Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - JUNIPER PHARMACEUTICALS INC | y83086e8vk.htm |
| EX-99.2 - EX-99.2 - JUNIPER PHARMACEUTICALS INC | y83086exv99w2.htm |
Exhibit 99.1

| Today's Announcement & The Plan Going ForwardAn Investor/Market UpdateMarch 4, 2010 |

| Today's Agenda Partnering ObjectivesTransaction HighlightsGoing Forward StrategyPost-transaction Financial HighlightsQ&A |

| Partnering Objectives Strengthen balance sheet immediatelyPreserve upside of preterm birth opportunity for stockholdersPartner should bring:Financial strengthStrong marketing capabilitySynergistic development & regulatory capabilitiesAllow aggressive lifecycle management activitiesFacilitate funding of new product development |
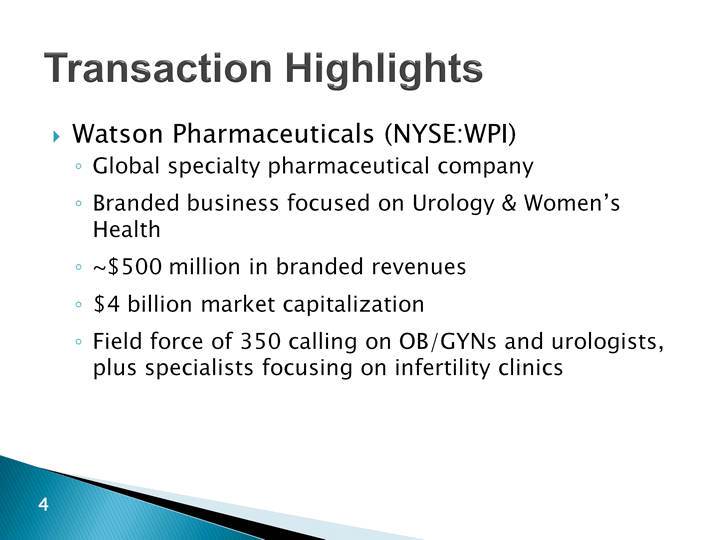
| Transaction Highlights Watson Pharmaceuticals (NYSE:WPI)Global specialty pharmaceutical companyBranded business focused on Urology & Women's Health~$500 million in branded revenues$4 billion market capitalizationField force of 350 calling on OB/GYNs and urologists, plus specialists focusing on infertility clinics |
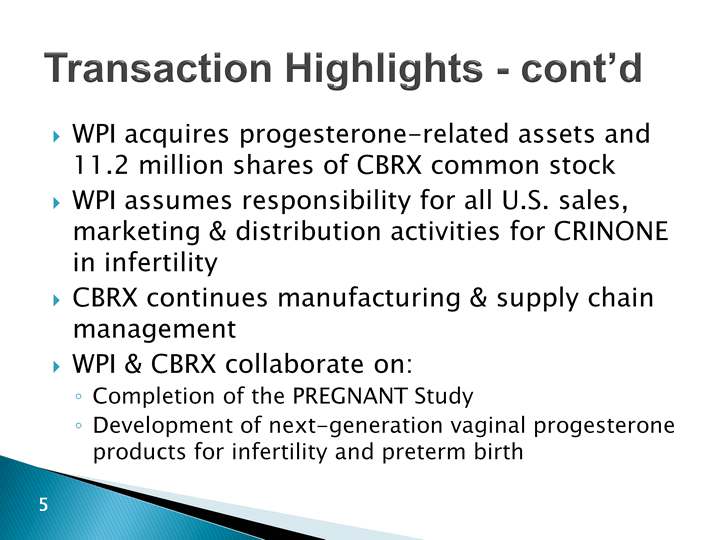
| Transaction Highlights - cont'd WPI acquires progesterone-related assets and 11.2 million shares of CBRX common stockWPI assumes responsibility for all U.S. sales, marketing & distribution activities for CRINONE in infertilityCBRX continues manufacturing & supply chain managementWPI & CBRX collaborate on: Completion of the PREGNANT StudyDevelopment of next-generation vaginal progesterone products for infertility and preterm birth |
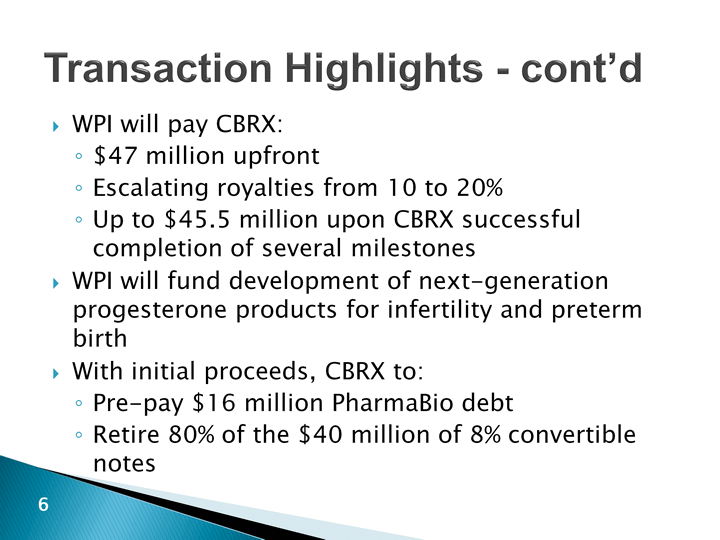
| Transaction Highlights - cont'd WPI will pay CBRX:$47 million upfrontEscalating royalties from 10 to 20%Up to $45.5 million upon CBRX successful completion of several milestonesWPI will fund development of next-generation progesterone products for infertility and preterm birthWith initial proceeds, CBRX to:Pre-pay $16 million PharmaBio debtRetire 80% of the $40 million of 8% convertible notes |

| Approval Process No immediate action required of stockholdersColumbia's Board of Directors recommends a vote in favor of this transactionTransaction subject to stockholder approvalSpecial meeting will be heldNeeds >50% of voting power in favor |

| CBRX Near-term Strategy Close WPI transactionSupport continued sales growth of Crinone in infertilityComplete PREGNANT StudyPursue FDA approval of Crinone/Prochieve for preterm birthPartner Striant in effort to grow sales and expand labeling |
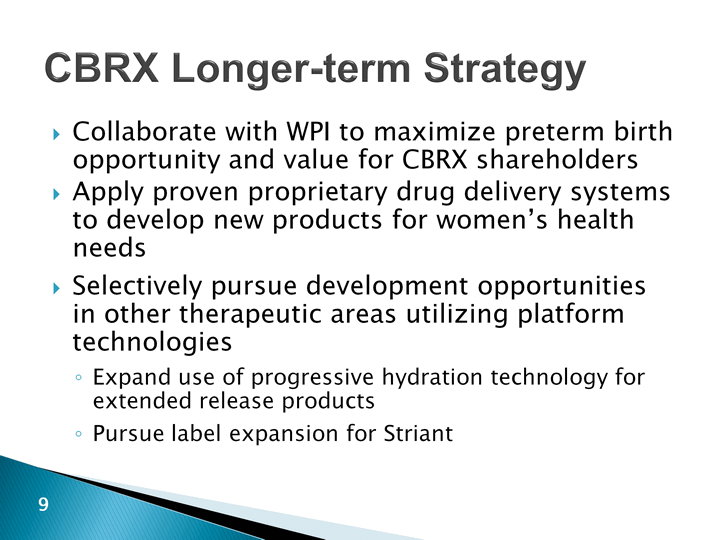
| CBRX Longer-term Strategy Collaborate with WPI to maximize preterm birth opportunity and value for CBRX shareholdersApply proven proprietary drug delivery systems to develop new products for women's health needsSelectively pursue development opportunities in other therapeutic areas utilizing platform technologiesExpand use of progressive hydration technology for extended release productsPursue label expansion for Striant |
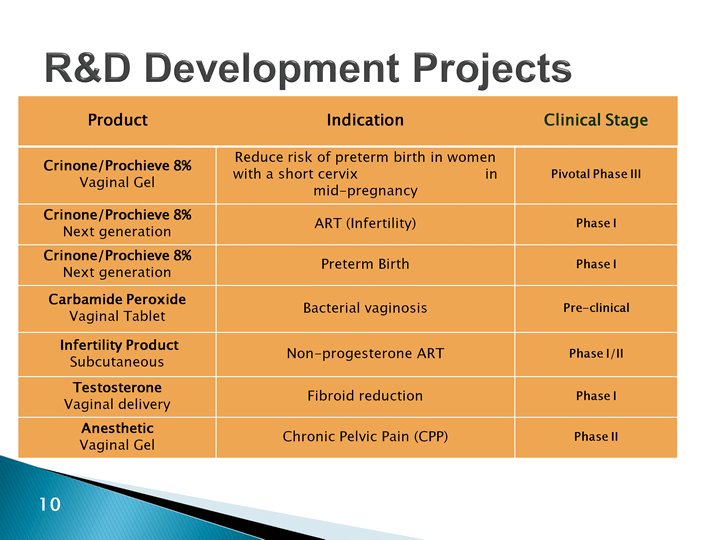
| R&D Development Projects Product Indication Clinical Stage Crinone/Prochieve 8%Vaginal Gel Reduce risk of preterm birth in women with a short cervix in mid-pregnancy Pivotal Phase III Crinone/Prochieve 8%Next generation ART (Infertility) Phase I Crinone/Prochieve 8%Next generation Preterm Birth Phase I Carbamide Peroxide Vaginal Tablet Bacterial vaginosis Pre-clinical Infertility ProductSubcutaneous Non-progesterone ART Phase I/II TestosteroneVaginal delivery Fibroid reduction Phase I AnestheticVaginal Gel Chronic Pelvic Pain (CPP) Phase II |
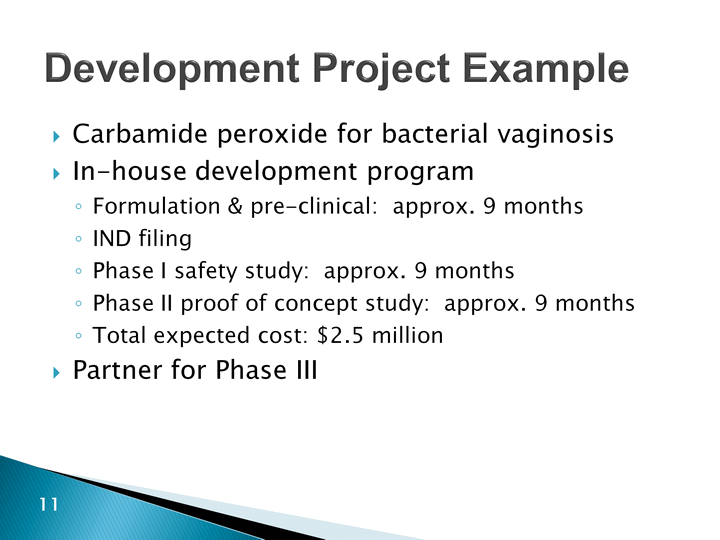
| Development Project Example Carbamide peroxide for bacterial vaginosisIn-house development programFormulation & pre-clinical: approx. 9 monthsIND filingPhase I safety study: approx. 9 monthsPhase II proof of concept study: approx. 9 monthsTotal expected cost: $2.5 millionPartner for Phase III |
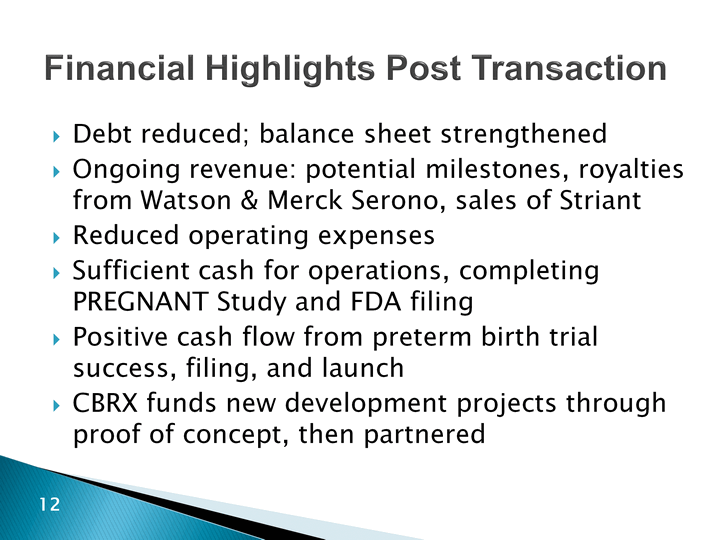
| Financial Highlights Post Transaction Debt reduced; balance sheet strengthenedOngoing revenue: potential milestones, royalties from Watson & Merck Serono, sales of StriantReduced operating expensesSufficient cash for operations, completing PREGNANT Study and FDA filingPositive cash flow from preterm birth trial success, filing, and launchCBRX funds new development projects through proof of concept, then partnered |

| Q & A |

| Summary Watson is a strong partner positioned toMaximize current & future revenues in infertilityDeliver results from preterm birth indicationUp to $92.5 million in upfront and potential milestone payments plus double-digit royalties on sales Strengthened balance sheet Majority of debt will be retiredPreserves upside potential from preterm birth opportunityColumbia becomes a focused business with a clearer path to profitability |

| Today's Announcements & The Plan Going ForwardAn Investor/Market UpdateMarch 4, 2010 |
