Attached files
| file | filename |
|---|---|
| EX-23.1 - SAVIENT PHARMACEUTICALS INC | c60440_ex23-1.htm |
| EX-21.1 - SAVIENT PHARMACEUTICALS INC | c60440_ex21-1.htm |
| EX-31.1 - SAVIENT PHARMACEUTICALS INC | c60440_ex31-1.htm |
| EX-31.2 - SAVIENT PHARMACEUTICALS INC | c60440_ex31-2.htm |
| EX-32.1 - SAVIENT PHARMACEUTICALS INC | c60440_ex32-1.htm |
| EX-32.2 - SAVIENT PHARMACEUTICALS INC | c60440_ex32-2.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
|
|
|
R |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
For the fiscal year ended December 31, 2009 |
|
OR |
||
£ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
For the transition period from to |
|
Commission File Number 0-15313
SAVIENT PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
|
|
Delaware |
13-3033811 |
|
Registrant’s telephone number, including area code: |
||
Securities registered pursuant to Section 12(b) of the Act: |
||
Common Stock, $.01 par value |
Nasdaq Global Market |
|
Securities registered pursuant to Section 12(g) of the Act: |
||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes R No £
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes £ No R
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes R No £
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. £
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes £ No £
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
|
|
|
|
|
|
Large accelerated filer R |
Accelerated filer £ |
Non-accelerated filer £ |
Smaller reporting company £ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes £ No R
The aggregate market value of Common Stock held by non-affiliates of the registrant (based on the closing price of these securities as reported by The Nasdaq Global Market on June 30, 2009) was approximately $535,481,000. Shares of Common Stock held by executive officers and directors of the registrant are not included in the computation. However, the registrant has made no determination that such individuals are “affiliates” within the meaning of Rule 405 under the Securities Act of 1933.
As of February 24, 2010, the number of shares of Common Stock outstanding was 66,953,873.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the Registrant’s definitive proxy statement for its 2010 annual meeting of stockholders are incorporated by reference into Part III of this report.
TABLE OF CONTENTS
3 PART I
BUSINESS
4
26
RISK FACTORS
27
UNRESOLVED STAFF COMMENTS
45
PROPERTIES
45
LEGAL PROCEEDINGS
46
[RESERVED]
46 PART II
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
47
SELECTED CONSOLIDATED FINANCIAL DATA
49
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
51
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
68
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
69
71
SAVIENT PHARMACEUTICALS, INC. CONSOLIDATED BALANCE SHEETS (In thousands, except share data)
73
74
75
SAVIENT PHARMACEUTICALS, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS (In thousands)
78
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
80
116
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
117
CONTROLS AND PROCEDURES
117
OTHER INFORMATION
117 PART III
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
118
EXECUTIVE COMPENSATION
118
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
118
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
118
PRINCIPAL ACCOUNTANT FEES AND SERVICES
118 PART IV
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
119
122 2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This Annual Report on Form 10-K and the documents incorporated by reference herein contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, that involve substantial risks and uncertainties.
All statements, other than statements of historical fact, including statements regarding our strategy, future operations, future financial position, future results of operations, future cash flows, projected costs, financing plans, product development, possible strategic alliances, competitive position, prospects, plans and
objectives of management, are forward-looking statements. We often use words such as “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “may,” “predict,” “will,” and “would,” and similar expressions, to identify forward-looking statements, although not all forward-looking statements contain
these identifying words. These forward-looking statements include, among other things, statements about:
•
our efforts to respond to the Complete Response Letter received from the US Food and Drug Administration, or FDA, on July 31, 2009 with respect to our Biologic License Application, or BLA, for our drug product candidate, KRYSTEXXA, • our efforts to revert to and validate the original manufacturing process used to produce KRYSTEXXA drug product for the Phase 3 clinical trials and to make certain other revisions to the manufacturing process, • the efforts of our primary third-party active pharmaceutical ingredient, or API, drug substance manufacturer, Bio-Technology General (Israel) Ltd, or BTG, to address the deficiencies and other observations identified by the FDA during its pre-approval inspection of BTG’s manufacturing facility, • our efforts to resubmit our BLA to the FDA, • the FDA’s consideration of our BLA for KRYSTEXXA, and our ability to obtain necessary FDA and foreign regulatory approvals, • our ability to complete the development of and execute our commercial strategy for KRYSTEXXA, • our ability to collaborate with a partner for the commercialization of KRYSTEXXA in the United States or internationally or complete a broader strategic transaction involving our company, • our ability to achieve profitability and raise the additional capital needed to achieve our business objectives, and • the market size for KRYSTEXXA and its degree of market acceptance. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking
statements that we make. We have included important factors in various cautionary statements included in this Annual Report on Form 10-K, particularly in the “Risk Factors” section, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-
looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make. You should read this Annual Report on Form 10-K, including the documents that we have incorporated by reference herein and filed as exhibits hereto, completely and with the understanding that our actual future results may be materially different from what we expect. We undertake no obligation to update any
forward-looking statements. 3
PART I ITEM 1. BUSINESS Overview We are a specialty biopharmaceutical company focused on developing KRYSTEXXA™ (pegloticase), or KRYSTEXXA, for the treatment of chronic gout in patients refractory to conventional therapy. Chronic gout that is refractory to conventional therapy occurs in patients who have failed to normalize serum
uric acid and whose signs and symptoms are inadequately controlled with xanthine oxidase inhibitors at the maximum medically appropriate dose or for whom these drugs are contraindicated. KRYSTEXXA is not recommended for the treatment of asymptomatic hyperuricemia. In our replicate randomized,
double-blind, placebo-controlled, Phase 3 clinical trials, KRYSTEXXA, a biologic PEGylated uricase enzyme, demonstrated the normalization of uric acid, complete resolution of tophi, improvement in chronic pain, improvement of physical functioning and decreased frequency of gout flares in patients with chronic
gout. Our strategic plan is to seek approval from the U.S. Food and Drug Administration, or FDA, of our Biologic License Application, or BLA, filing for KRYSTEXXA and to develop a program of regulatory filings and review of KRYSTEXXA in other countries. We continue to believe that a worldwide
partnership for the commercialization of KRYSTEXXA or broader strategic transaction involving our company would serve to maximize the commercial prospects of KRYSTEXXA in the United States, Europe and other foreign countries. If we are not able to consummate a worldwide partnership or broader
strategic transaction on terms acceptable to us, we intend to independently pursue the commercialization of KRYSTEXXA in the United States. Currently, we sell and distribute branded and generic versions of oxandrolone, a drug used to promote weight gain following involuntary weight loss. We launched our authorized generic version of oxandrolone in December 2006 in response to the approval and launch of generic competition to Oxandrin®. The
introduction of oxandrolone generics has led to significant decreases in demand for Oxandrin and our authorized generic version of oxandrolone. We believe that revenues from Oxandrin and our authorized generic version of oxandrolone will remain flat or continue to decrease in future periods. We currently operate within one “Specialty Pharmaceutical” segment, which includes sales of Oxandrin and oxandrolone and the research and development activities of KRYSTEXXA. Total revenues from continuing operations were $3.0 million in 2009, a decrease of $0.2 million, or 7%, from $3.2 million in
2008. KRYSTEXXA™ (pegloticase) KRYSTEXXA is a biologic PEGylated uricase being developed as a treatment for chronic gout in patients refractory to conventional therapy. Chronic gout that is refractory to conventional therapy occurs in patients who have failed to normalize serum uric acid and whose signs and symptoms are inadequately
controlled with xanthine oxidase inhibitors at the maximum medically appropriate dose or for whom these drugs are contraindicated. KRYSTEXXA would not be indicated for the treatment of asymptomatic hyperuricemia. We produce uricase, using a third-party manufacturer, through a recombinant process in
which genetically engineered bacteria produce uricase. We then PEGylate the resulting uricase in order to decrease the rate at which the human body would otherwise clear the uricase. PEGylation refers to a process in which a polyethylene glycol polymer is attached to a molecule. With KRYSTEXXA, we
PEGylate uricase by attaching methoxypolyethylene glycol, or mPEG, to it. Gout develops when urate, which is a derivative of uric acid, accumulates in the tissues and joints as a result of elevation of blood concentration of urate. Gout is usually associated with bouts of severe joint pain and disability, or gout flares, and tissue deposits of urate which may occur in concentrated forms, or
gout tophi. Patients with severe gout have an associated increased risk of kidney failure and increased risk of cardiovascular disease. Uricase is an enzyme that is not naturally expressed in humans, but is present in almost all other mammals. Uricase eliminates uric acid from the body by converting it 4
to allantoin, which is easily excreted by the kidney. Uric acid is poorly soluble in blood and tends to precipitate when the blood concentration is too high. In contrast, allantoin is easily soluble in blood and does not tend to precipitate in the body tissues or in urine. We believe that treatment with KRYSTEXXA will
control hyperuricemia by eliminating uric acid in the blood and tissue deposits of urate, thereby providing clinical benefits in addition to controlling hyperuricemia. Although the size of the market for KRYSTEXXA is difficult to predict at this time, we currently expect, based on our research, that there are approximately 100,000 to 170,000 patients who have chronic gout that is refractory to conventional therapy. KRYSTEXXA received “orphan drug” designation from the FDA in 2001. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process; however, it does make the product eligible for orphan drug exclusivity and specific tax credits in the
United States. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the use for which it has such designation, the product is entitled to orphan drug exclusivity. In such a case, later applications by other companies to market the same drug for the same use
may not be approved in the United States for a period of seven years, except in limited circumstances, such as if a competitive product is shown to be clinically superior to the original product. Orphan drug designation of KRYSTEXXA does not prevent competitors from introducing different competing drugs for
the same orphan indication that is covered by KRYSTEXXA’s orphan designation or the same drug for a different indication. Phase 1 Trials In 2001, we conducted the first of two Phase 1 clinical trials at the Duke University Medical Center, using subcutaneous delivery of KRYSTEXXA. The results demonstrated that over the three-week period of observation, uric acid levels decreased as the dosage of KRYSTEXXA increased. Two of 13
individuals participating in the study experienced allergic-type reactions. Therefore, we terminated the trial and abandoned the subcutaneous delivery approach. We completed a second Phase 1 clinical trial in 2003 in which KRYSTEXXA was administered intravenously. The results of this trial demonstrated that plasma uric acid levels decreased as blood level of KRYSTEXXA enzyme activity increased. This trial allowed us to identify a minimum effective dosage of
KRYSTEXXA and the dosage at which plasma uric acid levels were no longer improved by increasing the dosage of KRYSTEXXA, which is referred to as a dose-response plateau. In this second Phase 1 intravenous trial, no clinical allergic responses were observed, no infusion reactions were reported, and no
adverse reactions at the site of intravenous infusion were noted. Phase 2 Trials In 2004, we conducted an open label, randomized, three-month Phase 2 safety and efficacy clinical trial of KRYSTEXXA to determine an appropriate dose and dose regimen and to support further testing in pivotal Phase 3 trials. There were 41 participants in this trial. The results of the study confirmed and
extended the single dose data obtained in Phase 1, again showing a minimum effective dose and a dose-response plateau in terms of plasma uric acid normalization. These results supported our view as to KRYSTEXXA’s potential efficacy in reducing circulating uric acid. The most common adverse events observed
in the Phase 2 trial were gout flares. In addition, 44% of patients experienced one or more adverse events that we determined may have been related to the study infusion. No other important safety concerns arose from the Phase 2 data set. Phase 3 Trials Based on the results of our end-of-Phase 2 meeting with the FDA and post-meeting interactions, in December 2005, we submitted a protocol to the FDA under a Special Protocol Assessment, or SPA, for a Phase 3 clinical trial for KRYSTEXXA. In May 2006, we received written notification from the FDA
concurring that the Phase 3 protocols that we had specified were adequate to address the objectives of our Phase 3 clinical program. In June 2006, we began patient dosing in our two replicate Phase 3 clinical 5
trials of KRYSTEXXA for the treatment of chronic gout in patients refractory to conventional therapy, and patient recruitment was completed in March 2007. These international trials were multicenter, randomized, double-blind, placebo-controlled trials comparing KRYSTEXXA 8 mg intravenously every two weeks or every four weeks to placebo (every two weeks) in patients with chronic gout refractory to conventional therapy. Patients were randomized to the
two treatment groups and placebo in a 2:2:1 ratio, meaning that for every one patient in the placebo group, there were two patients in the every two weeks dosing group and two patients in the every four weeks dosing group. Patients were stratified by presence or absence of tophi. In order to maintain the blinded
nature of the studies, patients in the every four weeks dosing group received a placebo dose two weeks after each dose. The duration of each study was 26 weeks, including two weeks for screening and 24 weeks of treatment. The trials enrolled male and female patients at least 18 years old, hyperuricemic (with a serum uric acid level of at least 8 mg/dL) with symptomatic gout (at least three gout flares experienced in the 18
months prior to entry, or at least one gout tophus, or gouty arthritis) and for whom conventional urate-lowering therapy was contraindicated or had been ineffective. After completion of each study, patients were offered the option of continuing active treatment for up to an additional 24 months in an open label
extension, or OLE, study. In the first clinical trial of the two replicate studies conducted in the United States and Canada, there were 104 patients dosed (comprising the intent-to-treat population): 43 in the KRYSTEXXA every two weeks dose group, 41 in the KRYSTEXXA every four weeks dose group, and 20 in the placebo group.
In the second clinical trial of the two replicate studies conducted in the United States and Mexico, there were 108 patients dosed (comprising the intent-to-treat population): 42 in the KRYSTEXXA every two weeks dose group, 43 in the KRYSTEXXA every four weeks dose group, and 23 in the placebo group. We completed the treatment portion of our Phase 3 clinical trials of KRYSTEXXA in October 2007. The results from the Phase 3 trials showed that KRYSTEXXA 8 mg administered by a two-hour intravenous infusion every two weeks met the primary efficacy endpoint (statistically significant as compared to
placebo) in the intent-to-treat population (received at least one dose of study drug) analyses in each of the two replicate, six-month Phase 3 clinical trials. The every four weeks dose group also met the primary efficacy endpoint (statistically significant as compared to placebo). The primary efficacy endpoint
specified in the protocol was the normalization of plasma uric acid for more than 80% of the time during months three and six of the clinical trials. In the first clinical trial of the two replicate studies, the proportion of patients who met the primary endpoint for the every two weeks dose group was 47% (p is less than 0.001 as compared to placebo), and the proportion of patients who met the primary endpoint for the every four weeks dose group was 20%
(p is equal to 0.044 as compared to placebo). In the second clinical trial of the two replicate studies, the proportion of patients who met the primary endpoint for the every two weeks dose group was 38% (p is less than 0.001 as compared to placebo), and the proportion of patients who met the primary endpoint for
the every four weeks dose group was 49% (p is less than 0.001 as compared to placebo). Pooled across both studies, the proportion of patients who met the primary endpoint for the every two weeks dose group was 42% (p is less than 0.001 as compared to placebo), and the proportion of patients who met the
primary endpoint for the every four weeks dose group was 35% (p is less than 0.001 as compared to placebo). The proportion of patients who met the primary endpoint for the placebo group was zero in both trials. We assessed the following secondary endpoints using pre-specified pooled analyses (both studies combined) of each KRYSTEXXA treatment group as compared to placebo:
•
the proportion of patients with a tophus complete response, which is the elimination of a tophus without worsening of any other tophus or the appearance of a new tophus, • reduction in number of tender joints, • reduction in number of swollen joints, • improvement in the clinician’s global assessment of disease activity, 6
• improvement in patient-reported outcomes as measured by patient’s assessment of pain, patient’s global assessment of disease activity and patient’s assessment of disability using the Health Assessment Questionnaire, • improvement in patient-reported outcomes as measured by patient’s assessment of health-related quality of life using the Medical Outcomes Survey Short Form 36, or SF-36, and • reduction in the incidence and frequency of gout flares. The following endpoints for the KRYSTEXXA every two weeks dose groups were statistically significant as compared to placebo:
•
elimination of gout tophi in 40% of patients who received KRYSTEXXA as compared to 7% who received placebo. This endpoint also achieved statistical significance in each of the two studies individually, • reduction in the number of tender joints and improvement in the clinician’s global assessment of disease activity, • improvement in the patient-reported outcomes as measured by patient’s global assessment of disease activity and patient’s assessment of disability using the Health Assessment Questionnaire, • improvement in patient-reported outcomes as measured by patient’s assessment of overall health-related quality of life and pain as measured by the SF-36 physical component summary and bodily pain domain, and • reduction in the incidence and frequency of gout flares. As expected and is common with initiation of any urate lowering therapy, we observed an increase in the incidence and frequency of gout flares in months one through three of the trial as compared to placebo, followed by a reduction in the incidence
and frequency of gout flares in months four through six of the trial as compared to placebo. The following endpoints in the KRYSTEXXA every two weeks dose groups were not statistically significant as compared to placebo:
•
reduction in swollen joints, and • the mental component summary and the other dimensions (other than bodily pain) as measured by the SF-36 physical component summary. The primary endpoints were also met in the every four weeks dose group, though fewer of the secondary endpoints were met than in the every two weeks dose group. As discussed further below, the every two weeks dose regimen, we believe, presents a much clearer path toward FDA approval, so we have
determined to focus exclusively on this dosing regimen. The most common adverse events in the trials were gout flares, which occurred in 76% of patients in the every two weeks dose group, 83% of patients in the every four weeks dose group, and 81% of patients in the placebo group. An initial increase in gout flares (followed by a later decrease in gout flares) is
an expected side effect of urate-lowering therapies. Standard gout flare medicines, such as colchicine or non-steroidal anti-inflammatory drugs, were administered to help prevent gout flares. The second most common adverse events in the trials were infusion reactions, which occurred in 26% of patients in the every
two weeks dose group, 40% of patients in the every four weeks dose group and 5% in the placebo group. Most infusion reactions were managed by slowing the infusion or by stopping and restarting the infusion at a slower rate. Sometimes the physician also administered intravenous fluid or additional antihistamine
or anti-inflammatory drugs to treat the infusion reaction. As a prophylactic measure, all patients received a standardized regimen of glucocorticoids, antihistamines and acetaminophen to help mitigate the occurrence and severity of infusion reactions. Eleven of 169 KRYSTEXXA-treated patients in the Phase 3
clinical trials, or 6.5%, had an infusion reaction reported as a severe adverse event, and 12% withdrew because of infusion reactions. In late January 2009, under the auspices of the BLA Oversight Committee of our Board of Directors, we completed a formal blinded and unblinded post-hoc adjudication of all cardiovascular events included in reports of serious adverse events and serious infusion reactions from the Phase 3 trial. Our
evaluation was predicated on the Anti-Platelet Trialist Collaborative, or APTC, assessment 7
criteria, which is a standardized approach that the FDA has widely accepted as a method of evaluating cardiovascular risk. APTC cardiovascular events include deaths, nonfatal myocardial infarction and nonfatal stroke. As a result of the review, we concluded that there were three APTC cardiovascular events in
the KRYSTEXXA treatment arms of the Phase 3 trials (not including the OLE study) and none in the comparator placebo treatment arm. We also found that there were 10 non-APTC cardiovascular events in the treatment arms and no non-APTC cardiovascular events in the comparator placebo treatment arm. In
addition, we evaluated cardiovascular events in the Phase 2 trial and the OLE study. However, because there was no placebo arm in the Phase 2 trial or the OLE study, there were no comparative results between the treatment group and the placebo group. In late January 2009, we completed an additional analysis by reviewing all cause mortality in the Phase 3 trials and the OLE study. In that review, we noted that in the Phase 3 trials, there was a 2:2:1 randomization used, meaning that four times as many patients were receiving KRYSTEXXA treatment than
placebo. We determined that five deaths were in the KRYSTEXXA treatment arm of the combined Phase 3 trials and the OLE study, and three deaths were in the placebo comparator arm of the Phase 3 trials. On June 16, 2009, the FDA convened the Arthritis Advisory Committee, or AAC, in order to seek advice as part of the FDA’s regulatory decision-making process of the safety and efficacy data for KRYSTEXXA. The AAC reviewed separate data presentations by us and the FDA and discussed specific
questions asked by the FDA. The AAC recommended by a vote of 14 to 1 that KRYSTEXXA be granted marketing approval by the FDA for the treatment of chronic gout in patients refractory to conventional therapy. Open Label Extension Study The purpose of the OLE Study was to better understand the long-term safety of KRYSTEXXA in all patients who have completed the Phase 3 trials including those patients that received placebo during the Phase 3 trials. Patients did not know their Phase 3 group assignment and were permitted to choose to
go on or continue on KRYSTEXXA every two weeks or every four weeks or enter an observation group. One hundred fifty-seven patients completed one of the replicate double-blind pivotal Phase 3 clinical trials. One hundred fifty-one patients decided to enroll in the OLE study, 82 of whom decided to initially
receive pegloticase 8 mg treatment every two weeks, 67 of whom decided to initially receive pegloticase 8 mg treatment every four weeks and two of whom decided to be observed without further infusions. In the OLE study, the frequency of safety data collection was identical to the measures used in the Phase 3 trials while efficacy assessments were less frequent. Although there is no concurrent placebo comparator group, the interim efficacy and safety data of KRYSTEXXA in the OLE study appear to be
consistent with the randomized controlled Phase 3 clinical trials. In early July 2009, we completed the patient dosing phase of the OLE study, and in January 2010, we completed a protocol-specified six-month observation period for patients enrolled in this study. Re-Exposure Study A common issue with biologics is that there may be a more pronounced immune response upon re-exposure to the biologic after a prolonged drug-free period. This can lead to reduced effectiveness of the biologic upon a later re-exposure to the drug, and potentially present safety issues. We conducted a re-
exposure study in seven patients at four clinical sites that participated in KRYSTEXXA early development clinical studies. The goal of this study was to determine whether patients who are re-exposed to intravenous KRYSTEXXA treatment after a prolonged KRYSTEXXA-free interval could tolerate the drug.
The early development dose, schedule and number of KRYSTEXXA infusions as well of length of interval from last dose varied amongst these seven patients. These patients had not received KRYSTEXXA treatment since completing the Phase 1 or Phase 2 study in which they participated. The dosing phase of
this study has been completed and we are currently evaluating the results of this small study which may be useful in the design of future clinical studies to better understand re-exposure after finite periods of KRYSTEXXA therapy. 8
Effective July 1, 2009 there are no longer any patients who are receiving KRYSTEXXA in either the re-exposure study or the OLE study. BLA Filings In October 2008, we submitted our BLA to the FDA seeking approval to market KRYSTEXXA in the United States, and in December 2008, we were notified that the BLA was accepted with priority review status. The FDA also notified us of the acceptance, subject to final approval, of the trade name
KRYSTEXXA as the proprietary name for pegloticase in January 2009. We also received acceptance by the European Medicines Agency, or EMEA, of the trade name KRYSTEXXA for the European Union. During the course of the FDA’s review of our BLA, we submitted amendments to our BLA in late January 2009 to strengthen and clarify the data included in the BLA and to address review-related questions from the FDA. The key amendments to the BLA include:
•
Clearer Data on Cardiovascular Profile. We provided the results of our formal blinded and unblinded post-hoc adjudication of all cardiovascular events included in reports of serious adverse events and serious infusion reactions from the Phase 2 and Phase 3 trials and the OLE study. • Updated Immunogenicity Guidance. We performed additional review and analyses of the immunogenicity data in the Phase 3 trials to further understand the immune response to the drug. We found that antibody development followed a very predictable pattern, developing early following the initiation of
therapy and, in most cases, by month four, and then remaining consistent throughout the patient’s participation in the Phase 3 trials and the OLE study. Those patients who developed high antibody titers were found to have a higher incidence of infusion reactions. We also concluded that patients who
developed high antibody titers also had a corresponding risk of losing a uric acid response. We concluded that the effect of immunogenicity can be determined by a loss of uric acid response within the first four months of treatment. Loss of uric acid control can be readily detected by physicians monitoring a
standard laboratory serum uric acid level. As a result, patients who should not continue to receive KRYSTEXXA can be identified within the first four months of treatment by a standard single tool. Based on these findings and conclusions, we are proposing labeling that provides practical guidance for
physicians to manage both the benefit of the therapy and potential risk related to immunogenicity and infusion reactions. • Revised REMS Program and Postmarket Study. Based on these new analyses and data, we determined that our BLA for KRYSTEXXA would be strengthened by revising the Risk Evaluation and Mitigation Strategy, or REMS, program for KRYSTEXXA. The revised REMS program that we proposed is
based on an Elements to Assure Safe Use program. This program is based on a performance linked access program for prescribers, infusion sites and patients that will be required to enroll in this annual program and be certified in order to prescribe, administer or receive KRYSTEXXA. We also revised our
previously proposed 1,000 patient observational study so that it would instead comprise an expanded voluntary registry of up to 3,000 patients taking KRYSTEXXA for up to five years. The registry would capture more detailed information on adverse events than can be obtained through spontaneous adverse
event reporting. It may also capture information regarding the long-term effectiveness of KRYSTEXXA, prescribing patterns and quality of life outcomes for patients on KRYSTEXXA therapy, as well as additional information about cardiovascular and infusion reaction adverse events. • Revised Recommended Dosing Regimen and Indication. We are recommending pegloticase 8 mg every two weeks as a treatment for patients with chronic gout refractory to conventional therapy to control hyperuricemia and to manage signs and symptoms of gout, including the improvement of tophi,
improvement in chronic pain, improvement of physical functioning and decrease in frequency of flares. Although both the every two weeks dose regimen and every four weeks dose regimen for KRYSTEXXA met the primary efficacy endpoint in the Phase 3 trials, the every two weeks dose 9
regimen, we believe, presents a much clearer path toward FDA approval due to its more robust risk-to-benefit profile. As a result, we have determined to focus exclusively on the every two weeks dose regimen and have recommended only this dosage for approval with the amended BLA. We have determined not
to seek FDA approval for the every four weeks dose in the amended BLA. The FDA determined that the amendments to the BLA constituted major amendments, and so the FDA elected under applicable procedures to extend its review period of the BLA for KRYSTEXXA by three months, establishing a revised Prescription Drug User Fee Act action, or PDUFA, date of August 1,
2009. Complete Response Letter and Type A Meeting On July 31, 2009, the FDA issued a complete response letter, or CRL, notifying us that it was not in a position to approve the BLA as a treatment for chronic gout in patients refractory to conventional therapy unless and until a resubmission was provided to the FDA that addressed:
•
deficiencies with the chemistry, manufacturing and controls, or CMC, section of the BLA, • remediation of observations arising from the FDA pre-approval inspection of the manufacturing facility of our primary third-party active pharmaceutical ingredient, or API, manufacturer, Bio-Technology General (Israel) Ltd, or BTG, • a REMS program (consisting of a Medication Guide and a Communication Plan), and • a safety update focused on new clinical and pre-clinical data of KRYSTEXXA since the 120-Day Safety Update. One of the issues raised by the FDA in the CRL addressed a change in PEGylation concentration that we made in the proposed process for manufacturing pegloticase API drug substance for commercial use of KRYSTEXXA final drug product. The FDA concluded that the comparability data that we
submitted for the material manufactured using the proposed commercial manufacturing process was not adequate to demonstrate that it was representative of the material used to establish the safety and efficacy of KRYSTEXXA in the Phase 3 clinical trials. The FDA stated that we had the option of either
reverting to and validating the manufacturing process used to produce pegloticase for the Phase 3 clinical trials or conducting additional comparability clinical trials to support the use of KRYSTEXXA manufactured using the proposed commercial manufacturing process. The CRL also stated that the FDA determined that a REMS program is necessary for KRYSTEXXA consisting of:
•
a Medication Guide to ensure the safe and effective use of KRYSTEXXA by patients, alert and warn healthcare providers and patients about the risks of infusion reactions and anaphylaxis associated with KRYSTEXXA therapy, alert and warn healthcare providers not to use KRYSTEXXA in individuals
with glucose 6-phosphate dehydrogenase (G6PD) deficiency, and inform and educate healthcare providers to exercise caution when prescribing KRYSTEXXA to patients with major cardiovascular diseases, • a Communication Plan directed to healthcare providers likely to prescribe KRYSTEXXA to provide for the dissemination of information about the risks of severe infusion reactions and anaphylaxis, severe events associated with the use of KRYSTEXXA in individuals with G6PD deficiency, and major
cardiovascular events, and • an Assessment Plan to survey patients’ and providers’ understanding of the serious risks of KRYSTEXXA. The CRL also included the latest FDA comments on our draft label language as well as additional CMC comments focused on tightening a number of analytical methods used to assess manufacturing and narrowing analytical specifications associated with commercial production of pegloticase. Following our review of the CRL, we requested a “Type A” meeting with the FDA, which occurred on September 14, 2009, to determine defined resolutions with respect to the necessary requirements for approval of our BLA. At this meeting, we gained clarification from the FDA on the contents of the CRL
and proposed a resubmission plan to fully address all deficiencies and issues 10
identified therein. In response to the deficiencies cited by the FDA with the CMC section of our BLA, we proposed to revert to and validate the original manufacturing process used to produce KRYSTEXXA drug product for the Phase 3 clinical trials and to make certain revisions, such as the inclusion of
additional 0.2 micron filters, to the manufacturing process. The FDA indicated that, in its view, our plan was a reasonable approach that would be expected to produce pegloticase API drug substance that is representative of that used to establish safety and efficacy in the Phase 3 clinical trials. The FDA also stated
that it expects that the comparability between the material produced with this validated Phase 3 process that includes the additional filters and the Phase 3 clinical trial material used in the replicate clinical trials to establish safety and efficacy can be sufficiently established by quality criteria alone without the need
to conduct additional clinical studies, provided no significant differences between products are observed. The FDA also agreed at this meeting with the methods and criteria proposed by us to tighten a number of analytical methods used to assess manufacturing and acceptance criteria for all manufacturing steps in the final commercial production process for pegloticase. However, some of these final analytical
methods and acceptance criteria are subject to being set based upon the data from historical manufacturing and validation batches that will be included in the resubmission. Additionally, the FDA informed us that the review cycle for resubmission of our BLA for KRYSTEXXA would include the review of all data
to fully address all issues identified in the CRL, including the final product labeling and the REMS program materials. Since the resubmission will include REMS program materials, it is subject to a Class 2 review cycle, meaning simultaneous approval of all components of our filing within six months of the date of
resubmission. We expect to file our KRYSTEXXA BLA resubmission in March 2010. Manufacturing We do not own or operate our own manufacturing facilities and have historically used third-party contract manufacturers to manufacture our products under the oversight and supervision of our technical operations personnel. Although we intend to continue to rely on contract manufacturers to produce our
products, we have recruited personnel with manufacturing experience to oversee the production and release of KRYSTEXXA. The manufacturing process for pegloticase, which is the API drug substance of KRYSTEXXA, consists of the production of uricase through a recombinant process in which a genetically-engineered bacteria produces uricase, followed by its purification, PEGylation, further purification and formulation to
produce the bulk liquid API. The bulk API is then aseptically filled into single dose vials. The manufacturing process and the analytical methods used to test KRYSTEXXA and its intermediates have been validated as required by regulatory agencies prior to licensure. All clinical supplies of the API were produced by BTG, with aseptic filling being performed either by BTG, Wasserburger Arzneimittelwerk or Sigma-Tau PharmaSource, Inc., or Sigma-Tau (formerly known as Enzon Pharmaceuticals, Inc., which was acquired by Sigma-Tau in January 2010). If we obtain FDA
approval of our BLA filing, we plan to launch KRYSTEXXA with API drug substance produced at BTG, but with aseptic filling and finishing performed by Sigma-Tau. In support of these plans, we have transferred the filling and finishing process to Sigma-Tau and have submitted data in the BLA from validation
batches filled at Sigma-Tau from API manufactured at BTG. The production of the API is based on a recombinant microorganism, the subject of an issued patent owned by Duke University, or Duke, and licensed exclusively to us, as well as published patent applications owned directly by us. The manufacturing processes used in the production of the API drug substance
consist in most part of standard biotechnological techniques, except for the PEGylation step. The manufacturing process steps are covered by published patent applications owned directly by us and by an issued patent exclusively licensed to us by Mountain View Pharmaceuticals, or MVP. On July 31, 2009, the FDA issued a CRL notifying us that it would not approve the BLA as a treatment for chronic gout in patients refractory to conventional therapy unless and until a resubmission was provided to the FDA that addressed, among other items, deficiencies with the CMC section of the BLA.
The CRL also required correction of deficiencies and observations cited by the FDA during its June 2009 pre-approval inspection of BTG’s manufacturing facilities. While remediation of the 11
deficiencies and observations arising from the FDA pre-approval inspection had been underway since the June 2009 inspection in accordance with two work plans submitted to the FDA by BTG in June and July 2009, in October 2009 the FDA provided notification that these original work plans were not adequate
and identified additional corrective actions that would be required to successfully remediate the deficiencies and observations noted in their facility. Since October 2009, BTG has been addressing additional chemistry and manufacturing controls issues with their facility. At our September 2009 “Type A” meeting with the FDA, we proposed a resubmission plan to fully address all deficiencies and issues identified in the CRL. Specifically, we proposed to revert to and validate the original manufacturing process used to produce KRYSTEXXA drug product for the Phase 3
clinical trials and to make certain revisions, such as the inclusion of additional 0.22 micron filters, to the manufacturing process. The FDA indicated that, in its view, our plan was a reasonable approach that would be expected to produce pegloticase API drug substance that is representative of that used to establish
safety and efficacy in the pivotal Phase 3 clinical trials. The FDA also agreed at this meeting with the methods and criteria proposed by us to tighten a number of analytical methods used to assess manufacturing and acceptance criteria for all manufacturing steps in the final commercial production process for
pegloticase. However, some of these final analytical methods and acceptance criteria are subject to being set based upon the data from historical manufacturing and validation batches that will be included in the resubmission. In late October 2009, as part of our validation campaign, we completed the manufacture of three consecutive batches of pegloticase API drug substance at BTG using the manufacturing process used to manufacture pegloticase API drug substance for the Phase 3 clinical trials. These batches were manufactured
into final KRYSTEXXA drug product in November 2009 and samples from each batch were placed on stability testing by early December 2009. Our third-party testing laboratories then performed in-process and final release analytical testing on each batch of pegloticase API drug substance and final drug product
to validate their comparability to the drug substance and final drug product used in the Phase 3 clinical trials. We have received the analytical test results performed on these batches. Based on these results, we believe that we have successfully reverted to the Phase 3 manufacturing process and that the pegloticase API drug substance and final drug product produced in this validation campaign are comparable to the
material used in the Phase 3 trial. BTG submitted to the FDA in November and December 2009 reports of the steps that BTG has completed to date with the goal of addressing the deficiencies and other observations identified by the FDA during its pre-approval inspection of BTG’s manufacturing facility in June 2009. In addition, BTG
included in its submissions its plans and framework for implementing a broad, continuous quality improvement plan. In early February 2010, BTG received a letter from the FDA stating that the corrective actions implemented by BTG and the additional commitments made by BTG appear to address the FDA’s
concerns. The FDA also stated in its letter that it will verify these corrective actions and additional commitments during the FDA’s next inspection of BTG’s facility. The FDA may nevertheless continue to raise additional issues, or reassert previously raised issues, with BTG’s facility at any time during its review of
our BLA resubmission. It is also possible that the FDA could require us to repeat our manufacturing validation campaign once the FDA determines that BTG has resolved all of these issues to the satisfaction of the FDA, though we do not expect the FDA to take this step. We expect that the FDA will schedule its
next reinspection of BTG’s facility for a time prior to making its decision on our BLA resubmission. If so, the FDA may, at that reinspection, determine that it is or is not satisfied with BTG’s corrective actions and additional commitments, or it may identify new deficiencies with BTG’s facility. We are advancing our efforts to complete the validation of manufacturing processes for the pegloticase API drug substance by a second contract manufacturing organization, Diosynth RTP, or Diosynth, which is located in the United States. However, we will continue to rely on a single source of supply, NOF
Corporation of Japan, or NOF, for mPEG, the PEGylation reagent. We have commenced efforts to engage a secondary third-party fill and finish manufacturer for KRYSTEXXA, but at this time no contract agreement is in place for this secondary third-party function. As such, we also plan to continue to rely on a
single provider of fill and finish services, Sigma-Tau, for the foreseeable future. In 12
support of our plans to commercialize KRYSTEXXA, we have entered into agreements with BTG and Sigma-Tau for the supply of commercial materials and with NOF for supply of the PEGylation reagent. For more information on our agreements with BTG, Diosynth, NOF and Sigma-Tau, please see “Manufacturing, Supply, Distribution and Other Arrangements”. Sales and Marketing If the BLA for KRYSTEXXA receives FDA approval, we may seek to commercialize KRYSTEXXA ourselves, or collaborate with a third party to commercialize KRYSTEXXA in the United States. We would expect to collaborate with a third party in any event to commercialize KRYSTEXXA outside of
the United States. If we commercialize KRYSTEXXA in the United States ourselves, rheumatologists will be our primary target audience for our marketing and sales activities relating to KRYSTEXXA. Although the majority of gout patients are seen in primary care settings, the most difficult cases are typically referred to
rheumatologists. There are approximately 4,500 rheumatologists in the United States. Administration of biologic therapy via intravenous infusion is well-established in clinical practice in the rheumatology setting as there are two widely used pharmaceutical products used for the treatment of rheumatoid arthritis
that are administered via intravenous infusion. The key opinion leaders in the gout field are rheumatologists, many of whom have been involved in the clinical development program for KRYSTEXXA. A subset of nephrologists see a high volume of complicated gout patients, due to co-morbidity of gout with renal disease, and the use of calcineurin inhibitors (which exacerbate hyperuricemia) in kidney transplant recipients. Nephrologists mainly administer infused therapies in the hospital setting. If we
commercialize KRYSTEXXA ourselves in the United States, we plan to target these approximately 1,000 specialized nephrologists who we have determined have potential for adoption of KRYSTEXXA. Infusion nurses play a critical role for any infused product because they both perform the administration and are with the patient throughout the entire time of the infusion, providing a potential educational channel to patients. If we commercialize KRYSTEXXA ourselves in the United States, we also plan to
target infusion nurses for field force calls. We do not currently have an existing field force, as we do not actively promote any products at this time. In preparation for the launch of KRYSTEXXA, we have conducted field force sizing analyses based on these target audiences and presently believe that a field force of 60 specialty representatives will be
adequate and cost-effective for a commercial launch. If we commercialize KRYSTEXXA ourselves in the United States, in hiring these specialty representatives, our hiring criteria will stress experience in selling infused biologics, especially in the rheumatology field. We plan to time these hires so that our
commitments increase only as our expectations regarding BLA approval become more certain. Oxandrin and oxandrolone Oxandrin is an oral synthetic derivative of testosterone used to promote weight gain following involuntary weight loss related to disease or medical condition. We sell Oxandrin in both 2.5 mg and 10 mg tablets. We first introduced Oxandrin in the 2.5 mg strength in December 1995 and followed with the 10 mg
tablet in October 2002. We introduced the 10 mg strength to reduce the number of tablets required to be taken by patients taking 20 mg a day, which is a common dosage. For the years ended December 31, 2009 and 2008, approximately 46% and 40%, respectively, of all Oxandrin prescriptions were being filled
with the 10 mg tablet. Since our 1995 launch of Oxandrin, our Oxandrin sales have been primarily for the treatment of patients suffering from HIV/AIDS-related weight loss. Our financial results have been partially dependent on sales of Oxandrin since its launch in December 1995. Generic competition for Oxandrin began in December 2006. The introduction of generic products has caused a significant decrease in our Oxandrin revenues, which has had an adverse affect on our
financial results and cash flow. In response to the generic competition, we scaled back some of our business activities and eliminated our sales force related to the product. As a result, Oxandrin has become a less significant product for our future operating results. 13
In response to the December 2006 introduction of generic competition for Oxandrin, we, through our distribution partner Watson Pharma, Inc., or Watson, began distributing an authorized generic version of oxandrolone tablets, USP C-III, an Oxandrin brand equivalent product. The authorized generic version
of oxandrolone tablets has met all quality control standards of the Oxandrin brand and contains the same active and inactive pharmaceutical ingredients. We have a supply and distribution agreement in effect with Watson which provides for us to receive a significant portion of the gross margin earned by Watson on
sales of oxandrolone. Sales of Oxandrin and our authorized generic version of oxandrolone accounted for 100%, 95% and 99% of our 2009, 2008 and 2007 revenues, respectively. Manufacturing, Supply, Distribution and Other Arrangements KRYSTEXXA Bio-Technology General (Israel) Ltd. (a subsidiary of Ferring Pharmaceuticals) In March 2007, we entered into commercial supply and development agreements with BTG, pursuant to which BTG serves as the manufacturer and commercial supplier of the API drug substance for KRYSTEXXA and provides development, manufacturing and other services in relation to the product. Under
the agreements, BTG also provides support with respect to our BLA for KRYSTEXXA. Under our development agreement with BTG, BTG has agreed to provide development services and documentation needed for our regulatory filing in accordance with a development plan, including (i) validating the production facility and process; (ii) managing the contract production; and (iii) performing, and
allowing us to perform, quality assurance. The main purpose of the development agreement is to achieve validation of the manufacturing process which is defined, in part, as the successful validation of facilities, equipment and critical process steps in compliance with FDA requirements. Additionally, the
development agreement provides that it expires on the later of a) the completion of the development Plan, or b) first regulatory approval. Under our commercial supply agreement with BTG, as amended in June 2008, BTG has agreed to provide (i) all information and assistance necessary for the preparation of comprehensive and complete BLAs, and (ii) access to its facility to FDA inspectors conducting pre-approval inspections. We are obligated
to provide BTG with a rolling forecast on a monthly basis setting forth the total quantity of API drug substance that we expect to require for commercial supply in the following 18 months. The first six months of each forecast represent a rolling firm irrevocable order, and we may only increase or decrease our
forecast for the next 12 months within specified limits. As of December 31, 2009, based on our latest forecast, we were obligated to purchase an aggregate of approximately $5.0 million of API during 2010. During 2009 and 2008, we paid to BTG non-refundable fees of $0 and $2.2 million, respectively, to reserve
manufacturing capacity relating to our potential future orders of KRYSTEXXA. We recorded these capacity reservation fees, which may be credited as a discount against future orders of KRYSTEXXA, as research and development expenses as they were incurred. Under the commercial supply agreement, BTG is
obligated to manufacture for us our firmly forecasted commercial supply of KRYSTEXXA and we are obligated to purchase from BTG at least 80% of our worldwide requirements of API drug substance. However, if BTG produces specified numbers of failed batches of API within one or more calendar quarters,
then we may purchase all of our KRYSTEXXA requirements from other suppliers until BTG demonstrates to our reasonable satisfaction that it has remedied its supply failure. In addition, if our product forecasts are reasonably anticipated to exceed BTG’s processing capacity, then we may purchase from other
suppliers our KRYSTEXXA requirements that exceed BTG’s capacity. Beginning on the seventh anniversary of BTG’s first delivery of API under the commercial supply agreement, which may be as early as December 2015, either we or BTG may provide three years advance notice to terminate the commercial supply agreement. The commercial supply agreement may also be
terminated in the event of insolvency or uncured material breach by either party. 14
Diosynth RTP, Inc. In August 2007, we entered into a services agreement with Diosynth, pursuant to which Diosynth is preparing to serve as our secondary source supplier in the United States of API drug substance for KRYSTEXXA. Under the agreement, we are obligated to make specified milestone payments related to the
technology transfer, and subsequent performance, of the manufacturing and supply process, which was initiated in August 2007 with BTG’s cooperation. In November 2009, we entered into a revised services agreement with Diosynth, pursuant to which we delayed the 2009 conformance batch production campaign
until 2010. As of December 31, 2009, we were obligated to make approximately $7.9 million of remaining cash payments to Diosynth in 2010 under the agreement primarily related to the conformance batch production campaign coupled with other technology transfer services. We incurred total costs of $8.2 million
in 2009 for services rendered under the agreement. Included in the total costs incurred in 2009 was a $2.0 million fee for the reservation of manufacturing capacity required for the production of the conformance batches in 2010 and an additional $2.5 million idle and down-time fee imposed under the agreement
because we delayed the originally scheduled conformance batch production campaign from 2009 to 2010. We recorded the fees for capacity reservation, idle and down-time and other technology transfer services rendered by Diosynth as research and development expenses as they were incurred. Either we or Diosynth may terminate this agreement in the event of an uncured material breach by the other party. In addition, we may terminate the agreement at any time upon 45 days advance notice. If we terminate the agreement other than for Diosynth’s breach, or if Diosynth terminates the agreement
for our breach, we must pay Diosynth a termination fee based on the value of then remaining unbilled activities under the agreement. Either party may also terminate the agreement within 30 days after any written notice from Diosynth that, in its reasonable judgment and based on a change in the assumptions or
objectives for the project, it cannot continue to perform its obligations without a change in the scope, price or payment schedule for the project. We estimate that Diosynth will be able to commence its commercial supply of API for KRYSTEXXA as our secondary source supplier during the second half of 2011, at the earliest. This estimate assumes the validation batches are scheduled for production and testing in Q2 and Q3 of 2010, the execution of
validation batches meeting the same quality standards as those produced by BTG, the FDA’s satisfactory inspection and regulatory approval of the Diosynth manufacturing facilities and our completion and execution of a commercial supply agreement with Diosynth. We and Diosynth will jointly make a go/no-go
decision to have Diosynth proceed with commercial manufacturing following the evaluation of various preliminary manufacturing runs. NOF Corporation (Japan) In May 2007, we entered into a supply agreement with NOF pursuant to which NOF serves as our exclusive supplier of mPEG, which is used in the PEGylation process to produce KRYSTEXXA. We must purchase our entire supply of mPEG from NOF unless NOF fails to supply at least 75% of our firm
orders, in which case we may obtain mPEG from a third party until NOF’s supply failure is remedied to our reasonable satisfaction. Under the agreement, we are obligated to make specified minimum purchases of mPEG from NOF. We must provide NOF with a rolling forecast on a quarterly basis setting forth the total quantity of mPEG that we expect to require in the following 18 months. The first six months of each forecast represent a
rolling firm irrevocable order, and we may only increase or decrease our forecast for the next 12 months within specified limits. As of December 31, 2009, we were obligated to purchase mPEG at an aggregate cost of approximately $2.3 million and $2.3 million during 2010 and 2011, respectively. For any given year,
upon three months advance notice, we may terminate our minimum purchase obligation for the entire year or the remainder of that year by paying NOF 50% of the minimum purchase obligation for that year or the remainder of that year. Under the agreement, our minimum purchase obligations may be adjusted
or delayed upon mutual agreement of the parties in the event of a delay in FDA approval or filing of the BLA beyond March 2008. In accordance with these terms, we are in the process of renegotiating our minimum purchase obligations. Under the agreement, NOF was obligated to and did supply us with three
mPEG validation batches during 2007 and prepared a Type II 15
Drug Master File, or DMF, for submission to the FDA. NOF filed its Type II DMF with the FDA in December 2007 and it remains obligated under the agreement to use commercially reasonable efforts to submit a DMF, or its equivalent, to the appropriate regulatory agency in one additional country or the
European Union. Our agreement with NOF has an initial term ending in May 2017 and may be extended for an additional 10 years by mutual agreement of the parties at least 12 months before the expiration of the initial term. Prior to the expiration of the term, either we or NOF may terminate the agreement for convenience
upon 24 months advance notice. Either we or NOF may terminate the agreement in the event of the other party’s insolvency or uncured material breach. In the event that NOF terminates the agreement for convenience or if we terminate the agreement for NOF’s breach or bankruptcy, we may require NOF to
continue to supply mPEG for up to two years following the termination date. If we terminate the agreement for convenience or NOF terminates the agreement for our breach, we must pay NOF 50% of the minimum purchase obligation for the period from the termination date until the date on which the
agreement would have expired. Sigma-Tau PharmaSource, Inc. (formerly known as Enzon Pharmaceuticals, Inc., which was acquired by Sigma-Tau) In October 2008, we entered into a non-exclusive commercial supply agreement with Sigma-Tau, which replaced our 2006 service agreement with Sigma-Tau. Under the terms of the commercial supply agreement, Sigma-Tau has agreed to fill, inspect, package, test and provide specified product support services
for the final KRYSTEXXA product. In return, if KRYSTEXXA receives FDA marketing approval from the FDA, we will purchase from Sigma-Tau, product and product support services during 2010 and 2011 at an aggregate cost of approximately $1.7 million and $2.8 million, respectively. These purchase
obligations are based on a rolling forecast that we have agreed to provide to Sigma-Tau on a quarterly basis setting forth the total amount of final product that we expect to require in the following 24 months. The first six months of each forecast will represent a rolling firm irrevocable order, and we may only
increase or decrease our forecast for the next 18 months within specified limits. If we cancel batches subject to a firm order, we must pay Sigma-Tau a fee. Under the agreement, we are also obligated to pay Sigma-Tau a rolling, non-refundable capacity reservation fee, which may be credited against the fees for
Sigma-Tau’s production of the final product. During 2009, we incurred $0.3 million in capacity reservation fees. We recorded this capacity reservation fee as research and development expenses as incurred. In October 2009, we entered into an amendment agreement with Sigma-Tau modifying the payment terms for the filling, packaging, testing and other product support services that they will render for us. Under the terms of the amendment agreement, upon Sigma-Tau’s receipt of API drug substance from us,
Sigma-Tau will invoice us for an upfront non-refundable fee for their filling, packaging and testing services to be performed on the API. We will be responsible to pay for these services within 15 days of the invoice date even if we decide to delay the filling and packaging of the API drug substance material. After October 2009, either we or Sigma-Tau may terminate the agreement upon 24 months advance notice given 30 days before each year’s anniversary date of the agreement. If we terminate the agreement, we would be obligated to pay Sigma-Tau a fee based on the previously submitted rolling forecasts.
Either we or Sigma-Tau may also terminate the agreement in the event of insolvency or uncured material default in performance by either party, or in the event that the FDA does not approve our BLA for KRYSTEXXA and such disapproval is final or not appealed by us. In addition, if the FDA delays approval
of our BLA, the obligations of both parties will be held in abeyance for up to 18 months pending such approval, after which Sigma-Tau may terminate the agreement immediately and without penalty. We believe that our current arrangements for the supply of clinical and commercial quantities of pegloticase API drug substance and finished form KRYSTEXXA will be adequate to complete our clinical development program and to satisfy our currently forecasted commercial requirements of KRYSTEXXA. 16
Mountain View Pharmaceuticals, Inc. and Duke University We are party to an exclusive royalty bearing license agreement with MVP and Duke, originally entered into in August 1997 and amended in November 2001, granting us rights under technology relating to mammalian and non-mammalian uricases, and MVP’s technology relating to mPEG conjugates of these
uricases, as well as patents and pending patent applications covering this technology, to make, use and sell, for human treatment, products that use this technology. These patents and pending patent applications constitute the fundamental composition of matter and underlying manufacturing patents for
KRYSTEXXA. Under this agreement, we also have the exclusive license to the trademark Puricase®, which is a registered trademark of MVP and was available for potential use as the proprietary name of the product candidate we now refer to as KRYSTEXXA. However, if we elect not to use the trademark
Puricase, or if we otherwise fail to use the trademark Puricase within one year after the first sale of any product which uses the licensed technology, then MVP would retain all rights to use the trademark Puricase. Under the agreement, we are required to use best efforts to bring to market and diligently market products that use the licensed technology. The agreement requires us to pay to MVP and Duke quarterly royalty payments within 60 days after the end of each quarter based on KRYSTEXXA net sales we make
in that quarter. The royalty rate for a particular quarter ranges between 8% and 12% of net sales based on the amount of cumulative net sales made by us or our sub-licensees. Under the agreement, we are also required to pay royalties of 20% of any revenues or other consideration we receive from sub-licensees
during any quarter. During the year ended December 31, 2008, we made aggregate milestone payments to MVP and Duke of $0.5 million upon the filing of our BLA. We are also required to pay up to an aggregate of approximately $1.8 million to MVP and Duke if we successfully commercialize KRYSTEXXA
and attain specified KRYSTEXXA sales targets. In addition, if we obtain regulatory approval in one of five major global markets, which includes FDA approval in the United States, we will be required to make aggregate milestone payments of approximately $0.8 million to MVP and Duke. As of December 31,
2009, we have made aggregate payments of approximately $1.7 million to MVP and Duke for the achievement of milestones under this agreement. The agreement remains in effect, on a country-by-country basis, for the longer of 10 years from the date of first sale of KRYSTEXXA in such country, or the date of expiration of the last-to-expire patent covered by the agreement in such country. We may terminate this agreement in one or more countries with
six months prior notice, and we may also terminate the agreement with respect to any country in which the licensed patents are infringed by a third party or in which the manufacture, use or sale of KRYSTEXXA infringes a third party’s intellectual property rights. Either we or the licensors may also terminate the
agreement, with respect to the countries affected, upon the other party’s material breach, if not cured within a specified period of time, or immediately upon the other party’s third or subsequent material breach of the agreement or the other party’s fraud, willful misconduct or illegal conduct. Either party may also
terminate the agreement for the other party’s bankruptcy or insolvency. Upon a termination of the agreement in one or more countries, all intellectual property rights conveyed to us with respect to the terminated countries under the agreement, including regulatory applications and pre-clinical and clinical data,
revert to MVP and Duke and we are permitted to sell off any remaining inventory of KRYSTEXXA for such countries. Oxandrin Gedeon Richter Ltd. In February 1999, we entered into a supply agreement for oxandrolone bulk API with Gedeon Richter Ltd., or GRL, pursuant to which GRL served as our exclusive manufacturer and distributor of oxandrolone bulk API in the United States. Under the agreement, we were required to make specified minimum
purchases. In April 2007, in response to the introduction of generic forms of oxandrolone tablets by competitors, we and GRL mutually agreed to amend the supply agreement. Under the terms of the amended agreement, we and GRL eliminated our future purchase commitment obligations and the provisions for
supply exclusivity, and we purchased GRL’s remaining inventory of oxandrolone bulk API. The initial term of the amended agreement will expire in December 2010 and automatically 17
renews for successive one-year periods until such time as we or GRL terminate the agreement with advance notice of one year. DSM Pharmaceuticals, Inc. In December 2002, we entered into a supply agreement with DSM Pharmaceuticals, Inc., or DSM, for the manufacture of 2.5 mg and 10 mg Oxandrin and our authorized generic oxandrolone tablets. Under the agreement, which was amended in April 2007, we are obligated to supply DSM with sufficient
quantities of oxandrolone bulk API, which we acquire from GRL, for DSM’s production of the Oxandrin and authorized generic oxandrolone tablets. We are obligated to submit a rolling forecast to DSM on a monthly basis setting forth the total quantity of Oxandrin and authorized generic oxandrolone tablets that
we expect to require in the following 18 months. The first six months of each forecast represent a rolling firm irrevocable order. Our agreement with DSM will expire in December 2010 unless earlier terminated by mutual agreement of the parties. Either we or DSM may terminate the agreement at any time in the
event of insolvency or uncured material breach by the other party. During 2009, we were notified by DSM of its intent to terminate the supply agreement as of December 31, 2010. At this time, we do not intend to seek an alternative manufacturer for Oxandrin and authorized generic oxandrolone tablets. We expect DSM to manufacture sufficient quantities of Oxandrin and
authorized generic oxandrolone in 2010 prior to the termination of the supply agreement to adequately supply the products to our customers for at least the next two years. Oxandrolone Watson Pharma, Inc. In June 2006, we entered into a supply and distribution agreement with Watson, pursuant to which Watson serves as the exclusive U.S. distributor of our authorized generic version of oxandrolone tablets, an Oxandrin-brand equivalent product that we supply to Watson. Under the agreement, we began
providing oxandrolone to Watson for U.S. distribution in December 2006. We receive a significant portion of the gross margin earned by Watson on the sale of the product. The agreement has an initial term ending in June 2016, after which it will automatically renew for successive two-year periods unless we or Watson terminate upon one year’s advance notice. Either we or Watson may terminate the agreement upon one year’s advance notice, provided that such notice be
delivered no sooner than December 2010. Either we or Watson may terminate the agreement at any time in the event of insolvency or uncured material breach by the other party. Sales and Distribution We sell Oxandrin in the United States through our distributor, Integrated Commercialization Services, Inc., or ICS, mainly to three drug wholesaler customers: Cardinal Health, AmerisourceBergen Corp. (the parent of ICS) and McKesson Corp. Our sales to these three wholesalers as a percentage of our total
gross sales of Oxandrin relating to continuing operations were 74%, 83% and 82% for the years ended December 31, 2009, 2008 and 2007, respectively. In December 2006, we began selling our authorized generic version of oxandrolone to Watson for distribution in the United States. Sales of oxandrolone through Watson accounted for approximately 59%, 44% and 34% of our 2009, 2008 and 2007 total gross sales respectively. Research and Development Our research and development expenses related to continuing operations were $51.7 million, $55.5 million and $50.9 million for the years ended December 31, 2009, 2008 and 2007, respectively, all of which were incurred in the development of KRYSTEXXA. Research and development costs are expensed as
incurred and include salaries and benefits, stock-based compensation, and costs paid to third-party contractors to perform research, conduct clinical trials, and develop drug materials. 18
Manufacturing cost is a significant component of research and development expenses and include costs associated with third-party contractors for validation batch production, process technology transfer, quality control and stability testing, raw material purchases, overhead expenses and facilities costs. We
expense these manufacturing-related expenses as research and development as incurred because these costs do not meet the definition of an asset, as future use cannot be determined based upon the uncertainty of whether KRYSTEXXA will be approved for marketing by the FDA. Clinical trial cost is another
significant component of research and development expenses and most of our clinical studies are performed by third-party contract research organizations, or CROs. We accrue costs for clinical studies performed by CROs that are milestone or event driven in nature and based on reports and invoices submitted by
the CRO. These expenses are based on patient enrollment as well as costs consisting primarily of payments made to the CRO, clinical sites, investigators, testing facilities and patients for participating in our clinical trials. Non-refundable advance payments for future research and development activities are deferred
and capitalized. Such amounts are recognized as an expense as the goods are delivered or the related services are performed. Consistent with the consensus, we have deferred approximately $0 and $1.3 million of these costs as of December 31, 2009 and 2008, respectively, and have amortized approximately $0.7
million and $0.6 million for the years ended December 31, 2009 and 2008, respectively, based on services performed. The net deferred balance of $0 and $0.7 million as of December 31, 2009 and 2008, respectively, is included in Prepaid Expenses and Other Current Assets on our consolidated balance sheet. All
other research and development costs are charged to expense as incurred, including $2.3 million and $2.5 million of fees incurred during the years ended December 31, 2009 and 2008, respectively, to reserve manufacturing capacity at our third-party suppliers’ manufacturing facilities, for future potential orders of
KRYSTEXXA. We have received financial grants in support of research and development from the Office of the Chief Scientist of the State of Israel, or OCS, and the Israel-United States Bi-national Industrial Research and Development Foundation, or BIRD, of approximately $2.0 million and $0.6 million, respectively, for
the development of KRYSTEXXA. These grants are subject to repayment through royalties on the commercial sale of KRYSTEXXA if and when commercial sale commences. The OCS grants were received by our former subsidiary, BTG, and upon our divestiture of BTG to Ferring Pharmaceuticals, or Ferring,
we agreed to remain obligated to reimburse BTG for its repayments to OCS that relate to the KRYSTEXXA financial grants. In addition, under the Israeli Law of Encouragement of Research and Development in Industry, as amended, as a result of the funding received from OCS, if we do not manufacture 100%
of our annual worldwide bulk product requirements in Israel, we may be subject to total payments ranging from 120% to 300% of the repayment obligation plus interest, based upon the percentage of manufacturing that does not occur in Israel. Our aggregate principal repayment obligation plus interest to OCS and
BIRD if we successfully launch KRYSTEXXA and, as per our agreements with BTG and Diosynth, manufacture more than 20% of our annual worldwide bulk product requirements outside of Israel, would be between $4.0 million and $8.8 million at December 31, 2009. Governmental Regulation Regulatory Compliance Regulation by United States and foreign governmental authorities is a significant factor affecting our ability to commercialize KRYSTEXXA, the timing of such commercialization and our ongoing research and development activities. The commercialization of KRYSTEXXA requires regulatory approval by
governmental agencies prior to commercialization. Various laws and regulations govern or influence the research and development, non-clinical and clinical testing, manufacturing, processing, packing, validation, safety, labeling, storage, record keeping registration, listing, distribution, advertising, sale, and
marketing, and postmarketing commitments of our products. The lengthy process of seeking these approvals, and the subsequent compliance with applicable laws and regulations, require expending substantial resources. Any failure by us to obtain or maintain, or any delay in obtaining or maintaining, regulatory
approvals could materially adversely affect our business. Pharmaceutical products such as KRYSTEXXA may not be commercially marketed without prior approval from the FDA and comparable agencies in foreign countries. In the United States, the process 19
for obtaining FDA approval for products like KRYSTEXXA typically includes pre-clinical studies, the filing of an Investigational New Drug application, or IND, human clinical trials and filing and approval of either a New Drug Application, or NDA, for chemical pharmaceutical products, or a BLA for biological
pharmaceutical products. The results of pre-clinical testing, which include laboratory evaluation of product chemistry and formulation, animal studies to assess the potential safety and efficacy of the product and its formulations, details concerning the drug manufacturing process and its controls, and a proposed
clinical trial protocol and other information must be submitted to the FDA as part of an IND that must be reviewed and become effective before clinical testing can begin. The study protocol and informed consent information for patients in clinical trials must also be submitted to an independent institutional review
board, or IRB, for approval. Once a sponsor submits an IND, the sponsor must wait 30 calendar days before initiating any clinical trials, during which time the FDA has an opportunity to review the IND and raise concerns or questions relating to the proposed clinical trials outlined in the IND. If the FDA has
comments or questions, they must be resolved to the satisfaction of the FDA before clinical trials can begin. In addition, the FDA, an IRB or we may, at any time, impose a clinical hold on ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA
authorization and then only under terms authorized by the FDA. Our non-clinical and clinical studies must conform to the FDA’s Good Laboratory Practice, or GLP, and Good Clinical Practice, or GCP, requirements, which are designed to ensure the quality and integrity of submitted data and protect the rights
and well-being of study patients. Information for certain clinical trials also must be publicly disclosed within certain time limits on the clinical trial registry and results databank maintained by the National Institutes of Health, or NIH. Typically, clinical testing involves a three-phase process, however, the phases may overlap or be combined:
•
Phase 1 clinical trials are conducted in a small number of volunteers or patients to assess the early tolerability and safety profile, and the pattern of drug absorption, distribution and metabolism, • Phase 2 clinical trials are conducted in a limited patient population afflicted with a specific disease in order to assess appropriate dosages and dose regimens, expand evidence of the safety profile and evaluate preliminary efficacy, and • Phase 3 larger scale, multicenter, well-controlled clinical trials are conducted on patients with a specific disease to generate enough data to statistically evaluate the efficacy and safety of the product for approval, as required by the FDA, to establish the overall benefit-risk relationship of the drug and to
provide adequate information for the labeling of the drug. The results of the pre-clinical and clinical testing, chemistry, manufacturing and control information and proposed labeling are then submitted to the FDA in the form of either an NDA or BLA for review and potential approval to commence commercial sales. In responding to an NDA or BLA, the FDA may
grant marketing approval, request additional information in a complete response letter, or deny the approval if it determines that the NDA or BLA does not provide an adequate basis for approval. A complete response letter generally contains a statement of specific conditions that must be met in order to secure
final approval of an NDA or BLA and may require additional testing. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter, which authorizes commercial marketing of the product with specific prescribing information for specific indications, and
sometimes with specified postmarketing commitments. Any approval required from the FDA might not be obtained on a timely basis, if at all. Among the conditions for an NDA or BLA approval is the requirement that the manufacturing operations conform on an ongoing basis with current Good Manufacturing Practices, or cGMPs. In complying with cGMPs, we must expend time, money and effort in the areas of training, production and quality
control within our own organization and at our contract manufacturing laboratories. A successful inspection of the manufacturing facility by the FDA is a prerequisite for final approval of a biological product like KRYSTEXXA. Following approval of the NDA or BLA, we and our third-party manufacturers
remain subject to periodic inspections by the FDA. We also face similar inspections coordinated by the EMEA by inspectors from particular E.U. member countries that conduct inspections on behalf of the European Union and from other foreign regulatory authorities. Any 20
determination by the FDA or other regulatory authorities of manufacturing or other deficiencies could materially adversely affect our business. Regulatory requirements and approval processes in E.U. countries are similar in principle to those in the United States and can be at least as costly and uncertain. The European Union has established a unified centralized filing and approval system administered by the Committee for Medicinal Products for
Human Use designed to reduce the administrative burden of processing applications for pharmaceutical products derived from new technologies. In addition to obtaining regulatory approval of products, it is generally necessary to obtain regulatory approval of the facility in which the product will be manufactured. We use and plan to continue to use third-party manufacturers to produce our products in clinical and commercial quantities. Future FDA inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to
correct. In addition, discovery of problems with a product, including new safety risks, or the failure to comply with requirements may result in restrictions on a product, manufacturer or holder of an approved NDA or BLA, including withdrawal or recall of the product from the market or other voluntary or FDA-
initiated action that could delay further marketing. Newly discovered or developed safety or efficacy data may require changes to an approved product’s approved labeling, including the addition of new warnings and contraindications, the imposition of additional mandatory postmarket studies or clinical trials, or
the imposition of or revisions to a REMS program, including distribution and/or use restrictions. Also, new government requirements may be established that could delay or prevent the regulatory approval of KRYSTEXXA. Once an NDA or BLA is approved, a product will be subject to certain post-approval requirements, including requirements for adverse event reporting and submission of periodic reports to the FDA, recordkeeping, product sampling and distribution, and, as discussed above, may be subject to mandatory
postmarket study and REMS requirements. In addition, the FDA strictly regulates the promotional claims that may be made about prescription drug products and biologics. In particular, a drug or biologic may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved
labeling. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. The FDA also requires substantiation of any claims of superiority of one product
over another, including the requirement that such claims be proven by adequate and well-controlled head-to-head clinical trials. The FDA also requires all promotional materials that discuss the use or effectiveness of a prescription drug or biologic to disclose in a balanced manner the risks and safety profile of the
product. In addition, the distribution of prescription pharmaceutical products in the United States is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution and recordkeeping requirements for drugs and drug samples at the federal level, and sets minimum standards for the
registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution. We are also subject to various federal and state laws pertaining to health care “fraud and abuse” issues, including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive, or pay any remuneration in exchange for, or to induce, the
referral of business, including the purchase or prescribing of a particular drug. False claims laws prohibit anyone from knowingly and willfully presenting, or causing to be presented for payment to the United States government, including Medicare and Medicaid, claims for reimbursed drugs or services that are false
or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. We have adopted the Pharmaceutical Research and Manufacturers of America Code on Interactions With Healthcare Professionals, which is an industry code developed to govern interactions
with and communications to healthcare professionals and we have adopted processes that we believe enhance compliance with this code and applicable federal and state laws. 21
We are also subject to various laws and regulations relating to safe working conditions, clinical, laboratory and manufacturing practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents,
used in connection with our research and development activities. Reimbursement and Pricing Controls In many markets today, including those in which we or any potential collaborator would commercialize KRYSTEXXA if we obtain marketing approval to do so, the prices of pharmaceuticals are constantly being subjected to direct and indirect price controls by a multitude of laws and reimbursement programs
with varying price control mechanisms. In addition, where pricing of a pharmaceutical product varies from jurisdiction to jurisdiction, parallel importing in which product sold at a lower price in one jurisdiction may be legally or illegally imported into higher priced jurisdictions may undercut pricing in that higher
priced jurisdiction. After we set a price point, private and public payors will determine their interest in providing access to KRYSTEXXA. They will also determine what requirements or hurdles, such as prior authorization, will be put in place for patients to qualify for therapy and ultimately what level of reimbursement they will
provide, as well as the level of contribution required from the patient. These access decisions are closely linked to the price of KRYSTEXXA and our ability to demonstrate clinical and economic benefits for a payor’s covered populations. Given that KRYSTEXXA is an orphan drug targeting a relatively small patient population without alternative approved therapies, we expect that KRYSTEXXA will be reimbursed by most major plans. However, we anticipate usage to be restricted to approved labeling or any relevant published treatment
guidelines. In the United States, we expect limitations to be imposed through prior authorization and possibly through step therapy, by which patients would be required to fail both allopurinol and febuxostat before KRYSTEXXA would be reimbursed. We expect KRYSTEXXA to be covered mainly as a medical
benefit as it will be administered by healthcare professionals in medical specialist offices, hospital infusion suites and independent infusion centers. In the European Community, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems
under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very
intense. As a result, increasingly high barriers are being erected to the entry of new products. Patents and Proprietary Rights Our scientific staff, contract manufacturing partners and consultants continually work to develop proprietary techniques, processes and products. We protect our intellectual property rights in this work by a variety of means, including assignments of inventions from our scientific staff, contract manufacturing
partners and consultants, and filing patent and trademark applications in the United States, Europe and other major industrialized countries. We also rely upon trade secrets and improvements, unpatented proprietary know-how and continuing technological innovation to develop and maintain our competitive
position. As of February 1, 2010, we maintained 18 issued U.S. patents either owned or exclusively licensed by us, and 80 foreign issued patents in a number of jurisdictions. Additionally, as of February 1, 2010, we owned or have exclusively licensed to us, 28 pending patent applications in the United States, and 156
foreign counterpart filings to these patent applications in a number of jurisdictions. However, our patent applications might not result in issued patents and issued patents might be circumvented or invalidated. The expiration or loss of patent protection resulting from legal challenge normally results in significant
competition from generic products against the originally patented product and can result in significant reduction in sales of that product in a very short time. For example, generic competition for 22
Oxandrin began in December 2006 and the introduction of generic products has caused a significant decrease in our Oxandrin revenues, which has had an adverse affect on our financial results and cash flow. We believe that important legal issues remain to be resolved as to the extent and scope of patent protection,
and we expect that in certain cases litigation may be necessary to determine the validity and scope of our and others proprietary rights. Such litigation may consume substantial amounts of our resources. We are aware of patents and patent applications issued to and filed by other companies with respect to technology that is potentially useful to us and, in some cases, related to products and processes being developed by us. We cannot currently assess the effect, if any, that these patents may have on our
operations. In the aggregate, our patent and related rights are of material importance to our business. We have exclusively licensed from MVP and Duke issued patents and pending patent applications in numerous countries worldwide, including the United States, all of the countries included in Europe, China and Japan,
directed to PEGylated urate oxidase, as well as methods for making and using PEGylated urate oxidase. We have also jointly filed U.S. patent applications with Duke directed to administration of PEGylated urate oxidase. In addition, we are the sole owner of several pending patent applications filed in numerous
countries worldwide, including the United States, all of the countries included in Europe, China and Japan, directed to urate oxidase. Our licensed, co-owned and solely-owned issued patents and pending patent applications relating to PEGylated urate oxidase, if issued, would expire between 2019 and 2028, not
including any possible patent term extensions that may be available upon completion of the FDA approval process for KRYSTEXXA. Trademarks We currently own United States and several foreign registrations or applications for trademarks for Savient, KRYSTEXXA, Oxandrin and URIXIV, which are used or proposed for use in connection with pharmaceutical products containing the drugs oxandrolone and PEG-uricase. All of these trademarks are
covered by registrations or pending applications for registration in the U.S. Patent and Trademark Office and in the patent and trademark offices of many other countries. The United States registrations for issued trademarks will be valid and will not expire for so long as we continue to use and properly maintain
the registrations, for example, by obtaining renewals at the appropriate times and paying appropriate fees, and provided they are not otherwise successfully challenged. Competition In general, the pharmaceutical and biotechnology industries are intensely competitive. The technological areas in which we work continue to evolve at a rapid pace. Competition from pharmaceutical, chemical and biotechnology companies, universities and research institutions is intense and we expect it to
increase. Many of these competitors are substantially larger than we are and have substantially greater capital resources, research and development capabilities and experience, manufacturing, marketing, financial and managerial resources than we do. Acquisitions of competing companies by large pharmaceutical
companies or other companies could enhance the financial, marketing and other resources available to these competitors. An important factor in our ability to compete will be the timing of market introduction of competitive products. Accordingly, the relative speed with which we and competing companies can develop products, complete the clinical testing and approval processes, and supply commercial quantities of the products
to the market will be an important element of market success. Other significant competitive factors include:
•
product safety and efficacy, • timing and scope of regulatory approval, • product availability, • marketing and sales capabilities, • reimbursement coverage from insurance companies and others, 23
• the extent of clinical benefits and side effects of our products relative to their cost, • the method of administering a product, • price, • patent protection, and • capabilities of partners with whom we may collaborate. We are seeking approval for KRYSTEXXA as a treatment for chronic gout in patients refractory to conventional therapy. There is no other drug or biologic approved for this patient population. Currently, by far the most prevalent treatment for patients with gout is allopurinol, which can lower uric acid levels
by inhibiting uric acid formation. Additionally, a small number of gout patients are treated with probenecid, which can lower uric acid levels by increasing excretion of uric acid. Febuxostat is a new treatment for gout that received FDA approval in February 2009. Febuxostat was launched in the U.S. market in
March 2009 under the trade name Uloric®. Febuxostat can lower uric acid levels by inhibiting uric acid formation through the same mechanism of action as allopurinol. Although febuxostat at the 80 mg dose has demonstrated superior uric acid control as compared to the most commonly used dose of allopurinol in
randomized head-to-head clinical trials, febuxostat has not demonstrated superior clinical benefits in comparison to allopurinol. If febuxostat is able to adequately treat some gout patients who are unable to be adequately treated by allopurinol, it will reduce the population of patients with chronic gout that is
refractory to conventional therapy and therefore our target market. Febuxostat was developed by Teijin Pharma Limited in Japan and licensed to Takeda Pharmaceuticals North America, Inc. in the United States and to Ipsen Pharmaceuticals, or Ipsen, in Europe. In October 2009, Ipsen subsequently granted
exclusive license rights in 41 countries to the Menarini Group. Since KRYSTEXXA will be indicated for patients refractory to conventional therapy, we do not expect KRYSTEXXA to face competition from these existing treatments. Legislation has been introduced in the U.S. Congress that, if enacted, would permit more widespread reimportation of drugs and biologics from foreign countries into the United States. This may include reimportation from foreign countries where the drugs are sold at lower prices than in the United States.
Such legislation, or similar regulatory changes, if enacted, could be an additional competitive factor for any approved products that we market. Legislation also has been introduced in the U.S. Congress that, if enacted, would permit the FDA to approve biosimilar versions of biological products like KRYSTEXXA
through an abbreviated approval pathway. Such legislation, or similar regulatory changes, if enacted could result in earlier entry of similar, competing products and be another competitive factor for KRYSTEXXA In the United States, Oxandrin and our authorized generic version of oxandrolone compete against several other anabolic agents and appetite stimulants. Each of these competing products has different strategies and resources allocated to its promotion as compared to Oxandrin and oxandrolone. In addition,
Oxandrin faces competition from oxandrolone generics, including our authorized generic version of oxandrolone. Generic competition for Oxandrin began in December 2006. Our generic competitors are Sandoz Pharmaceuticals, Upsher-Smith Laboratories, Par Pharmaceuticals and Roxane Laboratories. Recent Changes in Our Senior Management On January 17, 2009, we and Christopher Clement entered into an agreement, based on his resignation in November 2008, providing for the termination of his employment as our President and Chief Executive Officer on mutually agreed terms. On February 12, 2009, Brian Hayden was terminated from his position as our Senior Vice President, Chief Financial Officer and Treasurer. On February 12, 2009, Dr. Robert Lamm resigned from his position as our Senior Vice President of Quality and Regulatory Affairs. On August 17, 2009, Dr. Zebulun Horowitz resigned from his position as Senior Vice President, Chief Medical Officer. 24
Employees As of February 1, 2010, we had 43 employees. All employees are located in the United States. There are 18 employees engaged in research and development activities including clinical, regulatory, quality assurance and control, and manufacturing or production activities; 20 employees are engaged in general
and administrative activities including executive, finance, legal, human resources, investor relations, information technology, and operations; and five employees are engaged in sales and marketing activities including business development, commercial operations, sales operations and marketing. On September 30, 2009, we completed a plan of termination pursuant to which we reduced our workforce by 25 employees, or approximately 37%. This termination was in addition to a previous termination of nine employees in August 2009. We took these actions in order to reduce costs and streamline
operations in order to focus our efforts on the tasks critical to the resubmission of the BLA for KRYSTEXXA to the FDA for approval. The reduction in staff was focused in our commercial, medical, financial, operational and sales departments with minimal effects in the regulatory, quality and manufacturing
departments. These terminations were a result of having completed clinical and pre-commercial activities that we concluded for KRYSTEXXA. Our Corporate Information We were incorporated as Bio-Technology General Corp. under the laws of Delaware in 1980. Our principal executive offices are located at One Tower Center, 14th Floor, East Brunswick, New Jersey 08816, and our telephone number is (732) 418-9300. Available Information We file annual, quarterly, and current reports, proxy statements and other documents with the Securities and Exchange Commission, or SEC, under the Securities Exchange Act of 1934, as amended, or Exchange Act. The SEC maintains an Internet web site that contains reports, proxy and information
statements, and other information regarding issuers, including us, that file electronically with the SEC. The public can obtain any documents that we file with the SEC at http://www.sec.gov. Our Internet website is http://www.savientpharma.com. We make available free of charge through our website our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Sections 13(a) and 15(d) of the
Exchange Act. We have made these reports available through our website during the period covered by this report at the same time that they become available from the SEC. Our Internet website is not incorporated by reference into this Annual Report on Form 10-K. Our corporate governance guidelines, code of ethics and whistleblower policy, and the charters of the Audit and Finance Committee, Compensation Committee and Nominating and Corporate Governance Committee are each available on the corporate governance section of our website. Stockholders may
request a free copy of any of these documents by writing to Investor Relations, Savient Pharmaceuticals, Inc., One Tower Center, Fourteenth Floor, East Brunswick, New Jersey 08816. 25
Our current executive officers are as follows:
Name
Age
Positions Paul Hamelin
55
President Philip K. Yachmetz
53
Senior Vice President, General Counsel and Secretary David G. Gionco
49
Group Vice President, Chief Financial Officer and Treasurer Paul Hamelin was appointed President in November 2008. From May 2006 to November 2008, Mr. Hamelin was our Senior Vice President, Commercial Operations. From March 2006 until May 2006, Mr. Hamelin was Managing Director of our former United Kingdom subsidiary, Rosemont Pharmaceuticals,
and from June 2005 to March 2006, he served as a consultant to us regarding the Rosemont business. From 2004 to June 2005, Mr. Hamelin served as President and Chief Operating Officer of Algorx Pharmaceuticals. From 2002 to 2004, he was President and Chief Executive Officer of Elitra Pharmaceuticals, and
from 2000 to 2002, he was Senior Vice President, Global Commercial Operations of Millennium Pharmaceuticals. Mr. Hamelin has also held marketing, sales and commercial operations management positions at Pharmacia/Searle, Abbott Labs and Eli Lilly, all of which are pharmaceutical companies, and has
worked in the pharmaceutical industry for over 30 years. Mr. Hamelin has participated in the launch of more than a dozen different drug products and is a registered pharmacist. Philip K. Yachmetz has been our Senior Vice President, General Counsel and Secretary since November 2008, having previously served as our Executive Vice President and Chief Business Officer from February 2006 through November 2008 and as our Senior Vice President—Corporate Strategy, General
Counsel and Secretary from May 2004 until February 2006. From 2000 until 2004, Mr. Yachmetz was Vice President, General Counsel and Secretary of Progenics Pharmaceuticals. From 1998 to 2000, he was Senior Vice President, Business Development, General Counsel and Secretary at CytoTherapeutics (now
StemCells), a stem cell therapeutics discovery company. Mr. Yachmetz founded and served from 1997 to 1998 as Managing Director of Millennium Venture Management, a business consulting and advisory firm to the pharmaceutical, healthcare and high technology industries. From 1989 to 1996, he held legal
positions of increasing responsibility at Hoffmann-La Roche, a pharmaceutical company. Mr. Yachmetz is an attorney admitted to the bar in New Jersey and New York. David G. Gionco has been our Group Vice President, Chief Financial Officer and Treasurer since February 2009, having previously served as our Vice President of Finance and Controller from February 2006. From August 2004 until February 2006, he was Acting Chief Financial Officer/Director of Finance
and Controller for Odyssey Pharmaceuticals. From July 2003 to August 2004, Mr. Gionco was Director of Finance for Progenics Pharmaceuticals. Mr. Gionco has spent more than 19 years in the pharmaceutical industry, including various audit, corporate accounting, financial planning, finance and controller roles of
increasing responsibility at pharmaceutical companies including Medco, Merck & Co., Progenics and Odyssey. Mr. Gionco is a Certified Public Accountant in the State of New York and has seven years of financial auditing experience with Edward Isaacs & Company, a public accounting firm that was acquired in 2000
by McGladrey & Pullen. Mr. Gionco holds a B.S. in Accounting from Fairleigh Dickinson University and an MBA from Rutgers University. 26
ITEM 1A. RISK FACTORS Risks Relating to Development of KRYSTEXXA and Our Ability to Accomplish Our Future Business Objectives Our business depends on our success in gaining FDA approval of KRYSTEXXA, which requires that we and our third-party manufacturer, BTG, address several issues raised by the FDA. If these issues are not satisfactorily addressed, we will not gain FDA approval of KRYSTEXXA and our business will be
materially harmed. On July 31, 2009, we received a CRL from the FDA stating that the FDA cannot at this time approve our BLA for KRYSTEXXA as a treatment for chronic gout in patients refractory to conventional therapy. The CRL:
•
cited deficiencies with the CMC section of the BLA, • required a tightening of a number of analytical methods used to assess manufacturing and narrowing of analytical specifications associated with commercial production of pegloticase, • required that BTG, remediate observations arising from the FDA pre-approval inspection of its manufacturing facility, • specified the requirements of a REMS program, and • required that we provide a follow-up pre-clinical and clinical safety update to the FDA. One of the issues raised by the FDA in the CRL addressed a change in PEGylation concentration that we made in the proposed process for manufacturing pegloticase API drug substance for commercial use of KRYSTEXXA final drug product. The FDA concluded that the comparability data that we
submitted for the material manufactured using the proposed commercial manufacturing process was not adequate to demonstrate that it was representative of the material used to establish the safety and efficacy of KRYSTEXXA in the Phase 3 clinical trials. The FDA stated that we had the option of either
reverting to and validating the manufacturing process used to produce pegloticase for the Phase 3 clinical trials or conducting additional comparability clinical trials to support the use of KRYSTEXXA manufactured using the proposed commercial manufacturing process. We opted to pursue a reversion to the
manufacturing processes used to manufacture pegloticase API drug substance for the Phase 3 trials, which we communicated to the FDA during our September 2009 Type “A” meeting. In late October 2009, as part of our validation campaign, we completed the manufacture of three consecutive batches of
pegloticase API drug substance at BTG using the manufacturing process used to manufacture pegloticase API drug substance for the Phase 3 clinical trials. These batches were then manufactured into final KRYSTEXXA drug product in November 2009 and samples from each batch were placed on stability testing
by early December 2009. Our third-party testing laboratories then performed in-process and final release analytical testing on each batch of pegloticase API drug substance and final drug product to validate their comparability to the drug substance and final drug product used in the Phase 3 clinical trials. We have
received the analytical test results performed on these batches and, based on these results, we believe that we have successfully reverted to the Phase 3 manufacturing process and that the pegloticase API drug substance and final drug product produced in this validation campaign are comparable to the material
used in the Phase 3 trial. It is possible that the FDA will disagree with our interpretation of the analytical testing results or will require us to redo the validation process used to produce pegloticase for the Phase 3 clinical trials. The CRL also required correction of deficiencies and observations cited by the FDA during its June 2009 pre-approval inspection of BTG’s manufacturing facilities. While remediation of the deficiencies and observations arising from the FDA pre-approval inspection had been underway since the June 2009
inspection in accordance with two work plans submitted to the FDA by BTG in June and July 2009, in October 2009 the FDA provided notification that these original work plans were not adequate and identified additional corrective actions that would be required to successfully remediate the deficiencies and
observations noted in their facility. Since October 2009, BTG has been addressing additional chemistry and manufacturing controls issues with their facility. BTG submitted to the FDA in November and December 2009, reports of the steps that BTG has completed to date with the goal of addressing the deficiencies
and other observations identified by the FDA during its pre-approval 27
inspection of BTG’s manufacturing facility in June 2009. In addition, BTG included in its submissions its plans and framework for implementing a broad, continuous quality improvement plan. BTG has received from the FDA a letter, dated early February 2010, stating that the corrective actions implemented by
BTG and the additional commitments made by BTG appear to address the FDA’s concerns. The FDA also stated in its letter that it will verify these corrective actions and additional commitments during the FDA’s next inspection of BTG’s facility. The FDA may nevertheless continue to raise additional issues, or
reassert previously raised issues, with BTG’s facility at any time during its review of our BLA resubmission. It is also possible that the FDA could require us to repeat our manufacturing validation campaign once the FDA determines that BTG has resolved all of these issues to the satisfaction of the FDA, though
we do not expect the FDA to take this step. We expect that the FDA will schedule its next reinspection of BTG’s facility for a time prior to making its decision on our BLA resubmission; however, delays in FDA’s reinspection of BTG’s facility, which is located in Israel, may delay FDA’s decision on our BLA. If
the FDA reinspects BTG’s facility, the FDA may, at that reinspection, determine that it is or is not satisfied with BTG’s corrective actions and additional commitments, or it may identify new deficiencies with BTG’s facility. Completing our efforts to address each of the issues identified by the FDA in the CRL will require additional time and expenditure of funds. Despite our efforts, we and BTG may not be able to adequately address the FDA’s concerns or the FDA may disagree with our conclusions that we have completely
addressed its concerns such that KRYSTEXXA is ultimately approved without further delays, if at all. If KRYSTEXXA is not approved or if approval of KRYSTEXXA is substantially delayed, our business will be materially harmed. We may not be able to execute upon our commercial strategy for KRYSTEXXA, or the commercialization of KRYSTEXXA may be significantly delayed, if the FDA requires us to conduct additional clinical trials prior to approval. In addition, we may elect to perform additional clinical trials for other
indications or in support of applications for regulatory marketing approval in jurisdictions outside the United States. These supplemental trials could be costly and could result in findings inconsistent with or contrary to the data from the clinical trials that supported our United States filings with the FDA, which
could restrict our marketing approval of KRYSTEXXA. Any of these results would materially harm our business. Before obtaining regulatory approval for the sale of KRYSTEXXA in their respective jurisdictions, we must provide the FDA and similar foreign regulatory authorities with clinical data to demonstrate that KRYSTEXXA is safe and effective. Clinical trials of KRYSTEXXA must comply with regulation by
numerous federal, state and local government authorities in the United States, principally the FDA, and by similar agencies in other countries. We may decide, or be required by regulators, to conduct additional clinical trials or testing of KRYSTEXXA. Clinical testing is expensive and difficult to design and
implement. Clinical testing can also take many years to complete, and the outcome of such testing is uncertain. Success in pre-clinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. The FDA has told
us that it does not expect to require additional clinical trials in connection with our application for regulatory approval for the sale of KRYSTEXXA. However, the FDA may continue its evaluation of the safety data from our clinical trials before granting regulatory approval for the sale of KRYSTEXXA, and may
nevertheless conclude that additional clinical trials are necessary to demonstrate its safety and efficacy. If the FDA were to determine that additional clinical trials of KRYSTEXXA are necessary, this would significantly delay the timeline for the FDA’s determination of whether to approve our BLA for
KRYSTEXXA. We may also be required, or we may elect, to conduct additional clinical trials or pre-clinical animal studies for or in support of our applications for regulatory marketing approval in jurisdictions outside the United States, such as the EMEA. Regulatory authorities in jurisdictions outside the United States may
require us to submit data from supplemental clinical trials, or pre-clinical animal studies, in addition to data from the clinical trials that supported our United States filings with the FDA. Any requirements to conduct supplemental trials would add to the cost of developing KRYSTEXXA, and we may not be able to
complete such supplemental trials. Additional trials could also produce findings that are inconsistent with the trial results we have previously submitted to the FDA, in which case we would be obligated to report those findings to the FDA. This could result in additional restrictions on 28
the marketing approval of KRYSTEXXA if it is obtained. Inconsistent trial results could also lead to delays in obtaining marketing approval in the United States for other indications for KRYSTEXXA, could cause regulators to impose restrictive conditions on marketing approvals and could even cause our
marketing approval to be revoked. Any of these results would materially harm our business and impair our ability to generate revenues and achieve or maintain profitability. Even if the FDA grants marketing approval for KRYSTEXXA, the FDA may decide to approve the labeling for indications that are not as broad as we requested. In addition, if the FDA approves our BLA, the final label may prescribe safety limits or warnings, and we will be required to implement a
REMS program to minimize the potential risks of the treatment of KRYSTEXXA. Such additional obligations and commitments may increase the cost of commercializing KRYSTEXXA, limit the commercial success of KRYSTEXXA and result in lower than expected future earnings. The FDA included its latest comments on the draft of the label language for KRYSTEXXA in its July 31, 2009 CRL for our KRYSTEXXA BLA. This language is subject to change until we receive final approval of the BLA. In the event that the FDA provides marketing approval for KRYSTEXXA, the final
label language may not be for an indication as broad as that set forth in the draft label language. Additionally, the approved label may prescribe safety limits or warnings that reduce the market for the product or increase the costs associated with the marketing and sale of the product. In addition, if the FDA
approves our BLA, we will be required to implement a REMS program to minimize the potential risks of KRYSTEXXA treatment. The REMS program will include a Medication Guide for patients, a Communication Plan for health care providers, and an Assessment Plan to survey patients’ and providers’
understanding of the serious risks of KRYSTEXXA. The REMS program is subject to further negotiation and final approval by the FDA. Until a final label is approved, the FDA may further revise the REMS program to impose significant additional obligations and commitments on us in the future or may require
post-approval clinical or pre-clinical studies. If imposed, such additional obligations and commitments may increase the cost of commercializing KRYSTEXXA, limit the commercial success of KRYSTEXXA and result in lower than expected future revenues. Unless we are successful in consummating a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving our company, we may need to raise substantial additional capital to complete the development of and execute upon our commercial strategy for
KRYSTEXXA. Such financing may only be available on terms unacceptable to us, or not at all. If we are unable to obtain financing on favorable terms, our business, results of operations and financial condition may be materially adversely affected. As of December 31, 2009, we had $108.2 million in cash and short-term investments, after giving effect to our October 2009 underwritten public offering of 4,945,000 shares of our common stock that yielded net proceeds of $61.4 million in cash, as compared to $78.6 million as of December 31, 2008. As of
December 31, 2009, we had an accumulated deficit of $242.5 million. The development and commercialization of pharmaceutical products requires substantial funds, and we currently have no committed external sources of capital. In recent periods, we have satisfied our cash requirements primarily through product
sales and the divestiture of assets that were not core to our strategic business plan. However, our product sales of Oxandrin and our authorized generic Oxandrin brand equivalent product, oxandrolone, have declined substantially in recent years. Although we may consider divesting Oxandrin, any proceeds of that
divestiture would not significantly improve our capital position, and we do not have further non-core assets to divest. Our strategic plan is to seek approval from the FDA of KRYSTEXXA and to develop a program of regulatory filings and review of KRYSTEXXA in other countries. We continue to believe that a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving
our company would serve to maximize the commercial prospects of KRYSTEXXA in the United States, Europe and other foreign countries. If we are not able to consummate a worldwide partnership or broader strategic transaction on terms acceptable to us, we intend to independently pursue the commercialization of KRYSTEXXA in the United States. Our future capital requirements will depend on many factors, including: 29
•
the timing of, and the costs involved in, obtaining regulatory approvals for KRYSTEXXA, • the cost of manufacturing activities, • the cost of commercialization activities, including product marketing, sales and distribution, and • our ability to establish and maintain additional collaborative arrangements relating to the commercialization of KRYSTEXXA in the United States and internationally. If the FDA does not approve or materially delays approval of our BLA for KRYSTEXXA, or if we are not successful in consummating a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving our company, our cash needs will increase, and we may need
to seek additional funding. We may not be able to obtain additional funds or, if such funds are available, such funding may not be on terms that are acceptable to us, particularly in light of current macroeconomic conditions. If we raise additional funds by issuing equity securities, dilution to our then-existing
stockholders will result. If we issue preferred stock, it would likely include a liquidation preference and other terms that would adversely affect our stockholders. If we raise additional funds through the issuance of debt securities or borrowings, we may incur substantial interest expense and could become subject to
financial and other covenants that could restrict our ability to take specified actions, such as incurring additional debt or making capital expenditures. If additional funds are not available on favorable terms, or at all, our business, results of operations and financial condition may be materially adversely affected, and
we may be required to curtail or cease operations. Our business focuses primarily on the development of a single product, KRYSTEXXA. If we are unable to complete the development of and execute upon our commercial strategy for KRYSTEXXA, or if we experience significant delays or unanticipated costs in doing so, our ability to generate product
revenue and our likelihood of success will be materially harmed. KRYSTEXXA is our only product candidate in development and our ability to generate product revenue will depend on the successful commercialization of this product. We do not have any other material assets or alternative business plans aside from executing our commercial strategy for KRYSTEXXA. As
a result of our reliance on the development of this single product, much of our near term results and value as a company depend on the FDA granting marketing approval for KRYSTEXXA and our ability to execute our commercial strategy for this product. The failure of the FDA to grant marketing approval for
KRYSTEXXA, or any material delays in the approval process, or our inability to execute our commercial strategy for KRYSTEXXA will substantially harm our ability to generate revenue, which would have a material adverse effect on our business. If we do not gain marketing approval for KRYSTEXXA or are
unsuccessful in our efforts to execute our commercial strategy for this product, our business, results of operations and financial condition may be materially adversely affected, and we may be required to curtail or cease operations. We have limited marketing and no sales capabilities. If we are unable to expand our sales and marketing capabilities or enter into sales and marketing agreements with third parties, we may be unable to generate product sales revenue from sales to customers. To achieve commercial success for KRYSTEXXA, we must either develop a sales and marketing organization, outsource these functions to third parties, or enter into a worldwide partnership with a third party to commercialize KRYSTEXXA. We currently have limited marketing and no sales capabilities. As
a result, if we obtain marketing approval for KRYSTEXXA, and independently launch and market KRYSTEXXA in the United States, we will need to rapidly expand our sales and marketing organization. This expansion will be difficult, expensive and time consuming. We may not be able to attract, hire, train and
retain qualified sales and marketing personnel to build a significant or effective sales and marketing force for the sale of KRYSTEXXA. Alternatively, we may seek to enter into sales and marketing arrangements with third parties, which may not be on terms favorable to us. In addition, such third parties may not
successfully perform such functions for us. If we are not successful in our efforts to rapidly expand our internal sales and marketing capabilities or to enter into third-party sales and marketing arrangements, our ability to market and sell KRYSTEXXA and generate product sales revenue from sales to customers will be impaired. 30
The
commercial success of KRYSTEXXA will depend upon the degree of market
acceptance by physicians, patients, health care payors and others in
the medical community. If KRYSTEXXA does not
achieve an adequate level of acceptance, we may not generate sufficient
additional revenues to achieve or maintain profitability. Even if we are able to bring KRYSTEXXA to market, it may not gain or maintain market acceptance by physicians, patients, health care payors or others in the medical community. We believe the degree of market acceptance of KRYSTEXXA, if approved for commercial sale, will depend on a number of
factors, including, but not limited to the:
•
prevalence and severity of any side effects that we have observed to date or that we observe in the future,
efficacy and potential advantages over alternative treatments, • ability to offer KRYSTEXXA for sale at competitive prices, • final labeling language, • terms of the final REMS program, • relative convenience and ease of administration of KRYSTEXXA compared with other alternatives, • timing of the release of KRYSTEXXA to the public compared to alternative products or treatments, • willingness of the target patient population to try KRYSTEXXA and of physicians to prescribe it, • strength of marketing and distribution support, and • whether third-party and government payors cover or reimburse for KRYSTEXXA, and if so, to what extent. For example, Uloric® (febuxostat) is a new treatment that received FDA approval in February 2009. Febuxostat lowers uric acid levels by inhibiting uric acid formation through the same mechanism of action as allopurinol. While febuxostat is labeled for the chronic management of hyperuricemia in patients with
gout, we may experience reduced demand for KRYSTEXXA in the event febuxostat is used to treat patients who failed on or were contraindicated to allopurinol, or if patients are otherwise treated with febuxostat prior to being treated with KRYSTEXXA. If KRYSTEXXA does not achieve an adequate level of
acceptance, we may not generate sufficient additional revenues to achieve or maintain profitability. Our business may be harmed if we do not adequately predict the market size or customer demand for KRYSTEXXA. The market size, and the timing and amount of customer demand, for KRYSTEXXA will be difficult to predict. Based on the results of our Phase 3 trials, interim OLE study data and the draft labeling for KRYSTEXXA, we have conducted internal and commissioned external quantitative market research to
forecast the size of the market for KRYSTEXXA. Ultimately, if we are able to bring KRYSTEXXA to market, the size of the market and demand for KRYSTEXXA would be based on the final approved label language, which may be different than the draft language most recently provided by the FDA. If the
FDA provides marketing approval for KRYSTEXXA, but with labeling that is materially different than the current draft labeling, the market for KRYSTEXXA could be significantly smaller than we expect. In addition, our REMS program is subject to final approval, and could limit our commercial success and
result in lower than expected future revenues. Moreover, if the actual rate at which physicians prescribe KRYSTEXXA is lower than we have forecasted based upon our market research, then our revenues would also be lower than expected. In addition, demand for KRYSTEXXA may be harmed as a result of the introduction of new products to treat gout. If we overestimate market size or customer demand, we could incur significant unrecoverable costs from creating excess manufacturing or commercial marketing capacity. In addition, if KRYSTEXXA is approved and it receives a limited shelf life at the time of the product launch, we will incur lost revenues to
the extent that any manufactured product is not sold and administered prior to its expiration. Any of these results could materially harm our business. 31
•
We face substantial competition, and our competitors may develop or commercialize alternative technologies or products more successfully than we do. The pharmaceutical and biotechnology industries are intensely competitive. We face competition with respect to KRYSTEXXA from major pharmaceutical companies and biotechnology companies worldwide. Potential competitors also include academic institutions and other public and private research
institutions that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization. Our competitors may develop products that are safer, are more effective, have fewer side effects, are more convenient or are less costly than
KRYSTEXXA. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for KRYSTEXXA. We are seeking approval for KRYSTEXXA for the treatment of chronic gout in patients refractory to conventional therapy, a subset of the broader population of patients with gout. By far the most prevalent treatment for gout today is allopurinol, which can lower uric acid levels by inhibiting uric acid
formation. A small number of patients with gout are treated with probenecid, which can lower uric acid levels by promoting excretion of uric acid. In addition, febuxostat was recently approved by the FDA for the chronic management of hyperuricemia in patients with gout. Legislation has been introduced in the U.S. Congress that, if enacted, would permit more widespread reimportation of drugs from foreign countries into the United States. This may include reimportation from foreign countries where the drugs are sold at lower prices than in the United States. Such legislation,
or similar regulatory changes, could decrease the revenue we receive for any approved products, which, in turn, could adversely affect our operating results and our overall financial condition. Legislation also has been introduced in the U.S. Congress that, if enacted, would permit the FDA to approve biosimilar versions of biological products like KRYSTEXXA through an abbreviated approval pathway. Such legislation, or similar regulatory changes, could result in earlier entry of similar, competing
products that could decrease the revenue we receive for any approved products, which, in turn, could adversely affect our operating results and our overall financial condition. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing and distributing approved products than we do. Smaller or early stage companies may
also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring products, product candidates and technologies
complementary to, or necessary for, our programs or advantageous to our business. If we fail to attract and retain senior management and other key personnel, we may not be able to complete the development of or execute upon our commercial strategy for KRYSTEXXA. We depend on key members of our management team. As a result of changes in our senior management, including the resignation in November 2008 of our former President and Chief Executive Officer on mutually agreed terms, the termination in February 2009 of our former Chief Financial Officer and the
resignation in August 2009 of our Senior Vice President, Chief Medical Officer, we rely more heavily on our Board of Directors, particularly our Chairman, Stephen O. Jaeger, and Lee S. Simon, M.D., who has been providing consulting services to us since January 2009. The loss of the services of Mr. Jaeger or Dr.
Simon, or any member of our senior management team, could harm our ability to complete the development of and execute our commercial strategy for KRYSTEXXA. We have employment agreements with certain key members of our management team and a consulting agreement with Dr. Simon, but these
agreements are terminable by the individuals on short or no notice at any time without penalty. In addition, we do not maintain, and have no current intention of obtaining, “key man” life insurance on any member of our management team. Recruiting and retaining qualified scientific and commercial personnel, including clinical development, regulatory, sales and marketing executives and field personnel, is also critical to our success. If we fail to recruit and then retain these personnel, our ability to pursue the development of and execute our
commercial strategy for KRYSTEXXA may be compromised. We may not be able to 32
attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. If we cannot continue to attract and
retain, on acceptable terms, the qualified personnel necessary for our business, we may not be able to complete the development of or execute upon our commercial strategy for KRYSTEXXA. If it is approved for sale, KRYSTEXXA could be subject to restrictions or withdrawal from the market, and we may be subject to penalties, if we fail to comply with regulatory requirements or experience unanticipated problems with the product, which would materially harm our business. If we obtain marketing approval for KRYSTEXXA, such approval, along with the manufacturing processes, reporting of safety or adverse events, post-approval clinical data, labeling, advertising and promotional activities, and REMS program, will be subject to continual requirements of, and review by, the
FDA and other regulatory authorities, including thorough inspections of third-party manufacturing facilities. These requirements include submission of safety and other post-marketing information and reports, registration requirements, current Good Manufacturing Practices, or cGMP, relating to quality control, quality assurance and corresponding maintenance of records and documents, and recordkeeping. The FDA
enforces cGMP and other requirements through periodic unannounced inspections of manufacturing facilities. The FDA is authorized to inspect drug manufacturing facilities, records, files, papers, processes, and controls at reasonable times and within reasonable limits and in a reasonable manner, and we cannot
refuse to permit entry or inspection. If, in connection with any future inspection, the FDA finds that we or any of our third-party manufacturers are not in substantial compliance with cGMP requirements, the FDA may undertake enforcement action against us. Even if regulatory approval for KRYSTEXXA is granted, the approval may be subject to limitations on the indicated uses for which KRYSTEXXA may be marketed or to the conditions of approval. The approval may also contain requirements for costly post-marketing testing and surveillance to monitor
KRYSTEXXA’s safety or efficacy. Later discovery of previously unknown problems with KRYSTEXXA or its manufacturing processes, such as known or unknown safety or adverse events, or failure to comply with regulatory requirements, may result in:
•
revisions of or adjustments to the product labeling, • restrictions on the marketing or manufacturing of KRYSTEXXA, • imposition of postmarket study or postmarket clinical trial requirements, • imposition of new or revised REMS requirements, including distribution and use restrictions, • public notice of regulatory violations, • costly corrective advertising, • warning letters, • withdrawal of KRYSTEXXA from the market, • refusal to approve pending applications or supplements to approved applications, • voluntary or mandatory product recall, • fines or disgorgement of profits or revenue, • suspension or withdrawal of regulatory approvals, including license revocation, • shutdown, or substantial limitations on the operations, of manufacturing facilities, • refusal to permit the import or export of products, • product seizure, • debarment from submitting certain abbreviated applications, and • injunctions or the imposition of civil or criminal penalties. If any of these events were to occur, our business would be materially harmed. 33
If we are unable to obtain adequate reimbursement from third-party payors, or acceptable prices, for KRYSTEXXA, our revenues and prospects for profitability will suffer. Our future revenues and ability to become profitable will depend heavily upon the availability of adequate reimbursement for the use of KRYSTEXXA from government-funded and private third-party payors. Reimbursement by a third-party payor may depend upon a number of factors, including the third-
party payor’s determination that use of a product is:
•
a covered benefit under its health plan,
safe, effective and medically necessary, • appropriate for the specific patient, • cost effective, and • neither experimental nor investigational. If our BLA for KRYSTEXXA is approved by the FDA, obtaining reimbursement approval for KRYSTEXXA from each government-funded and private third-party payor will be a time-consuming and costly process, which could require us to provide to each payor supporting scientific, clinical and cost-
effectiveness data for KRYSTEXXA’s use. We may not be able to provide data sufficient to gain acceptance with respect to reimbursement. Even when a payor determines that a product is eligible for reimbursement, the payor may impose coverage limitations that preclude payment for some product uses that are approved by the FDA or similar authorities. Moreover, eligibility for coverage does not imply that any product will be reimbursed in all
cases or at a rate that allows us to make a profit or even cover our costs. If we are not able to obtain coverage and profitable reimbursement promptly from government-funded and private third-party payors for our products, our ability to generate revenues and become profitable will be compromised. Federal legislation enacted in December 2003 has altered the way in which physician-administered drugs and biologics, such as KRYSTEXXA, are covered by Medicare and are reimbursed. Under this reimbursement methodology, physicians are reimbursed based on a product’s “average sales price.” This
reimbursement methodology has generally led to lower reimbursement levels. This legislation also added an outpatient prescription drug benefit to Medicare, which went into effect in January 2006. These benefits will be provided primarily through private entities, which we expect will attempt to negotiate price
concessions from pharmaceutical manufacturers. Moreover, the U.S. Congress is currently considering legislation that would dramatically overhaul the health care system, including the possibility of creating a government health care plan. As part of this legislative initiative, Congress is considering a number of proposals that are intended to reduce or limit the
growth of health care costs, which could significantly change the market for pharmaceuticals and biological products. If such proposals are enacted, they could, among other things, increase pressure on drug pricing or make it more costly for patients to gain access to prescription drugs like KRYSTEXXA at affordable prices. This could lead to fewer prescriptions for KRYSTEXXA and could force individuals who are
prescribed KRYSTEXXA to pay significant out-of-pocket costs or pay for the prescription entirely by themselves. As a result of such initiatives, market acceptance and commercial success of our product may be limited and our business may be harmed. If the FDA provides marketing approval for KRYSTEXXA, this product may also be eligible for reimbursement under Medicaid. If state-specific Medicaid programs do not provide adequate coverage and reimbursement for KRYSTEXXA, it may have a negative impact on our operations. The scope of coverage and payment policies varies among private third-party payors, including indemnity insurers, employer group health insurance programs and managed care plans. These third-party carriers may base their coverage and reimbursement on the coverage and reimbursement rate paid by
carriers for Medicare beneficiaries. Furthermore, many such payors are investigating or implementing methods for reducing health care costs, such as the establishment of capitated or prospective payment systems. Cost containment pressures have led to an increased emphasis on the use of cost-effective products by
health care providers. If third-party payors do not provide adequate 34
•
coverage or reimbursement for KRYSTEXXA, it could have a negative effect on our revenues and results of operations. Failure to obtain regulatory approval in foreign jurisdictions would prevent us from marketing our products abroad and could materially adversely affect our business. We intend to have our products marketed outside the United States. In order to market our products in the European Union and many other foreign jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. While we have been unable to do so
to date, if we are able to establish a development and commercialization partnership for KRYSTEXXA for markets outside the United States, third parties may be responsible for obtaining regulatory approvals outside the United States. If this occurs, we will depend on such third parties to obtain these approvals.
The approval procedure varies among countries and can involve additional testing or pre-filing requirements such as a pediatric investigational plan. The EMEA informed us that we must file such a plan prior to the filing of a Marketing Authorization Application. The time required to obtain foreign regulatory
approval may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory
authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. We and our collaborators may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our
products in any market. The failure to obtain these approvals could materially adversely affect our business, financial condition and results of operations. Foreign governments tend to impose strict price controls, which may adversely affect our revenues. In some foreign countries, particularly the countries of the European Union and Canada, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take, at a minimum, an additional six to 12 months after the receipt
of marketing approval for a product. To obtain reimbursement or pricing approval for KRYSTEXXA in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. The conduct of such a clinical trial would be expensive
and result in delays in commercialization of KRYSTEXXA in such markets. If reimbursement is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected. Additionally, legislation has been introduced in the U.S. Congress that, if enacted, would permit more widespread reimportation of drugs from foreign countries into the United States. This may include reimportation from foreign countries where the drugs are sold at lower prices than in the United States. Such
legislation, or similar regulatory changes, could decrease the revenue we receive for any approved products, which, in turn, could adversely affect our operating results and our overall financial condition. Risks Relating to Our Reliance on Third Parties We have no manufacturing capabilities and limited manufacturing personnel. We depend on third parties to manufacture KRYSTEXXA. If these manufacturers fail to meet our manufacturing requirements at acceptable quality levels and at acceptable cost, and if we are unable to identify suitable
replacements, our commercialization efforts may be materially harmed. We have limited personnel with experience in, and we do not own facilities for, the manufacturing of any of our products. We depend on third parties to manufacture KRYSTEXXA. We have entered into commercial supply agreements with third-party manufacturers, including NOF for the supply of mPEG-
NPC, a key raw material in the manufacture of the pegloticase drug substance, BTG for the production of the pegloticase drug substance, and Sigma-Tau, located in Indianapolis, Indiana, for the production of the KRYSTEXXA drug product. These companies are our sole source suppliers for the mPEG-NPC, the
pegloticase drug substance and the KRYSTEXXA drug product. Our third-party manufacturers have not yet manufactured KRYSTEXXA on a sustained basis and at a capacity that would support our market forecasts if KRYSTEXXA receives FDA approval. In 35
addition, in order to produce KRYSTEXXA in the quantities necessary to meet anticipated market demand if the product is approved, we and our contract manufacturers will need to increase the overall manufacturing capacity for the pegloticase drug substance. If we are unable to increase our manufacturing
capacity or qualify an additional supplier, or if the cost of the increased capacity is uneconomical to us, we may not be able to produce KRYSTEXXA in a sufficient quantity to meet future demand, which would adversely affect our projected revenues and gross margins. BTG underwent a pre-approval inspection by the FDA in June 2009, as a result of which the FDA identified certain deficiencies and violations by BTG of cGMP. Some of these deficiencies are significant and will require significant effort to remedy. The FDA has required that these deficiencies be corrected
prior to the approval of the KRYSTEXXA BLA. While remediation of the deficiencies and observations arising from the FDA pre-approval inspection had been underway since the June 2009 inspection in accordance with two work plans submitted to the FDA by BTG in June and July 2009, we were advised by
BTG at the end of October 2009 that the FDA had recently notified BTG that BTG’s proposed work plan was not satisfactory to the FDA, and identified to BTG actions that the FDA will require for BTG to successfully remediate these deficiencies and observations noted in their facility, and since October 2009,
BTG has been addressing additional CMC issues with their facility. BTG submitted to the FDA in November and December 2009, reports of the steps that BTG has completed to date with the goal of addressing the deficiencies and other observations identified by the FDA during its pre-approval inspection of BTG’s manufacturing facility in June 2009. In addition, BTG
included in its submissions its plans and framework for implementing a broad, continuous quality improvement plan. BTG has recently, in February of 2010, received from the FDA a letter stating that the corrective actions implemented by BTG and the additional commitments made by BTG appear to address the
FDA’s concerns. The FDA also stated in its letter that it will verify these corrective actions and additional commitments during the FDA’s next inspection of BTG’s facility. The FDA may nevertheless continue to raise additional issues, or reassert previously raised issues, with BTG’s facility at any time during its
review of our BLA resubmission. It is also possible that the FDA could require us to repeat our manufacturing validation campaign once the FDA determines that BTG has resolved all of these issues to the satisfaction of the FDA, though we do not expect the FDA to take this step. We expect that the FDA will
schedule its next reinspection of BTG’s facility for a time prior to making its decision on our BLA resubmission. If the FDA’s reinspection of BTG’s facility is delayed for any reason, it is likely FDA’s decision on our BLA resubmission likewise will be delayed. If the FDA is able to reinspect BTG’s facility, the
FDA may, at that reinspection, determine that it is not satisfied with BTG’s corrective actions and additional commitments, or it may identify new deficiencies with BTG’s facility. We continue to closely monitor the efforts by BTG and its parent company Ferring B.V. to satisfactorily address and correct these deficiencies in line with the direction provided by the FDA. A satisfactory inspection by the FDA verifying the remediation of the deficiencies and other observations is required
by the FDA prior to its approval of the KRYSTEXXA BLA. Failure by BTG to adequately and timely remediate these deficiencies may delay the issuance of the regulatory approvals necessary to market KRYSTEXXA in the United States, or prevent such approvals from being obtained at all. If a reinspection by
the FDA of the BTG facility cannot be completed prior to the PDUFA date for our BLA, or the FDA raises significant additional issues in such reinspection, this could cause further delays in the issuance of such regulatory approvals. In addition, future hostilities in Israel could harm BTG’s ability to supply us with the pegloticase drug substance and could harm our commercialization efforts. Moreover, the scheduling of such reinspection could be delayed in the event of future hostilities in Israel. Prior inspections of BTG scheduled by the
FDA were in fact delayed for this reason. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including:
•
reliance on the third party for regulatory compliance, quality assurance and adequate training in management of manufacturing staff,
the possible breach of the manufacturing agreement by the third party because of factors beyond our control, and
36
•
• the possibility of termination or non-renewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us. Any of these risks could cause us to be unable to obtain sufficient quantities of KRYSTEXXA to meet future demand, which would adversely affect our projected revenues and gross margins. The manufacture and packaging of pharmaceutical products such as KRYSTEXXA are subject to the requirements of the FDA and similar foreign regulatory bodies. If we or our third-party manufacturers fail to satisfy these requirements, our product development and commercialization efforts may be
materially harmed. The manufacture and packaging of pharmaceutical products, such as KRYSTEXXA, are regulated by the FDA and similar foreign regulatory bodies and must be conducted in accordance with the FDA’s cGMPs and comparable requirements of foreign regulatory bodies. Failure by us or our third-party
manufacturers to comply with applicable regulations, requirements, or guidelines could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of our product, delays, suspension or withdrawal of approvals, license revocation,
seizures or recalls of product, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business. Prior to the approval of our BLA, our third-party manufacturers, including BTG, Sigma-Tau and NOF, are subject to pre-approval inspection by the FDA and any delays in inspection or any unsatisfactory results of such pre-approval inspections may delay the issuance of the regulatory approvals that are
necessary to market KRYSTEXXA. As previously discussed, BTG is currently remediating observations arising from the FDA pre-approval inspection of its manufacturing facility. If our third-party manufacturers do not pass such FDA or other regulatory inspections for any reason, including equipment failures,
labor difficulties, failure to meet stringent manufacturing, quality control or quality assurance practices, or natural disaster, our ability to execute upon our commercial strategy for KRYSTEXXA will be jeopardized. Qualifying Diosynth as a global secondary source supplier of pegloticase API, any other change to any of our third-party manufacturers for KRYSTEXXA or any change in the location where KRYSTEXXA is manufactured would require prior FDA review, approval and validation of the manufacturing
process and procedures for KRYSTEXXA. This review, approval and validation would be costly and time consuming and could delay or prevent the manufacture of KRYSTEXXA at such facility. We have engaged Diosynth, which is located in North Carolina, as a secondary source supplier of the pegloticase API drug substance used in KRYSTEXXA. In connection with the FDA’s consideration of Diosynth as a secondary source supplier of pegloticase, Diosynth is required to produce validation
batches of the pegloticase API drug substance to demonstrate to the FDA that the materials produced at Diosynth are comparable to those produced at BTG. If the FDA approves BTG as a manufacturer of pegloticase drug substance used in KRYSTEXXA, and if we cannot establish to the satisfaction of the FDA
that the pegloticase drug substance manufactured at Diosynth is comparable to the drug substance manufactured at BTG, we may not be permitted to use the pegloticase drug substance manufactured at Diosynth in the formulation of KRYSTEXXA. We do not expect FDA approval of the Diosynth manufacturing
facility to be completed until the second half of 2011 at the earliest. If we elect to manufacture the pegloticase drug substance used in KRYSTEXXA at the facility of another third party, if we elect to utilize a new facility to fill and finish KRYSTEXXA or if we change the location where KRYSTEXXA is manufactured, we would need to ensure that the new facility and the
manufacturing process are in substantial compliance with the FDA’s cGMP. Any such new facility would also be subject to a pre-approval inspection by the FDA, and a successful technology transfer and subsequent validation by the FDA would be required, all of which are expensive and time-consuming
endeavors. Any delays or failures in satisfying these requirements could delay our ability to manufacture KRYSTEXXA. 37
If the company from which we source our mPEG-NPC is unable to supply us with product, our business may suffer. We procure mPEG-NPC, a key raw material in the manufacture of pegloticase drug substance, from a single supplier, NOF. Our contract with NOF requires us to purchase this material on an exclusive basis from NOF and, while we have a contractual right to procure this material from another supplier in the
event of a supply failure, procuring this material from another source would require time and effort which may interrupt the supply of mPEG-NPC and thereby cause an interruption of the supply of pegloticase drug substance and KRYSTEXXA to the marketplace. Any interruption of supply of mPEG-NPC could
cause harm to our business. If the company on which we plan to rely for fill and finish services for KRYSTEXXA is unable to perform these services for us, our business may suffer. We have outsourced the operation for KRYSTEXXA fill and finish services to a single company, Sigma-Tau. We have commenced efforts to engage a secondary third-party fill and finish manufacturer for KRYSTEXXA, however at this time we do not have redundancy in our supply chain for these fill and
finish functions and currently have no substitute that can provide these services. If we successfully commercialize KRYSTEXXA and if Sigma-Tau is unable to perform these services for us, we would need to identify and engage an alternative company or develop our own fill and finish capabilities. Any new
contract fill and finish manufacturer or capabilities that we acquire or develop will need to obtain FDA approval. Identifying and engaging a new contract fill and finish manufacturer or developing our own capabilities and obtaining FDA approval could involve significant cost and delay. As a result, we might not be
able to deliver KRYSTEXXA orders on a timely basis, and our business would be harmed. We rely on third parties to conduct our clinical development activities for KRYSTEXXA and those third parties may not perform satisfactorily, which could delay regulatory approval and commercialization of KRYSTEXXA. We do not independently conduct clinical development activities for KRYSTEXXA, including any additional clinical trials that may be required, or we may elect, to conduct in the future. We rely on third parties, such as contract research organizations, clinical data management organizations, medical
institutions and clinical investigators, to perform these activities. We use multiple contract research organizations to coordinate the efforts of our clinical investigators and to accumulate the results of our trials. Our reliance on these third parties for clinical development activities reduces our control over these
activities. We are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocol for the trial. Moreover, the FDA requires us and third parties acting on our behalf to comply with good clinical practices, or cGCP, for conducting, recording and
reporting the results of clinical trials to ensure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Furthermore,
these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual obligations, meet expected deadlines or conduct our clinical development activities in accordance with regulatory requirements or our stated protocols, we may not be able to obtain, or may be delayed in obtaining, regulatory approvals for
KRYSTEXXA and may not be able to, or may be delayed in our efforts to, successfully execute upon our commercial strategy for KRYSTEXXA. We also may be subject to fines and other penalties for failure to comply with requirements applicable to the public reporting of clinical trial information on the registry
and results database maintained by the NIH. We also rely on third parties to store and distribute drug supplies for our clinical development activities. Any performance failure on the part of such third parties could delay regulatory approval or commercialization of KRYSTEXXA, causing us to incur additional expenses and harming our ability to generate
additional revenue. 38
We are seeking a worldwide partner for the further development and commercialization of KRYSTEXXA, and we expect to continue to depend on collaborations with third parties. If we are not successful in these efforts, we may fail to meet our business objectives. We currently depend on third parties in many aspects of the development and commercialization of KRYSTEXXA. Although we are seeking to establish a development and commercialization partnership or other strategic transaction for KRYSTEXXA as part of our strategic business plan, to date we have
not been able to enter into such a partnership or transaction. If we are unable to reach agreements with suitable partners in a timely fashion or at all, we may fail to meet our business objectives for KRYSTEXXA. We face significant competition in seeking appropriate partners. Such arrangements may not be scientifically or commercially successful. The termination of such an arrangement might adversely affect our ability to develop, commercialize and market our products. The success of any collaboration arrangements will depend heavily on the efforts and activities of any potential collaborators. Any potential collaborators will have significant discretion in determining the efforts and resources that they will apply to such collaborations. The risks that we face in connection with
potential collaborations include the following:
collaboration agreements are generally for fixed terms and subject to termination under various circumstances, including, in many cases, on short notice without cause, • we expect to be required in any collaboration agreements not to conduct specified types of research and development in the field that is the subject of the collaboration. These agreements may have the effect of limiting the areas of research and development that we may pursue, either alone or in cooperation
with third parties,
•
collaborators may develop and commercialize, either alone or with others, products and services that are similar to or competitive with our products that are the subject of the collaboration with us, and • collaborators may change the focus of their development and commercialization efforts. Pharmaceutical and biotechnology companies historically have re-evaluated their priorities following mergers and consolidations, which have been common in recent years in our industry. The ability of KRYSTEXXA to
reach its potential could be limited if any potential collaborators decrease or fail to increase spending related to any collaboration. Collaborations with pharmaceutical companies and other third parties often are terminated or allowed to expire by the other party. Such terminations or expirations can adversely affect us financially as well as harm our business reputation. Risks Relating to Intellectual Property If we fail to comply with our obligations in our intellectual property licenses with third parties, we could lose license rights that are important to our business. We are party to various license agreements and we may enter into additional license agreements in the future. For example, we license exclusive worldwide rights to patents and pending patent applications that constitute the fundamental composition of matter and underlying manufacturing patents for
KRYSTEXXA from Mountain View Pharmaceuticals, Inc., or MVP, and Duke University, or Duke. Under the agreement, we are required to use best efforts to bring to market and diligently market products that use the licensed technology. We also must provide MVP and Duke with specified information relating
to the development of KRYSTEXXA. The agreement requires us to pay to MVP and Duke quarterly royalty payments within sixty days after the end of each quarter based on net sales we make in that quarter. The royalty rate for a particular quarter ranges between 8% and 12% of net sales of KRYSTEXXA
based on the amount of cumulative net sales made by us or our sub-licensees. Under the agreement, we are also required to pay royalties of 20% of any revenues or other consideration we receive from sub-licensees during any quarter. As of December 31, 2009, we had made aggregate payments of approximately
$1.7 million to MVP and Duke for the achievement of milestones under this agreement. If we obtain regulatory approval in one of five major global markets, which 39
•
includes FDA approval in the United States, we will be required to make aggregate milestone payments of approximately $0.8 million under this agreement. The agreement with MVP and Duke remains in effect, on a country-by-country basis, for the longer of 10 years from the date of first sale of KRYSTEXXA in such country or the date of expiration of the last-to-expire patent covered by the agreement in such country. The licensors may terminate the
agreement with respect to the countries affected upon our material breach, if not cured within a specified period of time, immediately after our third or subsequent material breach of the agreement or our fraud, willful misconduct or illegal conduct. The licensors may also terminate the agreement in the event of our
bankruptcy or insolvency. Upon a termination of the agreement in one or more countries, all intellectual property rights conveyed to us under the agreement with respect to the terminated countries, including regulatory applications and pre-clinical and clinical data, revert to MVP and Duke and we are permitted to
sell off any remaining inventory of KRYSTEXXA for such countries. In addition, we could have disputes with our current and future licensors regarding, for example, the interpretation of terms in our agreements. Any such disagreements could lead to delays in the development or commercialization of any potential products or could result in time-consuming and expensive
litigation or arbitration, which may not be resolved in our favor. If we fail to comply with our obligations under the agreement, we could lose the ability to develop and commercialize KRYSTEXXA, which could require us to curtail or cease our operations. If we are unable to obtain and maintain protection for the intellectual property relating to our technology and products, the value of our technology and products will be adversely affected. Our success will depend in large part on our ability to obtain and maintain protection in the United States and other countries for the intellectual property covering or incorporated into our technology and products. The patent situation in the field of biotechnology and pharmaceuticals is highly uncertain and
involves complex legal and scientific questions. We may not be able to obtain additional issued patents relating to our technology or products. Even if issued, patents may be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar products or limit
the length or term of patent protection we may have for our products. Generic forms of our product Oxandrin were introduced to the market in late 2006. As a result, our results of operations have been harmed. The patents and, if issued, patent applications relating to KRYSTEXXA, would expire between 2019
and 2026, unless a patent term extension is obtained. Changes in either patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection. Our patents also may not afford us protection against numerous competitors with similar technology. Patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, and in some cases not at all. Therefore, because publications of discoveries in the
scientific literature often lag behind actual discoveries, neither we nor our licensors can be certain that we or they were the first to develop the inventions claimed in issued patents or pending patent applications, or that we or they were the first to file for protection of the inventions set forth in these patent
applications. Even in the event that our patents are upheld as valid and enforceable, they may not foreclose potential competitors from developing new technologies or “workarounds” that circumvent our patent rights. This means that our patent portfolio may not prevent the entry of a competitive product into the
market. In addition, patents generally expire, regardless of their date of issue, 20 years from the earliest claimed non-provisional filing date. As a result, the time required to obtain regulatory approval for a product candidate may consume part or all of the patent term. We are not able to accurately predict the
remaining length of the applicable patent term following regulatory approval of any of our product candidates. If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected. In addition to patented technology, we rely upon unpatented proprietary technology, processes and know-how. We seek to protect this information in part through confidentiality agreements with our employees, consultants and third parties. If any of these agreements are breached, we may not have adequate
remedies for any such breach. In addition, any remedies we may seek may prove costly. 40
Furthermore, our trade secrets may otherwise become known or be independently developed by competitors. If we are unable to protect the confidentiality of our proprietary information and know-how, competitors may be able to use this information to develop products that compete with our products, which
could adversely affect our business. If we infringe or are alleged to infringe intellectual property rights of third parties, our business may be adversely affected. Our development and commercialization activities, as well as any product candidates or products resulting from these activities, may infringe or be claimed to infringe patents or patent applications under which we do not hold licenses or other rights. We are aware of patent applications filed by, and patents
issued to, other entities with respect to technology potentially useful to us and, in some cases, related to products and processes being developed by us. Third parties may own or control these patents and patent applications in the United States and abroad. These third parties could bring claims against us, our
licensors or our collaborators that would cause us to incur substantial expenses. If such third party claims are successful, we could be liable for substantial damages. Further, if a patent infringement suit were brought against us, our licensors or our collaborators, we or they could be forced to stop or delay research,
development, manufacturing or sales of the product or product candidate that is the subject of the suit. As a result of patent infringement claims, or in order to avoid potential claims, we, our licensors or our collaborators may choose or be required to seek a license from the third party and be required to pay license fees, royalties or both. These licenses may not be available on acceptable terms or at all. Even if
we, our licensors or our collaborators were able to obtain a license, our rights may be non-exclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business
operations if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. This could harm our business significantly. The pharmaceutical and biotechnology industries have experienced substantial litigation and other proceedings regarding patent and other intellectual property rights. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference
proceedings declared by the U.S. Patent and Trademark Office and opposition proceedings in the European Patent Office or in another patent office, regarding intellectual property rights with respect to our products and technology. The costs to us of any patent litigation or other proceeding, even if resolved in our
favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could adversely affect our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time. In the future we may be involved in costly legal proceedings to enforce or protect our intellectual property rights or to defend against claims that we infringe the intellectual property rights of others. Litigation is inherently uncertain and an adverse outcome could subject us to significant liability for damages or invalidate our proprietary rights and adversely impact our ability to develop and market KRYSTEXXA. Legal proceedings that we initiate to protect our intellectual property rights could also result
in counterclaims or countersuits against us. Any litigation, regardless of its outcome, could be time consuming and expensive to resolve and could divert our management’s time and attention. Any intellectual property litigation also could force us to take specific actions, including any of the following:
•
cease selling products or undertaking processes that are claimed to be infringing a third party’s intellectual property, • obtain licenses to make, use, sell, offer for sale or import the relevant technologies from the intellectual property’s owner, which licenses may not be available on reasonable terms or at all, • redesign products or processes that are claimed to be infringing a third party’s intellectual property, or 41
• pursue legal remedies with third parties to enforce our indemnification rights, which may not adequately protect our interests. We have been involved in several lawsuits and disputes regarding intellectual property in the past. We could be involved in similar disputes or litigation in the future. An adverse decision in any intellectual property litigation could have a material adverse effect on our business, results of operations and
financial condition. Risks Relating to Our Results of Operations and Your Investment in Our Common Stock We may not be able to achieve profitability. We have incurred operating losses from continuing operations since 2004 and anticipate that we will incur operating losses from continuing operations for the foreseeable future, particularly as a result of increasing expenses related to our development and, if our
BLA is approved by the FDA, commercialization of KRYSTEXXA. We continue to incur substantial operating losses from continuing operations. Our operating losses from continuing operations were $81.2 million and $89.0 million for the years ended December 31, 2009 and 2008, respectively. Our operating losses from continuing operations are the result of two factors:
increasing operating costs and decreasing product revenues. We have incurred and expect to continue to incur significant costs in connection with our research and development activities, including clinical trials, and from selling, general and administrative expenses associated with our operations. Product sales of
Oxandrin and oxandrolone, on which our continuing operations have been substantially dependent, have declined substantially in recent years. Sales of Oxandrin and, since December 2006, oxandrolone have accounted for 100% of our continuing net product sales. Our ability to achieve operating profitability in the future depends on the successful commercialization of KRYSTEXXA. If we do not receive regulatory approval for KRYSTEXXA, or experience significant delays or unanticipated costs in doing so, or if sales revenue from KRYSTEXXA, if it receives
marketing approval, is insufficient, we may never achieve operating profitability. If we do receive regulatory approval for KRYSTEXXA and have not been successful in consummating a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving our company, we
would expect to incur significant expenditures in connection with its commercialization. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis. We expect sales of Oxandrin and oxandrolone to remain flat or continue to decrease, which may continue to harm our results of operations. Sales of Oxandrin and oxandrolone have declined substantially in recent years due to generic competition. Our sales of Oxandrin and oxandrolone in the United States are also affected by fluctuations in the buying patterns of the three major drug wholesalers to which we principally sell these products. In the
past, wholesalers have reduced their inventories of Oxandrin and oxandrolone. We expect that wholesalers will continue to reduce such inventories as a result of generic competition, further decreasing our revenues from these products. Sales of Oxandrin and oxandrolone have also decreased as a result of the elimination of reimbursement, or limited reimbursement practices, by some states under their AIDS Drug Assistance Programs via their state Medicaid programs for HIV/AIDS prescription drugs, including Oxandrin and oxandrolone.
Other state formularies may follow suit. We have considered the demand deterioration of Oxandrin and oxandrolone in estimating future product returns. However, our demand forecasts are based upon our management’s best estimates. Future product returns in excess of our historical reserves could reduce our revenues even further and adversely
affect our results of operations. In addition, we do not have the ability to independently distribute our oxandrolone tablets and depend on our distribution partner, Watson, to distribute this product for us. If Watson fails to carry out its contractual obligations, does not devote sufficient resources to the distribution of oxandrolone, or does not
carry out its responsibilities in the manner we expect, our oxandrolone product may not compete successfully against other generics, and our results of operations could be further harmed. In addition, we rely on a third-party manufacturer to produce Oxandrin and oxandrolone tablets. If it is 42
unable to fulfill its manufacturing obligations to us, our ability to supply the market with Oxandrin and oxandrolone may be materially diminished and our existing market share may decrease. Our stock price is volatile, which could adversely affect your investment. Our stock price has been, and is likely to continue to be, volatile. Between January 1, 2008 and December 31, 2009, our common stock has traded as high as $28.42 per share and as low as $2.80 per share. The stock market in general, and the market for biotechnology companies in particular, has recently
experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price of our common stock may be influenced by many factors, including:
•
our ability to sustain our operations with our existing cash reserves,
whether we obtain regulatory approval for KRYSTEXXA, and the timing of such approval, • if we obtain regulatory approval for KRYSTEXXA, whether it achieves market acceptance, • the nature of post-marketing commitments, • the disclosure of information relating to the efficacy or safety of KRYSTEXXA, • announcements of technological innovations or developments relating to competitive products or product candidates,
•
the costs of commercialization activities, including product marketing, manufacturing, sales and distribution, • our ability to obtain adequate capital resources to execute our business strategy, • market conditions in the pharmaceutical and biotechnology industries and the issuance of new or revised securities analyst reports or recommendations, • period-to-period fluctuations in our financial results, • regulatory developments in the United States and foreign countries, • our issuance of additional shares of our common stock in capital raising transactions, or upon the exercise of outstanding warrants to purchase shares of our common stock, • general economic, industry and market conditions, and • other factors described in this “Risk factors” section. The volatility of our common stock imposes a greater risk of capital losses for our stockholders than a less volatile stock would. In addition, volatility makes it difficult to ascribe a stable valuation to a stockholder’s holdings of our common stock. We expect our quarterly results to fluctuate, which may cause volatility in our stock price. Our revenues from product sales of Oxandrin and oxandrolone have in the past displayed and may in the future continue to display significant variations. These variations may result from a variety of factors, including:
•
increased competition from new or existing products, including generic products, • the amount and timing of product sales, • changing demand for our products, • our inability to provide adequate supply for our products, • changes in wholesaler buying patterns, • returns of expired products, • changes in government or private payor reimbursement policies for our products, • the timing of the introduction of new products, • the timing and realization of milestone and other payments to licensees, • the timing and amount of expenses relating to research and development, product development and manufacturing activities, 43
•
• the timing and amount of expenses relating to sales and marketing, • the timing and amount of expenses relating to general and administrative activities, • the extent and timing of costs of obtaining, enforcing and defending intellectual property rights, and • any charges related to acquisitions. In addition, our expenses have fluctuated and are likely to increase significantly in the future in connection with our development and commercialization efforts related to KRYSTEXXA. As a result, any decrease in revenues or increase in expenses will likely adversely affect our earnings in a significant manner
until revenues can be increased or expenses reduced. We expect that our revenues and earnings from sales of Oxandrin and oxandrolone will remain flat or continue to decrease. Because of fluctuations in revenues and expenses, it is possible that our operating results for a particular quarter or quarters will not meet
the expectations of public market analysts and investors, which could cause the market price of our common stock to decline. We are a party to a stockholder lawsuit regarding the adequacy of our public disclosure, which could have a material adverse affect on our business, results of operations and financial condition. In November 2008, Richard Sagall, an alleged stockholder, commenced an action in the U.S. District Court for the Southern District of New York seeking to certify a class of shareholders who held Savient securities between December 13, 2007 and October 24, 2008. The suit alleges that we made false and
misleading statements relating to the GOUT1 and GOUT2 Phase 3 clinical trials and that we failed to disclose in a timely manner serious adverse events which occurred in five patients in these trials. A lead plaintiff was appointed in March 2009 resulting in the re-captioning of the action as Lawrence J. Koncelik,
Jr. vs. Savient Pharmaceuticals, et al. An amended compliant was filed in this matter in April 2009 and we filed a motion to dismiss in June 2009. The lead plaintiff subsequently filed an opposition of the motion to dismiss and we filed our reply in October 2009. Oral arguments were heard by the Court in February
2010 relating to our motion to dismiss and we are awaiting the Court’s decision. We intend to vigorously defend against this action. We expect that the costs related to this suit will be significant and we can provide no assurance as to its outcome. If we are not successful in defending this action, we may be required to pay substantial damages to the plaintiffs. As a result, our business, results of operations and financial condition could be
materially adversely affected. In addition, even if we are successful, the defense of this action will continue to divert the attention of our management and other resources that would otherwise be engaged or utilized in operating our business. Our outstanding warrants may be exercised in the future, which would increase the number of shares of our common stock in the public market and result in dilution to our stockholders. In April 2009, we completed a financing in which we issued 5,927,343 shares of our common stock and warrants to purchase up to 5,038,237 shares of our common stock. The warrants are exercisable at any time on or after the date of issuance and expire on November 2, 2010. Pursuant to the terms of the warrants, the exercise price for the warrants is $10.46 per share. We may, at our or the warrant holder’s election, issue net shares in lieu of a cash payment of the exercise price by the warrant holder upon exercise. In the event that we enter into a merger or change of control transaction, the holders of the warrants will be entitled to receive consideration as if they had exercised the warrant immediately prior to such transaction or may instead require us to purchase the warrant for cash at the Black-Scholes value of the
warrant on the date of such transaction. Effecting a change of control of our company could be difficult, which may discourage offers for shares of our common stock. Our certificate of incorporation and the Delaware General Corporation Law, or the DGCL, contain provisions that may delay or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your
shares. These provisions include the requirements of Section 203 of the DGCL. Section 203 prohibits a publicly held Delaware corporation from engaging in a business combination 44
with an “interested stockholder,” generally deemed a person that, together with its affiliates, owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
•
our Board of Directors approves the transaction before the third party acquires 15% of our stock,
the third party acquires at least 85% of our stock at the time its ownership exceeds the 15% level, or • our Board of Directors and the holders of two-thirds of the shares of our common stock not held by the third-party vote in favor of the transaction. Our certificate of incorporation also authorizes us to issue up to 4,000,000 shares of preferred stock in one or more different series with terms fixed by our Board of Directors. Stockholder approval is not necessary to issue preferred stock in this manner. Issuance of these shares of preferred stock could have the
effect of making it more difficult for a person or group to acquire control of our company. No shares of our preferred stock are currently outstanding. Although our Board of Directors has no current intention or plan to issue any preferred stock, issuance of these shares could also be used as an anti-takeover device. Product liability lawsuits could cause us to incur substantial liabilities. We face an inherent risk of product liability exposure related to product sales of Oxandrin and oxandrolone. We also face the risk of product liability exposure related to the testing and, if approved by the FDA, future product sales of KRYSTEXXA. If we cannot successfully defend ourselves against claims
that our products or product candidates caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
•
decreased demand for KRYSTEXXA,
injury to our reputation, • withdrawal of clinical trial participants, • withdrawal or recall of a product from the market, • modification to product labeling that may be unfavorable to us, • costs to defend the related litigation, • substantial monetary awards to trial participants or patients, and • loss of revenue. We currently have product liability insurance coverage in place, which is subject to coverage limits and deductibles. The amount of insurance that we currently hold may not be adequate to cover all liabilities that may occur. Product liability insurance is difficult to obtain and increasingly expensive. We may not
be able to maintain insurance coverage at a reasonable cost and we may not be able to obtain insurance coverage with policy limits that will be adequate to satisfy any liability that may arise. ITEM 1B. UNRESOLVED STAFF COMMENTS None. ITEM 2. PROPERTIES Our corporate headquarters are located in East Brunswick, New Jersey, where we lease approximately 53,000 square feet of office space. The lease has a base average annual rental expense of approximately $1.9 million and expires in March 2013. We have two five-year renewal options under the lease. In connection with this lease arrangement, we were required to provide a $1.3 million security deposit by way of an irrevocable letter of credit, which is secured by a cash deposit of $1.3 million and is reflected in Other Assets (as restricted cash) on our consolidated balance sheets at December 31, 2009 and
2008. 45
•
•
ITEM 3. LEGAL PROCEEDINGS Intellectual Property-Related Litigation In 2007, Joseph R. Berger filed a complaint against us in the Fayette County Circuit Court in Kentucky alleging breach of contract in connection with the assignment of certain inventions and patent rights related to the method of using oxandrolone to treat HIV/AIDS patients. The complaint alleged several
causes of action, each of which was premised on the existence of an oral agreement between us and Berger, which Berger alleges we breached. Berger sought, among other things, damages and rescission of the assignment of the inventions. In March 2008, Berger filed an amended complaint that abandoned several
of his claims, but continued to seek damages and rescission of the invention assignments. In March 2009, we filed a motion for summary judgment on Berger’s claims and thereafter Berger filed a cross-motion for summary judgment. Subsequently, oral argument on the summary judgment motions was held and on
August 17, 2009, the Court issued an order granting our motion for summary judgment, denying Berger’s cross-motion and dismissing the complaint and amended complaint with prejudice. On September 11, 2009, counsel for Berger filed a notice of appeal of the decision of the trial court, in response to which we
filed a notice of cross-appeal solely with respect to the decision of the trial court to apply Kentucky law to the facts of the case. The matter is currently pending the issuance of an appellate schedule. We intend to vigorously defend this action and to contest the appeal by Berger of the granted summary judgment
and dismissal. Other Litigation In November 2008, Richard Sagall, an alleged stockholder, commenced an action in the U.S. District Court for the Southern District of New York seeking to certify a class of shareholders who held Savient securities between December 13, 2007 and October 24, 2008. The suit alleges that we made false and
misleading statements relating to the GOUT1 and GOUT2 phase 3 clinical trials, and that we failed to disclose in a timely manner serious adverse events which occurred in five patients in these trials. In March 2009, the Court issued an order appointing a lead plaintiff and the law firm Pomerantz Haudek Block
Grossman & Gross LLP as lead counsel. Thereafter the lead plaintiff filed his amended complaint in April 2009, seeking unspecified monetary damages. In June 2009, we and the other named defendants moved to dismiss the complaint. The lead plaintiff subsequently filed an opposition of the motion to dismiss and
we filed our reply in October 2009. Oral arguments were heard by the Court in February 2010 relating to our motion to dismiss and we are awaiting the Court’s decision. We intend to vigorously defend against this action. From time to time we are subject to legal proceedings and claims in the ordinary course of business. Such claims, even if without merit, could result in the significant expenditure of our financial and managerial resources. ITEM 4. [RESERVED] 46
PART II ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES Our common stock is quoted on The Nasdaq Global Market under the symbol “SVNT”. The following table sets forth, for the periods indicated, the high and low sales prices per share of our common stock from January 1, 2008 through December 31, 2009 as reported by The Nasdaq Global Market.
High
Low 2008 First Quarter
$
24.55
$
16.71 Second Quarter
28.10
18.61 Third Quarter
28.42
14.50 Fourth Quarter
15.11
2.80 2009 First Quarter
$
8.27
$
3.45 Second Quarter
14.54
4.38 Third Quarter
16.62
10.00 Fourth Quarter
15.30
12.20 The number of stockholders of record of our common stock on February 24, 2010 was approximately 788. We have never declared or paid a cash dividend on our common stock, and we do not expect to pay cash dividends to the holders of our common stock in the foreseeable future. 47
STOCK PERFORMANCE GRAPH The following graph compares the cumulative total return of our common stock with the cumulative total returns on the (i) NASDAQ Composite, (ii) Nasdaq Pharmaceutical and (iii) Nasdaq Biotechnology indices for the period from December 31, 2004 through December 31, 2009. The graph assumes (a) $100
was invested on December 31, 2004 in our common stock and the stocks in each of the indices and (b) the reinvestment of dividends. The comparisons in the graph below are based on historical data and are not indicative of, or intended to forecast, possible future performance of our common stock. COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN* *100 invested on 12/31/04 in stock or index, including reinvestment of dividends.
12/04
12/05
12/06
12/07
12/08
12/09 Savient Pharmaceuticals, Inc.
100.00
138.01
413.65
847.60
213.65
502.21 NASDAQ Composite
100.00
101.41
114.05
123.94
73.43
105.89 NASDAQ Pharmaceutical
100.00
102.23
105.16
99.56
91.99
98.21 NASDAQ Biotechnology
100.00
117.54
117.37
121.37
113.41
124.58 48
Among Savient Pharmaceuticals, Inc., The NASDAQ Composite Index,
The NASDAQ Pharmaceutical Index and The NASDAQ Biotechnology Index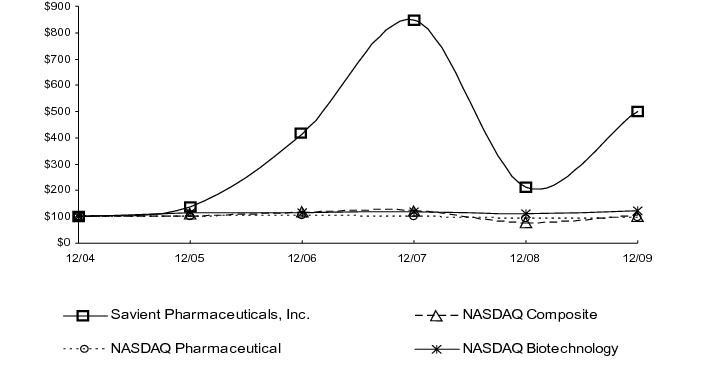
Fiscal year ending December 31.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA The consolidated statements of operations data for each of the years in the five-year period ended December 31, 2009 and the consolidated balance sheet data as of December 31, 2009, 2008, 2007, 2006 and 2005 are derived from our audited consolidated financial statements. The selected consolidated financial
data should be read in conjunction with our consolidated financial statements and the Notes to the consolidated financial statements and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”.
Year Ended December 31,
2009
2008
2007
2006
2005
(In thousands, except per share data) Statement of Operations Data (1): Product sales, net
$
2,956
$
3,028
$
13,825
$
47,351
$
48,043 Other revenues
4
153
199
163
1,452 Total revenues
2,960
3,181
14,024
47,514
49,495 Cost of goods sold and expenses
84,122
92,192
83,198
64,519
63,975 Operating loss from continuing operations
(81,162
)
(89,011
)
(69,174
)
(17,005
)
(14,480
) Other income (expense), net and investment income
(11,762
)
753
8,212
15,566
14,157 Income tax expense (benefit)
(2,071
)
(5,017
)
(11,807
)
25
146 Loss from continuing operations.
(90,853
)
(83,241
)
(49,155
)
(1,464
)
(469
) Income (loss) from discontinued operations
—
(928
)
487
61,789
6,437 Net income (loss)
$
(90,853
)
$
(84,169
)
$
(48,668
)
$
60,325
$
5,968 Loss per common share from continuing operations: Basic and diluted
$
(1.51
)
$
(1.55
)
$
(0.94
)
$
(0.03
)
$
(0.01
) Earnings (loss) per common share from discontinued operations: Basic and diluted
$
—
$
(0.02
)
$
0.01
$
1.06
$
0.11 Earnings (loss) per common share: Basic and diluted
$
(1.51
)
$
(1.57
)
$
(0.93
)
$
1.03
$
0.10 Weighted-average number of common and common equivalent shares: Basic
59,997
53,533
52,461
58,538
60,837
(1)
Selected consolidated financial data includes retrospective reclassifications from continuing operations to discontinued operations as a result of certain divestitures (BTG in 2005 and Rosemont in 2006) as disclosed in the footnotes to our consolidated financial statements.
49
As of December 31,
2009
2008
2007
2006
2005
(In thousands) Balance Sheet Data (2): Cash, cash equivalents and short-term investments.
$
108,175
$
78,597
$
142,422
$
179,396
$
75,372 Accounts receivable, net
352
822
1,490
3,517
11,716 Inventories, net.
585
1,892
2,636
4,203
9,419 Total current assets.
112,520
89,619
158,934
194,858
105,863 Goodwill
—
—
—
—
40,121 Other intangibles, net
—
—
—
—
67,638 Total assets
119,008
98,222
167,173
197,893
222,691 Total current liabilities
44,700
24,989
19,184
20,164
20,866 Long-term liabilities
10,109
9,809
8,924
43
— Accumulated deficit
(242,467
)
(151,614
)
(67,445
)
(14,316
)
(41,519
) Stockholders’ equity
64,199
63,424
139,065
177,686
181,394
(2)
Selected consolidated balance sheet data includes BTG and Rosemont for year ends prior to their date of divestiture.
50
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS Overview We are a specialty biopharmaceutical company focused on developing KRYSTEXXA™ (pegloticase) for the treatment of chronic gout in patients refractory to conventional therapy. Chronic gout that is refractory to conventional therapy occurs in patients who have failed to normalize serum uric acid and whose
signs and symptoms are inadequately controlled with xanthine oxidase inhibitors at the maximum medically appropriate dose or for whom these drugs are contraindicated. KRYSTEXXA is not recommended for the treatment of asymptomatic hyperuricemia. Based on the progress we have made to date, we believe that we will file our BLA resubmission for KRYSTEXXA in March 2010. Our strategic plan is to seek approval from the FDA of our BLA filing for KRYSTEXXA and to develop a program of regulatory filings and review of KRYSTEXXA in other countries. We continue to believe that a worldwide partnership for the commercialization of KRYSTEXXA or broader strategic
transaction involving our company would serve to maximize the commercial prospects of KRYSTEXXA in the United States, Europe and other foreign countries. If we are not able to consummate a worldwide partnership or broader strategic transaction on terms acceptable to us, we intend to independently pursue
the commercialization of KRYSTEXXA in the United States. Currently, we sell and distribute branded and generic versions of oxandrolone, a drug used to promote weight gain following involuntary weight loss. We launched our authorized generic version of oxandrolone in December 2006 in response to the approval and launch of generic competition to Oxandrin. The
introduction of oxandrolone generics has led to significant decreases in demand for Oxandrin and our authorized generic version of oxandrolone. We believe that revenues from Oxandrin and our authorized generic version of oxandrolone will remain flat or continue to decrease in future periods. We currently operate within one “Specialty Pharmaceutical” segment, which includes sales of Oxandrin and oxandrolone and the research and development activities of KRYSTEXXA. Discontinued Operations In August 2006, we sold Rosemont Pharmaceuticals, Ltd., or Rosemont, our former United Kingdom subsidiary that marketed more than 100 oral liquid pharmaceuticals in the United Kingdom, Europe and part of Asia, to Ingleby (1705) Limited, a Close Brothers Private Equity Company, or Close Brothers.
Under the terms of the sale, Close Brothers paid us an aggregate purchase price of $176.0 million for the issued share capital of Rosemont’s parent company and certain other related assets. Net proceeds from the transaction after selling costs and taxes were $151.6 million. We recorded a pre-tax gain of $77.2
million on the disposition of Rosemont in 2006. In July 2005, we sold BTG, a biologics manufacturing business which primarily operates in Israel, to Ferring. Under the terms of the sale, Ferring paid us an aggregate purchase price of $80 million in cash and assumed specified liabilities related to the BTG business. We recorded a loss of $4,000 on the
disposition of the BTG business in 2005. Revenue, operating income and income (loss) from discontinued operations, which consisted of the Rosemont and BTG businesses, for the years ended December 31, 2009, 2008 and 2007 are as follows:
Year Ended December 31,
2009
2008
2007
(In thousands) Revenues from discontinued operations
$
—
$
—
$
— Operating income from discontinued operations
$
—
$
—
$
— Income (loss) from discontinued operations
$
—
$
(928
)
$
487 During the year ended December 31, 2008, we incurred a $0.9 million tax assessment as a result of a tax audit for the 2003 through 2005 tax years that related to corporate income tax returns filed in 51
Israel by BTG. Discontinued operations for 2007 include tax refunds related to BTG that we received from the Israel taxing authorities and foreign currency exchange gains on these refunds. The tax refund was due to an overpayment of estimated taxes in Israel for the 2005 short tax year. We did not have any revenues or operating income from discontinued operations for the years ended December 31, 2009, 2008 and 2007. These results of discontinued operations are not included in the discussion below under “Results of Operations.” Critical Accounting Policies and Estimates Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States. Applying these principles requires our judgment in determining the
appropriateness of acceptable accounting principles and methods of application in diverse and complex economic activities. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of revenues, expenses, assets and liabilities, and related disclosure
of contingent assets and liabilities. We base our estimates on historical experience and other assumptions that we believe are reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are more fully described in Note 1 to our consolidated financial statements included in Item 8 of this Annual Report on Form 10-K, we believe the following accounting policies include management estimates that are most critical to our reported financial results: Research and Development. Research and development costs are expensed as incurred and include salaries and benefits, stock-based compensation, and costs paid to third-party contractors to perform research, conduct clinical trials, and develop drug materials. Manufacturing costs are a significant component of research and development expenses and include costs associated with third-party contractors for validation batch production, process technology transfer, quality control and stability testing, raw material purchases, overhead expenses and facilities costs. We
expense these manufacturing-related expenses as research and development as incurred because these costs do not meet the definition of an asset, as future use cannot be determined based upon the uncertainty of whether KRYSTEXXA will be approved for marketing by the FDA. Clinical trial costs are another significant component of research and development expenses and most of our clinical studies are performed by third-party CROs. We accrue costs for clinical studies performed by CROs that are milestone or event driven in nature and based on reports and invoices submitted by
the CRO. These expenses are based on patient enrollment as well as costs consisting primarily of payments made to the CRO, clinical sites, investigators, testing facilities and patients for participating in our clinical trials. Non-refundable advance payments for future research and development activities are deferred and capitalized. Such amounts are recognized as an expense as the goods are delivered or the related services are performed. Consistent with the consensus, we have deferred approximately $0 and $1.3 million of
these costs as of December 31, 2009 and 2008, respectively, and have amortized approximately $0.7 million and $0.6 million for the years ended December 31, 2009 and 2008, respectively, based on services performed. The net deferred balance of $0 and $0.7 million as of December 31, 2009 and 2008, respectively, is
included in Prepaid Expenses and Other Current Assets on our consolidated balance sheet. All other research and development costs are charged to expense as incurred, including $2.3 million and $2.5 million of fees incurred during the years ended December 31, 2009 and 2008, respectively, to reserve
manufacturing capacity at our third-party suppliers’ manufacturing facilities, for future potential orders of KRYSTEXXA. Share-Based Compensation. We have share-based compensation plans in place and record the associated stock-based compensation expense over the requisite service period. The share-based compensation plans are described in Note 10 to the consolidated financial statements. 52
Compensation cost for stock options that contain service conditions is charged against income on a straight-line basis between the grant date for the option and the vesting period. We estimate the fair value of all stock option awards that contain service conditions as of the grant date by applying the Black-
Scholes pricing valuation model. The application of this valuation model involves assumptions that are highly subjective, judgmental and sensitive in the determination of compensation cost. Total unrecognized compensation cost will be adjusted for future changes in estimated forfeitures. In addition, as future
grants are made, we expect to incur additional compensation costs. Restricted stock awards that contain service conditions are recorded as deferred compensation and amortized into compensation expense, on a straight-line basis over the vesting period, which ranges from one to four years in duration. Compensation cost for restricted stock awards that contain service
conditions is based on the grant date fair value of the award, which is the closing market price of our common stock on the grant date multiplied by the number of shares awarded. Compensation cost for restricted stock and stock option awards that contain performance or market conditions is based on the grant date fair value of the award. Compensation expense is recorded over the implicit or explicit requisite service period based on management’s best estimate as to whether it is
probable that the shares awarded are expected to vest. For purposes of recording compensation expense, we consider performance conditions that depend on a change in control event or an FDA approval, once the transaction is consummated or the event occurs. Previously recognized compensation expense is fully
reversed if performance targets are not satisfied. We assess the probability of the performance indicators being met on a continuous basis and record compensation expense from that date, over the remainder of the requisite service period. We grant stock options that contain service conditions to employees and Directors with exercise prices equal to the fair market value of the underlying shares of our common stock on the date that the options are granted. Options granted have a term of 10 years from the grant date. Options granted to
employees vest over a four-year period and options granted to Directors vest in equal quarterly installments over a one-year period, from the date of grant. Options to Directors are granted on an annual basis and represent compensation for service performance on the Board of Directors. Compensation cost for
stock options is charged against income on a straight-line basis between the grant date for the option and each vesting date. We estimate the fair value of all stock option awards at the closing price on the grant date by applying the Black-Scholes pricing valuation model. The application of this valuation model
involves assumptions that are highly subjective, judgmental and sensitive in the determination of compensation cost. During the years ended December 31, 2009, 2008 and 2007, we issued 1,110,000 shares, 378,000 shares, and 607,000 shares, respectively, of our common stock upon the exercise of outstanding stock
options and received proceeds of $6.7 million, $1.8 million, and $3.5 million, respectively. For the year ended December 31, 2009, we realized no tax benefit from the exercise of stock options. For the years ended December 31, 2009, 2008 and 2007, approximately $4.0 million, $3.5 million and $3.3 million,
respectively, of stock option compensation cost has been charged against income. As of December 31, 2009, there was $2.0 million of unrecognized compensation cost, adjusted for estimated forfeitures, related to unamortized stock option compensation which is expected to be recognized over a remaining weighted-
average period of approximately 1.9 years. Total unrecognized compensation cost will be adjusted for future changes in estimated forfeitures. During the year ended December 31, 2009, we recorded compensation expense of $0 related to stock option awards to senior management personnel that contain performance or market conditions, the vesting of which is contingent upon the achievement of certain specific strategic objectives by December 31,
2010, including FDA marketing approval of KRYSTEXXA and other business development objectives. At December 31, 2009, approximately 102,000 potential option shares with performance or market conditions remain unvested. Stock option awards with performance or market conditions encompass
performance targets set for senior management personnel through the end of 2010 and could result in approximately $0.8 million of additional compensation expense if the performance targets are met or expected to be achieved. We grant restricted stock awards that contain service conditions to our employees and to our Directors. Restricted stock awards are recorded as deferred compensation and amortized into compensation expense, on a straight-line basis over the vesting period, which ranges from one to four 53
years in duration. Restricted stock awards to Directors are granted on a yearly basis and represent compensation for services performed on the Board of Directors. Restricted stock awards to Directors vest in equal quarterly installments over a one-year period from the grant date. Compensation cost for restricted
stock awards that contain service conditions is based on the award’s grant date fair value, which is the closing market price of our common stock on the grant date, multiplied by the number of shares awarded. For the year ended December 31, 2009, we issued 244,000 shares of restricted stock at a weighted-average
grant date fair value of $9.28 per share amounting to $2.3 million in total aggregate fair market value. During the years ended December 31, 2009, 2008 and 2007, approximately $2.7 million, $3.5 million and $2.7 million, respectively, of deferred restricted stock compensation cost has been charged against income.
At December 31, 2009, approximately 500,000 shares remained unvested and there was approximately $3.8 million of unrecognized compensation cost related to restricted stock that contain service conditions. During the year ended December 31, 2009, we recorded compensation expense of $0 related to restricted stock awards to senior management personnel that contain performance or market conditions the vesting of which is contingent upon the achievement of certain specific strategic objectives by December
31, 2010, including FDA marketing approval of KRYSTEXXA and other business development objectives. At December 31, 2009, approximately 168,000 potential shares of restricted stock awards with performance or market conditions remain unvested, and could result in approximately $2.3 million of additional
compensation expense if the performance targets are met or expected to be achieved. During 2008, we reversed approximately $0.3 million of compensation expense that was previously charged against income and for the year ended December 31, 2007, $2.4 million of compensation cost was charged against income related to restricted stock awards issued in previous
years that contain performance or market conditions. At December 31, 2009, approximately 49,000 potential shares of these restricted stock awards issued in previous years with performance or market conditions remain unvested. Restricted stock awards with performance conditions issued in previous years
encompass performance targets set for senior management personnel through 2012 and could result in approximately $0.9 million of additional compensation expense if the performance targets are met or expected to be achieved. During the year ended December 31, 2007, we issued a restricted stock award to our former President and Chief Executive Officer, the vesting of which was contingent upon the price of our common stock achieving a certain pre-established stock price target. We used a Monte Carlo simulation model to calculate both the grant date fair
value and the derived requisite service period of the award. Based on the simulation, the grant date fair value of the award was calculated to be $8.98 per share and compensation cost was being charged against income ratably over a two-year derived requisite service period. For restricted stock awards that contain
market conditions, compensation cost is charged against income regardless of whether the market condition is ever achieved and is reversed only if the derived requisite service period is not met by the recipient of the award. As a result of the resignation of employment of our former President and Chief Executive
Officer in November 2008, the derived requisite service period for this award was not met, and therefore, we reversed in 2008, $1.5 million of compensation expense that was previously charged against income during 2007 and 2008. Income taxes. In 2009, we generated a net operating loss of approximately $80.3 million for federal income tax purposes and recorded an income tax benefit of $2.1 million. This benefit reflects the expected tax effect of carrying back an increased portion of our 2008 alternative minimum tax net operating loss
to our 2003 and 2006 tax years to recover the remaining alternative minimum income tax liability of $2.0 million that is eligible to be refunded for those tax years, as well as a $0.1 million benefit due to a reversal of a liability for unrecognized tax benefit caused by a lapse in statute. The opportunity for carryback
became available due to Section 172 (b) (1) (H) and 810(b) of the Internal Revenue Code which was amended under Section 13 of the Worker, Homeownership, and Business Assistance Act of 2009. A refund of $2.0 million was received in February of 2010 relating to this income tax benefit. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and 54
net operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax
rates is recognized in income in the period that includes the enactment date. We regularly review our deferred tax assets for recoverability and establish a valuation allowance based on historical taxable income, projected future taxable income, applicable tax strategies, and the expected timing of the reversals of
existing temporary differences. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. In forming our judgment regarding the recoverability of deferred tax assets related to deductible temporary differences and tax attribute carry
forwards, we give weight to positive and negative evidence based on the extent to which the forms of evidence can be objectively verified. We attach the most weight to historical earnings due to its verifiable nature. Weight is attached to tax planning strategies if the strategies are prudent and feasible and
implementable without significant obstacles. Less weight is attached to forecasted future earnings due to its subjective nature. In 2009, based on the net operating loss generated in 2009 and historical losses and the uncertainty of profitability in the near future, we concluded that we would maintain a full valuation
allowance on all of our deferred tax assets except those assets that are reserved by a liability for unrecognized tax benefits. We maintained a valuation allowance as of December 31, 2009 of $125.8 million. The increase in valuation allowance from 2009 compared to 2008 of approximately $39.0 million is primarily
due to an increase in net operating loss carry forwards and tax credits and the uncertainty that these additional deferred tax assets will be realized. We use judgment in determining income tax provisions and in evaluating our tax positions. Additional provisions for income taxes are established when, despite the belief that tax positions are fully supportable, there remain certain positions that do not meet the minimum probability threshold, which is a tax
position that is more-likely-than-not to be sustained upon examination by the applicable taxing authority. We are examined by Federal and state tax authorities. We regularly assess the potential outcomes of these examinations and any future examinations for the current or prior years in determining the adequacy
of our provision for income taxes. We continually assess the likelihood and amount of potential adjustments and adjust the income tax provision, the current tax liability and deferred taxes in the period in which the facts that give rise to a revision become known. The total amount of federal, state, local and foreign liabilities for unrecognized tax benefits was $10.0 million as of December 31, 2009, including accrued penalties and interest. The net increase of $5.4 million in the liability for unrecognized tax benefits subsequent to adoption of the new accounting guidance
on accounting for uncertainties in income taxes as codified under FASB ASC 640, Income Taxes, resulted in a corresponding decrease to the income tax benefit within our consolidated statements of operations as well as an increase to the deferred tax asset for which no tax benefit will be recognized in our
consolidated statements of operations. Disclosures about Fair Values of Financial Instruments. We adopted a new accounting standard that defines fair value and establishes a framework for fair value measurements effective January 1, 2008 for financial assets and liabilities and effective January 1, 2009 for non-financial assets and liabilities that are
not re-measured on a recurring basis. Under this standard, fair value is generally defined as the price that would be received to sell an asset or paid to transfer a liability (or “exit price”) in an orderly transaction between market participants at the measurement date. The adoption of this accounting standard did not
have a material impact on our condensed consolidated results of operations, financial position or cash flows. We categorize our financial instruments into a three-level fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1) and
the lowest priority to unobservable inputs (Level 3). If the inputs used to measure fair value fall within different levels of the hierarchy, the category level is based on the lowest priority level input that is significant to the fair value measurement of the instrument. Financial assets recorded at fair value on our consolidated balance sheets are categorized as follows: Level 1: Unadjusted quoted prices for identical assets in an active market. 55
Level 2: Quoted prices in markets that are not active or inputs that are observable either directly or indirectly for substantially the full-term of the asset. Level 2 inputs include the following:
•
Quoted prices for similar assets in active markets, • Quoted prices for identical or similar assets in non-active markets, • Inputs other than quoted market prices that are observable, and • Inputs that are derived principally from or corroborated by observable market data through correlation or other means. Level 3: Prices or valuation techniques that require inputs that are both unobservable and significant to the overall fair value measurement. They reflect management’s own assumptions about the assumptions a market participant would use in pricing the asset. During the three months ended June 30, 2009, we adopted two new accounting standards that require disclosures at each interim balance sheet date of the fair value of financial instruments and valuation techniques used to determine fair value. Previously, these disclosures were only required annually. One of
these accounting standards also provides additional guidance in estimating fair value when the market volume and level of activity for an asset or liability have significantly decreased and identifying circumstances that indicate a transaction may not be orderly. The adoption of these two accounting standards did not
have a material impact on our consolidated results of operations, financial position or cash flows. The carrying amounts of Cash and Cash Equivalents, Notes Receivable, Accounts Receivable, Accounts Payable and Other Current Liabilities approximate fair value. See Note 2 to our consolidated financial statements for further discussion of the fair value of financial instruments. Other-Than-Temporary Impairment Losses on Investments. We regularly monitor our available-for-sale portfolio to evaluate the necessity of recording impairment losses for other-than-temporary, or OTT, decreases in the fair value of investments. During the three months ended June 30, 2009, we adopted a
new accounting standard that modifies the guidance used in determining whether the impairment of a debt security is OTT. The impairment of a debt security is considered OTT if an entity concludes that it intends to sell the impaired security, that it is more likely than not that it will be required to sell the security
before the recovery of its cost basis or that it does not otherwise expect to recover the entire cost basis of the security. This accounting standard also amends the presentation requirements of OTT impaired debt securities and expands disclosure requirements in the financial statements for investments in both debt
and equity securities. The adoption of this accounting standard did not have a material impact on our consolidated results of operations, financial position or cash flows. Management makes determinations relating to recording impairment losses for OTT through the consideration of various factors such as management’s intent to sell an investment before the recovery of its cost basis. OTT impairment losses result in a permanent reduction to the cost basis of the investment.
For the years ended December 31, 2009, 2008 and 2007, we recorded $0, $0.5 million and $0.3 million, respectively, of realized investment losses due to OTT declines in fair value. Product revenue recognition. Product sales are generally recognized when title to the product has transferred to our customers in accordance with the terms of the sale. We ship our authorized generic oxandrolone to our distributor and account for these shipments on a consignment basis until product is sold
into the retail market. We defer the recognition of revenue related to these shipments until we confirm that the product has been sold into the retail market and all other revenue recognition criteria have been met. Revenue is not recognized until it is realized or realizable and earned. Revenue from sales transactions where the buyer has the right to return the product is recognized at the time of sale only if (i) the seller’s price to the buyer is substantially fixed or determinable at the date of sale, (ii) the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is
not contingent on resale of the product, (iii) the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, (iv) the buyer acquiring the product for resale has economic substance apart from that provided by the seller, (v) the 56
seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (vi) the amount of future returns can be reasonably estimated. Our net product revenues represent total product revenues less allowances for returns, Medicaid rebates, other government rebates, discounts, and distribution fees. Allowances for returns. In general, we provide credit for product returns that are returned six months prior to or up to 12 months after the product expiration date. Our product sales in the United States primarily relate to the following products:
Product
Expiration Oxandrin and oxandrolone 2.5 mg
5 Oxandrin and oxandrolone 10 mg
3-4 Upon sale, we estimate an allowance for future returns. We provide additional reserves for contemporaneous events that were not known or knowable at the time of shipment. In order to reasonably estimate future returns, we analyze both quantitative and qualitative information including, but not limited to,
actual return rates by lot productions, the level of product manufactured by us, the level of product in the distribution channel, expected shelf life of the product, current and projected product demand, the introduction of new or generic products that may erode current demand, and general economic and industry-
wide indicators. The aggregate net product return allowance reserve was $1.0 million as of December 31, 2009, and $1.2 million as of December 31, 2008. A tabular roll-forward of the activity related to the allowance for product returns is as follows:
Description
Balance at
Expense Provisions
Actual Deductions
Other
Balance at
Related to
Related to
Related to
Related to
(In thousands) Allowance for sales returns: 2009
$
1,163
194
1,972
—
(2,296
)
—
$
1,033 2008
$
905
175
2,673
—
(2,590
)
—
$
1,163 Allowances for Medicaid and other government rebates. Our contracts with Medicaid and other government agencies such as the Federal Supply System commit us to providing those agencies with our most favorable pricing. This ensures that our products remain eligible for purchase or reimbursement under
these government-funded programs. Based upon our contracts and the most recent experience with respect to sales through each of these channels, we provide an allowance for rebates. We monitor the sales trends and adjust the rebate percentages on a regular basis to reflect the most recent rebate experience. The
aggregate net rebate accrual balances were $0.3 million as of December 31, 2009 and $0.6 million as of December 31, 2008. A tabular roll-forward of the activity related to the allowances for Medicaid and other government rebates is as follows:
Description
Balance at
Expense Provisions
Actual Deductions
Other
Balance at
Related to
Related to
Related to
Related to
(In thousands) Allowance for rebates: 2009
$
643
708
(208
)
(446
)
(436
)
—
$
261 2008
$
1,003
1,754
(183
)
(1,111
)
(820
)
—
$
643 Inventory valuation. We state inventories at the lower of cost or market. Cost is determined based on actual cost. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, we record reserves for the difference between the cost and market value. We
determine these reserves based on estimates. 57
(in years)
Beginning
of Period
Deductions
End of Period
Current
Year
Sales
Prior
Period
Sales
Current
Year
Sales
Prior
Period
Sales
Beginning
of Period
Deductions
End of Period
Current
Year
Sales
Prior
Period
Sales
Current
Year
Sales
Prior
Period
Sales
We continually analyze the impact of generic competition on our inventory reserves considering the Oxandrin inventory currently on hand, inclusive of raw materials and finished goods, and the current demand forecasts. The aggregate net inventory valuation reserves were $1.3 million and $6.3 million as of
December 31, 2009 and 2008, respectively. Results of Operations During 2009, 2008 and 2007, we incurred substantial expenses relating to the clinical development of KRYSTEXXA. We expect to continue to incur significant losses in 2010, as we anticipate continued substantial expenses relating to the development and, if approved, the commercialization of KRYSTEXXA.
Our expenses relating to the development and commercialization of KRYSTEXXA will depend on many factors, including:
•
the timing of, and the costs involved in, obtaining regulatory approvals for KRYSTEXXA, • the cost of manufacturing activities, • the cost of commercialization activities, including product marketing, sales and distribution, and • our ability to establish and maintain additional collaborative arrangements relating to the commercialization of KRYSTEXXA in the United States and internationally. During 2009, the expenses associated with our regulatory, clinical, manufacturing and commercial development of KRYSTEXXA were the most significant factors affecting our results of operations. The following table summarizes our cost of expenses and indicates the significance of research and development
costs related to our development of KRYSTEXXA as well as percentage of total cost of expenses for the periods indicated:
Year Ended December 31,
2009
2008
2007
(In thousands) Cost of goods sold
$
1,606
1.9
%
$
1,154
1.2
%
$
1,205
1.5
% Research and development
51,726
61.5
%
55,488
60.2
%
50,870
61.1
% Selling, general and administrative
30,790
36.6
%
35,550
38.6
%
31,123
37.4
% Total costs and expenses
$
84,122
100.0
%
$
92,192
100.0
%
$
83,198
100.0
% Our revenues for 2009, 2008 and 2007 were derived primarily from Oxandrin and oxandrolone. Our product revenues and expenses have in the past displayed, and may in the future continue to display, significant variations. These variations may result from a variety of factors, including:
•
increased competition from new or existing products, including generic products, • the amount and timing of product sales, • changing demand for our products, • our inability to provide adequate supply for our products, • changes in wholesaler buying patterns, • returns of expired product, • changes in government or private payor reimbursement policies for our products, • the timing of the introduction of new products, • the timing and realization of milestone and other payments to licensees,
•
the timing and amount of expenses relating to research and development, product development and manufacturing activities, • the timing and amount of expenses relating to sales and marketing, • the timing and amount of expenses relating to general and administrative activities, • the extent and timing of costs of obtaining, enforcing and defending intellectual property rights, and • any charges related to acquisitions. 58
Our future revenues depend on our success in completing development and commercialization of KRYSTEXXA. The commercial success of KRYSTEXXA will depend on many factors including:
•
receiving, and the timing of, marketing approvals from the FDA and similar foreign regulatory authorities, • receiving FDA and other foreign regulatory approvals of the third-party manufacturing facilities used to produce the active pharmaceutical and other ingredients used in KRYSTEXXA, as well as for facilities used by our third-party fill and finish provider, • successful completion of the OLE and re-exposure studies and any other clinical trials we may undertake, • producing batches of pegloticase API drug substance and other ingredients used in KRYSTEXXA in commercial quantities through a validated process, • maintaining and, as appropriate, entering into additional, commercial manufacturing arrangements for KRYSTEXXA, • launching commercial sales of the product, whether alone or in collaboration with others, • acceptance of KRYSTEXXA by physicians, patients, healthcare payors and others in the medical community, and, • our ability to raise substantial additional capital. The following table summarizes net product sales of our commercialized products and their percentage of total net product sales for the periods indicated:
Year Ended December 31,
2009
2008
2007
(In thousands) Oxandrin
$
(366
)
-12.4
%
$
(357
)
-11.8
%
$
8,425
60.9
% Oxandrolone
3,322
112.4
3,385
111.8
5,400
39.1
$
2,956
100.0
%
$
3,028
100.0
%
$
13,825
100.0
% Our financial results have been dependent on sales of Oxandrin since its launch in December 1995. Generic competition for Oxandrin began in December 2006 and the introduction of generic products has caused a significant decrease in our Oxandrin revenues, which has adversely affected us financially and
has required us to scale back some of our business activities related to the product. As a result, we anticipate that Oxandrin will be a much less significant product for our future operating results. In September 2009, we completed a plan of termination pursuant to which we reduced our workforce by 25 employees. This termination was in addition to a previous termination of nine employees in August 2009. In connection with the plan of termination, we incurred charges of $2.3 million. We recorded $1.2
million in severance-related expenses during the quarter ended September 30, 2009 and an additional $1.1 million of stock-based compensation expense during the quarter ended December 31, 2009, as a result of modifications to stock option awards previously granted to the terminated employees. The
modifications included extending the term of exercisability of the vested portion of the stock options from three months following cessation of employment to six months following cessation of employment, coupled with accelerating the vesting to immediately vest any unvested stock options. The severance-related
expenses resulted in cash expenditures of $0.7 million during the quarter ended December 31, 2009 and will result in an additional $0.5 million in cash expenditures during the first half of 2010. Results of Operations for the Years Ended December 31, 2009 and December 31, 2008 Revenues Total revenues from continuing operations decreased $0.2 million, or 7%, to $3.0 million in 2009, from $3.2 million in 2008. This decrease resulted primarily from lower net product sales of Oxandrin and oxandrolone. Gross sales of Oxandrin decreased by $2.0 million, or 46%, to $2.3 million in 2009, from $4.3
million in 2008. The decrease is due to lower overall demand for the product as a result of 59
increase generic competition. Net sales of Oxandrin were flat as compared to the prior year due to higher prior year experience adjustments relating to reserves for product returns and government and customer rebates. Oxandrolone net sales were lower by $0.1 million, or 3%, to $3.3 million in 2009, from $3.4
million in 2008. We expect that sales of Oxandrin will remain flat or continue to decline in future periods due to the continued impact of generic competition. Cost of goods sold Cost of goods sold increased $0.4 million, or 39%, to $1.6 million in 2009, from $1.2 million in 2008. The increase in cost of goods sold was primarily due to an increase in gross sales of our authorized generic version of oxandrolone. Research and development expenses Research and development expenses decreased $3.8 million, or 7%, to $51.7 million in 2009, from $55.5 million in 2008. The decrease was primarily due to $2.0 million in lower manufacturing-related expenses including $4.3 million of decreased technology transfer expenses at Diosynth as the majority of the
work was completed in the prior year. Partially offsetting the lower technology transfer expenses were higher costs of $2.8 million for commercial batch production at BTG. The decrease in research and development expenses was also due to $1.9 million in lower clinical studies and research as a result of the wind down of the OLE study in 2009 coupled with a toxicology study performed in the prior year. Additionally, we incurred $1.1 million in lower compensation expenses due
to decreased headcount resulting from the plan of termination implemented in September 2009 and $1.0 million of lower consulting, medical writing and data management services associated with the preparation of our BLA filing for KRYSTEXXA. Partially offsetting these decreases was a $2.5 million increase in
severance costs related to the plan of termination in September 2009 and other terminated employees during 2009. If and when our BLA for KRYSTEXXA is approved by the FDA, we will no longer record manufacturing-related costs for KRYSTEXXA as research and development expenses and will instead capitalize those costs as inventory. Selling, general and administrative expenses Selling, general and administrative expenses decreased $4.8 million, or 13%, to $30.8 million in 2009, from $35.6 million in 2008. This decrease was primarily due to a $3.1 million decrease in legal expenses as a result of our patent infringement litigation with Upsher-Smith Laboratories, or Upsher-Smith, and
the settlement of a lawsuit with a former executive relating to his entitlement to severance payments and benefits upon termination of his employment, both occurring in 2008. Additionally, marketing expenses for the preparation of the commercial launch of KRYSTEXXA decreased by approximately $1.6 million
as these expenses wound down as a result of the previous expectation and preparation for a commercial launch in September 2009. Investment income Investment income decreased $0.8 million, or 75%, to $0.3 million in 2009, from $1.1 million in 2008. The decrease was primarily due to decreases in dividend and interest income driven by lower average cash, cash equivalent and investment balances and as a result of lower yields earned on these investments
due to a change in the investment strategy focused on capital retention. We expect these lower yields on investments to continue over the next 12 months. Other expense, net Other expense, net increased $11.7 million primarily as a result of the mark-to-market valuation adjustment to our warrant liability in the current year of $11.7 million, to record a loss due to the appreciation in fair market value of our warrants as a result of a higher underlying common stock price since the
date of issuance of the warrants. 60
Income tax benefit The income tax benefit decreased $2.9 million, to a benefit of $2.1 million in 2009, from a benefit of $5.0 million in 2008. The 2009 income tax benefit reflects the expected tax effect of carrying back an increased portion of our 2008 alternative minimum tax net operating loss to our 2003 and 2006 tax years to
recover the remaining alternative minimum income tax liability of $2.0 million that is eligible to be refunded for those tax years, as well as a $0.1 million benefit due to a reversal of a liability for unrecognized tax benefit caused by a lapse in statute. The opportunity for carry back became available due to Section
172 (b) (1) (H) and 810(b) of the Internal Revenue Code which was amended under Section 13 of the Worker, Homeownership, and Business Assistance Act of 2009. Results of Operations for the Years Ended December 31, 2008 and December 31, 2007 Revenues Total revenues from continuing operations decreased $10.8 million, or 77%, to $3.2 million in 2008, from $14.0 million in 2007. This decrease resulted primarily from lower product sales of Oxandrin and oxandrolone. We expect that sales of Oxandrin and oxandrolone will remain flat or continue to decrease in
future periods due to the continued impact of generic competition. We recorded Oxandrin negative net product sales of $0.4 million for the year ended December 31, 2008, compared with $8.4 million of net product sales for the year ended December 31, 2007. This decrease was primarily due to lower gross sales as a result of increased generic competition and an increase in our
allowance for product returns due to higher actual historical rates of return for the product. Revenues from oxandrolone decreased $2.0 million, or 37%, to $3.4 million in 2008, from $5.4 million in 2007 due to increased generic competition. Cost of goods sold Cost of goods sold remained unchanged at $1.2 million in both 2008 and 2007. Our cost of goods sold did not vary significantly from period to period. Research and development expenses Research and development expenses increased $4.6 million, or 9%, to $55.5 million in 2008, from $50.9 million in 2007. The increase was mainly due to a $5.5 million increase in manufacturing-related expenses for the completion of the first commercial batches of KRYSTEXXA and from the process validation
and technology transfer to our secondary source supplier of pegloticase API drug substance. The increase in expenses was also due to $3.1 million in higher consulting, medical writing and data management services associated with the preparation of our BLA filing for KRYSTEXXA, $2.0 million in higher
compensation expenses due to increased headcount and service-based restricted stock awards, $1.6 million from toxicology expenses and other clinical research studies for KRYSTEXXA and a $0.5 million license milestone payment resulting from the filing of our BLA in October 2008. Partially offsetting these
increases was an $8.0 million decrease in clinical trial expenses as our Phase 3 clinical trials for KRYSTEXXA were completed in late 2007. If and when our BLA for KRYSTEXXA is approved by the FDA, we will no longer record manufacturing-related costs for KRYSTEXXA as research and development expenses and will instead capitalize those costs as inventory. Selling, general and administrative expenses Selling, general and administrative expenses increased $4.5 million, or 14%, to $35.6 million in 2008, from $31.1 million in 2007. This increase was primarily due to a $3.9 million increase in legal expenses as a result of our patent infringement litigation with Upsher-Smith Laboratories, or Upsher-Smith, and the
settlement of a lawsuit with a former executive relating to his entitlement to severance payments and benefits upon termination of his employment. Additionally, marketing expenses for the 61
preparation of a possible commercial launch of KRYSTEXXA increased by approximately $2.0 million. Also contributing to the increase was approximately $1.9 million in accrued severance expenses related to the separation agreement with our former President and Chief Executive Officer. Partially offsetting the
increases was the reversal of $1.5 million in stock-based compensation expense that was previously charged against income related to a restricted stock award that contained a market condition for which the derived service period was not met, as a result of the resignation of our former President and Chief
Executive Officer. Also contributing to the offset was a decrease of approximately $0.7 million in audit fees and $1.0 million in decreased bonuses for senior executives. Investment income Investment income decreased $7.7 million, or 87%, to $1.1 million in 2008, from $8.8 million in 2007. The decrease was due to decreases in dividend and interest income driven by lower cash, cash equivalent and investment balances in addition to lower yields earned on these investments. The decrease is also
attributable to approximately $1.0 million of realized losses resulting from redemptions and impairment write-downs on our investment in the Columbia Strategic Cash Portfolio, or the Portfolio, during 2008. Income tax benefit The income tax benefit decreased $6.8 million, to a benefit of $5.0 million in 2008, from a benefit of $11.8 million in 2007. The 2008 income tax benefit reflects the expected tax effect of carrying back a portion of our 2008 net operating loss to our 2006 tax year to recover the remaining income tax liability that
is eligible to be refunded for that tax year and the reversal of a deferred tax benefit related to a liability for an unrecognized tax benefit. The decrease in the tax benefit from 2007 resulted from having significantly less 2006 taxes available to be refunded as a result of carrying back 2008 net operating losses than
were available to be refunded as a result of carrying back 2007 net operating losses. Liquidity and Capital Resources As of December 31, 2009, we had $108.2 million in cash, cash equivalents and short-term investments as compared to $78.6 million as of December 31, 2008. We primarily invest our cash equivalents and short-term investments in highly liquid, interest-bearing, U.S. Treasury money market funds in order to
preserve principal. Our strategic plan is to seek approval from the FDA of KRYSTEXXA and to develop a program of regulatory filings and review of KRYSTEXXA in other countries. We continue to believe that a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving
our company would serve to maximize the commercial prospects of KRYSTEXXA in the United States, Europe and other foreign countries. If we are not able to consummate a worldwide partnership or broader strategic transaction on terms acceptable to us, we intend to independently pursue the
commercialization of KRYSTEXXA in the United States. Based on our current strategic plan, we believe that our available cash and cash equivalents will be sufficient to fund anticipated levels of operations without KRYSTEXXA approval for at least the next 20 months. However, if we independently pursue the
commercialization of KRYSTEXXA in the United States, we believe that our available cash and cash equivalents will be sufficient to fund anticipated levels of operations for at least the next 14 months. In addition, in light of the FDA’s July 31, 2009 CRL to our KRYSTEXXA BLA, we are continuing to engage in
an active effort to reassess our expenditures and align our cash outlays more closely with the FDA approval process to conserve cash. On April, 8 2009, we raised $31.0 million from a registered direct offering, which yielded approximately $29.0 million in cash net of approximately $2.0 million of offering costs, which were charged to additional paid-in-capital. We issued 5,927,343 shares of our common stock to existing and new institutional
investors as part of the offering. The investors also received warrants to purchase up to 5,038,237 shares of our common stock at an initial exercise price of $10.46 per share. The warrants are exercisable at any time on or after the date of issuance and expire on November 2, 2010. We may, at our or the warrant holder’s election, issue net shares in lieu of a cash payment of 62
the exercise price by the warrant holder upon exercise. In the event that we enter into a merger or change of control transaction, the holders of the warrants will be entitled to receive consideration as if they had exercised the warrant immediately prior to such transaction, or they may require us to purchase the
warrant at the Black-Scholes value at the time of such transaction. In June 2009, we received a U.S. federal income tax refund of approximately $5.5 million which was previously reflected in Recoverable Income Taxes on our consolidated balance sheet as of December 31, 2008. The refund is the result of the carry back of our 2008 federal net operating losses against 2006
taxable income. In October 2009, we completed an underwritten public offering of 4,945,000 shares of our common stock for net proceeds of $61.4 million. We are using the net proceeds to complete our ongoing development effort to seek FDA approval of KRYSTEXXA, to develop a program of regulatory filings and review
of KRYSTEXXA in other countries, to engage a global secondary source supplier and a secondary fill and finish manufacturer for KRYSTEXXA and for working capital and other general corporate purposes As a result of the deficiencies cited by the FDA concerning our BLA filed for KRYSTEXXA and our decision to revert to the Phase 3 manufacturing process, launch quantity finished product batches previously manufactured will not be available for commercial distribution. Therefore, except for portions of
these batches being utilized for stability and other analytical testing, the remaining quantities of this finished product were destroyed. We believe we may incur approximately $5.1 million of cash outlays in the future to replace these launch quantities of finished product. The costs for these finished product batches
were expensed as incurred as research and development expenses within our consolidated statements of operations when the batches were manufactured. Tax Benefits As of December 31, 2009, we had a net operating loss for federal income taxes of $80.3 million. We currently don’t benefit from this net operating loss as we will carry forward these losses until we become profitable. In February 2010, we received an additional $2.0 million refund of federal income taxes paid in
prior years. The recovery in February 2010 of prior alternative minimum income taxes is the result of an amendment of Section 172(b)(1)(H) and 810(b) of the Internal Revenue Code which was amended under Section 13 of the Worker, Homeownership, and Business Assistance Act of 2009. Cash Flows Cash used in operating activities of $69.6 million for the year ended December 31, 2009 reflects our net loss for the period of $90.9 million partially offset by $5.5 million in cash received from our U.S. federal income tax refund as a result of the carry back of our 2008 federal net operating losses against 2006
taxable income and a non-cash charge of $11.7 million as a result of the mark-to-market valuation of our warrant liability. We received $4.1 million from investment activities resulting primarily from the full liquidation of our investment in the Portfolio, in 2009. The proceeds from the redemptions were re-invested
in cash and cash equivalents and used to fund operations. We also received $97.4 million in cash from financing activities, $29.0 million resulting from net proceeds from our registered direct offering in April 2009, $61.4 million resulting from net proceeds from our underwritten public offering of 4.9 million shares of
our common stock in October 2009, and $7.1 million in proceeds from the issuance of common stock, primarily due to the exercise of stock options. For the year ended December 31, 2008, we used net cash of $64.5 million in operating activities. This consisted of a net loss for the period of $84.2 million, offset in part by $5.0 million of federal income tax benefits realized and $7.4 million of non-cash stock compensation expense. We received $13.5 million
from investment activities resulting primarily from redemptions of our investment in the Portfolio. The proceeds from the redemptions were re-invested in cash and cash equivalents and used to fund operations. In November 2007, we used $20.0 million in cash to purchase an investment in the Portfolio, which is classified on our consolidated balance sheets as restricted Short-Term and Long-Term Investments. In December 2007, Columbia Management, or Columbia, a unit of Bank of America, or BOA, closed the
Portfolio to new investments and redemptions and began an orderly liquidation and dissolution of the 63
Portfolio’s assets for distribution to the unit holders, thereby restricting our potential to invest in and withdraw from the Portfolio. As of December 31, 2008, approximately $14.7 million, or 74%, of our original $20.0 million investment has been redeemed and re-invested in cash and cash equivalents and used to
fund operations. At December 31, 2008, the fair market value of restricted investment securities was $4.0 million, $2.3 million of which is reflected in our consolidated balance sheets as a component of Short-Term Investments and $1.7 million of which is reflected in our consolidated balance sheets as a component
of Long-Term Other Assets. During 2009, the remaining $4.0 million of this investment was redeemed and re-invested in cash and cash equivalents and used to fund operations. Funding Requirements Our strategic plan is to seek approval from the FDA of KRYSTEXXA and to develop a program of regulatory filings and review of KRYSTEXXA in other countries. We continue to believe that a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving
our company would serve to maximize the commercial prospects of KRYSTEXXA in the United States, Europe and other foreign countries. If we are not able to consummate a worldwide partnership or broader strategic transaction on terms acceptable to us, we intend to independently pursue the commercialization of KRYSTEXXA in the United States. Our future capital requirements will depend on many factors, including:
•
the timing of, and the costs involved in, obtaining regulatory approvals for KRYSTEXXA, • the cost of manufacturing activities, • the cost of commercialization activities, including product marketing, sales and distribution, and • our ability to establish and maintain additional collaborative arrangements relating to the commercialization of KRYSTEXXA in the United States and internationally. If the FDA does not approve or materially delays approval of our BLA for KRYSTEXXA, or if we are not successful in consummating a worldwide partnership for the commercialization of KRYSTEXXA or a broader strategic transaction involving our company, our cash needs will increase, and we may need
to seek additional funding. We may not be able to obtain additional funds or, if such funds are available, such funding may not be on terms that are acceptable to us, particularly in light of current macroeconomic conditions. If we raise additional funds by issuing equity securities, dilution to our then-existing
stockholders will result. If we issue preferred stock, it would likely include a liquidation preference and other terms that would adversely affect our stockholders. If we raise additional funds through the issuance of debt securities or borrowings, we may incur substantial interest expense and could become subject to
financial and other covenants that could restrict our ability to take specified actions, such as incurring additional debt or making capital expenditures. If additional funds are not available on favorable terms, or at all, our business, results of operations and financial condition may be materially adversely affected, and
we may be required to curtail or cease operations. Contractual Obligations Below is a table that presents our contractual obligations and commitments as of December 31, 2009: Payments Due by Period
Contractual Obligations
Total
Less
1-3 Years
3-5 Years
More
(In thousands) Capital lease obligations
$
181
$
80
$
101
$
—
$
— Operating lease obligations
6,148
1,916
4,232
— Purchase commitment obligations (1)
43,268
16,916
12,132
5,688
8,532 Other Commitments (2)
3,190
2,895
295
—
— Total
$
52,787
$
21,807
$
16,760
$
5,688
$
8,532 64
Than
One Year
Than
5 Years
(1)
Purchase commitment obligations represent our contractually obligated minimum purchase requirements based on our current manufacturing and supply and other agreements in place with third parties. The table does not include potential future purchase commitments for which the amounts and timing of
payments cannot be reasonably predicted. (2) Other commitments represent obligations related to potential developmental milestone payments and other commitments that we believe are probable to occur during the KRYSTEXXA research and development process up through and including regulatory submission. Developmental and sales-based
milestone payments that occur upon or subsequent to regulatory approval have not been included in the table due to the uncertainty of achieving regulatory approval. In the event that KRYSTEXXA receives regulatory approval, the resulting milestone payment obligation would be approximately $0.8 million.
If we successfully commercialize KRYSTEXXA and achieve certain sales levels, sales-based milestone payments could reach approximately $1.8 million. Other Commitments also includes accrued severance costs related to General Release and Separation agreements with our former President and Chief
Executive Officer, Chief Financial Officer, Chief Medical Officer, the head of Quality and Regulatory Affairs and other employees whose service was terminated during 2009. The table does not include potential future payment obligations, including uncertain tax liabilities, for which the amounts and timing of
payments cannot be reasonably predicted. We have received financial grants in support of research and development from the Office of the Chief Scientist of the State of Israel, or OCS, and the Israel-United States Bi-national Industrial Research and Development Foundation, or BIRD, of approximately $2.0 million and $0.6 million, respectively for
the development of KRYSTEXXA. These grants are subject to repayment through royalties on the commercial sales of KRYSTEXXA if and when they commence. The OCS grants were received by our former subsidiary, BTG, and upon our divestiture of BTG to Ferring, we agreed to remain obligated to
reimburse BTG for its repayments to OCS that relate to the KRYSTEXXA financial grants. In addition, under the Israeli Law of Encouragement of Research and Development in Industry, as amended, as a result of the funding received from OCS, if we do not manufacture 100% of our KRYSTEXXA annual
worldwide bulk product requirements in Israel, we may be subject to total payments ranging from 120% to 300% of the repayment obligation plus interest, based upon the percentage of manufacturing that does not occur in Israel. Our aggregate principle repayment obligation plus interest to OCS and BIRD if we
successfully launch KRYSTEXXA and, as per our agreements with BTG and Diosynth, manufacture more that 20% of our annual worldwide bulk product requirements outside of Israel, would range between $4.0 million and $8.8 million at December 31, 2009. These payments have been excluded from the table
above due to the uncertainties surrounding the regulatory approval and commercialization of KRYSTEXXA. We have a liability for unrecognized tax benefits of $10.0 million as of December 31, 2009. We don’t believe any of this liability will be paid within the next 12 months and are unable to estimate the amount or the timing of payments, if any, relating to this liability. Off-Balance Sheet Arrangements We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors. Accounting Pronouncements Adopted In
January 2010, the FASB issued ASU 2010-06, Improving Disclosures about
Fair Value Measurements. ASU 2010-06 amends ASU 820 to require a number
of additional disclosures regarding fair value measurements. The amended
guidance requires entities to disclose the amounts of significant transfers
between Level 1 and Level 2 of the fair value hierarchy and the reasons for
these transfers, the reasons for any transfers in or out of Level 3, and
information in the reconciliation of recurring Level 3 measurements about
purchases, sales, issuances and settlements on a gross basis. The ASU also
clarifies the requirement for entities to disclose information about both
the valuation techniques and inputs 65
used in estimating Level 2 and Level 3 fair value measurements. The amended guidance is effective for interim and annual financial periods beginning after December 15, 2009. ASU 2010-06 is not expected to have a significant effect on the Company’s consolidated financial statements. In August 2009, the FASB issued ASU 2009-05, Measuring Liabilities at Fair Value. FASB ASU 2009-05 provides amendments to FASB ASC 820, Fair Value Measurements and Disclosures, for the fair value measurement of liabilities. FASB ASU 2009-05 provides clarification that in circumstances in which a
quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using a valuation technique that uses the quoted price of the identical liability when traded as an asset, the quoted prices for similar liabilities or similar liabilities when traded as assets or
another valuation technique that is consistent with the principles of FASB ASC 820. The amendments in the update also clarify that when estimating the fair value of a liability, a reporting entity is not required to include a separate input or adjustment to other inputs relating to the existence of a restriction that
prevents the transfer of the liability. The amendments in the update also clarify that both a quoted price in an active market for the identical liability at the measurement date and the quoted price for the identical liability when traded as an asset in an active market when no adjustments to the quoted price of the
asset are required are Level 1 fair value measurements. The guidance provided in FASB ASU 2009-05 is effective for the first reporting period (including interim periods) beginning after issuance. The adoption of FASB ASU 2009-05 did not have a material effect on our consolidated financial statements. In June 2009, the FASB issued a new accounting statement on Accounting Standards Codification. The new statement establishes FASB Accounting Standards Codification, or Codification, as the single source of authoritative non-governmental GAAP and is codified under FASB ASC 105, Generally Accepted
Accounting Principles. The Codification does not change current GAAP, but is intended to simplify user access to all authoritative GAAP by providing all the authoritative literature related to a particular topic in one place. All existing accounting standard documents will be superseded and all other accounting
literature not included in the Codification will be considered non-authoritative. The Codification is effective for interim and annual periods ending after September 15, 2009. The Codification is for disclosure only and did not impact our financial condition or results of operations. In May 2009, the FASB issued a new accounting statement on Subsequent Events as codified under FASB ASC 855, Subsequent Events. The new statement establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued.
The new statement requires the disclosure of the date through which an entity has evaluated subsequent events and the basis for that date and should alert all users of financial statements that an entity has not evaluated subsequent events after that date in the set of financial statements being presented. The new
statement shall be applied to the accounting for and disclosure of subsequent events not addressed in other applicable GAAP. Other applicable GAAP may address the accounting treatment of events or transactions that occur after the balance sheet date but before the financial statements are issued. If an event or
transaction is within the scope of other applicable GAAP, then an entity shall follow the guidance in that applicable GAAP, rather than the guidance in the new statement. The new statement is effective for interim or annual financial periods ending after June 15, 2009, and is applied prospectively. The adoption of
the new statement did not have a material effect on our recognition or disclosures in our consolidated financial statements. We evaluate all subsequent events as of the issuance date of our financial statements. In April 2009, the FASB issued new accounting guidance on determining fair value when the volume and level of activity for the asset or liability have significantly decreased and identifying transactions that are not orderly as codified under FASB ASC 820, Fair Value Measurements and Disclosures. The
guidance provides additional options for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased. The guidance emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the
valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date
under current market conditions. The guidance addresses the recommendations of specific issues in the Securities and Exchange Commission’s, or SEC’s, study on mark-to-market 66
accounting and provides additional guidance on determining fair value when the volume and level of activity for the asset or liability has significantly decreased when compared with normal market activity for the asset or liability (or similar assets or liabilities). The guidance is effective for interim and annual
reporting periods ending after June 15, 2009 and shall be applied prospectively. The adoption of the guidance did not have a material effect on our consolidated financial statements. In April 2009, the FASB issued new accounting guidance on interim disclosures about the fair value of financial instruments as codified under FASB ASC 825, Financial Instruments. The guidance requires disclosures about fair value of financial instruments for interim reporting periods of publicly traded
companies as well as in annual financial statements. The guidance is effective for interim and annual reporting periods ending after June 15, 2009 with early adoption permitted for periods ending after March 15, 2009. The adoption of this guidance did not have a material effect on our consolidated financial
statements. In April 2009, the FASB issued new accounting guidance on the Recognition and Presentation of Other-Than-Temporary Impairments as codified under FASB ASC 320, Investments-Debt and Equity Securities. The guidance amends the OTT impairment guidance in GAAP for debt securities to make the
guidance more operational and improves the presentation and disclosure of OTT impairments on debt and equity securities in the financial statements. The guidance does not amend existing recognition and measurement guidance related to OTT impairments of equity securities. The guidance expands and increases
the frequency of existing disclosures about OTT impairments for debt and equity securities and requires a more detailed, risk oriented breakdown by major security types and related information. In addition, the guidance requires that the annual disclosures in existing GAAP related to OTT impairments of equity
securities be made for interim periods. The guidance is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. Early adoption for periods ending before March 15, 2009, is not permitted. The adoption of the guidance did
not have a material effect on our consolidated financial statements. In February 2007, the FASB issued new accounting guidance on the effective date of fair value measurements for non-financial assets and non-financial liabilities, as codified under FASB ASC 820, Fair Value Measurements and Disclosures. The guidance delayed the effective date to fiscal years beginning after
November 15, 2008 and interim periods within those fiscal years for non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis and at least annually. Since we do not carry any non-financial instruments that are
measured at fair value on a non-recurring basis, the conclusion of the deferral period did not have an effect on our financial statements. In July 2006,
the FASB issued new accounting guidance on accounting for uncertainties in
income taxes as codified under FASB ASC 640, Income Taxes. The guidance
clarifies the accounting for uncertainty in income taxes recognized in an
enterprise’s financial statements and prescribes a recognition threshold
and measurement attribute for the financial statement recognition and
measurement of a tax position taken or expected to be taken in a tax return. The
guidance also provides clarity on de-recognition of tax benefits, classification
on the consolidated balance sheet, interest and penalties, accounting in interim
periods, disclosure, and transition. We adopted this guidance effective January
1, 2007 and as a result, we recorded a $4.5 million increase in the liability
for unrecognized tax benefits, including $0.2 million of accrued interest and
penalties, which is included in Other Liabilities on our consolidated balance
sheet. This increase in liability resulted in a corresponding increase to
Accumulated Deficit. The total amount of federal, state, local and foreign
liabilities for unrecognized tax benefits was $10.0 million as of December 31,
2009, including accrued penalties and interest. The net increase of $5.4 million
in the liability for unrecognized tax benefits subsequent to adoption resulted
in a corresponding decrease to the income tax benefit within our consolidated
statements of operations as well as an increase to the deferred tax asset for
which no tax benefit will be recognized in our consolidated statements of
operations. 67
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK Market risk is the exposure to loss resulting from changes in interest rates, foreign currency exchange rates, commodity prices and equity prices. To date our exposure to market risk has been limited. We do not currently hedge any market risk, although we may do so in the future. We do not hold or issue any derivative financial instruments for trading or other speculative purposes. Interest Rate Risk Our interest bearing assets consist of cash and cash equivalents which primarily consist of money market funds. Our interest income is sensitive to changes in the general level of interest rates primarily in the United States, and other market conditions. 68
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA Index to Consolidated Financial Statements
Page
70
71 Consolidated Financial Statements: Consolidated Balance Sheets as of December 31, 2009 and 2008
73 Consolidated Statements of Operations for the years ended December 31, 2009, 2008
74
75 Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2008
78
80
116 69
and 2007
and 2007
Management’s Report on Internal Control Over Financial Reporting Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or
under the supervision of, the Company’s principal executive and principal financial officers and is affected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles (GAAP) and includes those policies and procedures that:
•
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company, • Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company, and • Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered
relative to their costs. Because of the inherent limitations in a cost-effective control system, no evaluation of internal control over financial reporting can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within our Company
have been detected. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Management does not expect that the Company’s disclosure controls and procedures or its internal control over financial reporting
will prevent or detect all errors and all fraud. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the
controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future
periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2009 based on the criteria described in the Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment under the framework in the Internal Control—Integrated Framework, management, including the Company’s President and Chief Financial Officer, concluded that the Company’s internal control over financial reporting was effective as of December 31, 2009. The effectiveness of the Company’s internal control over financial reporting as of December 31, 2009 has been audited by McGladrey & Pullen, LLP, an independent registered public accounting firm, as stated within their report herein. 70
Report of Independent Registered Public Accounting Firm To the Board of Directors and Stockholders We have audited the accompanying consolidated balance sheets of Savient Pharmaceuticals, Inc. and subsidiaries as of December 31, 2009 and 2008, and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for each of the three years in the period ended December
31, 2009. Our audits also included the financial statement schedule of Savient Pharmaceuticals, Inc. listed in Item 15(a). These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on
our audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion. In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Savient Pharmaceuticals, Inc. and subsidiaries as of December 31, 2009 and 2008, and the results of their operations and their cash flows for each of the three years in the period
ended December 31, 2009, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly in all material respects the information set forth
therein. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Savient Pharmaceuticals, Inc. and subsidiaries’ internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework
issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2010 expressed an unqualified opinion on the effectiveness of Savient Pharmaceuticals, Inc. and subsidiaries’ internal control over financial reporting. /s/ McGladrey & Pullen, LLP New York, NY 71
Savient Pharmaceuticals, Inc.
March 1, 2010
Report of Independent Registered Public Accounting Firm To the Board of Directors and Stockholders We have audited Savient Pharmaceuticals, Inc. and subsidiaries internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Savient Pharmaceuticals,
Inc. and subsidiaries’ management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our
responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all
material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. We believe that our audit provides a
reasonable basis for our opinion. A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control
over financial reporting includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (b) provide reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (c) provide reasonable assurance regarding prevention or
timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of
compliance with the policies or procedures may deteriorate. In our opinion, Savient Pharmaceuticals, Inc. and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission. We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Savient Pharmaceuticals, Inc. and subsidiaries as of December 31, 2009 and 2008 and the related consolidated statements of operations, changes in
stockholders’ equity and cash flows for each of the years in the three year period ended December 31, 2009 and our report dated March 1, 2010 expressed an unqualified opinion. /s/ McGladrey & Pullen, LLP New York, NY 72
Savient Pharmaceuticals, Inc.
March 1, 2010
CONSOLIDATED BALANCE SHEETS
December 31,
December 31, ASSETS Current Assets: Cash and cash equivalents
$
108,172
$
76,315 Short-term investments (including restricted investments)
3
2,282 Accounts receivable, net
352
822 Inventories, net
585
1,892 Recoverable income taxes
2,006
5,526 Prepaid expenses and other current assets
1,402
2,782 Total current assets
112,520
89,619 Deferred income taxes, net
4,200
4,200 Property and equipment, net
993
1,393 Other assets (including restricted cash and investments)
1,295
3,010 Total assets
$
119,008
$
98,222 LIABILITIES AND STOCKHOLDERS’ EQUITY Current Liabilities: Accounts payable
$
7,915
$
5,888 Deferred revenues
73
451 Warrant liability
24,239
— Other current liabilities
12,473
18,650 Total current liabilities
44,700
24,989 Other liabilities
10,109
9,809 Commitments and contingencies Stockholders’ Equity: Preferred stock—$.01 par value 4,000,000 shares authorized; no shares issued
—
— Common stock—$.01 par value 150,000,000 shares authorized; issued and outstanding 66,933,000 in 2009; 54,654,000 in 2008
669
547 Additional paid-in-capital
305,994
214,467 Accumulated deficit
(242,467
)
(151,614
) Accumulated other comprehensive income
3
24 Total stockholders’ equity
64,199
63,424 Total liabilities and stockholders’ equity
$
119,008
$
98,222 The accompanying notes are an integral part of these consolidated financial statements. 73
(In thousands, except share data)
2009
2008
CONSOLIDATED STATEMENTS OF OPERATIONS
Year Ended December 31,
2009
2008
2007 Revenues: Product sales, net
$
2,956
$
3,028
$
13,825 Other revenues
4
153
199
2,960
3,181
14,024 Cost and expenses: Cost of goods sold
1,606
1,154
1,205 Research and development
51,726
55,488
50,870 Selling, general and administrative
30,790
35,550
31,123
84,122
92,192
83,198 Operating loss from continuing operations
(81,162
)
(89,011
)
(69,174
) Investment income, net
289
1,146
8,755 Other expense, net
(12,051
)
(393
)
(543
) Loss from continuing operations before income taxes
(92,924
)
(88,258
)
(60,962
) Income tax benefit
(2,071
)
(5,017
)
(11,807
) Loss from continuing operations
(90,853
)
(83,241
)
(49,155
) Income (loss) from discontinued operations, net of income taxes
—
(928
)
487 Net loss
$
(90,853
)
$
(84,169
)
$
(48,668
) Loss per common share from continuing operations: Basic and diluted
$
(1.51
)
$
(1.55
)
$
(0.94
) Earnings (loss) per common share from discontinued operations: Basic and diluted
$
—
$
(0.02
)
$
0.01 Loss per common share: Basic and diluted
$
(1.51
)
$
(1.57
)
$
(0.93
) Weighted-average number of common and common equivalent shares: Basic and diluted
59,997
53,533
52,461 The accompanying notes are an integral part of these consolidated financial statements. 74
(In thousands, except per share data)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Common Stock
Accumulated
Accumulated
Total
Shares
Par Value
Additional Balance, December 31, 2006
52,309
$
523
$
189,496
$
(14,316
)
$
1,983
$
177,686 Comprehensive loss: Net loss
—
—
—
(48,668
)
—
(48,668
) Unrealized loss on marketable securities, net
—
—
—
—
(669
)
(669
) Total comprehensive loss
—
—
—
—
—
(49,337
) Cumulative impact of change in accounting related to the adoption of FIN 48
—
—
—
(4,461
)
—
(4,461
) Restricted stock grants
633
6
(5
)
—
—
1 Amortization of deferred compensation
—
—
5,155
—
—
5,155 Forfeiture of restricted stock grants
(14
)
—
(1
)
—
—
(1
) Issuance of common stock
177
2
656
—
—
658 ESPP compensation expense
—
—
249
—
—
249 Stock option compensation expense
—
—
3,338
—
—
3,338 Tax benefit of share-based compensation
—
—
2,240
—
—
2,240 Exercise of stock options
607
6
3,531
—
—
3,537 Balance, December 31, 2007
53,712
$
537
$
204,659
$
(67,445
)
$
1,314
$
139,065 The accompanying notes are an integral part of these consolidated financial statements. 75
(In thousands)
Deficit
Other
Comprehensive
Income
Stockholders’
Equity
Paid-in-
Capital
SAVIENT PHARMACEUTICALS, INC. CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Common Stock
Accumulated
Accumulated
Total
Shares
Par Value
Additional Balance, December 31, 2007
53,712
$
537
$
204,659
$
(67,445
)
$
1,314
$
139,065 Comprehensive loss: Net loss
—
—
—
(84,169
)
—
(84,169
) Unrealized loss on marketable securities, net
—
—
—
—
(1,290
)
(1,290
) Total comprehensive loss
—
—
—
—
—
(85,459
) Restricted stock grants
517
5
(5
)
—
—
— Amortization of deferred compensation
—
—
3,214
—
—
3,214 Forfeiture of restricted stock grants
(4
)
—
—
—
—
— Issuance of common stock
90
1
799
—
—
800 ESPP compensation expense
—
—
658
—
—
658 Stock option compensation expense
—
—
3,517
—
—
3,517 Shares repurchased and cancelled
(39
)
—
(208
)
—
—
(208
) Exercise of stock options
378
4
1,833
—
—
1,837 Balance, December 31, 2008
54,654
$
547
$
214,467
$
(151,614
)
$
24
$
63,424 The accompanying notes are an integral part of these consolidated financial statements. 76
(In thousands)
Deficit
Other
Comprehensive
Income
Stockholders’
Equity
Paid-in-
Capital
SAVIENT PHARMACEUTICALS, INC. CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Common Stock
Accumulated
Accumulated
Total
Shares
Par Value
Additional Balance, December 31, 2008
54,654
$
547
$
214,467
$
(151,614
)
$
24
$
63,424 Comprehensive loss: Net loss
—
—
—
(90,853
)
—
(90,853
) Unrealized loss on marketable securities, net
—
—
—
—
(21
)
(21
) Total comprehensive loss
—
—
—
—
—
(90,874
) Restricted stock grants
723
7
(7
)
—
—
— Amortization of deferred compensation
—
—
2,661
—
—
2,661 Forfeiture of restricted stock grants
(507
)
(5
)
5
—
—
— Issuance of common stock
81
1
352
—
—
353 ESPP compensation expense
—
—
82
—
—
82 Stock option compensation expense
—
—
3,983
—
—
3,983 Issuance of common stock for cash with underwritten public offering, net of expenses
4,945
50
61,316
—
—
61,366 Issuance of common stock for cash on registered direct offering, net of expenses
5,927
59
28,960
29,019 Issuance of warrants in connection with registered direct offering, net of expenses
—
—
(12,561
)
—
—
(12,561
) Exercise of stock options
1,110
10
6,736
—
—
6,746 Balance, December 31, 2009
66,933
$
669
$
305,994
$
(242,467
)
$
3
$
64,199 The accompanying notes are an integral part of these consolidated financial statements. 77
(In thousands)
Deficit
Other
Comprehensive
Income
Stockholders’
Equity
Paid-in-
Capital
CONSOLIDATED STATEMENTS OF CASH FLOWS
Year Ended December 31,
2009
2008
2007 Cash flows from operating activities: Net loss
$
(90,853
)
$
(84,169
)
$
(48,668
) Adjustments to reconcile net loss to net cash used in operating activities: Depreciation
471
474
458 Change in valuation of warrant liability
11,678
—
— Unrecognized tax benefit liability
(1,199
)
2,509
4,240 Deferred income taxes
—
(642
)
(3,558
) Net realized (gains) losses from sale of investments
(182
)
889
(6
) Amortization of deferred compensation related to restricted stock (including performance shares)
2,661
3,214
5,155 Stock compensation expense
4,065
4,175
3,587 Common stock issued as payment for services
—
—
51 Changes in: Accounts receivable, net
470
668
2,027 Inventories, net
1,307
744
1,567 Recoverable income taxes
3,520
3,111
(8,637
) Prepaid expenses and other current assets
1,380
323
3,993 Accounts payable
2,027
1,836
52 Income taxes payable
—
294
(846
) Other current liabilities
(4,612
)
2,957
(1,096
) Deferred revenues
(378
)
(847
)
882 Net cash used in operating activities
(69,645
)
(64,464
)
(40,799
) Cash flows from investing activities: Proceeds from sale of available-for-sale securities (restricted)
4,170
13,365
1,785 Capital expenditures
(71
)
(268
)
(918
) Purchases of available-for-sale securities (restricted)
(25
)
(323
)
(20,140
) Changes in other long-term assets
10
(25
)
— Proceeds from sales of available-for-sale securities
—
151
436 Proceeds from sale of Delatestryl
—
644
644 Net cash provided by (used in) investing activities
4,084
13,544
(18,193
) The accompanying notes are an integral part of these consolidated financial statements. 78
(In thousands)
SAVIENT PHARMACEUTICALS, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS
Year Ended December 31,
2009
2008
2007 Cash flows from financing activities: Proceeds from issuance of common stock for cash on registered direct offering, net of expenses
$
29,019
$
—
$
— Proceeds from issuance of common stock for cash on underwritten public offering, net of expenses
61,366
—
— Proceeds from issuance of common stock
7,099
2,637
4,144 Changes in other long-term liabilities
(66
)
(59
)
180 Repurchase and retirement of common stock
—
(208
)
— Additional paid-in-capital excess tax benefit
—
—
2,240 Net cash provided by financing activities
97,418
2,370
6,564 Net increase (decrease) in cash and cash equivalents
31,857
(48,550
)
(52,428
) Cash and cash equivalents at beginning of period
76,315
124,865
177,293 Cash and cash equivalents at end of period
$
108,172
$
76,315
$
124,865 Supplementary Information Other information: Income tax paid
$
1,237
$
44
$
139 Interest paid
$
365
$
29
$
21 The accompanying notes are an integral part of these consolidated financial statements. 79
(In thousands)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS Note 1—Organization and Summary of Significant Accounting Policies Savient Pharmaceuticals, Inc., and its wholly-owned subsidiaries (“Savient” or “the Company”), is a specialty biopharmaceutical company focused on developing KRYSTEXXA™ (pegloticase), or KRYSTEXXA, for the treatment of chronic gout in patients refractory to conventional therapy. The Company’s strategic plan is to seek approval from the U.S. Food and Drug Administration (“FDA”) of its Biologic License Application (“BLA”) filing for KRYSTEXXA and to develop a program of regulatory filings and review of KRYSTEXXA in other countries. The Company continues to believe
that a worldwide partnership for the commercialization of KRYSTEXXA or broader strategic transaction involving the company would serve to maximize the commercial prospects of KRYSTEXXA in the United States, Europe and other foreign countries. If the Company is not able to consummate a worldwide
partnership or broader strategic transaction on terms acceptable to it, the Company intends to independently pursue the commercialization of KRYSTEXXA in the United States. Currently, the Company sells and distributes branded and generic versions of oxandrolone, a drug used to promote weight gain following involuntary weight loss. The Company launched its authorized generic version of oxandrolone in December 2006 in response to the approval and launch of generic
competition to Oxandrin®. The introduction of oxandrolone generics has led to significant decreases in demand for Oxandrin and the Company’s authorized generic version of oxandrolone. The Company believes that revenues from Oxandrin and its authorized generic version of oxandrolone will remain flat or
continue to decrease in future periods. Savient was founded in 1980 to develop, manufacture and market products through the application of genetic engineering and related biotechnologies. On September 30, 2002, Savient, through its wholly-owned subsidiary Acacia Biopharma Limited, acquired Rosemont Pharmaceuticals Limited (“Rosemont” or
“oral liquid pharmaceutical business”), a specialty pharmaceutical company located in the United Kingdom that develops, manufactures and markets pharmaceutical products in oral liquid form. On June 6, 2006, Savient contributed 100% of the stock in Acacia Biopharma Limited into Savient Pharma Holdings,
Inc. (“Holdings”), a wholly-owned subsidiary of Savient. Additionally, Myelos Corporation (“Myelos”) contributed certain other intellectual property assets into Holdings. On July 18, 2005, the Company sold its global biologics manufacturing business, Bio-Technology General (Israel) Ltd. (“BTG”) to Ferring B.V.
and Ferring International Centre S.A. (“Ferring”). On August 4, 2006, the Company sold Rosemont to Ingleby (1705) Limited, a Close Brothers Private Equity Company (“Close Brothers”) (see Note 15 to the consolidated financial statements). The Company currently operates within one “Specialty Pharmaceutical” segment which includes sales of Oxandrin and oxandrolone, and the research and development activities of KRYSTEXXA. The results of the Company’s former Rosemont and BTG subsidiaries are included as part of discontinued
operations in the Company’s consolidated financial statements. The Company conducts its administration, finance, business development, clinical development, sales, marketing, quality assurance and regulatory affairs activities primarily from its headquarters in East Brunswick, New Jersey. Basis of consolidation The consolidated financial statements include the accounts of Savient and its subsidiaries. In addition, discontinued operations include the Company’s former subsidiaries, Rosemont and BTG. Certain prior period amounts have been reclassified to conform to current year presentations. There was no effect on net loss or equity related to these reclassifications. 80
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Cash and cash equivalents At December 31, 2009 and 2008, cash and cash equivalents included cash on hand and money market funds with weighted-average maturities at the date of purchase of 90 days or less. All cash and cash equivalents are in U.S. dollar accounts. Restricted cash The Company’s restricted cash represents a required security deposit in connection with the lease arrangement for the Company’s administrative offices located in East Brunswick, New Jersey. At the inception of the lease, the Company was required to provide a security deposit by way of an irrevocable letter
of credit for $1.3 million, which is secured by a cash deposit of $1.3 million and reflected in non-current Other Assets (as restricted cash) in the Company’s consolidated balance sheets at December 31, 2009 and 2008. Fair value of financial instruments The Company adopted a new accounting standard that defines fair value and establishes a framework for fair value measurements effective January 1, 2008 for financial assets and liabilities and effective January 1, 2009 for non-financial assets and liabilities that are not re-measured on a recurring basis. Under
this standard, fair value is generally defined as the price that would be received to sell an asset or paid to transfer a liability (the “exit price”) in an orderly transaction between market participants at the measurement date. The adoption of this accounting standard did not have a material impact on the Company’s
consolidated results of operations, financial position or cash flows. During the three months ended June 30, 2009, the Company adopted two new accounting standards that require disclosures at each interim balance sheet date of the fair value of financial instruments and valuation techniques used to determine fair value. Previously, these disclosures were only required
annually. One of these accounting standards also provides additional guidance in estimating fair value when the market volume and level of activity for an asset or liability have significantly decreased and identifying circumstances that indicate a transaction may not be orderly. The adoption of these two accounting
standards did not have a material impact on the Company’s consolidated results of operations, financial position or cash flows. The carrying amounts of Cash and Cash Equivalents, Notes Receivable, Accounts Receivable, Accounts Payable and Other Current Liabilities approximate fair value. See Note 2 to the Company’s consolidated financial statements for further discussion of the fair value of financial instruments. Investments The Company classifies investments as “available-for-sale securities” or “trading securities.” Investments that are purchased and held principally for the purpose of selling them in the near-term are classified as trading securities and marked to fair value through earnings. Investments not classified as trading
securities are considered to be available-for-sale securities. Changes in the fair value of available-for-sale securities are reported as a component of accumulated other comprehensive income in the consolidated statements of stockholders’ equity and are not reflected in the consolidated statements of operations until
a sale transaction occurs or when declines in fair value are deemed to be other-than-temporary (“OTT”). Restricted investments The Company classifies its restricted investments as “available-for-sale securities.” Changes in the fair value are reported as a component of accumulated other comprehensive income in the consolidated statements of stockholders’ equity and are not reflected in the consolidated statements of operations until a
sale transaction occurs or when declines in fair value are deemed to be OTT. 81
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) The Company’s restricted investments have consisted of its investment in the Columbia Strategic Cash Portfolio (“the Portfolio”). During the fourth quarter of 2007, Columbia Management (“Columbia”), a unit of Bank of America (“BOA”), closed the Portfolio to new investments and redemptions and began
an orderly liquidation and dissolution of the Portfolio’s assets for distribution to the unit holders, therefore restricting the Company’s potential to invest in and withdraw from the Portfolio. At December 31, 2009, the Portfolio has been fully redeemed to the Company. At December 31, 2008, the fair market value of
restricted investment securities was $4.0 million, $2.3 million of which is reflected in the Company’s consolidated balance sheets as a component of Short-Term Investments and $1.7 million of which is reflected in the Company’s consolidated balance sheets as a component of Other Assets. See Note 2 to the
Company’s consolidated financial statements for further discussion of the Company’s restricted investments. Other-Than-Temporary Impairment Losses on Investments. The Company regularly monitors its available-for-sale portfolio to evaluate the necessity of recording impairment losses for OTT declines in the fair value of investments. During the three months ended June 30, 2009, the Company adopted a new accounting standard that modifies the guidance used in
determining whether the impairment of a debt security is OTT. The impairment of a debt security is considered OTT if an entity concludes that it intends to sell the impaired security, that it is more likely than not that it will be required to sell the security before the recovery of its cost basis or that it does not
otherwise expect to recover the entire cost basis of the security. This accounting standard also amends the presentation requirements of OTT impaired debt securities and expands disclosure requirements in the financial statements for investments in both debt and equity securities. The adoption of this accounting
standard did not have a material impact on the Company’s consolidated results of operations, financial position or cash flows. Management makes this determination through the consideration of various factors such as management’s intent and ability to retain an investment for a period of time sufficient to allow for any anticipated recovery in market value. OTT impairment losses result in a permanent reduction of the cost basis of an
investment. For the years ended December 31, 2009, 2008 and 2007, the Company recorded realized investment losses due to OTT declines in fair value of $0, $0.5 million and $0.3 million, respectively. Accounts receivable, net The Company extends credit to customers based on its evaluation of the customer’s financial condition. The Company generally does not require collateral from its customers when credit is extended. Accounts receivable are usually due within 30 days and are stated at amounts due from customers net of an
allowance for doubtful accounts. Accounts outstanding longer than the contractual payment terms are considered past due. The Company assesses the need for an allowance by considering a number of factors, including the length of time trade accounts receivable are past due, previous loss history, the customer’s
current ability to pay its obligation and the condition of the general economy and the industry as a whole. The Company will write off accounts receivable when they become uncollectible, and payments subsequently received on such receivables are credited to recovery of accounts written off. In general, the
Company has experienced minimal collection issues with its large customers. At December 31, 2009 and 2008, the balance of the Company’s allowance for doubtful accounts was $18,000 and $2,000, respectively. Inventories, net Inventories are stated at the lower of cost or market. Cost is determined based on actual cost. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, reserves are recorded for the difference between cost and market value. These reserves are determined
based on estimates. 82
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Property and equipment, net of accumulated depreciation and amortization Property and equipment are stated at cost. Depreciation has been calculated using the straight-line method over the estimated useful lives of the assets, ranging from three to 10 years. Leasehold improvements are amortized over the lives of the respective leases, which are shorter than the useful life. Revenue recognition Product sales are generally recognized when title to the product has transferred to the Company’s customers in accordance with the terms of the sale. The Company ships its authorized generic version of oxandrolone to its distributor and accounts for these shipments on a consignment basis until product is sold
into the retail market. The Company defers the recognition of revenue related to these shipments until it confirms that the product has been sold into the retail market and all other revenue recognition criteria has been met. Revenue is not recognized until it is realized or realizable and earned. Revenue from sales transactions where the buyer has the right to return the product is recognized at the time of sale only if (i) the seller’s price to the buyer is substantially fixed or determinable at the date of sale, (ii) the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is
not contingent on resale of the product, (iii) the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, (iv) the buyer acquiring the product for resale has economic substance apart from that provided by the seller, (v) the seller does not have
significant obligations for future performance to directly bring about resale of the product by the buyer, and (vi) the amount of future returns can be reasonably estimated. The Company’s net product revenues represent total product revenues less allowances for returns, Medicaid rebates, other government rebates, other rebates, discounts, and distribution fees. Allowance for returns In general, the Company provides credit for product returns that are returned six months prior to and up to 12 months after the product expiration date. The Company’s product sales in the United States primarily relate to Oxandrin and its authorized generic version of oxandrolone. Upon sale, the Company
estimates an allowance for future product returns. The Company provides additional reserves for contemporaneous events that were not known or knowable at the time of shipment. In order to reasonably estimate future returns, the Company analyzed both quantitative and qualitative information including, but
not limited to, actual return rates by lot productions, the level of product manufactured by the Company, the level of product in the distribution channel, expected shelf life of the product, current and projected product demand, the introduction of new or generic products that may erode current demand, and
general economic and industry-wide indicators. The allowance for product returns at December 31, 2009 and 2008 was $1.0 million and $1.2 million, respectively. This allowance is included in Other Current Liabilities on the Company’s consolidated balance sheets. Allowances for Medicaid, other government rebates and other rebates The Company’s contracts with Medicaid, other government agencies such as the Federal Supply System and other non-governmental entities commit it to providing those entities with the Company’s most favorable pricing. This ensures that the Company’s products remain eligible for purchase or
reimbursement under these programs. Based upon the Company’s contracts and the most recent experience with respect to sales through each of these channels, the Company provides an allowance for rebates. The Company monitors the sales trends and adjusts the rebate percentages on a regular basis to reflect
the most recent rebate experience. The allowance for rebates at December 31, 2009 and 2008 was $0.3 million and $0.6 million, respectively. This allowance is included in Other Current Liabilities within the Company’s consolidated balance sheets. 83
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Commercial discounts The Company sells directly to drug wholesalers. Terms of these sales vary, but generally provide for invoice discounts for prompt payment. These discounts are recorded by the Company at the time of sale. Gross product revenue is also reduced for promotion and pricing incentives. Distribution fees The Company has a distribution arrangement with a third-party logistics provider which includes payment terms equal to a flat monthly fee plus a per transaction fee for specified services. The Company also records distribution fees as incurred associated with wholesaler distribution services from two of its
largest customers. Other revenues Other revenues primarily represent royalty income which is recognized as earned upon receipt of confirmation of the income amount or payment from contracting third parties. Share-based compensation The Company has share-based compensation plans in place and records the associated share-based compensation expense over the requisite service period. The share-based compensation plans are described in Note 10 to the consolidated financial statements. Compensation cost for stock options that contain service conditions is charged against income on a straight-line basis between the grant date for the option and the vesting period. The Company estimates the fair value of all stock option awards that contain service conditions as of the grant date by applying the
Black-Scholes pricing valuation model. The application of this valuation model involves assumptions that are highly subjective, judgmental and sensitive in the determination of compensation cost. Total unrecognized compensation cost will be adjusted for future changes in estimated forfeitures. In addition, as future
grants are made, the Company expects to incur additional compensation costs. Restricted stock awards that contain service conditions are recorded as deferred compensation and amortized into compensation expense, on a straight-line basis over the vesting period, which ranges from one to four years in duration. Compensation cost for restricted stock awards that contain service
conditions is based on the grant date fair value of the award, which is the closing market price of the Company’s common stock on the grant date multiplied by the number of shares awarded. Compensation cost for restricted stock and stock option awards that contain performance or market conditions is based on the grant date fair value of the award. Compensation expense is recorded over the implicit or explicit requisite service period based on management’s best estimate as to whether it is
probable that the shares awarded are expected to vest. For purposes of recording compensation expense, the Company considers performance conditions that depend on a change in control event or an FDA approval, once the transaction is consummated or the event occurs. Previously recognized compensation
expense is fully reversed if performance targets are not satisfied. The Company assesses the probability of the performance indicators being met on a continuous basis and records compensation expense from that date, over the remainder of the requisite service period. Research and development Research and development costs are expensed as incurred and include salaries and benefits, stock-based compensation, and costs paid to third-party contractors to perform research, conduct clinical trials, and develop drug materials. Manufacturing cost is a significant component of research and development expenses and includes costs associated with third-party contractors for validation batch production, process technology transfer, quality control and stability testing, raw material purchases, overhead expenses and facilities 84
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) costs. The Company expenses these manufacturing-related expenses as research and development as incurred because these costs do not meet the definition of an asset, as future use cannot be determined based upon the uncertainty of whether KRYSTEXXA will be approved for marketing by the FDA. Clinical trial cost is another significant component of research and development expenses and most of the Company’s clinical studies are performed by third-party contract research organizations (“CRO”). The Company accrues costs for clinical studies performed by CROs that are milestone or event driven in
nature and based on reports and invoices submitted by the CRO. These expenses are based on patient enrollment as well as costs consisting primarily of payments made to the CRO, clinical sites, investigators, testing facilities and patients for participating in the Company’s clinical trials. Non-refundable advance payments for future research and development activities are deferred and capitalized. Such amounts are recognized as an expense as the goods are delivered or the related services are performed. Consistent with the consensus, the Company has deferred approximately $0 and $1.3
million of these costs as of December 31, 2009 and 2008, respectively, and has amortized approximately $0.7 million and $0.6 million for the years ended December 31, 2009 and 2008, respectively, based on services performed. The net deferred balance of $0 and $0.7 million as of December 31, 2009 and 2008,
respectively, is included in Prepaid Expenses and Other Current Assets on the Company’s consolidated balance sheet. All other research and development costs are charged to expense as incurred, including $2.3 million and $2.5 million of fees incurred during the years ended December 31, 2009 and 2008,
respectively, to reserve manufacturing capacity at the Company’s third-party suppliers’ manufacturing facilities, for future potential orders of KRYSTEXXA. Income taxes In 2009 the Company generated a net operating loss of approximately $80.3 million for federal income tax purposes and recorded an income tax benefit of $2.1 million. This benefit reflects the expected tax effect of carrying back an increased portion of the Company’s 2008 alternative minimum tax net
operating loss to the Company’s 2003 and 2006 tax years to recover the remaining alternative minimum income tax liability of $2.0 million that is eligible to be refunded for those tax years, as well as a $0.1 million benefit due to a reversal of a liability for unrecognized tax benefit caused by a lapse in statute. The
opportunity for carryback became available due to Section 172 (b) (1) (H) and 810(b) of the Internal Revenue Code which was amended under Section 13 of the Worker, Homeownership, and Business Assistance Act of 2009. A refund of $2.0 million was received in February 2010 relating to this income tax
benefit. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and net operating loss
and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in
income in the period that includes the enactment date. The Company regularly reviews the Company’s deferred tax assets for recoverability and establishes a valuation allowance based on historical taxable income, projected future taxable income, applicable tax strategies, and the expected timing of the reversals of
existing temporary differences. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. In forming the Company’s judgment regarding the recoverability of deferred tax assets related to deductible temporary differences and tax attribute
carryforwards, the Company gives weight to positive and negative evidence based on the extent to which the forms of evidence can be objectively verified. The Company attaches the most weight to historical earnings due to its verifiable nature. Weight is attached to tax planning strategies if the strategies are
prudent and feasible and implementable without significant obstacles. Less weight is attached to forecasted future earnings due to its subjective nature. In 2009, based on the net operating loss generated in 2009 and historical 85
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) losses and the uncertainty of profitability in the near future, the Company concluded that it would maintain a full valuation allowance on all of the Company’s deferred tax assets except those assets that are reserved by a liability for unrecognized tax benefits. The Company maintained a valuation allowance as of
December 31, 2009 of $125.8 million. The increase in the valuation allowance from 2009 compared to 2008 of approximately $39.0 million is primarily due to an increase in net operating loss carryforwards and tax credits and the uncertainty that these additional deferred tax assets will be realized. The Company uses judgment in determining income tax provisions and in evaluating the Company’s tax positions. Additional provisions for income taxes are established when, despite the belief that tax positions are fully supportable, there remain certain positions that do not meet the minimum probability
threshold, which is a tax position that is more-likely-than-not to be sustained upon examination by the applicable taxing authority. The Company is examined by federal and state tax authorities. The Company regularly assesses the potential outcomes of these examinations and any future examinations for the
current or prior years in determining the adequacy of its provision for income taxes. The Company continually assesses the likelihood and amount of potential adjustments and adjusts the income tax provision, the current tax liability and deferred taxes in the period in which the facts that give rise to a revision
become known. The total amount of federal, state, local and foreign liabilities for unrecognized tax benefits was $10.0 million as of December 31, 2009, including accrued penalties and interest. The net increase of $5.4 million in the liability for unrecognized tax benefits subsequent to adoption of the new accounting guidance
on accounting for uncertainties in income taxes as codified under FASB ASC 640, Income Taxes, resulted in a corresponding decrease to the income tax benefit within the Company’s consolidated statements of operations as well as an increase to the deferred tax asset for which no tax benefit will be recognized in
the Company’s consolidated statements of operations. The Company’s former subsidiaries, BTG and Rosemont, filed separate income tax returns and provided for taxes under their respective local laws. Income tax expense related to these former subsidiaries is included in discontinued operations. Accumulated other comprehensive income Accumulated other comprehensive income consisted of unrealized gains (losses) on available-for-sale marketable securities. Earnings (loss) per common share The Company accounts for and discloses net earnings (loss) per share using the treasury stock method. Net earnings (loss) per common share, or basic earnings (loss) per share, is computed by dividing net earnings (loss) by the weighted-average number of common shares outstanding. Net earnings (loss) per
common share assuming dilutions, or diluted loss per share, is computed by reflecting the potential dilution from the exercise of in-the-money stock options and non-vested restricted stock and restricted stock units. See Note 9 to the Company’s consolidated financial statements for further discussion of the
Company’s earnings (loss) per common share. Use of estimates in preparation of financial statements The preparation of financial statements in conformity with Generally Accepted Accounting Principles (“GAAP”) in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date
of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, the Company evaluates its estimates, including those related to investments, accounts receivable, reserve for product returns, inventories, rebates, property and equipment, share-based
compensation and income taxes. The estimates are based on historical 86
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Results may differ from these estimates due to actual
outcomes being different from those on which the Company bases its assumptions. Concentration of credit risk Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, short-term and long-term investments and accounts receivable. The Company places its cash and cash equivalents and short-term investments with high quality financial
institutions and limits the amount of credit exposure to any one institution, except for U.S. Treasury or Government Agency issues. Concentration of credit risk with respect to accounts receivable is discussed in Note 16. Generally, the Company does not require collateral from its customers; however, collateral or
other security for accounts receivable may be obtained in certain circumstances when considered necessary. Accounting Pronouncements Adopted In January 2010, the FASB issued ASU 2010-06, Improving Disclosures about Fair Value Measurements. ASU 2010-06 amends ASC 820 to require a number of additional disclosures regarding fair value measurements. The amended guidance requires entities to disclose the amounts of significant transfers
between Level 1 and Level 2 of the fair value hierarchy and the reasons for these transfers, the reasons for any transfers in or out of Level 3, and information in the reconciliation of recurring Level 3 measurements about purchases, sales, issuances and settlements on a gross basis. The ASU also clarifies the
requirements for entities to disclose information about both the valuation techniques and inputs used in estimating Level 2 and Level 3 fair value measurements. The amended guidance is effective for interim and annual financial periods beginning after Decemer 15, 2009. ASU 2010-06 is not expected to have a
significant effect on the Company’s consolidated financial statements. In August 2009, the FASB issued ASU 2009-05, Measuring Liabilities at Fair Value. FASB ASU 2009-05 provides amendments to FASB ASC 820, Fair Value Measurements and Disclosures, for the fair value measurement of liabilities. FASB ASU 2009-05 provides clarification that in circumstances in which a
quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using a valuation technique that uses the quoted price of the identical liability when traded as an asset, the quoted prices for similar liabilities or similar liabilities when traded as assets or
another valuation technique that is consistent with the principles of FASB ASC 820. The amendments in the update also clarify that when estimating the fair value of a liability, a reporting entity is not required to include a separate input or adjustment to other inputs relating to the existence of a restriction that
prevents the transfer of the liability. The amendments in the update also clarify that both a quoted price in an active market for the identical liability at the measurement date and the quoted price for the identical liability when traded as an asset in an active market when no adjustments to the quoted price of the
asset are required are Level 1 fair value measurements. The guidance provided in FASB ASU 2009-05 is effective for the first reporting period (including interim periods) beginning after issuance. The adoption of FASB ASU 2009-05 did not have a material effect on the Company’s consolidated financial
statements. In June 2009, the FASB issued a new accounting statement on Accounting Standards Codification. The new statement establishes FASB Accounting Standards Codification (“the Codification”) as the single source of authoritative non-governmental GAAP and is codified under FASB ASC 105, Generally
Accepted Accounting Principles. The Codification does not change current GAAP, but is intended to simplify user access to all authoritative GAAP by providing all the authoritative literature related to a particular topic in one place. All existing accounting standard documents will be superseded and all other
accounting literature not included in the Codification will be considered non-authoritative. 87
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) The Codification is effective for interim and annual periods ending after September 15, 2009. The Codification is for disclosure only and did not impact the Company’s financial condition or results of operations. In May 2009, the FASB issued a new accounting statement on Subsequent Events as codified under FASB ASC 855, Subsequent Events. The new statement establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued.
The new statement requires the disclosure of the date through which an entity has evaluated subsequent events and the basis for that date and should alert all users of financial statements that an entity has not evaluated subsequent events after that date in the set of financial statements being presented. The new
statement shall be applied to the accounting for and disclosure of subsequent events not addressed in other applicable GAAP. Other applicable GAAP may address the accounting treatment of events or transactions that occur after the balance sheet date but before the financial statements are issued. If an event or
transaction is within the scope of other applicable GAAP, then an entity shall follow the guidance in that applicable GAAP, rather than the guidance in the new statement. The new statement is effective for interim or annual financial periods ending after June 15, 2009, and is applied prospectively. The adoption of
the new statement did not have a material effect on the Company’s recognition or disclosures in its consolidated financial statements. The Company evaluates all subsequent events as of the issuance date of its financial statements. In April 2009, the FASB issued new accounting guidance on determining fair value when the volume and level of activity for the asset or liability have significantly decreased and identifying transactions that are not orderly as codified under FASB ASC 820, Fair Value Measurements and Disclosures. The
guidance provides additional options for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased. The guidance emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the
valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date
under current market conditions. The guidance addresses the recommendations of specific issues in the Securities and Exchange Commission’s (“SEC’s”) study on mark-to-market accounting and provides additional guidance on determining fair value when the volume and level of activity for the asset or liability
has significantly decreased when compared with normal market activity for the asset or liability (or similar assets or liabilities). The guidance is effective for interim and annual reporting periods ending after June 15, 2009 and shall be applied prospectively. The adoption of the guidance did not have a material effect
on the Company’s consolidated financial statements. In April 2009, the FASB issued new accounting guidance on interim disclosures about the fair value of financial instruments as codified under FASB ASC 825, Financial Instruments. The guidance requires disclosures about fair value of financial instruments for interim reporting periods of publicly traded
companies as well as in annual financial statements. The guidance is effective for interim and annual reporting periods ending after June 15, 2009 with early adoption permitted for periods ending after March 15, 2009. The adoption of this guidance did not have a material effect on the Company’s consolidated
financial statements. In April 2009, the FASB issued new accounting guidance on the Recognition and Presentation of Other-Than-Temporary Impairments as codified under FASB ASC 320, Investments-Debt and Equity Securities. The guidance amends the OTT impairment guidance in GAAP for debt securities to make the
guidance more operational and improves the presentation and disclosure of OTT impairments on debt and equity securities in the financial statements. The guidance does not amend existing recognition and measurement guidance related to OTT impairments of equity securities. The guidance expands and increases
the frequency of existing disclosures about OTT impairments for debt and equity securities and requires a more detailed, risk oriented breakdown by major security types and related information. In addition, the guidance requires that the annual disclosures in existing GAAP related to OTT 88
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) impairments of equity securities be made for interim periods. The guidance is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. Early adoption for periods ending before March 15, 2009, is not permitted. The adoption
of the guidance did not have a material effect on the Company’s consolidated financial statements. In February 2007, the FASB issued new accounting guidance on the effective date of fair value measurements for non-financial assets and non-financial liabilities, as codified under FASB ASC 820, Fair Value Measurements and Disclosures. The guidance delayed the effective date to fiscal years beginning after
November 15, 2008 and interim periods within those fiscal years for non-financial assets and non-financial liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis and at least annually. Since the Company does not carry any non-financial instruments
that are measured at fair value on a non-recurring basis, the conclusion of the deferral period did not have an effect on the Company’s financial statements. In July 2006, the FASB issued new accounting guidance on accounting for uncertainties in income taxes as codified under FASB ASC 640, Income Taxes. The guidance clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition
threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The guidance also provides clarity on de-recognition of tax benefits, classification on the consolidated balance sheet, interest and penalties, accounting in
interim periods, disclosure, and transition. The Company adopted this guidance effective January 1, 2007 and as a result, the Company recorded a $4.5 million increase in the liability for unrecognized tax benefits, including $0.2 million of accrued interest and penalties, which is included in Other Liabilities on the
Company’s consolidated balance sheet. This increase in liability resulted in a corresponding increase to Accumulated Deficit. The total amount of federal, state, local and foreign liabilities for unrecognized tax benefits was $10.0 million as of December 31, 2009, including accrued penalties and interest. The net
increase of $5.4 million in the liability for unrecognized tax benefits subsequent to adoption resulted in a corresponding decrease to the income tax benefit within the Company’s consolidated statements of operations as well as an increase to the deferred tax asset for which no tax benefit will be recognized in the
Company’s consolidated statements of operations. Note 2—Fair Value of Financial Instruments and Investments The Company categorizes its financial instruments into a three-level fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities
(Level 1) and the lowest priority to unobservable inputs (Level 3). If the inputs used to measure fair value fall within different levels of the hierarchy, the category level is based on the lowest priority level input that is significant to the fair value measurement of the instrument. Financial assets recorded at fair value on the Company’s consolidated balance sheets are categorized as follows: Level 1: Unadjusted quoted prices for identical assets in an active market. Level 2: Quoted prices in markets that are not active or inputs that are observable either directly or indirectly for substantially the full-term of the asset. Level 2 inputs include the following:
•
Quoted prices for similar assets in active markets, • Quoted prices for identical or similar assets in non-active markets, • Inputs other than quoted market prices that are observable, and • Inputs that are derived principally from or corroborated by observable market data through correlation or other means. 89
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Level
3: Prices or valuation techniques that require inputs that are both unobservable
and significant to the overall fair value measurement. They reflect management’s
own assumptions about the assumptions a market participant would use in pricing
the asset. The following table presents the Company’s hierarchy for its financial instruments measured at fair value on a recurring basis as of December 31, 2009:
Level 1
Level 2
Level 3
Total
(In thousands) Cash and cash equivalents (1)
$
108,172
$
—
$
—
$
108,172 Short-term investments (available-for-sale) (2)
—
3
—
3 Total cash and cash equivalents and short-term investments
108,172
3
—
108,175 Restricted cash (1)
1,280
—
—
1,280 Total long-term investments
1,280
—
—
1,280 Total assets measured at fair value on a recurring basis
$
109,452
$
3
$
—
$
109,455 Warrant liability
$
—
$
—
$
24,239
$
24,239 Total liabilities measured at fair value on a recurring basis
$
—
$
—
$
24,239
$
24,239 The cost and estimated fair value of the Company’s available-for-sale investments at December 31, 2009 and 2008 were as follows:
2009
Cost
Gross
Gross
Estimated
(In thousands) Equity securities (short-term) (2)
$
—
$
3
$
—
$
3 Total
$
—
$
3
$
—
$
3
2008
Cost
Gross
Gross
Estimated
(In thousands) Equity securities (2)
$
—
$
24
$
—
$
24 Columbia strategic cash portfolio (restricted short-term portion) (3)
2,258
—
—
2,258 Total short-term investments
2,258
24
—
2,282 Columbia strategic cash portfolio (restricted long-term portion) (3)
1,705
—
—
1,705 Total
$
3,963
$
24
$
—
$
3,987
(1)
The estimated fair value of cash and cash equivalents and restricted cash approximates the carrying value. (2) Short-term investments at December 31, 2009 and 2008 were comprised of the Company’s investment in common shares of Neuro-Hitech, Inc. The fair value of this investment is obtained from quoted prices in an inactive market. The Company considers the market for Neuro-Hitech, Inc. shares to be inactive
due to its low trading volume and infrequency of trading. The Company purchased $1.0 million of Marco Hi-Tech JV, Ltd. (“Marco”) preferred stock in 2003 and 2004. In March 2004, the Company’s holdings of Marco preferred stock were converted into 654,112 shares of Marco common stock, or
approximately 8% of Marco’s fully-diluted outstanding common stock. In January, 2006, Marco entered into a merger agreement with Neurotech Pharmaceuticals, Inc. 90
Unrealized
Gains
Unrealized
Losses
Fair Value
Unrealized
Gains
Unrealized
Losses
Fair Value
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
which later changed its name to Neuro-Hitech, Inc. (“Neuro-Hitech”). Pursuant to the terms of this merger agreement, each share of Marco common stock converted into 0.583033 common shares of Neuro-Hitech, which resulted in the Company receiving 381,362 shares of Neuro-Hitech. Neuro-Hitech executed
its initial public offering in February 2006 and trades on the Over the Counter Bulletin Board stock market as of December 31, 2009. (3) The Company’s restricted investments consisted of its former investment in the Portfolio. During the fourth quarter of 2007, Columbia, a unit of BOA, closed the Portfolio to new investments and redemptions and began an orderly liquidation and dissolution of the Portfolio’s assets for distribution to the unit
holders, thereby restricting the Company’s potential to invest in and withdraw from the Portfolio. During 2009, the Portfolio redeemed all of the Company’s remaining cash investment in the Portfolio and the Company therefore was no longer invested in this fund as of December 31, 2009. At December 31,
2008, approximately $14.7 million, or 74%, of the Company’s original $20.0 million investment had been redeemed and re-invested in cash and cash equivalents and used to fund operations. At December 31, 2008, the fair market value of restricted investment securities was $4.0 million, $2.3 million of which is
reflected in the Company’s consolidated balance sheets as a component of Short-Term Investments and $1.7 million of which is reflected in the Company’s consolidated balance sheets as a component of Other Assets. The fair value of the securities held within the Portfolio were independently priced by third-
party pricing services that also provide valuations of other Columbia funds. The Portfolio’s valuation process was covered by Columbia’s Mutual Fund Valuation Committee and its Valuation Group which is subject to various reviews on a daily, monthly and ad-hoc basis. The remaining investment was
redeemed during 2009 and reinvested in cash and cash equivalents and used to fund operations. Level 3 Valuation Financial assets or liabilities are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. The following table provides a summary of the changes in fair value
of the Company’s financial liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during 2009:
Warrant Liability
Fair Value Balance, December 31, 2008
$
— Valuation of warrant liability on April 8, 2009
12,561 Total realized loss for three months ended June 30, 2009 (mark-to-market of warrant liability at June 30, 2009)
35,881 Valuation of warrant liability as of June 30, 2009
48,442 Total realized gain for the three months ended September 30, 2009 (mark-to-market of warrant liability at September 30, 2009)
(11,825
) Valuation of warrant liability as of September 30, 2009
36,617 Total realized gain for the three months ended December 31, 2009 (mark-to-market of warrant liability at December 31, 2009)
(12,378
) Balance, December 31, 2009
$
24,239 The warrant liability is marked-to-market each reporting period with the change in fair value recorded as a gain or loss within Other Expense, net on the Company’s consolidated statement of operations until the warrants are exercised, expire or other facts and circumstances lead the warrant liability to be
reclassified as an equity instrument. The fair value of the warrant liability is determined at 91
Measurements
Using Significant
Unobservable
Inputs (Level 3)
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) each reporting period by utilizing a Monte Carlo simulation model that takes into account estimated probabilities of possible outcomes provided by the Company (unobservable inputs). The Company does not carry any financial instruments or non-financial instruments that are measured at fair value on a non-recurring basis. There were no securities in a continuous unrealized loss position for greater than 12 months for the year ended December 31, 2009. Net unrealized losses included in accumulated other comprehensive income, net of taxes, at December 31, 2009 and 2008 were as follows:
December 31,
2009
2008 Net unrealized losses arising during the period
$
(21
)
$
(2,179
) Reclassification adjustment for (gains) losses included in earnings
—
889 Unrealized losses included in comprehensive income
$
(21
)
$
(1,290
) Proceeds from the sales of available-for-sale securities and the gross realized investment gains and losses on those sales reclassified out of accumulated other comprehensive income for the years ended December 31, 2009, 2008 and 2007 were as follows:
December 31,
2009
2008
2007 Proceeds
$
4,170
$
13,516
$
2,221 Gross realized investment gains
834
141
337 Gross realized investment losses
$
652
$
1,030
$
331 Note 3—Inventories, Net At December 31, 2009 and 2008, inventories at cost, net of reserves, were as follows:
December 31,
2009
2008
(In thousands) Raw materials
$
1,518
$
2,596 Finished goods
324
5,619 Inventory at Cost
1,842
8,215 Inventory reserves
(1,257
)
(6,323
) Total
$
585
$
1,892 An allowance is established when management determines that certain inventories may not be saleable. The Company states inventories at the lower of cost or market. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, the Company records
reserves for the difference between the cost and the market value. These reserves are recorded based on estimates of expected sales demand for Oxandrin and oxandrolone. Note 4—Property and Equipment, Net
December 31,
2009
2008
(In thousands) Office equipment
$
2,412
$
8,190 Office equipment—capital leases
313
313 Leasehold improvements
1,546
1,536
4,271
10,039 Accumulated depreciation and amortization
(3,278
)
(8,646
) Total
$
993
$
1,393 92
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Depreciation and amortization expense was $0.5 million for each of the years ended December 31, 2009, 2008 and 2007, respectively. Capital lease obligations associated with the capital lease office equipment are included in Other Current Liabilities and non-current Other Liabilities. See Note 6 to the consolidated financial statements for more details. Note 5—Other Current Liabilities The components of Other Current Liabilities for the years ended December 31, 2009 and 2008 are as follows:
December 31,
2009
2008
(In thousands) Severance
$
3,191
$
1,877 Manufacturing and technology transfer services
2,464
3,339 Returned product liability
2,385
818 Allowance for product returns
1,033
1,163 Clinical research organization expenses
805
2,121 Accrued taxes
760
760 Litigation, legal and professional fees
388
1,456 Allowance for product rebates
303
709 Salaries and related expenses
239
2,324 Unrecognized tax benefit—BTG tax assessment
—
1,565 Other
905
2,518 Total
$
12,473
$
18,650 On September 30, 2009, the Company completed a plan of termination pursuant to which it reduced its workforce by 25 employees, or approximately 37%. This termination was in addition to a previous termination of nine employees in August 2009. The Company took these actions in order to reduce costs
and streamline operations in order to focus its efforts on the tasks critical to the resubmission of the KRYSTEXXA BLA to the FDA. The reduction in staff was focused in the Company’s commercial, medical, financial, operational and sales departments with minimal effects in the regulatory, quality and
manufacturing departments. These terminations were a result of the Company’s having completed clinical and pre-commercial activities for KRYSTEXXA. In connection with this plan of termination, the Company incurred charges of $2.3 million during the year ended December 31, 2009 and recorded expense of $1.2 million and $1.1 million to Research and Development and Selling, General and Administrative, respectively, in the Company’s statement of
operations. The Company had a restructuring reserve of $0.5 million relating to the plan of termination as of December 31, 2009. Note 6—Other Liabilities (Non-Current) The components of other liabilities for the periods ended December 31, 2009 and 2008 are as follows:
December 31,
2009
2008
(In thousands) Unrecognized tax benefit (1)
$
10,011
$
9,645 Capital leases (2)
98
164 Total
$
10,109
$
9,809
(1)
See Note 14 to consolidated financial statement for further discussion of unrecognized tax benefits.
93
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) (2) The Company maintains capital leases for office equipment used at its corporate headquarters in East Brunswick, New Jersey. The leases range in terms from 34 to 60 months and were entered into between 2007 and December 31, 2009. Note 7—Commitments and Contingencies Commitments The Company’s corporate headquarters are located in East Brunswick, New Jersey, where it leases approximately 53,000 square feet of office space. The lease has a base average annual rental expense of approximately $1.9 million and expires in March 2013. The Company has two five-year renewal options
under the lease. In connection with this lease arrangement, the Company was required to provide a $1.3 million security deposit by way of an irrevocable letter of credit, which is secured by a cash deposit of $1.3 million and is reflected in Other Assets (as restricted cash) on the Company’s consolidated balance
sheets at December 31, 2009 and 2008. The Company subleased approximately 7,090 square feet at a base average annual rental of $0.2 million through February 2009. In March 2009, the sublease agreement was terminated and as a result, the Company’s net annual rental expense increased to approximately $1.9
million. The Company is also obligated to pay its share of operating maintenance and real estate taxes with respect to its leased property. Rent expense from continuing operations was approximately $2.0 million, $1.7 million and $1.7 million, for each of the years ended December 31, 2009, 2008 and 2007. Rent expense is presented net of the sublease arrangement, within Research and Development and Selling, General and Administrative
expense in the consolidated statement of operations for the years ended December 31, 2009, 2008 and 2007. The future annual minimum rentals (exclusive of amounts for real estate taxes, maintenance, etc.) for each of the following years are:
(in thousands) 2010
$
1,854 2011
$
1,867 2012
$
1,867 2013
$
467 2014
$
— In September 2009, the Company completed a plan of termination pursuant to which it reduced its workforce by 25 employees. This termination was in addition to a previous termination of nine employees in August 2009. In connection with the plan of termination, the Company incurred charges of $2.3
million. The Company recorded $1.2 million in severance-related expenses during the quarter ended September 30, 2009 and an additional $1.1 million of stock-based compensation expense during the quarter ended December 31, 2009, as a result of modifications to stock option awards previously granted to the
terminated employees. The modifications included extending the term of exercisability of the vested portion of the stock options from three months following cessation of employment to six months following cessation of employment, coupled with accelerating the vesting to immediately vest any unvested stock
options. The severance-related expenses resulted in cash expenditures of $0.7 million during the quarter ended December 31, 2009 and will result in an additional $0.5 million in cash expenditures during the first half of 2010. At December 31, 2009, the Company had employment agreements with three senior officers. Under these agreements, the Company has committed to total aggregate base compensation per year of approximately $1.1 million plus other fringe benefits and bonuses. These employment agreements generally have
an initial term of three years and are automatically renewed thereafter for successive one-year periods unless either party gives the other notice of non-renewal. In March 2007, the Company entered into commercial supply and development agreements with BTG, pursuant to which BTG serves as the manufacturer and commercial supplier of the API drug substance for KRYSTEXXA and provides development, manufacturing and other services in relation to 94
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) the product. Under the
agreements, BTG also provides support with respect to the Company’s
BLA for KRYSTEXXA. Under the development agreement with BTG, BTG has agreed
to provide development services and documentation needed for the Company’s
regulatory filing in accordance with a development plan, including (i) validating
the production facility and process; (ii) managing the contract production;
and (iii) performing, and allowing the Company to perform, quality assurance.
Under the commercial supply agreement with BTG, as amended in June 2008,
BTG has agreed to provide (i) all information and assistance necessary for
the preparation of comprehensive and complete BLAs, and (ii) access to its
facility to FDA inspectors conducting pre-approval inspections. The Company
is obligated to provide BTG with a rolling forecast on a monthly basis setting
forth the total quantity of API drug substance it expects to require for
commercial supply in the following 18 months. The first six months of each
forecast represent a rolling firm irrevocable order, and the Company may
only increase or decrease its forecast for the next 12 months within specified
limits. As of December 31, 2009, based on the Company’s latest forecast,
the Company is obligated to purchase an aggregate of approximately $5.0 million
of API during 2010. During 2009, 2008 and 2007, the Company paid to BTG non-refundable
fees of $0, $2.2 million and $4.6 million, respectively, to reserve manufacturing
capacity relating to the Company’s potential future orders of
KRYSTEXXA. The Company recorded these capacity reservation fees, which may
be credited as a discount against future orders of KRYSTEXXA, as research
and development expenses as they were incurred. Under the commercial supply
agreement, BTG is obligated to manufacture the Company’s firmly
forecasted commercial supply of KRYSTEXXA and the Company is obligated to purchase
from BTG at least 80% of its worldwide requirements of API drug substance.
However, if BTG produces specified numbers of failed batches of API within
one or more calendar quarters, then the Company may purchase all of its KRYSTEXXA
requirements from other suppliers until BTG demonstrates to the Company reasonable
satisfaction that it has remedied its supply failure. In addition, if the
Company’s product forecasts are reasonably anticipated to exceed BTG’s
processing capacity, then the Company may purchase from other suppliers its
KRYSTEXXA requirements that exceed BTG’s capacity. Beginning on the
seventh anniversary of BTG’s first delivery of API under the commercial
supply agreement, which may be as early as December 2015, either the Company
or BTG may provide three years advance notice to terminate the commercial
supply agreement. The commercial supply agreement may also be terminated
in the event of insolvency or uncured material breach by either party. In May 2007, the Company entered into a supply agreement with NOF Corporation of Japan (“NOF”), pursuant to which NOF serves as the Company’s exclusive supplier of mPEG, which is used in the PEGylation process to produce KRYSTEXXA. The Company must purchase its entire supply of mPEG
from NOF unless NOF fails to supply at least 75% of the Company’s firm orders, in which case the Company may obtain mPEG from a third party until NOF’s supply failure is remedied to the Company’s reasonable satisfaction. Under the agreement, the Company is obligated to make specified minimum
purchases of mPEG from NOF. The Company must provide NOF with a rolling forecast on a quarterly basis setting forth the total quantity of mPEG that it expects to require in the following 18 months. The first six months of each forecast represent a rolling firm irrevocable order, and the Company may only
increase or decrease its forecast for the next 12 months within specified limits. As of December 31, 2009, the Company was obligated to purchase mPEG at an aggregate cost of approximately $2.3 million and $2.3 million during 2010 and 2011, respectively. For any given year, upon three months advance notice, the
Company may terminate its minimum purchase obligation for the entire year or the remainder of that year by paying NOF 50% of the minimum purchase obligation for that year or the remainder of that year. The Company is in the process of renegotiating the minimum purchase obligation of this agreement.
Under the agreement, NOF was obligated and did supply the Company with three mPEG validation batches during 2007 and prepare a Type II Drug Master File (“DMF”) for submission to the FDA. NOF filed its Type II DMF with the FDA in December 2007 and it remains obligated under the agreement to use
commercially reasonable efforts to submit a DMF, or its equivalent, to the appropriate regulatory agency in one additional country or the European Union. The Company’s agreement with NOF has an initial term ending in May 2017 and may 95
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) be extended for an additional 10 years by mutual agreement of the parties at least 12 months before the expiration of the initial term. Prior to the expiration of the term, either the Company or NOF may terminate the agreement for convenience upon 24 months advance notice. Either the Company or NOF may
terminate the agreement in the event of the other party’s insolvency or uncured material breach. In the event that NOF terminates the agreement for convenience or if the Company terminates the agreement for NOF’s breach or bankruptcy, the Company may require NOF to continue to supply mPEG for up to
two years following the termination date. If the Company terminates the agreement for convenience or NOF terminates the agreement for the Company’s breach, the Company must pay NOF 50% of the minimum purchase obligation for the period from the termination date until the date on which the agreement
would have expired. In October 2008, the Company entered into a non-exclusive commercial supply agreement with Sigma-Tau PharmaSource, Inc. (“Sigma-Tau”) (formerly known as Enzon Pharmaceuticals, Inc., which was acquired by Sigma-Tau in January 2010), which replaced its 2006 service agreement with Sigma-Tau.
Under the terms of the commercial supply agreement, Sigma-Tau has agreed to fill, inspect, package, test and provide specified product support services for the final KRYSTEXXA product. In return, if KRYSTEXXA receives FDA marketing approval from the FDA, the Company will purchase from Sigma-Tau,
product and product support services during 2010 and 2011 at an aggregate cost of approximately $1.7 million and $2.8 million, respectively. These purchase obligations are based on a rolling forecast that the Company has agreed to provide to Sigma-Tau on a quarterly basis setting forth the total amount of final
product that it expects to require in the following 24 months. The first six months of each forecast will represent a rolling firm irrevocable order, and the Company may only increase or decrease its forecast for the next 18 months within specified limits. If the Company cancels batches subject to a firm order, it must
pay Sigma-Tau a fee. Under the agreement, the Company is also obligated to pay Sigma-Tau a rolling, non-refundable capacity reservation fee, which may be credited against the fees for Sigma-Tau’s production of the final product. During 2009, the Company incurred $0.3 million in capacity reservation fees. The
Company recorded this capacity reservation fee as research and development expenses as incurred. In October 2009, the Company entered into an amendment agreement with Sigma-Tau modifying the payment terms for the filling, packaging, testing and other product support services that they will render for the
Company. Under the terms of the amendment agreement, upon Sigma-Tau’s receipt of API drug substance from the Company, Sigma-Tau will invoice the Company for an upfront non-refundable fee for their filling, packaging and testing services to be performed on the API. The Company will be responsible to
pay for these services within 15 days of the invoice date even if it decides to delay the filling and packaging of the API drug substance material. After October 2009, either the Company or Sigma-Tau may terminate the agreement upon 24 months advance notice given 30 days before each year’s anniversary date of
the agreement. If the Company terminates the agreement, it would be obligated to pay Sigma-Tau a fee based on the previously submitted rolling forecasts. Either the Company or Sigma-Tau may also terminate the agreement in the event of insolvency or uncured material default in performance by either party, or
in the event that the FDA does not approve the Company’s BLA for KRYSTEXXA and such disapproval is final or not appealed by the Company. In addition, if the FDA delays approval of the Company’s BLA, the obligations of both parties will be held in abeyance for up to 18 months pending such approval,
after which Sigma-Tau may terminate the agreement immediately and without penalty. The Company believes that its current arrangements for the supply of clinical and commercial quantities of pegloticase API drug substance and finished form KRYSTEXXA will be adequate to complete its clinical development
program and to satisfy its currently forecasted commercial requirements of KRYSTEXXA. The Company is a party to an exclusive royalty bearing license agreement with MVP and Duke, originally entered into in August 1997 and amended in November 2001, granting the Company rights under technology relating to mammalian and non-mammalian uricases, and MVP’s technology relating to mPEG
conjugates of these uricases, as well as patents and pending patent applications covering this technology, to make, use and sell, for human treatment, products that use this technology. These 96
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) patents and pending patent applications constitute the fundamental composition of matter and underlying manufacturing patents for KRYSTEXXA. Under this agreement, the Company also has the exclusive license to the trademark Puricase®, which is a registered trademark of MVP and was available for potential
use as the proprietary name of the product candidate the Company now refers to as KRYSTEXXA. However, if the Company elects not to use the trademark Puricase, or if the Company otherwise fails to use the trademark Puricase within one year after the first sale of any product which uses the licensed
technology, then MVP would retain all rights to use the trademark Puricase. Under the agreement, the Company is required to use best efforts to bring to market and diligently market products that use the licensed technology. The agreement requires the Company to pay to MVP and Duke quarterly royalty
payments within 60 days after the end of each quarter based on KRYSTEXXA net sales made in that quarter. The royalty rate for a particular quarter ranges between 8% and 12% of net sales based on the amount of cumulative net sales made by the Company or its sub-licensees. Under the agreement, the
Company is also required to pay royalties of 20% on any revenues or other consideration it receives from sub-licensees during any quarter. During the years ended December 31, 2009 and 2008, the Company made aggregate milestone payments to MVP and Duke of $0 and $0.5 million, respectively. The milestone
payment made in 2008 resulted from the Company’s filing of the BLA for KRYSTEXXA. The Company is also required to pay up to an aggregate of approximately $1.8 million to MVP and Duke if it successfully commercializes KRYSTEXXA and attains specified KRYSTEXXA sales targets. In addition, if the
Company obtains regulatory approval in one of five major global markets, which includes FDA approval in the United States, it will be required to make aggregate milestone payments of approximately $0.8 million to MVP and Duke. As of December 31, 2009, the Company has made aggregate payments of
approximately $1.7 million to MVP and Duke for the achievement of milestones under this agreement. The agreement remains in effect, on a country-by-country basis, for the longer of 10 years from the date of first sale of KRYSTEXXA in such country, or the date of expiration of the last-to-expire patent covered
by the agreement in such country. The Company may terminate this agreement in one or more countries with six months prior notice, and it may also terminate the agreement with respect to any country in which the licensed patents are infringed by a third party or in which the manufacture, use or sale of
KRYSTEXXA infringes a third party’s intellectual property rights. Either the Company or the licensors may also terminate the agreement, with respect to the countries affected, upon the other party’s material breach, if not cured within a specified period of time, or immediately upon the other party’s third or
subsequent material breach of the agreement or the other party’s fraud, willful misconduct or illegal conduct. Either party may also terminate the agreement for the other party’s bankruptcy or insolvency. Upon a termination of the agreement in one or more countries, all intellectual property rights conveyed to the
Company with respect to the terminated countries under the agreement, including regulatory applications and pre-clinical and clinical data, revert to MVP and Duke and the Company is permitted to sell off any remaining inventory of KRYSTEXXA for such countries. The Company has received financial grants in support of research and development from the Office of the Chief Scientist of the State of Israel (“OCS”), and the Israel-United States Bi-national Industrial Research and Development Foundation (“BIRD”), of approximately $2.0 million and $0.6 million,
respectively, for the development of KRYSTEXXA. These grants are subject to repayment through royalties on the commercial sale of KRYSTEXXA if and when commercial sale commences. The OCS grants were received by the Company’s former subsidiary, BTG, and upon the Company’s divestiture of BTG
to Ferring, it agreed to remain obligated to reimburse BTG for its repayments to OCS that relate to the KRYSTEXXA financial grants. In addition, under the Israeli Law of Encouragement of Research and Development in Industry, as amended, as a result of the funding received from OCS, if the Company does
not manufacture 100% of its annual worldwide bulk product requirements in Israel, the Company may be subject to total payments ranging from 120% to 300% of the repayment obligation plus interest, based upon the percentage of manufacturing that does not occur in Israel. The Company’s aggregate principal
repayment obligation plus interest to OCS and BIRD if it successfully launches KRYSTEXXA and, as per the Company’s agreements with BTG and Diosynth, 97
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) manufacture more than 20% of its annual worldwide bulk product requirements outside of Israel, would be between $4.0 million and $8.8 million at December 31, 2009. Contingencies On December 5, 2006, the Company filed a petition for reconsideration with the FDA regarding the rejection of its Citizen Petitions on the basis that the FDA failed to adequately consider the significant safety and legal issues raised by permitting approval of generic oxandrolone drug products without the
inclusion of labels that contain full geriatric dosing and safety information to date. Having not received a decision or other communication regarding this petition for reconsideration, the Company withdrew its petition for reconsideration on July 24, 2008. In May 2007, the Company filed a notice of appeal with the New Jersey Division of Taxation contesting a New Jersey Sales & Use Tax assessment of $1.2 million for the tax periods 1999 through 2003. The Company believes it is not subject to taxes on services that were provided to the Company. An
acknowledgement was received from The Conference and Appeals Branch and an appeal conference is expected to be scheduled in the future. In 2007, Joseph R. Berger filed a complaint against the Company in the Fayette County Circuit Court in Kentucky alleging breach of contract in connection with the assignment of certain inventions and patent rights related to the method of using oxandrolone to treat HIV/AIDS patients. The complaint alleged
several causes of action, each of which was premised on the existence of an oral agreement between the Company and Berger, which Berger alleges the Company breached. Berger sought, among other things, damages and rescission of the assignment of the inventions. In March 2008, Berger filed an amended
complaint that abandoned several of his claims, but continued to seek damages and rescission of the invention assignments. In March 2009, Savient filed a motion for summary judgment on Berger’s claims and thereafter Berger filed a cross-motion for summary judgment. Subsequently, oral argument on the
summary judgment motions was held and on August 17, 2009, the court issued an order granting the Company’s motion for summary judgment, denying Berger’s cross-motion and dismissing the complaint and amended complaint with prejudice. On September 11, 2009, counsel for Berger filed a notice of appeal of
the decision of the trial court, in response to which Savient filed a notice of cross-appeal solely with respect to the decision of the trial court to apply Kentucky law to the facts of the case. The matter is currently pending the issuance of an appellate schedule. The Company intends to vigorously defend this action
and to contest the appeal by Berger of the granted summary judgment and dismissal. In November 2008, Richard Sagall, an alleged stockholder, commenced an action in the U.S. District Court for the Southern District of New York seeking to certify a class of shareholders who held Savient securities between December 13, 2007 and October 24, 2008. The suit alleges that the Company made
false and misleading statements relating to the GOUT1 and GOUT2 phase 3 clinical trials, and that the Company failed to disclose in a timely manner serious adverse events which occurred in five patients in these trials. In March 2009, the Court issued an order appointing a lead plaintiff and the law firm
Pomerantz Haudek Block Grossman & Gross LLP as lead counsel. Thereafter, the lead plaintiff filed his amended complaint in April 2009, seeking unspecified monetary damages. In June 2009, Savient and the other named defendants moved to dismiss the complaint. The lead plaintiff subsequently filed an
opposition to the Company’s motion to dismiss and the Company filed its reply in October 2009. Oral arguments were heard by the Court in February 2010 relating to the Company’s motion to dismiss and the Company is awaiting the Court’s decision. The Company intends to vigorously defend against this action. During the years ended December 31, 2009 and 2008, one of the Company’s
customers requested a total of $1.3 million in reimbursements relating to
Oxandrin returned goods that were not in compliance with the Company’s returned goods policy. The Company is in the process of contesting this matter. 98
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) From time to time, the Company becomes subject to legal proceedings and claims in the ordinary course of business. Such claims, even if without merit, could result in the significant expenditure of the Company’s financial and managerial resources. The Company is not aware of any legal proceedings or claims
that it believes will, individually or in the aggregate, materially harm its business, results of operations, financial condition or cash flows. The Company is obligated under certain circumstances to indemnify certain customers for certain or all expenses incurred and damages suffered by them as a result of any infringement of third-party patents. In addition, the Company is obligated to indemnify its officers and directors against all reasonable costs
and expenses related to stockholder and other claims pertaining to actions taken in their capacity as officers and directors which are not covered by the Company’s directors and officers insurance policy. These indemnification obligations are in the regular course of business and in most cases do not include a limit
on maximum potential future payments, nor are there any recourse provisions or collateral that may offset the cost. As of December 31, 2009, the Company has not recorded a liability for any obligations arising as a result of these indemnification obligations. Note 8—Stockholders’ Equity On April 8, 2009, the Company raised $31.0 million from a registered direct offering, which yielded approximately $29.0 million in cash, net of approximately $2.0 million of offering costs which were charged to additional paid-in-capital. The Company issued 5,927,343 shares of its common stock to existing and
new institutional investors as part of the offering. The investors also received warrants to purchase up to 5,038,237 shares of its common stock at an initial exercise price of $10.46 per share. The warrants are exercisable at any time on or after the date of issuance and expire on November 2, 2010. Pursuant to the terms of the warrants, the exercise price per share for the warrants is $10.46, which is equal to the dollar volume weighted-average price of the Company’s common stock for the five
trading days immediately preceding August 17, 2009. The Company may, at it’s or the warrant holder’s election, issue net shares in lieu of a cash payment of the exercise price by the warrant holder upon exercise. In the event that the Company enters into a merger or change of control transaction, the holders of the warrants will be entitled to receive consideration as if they had exercised the warrant immediately prior to such transaction, or they may require the Company to purchase the warrant at the Black-Scholes
value of the warrant on the date of such transaction. The Company’s warrant liability is marked-to-market each reporting period with the change in fair value recorded as a gain or loss within Other Expense, net on the Company’s consolidated statement of operations until the warrants are exercised, expire or other facts and circumstances lead the warrant
liability to be reclassified as an equity instrument. The fair value of the warrant liability is determined at each reporting period by utilizing a Monte Carlo simulation model that takes into account estimated probabilities of possible outcomes provided by the Company. At the date of the transaction, the fair value of
the warrant liability was $12.6 million. At December 31, 2009, the fair value of the warrant liability determined utilizing a Monte Carlo simulation model was approximately $24.2 million. The increase in the fair value of the warrants from April 8, 2009 to December 31, 2009 mainly reflects the increase in the value of the Company’s common stock
price subsequent to the April 8, 2009 issuance of the warrants. During the year ended December 31, 2009, the Company recorded a charge of $11.7 million within Other Income (Expense), net on its consolidated statement of operations to reflect the increase in the valuation of the warrants. In October 2009, the Company completed an underwritten public offering of 4,945,000 shares of its common stock for net proceeds of $61.4 million, pursuant to a shelf registration statement on Form S-3 99
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) that the Company filed with the Securities and Exchange Commission on September 24, 2007 and that became effective on October 10, 2007. Note 9—Earnings (Loss) per Common Share The Company accounts for and discloses net earnings (loss) per share using the treasury stock method. Net earnings (loss) per common share, or basic earnings (loss) per share, is computed by dividing net earnings (loss) by the weighted-average number of common shares outstanding. Net earnings (loss) per
common share assuming dilutions, or diluted earnings (loss) per share, is computed by reflecting the potential dilution from the exercise of in-the-money stock options, non-vested restricted stock and non-vested restricted stock units. A reconciliation between the numerators and denominators of the basic and diluted EPS computations for continuing operations is as follows:
Year Ended December 31, 2009
Year Ended December 31, 2008
Year Ended December 31, 2007
Income
Shares
Per
Income
Shares
Per
Income
Shares
Per
(In thousands, except per share data) Loss from continuing operations
$
(90,853
)
$
(83,241
)
$
(49,155
) Basic EPS Loss from continuing operations attributable to common stock
(90,853
)
59,997
$
(1.51
)
(83,241
)
53,533
$
(1.55
)
(49,155
)
52,461
$
(0.94
) Effect of Dilutive Securities Common stock equivalents
—
—
— Diluted EPS Loss from continuing operations attributable to common stock and common stock equivalents
$
(90,853
)
59,997
$
(1.51
)
$
(83,241
)
53,533
$
(1.55
)
$
(49,155
)
52,461
$
(0.94
) The difference between basic and diluted weighted-average common shares generally results from the assumption that dilutive stock options outstanding were exercised and dilutive restricted stock and restricted stock units have vested. For the years ended 2009, 2008, and 2007, the Company reported a loss
from continuing operations and therefore, all potentially dilutive stock options, restricted stock and restricted stock units as of such date were excluded from the computation of diluted net loss per share as their effect would have been anti-dilutive. The total shares of stock options, restricted stock and restricted
stock units that could potentially dilute earnings per share in the future, but which were not included in the calculation of diluted net loss per share because their affect would have been anti-dilutive, were 3.2 million, 4.8 million and 4.3 million for the years ended December 31, 2009, 2008 and 2007, respectively. 100
(Numerator)
(Denominator)
Share
Amounts
(Numerator)
(Denominator)
Share
Amounts
(Numerator)
(Denominator)
Share
Amounts
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Note 10—Share-Based Compensation In 2001, the Company adopted the 2001 Stock Option Plan (the “2001 Stock Option Plan”). The 2001 Stock Option Plan permits the granting of options to purchase up to an aggregate of 10 million shares of the Company’s common stock to employees (including employees who are directors), non-employee
directors (“Directors”) and consultants of the Company. Under the 2001 Stock Option Plan, the Company may grant either incentive stock options, at an exercise price of not less than 100% of the fair market value of the underlying shares on the date of grant, or non-qualified stock options, at an exercise price not
less than 85% of the fair market value of the underlying shares on the date of grant. Options generally become exercisable ratably over two or four-year periods, with unexercised options expiring after the earlier of 10 years or shortly after termination of employment. Terminated options are available for
reissuance. In 2004, the Company adopted the 2004 Incentive Plan which superseded the 2001 Stock Option Plan. The 2004 Incentive Plan allows the Compensation Committee of the Company’s Board of Directors to award stock appreciation rights, restricted stock awards, restricted stock unit awards performance-based
awards and other forms of equity-based and cash incentive compensation, in addition to stock options. Under this plan, 2.5 million shares remain available for future grant at December 31, 2009. Total compensation cost that has been charged against income related to the above plans was $6.6 million, $6.7 million and $8.5 million for the years ended December 31, 2009, 2008 and 2007, respectively. The exercise of stock options and the vesting of restricted stock during the year ended December 31, 2009
generated an income tax deduction of approximately $10.6 million. The Company does not recognize a tax benefit with respect to an excess stock compensation deduction until the deduction actually reduces the Company’s income tax liability. At such time, the Company utilizes the net operating losses generated
by excess stock-based compensation to reduce its income tax payable, the tax benefit will be recorded as an increase in additional paid-in-capital. The total income tax benefit recognized in the consolidated statements of operations for share-based compensation arrangements was $0, $0 and $1.4 million for the years
ended December 31, 2009, 2008 and 2007, respectively. The following table summarizes stock-based compensation by expense category for the years ended December 31, 2009, 2008 and 2007:
Year Ended December 31,
2009
2008
2007
(In thousands) Research and development
$
3,188
$
3,153
$
2,854 Selling, general and administrative
3,456
3,578
5,639 Total non-cash compensation expense related to share-based compensation included in operating expense
$
6,644
$
6,731
$
8,493 Stock Options The Company grants stock options to employees and Directors with exercise prices equal to the fair market value of the underlying shares of the Company’s common stock on the date that the options are granted. Options granted have a term of 10 years from the grant date. Options granted to employees vest
over a four-year period and options granted to Directors vest in equal quarterly installments over a one-year period, from the date of grant. Options to Directors are granted on an annual basis and represent compensation for service performance on the Board of Directors. Compensation cost for stock options is
charged against income on a straight-line basis between the grant date for the option and each vesting date. The Company estimates the fair value of all stock option awards at the closing price on the grant date by applying the Black-Scholes option pricing valuation model. The application of this valuation model
involves assumptions that are highly 101
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) subjective, judgmental and sensitive in the determination of compensation cost. The weighted-average key assumptions used in determining the fair value of options granted during the years ended December 31, 2009, 2008 and 2007, are as follows:
Year Ended December 31,
2009
2008
2007 Weighted-average volatility
77
%
72
%
61
% Weighted-average risk-free interest rate
2.5
%
2.4
%
4.6
% Weighted-average expected life in years
6.6
6.2
6.0 Dividend yield
0.0
%
0.0
%
0.0
% Weighted-average grant date fair value
$
5.75
$
6.19
$
8.33 Historical information is the primary basis for the selection of the expected volatility and expected dividend yield. The expected terms of options granted prior to December 31, 2007 were based upon the simplified method. The simplified method estimates the expected term as the midpoint between vesting and
the grant contractual life. The expected terms of options granted subsequent to December 31, 2007 are based upon the Company’s historical experience for similar types of stock option awards. The risk-free interest rate is selected based upon yields of United States Treasury issues with a term equal to the expected
life of the option being valued. During the years ended December 31, 2009, 2008 and 2007, the Company issued 1,110,000 shares, 378,000 shares, and 607,000 shares, respectively, of the Company’s common stock upon the exercise of outstanding stock options and received proceeds of $6.7 million, $1.8 million, and $3.5 million, respectively.
For the year ended December 31, 2009, the Company realized no tax benefit from the exercise of stock options. For the years ended December 31, 2009, 2008 and 2007, approximately $4.0 million, $3.5 million and $3.3 million, respectively, of stock option compensation cost has been charged against income. As of
December 31, 2009, there was $2.0 million of unrecognized compensation cost, adjusted for estimated forfeitures, related to unamortized stock option compensation which is expected to be recognized over a remaining weighted-average period of approximately 1.9 years. Total unrecognized compensation cost will
be adjusted for future changes in estimated forfeitures. Stock option activity during the year ended December 31, 2009 was as follows:
Number of
Weighted-
Weighted-
Aggregate
(In thousands, except weighted-average data) Outstanding at December 31, 2008
3,563
$
8.29
7.27
$
3,242 Granted
694
8.38 Exercised
(1,110
)
6.08 Cancelled
(741
)
12.40 Outstanding at December 31, 2009
2,406
$
8.07
7.46
$
14,592 Exercisable at December 31, 2009
1,408
$
8.49
6.57
$
7,986 The weighted-average grant date per share fair value for options granted during the years ended December 31, 2009, 2008 and 2007 was $5.75, $6.19 and $8.33, respectively. The aggregate intrinsic value in the previous table reflects the total pretax intrinsic value (the difference between the Company’s closing
stock price on the last trading day of the period and the exercise price of the options, multiplied by the number of in-the-money stock options) that would have been received by the option holders had all option holders exercised their options on December 31, 2009. The intrinsic value of the Company’s stock
options changes based on the closing price of the Company’s common stock. The total intrinsic value of options exercised (the difference in the market price of the Company’s common stock on the exercise date and the price paid by the optionee to exercise the option) was approximately $6.9 million, $6.3 million
and $5.2 million for the years ended December 31, 2009, 2008 102
Shares
Average
Exercise
Price
Per Share
Average
Remaining
Contractual
Term (in yrs)
Intrinsic
Value of
In-the-Money
Options
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) and 2007, respectively. The closing price per share of the Company’s common stock was $13.61, $5.79 and $22.97 on December 31, 2009, 2008 and 2007, respectively. In September 2009, the Company completed a plan of termination pursuant to which it reduced its workforce by 25 employees. This termination was in addition to a previous termination of nine employees in August 2009. In connection with the plan of termination, the Company incurred charges of $1.1 million
in stock based compensation expense during the quarter ended December 31, 2009, as a result of modifications to stock option awards previously granted to the terminated employees. The modifications included extending the term of exercisability of the vested position of the stock options from three months
following cessation of employment to six months following cessation of employment, coupled with accelerating the vesting to immediately vest any unvested stock options. Stock Options Awards that Contain Performance or Market Conditions Performance or Market Conditions During the year ended December 31, 2009, the Company recorded compensation expense of $0 related to stock option awards to senior management personnel that contain performance or market conditions, the vesting of which is contingent upon the achievement of various specific strategic objectives by
December 31, 2010, including FDA marketing approval of KRYSTEXXA and other business development objectives. At December 31, 2009, approximately 102,000 potential option shares with performance or market conditions remain unvested. Stock option awards with performance or market conditions
encompass performance targets set for senior management personnel through the end of 2010 and could result in approximately $0.8 million of additional compensation expense if the performance targets are met or expected to be attained. The weighted-average key assumptions used in determining the fair value of stock option awards with performance or market conditions granted during the year ended December 31, 2009 were as follows:
Year Ended
2009 Weighted-average volatility
111
% Weighted-average risk-free interest rate
1.3
% Weighted-average expected life in years
3.1 Dividend yield
0.0
% Weighted-average grant date fair value
$
7.43 A summary of the status of the Company’s non-vested stock option awards that contain performance or market conditions as of December 31, 2009, and changes during the year ended December 31, 2009, is presented below:
Number of
Weighted-Average Grant
(In thousands) Non-vested at December 31, 2008
—
— Granted
340
7.91 Vested
—
— Forfeited
(238
)
5.37 Non-vested at December 31, 2009
102
$
13.81 The weighted-average grant date per share fair value for stock option awards that contain performance or market conditions granted during the year ended December 31, 2009 was $7.91. The 103
December 31,
Shares
Date Fair Value
Per Share
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) total fair value of stock option awards that contain performance or market conditions that vested during the year ended December 31, 2009 was $0. Restricted Stock The
Company grants restricted stock and restricted stock unit awards to its employees
and to its Directors. Restricted stock and restricted stock unit awards are
recorded as deferred compensation and amortized into compensation expense,
on a straight-line basis over the vesting period, which ranges from one to
four years in duration. Restricted stock and restricted stock unit awards
to Directors are granted on a yearly basis and represent compensation for
services performed as members of the Board of Directors. Restricted stock
awards to Directors vest in equal quarterly installments over a one-year
period from the grant date. Restricted stock unit awards to Directors vest
on the one year and 31 day anniversary of the grant date. Compensation cost
for restricted stock and restricted stock unit awards is based on the award’s
grant date fair value, which is the closing market price of the Company’s
common stock on the grant date, multiplied by the number of shares awarded.
For the year ended December 31, 2009, the Company issued 244,000 shares of
restricted stock and restricted stock units at a weighted-average grant date
fair value of $9.28 per share amounting to $2.3 million in total aggregate
fair market value. During the years ended December 31, 2009, 2008 and 2007,
approximately $2.7 million, $3.5 million and $2.7 million, respectively,
of deferred restricted stock compensation cost has been charged against income.
At December 31, 2009, approximately 500,000 shares remained unvested and
there was approximately $3.8 million of unrecognized compensation cost related
to restricted stock. A summary of the status of the Company’s non-vested restricted stock and restricted stock units as of December 31, 2009, and changes during the year ended December 31, 2009, is presented below:
Number of
Weighted-Average Grant
(In thousands) Non-vested at December 31, 2008
875
$
11.24 Granted
244
9.28 Vested
(412
)
10.54 Forfeited
(207
)
12.01 Non-vested at December 31, 2009
500
$
10.55 The weighted-average grant date per share fair value for restricted stock and restricted stock unit awards granted during the years ended December 31, 2009, 2008 and 2007 was $9.28, $9.98 and $14.41, respectively. The total fair value of restricted stock and restricted stock units vested during the years ended
December 31, 2009, 2008 and 2007, was $3.6 million, $3.1 million and $2.9 million, respectively. Restricted Stock Awards that Contain Performance or Market Conditions Performance Conditions During the year ended December 31, 2009, the Company recorded compensation expense of $0 related to restricted stock awards to senior management personnel that contain performance or market conditions, the vesting of which is contingent upon the achievement of various specific strategic objectives by
December 31, 2010, including FDA marketing approval of KRYSTEXXA and other business development objectives. At December 31, 2009, approximately 168,000 potential shares of restricted stock awards with performance or market conditions that were awarded in 2009 remain unvested, and could result in
approximately $2.3 million of additional compensation expense if the performance targets are met or expected to be achieved. During the year ended December 31, 2008, the Company reversed approximately $0.3 million of compensation expense that was previously charged against income, and for the year ended December 31, 2007, $2.4 million of compensation cost was charged against income related to restricted stock 104
Shares
Date Fair Value
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) awards issued in previous years that contain performance or market conditions. At December 31, 2009, approximately 49,000 potential shares of these restricted stock awards issued in previous years with performance or market conditions remain unvested. Restricted stock awards with performance conditions issued
in previous years encompass performance targets set for senior management personnel through 2011 and could result in approximately $0.9 million of additional compensation expense if the performance targets are met or expected to be achieved. A summary of the status of the Company’s non-vested restricted stock awards that contain performance or market conditions as of December 31, 2009, and changes during the year ended December 31, 2009, is presented below:
Number of
Weighted-Average Grant
(In thousands) Non-vested at December 31, 2008
351
$
15.68 Granted
168
13.56 Vested
—
— Forfeited
(302
)
15.23 Non-vested at December 31, 2009
217
$
14.67 The weighted-average grant date per share fair value for restricted stock awards that contain performance or market conditions granted during the years ended December 31, 2009, 2008 and 2007 was $13.56, $20.59 and $11.77, respectively. The total fair value of restricted stock awards that contain performance
conditions that vested during the years ended December 31, 2009, 2008 and 2007 was $0, $2.2 million and $2.2 million, respectively. Market Conditions During 2007, the Company issued a restricted stock award to its former President and Chief Executive Officer, the vesting of which was contingent upon the price of the Company’s common stock achieving a certain pre-established stock price target. The Company used a Monte Carlo simulation model to
calculate both the grant date fair value and the derived requisite service period of the award. Based on the simulation, the grant date fair value of the award was calculated to be $8.98 per share and compensation cost was being charged against income ratably over a two-year derived requisite service period. For
restricted stock awards that contain market conditions, compensation cost is charged against income regardless of whether the market condition is ever achieved and is reversed only if the derived requisite service period is not met by the recipient of the award. As a result of the resignation of employment of the
Company’s former President and Chief Executive Officer in November 2008, the derived requisite service period for this award was not met, and therefore, the Company reversed approximately $1.5 million of compensation expense that was previously charged against income during 2007 and 2008. The key assumptions used in determining the grant date fair value and the requisite service period for the restricted stock award that contained a market condition were as follows:
Period
Interest Rate
Historical
Cost of 2.00 years
4.9
%
46.4
%
14.9
% Employee Stock Purchase Plan In April 1998, the Company adopted its 1998 Employee Stock Purchase Plan (the “1998 ESPP”). The 1998 ESPP is qualified as an employee stock purchase plan under Section 423 of the Internal Revenue Code of 1986, as amended (the “IRC”). Prior to the adoption of FASB ASC 718, Share-Based Payment,
and under the accounting guidance that preceded FASB ASC 718, the 1998 ESPP was 105
Shares
Date Fair Value
Term
Structure
Volatility
Calculation
Equity
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) considered to be non-compensatory. Under the 1998 ESPP, the Company will grant rights to purchase shares of common stock under the 1998 ESPP (“Rights”) at prices not less than 85% of the lesser of (i) the fair market value of the shares on the date of grant of such Rights or (ii) the fair market value of the
shares on the date such Rights are exercised. Therefore, the 1998 ESPP is considered compensatory under FASB ASC 718 since, along with other factors, it includes a purchase discount of greater than 5%. During the years ended December 31, 2009, 2008 and 2007, the Company recorded approximately $0.1
million, $0.7 million and $0.2 million, respectively, of compensation expense related to participation in the 1998 ESPP. Additional Paid-In-Capital Excess Tax Benefit Pool Excess tax benefits are used to create an additional paid-in-capital pool and any tax deficiencies are used to offset the pool, if available, or recorded as an additional charge to tax expense during the year. As of December 31, 2009, the additional paid-in-capital excess tax benefit pool available to absorb future
tax deficiencies was approximately $2.5 million. Note 11—Employee Benefits 401(k) Profit-Sharing Plan The Company has a 401(k) profit-sharing plan. During 2009, the 401(k) plan permitted employees who meet age and service requirements to contribute up to $16,500 of their total compensation on a pretax basis, which was 25% Company matched. For 2010, employees will be permitted to contribute up to
$16,500 of their total compensation on a pretax basis, which will be 50% Company matched. The Company’s contribution to the plan amounted to approximately $0.2 million, $0.5 million, and $0.4 million for the years ended December 31, 2009, 2008 and 2007, respectively. Note 12—Investment Income, Net
Year Ended December 31,
2009
2008
2007
(In thousands) Interest and dividend income from cash equivalents
$
107
$
2,035
$
8,749 Realized gains on sales of short-term investments
834
141
337 Realized losses on redemptions of short-term investments
(652
)
(507
)
(21
) Realized losses resulting from OTT impairment write-downs of short-term investments
—
(523
)
(310
) Total investment income, net
$
289
$
1,146
$
8,755 Note 13—Other Income (Expense), Net
Year Ended December 31,
2009
2008
2007
(In thousands) Realized loss on change in valuation of warrant liability
$
(11,678
)
$
—
$
— Other non-operating expenses
(373
)
(393
)
(543
) Total other income (expense), net
$
(12,051
)
$
(393
)
$
(543
) Note 14—Income Taxes The domestic components of loss from continuing operations before income taxes were $92.9 million, $88.3 million and $61.0 million for the years ended December 31, 2009, 2008 and 2007, respectively. 106
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) The components of current and deferred income tax benefit from continuing operations are as follows:
Year Ended December 31,
2009
2008
2007
(In thousands) Current State
$
(65
)
$
(221
)
$
(39
) Federal
(2,006
)
(5,650
)
(11,210
)
(2,071
)
(5,871
)
(11,249
) Deferred Federal
—
854
(558
) Income tax benefit from continuing operations
$
(2,071
)
$
(5,017
)
$
(11,807
) Deferred income tax assets (liabilities) are recognized for the tax consequences of temporary differences by applying the enacted statutory tax rates to differences between the financial statement carrying amounts and the tax basis of existing assets and liabilities and for capital and net operating losses and tax
credit carryforwards. Deferred tax assets are items that can be used as a tax deduction or credit in future periods. Deferred tax liabilities are items for which the Company receives a tax deduction which has not yet been recorded in the consolidated statement of operations. A valuation allowance is provided for deferred tax assets if, in management’s judgment, it is more likely than not that some portion or all of a deferred tax asset will not be realized. There are multiple factors that are considered when assessing the likelihood of future realization of deferred tax assets, including
historic operating results, expectations of future taxable income, the carryforward periods available to utilize tax attributes and other relevant factors. In making this assessment, substantial judgment is required. The components of deferred income tax assets/liabilities are as follows:
Year Ended December 31,
2009
2008
Assets
Liabilities
Assets
Liabilities
(In thousands) Net operating loss carryforward
$
42,327
$
—
$
14,121
$
— Research and experimental credits
52,497
—
42,525
— State NOL carryforward
17,486
—
11,513
— Employee-based compensation
4,736
—
5,005
— Foreign tax credits
4,026
—
4,026
— Inventories
3,588
—
6,428
— State tax reserves
1,428
—
1,452
— Accrued amounts
1,405
—
1,274
— Capital loss carryforwards
1,078
—
814
— Other tax credits
676
—
2,639
— State credits
26
—
26
— Other
926
—
1,372
— Prepaids
—
(157
)
—
(177
)
$
130,199
$
(157
)
$
91,195
$
(177
) Valuation allowance
(125,842
)
—
(86,818
)
— Total deferred income tax assets/(liabilities)
4,357
(157
)
4,377
(177
) Net deferred income tax asset*
$
4,200
$
4,200 107
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
*
As of December 31, 2009 and 2008, $4.2 million and $4.2 million, respectively, of the net deferred tax assets were offset by an unrecognized tax benefit reserve, recorded as components of Long-Term Other Liabilities on the Company’s consolidated balance sheets.
Reconciliation of income taxes between the Company’s effective tax rate on loss before income taxes and the statutory tax rate is as follows:
Year Ended December 31,
2009
2008
2007
(In thousands) Income tax at US statutory rate
$
(32,523
)
$
(30,890
)
$
(21,337
) State and local income taxes (net of federal benefit)
(5,366
)
(144
)
(25
) Non-deductible expenses
6,806
4,067
382 Research and experimental credits
(9,948
)
(10,592
)
(13,454
) Foreign taxes (net of foreign tax credits)
—
—
(1,863
) State NOL and tax credits
—
—
(3,046
) Benefit for unrecognized tax benefits
(64
)
(125
)
(1,561
) Change in valuation allowance
39,024
32,690
29,196 Other
—
(23
)
(99
) Income tax benefit from continuing operations
$
(2,071
)
$
(5,017
)
$
(11,807
) For the year ended December 31, 2009, the Company generated a net operating loss of approximately $80.3 million for federal income tax purposes and had federal net operating loss carryforwards of approximately $120.9 million, $4.4 million of which is subject to a separate company IRC Section 382
limitation. The Company also had combined state operating loss carryforwards of approximately $194.3 million. The federal and state net operating loss carryforwards expire at various dates from 2011 through 2029. Included in the net operating loss deferred tax assets above is approximately $5.6 million of deferred
tax asset attributable to excess stock option deductions. Due to a provision within FASB ASC 718 concerning when tax benefits related to excess stock option deductions can be credited to paid-in-capital, the related valuation allowance cannot be reversed even if the facts and circumstances indicate that it is more
likely than not that the deferred tax assets can be realized. The valuation allowance will only be reversed as the related deferred tax asset is applied to reduce taxes payable. The Company follows FASB ASC 740 ordering to determine when such net operating losses have been realized. At December 31, 2009, the Company also had federal tax credit carryforwards of approximately $54.3 million, which are comprised primarily of credits related to orphan drug and research and development expenses and foreign tax payments. These credits are available to reduce future income taxes and expire
at various times through 2029. Due to the change of ownership provisions of the Tax Reform Act of 1986, utilization of a portion of the Company’s domestic net operating loss and tax credit carryforwards may be subject to an annual limitation in the event of a change in ownership as defined by Section 382 of the Internal Revenue Code
and similar state provisions. Further, a portion of the carryforwards may expire before being applied to reduce future income tax liabilities. Based upon the uncertainty of the Company’s business and history of operating losses, the likelihood that the Company will be able to realize a benefit on its deferred tax assets is uncertain. Due to this uncertainty, the Company maintained a valuation allowance as of December 31, 2009 of $125.8 million
against its deferred tax assets except for those assets that are reserved by a liability for unrecognized tax benefits. The increase in valuation allowance from 2009 compared to 2008 of approximately $39.0 million is primarily due to an increase in net operating loss carryforwards and tax credits and the uncertainty
that the additional deferred tax assets will be realized. 108
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) The Company files income tax returns in the United States and various state jurisdictions. The United States federal income tax returns for the fiscal years ended December 31, 2008, 2007 and 2006 are currently under examination by the Internal Revenue Service. State income tax returns are generally subject
to examination for a period of three to five years subsequent to the filing of the respective tax return. The State of New Jersey has recently contacted the Company about beginning an income tax audit for the 2005 through 2008 tax years. This audit will also encompass a review of the payroll tax and sales and use
tax returns for those tax years. While the Company believes that its reserves reflect the probable outcome of identified contingencies, it is reasonably possible that the ultimate resolution of any tax matter may be more or less than the amount accrued. However, the Company believes that the result of these audits
will not have a material effect on its financial position. In 2009, BTG settled its examination with the Israeli Taxing Authorities for the tax years 2003 through 2005. The result of this examination required the Company to pay the new owners of BTG approximately $1.6 million, including interest. This liability had been accrued for as part of the current liabilities for
unrecognized tax benefits as of December 31, 2008 and was paid in 2009. Unrecognized tax benefits represented by liabilities on the balance sheet, arise when the estimated tax benefit in the financial statements differs from the amount taken or expected to be taken on a tax return due to the uncertainty of tax positions. The Company regularly evaluates its tax positions for additional unrecognized tax benefits and associated interest and penalties, if applicable. The Company believes that its accrual for tax liabilities is adequate for all open years. There are many factors that are considered when evaluating these tax positions
including; interpretation of tax laws, recent tax litigation on a position, past audit or examination history, and subjective estimates and assumptions. In making these estimates and assumptions, the Company relies on advice from industry and subject matter experts, analyzes actions taken by taxing authorities, as well
as the Company’s industry and audit experience. These evaluations are based on estimates and assumptions that have been deemed reasonable by management. However, if management’s estimates are not representative of actual outcomes, the Company’s results could be materially impacted. Reconciliation of the beginning and ending amount of unrecognized tax benefits:
2009
2008
2007
(In thousands) Unrecognized Tax Benefits—January 1,
$
11,211
$
8,701
$
4,586 Increases related to tax positions in prior period
471
568
2,901 Decreases related to tax positions in prior period
(5
)
(541
)
(386
) Increases related to tax positions in current period
—
2,614
1,600 Decreases related to tax positions in current period
—
(6
)
— Settlements/Lapses
(1,666
)
(125
)
— Unrecognized Tax Benefits—December 31
$
10,011
$
11,211
$
8,701 Included in the unrecognized tax benefit liability of $10.0 million as of December 31, 2009, $11.2 million as of December 31, 2008 and $8.7 million as of December 2007, is $5.8 million, $7.0 million and $5.7 million, respectively, of tax benefit that if recognized would impact the Company’s effective tax rate. The
difference of $4.2 million, $4.2 million and $3.0 million as of December 31, 2009, 2008 and 2007, respectively, is due to deferred tax accounting, in which a portion of the Company’s uncertain tax positions would result in adjustments to the Company’s deferred tax assets. This would be offset by adjustments to
recorded valuation allowances to provide no effect on the Company’s effective tax rate. The Company does not expect the unrecognized tax benefits to materially increase or decrease in the next 12 months. The Company accounts for interest and penalties related to unrecognized tax benefits as part of Other Income (Expense), net on the consolidated statements of operations. During the fiscal year 109
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) ended December 31, 2009, the Company recorded an increase in interest expense and penalties of $0.3 million and $0.2 million, respectively. As of December 31, 2009, the Company has accrued approximately $1.1 million of interest expense and $0.6 million of penalties related to the unrecognized tax benefit
liability which was recorded within Other Liabilities on the consolidated balance sheet. Note 15—Discontinued Operations A summary statement of discontinued operations of the former BTG and Rosemont businesses for the years ended 2009, 2008, and 2007, as it was included in the consolidated financial statements of the Company, is shown below.
Year Ended December 31,
2009
2008
2007
(In thousands) Revenues: Product sales, net
$
—
$
—
$
— Other revenues
—
—
—
—
—
— Cost and expenses: Cost of goods sold
—
—
— Research and development
—
—
— Selling and marketing
—
—
— General and administrative
—
—
— Amortization of intangibles
—
—
— Commissions and royalties
—
—
—
—
—
— Operating income from discontinued operations
—
—
— Other income (expense), net
—
(327
)
298 Income (loss) from discontinued operations before income taxes
—
(327
)
298 Income tax expense (benefit)
—
601
(189
) Income (loss) from discontinued operations
$
—
$
(928
)
$
487 During the year ended December 31, 2008, the Company incurred a $0.9 million tax assessment as a result of a tax audit for the 2003 through 2005 tax years that related to corporate income tax returns filed in Israel by BTG, the Company’s former global biologics manufacturing business in Israel, which the
Company sold to Ferring in 2005. Discontinued operations for 2007 includes tax refunds related to BTG that the Company received from the Israeli taxing authorities and foreign currency exchange gains on those tax refunds. The tax refund was due to an overpayment of estimated taxes to Israel for the 2005 short
tax year. The Company did not have any revenues or operating income from discontinued operations for the years ended December 31, 2009, 2008 and 2007. Note 16—Concentrations In the United States, the Company sells Oxandrin and oxandrolone, which accounted for 100% of net product sales from continuing operations for each of the years ended 2009, 2008 and 2007, respectively. The Company distributes Oxandrin through Integrated Commercialization Services, Inc. (“ICS”), a
third-party logistics organization. Oxandrolone is distributed through an agreement with Watson Pharma Inc. (“Watson”) in the United States. The Company sells Oxandrin in the United States mainly to three drug wholesaler customers; AmerisourceBergen Corp., the parent of ICS, Cardinal Health and McKesson
Corp. The Company’s gross sales to AmerisourceBergen Corp were 30%, 36% and 31% of total gross sales in 2009, 2008 and 2007, respectively. The Company’s gross sales to Cardinal Health were 19%, 29% and 32% of total gross sales in 2009, 2008 and 2007, respectively. 110
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) The Company’s gross sales to McKesson Corp. were 25%, 19% and 19% of total gross sales in 2009, 2008 and 2007, respectively. Generic competition for Oxandrin began in December 2006 and the introduction of generic products has produced significant decreases in the Company’s Oxandrin revenues, which has had a material adverse effect on the Company’s results of operations, cash flows, financial condition and profitability. The Company is dependent on third parties for the manufacture of Oxandrin. The Company’s dependence upon third parties for the manufacture of this product may adversely impact its profit margins or result in unforeseen delays or other problems beyond its control. During 2009, the Company was notified
by DSM Pharmaceuticals, Inc. (“DSM”), the primary manufacturer for Oxandrin and authorized generic oxandrolone tablets, of its intent to terminate the commercial supply agreement with the Company, as of December 31, 2010. At this time, the Company does not intend to seek an alternate manufacturer for
Oxandrin and authorized generic oxandrolone tablets. The Company has planned on manufacturing and stockpiling sufficient quantities of Oxandrin and authorized generic oxandrolone in 2010 prior to the termination of the supply agreement to adequately commercial supply the products to its customers for at
least the next two years. If for any reason the Company is unable to retain these third-party distributors and manufacturers, or obtain alternate third-party distributors and manufacturers on commercially acceptable terms, the Company may not be able to distribute its products as planned. If the Company encounters delays or
difficulties with contract manufacturers in producing this product, the sale of this product would be adversely affected. Note 17—Segment Information The Company currently operates within one “Specialty Pharmaceutical” segment which includes sales of Oxandrin and oxandrolone and the research and development activities of KRYSTEXXA. 111
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued) Note 18—Quarterly Data (Unaudited) Following are the quarterly results of operations for the years ended December 31, 2009 and 2008.
Quarter Ended March 31,
2009
2008
(In thousands, except per Revenues: Product sales, net
$
1,085
$
1,144 Other revenues
3
44
1,088
1,188 Cost and expenses: Cost of goods sold
491
333 Research and development
12,763
11,161 Selling general and administrative
9,468
9,264
22,722
20,758 Operating loss from continuing operations
(21,634
)
(19,570
) Investment income (expense), net
(202
)
953 Other expense, net
(113
)
(150
) Loss from continuing operations before income taxes
(21,949
)
(18,767
) Income tax benefit
—
(1,215
) Net loss
$
(21,949
)
$
(17,552
) Loss per common share: Basic and diluted
$
(0.41
)
$
(0.33
) Weighted-average number of common and common equivalent shares: Basic and diluted.
53,983
53,276 112
share data)
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Quarter Ended June 30,
2009
2008
(In thousands, except per Revenues: Product sales, net
$
679
$
315 Other revenues
1
38
680
353 Cost and expenses: Cost of goods sold
372
219 Research and development
11,638
15,726 Selling, general and administrative
7,397
10,450
19,407
26,395 Operating loss from continuing operations
(18,727
)
(26,042
) Investment income (expense), net
(27
)
411 Other expense, net
(36,055
)
(162
) Loss from continuing operations before income taxes
(54,809
)
(25,793
) Income tax benefit
—
(1,595
) Net loss
$
(54,809
)
$
(24,198
) Loss per common share: Basic and diluted
$
(0.92
)
$
(0.45
) Weighted-average number of common and common equivalent shares: Basic and diluted
59,594
53,542 113
share data)
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Quarter Ended
2009
2008
(In thousands, except
per share data) Revenues: Product sales, net
$
328
$
499 Other revenues
—
43
328
542 Cost and expenses: Cost of goods sold
374
303 Research and development
17,670
10,875 Selling, general and administrative
8,538
7,563
26,582
18,741 Operating loss from continuing operations
(26,254
)
(18,199
) Investment income, net
502
340 Other income (expense), net
11,812
(148
) Loss from continuing operations before income taxes
(13,940
)
(18,007
) Income tax benefit
(64
)
(863
) Loss from continuing operations
(13,876
)
(17,144
) Loss from discontinued operations, net of income taxes
—
(1,070
) Net loss
$
(13,876
)
$
(18,214
) Loss per common share from continuing operations: Basic and diluted
$
(0.23
)
$
(0.32
) Loss per common share from discontinued operations: Basic and diluted
$
—
$
(0.02
) Loss per common share: Basic and diluted
$
(0.23
)
$
(0.34
) Weighted-average number of common and common equivalent shares: Basic and diluted
60,922
53,617 114
September 30,
SAVIENT PHARMACEUTICALS, INC. NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Quarter Ended December 31,
2009
2008
(In thousands, except per Revenues: Product sales, net
$
864
$
1,070 Other revenues
—
28
864
1,098 Cost and expenses: Cost of goods sold
369
299 Research and development
9,655
17,726 Selling, general and administrative
5,387
8,273
15,411
26,298 Operating loss from continuing operations
(14,547
)
(25,200
) Investment income (expense), net
16
(558
) Other income, net
12,305
67 Loss from continuing operations before income taxes
(2,226
)
(25,691
) Income tax benefit
(2,007
)
(1,344
) Loss from continuing operations
(219
)
(24,347
) Income from discontinued operations, net of income taxes
—
142 Net loss
$
(219
)
$
(24,205
) Loss per common share from continuing operations: Basic and diluted
$
—
$
(0.45
) Earnings (loss) per common share from discontinued operations: Basic and diluted
$
—
$
— Loss per common share: Basic and diluted
$
—
$
(0.45
) Weighted-average number of common and common equivalent shares: Basic and diluted
65,353
53,694 115
share data)
SAVIENT PHARMACEUTICALS, INC. Schedule II—Valuation and Qualifying Accounts
Description
Balance at
Charged to
Charged
Deductions
Balance at
(In thousands) Allowance for inventory obsolescence 2009
$
6,323
$
88
—
$
(5,154
)
$
1,257 2008
$
7,917
$
409
$
(363
)
$
(1,640
)
$
6,323 2007
$
8,305
$
67
—
$
(455
)
$
7,917 Allowance for sales returns (1) 2009
1,163
2,166
—
(2,296
)
1,033 2008
905
2,848
—
(2,590
)
1,163 2007
2,452
(193
)
—
(1,354
)
905 Allowance for rebates (1) 2009
643
374
126
(882
)
261 2008
1,003
1,599
(28
)
(1,931
)
643 2007
1,253
1,895
(138
)
(2,007
)
1,003 Allowance for doubtful accounts 2009
2
16
—
—
18 2008
206
(160
)
(49
)
5
2 2007
1,036
175
(699
)
(306
)
206 Valuation allowance—Deferred income taxes short-term 2009
4,900
(1,637
)
—
—
3,263 2008
4,981
(81
)
—
—
4,900 2007
6,005
(1,024
)
—
—
4,981 Valuation allowance—Deferred income taxes long-term 2009
81,918
40,661
—
—
122,579 2008
35,989
45,929
—
—
81,918 2007
5,770
30,219
—
—
35,989 Restructuring Reserve 2009
—
2,330
—
(1,838
)
492 2008
—
—
—
—
— 2007
—
—
—
—
—
(1)
Included within Other Current Liabilities in the Company’s consolidated balance sheets.
116
Beginning
of
Period
Costs and
Expenses
to other
Accounts
End of
Period
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE None ITEM 9A. CONTROLS AND PROCEDURES (a). Evaluation of Disclosure Controls and Procedures We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is recorded, processed, summarized and reported within the time periods specified in the SEC’s
rules and forms, and that such information is accumulated and communicated to management, including our President and Chief Financial Officer (“CFO”) as appropriate, to allow timely decisions regarding required disclosure. Management, with the participation of our President and CFO, evaluated the effectiveness of our disclosure controls and procedures. Based on their evaluation, as of the end of the period covered by this Form 10-K, our President and CFO have concluded that our disclosure controls and procedures (as defined
in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) were effective. (b). Management’s Report on Internal Control Over Financial Reporting Management’s
report on our internal control over financial reporting is included on page
70. (c). Changes in Internal Control Over Financial Reporting No changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the quarter ended December 31, 2009 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. (d). Report of Independent Registered Accounting Firm The
report of our independent registered public accounting firm related to their
assessment of internal control over financial reporting is included on page
72. ITEM 9B. OTHER INFORMATION None 117
PART III ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE Directors The information concerning our directors required under this Item is incorporated herein by reference from our definitive proxy statement, to be filed pursuant to Regulation 14A, related to our 2010 Annual Meeting of Stockholders to be held on May 10, 2010 (our “2010 Proxy Statement”). Information regarding our director nomination process, audit committee and audit committee financial expert as required by the SEC’s Regulation S-K 407(c)(3), 407(d)(4) and 407(d)(5) is set forth in our 2010 Proxy Statement and is incorporated herein by reference. Executive Officers The information concerning our executive officers required under this Item is contained in the discussion under the heading Our Executive Officers in Part I of this Annual Report on Form 10-K. Section 16(a) Compliance Information concerning compliance with Section 16(a) of the Exchange Act is set forth in the Section 16(a) Beneficial Ownership Reporting Compliance segment of our 2010 Proxy Statement and is incorporated herein by reference. Code of Ethics We have adopted a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer and all other Savient employees performing similar functions. This code of ethics has been posted on our website, which can be found at http://www.savientpharma.com. We
intend to satisfy the disclosure requirement under Item 5.05(c) of Form 8-K regarding an amendment to or waiver from a provision of our code of ethics by posting such information on our website at the address specified above. We have filed as exhibits to this Annual Report on Form 10-K for the year ended December 31, 2009, the certifications of our Principal Executive Officer and Principal Financial Officer required pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. ITEM 11. EXECUTIVE COMPENSATION The information concerning executive compensation required under this Item is incorporated herein by reference from our 2010 Proxy Statement. ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS The information concerning security ownership of certain beneficial owners and management required under this Item is incorporated herein by reference from our 2010 Proxy Statement. ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE The information concerning certain relationships and related transactions required under this Item is incorporated herein by reference from our 2010 Proxy Statement. ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES. The information concerning principal accountant fees and services required under this Item is incorporated herein by reference from our 2010 Proxy Statement. 118
PART IV ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES (a) Financial Statements (1) and (2) See “Index to Consolidated Financial Statements” contained in Item 8 of this Annual Report on Form 10-K. (b) Exhibits Certain exhibits presented below contain information that has been granted or is subject to a request for confidential treatment. Such information has been omitted from the exhibit. Exhibit Nos. 10.4 through 10.11, 10.13 through 10.23 and 10.29 through 10.35 are management contracts, compensatory plans or
arrangements.
Exhibit
Description 2.1
Agreement and Plan of Reorganization, dated as of February 21, 2001, by and among Bio-Technology General Corp., MYLS Acquisition Corp. and Myelos Corporation.*(1) 2.2
Share Purchase Agreement, dated September 20, 2002, relating to Rosemont Pharmaceuticals Limited between NED-INT Holdings Ltd, Akzo Nobel N.V. and the Company.*(2) 2.3
Share Purchase Agreement, dated March 23, 2005, between the Company and Ferring B.V.*(3) 2.4
Asset Purchase Agreement, dated March 23, 2005, between the Company and Ferring International Centre SA.*(3) 3.1
Certificate of Incorporation of the Company, as amended.*(4) 3.2
By-laws of the Company, as amended.*(5) 4.1
Certificate of Designations of the Series A Junior Participating Cumulative Preferred Stock.*(5) 10.1
Letter from the Chief Scientist to Bio-Technology General (Israel) Ltd.*(6) 10.2
Agreement, dated January 20, 1984, between Bio-Technology General (Israel) Ltd. and the Chief Scientist with regard to certain projects.*(7) 10.3
Form of Indemnity Agreement between the Company and its directors and officers.*(8) 10.4
Bio-Technology General Corp. Stock Compensation Plan for Outside Directors, as amended.*(9) 10.5
Bio-Technology General Corp. Stock Option Plan for New Directors, as amended.*(9) 10.6
Bio-Technology General Corp. 1992 Stock Option Plan, as amended.*(10) 10.7
Bio-Technology General Corp. 1997 Stock Option Plan for Non-Employee Directors.*(10) 10.8
Savient Pharmaceuticals, Inc. 1998 Employee Stock Purchase Plan Amended and Restated as of May 23, 2006.*(11) 10.9
Bio-Technology General Corp. 2001 Stock Option Plan.*(12) 10.10
Employment Agreement, dated as of May 14, 2002, by and between the Company and Christopher Clement.*(13) 10.11
Employment Agreement, dated as of March 24, 2003, by and between the Company and Zebulun D. Horowitz, M.D.*(14) 10.12
Lease and Lease Agreement, dated as of June 11, 2002, between SCV Partners and the Company, as amended.*(15) 10.13
Employment Agreement, dated as of May 28, 2004, between the Company and Philip K. Yachmetz.*(16) 10.14
Employment Agreement, dated as of February 15, 2006, by and between the Company and Robert Lamm.*(17) 119
No.
Exhibit
Description 10.15
Amendment, dated February 15, 2006, to Employment Agreement dated May 28, 2004 by and between the Company and Philip K. Yachmetz.*(17) 10.16
Amendment, dated July 12, 2004, to Employment Agreement dated May 14, 2002 by and between the Company and Christopher Clement.*(17) 10.17
Amendment, dated December 7, 2006, to Employment Agreement dated as of March 24, 2003 by and between the Company and Zebulun D. Horowitz, M.D.*(18) 10.18
Form of Savient Pharmaceuticals, Inc. Senior Management Incentive Stock Option Agreement.*(18) 10.19
Form of Savient Pharmaceuticals, Inc. Senior Management Non-Qualified Stock Agreement.*(18) 10.20
Form of Savient Pharmaceuticals, Inc. Senior Management Restricted Stock Agreement.*(18) 10.21
Form of Savient Pharmaceuticals, Inc. Senior Management Performance Share Agreement.*(18) 10.22
Form of Savient Pharmaceuticals, Inc. Board of Directors Non-Qualified Stock Agreement.*(18) 10.23++
License Agreement, dated August 12, 1998, by and among Mountain View Pharmaceuticals, Inc., Duke University, and the Company, as amended on November 12, 2001.*(18) 10.24++
Supply Agreement, dated as of June 12, 2006, by and between Watson Pharma Inc. and the Company.*(18) 10.25++
Commercial Supply Agreement, dated October 16, 2008, by and between Enzon Pharmaceuticals, Inc. and the Company.*(19) 10.26++
Commercial Supply Agreement, dated March 20, 2007, between the Company and Bio-Technology General (Israel) Ltd.*(20) 10.27++
Supply Agreement, dated May 23, 2007, between the Company and NOF Corporation.*(20) 10.28
Amendment, dated December 19, 2008, to Employment Agreement dated March 23, 2006, by and between the Company and Paul Hamelin, as amended.*(21) 10.29
Amendment, dated February 15, 2008, to Employment Agreement dated March 23, 2006 by and between the Company and Paul Hamelin.* (22) 10.30
Amendment, dated February 15, 2008, to Employment Agreement dated May 28, 2004 by and between the Company and Philip K. Yachmetz.* (22) 10.31
Amendment, dated February 15, 2008, to Employment Agreement dated March 23, 2003 by and between the Company and Zebulun D. Horowitz, M.D.* (22) 10.32
Amendment, dated February 15, 2008, to Employment Agreement dated May 14, 2002 by and between the Company and Christopher Clement.* (22) 10.33
Amendment, dated February 15, 2008, to Employment Agreement dated July 5, 2006 by and between the Company and Brian Hayden.* (22) 10.34
Amendment, dated February 15, 2008, to Employment Agreement dated February 15, 2006 by and between the Company and Robert Lamm.* (22) 10.35
Amended and Restated Employment Agreement dated February 12, 2009 by and between the Company and David Gionco.* (23) 10.36
Consulting Services Agreement between the Company and Lee S. Simon, MD.* (24) 10.37
Form of Subscription Agreement executed in connection with the Company’s April 2009 registered direct offering.* (25) 10.38
Form of Warrant issued in connection with the Company’s April 2009 registered direct offering.* (25) 21.1
Subsidiaries of the Company. 120
No.
Exhibit
Description 23.1
Consent of McGladrey & Pullen, LLP 31.1
Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. 31.2
Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. 32.1
Certification of the Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. 32.2
Certification of the Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. Stockholders of the Company will be provided with copies of these exhibits upon written request to the Company.
++
Confidential treatment requested as to certain portions, which portions are omitted and filed separately with the SEC. * Previously filed with the SEC as Exhibits to, and incorporated herein by reference from, the following documents: (1) Company’s Current Report on Form 8-K, dated March 19, 2001. (2) Company’s Current Report on Form 8-K, dated September 30, 2002. (3) Company’s Current Report on Form 8-K, dated March 23, 2005. (4) Registration Statement on Form S-3 (File No. 333-146257). (5) Company’s Current Report on Form 8-K, dated October 9, 1998. (6) Registration Statement on Form S-1 (File No. 2-84690). (7) Registration Statement on Form S-1 (File No. 033-02597). (8) Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 1987. (9) Company’s Annual Report on Form 10-K for the year ended December 31, 1991. (10) Company’s Annual Report on Form 10-K for the year ended December 31, 1997. (11) Company’s Annual Report on Form 10-K for the year ended December 31, 2007. (12) Company’s Annual Report on Form 10-K for the year ended December 31, 2001. (13) Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2002. (14) Company’s Annual Report on Form 10-K for the year ended December 31, 2002. (15) Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2002. (16) Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004. (17) Company’s Annual Report on Form 10-K for the year ended December 31, 2005. (18) Company’s Annual Report on Form 10-K for the year ended December 31, 2006. (19) Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2008. (20) Company’s Current Report on Form 8-K, filed February 10, 2009. (21) Company’s Current Report on Form 8-K, dated December 19, 2008. (22) Company’s Annual Report on Form 10-K for the year ended December 31, 2008. (23) Company’s Current Report on Form 8-K, dated February 19, 2009. (24) Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2009. (25) Company’s Current Report on Form 8-K, dated April 2, 2009. 121
No.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. SAVIENT PHARMACEUTICALS, INC.
By: /s/ PAUL HAMELIN Paul Hamelin President March 1, 2010 Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature
Title
Date
/s/ PAUL HAMELIN Paul Hamelin President (Principal Executive Officer) March 1, 2010 /s/ HERBERT CONRAD Herbert Conrad Director March 1, 2010 /s/ GINGER D. CONSTANTINE Ginger D. Constantine Director March 1, 2010 /s/ ALAN L. HELLER Alan L. Heller Director March 1, 2010 /s/ STEPHEN O. JAEGER Stephen O. Jaeger Director March 1, 2010 /s/ JOSEPH KLEIN III Joseph Klein III Director March 1, 2010 /s/ LEE S. SIMON, M.D. Lee S. Simon, M.D. Director March 1, 2010 /s/ VIRGIL THOMPSON Virgil Thompson Director March 1, 2010 /s/ DAVID G. GIONCO David G. Gionco Group Vice President, March 1, 2010 122
(Registrant)
Chief Financial Officer and Treasurer
(Principal Financial Officer)
