Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT 31.1 - Winston Pharmaceuticals, Inc. | c97200exv31w1.htm |
| EX-32.1 - EXHIBIT 32.1 - Winston Pharmaceuticals, Inc. | c97200exv32w1.htm |
| EX-31.2 - EXHIBIT 31.2 - Winston Pharmaceuticals, Inc. | c97200exv31w2.htm |
| EX-21.1 - EXHIBIT 21.1 - Winston Pharmaceuticals, Inc. | c97200exv21w1.htm |
| EX-32.2 - EXHIBIT 32.2 - Winston Pharmaceuticals, Inc. | c97200exv32w2.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
| o | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 000-51314
Winston Pharmaceuticals, Inc.
(Name of registrant as specified in its charter)
| Delaware | 30-0132755 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 100 North Fairway Drive, Suite 134 Vernon Hills, IL | 60061 | |
| (Address of principal executive offices) | (Zip Code) |
Issuer’s telephone number (847) 362-8200
Securities registered under Section 12(b) of the Exchange Act: None
Securities registered pursuant to Section 12(g) of the Act:
Title of each class:
Common Stock, $.001 par value per share
Common Stock, $.001 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined
in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate whether the registrant (1) filed all reports required to be filed by
Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such
shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and
posted on its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter)
during the preceding 12 months (or for such shorter period that the registrant was
required to submit and post such files) (the Registrant is not yet required to submit
Interactive Data). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained in this herein, and will not be contained, to the best of
registrant’s knowledge, in definitive proxy or information statements incorporated by
reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See the
definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting
company in Rule 12b-2 of the Exchange Act.
| o Large Accelerated Filer | o Accelerated Filer | o Non-accelerated Filer | þ Smaller reporting company |
Indicate by check mark whether the registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act). Yes o No þ
As of March 1, 2010, 77,408,893 shares of the registrant’s Common Stock were
outstanding. As of June 30, 2009, the aggregate market value of the registrant’s Common
Stock held by non-affiliates of the registrant (without admitting that such person whose
shares are not included in such calculation is an affiliate) was $12,326,041 based on the
last sale price as reported on the over-the-counter bulletin board on such date.
Document incorporated by reference: None
WINSTON PHARMACEUTICALS, INC.
FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2009
FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2009
INDEX
| Page | ||||||||
| 1 | ||||||||
| 16 | ||||||||
| 16 | ||||||||
| 16 | ||||||||
| 16 | ||||||||
| 16 | ||||||||
| 17 | ||||||||
| 18 | ||||||||
| 18 | ||||||||
| 28 | ||||||||
| 28 | ||||||||
| 28 | ||||||||
| 28 | ||||||||
| 29 | ||||||||
| 30 | ||||||||
| 33 | ||||||||
| 37 | ||||||||
| 40 | ||||||||
| 42 | ||||||||
| 43 | ||||||||
| F-1 | ||||||||
| Exhibit 21.1 | ||||||||
| Exhibit 31.1 | ||||||||
| Exhibit 31.2 | ||||||||
| Exhibit 32.1 | ||||||||
| Exhibit 32.2 | ||||||||
Table of Contents
PART I
Item 1. Business
Cautionary Note Regarding Forward Looking Statements
This Annual Report on Form 10-K contains certain statements that are
“forward-looking” within the meaning of Section 27A of the Securities Act of 1933, as
amended, Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), and the Private Securities Litigation Reform Act of 1995, including statements
regarding our expected financial position and business and financing plans. These forward
looking statements and other information are based on our beliefs as well as assumptions
made by us using information currently available.
The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “will,” “should”
and similar expressions, as they relate to us, are intended to identify forward-looking
statements. Such statements reflect our current views with respect to future events and
are subject to certain risks, uncertainties and assumptions. Should one or more of these
risks or uncertainties materialize, or should underlying assumptions prove incorrect,
actual results may vary materially from those described herein as anticipated, believed,
estimated, expected, intended or using other similar expressions.
We are making investors aware that such forward-looking statements, because they
relate to future events, are by their very nature subject to many important factors that
could cause actual results to differ materially from those contemplated by the
forward-looking statements contained in this Report on Form 10-K. For example, we may
encounter competitive, technological, pharmacological, financial and business challenges
making it more difficult than expected to continue to develop and market our products;
the market may not accept our existing and future products; we may not be able to retain
our customers; we may be unable to retain existing key management personnel; and there
may be other material adverse changes in our operations or business. In addition,
assumptions relating to budgeting, marketing, product development and other management
decisions are subjective in many respects and thus susceptible to interpretations and
periodic revisions based on actual experience and business developments, the impact of
which may cause us to alter our marketing, expenditure or other budgets, which may in
turn affect our financial position and results of operations. For all of these reasons,
the reader is cautioned not to place undue reliance on forward-looking statements
contained herein, which speak only as of the date hereof. We assume no responsibility to
update any forward-looking statements as a result of new information, future events, or
otherwise, except as required by law.
Going Concern Audit Opinion
As
described herein under Item 7. Management’s Discussion and
Analysis of Financial Condition and Results of Operations and the Financial Statements filed as part of this Form 10-K, the Report of
Independent Registered Public Accounting Firm contains an emphasis of matter paragraph
which indicates that there is substantial doubt about the Company’s ability to continue
as a going concern. As described herein under Item 7. Management’s Discussion and
Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources, the Company plans to
raise additional funds early in the second quarter of 2010 in order to support its
operations through December 31, 2010.
Executive Overview
On September 25, 2008, Getting Ready Corporation (the “Company”) completed its
merger transaction (the “Merger”) with Winston Laboratories, Inc., a Delaware corporation
(“Winston Labs”), by merging the Company’s wholly-owned subsidiary into Winston Labs. The
Company is carrying on the business of Winston Labs as its sole line of business and it
has retained all of Winston Labs’s management. The Merger was accounted for as a reverse
merger for accounting purposes with Winston as the accounting acquirer. Effective as of
November 17, 2008, the Company changed its name to Winston Pharmaceuticals, Inc. Unless
the context otherwise requires, all references to “we,” “us,” “our” or the “Company”
shall mean the Company and Winston Labs, collectively.
Our common stock is currently quoted on the OTC Bulletin Board, or OTCBB, sponsored
by the National Association of Securities Dealers, Inc. under the symbol “WPHM.” The
OTCBB is a network of security dealers who buy and sell stock. The dealers are connected
by a computer network that provides information on current “bids” and “asks,” as well as
volume information.
Winston Labs was incorporated in July 1998 as a specialty pharmaceutical company and is
engaged in the discovery and development of products for pain management. Its core
proprietary technology affects the functioning of certain neurotransmitters that control
and mediate pain transmission. Based on this technology, We are developing products that
reduce pain transmission from peripheral receptors along nerve pathways to the brain. We
focus on major pain indications such as neuropathic pain syndromes, chronic daily
headache, migraine headache, and osteoarthritis, which have estimated worldwide market
sizes of $4 billion (Datamonitor, Commercial Insight: Neuropathic Pain, July 2006),
$5 billion (Analyst Research),
$4 billion (Analyst Research) and $10 billion (Analyst Research), respectively, as well
as niche indications that have limited or no products currently available.
1
Table of Contents
Pain Management Market
Pain is a significant, costly, and growing health care issue. In the United States,
76.5 million Americans (26% of adults age 20 or older) reported a problem with pain
lasting for more than 24 hours. The majority of adults in the United States (57% or 170
million) have experienced chronic or recurrent pain in the past year. The economic cost
of pain, including health care costs as well as lost productivity due to pain, is
estimated at $100 billion annually in the United States. Some $61 billion of this amount
is lost productivity attributable to pain, with an average loss of five hours per week
for workers experiencing reduced performance or work absences. Among pain conditions,
headache is the primary reason for lost productive time.
The market for pain management products is large. According to IMS Health data, the
total U.S. market for prescription pain management pharmaceuticals, including those for
arthritis or headache pain, exceeded $25 billion in 2008 (at a compound annual growth
rate of 4% between 2003 and 2008). Seven (7) of the top 50 prescription drugs ranked by
worldwide sales in 2008 had pain management indications.
Recent developments regarding serious side effects of certain non-steroidal
anti-inflammatory drugs (NSAIDs), coupled with the removal of several COX-2 inhibitors
from the market and a focus on the hepatoxicity of acetaminophen and acetaminophen/opiate
combinations, along with the FDA’s subsequent attention to this matter, have created
significant market opportunities for alternative pain therapy medications and has
generated a heightened consumer caution and wariness of current marketed products.
Similarly, potential for abuse of some existing prescription medications for
moderate-to-severe pain, such as opioid-based analgesics, establishes a need among
consumers for medications with less risk for addiction.
We believe that as recognition of the importance of pain diagnosis and treatment
becomes greater, and as the FDA continues to issue health advisories and tighten
restrictions on marketed opioids and NSAIDs including acetaminophen, the increasing need
for effective pain medications with fewer side effects and less dependency concerns will
create significant market opportunities for new products.
Several trends are also expected to drive growth and create opportunities for new
products for pain management:
Aging of the population
The populations in the United States, Europe, and Japan are aging rapidly. By 2025,
it is estimated that in the United States, one in four people will be 60 years of age or
older (versus one in six in 2000). In Europe and Japan, the aging of the population is
expected to be even more pronounced. As people age, they tend to be afflicted with more
chronic pain conditions and to undergo more surgical procedures which require
postoperative pain control.
Greater recognition of the importance of pain diagnosis and treatment
Medical and health care practitioners are increasingly aware of the importance of
diagnosing and treating pain. Whereas pain may have once been considered a natural or
inevitable consequence of aging or certain medical conditions, it is now considered a patient’s
“fifth vital sign,” which is essential to monitor and treat. The Joint
Commission on Accreditation of Healthcare Organizations has established standards that
require health care facilities to assess and treat pain.
Significant unmet medical needs in pain management
Almost 50% of those surveyed said their pain is not under control. More than 75% of
those surveyed strongly agreed that new options are needed to treat their pain. Even
among those patients who do receive treatment for pain, many do not achieve significant
relief. In fact, one survey found that respondents were so dissatisfied with the
efficacy of their prescription and over-the-counter (OTC) pain control medications that
78% were willing to try new treatments, and 43% said they would spend a substantial
portion of their financial resources on a treatment if they thought it would work.
2
Table of Contents
Shortcomings of Current Pain Management
Despite widespread clinical use of drugs for pain, pain management remains less than
optimal due to a variety of factors, including:
| • | Insufficient efficacy. Opioids, the current standard of care for severe pain,
reduce pain less than 50 percent in a majority of situations. Neuropathic pain
is difficult to treat with existing analgesics because of the differing types of
nerves and organs involved in, and types of injuries causing, this kind of pain.
Neuropathic pain does not respond to treatment with NSAIDs and responds poorly
to treatment with opioids at doses that do not impair the ability of patients to
live reasonably active lifestyles. |
| • | Lack of site specificity. Most analgesics, including opioids and NSAIDs, are
given orally or by intravenous infusion and thereby subject the patient to high
circulating concentrations of drug, even though most types of pain are
experienced in discrete parts of the body. Circulating drugs cause side effects
at parts of the body unrelated to the perception of pain. Although there are
currently means of delivering site-specific analgesia, such as by injection of
short-acting anesthetics into joints such as the ankle or knee, these techniques
are reserved to provide relatively short-term anesthesia prior to surgery and
are not appropriate for long-term pain relief. |
| • | Occurrence of side effects. NSAIDs can cause gastrointestinal ulcers, with
between 10,000 and 20,000 patients dying each year from gastrointestinal
bleeding believed to be related to the use of NSAIDs. Use of opioids is
associated with nausea and vomiting in many patients. High-dose opioids cause
sedation and may also cause respiratory depression, or a decrease in the ability
to breathe spontaneously. Opioids used chronically can cause severe constipation
that leads many patients to stop using them, and opioids may sometimes cause
severe itching. Drugs used to treat neuropathic pain frequently cause sedation
and problems with coordination. |
| • | Need for frequent dosing. Drugs used to treat neuropathic pain require
frequent dosing that makes their use inconvenient, often leading to reduced
patient compliance. |
| • | Potential to cause physical dependence. Opioids, when used chronically, can
cause physical dependence. Fear of physical dependence often influences
clinicians to prescribe less than adequate doses of opioid analgesics. Similar
fears lead many patients to refuse opioid analgesics. |
Given doctors’ and patients’ desire to achieve adequate control of pain, and the
significant shortcomings associated with existing treatments, doctors and patients often
struggle to find an appropriate balance between pain relief and adverse side effects.
With both over- and under-treatment of pain, patients may be suffering unnecessarily,
have poor quality of life and have difficulty meeting their social, familial and
work-related commitments.
3
Table of Contents
Winston Product Pipeline
We have developed multiple proprietary formulations and uses of Civamide and doxepin
hydrochloride, two agents that affect a variety of neuroactive chemical transmitters that
convey primary sensory input (e.g., pain) from the periphery to the central nervous
system. Our focus on neurotransmitter-active compounds has generated several of our most
important product candidates. A common feature of these product candidates is the use of
an active ingredient that depletes or interferes with the action of a neuroactive
transmitter (e.g., substance P (SP), calcitonin gene-related peptide (CGRP), histamine
and serotonin), thereby decreasing the ability of sensory fibers or nerve ganglia to
transmit or receive sensory stimuli and mediate inflammatory reactions. SP is believed to
be the principal neurotransmitter of pain.
Civamide is a proprietary TRPV-1 (transient receptor potential vanilloid-1) receptor
modulator, and doxepin hydrochloride is a potent histamine receptor blocker and serotonin
reuptake inhibitor. Civamide is being developed in several different dosage forms for a
variety of painful conditions. Doxepin is being developed in a nasal solution for
prophylaxis and treatment of chronic daily headache.
The following table summarizes the late stage pain control products in our
portfolio:
| MARKETING | ||||||||
| RIGHTS IN | ||||||||
| PRODUCT/ | PRODUCT | DEVELOPMENT | NORTH | |||||
| COMPOUND | DOSAGE FORM | INDICATION(S) | STATUS IN U.S. | AMERICA | ||||
Civamide
|
Patch | Postherpetic Neuralgia | Phase III | Winston | ||||
Civamide
|
Nasal Spray | Episodic Cluster Headache
PHN of Trigeminal Nerve |
Phase III Phase II |
Winston Winston |
||||
Doxepin
|
Nasal Spray | Chronic Daily Headache | Phase II | Winston | ||||
Civamide
|
Cream | Osteoarthritis | Phase III Completed NDA 2010 |
Winston Sanofi Aventis (Canada) |
||||
OTHER PRODUCTS |
||||||||
Civamide
|
Capsule | Crohn’s Disease | Phase II | Winston | ||||
Civamide
|
Nasal Spray | Keratoconjunctivitis Sicca | Phase II | Winston |
4
Table of Contents
Civamide
Civamide, a synthetically produced, proprietary new chemical entity, is the active
ingredient in several of our product candidates. The chemical structure is shown below:
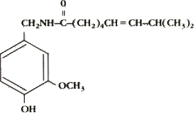
Civamide
(cis-8-methyl-N-vanilly1-6-nonenamide)
Civamide produces analgesia by decreasing the activity of sensory neurons, shown
below. Civamide appears to affect preferentially type-C neurons by specific binding to a
membrane receptor, the TRPV-1 receptor, which is coupled to a cation channel. The
specificity of Civamide for type-C neurons limits its sensory effects to the inhibition
of pain transmission unlike local anesthetics, which act to block the activity of all
sensory neurons, thereby impairing all sensations including touch, pressure, heat, and
vibration, in addition to pain signals.
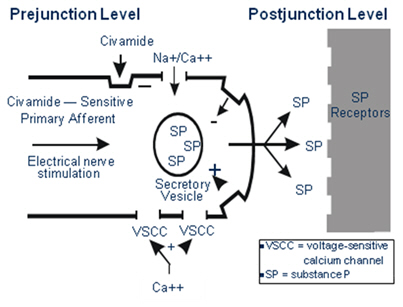
5
Table of Contents
Initial applications of Civamide cause a transient release of SP from type-C neurons
which may produce the transient burning sensation that is often felt following initial
applications of Civamide. Civamide has also been shown to inhibit the synthesis of SP and
reduce axonal transport of granules containing SP from the cell body to the nerve
terminals. These effects may likely contribute to the analgesic effects of Civamide by
reducing the amount of SP available for release. The analgesic effect of Civamide is
completely reversible, since after administration of Civamide is discontinued, SP stores
gradually return to normal and neuronal sensitivity to noxious stimuli returns. However,
in animal and human studies, Civamide has a very long duration of analgesic activity,
with a single dose or one-week course of treatment providing weeks of effective analgesic
activity.
We have extensive preclinical and clinical experience with Civamide, with over 1,600
patients having been treated with the drug in Phase I to Phase III clinical trials in
different formulations including the patch, nasal spray, oral capsule, and topical cream.
The most significant adverse event noted has been localized burning sensation at the
site of application. Because of these attributes, we believe Civamide represents a
clinically significant treatment advance for a number of painful conditions, including
neuropathic pain, cancer pain, headache pain, and arthritis pain.
Civamide Patch
Civamide Patch is an ultra-thin patch containing Civamide in a concentration of
0.015% which is being developed for postherpetic neuralgia (“PHN”) of the trunk. PHN is a
condition that affects the nerve fibers in the skin. It is the most common complication
of shingles and results from damage to nerve fibers during shingles. The rash and pain
from shingles usually lasts about a month. But in people with PHN, the pain from
shingles may last for months, and sometimes years, after the shingles rash has healed.
Capsaicinoids in a topical cream formulation have been shown to have efficacy in treating
PHN and other neuropathic pain syndromes in numerous studies. The development of
Civamide Patch for PHN would also offer a substantial improvement in the delivery of
medication to the cutaneous nerves involved in PHN.
Civamide Patch can be applied to the skin for up to 24 hours daily and the patient
can bathe or shower without removing it. As there is no measurable systemic absorption of
Civamide from the patch, we believe it is safer than its competitors, i.e. oral
medications including Cymbalta® and Lyrica® as well as the Lidoderm® Patch. Compared to
Lidoderm, the Civamide Patch will not have the potential of cardiovascular side effects
or drug interactions that Lidoderm® has. Civamide Patch is also a thinner, more flexible
patch and thus will have better adherence to the skin and will only require a single
patch to treat a dermatome affected by PHN, while Lidoderm requires three or four
patches.
6
Table of Contents
On February 9, 2009, we announced positive top line results from Study WL1001-04-01,
a Phase I clinical trial evaluating the tolerability and pharmacokinetic profile of two
concentrations of Civamide Patch 0.0075% and 0.015% vs. placebo. The study successfully
demonstrated the tolerability of the two concentrations tested for continuous application
to the skin for a period of one week. Additionally, there was no systemic absorption of
Civamide detected in any patients, supporting its local use for the topical treatment of
postherpetic neuralgia. In this 38 patient study, a patch was applied once daily to the
same area of the abdomen, and worn for 24 hours before replacing with a new patch, during
a 7 day study. Both strengths of Civamide Patch
were tolerable, with all subjects completing the study and with all but one subject being
able to wear the patch for the entire 7 day period. Subjects applying active Civamide
Patches experienced transient burning sensations which progressively lessened or resolved
with each application during the study. Based upon the favorable results of this study,
we conducted Study WL1001-04-03, a Phase II study of the 0.015% Civamide Patch for
treatment of postherpetic neuralgia.
On February 24, 2009, we received orphan drug designation from the FDA for Civamide
Patch for the treatment of postherpetic neuralgia. An orphan designation grants special
status to a product to treat a rare disease or condition upon request of a sponsor. This
is a key designation as the marketing application for a prescription drug product that
has been designated as a drug for a rare disease or condition is not subject to a
prescription drug user fee unless the application includes an indication for other than a
rare disease or condition. The product also receives seven year market exclusivity and
tax credits for the sponsor.
In September, 2009, we completed Study WL-1001-04-03, a Phase II clinical trial
evaluating the efficacy and safety of Civamide Patch 0.015% in the treatment of PHN.
Twenty-two patients with at least a 6 month history of PHN already on stable doses of
medication for PHN, but with recalcitrant pain of at least a 4 on a 0-10 categorical
scale during the week prior to enrollment, were enrolled in the study. Civamide Patch
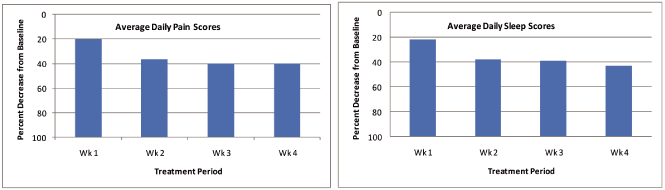
0.015% was applied over a 4 week Treatment Period, for up to 24 hours daily. Daily Pain
and Sleep Scores were assessed as the primary efficacy variables. There was a 2 week
Post-Observation Treatment Period during which no patch was applied, but patients
continued to record efficacy and safety parameters. Results demonstrated that by Week 2
and continuing throughout the Treatment Period and Post Treatment Observation Period,
there was approximately a 40% improvement from Baseline in both Daily Pain Scores and
Sleep Scores, seen below.
Civamide Patch Phase II
Study WL-1001-04-03
Adverse events were primarily transient self-limited application site burning
sensations. There were no systemic adverse events. The lack of systemic absorption should
permit the use of the Civamide Patch as both monotherapy and adjunctively with systemic
medications such as Cymbalta® (duloxetine) and Lyrica® (pregabalin)
without the risk of drug-drug interactions.
Civamide Nasal Spray
Civamide Nasal Spray contains Civamide in a concentration of 0.01% and is being
developed for the prophylaxis and treatment of Episodic Cluster Headache (“ECH”). Cluster
headache, nicknamed “suicide headache”, is a neurological disease that involves, as its
most prominent feature, an immense degree of pain. “Cluster” refers to the tendency of
these headaches to occur periodically, with active periods interrupted by spontaneous
remissions. The cause of the disease is currently unknown. There are currently no
approved products for this indication except injectable sumatriptan, which is approved
for the treatment of individual cluster headaches, but not for the prevention of others
occurring within the cluster period. There is no systemic absorption of Civamide from
this formulation making Civamide Nasal Spray safer to use than other medications which
might be used off label.
7
Table of Contents

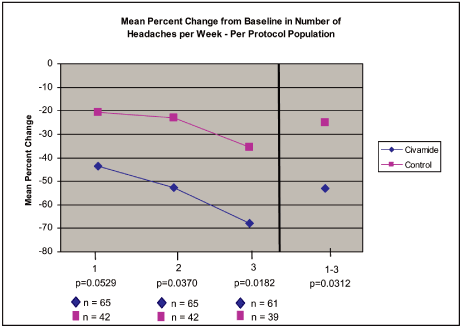
We have completed a Phase II as well two Phase III clinical trials. A meta-analysis
of the data was performed. After a single week of treatment with Civamide Nasal Spray
there was a decrease of approximately 70% vs. 35% in the control group (by the third week
post-treatment). The per protocol results are shown below.
Civamide Nasal Spray
Phase II and Phase III Meta-analysis
Episodic Cluster Headache
The FDA approved a Special Protocol Assessment (an “SPA”) for a Phase III pivotal
study of Civamide Nasal Spray for prophylaxis and treatment of episodic cluster
headache. The SPA is a process that provides for an official FDA evaluation of Phase III
clinical study protocols. The SPA provides trial sponsors with a binding written
agreement that the design and analysis of the studies are adequate to support a license
application submission if the study is performed according to the SPA and the study is
successful. The FDA has agreed that if this pivotal study is robustly positive, it would
be the final study necessary to support approval of Civamide Nasal Spray 0.01% for
this indication. Civamide Nasal Spray has been studied in several additional
indications, and there is positive Phase II data for acute migraine treatment and for
treatment of vasomotor rhinitis, a nonallergic condition that involves a constant runny
nose, sneezing, and nasal congestion. Civamide Nasal Spray has received orphan
designation from FDA for treatment of PHN of the trigeminal nerve.
8
Table of Contents
Civamide Cream
Civamide Cream is a cream formulation containing Civamide in a concentration of
0.075% being developed for osteoarthritis, the most common form of arthritis, which
occurs when cartilage in the joints wears down over time. The lack of systemic absorption
of Civamide from this formulation makes it safer to use than any oral COX-2 or NSAID.
i.e., without the risk of cardiovascular and gastrointestinal side effects, or the risks
of drug-drug interactions. Additionally, Civamide Cream 0.075% would also be without the
safety concerns of topical NSAIDs, e.g. Volteren® Gel, which because its use results in
diclofenac absorption, has similar safety concerns to that of the oral products.
We believe that we have completed all studies required for a new drug application
(an “NDA”) submission to the Food and Drug Administration (“FDA”) on Civamide Cream for
osteoarthritis. In a pivotal 695 patient Phase III trial in osteoarthritis, Civamide
Cream demonstrated statistically significant reductions in all three co-primary efficacy
parameters and in important secondary parameters.
Table 1: TWA and Day 84 P-Values for Co-Primary Efficacy Variables in Favor of
CIVANEX Over Control Study WL-1001-05-01 (ITT Population)
| Primary Efficacy Variable | TWA | Day 84 | ||||||
WOMAC® Pain Subscale |
0.0089 | 0.0126 | ||||||
WOMAC® Physical Function Subscale |
<0.0001 | 0.0005 | ||||||
Subject Global Evaluation |
0.0080 | 0.0485 | ||||||
| * | For the WOMAC® Pain and WOMAC® Physical Function Subscales,
these p-values correspond to treatment differences for Baseline scores >10 and,
>39, respectively, as a result of investigating the significant Baseline-by-Treatment
interaction. For SGE these p-values correspond to treatment differences for Baseline
scores ≤1(TWA) and 0 (Day 84). |
The filing of an NDA 505(b)(2) in the United States had been delayed pending
approval of a User Fee Waiver of the approximately $1.4 million filing fee. The User Fee
Waiver was granted to the Company on February 12, 2010. Consequently, the Company
intends to file an NDA 505(b)(2) in the second quarter, 2010. The Marketing Authorization
Applications (MAA) in Europe for Civamide Cream filed January 2008 was not approved in
July 2009 and an appeal has been filed in the United Kingdom and Sweden. A new drug
submission (“NDS”) submitted in Canada in October 2008 was issued a Notice of
Noncompliance in October, 2009, which decision is currently being appealed by the
Company. We have licensed the marketing rights for Civamide Cream to Sanofi-Aventis in
Canada and continue discussions with several pharmaceutical companies regarding the
marketing rights in the United States, Europe and the Pacific Rim.
Doxepin
Doxepin Nasal Spray
We are also developing doxepin hydrochloride, a potent histamine receptor blocker
and serotonin reuptake inhibitor in a patented nasal solution formulation in a
concentration of 0.4%, for the prophylaxis and treatment of chronic daily headache. There
are approximately 12 million patients in the United States with chronic daily headaches,
for which there are no FDA-approved treatment options. This product appears to interfere
with the release/actions of histamine and serotonin as well as modulating neuronal sodium
ion channels involved in the pathogenesis of this disorder.
Doxepin Nasal Spray would have advantages over oral tricyclics that may be used
off-label for this indication. Unlike systemically administered doxepin, there are
virtually no sedative or anti-cholinergic side affects which accompany nasal
administration of doxepin nasal spray.
9
Table of Contents
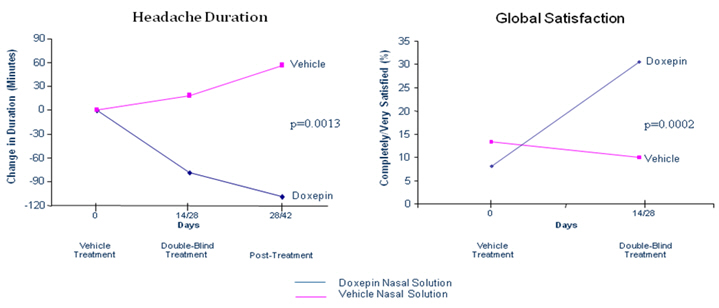
A successful Phase IIa trial of Doxepin Nasal Spray in chronic daily headache has
been completed. There were 210 patients enrolled in a 28-day lead-in Vehicle Treatment
Period to eliminate placebo responders. Of these patients, 92 continued in either a 14-
or 28-day Double-blind Treatment Period. There was statistical significant improvement
seen in decreases in headache duration and increases in global satisfaction, seen below.
Our next step in the clinical development of Doxepin Nasal Spray is to conduct a
Phase II/III study of longer duration.
Other Products
Civamide Capsule
Civamide Capsules are being developed for the treatment of Crohn’s disease. Crohn’s
disease is an inflammatory disease of the intestines that may affect any part of the
gastrointestinal tract from anus to mouth, causing a wide variety of symptoms. It
primarily causes abdominal pain, diarrhea (which may be bloody), vomiting, or weight
loss, but may also cause complications outside of the gastrointestinal tract such as skin
rashes, arthritis and inflammation of the eye. If effective for this indication, it would
represent a breakthrough therapy as it would be without the systemic side effects of
systemic medications currently used, including systemic steroids, immunosuppressives, and
biologics.
An investigational new drug application (“IND”) was filed in the United States in
the second quarter of 2008 for the Civamide soft-gel oral capsule. One Phase I
pharmacokinetic study has been completed which demonstrated that a single dose of the
enteric-coated capsule 5mg or 10mg, either in a fasting or fed state was safe and did not
provide measurable blood levels of Civamide. Since recent data have strongly suggested
that pain and inflammation in Crohn’s disease may be related to an accumulation of
substance P in nerve fibers in all layers of the intestinal wall, we are planning to
initiate a Phase II double-blind evaluation of several dosages of our Civamide Capsule
for relief of pain and inflammation in Crohn’s disease.
10
Table of Contents
Civamide Nasal Spray
Civamide Nasal Spray in a concentration of 0.01%, is also being developed for the
treatment of keratoconjuctivitis sicca, also known as dry eye syndrome, in addition to
its development for the prophylaxis and treatment of Episodic Cluster Headache described
above. The product was licensed to Opko Health in September 2007. On February 24, 2010,
the Company received communications from Opko that it was terminating the license
agreement effective May 24, 2010. As a result, all of Opko’s rights to certain products,
as defined in the agreement, will revert back to the Company. The only product approved
for this indication in the U.S. is Restasis® which had sales of over $400 million in
2008. Our product has several important advantages over Restasis, and if successfully
developed and approved could become the U.S. market leader for this condition.
Manufacturing and Raw Materials
All of our manufacturing is performed under contract by outside vendors. Currently,
our primary suppliers of contract manufacturing services are DPT Laboratories, Ltd.,
Patheon, Inc., Catalant Pharma Solutions, and Aveva Drug Delivery Systems. All of our
current products may be manufactured only at a site approved by the FDA specifically for
the individual product, as specified in the NDA for the product.
We believe that adequate and reliable sources of raw materials for our products are
currently available. In particular, Civamide is manufactured for us by Sigma-Aldrich Fine
Chemicals. Since some of the raw materials and finished drug products are purchased from
only one supplier, Winston’s operations may be adversely affected if certain suppliers
are unable to provide raw materials or to manufacture drug substance or finished products
on its behalf.
Commercialization Strategy
We currently have no marketing sales or distribution capabilities. In order to
commercialize our product candidates, we must develop sales, marketing and distribution
capabilities or make arrangements with other parties to perform these services for us.
As part of our commercialization plan, we will conduct a variety of promotional and
educational programs aimed at establishing awareness of our products in the physician
community, and will focus on differentiating our products from other competitive
products. These programs are planned to include sales representative promotion,
publications in medical journals, continuing medical education, symposia, regional
speaker programs and medical conference exhibits.
Our current plans call for us to market only those products of ours and those we
might obtain by acquisition or license, that are for pain control and which can be
successfully served with a focused specialty sales force. Consequently, we plan to build
our own U.S. sales force to market our Civamide Patch for PHN, our Civamide Nasal Spray
for ECH and our Doxepin Nasal Spray for chronic daily headache. This sales force will
call on the approximately 5,000 pain centers and 10,000 neurologists and other
specialists who specialize in treating chronic pain in the U.S.
For those products of ours which address large generalist medical markets, as our
Civamide Cream for osteoarthritis, or large markets unrelated to pain control as our
product for Crohn’s disease, we intend to seek U.S. marketing partners to handle the
primary commercialization of these products.
Outside of the United States, and subject to marketing approval in the relevant
countries, we intend to engage sales, marketing and distribution partners in Europe, Asia
and Latin America.
We attempt, to the extent practicable, to use patent protection in the
commercialization of our owned and licensed technology. We actively seek patent
protection for our proprietary technology in the United States and, as appropriate and
cost-effective, in other countries. We currently have five issued U.S. patents and 25
issued foreign patents and several pending foreign patent applications. These patents and
patent applications are principally composition of matter and method of use claims. The
range of expiration dates of our issued U.S. patents is from March 25, 2010 to October
16, 2023 with the patents most material to our business expiring January 28, 2019 and
October 16, 2023. Upon regulatory approval of any of our patent-protected products, we
plan to seek patent extension, where available, under existing U.S. and foreign
regulations permitting extension of a pharmaceutical patent. In the United States, the
Hatch-Waxman Act provides for patent term extension calculated as a portion of time
during which the drug was in clinical development or under review at the FDA with a
maximum extension of five years. Additionally, under the Hatch-Waxman Act, all new
molecular entities (“NMEs”) such as Civamide automatically receive five (5) years data
exclusivity upon approval. We believe that we are eligible for the full five-year patent
term extension period on patents covering Civamide. Also, we expect to employ product
life-cycle management, including the development of new formulations, new indications,
and other significant product improvements, in order to maximize the value of our
intellectual property.
11
Table of Contents
Set forth below, please find a table of our current trademarks:
| Trademark Name/Reg. No. | Owner | Filed (US) | Registered | |||
CIVANEX 2,729,437 |
Winston Labs | February 16, 1999 | June 24, 2003 | |||
MICANOL 3,283,446 |
Winston Labs | March 27, 2003 | August 21, 2007 | |||
WINSTON LABORATORIES, The Pain Company 2,856,161 |
Winston Labs | October 13, 1999 | June 22, 2004 | |||
WINSTON LABORATORIES Illinois State Trademark 73000 |
Winston Labs | August 13, 1993 | ||||
AXSAIN 2,925,798 |
Winston Labs | November 14, 2002 | February 8, 2005 | |||
ANTHRODERM
|
Winston Labs | February 1, 2010 | ||||
RHEUMODERM
|
Winston Labs | February 1, 2010 | ||||
CIVADERM
|
Winston Labs | February 1, 2010 | ||||
NEURODERM
|
Winston Labs | February 1, 2010 | ||||
DOLORAC
|
Winston Labs | November 10, 2009 |
In addition to seeking the protection of patents, licenses and trademarks, we also
rely on unpatented proprietary technology and know-how to maintain its competitive
position. To protect its rights in these areas, we have has a policy of requiring its
employees, consultants, advisors, prospective licensees and business partners to enter
into confidentiality agreements.
Competition
In general, a company’s ability to compete successfully in the pharmaceutical
industry is based on: relative product performance (efficacy, safety, ease of use);
price; acceptance by physicians, patients, and third-party payers; marketing support and
distribution; availability of patent protection; and the ability to obtain and maintain
FDA approval for testing, manufacturing and marketing.
Our products and technologies face competition from various classes of drugs,
currently marketed and in development, across a range of therapeutic categories, from
headache to neuropathic pain. For some indications, such as prophylaxis of episodic
cluster headache, chronic daily headache and PHN of the trigeminal nerve, there are no
existing FDA-approved products, and the Company is not aware of any other drugs presently
in development for these conditions.
For other markets such as PHN, Crohn’s disease and osteoarthritis, our products
targeted delivery combined with their lack of systemic side effects or potential for drug
interactions, conveys substantial competitive advantage over competitors products for
these conditions.
12
Table of Contents
Our primary competitors include: small companies focused on pain management, such
as Adolor, NeurogesX, NicOx, Pain Therapeutics, and Pozen; mid-sized companies with
significant pain franchises, such as Endo Pharmaceuticals, King Pharmaceuticals, Purdue
Pharma, and Valeant Pharmaceuticals; and very large, diversified pharmaceutical companies
including GlaxoSmithKline, Merck, and Pfizer. A summary of what we believe to be the
competitive advantages of our lead products as compared to the principal competing
products currently in the market, if any, is presented below:
| Competitive Advantages Summary | ||||||
| Our Product | Indication | Competitor Product(s) | Our Competitive Advantages | |||
Civamide Patch
|
Postherpetic Neuralgia | Lyrica® Neurontin® Opiates Lidoderm® Patch |
No Systemic Side Effects or Drug Interactions Can be Used Adjunctively No Disposal Issues (as with Lidoderm) Can be Bathed or Showered with (in Contrast to Lidoderm) |
|||
Civamide Nasal Spray |
Episodic Cluster Headache |
None | No Approved Competitor | |||
| Trigeminal PHN | None | No Systemic Side Effects or Drug Interactions | ||||
Civamide Capsule |
Crohn’s Disease | Aminosalicylates (e.g. sulfasalazine, mesalamine); Oral corticosteroids; Biologic Therapies (e.g. adalimumab (Humira®), infliximab (Remicade®)) | Targeted Unique Action No Systemic Side Effects or Drug Interactions | |||
Civamide Cream |
Osteoarthritis | Oral NSAIDs (e.g. Mobic®, Naprosyn®) and COX2 Inhibitors (e.g. Celebrex®) Topical NSAIDs (e.g. Volteran®) | No Approved Competitor No Systemic Side Effects or Drug Interactions | |||
Doxepin Nasal Spray |
Chronic Daily Headache |
None | No Approved Competitor No Systemic Side Effects or Drug Interactions | |||
In the case of certain of our products, particularly those targeting large markets
such as osteoarthritis and Crohn’s disease, thus requiring a very large sales force, we
intend to pursue collaborations with larger pharmaceutical companies to market such
products most effectively.
Government Regulation
Government authorities in the United States, at the federal, state, and local level,
and other countries extensively regulate, among other things, the safety, efficacy,
research, development, testing, manufacture, storage, record-keeping, labeling,
promotion, advertising, distribution, marketing and export and import of pharmaceutical
products such as those Winston is developing.
13
Table of Contents
United States Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and
Cosmetic Act and implements regulations. If we fail to comply with the applicable United
States requirements at any time during the product development process, clinical
testing, and the approval process or after approval, we may become subject to
administrative or judicial sanctions. These sanctions could include the FDA’s refusal to
approve pending applications, license suspension or revocation, withdrawal of an
approval, warning letters, product recalls, product seizures, total or partial suspension
of production or distribution, injunctions, fines, civil penalties or criminal
prosecution. Any agency enforcement action could have a material adverse effect on the Company.
The Company’s products are considered by the FDA to be drugs. The drugs are subject to
FDA review and approval or clearance. If the FDA denies approval or clearance of the
drugs, Winston’s ability to market its products could be significantly delayed or
precluded.
The steps required before a drug may be marketed in the United States include:
| • | completion of preclinical laboratory tests, animal studies and formulation studies under the FDA’s
good laboratory practices regulations; |
||
| • | submission to the FDA of an Investigational New Drug, or IND, application for human clinical
testing, which must become effective before human clinical trials may begin; |
||
| • | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of
the product for each proposed indication; |
||
| • | submission to the FDA of an NDA; |
||
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which
the product is produced to assess compliance with current good manufacturing practice, or cGMP; and |
||
| • | FDA review and approval of the NDA before any commercial marketing, sale or shipment of the product. |
Preclinical tests include laboratory evaluations of product chemistry, toxicity, and
formulation, as well as animal studies. The results of the preclinical tests, together
with manufacturing information and analytical data, are submitted to the FDA as part of
an IND application. The FDA requires a 30-day waiting period after the filing of each IND
application before clinical tests may begin, in order to ensure that human research
subjects will not be exposed to unreasonable health risks. An IND will automatically
become effective 30 days after receipt by the FDA, unless before that time the FDA has
placed the IND on clinical hold. In that case, the IND sponsor and the FDA must resolve
any outstanding FDA concerns or questions before clinical trials can proceed. Submission
of an IND may result in the FDA not allowing clinical trials to commence or not allowing
the trial to commence on the terms originally specified in the IND.
Clinical trials involve the administration of the investigational product to human
subjects under the supervision of qualified investigators. Clinical trials are conducted
under protocols detailing, among other things, the objectives of the study, the
parameters to be used in monitoring safety and the effectiveness criteria to be
evaluated. Each protocol must be submitted to the FDA as part of the IND. Each trial must
be reviewed and approved by an independent Institutional Review Board, or IRB, before it
can begin and the trial is subject to IRB oversight. The FDA, the IRB or the Company may
discontinue a clinical trial at any time for various reasons, including a belief that the
subjects are being exposed to an unacceptable health risk. Clinical testing also must
satisfy extensive good clinical practice requirements and the requirements for informed
consent.
Clinical trials typically are conducted in three sequential phases, but the phases
may overlap or be combined. Phase I trials usually involve the initial introduction of
the investigational drug into humans to evaluate the product’s safety, dosage tolerance,
pharmacodynamics, and, if possible, to gain an early indication of its effectiveness.
Phase II trials usually involve controlled trials in a limited patient population
to: (a) evaluate dosage tolerance and appropriate dosage; (b) identify possible adverse
effects and safety risks; and (c) evaluate preliminary the efficacy of the drug for
specific indications.
Phase III trials usually further evaluate clinical efficacy and test further for
safety in an expanded patient population. Phase I, Phase II and Phase III testing may not
be completed successfully within any specified period, if at all. Furthermore, the FDA or
the Company may suspend or terminate clinical trials at any time on various grounds,
including a finding that the subjects or patients are being exposed to an unacceptable
health risk.
14
Table of Contents
Assuming successful completion of the required clinical testing, the results of the
preclinical studies and of the clinical studies, together with other detailed
information, including extensive manufacturing information and information on the
composition of the product, are submitted to the FDA in the form of an NDA requesting
approval to market the product for one or more specified indications. An NDA may also be
submitted in the format of an electronic Common Technical Document, or eCTD, which under
ICH guidelines, is acceptable to the FDA and many foreign regulatory authorities. The FDA
reviews an NDA or eCTD to determine, among other things, whether a product is safe and
effective for its intended use.
Before approving an application, the FDA will inspect the facility or the facilities
at which the product is manufactured, and will not approve the product unless cGMP
compliance is satisfactory. FDA will also inspect the clinical sites at which the trials
were conducted to assess their compliance, and will not approve the product unless
compliance with Good Clinical Practice requirements is satisfactory. If the FDA
determines the application demonstrates that the product is safe and effective for the
proposed indication and that the manufacturing process and the manufacturing facilities
are acceptable, the FDA will issue an approval letter. If the FDA determines the
application, manufacturing process or manufacturing facilities are not acceptable, the
FDA will outline the deficiencies in the submission and often will request additional
testing or information. Notwithstanding the submission of any requested additional
information, the FDA ultimately may decide that the application does not satisfy the
regulatory criteria for approval and may deny the application, limit the indication for
which the drug is approved or require additional post-approval testing or other
requirements.
The testing and approval process requires substantial time, effort, and financial
resources, and each may take several years to complete. The FDA may not grant approval on
a timely basis, or at all. We may encounter difficulties or unanticipated costs in
its efforts to secure necessary governmental approvals, which could delay or preclude it
from marketing its products. The FDA may limit the indications for use or place other
conditions on any approvals that could restrict the commercial application of the
products. After approval, certain changes to the approved product, such as adding new
indications, manufacturing changes, or additional labeling claims are subject to further
FDA review and approval.
If and when regulatory approval of a product is obtained, we will be required
to comply with a number of post-approval requirements. We also must comply with
other regulatory requirements, including cGMP regulations and adverse event reporting.
Holders of an approved NDA are required to report certain adverse reactions and
production problems, if any, to the FDA, to provide updated safety and efficacy
information and to comply with requirements concerning advertising and promotional
labeling for their products. Also, quality control and manufacturing procedures must
continue to conform to cGMP after approval. The FDA periodically inspects manufacturing
facilities to assess compliance with cGMP. Accordingly, manufacturers must continue to
expend time, money, and effort in the area of production and quality control to maintain
compliance with cGMP and other aspects of regulatory compliance.
We use, and will continue to use at least in the near term, third-party
manufacturers to produce its products in clinical and commercial quantities. Future FDA
inspections may identify compliance issues at its facilities or at the facilities of its
contract manufacturers that may disrupt production or distribution, or require
substantial resources to correct. In addition, discovery of problems with a product or
the failure to comply with requirements may result in restrictions on a product,
manufacturer, or holder of an approved NDA, including withdrawal or recall of the product
from the market or other voluntary or FDA-initiated action that could delay further
marketing. Also, new government requirements may be established that could delay or
prevent regulatory approval of our products under development.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of
foreign regulations governing clinical trials and commercial sales and distribution of
its products. Whether or not we obtain FDA approval for a product, it must obtain
approval of a product by the comparable regulatory authorities of foreign countries
before it can commence clinical trials or marketing of the product in those countries.
The approval process varies from country to country, and the time may be longer or
shorter than that required for FDA approval. The requirements governing the conduct of
clinical trials, product licensing, pricing and reimbursement vary greatly from country
to country.
Under European Union regulatory systems, marketing authorizations may be submitted
either under a centralized or mutual recognition procedure. The centralized procedure
provides for the grant of a single marketing authorization that is valid for all European
Union member states. The mutual recognition procedure provides for mutual recognition of
national approval decisions. Under this procedure, the holder of a national marketing
authorization may submit an application to the remaining member states. Within 90 days of
receiving the applications and assessment report, each member state must decide whether
to recognize approval.
In addition to regulations in Europe and the United States, we will be subject
to a variety of foreign regulations governing clinical trials and commercial distribution
of its products.
15
Table of Contents
Product Liability
We maintain product liability insurance on our products and clinical trials that
provides coverage in the amount of $5 million per incident and $5 million in the annual
aggregate.
Executive Officers and Key Employees of the Registrant
Information concerning our executive officers and key employees, including their
names, ages and certain biographical information can be found in
Part III, Item 10 under
the caption, “Directors, Executive Officers and Corporate Governance”. This information
is incorporated by reference into Part I of this report.
Human Resources
As of March 1, 2010 we employed 9 persons, including 4 engaged in research and
development activities, including clinical development, and regulatory affairs, and 5 in
general and administrative functions such as human resources, finance, accounting,
business development and investor relations. Our staff includes 2 employees with Ph.D. or
M.D. degrees. Our employees are not represented by any collective bargaining unit. We
believe that we maintain good relations with our employees.
Financial Information about Segments
We operate in a single accounting segment — the development and commercialization of
novel treatments that target the central nervous system. Refer to Note 2, “Segment
Information” in the Notes to the Consolidated Financial Statements.
Item 1A. Risk Factors
We are a “smaller reporting company” as such term is defined in Item 10(f)(1) of
Regulation S-K and are exempt from making the disclosures required by this item pursuant
to the instructions to Item 1A of Form 10-K.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We maintain a 7,300 square foot leased office and warehouse facility in Vernon
Hills, Illinois (a suburb of Chicago), which serves as our headquarters.
Item 3. Legal Proceedings
As previously reported in the Company’s quarterly reports on Form 10-Q for the quarters
ended June 30, 2009 and September 30, 2009, the Company filed suit against the FDA in the United
States District Court for Northern District of Illinois for declaratory and injunctive relief
with respect to the FDA’s refusal to waive it’s application user fee under small business waiver
rules relating to the Company’s submission of the Civamide Cream NDA. In September, 2009, the
FDA filed a motion to dismiss the suit. In October 2009, the Company filed a response in
opposition to the FDA’s motion to dismiss and in December 2009, the court ruled in the Company’s
favor. Consequently, on February 12, 2010, the Company was granted the user fee waiver.
There are no other pending legal proceedings.
Item 4.
(Removed and Reserved)
16
Table of Contents
PART II
Item 5. Market for Registrant’s Common Equity, Related
Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is quoted on the Over-The-Counter Bulletin Board (the “OTCBB”)
under the symbol “WPHM.” The following table sets forth the high and low bid prices for
our common stock for each of the last two fiscal years, as reported on the OTCBB.
For the Year Ended December 31, 2009:
| High | Low | |||||||
First Quarter |
3.40 | 0.25 | ||||||
Second Quarter |
2.80 | 0.25 | ||||||
Third Quarter |
2.25 | 0.45 | ||||||
Fourth Quarter |
1.65 | 0.28 | ||||||
For the Year Ended December 31, 2008:
| High | Low | |||||||
First Quarter |
1.50 | 1.05 | ||||||
Second Quarter |
1.25 | 0.75 | ||||||
Third Quarter |
1.09 | 0.51 | ||||||
Fourth Quarter |
1.00 | 0.30 | ||||||
Holders
As of January 29, 2010, there were approximately 177 stockholders of record and 274
beneficial owners of our common stock. The last sale price as reported by the OTCBB on
March 1, 2010, was $0.20.
Dividends
We have never declared or paid dividends on our common stock and we do not
anticipate paying any cash dividends in the foreseeable future. We currently intend to
retain future earnings, if any, to fund the development and growth of our business.
Except for the rights of holders of shares of preferred stock that are authorized and
which may be issued in the future to receive dividends, any future determination to pay
dividends will be at the discretion of our Board of Directors and will be dependent upon
our financial condition, operating results, capital requirements, applicable contractual
restrictions and other such factors as our Board of Directors may deem relevant.
2009 Equity Compensation Plan Information
Information regarding securities authorized for issuance under our equity
compensation plans is disclosed in Part III Item 12 under the caption “Security Ownership
of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Recent Sales of Unregistered Securities
None.
17
Table of Contents
Performance Graph
We are a “smaller reporting company” as such term is defined in Item 10(f)(1) of
Regulation S-K and are exempt from making the disclosures required by this item pursuant
to instruction 6 to Item 201(e) of Regulation S-K.
Item 6. Selected Financial Data
We are a “smaller reporting company” as such term is defined in Item 10(f)(1) of
Regulation S-K and are exempt from making the disclosures required by this item pursuant
to paragraph (c) of Item 301 of Regulation S-K.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
THE FOLLOWING DISCUSSION SHOULD BE READ TOGETHER WITH THE INFORMATION CONTAINED IN
THE FINANCIAL STATEMENTS AND RELATED NOTES INCLUDED ELSEWHERE IN THIS ANNUAL REPORT ON
FORM 10-K.
On September 25, 2008, Getting Ready Corporation completed a merger transaction (the
“Merger”) with Winston Laboratories, Inc., a Delaware corporation (“Winston Labs”) by
merging a wholly-owned subsidiary of Getting Ready Corporation into Winston Labs.
Effective November 17, 2008, Getting Ready Corporation changed its name to Winston
Pharmaceuticals, Inc. (hereafter referred to as “we,” “us,” “our” or the “Company”). The
Company has carried on the business of Winston Labs as its sole line of business and it
has retained all of Winston’s management. The Merger was accounted for as a reverse
merger for accounting purposes with Winston Labs as the accounting acquirer.
This discussion contains forward-looking statements, within the meaning of
Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities
Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of
1995, including statements regarding our expected financial position and business and
financing plans. These statements involve risks and uncertainties. Our actual results
could differ materially from the results described in or implied by these forward-looking
statements as a result of various factors. See Item 1. Business —
Cautionary Note Regarding Forward-Looking Statements.
Executive Overview
Winston Labs is the successor to a research and development partnership, Cisco Ltd.
(“Cisco”), formed in 1992 to develop a specific novel neuroactive compound, civamide.
Civamide was discovered and patented by Joel E. Bernstein, M.D. (“Dr. Joel Bernstein”),
the managing general partner of Cisco and subsequently, the founder and Chief Executive
Officer, or CEO, of Winston Labs. In 1997, Cisco became Winston Laboratories LLC, which
in 1998 was converted to a “C” Corporation. Winston’s initial operating funds were
obtained from a rights offering in 1997 and a private equity placement in 1998. In 1999,
Winston Labs sold approximately 19% of its common stock to Bioglan Pharma Plc (“Bioglan”)
for $25 million; in 2004, Bioglan’s stock ownership was subsequently reduced below 7%
when it did not participate in Winston Labs offering of additional shares of its common
stock. In September 2007, Winston Labs purchased all of Bioglan’s outstanding ownership
in Winston Labs from Bioglan for $225,000. Subsequent to this transaction, Winston Labs
retired all of the shares purchased from Bioglan.
In 2000, Winston Labs established a subsidiary, Rodlen Laboratories, Inc. (“Rodlen,”
formerly Oncovir Corporation). In 2001, Bioglan purchased approximately 18% of Rodlen’s
outstanding common stock for $13.3 million. In 2002, Bioglan’s stock ownership in Rodlen
was reduced below 3% as a result of Bioglan’s election not to participate in a Rodlen
rights offering. The proceeds of this rights offering were used to acquire the Zostrix®
line of over-the-counter topical analgesics. Rodlen marketed the Zostrix® product line
until July 2005, when it sold the product line to Hi-Tech Pharmacal Co. (“Hi-Tech”). In
October 2004, Rodlen launched the marketing and sale of another topical analgesic,
Axsain®, which it promoted to physicians and other health-care professionals. In June 2005,
based on disappointing sales, Rodlen sharply reduced the promotion of the Axsain®
product and in March 2006, Rodlen discontinued selling this product altogether. In
February 2007, Rodlen licensed certain technology underlying the Axsain® product to
Hi-Tech. Rodlen operated as a “virtual company” with no employees of its own. From
inception to September, 2007, Rodlen was consolidated into the financial statements of
Winston Labs. In September 2007, Winston Labs purchased all of the then outstanding
ownership in Rodlen from Bioglan for $10,000. Subsequent to this transaction, all of the
shares purchased from Bioglan were retired. On September 21, 2007, Rodlen was merged into
Winston Labs.
18
Table of Contents
In 2005, Winston Labs established a wholly owned UK subsidiary, Winston Laboratories
Limited (“UK Ltd.”). UK Ltd. was established for the purpose of conducting work with
European drug regulatory authorities, who typically require a European
entity. UK Ltd. has no employees or material assets. The consolidated entity of
Winston Labs, Rodlen, and UK Ltd. is hereinafter referred to as “Winston Labs.”
Gideon Pharmaceuticals, Inc. (“Gideon”) is a corporation whose chairman and sole
director is Joel E. Bernstein, M.D., president and CEO of the Company. Gideon reimburses
the Company for certain expenses that the Company incurs on behalf of Gideon. Amounts
due from Gideon of $12,675 and $0 as of December 31, 2009 and 2008, respectively, are
included in related party receivable on the Consolidated Balance Sheets.
On January 30, 2006, Winston Labs licensed to Sirius Laboratories, Inc., a company
founded by Dr. Joel Bernstein, the rights to market products containing anthralin owned
by Winston Labs, including a marketed 1% anthralin cream trade name Psoriatec® . The
license had a two-year term which expired on January 31, 2008 and provided for the
following key terms: (i) a 25% royalty on net sales; (ii) a $300,000 minimum royalty; and
(iii) a $750,000 purchase option. This agreement was assigned by Sirius to DUSA
Pharmaceuticals, Inc. following DUSA’s purchase of Sirius. This license had been extended
until September 30, 2008 by mutual written consent of the parties and the extension
provided for continuation of the 25% royalty on net sales but eliminated the minimum
royalty and purchase option. This agreement expired on September 30, 2008. Under the
technology license agreement, the Company recorded royalty revenue of $0 and $34,861 for
the years ended December 31, 2009 and 2008, respectively. In July 2008, the Company
learned that DUSA had breached a license agreement with Winston Labs. Winston Labs
initiated arbitration in October 2008 to recover the damages caused by this breach. In
June 2009, DUSA settled this matter by agreeing to pay $75,000 in damages. This
settlement is included in other income in the Consolidated Statements of Operations.
On August 14, 2007, Winston Labs entered into an exclusive technology license
agreement with Exopharma, Inc., now known as Elorac, Inc. (“Elorac”). Dr. Joel Bernstein
is a majority owner and President and Chief Executive Officer of Elorac. Under the terms
of the license agreement, Winston Labs granted Elorac an exclusive license to the
proprietary rights of certain products (£ 0.025% civamide with the stated
indication of psoriasis of the skin). In exchange, Elorac paid Winston Labs a license fee
of $100,000 and is required to pay a 9% royalty on sales of the product. In addition, the
agreement required Elorac to pay Winston Labs a non-refundable payment of $250,000 upon
approval of a marketing authorization by Elorac on the product(s) described in the
agreement. On October 27, 2008 the Winston Labs and Elorac mutually terminated the above
license agreement. As a result of this mutual termination, the Winston Labs agreed to pay
Elorac the $105,000 in exchange for Winston Labs retaining all the proprietary rights
under the original agreement. Since inception, Elorac has been located in the same
offices as Winston Labs and therefore has shared in certain of Winston Labs expenses such
as rent, utilities, internet usage, etc. The amount of Elorac’s share of such expenses is
based on various allocation factors related to particular expense. Winston Labs received
$162,977 and $128,323 in 2009 and 2008, respectively for such services, which are
included as a reduction of Winston Labs expenses on the Consolidated Statement of
Operations. Amounts due from Elorac of $2,601 and $0 as of December 31, 2009 and 2008,
respectively, are included in related party receivables in the Company’s Consolidated
Balance Sheets.
On September 19, 2007, Winston Labs entered into an exclusive technology license
agreement with Opko Ophthalmologics, LLC, (“OPKO”). Under the terms of the license
agreement, Winston Labs granted OPKO an exclusive license to the proprietary rights of
certain products (pharmaceutical compositions or preparations containing the active
ingredient civamide in formulations suitable for use in the therapeutic or preventative
treatment of ophthalmic conditions in humans). In exchange, OPKO paid Winston Labs a
license fee of $100,000 and is required to pay a 10% royalty on sales of the products. In
addition, the agreement requires OPKO to pay Winston Labs a non-refundable payment of
$5,000,000 upon approval of a marketing authorization by OPKO on the product described in
the agreement. In addition, under the terms of the agreement, OPKO and Winston Labs
agreed to equally share the cost related to manufacturing and clinical supplies of
Civamide Nasal Spray. During 2009 and 2008, OPKO reimbursed Winston Labs approximately
$111,539 and $80,000, respectively for such costs. In addition, the agreement calls for
OPKO to reimburse Winston Labs for certain legal expenses Winston Labs has incurred
related to the use of the licensed products to treat keratoconjunctivitis. In 2009 and
2008, approximately $38,800 and $38,000, respectively of legal fees were billed to OPKO,
of which approximately $7,000 and $38,000 were outstanding as of December 31, 2009 and
2008 and are included in related party receivable on the Consolidated Balance Sheets.
Subsequent to December 31, 2009 and 2008, respectively, OPKO paid $7,000 and $38,000 of
the balance due. Phillip Frost, M.D. is the Chairman and Chief Executive Officer of
OPKO’s parent company, Opko Health, Inc. (“Opko Health”), and as of December 31, 2009 was
the beneficial owner of more than 50% of Opko Health’s common stock.
On February 24, 2010, the Company received communications from Opko that it was
terminating the license agreement effective May 24, 2010. As a result, all of Opko’s
rights to certain products, as defined in the agreement, will revert back to the Company.
As of December 31, 2009, Dr. Frost is also the indirect beneficial owner of
26,574,659 shares of the Company’s capital stock on a fully diluted basis (29.7% of the
total issued and outstanding shares), including, 8,779,797 shares of common stock
underlying warrants. Furthermore, Subbarao Uppaluri, Ph.D., a director of the Company and
the Senior Vice President — Chief Financial Officer of Opko Health, is the beneficial owner of 326,538 shares of the
Company’s capital stock on a fully-diluted basis, including 27,500 shares of common stock
underlying options and 89,589 shares of common stock underlying warrants.
19
Table of Contents
On November 13, 2007, the Company entered into a merger agreement with Winston Labs
and Winston Acquisition Corp., the Company’s wholly-owned subsidiary, pursuant to which,
upon completion of the Merger on September 25, 2008, Winston Labs became our wholly-owned
subsidiary and:
| • | all of the issued and outstanding capital stock of Winston Labs,
consisting of 23,937,358 shares of common stock, par value $0.001 per
share, 5,815,851 shares of the Winston Labs Series A Convertible
Preferred Stock, par value $0.001 per share (“Winston Labs Series A
Preferred Stock”), and 4,187,413 shares of the Winston Labs Series B
Convertible Preferred Stock, par value $0.001 per share (“Winston Labs
Series B Preferred Stock”), was exchanged for 422,518,545 shares of
our common stock, par value $0.001 per share (at an exchange ratio of
17.65101 shares of our common stock per share of Winston Labs common
stock), 101,849 shares of our Series A Preferred Stock and 73,332
shares of our Series B Preferred Stock (at an exchange ratio of
.01751238 shares of our preferred stock per share of Winston Labs
preferred stock); |
||
| • | we assumed Winston Labs’s stock option plans; |
||
| • | Winston Labs’s outstanding 1,643,750 options to purchase 1,643,750
shares of Winston Labs’s common stock were converted to options to
purchase 29,013,848 shares of our common stock; and |
||
| • | outstanding warrants to purchase Winston Labs Series A Preferred Stock
were assumed by us and converted into the right to acquire, expiring
November 13, 2012, upon the exercise of such warrants, an aggregate of
71,672 shares of our Series A Preferred Stock at a price per share of
$49.09. |
Immediately following the closing of the Merger, we had outstanding 440,851,441
shares of common stock. Our Board of Directors recommended and our stockholders approved
an amendment to our charter to increase the number of authorized shares of our common
stock from 499,000,000 shares to 900,000,000 shares to permit the conversion of all
outstanding shares of our preferred stock, including shares of our preferred stock
underlying outstanding warrants.
Subsequent to the completion of the Merger, the Company has carried on the business
of Winston Labs as its sole line of business and has retained all of Winston Labs’s
management. Our executive offices are located at 100 North Fairway Drive, Suite 134,
Vernon Hills, Illinois 60061 and our telephone number is (847) 362-8200. Effective
November 17, 2008, we changed our name from Getting Ready Corporation to “Winston
Pharmaceuticals, Inc.” and have discontinued any and all prior business operations in
favor of the business plan and operations of Winston, which has been our only significant
operation as a result of the merger with Winston Labs.
On October 29, 2008, Winston Labs filed a new drug submission (NDS) in Canada, for
CIVANEX® Cream (civamide cream 0.075%) for the treatment of signs and symptoms of
osteoarthritis, the first product Winston Labs has developed under its transient receptor
potential vanilloid (TRPV) channel technology. On October 30, 2008, Winston Labs entered
into a License Agreement (the “License Agreement”) with sanofi-aventis Canada Inc.
(“sanofi-aventis Canada”) pursuant to which Winston Labs granted sanofi-aventis an
exclusive license to the Canadian rights to Winston Labs proprietary transient receptor
potential vanilloid (TRPV-1) modulator in formulations for topical application. Under the
terms of the License Agreement, sanofi-aventis Canada owns the rights to manufacture,
develop and commercialize Civamide Cream in Canada along with a second generation cream
that is currently in development. In return for granting sanofi-aventis Canada the
Canadian rights to Civamide Cream, Winston Labs received an upfront payment of
$1.9 million (US), and will receive an additional $2 million (CAD) upon regulatory
approval of Civamide Cream in Canada, certain milestone payments, and future royalties on
net sales of Civamide or the related second generation cream in Canada.
On December 15, 2008 the Company amended its Certificate of Incorporation to provide
for the reduction of the total number of issued and outstanding shares of the Company’s
common stock, par value $.001 per share (“Common Stock”) and its preferred stock, par
value $.001 per share (“Preferred Stock”), by exchanging each eight (8) shares of such
issued and outstanding shares of Common Stock and Preferred Stock for one (1) share of
Common Stock or Preferred Stock, respectively.
Following a 1-for-8 reverse split of the
Company’s common and preferred stock on December 15, 2008, 12,730 shares of its Series A Preferred Stock and
9,157 shares of its Series B Preferred Stock were issued and outstanding. On September 24, 2009, each outstanding share of Company
Series A Preferred Stock and Series B Preferred Stock automatically converted into 1,000 fully-paid, non-assessable shares of
the Company’s Common Stock. In addition, in connection with such conversion, each outstanding warrant to purchase shares of
Series A Preferred Stock automatically converted into the right to acquire 1,000 shares of Common Stock upon the exercise of
such warrant, at an exercise price of $0.39 per share of Common Stock.
Winston Labs submitted a Marketing Authorization Application (“MAA”) in Europe for
CIVANEX in January 2008. On July 10, 2009 the Company received a negative opinion letter
from the Swedish Medical Products Administration (“MPA”) as the Reference Member State on
behalf of the Concerned Member States, the United Kingdom and Spain on the Company’s MAA
for CIVANEX in the decentralized procedure to obtain mutual recognition of CIVANEX by
such states. CIVANEX is an investigational therapy in development for the signs and
symptoms of, including the pain associated with, osteoarthritis. In its letter to the
Company, MPA cited lack of data relating to the full risk assessment of the carcinogenic
potential of CIVANEX. The Company’s management disagrees with several of the conclusions
in the opinion letter and has filed an appeal of the
negative opinion of the Swedish review team with the MPA. On November 2, 2009, the
Company filed a separate appeal of the opinion with the Medicines and Healthcare products
Regulatory Agency in the United Kingdom. The Company believes that the
current application containing safety and efficacy data support the approval of CIVANEX
for use under the proposed labeling. The Company is currently considering whether to
conduct another carcinogenesis study of CIVANEX.
20
Table of Contents
In May 2009, the Company completed a Phase I pharmacokinetic and safety study of
oral civamide in an enteric coated soft gel capsule with no systemic absorption detected.
The Company intends to conduct a new study designed to take advantage of this lack of
systemic absorption in a proof of concept study for the treatment of the inflammatory
bowel disorder, Crohn’s Disease.
As previously reported in the Company’s quarterly report on Form 10-Q for the
quarter ended June 30, 2009, in July 2009, the Company filed suit against the FDA in the
United States District Court for Northern District of Illinois for declaratory and
injunctive relief with respect to the FDA’s refusal to waive its application user fee
under small business waiver rules relating to the Company’s submission of the civamide
cream application. In September, 2009, the FDA filed a motion to dismiss the suit. In
October, 2009, the Company filed a response in opposition to the FDA’s motion to dismiss
and in December 2009, the court ruled in the Company’s favor. Consequently, on February
12, 2010, the Company was granted the user fee waiver.
In September, 2009, we completed Study WL-1001-04-03, a Phase II clinical trial
evaluating the efficacy and safety of Civamide Patch 0.015% in the treatment PHN.
Twenty-two patients with at least a 6 month history of PHN already on stable doses of
medication for PHN, but with recalcitrant pain of at least a 4 on a 0-10 categorical
scale during the week prior to enrollment, were enrolled in the study. Civamide Patch
0.015% was applied over a 4 week Treatment Period, for up to 24 hours daily. Daily Pain
and Sleep Scores were assessed as the primary efficacy variables. There was a 2 week
Post-Observation Treatment Period during which no patch was applied, but patients
continued to record efficacy and safety parameters. Results demonstrated that by Week 2
and continuing throughout the Treatment Period and Post Treatment Observation Period,
there was approximately up to a 40% improvement from Baseline in both Daily Pain Scores
and Sleep Scores as discussed in further detail in Part I, Item I, Business, elsewhere in
this Annual Report on Form 10-K.
Effective September 22, 2009, Robert Yolles succeeded Dr. Joel E. Bernstein as
Chairman of the Board of the Company’s Board of Directors.
On October 15, 2009, Winston Labs announced that it received a Notice of
Non-compliance (“NON”) from the Therapeutics Drug Directorate, Health Canada (the
“Directorate”) for its New Drug Submission (NDS) for CIVANEX (zucapsaicin cream 0.075%)
for the treatment of the signs and symptoms of osteoarthritis. The Directorate remarked
that the analysis of the pivotal trial did not support the requested indication. Winston
Labs had a period of ninety days to submit a response to the Directorate’s NON, which it
did on January 29, 2010. In the event that the Company is not be able to obtain
regulatory approval in Canada by June 30, 2010, the sanofi-aventis payment due upon
regulatory approval will be reduced by $250,000 (CAD) to $1,750,000 (CAD).
On February 12, 2010, Pharmaceutical Financial Syndicate, LLC (“PFS”), whose members
include Dr. Joel Bernstein, the Company’s President and Chief Executive Officer and
Robert A. Yolles and Neal S. Penneys, directors of the Company, executed a letter of
intent (“LOI”) with Frost Gamma Investments Trust, Subbarao Uppaluri, a director of the
company, and certain other shareholders of the Company (collectively, the “Frost Group”),
to acquire 100% of the Company’s capital stock (the “Acquisition”) beneficially owned by
all of the members of the Frost Group consisting of an aggregate of 18,399,271
outstanding shares of common stock and warrants to purchase an aggregate of 8,958,975
shares of common stock (collectively, the “Acquired Securities”).
As provided in the LOI, consummation of the Acquisition is subject to a number of
conditions, including the parties entering into a definitive acquisition agreement. The
LOI also provides that simultaneous with the completion of the Acquisition, three of the
Company’s directors, namely Subbarao Uppaluri, Glenn Halpryn and Curtis Lockshin, shall
resign as directors of the Company, and each of Messrs. Uppaluri, Halpryn and Lockshin
have advised the Company that they have consented to resign upon completion of the
Acquisition. Upon completion of the Acquisition, as the manager of PFS, Dr. Bernstein
would be deemed to be the beneficial owner of all of the Acquired Securities, however Dr.
Bernstein intends to disclaim beneficial ownership of those Acquired Securities in which
he does not have a pecuniary interest through PFS.
Winston Labs does not currently market any products. In the past, Winston Labs
marketed certain products revenues from which were used to help fund its research
programs. Winston Labs is engaged in the development of innovative products for managing
and alleviating pain. After discontinuing the Zostrix® and Axsain® product lines,
Winston Labs has devoted most of its resources to research and development. Winston Labs
has spent $2,062,933 and, $3,496,150, each of the years 2009 and 2008, respectively, on
research and development activities. Winston Labs has incurred significant operating
losses since the initiation of operations in 1997 and as of December 31, 2009, had an
accumulated deficit of approximately $49.3 million.
21
Table of Contents
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is
based upon our consolidated financial statements prepared in accordance with accounting
principles generally accepted in the United States. The preparation of these financial
statements requires us to make a number of assumptions and estimates that affect the
reported amounts of assets and liabilities, the disclosure of contingent assets and
liabilities at the date of the consolidated financial statements and the reported amounts
of revenues and expenses during the reported periods. Significant estimates and
assumptions are required in the determination of revenue recognition and sales deductions
for certain royalties and returns and losses. Significant estimates and assumptions are
also required in the appropriateness of capitalization and amortization periods for
identifiable intangible assets, income taxes, contingencies, and stock-based
compensation. We base our estimates on historical experience and various other
assumptions, available at that time, that we believe to be reasonable under the
circumstances. Some of these judgments can be subjective and complex. For any given
individual estimate or assumption made by us, there may also be other estimates or
assumptions that are reasonable. These items are monitored and analyzed by management for
changes in facts and circumstances, and material changes in these estimates could occur
in the future. Changes in estimates are recorded in the period in which they become
known. Actual results may differ from our estimates if past experience or other
assumptions do not turn out to be substantially accurate.
Revenue Recognition
The Company has historically generated revenues from product sales, collaborative
research and development arrangements, and other commercial arrangements such as,
royalties, and sales of technology rights. Payments received under such arrangements may
include non-refundable fees at the inception of the arrangements, milestone payments for
specific achievements designated in the agreements, royalties on sales of products
resulting from collaborative arrangements, and payments for the sale of rights to future
royalties.
The Company recognizes revenue in accordance with the SEC’s Staff Accounting
Bulletin Topic 13 (“Topic 13”), “Revenue Recognition.” Revenue is recognized when all of
the following criteria are met: (1) persuasive evidence of an arrangement exists;
(2) delivery has occurred or services have been rendered; (3) the seller’s price to the
buyer is fixed and determinable; and (4) collectability is reasonably assured.
Certain sales transactions include multiple deliverables. The Company allocates
amounts to separate elements in multiple element arrangements in accordance with ASC
Topic 605, Revenue Recognition. Revenues are allocated to a delivered product or service
when all of the following criteria are met: (1) the delivered item has value to the
customer on a standalone basis; (2) there is objective and reliable evidence of the fair
value of the undelivered item; and (3) if the arrangement includes a general right of
return relative to the delivered item, delivery or performance of the undelivered item is
considered probable and substantially in the Company’s control. The Company uses the
relative fair values of the separate deliverables to allocate revenue. For arrangements
with multiple elements that are separated, the Company recognizes revenues in accordance
with Topic 13.
Multiple Element Arrangements
The Company has arrangements whereby it delivers to the customer multiple elements
including technology and/or services. Such arrangements have generally included some
combination of the following: licensed rights to technology, patented products,
compounds, data and other intellectual property; and research and development services.
The Company analyzes its multiple element arrangements to determine whether the elements
can be separated. The Company performs its analysis at the inception of the arrangement
and as each product or service is delivered. If a product or service is not separable,
the combined deliverables will be accounted for as a single unit of accounting.
When a delivered element meets the criteria for separation, the Company allocates
amounts based upon the relative fair values of each element. The Company determines the
fair value of a separate deliverable using the price it charges other customers when it
sells that product or service separately; however, if the Company does not sell the
product or service separately, it uses third-party evidence of fair value. The Company
considers licensed rights or technology to have standalone value to its customers if it
or others have sold such rights or technology separately or its customers can sell such
rights or technology separately without the need for the Company’s continuing
involvement.
License Arrangements. License arrangements may consist of non-refundable upfront
license fees, data transfer fees, research reimbursement payments, exclusive licensed
rights to patented or patent pending compounds, technology access fees, various
performance or sales milestones. These arrangements are often multiple element
arrangements.
22
Table of Contents
Non-refundable, up-front fees that are not contingent on any future performance by
the Company, and require no consequential continuing involvement on its part, are
recognized as revenue when the license term commences and the licensed data, technology
and/or compound is delivered. Such deliverables may include physical quantities of
compounds, design of the
compounds and structure-activity relationships, the conceptual framework and mechanism of
action, and rights to the patents or patents pending for such compounds. The Company
defers recognition of non-refundable upfront fees if it has continuing performance
obligations without which the technology, right, product or service conveyed in
conjunction with the non-refundable fee has no utility to the licensee that is separate
and independent of the Company’s performance under the other elements of the arrangement.
In addition, if the Company has required continuing involvement through research and
development services that are related to its proprietary know-how and expertise of the
delivered technology, or can only be performed by the Company, then such up-front fees
are deferred and recognized over the period of continuing involvement. Under the license
agreement with sanofi-aventis Canada, Inc., referred to under “Results of Operations —
Revenues”, and described under “Liquidity and Capital Resources — Financing Activities”,
the obligation period was not contractually defined in relation to the $1.9 million
upfront fee. Under the circumstances, management exercised judgment in estimating the
period of time over which certain deliverables will be provided to enable the licensee to
utilize the license, which was determined to be 18 months. The estimated period of
18 months was primarily determined based on the management’s estimate of the maximum
amount of time it would take the Canadian regulatory authorities to approve the Company’s
new drug submission (NDS).
Payments related to substantive, performance-based milestones in a research and
development arrangement are recognized as revenues upon the achievement of the milestones
as specified in the underlying agreements when they represent the culmination of the
earnings process.
Research Services Arrangements. Revenues from research services are recognized
during the period in which the services are performed and are based upon the number of
full-time-equivalent personnel working on the specific project at the agreed-upon rate.
Reimbursements from collaborative partners for agreed upon direct costs including direct
materials and outsourced services, or subcontracted, pre-clinical studies are classified
as revenues in accordance with ASC Topic 605 and recognized in the period the
reimbursable expenses are incurred. Payments received in advance are deferred until the
research services are performed or costs are incurred.
Royalty Arrangements. The Company recognizes royalty revenues from licensed products
when earned in accordance with the terms of the license agreements. Net sales amounts
generally required to be used for calculating royalties include deductions for returned
product, pricing allowances, cash discounts, freight and warehousing.
Certain royalty arrangements require that royalties are earned only if a sales
threshold is exceeded. Under these types of arrangements, the threshold is typically
based on annual sales. The Company recognizes royalty revenue in the period in which the
threshold is exceeded.
Research and development expenses
The guidance in ASC Topic 730, Research and Development, requires the Company to
defer and capitalize nonrefundable advance payments made for goods or services to be used
in research and development activities until the goods have been delivered or the related
services have been performed. If the goods are no longer expected to be delivered or the
services are no longer expected to be performed, the Company would be required to expense
the related capitalized advance payments.
Research and development expenses consist of expenses incurred in performing
research and development activities including salaries and benefits, facilities and other
overhead expenses, clinical trials, contract services and outsource contracts. Research
and development expenses are charged to operations as they are incurred.
Acquired contractual rights. Payments to acquire contractual rights to a licensed
technology or drug candidate are expensed as incurred when there is uncertainty in
receiving future economic benefits from the acquired contractual rights. The Company
considers the future economic benefits from the acquired contractual rights to a drug
candidate to be uncertain until such drug candidate is approved by the FDA or when other
significant risk factors are abated.
Share-Based Compensation Expense
ASC Topic 718, Stock Compensation, requires that the compensation cost relating to
share-based payment transactions be recognized in financial statements, based on the fair
value of the instruments issued. ASC Topic 718 covers a wide range of share-based
compensation including stock options, restricted stock, performance-based awards, share
appreciation rights and employee share purchase plans. Prior to January 1, 2006, the
Company accounted for stock-based compensation arrangements using the intrinsic value
method.
23
Table of Contents
The fair value of each option award is estimated on the date of grant using a
Black-Scholes-Merton option pricing model (“Black-Scholes model”) that uses assumptions
regarding a number of complex and subjective variables. These variables
include, but are not limited to, our expected stock price volatility, actual and
projected employee stock option exercise behaviors, risk-free interest rate and expected
dividends. Expected volatilities are based on historical volatility of our common stock
and other factors. The expected terms of options granted are based on analyses of
historical employee termination rates and option
exercises. The risk-free interest rates are based on the U.S. Treasury yield in effect at
the time of the grant. Since we do not expect to pay dividends on our common stock in the
foreseeable future, we estimated the dividend yield to be 0%. ASC Topic 718 requires
forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent
periods if actual forfeitures differ from those estimates. We estimate pre-vesting
forfeitures based on our historical experience.
If factors change and we employ different assumptions in the application of ASC Topic 718
in future periods, the compensation expense that we record under ASC Topic 718 may differ
significantly from what we have recorded in the current period. There is a high degree of
subjectivity involved when using option pricing models to estimate share-based
compensation under ASC Topic 718. Because changes in the subjective input assumptions can
materially affect our estimates of fair values of our share-based compensation, in our
opinion, existing valuation models, including the Black-Scholes and lattice binomial
models, may not provide reliable measures of the fair values of our share-based
compensation. Consequently, there is a risk that our estimates of the fair values of our
share-based compensation awards on the grant dates may bear little resemblance to the
actual values realized upon the exercise, early termination or forfeiture of those
share-based payments in the future. Certain share-based payments, such as employee stock
options, may expire worthless or otherwise result in zero intrinsic value as compared to
the fair values originally estimated on the grant date and reported in our financial
statements. For awards with a longer vesting period, such as the three-year cliff vesting
awards issued to certain officers, the actual forfeiture rate and related expense may not
be known for a longer period of time, which can result in more significant accounting
adjustments once the awards are either vested or forfeited.
Alternatively, values may be realized from these instruments that are significantly
in excess of the fair values originally estimated on the grant date and reported in our
financial statements. There is no current market-based mechanism or other practical
application to verify the reliability and accuracy of the estimates stemming from these
valuation models, nor is there a means to compare and adjust the estimates to actual
values. Although the fair value of employee share-based awards is determined in
accordance with ASC Topic 718 and SAB 107 using an option-pricing.
Nature of Operating Expenses
In fiscal 2009, our operating expenses consisted mainly of our Phase, I, Phase II,
and Phase III clinical trial expenses (research costs) and general and administrative
expenses such as human resources, legal and accounting, primarily related to being a
public company. In fiscal 2008, our operating expenses consisted mainly of our Phase II
and Phase III clinical trial expenses (research costs) and general and administrative
expenses such as human resources, legal and accounting, primarily related with the
Merger.
Our business is exposed to significant risks which may result in additional
expenses, delays and lost opportunities that could have a material adverse effect on our
results of operations and financial condition.
Effects of Inflation
We believe the impact of inflation and changing prices on net revenues and on loss
from operations has been minimal during the past two years.
Results of Operations
We operate our business on the basis of a single reportable segment, which is the
discovery and development of products for pain management. Our chief operating
decision-maker is our Chief Executive Officer, who evaluates our company as a single
operating segment.
We categorize revenues by type of revenue in two different categories: 1) licensing
revenue and, 2) royalty revenue. All long-lived assets for fiscal 2009 and 2008 are
located in the United States.
24
Table of Contents
For the year ended December 31, 2009 compared to the year ended December 31, 2008
| Year Ended December 31, | ||||||||||||||||
| 2009 | 2008 | $ Change | % Change | |||||||||||||
REVENUES |
||||||||||||||||
License Revenue |
$ | 1,266,825 | $ | 316,706 | $ | 950,119 | 300 | % | ||||||||
Royalty Revenue |
127,762 | 135,836 | (8,074 | ) | (6 | )% | ||||||||||
| 1,394,587 | 452,542 | 942,045 | 208 | % | ||||||||||||
EXPENSES |
||||||||||||||||
Research and development |
2,062,933 | 3,496,150 | (1,433,217 | ) | (41 | )% | ||||||||||
General and administrative |
2,063,879 | 2,182,012 | (118,133 | ) | (5 | )% | ||||||||||
Depreciation and amortization |
9,007 | 8,651 | 356 | 4 | % | |||||||||||
Total Operating Expenses |
4,135,819 | 5,686,813 | (1,550,994 | ) | (27 | )% | ||||||||||
Loss from Operations |
(2,741,232 | ) | (5,234,271 | ) | 2,493,039 | (48 | )% | |||||||||
Interest income |
10,487 | 90,614 | (80,127 | ) | (88 | )% | ||||||||||
Other income |
75,429 | 1,911 | 73,518 | 3,847 | % | |||||||||||
Other income (expenses) |
85,916 | 92,525 | (6,609 | ) | (7 | )% | ||||||||||
Loss before income taxes |
(2,655,316 | ) | (5,141,746 | ) | 2,486,430 | (48 | )% | |||||||||
Income Taxes |
||||||||||||||||
Current |
— | — | — | — | ||||||||||||
Deferred |
— | — | — | — | ||||||||||||
Income Taxes |
— | — | — | — | ||||||||||||
NET LOSS |
$ | (2,655,316 | ) | $ | (5,141,746 | ) | $ | 2,486,430 | (48 | )% | ||||||
Revenues
Revenues from licenses increased to $1,266,825 for the fiscal year ended
December 31, 2009 compared to $316,706 for the fiscal year ended December 31, 2008. The
$1,226,825 and $316,706 license revenue in 2009 and in 2008, respectively, represents 12
and 3 months of revenue recognition related to a $1.9 million upfront cash payment
received by the Company from sanofi-aventis Canada Inc. in accordance with a license
agreement entered into by the Company in the fourth quarter of 2008.
Revenues from royalties declined to $127,762 for the fiscal year ended December 31,
2009 compared to $135,826 for the fiscal year ended December 31, 2008. The decline was
primarily due to $34,861 of royalties generated in 2008 from the License Agreement with
DUSA that expired on September 30, 2008. Royalties from Hi-Tech increased from $100,975
in 2008 to $127,762 for the same period in 2009. On October 30, 2009, the Company
received communication from Hi-Tech that is has discontinued the sale of the product as
defined in the license agreement. As a result, the Company does not expect to receive
any more royalties from Hi-Tech in the future.
Research & Development Expenses
Research and development expenses decreased to $2,062,933 for the fiscal year ended
December 31, 2009 compared to $3,496,150 for the fiscal year ended December 31, 2008. The
decrease was primarily due to decreased spending on various European and Canadian filing
fees and the timing of certain R&D projects.
25
Table of Contents
General and Administrative Expenses
General and administrative expenses decreased to $2,063,879 for the fiscal year
ended December 31, 2009 compared to $2,182,012 for the fiscal year ended December 31,
2008. The decrease is due largely to a decrease in spending on accounting, legal and
recruiting fees which countered with a slight increase in payroll due to hiring of
additional administrative staff.
Interest Income
Interest income decreased by $80,127 to $10,487 for the year ended December 31, 2009
from $90,614 for the same period in 2008 due to decreased amounts of funds on deposit.
Net Loss
Net loss was $2.6 million, or $0.04 per share, for the fiscal year ended
December 31, 2009, compared to a net loss of $5.2 million, or $0.10 per share for the
fiscal year ended December 31, 2008. The decrease in net loss is primarily attributed to
an increase in revenues of $0.9 million and a decrease in operating expenses totaling
$1.5 million, consisting of a decrease in research and development expenses totaling
$1.4 million and a decrease in general and administrative expenses totaling $0.1 million.
Liquidity and Capital Resources
Since
Winston Labs’s inception, it has financed its operations through the private
placement of equity securities and, to a lesser extent, through licensing revenues and
product sales. Through December 31, 2009, Winston Labs has raised approximately $54 million
from the private placement of Winston Labs and Rodlen common shares.
While the focus going forward is to improve our financial performance, we expect
operating losses and negative cash flow to continue for the foreseeable future. We
anticipate that our losses may increase from current levels because we expect to incur
significant additional costs and expenses related to being a public company, continuing
our research and development activities, filing with regulatory agencies (e.g. FDA) as
well as developing new compounds and products, advertising, marketing and promotional
activities, all of which will involve employing additional personnel as our business
expands. Our ability to become profitable depends on our ability to develop products and
to generate and sustain substantial revenue related to those products through new license
and distribution agreements while maintaining reasonable expense levels.
We assess our liquidity by our ability to generate cash to fund future operations.
Key factors in the management of our liquidity are: cash required to fund operating
activities including expected operating losses and the levels of accounts receivable and
accounts payable; the timing and extent of cash received from milestone payments under
license agreements; and financial flexibility to attract long-term equity capital on
favorable terms. Historically, cash required to fund on-going business operations has
been provided by financing activities and used to fund operations, working capital
requirements and investing activities.
Cash, cash equivalents and investments, as well as, net cash provided by or used for
operating, investing and financing activities, are summarized in the table below.
| Increase | ||||||||||||
| December 31, | (Decrease) | December 31, | ||||||||||
| 2009 | During Period | 2008 | ||||||||||
Cash and cash equivalents |
$ | 1,562,848 | $ | (4,064,065 | ) | $ | 5,626,913 | |||||
Net working capital |
$ | 457,356 | $ | (2,734,246 | ) | $ | 3,191,602 | |||||
| Year Ended | Change | Year Ended | ||||||||||
| December 31, | Between | December 31, | ||||||||||
| 2009 | Periods | 2008 | ||||||||||
Net cash used in operating activities |
$ | (4,198,636 | ) | $ | (1,136,416 | ) | $ | (3,062,220 | ) | |||
Net cash provided by (used in) investing activities |
141 | 16,475 | (16,334 | ) | ||||||||
Net cash provided by financing activities |
134,430 | (4,089,426 | ) | 4,223,856 | ||||||||
Net increase (decrease) in cash and cash equivalents |
$ | (4,064,065 | ) | $ | (5,209,367 | ) | $ | 1,145,302 | ||||
26
Table of Contents
Operating activities. Net cash used in operating activities was $4.2 million in the
fiscal year 2009 compared to $3.0 million in the fiscal year 2008. The increase in cash
used in operating activities resulted from a decrease in unearned revenue resulting from
the upfront payment of $1.9 million in connection with the licensing agreement with
sanofi aventis Canada.
Investing activities. Net cash provided by investing activities was $0.01 million in
the fiscal year 2009. Our capital expenditures have historically been a small fraction of
our overall expenses, since we outsource manufacturing and other capital-intensive
functions. The highest annual amount of capital expenditures was $56,000 in the year
ended December 31, 2004, reflecting expenditures on computer equipment, phone equipment
and furniture to support a field force and increased headcount associated with an
unsuccessful product launch. We do not anticipate making significant capital expenditures
in the near future.
Historically, our investing activities have included the acquisition or purchase of
product rights, such as Psoriatec® in 2001 and Zostrix® in 2002, the divestment of
product rights, such as Zostrix® in 2005, and the acquisition or redemption of holdings
in other companies, such as the preferred shares in Ovation that we redeemed in 2005.
Financing activities. Net cash provided by financing activities was $0.1 million in
the fiscal year 2009, compared to $4.2 million in fiscal 2008.
On November 13, 2007, Winston Labs issued 5,815,851 shares of Winston Labs Series A Preferred
Stock and warrants to purchase 4,092,636 shares of Winston Labs Series A Preferred Stock in a
private placement for an aggregate purchase price of $5.1 million. Immediately prior to
consummation of the Merger, Winston Labs issued 4,187,413 shares of Winston Labs Series B Preferred
Stock in a private placement for an aggregate purchase price of $4.0 million. All of the
Winston Labs shares and warrants issued in these transactions were exchanged for shares of the
Company’s Series A and B Preferred Stock and warrants to purchase the Company’s Series A
Preferred Stock upon consummation of the Merger.
On October 30, 2008, the Company and sanofi-aventis Canada Inc. entered into a
licensing agreement for the Canadian rights to Civamide, Winston Labs transient receptor
potential vanilloid (TRPV-1) modulator, in formulations for topical application. Under
the terms of the agreement, sanofi-aventis Canada Inc. owns the rights to develop,
manufacture and commercialize Civamide Cream in Canada along with a second generation
cream that is currently in development. In return for granting sanofi-aventis Canada Inc.
the Canadian rights, Winston Labs received an upfront payment of $1.9 million (US) and will
receive an additional $2 million (CAD) upon regulatory approval of Civamide Cream in
Canada, certain milestone payments and future royalties on net sales of Civamide or the
related second generation cream in Canada. In connection with this agreement, Winston Labs is
recognizing the upfront payment of $1.9 million over 18 months. As such, approximately
$1,264,000 and $316,000 has been recognized as revenue in 2009 and in 2008, respectively,
with the remainder being treated as unearned revenue on our Consolidated Balance Sheet as
of December 31, 2009 and 2008.
The Company has incurred significant losses from operations since its inception and
expects losses to continue for the foreseeable future. The Company’s success depends
primarily on the development and regulatory approval of its product candidates. From
inception through December 31, 2009, the Company has incurred cumulative net losses of
$49.4 million. Net losses may continue for at least the next several years as the
Company proceeds with the development of its product candidates and programs. The size
of these losses will depend on the receipt of revenue from its products candidates and
programs, if any, and on the level of the Company’s expenses. To achieve profitable
operations, the Company must successfully identify, develop, partner and/or commercialize
its product candidates and programs. Product candidates developed by the Company will
require approval of the U.S. Food and Drug Administration (FDA) or a foreign regulatory
authority prior to commercial sales. The regulatory approval process is expensive, time
consuming and uncertain, and any denial or delay of approval could have a material
adverse effect on the Company’s ability to become profitable or continue operations.
Even if approved, the Company’s product candidates may not achieve market acceptance and
could face competition.
The Company’s cash and cash equivalents have decreased from $5.6 million as of
December 31, 2008 to $1.6 million as of December 31, 2009. The Company will need to
raise additional funds in the immediate future to support its operations through December
31, 2010. The Company seeks additional sources of financing through collaborations with
third parties, or public or private debt or equity financings. There can be no assurance
that additional funds will be available on satisfactory terms, or at all. If adequate
funds are not available, the Company may be required to significantly reduce expenses
related to its operations and/or delay or reduce the scope of its development programs.
27
Table of Contents
As
described herein under Item 7. Management’s Discussion and
Analysis of Financial Condition and Results of Operations and the Financial Statements filed as part of this Form 10-K, the Report of
Independent Registered Public Accounting Firm contains an emphasis of matter paragraph
which indicates that there is substantial doubt about the Company’s ability to continue
as a going concern. As described herein under Item 7. Management’s Discussion and
Analysis of Financial Condition and Results of Operations — Liquidity and Capital
Resources, the Company plans to raise additional funds early in the second quarter
of 2010 in order to support its operations through December 31, 2010.
Contractual Obligations
| Contractual Obligations | Total | Less than 1 year | ||||||
Operating Lease Obligations |
$ | 3,000 | $ | 3,000 | ||||
We lease our facilities on a month-to-month basis and certain equipment under
operating leases that expire in 2010 and require payments totaling approximately $3,000.
Rental expense for the years ended December 31, 2009 and 2008 was $93,944 and $120,649,
respectively.
We enter into contracts in the normal course of business with clinical research
organizations and clinical investigators, for third party manufacturing and formulation
development. These contracts generally provide for termination with notice, and
therefore, our management believes that our non-cancelable obligations under these
agreements are not material.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, with the exception of the above
noted operating leases.
Recent Accounting Pronouncements
See Note 2, “Summary of Accounting Policies” in the Notes to Consolidation Financial
Statements for a discussion of recent accounting pronouncements and their effect, if any,
on the Company.
Item 7A. Quantitative and Qualitative Disclosure About Market Risk
We are a smaller reporting company, as defined by Item 10(f)(1) of Regulation S-K,
and we are not required to make the disclosures required by this item pursuant to Item
305(e) of Regulation S-K.
Item 8. Financial Statements and Supplementary Data
Our Financial statements are annexed to this report beginning on page F-1 and are
hereby incorporated by reference.
Item 9. Changes In and Disagreements With Accountants on Accounting and Financial
Disclosure
None.
Item 9A(T). Controls and Procedures
Disclosure Controls and Procedures. Our management, with the participation of our
Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of
our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and
15d-15(e) under the Exchange Act) as of the end of the period covered by this report.
Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have
concluded that, as of the end of such period, our disclosure controls and procedures are
effective in recording, processing, summarizing and reporting, on a timely basis,
information required to be disclosed by us in the reports that we file or submit under
the Exchange Act, including, without limitation, controls and procedures designed to
ensure that information required to be disclosed by us in the reports that we file or
submit under the Exchange Act is accumulated and communicated to our management,
including our Chief Executive Officer and Chief Financial Officer, to allow timely
decisions regarding required disclosure.
28
Table of Contents
Management’s Annual Report on Internal Control Over Financial Reporting. Our
management is responsible for establishing and maintaining adequate internal control over
financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities
Exchange Act of 1934). A company’s internal control over financial reporting is a process
designed to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with U.S.
generally accepted accounting principles. Internal control over financial reporting
includes those policies and procedures that (i) pertain to the maintenance of records
that, in reasonable detail, accurately and fairly reflect the transactions and
dispositions of the assets of the company; (ii) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that receipts and
expenditures of the company are being made only in accordance with authorizations of
management and directors of the company; and (iii) provide reasonable assurance regarding
prevention or timely detection of unauthorized
acquisition, use, or disposition of the company’s assets that could have a material
effect on the interim or annual consolidated financial statements. Because of its
inherent limitations, internal control over financial reporting may not prevent or detect
misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions,
or that the degree of compliance with the policies or procedures may deteriorate.
Our management, with the participation of our Chief Executive Officer and Chief
Financial Officer, conducted an evaluation of the effectiveness of our internal control
over financial reporting as of December 31, 2009 based on the framework in Internal
Control Integrated Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission. Based on this assessment, our management concluded that, as of
December 31, 2009, our internal control over financial reporting was effective based on
those criteria.
This annual report on Form 10-K does not include an attestation report of our
independent registered public accounting firm regarding internal control over financial
reporting. Management’s report was not subject to attestation by our registered public
accounting firm pursuant to temporary rules of the Securities and Exchange Commission
that permit the Company to provide only management’s report in this annual report.
Internal Control Over Financial Reporting. There have not been any changes in our
internal control over financial reporting (as such term is defined in Rules 13a-15(f) and
15d-15(f) under the Exchange Act) during the fiscal quarter to which this report relates
that have materially affected, or are reasonably likely to materially affect, our
internal control over financial reporting.
Item 9B. Other Information
None.
29
Table of Contents
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Management
As of March 1, 2010, the Company employed 9 full-time individuals, including 4
engaged in research and development activities, and 5 in general and administrative
functions. Most of the Company’s management and professional employees have substantial
prior experience with other pharmaceutical, biotechnology or medical products and service
companies. None of the Company’s employees is a member of a labor union. The Company
believes that it maintains good relations with its employees.
The table and text below provide certain descriptive information about our senior
management and directors following the Merger.
| Name | Age | Position | First Elected | Term Expires | ||||||||
Joel E. Bernstein, M.D. |
67 | President and Chief Executive Officer; Director | 2008 | 2010 | ||||||||
Scott B. Phillips, M.D. |
49 | Senior Vice President, Scientific Affairs; Director | 2008 | 2010 | ||||||||
David Starr |
40 | Vice President, Chief Financial Officer, Secretary | — | — | ||||||||
Robert A. Yolles |
69 | Director, Audit Committee Chair, Chairman of the Board | 2008 | 2010 | ||||||||
Glenn L. Halpryn |
49 | Director | 2008 | 2010 | ||||||||
Curtis Lockshin, Ph.D. |
49 | Director | 2006 | 2010 | ||||||||
Neal S. Penneys, M.D., Ph.D. |
68 | Director, Audit Committee Member | 2008 | 2010 | ||||||||
Subbarao Uppaluri, Ph.D. |
60 | Director, Audit Committee Member | 2008 | 2010 | ||||||||
Executive Officers
Joel E. Bernstein, M.D., a director of the Company and its President and Chief
Executive Officer since the Merger is also Winston Labs’s founder and has served as
Winston Labs’s Chief Executive Officer since 1998 and prior to the Merger as a director
of Winston Labs since 1998. Dr. Bernstein is also the founder and Chief Executive
Officer of Elorac, Inc. and Gideon Pharmaceuticals, Inc. Before founding Winston Labs,
Dr. Bernstein was the founder, Chairman, and Chief Executive Officer of GenDerm
Corporation, a pharmaceutical company that was acquired by Medicis Pharmaceutical
Corporation in 1997. Previously, Dr. Bernstein was head of dermatopharmacology at
Northwestern University Medical School and the University of Chicago Pritzker School of
Medicine. Dr. Bernstein has also held senior scientific positions at Abbott Laboratories
and Schering-Plough Corporation. He has authored more than 125 scientific publications
and holds over 150 patents. Dr. Bernstein received a B.A. from Carleton College and an
M.D. from the University of Chicago Pritzker School of Medicine, where he received the
Roche Award for ranking first in his class. He completed specialty training programs in
both dermatology and clinical pharmacology at the University of Chicago. Dr. Bernstein
is a past president of the University of Chicago Medical and Biological Alumni
Association. In 1988, he was chosen as the Illinois high-tech entrepreneur of the year
by KPMG and the State of Illinois. Dr. Bernstein was also the founder and non-executive
chairman of Sirius Laboratories, Inc., (acquired in 2006 by DUSA Pharmaceuticals) and a
co-founder of Ovation Pharmaceuticals (acquired by Lundbeck in 2009). Based on Dr. Bernstein’s
familiarity with the Company as an incumbent member of the
Company’s Board of Directors and an executive officer of the Company and his knowledge
and expertise in the pharmaceutical industry, the Corporate Governance and Nominating
Committee of the Board of Directors concluded that Dr. Bernstein has the requisite
experience, qualifications, attributes and skill necessary to serve as a member of the Board of Directors.
Scott B. Phillips, M.D., a director of the Company and its Senior Vice President,
Scientific Affairs since the Merger has also been Winston Labs’s Senior Vice President,
Scientific Affairs since April 1999. Previously Director of Drug Discovery at GenDerm
Corporation, Dr. Phillips has 15 years of experience in the pharmaceutical industry. In
addition, he has been Chief of the Clinical Investigations Unit at Harvard Medical School
and Clinical Assistant Professor of Dermatology and Medicine at the University of Chicago
Pritzker School of Medicine. He received a B.A. in biology from Cornell University and
an M.D. from Harvard University. Based on Dr. Phillips’s familiarity with the Company as an
incumbent member of the Company’s Board of Directors and an executive
officer of the Company and his knowledge and expertise in the pharmaceutical
industry, the Corporate Governance and Nominating Committee of the Board of
Directors concluded that Dr. Phillips has the requisite experience, qualifications,
attributes and skill necessary to serve as a member of the Board of Directors.
30
Table of Contents
David Starr, the Company’s Vice President and Chief Financial Officer since the
Merger has also been Winston Labs’s Vice President and Chief Financial Officer since
November 2007 and he has served as Winston Lab’s sole director since the Merger. From
August 2005 to October 2007, Mr. Starr was a Chief Financial Officer of DayOne Health,
which set up and managed bariatric surgery programs for ambulatory surgery centers. From
October 2003 to August 2005, Mr. Starr was a Chief Financial
Officer and Director of Operations Research of MSO Medical, a national obesity disease
management company serving hospitals with a proprietary version of the gastric bypass
surgery. From March 1998 to September 2003, Mr. Starr served in senior management
positions at several technology companies. From September 1991 to March 1998, Mr. Starr
was an audit manager with Arthur Andersen’s Enterprise Group. Mr. Starr has an MBA from
Northwestern’s Kellogg Graduate School of Business and a BS in Accounting from Indiana
University, Bloomington.
Outside Directors
Robert A. Yolles, J.D., the chairman of the Company’s board of directors, served as
a director of Winston Labs prior to the Merger since 2002, and is a retired partner of
the international law firm Jones Day. While a partner, he served as the chair of the
Firm’s domestic and international finance practices and, previously, co-chair of the
Firm’s corporate practice. He received a B.A. and J.D. from Northwestern University. Based on Mr. Yolles’s familiarity
with the Company as an incumbent member of the Company’s Board of Directors
and as a Chairperson of its Audit Committee and his knowledge and expertise
in numerous aspects of managing a rapidly-growing enterprise, the Corporate
Governance and Nominating Committee of the Board of Directors concluded that
Mr. Yolles has the requisite experience, qualifications, attributes and skill necessary to serve as a member of the Board of Directors.
Glenn L. Halpryn, was Chairman of the Board and Chief Executive Officer of Getting
Ready Corporation prior to the Merger. Mr. Halpryn has been a Director of the Company
since the Merger. Mr. Halpryn was a Director of Ivax Diagnostics, Inc., an AMEX listed
corporation specializing in the manufacturing and distribution of diagnostic products
from October 2002 until September 2008. Mr. Halpryn currently serves on the board of
directors of Sorrento Therapeutics (OTCBB: SRNE), a biopharmaceutical company focused on
applying a proprietary technology platform for the discovery and development of human
therapeutic antibodies for the treatment of a variety of disease conditions, Castle
Brands, Inc., (AMEX: ROX), a developer and international marketer of premium branded
spirits, and SearchMedia Holdings Limited, the second largest outdoor billboard and
in-elevator advertiser in China Mr. Halpryn received a B.S. in Business Administration
from the University of Florida. Based on Mr. Halpryn’s familiarity with the Company as an incumbent member of the
Company’s Board of Directors and as a Chairperson of its Compensation Committee,
the Corporate Governance and Nominating Committee of the Board of Directors
concluded that Mr. Halpryn has the requisite experience, qualifications,
attributes and skill necessary to serve as a member of the Board of Directors.
Curtis Lockshin, Ph.D. was a director of Getting Ready Corporation prior to the
Merger. Dr. Lockshin has been a Director of the Company since the Merger. Since 2003,
Dr. Lockshin has been an independent pharmaceutical and life sciences consultant, focused
on small companies that seek to leverage their technology assets inside healthcare,
biotechnology and security sectors. At Sepracor Inc. from 1998 to 2002, as a Scientist,
Associate Director, and Director of Discovery Biology & Informatics, Dr. Lockshin was
instrumental in establishing the New Leads program, which delivered novel chemical
entities into the preclinical pipeline. In 2002-2003, while Director of Discovery
Biology at Beyond Genomics, Inc., Dr. Lockshin co-developed strategies for utilizing
proprietary technology platforms in clinical trial optimization and prediction of
off-target drug activities. Since 2004, Dr. Lockshin has served on the Board of
Directors of the Ruth K. Broad Biomedical Research Foundation, a Duke University support
corporation, which supports basic research related to Alzheimer’s disease and
neurodegeneration via intramural, extramural, and international grants. From July, 2006
to December, 2006, Dr. Lockshin was a director of Orthodontix, a publicly traded shell
company that effected a reverse merger with a biotechnology company, Protalix (AMEX:
PLX). From September, 2007 to September, 2008, Dr. Lockshin was a director of
clickNsettle.com, a publicly traded shell company that effected a reverse merger with an
orthopedic medical device company, Cardo Medical, Inc. (OTCBB: CDOM). From July 2008 to
September, 2009, Dr. Lockshin was a director of QuikByte Software, Inc., a publicly
traded shell company that effected a reverse merger with a biopharmaceutical company,
Sorrento Therapeutics (OTCBB: SRNE), where he continues to serve as a director. Dr.
Lockshin is a co-inventor on several U.S. patents covering pharmaceuticals, biomaterials,
and optics for remote biochemical sensing. He holds a Bachelor’s degree in Life Sciences
and a Ph.D. in Biological Chemistry, both from the Massachusetts Institute of Technology. Based on Dr. Lockshin’s familiarity with the Company as an incumbent member of the
Company’s Board of Directors and his knowledge of the pharmaceutical industry,
the Corporate Governance and Nominating Committee of the Board of Directors
concluded that Dr. Lockshin has the requisite experience, qualifications,
attributes and skill necessary to serve as a member of the Board of Directors.
Neal S. Penneys, M.D., Ph.D., is currently a dermatopathologist with AmeriPath,
American Laboratories, Fort Lauderdale, Florida. Prior to joining AmeriPath, from
1999-2001 Dr. Penneys served as Associate Dean, Chief Operating Officer, Saint Louis
University Health Sciences Center, and from 1995-1999 as Chairperson, Department of
Dermatology, Saint Louis University School of Medicine. Dr. Penneys has served on many
Boards, including those of the audit committee of the American Academy of Dermatology,
the American Society of Dermatopathology, GenDerm Corporation, DUSA Pharmaceuticals, Inc.
(NASDAQ: DUSA) and Sirius Laboratories, Inc. He was a senior consultant to the FDA from
1989-2006, and from 1985-2004 served on several FDA Advisory Panels including those for
Dermatologic Drugs and for Orphan Drugs. Dr. Penneys received a B.A. from Franklin and
Marshall College, an M.D. from the University of Pennsylvania, a Ph.D. from the
University of Miami, and an M.B.A. from St. Louis University. Based on Dr. Penneys’s
familiarity with the Company as an incumbent member of the
Company’s Board of Directors and his knowledge of the pharmaceutical industry,
the Corporate Governance and Nominating Committee of the Board of Directors
concluded that Dr. Penneys has the requisite experience, qualifications,
attributes and skill necessary to serve as a member of the Board of Directors.
Subbarao Uppaluri, Ph.D., Dr. Uppaluri has served as Senior Vice President and Chief
Financial Officer of OPKO Health Inc. since May 2007. Dr. Uppaluri served as the Vice
President, Strategic Planning and Treasurer of IVAX from 1997 until December 2006.
Before joining IVAX, from 1987 to August 1996, Dr. Uppaluri was Senior Vice President,
Senior Financial Officer and Chief Investment Officer with Intercontinental Bank, a
publicly traded commercial bank in Florida. In addition, he served in various positions,
including Senior Vice President, Chief Investment Officer and Controller, at Peninsula
Federal Savings & Loan Association, a publicly traded Florida S&L, from October 1983 to
1987. His prior employment, during 1974 to 1983, included engineering, marketing and
research positions with multinational companies and research institutes in
31
Table of Contents
India
and the United States. Dr. Uppaluri currently serves on the board of directors of Non-Invasive
Monitoring Systems, Inc., (OTCBB:NIMU), a medical device company, Kidville, Inc.
(OTCBB:KVIL), which operates large, upscale facilities, catering to
newborns through five-year-old children and their families and offers a wide range of
developmental classes for newborns- 5 years old and Cardo Medical, Inc., an early-stage
orthopedic medical device company specializing in designing, developing and marketing
reconstructive joint devices and spinal surgical devices (OTCBB:CDOM). From June 1, 2007
to October 30, 2009, Dr. Uppaluri was a director of Ideation Acquisition Corporation, a
publicly traded SPAC (Special Purpose Acquisition Corporation), seeking to effect a
merger in the digital media sector. Based on Dr. Uppaluri’s familiarity with the Company
as an incumbent member of the Company’s Board of Directors
and his knowledge and expertise in the pharmaceutical industry,
the Corporate Governance and Nominating Committee of the Board of Directors
concluded that Dr. Uppaluri has the requisite experience, qualifications,
attributes and skill necessary to serve as a member of the Board of Directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our executive
officers, directors and persons who beneficially own more than 10% of a registered class
of our securities to file reports of ownership and changes in ownership with the SEC.
Based solely on a review of copies of such forms submitted to the Company, we believe
that all persons subject to the requirements of Section 16(a) filed such reports on a
timely basis in 2009, except as follows: (1) a Form 4 reporting an exercise of common
stock options by Dr. Joel Bernstein was filed after the due date; (2) Forms 4, each
reporting a grant of common stock options to Joel E. Bernstein, M.D., Scott B. Phillips,
M.D. and David Starr, respectively, were filed after the due date; and (3) Forms 4, each
reporting a grant of common stock options to Dr. Curtis Lockshin, Ph.D., Robert A.
Yolles, Glenn L. Halpryn, Subbarao Uppaluri, Ph.D. and Neal S. Penneys, M.D., Ph.D.,
respectively, were filed after the due date.
Code of Business Conduct and Ethics
The Company has adopted a code of business conduct and ethics within the meaning of
Item 406 of Regulation S-K that applies to its officers (including our principal
executive officer and principal financial officer) and employees. The code of ethics is
posted on the Company’s website at www.winstonlabs.com. The Company shall include on its
website any amendments to, or waivers from, a provision of its code of ethics that
applies to the Company’s chief executive officer and chief financial officer, or persons
performing similar functions, that relate to any element of the code of business conduct
and ethics.
Stockholder Communications with the Board of Directors
All stockholder communications must (i) be addressed to the Chief Financial Officer
of the Company, Winston Pharmaceuticals, Inc., 100 North Fairway Drive, Suite 134, Vernon
Hills, Illinois, 60061, or at the CFO’s internet e-mail address at
dstarr@winstonlabs.com, (ii) be in writing either in print or electronic format, (iii) be
signed by the stockholder sending the communication, (iv) indicate whether the
communication is intended for a specific director(s), the entire Board of Directors, the
Nominating and Governance Committee, or all non- management directors, (v) if the
communication relates to a stockholder proposal or director nominee, identify the number
of shares held by the stockholder, the length of time such shares have been held, and the
stockholder’s intention to hold or dispose of such shares, provided that the Board of
Directors and the Nominating and Governance Committee will not entertain stockholder
proposals or stockholder nominations from stockholders who do not meet the eligibility
and procedural criteria for submission of shareholder proposals under SEC Rule 14a-8 of
Regulation 14A under the Exchange Act, and (vi) if the communication relates to a
director nominee being recommended by the stockholder, must include appropriate
biographical information of the candidate.
Upon receipt of a stockholder communication that is compliant with the requirements
identified above, the Chief Financial Officer shall promptly deliver such communication
to the appropriate board or committee member(s) identified by the stockholder as the
intended recipient of such communication by forwarding the communication to either the
Chairman of the Board of Directors with a copy to the chief executive officer, the
Chairman of the Nominating and Governance Committee, or the non-management directors, as
the case may be.
The Chief Financial Officer may, in his sole discretion and acting in good faith,
provide copies of any such stockholder communication to any one or more directors and
executive officers of the Company, except that in processing any stockholder
communication addressed to the non-management directors, the Chief Financial Officer may
not copy any member of management in forwarding such communication to such directors.
Audit Committee
The Board of Directors has established an audit committee in accordance with Section
3(a)(58)(A) of the Exchange Act. The Board has designated from among its members Robert
A. Yolles, Neal S. Penneys and Subbarao Uppaluri as the members of the Audit Committee.
The primary functions of the Audit Committee are to represent and assist the Board of
Directors with the oversight of:
| • | appointing, approving the compensation of, and assessing the independence of the Company’s
independent auditors; |
||
| • | overseeing the work of the Company’s independent auditors, including through the receipt and
consideration of certain reports from the independent auditors; |
32
Table of Contents
| • | reviewing and discussing with management and the independent auditors the Company’s annual and
quarterly financial statements and related disclosures; |
||
| • | coordinating the Board of Director’s oversight of the Company’s internal control over financial
reporting, disclosure controls and procedures and code of business conduct and ethics; |
||
| • | establishing procedures for the receipt and retention of accounting related complaints and concerns; |
||
| • | meeting independently with the Company’s independent auditors and management; and |
||
| • | preparing the audit committee report required by SEC rules. |
The Board of Directors has determined that Subbarao Uppaluri is an “audit committee
financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K.
Item 11. Executive Compensation
Summary Compensation Table
The following table sets forth information concerning the compensation paid by
Winston Labs and/or the Company, as applicable, during the fiscal years ended December 31,
2009 and 2008 to the Company’s Chief Executive Officer, the Company’s former Chief
Executive Officer and the next two most highly compensated executive officers whose
salary and bonus for the year exceeded $100,000 and who served as an executive officer of
each of the respective companies as of, or during the fiscal year ended, December 31,
2009 (each, a “Named Executive Officer”).
| Option | All Other | Total | ||||||||||||||||||||||
| Name and Principal | Salary | Bonus | Awards | Compensation | Compensation | |||||||||||||||||||
| Position | Year | ($) | ($) | ($)(1) | ($)(2) | ($) | ||||||||||||||||||
Joel E. Bernstein, M.D. |
2009 | 265,307 | — | 218,000 | 23,593 | 506,900 | ||||||||||||||||||
President and Chief |
2008 | 242,000 | 25,000 | — | 23,570 | 290,570 | ||||||||||||||||||
Executive Officer of
the Company and;
Director of the
Company |
||||||||||||||||||||||||
Scott B. Phillips, M.D. |
2009 | 250,000 | — | 109,000 | 22,588 | 381,588 | ||||||||||||||||||
Senior Vice President, |
2008 | 221,000 | 14,500 | — | 20,840 | 256,340 | ||||||||||||||||||
Scientific Affairs of
the Company and;
Director of the
Company |
||||||||||||||||||||||||
David Starr, Vice |
2009 | 200,000 | — | 163,500 | 11,530 | 375,030 | ||||||||||||||||||
President, Chief |
2008 | 200,000 | 14,500 | — | 3,883 | 218,383 | ||||||||||||||||||
Financial Officer,
Secretary of the
Company and; Director
of Winston |
||||||||||||||||||||||||
| (1) | Represents aggregate grant date fair value pursuant to ASC Topic 718 for the respective year
for restricted stock options granted as long term incentive pursuant to the Company’s Omnibus
Incentive Plan. No options were granted to the above officers for fiscal year 2008. |
|
| (2) | Dr. Bernstein, Dr. Phillips and Mr. Starr received additional compensation in 2009 and 2008,
comprised of the following components: 401(k) matching contributions in 2009 of $10,612,
$10,000, and $0, respectively and in 2008 of $9,665, $9,405, and $0; insurance premium
payments in 2009 in amounts of $12,981, $12,588, and $11,530, respectively, and in 2008 in
amounts of $13,905, $11,435, and $3,883, respectively. |
33
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding exercisable and unexercisable
option and stock awards held by the Named Executive Officers as of December 31, 2009.
| Number of | ||||||||||||
| Securities | ||||||||||||
| Underlying | ||||||||||||
| Unexercised | ||||||||||||
| Options | Option | Option | ||||||||||
| (#) | Exercise | Expiration | ||||||||||
| Name | Exercisable | Price ($) | Date | |||||||||
Joel E. Bernstein, M.D. |
661,913 | 0.37 | 4/6/2010 | |||||||||
| 100,000 | 1.53 | 4/7/2019 | ||||||||||
Scott B. Phillips, M.D. |
205,193 | 0.33 | 12/10/2012 | |||||||||
| 231,670 | 0.33 | 1/12/2014 | ||||||||||
| 661,913 | 0.34 | 4/6/2015 | ||||||||||
| 50,000 | 1.53 | 4/7/2019 | ||||||||||
David Starr |
75,000 | 1.53 | 4/7/2019 | |||||||||
Narrative Disclosure to Summary Compensation Table; Additional Narrative Disclosure
Employment and Consulting Agreements; Termination, Severance and Change-in-Control
Matters
The Company and Winston Labs have entered into certain employment and severance
agreements with certain of our Named Executive Officers. Such agreements are summarized
below. Such summaries are intended solely as a synopsis of the material terms of such
agreements and are qualified in their entirety by the terms and provisions of such
agreements.
Joel E. Bernstein, M.D. Employment Agreement
In connection with the Merger, we entered into an employment agreement with Dr. Joel
Bernstein, effective on the date of the Merger. The agreement provides that Dr. Bernstein
will serve as President and Chief Executive Officer, or Vice Chairman in the event that
the Board hires a new Chief Executive, for a term of two years. His base salary under the
agreement is $260,000 for the first year of the agreement and $280,000 for the second.
Dr. Bernstein will be eligible to receive bonuses in such amounts and in such form as may
be determined by the Board if awarded by the Board, as well as medical insurance, life
insurance, 401(k) participation and six weeks of vacation annually.
Under the agreement, Dr. Bernstein is allowed to pursue other employment during the
period he is employed by us, provided he devotes at least 60% of his working time to us.
He has also assigned to Winston Labs all patents relating to pharmaceutical products that he
invents following the date of the agreement, except those related to dermatological and
ophthalmic products.
The agreement provides that we may terminate Dr. Bernstein for any reason; however,
if such termination is not due to Cause, death or Disability, we will be required to pay
the following:
| • | the base salary in effect on the date of termination for the twelve months following termination; |
||
| • | life insurance benefits, to the same extent as provided to similarly
situated employees, for the twelve months following termination; |
||
| • | medical insurance continuation coverage under COBRA up to twelve months following termination; and |
||
| • | all benefits not fully vested will vest on the date of termination. |
34
Table of Contents
Dr. Bernstein will not be entitled to any such compensation or benefits if he
breaches any of the covenants in the agreement relating to the protection of confidential
information, non-disclosure, non-competition and non-solicitation. He will also not be
entitled to any compensation or other benefits under the Agreement if his employment is
terminated for Cause.
As used in Dr. Bernstein’s employment agreement, the following terms have the
following definitions:
“Cause” means the following: (i) the conviction of Dr. Bernstein of, or
the entry of a plea of guilty or nolo contendere by Dr. Bernstein to, any
misdemeanor involving moral turpitude or any felony; (ii) fraud, misappropriation
or embezzlement by Dr. Bernstein with respect to the Company or any subsidiary or
affiliate thereof, including without limitation Winston Labs; (iii) Dr. Bernstein’s
willful failure, gross negligence or gross misconduct in the performance of his
assigned duties for the Company or any subsidiary or affiliate thereof, including
without limitation Winston Labs; (iv) Dr. Bernstein’s material breach of a fiduciary
duty to the Company or any subsidiary or affiliate thereof, including without
limitation Winston Labs; (v) any wrongful act or omission of Dr. Bernstein not at the
express direction of the Board of Directors of the Company or any subsidiary or
affiliate thereof, including without limitation Winston Labs, that reflects materially
and adversely on the integrity and reputation for honesty and fair dealing of the
Company or any subsidiary or affiliate thereof, including without limitation
Winston Labs, or has a material detrimental effect on the Company’s financial
condition, position or business, or the financial condition, position or business
of any subsidiary or affiliate thereof, including without limitation Winston Labs; or
(vi) the breach by Dr. Bernstein of any material term of his employment agreement
(provided that in the case of clauses (iii),(iv),(v) and (vi) (but
excluding breaches of Section 6 or 7 (i.e., confidentiality, non-solicitation and
non-competition provisions), the Company shall have provided Dr. Bernstein with
written notice of the acts, breaches or other events that would otherwise
constitute “Cause” thereunder and Dr. Bernstein shall have failed to cure or
remedy such acts, breaches or other events within ten (10) days following receipt
of such notice, and provided further that the failure of the
Company or any subsidiary to achieve any financial objective shall not serve as
the basis for Cause hereunder).
“Disability” means the incapacity of Dr. Bernstein due to physical or
mental illness where Dr. Bernstein has been unable to perform his duties during
the preceding 90 days, or where said incapacity has been determined to exist or
have existed such that he is or was unable to perform his previously assigned
duties, and that such incapacity continued, has continued and/or will continue for
such period of time for at least 90 days during any consecutive 365 day period by
either (i) the liability insurance carrier for the Company or its subsidiaries or
(ii) the concurring opinions of two board certified, licensed physicians (as
selected one by the Company and one by Dr. Bernstein); provided that Dr.
Bernstein shall, within 15 days after the written request of the Company or any
subsidiary or affiliate thereof, including without limitation Winston Labs, submit to a
physical and/or mental examination for purposes of determining Disability.
Scott B. Phillips, M.D. — Severance Agreements
Winston Labs has entered into two separate agreements dated as of October 8, 2003 (the
“Phillips Change in Control Agreement”) and January 26, 2006 (the “Phillips Severance
Letter”, and collectively with the Change in Control Agreement, the “Phillips Severance
Agreements”) with Scott B. Phillips, its Senior Vice President and Chief Scientific
Officer, which together outline the terms upon which Dr. Phillips’ employment with
Winston Labs may be terminated and the conditions upon which certain severance payments will
be made to Dr. Phillips in the event of such termination, including termination of his
employment in connection with a change in control of Winston Labs.
Pursuant to the terms of the Phillips Severance Letter, Winston Labs may terminate
Dr. Phillips’ employment at any time upon thirty (30) days written notice. Dr. Phillips
may terminate his employment with Winston Labs at any time upon fourteen (14) days written
notice. If Dr. Phillips’ employment is terminated by Winston Labs, then Dr. Phillips is
entitled to receive by reason of such termination his base salary and life insurance
benefits in effect at the time of such termination for an additional six (6) months from
the termination date. Any COBRA benefits shall begin at the conclusion of such six
(6) month period. All other benefits that are not fully vested on the termination date
shall cease and be extinguished on such date. If Dr. Phillips terminates his employment
for any reason, all salary and benefits that are not fully-vested, including any and all
unvested bonuses, shall cease and be extinguished on the termination date.
Pursuant to the terms of the Phillips Change in Control Agreement, in the event
Dr. Phillips’ employment with Winston Labs is terminated by Winston Labs following a change in
control of Winston Labs, Dr. Phillips shall be entitled to receive the following severance
compensation (“Change in Control Severance”): (i) a lump-sum severance payment equal to
two times his annual salary at the highest rate in effect at any time prior to such
termination, plus incentive pay in an amount not less than the highest incentive, bonus
or other cash payment made to Dr. Phillips in addition to his salary in any of the three
years immediately preceding the year in which the change in control occurred and (ii) for
a period of twenty-four (24) months following the termination date, welfare benefits (but
excluding stock option, stock purchase, stock appreciation and similar compensatory
benefits) substantially similar to those which Dr. Phillips was entitled to receive
immediately prior to the termination date, reduced to the extent comparable welfare
benefits are actually received by Dr. Phillips from another employer during such period.
35
Table of Contents
Dr. Phillips shall also be entitled to receive Change in Control Severance following
a change in control of Winston Labs, if he terminates his employment following: (i) failure by
Winston Labs to elect or re-elect Dr. Phillips to the position (or a substantially equivalent
position) he held immediately prior to the change in control, or the removal of
Dr. Phillips as a director of Winston Labs if he was a director immediately prior to the
change in control; (ii) a significant adverse change in the nature or scope of
Dr. Phillips’ duties, or a reduction in his base pay, incentive pay or benefits; (iii) a
good faith determination by Dr. Phillips that a change in circumstances (e.g. a change in
the scope of business or Dr. Phillips’ responsibilities) has occurred following a change
in control; (iv) the liquidation, dissolution, merger, consolidation, or reorganization
or sale of substantially all of Winston Labs’s assets, unless the successor assumes all duties
and obligations under the Phillips Change in Control Severance Agreement; (v) the
relocation of Winston Labs’s principal executive offices or of Dr. Phillips’ principal
location of work in excess of 25 miles from the location thereof immediately prior to the
change in control, or the requirement that Dr. Phillips travel away from his office in
the course of discharging his duties to Winston Labs at least 20% more often than was required
immediately prior to the change in control; and (vi) the material breach of the Phillips
Change in Control Agreement by Winston Labs or its successor. Dr. Phillips will not be
entitled to receive Change in Control Severance if his employment is terminated for Cause
or due to Dr. Phillips’ death or permanent disability.
As used in the Phillips Change in Control Agreement, “Cause” means:
| (i) | an intentional act of fraud, embezzlement or theft in connection with Dr.
Phillips’ duties or in the course of his employment with Winston Labs or a
subsidiary thereof; |
||
| (ii) | intentional wrongful damage to the property of Winston Labs or a subsidiary thereof; |
||
| (iii) | intentional wrongful disclosure of secret processes or confidential
information of Winston Labs or a subsidiary thereof; or |
||
| (iv) | intentional wrongful engagement by Dr. Phillips in the management of
any business enterprise that engages in substantial and direct
competition with Winston Labs and such enterprise’s sales of any product
or service competitive with any of Winston Labs’s products or services
amounted to 10% of the net sales of such enterprise and of Winston Labs
for their respective most recently-completed fiscal years. |
Compensation of Directors
The following table provides information regarding compensation of directors for the
year ended December 31, 2009.
| Fees | ||||||||||||
| earned or | ||||||||||||
| Paid in | Option | |||||||||||
| Cash | Awards | Total | ||||||||||
| Name | ($) | ($)(1) | ($) | |||||||||
Robert A. Yolles |
5,000 | 43,830 | 48,830 | |||||||||
Glenn L. Halpryn |
5,000 | 43,830 | 48,830 | |||||||||
Curtis Lockshin Ph.D. |
5,000 | 40,178 | 45,178 | |||||||||
Neal S. Penneys, M.D., Ph.D. |
5,000 | 40,178 | 45,178 | |||||||||
Subbarao Uppaluri, Ph.D. |
5,000 | 40,178 | 45,178 | |||||||||
| (1) | Represents aggregate grant date fair value pursuant to ASC Topic 718
for the respective year for restricted stock options granted as long term incentive
pursuant to the Company’s Omnibus Incentive Plan. |
Each of our non-employee directors currently receives $1,250 per quarter. Directors
do not receive additional compensation for attendance at meetings. Directors who are also
employees do not receive compensation for their service on the Board.
Compensation Committee Interlocks and Insider participation; Compensation Committee
Report
We are a “smaller reporting company” as defined by Item 10(f)(1) of Regulation S-K
and, pursuant to Item 407(g)(2) of Regulation S-K, we are not required to make the
disclosures otherwise required by Item 407(e)(4) and (e)(5) of
Regulation S-K.
36
Table of Contents
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
2009 Equity Compensation Plan Information
| Number of | ||||||||||||
| securities | ||||||||||||
| remaining | ||||||||||||
| available for | ||||||||||||
| future issuance | ||||||||||||
| Number of | Weighted | under | ||||||||||
| securities to | average | equity | ||||||||||
| be issued | exercise price | compensation | ||||||||||
| upon exercise | of | plans | ||||||||||
| of outstanding | outstanding | (excluding | ||||||||||
| options, | options, | securities | ||||||||||
| warrants and | warrants and | reflected in | ||||||||||
| rights | rights | column (a) | ||||||||||
| (a) | (b) | (c) | ||||||||||
Equity compensation plans approved by security holders |
3,041,985 | $ | 0.50 | 6,666,070 | ||||||||
Effective April 1, 2009, the Company’s Board of Directors adopted the Company’s
Omnibus Incentive Plan (the “Plan”). The Plan was established by amending, restating and
merging the Company’s existing Stock Option Plan for Non-Employee directors, and the 1999
Stock Option Plan (collectively, the “Prior Plans”), with and into the Plan. The Plan was
approved by the Company’s shareholders at the Company’s annual meeting held on June 17,
2009.
The Plan provides for a broad range of awards to attract, motivate and retain
qualified and talented employees, directors, consultants and other persons who provide
services to the Company (“Participants”), including stock options, stock appreciation
rights, restricted stock, restricted stock units, performance shares, performance units,
and other stock-based awards and cash-based awards (“Awards”). The Plan promotes the
success and enhances the value of the Company by linking the personal interests of
Participants to those of the Company’s stockholders, and by providing Participants with
an incentive for outstanding performance.
Awards under the Plan will be determined by a committee of the Board of Directors
(the “Committee”), the members of which are selected by the Board of Directors. Currently
the Plan provides that the Compensation Committee will serve as the administrator. The
number of shares of Company common stock (“Shares”) as to which an Award is granted and
to whom any Award is granted shall be determined by the Committee, subject to the
provisions of the Plan. Awards may be made exercisable or settled at such prices and may
be made terminable under such terms as are established by the Committee, to the extent
not otherwise inconsistent with the terms of the Plan.
Prior to the merger of the Prior Plans with and into the Plan, there were 9,708,055
Shares reserved for issuance for awards under the Prior Plans, including 3,196,487 Shares
reserved for outstanding awards, and 5,922,466 Shares for future awards. Upon the merger
of the Prior Plans with and into the Plan, effective April 1, 2009, there was no change
to such numbers, with 9,708,055 Shares reserved for issuance for Awards under the Plan,
of which 3,196,487 Shares were reserved for outstanding Awards, and 5,922,466 Shares for
future Awards.
On April 7, 2009, pursuant to the terms of the Plan, the Compensation Committee of
the Board of Directors granted 267,000 non-qualified stock options to purchase Shares to
employees of the Company, including 225,000 options to key officers of the Company. All
of the options expire on April 7, 2019, vest in five equal installments commencing
April 7, 2010, and have an exercise price of $1.53, as determined by the Compensation
Committee of the Board of Directors in accordance with the terms of the Plan.
On September 25, 2009 pursuant to the terms of the Plan, the Compensation committee
of the Board of Directors granted 142,500 non-qualified stock options to purchase shares
to non-employee directors of the Company. All of the options expire on September 25,
2019, vest in five equal installments commencing September 25, 2010 and have an exercise
price of $1.36 as determined by the Compensation Committee of the Board of Directors in
accordance with the terms of the Plan.
37
Table of Contents
Voting Securities and Principal Stockholders
Prior to the 8-1 reverse stock split described below, our voting securities consisted of
our common stock, par value $0.001 per share, of which 440,851,441 shares were
outstanding, our Series A Convertible Preferred Stock, of which 101,849 shares were
outstanding, and our Series B Convertible Preferred Stock, of which 73,332 shares were
outstanding. Each share of preferred stock was convertible into 1,000 shares of common
stock. The holders of our voting securities are entitled to one vote for each outstanding
share of common stock, including outstanding shares of preferred stock on an as-converted
basis, on all matters submitted to our stockholders.
On December 15, 2008 the Company amended its Certificate of Incorporation to provide
for the reduction of the total number of issued and outstanding shares of the Company’s
common stock, par value $.001 per share (“Common Stock”) and its preferred stock, par
value $.001 per share (“Preferred Stock”), by exchanging each eight (8) shares of such
issued and outstanding shares of Common Stock and Preferred Stock for one (1) share of
Common Stock or Preferred Stock, respectively.
On September 24, 2009, each outstanding share of Company Series A Convertible
Preferred Stock (12,730) and Series B Convertible Preferred Stock (9,157) automatically
converted into 1,000 fully-paid, non-assessable shares of the Company’s common stock, par
value $.001 per share. In addition, in connection with such conversion, each outstanding
warrant to purchase shares of Series A Convertible Preferred Stock automatically
converted into the right to acquire 1,000 shares of Common Stock upon the exercise of
such warrant, at an exercise price of $0.39 per share of Common Stock.
As of March 1, 2010, our voting securities consist of our common stock, par value
$0.001 per share, of which 77,408,893 shares are outstanding. The following tables
contain information regarding record ownership of our common stock as or March 1, 2010
held by:
| • | persons who own beneficially more than 5% of our outstanding voting securities, |
||
| • | our directors, |
||
| • | named executive officers, and |
||
| • | all of our directors and officers as a group. |
38
Table of Contents
| Management and Directors: | Shares Beneficially Owned (14) | Percentage Ownership | ||||||
Joel E. Bernstein, M.D. (1) |
26,498,432 | 29.63 | % | |||||
Scott B. Phillips, M.D. (2) |
1,810,433 | 2.02 | % | |||||
David Starr (3) |
75,000 | * | ||||||
Curtis Lockshin, Ph.D. (4) |
28,750 | * | ||||||
Robert A. Yolles (5) |
398,665 | * | ||||||
Glenn L. Halpryn (6) |
384,781 | * | ||||||
Neal Penneys, M.D., Ph.D. (7) |
439,362 | * | ||||||
Subbarao Uppaluri, Ph.D. (8) |
326,538 | * | ||||||
All officers and directors
As a group (8 people) |
29,961,961 | 33.51 | % | |||||
| 5% Stockholders | Shares Beneficially Owned | Percentage Ownership | ||||||
Frost Gamma Investments (9), (10) |
26,574,659 | 29.72 | % | |||||
Jeffrey Bernstein (11) |
5,170,824 | 5.78 | % | |||||
David Bernstein (12) |
4,982,129 | 5.57 | % | |||||
Rebecca Zelken (13) |
4,983,829 | 5.57 | % | |||||
| * | Less than 1%. |
|
| (1) | Includes 761,913 shares of common stock underlying options. Also includes 12,709,386 shares
of common stock that are beneficially owned by Dr. Bernstein’s wife, with respect to which
Dr. Bernstein disclaims any beneficial ownership. |
|
| (2) | Includes 1,148,776 shares of common stock underlying options. |
|
| (3) | Represents 75,000 shares of common stock underlying options. |
|
| (4) | Includes 27,500 shares of common stock underlying options. |
|
| (5) | Includes 311,315 shares of common stock underlying options. Also includes 4,350 shares of common stock that
are beneficially owned by Mr. Yolles’s wife, with respect to which Mr. Yolles disclaims any beneficial ownership. |
|
| (6) | Includes 30,000 shares of common stock underlying options. |
|
| (7) | Includes 27,500 shares of common stock underlying options. |
|
| (8) | Includes 27,500 shares of common stock underlying options and 89,589 shares of common stock underlying
warrants. |
|
| (9) | Includes 8,779,797 shares of common stock underlying warrants. |
|
| (10) | As the sole trustee of the Frost Gamma Investment Trust, Dr. Phillip Frost may be deemed the beneficial owner
of all shares owned by the trust by virtue that his power to vote or direct the vote of such shares or to dispose or
direct the disposition of such shares owned by the trust. |
39
Table of Contents
| (11) | Includes 250 and 1,500 shares of common stock that are beneficially owned by Mr. Bernstein’s
wife and his children, respectively, with respect to which, Mr. Bernstein disclaims any beneficial
ownership. Jeffrey Bernstein is the son of Dr. Joel E. Bernstein. |
|
| (12) | Includes 250 and 500 shares of common stock that are beneficially owned by Mr. Bernstein’s wife
and his child, respectively, with respect to which, Mr. Bernstein disclaims any beneficial ownership.
David Bernstein is the son of Dr. Joel E. Bernstein. |
|
| (13) | Includes 950 and 1,500 shares of common stock that are beneficially owned by Mrs. Zelken’s
husband and her children, respectively, with respect to which, Mrs. Zelken disclaims any beneficial
ownership. Rebecca Zelken is the daughter of Dr. Joel E. Bernstein. |
|
| (14) | On February 12, 2010, Pharmaceutical Financial Syndicate, LLC (“PFS”), whose members include Dr.
Joel E. Bernstein, the President and Chief Executive Officer and Robert A. Yolles and Neal S. Penneys,
directors of the Company, executed a letter of intent (“LOI”) with Frost Gamma Investments Trust,
Subbarao Uppaluri, a director of the company, and certain other shareholders of the Company
(collectively, the “Frost Group”), to acquire 100% of the Company’s capital stock (the “Acquisition”)
beneficially owned by all of the members of the Frost Group consisting of an aggregate of 18,399,271
outstanding shares of common stock and warrants to purchase an aggregate of 8,958,975 shares of common
stock (collectively, the “Acquired Securities”). As of the date of this filing, the Acquisition has
not taken place and its effect on the shares beneficially owned is not reflected in the table above. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Certain Relationships and Related Person Transactions
PFS Acquisition
On February 12, 2010, Pharmaceutical Financial Syndicate, LLC (“PFS”), whose members
include Dr. Joel E. Bernstein, the President and Chief Executive Officer and Robert A.
Yolles and Neal S. Penneys, directors of the Company, executed a letter of intent (“LOI”)
with Frost Gamma Investments Trust, Subbarao Uppaluri, a director of the company, and
certain other shareholders of the Company (collectively, the “Frost Group”), to acquire
100% of the Company’s capital stock (the “Acquisition”) beneficially owned by all of the
members of the Frost Group consisting of an aggregate of 18,399,271 outstanding shares of
common stock and warrants to purchase an aggregate of 8,958,975 shares of common stock
(collectively, the “Acquired Securities”).
As provided in the LOI, consummation of the Acquisition is subject to a number of
conditions, including the parties entering into a definitive acquisition agreement. The
LOI also provides that simultaneous with the completion of the Acquisition, three of the
Company’s directors, namely Subbarao Uppaluri, Glenn Halpryn and Curtis Lockshin, shall
resign as directors of the Company, and each of Messrs. Uppaluri, Halpryn and Lockshin
have advised the Company that they have consented to resign upon completion of the
Acquisition. Upon completion of the Acquisition, as the manager of PFS, Dr. Bernstein
would be deemed to be the beneficial owner of all of the Acquired Securities, however Dr.
Bernstein intends to disclaim beneficial ownership of those Acquired Securities in which
he does not have a pecuniary interest through PFS.
Opko License
On September 19, 2007, Winston Labs entered into an exclusive technology license
agreement with Opko Ophthalmologics, LLC (“OPKO”). Under the terms of the license
agreement, Winston Labs granted OPKO an exclusive license to the proprietary rights of
certain products (pharmaceutical compositions or preparations containing the active
ingredient civamide in formulations suitable for use in the therapeutic or preventative
treatment of ophthalmic conditions in humans). In exchange, OPKO paid Winston Labs a
license fee of $100,000 and is required to pay a 10% royalty on sales of the product. In
addition, the agreement requires OPKO to pay Winston Labs a non-refundable payment of
$5,000,000 upon approval of a marketing authorization by OPKO on the product described in
the agreement. In addition, under the terms of the agreement, OPKO and Winston Labs
agreed to equally share the cost related to manufacturing and clinical supplies of
Civamide Nasal solution. During 2009 and 2008, OPKO reimbursed Winston Labs approximately
$111,539 and $80,000, respectively for such costs. In addition, the agreement calls for
OPKO to reimburse Winston for certain legal expenses Winston Labs has incurred related to
keratoconjunctivitis. In 2009 and 2008, approximately $38,800 and $38,000, respectively,
of legal fees were billed to OPKO, of which approximately $7,000 and $38,000 is included
in related party receivable on the Consolidated Balance Sheets as of December 31, 2009
and 2008. Subsequent to December 31, 2009 and 2008, respectively, OPKO paid $7,000 and
$38,000 of the balance due. Phillip Frost, M.D. is the Chairman and Chief Executive
Officer of OPKO’s parent company, Opko Health, Inc. (“Opko Health”), and as of December
31, 2009 was the beneficial owner of more than 50% of Opko Health’s common stock. As of
December 31, 2009, Dr. Frost is also the indirect beneficial owner of 26,574,659 shares
of the Company’s capital
stock on a fully diluted basis (29.7% of the total issued and outstanding shares),
including 8,779,797 shares of common stock underlying warrants. Furthermore, Subbarao
Uppaluri, Ph.D., a director of the Company and the Senior Vice President — Chief
Financial Officer of Opko Health, is the beneficial owner of 326,538 shares of the
Company’s capital stock on a fully-diluted basis, including 27,500 shares of common stock
underlying options and 89,589 shares of common stock underlying warrants.
40
Table of Contents
On February 24, 2010, the Company received communications from Opko that it was
terminating the license agreement effective May 24, 2010. As a result, all of Opko’s
rights to certain products, as defined in the agreement, will revert back to the Company.
Elorac License
On August 14, 2007, Winston Labs entered into an exclusive technology license
agreement with Exopharma, Inc., now known as Elorac, Inc. (“Elorac”). Robert Yolles, a
director of the Company is also a director of Elorac. In addition, Elorac is an entity
that is controlled by Dr. Bernstein, CEO of Winston Labs and the Company. Under the terms
of the license agreement, Winston Labs granted Elorac an exclusive license to the
proprietary rights of certain products (£ 0.025% civamide with the stated
indication of psoriasis of the skin). In exchange, Elorac paid Winston Labs a license fee
of $100,000 and is required to pay a 9% royalty on sales of the product. In addition, the
agreement requires Elorac to pay Winston Labs a non-refundable payment of $250,000 upon
approval of a Marketing Authorization by Elorac on the product(s) described in the
agreement. On October 27, 2008 Winston Labs and Elorac mutually terminated the above
license agreement. As a result of this mutual termination, Winston Labs paid Elorac
$105,000 in November 2007, in exchange for Winston Labs retaining all the proprietary
rights under the original agreement. Since inception, Elorac has been located in the same
offices as Winston Labs and therefore has shared in certain of Winston Labs expenses such
as rent, utilities, internet usage, etc. The amount of Elorac’s share of such expenses is
based on various allocation factors related to a particular expense. Winston Labs has
received approximately $162,977 and $128,323 in 2009 and in 2008, respectively for such
services, which are included as a reduction of Winston Labs expenses on the Consolidated
Statement of Operations. Amounts due from Elorac of $2,601 and $0 as of December 31, 2009
and 2008, respectively, are included in related party receivables in the Company’s
Consolidated Balance Sheets.
Gideon Pharmaceuticals, Inc.
Gideon Pharmaceuticals, Inc. (“Gideon”) is a corporation whose chairman and sole
director is Joel E. Bernstein, M.D., president and CEO of the Company. Gideon reimburses
the Company for certain expenses that the Company incurs on behalf of Gideon. Amounts
due from Gideon of $12,675 and $0 as of December 31, 2009 and 2008, respectively, are
included in related party receivable on the Consolidated Balance Sheets.
Sirius (DUSA) License
On January 30, 2006, Winston Labs licensed to Sirius Laboratories, Inc., a company
founded by Dr. Bernstein, the rights to market products containing anthralin owned by
Winston Labs, including a marketed 1% anthralin cream trade name Psoriatec® . The
license had a two-year term which expired on January 31, 2008 and provided for the
following key terms: (i) a 25% royalty on net sales; (ii) a $300,000 minimum royalty; and
(iii) a $750,000 purchase option.
This agreement was assigned by Sirius to DUSA Pharmaceuticals, Inc. following DUSA’s
purchase of Sirius. This license had been extended until September 30, 2008 by mutual
written consent of the parties and the extension provides for continuing of the 25%
royalty on net sales but eliminates the minimum royalty and purchase option. This
agreement expired on September 30, 2008. Under the technology license agreement, Winston
Labs recorded royalty revenue of $0 and $34,861 for the years ended December 31, 2009 and
2008, respectively. In July 2008, the Company learned that DUSA had breached a license
agreement with Winston Labs. Winston Labs initiated arbitration in October 2008 to
recover the damages caused by this breach. In June 2009, DUSA settled this matter by
agreeing to pay $75,000 in damages. This settlement is included in other income in the
Consolidated Statements of Operations.
Packer’s-Pine Corporation
In 2006 and 2007, Winston Labs assisted Packer’s-Pine Corporation (“Packer’s”) with
distribution of certain personal care products and administration. Packer’s is a
corporation whose chairman is Dr. Bernstein, president and CEO of the Company and two
directors of the Company served on the board of directors of Packer’s. Under this
arrangement, Packer’s reimbursed Winston Labs for direct costs. In 2008, Packer’s merged
into Elorac.
41
Table of Contents
Consulting Agreement with Jeffrey Bernstein
On September 30, 2007, Dr. Jeffrey Bernstein resigned as Senior Vice President and
Chief Operating Officer of Winston Labs, and entered into a consulting agreement with
that company, effective as of October 1, 2007. Dr. Bernstein’s responsibilities as set
forth in his consulting agreement include assisting with the Merger, interfacing with
various of Winston Labs’s service providers, aiding in the completion of audits of
Winston Labs financial statements for fiscal years 2006 and 2007,
and working with Winston Labs’s new Chief Financial Officer, David Starr. Pursuant to the
terms of his consulting agreement, Winston Labs agreed to compensate Dr. Bernstein at a
rate of $150 per hour. Further, Winston Labs agreed to reimburse Dr. Bernstein for all
reasonable out-of-pocket expenses incurred in the course of his services to Winston Labs,
including, but not limited to, reasonable expenses related to travel, telephone, postage,
and office supplies. The consulting agreement is terminable by either Dr. Jeffrey
Bernstein or by Winston upon thirty (30) days notice to the other party. Dr. Jeffrey
Bernstein is the son of Dr. Joel E. Bernstein, the President and Chief Executive Officer
of the Company. Winston incurred $12,213 and $21,488 of expenses under the consulting
agreement for years ended December 31, 2009 and 2008, respectively, of which
approximately $7,900 was outstanding as of December 31, 2009 and is included in accrued
expenses and other current liabilities on the Consolidated Balance Sheets.
Independence of Directors
The Board of Directors has determined that the following individuals are independent
as defined by the listing standards of the NYSE Amex Market and the NASDAQ Stock Market:
Messrs. Yolles, and Halpryn and Drs. Lockshin, Penneys and Uppaluri. In reaching this
conclusion, the Board of Directors considered family and employment relationships that
such directors have with the members of the Board of Directors.
Item 14. Principal Accounting Fees and Services
Pre-Approval Policies and Procedures
Pursuant to its charter, the Audit Committee has the sole authority to select,
evaluate and replace the Company’s independent registered public accountants (subject, if
applicable, to stockholder ratification). The Audit Committee is directly responsible for
the compensation and oversight of the work of the Company’s independent registered public
accountants (including resolution of disagreements between management and the Company’s
independent registered public accountants regarding financial reporting) for the purpose
of preparing or issuing an audit report or related work. The Company’s independent
registered public accountants are engaged by, and report directly to, the Audit
Committee.
The Audit Committee pre-approves all auditing services and permitted non-audit
services (including the fees and terms thereof) to be performed for the Company by its
independent registered public accountants, subject to the de minimis exceptions for
non-audit services described in Section 10A(i)(1)(B) of the Exchange Act and SEC
Rule 2-01(c)(7)(i)(C) of Regulation S-X, all of which are approved by the Audit Committee
prior to the completion of the audit. In the event pre-approval for such auditing
services and permitted non-audit services cannot be obtained as a result of inherent time
constraints in the matter for which such services are required, the Chairman of the Audit
Committee has been granted the authority to pre-approve such services provided that the
Chairman of the Audit Committee presents for ratification such pre-approval to the Audit
Committee at its next scheduled meeting. The Audit Committee has complied with the
procedures set forth above, and has otherwise complied with the provisions of its
charter.
Audit Fees
The aggregate fees incurred by the Company and its consolidated subsidiaries for
fiscal years ended December 31, 2009 and 2008 for professional services rendered by
McGladrey & Pullen LLP in connection with (i) the audit of the Company’s annual financial
statements, (ii) the reviews of the financial statements included in the Company’s
Quarterly Reports on Form 10-Q for the quarters ended March 31, 2009, June 30, 2009,
September 30, 2009 and September 30, 2008 and (iii) a consent issued in connection with
the Company’s Current Report on Form 8-K as filed with the SEC on October 1, 2008, were
$138,062 and $148,125, respectively.
Audit-Related Fees
The Company did not incur fees for fiscal years ended December 31, 2009 and 2008 for
assurance and related services rendered by McGladrey & Pullen LLP in connection with
(i) the performance of the audit or review of the Company’s financial statements,
including 401(k) plan audits, (ii) due diligence assistance and (iii) assistance with
compliance with Section 404 of the Sarbanes-Oxley Act of 2002.
42
Table of Contents
Tax Fees
The aggregate fees incurred by the Company for fiscal years ended December 31, 2009
and 2008 for professional services rendered by RSM McGladrey, Inc. for tax compliance,
tax advice and tax planning were $20,000 and $54,750, respectively. These services
consisted of preparing the Company’s tax returns.
All Other Fees
The Company did not incur any other fees for fiscal years ended December 31, 2009
and 2008 for services rendered by RSM McGladrey, Inc. or McGladrey & Pullen LLP.
PART IV
Item 15. Exhibits, Financial Statement Schedules
The following are filed as part of this Annual Report on Form 10-K:
| (1) | Financial Statements: For a list of financial statements
which are filed as part of this Form 10-K, See Item 8.
Financial Statements and Supplementary Data on Page 28. |
|
| (2) | Exhibits |
| 2.1 | Merger Agreement and Plan of Reorganization
dated as of November 13, 2007 by and among
Getting Ready Corporation, Winston
Laboratories, Inc. and Winston Acquisition
Corp. (filed as Exhibit 2.1 to the Company’s
Current Report on Form 8-K dated November
13, 2007 and incorporated herein by
reference). |
|||
| 2.2 | First Amendment to Merger Agreement and Plan
of Reorganization dated as of May 30, 2008
by and among Winston Laboratories, Inc., a
Delaware corporation, Getting Ready
Corporation, a Delaware corporation, and
Winston Acquisition Corp., a Delaware
corporation (filed as Exhibit 2.1 to the
Company’s Current Report on Form 8-K dated
May 30, 2008 and incorporated herein by
reference). |
|||
| 2.3 | Second Amendment to Merger Agreement and
Plan of Reorganization dated as of June 23,
2008 by and among Winston Laboratories,
Inc., a Delaware corporation, Getting Ready
Corporation, a Delaware corporation, and
Winston Acquisition Corp., a Delaware
corporation (filed as Exhibit 2.1 to the
Company’s Current Report on Form 8-K dated
June 23, 2008 and incorporated herein by
reference). |
|||
| 3.1 | Certificate of Incorporation (filed as
Exhibit 3.1A to the Company’s Registration
Statement on Form SB-2 dated September 15,
2004 and incorporated herein by reference). |
|||
| 3.2 | Certificate of Amendment of Certificate of
Incorporation (filed as Exhibit 3.1B to the
Company’s Registration Statement on
Form SB-2 dated September 15, 2004 and
incorporated herein by reference). |
|||
| 3.3 | Certificate of Amendment of Certificate of
Incorporation (filed as Exhibit 1 to the
Company’s Form PRE14C dated December 7, 2007
and incorporated herein by reference). |
|||
| 3.4 | Certificate of Amendment to Certificate of
Incorporation (filed as Annex A to the
Company’s DEF 14C dated September 22, 2008
and incorporated herein by reference). |
|||
| 3.5 | Certificate of Designation, Preferences and
Rights of Series A Convertible Preferred
Stock of Getting Ready Corporation (filed as
Exhibit 3.5 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 3.6 | Certificate of Designation, Preferences and
Rights of Series B Convertible Preferred
Stock of Getting Ready Corporation (filed as
Exhibit 3.6 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 3.7 | Certificate of Amendment to Certificate of
Incorporation (form of amendment filed in
the Company’s DEF 14C dated October 22, 2008
and incorporated herein by reference). |
|||
| 3.8 | Certificate of Amendment to Certificate of
Incorporation (form of amendment filed in
the Company’s DEF 14C dated November 25,
2008 and incorporated herein by reference). |
43
Table of Contents
| 3.9 | By-laws (filed as Exhibit 3.7 to the
Company’s Current Report on Form 8-K as
filed with the SEC on October 1, 2008 and
incorporated herein by reference). |
|||
| 4.1 | Form of Common Stock Certificate (filed as
Exhibit 4.1 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 4.2 | Form of Warrant to Purchase Series A
Convertible Preferred Stock (filed as
Exhibit 4.2 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 4.3 | Form of Lockup Agreement (filed as
Exhibit 4.3 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 10.1 | + | License Agreement dated October 30, 2008 by
and between Winston Laboratories, Inc. and
sanofi-aventis Canada Inc. (filed as
Exhibit 10.1 to the Company’s Quarterly
Report on Form 10-Q for the quarter ended
September 30, 2008 and incorporated herein
by reference). |
||
| 10.2 | Voting Agreement dated as of September 25,
2008 between Getting Ready Corporation and
certain stockholders of the Company (filed
as Exhibit 10.1 to the Company’s Current
Report on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 10.3 | Employment Agreement dated as of
September 25, 2008 between Getting Ready
Corporation and Joel E. Bernstein, M.D
(filed as Exhibit 10.2 to the Company’s
Current Report on Form 8-K as filed with the
SEC on October 1, 2008 and incorporated
herein by reference). |
|||
| 10.4 | Secrecy, Invention and Non-Competition
Agreement dated as of October 5, 2007 by and
between Winston Laboratories, Inc. and Joel
E. Bernstein, M.D. (filed as Exhibit 10.3 to
the Company’s Current Report on Form 8-K as
filed with the SEC on October 1, 2008 and
incorporated herein by reference). |
|||
| 10.5 | Employment Letter Agreement dated October 3,
2007 between Winston Laboratories, Inc. and
David Starr (filed as Exhibit 10.4 to the
Company’s Current Report on Form 8-K as
filed with the SEC on October 1, 2008 and
incorporated herein by reference). |
|||
| 10.6 | Secrecy, Invention and Non-Competition
Agreement dated as of October 3, 2007 by and
between Winston Laboratories, Inc. and David
Starr (filed as Exhibit 10.5 to the
Company’s Current Report on Form 8-K as
filed with the SEC on October 1, 2008 and
incorporated herein by reference). |
|||
| 10.7 | Severance Agreement dated as of October 8,
2003 between Winston Laboratories, Inc. and
Scott B. Phillips, M.D. (filed as
Exhibit 10.6 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 10.8 | General Severance Letter Agreement dated
January 26, 2006 between Winston
Laboratories, Inc. and Scott B. Phillips,
M.D. (filed as Exhibit 10.7 to the Company’s
Current Report on Form 8-K as filed with the
SEC on October 1, 2008 and incorporated
herein by reference). |
|||
| 10.9 | Secrecy, Invention and Non-Competition
Agreement dated as of October 5, 2007 by and
between Winston Laboratories, Inc. and Scott
B. Phillips, M.D. (filed as Exhibit 10.8 to
the Company’s Current Report on Form 8-K as
filed with the SEC on October 1, 2008 and
incorporated herein by reference). |
|||
| 10.10 | Consultation Agreement dated September 30,
2007 between Winston Laboratories, Inc. and
Jeffrey R. Bernstein, Ph.D. (filed as
Exhibit 10.9 to the Company’s Current Report
on Form 8-K as filed with the SEC on
October 1, 2008 and incorporated herein by
reference). |
|||
| 10.11 | Winston Pharmaceuticals Inc. Omnibus
Inventive Plan (filed as Exhibit 10.1 to the
Company’s Current Report on Form 8-K dated
April 17, 2009 and incorporated herein by
reference). |
|||
| 10.12 | Form of Nonqualified Stock Option Agreement
(filed as Exhibit 10.2 to the Company’s
Current Report on Form 8-K dated April 17,
2009 and incorporated herein by reference). |
|||
| 10.13 | Form of Nonqualified Stock Option Agreement
(filed as Exhibit 10.1 to the Company’s
Quarterly Report on Form 10-Q for the
quarter ended September 30, 2009 and
incorporated herein by reference). |
44
Table of Contents
| 10.14 | Letter of Intent Between Pharmaceutical
Financial Syndicate, LLC, Frost Gamma
Investments Trust, Subbarao Uppaluri, Steven
D. Rubin and Jane Hsiao, Ph.D. (filed as
Exhibit 10.1 to the Company’s Current Report
on Form 8-K dated February 12, 2010 and
incorporated herein by reference). |
|||
| 21.1 | * | Subsidiaries of Winston Pharmaceuticals, Inc. |
||
| 31.1 | * | Certification of the Company’s President and
Chief Executive Officer, Joel E. Bernstein,
M.D., pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002. |
||
| 31.2 | * | Certification of the Company’s Vice
President and Chief Financial Officer, David
Starr, pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002. |
||
| 32.1 | * | Certification of the Company’s President and
Chief Executive Officer, Joel E. Bernstein,
M.D., pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002. |
||
| 32.2 | * | Certification of the Company’s Vice
President and Chief Financial Officer, David
Starr, pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002. |
| * | filed herewith. |
|
| + | Portions of this exhibit have been omitted pursuant to a request for
confidential treatment under Rule 24b-2 of the Securities Exchange Act
of 1934, amended, and the omitted material has been separately filed
with the Securities and Exchange Commission. |
45
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act
of 1934, as amended, the registrant has duly caused this report to be signed on its
behalf by the undersigned, thereunto duly authorized.
| Dated: March 5, 2010 | Winston Pharmaceuticals, Inc. | |||||
| (Registrant) | ||||||
| By: | /s/ Joel E. Bernstein
|
|||||
| President and Chief Executive Officer | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report
has been signed below by the following persons on behalf of the registrant and in the
capacities and on the dates indicated.
Dated: March 5, 2010
|
/s/ Joel E. Bernstein
|
|||
| President, Chief Executive Officer | ||||
| (Principal Executive Officer) | ||||
Dated: March 5, 2010
|
/s/ David Starr
|
|||
| Vice President, Chief Financial Officer | ||||
| (Principal Accounting Officer and | ||||
| Principal Financial Officer) | ||||
Dated: March 5, 2010
|
/s/ Robert A. Yolles
|
|||
| Director, Chairman of the Board |
46
Table of Contents
Dated: March
5, 2010
|
/s/ Glenn L. Halpryn
|
|||
| Director | ||||
Dated: March
5, 2010
|
/s/ Curtis Lockshin
|
|||
| Director | ||||
Dated: March
5, 2010
|
/s/ Neal S. Penneys
|
|||
| Director | ||||
Dated: March
5, 2010
|
/s/ Scott B. Phillips
|
|||
| Senior Vice President, | ||||
| Clinical Affairs and Director | ||||
Dated: March
5, 2010
|
/s/ Subbarao Uppaluri
|
|||
| Director |
47
Table of Contents
Index to Financial Statements
Winston Pharmaceuticals, Inc. and Subsidiaries
| Page | ||
Audited Financial Statements as of and for the Years Ended December 31, 2009 and 2008 |
||
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-7 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Winston Pharmaceuticals, Inc. and
Subsidiaries
We have audited the accompanying consolidated balance sheets of Winston
Pharmaceuticals, Inc. and Subsidiaries (the “Company”) as of December 31, 2009 and 2008,
and the related consolidated statements of operations, stockholders’ equity and cash
flows for the years then ended. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these financial
statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan and
perform the audit to obtain reasonable assurance about whether the financial statements
are free of material misstatement. An audit includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements. An audit also
includes assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement presentation. We
believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present
fairly, in all material respects, the financial position of Winston Pharmaceuticals, Inc.
and Subsidiaries as of December 31, 2009 and 2008, and the results of their operations
and their cash flows for the years then ended, in conformity with U.S. generally accepted
accounting principles.
We were not engaged to examine management’s assessment of the effectiveness of the
Company’s internal control over financial reporting as of December 31, 2009, included in
the accompanying Controls and Procedures and, accordingly, we do not express an opinion
thereon.
The accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. As discussed in Note 1 to the financial statements,
the Company has suffered significant recurring losses from operations and cash reserves
have fallen below recent cash flows from operations. This raises substantial doubt about
the Company’s ability to continue as a going concern. Management’s plans in regard to
these matters are also described in Note 1. The financial statements do not include any
adjustments that might result from the outcome of this uncertainty.
/s/ McGladrey & Pullen, LLP
|
||
March
5, 2010 |
F-2
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2009 AND 2008
| December 31, | December 31, | |||||||
| 2009 | 2008 | |||||||
ASSETS |
||||||||
CURRENT ASSETS |
||||||||
Cash and cash equivalents |
$ | 1,562,848 | $ | 5,626,913 | ||||
Accounts receivable |
15,653 | 17,498 | ||||||
Related party receivable |
22,395 | 38,142 | ||||||
Prepaid and other current assets |
43,944 | 68,465 | ||||||
Total current assets |
1,644,840 | 5,751,018 | ||||||
EQUIPMENT, net of accumulated depreciation of $141,433 at
December 31, 2009 and $161,907 at December 31, 2008 |
13,760 | 18,823 | ||||||
INTANGIBLE ASSETS, NET |
17,455 | 21,540 | ||||||
TOTAL ASSETS |
$ | 1,676,055 | $ | 5,791,381 | ||||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
CURRENT LIABILITIES |
||||||||
Accounts payable |
$ | 724,140 | $ | 1,058,980 | ||||
Accrued expenses and other current liabilities |
146,638 | 233,611 | ||||||
Unearned revenue — current portion |
316,706 | 1,266,825 | ||||||
Total current liabilities |
1,187,484 | 2,559,416 | ||||||
Unearned revenue — long-term portion |
— | 316,706 | ||||||
Total liabilities |
1,187,484 | 2,876,122 | ||||||
Commitments and Contingencies |
||||||||
Stockholders’ equity |
||||||||
Preferred Stock, $.001 par value, 250,000,000 shares authorized |
||||||||
Series A, Convertible 0 and 12,730 shares issued and
outstanding at December 31, 2009 and at December 31, 2008,
respectively |
— | 13 | ||||||
Series B, Convertible 0 and 9,157 shares issued and
outstanding at December 31, 2009 and December 31, 2008,
respectively |
— | 9 | ||||||
Common stock, $.001 par value, 900,000,000 shares authorized
77,408,893 and 55,106,364 shares issued and outstanding at
December 31, 2009 and December 31, 2008, respectively |
77,409 | 55,106 | ||||||
Additional paid-in capital |
49,794,260 | 49,587,913 | ||||||
Accumulated deficit |
(49,383,098 | ) | (46,727,782 | ) | ||||
Total stockholders’ equity |
488,571 | 2,915,259 | ||||||
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
$ | 1,676,055 | $ | 5,791,381 | ||||
See accompanying notes
F-3
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF OPERATIONS
Years Ended December 31, 2009 and 2008
| 2009 | 2008 | |||||||
REVENUE |
||||||||
License revenue |
$ | 1,266,825 | $ | 316,706 | ||||
Royalty revenue |
127,762 | 135,836 | ||||||
| 1,394,587 | 452,542 | |||||||
EXPENSES |
||||||||
Research and development |
2,062,933 | 3,496,150 | ||||||
General and administrative |
2,063,879 | 2,182,012 | ||||||
Depreciation and amortization |
9,007 | 8,651 | ||||||
Total operating expenses |
4,135,819 | 5,686,813 | ||||||
Loss from operations |
(2,741,232 | ) | (5,234,271 | ) | ||||
Interest income |
10,487 | 90,614 | ||||||
Other income |
75,429 | 1,911 | ||||||
| 85,916 | 92,525 | |||||||
Loss before income taxes |
(2,655,316 | ) | (5,141,746 | ) | ||||
Income Taxes |
||||||||
Current |
— | — | ||||||
Deferred |
— | — | ||||||
| — | — | |||||||
NET LOSS |
$ | (2,655,316 | ) | $ | (5,141,746 | ) | ||
Loss per share, basic and diluted |
$ | (0.04 | ) | $ | (0.10 | ) | ||
Weighted average number of shares outstanding basic and diluted |
61,258,364 | 53,422,158 | ||||||
See accompanying notes
F-4
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Years Ended December 31, 2009 and 2008
| Additional | Total | |||||||||||||||||||||||||||||||||||
| Common Stock | Preferred Stock A | Preferred Stock B | Paid-in | Accumulated | Stockholders’ | |||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Deficit | Equity (Deficit) | ||||||||||||||||||||||||||||
Balance at January 1, 2008 |
52,814,818 | $ | 52,814 | — | — | — | — | $ | 40,573,523 | $ | (41,586,036 | ) | $ | (959,699 | ) | |||||||||||||||||||||
Reclassification of Series A
Convertible Preferred Stock
(net of cost of $194,648) |
12,731 | $ | 13 | 4,805,339 | 4,805,352 | |||||||||||||||||||||||||||||||
Issuance of Series B
Convertible Preferred Shares
(net of cost of $3,211) |
9,166 | $ | 9 | 3,996,780 | 3,996,789 | |||||||||||||||||||||||||||||||
Merger with Getting Ready
Corporation (net of cost of
$250,000) |
2,291,612 | 2,292 | 233,615 | 235,907 | ||||||||||||||||||||||||||||||||
Redemption of fractional shares |
(66 | ) | (1 | ) | (9 | ) | (21,344 | ) | (21,344 | ) | ||||||||||||||||||||||||||
Net loss |
(5,141,746 | ) | (5,141,746 | ) | ||||||||||||||||||||||||||||||||
Balance at December 31, 2008 |
55,106,364 | 55,106 | 12,730 | 13 | 9,157 | 9 | 49,587,913 | (46,727,782 | ) | 2,915,259 | ||||||||||||||||||||||||||
Issuance of common stock upon
exercise of stock options |
415,529 | 416 | 134,014 | 134,430 | ||||||||||||||||||||||||||||||||
Conversion of Series A
Convertible Preferred Shares
and Series B Convertible
Preferred Shares to common
shares |
21,887,000 | 21,887 | (12,730 | ) | (13 | ) | (9,157 | ) | (9 | ) | (21,865 | ) | — | |||||||||||||||||||||||
Compensation with respect to
employee and non-employee
stock option grants |
94,198 | 94,198 | ||||||||||||||||||||||||||||||||||
Net loss |
$ | (2,655,316 | ) | (2,655,316 | ) | |||||||||||||||||||||||||||||||
Balance at December 31, 2009 |
77,408,893 | $ | 77,409 | — | $ | — | — | $ | — | $ | 49,794,260 | $ | (49,383,098 | ) | $ | 488,571 | ||||||||||||||||||||
See accompanying notes
F-5
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years Ended December 31, 2009 and 2008
| 2009 | 2008 | |||||||
Cash flows from operating activities |
||||||||
Net loss |
$ | (2,655,316 | ) | $ | (5,141,746 | ) | ||
Depreciation and amortization |
9,007 | 8,651 | ||||||
Stock option expense for non-employee directors and employees |
94,198 | — | ||||||
Changes in: |
||||||||
Accounts receivable |
17,592 | 9,145 | ||||||
Prepaid and other current assets |
24,521 | (51,552 | ) | |||||
Other assets |
— | 4,314 | ||||||
Unearned revenue |
(1,266,825 | ) | 1,583,531 | |||||
Accounts payable |
(334,840 | ) | 554,920 | |||||
Accrued expenses and other current liabilities |
(86,973 | ) | (29,483 | ) | ||||
Net cash used in operating activities |
(4,198,636 | ) | (3,062,220 | ) | ||||
Cash flows from investing activities |
||||||||
Proceeds from sale of trademarks |
1,154 | — | ||||||
Purchases of equipment |
(738 | ) | (14,604 | ) | ||||
Purchases of intangibles |
(275 | ) | (1,730 | ) | ||||
Net cash provided by (used in) investing activities |
141 | (16,334 | ) | |||||
Cash flows from financing activities |
||||||||
Issuance of common stock upon exercise of stock options |
134,430 | — | ||||||
Issuance of preferred stock, net of costs |
— | 3,996,789 | ||||||
Cash received upon consummation of merger |
— | 477,067 | ||||||
Cost incurred in connection with merger |
— | (250,000 | ) | |||||
Net cash provided by financing activities |
134,430 | 4,223,856 | ||||||
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS |
(4,064,065 | ) | 1,145,302 | |||||
Cash and cash equivalents at beginning of year |
5,626,913 | 4,481,611 | ||||||
Cash and cash equivalents at end of year |
$ | 1,562,848 | $ | 5,626,913 | ||||
Supplemental disclosures of cash flow information |
||||||||
Interest paid |
$ | — | $ | — | ||||
Income taxes paid |
$ | — | $ | — | ||||
Supplemental disclosures of non-cash investing and financing information |
||||||||
Accrual for redemption of fractional shares |
$ | — | $ | 21,344 | ||||
The Merger transaction among Getting Ready Corporation, Winston Acquisition Corp.
and Winston Laboratories, Inc. was consummated on September 25, 2008. The Merger was
accounted for as a “reverse merger,” since as a result of the Merger the shareholders of
Winston own a majority of the outstanding shares of the common stock of the Company. At
the date of consummation of the Merger, Getting Ready Corporation had $477,067 in cash,
approximately $8,800 in assets and no liabilities. The Merger resulted in additional
equity of $235,907 (net of $250,000 of transaction costs). For further information,
please refer to Note 1.
See accompanying notes
F-6
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2000 and 2008
NOTE 1 — COMPANY FORMATION, BACKGROUND AND ABILITY TO CONTINUE AS A GOING CONCERN
Organization
Winston Pharmaceuticals, Inc. (“Winston” or the “Company”) is a research-based
specialty pharmaceutical company engaged in the discovery, development and
commercialization of pain-management products.
Winston Laboratories, Inc. (“Winston Labs”) is the successor to a research and
development partnership, Cisco Ltd. (“Cisco”) formed in 1992 to develop a specific novel
neuroactive compound, civamide. Civamide was discovered and patented by Joel E.
Bernstein, M.D., the managing general partner of Cisco and subsequently, the founder and
CEO of Winston Labs. In 1997, Cisco became Winston Laboratories LLC, which in 1998 became
a C Corporation. Winston Labs’ initial operating funds came from a rights offering in
1997 and a private equity placement in 1998. In 1999, Winston Labs sold approximately 19%
of its common stock to Bioglan Pharma Plc (“Bioglan”) for $25 million; Bioglan’s stake
was subsequently reduced below 7% when it did not participate in a 2004 Winston Labs
rights offering. In September 2007, Winston Labs purchased all of the then outstanding
ownership in Winston Labs from Bioglan for $225,000. Subsequent to this transaction, all
of the then purchased shares were retired.
In 2000, Winston Labs established a subsidiary, Rodlen Laboratories, Inc. (“Rodlen,”
formerly Oncovir Corporation), approximately 18% of which was sold to Bioglan for
$13.3 million in 2001. In 2002, Bioglan’s stake in Rodlen was reduced below 3% as a
result of Bioglan’s election not to participate in a Rodlen rights offering. The proceeds
of this rights offering were used to acquire the Zostrix® line of over-the-counter
topical analgesics. Rodlen marketed the Zostrix® product line until July 2005, when it
sold the product to Hi-Tech Pharmacal Co. (“Hi-Tech”). In October 2004, Rodlen launched
another topical analgesic, Axsain® , which it promoted to physicians and other
health-care professionals. In June 2005, based on disappointing sales, Rodlen sharply
reduced promotion of the Axsain® product and in March 2006, Rodlen discontinued selling
this product altogether. In February 2007, Rodlen licensed certain technology underlying
the Axsain® product to Hi-Tech (Note 4). Rodlen operated as a “virtual company” with no
employees of its own. In September 2007, Winston Labs purchased all of the then
outstanding ownership in Rodlen from Bioglan for $10,000. Subsequent to this transaction,
all of the then purchased shares were retired. On September 21, 2007, Rodlen was merged
into Winston Labs.
In 2005, Winston Labs established a wholly owned UK subsidiary, Winston Laboratories
Limited (“UK Ltd.”). UK Ltd. was established for the purposes of conducting work with
European drug regulatory authorities, who typically require a European entity. UK Ltd.
has no employees or material assets.
Effective November 13, 2007, Getting Ready Corporation (“GRC”), entered into a
definitive Merger Agreement and Plan of Reorganization (the “Merger Agreement”) with
Winston Labs., and Winston Acquisition Corp., a Delaware corporation (“Merger Sub”),
which was a wholly-owned subsidiary of GRC. Pursuant to the Merger Agreement, on
September 25, 2008, Winston Labs merged with and into Merger Sub and became a
wholly-owned subsidiary of GRC (the “Merger”). At the closing of the Merger (the numbers
of shares discussed in this note reflects the number of shares prior to the 8-for-1
reverse stock split that took place on December 15, 2008):
| • | all of the issued and outstanding capital stock of Winston Labs,
consisting of 23,937,358 shares of common stock, par value $0.001 per
share, 5,815,851 shares of the Winston Labs Series A Convertible
Preferred Stock, par value $0.001 per share (“Series A Preferred
Stock”), and 4,187,413 shares of the Winston Labs Series B Convertible
Preferred Stock, par value $0.001 per share (“Series B Preferred
Stock”), was exchanged for 422,518,545 shares of the GRC’s common
stock, par value $0.001 per share (at an exchange ratio of 17.65101
shares of GRC common stock per share of Winston Labs common stock),
101,849 shares of the GRC’s Series A and 73,332 shares of the GRC’s
Series B Preferred Stock (at an exchange ratio of .01751238 shares of
GRC preferred stock per share of Winston Labs’ preferred stock); |
||
| • | GRC assumed Winston Labs’ stock option plans; |
||
| • | Winston Labs’ outstanding 1,643,750 options to purchase 1,643,750
shares of Winston Labs’ common stock were converted to options to
purchase 29,013,848 shares of GRC’s common stock; and |
||
| • | all outstanding warrants to purchase Winston Labs Series A Preferred
Stock were assumed by GRC and converted into the right to acquire,
expiring November 13, 2012, upon the exercise of such warrants, an
aggregate of 71,672 shares of GRC’s Series A Preferred Stock at a
price per share of $49.09. |
F-7
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Prior to the closing of the Merger, GRC had 18,332,896 shares of common stock issued
and outstanding and, subsequent to the Merger, GRC had 440,851,441 shares of common stock
issued and outstanding. GRC had no shares of Series A or Series B Preferred Stock
outstanding prior to the Merger.
Winston Labs was the accounting acquirer in the merger which was accounted for as a
reverse merger among Winston Labs, Merger Sub and GRC (a public shell company). For
accounting purposes, Winston Labs has been treated as the continuing registrant. As a
result, all post merger comparative historical financial statements filed by the Company
will be those of Winston Labs. Further, Winston Labs’ historical stockholders’ equity
prior to the merger has been retroactively restated (recapitalized) for the equivalent
number of shares received in the reverse merger. Earnings and loss per share calculations
have also been retroactively restated to give effect to the recapitalization for all
periods presented. Lastly, the merger involving Winston Labs and GRC has been accounted
for as a capital transaction equivalent to the issuance of capital stock by Winston Labs
for the net monetary assets of GRC. Upon completion of the Merger, GRC changed its name
to Winston Pharmaceuticals, Inc.
The Company has incurred significant losses from operations since its inception and
expects losses to continue for the foreseeable future. The Company’s success depends
primarily on the development and regulatory approval of its product candidates. From
inception through December 31, 2009, the Company has incurred cumulative net losses of
$49.4 million. Net losses may continue for at least the next several years as the
company proceeds with the development of its product candidates and programs. The size
of these losses will depend on the receipt of revenue from its products candidates and
programs, if any, and on the level of the Company’s expenses. To achieve profitable
operations, the Company must successfully identify, develop, partner and/or commercialize
its product candidates and programs. Product candidates developed by the Company will
require approval of the U.S. Food and Drug Administration (FDA) or a foreign regulatory
authority prior to commercial sales. The regulatory approval process is expensive, time
consuming and uncertain, and any denial or delay of approval could have a material
adverse effect on the Company’s ability to become profitable or continue operations.
Even if approved, the Company’s product candidates may not achieve market acceptance and
could face competition.
The Company’s cash and cash equivalents have decreased from $5.6 million as of
December 31, 2008 to $1.6 million as of December 31, 2009. The Company will need to
raise additional funds in the immediate future to support its operations through December
31, 2010. The Company seeks additional sources of financing through collaborations with
third parties, or public or private debt or equity financings. There can be no assurance
that additional funds will be available on satisfactory terms, or at all. If adequate
funds are not available, the Company may be required to significantly reduce expenses
related to its operations and/or delay or reduce the scope of its development programs.
The accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern, which contemplates the realization of
assets and the settlement of liabilities and commitments in the normal course of
business. The financial statements for the year ended December 31, 2009 do not include
any adjustments to reflect the possible future effects on the recoverability and
classification of assets or the amounts and classification of liabilities that may result
from uncertainty related to the Company’s ability to continue as a going concern.
In June 2009, the Financial Accounting Standards Board (FASB) issued Accounting
Standards Codification Topic 105, Generally Accepted Accounting Principle, which
established the FASB Accounting Standards Codification (ASC) as the sole source of
authoritative generally accepted accounting principals. Pursuant to the provisions of
ASC Topic 105, the Company has updated references to GAAP in its financial statements
issued for the period ended December 31, 2009. The adoption of ASC Topic 105 did no have
a material impact on the Company’s financial position, results of operations or cash
flows.
NOTE 2 — SUMMARY OF ACCOUNTING POLICIES
Reverse Stock Split
On December 15, 2008, the Company effected a 1-for-8 reverse split of its common and
preferred stock. Except where otherwise noted, all common stock and preferred stock share
and per share amounts have been retroactively restated to reflect the reverse stock split
in the accompanying consolidated financial statements and notes for all periods
presented. A $21,344 accrual related to a payout for fractional shares in connection with
the reverse stock split is included in accrued expenses and other current liabilities in
the Consolidated Balance Sheet as of December 31, 2008.
F-8
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Principles of Consolidation
The consolidated financial statements of the Company include the accounts of Winston
Labs and its Subsidiaries, UK Ltd., as of and for the years ended December 31, 2009 and
2008 and the accounts of GRC from September 25, 2008 through December 31, 2008. All
intercompany balances and transactions have been eliminated.
Use of Estimates
In preparing financial statements in conformity with accounting principles generally
accepted in the United States of America, management is required to make estimates and
assumptions that affect the reported amounts of assets and liabilities and the disclosure
of contingent assets and liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting period. Actual results
could differ from those estimates.
Cash and Cash Equivalents
For purposes of reporting cash flows, the Company considers all instruments with
original maturities of three months or less to be cash equivalents. Included in cash and
cash equivalents at December 31, 2008 is approximately $1.9 million in a U.S. Treasury
mutual fund. There are no cash equivalents as of December 31, 2009. It is the Company’s
policy to include investments in mutual funds with a $1 carrying value as a cash
equivalent.
Accounts Receivable
Accounts receivable are carried at original amount due less an estimate made for
doubtful accounts. Accounts outstanding longer than the payment terms are considered past
due. The Company determines its allowances by considering a number of factors, including
the length of time trade accounts receivable are past due, the Company’s previous loss
history, the customer’s current ability to pay its obligation to the Company, projected
returns based on historical trends and anticipated events at particular customers, and
the condition of the general economy and industry as a whole. The Company writes off
accounts receivable when they become uncollectible, and payments subsequently received on
such receivables are credited to the allowance for doubtful accounts. The allowance for
doubtful accounts was $0 at December 31, 2009 and 2008, respectively.
Equipment
Equipment consists primarily of furniture and office equipment with estimated lives
of 3-7 years. Depreciation is provided for in amounts sufficient to relate the cost of
depreciable assets to operations over their estimated service lives, principally on an
accelerated basis.
Impairment of Long-Lived Assets
The Company accounts for long-lived assets in accordance with ASC Topic 350,
Intangibles-Goodwill and Other. The long-lived assets are being reviewed for impairment
whenever events or changes in circumstances indicate that the carrying amount of an asset
may not be recoverable. Recoverability of assets is measured by a comparison of the
carrying amount of an asset to future undiscounted cash flows expected to be generated by
the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an
impairment charge is recognized by the amount by which the carrying amount of the asset
exceeds the fair value of the asset.
Fair Value of Financial Instruments
The carrying amounts of financial instruments, including cash and cash equivalents,
accounts receivable and accounts payable approximate fair value due to the short term
maturity of these instruments.
ASC Topic 820, Fair Value Measurements and Disclosures, provides a framework for
measuring fair value of assets and liabilities in accordance with GAAP, and establishes a
three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair
value. These tiers include: Level 1, defined as observable inputs such as quoted prices
in active markets; Level 2, defined as inputs other than quoted prices in active markets
that are either directly or indirectly observable; and Level 3, defined as unobservable
inputs in which little or no market data exists, therefore requiring an entity to develop
its own assumptions.
F-9
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company’s financial assets measured at fair value on a recurring basis, subject
to the disclosure requirements of ASC Topic 820, are as follows:
| Fair Value Measurements at December 31, 2008 | ||||||||||||||||
| Quoted | ||||||||||||||||
| Prices in | ||||||||||||||||
| Active | Significant | |||||||||||||||
| Markets | Other | Significant | ||||||||||||||
| for Identical | Observable | Unobservable | ||||||||||||||
| Assets | Inputs | Inputs | ||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||
Assets: |
||||||||||||||||
Mutual fund |
$ | 1,961,059 | $ | — | $ | — | $ | 1,961,059 | ||||||||
Total |
$ | 1,961,059 | $ | — | $ | — | $ | 1,961,059 | ||||||||
The above investment in a mutual fund is included in cash and cash equivalents on
the Consolidated Balance Sheet as of December 31, 2008. The Company did not have any
financial assets measured at fair value on a recurring basis, subject to the disclosure
requirements of ASC Topic 820 as of December 31, 2009.
Revenue recognition
The Company has historically generated revenues from product sales, collaborative
research and development arrangements, and other commercial arrangements such as,
royalties, and sales of technology rights. Payments received under such arrangements may
include non-refundable fees at the inception of the arrangements, milestone payments for
specific achievements designated in the agreements, royalties on sales of products
resulting from collaborative arrangements, and payments for the sale of rights to future
royalties.
The Company recognizes revenue in accordance with the SEC’s Staff Accounting
Bulletin Topic 13 (“Topic 13”), “Revenue Recognition.” Revenue is recognized when all of
the following criteria are met: (1) persuasive evidence of an arrangement exists;
(2) delivery has occurred or services have been rendered; (3) the seller’s price to the
buyer is fixed and determinable; and (4) collectability is reasonably assured.
F-10
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Certain sales transactions include multiple deliverables. The Company allocates
amounts to separate elements in multiple element arrangements in accordance with ASC
Topic 605, Revenue Recognition. Revenues are allocated to a delivered product or service
when all of the following criteria are met: (1) the delivered item has value to the
customer on a standalone basis; (2) there is objective and reliable evidence of the fair
value of the undelivered item; and (3) if the arrangement includes a general right of
return relative to the delivered item, delivery or performance of the undelivered item is
considered probable and substantially in the Company’s control. The Company uses the
relative fair values of the separate deliverables to allocate revenue. For arrangements
with multiple elements that are separated, the Company recognizes revenues in accordance
with Topic 13.
Multiple Element Arrangements
The Company has arrangements whereby it delivers to the customer multiple elements
including technology and/or services. Such arrangements have generally included some
combination of the following: licensed rights to technology, patented products,
compounds, data and other intellectual property; and research and development services.
The Company analyzes its multiple element arrangements to determine whether the elements
can be separated. The Company performs its analysis at the inception of the arrangement
and as each product or service is delivered. If a product or service is not separable,
the combined deliverables will be accounted for as a single unit of accounting.
When a delivered element meets the criteria for separation, the Company allocates
amounts based upon the relative fair values of each element. The Company determines the
fair value of a separate deliverable using the price it charges other customers when it
sells that product or service separately; however, if the Company does not sell the
product or service separately, it uses third-party evidence of fair value. The Company
considers licensed rights or technology to have standalone value to its customers if it
or others have sold such rights or technology separately or its customers can sell such
rights or technology separately without the need for the Company’s continuing
involvement.
License Arrangements. License arrangements may consist of non-refundable upfront
license fees, data transfer fees, research reimbursement payments, exclusive licensed
rights to patented or patent pending compounds, technology access fees, various
performance or sales milestones. These arrangements are often multiple element
arrangements.
Non-refundable, up-front fees that are not contingent on any future performance by
the Company, and require no consequential continuing involvement on its part, are
recognized as revenue when the license term commences and the licensed data, technology
and/or compound is delivered. Such deliverables may include physical quantities of
compounds, design of the compounds and structure-activity relationships, the conceptual
framework and mechanism of action, and rights to the patents or patents pending for such
compounds. The Company defers recognition of non-refundable upfront fees if it has
continuing performance obligations without which the technology, right, product or
service conveyed in conjunction with the non-refundable fee has no utility to the
licensee that is separate and independent of the Company’s performance under the other
elements of the arrangement. In addition, if the Company has required continuing
involvement through research and development services that are related to its proprietary
know-how and expertise of the delivered technology, or can only be performed by the
Company, then such up-front fees are deferred and recognized over the period of
continuing involvement. Under the license agreement with sanofi-aventis Canada, Inc.
(Note 4), the obligation period was not contractually defined in relation to the
$1.9 million upfront fee. Under the circumstances, management exercised judgment in
estimating the period of time over which certain deliverables will be provided to enable
the licensee to utilize the license, which was determined to be 18 months. The estimated
period of 18 months was primarily determined based on management’s estimate of the
maximum amount of time it would take the Canadian regulatory authorities to approve the
Company’s new drug submission (NDS).
Payments related to substantive, performance-based milestones in a research and
development arrangement are recognized as revenues upon the achievement of the milestones
as specified in the underlying agreements when they represent the culmination of the
earnings process.
Research Services Arrangements. Revenues from research services are recognized
during the period in which the services are performed and are based upon the number of
full-time-equivalent personnel working on the specific project at the agreed-upon rate.
Reimbursements from collaborative partners for agreed upon direct costs including direct
materials and outsourced services, or subcontracted, pre-clinical studies are classified
as revenues in accordance with ASC Topic 605, and recognized in the period the
reimbursable expenses are incurred. Payments received in advance are deferred until the
research services are performed or costs are incurred.
F-11
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Royalty Arrangements. The Company recognizes royalty revenues from licensed products
when earned in accordance with the terms of the license agreements. Net sales amounts
generally required to be used for calculating royalties include deductions for returned
product, pricing allowances, cash discounts, freight and warehousing.
Certain royalty arrangements require that royalties are earned only if a sales
threshold is exceeded. Under these types of arrangements, the threshold is typically
based on annual sales. The Company recognizes royalty revenue in the period in which the
threshold is exceeded.
Research and Development Expenses
The guidance in ASC Topic 730, Research and Development, requires the Company to
defer and capitalize nonrefundable advance payments made for goods or services to be used
in research and development activities until the goods have been delivered or the related
services have been performed. If the goods are no longer expected to be delivered or the
services are no longer expected to be performed, the Company would be required to expense
the related capitalized advance payments.
Research and development expenses consist of expenses incurred in performing
research and development activities including salaries and benefits, facilities and other
overhead expenses, clinical trials, contract services and outsource contracts. Research
and development expenses are charged to operations as they are incurred.
Acquired contractual rights. Payments to acquire contractual rights to a licensed
technology or drug candidate are expensed as incurred when there is uncertainty in
receiving future economic benefits from the acquired contractual rights. The Company
considers the future economic benefits from the acquired contractual rights to a drug
candidate to be uncertain until such drug candidate is approved by the FDA or when other
significant risk factors are abated.
Research and development costs totaled $2,062,933 and $3,496,150 in 2009 and 2008,
respectively.
Stock-Based Compensation
ASC Topic 718, Stock Compensation, requires that the compensation cost relating to
share-based payment transactions be recognized in financial statements, based on the fair
value of the instruments issued. ASC Topic 718 covers a wide range of share-based
compensation including stock options, restricted stock, performance-based awards, share
appreciation rights and employee share purchase plans. Prior to January 1, 2006, the
Company accounted for stock-based compensation arrangements using the intrinsic value
method.
Income Taxes
The Company files a consolidated tax return that includes all subsidiaries. Deferred
income taxes are recognized for the tax consequences in future years of differences
between the tax bases of assets and liabilities and their financial reporting amounts at
each year-end, based on enacted tax laws and statutory tax rates applicable to the
periods in which the differences are expected to affect taxable income. Valuation
allowances are established when necessary to reduce deferred income tax assets to the
amount expected to be realized.
ASC Topic 740, Income Taxes, prescribes a recognition threshold and measurement
attribute for the financial statement recognition and measurement of a tax position taken
or expected to be taken in a tax return. ASC Topic 740 provides a two-step process to
evaluate a tax position and also provides guidance on derecognition, classification,
interest and penalties, accounting in interim periods, disclosure, and transition. The
Company has not recorded a reserve for any tax positions for which the ultimate
deductibility is highly certain but for which there is uncertainty about the timing of
such deductibility. The Company files tax returns in all appropriate jurisdictions. The
open tax years are those years ending December 31, 2006 to December 31, 2009, which
statutes expire in 2010-2013. As of December 31, 2009 and December 31, 2008, the Company
has no liability for unrecognized tax benefits. The Company recognizes interest and
penalties related to uncertain tax positions as income tax expense as incurred. No
expense for interest and penalties was recognized for the years ended December 31, 2009
and 2008.
F-12
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Reclassification
Certain other reclassifications have been made to the prior year financial
statements to conform to the current year presentation. These classifications had no
effect on reported net loss or stockholders’ equity.
Segment Information
The Company is operated on the basis of a single reportable segment, which is the
business of discovery and development of products for pain management. The Company’s
chief operating decision-maker is the Chief Executive Officer, who evaluates the company
as a single operating segment.
Subsequent Events
In May 2009, the FASB issued ASC Topic 855, Subsequent Events, which established
general accounting standards and disclosure for subsequent events. The Company adopted
ASC Topic 855 during the second quarter of 2009. In accordance with ASC Topic 855, the
Company has evaluated subsequent events through the date and time the financial
statements were issued.
Recent Accounting Pronouncements
In December 2007, the FASB issued a new standard on Business Combinations which is
part of ASC Topic 805. This Statement provides greater consistency in the accounting and
financial reporting for business combinations. ASC Topic 805 establishes disclosure
requirements and, among other things, requires the acquiring entity in a business
combination to record contingent consideration payable, to expense transaction costs, and
to recognize all assets acquired and liabilities assumed at acquisition-date fair value.
The Company adopted this new standard on January 1, 2009 and such adoption had no effect
on the Company’s results of operations, net loss or basic diluted loss per share for the
year ended December 31, 2009.
In December 2007, the FASB issued ASC Topic 810, Noncontrolling Interests in
Consolidated Financial Statements (“ASC Topic 810”). ASC Topic 810 establishes accounting
and reporting standards for the minority or noncontrolling interests in a subsidiary or
variable interest entity and for the deconsolidation of a subsidiary or variable interest
entity. Minority interests will be recharacterized as noncontrolling interests and
classified as a component of equity. It also establishes a single method of accounting
for changes in a parent’s ownership interest in a subsidiary and requires expanded
disclosures. The Company adopted ASC Topic 810 on January 1, 2009. The adoption of ASC
Topic 810 had no effect on the Company’s results of operations, net loss or basic diluted
loss per share for the year ended December 31, 2009.
In March 2008, the FASB issued a new pronouncement on Disclosures about Derivative
Instruments and Hedging Activities. This statement establishes the requirements for the
disclosure of derivative instruments and hedging activities that include the reasons a
company uses derivative instruments, how derivative instruments and related hedged items
are accounted under ASC Topic 815 and how derivative instruments and related hedged items
affect a company’s financial position, financial performance and cash flows. The Company
adopted this new pronouncement on January 1, 2009 and such adoption had no effect on the
Company’s results of operations, net loss or basic diluted loss per share for the year
ended December 31, 2009.
In June 2008, the FASB ratified a new pronouncement on Determining Whether an
Instrument (or Embedded Feature) is Indexed to an Entity’s Own Stock. This new
pronouncement mandates a two-step process for evaluating whether an equity-linked
financial instrument or embedded feature is indexed to the entity’s own stock. It is
effective for fiscal years beginning after December 15, 2008, and interim periods within
those fiscal years, which is our first quarter of 2009. The adoption of this new
pronouncement did not have a material effect on the Company’s financial position, results
of operations, or cash flow.
In June 2009, the FASB issued a new pronouncement relating to “Amendments to
variable interest entities (VIE)”, which changes the approach to determining the primary
beneficiary of a VIE and requires companies to more frequently assess whether they must
consolidate VIEs. This new standard is effective for the Company beginning on January 1,
2010. The adoption of this new pronouncement is not expected to have a material impact on
the Company’s financial position, results of operations or cash flows.
F-13
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In October 2009, the FASB issued ASU 2009-13, Revenue Recognition (Topic 605)-
Multiple-Deliverable Revenue Arrangements, which amends ASC 605, Revenue Recognition, to
require companies to allocate revenue in multiple-element arrangements based on an
element’s estimated selling price if vendor-specific or other third-party evidence of
value is not available. ASU 2009-13 is effective prospectively for revenue arrangements
entered into or materially modified in fiscal years beginning on or after June 15, 2010.
Earlier application is permitted. The Company is currently evaluating both the timing
and the impact of the pending adoption of the ASU on its consolidated financial
statements.
NOTE 3 — EARNINGS PER SHARE
Basic EPS is computed by dividing income (loss) attributable to common stockholders
by the weighted average number of commons shares outstanding for the period. Diluted EPS
is computed giving effect to all dilutive potential common shares that were outstanding
during the period. Dilutive potential common shares consist of the incremental common
shares issuable upon conversion of convertible preferred shares and the exercise of stock
options and warrants and unvested shares granted to employees.
| Fiscal 2009 | Fiscal 2008 | |||||||
Denominator: |
||||||||
Weighted average common shares outstanding — basic and diluted |
61,258,364 | 53,422,158 | ||||||
Loss per common share — basic and diluted |
$ | (0.04 | ) | $ | (0.10 | ) | ||
The Company’s Basic EPS and Diluted EPS is identical as inclusion of the incremental
common shares attributable upon conversion of convertible preferred shares and the
exercise of stock options and warrants and unvested shares granted to employees would
have been anti-dilutive.
NOTE 4 — TECHNOLOGY LICENSE AGREEMENTS
In January 2006, the Company entered into a technology license agreement with Sirius
Laboratories, Inc. (“Sirius”). Several large stockholders in the Company had significant
stock holdings in Sirius until March 10, 2006, when Sirius was acquired by DUSA
Pharmaceuticals, Inc. Two officers of the Company served on Sirius’ board of directors
but resigned their directorships in 2007. Under the terms of the license agreement, the
Company granted Sirius an exclusive license to the proprietary rights of certain products
(first marketed as Micanol® and then later as Psoriatec®) containing the active
pharmaceutical ingredient, anthralin. The agreement provided for minimum annual royalties
of $300,000 through February, 2008 and an option to purchase all rights to the product
for $750,000. In January 2008, the license agreement was extended until September 30,
2008 by mutual consent and the extension and provides for a continuation of the 25%
royalty on net sales, but eliminates the minimum royalty and the purchase option. Under
the technology license agreement, the Company recorded royalty revenue of $0 and $34,861
for the years ended December 31, 2009 and 2008, respectively. In July 2008, the Company
learned that DUSA had breached a license agreement with Winston Labs. Winston Labs
initiated arbitration in October 2008 to recover the damages caused by this breach. In
June 2009, DUSA settled this matter by agreeing to pay $75,000 in damages. This
settlement is included in other income in the Consolidated Statements of Operations.
In February 2007, Rodlen licensed a patent and certain other technology to Hi-Tech
Pharmacal Co. (“Hi-Tech”) for cash consideration of two payments totaling $300,000, as
well as a 10% royalty on net sales of products sold by Hi-Tech that utilize the
intellectual property licensed from Rodlen for the remaining life of the related patent,
reducing to 5% of net sales for four years following the expiration of the patent. In
2009 and in 2008, the Company recorded $127,762 and $100,975 of royalties under this
agreement. On October 30, 2009, the Company received communication from Hi-Tech that it
has discontinued the sale of the product as defined in the license agreement. As a
result, the Company does not expect to receive any more royalties from Hi-Tech in the
future.
On August 14, 2007, the Company entered into an exclusive technology license
agreement with Exopharma, Inc., now known as Elorac, Inc. (“Elorac”) an entity that is
majority owned by the CEO of the Company and his affiliates (see Note 5). Under the terms
of the license agreement, the Company granted Elorac an exclusive license to the
proprietary rights of certain products (£ 0.025% Civamide with the stated
indication of psoriasis of the skin). In exchange, Elorac paid the Company a license fee
of $100,000 and was required to pay a 9% royalty on sales of the product. In addition,
the agreement required Elorac to pay the Company a non-refundable payment of $250,000 upon approval of a Marketing
Authorization by Elorac on the product(s) described in the agreement. On October 27, 2008
Winston and Elorac mutually terminated the above license agreement. As a result of this
mutual termination, Winston paid Elorac $105,000 in exchange for Winston retaining all
the property rights under the agreement.
F-14
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On October 30, 2008, the Company and sanofi-aventis Canada Inc. entered into a
licensing agreement for the Canadian rights to Civamide, Winston’s transient receptor
potential vanilloid (TRPV-1) modulator in formulations for topical application. Under the
terms of the agreement, sanofi-aventis Canada Inc. owns the rights to develop,
manufacture and commercialize Civamide Cream in Canada along with a second generation
cream that is currently in development. In return for granting sanofi-aventis Canada Inc.
the Canadian rights, Winston received an upfront payment of $1.9 million (US) and will
receive an additional $2 million (CAD) upon regulatory approval of Civamide Cream in
Canada, certain milestone payments, and future royalties on net sales of Civamide or the
related second generation cream in Canada. In connection with this agreement, Winston is
recognizing the upfront payment of $1.9 million over 18 months. As such approximately
$1,267,000 and $316,000 has been recognized as revenue in 2009 and in 2008, respectively,
with the remainder being treated as unearned revenue on the Consolidated Balance Sheet as
of December 31, 2009.
NOTE 5 — RELATED-PARTY TRANSACTIONS
Elorac is an entity controlled
by Dr. Bernstein, CEO of Winston Labs and the Company. In addition, Robert Yolles, a director of the Company, is also
a director of Elorac. Since inception, Elorac has been located in the same offices as Winston and
therefore has shared in certain of Winston’s expenses such as rent, utilities, internet
usage, etc. The amount of Elorac’s share of such expenses is based on various allocation
factors related to a particular expense. Winston received $162,977 and $128,323 in 2009
and in 2008 for such services, which are included as a reduction of Winston’s expenses on
the Consolidated Statement of Operations. Amounts due from Elorac of $2,601 and $0 as of
December 31, 2009 and 2008, respectively, are included in related party receivables in
the Company’s Balance Sheet. On February 28, 2009 Elorac purchased certain trademarks
from the Company for approximately $1,154.
Gideon Pharmaceuticals, Inc. (“Gideon”) is a corporation whose chairman and sole
director is Joel E. Bernstein, M.D., president and CEO of the Company. Gideon reimburses
the Company for certain expenses that the Company incurs on behalf of Gideon. Amounts due
from Gideon of $12,675 and $0 as of December 31, 2009 and 2008, respectively, are
included in related party receivable on the Consolidated Balance Sheets.
On September 19, 2007, Winston Labs entered into an exclusive technology license
agreement with Opko Ophthalmologists, LLC, (“OPKO”). The CEO and Chairman of OPKO is also
the indirect beneficial owner of 26,574,659 shares of the Company’s capital stock on a
fully diluted basis (29.7% of the total issued and outstanding shares), including
8,779,797 shares of common stock underlying warrants. Furthermore, Subbarao Uppaluri,
Ph.D., a director of the Company and the Senior Vice President — Chief Financial Officer
of Opko Health, is the beneficial owner of 326,538 shares of the Company’s capital stock
on a fully-diluted basis, including 27,500 shares of common stock underlying options and
89,589 shares of common stock underlying warrants. Under the terms of the license
agreement, Winston Labs granted OPKO an exclusive license to the proprietary rights of
certain products (pharmaceutical compositions or preparations containing the active
ingredient Civamide in formulations suitable for use in the therapeutic or preventative
treatment of ophthalmic conditions in humans). In exchange, OPKO paid Winston Labs a
license fee of $100,000 and is required to pay a 10% royalty on sales of the product. In
addition, the agreement requires OPKO to pay Winston Labs a non-refundable payment of
$5,000,000 upon approval of a Marketing Authorization by OPKO on the product described in
the agreement. Under the terms of the agreement, OPKO and Winston Labs agreed to equally
share the cost related to manufacturing and clinical supplies of Civamide Nasal Spray.
During 2008, OPKO reimbursed Winston Labs approximately $80,000 for such costs. In
addition, the agreement calls for OPKO to reimburse Winston Labs for certain legal
expenses Winston Labs has incurred related to keratoconjunctivitis. In each of 2009 and
2008, approximately $38,800 and $38,000, respectively, of legal fees were billed to OPKO,
of which approximately $7,000 and $38,000 were outstanding as of December 31, 2009 and
2008, respectively, and are included in related party receivable on the Consolidated
Balance Sheets. Subsequent to December 31, 2009 and 2008, respectively, OPKO paid $7,000
and $38,000 of the balance due.
On February 24, 2010, the Company received communications from Opko that it was
terminating the license agreement effective May 24, 2010. As a result, all of Opko’s
rights to certain products, as defined in the agreement, will revert back to the Company.
F-15
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On September 30, 2007, Dr. Jeffrey Bernstein resigned as Senior Vice President and
Chief Operating Officer of Winston and entered into a consulting agreement with Winston,
effective as of October 1, 2007. Pursuant to the terms of his consulting agreement,
Winston agreed to compensate Dr. Bernstein at a rate of $150 per hour. Further, Winston
agreed to reimburse Dr. Bernstein for all reasonable out-of-pocket expenses incurred in the course of his
services to Winston, including, but not limited to, reasonable expenses related to
travel, telephone, postage, and office supplies. The consulting agreement is terminable
by either Dr. Jeffrey Bernstein or by Winston upon thirty (30) days notice to the other
party. Dr. Jeffrey Bernstein is the son of Dr. Joel E. Bernstein, the President and
Chief Executive Officer of the Company and is the beneficial owner of 5,165,324 shares of
the Company’s capital stock on a fully diluted basis (5.7% of the total issued and
outstanding shares). Winston incurred $12,213 and $21,488 of expenses under the
consulting agreement for years ended December 31, 2009 and 2008, respectively, of which
approximately $7,900 was outstanding as of December 31, 2009 and is included in accrued
expenses and other current liabilities on the Consolidated Balance Sheets.
Each of the Company’s non-employee directors currently receives $1,250 per quarter.
Directors do not receive additional compensation for attendance of meetings. Directors
who are also employees do not receive compensation for this service on the Board. As of
December 31, 2009 and 2008, respectively, $18,750 and $6,250, respectively, was due to
the Company’s non-employee directors, which is included in the accounts payable on the
Consolidated Balance Sheets.
NOTE 6 — INTANGIBLE ASSETS
Intangible assets consist of intangible assets with finite useful lives. As of
December 31, 2009 and 2008, intangible assets were as follows:
| 2009 | 2008 | |||||||
Patents and trademarks |
$ | 41,426 | $ | 42,305 | ||||
Less: accumulated amortization |
(23,971 | ) | (20,765 | ) | ||||
| $ | 17,455 | $ | 21,540 | |||||
Future annual amortization expense at December 31, 2009 is as follows:
2010 |
$ | 2,766 | ||
2011 |
2,678 | |||
2012 |
2,678 | |||
2013 |
2,678 | |||
2014 |
2,678 | |||
Thereafter |
3,977 | |||
| $ | 17,455 | |||
Weighted average amortization period for the Company’s intangible asset is
approximately 10 years. Amortization expense related to amortizable intangible assets was
approximately $3,200 and $4,000 for each of 2009 and 2008, respectively.
F-16
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTE 7 — INCOME TAXES
The provision for income taxes differs from the amount computed by applying the
statutory federal income tax rate of 34.0% to pre-tax loss as follows:
| 2009 | 2008 | |||||||
Tax benefit at U.S. federal statutory rate |
$ | (903,000 | ) | $ | (1,748,000 | ) | ||
State tax benefit, net of federal benefit |
(128,000 | ) | (248,000 | ) | ||||
Change in valuation allowance |
1,132,000 | 2,180,000 | ||||||
Permanent differences |
1,000 | 1,000 | ||||||
Research and development credits |
(104,000 | ) | (179,000 | ) | ||||
Other, net |
2,000 | (6,000 | ) | |||||
Income tax expense (benefit) |
$ | — | $ | — | ||||
The Company’s deferred taxes consist of the following at December 31, 2009 and 2008:
| 2009 | 2008 | |||||||
Deferred tax assets |
||||||||
Net operating loss carryforward |
$ | 18,604,000 | $ | 16,929,000 | ||||
Research and development credits |
1,483,000 | 1,379,000 | ||||||
Intangible |
1,261,000 | 1,446,000 | ||||||
Unearned revenue |
123,000 | 615,000 | ||||||
Property and equipment |
23,000 | 22,000 | ||||||
Accruals |
6,000 | 14,000 | ||||||
Other |
104,000 | 67,000 | ||||||
Total deferred tax assets |
21,604,000 | 20,472,000 | ||||||
Valuation allowance for deferred tax assets |
(21,604,000 | ) | (20,472,000 | ) | ||||
Deferred taxes, net |
$ | — | $ | — | ||||
At December 31, 2009, the Company had Federal and state net operating loss
carryforwards of approximately $47,927,000 which begin expiring in 2018, unless
previously utilized. In addition, the Company has federal and state net operating loss
carryforwards of approximately $1,692,000, that originated from GRC prior to the merger,
that has substantial limitations on its use and begin expiring in 2026. Pursuant to
Internal Revenue Code Sections 382 and 383, certain substantial changes in the Company’s
ownership may limit the amount of the net operating loss carryforwards, which could be
utilized to offset future taxable income and income tax liabilities. The Company also had
Federal research and development credit carryforwards of approximately $1,483,000. The
Company has provided a valuation allowance for the full amount of its net deferred tax
assets since realization of any future benefit from deductible temporary differences, net
operating loss and tax credit carryforwards is uncertain.
F-17
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTE 8 — STOCK OPTION PLAN
Effective April 1, 2009, the Company’s Board of Directors adopted the Company’s
Omnibus Incentive Plan (the “Plan”). The Plan was established by amending, restating and
merging the Company’s existing Stock Option Plan for Non-Employee directors, and the 1999
Stock Option Plan (collectively, the “Prior Plans”), with and into the Plan. The Plan was
approved by the Company’s shareholders at the Company’s annual meeting held on June 17,
2009.
The Plan provides for a broad range of awards to attract, motivate and retain
qualified and talented employees, directors, consultants and other persons who provide
services to the Company (“Participants”), including stock options, stock appreciation
rights, restricted stock, restricted stock units, performance shares, performance units,
and other stock-based awards and cash-based awards (“Awards”). The Plan promotes the
success and enhances the value of the Company by linking the personal interests of
Participants to those of the Company’s stockholders, and by providing Participants with
an incentive for outstanding performance.
Awards under the Plan will be determined by a committee of the Board of Directors
(the “Committee”), the members of which are selected by the Board of Directors. Currently
the Plan provides that the Compensation Committee will serve as the administrator. The
number of shares of Company common stock (“Shares”) as to which an Award is granted and
to whom any Award is granted shall be determined by the Committee, subject to the
provisions of the Plan. Awards may be made exercisable or settled at such prices and may
be made terminable under such terms as are established by the Committee, to the extent
not otherwise inconsistent with the terms of the Plan.
Prior to the merger of the Prior Plans with and into the Plan, there were 9,708,055
Shares reserved for issuance for awards under the Prior Plans, including 3,196,487 Shares
reserved for outstanding awards, and 5,922,466 Shares for future awards. Upon the merger
of the Prior Plans with and into the Plan, effective April 1, 2009, there was no change
to such numbers, with 9,708,055 Shares reserved for issuance for Awards under the Plan,
of which 3,196,487 Shares were reserved for outstanding Awards, and 5,922,466 Shares for
future Awards.
On April 7, 2009, pursuant to the terms of the Plan, the Compensation Committee of
the Board of Directors granted 267,000 non-qualified stock options to purchase Shares to
employees of the Company, including 225,000 options to key officers of the Company. All
of the options expire on April 7, 2019, vest in five equal installments commencing
April 7, 2010, and have an exercise price of $1.53, as determined by the Compensation
Committee of the Board of Directors in accordance with the terms of the Plan.
On September 25, 2009 pursuant to the terms of the Plan, the Compensation Committee
of the Board of Directors granted 142,500 non-qualified stock options to purchase shares
to non-employee directors of the Company. All of the options expire on September 25, 2019
vest in five equal installments commencing September 25, 2010 and have an exercise price
of $1.36 as determined by the Compensation Committee of the Board of Directors in
accordance with the terms of the Plan.
The Company did not grant any options in 2008. In July 2008, 446,791 options were
forfeited by a past Director of the Company when rights to exercise those options lapsed.
ASC Topic 718 requires companies to estimate the fair value of share-based payment
awards on the date of grant using an option pricing model and to recognize this expense.
Stock-based compensation expense recognized for the years ended December 31, 2009 and
2008 was $94,198 and $0, respectively, and is included in general and administrative
expenses in the statement of operations. This expense was calculated based on the
Black-Scholes single-option pricing model, using the following assumptions:
| 2009 | ||
Expected dividend yield |
0.00% | |
Expected stock price volatility |
76.57% | |
Risk-free interest rate range |
1.87% – 2.36% | |
Expected life of options (years) |
6.5 years | |
Fair value of options granted |
$1.43 – $2.14 |
F-18
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The risk-free interest rate is based on the implied yield available on U.S. treasury
zero-coupon issues with an equivalent remaining term. Since the Company does not have
sufficient historical experience on which to estimate expected term, the simplified
method was used to generate the expected option life. Using the simplified method, the
expected term is calculated by taking the average of the vesting term and the original
contractual term. In a similar manner, the Company does not have a history of stock price
volatility, so it identified nine appropriate comparables for use as a benchmark. These
comparables (all publically traded companies) are in the same industry (specialty
pharmaceuticals) and focused on development of the same types of products (pain control
and central nervous system) as the Company. To generate the estimated stock price
volatility shown above, a weighted average of the nine comparable companies’ expected
volatility (which ranged from 71% to 82%) was employed for a period equal to the expected
life of the options.
The following table summarizes stock option activity under the Plans during the
years ended December 31, 2009 and 2008:
| Weighted | ||||||||||||||||||||
| Weighted- | Weighted- | Average | ||||||||||||||||||
| Average | Average | Aggregate | Remaining | |||||||||||||||||
| Exercise | Grant Date | Intrinsic | Contractual | |||||||||||||||||
| Shares | Price | Fair Value | Value | Life | ||||||||||||||||
Options at December 31, 2007 |
4,073,522 | $ | 0.34 | |||||||||||||||||
Granted |
— | $ | — | $ | — | |||||||||||||||
Exercised |
— | $ | — | |||||||||||||||||
Forfeitures |
(446,791 | ) | $ | 0.32 | ||||||||||||||||
Options at December 31, 2008 |
3,626,731 | $ | 0.34 | |||||||||||||||||
Granted |
409,500 | $ | 1.47 | $ | 1.89 | |||||||||||||||
Exercised |
(415,529 | ) | $ | 0.32 | ||||||||||||||||
Forfeitures |
(578,717 | ) | $ | 0.28 | ||||||||||||||||
Options at December 31, 2009 |
3,041,985 | $ | 0.50 | $ | — | 4.20 | ||||||||||||||
There is $678,615 in unrecognized compensation expense to be recognized over
remaining weighted average period of 4.4 years. The aggregate intrinsic value in the
table above is before income taxes, based on the fair value of a share of the Company’s
common stock of $0.28 at December 31, 2009. The aggregate intrinsic value of options
outstanding and exercisable during 2009 was $0. Cash proceeds received from the exercise
of options for 2009 was approximately $134,000. The total intrinsic value of options
exercised during 2009 was approximately $1,349,067. As of December 31, 2009 there were
2,632,485 exercisable options. The realized tax benefit from stock options and other
share-based payments for 2009 was $0, based on the Company’s election of the “with and
without” approach.
On January 12, 2009, an executive of the Company exercised 231,670 options at $0.36
for approximately $83,000. In March, 2009, 198,574 options were forfeited by a past
director of the Company when rights to exercise those options lapsed. On May 1, 2009, an
executive of the Company exercised 158,859 options at an exercise price of $0.28 for
approximately $44,000. On October 16, 2009, an executive of the Company exercised 25,000
options at an exercise price of $0.28 for approximately $7,000 and forfeited the
remaining 372,147 options that expired on October 18, 2009. In addition 7,996 options
were forfeited in 2009 by past employees of the Company when rights to exercise those
options lapsed.
F-19
Table of Contents
Winston Pharmaceuticals, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
NOTE 9 — 401(k) PLAN
The Company has adopted a qualified employee savings and retirement plan under
Section 401(k) of the U.S. Internal Revenue Code (the “401(k) Plan”). All of the
Company’s full-time employees are eligible to participate in the 401(k) Plan by enrolling
in the 401(k) Plan and electing to reduce their current compensation by up to the
statutorily prescribed annual limit, and having the amount of the reduction contributed
to the 401(k) Plan. The Company may also make discretionary matching contributions to the
401(k) Plan for employees. The Company made discretionary matching contributions to the
401(k) Plan of $54,602 and $40,449 in 2009 and 2008, respectively.
NOTE 10 — COMMITMENTS AND CONTINGENCIES
The Company leases its facilities on a month-to-month basis and certain equipment
under operating leases that expire in 2010 and require payments totaling approximately
$3,000. Rental expense for the years ended December 31, 2009 and 2008 was $93,944 and
$120,649, respectively.
NOTE 11 — PREFERRED STOCK AND WARRANTS
In connection with the Merger on September 25, 2008, the Company issued 101,849
shares of its Series A Convertible Preferred Stock, par value $.001 per share (“Series A
Preferred Stock”), and 73,332 shares of its Series B Convertible Preferred Stock, par
value $.001 per share (“Series B Preferred Stock”). Following a 1-for-8 reverse split of
the Company’s common and preferred stock on December 15, 2008, 12,730 shares of its
Series A Preferred Stock and 9,157 shares of its Series B Preferred Stock were issued and
outstanding. In addition, the Series A Preferred Stock shareholders also hold warrants to
purchase an aggregate of an additional 8,959 shares of the Company’s Series A Convertible
Preferred Stock at a price of $392.72. The warrants expire on November 13, 2012 and have
certain adjustment provisions as defined in the related warrant agreements. On
September 24, 2009, each outstanding share of Company Series A Preferred Stock and
Series B Preferred Stock automatically converted into 1,000 fully-paid, non-assessable
shares of the Company’s common stock, par value $.001 per share (“Common Stock”). In
addition, in connection with such conversion, each outstanding warrant to purchase shares
of Series A Preferred Stock automatically converted into the right to acquire 1,000
shares of Common Stock upon the exercise of such warrant, at an exercise price of $0.39
per share of Common Stock.
F-20
Table of Contents
EXHIBIT INDEX
| Exhibit | ||
| Number | Description | |
| 21.1* | Subsidiaries of Winston Pharmaceuticals, Inc. |
|
| 31.1* | Certification of the Company’s President and Chief
Executive Officer, Joel E. Bernstein, M.D.,
pursuant to Section 302 of the Sarbanes-Oxley Act
of 2002. |
|
| 31.2* | Certification of the Company’s Vice President and
Chief Financial Officer, David Starr, pursuant to
Section 302 of the Sarbanes-Oxley Act of 2002. |
|
| 32.1* | Certification of the Company’s President and Chief
Executive Officer, Joel E. Bernstein, M.D.,
pursuant to Section 906 of the Sarbanes-Oxley Act
of 2002. |
|
| 32.2* | Certification of the Company’s Vice President and
Chief Financial Officer, David Starr, pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002. |
| * | filed herewith. |
