Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Riot Blockchain, Inc. | appy_8k.htm |
Exhibit 99.1

AspenBio Pharma
Company Overview
“Advancing Medical Technology”
Lazard Medical Technology
Conference
February,
2010

Safe
Harbor Statement
Certain statements
made in this presentation include historical
information and forward looking actions that AspenBio Pharma
anticipates based on certain assumptions. These statements are
indicated by words such as “expect”, “anticipate”, “should” and similar
words are indicating uncertainty in facts, figures and outcomes. While
AspenBio Pharma believes that the expectations reflected in such
forward-looking statements are reasonable, it can give no assurance that
such statements will prove to be correct. The risks associated with the
company are detailed in the Company’s Annual Report on Form 10-K for
the year ended December 31, 2008 and other reports filed by the
Company with the Securities and Exchange Commission.
information and forward looking actions that AspenBio Pharma
anticipates based on certain assumptions. These statements are
indicated by words such as “expect”, “anticipate”, “should” and similar
words are indicating uncertainty in facts, figures and outcomes. While
AspenBio Pharma believes that the expectations reflected in such
forward-looking statements are reasonable, it can give no assurance that
such statements will prove to be correct. The risks associated with the
company are detailed in the Company’s Annual Report on Form 10-K for
the year ended December 31, 2008 and other reports filed by the
Company with the Securities and Exchange Commission.
2

AspenBio
Focus
Molecular
Ingenuity Creating Novel Clinical Solutions!
•Blood
based
•Rapid
•IP
Protected
• Dedicated
Instrument
and Consumables
and Consumables
Veterinary
Science
•Animals of
economic
importance
importance
•IP
Protected
•Recombinant
Protein
Drug platform
Drug platform
•Enhances
reproductive
efficiency
efficiency
AspenBio Pharma’s
mission is to
be a leader in the development
and commercialization of
innovative products that address
unmet diagnostic and therapeutic
needs in both Human and Animal
Health.
be a leader in the development
and commercialization of
innovative products that address
unmet diagnostic and therapeutic
needs in both Human and Animal
Health.
Our
Animal Health Division is
focused on therapeutic proteins
that support reproductive
efficiency in non-companion
animals.
focused on therapeutic proteins
that support reproductive
efficiency in non-companion
animals.
3

Jan
•Announced
Pivotal
Trial Results
Trial Results
•D Faulkner
joined
ABP
ABP
•Reanalyzed PT
Data and decided
to file 510(k).
Data and decided
to file 510(k).
•Robert Caspari
MD
joined Aspen
joined Aspen
•Roth Health
Care
Conference
Conference
•Engaged Becker
and
Associates
Associates
March
July
May
Sept
June
April
Aug
Feb
Oct
•Formed
Medical
Advisory Board
Advisory Board
•AppyScore
paper
approved and
presented at American
Academy of
Emergency Medicine
approved and
presented at American
Academy of
Emergency Medicine
•Filed
510(k)
with FDA
510(k)
with FDA
•Completed
initial
Priciing Analysis in
US and Europe
Priciing Analysis in
US and Europe
•Initiate
Supplemental
Trials at 13 National
Institutions
Trials at 13 National
Institutions
•Completed
Initial
Strategic Analysis of
Market Opportunity and
initial Business Plan
Strategic Analysis of
Market Opportunity and
initial Business Plan
•Received letter
from
FDA requesting
additional information
FDA requesting
additional information
•Canaccord
Health
Care Conference
Care Conference
•Dr. Andy
Peters
Joined ABP, began
Strategic analysis of
AH Business
Joined ABP, began
Strategic analysis of
AH Business
•Completed
$8.8M
financing to support
development work.
financing to support
development work.
•Greg Bennett
joined
ABP
ABP
•Think Equity
Health
Care Conference
Care Conference
•Rodman and
Renshaw and Baird
Health Care
Conference
Renshaw and Baird
Health Care
Conference
•Spec.’s lock
and
manufacturing
partnerships
formalized
manufacturing
partnerships
formalized
•Lazard Health
Care Conference
Care Conference
2009
AspenBio Year in Review…
building for the future!
building for the future!
Nov
4

What is
Appendicitis?
§ Inflammation
of the appendix usually
resulting from a bacterial infection within the
lumen.
resulting from a bacterial infection within the
lumen.
§ Late
teens peak age group
Appendicitis
is the Most Common Reason for Abdominal Surgery
Prevalence
Data
§ Effects
9% men and 7% women in
their lifetime.
their lifetime.
§ More
than 300K emergency
surgeries in US/year
surgeries in US/year
5

Current
estimate ~320K appendectomies per year
in the US*
in the US*
8-10%
of surgeries remove a normal appendix
(~24,000)
(~24,000)
Average
of 18% of patients mis-diagnosed and
sent home with appendicitis**
sent home with appendicitis**
Many
reports indicate 25-30% of appendicitis
cases not diagnosed in time, resulting in
perforated appendix & emergency surgery
cases not diagnosed in time, resulting in
perforated appendix & emergency surgery
Gynecological
issues makes female diagnosis
difficult resulting in 2x as many
appendectomies as men, with ~ 50% eventually
confirmed NOT having appendicitis
difficult resulting in 2x as many
appendectomies as men, with ~ 50% eventually
confirmed NOT having appendicitis
**Graff, et al,
study
Diagnostic
Process
Symptoms
Migratory
right iliac fossa pain
Nausea
/ Vomiting
Anorexia
Signs
Tenderness
in right iliac fossa
Rebound
tenderness in right iliac fossa
Elevated
temperature
Laboratory
findings
Elevated
WBC
Increased
neutrophils
CT
Scan
ED…quick
decisions with imperfect information!
10M
patients/year enter ED
w/Abdominal
w/Abdominal
pain
*CDC
2006 data
6

Trends in
Appendicitis Management
1. Historically,
appendicitis was a clinical
diagnosis with a negative appendectomy
rate (NAR) of ~16%.
diagnosis with a negative appendectomy
rate (NAR) of ~16%.
2. In 1998, the use of
CT was incorporated
into the diagnostic algorithm.
into the diagnostic algorithm.
3. Use of CT grows
exponentially but
incremental benefits are minimal.
incremental benefits are minimal.
4. Risks and
limitations of CT identified;
however few alternatives exist for ED
physicians and surgeons.
however few alternatives exist for ED
physicians and surgeons.
Source: American
Journal of Emergency Medicine (2008) 26, 39-44
CT
being over-prescribed
because of the lack of an
effective “screening” alternative.
because of the lack of an
effective “screening” alternative.
7

Despite
Advances…
Cost
Cost
for abdominal
CT ranges from
CT ranges from
$200 -
$2000****
Safety
0.4% -
2% of all cancers
in the US will be caused
by CT***
in the US will be caused
by CT***
…There
is a Need for a More Effective Screening Tool!
Sources:
*American
Journal of Emergency Medicine 2008; 26, 39-44
**Surgery
2008;144:276-82
***N
Engl J Med 2007;357:2277-84
****Emerg Radiol
2008;15:23-28 and Company estimates
Indiscriminant
Use
CT in
diagnosing
appendicitis is
overused with
diminishing
appendicitis is
overused with
diminishing
benefits
.**
CT
initially reduced
Negative
Appendectomy Rate,
but trend flattened in
recent years.*
Negative
Appendectomy Rate,
but trend flattened in
recent years.*
8

FDA
Initiative to Reduce
Unnecessary Radiation Imaging
Exposure
Unnecessary Radiation Imaging
Exposure
February
2010
“ The
National Council on Radiation
Protection estimates 67million CT
procedures in 2006.”
Protection estimates 67million CT
procedures in 2006.”
“The
adult effective dose from a CT exam
of the abdomen…is equivalent to roughly
400 chest x-rays.”
of the abdomen…is equivalent to roughly
400 chest x-rays.”
“While
CT scans make up app. 26% of
the imaging procedures…they contribute
89% of the total yearly exposure to
radiation from medical imaging.”
the imaging procedures…they contribute
89% of the total yearly exposure to
radiation from medical imaging.”
Studies
“estimate that app. 29,000
cancers could be related to CT scans
performed in the US in 2007”
cancers could be related to CT scans
performed in the US in 2007”
This
new guideline speaks to the
strength of a test like AppyScore and
is attuned to today’s political, health
care cost and safety environments.
strength of a test like AppyScore and
is attuned to today’s political, health
care cost and safety environments.
9

Our
Science
Appendicitis
Progression
Myeloid-related
protein
(MRP) 8/14
(MRP) 8/14
AspenBio
has patented this marker as a
“aid in evaluating Appendicitis”
“aid in evaluating Appendicitis”
AspenBio
did exhaustive proteomics differential
screens and identified >400 up-regulated proteins in
diseased appendix tissue.
screens and identified >400 up-regulated proteins in
diseased appendix tissue.
10

How AppyScore
Fits in the Workflow
Patient
experiencing acute
abdominal pain
experiencing acute
abdominal pain
Emergency
Department
Department
10.3
M Patients
X
AppyScore
Standard
Lab
Workup
Workup
5.6
M Patients
Abdominal
CT
1.5
M Patients
Appendectomies
320k
Patients
256
k CT’s
Other
Diagnosis
6.4M
Patients
1.2
M CT’s
X
+
Idiopathic
3.6M
Patients
0
CT’s
Source:
Calendar year 2005-2006 visits from the National Hospital Ambulatory Care Survey
(NHAMCS).
Counts
are annualized over the two-year period.
Patient
experiencing acute
abdominal pain
experiencing acute
abdominal pain
Emergency
Department
Department
10.3
M Patients
Standard
Lab
Workup
Workup
5.6
M Patients
Abdominal
CT
2.5
M Patients
Appendectomies
320k
Patients
256
k CT’s
Other
Diagnosis
6.4M
Patients
1.2
M CT’s
Idiopathic
3.6M
Patients
1.0
M CT’s
Current
Standard of Care
New
Standard of Care with AppyScore
Opportunity
to take out significant
portion of these CT Scans!
portion of these CT Scans!
-
11
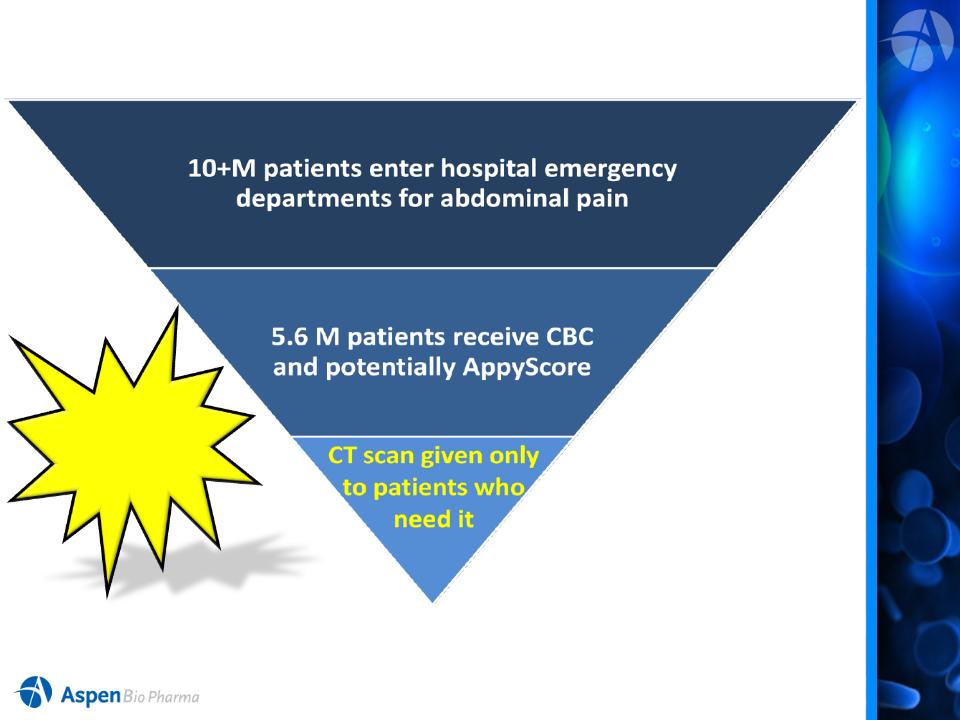
US Market
Opportunity
US
Market Estimates for Hospital Segment is 5.6 M Tests!
(Excludes
Primary Physician, OB/GYN, and Urgent Care Channels)
AppyScore
will help
manage this
funnel!
will help
manage this
funnel!
12

Total
Available Market
(TAM)Estimates
Based
on 5.6M Tests in the US and Global IVD Market Share (Kalorama)
5.6
3.3
1.4
2.4
12.7
13

Product
Acceptance and Value
Aspenbio
engaged
PriceSpective, experts in
biopharmaceutical pricing
and value capturing
strategies
PriceSpective, experts in
biopharmaceutical pricing
and value capturing
strategies
Utilizing the Appy
product
specifications PS
conducted 32 interviews in
the US and 15 in main
European countries
specifications PS
conducted 32 interviews in
the US and 15 in main
European countries
Extensive
“blinded”
Interviews
“blinded”
Interviews
Interviewees included
many highly published key opinion leaders in their
respective area of expertise including appendicitis management.
respective area of expertise including appendicitis management.
14

Market
Research in the US and
EU Confirms the Need for AppyScore
EU Confirms the Need for AppyScore
“There
are problems with the whole system; CT scan with contrast is taking too long; it
is a 5 hour
ordeal. CT without contrast is much faster, but the sensitivity is low; most of radiologists don’t do
that. There is a need for a more specific rule in/out test.”
ordeal. CT without contrast is much faster, but the sensitivity is low; most of radiologists don’t do
that. There is a need for a more specific rule in/out test.”
- ED
Physician
“The
current unmet need is in accuracy of diagnosis; it takes time to perform CT scan
and the
cost of CT is high.”
cost of CT is high.”
-
Surgeon
“Principal
advantages of [test] will be better discrimination of patients who require
surgical
intervention from those who do not; reduced healthcare costs in achieving this better
discrimination from fewer unnecessary surgeries, and less chance of medical errors.”
intervention from those who do not; reduced healthcare costs in achieving this better
discrimination from fewer unnecessary surgeries, and less chance of medical errors.”
- Lab
Director
“If
they are accepted [by physicians] as necessary tests, we will cover
them.”
-
Payer
15

Findings from
PriceSpectives
Market Research
Market Research
Physician
value rating range from 2.5 to 7, with an
average of 5.7 on the 1 to 7 scale
average of 5.7 on the 1 to 7 scale
§ The high ratings are
based on convenience, a blood
-based assay and clinical specificity
-based assay and clinical specificity
§ The two lower
ratings, were based on the desire for
more information on patient demographics
more information on patient demographics
Physicians
say they would initially use it in
patients that are more difficult to diagnose,
…young children, women, and the elderly
patients that are more difficult to diagnose,
…young children, women, and the elderly
Payers’
value rating ranged from 2 to 6.5 with an
average of 4.5 on a 1 to 7 scale
average of 4.5 on a 1 to 7 scale
Unprompted,
payers state that they would not
manage the use of the AppyScore™
manage the use of the AppyScore™
§ It would be included
in bundled DRG payments
§ Managing emergency
care utilization is difficult
because payers are reluctant to interfere in life-
threatening situations
because payers are reluctant to interfere in life-
threatening situations
Very
Valuable
Valuable
Not
Valuable
1
7
Physician Average
(5.7)
4
Please
rank the value of this product on a scale of 1-7, where 1 is
not valuable at all and 7 is very valuable.
not valuable at all and 7 is very valuable.
Max
Min
Payer
Average (4.5)
16

AppyScore
ELISA Trial Results*
Analysis
of 15 other diagnostic tests
approved as an “aid in the diagnosis of…”
approved as an “aid in the diagnosis of…”
•Sensitivity
Ranges of 60-96%
•Specificity
Ranges of 32-100%
•NPV
Ranges of 80-96%
Our
primary research indicated strong acceptance of the clinical utility of
AppyScore.
Experts
have indicated that identification of a low-risk patient group which can
be
observed without the need for CT, will be an important advance.
observed without the need for CT, will be an important advance.
* Data
are from AspenBio Clinical Trial in 2008 and represent the population in that
study.
17
Regulatory
Update and Milestones
• Initial
trial was completed in Dec 2008. (Utilizing
ELISA format)
ELISA format)
Trial
Demonstrated:
ü Fundamental
analytical performance to be
robust and repeatable
robust and repeatable
ü Clinical
utility that supports the intended use
claim
claim
• Original
510(k) filed in June 2009
• FDA
requested additional data in August 2009 letter
• Supplemental trial
commenced in July to provide
additional data clinical data
additional data clinical data
• Interim
analysis completed in January 2010 to
confirm trial sample size at 800
confirm trial sample size at 800
• Expected
completion of enrollment in March 2010
• Trial
data analysis, study report and 510(k)
submission in Q2 2010.
submission in Q2 2010.
Probable label intended
use:
use:
“to be used to evaluate
patients with abdominal
pain suspicious for
acute appendicitis”
patients with abdominal
pain suspicious for
acute appendicitis”
18

Clinical/Regulatory
Timeline
July
2009
Supplemental
Study
First
IRB approval
First
Subject Enrolled
Trial
Completed
1.Data
base locked
2.Study
Report drafted
Final
Study Report
Submission
of new
510(k) FDA
510(k) FDA
Q2
2010
Dates
in Red based on current understanding of work/requirements
FDA
Clearance
of ELISA
Clearance
of ELISA
2nd
half
2010
half
2010
March
2010
1. 800
Patient Clinical Trial
2. 13 Academic
sites
3. Central
sample processing
4. Electronic
data monitoring
19

Second-Generation
Product
Assay
Platform for Emergency
Department or Central Lab
Department or Central Lab
Simplified
for operator use
Rapid
results ~15 minutes
Product
designed to significantly
reduce operator dependence
reduce operator dependence
Data
captured and reported through
hospital LIS interface
hospital LIS interface
Designed
to accept additional
markers/assays
markers/assays
CE
Marking enables early launch in
Europe
Europe
Design
Features
20

Second-Generation
Product
Development
Update
ü Instrument
manufacturing partner (LRE)
ü Cassette
development and manufacturing partner
ü 37
instruments delivered
ü Validation
and certification work progress with clinical
samples from company’s previous trial work.
samples from company’s previous trial work.
ü ELISA
correlation study scheduled for Q1 2010
European
launch
ü Evaluating
RUO use of instrument
for
early revenue generation
21

Strategic
Alliance with
LRE Medical, GmbH
LRE Medical, GmbH
• Aligns AspenBio with
a proven leader…
ü 40 years of
experience in manufacturing
medical devices
medical devices
ü Expertise in
instruments for point of care and
near patient testing
near patient testing
• Pioneer in
developing diagnostic instruments
for use in emergency and critical care
settings
for use in emergency and critical care
settings
• Agreement solidifies
relationship that began
in 2008 and appoints LRE as the exclusive
manufacturer.
in 2008 and appoints LRE as the exclusive
manufacturer.
• ISO 13485 certified
and registered with FDA
as a manufacturer for medical devices
as a manufacturer for medical devices
22

“Build
a Company”
• Market own
brand
• Build/Lease sales
force
License
• Assay adapted
for
other platforms
other platforms
Sales
& Distribution
Partnership
Partnership
• Marketing
partnerships
Commercial
Options
Value
Drivers
Support
Option A
Drivers
Support
Option A
A
B
C
• Target Market
easily
reachable with
moderate sized sales force.
moderate sized sales force.
• Top 30
US metropolitan areas
represent >40% of US Market.
represent >40% of US Market.
23

Key
Statistics: APPY (NASDAQ
CM)
24
Mrq
estimates as of Sept 2009
|
Stock
Price (2/16/10)
|
$2.04
|
|
Avg. Daily
Vol. (3mo.)
|
150,000
|
|
Shares
Out. (mrq)(1)
|
37.5M
|
|
Fully-diluted(1)
|
42.2M
|
|
Public Float,
est.
(1)
|
34.6M
|
|
Institutional
Holdings, est.
(1) |
48%
|
|
Insider
/ 5% Holders, est. (1)
|
20%
|
|
Cash
& Equiv. (est.
2/10)
|
~$12M
|
|
Total Assets
(mrq)
|
$15.7M
|
|
Total
Liabilities (mrq)
|
$6.5M
|
|
|
|
|
Cash
Burn Avg.
(3
mo.)
|
~$1.2M
|
(mrq)
- most
recent reported quarter
(1)
As
adjusted for Oct. ‘09 Public Offering

Key
Milestones
• Complete
Clinical Trial
• Finalize
Study Reports and file
510(k)
510(k)
• Achieve
ELISA clearance
• Reader/Cassette
• Complete
development
• Initiate
trials
• CE
Marking
• Possible
Launch in Europe
• Finalize
US commercial launch plans
2010
Next
Year…
Consistently
executing towards commercialization!
US
Launch of
AppyScore
AppyScore
25

Significant
Appendicitis
Domain
Expertise
Appendicitis
Domain
Expertise
AspenBio
has built a solid foundation
for success…
for success…
26
Clinical/Regulatory
Support Team
Support Team
Medical
Advisory Board
and
Emergency
Medicine
Expert Panel
Expert Panel
13
Established
Emergency Medicine
Clinical Sites
Emergency Medicine
Clinical Sites
Extensive
Pre-Clinical
and Clinical Trial
Database and Sample
Repository
and Clinical Trial
Database and Sample
Repository
Robust
and Expandable
Assay
Platform

Daryl
J. Faulkner Chief
Executive Officer, Executive Chairman and Director
Daryl Faulkner has more than 25 years experience in developing and commercializing medical devices, drug and drug delivery systems, life science research tools and molecular
diagnostics. He most recently served as president, CEO and member of the board of directors of Digene Corporation, acquired by Qiagen in July 2008. He has continued to serve
as a consultant to Qiagen. Prior to joining Digene, Mr. Faulkner spent eight years with Invitrogen (now merged as Life Technologies Corp., a Nasdaq-traded company) . Mr.
Faulkner’s career also includes15 years with the Fortune 100 company Abbott Laboratories. Mr. Faulkner currently serves as a member of the board of directors
Daryl Faulkner has more than 25 years experience in developing and commercializing medical devices, drug and drug delivery systems, life science research tools and molecular
diagnostics. He most recently served as president, CEO and member of the board of directors of Digene Corporation, acquired by Qiagen in July 2008. He has continued to serve
as a consultant to Qiagen. Prior to joining Digene, Mr. Faulkner spent eight years with Invitrogen (now merged as Life Technologies Corp., a Nasdaq-traded company) . Mr.
Faulkner’s career also includes15 years with the Fortune 100 company Abbott Laboratories. Mr. Faulkner currently serves as a member of the board of directors
of
Osmetech, an emerging molecular diagnostics company.
Greg
Bennett Vice
President of Product Development and Manufacturing
Mr. Bennett has 25 years in product design and development focused in cassette and instrument test formats, including point-of-care (“POC”) and home test products. He
recently served as general manager of Cholestech’s operations following the company’s acquisition by Inverness Medical Innovations, Inc.,. At Cholestech, Mr. Bennett served
as vice president of research and development where he was responsible for the development and launch of its cholesterol instrument and cartridge system for POC use. Prior to
his six years with Cholestech / Inverness, Bennett spent 12 years with LifeScan, Inc., a Johnson & Johnson Company, where he served in increasing levels of responsibility and
lastly as director of process development engineering. At LifeScan, he led the group responsible for the process development, scale-up and commercialization of several blood
glucose monitoring devices. Bennett earned his B.S. in Mechanical Engineering from the University of Wisconsin, and has received specialized training in Process
Mr. Bennett has 25 years in product design and development focused in cassette and instrument test formats, including point-of-care (“POC”) and home test products. He
recently served as general manager of Cholestech’s operations following the company’s acquisition by Inverness Medical Innovations, Inc.,. At Cholestech, Mr. Bennett served
as vice president of research and development where he was responsible for the development and launch of its cholesterol instrument and cartridge system for POC use. Prior to
his six years with Cholestech / Inverness, Bennett spent 12 years with LifeScan, Inc., a Johnson & Johnson Company, where he served in increasing levels of responsibility and
lastly as director of process development engineering. At LifeScan, he led the group responsible for the process development, scale-up and commercialization of several blood
glucose monitoring devices. Bennett earned his B.S. in Mechanical Engineering from the University of Wisconsin, and has received specialized training in Process
Excellence/Six
Sigma and Stanford Executive Training - Corporate Finance and Portfolio
Management.
Robert
F. Caspari, MD Chief
Operating Officer / Chief Medical Officer
Robert Caspari has more than 25 years of experience in drug and diagnostic product development and commercialization. He most recently served as CEO of Living Cell
Technologies, a publicly traded biotech company focused on cellular therapy for Type I diabetes and neurological disorders. He was previously president and CEO of Aurogen, a
privately held biotech company involved in drug development for neurological disorders. Dr. Caspari has also served as senior vice president of commercial operations and
medical affairs at Myogen (now a unit of Gilead Sciences, traded on the Nasdaq), and as vice president and general manager of biopharmaceuticals at Novo
Robert Caspari has more than 25 years of experience in drug and diagnostic product development and commercialization. He most recently served as CEO of Living Cell
Technologies, a publicly traded biotech company focused on cellular therapy for Type I diabetes and neurological disorders. He was previously president and CEO of Aurogen, a
privately held biotech company involved in drug development for neurological disorders. Dr. Caspari has also served as senior vice president of commercial operations and
medical affairs at Myogen (now a unit of Gilead Sciences, traded on the Nasdaq), and as vice president and general manager of biopharmaceuticals at Novo
Nordisk
Pharmaceuticals. Dr. Caspari received a B.A. in psychology from UCLA and his
medical degree from Georgetown University.
Jeffrey
G. McGonegal Chief
Financial Officer
Jeffrey McGonegal joined AspenBio Pharma as CFO in 2003. He has more than 30 years experience in accounting and developing public companies. Mr. McGonegal devotes
limited time to PepperBall Technologies, Inc. as its CFO. Mr. McGonegal serves as Senior Vice President — Finance of Cambridge Holdings, Ltd., with limited activities. Since
1997, Mr. McGonegal has served as Managing Director of McGonegal and Co., a company engaged in providing accounting and business consulting services. For 23 years he
was in accounting with BDO Seidman LLP and his last position with that firm was managing partner of the Denver, Colorado office . Mr. McGonegal graduated from Florida
State University with a B.A. in accounting
Jeffrey McGonegal joined AspenBio Pharma as CFO in 2003. He has more than 30 years experience in accounting and developing public companies. Mr. McGonegal devotes
limited time to PepperBall Technologies, Inc. as its CFO. Mr. McGonegal serves as Senior Vice President — Finance of Cambridge Holdings, Ltd., with limited activities. Since
1997, Mr. McGonegal has served as Managing Director of McGonegal and Co., a company engaged in providing accounting and business consulting services. For 23 years he
was in accounting with BDO Seidman LLP and his last position with that firm was managing partner of the Denver, Colorado office . Mr. McGonegal graduated from Florida
State University with a B.A. in accounting
Gregory
Pusey Vice
Chairman / Vice President Investor Relations
Gregory Pusey became a director of AspenBio Pharma, Inc. in February 2002, Chairman in May 2003 and in January 2009 became the Vice Chairman , a newly created position.
Mr. Pusey has been helping develop and advance small public companies for approximately 30 years. Mr. Pusey is a director (previously Chairman ) of PepperBall Technologies,
Inc., a publicly held provider of non-lethal technology. Since 1988, Mr. Pusey has been the President and a director of Cambridge Holdings, Ltd. which has limited
Gregory Pusey became a director of AspenBio Pharma, Inc. in February 2002, Chairman in May 2003 and in January 2009 became the Vice Chairman , a newly created position.
Mr. Pusey has been helping develop and advance small public companies for approximately 30 years. Mr. Pusey is a director (previously Chairman ) of PepperBall Technologies,
Inc., a publicly held provider of non-lethal technology. Since 1988, Mr. Pusey has been the President and a director of Cambridge Holdings, Ltd. which has limited
activities..
Mr. Pusey graduated from Boston College with a BS degree in finance.
Mark
Colgin, PhD Chief
Scientific Officer
Mark Colgin was appointed Chief Scientific Officer of the Company in February 2009. Dr. Colgin joined the Company in September 2000 and served as Director of Recombinant
Technology until he was promoted to Chief Scientist in January 2003. Prior to joining the Company, his areas of research included the characterization and artificial synthesis of
spider silk proteins, regulation of gene expression, neurovirology and gene delivery systems. Dr. Colgin received a B.S. in Biochemistry and a Ph.D. in Molecular Biology from
the University of Wyoming.
Mark Colgin was appointed Chief Scientific Officer of the Company in February 2009. Dr. Colgin joined the Company in September 2000 and served as Director of Recombinant
Technology until he was promoted to Chief Scientist in January 2003. Prior to joining the Company, his areas of research included the characterization and artificial synthesis of
spider silk proteins, regulation of gene expression, neurovirology and gene delivery systems. Dr. Colgin received a B.S. in Biochemistry and a Ph.D. in Molecular Biology from
the University of Wyoming.
Executive
Team
Andy
Peters, PhD Vice
President of Animal Health
Dr.
Peters owns a consultancy company, Arpexas
Ltd, providing support to
the animal health Industry. He is also a the Director Translational Research,
Royal School of Veterinary
Studies, University of Edinburgh. He has also acted as Chief Scientific Adviser to GALVmed, a charity dedicated to provision of medicines for poor livestock farmers in developing
countries. Until November 2005 Dr. Peters was Head of European vaccine R&D with Pfizer Animal Health, during which time he acquired extensive multicultural leadership
experience. Between 1993 and 1998 he was Professor of Animal Health and Production at the Royal Veterinary College, University of London and previously Regulatory Manager
with Hoechst Animal Health. Dr. Peters is a member of the UK Veterinary Products Committee and of the DEFRA Advisory Committee on Releases (GMO’s) into the Environment.
He is veterinarian with PhD and DSc degrees in animal science and holds a special Professorship with the University of Nottingham. Dr. Peters has published some 145 papers in
physiology, animal science and vaccine development and has been a frequent speaker at scientific and industry conferences. Dr. Peters has also published two books viz.
Reproduction in Cattle and Vaccines for Veterinary Applications.
Studies, University of Edinburgh. He has also acted as Chief Scientific Adviser to GALVmed, a charity dedicated to provision of medicines for poor livestock farmers in developing
countries. Until November 2005 Dr. Peters was Head of European vaccine R&D with Pfizer Animal Health, during which time he acquired extensive multicultural leadership
experience. Between 1993 and 1998 he was Professor of Animal Health and Production at the Royal Veterinary College, University of London and previously Regulatory Manager
with Hoechst Animal Health. Dr. Peters is a member of the UK Veterinary Products Committee and of the DEFRA Advisory Committee on Releases (GMO’s) into the Environment.
He is veterinarian with PhD and DSc degrees in animal science and holds a special Professorship with the University of Nottingham. Dr. Peters has published some 145 papers in
physiology, animal science and vaccine development and has been a frequent speaker at scientific and industry conferences. Dr. Peters has also published two books viz.
Reproduction in Cattle and Vaccines for Veterinary Applications.
27

Medical
Advisory Board
David
Flum, MD, MPH is a leading
gastrointestinal surgeon and outcomes researcher at the University of
Washington. He holds the rank of
Professor in the Schools of Medicine and Public Health and serves as the Director of the Surgical Outcomes Research Center (SORCE)
at the University of Washington. He has a Masters in Public Health in the field of health services research. Dr. Flum serves as Medical Director
of the Surgical Care and Outcomes Assessment Program (SCOAP), a quality of care improvement program providing hospital-specific data
feedback and best practices regarding processes of care and outcomes across the Pacific Northwest. He is also one of the Principal Investigators
of the Longitudinal Assessment of Bariatric Surgery (LABS) study-the first NIH-funded study in bariatric surgery aimed at addressing fundamental
issues in the field. He is the contributing editor for surgery at the Journal of the American Medical Association, serves on the editorial board of the
journal Surgery for Obesity and Related Disease, and is a member of the Executive Leadership of the American College of Surgeons Research
Committee.
Professor in the Schools of Medicine and Public Health and serves as the Director of the Surgical Outcomes Research Center (SORCE)
at the University of Washington. He has a Masters in Public Health in the field of health services research. Dr. Flum serves as Medical Director
of the Surgical Care and Outcomes Assessment Program (SCOAP), a quality of care improvement program providing hospital-specific data
feedback and best practices regarding processes of care and outcomes across the Pacific Northwest. He is also one of the Principal Investigators
of the Longitudinal Assessment of Bariatric Surgery (LABS) study-the first NIH-funded study in bariatric surgery aimed at addressing fundamental
issues in the field. He is the contributing editor for surgery at the Journal of the American Medical Association, serves on the editorial board of the
journal Surgery for Obesity and Related Disease, and is a member of the Executive Leadership of the American College of Surgeons Research
Committee.
Douglas
K. Owens, MD, MS is a general
internist and a Professor of Medicine and of Health Research and Policy at
Stanford University,
where he directs the Program on Clinical Decision Making and Guideline Development at the Center for Primary Care and Outcomes Research
(PCOR). Dr. Owens also directs the Stanford University-UCSF Evidence-Based Practice Center funded by the Agency for Healthcare Research
and Quality (AHRQ). Dr. Owens’ research interests include diagnostic test evaluation, evidence synthesis, technology assessment, cost-
effectiveness analysis and guideline development. Dr. Owens has been principal investigator on grants funded by the National Institutes of
Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention (CDC), among others. From 2005
to 2009, Dr. Owens was Chair of the Clinical Efficacy Assessment Subcommittee (CEAS) of the American College of Physicians (ACP), which
develops clinical practice guidelines for the ACP. Dr. Owens is a past President of the Society for Medical Decision Making. He was elected to
the American Society for Clinical Investigation (ASCI), and the Association of American Physicians (AAP), societies that recognize excellence in
clinical research.
where he directs the Program on Clinical Decision Making and Guideline Development at the Center for Primary Care and Outcomes Research
(PCOR). Dr. Owens also directs the Stanford University-UCSF Evidence-Based Practice Center funded by the Agency for Healthcare Research
and Quality (AHRQ). Dr. Owens’ research interests include diagnostic test evaluation, evidence synthesis, technology assessment, cost-
effectiveness analysis and guideline development. Dr. Owens has been principal investigator on grants funded by the National Institutes of
Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention (CDC), among others. From 2005
to 2009, Dr. Owens was Chair of the Clinical Efficacy Assessment Subcommittee (CEAS) of the American College of Physicians (ACP), which
develops clinical practice guidelines for the ACP. Dr. Owens is a past President of the Society for Medical Decision Making. He was elected to
the American Society for Clinical Investigation (ASCI), and the Association of American Physicians (AAP), societies that recognize excellence in
clinical research.
David
A. Talan, MD, FACEP, FIDSA is
Chairman of the Department of Emergency Medicine and faculty in the Division of
Infectious
Diseases at Olive View-UCLA Medical Center, and Professor of Medicine in Residence at UCLA School of Medicine. He is a Fellow of the
American College of Emergency Physicians and the Infectious Diseases Society of America, and a member of the Society for Academic
Emergency Medicine and the American Society for Microbiology. Dr. Talan received his medical degree from the University of Illinois Medical
College in Chicago. He completed his residencies in Internal and Emergency Medicine and fellowship in Infectious Diseases at the University of
California Los Angeles (UCLA) and its associated medical centers. He is board certified in Internal Medicine, Emergency Medicine, and Infectious
Diseases. Dr. Talan serves on the editorial boards of the Annals of Emergency Medicine, Emergency Medicine News, and Pediatric Emergency
Care and is a reviewer for Clinical Infectious Diseases, Journal of the American Medical Association, and The Medical Letter.
Diseases at Olive View-UCLA Medical Center, and Professor of Medicine in Residence at UCLA School of Medicine. He is a Fellow of the
American College of Emergency Physicians and the Infectious Diseases Society of America, and a member of the Society for Academic
Emergency Medicine and the American Society for Microbiology. Dr. Talan received his medical degree from the University of Illinois Medical
College in Chicago. He completed his residencies in Internal and Emergency Medicine and fellowship in Infectious Diseases at the University of
California Los Angeles (UCLA) and its associated medical centers. He is board certified in Internal Medicine, Emergency Medicine, and Infectious
Diseases. Dr. Talan serves on the editorial boards of the Annals of Emergency Medicine, Emergency Medicine News, and Pediatric Emergency
Care and is a reviewer for Clinical Infectious Diseases, Journal of the American Medical Association, and The Medical Letter.
Steven
E. Wolf, MD is the Bob
and Betty
Kelso Distinguished Chair in Burn and Trauma Surgery, Vice Chairman for Research
and
Professor in the Department of Surgery at the University of Texas Health Science Center at San Antonio (UTHSCSA). He serves as Chair
for the Institutional Review Board UTHSCSA. He is Burn Surgeon and Chief of Clinical Research at the United States Army Institute of Surgical
Research at Fort Sam Houston and Pediatric Burn Program Director at University Hospital. Dr. Wolf received his medical degree from UTMB
Galveston and completed his post graduate training at the University of Missouri-Kansas City and at Shriners Hospital for Children in Galveston.
Dr. Wolf has previously held the position of Director of the US Army Institute of Surgical Research Burn Center at Brooke Army Medical Center
and Assistant Chief of Staff at Shriners Hospital for Children. He has authored over 150 peer reviewed publications and is nationally and
internationally recognized for his work in burn and trauma care and regenerative medicine. Dr. Wolf received the 2009 Health Care Heroes
Outstanding Physician Award. Dr. Wolf is Editor-in-Chief of Burns and on the editorial boards of Journal of Burn Care and Rehabilitation and
Surgery News.
Professor in the Department of Surgery at the University of Texas Health Science Center at San Antonio (UTHSCSA). He serves as Chair
for the Institutional Review Board UTHSCSA. He is Burn Surgeon and Chief of Clinical Research at the United States Army Institute of Surgical
Research at Fort Sam Houston and Pediatric Burn Program Director at University Hospital. Dr. Wolf received his medical degree from UTMB
Galveston and completed his post graduate training at the University of Missouri-Kansas City and at Shriners Hospital for Children in Galveston.
Dr. Wolf has previously held the position of Director of the US Army Institute of Surgical Research Burn Center at Brooke Army Medical Center
and Assistant Chief of Staff at Shriners Hospital for Children. He has authored over 150 peer reviewed publications and is nationally and
internationally recognized for his work in burn and trauma care and regenerative medicine. Dr. Wolf received the 2009 Health Care Heroes
Outstanding Physician Award. Dr. Wolf is Editor-in-Chief of Burns and on the editorial boards of Journal of Burn Care and Rehabilitation and
Surgery News.
28
