Attached files
| file | filename |
|---|---|
| 8-K - SINOBIOPHARMA 8-K 3-8-2010 - Sinobiopharma, Inc. | sino_8k.htm |

Sinobiopharma, Inc.
Corporate Presentation

Disclaimer
"Safe Harbor" statement under the Private Securities Litigation Reform Act of 1995:
This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. These forward-looking statements
are based on current expectations or beliefs, including, but not limited to,
statements concerning the Company's operations, financial performance and
condition. For this purpose, statements that are not statements of historical fact may
be deemed to be forward-looking statements. The Company cautions that these
statements by their nature involve risks and uncertainties, and actual results may
differ materially depending on a variety of important factors, including those
discussed in the Company's reports filed with the Securities and Exchange
Commission from time to time. In addition, the company disclaims any obligation to
update any forward-looking statements to reflect events or circumstances after the
date hereof.
Private Securities Litigation Reform Act of 1995. These forward-looking statements
are based on current expectations or beliefs, including, but not limited to,
statements concerning the Company's operations, financial performance and
condition. For this purpose, statements that are not statements of historical fact may
be deemed to be forward-looking statements. The Company cautions that these
statements by their nature involve risks and uncertainties, and actual results may
differ materially depending on a variety of important factors, including those
discussed in the Company's reports filed with the Securities and Exchange
Commission from time to time. In addition, the company disclaims any obligation to
update any forward-looking statements to reflect events or circumstances after the
date hereof.
No securities regulatory authority has either approved or disapproved the contents
of this presentation.
of this presentation.

* Adjusted to exclude stock option amortization expense
Equity Snapshot
|
Figures as of February 28, 2010
|
|
|
Symbol:
|
SNBP.OB
|
|
Price:
|
$0.26
|
|
Market Cap:
|
$30.6 million
|
|
Shares Outstanding:
|
117,587,608
|
|
Fiscal Year End
|
May 31
|
|
Fiscal 2009 Revenue:
|
$3.85 million
|
|
Fiscal 2009 Net Income*:
|
$0.76 million
|
|
Projected Fiscal 2010 Revenue:
|
$7.7 million
|
|
Projected Fiscal 2010 Net Income:
|
$3.5 million
|

Profitable, Fast Growing Chinese Specialty Pharma
Multiple commercialized and pipeline products in muscle relaxant and cardiovascular markets
drive sustainable revenue and profit growth
Fiscal 2010 guidance (fiscal year ending May 2010)
$7.7m revenues (103% revenue increase over prior year)
$3.5m net income, $0.030 EPS
High Margin Commercial Business
Lead product KuTai uniquely differentiated as only muscle relaxant stored at room temperature
Current gross margin above 70% and net margin above 40%
Expected sales of $7.3 million in fiscal 2010 (100% increase over prior year)
Strong YiTai product launch in the cardiovascular ACE inhibitor market
Market exclusivity and price premium as first-to-market product
Expected sales of $4 million in fiscal 2011 (launched in fiscal H3:2010)
Robust Product Pipeline
Rocuronium: Next-generation muscle relaxant.
Eplerenone: Aldosterone inhibitor for congestive heart failure.
Clevidipine Butyrate: Dihydropyridine calcium channel blocker
Gantacurium chloride: New non-depolarizing ultra-short acting neuromuscular blocker
Investment Summary

* Net income number exclude the stock option amortization charge
Outstanding Financial Performance
Profitable, Fast Growing Specialty Pharma

A fully integrated specialty pharmaceutical
company established in 2004 in Nantong,
Jiangsu. Headquartered in Nanjing, Jiangsu.
Listed on OTCBB in 2008.
company established in 2004 in Nantong,
Jiangsu. Headquartered in Nanjing, Jiangsu.
Listed on OTCBB in 2008.
Specializing in muscle relaxant and
cardiovascular drugs; each category has total
global market of more than $1 billion
cardiovascular drugs; each category has total
global market of more than $1 billion
3 marketed products and 4 major new
products in pipeline planned for
commercialization
products in pipeline planned for
commercialization
SFDA GMP-certified production facilities and
established distribution network
established distribution network
Company Overview

Expanding in fast-growing Chinese healthcare market
Government support includes no-interest loan, tax advantages for five years, and
high-tech corporation benefits
high-tech corporation benefits
Strong R&D team lead by our Chairman & CEO Dr. Lee Huang, who headed research
team at Bayer Pharmaceutical (USA for more than 12 years
team at Bayer Pharmaceutical (USA for more than 12 years
Proprietary technology enables low cost, high quality API and innovative
formulations for unstable drugs
formulations for unstable drugs
Experience with Chinese FDA expedites fast approval of first-to-market (FTM) drugs
Competitive Advantages
Experienced Management
Dr. Lee Huang Chairman & Chief Executive Officer
More than 12 years research and managerial experience at Bayer Pharmaceutical, USA
Published more than 30 peer-reviewed articles in scientific journals
Holds numerous patents
Earned Ph.D. in Chemistry, Iowa State University, 1987; B.S. Organic Chemistry (Nanjing University, 1981)
James Mu Director & Chief Financial Officer
Director of China Infrastructure Investment Corporation (Nasdaq: CIIC)
Former Chief Financial Officer of Jingwei International Limited (OTCBB: JNGW.OB)
B.S. at Hebei University of Science and Technology (Chemical Engineering)
M.B.A at Baruch College, City University of New York
Xuejun Chen Director & Vice President
Former Vice-President of sales at Nanjing Langkun Medicine Co., Ltd.
Marketing Director of Jiangsu Province for both Xiamen Beidazhilu Biotech Co., Ltd. and Shanxi Dongshen
Medicine Co., Ltd.
Medicine Co., Ltd.
M.B.A, Nanjing University, 2007; B.S., Lanzhou University

Population of 1.3 billion consumers with increasing disposable incomes
Chinese government will spend RMB124 billion in the next three years on
healthcare reform
healthcare reform
China’s entire drug market estimated at $122B in 2008; projected to be the 5th
largest pharmaceutical market by 2010 and the largest by 2050
largest pharmaceutical market by 2010 and the largest by 2050
Expanding government-backed healthcare policies and reimbursement systems
Growing elderly population is accelerating demand for drugs for seniors
11% of population is over 60 years old; to reach approximately 30% by 2045
China Market Opportunity

High Margin Business Model (>65%)
Drug Selection Criteria
Development
Commercialization
Distribution →Increasing Market Share
Established networks, Sales infrastructure expansion
>=$1B global sales
High-incidence,
high-mortality
diseases
high-mortality
diseases
Potential market
exclusivity
exclusivity
New drugs
First-to-market
branded generic and
innovative drugs
branded generic and
innovative drugs
Experience with
SFDA process
GMP certified
manufacturing
lines
manufacturing
lines
Launch/marketing
expertise
expertise
High margin/low
cost provider
cost provider
High Margin Commercial Business
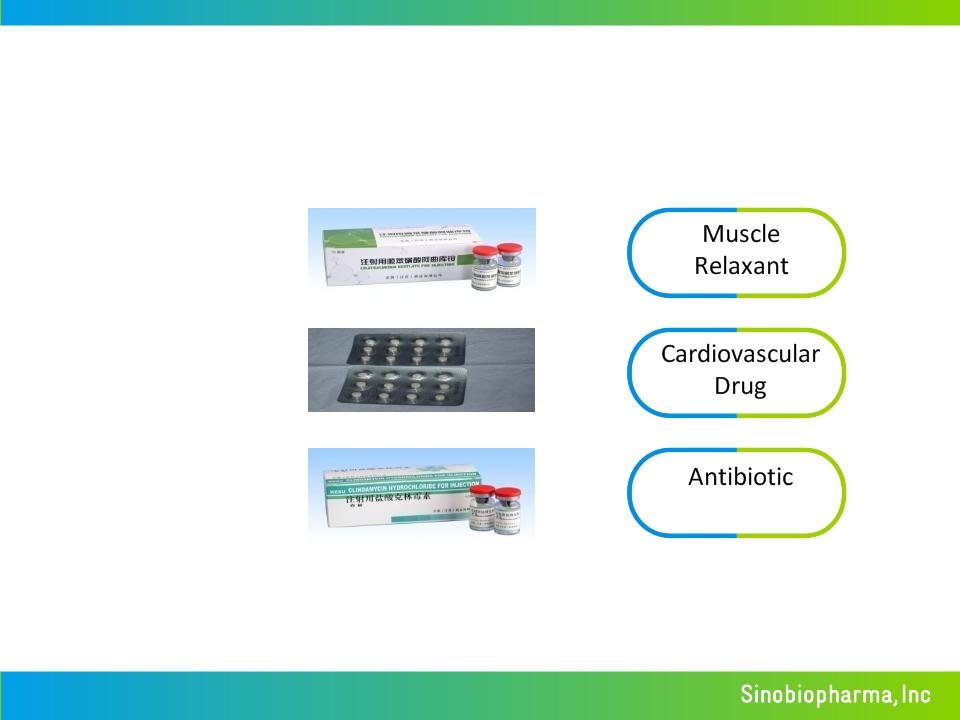
KuTai ®
(cisatracurium)
(cisatracurium)
YiTai ®
(perindopril)
(perindopril)
KeSu ®
(Clindamycin)
(Clindamycin)
Marketed Products

Muscle relaxants used in major surgery with anesthesia, and pain suppression
Total Chinese muscle relaxant market has CAGR 25 percent growth annually due to
increase in general anesthesia surgeries
increase in general anesthesia surgeries
Total market value in China is over RMB 1 billion (~$130 million)
Four main types of muscle relaxants in use; Kutai (cisatracurium) has the fewest and
mildest side effects of the four
mildest side effects of the four
Market numbers demonstrate Cisatracurium category is rapidly replacing other
muscle relaxants; will soon lead all muscle relaxants currently in use
muscle relaxants; will soon lead all muscle relaxants currently in use
Two Competitors (Jingsu Hengrui & GSK) both have lower sales; Kutai is the leader in
Cisatracurium category for total sales
Cisatracurium category for total sales
Market for Muscle Relaxants

Launched in 2007, KuTai is sales leader in
Cisatracurium category in Chinese market
Cisatracurium category in Chinese market
World’s first to be stored at room temperature;
convenient for surgeons
convenient for surgeons
Safer, more stable and more effective than
other relaxants
other relaxants
Much lower cost in China compared to Nimbex,
made by GSK
made by GSK
Sales revenue from Kutai was $3.6 million in FY
2009; $1.4 million in FY 2008, an increase of
157%
2009; $1.4 million in FY 2008, an increase of
157%
Expected sales revenue in FY 2010 is $7.3
million, an increase of 100%
million, an increase of 100%
Flagship Product - Ku Tai
Cardiovascular (CV) disease is leading cause of death in USA
Causes an estimated 17.5 million deaths globally every year; about one third of total
annual deaths
annual deaths
According to American Decision Resources research, total Chinese market in 2010 for
cardiovascular drug is $800M
cardiovascular drug is $800M
Multiple drugs for treatment of cardiovascular disease in Chinese market;
effectiveness varies by patient types
effectiveness varies by patient types
Main competitor in Chinese market is Acertil from French company Servier
Market for Cardiovascular Drugs
Launched in third quarter of FY 2010, estimated
sales for FY 2011 is $4 million
sales for FY 2011 is $4 million
A first-to-market drug which; SNBP enjoys
market exclusivity
market exclusivity
Chinese health insurance covers drug
For some patients, more effective when taken
with other cardiovascular drugs
with other cardiovascular drugs
Protects blood vessels and kidneys
Our CV Flagship Product - Yi Tai
Size:129×49×18mm

Extensive Distribution Network
Established nationwide sales and marketing network through distributors
Sales force covering all regions
Long-term relationships with hospitals and doctors
Distributes products in 30 provinces
and key major cities

Robust Product Pipeline

Products in Pipeline
Eplerenone
• Cardiovascular drug for high blood pressure and vascular disease
• No comparable drug produced and marketed in China
• First company to receive approval for, and first to start clinical trial
Rocuronium
• Fast-acting, injectable muscle relaxant drug
• Can be used in cocktail with Kutai; recognized by doctors
Gantacurium Chloride
• Ultra short acting, rapid onset muscle relaxant
• New generation drug; not yet produced anywhere
Clevidipine Butyrate
• Rapidly reduces blood pressure; controls blood pressure in patients with
acute hypertension when use of oral therapy is neither feasible nor desirable
acute hypertension when use of oral therapy is neither feasible nor desirable

Top tier R&D team lead by Dr. Lee Huang
Patented technology and production provide highest quality and safety levels for our
products
products
Long-term cooperative relationships with a number of world class research
institutions and universities in both China and USA
institutions and universities in both China and USA
Access to advanced research lab and devices
R&D Partnership
Nanjing University
China Pharmaceutical University
Nanjing Medical University
Cornell University
Strong R&D Capabilities

30,000 sq. meter SFDA GMP-certified production facilities
Location in Nantong Economic & Technology Development Zone confers extensive
tax benefits
tax benefits
6 GMP-Certified Production Lines of tablet, capsule, injectable, granule, dry powder,
and chemical APTs.
and chemical APTs.
50% capacity used at current production levels
Modern, Scalable Manufacturing Facilities

Financials Show Strong Growth

Balance Sheet
|
($ in thousands)
|
November 30, 2009
(Q2 FY2010)
|
May 31, 2009
(FY Y-End 2009)
|
|
Cash & Cash Equivalents
|
$ 365
|
$ 891
|
|
Total Current Assets
|
$ 1,880
|
$ 1,692
|
|
Total Assets
|
$ 6,928
|
$ 5,956
|
|
Total Current Liability
|
$ 4,446
|
$ 5,179
|
|
Owner’s Equity
|
$ 2,482
|
$ 777
|

Income Statement
|
($ in thousands)
|
Q1 FY 2010**
(Ends Aug 31, 2009)
|
Q2 FY 2010
( Ends Nov. 30, 2009)
|
1st 6 Months of**
FY 2010
(Ends Nov. 30, 2009)
|
|
Revenue
|
$ 1,293
|
$ 2,137
|
$ 3,431
|
|
Gross Income
|
$ 943
|
$ 1,720
|
$ 2,683
|
|
Gross Margin
|
73%
|
80%
|
78%
|
|
Operating
expenses |
$ 347
|
$ 596
|
$ 1,267
|
|
Operating Income
|
$ 616
|
$ 1,124
|
$ 1,740
|
|
Net Income
|
$ 561
|
$ 1,074
|
$ 1,635
|
|
Net Margin
|
43%
|
50%
|
48%
|
** Income number exclude the stock option amortization charge

Ambitious Growth Strategy
Leverage patented technology for fast, low-cost drug synthesis and new drug
formulation
formulation
Short-term focus on launching high margin, new drugs to Chinese and other large
markets
markets
Medium-term focus on exporting API to US market
Long-term focus on applying the technology platform to identify candidate
compounds from Traditional Chinese Medicines
compounds from Traditional Chinese Medicines
Become the “Chinese Teva Pharma”

Contact Information
Sinobiopharma, Inc. trades on OTC BB
Ticker Symbol: SNBP
Shares outstanding: 120 million
www.sinobiopharma.com
Tel: 86 139 0295 7267
Email: ximu@sinobiopharma.com

Thank You!
Sinobiopharma, Inc.
OTC BB: SNBP
