Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - THORATEC CORP | f54943exv32w1.htm |
| EX-31.2 - EX-31.2 - THORATEC CORP | f54943exv31w2.htm |
| EX-10.25 - EX-10.25 - THORATEC CORP | f54943exv10w25.htm |
| EX-31.1 - EX-31.1 - THORATEC CORP | f54943exv31w1.htm |
| EX-23.1 - EX-23.1 - THORATEC CORP | f54943exv23w1.htm |
| EX-32.2 - EX-32.2 - THORATEC CORP | f54943exv32w2.htm |
| EX-10.24 - EX-10.24 - THORATEC CORP | f54943exv10w24.htm |
| EX-10.23 - EX-10.23 - THORATEC CORP | f54943exv10w23.htm |
| EX-10.26 - EX-10.26 - THORATEC CORP | f54943exv10w26.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark one)
| þ | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended January 2, 2010
| o | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 000-49798
Thoratec Corporation
(Exact Name of Registrant as Specified in Its Charter)
| California (State or Other Jurisdiction of Incorporation or Organization) |
94-2340464 (I.R.S. Employer Identification No.) |
|
| 6035 Stoneridge Drive, Pleasanton, California (Address of Principal Executive Offices) |
94588 (Zip Code) |
Registrant’s telephone number, including area code: (925) 847-8600
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of Each Class | Name of Each Exchange of which Registered | |
| Common Stock, no par value per share | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule
405 of the Securities Act.
Yes þ No o
Indicate by a check mark if the registrant is not required to file reports pursuant to Section
13 or Section 15(d) of the Exchange Act. Yes o No þ
Indicate by a check mark whether the registrant: (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has
been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such files). Yes o No o
Indicate by a check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation
S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large
accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act. (Check one):
| Large accelerated filer þ | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller Reporting Company o |
Indicate by a check mark whether the registrant is a shell company (as defined in Exchange Act
Rule 12(b)-2) Yes o No þ
The aggregate market value of the voting stock held by non-affiliates computed by reference to
the last sale reported of such stock on July 4, 2009, the last business day of the Registrant’s
second fiscal quarter, was $1,291,151,553.
As of January 30, 2010, the Registrant had 57,067,248 shares of common stock outstanding.
TABLE OF CONTENTS
| Page | ||||||||
| 3 | ||||||||
| 17 | ||||||||
| 32 | ||||||||
| 32 | ||||||||
| 33 | ||||||||
| 33 | ||||||||
| 34 | ||||||||
| 37 | ||||||||
| 38 | ||||||||
| 49 | ||||||||
| 50 | ||||||||
| 86 | ||||||||
| 86 | ||||||||
| 87 | ||||||||
| 87 | ||||||||
| 87 | ||||||||
| 87 | ||||||||
| 88 | ||||||||
| 88 | ||||||||
| 88 | ||||||||
| 90 | ||||||||
| EX-10.23 | ||||||||
| EX-10.24 | ||||||||
| EX-10.25 | ||||||||
| EX-10.26 | ||||||||
| EX-23.1 | ||||||||
| EX-31.1 | ||||||||
| EX-31.2 | ||||||||
| EX-32.1 | ||||||||
| EX-32.2 | ||||||||
DOCUMENTS INCORPORATED BY REFERENCE
Designated portions of Thoratec’s definitive proxy statement for its 2010 annual meeting of
shareholders are incorporated by reference into Part III of this Form 10-K.
Thoratec, the Thoratec logo, Thoralon, TLC-II, HeartMate, and HeartMate II are registered
trademarks of Thoratec Corporation, and IVAD is a trademark of Thoratec Corporation.
CentriMag is a registered trademark of Levitronix LLC.
ITC, A-VOX Systems, AVOXimeter, HEMOCHRON, ProTime, Surgicutt, Tenderlett, Tenderfoot, and
IRMA are registered trademarks of International Technidyne Corporation, our wholly-owned
subsidiary.
2
Table of Contents
PART I
| Item 1: | Business |
OVERVIEW
Thoratec Corporation (“we,” “our,” “us,” or the “Company”) is a world leader in mechanical
circulatory support with a product portfolio to treat the full range of clinical needs for advanced
heart failure patients. We develop, manufacture and market proprietary medical devices used for
circulatory support. We also develop, manufacture and market point-of-care diagnostic test systems
and skin incision products. Our business is comprised of two operating divisions: Cardiovascular
and International Technidyne Corporation (“ITC”), a wholly-owned subsidiary.
Incorporated in the State of California in 1976, Thoratec Corporation trades on the NASDAQ
Global Select Market under the ticker symbol THOR and is headquartered in Pleasanton, California.
OUR PRODUCTS
Cardiovascular Division
For advanced heart failure (“HF”), our Cardiovascular division develops, manufactures and
markets proprietary medical devices used for mechanical circulatory support (“MCS”). Our primary
product lines are our ventricular assist devices (“VADs”): the HeartMate II Left Ventricular Assist
System (“HeartMate II”), the HeartMate Left Ventricular Assist System (“HeartMate XVE”), the
Thoratec Paracorporeal Ventricular Assist Device (“PVAD”), and the Thoratec Implantable Ventricular
Assist Device (“IVAD”). We refer to the HeartMate II and the HeartMate XVE collectively as the
“HeartMate product line,” and we refer to the PVAD and the IVAD collectively as the “Thoratec
product line.” In addition, for acute HF we market the CentriMag Blood Pumping System
(“CentriMag”), which is manufactured by Levitronix LLC (“Levitronix”) and distributed by us in the
U.S. under a distribution agreement with Levitronix. We also manufacture a vascular access graft
for renal dialysis.
VADs supplement the pumping function of the heart in patients with advanced HF. In most cases,
a cannula connects the left ventricle of the heart to a blood pump. Blood flows from the left
ventricle to the pump chamber via the cannula, powered by an electric or air driven mechanism that
drives the blood through another cannula into the aorta. From the aorta, the blood then circulates
throughout the body. Mechanical or tissue valves enable unidirectional flow in some devices.
Currently, the power source remains outside the body for all FDA-approved VADs.
Certain VADs are implanted internally, while others are placed immediately adjacent to the
body (paracorporeal).
Our product portfolio of VADs, blood pumping systems and graft products is described below.
The HeartMate II
The HeartMate II is an implantable, electrically powered, continuous flow, left ventricular
assist device consisting of a miniature rotary blood pump designed to provide intermediate and
long-term MCS. The HeartMate II is designed to improve survival and quality of life and to provide
five to ten years of circulatory support for a broad range of advanced HF patients. Significantly
smaller than the HeartMate XVE and with only one moving part, the HeartMate II is simpler and
designed to operate more quietly than pulsatile devices. Effective January 20, 2010, the HeartMate
II can be used in patients with New York Heart Association Class IIIB and IV end-stage left
ventricular failure who have received optimal medical therapy for at least forty-five of the last
sixty days, and who are not candidates for cardiac transplantation.
The HeartMate II received FDA approval in April 2008 for bridge-to-transplantation (“BTT”) and
received FDA approval for use in HF patients who are not eligible for heart transplantation
(“Destination Therapy” or “DT”) in January 2010. In November 2005, the HeartMate II received CE
Mark approval, allowing for its commercial sale in Europe. In May 2009, the HeartMate II was
approved in Canada.
During the third quarter of 2009 we launched our new HeartMate external peripherals (Go Gear),
including new batteries, charger and power module, which are designed to provide an enhanced
quality of life for HeartMate patients by providing them more freedom and mobility and the ability
to more easily resume many aspects of a normal lifestyle.
3
Table of Contents
The
HeartMate XVE
The HeartMate XVE is an implantable, pulsatile, left ventricular assist device for
intermediate and longer-term MCS. Patients with a HeartMate XVE do not require anticoagulation
drugs, other than aspirin, because of the product’s incorporation of proprietary textured surfaces
and tissue valves. The system is comprised of the blood pump and a wearable controller and
batteries providing a high degree of patient freedom and mobility.
The HeartMate XVE received FDA approval for BTT in December 2001 and for Destination Therapy
in April 2003. In June 2003, the HeartMate XVE received CE Mark approval, allowing for its
commercial sale in Europe. In June 2004, the HeartMate XVE was approved in Canada.
The
Paracorporeal Ventricular Assist Device
The PVAD is an external, pulsatile, ventricular assist device, FDA approved for BTT, including home discharge, and
post-cardiotomy myocardial recovery and provides left, right and biventricular MCS. The PVAD is a
paracorporeal device that is less invasive than implantable VADs since only the cannula is
implanted. The paracorporeal nature of the PVAD has several benefits including shorter implantation
times (approximately two hours) and the ability to use the device in smaller patients.
A pneumatic power source drives the PVAD. It is designed for short-to-intermediate duration
use of a few weeks to several months, although this device has supported numerous patients for nine
to eighteen months. Offering left, right or biventricular support, the PVAD and the IVAD, described
below, are the only biventricular support systems approved for use as BTT. This characteristic is
significant since approximately 50% of BTT patients treated with the PVAD and the IVAD require
right as well as left-sided ventricular assistance. The PVAD and the IVAD are also the only devices
approved for both BTT and recovery following cardiac surgery. The PVAD incorporates our proprietary
biomaterial, Thoralon, which has excellent tissue and blood compatibility and is resistant to blood
clots.
The PVAD received FDA approval for BTT in December 1995 and for recovery (post-cardiotomy) in
May 1998. In June 1998, the PVAD received CE Mark approval, allowing for its commercial sale in
Europe. In November 1994, the PVAD was approved in Canada.
The
Implantable Ventricular Assist Device
The IVAD is an implantable, pulsatile, ventricular assist device, FDA approved for BTT, including home discharge,
and post-cardiotomy myocardial recovery and provides left, right or biventricular MCS. The IVAD
maintains the same blood flow path, valves and blood pumping mechanism as the PVAD, but has an
outer housing made of a titanium alloy that makes it suitable for implantation.
The IVAD received FDA approval for BTT and recovery (post-cardiotomy) in August 2004. In June
2003, the IVAD received CE Mark approval, allowing for its commercial sale in Europe. In November
2004, the IVAD was approved in Canada.
The
CentriMag
The CentriMag is manufactured by Levitronix and is based on their magnetically levitated
bearingless motor technology. We entered into a distribution agreement with Levitronix in August
2007, effective through December 2011, to distribute the CentriMag in the U.S. The CentriMag is
510(k) cleared by the FDA for use up to six hours in patients requiring short-term extracorporeal
circulatory support during cardiac surgery. Additionally, CentriMag is approved under an FDA
humanitarian device exemption to be used as a right ventricular assist device for periods of
support up to thirty days in patients in cardiogenic shock due to acute right ventricular failure.
In May 2008, Levitronix received approval to commence a U.S. pivotal trial to demonstrate safety
and effectiveness of the CentriMag for periods of support up to thirty days. Levitronix has CE Mark
approval in Europe to market the product to provide support for up to thirty days.
Vascular
Graft Products
The Vectra Vascular Access Graft (“Vectra”) was approved for sale in the U.S. in December 2000
and in Europe in January 1998. It is designed for use as a shunt between an artery and a vein,
primarily to provide access to the bloodstream for renal hemodialysis patients requiring frequent
needle punctures during treatment.
4
Table of Contents
ITC Division
Our ITC division develops, manufactures and markets two product lines: point-of-care
diagnostic test systems for hospital point-of-care and alternate site point-of-care markets,
including diagnostic test systems that monitor blood coagulation while a patient is being
administered certain anticoagulants, and that monitor blood gas/electrolytes, oxygenation and
chemistry status; and incision products including devices used to obtain a patient’s blood sample
for diagnostic testing and screening for platelet function.
Our product portfolio of point-of-care diagnostic test systems and incision products includes
the following:
Hospital point-of-care
The HEMOCHRON Whole Blood Coagulation System
The HEMOCHRON Whole Blood Coagulation System (“HEMOCHRON”) is used to quantitatively monitor a
patient’s coagulation status while the patient is being administered anticoagulants. It may be used
in various hospital settings. For instance, it is used in the cardiovascular operating room and
cardiac catheterization lab to monitor the drug Heparin, and in the anticoagulation clinic to
monitor the drug warfarin. The system consists of a small portable instrument and disposable test
cuvettes or tubes and delivers results in minutes.
The IRMA TRUpoint Blood Analysis System
The IRMA TRUpoint Blood Analysis System (“IRMA”) is used to quantitatively monitor a patient’s
blood gas, electrolyte and chemistry status. This instrument is a self-contained, portable system
which uses disposable test cartridges and delivers results in minutes.
The AVOXimeter Whole Blood Co-Oximeter/Oximeter System
The AVOXimeter Whole Blood Co-Oximeter/Oximeter System (“AVOXimeter”) is used to assess a
patient’s oxygenation status and is commonly used in the cardiac catherization lab, the intensive
care unit, the neonatal intensive care unit and the emergency department. This portable instrument
uses small, single-use test cuvettes and delivers results in less than ten seconds.
Our integrated data management system connects the HEMOCHRON, IRMA and AVOXimeter products.
Alternate site point-of-care
The ProTime Microcoagulation System
The ProTime Microcoagulation System (“ProTime”) is designed to safely monitor blood clotting
activity in patients on anticoagulation therapy, specifically warfarin. The system can be
prescribed for patient use at home or can be used in the physician’s office or clinic. The system
consists of a portable, quantitative instrument and disposable test cuvettes and delivers results
in minutes.
The Hgb Pro Professional Hemoglobin Testing System
The Hgb Pro Professional Hemoglobin Testing System (“Hgb Pro”) is used by professionals,
mainly in the physician’s office, to test for anemia. Hgb Pro delivers quick results from a small
blood sample placed on a disposable test strip inserted into a hand-held test meter.
The ProTime and Hgb Pro products are sold into the alternate site non-hospital point-of-care
segment of the market comprised of physicians’ offices, long-term care facilities, clinics,
visiting nurse associations and home healthcare companies.
5
Table of Contents
Incision Products
The Tenderfoot Heel Incision Device (“Tenderfoot”), the Tenderlett Finger Incision Device
(“Tenderlett”) and the Surgicutt Bleeding Time Device (“Surgicutt”) are used by medical
professionals to obtain a patient’s blood sample for diagnostic testing. The Tenderfoot is a “heel
stick” used for infant testing, the Tenderlett is used for finger incisions and the Surgicutt is
used to perform screening tests to determine platelet function. These devices feature permanently
retracting blades for safe incision with minimal pain, as compared to traditional lancets, which
puncture the skin.
These products are sold to both the hospital point-of-care and alternate site point-of-care
segments of the market. These products offer certain advantages, command a premium over the
competition and are sold in the higher end of the market.
PRODUCT SEGMENTS
Our Cardiovascular division represented 75%, 69% and 61% of our product sales in 2009, 2008
and 2007, respectively. Our ITC division represented 25%, 31% and 39% of our total product sales in
2009, 2008 and 2007, respectively. For financial information related to our segments for each of
the past three years, please see Item 8, Note 15, “Enterprise and Related Geographic Information,” in our consolidated financial statements.
OUR MARKETS
Cardiovascular Division
Our VAD products primarily serve patients suffering from late-stage HF. HF is a chronic
disease that occurs when degeneration of the heart muscle reduces the pumping power of the heart,
causing the heart to become too weak to pump blood at a level sufficient to meet the body’s
demands. The condition can be caused by arterial and valvular diseases or a cardiomyopathy, which
is a disease of the heart muscle itself. Other conditions, such as high blood pressure or diabetes,
also can lead to HF.
According to estimates by the American Heart Association, 5.0 million people suffer from HF in
the U.S. and approximately 550,000 new cases are diagnosed each year. While the number of treatment
options for earlier stage HF has increased in recent years, pharmacologic therapies remain the most
widely used approach for treatment of HF. These drug therapies include angiotensin-converting
enzyme (“ACE”) inhibitors, anti-coagulants and beta-blockers, which facilitate blood flow, thin the
blood or help the heart work in a more efficient manner. In addition to the use of VADs, other
procedures addressing HF include angioplasty, biventricular pacing, valve replacement, bypass and
left ventricular reduction surgery.
Despite attempts to manage HF through drug therapy, the only curative treatment for
late-stages of the disease is heart transplantation. Unfortunately, the number of donor hearts
available each year can meet the needs of only a small number of patients who could benefit from
transplantation. The United Network for Organ Sharing reported that there were approximately 2,300
hearts available for transplant in the U.S. in the most recent twelve months reported. At any given
time, approximately 2,700 patients are on the U.S. national transplant waiting list, and we believe
a comparable number of patients are waiting in Europe. The median wait time for a donor heart is
approximately nine months; many patients have to wait as long as two years.
In the U.S., there are currently two FDA-approved indications for the long-term use of VADs in
patients with HF: as Destination Therapy and as a BTT. In addition to the chronic HF markets, MCS
devices are also approved for use for acute HF following and during cardiac surgery. All four
indications are summarized below.
Destination Therapy
On January 20, 2010, we received approval to market the HeartMate II for DT. The National
Institute for Health estimated that the Destination Therapy application represents a market
opportunity of up to 100,000 patients in the U.S. For these late-stage HF patients, drug therapy is
currently the only other treatment available. With drug therapy, the two-year survival rate for
these patients is approximately 8%. We believe that the success in transitioning this market from
maximum drug therapy to VADs is partially dependent on the development of the market for our
HeartMate product line.
6
Table of Contents
In April 2009, we filed a premarket approval (“PMA”) Supplement with the FDA seeking HeartMate
II approval for Destination Therapy, HF patients who are not eligible for heart transplantation
that included two-year data on a pivotal study cohort of 200 randomized patients enrolled at 38
centers. Patients in the HeartMate II Destination Therapy trial were randomized to the HeartMate
II or the HeartMate XVE on a 2:1 basis, respectively. The filing included data on adjunctive
cohorts totaling an additional 409 patients, including those who had been originally supported by a
HeartMate XVE, and based on a need for a device replacement, elected to receive a HeartMate II, was
approved for use in patients enrolled under continuous access protocols (“CAP”). Effective January
20, 2010 the HeartMate II can be used in patients with New York Heart Association Class IIIB and
IV end-stage left ventricular failure who have received optimal medical therapy for at least
forty-five of the last sixty days, and who are not candidates for cardiac transplantation.
Bridge-to-Transplantation
VADs provide additional cardiac support for patients with late-stage HF waiting for a donor
heart. Approximately 30%-40% of the patients on the waiting list for a heart transplant in the U.S.
receive a VAD. We believe that the percentage of patients bridged to transplant will continue to
increase as surgeons’ level of comfort with the technology increases, particularly for longer-term
support cases. There are currently five devices approved in the U.S. as a BTT in adults that are
commercially marketed, four of which are Thoratec devices.
Post-Cardiotomy Myocardial Recovery Following Cardiac Surgery
In addition to chronic HF, our devices are also used for patients who suffer from acute
cardiac failure after undergoing cardiac surgery. Some patients have difficulty being weaned off
heart/lung machines after surgery, a complication that arises in open-heart procedures. Many of
these patients ultimately die from HF when the heart, weakened by disease and the additional trauma
of surgery, fails to maintain adequate blood circulation. We believe that only a small portion of
this market is currently being treated with VADs and that this patient population could benefit
substantially from the use of our FDA-approved PVAD and IVAD products.
Cardiac Surgery Support
In addition to the longer term mechanical circulatory support indications, the CentriMag is
approved to provide MCS for periods appropriate to cardiopulmonary bypass and for circulatory
support when complete cardiopulmonary bypass is not necessary, for example during valvuloplasty,
mitral valve reoperation, surgery of the vena cava or aorta, or liver transplants.
ITC Division
Point-of-Care Diagnostics Products
Our point-of-care blood diagnostic test systems provide fast, accurate blood test results to
improve patient management, reduce healthcare costs and improve patient outcomes. These products
are sold into the hospital point-of-care and alternate site point-of-care segments of the market
including directly to patients. We believe that the market growth for point-of-care diagnostic
products is driven by greater convenience and ease of use for the clinician and patient. In
addition, in the case of the ProTime monitoring of oral anticoagulants, clinical studies have shown
that more frequent monitoring results in patients staying in their therapeutic range. More frequent
monitoring is made possible by patients testing themselves at home, in addition to being tested in
a doctor’s office, when appropriate.
Incision Products
Our incision products are used by professionals to obtain a patient’s blood sample for
diagnostic testing. Our incision products are sold into both the hospital point-of-care and the
alternate site point-of-care segments of the market. All products feature permanently retracting
blades for a safe, less painful incision as compared to traditional lancets, which puncture the
skin.
7
Table of Contents
OUR STRATEGY
Our strategy to maintain and expand our leadership position is comprised of the following
market and product development activities:
Focus on and partner with leading heart centers. We have developed long-standing relationships
with leading cardiovascular surgeons, heart failure cardiologists and heart centers worldwide. We
believe that no other cardiac assist company enjoys the same depth of relationships and access to
these customers. These relationships are an important part of our growth strategy, particularly for
the development and introduction of new products and the pursuit of additional indications for our
existing products. We continue our investment in building these relationships through cardiology
education outreach programs, including those in our Heart Hope Program. Our Market Development
Managers work in partnership with our VAD centers to increase the awareness of MCS and VADs in the
cardiology community.
Expand the use of VADs in existing segments. We plan to treat a greater number of patients in
advanced stage HF. To accomplish this, we are building upon our existing relationships with leading
cardiac surgeons and heart failure cardiologists in both transplant and open heart centers and
using our existing sales channels to gain acceptance and adoption of our products in the major
hospitals that perform open heart surgery.
| • | Destination Therapy Expansion. On January 20, 2010, we received approval to market the HeartMate II for Destination Therapy in the treatment of late-stage HF patients who are not candidates for heart transplant. In October 2003, the Centers of Medicare and Medicaid Services (“CMS”) issued a favorable National Coverage decision covering reimbursement for the use of left ventricular assist systems that are approved by the FDA for treating Destination Therapy in late-stage HF patients. | ||
| • | Mechanical Circulatory Support Expansion. We continue to expand awareness of MCS through education and outreach programs. We continue to enhance current products and develop new products including a miniaturized pump based on the HeartMate II platform and the HeartMate III, a magnetically levitated centrifugal continuous pump. | ||
| • | Center Expansion. In 2009, we added nineteen new HeartMate II centers, bringing the total to 120 centers in North America. Outside of North America, we added fourteen new HeartMate II centers, bringing the total to 91 centers. |
Increase
our market presence through strategic alliances, joint ventures and
acquisitions. In addition to increasing our presence in the HF, cardiovascular disease,
point-of-care and incision markets through internal growth, we continue to evaluate strategic
alliances, joint ventures, acquisitions and related business development opportunities.
| • | Acute HF segment. In August 2006, we entered into a distribution agreement with Levitronix to distribute the CentriMag in the U.S. This agreement allows us to expand more broadly beyond transplant centers and enables us to better address opportunities in short-term patient recovery. | ||
| • | Point-of-Care segment. In October 2006, we acquired A-VOX Systems, Inc. (“Avox”), a point-of-care company that developed and manufactured portable, bedside AVOXimeter systems to assist clinicians in assessing a patient’s oxygenation status. We sell these systems along with our HEMOCHRON and IRMA point-of-care products and our data management system that connects all of these systems together. | ||
| • | Chronic HF segment. In December 2007, we made a nominal investment in Acorn Cardiovascular, Inc., a medical device company that has intellectual property rights to the CorCapTM Cardiac Support Device (“CSD”), to support patients with heart failure. The CorCap CSD is a mesh wrap that is placed around the heart to support and relieve stress on the heart muscle. The CorCap CSD is intended to improve the heart’s size, shape and function. The CorCap CSD is CE Mark approved in Europe and is in clinical trials in the U.S. |
Obtain approval for new products. On January 20, 2010, we received FDA approval of our PMA
allowing the use of our HeartMate II for Destination Therapy.
In November 2008, Levitronix received approval from the FDA for a humanitarian device
exemption for the CentriMag Right Ventricular Assist System for temporary circulatory support up to
thirty days for patients in cardiogenic shock due to acute right ventricular failure.
8
Table of Contents
Offer a broad range of products. Our MCS devices provide circulatory support for the heart and
have been clinically proven to improve patient survival and quality of life. We currently offer the
widest range of MCS devices to cover indications for use ranging from acute to long-term support.
We believe that our broad and diverse product offering represents an important competitive
advantage because it allows us to address the various preferences of surgeons, the clinical needs
of a wide variety of patients, and the economic requirements of third-party payors. We intend to
further broaden our product line through internal development, acquisition and licensing.
Develop new products. We continue to invest aggressively in key technology innovations
including the ongoing introduction of the new HeartMate Go Gear peripherals. In addition, we
announced the development program for a smaller HeartMate II. This program will leverage our
already proven HeartMate II platform, but represents a dramatic reduction in the size of the device
that will facilitate flexibility for implantation and address the need for either full or partial
flow. We plan to conduct an initial animal implant with this technology during the first quarter
of 2010. We believe this will have a significant impact on continuing to advance the HeartMate II
and enable us to address a broadening population of advanced-stage HF patients in the coming years.
In the third quarter of 2009 we launched our new external peripherals, Go Gear, for the
HeartMate product line, including new batteries, charger and power module. These enhancements are
designed to provide an improved quality of life to patients by offering them additional freedom and
mobility.
We are also focused on the HeartMate III, which is a magnetically levitated, centrifugal,
continuous flow pump. We are continuing to advance the development of the system, combining the
benefits of full magnetic levitation in a smaller pump capable of being implanted less invasively,
which we believe will have important clinical benefits including reduced rates of adverse events.
We are currently working on our second iteration of this smaller HeartMate III platform and expect
to perform our first animal implant in the first quarter of 2010.
As part of our focus on the continued development of new and innovative mechanical support
solutions, we acquired intellectual property assets of Orqis Medical in the fourth quarter of 2009
and we purchased certain assets from Getinge AB and Ventracor in the first quarter of 2010.
Increase cost effectiveness of the therapies that employ our products. While Medicare data
indicates the cost of implanting a VAD for Destination Therapy is tracking similarly to that of a
heart, liver or other major organ transplant, cost remains a concern for our customers. In October
2003, CMS issued a favorable National Coverage decision covering reimbursement for the use of left
ventricular assist systems that are approved by the FDA for treating Destination Therapy in
late-stage HF patients. We work closely with VAD centers to develop the Destination Therapy market
through either previous recognition by Medicare or the Joint Commission certification program for
Destination Therapy, which we believe will ultimately improve the cost effectiveness of this
therapy. We also are expanding our market education and training programs, and will continue to
make improvements that enhance the performance and cost effectiveness of our products.
SALES AND MARKETING
Mechanical Circulatory Support Products
Hospitals that perform open heart surgery and heart transplants are the potential customers
for our MCS products. We estimate that 110 of the approximately 1,000 hospitals in the U.S. that
perform open-heart surgery also perform heart transplants. We actively market to heart transplant
hospitals and large cardiac surgery centers as well as to approximately 120 heart transplant
hospitals in Europe.
We have recruited and trained experienced cardiovascular sales specialists who sell our
circulatory support systems throughout the world. Our sales force is complemented by direct
clinical specialists and Market Development Managers. The clinical specialists conduct clinical
educational seminars, assist with VAD implants and resolve clinical questions or issues. Our Market
Development Managers work with our leading VAD centers to generate referrals and increase awareness
in the cardiology community regarding MCS. We partner with universities, experienced clinicians and
opinion leaders to assist with expanding clinical educational needs.
In addition to our direct selling efforts, we have a network of international distributors who
cover certain markets representing the majority of our remaining VAD sales potential. Our sales and
marketing initiatives include direct mail, education seminars, symposia, equipment purchase and
rental programs and journal advertisements, all common in the cardiovascular device market.
9
Table of Contents
The time from the initial contact with the cardiac surgeon until purchase is generally between
nine and eighteen months, due to the expense of the product and common hospital capital equipment
acquisition procedures. Upon receipt of a purchase order, we usually ship the product within thirty
days to meet the surgeon’s requirements. Hospitals and other medical institutions that acquire a
VAD system generally purchase VAD pumps, related disposables and training materials, and purchase
or rent two of the associated pump drivers (to ensure that a backup driver is available).
The introduction of a VAD system in a hospital or other medical facility requires that the
surgical and clinical support personnel possess certain product expertise. We provide initial
training and “best practice” instruction for these personnel, along with a variety of training
materials that accompany the initial delivery of our VAD products, including instructions for use,
patient management manuals and assorted videos. We provide clinical support during implants and
provide twenty-four hour access to clinically trained personnel. In addition, our sales force helps
customers understand and manage reimbursement from third-party payors. We believe that these
VAD-related services are an important part of the value that we provide to hospitals and patients.
Vascular Graft Products
We market Vectra through distributors in the U.S., and selected countries in Europe, the
Middle East, Northern Africa and Japan.
Point-of-Care Diagnostics
We currently sell in the U.S. directly to hospitals. In the alternate site market segment we
sell our products through national and regional distributors, such as Cardinal Health, Inc.,
Physician Sales and Service, Inc., IDEXX Laboratories, Inc. and Quality Assured Services, Inc.
Outside the U.S., our European staff sells principally to third party distributors.
Incision Products
Our incision products are sold worldwide by distributors. In 2009, our largest incision
distributor in the U.S. market was Cardinal Healthcare, Inc. and our largest distributor in the
International market was Izasa, S.A.
COMPETITION
Competition from pharmaceutical companies, gene- and cell-based therapies, medical device
companies and medical device divisions of healthcare companies is intense and is expected to
increase. The vast majority of VAD-eligible patients still receives pharmacological treatment
instead of a VAD. In our Cardiovascular division, we therefore continue to expect new competitors
both from the pharmacological and the medical device space. Among the medical device competitors
are Terumo Heart, Inc., HeartWare International Inc., AbioMed, Inc., Jarvik Heart, Inc., MicroMed
Technology, Inc., SynCardia Systems, Inc., CircuLite, Inc., and WorldHeart Corporation in the U.S.
and Europe and Berlin Heart GmbH in Europe. Principal competitors to our ITC division include the
Diagnostic Division of Abbott Laboratories, Becton Dickinson and Company, Helena Laboratories
Corp., Instrumentation Laboratory Company, Inverness Medical Innovation, Inc., the Cardiac Surgery
Division of Medtronic, Inc., and Roche Diagnostics.
We believe that key competitive factors include the relative speed with which we can
develop products, complete clinical testing, receive regulatory approvals, achieve market
acceptance, provide high-quality, ongoing support, and manufacture and sell commercial quantities
of our products.
Large medical device companies dominate the markets in which our ITC division competes. We
expect that our growth in this market will be generated by gaining market share and from a shift of
testing from central laboratories to the hospital and alternate site point-of-care. However, the
point-of-care space is very competitive, and includes the following potential drivers:
| • | New competitors might enter the market with broader test menus. To address this risk, in the fourth quarter of 2006, we acquired Avox, which increased our test menu offering, and has also offered us the opportunity to develop the next generation system that combines blood gas, electrolyte and oxygenation testing in one machine. | ||
| • | New drug therapies under development may not require the intense monitoring of a patient’s coagulation necessary with the current anticoagulation drug of choice, Heparin. To try to mitigate this risk, we participate in clinical trials with key pharmaceutical companies to provide the hemostasis monitoring that will ultimately be required for new drug therapies. |
10
Table of Contents
PATENTS AND PROPRIETARY RIGHTS
We seek to patent certain aspects of our technology. We hold, or have exclusive rights to,
various U.S. and foreign patents. Except for the patents mentioned below and one patent pertaining
to the TLC-II, our VAD products are not protected by any other patents. We do not believe that this
lack of patent protection will have a material adverse effect on our ability to sell our VAD
systems because of the lengthy regulatory period required to obtain approval of a VAD. Several
patents cover aspects of our HeartMate product line.
We hold several patents on the HeartMate II axial blood flow pump and transcutaneous energy
transmission technology, the remaining duration of which ranges from five to twelve years. In
August 1998, we obtained a license to incorporate technology developed by Sulzer Electronics Ltd.
(“Sulzer”) and Lust Antriebstechnik GmbH (“Lust”) into the HeartMate III. HeartMate III is a
miniature centrifugal pump featuring a magnetically levitated rotor with a bearingless motor that
was originally developed by Sulzer and Lust. The license from Sulzer and Lust gives us the
exclusive right to use in our HeartMate products technology protected by several U.S. and foreign
patents covering implantable bearingless motors for the duration of those patents, subject to our
payment of royalties. In December 2000, we were informed by Sulzer that it had sold all of its
business in the bearingless motor and magnetic bearing fields to Levitronix GmbH and had assigned
its portion of the agreements between Sulzer and us to Levitronix GmbH. We believe that the license
remains in full force and effect.
In addition, aspects of our blood coagulation, blood gas, blood electrolytes, blood chemistry
and skin incision device products are covered by patents directed to tube-and-micro-coagulation
whole blood analysis, including test methods, reagents and integral (on-board) controls, thick film
electrochemical analysis of blood gases, blood electrolytes, and blood chemistry, and low trauma
skin incision devices for capillary blood sampling, and methods of manufacturing such devices.
Several patents cover aspects of our proprietary biomaterials technology and skin incision products
from two to thirteen years. The duration remaining on some of our skin incision related patents
ranges from two to thirteen years, and two to thirteen years on our blood coagulation, blood gas,
blood electrolytes, oxygenation and blood chemistry patents. In addition, the duration remaining on
our graft related patents range from five to fourteen years. During the term of our patents, we
have the right to prevent third parties from manufacturing, marketing or distributing products that
infringe upon our patents.
We also hold, or have exclusive rights to, several international patents.
We have developed technical knowledge that, although non-patentable, we consider to be
significant in enabling us to compete. It is our policy to enter into confidentiality agreements
with each of our employees prohibiting the disclosure of any confidential information or trade
secrets. In addition, these agreements provide that any inventions or discoveries by employees
relating to our business will be assigned to us and become our sole property.
Despite our patent rights and policies with regard to confidential information, trade secrets
and inventions, we may be subject to challenges to the validity of our patents, claims that our
products allegedly infringe the patent rights of others and the disclosure of our confidential
information or trade secrets. These and other related risks are described more fully under the
heading “Our inability to protect our proprietary technologies or an infringement of others’
patents could harm our competitive position” in the “Risk Factors” section of this Annual Report on
Form 10-K.
At this time, we are not a party to any material legal proceedings that relate to patents or
proprietary rights.
GOVERNMENT REGULATIONS
Regulation by governmental authorities in the U.S. and foreign countries is a significant
factor in the manufacture and marketing of our current and future products and in our ongoing
product research and development activities. All of our proposed products will require regulatory
approval prior to commercialization. In particular, medical devices are subject to rigorous
pre-clinical testing as a condition of approval by the FDA and by similar authorities in foreign
countries.
11
Table of Contents
U.S. Regulations
In the U.S., the FDA regulates the design, manufacture, distribution and promotion of medical
devices pursuant to the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its regulations. Our MCS
systems, blood coagulation testing devices, skin incision devices, and Vectra graft products are
regulated as medical devices. To obtain FDA approval to market VADs similar to those under
development, the FDA requires proof of safety and efficacy in human clinical trials performed under
an Investigational Device Exemption (“IDE”). An IDE application must contain pre-clinical test data
supporting the safety of the product for human investigational use, information on manufacturing
processes and procedures, proposed clinical protocols and other information. If the IDE application
is accepted, human clinical trials may begin. The trials must be conducted in compliance with FDA
regulations and with the approval of one or more institutional review boards. Clinical trials are
subject to central registration requirements, such as on
www.clinicaltrials.gov (although none of
this information is, or should be deemed to be, incorporated by reference into this Annual Report
on Form 10-K). The results obtained from these trials, if satisfactory, are accumulated and
submitted to the FDA in support of either a PMA application, a PMA Supplement or a 510(k) premarket
notification. There are substantial user fees that must be paid at the time of PMA, PMA Supplement
or 510(k) submission to the FDA to help offset the cost of scientific data review that is required
before the FDA can determine if the device is approvable.
A PMA Supplement is required to make modifications to a device or application approved by a
PMA. A PMA Supplement must be supported by extensive preclinical data, and sometimes human clinical
data, to prove the safety and efficacy of the device with respect to the modifications disclosed in
the supplement. By regulation, the FDA has 180 days to review a PMA application, during which time
an FDA advisory committee of outside experts may be required to evaluate the application and
provide recommendations to the FDA. While the FDA has approved PMA applications within the allotted
time period, reviews can occur over a significantly protracted period, in some cases up to eighteen
months or longer, and a number of devices have never been cleared for marketing. This is a lengthy
and expensive process and there can be no assurance that FDA approval will be obtained.
Under the FDA’s requirements, if a manufacturer can establish that a newly developed device is
“substantially equivalent” to a legally marketed predicate device, the manufacturer may seek
marketing clearance from the FDA to market the device by filing with the FDA a 510(k) premarket
notification. This is the process that is used to gain FDA market clearance for most of ITC’s
products. The 510(k) premarket notification must be supported by data establishing the claim of
substantial equivalence to the satisfaction of the FDA. The process of obtaining a 510(k) clearance
typically can take several months to a year or longer. If substantial equivalence cannot be
established, or if the FDA determines that the device should be subjected to a more rigorous
review, the FDA will require that the manufacturer submit a PMA application that must be approved
by the FDA prior to marketing the device in the U.S.
Both a 510(k) and a PMA, if approved, may include significant limitations on the indicated
uses for which a product may be marketed. FDA enforcement policy prohibits the promotion of
approved medical devices for unapproved uses. In addition, product approvals can be withdrawn for
failure to comply with regulatory requirements or the occurrence of unforeseen problems following
initial marketing.
On October 26, 2002, the FDA signed into law The Medical Device User Fee and Modernization Act
(“MDUFMA”) of 2002. On September 28, 2007, MDUFMA was reauthorized for fiscal years 2008-2012. This
law amends the FDCA and regulations to provide, among other things, the ability of the FDA to
impose user fees for medical device reviews. Our activities require that we make many filings with
the FDA that are subject to this fee structure. Although the precise amount of fees that we will
incur each year will be dependent upon the specific quantity and nature of our filings, these fees
could be a significant amount per year.
In addition, any products distributed pursuant to the above authorizations are subject to
continuing regulation by the FDA. Products must be manufactured in registered establishments and
must be manufactured in accordance with Quality System Regulations (“QSR”). The Medical Device
Reporting (“MDR”) regulations require that we report to the FDA any incident in which our products
may have caused or contributed to a death or serious injury or in which our product malfunctioned
and, if the malfunction were to recur, would likely cause or contribute to death or serious injury.
Furthermore, the FDA may at any time inspect our facilities to determine whether we have adequate
compliance with FDA regulations, including the QSR, which requires manufacturers to follow
stringent design, testing, control, documentation and other quality assurance procedures during all
aspects of the design and manufacturing process. Following an inspection of our facilities, in
January 2009, ITC received an FDA Form 483 that listed a significant number of observations related
to the adequacy of ITC’s quality system. In March 2009, ITC submitted a response to the FDA Form
483 and then met with the FDA to discuss its implementation. We are providing updates to the FDA
on a regular basis outlining our progress and we continue to implement the preventative and
corrective actions to address the observations raised in the report.
12
Table of Contents
We are also subject to regulation by various state authorities, which may inspect our
facilities and manufacturing processes and enforce state regulations. Failure to comply with
applicable state regulations may result in seizures, injunctions or other types of enforcement
actions.
Healthcare Regulation
Our business is subject to extensive federal and state government regulation. This includes
the federal Anti-Kickback Law and similar state anti-kickback laws, the federal False Claims Act,
and the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) and similar state
laws addressing privacy and security. Although we believe that our operations materially comply
with the laws governing our industry, it is possible that non-compliance with existing laws or the
adoption of new laws or interpretations of existing laws could adversely affect our financial
performance.
Fraud and Abuse Laws
The healthcare industry is subject to extensive federal and state regulation. In particular,
the federal Anti-Kickback Law prohibits persons from knowingly and willfully soliciting, receiving,
offering or providing remuneration, directly or indirectly, to induce either the referral of an
individual, or the furnishing, recommending or arranging for a good or service, for which payment
may be made under a federal healthcare program such as the Medicare and Medicaid programs. The
definition of “remuneration” has been broadly interpreted to include anything of value, including
for example gifts, discounts, the furnishing of supplies or equipment, credit arrangements,
payments of cash, waivers of payments, ownership interests, and providing anything at less than its
fair market value. In addition, there is no one generally accepted definition of intent for
purposes of finding a violation of the Anti-Kickback Law. For instance, one court has stated that
an arrangement will violate the Anti-Kickback Law where any party has the intent to unlawfully
induce referrals. In contrast, another court has opined that a party must engage in the proscribed
conduct with the specific intent to disobey the law in order to be found in violation of the
Anti-Kickback Law. The lack of uniform interpretation of the Anti-Kickback Law makes compliance
with the law difficult. The penalties for violating the Anti-Kickback Law can be severe. These
sanctions include criminal penalties and civil sanctions, including fines, imprisonment and
possible exclusion from the Medicare and Medicaid programs.
The Anti-Kickback Law is broad, and it prohibits many arrangements and practices that are
lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Law is
broad and may technically prohibit many innocuous or beneficial arrangements within the healthcare
industry, the U.S. Department of Health and Human Services issued regulations in July of 1991,
which the Department has referred to as “safe harbors.” These safe harbor regulations set forth
certain provisions which, if met in form and substance, will assure healthcare providers and other
parties that they will not be prosecuted under the federal Anti-Kickback Law. Additional safe
harbor provisions providing similar protections have been published intermittently since 1991. Our
arrangements with physicians, physician practice groups, hospitals and other persons or entities
who are in a position to refer may not fully meet the stringent criteria specified in the various
safe harbors. Although full compliance with these provisions ensures against prosecution under the
federal Anti-Kickback Law, the failure of a transaction or arrangement to fit within a specific
safe harbor does not necessarily mean that the transaction or arrangement is illegal or that
prosecution under the federal Anti-Kickback Law will be pursued. Conduct and business arrangements
that do not fully satisfy one of these safe harbor provisions may result in increased scrutiny by
government enforcement authorities such as the U.S. Department of Health and Human Services Office
of Inspector General (“OIG”).
Many states have adopted laws similar to the federal Anti-Kickback Law. Some of these state
prohibitions apply to referral of patients for healthcare services reimbursed by any source, not
only the Medicare and Medicaid programs. Although we believe that we comply with both federal and
state anti-kickback laws, any finding of a violation of these laws could subject us to criminal and
civil penalties or possible exclusion from federal or state healthcare programs. Such penalties
would adversely affect our financial performance and our ability to operate our business.
13
Table of Contents
HIPAA created new federal statutes to prevent healthcare fraud and false statements relating
to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a
scheme to defraud any healthcare benefit program, including private payors. A violation of this
statute is a felony and may result in fines, imprisonment or exclusion from government sponsored
programs such as the Medicare and Medicaid programs. The false statements statute prohibits
knowingly and willfully falsifying, concealing or covering up a material fact or making any
materially false, fictitious or fraudulent statement in connection with the delivery of or payment
for healthcare benefits, items or services. A violation of this statute is a felony and may result
in fines or imprisonment or exclusion from government sponsored programs. Both federal and state
government agencies are continuing heightened and coordinated civil and criminal enforcement
efforts. As part of announced enforcement agency work plans, the federal government will continue
to scrutinize, among other things, the billing practices of hospitals and other providers of
healthcare services. The federal government also has increased funding to fight healthcare fraud,
and it is coordinating its enforcement efforts among various agencies, such as the U.S. Department
of Justice, the OIG and state Medicaid fraud control units. We believe that the healthcare industry
will continue to be subject to increased government scrutiny and investigations.
Federal False Claims Act
Another trend affecting the healthcare industry is the increased use of the federal False
Claims Act and, in particular, actions under the False Claims Act’s “whistleblower” provisions.
Those provisions allow a private individual to bring actions on behalf of the government alleging
that the defendant has defrauded the federal government. After the individual has initiated the
lawsuit, the government must decide whether to intervene in the lawsuit and to become the primary
prosecutor. If the government declines to join the lawsuit, then the individual may choose to
pursue the case alone, in which case the individual’s counsel will have primary control over the
prosecution, although the government must be kept apprised of the progress of the lawsuit. Whether
or not the federal government intervenes in the case, it will receive the majority of any recovery.
If the litigation is successful, the individual is entitled to no less than 15%, but no more than
30%, of whatever amount the government recovers. The percentage of the individual’s recovery
varies, depending on whether the government intervened in the case and other factors. Recently, the
number of suits brought against healthcare providers by private individuals has increased
dramatically. In addition, various states are considering or have enacted laws modeled after the
federal False Claims Act. Under the Deficit Reduction Act of 2005 (“DRA”) states are being
encouraged to adopt false claims acts similar to the federal False Claims Act, which establish
liability for submission of fraudulent claims to the State Medicaid program and contain
whistleblower provisions. Even in instances when a whistleblower action is dismissed with no
judgment or settlement, we may incur substantial legal fees and other costs relating to an
investigation. Future actions under the False Claims Act may result in significant fines and legal
fees, which would adversely affect our financial performance and our ability to operate our
business.
Further, on May 20, 2009, President Obama signed into law the Fraud Enforcement and Recovery
Act of 2009, which greatly expanded the types of entities and conduct subject to the False Claims
Act. We strive to ensure that we meet applicable billing requirements. However, the costs of
defending claims under the False Claims Act, as well as sanctions imposed under the Act, could
significantly affect our financial performance.
Health Insurance Portability and Accountability Act of 1996
In addition to creating the new federal statutes discussed above, HIPAA also establishes
uniform standards governing the conduct of certain electronic healthcare transactions and
protecting the security and privacy of individually identifiable health information maintained or
transmitted by healthcare providers, health plans and healthcare clearinghouses. Three standards
have been promulgated under HIPAA with which we currently are required to comply. We must comply
with the Standards for Privacy of Individually Identifiable Health Information (“Privacy
Standards”), which restrict our use and disclosure of certain individually identifiable health
information. We have been required to comply with the Privacy Standards since April 14, 2003.
14
Table of Contents
The American Recovery and Reinvestment Act of 2009, commonly referred to as the economic
stimulus package signed into law on February 17, 2009, dramatically expanded, among other things,
(1) the scope of HIPAA to also include “business associates,” or independent contractors who
receive or obtain protected health information (“PHI”) in connection with providing a service to
the covered entity, (2) substantive security and privacy obligations, including new federal
security breach notification requirements to affected individuals and Department of Health and
Human Services and potentially media outlets, (3) restrictions on marketing communications and a
prohibition on covered entities or business associates from receiving remuneration in exchange for
PHI, and (4) the civil and criminal penalties that may be imposed for HIPAA violations, increasing
the annual cap in penalties from $25,000 to $1.5 million per year. We believe that we are not
generally a business associate under HIPAA and we believe that we are in compliance with all of the
applicable HIPAA standards, rules and regulations. However, if we fail to comply with these
standards, we could be subject to criminal penalties and civil sanctions. In addition to federal
regulations issued under HIPAA, some states have enacted privacy and security statutes or
regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases
it may be necessary to modify our operations and procedures to comply with the more stringent state
laws, which may entail significant and costly changes for us. We believe that we are in compliance
with such state laws and regulations. However, if we fail to comply with applicable state laws and
regulations, we could be subject to additional sanctions.
International Regulations
We are also subject to regulation in each of the foreign countries where our products are
sold. These regulations relate to product standards, packaging and labeling requirements, import
restrictions, tariff regulations, duties and tax requirements. Many of the regulations applicable
to our products in these countries are similar to those of the FDA. The national health or social
security organizations of certain countries require our products to be qualified before they can be
marketed in those countries.
In order to be positioned for access to European and other international markets, we sought
and obtained certification under the International Standards Organization (“ISO”) 13485 standards.
ISO 13485 is a set of integrated requirements, which when implemented, form the foundation and
framework for an effective quality management system. These standards were developed and published
by the ISO, a worldwide federation of national bodies, founded in Geneva, Switzerland in 1947. ISO
has more than 90 member countries and ISO certification is widely regarded as essential to enter
Western European markets. We obtained ISO 13485:2003 Certification in February 2006. Since 1998,
all companies are required to obtain CE Marks for medical devices sold or distributed in the
European Union. The CE Mark is an international symbol of quality. With it, medical devices can be
distributed within the European Union. A prerequisite for obtaining authority to CE Mark products
is to achieve full quality system certification in accordance with ISO 13485 and European
Directives, such as the Medical Device Directive (“MDD”), In-Vitro Device Directive (“IVDD”) and
the Active Implantable Medical Device Directive (“AIMD”). These are quality standards that cover
design, production, installation and servicing of medical devices manufactured by us. We have the
ISO 13485 and appropriate MDD, IVDD or AIMD certification and authority to CE Mark all our devices
in commercial distribution, including our skin incision devices, blood coagulation testing devices,
Vectra graft and our VAD systems. We are also certified to be in compliance with the requirements
of the Canadian Medical Device Regulations at all Thoratec manufacturing sites, which certification
is required to sell medical devices in Canada.
Other Regulations
We are also subject to various federal, state and local laws and regulations relating to such
matters as safe working conditions, laboratory and manufacturing practices and the use, handling
and disposal of hazardous or potentially hazardous substances used in connection with our research
and development and manufacturing activities. Specifically, the manufacture of our biomaterials is
subject to compliance with federal environmental regulations and by various state and local
agencies. Although we believe we are in compliance with these laws and regulations in all material
respects, we cannot provide assurance that we will not be required to incur significant costs to
comply with environmental laws or regulations in the future.
THIRD PARTY COVERAGE AND REIMBURSEMENT
Our products are purchased primarily by customers, such as hospitals, who then bill various
third party payors for the services provided to the patients. These payors, which include Medicare,
Medicaid, private health insurance companies and managed care organizations, reimburse our
customers based on established payment formulas that take into account part or all of the expenses
associated with these devices and the related procedures performed.
15
Table of Contents
The CMS, the agency responsible for administering the Medicare program, and a majority of
private insurers with whom we have dealt have approved reimbursement for our VADs and our
diagnostic and vascular graft products. Effective October 1, 2003, CMS issued a National Coverage
Determination for the use of the HeartMate XVE for treating Destination Therapy in late-stage HF
patients. Seventy-six centers are now covered and reimbursed for providing Destination Therapy
through either previous recognition by Medicare or the Joint Commission certification program for
Destination Therapy. With approval by the FDA on January 20, 2010, the HeartMate II will also be
covered by CMS. Prior to issuance of the 2003 national coverage determination, CMS covered VADs
only when used for BTT under certain circumstances.
In addition, since FDA approval of the HeartMate XVE for Destination Therapy, the majority of
private payors also reimburse for Destination Therapy which is reflected in their coverage
policies. In 2010, with the FDA approval of the HeartMate II for Destination Therapy, the majority
of the private payors will also reimburse for Destination Therapy which will be reflected in the
coverage policies. In December 2002, Blue Cross/Blue Shield Technology Evaluation Center agreed to
cover the use of VADs for Destination Therapy. The majority of local Blue Cross and Blue Shield
plans cover procedures for both BTT and long-term therapy indications. Since December 2002, the
majority of national insurance carriers, including Aetna, Cigna, Humana, United Health Group and
UNICARE, have policies covering the use of ventricular assist devices for FDA-approved indications,
including DT.
Effective March 2008, CMS approved coverage for the use of home Prothombin Time and
International Normalized Ratio monitoring for patients with mechanical heart valves, chronic atrial
fibrillation or venous thromboembolism on warfarin. Previously, only mechanical heart valve
patients were covered. This expanded coverage decision increases the market opportunity for our
ProTime system.
Healthcare laws in the U.S. are subject to ongoing changes, including changes to the amount of
reimbursement for hospital services. Currently, there are a number of pending federal legislative
proposals that could substantially change the way healthcare is financed by both governmental and
private insurers and may negatively impact payment rates for our products. We are unable to predict
whether any currently circulating congressional proposals will become law or in what form. Also,
from time to time there are a number of legislative, regulatory and other proposals both at the
federal and state levels; it remains uncertain whether there will be any future changes that will
be proposed or finalized and what effect, if any, such legislation or regulations would have on our
business.
MANUFACTURING
VADs and grafts for the Cardiovascular division are manufactured at our facility located in
Pleasanton, California. This facility has been inspected, approved and licensed by the FDA, State
of California Department of Health Services Food and Drug Section for the manufacture of medical
devices, and has received the ISO 13485:2003 Quality Systems certification. The manufacturing
processes consist of utilizing precision components fabricated from a variety of materials and
assembling these components into specific configurations governed by the VAD design requirements.
During the manufacturing process, the VAD assemblies are rigorously tested to meet rigid
operational and quality standards.
The manufacturing process relies on single sources of supply for several of the components
used to manufacture VADs. We are working to identify and validate alternate sources of supply for
critical components. Where alternate sources are not available, we are working to develop strategic
alliances with the supplier and closely manage inventories to assure the ongoing supply of product.
During 2009, the Cardiovascular division expanded the manufacturing facility located in
Pleasanton, California. The main focus of the expansion project was to provide adequate
manufacturing capacity to meet the proposed volumes created by FDA DT approval of the HeartMate II
product line. The renovated facility will have the necessary capacity to meet the requirements for
our VAD products for the next five to seven years.
The CentriMag product line is manufactured by Levitronix and distributed from our
manufacturing facility located in Pleasanton, California.
Our ITC division blood coagulation testing, blood oxygenation testing and skin incision
devices are manufactured in Edison, New Jersey, with the exception of the ProTime instrument and
the hemoglobin monitor, which are manufactured through single source third-party contract
manufacturers in China and Germany, respectively. Our blood gas analyzer devices are manufactured
in Roseville, Minnesota. The New Jersey and Minnesota facilities have been inspected, approved and
licensed by the FDA and applicable state regulators. In addition, these facilities maintain ISO
9001, ISO 13485 and Canadian (CMDCAS) ISO certifications.
16
Table of Contents
A significant amount of our ITC division manufacturing at these facilities is vertically
integrated, with only limited reliance on third parties, such as for the manufacture of printed
circuit boards and the sterilization and testing of products. We rely on single sources of supply
for some components manufactured at our New Jersey and Minnesota facilities, and use safety stocks
where there might be risk in qualifying a second supplier in a timely manner.
Both Cardiovascular and ITC have typically been able to fill orders from inventory and
historically have not had significant order backlogs. With the expanded manufacturing capacity for
both Cardiovascular and ITC, we will be in a position to accommodate the increased demand for our
products. Total backlog as of the end of fiscal 2009 and 2008 was none and $0.2 million,
respectively, for our Cardiovascular division, and $0.4 million and $1.7 million, respectively, for
our ITC division.
RESEARCH AND DEVELOPMENT
Our
research and development expenses in fiscal years 2009, 2008 and 2007 totaled $54.2 million, $52.9
million and $43.8 million, respectively. Research and development costs are largely project driven,
and fluctuate based on the level of project activity planned and subsequently approved and
conducted. The primary component of our research and development costs is employee salaries and
benefits. Projects related to our Cardiovascular division include advancing the HeartMate II
platform, such as efforts to improve the operation and performance of our VAD products and
accessories, along with efforts to develop new products, such as the development of a miniaturized
pump based on the HeartMate II platform and the development program for the HeartMate III. ITC
research and development projects typically involve developing instruments and disposable test
cuvettes or cartridges that will be used at the point-of-care. Research and development costs for
both divisions also include regulatory and clinical costs associated with our compliance with FDA
regulations and clinical trials such as the recently completed HeartMate II DT pivotal trial.
MAJOR CUSTOMERS AND FOREIGN SALES
We sell our products primarily to large hospitals and distributors. No customer accounted for
more than 10% of total product sales in fiscal years 2009, 2008 and 2007.
Sales originating outside the U.S. and U.S. export sales accounted for approximately 25%, 26%
and 28% of our total product sales for fiscal years 2009, 2008 and 2007, respectively. No
individual foreign country accounted for more than 10% of our net sales in any of the last three
fiscal years.
EMPLOYEES
As of January 2, 2010, we had a total of 1,258 employees, consisting of 1,163 full-time
employees and 95 temporary employees. Of our total employees, 1,228 are employed in the U.S. and 30
are employed in the U.K. and other European countries. None of our employees are covered by a
collective bargaining agreement. We consider relations with our employees to be good.
ADDITIONAL INFORMATION
Additional information about Thoratec is available on our website at http://www.thoratec.com
(although none of this information is, or should be deemed to be, incorporated by reference into
this Annual Report on Form 10-K). We make filings of our periodic reports to the Securities and
Exchange Commission (“SEC”), including annual reports on Form 10-K, quarterly reports on Form 10-Q
and current reports on Form 8-K, as well as amendments to those reports, available free of charge
on our website as soon as reasonably practicable following electronic filing of those reports with
the SEC.
| Item 1A. | Risk Factors |
Our businesses face many risks. The risks described below are what we believe to be the
material risks facing our company, however, they may not be the only risks we face. Additional
risks that we do not yet know of or that we currently believe are immaterial may also impair our
business operations. If any of the events or circumstances described in the following risk factors
actually occur, our business, financial condition or results of operations could suffer, and the
trading price of our common stock could decline significantly. Investors should consider the
following risks, as well as the other information included in this Annual Report on Form 10-K, and
other documents we file from time to time with the SEC, such as our quarterly reports on Form 10-Q,
our current reports on Form 8-K and any public announcements we make from time to time.
17
Table of Contents
If we fail to obtain approval from the FDA and from foreign regulatory authorities, we cannot
market and sell our products under development in the U.S. and in other countries, and if we fail
to comply with government regulations, including FDA Quality System Regulations, or our products
experience certain adverse events, the FDA or foreign regulatory authorities may withdraw our
market clearance or take other enforcement action.
Before we can market new products in the U.S., we must obtain PMA approval or 510(k) clearance
from the FDA. This process is lengthy and uncertain. In the U.S., one must obtain clearance from
the FDA of a 510(k) pre-market notification or approval of a more extensive submission known as a
PMA application. If the FDA concludes that any of our products do not meet the requirements to
obtain clearance under Section 510(k) of the FDCA, then we will be required to file a PMA
application. The process for a PMA application is lengthy, expensive and typically requires
extensive pre-clinical and clinical trial data.
We may not obtain clearance of a 510(k) notification or approval of a PMA application with
respect to any of our products on a timely basis, if at all. If we fail to obtain timely clearance
or approval for our products, we will not be able to market and sell them, thereby harming our
ability to generate sales. The FDA also may limit the claims that we can make about our products.
We also may be required to obtain clearance of a 510(k) notification, a new PMA, or a PMA
Supplement from the FDA before we can market products which have already been cleared, but which
have since been modified or we subsequently wish to market for new disease indications.
In addition, our medical device products and operations are subject to extensive regulation by
the FDA pursuant to the FDCA and various other federal, state and foreign governmental authorities.
Government regulations and foreign requirements specific to medical devices are wide ranging and
govern, among other things, design, development, manufacture, testing, labeling, storage,
marketing, distribution, promotion, record keeping, and approval or clearance. The FDA requires us
to adhere to Quality System Regulations (“QSR”), which include production design controls, testing,
quality control, labeling, packaging, sterilization, and storage and documentation procedures. The
FDA may at any time inspect our facilities to determine whether we have adequate compliance with
the FDA’s QSR and other regulatory requirements. Compliance with QSR for medical devices is
difficult and costly. In addition, we may not be found compliant as a result of future changes in,
or interpretations of, regulations by the FDA or other regulatory agencies.
Following an inspection of our facilities in January 2009, ITC received an FDA Form 483 that
listed a significant number of observations relating to the adequacy of ITC’s quality system. In
March 2009, ITC submitted a response to the FDA Form 483 and then met with the FDA to discuss its
implementation. We are providing updates to the FDA on a regular basis outlining our progress. If
we do not adequately address the FDA’s concerns, or we do not maintain compliance with the FDA’s
QSR in the future, the FDA may issue untitled letters, warning letters, impose fines, injunctions,
consent decrees, civil or criminal penalties, withdraw marketing clearance or approvals, require
product recalls, or take other enforcement action against ITC, which in each case would harm our
business.
Sales of our products outside the U.S. are subject to foreign regulatory requirements that
vary from country to country. The time required to obtain approvals from foreign countries may be
longer or shorter than that required for FDA approval, and requirements for foreign licensing may
differ from FDA requirements. In any event, if we fail to obtain the necessary approvals to sell
any of our products in a foreign country, or if any obtained approval is revoked or suspended, we
will not be able to sell those products there.
The federal, state and foreign laws and regulations regarding the manufacture and sale of our
products are subject to future changes, as are administrative interpretations and policies of
regulatory agencies. If we fail to comply with applicable federal, state or foreign laws or
regulations, we could be subject to enforcement actions. Enforcement actions could include product
seizures, recalls, withdrawal of clearances or approvals, and civil and criminal penalties, which
in each case would harm our business.
18
Table of Contents
If hospitals do not conduct Destination Therapy procedures using our VADs, market opportunities for
our product will be diminished.
The use of certain of our VADs as long-term therapy in patients who are not candidates for
heart transplantation (i.e., Destination Therapy patients) was approved by the FDA in 2002, and was
approved for coverage and reimbursement by the CMS, the agency responsible for administering the
Medicare program, in late 2003. We received FDA approval for the HeartMate II in Destination
Therapy on January 20, 2010.
The number of Destination Therapy procedures actually performed depends on many factors, many
of which are out of our direct control, including, but not limited to, the following:
| • | the number of CMS sites approved for Destination Therapy; | ||
| • | the clinical outcomes of Destination Therapy procedures relative to pharmacological, gene- and cell-based therapies, and other device-based alternatives; | ||
| • | cardiologists’ and referring physicians’ education regarding, and their commitment to, Destination Therapy; | ||
| • | the economics of the Destination Therapy procedure for individual hospitals, which include the costs of the VAD and related pre- and post-operative procedures and their reimbursement; | ||
| • | the impact of changes in reimbursement rates on the timing of purchases of VADs for Destination Therapy; and | ||
| • | the economics for individual hospitals of not conducting a Destination Therapy procedure, including the costs and related reimbursements of long-term hospitalization. |
The different outcomes of these and other factors, and their timing, will have a significant
impact on our future Cardiovascular product sales.
Physicians may not accept or continue to accept our current products and products under
development.
The success of our current and future products will require acceptance or continued acceptance
by cardiovascular and vascular surgeons, and other medical professionals. Such acceptance will
depend on clinical results and the conclusion by these professionals that our products are safe,
cost-effective and acceptable methods of treatment. Even if the safety and efficacy of our future
products are established, physicians may elect not to use them for a number of reasons. These
reasons could include the high cost of our VAD systems, restrictions on insurance coverage,
unfavorable reimbursement from healthcare payors, or use of alternative therapiesincluding
pharmacological, gene- and cell-based therapies, and other device based alternatives. Also,
economic, psychological, ethical and other concerns may limit general acceptance of our ventricular
assist, graft and other products.
We rely on specialized suppliers for certain components and materials in our products and
alternative suppliers may not be available.
We depend on a number of custom-designed components and materials supplied by other companies
including, in some cases, single source suppliers for components, instruments and materials used in
our VAD products and blood testing products. For example, single source suppliers currently
manufacture and supply our ProTime and Hemoglobin instruments and the heart valves used in our
HeartMate XVE product. The suppliers of our ProTime and Hemoglobin products are located in China
and Germany, respectively. We do not have long-term written agreements with most of our vendors and
receive components from these vendors on a purchase order basis only. If we need alternative
sources for key raw materials or component parts for any reason, such alternative sources may not
be available and our inventory may not be sufficient to fill orders before we find alternative
suppliers or begin manufacturing these components or materials ourselves. Cessation or interruption
of sales of circulatory support products or our point-of-care products may seriously harm our
business, financial condition and results of operations.
Alternative suppliers, if available, may not agree to supply us. In addition, FDA approval may
be required before using new suppliers or manufacturing our own components or materials which can
take additional time to procure. Existing suppliers could also become subject to an FDA enforcement
action, which could also disrupt our supplies. If alternative suppliers are not available, we may
not have the expertise or resources necessary to produce these materials or component parts
internally.
19
Table of Contents
Because of the long product development cycle in our business, suppliers may discontinue
components upon which we rely before the end of life of our products. In addition, the timing of
the discontinuation may not allow us time to develop and obtain FDA approval for a replacement
component before we exhaust our inventory of the legacy component.
If suppliers discontinue components on which we rely, we may have to:
| • | pay premium prices to our suppliers to keep their production lines open or to obtain alternative suppliers; | ||
| • | buy substantial inventory to last through the scheduled end of life of our product, or through such time that we expect to have a replacement product developed and approved by the FDA; or | ||
| • | stop shipping the product in which the legacy component is used once our inventory of the discontinued component is exhausted. |
Any of these interruptions in the supply of our materials could result in substantial
reductions in product sales and increases in our production costs.
We may encounter problems manufacturing our products.
We may encounter difficulties manufacturing products in quantities sufficient to meet demand.
We do not have experience in manufacturing some of our products in the commercial quantities that
might be required with FDA approval of those products and indications currently under development,
including the HeartMate II. If we have difficulty manufacturing any of our products, our sales may
prove lower than would otherwise be the case and our reputation, business, financial condition and
results of operations could be harmed.
Identified quality problems can result in substantial costs and write-downs.
FDA regulations require us to track materials used in the manufacture of our products, so that
any problems identified in a finished product can easily be traced back to other finished products
containing the defective materials. In some instances, identified quality issues require scrapping
or expensive rework of the affected lot(s), not just the tested defective product, and could also
require us to stop shipments.
In addition, because some of our products are used in situations where a malfunction can be
life threatening, identified material deficiencies or defects in design or manufacture or labeling
can result in the recall and replacement, generally free of charge, of substantial amounts of
product already implanted or otherwise in the marketplace. The FDA and similar foreign governmental
authorities have the authority to require the recall of commercialized products. In the case of
the FDA, the authority to require a recall must be based on an FDA finding that there is a
reasonable probability that the device would cause serious injury or death. Manufacturers may,
under their own initiative, recall a product if any material deficiency in a device is found. A
government-mandated or voluntary recall by us or one of our distributors could occur as a result of
component failures, manufacturing errors, design or labeling defects or other deficiencies and
issues. Recalls of any of our products would divert managerial and financial resources and have an
adverse effect on our financial condition and results of operations. We may initiate certain
voluntary recalls involving our products in the future. Companies are required to maintain certain
records of recalls, even if they are not reportable to the FDA. If we determine that certain of
those recalls do not require notification of the FDA, the FDA may disagree with our determinations
and require us to report those actions as recalls. A future recall announcement could harm our
reputation with customers, negatively affect our sales, and subject us to additional FDA
enforcement actions.
Any identified quality issue can therefore both harm our business reputation and result in
substantial costs and write-offs, which in either case could materially harm our business and
financial results.
If we fail to successfully introduce new products, our future growth may suffer.
As part of our growth strategy, we intend to develop and introduce a number of new products
and product improvements. We also intend to develop new indications for our existing products. For
example, we are currently developing updated versions of our HeartMate and point-of-care blood
diagnostics products. If we fail to commercialize any of these new products, product improvements
and new indications on a timely basis, or if they are not well accepted by the market, our future
growth may suffer.
20
Table of Contents
If we are unable to successfully complete the pre-clinical studies or clinical trials necessary to
support our PMA applications or PMA supplements, our ability to obtain new approvals will be
limited.
Before submitting a PMA application, we must successfully complete pre-clinical studies and
clinical trials to demonstrate that the product is safe and effective. Product development,
including pre-clinical studies and clinical trials, is a long, expensive and uncertain process and
is subject to delays and failure at any stage. Furthermore, the data obtained from the trial may be
inadequate to support approval of a PMA application. The commencement or completion of any of our
clinical trials may be delayed or halted, or be inadequate to support approval of a PMA
application, for numerous reasons, including, but not limited to, the following:
| • | the FDA or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold; | ||
| • | patients do not enroll in clinical trials at the rate we expect; | ||
| • | patients do not comply with trial protocols; | ||
| • | patient follow-up is not at the rate we expect; | ||
| • | patients experience adverse side effects; | ||
| • | patients die during a clinical trial, even though their death may not be related to our product candidates; | ||
| • | institutional review boards and third-party clinical investigators may delay or reject our trial protocol; | ||
| • | third-party clinical investigators decline to participate in a trial or do not perform a trial on our anticipated schedule or consistent with the clinical trial protocol, good clinical practices or other regulatory requirements; | ||
| • | third-party organizations do not perform data collection and analysis in a timely or accurate manner; | ||
| • | regulatory inspections of our clinical trials or manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials; | ||
| • | changes in governmental regulations or administrative actions; | ||
| • | the interim or final results of a clinical trial are inconclusive or unfavorable as to safety or efficacy; and | ||
| • | the FDA concludes that our trial design is inadequate to demonstrate safety and efficacy. |
The results of pre-clinical studies do not necessarily predict future clinical trial results,
and predecessor clinical trial results may not be repeated in subsequent clinical trials.
Additionally, the FDA may disagree with our interpretation of the data from our pre-clinical
studies and clinical trials, or may find the clinical trial design, conduct or results inadequate
to prove safety or efficacy, and may require us to pursue additional pre-clinical studies or
clinical trials, which could further delay the approval of our products. If we are unable to
demonstrate the safety and efficacy of our products in our clinical trials, we will be unable to
obtain regulatory approval to market our products. The data we collect from our current clinical
trials, our pre-clinical studies and other clinical trials may not be sufficient to support FDA
approval.
21
Table of Contents
Our inability to protect our proprietary technologies or an infringement of others’ patents could
harm our competitive position.
We rely on patents, trade secrets, copyrights, know-how, trademarks, license agreements and
contractual provisions to establish our intellectual property rights and protect our products.
These legal means, however, afford only limited protection and may not adequately protect our
rights. In addition, we cannot assure you that any of our pending patent applications will issue.
The U.S. Patent and Trademark Office may deny or significantly narrow claims made under patent
applications and the issued patents, if any, may not provide us with commercial protection. We
could incur substantial costs in proceedings before the U.S. Patent and Trademark Office or in any
future litigation to enforce our patents in court. These proceedings could result in adverse
decisions as to the validity and/or enforceability of our patents. In addition, the laws of some of
the countries in which our products are or may be sold may not protect our products and
intellectual property to the same extent as U.S. laws, if at all. We may be unable to protect our
rights in trade secrets and unpatented proprietary technology in these countries.
A majority of our VAD products generally are not protected by any patents. We rely principally
on trade secret protection and, to a lesser extent, patents to protect our rights to the HeartMate
product line. We rely principally on patents to protect our coagulation testing equipment, skin
incision devices, HEMOCHRON disposable cuvettes, IRMA analyzer, IRMA disposable cartridges,
AVOXimeter and Hgb Pro disposable test strips.
We seek to protect our trade secrets and unpatented proprietary technology, in part, with
confidentiality agreements with our employees and consultants. Although it is our policy to require
that all employees and consultants sign such agreements, we cannot assure you that every person who
gains or has gained access to such information has done or will do so. Moreover, these agreements
may be breached and we may not have an adequate remedy.
Our products may be found to infringe prior or future patents owned by others. We may need to
acquire licenses under patents belonging to others for technology potentially useful or necessary,
and such licenses may not be available to us. We could incur substantial costs in defending suits
brought against us on such patents or in bringing suits to protect our patents or patents licensed
by us against infringement.
Our future disposable cuvette test product sales by ITC could be affected by changes in monitoring
requirements for medical procedures.
ITC product sales are generated by medical procedures that require monitoring of coagulation
and blood gas parameters done in cardiovascular operating rooms and cardiac catheterization labs.
The sales of our disposable test products could decline if there were a significant reduction in
those medical procedures, which could harm our ITC business.
Since we depend upon distributors, if we lose a distributor or a distributor fails to perform, our
operations may be harmed.
With the exception of Canada and the larger countries in Europe, we sell our Thoratec and
HeartMate product lines in foreign markets through distributors. In addition, we sell our vascular
access graft products through the Bard Peripheral Vascular division of C.R. Bard Corporation (which
is also a competitor of ours) in the U.S. and selected countries in Europe, the Middle East and
Africa, and through Goodman Co. Ltd. in Japan. Substantially all of the international operations
and a large portion of the alternate site domestic operations of ITC are conducted through
distributors. For the year ended January 2, 2010, approximately 48% of ITC’s total product sales
were through distributors.
To the extent we rely on distributors, our success will depend upon the efforts of others,
over whom we may have little or no control. If we lose a distributor or a distributor fails to
perform to our expectations, our product sales and results of operations may be harmed.
22
Table of Contents
If we fail to compete successfully against our existing or potential competitors, our product sales
or operating results may be harmed.
Competition from pharmaceutical companies, gene- and cell-based therapies, medical device
companies and medical device divisions of healthcare companies is intense and is expected to
increase. The vast majority of VAD-eligible patients still receives pharmacological treatment
instead of a VAD. In our Cardiovascular division, we therefore continue to expect new competitors
both from the pharmacological and the medical device space. Among the medical device competitors
are Terumo Heart, Inc., HeartWare International Inc., AbioMed, Inc., Jarvik Heart, Inc., MicroMed
Technology, Inc., SynCardia Systems, Inc., CircuLite, Inc., and WorldHeart Corporation in the U.S.
and Europe and Berlin Heart GmbH in Europe. Principal competitors to our ITC division include the
Diagnostic Division of Abbott Laboratories, Becton Dickinson and Company, Helena Laboratories
Corp., Instrumentation Laboratory Company, Inverness Medical Innovation, Inc., the Cardiac Surgery
Division of Medtronic, Inc., and Roche Diagnostics.
Some of our competitors have substantially greater financial, technical, distribution,
marketing and manufacturing resources than we do, while other competitors have different
technologies that may achieve broader customer acceptance or better cost structures than our
products. Accordingly, our competitors may be able to develop, manufacture and market products more
efficiently, at a lower cost and with more market acceptance than we can. In addition, new drugs or
other devices may provide additional alternatives to VADs. We expect that the key competitive
factors will include the relative speed with which we can:
| • | develop products; | ||
| • | complete clinical testing; | ||
| • | receive regulatory approvals; | ||
| • | achieve market acceptance; and | ||
| • | manufacture and sell commercial quantities of products. |
Large medical device companies dominate the markets in which ITC competes. We expect that any
growth in this market will come from testing being shifted away from central laboratories to the
hospital and alternate site point-of-care. However, this space is very competitive and includes the
following potential drivers:
| • | New drug therapies under development may not require the intense monitoring of a patient’s coagulation that the current anti-coagulation drug of choice (Heparin) requires. | ||
| • | New competitors might enter the market with broader test menus. |
Any of the devices of our competitors in clinical trials and in development could prove to be
clinically superior, easier to implant, and/or less expensive than current commercialized devices,
thereby impacting Thoratec’s market share.
Our non-U.S. sales present special risks.
A substantial portion of our total sales occurs outside the U.S. We anticipate that sales
outside the U.S. and U.S. export sales will continue to account for a significant percentage of our
product sales and we intend to continue to expand our presence in international markets. Non-U.S.
sales are subject to a number of special risks. For example:
| • | we sell some of our products at a lower price outside the U.S.; | ||
| • | sales agreements with foreign customers may be difficult to enforce; | ||
| • | receivables may be difficult to collect through a foreign country’s legal system; | ||
| • | foreign customers may have longer payment cycles; | ||
| • | foreign countries may impose additional withholding taxes or otherwise tax our foreign income, impose tariffs or adopt other restrictions on foreign trade; |
23
Table of Contents
| • | U.S. export licenses may be difficult to obtain; | ||
| • | intellectual property rights may be (and often are) more difficult to enforce in foreign countries; | ||
| • | terrorist activity or war may interrupt distribution channels or adversely impact our customers or employees; and | ||
| • | fluctuations in exchange rates may affect product demand and adversely affect the profitability, in U.S. dollars, of products sold in foreign markets where payments are made in local currencies. |
Any of these events could harm our operations or financial results.
Fluctuations in foreign currency exchange rates could result in declines in our reported sales and
earnings.
Because some of our international sales are denominated in local currencies and not in U.S.
dollars, our reported sales and earnings are subject to fluctuations in foreign currency exchange
rates. At present, we use forward foreign currency contracts to protect the gains and losses
created by the re-measurement of non-functional currency denominated assets and liabilities.
However, we do not hedge foreign currency exposures that will arise from future sales. As a result,
sales occurring in the future that are denominated in foreign currencies may be translated into
U.S. dollars at a less favorable rate than our current exchange rate resulting in reduced revenues
and earnings.
The long and variable sales and deployment cycles for our VAD systems may cause our product sales
and operating results to vary significantly, which increases the risk of an operating loss for any
given fiscal period.
Our VAD systems have lengthy sales cycles and we may incur substantial sales and marketing
expenses and expend significant effort without making a sale. Even after making the decision to
purchase our VAD systems, our customers often deploy our products slowly. For example, the length
of time between initial contact with potential customers and the purchase of our VAD systems is
generally between nine and eighteen months. In addition, cardiac centers that buy the majority of
our products are usually led by cardiac surgeons who are heavily recruited by competing centers or
by centers looking to increase their profiles. When one of these surgeons moves to a new center we
sometimes experience a temporary but significant reduction in purchases by the departed center
while it replaces its lead surgeon. As a result, it is difficult for us to predict the quarter in
which customers may purchase our VAD systems and our product sales and operating results may vary
significantly from quarter to quarter, which increases the risk of an operating loss for us for any
given quarter. In particular, sales of our HeartMate XVE for Destination Therapy have been lower
than we had originally anticipated, and we cannot predict what level of revenues our HeartMate II
product will generate.
24
Table of Contents
Since our physician and hospital customers depend on third party reimbursement, if third party
payors fail to provide appropriate levels of reimbursement for our products, our results of
operations will be harmed.
Significant uncertainty exists as to the reimbursement status of newly approved healthcare
products such as VADs and vascular grafts. This uncertainty could delay or prevent adoption by
hospitals of these products in volume. Government and other third party payors are increasingly
attempting to contain healthcare costs. Payors are attempting to contain costs by, for example,
limiting coverage and the level of reimbursement of new therapeutic products. Payors are also
attempting to contain costs by refusing, in some cases, to provide any coverage for uses of
approved products for disease indications other than those for which the FDA has granted marketing
approval.
To date, a majority of private insurers with whom we have been involved and the CMS have
determined to reimburse some portion of the cost of our VADs and our diagnostic and vascular graft
products, but we cannot estimate what portion of such costs will be reimbursed, and our products
may not continue to be approved for reimbursement. In addition, changes in the healthcare system
may affect the reimbursability of future products. If coverage were partially or completely
reduced, our revenues and results of operations would be harmed.
Healthcare laws and regulations may change significantly in the future which could adversely
affect our financial condition and results of operations. We continuously monitor these
developments and modify our operations from time to time as the legislative and regulatory
environment changes. Currently, there are a number of pending federal legislative proposals that
could substantially change the way healthcare is financed by both governmental and private insurers
and may negatively impact payment rates for our products. We are unable to predict whether any
currently circulating congressional proposals will become law or in what form, whether any
additional or similar changes to statutes or regulations (including interpretations) will occur in
the future, or what effect any such legislation or regulation would have on our business. The
federal government may, however, have greater involvement in the healthcare industry than in prior
years, and such greater involvement may adversely affect our financial condition and results of
operations.
Complying with federal and state regulations is an expensive and time-consuming process, and any
failure to comply could result in substantial penalties.
We are directly or indirectly through our clients subject to extensive regulation by both the
federal government and the states in which we conduct our business, including the federal
Anti-Kickback Law and similar state anti-kickback laws, the federal False Claims Act, HIPAA and
similar state laws addressing privacy and security, state unlawful practice of medicine and fee
splitting laws, state certificate of need laws, the Medicare and Medicaid statutes and regulations,
and the Medicare Prescription Drug, Improvement and Modernization Act of 2003.
Both federal and state government agencies have heightened and coordinated civil and criminal
enforcement efforts as part of numerous ongoing investigations of healthcare companies, as well as
their executives and managers. These investigations relate to a wide variety of matters, including
referral and billing practices. The OIG and the Department of Justice have, from time to time,
established national enforcement initiatives that focus on specific billing practices or other
suspected areas of abuse. Some of our activities could become the subject of governmental
investigations or inquiries.
If our operations are found to be in violation of any of the laws and regulations to which we
are subject, we may be subject to the applicable penalty associated with the violation, including
civil and criminal penalties, damages, fines and the curtailment of our operations. Any penalties,
damages, fines or curtailment of our operations, individually or in the aggregate, could adversely
affect our ability to operate our business and our financial results. Our risk of being found in
violation of these laws and regulations is increased by the fact that many of them have not been
fully interpreted by the regulatory authorities or the courts, and their provisions are open to a
variety of interpretations. Any action against us for violation of these laws or regulations, even
if we successfully defend against it, could cause us to incur significant legal expenses and divert
management’s attention from the operation of our business. For a more detailed discussion of the
various state and federal regulations to which we are subject see
“Business—Government
Regulations” and “Business—Third Party Coverage and Reimbursement.”
25
Table of Contents
We depend on HeartMate II for a significant portion of our Cardiovascular division revenues.
We derive, and expect to continue to derive, a significant portion of our Cardiovascular
division revenues from sales of our HeartMate II product. We anticipate that HeartMate II pump
sales will continue to account for a significant portion of our revenues in the foreseeable future,
and we anticipate that sales of this product will likely represent an even greater portion of our
revenues now that we have received FDA approval for HeartMate II for Destination Therapy.
Implementation of our strategy depends on continued sales of our HeartMate II product. Sales of
our HeartMate II product are subject to the factors described in this “Risk Factors” section,
including, but not limited to, the following:
| • | failure to obtain approval from the FDA and foreign regulatory authorities or to comply with government regulations, or the withdrawal of market clearance or other the taking of other enforcement actions; | ||
| • | lack of Destination Therapy procedures conducted by hospitals using our VADs; | ||
| • | lack of acceptance or continued acceptance by physicians; | ||
| • | reliance on specialized suppliers for certain components and materials; | ||
| • | manufacturing problems; | ||
| • | any identified quality problems; | ||
| • | inability to protect our proprietary technologies or an infringement of others’ patents; | ||
| • | loss of a distributor or distributor failure to perform; | ||
| • | failure to compete successfully against our existing or potential competitors; | ||
| • | special risks associated with non-U.S. sales; | ||
| • | long and variable sales and deployment cycles; | ||
| • | failure by third party payors to provide appropriate levels of reimbursement; | ||
| • | failure to comply with federal and state regulations; and | ||
| • | product liability claims. |
The outcomes of these and other factors will have a significant impact on our future HeartMate
II product sales.
26
Table of Contents
Federal and state anti-kickback laws may adversely affect our operations and income.
Various federal and state laws govern financial arrangements among healthcare providers. The
federal Anti-Kickback Law prohibits the knowing and willful offer, payment, solicitation or receipt
of any form of remuneration in return for, or to induce, the referral of Medicare, Medicaid or
other federal healthcare program patients, or in return for, or to induce, the purchase, lease or
order of items or services that are covered by Medicare, Medicaid or other federal healthcare
programs. Many state laws also prohibit the solicitation, payment or receipt of remuneration in
return for, or to induce, the referral of patients in private as well as government programs.
Violation of these laws may result in substantial civil or criminal penalties and/or exclusion from
participation in federal or state healthcare programs. While we believe that we are operating in
compliance with applicable laws and believe that our arrangements with providers would not be found
to violate the federal and state anti-kickback laws it is possible that these laws could be
interpreted in a manner that could have an adverse effect on our operations.
In addition, under the DRA, states are encouraged to adopt false claims acts, similar to the
federal False Claims Act, which establish liability for submission of fraudulent claims to the
State Medicaid program and contain qui tam or whistleblower provisions. States enacting such false
claims statutes will receive an increased percentage of any recovery from a State Medicaid judgment
or settlement.
Adoption of new false claims statutes in states where we operate may impose additional
requirements or burdens on us.
Our debt obligations expose us to risks that could adversely affect our business, operating results
and financial condition.
We have a substantial level of debt in the form of our senior subordinated convertible notes.
The terms of our senior subordinated convertible notes do not restrict our ability to incur
additional indebtedness, including indebtedness senior to the convertible notes. Our current level
of indebtedness could, among other things:
| • | make it difficult for us to make payments on our debt; | ||
| • | make it difficult for us to obtain any necessary financing in the future for working capital, capital expenditures, debt service, acquisitions or general corporate purposes; | ||
| • | limit our flexibility in planning for or reacting to changes in our business; | ||
| • | reduce funds available for use in our operations; | ||
| • | make us more vulnerable in the event of a downturn in our business or an increase in interest rates; | ||
| • | impair our ability to incur additional debt because of financial and other restrictive covenants proposed for any such additional debt; or | ||
| • | place us at a possible competitive disadvantage relative to less leveraged competitors and competitors that have better access to capital resources. |
If we experience a decline in product sales due to any of the factors described in this “Risk
Factors” section or otherwise, we could have difficulty paying interest or principal amounts due on
our indebtedness. If we are unable to generate sufficient cash flow or otherwise obtain funds
necessary to make required payments, or if we fail to comply with the various requirements of our
indebtedness, including the convertible notes, we would be in default, which would permit the
holders of our indebtedness to accelerate the maturity of the indebtedness and could cause defaults
under our other indebtedness. Any default under our indebtedness could have a material adverse
effect on our business, operating results and financial condition.
27
Table of Contents
We may be unable to repay or repurchase our senior subordinated convertible notes or our other
indebtedness.
At maturity, the entire outstanding principal amount of our senior subordinated convertible
notes will become due and payable. Holders of the convertible notes may also require us to
repurchase the convertible notes on May 16 in each of 2011, 2014, 2019, 2024 and 2029. In addition,
if certain fundamental changes to our company occur, the holders of the convertible notes may
require us to repurchase all or any portion of their convertible notes. We may not have sufficient
funds or may be unable to arrange for additional financing to pay the principal amount due at
maturity or the repurchase price of the convertible notes. Any such failure would constitute an
event of default under the indenture for the senior subordinated convertible notes, which could, in
turn, constitute a default under the terms of any other indebtedness we may have incurred. Any
default under our indebtedness could have a material adverse effect on our business, operating
results and financial condition.
Conversion of the senior subordinated convertible notes or other future issuances of our stock will
dilute the ownership interests of existing shareholders.
Commencing October 1, 2008, holders of the senior subordinated convertible notes may convert
their notes through the final maturity date of the notes into shares of our common stock. If
holders elect conversion, we may at our option deliver shares of common stock, pay a holder in
cash, or deliver a combination of shares and cash, as determined pursuant to the terms of the
notes. The conversion of some or all of the senior subordinated convertible notes into shares of
our common stock will dilute the ownership interest of our existing shareholders. Any sales in the
public market of the common stock issuable upon such conversion could adversely affect prevailing
market prices of our common stock. In addition, future sales of substantial amounts of our stock in
the public market, or the perception that such sales could occur, could adversely affect the market
price of our stock. Further, the existence of the convertible notes may encourage short selling of
our common stock by market participants because the conversion of the convertible notes could
depress the price of our common stock.
Amortization of our intangible assets, which represent a significant portion of our total assets,
will adversely affect our net income and we may never realize the full value of our intangible
assets.
A substantial portion of our assets is comprised of goodwill and purchased intangible assets,
recorded as a result of our merger with Thermo Cardiosystems, Inc. in 2001. We may not receive the
recorded value for our intangible assets if we sell or liquidate our business or assets. The
material concentration of intangible assets increases the risk of a large charge to earnings if
recoverability of these intangible assets is impaired. For example, in the first quarter of 2004,
we completed an assessment of the final results from the feasibility clinical trial for the Aria
CABG graft, which was ongoing through fiscal 2003. Based on the clinical trial results, we decided
not to devote additional resources to development of the Aria graft. Upon the decision to
discontinue product development, we recorded an impairment charge of approximately $9 million as of
January 3, 2004 to write off purchased intangible assets related to the Aria graft. In the event of
another such charge to net income, the market price of our common stock could be adversely
affected.
Product liability claims could damage our reputation and hurt our financial results.
Our business exposes us to an inherent risk of potential product liability claims related to
the manufacturing, marketing and sale of medical devices. We maintain a limited amount of product
liability insurance. Our insurance policies generally must be renewed on an annual basis. We may
not be able to maintain or increase such insurance on acceptable terms or at reasonable costs, and
such insurance may not provide us with adequate coverage against all potential liabilities. A
successful claim brought against us in excess, or outside, of our insurance coverage could
seriously harm our financial condition and results of operations. Claims against us, regardless of
their merit or potential outcome, may also reduce our ability to obtain physician acceptance of our
products or expand our business.
28
Table of Contents
The competition for qualified personnel is particularly intense in our industry. If we are unable
to retain or hire key personnel, we may not be able to sustain or grow our business.
Our ability to operate successfully and manage our potential future growth depends
significantly upon retaining key research, technical, clinical, regulatory, sales, marketing,
managerial and financial personnel, and attracting and retaining additional highly qualified
personnel in these areas. We face intense competition for such personnel, and we may not be able to
attract and retain these individuals. We compete for talent with numerous companies, as well as
universities and nonprofit research organizations, throughout all our locations. The loss of key
personnel for any reason or our inability to hire and retain additional qualified personnel in the
future could prevent us from sustaining or growing our business. Our success will depend in large
part on the continued services of our research, managerial and manufacturing personnel. We cannot
assure you that we will continue to be able to attract and retain sufficient qualified personnel.
The price of our common stock may fluctuate significantly.
The price of our common stock has been, and is likely to continue to be, volatile, which means
that it could decline substantially within a short period of time. For example, our closing stock
price ranged from $20.40 to $31.72 during the twelve months ended January 2, 2010. The price of our
common stock could fluctuate significantly for many reasons, including but not limited to the
following:
| • | future announcements concerning us or our competitors; | ||
| • | regulatory developments, including ongoing healthcare reform initiatives, enforcement actions bearing on advertising, marketing or sales, and disclosure regarding completed ongoing or future clinical trials; | ||
| • | quarterly variations in operating results, which we have experienced in the past and expect to experience in the future; | ||
| • | introduction of new products or changes in product pricing policies by us or our competitors; | ||
| • | acquisition or loss of significant customers, distributors or suppliers; | ||
| • | reaction to estimates of business operations, product development or financial performance made public by our management; | ||
| • | business acquisitions or divestitures; | ||
| • | changes in earnings estimates by analysts; | ||
| • | changes in third party reimbursement practices; | ||
| • | charges, amortization and other financial effects relating to our business; and | ||
| • | fluctuations in the economy, world political events or general market conditions, such as the current economic recession. |
In addition, stock markets in general and the market for shares of healthcare stocks in
particular, have experienced extreme price and volume fluctuations, including recently as a result
of the current global financial crisis. These fluctuations can be unrelated to the operating
performance of the affected companies. These broad market fluctuations may adversely affect the
market price of our common stock. The market price of our common stock could decline below its
current price and the market price of our stock may fluctuate significantly in the future. These
fluctuations may be unrelated to our performance.
Shareholders often have instituted securities class action litigation after periods of
volatility in the market price of a company’s securities. Securities class action suits have been
filed against us in the past, and if other such suits are filed against us in the future we may
incur substantial legal fees and our management’s attention and resources may be diverted from
operating our business in order to respond to the litigation.
29
Table of Contents
Current global economic conditions could harm our business and liquidity.
Recent global market and economic conditions have been unprecedented and challenging with
tighter credit conditions and recession in most major economies. Continued concerns about the
systemic impact of potential long-term and wide-spread recession, energy costs, geopolitical
issues, the availability and cost of credit, and the global housing and mortgage markets have
contributed to increased market volatility and diminished expectations for western and emerging
economies. As a result of these market conditions, the cost and availability of credit has been and
may continue to be adversely affected by illiquid credit markets and wider credit spreads. These
factors have lead to a decrease in spending by businesses and consumers alike. Continued turbulence
in the U.S. and international markets and economies and prolonged declines in spending may
adversely affect our liquidity and financial condition, and the liquidity and financial condition
of our customers and suppliers, including our ability to refinance maturing liabilities and access
the capital markets to meet liquidity needs.
If we make acquisitions or divestitures, we could encounter difficulties that harm our business.
We may acquire companies, products or technologies that we believe to be complementary to our
business such as our proposed and ultimately abandoned acquisition of HeartWare. If we do so, we
may have difficulty integrating the acquired personnel, operations, products or technologies and we
may not realize the expected benefits of any such acquisition. In addition, acquisitions may dilute
our earnings per share, disrupt our ongoing business, distract our management and employees and
increase our expenses, any of which could harm our business. We may also sell businesses or assets
as part of our strategy or if we receive offers from third parties. If we do so, we may sell an
asset or business for less than its carrying value.
The occurrence of a catastrophic disaster or other similar events could cause damage to our
facilities and equipment, which would require us to cease or curtail operations.
We are vulnerable to damage from various types of disasters, including earthquakes, fires,
terrorist acts, floods, power losses, communications failures and similar events. For example, in
October 1989, a major earthquake that caused significant property damage and a number of fatalities
struck near the area in which our Pleasanton, California facility is located. If any such disaster
were to occur, we may not be able to operate our business at our facilities, in particular because
our premises require FDA approval, which could result in significant delays before we could
manufacture products from a replacement facility. The insurance we maintain may not be adequate to
cover our losses resulting from disasters or other business interruptions. Therefore, any such
catastrophe could seriously harm our business and results of operations.
We have a history of net losses.
We were founded in 1976 and we have had some history of incurring losses from operations. We
anticipate that our expenses will increase as a result of research and development and selling,
general and administrative expenses. We could also incur significant additional costs in connection
with our business development activities and the development and marketing of new products and
indicated uses for our existing products, as well as litigation and share-based compensation costs.
Such costs could prevent us from maintaining profitability in future periods.
We have experienced rapid growth and changes in our business, and our failure to manage this and
any future growth could harm our business.
The number of our employees has substantially increased during the past several years. We
expect to continue to increase the number of our employees, and our business may suffer if we do
not manage and train our new employees effectively. Our product sales may not continue to grow at a
rate sufficient to support the costs associated with an increasing number of employees. Any future
periods of rapid growth may place significant strains on our managerial, financial and other
resources. The rate of any future expansion, in combination with our complex technologies and
products, may demand an unusually high level of managerial effectiveness in anticipating, planning,
coordinating and meeting our operational needs, as well as the needs of our customers. If we are
unable to meet these demands our reputation, revenue and results of operations could be harmed.
30
Table of Contents
Revisions to accounting standards, financial reporting and corporate governance requirements and
tax laws could result in changes to our standard practices and could require a significant
expenditure of time, attention and resources, especially by senior management.
We must follow accounting standards, financial reporting and corporate governance requirements
and tax laws set by the governing bodies and lawmakers in the U.S. and U.K. where we do business.
From time to time, these governing bodies and lawmakers implement new and revised rules and laws.
These new and revised accounting standards, financial reporting and corporate governance
requirements and tax laws may require changes to our financial statements, the composition of our
Board of Directors, the responsibility and manner of operation of various board-level committees,
the information filed by us with the governing bodies and enforcement of tax laws, against us.
Implementing changes required by new standards, requirements or laws likely will require a
significant expenditure of time, attention and resources. It is impossible to completely predict
the impact, if any, on us of future changes to accounting standards, financial reporting and
corporate governance requirements and tax laws.
Our accounting principles that recently have been or may be affected by changes in the
accounting principles are as follows:
| • | fair value measurement; | ||
| • | accounting for convertible debt instruments; | ||
| • | accounting for income taxes; and | ||
| • | accounting for business combinations. |
We are subject to taxation in a number of jurisdictions and changes to the corporate tax rate and
laws of any of these jurisdictions could increase the amount of corporate taxes we have to pay.
We pay taxes principally in the U.S., U.K., Germany and France and these tax jurisdictions
have in the past and may in the future make changes to their corporate tax rates and other tax
laws, which changes could increase our future tax obligations.
Unanticipated changes in our tax rates could affect our future results of operations. Our
future effective tax rates could be unfavorably affected by changes in tax laws or the
interpretation of tax laws, by unanticipated decreases in the amount of revenue or earnings in
states with low statutory tax rates, or by changes in the valuation of our deferred tax assets and
liabilities. In addition, we are subject to the continual examination of our income tax returns by
the Internal Revenue Service and other domestic and foreign tax authorities, primarily related to
our intercompany transfer pricing. We regularly assess the likelihood of outcomes resulting from
these examinations to determine the adequacy of our provision for income taxes and our reserves for
potential adjustments, including tax credits and other tax benefits that can be challenged under
audit by various taxing authorities resulting in potential reduction in the amount of credits or
other benefits eventually realized. We believe such estimates to be reasonable; however, there can
be no assurance that the final determination of any of these examinations will not have an adverse
effect on our operating results and financial position.
Future levels of research and development spending, capital investment and export sales may
impact our entitlement to related tax credits and benefits which have the effect of lowering our
tax rate.
Any claims relating to improper handling, storage or disposal of hazardous chemicals and
biomaterials could be time consuming and costly.
Manufacturing and research and development of our products require the use of hazardous
materials, including chemicals and biomaterials. We cannot eliminate the risk of accidental
contamination or discharge and any resultant injury from these materials.
We could be subject to both criminal liability and civil damages in the event of an improper
or unauthorized release of, or exposure of individuals to, hazardous materials. In addition,
claimants may sue us for injury or contamination that results from our use or the use by third
parties of these materials, and our liability may exceed our total assets. Compliance with
environmental laws and regulations is expensive, and current or future environmental regulations
may impair our research, development or production efforts or harm our operating results.
31
Table of Contents
Anti-takeover defenses in our governing documents could prevent an acquisition of our company or
limit the price that investors might be willing to pay for our common stock.
Our governing documents could make it difficult for another company to acquire control of our
company. For example:
| • | Our Articles of Incorporation allow our Board of Directors to issue, at any time and without shareholder approval, preferred stock with such terms as it may determine. No shares of preferred stock are currently outstanding. However, the rights of holders of any of our preferred stock that may be issued in the future may be superior to the rights of holders of our common stock. | ||
| • | We have a shareholder rights plan, commonly known as a “poison pill,” which would make it difficult for someone to acquire us without the approval of our Board of Directors. |
All or any one of these factors could limit the price that certain investors would be willing
to pay for shares of our common stock and could delay, prevent or allow our Board of Directors to
resist an acquisition of our company, even if the proposed transaction was favored by a majority of
our independent shareholders.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We are headquartered in Pleasanton, California, where we own an approximately 67,000 square-foot
building that is Thoratec’s corporate office building. We also own approximately 66,000 square feet
of office, manufacturing and research facilities in Edison, New Jersey. We consider our operating
properties to be in satisfactory condition and adequate to meet our needs for the next several
years without making capital expenditures materially higher than historical levels.
Additionally, we lease the following facilities:
| • | Approximately 62,000 square feet of office, manufacturing and research facilities in Pleasanton, California, expiring in 2012. | ||
| • | Approximately 6,400 square feet of warehouse space in Dublin, California, expiring in 2011. | ||
| • | Approximately 21,800 square feet of warehouse space in San Ramon, California, expiring in 2016. | ||
| • | Approximately 11,000 square feet of office and research facilities in Rancho Cordova, California, expiring in 2012. | ||
| • | Approximately 45,000 square feet of office, manufacturing, warehouse and research facilities in Edison, New Jersey, expiring through 2017. | ||
| • | Approximately 53,500 square feet of office and research facilities in Piscataway, New Jersey, expiring in 2014. | ||
| • | Approximately 35,000 square feet of office, manufacturing and research facilities in Roseville, Minnesota, expiring in 2013. | ||
| • | Approximately 39,000 square feet of office and research facilities in Burlington, Massachusetts, expiring in 2011. | ||
| • | Approximately 8,700 square feet of office and warehouse facilities in the U.K. expiring in 2022. |
Each of our manufacturing areas has been inspected, approved and licensed for the manufacture
of medical devices by the FDA. Additionally, the Pleasanton facility is subject to inspections,
approvals and licensing by the State of California Department of Health Services (Food and Drug
Section). The Edison facility is subject to inspections, approvals and licensing by the State of
New Jersey Department of Health.
The Cardiovascular division utilizes all of the facilities in California, Massachusetts and in
the U.K. The ITC segment utilizes all of the facilities in New Jersey and Minnesota.
32
Table of Contents
Item 3. Legal Proceedings
None.
Item 4. Submission of Matters to a Vote of Security Holders
No matters were submitted to a vote of security holders during the quarter ended January 2,
2010.
Our Executive Officers
Gerhard F. Burbach, 47, President, Chief Executive Officer and Director, joined our company as
President, Chief Executive Officer and a director, in January 2006. Prior to joining us, Mr.
Burbach served as the President and Chief Executive Officer of Digirad Corporation, a leading
provider of solid-stage imaging products and services to cardiologist offices, hospitals and
imaging centers from April 2005 to January 2006. He continues to serve on the Digirad Board of
Directors. Before that he served for two years as president and chief executive officer of Bacchus
Vascular Inc., a developer of interventional cardiovascular devices. Previously, he served for
three years as chief executive officer of Philips Nuclear Medicine, a division of Philips Medical
Systems specializing in nuclear medicine imaging systems. Until its acquisition by Philips Medical
Systems, he spent four years at ADAC Laboratories, a provider of nuclear medicine imaging equipment
and radiation therapy planning systems, where he became president and general manager of the
nuclear medicine division. He also spent six years with the consulting firm of McKinsey & Company,
primarily within the firm’s healthcare practice.
David V. Smith, 50, Executive Vice President and Chief Financial Officer, joined our company
on December 29, 2006 as Executive Vice President and Chief Financial Officer. Prior to joining us,
Mr. Smith was Vice President, Chief Financial Officer of Chiron Corporation, a global
pharmaceutical company, from April 2003 until April 2006. Mr. Smith served as Chiron’s Vice
President, Finance from February 2002 until April 2003 and as Chiron’s Vice President and Principal
Accounting Officer from February 1999 until February 2002. Mr. Smith served as the Vice President,
Finance and Chief Financial Officer of Anergen, Inc. from 1997 until he joined Chiron. From 1988 to
1997, Mr. Smith held various financial management positions with Genentech, Inc., in both the U.S.
and Europe.
Lawrence Cohen, 60, President of ITC, joined our company in May 2001 as President of ITC.
Prior to joining ITC, Mr. Cohen served as CEO of HemoSense, Inc., a developer of medical diagnostic
products, from August 1998 to April 2001. From October 1989 to March 1998, Mr. Cohen held the
positions of Vice President Marketing and Sales, Vice President International and Worldwide
Executive Vice President at Ortho-Clinical Diagnostics, a Johnson & Johnson company. From 1980 to
1989, Mr. Cohen held executive management positions at Instrumentation Laboratory and Beckman
Coulter Corporation. He is a past president of the Biomedical Marketing Association and was on the
Board of Trustees of the National Blood Foundation from 1998 to 2004.
David A. Lehman, 49, Senior Vice President, General Counsel and Secretary, joined our company
as Vice President and General Counsel in May 2003. Mr. Lehman was appointed as Secretary in
December 2004 and became Senior Vice President in February 2007. Prior to joining us, Mr. Lehman
served as Vice President and General Counsel of Brigade Corporation, a provider of business process
outsourcing services, from June 2000 to May 2003. From November 1997 to June 2000, Mr. Lehman was
Assistant General Counsel at Bio-Rad Laboratories, Inc., a diagnostic and life science products
company. Prior to November 1997, Mr. Lehman was in the legal department of Mitsubishi International
Corporation, in New York and Tokyo, for more than seven years. Mr. Lehman started his career as an
associate attorney at the law firm of Hall, Dickler, Kent, Friedman and Wood.
33
Table of Contents
PART II
Item 5. Market for Common Equity, Related Stockholder Matters and Issuer Purchases of Equity
Securities
Our common stock is traded on the NASDAQ Global Select Market under the symbol “THOR.” The
following table sets forth, for the periods indicated, the high and low closing sales price per
share of our common stock, as reported by the NASDAQ Global Select Market. As of January 30, 2010
there were 57,067,248 shares of our common stock outstanding with approximately 470 holders of
record, including multiple beneficial holders at depositories, banks and brokerages listed as a
single holder in the “street” name of each respective depository, bank or broker.
| High | Low | |||||||
Fiscal Year 2008 |
||||||||
First Quarter |
$ | 18.44 | $ | 13.09 | ||||
Second Quarter |
18.50 | 14.08 | ||||||
Third Quarter |
28.87 | 16.67 | ||||||
Fourth Quarter |
32.49 | 19.48 | ||||||
Fiscal Year 2009 |
||||||||
First Quarter |
$ | 31.72 | $ | 20.40 | ||||
Second Quarter |
30.38 | 23.83 | ||||||
Third Quarter |
31.08 | 23.96 | ||||||
Fourth Quarter |
31.06 | 26.26 | ||||||
We have not declared or paid any dividends on our common stock and we do not anticipate doing
so in the foreseeable future.
There were no unregistered sales of our equity securities during the three months ended
January 2, 2010.
34
Table of Contents
Stock Price Performance Graph
The graph below compares the cumulative total shareholder return on an investment in our
common stock, the NASDAQ Composite Index (U.S. companies only) and the NASDAQ Medical Equipment
Index for the five-year period ended December 31, 2009, the last trading day in our 2009 fiscal
year.
The graph assumes the value of an investment in our common stock and each index was $100 at
December 31, 2004 and the reinvestment of all dividends, if any.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Thoratec Corporation, The NASDAQ Composite Index
And The NASDAQ Medical Equipment Index
Among Thoratec Corporation, The NASDAQ Composite Index
And The NASDAQ Medical Equipment Index
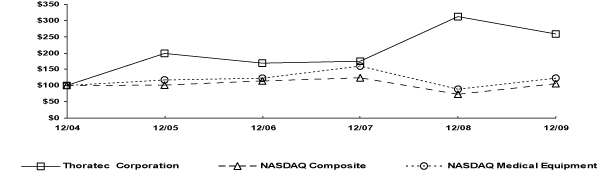
| * | $100 invested on December 31, 2004 in stock or index-including reinvestment of dividends. |
| 12/04 | 12/05 | 12/06 | 12/07 | 12/08 | 12/09 | |||||||||||||||||||
Thoratec Corporation |
100.00 | 198.56 | 168.71 | 174.57 | 311.80 | 258.35 | ||||||||||||||||||
NASDAQ Composite |
100.00 | 101.33 | 114.01 | 123.71 | 73.11 | 105.61 | ||||||||||||||||||
NASDAQ Medical Equipment |
100.00 | 117.06 | 122.50 | 159.63 | 88.67 | 122.59 | ||||||||||||||||||
35
Table of Contents
Issuer Purchases of Equity Securities
The following table sets forth certain information about our common stock repurchased during
the three months ended January 2, 2010:
| Total number | ||||||||||||||||
| of shares | Approximate dollar | |||||||||||||||
| purchased | value of shares | |||||||||||||||
| as part of | that may yet be | |||||||||||||||
| Total number | publicly | purchased under | ||||||||||||||
| of shares | Average price | announced plans or | the plans or | |||||||||||||
| purchased | paid per share | programs (1) | programs | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
October 4, 2009 through October 31, 2009 |
3.1 | $ | 27.54 | — | $ | — | ||||||||||
November 1, 2009 through November 28, 2009 |
2.1 | 28.36 | — | — | ||||||||||||
November 29, 2009 through January 2, 2010 |
3.8 | 27.42 | — | — | ||||||||||||
Total |
9.0 | (2) | $ | 27.68 | — | $ | — | |||||||||
| (1) | Our share repurchase programs, which authorized us to repurchase up to a total of $130 million of the Company’s common shares, were announced on February 11, 2004 as a $25 million program, on May 12, 2004 as a $60 million program, on July 29, 2004 as a $25 million program and on February 2, 2006 as a $20 million program. These programs authorize us to acquire shares in the open market or in privately negotiated transactions and do not have an expiration date. No shares were repurchased under these programs during the three months ended January 2, 2010. As of January 2, 2010, we have $10.1 million remaining on our share repurchase programs. | |
| (2) | Shares purchased that were not part of our publicly announced repurchase programs represent the surrender value of shares of restricted stock awards and restricted stock units used to pay income taxes due upon vesting, and do not reduce the dollar value that may yet be purchased under our publicly announced repurchase programs. |
36
Table of Contents
Item 6. Selected Consolidated Financial Data
The selected consolidated financial data presented below for the five fiscal years ended
January 2, 2010 are derived from our audited financial statements. The data set forth below should
be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and
Results of Operations” below and our audited consolidated financial statements and notes thereto
appearing elsewhere in this Annual Report on Form 10-K in Item 7.
We report on a 52-53 week fiscal year, which ends on the Saturday closest to December 31.
Accordingly, our fiscal year contains more or less than 365 days. For example, fiscal 2005 ended
December 31, 2005, fiscal 2006 ended December 30, 2006, fiscal 2007 ended December 29, 2007 and
each contained 52 weeks and fiscal 2008 ended January 3, 2009 and contained 53 weeks. Our fiscal
2009 contained 52 weeks and ended on January 2, 2010 and our fiscal year 2010 will include 52
weeks and will end on January 1, 2011.
| Fiscal Years | ||||||||||||||||||||
| 2009(1)(2) | 2008(1)(2) | 2007(1)(2) | 2006(1)(2) | 2005(1) | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
Statement of Operations: |
||||||||||||||||||||
Product sales |
$ | 373,937 | $ | 313,564 | $ | 234,780 | $ | 214,133 | $ | 201,712 | ||||||||||
Gross profit |
219,834 | 185,998 | 136,264 | 125,485 | 123,340 | |||||||||||||||
Amortization of purchased intangible assets |
10,663 | 13,183 | 12,582 | 12,055 | 11,204 | |||||||||||||||
In-process research and development |
— | — | — | 1,120 | — | |||||||||||||||
Litigation, merger, restructuring and other costs |
— | — | — | 447 | 95 | |||||||||||||||
Net income (loss) |
$ | 28,584 | $ | 18,331 | $ | (603 | ) | $ | 470 | $ | 9,994 | |||||||||
Basic net income (loss) per common share |
$ | 0.50 | $ | 0.33 | $ | (0.01 | ) | $ | 0.01 | $ | 0.20 | |||||||||
Diluted net income (loss) per common share |
$ | 0.49 | $ | 0.33 | $ | (0.01 | ) | $ | 0.01 | $ | 0.20 | |||||||||
Cash and cash equivalents and short term
available-for-sale investments |
$ | 305,635 | $ | 248,651 | $ | 218,350 | $ | 194,478 | $ | 210,936 | ||||||||||
Working capital |
412,155 | 332,378 | 301,736 | 265,691 | 269,293 | |||||||||||||||
Total assets |
746,557 | 684,085 | 613,009 | 590,215 | 572,792 | |||||||||||||||
Subordinated convertible debentures |
131,929 | 124,115 | 116,959 | 110,407 | 104,406 | |||||||||||||||
Long-term deferred tax liability (3) |
31,720 | 38,842 | 46,254 | 59,226 | 63,862 | |||||||||||||||
Total shareholders’ equity (3) |
$ | 525,128 | $ | 466,279 | $ | 413,809 | $ | 384,691 | $ | 371,268 | ||||||||||
| (1) | On January 4, 2009, we adopted Financial Accounting Standards (“FASB”) issued Accounting Standards Codification (“ASC”) 470-20, Debt, and retrospectively included the accounting for convertible debt instruments that may be settled in cash upon conversion, including partial settlements. | |
| (2) | On January 1, 2006, we adopted ASC 718, Share-Based Payment, and included share-based compensation for employee stock-based awards in our results of operations. | |
| (3) | On December 31, 2006, we adopted ASC 740, Income Taxes, and as a result we reported a cumulative effect adjustment of $0.5 million, which increased our December 31, 2006 “Accumulated deficit” balance offset by a “Long-term deferred tax liability” balance. |
37
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Annual Report on Form 10-K, including the documents incorporated by reference in this
Annual Report, includes forward-looking statements within the meaning of Section 27A of the
Securities Act of 1933, as amended, and Section 21E on Form 10-K of the Securities Exchange Act of
1934, as amended. These statements can be identified by the words “expects,” “projects,” “hopes,”
“believes,” “intends,” “should,” “estimate,” “will,” “would,” “may,” “anticipates,” “plans,”
“could” and other similar words. Actual results, events or performance could differ materially from
these forward-looking statements based on a variety of factors, many of which are beyond our
control. Therefore, readers are cautioned not to put undue reliance on these statements. Factors
that could cause actual results or conditions to differ from those anticipated by these and other
forward-looking statements include those more fully described in the “Risk Factors” section of this
Annual Report and in other documents we file with the SEC. These forward-looking statements speak
only as of the date hereof. We undertake no obligation to publicly release the results of any
revisions to these forward-looking statements that may be made to reflect events or circumstances
after the date hereof, or to reflect the occurrence of unanticipated events.
The following presentation of management’s discussion and analysis of our financial condition
and results of operations should be read together with our consolidated financial statements
included elsewhere in this Annual Report on Form 10-K.
Overview
We are a leading manufacturer of mechanical circulatory support products for use by patients
with HF. Our VADs are used primarily by HF patients to perform some or all of the pumping function
of the heart. We currently offer the widest range of products to serve this market. We believe that
our long-standing reputation for quality and innovation, and our excellent relationships with
leading cardiovascular surgeons and HF cardiologists worldwide, position us to capture growth
opportunities in the expanding HF market. Through our wholly-owned subsidiary ITC, we design,
develop, manufacture and market point-of-care diagnostic test systems and incision products that
provide fast and accurate blood test results to improve patient management, reduce healthcare costs
and improve patient outcomes.
Our Business Model
Our business is comprised of two operating divisions: Cardiovascular and ITC.
The product line of our Cardiovascular division is:
| • | Circulatory Support Products. Our mechanical circulatory support products include the HeartMate II, HeartMate XVE, PVAD, IVAD and CentriMag for acute, intermediate and long-term mechanical circulatory support for patients with advanced HF. We also manufacture and sell small diameter grafts using our proprietary materials to address the vascular access market for hemodialysis. |
The product lines of our ITC division are:
| • | Point-of-Care Diagnostics. Our point-of-care products include diagnostic test systems that monitor blood coagulation while a patient is being administered certain anticoagulants, as well as monitor blood gas/electrolytes, oxygenation and chemistry status. | ||
| • | Incision. Our incision products include devices used to obtain a patient’s blood sample for diagnostic testing and screening for platelet function. |
Critical Accounting Policies and Estimates
We have identified the policies and estimates below as critical to our business operations and
the understanding of our results of operations. The impact of, and any associated risks related to,
these policies and estimates on our business operations are discussed below. Preparation of
financial statements in accordance with accounting principles generally accepted in the United
States of America requires management to make estimates and assumptions that affect the reported
amount of assets, liabilities, revenue and expenses and the disclosure of contingent assets and
liabilities. There can be no assurance that actual results will not differ from those estimates and
assumptions.
38
Table of Contents
Revenue Recognition
We recognize revenue from product sales for our Cardiovascular and ITC business divisions when
evidence of an arrangement exists, title has passed (generally upon shipment) or services have been
rendered, the selling price is fixed or determinable and collectability is reasonably assured.
Sales to distributors are recorded when title transfers.
We recognize sales of certain Cardiovascular division products to first-time customers when it
has been determined that the customer has the ability to use the products. These sales frequently
include the sale of products and training services under multiple element arrangements. Training is
not considered essential to the functionality of the products. Revenue under these arrangements is
allocated to training based upon fair market value of the training, which is typically performed on
our behalf by third party providers. Under this method, the total value of the arrangement is
allocated to the training and the Cardiovascular division products based on the relative fair
market value of the training and products.
In determining when to recognize revenue, management makes decisions on such matters as the
fair values of the product and training elements when sold together, customer credit worthiness and
warranty reserves. If any of these decisions proves incorrect, the carrying value of these assets
and liabilities on our consolidated balance sheets or the recorded product sales could be
significantly different, which could have a material adverse effect on our results of operations
for any fiscal period.
Reserves
We maintain allowances for doubtful accounts for estimated losses resulting from the inability
of our customers to make payments owed to us for product sales and training services. If the
financial condition of our customers were to deteriorate, resulting in an impairment of their
ability to make payments, additional allowances may be required.
The majority of our products are covered by up to a two-year limited manufacturer’s warranty
from the date of shipment or installation. Estimated contractual warranty obligations are recorded
when the related sales are recognized and any additional amounts are recorded when such costs are
probable and can be reasonably estimated, at which time they are included in “Cost of product
sales” in our consolidated statements of operations. In determining the warranty reserve estimate,
management makes judgments and estimates on such matters as repair costs and probability of
warranty obligations.
Estimated excess and obsolete inventory charges are recorded when inventory levels exceed
projected sales volume for a certain period of time. In determining the excess obsolete charges,
management makes judgments and estimates on matters such as forecasted sales volume. If sales
volume does not meet projections, additional write-downs may be required.
Management must make estimates and judgments to determine the amount of reserves to accrue. If
any of these decisions proves incorrect, our consolidated financial statements could be materially
and adversely affected.
Income Taxes
We make certain estimates and judgments in determining income tax expense for financial
statement purposes. These estimates and judgments occur in the calculation of tax credits, benefits
and deductions, such as tax benefits from our non-U.S. operations and in the calculation of certain
tax assets and liabilities, which arise from differences in the timing of revenue and expense for
tax and financial statement purposes.
We record a valuation allowance to reduce our deferred tax assets to an amount that
more-likely-than-not will be realized. While we have considered future taxable income and ongoing
prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in
the event we were to determine that we would be able to realize our deferred tax assets in the
future in excess of our net recorded amount, an adjustment to the allowance for the deferred tax
asset would increase income in the period such determination was made. Likewise, should we
determine that we would not be able to realize all or part of our net deferred tax asset in the
future, an adjustment to the valuation allowance for the deferred tax asset would be charged to
income in the period such determination was made.
39
Table of Contents
We believe we have provided adequate reserves for uncertain tax positions for anticipated
audit adjustments by U.S. federal, state and local, as well as foreign, tax authorities based on
our estimate of whether, and the extent to which, additional taxes, interest and penalties may be
due. If events occur which indicate payment of these amounts is unnecessary, the reversal of the
liabilities would result in tax benefits being recognized in the period when we determine the
accrued liabilities are no longer warranted. If our estimate of tax liabilities proves to be less
than the ultimate assessment, a further charge to expense would result.
Evaluation of Purchased Intangible Assets and Goodwill for Impairment
We periodically evaluate the carrying value of long-lived assets to be held and used,
including intangible assets subject to amortization, when events or circumstances warrant such a
review. The carrying value of a long-lived asset to be held and used is considered impaired when
the anticipated separately-identifiable undiscounted cash flows to be generated by such an asset
are less than the carrying value of the asset. In that event, a loss is recognized based on the
amount by which the carrying value exceeds the fair value of the long-lived asset. Fair value is
determined primarily using the anticipated cash flows discounted at a rate commensurate with the
risk involved. Management must make estimates of these future cash flows, and if necessary, the
approximate discount rate, and if any of
these estimates proves incorrect, the carrying value of these assets on our consolidated
balance sheets could become significantly impaired.
Purchased intangible assets with indefinite lives and goodwill are not amortized but are
subject to annual impairment tests. If there is an impairment, a new fair value would be
determined. If the new fair value is less than the carrying amount, an impairment loss would be
recognized.
Valuation of Share-Based Awards
Share-based compensation expense is measured at the grant date based on the value of the award
and is recognized as expense over the vesting period. Determining the fair value of option awards
at the grant date requires judgment, including estimating the expected term of stock options, the
expected volatility of our stock, expected forfeitures and expected dividends. The computation of
the expected volatility assumption used in the Black-Scholes option pricing model for option grants
is based on our historical volatility. When establishing the expected life assumption, we review
annual historical employee exercise behavior of option grants with similar vesting periods. In
addition, judgment is also required in estimating the amount of share-based awards that are
expected to be forfeited. If actual forfeitures differ significantly from these estimates,
share-based compensation expense and our results of operations could be materially affected.
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer
a liability (“exit price”) in an orderly transaction between market participants at the measurement
date.
In determining fair value, we use various approaches, including market, income and/or cost
approaches, and each of these approaches requires certain inputs. Fair value measurement standards
establish a hierarchy for inputs used in measuring fair value that maximizes the use of observable
inputs and minimizes the use of unobservable inputs by requiring that observable inputs be used
when available. Observable inputs are inputs that market participants would use in pricing the
asset or liability based on market data obtained from sources independent of us. Unobservable
inputs are inputs that reflect our assumptions as compared to the assumptions market participants
would use in pricing the asset or liability based on the best information available in the
circumstances. The hierarchy is broken down into three levels based on the reliability of inputs as
follows:
| • | Level 1 — Valuations based on quoted prices in active markets for identical assets or liabilities that we have the ability to access. Assets and liabilities utilizing Level 1 inputs include broker-dealer quoted securities that are traded in an active market. Since valuations are based on quoted prices that are readily and regularly available in an active market, a significant degree of judgment is not required. | ||
| • | Level 2 — Valuations based on quoted prices of similar investments in active markets, of similar or identical investments in markets that are not active or model based valuations for which all significant inputs and value drivers are observable, directly or indirectly. Assets and liabilities utilizing Level 2 inputs primarily include municipal bonds, variable demand notes, corporate bonds and our senior subordinated convertible notes, except the make-whole provision, which uses Level 3 inputs, described below. |
40
Table of Contents
| • | Level 3 — Valuations based on inputs that are unobservable and significant to the overall fair value measurement. Assets and liabilities utilizing Level 3 inputs include auction rate securities, our convertible debenture with Levitronix, our purchased intangible asset valuations and the make-whole feature of our senior subordinated convertible notes. Given the current credit market illiquidity for auction rate securities, our estimates are subject to significant judgment by management. |
Fair value is a market-based measure considered from the perspective of a market participant
who holds the asset or owes the liability rather than an entity-specific measure. Therefore, even
when market assumptions are not readily available, our own assumptions are developed to reflect
those that market participants would use in pricing the asset or liability at the measurement date.
See Note 3 “Fair Value Measurements,” to the consolidated financial statements for further
information about our financial assets that are accounted for at fair value.
Due to the uncertainty inherent in the valuation process, estimates of fair value may differ
significantly from the values that would have been obtained had an active market for the securities
existed, and the differences could be material. After determining the fair value of our
available-for-sale securities, gains or losses on these investments are recorded to other
comprehensive income, until either the investment is sold or we determine that the decline in value
is other-than-temporary. Determining whether the decline in fair value is other-than-temporary
requires management judgment based on the specific facts and circumstances of each investment. For
investments in available-for-sale securities, these judgments primarily consider: our ability and
intent to hold the investment to maturity, more-likely-than-not that we would be required to sell
the investment before recovery of the investments amortized cost basis and whether we expect to
recover the amortized cost basis of the investment. Given the current market conditions, these
judgments could prove to be incorrect, and companies with relatively high credit ratings and solid
financial conditions may not be able to fulfill their obligations. In addition, if we decide not to
hold an investment until its value recovers it may result in the recognition of an
other-than-temporary impairment.
Results of Operations
The following table sets forth selected consolidated statements of operations data for the
years indicated and as a percentage of total product sales:
| Fiscal Years | ||||||||||||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| (in thousands, except percentages) | ||||||||||||||||||||||||
Product sales |
$ | 373,937 | 100 | % | $ | 313,564 | 100 | % | $ | 234,780 | 100 | % | ||||||||||||
Cost of product sales |
154,103 | 41 | 127,566 | 41 | 98,516 | 42 | ||||||||||||||||||
Gross margin |
219,834 | 59 | 185,998 | 59 | 136,264 | 58 | ||||||||||||||||||
Operating expenses: |
||||||||||||||||||||||||
Selling, general and administrative |
107,927 | 29 | 94,142 | 30 | 82,044 | 35 | ||||||||||||||||||
Research and development |
54,227 | 14 | 52,943 | 17 | 43,835 | 19 | ||||||||||||||||||
Amortization of purchased intangible assets |
10,663 | 3 | 13,183 | 4 | 12,582 | 5 | ||||||||||||||||||
Total operating expenses |
172,817 | 46 | 160,268 | 51 | 138,461 | 59 | ||||||||||||||||||
Income (loss) from operations |
47,017 | 13 | 25,730 | 8 | (2,197 | ) | (1 | ) | ||||||||||||||||
Other income and (expense): |
||||||||||||||||||||||||
Interest expense (1) |
(12,307 | ) | (3 | ) | (10,984 | ) | (4 | ) | (10,428 | ) | (4 | ) | ||||||||||||
Interest income and other |
6,043 | 2 | 9,146 | 3 | 8,624 | 4 | ||||||||||||||||||
Income (loss) before taxes |
40,753 | 12 | 23,892 | 7 | (4,001 | ) | (1 | ) | ||||||||||||||||
Income tax expense (benefit) |
12,169 | 3 | 5,561 | 2 | (3,398 | ) | (1 | ) | ||||||||||||||||
Net income (loss) |
$ | 28,584 | 9 | % | $ | 18,331 | 5 | % | $ | (603 | ) | — | % | |||||||||||
| (1) | Includes non-cash interest expense of $7.8 million, $7.2 million and $6.6 million for fiscal years 2009, 2008 and 2007, respectively. |
41
Table of Contents
Product Sales
Product sales consisted of the following:
| Fiscal Years | Annual Percentage Change | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009/2008 | 2008/2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Cardiovascular |
$ | 279,967 | $ | 214,976 | $ | 144,220 | 30.2 | % | 49.1 | % | ||||||||||
ITC |
93,970 | 98,588 | 90,560 | 4.7 | 8.9 | |||||||||||||||
Total product sales |
$ | 373,937 | $ | 313,564 | $ | 234,780 | 19.3 | 33.6 | ||||||||||||
In 2009 as compared to 2008, Cardiovascular product sales increased by $65.0 million primarily
due to higher sales driven by increased HeartMate II volume in North America and Europe and the
launch of Go Gear peripherals in the third quarter of 2009, partially offset by the decline in
stocking revenues as nineteen new HeartMate II centers were added in 2009 as compared to fifty-five
new HeartMate II centers in 2008. The increases in product sales were partially offset by a decline
in the sales of the HeartMate XVE and Thoratec product lines as a result of cannibalization by
HeartMate II and unfavorable foreign exchange rates. ITC product sales decreased by $4.6 million
primarily due to delayed capital purchases by hospitals due to current economic conditions and
competitive pressures on the ProTime and skin incision businesses, partially offset by an increase
in sales to customers for pharmaceutical clinical trials.
In 2008 as compared to 2007, Cardiovascular product sales increased by $70.8 million primarily
due to higher sales from our HeartMate product line. The higher sales resulted from increased
HeartMate II volume in North America and Europe, a commercial price increase for the HeartMate II
in North America, and higher stocking revenue associated with the addition of fifty-five new
HeartMate II centers. Also, product sales increased because of favorable foreign currency
translation and higher CentriMag sales due to increased implant activity. This increase in product
sales was partially offset by a decline in the sales of the Thoratec product line due to HeartMate
II cannibalization. ITC product sales increased by $8.0 million primarily due to higher domestic
and international sales of our HEMOCHRON product line and higher international sales of our
Alternate Site product line, partly offset by the decrease of our incision product line sales due
to competitive offerings affecting both volume and selling price.
Sales originating outside of the U.S. and U.S. export sales accounted for approximately 25%,
26% and 28% of our total product sales in 2009, 2008 and 2007, respectively.
Gross Profit
Gross
profit and gross margin were as follows:
| Fiscal Years | Annual Percentage Change | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009/2008 | 2008/2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Total gross profit |
$ | 219,834 | $ | 185,998 | $ | 136,264 | 18.2 | % | 36.5 | % | ||||||||||
Total gross margin |
58.8 | % | 59.3 | % | 58.0 | % | 0.5 | % | 1.3 | % | ||||||||||
In 2009 as compared to 2008, the Cardiovascular gross margin percentage decreased by 1.3%
primarily due to an increase in inventory write-downs, warranty reserves and unfavorable foreign
exchange rates partially offset by increased HeartMate II volume and favorable manufacturing
variances. ITC gross margin percentage decreased by 4.5% due to unfavorable geographic and product
mix and increase in unfavorable manufacturing variances. The division specific gross margin decreases were partially offset by a mix shift between the Cardiovascular and ITC division as the Cardiovascular division becomes a larger percentage of revenue.
In 2008 as compared to 2007, Cardiovascular gross margin percentage increased by 2.2%
primarily due to increased HeartMate II prices in North America and favorable foreign currency
translations, partially offset by the increased percentage of non-pump revenue and unfavorable
manufacturing variances. ITC gross margin percentage decreased by 4.8% due to unfavorable
geographic and product mix and increased unfavorable manufacturing variances.
42
Table of Contents
Selling, General and Administrative
Selling, general and administrative expenses were as follows:
| Fiscal Years | Annual Percentage Change | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009/2008 | 2008/2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Total selling, general and administrative expenses |
$ | 107,927 | $ | 94,142 | $ | 82,044 | 14.6 | % | 14.7 | % | ||||||||||
In 2009 as compared to 2008, Cardiovascular costs increased by $2.6 million, primarily due to
market development initiatives associated with the launch of Go Gear peripherals and the
preparation for HeartMate II Destination Therapy approval and higher stock-based compensation
expense partially offset by lower bonus and commission expense. ITC costs increased by $1.1
million, primarily due to higher costs for quality system improvements offset by lower personnel
costs. Corporate costs increased by $10.1 million, primarily due to $12.3 million related to the
HeartWare transaction, partially offset by lower compensation costs.
In 2008 as compared to 2007, Cardiovascular costs increased by $8.6 million, primarily due to
market development initiatives and commercialization efforts associated with the HeartMate II and
higher compensation expense. ITC costs increased by $1.0 million, primarily due to higher sales and
marketing personnel and travel costs. Corporate costs increased by $2.5 million, primarily due to
higher compensation, and other corporate expenses.
Research and Development
Research and development expenses were as follows:
| Fiscal Years | Annual Percentage Change | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009/2008 | 2008/2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Total research and development expenses |
$ | 54,227 | $ | 52,943 | $ | 43,835 | 2.4 | % | 20.8 | % | ||||||||||
Research and development costs are largely project driven, and fluctuate based on the level of
project activity planned and subsequently approved and conducted.
In 2009 as compared to 2008, research and development costs increased $1.3 million.
Cardiovascular costs increased by $2.7 million, primarily due to increased research and development
costs associated with the HeartMate product line peripheral enhancements and new product
technology. ITC costs decreased by $1.4 million due to lower spending related to new product
development activities.
In 2008 as compared to 2007, research and development costs increased by $9.1 million.
Cardiovascular costs increased by $6.9 million, primarily due to increased research and development
costs associated with our HeartMate product line peripheral enhancements and new product
technology. ITC costs increased by $2.2 million, primarily due to new product development.
Amortization of Purchased Intangible Assets
Amortization of purchased intangible assets in 2009 was $10.7 million as compared to $13.2
million in 2008. The decrease in amortization of $2.5 million is attributable to certain intangible
assets at the Cardiovascular division being fully amortized in the first quarter of 2009.
Amortization of purchased intangible assets in 2008 was $13.2 million as compared to $12.6
million in 2007. The $0.6 million increase resulted from decreasing the estimated useful lives of
certain of our intangible assets at our Cardiovascular division in 2007.
43
Table of Contents
Interest Expense
Interest expense primarily relates to interest on the senior subordinated convertible notes as
follows:
| Fiscal Years | Annual Percentage Change | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009/2008 | 2008/2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Interest expense |
$ | 11,897 | $ | 10,574 | $ | 10,018 | 12.5 | % | 5.6 | % | ||||||||||
Amortization of debt
issuance costs
related to senior
subordinated
convertible notes |
410 | 410 | 410 | — | — | |||||||||||||||
Total interest expense |
$ | 12,307 | $ | 10,984 | $ | 10,428 | 12.0 | 5.3 | ||||||||||||
Interest Income and Other
Interest
income and other consisted of the following:
| Fiscal Years | Annual Percentage Change | |||||||||||||||||||
| 2009 | 2008 | 2007 | 2009/2008 | 2007/2006 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Interest income |
$ | 5,713 | $ | 8,744 | $ | 8,063 | 34.7 | % | 8.4 | % | ||||||||||
Foreign currency, net |
(628 | ) | 73 | 347 | 960.3 | 79.0 | ||||||||||||||
Other |
958 | 329 | 214 | 191.1 | 53.7 | |||||||||||||||
Total interest income and other |
$ | 6,043 | $ | 9,146 | $ | 8,624 | 33.9 | 6.1 | ||||||||||||
In 2009, interest income declined by $3.0 million as compared to 2008, primarily due to
decline in market interest rates and shortened maturities on our investment portfolio, partially
offset by higher investment balances. Foreign currency decreased by $0.7 million in 2009 as
compared to 2008 due to certain foreign currency transactions related to inventory for our foreign
operations, which are not hedged in our foreign currency contracts. Other income increased by $0.6
million in 2009 as compared to 2008 primarily due to an increase in royalty income.
In 2008, interest income increased by $0.7 million as compared to 2007, primarily due to
higher investment balances in our portfolio and increase in market interest rates. Foreign
currency decreased by $0.3 million in 2008 as compared to 2007 due to certain foreign currency
transactions related to inventory for our foreign operations, which are not hedged in our foreign
currency contracts.
Income Taxes
Our effective tax rate was an expense of 30% in 2009 compared to an expense of 23% in 2008.
This increase in the annual effective tax rate of 7% was primarily due to an increase in pre-tax
income, lower tax-exempt interest and non-deductible compensation, in part offset by a change in
state apportionment rates.
Our effective tax rate was an expense of 23% in 2008 compared to a benefit of 85% in 2007. The
increase in our annual effective tax rate of 108% on a comparative basis was primarily due to a
significant increase in operating profit, and an increase in reserves related to domestic income
tax positions.
Liquidity and Capital Resources
Cash, Cash Equivalents and Investments
Cash and cash equivalents include highly liquid financial instruments that are readily
convertible to cash and have maturities of 90 days or less from the date of purchase.
Investments classified as short-term consist of various financial instruments such as
commercial paper, U.S. government agency obligations and corporate notes. Bonds with high credit
quality with maturities of greater than 90 days when purchased are classified as
available-for-sale. Investments classified as long-term consist of our investments in auction rate
securities.
44
Table of Contents
Following is a summary of our cash, cash equivalents and investments:
| January 2, 2010 | January 3, 2009 | December 29, 2007 | ||||||||||
| (in thousands) | ||||||||||||
Cash and cash equivalents |
$ | 26,461 | $ | 107,053 | $ | 20,689 | ||||||
Short-term available-for-sale investments |
279,174 | 141,598 | 197,661 | |||||||||
Long-term available-for-sale investments |
24,634 | 29,959 | — | |||||||||
Total cash and equivalents and available-for-sale investments |
$ | 330,269 | $ | 278,610 | $ | 218,350 | ||||||
We believe that cash and cash equivalents, short-term available-for-sale investments on hand
and expected cash flows from operations will be sufficient to fund our operations, capital
requirements and stock repurchase programs for at least the next twelve months.
As of January 2, 2010, we owned approximately $27.8 million face amount of auction rate
securities, of which $0.1 million was classified as short-term and $27.7 million was classified as
long-term. The assets underlying these investments are student loans backed by the U.S. government
under the Federal Family Education Loan Program or by private insurers and are rated between A- and
AAA. Historically, these securities have provided liquidity through a Dutch auction process that
resets the applicable interest rate periodically every seven to thirty-five days. Beginning in
February of 2008, these auctions began to fail. The principal amount of these auction rate
securities will not be accessible until future auctions for these securities are successful, a
secondary market is established, these securities are called for redemption, or they are paid at
maturity.
We recorded an estimated cumulative unrealized loss of $3.1 million ($1.9 million, net of tax)
related to the temporary impairment of the auction rate securities, which was included in
accumulated other comprehensive gain/loss within shareholders’ equity. In addition, our management
reviews impairments and credit loss associated with its investments, including auction rate
securities to determine the classification of the impairment as “temporary” or
“other-than-temporary” and to bifurcate the credit and non-credit component of an
other-than-temporary impairment event. We do not intend to sell any of the auction rate securities
prior to maturity at an amount below the original purchase value; intend to hold the investment to
recovery and based on a more-likely-than-not probability assessment we will not be required to sell
the security before recovery; and deem that it is not probable that we will receive less than 100%
of the principal and accrued interest from the issuer. Therefore, 100% of the impairment was
charged to other comprehensive income. Further, we continue to liquidate investments in auction
rate securities as opportunities arise. During the fiscal year ended January 2, 2010, $9.4 million
in auction rate securities were liquidated at par in connection with issuer calls.
We continue to monitor the market for auction rate securities and consider its impact (if any)
on the fair value of our investments. If the current market conditions deteriorate further, or the
anticipated recovery in fair values does not occur, we may be required to record additional
unrealized losses in other comprehensive income or other-than-temporary impairment charges to the
consolidated statements of operations in future periods.
We intend and have the ability to hold these auction rate securities until the market recovers
or until maturity. We do not anticipate having to sell these securities in order to operate our
business. We believe that, based on our current unrestricted cash, cash equivalents and short-term
marketable security balances of $305.6 million as of January 2, 2010, the current lack of liquidity
in the credit and capital markets will not have an impact on our liquidity, our cash flow or our
ability to fund our operations. If the issuers of the auction rate securities are unable to
successfully complete future auctions and their credit ratings deteriorate, we may in the future be
required to record an impairment charge on these investments. It could conceivably take until the
final maturity of the underlying notes (up to 30 years) to realize our investments’ recorded value.
45
Table of Contents
Long-term obligation
In 2004, we completed the sale of $143.8 million initial principal amount of senior
subordinated convertible notes due in 2034. The senior subordinated convertible notes were issued
at an issue price of $580.98 per note, which is 58.098% of the principal amount at maturity of the
notes. The senior subordinated convertible notes bear interest at a rate of 1.3798% per year on the
principal amount at maturity, payable semi-annually in arrears in cash on May 16 and November 16 of
each year, from November 16, 2004 until May 16, 2011. We have applied, retrospectively, the
adoption of recent guidance for the accounting for convertible debt instruments that may be settled
in cash upon conversion, including partial settlements, which increases non-cash interest expense
based on the assumed market rate of 9% percent per annum as compared to the cash coupon rate of
2.375% as further discussed in Note 10, “Long-Term Debt” of the notes to the consolidated financial
statements. Holders of the senior subordinated convertible notes may convert their convertible
notes into shares of our common stock at a conversion rate of 29.4652 shares per $1,000 principal
amount of senior subordinated convertible notes, which represents a conversion price of $19.72 per
share, subject to adjustments upon the occurrence of certain events as set forth in the indenture.
Holders have been and are able to convert their convertible notes at any point after the close of
business on October 30, 2004 if, as of the last day of the preceding calendar quarter, the closing
price of our common stock for at least 20 trading days in a period of 30 consecutive trading days
ending on the last trading day of such preceding calendar quarter is more than 120% of the accreted
conversion price per share of our common stock. Commencing October 1, 2008, this market price
conversion feature was satisfied, such that holders of the senior subordinated convertible notes
may convert their notes through the final maturity date of the notes into shares of our common
stock at a conversion rate of 29.4652 shares per $1,000 principal amount of senior subordinated
convertible notes, subject to adjustments as provided in the indenture. If holders elect
conversion, we may, at our option, deliver shares of common stock, pay a holder in cash, or deliver
a combination of shares and cash, as determined pursuant to the terms of the notes.
Cash Flow Activities
Following is a summary of our cash flow activities:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Net cash provided by operating activities |
$ | 49,074 | $ | 51,040 | $ | 14,364 | ||||||
Net cash (used in) provided by investing activities |
(141,249 | ) | 7,331 | (78,041 | ) | |||||||
Net cash provided by financing activities |
11,727 | 28,064 | 16,960 | |||||||||
Effect of exchange rate changes on cash and cash equivalents |
(144 | ) | (71 | ) | (47 | ) | ||||||
Net (decrease) increase in cash and cash equivalents |
$ | (80,592 | ) | $ | 86,364 | $ | (46,764 | ) | ||||
Cash Provided by Operating Activities
In 2009, cash provided by operating activities was $49.1 million. This amount included net
income of $28.6 million increased by positive non-cash adjustments to net income of $36.0 million
primarily comprised of $11.0 million related to depreciation, $10.7 million related to
amortization, $13.9 million related to share-based compensation expenses, and $3.9 million of tax
benefit related to the exercise of stock options. These positive non-cash contributions were
partially offset by a decrease of $3.2 million related to excess tax benefits from share-based
compensation and a decrease of $12.5 million in our net deferred tax liability. Changes in assets
and liabilities used additional cash of $15.5 million primarily due to the increase in receivables,
inventory and a decrease in accounts payable, partially offset by an increase in income taxes.
Cash Provided by or Used in Investing Activities
In 2009, cash used in investing activities was $141.2 million, due to net purchases of
investments of $131.0 million, issuance of HeartWare loan of $20.0 million, purchase of a patent
portfolio for $1.4 million, and purchases of property, plant and equipment of $11.8 million, net of
$2.6 million in transfers of rental drivers and demonstration equipment from inventory into fixed
assets. This was offset in part by $23.0 million of loan collections. The purchased property,
plant and equipment included $8.7 million primarily for leasehold and building improvements related
to the expansion of our manufacturing facility and the office building at our Pleasanton
headquarters and purchases of management information systems equipment at our Cardiovascular
division and $3.1 million for manufacturing equipment and management information systems equipment
at our ITC division.
46
Table of Contents
Cash Provided by Financing Activities
In 2009, cash provided by financing activities was $11.7 million, which was primarily
comprised of $12.1 million in proceeds related to stock option exercises and purchases under our
Employee Stock Purchase Plan and $3.2 million from excess tax benefits from share-based compensation.
This was partially offset by $3.5 million of restricted stock purchased for payment of income tax
withholding due upon vesting.
Off Balance Sheet Arrangements
Letter of Credit — We maintain an Irrevocable Standby Letter of Credit as part of our
workers’ compensation insurance program. The Letter of Credit is not collateralized. The Letter of
Credit automatically renews on June 30th of each year, unless terminated by one of the parties. As
of January 2, 2010, our Letter of Credit balance was approximately $0.8 million.
Contractual Obligations
As of January 2, 2010, we had the following contractual obligations:
| Total | 2010 | 2011 | 2012 | 2013 | 2014 | Thereafter | ||||||||||||||||||||||
| (in millions) | ||||||||||||||||||||||||||||
Long-Term Debt Obligations (a) |
$ | 252.5 | $ | 3.4 | $ | 1.7 | $ | — | $ | — | $ | — | $ | 247.4 | ||||||||||||||
Operating Lease Obligations (b) |
31.2 | 3.7 | 3.9 | 3.7 | 3.7 | 2.6 | 13.6 | |||||||||||||||||||||
Purchase Obligations (c) |
78.2 | 47.5 | 11.1 | 9.8 | 9.8 | — | — | |||||||||||||||||||||
Total |
$ | 361.9 | $ | 54.6 | $ | 16.7 | $ | 13.5 | $ | 13.5 | $ | 2.6 | $ | 261.0 | ||||||||||||||
| (a) | Includes interest of $5.1 million and original issue discount of $103.7 million. See note 10 to our consolidated financial statements included in this Annual Report on Form 10-K related to our long-term debt. | |
| (b) | Our operating lease obligations of $31.2 million were comprised of our various leased facilities and office equipment. | |
| (c) | Our purchase obligations include $78.2 million of supply agreements in effect at January 2, 2010. |
As of January 2, 2010, the liability for uncertain tax positions was $11.0 million including
interest and penalties. Due to the high degree of uncertainty regarding the timing of potential
future cash flows associated with these liabilities, we are unable to make a reasonably reliable
estimate of the amount and period in which these liabilities might be paid.
Recently Issued Accounting Pronouncements
In January 2010, the FASB issued Accounting Standards Updates (“ASU”) No. 2010-06, Fair Value
Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements. ASU
No. 2010-06 amends ASC 820 and clarifies and provides additional disclosure requirements related to
recurring and non-recurring fair value measurements and employers’ disclosures about postretirement
benefit plan assets. This ASU became effective for us on January 1, 2010. We do not currently
anticipate that this ASU will have a material impact on our consolidated financial statements upon
adoption.
In October 2009, the FASB issued ASU No. 2009-13, Revenue Recognition (Topic 605):
Multiple-Deliverable Revenue Arrangements (a consensus of the FASB Emerging Issues Task Force),
which amends ASC 605-25, Revenue Recognition: Multiple-Element Arrangements. ASU No. 2009-13
addresses how to determine whether an arrangement involving multiple deliverables contains more
than one unit of accounting and how to allocate consideration to each unit of accounting in the
arrangement. This ASU replaces all references to fair value as the measurement criteria with the
term selling price and establishes a hierarchy for determining the selling price of a deliverable.
ASU No. 2009-13 also eliminates the use of the residual value method for determining the allocation
of arrangement consideration. Additionally, ASU No. 2009-13 requires expanded disclosures. This ASU
will become effective for revenue arrangements entered into or materially modified after the fiscal
year 2010. Earlier application is permitted with required transition disclosures based on the
period of adoption. We are currently evaluating the application date and the impact of this
standard on our consolidated financial statements.
47
Table of Contents
In September 2009, the FASB issued ASU No. 2009-12, Fair Value Measurements and Disclosures
(Topic 820): Investments in Certain Entities That Calculate Net Asset Value per Share (or Its
Equivalent), which amends ASC 820-10, Fair Value Measurements and Disclosures—Overall. ASU No.
2009-12 permits a reporting entity to measure the fair value of certain alternative investments
that do not have a readily determinable fair value on the basis of the investments’ net asset value
per share or its equivalent. This ASU also requires expanded disclosures. This guidance became
effective for us on October 1, 2009 and did not have a material impact on our consolidated
financial statements upon adoption; however, it may impact the valuation of our future investments.
On July 1, 2009, we adopted ASU No. 2009-1, Topic 105 — Generally Accepted Accounting
Principles, which amended ASC 105, Generally Accepted Accounting Principles, to establish the ASC
as the source of authoritative GAAP recognized by the FASB to be applied by nongovernmental
entities. Rules and interpretive releases of the SEC under authority of federal securities laws are
also sources of authoritative GAAP for SEC registrants. On the effective date, the ASC superseded
all then-existing non-SEC accounting and reporting standards. All previous references to the
superseded standards in our consolidated financial statements have been replaced by references to
the applicable sections of the ASC. The adoption of these sections did not have an impact on our
consolidated financial statements.
In April 2009, we adopted ASC 320, Investments — Debt and Equity Securities, to modify the
existing impairment model with respect to debt securities falling within the scope of investments
for debt and equity securities. ASC 320 defines whether an other-than-temporary impairment (“OTTI”)
will have occurred when either: (i) an entity has the intent to sell an impaired security; (ii) it
is more likely than not that an entity will be required to sell an impaired security prior to its
anticipated recovery in value; or (iii) an entity does not expect to recover the entire cost basis
of an impaired security. In addition, ASC 320 modifies the manner in which an OTTI is measured and
presented on the statement of operations and requires expanded disclosures. ASC 320 was effective
for interim and annual reporting periods ending after June 15, 2009. The adoption of this standard
did not have a material impact on our consolidated financial statements, but resulted in additional
disclosure requirements about our investments. See Note 2, “Investments” for further discussion.
On January 4, 2009, we adopted ASC 820, Fair Value Measurements and Disclosures, which
provides a consistent definition of fair value that focuses on exit price, prioritizes the use of
market-based inputs over entity-specific inputs for measuring fair value and establishes a
three-level hierarchy for fair value measurements. On January 4, 2008, we adopted the applicable
sections of ASC 820 for financial assets and financial liabilities and for nonfinancial assets and
nonfinancial liabilities that are remeasured at least annually. At that time, we elected to defer
adoption of ASC 820 for one year for nonfinancial assets and nonfinancial liabilities that are
recognized or disclosed at fair value in the financial statements on a nonrecurring basis. On
January 4, 2009, we adopted the sections of ASC 820 regarding nonfinancial assets and
nonfinancial liabilities that are recognized or disclosed at fair value in the financial statements
on a nonrecurring basis. The applicable sections of ASC 820 were applied prospectively. The
adoption of the various sections of ASC 820 is disclosed in Note 3, “Fair Value Measurements.”
On January 4, 2009, we adopted the applicable sections of ASC 805, Business Combinations,
which provides revised guidance for recognizing and measuring identifiable assets and goodwill
acquired, liabilities assumed and any noncontrolling interest in the acquiree in a business
combination. Additionally, this ASC provides disclosure requirements to enable users of the
financial statements to evaluate the nature and financial effects of the business combination. ASC
805 amends the applicable sections of ASC 740, Income Taxes, such that adjustments made to
valuation allowances on deferred taxes and acquired tax contingencies related to acquisitions made
prior to January 4, 2009 also fall within the scope of these sections. During the fiscal year ended
2009, we expensed approximately $12.3 million of transaction costs recorded to “Selling, general
and administrative” expenses in the consolidated statements of operations related to the merger
agreement with HeartWare International Inc. (“HeartWare”). The Company and HeartWare mutually
agreed effective July 31, 2009 to terminate the definitive merger agreement pursuant to which we
would have acquired HeartWare.
On January 4, 2009, we adopted the applicable sections of ASC 275, Risks and Uncertainties,
and ASC 350, Intangibles — Goodwill and Other, that address the determination of the useful life
of intangible assets. These sections address the factors that should be considered in developing
renewal or extension assumptions used to determine the useful life of a recognized intangible
asset. The adoption of these applicable sections did not have a material impact our consolidated
financial statements.
48
Table of Contents
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
Our investment portfolio is made up of marketable investments in money market funds, auction
rate securities, U.S. Treasury securities and debt instruments of government agencies, local
municipalities, and high quality corporate issuers. All investments are carried at market value and
are treated as available-for-sale. Investments with maturities beyond one year may be classified as
short-term based on their highly liquid nature due to the frequency with which the interest rate is
reset and because such marketable securities represent the investment of cash that is available for
current operations. Our holdings of the securities of any one issuer, except government agencies,
do not exceed 10% of the portfolio. If interest rates rise, the market value of our investments may
decline, which could result in a loss if we choose or are forced to sell an investment before its
scheduled maturity. If interest rates were to rise or fall from current levels by 25 basis points,
the change in our net unrealized loss on investments would be $0.5 million. We do not utilize
derivative financial instruments to manage interest rate risks.
Our senior subordinated convertible notes do not bear interest rate risk as the notes were
issued at a fixed rate of interest.
As of January 2, 2010 we owned approximately $27.8 million of auction rate securities, which
is part of our investment portfolio. The assets underlying these auction rate securities are
student loans which are AAA or A- rated, and backed by the U.S. government under the Federal Family
Education Loan Program or private insurers. Historically, these securities have provided liquidity
through a Dutch auction process that resets the applicable interest rate, periodically every seven
to thirty-five days. Beginning in February of 2008, these auctions began to fail. Although we have
realized at or below market interest rates for many of these auction rate securities than we
otherwise would have, the principal amount will not be accessible until future auctions for these
securities are successful, a secondary market is established, or these securities are called for
redemption. Therefore, our auction rate securities are classified as long-term and are valued at
$24.6 million, net of a $3.1 million impairment, using significant unobservable inputs, with the exception of $0.1 million which was
classified as short-term investment. Based on our expected operating cash flows, and our other
sources of cash, we do not anticipate the potential lack of liquidity of these investments will
affect our ability to execute our current business plan.
Foreign Currency Rate Fluctuations
We use forward foreign currency contracts to mitigate the gains and losses generated by the
re-measurement of non-functional currency assets and liabilities (primarily assets and liabilities
on our U.K. subsidiary’s consolidated balance sheet that are not denominated in U.K. pounds). Our
contracts typically have maturities of three months or less.
As of January 2, 2010, we had forward contracts to sell euros with a notional value of €1.6
million and to sell U.K. pounds with a notional value of £0.3 million, and as of January 3, 2009,
we had forward contracts to sell euros with a notional value of €7.1 million and purchase U.K.
pounds with a notional value of £4.7 million. As of January 2, 2010, our forward contracts had an
average exchange rate of one U.S. dollar to 0.695 euros and one U.S. dollar to 0.628 U.K. pounds.
The potential fair value loss for a hypothetical 10% adverse change in foreign currency exchange
rates as of January 2, 2010 would be approximately $0.3 million.
49
Item 8. Financial Statements and Supplementary Data
THORATEC CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
Financial Statements: |
||||
| 51 | ||||
| 53 | ||||
| 54 | ||||
| 55 | ||||
| 56 | ||||
| 57 | ||||
50
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Thoratec Corporation:
We have audited the accompanying consolidated balance sheets of Thoratec Corporation and its
subsidiaries (the “Company”) as of January 2, 2010 and January 3, 2009, and the related
consolidated statements of operations, shareholders’ equity, and cash flows for each of the three
fiscal years in the period ended January 2, 2010. Our audits also included the consolidated
financial statement schedule listed in the Index at Item 15(a)2. These financial statements and
financial statement schedule are the responsibility of the Company’s management. Our responsibility
is to express an opinion on the financial statements and financial statement schedule based on our
audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects,
the financial position of Thoratec Corporation and subsidiaries as of January 2, 2010 and January
3, 2009, and the results of their operations and their cash flows for each of the three fiscal
years in the period ended January 2, 2010, in conformity with accounting principles generally
accepted in the United States of America. Also, in our opinion, such consolidated financial
statement schedule, when considered in relation to the basic consolidated financial statements
taken as a whole, presents fairly, in all material respects, the information set forth therein.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the Company’s internal control over financial reporting as of January 2,
2010, based on the criteria established in Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 24,
2010 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/
DELOITTE & TOUCHE LLP
San Francisco, CA
February 24, 2010
February 24, 2010
51
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Thoratec Corporation:
We have audited the internal control over financial reporting of Thoratec Corporation and its
subsidiaries (the “Company”) as of January 2, 2010, based on criteria established in Internal
Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway
Commission. The Company’s management is responsible for maintaining effective internal control over
financial reporting and for its assessment of the effectiveness of internal control over financial
reporting, included in the accompanying Management’s Report on Internal Control over Financial
Reporting. Our responsibility is to express an opinion on the Company’s internal control over
financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control over financial reporting was
maintained in all material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness exists, testing and
evaluating the design and operating effectiveness of internal control based on the assessed risk,
and performing such other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the
supervision of, the company’s principal executive and principal financial officers, or persons
performing similar functions, and effected by the company’s Board of Directors, management, and
other personnel to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with generally
accepted accounting principles. A company’s internal control over financial reporting includes
those policies and procedures that (1) pertain to the maintenance of records that, in reasonable
detail, accurately and fairly reflect the transactions and dispositions of the assets of the
company; (2) provide reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted accounting principles,
and that receipts and expenditures of the company are being made only in accordance with
authorizations of management and directors of the company; and (3) provide reasonable assurance
regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the
possibility of collusion or improper management override of controls, material misstatements due to
error or fraud may not be prevented or detected on a timely basis. Also, projections of any
evaluation of the effectiveness of the internal control over financial reporting to future periods
are subject to the risk that the controls may become inadequate because of changes in conditions,
or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over
financial reporting as of January 2, 2010, based on the criteria established in Internal Control —
Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway
Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the consolidated financial statements and consolidated financial statement
schedule as of and for the fiscal year ended January 2, 2010 of the Company and our report dated
February 24, 2010 expressed an unqualified opinion on those financial statements and financial
statement schedule.
/s/
DELOITTE & TOUCHE LLP
San Francisco, CA
February 24, 2010
February 24, 2010
52
Table of Contents
THORATEC CORPORATION
CONSOLIDATED BALANCE SHEETS
(In thousands)
(In thousands)
| January 2, 2010 | January 3, 2009 | |||||||
ASSETS |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 26,461 | $ | 107,053 | ||||
Short-term available-for-sale investments |
279,174 | 141,598 | ||||||
Receivables, net of allowances of $844 in 2009 and $947 in 2008 |
66,088 | 55,065 | ||||||
Inventories |
66,935 | 61,373 | ||||||
Deferred tax assets |
12,261 | 8,397 | ||||||
Short-term income taxes receivable |
1,234 | 2,514 | ||||||
Prepaid expenses and other assets |
5,713 | 4,901 | ||||||
Total current assets |
457,866 | 380,901 | ||||||
Property, plant and equipment, net |
51,852 | 50,138 | ||||||
Goodwill |
99,287 | 99,287 | ||||||
Purchased intangible assets, net |
99,859 | 108,584 | ||||||
Long-term available-for-sale investments |
24,634 | 29,959 | ||||||
Other long-term assets |
13,059 | 15,216 | ||||||
Total Assets |
$ | 746,557 | $ | 684,085 | ||||
LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 8,532 | $ | 10,563 | ||||
Accrued compensation |
22,407 | 25,550 | ||||||
Other accrued liabilities |
14,772 | 12,410 | ||||||
Total current liabilities |
45,711 | 48,523 | ||||||
Senior subordinated convertible notes |
131,929 | 124,115 | ||||||
Long-term deferred tax liability |
31,720 | 38,842 | ||||||
Other long-term liabilities |
12,069 | 6,326 | ||||||
Total Liabilities |
221,429 | 217,806 | ||||||
Contingencies (Note 9) |
||||||||
Shareholders’ equity: |
||||||||
Common shares: no par, authorized 100,000; issued and
outstanding 57,043 in 2009 and 56,395 in 2008 |
— | — | ||||||
Additional paid-in-capital |
557,418 | 528,657 | ||||||
Accumulated deficit |
(30,321 | ) | (56,634 | ) | ||||
Accumulated other comprehensive income (loss): |
||||||||
Unrealized loss on investments |
(648 | ) | (3,337 | ) | ||||
Cumulative translation adjustments |
(1,321 | ) | (2,407 | ) | ||||
Total accumulated other comprehensive loss |
(1,969 | ) | (5,744 | ) | ||||
Total Shareholders’ Equity |
525,128 | 466,279 | ||||||
Total Liabilities and Shareholders’ Equity |
$ | 746,557 | $ | 684,085 | ||||
See notes to consolidated financial statements.
53
Table of Contents
THORATEC CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
(In thousands, except per share data)
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Product sales |
$ | 373,937 | $ | 313,564 | $ | 234,780 | ||||||
Cost of product sales |
154,103 | 127,566 | 98,516 | |||||||||
Gross profit |
219,834 | 185,998 | 136,264 | |||||||||
Operating expenses: |
||||||||||||
Selling, general and administrative |
107,927 | 94,142 | 82,044 | |||||||||
Research and development |
54,227 | 52,943 | 43,835 | |||||||||
Amortization of purchased intangible assets |
10,663 | 13,183 | 12,582 | |||||||||
Total operating expenses |
172,817 | 160,268 | 138,461 | |||||||||
Income (loss) from operations |
47,017 | 25,730 | (2,197 | ) | ||||||||
Other income and (expense): |
||||||||||||
Interest expense |
(12,307 | ) | (10,984 | ) | (10,428 | ) | ||||||
Interest income and other |
6,043 | 9,146 | 8,624 | |||||||||
Income before taxes |
40,753 | 23,892 | (4,001 | ) | ||||||||
Income tax expense (benefit) |
12,169 | 5,561 | (3,398 | ) | ||||||||
Net income (loss) |
$ | 28,584 | $ | 18,331 | $ | (603 | ) | |||||
Net income(loss) per common share (1): |
||||||||||||
Basic |
$ | 0.50 | $ | 0.33 | $ | (0.01 | ) | |||||
Diluted |
$ | 0.49 | $ | 0.33 | $ | (0.01 | ) | |||||
Shares used to compute net income (loss) per common share (1): |
||||||||||||
Basic |
55,910 | 54,144 | 52,777 | |||||||||
Diluted |
57,322 | 55,243 | 52,777 | |||||||||
(1) See Note 16, “Net Income (Loss) Per Share,” for the computation of basic and diluted
calculation using the two-class method.
See notes to consolidated financial statements.
54
Table of Contents
THORATEC CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(In thousands)
(In thousands)
| Accumulated | ||||||||||||||||||||||||
| Other | Total | Total | ||||||||||||||||||||||
| Common | Additional | Accumulated | Comprehensive | Shareholders’ | Comprehensive | |||||||||||||||||||
| Shares | Paid-in Capital | Deficit | Income (Loss) | Equity | Income (Loss) | |||||||||||||||||||
BALANCE, DECEMBER 31, 2006,
as previously reported |
52,329 | $ | 427,941 | $ | (63,675 | ) | $ | 807 | $ | 365,073 | ||||||||||||||
Retrospective application of FSP
APB 14-1, net of taxes |
28,462 | (8,844 | ) | 19,618 | ||||||||||||||||||||
BALANCE, DECEMBER 31, 2006, as
adjusted |
52,329 | $ | 456,403 | $ | (72,519 | ) | $ | 807 | $ | 384,691 | ||||||||||||||
Cumulative adoption effect of FIN 48 |
(534 | ) | (534 | ) | ||||||||||||||||||||
Exercise of common stock options
for cash |
1,178 | 14,036 | 14,036 | |||||||||||||||||||||
Issuance of common shares under
Employee Stock Purchase Plan |
137 | 1,958 | 1,958 | |||||||||||||||||||||
Tax benefit related to employees’
and directors’ stock plans |
3,327 | 3,327 | ||||||||||||||||||||||
Repurchase of common shares, net |
464 | (411 | ) | (603 | ) | (1,014 | ) | |||||||||||||||||
Share-based compensation |
11,532 | 11,532 | ||||||||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||
Unrealized gain on
available-for-sale investments (net
of taxes of $222) |
333 | 333 | 333 | |||||||||||||||||||||
Foreign currency translation
adjustment |
83 | 83 | 83 | |||||||||||||||||||||
Net loss |
(603 | ) | (603 | ) | (603 | ) | ||||||||||||||||||
Total comprehensive income (loss) |
$ | (187 | ) | |||||||||||||||||||||
BALANCE, DECEMBER 29, 2007 |
54,108 | $ | 486,845 | $ | (74,259 | ) | $ | 1,223 | $ | 413,809 | ||||||||||||||
Exercise of common stock options
for cash |
1,768 | 22,914 | 22,914 | |||||||||||||||||||||
Issuance of common shares under
Employee Stock Purchase Plan |
149 | 2,062 | 2,062 | |||||||||||||||||||||
Tax benefit related to employees’
and directors’ stock plans |
6,926 | 6,926 | ||||||||||||||||||||||
Repurchase of common shares, net |
370 | (715 | ) | (706 | ) | (1,421 | ) | |||||||||||||||||
Share-based compensation |
10,625 | 10,625 | ||||||||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||
Unrealized loss on
available-for-sale investments (net
of taxes of $2,436) |
(3,654 | ) | (3,654 | ) | (3,654 | ) | ||||||||||||||||||
Foreign currency translation
adjustment |
(3,313 | ) | (3,313 | ) | (3,313 | ) | ||||||||||||||||||
Net income |
18,331 | 18,331 | 18,331 | |||||||||||||||||||||
Total comprehensive income |
$ | 11,364 | ||||||||||||||||||||||
BALANCE, JANUARY 3, 2009 |
56,395 | $ | 528,657 | $ | (56,634 | ) | $ | (5,744 | ) | $ | 466,279 | |||||||||||||
Exercise of common stock options
for cash |
652 | 9,184 | 9,184 | |||||||||||||||||||||
Issuance of common shares under
Employee Stock Purchase Plan |
133 | 2,898 | 2,898 | |||||||||||||||||||||
Tax benefit related to employees’
and directors’ stock plans |
3,932 | 3,932 | ||||||||||||||||||||||
Repurchase of common shares, net |
(137 | ) | (1,236 | ) | (2,271 | ) | (3,507 | ) | ||||||||||||||||
Share-based compensation |
13,983 | 13,983 | ||||||||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||
Unrealized gain on
available-for-sale investments (net
of taxes of $1,753) |
2,689 | 2,689 | 2,689 | |||||||||||||||||||||
Foreign currency translation
adjustment |
1,086 | 1,086 | 1,086 | |||||||||||||||||||||
Net income |
28,584 | 28,584 | 28,584 | |||||||||||||||||||||
Total comprehensive income |
$ | 32,359 | ||||||||||||||||||||||
BALANCE, JANUARY 2, 2010 |
57,043 | $ | 557,418 | $ | (30,321 | ) | $ | (1,969 | ) | $ | 525,128 | |||||||||||||
See notes to consolidated financial statements.
55
Table of Contents
THORATEC CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(In thousands)
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Cash flows from operating activities: |
||||||||||||
Net income (loss) |
$ | 28,584 | $ | 18,331 | $ | (603 | ) | |||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
||||||||||||
Depreciation and amortization |
21,681 | 23,731 | 21,836 | |||||||||
Investment premium amortization (net) |
3,115 | 2,307 | 977 | |||||||||
Non-cash interest income and other |
1,050 | 893 | 424 | |||||||||
Non-cash interest expense |
7,814 | 7,156 | 6,554 | |||||||||
Write-down of investment |
— | — | 500 | |||||||||
Write-down of capitalized costs |
— | 490 | 161 | |||||||||
Tax benefit related to stock options |
3,932 | 6,926 | 3,327 | |||||||||
Share-based compensation expense |
13,898 | 10,866 | 11,414 | |||||||||
Excess tax benefits from share-based compensation |
(3,152 | ) | (4,509 | ) | (1,980 | ) | ||||||
Loss on disposal of asset |
233 | 573 | 254 | |||||||||
Change in net deferred tax liability |
(12,538 | ) | (9,512 | ) | (12,015 | ) | ||||||
Changes in assets and liabilities: |
||||||||||||
Receivables |
(10,668 | ) | (12,635 | ) | (3,373 | ) | ||||||
Inventories |
(7,760 | ) | (10,433 | ) | (8,804 | ) | ||||||
Prepaid expenses and other assets |
(1,609 | ) | 1,069 | (1,124 | ) | |||||||
Accounts payable |
(2,141 | ) | 998 | (3,661 | ) | |||||||
Accrued compensation and other accrued liabilities |
602 | 15,191 | 3,587 | |||||||||
Accrued income taxes |
6,033 | (402 | ) | (2,976 | ) | |||||||
Other |
— | — | (134 | ) | ||||||||
Net cash provided by operating activities |
49,074 | 51,040 | 14,364 | |||||||||
Cash flows from investing activities: |
||||||||||||
Purchases of available-for-sale investments |
(346,715 | ) | (153,238 | ) | (257,668 | ) | ||||||
Sales of available-for-sale investments |
156,398 | 96,471 | 175,475 | |||||||||
Maturities of available-for-sale |
59,333 | 74,475 | 12,803 | |||||||||
Issuance of HeartWare loan |
(20,000 | ) | — | — | ||||||||
Investment in convertible debentures and preferred shares |
— | — | (2,000 | ) | ||||||||
Purchased intangibles |
(1,440 | ) | — | — | ||||||||
Loan collections |
23,000 | — | — | |||||||||
Purchases of property, plant and equipment, net |
(11,825 | ) | (10,377 | ) | (6,651 | ) | ||||||
Net cash (used in) provided by investing activities |
(141,249 | ) | 7,331 | (78,041 | ) | |||||||
Cash flows from financing activities: |
||||||||||||
Proceeds from stock option exercises |
9,184 | 22,914 | 14,036 | |||||||||
Proceeds from stock issued under employee stock purchase plan |
2,898 | 2,062 | 1,958 | |||||||||
Excess tax benefits from share-based compensation |
3,152 | 4,509 | 1,980 | |||||||||
Repurchase and retirement of common shares |
(3,507 | ) | (1,421 | ) | (1,014 | ) | ||||||
Net cash provided by financing activities |
11,727 | 28,064 | 16,960 | |||||||||
Effect of exchange rate changes on cash and cash equivalents |
(144 | ) | (71 | ) | (47 | ) | ||||||
Net (decrease) increase in cash and cash equivalents |
(80,592 | ) | 86,364 | (46,764 | ) | |||||||
Cash and cash equivalents at beginning of fiscal year |
107,053 | 20,689 | 67,453 | |||||||||
Cash and cash equivalents at end of fiscal year |
$ | 26,461 | $ | 107,053 | $ | 20,689 | ||||||
Supplemental disclosure of cash flow information: |
||||||||||||
Cash paid for taxes |
$ | 15,178 | $ | 8,947 | $ | 9,345 | ||||||
Cash paid for interest |
$ | 3,414 | $ | 3,414 | $ | 3,414 | ||||||
Supplemental disclosure of non-cash investing and financing activities: |
||||||||||||
Transfers of equipment from inventory |
$ | 2,642 | $ | 3,055 | $ | 3,698 | ||||||
Purchases of property, plant and equipment through accounts payable and other
accrued liabilities |
$ | 1,573 | $ | 1,938 | $ | 170 | ||||||
Purchase of intangibles through other accrued liabilities |
$ | 500 | $ | — | $ | — | ||||||
See notes to consolidated financial statements.
56
Table of Contents
THORATEC CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Operations and Significant Accounting Policies
The Company and Basis of Presentation
Thoratec Corporation (referred to in these Notes as “we,” “our,” “us,” “Thoratec” or the
“Company”), is headquartered in Pleasanton, California and is a manufacturer of mechanical
circulatory support products for use by patients with heart failure (“HF”). We develop, manufacture
and market products that are used by physicians and hospitals for cardiac assist, vascular and
diagnostic applications. We organize and manage our business by functional operating entities,
which operate in two business segments: Cardiovascular and International Technidyne Corporation
(“ITC”). Our Cardiovascular division develops, manufactures and markets proprietary medical devices
used for circulatory support and vascular graft applications. Our ITC division designs, develops,
manufactures and markets point-of-care diagnostic test systems and incision products. We conduct
business both domestically and internationally.
We report on a 52-53 week fiscal year, which ends on the Saturday closest to December 31. The
fiscal year ended December 29, 2007 (“2007”) included 52 weeks and the fiscal year ended January 3,
2009 (“2008”) included 53 weeks and the fiscal year ended January 2, 2010 (“2009”) included 52
weeks.
We evaluated subsequent events for the period from January 2, 2010, the date of these
financial statements, through February 24, 2010, which represents the date these financial
statements are being filed with the Securities Exchange Commission (“SEC”). There were no
subsequent events which require reporting in these consolidated financial statements except as
noted in Note 18, “Subsequent Event.”
Principles of Consolidation
Our consolidated financial statements include the financials statements of our wholly-owned
subsidiaries. Intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting
principles requires us to make estimates and assumptions that affect the reported amounts in the
consolidated financial statements and the accompanying notes. The actual amounts could differ from
those estimated amounts.
Major Customers and Concentration of Credit Risk
We primarily sell our products to large hospitals and distributors. No customer accounted for
more than 10% of total product sales in fiscal years 2009, 2008 or 2007. No customer had an accounts
receivable balance greater than 10% of total accounts receivable at
the end of fiscal year 2009 or
2008.
Financial instruments that potentially expose us to concentrations of credit risk consist
primarily of cash and cash equivalents, short and long-term investments, and trade accounts
receivable. Cash and cash equivalents held with financial institutions may exceed the amount of
insurance provided by the Federal Deposit Insurance Corporation on such deposits. Investments in
municipal bonds, variable demand notes and auction rate securities, backed by U.S Government or
private insurers, are subject to credit risk. However, we invest in high-grade instruments and
limit our exposure to any one issuer. In addition, we have recorded an impairment loss on our
auction rate securities. Concentration of credit risk with respect to our trade accounts receivable
to our customers is limited to large hospitals and distributors. Credit is extended to our
customers, based on an evaluation of a customer’s financial condition and generally collateral is
not required. To date, credit losses have not been significant; however, we maintain allowances for
potential credit losses.
57
Table of Contents
Certain Risks and Uncertainties
We are subject to certain risks and uncertainties and believe that changes in any of the
following areas could have a material adverse effect on our future financial position or results of
operations: counterparty credit risk in the current market environment; the ability to receive and
maintain U.S. Food and Drug Administration (“FDA”) and foreign regulatory authorities approval to
manufacture, market and sell our products; our ability to adequately and timely address issues
raised by the FDA inspections; the ability to direct and manage current and future growth and
physician acceptance of our current or future products; our reliance on specialized suppliers; the
ability to manufacture products on an efficient and timely basis and at a reasonable cost and in
sufficient volume, including the ability to obtain timely deliveries of parts from suppliers; our
ability to identify and correct quality issues in a timely manner and at a reasonable cost; new
product development and introduction, including FDA approval and market receptiveness; the ability
to protect our proprietary technologies or an infringement by us of others’ patents; the number of
heart transplants conducted; any reduction in the number of medical procedures requiring certain
types of blood monitoring; our dependence upon distributors and any changes made to our method of
distribution; competition from other products; worldwide demand for circulatory support and graft
products and blood coagulation testing and skin incision devices and the management of risks
inherent in selling in foreign countries; foreign currency fluctuations; the long and variable
sales and deployment cycle of our ventricular assist device (“VAD”) products; the willingness of
third party payors to cover and provide appropriate levels of reimbursement for our products; our
subordinated convertible notes, their repayment and potential related dilution from conversion; the
ability to realize the full value of our intangible assets; product liability or other claims; the
ability to attract and retain talented employees; stock price volatility due to general economic
conditions or future issuances and sales of our stock; the integration of any current and future
acquisitions of companies or technologies; the occurrence of catastrophic disasters; the ability to
achieve and maintain profitability; claims relating to the handling, storage or disposal of
hazardous chemicals and biomaterials; changes in legal and accounting regulations and standards;
changes in tax regulations; and limitations on potential acquisitions and stock pricing.
Cash and Cash Equivalents
Cash equivalents are defined as short-term, highly liquid investments with original maturities
of 90 days or less at the date of purchase. The fair value of these investments was determined by
using quoted prices for identical investments in active markets which are measured at Level 1
inputs under ASC 820, Fair Value Measurements and Disclosures. The carrying value of all other
cash equivalents approximates fair value due to their relatively short-term nature.
Investments
We hold investments in short-term and long-term available-for-sale securities. Investments in
short-term investments consist primarily of municipal bonds, variable demand notes and corporate
bonds and investments in long-term investments consist of auction rate securities.
Our investments with unrealized gains and losses are included in accumulated other
comprehensive income in stockholders’ equity. Unrealized losses are charged against “Interest and
other income, net” when a decline in fair value is determined to be other-than-temporary. We review
several factors to determine whether a loss is other-than-temporary. These factors include but are
not limited to: (i) our ability and intent to hold the
investment to maturity, (ii) whether it is more-likely-than-not we would be required to sell the investment before recovery of the
investments amortized cost basis and (iii) whether we expect to recover the amortized cost basis of
the investment. Auction rate securities are classified as long-term.
If the impairment is considered to be other-than-temporary, the security is written down to
its estimated fair value. Other-than-temporary declines in estimated fair value of all marketable
securities are charged to “Interest and other income, net.” The cost of all securities sold is
based on the specific identification method.
58
Table of Contents
Fair Value Measurement
The carrying amounts of certain of our financial instruments including cash and cash
equivalents, accounts receivable, accounts payable and accrued liabilities approximate fair value
due to their short maturities. Short-term investments are comprised of available-for-sale
securities, which are carried at fair value. Other non-current assets, which include auction rate
securities and deferred compensation plan assets, are carried at fair value and our convertible
debt and our Levitronix receivable is disclosed at fair value. The recorded carrying amount of our
other long-term obligations approximates fair value as of January 2, 2010. Foreign exchange
contracts are stated at fair value based on prevailing financial market information.
See Note 3, “Fair Value Measurement” for further information on fair value measurement of our
financial and nonfinancial assets and liabilities.
Inventories
Inventories are stated at the lower of cost or market. Cost is based on the first in, first
out method.
Property, Plant and Equipment
Property, plant and equipment is stated at cost, net of accumulated depreciation. Depreciation
is computed using the straight-line method based on estimated useful lives of two to thirty years.
Leasehold improvements are amortized over the lesser of the useful life or the remaining term of
the lease. Property, plant and equipment includes certain medical devices rented to customers.
Depreciation expense of all rental equipment included in our rental program is recognized ratably
over two to three years and is recorded in cost of product sales.
Capitalized Software Held for Internal Use
We capitalize costs of software held for internal use during the application development.
Capitalized computer software costs consist of purchased software licenses, implementation costs
and consulting for certain projects that qualify for capitalization. We expense costs related to
preliminary project assessment, research and development, re-engineering, training and application
maintenance as incurred. Completed projects are amortized after reaching the point of general
availability using a straight-line method based on the estimated useful life ranging from three to
eight years. As of January 2, 2010 and January 3, 2009, capitalized software held for internal use
was $7.0 million and $6.4 million, net of accumulated amortization of $1.9 million and $2.2
million, respectively, and was included in “Property, plant and equipment, net” on the consolidated
balance sheets.
Valuation of Long-Lived Assets and Purchased Intangible Assets
We evaluate the carrying value of long-lived assets, including intangible assets, whenever
events or changes in business circumstances or our planned use of long-lived assets indicate that
their carrying amounts may not be fully recoverable or that their useful lives are no longer
appropriate. Reviews are performed to determine whether the carrying values of long-lived assets
are impaired based on a comparison to the undiscounted expected future net cash flows. If the
comparison indicates that impairment exists, long-lived assets are written down to their respective
fair values. Significant management judgment is required in the forecast of future operating
results that is used in the preparation of expected undiscounted cash flows.
Purchased intangible assets are subject to amortization and are amortized over their estimated
period of benefit, ranging from one to fourteen years. We evaluate the recoverability of
intangible assets periodically, and take into account events or circumstances that warrant revised
estimates of useful lives or indicate that impairment exists, such as when the anticipated
identifiable undiscounted cash flows expect to be generated from an intangible asset is less than
the carrying value of the asset. In that event, a loss is recognized based on the amount by which
the carrying value exceeds the fair value of intangible asset. Fair value is determined primarily
using the anticipated cash flows discounted at a rate commensurate with the risk involved. No
impairments of purchased intangible assets have been identified during the years presented.
59
Table of Contents
Goodwill
Goodwill is tested for impairment on an annual basis or more frequently if indicators for
potential impairment exist. Impairment testing is conducted at the reporting unit level, for which
discrete financial information is available and for which the reporting units management regularly
reviews the operating results thereof.
Impairment testing requires that we first compare the carrying value of net assets to the
estimated fair value of net assets for the reporting units. If the carrying value exceeds the fair
value, a second step is performed to calculate the amount of impairment, which would be recorded as
a charge in the consolidated statements of operations. The fair value of a reporting unit is based
upon a number of considerations including projections of revenues, earnings and discounted cash
flows. The discount rate used for cash flows reflects capital market conditions and the specific
risks associated with the business. In addition, we compare the aggregate of the reporting unit’s
fair values to the Company’s market capitalization as a further corroboration of the fair value.
The testing requires a complex series of assumptions and judgment by management in projecting
future operating results and assessing risks. The use of alternative assumptions and estimates
could affect the fair values and change the impairment determinations. There have been no goodwill
impairments during the years presented.
Deferred Compensation Plan
We established a non-qualified, unfunded deferred compensation plan for certain management
employees and our Board of Directors. Amounts deferred and contributed under the deferred
compensation plan are credited or charged with the performance of investment options offered under
the plan as elected by the participants. The liability for compensation deferred under this plan is
included in “Other long-term liabilities” on our consolidated balance sheets. We manage the risk of
changes in the fair value of the liability for deferred compensation by electing to match our
liability under the plan with an investment that offsets a substantial portion of the Company’s
exposure. The cash value of the investment vehicle, which includes funding for future deferrals, is
included in “Other long-term assets” on our consolidated balance sheets.
Debt Issuance Costs
Costs incurred in connection with the issuance of our senior subordinated convertible notes
have been allocated between the liability component and the equity component as further discussed
in Note 10, “Long-Term Debt.” The liability component of the debt issuance costs incurred are
capitalized and are included in “Other long-term assets” on the consolidated balance sheets. These
costs are amortized using the effective interest method until May 2011, the point at which we can
redeem the debt, and such amortization expense is reflected in “Interest expense” on our
consolidated statements of operations.
Foreign Currency Translation
Our international operations consist primarily of sales and service personnel for our
Cardiovascular division who report to our U.S. sales and marketing group. The functional currency
is the local currency. All assets and liabilities of our non-U.S. operations are translated into
U.S. dollars at the period-end exchange rates and the resulting translation adjustments are
included in other comprehensive income. The period-end translation of the non-functional currency
assets and liabilities (primarily assets and liabilities on our U.K. subsidiary’s consolidated
balance sheet that are not denominated in U.K. pounds) at the period-end exchange rates result in
foreign currency gains and losses, which are included in “Interest income and other.”
Revenue Recognition and Product Warranty
We recognize revenue from product sales of our Cardiovascular and ITC divisions when evidence
of an arrangement exists, and title has passed (generally upon shipment) or services have been
rendered, the selling price is fixed or determinable and collectability is reasonably assured.
Sales to distributors are recorded when title transfers.
60
Table of Contents
The majority of our products are covered by up to a two-year limited manufacturer’s warranty.
Estimated contractual warranty obligations are recorded when related sales are recognized and any
additional amounts are recorded when such costs are probable, can be reasonably estimated and are
included in “Cost of product sales.” The change in accrued warranty expense is summarized in the
following table:
| Balance | Accruals | Balance | ||||||||||||||
| Beginning | for Warranties | Settlements | End | |||||||||||||
| of Year | Issued | Made | of Year | |||||||||||||
| ( in thousands) | ||||||||||||||||
Fiscal 2009 |
$ | 1,071 | $ | 4,537 | $ | (3,136 | ) | $ | 2,472 | |||||||
Fiscal 2008 |
$ | 1,006 | $ | 1,925 | $ | (1,860 | ) | $ | 1,071 | |||||||
Fiscal 2007 |
$ | 1,032 | $ | 634 | $ | (660 | ) | $ | 1,006 | |||||||
Research and Development Expense
Research and development costs are charged to expense when incurred. Major components of
research and development expenses consist of personnel costs, including salaries and benefits, and
regulatory and clinical costs associated with our compliance with FDA regulations. Research and
development costs are largely project driven, and the level of spending depends of the level of
project activity planned and subsequently approved and conducted.
Share-Based Compensation
Share-based compensation expense is measured based on the grant-date fair value of the
share-based awards. We recognize share-based compensation expense for the portion of the award that
will ultimately be expected to vest over the requisite service period for those awards with graded
vesting and service conditions. We develop an estimate of the number of share-based awards which
will ultimately vest, primarily based on historical experience. The estimated forfeiture rate is
reassessed periodically throughout the requisite service period. Such estimates are revised if they
differ materially from actual forfeitures. As required, the forfeiture estimates will be adjusted
to reflect actual forfeitures when an award vests.
We use the Black-Scholes option pricing model as the method for determining the estimated fair
value of stock options and purchase rights under the ESPP. The Black-Scholes model requires the use
of highly subjective and complex assumptions which determine the fair value of share-based awards,
including the option’s expected term and the price volatility of the underlying stock.
For restricted stock awards and restricted stock units, compensation expense is calculated
based on the fair value of our stock at the grant date.
See Note 11, “Share-Based Compensation” for further information on our equity incentive plans.
Income Taxes
We make certain estimates and judgments in determining income tax expense for financial
statement purposes. These estimates and judgments occur in the calculation of tax credits, benefits
and deductions, such as tax benefits from our non-U.S. operations and in the calculation of certain
tax assets and liabilities, which arise from differences in the timing of revenue and expense for
tax and financial statement purposes.
We record a valuation allowance to reduce our deferred tax assets to an amount that
more-likely-than-not will be realized. While we have considered future taxable income and ongoing
prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in
the event we were to determine that we would be able to realize our deferred tax assets in the
future in excess of our net recorded amount, an adjustment to the allowance for the deferred tax
asset would increase income in the period such determination was made. Likewise, should we
determine that we would not be able to realize all or part of our net deferred tax asset in the
future, an adjustment to the valuation allowance for the deferred tax asset would be charged to
income in the period such determination was made.
61
Table of Contents
We recognize liabilities for uncertain tax positions based on a two-step process. The first
step is to evaluate the tax position for recognition by determining if the weight of available
evidence indicates that it is more likely than not that the position will be sustained on audit,
including resolution of related appeals or litigation processes, if any. If we determine that a tax
position will more likely than not be sustained on audit, the second step requires us to estimate
and measure the tax benefit as the largest amount that is more than 50% likely to be realized upon
ultimate settlement. It is inherently difficult and subjective to estimate such amounts, as we have
to determine the probability of various possible outcomes. We reevaluate these uncertain tax
positions on a quarterly basis. This evaluation is based on factors including, but not limited to,
changes in facts or circumstances, changes in tax law and settled and effectively settled issues
under audit. Such a change in recognition or measurement would result in the recognition of a tax
benefit or an additional charge to the tax provision.
See Note 14, “Taxes on Income” for further information on our tax position.
Other Comprehensive Income
Other comprehensive income includes unrealized gains and losses on available-for-sale
investments and foreign currency translation adjustments.
Letter of Credit
We maintain an Irrevocable Standby Letter of Credit as part of our workers’ compensation
insurance program. The Letter of Credit is not collateralized. The Letter of Credit automatically
renew on June 30 of each year, unless terminated by one of the parties. As of January 2, 2010, our
Letters of Credit balance was approximately $0.8 million.
Recently Issued Accounting Pronouncements
In January 2010, the FASB issued Accounting Standards Updates (“ASU”) No. 2010-06, Fair Value
Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements. ASU
No. 2010-06 amends ASC 820 and clarifies and provides additional disclosure requirements related to
recurring and non-recurring fair value measurements and employers’ disclosures about postretirement
benefit plan assets. This ASU became effective for us on January 1, 2010. We do not currently
anticipate that this ASU will have a material impact on our consolidated financial statements upon
adoption.
In October 2009, the FASB issued ASU No. 2009-13, Revenue Recognition (Topic 605):
Multiple-Deliverable Revenue Arrangements (a consensus of the FASB Emerging Issues Task Force),
which amends ASC 605-25, Revenue Recognition: Multiple-Element Arrangements. ASU No. 2009-13
addresses how to determine whether an arrangement involving multiple deliverables contains more
than one unit of accounting and how to allocate consideration to each unit of accounting in the
arrangement. This ASU replaces all references to fair value as the measurement criteria with the
term selling price and establishes a hierarchy for determining the selling price of a deliverable.
ASU No. 2009-13 also eliminates the use of the residual value method for determining the allocation
of arrangement consideration. Additionally, ASU No. 2009-13 requires expanded disclosures. This ASU
will become effective for us for revenue arrangements entered into or materially modified after
fiscal year 2010. Earlier application is permitted with required transition disclosures based on
the period of adoption. We are currently evaluating the application date and the impact of this
standard on our consolidated financial statements.
In September 2009, the FASB issued ASU No. 2009-12, Fair Value Measurements and Disclosures
(Topic 820): Investments in Certain Entities That Calculate Net Asset Value per Share (or Its
Equivalent), which amends ASC 820-10, Fair Value Measurements and Disclosures—Overall. ASU No.
2009-12 permits a reporting entity to measure the fair value of certain alternative investments
that do not have a readily determinable fair value on the basis of the investments’ net asset value
per share or its equivalent. This ASU also requires expanded disclosures. This guidance became
effective for us on October 1, 2009 and did not have a material impact on our consolidated
financial statements upon adoption; however, it may impact the valuation of our future investments.
On July 1, 2009, we adopted ASU No. 2009-1, Topic 105 — Generally Accepted Accounting
Principles, which amended Accounting Standards Codification (“ASC”) 105, Generally Accepted
Accounting Principles, to establish the ASC as the source of authoritative GAAP recognized by the
FASB to be applied by nongovernmental entities. Rules and interpretive releases of the SEC under
authority of federal securities laws are also sources of authoritative GAAP for SEC registrants. On
the effective date, the ASC superseded all then-existing non-SEC accounting and reporting
standards. All previous references to the superseded standards in our consolidated financial
statements have been replaced by references to the applicable sections of the ASC. The adoption of
these sections did not have an impact on our consolidated financial statements.
62
Table of Contents
In April 2009, we adopted ASC 320, Investments — Debt and Equity Securities, to modify the
existing impairment model with respect to debt securities falling within the scope of investments
for debt and equity securities. ASC 320 defines whether an other-than-temporary impairment (“OTTI”)
will have occurred when either: (i) an entity has the intent to sell an impaired security; (ii) it
is more likely than not that an entity will be required to sell an impaired security prior to its
anticipated recovery in value; or (iii) an entity does not expect to recover the entire cost basis
of an impaired security. In addition, ASC 320 modifies the manner in which an OTTI is measured and
presented on the statement of operations and requires expanded disclosures. ASC 320 was effective
for interim and annual reporting periods ending after June 15, 2009. The adoption of this standard
did not have a material impact on our consolidated financial statements, but resulted in additional
disclosure requirements about our investments. See Note 2, “Investments” for further discussion.
On January 4, 2009, we adopted ASC 820, Fair Value Measurements and Disclosures, which
provides a consistent definition of fair value that focuses on exit price, prioritizes the use of
market-based inputs over entity-specific inputs for measuring fair value and establishes a
three-level hierarchy for fair value measurements. On January 4, 2008, we adopted the applicable
sections of ASC 820 for financial assets and financial liabilities and for nonfinancial assets and
nonfinancial liabilities that are remeasured at least annually. At that time, we elected to defer
adoption of ASC 820 for one year for nonfinancial assets and nonfinancial liabilities that are
recognized or disclosed at fair value in the financial statements on a nonrecurring basis. On
January 4, 2009, we adopted the sections of ASC 820 regarding nonfinancial assets and nonfinancial
liabilities that are recognized or disclosed at fair value in the financial statements on a
nonrecurring basis. The applicable sections of ASC 820 were applied prospectively. The adoption of
the various sections of ASC 820 is disclosed in Note 3, “Fair
Value Measurements.”
On January 4, 2009, we adopted the applicable sections of ASC 805, Business Combinations,
which provides revised guidance for recognizing and measuring identifiable assets and goodwill
acquired, liabilities assumed and any noncontrolling interest in the acquiree in a business
combination. Additionally, this ASC provides disclosure requirements to enable users of the
financial statements to evaluate the nature and financial effects of the business combination. ASC
805 amends the applicable sections of ASC 740, Income Taxes, such that adjustments made to
valuation allowances on deferred taxes and acquired tax contingencies related to acquisitions made
prior to January 4, 2009 also fall within the scope of these sections. During the fiscal year 2009,
we expensed approximately $12.3 million of transaction costs recorded to “Selling, general and
administrative” expenses in the consolidated statements of operations related to the merger
agreement with HeartWare International Inc. (“HeartWare”). The Company and HeartWare mutually
agreed effective July 31, 2009 to terminate the definitive merger agreement pursuant to which we
would have acquired HeartWare.
On January 4, 2009, we adopted the applicable sections of ASC 275, Risks and Uncertainties,
and ASC 350, Intangibles — Goodwill and Other, that addresses the determination of the useful life
of intangible assets. These sections address the factors that should be considered in developing
renewal or extension assumptions used to determine the useful life of a recognized intangible
asset. The adoption of these applicable sections did not have a material impact our consolidated
financial statements.
2. Investments
Our investment portfolio is comprised of short-term and long-term investments. Investments
classified as short-term available-for-sale consist primarily of municipal bonds, corporate bonds
and variable demand notes. Investments classified as long-term available-for-sale consist of
auction rate securities, whose underlying assets are student loans.
Our investments in available-for-sale securities are recorded at estimated fair value on our
financial statements, and the temporary differences between cost and estimated fair value are
presented as a separate component of accumulated other comprehensive income.
As of January 2, 2010, we had gross unrealized gains from our investment in municipal bonds,
variable demand notes and corporate bonds of $1.9 million and gross unrealized losses from our
auction rate securities of $3.1 million.
63
Table of Contents
The aggregate market value, cost basis and gross unrealized gains and losses of
available-for-sale investments for fiscal 2009 and 2008 by major security type are as follows:
| Gross | ||||||||||||
| Amortized | Unrealized | Fair | ||||||||||
| Cost | Gains (Losses) | Value | ||||||||||
| (in thousands) | ||||||||||||
As of January 2, 2010: |
||||||||||||
Short-term investments: |
||||||||||||
Municipal bonds, variable demand notes and corporate bonds |
$ | 277,300 | $ | 1,874 | $ | 279,174 | ||||||
Long-term investments: |
||||||||||||
Auction rate securities |
$ | 27,700 | $ | (3,066 | ) | $ | 24,634 | |||||
As of January 3, 2009: |
||||||||||||
Short-term investments: |
||||||||||||
Municipal bonds |
$ | 139,931 | $ | 1,667 | $ | 141,598 | ||||||
Long-term investments: |
||||||||||||
Auction rate securities |
$ | 37,200 | $ | (7,241 | ) | $ | 29,959 | |||||
The contractual maturities of our available-for-sale investments are as follows:
| Amortized | Fair | |||||||
| Cost | Value | |||||||
| (in thousands) | ||||||||
As of January 2, 2010: |
||||||||
Maturing within one year |
$ | 179,998 | $ | 180,620 | ||||
Due after one year through two years |
97,302 | 98,554 | ||||||
Short-term available-for sale investments |
277,300 | 279,174 | ||||||
Auction rate securities maturing within five years or greater |
27,700 | 24,634 | ||||||
| $ | 305,000 | $ | 303,808 | |||||
As of January 3, 2009: |
||||||||
Maturing within one year |
$ | 55,552 | $ | 56,093 | ||||
Due after one year through two years |
84,379 | 85,505 | ||||||
Short-term available-for sale investments |
139,931 | 141,598 | ||||||
Auction rate securities maturing within five years or greater |
37,200 | 29,959 | ||||||
| $ | 177,131 | $ | 171,557 | |||||
64
Table of Contents
As of January 2, 2010 we owned approximately $27.8 million face amount of auction rate
securities, of which $0.1 million was classified as short-term and $27.7 million was classified as
long-term. The assets underlying these investments are student loans backed by the U.S. government
under the Federal Family Education Loan Program or by private insurers and are rated between A- and
AAA. Historically, these securities have provided liquidity through a Dutch auction process that
resets the applicable interest rate periodically every seven to thirty-five days. Beginning in
February of 2008, these auctions began to fail. The principal amount of these auction rate
securities will not be accessible until future auctions for these securities are successful, a
secondary market is established, these securities are called for redemption, or they are paid at
maturity.
As a result of these auction failures, these auction rate securities do not have a readily
determinable market value. Consistent with the fair value methodology used on January 3, 2009, we
estimated fair values at January 2, 2010, using a discounted cash flow model based on estimated
interest rates, the present value of future principal and interest payments discounted at rates
considered to reflect current market conditions, and the credit quality of the underlying
securities. Specifically, our management estimated the future cash flows over a five-year period,
and applied a credit default rate to reflect the risk in the marketplace for these investments that
has arisen due to the lack of an active market. As a result of feedback from outside consultants,
and government activities including recent settlement agreements, management’s assumption on the
expected recovery was modified to five years beginning at January 3, 2009 and this assumption
continues to be applicable as of January 2, 2010.
As of January 2, 2010, we have recorded an estimated cumulative unrealized loss of $3.1
million ($1.9 million, net of tax) related to the temporary impairment of the auction rate
securities, which was included in accumulated other comprehensive income (loss) within
shareholders’ equity. In addition, our management reviews impairments and credit loss associated
with its investments, including auction rate securities to determine the classification of the
impairment as “temporary” or “other-than-temporary” and to birfurcate the credit and non-credit
component of an other-than-temporary impairment event. We (i) do not intend to sell any of the
auction rate securities prior to maturity at an amount below the original purchase value; (ii)
intend to hold the investment to recovery and based on a more-likely-than-not probability
assessment that will not be required to sell the security before recovery; and (iii) deem that it
is not probable that we will receive less than 100% of the principal and accrued interest from the
issuer. Therefore, 100% of the impairment was charged to other comprehensive income (loss). Our
auction rate securities are classified as long-term and are valued at $24.6 million using
significant unobservable inputs. Further, we continue to liquidate investments in auction rate
securities as opportunities arise. During fiscal year 2009 $9.4 million in auction rate securities
were liquidated at par in connection with issuer calls.
If the issuers of the auction rate securities are unable to successfully complete future
auctions and their credit ratings deteriorate, we may in the future be required to record an
impairment charge to earnings on these investments. It could conceivably take until the final
maturity of the underlying notes (up to 30 years) to realize the investments’ carrying value.
3. Fair Value Measurement
On December 30, 2007, we adopted ASC 820, Fair Value Measurements and Disclosure. ASC 820
defines fair value as the price that would be received to sell an asset or paid to transfer a
liability (“exit price”) in an orderly transaction between market participants at the measurement
date. In determining fair value, we used various approaches, including market, income and/or cost
approaches, and each of these approaches requires certain inputs. Fair value measurement
establishes a hierarchy for inputs used in measuring fair value that maximizes the use of
observable inputs and minimizes the use of unobservable inputs by requiring that observable inputs
be used when available. Observable inputs are inputs that market participants would use in pricing
the asset or liability based on market data obtained from sources independent of us. Unobservable
inputs are inputs that reflect our assumptions as compared to the assumptions market participants
would use in pricing the asset or liability based on the best information available in the
circumstances.
We value our financial and nonfinancial assets and liabilities based on the observability of
inputs used in the valuation of such assets and liabilities using the following fair value
hierarchy. The fair value hierarchy ranks the quality and reliability of the information used to
determine fair values. Financial and nonfinancial assets and liabilities carried or disclosed at
fair value were classified and disclosed in one of the following three categories:
65
Table of Contents
| Level 1: | Quoted prices in active markets for identical assets or liabilities. |
| Level 2: | Quoted prices of similar investments in active markets, of similar or identical investments in markets that are not active or model based valuations for which all significant inputs and valuable drivers are observable, directly or indirectly. |
| Level 3: | Inputs that are unobservable and significant to the overall fair value measurement. |
The following table represents the fair value hierarchy for our financial assets and financial
liabilities measured at fair value on a recurring basis:
| January 2, 2010 | ||||||||||||||||||||
| Assets and | Significant | |||||||||||||||||||
| liabilities at | Quoted prices in | Other | Significant | |||||||||||||||||
| carrying | Total | active markets for | observable | unobservable | ||||||||||||||||
| value | fair value | identical assets | inputs | inputs | ||||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Assets |
||||||||||||||||||||
Short-term investments — municipal bonds, variable
demand notes and corporate bonds |
$ | 279,174 | $ | 279,174 | $ | — | $ | 279,174 | $ | — | ||||||||||
Long-term investments — auction rate securities |
24,634 | 24,634 | — | — | 24,634 | |||||||||||||||
Mark to market on foreign exchange instruments (Note 4) |
8 | 8 | — | 8 | — | |||||||||||||||
Cash value of investment vehicle — deferred
compensation plan (Note 13) |
2,436 | 2,436 | — | 2,436 | — | |||||||||||||||
Convertible debenture with Levitronix LLC (disclosure
only) |
2,812 | 3,000 | — | — | 3,000 | |||||||||||||||
Liabilities |
||||||||||||||||||||
Make-whole provision (Note 10) |
23 | 23 | — | — | 23 | |||||||||||||||
Senior subordinated convertible notes (fair value for
purposes of disclosure in Note 10) |
131,929 | 205,364 | — | 205,364 | — | |||||||||||||||
| January 3, 2009 | ||||||||||||||||||||
| Assets and | Significant | |||||||||||||||||||
| liabilities at | Quoted prices in | Other | Significant | |||||||||||||||||
| carrying | Total | active markets for | observable | unobservable | ||||||||||||||||
| value | fair value | identical assets | inputs | inputs | ||||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Assets |
||||||||||||||||||||
Short-term investments — municipal bonds |
$ | 141,598 | $ | 141,598 | $ | — | $ | 141,598 | $ | — | ||||||||||
Long-term investments — auction rate securities |
29,959 | 29,959 | — | — | 29,959 | |||||||||||||||
Cash value of investment vehicle — deferred
compensation plan (Note 13) |
1,708 | 1,708 | — | 1,708 | — | |||||||||||||||
Convertible debenture with Levitronix LLC (disclosure
only) |
5,711 | 4,200 | — | — | 4,200 | |||||||||||||||
Liabilities |
||||||||||||||||||||
Mark to market on foreign exchange instruments (Note 4) |
73 | 73 | — | 73 | — | |||||||||||||||
Make-whole provision (Note 10) |
46 | 46 | — | — | 46 | |||||||||||||||
Senior subordinated convertible notes (fair value for
purposes of disclosure in Note 10) |
124,115 | 215,880 | — | 215,880 | — | |||||||||||||||
Assets measured at fair value, on a recurring basis using significant unobservable Level 3
inputs consist of securities with an auction reset feature (“auction rate securities”) whose
underlying assets are student loans issued by various tax-exempt state agencies, most of which are
supported by federal government guarantees and some of which are supported by private insurers. In
addition, we are using significant unobservable Level 3 inputs for our disclosure of the fair value
of our convertible debenture with Levitronix LLC (“Levitronix”).
66
Table of Contents
The following table provides a reconciliation of the beginning and ending balances for the
assets and liabilities measured at fair value using significant unobservable inputs (Level 3):
| Fair Value Measurement Using | ||||||||
| Significant Unobservable Inputs | ||||||||
| (Level 3) | ||||||||
| Auction | ||||||||
| Rate | ||||||||
| Securities | Liabilities | |||||||
| (in thousands) | ||||||||
Balance as of December 29, 2007 |
$ | — | $ | 96 | ||||
Transfer to Level 3 |
46,050 | — | ||||||
Settlements at par |
(8,850 | ) | — | |||||
Unrealized holding gain on
make-whole provision, included in
interest income and other |
— | (50 | ) | |||||
Unrealized holding loss on auction
rate securities, included in other
comprehensive income(loss) |
(7,241 | ) | — | |||||
Balance as of January 3, 2009 |
$ | 29,959 | $ | 46 | ||||
Settlements at par |
(9,400 | ) | — | |||||
Transfer to Level 2 |
(100 | ) | — | |||||
Unrealized holding gain on
make-whole provision, included in
interest income and other |
— | (23 | ) | |||||
Unrealized holding gain on auction
rate securities, included in other
comprehensive income (loss) |
4,175 | — | ||||||
Balance as of January 2, 2010 |
$ | 24,634 | $ | 23 | ||||
The fair value, calculated using Level 3 inputs, for disclosure purposes on the Levitronix
loan receivable as of January 2, 2010 was $3.0 million and as of January 3, 2009 was $4.2 million.
We continue to monitor the market for auction rate securities and consider its impact (if any)
on the fair value of our investments. If the current market conditions deteriorate further, or the
anticipated recovery in fair values does not occur, we may be required to record additional
unrealized losses in other comprehensive income or other-than-temporary impairment charges to the
consolidated statement of operations in future periods.
4. Foreign Exchange Instruments
We utilize foreign currency forward exchange contracts and options to mitigate against future
movements in foreign exchange rates that affect certain existing and forecasted foreign currency
denominated sales and purchase transactions (primarily assets and liabilities on our U.K.
subsidiary’s consolidated balance sheet that are not denominated in U.K. pounds). We do not use
derivative financial instruments for speculative or trading purposes. We routinely hedge our
exposure to certain foreign currencies with various financial institutions in an effort to minimize
the impact of certain currency exchange rate fluctuations. If a financial counterparty to any of
our hedging arrangements experiences financial difficulties or is otherwise unable to honor the
terms of the foreign currency forward contract, we may experience material financial losses.
On January 4, 2009, we adopted the accounting pronouncement that requires the disclosure about
our objective of using derivative instruments for our forward foreign currency contracts that
qualify as derivatives which is incorporated in ASC 815, Derivatives and Hedging, and do not
qualify for hedge accounting. The impacts of the outstanding foreign currency contracts with a
maximum maturity of three months were as follows:
| Notional Amounts | ||||||||
| Fiscal Years | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
Purchases |
$ | — | $ | 7,250 | ||||
Sales |
2,707 | 10,138 | ||||||
67
Table of Contents
As of January 2, 2010, we had forward contracts to sell euros with a notional value of
€1.6 million and to sell U.K. pounds with a notional value of £0.3 million, and as of January 3,
2009, we had forward contracts to sell euros with a notional value of €7.1 million and to purchase
U.K. pounds with a notional value of £4.7 million. As of January 2, 2010, our forward contracts had
an average exchange rate of one U.S. dollar to 0.695 euros and one U.S. dollar to 0.628 U.K.
pounds. As of January 2, 2010 and January 3, 2009, the estimated fair value of these foreign
currency contracts was $8,000 and $73,000 respectively, which was reported in “Prepaid expenses and
other assets” on the consolidated balance sheets.
The following represents our realized fair value of the forward currency contracts and offsets
to the foreign currency exchange gains and losses which were included in “Interest income and
other” in the consolidated statements of operations:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Foreign currency exchange gain (loss) on foreign currency contracts |
$ | 334 | $ | (1,984 | ) | $ | (702 | ) | ||||
Foreign currency exchange (loss) gain on foreign translation adjustments |
(961 | ) | 2,057 | 1,049 | ||||||||
5. Inventories
Inventories consisted of the following:
| January 2, 2010 | January 3, 2009 | |||||||
| (in thousands) | ||||||||
Finished goods |
$ | 18,003 | $ | 24,373 | ||||
Work-in-process |
10,418 | 9,174 | ||||||
Raw materials |
38,514 | 27,826 | ||||||
Total |
$ | 66,935 | $ | 61,373 | ||||
6. Purchased Intangible Assets and Goodwill
The carrying amount of goodwill was $99.3 million as of January 2, 2010 and January 3, 2009.
The components of goodwill at January 2, 2010 and January 3, 2009 were $95.0 million attributable
to the Cardiovascular division and $4.3 million attributable to the ITC acquisition of the
outstanding common shares of privately held A-VOX Systems, Inc. (“Avox”).
In October 2009, we purchased patents at a fair value of $1.9 million, which we capitalized
under ASC 350, Intangibles — Goodwill and Other. These patents have an estimated useful life of
nine years.
In October 2006, ITC, our wholly-owned subsidiary, completed the acquisition of all of the
outstanding common shares of privately held Avox based in San Antonio, Texas. The assets and
liabilities of Avox were accounted for under the purchase method of accounting and recorded at
their fair values at October 3, 2006. The excess of the purchase price over the estimated fair
values of the net assets acquired was recorded as an increase in goodwill. The results of
operations of Avox have been included in the consolidated statement of operations beginning as of
October 3, 2006.
In February 2001, we merged with Thermo Cardiosystems, Inc. (“TCA”). Prior to the merger with
TCA, TCA was a subsidiary of Thermo Electron Corporation. The components of identifiable
intangible assets related to the merger include: patents and trademarks, core technology (Thoralon,
our proprietary bio-material), and developed technology (patented technology, other than core
technology, acquired in the merger). The components of intangible assets related to the October
2006 Avox acquisition include: patents and trademarks, developed technology, and customer and
distributor relationships and other.
The purchased intangibles on the consolidated balance sheets are summarized as follows:
| Fiscal Year 2009 | ||||||||||||
| Gross Carrying | Accumulated | |||||||||||
| Amount | Amortization | Net Carrying Amount | ||||||||||
| (in thousands) | ||||||||||||
Patents and trademarks |
$ | 40,454 | $ | (30,008 | ) | $ | 10,446 | |||||
Core technology |
37,485 | (15,737 | ) | 21,748 | ||||||||
Developed technology |
125,742 | (58,448 | ) | 67,294 | ||||||||
Customer and distributor relationships and other |
897 | (526 | ) | 371 | ||||||||
Total purchased intangible assets |
$ | 204,578 | $ | (104,719 | ) | $ | 99,859 | |||||
68
Table of Contents
| Fiscal Year 2008 | ||||||||||||
| Gross Carrying | Accumulated | |||||||||||
| Amount | Amortization | Net Carrying Amount | ||||||||||
| (in thousands) | ||||||||||||
Patents and trademarks |
$ | 38,515 | $ | (28,803 | ) | $ | 9,712 | |||||
Core technology |
37,485 | (13,765 | ) | 23,720 | ||||||||
Developed technology |
125,742 | (51,098 | ) | 74,644 | ||||||||
Customer and distributor relationships and other |
897 | (389 | ) | 508 | ||||||||
Total purchased intangible assets |
$ | 202,639 | $ | (94,055 | ) | $ | 108,584 | |||||
Amortization expense related to identifiable intangible assets for fiscal 2009, 2008 and 2007
was $10.7 million, $13.2 million and $12.6 million, respectively.
Patents and trademarks have useful lives ranging from three to fourteen years, core and
developed technology assets have useful lives ranging from one to twelve years and customer and
distributor relationships and other have useful lives ranging from one to five years.
Estimated amortization expense for the next five fiscal years and all years thereafter are as
follows:
| Fiscal year: | (in thousands) | |||
2010 |
$ | 10,452 | ||
2011 |
9,552 | |||
2012 |
9,329 | |||
2013 |
8,935 | |||
2014 |
8,831 | |||
Thereafter |
52,760 | |||
Total |
$ | 99,859 | ||
7. Property, Plant and Equipment, Net
Property, plant and equipment, net consisted of the following:
| January 2, 2010 | January 3, 2009 | |||||||
| (in thousands) | ||||||||
Land, building and improvements |
$ | 22,740 | $ | 21,187 | ||||
Equipment and capitalized software |
73,746 | 68,029 | ||||||
Furniture and leasehold improvements |
23,753 | 22,372 | ||||||
Total |
120,239 | 111,588 | ||||||
Less accumulated depreciation |
(68,387 | ) | (61,450 | ) | ||||
Total |
$ | 51,852 | $ | 50,138 | ||||
Depreciation expense in fiscal years 2009, 2008 and 2007 was $11.0 million, $10.5 million and
$9.3 million, respectively.
69
Table of Contents
8. Other Assets
Levitronix Convertible Debenture:
On August 23, 2006, we purchased a $5.0 million convertible debenture from Levitronix, a
company with which we have a distribution arrangement to sell Levitronix products. The convertible
debenture is a long-term note receivable with an annual interest rate of 5.7%, to be accrued
monthly and at the option of Levitronix, paid in cash or in-kind semi-annually on February 23 and
August 23 until its maturity on August 23, 2013. We may convert the debenture at any time at our
option into membership interests of Levitronix at a conversion price of $4.2857, which may be
adjusted as a result of certain corporate events. This conversion feature is not an embedded
derivative because the membership interests of the issuer are not readily convertible to cash. If
we had converted the debenture as of January 2, 2010, our ownership in Levitronix would have been
less than 5%.
As of January 2, 2010, the convertible debenture of $2.0 million plus accrued interest of $0.8
million was included in “Other long-term assets” on our consolidated balance sheets. We received a
principal payment of $3.0 million during the fourth quarter of 2009. The fair value of the
convertible debenture, based on a discounted cash flows valuation approach, was $3.0 million at
January 2, 2010.
HeartWare Loan Agreement:
On February 12, 2009, we entered into a definitive merger agreement with HeartWare, pursuant
to which we intended to acquire HeartWare. The Company and HeartWare mutually agreed effective July
31, 2009 to terminate the definitive merger agreement pursuant to which we would have acquired
HeartWare. As announced on July 29, 2009, the Federal Trade Commission (“FTC”) informed HeartWare and us that it would file a complaint in U.S. Federal District Court to challenge our
proposed acquisition of HeartWare. HeartWare and our decision to terminate the definitive merger
agreement was in response to the FTC’s determination to challenge the proposed acquisition.
Pursuant to the HeartWare loan agreement, we deposited $20.0 million (the “Loan Amount”) into
an escrow account on February 13, 2009 and agreed to loan such funds to HeartWare. Despite the
mutual termination of the definitive merger agreement, the Loan Amount continued to remain
available for borrowing by HeartWare at any time prior to the earlier of (i) November 1, 2011, (ii)
the date on which the outstanding portion of the Loan Amount borrowed by HeartWare, including any
accrued and unpaid interest, as well as the portion of the Loan Amount remaining in the escrow
account that have not been loaned to HeartWare, are converted into shares of HeartWare’s common
stock, as further described below, or (iii) the date on which the outstanding principal of the Loan
Amount borrowed by HeartWare becomes due and payable in full, whether by acceleration or otherwise,
pursuant to the terms of the loan agreement. Beginning as of May 1, 2009, HeartWare was able to
borrow up to an aggregate of $12.0 million and beginning as of July 31, 2009, HeartWare was able to
borrow up to an aggregate of $20.0 million, under certain conditions provided in the loan
agreement. The loan to HeartWare bore interest at a rate per annum equal to 10%. The principal
amount, together with any accrued and unpaid interest on the principal amount, would be due and
payable in full in cash on the earlier of (i) November 1, 2011 or (ii) the date on which the
outstanding principal of the Loan Amount borrowed by HeartWare became due and payable in full,
whether by acceleration or otherwise, pursuant to the terms of the loan agreement. Following the
mutual termination of the definitive merger agreement on July 31, 2009 and pursuant to the terms of
the loan agreement, we could convert the Loan Amount including the amounts drawn down by HeartWare
and accrued but unpaid interest thereon, into shares of HeartWare’s common stock, at our option.
The Loan Amount was convertible into shares of HeartWare’s common stock, at the conversion price
equal to $35.00 Australian dollars per share of HeartWare common stock, as adjusted pursuant to the
terms of the loan agreement.
On August 5, 2009, HeartWare borrowed $4.0 million from the escrow account leaving a balance
of $16.0 million. In November, HeartWare repaid the $4.0 million and in December 2009, HeartWare
borrowed and repaid $16.0 million, extinguishing the escrow facility and eliminating any further
obligations under this agreement as the Loan Amount was borrowed and repaid. Also in the fourth
quarter, the conversion option gain of $5.2 million, recorded in the third quarter of 2009, was
reversed and there was no option value as of the fiscal year ended January 2, 2010.
70
Table of Contents
9. Commitments and Contingencies
Legal Proceedings
From time to time we are involved in litigation arising out of claims in the normal course of
business. Based on the information presently available, management believes that there are no
claims or actions pending or threatened against us, the ultimate resolution of which will have a
material adverse effect on our financial position, liquidity or results of operations, although the
results of litigation are inherently uncertain and adverse outcomes are possible.
Leases
We lease manufacturing, office and research facilities and equipment under various operating
lease agreements. Future minimum lease payments for the next five years and thereafter are as
follows:
| Fiscal year ended: | (in thousands) | |||
2010 |
$ | 3,718 | ||
2011 |
3,890 | |||
2012 |
3,726 | |||
2013 |
3,652 | |||
2014 |
2,568 | |||
Thereafter |
13,626 | |||
Total |
$ | 31,180 | ||
Rent expense for all operating leases was $3.3 million in 2009, $3.2 million in 2008 and $3.0
million in 2007.
Commitments
We had purchase order commitments, including both supply and inventory related agreements,
totaling approximately $78.2 million and $90.3 million as of the end of fiscal 2009 and 2008,
respectively.
10. Long-Term Debt
In 2004, we completed the sale of $143.8 million initial principal amount of senior
subordinated convertible notes due in 2034. The convertible notes were sold to Qualified
Institutional Buyers pursuant to the exemption from the registration requirements of the Securities
Act of 1933, as amended, provided by Rule 144A thereunder. A portion of the proceeds was used to
repurchase 4.2 million shares of our outstanding common stock for $60 million. The balance of the
proceeds has been and will be used for general corporate purposes, which may include additional
stock repurchases, strategic investments or acquisitions. The principal amount of the convertible
notes at maturity is $247.4 million which, when offset by the original issue discount of $103.7
million and net debt issuance costs of $4.3 million, equaled net proceeds of $139.4 million.
The senior subordinated convertible notes were issued at an issue price of $580.98 per note,
which is 58.098% of the principal amount at maturity of the notes. The senior subordinated
convertible notes bear interest at a rate of 1.3798% per year on the principal amount at maturity,
payable semi-annually in arrears in cash on May 16 and November 16 of each year, from November 16,
2004 until May 16, 2011. Beginning on May 16, 2011, the original issue discount will accrue daily
at a rate of 2.375% per year on a semi-annual bond equivalent basis and, on the maturity date, a
holder will receive $1,000 per note. As a result, the aggregate principal amount of the notes at
maturity will be $247.4 million.
71
Table of Contents
Holders of the senior subordinated convertible notes may convert their convertible notes into
shares of our common stock at a conversion rate of 29.4652 shares per $1,000 principal amount of
senior subordinated convertible notes, which represents a conversion price of $19.72 per share,
subject to adjustments upon the occurrence of certain events as set forth in the indenture. Holders
have been and are able to convert their convertible notes at any point after the close of business
on October 30, 2004 if, as of the last day of the preceding calendar quarter, the closing price of
our common stock for at least 20 trading days in a period of 30 consecutive trading days ending on
the last trading day of such preceding calendar quarter is more than 120% of the accreted
conversion price per share of our common stock. Commencing October 1, 2008, this market price
conversion feature was satisfied, such that holders of the senior subordinated convertible notes
may convert their notes through the final maturity date of the notes into shares of the Company’s
common stock at a conversion rate of 29.4652 shares per $1,000 principal amount of senior
subordinated convertible notes, subject to adjustments as provided in the indenture. If holders
elect conversion, we may, at our option, deliver shares of common stock, pay a holder in cash, or
deliver a combination of shares and cash, as determined pursuant to the terms of the notes. As of
January 2, 2010, no notes had been converted.
Holders may require us to repurchase all or a portion of their senior subordinated convertible
notes on each of May 16, 2011, 2014, 2019, 2024 and 2029 at a repurchase price equal to 100% of the
issue price, plus accrued original issue discount, if any. In addition, if we experience a change
in control or a termination of trading of our common stock each holder may require the Company to
purchase all or a portion of such holder’s notes at the same price, plus, in certain circumstances,
to pay a make-whole premium. This premium is considered an embedded derivative and has been
bifurcated from the senior subordinated convertible notes and recorded at its estimated fair value,
$23,000 as of January 2, 2010. There are significant variables and assumptions used in valuing the
make-whole provision including, but not limited to, our stock price, the volatility of our stock,
the probability of us being acquired and the probability of the type of consideration used by a
potential acquirer.
The senior subordinated convertible notes are subordinated to all of our senior indebtedness
and structurally subordinated to all indebtedness of our subsidiaries. Therefore, in the event of a
bankruptcy, liquidation or dissolution of the Company or one or more of our subsidiaries and
acceleration of or payment default on our senior indebtedness, holders of the convertible notes
will not receive any payment until holders of any senior indebtedness we may have outstanding have
been paid in full.
In accordance with ASC 470-20, Debt, which applies to certain convertible debt instruments
that may be settled in cash or other assets, or partially in cash, upon conversion, we recorded the
long-term debt and equity components on the senior subordinated convertible notes separately. This accounting pronouncement increased interest expense associated with
our senior subordinated convertible notes by adding a non-cash component to amortize a debt discount calculated based on the
difference between the cash coupon rate (2.375% per year) of the senior subordinated convertible notes and the effective interest rate on debt borrowing (9% per year). The
discount, which represents the non-cash interest expense, classified as interest expense on the
statements of operations, is being amortized to interest expense over a seven-year period ending
May 16, 2011 (the expected life of the liability component) using the effective interest method.
Additionally, we allocated transaction costs on the same percentage as the liability and equity
component, such that a portion of the deferred debt issuance costs is allocated to the liability
component to be amortized using the effective interest method until May 16, 2011, and the equity
component to be included in additional paid-in capital.
Interest
expense primarily includes interest and amortization of discount related to
senior subordinated convertible notes as follows:
| Fiscal Years | |||||||||||
| 2009 | 2008 | 2007 | |||||||||
Interest expense — cash component |
$ | 3,414 | $ | 3,414 | $ | 3,414 | |||||
Interest expense — non-cash component |
8,224 | 7,566 | 6,964 | ||||||||
The long-term debt and equity component
(recorded in additional paid-in-capital, net of income tax benefit) consisted of the following:
| January 2, 2010 | January 3, 2009 | |||||||
| (in thousands) | ||||||||
Long-term debt |
||||||||
Principal amount |
$ | 143,750 | $ | 143,750 | ||||
Unamortized discount |
(11,821 | ) | (19,635 | ) | ||||
Net carrying amount |
$ | 131,929 | $ | 124,115 | ||||
Equity component, net of income tax benefit |
$ | 28,462 | $ | 28,462 | ||||
We may redeem either in whole or in part any of the senior subordinated convertible notes at
any time beginning May 16, 2011, by giving the holders at least 30 days notice, at a redemption
price equal to the sum of the issue price and the accrued original issue discount. If the holders
converted the senior subordinated convertible notes into shares of our stock as of January 2, 2010,
the if-converted value would be $196.3 million, based on our stock price of $26.92 per share on
December 31, 2009, which amount exceeds the original value of $143.8 million by $52.5 million. This
if-converted value is $51.1 million less than the $247.4 million face amount at maturity in 2034.
The aggregate fair value of the senior subordinated convertible notes at January 2, 2010 was
$205.4 million.
72
Table of Contents
11. Share-Based Compensation
Share-based compensation expense is measured based on the grant-date fair value of the
share-based awards. We recognize share-based compensation expense for the portion of the awards
that will ultimately be expected to vest, on a straight-line basis over the requisite service
period for those awards with graded vesting and service conditions. We develop an estimate of the
number of share-based awards, which will ultimately vest primarily based on historical experience.
The estimated forfeiture rate is re-assessed periodically throughout the requisite service period.
Such estimates are revised if they differ materially from actual forfeitures. As required, the
forfeiture estimates will be adjusted to reflect actual forfeitures when an award vests.
Share-based compensation expense has been classified in the income statement or capitalized on
the balance sheet in the same manner as compensation that is paid to our employees and consists of
the following:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Cost of product sales |
$ | 2,023 | $ | 1,749 | $ | 1,598 | ||||||
Selling, general and administrative |
8,383 | 6,491 | 7,232 | |||||||||
Research and development |
3,492 | 2,626 | 2,584 | |||||||||
Total share-based compensation expense before tax |
13,898 | 10,866 | 11,414 | |||||||||
Tax benefit for share-based compensation expense |
5,348 | 4,351 | 3,525 | |||||||||
Total share-based compensation expense (net of taxes) |
$ | 8,550 | $ | 6,515 | $ | 7,889 | ||||||
Share-based compensation expense of $0.5 million and $0.4 million was capitalized to inventory
as of January 2, 2010 and January 3, 2009, respectively.
We receive a tax deduction for certain stock option exercises during the period the options
are exercised, generally for the excess of the fair market value of the options at the date of
exercise over the exercise prices of the options. Our consolidated statements of cash flows
presentation reports the excess tax benefits (i.e., windfalls only
for tax deductions in excess of the share-based compensation expense recognized) as financing cash
flows of $3.2 million, $4.5 million and $2.0 million for fiscal years 2009, 2008 and 2007,
respectively.
Cash proceeds from the exercise of stock options were $9.2 million and cash proceeds from our
employee stock purchase plan were $2.9 million for the fiscal year ended January 2, 2010. Cash
proceeds from the exercise of stock options were $22.9 million and cash proceeds from our employee
stock purchase plan were $2.1 million for the fiscal year ended January 3, 2009. Cash proceeds from
the exercise of stock options were $14.0 million and cash proceeds from our employee stock purchase
plan were $2.0 million for the fiscal year ended December 29, 2007. The actual income tax benefit
realized from stock option exercises was $3.9 million, $6.9 million and $3.3 million for fiscal
years 2009, 2008 and 2007, respectively.
Equity Plans
In 1996, the Board of Directors and our shareholders approved the 1996 Non-employee Directors
Stock Option Plan (“Directors Option Plan”). The Directors Option Plan was amended by the Board of
Directors in November 1996, amended again by approval of our shareholders in May 1997, amended
again by approval of our shareholders in May 1999, amended again by the Board of Directors in
February 2003, amended again by approval of our shareholders in May 2003, and amended again by the
Board of Directors in October 2003. The Directors Option Plan expired in February 2006 and no
options were granted under the Directors Option Plan in 2009.
In 1997, the Board of Directors adopted the 1997 Stock Option Plan (“1997 SOP”). The 1997 SOP
was amended by approval of our shareholders in February 2001, amended by the Board of Directors in
December 2001, amended again by approval of our shareholders in May 2003, and amended again by the
Board of Directors in March 2006. The 1997 SOP allowed us to grant up to a total of 13.7 million
shares of common stock in the form of stock options, restricted stock awards and stock bonuses.
This plan expired in May 2006 and no options were granted under the 1997 SOP in 2009.
73
Table of Contents
In April 2006, the Board of Directors approved the 2006 Incentive Stock Plan (“2006 Plan”), in
May 2006 the 2006 Plan was amended by the Board of Directors and approved by our shareholders and
in May 2008 the 2006 Plan was further amended by the Board of Directors and approved by our
shareholders. The 2006 Plan allows us to grant to employees and directors of, and consultants to,
the Company up to a total of 5.4 million shares of stock. Each share issued from and after May 20,
2008 as restricted stock bonuses, restricted stock units, phantom stock units, performance share
bonuses, or performance share units reduces the number of shares available for issuance under the 2006 Plan by one and seventy-four hundredths (1.74)
shares, and each share issued as stock options, restricted stock purchases or stock appreciation
rights reduces the shares available for issuance under the 2006 Plan on a share-for-share basis.
During the fiscal year ended January 2, 2010, approximately 344,900 options were granted under the
2006 Plan at an exercise price equal to the fair market value on the date of grant, and
approximately 498,100 shares of restricted stock units were granted under the 2006 Plan. As of
January 2, 2010, 2.1 million shares remained available for grant under the 2006 Plan.
Stock Options
Upon approval in May 2006, the 2006 Plan replaced our previous common stock option plans and
equity incentive plans. As of January 2, 2010, we had 3.9 million options outstanding under the
2006 Plan and the replaced plans. Options under the 2006 Plan may be granted by the Board of
Directors at the fair market value on the date of grant and generally become fully exercisable
within four years after the grant date and expire between five and ten years from the date of
grant. Vesting on some options granted to officers may be accelerated in certain circumstances
following a change in control of the Company.
The fair value of each option is estimated at the date of grant using the Black-Scholes option
pricing model with the following assumptions:
| Fiscal Years | |||||||||||||
| 2009 | 2008 | 2007 | |||||||||||
Risk-free interest rate |
2.34% | 3.25% | 4.79% | ||||||||||
Expected volatility |
53% | 40% | 40% | ||||||||||
Expected option life |
4.89-6.03 years | 5.08-6.07 years | 5.08-6.05 years | ||||||||||
Dividends |
None | None | None | ||||||||||
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of
grant. The expected term of options represents the period of time that options are expected to be
outstanding. We use separate assumptions for groups of employees (for example, officers) that have
similar historical exercise behavior. The range above reflects the expected option impact of these
separate groups. We base the expected volatility on historical volatility trends, because we have
determined that the historical volatility trends are reflective of market conditions.
As of January 2, 2010, there was $3.0 million of unrecognized compensation expense, net of
estimated forfeitures, related to stock options which expense we expect to recognize over a
weighted average period of 0.94 years. The aggregate intrinsic value of in-the-money options
outstanding was $37.1 million, based on the closing price of our common stock on December 31, 2009,
the last trading day in the fiscal year ended January 2, 2010, of $26.92. As of January 2, 2010,
the intrinsic value of options currently exercisable was $28.9 million and the intrinsic value of
options vested and expected to vest was $36.7 million.
The
total intrinsic value of options exercised for the fiscal years 2009,
2008, 2007 was $9.2 million, $20.7 million and $9.1million, respectively.
74
Table of Contents
Stock option activity is summarized as follows:
| Number of | Weighted | Weighted Average | ||||||||||
| Options | Average Exercise | Remaining Contract | ||||||||||
| (in thousands) | Price Per Share | Life (years) | ||||||||||
Outstanding as of December 30, 2006 (4,064 exercisable at $12.75
weighted average price per share) |
6,585 | $ | 14.65 | 6.74 | ||||||||
Granted |
583 | 18.32 | ||||||||||
Cancelled and expired |
(242 | ) | 17.70 | |||||||||
Exercised |
(1,178 | ) | 11.91 | |||||||||
Outstanding as of December 29, 2007 (3,940 exercisable at $13.72
weighted average price per share) |
5,748 | $ | 15.46 | 6.19 | ||||||||
Granted |
383 | 14.98 | ||||||||||
Cancelled and expired |
(104 | ) | 18.76 | |||||||||
Exercised |
(1,768 | ) | 12.96 | |||||||||
Outstanding as of January 3, 2009 (2,775 exercisable at $15.23
weighted average price per share) |
4,259 | $ | 16.37 | 5.98 | ||||||||
Granted |
345 | 24.03 | ||||||||||
Cancelled and expired |
(95 | ) | 22.44 | |||||||||
Exercised |
(652 | ) | 14.08 | |||||||||
Outstanding as of January 2, 2010 (2,687 exercisable at $16.17
weighted average price per share) |
3,857 | $ | 17.29 | 5.60 | ||||||||
Outstanding options vested at fiscal year end 2009 and expected to vest |
3,762 | $ | 17.22 | 5.53 | ||||||||
Weighted average remaining contract life for options exercisable was 4.68 years.
The weighted average grant-date fair value of options granted during the fiscal years 2009,
2008 and 2007 was $12.07 per share, $6.44 per share and $8.12 per share, respectively.
Options outstanding as of January 2, 2010 are summarized as follows:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| (in thousands, except contractual life and exercise price) | ||||||||||||||||||||
| Weighted | ||||||||||||||||||||
| Average | Weighted | Weighted | ||||||||||||||||||
| Exercise | Remaining | Average | Average | |||||||||||||||||
| Price | Contractual Life | Exercise | Exercise | |||||||||||||||||
| Range | Number | (In Years) | Price | Number | Price | |||||||||||||||
$5.60 —$11.80 |
236 | 2.79 | $ | 9.68 | 236 | $ | 9.68 | |||||||||||||
11.88 — 12.45 |
603 | 4.26 | 12.43 | 603 | 12.43 | |||||||||||||||
12.61 — 14.97 |
624 | 6.16 | 14.56 | 347 | 14.25 | |||||||||||||||
14.98 — 15.92 |
400 | 2.74 | 15.71 | 387 | 15.71 | |||||||||||||||
15.95 — 17.84 |
223 | 5.47 | 16.76 | 196 | 16.67 | |||||||||||||||
17.91 — 17.91 |
393 | 7.06 | 17.91 | 180 | 17.91 | |||||||||||||||
18.01 — 19.90 |
54 | 5.83 | 19.31 | 33 | 19.43 | |||||||||||||||
20.34 — 20.34 |
453 | 6.14 | 20.34 | 312 | 20.34 | |||||||||||||||
20.60 — 23.62 |
471 | 6.12 | 23.15 | 343 | 23.20 | |||||||||||||||
23.64 — 29.00 |
400 | 8.64 | 24.06 | 50 | 24.09 | |||||||||||||||
| 3,857 | 5.60 | 17.29 | 2,687 | 16.17 | ||||||||||||||||
Restricted Stock Awards and Units
The 2006 Plan allows for the issuance of restricted stock awards and restricted stock units,
which awards or units may not be sold or otherwise transferred until certain restrictions have
lapsed. The unearned share-based compensation related to these awards is being amortized to
compensation expense over the period of the restrictions, generally four years. The expense for
these awards was determined based on the market price of our shares on the date of grant applied to
the total number of shares that were granted.
75
Table of Contents
Restricted Stock Awards
Share-based compensation expense related to restricted stock awards was $5.5 million for the
fiscal year ended January 2, 2010. As of January 2, 2010, we had $5.3 million of unrecognized
compensation expense, net of estimated forfeitures, related to restricted stock awards, which
amount we expect to recognize over 1.6 years. There were no restricted stock awards granted during
fiscal year 2009.
Restricted stock award activity is summarized as follows:
| Number of | Weighted Average | |||||||
| Shares | Grant Date Fair | |||||||
| (in thousands) | Value | |||||||
Outstanding unvested restricted stock as of December 30, 2006 |
422 | $ | 17.63 | |||||
Granted |
570 | 18.39 | ||||||
Vested |
(165 | ) | 16.93 | |||||
Forfeited or expired |
(59 | ) | 18.31 | |||||
Outstanding unvested restricted stock as of December 29, 2007 |
768 | 18.29 | ||||||
Granted |
496 | 15.41 | ||||||
Vested |
(231 | ) | 18.40 | |||||
Forfeited or expired |
(50 | ) | 17.90 | |||||
Outstanding unvested restricted stock as of January 3, 2009 |
983 | 16.83 | ||||||
Granted |
— | — | ||||||
Vested |
(326 | ) | 17.12 | |||||
Forfeited or expired |
(48 | ) | 17.36 | |||||
Outstanding unvested restricted stock as of January 2, 2010 |
609 | 16.63 | ||||||
Restricted Stock Units
Share-based compensation expense related to restricted stock units was $3.2 million for the
fiscal year ended January 2, 2010. Unrecognized compensation expense as of January 2, 2010 was $7.1
million, net of estimated forfeitures, related to restricted stock units, which amount we expect to
recognize over 3.12 years. The aggregate intrinsic value of the units outstanding, based on our
stock price on January 2, 2010 was $12.5 million.
Restricted stock units activity is summarized as follows:
| Number of | Weighted Average | Weighted Average | ||||||||||
| Units | Grant Date Fair | Remaining Contract | ||||||||||
| (in thousands) | Value | (in Years) | ||||||||||
Outstanding units as of December 30, 2006 |
10 | $ | 19.08 | 1.74 | ||||||||
Granted |
15 | 18.27 | ||||||||||
Released |
(3 | ) | 19.10 | |||||||||
Forfeited or expired |
(1 | ) | 18.14 | |||||||||
Outstanding units as of December 29, 2007 |
21 | 18.58 | 2.82 | |||||||||
Granted |
15 | 15.00 | ||||||||||
Released |
(7 | ) | 18.68 | |||||||||
Forfeited or expired |
(1 | ) | 18.05 | |||||||||
Outstanding units as of January 3, 2009 |
28 | 16.66 | 2.46 | |||||||||
Granted |
498 | 24.63 | ||||||||||
Released |
(49 | ) | 24.70 | |||||||||
Forfeited or expired |
(14 | ) | 23.93 | |||||||||
Outstanding units as of January 2, 2010 |
463 | 24.17 | 3.12 | |||||||||
76
Table of Contents
Employee Stock Purchase Plan
In May 2002, our shareholders approved the Company’s Employee Stock Purchase Plan (“ESPP”)
under which 500,000 shares of common stock was reserved for issuance. In addition, the ESPP
provides for an annual, automatic increase of up to 250,000 shares in the total number of shares
available for issuance thereunder on March 1 of each year, unless our Board of Directors specifies
a smaller increase or no increase. Under this provision, an additional 250,000 shares were reserved
for issuance under the ESPP on each of March 1, 2006, March 1, 2008 and March 1, 2009; our Board of
Directors specified no increase as of each other year. Eligible employees may purchase a limited
number of shares, over a six month period, of our common stock at 85% of the lower of the market
value on the offering date or the market value on the purchase date. During the fiscal year ended
January 2, 2010, approximately 132,711 shares of common stock were issued under the ESPP. As of
January 2, 2010, approximately 299,700 shares remained available for issuance under this plan.
The estimated subscription date fair value of the offering under the ESPP for fiscal years
2009, 2008 and 2007 was approximately $0.6 million, $0.5 million and $0.3 million respectively,
using the Black-Scholes option pricing model and the following assumptions:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Risk-free interest rate |
0.17 | % | 1.07 | % | 4.80 | % | ||||||
Expected volatility |
40 | % | 60 | % | 40 | % | ||||||
Expected option life |
0.50 years | 0.50 years | 0.50 years | |||||||||
Dividends |
None | None | None | |||||||||
At January 2, 2010, there was approximately $0.4 million of unrecognized compensation expense
related to ESPP subscriptions that began on November 1, 2009, which amount we expect to recognize
during the first four months of 2010.
12. Common and Preferred Stock
We have authorized 100 million shares of no par common stock, and 2.5 million shares of no par
preferred stock, of which 540,541 shares have been designated Series A, 500,000 shares have been
designated Series B and 100,000 shares have been designated Series RP.
Our share repurchase programs, which authorized us to repurchase up to a total of $130 million
of our common stock, were announced on February 11, 2004 as a $25 million program, on May 12, 2004
as a $60 million program, on July 29, 2004 as a $25 million program and on February 2, 2007 as a
$20 million program. No shares of our common stock were repurchased under our publicly announced
repurchase programs during the fiscal years ended January 2, 2010 and January 3, 2009. All
repurchased shares have been retired and are not included in net income per common share. As of
January 2, 2010, we have $10.1 million remaining on our share repurchase programs.
The Series A preferred stock is entitled to cumulative annual dividends of $1.30 per share and
has a liquidation preference of $9.25 per share plus cumulative unpaid dividends. We may redeem the
Series A preferred stock at any time for its liquidation preference. Each share of Series A
preferred stock is convertible into one-third of a share of common stock, after adjusting for
earned but unpaid dividends. As of January 2, 2010, no shares of Series A preferred stock were
outstanding.
The Series B preferred stock is senior to the Series A in all preferences. The Series B
preferred stock is entitled to cumulative annual dividends of $0.96 per share and has a liquidation
preference of $8.00 per share plus cumulative unpaid dividends. The Series B preferred stock is
redeemable by us five years after its issuance for its liquidation preference. Each share of Series
B preferred stock is convertible at any time into three and one-third shares of common stock and
has certain anti-dilution provisions. Series B preferred shares vote on an as-converted basis. As
of January 2, 2010, no shares of Series B preferred stock were outstanding.
77
Table of Contents
On May 2, 2002, we adopted a shareholder rights plan, which we call the Rights Plan. Under the
Rights Plan, we distributed one purchase right for each share of common stock outstanding at the
close of business on May 17, 2002. If a person or group acquires 15% or more of our common stock in
a transaction not pre-approved by our Board of Directors, each right will entitle its holder, other
than the acquirer, to buy our common stock at 50% of its market value for the right’s then current
exercise price (initially $70.00). In addition, if an unapproved party acquires more than 15% of
our common stock, and our Company or our business is later acquired by the unapproved party or in a
transaction in which all shareholders are not treated alike, shareholders with unexercised rights,
other than the unapproved party, will be entitled to purchase common stock of the acquirer with a
value of twice the exercise price of the rights. Each right also becomes exercisable for one
one-thousandth of a share of our Series RP preferred stock at the right’s then current exercise
price ten days after an unapproved third party makes, or announces an intention to make, a tender
offer or exchange offer that, if completed, would result in the unapproved party acquiring 15% or
more of our common stock. Our Board of Directors may redeem the rights for a nominal amount at any
time before an event that causes the rights to become exercisable. The rights will expire on May 2,
2012.
In connection with the Rights Plan, we designated 100,000 no par shares of Series RP preferred
stock. These shares, if issued, will be entitled to receive quarterly dividends and liquidation
preferences. There are no shares of Series RP preferred stock issued and outstanding and we do not
anticipate issuing any shares of Series RP preferred stock except as may be required under the
Rights Plan.
13. Retirement Savings Plans
Substantially all of our full-time employees are eligible to participate in a 401(k)
retirement savings plan (the “Retirement Plan”). Under the Retirement Plan, employees may elect to
contribute up to 100% of their eligible compensation to the Retirement Plan with Thoratec making
discretionary matching contributions, subject to certain IRS limitations. In each of fiscal 2009,
2008 and 2007, our matching contribution was 50%, up to the first 6% of eligible employee plan
contribution. Employees vest in our matching contribution to the Retirement Plan at the rate of 25%
per year, with full vesting after four years of service with us. In fiscal 2009, 2008 and 2007, we
made contributions to the Retirement Plan of approximately $1.2 million, $1.0 million and $1.0
million, respectively.
In 2004, we established a non-qualified, unfunded deferred compensation plan for certain
management employees and our Board of Directors. Amounts deferred and contributed under the
deferred compensation plan are credited or charged with the performance of investment options
offered under the plan and elected by the participants. The liability for compensation deferred
under this plan was $2.8 million and $1.4 million at January 2, 2010 and January 3, 2009,
respectively, and is included in “Other long-term liability” on our consolidated balance sheets. We
manage the risk of changes in the fair value of the liability for deferred compensation by electing
to match our liability under the plan with an investment that offsets a substantial portion of the
Company’s exposure. The cash value of the investment vehicle was $2.4 million as of January 2, 2010
and $1.7 million as of January 3, 2009, and is included in “Other long-term assets” on our
consolidated balance sheets.
14. Taxes on Income
We account for income taxes using the asset and liability approach under which deferred income
taxes are based upon enacted tax laws and rates applicable to the periods in which taxes become
payable.
The provisions for income tax expense (benefit) were as follows:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Current: |
||||||||||||
Federal |
$ | 18,721 | $ | 11,578 | $ | 5,284 | ||||||
State |
3,636 | 3,192 | 1,968 | |||||||||
Foreign |
415 | 2,122 | 1,179 | |||||||||
| 22,772 | 16,892 | 8,431 | ||||||||||
Deferred: |
||||||||||||
Federal |
(7,010 | ) | (8,843 | ) | (10,366 | ) | ||||||
State |
(3,373 | ) | (2,281 | ) | (1,585 | ) | ||||||
Foreign |
(220 | ) | (207 | ) | 122 | |||||||
| (10,603 | ) | (11,331 | ) | (11,829 | ) | |||||||
Total income tax expense (benefit) |
$ | 12,169 | $ | 5,561 | $ | (3,398 | ) | |||||
78
Table of Contents
Income before taxes are generated from the following geographic areas:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Domestic |
$ | 38,185 | $ | 17,990 | $ | (7,962 | ) | |||||
Foreign |
2,568 | 5,902 | 3,961 | |||||||||
Income before taxes |
$ | 40,753 | $ | 23,892 | $ | (4,001 | ) | |||||
The provision for income tax benefit (expense) in the accompanying statements of operations
differs from the provision calculated by applying the U.S. federal statutory income tax rate of 35%
to income before taxes due to the following:
| Fiscal Years | ||||||||||||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| (in thousands, except percentages) | ||||||||||||||||||||||||
U.S. federal statutory income tax expense (benefit) |
$ | 14,264 | 35.0 | % | $ | 8,362 | 35.0 | % | $ | (1,400 | ) | 35.0 | % | |||||||||||
State income tax expense/(benefit), net of federal tax
expense or benefit |
750 | 1.8 | (144 | ) | (0.6 | ) | (700 | ) | (17.5 | ) | ||||||||||||||
Share-based compensation |
161 | 0.4 | (4 | ) | — | 910 | 22.7 | |||||||||||||||||
Non-deductible expenses |
326 | 0.8 | 527 | 2.2 | 291 | 7.3 | ||||||||||||||||||
Research and development credits |
(1,203 | ) | (3.0 | ) | (1,097 | ) | (4.6 | ) | (436 | ) | (10.9 | ) | ||||||||||||
Foreign earnings permanently reinvested |
(138 | ) | (0.3 | ) | (99 | ) | (0.4 | ) | (58 | ) | (1.4 | ) | ||||||||||||
Tax advantaged investment income |
(1,599 | ) | (3.9 | ) | (2,575 | ) | (10.8 | ) | (2,560 | ) | (64.0 | ) | ||||||||||||
Return-to-provision true-up |
448 | 1.1 | (528 | ) | (2.2 | ) | 552 | 13.8 | ||||||||||||||||
CA rate change |
(927 | ) | (2.3 | ) | — | — | — | — | ||||||||||||||||
Purchased intangible rate change |
(973 | ) | (2.4 | ) | — | — | — | — | ||||||||||||||||
Section 162(m) write-down |
1,424 | 3.5 | — | — | — | — | ||||||||||||||||||
Prior period purchase price correction |
— | — | (36 | ) | (0.1 | ) | — | — | ||||||||||||||||
Domestic production activities |
(1,268 | ) | (3.1 | ) | (304 | ) | (1.3 | ) | (123 | ) | (3.1 | ) | ||||||||||||
Other |
118 | 0.4 | — | — | — | — | ||||||||||||||||||
Tax reserves |
786 | 1.9 | 1,459 | 6.1 | 126 | 3.1 | ||||||||||||||||||
| $ | 12,169 | 29.9 | % | $ | 5,561 | 23.3 | % | $ | (3,398 | ) | (85.0 | )% | ||||||||||||
Deferred income taxes reflect the net tax effects of: (a) temporary differences between the
carrying amounts of assets and liabilities for financial reporting purposes and the amounts used
for income tax purposes, and (b) operating loss and tax credit carryforwards.
79
Table of Contents
Significant components of deferred tax assets and liabilities are as follows:
| January 2, 2010 | January 3, 2009 | |||||||
| (in thousands) | ||||||||
Deferred tax assets: |
||||||||
Write-off of acquired technology |
$ | 247 | $ | 376 | ||||
Reserves and accruals |
4,699 | 3,119 | ||||||
Depreciation and amortization |
2,645 | 1,746 | ||||||
Inventory basis difference |
6,469 | 2,583 | ||||||
Share-based compensation |
5,618 | 5,170 | ||||||
Research and development and other credit carryforwards |
2,835 | 5,861 | ||||||
Net operating loss carryforwards |
— | 59 | ||||||
Other, net |
674 | 2,348 | ||||||
Total deferred tax assets |
23,187 | 21,262 | ||||||
Deferred tax liabilities: |
||||||||
Purchased intangibles |
(37,009 | ) | (42,779 | ) | ||||
Interest expense |
(4,430 | ) | (7,557 | ) | ||||
Other, net |
(46 | ) | (9 | ) | ||||
Total deferred tax liabilities |
(41,485 | ) | (50,345 | ) | ||||
Net deferred tax liabilities |
$ | (18,298 | ) | $ | (29,083 | ) | ||
Reported As: |
||||||||
Net current deferred tax assets |
$ | 12,261 | $ | 8,397 | ||||
Net long-term deferred tax assets (included in “Other long-term assets”) |
1,161 | 1,362 | ||||||
Net long-term deferred tax liabilities |
(31,720 | ) | (38,842 | ) | ||||
Net deferred tax liabilities |
$ | (18,298 | ) | $ | (29,083 | ) | ||
At the end of 2009, we had no remaining federal net operating loss, research and development
credit or alternative minimum tax credit carryforwards.
At the end of 2009, we had research and development tax credit carryovers for state purposes
of approximately $6.5 million. These state credits generally may be carried forward indefinitely.
We believe realization of all of our net deferred tax assets as of January 2, 2010 is more
likely than not based on the future reversal of temporary tax differences and upon future taxable
earnings exclusive of reversing temporary differences.
In the first quarter of 2009, we recorded a discrete benefit of approximately $0.9 million to
reflect the effect of a change in California tax law which will permit us to make a beneficial
apportionment election beginning in 2011. This election will impact the California state tax rate
for certain of our existing long-term deferred tax assets and liabilities which are anticipated to
reverse subsequent to 2010.
In the fourth quarter of 2009, we wrote off approximately $1.4 million of deferred tax assets
related to share-based officer compensation. Based upon newly available fourth quarter projections
of future compensation, restricted stock vesting schedules and the anticipated timing of option
exercises, we anticipate the deductions associated with these deferred tax assets will be
disallowed in future periods under certain Internal Revenue Code provisions for excessive employee
remuneration.
In addition, as a result of the abandonment of certain state restructuring plans in the fourth
quarter of 2009, we recorded a discrete benefit of approximately $1.0 million to reflect the effect
of a change in the state rate anticipated to apply in future years to certain of our long term
deferred tax liabilities associated with purchased intangibles.
We have utilized the “short-cut” method for purposes of determining our hypothetical stock
option pool of excess tax benefits. As of January 2, 2010 the stock option pool of excess tax
benefits was $15.1 million.
The federal, state and foreign provisions do not reflect certain tax savings resulting from
tax benefits associated with our various stock option plans. The savings were credited to
additional paid-in-capital for $3.9 million, $6.9 million and $3.3 million in fiscal 2009, 2008 and
2007, respectively.
80
Table of Contents
We provide U.S. income taxes on the earnings of foreign subsidiaries unless such earnings are
considered permanently reinvested in their respective foreign jurisdictions. As of January 2, 2010
the cumulative earnings on which U.S. income taxes have not been provided were approximately $8.3
million. A determination of the potential deferred tax liability which would result from these
earnings is not practicable at this time. Foreign earnings were considered to be permanently
reinvested in operations outside the U.S.
We remain subject to audit in California, for the tax years 2003 to 2008, New Jersey, for the
tax years 2005 to 2008, and U.K., for the tax years 2007 through 2008. Additionally, we are
subject to audit for the tax years 2006 through 2008 for U.S. federal purposes and in certain
other jurisdictions. However, certain items attributed to closed years remain subject to
adjustment by the relevant tax authority through an adjustment to tax attributes carried forward to
open years.
We are also under audit by the state of California for the tax years from 2003 to 2007.
Although the ultimate outcome and the timing of the conclusion of this examination is unknown, we
believe that adequate amounts have been provided for any adjustments that may result from the
current examination and that the final outcome will not have a material adverse effect on our
consolidated statements of operations.
We evaluate tax positions for recognition using a more-likely-than-not recognition threshold,
and those tax positions eligible for recognition are measured as the largest amount of tax benefit
that is greater than 50% likely of being realized upon the effective settlement with a taxing
authority that has full knowledge of all relevant information.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as
follows:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Balance at beginning of fiscal year |
$ | 8,860 | $ | 6,853 | $ | 9,300 | ||||||
Additions based on tax positions related to the current year |
732 | 725 | 445 | |||||||||
Additions for tax positions, related to prior years |
725 | 1,406 | 264 | |||||||||
Reductions for tax positions, related to prior years |
(145 | ) | (29 | ) | (2,627 | ) | ||||||
Settlements |
(9 | ) | (9 | ) | (527 | ) | ||||||
Reductions for statute of limitations lapses |
(144 | ) | (86 | ) | (2 | ) | ||||||
Balance at end of fiscal year |
$ | 10,019 | $ | 8,860 | $ | 6,853 | ||||||
Included in the unrecognized tax benefits balance at January 2, 2010, January 3, 2009, and
December 29, 2007 was $5.2 million, $4.5 million and $2.5 million, respectively, which, if
recognized, would impact our effective tax rate.
Our policy for classifying interest and penalties associated with unrecognized income tax
benefits is to include the following items in income tax expense
(benefit):
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Interest |
$ | 126 | $ | 148 | $ | 141 | ||||||
Penalties |
(6 | ) | 47 | 10 | ||||||||
We accrued the following interest and penalties in our consolidated balance sheets:
| January 2, 2010 | January 3, 2009 | December 29, 2007 | ||||||||||
| (in thousands) | ||||||||||||
Interest |
$ | 765 | $ | 638 | $ | 490 | ||||||
Penalties |
188 | 195 | 149 | |||||||||
We
file tax returns in the U.S. for federal purposes, U.K., and state tax
returns in California, New Jersey and other domestic and foreign jurisdictions. The years 2006 through 2008 remain open to examination
for U.S. purposes, 2007 through 2008 for U.K. purposes, 2005 through
2008 for New Jersey purposes, and 2003 through 2008 for California purposes. In 2010, it is reasonably possible that we will settle existing audits or
close certain years to examination under the relevant statute of limitations or reserve additional
amounts for tax positions taken throughout the year. This may further decrease our liability for
unrecognized tax benefits by approximately $5.0 million. Less than 30% of this decrease would
result from the settlement of outstanding audits.
81
Table of Contents
We believe we have provided adequate amounts for anticipated tax audit adjustments in the
U.S., state and other foreign tax jurisdictions based on our estimate of whether, and the extent to
which, additional taxes and interest may be due. If events occur which indicate payment of these
amounts are unnecessary, the reversal of the liabilities would result in tax benefits being
recognized in the period when we determine the liabilities are no longer necessary. If our estimate
of tax liabilities proves to be less than the ultimate assessment, a further charge to expense
would result.
15. Enterprise and Related Geographic Information
We organize and manage our business by functional operating entities. Our functional entities
operate in two segments: Cardiovascular and ITC. The Cardiovascular segment develops, manufactures
and markets proprietary medical devices used for circulatory support and vascular graft
applications. The ITC segment designs, develops, manufactures and markets point-of-care diagnostic
test systems and incision devices. Long-lived asset are primarily held in U.S.
Business segments:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Product sales: |
||||||||||||
Cardiovascular |
$ | 279,967 | $ | 214,976 | $ | 144,220 | ||||||
ITC |
93,970 | 98,588 | 90,560 | |||||||||
Total product sales |
$ | 373,937 | $ | 313,564 | $ | 234,780 | ||||||
Income (loss) before taxes: |
||||||||||||
Cardiovascular(a)(c) |
$ | 80,311 | $ | 39,156 | $ | 5,188 | ||||||
ITC(a)(c) |
(3,489 | ) | 2,614 | 6,787 | ||||||||
Corporate (b)(c) |
(29,805 | ) | (16,040 | ) | (14,172 | ) | ||||||
Total operating income (loss) |
47,017 | 25,730 | (2,197 | ) | ||||||||
Other income and (expense): |
||||||||||||
Interest expense |
(12,307 | ) | (10,984 | ) | (10,428 | ) | ||||||
Interest income and other |
6,043 | 9,146 | 8,624 | |||||||||
Total income (loss) before taxes |
$ | 40,753 | $ | 23,892 | $ | (4,001 | ) | |||||
Depreciation and amortization: |
||||||||||||
Cardiovascular |
$ | 16,481 | $ | 18,277 | $ | 17,218 | ||||||
ITC |
4,348 | 4,589 | 4,174 | |||||||||
Corporate (b) |
852 | 865 | 444 | |||||||||
Total depreciation and amortization |
$ | 21,681 | $ | 23,731 | $ | 21,836 | ||||||
| January 2, 2010 | January 3, 2009 | December 29, 2007 | ||||||||||
| (in thousands) | ||||||||||||
Total assets: |
||||||||||||
Cardiovascular |
$ | 327,038 | $ | 321,605 | $ | 312,691 | ||||||
ITC |
61,145 | 61,552 | 62,642 | |||||||||
Corporate (b) |
358,374 | 300,928 | 237,676 | |||||||||
Total assets |
$ | 746,557 | $ | 684,085 | $ | 613,009 | ||||||
Capital expenditures (d): |
||||||||||||
Cardiovascular |
$ | 5,644 | $ | 7,128 | $ | 5,654 | ||||||
ITC |
3,127 | 4,412 | 4,435 | |||||||||
Corporate (b) |
1,481 | 774 | 99 | |||||||||
Total capital expenditures |
$ | 10,252 | $ | 12,314 | $ | 10,188 | ||||||
82
Table of Contents
| (a) | Includes amortization expense of $9.9 million, $12.4 million and $11.7 million for fiscal 2009, 2008 and 2007 respectively, related to the Cardiovascular division. The ITC division had amortization expense of $0.8 million, $0.8 million and $0.9 million for fiscal ended 2009, 2008 and 2007, respectively. | |
| (b) | Represents unallocated items, not specifically identified to any particular business segment. | |
| (c) | Includes share-based compensation expense of $8.3 million, $4.0 million and $1.6 million for Cardiovascular, ITC and Corporate, respectively, for fiscal 2009, $6.4 million, $2.9 million and $1.6 million for Cardiovascular, ITC and Corporate, respectively, for fiscal 2008 and $6.9 million, $3.0 million and $1.5 million for Cardiovascular, ITC and Corporate, respectively, for fiscal 2007. | |
| (d) | Capital expenditures include inventory transfers of $2.2 million, $2.5 million and $2.7 million for fiscal 2009, 2008 and 2007 respectively, related to our Cardiovascular division and $0.4 million, $0.6 million and $1.0 million for fiscal 2009, 2008 and 2007 respectively, related to our ITC division. |
Geographic Areas:
Revenue attributed to a country or region includes product sales to hospitals, physicians and
distributors and is based on the final destination where the products are sold. During fiscal 2009, 2008 and 2007, no customer or international country represented individually greater than 10%
of our total product sales. The geographic composition of our product sales was as follows:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Product sales: |
||||||||||||
Domestic |
$ | 281,762 | $ | 232,567 | $ | 169,786 | ||||||
International |
92,175 | 80,997 | 64,994 | |||||||||
Total |
$ | 373,937 | $ | 313,564 | $ | 234,780 | ||||||
16. Net Income (Loss) Per Share
We adopted authoritative accounting guidance that requires participating securities to be
included in the calculation of the net income per share using the two-class method. Our restricted
shares awards subject to repurchase and settled in shares of common stock upon vesting have
non-forfeitable rights to receive dividends on an equal basis with common stock and therefore are
considered participating securities. Under the two-class method, basic and diluted net income per
common share is determined by calculating net income per share for common stock and participating
securities based on participation rights in undistributed earnings. Dilutive net income per common
share also considers the dilutive effect of the in-the-money stock options and restricted stock
units, calculated using the treasury stock method. Under the treasury stock method, the amount of
assumed proceeds from unexercised stock options and restricted stock units includes the amount of
unrecognized compensation cost attributable to future services, assumed proceeds from the exercise
of the options, and the incremental income tax benefit or liability that would be recorded in
additional-paid-in capital when the award becomes deductible.
83
Table of Contents
Basic and diluted net income (loss) per common share attributable to common shareholders under
the two-class method were calculated as follows:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands, except per share data) | ||||||||||||
Basic net income per common share calculation |
||||||||||||
Net income (loss) |
$ | 28,584 | $ | 18,331 | $ | (603 | ) | |||||
Net income (loss) allocated to participating securities |
356 | 317 | (8 | ) | ||||||||
Net income (loss) attributable to common shareholders |
$ | 28,228 | $ | 18,014 | $ | (595 | ) | |||||
Weighted average number of common shares used to
compute basic net income (loss) per common share |
55,910 | 54,144 | 52,777 | |||||||||
Basic net income (loss) per common share |
$ | 0.50 | $ | 0.33 | $ | (0.01 | ) | |||||
Diluted net income per common share calculation |
||||||||||||
Net income (loss) |
$ | 28,584 | $ | 18,331 | $ | (603 | ) | |||||
Net income (loss) allocated to participating securities |
347 | 311 | (8 | ) | ||||||||
Net income (loss) attributable to common shareholders |
$ | 28,237 | $ | 18,020 | $ | (595 | ) | |||||
Weighted average number of common shares used to
compute basic net income (loss) per common share
attributable to common shares |
55,910 | 54,144 | 52,777 | |||||||||
Dilutive effect of stock-based compensation plans |
1,412 | 1,099 | — | |||||||||
Weighted average number of common shares used to
compute diluted net income (loss) per common share |
57,322 | 55,243 | 52,777 | |||||||||
Diluted net income (loss) per common share |
$ | 0.49 | $ | 0.33 | $ | (0.01 | ) | |||||
The weighted average unvested restricted stock awards outstanding were 704,673, 952,784, and
716,512 for the fiscal years 2009, 2008, and 2007, respectively.
Potential common share equivalents have been excluded where the inclusion would be
anti-dilutive are as follows:
| Fiscal Years | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Options to purchase shares not
included in the computation of diluted net
income per common share because their
inclusion would be antidilutive |
294 | 1,284 | 3,215 | |||||||||
The computation of diluted net income (loss) per common share for fiscal years 2009, 2008 and
2007 excludes the effect of assuming the conversion of our senior subordinated convertible notes,
which are convertible at $19.72 per share into 7.3 million shares of common stock, because the
effect would have been antidilutive.
84
Table of Contents
17. Quarterly Results of Operations (Unaudited)
The following is a summary of our unaudited quarterly results of operations for the fiscal
years 2009 and 2008:
| First | Second | Third | Fourth | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
Fiscal Year 2009: |
||||||||||||||||
Product sales |
$ | 89,466 | $ | 92,059 | $ | 87,917 | $ | 104,495 | ||||||||
Gross profit |
54,027 | 50,755 | 53,732 | 61,320 | ||||||||||||
Net income |
5,627 | 1,832 | 11,777 | (1) | 9,348 | (1) | ||||||||||
Net income
per common share: |
||||||||||||||||
Basic |
$ | 0.10 | $ | 0.03 | $ | 0.21 | $ | 0.16 | ||||||||
Diluted |
$ | 0.10 | $ | 0.03 | $ | 0.20 | $ | 0.16 | ||||||||
Fiscal Year 2008: |
||||||||||||||||
Product sales |
$ | 64,427 | $ | 82,648 | $ | 80,815 | $ | 85,674 | ||||||||
Gross profit |
35,837 | 50,823 | 48,770 | 50,568 | ||||||||||||
Net income (loss) |
(678 | ) | 7,624 | 6,108 | 5,277 | |||||||||||
Net income
(loss) per common share: |
||||||||||||||||
Basic |
$ | (0.01 | ) | $ | 0.14 | $ | 0.11 | $ | 0.10 | |||||||
Diluted |
$ | (0.01 | ) | $ | 0.14 | $ | 0.11 | $ | 0.09 | |||||||
| (1) | Net income (loss) per share in the third quarter of 2009 includes the fair value of a conversion option gain of $5.2 million ($3.1 million, net of tax) related to the intended HeartWare agreement, which was reversed in the fourth quarter of 2009, upon the extinguishment of the HeartWare loan agreement. For further details related to the termination of the merger agreement with HeartWare and the extinguishment of the loan agreement, please refer to Note 8, “Other Assets.” |
18. Subsequent Event
On January 25, 2010, we purchased technology from Getinge AB for an aggregate purchase price
of $8.5 million.
85
Table of Contents
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Attached as exhibits to this Annual Report on Form 10-K are certifications of our Chief
Executive Officer and Chief Financial Officer, which are required in accordance with Rule 13a-14 of
the Securities Exchange Act of 1934, as amended (the “Exchange Act”). This “Controls and
Procedures” section includes information concerning the controls and controls evaluation referred
to in the certifications.
Disclosure Controls and Procedures
An evaluation was performed under the supervision and with the participation of our
management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness
of the design and operation of our disclosure controls and procedures, as defined in Rule 13a-15(e)
under the Exchange Act, as of January 2, 2010. The evaluation of our disclosure controls and
procedures included a review of our processes and implementation and the effect on the information
generated for use in this Annual Report on Form 10-K. In the course of this evaluation, we sought
to identify any significant deficiencies or material weaknesses in our disclosure controls and
procedures, to determine whether we had identified any acts of fraud involving personnel who have a
significant role in our disclosure controls and procedures, and to confirm that any necessary
corrective action, including process improvements, was taken. This type of evaluation is done
quarterly so that our conclusions concerning the effectiveness of these controls can be reported in
our periodic reports filed with the SEC. The overall goals of these evaluation activities are to
monitor our disclosure controls and procedures and to make modifications as necessary. We intend to
maintain these disclosure controls and procedures, modifying them as circumstances warrant.
Based on that evaluation, our management, including the Chief Executive Officer and Chief
Financial Officer, concluded that as of January 2, 2010 the Company’s disclosure controls and
procedures, as defined in Rule 13a-15(e) under the Exchange Act, were effective to provide
reasonable assurance that information required to be disclosed by us in the reports that we file or
submit under the Exchange Act is recorded, processed, summarized, and reported within the time
periods specified in the SEC’s rules and forms, and to provide reasonable assurance that such
information is accumulated and communicated to our management, including our principal executive
officer, as appropriate to allow timely decisions regarding required disclosures.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over
financial reporting to provide reasonable assurance regarding the reliability of our financial
reporting and the preparation of financial statements for external purposes in accordance with
generally accepted accounting principles.
Management assessed our internal control over financial reporting as of January 2, 2010, the
end of our fiscal year. Management based its assessment on criteria
established in “Internal
Control-Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway
Commission. Management’s assessment included evaluation of such elements as the design and
operating effectiveness of key financial reporting controls, process documentation, accounting
policies, and our overall control environment. This assessment is supported by testing and
monitoring performed by our internal accounting and finance organization.
Based on our assessment, management has concluded that our internal control over financial
reporting was effective as of January 2, 2010. The results of management’s assessment were
reviewed with the Audit Committee.
Our independent registered public accounting firm, Deloitte & Touche LLP, has issued a report
on our internal control over financial reporting, which is included in Item 8 of this Annual Report
on Form 10-K.
86
Table of Contents
Changes to Internal Controls
There have been no changes in our internal controls over financial reporting during the
quarter ended January 2, 2010 that have materially affected or are reasonably likely to materially
affect our internal control over financial reporting.
Inherent Limitations on Controls and Procedures
Our management, including the Chief Executive Officer and the Chief Financial Officer, does
not expect that our disclosure controls and procedures and our internal controls will prevent all
error and all fraud. A control system, no matter how well designed and operated, can only provide
reasonable assurances that the objectives of the control system are met. The design of a control
system reflects resource constraints; the benefits of controls must be considered relative to their
costs. Because there are inherent limitations in all control systems, no evaluation of controls can
provide absolute assurance that all control issues and instances of fraud, if any, within the
Company have been or will be detected. As these inherent limitations are known features of the
financial reporting process, it is possible to design into the process safeguards to reduce, though
not eliminate, these risks. These inherent limitations include the realities that judgments in
decision-making can be faulty and that breakdowns occur because of simple error or mistake.
Controls can be circumvented by the individual acts of some persons, by collusion of two or more
people, or by management override of the control. The design of any system of controls is based in
part upon certain assumptions about the likelihood of future events. While our disclosure controls
and procedures are designed to provide reasonable assurance of achieving their objectives, there
can be no assurance that any design will succeed in achieving its stated goals under all future
conditions. Over time, controls may become inadequate because of changes in conditions or
deterioration in the degree of compliance with the policies or procedures. Because of the inherent
limitations in a cost-effective control system, misstatements due to error or fraud may occur and
not be detected.
We intend to review and evaluate the design and effectiveness of our disclosure controls and
procedures on an ongoing basis and to improve our controls and procedures over time and to correct
any deficiencies that we may discover in the future. While our Chief Executive Officer and Chief
Financial Officer have concluded that, as of January 2, 2010, the design of our disclosure controls
and procedures, as defined in Rule 13a-15(e) under the Exchange Act, was effective, future events
affecting our business may cause us to significantly modify our disclosure controls and procedures.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Certain information regarding our executive officers is included in Part I of this Annual
Report on Form 10-K under the caption “Our Executive Officers.” All other information regarding
directors, executive officers and corporate governance required by Item 10 is incorporated herein
by reference from the information under the captions “Board of Directors Structure and
Compensation,” “Election of Directors,” “Section 16(a) Beneficial Ownership Reporting Compliance,”
“Code of Ethics and Corporate Governance,” and in other applicable sections in the definitive proxy
statement to be filed with the Securities and Exchange Commission pursuant to Regulation 14A for
our 2010 annual meeting of shareholders.
Item 11. Executive Compensation
The information required by Item 11 is incorporated herein by reference from the information
under the captions “Board of Directors Structure and Compensation,” “Compensation Discussion and
Analysis,” “Report of the Compensation and Option Committee of the Board of Directors” and
“Executive Compensation” in the definitive proxy statement to be filed with the Securities and
Exchange Commission pursuant to Regulation 14A for our 2010 annual meeting of shareholders.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholders Matters
The information required by Item 12 is incorporated herein by reference from the information
under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Securities
Authorized for Issuance Under Equity Compensation Plans” in the definitive proxy statement to be
filed with the Securities and Exchange Commission pursuant to Regulation 14A for our 2010 annual
meeting of shareholders.
87
Table of Contents
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by Item 13 is incorporated herein by reference from the information
under the caption “Certain Transactions” in the definitive proxy statement to be filed with the
Securities and Exchange Commission pursuant to Regulation 14A for our 2010 annual meeting of
shareholders.
Item 14. Principal Accounting Fees and Services
The information required by Item 14 is incorporated herein by reference from the information
under the caption “Fees Paid to Accountants for Services Rendered During Fiscal Years 2009 and
2008” in the definitive proxy statement to be filed with the Securities and Exchange Commission
pursuant to Regulation 14A for our 2010 annual meeting of shareholders.
PART IV
Item 15. Exhibit and Financial Statement Schedules
| (a) | List of documents filed as part of this report: | |
| 1. | Financial Statements and Reports of Independent Registered Public Accounting Firm. | |
| Reference is made to the Index to Financial Statements under Item 8 of Part II of this Annual Report on Form 10-K, where these documents are included. | ||
| 2. | Financial Statement Schedules | |
| Schedule II — Valuation and Qualifying Accounts and Reserves for each of the three fiscal years ended January 2, 2010, January 3, 2009 and December 29, 2007. Other financial statement schedules are not included either because they are not required or the information is otherwise shown in our audited consolidated financial statements or the notes thereto. | ||
| 3. | Exhibits | |
| Reference is made to the Exhibit Index on page 90 of this Annual Report on Form 10-K, where these documents are included. |
88
Table of Contents
THORATEC CORPORATION
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
For Each of the Three Fiscal Years:
| Balance | Additions | Balance | ||||||||||||||
| Beginning | (charges | End of | ||||||||||||||
| of Year | to expense) | Deductions | Year | |||||||||||||
| (in thousands) | ||||||||||||||||
Year Ended January 2, 2010: |
||||||||||||||||
Allowance for doubtful accounts |
$ | 947 | $ | 699 | $ | (802 | )(1) | $ | 844 | |||||||
Year Ended January 3, 2009: |
||||||||||||||||
Allowance for doubtful accounts |
$ | 861 | $ | 298 | $ | (212 | )(1) | $ | 947 | |||||||
Year Ended December 29, 2007: |
||||||||||||||||
Allowance for doubtful accounts |
$ | 491 | $ | 690 | $ | (320 | )(1) | $ | 861 | |||||||
| (1) | Accounts written off. |
89
Table of Contents
EXHIBIT INDEX
| Exhibit | ||
| Number | Exhibit | |
3.1
|
Thoratec’s Articles of Incorporation, as amended.(1) | |
3.2
|
Thoratec’s By-Laws, as amended February 25, 2005.(2) | |
4.1
|
Rights Agreement between Thoratec Corporation and Computershare Trust Company, Inc. as Rights Agent dated as of May 2, 2002.(3) | |
4.2
|
Indenture, dated as of May 24, 2004, by and between Thoratec Corporation and U.S. Bank, National Association, as Trustee.(4) | |
4.3
|
Form of Senior Subordinated Convertible Note due 2034.(5) | |
4.4
|
Pledge Agreement, dated as of May 24, 2004, between Thoratec Corporation and U.S. Bank, National Association, and Pledge Agreement Supplement, dated as of June 7, 2004.(4) | |
4.5
|
Control Agreement, dated as of May 24, 2004, between Thoratec Corporation and U.S. Bank, National Association, and Control Agreement Amendment, dated as of June 7, 2004.(4) | |
4.6
|
Registration Rights Agreement, dated May 24, 2004, by and among Thoratec Corporation and Merrill Lynch Pierce Fenner & Smith Incorporated as Initial Purchaser of the Senior Subordinated Convertible Notes due 2034.(4) | |
10.1
|
Form of Indemnification Agreement between Thoratec Cardiosystems and its officers and directors.(6) | |
10.2
|
Thoratec’s 1996 Nonemployee Directors Stock Option Plan, as amended.(7) | |
10.3
|
Lease Agreement dated July 25, 1996, between Main Street Associates and Thoratec, as amended.(8) | |
10.4
|
First Amendment to Lease Agreement originally between Main Street Associates and Thoratec dated July 25, 1996.(9) | |
10.5
|
Second Amendment to Lease Agreement originally between Main Street Associates and Thoratec dated July 25, 1996.(10) | |
10.6
|
Thoratec’s 1997 Stock Option Plan, as amended.(11) | |
10.7
|
Lease agreement dated August 16, 1995, between International Technidyne Corporation and BHBMC, as amended.(12) | |
10.8
|
Thoratec’s 2002 Employee Stock Purchase Plan.(13) | |
10.9
|
Grantor Trust Agreement between Thoratec and Wachovia Bank, National Association effective as of November 21, 2003.(7) | |
10.10
|
Commercial Lease between International Technidyne Corporation and Roseville Properties Management Company dated September 26, 2003.(7) | |
10.11
|
Lease Agreement between International Technidyne Corporation and NJ Mortgage Association dated February 21, 2003.(15) | |
10.12
|
Description of the Executive Disability Income Protection Program.(14) | |
10.13
|
Amended and Restated Thoratec Corporation 2006 Incentive Stock Plan.(16) | |
10.14
|
Amended and Restated Employment Agreement by and between Thoratec and Gerhard F. Burbach, dated April 23, 2007.(17)* | |
10.15
|
Amended and Restated Employment Agreement by and between Thoratec and Lawrence Cohen, dated April 23, 2007.(17)* | |
10.16
|
Amended and Restated Separation Benefits Agreement by and between Thoratec and David A. Lehman, dated April 23, 2007.(17)* | |
10.17
|
Amended and Restated Separation Benefits Agreement by and between Thoratec and David V. Smith, dated April 23, 2007.(17)* | |
10.18
|
Thoratec Corporation Amended and Restated Deferred Compensation Plan Effective January 1, 2005.(18) | |
10.19
|
First Amendment to Lease between International Technidyne Corporation and Roseville Properties Management Company effective January 1, 2009.(19) | |
10.20
|
Description of Director Compensation Program.(19) | |
10.21
|
Thoratec Corporation Corporate Executive Incentive Plan FY 2009, effective for certain executive officer of the Company.(20)* | |
10.22
|
International Technidyne Corporation Executive Incentive Plan FY 2009, effective for certain executive officers of the Company.(20)* | |
10.23
|
Amendment to the Amended and Restated Employment Agreement by and between Thoratec and Gerhard F. Burbach, dated November 16, 2009. * | |
10.24
|
Amendment to the Amended and Restated Employment Agreement by and between Thoratec and Lawrence Cohen, dated November 16, 2009. * | |
10.25
|
Amendment to the Amended and Restated Separation Benefits Agreement by and between Thoratec and David A. Lehman, dated November 16, 2009. * | |
10.26
|
Amendment to the Amended and Restated Separation Benefits Agreement by and between Thoratec and David V. Smith, dated November 16, 2009. * | |
21
|
Subsidiaries of Thoratec.(21) | |
23.1
|
Consent of Independent Registered Public Accounting Firm. | |
24
|
Power of Attorney — Reference is made to page 93 hereof. | |
31.1
|
Section 302 Certification of Chief Executive Officer | |
31.2
|
Section 302 Certification of Chief Financial Officer | |
32.1
|
Section 906 Certification of Chief Executive Officer | |
32.2
|
Section 906 Certification of Chief Financial Officer | |
90
Table of Contents
| (1) | Filed as an Exhibit to Thoratec’s Annual Report on Form 10-K for the fiscal year ended December 28, 2002 filed with the SEC on March 20, 2003 and incorporated herein by reference. | |
| (2) | Filed as an Exhibit to Thoratec’s Form 8-K filed with the SEC on March 3, 2005. | |
| (3) | Filed as an Exhibit to Thoratec’s Form 8-A12G filed with the SEC on May 3, 2002 (Registration No. 000-49798), and incorporated herein by reference. | |
| (4) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended July 3, 2004 filed with the SEC on August 12, 2004, and incorporated herein by reference. | |
| (5) | Included as an exhibit to Exhibit 4.2. | |
| (6) | Filed as an Exhibit to Thoratec Cardiosystems’ Registration Statement on Form S-1 (Registration No. 33-25144) and incorporated herein by reference. | |
| (7) | Filed as an Exhibit to Thoratec’s Annual Report on Form 10-K for the fiscal year ended January 3, 2004 filed with the SEC on March 17, 2004 and incorporated herein by reference. | |
| (8) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 29, 1996, filed with the SEC on August 13, 1996, and incorporated herein by reference. | |
| (9) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 1997, filed with the SEC on July 30, 1997, and incorporated herein by reference. | |
| (10) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 1997 filed with the SEC on November 12, 1997, and incorporated herein by reference. | |
| (11) | Filed as an Exhibit to Thoratec’s Registration Statement on Form S-8 filed with the SEC on June 18, 2003 (Registration No. 333-106238), and incorporated herein by reference. | |
| (12) | Filed as an Exhibit to Thoratec’s Form 10-K405 filed with the SEC on March 15, 2002 (Registration No. 033-72502), and incorporated herein by reference. | |
| (13) | Filed as an Exhibit to Thoratec’s Form S-8 POS filed with the SEC on July 1, 2002 (Registration No. 333-90768), and incorporated herein by reference. | |
| (14) | Filed as an Exhibit to Thoratec’s Annual Report on Form 10-K for the fiscal year ended January 1, 2005 filed with the SEC on March 16, 2005 and incorporated herein by reference. | |
| (15) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 29, 2003 filed with the SEC on May 13, 2003, and incorporated herein by reference. | |
| (16) | Filed as an Exhibit to Thoratec’s Form 8-K filed with the SEC on May 22, 2008. | |
| (17) | Filed as an Exhibit to Thoratec’s Form 8-K filed with the SEC on April 27, 2007. | |
| (18) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2007 filed with the SEC on August 9, 2007 and incorporated herein by reference. | |
| (19) | Filed as an Exhibit to Thoratec’s Annual Report on Form 10-K for the fiscal year ended January 3, 2009 filed with the SEC on February 27, 2009 and incorporated herein by reference. | |
| (20) | Filed as an Exhibit to Thoratec’s Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009 filed with the SEC on May 14, 2009 and incorporated herein by reference. | |
| (21) | Filed as an Exhibit to Thoratec’s Annual Report on Form 10-K for the fiscal year ended December 29, 2001 filed with the SEC on March 15, 2002 and incorporated herein by reference. | |
| * | Indicates a management contract or compensatory plan. |
91
Table of Contents
SIGNATURES
In accordance with Section 13 or Section 15(d) of the Exchange Act, as amended, the Registrant
has duly caused this Form 10-K to be signed on its behalf by the undersigned, thereunto duly
authorized on this 24th day of February 2010.
| THORATEC CORPORATION |
||||
| By: | /s/ Gerhard F. Burbach | |||
| Gerhard F. Burbach | ||||
| President and Chief Executive Officer | ||||
Date: February 24, 2010
92
Table of Contents
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each person whose signature appears below constitutes
and appoints Gerhard F. Burbach and David Lehman, and each of them, his or her true and lawful
attorney-in-fact, with full power of substitution and resubstitution, to act for him or her and in
his or her name, place and stead, in any and all capacities to sign any and all amendments to this
annual report on Form 10-K and to file the same, with all exhibits thereto, and all documents in
connection therewith, with the Securities and Exchange Commission, granting unto said
attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and
every act and thing which they, or any of them, may deem necessary or advisable to be done in
connection with this annual report as fully to all intents and purposes as he might or could do in
person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them,
or any substitute or substitutes for any or all of them, may lawfully do or cause to be done by
virtue hereof.
In accordance with the Exchange Act, this report has been signed below by the following
persons on behalf of the Thoratec Corporation and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
/s/ Gerhard F. Burbach
|
Chief Executive Officer, President and Director | February 24, 2010 | ||
Gerhard F. Burbach
|
(Principal Executive Officer) | |||
/s/ David V. Smith
|
Executive Vice President and Chief Financial Officer | February 24, 2010 | ||
David V. Smith
|
(Principal Financial and Accounting Officer) | |||
/s/ Neil F. Dimick
|
Director and Chairman of the Board of Directors | February 24, 2010 | ||
Neil F. Dimick |
||||
/s/ J. Daniel Cole
|
Director | February 24, 2010 | ||
J. Daniel Cole |
||||
/s/ Steven H. Collis
|
Director | February 24, 2010 | ||
Steven H. Collis |
||||
/s/ Elisha W. Finney
|
Director | February 24, 2010 | ||
Elisha W. Finney |
||||
/s/ D. Keith Grossman
|
Director | February 24, 2010 | ||
D. Keith Grossman |
||||
/s/ Paul A. LaViolette
|
Director | February 24, 2010 | ||
Paul A. LaViolette |
||||
/s/ Daniel M. Mulvena
|
Director | February 24, 2010 | ||
Daniel M. Mulvena |
93
