Attached files
| file | filename |
|---|---|
| EX-23 - EX-23 - ATS MEDICAL INC | c56845exv23.htm |
| EX-21 - EX-21 - ATS MEDICAL INC | c56845exv21.htm |
| EX-32.2 - EX-32.2 - ATS MEDICAL INC | c56845exv32w2.htm |
| EX-31.1 - EX-31.1 - ATS MEDICAL INC | c56845exv31w1.htm |
| EX-12.1 - EX-12.1 - ATS MEDICAL INC | c56845exv12w1.htm |
| EX-32.1 - EX-32.1 - ATS MEDICAL INC | c56845exv32w1.htm |
| EX-31.2 - EX-31.2 - ATS MEDICAL INC | c56845exv31w2.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2009
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 0-18602
ATS MEDICAL, INC.
(Exact name of registrant as specified in its charter)
| Minnesota | 41-1595629 | |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) | |
| incorporation or organization) |
| 3905 Annapolis Lane North Minneapolis, Minnesota |
55447 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (763) 553-7736
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common stock, $.01 par value | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of
the Securities Act.
o Yes þ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or
Section 15(d) of the Act.
o Yes þ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by
Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for
such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days.
þ Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant
to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files):
o Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is
not contained herein, and will not be contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated
filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act
(Check one):
| Large accelerated filer o | Accelerated filer þ | Non-accelerated filer o | Smaller reporting company o | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Act).
o Yes þ No
The aggregate market value of voting and non-voting stock held by non-affiliates of the registrant
as of June 30, 2009, was approximately $156,726,088 (based on the last sale price of such stock as
reported by the NASDAQ Global Market on such date).
The number of shares outstanding of the registrant’s common stock, $.01 par value per share, as of
February 26, 2010, was 78,353,218 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Pursuant to General Instruction G, the responses to
Items 10, 11, 12, 13 and 14 of Part III of this Form 10-K
are incorporated herein by reference to certain
information contained in the registrant’s definitive Proxy
Statement for its 2010 Annual Meeting of
Shareholders.
TABLE OF CONTENTS
Table of Contents
PART I
ITEM 1. BUSINESS
OVERVIEW
ATS Medical, Inc. (hereinafter the “Company,” “ATS,” “we,” “us,” or “our”) is a Minnesota
corporation established in 1987. Our common shares are traded on the NASDAQ Global Market under
the symbol ATSI.
We develop, manufacture, and market medical devices for the treatment of structural heart disease.
Our product offerings are focused on heart valve therapy and the surgical treatment of cardiac
arrhythmias. Our core mission is to build a company with a diversified product portfolio focused
exclusively on the cardiac surgeon. Our objective is to establish ATS products as the standard of
care for patients with structural heart disease.
From our founding until 2004, 100% of our revenue was from our legacy product – a mechanical heart
valve. Sales of our mechanical heart valves represented approximately 60% of our revenue in 2009.
Beginning in 2004, we began to execute a diversification strategy and since have added several
product lines through distribution agreements or acquisitions. The most significant of these are:
| • | Surgical Treatment of Cardiac Arrythmias. In 2004, we entered into a distribution agreement with CryoCath Technologies, Inc. (“CryoCath”) to distribute a set of products for the surgical treatment of cardiac arrythmias, the most prevalent of which is atrial fibrillation. In 2007, we acquired this business from CryoCath. We have increased revenue from these products to approximately $18.9 million (25% of total revenue) in 2009, compared to $4.6 million (11% of total revenue) in 2006. | ||
| • | Heart Valve Repair. In 2005, we entered into a development and distribution agreement with Genesee BioMedical, Inc. (“GBI”), under which we co-developed a novel line of mitral valve repair rings and bands. We have increased revenue from these products to approximately $4.6 million (6% of total revenue) in 2009, compared to $1.1 million (less than 3% of total revenue) in 2006. | ||
| • | Tissue Heart Valves. In 2006, we acquired 3F Therapeutics, Inc. (“3F Therapeutics” or “3F”), which had developed a line of tissue heart valves based on a unique tubular design. U.S. Food and Drug Administration (“FDA”) pre-market approval of our first generation tissue valve was received in October 2008. European CE Mark approval on our first minimally invasive sutureless platform product was received in December 2009. In addition, a minimally invasive “beating heart” valve replacement platform is in development with human feasibility studies planned for 2010. Tissue heart valve revenues have increased to approximately $5.6 million (7% of total revenue) in 2009, compared to $0.2 million (less than 1% of total revenue) in 2006. |
The marketing and sales of these new non-mechanical valve products leverage our sales and marketing
infrastructure and broaden our relationships with cardiac surgeons. Sales from these and other new
non-mechanical valve products have grown over the last five years from no revenue in 2004 to
approximately 40% of our total revenue in 2009.
Net sales by product group for 2009, 2008 and 2007 are discussed in Item 7 of this Form 10-K.
BUSINESS STRATEGY
The key components of our business strategy to create a profitable, diversified, cardiac
surgery-focused company include:
| • | Increase market share of all our core products, including the ATS Open Pivot® Heart Valve, ATS CryoMaze® Surgical Ablation products, ATS Simulus® annuloplasty repair rings and bands and the ATS 3f® Aortic Bioprosthesis and ATS 3f Enable® Aortic Bioprosthesis tissue heart valves. |
1
Table of Contents
| • | Develop and introduce heart valve therapy and surgical ablation products that enable less invasive surgery without compromising outcomes compared to traditional therapies. | ||
| • | Broaden our relationships with cardiac surgeons by selectively adding new medical devices to our product portfolio. | ||
| • | Leverage our investments in our marketing and sales infrastructure. |
OUR MARKETS AND PRODUCTS
Heart Valve Therapy
Heart valve therapy revenue consists of prosthetic heart valves, heart valve repair products and
allograft tissue valves, which we discontinued selling at the end of 2007. These products
primarily relate to the repair or replacement of the aortic or mitral heart valves. For 2009,
heart valve therapy revenue was $56.0 million, or 74% of revenue, compared to $47.6 million, or 72%
of revenue in 2008 and $38.6 million, or 78% of revenue, in 2007. We estimate that the current
total worldwide heart valve therapy market approximates $1.5 billion. The heart valve therapy
market has two segments: 1) the established conventional surgical market which involves placing the
patient on cardio pulmonary bypass and removing the diseased valve and 2) the emerging market for
beating heart valve replacement for patients considered too sick for surgery. The conventional
surgical market is estimated to be approximately $1.3 billion and the beating heart valve market is
estimated to be approximately $220 million.
Prosthetic Heart Valve Market
Overview. There are two types of prosthetic heart valves: mechanical and tissue. Mechanical
valves are made from highly durable materials such as metals and pyrolytic carbon with implant
longevity well in excess of any patient’s lifetime. Tissue valves are made primarily from animal
or cadaver tissue. Tissue valves have a finite durability and over time may experience structural
valve deterioration requiring a re-operation to replace the failing valve. Tissue valves are
typically prescribed for patients less able to tolerate anti-coagulants, those who have a life
expectancy less than the projected longevity of tissue valves, or women in their childbearing
years.
Cardiac surgeons choose a valve through consideration of valve selection criteria and a patient’s
life expectancy, medical conditions, and lifestyle preferences. Besides durability, a valve’s
design and materials determine its thrombogenicity, which is the tendency to contribute to the
formation of thrombus or blood clots. Thrombus can impair the performance of a valve. If the
thrombus detaches and begins to move through the bloodstream (embolus), it may create an arterial
blockage leading to stroke or infarction. Mechanical valve recipients must take anticoagulants to
reduce and control thrombogenicity, while tissue valves do not usually require anticoagulant
therapy. Hemodynamics, the measure of how efficiently blood flows through a prosthetic valve, is
an important selection criteria. Blood must flow easily through the valve with minimal pressure
required to open the valve leaflets and limited backflow of blood when the leaflets close. The
valve should exert minimal force on the blood so that damage to fragile blood cells is limited.
Other factors that are important in a surgeon’s choice of a prosthetic valve are the ease of
implantation, patient quality of life and the physician’s familiarity with and confidence in the
valve.
In addition to cardiac surgeons, administrators or business managers at hospitals and clinics have
become increasingly influential in the purchase decision-making process in recent years. The
increasing emphasis on medical cost containment in most world markets has elevated the
decision-making power of the administrator. The administrator tends to focus on cost-effectiveness
of the prosthesis compared to alternatives and, in some markets, primarily on the cost of the
valve.
Over time, the mix between mechanical and tissue prosthesis has varied significantly. We estimate
that the current worldwide market for prosthetic heart valves is approximately $1.3 billion and in
aggregate is projected to grow at 6% per year. We also estimate that the current prosthetic heart
valve market consists of a worldwide mechanical heart valve market of approximately $313 million
declining at about 1 to 2% per year, a worldwide tissue heart valve market of approximately $810
million growing at 5% per year, and a worldwide beating heart valve replacement market of
approximately $220 million growing at greater than 50% per year.
2
Table of Contents
Our Mechanical Heart Valve Products. Our ATS Open Pivot Heart Valve was designed to improve upon
existing mechanical heart valves by combining a proprietary open pivot design and other innovative
features with the widely accepted biocompatibility and durability of pyrolytic carbon.
The major design features of the ATS Open Pivot Heart Valve include:
| • | Open pivot areas that are exposed to the washing action of flowing blood with each cardiac cycle | ||
| • | A thin but durable pyrolytic carbon orifice surrounded by a titanium strengthening band | ||
| • | Low profile design | ||
| • | Multiple sewing cuff options | ||
| • | Bileaflet valve design | ||
| • | Enhanced radiopacity |
The ATS Open Pivot Heart Valve provides the following advantages over other currently available
mechanical heart valves:
| • | Open pivot washing contributes to low thromboembolic complications | ||
| • | Improved patient quality of life through lower noise levels | ||
| • | Improved hemodynamic efficiencies | ||
| • | Ease of implantation and valve rotation | ||
| • | Improved follow-up diagnostic capability |
We have a development project called “ForceField” which utilizes electrical fields present at the
time a valve (or other device) is introduced into the blood stream to create a biocompatible
interface between the valve and the patient’s blood. We believe that by enhancing, accelerating
and modifying the naturally occurring protein layers, the valve will look natural to blood as it
passes through the valve, thus inhibiting platelet aggregation that may later lead to embolism or
blood clots. We have performed several preclinical tests of this technology and have developed the
commercial product configuration and our clinical strategy. We expect to begin clinical studies of
ForceField treated mechanical heart valves in 2010. Ultimately, if the ForceField technology is
successful, we would have the only mechanical valve that does not require concomitant
anti-coagulation therapy as well as a platform technology which could be applied to other
conductive medical devices.
CarboMedics, Inc. (“CarboMedics,” f/k/a Sulzer CarboMedics) developed the basic design from which
the ATS Open Pivot Heart Valve evolved. In 1990 we entered into a license agreement with
CarboMedics under which we eventually held an exclusive, royalty-free, worldwide license to
CarboMedic’s open pivot, bileaflet mechanical heart valve design. After making some design changes
in the valve, we finalized the design of the ATS Open Pivot Heart Valve and filed and received our
own U.S. patent covering the design of the ATS Open Pivot Heart Valve. The design modifications
and the resulting U.S. patent covering the new design are the exclusive property of ATS.
In connection with the execution of the license agreement, we were also required to enter into a
long-term supply agreement with CarboMedics under which we were obligated to purchase pyrolytic
carbon components for the ATS Open Pivot Heart Valve from CarboMedics. In 1999 we entered into a
carbon technology agreement with CarboMedics under which we obtained an exclusive, worldwide right
and license to use CarboMedics’ pyrolytic carbon technology to manufacture components for the ATS
Open Pivot Heart Valve. Under the agreement, CarboMedics also assisted us in establishing our own
pyrolytic carbon component production facility in Minneapolis, Minnesota.
Our Tissue Heart Valve Products. In 2006, ATS Medical acquired 3F Therapeutics, a medical device
company based in Lake Forest, California. 3F was an early stage privately-held medical device
company at the forefront of the emerging field of minimally invasive stopped and beating heart
tissue valve replacement. The acquisition of 3F was a major step in the execution of our
long-standing vision of obtaining a leadership position in the major segments of the cardiac
surgery market.
Our first generation tissue valve product, the ATS 3f Aortic Bioprosthesis, is a biological
replacement aortic heart valve that has demonstrated characteristics that compare favorably with
other biological valves presently in the market. The ATS 3f Aortic Bioprosthesis received the CE
Mark in 2004 for commercial
release in Europe and other foreign countries. We received FDA approval to market this product in
the United States in October 2008.
3
Table of Contents
The major design features of the ATS 3f Aortic Bioprosthesis include:
| • | Tubular valve design, which mimics a native aortic valve in form and function | ||
| • | Stentless tissue valve design | ||
| • | Pericardial tissue |
The ATS 3f Aortic Bioprosthesis provides the following advantages over other currently available
tissue valves:
| • | Low pressure gradients | ||
| • | Large effective orifice areas | ||
| • | Maintains continuity between the annulus and the sinotubular junction | ||
| • | Preserves the aortic sinuses | ||
| • | Restores physiologic flow | ||
| • | Restores native valve stress distribution | ||
| • | No long-term anticoagulation required |
We believe that substantial growth in the future within the heart valve industry will be the result
of the introduction of minimally invasive and off-pump beating heart valve replacement products.
To address this future demand, we are currently developing minimally invasive and beating heart
aortic valve products.
Our first product in this arena is the sutureless ATS 3f Enable Aortic Bioprosthesis, which is
intended to enable less invasive aortic valve replacement and reduce cardio-pulmonary bypass time.
The Enable Aortic Heart Valve is the ATS 3f Aortic Bioprosthesis mounted on a nitinol
self-expanding frame. The Enable valve is designed to eliminate the traditional suturing required
to replace a patient’s diseased aortic heart valve. If suturing can be eliminated from the
procedure, surgeons can potentially reduce procedure time and offer less invasive options for the
treatment of aortic valve disease. In addition, the elimination of suturing offers the potential to
significantly improve valve related hemodynamics by allowing the surgeon to provide a replacement
valve of a size larger than what is traditionally possible with conventionally sutured heart
valves. We received European CE Mark approval for commercial release of the Enable valve in
December 2009.
We are also developing an aortic tissue valve for use in beating heart procedures based in part on
the characteristics of the next generation of the Enable valve. First in-human studies of this
technology is targeted for 2010, and if successful, commercialization of a beating heart solution
could occur within one to two years thereafter. An Enable valve that is compatible in beating
heart procedures would enlarge our market opportunity by providing a solution for those patients
who are poor candidates to endure conventional surgery or prefer a more minimally invasive
solution.
Prosthetic Heart Valve Market Competition. The prosthetic heart valve market is highly competitive
with St. Jude Medical, Inc. as the mechanical valve market share leader and Edwards Lifesciences
Corporation as the tissue valve market leader. Other companies that sell mechanical valves include
Medtronic, Inc., CarboMedics, Sorin Biomedica (only outside the United States), and Medical Carbon
Research Institute LLC. Other companies that sell tissue valves include St. Jude Medical,
Medtronic, Sorin Biomedica and CryoLife, Inc.
We are aware of several companies that are developing new prosthetic heart valves. Several
companies are developing new beating heart tissue valves designed to enable minimally invasive
valve replacement. Additionally, there are companies testing new autologous (created from the
patient’s own tissue) valves, potentially more durable tissue valves and new bileaflet and
trileaflet mechanical designs. Other companies are pursuing biocompatible coatings to be applied to
mechanical valves in an effort to reduce the incidence of thromboembolic events and to treat tissue
valves to forestall or eliminate calcific degeneration in these valves. Product differentiation
within the prosthetic heart valve market is based on, among other things, clinical performance
record, minimizing complications, ease-of-use for the surgeon, patient comfort and quality of life
and cost-effectiveness.
4
Table of Contents
We believe that the most important factors in a heart surgeon’s selection of a particular
prosthetic valve are the perceived benefits of the valve and the heart surgeon’s confidence in the
valve design. As a result, valves that have developed a favorable clinical performance record have
a significant marketing advantage over new valves. In addition, negative publicity resulting from
isolated incidents can have a significant negative effect on a valve’s overall acceptance. Our
success is dependent upon the surgeon’s willingness to use a new prosthetic heart valve as well as
the future clinical performance of the ATS Open Pivot Heart Valve and the ATS 3f tissue heart
valves compared with the more established competition.
Heart Valve Repair Market
Overview. Depending on the type and severity of a patient’s heart valve disease, it may be
preferable to repair their damaged valve as opposed to complete replacement with either a
mechanical or tissue heart valve. We estimate the worldwide market for heart valve repair is at
$152 million and growing approximately 5% per year.
Our Heart Valve Repair Products. We commenced development and manufacturing of a line of cardiac
surgical products in 2005 pursuant to our exclusive worldwide development, supply and distribution
agreement with GBI. Our partnership with GBI provides us with access to a portfolio of patents,
intellectual property and important manufacturing and product development experience specific to
heart valve repair and the related tools and accessories for entry into this segment of the heart
valve therapy market.
In 2006, we began to market and sell the first of these products, the ATS Simulus Flexible
annuloplasty repair rings and bands. This is a fully flexible ring that conforms to a patient’s
unique anatomy. In 2007, we received FDA clearance for our ATS Simulus Semi-Rigid annuloplasty
ring and our ATS Simulus Adjustable repair ring. In 2009, we received FDA clearance for our ATS
Simulus Semi-Rigid and Adjustable annuloplasty repair bands. Semi-rigid rings and bands feature a
unique Flex-Zone™ anterior segment, which respects the natural motion of the mitral
annulus and its proximity to the aortic valve, allowing for a more physiologic valve repair.
Adjustable rings and bands incorporate many of the same features of flexible rings and bands while
allowing the surgeon to precisely accommodate individual patient anatomies by adjusting the
positioning and shape of the ring or band after implantation.
The major design features of the ATS Simulus annuloplasty rings and bands include:
| • | Generous suture target area | ||
| • | Semi-rigid ring that employs unique Flex-zone flexible anterior segment with semi-rigid posterior segment | ||
| • | Adjustable flexible ring that can be adjusted symmetrically or asymmetrically after implantation |
The ATS Simulus annuloplasty rings and bands provide the following advantages over other currently
available annuloplasty rings and bands:
| • | Ease of suture placement in traditional open chest repair procedures | |
| • | Readily accommodates robotic or minimally invasive surgical approaches | |
| • | Semi-rigid rings and bands accommodate anterior annular movement while allowing for posterior annular remodeling | |
| • | Adjustable rings and bands allow precise matching with individual patient anatomy |
Heart Valve Repair Market Competition. Advancements are being made in surgical procedures such as
mitral valve reconstruction, whereby the natural mitral valve is repaired, delaying the need for a
replacement valve. Developments include continued expansion of the available repair rings and
bands to fit physician needs and specific patient anatomies and to enable less invasive surgery.
The heart valve repair market is very competitive. Edwards Lifesciences is the market leader.
Medtronic, St Jude Medical and Sorin Biomedical also participate in the heart valve repair market.
Product differentiation within the heart valve repair market is based on, among other things,
clinical performance record, minimizing complications, ease-of-use for the surgeon, patient comfort
and quality of life and cost-effectiveness.
5
Table of Contents
Surgical Cardiac Ablation Market
Overview. Surgical treatment of atrial fibrillation products consist of tools used to create
lesions on cardiac tissue to inhibit abnormal electrical impulses in the upper chambers of the
heart. Atrial fibrillation (“AF”) is the most common type of irregular heartbeat. It is found in
about three million Americans and the incidence increases with age. When a person has AF, the
electrical impulses that control the natural heartbeat travel erratically. The result is a very
rapid and disorganized atrial heartbeat. Because the atria are beating rapidly and irregularly,
blood does not flow through the atria as quickly or efficiently. The inefficient beating can cause
clots to form. If a clot is pumped out of the heart, it can travel to the brain, resulting in a
stroke. The likelihood of a stroke in people with AF is 5 to 7 times higher than in the general
population. AF combined with a prolonged rapid heart rate can also lead to heart failure.
Cryoablation involves the use of extremely cold temperatures to stop electrical conductivity in
certain areas of the heart while leaving underlying connective tissues largely unaffected.
Additionally, by placing lines of ablation in a specific anatomic pattern, the surgeon can direct
the path of electrical impulses to restore sinus conduction.
Historically, the ablation pattern that has the greatest success in restoring sinus rhythm is the
original cut-and-sew Maze procedure established by Dr. James L. Cox. The Maze procedure has
demonstrated freedom from AF with over 15 years of follow-up data. Cryoablation allows surgeons to
perform the Maze procedure with a less invasive technique.
We estimate the market for the surgical cardiac ablation at $135 million and growing at
approximately 14% per year.
Our Surgical Cryotherapy Products. We market and sell surgical cryoablation products for the
treatment of cardiac arrhythmias acquired through our 2007 acquisition of the surgical cryoablation
business of CryoCath.
We started marketing and selling this technology during the first half of 2005 under a November
2004 global partnership agreement with CryoCath. Pursuant to this partnership, we were granted
co-promotion rights in the United States, earning an agency commission on sales to accounts as
specified in the partnership agreement, and distribution rights in the rest of the world. This
partnership agreement was in effect until our acquisition of CryoCath’s surgical cryoablation
business in June 2007.
We currently market and sell several surgical cryotherapy products, including the ATS CryoMaze
Surgical Ablation Probe (7 and 10 cm sizes and the 10S rigid Probe), the ATS CryoMaze Surgical
Ablation Clamp and the ATS CryoMaze Surgical Ablation Console. Most of our cryotherapy product
revenue is derived from probes, which are single-use devices used for freezing tissue in seconds
and which are very malleable to conform to an individual’s anatomy. To date, our cryotherapy
product revenues have been primarily derived from open chest surgical procedures performed
concomitantly with other cardiac surgery procedures.
The major design features of the ATS CryoMaze products include:
| • | The ablation probe is malleable to conform to the shape of the tissue. | ||
| • | The probes feature a sleeve to adjust the lesion length. | ||
| • | Argon-based cryoablation freezes rapidly and can reach temperatures as low as -160C. | ||
| • | The size and malleability of the probe allow it to be easily used in minimally invasive access sites and robotic manipulation. |
Cryotherapy provides the following advantages over the more prevalent heat-based therapies:
| • | Cryoablation adheres to the heart tissue during therapy, keeping the device in place. | ||
| • | Cryoablation does not produce thrombus. | ||
| • | Cryoablation preserves the integrity of the heart’s collagen matrix. | ||
| • | Cryoablation can safely create all of the lesion lines of the Maze procedure. |
6
Table of Contents
Advancements in the market for surgical ablation include tools to enable safer, quicker, and more
effective lesions creation in cardiac tissue. The market today consists primarily of AF treatment
in conjunction with or concomitant to another cardiac surgery procedure and done with an open
chest. We are aware of several companies that are developing new ablation tools. The majority of
these tools utilize heat-based energy and will therefore not be able to safely complete the Maze
lesion set. There has been a recent trend among cardiac surgeons to hold surgical ablation
technologies to a higher level of scrutiny with advanced long-term monitoring to ensure freedom
from AF. We believe these new tools will be held to this new standard of scrutiny.
Significant efforts are currently being developed to enable a sole therapy solution consisting of a
full Maze lesion set that can be achieved with a closed chest with port access on a beating heart.
Surgical Cardiac Ablation Market Competition. Competition in the surgical AF market consists
primarily of heat-based energy sources from AtriCure, Inc. and Medtronic. ATS cryoablation
products hold a third-place position in the market. Other companies that produce surgical AF
ablation technologies include St Jude Medical and Estech. Product differentiation within the
surgical cardiac ablation market is based on, among other things, clinical performance record,
minimizing complications, ease-of-use for the surgeon, patient comfort and quality of life and
cost-effectiveness.
MARKETING, SALES AND DISTRIBUTION
Overview. A key component of our business strategy is to leverage the investments we have made in
our marketing, sales and distribution resources through higher sales of new products in addition to
increased sales of our ATS Open Pivot Heart Valve. We have been steadily building both our
domestic and international sales and marketing infrastructure. Because sales prices in the United
States exceed sales prices in most other markets, we believe our future success will, in part,
depend on achieving increased market share and leveraging our sales force through the introduction
of new products in the United States. Our U.S. sales as a percent of overall sales have increased
from 4% in 2000 to 39% of overall sales in 2009. Because of quicker regulatory and approval
timelines in European markets compared to the United States, European markets will be the first to
commercialize our new products, namely our sutureless and beating heart tissue valve products. As
such, we plan to add organizational structure in Europe to support the launch of these products.
U.S. Marketing and Sales. Our sales organization in the United States consists of a Vice President
of Sales and four area directors managing 31 sales territories. Our representation within these
territories consists of both direct sales representatives and independent agents. We focus our
sales and marketing efforts on increasing awareness of our products in the approximately 1,000 U.S.
open heart centers.
International Marketing, Sales and Distribution. We have direct sales operations in France (since
2003), Germany (since 2005), the United Kingdom (since 2006), Belgium and the Netherlands (since
2007) and Switzerland (also since 2007) as well as direct marketing organizations in China (since
2004) and India (since 2005). For our European direct selling operations, we maintain consignment
inventories at in-country hospitals. In 2008, we established an administrative and European
headquarters office in Belgium, which we utilize as a European support center for our current and
future direct selling operations in Europe.
We sell through independent distribution networks in other markets throughout the world. We
believe that our distribution partners have provided a rapid and cost-efficient means of increasing
market penetration and commercial acceptance of our products in key international markets. We have
been able to attract experienced medical device sales organizations and people familiar with local
markets and customs to serve as our representatives. Each of our independent distributors has the
exclusive right to sell certain ATS products within a defined territory. These distributors, in
some instances, also market other medical products, although they have agreed not to sell products
which directly compete with our products. Under most of the distributor agreements, we may, at our
option, terminate the agreement upon the departure of certain key employees of the distributor, if
we experience a change in control or if key performance criteria, including sales quotas, are not
met. Our sales, marketing and customer service personnel provide professional sales, marketing and
promotional support to our independent distributors. We sell our products to international
distributors F.O.B. Minneapolis, Minnesota, denominated in U.S. dollars. See Note 11 of “Notes to
Consolidated Financial Statements” in Item 8 of this Form 10-K for information on
our net sales by geographic region. Net sales both inside and outside the United States are also
discussed in Item 7 of this Form 10-K.
7
Table of Contents
Medical Device Industry Competition. Competition in the medical device industry is intense and is
characterized by extensive research efforts and rapid technological progress. We believe the
primary competitive factors include quality, technical capability, innovation, distribution
capabilities and price. Many of our competitors in the heart valve market have greater resources,
more widely-accepted products, greater technical capabilities and stronger name recognition than we
do. Our competitive capability is affected by our ability to support our products, ensure
regulatory compliance for our products, protect the proprietary technology of our products and
their manufacturing processes, effectively market our products, and maintain and establish
distribution relationships. In order to maintain these capabilities, ATS must continuously attract
and retain skilled and dedicated employees and develop and maintain excellent relationships with
physicians and suppliers.
Cardiac surgery products are currently being marketed to hospitals at prices that vary
significantly from country to country due to market conditions, currency valuations, distributor
mark-ups and government regulations. In many markets, government agencies are imposing or proposing
price controls or restrictions on medical products. We work with our independent distributors to
price our products in each market to meet these limitations. In addition, our primary competitors
have the ability, due to economies of scale, to manufacture their valves at a lower cost than we
can currently manufacture the ATS Open Pivot and ATS 3f heart valves. The market leader has
occasionally used price as a method to compete in several markets.
MANUFACTURING AND SUPPLY
Our mechanical heart valves are manufactured in ISO 13485:2003 certified facilities. We have two
mechanical heart valve production facilities in close proximity in Plymouth, a suburb of
Minneapolis, Minnesota, for our manufacturing activities. Our pyrolytic carbon mechanical valve
components are manufactured in one facility. In the other facility, we assemble our mechanical
valves in controlled clean room environments. Most of the materials we purchase for our products
are supplied by a limited number of vendors. We are currently operating two manufacturing shifts
at our valve assembly facility. At our pyrolytic carbon facility, most processes are operating one
manufacturing shift while some operate up to three manufacturing shifts. We established our
pyrolytic carbon manufacturing facility in 2004 under an initiative to become a low-cost,
self-supplier of the critical carbon components necessary to meet demand for our mechanical heart
valves. While this initiative resulted in ramp-up and start-up expenses, low initial production
yields, and higher-than-normal scrap costs in 2004 and 2005, our gross margins have improved
consistently since 2005.
The production of surgical cryotherapy products are manufactured in the same ISO 13485:2003
certified facility in Plymouth as our mechanical heart valve, again in controlled clean room
environments. The Plymouth manufacturing facility has also been approved by international
regulatory agencies to manufacture surgical cryotherapy products.
Our ATS 3f tissue heart valves are manufactured in a controlled clean room environment in an ISO
13485:2003 certified facility in Lake Forest, California. Most of the materials used to
manufacture our valves are supplied by a limited number of qualified vendors. We currently operate
one manufacturing shift at the Lake Forest facility. We have been ramping up our tissue valve
production in connection with the market launch of the ATS 3f Aortic Bioprosthesis, approved for
commercialization in the United States by the FDA in October 2008 and in preparation for a market
launch of the ATS 3f Enable Aortic Bioprosthesis after its European CE Mark approval in December
2009. In addition, we also manufacture significant quantities of our next generation tissue valves
for use in pre-clinical and clinical testing. These initiatives have resulted in low production
yields and inefficiencies. As our tissue valve volume increases, we expect our yields will
increase and our process will become more efficient.
For our heart valve repair and surgical tools and accessories franchises, we do not manufacture or
produce the products we sell, and we only service some of these products.
We believe our facilities are adequate to serve our business operations for the foreseeable future.
We warehouse our finished products at our Plymouth, Minnesota facilities, our sales offices in
France and Germany and at a third party warehouse in the Netherlands.
8
Table of Contents
We maintain a comprehensive quality assurance and quality control program, which includes
documentation of all material specifications, operating procedures, equipment maintenance and
quality control test methods. Our documentation systems comply with the FDA Quality System
Regulation (“QSR”) and ISO 13485:2003 requirements.
RESEARCH AND DEVELOPMENT
Our research and development (“R & D”) activities include developing new products, improving our
current products, and the clinical and regulatory activities to support our products. These
activities are carried out in our Plymouth and Lake Forest facilities, although we work with
physicians, research hospitals and universities around the world. None of this work is funded by
customers or other outside institutions. The development process for any new product can range
from several months to several years, primarily depending on the regulatory pathway required for
approval. R & D expenses totaled $8.9 million in 2009, $8.2 million in 2008, and $7.5 million in
2007 (excluding $3.5 million of in-process research and development related to the CryoCath asset
acquisition). At December 31, 2009, our R & D headcount totaled 30 employees, including our
regulatory and clinical affairs groups.
FINANCIAL INFORMATION ABOUT SEGMENTS
Since our inception, we have operated in the single industry segment of developing, manufacturing
and marketing medical devices.
SEASONALITY
Our sales and operating results have varied and are expected to continue to vary significantly from
quarter to quarter as a result of seasonal patterns. We expect that our business will be seasonal,
with the third quarter of each year typically having the lowest sales, due to vacation and time-off
periods in our international markets, especially Europe.
PATENTS AND PROPRIETARY TECHNOLOGY
Our policy is to protect our proprietary position by securing U.S. and foreign patents that cover
the technology, inventions and improvements important to our business. The original patent
obtained by CarboMedics under which our valve was developed expired in 2004. We subsequently made
modifications to the basic design. We obtained a U.S. patent covering the improvements to the ATS
Open Pivot Heart Valve in October 1994. This patent expires in 2011. We have also obtained issued
patents in Japan, Belgium, France, Germany, the Netherlands, Spain, Switzerland and the United
Kingdom relating to these improvements. We cannot be certain that any patents will not be
challenged or circumvented by competitors.
Our ATS 3f tissue valve platforms are supported by an extensive intellectual property portfolio.
We own 83 issued U.S. and foreign patents and 51 U.S. and foreign patent applications that protect
our core technology in the tissue valve market. These patents expire in various years ranging from
2011 to 2028, with 17 of the patents expiring between 2011 and 2013 and 66 of the patents expiring
between 2014 and 2028. We also hold co-exclusive rights to certain intellectual property,
including the “Anderson Patents” for minimally invasive valve deployment.
Our ATS cryoablation platforms for treatment of AF are also supported by a licensed intellectual
property portfolio comprising at least 16 issued U.S. and foreign patents and at least 17 U.S. and
foreign patent applications that protect our core technology in the market. These patents expire
in various years ranging from 2016 to 2023.
The effect of these patents is to give us the ability to practice certain technologies and the
right to preclude third parties from making, using, selling or offering to sell products which
infringe upon the claims made in each of these patents within the jurisdiction of the country where
the patent is issued. We believe the claims covered by the issued patents are broad and cover many
unique attributes of the products we plan for commercialization and the processes we use to
fabricate these products.
9
Table of Contents
We also rely on trade secrets and technical know-how in the manufacture and marketing of both our
mechanical and tissue heart valves. We typically require our employees, consultants and contractors
to execute confidentiality agreements with respect to our proprietary information.
We claim trademark protection on ATS Medical™, ATS Open Pivot®, ATS AP 360®, ATS 3f® Aortic
Bioprosthesis, ATS 3f Enable® Aortic Bioprosthesis, ATS CryoMaze® and ATS Simulus® and
either claim or have applied for trademark or tradename protection on most of our product offering
names. U.S. trademark and service mark registrations are generally for a term of 10 years,
renewable every 10 years so long as the trademark is used in the regular course of trade. We have
also been granted rights by certain partners to use their trademark(s) in our sales and marketing
activities of their products and services.
GOVERNMENT REGULATION
United States. Numerous governmental authorities, principally the FDA and corresponding state
regulatory agencies, strictly regulate our products and research and development activities. The
Federal Food, Drug, and Cosmetic Act, the regulations promulgated under this act, and other federal
and state statutes and regulations, govern, among other things, the pre-clinical and clinical
testing, design, manufacture, safety, efficacy, labeling, storage, record keeping, advertising and
promotion of medical devices. The FDA classifies our ATS heart valves as a Class III device, which
is subject to the highest level of controls.
Generally, before we can market a new medical device, we must obtain marketing clearance through a
510(k) pre-market notification, approval of a pre-market approval application (“PMA”) or approval
of product development protocol (“PDP”). A PMA or PDP application must be submitted if a proposed
device does not qualify for a 510(k) pre-market clearance procedure. It generally takes several
months from the date of a 510(k) submission to obtain clearance, but it may take longer,
particularly if a clinical trial is required. The FDA and Institute of Medicine are presently
evaluating potential modifications to the 510(k) process, which could make it more difficult to
obtain marketing clearance from the FDA using the 510(k) pre-market notification procedure. The
PMA and PDP process can be expensive, uncertain, require detailed and comprehensive data and
generally take significantly longer than the 510(k) process.
If human clinical trials of a device are required, either for a 510(k) submission or a PMA
application, the sponsor of the trial, usually the manufacturer or the distributor of the device
must file an investigational device exemption (“IDE”) application prior to commencing human
clinical trials. The IDE application must be supported by data, typically including the results of
animal and/or laboratory testing. If the IDE application is approved by the FDA and one or more
appropriate institutional review boards (“IRBs”), human clinical trials may begin at a specific
number of investigational sites with a specific number of patients, as approved by the FDA. If the
device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after
obtaining approval for the study by the IRBs without separate approval from the FDA. Submission of
an IDE does not give assurance that the FDA will approve the IDE and, if it is approved, there can
be no assurance the FDA will determine that the data derived from the studies support the safety
and efficacy of the device or warrant the continuation of clinical trials. An IDE supplement must
be submitted to and approved by the FDA before a sponsor or investigator may make a change to the
investigational plan that may affect its scientific soundness, study indication or the rights,
safety or welfare of human subjects.
We are also subject to the FDA QSR concerning manufacturing processes and reporting obligations.
These regulations require that manufacturing steps be performed according to FDA standards and in
accordance with documentation, control and testing standards. The FDA monitors compliance with its
good manufacturing practices regulations by conducting periodic inspections. We are required to
provide information to the FDA on adverse incidents as well as maintain a detailed record keeping
system in accordance with FDA guidelines.
The advertising of our products is also subject to both FDA and U.S. Federal Trade Commission
regulations. In addition, we are subject to the “fraud and abuse” laws and regulations promulgated
by the U.S. Department of Health and Human Services and the U.S. Centers for Medicare and Medicaid
Services if we sell ATS products to Medicare or Medicaid patients. Under these regulations, it is
a criminal offense (subject to certain exceptions) to knowingly or willfully offer, pay, solicit or
receive remuneration in order to induce business for which reimbursement may be provided under a
federal healthcare program.
10
Table of Contents
If the FDA believes we are not in compliance with law, it can institute proceedings to detain or
seize products, issue a recall, enjoin future violations and assess civil and criminal penalties
against us and our officers and employees. If we fail to comply with these regulatory requirements,
our business, financial condition and operating results could be harmed. In addition, regulations
regarding the manufacture and sale of our products are subject to change. We cannot predict the
effect, if any, that these changes might have on our business, financial condition and operating
results.
International. In order to market our products in European and other foreign countries, we must
obtain required regulatory approvals and comply with extensive regulations governing product
safety, quality and manufacturing processes. These regulations vary significantly from country to
country and with respect to the nature of the particular medical device. The time required to
obtain these foreign approvals to market our products may be longer or shorter than in the United
States, and requirements may differ from FDA requirements.
For example, in order to market our products in the 27 member countries of the European Union, we
are required to comply with the European Union Medical Devices Directive and obtain CE mark
certification. The CE mark denotes conformity with European standards for safety and allows
certified devices to be sold in all European Union countries.
THIRD-PARTY REIMBURSEMENT
In the United States, healthcare providers that purchase medical devices, including our products,
generally rely on third-party payers, including Medicare, Medicaid, private health insurance
carriers and managed care organizations, to reimburse all or part of the cost and fees associated
with the procedures performed using these devices. The commercial success of our products will
depend on the ability of healthcare providers to obtain adequate reimbursement from third-party
payers for the surgical procedures in which our products are used. Third-party payers are
increasingly challenging the coverage and pricing of medical products and procedures. Even if a
procedure is eligible for reimbursement, the level of reimbursement may not be adequate. In
addition, third-party payers may deny reimbursement if they determine that the device used in the
treatment was not cost-effective or was used for a non-approved indication.
In international markets, market acceptance of our products also depends in part upon the
availability of reimbursement from healthcare payment systems. Reimbursement and healthcare
payment systems in international markets vary significantly by country. The main types of
healthcare payment systems in international markets are government-sponsored healthcare and private
insurance. Countries with government-sponsored healthcare, such as the United Kingdom, have a
centralized, nationalized healthcare system. New devices are brought into the system through
negotiations between departments at individual hospitals at the time of budgeting. In many of the
countries where we market our products, the government sets an upper limit of reimbursement for
various valve types. In most foreign countries, there are also private insurance systems that may
offer payments for alternative devices.
We have pursued reimbursement for our products internationally through our independent
distributors. While the healthcare financing issues in these countries are substantial, our
distributors have been able to sell our products to private clinics and nationalized hospitals in
each of these countries.
All third-party reimbursement programs, whether government-funded or insured commercially, inside
the United States or outside, are developing increasingly sophisticated methods of controlling
health care costs through prospective reimbursement and capitation programs, group purchasing,
redesign of benefits, second opinions required prior to major surgery, careful review of bills,
encouragement of healthier lifestyles and exploration of more cost-effective methods of delivering
healthcare. These types of programs can potentially limit the amount that healthcare providers may
be willing to pay for medical devices.
PRODUCT LIABILITY AND INSURANCE
Cardiovascular device companies are subject to an inherent risk of product liability and other
liability claims in the event that the use of their products results in personal injury. Heart
valves are life-sustaining devices, and the failure of any heart valve usually results in the death
of the patient. We have not received
11
Table of Contents
any reports of mechanical failure of our valves implanted to date. Any product liability claim
could subject us to costly litigation, damages and adverse publicity.
We currently maintain a product liability insurance policy with an annual coverage limit of $25
million in the aggregate. We are financially responsible for any uninsured claims or claims which
exceed the insurance policy limits. Product liability insurance is expensive for mechanical
valves. If insurance becomes completely unavailable, we must either develop a self-insurance
program or sell without insurance. The development of a self-insurance program would require
significant capital.
EMPLOYEES
As of December 31, 2009, we employed 321 full-time and part-time employees worldwide. Our
employees are vital to our success. We believe we have been successful in attracting and retaining
qualified personnel. We believe our employee relations are good.
EXECUTIVE OFFICERS OF THE REGISTRANT
Our executive officers are as follows:
| Name | Age | Position | ||||
Michael D. Dale
|
50 | Chairman, Chief Executive Officer and President | ||||
Michael R. Kramer
|
34 | Chief Financial Officer | ||||
Astrid M. Berthe
|
46 | Vice President, Regulatory Affairs and Quality Assurance | ||||
Thaddeus Coffindaffer
|
48 | Vice President, Sales | ||||
David R. Elizondo
|
42 | Vice President, Research and Development | ||||
Michael E. Reinhardt
|
51 | Vice President, Global Marketing | ||||
Craig A. Swandal
|
49 | Vice President, Operations | ||||
Xavier K. Bertrand
|
38 | Vice President and General Manager, Europe, Middle East and Africa | ||||
Michael D. Dale has served as our Chairman of the Board since April 2003 and as our Chief Executive
Officer and President since October 2002. From 1998 to 2002, Mr. Dale was Vice President of
Worldwide Sales and Marketing at Endocardial Solutions, Inc., a company that developed and marketed
an advanced cardiac mapping and catheter navigation system for the diagnosis and treatment of
cardiac arrhythmias. From 1996 to 1998, Mr. Dale was Vice President of Global Sales for
Cyberonics, Inc., a neuromodulation medical device company, and additionally was Managing Director
of Cyberonics Europe S.A. From 1988 to 1996, Mr. Dale served in several capacities at
cardiovascular medical device manufacturer and marketer St. Jude Medical, most recently as the
Business Unit Director for St. Jude Medical Europe. Mr. Dale is on the board of directors of
Neuronetics, a world leader in Transcranial Magnetic Stimulation (TMS) Therapy, which involves the
use of MRI-strength magnetic fields to stimulate nerve cells in the brain for the treatment of
patients suffering from depression. Mr. Dale also serves on the Board of Directors of Rhythmia,
Inc., an early stage medical technology company, and the Advanced Medical Technology Association
(AdvaMed).
Michael R. Kramer has served as our Chief Financial Officer since August 2007. Mr. Kramer joined
ATS as our Senior Director of Finance in September 2006 and was appointed Acting Chief Financial
Officer in February 2007. During 2006, prior to joining ATS, Mr. Kramer was engaged by ATS as an
independent financial consultant. From February 2005 to May 2006, Mr. Kramer served as Controller
at CABG Medical, Inc., a cardiovascular device manufacturer. During 2004, Mr. Kramer was a
Corporate Finance Manager at Ecolab, Inc., a developer and marketer of products and services to the
hospital, foodservice, healthcare and industrial markets. From December 1999 through July 2004,
Mr. Kramer worked at Ernst & Young LLP, a global professional services firm, where he served as a
manager in the assurance and advisory services practice from September 2002 until July 2004.
Astrid M. Berthe was appointed an executive officer of ATS in June 2008. Ms. Berthe joined ATS in
September 2006 as a result of our acquisition of 3F. Ms. Berthe has served as both a Director and
Vice President of Regulatory Affairs and Quality Assurance for 3F and ATS since 2004. Prior to 3F,
Ms. Berthe served as the Director of Quality Assurance and Regulatory Compliance from April 2001 to
November 2003 at Medtronic Cardiac Surgery, a business unit of Medtronic, Inc., a worldwide
developer and
12
Table of Contents
manufacturer of medical devices and technologies. During her career, Ms. Berthe has held various
other leadership positions in the areas of quality systems, regulatory, compliance, engineering and
manufacturing operations.
Thaddeus Coffindaffer was appointed an executive officer of ATS in June 2008. Mr. Coffindaffer
joined ATS in August 2003 and served as an Area Sales Director until he was appointed Vice
President-Sales in April 2007. Prior to joining ATS, Mr. Coffindaffer served as Western Region
Sales Manager for Thoratec, a manufacturer and marketer of ventricular assist devices, from June
2000 to August 2003.
David R. Elizondo has served as our Vice President of Research and Development, since September
2006. From July 2000 to August 2006, Mr. Elizondo served in several capacities at Boston
Scientific Corporation, a developer of technologies and products for interventional and surgical
procedures, and most recently served as the Director of New Business Development for Boston
Scientific’s Cardiology Division from 2005 to August 2006.
Michael E. Reinhardt has served as our Vice President, Global Marketing since May 2009. Prior to
joining ATS, Mr. Reinhardt was an independent consultant from November 2006 to April 2009. From
April 2005 to October 2006, Mr. Reinhardt served as Vice President of Global Marketing for St. Jude
Medical. From August 2003 to March 2005, Mr. Reinhardt served as Vice President of Global
Marketing for C.R. Bard, a manufacturer and marketer of implantable and disposable medical devices.
Craig A. Swandal has served as our Vice President, Operations since joining ATS in August 2008.
Prior to joining ATS, Mr. Swandal served as Senior Vice President of Corporate Operations for Gyrus
ACMI, a leader in the development and manufacture of minimally invasive surgical instruments and
visualization systems, from September 2005 to August 2008. From August 2001 to September 2005, Mr.
Swandal served as Vice President of Operations for Gyrus.
Xavier K. Bertrand was appointed Vice President and General Manager for Europe, Middle East and
Africa and effective March 1, 2010. Prior to joining ATS, Mr. Bertrand served in executive sales
and marketing management positions for Biosensors International, a manufacturer and marketer of
medical devices for interventional cardiology, from July 2008 to February 2010. From April 1999 to
June 2008, Mr. Bertrand in various operations, marketing and finance positions for Boston
Scientific Corporation, most recently as Business Unit Director-France (February 2007 to June
2008), Marketing Director-France (August 2005 to February 2007) and Group Marketing Manager-Europe
(December 2002 to August 2005).
AVAILABLE INFORMATION
Copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form
8-K and amendments to those reports filed or furnished to the Securities and Exchange Commission
(the “SEC”) pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (the
“Exchange Act”) are available free of charge through our website (www.atsmedical.com) as soon as
reasonably practicable after we electronically file the material with, or furnish it to, the SEC.
ITEM 1A. RISK FACTORS
Our business faces many risks. Any of the risks discussed below could have a material impact
on our business, financial condition or operating results.
We have historically relied on the ATS mechanical heart valve as our primary source of revenue. If
we are unable to maintain our current sales levels of mechanical heart valves, or if we are unable
to successfully market our other products on a broad basis, including in Europe, our operating
results may be harmed and we may not achieve profitability.
Sales of our mechanical heart valves accounted for approximately 60% of our net sales for the year
ended December 31, 2009. In addition, we depend upon sales outside the United States and expect
that international sales will account for a substantial majority of our net sales until our
products receive wider market acceptance from U.S. customers. In fiscal 2009 approximately 61% of
our net sales were derived outside of the United States. Accordingly, our success will depend in
large part on the medical community’s continued acceptance of our mechanical heart valves, and on
our ability to expand worldwide,
13
Table of Contents
and particularly in Europe, market acceptance of our other existing and new products, including our
sutureless heart valves. If we encounter difficulties in maintaining existing markets for our
products or in expanding the market for our existing and new products in Europe, we may not be able
to increase our revenue enough to achieve profitability, and our business and operating results
will be seriously harmed.
Recently there has been an increase in the use of tissue valves. We estimate that mechanical heart
valves are currently being used in 20% to 65% of all heart valve replacements, depending on the
geographic market, down from 65% to 75% approximately ten years ago. We believe that the tissue
manufacturers’ claims of improvements in tissue valve longevity and an increase in the average age
of valve patients have contributed to the recent increase in the use of tissue valves. There can
be no assurance that use of mechanical heart valves will not decline or that we will be able to
maintain our current levels of mechanical heart valve sales. There can also be no guarantee that
we will be able to successfully market and sell our tissue heart valves, that our tissue heart
valves will be approved or gain broad market acceptance, or that our other products will decrease
our dependence on sales of mechanical heart valves. The acceptance of our products in medical
communities will depend upon our ability to demonstrate the safety and efficacy, advantages,
long-term clinical performance and cost-effectiveness of our products as compared to other
products, as well as our ability to successfully train medical communities on the proper use and
deployment of our products. We cannot predict whether medical communities will accept ATS products
or, if accepted, the extent of their respective uses. In addition, if members of a medical
community are not properly trained, they may misuse or ineffectively use our products. Negative
publicity resulting from isolated incidents involving ATS products or other products related to
those we sell could have a significant adverse effect on the overall acceptance of our heart valves
and other product and technology offerings. In addition, increasing revenues from new products
cannot be guaranteed, and even if we were to develop additional products, regulatory approval would
likely be required to sell them. Clinical testing and the approval process itself are very
expensive and can take many years. Adverse rulings by regulatory authorities, product liability
lawsuits, the loss of market acceptance or the failure to expand market acceptance, or other
adverse publicity, may significantly and adversely affect sales of our products and, as a result,
would adversely affect our business, financial condition and operating results.
Further, we will need to expand our infrastructure in Europe in order to successfully execute our
growth plan in that region. We may not be able to sufficiently expand our infrastructure in Europe
efficiently or in a timely manner in order to execute our European growth plan, and given our
dependence on sales outside the United States, any material decrease in foreign sales may
materially and adversely affect our operating results.
We have a history of net losses and of regularly raising funds and incurring debt to fund our net
losses. If our current cash and investment balances are inadequate to carry us to profitability,
we may need to raise equity or incur additional debt in the future. If we are unable to obtain
adequate financing, we may be unable to continue our operations.
We are not currently profitable and have a very limited history of profitability. We had net
losses of approximately $6.3 million for 2009, $19.3 million for 2008, and $23.0 million for 2007.
As of December 31, 2009, we had an accumulated deficit of approximately $158.2 million, compared to
approximately $151.9 million as of December 31, 2008. We expect to incur significant expenses over
the next several years as we continue to devote substantial resources to the commercialization and
marketing of our products both in the United States and in many foreign countries, and we may in
the future be required to raise equity or issue additional debt to fund acquisitions and our
operations if our future operations require more cash than our current balances. In addition, we
will not generate net income unless we are able to significantly increase revenue from sales. If
we continue to sustain losses, we may not be able to continue our operations. Furthermore, there
may be delays in obtaining necessary governmental approvals of our products or introducing products
to market or other events that may cause actual cash requirements to exceed those for which we have
budgeted. In such event, we would need additional financing. Lastly, the holders of our 6%
Convertible Senior Notes due 2025 (“Convertible Senior Notes” or “Notes”) have the option to
require us to repurchase the Convertible Senior Notes in October 2010. While we have recently
obtained a commitment for term debt financing which will be used to call and retire the Notes, if
this financing is not consummated and the Note holders exercise their repurchase option, we would
need to raise capital, issue debt securities and/or borrow to finance the repurchase of these
Notes.
14
Table of Contents
We do not know what impact the current unprecedented volatility in worldwide credit and equity
markets may have on our ability to obtain future financing. Beginning in the fall of 2008, the
world experienced unprecedented turmoil in equity and credit markets that has resulted in
record-setting losses in the stock markets, dramatic decreases of liquidity in the credit markets,
bank failures, hedge fund closures and massive market intervention by the United States and foreign
governments. Because of the unprecedented nature of these market events, and because the markets
remain highly volatile, we cannot predict what effect these events will have on our ability to
obtain debt or equity financing in the future. If we are unable to raise sufficient capital, it
will have a material adverse effect on our financial condition and our ability to remain in
business.
The recent U.S. and global economic downturn could have adverse effects on our business and our
company.
The economic turmoil discussed above has also negatively impacted world economies, businesses, and
markets, and could adversely impact our company in the future. We cannot predict what effect these
events will have on our sales, collectibility of receivables, debt covenants, ability to attract
additional investors, and company stock price. Increased volatility in our stock price
attributable to the economic downtown could negatively impact our future reported operating
results, including the potential for impairments to our recorded goodwill and higher stock
compensation expense.
We are subject to extensive governmental regulation, which is costly, time consuming and can
subject us to unanticipated delays or could ultimately preclude us from marketing and selling our
products, and we are subject to risks relating to non-FDA-approved, or off-label, use of our
products.
Our heart valves, surgical cryoablation products and other products and our manufacturing
activities are subject to extensive regulation by a number of governmental agencies, including the
FDA and comparable international agencies, as well as other federal, state, local and international
authorities. We are required to:
| • | obtain the approval of the FDA or international regulatory authorities where our products are not yet marketed; | ||
| • | after obtaining approval or clearance of the FDA or international regulatory authorities, maintain the approval of the FDA and international regulatory authorities to continue selling and manufacturing our products; | ||
| • | satisfy content requirements for all of our labeling, sales and promotional materials; | ||
| • | comply with manufacturing and reporting requirements; and | ||
| • | undergo rigorous inspections by these agencies. |
Compliance with the regulations of these governmental authorities may delay or prevent us from
introducing any new or improved products. The governmental authorities charged with making and
implementing these laws or related regulations may change the laws, impose additional restrictions,
or adopt interpretations of existing laws or regulations that could have a material adverse effect
on us. Violations of these laws or regulatory requirements may result in fines, marketing
restrictions, product recall, withdrawal of approvals and civil and criminal penalties. We also
may incur substantial costs associated with complying and overseeing compliance with the laws and
regulations of these governmental authorities.
We ultimately may not be able to obtain the necessary governmental approvals or clearances in the
United States or other jurisdictions, including FDA and CE approvals and clearances, for products
that are now under development, including, but not limited to, our ATS 3f Enable Aortic
Bioprosthesis tissue heart valve product, our closed-chest beating heart aortic tissue heart valve
product, and the ForceField process. Obtaining these governmental approvals or clearances is
uncertain, and the regulatory approval process is likely to be time-consuming and expensive. If we
are unable to obtain such governmental approvals or clearances, then our ability to market and sell
products currently under development may be delayed or may never occur. Our potential inability to
market and sell our products currently under development, together with the potential expenses
associated with obtaining the necessary governmental approvals or
clearances, may cause us to suffer financial difficulties, which could have a material adverse
effect on our business, financial condition and prospects.
15
Table of Contents
In addition, the use of our products outside the indications cleared for use, or “off-label use,”
may increase the risk of injury to patients. Because the FDA does not restrict or regulate a
doctor’s choice of treatment within the practice of medicine, doctors may use our products for
off-label uses. Off-label use of our products may increase the risk of product liability claims,
which are expensive to defend, would divert our management’s attention and could result in
substantial damage awards against us. In addition, the FDA and other regulatory agencies actively
enforce regulations prohibiting promotion of off-label uses and the promotion of products for which
marketing clearance has not been obtained. Any investigation of us concerning the promotion of
off-label uses could be expensive, disruptive and burdensome to defend, and could generate negative
publicity. If the FDA or another regulatory agency determines that our promotional materials or
training constitutes promotion of an unapproved use, it could request that we modify our training
or promotional materials or subject us to regulatory enforcement actions, including the issuance of
a warning letter, injunction, seizure, civil fine and criminal penalties. Although our policy is
not to use any statements that could be considered off-label promotion of our products, the FDA or
another regulatory agency could disagree and conclude that we have engaged in off-label promotion,
which could have a material adverse effect on our business and operating results.
Healthcare reform legislation could adversely affect our revenue and financial condition.
In recent years, there have been numerous initiatives on the federal and state levels for
comprehensive reforms affecting the payment for, the availability of and reimbursement for
healthcare services in the United States. These initiatives have ranged from proposals to
fundamentally change federal and state healthcare reimbursement programs, including providing
comprehensive healthcare coverage to the public under governmental funded programs, to minor
modifications to existing programs. Recently, President Obama and members of Congress have
proposed significant reforms to the U.S. healthcare system. Both houses of Congress have conducted
hearings about U.S. healthcare reform and a number of bills have been proposed in Congress. A
leading proposal includes an excise tax on the medical device industry that would be payable based
on revenue, not income. In addition, recent legislation and many of these proposed bills include
funding to assess the comparative effectiveness of medical devices. Although Congress has
indicated that this funding is intended to improve the quality of health care, it is unclear what
impact this assessment will have on coverage, reimbursement or other third-party payor policies, or
what effect that assessment and the excise tax proposal would have on our products or our financial
results. To the extent these or other reform measures affect the coverage and reimbursement of our
current or future products, there could be a material adverse affect on our financial condition and
operating results.
The ultimate content or timing of any future healthcare reform legislation, and its impact on
medical device companies such as ATS, is impossible to predict. If significant reforms are made to
the healthcare system in the United States, or in other jurisdictions, those reforms could
materially impact our business, financial condition and operating results.
Our business could be seriously harmed if third-party payers do not reimburse the costs for our
products.
Our ability to successfully commercialize the ATS mechanical heart valve, our tissue heart valves,
surgical cryoablation devices and other products depends on the extent to which reimbursement for
the cost of our product and the related surgical procedure is available from third-party payers,
such as governmental programs, private insurance plans and managed care organizations. Third-party
payers are increasingly challenging the pricing of medical products and procedures that they
consider not to be cost-effective or that are used for a non-approved indication. The failure by
physicians, hospitals and other users of our products to obtain sufficient reimbursement from
third-party payers would seriously harm our business, financial condition and operating results.
As noted above, in recent years there have been numerous proposals to change the health care system
in the United States. Some of these proposals have included measures that would limit or eliminate
payment for medical procedures or treatments. In addition, government and private third-party
payers are increasingly attempting to contain health care costs by limiting both the coverage and
the level of reimbursement. In international markets, reimbursement and health care payment
systems vary significantly by country.
16
Table of Contents
Furthermore, we have encountered price resistance from government-administered health programs.
Significant changes in the health care system in the United States or elsewhere, including changes
resulting from adverse trends in third-party reimbursement programs, could have a material adverse
effect on our business, financial condition and operating results.
Foreign currency fluctuations and other risks inherent in doing business in international markets
could adversely affect our operating results.
In certain countries in Europe we maintain direct sales operations and sell in local currency. In
addition, we sell in U.S. dollars to our international distributors and European subsidiaries
abroad. An increase in the value of the U.S. dollar in relation to other currencies can adversely
affect our sales volume outside of the United States as well as increase our exposure to foreign
exchange losses on subsidiary intercompany payments and payables. Given our dependence on sales
outside of the United States and our expectation that international sales will account for a
substantial majority of our net sales until our products receive wider market acceptance from U.S.
customers, we believe we will continue to be exposed to U.S. dollar currency fluctuations for the
foreseeable future.
Our future operating results could also be harmed by risks inherent in doing business in
international markets, including:
| • | unforeseen changes in regulatory requirements and government health programs; | ||
| • | weaker intellectual property rights protection in some countries; | ||
| • | new export license requirements, changes in tariffs or trade restrictions; | ||
| • | political and economic instability in our target markets; | ||
| • | greater difficulty in collecting payments from product sales; | ||
| • | lengthy/extended credit terms; and | ||
| • | competitive price pressure. |
The markets in which we compete are highly competitive, and a number of our competitors are larger
and have more financial resources. If we do not compete effectively, our business will be harmed.
The medical device industry, including the market for prosthetic heart valves and the surgical AF
market, is highly competitive, subject to rapid technological change and significantly affected by
new product introductions and promotional activities of other participants. We expect that
competition will intensify as additional companies enter the market or modify their existing
products to compete directly with us. Our primary competitor in the mechanical heart valve market
is St. Jude Medical, Inc., our primary competitor in the tissue heart valve market is Edwards
Lifesciences Corporation, and our primary competitor in the surgical ablation market is AtriCure,
Inc. All of these companies currently hold a significantly higher market share position than ATS.
Most of our heart valve competitors have long-standing FDA approval for their valves and extensive
clinical data demonstrating the performance of their valves. In addition, they have greater
financial, manufacturing, marketing and research and development capabilities than we have. For
example, many of our competitors have the ability, due to economies of scale, to manufacture their
heart valves at a lower cost than we can manufacture our valves. Our competitors have also used
price as a method to compete in several international markets. If heart valve prices decline
significantly, we might not be able to compete successfully, which would harm our business,
financial condition and operating results. In addition, we cannot assure you that our surgical
ablation products will compete effectively against other ablation systems or other surgical
treatments. A number of companies are promoting devices for the ablation of cardiac tissue, and we
anticipate that new or existing competitors may develop competing products, procedures or clinical
solutions. The introduction of new products, procedures, clinical solutions, or a competitor
obtaining FDA approval of a product for the treatment of AF, may result in price reductions,
reduced margins or loss of market share and may render our products obsolete, which could adversely
affect our operating results and prospects.
17
Table of Contents
Without the timely introduction of new and improved products, new products or technologies could
emerge that render our products noncompetitive and obsolete.
The medical device industry is characterized by significant technological advances, frequent new
product introductions, and evolving industry standards. Several companies are developing new
prosthetic heart valves based on new or potentially improved technologies. Significant advances
are also being made in surgical procedures, which may delay the need for replacement heart valves.
Without the timely introduction of new and improved products, new products or technologies could
emerge that render our products noncompetitive and obsolete. This could materially harm our
operating results or force us to cease doing business altogether. Even if we are able to develop
new products, our ability to market them could be limited by the need for regulatory clearance,
restrictions imposed on approved indications, entrenched patterns of clinical practice, uncertainty
over third party reimbursement, or other factors.
We ultimately may experience a delay in introducing, or may not successfully complete the
development of, products that are currently under development, resulting in harm to our business.
Technical innovations often require substantial time and investment before we can determine their
commercial viability. We are in the process of developing certain products, including, but not
limited to, the ATS 3f Enable Aortic Bioprosthesis tissue heart valve product, a closed-chest
beating heart aortic tissue heart valve product and the ForceField process. U.S. clinical trials
for the Enable product are expected to commence during 2010. The closed-chest aortic tissue heart
valve product is still under development. Successfully completing the development of these
products and technologies presents substantial technical, medical and engineering challenges, as
well as clinical and regulatory hurdles. We may not successfully complete the development of these
products, they may fail to work in the manner intended, or even if we are able to successfully
develop these products, they may not produce revenue in excess of the costs of development. If we
are unable to successfully develop the products that are currently under development, we may suffer
financial difficulties, which may have a material adverse effect on our business, financial
condition and operating results.
If we are unsuccessful in clinical trials, our lack of success could have a material adverse effect
on our prospects.
The development of new products requires extensive clinical trials and procedures. Such clinical
trials are inherently uncertain and there can be no assurance that these trials or procedures will
be completed in a timely or cost effective manner or result in a commercially viable product.
Failure to successfully complete these trials or procedures in a timely and cost effective manner
could have a material adverse effect on our prospects. Clinical trials may experience significant
setbacks even after earlier trials have shown promising results. In addition, results from our
clinical trials or procedures may not be supported by actual long-term studies or clinical
experience. If initial results cannot be supported by actual long-term studies or clinical
experience, our business could be adversely affected. Clinical trials may be suspended or
terminated by us, the FDA, or other regulatory authorities at any time if it is believed that the
trial participants face unacceptable health risks.
If we acquire other companies or businesses, we will be subject to risks that could hurt our
business.
We may pursue acquisitions to obtain complementary businesses, products or technologies. Any such
acquisition may not produce the revenues, earnings or business synergies that we anticipate and an
acquired business, product or technology might not perform as we expect. Our management could
spend a significant amount of time, effort and expense to identify, pursue and complete an
acquisition. If we complete an acquisition, we may encounter significant difficulties and incur
substantial expenses in integrating the operations and personnel of the acquired company into our
operations while striving to preserve the goodwill of the acquired company. In particular, we may
lose the services of key employees of the acquired company and we may make changes in management
that impair the acquired company’s relationships with employees and customers.
Any of these outcomes could prevent us from realizing the anticipated benefits of an acquisition.
In addition, to fund an acquisition we might use stock or cash. In the alternative we might borrow
money from a bank or other lender. If we use stock, our stockholders would experience dilution of
their ownership
18
Table of Contents
interests. If we use cash or debt financing, our financial liquidity would be reduced. We may
also be required to capitalize a significant amount of intangibles, including goodwill, which may
lead to significant amortization or write-off charges. These amortization charges and write-offs
could decrease our future earnings or increase our future losses.
Any termination of our right to manufacture pyrolytic carbon components under our carbon technology
agreement with Carbomedics, or any delay or interruption in our manufacturing of these components,
could delay product delivery or force us to cease operations.
We license patented technology and other proprietary rights from CarboMedics. Under our agreement
with Carbomedics, we have obtained a license to use CarboMedics’ pyrolytic carbon technology to
manufacture components for the ATS mechanical heart valve. The license permits us to use and sell
only valves with a specific configuration as described in the agreement, and to manufacture
components only for these valves. It does not include the right to sublicense the technology or to
manufacture components for third parties. If our use of the licensed technology is deemed to fall
outside the scope of the agreement, or if the agreement is otherwise breached or alleged to have
been breached or if it is terminated, we would lose our right to manufacture components for the ATS
mechanical heart valve. In addition, although we anticipate that our manufacturing capacity will
be sufficient to meet our current and foreseeable carbon component needs, it is possible that our
inventory could be exhausted or that we could become unable to manufacture carbon components. In
any of these cases, it is unlikely that we will be able to obtain the necessary carbon components
from any other source. If we are unable to obtain these carbon components from other sources, we
could be forced to reduce or cease operations.
We may encounter litigation that could have a material impact on our business.
We may be subject to product liability claims, intellectual property infringement claims or other
lawsuits, proceedings and claims arising in the ordinary course of business or otherwise. Although
we do not believe that any lawsuits, claims or proceedings arising in the ordinary course of
business will have a material adverse impact on our business, operating results or financial
condition, it is possible that unfavorable resolutions of any lawsuits, claims or proceedings could
have an adverse effect on our business, results of operation or financial condition because of the
uncertainty inherent in litigation.
We may face product liability claims, which could result in losses in excess of our insurance
coverage and which could negatively affect our ability to attract and retain customers.
The manufacture and sale of mechanical heart valves, tissue heart valves and surgical cryoablation
products entails significant risk of product liability claims and product recalls. Mechanical
heart valves, tissue heart valves and valve repair products are life-sustaining devices, and the
failure of any valve or repair product usually results in the patient’s death or need for
re-operation. In addition, our tissue heart valves are made primarily from animal or cadaver
tissue. While we closely monitor the sources from which we obtain the tissue used in our tissue
heart valves and are not aware of any issues relating to the transmission of infection due to the
use of our tissue heart valves, there is a risk of potential transmission of infection to a patient
if the tissue used in the valve is infected. Further, the use of products we sell for the
treatment of arrhythmia may result in a number of serious complications, including damage to the
heart, internal bleeding or other complications, including death. A product liability claim or
product recall, regardless of the ultimate outcome, could require us to spend significant time and
money in litigation or to pay significant damages and could seriously harm our business. We
currently maintain product liability insurance coverage in an aggregate amount of $25 million.
However, we cannot be assured that our current insurance coverage is adequate to cover the costs of
any product liability claims made against us. Product liability insurance is expensive and does
not cover the costs of a product recall. In the future, product liability insurance may not be
available at satisfactory rates or in adequate amounts. A product liability claim or product
recall could also materially and adversely affect our ability to attract and retain customers.
Our business would be adversely affected if we are not able to protect our intellectual property
rights.
Our success depends in part on our ability to maintain and enforce our patents and other
proprietary rights. We rely on a combination of patents, trade secrets, know-how and
confidentiality agreements to protect the proprietary aspects of our technology. These measures
afford only limited protection, and competitors may
19
Table of Contents
gain access to our intellectual property and proprietary information. The patent positions of
medical device companies are generally uncertain and involve complex legal and technical issues.
Litigation may be necessary to enforce our intellectual property rights, to protect our trade
secrets and to determine the validity and scope of our proprietary rights. Any litigation could be
costly and divert our attention from the growth of the business. We cannot assure you that our
patents and other proprietary rights will not be successfully challenged, or that others will not
independently develop substantially equivalent information and technology or otherwise gain access
to our proprietary technology.
We may be sued by third parties claiming that our products infringe on their intellectual property
rights. Any such suits could result in significant litigation or licensing expenses or we might be
prevented from selling our products.
We may be exposed to future litigation by third parties based on intellectual property infringement
claims. Any claims or litigation against us, regardless of the merits, could result in substantial
costs and could harm our business. In addition, intellectual property litigation or claims could
force us to:
| • | cease manufacturing and selling our product, which would seriously harm us; | ||
| • | obtain a license from the holder of the infringed intellectual property right, which license may not be available on reasonable terms, if at all; or | ||
| • | redesign our product, which could be costly and time-consuming. |
If the value of our goodwill or other intangible assets become impaired, it could materially reduce
the value of our assets and reduce our net income for the year in which the related impairment
charges occur.
We apply the applicable accounting principles set forth in the U.S. Financial Accounting Standards
Board’s Accounting Standards Codification to our intangible assets (including goodwill), which
prohibits the amortization of intangible assets with indefinite useful lives and requires that
these assets be reviewed for impairment at least annually. The test for impairment requires our
management to make estimates about fair value that are based either on the expected undiscounted
future cash flows or on other measures of value such as the market capitalization of ATS. If the
carrying amount of the assets is greater than the measures of fair value, impairment is considered
to have occurred and a write-down of the asset is recorded. Our management completed impairment
testing on all of the Company’s intangible assets (including goodwill) as of the end of 2009 and
determined that our goodwill and other intangible assets were not impaired. We may, however, have
future impairment adjustments to the recorded values of our intangible assets, including goodwill.
Any finding that the value of our intangible assets has been impaired would require us to
write-down the impaired portion, which could materially reduce the value of our assets and reduce
our net income for the year in which the related impairment charges occur.
As of December 31, 2009, we had a net carrying value of approximately $61.1 million in goodwill and
other definite-lived intangible assets recorded, which largely represents intangible assets
connected with our acquisitions of 3F and the surgical cryoablation business of CryoCath. The
amount of the purchase price allocated to goodwill and other intangible assets in connection with
the acquisition of 3F is approximately $27.4 million. The amount of the purchase price allocated
to goodwill and other intangible assets in connection with the purchase of the surgical
cryoablation business of CryoCath is approximately $23.7 million. Our estimates of useful lives on
definite-lived intangibles are based upon available information and assumptions that we believe are
reasonable. However, there can be no assurance that the actual useful lives will not differ
significantly from our estimates. The amortization of definite-lived intangible assets could
result in net losses for ATS for the foreseeable future, which could have a material adverse effect
on the market value of our common stock.
We use a combination of direct sales persons and independent sales representatives to sell our
products. If our sales strategy is not successful, we may not be able to continue our operations.
The sales approach for the sale of our products consists primarily of direct salespersons with a
few independent sales representatives. We will need to continue to expend significant funds and
management resources to develop and maintain this hybrid sales force. We believe that there is
significant competition for sales personnel and independent sales representatives with the advanced
sales skills and technical
20
Table of Contents
knowledge we need. If we are unable to recruit, retain and motivate qualified personnel and
representatives, sales of our products could be adversely affected. The loss of key salespersons
or independent sales representatives could have a material adverse effect on our sales or potential
sales to current customers and prospects serviced by such salespersons or representatives.
Further, we cannot assure the successful expansion of our network of independent sales
representatives on terms acceptable to ATS, if at all, or the successful marketing of our products
by our hybrid sales force. To the extent we rely on sales through independent sales
representatives, any revenues we receive will depend primarily on the efforts of these parties. We
do not control the amount and timing of marketing resources that these third parties devote to our
product. If our sales strategy is not successful, we may be forced to change it. Any such change
could disrupt our sales. Further, any change in our sales strategy could be expensive and would
likely have a material adverse impact on our operating results.
We currently depend on the marketing and sales efforts of international independent distributors.
In many countries our products are sold through independent distributors. The loss of an
international distributor could seriously harm our business and operating results if a new
distributor could not be found on a timely basis in the relevant geographic market. We do not
control the amount and timing of marketing resources that these third party distributors devote to
our product. Furthermore, to the extent we rely on sales through independent distributors, any
revenues we receive will depend primarily on the efforts of these parties.
Because we have limited manufacturing experience with some of our products, we may not realize the
expected cost savings related to manufacturing our own products. In addition, we could experience
production delays and significant additional costs.
We have limited experience manufacturing tissue heart valves and surgical cryoablation products.
Our inability to manufacture these products in a cost-effective manner could adversely affect our
business and results of operations. We cannot be certain that we will be able to manufacture
commercial quantities of tissue heart valves or fully develop internal manufacturing capabilities
for surgical cryoablation products in a cost-effective manner. In addition, in the future, as we
continue to increase production, we may encounter difficulties in maintaining and expanding our
manufacturing, including problems involving:
| • | production yields; | ||
| • | quality control; | ||
| • | per unit manufacturing costs; | ||
| • | shortages of qualified personnel; and | ||
| • | compliance with FDA and international regulations and requirements regarding good manufacturing practices. |
Difficulties encountered by us in establishing or maintaining commercial-scale manufacturing
facilities may limit our ability to manufacture our products and therefore could seriously harm our
business, financial condition and operating results.
The price of our common stock has been volatile, which may result in losses to investors.
The trading price of our common stock has been and may continue to be subject to wide fluctuations.
The market price of our common stock could be impacted by the following:
| • | general market conditions; | ||
| • | actual or anticipated sales of common stock by existing shareholders, whether in the market or in subsequent public offerings; | ||
| • | capital commitments; | ||
| • | the success of our management in operating ATS effectively; |
21
Table of Contents
| • | the failure of our heart valves and other products to gain market acceptance in the United States; | ||
| • | announcements of technical innovations or new products by our competitors; | ||
| • | the status of component supply arrangements; | ||
| • | changes in reimbursement policies; | ||
| • | government regulation; | ||
| • | developments in patent or other proprietary rights; | ||
| • | public concern as to the safety and efficacy of products developed by us or others; and | ||
| • | armed conflict, war or terrorism. |
In addition, due to one or more of the foregoing factors, in future years our operating results may
fall below the expectations of securities analysts and investors. In that event, the market price
of our common stock could be materially and adversely affected. Finally, in recent years the stock
market has experienced extreme price and volume fluctuations. This volatility has had a
significant effect on the market prices of securities issued by many companies for reasons
unrelated to their operating performance. These broad market fluctuations may materially adversely
affect our stock price, regardless of our operating results. The stock markets in general have
experienced extreme volatility that has at times been unrelated to the operating performance of
particular companies. These broad market fluctuations may adversely affect the trading price of
our common stock, make it difficult to predict the market price of our common stock in the future
and cause the value of an investment in our common stock to decline.
Our charter documents and Minnesota law may discourage and could delay or prevent a takeover of our
company.
Provisions of our articles of incorporation, bylaws and Minnesota law could make it more difficult
for a third party to acquire us, even if doing so would be beneficial to our shareholders. These
provisions include the following:
| • | No cumulative voting by shareholders for directors; | ||
| • | The ability of our Board of Directors to control its size, to create new directorships and to fill vacancies; | ||
| • | The ability of our Board of Directors, without shareholder approval, to issue preferred stock, which may have rights and preferences that are superior to our common stock; | ||
| • | The ability of our Board of Directors to amend the bylaws; and | ||
| • | Restrictions under Minnesota law regarding mergers or other business combinations between us and any holder of 10% or more of our outstanding common stock. |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not Applicable.
| ITEM 2. | PROPERTIES |
We lease approximately 64,000 square feet of space in two adjacent buildings in Plymouth,
Minnesota. The first building lease, covering approximately 39,000 square feet, expires on July
31, 2010 and is used for administrative, production and engineering purposes. The lease on the
second building (25,000 square feet) also expires July 31, 2010 and is used for carbon
manufacturing and research and development. Both of our Minnesota leases carry a three-year
renewal option. We also lease approximately 16,000 square feet of space in Lake Forest,
California. This lease expires on September 30, 2012 and is used for research and development and
manufacturing purposes. Outside the United States, we lease sales and marketing offices in China,
France, Germany and Belgium. We believe that our facilities are adequate for our current needs.
22
Table of Contents
| ITEM 3. | LEGAL PROCEEDINGS |
Abbey Litigation
In January 2006, following execution of a Merger Agreement between the Company and 3F Therapeutics,
Inc. (“3F”), 3F was informed of a summons and complaint which was filed in the U.S. District Court
in the Southern District of New York by Arthur N. Abbey (“Abbey”) against 3F Partners Limited
Partnership II (a major stockholder of 3F, “3F Partners II”), Theodore C. Skokos (the then chairman
of the board and a stockholder of 3F), 3F Management II, LLC (the general partner of 3F Partners
II), and 3F (collectively, the “Defendants”) (the “Abbey I Litigation”). The summons and complaint
alleges that the Defendants committed fraud under federal securities laws, common law fraud and
negligent misrepresentation in connection with the purchase by Abbey of certain securities of 3F
Partners II. In particular, Abbey claims that the Defendants induced Abbey to invest $4 million in
3F Partners II, which, in turn, invested $6 million in certain preferred stock of 3F, by allegedly
causing Abbey to believe, among other things, that such investment would be short-term. Pursuant to
the complaint, Abbey is seeking rescission of his purchase of his limited partnership interest in
3F Partners II and return of the amount paid therefor (together with pre-and post-judgment
interest), compensatory damages for the alleged lost principal of his investment (together with
interest thereon and additional general, consequential and incidental damages), general damages for
all alleged injuries resulting from the alleged fraud in an amount to be determined at trial and
such other legal and equitable relief as the court may deem just and proper. Abbey did not
purchase any securities directly from 3F and is not a stockholder of 3F. In March 2006, 3F filed a
motion to dismiss the complaint. In August 2007, the Court granted 3F’s motion to dismiss the
complaint based on plaintiff’s failure to state a claim upon which relief may be granted and
ordered the Clerk of the Court to close the case. Abbey filed a Notice of Appeal with the United
States Court of Appeals for the Second Circuit seeking to reverse the District Court’s August 2007
Order dismissing the case. In December 2008, the Second Circuit issued a Summary Order that
affirmed the District Court’s judgment of dismissal finding that Abbey failed to state a claim
against 3F. However, the Second Circuit remanded the case to the District Court to allow Abbey a
chance to replead his claims. On or about February 13, 2009, Abbey filed an amended complaint
which purports to allege additional facts to support the same claims against 3F that were asserted
and dismissed in the original complaint. On or about March 31, 2009, 3F served and filed its
motion to dismiss the amended complaint with prejudice. 3F’s motion to dismiss was fully submitted
on June 8, 2009.
In June 2006, Abbey commenced a second civil action in the Court of Chancery in the State of
Delaware by serving 3F with a complaint naming both 3F and Mr. Skokos as defendants (the “Abbey II
Litigation”). The complaint alleges, among other things, fraud and breach of fiduciary duties in
connection with the purchase by Abbey of his partnership interest in 3F Partners II. The Delaware
action seeks: (1) a declaration that (a) for purposes of the merger, Abbey was a record stockholder
of 3F and was thus entitled to withhold his consent to the merger and seek appraisal rights after
the merger was consummated and (b) the irrevocable stockholder consent submitted by 3F Partners II
to approve the merger be voided as unenforceable; and (2) damages based upon allegations that 3F
aided and abetted Mr. Skokos in breaching Mr. Skokos’s fiduciary duties of loyalty and faith to
Abbey. On July 17, 2006, 3F filed a motion to dismiss the complaint in the Abbey II Litigation,
or, alternatively, to stay the action pending adjudication of the Abbey I Litigation. In October
2006, the Delaware Chancery Court entered an order staying the Delaware action pending the outcome
of the Abbey I litigation. In August 2007, the parties informed the Delaware Chancery Court that
they would consent to the continued stay of the Delaware action pending the outcome of Abbey’s
appeal of the Abbey I Litigation. The stay remains in effect pending the outcome of 3F’s motion to
dismiss the amended complaint. By Order entered December 2, 2009, the District Court denied 3F’s
motion to dismiss on the ground of lack of standing and converted the remainder of 3F’s motion to
dismiss for failure to state a claim into a motion for summary judgment. Accordingly, the Court
ordered “limited discovery” to be completed by February 26, 2010 and additional briefings on
summary judgment to be completed by April 9, 2010. By Stipulation and Order, this schedule has
been modified and discovery is to be completed by April 2, 2010 and additional briefings on summary
judgment are to be fully submitted by May 17, 2010.
3F has been notified by its director and officer insurance carrier that such carrier will defend
and cover all defense costs as to 3F and Mr. Skokos in the Abbey I Litigation and Abbey II
Litigation, subject to policy terms and full reservation of rights. In addition, under the Merger
Agreement, 3F and the 3F stockholder
23
Table of Contents
representative have agreed that the Abbey I Litigation and Abbey II Litigation are matters for
which express indemnification is provided. As a result, certain escrow shares and contingent
shares, if any, which may in the future be issued under the Merger Agreement, may be used by ATS to
satisfy, in part, ATS’s set-off rights and indemnification claims for damages and losses incurred
by 3F or ATS, and their directors, officers and affiliates, that are not otherwise covered by
applicable insurance arising from the Abbey I Litigation and Abbey II Litigation. See Note 5 of
“Notes to Consolidated Financial Statements” in this Form 10-K for a description of the contingent
shares. The Company believes the Abbey I Litigation and Abbey II Litigation will not have a
material impact on the Company’s financial position or operating results.
| ITEM 4. | (REMOVED AND RESERVED) |
This item was removed and reserved pursuant to SEC Release No. 33-9089A issued on February 23, 2010.
24
Table of Contents
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on the NASDAQ Global Market under the symbol “ATSI.” The following
table sets forth, for the periods indicated, the high and low closing sales prices per share of our
common stock as reported on the NASDAQ Global Market. These prices do not include adjustments for
retail mark-ups, mark-downs, or commissions.
| Fiscal Year 2008: | High | Low | ||||||
First Quarter |
$ | 2.18 | $ | 1.41 | ||||
Second Quarter |
$ | 2.34 | $ | 1.41 | ||||
Third Quarter |
$ | 3.24 | $ | 2.06 | ||||
Fourth Quarter |
$ | 2.89 | $ | 1.98 | ||||
| Fiscal Year 2009: | High | Low | ||||||
First Quarter |
$ | 2.84 | $ | 2.11 | ||||
Second Quarter |
$ | 3.37 | $ | 2.35 | ||||
Third Quarter |
$ | 3.50 | $ | 2.58 | ||||
Fourth Quarter |
$ | 3.26 | $ | 2.52 | ||||
Holders
As of February 26, 2010, we had approximately 549 holders of record of our common stock.
Dividends
We are currently restricted from declaring or paying dividends on our common stock under our loan
agreements with Silicon Valley Bank (the “Bank”). We have never declared or paid cash dividends in
the past and intend to retain all future earnings for the operation and expansion of our business.
Repurchases of Common Stock
We did not repurchase any of our securities during the fourth quarter of 2009.
Sales of Unregistered Securities
We had no sales of unregistered securities during 2009 that have not been previously disclosed in a
Current Report on Form 8-K or Quarterly Report on Form 10-Q.
25
Table of Contents
Performance Graph
The graph below compares the cumulative total shareholder return on our common stock since December
31, 2004 with the cumulative return of the Standard & Poor’s 500 Stock Index and the NASDAQ Medical
Devices, Instruments and Supplies Index over the same period (assuming the investment of $100 in
each vehicle on December 31, 2004 and reinvestment of all dividends).
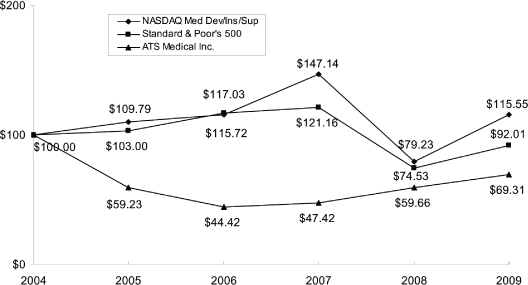
| Name | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | ||||||||||||||||||
NASDAQ Medical Dev/Ins/Sup |
$ | 100.00 | $ | 109.79 | $ | 115.72 | $ | 147.14 | $ | 79.23 | $ | 115.55 | ||||||||||||
Standard & Poor’s 500 Stock Index |
100.00 | 103.00 | 117.03 | 121.16 | 74.53 | 92.01 | ||||||||||||||||||
ATS Medical, Inc. |
100.00 | 59.23 | 44.42 | 47.42 | 59.66 | 69.31 | ||||||||||||||||||
26
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
| Year Ended December 31, | ||||||||||||||||||||
| (in thousands, except per share data) | 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||||
Statement of Operations Data: |
||||||||||||||||||||
Net sales |
$ | 75,710 | $ | 65,821 | $ | 49,587 | $ | 40,449 | $ | 34,636 | ||||||||||
Cost of sales |
26,821 | 25,267 | 21,348 | 19,568 | 22,828 | |||||||||||||||
Gross profit |
48,889 | 40,554 | 28,239 | 20,881 | 11,808 | |||||||||||||||
Operating expenses: |
||||||||||||||||||||
Sales and marketing |
30,617 | 27,373 | 24,633 | 21,008 | 18,948 | |||||||||||||||
Research and development |
8,863 | 8,215 | 7,546 | 3,381 | 1,733 | |||||||||||||||
Acquired in-process research
and development |
— | — | 3,500 | 14,400 | — | |||||||||||||||
General and administrative |
9,905 | 10,509 | 10,417 | 8,786 | 7,314 | |||||||||||||||
Litigation settlement |
— | 7,500 | — | — | — | |||||||||||||||
Amortization of intangibles |
3,224 | 3,489 | 2,516 | 106 | — | |||||||||||||||
Intangible asset impairment |
— | — | 755 | — | — | |||||||||||||||
Distributor termination expense |
— | — | — | 733 | — | |||||||||||||||
Total operating expenses |
52,609 | 57,086 | 49,367 | 48,414 | 27,995 | |||||||||||||||
Operating loss |
(3,720 | ) | (16,532 | ) | (21,128 | ) | (27,533 | ) | (16,187 | ) | ||||||||||
Interest income (expense), net |
(2,696 | ) | (2,739 | ) | (1,822 | ) | (1,669 | ) | (338 | ) | ||||||||||
Other income, net |
391 | 413 | 61 | 1,528 | 2,131 | |||||||||||||||
Net loss before income taxes |
(6,025 | ) | (18,858 | ) | (22,889 | ) | (27,674 | ) | (14,394 | ) | ||||||||||
Income tax expense |
288 | 481 | 119 | — | — | |||||||||||||||
Net loss |
($ | 6,313 | ) | ($ | 19,339 | ) | ($ | 23,008 | ) | ($ | 27,674 | ) | ($ | 14,394 | ) | |||||
Net loss per share: |
||||||||||||||||||||
Basic |
($ | 0.09 | ) | ($ | 0.31 | ) | ($ | 0.44 | ) | ($ | 0.83 | ) | ($ | 0.46 | ) | |||||
Diluted |
($ | 0.09 | ) | ($ | 0.31 | ) | ($ | 0.44 | ) | ($ | 0.83 | ) | ($ | 0.46 | ) | |||||
Weighted average number of shares outstanding: |
||||||||||||||||||||
Basic |
72,114 | 61,440 | 52,589 | 33,537 | 31,009 | |||||||||||||||
Diluted |
72,114 | 61,440 | 52,589 | 33,537 | 31,009 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| (in thousands) | 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||
Cash, cash equivalents and short-term
investments |
$ | 14,235 | $ | 20,895 | $ | 14,669 | $ | 10,704 | $ | 21,709 | ||||||||||
Working capital |
20,951 | 37,976 | 30,121 | 32,976 | 46,417 | |||||||||||||||
Total assets |
121,255 | 114,981 | 105,897 | 85,840 | 85,443 | |||||||||||||||
Long-term liabilities, excluding current maturities |
2,113 | 21,789 | 25,321 | 18,588 | 19,679 | |||||||||||||||
Shareholders’ equity |
88,872 | 74,575 | 64,956 | 57,890 | 57,529 | |||||||||||||||
27
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Executive Overview
ATS Medical, Inc. (hereinafter the “Company”, “ATS”, “we”, “us” or “our”) develops, manufactures,
and markets medical devices for the treatment of structural heart disease used by cardiovascular
surgeons in the cardiac surgery operating theater. Currently, we participate in the markets for
heart valve therapy including mechanical bileaflet replacement heart valves, tissue heart valves
and valve repair products and the surgical treatment of cardiac arrhythmias, primarily the
treatment of atrial fibrillation. Additionally, a small portion of our business is comprised of
surgical tools and accessories used by the cardiac surgeon for the treatment of structural heart
disease.
In 1990, we licensed a patented and partially developed mechanical heart valve from CarboMedics,
Inc. Under the terms of the license, we would complete the development of the valve and agreed to
purchase carbon components from CarboMedics. As a result, ATS now holds an exclusive,
royalty-free, worldwide license to an open pivot, bileaflet mechanical heart valve design owned by
CarboMedics. In addition, we have an exclusive, worldwide right and license to use CarboMedics’
pyrolytic carbon technology to manufacture components for the ATS mechanical heart valve. We
commenced selling the ATS mechanical heart valve in international markets in 1992. In 2000, we
received FDA approval to sell the ATS Open Pivot mechanical heart valve and commenced sales and
marketing of our valve in the United States.
During 2002, we reorganized the Company and began the process of rebuilding our sales and marketing
teams, both in the United States and internationally. This rebuilding has been a significant
factor in our operating expense levels since 2002. During 2004 and 2005, we developed and
implemented a plan to ramp-up our own manufacturing facility for pyrolytic carbon. By the end of
2005, this process was substantially complete.
During 2004, we made our first investments outside the mechanical heart valve market. We completed
a global partnership agreement with CryoCath Technologies, Inc. to market CryoCath’s surgical
cryotherapy products for the ablation of cardiac arrhythmias. CryoCath developed a portfolio of
novel products marketed under the SurgiFrost® and FrostByte® trade names which are used by cardiac
surgeons to treat cardiac arrhythmias. Treatment is accomplished through the creation of an
intricate pattern of lesions on the surface of the heart to block inappropriate electrical
conduction circuits which cause the heart to be less effective when pumping blood and can lead to
stroke, heart failure and death. Unique to this technology is the use of cryothermy (cold) to
create lesions. The agreement with CryoCath has resulted in revenues for ATS since 2005.
During 2005, we continued to expand our business outside the mechanical heart valve market. We
entered into an exclusive development, supply and distribution agreement with Genesee BioMedical,
Inc. under which GBI develops, supplies and manufactures cardiac surgical products to include
annuloplasty repair rings and bands and accessories, and we have exclusive worldwide rights to
market and sell such products. Our agreement with GBI has produced revenues for us since 2006.
In 2006, we completed the acquisition of all the voting and non-voting stock of 3F Therapeutics,
Inc., an early stage privately-held medical device company at the forefront of the emerging field
of minimally invasive open- and closed-chest tissue valve replacement. The acquisition of 3F was a
major step in the execution of our long-standing vision of obtaining a leadership position in the
major segments of the cardiac surgery market. The acquisition was consummated pursuant to an
agreement and plan of merger, as amended (“the Merger Agreement”). Under the terms of the Merger
Agreement, upon closing, we paid each 3F stockholder its pro-rata portion of an initial payment of
9,000,000 shares of our common stock, subject to certain adjustments. In December 2009, we issued
5,000,000 shares of common stock to 3F stockholders under the Merger Agreement upon obtaining the
European CE mark on our Enable Aortic Bioprosthesis sutureless tissue valve product. In addition,
we are obligated under the Merger Agreement to make an additional contingent payment to 3F
stockholders of up to 5,000,000 shares of our common stock with shares issuable upon obtaining U.S.
FDA approval on the Enable product or FDA or European CE mark approval for certain future key
products acquired in the 3F acquisition on or prior to December 31, 2013. Milestone share payments
may be accelerated upon completion of certain transactions involving these future key products.
The first generation tissue valve of the 3F portfolio, the ATS 3f Aortic
28
Table of Contents
Bioprosthesis, has received both FDA approval and the European CE mark and is available for sale in
the United States, Europe and certain other international markets. In December 2009, we received
European CE Mark approval of our second tissue valve, the sutureless ATS 3f Enable Aortic
Bioprosthesis, which is intended to enable less invasive aortic valve replacement. We are also
developing an aortic tissue valve for use in beating heart procedures based in part on the
characteristics of the next generation of Enable valves. First in-human studies of this technology
is targeted for 2010, and if successful, commercialization of a beating heart solution could occur
within one to two years thereafter.
Also in 2006, we entered into an exclusive distribution agreement with Novare Surgical Systems,
Inc. (“Novare”). Novare is the owner of the Enclose II® cardiac anastomosis assist device, which
is a device used by cardiac surgeons to attach a bypass vessel to the aorta during coronary artery
bypass graft surgery. Under the terms of the agreement, we held the exclusive right to market,
sell and distribute the Enclose II product in the United States, Germany, France and the United
Kingdom. We discontinued selling the Enclose II device in August 2009.
In June 2007, we acquired the cryoablation surgical device business of CryoCath. The acquisition
included the SurgiFrost, FrostByte and SurgiFrost XL family of products for which we had served as
CryoCath’s exclusive agent in the United States and distributor in certain international markets.
Under the acquisition agreement, we paid CryoCath $22.0 million upon closing of the transaction
(reduced by $0.9 million subsequent to closing) and $2.0 million during 2008 upon the achievement
of certain manufacturing transition milestones. We also paid $2.0 million in June 2009 (two years
after closing) and agreed to pay up to $4.0 million in contingent payments based on future sales of
Surgifrost XL, an FDA cleared and CE Marked product designed to enable less-evasive ablations.
Based on the results of extensive bench and pre-clinical testing as well as human feasibility
studies, the Company discontinued development of the SurgiFrost XL product in late 2008. The
Company continues to develop less invasive procedures utilizing its existing set of tools. The
acquisition of the cryoablation surgical device business of CryoCath enables us to leverage our
current operating infrastructure and allows us to better address the rapidly growing cardiac
arrhythmia market within cardiac surgery.
Critical Accounting Policies and Estimates
We base our estimates on historical experience and on various other assumptions that we believe to
be reasonable under the circumstances. This discussion and analysis of our financial condition and
results of operations is based upon our consolidated financial statements, which have been prepared
in accordance with generally accepted accounting principles in the United States as set forth by
the Financial Accounting Standards Board (“FASB”) in its Accounting Standards Codification (“ASC”),
and applicable rules and regulations of the SEC. The preparation of these financial statements
requires us to make estimates and judgments that affect (1) the reported amounts of assets,
liabilities, revenues, and expenses and (2) the related disclosure of contingent liabilities. At
each balance sheet date, we evaluate our estimates, including but not limited to, those related to
accounts receivable, inventories, long-lived and intangible assets and income taxes. The critical
accounting policies that are most important in fully understanding and evaluating the financial
condition and results of operations are discussed below.
Revenue Recognition Policy. A significant portion of our revenue in the United States and in our
direct European sales operations is generated from consigned inventory maintained at hospitals or
with field representatives. In these situations, revenue is recognized at the time that the
product has been implanted or used. In all other instances, revenue is recognized at the time
product is shipped. Certain independent distributors in select international markets receive
rebates against invoiced sales amounts. In these situations, we accrue for these rebates at the
time of the original sale. These rebates are treated as a reduction of revenue and have not been
significant. We include shipping and handling costs in cost of goods sold.
Allowance for Doubtful Accounts. We maintain an allowance for doubtful accounts that is calculated
using subjective judgments and estimates to establish this valuation account. Our distribution in
international markets through independent distributors concentrates relatively large amounts of
receivables in relatively few customer accounts. We have successfully done business with most of
these distributors for many years. We monitor amounts that are not paid according to terms. We
attempt to accrue for potential losses due to non-payment. Financial conditions in international
markets can change very quickly and our allowance for doubtful accounts cannot anticipate all
potential changes. Our allowance for
29
Table of Contents
doubtful accounts was approximately $0.4 million at December 31, 2009 and 2008. The allowance as a
percentage of total accounts receivable was 2.8% and 2.4% at December 31, 2009 and 2008,
respectively.
Inventory Valuation. Inventories are recorded and relieved at the lower of manufacturing cost
(first-in, first-out basis) or market (net realizable value). We maintain allowances and reserves
against certain finished goods and work-in-process inventories to cover obsolete and short
shelf-life product, scrap and rework costs and resterilization costs for expired or near-expired
items. These allowances and reserves totaled $1.1 million and $1.0 million at December 31, 2009
and 2008, respectively.
Goodwill and Intangible Assets. We assess the carrying value of goodwill, our only
indefinite-lived intangible asset, in accordance with the provisions of FASB ASC 350,
Intangibles-Goodwill and Other. We review goodwill for impairment annually as of the last day of
the second quarter, or more frequently if a change in circumstances or occurrence of events
suggests the remaining value may not be recoverable. The test for impairment requires management
to make estimates about fair-value which are based either on the expected present value of future
cash flows or on other measures of value such as the market capitalization of the Company. If the
carrying amount of the assets is greater than the measures of fair value, impairment is considered
to have occurred and a write-down of the asset is recorded.
Definite-lived intangible assets consist of purchased technology and patents, technology licenses
and agreements, trademarks, trade names and distribution intangibles, which are carried at
amortized cost. The Company assesses definite-lived intangible asset impairment in accordance with
FASB ASC 360-10-35, Property, Plant and Equipment-Overall-Subsequent Measurement. If a triggering
event occurs, the Company assesses the recoverability of definite-lived intangible assets by
reference to future gross profit cash flows from the underlying products utilizing the capitalized
intangible assets.
As of December 31, 2009, we completed impairment testing on all of our intangible assets and
determined that the carrying value of our intangible assets, including goodwill, were not impaired,
were recoverable and that no impairment charges were necessary. During 2007, we recorded an
impairment charge of $0.8 million related to licensing fee and development milestone payments made
to a Swedish research firm related to blood filtration technology for cardiac surgery procedures.
Convertible Debt and Derivative Instruments. We account for embedded derivatives related to our
Convertible Senior Notes under FASB ASC 815, Derivatives and Hedging, and applicable SEC rules,
which require certain embedded derivative financial instruments to be bifurcated from the debt
agreement and accounted for as a liability. Our Convertible Senior Notes contain several embedded
derivatives. The valuation of derivatives requires management to make certain judgments and
estimates, including the potential future fair value of our common stock, the probability of a
change in control of the Company and the probability that the debt may be put back to or called by
us.
Fair Value Measurements. We apply fair value measurement accounting principles related to our
Convertible Senior Notes derivative liability, goodwill and certain other assets and liabilities
pursuant to FASB ASC 820, Fair Value Measurements and Disclosures (“ASC 820”). ASC 820 establishes
a framework for measuring fair value in accordance with GAAP and expands disclosures about fair
value measurements. ASC 820 defines fair value as the price that would be received for an asset or
paid to transfer a liability (an exit price) in the principal or most advantageous market for the
asset or liability in an orderly transaction between market participants on the measurement date.
ASC 820 also describes three levels of inputs that may be used to measure fair value. See Note 13
of “Notes to Consolidated Financial Statements” in this Form 10-K for a discussion of the
application of ASC 820 to specific assets and liabilities.
Deferred Tax Assets. We have incurred cumulative tax losses of approximately $167 million. The
losses are carried forward for U.S. and state corporate income taxes and can be used to reduce
future taxable income. As a result, at December 31, 2009, we had net deferred tax assets totaling
approximately $64.2 million. We have recorded a full valuation allowance against these assets
because of the limited lives of the carryforwards and our lack of earnings history, which has
resulted in our conclusion that it is not more than likely we will be able to utilize our loss
carryforwards. The ability to utilize a portion of our cumulative tax losses to offset future
taxable income is subject to certain limitations under Section 382 and 383 of the Internal Revenue
Code due to changes in the equity ownership of the Company. In addition, 3F’s tax loss
carryforwards may also be limited by separate return limitation year rules.
30
Table of Contents
Stock-Based Compensation. We account for our stock-based employee compensation plans under
the recognition and measurement principles of FASB ASC 718, Stock Compensation, which requires all
share-based payments to be recognized in the income statement based on their fair values. We issue
both stock options and restricted stock unit awards (“RSUs”) to our employees. The fair value of
stock option grants is determined based on the Black-Scholes-Merton (“Black-Scholes”) option
pricing model. The fair value of RSUs is determined based on the closing market price on the award
date.
Recently Issued Accounting Pronouncements. See Note 14 of “Notes to Consolidated Financial
Statements” in this Form 10-K for a discussion of recently issued accounting pronouncements
impacting our Company.
Results of Operations
The following table provides the dollar and percentage change in our Statements of Operations for
2009 compared to 2008 and 2008 compared to 2007 (dollars in thousands).
| Increase (Decrease) | Increase (Decrease) | |||||||||||||||||||||||||||||||
| 2009 | 2008 | $ | % | 2008 | 2007 | $ | % | |||||||||||||||||||||||||
Net sales |
$ | 75,710 | $ | 65,821 | $ | 9,889 | 15.0 | % | $ | 65,821 | $ | 49,587 | $ | 16,234 | 32.7 | % | ||||||||||||||||
Cost of goods sold |
26,821 | 25,267 | 1,554 | 6.2 | % | 25,267 | 21,348 | 3,919 | 18.4 | % | ||||||||||||||||||||||
Gross profit |
48,889 | 40,554 | 8,335 | 20.6 | % | 40,554 | 28,239 | 12,315 | 43.6 | % | ||||||||||||||||||||||
Operating expenses: |
||||||||||||||||||||||||||||||||
Sales and marketing |
30,617 | 27,373 | 3,244 | 11.9 | % | 27,373 | 24,633 | 2,740 | 11.1 | % | ||||||||||||||||||||||
Research and development |
8,863 | 8,215 | 648 | 7.9 | % | 8,215 | 7,546 | 669 | 8.9 | % | ||||||||||||||||||||||
Acquired in-process R&D |
— | — | — | 0.0 | % | — | 3,500 | (3,500 | ) | (100.0 | )% | |||||||||||||||||||||
General and administrative |
9,905 | 10,509 | (604 | ) | (5.7 | )% | 10,509 | 10,417 | 92 | 0.9 | % | |||||||||||||||||||||
Litigation settlement |
— | 7,500 | (7,500 | ) | (100.0 | )% | 7,500 | — | 7,500 | — | ||||||||||||||||||||||
Amortization of intangibles |
3,224 | 3,489 | (265 | ) | (7.6 | )% | 3,489 | 2,516 | 973 | 38.7 | % | |||||||||||||||||||||
Impairment of intangibles |
— | — | — | 0.0 | % | — | 755 | (755 | ) | (100.0 | )% | |||||||||||||||||||||
Total operating expenses |
52,609 | 57,086 | (4,477 | ) | (7.8 | )% | 57,086 | 49,367 | 7,719 | 15.6 | % | |||||||||||||||||||||
Operating loss |
(3,720 | ) | (16,532 | ) | (12,812 | ) | (77.5 | )% | (16,532 | ) | (21,128 | ) | (4,596 | ) | (21.8 | )% | ||||||||||||||||
Interest expense, net |
(2,696 | ) | (2,739 | ) | (43 | ) | (1.6 | )% | (2,739 | ) | (1,822 | ) | 917 | 50.3 | % | |||||||||||||||||
Other income, net |
391 | 413 | (22 | ) | (5.3 | )% | 413 | 61 | 352 | 577.0 | % | |||||||||||||||||||||
Net loss before income taxes |
(6,025 | ) | (18,858 | ) | (12,833 | ) | (68.1 | )% | (18,858 | ) | (22,889 | ) | (4,031 | ) | (17.6 | )% | ||||||||||||||||
Income tax expense |
288 | 481 | (193 | ) | (40.1 | )% | 481 | 119 | 362 | 304.2 | % | |||||||||||||||||||||
Net loss |
($ | 6,313 | ) | ($ | 19,339 | ) | ($ | 13,026 | ) | (67.4 | )% | ($ | 19,339 | ) | ($ | 23,008 | ) | ($ | 3,669 | ) | (15.9 | )% | ||||||||||
31
Table of Contents
The following table presents our Statements of Operations as a percentage of net sales for
2009, 2008 and 2007.
| 2009 | 2008 | 2007 | ||||||||||
Net sales |
100.0 | % | 100.0 | % | 100.0 | % | ||||||
Cost of goods sold |
35.4 | % | 38.4 | % | 43.1 | % | ||||||
Gross profit |
64.6 | % | 61.6 | % | 56.9 | % | ||||||
Operating expenses: |
||||||||||||
Sales and marketing |
40.4 | % | 41.6 | % | 49.7 | % | ||||||
Research and development |
11.7 | % | 12.5 | % | 15.2 | % | ||||||
Acquired in-process R&D |
0.0 | % | 0.0 | % | 7.1 | % | ||||||
General and administrative |
13.1 | % | 16.0 | % | 21.0 | % | ||||||
Litigation settlement |
0.0 | % | 11.4 | % | 0.0 | % | ||||||
Amortization of intangibles |
4.3 | % | 5.3 | % | 5.1 | % | ||||||
Impairment of intangibles |
0.0 | % | 0.0 | % | 1.5 | % | ||||||
Total operating expenses |
69.5 | % | 86.7 | % | 99.6 | % | ||||||
Operating loss |
(4.9 | )% | (25.1 | )% | (42.6 | )% | ||||||
Interest expense, net |
(3.6 | )% | (4.2 | )% | (3.7 | )% | ||||||
Other income, net |
0.5 | % | 0.6 | % | 0.1 | % | ||||||
Net loss before income taxes |
(8.0 | )% | (28.7 | )% | (46.2 | )% | ||||||
Income tax expense |
(0.4 | )% | (0.7 | )% | (0.2 | )% | ||||||
Net loss |
(8.3 | )% | (29.4 | )% | (46.4 | )% | ||||||
Net Sales
The following table provides the dollar and percentage change in our net sales inside and outside
the United States for 2009 compared to 2008 and 2008 compared to 2007 (dollars in thousands).
| Increase | Increase | |||||||||||||||||||||||||||||||
| 2009 | 2008 | $ | % | 2008 | 2007 | $ | % | |||||||||||||||||||||||||
United States |
$ | 29,775 | $ | 25,139 | $ | 4,636 | 18.4 | % | $ | 25,139 | $ | 18,653 | $ | 6,486 | 34.8 | % | ||||||||||||||||
Outside United States |
45,935 | 40,682 | 5,253 | 12.9 | % | 40,682 | 30,934 | 9,748 | 31.5 | % | ||||||||||||||||||||||
Total |
$ | 75,710 | $ | 65,821 | $ | 9,889 | 15.0 | % | $ | 65,821 | $ | 49,587 | $ | 16,234 | 32.7 | % | ||||||||||||||||
The following table provides our net sales inside and outside the United States as a
percentage of total net sales for 2009, 2008 and 2007.
| 2009 | 2008 | 2007 | ||||||||||
United States |
39.3 | % | 38.2 | % | 37.6 | % | ||||||
Outside United States |
60.7 | % | 61.8 | % | 62.4 | % | ||||||
Total |
100.0 | % | 100.0 | % | 100.0 | % | ||||||
32
Table of Contents
The following table provides our net sales by product group for 2009 compared to 2008 and 2008
compared to 2007 (dollars in thousands).
| Increase (Decrease) | Increase | |||||||||||||||||||||||||||||||
| 2009 | 2008 | $ | % | 2008 | 2007 | $ | % | |||||||||||||||||||||||||
Heart valve therapy |
$ | 56,015 | $ | 47,576 | $ | 8,439 | 17.7 | % | $ | 47,576 | $ | 38,560 | $ | 9,016 | 23.4 | % | ||||||||||||||||
Surgical arrhythmia therapy |
18,881 | 16,888 | 1,993 | 11.8 | % | 16,888 | 9,690 | 7,198 | 74.3 | % | ||||||||||||||||||||||
Surgical tools and
accessories |
814 | 1,357 | (543 | ) | (40.0 | )% | 1,357 | 1,337 | 20 | 1.5 | % | |||||||||||||||||||||
Total |
$ | 75,710 | $ | 65,821 | $ | 9,889 | 15.0 | % | $ | 65,821 | $ | 49,587 | $ | 16,234 | 32.7 | % | ||||||||||||||||
Heart valve therapy sales, our largest product group, consists of mechanical and tissue heart
valves and heart valve repair products. Our mechanical heart valve products continue to be our
primary product line and comprised approximately 61%, 65% and 72% of our total worldwide sales for
2009, 2008 and 2007, respectively, and 82%, 90% and 93% of total heart valve therapy revenues in
2009, 2008 and 2007, respectively. Surgical arrhythmia therapy products consist of cryotherapy
products for the ablation of cardiac arrhythmias. We acquired this business from CryoCath in June
2007. Surgical tools and accessories consist primarily of cardiac anastomosis assist devices
(which we discontinued selling in August 2009) and thoracic port systems.
Net sales for all periods have been favorably impacted by revenue from the acquisitions, new
products and new business initiatives and partnerships discussed above. Approximately 39% of our
worldwide revenue in 2009 was derived from products other than mechanical heart valves, up from
approximately 35% in 2008 and 28% in 2007.
2009 compared to 2008. Worldwide heart valve therapy revenue increased 18% in 2009. A
primary driver of this increase was tissue heart valve revenue, which increased 276% in
2009 to $5.6 million (compared to $1.5 million in 2008) due primarily to the limited U.S.
commercial launch of the Company’s first generation tissue valve product, the ATS 3f Aortic
Bioprosthesis, approved by the FDA in October 2008. U.S. tissue valve revenue comprised
approximately 55% of total tissue valve revenue for 2009. Worldwide mechanical heart valve revenue
for 2009 increased 7% from 2008 due to stronger mechanical heart valve sales in Asia and certain
developing markets. Heart valve repair revenue increased 45% in 2009 compared to 2008 due to the
introduction of our semi-rigid line of repair rings in 2008 as well as to continued sales growth of
our existing repair ring products.
Surgical arrhythmia therapy revenue for 2009 increased 12% compared to 2008. Cryotherapy products
in general and CryoMaze procedural growth in particular has benefited from the overall growth of
the surgical ablation market and increased market acceptance of cryo-energy (cold) as a preferred
technology to perform Cox-Maze lesion sets.
Our total net sales increase for 2009 of 15% was negatively impacted by approximately 210 basis
points related to lower foreign currency exchange rates against the U.S. dollar during 2009.
Approximately 20% of our total sales for 2009 were invoiced in Euros or other local currencies in
European markets where we sell our products directly to hospitals.
2008 compared to 2007. Heart valve therapy revenue increased 23% in 2008 compared to 2007 due
primarily to higher international mechanical heart valve revenue, which increased approximately 26%
in 2008, attributable to the expansion of direct selling operations in certain international
markets and to stronger mechanical heart valve sales in developing markets. U.S. mechanical heart
valve revenue in 2008 increased 2% from 2007, the result of the introduction of a new mechanical
valve offering in 2008, the AP 360 valve, which allowed us to take market share from competitors.
Tissue heart valve revenue increased 173% in 2008 to $1.5 million, driven primarily by a limited
commercial launch in certain western European markets. Heart valve repair revenue increased 80% in
2008 to $3.2 million, due to the introduction of our semi-rigid line of repair rings in the first
quarter of 2008 as well as to continued sales growth of our existing repair ring products.
33
Table of Contents
Surgical arrhythmia therapy revenue in 2008 of $16.9 million increased 74% compared to 2007. The
most significant driver of the revenue increase was our acquisition of the surgical cryoablation
business of CryoCath in June 2007. Prior to this date we served as an agent and received a
commission on the majority of the sales transactions. After the acquisition our revenue is
representative of end user pricing and the addition of more direct customers and geographies where
CryoCath had previously maintained direct distribution. CryoMaze procedural growth has benefited
from the overall growth of the surgical ablation market and increased market acceptance of
cryo-energy as a preferred technology to perform Cox-Maze lesion sets.
Approximately 200 basis points of our 32.7% increase in net sales for 2008 compared to 2007 is
attributable to higher average foreign currency exchange rates against the U.S. dollar.
Approximately 23% of our total sales in 2008 were invoiced in Euros or other local currencies in
European markets where we sell our products direct to hospitals.
Cost of Goods Sold and Gross Profit
2009 compared to 2008. Our 2009 gross profit percentage of net sales improved to 64.6%,
an increase of 300 basis points compared to 61.6% in 2008. Our 2009 gross profit benefited
significantly from lower product manufacturing costs, particularly for mechanical heart valves.
The cost declines are attributable primarily to higher manufacturing volumes as a result of
increased demand enabling greater leverage of overhead and fixed costs and to product cost
reduction initiatives. Lower product costs increased our 2009 gross profit percentage of net sales
by approximately 500 basis points compared to 2008. Also contributing to the higher 2009 gross
profit percentage were higher U.S. average selling prices, attributable in large part to tissue
valves, which increased our 2009 gross profit percentage of net sales by approximately 10 basis
points compared to 2008.
Partially offsetting the improved 2009 gross profit percentage of net sales were lower
international selling prices due to foreign currency exchange rate changes and geographical sales
mix shifts resulting from higher sales growth in lower-margin developing countries, which had a net
unfavorable impact on our 2009 gross profit percentage of net sales of approximately 210 basis
points compared to the prior year.
2008 compared to 2007. Our 2008 gross profit was 61.6% of revenue, which represents an
increase of 470 basis points compared to 56.9% in 2007. Our 2008 gross profit, both in
dollars and in percentage of net sales, benefited from lower cost internally-manufactured
mechanical heart valves as production significantly increased in 2008 to meet increased mechanical
heart valve demand, primarily in international markets. Greater leverage of manufacturing labor
and overhead in 2008 resulted in a 15% reduction in our mechanical heart valve cost of goods sold
per unit compared to 2007 and contributed to an overall 220 basis points increase in gross profit
percentage of net sales. Our 2008 gross profit percentage of net sales also benefited, by
approximately 90 basis points, from higher international average selling prices associated with our
expansion of direct selling efforts in certain European markets and appreciation of the Euro
against the dollar compared to 2007. Direct sales of surgical cryotherapy products after our
acquisition of the surgical cryoablation business of CryoCath in late June 2007 contributed 260
basis points to our 2008 increase in gross profit percentage of net sales. These sales included
the gross-up on certain sales for which we had served as an agent and received a commission prior
to the acquisition and the addition of direct sales to other CryoCath corporate customers.
Partially offsetting the improved 2008 gross profit percentage of net sales were shifts in the
overall sales mix, both geographic and product-related. The most significant sales mix impact was
related to product mix shifts during 2008 from higher margin mechanical heart valve products to
lower margin cryotherapy and repair ring products, which negatively impacted the 2008 overall gross
profit percentage of net sales by approximately 130 basis points compared to 2007.
Sales and Marketing
2009 compared to 2008. In the United States, our sales and marketing costs for 2009 increased
12.8% over 2008 to $18.7 million. The increase reflects higher marketing program costs,
particularly related to the launch of the ATS 3f Aortic Bioprosthesis tissue valve in the United
States and marketing programs for our cryoablation business, as well as additional marketing
personnel. Field selling costs in the United States were higher by 8.8% for 2009 compared to 2008,
reflecting hospital sales program costs as well as higher
34
Table of Contents
independent sales agent and territory manager commissions connected with increased 2009 sales in
the United States.
Internationally, our sales and marketing costs for 2009 increased 10.4% over 2008 to $11.9 million.
The increase is attributable primarily to 2009 severance and restructuring charges for our
international sales organization of approximately $1.1 million and to higher costs associated with
our continuing investment in international markets, evidenced by the establishment of a European
support office in Belgium during the second half of 2008 to continue the support and expansion of
our direct sales operations in Europe. These increases were offset in part by the impact of lower
Euro-to-U.S. dollar foreign exchange rates during 2009 than 2008. More than 80% of our 2009
international sales and marketing costs are denominated in Euros.
Also offsetting the increases in sales and marketing costs discussed above were lower 2009
corporate incentive compensation accruals of $0.7 million.
2008 compared to 2007. In the United States, our sales and marketing costs in 2008
increased approximately 10% over the prior year, to $16.6 million. The increase reflects costs for
additional marketing personnel ($0.6 million), higher marketing program costs ($0.3 million) and
higher achievement under incentive compensation plans ($0.5 million). Field selling costs in the
United States were largely flat in 2008 compared to the prior year, reflecting the 2007 turnover
and reduction in field sales personnel offset by higher sales commissions in 2008.
Internationally, our sales and marketing costs in 2008 increased approximately 12% over 2007 to
$10.7 million. The increase reflects our continued investment in international markets, including
the establishment of a European support office during the second half of 2008 to support the
expansion of our direct sales operations in Europe ($0.2 million) and the commencement of direct
sales activities in Switzerland in the third quarter of 2007 ($0.5 million), and higher achievement
under incentive compensation plans ($0.3 million). Our higher 2008 international sales and
marketing costs were also attributable to rising Euro-to-U.S. dollar foreign exchange rates, which
accounted for approximately one-half of the year-over-year increase. More than 75% of our
international sales and marketing costs in 2008 were denominated in Euros.
Research and Development
2009 compared to 2008. Research and development (“R & D”) expenses for 2009 increased 7.9% over
2008 to $8.9 million. The increase reflects stepped up investment in new product and technology
programs, particularly for minimally invasive tissue heart valve platforms and the Forcefield
program, as well as headcount additions and the allocation of stock compensation expense to R & D
beginning in 2009. These cost increases were partially offset by lower clinical affairs expenses
connected with the timing of clinical trial enrollments for tissue valves and lower 2009 corporate
incentive compensation accruals of $0.5 million. R & D expense fluctuations are generally related
to the timing and stages of development project cycles.
2008 compared to 2007. R & D expenses for 2008 increased approximately 9% compared to the prior
year to $8.2 million. The increase in R & D reflects higher clinical program and product approval
costs for tissue heart valves ($0.9 million) primarily related to the U.S. approval of our first
tissue valve offering, the ATS 3f Aortic Bioprosthesis, and the enrollment ramp-up in our Enable
clinical trial during 2008. Also contributing to the higher R & D costs were corporate bonus plan
accruals ($0.6 million) and increases in both R & D personnel and programs ($0.7 million).
Partially offsetting these increases in 2008 R & D spending was a decline in tissue valve R & D
spending ($1.1 million) and transfers of costs related to R & D clinical product builds, prototypes
and testing devices ($0.3 million) as our tissue valve products advanced through the regulatory
approval process.
Acquired In-Process R & D
In connection with our 2007 acquisition of the surgical cryoablation business of CryoCath, we
recorded a non-recurring in-process R & D (“IPR&D”) charge of $3.5 million. See Note 2 of “Notes
to Consolidated Financial Statements” in this Form 10-K for additional information regarding the
CryoCath acquisition, including the purchase price and the allocation of the purchase price. The
IPR&D relates to SurgiFrost XL, a product designed to enable less invasive stand alone or sole
therapy solutions to treat atrial fibrillation.
35
Table of Contents
Based on the results of extensive bench and pre-clinical testing as well as human feasibility
studies, we discontinued development of SurgiFrost XL product in late 2008.
General and Administrative
2009 compared to 2008. General and administrative (“G & A”) expenses for 2009 decreased 5.7% from
2008. The primary driver behind this result was significantly lower legal fees in 2009 ($1.8
million) after the fourth quarter 2008 settlement of the litigation with CarboMedics. Also
contributing to lower 2009 G & A expense was lower corporate incentive compensation accruals of
$0.5 million. Partially offsetting these decreases were higher consulting and outside services
costs ($0.7 million) and higher compensation and benefits expenses ($0.3 million).
In 2009 and 2008, we recognized total stock compensation expense of $2.6 million and $1.7 million,
respectively. Stock compensation expense has been allocated to cost of goods sold, sales and
marketing, R & D and G & A expenses as indicated in Note 7 of “Notes to Consolidated Financial
Statements” in this Form 10-K. The increase in stock compensation expense is attributable
primarily to new equity grants and awards and to fewer forfeitures.
2008 compared to 2007. G & A expenses for 2008 increased $0.1 million over 2007 to $10.5 million.
Major cost increases in G & A expenses in 2008 were for legal fees (primarily related to the
CarboMedics litigation) of $1.2 million and corporate incentive compensation plan accruals of $0.3
million. These cost increases were offset by $0.7 million in employee severance costs and $0.4
million of business development expenses incurred in 2007 which did not repeat in 2008, as well as
$0.3 million of lower consulting and outside services fees in 2008.
In 2008 and 2007, we recognized total stock compensation expense of $1.7 million and $1.5 million,
respectively. For 2008 and 2007, stock compensation expense was allocated to sales and marketing
and G & A expenses as indicated in Note 7 of “Notes to Consolidated Financial Statements” in
this Form 10-K. The increase in stock compensation expense for 2008 over 2007 reflects primarily
an increase in equity grants and awards in 2008 at higher closing stock prices.
Litigation Settlement
In December 2008, we settled ongoing litigation with CarboMedics which began in 2007 and was
related to our supply agreement with CarboMedics for certain mechanical heart valve components. As
part of the settlement, we paid $7.5 million to CarboMedics, $3.0 million of which was paid in
December 2008 and $4.5 million of which was paid in April 2009. Under the terms of the settlement,
we maintain all rights to manufacture, market and sell our ATS Open Pivot mechanical heart valve.
Satisfaction of the settlement terms will conclude all related matters with CarboMedics and
preclude any future litigation on the matter in question. See Note 15 of “Notes to Consolidated
Financial Statements” in this Form 10-K for more information regarding the CarboMedics litigation
and settlement.
Amortization of Intangibles
Amortization expense includes amortization of our pyrolytic carbon technology license with
CarboMedics and amortization of the definite-lived intangible assets acquired in our 2007 purchase
of the surgical cryoablation business of CryoCath and our 2006 acquisition of 3F. See Note 5 of
“Notes to Consolidated Financial Statements” in this Form 10-K for more information regarding our
intangible assets and amortization of our definite-lived intangible assets. We estimate
amortization expense for 2010 to total approximately $3.2 million.
Impairment of Intangibles
We made licensing fee and development milestone payments to ErySave AB (“ErySave”), a Swedish
research firm, under an exclusive development and licensing agreement, executed in 2004, for
worldwide rights to ErySave’s PARSUS filtration technology for cardiac surgery procedures. In July
2007, we were informed that ErySave was in the process of declaring bankruptcy and they could not
continue development work. Accordingly, the $0.8 million ErySave license payments intangible asset
was written off during fiscal 2007.
36
Table of Contents
Net Interest Expense
The primary components of our net interest expense are: (1) commitment fees and amortization of
deferred financing costs related to the June 2008 Subordinated Credit Agreement (“Credit
Agreement”) and Revolving Credit Facility (“Credit Facility”) with Theodore C. Skokos, (2) interest
on the $8.6 million Term Loan (“Term Loan”) with Silicon Valley Bank obtained in connection with
the June 2007 acquisition of the surgical cryoablation business of CryoCath, and (3) interest on
the $22.4 million aggregate principal amount of 6% Convertible Senior Notes issued in 2005.
Interest expense on the Convertible Senior Notes includes amortization of (a) deferred financing
costs, (b) the discount related to the implied value of common stock warrants sold with the
Convertible Senior Notes, and (c) the discounts related to the bifurcated Convertible Senior Notes
derivatives. See Note 6 of “Notes to Consolidated Financial Statements” in this Form 10-K for more
information regarding all of these debt instruments.
2009 compared to 2008. Net interest expense for 2009 was relatively flat compared to
2008, reflecting lower interest on the declining principal balance of our loan with Silicon Valley
bank offset by lower interest income on cash and investment balances and higher commitment fee and
deferred financing costs related to the June 2008 Credit Facility with Mr. Skokos.
2008 compared to 2007. Net interest expense for 2008 increased $0.9 million over the
prior year. The 2008 increase was attributable in large part to a decrease in interest income on
declining cash and investment balances during 2008. Interest income in 2008 and 2007 was $0.2
million and $0.7 million, respectively. Also contributing to the 2008 increase in net interest
expense was interest on the June 2007 Term Loan with Silicon Valley Bank obtained in connection
with the CryoCath asset acquisition.
Net Other Income
The following table summarizes our net other income for the years ended December 31, 2009, 2008 and
2007 (in thousands).
| Year ended December 31: | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Alta Partners VIII, L.P. (“Alta”) warrant liability gain (loss) |
$ | — | $ | 243 | ($652 | ) | ||||||
Convertible Senior Notes derivative liability gain |
34 | 51 | 98 | |||||||||
Net realized foreign currency transaction gains |
246 | 472 | 303 | |||||||||
Unrealized foreign currency gain (loss) related to
intercompany balances with foreign subsidiaries |
54 | (353 | ) | 312 | ||||||||
Other |
57 | — | — | |||||||||
Net other income |
$ | 391 | $ | 413 | $ | 61 | ||||||
In our June 2007 private equity placement in connection with the acquisition of the surgical
cryoablation business of CryoCath, we sold to Alta, a life sciences venture capital firm, 9,800,000
shares of our common stock and a seven-year warrant to purchase up to 1,960,000 shares of our
common stock at an exercise price of $1.65 per share. The Company was required to treat the
warrant as a liability pending approval of its shareholders at the Company’s 2008 annual meeting of
shareholders to provide shares of common stock issuable to Alta upon exercise of the warrant.
Accordingly, the fair value of the warrant was recorded as a liability on the date of issuance and
marked-to-market at each quarter-end. At the annual meeting of shareholders in May 2008, we
received shareholder approval to issue shares of our common stock upon exercise of the warrant.
Consequently, the warrant liability was marked-to-market through the date of shareholder approval
and the remaining liability was credited to additional paid-in capital.
Since 2005 we have recorded non-operating other income for the change in fair value of the
Convertible Senior Notes derivative liability. See Note 6 of “Notes to Consolidated Financial
Statements” in this Form 10-K for more information regarding the Convertible Senior Notes
derivative liability.
Net other income also includes net foreign currency transaction gains and losses, including
unrealized foreign currency gains and losses related to short-term intercompany balances with
foreign subsidiaries. These gains and losses have increased since 2006 due to larger fluctuations in foreign currency
exchange rates.
37
Table of Contents
Income Taxes
Our income tax expense for 2009, 2008 and 2007 relates to: (1) deferred income taxes connected with
the deductibility of goodwill from the CryoCath acquisition for tax purposes, but not for book
purposes, and the uncertainty of the timing of its reversal for book purposes, (2) current income
taxes for certain of our European sales and distribution subsidiaries, and (3) provisions for taxes
resulting from open international tax audits. In future years, we will continue recognizing
deferred income tax expense related to the CryoCath goodwill over its tax life as long as there is
no impairment of the goodwill’s recorded value.
Through 2009 we have accumulated approximately $167 million of net operating loss (“NOL”)
carryforwards for U.S. tax purposes ($59 million related to 3F). We believe that our ability to
fully utilize the existing NOL carryforwards could be restricted on a portion of the NOL by changes
in control that may have occurred or may occur in the future and by our ability to generate net
income. We are conducting a formal study of whether, or to what extent, past changes in control of
ATS impair our NOL carryforwards. We have recorded no net deferred tax asset related to our NOL
carryforwards and other deferred items as we currently cannot determine that it is more likely than
not that this asset will be realized and we, therefore, have provided a valuation allowance for the
entire asset.
Net Loss
Our net losses in 2009, 2008 and 2007 were $6.3 million, $19.3 million and $23.0 million,
respectively. Our decrease in net loss in 2009 compared to 2008 was due to significantly higher
sales and gross profit, the absence of the 2008 CarboMedics litigation settlement expense and
smaller changes in operating expenses and non-operating income, all of which are described in
detail above. Our decrease in net loss in 2008 compared to 2007 was also due to significantly
higher sales and gross profit as well as to the absence of acquisition-related IPR&D in 2008,
partially offset by higher operating expenses and the settlement of the CarboMedics litigation, all
of which are described in detail above.
Liquidity and Capital Resources
Cash, cash equivalents, and short-term investments totaled $14.2 million and $20.9 million at
December 31, 2009 and December 31, 2008, respectively.
Operating Activities
During 2009, we received cash payments from customers of approximately $75.9 million and made
payments to employees and suppliers of approximately $87.0 million. Included in our cash payments
for 2009 was a $4.5 million payment related to the settlement of the CarboMedics litigation.
During 2008, we received cash payments from customers of approximately $62.5 million and made
payments to employees and suppliers of approximately $72.0 million.
Since 2002, we have incurred significant expenses to support the commercialization of ATS products
both in the United States and international markets, have invested in new products and technologies
and have completed strategic acquisitions and business partnerships to diversify our product
portfolio. As we grow sales in future periods, better leverage our operating expenses and continue
to drive declines in our product manufacturing costs, we believe our operating losses will continue
to decrease and we will move toward a cash flow breakeven on sales and eventually to full-year
profitability.
Investing Activities
We purchased leasehold improvements, property and equipment totaling $2.4 million, $1.4 million and
$0.7 million during 2009, 2008 and 2007, respectively. Capital spending in 2009 focused on
information technology, manufacturing improvement projects and capitalized cryoablation hardware
placed at hospitals. A significant portion of our capital spending in 2008 was related to the
addition of a surgical cryoablation production clean room at our Plymouth, Minnesota location
following the 2007 acquisition of the surgical cryoablation business of CryoCath.
38
Table of Contents
Our major investing activity since the beginning of 2007 has been the acquisition of the assets of
the surgical cryoablation business of CryoCath. We paid $22 million at the closing in June 2007
(subsequently reduced by $0.9 million) and paid approximately $1.8 million in transaction costs.
In June and August 2008, we paid to CryoCath contingent acquisition payments totaling $2.0 million
due upon the successful transition of manufacturing operations from CryoCath to us. In June 2009,
we made a $2.0 million acquisition payment to CryoCath due two years after the closing of the
acquisition. See Note 2 of “Notes to Consolidated Financial Statements” in this Form 10-K for
additional details regarding this acquisition.
During 2009 we converted a long-term restricted investment of $0.5 million to cash. During 2007 we
invested $0.3 million for the purchase of patents, patent rights and other intellectual property.
See Note 5 of “Notes to Consolidated Financial Statements” in this Form 10-K for more information
regarding these intangible asset purchases.
Financing Activities
Equity Financing. During 2009, we received $2.5 million from the issuance of common stock related
to the exercise of a warrant on 1,000,000 shares of our common stock issued in connection with the
December 2008 private placement discussed below. We also received net proceeds of approximately
$0.3 million during 2009 from other issuances of common stock, primarily through purchases under
our employee stock purchase plan.
During 2008, we raised $23.9 million from the issuance of our common stock, including $19.8 million
received in connection with a December 2008 private placement sale of 8,510,639 shares of our
common stock and issuance of warrants to purchase 2,533,192 shares of our common stock. The
warrants are exercisable at $2.475 per share during the first year after the closing of the stock
sale, $2.85 per share during the second year, and $3.10 thereafter. We also received $3.2 million
upon the 2008 exercise of a warrant on 1,960,000 shares of our common stock issued in connection
with the June 2007 private placement discussed below. We received net proceeds of approximately
$0.8 million during 2008 from other issuances of common stock, primarily through exercises of stock
options and warrants as well as purchases under our employee stock purchase plan.
During 2007, we raised $30.6 million, net of offering costs, through two private placement sales of
our common stock. The first, in March 2007, raised $15.3 million, net of offering costs, through
the sale of 8,125,000 shares of our common stock at a price of $2.00 per share and warrants to
purchase 3,250,000 shares of our common stock at an exercise price of $2.40 per share. The second,
in June 2007, raised $15.3 million, net of offering costs, through the sale of 9,800,000 shares of
our common stock at a price of $1.65 per share and a seven-year warrant to purchase up to 1,960,000
shares of our common stock at an exercise price of $1.65 per share. We also received net proceeds
of $0.7 million during 2007 from the issuance of common stock through exercises of stock options
and purchases under our employee stock purchase plan.
Debt Financing. In June 2008, we entered into a Credit Agreement with Theodore C. Skokos, a member
of our Board of Directors, for a two-year, $5 million Credit Facility. Advances under the Credit
Facility carry interest at 15% per annum payable quarterly. The Credit Facility also carries an
annual commitment fee of 1% of the average unused Revolving Commitment Amount, payable annually.
We paid the first annual commitment fee of $0.05 million to Mr. Skokos in July 2009. As of
December 31, 2009, no amounts had been drawn under the Credit Facility. Our obligations to Mr.
Skokos under the Credit Agreement are subordinate to (1) our obligations to the holders of our
Convertible Senior Notes and (2) our obligations to Silicon Valley Bank. All assets are pledged as
collateral on the Credit Facility. In connection with the execution of the Credit Agreement, we
issued to Mr. Skokos a warrant to purchase 245,098 shares of our common stock at $2.04 per share
until June 29, 2015. In July 2008, Mr. Skokos exercised this warrant in full and we received $0.5
million from the exercise. We are obligated to issue additional seven-year warrants to Mr. Skokos
in the future based on the total amount of advances under the Credit Facility.
Since 2004 we have maintained a Loan and Security Agreement (“Loan Agreement”) with Silicon Valley
Bank. Under the Loan Agreement, as amended, all ATS assets are pledged as collateral and we are
subject to certain financial covenants. In June 2007, we entered into an Amendment to the Loan
Agreement whereby the Bank provided for an $8.6 million Term Loan, which we used to repay then
outstanding term loans and advances from the Bank and to purchase the surgical cryoablation business from CryoCath.
39
Table of Contents
Under the Term Loan, as amended, we made monthly payments of interest only from July 2007 through
March 2008, and we began making monthly payments of principal plus interest effective April 2008
that continue until June 2011. Accordingly, we repaid $2.6. million and $2.0 million of principal
on the Term Loan during 2009 and 2008, respectively. We have the right to prepay all, but not less
than all, of the outstanding Term Loan at any time so long as no event of default has occurred.
Interest on the Term Loan accrues at a fixed rate per annum of 9.5%, equal to 1.25% above the Prime
Rate in effect as of the funding date of the Term Loan.
The June 2007 Amendment also made certain changes to the liquidity ratio covenant set forth in the
Loan Agreement, as amended. The liquidity ratio was changed to require that we maintain, at all
times, on a consolidated basis, a ratio of (1) the sum of (a) our unrestricted cash (and
equivalents) on deposit with the Bank plus (b) 50% of the our accounts receivable arising from the
sale or lease of goods, or provision of services, in the ordinary course of business, divided by
(2) our indebtedness to the Bank for borrowed money, equal to or greater than 1.4 to 1.0. In June
2008, the Company entered into an Amendment to the Loan Agreement whereby, for the balance of 2008,
the 1.4 to 1.0 required liquidity ratio was reduced to 1.1 to 1.0 for intra-quarter months only.
The liquidity ratio remained at 1.4 to 1.0 for quarter-end months and reverted to 1.4 to 1.0 for
all months beginning in 2009. In December 2008, we entered into an Amendment to the Loan Agreement
whereby the liquidity ratio was raised to 2.0 to 1.0 until a $4.5 million litigation settlement
payment was made to CarboMedics and a related security interest granted to CarboMedics was
released. Upon such payment, the Bank agreed to return the liquidity ratio requirement to 1.4 to
1.0. In April 2009, we made the $4.5 million settlement payment. At December 31, 2009, we were in
compliance with all financial covenants set forth in the Loan Agreement, as amended.
In October 2005, we sold a combined $22.4 million aggregate principal amount of 6% Convertible
Senior Notes due in 2025, warrants to purchase 1,344,000 shares of our common stock (“Warrants”)
and certain embedded derivatives. The Warrants are exercisable at $4.40 per share and expire in
2010. We used the proceeds from the Convertible Senior Notes for general corporate purposes,
working capital, capital expenditures and to fund business development opportunities. Interest on
the Convertible Senior Notes is due semi-annually in April and October. The Convertible Senior
Notes are convertible into common stock at any time at a fixed conversion price of $4.20 per share,
subject to certain adjustments. If fully converted, the Convertible Senior Notes would convert
into approximately 5,333,334 shares of our common stock. We have the right to redeem the
Convertible Senior Notes at 100% of the principal amount plus accrued interest at any time on or
after October 20, 2008, and the investors have the right to require us to repurchase the
Convertible Senior Notes at 100% of the principal amount plus accrued interest on October 15 in
2010, 2015 and 2020. Accordingly, we have reclassified the Convertible Senior Notes from long-term
to current liabilities on our balance sheet at December 31, 2009.
On February 25, 2010, we received a commitment letter for four-year (interest only first year) term
debt financing of approximately $30 million from one of our directors, Theodore C. Skokos, and The
Ted and Shannon Skokos Foundation. This financing will be used to call and retire the Convertible
Senior Notes and bank Term Loan together totaling approximately $26 million as well as to provide
general corporate working capital. We expect to close the financing during the second quarter of
2010. When the Notes are retired we expect to recognize a non-cash debt restructuring charge of
approximately $4.5 to $5.0 million related primarily to the unamortized discount on the Notes.
Cash Management and Funding of Future Operations
We anticipate that operating costs will remain relatively high in comparison to sales during 2010
and will continue to require the use of cash to fund operations. Based upon the current forecast
of sales and operating expenses, we anticipate having sufficient cash to fund our operations.
However, we may need to raise additional cash in or after 2010 to fund our strategic investments,
refinance long-term obligations or to opportunistically add accretive products to our distribution
network. As identified in Item 1A of this Form 10-K, global economic turmoil may adversely impact
our revenue, access to the capital markets or future demand for our products, all of which could
affect our long-term viability. Approximately 60% of our revenue is derived from markets outside
the United States and this revenue may be adversely impacted by large swings in foreign currency
exchange rates and the availability of credit for our distributors in emerging markets.
Maintaining adequate levels of working capital depends in part upon the success of our products in the marketplace, the relative profitability of those products and our ability to
control operating and capital expenses.
40
Table of Contents
Funding of our operations in future periods may require additional investments in ATS in the form
of equity or debt. In addition, as discussed above, the holders of our $22.4 million Convertible
Senior Notes have the option to require us to repurchase the Convertible Senior Notes in October
2010. As discussed above, we have recently obtained a commitment for term debt financing which
will be used to call and retire the Notes. However, if this financing is not consummated and the
Note holders exercise their repurchase option, we would need to raise capital, issue debt
securities and/or borrow to finance the repurchase of these Notes.. Any sale of additional equity
or issuance of debt securities may result in dilution to our stockholders, and we cannot be certain
that additional public or private financing will be available in amounts or on terms acceptable to
us, or at all. If we are unable to obtain this additional financing when needed, we may be
required to delay, reduce the scope of, or eliminate one or more aspects of our business
development activities, which could harm the growth of our business.
Off-Balance Sheet Arrangements
We do not have any “off-balance sheet arrangements” (as such term is defined in Item 303 of
Regulation S-K) that are reasonably likely to have a current or future effect on our financial
condition, changes in financial condition, revenues or expenses, operating results, liquidity,
capital expenditures or capital resources.
Contractual Obligations
The following table sets forth our future payment obligations (in thousands):
| Payments Due By Period | ||||||||||||||||||||
| Less | More | |||||||||||||||||||
| Than | 1-3 | 3-5 | Than 5 | |||||||||||||||||
| Total | 1 year | Years | Years | Years (2) | ||||||||||||||||
Convertible Senior Notes payable (1) |
$ | 43,904 | $ | 1,344 | $ | 4,032 | $ | 4,032 | $ | 34,496 | ||||||||||
Bank Term Loan payable (1) |
4,319 | 2,943 | 1,376 | — | — | |||||||||||||||
Leases and other |
1,902 | 851 | 979 | 72 | — | |||||||||||||||
Total |
$ | 50,125 | $ | 5,138 | $ | 6,387 | $ | 4,104 | $ | 34,496 | ||||||||||
| (1) | Includes interest payments. | |
| (2) | The holders of our Convertible Senior Notes have the option to require us to repurchase the Convertible Senior Notes in October 2010. Therefore, the $22.4 million face amount of the Convertible Senior Notes has been classified on our balance sheet at December 31, 2009 as a current liability. |
Cautionary Statements
This document contains forward-looking statements within the meaning of federal securities laws
that may include statements regarding intent, belief or current expectations of ATS and our
management. The Private Securities Litigation Reform Act of 1995 provides a “safe harbor” for
forward-looking statements to encourage companies to provide prospective information without fear
of litigation so long as those statements are identified as forward-looking and are accompanied by
meaningful cautionary statements identifying important factors that could cause actual results to
differ materially from those projected in the statement. We desire to take advantage of these “safe
harbor” provisions. Accordingly, we have identified in Item 1A of this Form 10-K important risk
factors which could cause our actual results to differ materially from any such results which may
be projected, forecast, estimated or budgeted by us in forward-looking statements made by us from
time to time in reports, proxy statements, registration statements and other written
communications, or in oral forward-looking statements made from time to time by our officers and
agents. We do not intend to update any of these forward-looking statements after the date of this
Form 10-K to conform them to actual results.
41
Table of Contents
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
The primary objective of our investment activities is to preserve principal while at the
same time maximizing the income we receive from our investments without significantly
increasing risk. Some of the securities that we invest in may have market risk. This means
that a change in prevailing interest rates may cause the fair market value of the principal
amount of the investment to fluctuate. For example, if we hold a security that was issued with
a fixed interest rate at the then prevailing rate and the prevailing interest rate later rises,
the fair value of the principal amount of our investment will probably decline. To minimize
this risk, our portfolio of cash equivalents and short-term investments (if any) may be
invested in a variety of securities, including commercial paper, money market funds, and both
government and non-government debt securities. The average duration of our investments has
generally been less than one year. Due to the short-term nature of these investments, we
believe we have no material exposure to interest rate risk arising from our investments.
In the United States, the United Kingdom, France, Germany, Belgium, the Netherlands and
Switzerland, we sell our products directly to hospitals. In other international markets, we
sell our products to independent distributors who, in turn, sell to medical hospitals. Loss,
termination, or ineffectiveness of distributors to effectively promote our product would have a
material adverse effect on our financial condition and results of operations.
Transactions with U.S. and non-U.S. customers and distributors, other than in our direct
selling markets in Europe, are entered into in U.S. dollars, precluding the need for foreign
currency hedges on such sales. Sales through our French, German and Belgian distribution
subsidiaries are generally denominated in Euros. Sales to the United Kingdom and Switzerland
are also made through our Belgian distribution company and are denominated in pounds and Swiss
francs, respectively. Therefore, we are subject to profitability risk arising from exchange
rate movements. We have not used foreign exchange contracts or similar devices to reduce this
risk. We will evaluate the need to use foreign exchange contracts or similar devices if sales
in our European direct markets increase substantially.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Our Consolidated Financial Statements and the reports of our independent registered public
accounting firm are included in this Form 10-K beginning on page F-1. The index to these
reports and the financial statements is included in Item 15 below.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not Applicable.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures, which are designed to ensure that information
required to be disclosed in the reports we file or submit under the Exchange Act is recorded,
processed, summarized and reported within the time periods specified in the SEC’s rules and forms,
and that such information is accumulated and communicated to our management, including our chief
executive officer, or CEO, and chief financial officer, or CFO, as appropriate to allow timely
decisions regarding required disclosure.
Under the supervision and with the participation of our management, including our CEO and CFO, we
evaluated the effectiveness of the design and operation of our disclosure controls and procedures
(as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this
annual
42
Table of Contents
report. Based on that evaluation, our management, including the CEO and CFO, concluded that
our disclosure controls and procedures were effective as of December 31, 2009.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over
financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control
system was designed to provide reasonable assurance to our management and Board of Directors
regarding the preparation and fair presentation of our published financial statements. All internal
control systems, no matter how well designed, have inherent limitations. Therefore, even those
systems determined to be effective can provide only reasonable assurance with respect to financial
statement preparation and presentation.
Under the supervision and with the participation of our management, including our CEO and CFO, we
conducted an evaluation of the effectiveness of our internal control over financial reporting based
on the framework in “Internal Control — Integrated Framework” issued by the Committee of
Sponsoring Organizations of the Treadway Commission (“COSO”). Based on our evaluation under the
framework in “Internal Control — Integrated Framework,” our management concluded that our internal
control over financial reporting was effective as of December 31, 2009.
The effectiveness of our internal control over financial reporting as of December 31, 2009, has
been audited by Grant Thornton LLP, the independent registered public accounting firm who also has
audited our consolidated financial statements as of and for the year ended December 31, 2009,
included in this Form 10-K. Grant Thornton’s attestation report on the effectiveness of our
internal control over financial reporting appears on page F-2 of this Form 10-K.
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rule
13a-15(f) under the Exchange Act) during the fiscal quarter ended December 31, 2009 that have
materially affected, or are reasonably likely to materially affect, our internal control over
financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
43
Table of Contents
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
See Item 1 of this Form 10-K for certain information regarding our executive officers.
Reference is made to information contained under the headings “Proposal 1 — Election of Directors,”
“Committees of the Board of Directors and Attendance,” “Nominations,” and “Section 16(a) Beneficial
Ownership Reporting Compliance” in our definitive proxy statement for our 2010 Annual Meeting of
Shareholders to be filed with the SEC on or before April 30, 2010 (our “2010 Proxy Statement”),
which information is incorporated herein.
In 2004, we adopted a Code of Conduct for our employees, including our principal executive officer,
principal financial officer and principal accounting officer, which is posted on our website
(www.atsmedical.com). We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K
regarding an amendment to, or waiver from, a provision of the Code of Conduct by posting such
information on our website at the address specified above.
| ITEM 11. | EXECUTIVE COMPENSATION |
Reference is made to information contained under the headings “Executive Compensation” and
“Compensation of Directors” in our 2010 Proxy Statement, which information is incorporated herein.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Reference is made to information contained under the headings “Security Ownership of Certain
Beneficial Owners and Management” and “Equity Compensation Plans” in our 2010 Proxy Statement,
which information is incorporated herein.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Reference is made to information contained under the headings “Director Independence” and
“Related Person Transactions” in our 2010 Proxy Statement, which information is incorporated
herein.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Reference is made to information contained under the heading “Independent Registered Public
Accounting Firm Fees” in our 2010 Proxy Statement , which information is incorporated herein.
44
Table of Contents
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
Financial Statements
Our Consolidated Financial Statements and the Independent
Registered Public Accounting Firm’s Reports thereon are
included herein (page numbers refer to pages following the
signature page of this Annual Report on Form 10-K): |
||||
Reports of Independent Registered Public Accounting Firm |
Page F-1 through F-2 | |||
Consolidated Balance Sheets as of December 31, 2009 and 2008 |
Page F-3 | |||
Consolidated Statements of Operations for the years ended
December 31, 2009, 2008, and 2007 |
Page F-4 | |||
Consolidated Statement of Changes in Shareholders’ Equity
for the years ended December 31, 2009, 2008, and 2007 |
Page F-5 | |||
Consolidated Statements of Cash Flows for the years ended
December 31, 2009, 2008, and 2007 |
Page F-6 | |||
Notes to Consolidated Financial Statements for the years
ended December 31, 2009, 2008, and 2007 |
Page F-7 through F-28 | |||
Financial Statement Schedules
ATS MEDICAL, INC.
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
(in thousands)
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
(in thousands)
| Additions - | ||||||||||||||||||||
| Charged to | ||||||||||||||||||||
| Balance at | Charged to | Other | Balance at | |||||||||||||||||
| Beginning | Costs and | Accounts - | Deductions - | End of | ||||||||||||||||
| Description | of Period | Expenses | Describe | Describe | Period | |||||||||||||||
Valuation Accounts: |
||||||||||||||||||||
Deducted from asset accounts: |
||||||||||||||||||||
Year ended December 31, 2009: |
||||||||||||||||||||
Allowance for doubtful accounts |
$ | 364 | $ | 323 | $ | — | $ | (267 | )(1) | $ | 420 | |||||||||
Inventory reserves and allowances |
1,006 | 386 | (86 | )(2) | (256 | )(3) | 1,050 | |||||||||||||
Year ended December 31, 2008: |
||||||||||||||||||||
Allowance for doubtful accounts |
225 | 224 | — | (85 | )(1) | 364 | ||||||||||||||
Inventory reserves and allowances |
634 | 933 | 211 | (2) | (772 | )(3) | 1,006 | |||||||||||||
Year ended December 31, 2007: |
||||||||||||||||||||
Allowance for doubtful accounts |
537 | 250 | — | (562 | )(1) | 225 | ||||||||||||||
Inventory reserves and allowances |
786 | 43 | — | (195 | )(3) | 634 | ||||||||||||||
| (1) | Uncollectible accounts written off, net of recoveries. | |
| (2) | Adjustments for standard manufacturing cost increases to keep net carrying value of reserved-for inventories at zero. | |
| (3) | Inventory disposals and write-offs due to obsolescence, scrap, etc. |
All other schedules have been omitted because of absence of conditions under which they are
required or because the required information is included in the financial statements or notes
thereto.
45
Table of Contents
Exhibits
| Exhibit | ||
| Number | Description | |
2.1**
|
Agreement and Plan of Merger, dated as of January 23, 2006, by and among ATS Medical, Inc., Seabiscuit Acquisition Corp., 3F Therapeutics, Inc. and Boyd D. Cox, as Stockholder Representative (Incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed on January 26, 2006). | |
2.2
|
Amendment No. 1 to Agreement and Plan of Merger, dated as of June 13, 2006, by and among ATS Medical, Inc., Seabiscuit Acquisition Corp., 3F Therapeutics, Inc. and Boyd D. Cox, as Stockholder Representative (Incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on June 19, 2006). | |
2.3
|
Amendment No. 2 to Agreement and Plan of Merger, dated as of August 10, 2006, by and among the Company, Seabiscuit Acquisition Corp., 3F Therapeutics, Inc. and Boyd D. Cox, as Stockholder Representative (Incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on August 15, 2006). | |
2.4
|
Escrow Agreement, effective as of September 29, 2006, by and among the Company, Boyd D. Cox, as Stockholder Representative and Wells Fargo Bank, N.A. (Incorporated by reference to Exhibit 2.4 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006). | |
2.5**
|
Option and Asset Purchase Agreement, dated as of May 31, 2005, by and among ATS Medical, Inc., em Vascular, Inc., Keith L. March, M.D., John Hauck, Walter L. Sembrowich and James E. Shapland II (Incorporated by reference to Exhibit 2.5 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006). | |
2.6
|
Letter Amendment, dated as of November 29, 2006, to the Option and Asset Purchase Agreement, dated as of May 31, 2005, by and among ATS Medical, Inc., em Vascular, Inc., Keith L. March, M.D., John Hauck, Walter L. Sembrowich and James E. Shapland II, (Incorporated by reference to Exhibit 2.6 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006). | |
2.7**
|
Asset Purchase Agreement dated June 18, 2007 by and between ATS Medical, Inc. and CryoCath Technologies Inc. (Incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on June 25, 2007). | |
3.1
|
Third Restated Articles of Incorporation of ATS Medical, Inc. (Incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 28, 2008 (the “June 2008 Form 10-Q”)). | |
3.2
|
Bylaws of the Company, as amended February 13, 2007 (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on February 20, 2007). | |
4.1
|
Specimen certificate for shares of common stock of the Company (Incorporated by reference to Exhibit 4.1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1997 (the “1997 Form 10-K”)). | |
4.2
|
Indenture, dated as of October 7, 2005, between ATS Medical, Inc. and Wells Fargo Bank, National Association, as Trustee (Incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed on October 12, 2005 (the “October 12, 2005 Form 8-K”)). | |
4.3
|
First Supplemental Indenture, dated October 13, 2005, to the Indenture dated as of October 7, 2005, by and between ATS Medical, Inc. and Wells Fargo Bank, National |
46
Table of Contents
| Exhibit | ||
| Number | Description | |
| Association, as Trustee (Incorporated by reference to Exhibit 4.3 of the Company’s October 18, 2005 Form 8-K). | ||
4.4
|
Form of 6% Convertible Senior Notes due 2025 (Incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed on October 18, 2005 (the “October 18, 2005 Form 8-K”)). | |
4.5
|
Form of Warrant (Incorporated by reference to Exhibit 4.2 of the Company’s October 18, 2005 Form 8-K). | |
4.6
|
Form of Warrant (Incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on March 16, 2007). | |
4.7
|
Form of Warrant, dated December 19, 2008, issued by ATS Medical, Inc. to each of the Investors (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on December 23, 2008 (the “December 2008 Form 8-K”)). | |
10.1*
|
1987 Stock Option and Stock Award Plan, as restated and amended to date (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 1997). | |
10.2*
|
ATS Medical, Inc. 2000 Stock Incentive Plan, as amended (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 13, 2008). | |
10.3
|
Lease Agreement between the Company and Crow Plymouth Land Limited Partnership dated December 22, 1987 (Incorporated by reference to Exhibit 10(d) to the Company’s Registration Statement on Form S-18, File No. 33-34785-C (the “Form S-18”)). | |
10.4
|
Amendment No. 1 to Lease Agreement between the Company and Crow Plymouth Land Limited Partnership, dated January 5, 1989 (Incorporated by reference to Exhibit 10(e) to the Form S-18). | |
10.5
|
Amendment No. 2 to Lease Agreement between the Company and Crow Plymouth Land Limited Partnership, dated January 1989 (Incorporated by reference to Exhibit 10(f) to the Form S-18). | |
10.6
|
Amendment No. 3 to Lease Agreement between the Company and Crow Plymouth Land Limited Partnership, dated June 14, 1989 (Incorporated by reference to Exhibit 10(g) to the Form S-18). | |
10.7
|
Amendment No. 4 to Lease Agreement between the Company and Plymouth Business Center Limited Partnership, dated February 10, 1992 (Incorporated by reference to Exhibit 10.8 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1996 (the “1996 Form 10-K”)). | |
10.8
|
License Agreement dated September 24, 1990, with CarboMedics, Inc. (Incorporated by reference to Exhibit 10.11 to the 1996 Form 10-K). | |
10.9*
|
Employment Agreement between the Company and Michael D. Dale dated September 18, 2002 (Incorporated by reference to Exhibit 10.12 to the Company’s Form 10-K for the year ended 2002 (the “2002 Form 10-K”)). | |
10.10
|
Amendment 1 to License Agreement dated December 16, 1993, with CarboMedics, Inc. (Incorporated by reference to Exhibit 10.17 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1993 (the “1993 Form 10-K)). | |
10.11
|
Letter Agreement between the Company and Sulzer CarboMedics, Inc., dated June 27, 2002 (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 20, 2002). |
47
Table of Contents
| Exhibit | ||
| Number | Description | |
10.12
|
Form of International Distributor Agreement (Incorporated by reference to Exhibit 10.22 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1994). | |
10.13
|
Amendment No. 5 to Lease Agreement between the Company and St. Paul Properties, Inc., dated May 30, 1996 (Incorporated by reference to Exhibit 10.22 to the 1996 Form 10-K). | |
10.14
|
Amendment No. 6 to Lease Agreement between the Company and St. Paul Properties, Inc., dated November 25, 1997 (Incorporated by reference to Exhibit 10.23 to the 1997 Form 10-K). | |
10.15
|
1998 Employee Stock Purchase Plan, as amended through September 25, 2006 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 29, 2006). | |
10.16
|
Carbon Agreement by and between Sulzer CarboMedics, Inc. and ATS Medical, Inc., dated December 29, 1999 (Incorporated by reference to Exhibit 99.1 to the Current Report on Form 8-K filed on January 13, 2000 (the “January 2000 Form 8-K”). | |
10.17
|
Amendment 2 to License Agreement by and between Sulzer CarboMedics, Inc. and ATS Medical, Inc., dated December 29, 1999 (Incorporated by reference to Exhibit 99.3 to the January 2000 Form 8-K). | |
10.18
|
Amendment No. 7 to Lease Agreement between the Company and St. Paul Properties, Inc., dated May 18, 2000 (Incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2000). | |
10.19
|
Lease Agreement between the Company and St. Paul Properties, Inc., dated April 29, 2000 (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2000). | |
10.20
|
Amendment No. 8 to Lease Agreement between the Company and St. Paul Properties, Inc., dated December 14, 2000 (Incorporated by reference to Exhibit 10.32 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2000 (the “2000 Form 10-K”)). | |
10.21
|
Form of U.S. Distribution Agreement (Incorporated by reference to Exhibit 10.34 to the 2002 Form 10-K). | |
10.22
|
Amendment No. 9 to Lease Agreement between the Company and St. Paul Properties, Inc., dated September 8, 2003 (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2003). | |
10.23*
|
Form of Employee Stock Option Agreement under the Company’s 2000 Stock Incentive Plan (Incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2004 (the “September 2004 Form 10-Q”)). | |
10.24*
|
Form of Incentive Stock Option Agreement for option grants made under the Company’s 2000 Stock Incentive Plan beginning April 2009 (Incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended April 4, 2009 (the “First Quarter 2009 Form 10-Q”)). | |
10.25*
|
Form of Non-Qualified Stock Option Agreement for option grants made under the Company’s 2000 Stock Incentive Plan beginning April 2009 (Incorporated by reference to Exhibit 10.3 to the Company’s First Quarter 2009 Form 10-Q). |
48
Table of Contents
| Exhibit | ||
| Number | Description | |
10.26*
|
Form of Non-Plan Non-Qualified Stock Option Agreement (Incorporated by reference to Exhibit 10.4 to the Company’s September 2004 Form 10-Q). | |
10.27
|
Credit Agreement between Silicon Valley Bank and the Company, dated July 28, 2004 (Incorporated by reference to Exhibit 10.1 to the Company’s September 2004 Form 10-Q). | |
10.28
|
Amendment No. 10 to Lease Agreement between the Company and St. Paul Properties, Inc. dated as of October 1, 2004 (Incorporated by reference to Exhibit 10.40 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004 (the “2004 Form 10-K”)). | |
10.29
|
Letter Agreement between the Company and Centerpulse USA Holding Co. dated July 9, 2003 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 26, 2003). | |
10.30
|
Exclusive Development, Supply and Distribution Agreement with Genesee BioMedical, Inc., dated June 23, 2005 (Incorporated by reference to Exhibit 10.44 of the 2005 Form 10-K). | |
10.31
|
Amendment Agreement, dated March 24, 2005, to the Credit Agreement between Silicon Valley Bank and the Company, dated July 28, 2004 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 30, 2005). | |
10.32
|
Securities Purchase Agreement, dated as of October 6, 2005, by and among ATS Medical, Inc. and the Buyers listed on the Schedule of Buyers attached thereto as Exhibit A (Incorporated by reference to Exhibit 10.1 of the Company’s October 12, 2005 Form 8-K). | |
10.33
|
Amendment No. 1, dated October 12, 2005, to the Securities Purchase Agreement by and among ATS Medical, Inc. and the Buyers listed therein, dated as of October 6, 2005 (Incorporated by reference to Exhibit 10.1 of the Company’s October 18, 2005 Form 8-K). | |
10.34
|
Registration Rights Agreement, dated as of October 7, 2005, by and among ATS Medical, Inc. and the Buyers listed on the Schedule of Buyers attached thereto as Exhibit A (Incorporated by reference to Exhibit 10.2 of the Company’s October 12, 2005 Form 8-K). | |
10.35
|
Amendment No. 1, dated October 13, 2005, to the Registration Rights Agreement by and among ATS Medical, Inc. and the Buyers, as defined therein, dated as of October 7, 2005 (Incorporated by reference to Exhibit 10.2 of the Company’s October 18, 2005 Form 8-K). | |
10.36
|
Warrant Agent Agreement, dated as of October 7, 2005, between ATS Medical, Inc. and Wells Fargo Bank, National Association, as Warrant Agent (Incorporated by reference to Exhibit 10.3 of the Company’s October 12, 2005 Form 8-K). | |
10.37*
|
Form of Lock-Up Agreement with Executive Officers (Incorporated by reference to Exhibit 99.1 of the Company’s Current Report on Form 8-K filed on December 29, 2005). | |
10.38*
|
Form of Restricted Stock Unit Agreement under the Company’s 2000 Stock Incentive Plan (Incorporated by reference to Exhibit 10.53 of the 2005 Form 10-K). | |
10.39
|
Amendment, dated March 29, 2006, to the Loan and Security Agreement between Silicon Valley Bank and the Company, dated July 28, 2004 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 3, 2006). |
49
Table of Contents
| Exhibit | ||
| Number | Description | |
10.40*
|
Form of Change in Control Agreement executed by executive officers of the Company (Incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2006). | |
10.41
|
Amendment No. 2 dated September 1, 2006, to Original Lease Agreement dated April 29, 2000, by and between the Company and St. Paul Properties, Inc. (Incorporated by reference to Exhibit 10.2 to the Company’s September 2006 Form 10-Q). | |
10.42
|
Amendment No. 11 dated September 1, 2006, to Original Lease Agreement dated December 22, 1987, by and between the Company and St. Paul Properties, Inc. (Incorporated by reference to Exhibit 10.3 to the Company’s September 2006 Form 10-Q). | |
10.43
|
Amendment, dated August 15, 2006, to the Loan and Security Agreement between Silicon Valley Bank and the Company, dated July 28, 2004 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on August 17, 2006). | |
10.44*
|
Form of Restricted Stock Unit Award Agreement for awards to Non-Employee Directors under 2000 Stock Incentive Plan (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 20, 2007). | |
10.45
|
Amendment No. 4, dated February 15, 2007, to the Loan and Security Agreement between Silicon Valley Bank and the Company, dated July 28, 2004 (Incorporated by reference to the Company’s Current Report on Form 8-K filed on February 23, 2007). | |
10.46
|
Securities Purchase Agreement, dated March 15, 2007, between the Company and Certain Investors (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 16, 2007). | |
10.47
|
Registration Rights Agreement, dated March 15, 2007, between the Company and Certain Investors (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on March 16, 2007). | |
10.48
|
Letter Agreement, dated June 7, 2007, by and among Endocare, Inc., CryoCath Technologies Inc. and ATS Medical, Inc. (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 25, 2007 (the “June 2007 Form 8-K”)). | |
10.49
|
License Agreement, dated June 28, 2007, by and between ATS Acquisition Corp. and CryoCath Technologies Inc. (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 5, 2007 (the “July 2007 Form 8-K”)). | |
10.50
|
Manufacturing Agreement, dated June 28, 2007, by and between ATS Acquisition Corp. and CryoCath Technologies Inc. (Incorporated by reference to Exhibit 10.2 to the July 2007 Form 8-K). | |
10.51
|
Termination Agreement, dated June 28, 2007, by and between ATS Medical, Inc. and CryoCath Technologies Inc. (Incorporated by reference to Exhibit 10.3 to the July 2007 Form 8-K). | |
10.52
|
Common Stock and Warrant Purchase Agreement, dated as of June 19, 2007, by and between ATS Medical, Inc. and Alta Partners VIII, L.P. (Incorporated by reference to Exhibit 10.2 to the June 2007 Form 8-K). | |
10.53
|
Registration Rights Agreement, dated June 28, 2007, by and between ATS Medical, Inc. and Alta Partners VIII, L.P. (Incorporated by reference to Exhibit 10.5 to the July 2007 Form 8-K). |
50
Table of Contents
| Exhibit | ||
| Number | Description | |
10.54
|
Amendment, dated June 18, 2007, to the Loan and Security Agreement between Silicon Valley Bank and the Company dated July 28, 2004 (Incorporated by reference to Exhibit 10.3 to the June 2007 Form 8-K). | |
10.55
|
First Amendment, dated February 29, 2008, to the Loan and Security Agreement between Silicon Valley Bank and the Company dated July 28, 2004 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 6, 2008 (the “March 2008 Form 8-K”)). | |
10.56
|
Second Amendment, dated February 29, 2008, to the Loan and Security Agreement between Silicon Valley Bank and the Company dated July 28, 2004 (Incorporated by reference to Exhibit 10.2 to the March 2008 Form 8-K). | |
10.57
|
Unconditional Guaranty, dated February 29, 2008, entered into by 3F Therapeutics and ATS Acquisition Corp., in favor of Silicon Valley Bank (Incorporated by reference to Exhibit 10.3 to the March 2008 Form 8-K). | |
10.58
|
Security Agreement, dated February 29, 2008, by and between Silicon Valley Bank, 3F Therapeutics, Inc. and ATS Acquisition Corp. (Incorporated by reference to Exhibit 10.4 to the March 2008 Form 8-K). | |
10.59
|
Amendment No. 3 dated April 30, 2008, to Original Lease Agreement dated April 29, 2000, by and between the Company and St. Paul Properties, Inc. (Incorporated by reference to Exhibit 10.2 to the Company’s June 2008 Form 10-Q). | |
10.60
|
Subordinated Credit Agreement, dated June 29, 2008, by and between ATS Medical, Inc. and Theodore C. Skokos (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 2, 2008 (the “July 2008 Form 8-K”)). | |
10.61
|
Amendment, dated June 30, 2008, to the Loan and Security Agreement between Silicon Valley Bank and the Company dated July 28, 2004 (Incorporated by reference to Exhibit 10.3 to the July 2008 Form 8-K). | |
10.62
|
Common Stock and Warrant Purchase Agreement, dated December 19, 2008, by and between ATS Medical, Inc. and each of the Investors named therein (Incorporated by reference to Exhibit 10.1 to the December 2008 Form 8-K). | |
10.63
|
Registration Rights Agreement, dated December 19, 2008, by and between ATS Medical, Inc. and each of the Investors named therein (Incorporated by reference to Exhibit 10.3 to the December 2008 Form 8-K). | |
10.64
|
Amendment to and Consent, dated December 19, 2008, regarding Loan and Security Agreement between Silicon Valley Bank and the Company dated July 28, 2004 (Incorporated by reference to Exhibit 10.79 of the Company’s Annual Report on Form 10-K for the year ended December 31, 2008 (the “2008 Form 10-K”)). | |
10.65
|
Confidential Settlement and Mutual Release Agreement dated December 1, 2008, by and between CarboMedics, Inc. and ATS Medical, Inc. (Incorporated by reference to Exhibit 10.80 of the Company’s 2008 Form 10-K). | |
10.66*
|
2009 ATS Medical Management Incentive Compensation Plan (Incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K filed on February 23, 2009). | |
10.67
|
Amendment, dated June 2, 2009, to the Loan and Security Agreement between Silicon Valley Bank and the Company dated July 28, 2004 (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended July 4, 2009). |
51
Table of Contents
| Exhibit | ||
| Number | Description | |
10.68*
|
2010 ATS Medical Management Incentive Compensation Plan (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 22, 2010). | |
12.1
|
Computation of Ratio of Earnings to Fixed Charges, filed herewith. | |
21
|
List of Subsidiaries, filed herewith. | |
23
|
Consent of Grant Thornton LLP, filed herewith. | |
31.1
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, filed herewith. | |
31.2
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, filed herewith. | |
32.1
|
Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, filed herewith. | |
32.2
|
Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, filed herewith |
| * | Represents a management contract or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15 of Form 10-K. | |
| ** | Exhibits and Schedules to the acquisition agreement have been omitted but will be provided supplementally to the Securities and Exchange Commission upon request. |
52
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| Dated: March 12, 2010 | ATS MEDICAL, INC. |
|||
| By /s/ Michael D. Dale
|
||||
Michael D. Dale |
||||
Chief Executive Officer |
||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed
below by the following persons on behalf of the registrant and in the capacities indicated on March
12, 2010.
| Signature | Title | |
/s/ Michael D. Dale
|
Chief Executive Officer, | |
Michael D. Dale |
President and Chairman of the Board (principal executive officer) |
|
/s/ Michael R. Kramer
|
Chief Financial Officer
(principal financial and accounting officer) |
|
Michael R. Kramer |
||
/s/ Steven M. Anderson
|
Director | |
Steven M. Anderson |
||
/s/ Robert E. Munzenrider
|
Director | |
Robert E. Munzenrider |
||
/s/ Guy P. Nohra
|
Director | |
Guy P. Nohra |
||
/s/ Eric W. Sivertson
|
Director | |
Eric W. Sivertson |
||
/s/ Theodore C. Skokos
|
Director | |
Theodore C. Skokos |
||
/s/ Martin P. Sutter
|
Director | |
Martin P. Sutter |
53
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
ATS Medical, Inc.
ATS Medical, Inc.
We have audited the accompanying consolidated balance sheets of ATS Medical, Inc. and subsidiaries
(the “Company”) as of December 31, 2009 and 2008, and the related consolidated statements of
operations, changes in shareholders’ equity, and cash flows for each of the three years in the
period ended December 31, 2009. Our audits of the basic consolidated financial statements included
the financial statement schedule listed in the index appearing under Item 15. These financial
statements and financial statement schedule are the responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial statements and financial statement
schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all
material respects, the consolidated financial position of ATS Medical, Inc. and subsidiaries as of
December 31, 2009 and 2008, and the consolidated results of their operations and their cash flows
for each of the three years in the period ended December 31, 2009 in conformity with accounting
principles generally accepted in the United States of America. Also in our opinion, the related
financial statement schedule, when considered in relation to the basic consolidated financial
statements taken as a whole, presents fairly, in all material respects, the information set forth
therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), ATS Medical, Inc. and subsidiaries’ internal control over financial
reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated
Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO),
and our report dated March 12, 2010 expressed an unqualified opinion on the effectiveness of the
Company’s internal control over financial reporting.
| /s/ GRANT THORNTON LLP | ||||
| Minneapolis, Minnesota | ||||
| March 12, 2010 | ||||
F - 1
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
ATS Medical, Inc.
ATS Medical, Inc.
We have audited ATS Medical, Inc. and subsidiaries’ (the “Company”) internal control over financial
reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated
Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
The Company’s management is responsible for maintaining effective internal control over financial
reporting and for its assessment of the effectiveness of internal control over financial reporting,
included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our
responsibility is to express an opinion on the Company’s internal control over financial reporting
based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control over financial reporting was
maintained in all material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness exists, testing and
evaluating the design and operating effectiveness of internal control based on the assessed risk,
and performing such other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted accounting principles. A
company’s internal control over financial reporting includes those policies and procedures that (1)
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the
transactions and dispositions of the assets of the company; (2) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of management and directors of the company;
and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized
acquisition, use, or disposition of the company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
In our opinion, ATS Medical, Inc. and subsidiaries maintained, in all material respects, effective
internal control over financial reporting as of December 31, 2009, based on criteria established in
Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the consolidated balance sheets of ATS Medical, Inc. and subsidiaries as of
December 31, 2009 and 2008, and related consolidated statements of operations, changes in
shareholders’ equity, and cash flows for each of the three years in the period ended December 31,
2009, and our report dated March 12, 2010 expressed an unqualified opinion on those financial
statements.
| /s/ GRANT THORNTON LLP | ||||
| Minneapolis, Minnesota | ||||
| March 12, 2010 | ||||
F - 2
Table of Contents
ATS Medical, Inc.
Consolidated Balance Sheets
(In Thousands, Except Share and Per Share Data)
(In Thousands, Except Share and Per Share Data)
| December 31 | ||||||||
| 2009 | 2008 | |||||||
Assets |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 14,235 | $ | 20,895 | ||||
Accounts receivable, less allowance of $420 in 2009
and $364 in 2008 |
14,398 | 14,532 | ||||||
Inventories, net |
20,814 | 20,208 | ||||||
Prepaid expenses |
1,774 | 958 | ||||||
Total current assets |
51,221 | 56,593 | ||||||
Leasehold improvements, furniture, and equipment, net |
7,659 | 7,031 | ||||||
Goodwill |
32,166 | 17,016 | ||||||
Other intangible assets |
28,910 | 32,115 | ||||||
Other assets |
1,299 | 2,226 | ||||||
Total assets |
$ | 121,255 | $ | 114,981 | ||||
Liabilities and shareholders’ equity |
||||||||
Current liabilities: |
||||||||
Current portion of bank loan payable |
$ | 2,646 | $ | 2,646 | ||||
Accounts payable |
4,995 | 4,054 | ||||||
Accrued compensation |
2,076 | 3,537 | ||||||
Convertible senior notes payable, net of unamortized
discount and bifurcated derivatives of $4,741 |
17,659 | — | ||||||
Payable to CryoCath Technologies, Inc. |
— | 1,910 | ||||||
Payable to CarboMedics, Inc. |
— | 4,500 | ||||||
Other accrued liabilities |
2,894 | 1,970 | ||||||
Total current liabilities |
30,270 | 18,617 | ||||||
Convertible senior notes payable, net of unamortized
discount and bifurcated derivatives of $4,867 |
— | 17,533 | ||||||
Bank loan payable |
1,323 | 3,969 | ||||||
Other long-term liabilities |
790 | 287 | ||||||
Shareholders’ equity: |
||||||||
Common stock, $0.01 par value: |
||||||||
Authorized shares – 150,000,000 |
||||||||
Issued and outstanding shares – 78,187,741 in
2009 and 71,077,458 in 2008 |
782 | 711 | ||||||
Additional paid-in capital |
246,024 | 225,657 | ||||||
Accumulated deficit |
(158,229 | ) | (151,916 | ) | ||||
Accumulated other comprehensive income |
295 | 123 | ||||||
Total shareholders’ equity |
88,872 | 74,575 | ||||||
Total liabilities and shareholders’ equity |
$ | 121,255 | $ | 114,981 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
F - 3
Table of Contents
ATS Medical, Inc.
Consolidated Statements of Operations
(In Thousands, Except Per Share Amounts)
(In Thousands, Except Per Share Amounts)
| Year Ended December 31 | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Net sales |
$ | 75,710 | $ | 65,821 | $ | 49,587 | ||||||
Cost of goods sold |
26,821 | 25,267 | 21,348 | |||||||||
Gross profit |
48,889 | 40,554 | 28,239 | |||||||||
Operating expenses: |
||||||||||||
Sales and marketing |
30,617 | 27,373 | 24,633 | |||||||||
Research and development |
8,863 | 8,215 | 7,546 | |||||||||
Acquired in-process research and development |
— | — | 3,500 | |||||||||
General and administrative |
9,905 | 10,509 | 10,417 | |||||||||
Litigation settlement |
— | 7,500 | — | |||||||||
Amortization of intangibles |
3,224 | 3,489 | 2,516 | |||||||||
Intangible asset impairment |
— | — | 755 | |||||||||
Total operating expenses |
52,609 | 57,086 | 49,367 | |||||||||
Operating loss |
(3,720 | ) | (16,532 | ) | (21,128 | ) | ||||||
Interest income |
27 | 184 | 720 | |||||||||
Interest expense |
(2,723 | ) | (2,923 | ) | (2,542 | ) | ||||||
Other income, net |
391 | 413 | 61 | |||||||||
Net loss before income tax expense |
(6,025 | ) | (18,858 | ) | (22,889 | ) | ||||||
Income tax expense |
288 | 481 | 119 | |||||||||
Net loss |
$ | (6,313 | ) | $ | (19,339 | ) | $ | (23,008 | ) | |||
Net loss per share: |
||||||||||||
Basic and diluted |
$ | (0.09 | ) | $ | (0.31 | ) | $ | (0.44 | ) | |||
Weighted average number of shares outstanding: |
||||||||||||
Basic and diluted |
72,114 | 61,440 | 52,589 | |||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F - 4
Table of Contents
ATS Medical, Inc.
Consolidated Statement of Changes in Shareholders’ Equity
(In Thousands)
(In Thousands)
| Accumulated Other | ||||||||||||||||||||||||
| Common Stock | Additional | Comprehensive | ||||||||||||||||||||||
| Shares | Amount | Paid-In Capital | Accumulated Deficit | Income (Loss) | Total | |||||||||||||||||||
Balance at December 31, 2006 |
40,320 | $ | 403 | $ | 166,411 | $ | (109,569 | ) | $ | 645 | $ | 57,890 | ||||||||||||
Stock issued under the Employee Stock Purchase Plan |
130 | 2 | 208 | — | — | 210 | ||||||||||||||||||
Stock options exercised |
524 | 5 | 475 | — | — | 480 | ||||||||||||||||||
Restricted stock units issued |
389 | 4 | (59 | ) | — | — | (55 | ) | ||||||||||||||||
Stock issued in private placement sales, net of
offering costs |
17,925 | 179 | 30,382 | — | — | 30,561 | ||||||||||||||||||
Stock issued for purchase of intangible assets |
224 | 2 | 498 | — | — | 500 | ||||||||||||||||||
Warrant issued in connection with private
placement stock sale |
— | — | (3,261 | ) | — | — | (3,261 | ) | ||||||||||||||||
Stock compensation expense |
— | — | 1,454 | — | — | 1,454 | ||||||||||||||||||
Comprehensive loss: |
||||||||||||||||||||||||
Change in foreign currency translation |
— | — | — | — | 185 | 185 | ||||||||||||||||||
Net loss for the year |
— | — | — | (23,008 | ) | — | (23,008 | ) | ||||||||||||||||
Comprehensive loss |
(22,823 | ) | ||||||||||||||||||||||
Balance at December 31, 2007 |
59,512 | 595 | 196,108 | (132,577 | ) | 830 | 64,956 | |||||||||||||||||
Stock issued under the Employee Stock Purchase Plan |
123 | 2 | 202 | — | — | 204 | ||||||||||||||||||
Stock options exercised |
103 | 1 | 132 | — | — | 133 | ||||||||||||||||||
Restricted stock units issued |
600 | 6 | (6 | ) | — | — | — | |||||||||||||||||
Stock issued in private placement sales, net of
offering costs |
8,511 | 85 | 19,739 | — | — | 19,824 | ||||||||||||||||||
Exercise of common stock warrants |
2,205 | 22 | 3,712 | — | — | 3,734 | ||||||||||||||||||
Stock issued for services rendered |
23 | — | 48 | — | — | 48 | ||||||||||||||||||
Transfer of private placement warrant liability |
— | — | 3,670 | — | — | 3,670 | ||||||||||||||||||
Credit facility warrants issued |
— | — | 376 | — | — | 376 | ||||||||||||||||||
Stock compensation expense |
— | — | 1,676 | — | — | 1,676 | ||||||||||||||||||
Comprehensive loss: |
||||||||||||||||||||||||
Change in foreign currency translation |
— | — | — | — | (707 | ) | (707 | ) | ||||||||||||||||
Net loss for the year |
— | — | — | (19,339 | ) | — | (19,339 | ) | ||||||||||||||||
Comprehensive loss |
(20,046 | ) | ||||||||||||||||||||||
Balance at December 31, 2008 |
71,077 | 711 | 225,657 | (151,916 | ) | 123 | 74,575 | |||||||||||||||||
Stock issued under the Employee Stock Purchase Plan |
125 | 1 | 274 | — | — | 275 | ||||||||||||||||||
Stock options exercised |
3 | — | 3 | — | — | 3 | ||||||||||||||||||
Restricted stock units issued |
983 | 10 | (10 | ) | — | — | — | |||||||||||||||||
Exercise of common stock warrants |
1,000 | 10 | 2,465 | — | — | 2,475 | ||||||||||||||||||
Stock issued upon achievement of acquisition
milestone, net of issuance costs |
5,000 | 50 | 15,050 | — | — | 15,100 | ||||||||||||||||||
Adjustment to private placement sale offering costs |
— | — | 28 | — | — | 28 | ||||||||||||||||||
Stock compensation expense |
— | — | 2,557 | — | — | 2,557 | ||||||||||||||||||
Comprehensive loss: |
||||||||||||||||||||||||
Change in foreign currency translation |
— | — | — | — | 172 | 172 | ||||||||||||||||||
Net loss for the year |
— | — | — | (6,313 | ) | — | (6,313 | ) | ||||||||||||||||
Comprehensive loss |
(6,141 | ) | ||||||||||||||||||||||
Balance at December 31, 2009 |
78,188 | $ | 782 | $ | 246,024 | $ | (158,229 | ) | $ | 295 | $ | 88,872 | ||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F - 5
Table of Contents
ATS Medical, Inc.
Consolidated Statements of Cash Flows
(In Thousands)
(In Thousands)
| Year Ended December 31 | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Operating activities: |
||||||||||||
Net loss |
$ | (6,313 | ) | $ | (19,339 | ) | $ | (23,008 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
Depreciation and amortization |
5,262 | 5,623 | 4,622 | |||||||||
Non-cash interest expense |
768 | 743 | 546 | |||||||||
Stock based compensation expense |
2,557 | 1,676 | 1,454 | |||||||||
Change in value of warrant liability and derivative liability
bifurcated from convertible senior notes |
(34 | ) | (294 | ) | 554 | |||||||
In-process research and development related to acquisitions |
— | — | 3,500 | |||||||||
Impairment of intangibles |
— | — | 755 | |||||||||
Deferred income taxes |
261 | 192 | 95 | |||||||||
Changes in operating assets and liabilities, net of acquisition: |
||||||||||||
Accounts receivable |
158 | (3,446 | ) | 1,117 | ||||||||
Inventories |
(592 | ) | (2,500 | ) | 669 | |||||||
Accounts payable and accrued expenses |
(4,134 | ) | 4,782 | 121 | ||||||||
Other |
(789 | ) | 159 | 32 | ||||||||
Net cash used in operating activities |
(2,856 | ) | (12,404 | ) | (9,543 | ) | ||||||
Investing activities: |
||||||||||||
Purchases of short-term investments |
— | (938 | ) | (4,140 | ) | |||||||
Maturities of short-term investments |
— | 5,127 | 6,043 | |||||||||
Payments for business acquisitions |
(2,000 | ) | (2,000 | ) | (21,074 | ) | ||||||
Business acquisition costs, net of cash acquired |
— | — | (1,791 | ) | ||||||||
Payments for other intangibles |
(19 | ) | — | (277 | ) | |||||||
Purchases of leasehold improvements, furniture, and equipment |
(2,414 | ) | (1,440 | ) | (748 | ) | ||||||
Other |
479 | — | (36 | ) | ||||||||
Net cash (used in) provided by investing activities |
(3,954 | ) | 749 | (22,023 | ) | |||||||
Financing activities: |
||||||||||||
Advances on bank notes payable |
— | — | 8,600 | |||||||||
Payments on bank notes payable |
(2,646 | ) | (1,985 | ) | (2,327 | ) | ||||||
Proceeds from issuance of common stock, net of issuance costs |
2,781 | 23,943 | 31,196 | |||||||||
Other |
(20 | ) | 162 | 168 | ||||||||
Net cash provided by financing activities |
115 | 22,120 | 37,637 | |||||||||
Effect of exchange rate changes on cash |
35 | (50 | ) | (203 | ) | |||||||
(Decrease) increase in cash and cash equivalents |
(6,660 | ) | 10,415 | 5,868 | ||||||||
Cash and cash equivalents at beginning of year |
20,895 | 10,480 | 4,612 | |||||||||
Cash and cash equivalents at end of year |
$ | 14,235 | $ | 20,895 | $ | 10,480 | ||||||
Supplemental cash flow information: |
||||||||||||
Net cash paid during the year for interest |
$ | 1,945 | $ | 2,139 | $ | 2,241 | ||||||
Net cash paid during the year for income taxes |
107 | — | — | |||||||||
Significant non-cash transactions: |
||||||||||||
Issuance of common stock for acquisition contingent payment |
$ | 15,150 | — | — | ||||||||
Equipment purchased under capital lease |
242 | — | — | |||||||||
Transfer of warrant liability to additional paid-in capital |
— | $ | 3,670 | — | ||||||||
Credit facility warrants issued |
— | 376 | — | |||||||||
Issuance of common stock for acquisition of intangible assets |
— | — | $ | 500 | ||||||||
Assumption of liabilities in connection with asset acquisition |
— | — | 2,429 | |||||||||
License agreement intangible asset tendered in asset acquisition |
— | — | 1,765 | |||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F - 6
Table of Contents
ATS Medical, Inc.
Notes to Consolidated Financial Statements
1. Summary of Significant Accounting Policies
Business Activity
ATS Medical, Inc. (the “Company”) develops, manufactures, and markets medical devices for the
treatment of structural heart disease. The Company operates in one business segment and its
interest lies with devices used by cardiovascular surgeons in the cardiac surgery operating
theater. Currently, the Company participates in the markets for mechanical and tissue replacement
heart valves, heart valve repair, the surgical treatment of atrial fibrillation, and other cardiac
surgery devices, tools and accessories.
Principles of Consolidation
The consolidated financial statements have been prepared in accordance with United States Generally
Accepted Accounting Principles (“GAAP”) as set forth by the Financial Accounting Standards Board
(“FASB”) in its Accounting Standards Codification (“ASC”) as well as applicable rules and
regulations of the Securities and Exchange Commission (“SEC”). The consolidated financial
statements include the accounts of the Company and wholly owned sales and distribution subsidiaries
in France, Germany, Belgium and Austria, after elimination of intercompany accounts and
transactions.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to
make estimates and assumptions that affect the amounts reported in the consolidated financial
statements and accompanying notes. Actual results could differ from those estimates.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the
time of purchase to be cash equivalents. Cash equivalents are carried at cost which approximates
market value and includes $1.7 million and $1.1 million in primarily Euro-denominated balances in
foreign banks at December 31, 2009 and 2008, respectively. Cash amounts typically are in excess of
federally insured limits.
Accounts Receivable
Credit is extended based on evaluation of a customer’s financial condition and, generally,
collateral is not required. Accounts receivable are generally due within 30-180 days and are stated
at amounts due from customers net of an allowance for doubtful accounts. Accounts receivable
outstanding longer than the contractual payment terms are considered past due. The Company
determines its allowance by considering a number of factors, including the length of time trade
accounts receivable are past due, the Company’s previous loss history, the customer’s current
ability to pay its obligation to the Company, and the condition of the general economy and the
industry as a whole. The Company writes off accounts receivable when they become uncollectible, and
payments subsequently received on such receivables are credited to the allowance for doubtful
accounts.
Inventories
The Company carries and relieves inventories at the lower of manufacturing cost (first-in,
first-out basis) or market (net realizable value). In addition, the Company maintains allowances
and reserves against certain finished goods and work-in-process inventories to cover obsolete and
short shelf-life product, scrap and rework costs and resterilization costs for expired or
near-expired items.
F - 7
Table of Contents
At December 31, 2009 and 2008, inventories consisted of the following (in thousands):
| 2009 | 2008 | |||||||
Raw materials |
$ | 2,639 | $ | 4,712 | ||||
Work-in-process |
6,390 | 4,880 | ||||||
Finished goods |
12,685 | 11,416 | ||||||
Total inventories |
21,714 | 21,008 | ||||||
Less: non-current inventories |
(900 | ) | (800 | ) | ||||
Inventories, net |
$ | 20,814 | $ | 20,208 | ||||
A portion of the Company’s finished goods inventories was in excess of its current requirements
based on the historical and anticipated level of sales. Management believes that these excess
quantities will be utilized over the next two to three years. The Company therefore included $0.9
million and $0.8 million of inventories in non-current other assets on the balance sheet at
December 31, 2009 and 2008, respectively.
Other Assets
Included in other assets are deferred financing costs (unamortized balance of $0.4 million and
$0.9 million at December 31, 2009 and 2008, respectively) in connection with the 6% Convertible
Senior Notes and Subordinated Credit Agreement, both discussed in Note 6 below, which are being
amortized to interest expense over five years and two years, respectively. Amortization of the
remaining balance of unamortized deferred financing costs at December 31, 2009 will occur in 2010.
Leasehold Improvements, Furniture, and Equipment
Leasehold improvements, furniture, and equipment are stated at cost. Depreciation is provided using
the straight-line method over the estimated useful lives of the assets as follows:
Furniture and fixtures
|
7 years | |
Equipment
|
5 to 17 years | |
Computer hardware and software
|
3 years |
Leasehold improvements are amortized over the remaining related lease term or estimated useful
life, whichever is shorter.
Intangible Assets
Indefinite-lived intangible assets consist of goodwill, which is carried at cost. The Company
applies the accounting principles set forth in FASB ASC 350, Intangibles-Goodwill and Other, which
prohibits the amortization of intangible assets with indefinite useful lives and requires that
these assets be reviewed for impairment at least annually. Management reviews goodwill for
impairment annually, or more frequently if a change in circumstances or occurrence of events
suggests the remaining value may not be recoverable. The test for impairment requires management
to make estimates about fair-value which are based either on the expected present value of future
cash flows or on other measures of value such as the market capitalization of the Company. If the
carrying amount of the assets is greater than the measures of fair value, impairment is considered
to have occurred and a write-down of the asset is recorded.
Definite-lived intangible assets consist of purchased technology and patents, technology licenses
and agreements, trademarks, tradenames and distribution intangibles, which are carried at amortized
cost. The Company assesses definite-lived intangible asset impairment in accordance with FASB ASC
360-10-35, Property, Plant and Equipment-Overall-Subsequent Measurement. If a triggering event
occurs, the Company assesses the recoverability of definite-lived intangible assets by reference to
future gross profit cash flows from the underlying products utilizing the capitalized intangible
assets.
Impairment testing on all of the Company’s intangible assets was completed by management as of July
2, 2009 and December 31, 2009. Management determined that the Company’s intangible assets,
including goodwill, were not impaired. In performing its year-end impairment test for goodwill,
the Company used a market capitalization approach based on the closing price of the Company’s
common stock on the date of
F - 8
Table of Contents
measurement. This market capitalization value was compared to the net book value of the Company
(i.e. total assets, including goodwill, minus total liabilities). The total market capitalization
exceeded the net book value of the Company; therefore, no goodwill impairment was indicated. In
performing its year-end impairment review for definite-lived intangible assets, the Company
assessed the recoverability of definite-lived intangible assets by reference to future gross profit
cash flows from the underlying products using the capitalized technology and trademarks. Based on
this assessment, no impairment of definite-lived intangible assets was indicated.
Convertible Debt and Derivative Instruments
The Company accounts for embedded derivatives related to its convertible senior notes and certain
common stock warrants under FASB ASC 815, Derivatives and Hedging, and applicable SEC rules, which
require certain embedded derivative financial instruments to be bifurcated from the debt or equity
agreement and accounted for as a liability. The Company determines the fair value of these
derivatives by making judgments and estimates of the probability that future conditions giving rise
to such derivatives may occur.
Fair Value Measurements
The Company applies fair value measurement accounting principles related to its convertible debt
derivative liability, goodwill and certain other assets and liabilities pursuant to FASB ASC 820,
Fair Value Measurements and Disclosures (“ASC 820”). See Note 13 below for a discussion of the
application of ASC 820 to specific assets and liabilities.
The Company has estimated the fair value of its financial instruments using available market
information and appropriate valuation methods. However, considerable judgment is required in
formulating fair value and the estimated fair value amounts may not be indicative of amounts that
would be required in market transactions. All financial instruments’ carrying values approximate
fair value.
Revenue Recognition
The majority of the Company’s revenue in the United States and direct European countries is
generated from consigned inventory maintained at hospitals or with field representatives. In these
situations, revenue is recognized at the time that the product has been implanted or used. In all
other instances, revenue is recognized, net of any applicable sales and value added taxes invoiced,
at the time product is shipped. Certain independent distributors in select international markets
receive rebates against invoiced sales amounts. In these situations, the Company accrues for these
rebates at the time of the original sale. These accrued rebates are treated as a reduction of
revenue and have not been significant. The Company includes shipping and handling costs in cost of
goods sold.
Advertising and Promotional Costs
Advertising and promotional costs are charged to operations in the year incurred. Advertising and
promotional costs charged to operations for each of 2009, 2008 and 2007 were $0.1 million.
Foreign Currency Translation and Transaction Gains and Losses
The financial statements for the Company’s European operations are maintained in Euros. All assets
and liabilities of the Company’s international subsidiaries are translated to U.S. dollars at
year-end exchange rates, while the statement of operations is translated at average exchange rates
in effect during the year. Translation adjustments arising from the use of differing exchange
rates are included in accumulated other comprehensive income (loss) in shareholders’ equity.
Net gains on foreign currency transactions were $0.3 million in 2009, $0.1 million in 2008 and $0.6
million in 2007 and includes gains and losses on short-term intercompany payables (denominated in
U.S. dollars), while gains and losses on long-term intercompany payables are recognized in
accumulated other comprehensive income (loss).
F - 9
Table of Contents
The Company reclassified foreign exchange transaction gains originally recorded as a reduction of
sales and marketing expenses in the first, second and third quarters of 2007 to other income. These
reclassifications totaled $0.2 million and had no impact on 2007 quarterly net losses as previously
reported.
Income Taxes
The Company accounts for income taxes under FASB ASC 740, Income Taxes (“ASC 740”). Deferred taxes
are provided on the asset and liability method whereby deferred tax assets are recognized for
deductible temporary differences and operating loss and tax credit carry forwards and deferred tax
liabilities are recognized for taxable temporary differences. Temporary differences are the
differences between the amounts of assets and liabilities recorded for income tax and financial
reporting purposes. Deferred tax assets are reduced by a valuation allowance when, in the opinion
of management, it is more likely than not that some portion or all of the deferred tax assets will
not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in
tax laws and rates on the date of enactment.
As required by ASC 740, the Company recognizes the financial statement benefit of a tax position
only after determining that the relevant tax authority would more likely than not sustain the
position. For tax positions meeting the more likely than not threshold, the amount recognized in
the financial statements is the largest benefit that has a greater than 50 percent likelihood of
being realized upon ultimate settlement with the relevant tax authority. At the adoption date, the
Company applied ASC 740 to all tax positions for which the statute of limitations remained open.
Warranties
The Company adheres to FASB ASC 460, Guarantees (“ASC 460”). ASC 460 requires disclosures
concerning the Company’s obligations under certain guarantees. The Company sells service
agreements on cryoablation consoles, for which it defers the related service revenue and recognizes
it over the service period. Revenue and warranty costs under these service agreements are minimal.
Stock-Based Compensation
The Company has stock-based employee compensation plans, which are described more fully in Note 7.
The Company accounts for its stock-based employee compensation plans under the recognition and
measurement principles FASB ASC 718, Stock Compensation, which requires all share-based payments to
be recognized in the income statement based on their fair values.
Net Loss Per Share
Basic net loss per share is computed by dividing net loss by the weighted average shares
outstanding and excludes any dilutive effects of restricted stock units, options, warrants, and
convertible securities. For all periods presented, diluted net loss per share is equal to basic net
loss per share because the effect of including potential common shares for stock options,
restricted stock units and warrants outstanding would have been anti-dilutive. Had net income been
achieved, approximately 2,738,000, 1,374,000, and 815,000 shares of common stock equivalents would
have been included in the computation of diluted net income per share for the years ended December
31, 2009, 2008 and 2007, respectively.
Reclassifications
Certain amounts in the fiscal year 2008 consolidated financial statements have been reclassified to
conform to the fiscal year 2009 presentation. These reclassifications had no effect on net
earnings as previously reported.
2. Acquisition of Surgical Cryoablation Business from CryoCath Technologies, Inc.
In June 2007, the Company completed the acquisition of the cryoablation surgical device business of
CryoCath Technologies, Inc. (“CryoCath”). Pursuant to the Asset Purchase Agreement between the
Company and CryoCath, the Company paid CryoCath $22.0 million at closing, $2.0 million in 2008
after the successful transition of manufacturing operations from CryoCath to the Company and an
additional $2.0 million non-contingent cash payment made in June 2009, 24 months after closing.
Each of these $2.0
F - 10
Table of Contents
million payments was recorded as additional goodwill. The $2.0 million payment paid 24 months
after closing was discounted to present value and was shown on the balance sheet as Payable to
CryoCath Technologies, Inc. prior to payment in 2009 The increase in present value of this payment
($0.1 million in 2009, $0.2 million in 2008 and $0.1 million in 2007) was charged to interest
expense. The Company also agreed to pay up to an additional $4.0 million in contingent payments
based upon the Company reaching certain levels of sales in 2009 and 2010 of SurgiFrost® XL, a
product then in the early development stages and designed to enable less invasive stand alone or
sole therapy solutions to treat atrial fibrillation. Based on the results of extensive bench and
pre-clinical testing as well as human feasibility studies, the Company discontinued development of
SurgiFrost XL product in late 2008.
The Company and CryoCath also entered into (1) a License Agreement, which provides the Company with
an exclusive, perpetual, royalty-free, worldwide license to use CryoCath’s intellectual property
related to the cryoablation surgical device business, (2) a Manufacturing Agreement, pursuant to
which CryoCath agreed to manufacture, assemble and supply products relating to the cryoablation
surgical business to the Company for a period of up to one year, and (3) a Termination Agreement,
which terminated the Distribution Agreement and Agent Agreement, each dated November 9, 2004,
between the Company and CryoCath.
Purchase Price. The Company accounted for the acquisition of the surgical cryoablation business of
CryoCath as a purchase under U.S. generally accepted accounting principles. Under the purchase
method of accounting, the assets and liabilities acquired were recorded as of the acquisition date,
at their respective fair values, and consolidated with those of the Company. The purchase price
allocation was based upon estimates and valuations of the fair value of assets acquired and
liabilities assumed. The valuations required the use of significant assumptions and estimates.
Critical estimates included, but were not limited to, future expected cash flows and the applicable
discount rates. These estimates were based on assumptions that the Company believed to be
reasonable.
The following table summarizes the purchase price of the surgical cryoablation business of CryoCath
(amounts in thousands):
Cash paid (includes $0.9 million post-closing purchase price reduction
and $2.0 million manufacturing transition payments) |
$ | 23,074 | ||
License payments made under prior Distribution and Agent Agreements |
1,765 | |||
Non-contingent cash payment made 24 months after closing
(discounted to present value using discount rate of 9.25%) |
1,663 | |||
Acquisition-related costs |
1,791 | |||
Total purchase price |
$ | 28,293 | ||
Purchase Price Allocation. The following table summarizes the purchase price allocation for the
acquisition of the surgical cryoablation business of CryoCath (amounts in thousands):
Current assets |
$ | 951 | ||
Fixed assets |
761 | |||
Definite-lived intangible assets subject to amortization |
11,800 | |||
Goodwill |
11,888 | |||
Acquired in-process research and development |
3,500 | |||
Current liabilities |
(607 | ) | ||
Total purchase price allocation |
$ | 28,293 | ||
F - 11
Table of Contents
The excess of the purchase price over the fair value of net tangible assets acquired was allocated
to specific intangible asset categories and in-process research and development as follows:
| Amount | Weighted Average | |||||||
| (in thousands) | Assigned | Amortization Period | ||||||
Definite-lived intangible assets: |
||||||||
Existing technology – core |
$ | 4,400 | 16 years | |||||
Existing technology – developed |
5,600 | 5 years | ||||||
Distributor relationships |
1,500 | 12 years | ||||||
Product trademarks |
300 | 10 years | ||||||
Total definite-lived intangible assets |
$ | 11,800 | 10 years | |||||
Goodwill |
$ | 11,888 | ||||||
Acquired in-process research and development |
$ | 3,500 | ||||||
The Company believes the intangible assets as determined represent the fair value at the date of
acquisition. The Company used various cash flow methodologies to determine the value of the
individual definite-lived intangible assets. The assumptions as to the level of cash flows
generated and the commercial life over which cash flows occur were based on the Company’s
expectations of the commercial viability and expected usage of the underlying technology or asset,
the competitive environment in the market and the lives of similar assets or technologies
Estimated useful lives were determined to be at the point at which the majority of the discounted
cash flows were achieved. The product trademarks amortization period was subsequently reduced to
15 months in the fourth quarter of 2007, due to changing product trade names and trademarks.
The $3.5 million acquired in-process research and development (“IPR&D”) associated with the
acquisition related to SurgiFrost XL. While the Company discontinued development of the SurgiFrost
XL product line as discussed above, it is working on procedural development with its existing
related products to enable less invasive ablation surgery. The IPR&D was recorded as a
non-recurring charge to operations in the second quarter of 2007. The Company used the income
approach to determine the fair value of the IPR&D, applying a risk adjusted discount rate of 30% to
the development project’s projected cash flows.
Pro Forma Results of Operations
The following unaudited pro forma financial information presents a summary of consolidated results
of operations of the Company as if the acquisition of CryoCath’s surgical cryoablation business had
occurred at the beginning of the earliest period presented. The historical consolidated financial
information has been adjusted to give effect to pro forma events that are directly attributable to
the acquisitions and are factually supportable. The unaudited pro forma condensed consolidated
financial information is presented for informational purposes only. The pro forma information is
not necessarily indicative of what the results of operations actually would have been had the
acquisitions been completed at the dates indicated. In addition, the unaudited pro forma condensed
consolidated financial information does not purport to project the future operating results of the
Company after completion of the acquisitions.
For the years ended December 31, 2009 and 2008, CryoCath’s surgical cryoablation business has been
included in the Company’s consolidated results of operations; consequently, no pro forma financial
information for these periods is presented. For purposes of preparing the unaudited pro forma
financial information for the year ended December 31, 2007, CryoCath’s surgical cryoablation
business unaudited Statement of Sales and Direct Operating Expenses for the six-month period ended
March 31, 2007 was combined with the Company’s consolidated Statement of Operations for the year
ended December 31, 2007, which includes six months of post-CryoCath asset acquisition operating
results. All periods used in preparing the unaudited pro forma financial information represent the
most recent financial information available. The CryoCath financial statements referenced above
have been summarized in a format similar to the financial statements of the Company and translated
to U.S. dollars in accordance with U.S. generally accepted accounting principles.
F - 12
Table of Contents
| Year ended | ||||
| December 31, | ||||
| (Unaudited pro forma data in thousands, except per share data) | 2007 | |||
Total revenue |
$ | 53,951 | ||
Net loss |
$ | (19,238 | ) | |
Net loss per share – basic and diluted |
$ | (0.37 | ) | |
The unaudited pro forma net loss includes (1) amortization of purchased intangible assets acquired,
(2) an increase in depreciation expense related to the step-up of fixed assets to fair value, (3)
adjustments to eliminate intercompany sales, commission and distribution rights income and
commission expense resulting from sales of CryoCath products, (4) the elimination of certain
license amortization recorded by the surgical cryoablation division of Cryocath which does not
apply to the combined entity, (5) the estimated impact of the ongoing supply arrangement between
CryoCath and ATS and (6) estimated additional interest expense on a pro forma basis due to the
additional bank borrowing completed to finance the asset acquisition.
The unaudited pro forma financial information excludes the non-recurring IPR&D charge of $3.5
million recorded in connection with the acquisition.
3. Leasehold Improvements, Furniture, and Equipment, net
At December 31, 2009 and 2008, leasehold improvements, furniture, and equipment consisted of the
following (in thousands):
| 2009 | 2008 | |||||||
At cost: |
||||||||
Furniture and fixtures |
$ | 738 | $ | 599 | ||||
Equipment |
16,226 | 13,604 | ||||||
Leasehold improvements |
3,795 | 3,678 | ||||||
Construction in progress |
316 | 556 | ||||||
| 21,075 | 18,437 | |||||||
Less accumulated depreciation |
13,416 | 11,406 | ||||||
| $ | 7,659 | $ | 7,031 | |||||
4. Private Placements of Common Stock
In December 2008, the Company sold 8,510,639 shares of its common stock to Essex Woodlands Health
Ventures (“Essex”) at $2.35 per share and received $19.8 million, net of offering costs. In
connection with the financing, the Company issued to Essex warrants to purchase 2,533,192 shares of
common stock at an exercise price of $2.475 per share until December 19, 2009, $2.85 per share
between December 19, 2009 and December 18, 2010, and $3.10 thereafter. The warrants expire on
December 19, 2015. In December 2009, Essex exercised the warrant on 1,000,000 shares of common
stock at an exercise price of $2.475 per share. The Company received $2.5 million as a result of
the exercise. In connection with the original stock sale in 2008, a co-founder and managing
director of Essex was appointed to the Company’s Board of Directors.
In June 2007, the Company sold to Alta Partners VIII, L.P. (“Alta”) 9,800,000 shares of its common
stock and a seven-year warrant to purchase up to 1,960,000 shares of common stock at an exercise
price of $1.65 per share. The Company was required to treat the Alta warrant as a liability
pending approval of its shareholders at the Company’s 2008 annual meeting of shareholders (or any
subsequent annual meeting) to issue shares of common stock to Alta upon exercise of the warrant.
Accordingly, the fair value of the warrant was recorded as a liability on the date of issuance and
marked-to-market quarterly, resulting in change in valuation gain or loss. At the Company’s annual
meeting of shareholders in May 2008, the Company received shareholder approval to issue shares of
common stock upon exercise of the warrant. Consequently, the liability was marked-to-market
through the date of shareholder approval, with the remaining warrant liability balance of $3.7
million credited to additional paid-in capital.
F - 13
Table of Contents
Alta warrant liability activity for 2008 is summarized below (in thousands):
Warrant liability balance on January 1, 2008 |
$ | 3,913 | ||
Change in fair value (gain) loss included in other (income) expense: |
||||
First quarter 2008 |
(1,521 | ) | ||
Second quarter 2008 |
1,278 | |||
Transfer to additional-paid-in-capital |
(3,670 | ) | ||
Warrant liability balance on December 31, 2008 |
$ | — | ||
In June 2008, Alta exercised the warrant on all 1,960,000 shares of common stock. The Company
received $3.2 million as a result of the exercise.
In March 2007, the Company sold 8,125,000 shares of its common stock to certain institutional
investors and received $15.3 million, net of offering costs. The private placement included the
issuance of warrants to purchase 3,250,000 shares of the Company’s common stock at an exercise
price of $2.40 per share, subject to adjustment upon certain events. The warrants expire on March
15, 2012.
5. Goodwill and Other Intangible Assets
Goodwill and intangible asset balances are summarized as follows (in thousands):
| Assets Not Subject | ||||||||||||||||||||||||
| Assets Subject to Amortization | to Amortization | |||||||||||||||||||||||
| Other | ||||||||||||||||||||||||
| Tissue Valve | Cryoablation | Technology | ||||||||||||||||||||||
| Technology | Technology | Carbon Technology | Licenses and | |||||||||||||||||||||
| and Trademarks | and Trademarks | License | Agreements | Total | Goodwill | |||||||||||||||||||
Balance at December 31, 2008: |
||||||||||||||||||||||||
Gross carrying amount (cost) |
$ | 7,150 | $ | 11,800 | $ | 18,500 | $ | 777 | $ | 38,227 | $ | 17,016 | ||||||||||||
Accumulated amortization |
(956 | ) | (2,580 | ) | (2,467 | ) | (109 | ) | (6,112 | ) | — | |||||||||||||
Net carrying amount |
$ | 6,194 | $ | 9,220 | $ | 16,033 | $ | 668 | $ | 32,115 | $ | 17,016 | ||||||||||||
Balance at December 31, 2009: |
||||||||||||||||||||||||
Gross carrying amount (cost) |
$ | 7,150 | $ | 11,800 | $ | 18,500 | $ | 797 | $ | 38,247 | $ | 32,166 | ||||||||||||
Accumulated amortization |
(1,381 | ) | (4,100 | ) | (3,700 | ) | (156 | ) | (9,337 | ) | — | |||||||||||||
Net carrying amount |
$ | 5,769 | $ | 7,700 | $ | 14,800 | $ | 641 | $ | 28,910 | $ | 32,166 | ||||||||||||
Goodwill and intangible assets activity is summarized as follows (in thousands):
| Assets Not Subject | ||||||||||||||||||||||||
| Assets Subject to Amortization | to Amortization | |||||||||||||||||||||||
| Other | ||||||||||||||||||||||||
| Tissue Valve | Cryoablation | Technology | ||||||||||||||||||||||
| Technology | Technology | Carbon Technology | Licenses and | |||||||||||||||||||||
| and Trademarks | and Trademarks | License | Agreements | Total | Goodwill | |||||||||||||||||||
Balance at December 31, 2007 |
$ | 6,619 | $ | 10,959 | $ | 17,267 | $ | 759 | $ | 35,604 | $ | 15,175 | ||||||||||||
Acquisition of CryoCath
surgical cryoablation business |
— | — | — | — | — | 2,000 | ||||||||||||||||||
CryoCath acquisition adjustments |
— | — | — | — | — | (159 | ) | |||||||||||||||||
Amortization |
(425 | ) | (1,739 | ) | (1,234 | ) | (91 | ) | (3,489 | ) | - | |||||||||||||
Balance at December 31, 2008 |
6,194 | 9,220 | 16,033 | 668 | 32,115 | $ | 17,016 | |||||||||||||||||
Contingent acquisition
common stock issued |
— | — | — | — | — | 15,150 | ||||||||||||||||||
Technology acquisition payments |
— | — | — | 19 | 19 | - | ||||||||||||||||||
Amortization |
(425 | ) | (1,520 | ) | (1,233 | ) | (46 | ) | (3,224 | ) | - | |||||||||||||
Balance at December 31, 2009 |
$ | 5,769 | $ | 7,700 | $ | 14,800 | $ | 641 | $ | 28,910 | $ | 32,166 | ||||||||||||
F - 14
Table of Contents
Aggregate amortization of intangible assets over the next five years is as follows (in
thousands):
2010 |
$ | 3,234 | ||
2011 |
3,189 | |||
2012 |
2,629 | |||
2013 |
2,067 | |||
2014 |
2,062 | |||
| $ | 13,181 | |||
Tissue Valve Technology and Trademarks
The Company acquired goodwill and certain other definite-lived intangible assets related to its
tissue valve business through the 2006 acquisition of 3F Therapeutics, Inc. (“3F”). In December
2009, the Company issued 5,000,000 shares of common stock to 3F stockholders under the terms of the
2006 agreement and plan of merger upon the Company’s obtaining the European CE mark on its Enable®
Aortic Bioprosthesis sutureless tissue valve product. The Company recorded the 5,000,000 shares
issued as additional goodwill from the 3F acquisition. The goodwill was valued using the Company’s
closing stock price on the date of the announcement of CE mark approval of $3.03 per share. The
Company is obligated to make an additional contingent payment to 3F stockholders of up to 5,000,000
shares of common stock upon obtaining U.S. Food and Drug Administration (“FDA”) approval for the
Enable product or FDA or European CE mark approval for certain future key products acquired in the
3F acquisition on or prior to December 31, 2013. This contingent share payment may be accelerated
upon completion of certain transactions involving these key products and is subject to certain
rights of offset for indemnification claims and certain other events.
Cryoablation Technology and Trademarks
As disclosed in Note 2 above, the Company acquired goodwill and certain other definite-lived
intangible assets through the June 2007 acquisition of the surgical cryoablation business of
CryoCath Technologies, Inc.
Carbon Technology License
The Company holds an exclusive, worldwide right and license to use CarboMedics, Inc.’s
(“CarboMedics”) pyrolytic carbon technology. The license was originally obtained in 1999 and had a
carrying value of $18.5 million at December 31, 2006. Based on the Company’s periodic review of
its indefinite-lived intangibles, the Company determined that this carbon technology license has a
finite life and began amortizing this asset over a 15-year life commencing January 1, 2007. The
Company expects amortization expense on this technology license to be approximately $1.2 million
per year through 2021.
Other Technology Licenses and Agreements
In October 2007, the Company acquired certain patent rights and intellectual property related to a
heart valve holder, a heart valve folding and delivery device, and other ancillary devices. The
Company paid an up-front license fee of $0.1 million and will be obligated to make royalty payments
on future sales of products related to certain of the patent rights transferred.
In September 2007, the Company acquired a fully paid-up license for $0.2 million related to a
thoracic port surgical device which the Company has been selling and for which the Company had
previously been paying royalties based on product sales.
In January 2007, the Company issued 224,416 shares of its common stock pursuant to the exercise of
its option to purchase certain assets of EM Vascular, Inc. (“EM Vascular”), under a May 2005 Option
and Asset Purchase Agreement (“Option Agreement”). The payment in shares was at the option of the
Company and was in lieu of a $0.5 million cash payment. The most significant asset acquired as
part of this purchase is technology that may potentially allow for a non-invasive, non-pharma
therapy for the treatment of such disorders as atherosclerotic plaque and blood
hyper-cholesterolemia. Under the terms of the Option Agreement, the Company will also be obligated
to make additional contingent payments to EM
F - 15
Table of Contents
Vascular of up to $2.2 million in the form of ATS common stock upon the attainment of certain
milestone events and to pay royalties on applicable product sales.
Intangible Asset Impairment
The Company made licensing fee and development milestone payments to ErySave AB (“ErySave”), a
Swedish research firm, under an exclusive development and licensing agreement, executed in 2004,
for worldwide rights to ErySave’s PARSUS filtration technology for cardiac surgery procedures. In
July 2007, the Company was informed that ErySave was in the process of declaring bankruptcy and
they could not continue development work. Accordingly, the $0.8 million ErySave license payments
intangible asset was written off during the year ended December 31, 2007.
Goodwill
Goodwill is not subject to amortization for book purposes, but must be analyzed for impairment on
at least an annual basis. For tax purposes, the goodwill recognized in connection with the
CryoCath asset acquisition ($11.9 million) is deductible over a 15-year period. The remaining
goodwill is not tax deductible.
6. Debt
Convertible Notes Payable
In 2005, the Company sold a combined $22.4 million aggregate principal amount of 6% Convertible
Senior Notes due 2025 (“Convertible Senior Notes” or “Notes”), warrants to purchase 1,344,000
shares of the Company’s common stock (“Warrants”), and related embedded derivatives. Interest is
payable under the Notes each April and October.
The Convertible Senior Notes are convertible into common stock at any time at a fixed conversion
price of $4.20 per share, subject to adjustment under certain circumstances including, but not
limited to, the payment of cash dividends on common stock. If fully converted, the Notes would
convert into 5,333,334 shares of the Company’s common stock.
The Warrants are exercisable at $4.40 per share and expire in 2010. The Company has reserved 105%
of the shares necessary for the exercise of the warrants. The Warrants were valued at $1.13 per
share using the Black-Scholes valuation model. The total value of the Warrants on the date of
issuance was $1.5 million and was recorded as a discount on the Notes and is being amortized to
interest expense over the 20 year life of the Notes using the effective interest method.
The Convertible Senior Note holders have the right to require the Company to repurchase the Notes
at 100% of the principal amount plus accrued and unpaid interest on October 15 in 2010, 2015 and
2020 or in connection with certain corporate change of control transactions. Accordingly, the
Company has reclassified the Notes from long-term to current liabilities on its balance sheet as of
December 31, 2009. If the Note holders elect to convert the Notes prior to October 15, 2010 in
connection with certain corporate change of control transactions, the Company will increase the
conversion rate for the Notes surrendered for conversion by a number of additional shares based on
the stock price of the Company on the date of the change of control.
The Company has the right to redeem the Notes at 100% of the principal amount plus accrued and
unpaid interest at any time on or after October 20, 2008. Subsequent to December 31, 2009, the
Company obtained a commitment for $30 million in term debt financing which, if obtained, will be
used to call and retire the Notes. See Note 16 below. At any time prior to maturity, the Company
may also elect to automatically convert some or all of the Notes into shares of its common stock if
the closing price of the common stock exceeds $6.40 for a period as specified in the indenture.
The Company has bifurcated embedded derivatives from the Convertible Senior Notes as required by
FASB ASC 815, Derivatives and Hedging, and applicable SEC rules. The Company recorded a $5.5
million derivative liability on the date of issuance of the Notes, with an offsetting discount on
the Notes. The most significant derivative related to the lack of authorized shares to cover the
potential conversion
F - 16
Table of Contents
and warrant exercise. That derivative was marked to market until appropriate shareholder approval
was obtained, at which time the balance of the liability was offset against the remaining discount
on the debt. The other derivatives identified were not material. The derivative liability
includes certain time-based provisions of the Notes which expire over time, the latest in 2011.
The derivative liability is adjusted to fair value on a quarterly basis.
Derivative liability activity is summarized below (in thousands):
| 2009 | 2008 | 2007 | ||||||||||
Derivative liability balance at beginning of year |
$ | 90 | $ | 141 | $ | 239 | ||||||
Change in valuation gain included in other income |
(34 | ) | (51 | ) | (98 | ) | ||||||
Derivative liability balance at end of year |
$ | 56 | $ | 90 | $ | 141 | ||||||
The total discount on the Notes is being amortized to interest expense over the 20 year life of the
Notes, using the effective interest method. Interest expense attributable to discount amortization
totaled approximately $0.2 million, $0.1 million and $0.1 million for the years ended December 31,
2009, 2008 and 2007, respectively. The remaining unamortized discount was $4.8 million at December
31, 2009. Should the Notes be called be by the Company or put back to the Company by the holders
in 2010, the Company would recognize a loss on debt extinguishment, which would include any
unamortized discount on the Notes.
Both the derivative liability and related discount are presented in the balance sheet within the
same line as the Convertible Senior Notes payable.
Bank Loan Payable
Since 2004, the Company has maintained a Loan and Security Agreement (“Loan Agreement”) with
Silicon Valley Bank (“Bank”). All Company assets are pledged as collateral under the Loan
Agreement. The Loan Agreement, as amended, subjects the Company to certain financial covenants and
restricts the Company from declaring or paying dividends.
In June 2007, the Company entered into an Amendment to the Loan Agreement (“June 2007 Amendment”)
whereby the Bank consented to (i) the Company’s purchase of the cryoablation surgical device
business of CryoCath (“CryoCath Assets”) and (ii) certain agreements related to the acquisition of
the CryoCath Assets. The June 2007 Amendment also provided for an $8.6 million term loan (the “Term
Loan”) to the Company, which was used to purchase a portion of the CryoCath Assets and to repay
then-outstanding term loans and advances from the Bank under the Loan Agreement.
Under the Term Loan, as amended, the Company made monthly payments of interest only from July 2007
until March 2008, and began making 39 monthly payments of principal plus interest effective April
1, 2008 and continuing on the first day of each successive month until June 1, 2011. The Company
also has the right to prepay all, but not less than all, of the outstanding Term Loan at any time
so long as no event of default has occurred. Subsequent to December 31, 2009, the Company obtained
a commitment for $30 million in term debt financing, a portion of which, if obtained, will be used
to prepay the remaining balance of the Term Loan. See Note 16 below. Interest on the Term Loan
accrues at a fixed rate per annum of 9.5%, equal to 1.25% above the Prime Rate in effect as of the
funding date of the Term Loan.
The June 2007 Amendment also made certain changes to the liquidity ratio covenant set forth in the
Loan Agreement, as amended. The liquidity ratio was changed to require that the Company maintain,
at all times, on a consolidated basis, a ratio of (a) the sum of (1) unrestricted cash (and
equivalents) of the Company on deposit with the Bank plus (2) 50% of the Company’s accounts
receivable arising from the sale or lease of goods, or provision of services, in the ordinary
course of business, divided by (b) the indebtedness of the Company to the Bank for borrowed money,
equal to or greater than 1.4 to 1.0. In June 2008, the Company entered into an Amendment to the
Loan Agreement whereby, for the balance of 2008, the 1.4 to 1.0 required liquidity ratio was
reduced to 1.1 to 1.0 for intra-quarter months only. The liquidity ratio remained at 1.4 to 1.0
for quarter-end months and reverted to 1.4 to 1.0 for all months beginning in 2009. In December
2008, the Company entered into an Amendment to the Loan Agreement (“December 2008 Amendment”)
whereby the liquidity ratio was raised to 2.0 to 1.0 until a $4.5 million litigation
F - 17
Table of Contents
settlement payment was made to CarboMedics (see Note 15 below) and a related security interest
granted to CarboMedics was released. Upon such payment, the Bank agreed to return the liquidity
ratio requirement to 1.4 to 1.0. In addition, the December 2008 Amendment required the Company to
maintain at least $4.5 million on deposit with the Bank at all times until the litigation
settlement payment of $4.5 million was made. On April 30, 2009, the Company made the $4.5 million
settlement payment. As of December 31, 2009, the Company was in compliance with the financial
covenants as set forth in the Loan Agreement, as amended.
Future maturities of bank notes payable are as follows (in thousands):
2010 |
$ | 2,646 | ||
2011 |
1,323 | |||
| $ | 3,969 | |||
Subordinated Credit Agreement
In June 2008, the Company entered into a Subordinated Credit Agreement (“Credit Agreement”) with
Theodore C. Skokos, a member of the Company’s Board of Directors, for a two-year, $5 million
Revolving Credit Facility (“Credit Facility”). Advances under the Credit Facility will carry
interest at 15% per annum payable quarterly. The Credit Facility also carries an annual commitment
fee of 1% of the average unused Revolving Commitment Amount, payable annually. In July 2009, the
first annual commitment fee of $50,000 was paid to Mr. Skokos. As of December 31, 2009, no
advances had been made and all $5 million was available for borrowing under the Credit Facility.
The Company’s obligations to Mr. Skokos under the Credit Agreement have been made subordinate to
(1) the Company’s obligations to the holders of its Convertible Senior Notes and (2) the Company’s
obligations to Silicon Valley Bank as provided in a Subordination Agreement executed by and between
the Bank and Mr. Skokos. All Company assets are pledged as collateral on the Credit Facility.
In connection with the execution of the Credit Agreement, the Company issued to Mr. Skokos a
warrant to purchase 245,098 shares of common stock of the Company at $2.04 per share until June 29,
2015 (“Effective Date Warrant”). In July 2008, Mr. Skokos exercised the Effective Date Warrant in
full and the Company received $0.5 million from the exercise. The Company is obligated to issue
additional seven-year warrants to Mr. Skokos in the future based on the total amount of advances
under the Credit Facility. If the aggregate unpaid principal amount of all advances (“Total
Outstanding Revolver Amount”) under the Credit Facility, after giving effect to each new advance,
is greater than the amount of the highest Total Outstanding Revolver Amount at any time prior to
the date of any such advance (“Maximum Total Outstanding Revolver Amount”), then the Company shall
issue a warrant to Mr. Skokos for a number of shares of common stock equal to 20% of (a) the
difference between (1) such Total Outstanding Revolver Amount less (2) the Maximum Total
Outstanding Revolver Amount, (b) divided by the warrant exercise price of $2.04 per share. The
maximum number of additional shares issuable pursuant to warrants issued under the Credit Facility
is 490,196 shares (not including the Effective Date Warrant), which represents 20% of the maximum
amount of advances under the $5 million Credit Facility divided by the $2.04 warrant exercise
price.
7. Stock-Based Compensation and Equity Plans
Stock-Based Compensation
The Company accounts for its stock-based employee compensation plans under the recognition and
measurement principles of FASB ASC 718, Stock Compensation (“ASC 718”), which requires all
share-based payments to be recognized in the income statement based on their fair values.
F - 18
Table of Contents
Fair Value. For stock option grants, the Company uses the Black-Scholes option pricing model as
its method for determining fair value. The weighted average per share fair value of these option
grants is shown below for 2009 and 2008 (there were no options granted in 2007) and was estimated
at the date of grant using the Black-Scholes model with the following weighted average assumptions:
| Assumptions used: | 2009 | 2008 | ||||||
Expected volatility |
0.77 | 1.06 | ||||||
Risk-free interest rate |
2.0 | % | 2.8 | % | ||||
Expected life |
5 years | 5 years | ||||||
Dividend yield |
0 | % | 0 | % | ||||
Weighted average per share fair value of options granted |
$ | 1.67 | $ | 1.22 | ||||
The expected volatility is a measure of the amount by which the Company’s stock price is expected
to fluctuate during the expected term of options granted. The Company determines the expected
volatility solely based upon the historical volatility of the Company’s common stock over a period
commensurate with the option’s expected life. The Company does not believe the future volatility
of its common stock over an option’s expected life is likely to differ significantly from the past.
The risk-free interest rate is the implied yield available on U.S. Treasury issues with a
remaining term equal to the option’s expected life on the grant date. The expected life of options
granted represents the period of time for which options are expected to be outstanding and is
derived from the Company’s historical stock option exercise experience and option expiration data.
For purposes of estimating the expected life, the Company has aggregated all individual option
awards into one group as the Company does not expect substantial differences in exercise behavior
among its employees. The dividend yield is zero since the Company has never declared or paid any
cash dividends on its common stock and does not expect to do so in the foreseeable future.
For restricted stock unit (“RSU”) awards, fair value is determined based on the closing market
price on the award date.
Stock Compensation Expense. The Company uses the single option (i.e. straight-line) method of
attributing the value of stock-based compensation expense for all stock option grants and RSU
awards. Stock compensation expense for all stock-based grants and awards is recognized over the
service or vesting period of each grant or award.
The following table summarizes stock compensation expense recognized in the statements of
operations for the years ended December 31, 2009, 2008 and 2007 (in thousands, except per share
data):
| 2009 | 2008 | 2007 | ||||||||||
Stock compensation expense included in: |
||||||||||||
Cost of goods sold |
$ | 112 | $ | — | $ | — | ||||||
Sales and marketing expenses |
1,319 | 973 | 805 | |||||||||
Research and development |
301 | — | — | |||||||||
General and administrative expenses |
825 | 703 | 649 | |||||||||
Total stock compensation expense |
$ | 2,557 | $ | 1,676 | $ | 1,454 | ||||||
Stock compensation expense per share |
$ | 0.04 | $ | 0.03 | $ | 0.03 | ||||||
Because the Company maintained a full valuation allowance on its U.S. deferred tax assets, the
Company did not recognize any net tax benefit related to its stock-based compensation expense for
the years ended December 31, 2009, 2008 and 2007.
Forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if
necessary, in subsequent periods if actual forfeitures differ from those estimates in order to
derive the Company’s best estimate of awards ultimately expected to vest. Forfeitures represent
only the unvested portion of a surrendered option or RSU and are typically estimated based on
historical experience. Based on an analysis of the Company’s historical data, the Company applied
average forfeiture rates of 9.56%, 10.79% and 8.95% for the years ended December 31, 2009, 2008 and
2007, respectively, to stock options and RSUs outstanding in determining its stock compensation
expense, which it believes are reasonable forfeiture estimates for these periods.
F - 19
Table of Contents
Equity Plans
Stock Incentive Plans. The Company has a Stock Incentive Plan (the “Plan”) under which stock
options to purchase common stock of the Company may be granted or RSUs may be awarded to employees
and non-employees of the Company. Stock options may be granted under the Plan as incentive stock
options (“ISO”) or as non-qualified stock options (“non-ISO”). The Company also has stock options
outstanding from a previous equity compensation plan as well as free-standing options not under any
plan. All stock issued under options exercised or RSUs awarded are new shares of the Company’s
common stock. Option grants generally carry vesting terms of five years and contractual terms of
up to ten years. RSU awards generally carry contractual terms of up to five years.
The Company had a total of 8,742,755 shares of common stock reserved for stock option grants and
RSU awards at December 31, 2009, of which 2,075,676 shares were available for future grants or
awards under the Plan.
Employee Stock Purchase Plan. The Company maintains an Employee Stock Purchase Plan (“ESPP”).
Under the terms of the ESPP, employees are eligible to purchase new shares of common stock of the
Company on a quarterly basis. Employees can purchase common stock at 85% of the lesser of the
market price of the common stock on the first day of the quarter or the last day of the quarter.
The ESPP is deemed to be a compensatory plan under ASC 718, and as such the related expense is
included in stock compensation expense.
The following table summarizes the shares issued and issuance prices under the ESPP:
| Number | ||||||||
| of Shares | Price Range | |||||||
2009 |
124,231 | $ | 2.14 — $2.29 | |||||
2008 |
123,278 | $ | 1.40 — $2.25 | |||||
2007 |
129,449 | $ | 1.50 — $1.84 | |||||
Stock Options. The following table summarizes the changes in stock options outstanding under the
Company’s stock-based compensation plans:
| Weighted Average | ||||||||||||||||||||
| Stock Options Outstanding | Option | |||||||||||||||||||
| Under the Plans | Exercise Price | |||||||||||||||||||
| ISO | Non-ISO | Non-Plan Options | Total | Per Share | ||||||||||||||||
Outstanding at December 31, 2006 |
917,625 | 299,000 | 2,532,700 | 3,749,325 | $ | 2.94 | ||||||||||||||
Options granted |
— | — | — | — | — | |||||||||||||||
Options exercised |
(21,500 | ) | — | (502,000 | ) | (523,500 | ) | 0.92 | ||||||||||||
Options canceled |
(226,250 | ) | — | (333,750 | ) | (560,000 | ) | 4.44 | ||||||||||||
Outstanding at December 31, 2007 |
669,875 | 299,000 | 1,696,950 | 2,665,825 | 3.02 | |||||||||||||||
Options granted |
125,900 | — | — | 125,900 | 1.57 | |||||||||||||||
Options exercised |
(24,750 | ) | — | (78,750 | ) | (103,500 | ) | 1.28 | ||||||||||||
Options canceled |
(128,375 | ) | — | (125,000 | ) | (253,375 | ) | 4.29 | ||||||||||||
Outstanding at December 31, 2008 |
642,650 | 299,000 | 1,493,200 | 2,434,850 | 2.89 | |||||||||||||||
Options granted |
157,250 | — | — | 157,250 | 2.64 | |||||||||||||||
Options exercised |
(3,100 | ) | — | — | (3,100 | ) | 1.05 | |||||||||||||
Options canceled |
(79,000 | ) | (10,000 | ) | — | (89,000 | ) | 4.27 | ||||||||||||
Outstanding at December 31, 2009 |
717,800 | 289,000 | 1,493,200 | 2,500,000 | $ | 2.82 | ||||||||||||||
F -20
Table of Contents
The following table summarizes the ranges of exercise prices for outstanding and exercisable stock
options at December 31, 2009:
| Options Outstanding at | Options Exercisable at | |||||||||||||||
| December 31, 2009 | December 31, 2009 | |||||||||||||||
| Weighted Average | Weighted Average | Weighted Average | ||||||||||||||
| Range of | Remaining | Exercise | Exercise | |||||||||||||
| Exercise Prices | Number Outstanding | Contractual Life | Price | Number Exercisable | Price | |||||||||||
$0.37 — $0.46 |
505,000 | 2.88 years | $ | 0.40 | 505,000 | $ | 0.40 | |||||||||
0.51 — 2.51 |
479,550 | 3.67 years | 1.90 | 396,430 | 1.97 | |||||||||||
2.64 — 3.36 |
528,950 | 4.22 years | 3.05 | 389,200 | 3.19 | |||||||||||
3.46 — 3.78 |
433,750 | 4.09 years | 3.65 | 433,750 | 3.65 | |||||||||||
3.80 — 5.34 |
439,750 | 3.99 years | 4.05 | 439,750 | 4.05 | |||||||||||
8.19 — 9.88 |
113,000 | 0.61 years | 8.63 | 113,000 | 8.63 | |||||||||||
$0.37 — $9.88 |
2,500,000 | 3.62 years | $ | 2.82 | 2,277,130 | $ | 2.88 | |||||||||
At December 31, 2009, the aggregate intrinsic value of options outstanding and exercisable was
approximately $2.2 million and $2.0 million, respectively. The aggregate intrinsic value of
options exercised for the year ended December 31, 2009 was not significant. The aggregate
intrinsic value of options exercised for the years ended December 31, 2008 and 2007 was
approximately $0.2 million and $0.7 million, respectively. The aggregate intrinsic value
represents the total pre-tax intrinsic value (the difference between the closing price of the
Company’s common stock on December 31, 2009, 2008 and 2007 ($3.23, $2.78 and $2.21 per share,
respectively) and the exercise price of each-in-the-money option that would have been received by
the option holders had all option holders exercised their options on those dates). At December 31,
2009, the Company had $0.2 million of total unrecognized compensation expense, net of estimated
forfeitures, related to stock options that will be recognized over a weighted average period of
approximately four years.
Restricted Stock Units. The following table summarizes RSU awards activity under the Company’s
stock-based compensation plans:
| Weighted | ||||||||||||
| Weighted Average | Average | |||||||||||
| Award Date | Remaining | |||||||||||
| Number of Shares | Fair Value | Contractual Term | ||||||||||
Unvested at December 31, 2006 |
1,169,522 | $ | 2.83 | 2.03 years | ||||||||
Awards granted |
1,537,184 | 1.74 | ||||||||||
Awards vested |
(419,055 | ) | 2.75 | |||||||||
Awards forfeited |
(250,113 | ) | 2.51 | |||||||||
Unvested at December 31, 2007 |
2,037,538 | 2.06 | 2.09 years | |||||||||
Awards granted |
2,158,634 | 2.02 | ||||||||||
Awards vested |
(600,166 | ) | 2.27 | |||||||||
Awards forfeited |
(204,263 | ) | 2.09 | |||||||||
Unvested at December 31, 2008 |
3,391,743 | 2.00 | 2.14 years | |||||||||
Awards granted |
2,032,236 | 2.27 | ||||||||||
Awards vested |
(982,999 | ) | 2.08 | |||||||||
Awards forfeited |
(273,901 | ) | 1.90 | |||||||||
Unvested at December 31, 2009 |
4,167,079 | $ | 2.12 | 1.92 years | ||||||||
At December 31, 2009, the aggregate intrinsic value of RSU awards outstanding was $13.5 million.
The aggregate intrinsic value represents the total pre-tax value of common stock that RSU holders
would have received (based on the closing price of the Company’s common stock on December 31, 2009
of $3.23 per share) had all RSUs vested and common stock been issued to the RSU holders on December
31, 2009. At December 31, 2009, the Company had approximately $5.5 million of total unrecognized
compensation expense, net of estimated forfeitures, related to RSU awards that will be recognized
over a weighted average period of approximately 3.5 years.
F -21
Table of Contents
8. Leases
The Company maintains operating leases for its facilities. These leases expire at various dates
through September 2012. Future minimum lease payments under these agreements are as follows (in
thousands):
| Year ending December 31: | ||||
2010 |
$ | 674 | ||
2011 |
310 | |||
2012 |
249 | |||
2013 |
24 | |||
| $ | 1,257 | |||
Rent expense was $1.1 million, $1.1 million and $1.0 million for 2009, 2008 and 2007, respectively.
Included in rent expense are several smaller operating leases covering miscellaneous office
equipment.
The Company has a capital lease for its corporate telephone system. The total amount capitalized
to fixed assets for the phone system was $0.2 million (net of $0.2 million representing interest),
which is being depreciated over the lease term of five years, commencing in 2010. The total lease
payments to be made is $0.4 million ($0.08 million per year). The equipment becomes the property of
the Company at the end of the lease. The capital lease liability of $0.2 million is reflected on
the balance sheet at December 31, 2009 in other long-term liabilities.
9. Income Taxes
Income tax expense from continuing operations for the years ended December 31, 2009, 2008 and 2007
consists of the following (in thousands):
| 2009 | 2008 | 2007 | ||||||||||
Current income taxes: |
||||||||||||
Federal |
$ | — | $ | — | $ | — | ||||||
State |
15 | 12 | — | |||||||||
Foreign |
12 | 277 | 24 | |||||||||
| 27 | 289 | 24 | ||||||||||
Deferred income taxes |
261 | 192 | 95 | |||||||||
| $ | 288 | $ | 481 | $ | 119 | |||||||
At December 31, 2009, the Company had federal net operating loss carryforwards of approximately
$167 million ($59 million related to 3F) and credits for increasing research and development costs
of approximately $1.1 million ($0.9 million related to 3F). The Company also had state net
operating loss carryforwards of approximately $84.5 million ($42.1 million related to 3F) and
research and development credits of approximately $1.0 million ($0.9 million related to 3F). The
net operating loss carryforwards are available to offset future taxable income or reduce taxes
payable through 2029. These loss carryforwards will begin expiring in 2010. The credits continue to
expire in 2011 through 2029.
Included as part of the Company’s net operating loss carryforwards are approximately $3.4 million
in tax deductions that resulted from the exercise of stock options. Should these loss
carryforwards be realized, the corresponding change in valuation allowance will be recorded as
additional paid-in capital.
The Company’s ability to utilize a portion of its net operating loss carryforwards and research and
development credits to offset future taxable income are subject to certain limitations under
Section 382 and 383 of the Internal Revenue Code due to changes in the equity ownership of the
Company. In addition, 3F’s net operating loss carryforwards may also be limited by separate return
limitation year rules.
In addition to the U.S. tax attributes discussed above, the Company had net operating loss
carryforwards outside the U.S. totaling approximately $0.7 million. These international net
operating loss carryforwards do not expire.
F -22
Table of Contents
The Company has not provided deferred taxes on unremitted earnings attributable to its Belgium
subsidiary that have been considered to be reinvested indefinitely. These earnings relate to
currency gains recorded as Other Comprehensive Income and are approximately $0.2 million at
December 31, 2009.
Components of deferred tax assets and liabilities are as follows (in thousands):
| December 31 | ||||||||
| 2009 | 2008 | |||||||
Current deferred tax assets: |
||||||||
Inventory |
$ | 569 | $ | 543 | ||||
Accrued vacation |
297 | 295 | ||||||
Other current deferred tax assets |
64 | 151 | ||||||
Net current deferred tax assets |
930 | 989 | ||||||
Long-term deferred tax assets (liabilities): |
||||||||
Net operating loss carryforwards |
60,861 | 59,376 | ||||||
Foreign net operating loss carryforwards |
225 | 319 | ||||||
Research and development credits |
1,799 | 1,605 | ||||||
Alternative minimum tax credits |
54 | 54 | ||||||
Depreciation |
1,225 | 1,188 | ||||||
Compensation accruals and reserves |
663 | 540 | ||||||
Deferred financing costs |
374 | 286 | ||||||
Technology license amortization |
(2,176 | ) | (1,857 | ) | ||||
Other intangible assets and goodwill |
(885 | ) | (914 | ) | ||||
Other |
1,086 | 766 | ||||||
Net long-term deferred tax assets |
63,226 | 61,363 | ||||||
Net deferred tax assets before valuation allowance |
64,156 | 62,352 | ||||||
Less valuation allowance |
(64,704 | ) | (62,639 | ) | ||||
Net deferred tax liability |
$ | (548 | ) | $ | (287 | ) | ||
The net deferred tax liability shown above has been included in other long-term liabilities on the
balance sheet. The Company has determined that a full valuation allowance is appropriate given the
uncertainty of the Company’s ability to utilize the deferred tax assets. The valuation allowance
shown above includes 3F net deferred tax assets (primarily net operating loss carryforwards) of $22
million. Under FASB ASC 805, adopted by the Company effective January 1, 2009, if realized, the 3F
tax assets will no longer be recorded first as a reduction to goodwill and intangible assets, but
will be recorded as income tax benefits.
Reconciliation of the statutory federal income tax rate to the Company’s effective tax rate for the
years ended December 31, 2009, 2008 and 2007 is as follows:
| 2009 | 2008 | 2007 | ||||||||||
Tax at statutory rate |
(34.0 | )% | (34.0 | )% | (34.0 | )% | ||||||
State income taxes |
(1.7 | ) | (2.0 | ) | (2.4 | ) | ||||||
Meals & entertainment |
1.8 | 0.6 | 0.5 | |||||||||
Other permanent differences |
1.4 | 0.4 | 1.0 | |||||||||
R&D credits |
(3.2 | ) | 0.5 | (2.7 | ) | |||||||
Uncertain tax positions |
(0.3 | ) | 0.7 | — | ||||||||
Other (including foreign taxes) |
0.3 | 0.5 | 1.5 | |||||||||
Impact of changes in valuation allowance |
40.5 | 35.8 | 36.6 | |||||||||
| 4.8 | % | 2.5 | % | 0.5 | % | |||||||
The Company applies the provisions of FASB ASC 740-10, Income Taxes (“ASC 740-10”) related to
accounting for uncertainty in income taxes. Under these provisions of ASC 740-10, the Company
recognizes the financial statement benefit of a tax position only after determining that the
relevant tax authority would more likely than not sustain the position. For tax positions meeting
the more likely than not threshold, the amount recognized in the financial statements is the
largest benefit that has a greater than
F -23
Table of Contents
50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority.
At the adoption date, the Company applied the provisions of FASB ASC 740-10 related to accounting
for uncertainty in income taxes to all tax positions for which the statute of limitations remained
open.
The total gross amount of unrecognized tax benefits as of December 31, 2009, 2008 and 2007 was
approximately $0.7, $0.8 million and $0.5 million, respectively. If recognized, none of the
unrecognized tax benefits would affect the tax rate. A reconciliation of unrecognized tax benefits
for the years ended December 31, 2009, 2008 and 2007 is as follows (in thousands):
Balance at January 1, 2007 |
$ | 540 | ||
Additions for tax positions of prior periods |
13 | |||
Reductions for tax positions related to the current period |
(23 | ) | ||
Balance at December 31, 2007 |
530 | |||
Additions for tax positions of prior periods |
166 | |||
Additions for tax positions related to the current period |
120 | |||
Balance at December 31, 2008 |
816 | |||
Reductions for settlements with taxing authorities |
(133 | ) | ||
Reductions for tax positions related to prior periods |
(33 | ) | ||
Additions for tax positions related to the current period |
59 | |||
Balance at December 31, 2009 |
$ | 709 | ||
Unrecognized tax benefits decreased by approximately $0.1 million during 2009 resulting from the
settlement of a tax examination of the Company’s France subsidiary for the 2005 through 2007 tax
years.
It is the Company’s practice to recognize penalties and/or interest related to income tax matters
in interest and penalties expense. As of December 31, 2009, the Company had no accrued interest
and penalties.
The Company is subject to income taxes in the U.S. federal jurisdiction, foreign jurisdictions and
various states jurisdictions. Tax regulations within each jurisdiction are subject to the
interpretation of the related tax laws and regulations and require significant judgment to apply.
With few exceptions, the Company is no longer subject to U.S. federal, foreign, state or local
income tax examinations by tax authorities for the years before 2006.
10. Benefit Plan
The Company has a defined contribution salary deferral plan covering substantially all employees
under Section 401(k) of the Internal Revenue Code. Under the plan, the Company contributes an
amount equal to 25% of the first 12% of each employee’s contribution. The Company recognized
expense for contributions to the plan of $0.3 million for 2009 and $0.2 million for each of 2008
and 2007.
11. Significant Customers and Concentration of Credit Risk
Since its inception, the Company has operated in a single industry segment: developing,
manufacturing, and marketing medical devices. As a result, the information disclosed herein
materially represents all of the financial information related to the Company’s principal operating
segment. The Company uses independent distributors to sell its products in many European and all
other international markets. The Company derived the following percentages of its net sales from
the following geographic regions:
| 2009 | 2008 | 2007 | ||||||||||
United States |
39 | % | 38 | % | 38 | % | ||||||
Europe |
30 | % | 36 | % | 33 | % | ||||||
Asia Pacific |
14 | % | 12 | % | 13 | % | ||||||
China |
12 | % | 9 | % | 9 | % | ||||||
Other Markets |
5 | % | 5 | % | 7 | % | ||||||
The Company had balances owing from two customers that aggregated 16% and 15% of its accounts
receivable balances at December 31, 2009 and 2008, respectively.
F -24
Table of Contents
12. Quarterly Financial Data (Unaudited)
Quarterly data for 2009 and 2008 was as follows (in thousands, except loss per share):
| Quarter | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
Year ended December 31, 2009: |
||||||||||||||||
Net sales |
$ | 18,403 | $ | 19,781 | $ | 18,827 | $ | 18,699 | ||||||||
Gross profit |
12,273 | 12,741 | 11,844 | 12,031 | ||||||||||||
Net loss |
(1,671 | ) | (186 | ) | (975 | ) | (3,481 | ) | ||||||||
Net basic and diluted loss per share |
$ | (0.02 | ) | $ | (0.00 | ) | $ | (0.01 | ) | $ | (0.05 | ) | ||||
Year ended December 31, 2008: |
||||||||||||||||
Net sales |
$ | 14,845 | $ | 16,900 | $ | 16,044 | $ | 18,032 | ||||||||
Gross profit |
8,948 | 10,290 | 9,474 | 11,842 | ||||||||||||
Net loss |
(2,411 | ) | (4,669 | ) | (3,746 | ) | (8,513 | ) | ||||||||
Net basic and diluted loss per share |
$ | (0.04 | ) | $ | (0.08 | ) | $ | (0.06 | ) | $ | (0.13 | ) | ||||
In connection with the settlement of litigation with CarboMedics discussed in Note 15 below, the
Company recorded a $7.5 million litigation settlement charge in the fourth quarter of 2008.
Note 13. Fair Value Measurements
The Company uses fair value measurement accounting principles related to its financial and
non-financial assets and liabilities as required by FASB ASC 820, Fair Value Measurements and
Disclosures (“ASC 820”). ASC 820 defines fair value, establishes a framework for measuring fair
value in accordance with U.S. GAAP and expands disclosures about fair value measurements. The
adoption of ASC 820 did not have a material impact on the Company’s financial condition or results
of operations.
ASC 820 defines fair value as the price that would be received for an asset or paid to transfer a
liability (an exit price) in the principal or most advantageous market for the asset or liability
in an orderly transaction between market participants on the measurement date. ASC 820 also
describes three levels of inputs that may be used to measure fair value:
| • | Level 1 — quoted prices in active markets for identical assets and liabilities. |
| • | Level 2 — observable inputs other than quoted prices in active markets for identical assets and liabilities. |
| • | Level 3 — unobservable inputs in which there is little or no market data available, which require the reporting entity to develop its own assumptions. |
The fair value of the Company’s warrant liability (described in Note 4 above) was determined based
on Level 2 inputs. The fair value of the Company’s Convertible Senior Notes derivative liability
(described in Note 6 above) was determined based on Level 3 inputs using discounted probability
cash flow valuation models. As discussed in Note 1 above, the Company’s goodwill is analyzed
annually for impairment by reference to fair value based on the Company’s market capitalization, a
Level 1 input. The Company assesses the potential impairment of definite-lived intangible assets
by reference to future gross profit cash flows from the underlying products using the capitalized
technology and trademarks, a Level 3 input.
The effective date for certain aspects of ASC 820 was deferred until January 1, 2009. Areas
impacted by the deferral relate to nonfinancial assets and liabilities that are measured at fair
value but are recognized or disclosed at fair value on a nonrecurring basis. The Company adopted
these aspects of the accounting for fair value measurements related to its nonfinancial assets and
liabilities effective January 1, 2009. This adoption applies to such items as nonfinancial
long-lived asset groups measured at fair value for an impairment assessment and determining the
fair value of a financial asset when the market for that asset is not active. The application of
these remaining aspects of ASC 820 did not have a material impact on the Company’s financial
condition or results of operations.
F -25
Table of Contents
In August 2009, the FASB issued Accounting Standards Update No. 2009-05 (“ASU 2009-05”), which
amends ASC 820 to provide guidance on fair value measurement of liabilities. ASU 2009-05 requires
the use of specific valuation techniques when measuring the fair value of liabilities and was
effective for the Company in the fourth quarter of 2009. The implementation of ASU 2009-05 did not
have a material impact on the Company’s financial condition or results of operations.
Note 14. Recently Issued Accounting Pronouncements
In June 2009, the FASB issued and established its Accounting Standards Codification as the
exclusive authoritative reference for nongovernmental U.S. GAAP for use in financial statements
issued for interim and annual periods ending after September 15, 2009, except for SEC rules and
interpretative releases, which are also authoritative for SEC registrants. Accordingly, the
Company adopted the ASC effective for its third quarter 2009 financial reporting. All references
to U.S. GAAP principles in this report have been changed to reference the relevant topic from the
FASB ASC. The adoption did not have a material impact on the Company’s consolidated financial
statements.
In May 2009, the FASB issued standards for accounting and reporting of subsequent events, included
in FASB ASC 855, Subsequent Events (“ASC 855”). ASC 855 sets forth general standards of accounting
for and disclosure of events that occur after the balance sheet date but before financial
statements are issued or are available to be issued, including:
| 1. | The period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements. |
| 2. | The circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements. |
| 3. | The disclosures that an entity should make about events or transactions that occurred after the balance sheet date. |
These standards for accounting and reporting of subsequent events were effective for interim or
annual periods ending after June 15, 2009, and were adopted by the Company as of that date. See
Note 16 below for information regarding subsequent events identified.
In December 2007, the FASB issued standards (included in FASB ASC 805, Business Combinations) which
established principles and requirements for how an acquirer recognizes and measures in its
financial statements the identifiable assets acquired, the liabilities assumed, and any
non-controlling interest in the acquired company and the goodwill acquired. These standards made
certain changes to then-existing accounting practices for business combinations. Among the changes
made were: transaction-related costs will be expensed; restructuring costs that the acquirer
expects but is not obligated (as of the acquisition date) to incur will not be included in the
measurement of the acquisition cost; and research and development assets will be capitalized. The
new standards also established disclosure requirements to enable the evaluation of the nature and
financial effects of the business combination. These new business combination accounting standards
were effective as of the beginning of an entity’s fiscal year that begins after December 15, 2008,
were adopted by the Company effective January 1, 2009, and did not have a material impact on the
Company’s financial condition or results of operations.
15. Litigation
Abbey Litigation
In January 2006, following execution of a Merger Agreement between the Company and 3F Therapeutics,
Inc. (“3F”), 3F was informed of a summons and complaint which was filed in the U.S. District Court
in the Southern District of New York by Arthur N. Abbey (“Abbey”) against 3F Partners Limited
Partnership II (a major stockholder of 3F, “3F Partners II”), Theodore C. Skokos (the then chairman
of the board and a stockholder of 3F), 3F Management II, LLC (the general partner of 3F Partners
II), and 3F (collectively, the “Defendants”) (the “Abbey I Litigation”). The summons and complaint
alleges that the Defendants committed fraud under federal securities laws, common law fraud and
negligent misrepresentation in connection with the purchase by Abbey of certain securities of 3F
Partners II. In particular, Abbey claims that the Defendants induced Abbey to invest $4 million in
3F Partners II, which, in turn, invested $6
F -26
Table of Contents
million in certain preferred stock of 3F, by allegedly causing Abbey to believe, among other
things, that such investment would be short-term. Pursuant to the complaint, Abbey is seeking
rescission of his purchase of his limited partnership interest in 3F Partners II and return of the
amount paid therefor (together with pre-and post-judgment interest), compensatory damages for the
alleged lost principal of his investment (together with interest thereon and additional general,
consequential and incidental damages), general damages for all alleged injuries resulting from the
alleged fraud in an amount to be determined at trial and such other legal and equitable relief as
the court may deem just and proper. Abbey did not purchase any securities directly from 3F and is
not a stockholder of 3F. In March 2006, 3F filed a motion to dismiss the complaint. In August
2007, the Court granted 3F’s motion to dismiss the complaint based on plaintiff’s failure to state
a claim upon which relief may be granted and ordered the Clerk of the Court to close the case.
Abbey filed a Notice of Appeal with the United States Court of Appeals for the Second Circuit
seeking to reverse the District Court’s August 2007 Order dismissing the case. In December 2008,
the Second Circuit issued a Summary Order that affirmed the District Court’s judgment of dismissal
finding that Abbey failed to state a claim against 3F. However, the Second Circuit remanded the
case to the District Court to allow Abbey a chance to replead his claims. On or about February 13,
2009, Abbey filed an amended complaint which purports to allege additional facts to support the
same claims against 3F that were asserted and dismissed in the original complaint. On or about
March 31, 2009, 3F served and filed its motion to dismiss the amended complaint with prejudice.
3F’s motion to dismiss was fully submitted on June 8, 2009. By Order entered December 2, 2009, the
District Court denied 3F’s motion to dismiss on the ground of lack of standing and converted the
remainder of 3F’s motion to dismiss for failure to state a claim into a motion for summary
judgment. Accordingly, the Court ordered “limited discovery” to be completed by February 26, 2010
and additional briefings on summary judgment to be completed by April 9, 2010. By Stipulation and
Order, this schedule has been modified and discovery is to be completed by April 2, 2010 and
additional briefings on summary judgment are to be fully submitted by May 17, 2010.
In June 2006, Abbey commenced a second civil action in the Court of Chancery in the State of
Delaware by serving 3F with a complaint naming both 3F and Mr. Skokos as defendants (the “Abbey II
Litigation”). The complaint alleges, among other things, fraud and breach of fiduciary duties in
connection with the purchase by Abbey of his partnership interest in 3F Partners II. The Delaware
action seeks: (1) a declaration that (a) for purposes of the merger, Abbey was a record stockholder
of 3F and was thus entitled to withhold his consent to the merger and seek appraisal rights after
the merger was consummated and (b) the irrevocable stockholder consent submitted by 3F Partners II
to approve the merger be voided as unenforceable; and (2) damages based upon allegations that 3F
aided and abetted Mr. Skokos in breaching Mr. Skokos’s fiduciary duties of loyalty and faith to
Abbey. On July 17, 2006, 3F filed a motion to dismiss the complaint in the Abbey II Litigation,
or, alternatively, to stay the action pending adjudication of the Abbey I Litigation. In October
2006, the Delaware Chancery Court entered an order staying the Delaware action pending the outcome
of the Abbey I litigation. In August 2007, the parties informed the Delaware Chancery Court that
they would consent to the continued stay of the Delaware action pending the outcome of Abbey’s
appeal of the Abbey I Litigation. The stay remains in effect pending the outcome of 3F’s motion to
dismiss the amended complaint.
3F has been notified by its director and officer insurance carrier that such carrier will defend
and cover all defense costs as to 3F and Mr. Skokos in the Abbey I Litigation and Abbey II
Litigation, subject to policy terms and full reservation of rights. In addition, under the Merger
Agreement, 3F and the 3F stockholder representative have agreed that the Abbey I Litigation and
Abbey II Litigation are matters for which express indemnification is provided. As a result,
certain escrow shares and contingent shares, if any, which may in the future be issued under the
Merger Agreement, may be used by ATS to satisfy, in part, ATS’s set-off rights and indemnification
claims for damages and losses incurred by 3F or ATS, and their directors, officers and affiliates,
that are not otherwise covered by applicable insurance arising from the Abbey I Litigation and
Abbey II Litigation. See Note 5 above for a description of the contingent shares. The Company
believes the Abbey I Litigation and Abbey II Litigation will not have a material impact on the
Company’s financial position or operating results.
CarboMedics Litigation Settlement
In November 2006, CarboMedics filed a complaint against the Company in the U.S. District Court in
the District of Minnesota. The complaint alleged that the Company breached certain contractual
obligations, including an alleged obligation to purchase $22 million of mechanical heart valve
carbon components under a long-term supply agreement with CarboMedics.
F -27
Table of Contents
CarboMedics initially sought specific performance and claimed damages of more than $20 million. In
February 2007, the Company filed its answer and counterclaims to the complaint. CarboMedics
subsequently withdrew its request for specific performance and revised its damages estimate to
$12.5 million before accounting for attorney fees and costs.
In May 2008, the court entered a formal stay to permit the parties to pursue alternative dispute
resolution. The parties were unable to reach an agreement and the stay was lifted on September 17,
2008. On the same date, the court denied both parties’ dispositive motions.
In December 2008, the parties executed a settlement agreement. CarboMedics agreed to release all
claims that were or could have been asserted in the case. In exchange, the Company agreed to pay
CarboMedics $7.5 million and to release its claims. The Company paid $3.0 million in December 2008
and paid $4.5 million in April 2009. Satisfaction of the settlement terms concludes all related
matters with CarboMedics and precludes any future litigation on the matter.
16. Subsequent Event — Debt Refinancing
On February 25, 2010, the Company received a commitment letter for four-year (interest only first
year) term debt financing of approximately $30 million from one of its directors, Theodore C.
Skokos, and The Ted and Shannon Skokos Foundation. This financing will be used to call and retire
the Company’s Convertible Senior Notes and bank Term Loan together totaling approximately $26
million as well as to provide general corporate working capital. The Company expects to close the
financing during the second quarter of 2010. When the Notes are retired the Company expects to
recognize a non-cash debt restructuring charge of approximately $4.5 to $5.0 million related
primarily to the unamortized discount on the Notes.
F -28
Table of Contents
EXHIBIT INDEX
| Exhibit | ||
| Number | Description | |
12.1
|
Computation of Ratio of Earnings to Fixed Charges. | |
21
|
List of Subsidiaries. | |
23
|
Consent of Grant Thornton LLP. | |
31.1
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. | |
31.2
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. | |
32.1
|
Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
32.2
|
Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
