Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2009
| ¨ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT 1934 |
For the transition period from to
Commission file number: 000-53554
DAIS ANALYTIC CORPORATION
(Exact name of registrant as specified in its charter)
| New York | 14-1760865 | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) | |
| 11552 Prosperous Drive Odessa, Florida |
33556 | |
| (Address of Principal Executive Offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (727) 375-8484
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12 (g) of the Act:
Common Stock, par value $0.01 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405) during the preceding 12 months. Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act): Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $1,568,919 as of the last business day of the registrant’s most recently completed second fiscal quarter, based upon the closing sale price on the OTC:BB reported for such date. Shares of common stock held by each officer and director and by each person who owns 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 30, 2010, the Registrant had 29,095,717 outstanding shares of its common stock, $0.01 par value.
Documents incorporated by reference: none
Table of Contents
FORM 10-K
TABLE OF CONTENTS
2
Table of Contents
PART I
INTRODUCTORY NOTE
Information contained or incorporated by reference in this Annual Report may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. This information may involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from the future results, performance or achievements expressed or implied by any forward-looking statements. Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend” or “project” or the negative of these words or other variations on these words or comparable terminology.
This Annual Report on Form 10-K contains forward-looking statements, including statements regarding, among other things:
| • | our ability to continue as a going concern; |
| • | our ability to achieve and maintain profitability; |
| • | the price volatility of the common stock; |
| • | the historically low trading volume of the common stock; |
| • | our ability to manage and fund our growth; |
| • | the short period of time we have employed certain of our executive officers; |
| • | our ability to attract and retain qualified personnel; |
| • | litigation; |
| • | our ability to do business overseas; |
| • | our ability to compete with current and future competitors; |
| • | our short operating history; |
| • | our ability to obtain additional financing; |
| • | general economic and business conditions; |
| • | other risks and uncertainties included in the section of this document titled “Risk Factors”; and |
| • | other factors discussed in our other filings made with the Commission. |
These statements may be found under “Management’s Discussion and Analysis” and “Description of Business,” as well as in other sections of this Annual Report generally. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this Annual Report generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this Annual Report will in fact occur. We have no obligation to publicly update or revise these forward-looking statements to reflect new information, future events, or otherwise, except as required by applicable Federal securities laws, and we caution you not to place undue reliance on these forward-looking statements.
3
Table of Contents
| ITEM 1. | BUSINESS |
Dais Analytic Corporation is a nano-structure polymer technology materials company which has developed and is commercializing applications using its materials. The first commercial product is called ConsERV™, a fixed plate energy recovery ventilator which we believe is useful in meeting building indoor fresh air requirements while saving energy in the Heating, Ventilation and Air Conditioning (“HVAC”) industry. We are developing other nano-structure polymer technology products including (i) “NanoAir”, a water based packaged heating and cooling system and (ii) “NanoClear”, a water clean-up process useful in the creation of potable water from sea, brackish or waste water. We further believe that our nano-structure polymer technology may be useful in developing an ultra-capacitor, a device which may be capable of greater energy density and power per pound than traditional capacitors or batteries.
History
We were incorporated as a New York corporation on April 8, 1993 as Dais Corporation. We subsequently changed our name to Dais Analytic Corporation on December 13, 1999. We were formed to develop new, cost-effective polymer materials for various applications, including providing a lower cost membrane material for Polymer Electrolyte Membrane (“PEM”) fuel cells. We believe our research on materials science has yielded technological advances in the field of selective ion transport polymer materials.
In December 1999, we purchased the assets of Analytic Power Corporation, which was founded in 1984 to provide fuel cell and fuel processor design and consulting services, systems integration and analysis services to develop integrated fuel cell power systems, and we were re-named Dais Analytic Corporation. Analytic Power Corporation developed a portfolio of fuel cell and related fuel cell component technologies, including fuel cell stack designs, a membrane electrode assembly process, and natural gas, propane, diesel and ammonia fuel processors for use in creating integrated fuel cell systems.
4
Table of Contents
In March 2002, we sold substantially all of our fuel cell assets to a large U.S. oil company for a combination of cash and the assumption by such company of certain of our obligations. After we sold a substantial portion of our fuel cell assets, we focused on expanding our nano-structured polymer platform, having already identified the Energy Recovery Ventilator (“ERV”) application as our first commercial product.
Financing
From December 2007 to January 2008, we issued (i) 9% secured convertible promissory notes in an aggregate principal amount of $2,950,000 (the principal amount of which may be converted into 14,750,000 shares of common stock, par value $.01 per share) and (ii) warrants to purchase up to 14,750,000 shares of common stock at an exercise price of $.25 per share (the “Financing”). Aggregate gross proceeds to the Company from this Financing were $2,950,000. The notes issued in the Financing matured 12 months from the date of issuance and permitted conversion of all principal and interest into common stock at the option of the holder any time prior to the maturity date at a conversion price of $0.20 per share. The notes include standard default provisions and price protection with regards to issuance by the Company of common stock and common stock equivalents. The warrants issued in the Financing have a five-year term, cashless exercise provisions and anti-dilution protection. The anti-dilution protection in the warrants includes protection for stock dividends or splits, reclassification or capital reorganization as well as protection with regards to additional issuances of common stock or common stock equivalents. During the term each warrant holder has, upon full conversion or payment prior to the maturity date (as defined in the note), the right to exercise the warrant for all warrant shares. However, until such pay-off or full conversion of the note each holder is limited to the extent to which such holder may exercise the warrant to the same percentage to which the holder has converted the note. The exercise price under the warrants is $0.25 per share of common stock.
Pursuant to the terms of the Financing and the Security Agreement entered into in connection with the Financing, we granted investors a first priority security interest in our patents and certain patent applications. The following are each an event of default under the Security Agreement: (a) any material misrepresentation relating to the Security Agreement or the convertible notes on our part, (b) any material noncompliance with or nonperformance of our obligations under the notes or the Security Agreement, (c) if we make (i) an assignment for the benefit of creditors, or are subject to (ii) an attachment or receivership of assets that is not dissolved, or (iii) are subject to the institution of Bankruptcy proceedings, whether voluntary or involuntary, and (d) any event of default under the Notes. Should an event of default occur, the secured parties are required to provide us with written notice detailing the event of default. We have 60 days from receipt of said notice to cure such default. Should we fail to cure within the prescribed time period, the secured parties may at any time thereafter declare the notes in default and all obligations secured thereby immediately due and payable and shall have the remedies of a secured party under the Uniform Commercial Code. There are no other liens currently in effect against such patents or patent applications. The Company was obligated to register the shares of common stock underlying the convertible principal amount of the convertible notes and warrants issued in this Financing pursuant to a registration rights agreement entered into with investors in this Financing. On August 11, 2008, we filed a registration statement on Form S-1 covering the resale of up to 18,110,782 shares of common stock. Such registration statement was declared effective November 12, 2008
Between December 11, 2008 and January 21, 2009, all amounts due under the Convertible Notes matured and became due and payable in full. We have from time to time proposed and are currently proposing that holders of matured Convertible Notes either (i) convert their notes into shares at this time in exchange for additional warrants or (ii) extend the maturity of the Convertible Notes and continue to accrue interest.
During the year ended December 31, 2009 eighteen holders converted the principal balance of $2,350,000 plus accrued interest of $361,600 on their Convertible Notes into 13,553,822 shares of common stock. Some of the said note holders converted their Convertible Notes during periods in which we were offering an additional warrant as an inducement to convert. Under said offers we issued additional warrants to purchase 1,665,000 shares of common
5
Table of Contents
stock, exercisable immediately at $0.25 per share and valued at $126,367 (using the Black-Scholes pricing model), and 575,000 warrants, exercisable immediately at $0.75 per share valued at $286,641 (using the Black-Scholes pricing model) which was recorded as interest expense during the twelve months ended December 31, 2009.
During 2009, four investors holding Convertible Notes with an aggregate outstanding principal balance of approximately $450,000 at December 31, 2008 notified the Company that they were asserting their rights to receive payment of the principal and interest pursuant to the terms of the Convertible Notes. In June of 2009, three of these investors, holding an aggregate principal note balance of $250,000, entered into a confession of judgment with the Company. Under that agreement the three investors had the right, should the Company fail to pay all principal and interest due pursuant to their Convertible Notes on or before September 11, 2009, to file the confession of judgment with the court and seek to secure a judgment against the Company in the amount of all principal and interest due under their Convertible Notes together with the reasonable cost and expense of collection. All interest and principal related to the three Convertible Notes, $289,803 in the aggregate, was paid in full by the Company on or before September 11, 2009. In July of 2009, the fourth investor, holding a Convertible Note in the principal amount of $200,000, agreed to extend said note to September 2009. In November of 2009, this investor and the Company modified the Convertible note to extend the maturity date of said note to July 2010, pay the principal amount due in eight monthly installments commencing December of 2009, end the accrual of interest as of November 20, 2009 and convert the $34,861 in interest due under the Convertible Note as of November 20, 2009 into 170,137 shares of Company’s common stock. As of December 31, 2009 the outstanding principal balance of said loan was $175,000.
As of December 31, 2009, $325,000 of principal on the Convertible Notes was outstanding, of which $175,000 has been extended to July of 2010 with the remaining notes currently in default and due and payable in full.
In July 2009 we secured a loan of $300,000 from an investor and issued the lender an unsecured promissory note for the principal amount on December 8, 2009. Pursuant to the terms of the note, we are to pay the note holder simple interest at the rate of seven percent per annum commencing on July 17, 2009 with all interest and principal due there under payable in cash on or before January 16, 2011. If an event of default were to occur the interest rate would increase to ten percent for the duration of said event. Should we not cure the default within 60 days of receiving notice the note holder may, at his option, declare all interest accrued and unpaid and principal outstanding immediately due and payable.
In December 2009 we secured a loan in the principal amount of $1,000,000 from an investor and issued the lender an unsecured promissory note. Pursuant to the terms of the note, we are to pay the holder simple interest at the rate ten percent per annum commencing on the date of issuance with all interest and principal due and payable in cash on or before June 17, 2010. The note has equal standing with all other existing notes with respect to seniority. We may not incur more than $500,000 in additional debt without the holder’s prior approval and said additional debt may not be senior to this promissory note. During the term of the note, the holder has the right to participate, by investing additional funds the total amount of which may not exceed the outstanding balance of the note, in any subsequent financings undertaken by Company. Any such participation shall be upon the same terms as provided for in the subsequent financing. If an event of default were to occur and said default is not cured within the allotted period, the holder may declare all principal and accrued and unpaid interest due and payable without presentment, demand, protest or notice. Further, in addition to all remedies available under law, holder may in the event of a default opt to convert the principal and interest outstanding under the note into any debt or equity security which the Company issued after the date of this note and prior to the date of full payment of this note in accordance with the same terms as the subsequent financing.
The Company secured loans from two of its investors in the principal amounts of $250,000 and $620,000. The loan amounts were received by the Company on December 31, 2009 and February 18, 2010, respectively, and the Company issued the lenders unsecured promissory notes with respect to said loans on February 19, 2010. Pursuant to the terms of the notes, the Company is to pay the holders simple interest at the rate ten percent per annum
6
Table of Contents
commencing on the date of issuance with all interest and principal due and payable in cash on or before June 30, 2010 and August 10, 2010, respectively. The notes have equal standing with all other existing notes with respect to seniority. After receipt of proceeds on the foregoing loans, we may not incur more than $500,000 in additional debt without the holders’ prior approval and said additional debt may not be senior to these promissory notes. During the term of the notes, each note holder has the right to participate, by investing additional funds the total amount of which may not exceed the outstanding balance of the holder’s note, in any subsequent financings undertaken by Company. Any such participation shall be upon the same terms as provided for in the subsequent financing. If an event of default were to occur and said default is not cured within the allotted period, the holders may declare all principal and interest due and payable without presentment, demand, protest or notice. Further, in addition to all remedies available under law, each holder may in the event of a default opt to convert the principal and interest outstanding under its note into any debt or equity security which the Company issued after the date of its note and prior to the date of full payment of its note in accordance with the same terms as the subsequent financing.
Technology
We use proprietary nano-technology to reformulate thermoplastic materials called polymers. Nano-technology involves studying and working with matter on an ultra-small scale. One nanometer is one-millionth of a millimeter and a single human hair is around 80,000 nanometers in width. Polymers are chemical, plastic-like compounds used in diverse products such as Dacron, Teflon, and polyurethane. A thermoplastic is a material that is plastic or deformable, melts to a liquid when heated and to a brittle, glassy state when cooled sufficiently.
These reformulated polymers have properties that allow them to be used in unique ways. We transform polymers from a hard, water impermeable substance into a material which water and similar liquids can, under certain conditions, diffuse (although there are no openings in the material) as molecules as opposed to liquid water. Water and similar liquids penetrate the thermoplastic material at the molecular level without oxygen and other atmospheric gases penetrating the material. It is believed this selectivity is dependent on the size and type of a particular molecule.
Products
ConsERV™
We currently sell one product, our ConsERV™ product, a HVAC energy conservation product which should, according to various tests, save an average of up to 30% on HVAC operating costs and allow HVAC equipment to be up to 30% smaller, reducing peak energy usage by up to 20% while simultaneously improving indoor air quality. This product makes HVAC systems operate more efficiently and results, in many cases, in energy and cost savings. ConsERV™ attaches onto existing HVAC systems, typically in commercial buildings, to provide ventilation within the structure. It pre-conditions the incoming air by passing through our nano-technology polymer which has been formed into a filter of sorts. The nano-technology ‘filter’ uses the stale building air that must be simultaneously exhausted to transfer heat and moisture into or out of the incoming air. For summer air conditioning, the “core” removes some of the heat and humidity from the incoming air, transferring it to the exhaust air stream thereby, under certain conditions, saving energy. For winter heating, the “core” transfers a portion of the heat and humidity into the incoming air from the exhaust air stream thereby often saving energy.
Our ConsERV™ product is the primary focus of our resources and commercialization efforts. When compared to similar competitive products, we believe based on test results conducted by the Air-Conditioning, Heating and Refrigeration Institute (“AHRI”), a leading industry association, ConsERV™ is twice as effective in managing latent and sensible heat. We expect ConsERV™ to continue to be our focused commercial product through 2010 with a growing emphasis on moving the development of the NanoClear and NanoAir technologies towards commercialization.
7
Table of Contents
How ConsERV™ Works
Most building codes mandate commercial structures to provide certain levels of ventilation determined by use and occupancy. ERVs are systems used by HVAC manufacturers to increase energy efficiencies in HVAC units by transferring heat and humidity between air flows. They do this by capturing a portion of the energy already used to heat or cool air that is being released to the outside and use such released air to condition the incoming air stream. In an air conditioning application, heat and humidity that are part of the incoming air stream are transferred to the cool, dry exhaust air, thereby “pre-conditioning” the incoming air before it reaches the building’s air conditioning system. By pre-conditioning the incoming air, ERVs should increase the operating efficiency of the HVAC unit, thereby lowering the overall costs associated with heating and cooling buildings and potentially reducing the size and initial capital cost of the overall HVAC unit.
ConsERV™ has a “core” component made using our nano-structured material and may be described as a high-performance ERV. It is used in conjunction with a building’s HVAC equipment. The ConsERV™ energy recovery ventilator employs nano-technology based materials to create an exchange of sensible (temperature) and latent (humidity) energy between the two air streams using HVAC equipment to provide building ventilation. The first air stream typically exits a building at the temperature and relative humidity level set by the buildings air conditioning and heating equipment. The second air stream comes from the outside environment at a different temperature and relative humidity level and is used to bring outdoor air to the occupants of the building. The ConsERV™ product uses the energy found in the first air stream (air already cooler or heated) to condition the second air stream (the outdoor air coming in) before the second air stream (outside air) enters the HVAC equipment. The ConsERV™ product may save energy, in that it often reduces the required energy and size of the HVAC equipment and thereby may lower the cost of providing ventilation. In addition, it may lower carbon dioxide emissions because the HVAC equipment may not need to be used as frequently and often times can be reduced in size to provide the same levels of comfort indoors. The process is shown in the picture below.
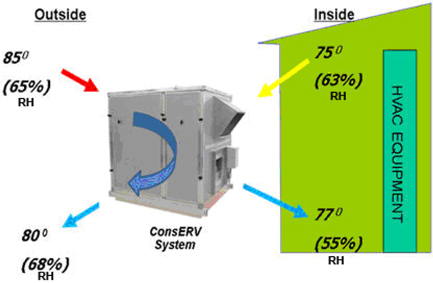
Given third-party test data, our ConsERV™ product, with its nano-structured materials, offers better total performance than other fixed plate ERV products of which we are aware, with no moving parts and little or no cross-air stream contamination.1 Our ConsERV™ core product has received UL 900 recognition and Air-Conditioning, Heating and Refrigeration Institute (“AHRI”) standard 1060 certification. Our ConsERV™ product is compatible with most commercial HVAC units and requires only a small amount of additional HVAC technical expertise to install. We believe the purchase and installation costs of our ConsERV™ product are comparable to the costs of many competing energy recovery products and our ConsERV™ product is more efficient in transferring moisture with lower life cycle maintenance costs.
| 1 | Air-Conditioning, Heating, and Refrigeration Institute (“AHRI”) – May 2008 test results. This study is publicly available and was not prepared for our benefit or funded by us. |
8
Table of Contents
Achieving increased sales revenue growth from our ConsERV™ product is predicated on the success in five key areas:
| • | Achieving continued technological improvements in key materials to lower our ‘per unit’ cost structure. |
| • | Implementing ‘Lean Manufacturing’ techniques for in-house assembly processes as well as monitoring existing outsourced manufacturing and assembly relationships which lower our ‘per unit’ cost structure. |
| • | Securing HVAC equipment manufacturers, as well as ERV Original Equipment Manufacturers (“OEM”) (or Licensees), with presence in existing and evolving sales channels as our customers or partners to sell worldwide “in-country or region”. |
| • | Recruiting and retaining the necessary people and infrastructure to support sales growth of ConsERV™ and other products as they are introduced into their respective sales channels. |
| • | Obtaining capital in a timely manner for the necessary steps outlined above to continue without interruption. |
Our Other Nano-Structured Products
We are devoting varying amounts of time to other uses of our nano-structured products in ways which are not disruptive to the key ConsERV™ effort. To date, insignificant revenues have been generated from these non-ConsERV™ related applications.
These product applications and activities include:
| • | Water Clean-up or “NanoClear”: We expect that this application would function to remove quantities of salt and other impurities from water to produce potable water using an environmentally friendly design that would use less energy and be less expensive than current methods. We have developed a functional table-top demonstration unit which highlights the basics of how this system works using the Company’s nano-structured materials to produce potable water. This demonstration unit is being used as the basis for the product’s next planned inflexion point: a 10m3 (26,500 gallons per day) pilot plant projected to be set up at a local County waste water treatment facility. The NanoClear product is currently in the late stages of prototype development. |
| • | Water Based packaged HVAC system or “NanoAir”: We expect this application would function to dehumidify and cool air in warm weather, or humidify and heat in cold weather. This NanoAir application may be capable of replacing a traditional refrigerant loop based heating/cooling system. The Company has a small prototype showing fundamental heating, cooling, humidification, and dehumidification operation of this evolving product. The NanoAir product is in the middle to late stages of prototype development. |
| • | Ultra-capacitor: Based on initial tests conducted by a third party, we believe that by applying a combination of our nano-materials we may be able to construct a device which stores energy similar to a battery with projected increases in energy density and lifetimes. We believe the key application for such a device would be in transportation. We have not invested significant resources to date in the development of this product. |
9
Table of Contents
As previously mentioned, aside from our ConsERV™ product, the Company has three additional products under development. The Company intends to sell polymer membrane or polymer membrane incorporated into an application appropriate form, or to license the application. We do not intend to build and market the entire product. The three product applications include:
| Application |
Current Stage |
Estimated Funding Required to Ready for Full Scale Commercialization 1 |
Estimated Time to Initial Market Entry (post funding) | |||
| Water Clean-up (NanoClean) - A process using a low temperature, low pressure approach to process brackish and salt water into potable water. |
3rd Stage Alpha/Beta | $8 Million | 12 – 30 months | |||
| Advanced Heating, Ventilating, and Air Conditioning (NanoAir)- A process using the nano-technology materials to create an advanced heating, ventilating, and air-conditioning system. |
3rd Stage Alpha/Beta | $6 Million | 12– 24 months | |||
| Ultracapacitor –if fully developed, may have a greater energy density and power per pound than traditional capacitors or the batteries on the market today. |
Base materials testing underway by third party to confirm the effectiveness of the Company’s materials in the application.
Current activities are moving us closer to the optimization of materials. An alpha prototype would then be required. |
$12 Million | 36 – 48 months | |||
The Company has identified other potential products for its materials and processes. Some have basic data to support the needed functionality and market differentiation of a product based on using our nano-technology based inventions. Such applications include immersion coatings and performance fabrics. These other products are based, in part, upon the known functionality of the Company’s materials and processes.
Patents
We own the rights to seven U.S. patents, five pending U.S. patent applications, and one Patent Cooperation Treaty (“PCT”) application. National stage applications based on the PCT application have been filed in the U.S., China and Europe. In addition, we co-own two PCT applications with Aegis Biosciences LLC, a biomaterials drug delivery technology company. National stage applications based on one of the co-owned PCT applications have been filed in the U.S., China and Europe, and a National stage application based on the other co-owned PCT application has been filed in the U.S. and China. These patents relate to, or are applications of, our nano-structured polymer materials that perform functions such as ion exchange and modification of surface properties. The polymers are selectively permeable to polar materials, such as water, in molecular form. Selective permeability allows these materials to function as a nano-filter in various transfer applications. These materials are made from base polymer resins available from a number of commercial firms worldwide and possess what we believe to be some unique and controllable properties, such as:
| • | Selectivity: Based on our research, we believe that when the polymer is made there are small channels created that are 5 to 30 nanometers in diameter. There are two types of these channels: hydrophilic (water permeable), and hydrophobic (water impermeable). The channels can be chemically tuned to be highly selective for the ions or molecules they transfer. The high selectivity of the polymer can be adjusted to efficiently transfer water molecules from one face to the other using these channels. |
| 1 | This step is defined as completing pilot or beta site performance and benchmark testing, obtaining (if needed) industry certification(s), and securing initial fundamentals to scale for production. |
10
Table of Contents
| • | High transfer rate: Based on in-house testing protocols and related results, we have found that the channels created when casting the materials into a nano-structured membrane have a transfer rate of water, or flux, greater than 90% of an equivalent area of an open tube. This feature is fundamental to the material’s ability to transfer moisture at the molecular level while substantially allowing or disallowing the transfer of certain other substances at a molecular level. |
| • | Unique surface characteristic: The materials offer a surface characteristic that we believe inhibits the growth of bacteria, fungus and algae and prevents adhesives from attaching. |
The molecular selectivity, transfer rate and surface coating properties, coupled with our ability to produce the nano-structured materials at what we believe is an affordable price, distinguishes our technology and value-added products. By incorporating our nano-structured materials into existing products, we strive to address current real-world market needs by offering what we believe to be higher efficiencies and improved price performance. For example, there are other energy recovery mechanisms available for HVAC that use coated paper or desiccant technology instead of our highly efficient nano-structured polymer materials.
Manufacturing
We do not have long term contractual relationships with any of our manufacturers or vendors. The only product or service which we could not have purchased elsewhere and used in the on-going growth of the ConsERV™ business is the plastic based sheet good. In progress is a project aimed at lessening the Company’s exposure in this sheet good area. All purchases to date of raw materials and related services have been on a purchase order basis using non-disclosure agreements. Our manufacturing process is described below.
Polymer Membrane
Commercially available styrene based polymer resin in flake form and industrial grade solvents are mixed together using a proprietary process involving heat, industrial mixers, and solvents. The resin and the solvents are commercially available from any number of chemical supply houses, or firms such as Dow and Kraton (formerly Shell Elastomers then part of Royal Dutch Shell). Our process changes the molecular properties of the starting styrene based polymer resins into a liquid material which we believe gives the attribute of being selective in what molecules it will allow through the plastic, which includes water molecules. This process, called ‘sulfonation’, is done at facilities around the world known as Toll Houses. These are firms which specialize in making small lot (by industry standards) runs of specialty chemicals.
Plastic Based Sheet Good
A thin coating of the liquid polymer material is applied on one side of the sheet good by a ‘tape casting’ firm of which there are many in the United States. The coated sheet good is heated to rapidly dry the liquid material thus bonding the polymer solution and rolled sheet good together. The resulting ‘modified sheet good’ is then re-coiled into rolls and shipped to us. Currently this is provided to us by one vendor. Additional vendors for this component have not been sought by the Company. However, we have identified other entities making similar types of products and believe such entities and products may provide alternatives should one be required. As noted above the Company is actively working one project to lower its exposure.
The “Core”
The modified sheet good is cut into defined dimensions and glued to a PVC formed spacer. This ‘spacer/glued modified sheet good’ is a single layer. Multiple layers are stacked one on top the other until a certain height is achieved. Once the proper height is achieved, these layers are then fitted with a galvanized sheet metal plate on the top and bottom of the stack along with galvanized sheet metal ‘Y’ shaped bracket on each of the four corners of the
11
Table of Contents
assembly. This assembly is called a ‘core’. The galvanized sheet metal is a world-wide commodity material formed to our specifications by local and out-of-town sheet metal forming companies. We have no long term contractual relationships with firms making the PVC spacers, supplying the glue, supplying rivets to hold the structure together, and the sheet metal firms making the top and bottom plate as well as the side rails.
Completion
For the complete ConsERV™ system, one or more cores are placed inside of aluminum or steel boxes built by us or a vendor. The box may or may not also be fitted with an electric motor, fan, electric relay, and electrical disconnect. Inclusion or exclusion of the electric motor and fan is dictated by the customers’ needs and current HVAC system. Once outfitted with cores, the product is complete. We have no long term contractual relationships with firms providing the aluminum or steel parts used to build the box, the motors, the fans, the relays, or the electrical disconnects.
Licensing
While we have earned licensing revenue under agreements licensing our technology in the past, we may not receive any material revenue from these agreements in the near or foreseeable future.
Research and Development
The Company has spent approximately $6,600 and $36,000 on research and development during each of the last two fiscal years.
Key Relationships
We have strategic relationships with leaders in the energy industry who have entered into sales, marketing, distribution and product development arrangements with us and, in some cases, hold equity in our Company. They include:
Electric Power Research Institute (“EPRI”)
We have an on-going relationship with a number of utilities through EPRI. The EPRI participants include Public Service Company of New Mexico, Kansas City Power & Light, Reliant Energy Incorporated, Alliant Energy Company, Omaha Public Power District, Wisconsin Public Service Corporation, Southern California Gas Company, EDF Electricite de France, Consolidated Edison of New York, Tokyo Gas Co., Ltd., CINERGY Corporation, Northern States Power Company, American Electric Power Company, Inc., Sierra Pacific Power Company, Public Service Electric & Gas Company (“PSE&G”), and Tennessee Valley Authority. The EPRI users group has been helpful in creating opportunities for us to define specifications and applications for our nano-structured materials that address existing energy related challenges while possibly opening new sources of revenue.
Genertec America, Inc.
On August 21, 2009, we entered into an Exclusive Distribution Agreement with Genertec America, Inc (“Genertec”), under which we are to supply and Genertec is to distribute, on an exclusive basis, three of our nanotechnology-based membrane products and related products in Great China, including main land China, Hong Kong, Macau and Taiwan. The agreement provides that during the initial term of the agreement, Genertec will order and purchase these products in the aggregate amount of Two Hundred Million U.S. Dollars. A minimum quantity of said products is to be purchased by Genertec during each contract year of the initial term. In the event Genertec fails to purchase the minimum amount of products in any given year, we may convert the exclusivity provided to Genertec to a non-exclusive or terminate the agreement. Genertec has agreed to engage and appoint authorized person(s) or firm(s), to install, engineer, perform maintenance, sell and use the products within the defined distribution area and neither Genertec nor its designated buyer is permitted to alter, decompile or modify our products in any way. As consideration for entering into this agreement, Genertec agreed to pay us a deposit in
12
Table of Contents
monthly installments beginning in September 2009 and continuing through April, 2010. Since September 2009, we have received three monthly deposit payments under this agreement. The third payment was received on December 14, 2009. All such payments are to be applied to products purchased by Genertec. During the initial term of the agreement, the parties are to negotiate in good faith a royalty bearing license agreement whereby Genertec may be granted a license to manufacture certain portions of the our products in the designated territory. The initial term of the agreement shall be for a period of five (5) years, commencing on August 21, 2009, unless earlier terminated. Unless notice of termination is delivered to the respective parties 180 days prior to the expiration of the initial term, the Agreement will automatically renew for consecutive one year periods. We may terminate this agreement in the event: (1) Genertec fails to pay the deposit as indicated, (2) Genertec does not purchase the minimum amount of our designated products during any contract year, (3) breach by Genertec of its obligations under the Agreement, or (4) at our discretion immediately upon the transfer of fifty percent (50%) or more of either the assets of the voting stock of Genertec to any third party. Genertec may not assign the Agreement to any party without our prior written consent.
ConsERV™ - Sales and Marketing Strategies
We market our ConsERV™ product in North America principally through alliances with local independent manufacturer representatives. We currently have 30 independent commercial sales representatives in various locations throughout North America selling the ConsERV™ product. We intend to increase the number of commercial independent sales representatives to approximately 40 to properly cover the North American commercial sales territory. We are also working to secure ongoing relationships with leading industry HVAC manufacturers and other ERV manufacturers. Other potential and targeted sales channels for the ConsERV™ product are energy service companies and HVAC product distributorships. We continue to leverage our relationship with EPRI and a group of 16 utility companies (consisting of EPRI members and some of our minority shareholders) into expected sources of future product sales through the introduction of demand reduction incentives. In January 2010, the Company hired David Longacre, who possesses 25 years of experience in the HVAC industry, as our Vice President of Sales.
Future Products – Sales and Marketing Strategies
Our intended sales and marketing strategy will require us to create alliances with companies having strong, existing channel presence in the target industries. We believe working with industry leaders at the development level allows us to better address the market’s needs and possibly accelerate the time to market cycle.
Competition and Barriers to Entry
We believe the efficacy of our value-added products and technology has the ability to decrease sales of competing products, thus taking business away from more established firms using older technology. We believe that our ConsERV™ product may become a functional component of newer, more efficient OEM products. Our key challenge is to educate channel decision makers of the benefits of products made using our materials and processes to overcome the strength of the current product sales.
There are a number of companies located in the United States, Canada, Europe and Asia that have been developing and selling technologies and products in the energy recovery industry, including but not limited to: Semco, Greenheck, Venmar, Bry-Air, dPoint, Renewaire and AirXchange.
We will experience significant competition regarding our products because certain competing companies possess greater financial and personal resources than us. Future product competitors include, but are not limited to:
| Products |
Current and Future Competitors | |
| ConsERV |
Semco, Greenheck, Venmar, Bry-Air, dPoint, Renewaire and AirXchange. | |
| NanoClear |
Dow, Siemens, , GE | |
| NanoAir |
AAON, Trane, Carrier, York, Hier | |
| Ultracapacitor |
EEstor Maxwell |
13
Table of Contents
We believe that the combination of our nano-material platform’s characteristics (high selectivity, high flux rate, manufacturability, et al.), growing patent position, and possible ‘first to market’ position, are competitive advantages, which may allow us time to execute our business plan. Competitors may experience barriers to entry in these markets primarily related to the lack of similarly performing proprietary materials and processes.
Intellectual Property
As stated above, we have seven granted U.S. patents, including patents covering the composition and structure of a family of ion conducting polymers and membranes and applications of the polymer. We believe some of these patents make reference to applications relating to the materials we are developing. Please see the “Risk Factors” Section of this Annual Report. A list of our existing patents follows:
| 1. | Patent No. 6,841,601- Cross-linked polymer electrolyte membranes for heat and moisture exchange devices. This patent was issued on January 11, 2005 and expires March 12, 2022. |
| 2. | Patent No. 6,413,298 – Water and ion-conducting membranes and uses thereof. This patent was issued on July 2, 2002 and expires July 27, 2020. |
| 3. | Patent No. 6,383,391 – Water and ion-conducting membranes and uses thereof. This patent was issued on May 7, 2002 and expires on July 27, 2020. |
| 4. | Patent No. 6,110,616 – Ion-conducting membrane for fuel cell. This patent was issued on August 29, 2000 and expires on January 29, 2018. |
| 5. | Patent No. 5,679,482 – Fuel Cell incorporating novel ion-conducting membrane. This patent was issued on October 21, 1997 and expires on October 20, 2014. |
| 6. | Patent No. 5,468,574 – Fuel Cell incorporating novel ion-conducting membrane. This patent was issued on October 21, 1995 and expires on May 22, 2014. |
| 7. | Patent No. 7,179,860 – Cross-linked polymer electrolyte membranes for heat, ion and moisture exchange devices. This patent was issued on February 20, 2007 and expires on March 11, 2022. |
We have provisional and patent applications in the following areas: Advanced Polymer Synthesis Processes, Reversible Liquid to Air Enthalpy Core Applications and Construction, Nanoparticle Ultra Capacitor and Water Treatment and Desalination.
The following is a partial list of the patent applications publicly visible:
| 1. | WO20080316678 – Nanoparticle Ultra Capacitor |
| 2. | WO/2008/039779 – Enhanced HVAC System and Method |
| 3. | WO/2008/089484 – Multiphase selective Transport Through a Membrane |
| 4. | WO/2008/141179 – Molecule Sulphonation Process * |
| 5. | WO/2009/002984 – Stable and Compatible Polymer Blends* |
| 6. | WO2009/002984 – Novel Coblock Polymers and Methods for Making Same |
| * | Patent applications jointly owned with Aegis Biosciences, LLC. |
Patents may or may not be granted on these applications. As noted above, two of these applications are jointly owned with Aegis Biosciences, LLC. We also seek to protect our proprietary intellectual property, including intellectual property that may not be patented or patentable, in part by confidentiality agreements with our current and prospective strategic partners and employees.
14
Table of Contents
Government Regulation
We do not believe the sale, installation or use of our nano-structured products will be subject to any government regulation, other than perhaps adherence to building codes, military specifications, and water safety regulations governing products used in HVAC, military clothing, immersion coatings, and water treatment and desalination. We do not believe that the cost of complying with such codes and regulations, to the extent applicable to our products, will be material.
We do not know the extent to which any existing or new regulations may affect our ability to distribute, install and service any of our products. Once our products reach the commercialization stage and we begin distributing them to our target markets, federal, state or local governmental entities may seek to impose regulations.
We are also subject to various international, federal, state and local laws and regulations relating to, among other things, land use, safe working conditions, and environmental regulations regarding handling and disposal of hazardous and potentially hazardous substances and emissions of pollutants into the atmosphere. Our business may expose us to the risk of harmful substances escaping into the environment, resulting in potential personal injury or loss of life, damage to or destruction of property, and natural resource damage. Depending on the nature of the claim, our current insurance policies may not adequately reimburse us for costs incurred in settling environmental damage claims, and in some instances, we may not be reimbursed at all. To date, we are not aware of any claims or liabilities under these existing laws and regulations that would materially affect our results of operations or financial condition.
Employees
As of December 31, 2009, we employed 15 full-time employees and one part time employee in our Odessa, Florida facility. Of the 16 employees, 4 are technicians, 2 product managers, 1 polymer chemist, 2 engineers, 1 development vice president, 2 administrative assistant, 1 administrator, 1 sales Vice President, 1 accountant and 1 president and chief executive officer. None of the employees are subject to a collective bargaining agreement. We consider our relations with our employees to be good.
Principal Offices
Our principal office is located at 11552 Prosperous Drive, Odessa, FL 33556.
| ITEM 1A. | RISK FACTORS |
You should carefully consider the risks described below. Our business, financial condition, results of operations or cash flows could be materially adversely affected by any of these risks. The valuation for the Company could also decline due to any of these risks, and you may lose all or part of your investment. This document also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of several factors, including the risks faced by us described below and elsewhere in this Annual Report. In assessing these risks, you should also refer to the other information contained in this Annual Report, including our financial statements and related notes.
Risks Related to Our Business
$325,000 of our 9% convertible secured promissory notes have matured, which may cause us to go out of business if Judgments are obtained.
At December 31, 2009, as a result of the Financing transaction, the Company had 3 note holders holding $325,000 in principal amount 9% convertible secured promissory notes together with the interest thereon outstanding. One of the note holders, whose note at year end accounted for $175,000 of the aforementioned aggregate principal amount, agreed to extend the term of his note to July 15, 2010. The two remaining notes have matured and remain
15
Table of Contents
outstanding. As of the date hereof the aggregate principal amount outstanding with respect to the two matured notes is $150,000. The Company has from time to time proposed and is currently proposing that holders of matured notes either (i) convert their notes into shares at this time in exchange for additional warrants or (ii) extend the maturity of the Convertible Notes and continue to accrue interest. If we are unable to reach an agreement with all the note holders and the note holders elect to obtain a judgment against the company, we may be forced out of business. The Company will find it difficult to continue its business, including financing its operations, where a default continues.
We may be unable to repay or secure an extension on the $2,170,000 in unsecured promissory notes outstanding as of the date hereof.
The Company does not currently have and does not expect to attain adequate funds for repayment from its current operations. Although the Company intends to continue to finance its operations, including the repayment of these notes, primarily through private sales of debt and equity securities it may not be able to secure additional financing to repay the notes on acceptable terms, if at all. Further, many of these notes contain a limitation on the amount of debt the Company can incur prior to repayment of the note. Hence, unless the note holders waive the debt limit we may not be able to avail ourselves of sufficient financing should it be available. Further many of these notes provide the holders with the option of participating in any additional financing we may undertake which could substantially dilute existing shareholders. If we are unable to secure financing to repay the notes we will seek to re-negotiate the notes. However, there is no guarantee that all note holders will accept any offer we may make and some or all of the note holders may request additional concessions from the Company for any accommodation we do secure. Any such additional consideration would likely be offered to all such note holders. Any terms we may be able to secure may not be favorable to the Company. Unfavorable terms would adversely impact our business, financial condition and/or results of operations. In the event we are unable to secure additional financing sufficient to pay the notes prior to the maturity date and we are not able to renegotiate the terms of the notes the note holders will have the option to foreclose which would likely result in the failure of our business or they may opt to convert the principal and interest outstanding under the note into any debt, equity or equity linked security which Company issued after the date of the note in accordance with the same terms as the subsequent financing which could result in substantial dilution to existing shareholders.
We may lose all our patents and the majority of our patent applications if we are unable to repay or convert the principal and interest on the outstanding 9% secured convertible notes issued in the Financing transaction or obtain an agreement for extension of the maturity dates.
The Company may not have adequate funds to repay the $325,000 of Convertible notes and does not expect to attain adequate funds for repayment from operations. The notes are secured by all of the Company’s patents and the majority of the Company’s patent applications. Our success depends, to a significant extent, on the technology that is incorporated in our product and the underlying patents and patent applications securing the notes. The Company intends to continue to finance its operations, including the repayment of the notes, primarily through private sales of debt and equity securities. The Company may not be able to secure additional financing to repay the notes on acceptable terms, if at all. There is no guarantee that all note holders will accept any offer the Company will make with regard to extending or converting these notes. Further, some note holders may request additional concessions from the Company in return for extending or converting their note. Any such additional consideration would likely be offered to all note holders. Any re-negotiated terms we may be able to secure may not be favorable to the Company. Unfavorable terms, in either a financing transaction or debt renegotiation, would adversely impact our business, financial condition and/or results of operations. In the event (i) we are unable to secure additional financing sufficient to pay the notes, (ii) the notes are not converted into shares of our common stock, or (iii) we are not able to negotiate extensions to the maturity dates of the notes, note holders will have the option to foreclose on all of our patents and those patent applications securing the notes, which would likely result in the failure of our business.
If we fail to raise additional capital we will be unable to continue our business.
Our commercialization and development efforts to date have consumed and will continue to require substantial amounts of capital in connection with our nano-technology material based products (including but not limited to ConsERV™, water desalination, immersion coatings, and performance fabrics). Our channel penetration and product development programs require substantial capital outlays in order to reach full product commercialization. As we enter into more advanced product development we will need significant funding to complete product development and to pursue product commercialization.
16
Table of Contents
Additionally, our auditors have expressed substantial doubt about our ability to continue as a going concern. Our ability to continue our business and our research, development and testing activities and commercialize our products in development is highly dependent on our ability to obtain additional sources of financing, including entering into and maintaining collaborative arrangements with third parties who have the resources to fund such activities. Any future financing may result in substantial dilution to existing shareholders, and future debt financing, if available, may include restrictive covenants or may require us to grant a lender security interest in our assets not already subject to an existing security interest. To the extent that we attempt to raise additional funds through third party collaborations and/or licensing arrangements, we may be required to relinquish some rights to our technologies or products currently in various stages of development, or grant licenses or other rights on terms that are not favorable to us. Any failure by us to timely procure additional financing or investment adequate to repay our outstanding promissory notes, including but not limited to the 9% convertible notes, and to fund our ongoing operations, including planned product development initiatives, clinical studies and commercialization efforts, will have material adverse consequences on our business operations, financial condition, results of operations and cash flows.
We have a history of operating losses, and we expect our operating losses to continue for the foreseeable future and we may not continue as a going concern.
We have incurred substantial losses since we were funded in 1993 and have not achieved profitability in any year to date. We have only one product developed and marketed, ConsERV™, and anticipate all other products will take at least 12 to 48 months to develop. We expect our operating losses to continue for the foreseeable future as we continue to expend substantial resources to expand the ConsERV™ business while working to bring additional products to the market including research and development, design and testing, obtaining third party validations, identifying and securing collaborative partnerships, and executing to enter into strategic relationships. Furthermore, even if we achieve our projection of selling a greater number of ConsERV™ units in 2010, we anticipate that we will continue to incur losses until we can cost-effectively produce and sell our products o a wider market. Our accumulated deficit was $32.0 million as of December 31, 2009. The reports from Cross, Fernandez and Riley, LLP, our independent registered public accounting firm, related to our December 31, 2009 financial statements contains their opinion that our net losses from operations, negative working capital and stockholder’s deficit raised substantial doubt about our ability to continue as a going concern. There has been no change in the Company’s position relative to the foregoing statements. It is possible that we will never generate sufficient revenue to achieve and sustain profitability. Even if we achieve profitability, we may not be able to sustain or increase profitability.
The Company financed its operations since inception primarily through private sales of its common and preferred stock, issuance of convertible promissory notes; issuance of unsecured promissory notes, cash received in connection with exercise of warrants, license agreements and the sale of certain fuel cell assets in 2002. As of December 31, 2009, the Company had $1,526,619 of current assets.
Even if the Company is successful in raising additional equity capital to fund its operations, the Company will still be required to raise an additional substantial amount of capital in the future to fund its development initiatives and to achieve profitability. The Company’s ability to fund its future operating requirements will depend on many factors, including the following:
| • | ability to obtain funding from third parties; |
| • | progress on research and development programs; |
| • | time and costs required to gain third party approvals; |
| • | costs of manufacturing, marketing and distributing its products; |
| • | costs of filing, prosecuting and enforcing patents, patent applications, patent claims and trademarks; |
17
Table of Contents
| • | status of competing products; and |
| • | market acceptance and third-party reimbursement of its products, if successfully developed. |
In the event the lease on our corporate office and production space is terminated, we may not be able to acquire a lease on another suitable property, or a lease on a suitable property at a comparable cost.
The lease on our corporate office and production space may be terminated upon 30 days prior written notice by either party. If this lease is terminated, we may not be able to acquire another lease for another suitable property or a lease on a suitable property at a comparable cost in a timely manner, which could materially disrupt our operations. Even if we are able to relocate into another suitable property at a comparable cost in a timely manner, we would incur significant moving expenses.
Our future indebtedness could adversely affect our financial health.
We have and may continue to incur a significant amount of indebtedness to finance our operations and growth. Any such indebtedness could result in negative consequences to us, including:
| • | increasing our vulnerability to general adverse economic and industry conditions; |
| • | requiring a portion of our cash flow from operations be used for the payment of interest on our debt, thereby reducing our ability to use our cash flow to fund working capital, capital expenditures and general corporate requirements; |
| • | limiting our ability to obtain additional financing to fund future working capital, capital expenditures and general corporate requirements; |
| • | limiting our flexibility in planning for, or reacting to, changes in our business; |
| • | placing us at a competitive disadvantage to competitors who have less indebtedness; and |
| • | as the majority of our assets are pledged to current debt holders, the failure to meet the terms and conditions of the debt instruments, or a failure to timely rearrange the current terms and conditions of the notes, if so required, will result in the Company having no access to certain portions of its own technology. |
The recent economic downturn has affected, and is likely to continue to adversely affect, our operations and financial condition potentially impacting our ability to continue as a going concern.
The recent economic downturn has resulted in a reduction in new construction and less than favorable credit markets, both of which may adversely affect the Company. Certain vendors from which we currently secure parts for our ConsERV product have and may continue to either reduce or eliminate payment terms. Hence, more capital is required to secure parts necessary to produce our products. In addition, our products are often incorporated in new construction which has experienced a decided down turn in project starts over the past year and such trend is expected for 2010. Although the portion of new construction most affected is home sales, which represents a minority of our sales, commercial construction has also experienced a reduction in starts with some projects being delayed and possibly eliminated. If the commercial construction market stagnates or decreases in volume or project size, our operations and financial condition could be negatively impacted. Expenditures under the United States economic stimulus plan appear to have targeted energy products. ConsERV™ may qualify under said program and the Company may potentially benefit from such program. However, when and if we will experience any increase in sales or investment due to this program is uncertain. In the interim, we may need additional capital to address these external conditions. An internal program to reduce personnel costs including the reduction of some salaries and limited furloughs was undertaken in 2009. Such a program may impact our ability to retain personnel and produce our products; however, we do not expect the impact of our internal program to be significant or long in duration. As noted above, we intend to continue to finance operations, including the repayment of all outstanding debt, including but not limited to the convertible notes and unsecured promissory notes, primarily through private sales of debt and equity securities. In light of the recent economic downturn the Company may not be able to secure additional financing on acceptable terms, if at all. Unfavorable terms for a financing transaction would adversely impact our business, financial condition and/or results of operations. In the event we are unable to secure additional financing our business may fail.
18
Table of Contents
If we fail to successfully address the challenges, risks and uncertainties associated with operating as a public company, our business, results of operations and financial condition would be materially harmed.
We have and will continue to incur a significant increase in costs as a result of operating as a public company, and our management has and will be required to devote substantial time to new compliance initiatives. Until November of 2008 we had never operated as a public company. In preparation for and since reporting as a public company, we have and expect to continue to incur significant legal, accounting and other expenses that we did not incur as a non-reporting company. In addition, the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), as well as new rules subsequently implemented by the Securities and Exchange Commission (the “SEC”) and various stock exchanges, has imposed many new requirements on public companies, including requiring changes in corporate governance practices. Our management and other personnel have and will continue to devote a substantial amount of time to these new compliance procedures.
We have and will continue to incur significant increased costs as a result of operation as a public company, and our management has and will continue to be required to devote substantial time to new compliance initiatives.
As a public company, we are now subject to the reporting requirements of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), the Sarbanes-Oxley Act and the rules promulgated by the SEC and the NASDAQ Global Market in response to the Sarbanes-Oxley Act. The requirements of these rules and regulations have and will continue to significantly increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and may strain our systems and resources. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls for financial reporting. In particular, commencing in 2010, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. We expect to incur significant expense and devote substantial management effort toward ensuring compliance with Section 404. As a result, management’s attention may be diverted from other business concerns, which could have a material adverse effect on our business, financial condition and results of operations.
Furthermore, we currently do not have an internal audit function, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal controls that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by SEC or other regulatory authorities, which would entail expenditure of additional financial and management resources.
These rules and regulations could also make it more difficult for us to attract and retain qualified independent members of our Board of Directors. Additionally, we have found these rules and regulations make it more difficult and more expensive for us to obtain director and officer liability insurance. We have, and may be required once again, to accept reduced policy limits and/or coverage or incur substantially higher costs to obtain the same or similar coverage. NASDAQ rules also require that a majority of our Board of Directors and all of the members of certain committees of the Board of Directors consist of independent directors. We cannot assure you that we will be able to expand our Board of Directors to include a majority of independent directors in a timely fashion to comply with the requirements of these rules.
19
Table of Contents
Our ConsERV™ product is in small volume production, we have no long term experience manufacturing our products on a commercial basis and may not be able to achieve cost effective large volume production.
Our ConsERV™ product is built in small volumes. Our ability to achieve commercial production of that product is subject to significant uncertainties, including: completion of necessary product automation, developing experience in manufacturing and assembly on a large commercial scale; assuring the availability of raw materials and key component parts from third party suppliers; and developing effective means of marketing and selling our product.
We are in the process of assembling our ConsERV™ product at our facility in Odessa, Florida. Initial production costs of this product line are high with no or a lower than desired profit margin. As a result, we believe we will need to reduce unit production costs, including the nano-structured materials themselves made to our specifications by third parties, over time in order to offer our products at a profitable basis on a commercial scale. Our ability to achieve cost reductions in all areas of nano-structured materials and value added products depends on entering into suitable manufacturing relationships with component suppliers, as well as increasing sales volumes so that we can achieve economies of scale. A failure to achieve a lower cost structure through economies of scale and improvements in engineering and manufacturing in a timely manner would have a material adverse effect on our business and financial results. There can be no assurance that we will obtain higher production levels or that the anticipated sales prices of our products will ever allow an adequate profit margin.
We may not be able to meet our product development and commercialization milestones.
We have established internal product and commercialization milestones and dates for achieving development goals related to technology and design improvements of our products. To achieve these milestones we must complete substantial additional research, development and testing of our products and technologies. Except for our ConsERV product, we anticipate that it will take at least 12 to 48 months to develop and ready for scaled production our other products. Product development and testing are subject to unanticipated and significant delays, expenses and technical or other problems. We cannot guarantee that we will successfully achieve our milestones. Our business strategy depends on acceptance by key market participants and end-users of our products.
Our plans and ability to achieve profitability depend on acceptance by key market participants, such as vendors and marketing partners, and potential end-users of our products. We continue to educate designers and manufacturers of HVAC equipment with respect to our ConsERV™ product. More generally, the commercialization of our products may also be adversely affected by many factors that are out of our control, including:
| • | willingness of market participants to try a new product and the perceptions of these market participants of the safety, reliability and functionality of our products; |
| • | emergence of newer, possibly more effective technologies; |
| • | future cost and availability of the raw materials and components needed to manufacture and use our products; |
| • | cost competitiveness of our products; and |
| • | adoption of new regulatory or industry standards which may adversely affect the use or cost of our products. |
Accordingly, we cannot predict with any certainty that there will be acceptance of our products on a scale sufficient to support development of mass markets for those products.
We are dependent on third party suppliers and vendors for the supply of key components for our products.
We are dependent on third parties to manufacture the key components needed for our nano-structured based materials and value added products made with these materials. Accordingly, a supplier’s failure to supply components in a timely manner, or to supply components that meet our quality, quantity and cost requirements, technical specifications, or the inability to obtain alternative sources of these components on a timely basis or on terms acceptable to us, would create delays in production of our products or increase unit costs of production. Certain of the components contain proprietary products of our suppliers, or the processes used by our suppliers to manufacture these components are proprietary. If we are required to replace any of our suppliers, while we should be able to obtain comparable components from alternative suppliers at comparable costs, this would create a delay in production. If we experience such delays or our third party suppliers and vendors fail to supply us with components that meet our quality, quantity, or cost standards, we may lose our customers or be subject to product liability claims.
20
Table of Contents
Our applications require extensive commercial testing and will take long periods of time to commercialize.
Our nano-structured materials and associated applications need to undergo extensive testing before becoming commercial products. Consequently, the commercialization of our products could be delayed significantly or rendered impractical. Moreover, much of the commercial process testing will be dependent on the efforts of others. Any failure in a manufacturing step or an assembly process may render a given application or our nano-structured material(s) unsuitable or impractical for commercialization. Testing and required development of the manufacturing process will take time and effort.
We have not devoted any significant resources towards the marketing and sale of our products, we expect to face intense competition in the markets in which we do business, and expect to rely, to a significant extent, on the marketing and sales efforts of third parties that we do not control.
To date, we primarily focused on the sale of the ConsERV™ and, since we have only sold limited quantities of our products, we have limited experience in the marketing and sale of products on a commercial basis. We expect that the marketing and sale of the ConsERV product will continue to be conducted by a combination of independent manufactures representatives, third-party strategic partners, distributors, or OEMs. Consequently, commercial success of our products will be dependent largely on the efforts of others. We intend to enter into additional strategic marketing and distribution agreements or other collaborative relationships to market and sell our nano-structured materials and value added product. However, we may not be able to identify or establish appropriate relationships in the future. Even if we enter into these types of relationships, we cannot assure you that the distributors or OEMs with which we form relationships will focus adequate resources on selling our products or will be successful in selling them. In addition, our chosen third-party distributors or OEMs may require us to provide volume price discounts and other allowances, customize our products or provide other concessions which could reduce the potential profitability of these relationships. To the extent any strategic relationships that we establish are exclusive, we may not be able to enter into other arrangements at a time when the distributor with which we form a relationship is not successful in selling our products or has reduced its commitment to marketing our products. Failure to develop sufficient distribution and marketing relationships in our target markets will adversely affect our commercialization schedule and, to the extent we enter into such relationships, the failure of our distributors and other third parties in assisting us with the marketing and distribution of our products may adversely affect our financial condition and results of operations.
We will face intense competition in the markets for our nano-structured materials and value-added products made from these materials. We will compete directly with currently available products, some of which may be less expensive. The companies that make these other products may have established sales relationships and more “name-brand” recognition in the market than we do. In addition, some of those companies may have significantly greater financial, marketing, manufacturing and other resources.
Our future results could be harmed by economic, political, regulatory and other risks associated with international sales and operations.
We intend to market, distribute and service our products on an international basis and expect to derive a significant portion of our revenue in coming years from international sales. If we fail to successfully sell our products internationally, our ability to increase our future revenue and grow our business would be impaired. We have limited experience developing, and no experience manufacturing, our products to comply with the commercial, regulatory and legal requirements of international markets. Our success in those markets will depend on our ability to secure relationships with foreign resellers and our ability to manufacture products that meet foreign regulatory and commercial requirements. In addition, our planned international operations could be harmed by a variety of factors, including but not limited to:
| • | difficulties in collecting international accounts receivable; |
| • | increased costs associated with maintaining international marketing efforts; |
21
Table of Contents
| • | compliance with potential United States Department of Commerce export controls; |
| • | increases in duty rates or other adverse changes in tax laws; |
| • | trade protection measures and import or export licensing requirements; |
| • | fluctuations in currency exchange rates; |
| • | political and economic instability in foreign countries; and |
| • | difficulties in securing and enforcing intellectual property rights, foreign (where filed and obtained) or domestic, and time and complexities of vetting and establishing relations with foreign resellers or licensees including but not limited to designing, validating and marketing a product geared specifically to a particular market segment. |
We depend on our intellectual property and failure to protect it could enable competitors to market products with similar features that may reduce demand for our products.
We currently have seven United States patents, six patent applications and co-own two patent applications, some of which apply to the composition and structure of a family of ion conducting polymers and membranes. These patents and patent applications often make reference to applications for, and in some instances, are application patents relating to materials we are developing. Our patent applications may or may not mature into issued patents.
Our success depends, to a significant extent, on the technology that is incorporated in our product. Although some of the inventions which we have obtained or applied for patent protection are no longer suitable for use with our planned products, we believe that some of the other inventions covered by the patents and patent applications are important to the success of our products. If we are unable to protect our intellectual property, competitors could use our intellectual property to market products similar to our products, which could reduce demand for our products. We may be unable to prevent unauthorized parties from attempting to copy or otherwise obtain and use our products or technology. Policing unauthorized use of our technology is difficult, and we may not be able to prevent misappropriation of our technology, particularly in foreign countries where the laws may not protect our intellectual property as fully as those in the United States. Others may circumvent trade secrets, trademarks and copyrights that we own or may own. Any such infringements, or any alleged infringements, could have a material adverse effect on our business, results of operations, and financial condition.
Any of the United States patents or foreign patents owned by us or subsequently issued to us may be invalidated, circumvented, challenged or rendered unenforceable. We may not be issued any patents as a result of our pending and future patent applications and any patents we are issued may not have the claim coverage sought by us or necessary to prevent others from introducing similar products. Any litigation surrounding our patent rights could force us to divert significant financial and other important resources away from our business operations.
Some of our intellectual property is not covered by any patent or patent application. We seek to protect this proprietary intellectual property, which includes intellectual property that may not be patented or patentable, in part by confidentiality agreements with our distributors and employees. These agreements afford only limited protection and may not provide us with adequate remedies for any breach or prevent other persons or institutions from asserting rights to intellectual property arising out of these relationships. In addition, we cannot assure you that these agreements will not be breached, that we will have adequate remedies for any such breach or that the parties to such agreements will not assert rights to intellectual property arising out of these relationships.
The members of our scientific advisory board are employed by entities other than us, some of which may compete with us. While we intend to enter into non-competition agreements with our scientific advisors, if any of them were to consult with or become employed by any of our competitors, our business could be negatively affected.
We have entered into agreements with various third parties that may affect our intellectual property rights.
We have entered into agreements with various third parties in connection with the development of various applications for our technology. Those agreements generally provide for the third party to own any resulting intellectual property rights and often provide for the grant of a license to us relating to those rights. We cannot assure you that the terms of those licenses will not limit our ability to apply such rights to specific applications in competition with the relevant third party, which may adversely affect our business.
22
Table of Contents
Our products employ technology that may unknowingly infringe on the proprietary rights of others, and, as a result, we could become liable for significant damages and suffer other harm.
We cannot assure you that our technologies and products do not or will not infringe on the proprietary rights of third parties or that third parties will not assert infringement claims against us in the future. We are aware of some patents in the nano-materials field held by potential competitors and other third parties. We cannot assure you that a third party will not claim infringement by us with respect to these patents, other patents or proprietary rights, or that we would prevail in any such proceeding. Any such infringement claim, whether meritorious or not, could:
| • | be time-consuming; |
| • | result in costly litigation or arbitration and the diversion of technical and management personnel, as well as the diversion of financial resources from business operations; |
| • | require us to develop non-infringing technology or seek to enter into royalty or licensing agreements; or |
| • | require us to cease use of any infringing technology. |
We may not be successful in developing non-infringing technologies. Royalty or licensing agreements, if required, may not be available on terms acceptable to us, or at all, and could significantly harm our business and operating results. A successful claim of infringement arising from the existence of a ‘submarine patent’ or another existing patent against us or our failure or inability to license the infringed or similar technology could require us to pay substantial damages and could harm our business. In addition, to the extent we agree to indemnify customers or other third parties against infringement of the intellectual property rights of others, a claim of infringement could disrupt or terminate their ability to use, market or sell our products.
We may not be able to control our warranty exposure, which could increase our expenses.
We currently offer and expect to continue to offer a warranty with respect to our ConsERV™ product and we expect to offer a warranty with each of our future products. If the cost of warranty claims exceed any reserves we may establish for such claims, our results of operations and financial condition could be adversely affected.
We may be exposed to lawsuits and other claims if our products malfunction, which could increase our expenses, harm our reputation and prevent us from growing our business.
Any liability for damages resulting from malfunctions of our products could be substantial, increase our expenses and prevent us from growing or continuing our business. Potential customers may rely on our products for critical needs, such as backup power. A malfunction of our products could result in warranty claims or other product liability. In addition, a well-publicized actual or perceived problem could adversely affect the market’s perception of our products. This could result in a decline in demand for our products, which would reduce revenue and harm our business. Further, since our products are used in devices that are manufactured by other manufacturers, we may be subject to product liability claims even if our products do not malfunction.
Our key employees are critical to our success and the loss of any key employees could impair our ability to execute our strategy and grow our business.
Our future success depends, to a significant extent, on the continued service of our executive officers and other key technical, sales and senior management personnel and their ability to execute our growth strategy, all of whom have non-compete agreements with the Company which may not withstand court review if litigation were to occur. The loss of the services of any of our senior level management or other key employees could harm our business. Our future performance will depend, in part, on our ability to retain management personnel and for our executive officers to work together effectively. Our executive officers may not be successful in carrying out their duties or running our Company. Any dissent among executive officers could impair our ability to make strategic decisions.
The Company has, when required, reduced the salaries of its employees. Such salary reductions may have an adverse effect on our ability to retain key employees.
23
Table of Contents
If we fail to attract, retain and motivate qualified employees, we may be unable to execute our business strategy.
Our future success will depend in part on our ability to attract and retain highly qualified individuals, including researchers, engineers, sales and marketing personnel and management. Competition for these individuals may become intense, and it may become increasingly difficult to attract, assimilate and retain these highly qualified persons. Competitors and others may attempt to recruit our employees. Should we experience attrition or need to augment our staff, the cost of securing personnel may be significantly higher than currently experienced and thus negatively impact our financial position.
Our failure to manage our growth could harm our business.
We may grow in the number of our employees, the size of our physical facilities and the scope of our operations. In addition, we intend to focus greater resource on ConsERV™ margins, sales/marketing activities and channel expansion, and marketplace education. Any expansion would likely place a significant strain on our senior management team and other internal and external resources. Furthermore, we may be required to hire additional senior management personnel. Our ability to manage growth will depend in part on our ability to continue to enhance our operating, financial and management information systems. Our personnel, systems and controls may be unable to support any growth we may experience and as a result, our financial results would suffer.
Any acquisitions we make could disrupt our business and harm our financial condition.
As part of our growth strategy we may review opportunities to acquire other businesses or technologies that would complement our products, expand the breadth of our target markets or enhance our technical capabilities. Acquisitions entail a number of risks that could materially and adversely affect our business and operating results, including but not limited to:
| • | problems integrating the acquired operations, technologies or products with our existing businesses and products; |
| • | constraints arising from increased expenses and working capital requirements; |
| • | constraints on our ability to incur debt; |
| • | dilution of our stock if we issue additional securities; |
| • | disruption of our ongoing business, diversion of capital and distraction of our management; |
| • | difficulties in retaining business relationships with suppliers and customers of acquired companies; |
| • | difficulties in coordinating and integrating overall business strategies, sales, marketing, research and development efforts; |
| • | potential liabilities in businesses and facilities acquired; |
| • | difficulties in maintaining corporate cultures, controls, procedures and policies; |
| • | difficulties evaluating risks associated with entering markets in which we lack prior experience; and |
| • | potential loss of key employees. |
Our revenue and operating results may fluctuate significantly as a result of factors outside of our control, which could cause the value of our Company to decline.
Unless and until we establish a predictable sales record for our products, we expect our revenue and operating results to vary significantly from quarter to quarter. As a result, quarterly comparisons of our financial results are not necessarily meaningful and you should not rely on them as an indication, in any manner, of our future performance. In addition, due to our stage of development, we cannot predict our future revenue or results of operations accurately. As a consequence, our operating results may fall below the expectations of investors, which could cause the valuation of our company to decline.
We expect to make significant investments in all areas of our business, particularly in research and product development and in expanding in-house or outsourced manufacturing capability. Because the investments associated with these activities are relatively fixed in the short-term, we may be unable to adjust our spending
24
Table of Contents
quickly enough to offset any unexpected shortfall in our revenue growth. In addition, because we are in the early stages of commercializing the ConsERV™ application and anticipate that it will take at least an additional 12 to 48 months to develop our other products for commercial sales, we expect our order flow to be uneven from period to period.
Risks Related to Our Industry
If our products fail to meet certain technical standards, we could be subject to claims, fines or other penalties and we may be curtailed from conducting our business operations.
Our nano-structured membrane products are designed for specific applications with specific technical objectives and standards. If these membranes, or the hardware device(s) used to make the membranes work, fail to meet those technical objectives and/or standards, we could be liable for potential personal injury, loss of life and damages (including consequential damages). Depending on the nature of the claim, our current insurance policies may not adequately reimburse us for costs incurred by reason of said claims, including, but not limited to, environmental damage claims, and in certain instances, we may not be reimbursed at all. Our business is subject to numerous federal, state and local laws, regulations and policies that govern environmental protection. These laws and regulations have changed frequently in the past and may continue to do so in the future. Our operations may not comply with such changes and we may be required to make significant unanticipated capital and operating expenditures to comply with such changes. If we fail to comply with any such applicable environmental laws and regulations, governmental authorities may seek to impose fines or other penalties on us or to revoke or deny the issuance or renewal of certain permits issued to us. Accordingly, we might be subject to damage claims or penalties, and we may be curtailed from conducting our business operations.
We could be liable for environmental damages resulting from our research, development and manufacturing operations.
Our business may expose us to the potential risk of harmful substances escaping into the environment, resulting in potential personal injury or loss of life, damage to or destruction of property, and natural resource damage. Depending on the nature of the claim, our current insurance policies may not adequately reimburse us for costs incurred in settling environmental damage claims, and in certain instances, we may not be reimbursed at all. Our business may be or become subject to numerous federal, state and local laws, regulations and policies that govern environmental protection. These laws and regulations have changed frequently in the past and may continue to do so in the future. Our operations may not comply with such changes and we may be required to make significant unanticipated capital and operating expenditures to comply with such changes. If we fail to comply with applicable environmental laws and regulations, governmental authorities may seek to impose fines or other penalties on us or to revoke or deny the issuance or renewal of certain permits issued to us. Accordingly, we might be subject to damage claims or penalties, and we may be curtailed from conducting our business operations.
Future government regulation may impair our ability to market and sell our products.
Our current and planned products are potentially subject to federal, state, local and foreign laws and regulations governing, among other things, emissions to air as well as laws relating to occupational health and safety. As these products are introduced commercially, it is possible that governmental authorities will adopt new regulations that will limit or curtail our ability to market and sell such products. We may also incur substantial costs or liabilities in complying with such new governmental regulations. Our potential customers and distributors, some of which operate in highly regulated industries, may also be required to comply with new laws and regulations applicable to products such as ours, which could adversely affect their interest in our products.
Alternatives to our technology could render our systems obsolete prior to commercialization.
Our nano-structured materials and their identified uses are one of a number of products being developed today as potential answers to perceived market needs such as additional water sources, energy and emissions savings with regard to HVAC operation, alternative energy storage and “clean” power sources. Improvements are also being made to the existing products. Technological advances in all fields and improvements in key targeted application areas with existing or different new technology may render our nano-structured material approach obsolete before or during commercialization.
25
Table of Contents
Risks Related to Investment in Our Securities
Future issuances or the conversion or exercise, as applicable, of our outstanding convertible securities and warrants could result in substantial dilution to the interests of our shareholders and downward pressure on the price of our common stock.
The issuance of any shares of our common stock, either pursuant to the conversion or exercise of our outstanding convertible securities and warrants or pursuant to any other agreement entered into by us, including but not limited to the existing unsecured promissory notes, or which may be entered into by us in the future, may result in substantial dilution to the interests of holders of our common stock. The sale of our shares of common stock in the market could cause the market price of our common stock to decline as a result of the increased supply of shares, which could in turn cause you to lose a portion of your investment. Such depression in the value of our common stock could also reduce or eliminate amounts that would otherwise have been available to pay dividends on our common stock or to make distributions upon our liquidation.
Furthermore, shares owned by our current shareholders, to the extent they may be transferred without registration pursuant to applicable exemptions under the Securities Act of 1933, as amended (the “Securities Act”) may be sold. Because of the perception by the investing public that a sale by such insiders may be reflective of their own lack of confidence in our prospects, the market price of our common stock could decline as a result of a sell-off following sales of substantial amounts of common stock, including but not limited to any such sales by our officers, directors and 5% or more beneficial owners, into the public market, or the mere perception that these sales could occur.
Our stock price is likely to be volatile.
Our common stock has been quoted on the Pink OTC Markets, Inc.’s Pink Sheets since November 15, 2005 and the Over the Counter Bulletin Board since November 24, 2008. The market price of our common stock has been and will likely continue to be subject to fluctuations. In addition, the stock market in general and the market for technology companies in particular, have from time to time experienced significant price and volume fluctuations that have been often unrelated or disproportionate to the operating performance of such companies. These broad market and industry factors may cause our common stock to materially decline, regardless of our operating performance. In the past, following periods of volatility in the stock market and the market price of a particular company’s securities, securities class action litigation has often been instituted against that company. Litigation of this type could result in substantial legal fees and other costs, potential liabilities and a diversion of management’s attention and resources.
We have not and do not intend to pay dividends on our common stock.
The payment of dividends upon our capital stock is solely within the discretion of our board of directors and dependent upon our financial condition, results of operations, capital requirements, restrictions contained in our future financing instruments and any other factors our board of directors may deem relevant. We have never declared or paid a dividend on our common stock and, because we have very limited resources, we do not anticipate declaring or paying any dividends on our common stock in the foreseeable future. Rather, we intend to retain any future earnings for the continued operation and expansion of our business. It is unlikely, therefore, that the holders of our common stock will have an opportunity to profit from anything other than potential appreciation in the value of our common shares held by them.
Our executive officers and directors have significant shareholdings, which may lead to conflicts with other shareholders over corporate governance matters.
As of March 30, 2010, our directors and officers, as a group, beneficially own approximately 31.6% of our outstanding common stock, including shares of common stock issuable upon exercise of warrants and options. Acting together, these shareholders would be able to significantly influence all matters that our shareholders vote upon, including the election of directors, mergers or other business combinations.
26
Table of Contents
As a company quoted on the OTC Bulletin Board, we are not subject to any minimum listing criteria or other eligibility requirements.
Companies that are listed on a national securities exchange, such as the NASDAQ Stock Market, American Stock Exchange or New York Stock Exchange, must meet certain qualitative and quantitative listing criteria, such as they must meet requirements with respect to operating results, net asset thresholds, corporate governance, trading price and minimums for their public float. Companies that are quoted on the OTC Bulletin Board, while not subject to listing requirements per se, must be registered with the SEC under Section 13 or 15(d) of the Exchange Act, and must remain current in their reporting requirements in order to remain eligible for quotation. As we are quoted on the OTC Bulletin Board, we do not need to meet minimum listing criteria and the information available regarding us, our financial condition, business or operations, may be more limited than those companies listed on a national securities exchange. Therefore you may find it more difficult to obtain accurate quotations as to the price of our securities or dispose of securities which you own.
Our securities are characterized as “microcap stock”, and are subject to a number of unique risks.
The term “microcap stock” applies to companies with low or “micro” capitalizations, meaning the total value of the company’s stock. Our securities are characterized as “microcap stock”, and are subject to a number of unique risks. Many microcap companies tend to be new and have no proven track record. Some of these companies have limited or no assets or operations. Others have products and services that are still in development or have yet to be tested in the market. Another risk that pertains to microcap stocks involves the low volumes of trades. Because microcap stocks trade in low volumes, any size of trade can have a large percentage impact on the price of the stock. While all investments involve risk, microcap stocks can be among the most risky.
Unless an active trading market develops for our securities, shareholders may have difficulty or be unable to sell their shares of common stock.
Our common stock is currently quoted on the OTC:BB under the symbol “DLYT.” However, currently there is not an active trading market for our common stock, meaning that the number of persons interested in purchasing shares of our common stock at or near ask prices at any given time may be relatively small or non-existent, and there can be no assurance that an active trading market may ever develop or, if developed, that it will be maintained. There are a number of factors that contribute to this situation, including, without limitation, the fact that we are a small development-stage company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven, development-stage company such as ours or purchase or recommend the purchase of shares of our common stock until such time we become more seasoned and viable.
As a consequence, our stock may be characterized by a lack of liquidity, sporadic trading, larger spreads between bid and ask quotations, and other conditions that may affect shareholders’ ability to re-sell our securities. Moreover, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. Unless an active trading market for our common stock is developed and maintained, shareholders may be unable to sell their common stock and any attempted sale of such shares may have the effect of lowering the market price of our common stock and a shareholder’s investment could be a partial or complete loss.
Since our common stock is thinly traded, it is more susceptible to extreme rises or declines in price and shareholders may not be able to sell their shares at or above the price paid.
Since our common stock is thinly traded, its trading price is likely to be highly volatile and could be subject to extreme fluctuations in response to various factors, many of which are beyond our control, including:
| • | the trading volume of our shares; |
27
Table of Contents
| • | the number of securities analysts, market-makers and brokers following our common stock; |
| • | new products or services introduced or announced by us or our competitors; |
| • | actual or anticipated variations in quarterly operating results; |
| • | conditions or trends in our business industries; |
| • | announcements by us of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | additions or departures of key personnel; |
| • | sales of our common stock; |
| • | general stock market price and volume fluctuations of publicly-quoted, and particularly microcap, companies; and |
| • | material legal action. |
Shareholders, including but not limited to those who hold shares as a result of the exercise or conversion of our outstanding convertible securities and warrants, may have difficulty reselling shares of our common stock, either at or above the price paid, or even at fair market value. The stock markets often experience significant price and volume changes that are not related to the operating performance of individual companies, and because our common stock is thinly traded it is particularly susceptible to such changes. These broad market changes may cause the market price of our common stock to decline regardless of how well we perform as a company. In addition, and as noted below, our shares are subject to the penny stock regulations. Price fluctuations in such shares are particularly volatile and subject to manipulation by market-makers, short-sellers and option traders.
Our common stock is subject to the “penny stock” regulations, which are likely to make it more difficult to sell.
Our common stock is considered a “penny stock,” which generally is a stock trading under $5.00 and not registered on a national securities exchange. The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. These rules generally have the result of reducing trading in such stocks, restricting the pool of potential investors for such stocks, and making it more difficult for investors to sell their shares once acquired. Prior to a transaction in a penny stock, a broker-dealer is required to:
| • | deliver to a prospective investor a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market; |
| • | provide the prospective investor with current bid and ask quotations for the penny stock; |
| • | explain to the prospective investor the compensation of the broker-dealer and its salesperson in the transaction; |
| • | provide investors monthly account statements showing the market value of each penny stock held in their account; and |
| • | make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. |
These requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that is subject to the penny stock rules. Since our common stock is subject to the penny stock rules, investors in our common stock may find it more difficult to sell their shares.
Our listing has moved to the OTC Bulletin Board as of November 24, 2008, subjecting us to additional regulatory requirements which may negatively affect our common stock’s trading price.
On November 24, 2008, the Company moved its listing to the OTC Bulletin Board where the trading price of our common stock has been and is expected to remain below $5.00 per share. As a result of this exchange relocation, trading in our common stock is subject to the requirements of certain rules promulgated under the Exchange Act. These rules require additional disclosure by broker-dealers in connection with any trades generally involving any non-NASDAQ equity security that has a market price of less than $5.00 per share, subject to certain exceptions. Such rules require the delivery, before any penny stock transaction, of a disclosure schedule explaining the penny stock market and the risks associated therewith, and impose various sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally institutions).
28
Table of Contents
For these types of transactions, the broker-dealer must determine the suitability of the penny stock for the purchaser and receive the purchaser’s written consent to the transaction before sale. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our common stock. As a consequence, the market liquidity of our common stock could be severely affected or limited by these regulatory requirements.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
We currently lease a 7,200 square feet of combined office and production space located at 11552 Prosperous Drive, Odessa, FL 33556. We lease the site from Ethos Business Ventures, LLC, a limited liability company in which our Chief Executive Officer, Timothy N. Tangredi, has a controlling financial interest.
The lease for our corporate headquarters began on March 18, 2005. The lease term will terminate upon 30 days’ written notice from either party. The current monthly rent is $3,800 per month. We also pay all taxes and utilities as well as most repairs relating to our office. Most of the Company functions are performed at this site including corporate, marketing, administration, on-going product and nano-structured polymer development, and product assembly and shipping. Key polymer synthesis and casting is out-sourced and not done at this facility.
We do not anticipate investing in real estate or interests in real estate, real estate mortgages, or securities of or interests in persons primarily engaged in real estate activities. We currently have no formal investment policy and do not intend to undertake investments in real estate as a part of our normal operations.
| ITEM 3. | LEGAL PROCEEDINGS |
We are not currently a party to any pending legal proceedings. In the ordinary course of business, we may become a party to various legal proceedings generally involving contractual matters, infringement actions, product liability claims and other matters.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
At the 2009 Annual Meeting of Stockholders, held on November 12, 2009, the stockholders elected the following individuals as directors, to serve until the 2010 Annual Meeting of Stockholders, and until their successors are elected and qualified:
| Name |
Votes For |
Votes Withheld | ||
| Timothy N. Tangredi |
16,263,298 | 327,720 | ||
| Raymond Kazyaka, Sr. |
16,213,298 | 377,720 | ||
| Robert W. Schwartz |
16,167,698 | 423,320 | ||
Also, at the 2009 Annual Meeting of Stockholders the stockholders:
| • | Approved the amendment of the Company’s Certificate of Incorporation to increase the amount of the Company’s authorized shares from 110,000,000 comprised of 100,000,000 shares of common stock, par value $.01 per share (the “Common Stock”) and 10,000,000 shares of preferred stock to 210,000,000 shares, comprised of 200,000,000 shares of Common Stock and 10,000,000 shares of preferred stock. There were 13,577,299 votes cast for the amendment, 102,688 votes cast against the amendment, and 12,000 abstentions. |
| • | Adopted the Company’s 2009 Long-Term Incentive Plan. There were 13,580,519 votes cast for adoption of the plan, 67,468 votes cast against adoption of the plan, and 44,000 abstentions. |
| • | Ratified and approved (i) a five year warrant to purchase 3,000,000 shares of Common Stock previously issued to Timothy N. Tangredi, the Company’s President, Chief Executive Officer and Chairman of the Board and (ii) a five-year warrant to purchase 250,000 shares of Common Stock previously issued to Scott Ehrenberg, the Company’s Chief Technology Officer and Secretary. There were 13,125,264 votes cast for ratification and approval of the warrants, 89,933 votes cast against ratification and approval of the warrants, and 476,790 abstentions. |
29
Table of Contents
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock was traded from November 15, 2005 to November 23, 2008 on the Pink Sheets and from November 24, 2008 to present on the Over the Counter Bulletin Board under the trading symbol “DLYT.” The following table sets forth the range of reported high and low sales prices of our common stock during the periods indicated. Such quotations reflect prices between dealers in securities and do not include any retail mark-up, mark-down or commission, and may not necessarily represent actual transactions. Trading in our common stock should not be deemed to constitute an “established trading market.”
| High | Low | |||||
| For the year ending December 31, 2009: |
||||||
| First Quarter |
$ | 0.20 | $ | 0.08 | ||
| Second Quarter |
$ | 0.19 | $ | 0.13 | ||
| Third Quarter |
$ | 2.60 | $ | 0.10 | ||
| Fourth Quarter |
$ | 0.95 | $ | 0.22 | ||
| For the year ending December 31, 2008: |
||||||
| First Quarter |
$ | 0.51 | $ | 0.15 | ||
| Second Quarter |
$ | 0.51 | $ | 0.24 | ||
| Third Quarter |
$ | 0.45 | $ | 0.16 | ||
| Fourth Quarter |
$ | 0.20 | $ | 0.07 | ||
Transfer Agent
Our transfer agent is Clear Trust Transfer located at 17961 Hunting Bow Circle, Suite 102, Lutz, Florida 33558, telephone (813) 235-4490.
Holders
As of March 30, 2010 there were approximately 184 shareholders of record of our common stock.
Dividend Policy
We have not declared or paid any dividends and do not intend to pay any dividends in the foreseeable future to the holders of our common stock. We intend to retain future earnings, if any, for use in the operation and expansion of our business. Any future decision to pay dividends on common stock will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors our board of directors may deem relevant.
30
Table of Contents
Equity Compensation Plan Information
The following table sets forth information regarding our 2000 Incentive Compensation Plan (the “2000 Plan”) and the 2009 Long-Term Incentive Plan (the 2009 Plan”) under which our securities are authorized for issuance as of December 31, 2009
| Plan Category |
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
(b) Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans Excluding Securities Reflected in Column (a) | ||||
| Equity compensation plans approved by security holders: |
12,348,882 | $ | 0.26 | 15,000,000 | |||
In June 2000 and November 2009, our board of directors adopted, and our shareholders approved, the 2000 Plan and 2009 Plan respectively (together the “Plans”). The Plans provide for the grant of stock options, incentive stock options, stock appreciation rights, restricted stock, restricted stock units and bonus stock and other awards to eligible persons, as defined in said plans, including, but not limited to, officers, directors and employees of the Company. Certain awards under the Plans may be subject to performance conditions.
Number of Shares of Common Stock Available Under the Plans. As of December 31, 2007, our board of directors approved and made available 6,093,882 shares of common stock to be issued pursuant to the 2000 Plan. Subsequently, our board of directors approved and made available an additional 5,000,000 shares of our common stock for issuance under the 2000 Plan. The 2000 Plan permits grants of options to purchase common shares authorized and approved by the Company’s Board of Directors and shareholders for issuance prior to the enactment of the 2000 Plan. On November 5, 2009, our board of directors approved and made available a total of 15,000,000 shares of common stock to be issued pursuant to the 2009 Plan.
Administration of the Plans. The Plans are administered by a committee of two or more directors designated by the board of directors to administer the Plans (the “Committee”) or, in the absence of such Committee, by the board of directors. Currently, the Plans are administered by our board of directors. The board of directors has the authority to select the participants to whom awards under Plans will be granted, grant awards, determine the type, number and other terms and conditions of, and all other matters relating to, awards granted under the Plans and to prescribe the rules and regulations for the administration of the Plans. No option or stock appreciation rights granted under the Plans shall be exercisable, however, more than ten years after the date of the grant.
Exercise Price. The Plans require the Committee to grant qualified options with an exercise price per share not less than the fair market price of a share of common stock on the date of grant of the option.
Transferability. Awards granted under the Plans are generally not transferable by the optionee otherwise than by will or the laws of descent and distribution and generally exercisable during the lifetime of the optionee only by the optionee.
Change in Control. All awards granted under the 2000 Plan which were not previously exercisable and vested shall become fully exercisable and vested upon a change of control of the Company, which includes the consummation of a merger or consolidation of the Company with or into any other entity, sale of all or substantially all of our assets, replacement of a majority of our board of directors, acquisition by any person of securities representing 20% or more of the voting power of our then outstanding securities (other than securities issued by us) or any other event which the board of directors determines would materially alter our structure or ownership.
31
Table of Contents
Options Granted to Non-Employee Directors. Non-employee directors of the Company are usually granted options each year, which generally become exercisable upon the date of grant, and generally expire on the earlier of ten years from the date of grant or up to three years after the date that the optionee ceases to serve as a director.
Stand-Alone Grants
Our board of directors may grant common share purchase options or warrants to selected directors, officers, employees, consultants and advisors in payment of goods or services provided by such persons on a stand-alone basis outside of any of our Plans. The terms of these grants may be individually negotiated.
RECENT SALES OF UNREGISTERED SECURITIES- USE OF PROCEEDS
We issued securities and reported these issuances, which were not registered under the Securities Act of 1933, as amended (the “Securities Act”) in our Forms 10-Q for the quarters ended March 31, 2009, June 30, 2009, and September 30, 2009. For the quarter ended December 31, 2009, we issued the following securities without registration under the “Securities Act”, pursuant to exemption from registration under Section 4(2) and Regulation D of the Securities Act:
In October 2009, four investors elected to convert their 9% secured convertible notes and the related accrued interest in the amounts of $174,349, $638,693, $28,859 and $57,989 into 871,746, 3,193,466, 144,295 and 289,945 shares of the Company’s Common Stock, respectively. Said investors also received an additional five-year warrant to purchase up to 75,000, 275,000, 12,500 and 25,000 shares, respectively, of Common Stock, at an exercise price of $0.75 per share in consideration for converting their 9% secured convertible note. The warrant is immediately exercisable and subject to adjustment for standard anti-dilution events. The Common Stock was issued pursuant to exemption from registration under Section 4(2) of the Securities Act.
Recent Repurchases of Common Stock
There were no repurchases of our common stock during the fourth quarter of 2009.
| ITEM 6. | SELECTED FINANCIAL DATA |
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act 1934, as amended, and are not required to provide the information under this item.
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes appearing elsewhere in this Report. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited to, those which are not within our control.
OVERVIEW
We have developed and patented a nano-structure polymer technology, which is being commercialized in products based on the functionality of these materials. We believe the applications of our technology have promise in a number of diverse market segments and products.
The initial product focus of the Company is ConsERV, an energy recovery ventilator. We also have new product applications in various developmental stages. We believe that three of these additional product applications, including an advanced air conditioning system which is projected to use less energy and emit fewer emissions than most current HVAC equipment, a sea-water desalination product and an electrical energy storage device, can be brought to market in the foreseeable future if we receive adequate capital funding.
32
Table of Contents
We expect ConsERV™ to continue to be our focused commercial product through 2010 with a growing emphasis on moving the development of the NanoClear and NanoAir technologies towards commercialization.
REVENUES
We generate our revenues primarily from the sale of our ConsERV™ products in residential and commercial HVAC markets. Sales channels for our ConsERV™ products include OEMs, distributors, retailers, and consumers. We also occasionally license our technology to strategic partners and sell various prototypes of other product applications that use our polymer technology.
Our revenue growth is dependent on continued sales from (i) more seasoned independent sales representatives, (ii) a greater number of independent sales representatives (iii) fulfilling the ventilation needs of the growing “energy consultant” marketplace which work to lower their client’s energy costs and emissions, and (iv) from the Company’s own ‘customer direct’ sales activities, all of which focus on the sale of product primarily into the commercial user marketplace with a growing emphasis on low rise structures (small commercial buildings, multi-purpose structures, and residences). In addition, the Company and its independent sales representative sales force will work to secure orders for ConsERV™ “core only” sales (i) from HVAC equipment manufacturers, (ii) from distribution firms servicing the equipment needs of the HVAC installer community, and (iii) creating license/supply relationships with HVAC or ERV OEMs preferably having a dominant presence in existing direct related sales channels.
COST OF SALES
Our cost of sales consists primarily of materials (including freight), direct labor, and outsourced manufacturing expenses incurred to produce our ConsERV™ products.
We are dependent on third parties to manufacture the key components needed for our nano-structured based materials and value added products made with these materials. Accordingly, a supplier’s failure to supply components in a timely manner, or to supply components that meet our quality, quantity and cost requirements or our technical specifications, or the inability to obtain alternative sources of these components on a timely basis or on terms acceptable to us, would create delays in production of our products or increase our unit costs of production. Certain of the components contain proprietary products of our suppliers, or the processes used by our suppliers to manufacture these components are proprietary. If we are required to replace any of our suppliers, while we should be able to obtain comparable components from alternative suppliers at comparable costs, it would create a delay in production.
Our cost of sales may fluctuate due to a number of factors, including, but not limited to:
| • | A change in key suppliers or the prices that they charge for the fundamental components of our ConsERV™ products; |
| • | An increase in the labor resources needed to expand the production of our ConsERV™ products; |
| • | Commercialization of new product applications of our polymer technology; |
| • | Continued technological improvements in key materials or configuration(s) to reduce our ‘per unit’ cost structure; and |
| • | Additional outsourcing of our manufacturing and assembly processes with strategic partners to reduce our ‘per unit’ cost structure. |
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
Our selling, general and administrative expenses consist primarily of payroll and related benefits, share-based compensation, professional fees, marketing and channel support costs, and other infrastructure costs such as insurance, information technology and occupancy expenses.
33
Table of Contents
Our selling, general and administrative expenses may fluctuate due to a variety of factors, including, but not limited to:
| • | Additional expenses as a result of becoming a reporting company including, but not limited to, director and officer insurance, director fees, SEC reporting and compliance expenses, transfer agent fees, additional staffing, professional fees and similar expenses; |
| • | Additional infrastructure needed to support the expanded commercialization of our ConsERV™ products and/or new product applications of our polymer technology for, among other things, administrative personnel, physical space, marketing and channel support and information technology; and |
| • | The fair value of new share-based awards, which is based on various assumptions including, among other things, the volatility of our stock price |
RESULTS OF OPERATIONS
The following table sets forth, for the periods indicated, certain data derived from our Statements of Operations:
| Year Ended December 31, |
||||||||
| 2009 | 2008 | |||||||
| Revenues |
$ | 1,531,215 | $ | 1,015,433 | ||||
| Percentage of revenues |
100.0 | % | 100.0 | % | ||||
| Cost of goods sold |
$ | 1,071,098 | $ | 796,217 | ||||
| Percentage of revenues |
70.0. | % | 78.4 | % | ||||
| Selling, general and administrative expenses |
$ | 3,224,592 | $ | 2,935,552 | ||||
| Percentage of revenues |
210.6 | % | 289.1 | % | ||||
| Interest Expense |
$ | 621,574 | $ | 3,282,768 | ||||
| Percentage of revenues |
40.6 | % | 323.3 | % | ||||
| Net loss |
$ | (3,385,382 | ) | $ | (5,979,446 | ) | ||
| Percentage of revenues |
(221.1 | )% | (588.9 | )% | ||||
DECEMBER 31, 2009 COMPARED TO DECEMBER 31, 2008
REVENUES: Total revenues for the year ended December 31, 2009 and 2008 were $1,531,215 and $1,015,433 respectively, an increase of $515,782, or 50.8%. The increase in revenues for 2009 is primarily attributable to the Company increasing the sales price of the ConsERV products, introducing a new product to the ConsERV line generating additional sales in a new price category and an increase in the number and size of its sales transactions in 2009. The Company also attributes the sales increase to a realignment of its independent sales representatives. During the year ended December 31, 2009 and 2008, five and four customers accounted for approximately 66% and 64% of revenues, respectively.
COST OF GOODS SOLD: Cost of goods sold was $1,071,098 and $796,217, or 70.0% and 78.4% of revenues for the years ended December 31, 2009 and 2008, respectively. The increase in 2009 of $274,881 is primarily due an increase in sales. The decrease in cost of goods sold as a percentage of revenue in 2009 is primarily attributable to a decrease in the cost of materials due to the implementation of improvements to the production process of certain product components and a decrease in the cost of both labor and materials through outsourcing a portion of the production.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES: Selling, general and administrative expenses were $3,224,592 for the year ended December 31, 2009, compared to $2,935,552 for the year ended December 31, 2008, an increase of $289,040 or 9.8%. This increase is primarily due to an increase in stock based compensation of approximately $396,000 and a $75,000 increase in professional fees, which was partially offset by a decrease in payroll expenses of approximately $177,700.
34
Table of Contents
INTEREST EXPENSE: Interest expense was $621,574 for the year ended December 31, 2009 compared to $3,282,768 for the same period of 2008, a decrease of $2,661,194 or 81.1%. During the year ended December 31, 2009, interest expense was primarily related to convertible notes issued from December 2007 to January 2008, and comprised of approximately $172,000 of stated interest expense on the notes, approximately $413,000 in expense relating to warrants issued to induce conversion of principal and $30,100 in expense related to the amortization of the discount and embedded beneficial conversion feature. The decrease in interest expense is due to the fact that the beneficial conversion feature and discount on the notes payable became fully amortized in January 2009 and the convertible debt has been reduced by $2,625,000 in 2009.
For the year ended December 31, 2008, interest expense was comprised of amounts related to convertible notes issued from December 2007 to January 2008, including approximately $197,000 of stated interest expense on the notes, loan cost amortization of approximately $102,000, and approximately $2,849,000 for the amortization of the discount and embedded beneficial conversion feature. During the year ended December 31, 2008, the Company also recognized approximately $65,000 of interest expense from the induced conversion of notes payable to the Robb Charitable Trust.
NET LOSS: Net loss for the year ended December 31, 2009 decreased by $2,594,064 to $3,385,382 from $5,979,446 for the year ended December 31, 2008. The decrease in net loss is primarily due to the increases in sale, and decreases in cost of sales and interest expense, which was partially offset by increases in selling, general and administrative expenses.
LIQUIDITY AND CAPITAL RESOURCES
The Company finances its operations primarily through sales of its ConsERV™ products, sales of its common stock, the issuance of convertible promissory notes, unsecured promissory notes and license agreements.
Our historical revenues have not been sufficient to sustain our operations. We have not achieved profitability in any year since inception and we expect to continue to incur net losses and negative cash flow from operations until we can produce sufficient revenues to cover our costs, which are not expected for several years. Furthermore, even if we achieve our goal of selling a greater number of ConsERV™ units, we anticipate that we will continue to incur losses until we can cost-effectively produce and sell our products to a wider market. Our profitability will require the successful commercialization of our ConsERV™ products and any future products we develop. No assurances can be given when this will occur.
During the year ended December 31, 2009 eighteen holders converted their Convertible Notes, having an aggregate outstanding principal balance of $2,350,000 plus accrued interest of $361,600, into 13,553,822 shares of common stock. Some of the holders converted during periods in which we were offering an additional warrant as an inducement to convert. In accordance with the offers we issued additional warrants to purchase 1,665,000 shares of common stock, exercisable immediately at $0.25 per share and valued at $126,367, and 575,000 warrants, exercisable immediately at $0.75 per share valued at $286,641 which was recorded as interest expense during the twelve months ended December 31, 2009.
During 2009, four investors holding Convertible Notes with an aggregate outstanding principal balance of approximately $450,000 at December 31, 2008 notified the Company that they were asserting their rights to receive payment of the principal and interest pursuant to the terms of the Convertible Notes. In June of 2009, three of these investors, holding an aggregate principal note balance of $250,000, entered into a confession of judgment with the Company. Under that agreement, the three investors had the right, should the Company fail to pay all principal and interest due pursuant to their Convertible Notes on or before September 11, 2009, to file the confession of judgment with the court and seek to secure a judgment against the Company in the amount of all principal and interest due under their Convertible Notes together with the reasonable cost and expense of collection. All interest and principal related to the three Convertible Notes, $289,600 in the aggregate, was paid in full by the Company on or before September 11, 2009. In July of 2009, the fourth investor, holding a Convertible Note in the principal amount of
35
Table of Contents
$200,000, agreed to extend said note to September 2009. In November of 2009, this investor and the Company modified the Convertible note to extend the maturity date of said note to July 2010, pay the principal amount due in eight monthly installments commencing December of 2009, end the accrual of interest as of November 20, 2009 and convert the $34,861 in interest due under the Convertible Note as of November 20, 2009 into 170,137 shares of Company’s common stock. As of December 31, 2009 the outstanding principal balance of said loan was $175,000.
As of December 31, 2009, $325,000 of principal on the Convertible Notes was outstanding, of which $175,000 has been extended to July of 2010 with the remaining notes currently in default and due and payable in full.
We cannot currently pay the convertible notes without severely impacting our ability to continue operations and the Company may not be able to secure additional financing to repay the notes on acceptable terms, if at all. As an alternative, management may attempt to renegotiate the repayment terms of the notes and seek extension of the maturity dates. There is no guarantee that any renegotiated terms we may be able to secure would be favorable to the Company. Unfavorable terms, in either a financing transaction or debt renegotiation, would adversely impact our business, financial condition and/or results of operations. In the event (i) we are unable to secure additional financing sufficient to pay the notes, (ii) the notes are not converted into shares of our common stock pursuant to their terms, (iii) we are not able to negotiate extensions to the maturity dates of the notes, or (iv) we are unable to generate sufficient funds from operations to repay these loans, the $325,000 Convertible note holders will have the option to foreclose on all of our patents and those patent applications securing the notes, which would likely result in the failure of our business.
In July 2009, we secured a loan of $300,000 from an investor. Pursuant to the terms of the note, we are to pay the note holder simple interest at the rate of seven percent per annum commencing on July 17, 2009 with all interest and principal due there under payable in cash on or before January 16, 2011.
In December 2009, we secured a loan in the principal amount of $1,000,000 from an investor. Pursuant to the terms of the note, we are to pay the holder simple interest at the rate of ten percent per annum commencing on the date of issuance with all interest and principal due and payable in cash on or before June 17, 2010.
The Company secured loans from two investors in the principal amounts of $250,000 and $620,000. The loan amounts were received by the Company on December 31, 2009 and February 18, 2010, respectively. Pursuant to the terms of the notes, the Company is to pay the holders simple interest at the rate of ten percent per annum commencing on the date of issuance with all interest and principal due and payable in cash on or before June 30, 2010 and August 10, 2010.
Any future financing may result in substantial dilution to existing shareholders, and future debt financing, if available, may include restrictive covenants or may require us to grant a lender a security interest in any of our assets not already subject to an existing security interest. To the extent that we attempt to raise additional funds through third party collaborations and/or licensing arrangements, we may be required to relinquish some rights to our technologies or products currently in various stages of development, or grant licenses or other rights on terms that are not favorable to us. Any failure by us to timely procure additional financing or investment adequate to fund our ongoing operations, including planned product development initiatives, clinical studies and commercialization efforts, will have material adverse consequences on our financial condition, results of operations and cash flows.
We will be dependent upon our existing cash of $1,085,628 at December 31, 2009, product sales and additional debt and equity issuances to finance our operations through the next 12 months. We need to raise additional capital of approximately $13 million to $18 million, net of costs, during the next nine to twelve months, the proceeds of which will be used to pay down existing debt, secure new patents for innovative applications of our core technology, purchase equipment, and fund our working capital requirements through December 2011. We currently have no commitments for any such funds. If we are unable to raise the funds we may delay development plans and reduce expenditures wherever possible.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. For the year ended December 31, 2009, the Company incurred a net loss of $3,385,382 and has incurred significant losses since inception. As of December 31, 2009, the Company has an accumulated deficit of $32,162,151, negative working capital of $2,265,370 and a stockholders’ deficit of $2,678,939. The Company used $818,378 and $1,824,510 of cash from operations during 2009 and 2008, respectively, which was funded by proceeds from debt and equity financings. There is no assurance that such financing will be available in the future. In view of these matters, there is substantial doubt that the Company will continue as a going concern. The Company is currently pursuing the following sources of short and long-term working capital:
| 1. | We are currently holding preliminary discussions with parties who are interested in licensing, purchasing the rights to, or establishing a joint venture to commercialize, certain applications of our technology. |
36
Table of Contents
| 2. | We are seeking growth capital from certain strategic and/or government (grant) related sources. In addition to said capital, these sources may, pursuant to any agreements that may be developed in conjunction with such funding, assist in the product definition and design, roll-out, and channel penetration of our products. As part of this step we will attempt to take advantage of key programs associated with the recently enacted American Recovery and Reinvestment Act of 2009. |
The Company’s ability to continue as a going concern is highly dependent on our ability to obtain additional sources of cash flow sufficient to fund our working capital requirements. However, there can be no assurance that the Company will be successful in its efforts to secure such cash flow. Any failure by us to timely procure additional financing or investment adequate to fund our ongoing operations, including planned product development initiatives and commercialization efforts, will have material adverse consequences on our financial condition, results of operations and cash flows.
The financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
Statements of Cash Flows
Cash and cash equivalents as of December 31, 2009 was $1,085,628 compared to $26,867 as of December 31, 2008. Cash is primarily used to fund our working capital requirements.
As of December 31, 2009, the Company had an increase in working capital of $1,536,639, resulting in a working capital deficit of $2,265,370 compared to $3,802,009 of working capital deficit as of December 31, 2008. During the year ended 2009, we used approximately $818,000 of cash to fund our operations, approximately $513,000 to repay debt, and approximately $41,000 to purchase patent licenses and equipment. These uses of cash are partially offset by approximately $643,000 of proceeds received during 2009 in connection with the sale of common stock and $1,787,900 of proceeds from the issuance of debt.
Net cash used in operating activities was approximately $818,000 for the year ended December 31, 2009 compared to approximately $1,825,000 for the same period in 2008. During the year ended December 31, 2009, we used additional cash to fund operating losses of approximately $3,385,000 and working capital requirements of approximately $1,116,000 compared to the same period in 2008.
Net cash used in investing activities was approximately $41,000 for the year ended December 31, 2009 compared to approximately $19,000 for the same period in 2008. During the year ended December 31, 2009, we used additional cash to purchase patent licenses and equipment.
Net cash provided by financing activities was approximately $1,918,000 for the year ended December 31, 2009 compared to approximately $1,366,000 for the same period in 2008. During the year ended December 31, 2009, we received net proceeds of $1,787,900 from the issuance of debt and $643,000 of proceeds from the sale of common stock.
ECONOMY AND INFLATION
We have not experienced any significant cancellation in orders due to the downturn in the economy and only a small number of customer requested delays in delivery or production of orders in process.
Our management believes that inflation has not had a material effect on our results of operations
37
Table of Contents
CONTRACTUAL OBLIGATIONS
As of December 31, 2009, we have contractual obligations of $1,900,117 as indicated below:
| Contractual Obligations |
Total | Less than 1 Year |
1-3 Years | 3-5 Years | ||||||||
| Long-term debt |
$ | 1,875,624 | $ | 1,575,624 | $ | 300,000 | $ | — | ||||
| Purchase Obligations |
24,493 | 24,493 | — | — | ||||||||
| Total |
$ | 1,900,117 | $ | 1,600,117 | $ | 300,000 | $ | — | ||||
The Company secured loans from two of its investors in the principal amounts of $250,000 on December 31, 2009 and $620,000 on February 18, 2010. The Company issued the lenders unsecured promissory notes with respect to said loans on February 19, 2010. The loan received in December of 2009 is included in the foregoing table. Pursuant to the terms of these notes, we are to pay the holders simple interest at the rate ten percent per annum.
OFF-BALANCE SHEET ARRANGEMENTS
We do not have any off balance sheet arrangements that are reasonably likely to have a current or future effect on our financial condition, revenues, results of operations, liquidity or capital expenditures.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of the accompanying financial statements and related disclosures in conformity with U.S. GAAP requires us to make judgments, assumptions and estimates that affect the amounts reported in the accompanying financial statements and the accompanying notes. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. When making these estimates and assumptions, we consider our historical experience, our knowledge of economic and market factors and various other factors that we believe to be reasonable under the circumstances. Actual results could differ from these estimates. The following critical accounting policies are significantly affected by judgments, assumptions and estimates used in the preparation of the financial statements:
The significant accounting policies followed are:
Revenue Recognition
Generally, the Company recognizes revenue for its products upon shipment to customers, provided no significant obligations remain and collection is probable. Our ConsERV product typically carries a warranty of two years for all parts contained therein with the exception of the energy recovery ventilator core which typically carries a 10 year warranty. The warranty includes replacement of defective parts. Warranty costs have been immaterial to our overall operations in the past and therefore, the Company has not recorded an accrual for any estimated warranty expenses. Revenue derived from the sale of licenses is deferred and recognized as revenue on a straight-line basis over the life of the license, or until the license arrangement is terminated. The Company recognized revenue of approximately $84,100 from license agreements for each of the years ended December 31, 2009 and 2008.
Impairment of Long-Lived and Intangible Assets
Long-lived and intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the book value of the asset may not be recoverable. The Company periodically evaluates whether events and circumstances have occurred that indicate possible impairment. When impairment indicators exist, the Company uses market quotes, if available or an estimate of the future undiscounted net cash flows of the related asset or asset group over the remaining life in measuring whether or not the asset values are recoverable. There have been no significant impairments of long-lived and intangible assets during the two-year period ended December 31, 2009.
38
Table of Contents
Stock-Based Compensation
The Company recognizes all share-based payments to employees, including grants of employee stock options, as compensation expense in the financial statements based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
The value of each grant is estimated at the grant date using the Black-Scholes option model with the following assumptions for options granted during the years ended December 31, 2009 and 2008:
| Years Ended December 31, | ||||||
| 2009 | 2008 | |||||
| Dividend rate |
0 | % | 0 | % | ||
| Risk free interest rate |
1.65% – 3.49 | % | 2.64% – 3.98 | % | ||
| Expected term |
5 – 10 years | 5 – 10 years | ||||
| Expected volatility |
92% – 106 | % | 80% – 114 | % | ||
The basis for the above assumptions are as follows: the dividend rate is based upon the Company’s history of dividends; the risk-free interest rate for periods within the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant; the expected term was calculated based on the Company’s historical pattern of options granted and the period of time they are expected to be outstanding; and expected volatility was calculated by review of a peer company’s historical stock prices.
Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Based on historical experience of forfeitures, the Company estimated forfeitures at 0% and 22% for the years ended December 31, 2009 and 2008, respectively, and incorporated this rate in the estimated fair value of employee option grants during 2009 and 2008.
Taxes
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due plus deferred taxes resulting from temporary differences. Such temporary differences result from differences in the carrying value of assets and liabilities for tax and financial reporting purposes. The deferred tax assets and liabilities represent the future tax consequences of those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
As of December 31, 2009, the Company had no unrecognized tax benefits or related interest and penalties. We will include future interest and penalties associated with any unrecognized benefits within provision for income taxes on the Statements of Operations. We do not anticipate any unrecognized benefits in the next 12 months that would result in a material change to our financial position.
RECENT ACCOUNTING PRONOUNCEMENTS
For a description of recent accounting standards, including the expected dates of adoption and estimated effects, if any, on our financial statements, see “Note 3: Significant Accounting Polices: Recent Accounting Standards” in Part II, Item 8 of this Form 10-K.
39
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
Our financial statements and the related notes begin on Page F-1, which are included in this Annual Report on Form 10-K.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
| ITEM 9A(T). | CONTROLS AND PROCEDURES. |
Disclosure Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Management conducted its evaluation based on the framework in Internal Control – Integrated Framework issued by the Committee on Sponsoring Organizations of the Treadway Commission (COSO). Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that, at December 31, 2009, such disclosure controls and procedures were effective.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rue 13a-15(f) under the Exchange Act). Internal control over financial reporting is a process, including policies and procedures, designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles. Our management assessed our internal control over financial reporting based on the Internal Control – Integrated Framework issued by the COSO. Based on the results of this assessment, our management concluded that our internal control over financial reporting was effective as of December 31, 2009 based on such criteria.
Limitations on the Effectiveness of Controls
Our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met. Because of inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Based on their evaluation as of the end of the period covered by this report, management concluded that our disclosure controls and procedures were sufficiently effective to provide reasonable assurance that the objectives of our disclosure control system were met.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the fourth fiscal quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Auditor’s Report on Internal Control over Financial Reporting
This Annual Report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
| ITEM 9B. | OTHER INFORMATION |
None.
40
Table of Contents
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The following table sets forth the names and ages of all of our directors and executive officers as of the date of this Annual Report. Also provided herein is a brief description of the business experience of each director and executive officer during the past five years and an indication of directorships held by each director in other companies subject to the reporting requirements under the Federal securities laws. All of the directors will serve until the next annual meeting of shareholders and until their successors are elected and qualified, or until their earlier death, retirement, resignation or removal. There are no arrangements or understandings between any director or executive officer and any other person pursuant to which the director or executive officer was selected.
| Name |
Age | Position | ||
| Timothy N. Tangredi | 54 | President, Chief Executive Officer and Chairman of the Board of Directors | ||
| Robert W. Brown | 60 | Vice President – Operations | ||
| Scott G. Ehrenberg | 56 | Chief Technology Officer and Secretary | ||
| Judith C. Norstrud | 41 | Chief Financial Officer and Treasurer | ||
| David Longacre | 51 | Vice President – Sales and Marketing | ||
| Robert W. Schwartz | 65 | Director | ||
| Raymond Kazyaka Sr. | 79 | Director |
Directors and Executive Officers
The following are the Company’s directors and executive officers:
Timothy N. Tangredi has been our Chief Executive Officer since 1996. Mr. Tangredi joined the Company in 1996, and was appointed a member of our board of directors in 1997. In 1999 and 2000, respectively, Mr. Tangredi initiated and executed the strategic purchases of Analytic Power and American Fuel Cell Corporation. From 1979 to 1990, Mr. Tangredi worked for AT&T, as a member of the Leadership Continuity Program working in technical marketing, network operations, and project management. Mr. Tangredi earned his BS from Siena College and MBA from Rensselaer Polytechnic Institute. He is a founder and member of the board of directors of Aegis BioSciences, LLC (“Aegis”). Aegis, created in 1995, is a licensee of the Company’s nano-structured intellectual property and materials in the biomedical and healthcare fields. Mr. Tangredi spends approximately one to two days per month on Aegis business and is compensated by Aegis for his time and contribution(s).
41
Table of Contents
Scott G. Ehrenberg, is a founder of the Company and has been our Chief Technology Officer since 1993 and Secretary since November 7, 2008. He has thirty years of experience developing along with others new materials and applications. These applications range from laser cutting systems, optical inspection technology, and new organic electronic packages for IBM to new polymer electrolytes for electrochemical and mass transport devices for the Company. His background includes 12 years at IBM plus two previous successful startups in the fields of electronic packaging and ultrasonic devices: Tessera of San Jose, CA and Sono-Tek of Milton, NY. He has 14 issued patents with 6 more pending along with numerous technical papers and presentations. Mr. Ehrenberg received his bachelor of science from Pennsylvania State University in 1976.
David E. Longacre has been Vice President of Sales and Marketing since January 2010. His background includes over 25 years of experience in the Heating, Ventilation and Air Conditioning (HVAC) industry. His career started with York International as a Sales Engineer, progressing to a Zone Manager over 17 years. He worked as an independent manufactures representative for two years before joining Trane, where he was a Strategic Account Manager and Team Leader for five years. He then worked with Siemens Building Technologies as their Service Sales Manager for a district, then became Branch Manager for Johnson Controls handling the profit and loss for both sales and operations. Mr. Longacre received his BS in Commerce and Engineering from Drexel University in 1980. He is also a LEED AP.
Robert W. Brown has been an officer of the Company since March 2003 and is currently Vice President of Operations. His background includes 28 years of experience in technical marketing and product management, technology commercialization, and many aspects of technology business start-up and growth. He has experience both onshore and internationally with utility and engineering organizations. From March 1994 to February 2003, as CEO of a subsidiary of Baymont Technologies Inc. Mr. Brown’s responsibilities included turn around management and financial restructuring. Mr. Brown received a Technical Diploma from Southern Alberta Institute of Technology in 1970.
Judith C. Norstrud, CPA was appointed Chief Financial Officer and Treasurer on October 14, 2009. In March 2002, Judith founded Norco Accounting & Consulting, Inc., a firm that provides various accounting and consulting services to small companies on an as needed basis. From July 1999 to June 2002, Judith served as a manager with Pender, Newkirk and Company, CPAs. While at Pender, Judith served a variety of companies from start up enterprises to mid-sized publicly traded companies. Previously, from August 1996 to July 1999, Judith was an Audit Senior with PricewaterhouseCoopers, LLP. Judith graduated from the University of South Florida’s College of Business Administration with a Master of Accountancy degree in 2002.
Non-Employee Directors
Raymond Kazyaka Sr. was appointed to our board of directors in 1995. Mr. Kazyaka is the former President (1976-2006) and a co-founder of Wright Malta Corporation, which was founded in 1972. Wright Malta, liquidated in 2005, owned and operated the Malta Test Station, which had performed military product development for various governmental and commercial organizations. Mr. Kazyaka has also served as a consultant to the Canadian National Defense on facility noise abatement. Prior to founding Wright Malta, Mr. Kazyaka worked for General Electric as a rocket engine design engineer and a manager. Mr. Kazyaka holds several patents on rocket engine components and noise abatement systems, and is a senior member of the American Institute of Aeronautics and Astronautics. Mr. Kazyaka graduated from Union College with a degree in Mechanical Engineering in 1953.
Robert W. Schwartz was appointed to our board of directors in 2001. Mr. Schwartz founded the Schwartz-Heslin Group (“SHG”) in 1985 and serves as one of its Managing Directors. Mr. Schwartz specializes in corporate planning, finance and development. Prior to starting SHG, he was a founder, President and Chief Executive Officer of a venture-funded high tech telecommunications company (Windsource, Inc.). In addition, he was the President and Chief Operating Officer of an American Stock Exchange listed company (Coradian Corporation). He was also the Chief Financial Officer of a major manufacturer of outdoor power equipment (Troy Built Products, Troy, NY). His earlier experience was with KPMG as a management consultant and with IBM. Mr. Schwartz received a Bachelor of Science from Cornell University in 1967 and his Masters Degree in Business from the University of New York Albany in 1969.
42
Table of Contents
Involvement in Certain Legal Proceedings
None of our directors or executive officers has been, during the past ten years:
(i) involved in any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer, either at the time of the bankruptcy or within two years prior to that time;
(ii) convicted of any criminal proceeding or subject to a pending criminal proceeding (excluding traffic violations and other minor offences);
(iii) subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoined, barred, suspended or otherwise limited from involvement in any type of business, securities, futures, commodities or banking activities;
(iv) found by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated
(v) found by a court of competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has not been subsequently reverse, suspended, or vacated;
(vii) subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, related to an alleged violation of securities or commodities law or regulation; any law or regulation respecting financial institutions or insurance companies; or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
(viii) the subject of, or a party to, any sanction or order, not subsequently reversed, suspending or vacated, of any self-regulatory any registered entity of the Commodity Exchange Act or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Director Independence
Our board of directors has determined that it currently has two members who qualify as “independent” as the term is used in Item 407 of Regulation S-K as promulgated by the SEC and as that term is defined under NASDAQ Rule 4200(a)(15). The independent directors are Raymond Kazyaka Sr. and Robert W. Schwartz.
Board Meetings and Committees; Annual Meeting Attendance
Our board of directors has not adopted any committees to the board of directors. Our board of directors held five formal meeting during the most recently completed fiscal year. Other proceedings of the board of directors were conducted by resolutions consented to in writing by all the directors and filed with the minutes of the proceedings of the directors. Such resolutions consented to in writing by the directors entitled to vote on that resolution at a meeting of the directors are, according to the corporate laws of the State of New York and our bylaws, as valid and effective as if they had been passed at a meeting of the directors duly called and held.
At each annual meeting of shareholders, directors will be elected by the holders of common stock to succeed those directors whose terms are expiring. Directors will be elected annually and will serve until successors are duly elected and qualified or until a director’s earlier death, resignation or removal. Our bylaws provide that the authorized number of directors may be changed by action of the majority of the board of directors or by a vote of the shareholders of our Company. Vacancies in our board of directors may be filled by a majority vote of the board of directors with such newly appointed director to serve until the next annual meeting of shareholders, unless sooner removed or replaced. We currently do not have a policy regarding the attendance of board members at the annual meeting of shareholders.
43
Table of Contents
Code of Ethics
We have adopted a code of ethics that applies to our officers, directors and employees in accordance with applicable federal securities laws. We have filed a copy of our code of ethics as an exhibit to our Annual Report on Form 10-K as filed on March 31, 2009. This document may be reviewed by accessing our public filings at the SEC’s web site at www.sec.gov. In addition, a copy of the code of ethics will be provided without charge upon request to us. We intend to disclose any amendments to or waivers of certain provisions of our code of ethics in a Current Report on Form 8-K
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Exchange Act requires our executive officers and directors, and persons who own more than 10% of any publicly traded class of our equity securities, to file reports of ownership and changes in ownership of our equity securities with the SEC. Officers, directors, and greater than ten percent stockholders are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms that they file.
Based solely on the reports received and on the representations of the reporting persons, we believe that these persons have complied with all applicable filing requirements of Section 16(a) of the Exchange Act during fiscal 2009.
| ITEM 11. | EXECUTIVE COMPENSATION |
The following executive compensation disclosure reflects all compensation awarded to, earned by or paid to the executive officers below for the fiscal years ended December 31, 2009 and 2008. The following table summarizes all compensation for fiscal years 2009 and 2008 received by our Chief Executive Officer, and most highly compensated executive officers in fiscal year 2009.
SUMMARY COMPENSATION TABLE
| Summary Compensation Table |
||||||||||||||||||||||
| Name and principal position | Year | Salary ($) |
Bonus ($) |
Stock ($)(2) |
Option ($)(2) |
Non-Equity Incentive |
Non-qualified ($) |
All other compensation ($) |
Total ($) | |||||||||||||
| (a) |
(b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||
| Timothy N. Tangredi |
2009 2008 |
$ $ |
170,000 170,000 |
— — |
— — |
$
|
1,134,425 752,450 |
(3) |
— — |
— — |
— — |
$ $ |
1,304,425 922,450 | |||||||||
| Robert W. Brown |
2009 2008 |
$ $ |
57,187 75,000 |
— — |
— — |
$ |
— 23,412 |
|
— — |
— — |
— — |
$ $ |
57,187 98,412 | |||||||||
| Scott G. Ehrenberg, |
2009 2008 |
$ $ |
67,100 88,000 |
—
|
— — |
$ |
— 84,337 |
(3) |
— — |
— — |
— — |
$ $ |
67,100 172,337 | |||||||||
| Judith C. Norstrud, |
2009 | $ | 13,447 | — | — | $ | 82,930 | (3) | — | — | — | $ | 96,377 | |||||||||
| (1) | Mr. Tangredi receives a salary of $170,000 per year, and a bonus in an amount not to exceed 100% of his salary, which bonus shall be measured by meeting certain performance goals as determined in the sole discretion of our board of directors. In 2009 and 2008, Mr. Tangredi was paid $55,350 and $117,500, respectively and has accrued unpaid salary of $114,650 for 2009 and $52,500 for 2008. Additional accruals have been made for the years prior to 2008. |
44
Table of Contents
| (2) | The amounts included in these columns are the aggregate dollar amounts of compensation expense recognized by us for financial statement reporting purposes in accordance with Accounting Standards Codification 718, Compensation-Stock Compensation, for the fiscal years ended December 31, 2009 and December 31, 2008, and thus include amounts from option awards granted in and prior to the indicated year. For information on the valuation assumptions used in calculating these dollar amounts, see Note 1 to our audited financial statements included in this Report for the fiscal years ended December 31, 2009 and December 31, 2008, each as filed with the SEC. These amounts reflect our accounting expense for these awards and do not correspond to the actual value that may be recognized by the individuals upon option exercise. During the fiscal year ended December 31, 2009, there were 472,733 option award forfeitures related to service-based vesting conditions. |
| (3) | In 2008 we issued Mr. Tangredi a warrant to purchase 3,000,000 shares of the Company’s common stock at an exercise price of $0.36 per share. The warrant had a five year term, vested upon issuance, provided for a cashless exercise and contained standard anti-dilution provisions. In the same year we issued Mr. Ehrenberg a warrant to purchase 250,000 shares of Company’s common stock at the exercise price of $0.30 per share with all other terms and conditions being the same as those of the Tangredi warrant. During 2009, we issued Ms. Norstrud an option to purchase 200,000 shares of the Company’s common stock at an exercise price of $0.45 per share. The option vests at a rate of 50,000 per quarter and has a term of five years. |
Narrative Disclosure to Summary Compensation Table
Employment Agreements
Officer Employment Agreement
Timothy N. Tangredi. We are party to an employment agreement with Mr. Tangredi, our President, Chief Executive Officer, and director. The employment agreement, as amended and restated on July 29, 2008, sets forth Mr. Tangredi’s compensation level and eligibility for salary increases, bonuses, benefits, royalty sharing for newer applications, and option grants. Mr. Tangredi’s employment agreement provided for an initial term of three years with the term extending on the second anniversary thereof for an additional one year period and on each subsequent anniversary of the agreement for an additional year period. The agreement sets forth Mr. Tangredi’s compensation level, conditions for certain option grants, benefits and the obligations of the Company in the event of termination. Mr. Tangredi’s base salary is $170,000, plus certain allowances as well as performance related payments and option issuances.
For each product for which the Company commences commercial sale or licensing during the term and receives more than $1 million of revenue during any 12 month period, Mr. Tangredi, in addition to any other compensation which he may receive under the agreement, shall be granted options to purchase 10,000 shares of the Company’s common stock at an exercise price equal to either (i) the lower of: (a) $2.50 per share or (b) the fair market value per share of the stock on the date of grant as determined in good faith by the Compensation Committee of the Board of Directors, if the Company has not conducted an initial public offering prior to the date of grant (as hereinafter defined), or (ii) at an exercise price equal to 75% of the market price of the common stock, if the Company has completed an initial public offering of its common stock prior to the date of grant (with the market price to be the average of the closing sale prices during the five trading days immediately preceding the date of grant of the option). Such options, as well as any other options granted to Mr. Tangredi during the term of his employment, shall be granted under the Company’s then existing stock option plan, shall be immediately exercisable, have a term of ten years, shall be exercisable for up to three years after termination of employment (unless termination is for cause, in which event they shall expire on the date of termination), shall have a “cashless” exercise feature, and shall be subject to such additional terms and conditions as are then applicable to options granted under such plan provided they do not conflict with the terms set forth in the agreement.
In the event that the fair market value of the Company’s common stock (the average of the closing prices of the common stock for any five consecutive trading days, as reported by the principal exchange or other stock market on
45
Table of Contents
which the commons stock is then traded) equals or exceeds 200% of the price at which the Company sells common stock in a public offering (the “Target Value”) at any time during the term of the agreement, Mr. Tangredi shall be granted options to purchase 50,000 shares of common stock at an exercise price equal to 75% of the Target Value, on terms identical to the options provided for above.
In the event Mr. Tangredi’s employment is terminated by the Company without cause or by Mr. Tangredi for good reason, death or disability, Mr. Tangredi shall be entitled to the following:
(i) An amount equal to the sum of (A) the greater of 200% of the base salary then in effect for Mr. Tangredi or $270,000 plus (B) the cash bonus, if any, awarded to Mr. Tangredi for the most recent year shall be payable by the Company in full within 10 days following termination;
(ii) The Company shall continue to provide Mr. Tangredi the health and life insurance, car allowance and other benefits set forth in the agreement until two years following termination of employment, and shall continue to offer any of such benefits to Mr. Tangredi beyond such two year period to the extent required by COBRA or similar statute which may then be in effect;
(iii) All stock options, to the extent they were not exercisable at the time of termination of employment, shall become exercisable in full; and
(iv) Any indebtedness of Mr. Tangredi to the Company shall thereupon be cancelled and of no further force and effect, and the Company shall pay to Mr. Tangredi, within ten days following receipt of a written demand therefore, any income or other taxes resulting from such cancellation.
In the event that Mr. Tangredi elects to terminate employment within one year following a change in control of the Company, he shall receive, within the later of ten days following the date on which the change in control occurs or the date on which he gives notice of his election to terminate employment, a lump sum payment equal to three times the greater of (i) his then current base salary plus the cash bonus, if any, awarded to him for the most recent year or (ii) $350,000 plus said cash bonus. In addition, he will be entitled to accelerated vesting of outstanding options and continuing benefits as described above.
Significant Employee
Patricia K. Tangredi. We are a party to an employment agreement with Ms. Tangredi. The agreement, provided for an initial term of 3 years beginning on January 1, 2001, with automatic extensions for subsequent one year terms, unless the Company or Ms. Tangredi provides the other party with written notice of intent not to renew. The agreement was subsequently amended and restated on July 29, 2008. The employment agreement set forth Ms. Tangredi’s compensation level and eligibility for salary increases, options, royalty sharing for newer applications, benefits and the obligations of the Company in the event of termination. A portion of Ms. Tangredi’s salary has been accrued and carried on the Company’s books since 2002.
In the event Ms. Tangredi’s employment is terminated by the Company without cause or by the Ms. Tangredi for good reason or by reason of death or disability, Ms. Tangredi shall be entitled to the following:
(i) the greater of 100% of the base salary then in effect for Employee or $115,000, which amount shall be payable by the Company in full within 10 days following termination;
(ii) the Company shall provide, at its sole cost, Ms. Tangredi with the medical benefits for one year following the date of termination. The Company shall continue to offer such benefits to Ms. Tangredi beyond such one year period to the extent required by COBRA or any similar statute which may then be in effect; and
(iii) all stock options granted to Ms. Tangredi at any time during the course of the term shall be exercisable in full.
In the event that Ms. Tangredi elects to terminate her employment within six months following a change in control of the Company, she shall receive, within the later of 10 days following the date on which the change in control occurs or the date on which she give notice of her election to terminate employment, a lump sum payment equal to the greater of three times her then current base salary or $235,000. In addition, she will be entitled to accelerated vesting of outstanding options and continuing medical benefits as described above.
46
Table of Contents
Outstanding Equity Awards
The following table summarizes outstanding unexercised options, unvested stocks and equity incentive plan awards held by each of our name executive officers and significant employees, as of December 31, 2009.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| OPTION AWARDS |
STOCK AWARDS | |||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) Exercisable |
Number of securities underlying unexercised options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities underlying unexercised unearned options (#) |
Option price |
Option expiration date |
Number of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | |||||||||||
| (a) |
(b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||
| Timothy N. Tangredi (1) |
825,000 | 0 | 0 | $ | .26 | 9/23/2014 | ||||||||||||||
| 150,000 | 0 | 0 | $ | .10 | 5/10/2015 | |||||||||||||||
| 120,000 | 0 | 0 | $ | .10 | 10/1/2015 | |||||||||||||||
| 40,000 | 0 | 0 | $ | .30 | 5/2/2016 | |||||||||||||||
| 110,000 | 0 | 0 | $ | .55 | 11/1/2016 | |||||||||||||||
| 140,000 | 0 | 0 | $ | .55 | 2/20/2017 | |||||||||||||||
| 300,000 | 0 | 0 | $ | .21 | 8/18/2017 | |||||||||||||||
| 350,000 | 0 | 0 | $ | .21 | 1/30/2018 | |||||||||||||||
| 3,000,000 | * | 0 | 0 | $ | .36 | 4/18/2013 | ||||||||||||||
| 75,000 | 0 | 0 | $ | .30 | 8/4/2018 | |||||||||||||||
| 100,000 | 0 | 0 | $ | .42 | 11/12/2019 | |||||||||||||||
| 3,540,058 | 0 | 0 | $ | .42 | 11/12/2019 | |||||||||||||||
| * Warrant |
||||||||||||||||||||
| Robert W. Brown (2) |
106,416 | 0 | 0 | $ | .26 | 9/23/2014 | ||||||||||||||
| 120,000 | 0 | 0 | $ | .10 | 5/10/2015 | |||||||||||||||
| 120,000 | 0 | 0 | $ | .10 | 10/1/2015 | |||||||||||||||
| 72,500 | 0 | 0 | $ | .55 | 11/1/2016 | |||||||||||||||
| 33,334 | 16,666 | 16,666 | $ | .21 | 8/18/2017 | |||||||||||||||
| 66,667 | 133,333 | 133,333 | $ | .30 | 8/4/2018 | |||||||||||||||
| Scott G. Ehrenberg (3) |
140,000 | 0 | 0 | $ | .26 | 9/23/2014 | ||||||||||||||
| 110,000 | 0 | 0 | $ | .10 | 5/10/2015 | |||||||||||||||
| 80,000 | 0 | 0 | $ | .10 | 10/1/2015 | |||||||||||||||
| 40,000 | 0 | 0 | $ | .55 | 11/1/2016 | |||||||||||||||
| 120,000 | 0 | 0 | $ | .55 | 2/20/2017 | |||||||||||||||
| 33,334 | 16,666 | 16,666 | $ | .21 | 8/18/2017 | |||||||||||||||
| 83,333 | 166,667 | 166,667 | $ | .30 | 8/4/2018 | |||||||||||||||
| *250,000 | 0 | 0 | $ | .30 | 8/4/2013 | |||||||||||||||
| * Warrant |
||||||||||||||||||||
| Judith C. Norstrud (4) |
0 | 200,000 | 200,000 | $ | .45 | 10/15/2019 | ||||||||||||||
| Patricia K. Tangredi (5) |
395,000 | 0 | 0 | $ | .26 | 9/23/2014 | ||||||||||||||
| 278,058 | 0 | 0 | $ | .10 | 5/10/2015 | |||||||||||||||
| 140,000 | 0 | 0 | $ | .10 | 10/1/2015 | |||||||||||||||
| 125,000 | 0 | 0 | $ | .55 | 11/1/2016 | |||||||||||||||
| 350,000 | 0 | 0 | $ | .21 | 8/18/2017 | |||||||||||||||
| 300,000 | 0 | 0 | $ | .21 | 1/30/2018 | |||||||||||||||
| 250,000 | 0 | 0 | $ | .30 | 8/4/2018 | |||||||||||||||
| 100,000 | 0 | 0 | $ | .42 | 11/12/2019 | |||||||||||||||
47
Table of Contents
| (1) | Mr. Tangredi receives a salary of $170,000 per year, and a bonus in an amount not to exceed 100% of his salary, which bonus shall be measured by meeting certain performance goals as determined in the sole discretion of our board of directors. The April 2008 warrant grant to Mr. Tangredi was made by the Board of Directors in recognition for Mr. Tangredi’s achievement of the following goals: negotiating conversion of the convertible notes issued in the Additional Financing, securing a release with respect to the consulting agreement with Gray Capital Partners, Inc., securing and closing upon the Financing. In 2009 and 2008, Mr. Tangredi has accrued unpaid salary of $114,650 for 2009 and $52,500 for 2008. Additional accruals have been made for the years prior to 2008. All stock options issued to Mr. Tangredi were issued under the 2000 Plan. |
| (2) | All stock options issued to Mr. Brown were issued under the 2000 Plan. |
| (3) | All stock options issued to Mr. Ehrenberg were issued under the 2000 Plan. |
| (4) | All stock options issued to Ms. Norstrud were issued under the 2000 Plan. |
| (5) | All stock options issued to Ms. Tangredi were issued under the 2000 Plan |
Director Compensation
The following table sets forth the compensation awarded to, earned by or paid to the directors during the fiscal year ended December 31, 2009.
DIRECTOR COMPENSATION
| Name | Fees Earned ($) |
Stock ($) |
Option ($) |
Non-Equity ($) |
Change in Value and ($) |
All Other ($) |
Total ($) | |||||||||
| (a) |
(b) | (c) | (d) | (e) | (f) | (g) | (h) | |||||||||
| Raymond Kazyaka Sr., Director(1) |
— | — | $ | 31,165 | — | — | — | $ | 31,165 | |||||||
| Robert W. Schwartz, Director(2) |
— | — | $ | 31,165 | — | — | — | $ | 31,165 | |||||||
| (1) | At fiscal year end December 31, 2009, Mr. Kazyaka had 504,600 option awards outstanding and no stock awards outstanding. |
| (2) | At fiscal year end December 31, 2009, Mr. Schwartz had 474,600 option awards outstanding and no stock awards outstanding. |
We do not have a plan pursuant to which our directors are compensated and directors do not receive cash compensation for their services on the Board of Directors although they do receive stock options as determined by the full board of directors. Timothy N. Tangredi, Raymond Kazyaka Sr. and Robert W. Schwartz were each granted
48
Table of Contents
an option on November 12, 2009 to purchase 100,000 shares of common stock at an exercise price of $0.42 per share, vesting immediately upon issuance and exercisable for a period of ten years. This option grant to Mr. Tangredi as a director is also contained in the table summarizing grants made to our officers.
Our non-employee directors are currently compensated with the issuance of stock options, which generally become exercisable upon the date of grant, and which generally expire on the earlier of ten years from the date of grant or up to three years after the date that the optionee ceases to serve as a director. Non-employee directors are also reimbursed for out-of-pocket expenses associated with attending to the Company’s business.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets forth information regarding our 2000 Incentive Compensation Plan (the “2000 Plan”) and our Long-Term Incentive Plan of 2009 (the “2009 Plan”) under which our securities are authorized for issuance as of December 31, 2009.
| Plan Category |
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans | |||
| Equity compensation plans approved by security holders |
12,348,882 | $.26 | 15,000,000 | |||
| Equity compensation plans not approved by security holders |
0 | 0 | 0 |
Security Ownership of Certain Beneficial Owners
The following table sets forth information as of the date of this prospectus as to each person or group who is known to us to be the beneficial owner of more than 5% of our outstanding voting securities and as to the security and percentage ownership of each of our executive officers and directors and of all of our officers and directors as a group.
Beneficial ownership is determined under the rules of the SEC and generally includes voting or investment power over securities. The number of shares shown as beneficially owned in the tables below are calculated pursuant to Rule 13d-3(d)(1) of the Exchange Act. Under Rule 13d-3(d)(1), shares not outstanding that are subject to options, warrants, rights or conversion privileges exercisable within 60 days are deemed outstanding for the purpose of calculating the number and percentage owned by such person, but not deemed outstanding for the purpose of calculating the percentage owned by each other person listed. Except in cases where community property laws apply or as indicated in the footnotes to this table, we believe that each shareholder identified in the table possesses sole voting and investment power over all of the shares of common stock shown as beneficially owned by the shareholder.
The address for each of the persons named below is 11552 Prosperous Drive, Odessa, FL 33556, unless otherwise indicated.
49
Table of Contents
Applicable percentage ownership in the following table is based on approximately 29,095,717 shares of common stock outstanding as of January 31, 2010, plus, for each individual, any securities that individual has the right to acquire within 60 days of January 31, 2010.
| Common Stock Beneficially Owned |
|||||
| Name of Beneficial Owner |
Number of Shares of Common Stock |
Percentage of Class |
|||
| Timothy N. Tangredi (Officer and Chairman) (1) |
10,835,916 | 27.2 | % | ||
| Robert W. Brown (Officer) (2) |
518,916 | 1.8 | % | ||
| Scott G. Ehrenberg(3) (Officer) |
939,466 | 3.1 | % | ||
| Judith Norstrud (Officer) (4) |
50,000 | .17 | % | ||
| David Longacre (Officer) |
0 | 0 | % | ||
| Raymond Kazyaka Sr. (Director) (5) |
504,600 | 1.7 | % | ||
| Robert W. Schwartz (Director) (6) |
474,600 | 1.6 | % | ||
| Executive officers and directors as a group (7 persons) |
13,323,498 | 31.58 | % | ||
| Brian A. Kelly 181C Hague Blvd. Glenmont, N.Y. 12077 |
2,254,085 | 7.7 | % | ||
| Michael Gostomski (7) 1666 Valley View Dr. Winnona, MN 55987 |
2,086,842 | 7.2 | % | ||
| Louis M. Jaffe (8) 1500 S. Ocean Blvd #5201 Boca Raton, FL 33432 |
3,631,646 | 12.0 | % | ||
| Michael Frederick Stone (9) 18 Ozone Avenue Venice, CA 90291 |
1,920,137 | 6.2 | % | ||
| Marisa Stadmauer (10) 26 Columbia Turnpike Florham Park, NJ 07932 |
1,835,373 | 6.1 | % | ||
| Mark Nordlich (11) 152 West 575th St. 4th Floor New York, NY 10019 |
3,324,740 | 11.4 | % | ||
| Erick Richardson (12) 10900 Wilshire Blvd. Suite 500 Los Angeles, CA 90024 |
1,863,677 | 6.2 | % | ||
| Leonard Samuels (13) 1011 Centennial Road Penn Valley, PA 19072 |
8,795,212 | 26.6 | % | ||
| Leah Kaplan Samuels (14) 1011 Centennial Road Penn Valley, PA 19072 |
2,576,746 | 8.6 | % | ||
| (1) | Includes 8,750,058 shares of common issuable upon exercise of stock options and warrants and 2,065,858 shares beneficially owned by Mr. Tangredi’s wife, Patricia Tangredi. 1,938,058 of Ms. Tangredi’s shares are issuable upon the exercise of stock options. |
| (2) | Includes 518,916 shares of common stock issuable upon exercise of stock options. |
| (3) | Includes 856,666 shares of common stock issuable upon the exercise of stock options and warrants and 41,400 shares beneficially owned by Mr. Ehrenberg’s wife, Linda Ehrenberg. |
| (4) | Includes 50,000 shares of common stock issuable upon exercise of stock options. |
| (5) | Includes 504,600 shares of common stock issuable upon exercise of stock options. |
| (6) | Includes 474,600 shares of common stock issuable upon exercise of stock options. |
50
Table of Contents
| (7) | Includes 18,750 common shares issuable upon exercise of certain warrant issued in connection with the conversion of notes issued in the Additional Financing. Also includes 499,875 shares of common stock issuable upon exercise of warrants issued in connection with the Financing and 288,462 shares of common stock issuable upon exercise of a certain warrant issued in connection with a March 2009 purchase of Company’s common stock. |
| (8) | Includes 666,500 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with the Financing to Louis M. Jaffe 2004 Intangible Asset Mgmt. TR U/A DTD 5/24/04 and 298,077 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with a purchase of Company’s common stock in 2009. Also includes 1,817,061 shares held by the aforementioned trust, 250,004 shares held by the Louis Jaffe TTEE Irrevocable Trust – Jennifer Jaffe and 250,004 shares held by the Louis Jaffe TTEE Irrevocable Trust – Lara Jaffe Taylor. The natural person with voting power and investment power on behalf each of the aforementioned trusts is Louis M. Jaffe. Also includes 100,000 shares held by the Diana G. Jaffe Revocable Trust Dated 8/4/99 of which Diana G. Jaffe, Louis M. Jaffe’s wife, is the natural person with voting power and investment power on behalf of the trust and 250,000 shares of common stock issuable on exercise of a certain outstanding warrant issued to Louis M. Jaffe pursuant to a consulting agreement. |
| (9) | Includes 750,000 shares of common stock issuable upon conversion of certain outstanding convertible notes and 1,000,000 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with the Financing. Also includes 170, 137 shares of common stock issued upon conversion of all interest due under the Convertible Note. |
| (10) | Includes 999,750 shares of common stock issuable upon exercise of warrants issued in connection with the Financing in the name of MSSRPS, LLC. The natural person with voting power and investment power on behalf of MSSRPS, LLC is Marisa Stadmauer. |
| (11) | Includes 3,324,740 shares of common stock issued upon conversion of convertible notes. The natural person with voting power and investment power on behalf of Platinum Montaur Life Sciences, LLC is Mark Nordlich. Not included above are 3,999,000 shares of common stock issuable upon exercise of warrants issued in connection with the Financing. The warrants, as amended, may not be exercised to the extent the shares resulting from such exercise, when aggregated with its other holdings of the our common stock as of the time of exercise, would result in Platinum Montaur Life Sciences, LLC holding in excess of 9.9% of all our common stock at the time of the exercise on a beneficially converted basis. |
| (12) | Includes 598,261 shares of common stock issuable upon conversion of certain outstanding convertible notes and 500,000 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with the Financing in the name of RP Capital LLC. Erick Richardson and Nimish Patel of Richardson & Patel LLP own RP Capital LLC. Also includes 373,108 shares in the name of Richardson & Patel LLP and warrants to purchase an additional 392,308 shares. Erick Richardson is a partner at Richardson & Patel LLP. The natural person with voting and investment control over the shares held by these entities is Erick Richardson. |
| (13) | Includes 825,000 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with the Financing and 80,000 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with a purchase of Company’s common stock in 2009. All of the foregoing warrants are held in the name of Leah Kaplan-Samuels and Leonard Samuels JTWROS. The natural persons with voting power and investment power on behalf of Leah Kaplan-Samuels and Leonard Samuels JTWROS are Leah Kaplan-Samuels and Leonard Samuels. Also includes 3,193,466 shares of common stock issued upon conversion of convertible notes and 3,025,000 shares of common stock issuable upon exercise of certain outstanding warrants in the Financing issued to shareholder RBC Dain – Custodian for Leonard Samuels IRA. |
| (14) | Includes 825,000 shares of common stock issuable upon exercise of warrants issued in connection with the Financing and 80,000 shares of common stock issuable upon exercise of certain outstanding warrants issued in connection with a purchase of Company’s common stock in 2009. All of the foregoing warrants are held in the name of Leah Kaplan-Samuels and Leonard Samuels JTWROS. The natural persons with voting power and investment power on behalf of Leah Kaplan-Samuels and Leonard Samuels JTWROS are Leah Kaplan-Samuels and Leonard Samuels. |
51
Table of Contents
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
Timothy N. Tangredi, the Company’s Chief Executive Officer and Chairman, is a founder and a member of the board of directors of Aegis BioSciences, LLC (“Aegis”). Aegis, created in 1995, is a licensee of the Company’s nano-structured intellectual property and materials in the biomedical and healthcare fields. Mr. Tangredi spends approximately one to two days per month on Aegis business and is compensated by Aegis for his time and contribution(s). We granted Aegis two exclusive, world-wide licenses, the first in 1995 and the second in 2005. Pursuant to these licenses, Aegis has the right to use and sell products containing our polymer technologies in biomedical and health care applications. The first license was entered into in 1995 and has been amended twice. In 2005, we agreed to accept $150,000 as payment in full of all royalties and no further license revenue will be forthcoming. The second license allows Aegis the use of our intellectual property in the field of health care. A one-time payment of $50,000 was made under this license in 2005. In addition, under the second license Aegis is to make royalty payments of 1.5% of the net sales price it receives with respect to any personal hygiene product, surgical drape or clothing products (the latter when employed in medical and animal related fields) and license revenue it receives should Aegis grant a sublicense to a third party. To date Aegis has sold no such products nor has it received any licensing fees requiring a royalty payment be made to us. All obligations for such payments will end on the earlier of June 2, 2015 or upon the aggregate of all sums paid to us by Aegis under the agreement reaching $1 million. The term of each respective license runs for the duration of the patented technology.
On May 21, 2009, to evidence a loan, Company issued its Chief Executive Officer a promissory note in the principal amount of $51,900. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all accrued interest is to be paid in full to the holder no later than July 31, 2009. This note was paid in full prior to July 31, 2009.
On June 10, 2009, to evidence a loan, the Company issued a promissory note in the principal amount of $10,000 to Ethos Business Ventures, an entity in which its Chief Executive Officer holds a position. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all interest accrued is to be paid in full to the holder no later than July 31, 2009. This note was paid in full prior to July 31, 2009
On September 11, 2009, to evidence a loan, Company issued its Chief Executive Officer a promissory note in the principal amount of $124,000. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all accrued interest is to be paid in full to the holder no later than October 15, 2009. This note was paid in full prior to October 15, 2009.
On September 11, 2009, to evidence a loan, the Company issued a promissory note in the principal amount of $37,000 to Ethos Business Ventures, an entity in which its Chief Executive Officer holds a position. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all interest accrued is to be paid in full to the holder no later than October 15, 2009. This note was paid in full prior to October 15, 2009
The Company rents a building on a month to month basis from a related party which is wholly owned by two shareholders of the Company, one of which is Timothy N. Tangredi, our Chief Executive Officer. The base monthly rent expense is $3,800 per month. The Company also pays the taxes, insurance and some repairs on the building. For the year ending December 31, 2009 and 2008, the Company recorded $49,604 and $48,792, in rent expense to this related party, respectively.
There are no material relationships between us and our directors or executive officers except as previously discussed herein.
Since the beginning of our last fiscal year, we have not been a participant in any transaction, or proposed transaction, not disclosed herein in which any related person had or will have a direct or indirect material interest and in which the amount involved exceeds the lesser of $120,000 or one percent of our total assets at year end for the last two completed fiscal years.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES. |
Audit Fees
The aggregate audit fees billed for the years ended December 31, 2009 was $53,000. Audit services include the audits of the financial statements included in the Company’s annual reports on Form 10-K and reviews of interim financial statements included in the Company’s quarterly reports on Form 10-Q.
Audit-Related Fees
None.
Tax Fees
None
All Other Fees
Aggregate other fees billed for the year ended December 31, 2009 was $4,000.
Audit Committee Policies and Procedures
As of the date of this Annual Report, the Company does not have an established audit committee. The appointment of Cross, Fernandez & Riley LLP was approved by the Board of Directors as the principal auditors for the Company. There are no board members that are considered to have significant financial experience. When independent directors with the appropriate financial background join the board, the board plans to establish an audit committee, which will then adopt an appropriate charter and pre-approval policies and procedures in connection with services to be rendered by the independent auditors.
52
Table of Contents
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES. |
| No. |
Exhibit | |
| 3.1 | Certificate of Incorporation of The Dais Corporation filed April 8, 1993* | |
| 3.2 | Certificate of Amendment of the Certificate of Incorporation of The Dais Corporation filed February 21, 1997* | |
| 3.3 | Certificate of Amendment of the Certificate of Incorporation of The Dais Corporation filed June 25, 1998* | |
| 3.4 | Certificate of Amendment of the Certificate of Incorporation of Dais Analytic Corporation filed December 13, 1999* | |
| 3.5 | Certificate of Amendment of the Certificate of Incorporation of Dais Analytic Corporation filed September 26, 2000* | |
| 3.6 | Certificate of Amendment of the Certificate of Incorporation of Dais Analytic Corporation filed September 28, 2000* | |
| 3.7 | Certificate of Amendment of the Certificate of Incorporation of Dais Analytic Corporation filed August 28, 2007* | |
| 3.8 | Certificate of Amendment of the Certificate of Incorporation of Dais Analytic Corporation filed March 20, 2008* | |
| 3.9 | Bylaws of The Dais Corporation* | |
| 4.0 | Certificate of Amendment to the Certificate of Incorporation filed December 17, 2009, incorporated by reference to the exhibits included with the Definitive Proxy Statement Form DEF14A as filed on October 9, 2009 | |
| 4.1 | Form of Non-Qualified Stock Option Agreement* | |
| 4.2 | Form of Non-Qualified Option Agreement* | |
| 4.3 | Form of Warrant (Daily Financing)* | |
53
Table of Contents
| 4.4 | Form of Warrant (Financing)* | |
| 4.5 | Form of Warrant (Robb Trust Note and Additional Financing)* | |
| 4.6 | Form of Placement Agent Warrant (Financing)* | |
| 4.7 | Form of 9% Secured Convertible Note (Financing)* | |
| 4.8 | Form of Note (Robb Trust Note)* | |
| 4.9 | Form of Amendment to Note (Robb Trust Note)* | |
| 4.10 | Form of Warrant (Note Conversion)** | |
| 4.11 | Form of Warrant (2009 Stock Purchases)** | |
| 4.12 | Unsecured Promissory Note (Gostomski) | |
| 4.13 | Unsecured Promissory Note from Platinum-Montaur, incorporated by reference to the exhibits included with the Current Report on Form 8-K as filed on December 22, 2009 | |
| 4.14 | Unsecured Promissory Note from Samuels, incorporated by reference to the exhibits included with the Current Report on Form 8-K as filed on February 23, 2010 | |
| 4.15 | Unsecured Promissory Note from RBC Capital Corp., incorporated by reference to the exhibits included with the Current Report on Form 8-K as filed on February 23, 2010. | |
| 10.1 | 2000 Equity Compensation Plan* | |
| 10.2 | Form of Employee Non-Disclosure and Non-Compete Agreement* | |
| 10.3 | Amended and Restated Employment Agreement between Dais Analytic Corporation and Timothy N. Tangredi dated July 29, 2008* | |
| 10.4 | Amended and Restated Employment Agreement between Dais Analytic Corporation and Patricia K. Tangredi dated July 29, 2008* | |
| 10.5 | Commercial Lease Agreement between Ethos Business Venture LLC and Dais Analytic Corporation dated March 18, 2005* | |
| 10.6 | First Amendment of Lease Agreement between Ethos Business Venture LLC and Dais Analytic Corporation dated November 15, 2005* | |
| 10.7 | Form of Subscription Agreement (Daily Financing)* | |
| 10.8 | Form of Subscription Agreement (Financing)* | |
| 10.9 | Form of Registration Rights Agreement (Financing)* | |
| 10.10 | Form of Secured Patent Agreement (Financing)* | |
| 10.11 | Placement Agent Agreement between Dais Analytic Corporation and Legend Merchant Group, Inc., dated October 5, 2007* | |
| 10.12 | Exclusive Distributorship Agreement between Genertec America, Inc. and Dais Analytic Corporation entered into August 21, 2009, incorporated by reference to the exhibits included with the Current Report on Form 8-K, as filed August 27, 2009. | |
| 10.13 | Employee Non-Disclosure and Non-Compete Agreement entered into between Judith Norstrud and Dais Analytic Corporation on October 15, 2009, incorporated by reference to the exhibits included with the Current Report on Form 8-K, as filed October 16, 2009. | |
| 10.14 | 2009 Long Term Incentive Plan, incorporated by reference to the exhibits included with the Definitive Proxy Statement Form DEF14A as filed on October 9, 2009 | |
| 14.1 | Code of Ethics incorporated by reference to the exhibits included with the Annual Report on Form 10-K as filed on March 31, 2009 | |
| 31.1 | Certification of Chief Executive Officer pursuant to section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification of Chief Financial Officer pursuant to section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| * | Incorporated by reference to the exhibits included with the Registration Statement on Form S-1, File No. 333-152940, as filed August 11, 2008. |
| ** | Incorporated by reference to the exhibits included with the Current Report on Form 8-K, as filed March 13, 2009. |
54
Table of Contents
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| DAIS ANALYTIC CORPORATION | ||||||
| Dated: March 30, 2010 | By: | /S/ TIMOTHY N. TANGREDI | ||||
| Timothy N. Tangredi | ||||||
| Chairman of the Board | ||||||
| Chief Executive Officer and Director | ||||||
| (Principal Executive Officer) | ||||||
| Dated: March 30, 2010 | By: | /S/ JUDITH C. NORSTRUD | ||||
| Judith C. Norstrud | ||||||
| Chief Financial Officer and Treasurer | ||||||
| (Principal Financial and Accounting Officer) | ||||||
| Signatures |
Title |
Date | ||
| /S/ TIMOTHY N. TANGREDI Timothy N. Tangredi |
Chairman of the Board, Chief Executive Officer and Director | March 30, 2010 | ||
| /S/ ROBERT W. SCHWARTZ Robert W. Schwartz |
Director |
March 30, 2010 | ||
| /S/ RAYMOND KAZYAKA SR. Raymond Kazyaka Sr. |
Director |
March 30, 2010 | ||
55
Table of Contents
Dais Analytic Corporation
Years Ended December 31, 2009 and 2008
Report of Independent Registered Public Accounting Firm
56
Table of Contents
Financial Statements
Years Ended December 31, 2009 and 2008
Contents
| Report of Independent Registered Public Accounting Firm for the year ended December 31, 2009 |
F-1 | |
| Report of Independent Registered Public Accounting Firm for the year ended December 31, 2008 |
F-2 | |
| Financial Statements: |
||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-8 | ||
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Dais Analytic Corporation
Odessa, Florida
We have audited the accompanying balance sheet of Dais Analytic Corporation (“the Company”) as of December 31, 2009, and the related statements of operations, stockholders’ equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Dais Analytic Corporation as of December 31, 2009, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2, the Company has incurred significant losses since inception and has a capital deficit and stockholders’ deficit of $2,265,370 and $32,162,151 at December 31, 2009. These factors, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also discussed in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Cross, Fernandez & Riley LLP
Tampa, Florida
March 29, 2010
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors
Dais Analytic Corporation
Odessa, Florida
We have audited the accompanying balance sheet of Dais Analytic Corporation as of December 31, 2008 and the related statements of operations, changes in stockholders’ deficit, and cash flows for the year then ended. These financial statements are the responsibility of the management of Dais Analytic Corporation. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required at this time, to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Dais Analytic Corporation as of December 31, 2008 and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2, the Company incurred a net loss of $5,979,446 during the year ended December 31, 2008, has an accumulated deficit of $28,776,769, has negative working capital of $3,802,009, and a stockholders’ deficit of $4,697,436 at December 31, 2008. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Pender Newkirk & Company LLP
Pender Newkirk & Company LLP
Certified Public Accountants
Tampa, Florida
March 23, 2009
F-2
Table of Contents
Balance Sheets
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 1,085,628 | $ | 26,867 | ||||
| Accounts receivable |
187,434 | 188,970 | ||||||
| Inventory |
149,986 | 147,128 | ||||||
| Loan costs, net of accumulated amortization |
— | 1,004 | ||||||
| Prepaid expenses and other current assets |
103,571 | 31,181 | ||||||
| Total current assets |
1,526,619 | 395,150 | ||||||
| Property and equipment, net |
19,383 | 26,933 | ||||||
| Other assets: |
||||||||
| Deposits |
2,280 | 2,280 | ||||||
| Patents, net of accumulated amortization of $107,319 and $96,389 at December 31, 2009 and 2008, respectively |
72,464 | 44,129 | ||||||
| Total other assets |
74,744 | 46,409 | ||||||
| $ | 1,620,746 | $ | 468,492 | |||||
| Liabilities and Stockholders’ Deficit |
||||||||
| Current liabilities: |
||||||||
| Accounts payable, including related party payables of $150,740 and $105,925 at December 31, 2009 and 2008, respectively |
$ | 385,955 | $ | 380,022 | ||||
| Accrued compensation and related benefits |
1,314,356 | 1,147,389 | ||||||
| Accrued expenses, other |
223,597 | 340,115 | ||||||
| Current portion of deferred revenue |
292,457 | 84,145 | ||||||
| Current portion of notes payable, net of unamortized discount of $0 and $23,171 at December 31, 2009 and 2008, respectively |
1,575,624 | 2,245,488 | ||||||
| Total current liabilities |
3,791,989 | 4,197,159 | ||||||
| Long-term liabilities: |
||||||||
| Long-term portion of notes payable |
300,000 | 675,000 | ||||||
| Deferred revenue, net of current portion |
207,696 | 293,769 | ||||||
| Total long-term liabilities |
507,696 | 968,769 | ||||||
| Stockholders’ deficit: |
||||||||
| Preferred stock; $.01 par value; 10,000,000 shares authorized; 0 shares issued and outstanding |
||||||||
| Common stock; $0.01 par value; 200,000,000 shares authorized; 29,352,930 and 12,162,398 shares issued and 29,095,717 and 11,905,185 shares outstanding at December 31, 2009 and 2008, respectively |
293,530 | 121,624 | ||||||
| Capital in excess of par value |
30,461,794 | 25,253,196 | ||||||
| Prepaid services paid for with common stock |
— | (23,375 | ) | |||||
| Accumulated deficit |
(32,162,151 | ) | (28,776,769 | ) | ||||
| (1,406,827 | ) | (3,425,324 | ) | |||||
| Treasury stock at cost, 257,213 shares |
(1,272,112 | ) | (1,272,112 | ) | ||||
| Total stockholders’ deficit |
(2,678,939 | ) | (4,697,436 | ) | ||||
| $ | 1,620,746 | $ | 468,492 | |||||
The Accompanying notes are an integral part of the financial statements
F-3
Table of Contents
Statements of Operations
| Year Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Revenue: |
||||||||
| Sales |
$ | 1,447,071 | $ | 931,289 | ||||
| License fees |
84,144 | 84,144 | ||||||
| 1,531,215 | 1,015,433 | |||||||
| Expenses: |
||||||||
| Cost of goods sold |
1,071,098 | 796,217 | ||||||
| Selling, general and administrative |
3,224,592 | 2,935,552 | ||||||
| 4,295,690 | 3,731,769 | |||||||
| Loss from operations |
(2,764,475 | ) | (2,716,336 | ) | ||||
| Other expense (income): |
||||||||
| Interest expense |
621,574 | 3,282,768 | ||||||
| Interest income |
(667 | ) | (19,658 | ) | ||||
| 620,907 | 3,263,110 | |||||||
| Net loss |
$ | (3,385,382 | ) | $ | (5,979,446 | ) | ||
| Net loss per common share, basic and diluted |
$ | (0.17 | ) | $ | (0.57 | ) | ||
| Weighted average number of common shares, basic and diluted |
19,960,150 | 10,522,511 | ||||||
The accompanying notes are an integral part of the financial statements.
F-4
Table of Contents
Statements of Stockholders’ Deficit
Years Ended December 31, 2009 and 2008
| Common Stock | Capital in Excess of |
Accumulated | Deferred Non-Cash |
Prepaid Services Paid for with Common |
Treasury | Total Stockholders’ |
|||||||||||||||||||||||
| Shares | Amount | Par Value | Deficit | Costs | Stock | Stock | Deficit | ||||||||||||||||||||||
| Balance, December 31, 2007 |
8,742,797 | $ | 87,428 | $ | 23,389,320 | $ | (22,797,323 | ) | $ | (55,000 | ) | — | $ | (1,266,112 | ) | $ | (641,687 | ) | |||||||||||
| Issuance of common stock for conversion of notes payable and related accrued interest |
439,293 | 4,393 | 104,147 | — | — | — | — | 108,540 | |||||||||||||||||||||
| Beneficial conversion feature |
— | — | 266,814 | — | — | — | — | 266,814 | |||||||||||||||||||||
| Issuance of warrants with convertible debt |
— | — | 298,005 | — | — | — | — | 298,005 | |||||||||||||||||||||
| Offering costs |
— | — | (17,340 | ) | — | — | — | — | (17,340 | ) | |||||||||||||||||||
| Issuance of common stock and warrants for offering costs |
392,308 | 3,923 | (3,923 | ) | — | — | — | — | — | ||||||||||||||||||||
| Write off of non-cash offering costs |
— | — | — | — | 55,000 | — | (6,000 | ) | 49,000 | ||||||||||||||||||||
| Issuance of common stock for services, net of amortization of $27,625 |
148,000 | 1,480 | 56,880 | — | — | $ | (23,375 | ) | — | 34,985 | |||||||||||||||||||
| Stock-based compensation expense |
— | — | 1,183,693 | — | — | — | — | 1,183,693 | |||||||||||||||||||||
| Issuance of common stock |
2,440,000 | 24,400 | (24,400 | ) | — | — | — | — | — | ||||||||||||||||||||
| Net loss |
— | — | — | (5,979,446 | ) | — | — | — | (5,979,446 | ) | |||||||||||||||||||
| Balance, December 31, 2008 |
12,162,398 | 121,624 | 25,253,196 | (28,776,769 | ) | — | (23,375 | ) | (1,272,112 | ) | (4,697,436 | ) | |||||||||||||||||
| Issuance of common stock for conversion of notes payable and related accrued interest |
13,553,822 | 135,538 | 2,576,062 | — | — | — | — | 2,711,600 | |||||||||||||||||||||
| Issuance of common stock and warrant for services |
344,692 | 3,448 | 105,029 | — | — | 23,375 | — | 131,852 | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | 1,504,669 | — | — | — | — | 1,504,669 | |||||||||||||||||||||
| Issuance of warrants for debt conversion |
413,008 | — | — | — | — | 413,008 | |||||||||||||||||||||||
| Issuance of common stock and warrants for cash |
2,490,385 | 24,904 | 613,596 | — | — | — | — | 638,500 | |||||||||||||||||||||
| Exercise of warrants and options |
801,633 | 8,016 | (3,766 | ) | — | — | — | — | 4,250 | ||||||||||||||||||||
| Net loss |
— | — | — | (3,385,382 | ) | — | — | — | (3,385,382 | ) | |||||||||||||||||||
| Balance, December 31, 2009 |
29,352,930 | $ | 293,530 | $ | 30,461,794 | $ | (32,162,151 | ) | $ | — | $ | — | $ | (1,272,112 | ) | $ | (2,678,939 | ) | |||||||||||
The accompanying notes are an integral part of the financial statements.
F-5
Table of Contents
Statements of Cash Flows
| Years Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Operating activities |
||||||||
| Net loss |
$ | (3,385,382 | ) | $ | (5,979,446 | ) | ||
| Adjustments to reconcile net loss to net cash used by operating activities: |
||||||||
| Depreciation and amortization |
19,826 | 16,187 | ||||||
| Amortization of deferred loan costs |
1,004 | 102,416 | ||||||
| Amortization of discount on convertible notes |
144 | 1,525,598 | ||||||
| Amortization of the beneficial conversion feature on convertible notes |
29,992 | 1,388,216 | ||||||
| Write off of deferred noncash offering costs |
— | 49,000 | ||||||
| Issuance of options and warrants for services |
110,316 | 34,985 | ||||||
| Stock based compensation expense |
1,504,669 | 1,183,693 | ||||||
| Issuance of common stock warrants to induce conversion of notes payable |
413,008 | — | ||||||
| (Increase) decrease in: |
||||||||
| Accounts receivable |
1,536 | (182,220 | ) | |||||
| Inventory |
(2,858 | ) | (73,499 | ) | ||||
| Prepaid expenses and other current assets |
(50,853 | ) | (7,263 | ) | ||||
| Increase (decrease) in: |
||||||||
| Accounts payable and accrued expenses |
251,014 | 150,091 | ||||||
| Accrued compensation and related benefits |
166,967 | 51,876 | ||||||
| Deferred revenue |
122,239 | (84,144 | ) | |||||
| Net cash used by operating activities |
(818,378 | ) | (1,824,510 | ) | ||||
| Investing activities |
||||||||
| Increase in patent costs |
(39,265 | ) | ||||||
| Purchase of property and equipment |
(1,346 | ) | (18,855 | ) | ||||
| Net cash used by investing activities |
(40,611 | ) | (18,855 | ) | ||||
| Financing activities |
||||||||
| Proceeds from issuance of notes payable |
1,565,000 | 500,000 | ||||||
| Proceeds received from escrow |
— | 1,000,000 | ||||||
| Payments on notes payable |
(290,000 | ) | (100,000 | ) | ||||
| Payments for offering costs |
— | (34,000 | ) | |||||
| Proceeds from advance from related party |
222,900 | — | ||||||
| Repayments of advance from related party |
(222,900 | ) | — | |||||
| Issuance of common stock and exercise of warrants for cash |
642,750 | — | ||||||
| Net cash provided by financing activities |
1,917,750 | 1,366,000 | ||||||
| Net increase (decrease) in cash and cash equivalents |
1,058,761 | (477,365 | ) | |||||
| Cash and cash equivalents, beginning of period |
26,867 | 504,232 | ||||||
| Cash and cash equivalents, end of period |
$ | 1,085,628 | $ | 26,867 | ||||
| Cash paid during the year for interest |
$ | 42,651 | $ | 10,100 | ||||
The accompanying notes are an integral part of the financial statements.
F-6
Table of Contents
Dais Analytic Corporation
Statements of Cash Flows
Supplemental disclosures of cash flow information
and noncash investing and financing activities:
During the years ended December 31, 2009 and 2008, the Company issued 13,553,822 and 439,293 shares of common stock in conversion of $2,350,000 and $100,000 of notes payable and $361,600 and $8,540 of accrued interest, respectively.
During the year ended December 31, 2009, the Company issued 344,692 shares of common stock and warrants for services valued at $110,316.
During the year ended December 31, 2008 the Company issued 540,308 shares of common stock valued at $229,991 as payment for services.
During the year ended December 31, 2008, the Company issued convertible notes payable with a beneficial conversion feature of $245,106 and a discount equivalent to the relative fair value of the accompanying warrants of $254,894.
The accompanying notes are an integral part of the financial statements.
F-7
Table of Contents
Notes to Financial Statements
Years Ended December 31, 2009 and 2008
1. Background Information
Dais Analytic Corporation (the “Company”), a New York corporation, has developed and is commercializing applications using its nano-structure polymer technology. The first commercial product is an energy recovery ventilator (ERV) (cores and systems) for use in commercial Heating, Ventilating, and Air Conditioning (HVAC) applications. In addition to direct sales, the Company licenses its nano-structured polymer technology to strategic partners in the aforementioned application and is in various stages of development with regard to other applications employing its base technologies. The Company was incorporated in April of 1993 with its corporate headquarters located in Odessa, Florida.
The Company is dependent on third parties to manufacture the key components needed for our nano-structured based materials and value added products made with these materials. Accordingly, a supplier’s failure to supply components in a timely manner, or to supply components that meet our quality, quantity and cost requirements or our technical specifications, or the inability to obtain alternative sources of these components on a timely basis or on terms acceptable to us, would create delays in production of our products or increase our unit costs of production. Certain of the components contain proprietary products of our suppliers, or the processes used by our suppliers to manufacture these components are proprietary. If we are required to replace any of our suppliers, while we should be able to obtain comparable components from alternative suppliers at comparable costs, this would create a delay in production.
For the years ended December 31, 2009 and 2008, five and four customers accounted for approximately 66% and 64% of the Company’s total revenue, respectively. At December 31, 2009 and 2008 amounts due from these customers was approximately 24.5% and 56.7% of total accounts receivable, respectively.
2. Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. For the year ended December 31, 2009, the Company incurred a net loss of $3,385,382 and has incurred significant losses since inception. As of December 31, 2009, the Company has an accumulated deficit of $32,162,151, negative working capital of $2,265,370 and a stockholders’ deficit of $2,678,939. The Company used $818,378 and $1,824,510 of cash from operations during 2009 and 2008, respectively, which was funded by proceeds from debt and equity financings. There is no assurance that such financing will be available in the future. In view of these matters, there is substantial doubt that the Company will continue as a going concern. The Company is currently pursuing the following sources of short and long-term working capital:
| 1. | We are currently holding preliminary discussions with parties who are interested in licensing, purchasing the rights to, or establishing a joint venture to commercialize certain applications of our technology. |
| 2. | We are seeking growth capital from certain strategic and/or government (grant) related sources. In addition to said capital, these sources may, pursuant to any agreements that may be developed in conjunction with such funding, assist in the product definition and design, roll-out, and channel penetration of our products. As part of this step we will attempt to take advantage of key programs associated with the recently enacted American Recovery and Reinvestment Act of 2009. |
The Company’s ability to continue as a going concern is highly dependent on our ability to obtain additional sources of cash flow sufficient to fund our working capital requirements. However, there can be no assurance that the Company will be successful in its efforts to secure such cash flow. Any failure by us to timely procure additional
F-8
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
financing or investment adequate to fund our ongoing operations, including planned product development initiatives and commercialization efforts, will have material adverse consequences on our financial condition, results of operations and cash flows.
The financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
3. Significant Accounting Policies
The significant accounting policies followed are:
FASB Codification - In June 2009, the FASB issued ASC 105, Generally Accepted Accounting Principles, effective for interim and annual reporting periods ending after September 15, 2009. This statement establishes the Codification as the source of authoritative accounting principles used in the preparation of financial statements in conformity with generally accepted accounting principles. The Codification does not replace or affect guidance issued by the SEC or its staff. As a result of the Codification, the references to authoritative accounting pronouncements included herein in this Annual Report on Form 10-K now refer to the Codification topic section rather than a specific accounting rule as was past practice.
Use of estimates - The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and cash equivalents - All cash, other than held in escrow, is maintained with a major financial institution in the United States. Deposits with this bank may exceed the amount of insurance provided on such deposits. Temporary cash investments with an original maturity of three months or less are considered to be cash equivalents.
Accounts receivable - Accounts receivable consist primarily of receivables from the sale of our ERV products. The Company regularly reviews accounts receivable for any bad debts based on an analysis of the Company’s collection experience, customer credit worthiness, and current economic trends. Based on management’s review of accounts receivable, no allowance for doubtful accounts is considered necessary at December 31, 2009 and 2008.
Debt issuance costs - Direct loan costs incurred with the issuance of long-term debt are deferred and amortized to interest expense over the life of the related debt. For the years ended December 31, 2009 and 2008, the Company recorded amortization of direct loan costs of $1,004 and $102,416, respectively.
Inventory - Inventory consists of raw materials and work-in-process and is stated at the lower of cost, determined by first-in, first-out method, or market. Market is determined based on the net realizable value, with appropriate consideration given to obsolescence, excessive levels, deterioration and other factors. At December 31, 2009 and 2008, the Company had $2,160 and $2,043 of in-process inventory, respectively. A reserve is recorded for any inventory deemed excessive or obsolete. No reserve is considered necessary at December 31, 2009 and 2008.
Property and equipment - Property and equipment are recorded at cost. Depreciation is calculated using accelerated methods over the estimated useful lives of the assets ranging from 5 to 7 years. Depreciation expense was approximately $8,900 and $8,500 for the years ended December 31, 2009 and 2008, respectively. Gains and losses upon disposition are reflected in the statement of operations in the period of disposition. Maintenance and repair expenditures are charged to expense as incurred.
Intangible assets - Identified intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. The Company’s existing intangible
F-9
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
assets consist solely of patents. Patents are amortized over their estimated useful or economic lives of 15 years. Patent amortization expense was approximately $10,100 and $9,300 for the years ended December 31, 2009 and 2008, respectively. Total patent amortization expense for the next five years is estimated to be approximately $12,000 per year.
Long-lived assets - Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the book value of the asset may not be recoverable. The Company periodically evaluates whether events and circumstances have occurred that indicate possible impairment. When impairment indicators exist, the Company uses market quotes, if available or an estimate of the future undiscounted net cash flows of the related asset or asset group over the remaining life in measuring whether or not the asset values are recoverable. There have been no significant impairments of long-lived assets during the two-year period ended December 31, 2009.
Research and development expenses - Expenditures for research, development, and engineering of products are expensed as incurred. For the years ended December 31, 2009 and 2008, the Company incurred research and development costs of approximately $6,600 and $36,000, respectively.
Stock issuance costs - Stock issuance costs are recorded as a reduction of the related proceeds through a charge to stockholders’ equity.
Common stock - The Company records common stock issuances when all of the legal requirements for the issuance of such common stock have been satisfied.
Revenue recognition - Generally, the Company recognizes revenue for its products upon shipment to customers, provided no significant obligations remain and collection is probable. Our ConsERV product typically carries a warranty of two years for all parts contained therein with the exception of the energy recovery ventilator core which typically carries a 10 year warranty. The warranty includes replacement of defective parts. Warranty cost has been immaterial to our overall operations in the past and therefore, the Company has not recorded an accrual for any estimated warranty expenses. Revenue derived from the sale of licenses is deferred and recognized as revenue on a straight-line basis over the life of the license, or until the license arrangement is terminated. The Company recognized revenue of approximately $84,100 from license agreements for each of the years ended December 31, 2009 and 2008.
Stock based compensation - The Company recognizes all share-based payments to employees, including grants of employee stock options, as compensation expense in the financial statements based on their fair values. That expense will be recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
The value of each grant is estimated at the grant date using the Black-Scholes option model with the following assumptions for options granted during the years ended December 31, 2009 and 2008:
| Years Ended December 31, | ||||||
| 2009 | 2008 | |||||
| Dividend rate |
0 | % | 0 | % | ||
| Risk free interest rate |
1.65% – 3.49 | % | 2.64% – 3.98 | % | ||
| Expected term |
5 – 10 years | 5 – 10 years | ||||
| Expected volatility |
92% – 106 | % | 80% – 114 | % | ||
F-10
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
The basis for the above assumptions are as follows: the dividend rate is based upon the Company’s history of dividends; the risk-free interest rate for periods within the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant; the expected term was calculated based on the Company’s historical pattern of options granted and the period of time they are expected to be outstanding; and expected volatility was calculated by review of a peer company’s historical activity.
Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Based on historical experience of forfeitures, the Company estimated forfeitures at 0% and 22% for the years ended December 31, 2009 and 2008, respectively, and incorporated this rate in the estimated fair value of employee option grants during 2009 and 2008.
Non-Employee Stock-Based Compensation - The Company accounts for stock based compensation awards issued to non-employees for services and financing arrangements, as prescribed by FASB ASC 505-50, Equity-Based Payments to Non-Employees, at either the fair value of the services rendered or the instruments issued in exchange for such services, whichever is more readily determinable. The fair value of common stock issued for services is based on the closing stock price on the date the common stock was issued. The fair value of warrants issued in 2009 and 2008 was calculated using the Black-Scholes model with the following assumptions: Expected life in years: 5-10 years and 5-10 years, respectively; Estimated volatility 94% - 105% and 80% - 114%, respectively; Risk-free interest rate: 2.02% - 3.21% and 2.64% - 3.98%, respectively; Dividend yield: 0%.
Financial instruments - In September 2006, the Financial Accounting Standards Board (FASB) introduced a framework for measuring fair value and expanded required disclosure about fair value measurements of assets and liabilities. The Company adopted the standard for those financial assets and liabilities as of the beginning of the 2008 fiscal year and the impact of adoption was not significant. FASB Accounting Standards Codification (ASC) 820 “Fair Value Measurements and Disclosures” (ASC 820) defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy are described below:
| • | Level 1 - Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
| • | Level 2 - Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly, including quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted prices that are observable for the asset or liability (e.g., interest rates); and inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
| • | Level 3 - Inputs that are both significant to the fair value measurement and unobservable. |
Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of December 31, 2009. The respective carrying value of certain on-balance-sheet financial instruments approximated their fair values due to the short-term nature of these instruments. These financial instruments include accounts receivable, other current assets, accounts payable, accrued compensation and accrued expenses. The fair value of the Company’s notes payable is estimated based on current rates that would be available for debt of similar terms which is not significantly different from its stated value.
F-11
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
On January 1, 2009, the Company applied ASC 820 for all non-financial assets and liabilities measured at fair value on a non-recurring basis. The adoption of ASC 820 for non-financial assets and liabilities did not have a significant impact on the Company’s financial statements.
Income taxes - Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due plus deferred taxes resulting from temporary differences. Such temporary differences result from differences in the carrying value of assets and liabilities for tax and financial reporting purposes. The deferred tax assets and liabilities represent the future tax consequences of those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company adopted the provisions of FASB ASC 740-10 “Uncertainty in Income Taxes” (ASC 740-10), on January 1, 2007. The Company has not recognized a liability as a result of the implementation of ASC 740-10. A reconciliation of the beginning and ending amount of unrecognized tax benefits has not been provided since there is no unrecognized benefit since the date of adoption. The Company has not recognized interest expense or penalties as a result of the implementation of ASC 740-10. If there were an unrecognized tax benefit, the Company would recognize interest accrued related to unrecognized tax benefits in interest expense and penalties in operating expenses.
Loss per share - Basic and diluted earnings per share are computed based on the weighted-average common shares and common share equivalents outstanding during the period. Common share equivalents consist of stock options, warrants and convertible notes payable. Common share equivalents of 30,003,977 and 25,722,521 were excluded from the computation of diluted earnings per share for the years ended December 31, 2009 and 2008, respectively, because their effect is anti-dilutive.
Reclassifications - Certain reclassifications have been made to the financial statements as of and for the year ended December 31, 2008 to conform to the presentation as of and for the year ended December 31, 2009.
Recent accounting pronouncements
In October 2009, the FASB issued Accounting Standard Update (“ASU”) No. 2009-13, Multiple-Deliverable Revenue Arrangements (“ASU 2009-13”) and No. 2009-14, Certain Revenue Arrangements that include Software Elements (“ASU 2009-14”). These standards update FASB ASC 605, Revenue Recognition (“ASC 605”) and FASB ASC 985, Software (“ASC 985”). The amendments to ASC 605 requires entities to allocate revenue in an arrangement using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The amendments to ASC 985 remove tangible products from the scope of software revenue guidance and provide guidance on determining whether software deliverables in an arrangement that includes a tangible product are covered by the scope of the software revenue guidance. These amendments to ASC 605 and ASC 985 should be applied on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, with early adoption permitted. The Company adopted these amendments on January 1, 2010. Management does not believe that the adoption of this standard will have a material impact on the Company’s financial statements.
In January 2010, the FASB issued ASU No. 2010-06, Fair Value Measurements and Disclosures (“ASU 2010-06”). This standard updates FASB ASC 820, Fair Value Measurements (“ASC 820”). ASU 2010-06 requires additional disclosures about fair value measurements including transfers in and out of Levels 1 and 2 and separate disclosures about purchases, sales, issuances, and settlements relating to Level 3 measurements. It also clarifies existing fair value disclosures about the level of disaggregation and about inputs and valuation techniques used to measure fair value. The standard is effective for interim and annual reporting periods beginning after December 15, 2009 except for the disclosures about purchases, sales, issuances and settlements which is effective for fiscal years beginning after December 15, 2010 and for interim periods within those fiscal years. The Company adopted ASU 2010-06 on January 1, 2010, which had no material impact on the financial statements.
Other recent accounting pronouncements issued by the FASB (including its EITF), the AICPA, and the SEC did not or are not believed by management to have a material impact on the Company’s present or future financial statements.
F-12
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
4. Property and Equipment
Property and equipment consist of the following:
| December 31, | ||||||
| 2009 | 2008 | |||||
| Furniture and fixtures |
$ | 33,530 | $ | 33,530 | ||
| Computer equipment |
57,344 | 106,260 | ||||
| Office and lab equipment |
194,429 | 194,429 | ||||
| 285,303 | 334,219 | |||||
| Less accumulated depreciation |
265,920 | 307,286 | ||||
| $ | 19,383 | $ | 26,933 | |||
5. Notes Payable
Notes payable consist of the following:
| December 31, | ||||||
| 2009 | 2008 | |||||
| Convertible notes payable; interest at 9.0%; $150,000 currently in default and $175,000 due July 2010; collateralized by the Company’s patents and patent applications, net of unamortized discount and beneficial conversion feature of $0 and $30,136 at 2009 and 2008, respectively |
$ | 325,000 | $ | 2,919,864 | ||
| Note payable to investor; 7% interest; unsecured; due January 16, 2011 |
300,000 | — | ||||
| Note payable to investor; interest at 10% per annum; due June 17, 2010 |
1,000,000 | — | ||||
| Note payable to an investor; 10% interest; unsecured; due June 30, 2010 |
250,000 | — | ||||
| Note payable; related party |
624 | 624 | ||||
| 1,875,624 | 2,920,488 | |||||
| Less amounts currently due |
1,575,624 | 2,245,488 | ||||
| $ | 300,000 | $ | 675,000 | |||
Convertible Notes
During December 2007 and January 2008, the Company issued convertible promissory notes (the “Convertible Notes”) and warrants to purchase common stock in exchange for proceeds totaling $2,950,000. The Convertible Notes bear interest at nine percent per annum and have stated maturity dates from December 2008 to January 2009. The Convertible Notes are repayable in cash or convertible into shares of the Company’s stock at a rate of one share per $0.20 of outstanding principal and interest. Warrants to purchase 14,750,000 shares of the Company’s common stock accompanying the Convertible Notes are, subject to certain limitations, exercisable at $0.25 per share, vest immediately, and expire between December 2012 and January 2013.
The Convertible Notes contain an embedded conversion feature. The Company accounted for this conversion feature and the detachable warrants by allocating the proceeds from issuance of the convertible notes to the beneficial conversion feature and the warrants based on their relative fair values. The Company concluded that the warrants should be recorded as a component of permanent equity based on applicable accounting guidance.
To recognize the fair value of the warrants, the Company discounted the notes and increased additional paid in capital. The fair value of the beneficial conversion feature of $1,383,437 and discount of $1,566,563 related to the warrants were amortized over the term of the Convertible Notes. For the years ended December 31, 2009 and 2008, the Company recognized interest expense from the amortization of the beneficial conversion feature and discount of $30,136 and $2,848,995, respectively.
F-13
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
During the year ended December 31, 2009 eighteen holders converted their Convertible Notes, having an aggregate principal balance of $2,350,000 plus accrued interest of $361,600, into 13,557,993 shares of common stock. Some of the holders converted during periods in which we were offering an additional warrant as an inducement to convert. In accordance with said offers we issued additional warrants to purchase 1,665,000 shares of common stock, exercisable immediately at $0.25 per share and valued at $126,367, and 575,000 warrants, exercisable immediately at $0.75 per share valued at $286,641 which was recorded as interest expense during the twelve months ended December 31, 2009.
During 2009, four investors holding Convertible Notes with an aggregate outstanding principal balance of approximately $450,000 at December 31, 2008 notified the Company that they were asserting their rights to receive payment of the principal and interest pursuant to the terms of the Convertible Notes. In June of 2009, three of these investors, holding an aggregate principal note balance of $250,000, entered into a confession of judgment with the Company. Under that agreement, the three investors had the right, should the Company fail to pay all principal and interest due pursuant to their Convertible Notes on or before September 11, 2009, to file the confession of judgment with the court and seek to secure a judgment against the Company in the amount of all principal and interest due under their Convertible Notes together with the reasonable cost and expense of collection. All interest and principal related to the three Convertible Notes, $289,803 in the aggregate, was paid in full by the Company on or before September 11, 2009. In July 2009, the fourth investor, holding a Convertible Note in the principal amount of $200,000, agreed to extend said note to September 2009. In November 2009, this investor and the Company modified the Convertible note to extend the maturity date of said note to July 2010, pay the principal amount due in eight monthly installments commencing December of 2009, end the accrual of interest as of November 20, 2009 and convert the $34,861 in interest due under the Convertible Note as of November 20, 2009 into 170,137 shares of Company’s common stock. As of December 31, 2009 the outstanding principal balance of said loan was $175,000.
As of December 31, 2009, $325,000 of principal on the Convertible Notes was outstanding, of which $175,000 has been extended to July 2010 with the remaining notes currently in default and due and payable in full.
Other Notes
In July 2009 we secured a loan of $300,000 from an investor and issued the lender an unsecured promissory note for the principal amount on December 8, 2009. Pursuant to the terms of the note, we are to pay the note holder simple interest at the rate seven percent per annum commencing on July 17, 2009 with all interest and principal due there under payable in cash on or before January 16, 2011. If an event of default were to occur the interest rate would increase to ten percent for the duration of the event. Should we not cure the default within 60 days of receiving notice the note holder may, at his option, declare all interest accrued and unpaid and principal outstanding immediately due and payable
In December 2009 we secured a loan in the principal amount of $1,000,000 from an investor and issued the lender an unsecured promissory note. Pursuant to the terms of the note, we are to pay the holder simple interest at the rate ten percent per annum commencing on the date of issuance with all interest and principal due and payable in cash on or before June 17, 2010. The note has equal standing with all other existing notes with respect to seniority. We may not incur more than $500,000 in additional debt without the holder’s prior approval and said additional debt may not be senior to this promissory note. During the term of the note, the holder has the right to participate, by investing additional funds the total amount of which may not exceed the outstanding balance of the note, in any subsequent financings undertaken by Company. Any such participation shall be upon the same terms as provided for in the subsequent financing. If an event of default were to occur and said default is not cured within the allotted period, the holder may declare all principal and interest due and payable without presentment, demand, protest or notice. Further, in addition to all remedies available under law holder may in the event of a default opt to convert the principal and accrued and unpaid interest outstanding under the note into any debt or equity security which the Company issued after the date of this note and prior to the date of full payment of this note in accordance with the same terms as the subsequent financing.
F-14
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
The Company secured loans from two investors in the principal amounts of $250,000 and $620,000. The loan amounts were received by the Company on December 31, 2009 and February 18, 2010, respectively, and the Company issued the lenders unsecured promissory notes with respect to said loans on February 19, 2010. Pursuant to the terms of the notes, the Company is to pay the holders simple interest at the rate ten percent per annum commencing on the date of issuance with all interest and principal due and payable in cash on or before June 30, 2010 and August 10, 2010. The notes have equal standing with all other existing notes with respect to seniority. After receipt of proceeds on the foregoing loans, we may not incur more than $500,000 in debt without the holders’ prior approval and said additional debt may not be senior to these promissory notes. During the term of the notes, each note holder has the right to participate, by investing additional funds the total amount of which may not exceed the outstanding balance of the holder’s note, in any subsequent financings undertaken by Company. Any such participation shall be upon the same terms as provided for in the subsequent financing. If an event of default were to occur and said default is not cured within the allotted period, the holders may declare all principal and accrued and unpaid interest due and payable without presentment, demand, protest or notice. Further, in addition to all remedies available under law, each holder may in the event of a default opt to convert the principal and interest outstanding under its note into any debt or equity security which Company issued after the date of its note and prior to the date of full payment of its note in accordance with the same terms as the subsequent financing.
Accrued interest on the notes was $13,501 and $268,453 at December 31, 2009 and 2008, respectively.
6. Related Party Transactions
Timothy Tangredi, the Company’s Chief Executive Officer and Chairman, is a founder and a member of the Board of Directors of Aegis Biosciences, LLS (“Aegis”). Aegis, created in 1995, is a licensee of the Company’s nano-structured intellectual property and materials in the biomedical and healthcare fields. Mr. Tangredi spends approximately one to two days per month on Aegis business and is compensated by Aegis for his time and contribution(s). We granted Aegis two exclusive, world-wide licenses, the first in 1995 and the second in 2005. Pursuant to these licenses, Aegis has the right to use and sell products containing our polymer technologies in biomedical and healthcare applications. The first license was entered into in 1995 has been amended twice. In 2005, we agreed to accept $150,000 as payment in full of all royalties and no further license revenue will be forthcoming. The second license allows Aegis the use of our intellectual property in the field of healthcare. A one-time payment of $50,000 was made under this license in 2005. In addition, under the second license Aegis is to make royalty payments of 1.5% of the net sales price it receives with respect to any personal hygiene product, surgical drape or clothing products (the latter when employed in medical and animal related fields) and license revenue it receives should Aegis grant a sublicense to a third party. To date Aegis has sold no such products nor has it received any licensing fees requiring a royalty payment be made to us. All obligations for such payments will end on the earlier of June 2, 2015 or upon the aggregate of all sums paid to us by Aegis under the agreement reaching $1 million. The term of each respective license runs for the duration of the patented technology.
The Company rents a building that is owned by two stockholders of the Company, one of which is the Chief Executive Officer. Rent expense for this building is $3,800 per month. The Company recognized rent expense of approximately $49,000 in each of years ended December 31, 2009 and 2008. At December 31, 2009 and 2008, $150,740 and $105,925, respectively, were included in accounts payable for amounts owed to these stockholders.
The Company also has accrued compensation due to the Chief Executive Officer and one other employee for deferred salaries earned and unpaid as of December 31, 2009 and 2008 of $1,314,356 and $1,147,389, respectively.
On May 21, 2009, to evidence a loan, the Company issued its Chief Executive Officer a promissory note in the principal amount of $51,900. The note is unsecured and bears a simple interest rate of 9% per annum. The principle amount plus all accrued interest is to be paid in full to the holder no later than July 31, 2009. This note was paid in full prior to maturity.
F-15
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
On June 10, 2009, to evidence a loan, the Company issued a promissory note in the principal amount of $10,000 to Ethos Business Ventures, an entity in which the Company’s Chief Executive Officer holds a controlling financial position. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all interest accrued is to be paid in full to the holder no later than July 31, 2009. This note was paid in full prior to July 31, 2009.
On September 11, 2009, to evidence a loan, Company issued its Chief Executive Officer a promissory note in the principal amount of $124,000. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all accrued interest is to be paid in full to the holder no later than October 15, 2009. This note was paid in full prior to October 15, 2009.
On September 11, 2009, to evidence a loan, the Company issued a promissory note in the principal amount of $37,000 to Ethos Business Ventures, an entity in which its Chief Executive Officer holds a position. The note is unsecured and bears a simple interest rate of 9% per annum. The principal amount plus all interest accrued is to be paid in full to the holder no later than October 15, 2009. This note was paid in full prior to October 15, 2009.
7. Authorized Shares
During the years ended December 31, 2008 and December 31, 2009, the Company’s board of directors approved proposals to amend the Articles of Incorporation to increase the number of authorized shares of common stock from 50,000,000 to 100,000,000 shares and from 100,000,000 to 200,000,000, respectively.
8. Preferred Stock
The Company’s Board of Directors has authorized 10,000,000 million shares of preferred stock with a par value of $0.01 to be issued in series with terms and conditions to be determined by the Board of Directors. The Company has designated 400,000 shares of Series A convertible preferred stock; 1,000,000 shares of Series B convertible preferred stock; 500,000 shares of Series C convertible preferred stock; and 1,100,000 shares of Series D convertible preferred stock. The Series A through D convertible preferred stock rank senior to the common stock as to dividends and liquidation. Each share of Series A through D convertible preferred stock is convertible into one share of common stock, except in specified circumstances as defined by the Company’s Certificate of Incorporation, and is automatically converted into common stock upon the occurrence of an initial public offering that meets certain criteria. No dividend or distribution may be paid on any shares of the Company’s common stock unless an equivalent dividend or distribution is paid on the Series A through D convertible preferred stock.
9. Stock Options and Warrants
In June 2000 and November 2009, our Board of Directors adopted, and our shareholders approved, the 2000 Plan and 2009 Plan, respectively (together the “Plans”). The Plans provide for the granting of options to qualified employees of the Company, independent contractors, consultants, directors and other individuals. As of December 31, 2009, the Company’s Board of Directors approved and made available 15,000,000 shares of common stock to be issued pursuant to the 2009 Plan. The Plans permit grants of options to purchase common shares authorized and approved by the Company’s Board of Directors.
The average fair value of options granted at market during 2009 and 2008 was $0.31 and $0.20 per option, respectively. The total intrinsic value of options exercised during the years ended December 31, 2009 and 2008 was $3,250 and $0, respectively.
F-16
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
The following summarizes the information relating to outstanding stock options activity with employees during 2009 and 2008:
| Common Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value | ||||||||
| Outstanding at December 31, 2007 |
6,376,889 | $ | 0.27 | 7.94 | $ | — | |||||
| Granted |
2,916,668 | $ | 0.26 | ||||||||
| Forfeited or expired |
(687,000 | ) | $ | 0.31 | |||||||
| Outstanding at December 31, 2008 |
8,606,556 | $ | 0.26 | 7.58 | $ | 38,294 | |||||
| Granted |
4,190,058 | $ | 0.21 | ||||||||
| Exercised |
(25,000 | ) | $ | 0.17 | $ | 3,250 | |||||
| Forfeited or expired |
(472,732 | ) | $ | 0.58 | |||||||
| Outstanding at December 31, 2009 |
12,298,882 | $ | 0.26 | 7.64 | $ | 1,052,839 | |||||
| Exercisable at December 31, 2009 |
11,951,021 | $ | 0.24 | 7.61 | $ | 1,034,594 | |||||
| Exercisable at December 31, 2008 |
7,329,993 | $ | 0.26 | 7.39 | $ | 35,727 | |||||
Stock compensation expense was approximately $1,580,000 for the year ended December 31, 2009, including approximately $75,000 that was accrued for warrants issued subsequent to year end, and $1,184,000 for the year ended December 31, 2008. The total fair value of shares vested during the years ended December 31, 2009 and 2008 was approximately $1,549,000 and $447,000, respectively.
As of December 31, 2009, there was approximately $158,000 of unrecognized employee stock-based compensation expense related to non vested stock options, of which $129,000, $27,000 and $2,000 is expected to be recognized for the years ended December 31, 2010, 2011 and 2012, respectively.
The following table represents our non vested share-based payment activity with employees for the year ended December 31, 2009 and 2008:
| Number of Options |
Weighted Average Grant Date Fair Value | |||||
| Nonvested options - December 31, 2007 |
941,340 | |||||
| Granted |
2,916,668 | $ | 0.27 | |||
| Forfeited |
(687,000 | ) | ||||
| Vested |
(1,894,445 | ) | ||||
| Nonvested options - December 31, 2008 |
1,276,563 | $ | 0.37 | |||
| Granted |
4,190,058 | $ | 0.31 | |||
| Vested |
(5,088,426 | ) | $ | 0.30 | ||
| Forfeited |
(30,334 | ) | $ | 0.17 | ||
| Nonvested options - December 31, 2009 |
347,861 | $ | 0.27 | |||
Warrants
At December 31, 2009, the Company had outstanding warrants to purchase the Company’s common stock which were issued in connection with multiple financing arrangements and consulting agreements. Information relating to these warrants is summarized as follows:
| Warrants |
Remaining Number Outstanding |
Weighted Average Remaining Life (Years) |
Weighted Average Exercise Price | ||||
| Warrants-Daily Financing |
197,055 | 1.98 | $ | 0.55 | |||
| Warrants-Additional Financing |
428,637 | 2.70 | $ | 0.40 | |||
| Warrants-Robb Trust Note |
50,000 | 2.42 | $ | 0.55 | |||
| Warrants-Financing |
14,750,000 | 2.99 | $ | 0.25 | |||
| Warrants-Placement Agent Warrants |
793,641 | 3.26 | $ | 0.25 | |||
| Warrants-Tangredi (a) |
3,000,000 | 3.25 | $ | 0.36 | |||
| Warrants-Ehrenberg (b) |
250,000 | 3.59 | $ | 0.30 | |||
| Warrants-Consulting Agreement (c) |
400,000 | 4.45 | $ | 0.35 | |||
| Warrants-Note Conversions |
2,240,000 | 4.38 | $ | 0.38 | |||
| Warrants-Stock Purchases 2009 |
758,270 | 4.40 | $ | 0.34 | |||
| Warrants-Mandelbaum |
50,000 | 4.33 | $ | 0.19 | |||
| Total |
22,917,603 | ||||||
F-17
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
| (a) | In April 2008, the Company’s Board of Directors issued a five year warrant to purchase 3,000,000 shares of common stock at an exercise price of $0.36 per share outside of the Plans. The fair value of warrants recognized as stock-based compensation during the year ended December 31, 2008 was $686,868 and was calculated using the Black-Scholes model with the following assumptions: Expected life in years: 5 years; Estimated volatility 81%; Risk-free interest rate: 2.72%; Dividend yield: 0%. |
| (b) | In August 2008, the Company’s Board of Directors issued a five year warrant to purchase 250,000 shares of common stock at an exercise price of $0.30 per share outside of the Plans. The fair value of warrants recognized as stock-based compensation during the year ended December 31, 2008 was $49,361 and was calculated using the Black-Scholes model with the following assumptions: Expected life in years: 5 years; Estimated volatility 95%; Risk-free interest rate: 3.23%; Dividend yield: 0%. |
| (c) | In April 2009, the Company issued a five year warrant to purchase 250,000 shares of common stock at an exercise price of $0.26 per share pursuant to a consulting agreement. The warrants are immediately exercisable and subject to adjustment for standard anti-dilution evenings, including, but not limited to stock dividends, split-up, reclassification or combination of the Company’s share, exchange of stock for other Company stock, or certain capital reorganizations or reclassification of the capital stock or consolidation, merger or sale of substantially all of the Company’s assets. The fair value of the warrants was calculated using the Black-Scholes model with the following assumptions: Expected life: 5 years; Estimated volatility: 94%; Risk-free interest rate: 2.02%; Dividend yield: 0%. In addition, subject to certain conditions, upon the per share market price of the common stock (as defined in warrant) being $1.50 per share for ten consecutive days, the Company may require the holder of the warrant to exercise the warrant or it will automatically terminate. |
The Company entered into a consulting agreement in August 2009 with an individual to provide marketing and advisory assistance services to the Company. The term of the agreement is two years and calls for the Company to issue the consultant a warrant to purchase 200,000 shares of the Company’s common stock at a $0.37 exercise price, five year term with one half of the warrant vesting immediately. The remaining 100,000 warrant shares become vested one year from the date of the Agreement dated August 18, 2009. The Company has valued the warrant at $12,609 and is amortizing the cost over the term of the service agreement. At December 31, 2009, the Company has included $10,245 in prepaid expenses on its balance sheet, representing the unamortized portion of the warrant value.
The Company entered into a consulting agreement in August 2009 with an individual to provide advisory services to the Company. The term of the agreement is two years and calls for the Company to issue the consultant a warrant to purchase 50,000 shares of the Company’s common stock at a $0.75 exercise price and a five year term. The Company has valued the warrant at $5,106 and is amortizing the cost over the term of the service agreement. At December 31, 2009, the Company has included $4,018 in prepaid expenses on its balance sheet, representing the unamortized portion of the warrant value.
Common Stock Issued For Services
The Company entered into an agreement in October 2009 with an individual to provide consulting services to the Company. The term of the agreement is for nine months and calls for the Company to issue the consultant 10,000 shares of common stock in each of the nine months of service. For the year ended December 31, 2009, the Company has recorded $10,800 as common stock payable and consulting expense on its statement of operations.
F-18
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
10. Deferred Revenue
The Company entered into a licensing agreement during the year ended December 31, 2003 and received an initial fee of $770,000. This fee is deferred and recognized on a straight-line basis over the life of the license agreement of 10 years. In addition, the Company received royalties of $100,000 in each of the first three years of the agreement. The Company recognized revenue of approximately $77,000 for this agreement during each of the years ended December 31, 2009 and 2008.
The Company entered into a licensing agreement with a biomedical entity during the year ended December 31, 2005 and received an initial license fee of $50,000. This fee is deferred and recognized on a straight-line basis over the life of the license agreement of 7 years. The Company recognized revenue of approximately $7,100 for this agreement during each of the years ended December 31, 2009 and 2008.
11. Commitments and Contingencies
The Company has employment agreements with some of its key employees and executives. These agreements provide for minimum levels of compensation during current and future years. In addition, these agreements call for grants of stock options and for payments upon termination of the agreements.
The Company entered into an agreement with the holders of the Convertible Notes to file a registration statement within 45 days of the first Note conversion and to have the registration statement declared effective within 150 days. The Company will incur penalties and damages of up to approximately $236,000 if it does not file and keep the registration statement effective pursuant to the terms of this agreement. As of December 31, 2009, the Company has recorded a liability of $41,000 in accrued expenses related to this agreement on its balance sheet.
The Company is not currently a party to any pending legal proceedings. In the ordinary course of business the Company may become a party to various legal proceedings generally involving contractual matters, infringement actions, product liability claims and other matters.
12. Income Taxes
There is no current or deferred income tax expense or benefit for the years ended December 31, 2009 and 2008.
The provision for income taxes is different from that which would be obtained by applying the statutory federal income tax rate to income before income taxes. The items causing this difference are as follows:
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Tax benefit at U.S. statutory rate |
$ | (1,151,000 | ) | $ | (2,033,100 | ) | ||
| State income tax benefit, net of federal benefit |
(123,000 | ) | (217,100 | ) | ||||
| Effect of non-deductible expenses |
1,000 | 3,000 | ||||||
| Stock-based compensation |
536,000 | 403,000 | ||||||
| Other adjustments |
825,000 | 1,383,000 | ||||||
| Change in valuation allowance |
(88,000 | ) | 461,200 | |||||
| $ | — | $ | — | |||||
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities are as follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Deferred tax assets (liabilities), current: |
||||||||
| Bonus payable |
$ | 108,300 | $ | 108,300 | ||||
| Accrued deferred compensation payable |
386,300 | 323,500 | ||||||
| Other |
49,100 | 49,100 | ||||||
| Deferred license revenue |
32,400 | 31,700 | ||||||
| Valuation allowance |
(576,100 | ) | (512,600 | ) | ||||
| $ | — | $ | — | |||||
| Deferred tax assets (liabilities), noncurrent: |
||||||||
| Deferred license revenue |
$ | 77,400 | $ | 110,600 | ||||
| Depreciation |
3,400 | 3,400 | ||||||
| Net operating loss carryforwards |
7,261,000 | 6,600,100 | ||||||
| Valuation allowance |
(7,341,800 | ) | (6,714,100 | ) | ||||
| $ | — | $ | — | |||||
F-19
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
As of December 31, 2009 and 2008, the Company had federal and state net operating loss carry-forwards totaling approximately $21,400,000 and $19,000,000, respectively, which begin expiring in 2012. The Company has established a valuation allowance to fully reserve all deferred tax assets at December 31, 2009 and 2008 because it is more likely than not that the Company will not be able to utilize these assets.
As of December 31, 2009, the Company has not performed an IRC Section 382 study to determine the amount, if any, of its net operating losses that may be limited as a result of the ownership change percentages during 2009. However, the Company will complete the study prior to the utilization of any of its recorded net operating losses.
13. Genertec Agreement
On August 21, 2009, the Company, entered into a Exclusive Distribution Agreement (the “Agreement”) with Genertec America, Inc., a California corporation (“Genertec”), to grant Genertec the exclusive right to obtain, distribute and market three of the Company’s nanotechnology-based membrane products and related products in Great China, including main land China, Hong Kong, Macau and Taiwan (the “Territory”). The Agreement provides that during the initial term of the Agreement, Genertec agreed to order and purchase the Company’s products in the aggregate amount of Two Hundred Million U.S. Dollars ($200,000,000). Certain terms of the agreement have been granted confidential treatment by the Securities and Exchange Commission.
Under the Agreement, the Company will supply and Genertec will distribute the Company’s products in the designated Territory on an exclusive basis. Genertec agreed to purchase from the Company a minimum of the Company’s products during any contract year. In the event Genertec fails to purchase such minimum in any given year, the Company may convert the exclusivity to Genertec into a non-exclusive basis or terminate the Agreement. Pursuant to the terms of the Agreement, Genertec will engage and appoint authorized person(s) or firm(s), to install, engineer, perform maintenance, sell and use the products within the Territory. Genertec nor its designated Buyer is permitted to alter, decompile or modify the Company’s products in any way. As consideration for entering into the Agreement, Genertec agreed to pay the Company a deposit in monthly installments beginning in September 2009 and continuing through April 2010. The deposit will be credited against future orders and is not refundable in the event the agreement is terminated. During the initial term of the Agreement, the Company and Genertec agreed to negotiate in good faith a royalty bearing license agreement whereby Genertec shall be granted a license to manufacture certain portions of the Company’s products in the Territory.
The initial term of the Agreement shall be for a period of five (5) years, commencing on August 21, 2009, unless earlier terminated. Unless notice of termination is delivered to the respective parties 180 days prior to the expiration of the initial term, the Agreement will automatically renew for consecutive one (1) year periods. The Company may terminate the Agreement in the event: (1) Genertec fails to pay the deposit as indicated, (2) that Genertec has not purchased a minimum amount of the Company’s products during any contract year, (3) breach by Genertec of its obligations under the Agreement, or (4) at the discretion of the Company, immediately upon the transfer of fifty percent (50%) or more of either the assets of the voting stock of Genertec to any third party. Genertec may not assign the Agreement to any party without the prior written consent of the Company.
14. Subsequent Events
The Company secured a loan from an investor in the principal amount of $620,000. The loan amount was received on February 18, 2010. Pursuant to the terms of the note, the Company is to pay the holder simple interest at the rate of ten percent per annum commencing on the date of issuance with all interest and principal due and payable in cash on August 10, 2010.
F-20
Table of Contents
Dais Analytic Corporation
Notes to Financial Statements—(Continued)
Years Ended December 31, 2009 and 2008
The Company has evaluated subsequent events through March 30, 2010, the date on which this Form 10-K was filed with the Securities and Exchange Commission.
F-21
