UNITED
STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.
20549
Form 10-K
| |
|
|
|
(Mark One)
|
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal
year ended December 31, 2009
|
|
or
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition period
from to
|
Commission file number
000-23092
NATIONAL DENTEX
CORPORATION
(Exact name of registrant as
specified in its charter)
| |
|
|
MASSACHUSETTS
(State or Other Jurisdiction
of
Incorporation or Organization)
|
|
04-2762050
(I.R.S. Employer
Identification No.)
|
2 Vision Drive,
Natick, MA
(Address of Principal
Executive Offices)
|
|
01760
(Zip
Code)
|
(508) 907-7800
(Registrant’s
Telephone No., including Area Code)
Securities
registered pursuant to Section 12(b) of the Act:
| |
|
|
|
Title of Each Class
|
|
Name of Exchange on Which Registered
|
|
|
|
Common Stock, par value $.01 per share
|
|
The NASDAQ Stock Market, LLC
|
Securities registered pursuant to Section 12(g) of the
Act:
None
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes o No þ
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any,
every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of
Regulation S-T
(§229.405 of this chapter) during the preceding
12 months (or for such shorter period that the registrant
was required to submit and post such
files). Yes o No o
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in
Part III of this
Form 10-K
or any amendment to this
Form 10-K. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in
Rule 12b-2
of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated filer o
|
|
Accelerated filer o
|
|
Non-accelerated filer þ
|
|
Smaller reporting company o
|
|
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|
Indicate by check mark whether the registrant is a shell company
as defined in
Rule 12b-2
of the Exchange
Act. Yes o No þ
As of June 30, 2009, the aggregate market value of the
5,512,656 outstanding shares of voting stock held by
non-affiliates of the registrant was $35,887,391, based upon the
closing price of the Common Stock on the NASDAQ Global Market on
such date.
As of March 11, 2010, 5,761,363 shares of the
registrant’s Common Stock, par value $.01 per share, were
issued and outstanding.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions of the proxy statement for the 2010 annual
stockholders’ meeting, which we plan to file with the SEC
no later than 120 days after the end of our fiscal year
ended December 31, 2009, are incorporated by reference into
Part III.
TABLE OF CONTENTS
PART I
General
We were founded in 1982 as H&M Laboratories Services, Inc.,
a Massachusetts corporation, which acquired six full-service
dental laboratories and related branch laboratories from
Healthco, Inc. In 1983, we changed our name to National Dentex
Corporation and acquired 20 additional full-service dental
laboratories and related branch laboratories from Lifemark
Corporation. Our acquisition strategy is to consolidate our
position within the dental laboratory industry and use our
financial and operational synergies to create a competitive
advantage. Over the last five years we have acquired the
following stand-alone laboratory facilities: in 2005,
Wornson-Polzin Dental Laboratory and Green Dental Laboratories;
in 2006, Impact Dental and the Keller Group; and in 2008, Dental
Art Laboratories. Impact, located in the Canadian province of
Ontario, was our first acquisition outside of the United States.
We currently own and operate 44 dental laboratories, consisting
of 40 full-service dental laboratories and 4 branch
laboratories located in 30 states throughout the United
States and in one province of Canada. Our dental laboratories
custom design and fabricate dentures, crowns and fixed bridges,
and other dental prosthetic appliances. Each dental laboratory
operates under its own business name. Our principal executive
offices are located at 2 Vision Drive, Natick, MA 01760,
telephone number
(508) 907-7800.
Our corporate web site is www.nationaldentex.com. We make
available free of charge through our website our annual report
on
Form 10-K,
quarterly reports on
Form 10-Q,
current reports on
Form 8-K,
and amendments to these reports filed or furnished pursuant to
Section 13(a) of the Securities Exchange Act of 1934 as
soon as practicable after we electronically file such material
with, or furnish it to, the Securities and Exchange Commission.
References to our website address are provided for convenience
only and do not constitute, or should be viewed as, an
incorporation by reference of the information contained therein.
Therefore, such information should not be considered a part of
this report.
Information
as to Industry and Operating Segments
Our business consists of a single industry segment, which is the
design, fabrication, marketing and sale of custom dental
prosthetic appliances for and to dentists. We report on three
reportable segments within this single industry segment. These
three segments are known as Green Dental, representing the
operations of Green Dental Laboratories, Inc. of Heber Springs,
Arkansas which was acquired in March 2005; Keller Group,
representing the operations of Keller Group, Incorporated with
laboratories in St. Louis, Missouri and Louisville,
Kentucky which was acquired in October, 2006; and NDX
Laboratories, which represents our remaining laboratories. You
will find information about these segments in Note 10 to
the Consolidated Financial Statements “Segment
Information”, which you will find in Part II,
Item 8 of this annual report.
Description
of Business
Our dental laboratories in all three of our reportable segments
design and fabricate custom dental prosthetic appliances such as
dentures, crowns and bridges. These products are produced by
trained technicians working in dental laboratories in accordance
with work orders and cases (consisting of impressions, models
and occlusal registrations of a patient’s teeth) provided
by the dentist. Dentists are the direct purchasers of our
products.
Our products are grouped into the following three main
categories:
Restorative Products. Restorative products
that our dental laboratories sell consist primarily of crowns
and bridges. A crown replaces the part of a tooth that is
visible, and is usually made of gold, porcelain or zirconia. A
bridge is a restoration of one or more missing teeth that is
permanently attached to the natural teeth or roots. In addition
to the traditional crown, we also make onlays, which are partial
crowns which do not cover all of the visible tooth; inlays,
which are restorations made to fit a prepared tooth cavity and
then cemented into place and precision crowns, which are
restorations designed to receive and connect a removable partial
denture. We also mill crowns and bridges from zirconia using
CAD-CAM (computer-assisted design and computer-assisted
manufacturing) technologies.
1
Reconstructive Products. Reconstructive
products sold by our dental laboratories consist primarily of
partial dentures and full dentures. Partial dentures are
removable dental prostheses that replace missing teeth and
associated structures. Full dentures are dental prostheses that
substitute for the total loss of teeth and associated
structures. We also sell precision attachments, which connect a
crown and an artificial prosthesis, and implants, which are
fixtures anchored securely in the bone of the mouth to which a
crown, a partial denture a or full denture is secured by means
of screws or clips.
Cosmetic Products. Cosmetic products sold by
our dental laboratories consist primarily of porcelain veneers
and ceramic crowns. Porcelain veneers are thin coverings of
porcelain cemented to the front of a tooth to enhance personal
appearance. Ceramic crowns are crowns made from ceramic
materials that most closely replicate natural teeth. We also
sell composite inlays and onlays, which replace silver fillings
for a more natural appearance, and orthodontic appliances, which
are products fabricated to move existing teeth to enhance
function and appearance.
Additionally, we manufacture and market under an exclusive
license in the United States and on a non-exclusive basis in
Canada, the laboratory version of the NTI-tss
plustm
device, an appliance that is an alternative to full-coverage
bite guards, which is also approved by the Food & Drug
Administration (FDA) for use in the treatment of medically
diagnosed migraine pain and jaw disorders. We hold exclusive
rights for the production of the laboratory fabricated version
of this product through March 2023.
Laboratory
and Corporate Operations
Our full-service dental laboratories design and manufacture a
full range of custom-made dental prosthetic appliances. These
custom products are manufactured from raw materials, such as
high noble, noble and predominantly base alloys, zirconia,
dental resins, composites and porcelain. There are different
production processes for the various types of prosthetic
appliances depending upon the product and the materials used in
the type of appliance being manufactured, each of which requires
different skills, technologies and levels of training. Our
dental laboratories perform numerous quality control checks
throughout the production cycle to improve the quality of our
products and to better ensure that the design and appearance
satisfy the needs of the dentist and the patient. Our branch
dental laboratories are smaller in size and offer a limited
number of products. When a branch receives an order that it
cannot fill, the branch refers the order to one of our
affiliated full-service dental laboratories.
We operate each of our full-service dental laboratories as
stand-alone facilities under the direction of a local manager
responsible for operation of the dental laboratory, supervision
of its technical and sales staff and delivery of quality
products and services. Most of our dental laboratories market
and sell their products through their own direct sales force,
while the remainder of our laboratories are supported by a team
of national client specialists. Corporate level sales
management, company-wide marketing programs and group managers
are additional resources for our laboratories to draw upon.
Employees at each dental laboratory have a direct stake in the
growth of the dental laboratory and improvement in earnings
through participation in our performance incentive plans.
Our corporate management provides our overall strategy,
direction and financial management and negotiates all dental
laboratory acquisitions. Corporate personnel also support the
operations of our dental laboratories by performing functions
that are not directly related to the production and sale of
dental laboratory products, such as processing payroll and
related benefit programs, obtaining insurance and procuring
financing. Our corporate management provides marketing,
financial and administrative services, negotiates national
purchasing arrangements, and sets quality and performance
standards for our dental laboratories. Finally, our corporate
management includes industry recognized technical experts who
guide and direct our investments in new technology and materials.
Sales
and Marketing
The majority of our local dental laboratories market and sell
their products through their own direct sales force, while the
remainder of our laboratories are supported by a team of
national client specialists. The sales force interacts with
dentists within its local market area, primarily through visits
to dentists’ offices, to introduce the dental
laboratory’s services and products offered, and to promote
new products and techniques that can assist dentists in
expanding their practices. Our dentist-focused marketing and
sales programs, are specifically designed to make choosing a
dental laboratory an easier decision for dentists and help
differentiate our laboratories from their
2
many competitors. We believe that our efforts to assist the
dentist and his or her staff to improve chair-time efficiencies
while providing exceptional service, superior quality and quick
and timely product delivery will enhance our ability to expand
our base of business by establishing lasting professional
relationships with our customers. Our laboratory sales force is
comprised of 31 local and national client specialists and 20
other team members, including inside sales and support
personnel. In addition, our dental laboratories, alone or with
local dental societies, dental schools or study clubs, sponsor
technical training clinics for dentists and their staffs on
topics such as advanced clinical techniques. Our dental
laboratories also exhibit at state and local dental conventions.
In addition to our local service strategy, since the acquisition
of the Keller Group, we now also market more directly to the
entire United States dental marketplace using a direct mail and
trade advertising approach that focuses upon products that can
generate strong revenue growth, such as the NTI-tss
plustm
device. We believe this additional product offering has helped
us to further diversify our business growth strategy.
Competition
The dental laboratory industry is highly competitive and
fragmented. A typical dental laboratory’s business
originates from dentists located within 50 miles of the
dental laboratory. We believe there are currently approximately
12,000 dental laboratories in the United States. We estimate
that our sales presently represent less than 3% of the total
sales of custom-made dental prosthetic appliances in the United
States. We face competition primarily from other dental
laboratories in the respective local market areas. The vast
majority of dental laboratories consist of single business
units, although we recognize that there are several other
multiple-location operators, including Dental Services Group,
Dental Technologies, Inc. and NovaDent. These groups compete
with us in several market areas. We also face competition from
various mail order dental laboratories, most notably Glidewell
Laboratories.
The domestic industry has experienced growing competition from
low-wage countries, particularly from laboratories manufacturing
in China. Competition for business in the low-price segment of
the marketplace has grown over the last three years. Dental
laboratories manufacturing in China, including Dentsply-Prident,
Dentalle, Exceldent, DentUSA, Beijing Dental Lab, Sun Dental Lab
and Trident operate large, modern facilities. In addition to
partnering with laboratories in the United States, some have
also reached agreements to provide laboratory services for some
of the larger, price focused, economy dental practice chains. In
addition, the number of smaller domestic competitors that seek
to take advantage of this segment of the market by using price
as the main differentiator has continued to grow. While our
business has not traditionally focused on this lower cost
segment of the market, certain customers are sensitive to price
competition. As a result, these competitive pressures have
constrained somewhat our ability to increase prices. Beginning
in 2008, we partnered with Dentsply-Prident to provide a high
quality, economical restoration manufactured in China with FDA
registered materials for those price-focused practices who
demand it. We believe that this product offering, which has been
made available in select marketplaces based solely upon
individual customer requests and is coupled with customer level
disclosures regarding country of origin, materials and our
satisfaction guarantee, provides these dentists with a
risk-free, offshore product while allowing us to maintain the
relationship to provide other U.S. manufactured products.
We continue to evaluate competitive threats as well as
opportunities arising from globalization, technology and
changing marketplaces to ensure our products and services can
continue to be provided at competitive prices.
Our ability to produce quality products locally, to deliver such
products on a timely basis, to provide convenience for dentists
through the breadth of our product line, to provide technical
assistance, and our sponsorship of educational clinics, all
provide us with what we consider to be a competitive advantage
over other dental laboratories in the local markets in which our
dental laboratories operate. Most dentists use a limited number
of dental laboratories. We believe they prefer and tend to rely
on those laboratories which produce quality products delivered
on a timely basis and which carry all of the products which they
may need, even if a particular item is a newer specialty product
used only sporadically by the dentist. While price is one of the
competitive factors in the dental laboratory industry, we
believe that most dentists consider product quality and
consistency, service, and breadth of product line to also be
important factors in selecting dental laboratories. We believe
that we compete favorably with respect to all of these factors.
Our ability to provide newer specialty products for dental
implants, adult orthodontics and cosmetic dentistry, which
require highly skilled technicians, more extensive inventories,
additional working capital, and investment in both training and
capital equipment, also distinguishes us from the
3
many other dental laboratories which do not have comparable
resources to provide these products. While such specialty
products presently represent less than 20% of our business, we
believe that the ability to offer these products will become
increasingly essential for dental laboratories to remain
competitive. Additionally, our ability to offer CAD-CAM
fabricated, metal-free restorations through our own centralized
milling centers allows us to participate fully in this fast
growing segment.
Employees
As of December 31, 2009, we had 1,745 full time employees,
1,684 of whom worked at individual laboratories. Corporate
management and administrative staff totaled 61 people. None
of our employees are covered by a collective bargaining
agreement. Management considers our employee relations to be
good.
Intellectual
Property
Our general technological know-how, experience and workforce are
important to the conduct of our business. Each of our dental
laboratories operates under its own trade name, and we consider
these trade names to be important to the conduct of our
business. Also important is the development and maintenance of
customer relationships. We expect that our continued focus on
ensuring that clients get a consistent product that is delivered
on time and meets or exceeds their quality expectations, will
continue to assist us in generating and maintaining customer
relationships and the goodwill of our dental laboratories. We
also have licensed long-term, exclusive manufacturing and
distribution rights to fabricate and market the laboratory
version of the NTI-tss
plustm
device in the United States. Finally, while we have several
other trademarks and licenses to use trademarks, we do not deem
these to be material to the overall conduct of our business.
Backlog
Due to the individualized and customized nature of most dental
products and a typical turnaround product cycle of less than
seven days, there was no significant backlog of orders existing
at December 31, 2009 and 2008.
Our business is subject to certain risks that could materially
affect our financial condition, results of operations, and the
value of our common stock. These risks include, but are not
limited to, the ones described below. Additional risks and
uncertainties that we are unaware of, or that we may currently
deem immaterial, may become important factors that harm our
business, financial condition, results of operations, or the
value of our common stock.
Our
success depends on economic and other external factors that
affect consumer decisions about whether and when to have dental
procedures performed.
Our business success depends in large measure on consumer
decisions to have dental procedures performed. In this respect,
demand for our products and our business results are sensitive
to external factors that, directly or indirectly, affect
consumer confidence, affect levels of disposable consumer
income, or otherwise lead consumers to defer or elect not to
have dental procedures performed. Examples of such external
factors include the timing, duration and effects of adverse
changes in overall economic conditions, including rates of job
loss or growth, rising food and energy prices, tightening
consumer credit and the resulting problems in the housing
market, and increases in medical and dental costs, nationally or
regionally in the markets we serve. Trends in the dental
industry towards managed care may also result in decreased
consumer access to dental services and thereby adversely affect
demand for our products and our sales and profitability. The
precise impact of these external factors is difficult to predict
in advance, but one or more of these factors could adversely
affect our business to the extent they adversely affect consumer
spending on dental procedures.
The US and world economy have experienced a recession that has
affected all sectors of the economy, resulting in declines in
economic growth and consumer confidence, increases in
unemployment rates and uncertainty about economic stability.
Recently, there have been some signs that the US economy is
recovering; however, the strength and duration of that recovery
is unpredictable. Recessions and other economic downturns,
especially prolonged downturns, as well as disruptions in the
credit and stock markets, can result in lower levels of economic
activity,
4
lower employment levels, less consumer disposable income and
lower economic confidence. Any of these factors can reduce
discretionary consumer spending. Many or our cosmetic dental
products may be considered discretionary spending by consumers.
If consumers reduce or delay spending on cosmetic dental
products, our business and financial performance may be
adversely affected.
We
operate in a highly competitive and fragmented market that is
increasingly global in scope.
The dental laboratory industry is highly competitive and
fragmented. We believe there are currently approximately 12,000
dental laboratories in the United States. We estimate that our
sales presently represent less than 3% of the total sales of
custom-made dental prosthetic appliances in the United States.
Competition is primarily from other dental laboratories in the
respective local market areas. The vast majority of dental
laboratories consist of single business units, although there
are several other multiple-location operators, including Dental
Services Group, Dental Technologies, Inc., and NovaDent. These
groups compete with us in several market areas. We also face
competition from various mail order dental laboratories, most
notably Glidewell Laboratories. Our success thus depends on our
ability to be competitive against many different competitors in
each market area we serve. If we fail to anticipate evolving
technological innovations and product offerings from our
competitors, particularly offerings that seek to leverage lower
labor costs available in foreign countries, or fail to offer
products that appeal to the changing needs and preferences of
our customers in the various markets we serve, demand for our
products could decline and our operating results would be
adversely affected. While the competitive importance of product
quality, price, service and innovation varies from product to
product, price is a factor, and we experience pricing pressures
from competitors in our markets.
We
face increased competitive pressures from foreign-sourced
products and technology-based solutions.
The industry in which we operate continues to change and evolve.
Increasing competitive pressures from offshore laboratories
based in China, India and elsewhere are impacting sales growth
and selling prices of certain core products, particularly
partial frames and traditional crowns. Technology-based dental
laboratory CAD-CAM solutions have required us to make additional
investments in capital equipment. While we expect these capital
investments to continue to benefit our operations in future
periods, there is no assurance that they will be able to do so.
Certain of these technology-based solutions also enable dentists
to fabricate restorations in their offices rather than
purchasing them from an off-site laboratory.
Price pressures from such new sources of competition could erode
our margins and cause our financial results of operations to
suffer. Our success depends on our ability to evaluate and
respond to the threats arising from growing foreign competition,
changing marketplaces and new technology and our ability to
identify ways in which we can competitively provide the products
and services demanded by our customers.
We
face increased competitive pressures from multiple location
operators and the consolidation in the industry.
The dental laboratory industry has continued to consolidate and
has drawn the attention of private equity investors. While the
consequences of these changes in the dental laboratory industry
are not yet fully known, these competitors may now have greater
financial and other resources than previously available to them,
which could increase competitive pressures on our operations.
These developments could impact the availability of suitable
acquisition candidates or otherwise increase the costs of
acquiring dental laboratories.
Our
failure to generate sufficient cash to meet our liquidity needs
may affect our ability to service our indebtedness and grow our
business.
Our ability to make payments on and to refinance our
indebtedness, principally the amounts borrowed under our credit
facility, and to fund planned capital expenditures, expansion
efforts and strategic acquisitions we may make in the future, if
any, will depend on our ability to generate cash in the future.
This, to a certain extent, is subject to general economic,
financial, competitive and other factors that are beyond our
control.
Based on our current level of operations, we believe our cash
flow from operations, together with available cash and available
borrowings under our senior credit facility, will be adequate to
meet future liquidity needs in the
5
coming year. However, we cannot assure you that our business
will generate sufficient cash flow from operations in the future
or that future borrowings will be available to us under the
senior credit facility in an amount sufficient to enable us to
service indebtedness, undertake strategic acquisitions to grow
our business, or to fund other liquidity needs. If we need to
refinance all or a portion of our indebtedness, we cannot assure
you that we will be able to do so on commercially reasonable
terms or at all. In the event that our financial performance was
to deteriorate, it could result in higher interest rates under
our senior credit facility, or a default under the facility, if
we are unable to maintain compliance with the financial ratio
and other covenants in the facility.
We may
be more leveraged than some of our competitors, which could
adversely affect our business plans.
We have incurred debt in pursuing our strategic acquisitions and
a portion of our cash flow is used to service this debt. This in
turn has reduced the funds we have available for working
capital, capital expenditures, additional acquisitions, and
other purposes and, a significant part of our growth strategy is
to acquire additional dental laboratories. Given current credit
conditions it may be more difficult for us to make additional
borrowings in the future, potentially affecting our growth
strategy.
Similarly, our debt levels may increase our vulnerability to,
and limit our flexibility in planning for, adverse economic and
industry conditions and create other competitive disadvantages
compared with other companies with relatively less leverage,
especially in times of industry consolidation.
Our
Senior Credit Facility contains a number of financial and other
covenants that may restrict our ability to engage in some
business transactions.
We have entered into a senior credit facility with Bank of
America, N.A. which includes a term loan facility and a line of
credit of $25 million. As of December 31, 2009, the
outstanding balance was $19.6 million on the term loan and
$6.5 million on the revolving line of credit. The credit
agreement restricts our ability and the ability of our
subsidiaries to, among other things, engage in a number of
actions including incurring or guaranteeing additional
indebtedness, merging or consolidating with, acquiring
substantially all of the stock or assets of any other companies,
acquiring additional dental laboratories, repurchasing our
common stock, making investments, transferring assets, incurring
or permitting to exist liens, and making any material changes to
the nature of our business. The credit facility also contains
other covenants that are typical for credit facilities of this
size, type and tenor, such as requirements that we meet
specified financial ratios and financial condition tests. Our
ability to make additional borrowings under the facility depends
upon satisfaction of these covenants. Our ability to meet these
covenants and requirements may be affected by events beyond our
control.
Our inability to comply with obligations could result in an
event of default under the credit facility. A default, if not
cured or waived, could permit acceleration of our indebtedness.
We cannot be certain that we will be able to remedy any default.
If our indebtedness is accelerated, we cannot be certain that we
will have funds available to pay the accelerated indebtedness or
that we will have the ability to refinance the accelerated
indebtedness on terms favorable to us or at all.
An
impairment in the carrying value of goodwill or other acquired
intangibles could negatively affect our operating results and
net worth.
General economic conditions, or other factors resulting in
changes in the industry in which we operate, competition,
advances in technology, or other factors may lead to reductions
in expected sales, profitability or cash flows that could result
in impairment of goodwill and other acquired intangible assets.
If the value of goodwill or other acquired intangibles is
impaired, our earnings, net worth and financial covenants could
be adversely affected.
The goodwill impairment analysis is a two-step process. The
first step is used to identify potential impairment and involves
comparing each reporting unit’s estimated fair value to its
carrying value, including goodwill. Fair value is determined by
using an income approach, consistent with our valuation of
dental laboratories acquired in purchase business combinations.
We determine fair value based on the estimated future cash flows
of each reporting unit, based on a multiple of annual earnings.
Determining the fair value of a reporting unit is judgmental in
nature and requires the use of significant estimates and
assumptions, including revenue growth rates and profit margin
percentages, and future market conditions, among others. Our
projections are based on an internal forecast and a
6
business review. If the estimated fair value of a reporting unit
exceeds its carrying value, goodwill is not considered to be
impaired. However, if the carrying value exceeds estimated fair
value, there is an indication of potential impairment and the
second step is performed to measure the amount of impairment.
The second step of the goodwill impairment process involves the
calculation of an implied fair value of goodwill for the
laboratories which step one indicated were impaired. The implied
fair value of goodwill is determined similar to how goodwill is
calculated in a business combination, by measuring the excess of
the estimated fair value of the reporting unit as calculated in
step one, over the estimated fair values of the individual
assets, liabilities and identifiable intangibles as if the
reporting unit was being acquired in a business combination. If
the carrying value of goodwill assigned to a reporting unit
exceeds the implied fair value of the goodwill, an impairment
charge is recorded for the excess. In determining the fair value
of assets we utilize valuations of certain intangible assets,
including trade names and customer relationships. The analysis
we completed for December 31, 2008 determined that the fair
value of ten dental laboratories in the NDX Laboratories
operating segment was less than their carrying value resulting
in goodwill impairment of $6,950,000. During the third quarter
of 2009, the Company recorded an impairment charge of $264,000,
comprising the entire goodwill balance of one of our smaller
laboratories, due to a significant decrease in revenues and
operating income during the third quarter of 2009 at this
laboratory. As of December 31, 2009, we had $69,616,000 of
goodwill remaining on our consolidated balance sheet. If the
conditions noted above were to significantly deteriorate, we may
need to record additional goodwill impairment charges, which
could adversely affect our earnings, net worth and debt
covenants.
Risks
associated with our strategic acquisitions could adversely
affect our business.
We have completed a number of acquisitions over the past five
years. Our acquisition strategy depends on our ability to
identify laboratories that are suitable acquisition candidates,
successfully negotiate and enter into transactions on acceptable
terms, and our capacity to integrate and successfully operate
newly acquired as well as our previously acquired laboratories.
If we fail to locate suitable acquisition candidates, reach
mistaken conclusions as to the suitability of laboratories as
acquisition candidates, enter into transactions on terms that
prove unfavorable to us, or fail to integrate new laboratories
following an acquisition, our ability to operate and grow our
business in the ways we would like could be materially and
adversely affected. While we will continue to consider
acquisitions as a means of enhancing shareowner value,
acquisitions involve risks and uncertainties, including:
|
|
|
| |
•
|
difficulties integrating the acquired company, retaining the
acquired laboratories’ customers, and achieving the
expected benefits of the acquisition, such as revenue increases,
cost savings, and increases in geographic or product presence,
in the desired time frames, if at all;
|
| |
| |
•
|
loss of key employees from the acquired company;
|
| |
| |
•
|
implementing and maintaining consistent standards, controls,
procedures, policies and information systems; and
|
| |
| |
•
|
diversion of management’s attention from other business
concerns.
|
Our long-term success depends, in part, on our ability to
acquire additional dental laboratories in a manner that achieves
appropriate returns on our capital invested. If we are unable to
generate the required sales or profit levels, as a result of
macroeconomic or operational challenges, we will not acquire new
dental laboratories and our future financial performance could
be materially and adversely affected. Additionally, future
acquisitions could cause us to incur additional debt, contingent
liabilities, increased interest expense and higher amortization
expense related to intangible assets, as well as experience
dilution in earnings per share. Impairment losses on goodwill
and intangible assets with an indefinite life, or restructuring
charges, could also occur as a result of acquisitions, which
could adversely affect our results of operations and cause us to
violate the covenants under our senior credit facility.
If we
fail to develop new or expand existing customer relationships,
our ability to grow our business will be impaired.
Our growth depends on our ability to develop new customer
relationships with dentists, maintain existing relationships,
and to expand existing relationships with our current customers.
We cannot guarantee that new customers will be found, that any
such new relationships will be successful when they are in
place, or that business
7
with current customers will increase. Failure to develop and
expand such relationships could have a material adverse effect
on our business, results of operations and financial condition.
If we
cannot continue to respond to technical innovations we may not
be able to compete effectively.
We believe that our future success will depend, in part, upon
our ability to continue to respond to technological innovations
by the dental industry and introduce innovative design
extensions for our existing products and to manufacture and
market new products. We cannot assure you that we will be
successful in the introduction, manufacturing and marketing of
any new products or product innovations, or develop and
introduce, in a timely manner, innovations to our existing
products that satisfy our dentist customers’ needs or
achieve market acceptance. Our failure to introduce new products
successfully and in a timely manner, and at favorable margins,
could harm our ability to successfully grow our business and
could have a material adverse effect on our business, results of
operations and financial condition.
Our
failure to attract and retain qualified personnel would
adversely affect our business.
Our success depends in part on the efforts and abilities of our
senior management team and key employees, a number of which are
approaching retirement age. Their skills, experience and
industry contacts significantly benefit our operations and
administration. The failure to attract, retain, and properly
motivate the members of our senior management team and key
employees, or to find suitable replacements for them in the
event of death, ill health, resignation, or retirement, could
have a negative effect on our operating results. In addition,
our success is also dependent upon our ability to retain
qualified employees at our dental laboratories, including dental
laboratory technicians who are able to manufacture our dental
prosthetics. In the future, if competition for these services
increases, the Company may not be able to continue to attract
and retain these individuals, which could have a material
adverse effect on our revenues.
Our
business results are adversely affected by increases in labor,
benefits and related costs.
The costs of medical and other benefits have increased in recent
years. The increased usage of medical benefits has intensified
medical inflation in the United States. If such trends continue,
then our business could be negatively affected. Changes in law
that may increase the funding of, and the expense reflected for,
employee benefits, could also adversely affect our financial
results of operations, financial position, and competitiveness.
Our
operating results can be adversely affected by changes in the
cost or availability of raw materials, particularly precious
metals like gold, platinum and palladium.
Pricing and availability of raw materials for use in our
businesses — most especially precious metals, like
gold, platinum and palladium which are components of many dental
alloys — can be volatile due to numerous factors
beyond our control, including domestic and international
economic and geopolitical conditions, production levels,
competition, consumer demand, and investor speculation. This
volatility can significantly affect the availability and cost of
raw materials for us, and may, therefore, have a material
adverse effect on our business, results of operations and
financial condition. During periods of rising prices of raw
materials, there can be no assurance that we will be able to
pass any portion of such increases on to customers. Prolonged
higher metal costs may thus have a negative impact on gross
profit percentages. Conversely, when raw material prices
decline, customer demands for lower prices could result in lower
sale prices and, to the extent we have existing inventory, lower
margins. As a result, fluctuations in raw material prices could
have a material adverse effect on our business, results of
operations and financial condition. The combination of higher
precious metal prices and increasing offshore competition has
made it more difficult for us to pass on these additional costs
without impacting our customer base.
Compliance
with changing regulation of corporate governance, public
disclosure, and accounting standards may result in additional
expenses and risks.
Changing laws, regulations and standards relating to corporate
governance, public disclosure and accounting practices, new
Securities and Exchange Commission regulations and evolving
rules applicable to publicly-traded
8
companies on the NASDAQ Global Market (Nasdaq), are creating
uncertainty, and hence risks, for companies such as ours. These
new or changed laws, regulations and standards are subject to
varying interpretations due to the fact that they are new and
there has not yet emerged a well-developed body of
interpretation. As a result, their application in practice may
evolve over time as new guidance is provided by regulatory and
governing bodies. This development could result in continuing
uncertainty regarding compliance matters and higher costs
necessitated by ongoing revisions to disclosure, governance and
accounting practices.
Our efforts to comply with evolving laws, regulations and
standards have resulted in, and are likely to continue to result
in, increased general and administrative expenses and an
investment of management time and attention from
revenue-generating activities to compliance activities. In
particular, our efforts to comply with Section 404 of the
Sarbanes-Oxley Act of 2002 and the related regulations regarding
our required assessment of our internal controls over financial
reporting and our external auditors’ audit of that
assessment has required the commitment of significant financial
and managerial resources. If our efforts to comply with new or
changed laws, regulations and standards differ from the
activities intended by regulatory or governing bodies, we could
face many material and adverse consequences, including, a
possible delisting of our common stock.
We are
subject to a number of continuing listing standard requirements
of Nasdaq Global Market. If we fail to comply with these listing
standards we may be subject to delisting.
Our common stock is currently listed on the NASDAQ Global Market
(Nasdaq). Nasdaq marketplace rules require the maintenance of a
number of continued listing standards. If we fail to comply with
any of the continued listing standards and are unable to cure
such defect within the allotted time following the receipt of
any notice from Nasdaq regarding our failure to maintain such
continued listing standard, Nasdaq might delist our common
stock. Delisting would have an adverse effect on the liquidity
of our common stock and, as a result, the market price for our
common stock might become more volatile. Delisting could also
make it more difficult for us to raise additional capital.
We may
face increased regulatory action by, among other governmental
entities, the United States Food and Drug Administration (the
“FDA”).
Since 2007, the industry has faced increased scrutiny from the
FDA and other governmental entities concerning the safety and
efficacy of dental products distributed in the United States
from both foreign and domestic laboratories. As a result of this
scrutiny, the FDA may propose possible registration,
certification, material content and point of origin disclosure.
Several states have passed legislation for a combination of
material content and point of origin disclosures. Potential
changes in laws, regulations and standards involving these
issues, while new and evolving, are creating uncertainty in
compliance requirements and could result in higher costs in
order to comply with any revisions or additions to them and may
create new legal liabilities if we fail to do so.
Our
business may be adversely affected by the actions of and risks
associated with our third-party suppliers.
If we experience declining operating performance, or if we
experience liquidity challenges, our suppliers may demand
accelerated payment of amounts due to them or require advance
payments or letters of credit before goods are shipped to us.
These demands could have a significant adverse impact on our
operating cash flow and result in a drain on our liquidity. In
addition, many of our suppliers may be significantly impacted by
current macroeconomic conditions. We may have no warning before
a supplier fails, which may have an adverse effect on our
business and results of operations. Further, we cannot control
the cost of our raw products, and cost increases must either be
passed along to our customers or will result in erosion of our
earnings.
Forward
Looking Statements
Certain statements in this Annual Report, particularly
statements contained in Item 7, “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” constitute “forward-looking
statements” within the meaning of Section 27A of the
Securities Act of 1933, as amended, and Section 21E of the
Securities Exchange Act of 1934, as amended. The words
“anticipate”, “believe”,
“estimate”, “expect”, “plan”,
“intend” and other similar expressions are intended to
identify these forward-looking statements, but are not the
9
exclusive means of identifying them. Forward-looking statements
included in this Annual Report or hereafter included in other
publicly available documents filed with the Securities and
Exchange Commission (“SEC”), reports to our
stockholders and other publicly available statements issued or
released by us involve known and unknown risks, uncertainties,
and other factors which could cause our actual results,
performance (financial or operating) or achievements to differ
from the future results, performance (financial or operating) or
achievements expressed or implied by such forward-looking
statements. Such future results are based upon our best
estimates based upon current conditions and the most recent
results of operations. We assume no obligation to update these
forward-looking statements contained in this report, whether as
a result of new information, future events, or otherwise.
Various risks, uncertainties and contingencies could cause our
actual results, performance or achievements to differ materially
from those expressed in, or implied by, the forward-looking
statements contained in this Annual Report. These include, but
are not limited to, those listed above in this Item 1A,
“Risk Factors.”
|
|
|
Item 1B.
|
Unresolved
Staff Comments
|
Not applicable.
We currently lease a total of approximately 361,000 square
feet of space. As of December 31, 2009, the future
aggregate minimum rent payable for all of our leased real
properties was approximately $16,092,000. We believe that
suitable substitute or replacement space is readily available at
reasonable rental rates. Our principal executive and
administrative offices occupy approximately 15,000 square
feet of space in Natick, Massachusetts. Our 38 leased dental
laboratories range in size from 1,000 to 40,000 square feet
and average approximately $95,000 in annual base rent.
As of December 31, 2009, we owned six of our dental
laboratory facilities at locations in Heber Springs, Arkansas;
Metairie, Louisiana; Shreveport, Louisiana; Addison, Texas;
Houston, Texas; and Waukesha, Wisconsin. These locations total
approximately 152,000 square feet and range in building
size from 10,000 to 41,000 square feet. In September 2009,
we sold our former Denver, Colorado facility comprising
approximately 6,000 square feet and recognized a gain on
the sale of $365,000.
All of our owned real property is used in connection with our
NDX Laboratories operating segment, except for our Heber
Springs, Arkansas facility, which is used by our Green Dental
operating segment. Our third operating segment, Keller, uses
leased property located in St. Louis, Missouri and
Louisville, Kentucky. We consider our leased and owned
properties to be modern, well maintained and suitable for our
purposes and believe that our current facilities are adequate to
meet our needs for the foreseeable future.
|
|
|
Item 3.
|
Legal
Proceedings
|
We are involved from time to time in litigation incidental to
our business. Our management believes that the outcome of
current litigation will not have a material adverse effect upon
our operations or financial condition and will not disrupt our
normal operations.
|
|
|
Item 4.
|
Removed
and Reserved
|
|
|
|
Item 5.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters
and Issuer Purchases of Equity Securities
|
10
PART II
Trading
Market
The NASDAQ Global Market (“Nasdaq”) is the principal
market for our common stock, where our shares are traded under
the symbol “NADX”. Our common stock has been publicly
traded since December 21, 1993.
The following table sets forth the range of high and low sale
prices for our common stock for each of the fiscal quarters of
2008 and 2009. The sale prices set forth below are based on
information provided by NASDAQ.
| |
|
|
|
|
|
|
|
|
|
|
|
Price
|
|
Quarter Ending
|
|
Low
|
|
High
|
|
|
|
03/31/08
|
|
$
|
11.50
|
|
|
$
|
16.84
|
|
|
06/30/08
|
|
$
|
10.05
|
|
|
$
|
13.25
|
|
|
09/30/08
|
|
$
|
6.01
|
|
|
$
|
12.60
|
|
|
12/31/08
|
|
$
|
4.19
|
|
|
$
|
6.90
|
|
|
03/31/09
|
|
$
|
1.25
|
|
|
$
|
5.35
|
|
|
06/30/09
|
|
$
|
3.78
|
|
|
$
|
8.00
|
|
|
09/30/09
|
|
$
|
6.25
|
|
|
$
|
8.93
|
|
|
12/31/09
|
|
$
|
6.00
|
|
|
$
|
12.73
|
|
Holders
The number of record holders of our common stock as of
March 1, 2010 was 612. The number of record owners was
determined from our stockholder records, and does not include
beneficial owners of our common stock whose shares are held in
the names of various security holders, dealers and clearing
agencies. We believe that the number of beneficial owners of our
common stock held by others in nominee names is approximately
1,200 beneficial holders.
Dividends
We have never paid a cash dividend on our shares of common stock
and have no expectation of doing so for the foreseeable future.
Recent
Sales of Unregistered Securities
None.
Purchases
of Equity Securities by the Issuer and Affiliated
Purchasers
In November 2002, we announced that our Board of Directors
approved the repurchase by us of up to 300,000 shares of
our common stock pursuant to a stock repurchase program. During
the year ended December 31, 2009, we did not repurchase any
shares of our common stock. We continue to consider repurchases
on the open market or in privately-negotiated transactions, at
management’s discretion, in each case subject to applicable
securities law. In addition, before making any repurchases, we
are required to obtain approval from our lender under the terms
of the credit facility. The following table provides information
about our repurchase activity during fiscal 2009 and the number
of shares that may yet be purchased under our stock repurchase
program.
Issuer
Purchases of Equity Securities
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum Number
|
|
|
|
|
|
|
|
Total Number of
|
|
of Shares that
|
|
|
|
|
|
|
|
Shares Purchased
|
|
May Yet Be
|
|
|
|
Total Number
|
|
Average
|
|
as Part of Publicly
|
|
Purchased Under
|
|
|
|
of Shares
|
|
Price Paid
|
|
Announced Plans
|
|
the Plans or
|
|
Fiscal Period
|
|
Purchased
|
|
per Share
|
|
or Programs
|
|
Programs
|
|
|
|
January 1, 2009 - December 31, 2009
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
|
206,700
|
|
11
Stock
Performance Graph
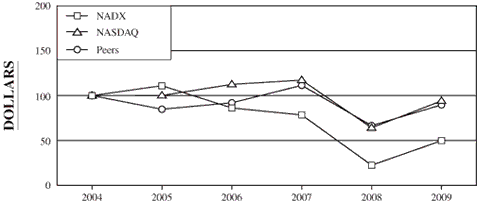
The following graph compares the cumulative total stockholder
return of our common stock during the five fiscal years ended
December 31, 2009 with the cumulative total return of the
NASDAQ Industrial Index and a peer group index described more
fully below.
This graph is not deemed to be “filed” with the SEC or
subject to the liabilities of Section 18 of the Securities
Exchange Act of 1934, and the graph shall not be deemed to be
incorporated by reference into any prior or subsequent filings
by us under the Securities Act of 1933 or the Securities
Exchange Act of 1934.
COMPARISON
OF CUMULATIVE TOTAL RETURN (1) AMONG NATIONAL DENTEX
(“NADX”),
NASDAQ INDUSTRIAL INDEX AND PEER GROUP INDEX (2)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12-31-04
|
|
|
|
12-31-05
|
|
|
|
12-31-06
|
|
|
|
12-31-07
|
|
|
|
12-31-08
|
|
|
|
12-31-09
|
|
|
NADX
|
|
|
|
100.00
|
|
|
|
|
111.03
|
|
|
|
|
86.20
|
|
|
|
|
78.42
|
|
|
|
|
22.41
|
|
|
|
|
49.75
|
|
|
NASDAQ Industrial Index
|
|
|
|
100.00
|
|
|
|
|
100.12
|
|
|
|
|
112.52
|
|
|
|
|
117.27
|
|
|
|
|
64.11
|
|
|
|
|
94.07
|
|
|
Peers
|
|
|
|
100.00
|
|
|
|
|
84.82
|
|
|
|
|
92.01
|
|
|
|
|
111.40
|
|
|
|
|
66.55
|
|
|
|
|
89.65
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Assumes $100 invested on December 31, 2004 in our common
stock, the NASDAQ Industrial Index and the Peer Group Index,
including reinvestment of any dividends paid on the investment. |
| |
|
(2) |
|
The Peer Group Index consists of Dentsply International, Inc.
and Patterson Companies, Inc. We believe that these companies
represent the other publicly traded companies within the dental
service community. |
12
|
|
|
Item 6.
|
Selected
Financial Data
|
The following selected financial data for the five years ended
December 31, 2009 are derived from our audited consolidated
financial statements. The data should be read in conjunction
with the consolidated financial statements and the related notes
included in this Report and in conjunction with Item 7,
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations.”
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2005
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
(Dollars in thousands, except per share data)
|
|
|
|
|
Consolidated Statements of Income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales
|
|
$
|
135,843
|
|
|
$
|
150,107
|
|
|
$
|
170,361
|
|
|
$
|
171,674
|
|
|
$
|
161,195
|
|
|
Cost of goods sold
|
|
|
78,381
|
|
|
|
88,269
|
|
|
|
97,739
|
|
|
|
102,184
|
|
|
|
93,119
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
57,462
|
|
|
|
61,838
|
|
|
|
72,622
|
|
|
|
69,490
|
|
|
|
68,076
|
|
|
Selling, general & administrative expenses
|
|
|
44,728
|
|
|
|
50,097
|
|
|
|
58,562
|
|
|
|
58,588
|
|
|
|
55,755
|
|
|
Goodwill impairment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,950
|
|
|
|
264
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
12,734
|
|
|
|
11,741
|
|
|
|
14,060
|
|
|
|
3,952
|
|
|
|
12,057
|
|
|
Other expense
|
|
|
646
|
|
|
|
786
|
|
|
|
771
|
|
|
|
747
|
|
|
|
745
|
|
|
Interest expense
|
|
|
665
|
|
|
|
1,523
|
|
|
|
2,803
|
|
|
|
2,110
|
|
|
|
1,257
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
11,423
|
|
|
|
9,432
|
|
|
|
10,486
|
|
|
|
1,095
|
|
|
|
10,055
|
|
|
Provision for income taxes
|
|
|
4,334
|
|
|
|
3,669
|
|
|
|
3,860
|
|
|
|
1,972
|
|
|
|
4,178
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
7,089
|
|
|
$
|
5,763
|
|
|
$
|
6,626
|
|
|
$
|
(877
|
)
|
|
$
|
5,877
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share — basic
|
|
$
|
1.33
|
|
|
$
|
1.05
|
|
|
$
|
1.20
|
|
|
$
|
(0.16
|
)
|
|
$
|
1.03
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share — diluted
|
|
$
|
1.27
|
|
|
$
|
1.01
|
|
|
$
|
1.17
|
|
|
$
|
(0.16
|
)
|
|
$
|
1.02
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding — basic
|
|
|
5,334
|
|
|
|
5,485
|
|
|
|
5,540
|
|
|
|
5,631
|
|
|
|
5,727
|
|
|
Weighted average shares outstanding — diluted
|
|
|
5,601
|
|
|
|
5,732
|
|
|
|
5,665
|
|
|
|
5,631
|
|
|
|
5,761
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Working capital
|
|
$
|
11,126
|
|
|
$
|
6,232
|
|
|
$
|
6,000
|
|
|
$
|
9,527
|
|
|
$
|
9,296
|
|
|
Total assets
|
|
|
117,119
|
|
|
|
148,490
|
|
|
|
155,639
|
|
|
|
161,515
|
|
|
|
155,295
|
|
|
Long-term debt, including current portion
|
|
|
18,701
|
|
|
|
35,458
|
|
|
|
29,695
|
|
|
|
39,258
|
|
|
|
26,848
|
|
|
Stockholders’ equity
|
|
$
|
76,074
|
|
|
$
|
82,794
|
|
|
$
|
91,192
|
|
|
$
|
90,492
|
|
|
$
|
97,790
|
|
|
|
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
The following discussion should be read in conjunction with
the Consolidated Financial Statements
and the related notes that appear elsewhere in this
document.
Certain statements in this Annual Report, particularly
statements contained in this Item 7,
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations” constitute
“forward-looking statements” within the meaning of
Section 27A of the Securities Act of 1933, as amended, and
Section 21E of the Securities Exchange Act of 1934, as
amended. The words “anticipate”, “believe”,
“estimate”, “expect”, “plan”,
“intend” and other similar expressions are intended to
identify these forward-looking statements, but are not the
exclusive means of identifying them. Forward-looking statements
included in this Annual Report or hereafter included in other
publicly available documents filed with the Securities and
Exchange Commission (“SEC”), reports to our
stockholders and other publicly available statements issued or
released by us involve known and unknown risks, uncertainties,
and other factors which could cause our actual results,
performance (financial or operating) or achievements to differ
from the future results, performance (financial or operating) or
achievements expressed or implied by such forward-looking
statements. Such future results are based upon our best
estimates based upon current conditions and the most recent
results of operations. Various risks, uncertainties and
contingencies could cause our actual results, performance or
achievements to differ materially from those
13
expressed in, or implied by, the forward-looking statements
contained in this Annual Report. These include, but are not
limited to, those described above under Item 1A, “Risk
Factors.” We assume no obligation to update these
forward-looking statements contained in this report, whether as
a result of new information, future events, or otherwise.
Overview
We own and operate 44 dental laboratories located in
30 states and one Canadian province, serving an active
customer base of over 24,000 dentists. Our business consists of
the design, fabrication, marketing and sale of custom dental
prosthetic appliances for dentists located primarily in the
domestic marketplace.
Our products are grouped into the following three main
categories:
Restorative Products. Restorative products
that our dental laboratories sell consist primarily of crowns
and bridges. A crown replaces the part of a tooth that is
visible, and is usually made of gold, porcelain or zirconia. A
bridge is a restoration of one or more missing teeth that are
permanently attached to the natural teeth or roots. In addition
to the traditional crown, we also make onlays, which are partial
crowns which do not cover all of the visible tooth; inlays,
which are restorations made to fit a prepared tooth cavity and
then cemented into place and precision crowns, which are
restorations designed to receive and connect a removable partial
denture. We also mill crowns and bridges from zirconia using
CAD-CAM (computer-assisted design and computer-assisted
manufacturing) technologies.
Reconstructive Products. Reconstructive
products sold by our dental laboratories consist primarily of
partial dentures and full dentures. Partial dentures are
removable dental prostheses that replace missing teeth and
associated structures. Full dentures are dental prostheses that
substitute for the total loss of teeth and associated
structures. We also sell precision attachments, which connect a
crown and an artificial prosthesis, and implants, which are
fixtures anchored securely in the bone of the mouth to which a
crown, partial denture or full denture is secured by means of
screws or clips.
Cosmetic Products. Cosmetic products sold by
our dental laboratories consist primarily of porcelain veneers
and ceramic crowns. Porcelain veneers are thin coverings of
porcelain cemented to the front of a tooth to enhance personal
appearance. Ceramic crowns are crowns made from ceramic
materials that most closely replicate natural teeth. We also
sell composite inlays and onlays, which replace silver fillings
for a more natural appearance, and orthodontic appliances, which
are products fabricated to move existing teeth to enhance
function and appearance.
Additionally, we manufacture and market under an exclusive
license in the United States and on a
non-exclusive
basis in Canada, the laboratory version of the NTI-tss
plustm
device, an appliance that is an alternative to full-coverage
bite guards, which is also approved by the FDA for use in the
treatment of medically diagnosed migraine pain and jaw disorders.
Recent
Trends
We believe that the recent economic recession in the United
States has negatively impacted the entire dental laboratory
industry, as price-sensitive consumers have postponed elective
dental work. Recent economic conditions, coupled with high
unemployment, tight credit and continued difficulties in the
housing market, dampened consumer confidence and purchasing
activities in 2009. Additionally, we believe that the low cost
segment for United States manufactured dental prosthetics
declined in 2009 as competition from offshore laboratories,
primarily those located in China, has become more intensive.
While our business has not traditionally focused on this low
cost segment of the market, certain customers are sensitive to
price competition. As a result, these increasing competitive
pressures have somewhat constrained our ability to increase
prices. Since 2007, these increasing competitive pressures in
the form of low price competition have been partially
responsible for decreasing revenues or revenue growth in several
marketplaces. While the growth in offshore restorations
moderated somewhat during 2009, the deployment of
technology-based solutions that allow dentists to fabricate
their own restorations without the use of a dental laboratory
has increased. These trends appear to be restraining industry
growth, and have impacted our results of operations.
14
The main components of our costs are labor and related employee
benefits as well as raw materials, including precious metals
such as gold and palladium. Beginning in the fourth quarter of
2008 and throughout 2009, we proactively reduced staffing levels
to improve profitability and eliminate excess capacity in
response to the economic recession and the decline in consumer
discretionary spending. As a result of reductions in staffing
levels, our costs for labor and related benefits for the year
ended December 31, 2009 were significantly lower than
incurred during the same period in 2008. We also focused on
reducing discretionary operating expenses and as a result our
operating expenses were reduced in the year ended
December 31, 2009 compared to the prior year.
In addition to our cost reduction efforts, we have made
additional investments in capital equipment for technology-based
dental laboratory CAD-CAM manufacturing solutions. Our ability
to afford and utilize these CAD-CAM systems provides us the
opportunity to centrally produce product for many of our
laboratories at more efficient and profitable levels. We believe
we have begun to recognize these efficiencies and will continue
to focus on more completely leveraging these and future
technology investments to reduce labor costs. Therefore, we
believe that these investments are critical to our long-term
business strategy.
As described in more detail below, our focus on controlling
costs has resulted in improved profitability, despite the
industry wide slowdown in revenues in 2009 primarily due to the
economic recession. As the economy recovers, we anticipate that
our new cost structure and improvements in productivity will
allow us to capitalize on the expected increase in demand for
dental services, as patients who deferred these types of
expenditures no longer do so.
Acquisitions
We continue to seek strategic acquisitions, which have played an
important role in helping us increase sales from $135,843,000 in
2005 to $161,195,000 in 2009. In March 2005, we completed the
acquisition of Green Dental Laboratories, Inc.
(“Green”). Green is treated as a separate reportable
segment for financial reporting purposes. In October 2006, we
completed our largest acquisition to date, that of Keller Group,
Incorporated (“Keller”) of St. Louis, Missouri.
Keller is also treated as a separate reportable segment for
financial reporting purposes. The acquisition of Keller has
broadened our marketing strategies and product offerings. In
recent years Keller has broadened its focus from local markets
in the Midwest to the national marketplace. In order to sustain
this strategy, Keller invests significantly in product
advertising, primarily in dental print publications and direct
mail, on products that can generate strong revenue growth. Most
recently, in September 2008, we completed the acquisition of
Dental Art Laboratories, Inc. (“Dental Art”) of
Lansing, Michigan.
We have generally used long-term debt to finance the purchase of
our dental laboratories, including Green, Keller and Dental Art.
Future acquisitions may also be funded using available debt
financing. As a result of these acquisitions, we became more
highly leveraged than we were previously. Our interest expense
had therefore become a more significant component of our pre-tax
earnings, yet has declined in recent years. Interest expense of
$2,803,000 in 2007 declined to $2,110,000 in 2008 primarily as a
result of lower interest rates. Interest expense in 2009 further
declined to $1,257,000 as a result of further decreases in
interest rates coupled with a reduction in outstanding debt of
$15,350,000 from $42,198,000 at December 31, 2008 to
$26,848,000 at December 31, 2009, as we did not acquire any
dental laboratories in 2009 increasing the pay down of our
long-term
debt.
Overview
of Results of Operations
Since the fourth quarter of 2008, the decline in discretionary
consumer spending resulting from the recessionary environment in
the United States negatively impacted our revenues and the
revenues of the entire dental laboratory industry, as
price-sensitive consumers postponed elective dental work. In
addition, competitive pressures from offshore laboratories were
partially responsible for declines in revenues or revenue growth
in several marketplaces.
For the year ended December 31, 2009, net sales decreased
$10,479,000 or 6.1% over year ended December 31, 2008. In
September 2008, we acquired Dental Art. Therefore, sales for the
year ending December 31, 2008 include only four months of
Dental Art sales. Net sales in 2009 included approximately
$5,067,000 as a result of eight additional months of sales
provided by the Dental Art acquisition, without which net sales
would have decreased approximately $15,546,000 or 9.1% for the
full year ended December 31, 2009 as compared to 2008.
Sales declined
15
$3,089,000, or 7.5%, to $38,175,000 for the fourth quarter of
2009 from $41,264,000 for the fourth quarter of 2008. By
operating segment, in the fourth quarter of 2009 compared to the
fourth quarter of 2008, sales for Keller declined 4.2%, compared
to 7.5% for the first nine months of 2009 as compared to the
same period in 2008, sales for Green declined 3.8% compared to
8.4% for the first nine months of 2009 as compared to the same
period in 2008, and sales for NDX declined 8.7% compared to
10.2% for the first nine months of 2009 as compared to the same
period in 2008, excluding acquired sales from Dental Art.
Despite the decline in revenues of $10,479,000, gross profit for
the year ended December 31, 2009 decreased by only
$1,414,000 from 2008, and our gross margins increased to 42.2%
for the year ended December 31, 2009 from 40.5% in 2008.
Within our cost of sales, production labor costs decreased by
$4,084,000 and production employee benefits costs decreased by
$1,110,000, as a result of reduced staffing levels and reduced
overtime. Materials costs declined by $3,302,000 in fiscal 2009
as compared to fiscal 2008, as a result of lower volume and
lower costs for precious metals. Laboratory overhead expenses
decreased $569,000 in fiscal 2009 as compared to fiscal 2008,
primarily as a result of cost reduction efforts.
Operating expenses decreased $2,833,000 or 4.8% for the year
ended December 31, 2009, including decreases in labor and
benefits of $2,418,000 at our laboratories, as a result of
reduced staffing levels, and lower levels of various other
expense items including delivery service, travel, and
advertising expenses, from cost reduction efforts and lower fuel
prices. As a result of improved profitability at many of our
laboratories, laboratory incentive compensation increased
$1,288,000 to $1,879,000 for the year ended December 31,
2009 from $591,000 for the year ended December 31, 2008.
Accruals for corporate incentive compensation increased
$1,040,000 to $1,090,000 for the year ended December 31,
2009 from $50,000 in 2008 as a result of the achievement of
defined earnings targets. Increases in the cash surrender value
of life insurance policies relative to large prior year
declines, net of increases in the related deferred compensation
accruals for our supplemental executive retirement plans
decreased operating expenses by $460,000. Professional
fees and consulting decreased by $655,000 to $1,513,000 for the
year ended December 31, 2009 from $2,168,000 for 2008. Also
in the third quarter of 2009, we recognized a gain of $365,000
resulting from the sale of real estate in Denver, Colorado that
had been held for sale since December 2008. Offsetting these
improvements in operating expenses, we recognized impairments of
$361,000 related to trade names in 2009 compared to $44,000 in
2008. As a result of these factors, our operating expenses
decreased $2,833,000 to $55,755,000 for the year ended
December 31, 2009 from $58,588,000 in 2008.
In the fourth quarter of 2008, we identified an impairment of
$6,950,000 related to ten laboratories in our NDX laboratory
segment as a result of the economic recession. We assessed
goodwill impairment on June 30, 2009, the date of our
annual impairment test, and determined that no impairment charge
was required. Although no laboratories were impaired at
June 30, 2009, we reassessed goodwill impairment at the end
of the third quarter of 2009. As a result of this analysis, we
recorded an impairment charge of $264,000, comprising the entire
goodwill of one of our smaller laboratories, due to a
significant decrease in revenues and operating income in the
third quarter at this laboratory compared to the previous
forecast at June 30, 2009. During the fourth quarter of
2009, there were no events that triggered a goodwill impairment
test or charge to goodwill impairment expense. We will continue
to closely monitor the financial results of certain other
locations in the NDX laboratories operating segment during
subsequent periods in comparison to forecasted financial results
and assumptions.
Our operating income increased to $12,057,000 for the year ended
December 31, 2009 compared to $3,952,000 for the year ended
December 31, 2008 primarily due to our goodwill impairment
of $6,950,000 in 2008 and our cost reductions in labor and other
expenses mentioned above.
Due to lower interest rates and debt levels, decreases in
interest expense contributed $853,000 to pre-tax earnings. As a
result of the factors discussed above, particularly goodwill
impairment, income before provision for income taxes increased
by $8,960,000 to $10,055,000 for the year ended
December 31, 2009 from $1,095,000 for the year ended
December 31, 2008.
Liquidity
and Capital Resources
On August 9, 2005, we entered into an amended and restated
financing agreement (the “Amended Agreement”) with
Bank of America, N.A. (the “Bank”). The Amended
Agreement included a revolving line of credit of $5,000,000, a
revolving acquisition line of credit of $20,000,000 and a term
loan facility of $20,000,000. The
16
interest rate on both revolving lines of credit and the term
loan was the prime rate or, at our option, LIBOR, a cost of
funds rate, or the Bank’s fixed rate plus a range of 1.25%
to 2.25% per annum, depending on the ratio of consolidated
funded debt to consolidated “EBITDA,” as defined in
the Amended Agreement. The Amended Agreement required monthly
payments of principal on the term loan, based on a seven-year
amortization schedule, with a final payment due on the fifth
anniversary of the Amended Agreement. The Amended Agreement
required compliance with certain covenants, including the
maintenance of specified net worth, income and other financial
ratios.
In October 2006 we borrowed against our acquisition line of
credit to finance our acquisition of Keller. In order to
refinance the borrowings incurred for the Keller acquisition, we
and the Bank executed a Second Amended and Restated Loan
Agreement as of November 7, 2006 (the “Second
Agreement”) comprising uncollateralized senior credit
facilities that at that time totaled $60,000,000. The Second
Agreement amended and restated the Amended Agreement (a) to
increase the term loan facility to an aggregate principal amount
of $35,000,000 and used the proceeds of the increase in the term
loan to repay the portion of the outstanding principal balance
under the acquisition line of credit and (b) to adjust the
allocation of availability under the lines of credit by
increasing the revolving line of credit to $10,000,000
($5,000,000 of which may be used for future acquisitions) and
decreasing the acquisition line of credit from $20,000,000 to
$15,000,000. The interest rate on both lines of credit and the
term loan was the prime rate or, at our option, LIBOR, a cost of
funds rate or the Bank’s fixed rate, plus, in each case, a
range of 1.25% to 3.00% per annum, depending on the ratio of
consolidated total funded debt to consolidated
“EBITDA,” as each is defined in the Second Agreement.
The term loan facility portion of the Second Agreement requires
monthly interest payments and monthly payments of principal,
based on a seven year amortization schedule, with a final
payment due on the fifth anniversary of the Second Agreement.
The Second Agreement requires compliance with certain covenants,
including the maintenance of specified net worth, minimum
consolidated total “EBITDA,” debt to income ratio and
other financial ratios.
The Second Agreement was amended on May 9, 2008, effective
March 31, 2008, to revise certain financial targets within
these covenants. Additionally, we and the Bank agreed to
consolidate the revolving line of credit with the acquisition
line of credit into a single line of credit of $25,000,000 to be
used by us for general corporate purposes, including potential
acquisitions. The Second Agreement was also amended on
September 2, 2008 in connection with the acquisition of
Dental Art, which increased our outstanding debt and therefore
required an adjustment to an affected financial covenant. We
further amended the agreement on December 16, 2008 to
extend the maturity of the line of credit to November 7,
2011. The amendment changed the interest rate on both the line
of credit and the term loan to prime rate or, at our option,
LIBOR, a cost of funds rate, or the Bank’s fixed rate,
plus, in each case, a range of 2.50% to 3.50% per annum,
depending on the ratio of consolidated total funded debt to
consolidated “EBITDA,” as each is defined in the
Second Agreement and also increased the commitment fee on the
unused portion of the line of credit from 0.125% to 0.50% per
annum. In addition, the amendment revised certain financial
targets within the covenants. Finally, on March 13, 2009,
we amended the Second Agreement to exclude the $6,950,000
goodwill impairment in the fourth quarter of 2008 discussed
previously from the calculation of “EBITDA,” used in
determining our compliance with certain financial covenants.
These amendments did not change the total availability under the
Second Agreement. While we were in compliance with our debt
covenants as of December 31, 2009, we continue to closely
monitor our compliance with these covenants in future periods,
particularly minimum consolidated total “EBITDA,”
which may be negatively impacted by, among other things,
potential declines in future earnings, including declines
attributable to additional goodwill impairment.
17
As of December 31, 2009, $18,471,000 was available under
the consolidated revolving line of credit.
Long-Term
Obligations:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Term note
|
|
$
|
24,583,000
|
|
|
$
|
19,583,000
|
|
|
Borrowings classified as long term under the revolving line of
credit
|
|
|
13,800,000
|
|
|
|
6,529,000
|
|
|
Borrowings classified as short term under the revolving line of
credit
|
|
|
2,940,000
|
|
|
|
—
|
|
|
Other long-term debt
|
|
|
875,000
|
|
|
|
736,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Total long-term debt
|
|
|
42,198,000
|
|
|
|
26,848,000
|
|
|
Less: current maturities
|
|
|
8,055,000
|
|
|
|
5,072,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt, less current portion
|
|
$
|
34,143,000
|
|
|
$
|
21,776,000
|
|
|
|
|
|
|
|
|
|
|
|
The table below reflects the expected repayment terms associated
with the Company’s long-term debt at December 31,
2009. The weighted average annual interest rate on all of our
borrowings was 3.2% as of December 31, 2009.
| |
|
|
|
|
|
|
|
December 31, 2009
|
|
|
|
|
Principal Due
|
|
|
|
|
Fiscal 2010
|
|
|
5,072,000
|
|
|
Fiscal 2011
|
|
|
21,187,000
|
|
|
Fiscal 2012
|
|
|
80,000
|
|
|
Fiscal 2013
|
|
|
85,000
|
|
|
Fiscal 2014
|
|
|
90,000
|
|
|
Thereafter
|
|
|
334,000
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
26,848,000
|
|
|
|
|
|
|
|
Liquidity:
Our working capital decreased by $231,000 to $9,296,000 at
December 31, 2009 from $9,527,000 at December 31,
2008. Operating activities provided $15,813,000 in cash flow for
the year ended December 31, 2009 compared to $13,327,000
during the year ended December 31, 2008, an increase of
$2,486,000. The increase in cash flow provided by operating
activities during 2009, as compared to the prior year period,
was due primarily to the following factors:
|
|
|
| |
•
|
increases in accrued expenses and accounts payable of $2,117,000
as a result of increases in payroll related accruals and the
timing of vendor payments;
|
| |
| |
•
|
decreases of other receivables of $841,000 primarily due to
collections of stop loss insurance for medical claims;
|
| |
| |
•
|
decreases in benefit for deferred income taxes of $957,000 due
to timing differences in the deductibility of depreciation and
goodwill impairment;
|
partially offset by:
|
|
|
| |
•
|
increases in inventory of $349,000 primarily due to increased
precious metal values;
|
| |
| |
•
|
increases in prepaid assets of $681,000 primarily due to the
timing of insurance payments, income taxes, and director fees;
and;
|
| |
| |
•
|
increases in other assets of $722,000 due to improved
performance of the cash surrender value of assets held for life
insurance policies related to our supplemental employee
retirement plans.
|
18
Investing activities consumed $1,415,000 in cash flow for the
year ended December 31, 2009 compared to $20,516,000 during
the year ended December 31, 2008, a decrease of
$19,101,000. Capital expenditures decreased $5,466,000 to
$1,558,000 for the year ended December 31, 2009 from
$7,024,000 for the year ended December 31, 2008, primarily
due to lower spending on new facilities as a multi-year phase of
laboratory construction had substantially concluded in 2008. In
addition, for the year ended December 31, 2008, acquisition
related cash outflows totaled $11,578,000, and cash outflows
related to notes receivable totaled $2,000,000, while
acquisition related cash outflows, consisting of a deferred
purchase price payment, totaled only $300,000 in 2009.
For the year ended December 31, 2009, financing activities
consumed $15,001,000 compared to providing $7,644,000 for the
year ended December 31, 2008. The increased use of cash in
financing activities of $22,645,000 is attributable to
repayments on our revolving line of credit primarily as a result
of greater availability of cash, due in large part to lower
investing activities and increases in cash provided by operating
activities, as discussed above.
We believe that cash flow from operations and available
financing will be sufficient to meet contemplated operating and
capital requirements such as those discussed below, for the
foreseeable future.
Commitments
and Contingencies
The following table represents a list of our contractual
obligations and commitments as of December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due By Period
|
|
|
|
|
|
|
|
Less Than
|
|
|
|
|
|
|
|
|
Greater Than
|
|
|
|
|
Total
|
|
|
1 Year
|
|
|
1 - 3 Years
|
|
|
4 - 5 Years
|
|
|
5 Years
|
|
|
|
|
Term Loan Facility
|
|
$
|
19,583,000
|
|
|
$
|
5,000,000
|
|
|
$
|
14,583,000
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Line of Credit
|
|
|
6,529,000
|
|
|
|
—
|
|
|
|
6,529,000
|
|
|
|
—
|
|
|
|
—
|
|
|
Capital Leases
|
|
|
736,000
|
|
|
|
71,000
|
|
|
|
156,000
|
|
|
|
175,000
|
|
|
|
334,000
|
|
|
Operating Leases:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Real Estate
|
|
|
18,386,000
|
|
|
|
3,829,000
|
|
|
|
6,629,000
|
|
|
|
4,165,000
|
|
|
|
3,763,000
|
|
|
Vehicles
|
|
|
444,000
|
|
|
|
444,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Equipment
|
|
|
236,000
|
|
|
|
111,000
|
|
|
|
98,000
|
|
|
|
26,000
|
|
|
|
1,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL
|
|
$
|
45,914,000
|
|
|
$
|
9,455,000
|
|
|
$
|
27,995,000
|
|
|
$
|
4,366,000
|
|
|
$
|
4,098,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank borrowings on the term loan facility, with repayment terms
greater than one year, are classified as long-term debt on the
balance sheet.
We are committed under various non-cancelable operating lease
agreements covering office space and dental laboratory
facilities, vehicles and certain equipment. Certain of these
leases also require us to pay maintenance, repairs, insurance
and related taxes.
19
Results
of Operations
Our results are reported within three operating segments, NDX
Laboratories, Green Dental and Keller. The following table sets
forth for the periods indicated the percentage of net sales
represented by certain items in our Consolidated Financial
Statements:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
2007
|
|
2008
|
|
2009
|
|
|
|
Net sales
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
Cost of goods sold
|
|
|
57.4
|
|
|
|
59.5
|
|
|
|
57.8
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
42.6
|
|
|
|
40.5
|
|
|
|
42.2
|
|
|
Selling, general and administrative expenses
|
|
|
34.3
|
|
|
|
34.2
|
|
|
|
34.5
|
|
|
Goodwill impairment
|
|
|
—
|
|
|
|
4.0
|
|
|
|
0.2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
8.3
|
|
|
|
2.3
|
|
|
|
7.5
|
|
|
Other expense
|
|
|
0.5
|
|
|
|
0.4
|
|
|
|
0.5
|
|
|
Interest expense
|
|
|
1.6
|
|
|
|
1.3
|
|
|
|
0.8
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
6.2
|
|
|
|
0.6
|
|
|
|
6.2
|
|
|
Provision for income taxes
|
|
|
2.3
|
|
|
|
1.1
|
|
|
|
2.5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
3.9
|
%
|
|
|
(0.5
|
)%
|
|
|
3.7
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year
Ended December 31, 2009 Compared with Year Ended
December 31, 2008
Net
Sales
Since the fourth quarter of 2008, the decline in discretionary
consumer spending resulting from the recessionary environment in
the United States has negatively impacted our revenues and the
revenues of the entire dental laboratory industry, as
price-sensitive consumers postponed elective dental work. In
addition, competitive pressures from offshore laboratories were
partially responsible for declines in revenues or revenue growth
in several marketplaces.
For the year ended December 31, 2009, net sales decreased
$10,479,000 or 6.1% over the year ended December 31, 2008.
We acquired Dental Art in September 2008. Therefore, sales for
the year ending December 31, 2008 include only four months
of Dental Art sales. Net sales in 2009 included approximately
$5,067,000 as a result of eight additional months of sales
provided by the Dental Art acquisition, without which net sales
would have decreased approximately $15,546,000 or 9.1% for the
full year ended December 31, 2009 as compared to 2008.
Sales declined $3,089,000 or 7.5% in the fourth quarter of 2009
compared to the fourth quarter of 2008. By operating segment, in
the fourth quarter of 2009 compared to the fourth quarter of
2008, sales for Keller declined 4.2%, compared to 7.5% for the
first nine months of 2009 as compared to the same period in
2008, sales for Green declined 3.8% compared to 8.4% for the
first nine months of 2009 as compared to the same period in 2008
, and sales for NDX declined 8.7% compared to 10.2% for the
first nine months of 2009 as compared to the same period in
2008, excluding acquired sales from Dental Art.
Cost of
Goods Sold
Our cost of goods sold decreased by $9,066,000 or 8.9% in the
year ended December 31, 2009 over the year ended
December 31, 2008. As a percentage of sales, cost of goods
sold decreased to 57.8% for the year ended December 31,
2009 from 59.5% in 2008, primarily resulting from decreases in
labor and related benefits and decreasing materials costs.
Production labor decreased by approximately $4,084,000 and
related benefits decreased by approximately $1,110,000 for the
year ended December 31, 2009 compared to the year ended
December 31, 2008. Excluding the acquisition of Dental Art,
production labor and related benefits decreased by approximately
$6,977,000 for the year ended December 31, 2009 compared to
the year ended December 31, 2008. Green’s production
labor and benefit costs of 28.6% of sales, as compared to 28.2%
in 2008, and Keller’s production labor and benefits costs
of 22.7% of sales, as compared to 22.6% in 2008, were fairly
consistent on a year to year basis.
20
Within the NDX Laboratories operating segment, production labor
and benefits decreased to 35.9% of sales for the year ended
December 31, 2009 from 37.3% for the year ended
December 31, 2008 primarily as a result of workforce
reductions.
The cost of raw materials as a percentage of sales decreased to
15.1% for the year ended December 31, 2009 from 16.1% for
the year ended December 31, 2008 as a result of lower
commodity costs. We benefited from the downward trend of the
average cost of palladium, a precious metal used as a component
of many dental alloys, in 2009, particularly in the first three
quarters when the average price was down 42.1% compared to the
first three quarters of 2008. However, due to increased global
demand for palladium, the average price of palladium increased
by 75.4% in the fourth quarter of 2009 compared to the fourth
quarter of 2008. Similarly, the average price of gold increased
40.6% in the fourth quarter of 2009 compared to the fourth
quarter of 2008. Although we are generally able to pass a
majority of precious metal cost increases on to our customers,
prolonged higher metal costs likely will have a negative impact
on gross profit percentages.
Selling,
General and Administrative Expenses
Operating expenses, consisting of selling, delivery and
administrative expenses both at the laboratory and corporate
level, decreased by $2,833,000 for the year ended
December 31, 2009 compared to 2008. Operating expenses
increased as a percentage of net sales to 34.6% in 2009 from
34.1% in 2008. As a percentage of net sales, delivery expenses
decreased to 9.2% for the year ended December 31, 2009 from
9.6% in 2008. Selling expenses remained consistent at 6.6% of
sales for the years ended December 31, 2009 and 2008.
Selling expenses in 2009 for the Keller segment were 14.5% of
sales, or $3,455,000, compared to 15.3% of sales, or $3,914,000
for 2008. Laboratory incentive compensation increased to 1.2% of
sales in 2009 from 0.3% of sales in 2008, as the amount
increased by $1,288,000 to $1,879,000 for the year ended
December 31, 2009 from $591,000 in 2008.
The net decrease of $2,833,000 in our operating expenses in 2009
was primarily attributable to the following decreases:
|
|
|
| |
•
|
Decreases in delivery costs, resulting from decreases of
$1,108,000 in fuel and delivery services and decreases of
$857,000 in compensation — $2,006,000;
|
| |
| |
•
|
Decreases in administrative expenses at the laboratory level,
including $1,095,000 in decreased compensation primarily
resulting from workforce reductions, decreases of $276,000 in
travel costs, decreases of $168,000 in recruiting and training
expenses, and $684,000 of other broad based expense
reductions — $2,223,000;
|
| |
| |
•
|
Decreases in selling expenses, including $261,000 in decreased
sales compensation, $241,000 in decreased marketing expense at
Keller, $228,000 in decreased travel related expenses, and
$134,000 in decreased promotional related expenses —
$886,000;
|
| |
| |
•
|
Decreases in consulting and professional fees —
$655,000;
|
| |
| |
•
|
Decrease in insurance expense resulting from increases in the
cash surrender value of insurance, net of increases in the
related deferred compensation accruals for our supplemental
executive retirement plans — $460,000;
|
| |
| |
•
|
The decrease in training and travel expenses at the corporate
level — $295,000; and additionally,
|
| |
| |
•
|
The gain from the sale of real estate in Denver,
Colorado — $365,000;
|
partially offset by the following increases:
|
|
|
| |
•
|
Increases in laboratory incentive compensation as a result of
additional operating profit — $1,239,000;
|
| |
| |
•
|
Additional operating and amortization expense associated with
the Dental Art acquisition — $1,083,000;
|
| |
| |
•
|
Increases in corporate incentive compensation accruals due to
our improved financial results — $1,040,000; and
|
| |
| |
•
|
Increase in amortization due primarily to $317,000 of trade name
impairment charges — $380,000.
|
21
Goodwill
Impairment
Our annual goodwill impairment assessment has historically been
completed at the end of the second quarter. In the fourth
quarter of 2008, management determined that circumstances had
changed enough to perform an additional goodwill impairment test
and we recorded goodwill impairment of $6,950,000 related to ten
laboratories in the NDX laboratory segment. We performed an
annual goodwill impairment test at the end of the second quarter
of 2009 and noted no impairment. We later recorded an impairment
charge of $264,000 in the third quarter of 2009, comprising the
entire goodwill of one of our smaller laboratories in our NDX
laboratory segment, due to a significant decrease in revenues
and operating income from those forecasted as of June 30,
2009. During the fourth quarter of 2009, there were no events
that would trigger an additional goodwill impairment test or
result in a charge to goodwill impairment expense. We continue
to closely monitor the financial results of certain other
locations in the NDX laboratory segment in subsequent periods in
comparison to forecast financial results and assumptions made at
June 30, 2009.
Operating
Income
As a result of the above factors, our operating income increased
by $8,105,000 to $12,057,000 for the year ended
December 31, 2009 from $3,952,000 in 2008. As a percentage
of net sales, operating income increased to 7.5% in 2009 from
2.3% in 2008.
Interest
Expense
Due to lower interest rates and lower debt levels, interest
expense declined to $1,257,000 for 2009 from $2,110,000 for 2008.
Provision
for Income Taxes
For the year ended December 31, 2009, the provision for
income taxes increased by $2,206,000 to $4,178,000 from
$1,972,000 for the year ended December 31, 2008. For the
year ended December 21, 2009, the effective tax rate was
41.6% compared to 180.1% for 2008. In 2008, goodwill impairment
recorded in the amount of $6,950,000 had an associated tax
benefit of only $833,000, as approximately 70% of the goodwill
impairment charge was not deductible for tax purposes. Without
the impact of goodwill impairment, our tax provision in 2008
would have resulted in an effective tax rate of 34.9%. The
increase in the effective tax rate before adjustment for
goodwill impairment was due in part to increased state taxes and
additional stock based compensation offset by the absence of
recognition of research and experimentation credits, which
equaled $604,000 in 2008.
Net
Income
As a result of the factors discussed above, particularly the
goodwill impairment charge recorded in the fourth quarter of
2008, net income increased $6,754,000 to $5,877,000 or $1.02 per
share on a diluted basis for the year ended December 31,
2009 from ($877,000) or ($0.16) per share on a diluted basis in
2008.
Operating
Segment Results
Our business consists of a single industry segment, which is the
design, fabrication, marketing and sale of custom dental
prosthetic appliances for and to dentists in North America. We
report on three operating segments within this single industry
segment. These three segments are known as Green Dental,
representing the operations of Green Dental Laboratories, Inc.
of Heber Springs, Arkansas, which we acquired in March 2005;
Keller, representing the operations of Keller Group,
Incorporated with laboratories in St. Louis, Missouri and
Louisville, Kentucky, which we acquired in October, 2006; and
NDX Laboratories, which represents our remaining laboratories.
We have identified both Green and Keller as separate reportable
segments based on the quantitative criteria for financial
reporting purposes.
22
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2009
|
|
|
$ Change
|
|
|
% Change
|
|
|
|
|
Revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
126,240,949
|
|
|
$
|
118,801,942
|
|
|
$
|
(7,439,007
|
)
|
|
|
(5.9
|
)%
|
|
Green Dental Laboratory
|
|
|
20,724,865
|
|
|
|
19,169,497
|
|
|
|
(1,555,368
|
)
|
|
|
(7.5
|
)%
|
|
Keller Group
|
|
|
25,987,992
|
|
|
|
24,248,404
|
|
|
|
(1,739,588
|
)
|
|
|
(6.7
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
172,953,806
|
|
|
|
162,219,843
|
|
|
|
(10,733,963
|
)
|
|
|
(6.2
|
)%
|
|
Less: Inter-segment Revenues:
|
|
|
1,279,371
|
|
|
|
1,024,639
|
|
|
|
(254,732
|
)
|
|
|
(19.9
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Sales
|
|
$
|
171,674,435
|
|
|
$
|
161,195,204
|
|
|
$
|
(10,479,231
|
)
|
|
|
(6.1
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratory Operating Income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
15,059,508
|
|
|
$
|
16,318,096
|
|
|
$
|
1,258,588
|
|
|
|
8.4
|
%
|
|
Green Dental Laboratory
|
|
|
4,795,136
|
|
|
|
4,418,446
|
|
|
|
(376,690
|
)
|
|
|
(7.9
|
)%
|
|
Keller Group
|
|
|
3,660,913
|
|
|
|
4,237,948
|
|
|
|
577,035
|
|
|
|
15.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratory Operating Income
|
|
$
|
23,515,557
|
|
|
$
|
24,974,490
|
|
|
$
|
1,458,933
|
|
|
|
6.2
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX
Laboratories
For the year ended December 31, 2009, before elimination of
inter-segment revenues, sales in this segment decreased by
$7,439,000 or 5.9%. Net of acquired sales of $5,067,000 from
Dental Art, sales decreased $12,506,000, or 9.9% for 2009 when
compared with 2008. In the fourth quarter of 2009 compared to
the fourth quarter of 2008, sales for NDX declined 8.7%. In
comparison, sales for the first nine months of 2009 declined by
10.2% compared to the same period in 2008, excluding acquired
sales from Dental Art. Gross profit as a percentage of sales
increased to 38.9% for the year ended December 31, 2009
from 36.7% for the year ended December 31, 2008. Cost of
goods sold decreased by $7,344,000. The decrease in cost of
sales is attributable to decreases in manufacturing labor and
benefits of approximately $6,205,000 resulting from labor force
reductions, decreases in materials costs of $3,288,000 due to
lower sales and lower materials costs, and decreased laboratory
overhead of $947,000 resulting from less expenditures on
supplies and less depreciation related to facilities, partially
offset by additional costs of $3,097,000 related to the
acquisition of Dental Art.
Laboratory operating income as a percentage of sales for NDX
Laboratories increased to 13.8% for the year ended
December 31, 2009 from 11.9% for the year ended
December 31, 2008 and increased by $1,259,000 or 8.4% as a
result of the factors discussed above.
Green
Dental Laboratory
For the year ended December 31, 2009, before elimination of
inter-segment revenues, sales in this segment decreased by
$1,555,000 or 7.5% as compared to fiscal 2008. In the fourth
quarter of 2009 compared to the fourth quarter of 2008, sales
for Green declined 3.8%. In comparison, sales for the first nine
months of 2009 declined by 8.4% compared to the same period in
2008. As a percentage of sales, gross profit remained flat at
48.4% for the year ended December 31, 2009 compared to
48.5% for 2008. Cost of goods sold decreased by $747,000,
resulting primarily from decreases in material costs of $475,000
and decreased overtime of $130,000.
As a result of the factors discussed above, laboratory operating
income as a percentage of sales for Green decreased slightly to
23.0% for the year ended December 31, 2009 compared to
23.1% for the year ended December 31, 2008 and decreased by
$377,000.
Keller
Group
For the year ended December 31, 2009, the sales decline
before elimination of inter-segment revenues in this segment was
$1,740,000 or 6.7%. In the fourth quarter of 2009 compared to
the fourth quarter of 2008, sales for Keller declined 4.2%. In
comparison, sales for the first nine months of 2009 declined by
7.5% compared to the same period in 2008. As a percentage of
sales, gross profit increased to 53.8% for the year ended
December 31, 2009 from
23
53.0% for the year ended December 31, 2008, primarily as a
result of decreased material costs, including precious metals.
Administrative costs decreased by $492,000 primarily due to
decreased labor and benefits of $176,000 and various other
expense reductions. Selling expense decreased by $459,000
primarily due to decreased labor and benefits of $227,000 and
decreased advertising expense of $241,000 for the year ended
December 31, 2009 compared to 2008. Delivery costs
decreased by $443,000 primarily due to decreased delivery
service charges due to lower volume and decreased fuel costs. As
a result, delivery costs as a percentage of sales decreased to
11.0% during the year ended December 31, 2009 from 12.0%
during the year ended December 31, 2008.
As a result of the factors discussed above, laboratory operating
income as a percentage of sales for Keller increased to 17.5%
for the year ended December 31, 2009 as compared to 14.1%
for the year ended December 31, 2008, an increase of
$577,000.
Year
Ended December 31, 2008 Compared with Year Ended
December 31, 2007
Net
Sales
In 2008, competitive pressures from offshore laboratories that
can produce crowns at fees lower than crowns manufactured in the
United States limited our ability to raise our prices,
particularly during a time when we experienced relatively higher
costs for precious metals used in manufacturing. In addition,
these competitive pressures are partially responsible for
declines in revenues or revenue growth in several marketplaces.
We also believe that in 2008, the recessionary environment in
the United States impacted our revenues and the revenues of the
entire dental laboratory industry, as price-sensitive consumers
postponed elective dental work. The impact of the recession on
our business was particularly noticeable in the fourth quarter
of 2008.
For the year ended December 31, 2008, net sales increased
$1,314,000 or 0.8% over year ended December 31, 2007. Net
sales increased by approximately $2,665,000 as a result of the
Dental Art acquisition in September 2008. Excluding the Dental
Art acquisition, net sales decreased approximately $1,351,000
for the full year ended December 31, 2008 as compared to
2007. Furthermore, approximately $550,000 of sales growth was
attributable to the effect of increased prices due to underlying
increases in the prices of precious metals passed through to
customers. The decline in sales for laboratories held more than
one year was primarily attributable to decreased patient demand,
particularly in the fourth quarter of 2008 as consumers started
to delay certain dental work due to economic uncertainties.
Excluding acquired sales from Dental Art, sales declined
$2,112,000 in the fourth quarter of 2008. Sales growth in the
fourth quarter of 2008 for Keller, relative to their performance
in the first nine months of 2008, was flat at $84,000 or 1.3%.
In the fourth quarter, sales declined $89,000 at Green and
$2,107,000 at the NDX Laboratories.
Cost of
Goods Sold
Our cost of goods sold increased by $4,446,000 or 4.5% in the
year ended December 31, 2008 over the year ended
December 31, 2007. As a percentage of sales, cost of goods
sold increased from 57.4% to 59.5%, primarily resulting from
increases in labor and related benefits, increases in laboratory
overhead and rising materials costs. Green’s labor costs of
28.2% of sales, as compared to 28.8% in 2007, and Keller’s
labor costs of 22.6% of sales, as compared to 22.9% in 2007,
lowered the overall percentage while the portion attributable to
NDX Laboratories increased to 37.6% of sales for the year ended
December 31, 2008 from 35.7% for the year ended
December 31, 2007. Included in the increase in the NDX
Laboratories segment is a reclassification of approximately
$995,000 in base pay increases related to modifications of the
Laboratory Plan, which is discussed in Note 7
“Employee Benefit Plans” of our Consolidated Financial
Statements included in Item 8 of this Annual Report.
Laboratory overhead increased $1,250,000, primarily as a result
of increased depreciation and rent for new facilities and
increases in technical training. Excluding the acquisition of
Dental Art, production labor and related benefits increased by
approximately $1,453,000 for the year ended December 31,
2008 compared to the year ended December 31, 2007.
The cost of raw materials as a percentage of sales increased
from 15.7% for the year ended December 31, 2007 to 16.1%
for the year ended December 31, 2008. For most of 2008, the
average cost of gold and palladium, precious metals components
of many dental alloys, was on the rise. However, by the end of
2008, due to decreased global demand for palladium, the average
price of palladium was essentially unchanged, while gold
increased by approximately 25% over average costs in the prior
year. Although we were able to pass a majority of precious
24
metal cost increases on to our customers, prolonged higher metal
costs have had and likely will continue to have a negative
impact on gross profit percentages.
Selling,
General and Administrative Expenses
Operating expenses, net of goodwill impairment and consisting of
selling, delivery and administrative expenses both at the
laboratory and corporate level, increased by $27,000 for the
year ended December 31, 2008 compared to 2007. Operating
expenses decreased as a percentage of net sales from 34.4% in
2007 to 34.1% in 2008. As a percentage of net sales, delivery
expenses increased from 9.2% in the year ended December 31,
2007 to 9.6% in 2008. Selling expenses increased from 6.2% of
sales for the year ended December 31, 2007 to 6.6% in 2008.
Selling expenses in 2008 for the Keller segment were 15.1% of
sales, or $3,914,000. Laboratory incentive compensation
decreased from 2.3% of sales in 2007 to 0.3% in 2008, as the
amount decreased by $3,281,000 from $3,872,000 for the year
ended December 31, 2007 to $591,000 in 2008.
The net increase of $27,000 in our operating expenses in 2008
was primarily attributable to the following increases:
|
|
|
| |
•
|
Additional operating and amortization expense associated with
the Dental Art acquisition — $501,000;
|
| |
| |
•
|
Increases in delivery costs, resulting primarily from cost
increases in fuel and delivery services — $694,000;
|
| |
| |
•
|
Increases in selling expenses, including $271,000 in increased
marketing expense at Keller and $454,000 in increased sales
compensation, offset by $273,000 of decreases in customer
rewards program expenses — $507,000;
|
| |
| |
•
|
Increases in administrative expenses at the laboratory level,
including $852,000 in increased compensation primarily resulting
from increases to base pay of $485,000 related to the change in
the Laboratory Plan, offset by $189,000 of decreases to
depreciation expense and losses on asset disposals —
$758,000;
|
| |
| |
•
|
Increases in salaries and benefits at the corporate
level— $649,000; and;
|
| |
| |
•
|
Decrease of the cash surrender value of life insurance policies
as a result of market value declines, net of decreases in the
related deferred compensation accruals for our supplemental
executive retirement plans — $730,000;
|
partially offset by:
|
|
|
| |
•
|
Decreases in laboratory incentive compensation as a result of
the Laboratory Plan restructuring — $3,281,000.
|
| |
| |
•
|
Decreases in executive incentive compensation accruals due to
our financial results — $400,000; and
|
| |
| |
•
|
Decrease in amortization due to fully amortized non-compete
agreements — $193,000.
|
Goodwill
Impairment
Our annual goodwill impairment assessment has historically been
completed at the end of the second quarter. Based on our initial
assessment for 2008, the fair value of our business units
exceeded their carrying value and therefore our goodwill was not
impaired. As economic conditions worsened in the fourth quarter
and our business performance and outlook was not as strong as
anticipated at the end of the second quarter, management
determined that circumstances had changed enough to perform an
additional goodwill impairment test as of December 31,
2008. Based on our evaluation of goodwill, we determined that
the fair value of ten dental laboratories in the NDX
Laboratories operating segment was less than their carrying
value, resulting in goodwill impairment of $6,950,000.
Operating
Income
As a result of the above factors, our operating income decreased
by $10,108,000 to $3,952,000 for the year ended
December 31, 2008 from $14,060,000 in 2007. As a percentage
of net sales, operating income decreased from 8.3% in 2007 to
2.3% in 2008.
25
Interest
Expense
Due primarily to lower interest rates, interest expense declined
from $2,803,000 for 2007 to $2,110,000 for 2008. Approximately
$180,000 of interest expense was related to the borrowings that
funded the acquisition of Dental Art.
Provision
for Income Taxes
For the year ended December 31, 2008, the provision for
income taxes decreased by $1,888,000 from $3,860,000 for the
year ended December 31, 2007 to $1,972,000 for the year
ended December 31, 2008. However, for the year ended
December 31, 2008, income before provision for income taxes
was $1,095,000, and the effective tax rate was therefore 180.1%.
The reason the tax provision exceeds income before provision for
income taxes is due to the tax impact of the impairment of
goodwill. Goodwill impairment recorded in the amount of
$6,950,000 has an associated tax benefit of only $833,000, as
approximately 70% of the goodwill impairment charge is not
deductible for tax purposes. Without the impact of goodwill
impairment, our tax provision would have resulted in an
effective tax rate of 34.9%, which would have represented a
decrease from the 36.8% effective tax rate for the year ended
December 31, 2007. The decrease in the effective tax rate
before adjustment for goodwill impairment was due in part to
recognition of research and experimentation credits of $604,000,
offset by increases in the amount of certain non-deductible
expenses.
Net
Income
As a result of all the factors discussed above, particularly the
goodwill impairment charge recorded in the fourth quarter, net
income decreased $7,503,000 to ($877,000) or ($0.16) per share
on a diluted basis for the year ended December 31, 2008
from $6,626,000 or $1.17 per share on a diluted basis in 2007.
Operating
Segment Results
The following is a summary of segment reporting for 2007 and
2008:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31
|
|
|
|
|
|
|
|
|
|
|
2007
|
|
|
2008
|
|
|
$ Change
|
|
|
% Change
|
|
|
|
|
Revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
127,388,588
|
|
|
$
|
126,240,949
|
|
|
$
|
(1,147,639
|
)
|
|
|
(0.9
|
)%
|
|
Green Dental
|
|
|
19,859,770
|
|
|
|
20,724,865
|
|
|
|
865,095
|
|
|
|
4.4
|
%
|
|
Keller Group
|
|
|
23,843,093
|
|
|
|
25,987,992
|
|
|
|
2,144,899
|
|
|
|
9.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
171,091,451
|
|
|
|
172,953,806
|
|
|
|
1,862,355
|
|
|
|
1.1
|
%
|
|
Less: Inter-segment Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
|
370,913
|
|
|
|
438,200
|
|
|
|
67,287
|
|
|
|
18.1
|
%
|
|
Green Dental
|
|
|
199,525
|
|
|
|
369,526
|
|
|
|
170,001
|
|
|
|
85.2
|
%
|
|
Keller Group
|
|
|
160,384
|
|
|
|
471,645
|
|
|
|
311,261
|
|
|
|
194.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Sales
|
|
$
|
170,360,629
|
|
|
$
|
171,674,435
|
|
|
$
|
1,313,806
|
|
|
|
0.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratory Operating Income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
17,572,491
|
|
|
$
|
15,059,508
|
|
|
$
|
(2,512,983
|
)
|
|
|
(14.3
|
)%
|
|
Green Dental
|
|
|
4,665,630
|
|
|
|
4,795,136
|
|
|
|
129,506
|
|
|
|
2.8
|
%
|
|
Keller Group
|
|
|
3,228,640
|
|
|
|
3,660,913
|
|
|
|
432,273
|
|
|
|
13.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratory Operating Income
|
|
$
|
25,466,761
|
|
|
$
|
23,515,557
|
|
|
$
|
(1,951,204
|
)
|
|
|
(7.7
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX
Laboratories
For the year ended December 31, 2008, before elimination of
inter-segment revenues, sales in this segment decreased by
$1,148,000 or 0.9%. Net of acquired sales of $2,665,000 from
Dental Art, sales decreased $3,813,000,
26
or 3.0% for 2008 when compared with 2007. The decrease in sales
in the fourth quarter accounted for $2,107,000 of this amount.
Gross profit as a percentage of sales decreased from 40.3% for
the year ended December 31, 2007 to 36.9% for the year
ended December 31, 2008. Cost of goods sold increased by
$3,244,000. The increase was primarily attributable to
additional costs of $1,549,000 related to the acquisition of
Dental Art, increases in manufacturing labor and benefits of
approximately $1,790,000, resulting from increases to base pay
of $995,000 related to changes in the Laboratory Plan and
increases in health insurance and other benefit costs of
$380,000, which were partially offset by lower labor costs
resulting from labor force reductions. Materials costs increased
approximately $242,000 and laboratory overhead increased by
approximately $1,053,000 resulting from depreciation and
increased rent for new facilities.
Laboratory operating income as a percentage of sales for NDX
Laboratories decreased from 13.9% for the year ended
December 31, 2007 to 12.0% for the year ended
December 31, 2008 and declined by $2,513,000 or 14.3% as a
result of the factors discussed above. Goodwill impairment of
$6,950,000 was recorded in the NDX Laboratories operating
segment but is not a component of laboratory operating income.
Green
Dental Laboratory
Sales growth before elimination of inter-segment revenues for
the year ended December 31, 2008 in this segment was
$865,000 or 4.4%. Sales increased during the first nine months
of 2008, however, in the fourth quarter, sales declined $89,000.
As a percentage of sales, gross profit increased from 47.4% for
the year ended December 31, 2007 to 47.6% for the year
ended December 31, 2008. Cost of goods sold increased by
$420,000. In addition to temporary increases in overtime of
$72,000 resulting primarily from training and implementation of
new production methods, benefit costs increased $107,000,
primarily resulting from increases in the cost of providing
health insurance. Materials costs increased $106,000 for the
year ended December 31, 2008 as compared to the year ended
December 31, 2007. Precious metals used declined as
precious metal-based unit volume was down in favor of
zirconia-based, CAD-CAM produced units. Increased expenses for
implant parts, porcelain and zirconia materials resulted from
this changing product mix. Laboratory overhead increased by
approximately $147,000.
As a result of the factors discussed above, laboratory operating
income as a percentage of sales for Green was essentially flat
at 23.5% for the year ended December 31, 2007 versus 23.1%
for the year ended December 31, 2008 and increased by
$130,000.
Keller
Group
For the year ended December 31, 2008, sales growth before
elimination of inter-segment revenues in this segment was
$2,145,000 or 9.0%. Sales growth in the fourth quarter of 2008
for Keller, relative to their performance in the first nine
months of 2008, was flat at $84,000 or 1.3%. As a percentage of
sales, gross profit increased from 51.0% for the year ended
December 31, 2007 to 52.0% for the year ended
December 31, 2008, primarily as a result of improved labor
efficiency, partially offset by increased materials cost,
including precious metals. Delivery costs increased by $483,000
which primarily is the result of increased delivery service
charges due to higher volume and increased fuel costs. As a
result, delivery costs as a percentage of sales rose to 11.8%
during the year ended December 31, 2008 from 10.8% during
the year ended December 31, 2007. Advertising expense
increased by $271,000 for the year ended December 31, 2008
compared to 2007 due to increased marketing activities.
As a result of the factors discussed above, laboratory operating
income as a percentage of sales for Keller increased to 14.1%
for the year ended December 31, 2008 as compared to 13.5%
for the year ended December 31, 2007, while increasing by
$432,000 due to higher sales volumes.
Critical
Accounting Policies
Financial Reporting Release No. 60 as released by the SEC
requires all companies to include a discussion of critical
accounting policies or methods used in the preparation of
financial statements. The preparation of our consolidated
financial statements requires the use of estimates and
assumptions that affect the reported amounts of assets and
liabilities and the reported amounts of expenses during the
reporting period. A summary of certain of our significant
accounting policies is presented below.
27
Summary
of Significant Accounting Policies
Revenue
Recognition
Revenue is recognized upon transfer of title and risk of loss,
generally as the dentists’ orders are shipped. We record
shipping and handling fees charged to customers as revenues in
accordance with U.S. Generally Accepted Accounting
Principles (GAAP). Shipping and handling costs totaled
approximately $15,617,000 in fiscal 2007, $16,463,000 in fiscal
2008 and $14,800,000 in fiscal 2009, and are included in
selling, general and administrative expense.
Goodwill
and Other Indefinite-Lived Intangible Assets Not Subject to
Amortization
We continually evaluate whether events and circumstances have
occurred that indicate that the value of goodwill has been
impaired. In addition, goodwill is evaluated for possible
impairment on an annual basis, based on a two-step process. We
have selected June 30th as the date for the annual assessment of
goodwill impairment.
The first step is used to identify potential impairment and
involves comparing each reporting unit’s estimated fair
value to its carrying value, including goodwill. A reporting
unit is an operating segment or one level below an operating
segment (referred to as a component). We have determined that
each individual laboratory is a reporting unit. The second step
of the goodwill impairment process involves the calculation of
an implied fair value of goodwill for the laboratories which
step one indicated were impaired. Fair value is determined by
using an income approach, consistent with our valuation of
dental laboratories acquired in purchase business combinations.
We assessed goodwill impairment as of June 30, 2009 and
determined that no impairment charge was required. However, our
impairment analysis incorporates forecasted financial results
and assumptions for several laboratories within the NDX
Laboratories operating segment. Although these laboratories were
not impaired at June 30, 2009, in the third quarter of
2009, we recorded an impairment charge of $264,000, comprising
the entire goodwill balance of one of our smaller laboratories
in the NDX laboratories operating segment, due to a significant
decrease in revenues and operating income from those forecasted
as of June 30, 2009. We continue to closely monitor the
financial results of certain other laboratories in the NDX
laboratories operating segment in subsequent periods in
comparison to forecasted financial results and assumptions made
at June 30, 2009 to determine whether any other impairment
charges are warranted.
Additionally, we also recognize the existence of value in trade
names acquired in business combinations and believe the useful
life of this intangible to be indefinite based on a long history
of utilizing the laboratory trade name. Accordingly, trade names
are also evaluated for impairment on an annual basis using a
single step method. Impairment charges related to trade names
are recognized when the fair value is less than the carrying
value of the asset. Impairment charges related to trade names
were recorded in the amount of $94,000, $44,000 and $361,000 for
the years ended December 31, 2007, 2008 and 2009,
respectively. Trade name impairment charges generally result
from a decline in forecasted revenue at specific laboratories in
comparison to revenue forecasts used in previous valuation
calculations.
Intangible
Assets Subject to Amortization
All acquisitions reflected in the accompanying consolidated
financial statements have been accounted for as purchase
business combinations. Non-competition agreements and customer
relationship intangibles arising from dental laboratory
acquisitions are amortized over their useful lives. The
acquisition date fair value of non-competition agreements are
deferred and amortized over their economic useful lives, in
accordance with the terms of the agreements, ranging from 2 to
15 years. The acquisition date fair value associated with
acquired customer relationships are amortized over their
estimated economic useful life, ranging from 9 to 12 years.
Inventories
Inventories, consisting principally of raw materials, are stated
at the lower of cost
(first-in,
first-out) or market. We use estimates based on specific
identification to maintain proper reserves for excess and
obsolete inventory. Additionally, we estimate work in process
inventories by applying current labor, materials and selected
overhead
28
expense rates to standard production schedules. We estimate the
value of unrefined precious metal scrap based on the application
of various return and refining statistics. Finished goods
inventory consists of completed orders that were shipped to
customers immediately subsequent to period end.
Property,
Plant and Equipment
Property, plant and equipment are stated at cost, less
accumulated depreciation. Depreciation is calculated using the
straight-line method over the following estimated depreciable
lives:
| |
|
|
|
|
|
Buildings
|
|
|
25 years
|
|
|
Furniture and fixtures
|
|
|
5 - 10 years
|
|
|
Laboratory equipment
|
|
|
5 - 20 years
|
|
|
Computer equipment
|
|
|
3 - 5 years
|
|
Leasehold improvements and capital leases are amortized over the
lesser of the assets’ estimated useful lives or the
remaining lease terms.
Gains and losses are recognized upon the disposal of property
and equipment, and the related accumulated depreciation and
amortization are removed from the accounts. Maintenance, repairs
and betterments that do not enhance the value of or increase the
life of the assets are charged to operations as incurred.
Depreciation expense totaled approximately $4,055,000 in fiscal
2007, $4,872,000 in fiscal 2008 and $4,569,000 in fiscal 2009.
Impairment
of Long-Lived Assets
At each balance sheet date, management evaluates the
recoverability of long-lived assets, including property and
equipment and intangible assets, using certain financial
indicators, such as historical and future ability to generate
income from operations. Our policy is to assess whether an
impairment exists in the period when it is determined that the
carrying amount of the asset may not be recoverable. The
determination is based on an evaluation of such factors as the
occurrence of a significant event, a significant change in the
environment in which the business operates or if the expected
future cash flows become less than the carrying amount of the
asset.
Cash
Surrender of Life Insurance
The cash surrender value of life insurance policies which are
owned by the Company are recorded at net realizable value.
Income
Taxes
Deferred tax assets and liabilities are recognized for the
expected future tax consequences of events that have been
included in the financial statements or tax returns. The amount
of deferred tax asset or liability is based on the difference
between the financial statement and tax basis of assets and
liabilities using enacted tax rates in effect for the year in
which the differences are expected to reverse. We have
considered our current financial characteristics as well as
current tax law and do not believe that the recoverability of
various tax assets and liabilities is impaired, and therefore
have recorded them at their full value.
Accounting for income taxes prescribes a recognition threshold
and measurement attribute for the financial statement
recognition and measurement of a tax position taken or expected
to be taken in a tax return. The minimum threshold is defined as
a tax position that is more likely than not to be sustained upon
examination by the applicable taxing authority, including
resolution of any related appeals or litigation processes, based
on the technical merits of the position. The tax benefit to be
recognized is measured as the largest amount of benefit that is
greater than fifty percent likely of being realized upon
ultimate settlement.
29
Fair
Value Measurements
Effective January 1, 2008, we adopted accounting standards
that defined fair value, established a framework for measuring
fair value and enhanced disclosures about fair value
measurements. Fair value is defined as the exchange price that
would be received for an asset or paid to transfer a liability
(an exit price) in the principal or most advantageous market for
the asset or liability in an orderly transaction between market
participants on the measurement date. These standards establish
a fair value hierarchy, which requires an entity to maximize the
use of observable inputs and minimize the use of unobservable
inputs when measuring fair value; describe three levels of
inputs that may be used to measure fair value which are provided
in the table below; and allow an entity the irrevocable option
to elect fair value for the initial and subsequent measurement
for certain financial assets and liabilities on a
contract-by-contract
basis. The adoption of these standards had no material impact on
our financial statements.
We use the market approach technique to value our financial
instruments and there were no changes in valuation techniques
during the year ended December 31, 2009. Our financial
assets and liabilities are primarily comprised of investments in
insurance contracts held as assets to satisfy outstanding
retirement liabilities.
Recent
Accounting Pronouncements
In April 2009, the FASB issued additional guidance for
estimating fair value when the volume and level of activity for
the asset or liability have significantly decreased and for
identifying circumstances that indicate a transaction is not
orderly. This guidance emphasizes that even if there has been a
significant decrease in the volume and level of activity for the
asset or liability and regardless of the valuation technique(s)
used, the objective of a fair value measurement remains the
same. Fair value is the price that would be received to sell an
asset or paid to transfer a liability in an orderly transaction
(that is, not a forced liquidation or distressed sale) between
market participants at the measurement date under current market
conditions. This guidance is effective for interim and annual
reporting periods ending after June 15, 2009 and its
adoption did not have an impact on our unaudited consolidated
financial statements.
In May 2009, the FASB issued a pronouncement on subsequent event
accounting. The guidance identifies the following: the period
after the balance sheet date during which management shall
evaluate events or transactions that may occur for potential
recognition or disclosure in the financial statements; the
circumstances under which an entity shall recognize events or
transactions occurring after the balance sheet date in its
financial statements; and the disclosures that an entity shall
make about events or transactions that occurred after the
balance sheet date. This statement was effective for our second
quarter 2009, and there was no effect from adoption.
In June 2009, the FASB issued guidance on The FASB
Accounting Standards
Codificationtm
and the Hierarchy of Generally Accepted Accounting
Principles, which is effective for financial statements
issued for interim and annual periods ending after
September 15, 2009. The adoption of this pronouncement did
not have an impact on our consolidated financial statements.
|
|
|
Item 7A.
|
Quantitative
and Qualitative Disclosures about Market Risk
|
Our market risk exposure includes potential price volatility of
commodities we use in our manufacturing processes. We purchase
dental alloys that contain gold, palladium and other precious
metals. We have not participated in hedging transactions. We
have relied on pricing practices that attempt to pass some
portion, if not all, of our increased costs on to our customers,
in conjunction with materials substitution strategies. Our
market risk exposure also includes investments in insurance
contracts held as assets to satisfy outstanding retirement
liabilities, a portion of which are subject to market value
fluctuations of the underlying investment. Cash surrender values
totaled approximately $5,479,000 for fiscal 2008 and $5,546,000
for fiscal 2009.
At December 31, 2009, we had variable rate debt of
$26,112,000 associated with our credit facility. Based on this
amount, the earnings and cash flows impact for the next year
resulting from a one percentage point increase in interest rates
would be approximately $153,000, net of tax, holding other
variables constant.
30
We have investments in a Canadian subsidiary. The net assets of
this subsidiary are exposed to volatility in current exchange
rates. Translation gains and losses related to the net assets of
this subsidiary are included in accumulated other comprehensive
income.
|
|
|
Item 8.
|
Financial
Statements and Supplementary Data
|
Quarterly
Results
The following table sets forth certain selected financial
information for the eight fiscal quarters in our two most
recently completed fiscal years. In our opinion, this unaudited
information has been prepared on the same basis as the audited
financial information and includes all adjustments (consisting
of only normal, recurring adjustments) necessary to present this
information fairly when reviewed in conjunction with our
Consolidated Financial Statements and notes thereto contained
herein.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
March 31,
|
|
June 30,
|
|
Sept. 30,
|
|
Dec. 31,
|
|
March 31,
|
|
June 30,
|
|
Sept. 30,
|
|
Dec. 31,
|
|
|
|
2008
|
|
2008
|
|
2008
|
|
2008
|
|
2009
|
|
2009
|
|
2009
|
|
2009
|
|
|
|
(Dollars in thousands except per share data)
|
|
|
|
Net sales
|
|
$
|
43,529
|
|
|
$
|
44,580
|
|
|
$
|
42,302
|
|
|
$
|
41,264
|
|
|
$
|
41,260
|
|
|
$
|
42,755
|
|
|
$
|
39,006
|
|
|
$
|
38,175
|
|
|
Gross profit
|
|
$
|
18,210
|
|
|
$
|
18,665
|
|
|
$
|
16,552
|
|
|
$
|
16,062
|
|
|
$
|
17,622
|
|
|
$
|
18,828
|
|
|
$
|
15,836
|
|
|
$
|
15,790
|
|
|
Gross margin
|
|
|
41.8
|
%
|
|
|
41.9
|
%
|
|
|
39.1
|
%
|
|
|
38.9
|
%
|
|
|
42.7
|
%
|
|
|
44.0
|
%
|
|
|
40.6
|
%
|
|
|
41.4
|
%
|
|
Operating income (loss)
|
|
$
|
3,389
|
|
|
$
|
3,861
|
|
|
$
|
2,183
|
|
|
$
|
(5,482
|
)
|
|
$
|
3,898
|
|
|
$
|
4,377
|
|
|
$
|
2,393
|
|
|
$
|
1,388
|
|
|
Operating margin
|
|
|
7.8
|
%
|
|
|
8.7
|
%
|
|
|
5.2
|
%
|
|
|
(13.3
|
)%
|
|
|
9.4
|
%
|
|
|
10.2
|
%
|
|
|
6.1
|
%
|
|
|
3.6
|
%
|
|
Net income (loss)
|
|
$
|
1,681
|
|
|
$
|
1,937
|
|
|
$
|
791
|
|
|
$
|
(5,286
|
)
|
|
$
|
2,059
|
|
|
$
|
2,330
|
|
|
$
|
1,059
|
|
|
$
|
429
|
|
|
Net income (loss) per diluted share
|
|
$
|
0.30
|
|
|
$
|
0.34
|
|
|
$
|
0.14
|
|
|
$
|
(0.93
|
)
|
|
$
|
0.36
|
|
|
$
|
0.40
|
|
|
$
|
0.18
|
|
|
$
|
0.07
|
|
Our results of operations have historically fluctuated on a
quarterly basis and are expected to be subject to quarterly
fluctuations in the future. As a result, we believe that the
results of operations for the interim periods are not
necessarily indicative of the results to be expected for any
future period or for a full year. Quarterly results are subject
to fluctuations resulting from a number of factors, including
the number of working days in the quarter for both dentists and
our employees, the number of paid vacation days and holidays in
the period, general economic conditions and consumer spending
patterns. Historically, the second quarter has generated the
highest quarterly net sales for the year and has been the most
profitable for us due to the greater number of working days in
the quarter and more patients scheduling visits with their
dentists before departing for summer vacation.
Location
of Financial Statements
The consolidated financial statements furnished in connection
with this Report are attached immediately following Part IV.
|
|
|
Item 9.
|
Changes
in and Disagreements with Accountants on Accounting and
Financial Disclosure
|
None.
|
|
|
Item 9A.
|
Controls
and Procedures
|
Conclusion
Regarding the Effectiveness of Disclosure Controls and
Procedures
We carried out an evaluation, with the participation of our
principal executive officer and principal financial officer, of
the effectiveness of our disclosure controls and procedures as
of December 31, 2009. In designing and evaluating our
disclosure controls and procedures, our management recognized
that any controls and procedures, no matter how well designed
and operated, can provide only reasonable assurance of achieving
their objectives, and our management necessarily applied its
judgment in evaluation of the cost-benefit relationship of
possible controls and procedures. Based on this evaluation, our
principal executive officer and principal financial officer
concluded that, as of December 31, 2009, our disclosure
controls and procedures, as defined in the Securities Exchange
Act of 1934 (the “Exchange Act”)
Rules 13a-15(e)
and
15d-15(e),
were effective to ensure that information required to be
31
disclosed by us in the reports that we file or submit under the
Exchange Act are recorded, processed, summarized and reported
within the time periods specified in the Securities and Exchange
Commission’s rules and forms and such information is
accumulated and communicated to management, including the CEO
and CFO, to allow timely decisions regarding required disclosure.
Management’s
Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining
adequate internal control over financial reporting, as defined
in Exchange Act
Rules 13a-15(f)
and
15d-15(f).
Under the supervision and with the participation of our
management, including our principal executive officer and
principal financial officer, we carried out an evaluation of the
effectiveness of our internal control over financial reporting
as of December 31, 2009 based on the Internal
Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission
(“COSO”). Based upon this evaluation, our management
concluded that our internal control over financial reporting was
effective as of December 31, 2009.
PricewaterhouseCoopers LLP, the independent registered public
accounting firm that audited our financial statements included
in this Annual Report on
Form 10-K,
has also audited the effectiveness of internal control over
financial reporting as of December 31, 2009, as stated in
their report which is included herein.
Changes
in Internal Control over Financial Reporting
No change in our internal control over financial reporting (as
defined in
Rules 13a-15(f)
and
15d-15(f)
under the Exchange Act) occurred during the fiscal quarter ended
December 31, 2009 that has materially affected, or is
reasonably likely to materially affect, our internal control
over financial reporting.
|
|
|
Item 9B.
|
Other
Information
|
None.
PART III
|
|
|
Item 10.
|
Directors,
Executive Officers and Corporate Governance
|
The information required by this Item will be contained in our
proxy statement for our 2010 annual meeting of stockholders,
which we plan to file with the SEC no later than 120 days
after the end of our fiscal year ended December 31, 2009
(the “2010 Proxy Statement”). Such information is
hereby incorporated by reference.
We have adopted a written code of business conduct and ethics
that applies to all our directors, officers and employees, a
copy of which is located on the Investor Relations page of our
website which is located at www.nationaldentex.com. We
intend to disclose any amendments to, or waivers from, our code
of business conduct and ethics on that same page of our website.
|
|
|
Item 11.
|
Executive
Compensation
|
The information required by this item will be included in our
2010 Proxy Statement and is incorporated herein by reference.
|
|
|
Item 12.
|
Security
Ownership of Certain Beneficial Owners and Management and
Related Shareholder Matters
|
The information required by this item will be included in our
2010 Proxy Statement and is incorporated herein by reference.
|
|
|
Item 13.
|
Certain
Relationships and Related Transactions
|
The information required by this item will be included in our
2010 Proxy Statement and is incorporated herein by reference.
32
|
|
|
Item 14.
|
Principal
Accountant Fees and Services
|
The information required by this item will be included in our
2010 Proxy Statement and is incorporated herein by reference.
PART IV
|
|
|
Item 15.
|
Exhibits
and Financial Statement Schedules
|
(a) 1. Financial statements:
For a listing of consolidated financial statements which are
included in this Report, see
page F-1.
2. Financial Statement Schedules:
All schedules for which provision is made under Item 15(a)
(2) are inapplicable and, therefore, have been omitted.
3. Exhibits:
The exhibits listed in the Exhibit Index immediately
preceding the exhibits are filed as part of this Annual Report
on
Form 10-K
and are incorporated herein by reference.
(b) Exhibits:
The exhibits listed in the Exhibit Index immediately
preceding the exhibits are filed as part of this Annual Report
on
Form 10-K
and are incorporated herein by reference.
(c) Not Applicable
33
NATIONAL
DENTEX CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS AND
SCHEDULE
| |
|
|
|
|
|
|
|
Page
|
|
|
|
Financial Statements:
|
|
|
|
|
|
The consolidated financial statements of National Dentex
Corporation included herein are as listed below:
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
F-2
|
|
|
Consolidated Balance Sheets as of December 31, 2008 and 2009
|
|
|
F-3
|
|
|
Consolidated Statements of Income for each of the three years in
the period ended December 31, 2009
|
|
|
F-4
|
|
|
Consolidated Statements of Stockholders’ Equity for each of
the three years in the period ended December 31, 2009
|
|
|
F-5
|
|
|
Consolidated Statements of Cash Flows for each of the three
years in the period ended December 31, 2009
|
|
|
F-6
|
|
|
Notes to Consolidated Financial Statements
|
|
|
F-7
|
|
F-1
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of National Dentex
Corporation:
In our opinion, the accompanying consolidated balance sheets and
the related consolidated statements of income, of
shareholders’ equity and of cash flows present fairly, in
all material respects, the financial position of National Dentex
Corporation and its subsidiaries at December 31, 2009 and
December 31, 2008, and the results of their operations and
their cash flows for each of the three years in the period ended
December 31, 2009 in conformity with accounting principles
generally accepted in the United States of America. Also in our
opinion, the Company maintained, in all material respects,
effective internal control over financial reporting as of
December 31, 2009, based on criteria established in
Internal Control — Integrated Framework issued
by the Committee of Sponsoring Organizations of the Treadway
Commission (COSO). The Company’s management is responsible
for these financial statements, for maintaining effective
internal control over financial reporting and for its assessment
of the effectiveness of internal control over financial
reporting, included in Management’s Report on Internal
Control over Financial Reporting appearing under Item 9A.
Our responsibility is to express opinions on these financial
statements and on the Company’s internal control over
financial reporting based on our integrated audits. We conducted
our audits in accordance with the standards of the Public
Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audits to obtain
reasonable assurance about whether the financial statements are
free of material misstatement and whether effective internal
control over financial reporting was maintained in all material
respects. Our audits of the financial statements included
examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by
management, and evaluating the overall financial statement
presentation. Our audit of internal control over financial
reporting included obtaining an understanding of internal
control over financial reporting, assessing the risk that a
material weakness exists, and testing and evaluating the design
and operating effectiveness of internal control based on the
assessed risk. Our audits also included performing such other
procedures as we considered necessary in the circumstances. We
believe that our audits provide a reasonable basis for our
opinions.
As discussed in Note 5 to the consolidated financial
statements, during the year ended December 31, 2007, the
Company changed the manner in which it accounts for uncertain
tax positions.
A company’s internal control over financial reporting is a
process designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. A company’s
internal control over financial reporting includes those
policies and procedures that (i) pertain to the maintenance
of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the
company; (ii) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of
financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of the
company are being made only in accordance with authorizations of
management and directors of the company; and (iii) provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements.
Also, projections of any evaluation of effectiveness to future
periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers
LLP
Boston, MA
March 11, 2010
F-2
NATIONAL
DENTEX CORPORATION
CONSOLIDATED BALANCE SHEETS
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
ASSETS
|
|
CURRENT ASSETS:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
2,109,943
|
|
|
$
|
1,510,046
|
|
|
Accounts receivable:
|
|
|
|
|
|
|
|
|
|
Trade, less allowance of $492,000 in 2008 and $583,000 in 2009
|
|
|
16,701,139
|
|
|
|
16,132,939
|
|
|
Other
|
|
|
2,527,168
|
|
|
|
1,728,591
|
|
|
Inventories
|
|
|
6,991,385
|
|
|
|
6,890,017
|
|
|
Prepaid expenses
|
|
|
3,688,057
|
|
|
|
3,784,470
|
|
|
Deferred tax assets
|
|
|
931,919
|
|
|
|
973,237
|
|
|
Property held for sale
|
|
|
69,822
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
33,019,433
|
|
|
|
31,019,300
|
|
|
|
|
|
|
|
|
|
|
|
|
PROPERTY, PLANT AND EQUIPMENT:
|
|
|
|
|
|
|
|
|
|
Land and buildings
|
|
|
7,535,015
|
|
|
|
7,535,015
|
|
|
Leasehold and building improvements
|
|
|
18,890,911
|
|
|
|
19,334,362
|
|
|
Laboratory equipment
|
|
|
22,503,086
|
|
|
|
20,459,600
|
|
|
Furniture and fixtures
|
|
|
8,721,724
|
|
|
|
8,337,527
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
57,650,736
|
|
|
|
55,666,504
|
|
|
Less — Accumulated depreciation and amortization
|
|
|
24,213,721
|
|
|
|
24,946,070
|
|
|
|
|
|
|
|
|
|
|
|
|
Net property, plant and equipment
|
|
|
33,437,015
|
|
|
|
30,720,434
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER ASSETS, net:
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
69,384,320
|
|
|
|
69,616,034
|
|
|
Trade names
|
|
|
9,977,917
|
|
|
|
9,490,645
|
|
|
Customer relationships
|
|
|
6,210,176
|
|
|
|
5,352,962
|
|
|
Non-competition agreements
|
|
|
1,583,895
|
|
|
|
1,291,824
|
|
|
Other assets
|
|
|
7,902,147
|
|
|
|
7,803,877
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other assets
|
|
|
95,058,455
|
|
|
|
93,555,342
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
161,514,903
|
|
|
$
|
155,295,076
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Revolving line of credit
|
|
$
|
2,939,978
|
|
|
$
|
—
|
|
|
Current portion of long-term debt
|
|
|
5,115,032
|
|
|
|
5,071,050
|
|
|
Accounts payable
|
|
|
3,541,996
|
|
|
|
3,495,044
|
|
|
Accrued liabilities:
|
|
|
|
|
|
|
|
|
|
Payroll and employee benefits
|
|
|
7,574,971
|
|
|
|
9,315,821
|
|
|
Current portion of deferred acquisition costs
|
|
|
300,000
|
|
|
|
—
|
|
|
Other accrued expenses
|
|
|
4,019,992
|
|
|
|
3,841,752
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
23,491,969
|
|
|
|
21,723,667
|
|
|
|
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Long-term obligations
|
|
|
34,142,891
|
|
|
|
21,776,455
|
|
|
Deferred compensation
|
|
|
6,114,609
|
|
|
|
6,739,265
|
|
|
Other accrued expenses
|
|
|
1,419,561
|
|
|
|
1,475,247
|
|
|
Deferred tax liabilities
|
|
|
5,853,821
|
|
|
|
5,789,956
|
|
|
|
|
|
|
|
|
|
|
|
|
Total long-term liabilities
|
|
|
47,530,882
|
|
|
|
35,780,923
|
|
|
|
|
|
|
|
|
|
|
|
|
COMMITMENTS AND CONTINGENCIES (Note 8)
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY:
|
|
|
|
|
|
|
|
|
|
Preferred stock, $.01 par value
|
|
|
|
|
|
|
|
|
|
Authorized — 500,000 shares
|
|
|
|
|
|
|
|
|
|
None issued and outstanding
|
|
|
—
|
|
|
|
—
|
|
|
Common stock, $.01 par value
|
|
|
|
|
|
|
|
|
|
Authorized — 8,000,000 shares
|
|
|
|
|
|
|
|
|
|
Issued and Outstanding — 5,663,749 shares at
December 31, 2008 and 5,761,363 shares at
December 31, 2009
|
|
|
56,637
|
|
|
|
57,613
|
|
|
Paid-in capital
|
|
|
19,522,536
|
|
|
|
20,374,197
|
|
|
Retained earnings
|
|
|
71,312,895
|
|
|
|
77,189,758
|
|
|
Accumulated other comprehensive (loss) income
|
|
|
(400,016
|
)
|
|
|
168,918
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
90,492,052
|
|
|
|
97,790,486
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
161,514,903
|
|
|
$
|
155,295,076
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
F-3
NATIONAL
DENTEX CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Net sales
|
|
$
|
170,360,629
|
|
|
$
|
171,674,435
|
|
|
$
|
161,195,204
|
|
|
Cost of goods sold
|
|
|
97,738,785
|
|
|
|
102,184,879
|
|
|
|
93,119,314
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
72,621,844
|
|
|
|
69,489,556
|
|
|
|
68,075,890
|
|
|
Selling, general and administrative expenses
|
|
|
58,561,368
|
|
|
|
58,587,890
|
|
|
|
55,755,023
|
|
|
Goodwill impairment
|
|
|
—
|
|
|
|
6,950,000
|
|
|
|
263,537
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
14,060,476
|
|
|
|
3,951,666
|
|
|
|
12,057,330
|
|
|
Other expense
|
|
|
771,660
|
|
|
|
746,633
|
|
|
|
744,867
|
|
|
Interest expense
|
|
|
2,802,944
|
|
|
|
2,110,113
|
|
|
|
1,257,184
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before provision for income taxes
|
|
|
10,485,872
|
|
|
|
1,094,920
|
|
|
|
10,055,279
|
|
|
Provision for income taxes
|
|
|
3,860,213
|
|
|
|
1,971,963
|
|
|
|
4,178,416
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
6,625,659
|
|
|
$
|
(877,043
|
)
|
|
$
|
5,876,863
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share — basic
|
|
$
|
1.20
|
|
|
$
|
(0.16
|
)
|
|
$
|
1.03
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share — diluted
|
|
$
|
1.17
|
|
|
$
|
(0.16
|
)
|
|
$
|
1.02
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding — basic
|
|
|
5,540,496
|
|
|
|
5,631,450
|
|
|
|
5,727,071
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding — diluted
|
|
|
5,665,042
|
|
|
|
5,631,450
|
|
|
|
5,760,785
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
F-4
NATIONAL
DENTEX CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’
EQUITY
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock
|
|
|
|
|
|
|
|
|
Cumulative
|
|
|
|
|
|
|
|
Number of
|
|
|
$.01 Par
|
|
|
Paid-in
|
|
|
Retained
|
|
|
Translation
|
|
|
|
|
|
|
|
Shares
|
|
|
Value
|
|
|
Capital
|
|
|
Earnings
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
BALANCE, December 31, 2006
|
|
|
5,509,412
|
|
|
$
|
55,094
|
|
|
$
|
17,296,170
|
|
|
$
|
65,564,279
|
|
|
$
|
(121,911
|
)
|
|
|
82,793,632
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of 42,450 shares of common stock under the stock
option plans
|
|
|
42,450
|
|
|
|
425
|
|
|
|
567,407
|
|
|
|
|
|
|
|
|
|
|
|
567,832
|
|
|
Issuance of 24,030 shares of common stock under the
employee stock purchase program
|
|
|
24,030
|
|
|
|
240
|
|
|
|
295,325
|
|
|
|
|
|
|
|
|
|
|
|
295,565
|
|
|
Tax benefit associated with exercise of stock options
|
|
|
|
|
|
|
|
|
|
|
15,859
|
|
|
|
|
|
|
|
|
|
|
|
15,859
|
|
|
Net income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6,625,659
|
|
|
|
|
|
|
|
6,625,659
|
|
|
Issuance of 6,227 shares of common stock as directors’
fees
|
|
|
6,227
|
|
|
|
62
|
|
|
|
165,302
|
|
|
|
|
|
|
|
|
|
|
|
165,364
|
|
|
Stock compensation expense
|
|
|
|
|
|
|
|
|
|
|
161,112
|
|
|
|
|
|
|
|
|
|
|
|
161,112
|
|
|
Cumulative translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
567,240
|
|
|
|
567,240
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE, December 31, 2007
|
|
|
5,582,119
|
|
|
|
55,821
|
|
|
|
18,501,175
|
|
|
|
72,189,938
|
|
|
|
445,329
|
|
|
|
91,192,263
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of 12,000 shares of common stock under the stock
option plans
|
|
|
20,250
|
|
|
|
202
|
|
|
|
188,799
|
|
|
|
|
|
|
|
|
|
|
|
189,001
|
|
|
Issuance of 33,375 shares of common stock under the
employee stock purchase program
|
|
|
33,375
|
|
|
|
334
|
|
|
|
371,308
|
|
|
|
|
|
|
|
|
|
|
|
371,642
|
|
|
Tax benefit associated with exercise of stock options
|
|
|
|
|
|
|
|
|
|
|
12,037
|
|
|
|
|
|
|
|
|
|
|
|
12,037
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(877,043
|
)
|
|
|
|
|
|
|
(877,043
|
)
|
|
Issuance of 28,005 shares of common stock as
directors’ fees
|
|
|
28,005
|
|
|
|
280
|
|
|
|
269,720
|
|
|
|
|
|
|
|
|
|
|
|
270,000
|
|
|
Stock compensation expense
|
|
|
|
|
|
|
|
|
|
|
179,497
|
|
|
|
|
|
|
|
|
|
|
|
179,497
|
|
|
Cumulative translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(845,345
|
)
|
|
|
(845,345
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE, December 31, 2008
|
|
|
5,663,749
|
|
|
|
56,637
|
|
|
|
19,522,536
|
|
|
|
71,312,895
|
|
|
|
(400,016
|
)
|
|
|
90,492,052
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of 93,102 shares of common stock under the
employee stock purchase program
|
|
|
93,102
|
|
|
|
934
|
|
|
|
315,567
|
|
|
|
|
|
|
|
|
|
|
|
316,501
|
|
|
Net income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,876,863
|
|
|
|
|
|
|
|
5,876,863
|
|
|
Issuance of 4,155 shares of common stock as directors’
fees
|
|
|
4,155
|
|
|
|
42
|
|
|
|
162,708
|
|
|
|
|
|
|
|
|
|
|
|
162,750
|
|
|
Stock compensation expense
|
|
|
|
|
|
|
|
|
|
|
373,386
|
|
|
|
|
|
|
|
|
|
|
|
373,386
|
|
|
Cumulative translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
568,934
|
|
|
|
568,934
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE, December 31, 2009
|
|
|
5,761,006
|
|
|
$
|
57,613
|
|
|
$
|
20,374,197
|
|
|
$
|
77,189,758
|
|
|
$
|
168,918
|
|
|
$
|
97,790,486
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
F-5
NATIONAL
DENTEX CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
6,625,659
|
|
|
$
|
(877,043
|
)
|
|
$
|
5,876,863
|
|
|
Adjustments to reconcile net income to net cash provided by
operating activities, net of effects of acquisitions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
5,338,432
|
|
|
|
6,011,564
|
|
|
|
5,825,730
|
|
|
Goodwill impairment
|
|
|
—
|
|
|
|
6,950,000
|
|
|
|
263,536
|
|
|
Loss (gain) on disposal of property, plant and equipment
|
|
|
150,109
|
|
|
|
(2,792
|
)
|
|
|
(311,472
|
)
|
|
Benefit for deferred income taxes
|
|
|
(265,149
|
)
|
|
|
(1,099,572
|
)
|
|
|
(142,638
|
)
|
|
Impairment of long-lived assets
|
|
|
94,000
|
|
|
|
44,000
|
|
|
|
361,443
|
|
|
Tax benefit associated with exercise of stock options
|
|
|
15,859
|
|
|
|
12,037
|
|
|
|
—
|
|
|
Provision for bad debts
|
|
|
151,330
|
|
|
|
148,068
|
|
|
|
274,337
|
|
|
Losses on write-down of inventories
|
|
|
107,791
|
|
|
|
219,255
|
|
|
|
124,583
|
|
|
Stock-based compensation expense
|
|
|
326,477
|
|
|
|
449,498
|
|
|
|
536,136
|
|
|
Other non-cash items
|
|
|
489,375
|
|
|
|
—
|
|
|
|
—
|
|
|
Changes in operating assets and liabilities, net of effects of
acquisitions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Increase) decrease in accounts receivable
|
|
|
(701,142
|
)
|
|
|
(5,886
|
)
|
|
|
1,159,268
|
|
|
(Increase) decrease in inventories
|
|
|
(226,117
|
)
|
|
|
355,532
|
|
|
|
6,632
|
|
|
(Increase) decrease in prepaid expenses
|
|
|
(2,363,317
|
)
|
|
|
586,798
|
|
|
|
(93,747
|
)
|
|
(Increase) decrease in other assets
|
|
|
(19,635
|
)
|
|
|
616,081
|
|
|
|
(106,294
|
)
|
|
Increase (decrease) in accounts payable and accrued liabilities
|
|
|
2,828,757
|
|
|
|
(80,844
|
)
|
|
|
2,038,549
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
12,552,429
|
|
|
|
13,326,696
|
|
|
|
15,812,926
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment for acquisitions, net of cash acquired
|
|
|
—
|
|
|
|
(10,000,000
|
)
|
|
|
—
|
|
|
Payment of deferred purchase price
|
|
|
(2,158,880
|
)
|
|
|
(1,577,720
|
)
|
|
|
(300,000
|
)
|
|
Increase in notes receivable
|
|
|
—
|
|
|
|
(2,000,000
|
)
|
|
|
—
|
|
|
Premiums paid for life insurance policies
|
|
|
(386,621
|
)
|
|
|
(324,505
|
)
|
|
|
(171,042
|
)
|
|
Proceeds received from life insurance policies
|
|
|
46,700
|
|
|
|
87,734
|
|
|
|
163,950
|
|
|
Additions to property, plant and equipment
|
|
|
(7,535,578
|
)
|
|
|
(7,024,076
|
)
|
|
|
(1,557,588
|
)
|
|
Cash proceeds from the disposition of property, plant, and
equipment
|
|
|
183,373
|
|
|
|
322,589
|
|
|
|
450,097
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(9,851,006
|
)
|
|
|
(20,515,978
|
)
|
|
|
(1,414,583
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Borrowings of revolving line of credit
|
|
|
55,370,014
|
|
|
|
55,555,496
|
|
|
|
52,894,765
|
|
|
Repayments of revolving line of credit
|
|
|
(52,166,141
|
)
|
|
|
(57,162,619
|
)
|
|
|
(63,106,023
|
)
|
|
Borrowings of long-term debt
|
|
|
—
|
|
|
|
13,800,000
|
|
|
|
—
|
|
|
Repayments of long-term debt
|
|
|
(5,775,105
|
)
|
|
|
(5,109,985
|
)
|
|
|
(5,106,371
|
)
|
|
Net proceeds from issuance of common stock
|
|
|
863,396
|
|
|
|
560,644
|
|
|
|
316,501
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by financing activities
|
|
|
(1,707,836
|
)
|
|
|
7,643,536
|
|
|
|
(15,001,128
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of Exchange rate changes on cash
|
|
|
47,539
|
|
|
|
(33,702
|
)
|
|
|
2,888
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
1,041,126
|
|
|
|
420,552
|
|
|
|
(599,897
|
)
|
|
Cash and cash equivalents at beginning of period
|
|
|
648,265
|
|
|
|
1,689,391
|
|
|
|
2,109,943
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of period
|
|
$
|
1,689,391
|
|
|
$
|
2,109,943
|
|
|
$
|
1,510,046
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid (net of capitalized interest of $37,000 in 2007,
$26,000 in 2008 and $3,000 in 2009)
|
|
$
|
2,853,706
|
|
|
$
|
2,211,714
|
|
|
$
|
1,306,242
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid
|
|
$
|
5,792,166
|
|
|
$
|
3,156,077
|
|
|
$
|
4,499,336
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental schedule of non-cash investing and financing
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital lease obligations
|
|
$
|
—
|
|
|
$
|
(881,000
|
)
|
|
$
|
—
|
|
|
The Company purchased the operations of certain dental
laboratories in 2008. In connection with these acquisitions,
liabilities were assumed as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of assets acquired including acquired cash
|
|
$
|
—
|
|
|
$
|
11,959,000
|
|
|
$
|
—
|
|
|
Cash purchase price
|
|
|
—
|
|
|
|
(10,112,000
|
)
|
|
|
—
|
|
|
Deferred purchase price at date of acquisition
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities assumed
|
|
$
|
—
|
|
|
$
|
1,847,000
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
F-6
NATIONAL
DENTEX CORPORATION
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS
December 31,
2009
National Dentex Corporation (the “Company”) owns and
operates 40 full-service dental laboratories and four branch
laboratories in 30 states throughout the United States and
one Canadian province as of December 31, 2009. Working from
dentists’ work orders, the Company’s dental
laboratories custom design and fabricate dentures, crowns and
fixed bridges, and other dental prosthetic appliances.
|
|
|
(2)
|
Summary
of Significant Accounting Policies
|
Principles
of Consolidation
The Company follows the guidance established by
U.S. Generally Accepted Accounting Principles (GAAP) in
presenting the consolidated financial statements. Acquisitions
are reflected from the date acquired by the Company (see
Note 3) to December 31, 2009. The accompanying
financial statements include the accounts of the Company and its
wholly and majority-owned subsidiaries. All material
inter-company balances and transactions have been eliminated in
consolidation.
Revenue
Recognition
Revenue is recognized upon transfer of title and risk of loss,
generally as the dentists’ orders are shipped. The Company
records shipping and handling fees charged to customers as
revenue. Shipping and handling costs totaling approximately
$15,617,000, $16,463,000 and $14,800,000 for the years ended
December 31, 2007, 2008 and 2009, respectively, are
included in selling, general and administrative expense.
Goodwill
and Other Indefinite-Lived Intangible Assets Not Subject to
Amortization
The Company evaluates goodwill for impairment whenever events
and circumstances have occurred that indicate that the value of
goodwill may be impaired. In addition, goodwill is evaluated for
possible impairment on an annual basis, based on a two-step
process. The Company has selected June 30th as the date for the
annual assessment of goodwill impairment.
The first step is used to identify potential impairment and
involves comparing each reporting unit’s estimated fair
value to its carrying value, including goodwill. A reporting
unit is an operating segment or one level below an operating
segment (referred to as a component). The Company has determined
that each individual laboratory is a reporting unit. The second
step of the goodwill impairment process involves the calculation
of an implied fair value of goodwill for the laboratories which
step one indicated were impaired. Fair value is determined by
using an income approach, consistent with our valuation of
dental laboratories acquired in purchase business combinations.
The Company assessed goodwill impairment as of June 30,
2009 and determined that no impairment charge was required. The
Company’s impairment analysis incorporates forecasted
financial results and assumptions for all laboratories within
the NDX Laboratories operating segment. Although these
laboratories were not impaired at June 30, 2009, in the
third quarter of 2009, the Company recorded an impairment charge
of $264,000, comprising the entire goodwill balance of one of
its smaller laboratories, due to a significant decrease in
revenues and operating income during the third quarter of 2009
at this laboratory. During the fourth quarter of 2009, there
were no events that triggered a goodwill impairment test or
charge to goodwill impairment expense. The Company will continue
to closely monitor the financial results of certain other
laboratories in subsequent periods in comparison to forecasted
financial results and assumptions made at June 30, 2009 to
determine whether any other impairment charges are warranted.
Additionally, the Company also recognizes the existence of value
in trade names acquired in business combinations and believes
the useful life of this intangible to be indefinite based on a
long history of utilizing the laboratory trade names.
Accordingly, trade names are also evaluated for impairment on an
annual basis using a
F-7
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
single step method. Impairment charges related to trade names
are recognized when the fair value is less than the carrying
value of the asset. Impairment charges related to trade names
were recorded in the amount of $94,000, $44,000 and $361,000 for
the years ended December 31, 2007, 2008 and 2009,
respectively. Trade name impairment charges generally result
from a decline in forecasted revenue at specific laboratories in
comparison to revenue forecasts used in previous valuation
calculations.
Intangible
Assets Subject to Amortization
All acquisitions reflected in the accompanying consolidated
financial statements have been accounted for as purchase
business combinations. Non-competition agreements and customer
relationship intangibles arising from dental laboratory
acquisitions are amortized over their useful lives. The
acquisition date fair value of non-competition agreements are
deferred and amortized over their economic useful lives, in
accordance with the terms of the agreements, over 2 to
15 years. The acquisition date fair value associated with
acquired customer relationships are amortized over their
estimated useful life, generally ranging over 9 to 12 years.
Advertising
and Promotional Costs
Advertising, promotional and marketing costs are charged to
earnings in the period in which they are incurred. These costs
were approximately $2,625,000, $2,773,000 and $2,468,000 for the
years ended December 31, 2007, 2008 and 2009, respectively.
Cash
and Cash Equivalents
The Company considers all highly liquid investments purchased
with maturities of 90 days or less to be cash equivalents.
At certain times the Company may have cash investments including
overnight repurchase agreements with financial institutions in
excess of the $250,000 insured limit of the Federal Deposit
Insurance Corporation.
Accounts
Receivable and Allowance for Doubtful Accounts
Trade accounts receivable are recorded at the invoiced amount.
Service charges are assessed on balances 60 days past due.
The allowance for doubtful accounts is the Company’s best
estimate of the amount of probable credit losses in its existing
accounts receivable. The Company determines the allowance based
on historical write-off experience. Past due balances over
90 days and over a specified amount are also reviewed
individually for collectability. Account balances are charged
off against the allowance when the Company determines it is
probable the receivable will not be recovered.
Receivables consist of the following at December 31, 2008
and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Trade
|
|
$
|
17,193,121
|
|
|
$
|
16,715,927
|
|
|
Allowance for doubtful accounts
|
|
|
(491,982
|
)
|
|
|
(582,988
|
)
|
|
Employee
|
|
|
298,893
|
|
|
|
50,088
|
|
|
Income Taxes
|
|
|
1,400,548
|
|
|
|
1,393,000
|
|
|
Other
|
|
|
827,727
|
|
|
|
285,503
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Receivables
|
|
$
|
19,228,307
|
|
|
$
|
17,861,530
|
|
|
|
|
|
|
|
|
|
|
|
F-8
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Following are the changes in the allowance for doubtful accounts
during the years ended December 31, 2007, 2008 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
Charged to
|
|
|
|
Acquired in
|
|
Balance at
|
|
|
|
Beginning
|
|
Costs and
|
|
|
|
Purchase Business
|
|
End of
|
|
|
|
of Period
|
|
Expenses
|
|
Write-offs
|
|
Combinations
|
|
Period
|
|
|
|
Allowance for Doubtful Accounts:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2007
|
|
|
289,992
|
|
|
|
151,330
|
|
|
|
82,770
|
|
|
|
—
|
|
|
|
358,552
|
|
|
December 31, 2008
|
|
|
358,552
|
|
|
|
148,069
|
|
|
|
84,639
|
|
|
|
70,000
|
|
|
|
491,982
|
|
|
December 31, 2009
|
|
|
491,982
|
|
|
|
274,337
|
|
|
|
183,331
|
|
|
|
—
|
|
|
|
582,988
|
|
Inventories
Inventories consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Raw Materials
|
|
$
|
5,783,468
|
|
|
$
|
5,726,274
|
|
|
Work in Process
|
|
|
985,278
|
|
|
|
945,196
|
|
|
Finished Goods
|
|
|
222,639
|
|
|
|
218,547
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
6,991,385
|
|
|
$
|
6,890,017
|
|
|
|
|
|
|
|
|
|
|
|
Inventories are stated at the lower of cost
(first-in,
first-out) or market. Work in process represents an estimate of
the value of specific orders in production yet incomplete at
period end. Finished goods consist of completed orders that were
shipped to customers immediately subsequent to period end.
Property,
Plant and Equipment
Property, plant and equipment are stated at cost, less
accumulated depreciation. Depreciation is calculated using the
straight-line method over the following estimated depreciable
lives:
| |
|
|
|
Buildings
|
|
25 years
|
|
Furniture and fixtures
|
|
5 - 10 years
|
|
Laboratory equipment
|
|
5 - 20 years
|
|
Computer equipment
|
|
3 - 5 years
|
Leasehold improvements and capital leases are amortized over the
lesser of the assets’ estimated useful lives or the
remaining lease term.
Gains and losses are recognized upon the disposal of property
and equipment, and the related accumulated depreciation and
amortization are removed from the accounts. Maintenance, repairs
and betterments that do not enhance the value of or increase the
life of the assets are charged to operations as incurred.
Interest costs, if incurred, should be capitalized as part of
the cost of acquiring or constructing qualifying assets. The
Company had two qualifying assets which required a period of
time to make ready for their intended use. Capitalized interest
which is classified as Leasehold and Building Improvements
totaled approximately $37,000, $26,000 and $3,000 for the years
ended December 31, 2007, 2008 and 2009, respectively.
Depreciation expense totaled approximately $4,055,000,
$4,872,000 and $4,569,000 for the years ended December 31,
2007, 2008 and 2009, respectively.
F-9
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Impairment
of Long-Lived Assets
At each balance sheet date, management evaluates the
recoverability of the long-lived assets, including property and
equipment and intangible assets, using certain financial
indicators, such as historical and future ability to generate
income from operations. The Company’s policy is to assess
long-lived asset impairment in the period when it is determined
that the carrying amount of the asset may not be recoverable.
The determination is based on an evaluation of such factors as
the occurrence of a significant event, a significant change in
the environment in which the business operates or if the
expected future undiscounted cash flows become less than the
carrying amount of the asset.
Cash
Surrender of Life Insurance
Life insurance policies, which are owned by the Company and are
presented as other assets, are recorded at their net realizable
value, which approximates the surrender value of the policy.
Income
Taxes
The Company has deferred tax assets and liabilities that are
recognized for the expected future tax consequences of events
that have been included in the financial statements or tax
returns. The amount of deferred tax asset or liability is based
on the difference between the financial statement and tax basis
of assets and liabilities using enacted tax rates in effect for
the year in which the differences are expected to reverse.
Accounting for income taxes prescribes a recognition threshold
and measurement attribute for the financial statement
recognition and measurement of a tax position taken or expected
to be taken in a tax return. The minimum threshold is defined as
a tax position that is more likely than not to be sustained upon
examination by the applicable taxing authority, including
resolution of any related appeals or litigation processes, based
on the technical merits of the position. The tax benefit to be
recognized is measured as the largest amount of benefit that is
greater than fifty percent likely of being realized upon
ultimate settlement.
Earnings
per Share
In accordance with disclosure requirements, basic earnings per
share is computed by dividing net income by the weighted average
number of shares outstanding and diluted earnings per share
reflects the dilutive effect of potential common shares. The
weighted average number of shares outstanding, the dilutive
effects of outstanding stock options and the shares under option
plans that were anti-dilutive for the years ended
December 31, 2007, 2008 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
2007
|
|
2008
|
|
2009
|
|
|
|
Weighted average number of shares used in basic earnings per
share calculation
|
|
|
5,540,496
|
|
|
|
5,631,450
|
|
|
|
5,727,071
|
|
|
Incremental shares under option and employee stock purchase plans
|
|
|
124,546
|
|
|
|
—
|
|
|
|
33,714
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares used in diluted earnings per
share calculation
|
|
|
5,665,042
|
|
|
|
5,631,450
|
|
|
|
5,760,785
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares under option plans excluded in computation of diluted
earnings per share due to anti-dilutive effects
|
|
|
1,215
|
|
|
|
591,486
|
|
|
|
621,511
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Use of
Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States of
America requires management to make estimates and assumptions
that affect the reported amounts
F-10
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
of assets and liabilities and disclosure of contingent assets
and liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting
period. Actual results could differ from those estimates.
Disclosures
about the Fair Value of Financial Instruments
The Company’s financial instruments mainly consist of cash
and cash equivalents, accounts receivable, accounts payable, and
current and long-term liabilities. The carrying amounts of the
Company’s cash and cash equivalents, accounts receivable
and accounts payable approximate their fair value due to the
short-term nature of these instruments. The carrying amount of
the long-term liabilities also approximates their fair value,
based on rates available to the Company for debt with similar
terms and remaining maturities.
Comprehensive
Income
Comprehensive income is defined as the change in equity of a
business enterprise during a period from transactions and other
events and circumstances from non-owner sources. The
Company’s total comprehensive income was as follows for the
periods presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Net income (loss)
|
|
$
|
6,625,659
|
|
|
$
|
(877,043
|
)
|
|
$
|
5,876,863
|
|
|
Foreign currency translation adjustments
|
|
|
567,240
|
|
|
|
(845,345
|
)
|
|
|
568,934
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income (loss)
|
|
$
|
7,192,899
|
|
|
$
|
(1,722,388
|
)
|
|
$
|
6,445,797
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income (loss) at
December 31, 2007, 2008 and 2009 of $445,329, ($400,016)
and $168,918, respectively, as presented in the equity section
of the consolidated balance sheet is entirely attributable to
accumulated foreign currency translation adjustments.
Disclosures
about Segments of an Enterprise
Accounting standards for reporting information regarding
operating segments in annual financial statements require
selected information for those segments to be presented in
interim financial reports issued to stockholders and also
establish standards for related disclosures about products and
services and geographic areas. Operating segments are identified
as components of an enterprise about which separate financial
information is available for the evaluation by the chief
operating decision maker, or decision-making group, in making
decisions how to allocate resources and assess performance.
In March 2005, the Company acquired Green Dental Laboratories,
Inc. of Heber Springs, Arkansas. In October 2006, the Company
acquired Keller Group, Incorporated, a privately-held dental
laboratory business with production facilities in both
St. Louis, Missouri and Louisville, Kentucky. The Company
identified Green and Keller as separate operating segments based
on the quantitative criterion of the accounting standard. As a
result, the Company has three reportable segments. The
accounting policies of these segments are consistent with those
described for the consolidated financial statements in the
summary of significant accounting policies.
Fair
Value Measurements
Effective January 1, 2008, the Company adopted accounting
standards that define fair value, establish a framework for
measuring fair value and enhances disclosures about fair value
measurements. Fair value is defined as the exchange price that
would be received for an asset or paid to transfer a liability
(an exit price) in the principal or most advantageous market for
the asset or liability in an orderly transaction between market
participants on the measurement date. These standards establish
a fair value hierarchy which requires an entity to maximize the
use of observable inputs and minimize the use of unobservable
inputs when measuring fair value; describe three levels of
F-11
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
inputs that may be used to measure fair value which are provided
in the table below; and allow an entity the irrevocable option
to elect fair value for the initial and subsequent measurement
for certain financial assets and liabilities on a
contract-by-contract
basis. The adoption of these standards had no material impact on
the Company’s financial statements.
The Company uses the market approach technique to value its
financial instruments and there were no changes in valuation
techniques during the year ended December 31, 2009. The
Company’s financial assets and liabilities are primarily
comprised of investments in insurance contracts held as assets
to satisfy outstanding retirement liabilities.
Assets and liabilities carried at fair value are classified and
disclosed in one of the following three categories:
Level 1: Quoted market prices in active
markets for identical assets or liabilities that the Company has
the ability to access.
Level 2: Observable market based inputs
or unobservable inputs that are corroborated by market data such
as quoted prices, interest rates, and yield curves.
Level 3: Inputs are unobservable data
points that are not corroborated by market data.
The following table presents information about the
Company’s financial assets measured at fair value on a
recurring basis as of December 31, 2009 and 2008. There
were no liabilities that required disclosure:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quoted Prices in
|
|
|
Significant Other
|
|
|
Significant
|
|
|
|
|
As of
|
|
|
Active Markets
|
|
|
Observable Inputs
|
|
|
Unobservable
|
|
|
Description
|
|
December 31, 2009
|
|
|
(Level 1)
|
|
|
(Level 2)
|
|
|
Inputs (Level 3)
|
|
|
|
|
Financial Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Surrender Value of Life Insurance
|
|
$
|
5,546,000
|
|
|
|
—
|
|
|
$
|
5,546,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Financial Assets
|
|
$
|
5,546,000
|
|
|
|
—
|
|
|
$
|
5,546,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quoted Prices in
|
|
|
Significant Other
|
|
|
Significant
|
|
|
|
|
As of
|
|
|
Active Markets
|
|
|
Observable Inputs
|
|
|
Unobservable
|
|
|
Description
|
|
December 31, 2008
|
|
|
(Level 1)
|
|
|
(Level 2)
|
|
|
Inputs (Level 3)
|
|
|
|
|
Financial Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Surrender Value of Life Insurance
|
|
$
|
5,479,000
|
|
|
|
—
|
|
|
$
|
5,479,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Financial Assets
|
|
$
|
5,479,000
|
|
|
|
—
|
|
|
$
|
5,479,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recent
Accounting Pronouncements
In April 2009, the FASB issued additional guidance for
estimating fair value when the volume and level of activity for
the asset or liability have significantly decreased and for
identifying circumstances that indicate a transaction is not
orderly. This guidance emphasizes that even if there has been a
significant decrease in the volume and level of activity for the
asset or liability and regardless of the valuation technique(s)
used, the objective of a fair value measurement remains the
same. Fair value is the price that would be received to sell an
asset or paid to transfer a liability in an orderly transaction
(that is, not a forced liquidation or distressed sale) between
market participants at the measurement date under current market
conditions. This guidance is effective for interim and annual
reporting periods ending after June 15, 2009 and its
adoption did not have an impact on the Company’s unaudited
consolidated financial statements.
In May 2009, the FASB issued a pronouncement on subsequent event
accounting. The guidance identifies the following: the period
after the balance sheet date during which management shall
evaluate events or transactions that may occur for potential
recognition or disclosure in the financial statements; the
circumstances under which an
F-12
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
entity shall recognize events or transactions occurring after
the balance sheet date in its financial statements; and the
disclosures that an entity shall make about events or
transactions that occurred after the balance sheet date. This
statement was effective for the Company’s second quarter
2009, and there was no effect from adoption.
In June 2009, the FASB issued guidance on The FASB
Accounting Standards
Codificationtm
and the Hierarchy of Generally Accepted Accounting
Principles, which is effective for financial statements
issued for interim and annual periods ending after
September 15, 2009. The adoption of this pronouncement did
not have an impact on the Company’s consolidated financial
statements.
The Company’s acquisition strategy is to consolidate within
the dental laboratory industry and use its financial and
operational synergies to create a competitive advantage. Certain
factors, such as the laboratory’s assembled workforce and
technical skills, result in the recognition of goodwill.
In certain transactions, the Company executes non-compete
agreements with the former owners and other key employees. The
fair value of these agreements is recognized in purchase
accounting as an identifiable intangible asset and is amortized
over the estimated economic life of the agreement. All
acquisitions have been reflected in the accompanying
consolidated financial statements from the date of acquisition
and have been accounted for as purchase business combinations.
Purchase price is allocated to acquired assets and liabilities
based on estimates of their related fair values.
There were no acquisitions of dental laboratories during the
year ended December 31, 2009. During 2008, the Company
acquired the following dental laboratory operations:
| |
|
|
|
|
|
|
|
Acquisition
|
|
Form of Acquisition
|
|
Location
|
|
Period Acquired
|
|
|
|
Dental Art Laboratories, Inc.
|
|
All Outstanding Capital Stock
|
|
Lansing, MI
|
|
September, 2008
|
The cost of the acquisition of Dental Art, net of cash acquired,
was $10,000,000. The total purchase price has been allocated to
the acquired assets and liabilities based on estimates of their
related fair values. The total purchase price was allocated as
follows as of December 31, 2008:
| |
|
|
|
|
|
|
|
Total
|
|
|
Dental Art Laboratories, Inc.
|
|
Acquired
|
|
|
|
|
Total Purchase Price
|
|
$
|
10,112,000
|
|
|
Less Fair Market Values Assigned to Tangible Assets and
Liabilities:
|
|
|
|
|
|
Cash
|
|
|
112,000
|
|
|
Accounts receivable
|
|
|
883,000
|
|
|
Inventories
|
|
|
252,000
|
|
|
Property, plant and equipment
|
|
|
332,000
|
|
|
Other assets
|
|
|
140,000
|
|
|
Accounts payable
|
|
|
(192,000
|
)
|
|
Accrued liabilities and other
|
|
|
(1,655,000
|
)
|
|
Less Fair Market Values Assigned to Intangible Assets:
|
|
|
|
|
|
Customer relationships
|
|
|
1,500,000
|
|
|
Trade names
|
|
|
920,000
|
|
|
Non-compete agreements
|
|
|
150,000
|
|
|
|
|
|
|
|
|
Goodwill
|
|
$
|
7,670,000
|
|
|
|
|
|
|
|
Acquired goodwill in certain situations may be tax deductible
over a fifteen-year period, as allowed under Internal Revenue
Code Section 197. However, acquired goodwill for Dental Art
is not tax deductible.
F-13
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The following unaudited pro forma operating results of the
Company assume the Dental Art acquisition had been made as of
January 1, 2007. Such information includes adjustments to
reflect additional depreciation, non-compete and customer
relationship amortization and interest expense, and is not
necessarily indicative of what the results of operations would
actually have been or of the results of operations in future
periods.
| |
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
December 31, 2007
|
|
December 31, 2008
|
|
|
|
Net sales
|
|
$
|
178,140,000
|
|
|
$
|
176,858,000
|
|
|
Net income (loss)
|
|
|
7,324,000
|
|
|
|
(413,000
|
)
|
|
Net income (loss) per share:
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
1.32
|
|
|
$
|
(0.07
|
)
|
|
Diluted
|
|
$
|
1.29
|
|
|
$
|
(0.07
|
)
|
(4) Goodwill
and Other Intangible Assets
The changes in the carrying amount of goodwill for the years
ended December 31, 2008 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
NDX Laboratories Segment
|
|
|
|
|
|
|
|
|
|
Goodwill, Balance as of January 1
|
|
$
|
37,636,000
|
|
|
$
|
44,983,000
|
|
|
Accumulated impairment losses
|
|
|
—
|
|
|
|
(6,950,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
37,636,000
|
|
|
|
38,033,000
|
|
|
Goodwill acquired during the year
|
|
|
7,501,000
|
|
|
|
—
|
|
|
Adjustments related to contingent consideration
|
|
|
300,000
|
|
|
|
—
|
|
|
Effects of exchange rate changes
|
|
|
(454,000
|
)
|
|
|
316,000
|
|
|
Impairment losses and other adjustments
|
|
|
(6,950,000
|
)
|
|
|
(84,000
|
)
|
|
Balance as of December 31
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
44,983,000
|
|
|
|
45,299,000
|
|
|
Accumulated impairment losses
|
|
|
(6,950,000
|
)
|
|
|
(7,034,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
38,033,000
|
|
|
$
|
38,265,000
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Green Dental Laboratory Segment
|
|
|
|
|
|
|
|
|
|
Goodwill balance
|
|
$
|
15,208,000
|
|
|
$
|
15,208,000
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Keller Group Segment
|
|
|
|
|
|
|
|
|
|
Goodwill balance
|
|
$
|
16,143,000
|
|
|
$
|
16,143,000
|
|
|
|
|
|
|
|
|
|
|
|
In connection with dental laboratory acquisitions, the Company
has identified certain intangible assets including trade names,
customer relationships and non-competition agreements.
F-14
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The Company continually evaluates whether events and
circumstances have occurred that indicate that the value of
goodwill has been impaired. In addition, goodwill is evaluated
for possible impairment on an annual basis, based on a two-step
process. The Company has selected June 30th as the date for the
annual assessment of goodwill impairment.
The first step is used to identify potential impairment and
involves comparing each reporting unit’s estimated fair
value to its carrying value, including goodwill. A reporting
unit is an operating segment or one level below an operating
segment (referred to as a component). The Company has determined
that each individual laboratory is a reporting unit. The second
step of the goodwill impairment process involves the calculation
of an implied fair value of goodwill for the laboratories which
step one indicated were impaired. Fair value is determined by
using an income approach, consistent with our valuation of
dental laboratories acquired in purchase business combinations.
The Company assessed goodwill impairment as of June 30,
2009 and determined that no impairment charge was required. The
Company’s impairment analysis incorporates forecasted
financial results and assumptions for its dental laboratories.
Although none of its laboratories were impaired at June 30,
2009, in the third quarter of 2009, the Company recorded an
impairment charge of $264,000, comprising the entire goodwill
balance of one of its smaller laboratories in the NDX
laboratories operating segment, due to a significant decrease in
revenues and operating income from those forecasted as of
June 30, 2009. During the fourth quarter of 2009, there
were no events that triggered an additional goodwill impairment
test or result in a charge to goodwill impairment expense. The
Company continues to closely monitor the financial results of
certain other laboratories in the NDX laboratories operating
segment in subsequent periods in comparison to forecasted
financial results and assumptions made at June 30, 2009 to
determine whether any other impairment charges are warranted for
these other laboratories. As of December 31, 2009, the
Company had $69,616,000 of goodwill remaining on its
consolidated balance sheet.
Trade
Names
Trade names, as acquired, are valued using the
relief-from-royalty valuation approach. Company practice is to
use existing and acquired trade names in perpetuity, and
consequently they have been treated as indefinite-lived
intangibles. While these assets are not subject to amortization,
they are tested for impairment on an annual basis, or as
circumstances may require. The Company uses the
relief-from-royalty valuation approach at each fiscal year end
to determine whether the trade names are impaired. Trade name
impairment charges resulted from a decline in forecasted revenue
at specific laboratories in comparison to revenue forecasts used
in previous valuation calculations. The Company recorded
impairment charges of $94,000, $44,000 and $361,000 in 2007,
2008 and 2009, respectively. Impairment charges are a component
of selling, general and administrative expense.
The changes in the carrying amount of trade names for the years
ended December 31, 2008 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Beginning of year
|
|
$
|
8,998,000
|
|
|
$
|
9,978,000
|
|
|
Trade names acquired during the year
|
|
|
1,100,000
|
|
|
|
—
|
|
|
Effects of exchange rate changes
|
|
|
(76,000
|
)
|
|
|
54,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names
|
|
|
10,022,000
|
|
|
|
10,032,000
|
|
|
Impairment charges and other adjustments
|
|
|
(44,000
|
)
|
|
|
(541,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Trade names — end of year, net
|
|
$
|
9,978,000
|
|
|
$
|
9,491,000
|
|
|
|
|
|
|
|
|
|
|
|
F-15
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Customer
Relationships
Acquired dental laboratories have customer relationships in
place with dentists within their market areas. The Company
recognizes customer relationship assets when established
relationships exist with customers through contract or other
contractual relationships such as purchase orders or sales
orders. Customer relationships are valued based on an analysis
of revenue and customer attrition data and amortized over their
useful life. The weighted-average amortization period for the
Dental Art acquisition completed in 2008 was 12 years. The
amounts assigned to customer relationships are amortized on a
straight-line basis over their useful lives. The Company has
determined that the straight-line method is appropriate based on
an analysis of customer attrition statistics.
| |
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Beginning of year, gross
|
|
$
|
7,993,000
|
|
|
$
|
9,439,000
|
|
|
Customer relationships acquired during the year
|
|
|
1,500,000
|
|
|
|
—
|
|
|
Effects of exchange rate changes
|
|
|
(54,000
|
)
|
|
|
35,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer relationships, gross
|
|
|
9,439,000
|
|
|
|
9,474,000
|
|
|
Less: Accumulated amortization
|
|
|
(3,229,000
|
)
|
|
|
(4,121,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Customer relationships, net — end of year
|
|
$
|
6,210,000
|
|
|
$
|
5,353,000
|
|
|
|
|
|
|
|
|
|
|
|
Amortization expense associated with customer relationships
totaled approximately $771,000, $811,000 and $892,000 for the
years ended December 31, 2007, 2008 and 2009, respectively,
and is recorded as operating expenses. Future amortization
expense of the current customer relationship balance will be
approximately:
| |
|
|
|
|
|
2010
|
|
$
|
894,000
|
|
|
2011
|
|
|
894,000
|
|
|
2012
|
|
|
825,000
|
|
|
2013
|
|
|
614,000
|
|
|
2014
|
|
|
483,000
|
|
|
Thereafter
|
|
|
1,643,000
|
|
|
|
|
|
|
|
|
|
|
$
|
5,353,000
|
|
|
|
|
|
|
|
Non-competition
Agreements
In connection with acquisitions, the Company has executed
non-compete agreements with certain individuals, ranging over
periods of 2 to 15 years. The weighted-average amortization
period, which is based on the estimated useful life of the
agreement, for the acquisition completed in 2008 was
7.5 years. The amounts assigned to non-competition
agreements are amortized on a straight-line basis over the
economic useful life of the agreement, and are recorded as
operating expenses.
F-16
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Beginning of year, gross
|
|
$
|
10,553,000
|
|
|
$
|
10,696,000
|
|
|
Non-competition agreements acquired during the year
|
|
|
150,000
|
|
|
|
—
|
|
|
Effects of exchange rate changes
|
|
|
(7,000
|
)
|
|
|
3,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-competition Agreements, gross
|
|
|
10,696,000
|
|
|
|
10,699,000
|
|
|
Less: Accumulated amortization
|
|
|
(9,112,000
|
)
|
|
|
(9,407,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Non-competition agreements, net
|
|
$
|
1,584,000
|
|
|
$
|
1,292,000
|
|
|
|
|
|
|
|
|
|
|
|
Amortization expense associated with non-competition agreements
totaled approximately $489,000, $303,000 and $296,000 for the
years ended December 31, 2007, 2008 and 2009, respectively.
Future amortization expense of non-competition agreements will
be approximately:
| |
|
|
|
|
|
2010
|
|
$
|
287,000
|
|
|
2011
|
|
|
247,000
|
|
|
2012
|
|
|
192,000
|
|
|
2013
|
|
|
170,000
|
|
|
2014
|
|
|
115,000
|
|
|
Thereafter
|
|
|
281,000
|
|
|
|
|
|
|
|
|
|
|
$
|
1,292,000
|
|
|
|
|
|
|
|
The following is a summary of the provision (benefit) for income
taxes:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Federal —
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
|
|
$
|
2,985,255
|
|
|
$
|
2,134,884
|
|
|
$
|
3,418,520
|
|
|
Deferred
|
|
|
(189,842
|
)
|
|
|
(851,093
|
)
|
|
|
(533,076
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,795,413
|
|
|
|
1,283,791
|
|
|
|
2,885,444
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
State —
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
|
|
|
860,500
|
|
|
|
646,717
|
|
|
|
1,168,660
|
|
|
Deferred
|
|
|
(25,700
|
)
|
|
|
(188,545
|
)
|
|
|
(105,688
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
834,800
|
|
|
|
458,172
|
|
|
|
1,062,972
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign —
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
|
|
|
237,848
|
|
|
|
240,108
|
|
|
|
251,460
|
|
|
Deferred
|
|
|
(7,848
|
)
|
|
|
(10,108
|
)
|
|
|
(21,460
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
230,000
|
|
|
|
230,000
|
|
|
|
230,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,860,213
|
|
|
$
|
1,971,963
|
|
|
$
|
4,178,416
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Through December 31, 2009, the Company has not provided
deferred income taxes on the undistributed earnings of its
foreign subsidiary because such earnings are intended to be
permanently reinvested outside the
F-17
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
U.S. Determination of the potential deferred income tax
liability on these undistributed earnings is not practicable. At
December 31, 2009, the Company had 1,511,000 of
undistributed earnings in its foreign subsidiary.
Deferred income taxes are comprised of the following at
December 31, 2008 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Deferred Tax Assets:
|
|
|
|
|
|
|
|
|
|
Non-compete agreements
|
|
$
|
900,252
|
|
|
$
|
811,491
|
|
|
Other liabilities
|
|
|
2,917,310
|
|
|
|
3,321,180
|
|
|
Vacation benefits
|
|
|
745,677
|
|
|
|
750,548
|
|
|
Inventory basis differences
|
|
|
251,687
|
|
|
|
234,018
|
|
|
Receivables basis differences
|
|
|
44,405
|
|
|
|
83,058
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
|
4,859,331
|
|
|
|
5,200,295
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred Tax Liabilities:
|
|
|
|
|
|
|
|
|
|
Depreciation differences
|
|
|
(2,146,150
|
)
|
|
|
(2,373,141
|
)
|
|
Intangible amortization differences
|
|
|
(7,635,082
|
)
|
|
|
(7,643,873
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax liabilities
|
|
|
(9,781,232
|
)
|
|
|
(10,017,014
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax asset/liability
|
|
$
|
(4,921,901
|
)
|
|
$
|
(4,816,719
|
)
|
|
|
|
|
|
|
|
|
|
|
A reconciliation between the provision for income taxes computed
at statutory rates and the amount reflected in the accompanying
statements of income is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
December 31,
|
|
December 31,
|
|
|
|
2007
|
|
2008
|
|
2009
|
|
|
|
Statutory federal income tax rate
|
|
|
35.0
|
%
|
|
|
34.0
|
%
|
|
|
34.0
|
%
|
|
State income tax, net of federal income tax benefit
|
|
|
4.9
|
|
|
|
27.6
|
|
|
|
6.9
|
|
|
Research and experimentation credit
|
|
|
—
|
|
|
|
(55.1
|
)
|
|
|
—
|
|
|
Non-deductible goodwill
|
|
|
—
|
|
|
|
151.3
|
|
|
|
0.9
|
|
|
Cash surrender value of life insurance
|
|
|
(.7
|
)
|
|
|
21.9
|
|
|
|
(.2
|
)
|
|
Domestic production deduction
|
|
|
(2.2
|
)
|
|
|
(13.2
|
)
|
|
|
(2.3
|
)
|
|
Other
|
|
|
(.2
|
)
|
|
|
13.6
|
|
|
|
2.3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effective income tax rate
|
|
|
36.8
|
%
|
|
|
180.1
|
%
|
|
|
41.6
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2007 the Company had recorded $2,033,000 of
unrecognized tax benefits and related interest and penalties of
$102,000. This liability related to ongoing tax filing positions
taken by the Company in its previously filed US Federal and
State tax returns. The Company determined this $2,135,000 did
not meet the recognition provisions of accounting for tax
uncertainties and no material adjustments were required upon
adoption. Interest and penalties, as appropriate, are recorded
as a component of the Company’s tax liability and tax
provision. Interest and penalties recorded for the year ended
December 31, 2009 were approximately $21,000.
F-18
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
A reconciliation of the beginning and ending amount of
unrecognized tax benefits, exclusive of accrued interest and
penalties of $154,000 at January 1, 2009 and $175,000 at
December 31, 2009, is as follows:
| |
|
|
|
|
|
Unrecognized tax benefits at January 1, 2008
|
|
$
|
2,033,000
|
|
|
Additions based on tax positions related to the current year
|
|
|
—
|
|
|
Subtractions for tax positions of prior years
|
|
|
(536,000
|
)
|
|
|
|
|
|
|
|
Unrecognized tax benefits at December 31, 2008
|
|
|
1,497,000
|
|
|
Additions based on tax positions related to the current year
|
|
|
—
|
|
|
Subtractions for tax positions of prior years
|
|
|
—
|
|
|
|
|
|
|
|
|
Unrecognized tax benefits at December 31, 2009
|
|
$
|
1,497,000
|
|
|
|
|
|
|
|
In 2008, tax benefits of $515,000 and the reversal of accrued
interest and penalties of $89,000 were recognized as a result of
the lapse of the statute of limitations for tax year 2002. The
remaining balances represent unrecognized tax benefits that
would decrease the Company’s effective tax rate upon
recognition. The Company believes that it is reasonably possible
unrecognized tax benefits of $1,497,000 and accruals for
interest and penalties of $86,000 will reverse in 2010 as a
result of the Internal Revenue Service examination of the
Company’s U.S. income tax returns for 2004 through
2006. Additionally, there is an amount included in prepaid
expenses related to advanced fees which will reverse when the
IRS audit is settled. As of December 31, 2009, the tax
years that remain subject to examination by the IRS and other
jurisdictions are 2004 to 2008.
|
|
|
(6)
|
Lines of
Credit and Term Loan Facility
|
On August 9, 2005, the Company entered into an amended and
restated financing agreement (the “Amended Agreement”)
with Bank of America, N.A. (the “Bank”). The Amended
Agreement included a revolving line of credit of $5,000,000, a
revolving acquisition line of credit of $20,000,000 and a term
loan facility of $20,000,000. The interest rate on both
revolving lines of credit and the term loan was the prime rate
or, at the Company’s option, LIBOR, a cost of funds rate or
the Bank’s fixed rate plus a range of 1.25% to 2.25% per
annum, depending on the ratio of consolidated funded debt to
consolidated “EBITDA,” as defined in the Amended
Agreement. The Amended Agreement required monthly payments of
principal, based on a seven-year amortization schedule, with a
final payment due on the fifth anniversary of the Amended
Agreement. The Amended Agreement required compliance with
certain covenants, including the maintenance of specified net
worth, income and other financial ratios.
In October 2006, the Company borrowed against its acquisition
line of credit to finance the acquisition of Keller Group,
Incorporated (“Keller”). In order to refinance the
borrowings incurred for the Keller acquisition, the Company and
the Bank executed a Second Amended and Restated Loan Agreement
as of November 7, 2006 (the “Second Agreement”)
comprised of uncollateralized senior credit facilities that at
that time totaled $60,000,000. The Second Agreement amended and
restated the Amended Agreement (a) to increase the term
loan facility to an aggregate principal amount of $35,000,000
and used the proceeds of the increase in the term loan to repay
the outstanding principal balance under the acquisition line of
credit and (b) to adjust the allocation of availability
under the lines of credit by increasing the revolving line of
credit to $10,000,000 ($5,000,000 of which may be used for
future acquisitions) and decreasing the acquisition line of
credit from $20,000,000 to $15,000,000. The interest rate on
both lines of credit and the term loan was the prime rate or, at
the Company’s option, LIBOR, a cost of funds rate or the
Bank’s fixed rate, plus, in each case, a range of 1.25% to
3.00% per annum, depending on the ratio of consolidated total
funded debt to consolidated “EBITDA,” as each is
defined in the Second Agreement. The term loan facility portion
of the Second Agreement requires monthly interest payments and
monthly payments of principal, based on a seven year
amortization schedule, with a final payment due on the fifth
anniversary of the Second Agreement. The Second Agreement
requires compliance with certain covenants, including the
maintenance of specified net worth, minimum consolidated total
“EBITDA,” debt to income ratio and other financial
ratios.
The Second Agreement was amended on May 9, 2008, effective
March 31, 2008, to revise certain financial targets within
these covenants. Additionally, the Bank and the Company agreed
to consolidate the revolving line of
F-19
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
credit with the acquisition line of credit into a single line of
credit of $25,000,000 to be used by the Company for general
corporate purposes, including potential acquisitions. The Second
Agreement was also amended on September 2, 2008 in
connection with the acquisition of Dental Art, which increased
the Company’s outstanding debt and therefore required an
adjustment to an affected financial covenant. The Company
further amended the agreement on December 16, 2008 to
extend the maturity of the line of credit to November 7,
2011. The amendment changed the interest rate on both the line
of credit and the term loan to prime rate or, at the
Company’s option, LIBOR, a cost of funds rate, or the
Bank’s fixed rate, plus, in each case, a range of 2.50% to
3.50% per annum, depending on the ratio of consolidated total
funded debt to consolidated “EBITDA,” as each is
defined in the Second Agreement and also increased the
commitment fee on the unused portion of the line of credit from
0.125% to 0.50% per annum. In addition, the amendment revised
certain financial targets within the covenants. Finally, on
March 13, 2009, the Second Agreement was further amended to
exclude $6,950,000 of goodwill impairment in the fourth quarter
of 2008 from the calculation of “EBITDA,” used in
determining compliance with certain financial covenants. These
amendments did not change the total availability under the
Second Agreement. While the Company was in compliance with its
debt covenants as of December 31, 2009, it continues to
closely monitor compliance with these covenants in future
periods, particularly minimum consolidated total
“EBITDA,” which may be negatively impacted by, among
other things, potential declines in future earnings, or declines
attributable to additional goodwill impairment.
As of December 31, 2009, $18,471,000 was available under
the consolidated revolving line of credit.
Long-Term Obligations:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Term note
|
|
$
|
24,583,000
|
|
|
$
|
19,583,000
|
|
|
Borrowings classified as long term under the revolving line of
credit
|
|
|
13,800,000
|
|
|
|
6,529,000
|
|
|
Borrowings classified as short term under the revolving line of
credit
|
|
|
2,940,000
|
|
|
|
—
|
|
|
Other long-term debt
|
|
|
875,000
|
|
|
|
736,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Total long-term debt
|
|
|
42,198,000
|
|
|
|
26,848,000
|
|
|
Less: current maturities
|
|
|
8,055,000
|
|
|
|
5,072,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt, less current portion
|
|
$
|
34,143,000
|
|
|
$
|
21,776,000
|
|
|
|
|
|
|
|
|
|
|
|
The table below reflects the expected repayment terms associated
with the long-term debt at December 31, 2009. The interest
rate associated with the Company’s borrowings as of
December 31, 2009 was 3.2%.
| |
|
|
|
|
|
|
|
December 31, 2009
|
|
|
|
|
Principal Due
|
|
|
|
|
Fiscal 2010
|
|
|
5,072,000
|
|
|
Fiscal 2011
|
|
|
21,187,000
|
|
|
Fiscal 2012
|
|
|
80,000
|
|
|
Fiscal 2013
|
|
|
85,000
|
|
|
Fiscal 2014
|
|
|
90,000
|
|
|
Thereafter
|
|
|
334,000
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
26,848,000
|
|
|
|
|
|
|
|
The Company has a qualified retirement plan under Internal
Revenue Code Sections 401(a) and 401(k) (the “401(k)
Plan”). The 401(k) Plan allows contributions of up to 10%
of a participant’s salary, a portion of which is
F-20
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
matched in cash by the Company. The Company contributes cash
once a year, within 120 days after December 31, the
401(k) Plan’s year-end. All employees are eligible to
participate in the 401(k) Plan after completing one year of
service with the Company and the attainment of age 21.
Participants are fully vested immediately in employee
contributions and become fully vested in the Company’s
matching contributions after six years of service or upon
attaining age 65. The Company has incurred charges to
operations of approximately $826,000, $849,000 and $847,000 to
match contributions for the years ended December 31, 2007,
2008 and 2009, respectively.
The Company had a cash incentive plan (the “Laboratory
Plan”) for dental laboratory management and other
designated key employees who directly influenced the financial
performance of an individual dental laboratory. Participant
eligibility was determined annually for each laboratory and each
participant was eligible to receive an amount based on the
achievement of certain earnings levels and other performance
metrics by the participant’s laboratory. The Company
incurred charges to operations of approximately $3,872,000 for
the year ended December 31, 2007 under the Laboratory Plan.
Beginning in 2008, a new incentive program was implemented to
provide incentives for growth in profits with participant
eligibility to be determined on an ongoing and discretionary
basis. The Company has incurred charges to operations of
approximately $591,000 and $1,879,000 for the years ended
December 31, 2008 and 2009, respectively, under this
program.
The Company has an executive bonus plan (the “Executive
Plan”) for key executives and management of the Company.
Eligibility to participate in this plan is determined annually.
Participants are eligible to receive a cash bonus, based on a
percentage of salary, dependent upon the achievement of earnings
targets, as defined. The bonus is distributed within
75 days after year-end. The Company has incurred aggregate
charges to operations of approximately $448,000, $50,000 and
$860,000, for the years ended December 31, 2007, 2008 and
2009, respectively, with respect to this plan.
The Company has established Supplemental Executive Retirement
Plans (“SERP”) for certain key employees. Benefits are
funded by life insurance contracts purchased and owned by the
Company. The retirement benefit is based on the value in the
individual life insurance policy grossed up for taxes and is
payable at the age of 65 or upon retirement, if later, in either
a lump sum or equal monthly installments over a period of
10 years. These benefits vest to the participating
employees over periods of up to ten years. In one particular
plan effective 2006, the retirement benefit is fixed at $125,000
per year for ten years, payable in equal monthly installments
upon the individual attaining the age of 75. This benefit vests
over five years. The charges to expense for the years ended
December 31, 2007, 2008 and 2009, were approximately
$727,000, $688,000 and $714,000, respectively and are recorded
as accrued liabilities.
|
|
|
(8)
|
Commitments
and Contingencies
|
Operating
Leases
The Company is committed under various non-cancelable operating
lease agreements covering its office space and dental laboratory
facilities and certain equipment. Certain of these leases also
require the Company to pay maintenance, repairs, insurance and
related taxes. The total rental expense for the years ended
December 31, 2007, 2008 and 2009 was approximately
$5,061,000, $4,888,000 and $4,778,000 respectively. The
approximate aggregate minimum lease commitments under these
operating leases as of December 31, 2009 are as follows:
| |
|
|
|
|
|
Year
|
|
Amount
|
|
|
|
|
2010
|
|
$
|
4,383,000
|
|
|
2011
|
|
|
3,691,000
|
|
|
2012
|
|
|
3,231,000
|
|
|
2013
|
|
|
2,459,000
|
|
|
2014
|
|
|
1,739,000
|
|
|
Thereafter
|
|
|
3,763,000
|
|
|
|
|
|
|
|
|
|
|
$
|
19,266,000
|
|
|
|
|
|
|
|
F-21
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Employment
Contracts and
Change-in-Control
Arrangements
In April 1995, January 2001, May 2004, October 2006 and August
2008 the Company entered into employment contracts and
change-in-control
arrangements with certain key executives. The initial term of
these employment contracts is three years and the contracts by
their terms renew automatically thereafter until termination by
the Company or the executive. The
change-in-control
arrangements provide certain severance benefits in the event
that the executive is terminated by the Company without cause or
the executive terminates his employment contract for certain
specified reasons.
The Company measures and recognizes in its consolidated
statement of income the expense associated with all share-based
payment awards made to employees and directors. Prior to
January 1, 2006, compensation cost was not recognized for
options granted because the exercise price of options granted
was equal to the market value of the Company’s common stock
on the measurement date and the Employees’ Stock Purchase
Plan (ESPP) was deemed non-compensatory. Our share-based
compensation programs include shares issued under our ESPP,
time-vested restricted stock and restricted stock units, and
incentive stock options issued under our 1992 Long Term
Incentive Plan (LTIP) and our Amended and Restated 2001 Stock
Plan (2001 Stock Plan). Compensation cost is measured on the
grant date of the option, which is the date the Company’s
Board of Directors approves the granting of the option.
Compensation cost on discounts associated with ESPP purchases is
estimated on the date that share rights are granted. To measure
the fair value of stock option grants, the Company utilizes the
Black-Scholes option valuation method. The requisite service
period for substantially all of the Company’s stock options
is the explicit vesting period included in the terms of the
stock option award. Accordingly, the Company estimates
compensation expense based on the number of options it believes
will ultimately vest, which includes an estimate of the number
of options expected to be forfeited. The estimated fair value of
stock options grants will be recognized on a straight line basis
over the requisite service period of the award. The Company
periodically reviews its estimate of forfeitures and revises the
estimate as facts and circumstances warrant. The following table
summarizes share-based compensation expense associated with each
of these programs:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Employee stock purchase plan
|
|
$
|
161,000
|
|
|
$
|
179,000
|
|
|
$
|
230,000
|
|
|
Restricted stock and restricted stock units
|
|
|
165,000
|
|
|
|
270,000
|
|
|
|
163,000
|
|
|
Performance-based stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Incentive stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
143,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total share-based compensation expense
|
|
$
|
326,000
|
|
|
$
|
449,000
|
|
|
$
|
536,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
No share-based compensation has been capitalized. Windfall tax
benefits from the exercises of stock options and ESPP options
and issuance of restricted stock and restricted stock units was
approximately $20,000, $35,000, and $183,000 for the years ended
December 31, 2007, 2008, and 2009 respectively. These
amounts have been calculated under the shortcut method.
Share-based
Compensation Plans
We have three share-based compensation plans pursuant to which
awards have been or are currently being made: (1) the 1992 LTIP,
as amended from which no additional options may be granted;
(2) the Amended and Restated 2001 Stock Plan from which
incentive stock options and restricted shares and share units
are granted; and (3) the Employees’ Stock Purchase
Plan (ESPP), as amended.
F-22
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Stock
Option Plans, Restricted Stock, and Restricted Stock
Units
In May 1992, the Company’s Board of Directors (the
“Board”) adopted the LTIP. Under the LTIP, the Board
may grant stock options, stock appreciation rights, restricted
stock, deferred stock, stock purchase rights and other
share-based payments to key employees, officers and directors of
the Company. As of May 2002, no additional options may be
granted under this plan. These options vested over three years
from date of grant and have a maximum term of ten years from the
date of grant.
In April 2001, the Company’s shareholders approved the 2001
Stock Plan, under which awards may be granted to key employees,
officers and directors in the form of stock options. The Board
reserved 450,000 shares of common stock for issuance under
the 2001 Stock Plan. In April 2004, the Board amended the 2001
Stock Plan to increase the number of shares of common stock
reserved for issuance under the plan from 450,000 to 825,000.
The Company’s shareholders approved an amendment to the
2001 Stock Plan on May 16, 2006 to allow for the issuance
of restricted stock and restricted stock units, subject to a
limit of 82,500. At December 31, 2009, options to purchase
a total of 590,586 shares were outstanding and 8,450
unvested restricted shares were outstanding. There were
89,051 shares available for grant under the 2001 Stock
Plan. Stock option awards granted under the 2001 Stock Plan
generally vest ratably over three years on the anniversary date
of the grants and are exercisable generally over a period of ten
years. Restricted stock and restricted stock units are granted
to directors and may vest anywhere from one to two years.
During 2008, the Company granted 275,000 performance-based stock
options, which would vest upon the achievement of specific
earnings per share targets during 2009, 2010 and 2011. The
Company has assumed that none of these performance-based awards
will vest and accordingly has not provided for compensation
expense associated with the awards. The Company periodically
evaluates the likelihood of reaching the performance
requirements and would be required to recognize aggregate
compensation expense of approximately $820,000 if the targets
for 2010 and 2011 are fully met. The fair value of the stock
option grants awarded was estimated as of the date of grant
using a Black-Scholes option valuation model that uses the
following weighted-average assumptions:
| |
|
|
|
|
|
Weighted-average grant date fair value
|
|
$
|
4.47
|
|
|
Risk-free interest rate
|
|
|
3.84
|
%
|
|
Expected volatility
|
|
|
30.19
|
%
|
|
Expected holding period in years
|
|
|
6.0
|
|
|
Expected dividends
|
|
|
None
|
|
The weighted average grant date fair value was calculated under
the Black-Scholes option-pricing model. The risk-free interest
rate is based on the yield of U.S. Treasury securities that
correspond to the expected holding period of the options. After
assessing all available information on either historical and
implied volatility, or both, the Company has concluded that a
combination of both historical and implied volatility provides
the best estimate of expected volatility. The expected holding
period of options granted is derived using assumed exercise
rates based on historical exercise patterns and represents the
period of time that options are expected to be outstanding. The
dividend yield was based on the Company’s expected dividend
rate.
During 2009, the Company granted 120,000 incentive stock
options. The estimated fair value of the options is recognized
over the options’ vesting periods. Of those 120,000
options, 90,000 options vest up to one third in 2010, 2011, and
2012 and 30,000 options vest up to one half in 2010 and 2011.
These options expire in April 2019. As of December 31,
2009, there was approximately $167,000 of unrecognized
compensation cost related to incentive stock options. That cost
will be recognized over a weighted average period of 10 months.
The fair value of the stock option grants awarded was estimated
as of the date of grant using the Black-Scholes option valuation
model using the following weighted-average assumptions:
| |
|
|
|
|
|
Weighted-average grant date fair value
|
|
$
|
2.58
|
|
|
Risk-free interest rate
|
|
|
2.91
|
%
|
|
Expected volatility
|
|
|
58.02
|
%
|
|
Expected holding period in years
|
|
|
8.0
|
|
|
Expected dividends
|
|
|
None
|
|
F-23
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
A summary of stock option activity and related information for
the year ended December 31, 2009 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LTIP
|
|
|
2001 Stock Plan
|
|
|
|
|
|
|
|
Weighted-
|
|
|
|
|
|
Weighted-
|
|
|
|
|
Shares
|
|
|
Average
|
|
|
Shares
|
|
|
Average
|
|
|
|
|
Underlying
|
|
|
Exercise
|
|
|
Underlying
|
|
|
Exercise
|
|
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
|
|
Outstanding December 31, 2008
|
|
|
154,330
|
|
|
$
|
12.18
|
|
|
|
563,000
|
|
|
$
|
13.25
|
|
|
Granted
|
|
|
—
|
|
|
|
—
|
|
|
|
120,000
|
|
|
|
4.07
|
|
|
Exercised
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Canceled
|
|
|
(3,405
|
)
|
|
|
11.52
|
|
|
|
(92,414
|
)
|
|
|
12.02
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding December 31, 2009
|
|
|
150,925
|
|
|
$
|
12.20
|
|
|
|
590,586
|
|
|
$
|
11.58
|
|
|
Exercisable at end of period:
|
|
|
150,925
|
|
|
$
|
12.20
|
|
|
|
287,250
|
|
|
$
|
14.45
|
|
The weighted-average remaining contractual term for options
exercisable as of December 31, 2009 was 1.6 years.
While, the intrinsic value of options exercised was $107,973 for
the year ended December 31, 2007, there was no intrinsic
value for options exercised or exercisable for the years ended
December 31, 2008 and 2009.
Time-vested
restricted stock and restricted stock units under the 2001 Stock
Plan
For the year ended December 31, 2009, there were no grants
of restricted stock or restricted stock units to our Board of
Directors. For the year ended December 31, 2008, the
Company granted 20,280 shares of restricted stock and
10,140 restricted stock units as directors’ fees under the
2001 Stock Plan, as amended in May 2006. For the year ended
December 31, 2007, the Company granted 9,002 shares of
restricted stock and 6,003 restricted stock units as
directors’ fees under the 2001 Stock Plan.
The following table summarizes restricted stock and restricted
stock unit awards for the year ended December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Stock
|
|
|
Restricted Stock Units
|
|
|
|
|
|
|
|
Weighted-Average
|
|
|
|
|
|
Weighted-Average
|
|
|
|
|
|
|
|
Grant Date Fair
|
|
|
|
|
|
Grant Date Fair
|
|
|
|
|
Number of Shares
|
|
|
Value
|
|
|
Number of Shares
|
|
|
Value
|
|
|
|
|
Nonvested December 31, 2006
|
|
|
1,596
|
|
|
$
|
22.55
|
|
|
|
3,192
|
|
|
$
|
22.55
|
|
|
Granted
|
|
|
9,002
|
|
|
|
17.99
|
|
|
|
6,003
|
|
|
|
17.99
|
|
|
Vested
|
|
|
1,596
|
|
|
|
22.55
|
|
|
|
3,192
|
|
|
|
22.55
|
|
|
Cancelled
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nonvested December 31, 2007
|
|
|
9,002
|
|
|
|
17.99
|
|
|
|
6,003
|
|
|
|
17.99
|
|
|
Granted
|
|
|
20,280
|
|
|
|
10.65
|
|
|
|
10,140
|
|
|
|
10.65
|
|
|
Vested
|
|
|
6,502
|
|
|
|
17.99
|
|
|
|
6,003
|
|
|
|
17.99
|
|
|
Cancelled
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nonvested December 31, 2008
|
|
|
22,780
|
|
|
|
11.46
|
|
|
|
10,140
|
|
|
|
10.65
|
|
|
Granted
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Vested
|
|
|
14,330
|
|
|
|
11.93
|
|
|
|
10,140
|
|
|
|
10.65
|
|
|
Cancelled
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nonvested December 31, 2009
|
|
|
8,450
|
|
|
$
|
10.65
|
|
|
|
—
|
|
|
$
|
—
|
|
As of December 31, 2009, there was $33,750 of total
unrecognized compensation cost for restricted stock. That cost
will be recognized over a weighted average period of
4.5 months. The total fair value of shares vested during
2007, 2008, and 2009 was $65,404, $134,405, and $172,447
respectively.
F-24
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Employees’
Stock Purchase Plan (ESPP)
Also, the Company has the ESPP, under which an aggregate of
450,000 shares of the Company’s common stock have been
reserved. Upon enrollment, employees purchase shares of the
Company’s common stock at the end of each plan year,
through payroll deductions, at a discount of 15% of the lower of
the market price on the date of grant or the date of exercise,
as quoted on NASDAQ. These shares may be purchased in the
current plan year, through a payroll deduction program, at a
price equal to 85% of the fair market value of the common stock
on either April 1, 2008 or March 31, 2009, whichever
is lower. Approximately 273,195 shares are available for
future purchases as of December 31, 2009. The number of
shares of common stock purchased through the Stock Purchase Plan
for 2007, 2008 and 2009 were 24,030, 33,375, and 93,102
respectively.
The following tables summarize the significant assumptions used
to estimate stock compensation costs for the ESPP for the
periods indicated:
| |
|
|
|
|
|
|
|
|
|
|
|
January 1-
|
|
April 1-
|
|
|
|
March 31,
|
|
December 31,
|
|
|
|
2009
|
|
2009
|
|
|
|
Weighted-Average Grant Date Fair Value
|
|
$
|
3.91
|
|
|
$
|
1.82
|
|
|
Risk-free Interest Rate
|
|
|
2.25
|
%
|
|
|
0.56
|
%
|
|
Expected Volatility
|
|
|
40.54
|
%
|
|
|
78.76
|
%
|
|
Expected Holding Period in Years
|
|
|
1.0
|
|
|
|
1.0
|
|
|
Expected Dividends
|
|
|
None
|
|
|
|
None
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
January 1-
|
|
April 1-
|
|
|
|
March 31,
|
|
December 31,
|
|
|
|
2008
|
|
2008
|
|
|
|
Weighted-Average Grant Date Fair Value
|
|
$
|
4.18
|
|
|
$
|
3.91
|
|
|
Risk-free Interest Rate
|
|
|
4.92
|
%
|
|
|
2.25
|
%
|
|
Expected Volatility
|
|
|
30.29
|
%
|
|
|
40.54
|
%
|
|
Expected Holding Period in Years
|
|
|
1.0
|
|
|
|
1.0
|
|
|
Expected Dividends
|
|
|
None
|
|
|
|
None
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
January 1-
|
|
April 1-
|
|
|
|
March 31,
|
|
December 31,
|
|
|
|
2007
|
|
2007
|
|
|
|
Weighted-Average Grant Date Fair Value
|
|
$
|
6.69
|
|
|
$
|
4.18
|
|
|
Risk-free Interest Rate
|
|
|
5.27
|
%
|
|
|
4.92
|
%
|
|
Expected Volatility
|
|
|
33.19
|
%
|
|
|
30.29
|
%
|
|
Expected Holding Period
|
|
|
1.0 Year
|
|
|
|
1.0 Year
|
|
|
Expected Dividends
|
|
|
None
|
|
|
|
None
|
|
F-25
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The risk-free interest rate is based on the yield of
U.S. Treasury securities that correspond to the expected
holding period of the options. The
1-year
historical volatility period was selected since that period
corresponds with the expected holding period. The expected
forfeiture rate was determined based on the historical ESPP
forfeiture data. The dividend yield was based on the
Company’s expected dividend rate.
The Company follows accounting standards for disclosing
information about reportable segments in financial statements.
Laboratory operating income includes the direct profits
generated by laboratories owned by the Company and excludes
general and administrative expenses of the Company’s
corporate location including amortization expenses associated
with the Company’s intangible assets as well as interest
expense.
In March 2005, the Company acquired Green Dental Laboratories,
Inc. of Heber Springs, Arkansas. In October 2006, the Company
acquired Keller Group, Incorporated, a privately-held dental
laboratory business with production facilities in both
St. Louis, Missouri and Louisville, Kentucky. The Company
has identified both Green and Keller as separate reportable
segments based on the quantitative criteria for financial
reporting purposes. All of the Company’s remaining
laboratories are included under the NDX Laboratories segment. As
a result, the Company has three reportable segments. The
accounting policies of this segment are consistent with those
described for the consolidated financial statements in the
summary of significant accounting policies.
F-26
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
The following table sets forth information about the
Company’s reportable segments for the years ended
December 31, 2007, 2008 and 2009. Prior to the fourth
quarter of 2006 the Company had two reportable segments and
prior to 2005 the Company had only one reportable segment.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
127,388,588
|
|
|
$
|
126,240,949
|
|
|
$
|
118,801,942
|
|
|
Green Dental Laboratory
|
|
|
19,859,770
|
|
|
|
20,724,865
|
|
|
|
19,169,497
|
|
|
Keller Group
|
|
|
23,843,093
|
|
|
|
25,987,992
|
|
|
|
24,248,404
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal
|
|
|
171,091,451
|
|
|
|
172,953,806
|
|
|
|
162,219,843
|
|
|
Inter-segment Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
|
370,913
|
|
|
|
438,200
|
|
|
|
328,783
|
|
|
Green Dental Laboratory
|
|
|
199,525
|
|
|
|
369,526
|
|
|
|
281,469
|
|
|
Keller Group
|
|
|
160,384
|
|
|
|
471,645
|
|
|
|
414,387
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Sales
|
|
$
|
170,360,629
|
|
|
$
|
171,674,435
|
|
|
$
|
161,195,204
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratory Operating Income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
17,572,491
|
|
|
$
|
15,059,508
|
|
|
$
|
16,318,096
|
|
|
Green Dental Laboratory
|
|
|
4,665,630
|
|
|
|
4,795,136
|
|
|
|
4,418,446
|
|
|
Keller Group
|
|
|
3,228,640
|
|
|
|
3,660,913
|
|
|
|
4,237,948
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
25,466,761
|
|
|
$
|
23,515,557
|
|
|
$
|
24,974,490
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
87,811,020
|
|
|
$
|
93,661,985
|
|
|
$
|
89,146,022
|
|
|
Green Dental Laboratory
|
|
|
26,756,893
|
|
|
|
26,140,524
|
|
|
|
25,758,671
|
|
|
Keller Group
|
|
|
25,769,930
|
|
|
|
25,634,168
|
|
|
|
25,132,647
|
|
|
Corporate
|
|
|
15,301,300
|
|
|
|
16,078,226
|
|
|
|
15,257,735
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
155,639,143
|
|
|
$
|
161,514,903
|
|
|
$
|
155,295,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital Expenditures:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
4,604,908
|
|
|
$
|
5,606,100
|
|
|
$
|
1,582,078
|
|
|
Green Dental Laboratory
|
|
|
463,763
|
|
|
|
161,415
|
|
|
|
34,073
|
|
|
Keller Group
|
|
|
756,581
|
|
|
|
420,628
|
|
|
|
86,039
|
|
|
Corporate
|
|
|
1,871,058
|
|
|
|
899,243
|
|
|
|
288,694
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
7,696,310
|
|
|
$
|
7,087,386
|
|
|
$
|
1,990,884
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation & Amortization on Property,
Plant & Equipment:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NDX Laboratories
|
|
$
|
2,579,090
|
|
|
$
|
3,114,183
|
|
|
$
|
2,853,540
|
|
|
Green Dental Laboratory
|
|
|
330,820
|
|
|
|
330,698
|
|
|
|
342,671
|
|
|
Keller Group
|
|
|
452,935
|
|
|
|
532,410
|
|
|
|
524,395
|
|
|
Corporate
|
|
|
691,769
|
|
|
|
894,591
|
|
|
|
848,332
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
4,054,614
|
|
|
$
|
4,871,882
|
|
|
$
|
4,568,938
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-27
NATIONAL
DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
Reconciliation of Laboratory Operating Income with reported
Consolidated Operating Income:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
|
|
Laboratory Operating Income
|
|
$
|
25,466,761
|
|
|
$
|
23,515,557
|
|
|
$
|
24,974,490
|
|
|
Less:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate Selling, General and Administrative Expenses
|
|
|
10,894,225
|
|
|
|
12,220,728
|
|
|
|
11,780,255
|
|
|
Amortization Expense — Intangible Assets
|
|
|
1,283,720
|
|
|
|
1,139,796
|
|
|
|
1,618,236
|
|
|
Goodwill Impairment
|
|
|
—
|
|
|
|
6,950,000
|
|
|
|
263,536
|
|
|
Add:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Expense
|
|
|
771,660
|
|
|
|
746,633
|
|
|
|
744,867
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated Operating Income
|
|
$
|
14,060,476
|
|
|
$
|
3,951,666
|
|
|
$
|
12,057,330
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(11) Subsequent
Events
The Company has assessed the impact of subsequent events and has
concluded that there were no such events that require adjustment
to the audited consolidated financial statements or disclosure
in the notes to the audited consolidated financial statements.
F-28
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the
Securities Exchange Act of 1934, the Registrant has duly caused
this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
NATIONAL DENTEX CORPORATION
David L. Brown, Chief Executive Officer
March 12, 2010
Pursuant to the requirements of the Securities Exchange Act of
1934, this report has been signed below by the following persons
on behalf of the registrant and in the capacities and on the
dates indicated.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ DAVID
V. HARKINS
David
V. Harkins
|
|
Director
|
|
March 12, 2010
|
|
|
|
|
|
|
|
/s/ JACK
R. CROSBY
Jack
R. Crosby
|
|
Director
|
|
March 12, 2010
|
|
|
|
|
|
|
|
/s/ THOMAS
E. CALLAHAN
Thomas
E. Callahan
|
|
Director
|
|
March 12, 2010
|
|
|
|
|
|
|
|
/s/ NORMAN
F. STRATE
Norman
F. Strate
|
|
Director
|
|
March 12, 2010
|
|
|
|
|
|
|
|
/s/ JAMES
E. MULVIHILL, D.M.D.
James
E. Mulvihill, D.M.D.
|
|
Director
|
|
March 12, 2010
|
|
|
|
|
|
|
|
/s/ DAVID
L. BROWN
David
L. Brown
|
|
Chairman and CEO
(Principal Executive Officer)
|
|
March 12, 2010
|
|
|
|
|
|
|
|
/s/ WAYNE
M. COLL
Wayne
M. Coll
|
|
Vice President & Chief Financial Officer (Principal
Financial Officer)
|
|
March 12, 2010
|
EXHIBIT INDEX
| |
|
|
|
|
|
Exhibit No.
|
|
Description of Exhibit
|
|
|
|
|
3
|
.1(2)
|
|
Restated Articles of Organization of the Company, filed with the
Massachusetts Secretary of State on October 14, 1993.
|
|
|
3
|
.2(2)
|
|
Articles of Amendment, filed with the Massachusetts Secretary of
State on September 26, 1995.
|
|
|
3
|
.3(17)
|
|
Amended and Restated By-Laws of the Company, as amended on
March 25, 2008.
|
|
|
10
|
.1(5)*
|
|
Amended & Restated 2001 Stock Plan, as amended on
May 16, 2006.
|
|
|
10
|
.1a(6)*
|
|
Form of Annual Director Fee Deferral and Restricted Stock/RSU
Subscription Agreement.
|
|
|
10
|
.1b(6)*
|
|
Form of Restricted Stock Unit Agreement for Employees and
Directors Under the Company’s Amended and Restated 2001
Stock Plan.
|
|
|
10
|
.1c(6)*
|
|
Form of Restricted Stock Agreement (Non-Employee Director).
|
|
|
10
|
.1d(18)*
|
|
Amended and Restated 2001 Stock Plan Form of Incentive Stock
Option Agreement for Employees.
|
|
|
10
|
.1e(18)*
|
|
Amended and Restated 2001 Stock Plan Form of Non-Qualified Stock
Option Agreement for Employees.
|
|
|
10
|
.1f(18)*
|
|
Amended and Restated 2001 Stock Plan Form of Restricted Stock
Agreement for Employees.
|
|
|
10
|
.1g(18)*
|
|
Amended and Restated 2001 Stock Plan Form of Restricted Stock
Unit Agreement for Employees.
|
|
|
10
|
.1h(25)*
|
|
Amended and Restated 2001 Stock Plan Form of Incentive Stock
Option Agreement for Employees with Extended Exercisability upon
Retirement.
|
|
|
10
|
.1i(25)*
|
|
Amended and Restated 2001 Stock Plan Form of Non-Qualified Stock
Option Agreement for Employees with Extended Exercisability upon
Retirement.
|
|
|
10
|
.2(20)*
|
|
Form of Amended and Restated Change of Control Severance
Agreement with David L. Brown, Richard F. Becker, Jr., and
Arthur B. Champagne.
|
|
|
10
|
.3(20)*
|
|
Form of Change of Control Severance Agreement with Wayne Coll
And John F. Green.
|
|
|
10
|
.4(1)*
|
|
1992 Long-Term Incentive Plan, as amended.
|
|
|
10
|
.5(1)*
|
|
Employment Agreement between the Company and Richard F. Becker,
Jr., dated April 1, 1995.
|
|
|
10
|
.5a(20)*
|
|
First Amendment to Employment Agreement between the Company and
Richard F. Becker, Jr., dated July 28, 2008.
|
|
|
10
|
.6(1)*
|
|
Employment Agreement between the Company and David L. Brown,
dated April 1, 1995.
|
|
|
10
|
.6b (20)*
|
|
First Amendment to Employment Agreement between the Company and
David L. Brown dated July 28, 2008.
|
|
|
10
|
.7(4)*
|
|
National Dentex Corporation Key Employee and Corporate Support
Group Incentive Compensation Plan.
|
|
|
10
|
.7a(26)*
|
|
Amended National Dentex Corporation Key Employee and Corporate
Support Group Incentive Compensation Plan.
|
|
|
10
|
.8(4)*
|
|
National Dentex Corporation Employees’ Stock Purchase Plan,
as amended effective April 4, 2000.
|
|
|
10
|
.8a(15)*
|
|
Second Amendment to National Dentex Corporation Employees’
Stock Purchase Plan.
|
|
|
10
|
.8b(27)*
|
|
Third Amendment to National Dentex Corporation Employees’
Stock Purchase Plan.
|
|
|
10
|
.9(9)
|
|
Second Amended and Restated Loan Agreement by and between Bank
of America, N.A., National Dentex Corporation and Green Dental
Laboratories, Inc. dated November 7, 2006.
|
|
|
10
|
.9a(14)
|
|
Amendment dated October 24, 2007 to Second Amended and
Restated Loan Agreement by and between Bank of America, N.A.,
National Dentex Corporation, and its subsidiaries listed therein.
|
|
|
10
|
.9b(13)
|
|
Loan Modification Agreement dated as of March 29, 2007 by
and between Bank of America, N.A., National Dentex Corporation,
Green Dental Laboratories, Inc., Keller Group, Inc., Keller
Laboratories, Incorporation — Midwest, and Keller
Laboratories, Incorporation — Southwest.
|
|
|
10
|
.9c(18)
|
|
Amendment No. 2 to Second Amended and Restated Loan
Agreement by and among Bank of America, N.A., National Dentex
Corporation and the subsidiaries of National Dentex Corporation
therein named.
|
|
|
10
|
.9d(21)
|
|
Amendment No. 3 to Second Amended and Restated Loan
Agreement by and between Bank of America, N.A., National Dentex
Corporation and the subsidiaries therein named dated
September 2, 2008.
|
| |
|
|
|
|
|
Exhibit No.
|
|
Description of Exhibit
|
|
|
|
|
10
|
.9e(22)
|
|
Amendment No. 4 to Second Amended and Restated Loan
Agreement by and between Bank of America, N.A., National Dentex
Corporation and the subsidiaries therein named dated
December 11, 2008.
|
|
|
10
|
.9f(26)
|
|
Amendment No. 5 to Second Amended and Restated Loan
Agreement by and between Bank of America, N.A., National Dentex
Corporation and the subsidiaries therein named dated
March 13, 2009.
|
|
|
10
|
.10(4)*
|
|
National Dentex Supplemental Executive Retirement Plan.
|
|
|
10
|
.10a(10)*
|
|
Amendment No. 1 to Supplemental Executive Retirement Plan
dated as of January 17, 2006.
|
|
|
10
|
.10b(10)*
|
|
Amendment No. 2 to Supplemental Executive Retirement Plan
dated as of January 17, 2006.
|
|
|
10
|
.10c(23)*
|
|
Amendment No. 3 to National Dentex Supplemental Executive
Retirement Plan, dated December 31, 2008.
|
|
|
10
|
.11(4)*
|
|
National Dentex Supplemental Laboratory Executive Retirement
Plan.
|
|
|
10
|
.11a(23)*
|
|
Amendment No. 2 to National Dentex Supplemental Laboratory
Executive Retirement Plan., dated December 31, 2008.
|
|
|
10
|
.12(3)
|
|
Stock Purchase Agreement by and among John W. Green IV, Richard
M. Nordskog and the Company dated as of March 1, 2005.
|
|
|
10
|
.13(7)*
|
|
Supplemental Executive Retirement Plan VI effective as of
August 11, 2006.
|
|
|
10
|
.13a(23)*
|
|
Amendment No. 1 to Supplemental Executive Retirement Plan
VI effective as of December 31, 2008.
|
|
|
10
|
.14(8)
|
|
Stock Purchase Agreement by and among William G. Keller, Thomas
A. Keller and the Company dated October 5, 2006.
|
|
|
10
|
.15*
|
|
Written Summary of Compensation Arrangements with Named
Executive Officers.
|
|
|
10
|
.16(24)*
|
|
Written Summary of Non-Employee Director Compensation
Arrangements.
|
|
|
10
|
.17(16)*
|
|
Retirement Agreement by and among National Dentex Corporation
and Donald Merz dated January 2, 2008.
|
|
|
21
|
(12)
|
|
Subsidiaries of the Company.
|
|
|
23
|
|
|
Consent of PricewaterhouseCoopers LLP.
|
|
|
31
|
.1
|
|
Certification pursuant to Section 302 of the Sarbanes-Oxley
Act (Chief Executive Officer).
|
|
|
31
|
.2
|
|
Certification pursuant to Section 302 of the Sarbanes-Oxley
Act (Chief Financial Officer).
|
|
|
32
|
.1
|
|
Certification pursuant to 18 U.S.C. Section 1350, as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act
(Chief Executive Officer).
|
|
|
32
|
.2
|
|
Certification pursuant to 18 U.S.C. Section 1350, as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act
(Chief Financial Officer).
|
|
Unless otherwise noted all exhibits are filed herewith. The file
number for our Exchange Act reports is
0-23092.
|
|
* These exhibits relate to a management contract or
to a compensatory plan or arrangement.
|
|
|
|
|
(1) |
|
Incorporated by reference from the
Form 10-K
for the fiscal year ended December 31, 2003 as filed with
the Commission on March 12, 2004. |
| |
|
(2) |
|
Incorporated by reference from the Annual Report on
Form 10-K
for the fiscal year ended December 31, 2004 as filed with
the Commission on May 24, 2005. |
| |
|
(3) |
|
Incorporated by reference from the Current Report on
Form 8-K/A
as filed with the Commission on January 26, 2006. |
| |
|
(4) |
|
Incorporated by reference from the Annual Report on
Form 10-K
for the fiscal year ended December 31, 2005 as filed with
the Commission on March 16, 2006. |
| |
|
(5) |
|
Incorporated by reference from the Proxy Statement filed on
Schedule 14A with the Commission on March 29, 2006. |
| |
|
(6) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on May 22, 2006. |
|
|
|
|
(7) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on August 14, 2006. |
| |
|
(8) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on October 6, 2006. |
| |
|
(9) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on November 8, 2006. |
| |
|
(10) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on December 12, 2006. |
| |
|
(11) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on January 29, 2007. |
| |
|
(12) |
|
Incorporated by reference from the
Form 10-K
for the fiscal year ended December 31, 2006 filed with the
Commission on March 13, 2007. |
| |
|
(13) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on April 2, 2007. |
| |
|
(14) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on October 30, 2007. |
| |
|
(15) |
|
Incorporated by reference from the Registrant’s
Registration Statement on
Form S-8
as filed on November 16, 2007. |
| |
|
(16) |
|
Incorporated by reference from the Current Report on
Form 8-K
as filed with the Commission on January 1, 2008. |
| |
|
(17) |
|
Incorporated by reference from the Current Report on
Form 8-K
filed with the Commission on March 27, 2008. |
| |
|
(18) |
|
Incorporated by reference from the Quarterly Report on
Form 10-Q
for the quarter ended March 31, 2008 as filed with the
Commission on May 12, 2008. |
| |
|
(19) |
|
Incorporated by reference from the Quarterly Report on
Form 10-Q
for the quarter ended June 30, 2008 as filed with the
Commission on August 8, 2008. |
| |
|
(20) |
|
Incorporated by reference from the Current Report on
Form 8-K
filed with the Commission on August 1, 2008. |
| |
|
(21) |
|
Incorporated by reference from the Current Report on
Form 8-K
filed with the Commission on September 8, 2008. |
| |
|
(22) |
|
Incorporated by reference from the Current Report on
Form 8-K
filed with the Commission on December 17, 2008. |
| |
|
(23) |
|
Incorporated by reference from the Current Report on
Form 8-K
filed with the Commission on January 6, 2009. |
| |
|
(24) |
|
Incorporated by reference from the
Form 10-K
for the fiscal year ended December 31, 2007 filed with the
Commission on March 12, 2008. |
| |
|
(25) |
|
Incorporated by reference from the Current Report on
Form 8-K
filed with the Commission on April 23, 2009. |
| |
|
(26) |
|
Incorporated by reference from the Annual Report on
Form 10-K
filed with the Commission on March 16, 2009. |
| |
|
(27) |
|
Incorporated by reference from the Proxy Statement filed on
Schedule 14A with the Commission on April 9, 2009. |