Attached files
| file | filename |
|---|---|
| 8-K - 8-K - BIOSPHERE MEDICAL INC | a10-5703_18k.htm |
Exhibit 99.1
|
|
March 2010 |
|
|
SAFE HARBOR This presentation contains statements about the Company’s future expectations, plans and prospects that constitute forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995, including statements concerning the Company’s strategic goals and objectives relating to, among other things: geographic and market expansion; new product opportunities in interventional oncology and interventional urology; sales and marketing initiatives; and research and development. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including risks relating to: the Company’s ability to successfully obtain and maintain regulatory approvals, develop and market, existing and new products, maintain key intellectual property rights, manage competitive pressures, successfully execute on its plans and strategies for growth, and overcome adverse economic and market conditions, as well as those factors described in “Risk Factors” and elsewhere in the Company's Annual Report on Form 10-K for the year ended December 31, 2008, Form 10-Q for the period ending September 30, 2009 and other filings the company makes with the SEC from time to time. The forward-looking statements included in this presentation represent the Company's views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. While the Company may elect to update these forward-looking statements in the future, the Company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation. |
|
|
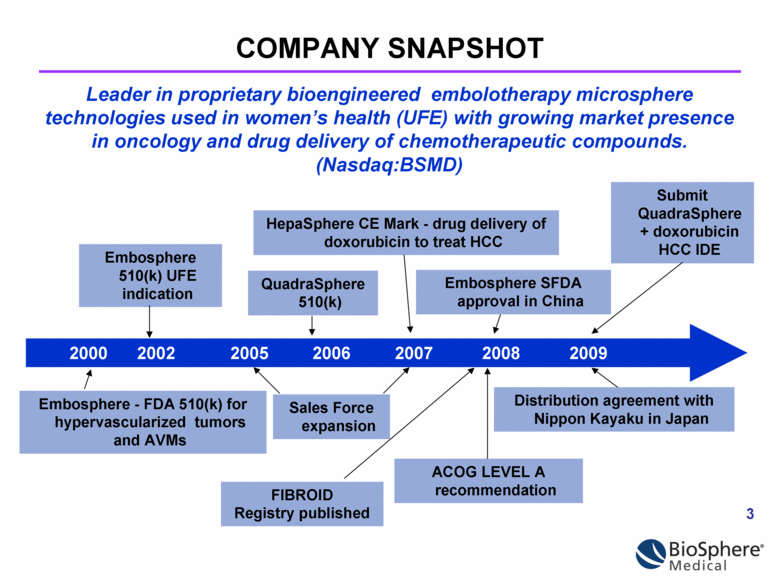
COMPANY SNAPSHOT Embosphere - FDA 510(k) for hypervascularized tumors and AVMs 2000 2002 2005 2006 2007 2008 2009 Embosphere 510(k) UFE indication Sales Force expansion QuadraSphere 510(k) HepaSphere CE Mark - drug delivery of doxorubicin to treat HCC ACOG LEVEL A recommendation Distribution agreement with Nippon Kayaku in Japan Submit QuadraSphere + doxorubicin HCC IDE Embosphere SFDA approval in China FIBROID Registry published Leader in proprietary bioengineered embolotherapy microsphere technologies used in women’s health (UFE) with growing market presence in oncology and drug delivery of chemotherapeutic compounds. (Nasdaq:BSMD) |
|
|
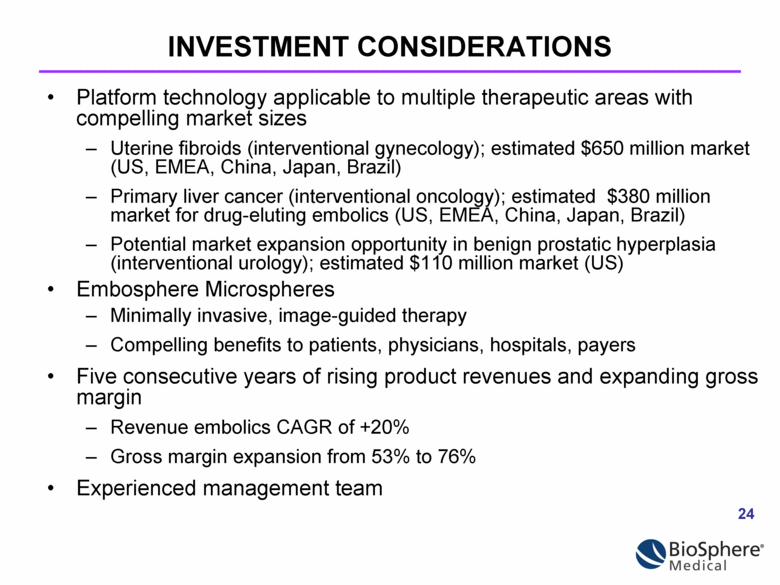
INVESTMENT CONSIDERATIONS Platform technology applicable to multiple therapeutic areas with compelling market sizes Uterine fibroids (interventional gynecology); estimated $650 million market (US, EMEA, China, Japan, Brazil) Primary liver cancer (interventional oncology); estimated $380 million market for drug-eluting embolics (US, EMEA, China, Japan, Brazil) Potential market expansion opportunity in benign prostatic hyperplasia (interventional urology); estimated $110 million market (US) Embosphere Microspheres Minimally invasive, image-guided therapy Compelling benefits to patients, physicians, hospitals, payers Five consecutive years of rising product revenues and expanding gross margin Revenue embolics CAGR of +20% Gross margin expansion from 53% to 76% Experienced management team |
|
|
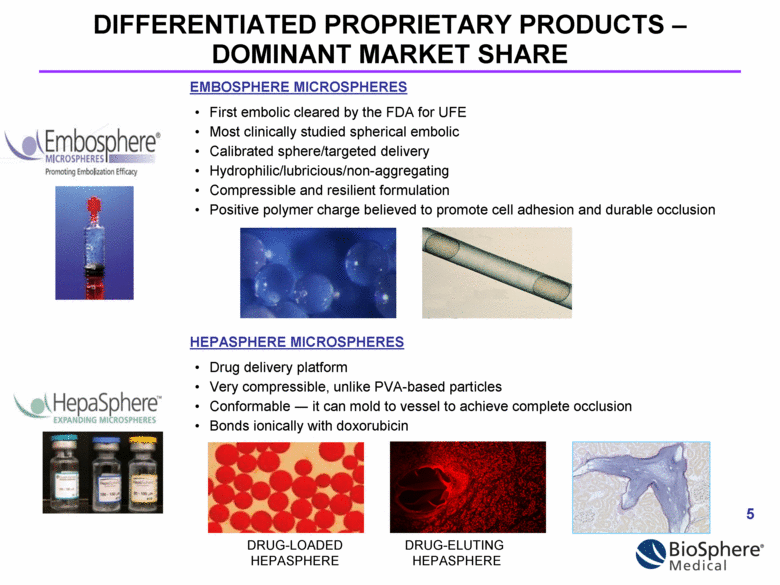
DIFFERENTIATED PROPRIETARY PRODUCTS – DOMINANT MARKET SHARE EMBOSPHERE MICROSPHERES First embolic cleared by the FDA for UFE Most clinically studied spherical embolic Calibrated sphere/targeted delivery Hydrophilic/lubricious/non-aggregating Compressible and resilient formulation Positive polymer charge believed to promote cell adhesion and durable occlusion HEPASPHERE MICROSPHERES Drug delivery platform Very compressible, unlike PVA-based particles Conformable — it can mold to vessel to achieve complete occlusion Bonds ionically with doxorubicin DRUG-LOADED DRUG-ELUTING HEPASPHERE HEPASPHERE |
|
|
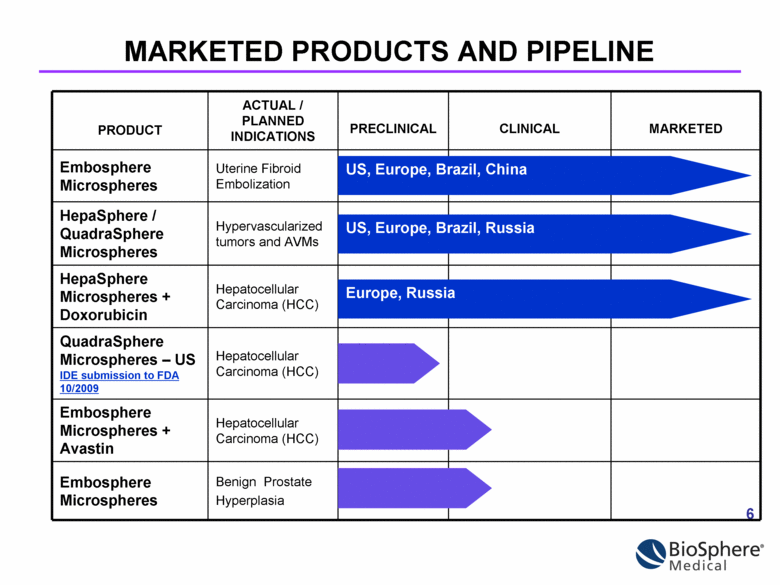
PRODUCT ACTUAL / PLANNED INDICATIONS PRECLINICAL CLINICAL MARKETED Embosphere Microspheres Uterine Fibroid Embolization HepaSphere / QuadraSphere Microspheres Hypervascularized tumors and AVMs HepaSphere Microspheres + Doxorubicin Hepatocellular Carcinoma (HCC) QuadraSphere Microspheres – US IDE submission to FDA 10/2009 Hepatocellular Carcinoma (HCC) Embosphere Microspheres + Avastin Hepatocellular Carcinoma (HCC) Embosphere Microspheres Benign Prostate Hyperplasia US, Europe, Brazil, China MARKETED PRODUCTS AND PIPELINE US, Europe, Brazil, Russia Europe, Russia |
|
|
Interventional Gynecology – Treatment of benign uterine fibroids – Uterine Fibroid Embolization (UFE) Interventional Oncology – Treatment of malignant tumors through targeted vascular occlusion and drug delivery PIONEER/LEADER IN EMBOLOTHERAPY |
|
|
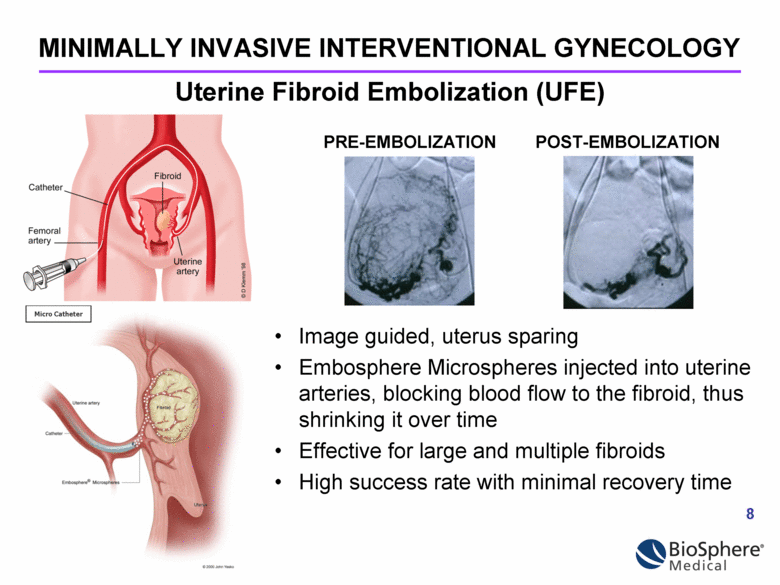
Image guided, uterus sparing Embosphere Microspheres injected into uterine arteries, blocking blood flow to the fibroid, thus shrinking it over time Effective for large and multiple fibroids High success rate with minimal recovery time MINIMALLY INVASIVE INTERVENTIONAL GYNECOLOGY Micro Catheter Uterine Fibroid Embolization (UFE) PRE-EMBOLIZATION POST-EMBOLIZATION |
|
|
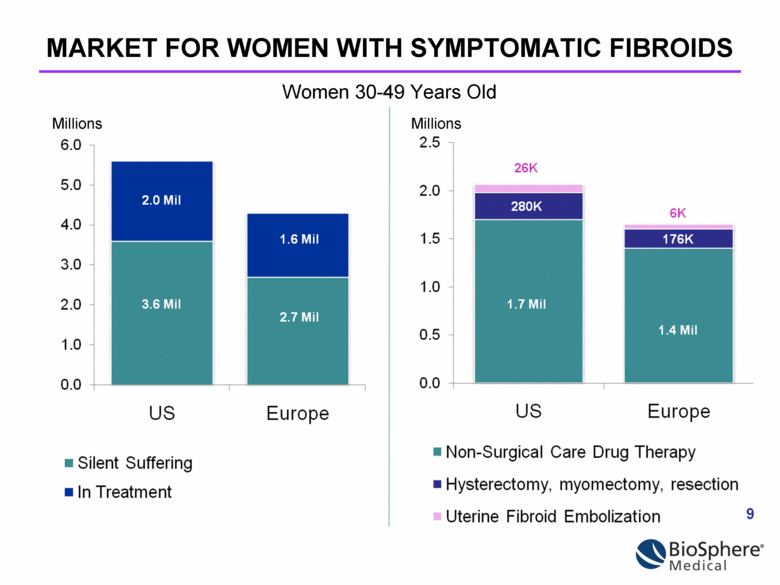
MARKET FOR WOMEN WITH SYMPTOMATIC FIBROIDS Millions Millions Women 30-49 Years Old |
|
|
CLINICAL RESULTS OF UFE Uterus-sparing, safe and effective symptom relief (2003-2008) ACOG Level A Recommendation Reproducible quality-of-life outcomes FIBROID Registry (December 2005, January 2008) 5-year durability (November 2005) Fertility “Pregnancy possible after UFE” (January 2005, November 2006) |
|
|
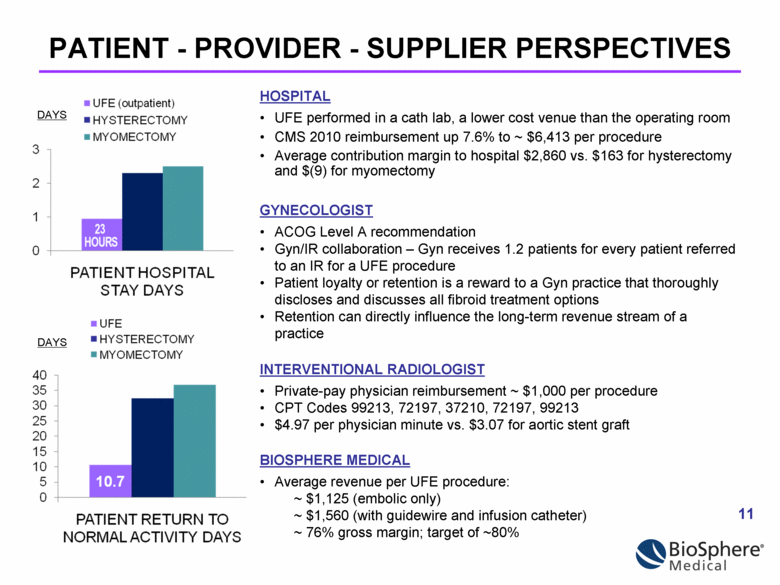
PATIENT - PROVIDER - SUPPLIER PERSPECTIVES DAYS DAYS HOSPITAL UFE performed in a cath lab, a lower cost venue than the operating room CMS 2010 reimbursement up 7.6% to ~ $6,413 per procedure Average contribution margin to hospital $2,860 vs. $163 for hysterectomy and $(9) for myomectomy INTERVENTIONAL RADIOLOGIST Private-pay physician reimbursement ~ $1,000 per procedure CPT Codes 99213, 72197, 37210, 72197, 99213 $4.97 per physician minute vs. $3.07 for aortic stent graft BIOSPHERE MEDICAL Average revenue per UFE procedure: ~ $1,125 (embolic only) ~ $1,560 (with guidewire and infusion catheter) ~ 76% gross margin; target of ~80% GYNECOLOGIST ACOG Level A recommendation Gyn/IR collaboration – Gyn receives 1.2 patients for every patient referred to an IR for a UFE procedure Patient loyalty or retention is a reward to a Gyn practice that thoroughly discloses and discusses all fibroid treatment options Retention can directly influence the long-term revenue stream of a practice |
|
|
Interventional Gynecology – Treatment of benign uterine fibroids – Uterine Fibroid Embolization (UFE) Interventional Oncology – Treatment of malignant tumors through targeted vascular occlusion and drug delivery PIONEER/LEADER IN EMBOLOTHERAPY |
|
|
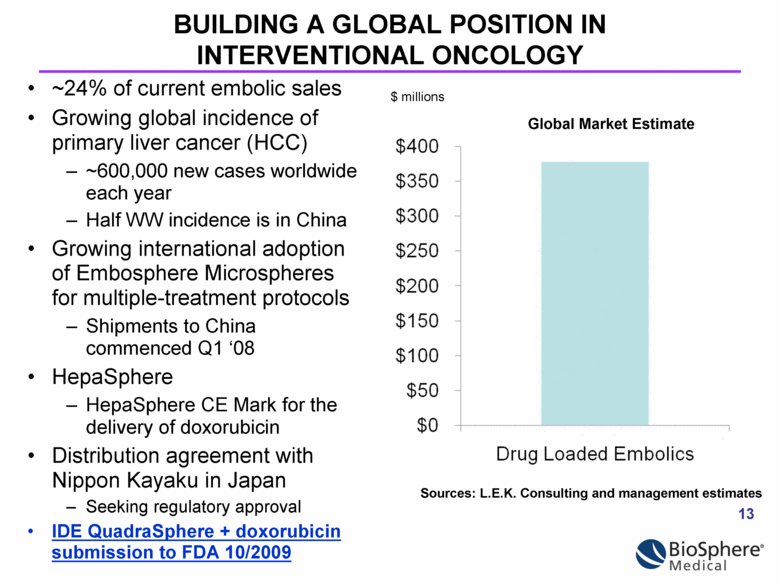
BUILDING A GLOBAL POSITION IN INTERVENTIONAL ONCOLOGY ~24% of current embolic sales Growing global incidence of primary liver cancer (HCC) ~600,000 new cases worldwide each year Half WW incidence is in China Growing international adoption of Embosphere Microspheres for multiple-treatment protocols Shipments to China commenced Q1 ‘08 HepaSphere HepaSphere CE Mark for the delivery of doxorubicin Distribution agreement with Nippon Kayaku in Japan Seeking regulatory approval IDE QuadraSphere + doxorubicin submission to FDA 10/2009 $ millions Sources: L.E.K. Consulting and management estimates Global Market Estimate |
|
|
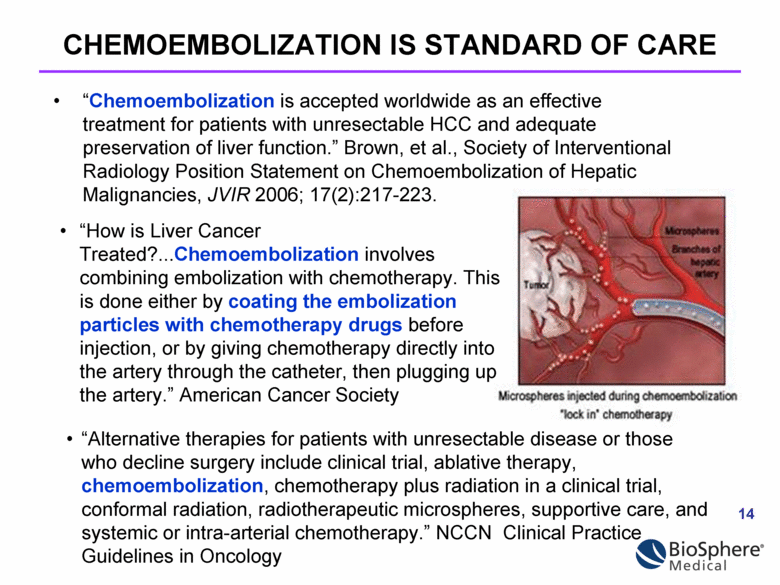
“Chemoembolization is accepted worldwide as an effective treatment for patients with unresectable HCC and adequate preservation of liver function.” Brown, et al., Society of Interventional Radiology Position Statement on Chemoembolization of Hepatic Malignancies, JVIR 2006; 17(2):217-223. CHEMOEMBOLIZATION IS STANDARD OF CARE “Alternative therapies for patients with unresectable disease or those who decline surgery include clinical trial, ablative therapy, chemoembolization, chemotherapy plus radiation in a clinical trial, conformal radiation, radiotherapeutic microspheres, supportive care, and systemic or intra-arterial chemotherapy.” NCCN Clinical Practice Guidelines in Oncology “How is Liver Cancer Treated?...Chemoembolization involves combining embolization with chemotherapy. This is done either by coating the embolization particles with chemotherapy drugs before injection, or by giving chemotherapy directly into the artery through the catheter, then plugging up the artery.” American Cancer Society |
|
|
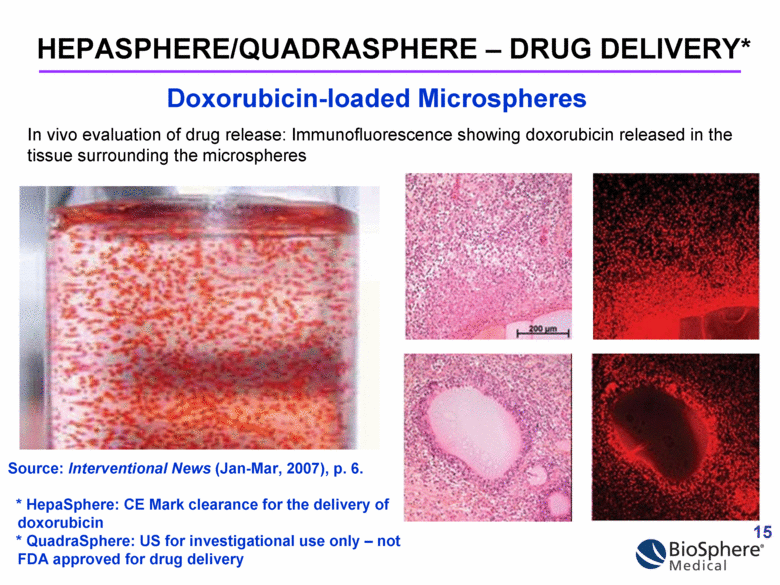
In vivo evaluation of drug release: Immunofluorescence showing doxorubicin released in the tissue surrounding the microspheres HEPASPHERE/QUADRASPHERE – DRUG DELIVERY* Doxorubicin-loaded Microspheres Source: Interventional News (Jan-Mar, 2007), p. 6. * HepaSphere: CE Mark clearance for the delivery of doxorubicin * QuadraSphere: US for investigational use only – not FDA approved for drug delivery 15 |
|
|
HiQuality Trial: QuadraSphere loaded with doxorubicin vs. conventional transarterial chemoembolization (cTACE) with doxorubicin Update on IDE Status IDE filed with FDA in October 2009 Discussions with FDA ongoing FDA has requested amended study protocol on primary endpoint (survival and quality-of-life measures vs. planned overall tumor response at 6 mos.) We have not reached final agreement with FDA as to the nature of the study; satisfactory resolution of IDE protocol issues is a condition to starting the trial QuadraSphere is not approved by the FDA for the delivery of doxoxrubicin in the treatment of hepatocellular carcinoma (HCC). PROPOSED HiQUALITY PIVOTAL TRIAL |
|
|
PIPELINE: EXPANDING EMBOLOTHERAPY PLATFORM Interventional Urology – Treatment of Benign Prostatic Hyperplasia (BPH) |
|
|
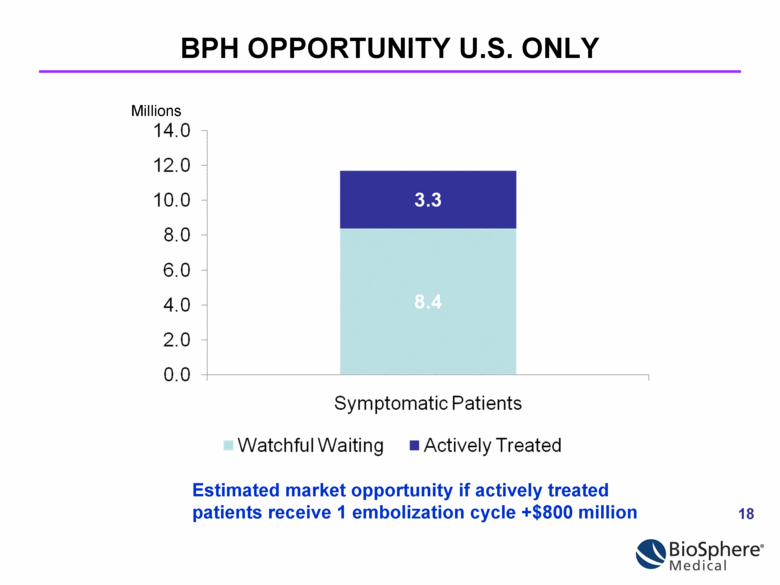
Millions Estimated market opportunity if actively treated patients receive 1 embolization cycle +$800 million BPH OPPORTUNITY U.S. ONLY |
|
|
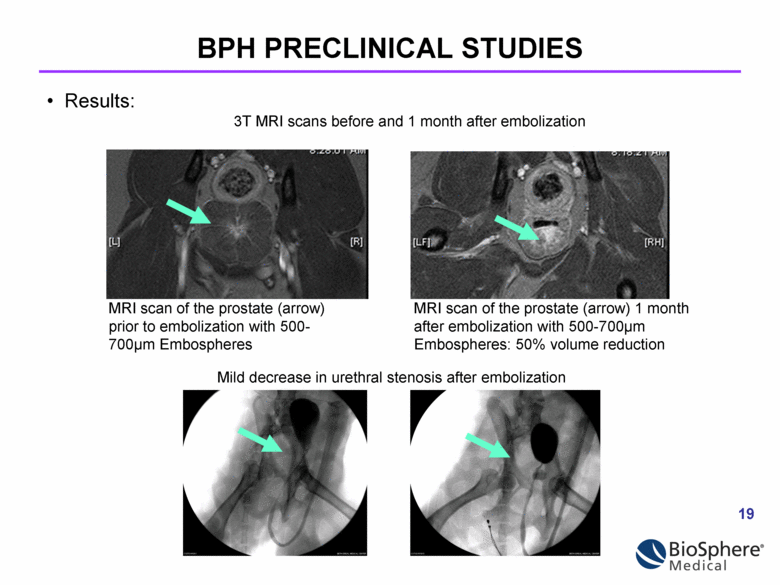
MRI scan of the prostate (arrow) MRI scan of the prostate (arrow) 1 month prior to embolization with 500- after embolization with 500-700µm 700µm Embospheres Embospheres: 50% volume reduction Mild decrease in urethral stenosis after embolization Results: 3T MRI scans before and 1 month after embolization BPH PRECLINICAL STUDIES |
|
|
Clinical Studies Initial clinical results (2 patients using 300-500µm Embosphere) Each patient exhibited marked (40% and 24%, respectively, at one month, and 48% and 28%, respectively, at 6 months) reduction in prostate volume post procedure by MRI Both patients demonstrated improvement in symptoms with less urinary retention, now sustained over 1 year post procedure Ethics committee approval to initiate more formal clinical study 10 patients from the TURP waiting list already identified 6 treated to date Estimate is that all 10 will be embolized using Embosphere Microspheres within 6 months, and results expected (with 6-month follow-up) in a year or less This study + preclinical work may be sufficient to obtain IDE BPH CLINICAL UPDATE |
|
|
EXPERIENCED EXECUTIVE LEADERSHIP TEAM Richard J. Faleschini, President and Chief Executive Officer American Medical Systems, Medtronic, Cordis Martin J. Joyce, Exec. Vice President, Chief Financial Officer Stratex Group, Serono, Millipore Melodie R. Domurad, PhD, Vice President of Regulatory, Medical Affairs and Quality Systems Matritech, Inc., Ergo Science, Center for the Study of Nutrition and Medicine at New England Deaconess Hospital Willard “Bill” W. Hennemann, PhD, Vice President, New Product and Business Development CryoCath Technologies, Intervascular, Medtronic, Cordis Peter C. Sutcliffe, Vice President of Manufacturing Whatman plc, HemaSure, Corning Costar, Millipore Joel B. Weinstein, Vice President of Global Marketing and Sales Hologic, Inc., Advanced Technology Laboratories (ATL) |
|
|
2010 STRATEGIC GOALS Interventional Gynecology Scale-up community health talks in 2010 to over ~200+ events Commence direct-to-employer education for UFE promoting the rapid return to work after a UFE procedure compared to surgical alternatives Interventional Oncology Obtain FDA approval to begin pivotal clinical trial of QuadraSphere + doxorubicin for the treatment of Hepatocellular Carcinoma (HCC) Plan follow-on clinical work based on the expected positive results of the Phase II, prospective, single-arm clinical trial combining Avastin, with (TACE), using Embosphere Microspheres, for unresectable hepatocellular carcinoma Conclude a partnership with big pharma for HepaSphere Interventional Urology Continue to advance clinical studies |
|
|
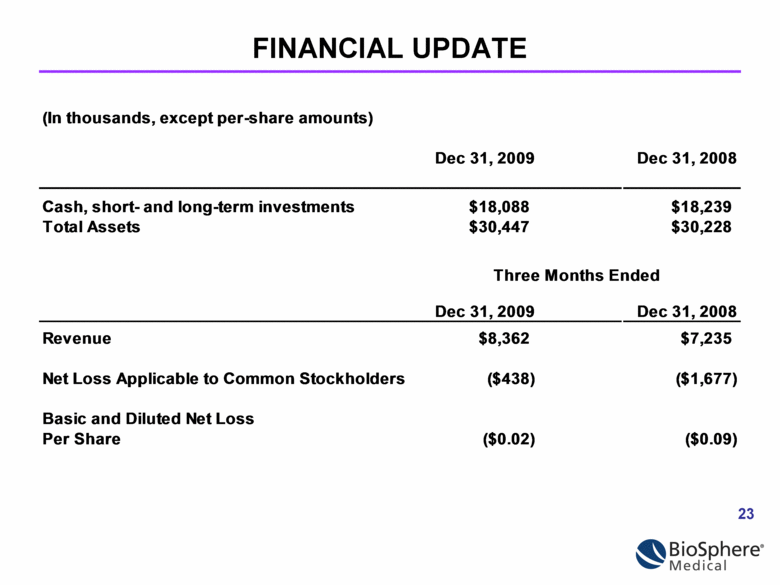
FINANCIAL UPDATE (In thousands, except per-share amounts) Dec 31, 2009 Dec 31, 2008 Cash, short- and long-term investments $18,088 $18,239 Total Assets $30,447 $30,228 Dec 31, 2009 Dec 31, 2008 Revenue $8,362 $7,235 Net Loss Applicable to Common Stockholders ($438) ($1,677) Basic and Diluted Net Loss Per Share Three Months Ended ($0.02) ($0.09) |
|
|
INVESTMENT CONSIDERATIONS Platform technology applicable to multiple therapeutic areas with compelling market sizes Uterine fibroids (interventional gynecology); estimated $650 million market (US, EMEA, China, Japan, Brazil) Primary liver cancer (interventional oncology); estimated $380 million market for drug-eluting embolics (US, EMEA, China, Japan, Brazil) Potential market expansion opportunity in benign prostatic hyperplasia (interventional urology); estimated $110 million market (US) Embosphere Microspheres Minimally invasive, image-guided therapy Compelling benefits to patients, physicians, hospitals, payers Five consecutive years of rising product revenues and expanding gross margin Revenue embolics CAGR of +20% Gross margin expansion from 53% to 76% Experienced management team |
|
|
March 2010 |