issued warrants to purchase 192,456 shares of common stock to investors with an exercise price of $15.45 per share. Approximately 13,000 warrants have been exercised and the remaining warrants expired in June 2007.
As a finders fee, the Company issued a warrant to the placement agent, for a total of 44,266 shares of common stock with an exercise price of $13.22 per share. The value ascribed to this warrant based on the Black-Scholes pricing model was $0.5 million and was charged to equity. This warrant expired in June 2008.
2004 Consulting Warrants
During 2004, the Company issued two two-year warrants to purchase up to a total of 12,000 shares of common stock at exercise prices of $10.15 and $11.75 per share. The warrants were issued for consulting services performed for the Company and expired during 2006.
The 2004 warrants were valued at a total of $108,280 using the Black-Scholes pricing model. The value of the warrants is amortized according to the vesting period which approximates the period over which the services are performed. In 2004, $102,860 was expensed and is included in the 2004 operating expenses. The additional $5,420 was expensed in 2005, over the remaining vesting period.
2005 Financing Warrants
In connection with the June 2005 subscription agreement with a single institutional investor, the Company issued a four-year warrant to purchase up to an additional 266,667 shares of common stock at an exercise price of $10.00 per share. The value ascribed to this warrant based on the Black-Scholes pricing model was $1.8 million and was charged to equity. This warrant expired in June 2009.
2006 Financing Warrants
In connection with the February 2006 financing agreement with institutional investors, the Company issued four-year warrants to purchase up to an additional 800,000 shares of common stock at an exercise price of $8.75 per share. The warrants became exercisable six months following issuance, and expired in February 2010.
In connection with the November 2006 financing agreement with institutional investors, the Company issued four-year warrants to purchase up to an additional 800,000 shares of common stock at an exercise price of $8.75 per share. The warrants became exercisable six months following issuance, and none have been exercised.
70
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
2006 Consulting Warrants
During 2006, the Company issued two two-year warrants to purchase up to a total of 14,000 shares of the Company’s common stock at exercise prices of $6.20 and $5.52 per share. The warrants were issued for consulting services performed for the Company. These warrants expired in September and December 2008.
The 2006 warrants were valued at a total of $40,320 using the Black-Scholes pricing model. The value of the warrants was amortized according to the vesting period that approximated the period over which the services were performed.
In December 2006, the Company issued a consulting warrant to purchase up to a total of 50,000 shares of the Company’s common stock at an exercise price of $5.52. The warrant was issued for consulting services performed for the Company. The warrant was valued at a total of $215,000 using the Black-Scholes pricing model. The warrant vested immediately and was expensed in 2006. The expense is included in the 2006 operating expenses. This warrant has a ten-year life and has not been exercised.
The following table summarizes stock warrant activity for 2006 through 2009 (in thousands, except per share amounts):
| Price Per Share | ||||||||
| Shares | Range | Weighted Average | ||||||
| Outstanding, December 31, 2005 |
1,652 | $ | 3.09-15.45 | $ | 9.96 | |||
| 2006 |
||||||||
| Issued |
1,664 | 5.52-8.75 | 8.63 | |||||
| Exercised |
10 | 3.09-6.23 | 3.22 | |||||
| Forfeited |
787 | 3.09-12.00 | 10.25 | |||||
| Outstanding, December 31, 2006 |
2,519 | $ | 5.52-15.45 | $ | 9.02 | |||
| 2007 |
||||||||
| Issued |
— | — | — | |||||
| Exercised |
— | — | — | |||||
| Forfeited |
470 | 6.17-15.45 | 9.91 | |||||
| Outstanding, December 31, 2007 |
2,049 | $ | 5.52-13.22 | $ | 8.81 | |||
| 2008 |
||||||||
| Issued |
— | — | — | |||||
| Exercised |
— | — | — | |||||
| Forfeited |
132 | 5.52-13.22 | 8.39 | |||||
| Outstanding, December 31, 2008 |
1,917 | $ | 5.52-10.00 | $ | 8.84 | |||
| 2009 |
||||||||
| Issued |
— | — | — | |||||
| Exercised |
— | — | — | |||||
| Forfeited |
267 | 10.00 | 10.00 | |||||
| Outstanding, December 31, 2009 |
1,650 | $ | 5.52-8.75 | $ | 8.65 | |||
71
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
For various price ranges, the following table summarizes the weighted average prices of outstanding warrants as of December 31, 2009 (in thousands, except per share amounts):
| Outstanding Warrants | Exercisable Warrants | |||||||||
| Range of Exercise Prices |
Shares |
Weighted average price |
Shares |
Weighted average price | ||||||
| $5.01-$ 8.00 |
50 | 5.52 | 50 | 5.52 | ||||||
| $8.01-$10.00 |
1,600 | 8.75 | 1,600 | 8.75 | ||||||
| Balance as of 12/31/2009 |
1,650 | $ | 8.65 | 1,650 | $ | 8.65 | ||||
| 11. | Employment Agreement |
Pursuant to an employment agreement between Hollis-Eden and Mr. Richard B. Hollis entered into in November 1996, as amended (the “Hollis Employment Agreement”), Mr. Hollis’ annual base salary was increased to $225,000 upon the consummation of the Merger, with bonuses, future salary increases and equity compensation as determined by the Hollis-Eden Pharmaceuticals Board of Directors. Effective January 1, 2008, Mr. Hollis’ base salary was increased for 2008 from $532,480 to $553,779. If Mr. Hollis’ employment is terminated “without cause,” “for insufficient reason” or pursuant to a “change in control” (as such terms are defined in the Hollis Employment Agreement), Mr. Hollis will receive as severance (i) an amount equal to five times his then current annual base salary plus five times the amount of the bonus awarded to him in the prior calendar year, (ii) immediate vesting of all unvested stock options of Hollis-Eden Pharmaceuticals (or the surviving corporation in a change in control, if applicable) held by him and (iii) continued benefits under all employee benefit plans and programs for a period of three years. All of such payments are to be made in one lump sum within 30 days of termination. If Mr. Hollis’ employment is terminated “with cause” or if Mr. Hollis resigns other than for “sufficient reason,” Mr. Hollis’ compensation and benefits will cease immediately and Mr. Hollis will not be entitled to severance benefits. Mr. Hollis was terminated with cause in March 2009.
| 12. | Leases |
Rental expenses for principal leased facilities under non-cancelable operating leases were approximately $1,349,000, $1,395,600 and $1,323,800 for 2009, 2008 and 2007 respectively. Future minimum payments for operating leases as of December 31, 2009 are as follows (in thousands):
| Operating Leases | |||
| 2010 |
$ | 202 | |
| 2011 |
3 | ||
| 2012 |
— | ||
| 2013 |
— | ||
| Total minimum lease payments |
$ | 205 | |
| 13. | Fair Value Measurement |
We adopted ASC Topic 820 as of January 1, 2008, for financial instruments measured at fair value on a recurring basis. ASC Topic 820 defines the fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the United States and expands disclosures about fair value measurements.
72
Table of Contents
HARBOR BIOSCIENCES, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC Topic 820 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). These tiers include:
| • | Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; |
| • | Level 2, defined as inputs other than quoted prices in active markets that are directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| • | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant value drivers are observable. |
We measure certain financial instruments at fair value on a recurring basis. Financial assets measured at fair value on a recurring basis are as follows at December 31, 2009.
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| In Thousands | ||||||||||||
| Money Market Accounts |
$ | 8,931 | $ | 0 | $ | 0 | $ | 8,931 | ||||
| Total |
$ | 8,931 | $ | 0 | $ | 0 | $ | 8,931 | ||||
| 14. | Supplementary Financial Data (Unaudited) |
Interim Financial Information
(Unaudited)
Quarterly and year-to-date computations of loss per share amounts are made independently. Therefore, the sum of the per share amounts for the quarter may not agree with the per share amounts for the year.
| Quarter - Ended | Total Year |
|||||||||||||||||||
| March | June | September | December | |||||||||||||||||
| (In thousands, except per share) | ||||||||||||||||||||
| Year Ended December 31, 2009 |
||||||||||||||||||||
| R&D operating expenses |
$ | 3,266 | $ | 2,583 | $ | 2,411 | $ | 1,663 | $ | 9,923 | ||||||||||
| G&A operating expenses |
1,755 | 1,241 | 738 | 716 | 4,450 | |||||||||||||||
| Non-cash charges |
342 | 462 | 270 | 248 | 1,322 | |||||||||||||||
| Net loss |
(5,295 | ) | (4,251 | ) | (3,413 | ) | (2,667 | ) | (15,626 | ) | ||||||||||
| Net loss per share |
(0.18 | ) | (0.14 | ) | (0.12 | ) | (0.09 | ) | (0.53 | ) | ||||||||||
| Cash and cash equivalents |
20,091 | 15,173 | 12,099 | 9,738 | 9,738 | |||||||||||||||
| Year Ended December 31, 2008 |
||||||||||||||||||||
| R&D operating expenses |
$ | 4,126 | $ | 4,173 | $ | 3,339 | $ | 3,454 | $ | 15,092 | ||||||||||
| G&A operating expenses |
1,484 | 1,400 | 1,128 | 1,013 | 5,025 | |||||||||||||||
| Non-cash charges |
553 | 665 | 598 | 674 | 2,490 | |||||||||||||||
| Net loss |
(5,748 | ) | (5,987 | ) | (4,855 | ) | (4,975 | ) | (21,565 | ) | ||||||||||
| Net loss per share |
(0.20 | ) | (0.21 | ) | (0.17 | ) | (0.17 | ) | (0.74 | ) | ||||||||||
| Cash and cash equivalents |
38,961 | 34,123 | 29,062 | 24,152 | 24,152 | |||||||||||||||
73
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Harbor BioSciences, Inc.
San Diego, California
We have audited the accompanying balance sheets of Harbor BioSciences, Inc. (the “Company”) (a development stage company) as of December 31, 2009 and 2008, and the related statements of operations, stockholders’ equity and cash flows for each of the years in the three-year period ended December 31, 2009 and for the period from inception (August 15, 1994) to December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Harbor BioSciences, Inc. as of December 31, 2009 and 2008, and the results of its operations and its cash flows for each of the years in the three year period ended December 31, 2009 and for the period from inception (August 15, 1994) to December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations resulting in accumulated net losses of approximately $252 million and has limited financial resources that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ BDO Seidman, LLP
San Diego, California
March 26, 2010
74
Table of Contents
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A(T). | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, the Company carried out an evaluation, under the supervision and with the participation of the Harbor BioSciences’ management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Exchange Act Rule 13a-15(e) 15d-15(e). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective in enabling the Company to record, process, summarize and report information required to be included in the Company’s periodic SEC filings within the required time period.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting for Harbor BioSciences, Inc. Harbor BioSciences Inc.’s internal control system was designed to provide reasonable assurance to Company management and board of directors regarding the preparation and fair presentation of published financial statements in accordance with accounting principles generally accepted in the United States (GAAP).
Management recognizes its responsibility for fostering a strong ethical climate so that the Company's affairs are conducted according to the highest standards of personal and corporate conduct.
The Company’s internal control over financial reporting includes those policies and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| • | provide reasonable assurance that transactions are recorded properly to allow for the preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and the Board of Directors of the Company; |
| • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company's assets that could have a material effect on the financial statements; and |
| • | provide reasonable assurance as to the detection of fraud. |
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Further, because of changing conditions, effectiveness of internal control over financial reporting may vary over time. The Company's processes contain self-monitoring mechanisms, and actions are taken to correct deficiencies as they are identified.
Management has assessed the effectiveness of Harbor BioSciences’ internal control over financial reporting as of December 31, 2009, based on the criteria for effective internal control described in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its assessment, management concluded that the Company's internal control over financial reporting was effective as of December 31, 2009.
This annual report on Form 10-K does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and
75
Table of Contents
Exchange Commission that permit the Company to provide only management’s report in this annual report on Form 10-K
Changes in Internal Control over Financial Reporting
There were no changes in Harbor BioSciences, Inc.’s internal controls over financial reporting that occurred during the quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information |
None.
76
Table of Contents
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
See the section entitled “Executive Officers and Senior Management” in Part I, Item 1 of this Annual Report on Form 10-K for information regarding executive officers and senior management.
The other information required by this Item 10 is set forth in Harbor BioSciences, Inc.’s definitive Proxy Statement which is expected to be filed with the Commission pursuant to Regulation 14A in connection with the Harbor BioSciences, Inc.’s 2009 Annual Meeting (the “Proxy Statement”) not later than 120 days after the end of our fiscal year ended December 31, 2009. This information is set forth in the Proxy Statement under the heading “Election of Directors” and is incorporated herein by reference to the Proxy Statement.
| Item 11. | Executive Compensation |
The information required by this Item 11 is set forth in the Proxy Statement under the heading “Executive Compensation” and is incorporated herein by reference to the Proxy Statement.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholders Matters |
The information required by this Item 12 is set forth in the Proxy Statement under the heading “Security Ownership of Certain Beneficial Owners an Management” and is incorporated by reference to the Proxy Statement.
The following table provides information as of December 31, 2009 with respect to all of our compensation plans under which we are authorized to issue equity securities of the company.
Equity Compensation Plan Information
| Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities in the first column) | ||||
| Stock option equity compensation plans approved by security holders |
3,319,188 | $ | 5.60 | 5,557,014 | |||
| Stock option equity compensation plans not approved by security holders |
— | — | — | ||||
| Warrant equity compensation plans not approved by security holders |
50,000 | $ | 5.52 | — | |||
| Total |
3,369,188 | 5,557,014 | |||||
The material features of each compensation plan or arrangement adopted without the approval of securities holders is included in Note 9 (“Stock Options—Non-Plan Options”) and Note 10 (“Common Stock Purchase Warrants”) in our Notes To Financial Statements.
Unregistered Sales of Equity Securities and Use of Proceeds
We made no unregistered sales of securities or repurchases of our securities during the quarter ended December 31, 2009.
77
Table of Contents
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item 13 is set forth in the Proxy Statement under the headings “Certain Transaction” and “Independence of the Board of Directors” and is incorporated by reference to the Proxy Statement.
| Item 14. | Principal Accountant Fees and Services |
The information concerning Principal Accountant Fees and Services is set forth in the Proxy Statement under the heading “Ratification of Selection of Independent Auditors” in the Proxy Statement which information is incorporated herein by reference.
78
Table of Contents
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
(a) The following documents have been filed as part of this Annual Report on Form 10-K:
| 1. | Financial Statements: The information required by this item is included in Item 8 of Part II of this report. |
| 2. | Financial Statement Schedules: Financial statement schedules required under the related instructions are not applicable for the three years ended December 31, 2009, and have therefore been omitted. |
| 3. | Exhibits: The exhibits listed in the Exhibit Index attached to this report are filed or incorporated by reference as part of this Annual Report. |
79
Table of Contents
SIGNATURES
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| HARBOR BIOSCIENCES, INC. | ||
| By: | /S/ JAMES M. FRINCKE | |
| James M. Frincke, President and Chief Executive Officer | ||
| Date: March 26, 2010 | ||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints ROBERT W. WEBER as his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments to this Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his substitute or substituted, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /S/ JAMES M. FRINCKE James M. Frincke |
President and Chief Executive Officer |
March 26, 2010 | ||
| /S/ ROBERT W. WEBER Robert W. Weber |
Chief Financial Officer and Secretary |
March 26, 2010 | ||
| /S/ JEROME M. HAUER Jerome M. Hauer |
Director |
March 26, 2010 | ||
| /S/ MARC R. SARNI Marc R. Sarni |
Director |
March 26, 2010 | ||
| /S/ SALVATORE J. ZIZZA Salvatore J. Zizza |
Director |
March 26, 2010 | ||
80
Table of Contents
INDEX TO EXHIBITS
| Exhibit |
Description of Document | |
| *3.1 | Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 4.1 to Registrant’s Registration Statement on Form S-4 (No. 333-18725), as amended (the “Form S-4”)). | |
| 3.2 | Bylaws of Registrant | |
| *3.3 | Certificate of Designation of Series B Junior Participating Preferred Stock (incorporated by reference to Exhibit 4.1 to Registrant’s Current Report on Form 8-K dated November 15, 1999). | |
| *3.4 | Certificates of Amendment of Certificate of Incorporation (incorporated by reference to Exhibit 3.4 to Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2001.) | |
| *4.1 | Amended and Restated Rights Agreement (the “Rights Agreement”) between the Company and American Stock Transfer and Trust Company, LLC, as Rights Agent, amending and restating the Rights Agreement dated as of November 15, 1999 (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K dated October 22, 2009). | |
| *†10.1 | Registrant’s 1997 Incentive Stock Option Plan (the “Option Plan”) as amended (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2002). | |
| *†10.2 | Forms of Incentive Stock Options and Nonstatutory Stock Options under the Option Plan (incorporated by reference to Exhibit 10.5 to the Form S-4). | |
| *†10.3 | Form of Nonstatutory Stock Options outside the Option Plan (including Annex I, identifying the officers and directors who are holders of such options and their respective option amounts and exercise prices), (incorporated by reference to Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2002). | |
| *†10.4 | Employment Agreement by and between Registrant and Richard B. Hollis dated November 1, 1996 (incorporated by reference to Exhibit 10.6 to the Form S-4). | |
| *†10.5 | Employment Agreement by and between Registrant and Robert W. Weber dated March 16, 1996 (incorporated by reference to Exhibit 10.9 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1998). | |
| *†10.8 | Nonstatutory Stock Option by and between Registrant and Terren S. Peizer effective as of February 6, 1997 (incorporated by reference to Exhibit 10.8 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2002). | |
| *†10.9 | Separation and Mutual Release Agreement by and between Registrant and Terren S. Peizer effective as of February 25, 1999 (incorporated by reference to Exhibit 10.10 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 1999). | |
| *†10.10 | Nonstatutory Stock Option by and between Registrant and Richard B. Hollis effective as of January 1, 1999 (incorporated by reference to Exhibit 10.10 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2002). | |
| *10.16 | Settlement and Mutual Release Agreement, dated January 20, 2000, among Registrant, Colthurst Limited, Edenland, Inc. and Patrick T. Prendergast (incorporated by reference to Exhibit 99.2 to Registrant’s Current Report on Form 8-K dated January 20, 2000). | |
| *10.17 | Technology Assignment Agreement, dated January 20, 2000, among Registrant, Colthurst Limited and Patrick T. Prendergast (incorporated by reference to Exhibit 99.3 to Registrant’s Current Report on Form 8-K dated January 20, 2000). | |
81
Table of Contents
| Exhibit |
Description of Document | |
| *10.18 | Common Stock and Warrant Agreement, dated January 20, 2000, among Registrant and Colthurst Limited (incorporated by reference to Exhibit 99.4 to Registrant’s Current Report on Form 8-K dated January 20, 2000). | |
| *10.19 | Warrant, dated January 20, 2000, issued to Colthurst Limited (incorporated by reference to Exhibit 99.5 to Registrant’s Current Report on Form 8-K dated January 20, 2000). | |
| *10.20 | Indemnification Agreement among Registrant and Executive Officers and Directors (incorporated by reference to Exhibit 10.17 to Registrant’s Registration Statement on Form S-1 (No. 333-69454). | |
| *10.26 | Sublease dated December 19, 2001 between Cooley Godward LLP and Registrant (incorporated by reference to Exhibit 10.16 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2001). | |
| *10.27 | Securities Purchase Agreement, dated as of February 25, 2003, by and between The Company and the purchasers identified therein (incorporated by reference to Exhibit 10.27 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
| *10.28 | Form of 7.5% Convertible Debenture issued to the purchasers listed on Schedule I attached thereto on February 25, 2003 (incorporated by reference to Exhibit 10.28 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
| *10.29 | Form of Stock Purchase Warrant issued to purchasers listed on Schedule I attached thereto on February 25, 2003 (incorporated by reference to Exhibit 10.29 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
| *10.30 | Registration Rights Agreement, dated February 25, 2003, by and between The Company and the purchasers identified therein (incorporated by reference to Exhibit 10.30 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
| *10.31 | Warrant, dated February 25, 2003, issued to SG Cowen Securities Corporation (incorporated by reference to Exhibit 10.30 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
| *10.34 | Warrant issued to SG Cowen Securities Corporation on June 19, 2003 (incorporated by reference to Exhibit 4.3 to the Registrant’s Registration Statement on Form S-3 (No. 333-106835)). | |
| *#10.35 | Study Funding Agreement, dated as of June 17, 2003, between the Registrant and Cystic Fibrosis Foundation Therapeutics, Inc (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003). | |
| *10.37 | Amended 401(k) Plan (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2003). | |
| *10.38 | First Amendment to Sublease dated December 19, 2001 between Cooley Godward LLP and Registrant (incorporated by reference to Exhibit 10.38 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2004). | |
| *10.39 | Form of Common Stock Purchase Warrant issued on June 1, 2005 (incorporated by reference to Exhibit 10.41 to the Registrant’s Current Report on Form 8-K dated June 2, 2005). | |
| *#10.40 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant entered into on December 3, 2003 (incorporated by reference to Exhibit 10.42 to the Registrant’s Amendment No. 1 on Form 10-Q/A to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005). | |
82
Table of Contents
| Exhibit |
Description of Document | |
| *#10.41 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant entered into on February 17, 2004 (incorporated by reference to Exhibit 10.43 to the Registrant’s Amendment No. 1 on Form 10-Q/A to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005). | |
| *#10.42 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated July 12, 2004 (incorporated by reference to Exhibit 10.44 to the Registrant’s Amendment No. 1 on Form 10-Q/A to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005). | |
| *#10.43 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated October 19, 2004 (incorporated by reference to Exhibit 10.45 to the Registrant’s Amendment No. 1 on Form 10-Q/A to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005). | |
| *#10.44 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated January 6, 2005 (incorporated by reference to Exhibit 10.46 to the Registrant’s Amendment No. 1 on Form 10-Q/A to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005). | |
| *#10.45 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated May 16, 2005 (incorporated by reference to Exhibit 10.47 to the Registrant’s Amendment No. 1 on Form 10-Q/A to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005). | |
| *†10.46 | 2005 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 (No. 333-130670), filed December 23, 2005). | |
| *†10.47 | Form of Option Agreement for use under 2005 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 (No. 333-130670), filed December 23, 2005). | |
| *†10.48 | Form of Restricted Stock Award Agreement for use under 2005 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 (No. 333-130670), filed December 23, 2005). | |
| *†10.49 | Form of Restricted Stock Unit Award Agreement for use under 2005 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 (No. 333-130670), filed December 23, 2005). | |
| *†10.50 | 2005 Non-Employee Directors’ Equity Incentive Plan, as amended (incorporated by reference to Exhibit 99.6 to the Registrants’ Registration Statement on Form S-8 (No. 333-130670), filed December 23, 2005). | |
| *†10.51 | Form of Option Agreement for use under 2005 Non-Employee Directors’ Equity Incentive Plan (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 (No. 333-130670), filed December 23, 2005). | |
| *#10.52 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated November 30, 2005 (incorporated by reference to Exhibit 10.52 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2005). | |
| *10.53 | Form of Stock Purchase Agreement dated as of February 2, 2006 (incorporated by reference to Exhibit 10.48 to Registrant’s Current Report on Form 8-K dated February 2, 2006) (the “February 2006 Purchase Agreement”). | |
83
Table of Contents
| Exhibit |
Description of Document | |
| *10.54 | Form of Stock Purchase Warrant, issued pursuant to the February 2006 Purchase Agreement (incorporated by reference to the Current Report on Form 8-K dated February 2, 2006). | |
| *10.55 | Form of Stock Purchase Agreement dated as of November 7, 2006 (incorporated by reference to Exhibit 10.49 to Registrant’s Current Report on Form 8-K dated November 7, 2006) (the “November 2006 Purchase Agreement”). | |
| *10.56 | Form of Common Stock Purchase Warrant, issued pursuant to the November 2006 Purchase Agreement (incorporated by reference to Exhibit 10.48 to Registrant’s Current Report on Form 8-K dated November 7, 2006). | |
| #10.58 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated December 11, 2006 (incorporated by reference to Exhibit 10.58 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2006). | |
| *†10.60 | Amendment to Employment Agreement dated as of December 7, 2007 between the Registrant and Richard B. Hollis (incorporated by reference to Exhibit 99.1 to the Registrant’s Current Report on Form 8-K Dated December 10, 2007). | |
| *†10.61 | Letter Agreement between Cystic Fibrosis Foundation Therapeutics, Inc. and the Registrant dated June 12, 2008 (incorporated by reference to Exhibit 10.61 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008). | |
| 23.1 | Consent of BDO Seidman, LLP. | |
| 31.1 | Rule 13a-14(a)/15d-14(a) Certification of James M. Frincke. | |
| 31.2 | Rule 13a-14(a)/15d-14(a) Certification of Robert W. Weber. | |
| 32.1 | Section 1350 Certifications of James M. Frincke and Robert W. Weber. | |
| * | Previously filed. |
| † | Management contract or compensatory plan, contract or arrangement to be filed as an exhibit pursuant to Item 14(c) of Form 10-K. |
| # | Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
84
