Attached files
| file | filename |
|---|---|
| 8-K - BIOMIMETIC THERAPEUTICS, INC. | v177133_8k.htm |
| EX-99.3 - BIOMIMETIC THERAPEUTICS, INC. | v177133_ex99-3.htm |
| EX-99.1 - BIOMIMETIC THERAPEUTICS, INC. | v177133_ex99-1.htm |

A Prospective, Randomized, Controlled, Multi-Center Pivotal
Human Clinical Trial to Evaluate the Safety and Effectiveness
of Augment™ Bone Graft as a Substitute for Autologous Bone
Christopher W. DiGiovanni, MD
Sheldon Lin, MD
Judith Baumhauer, MD
Timothy R. Daniels, MD
Alastair S. Younger, MD
Mark A. Glazebrook, MD
John G. Anderson, MD
Johnny T.C. Lau, MD
Peter Evangelista, MD
DaVinci

Investigator Acknowledgement
Nick Abidi
David Amirault
Eric Anctil
Jorge Acevedo
Robert Anderson
Donald Bohay
Wayne Berberian
Gregory Berlet
Christopher Bibbo
Bradley Brainard
Bruce Cohen
W. Hodges Davis
Jonathan Deland
Hugh Dougall
Jim DeOrio
Keith Donatto
Karl-Andre LaLonde
Ian Le
Thomas Lee
David Levine
Thomas Limbird
Richard Marks
John Maskill
Andrew Murphy
Steve Neufeld
Christopher Nicholson
James Nunley
William Obremskey
Martin O’Malley
Javid Parvis
Murray Penner
Terry Philbin
Mark Easley
Frankl Ebert
Andrew Elliott
Sam Flemister
Wm Granberry
Justin Greisberg
Steve Haddad
Christopher Hyer
Tony Hinz
Osarentin Idusuyi
Susan Ishikawa
Juha Jaakkola
Carroll Jones
Paul Juliano
David Katcherian
John Kennedy
Mickey Pinzur
Steve Raikin
Gr. Richardson
D. Richardson
Robt Rochman
Iain Russell
Lew Schon
James Sferra
Naomi Shields
RJ Sullivan
Michael Swords
Brian Thomson
Marc Tressler
Jaime Yun
Troy Watson
Kevin Wing

Study Background
FUSION well accepted Rx for severe arthritis of hindfoot/ankle
Conservatively, @ 10% incidence of delayed/non-union
rates: smokers, revisions, post-traumatics, AVN, co-morbidities
Autologous Bone Graft is frequently used in these fusions
Frey et al 1994, Easley et al 2000, Myerson et al 2000, Thordarson et al 2003, Haddad et al 2007

Are We Satisfied With Autograft?
Autograft Augmentation does work, BUT
Historical results ‘good’…certainly not great
Incur donor site pain and morbidity
Can add OR/recovery/anaesthesia time
How good is it, really? Variable quality and biologic activity
Limited supply
Cost
Why stand still? Promising Alternatives
Synthetics, Allografts
MSC technology, Platelet Gels
Growth Factors (eg. rhBMP, rhPDGF)

Augment™ Bone Graft
(rhPDGF-BB
+ ß-TCP)
Recombinant PDGF (rhPDGF-BB):
FDA approved for tissue regeneration applications
periodontal bone defects (GEM 21S®)
diabetic foot ulcers (Regranex™)
Previously evaluated as bone graft substitute
pilot studies: foot/ankle, wrist
Most recently: Augment™ Bone Graft studied in
pivotal clinical trial vs. ABG during hindfoot/ankle fusion

Augment™ North American Pivotal Trial
434 Randomized Patients, 37 centers (US/CAN)
Level I, Prospective, RCT
HYPOTHESIS: Augment
™ is non-inferior to ABG when used as a healing
adjunct during hindfoot and ankle fusion surgery
1° ENDPOINT: % patients fused by CT scan at 6 mos, as evaluated by
blinded
radiologist review (fusion defined as = 50% osseous bridging)
2° ENDPOINTS: Safety: AEs, SAEs, Surgical Complications
Efficacy: XR union, Clinical ‘healing’,
Composite success,
Delayed/Non-Union, Pain with WB, Graft site pain,
AOFAS Score, FFI Score, SF-12

Study Protocol
2:1 randomization, Augment ™: Autogenous
Autograft site @ surgeon discretion (IC, Gerdy’s, distal tib, calc)
Std joint preparation, ORIF using ≤ 3 screws/joint
Post-op immobilization for 12 wks, NWB first 6 wk
Standardized, high definition CT data
Independent CT evaluation: 9, 16, 24, 36 wks
Clinical assessment: pain, QOL, functional outcomes, (XRs):
1-3, 6, 9, 12, 16, 24, 36 and 52 wks
Serum screening for Ab formation: all pts (pre and post surgery)
Non-inferiority statistical analysis

STUDY SUMMARY
Substantial percentage of study patients (75%) had one or more risk factors
for poor healing (e.g. Diabetes, Smoking, Obesity, Prior Surgery)
Rigorous CT scan assessment as the 1° endpoint for bone healing
Results for radiologic, clinical, and functional assessments demonstrated
comparability to both autograft as well as published studies on
hindfoot/ankle fusion
Augment™ Bone Graft (rhPDGF –BB/ß-TCP) is a safe and effective
alternative to autograft

Patient Disposition and Evaluability
Screened Population
n=457 (456*)
*Less one subject screened twice
Randomized Population
n=435
ITT Population
n=434
Augment (285)/Autologous (149)
mITT Population
n=397
Augment (260)/Autologous (137)
Not randomized
n=21
Not randomized
prior to treatment
n=1
Excluded from mITT
n=17
Augment (12)/Autologous (5)
Safety Population
(Treated), n=414
Augment (272)/Autologous (142)
Not treated
n=20
Augment (13)/Autologous (7)

Demographics
All Patients
n= 434
Augment™
n= 285
Autograft
n=149
Sex (M/F)
49.8% / 49.1%
46.3% / 52.3%
56.4% / 43.0%
Age (Mean)
56.6 years
56.2 years
57.5 years
BMI (Mean)
30.8
30.7
31.1
Diagnosis
Post Traumatic OA
48.2%
48.8%
47.0%
Primary Arthritis
34.3%
32.6%
37.6%
Rheumatoid
6.7%
8.4%
3.4%
Other
9.7%
8.8%
11.4%
Risk Factors
Obesity (BMI>30)
48.4%
46.3%
52.3%
Smoking History
24.2%
24.9%
22.8%
Prior Surgery
23.3%
22.8%
24.2%
Diabetes
12.0%
11.2%
13.4%

Clinical Example, Ankle

Radiographic Assessment
CT Assessment
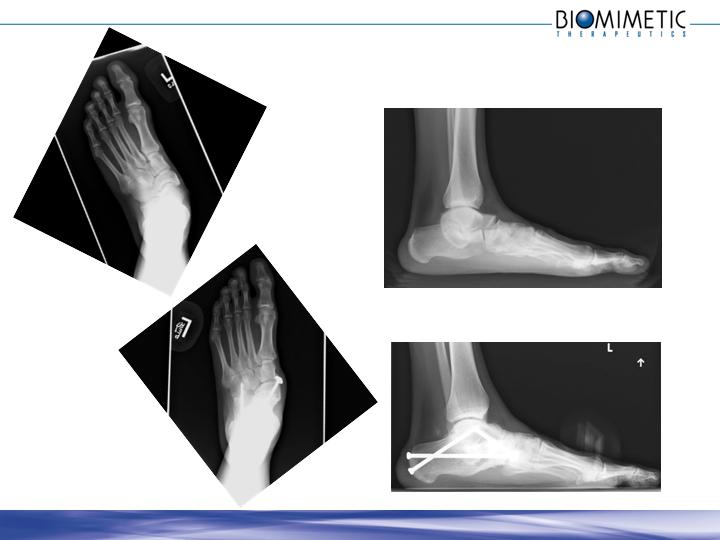
Example, Foot
13

6 mos CT
Assessment
14

Augment
(rhPDGF-BB/ß-TCP)
Autograft
Significance
P-value
(non-
inferiority)
Subjects
260
137
-
-
Joints
394
203
-
-
CT Fusion Rate ( 1º endpoint)
Full complement of joints
61.2%
62.0%
ND
0.038
All joints
66.5%
62.6%
ND
<0.001
Radiographic Fusion (3 aspects)
Full complement of joints
30.8%
32.8%
-
0.054
All joints
38.3%
37.9%
ND
0.007
Radiographic Fusion (2 aspects)
Full complement of joints
60.8%
66.4%
-
0.194
All joints
67.5%
70.9%
ND
0.049
Results @ 6 months: Radiographic Endpoints

Augment
(rhPDGF-BB/ß-TCP)
Autograft
Significance
P-value
(non-
inferiority)
Subjects
260
137
-
-
Joints
394
203
-
-
Clinically Healed (PI Assess)
83.1%
83.9%
ND
0.010
XR, Full Complement
82.3%
83.2%
ND
0.011
XR, All Joints
83.5%
83.3%
ND
<0.001
Delayed Union/Nonunion
8.8%
10.2%
ND
0.008
Composite Success
66.9%
66.4%
ND
0.017
Clinical Success
74.6%
78.1%
-
0.071
Therapeutic Failure
9.2%
10.9%
ND
<0.001
Results @ 6 months: Clinical Endpoints

Augment
(rhPDGF-BB/ß-TCP)
Autograft
Significance
P-value
(non-
inferiority)
Subjects
260
137
-
-
SF-12 (Mean PCS)
39.9
41.1
ND
<0.001
Foot Fusion Index (Mean Total)
27.4
22.3
ND
0.012
AOFAS Ankle-Hindfoot Scale
73.9
75.9
ND
<0.001
VAS Pain Scores
Fusion Site
18.9
16.5
ND
0.001
Weight Bearing
23.5
19.3
ND
0.016
Graft Site
N/A
8.1
<0.001
<0.001
Results @ 6 months: Functional/Pain Endpoints

Literature Comparison
Effectiveness Endpoint
Study Population
Literature
Reported
Rates
Augment™
Autograft
(control)
CT Fusion Rate
(6 months)
61.2%
62.0%
64%1
Nonunion/Delayed Union
8.8%
10.2%
10%2
16%3
41%4
AOFAS Hindfoot/Ankle
Overall Score
73.9
75.9
70.03
75.62
1. Coughlin MJ et al, Comparison of Radiographs and CT Scans in the Prospective Evaluation of the Fusion of Hindfoot Arthrodesis. Foot Ankle Int 2006,; 27(10): 780-7.
2. Haddad SL et al, Intermediate and Long-Term Outcomes of Total Ankle Arthroplasty and Ankle Arthrodesis: A Systematic Review of the Literature.
J Bone Joint Surg Am 2007; 89: 1899-905.
3. Easley M et al, Isolated Subtalar Arthrodesis, JBJS 2000; 82-A(5): 613-624.
4. Frey C, Halikus N, Vu-Rose T, Ebramzadeh, E: A Review of Ankle Arthrodesis: Predisposing Factors to Nonunion, FAI 1994; 15(11):581-584.

Augment
(rhPDGF-BB/ß-TCP)
Autograft
P-value
(Fisher Exact
Test)
Subjects
272
142
Pre-Tx Signs and Symptoms
3.7%
2.8%
0.779
Tx Emergent Adverse Events (TEAE)
72.4%
70.4%
0.730
Serious TEAE
7.7%
14.1%
0.055
Complications (Overall)
33.5%
38.0%
0.386
Complications (Surgically Related)
23.5%
29.6%
0.193
Complications (Serious)
4.4%
6.3%
0.480
Complications (Serious, Surgical)
4.0%
6.3%
0.337
Infections
7.7%
9.9%
0.462
Results @ 6 months: Safety Outcomes

Results Summary: Statistically Significant Non-Inferiority
Primary Endpoint:
CT Fusion Rate, Full Complement of Joints
CT Fusion Rate, All Joints
Secondary Endpoints:
XR union (3 aspects) for all joints
XR union (2 aspects) for all joints
Clinical healing: full comp/all joints
Non-Union/Delayed Union
Therapeutic Failure Rate
Composite Success Rate
SF-12 mean PCS
FFI mean total score
AOFAS mean total score

Perspective on Autograft Morbidity
Safety benefits trended in favor of Augment ™ group
Serious treatment emergent adverse events
Complication rates
Surgical complications
Infection (including one serious infection at ABG harvest site)
Augment ™ Bone Graft treated pts had no harvest site morbidity
55% ABG group @ wk 24 reported some pain at graft harvest site
12% clinically significant pain

Discussion
In trying to improve upon the many challenges of bone healing, we need to
pay more attention to basic bone biology
Currently there are few alternatives that have been shown to be equivalent
to autograft
in large, randomized controlled trials — and none
that are
indicated for use in the foot and ankle
There remains an existing need to eliminate morbidity associated with
autograft harvest
The results of this study establish Augment™ Bone Graft as a safe and
effective
alternative to autograft for ankle and hindfoot fusion surgery

Study Context
This study represents
Largest prospective RCT ever performed in the subspecialty of Foot and Ankle
First to use rigorous, independent, CT analysis as the 1° endpoint
Thanks to many people, and many AOFAS members
Sufficiently powered to test the hypothesis of non-inferiority to autograft
The results for plain XR healing, clinical assessment, and delayed/non-union
are comparable to published studies on hindfoot and ankle fusion
Even with these data, a substantial percentage of study patients (75%) had
one or more risk factors for poor healing

Osseous Bridging on CT as a Predictor of Clinical Healing
Osseous bridging =50% a strong predictor of clinical success
96% of patients with =50% osseous bridging on CT scan achieved
successful clinical healing (per investigator)
Failure to achieve =50% osseous bridging doesn’t necessarily
imply clinical failure
61% of patients with <50% osseous bridging on CT scan achieved
successful clinical healing (per investigator)
50% osseous bridging is a rigorous, sensitive, and
specific predictor of clinical healing

CONCLUSIONS
Augment ™
Bone Graft demonstrated statistical significance for
non-inferiority to autograft
Primary radiologic endpoint (CT fusion rate)
Key secondary clinical functional, & pain endpoints
Augment ™ patients experienced fewer adverse events &
complications
Radiologic, clinical, and functional assessments demonstrated
comparability to ABG and historic hindfoot/ankle fusion data
The results of this study establish Augment™ Bone Graft as a safe
and effective alternative to autograft
