Attached files
Table of Contents
As filed with the Securities and Exchange Commission on February 16, 2010
Registration No. 333-163041
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
PRE-EFFECTIVE AMENDMENT NO. 4 TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Dehaier Medical Systems Limited
(Exact Name of Registrant as Specified in its Charter)
| British Virgin Islands | 3841 | Not applicable | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
| Dehaier Medical Systems Limited 1223 Epoch Center No. 31 Zi Zhu Yuan Road Haidian District Beijing 100089 People’s Republic of China (8610) 5166-0080 |
CT Corporation System 111 Eighth Avenue New York, New York 10011 (800) 624-0909 | |
| (Address, including zip code, and telephone number, including area code, of principal executive offices) |
(Name, address, including zip code, and telephone number, including area code, of agent for service) |
Copies to:
Bradley A. Haneberg, Esq.
Anthony W. Basch, Esq.
Kaufman & Canoles, P.C.
Three James Center, 1051 East Cary Street, 12th Floor
Richmond, Virginia 23219
(804) 771-5700 – telephone
(804) 771-5777 – facsimile
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Table of Contents
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee | ||
| Common Shares(2) |
$13,500,000 | $753.30 | ||
| Placement Agent’s Warrants(3) |
$150 | $0.01 | ||
| Common Shares Underlying Placement Agent’s Warrants(3) |
$1,687,500 | $94.81 | ||
| Total |
$15,187,650 | $848.52(4) | ||
| (1) | The registration fee for securities is based on an estimate of the Proposed Maximum Aggregate Offering Price of the securities, assuming the sale of the maximum number of shares at the highest expected offering price, and such estimate is solely for the purpose of calculating the registration fee pursuant to Rule 457(o). Please note that the remainder of the registration statement assumes an offering price at the midpoint of the offering range. |
| (2) | In accordance with Rule 416(a), the Registrant is also registering an indeterminate number of additional common shares that shall be issuable pursuant to Rule 416 to prevent dilution resulting from share splits, share dividends or similar transactions. |
| (3) | We have agreed to issue, on the closing date of this offering, warrants to our placement agent, Anderson & Strudwick, Incorporated (the “Placement Agent”), to purchase up to 10% of the aggregate number of common shares sold by the Registrant (the “Placement Agent’s Warrants”). The price to be paid by the Placement Agent for the Placement Agent’s Warrants is $0.001 per warrant. The closing date will be a date mutually acceptable to the Placement Agent and the Registrant after the minimum offering has been sold; provided, however, that the closing date will be on or before May 31, 2010. Assuming a maximum placement and a maximum offering price of $9.00 per share, on the closing date the Placement Agent would receive 150,000 Placement Agent’s Warrants at an aggregate purchase price of $150. The exercise price of the Placement Agent’s Warrants is equal to 125% of the price of the common shares offered hereby. Assuming a maximum placement and an exercise price of $11.25 per share, we would receive, in the aggregate, $1,687,500 upon exercise of the Placement Agent’s Warrants. The common shares underlying the Placement Agent’s Warrants are exercisable within one year of the date of this registration statement and are deemed to commence simultaneously with the Placement Agent’s Warrants. |
| (4) | Previously paid. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED February 16, 2010
Registration Statement No. 333-163041

Dehaier Medical Systems Limited
Minimum Offering: 1,250,000 Common Shares
Maximum Offering: 1,500,000 Common Shares
This is the initial public offering of Dehaier Medical Systems Limited, a British Virgin Islands limited company. We are offering a minimum of 1,250,000 and a maximum of 1,500,000 of our common shares. None of our officers, directors or affiliates may purchase shares in this offering.
We expect that the offering price will be between $7.00 and $9.00 per common share. No public market currently exists for our common shares. We have applied for approval for quotation on the NASDAQ Capital Market under the symbol “DHRM” for the common shares we are offering.
Investing in these common shares involves significant risks. See “Risk Factors” beginning on page 12 of this prospectus.
| Per Common Share | Minimum Offering | Maximum Offering | |||||||
| Assumed public offering price |
$ | 8.00 | $ | 10,000,000 | $ | 12,000,000 | |||
| Placement discount |
$ | 0.56 | $ | 700,000 | $ | 840,000 | |||
| Proceeds to us, before expenses |
$ | 7.44 | $ | 9,300,000 | $ | 11,160,000 | |||
We expect our total cash expenses for this offering to be approximately $500,000, exclusive of the above commissions. In addition, we will pay our placement agent a non-accountable expense allowance of 1% of the amount of the offering, or up to $120,000 (maximum offering, exclusive of any shares registered under Rule 462(b)) or $100,000 (minimum offering). The placement agent must sell the minimum number of securities offered (1,250,000 common shares) if any are sold. The placement agent is only required to use its best efforts to sell the maximum number of securities offered (1,500,000 common shares). The offering will terminate upon the earlier of: (i) a date mutually acceptable to us and our placement agent after which the minimum offering is sold or (ii) May 31, 2010. Until we sell at least 1,250,000 shares, all investor funds will be held in an escrow account at SunTrust Bank, Richmond, Virginia. If we do not sell at least 1,250,000 shares by May 31, 2010, all funds will be promptly returned to investors (within one business day) without interest or deduction. If we complete this offering, net proceeds will be delivered to our company on the closing date. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. If we complete this offering, then on the closing date, we will issue common shares to investors in the offering and placement agent warrants to our placement agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of Common Shares sold in this offering.
These securities have not been approved or disapproved by the Securities and Exchange Commission or any state securities commission nor has the Securities and Exchange Commission or any state securities commission passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
Anderson & Strudwick,
Incorporated
Prospectus dated , 2010
Table of Contents

Table of Contents
Except where the context otherwise requires and for purposes of this prospectus only:
| • | the terms “we,” “us,” “our company” and “our” refer to (i) Dehaier Medical Systems Limited, a British Virgin Islands company, (ii) Beijing Dehaier Medical Technology Company Limited, a PRC company, (iii) De-haier Medical Systems (Hong Kong) Limited, a Hong Kong company and (iv) Beijing Dehaier Technology Company Limited, a PRC company. |
| • | “shares” and “common shares” refer to our common shares, $0.002731 par value per share; |
| • | “our branded products” refers to those products developed by our company; |
| • | “our distributed products” refers to those products developed by third parties and distributed by our company; |
| • | “our products” refers both to our branded products and our distributed products. |
| • | “China” and “PRC” refer to the People’s Republic of China, and for the purpose of this prospectus only, excluding Taiwan, Hong Kong and Macau; and |
| • | all references to “RMB,” “Renminbi” and “¥” are to the legal currency of China, and all references to “$” and “U.S. dollars” are to the legal currency of the United States. |
For purposes of clarity, where the context requires us to differentiate between the entities generally referred to collectively as “our company”, and for purposes of this prospectus only:
| • | “Dehaier” refers to Dehaier Medical Systems Limited, a British Virgin Islands business company with limited liability. |
| • | “DHK” refers to De-haier Medical Systems (Hong Kong) Limited, our wholly-owned subsidiary in Hong Kong. |
| • | “BDL” refers to Beijing Dehaier Medical Technology Company Limited, our PRC operating subsidiary, of which Dehaier owns 96.37% and BTL owns the remaining 3.63% of equity. |
| • | “BTL” refers to Beijing Dehaier Technology Company Limited, a PRC company controlled by our chief executive officer, Mr. Ping Chen. BTL owns approximately 3.63% of BDL’s equity. |
This prospectus contains translations of certain Renminbi amounts into U.S. dollars amounts at a specified rate solely for the convenience of the reader. Except as provided below or otherwise noted, all translations made in this prospectus are based upon a rate of ¥6.8262 to $1.00, which was the exchange rate on September 30, 2009. Unless otherwise stated, we have translated balance sheet amounts with the exception of equity at December 31, 2008 at ¥6.8225 to $1.00 as compared to ¥7.2946 to $1.00 at December 31, 2007. We have stated equity accounts at their historical rate. The average translation rates applied to income statement accounts for the year ended December 31, 2008 and the year ended December 31, 2007 were ¥6.9483 and ¥7.6040, respectively. The translation rates of DHK were not significant for the years ended December 31, 2008 and 2007.
We make no representation that the RMB or U.S. dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars or RMB, as the case may be, at any particular rate or at all. See “Risk Factors – Fluctuation of the Renminbi could materially affect our financial condition and results of operations” for discussions of the effects of fluctuating exchange rates on the value of our common shares. Any discrepancies in any table between the amounts identified as total amounts and the sum of the amounts listed therein are due to rounding.
This prospectus follows the English naming convention of first name followed by last name, whether an individual’s name is Chinese or English. For example, the name of the chief executive officer of Dehaier is presented as “Ping Chen,” even though, in Chinese, his name is presented as “Chen Ping.”
Unless otherwise indicated, all information in this prospectus assumes:
| • | no person will exercise any outstanding options; |
| • | the sale of 1,500,000 common shares, the maximum shares offered in this offering; and |
| • | an assumed initial public offering price of $8.00 per common share, the midpoint of the range set forth on the cover page of this prospectus. |
We have relied on statistics provided by a variety publicly-available sources regarding China’s expectations of growth and market potential in our industry, increased government spending on infrastructure and economic development. We did not, directly or indirectly, sponsor or participate in the publication of such materials.
i
Table of Contents
This summary highlights information that we present more fully in the rest of this prospectus. This summary does not contain all of the information you should consider before buying common shares in this offering. This summary contains forward-looking statements that involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “should,” “could,” and similar expressions. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements. You should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and the notes to those statements.
Our Company
Our business consists of the marketing and sale of home respiratory and oxygen homecare products and other medical devices in China. We develop and assemble some of our branded products ourselves from components manufactured by third parties according to our specifications. We do not manufacture these components ourselves. In addition to our branded products, we also sell a variety of products developed, manufactured and assembled by third parties, which we refer to in this prospectus as our distributed products.
Prior to 2009, our branded products have accounted for approximately one-third of our revenues, and our distributed products have accounted for approximately two-thirds of our revenues. In the nine months ended September 30, 2009, our branded products and distributed products have each accounted for approximately half of our revenues.
We sell the majority of our branded and distributed products to distributors, and a very small amount to other end users. Distributors, however, sell their products to the same end users. We estimate that approximately 90% of our sales are to distributors, 7% are to hospitals, 1% are to clinics, and 2% are to individuals. Based on the expected use of products sold to distributors, we estimate that they sell approximately 60% of our products to hospitals, 20% to clinics and 10% to individuals. As a result, we estimate that approximately 67% of our products (on a revenue basis, rather than unit basis) are sold to hospitals, approximately 21% to clinics and approximately 12% to individuals. We have contractual distribution relationships with over 2,000 independent distributors, and we employ 70 direct sales and sales support personnel.
Our Branded Products
We develop, assemble and market home respiratory and oxygen homecare products and other medical devices in China. We offer a broad range of approximately 30 products that can be used in the surgery room, patient room and at home, and we also provide technical service products to manufacturers and distributors. In recent years, we have placed a significant emphasis on our respiratory and oxygen homecare products. Through the integration of technology, customer input, and employee creativity, we seek to provide innovative, high quality and affordable products that improve the lives of people with sleep and respiratory disorders.
Our branded products include medical devices, technical service products and respiratory and oxygen homecare products:
Medical Devices
| • | Mobile Medical X-Ray Image Devices. We provide four types of DHR Explorer Series mobile and C-armed X-ray machines. X-ray is used for visualizing bone structures and other dense tissues such as tumors. These mobile and C-armed X-ray machines provide added convenience for use in hospitals and clinics. |
| • | Anesthesia Machines. We provide two types of DHR ORSA Series anesthesia machines. These machines are used by anesthesiologists to support the administration of anesthesia. These machines administer a precise and continuous supply of anesthetic gases and vapors to the patient at accurate and safe levels of pressure and flow. These machines maintain a continuous, closed-loop control over the pressure of gas within a patient’s mouth or respiratory according to the selected pressure input. |
| • | Patient Monitoring Devices. We provide two types of DHR 930 patient monitoring devices which monitor patients’ physiological parameters, such as heart rate, blood pressure, respiration and temperature. We also provide polysomnograph devices, which are used to monitor and record patients’ sleep data through multiple parameters. These machines are suitable for adult, pediatric and neonatal care and principally used by hospitals in intensive care units, operating rooms and emergency rooms. |
1
Table of Contents
Technical Service Products
| • | Ventilator Air Compressor. We provide two types of air compressors to support medical ventilators in surgery by supplying continuous airflow for the ventilator. Where a facility lacks a central pressured air supply system, our C250 and C280 air compressors provide a portable source of such pressured air. |
| • | Trolleys for Ventilators. We provide three types of trolleys to hold ventilators and their accessories for mobility. These trolleys can be fit with a monitor to further enhance the portability and utility. |
| • | Sterilizers for Ventilators. We provide one type of sterilizer to treat the air from patients in order to control cross-contamination and infection in a facility in general and for subsequent ventilator patients in particular. |
Respiratory and Oxygen Homecare Products
| • | Oxygen Concentrating Products. We provide two types of oxygen concentrator products, including, the DHR-3L/5L Oxi-Fairy and the DHR-3L/5L Oxi-Pioneer. These products use our patented advanced Pressure Swing Absorbing (“PSA”) technology to produce highly-concentrated, therapeutic-level oxygen (approximately 90% oxygen concentration) from air at normal temperatures. These products are used by patients with cardiovascular disease, respiratory diseases, such as chronic obstructive pulmonary disease, and geriatric patients. |
| • | Sleep Apnea Treatment Products. We have designed and expect to provide several products designed for obstructive sleep apnea (“OSA”) therapy. These products include our DHR CPAP C5, DHR Auto CPAP A8, and DHR Auto S-CPAP A9. Our DHR CPAP C5, Auto CPAP A8 and DHR S-CPAP A9 are in the process of obtaining PRC State Food and Drug Administration (SFDA) approval and will not be available for sale until we receive such approval. The SFDA is the competent authority on regulating the safety of medical devices in the PRC. While we expect to receive this approval within the first half of 2010, we cannot guarantee that we will obtain such SFDA approval in this timeframe or all. These products are all non-invasive therapy products that treat symptoms of sleep apnea. Our CPAP devices do not cure apnea but instead use air pressure to open customers’ airways to reduce snoring and apnea disturbances during sleep. Our automatic CPAP products provide air pressure at a customized, adjustable level, while our traditional CPAP products provide a constant level of air pressure. |
| • | Diagnostic Products. We have designed and expect to provide two types of screening and diagnosis products are portable sleep respiratory recording devices that can be used in a healthcare facility or in a patient’s home to assist physicians in determining whether the patient has obstructive sleep apnea requiring use of a CPAP device. We have applied to SFDA for approval of our DHR 998 and DHR 999 diagnostic products, and they will not be available for sale until we receive such approval. While we expect to receive this approval within the first half of 2010, we cannot guarantee that we will obtain such SFDA approval in this timeframe or all. |
| • | Thermotherapy Products. We have designed and expect to provide our thermotherapy device for patients with rhinitis and delivers atomized clean water at a therapeutic temperature to the affected site. We have not yet begun to market this device and cannot project when we will begin to market this device. |
Our Distributed Products
We serve as a significant distributor in China for several foreign producers of medical devices and respiratory and oxygen homecare products, including IMD, Timesco, JMS and ResMed. We believe this extensive platform allows us to be responsive to local market demand. We distribute medical devices and respiratory and oxygen homecare products for these companies.
Medical Devices
| • | Mobile Medical X-Ray Image Devices. We provide two types of X-ray machines developed by other companies: the IMD Radius Series mobile and C-armed X-ray machine and the IMD Compact Series mobile-X-ray machine. |
| • | Medical Ventilator. We provide three types of ventilators developed by other companies: the ResMed VS Serena, Ultra and Integra Ventilators. The VS Series of medical ventilators mechanically move breathable air to and from the lungs to support breathing support for patients who are physically unable to breathe or who are breathing insufficiently. |
| • | Laryngoscopes. We provide four types of laryngoscopes developed by other companies: the Optima, Optima XL and Eclipse lines of laryngoscopes from Timesco. Laryngoscopes are flexible lighted tubes that are used to look at the inside of the larynx. Anesthesiologists make use of laryngoscopes to assist with intubation in surgery. |
2
Table of Contents
Respiratory and Oxygen Homecare Products
| • | Sleep Apnea Treatment Products. We provide two types of products developed by other companies designed for obstructive sleep apnea (“OSA”) therapy. These products include the ResMed S6 and MAP MiniPAP. |
Our Distribution Network
To date, we have sold medical devices developed by our company and other producers to approximately 3,000 hospitals, clinics and other healthcare facilities and have sold respiratory and oxygen homecare products to over 35,000 families. We leverage our network of relationships with healthcare facilities and with distributors to drive sales of both our branded products and our distributed products. By growing our distribution network and product offerings, we are able to offer both branded and distributed products for sale, depending on the needs and budget of the customer.
Assembly
We assemble some of our branded product components and final branded products at our assembly facility in Beijing. We employ 40 full-time employees at this facility. Every year we assemble under ten thousand of our branded products at this assembly facility. In some cases, we assemble final branded products from components manufactured for us by our suppliers. No other companies distribute those branded products except for Friend of Health (Chuzhou) Medical Technology Co. Ltd. (“Friend of Health”), which assembles some of our branded products and is then allowed to sell them only in Chuzhou. For example, we assemble between approximately 40 and 50 C-arm X-ray machines each year from components supplied by our suppliers. In other cases, we assemble components that must be incorporated into final branded products. For example, we assemble the core component of our oxygen concentrators, the oxi-chip, in our assembly facility and then provide that oxi-chip to another company for inclusion in the oxygen concentrator assembled by that company. Depending on the component or branded product, we assemble between approximately 40 (C-arm X-ray machines) and more than 5,000 (oxi-chip) products or components annually.
Marketing
We focus our marketing efforts on two annual exhibitions of medical devices in China and on our Customer Experience Centers (“CECs”). We use the annual exhibitions to introduce our branded and distributed product offerings to distributors and end users. We have focused on meeting potential new distributors at these meetings and on showing our solutions to potential purchasers of those branded and distributed products.
We have used our CEC model as another method to introduce our branded and distributed products to potential purchasers. The CEC allows distributors, hospital and clinic representatives, individual purchasers and other interested parties to see how the products work in practice.
To date, we have not provided any market support to any distributors and have no current plans to do so. Distributors are responsible for meeting their sales goals, and we understand that some distributors may purchase advertising. Nevertheless, Dehaier does not currently reimburse any such costs for growing such distributors’ client bases.
Our Industry
In April 2009, the Chinese government implemented large-scale healthcare reform. The State Council allocated $123 billion as part of its New Medical Reform Plan. The plan, which is part of China’s stimulus package aimed at correcting the recent global economic down-turn, contemplates the development of a universal healthcare system that will cover 90% of China’s population by 2010. In addition, the plan includes significant improvements to health care facilities and expansion of China’s health related infrastructure.
Specifically, within three years, the Chinese government aims to improve the urban healthcare system by rebuilding and restructuring approximately 3,700 existing urban community health centers and 11,000 community health clinics. The plan will also accommodate the development of approximately 2,400 new urban health centers. In effect, the plan de-emphasizes the prevalence of large, magnet facilities in favor of smaller, more accessible clinics.
In addition, the plan is designed to dramatically improve medical services available for the 800 million rural poor in China. Through the plan, the Chinese government contemplates the development of clinics in every village and a hospital in every county in China by the end of 2011. If successfully implemented, the plan would result in at least 2,000 new county-level hospitals and 29,000 township hospitals and the upgrading of another 5,000 more.
Our Opportunity
We believe that we are well positioned to benefit from the rapid growth of expenditures on medical devices in China and the growing demand for domestic medical devices in China. A recent article published by the Wharton School of the University of Pennsylvania suggests that the expected value of China’s medical device market will reach $28 billion by 2014. As this market was valued at $12.14 billion in 2008, or roughly one-eighth of China’s overall healthcare market, there may still be significant potential for growth. China’s medical device market is projected to grow faster than the global medical device market. China is already the third largest medical device market in the world behind the United States and Japan, and within the next five to seven years China is expected to surpass Japan and become the second largest medical device market in the world. The respiratory and oxygen homecare market in China is also experiencing rapid growth. Market growth rates for 2009 are projected at 11.2%. Reasons for the rapid growth of China’s medical device market include:
| • | fast growing economy; |
| • | increasing percentage of gross domestic product, or GDP, expected to be spent on healthcare by the Chinese government; |
| • | increasing desire for and utilization of more advanced technologies in Chinese hospitals and clinics; |
| • | increasing availability of healthcare insurance; |
| • | higher degree of operating autonomy at hospitals and clinics; and |
| • | growing desire for better quality of care. |
Hospitals and clinics in China purchase almost all of their medical devices and supplies through distributors. These distributors tend to operate in small territories in China, and many focus on eastern coastal cities. As a result,
3
Table of Contents
medical device companies need to develop relationships with several distributors in different regions to be able to reach a broad end user base. We believe the ability to leverage local contacts and knowledge is vital in establishing an effective distribution network, constituting a significant barrier to entry for both smaller local companies and international competitors that lack a meaningful local presence in China.
Our Competitive Strengths
We believe we have the following principal competitive strengths, enabling us to attain a leading position in China’s medical device and respiratory and oxygen homecare industries:
| • | our established brand and market position in China’s medical device and respiratory and oxygen homecare industries; |
| • | our established distribution, sales and service network throughout China; |
| • | our research and development capabilities; |
| • | our established relationships with foreign medical device manufacturers; |
| • | our established relationships with approximately 3,000 hospital customers; and |
| • | dedicated customer support and service. |
Our Strategies
Our objective is to strengthen our position as one of China’s leaders in developing, assembling and marketing respiratory and oxygen homecare products and to develop a presence in certain select foreign markets, including, Europe and America. We intend to achieve our objective by implementing the following strategies:
| • | increasing our market share in China’s respiratory and oxygen homecare market; |
| • | increasing our focus on research and development in order to develop more advanced products and new product lines; |
| • | enhancing our market position and brand recognition in China as well as other certain select foreign markets; |
| • | maintaining our disciplined cost focus; |
| • | pursuing favorable opportunities to acquire complementary businesses; and |
| • | maintaining our distribution network through active management and regular reviews of distributor performance. |
Our Challenges and Risks
The successful execution of our strategies is subject to certain risks and uncertainties, including those related to:
| • | uncertainties in our development, introduction and marketing of new solution and services; |
| • | recruitment, training and retention of skilled engineers and other personnel; |
| • | our ability to respond to competitive pressures; |
| • | our ability to build our brand recognition in our existing and any future markets; |
| • | our ability to increase the market penetration of our products; |
| • | our ability to export our branded products to new markets in the future; |
| • | uncertainties in the fluctuation of the RMB; |
| • | uncertainties in the world economy, including potential continuance and recurrence of the current global economic slowdown; |
| • | our ability to purchase products from our international suppliers at competitive prices; |
| • | protection of our trade secrets and other valuable intellectual property; |
| • | our ability to identify and negotiate favorable opportunities to acquire complementary businesses; and |
| • | complexities of the regulatory environment in China. |
In addition, we face risks and uncertainties that may materially affect our business, financial condition, results of operations and prospects. Thus, you should consider the risks discussed in “Risk Factors” and elsewhere in this prospectus before investing in our common shares.
4
Table of Contents
Our Corporate Structure
Historical Overview
Our founder, Mr. Ping Chen, founded BTL on July 5, 2001 to develop and distribute medical devices. He currently owns approximately 86% of BTL, and his wife and employees of Dehaier own the remaining 14% of BTL. BTL leases some of our property to us and provides certain transportation and repair services to medical devices for which we are not obligated to perform warranty services, either because the warranty is expired or because the product was sold by another company. We do not receive any payment for such services from BTL. BTL served as the domestic partner to our joint venture pursuant to which we, a British Virgin Islands company, own the majority BDL, a PRC company in the medical device business. At the time of the formation of the joint venture, foreign enterprises were not permitted to own such companies without PRC partners.
In 2003, in order to continue to grow our business, BTL engaged in a corporate restructuring and Series A venture capital financing. As a result of those actions, Dehaier, BDL and DHK were established and a venture capital investor, De-haier Investment Holdings, Ltd., a British Virgin Islands company, became a shareholder in Dehaier. At the same time, Mr. Chen’s wholly-owned company, Chen Ping Ltd., became a shareholder in Dehaier, and we created the holding company structure that is currently in place.
Dehaier was incorporated as an international business company under the International Business Companies Act, 1984, in the British Virgin Islands on July 22, 2003 under the name “De-Haier Medical Systems Limited.” We changed this name to “Dehaier Medical Systems Limited” on June 3, 2005. Dehaier is a holding company. Dehaier does not conduct business in China and instead relies on BDL to conduct business in China.
On September 24, 2003, we established BDL. BDL conducts substantially all of our operations in China and is responsible for generating substantially all of our revenues. BDL was formed as a joint venture between a Chinese entity, BTL, and a foreign invested enterprise, Dehaier, in order to allow foreign investments to be used to grow our business. Because BDL is engaged in an encouraged industry under the Foreign Investment Industrial Guidance Catalogue, it was allowed to have foreign investments and to be established as a Chinese-foreign equity joint-venture. This structure allowed BDL access to foreign capital that would not have been available outside this structure. In addition to its engagement in an encouraged industry, the use of this joint venture structure allowed BDL to take advantage of favorable tax rates available prior to January 1, 2008. Before January 1, 2008, equity joint-ventures such as BDL could enjoy a preferential enterprise income tax rate of 24%. In addition to the lower standard rate, such equity joint-ventures were also allowed a two-year income tax exemption from the date they first became profitable and a 50% income tax reduction for the following three years after that time. After January 1, 2008, the income tax rates were unified at 25% under the PRC Enterprise Income Tax Law; however, BDL’s ability to pay lower income taxes prior to this date left it with more net income with which to grow its business. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Tax Matters Applicable to Our Company — PRC Enterprise Income Taxes.”
On October 15, 2003, we founded DHK and created a holding company structure by which we are the parent company of BDL and DHK. DHK was formed in anticipation of opportunities to make use of its status as a Hong Kong company to grow our business; we have not yet made significant use of DHK for such purposes and have no present plans to do so. BDL has been focused on the development and distribution of medical devices since its inception and began developing its respiratory and oxygen homecare business in 2006.
Effective as of January 5, 2007, we completed a Series B venture capital financing with Crystal East Group Limited, a British Virgin Islands company. Crystal East Group Limited subsequently completed the transfer of its shares to its individual shareholder, Yijen Chen.
Relationship among Dehaier, BTL and BDL
BTL is a PRC company established on July 5, 2001. Dehaier is a BVI company established on July 22, 2003. Dehaier and BTL jointly established BDL on September 24, 2003 as a Chinese-foreign equity joint-venture under the PRC laws. Dehaier has been and is BDL’s foreign shareholder, and BTL has been and is BDL’s domestic shareholder since BDL was established. Currently, Dehaier owns 96.37% of the contributions of BDL, and BTL owns 3.63% of the contributions of BDL. Under PRC laws, the shareholders of the equity joint-venture share the profits, risks and losses in proportion to their respective contributions of the equity joint-venture.
5
Table of Contents
As described above, the business of Dehaier was historically performed by BTL before we completed our Series A venture capital financing in 2003. At the time of the Series A venture capital financing, BTL’s founder, Mr. Ping Chen, sought capital to grow the business, and a foreign (non-Chinese) investor wanted to invest in BTL. Chinese law at the time required such foreign investments in companies like BTL to be in the form of a joint venture between a foreign company and a Chinese company. For this reason, our company was restructured to create a joint venture, with BTL as the Chinese party to the joint venture and Dehaier as the foreign party to the joint venture. Dehaier was considered the foreign party to the joint venture both because it is a British Virgin Islands company and because its shareholders at the time were also British Virgin Islands companies. In connection with the joint venture, BDL was formed as the operating entity. As a result of this restructuring, BDL conducted the business previously conducted by BTL.
Dehaier presently operates as a holding company, of which DHK is its wholly owned subsidiary and BDL is its 96.37%-owned subsidiary. BTL exists primarily as the Chinese joint venture party for the reasons described above. In addition, BTL performs certain limited functions, including leasing some of our property to us and providing certain transportation and repair services to medical devices for which we are not obligated to perform warranty services, either because the warranty is expired or because the product was sold by another company. BDL is our operating entity through which we sell all of our medical products and provide all of our warranty services. We develop, assemble and market our branded products through BDL and market our distributed products through BDL.
Financings and Restructurings
2003 Series A Financing
On October 23, 2003, Dehaier agreed to issue up to $2,400,000 in Series A Preferred Shares to De-haier Investment Holdings Ltd., a British Virgin Islands company. The investor received one-half of these shares in 2003 for $1,200,000 and had an option to acquire the other half of the shares for the remainder of the purchase price. The investor ultimately elected in 2006 not to purchase the other one-half of the Series A Preferred Shares. In connection with the transaction, BDL was formed as a Sino-foreign joint venture with Dehaier holding a majority of the registered capital and BTL holding a minority of the registered capital. In connection with closing, BTL’s assets were transferred to BDL. In connection with the Series A Financing, the parties entered into a Shareholder Agreement that granted certain rights to the Series A Preferred shareholders that terminated upon conversion of the Series A Preferred Shares to common shares.
The Series A financing was exempt from registration under U.S. Securities laws by virtue of exemptions provided by Regulation S and Section 4(2) of the Securities Act of 1933 and by falling outside the an offering covered by Section 5 of the Securities Act of 1933.
2007 Series B Financing
Effective January 5, 2007, Dehaier agreed to issue up to $2,000,000 in Series B Preferred Shares to Crystal East Group Limited, a British Virgin Islands company. In connection with the Series B Financing, the parties entered into a Shareholder Agreement that granted certain rights to the Series B Preferred shareholders that terminated upon conversion of the Series B Preferred Shares to common shares.
The Series B financing was exempt from registration under U.S. Securities laws by virtue of exemptions provided by Regulation S and Section 4(2) of the Securities Act of 1933 and by falling outside the an offering covered by Section 5 of the Securities Act of 1933.
2009 Restructuring
In preparation for the commencement of this initial public offering, our company completed a restructuring to accomplish two goals. First, we sought to convert all Series A and Series B Preferred Shares into common shares. Second, we completed a shares split so that we would have, in the aggregate, 3,000,000 common shares outstanding prior to commencement of the offering. As to the first goal, our Series A and Series B Preferred shareholders agreed to convert their respective Series A and Series B Preferred Shares into an equal number of our common shares. This 1:1 conversion was completed without consideration. As to the second goal, we completed a 3.66140766-for-1 share split of common shares completed of October 31, 2009. As a result of the conversions and share split, we have 3,000,000 common shares issued and outstanding prior to commencement of this offering and no issued and outstanding preferred shares.
6
Table of Contents
Our Structure
As a result of the above 2003 restructuring, rounds of financing completed in 2003 and 2007 and the 2009 conversion of outstanding preferred shares, the following diagram illustrates our current corporate structure as of the date of this prospectus.
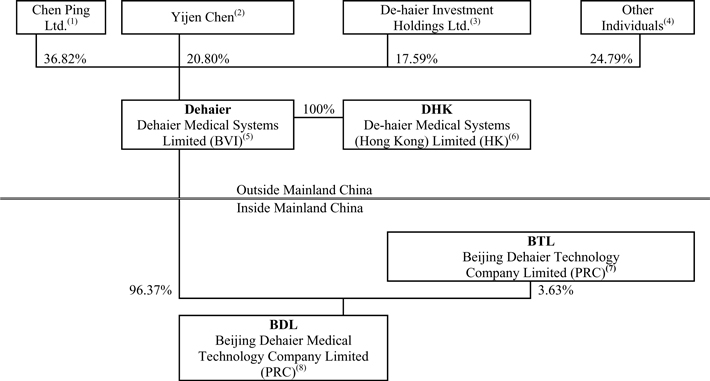
| (1) | Chen Ping Ltd. is a British Virgin Islands company beneficially owned by our Chief Executive Officer, Mr. Ping Chen. |
| (2) | These shares were initially acquired by Crystal East Group Limited, a British Virgin Islands company, in our second round of venture capital financing. Crystal East Group Limited transferred the Series B preferred shares to its shareholder, Ms. Chen, and such shares were converted into our common shares as of October 31, 2009. Ms. Chen is not related to our chief executive officer, Mr. Ping Chen. |
| (3) | De-haier Investment Holdings Ltd, a British Virgin Islands company, obtained these shares in our first round of venture capital financing. These Series A preferred shares were converted into our common shares as of October 31, 2009. |
| (4) | Such shareholders consist of employees and consultants to our company. |
| (5) | Dehaier is our British Virgin Islands holding company that seeks to register its common shares in this initial public offering. |
| (6) | DHK is a Hong Kong company owned by Dehaier, through which we may in the future conduct operations. We do not, however, have any present plans to do so. We do not currently receive any significant revenues through DHK. |
| (7) | BTL is a variable interest entity, and BDL is the primary beneficiary. BTL owns a building which is pledged as collateral for BDL’s bank loans. In exchange, BDL loans money to BTL to finance its operations. Dehaier, BDL and BTL are under common control, and BDL provides substantial financial support to BTL. This substantial financial support is primarily for working capital loans to finance BTL’s operations. At December 31, 2006, 2007, 2008 and September 30, 2009, BDL had advanced to BTL funds in the amount of $823,405, $786,816, $292,011 and $164,248, which represented 87%, 74%, 24% and 13% of BTL’s equity, respectively. Because BTL has insufficient equity to finance its operations, BTL then is required to obtain additional financial support to fund its operations. Accordingly, BTL’s sales are included in Dehaier’s consolidated income from operations. Mr. Chen owns 86% of BTL and his wife and employees of Dehaier own the remaining 14%. BTL performs certain out-of-warranty repair work for products we sell and provides transportation services related to such services. |
7
Table of Contents
| (8) | BDL is our Chinese operating entity. We develop, assemble, market and distribute our branded products through BDL, and we market and distribute our distributed products developed by other companies through BDL. |
The Offering
| Shares Offered: |
Minimum: 1,250,000 common shares | |
| Maximum: 1,500,000 common shares | ||
| Shares Outstanding Prior to Completion of Offering: |
3,000,000 common shares | |
| Shares to be Outstanding after Offering: |
Minimum: 4,250,000 common shares | |
| Maximum: 4,500,000 common shares | ||
| Assumed Offering Price per Share: |
$8.00 | |
| Gross Proceeds: |
Minimum: $10,000,000 | |
| Maximum: $12,000,000 | ||
| Proposed NASDAQ Capital Market Symbol: |
“DHRM” (CUSIP No. G27010 100) | |
| Transfer Agent: |
Computershare Trust Company, N.A. 250 Royall Street, Canton, Massachusetts 02021 | |
| Risk Factors: |
Investing in these securities involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section of this prospectus before deciding to invest in our common shares. | |
| Closing of Offering: |
The offering contemplated by this prospectus will terminate upon the earlier of: (i) a date mutually acceptable to us and our placement agent after the minimum offering is sold or (ii) May 31, 2010. If we complete this offering, net proceeds will be delivered to our company on the closing date (such closing date being the above mutually acceptable date on or before May 31, 2010, provided the minimum offering has been sold). We will not complete this offering unless our application to list on the NASDAQ Capital Market is approved. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. If we complete this offering, then on the closing date, we will issue shares to investors and placement agent warrants to our placement agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of common shares sold in this offering. | |
8
Table of Contents
Placement
We have engaged Anderson & Strudwick, Incorporated to conduct this offering on a “best efforts, minimum/maximum” basis. The offering is being made without a firm commitment by the placement agent, which has no obligation or commitment to purchase any of our common shares. Our placement agent is required to use only its best efforts to sell the securities offered. The offering will terminate upon the earlier of: (i) a date mutually acceptable to us and our placement agent after which at least 1,250,000 common shares are sold or (ii) May 31, 2010. Until we sell at least 1,250,000 common shares, all investor funds will be held in an escrow account at SunTrust Bank, Richmond, Virginia. If we do not sell at least 1,250,000 common shares by May 31, 2010, all funds will be promptly returned to investors (within one business day) without interest or deduction. If we complete this offering, net proceeds will be delivered to our company on the closing date. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. None of our officers, directors or affiliates may purchase shares in this offering. If we complete this offering, then on the closing date, we will issue shares to investors and placement agent warrants to our placement agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of Common Shares Sold in this offering.
Make-Good Escrow
We have agreed with our placement agent to value our company based on a multiple of approximately 6.6 times our targeted after-tax earnings for the year ending December 31, 2010, subject to the terms of a Make-Good Escrow Agreement to be executed before effectiveness of this registration statement and our ability to redeem a sufficient number of Make-Good Shares to meet our target audited after-tax earnings per share for the year ending December 31, 2010, as more fully discussed below. If we are unable to achieve these targeted after-tax earnings, then there is a risk that our company would be considered overvalued based on this multiple. In order to mitigate some of this risk, each of Ping Chen, Zheng (Rita) Liu, Weibing Yang, Jian Sun and Yong Wang has agreed to place, on a prorated basis, that number of beneficially owned common shares into escrow that is equal to 40% of the maximum number of shares to be sold in this offering. These shares will be placed into escrow prior to the time we request effectiveness of the registration statement. Upon closing of this offering, the escrow agent will return any shares in excess of 40% of the actual number of shares sold in the offering. Such escrowed shares are referred to as the “Make-Good Shares.” The Make-Good Shares will remain in escrow with SunTrust Bank or another bank acceptable to our placement agent pending the filing of our company’s Form 10-K for the year ending December 31, 2010.
To the extent our audited after-tax earnings per share for the year ending December 31, 2010 are less than the target of $0.80, excluding any expenses associated with releasing the Make-Good Shares back to the original owners, our company will redeem and cancel, pro rata, the Make-Good Shares without any consideration to the extent necessary to cause our audited after-tax earnings per share to be equal to the target of $0.80. We cannot guarantee that we will be able to redeem a sufficient number of Make-Good Shares to increase audited after-tax earnings per share to the target of $0.80 if our company either has low net income or any net losses in 2010.
Any remaining Make-Good Shares will be released from escrow to our initial shareholders upon the earlier of (i) one (1) business day after the termination of this offering without closing or (ii) thirty (30) calendar days after the filing of the Form 10-K for the year ending December 31, 2010 after redeeming any Make-Good Shares. Additionally, notwithstanding any other terms of the Make-Good Escrow, if our shares trade at or above 2.5 times the price of this offering for a period of five trading days within a ten day trading period, the Make-Good Escrow will terminate and the Make-Good Shares will be released to the initial shareholders. Any delay in redeeming the Make-Good Shares will delay the release of such remaining Make-Good Shares from escrow.
We believe the Make-Good Escrow arrangement benefits the shareholders of our company (other than those who may forfeit shares without consideration) because it is designed to increase the likelihood that our company will achieve the after-tax earnings per share upon which our valuation is based. To the extent Make-Good Shares are redeemed without cost, the after-tax per-share earnings will increase for all remaining outstanding shares. While we believe the Make-Good Escrow arrangement is a benefit to our shareholders, we may be unable to redeem enough Make-Good Shares to reach our targeted 2010 after-tax earnings per share. This could occur if we either have net losses or substantially lower than anticipated earnings. If this were to happen, our audited after-tax earnings after redemption of the Make-Good Shares could be less than the target of $0.80 per share. See “Risk Factors – A redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company.”
Placement Agent’s Warrants
In connection with this offering, we will, for a nominal amount, sell our placement agent warrants exercisable at a rate of one warrant per share to purchase up to ten percent of the shares sold in the offering. These warrants are exercisable for a period of five years from the date of issuance at a price equal to 125% of the price of the shares in this offering. If we complete the maximum offering, then on the closing date we will issue 150,000 warrants to the placement agent to purchase one common share each. During the term of the warrants, the holders thereof will be given the opportunity to profit from a rise in the market
9
Table of Contents
price of our common shares, with a resulting dilution in the interest of our other shareholders. The terms on which we could obtain additional capital during the life of these warrants may be adversely affected because the holders of these warrants might be expected to exercise them when we are able to obtain any needed additional capital in a new offering of securities at a price greater than the exercise price of the warrants. If the placement agent exercises all of its warrants, we would have between 2.94% (minimum offering) and 3.33% (maximum offering) more shares outstanding after the placement agent’s warrant exercise than at the conclusion of the offering, assuming no other issuances (including any issuances under the share incentive plan). See “Placement.”
Corporate Information
Our principal executive offices are located at 1223 Epoch Center, No. 31 Zi Zhu Yuan Road, Haidian District, Beijing, People’s Republic of China (100089). Our website address is http://www.chinadhr.com. Information contained on our website or any other website is not a part of this prospectus.
10
Table of Contents
Summary Financial Information
In the table below, we provide you with summary financial data of our company. This information is derived from our consolidated financial statements included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected for any future period. When you read this historical selected financial data, it is important that you read it along with the historical statements and notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| For the nine months ended |
For the fiscal year ended December 31, |
|||||||||||
| September 30, 2009 | 2008 | 2007 | ||||||||||
| (Unaudited) | ||||||||||||
| Revenue |
$ | 9,422,460 | $ | 9,414,430 | $ | 6,599,512 | ||||||
| Operating income |
2,586,824 | 1,496,086 | 970,622 | |||||||||
| Net income |
2,110,278 | 980,351 | 848,421 | |||||||||
| Non-controlling interest in income |
(38,502 | ) | (62,331 | ) | (53,214 | ) | ||||||
| Net income attributable to Dehaier |
2,071,776 | 918,020 | 795,207 | |||||||||
| Basic earnings per share (based on 1,891,930 shares outstanding on each of September 30, 2009, December 31, 2008 and 2007)(1) |
1.10 | 0.48 | 0.42 | |||||||||
| Diluted earnings per share (based on 3,000,000, 3,000,000 and 2,451,624 shares outstanding on September 30, 2009, December 31, 2008 and 2007, respectively)(1) |
0.69 | 0.31 | 0.32 | |||||||||
| Pro forma basic earnings per share (based on 1,291,930 shares outstanding on each of September 30, 2009 and December 31, 2008)(2) |
1.60 | 0.71 | N/A | |||||||||
| Pro forma diluted earnings per share (based on 2,400,000 shares outstanding on each of September 30, 2009 and December 31, 2008, respectively)(2) |
0.86 | 0.38 | N/A | |||||||||
| December 31, | ||||||||||||
| September 30, 2009 | 2008 | 2007 | ||||||||||
| (Unaudited) | ||||||||||||
| Total assets |
$ | 16,485,711 | $ | 13,046,400 | $ | 9,928,863 | ||||||
| Total current liabilities |
6,566,373 | 5,232,464 | 3,550,505 | |||||||||
| Total Dehaier shareholders’ equity |
8,676,245 | 6,608,704 | 5,310,499 | |||||||||
| Non-controlling interest |
1,243,093 | 1,205,232 | 1,067,859 | |||||||||
| Total liabilities and shareholders’ equity |
16,485,711 | 13,046,400 | 9,928,863 | |||||||||
| (1) | We have presented these basic and diluted earnings per share in Dehaier after giving retroactive effect to the 3.66140766-for-1 share split of common shares completed as of October 31, 2009. |
| (2) | We have presented these pro forma earnings per share after (a) giving retroactive effect to the 3.66140766-for-1 share split of our common shares completed as of October 31, 2009 and (b) assuming the redemption of all shares placed into escrow as described in the section entitled “Related Party Transactions – Make-Good Shares Subject to Redemption.” The number of escrowed shares is based on 600,000 common shares (40% of an assumed maximum of 1,500,000 common shares). Pro forma basic EPS for the nine months ended September 30, 2008 calculated on the foregoing assumptions and based on 1,291,930 Dehaier shares, is $0.40. Pro forma diluted EPS for the nine months ended September 30, 2008 calculated on the foregoing assumptions and based on 2,400,000 Dehaier shares, is $0.22. No pro forma numbers have been provided for the year ended December 31, 2007. |
11
Table of Contents
Investment in our securities involves a high degree of risk. You should carefully consider the risks described below together with all of the other information included in this prospectus before making an investment decision. The risks and uncertainties described below represent our known material risks to our business. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, you may lose all or part of your investment. You should not invest in this offering unless you can afford to lose your entire investment.
Risks Related to Our Business and Industry
Our quarterly revenues and operating results are difficult to predict and could fall below investor expectations, which could cause the trading price of our common shares to decline.
Our quarterly revenues and operating results have fluctuated in the past and may continue to fluctuate significantly depending upon numerous factors. In particular, during the period from April to May, we generally experience an increase in revenues associated with our attendance at the China International Medical Equipment Fair, the largest exhibition of medical equipment, related products and services in Asia-Pacific region. This fair occurs in the spring each year. In addition, we generally experience an increase in revenues in the period from September through November. This increase is associated with hospital purchasing designed to extinguish governmental budgets prior to the fiscal year end. We believe that our first quarter performance will generally decline as a result of the lack of business conducted during the Chinese Lunar New Year Holiday. To the extent our financial performance fluctuates significantly, investors may lose confidence in our business and the price of our common shares could decrease.
We may fail to effectively develop and commercialize new products, which could materially and adversely affect our business, financial condition, results of operations and prospects.
The respiratory and oxygen homecare market is developing rapidly and related technology trends are constantly evolving. This results in the frequent introduction of new products, short product life cycles and significant price competition. Consequently, our future success depends on our ability to anticipate technology development trends and identify, develop and commercialize in a timely and cost-effective manner the new and advanced products that our customers demand. New products contribute significantly to our revenues. In 2008, we developed three new products in the respiratory and oxygen homecare market. We expect the respiratory and oxygen homecare market to continue to evolve toward newer and more advanced products. Moreover, it may take an extended period of time for our new products to gain market acceptance, if at all. Furthermore, as the life cycle for a product matures, the average selling price generally decreases. In the future, we may be unable to offset the effect of declining average sales prices through increased sales volume and controlling product costs. Lastly, during a product’s life cycle, problems may arise regarding regulatory, intellectual property, product liability or other issues which may affect the product’s continued commercial viability. Whether we are successful in developing and commercializing new products will depend on our ability to:
| • | accurately assess technology trends and customer needs and meet market demands; |
| • | optimize our assembly and procurement processes to predict and control costs; |
| • | assemble and deliver products in a timely manner; |
| • | increase customer awareness and acceptance of our products; |
| • | minimize the time and costs required to obtain required regulatory clearances or approvals; |
| • | anticipate and compete effectively with other medical device developers, manufacturers and marketers; |
| • | price our products competitively; and |
| • | effectively integrate customer feedback into our research and development planning. |
Our revenues are highly dependent on a limited number of customers involved in China’s healthcare device industry.
For the years ended December 31, 2008 and 2007, and for the nine months ended September 2009 and 2008, approximately 11%, 17%, 8% and 7%, respectively, of our sales were from one customer, Poverty Aid Office.
We anticipate that our dependence on a limited number of customers will continue for the foreseeable future. Consequently, any one of the following events may cause material fluctuations or declines in our revenues:
| • | reduction, delay or cancellation of orders from one or more of our significant customers; |
12
Table of Contents
| • | loss of one or more of our significant customers and our failure to identify additional or replacement customers; and |
| • | failure of any of our significant customers to make timely payment for our products. |
To anticipate our client’s future needs, we must maintain a close relationship with our key customers. Any failure to maintain this close relationship, due to unsuccessful sales and marketing efforts, lack of suitable products, unsatisfactory performance or other reasons, could result in our losing a client and its business. If we lose a key customer or a portion of work we currently receive from it, a key customer significantly reduces its purchasing levels or delays a major purchase or we fail to attract additional major customers, our revenues could decline, and our operating results could be materially and adversely affected.
We sell our products primarily to distributors and our ability to add distributors will impact our revenue growth. Failure to maintain or expand our distribution network would materially and adversely affect our business.
With contractual relationships with over 2,000 independent distributors throughout China, we depend on sales to distributors for a significant majority of our revenues. Our distributors purchase all products ordered regardless of whether the products are ultimately sold. Products are not purchased by distributors on consignment, and distributors have no right to return unsold products. As our existing distributor agreements expire, we may be unable to renew such agreements on favorable terms or at all, and we do not own, employ or control these independent distributors. Furthermore, we actively manage our distribution network and regularly review the performance of each distributor. We may terminate agreements with distributors if we are not satisfied with their performance for any reason. We periodically terminate relationships with underperforming exclusive distributors. When an exclusive distributor in a particular geographic area fails to meet our expectations, then we are economically incentivized to replace that distributor with a new distributor so that area can be served as well as possible. We occasionally terminate a relationship with a non-exclusive distributor and are more likely to simply appoint another one; however, we have found that in some instances we are better served to replace an underperforming non-exclusive distributor with an exclusive distributor. Additionally, we have found that even in cases where there may not be an economic incentive to terminate a non-exclusive distributor, having the ability to replace a distributor often motivates distributors to increase their efforts to meet our expectations. This policy may make us less attractive to some distributors. In addition, we compete for distributors with other leading medical device companies who may enter into long-term distribution agreements, effectively preventing many distributors from selling our products. As a result, a significant amount of time and resources must be devoted to maintaining and growing our distribution network. Any disruption in our distribution network could have negative effects on our ability to sell our products, which would in turn materially and adversely affect our business, financial condition and results of operations.
We sell products for some of our competitors, some of which compete with our branded products.
We are the exclusive distributor in China for IMD, Timesco and JMS in the PRC. We are a non-exclusive regional distributor for ResMed. Our agreement with IMD does not prohibit us from selling X-ray units from other companies, and we provide four types of branded mobile and C-armed X-ray machines. Our agreements with Timesco, JMS, and ResMed contain provisions that limit our ability to sell products that compete with the products we sell for them. We do not currently sell any branded laryngoscopes, including any that would compete with those from Timesco. We also do not sell any infusion pumps or syringe pumps, including any that would compete with those from JMS. We do not currently sell any products to treat obstructive sleep apnea that would compete with those from ResMed; however, we have developed and are currently seeking SFDA approval for our DHR CPAP C5, DHR Auto CPAP A8 and DHR Auto S-CPAP A9. ResMed may claim that these products compete with the ResMed S6 and MAP MiniPAP and may seek to terminate our distribution agreement with them on that basis. The percentage of our total revenues attributable to the distribution agreement for the ResMed S6 and MAP MiniPAP was 1.86% and 0.58% for the years ended December 31, 2008 and 2007, respectively and 1.91% and 2.18% for the nine months ended September 30, 2009 and 2008, respectively.
In addition, our sales of X-ray machines that may compete with those from IMD and sleep apnea devices that may compete with those from ResMed could result in claims from our suppliers that we have favored our branded products over distributed products in marketing. Further, where our agreements with suppliers limit our ability to sell competing branded products, we may have to forego developing potentially profitable products. Any of these results could materially harm our business.
We rely on some of our competitors to supply component parts for our branded products.
We obtain some components from companies that are competitors in our market, including IMD and Spacelabs Medical Trading (Shanghai) Co., Ltd. None of these competitors supplies more than 4% of our components, and we are not reliant on them for such components. We do, however, provide detailed technical specifications to these competitors for use in producing components for our branded products. If these companies were to reverse-engineer or otherwise misappropriate such information, our business could be materially harmed.
13
Table of Contents
Although we do not own or control our distributors, the actions of these distributors may affect our business operations or our reputation in the marketplace.
Our distributors are independent from us, and as such, our ability to effectively manage their activities is limited. Distributors could take any number of actions that could have material adverse effects on our business. If we fail to adequately manage our distribution network or if distributors do not comply with our distribution agreements, our corporate image could be tarnished among end users, disrupting our sales. Furthermore, we could be liable for actions taken by our distributors, including any violations of applicable law in connection with the marketing or sale of our products, including China’s anti-corruption laws. Recently, the PRC government has increased its anti-bribery efforts in the healthcare sector to reduce improper payments received by hospital administrators and doctors in connection with the purchase of pharmaceutical products and medical devices. Our distributors may violate these laws or otherwise engage in illegal practices with respect to their sales or marketing of our products. If our distributors violate these laws, we could be required to pay damages or fines, which could materially and adversely affect our financial condition and results of operations. In addition, our brand and reputation, our sales activities or the price of our shares could be adversely affected if our company becomes the target of any negative publicity as a result of actions taken by our distributors.
We plan to expand our homecare and technical service products internationally and become a leader in selected international markets. Such expansion can be difficult and time consuming, and if unsuccessful our future profits would be materially and adversely affected.
While we currently operate exclusively in China, we envision competing in selected international markets with our homecare and technical service products. We intend to enter into markets in which we have limited or no experience and in which our brand may be less recognized. To the extent we are able to obtain approval to sell our branded products in the European Union, we currently plan to expand initially into Europe. We have not yet qualified or applied to do so, and we have no guarantee that we will be successful with any such application for approval. We plan to devote significant resources to marketing and promoting our brand internationally and attracting distributors in foreign markets. Success in international markets will depend on our ability to attract a sufficient number of distributors suitable for selling our branded products. Furthermore, in new markets we may fail to anticipate competitive conditions that are different from those in our existing markets. These competitive conditions may make it difficult or impossible for us to operate effectively in these markets.
Operation in international markets will also expose us to many other risks, including but not limited to:
| • | political instability; |
| • | economic instability and recessions; |
| • | changes in tariffs; |
| • | difficulties of administering foreign operations generally; |
| • | limited protection for intellectual property rights; |
| • | obligations to comply with a wide variety of foreign laws and other regulatory requirements; |
| • | financial condition, expertise and performance of international distributors; |
| • | export license requirements; |
| • | unauthorized re-export of our branded products; |
| • | inability to purchase our distributed products from international suppliers at competitive prices; |
| • | potentially adverse tax consequences; and |
| • | inability to effectively enforce contractual or legal rights. |
We are highly dependent on our key personnel such as key executives and research and development personnel.
We are highly dependent on the continued service of our key executives and other key personnel. In particular, we substantially rely on our chairman and chief executive officer Mr. Ping Chen to manage our business and operations. We also rely on key research and development personnel for the development of new products. In addition, we rely on customer service personnel for the installation and support of our products and on marketing and sales personnel, engineers and other personnel with technical and industry knowledge to market, sell, install and service our products. We have entered into standard one-year employment contracts with all of our officers and managers and other key personnel and one-year employment contracts with our other employees. These contracts prohibit our employees from engaging in any conduct or activity that would be competitive with our business during the course
14
Table of Contents
of their employment. Loss of any of our key personnel could severely disrupt our business. We may not be able to find suitable or qualified replacements, and will likely incur additional expenses in order to recruit and train any new personnel.
Competition for qualified management and key personnel in the medical technology field is intense and the pool of qualified candidates is limited. We not only compete with other medical device companies but also universities and other research institutions to attract and retain qualified personnel. This intense competition may force us to offer higher compensation and benefit packages in order to attract and retain the most qualified personnel. Our future success depends on our ability to attract and retain these individuals and failure to do so could result in severe disruptions to our business and growth.
Our business is subject to intense competition, which may reduce demand for our products and materially and adversely affect our business, financial condition, results of operations and prospects.
The medical device market is highly competitive, and we expect competition to intensify. Given the $585 billion stimulus initiative in China and its impact on healthcare, we expect the availability of healthcare to increase, as more hospitals and clinics are developed rurally.
We face direct competition from both domestic and international competitors across all product lines and price points. Our competitors also vary by product. Currently, our competitors include publicly traded and privately held multinational companies, such as Respironics, Inc., ResMed Inc., and Covidien, as well as domestic Chinese companies such as Beijing Aoji, Beijing Ya’ao, Jiangsu Yuyue and Zhejiang Longfei. As we expand into international sales, we expect our competitors will primarily be publicly traded and privately held multinational companies. We also expect to face competition in international sales from companies that have local operations in the markets in which we sell our products. Some of our larger competitors may have:
| • | greater financial and other resources; |
| • | larger variety of products; |
| • | more products that have received regulatory approvals; |
| • | greater pricing flexibility; |
| • | more extensive research and development and technical capabilities; |
| • | patent portfolios that may present an obstacle to our conduct of business; |
| • | greater knowledge of local market conditions where we seek to increase our international sales; |
| • | stronger brand recognition; and |
| • | larger sales and distribution networks. |
As a result, we may be unable to offer products similar to, or more desirable than, those offered by our competitors, market our products as effectively as our competitors or otherwise respond successfully to competitive pressures. In addition, our competitors may be able to offer discounts on competing products as part of a “bundle” of non-competing products, systems and services that they sell to our customers, and we may not be able to profitably match those discounts. Our competitors may develop technologies and products that are more effective than those we currently offer or that render our products obsolete or uncompetitive. The timing of the introduction of competing products into the market could affect the market acceptance and market share of our products. As we expect demand for our products to increase along with the availability of healthcare, we must continue to focus on competitive pricing and innovation by being at the forefront of market trends and improving our product and service offerings. Our failure to compete successfully could materially and adversely affect our business, financial condition, results of operation and prospects.
Some of our internationally-based competitors may follow in the footsteps of Covidien and establish production or research and development facilities in China, while others may enter into cooperative business arrangements with Chinese manufacturers. If we are unable to develop competitive branded products, obtain regulatory approval or clearance and supply sufficient quantities to the market as quickly and effectively as our competitors, market acceptance of our branded products may be limited, which could result in decreased sales. In addition, we may not be able to maintain our branded product cost advantages.
We believe that corrupt practices in the medical device industry in China still occur. To increase sales, certain manufacturers or distributors of medical devices may pay kickbacks or provide other benefits to hospital personnel who make procurement decisions. Our company policy prohibits these practices by our direct sales personnel and our distribution agreements require our distributors to comply with applicable law. As a result, as competition intensifies in the medical device industry in China, we may lose sales, customers or contracts to competitors.
15
Table of Contents
If we fail to accurately project demand for our products, we may encounter problems of inadequate supply or oversupply, which would materially and adversely affect our financial condition and results of operations, as well as damage our reputation and brand.
Our distributors typically order our products on a purchase order basis. We project demand for our products based on rolling projections from our distributors, our understanding of anticipated hospital procurement spending, and distributor inventory levels. Lack of significant order backlog and the varying sales and purchasing cycles of our distributors and other customers, however, make it difficult for us to forecast future demand accurately.
If we overestimate demand, we may purchase more distributed products or more unassembled parts or components for our branded products than we require. If we underestimate demand, our third party suppliers may have inadequate supply of distributed products or unassembled parts or product component inventories, which could interrupt the assembly process and delay shipments of our branded products, and could result in lost sales. In particular, we are seeking to reduce our procurement and inventory costs by matching our inventory closely with our projected product needs and by, from time to time, deferring our purchase of components in anticipation of supplier price reductions. As we seek to balance reduced inventory costs and assembly flexibility, we may fail to accurately forecast demand and coordinate our procurement and assembly to meet demand on a timely basis. Our inability to accurately predict our demand and to timely meet our demand could materially and adversely affect our financial conditions and results of operations as well as damage our reputation and corporate brand.
Failure to manage our growth could strain our management, operational and other resources, which could materially and adversely affect our business and prospects.
Our growth strategy includes building our brand, increasing market penetration of our existing products, developing new products, increasing our targeting of the home respiratory market in China, and increasing our exports. Pursuing these strategies has resulted in, and will continue to result in substantial demands on management resources. In particular, the management of our growth will require, among other things:
| • | continued enhancement of our research and development capabilities; |
| • | information technology system enhancement; |
| • | stringent cost controls and sufficient liquidity; |
| • | strengthening of financial and management controls and information technology systems; and |
| • | increased marketing, sales and support activities; and hiring and training of new personnel. |
If we are not able to manage our growth successfully, our business and prospects would be materially and adversely affected.
A redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company.
As described in greater detail in the sections entitled “Related Party Transactions – Make-Good Shares Subject to Redemption” and “Placement – Market and Pricing Considerations,” certain key members of our management have agreed to place, on a prorated basis, that number of common shares into escrow that is equal to 40% of the number of shares sold in this offering (such escrowed shares, the “Make-Good Shares”) pending determination of our audited net after-tax income for the year ending December 31, 2010. The Make-Good Shares will consist of a prorated allocation of shares beneficially owned by Ping Chen, Zheng (Rita) Liu, Weibing Yang, Jian Sun and Yong Wang, key members of our management team. Our company will redeem and cancel these Make-Good Shares pro rata without consideration to the extent necessary to cause our earnings per share to be at least $0.80, excluding any expenses associated with releasing the Make-Good Shares back to the original owners.
We cannot guarantee that we will be able to redeem a sufficient number of Make-Good Shares to increase audited after-tax earnings per share to the target of $0.80 if our company either has low net income or any net losses in 2010. To the extent there are an insufficient number of Make-Good Shares available for such redemption, our per-share after tax earnings may be less than the target of $0.80 for 2010.
16
Table of Contents
As noted above, the holders of the Make-Good Shares are integral to our company’s success. Prior to the commencement of this offering, they collectively own 58.61% of our issued and outstanding shares. Assuming a maximum offering, they would collectively hold approximately 39.07% of our shares upon completion of the offering. In the event all of the Make-Good Shares are redeemed, these members of management would collectively hold 23.44% of our shares, assuming a maximum offering. See “Risk Factors – We are substantially dependent upon our key personnel.”
Unauthorized use of our brand name by third parties, and the expenses incurred in developing and preserving the value of our brand name, may adversely affect our business.
We regard our brand name as critical to our success. Unauthorized use of our brand name by third parties may adversely affect our business and reputation, including the perceived quality and reliability of our branded products. We rely on trademark law, company brand name protection policies, and agreements with our employees, customers, business partners and others to protect the value of our brand name. Despite our precautions, we may be unable to prevent third parties from using our brand name without authorization. Moreover, litigation may be necessary in the future to protect our brand name. However, because the validity, enforceability and scope of protection of trademarks in the PRC are uncertain and still evolving, we may not be successful in prosecuting these cases. Future litigation could also result in substantial costs and diversion of our resources, and could disrupt our business, as well as have a material adverse effect on our financial condition and results of operations.
If we fail to obtain or maintain applicable regulatory clearances or approvals for our products, or if such clearances or approvals are delayed, we will be unable to commercially distribute and market our products at all or in a timely manner, which could significantly disrupt our business and materially and adversely affect our sales and profitability.
The sale and marketing of our products are subject to regulation in China. For a significant portion of our sales, we need to obtain and renew licenses and registrations with the SFDA. The processes for obtaining regulatory clearances or approvals can be lengthy and expensive, and the results are unpredictable. In addition, the relevant regulatory authorities may introduce additional requirements or procedures that have the effect of delaying or prolonging the regulatory clearance or approval for our existing or new products. If we are unable to obtain clearances or approvals needed to market existing or new branded products, or obtain such clearances or approvals in a timely fashion, our business would be significantly disrupted, and our sales and profitability could be materially and adversely affected. Similarly, if the third parties from which we buy our distributed products fail to obtain such clearance, we would be unable to sell such distributed products, and our sales and profitability could be materially and adversely affected.
We generate a significant portion of our revenues from a small number of products, and a reduction in demand for any of these products could materially and adversely affect our financial condition and results of operations.
We derive a substantial percentage of our revenues from a small number of products. Our three top selling product categories (mobile C arm X-ray system, general medical products and compressors) accounted, in the aggregate, for approximately 73%, 65%, 61% and 57% of our total net revenues in the years ended December 31, 2007 and 2008, and the nine months ended September 30, 2008 and 2009, respectively. We expect a small number of our key products will continue to account for a significant portion of our net revenues for the foreseeable future. As a result, continued market acceptance and popularity of these products is critical to our success, and a reduction in demand due to, among other factors, the introduction of competing products by our competitors, the entry of new competitors, or end-users’ dissatisfaction with the quality of these products could materially and adversely affect our financial condition and results of operations.
If we fail to protect our intellectual property rights, it could harm our business and competitive position.
We rely on a combination of patent, copyright, trademark and trade secret laws and non-disclosure agreements and other methods to protect our intellectual property rights. We own two patents in China covering our oxygen concentrators. We have applied for five additional patents related to our CPAP devices (2), portable sleep screening (2) and diagnostic services (1).
17
Table of Contents
The process of seeking patent protection can be lengthy and expensive, our patent applications may fail to result in patents being issued, and our existing and future patents may be insufficient to provide us with meaningful protection or commercial advantage. Our patents and patent applications may also be challenged, invalidated or circumvented.
We also rely on trade secret rights to protect our business through non-disclosure provisions in employment agreements with employees. If our employees breach their non-disclosure obligations, we may not have adequate remedies in China, and our trade secrets may become known to our competitors.
Implementation of PRC intellectual property-related laws has historically been lacking, primarily because of ambiguities in the PRC laws and enforcement difficulties. Accordingly, intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other western countries. Furthermore, policing unauthorized use of proprietary technology is difficult and expensive, and we may need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. Such litigation and an adverse determination in any such litigation, if any, could result in substantial costs and diversion of resources and management attention, which could harm our business and competitive position. See “Risk Factors – Unauthorized use of our brand name by third parties, and the expenses incurred in developing and preserving the value of our brand name, may adversely affect our business.”
We may be exposed to intellectual property infringement and other claims by third parties which, if successful, could disrupt our business and have a material adverse effect on our financial condition and results of operations.
Our success depends, in large part, on our ability to use and develop our technology and know-how without infringing third party intellectual property rights. If we sell our branded products internationally, and as litigation becomes more common in China, we face a higher risk of being the subject of claims for intellectual property infringement, invalidity or indemnification relating to other parties’ proprietary rights. Our current or potential competitors, many of which have substantial resources and have made substantial investments in competing technologies, may have or may obtain patents that will prevent, limit or interfere with our ability to make, use or sell our branded products in either China or other countries, including the United States and other countries in Asia. The validity and scope of claims relating to medical device technology patents involve complex scientific, legal and factual questions and analysis and, as a result, may be highly uncertain. In addition, the defense of intellectual property suits, including patent infringement suits, and related legal and administrative proceedings can be both costly and time consuming and may significantly divert the efforts and resources of our technical and management personnel. Furthermore, an adverse determination in any such litigation or proceedings to which we may become a party could cause us to:
| • | pay damage awards; |
| • | seek licenses from third parties; |
| • | pay ongoing royalties; |
| • | redesign our branded products; or |
| • | be restricted by injunctions, |
each of which could effectively prevent us from pursuing some or all of our business and result in our customers or potential customers deferring or limiting their purchase or use of our branded products, which could have a material adverse effect on our financial condition and results of operations.
We are subject to product liability exposure and have limited insurance coverage. Any product liability claims or potential safety-related regulatory actions could damage our reputation and materially and adversely affect our business, financial condition and results of operations.
The medical devices we assemble and sell can expose us to potential product liability claims if the use of these products causes or is alleged to have caused personal injuries or other adverse effects. Any product liability claim or regulatory action could be costly and time-consuming to defend. If successful, product liability claims may require us to pay substantial damages. We do not maintain product liability insurance to cover potential product liability arising from the use of our branded products because product liability insurance available in China offers only limited coverage compared to coverage offered in many other countries. As we expand our sales internationally and increase our exposure to these risks in many countries, we may be unable to obtain sufficient product liability
18
Table of Contents
insurance coverage on commercially reasonable terms, or at all. A product liability claim or potential safety-related regulatory action, with or without merit, could result in significant negative publicity and could materially and adversely affect the marketability of our branded products and our reputation, as well as our business, financial condition and results of operations.
Moreover, a material design, manufacturing or quality failure or defect in our branded products, other safety issues or heightened regulatory scrutiny could each warrant a product recall by us and result in increased product liability claims. Also, if these products are deemed by the authorities in the countries where we sell our branded products to fail to conform to product quality and safety requirements, we could be subject to regulatory action. In China, violation of PRC product quality and safety requirements may subject us to confiscation of related earnings, penalties, an order to cease sales of the violating product or to cease operations pending rectification. Furthermore, if the violation is determined to be serious, our business license to assemble or sell violating and other products could be suspended or revoked.
We may undertake acquisitions, which may have a material adverse effect on our ability to manage our business, and may end up being unsuccessful.
Our growth strategy may involve the acquisition of new technologies, businesses, products or services or the creation of strategic alliances in areas in which we do not currently operate. We do not have any understanding, commitment or agreement in place with regard to any such acquisitions at this time. These acquisitions could require that our management develop expertise in new areas, manage new business relationships and attract new types of customers. Furthermore, acquisitions may require significant attention from our management, and the diversion of our management’s attention and resources could have a material adverse effect on our ability to manage our business. We may also experience difficulties integrating acquisitions into our business and operations. Future acquisitions may also expose us to potential risks, including risks associated with:
| • | the integration of new operations, services and personnel; |
| • | unforeseen or hidden liabilities; |
| • | the diversion of resources from our existing businesses and technologies; our inability to generate sufficient revenue to offset the costs of acquisitions; and |
| • | potential loss of, or harm to, relationships with employees or customers, any of which could significantly disrupt our ability to manage our business and materially and adversely affect our business, financial condition and results of operations. |
We expect to allocate a portion of the net proceeds from this offering to such acquisitions, but we have not yet located any potential targets, and we may be unable to do so. Further, even if we find a target we believe to be suitable, we may be unable to negotiate acquisition terms that are satisfactory to us. In the event we are unable to complete acquisitions, we have reserved the right to reallocate such funds to our working capital. If this happens, we would have broad discretion over the ultimate us of such funds, and we could use such funds in ways with which investors might disagree.
We may need additional capital in the future, and we may be unable to obtain such capital in a timely manner or on acceptable terms, or at all.
In order for us to grow, remain competitive, develop new products, and expand our distribution network, we may require additional capital in the future. Our ability to obtain additional capital in the future is subject to a variety of uncertainties, including:
| • | our future financial condition, results of operations and cash flows; |
| • | general market conditions for capital raising activities by medical device manufacturers and other related companies; and |
| • | economic, political and other conditions in China and elsewhere. |
We may be unable to obtain additional capital in a timely manner or on acceptable terms or at all. Furthermore, the terms and amount of any additional capital raised through issuances of equity securities may result in significant shareholder dilution.
19
Table of Contents
We rely on technology and know-how, which we seek to protect, in part, by confidentiality provisions in contracts with our employees.
We rely on technology and know-how, which we seek to protect by confidentially provisions in contracts with our employees. There can be no assurance that the confidentially provisions in such contracts will not be breached, or that we will have adequate remedies for any breach, or that other parties may not obtain knowledge of our trade secrets and processes, technology and systems. Should these events occur, our business and hence, our profitability, will be adversely affected.
We are heavily dependent upon the services of experienced personnel who possess skills that are valuable in our industry, and we may have to actively compete for their services.
We are heavily dependent upon our ability to attract, retain and motivate skilled personnel to serve our customers. Many of our personnel possess skills that would be valuable to all companies engaged in our industry. Consequently, we expect that we will have to actively compete for these employees. Some of our competitors may be able to pay our employees more than we are able to pay to retain them. Our ability to profitably operate is substantially dependent upon our ability to locate, hire, train and retain our personnel. Although we have not experienced difficulty locating, hiring, training or retaining our employees to date, there can be no assurance that we will be able to retain our current personnel, or that we will be able to attract and assimilate other personnel in the future. If we are unable to effectively obtain and maintain skilled personnel, the development and quality of our services could be materially impaired. See “Our Business – Employees.”
If we experience a significant number of warranty claims, our costs could substantially increase and our reputation and brand could suffer.
We typically sell our branded products with warranty terms covering 12 months after purchase. Our branded product warranty requires us to repair all mechanical malfunctions and, if necessary, replace defective components. We accrue liability for potential warranty claims at the time of sale. If we experience an increase in warranty claims or if our repair and replacement costs associated with warranty claims increase significantly, we may have to accrue a greater liability for potential warranty claims. Moreover, an increase in the frequency of warranty claims could substantially increase our costs and harm our reputation and brand. Our business, financial condition, results of operations and prospects may suffer materially if we experience a significant increase in warranty claims on our branded products.
Foreign Operational Risks
Our operations could be adversely affected by changes in the political and economic conditions in the PRC.
We face risks related to conducting business in the PRC. Changes in the social, economic and political conditions of the PRC may adversely affect our business. Unfavorable changes in government policies, political unrest and economic developments may also have a negative impact on our operations.
Currently, all of our business operations are conducted in the PRC. Accordingly, any significant slowdown in the PRC economy may cause our customers to reduce expenditures. This may in turn lead to a decline in the demand for our products and services. That would have a material adverse effect on our business, financial condition and results of operations.
China’s economy experienced overall growth of only 9.6% in 2008, its lowest growth rate in seven years, down from a growth rate of 13% in 2007. The global financial crisis has significantly decreased demand for Chinese exports, resulting in factory closures and losses of millions of jobs. The falling demand for Chinese goods has caused the World Bank to predict economic growth of 7.2% for China in 2009, down 2.4% from the seven-year-low of 9.6% seen in 2008. This prediction is also 0.8% lower than the 8% rate the Chinese government had predicted and said would be necessary to avoid significant further job losses. While predictions for China’s growth rate have fallen, China’s growth rate remains well above the World Bank’s forecast for decline of 1.5% for the world economy in 2009.
Since the adoption of the “open door policy” in 1978 and the “socialist market economy” in 1993, the PRC government has been reforming and is expected to continue to reform its economic and political systems. Any changes in the political and economic policy of the PRC government may lead to changes in the laws and
20
Table of Contents
regulations or the interpretation of the same, as well as changes in the foreign exchange regulations, taxation and import and export restrictions, which may in turn adversely affect our financial performance. While the current policy of the PRC government seems to be one of imposing economic reform policies to encourage foreign investments and greater economic decentralization, there is no assurance that such a policy will continue to prevail in the future.
Our failure to obtain the prior approval of the China Securities Regulatory Commission of the listing and trading of our common shares on the NASDAQ Capital Market could have a material adverse effect on our business, operating results, reputation and trading price of our common shares, and may also create uncertainties for this offering.
On August 8, 2006, six PRC regulatory agencies, including the China Securities Regulatory Commission (“CSRC”), promulgated a regulation that became effective on September 8, 2006. This regulation, among other things, has some provisions that purport to require that an offshore special purpose vehicle, or SPV, formed for listing purposes and controlled directly or indirectly by PRC companies or individuals shall obtain the approval of the CSRC prior to the listing and trading of such SPV’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published on its official website procedures specifying documents and materials required to be submitted to it by SPVs seeking CSRC approval of their overseas listings.
We did not seek CSRC approval in connection with our initial public offering or this offering. However, the application of this PRC regulation remains unclear with no consensus currently existing among the leading PRC law firms regarding the scope and applicability of the CSRC approval requirement.
Our PRC counsel, Beijing Kang Da Law Firm, has advised us that because we completed our restructuring before September 8, 2006, the effective date of the new regulation, it was not and is not necessary for us to submit the application to the CSRC for its approval, and the listing and trading of our common shares on the NASDAQ Capital Market does not require CSRC approval. A copy of Beijing Kang Da Law Firm’s legal opinion regarding this PRC regulation is filed as an exhibit to our registration statement on Form S-1 in connection with this offering, which is available at the SEC’s website at www.sec.gov.
If the CSRC or another PRC regulatory agency subsequently determines that CSRC approval was required for our initial public offering or this offering, we may face regulatory actions or other sanctions from the CSRC or other PRC regulatory agencies. These regulatory agencies may impose fines and penalties on our operations in the PRC, limit our operating privileges in the PRC, delay or restrict the repatriation of the proceeds from our initial public offering into the PRC, or take other actions that could have a material adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our common shares. The CSRC or other PRC regulatory agencies also may take actions requiring us, or making it advisable for us, to halt this offering before settlement and delivery of the common shares offered hereby. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that settlement and delivery may not occur.
Also, if later the CSRC requires that we obtain its approval, we may be unable to obtain a waiver of the CSRC approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties and/or negative publicity regarding this CSRC approval requirement could have a material adverse effect on the trading price of our common shares.
We may become a passive foreign investment company, which could result in adverse U.S. tax consequences to U.S. investors.
Based upon the nature of our business activities, we may be classified as a passive foreign investment company (“PFIC”), by the U.S. Internal Revenue Service (“IRS”), for U.S. federal income tax purposes. Such characterization could result in adverse U.S. tax consequences to you if you are a U.S. investor. For example, if we are a PFIC, a U.S. investor will become subject to burdensome reporting requirements. The determination of whether or not we are a PFIC is made on an annual basis and will depend on the composition of our income and assets from time to time. Specifically, we will be classified as a PFIC for U.S. tax purposes if either:
| • | 75% or more of our gross income in a taxable year is passive income; or |
| • | the average percentage of our assets by value in a taxable year which produce or are held for the production of passive income (which includes cash) is at least 50%. |
21
Table of Contents
The calculation of the value of our assets is based, in part, on the then market value of our common shares, which is subject to change. In addition, the composition of our income and assets will be affected by how, and how quickly, we spend the cash we raise in this offering. We cannot assure you that we will not be a PFIC for any taxable year. See “Taxation – United States Federal Income Taxation – Passive Foreign Investment Company.”
We may be subject to foreign exchange controls in the PRC.
Our PRC subsidiary and affiliates are subject to PRC rules and regulations on currency conversion. In the PRC, the State Administration for Foreign Exchange (“SAFE”) regulates the conversion of the RMB into foreign currencies. Currently, foreign investment enterprises (“FIEs”) are required to apply to SAFE for “Foreign Exchange Registration Certificate for FIEs.” BDL is a FIE. With such registration certifications (which need to be renewed annually), FIEs are allowed to open foreign currency accounts including the “recurrent account” and the “capital account.” Currently, conversion within the scope of the “recurrent account” can be effected without requiring the approval of SAFE. However, conversion of currency in the “capital account” (e.g. for capital items such as direct investments, loans, securities, etc.) still requires the approval of SAFE.
We do not have business interruption, litigation or natural disaster insurance.
The insurance industry in China is still at an early state of development. In particular PRC insurance companies offer limited business products. As a result, we do not have any business liability or disruption insurance coverage for our operations in China. Any business interruption, litigation or natural disaster may result in our business incurring substantial costs and the diversion of resources.
The newly enacted Chinese enterprise income tax law will affect tax exemptions on the dividends we receive and increase the enterprise income tax rate applicable to us.
We are a holding company incorporated under the laws of the British Virgin Islands. We conduct substantially all of our business through our wholly owned Chinese subsidiaries and we derive all of our income from these subsidiaries. Prior to January 1, 2008, dividends derived by foreign legal persons from business operations in China were not subject to the Chinese enterprise income tax.
On March 16, 2007, the National People’s Congress of the PRC passed the PRC Enterprise Income Tax Law (the “EIT Law”), which took effect on January 1, 2008. Such tax exemptions ceased with the effectiveness of the EIT Law.
Under the EIT Law, if we are deemed to be a non-resident enterprise for Chinese tax purposes, a withholding tax at the rate of 10% would be applicable to any dividends paid by our Chinese subsidiaries to us. However, if we are deemed to have a “de facto management organization” in China, we would be classified as a resident enterprise for Chinese tax purposes and thus would be subject to an enterprise income tax rate of 25% on all of our income, including interest income on the proceeds from this offering on a worldwide basis. At present, the Chinese tax authority has not issued any guidance on the application of the EIT Law and its implementing rules on non-Chinese enterprises or group enterprise controlled entities whose structures are like ours. As a result, it is unclear what factors will be used by the Chinese tax authorities to determine whether we are a “de facto management organization” in China. However, as substantially all members of our management team are located in China, we may be deemed to be a resident enterprise and therefore subject to an enterprise income tax rate of 25% on our worldwide income, with the possible exclusion of dividends received directly from another Chinese tax resident. As a result of such changes, our historical operating results will not be indicative of our operating results for future periods and the value of our shares may be adversely affected.
We must remit the offering proceeds to China before they may be used to benefit our business in China, and this process may take a number of months.
BDL is reported to PRC authorities as a special purpose vehicle for financing. The proceeds of this offering must be sent back to the PRC, and the process for sending such proceeds back to the PRC may take several months after the closing of this offering. We may be unable to use these proceeds to grow our business until we receive such proceeds in the PRC. In order to remit the offering proceeds to China, we will take the following actions:
| • | First, we will open a special foreign exchange account for capital account transactions. To open this account, we must submit to SAFE certain application forms, identity documents, transaction documents, form of foreign exchange registration of overseas investments of the domestic residents, and foreign exchange registration certificate of the invested company. |
22
Table of Contents
| • | Second, we will remit the offering proceeds into this special foreign exchange account. |
| • | Third, we will apply for settlement of the foreign exchange. In order to do so, we must submit to SAFE certain application forms, identity documents, payment order to a designated person, and a tax certificate. |
The timing of the process is difficult to estimate because the efficiencies of different SAFE branches can vary significantly. Ordinarily the process takes several months but is required to be accomplished within 180 days of application by law.
We rely on dividends paid by BDL for our cash needs.
We rely primarily on dividends paid by BDL for our cash needs, including the funds necessary to pay dividends and other cash distributions, if any, to our shareholders, to service any debt we may incur and to pay our operating expenses. The payment of dividends by entities organized in China is subject to limitations. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. Dividend policy is subject to the discretion of our Board of Directors and will depend on, among other things, our future earnings, financial condition, capital requirements and other factors. Under British Virgin Islands law, we may only pay dividends from surplus (the excess, if any, at the time of the determination of the total assets of our company over the sum of our liabilities, as shown in our books of account, plus our capital), and we must be solvent before and after the dividend payment in the sense that we will be able to satisfy our liabilities as they become due in the ordinary course of business; and the realizable value of assets of our company will not be less than the sum of our total liabilities, other than deferred taxes as shown on our books of account, and our capital. If we determine to pay dividends on any of our common shares in the future, as a holding company, we will be dependent on receipt of funds from our operating subsidiary, BDL. See “Dividend Policy.”
Pursuant to the new PRC enterprise income tax law effective on January 1, 2008, dividends payable by a foreign investment entity to its foreign investors are subject to a withholding tax of up to 20%. At present, the Chinese tax authority has not issued any guidance on the application of the EIT Law and its implementing rules on non-Chinese enterprises or group enterprise controlled entities whose structures are like ours. In practice, the tax authorities typically impose the withholding tax rate of 10% rate, as prescribed in the implementation regulations; however, there can be no guarantee that this practice will continue as more guidance is provided by relevant government authorities. As a result, we are unable to predict whether payments from BDL to Dehaier will be subject to withholding tax because it is unclear whether Dehaier will be deemed to be a resident enterprise for Chinese tax purposes. If so, Dehaier will be subject to an enterprise income tax rate of 25% on all of its income, including interest income on the proceeds from this offering on a worldwide basis. However, if Dehaier is deemed to be a non-resident enterprise, then it will be subject to a withholding tax at the rate of 10% on any dividends paid by its Chinese subsidiaries to Dehaier.
The payment of dividends by entities organized in China is subject to limitations, procedures and formalities. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. BDL is also required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its compulsory reserves fund until the accumulative amount of such reserves reaches 50% of its registered capital.
The transfer to this reserve must be made before distribution of any dividend to shareholders. The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 25% of the registered capital. For the years ended December 31, 2008 and the years ended December 31, 2007, respectively, the Company made no appropriations to surplus reserve.
23
Table of Contents
BDL is also required to allocate a portion of its after-tax profits, as determined by its board of directors, to the general reserve, the staff welfare and bonus funds, and the enterprise expansion reserve, which may not be distributed to equity owners.
Pursuant to the Law of Chinese-Foreign Equity Joint Ventures, Chinese-foreign equity joint ventures are required to allocate a portion of their after-tax profits in accordance with their Articles of Association, to the general reserve, the staff welfare and bonus funds, and the enterprise expansion reserve. According to the Articles of Association of BDL, the amount of each reserve is determined by BDL’s board of directors. The general reserve is used to offset future extraordinary losses. The subsidiaries may, upon a resolution passed by the shareholders, convert the general reserve into capital. The employee welfare and bonus reserve is used for the collective welfare of the employees of the subsidiaries. The enterprise expansion reserve is used for the expansion of the subsidiaries’ operations and can be converted to capital subject to approval by the relevant authorities. These reserves represent appropriations of retained earnings determined according to PRC law.
As of the date of this prospectus, the amounts of these reserves have not yet been determined, and we have not committed to establishing such amounts at this time. Under current PRC laws, BDL is required to set aside reserve amounts, but has not yet done so. BDL has not done so because PRC authorities grant companies flexibility in making a determination. Chinese law requires such a determination to be made in accordance with the companies’ organizational documents and BDL’s organizational documents do not require the determination to be made within a particular timeframe. Although we have not yet been required by PRC authorities to make such determinations or set aside such reserves, PRC authorities may require BDL to rectify its noncompliance and we may be fined if we fail to do so after warning within the time period set in the warning.
PRC law requires allocation to the general reserve before distribution of the after-tax profits of foreign invested companies, which could prevent us from receiving the dividends from BDL.
PRC law requires that the after-tax profits of foreign invested companies be distributed after a portion of after-tax profits is allocated to the reserve; therefore if for any reason, the dividends from BDL cannot be repatriated to us or not in time, then it may detrimentally affect our cash flow and even cause us to become insolvent.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. We receive the majority of our revenues in Renminbi. Under our current corporate structure, our income is derived from payments from BDL. Shortages in the availability of foreign currency may restrict the ability of BDL to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency denominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from SAFE by complying with certain procedural requirements. However, approval from appropriate government authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of bank loans denominated in foreign currencies. The PRC government may also at is discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay dividends in foreign currencies to our shareholders. See “Our Business – Regulations on Foreign Exchange.”
Fluctuation of the Renminbi could materially affect our financial condition and results of operations.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in an appreciation of the Renminbi against the U.S. dollar. While the international reaction to the Renminbi revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. dollar. Any significant revaluation of Renminbi may materially and adversely affect our cash flows, revenues, earnings and financial position, and the value of, and any dividends payable on, our common shares in U.S. dollars. For example, an appreciation of Renminbi against the U.S. dollar would make any new Renminbi denominated investments or expenditures more costly to us, to the extent that we need to convert U.S. dollars into Renminbi for such purposes. See “Exchange Rate Information.”
Changes in China’s political and economic policies could harm our business.
China’s economy has historically been a planned economy subject to governmental plans and quotas and has, in certain aspects, been transitioning to a more market-oriented economy. Although we believe that the economic reform and the macroeconomic measures adopted by the Chinese government have had a positive effect on the
24
Table of Contents
economic development of China, we cannot predict the future direction of these economic reforms or the effects these measures may have on our business, financial position or results of operations. In addition, the Chinese economy differs from the economies of most countries belonging to the Organization for Economic Cooperation and Development (“OECD”). These differences include:
| • | economic structure; |
| • | level of government involvement in the economy; |
| • | level of development; |
| • | level of capital reinvestment; |
| • | control of foreign exchange; |
| • | methods of allocating resources; and |
| • | balance of payments position. |
As a result of these differences, our business may not develop in the same way or at the same rate as might be expected if the Chinese economy were similar to those of the OECD member countries. See “Our Business – Market Background.”
If PRC law were to phase out the preferential tax benefits currently being extended to certified high technology companies or if we were to fail to be certified to receive such a benefit, we would have to pay more taxes, which could have a material and adverse effect on our financial condition and results of operations.
Under PRC laws and regulations, a company may enjoy preferential tax benefits if it is certified as a high technology enterprise. As a certified high technology enterprise, we are subject to an enterprise income tax rate of 15% tax rate so long as we continue to be so certified. If the PRC law were to phase out preferential tax benefits currently granted to certified high technology enterprises or if we were to fail to be certified to receive such a benefit, we would be subject to the standard statutory tax rate, which currently is 25%.
If relations between the United States and China worsen, our share price may decrease and we may have difficulty accessing U.S. capital markets.
At various times during recent years, the United States and China have had disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China could adversely affect the market price of our common shares and our ability to access U.S. capital markets.
The Chinese government could change its policies toward private enterprise or even nationalize or expropriate private enterprises, which could result in the total loss of our investment in that country.
Our business is subject to significant political and economic uncertainties and may be adversely affected by political, economic and social developments in China. Over the past several years, the Chinese government has pursued economic reform policies including the encouragement of private economic activity and greater economic decentralization. The Chinese government may not continue to pursue these policies or may significantly alter them to our detriment from time to time with little, if any, prior notice.
Changes in policies, laws and regulations or in their interpretation or the imposition of confiscatory taxation, restrictions on currency conversion, restrictions or prohibitions on dividend payments to shareholders, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on our business. Nationalization or expropriation could even result in the total loss of our investment in China and in the total loss of your investment in us.
The PRC legal system embodies uncertainties that could limit the legal protections available to you and us.
The PRC legal system is a civil law system based on written statutes. Unlike common law systems, it is a system in which decided legal cases have limited precedential value. In 1979, the PRC government began to promulgate a comprehensive system of laws and regulations governing economic matters in general. The overall effect of legislation over the past three decades has significantly increased the protections afforded to various forms of foreign investment in China. Our PRC operating subsidiary, BDL, is a foreign-invested enterprise and is subject to laws and regulations applicable to foreign investment in China as well as laws and regulations applicable to foreign-invested enterprises. These laws and regulations change frequently, and their interpretation and enforcement involve
25
Table of Contents
uncertainties. For example, we may have to resort to administrative and court proceedings to enforce the legal protections that we enjoy either by law or contract. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties may also impede our ability to enforce the contracts we have entered into. As a result, these uncertainties could materially and adversely affect our business and operations.
Recent PRC regulations relating to offshore investment activities by PRC residents may increase the administrative burden we face and create regulatory uncertainties that could restrict our overseas and cross-border investment activity, and a failure by our shareholders who are PRC residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our PRC resident shareholders to liability under PRC law.
In October 2005, SAFE promulgated regulations that require PRC residents and PRC corporate entities to register with and obtain approvals from relevant PRC government authorities in connection with their direct or indirect offshore investment activities. These regulations apply to our shareholders who are PRC residents in connection with our prior and any future offshore acquisitions.
The October 2005 SAFE regulation required registration by March 31, 2006 of direct or indirect investments previously made by PRC residents in offshore companies prior to the implementation of the Notice on Issues Relating to the Administration of Foreign Exchange in Fund-Raising and Reverse Investment Activities of Domestic Residents Conducted via Offshore Special Purpose Companies on November 1, 2005. If a PRC shareholder with a direct or indirect stake in an offshore parent company fails to make the required SAFE registration, the PRC subsidiaries of such offshore parent company may be prohibited from making distributions of profit to the offshore parent and from paying the offshore parent proceeds from any reduction in capital, share transfer or liquidation in respect of the PRC subsidiaries. Furthermore, failure to comply with the various SAFE registration requirements described above could result in liability under PRC law for foreign exchange evasion.
We previously notified and urged our shareholders, and the shareholders of the offshore entities in our corporate group, who are PRC residents to make the necessary applications and filings, as required under this regulation. However, as these regulations are relatively new and there is uncertainty concerning their reconciliation with other approval requirements, it is unclear how they, and any future legislation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant government authorities. While we believe that these shareholders submitted applications with local SAFE offices, some of our shareholders may not comply with our request to make or obtain any applicable registrations or approvals required by the regulation or other related legislation. The failure or inability of our PRC resident shareholders to obtain any required approvals or make any required registrations may subject us to fines and legal sanctions, prevent us from being able to make distributions or pay dividends, as a result of which our business operations and our ability to distribute profits to you could be materially and adversely affected.
Because our operations are located in China, information about our operations are not readily available from independent third-party sources.
Because the BDL is based in China, our shareholders may have greater difficulty in obtaining information about it on a timely basis than would shareholders of a U.S.-based company. BDL’s operations will continue to be conducted in China and shareholders may have difficulty in obtaining information about it from sources other than BDL itself. Information available from newspapers, trade journals, or local, regional or national regulatory agencies such as issuance of construction permits and contract awards for development projects will not be readily available to shareholders and, where available, will likely be available only in Chinese. Shareholders will be dependent upon management for reports of their progress, development, activities and expenditure of proceeds.
Risks Associated with this Offering
There may not be an active, liquid trading market for our common shares.
Prior to this offering, there has been no public market for our common shares. An active trading market for our common shares may not develop or be sustained following this offering. You may not be able to sell your shares at the market price, if at all, if trading in our shares is not active. The initial public offering price was determined by negotiations between us and the placement agent based upon a number of factors. The initial public offering price may not be indicative of prices that will prevail in the trading market.
26
Table of Contents
Investors risk loss of use of funds allocated for purchases, with no right of return, during the offering period.
We cannot assure you that all or any shares will be sold. Anderson & Strudwick, our placement agent, is offering our shares on a “best efforts, minimum-maximum basis.” We have no firm commitment from anyone to purchase all or any of the shares offered. If offers to purchase a minimum of 1,250,000 shares are not received on or before May 31, 2010, escrow provisions require that all funds received be promptly refunded. If refunded, investors will receive no interest on their funds. During the offering period, investors will not have any use or right to return of the funds. None of our officers, directors or affiliates may purchase shares in this offering.
The market price for our common shares may be volatile, which could result in substantial losses to investors.
The market price for our common shares is likely to be volatile and subject to wide fluctuations in response to factors including the following:
| • | actual or anticipated fluctuations in our quarterly operating results; |
| • | changes in the Chinese economy; |
| • | announcements by our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | additions or departures of key personnel; or |
| • | potential litigation. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. As a result, to the extent shareholders sell our shares in negative market fluctuation, they may not receive a price per share that is based solely upon our business performance. We cannot guarantee that shareholders will not lose some of their entire investment in our common shares.
We have applied to list our common shares for trading on the NASDAQ Capital Market under an objective net income test. We currently meet this test, but if our financial condition deteriorates or if we experience a significantly protracted registration and offering period, we may not meet initial listing standards on the NASDAQ Capital Market.
We have applied to list our common shares for trading on the NASDAQ Capital Market. We have not yet been informed that our common shares will trade on the NASDAQ Capital Market and can provide no assurance that our NASDAQ Capital Market listing application will be approved. Additionally, we will not complete this offering unless our application to list on the NASDAQ Capital Market is approved. In order to qualify for initial listing on the NASDAQ Capital Market upon the completion of this offering, we must meet the following criteria:
| • | (i) We must have been in operation for at least two years, must have shareholder equity of at least $5,000,000 and must have a market value for our publicly held securities of at least $15,000,000; or (ii) we must have shareholder equity of at least $4,000,000, must have a market value for our publicly held securities of at least $15,000,000 and must have a market value of our listed securities of at least $50,000,000; OR (iii) we must have net income from continuing operations in our last fiscal year (or two of the last three fiscal years) of at least $750,000, must have shareholder equity of at least $4,000,000 and must have a market value for our publicly held securities of at least $5,000,000; and |
| • | The market value of our shares held by non-affiliates must be at least $1,000,000; |
| • | The market value of our shares must be at least $5,000,000; |
| • | The minimum bid price for our shares must be at least $4.00 per share; |
| • | We must have at least 300 round-lot shareholders; |
| • | We must have at least 3 market makers; and |
| • | We must have adopted NASDAQ-mandated corporate governance measures, including a Board of Directors comprised of a majority of independent directors, an Audit Committee comprised solely of independent directors and the adoption of a code of ethics among other items. |
Although we have applied to have our common shares trade on the NASDAQ Capital Market upon closing of this offering, investors should be aware that they will be required to commit their investment funds prior to the approval or disapproval of our listing application by the NASDAQ Capital Market. We will not close this offering unless our listing application is approved.
In addition, we have relied on an exemption to the blue sky registration requirements afforded to “covered securities”. Securities listed on the NASDAQ Capital Market are “covered securities.” If we were unable to meet the NASDAQ Capital Market’s listing standards, then we would be unable to rely on the covered securities exemption to blue sky registration requirements and we would need to register the offering in each state in which we planned to sell shares. Consequently, we will not complete this offering unless we meet the NASDAQ Capital Market’s listing requirements.
If we are listed on the NASDAQ Capital Market and our financial condition deteriorates, we may not meet continued listing standards on the NASDAQ Capital Market.
The NASDAQ Capital Market also requires companies to fulfill specific requirements in order for their shares to continue to be listed. In order to qualify for continued listing on the NASDAQ Capital Market, we must meet the following criteria:
| • | Our shareholders’ equity must be at least $2,500,000; or the market value of our listed securities must be at least $35,000,000; or our net income from continuing operations in our last fiscal year (or two of the last three fiscal years) must have been at least $500,000; |
27
Table of Contents
| • | The market value of our shares held by non-affiliates must be at least $500,000; |
| • | The market value of our shares must be at least $1,000,000; |
| • | The minimum bid price for our shares must be at least $1.00 per share; |
| • | We must have at least 300 shareholders; |
| • | We must have at least 2 market makers; and |
| • | We must have adopted NASDAQ-mandated corporate governance measures, including a Board of Directors comprised of a majority of independent directors, an Audit Committee comprised solely of independent directors and the adoption of a code of ethics among other items. |
If our shares are listed on the NASDAQ Capital Market but are delisted from the NASDAQ Capital Market at some later date, our shareholders could find it difficult to sell our shares. In addition, if our common shares are delisted from the NASDAQ Capital Market at some later date, we may apply to have our common shares quoted on the Bulletin Board or in the “pink sheets” maintained by the National Quotation Bureau, Inc. The Bulletin Board and the “pink sheets” are generally considered to be less efficient markets than the NASDAQ Capital Market. In addition, if our common shares are not so listed or is delisted at some later date, our common shares may be subject to the “penny stock” regulations. These rules impose additional sales practice requirements on broker-dealers that sell low-priced securities to persons other than established customers and institutional accredited investors and require the delivery of a disclosure schedule explaining the nature and risks of the penny stock market. As a result, the ability or willingness of broker-dealers to sell or make a market in our common shares might decline. If our common shares are not so listed or are delisted from the NASDAQ Capital Market at some later date or were to become subject to the penny stock regulations, it is likely that the price of our shares would decline and that our shareholders would find it difficult to sell their shares.
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, as well as new rules subsequently implemented by the SEC and NASDAQ, have required changes in corporate governance practices of public companies. We expect these new rules and regulations to significantly increase our legal, accounting and financial compliance costs and to make certain corporate activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements.
Our classified board structure may prevent a change in our control.
Our board of directors is divided into three classes of directors. The current terms of the directors expire in 2010, 2011 and 2012. Directors of each class are chosen for three-year terms upon the expiration of their current terms, and each year one class of directors is elected by the shareholders. The staggered terms of our directors may reduce the possibility of a tender offer or an attempt at a change in control, even though a tender offer or change in control might be in the best interest of our shareholders. See “Management – Board of Directors and Board Committees.”
28
Table of Contents
Shares eligible for future sale may adversely affect the market price of our common shares, as the future sale of a substantial amount of outstanding common shares in the public marketplace could reduce the price of our common shares.
The market price of our shares could decline as a result of sales of substantial amounts of our shares in the public market, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of our common shares. An aggregate of 3,000,000 shares will be outstanding before the consummation of this offering and 4,500,000 shares will be outstanding immediately after this offering, if the maximum offering is raised. All of the shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act. The remaining shares will be “restricted securities” as defined in Rule 144. These shares may be sold in the future without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act. See “Shares Eligible for Future Sale.”
You will experience immediate and substantial dilution.
The initial public offering price of our shares is expected to be substantially higher than the pro forma net tangible book value per share of our common shares. Therefore, assuming the completion of the maximum offering, if you purchase shares in this offering, you will incur immediate dilution of approximately $3.46 or approximately 43.25% in the pro forma net tangible book value per share from the price per share that you pay for the common shares. Assuming the completion of the minimum offering, if you purchase shares in this offering, you will incur immediate dilution of approximately $3.62 or approximately 45.25% in the pro forma net tangible book value per share from the price per share that you pay for the shares. Accordingly, if you purchase shares in this offering, you will incur immediate and substantial dilution of your investment. See “Dilution.”
We have not determined a specific use for a significant portion of the proceeds from this offering, and we may use the proceeds in ways with which you may not agree.
Our management and board of directors will have considerable discretion in the application of the net proceeds received by us. In addition, in the event we are unable to locate favorable targets for acquisitions, we have reserved the right to re-allocate funds currently allocated to that purpose to our general working capital. If that were to happen, then our management would have significant discretion over even more of the net proceeds to be received by our company in this offering. You will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate purposes that do not improve our efforts to achieve profitability or increase our share price. The net proceeds from this offering may be placed in investments that do not produce income or that lose value. See “Use of Proceeds.”
Entities controlled by our employees, officers, management and/or directors will control a significant percentage of our common shares, decreasing your influence on shareholder decisions.
Assuming the sale of the maximum offering, entities controlled by our employees, officers, management and/or directors will, in the aggregate, beneficially own approximately 39.07% of our outstanding shares. Assuming the sale of the minimum offering, entities controlled by our employees, officers, management and/or directors will, in the aggregate, beneficially own approximately 41.37% of our outstanding common shares. (Both numbers assume no redemption of shares under the Make-Good Share Escrow described under the sections entitled “Related Party Transactions – Make-Good Shares Subject to Redemption” and “Placement – Market and Pricing Considerations.”) As a result, our employees, officers and directors will possess substantial ability to impact our management and affairs and the outcome of matters submitted to shareholders for approval. These shareholders, acting individually or as a group, could exert control and substantial influence over matters such as electing directors and approving mergers or other business combination transactions. This concentration of ownership and voting power may also discourage, delay or prevent a change in control of our company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and might reduce the price of our common shares. These actions may be taken even if they are opposed by our other shareholders, including those who purchase shares in this offering. See “Principal Shareholders.”
29
Table of Contents
We will have an ongoing relationship with our placement agent that may impact our ability to obtain additional capital.
In connection with this offering, we will, for a nominal amount, sell our placement agent warrants exercisable at a rate of one warrant per share to purchase up to ten percent of the shares sold in the offering. These warrants are exercisable for a period of five years from the date of issuance at a price equal to 125% of the price of the shares in this offering. During the term of the warrants, the holders thereof will be given the opportunity to profit from a rise in the market price of our common shares, with a resulting dilution in the interest of our other shareholders. The term on which we could obtain additional capital during the life of these warrants may be adversely affected because the holders of these warrants might be expected to exercise them when we are able to obtain any needed additional capital in a new offering of securities at a price greater than the exercise price of the warrants. See “Placement.”
We will have an ongoing relationship with our placement agent that may impact our shareholders’ ability to impact decisions related to our operations.
In connection with this offering, we have agreed to allow our placement agent to designate two (2) non-voting observers to our Board of Directors until the earlier of the date that:
| (i) | the investors that purchase shares in this offering beneficially own less than ten percent (10%) of our outstanding shares; or |
| (ii) | the trading price per share is at least three (3) times the offering price for any consecutive 15 trading day period. |
Although our placement agent’s observers will not be able to vote, they may nevertheless be in position to influence the outcome of matters submitted to the Board of Directors for approval by virtue of their presence at such meetings and availability to provide opinions to the Board. We have agreed to reimburse the observers for their expenses for attending our Board meetings, subject to a maximum reimbursement of $6,000 per meeting and $12,000 annually per observer, such amount being not more than the reimbursement to be received by any of our directors. We will also pay our observers the same amount of compensation as our independent directors receive. As of the date of this prospectus, Mr. L. McCarthy Downs III and Mr. Ming Zhu are serving as our placement agent’s observers to our Board of Directors. See “Management – Board of Directors Observers.”
As the rights of shareholders under British Virgin Islands law differ from those under U.S. law, you may have fewer protections as a shareholder.
Our corporate affairs will be governed by our fourth amended and restated memorandum and articles of association, the British Virgin Islands Business Companies Act, 2004 (the “BVI Act”), and the common law of the British Virgin Islands. The rights of shareholders to take legal action against our directors, actions by minority shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law are to a large extent governed by the common law of the British Virgin Islands and by the BVI Act. The common law of the British Virgin Islands is derived in part from comparatively limited judicial precedent in the British Virgin Islands as well as from English common law, which has persuasive, but not binding, authority on a court in the British Virgin Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law are not as clearly established as they would be under statutes or judicial precedents in some jurisdictions in the United States. In particular, the British Virgin Islands has a less developed body of securities laws as compared to the United States, and some states (such as Delaware) have more fully developed and judicially interpreted bodies of corporate law.
As a result of all of the above, holders of our shares may have more difficulty in protecting their interests through actions against our management, directors or major shareholders than they would as shareholders of a U.S. company. For a discussion of significant differences between the provisions of the BVI Act and the laws applicable to companies incorporated in the United States and their shareholders, see “Description of Share Capital – Differences in Corporate Law.”
British Virgin Islands companies may not be able to initiate shareholder derivative actions, thereby depriving shareholders of the ability to protect their interests.
British Virgin Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States. The circumstances in which any such action may be brought, and the procedures and defenses that may be available in respect to any such action, may result in the rights of shareholders of a British Virgin
30
Table of Contents
Islands company being more limited than those of shareholders of a company organized in the United States. Accordingly, shareholders may have fewer alternatives available to them if they believe that corporate wrongdoing has occurred. The British Virgin Islands courts are also unlikely to recognize or enforce against us judgments of courts in the United States based on certain liability provisions of U.S. securities law; and to impose liabilities against us, in original actions brought in the British Virgin Islands, based on certain liability provisions of U.S. securities laws that are penal in nature. There is no statutory recognition in the British Virgin Islands of judgments obtained in the United States, although the courts of the British Virgin Islands will generally recognize and enforce the non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits. This means that even if shareholders were to sue us successfully, they may not be able to recover anything to make up for the losses suffered.
The laws of the British Virgin Islands provide little protection for minority shareholders, so minority shareholders will have little or no recourse if the shareholders are dissatisfied with the conduct of our affairs.
Under the law of the British Virgin Islands, there is little statutory law for the protection of minority shareholders other than the provisions of the BVI Act dealing with shareholder remedies. The principal protection under statutory law is that shareholders may bring an action to enforce the constituent documents of the corporation, our fourth amended and restated memorandum and articles of association. Shareholders are entitled to have the affairs of the company conducted in accordance with the general law and the articles and memorandum.
There are common law rights for the protection of shareholders that may be invoked, largely dependent on English company law, since the common law of the British Virgin Islands for business companies is limited. Under the general rule pursuant to English company law known as the rule in Foss v. Harbottle, a court will generally refuse to interfere with the management of a company at the insistence of a minority of its shareholders who express dissatisfaction with the conduct of the company’s affairs by the majority or the board of directors. However, every shareholder is entitled to have the affairs of the company conducted properly according to law and the constituent documents of the corporation. As such, if those who control the company have persistently disregarded the requirements of company law or the provisions of the company’s memorandum and articles of association, then the courts will grant relief. Generally, the areas in which the courts will intervene are the following: (1) an act complained of which is outside the scope of the authorized business or is illegal or not capable of ratification by the majority; (2) acts that constitute fraud on the minority where the wrongdoers control the company; (3) acts that infringe on the personal rights of the shareholders, such as the right to vote; and (4) where the company has not complied with provisions requiring approval of a special or extraordinary majority of shareholders, which are more limited than the rights afforded minority shareholders under the laws of many states in the United States.
31
Table of Contents
We have made statements in this prospectus, including under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Our Business” and elsewhere that constitute forward-looking statements. Forward-looking statements involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “should,” “could” and similar expressions. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements.
Examples of forward-looking statements include:
| • | the timing of the development of future products; |
| • | projections of revenue, earnings, capital structure and other financial items; |
| • | statements of our plans and objectives; |
| • | statements regarding the capabilities of our business operations; |
| • | statements of expected future economic performance |
| • | statements regarding competition in our market; and |
| • | assumptions underlying statements regarding us or our business. |
The ultimate correctness of these forward-looking statements depends upon a number of known and unknown risks and events. We discuss our known material risks under the heading “Risk Factors” above. Many factors could cause our actual results to differ materially from those expressed or implied in our forward-looking statements. Consequently, you should not place undue reliance on these forward-looking statements.
The forward-looking statements speak only as of the date on which they are made, and, except as required by law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
32
Table of Contents
Corporate Structure
We were incorporated as an international business company under the International Business Companies Act, 1984, in the British Virgin Islands on July 22, 2003 under the name “De-Haier Medical Systems Limited.” We changed our name to “Dehaier Medical Systems Limited” on June 3, 2005. On September 24, 2003, we established BDL. On October 15, 2003, we founded DHK and created a holding company structure by which we are the parent company of BDL and DHK. BDL has been focused on the development and distribution of medical devices since its inception and began developing its respiratory and oxygen homecare business in 2006.
The following diagram illustrates our current corporate structure as of the date of this prospectus. Further discussion of the history of our company from our chief executive officer’s initial founding in 2001 of our affiliate, BTL, through our two rounds of private fundraising, may be found in the section titled “Our Business – Corporate History.”
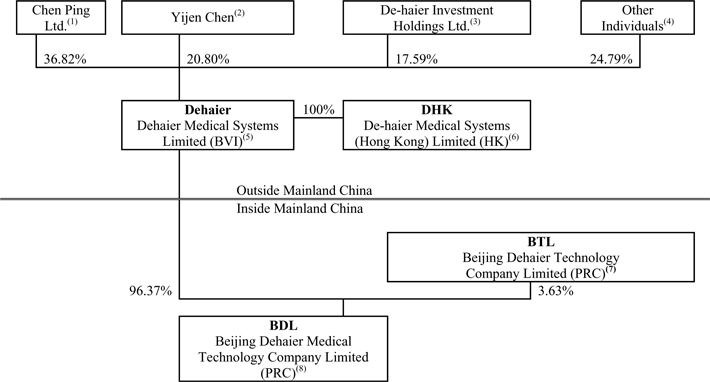
| (1) | Chen Ping Ltd. is a British Virgin Islands company beneficially owned by our Chief Executive Officer, Mr. Ping Chen. |
| (2) | These shares were initially acquired by Crystal East Group Limited, a British Virgin Islands company, in our second round of venture capital financing. Crystal East Group Limited transferred the Series B preferred shares to its shareholder, Ms. Chen, and such shares were converted into our common shares as of October 31, 2009. Ms. Chen is not related to our chief executive officer, Mr. Ping Chen. |
| (3) | De-haier Investment Holdings Ltd, a British Virgin Islands company, obtained these shares in our first round of venture capital financing. These Series A preferred shares were converted into our common shares as of October 31, 2009. |
| (4) | Such shareholders consist of employees and consultants to our company. |
| (5) | Dehaier is our British Virgin Islands holding company that seeks to register its common shares in this initial public offering. |
33
Table of Contents
| (6) | DHK is a Hong Kong company owned by Dehaier, through which we may in the future conduct operations. We do not, however, have any present plans to do so. We do not currently receive any significant revenues through DHK. |
| (7) | BTL is a variable interest entity, and BDL is the primary beneficiary. BTL owns a building which is pledged as collateral for BDL’s bank loans. In exchange, BDL loans money to BTL to finance its operations. Dehaier, BDL and BTL are under common control, and BDL provides substantial financial support to BTL. This substantial financial support is primarily for working capital loans to finance BTL’s operations. At December 31, 2006, 2007, 2008 and September 30, 2009, BDL had advanced to BTL funds in the amount of $823,405, $786,816, $292,011 and $164,248, which represented 87%, 74%, 24% and 13% of BTL’s equity, respectively. Because BTL has insufficient equity to finance its operations, BTL then is required to obtain additional financial support to fund its operations. Accordingly, BTL’s sales are included in Dehaier’s consolidated income from operations. Mr. Chen owns 86% of BTL and his wife and employees of Dehaier own the remaining 14%. BTL performs certain out-of-warranty repair work for products we sell and provides transportation services related to such services. |
| (8) | BDL is our Chinese operating entity. We develop, assemble, market and distribute our branded products through BDL, and we market and distribute products developed by other companies through BDL. |
In the opinion of our PRC legal counsel, (i) our ownership structure complies with, and immediately after this offering will comply with, current PRC laws and regulations and the business and (ii) the operations of BDL comply with current PRC laws and regulations.
After deducting the estimated placement discount and offering expenses payable by us, we expect to receive net proceeds of approximately $8,700,000 from this offering if the minimum offering is sold and approximately $10,540,000 if the maximum offering is sold. The net proceeds from this offering must be remitted to China before we will be able to use the funds to grow our business. The procedure to remit funds may take several months after completion of this offering, and we will be unable to use the funds in China until remittance is completed. See “Risk Factors – We must remit the offering proceeds to China before they may be used to benefit our business in China, and this process may take a number of months.”
We intend to use the net proceeds of this offering as follows after we complete the remittance process, and we have ordered the specific uses of proceeds in order of priority. We do not expect that our priorities for fund allocation would change if the amount we raise in this offering exceeds the size of the minimum offering but is less than the maximum offering.
| Description of Use |
Percentage of Net Proceeds |
||
| Product Research and Development |
25 | % | |
| Marketing |
30 | % | |
| Potential Acquisitions |
20 | % | |
| Working Capital |
25 | % | |
| Total |
100 | % |
In the event we do not locate any appropriate targets for acquisitions or are not able to negotiate such acquisitions on terms that are acceptable to us, we reserve the right to allocate such funds to our working capital purposes. There are no understandings, commitments or agreements with respect to any such transaction at this time.
We will use a portion of our working capital to retain a consulting firm during our transition to operating as a public company and an investor relations firm. Pending use of the net proceeds, we intend to invest our net proceeds in short-term, interest bearing, investment-grade obligations. These investments may have a material adverse effect on the U.S. federal income tax consequences of an investment in our common shares. It is possible that we may become a passive foreign investment company for U.S. federal income taxpayers, which could result in negative tax consequences to you. These consequences are described in more detail in “Taxation.”
34
Table of Contents
We have never declared or paid any cash dividends on our common shares. We anticipate that we will retain any earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future. Any future determination relating to our dividend policy will be made at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions and future prospects and other factors the Board of Directors may deem relevant.
Under British Virgin Islands law, we may only pay dividends from surplus (the excess, if any, at the time of the determination of the total assets of our company over the sum of our liabilities, as shown in our books of account, plus our capital), and we must be solvent before and after the dividend payment in the sense that we will be able to satisfy our liabilities as they become due in the ordinary course of business; and the realizable value of assets of our company will not be less than the sum of our total liabilities, other than deferred taxes as shown on our books of account, and our capital.
If we determine to pay dividends on any of our common shares in the future, as a holding company, we will be dependent on receipt of funds from our majority-owned subsidiary, BDL. Payments of dividends by BDL to our company are subject to the requirement that foreign invested enterprises may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business. Further, such remittances would require BDL to provide an application for remittance that includes, in addition to the application form, a foreign registration certificate, board resolution, capital verification report, audit report on profit and stock bonuses, and a tax certificate. There are no such similar foreign exchange restrictions in the British Virgin Islands.
35
Table of Contents
The following table sets forth our capitalization as of September 30, 2009 on a pro forma as adjusted basis giving effect to the sale of the minimum and maximum offering at an assumed public offering price of $8.00 per share and to reflect the application of the proceeds after deducting the estimated placement fees. You should read this table in conjunction with our financial statements and related notes appearing elsewhere in this prospectus and “Use of Proceeds” and “Description of Share Capital.”
Minimum Offering (1,250,000 Common shares)
U.S. Dollars
(unaudited)
September 30, 2009
| As Reported(1) | Pro Forma Adjusted for IPO(2) | |||||
| Common shares |
||||||
| Shares |
1,891,930 | 4,250,000 | ||||
| Amount |
$ | 5,167 | $ | 11,607 | ||
| Additional Paid-In Capital(3) |
$ | 3,196,974 | $ | 11,893,560 | ||
| Retained Earnings |
$ | 2,624,771 | $ | 2,624,771 | ||
| Accumulated Other Comprehensive Income |
$ | 778,766 | $ | 778,766 | ||
| Total |
$ | 6,605,678 | $ | 15,308,704 | ||
Maximum Offering (1,500,000 Common shares)
U.S. Dollars
(unaudited)
September 30, 2009
| As Reported(1) | Pro Forma Adjusted for IPO(2) | |||||
| Common shares |
||||||
| Shares |
1,891,930 | 4,500,000 | ||||
| Amount |
$ | 5,167 | $ | 12,290 | ||
| Additional Paid-In Capital(3) |
$ | 3,196,974 | $ | 13,732,877 | ||
| Retained Earnings |
$ | 2,624,771 | $ | 2,624,771 | ||
| Accumulated Other Comprehensive Income |
$ | 778,766 | $ | 778,766 | ||
| Total |
$ | 6,605,678 | $ | 17,148,704 | ||
| (1) | This column gives effect to the 3.66140766-for-1 share split of our common shares that was completed as of October 31, 2009 but does not give effect to the conversion of outstanding preferred shares on such date as reported. |
| (2) | Gives effect (i) to the conversion of outstanding preferred shares into common shares and simultaneous 3.66140766-for-1 share split of our common shares that was completed as of October 31, 2009, and (ii) to the sale of the minimum offering and the maximum offering, as applicable, at an assumed public offering price of $8.00 per share and to reflect the application of the proceeds after deducting the estimated underwriting discounts and our estimated offering expenses. |
| (3) | Pro forma adjusted for IPO additional paid in capital reflects the net proceeds we expect to receive, after deducting a 7% underwriting discount, 1% non-accountable expense allowance and approximately $500,000 in expenses. In a maximum offering, we expect to receive net proceeds of approximately $10,540,000 ($12,000,000 offering, less underwriting discount of $840,000, non-accountable expense allowance of $120,000 and offering expenses of $500,000). In a minimum offering, we expect to receive net proceeds of approximately $8,700,000 ($10,000,000 offering, less underwriting discount of $700,000, non-accountable expense allowance of $100,000 and offering expenses of $500,000). |
36
Table of Contents
Our business is primarily conducted in China, and the financial records of our PRC subsidiary and BTL are maintained in RMB, their functional currency. However, we use the U.S. dollar as our reporting and functional currency; therefore, periodic reports made to shareholders will include current period amounts translated into U.S. dollars using the then-current exchange rates, for the convenience of the readers. Our financial statements have been translated into U.S. dollars in accordance with Accounting Standards Codification (“ASC”) 830-10, “Foreign Currency Matters.” We have translated our asset and liability accounts using the exchange rate in effect at the balance sheet date. We translated our statements of operations using the average exchange rate for the period. We reported the resulting translation adjustments under other comprehensive income. Unless otherwise noted, we have translated balance sheet amounts with the exception of equity at December 31, 2008 at ¥6.8225 to $1.00 as compared to ¥7.2946 to $1.00 at December 31, 2007. The average translation rates applied to income statement accounts for the year ended December 31, 2008 and the year ended December 31, 2007 were ¥6.9483 and ¥7.6040, respectively.
We make no representation that any RMB or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, or at all. The PRC government imposes control over its foreign currency reserves in part through direct regulation of the conversion of RMB into foreign exchange and through restrictions on foreign trade. On February 12, 2010, the noon buying rate was ¥6.8327 to $1.00. The Company does not currently engage in currency hedging transactions.
The following table sets forth information concerning exchange rates between the RMB and the U.S. dollar for the periods indicated.
| Noon Buying Rate | ||||||||
| Period |
Period-End | Average(1) | Low | High | ||||
| (RMB per U.S. Dollar) | ||||||||
| 2004 |
8.2765 | 8.2768 | 8.2764 | 8.2774 | ||||
| 2005 |
8.0702 | 8.1940 | 8.0702 | 8.2765 | ||||
| 2006 |
7.8041 | 7.9723 | 7.8041 | 8.0702 | ||||
| 2007 |
7.2946 | 7.6040 | 7.2946 | 7.8127 | ||||
| 2008 |
6.8225 | 6.9483 | 6.7800 | 7.2946 | ||||
| 2009 |
6.8259 | 6.8307 | 6.8176 | 6.8470 | ||||
| July |
6.8319 | 6.8317 | 6.8300 | 6.8342 | ||||
| August |
6.8299 | 6.8323 | 6.8299 | 6.8358 | ||||
| September |
6.8262 | 6.8277 | 6.8247 | 6.8303 | ||||
| October |
6.8264 | 6.8267 | 6.8248 | 6.8292 | ||||
| November |
6.8265 | 6.8271 | 6.8255 | 6.8300 | ||||
| December |
6.8259 | 6.8275 | 6.8244 | 6.8299 | ||||
| 2010 |
||||||||
| January |
6.8268 | 6.8269 | 6.8258 | 6.8295 | ||||
| February (through February 12, 2010) |
6.8327 |
6.8280 |
6.8260 |
6.8328 | ||||
| (1) | Averages are calculated using the daily rates during the relevant period. |
37
Table of Contents
If you invest in our common shares, your interest will be diluted to the extent of the difference between the initial public offering price per common share and the pro forma net tangible book value per common share after the offering. Dilution results from the fact that the per common share offering price is substantially in excess of the book value per common share attributable to the existing shareholders for our presently outstanding common shares. Our unaudited net tangible book value attributable to shareholders at September 30, 2009 was $9,910,207 or approximately $3.30 per common share. Unaudited net tangible book value per common share as of September 30, 2009 represents the amount of total assets less intangible assets and total liabilities, divided by the number of common shares outstanding.
If the minimum offering is sold, we will have 4,250,000 common shares outstanding upon completion of the offering. Our post offering pro forma net tangible book value, which gives effect to receipt of the net proceeds from the offering and issuance of additional shares in the offering, but does not take into consideration any other changes in our net tangible book value after September 30, 2009, will be approximately $18,610,207 or $4.38 per common share. This would result in dilution to investors in this offering of approximately $3.62 per common share or approximately 45.25% from the assumed offering price of $8.00 per common share. Net tangible book value per common share would increase to the benefit of present shareholders by $1.08 per share attributable to the purchase of the common shares by investors in this offering.
If the maximum offering is sold, we will have 4,500,000 common shares outstanding upon completion of the offering. Our post offering pro forma net tangible book value, which gives effect to receipt of the net proceeds from the offering and issuance of additional shares in the offering, but does not take into consideration any other changes in our net tangible book value after September 30, 2009, will be approximately $20,450,207 or $4.54 per common share. This would result in dilution to investors in this offering of approximately $3.46 per common share or approximately 43.25% from the assumed offering price of $8.00 per common share. Net tangible book value per common share would increase to the benefit of present shareholders by $1.24 per share attributable to the purchase of the common shares by investors in this offering.
The following table sets forth the estimated net tangible book value per common share after the offering and the dilution to persons purchasing common shares based on the foregoing minimum and maximum offering assumptions.
| Minimum Offering(1) |
Maximum Offering(2) | |||||
| Assumed offering price per common share |
$ | 8.00 | $ | 8.00 | ||
| Net tangible book value per common share before the offering (unaudited) |
$ | 3.30 | $ | 3.30 | ||
| Increase per common share attributable to payments by new investors |
$ | 1.08 | $ | 1.24 | ||
| Pro forma net tangible book value per common share after the offering |
$ | 4.38 | $ | 4.54 | ||
| Dilution per common share to new investors |
$ | 3.62 | $ | 3.46 | ||
| (1) | Assumes gross proceeds from offering of 1,250,000 common shares. |
| (2) | Assumes gross proceeds from offering of 1,500,000 common shares. |
38
Table of Contents
The following charts illustrate our pro forma proportionate ownership, upon completion of the offering under alternative minimum and maximum offering assumptions, by present shareholders and investors in this offering, compared to the relative amounts paid by each. The charts reflect payment by present shareholders as of the date the consideration was received and by investors in this offering at the assumed offering price without deduction of commissions or expenses. The charts further assume no changes in net tangible book value other than those resulting from the offering and provide alternative scenarios depending on whether the Make-Good Shares are redeemed. See “Risk Factors – A redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company,” “Related Party Transactions – Make-Good Shares Subject to Redemption” and “Placement – Market and Pricing Considerations.”
Scenario 1: Pro forma presentation assuming no redemption of any Make-Good Shares
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Amount | Percent | Amount | Percent | |||||||||||
| MINIMUM OFFERING |
||||||||||||||
| Existing shareholders |
3,000,000 | 70.6 | % | $ | 9,910,207 | 49.8 | % | $ | 3.30 | |||||
| New investors |
1,250,000 | 29.4 | % | $ | 10,000,000 | 50.2 | % | $ | 8.00 | |||||
| Total |
4,250,000 | 100.0 | % | $ | 19,910,207 | 100.0 | % | $ | 4.68 | |||||
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Amount | Percent | Amount | Percent | |||||||||||
| MAXIMUM OFFERING |
||||||||||||||
| Existing shareholders |
3,000,000 | 66.7 | % | $ | 9,910,207 | 45.2 | % | $ | 3.30 | |||||
| New investors |
1,500,000 | 33.3 | % | $ | 12,000,000 | 54.8 | % | $ | 8.00 | |||||
| Total |
4,500,000 | 100.0 | % | $ | 21,910,207 | 100.0 | % | $ | 4.87 | |||||
Scenario 2: Pro forma presentation assuming redemption of all Make-Good Shares
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Amount | Percent | Amount | Percent | |||||||||||
| MINIMUM OFFERING |
||||||||||||||
| Existing shareholders |
2,400,000 | 65.8 | % | $ | 9,910,207 | 49.8 | % | $ | 4.13 | |||||
| New investors |
1,250,000 | 34.2 | % | $ | 10,000,000 | 50.2 | % | $ | 8.00 | |||||
| Total |
3,650,000 | 100.0 | % | $ | 19,910,207 | 100.0 | % | $ | 5.45 | |||||
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Amount | Percent | Amount | Percent | |||||||||||
| MAXIMUM OFFERING |
||||||||||||||
| Existing shareholders |
2,400,000 | 61.5 | % | $ | 9,910,207 | 45.2 | % | $ | 4.13 | |||||
| New investors |
1,500,000 | 38.5 | % | $ | 12,000,000 | 54.8 | % | $ | 8.00 | |||||
| Total |
3,900,000 | 100.0 | % | $ | 21,910,207 | 100.0 | % | $ | 5.62 | |||||
39
Table of Contents
CONSOLIDATED FINANCIAL AND OPERATING DATA
You should read the following selected financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes included elsewhere in this prospectus. The selected statements of operations data are for the fiscal years ended December 31, 2008 and 2007. The selected balance sheet data set forth below is as of December 31, 2008 and 2007. This selected financial data is derived from our consolidated financial statements and should be read in conjunction with the consolidated financial statements and notes thereto which are included elsewhere in this prospectus.
| For the nine months ended September 30, 2009 |
For the fiscal year ended December 31, |
|||||||||||
| 2008 | 2007 | |||||||||||
| (Unaudited) | ||||||||||||
| Revenue |
$ | 9,422,460 | $ | 9,414,430 | $ | 6,599,512 | ||||||
| Operating income |
2,586,824 | 1,496,086 | 970,622 | |||||||||
| Net income |
2,110,278 | 980,351 | 848,421 | |||||||||
| Non-controlling interest in income |
(38,502 | ) | (62,331 | ) | (53,214 | ) | ||||||
| Net income attributable to Dehaier |
2,071,776 | 918,020 | 795,207 | |||||||||
| Basic earnings per share (based on 1,891,930 shares outstanding on each of September 30, 2009, December 31, 2008 and 2007)(1) |
1.10 | 0.48 | 0.42 | |||||||||
| Diluted earnings per share (based on 3,000,000, 3,000,000 and 2,451,624 shares outstanding on September 30, 2009, December 31, 2008 and 2007, respectively)(1) |
0.69 | 0.31 | 0.32 | |||||||||
| Pro forma basic earnings per share (based on 1,291,930 shares outstanding on each of September 30, 2009 and December 31, 2008)(2) |
1.60 | 0.71 | N/A | |||||||||
| Pro forma diluted earnings per share (based on 2,400,000 shares outstanding on each of September 30, 2009 and December 31, 2008, respectively)(2) |
0.86 | 0.38 | N/A | |||||||||
| September 30, 2009 |
December 31, | ||||||||
| 2008 | 2007 | ||||||||
| (Unaudited) | |||||||||
| Total assets |
$ | 16,485,711 | $ | 13,046,400 | $ | 9,928,863 | |||
| Total current liabilities |
6,566,373 | 5,232,464 | 3,550,505 | ||||||
| Total Dehaier shareholders’ equity |
8,676,245 | 6,608,704 | 5,310,499 | ||||||
| Non-controlling interest |
1,243,093 | 1,205,232 | 1,067,859 | ||||||
| Total liabilities and shareholders’ equity |
16,485,711 | 13,046,400 | 9,928,863 | ||||||
| (1) | We have presented these basic and diluted earnings per share in Dehaier after giving retroactive effect to the 3.66140766-for-1 share split of common shares completed as of October 31, 2009. |
| (2) | We have presented these pro forma earnings per share after (a) giving retroactive effect to the 3.66140766-for-1 share split of our common shares completed as of October 31, 2009 and (b) assuming the redemption of all shares placed into escrow as described in the section entitled “Related Party Transactions – Make-Good Shares Subject to Redemption.” The number of escrowed shares is based on 600,000 common shares (40% of an assumed maximum of 1,500,000 common shares). Pro forma basic EPS for the nine months ended September 30, 2008 calculated on the foregoing assumptions and based on 1,291,930 Dehaier shares, is $0.40. Pro forma diluted EPS for the nine months ended September 30, 2008 calculated on the foregoing assumptions and based on 2,400,000 Dehaier shares, is $0.22. No pro forma numbers have been provided for the year ended December 31, 2007. |
40
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our audited historical consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We have been focused on the development and distribution of medical devices since our inception, and we began developing our respiratory and oxygen homecare business in 2006. We design, develop and market our branded products in China. We import the majority of the products and medical components we sell to our customers. We design and develop some of the medical components that are part of the branded products to be distributed. Some of these medical components are manufactured by contractors in China because we do not have a manufacturing facility. Most of our branded products we sell require light assembly.
In addition to our branded products, we also serve as a distributor of products designed and manufactured by other companies. We sell products from IMD (Italy), Timesco (UK), ResMed (Australia) and JMS (Japan).
We sell products primarily to distributors; however, we also sell products directly to hospitals, clinics and government health bureaus. We continually seek to broaden our market reach by introducing new and more advanced products and new product lines that address different end-user populations.
For the past two fiscal year periods ended December 31, 2007 and 2008, our total revenues amounted to approximately $6.60 million and $9.41 million, respectively. For the nine month periods ended September 30, 2008 and 2009, our total revenues amounted to approximately $6.53 million and $9.42 million, respectively. Our revenues are subject to value added tax (“VAT”), sales returns and trade discounts .We deduct these amounts from our gross revenues to arrive at our total revenues. Our net income attributable to Dehaier for the periods ended December 31, 2007 and 2008 was $0.79 million and $0.92 million, respectively, and $0.52 million and $2.07 million for the nine month periods ended September 30, 2008 and 2009, respectively.
Revenues
Our total revenues are derived from products we provide in our three product lines, (i) Medical Devices (ii) Respiratory and Oxygen Homecare Products and (iii) Technical Service Products. We are currently operating in one business segment for all of our products we distribute.
Medical Devices – Our Branded and Distributed Products
We derive revenues in our medical devices product line from the sale of C-arm X-ray systems, anesthesia machines, patient monitors and general hospital products. Our medical device line is our largest business line of products and has the most extensive market penetration of our three product lines. We anticipate that we will continue to experience revenue growth in our medical devices line as we further penetrate the market through the development and introduction of new advanced product offerings.
Respiratory and Oxygen Homecare Products – Our Branded and Distributed Products
We derive revenues in our respiratory and oxygen homecare line from sales of oxygen concentrators, CPAP devices, portable sleep screening and diagnostic devices and thermotherapy devices. We anticipate that, on a percentage basis, net revenues in our respiratory and oxygen homecare product line will increase more rapidly than total net revenues in the near term, as we introduce new and more advanced products in this product line. We expect to increase our market penetration in the respiratory and oxygen homecare market both in China and internationally through the use of distributors as well as through our direct sales platform.
41
Table of Contents
Technical Service Products – Our Branded Products
We derive revenues in our technical service products line from sales of our air compressors and ventilator trolleys. We anticipate continued growth in revenues from our technical service products as we further penetrate this market by increasing the number of our distributors and maintaining a competitive pricing model.
Our ability to increase our revenues depends in large part on our ability to (i) increase the market penetration of our existing products, (ii) successfully identify, develop, introduce and commercialize, in a timely and cost-effective manner, new and upgraded products and (iii) enter into international markets in the future. Generally, we choose to devote our resources to product development efforts that we believe are commercially feasible, can generate significant revenues and margins and can be introduced into the market quickly.
Factors Affecting Our Results of Operations – Generally
We believe the most significant factors that directly or indirectly affect our sales revenues and net income are:
| • | global economic conditions; |
| • | the changes in China’s macro-economic environment and healthcare-related government strategies and policies; |
| • | the level of acceptance of our products among hospitals and other healthcare facilities; |
| • | our ability to attract and retain distributors, key customers and our direct sales force; |
| • | new product introductions by us and our competitors; and |
| • | our ability to price our products at levels that provide favorable margins. |
Operating Costs and Expenses
Our operating costs and expenses consist of cost of revenues, general and administrative expenses, selling expenses and other expenses. Our total operating costs and expenses decreased as a percentage of our total revenues for the year ended December 31, 2008 compared to the year ended December 31, 2007 and for the nine months ended September 30, 2009 compared to the same period in 2008. These decreases were primarily due to increased economies of scale and an increase in revenue. The following table sets forth the components of our costs and expenses both in U.S. dollar amounts (in thousands) and as a percentage of total net revenues for the periods indicated.
| For the years ended December 31, | For the nine months ended September 30, | |||||||||||||||
| 2008 | 2007 | 2009 | 2008 | |||||||||||||
| $ | % | $ | % | $ | % | $ | % | |||||||||
| (in thousands) |
(in thousands) |
(in thousands) |
(in thousands) |
|||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Revenues |
9,414 | 100.00 | 6,600 | 100.00 | 9,422 | 100.00 | 6,532 | 100.00 | ||||||||
| Costs and expenses |
||||||||||||||||
| Cost of revenues |
5,931 | 63.00 | 4,280 | 64.85 | 5,688 | 60.37 | 4,111 | 62.94 | ||||||||
| General and administrative expense |
1,326 | 14.09 | 758 | 11.48 | 788 | 8.36 | 918 | 14.05 | ||||||||
| Selling expense |
974 | 10.35 | 881 | 13.35 | 544 | 5.77 | 785 | 12.02 | ||||||||
| Total costs and expenses |
8,231 | 87.44 | 5,919 | 89.68 | 7,020 | 74.50 | 5,814 | 89.01 | ||||||||
Cost of revenues
Cost of revenues primarily includes wages, parts for assembly, handling charges, and other expenses associated with the assembly and distribution of product.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and benefits and related costs for our administrative personnel and management, fees and expenses of our outside advisers, including legal, audit and valuation expenses, expenses associated with our administrative offices and the depreciation of equipment used for administrative purposes. We expect that our general and administrative expenses will increase, both on an absolute basis and as a percentage of revenue, as we hire additional personnel and incur costs related to the anticipated growth of our business. In addition, we expect to incur additional general and administrative expenses associated with becoming a public company.
42
Table of Contents
Selling Expenses
Selling expenses consist primarily of compensation and benefits for our sales and marketing staff, expenses for promotional, advertising, travel and entertainment activities, lease payments for our sales offices, and depreciation expenses related to equipment used for sales and marketing activities. Going forward, we expect our selling expenses to increase, both on an absolute basis and as a percentage of revenue, as we increase our efforts to promote our products, especially our new respiratory and oxygen homecare products.
Tax Matters Applicable to Our Company
Generally
Dehaier is a tax-exempt company incorporated in the British Virgin Islands. BDL and BTL were incorporated in the PRC and are governed by PRC laws. DHK is subject to Hong Kong profits tax rate.
Our company pays PRC enterprise income taxes, value added taxes and business taxes in China for revenues from BDL and is governed by British Virgin Islands tax laws as to Dehaier.
British Virgin Islands Tax
We are exempt from all provisions of the Income Tax Act of the British Virgin Islands, including with respect to all dividends, interests, rents, royalties, compensation and other amounts payable by or to persons who are not resident in the British Virgin Islands. Capital gains realized with respect to any of our shares, debt obligations or other securities by persons who are not resident in the British Virgin Islands are also exempt from all provisions of the Income Tax Act of the British Virgin Islands. No estate, inheritance tax succession or gift tax rate, duty, levy or other charge is payable by persons who are not resident in the British Virgin Islands with respect to any of our shares, debt obligations, or other securities. No stamp duty is payable in the British Virgin Islands in relation to a transfer of shares in a British Virgin Islands Business Company.
PRC Enterprise Income Taxes
PRC enterprise income tax is calculated based on taxable income determined under PRC accounting principles. According to the Foreign-invested Enterprises and Foreign Enterprises Income Tax Law (the “FIE Income Tax Law”) and the related implementing rules, both of which issued in 1991, foreign-invested enterprises established in China are generally subject to an income tax rate of 33% (consisting of 30% enterprise income tax and 3% local income tax). The FIE Income Tax Law and the related implementing rules provide certain favorable tax treatments to qualified foreign invested enterprises.
The FIE Income Tax Law was replaced by the Enterprise Income Tax Law (the “EIT Law”) as of January 1, 2008. Under the EIT Law, a unified enterprise income tax rate of 25% and unified tax deduction standards will be applied equally to both domestic-invested enterprises and foreign-invested enterprises. Enterprises established prior to March 16, 2007 eligible for preferential tax treatment in accordance with the currently prevailing tax laws and administrative regulations shall, under the regulations of the State Council, gradually become subject to the EIT Law rate over a five-year transition period starting from the date of effectiveness of the EIT Law. The details of the transitional arrangement for the five-year period from January 1, 2008 to December 31, 2012 applicable to enterprises approved for establishment prior to March 16, 2007, such as our company, were adopted in January 2008.
Furthermore, under the EIT Law, an enterprise established outside of the PRC with “de facto management bodies” within the PRC is considered a resident enterprise and will normally be subject to the enterprise income tax at the rate of 25% on its global income. If the PRC tax authorities subsequently determine that we or any of our non-PRC subsidiaries should be classified as a PRC resident enterprise, then such entity’s global income will be subject to PRC income tax at a tax rate of 25%. In addition, under the EIT Law, payments from BDL to us may be subject to a withholding tax. The EIT Law currently provides for a withholding tax rate of 20%. If Dehaier is deemed to be a non-resident enterprise, then it will be subject to a withholding tax at the rate of 10% on any dividends paid by its Chinese subsidiaries to Dehaier. In practice, the tax authorities typically impose the withholding tax rate of 10%
43
Table of Contents
rate, as prescribed in the implementation regulations; however, there can be no guarantee that this practice will continue as more guidance is provided by relevant government authorities. We are actively monitoring the proposed withholding tax and are evaluating appropriate organizational changes to minimize the corresponding tax impact.
The State Council issued the “Notice on Implementation of the Transition Period for Preferential Enterprise Income Tax,” or the “Transition Implementation Notice,” on December 26, 2007, which provides detailed rules on how preferential tax rates under previous income tax laws or regulations would transition to the uniform 25% EIT rate. In addition, entities that qualify as “high and new technology enterprises” will enjoy a 15% preferential tax rate under the EIT Law. The Ministry of Science and Technology, the Ministry of Finance and the State Administration of Taxation issued the “Measures on Qualification of High and New Technology Enterprises,” or “Circular 172,” on April 14, 2008, which provides detailed standards for “high and new technology enterprises.” In addition, according to the Notice on Prepayment of Enterprise Income Tax issued by the State Administration of Taxation, enterprises that have been certified as “high and new technology enterprises” shall pre-pay EIT at the rate of 25% temporarily until re-certified as “high and new technology enterprises” under Circular 172.
Under the current PRC laws, BDL and BTL are subject to EIT and VAT. BDL was classified as a high and new technology company and operates in an approved economic-technological development area. Given this classification, it was entitled to an EIT rate of 15%, compared to the statutory rate of 30% for most companies in China. This classification also exempted BDL from paying the EIT for the three calendar years ended December 31, 2004, 2005 and 2006 and reduced BDL’s EIT rate by 50% to 7.5% for the three calendar years ended December 31, 2007, 2008 and 2009.
Due to the PRC’s standardization of tax rates for all companies in China effective January 1, 2008 and promulgation of new standards for high technology companies, BDL paid the 25% tax rate in 2008. While the certification remained available in 2008, the standards were revised, requiring previously certified entities to apply for high technology certification again. BDL subsequently applied for and obtained such high technology certification approval online and expects to receive the confirmation certificate in the near future. As a result, we have calculated our 2009 estimated income tax expenses at the preferential tax rate of 15%. While we believe that BDL will continue to qualify for this preferential tax rate so long as it continues to conduct business as it currently does and the requirements for qualification are not changed, we cannot guarantee that we will be successful in remaining so certified. If we were to lose our preferential tax rate, we would need to calculate our estimated income tax expense at 25%, the standard enterprise tax rate in China.
PRC Value Added Tax
Pursuant to the Provisional Regulation of China on Value Added Tax and its implementing rules, issued in December 1993, all entities and individuals that are engaged in the businesses of sales of goods, provision of repair and placement services and importation of goods into China are generally subject to a VAT at a rate of 17% (with the exception of certain goods which are subject to a rate of 13%) of the gross sales proceeds received, less any VAT already paid or borne by the taxpayer on the goods or services purchased by it and utilized in the production of goods or provisions of services that have generated the gross sales proceeds.
PRC Business Tax
Companies in China are generally subject to business tax and related surcharges by various local tax authorities at rates ranging from 3% to 20% on revenue generated from providing services and revenue generated from the transfer of intangibles.
Critical Accounting Policies
Presentation in Accordance with US GAAP
We prepare consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). These accounting principles require us to make judgments, estimates and assumptions on the reported amounts of assets and liabilities at the end of each fiscal period, and the reported amounts of revenues and expenses during each fiscal period. We continually evaluate these judgments and estimates based on our own historical experience, knowledge and assessment of current business and other conditions, our expectations regarding the future based on available information and assumptions that we believe to be reasonable.
44
Table of Contents
Basis of Consolidation
The consolidated financial statements include the accounts of Dehaier, BDL, its majority-owned subsidiary, and its wholly-owned subsidiary, DHK as well as BTL. All significant inter-company transactions and balances are eliminated in consolidation.
A group of shareholders, including the Chief Executive Officer, holds more than 50% of the voting ownership interest of BDL and BTL. BTL is a variable interest entity, and Dehaier is the primary beneficiary. Dehaier, BDL and BTL are under common control, and Dehaier, through its 96.37% subsidiary, BDL, provides substantial finacial support to BTL. BTL owns a building which is pledged as collateral for BDL’s bank loans. In exchange, BDL loans money to BTL to finance its operations. BTL’s primary operation is to provide repairs and transportation services to BDL’s customers. Dehaier has included BTL in its consolidated financial statements since December 31, 2006 and presented the non-controlling interest of BTL as a separate component in Dehaier’s equity section on the accompanying consolidated balance sheets. BTL’s share of income (loss) is subtracted from (added to) consolidated income (loss) to arrive at Dehaier’s net income (loss).
Carrying amount and classification of BTL’s assets and liabilities included in the consolidated balance sheets are as follows:
| December 31, | September 30, | |||||
| 2008 | 2007 | 2009 | ||||
| $ | $ | $ | ||||
| Total current assets |
392,431 | 770,598 | 268,407 | |||
| Total assets |
1,573,073 | 1,988,906 | 1,380,680 | |||
| Total current liabilities |
367,842 | 921,047 | 137,587 | |||
| Total liabilities |
367,842 | 921,047 | 137,587 | |||
The accounts of BTL are consolidated in the accompanying financial statements pursuant to Accounting Standards Codification (“ASC”) 810-10, “Consolidation”. As a VIE, BTL’s revenues are included in the Company’s total revenues, and its income from operations is consolidated with the Company’s. Because of the arrangements, the Company had a pecuniary interest in BTL that requires consolidation of the Company’s and BTL’s financial statements.
On January 1, 2009, the Company adopted the transition rules within ASC 810-10-65, regarding accounting and reporting standards for the non-controlling interest in a subsidiary.
Use of Estimates
The preparation of the consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting periods. Estimates are adjusted to reflect actual experience when necessary. Significant accounting estimates reflected in the Company’s consolidated financial statements include revenue recognition, allowance for doubtful accounts and impairment of long-lived assets. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash on hand and highly liquid investments which are unrestricted as to withdrawal or use, and which have maturities of three months or less when purchased. Our company maintains uninsured cash and cash equivalents with various financial institutions mainly in the PRC and Hong Kong.
Accounts Receivable
Accounts receivable are recorded at net realizable value. Accounts receivable terms typically are net 60-180 days from the end of the month in which services are provided or goods are delivered. Our typical trade receivable terms vary based on the type of customer. We general require 100% prepayment before delivering our products to individual clients. Our contract terms general require 10%-30% prepayment for our hospital and healthcare center clients, and the trade receivable term in contracts for those clients is generally between 60 and 90 days. Our contract
45
Table of Contents
terms general require 10% prepayment from our distributor clients, and the trade receivable term in contracts for those clients is generally between 60 and 180 days. With the exception of the prepayments we require in some cases, our company generally does not require collateral or other security to support accounts receivable. An allowance, if required, is based on a combination of historical experience, aging analysis, and an evaluation of the collectability of specific accounts. At September 30, 2009, our accounts receivable aging was as follows:
| Total Accounts Receivable |
1 to 3 months | 3 to 6 months | 6 months to 1 year | 1 to 2 years | ||||||||
| $6,059,480 |
$ | 2,679,059 | $ | 1,771,944 | $ | 1,448,310 | $ | 160,167 | ||||
Receivables are considered past due after 3 years and written off. Management has determined this write-off policy by reviewing the decrease in likelihood of collection as a function of time and concluded that collectability of accounts receivable after three years was significantly impaired, compared with collectability prior to such time. At December 31, 2008 and 2007 and September 30, 2009, an allowance for doubtful accounts has been provided based on management’s analysis of both past bad debt allowances and performance and current aging of accounts receivable. Based on this analysis, management believes that its historical bad debt rates and long-term accounts receivable (over one year) are relatively low and that, as a result, the allowance for doubtful accounts is adequate. To the extent actual bad debts differ from management’s estimates by 10%, consolidated net income would be an estimated $3,000, $11,000 and $2,000 higher or lower for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009, respectively, depending on whether the actual write-offs are greater or lower than estimated.
Fair Value of Financial Instruments
The carrying amounts reported in the consolidated financial statements for current assets and current liabilities approximate fair value due to the short-term nature of these financial instruments.
In 2008, the Company adopted ASC 820-10, “Fair Value Measurements and Disclosures,” which establishes a single authoritative definition of fair value and a framework for measuring fair value and expands disclosure of fair value measurements for both financial and nonfinancial assets and liabilities. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flows) and the cost approach (cost to replace the service capacity of an asset or replacement cost). For purposes of ASC 820-10-15, nonfinancial assets and nonfinancial liabilities would include all assets and liabilities other than those meeting the definition of a financial asset or financial liability as defined in ASC 820-10-15-15-1A. Management elected the deferral option available for one year for nonfinancial assets and liabilities as permitted by ASC 820-10.
The Company decided not to elect the fair value option prescribed by Financial Accounting Standards Board (“FASB”) ASC 825-10 for its financial assets and liabilities.
Inventory
Inventory is stated at the lower of cost or market and consists of assembled and unassembled parts relating to medical devices. The Company reviews its inventory annually for possible obsolete goods and to determine if any reserves are necessary for potential obsolescence. At December 31, 2008 and 2007 and September 30, 2009, no reserve for obsolescence was considered necessary because the Company’s inventory consists of components that are assembled shortly prior to distribution. Because the Company orders components and ships products to order, it typically maintains very little inventory that could become obsolete.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may no longer be recoverable. When these events occur, the Company measures impairment by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to result from the use of the asset and eventual disposition. If the sum of the expected future cash flows is less than the carrying amount of the asset, an impairment loss, equal to the excess of the carrying amount over the fair market value of the asset, is recognized. Management has determined no impairment exists at the balance sheet dates.
46
Table of Contents
The Company reviews intangible assets for impairment. There was no impairment of intangible assets for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008. Although management believes the assumptions used in testing for impairment are reasonable, changes in any one of the assumptions could produce a significantly different result.
Revenue Recognition
The Company recognizes revenues when all the followings conditions have been satisfied:
| • | Persuasive evidence an arrangement exists; |
| • | Delivery and/or installation has occurred (e.g., risks and rewards of ownership have passed); |
| • | The sales price is fixed or determinable; and, |
| • | Collectibility is reasonably assured. |
All revenues are based on firm customer orders with fixed terms and conditions. Because the products are assembled to the customers’ specification, there is no right of return. The Company does not provide its customers with price protection or cash rebates. For products which include software, the software is an off-the-shelf package and an integral part of the products being delivered. The Company does not provide any significant post-sale customer support services and does not provide customers with upgrades. The software is incidental to the product as a whole. For products that do not require installation, revenues are recognized when the products are delivered. For products that require installation, revenues are recognized when the installation is completed.
In the PRC, a VAT rate of 17% of the invoice amount is collected in respect of the sales of goods on behalf of tax authorities. The VAT collected is not revenue of the Company; instead, the amount is recorded as a liability on the balance sheet until such VAT is paid to the authorities.
Service Income and Expense
Service income and expense represents activities related to repairs and transportation services provided for the customers by BTL.
Warranty Costs
The Company provides for the estimated cost of product warranties at the time revenue is recognized. Warranty costs are included in general and administrative expenses. The Company’s warranty obligation is affected by product failure rates and material usage and service delivery costs incurred in correcting product failure. Should actual product failure rates, material usage or service delivery costs differ from the Company’s estimates, the Company may be required to revise its estimated product warranty liability. The term of the product warranty is generally twelve months. Provision for warranty costs was $158,065, $98,964 and $199,727 at December 31, 2008 and 2007 and September 30, 2009, respectively. The provision for warranty costs is estimated based on historical cost. To the extent that actual warranty costs differ from management’s estimates by 10%, consolidated net income would be an estimated $6,000, $1,000, $4,000 and $4,000 higher or lower for the years ended December 31, 2008 and 2007 and the nine months ended September 30, 2009 and 2008, respectively, depending on whether the actual warranty costs are greater or less than estimated.
Value Added Tax
The Company reports revenues net of PRC’s value added tax for all the periods presented in the consolidated statements of operations.
47
Table of Contents
Income Taxes
In July 2006, the FASB issued ASC 740-10, “Accounting for Uncertainty in Income Taxes – an Interpretation of Accounting for Income Taxes.” It prescribes a recognition threshold and measurement attribute for the financial statement recognition of a tax position taken or expected to be taken on a tax return. Under ASC 740-10, a tax benefit from an uncertain tax position taken or expected to be taken may be recognized only if it is “more likely than not” that the position is sustainable upon examination, based on its technical merits. The tax benefit of a qualifying position under ASC 740-10 would equal the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all the relevant information. A liability (including interest and penalties, if applicable) is established in the financial statements to the extent a current benefit has been recognized on a tax return for matters that are considered contingent upon the outcome of an uncertain tax position. Related interest and penalties, if any, are included as components of income tax expense and income taxes payable. The Company is awaiting resolution of certain complex tax issues and has not yet filed its 2007 and 2008 Value Added Tax (“VAT”) tax returns for some of its customers. However, all the potential VAT liabilities on these VAT returns were accrued and included in the accompanying consolidated financial statements.
Results of Operations
Our limited operating history makes it difficult to predict future operating results. We believe that period-to-period comparisons of operating results should not be relied upon as indicative of future performance.
Nine Months Ended September 30, 2009 Compared to Nine Months Ended September 30, 2008
Revenues
Our total revenues increased by 44.25% from $6.53 million for the nine months ended September 30, 2008 to $9.42 million for the nine months ended September 30, 2009. Our top three selling product categories (mobile C arm X-ray system, general medical products and compressors) accounted for 57% of our total revenues for the nine months ended September 30, 2009. Our branded products increased by $2.65 million during this period. The increase in revenues was due to the increased acceptance of our products among hospitals and other healthcare facilities as many of our end users such as hospitals became repeat customers when they needed new medical equipment. In addition, in 2009, we developed our distribution network in China, which led to greater market penetration and more robust growth for the nine months ended September 30, 2009.
Cost of Revenues
Our cost of revenues increased 38.38% from $4.11 million for the nine months ended September 30, 2008 to $5.69 million for the nine months ended September 30, 2009. We had an increase in gross margin from 37.07% up to 39.63% for the nine months ended September 30, 2008 and 2009, respectively. Our cost of revenues grew in U.S. dollars as our revenues grew, but our gross margin increased largely because we were able to leverage a stronger relationship with an original equipment manufacturer, or OEM, supplier, and take advantage of economies of scale. We were able to purchase assembled products from this supplier for less than it would cost us to assemble them ourselves. As our business with this supplier grew, our gross margin subsequently increased.
Operating Expenses
Our operating expenses decreased by 25.52% from $1.82 million for the nine months ended September 30, 2008 to $1.45 million for the nine months ended September 30, 2009. This decrease was due to decrease in general and administrative and selling expenses, for the reasons described below.
Operating Expenses—General and Administrative Expenses
General and administrative expenses consist primarily of salaries and benefits and related costs for our administrative personnel and management, legal, audit and valuation expenses and expenses associated with our administrative offices and the depreciation of equipment used for administrative purposes.
Our general and administration expenses decreased by 14.18% from $0.92 million for the nine months ended September 30, 2008 to $0.79 million for the nine months ended September 30, 2009. This decrease was largely because our professional expenses for the nine months ended September 30, 2008 were $0.3 million consisting
48
Table of Contents
primarily of audit fees relating to our 2006 and 2007 financial statements, compared with professional expenses for the same period in 2009 of $0.18 million. In addition, our meeting and entertainment expense decreased by about $0.01 million for the nine months ended September 30, 2009 compared with the nine months ended September 30, 2008.
However, we expect that our general and administration expenses will increase in the near term as a result of Sarbanes-Oxley Section 404 compliance and business expansion upon completion of this offering. Upon completion of this offering, we anticipate using a portion of the net proceeds to expand our marketing efforts in order to continue to grow our revenues in China and internationally. Among other strategies to expand our business, we plan to open new CECs, in strengthen our market presence in China. These CECs give our potential customers an opportunity to experience our products first-hand in an environment that is similar to the environment in which they will use the products, whether that is a home or healthcare facility. See “Our Business – Our Strategies.”
Operating Expenses—Selling Expense
Our selling expense decreased by 30.65% from $0.79 million for the nine months ended September 30, 2008 to $0.54 million for the nine months ended September 30, 2009.
Our selling expense primarily consists of salaries and related expenses for personnel engaged in sales, marketing and customer support functions and costs associated with advertising and other marketing activities. Our selling expense decreased in both U.S. dollars and as a percentage of our total net revenues for the nine months ended September 30, 2009, mainly due to exhibition expenses to promote our products both inside and outside China for the nine months ended September 30, 2008 that did not recur in 2009.
Operating Income
As a result of the foregoing, we generated operating income of approximately $2.6 million for the nine months ended September 30, 2009, compared to approximately $0.90 million for the nine months ended September 30, 2008. Operating income increased 188.9% largely due to the increase of revenues and gross profit margin as stated above.
Taxation
Our income tax expenses were approximately $0.41 million for the nine months ended September 30, 2009, compared to approximately $0.27 million for the nine months ended September 30, 2008. Our taxable income increased primarily due to increased revenues while our tax rate decreased. For the nine months ended September 30, 2008, the income tax rate applicable for BDL is 25%. For the nine months ended September 30, 2009, BDL has obtained a high technology certification approval online and expects to receive the confirmation certificate in the near future. As a result, BDL uses a 15% income tax rate to calculate the income tax expense for the nine months ended 2009.
Net Income
As a result of the foregoing, we had net income of approximately $2.11 million for the nine months ended September 30, 2009, compared to approximately $0.6 million for the nine months ended September 30, 2008. After deduction of non-controlling interest in income, net income attributable to Dehaier was approximately $2.07 million and $0.52 million for the nine months ended September 30, 2009 and 2008, respectively.
Fiscal Year Ended December 31, 2008 Compared to Fiscal Year Ended December 31, 2007.
Revenues
Our total revenues increased by 42.64% from $6.6 million for the fiscal year ended December 31, 2007 to $9.41 million for the fiscal year ended December 31, 2008. The increase in revenues was due to the increased acceptance of our products among hospitals and other healthcare facilities as many of our end users such as hospitals became repeat customers when they needed new medical equipment. In addition, we developed our distribution network in China, which led to greater market penetration and more robust growth in 2008.
49
Table of Contents
Cost of Revenues
Our cost of revenues increased 38.59% from $4.28 million for the fiscal year ended December 31, 2007 to $5.93 million for the fiscal year ended December 31, 2008. Our cost of revenues grew in U.S. dollars as our revenues grew, but our gross margin increased largely because we were able to leverage a stronger relationship with an original equipment manufacturer, or OEM, supplier and take advantage of economies of scale. We were able to purchase assembled products from this supplier for less than it would cost us to assemble them ourselves. As our business with this supplier grew, our gross margin subsequently increased. We had an increase in gross margin of 1.85% from 35.15% up to 37.00% for the years ended December 31, 2007 and 2008, respectively. This result is largely due to the fact that our sales in 2008 consisted of relatively higher margin medical devices.
Operating Expenses
Our operating expenses increased by 40.12% from $1.72 million for the fiscal year ended December 31, 2007 to $2.41 million for the fiscal year ended December 31, 2008. This increase was mainly due to an increase in administrative expense, and, to a lesser extent, an increase in our selling expense.
Operating Expenses—General and Administrative Expenses
General and administrative expenses consist primarily of salaries and benefits and related costs for our administrative personnel and management and expenses associated with our research and development and the registration of foreign exchange certificate.
Our general and administrative expenses increased by 75.07% from $0.76 million for the fiscal year ended December 31, 2007 to $1.3 million for the fiscal year ended December 31, 2008. This increase was due primarily to increased headcount. During 2008, our headcount increased by about 20 full-time employees. Most of these employees are in sales.
However, we expect that our general and administration expenses will increase in the near term as a result of Sarbanes-Oxley Section 404 compliance and business expansion upon completion of this offering. Upon completion of this offering, we anticipate using a portion of the net proceeds to expand our marketing efforts in order to continue to grow our revenues in China and internationally. Among other strategies to expand our business, we plan to open new Customer Experience Centers, or CECs, in strengthen our market presence in China. See “Our Business – Our Strategies.”
Operating Expenses—Selling Expense
Our selling expense increased by 10.56% from $0.88 million for the fiscal year ended December 31, 2007 to $0.97 million for the fiscal year ended December 31, 2008.
Our selling expense primarily consists of salaries and related expenses for personnel engaged in sales, marketing and customer support functions and costs associated with advertising and other marketing activities. Our selling expense increased in both U.S. dollars and as a percentage of our total net revenues for the fiscal year ended December 31, 2008, mainly to promote our products.
Operating Income
As a result of the foregoing, we generated an operating income of approximately $1.5 million in 2008, compared to approximately $0.97 million in 2007. Operating income increased 54.64% largely due to the increase of revenues and gross profit margin.
Taxation
Our income tax expense was approximately $0.42 million in 2008, compared to approximately $0.07 million in 2007. The increase in income tax in 2008 was primarily due to the change in applicable tax rate discussed above in “PRC Enterprise Income Tax.” As a result, our applicable tax rate in 2008 was 25%, compared to 7.5% in 2007.
50
Table of Contents
Net Income
As a result of the foregoing, we had net income of approximately $0.98 million in 2008, compared to approximately $0.85 million in 2007. After deduction of non-controlling interest in income, net income attributable to Dehaier was approximately $0.92 million and $0.79 million in 2008 and 2007, respectively.
Liquidity and Capital Resources
Cash Flows and Working Capital
To date, we have financed our operations primarily through cash flows from operations and short-term borrowings. As of December 31, 2008, we had approximately $0.28 million in cash and cash equivalents. As a result of the total cash activities, net cash increased from $282,603 on December 31, 2008 to $937,970 on September 30, 2009. We believe that our currently available working capital of $5,819,136, including cash of $937,970, should be adequate to meet our anticipated cash needs and sustain our current operations for at least 12 months.
However, currently available working capital, especially cash, may not be sufficient to fund our anticipated expansion. In order to meet the working capital needs for our anticipated expansion, we may take the following actions: (i) continue to improve our collection of accounts receivable; (ii) if necessary, raise additional capital through sale of equity; and (iii) enter into new, or refinance existing, short- and/or long-term commercial loans. We cannot assure you that financing will be available in the amounts we need or on terms acceptable to us, if at all. The sale of additional equity securities, including convertible debt securities, would dilute our shareholders. The incurrence of debt would divert cash from working capital and capital expenditures to service debt obligations and could result in operating and financial covenants that would restrict our operations and our ability to pay dividends to our shareholders. If we are unable to obtain additional equity or debt financing as required, our business, operations and prospects may suffer.
Operating Activities
Net cash used in operating activities was $802,964 for the year ended December 31, 2008 as compared to $1,656,945 net cash used in operating activities for the same period in 2007. The decrease of net cash used in operating activities is a result of several factors, including
(i) Increase of $2,269,856 in accounts receivable. This increase was associated with a growth in sales in 2008 compared with 2007 and the development of relationships with additional sales distributors. Our historical accounts receivable term, which differs from the typical contractual term, for distributors (ordinarily within one year) was longer than the historical accounts receivable term for hospitals (usually three to six months).
(ii) Increase of $1,072,813 in prepayments and other current assets. Our prepayment balance refers to amounts paid to suppliers for inventory purchases, and the increase in the amount corresponded with an increase in purchase agreements in 2008 compared with 2007.
(iii) Increase of $1,267,130 in income tax payable. The increase in taxes payable is attributable to the growth in our income and the increase in our applicable tax rate.
These increases were mainly attributable to the growth of our business during 2008.
Net cash provided by operating activities was $1,573,390 for the nine months ended September 30, 2009 as compared to net cash used in operating activities of $978,152 for the same period in 2008. The cash generated from operating activities for the nine months ended September 30, 2009 was approximately $2.5 million more than the same period ended in the proceeding year primarily due to the following:
(i) Increase of $1,544,278 in net income. Our net income increased because of a growth in both sales volume and gross margin in 2009.
(ii) Increase of $1,050,453 in inventory. We have purchased more inventory in 2009 than we did in 2008 to meet management’s expected demand for our products in the future.
(iii) Increase of $642,788 in accounts receivable. The increase in accounts receivable is attributable to the growth in our income.
51
Table of Contents
(iv) Increase of $1,409,645 in tax payable. The increase in taxes payable is attributable to the growth in our income.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2008 was $637,811, compared to net cash used in investing activities of $542,401 in the year ended December 31, 2007. Net cash used in investing activities for the nine months ended September 30, 2009 was $913,717, compared to $597,476 for the nine months ended September 30, 2008. The cash used in investing activities was mainly attributable to capital expenditures for the purchase of new equipment.
Financing Activities
Net cash provided by financing activities in the year ended December 31, 2008 totaled $231,948, compared to net cash provided by financing activities of $2,849,378 in the year ended December 31, 2007. The cash provided by financing activities in the year ended December 31, 2008 was mainly attributable to proceeds received from a bank loan provided by ICBC in Beijing. The cash provided by financing activities for the year ended December 31, 2007 was mainly attributable to the proceeds from issuance of Series B preferred shares and proceeds from bank loans. As of October 31, 2009, the Series B preferred shares were converted into common shares on a 1-for-1 basis.
Net cash provided by financing activities for the nine months ended September 30, 2009 was nil mainly because we did not incur any new bank loan or other financing activities. Net cash provided by financing activities for the nine months ended September 30, 2008 totaled $238,984. The cash provided by financing activities for the nine months ended September 30, 2008 was mainly attributable to proceeds received from a bank loan.
Contractual Obligations and Commercial Commitments
The following table sets forth our contractual obligations as of December 31, 2008:
| Payments due by period | |||||||||||||
| Contractual obligations |
Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years | ||||||||
| Operating Lease Obligations |
$ | 19,855 | $ | 18,328 | $ | 1,527 | — | — | |||||
| Total |
$ | 19,855 | $ | 18,328 | $ | 1,527 | — | — | |||||
The leased properties are principally located in the PRC, and we use such properties for administration and research and development purposes. The leases are renewable subject to negotiation. Rental expenses for the years ended December 31, 2008 and 2007 and for the nine months ended September 30, 2009 and 2008 were $32,011, $28,399, $36,174 and $25,507, respectively. Rent expense paid to the spouse of the chief executive officer for the years ended December 31, 2008 and 2007 and for the nine months ended September 30, 2009 and 2008 was $0, $0, $17,418 and $0, respectively.
Capital Expenditures
We made capital expenditures of approximately $0.71 million and $0.51 million in 2008 and 2007, and $0.92 million and $0.67 million for the nine months ended September 30, 2009 and 2008, respectively, representing 7.53%, 7.74% and 9.72%, 10.23%, of our total revenues, respectively. In the past, our capital expenditures were used to purchase machines for our assembly line. Our capital expenditures may increase in the near term as our business continues to grow and as we expand and improve our financial and accounting systems and infrastructure.
Off-Balance Sheet Commitments and Arrangements
We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
52
Table of Contents
Recent Accounting Pronouncements
In June 2009, the FASB issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162.” The objective of this Statement is to replace SFAS No. 162 and to establish the FASB Accounting Standards Codification as the source of the authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative GAAP for SEC registrants. This Statement shall be effective for financial statements issued for interim and annual periods ending after September 15, 2009. On the effective date of this Statement, all then existing non-SEC accounting and reporting standards are superseded.
In June 2009, the FASB issued SFAS No. 167, an amendment to FASB Interpretation 46(R) “Consolidation of Variable Interest Entities.” The statement requires an entity to perform an analysis to determine whether the entity’s variable interest give it a controlling financial interest in a variable interest entity by rationalizing characteristics that would give it power to direct the activities of a variable interest entity and the obligation to absorb losses or the right to receive benefits from the entity that could potentially be significant to the variable interest entity. The statement is effective for years beginning after November 15, 2009 and is not expected to have a material effect on the Company’s consolidated financial statements.
In June 2009, the FASB issued SFAS No. 166 “Accounting for Transfers of Financial Assets” an amendment to SFAS 140 “Accounting for Transfers and Servicing of Financial Assets and Extinguishment of Liabilities.” The statement defines the term “participating interest” to establish specific conditions for reporting a transfer of financial assets as a sale and improves financial reporting by eliminating (a) the exception for qualifying special-purpose entities from consolidation guidance and (b) the exception that permitted sale accounting for certain mortgage securitizations when a transferor has not surrendered control over the transferred financial assets. The statement is effective for annual reports for years beginning after November 15, 2009 and is not expected to have a material effect on the Company’s consolidated financial statements.
On October 7, 2009, the FASB issued ASU No. 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force. The amendments to FASB ASC 605-25, Revenue Recognition: Multiple-Element Arrangements, permit vendors to account for products and services separately rather than as a combined unit. Any vendor who enters into multiple-deliverable arrangements with customers that are covered by Subtopic 605-25 will be affected, the FASB said. As a result of the changes, multiple-deliverable arrangements will be separated in more circumstances than under existing guidance. With the changes to Subtopic 605-25, the FASB is eliminating the residual method of allocation and instead requiring entities to allocate the arrangement consolidation at the inception of the arrangement to all deliverables using the relative selling price method. Vendors will be required to determine their best estimate of the selling price consistently with the method they use to determine the selling price when the good or service is sold separately. The changes in ASU No. 2009-13 will be effective for revenue arrangements that begin or are changed in fiscal years that start June 15, 2010, or later. Entities that adopt the changes before then will have to apply them to their results from the beginning of their fiscal years. The adoption of this accounting standard is not expected to have a material effect on the Company’s consolidated financial statements.
On August 26, 2009, the FASB issued ASU 2009-05, Measuring Liabilities at Fair Value, to clarify how entities should estimate the fair value of liabilities under the FASB ASC Topic 820, Fair Value Measurements and Disclosures. The amendments in ASU 2009-05 reduce potential ambiguity in financial reporting when measuring the fair value of liabilities. Therefore, preparers, investors, and other users of financial statements will have a better understanding of how the fair value of liabilities was measured, helping to improve consistency in the application of Topic 820. The FASB issued ASU 2009-05 as a result of expressed concern that there may be a lack of observable market information to measure the fair value of a liability. For example, in the hypothetical transfer of an asset subject to a restriction there will be no observable data available to measure the liability because it is restricted from being transferred. This guidance is effective for the first reporting period (including interim periods) beginning after issuance. The adoption of this accounting standard is not expected to have a material effect on the Company’s consolidated financial statements.
53
Table of Contents
Quantitative and Qualitative Disclosure about Market Risk
Interest Rate Risk
Our risk exposure from changes in interest rates relates primarily to the interest expenses associated with our historical bank borrowings, as well as the interest income generated by excess cash invested in demand and time deposits.
We have not historically used, and do not expect to use in the future, any derivative financial instruments to manage our interest risk exposure. Such interest-earning instruments and borrowings carry a degree of interest rate risk. We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates.
Foreign Exchange Risk
Accumulated translation adjustments amounted to $778,766, $398,581 and $774,531 as of December 31, 2008, 2007 and September 30, 2009, respectively. Asset and liability accounts at December 31, 2008, 2007 and September 30 2009 were translated at ¥6.8225 to $1.00, at ¥7.2946 to $1.00 and at ¥6.8263 to $1.00, respectively. The average translation rates applied to the statements of income for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008 were ¥6.9483, ¥7.6038, ¥6.8323 and ¥6.9848 to $1.00, respectively.
Inflation
Although China has experienced an increasing inflation rate, inflation has not had a material impact on our results of operations in recent years. According to the National Bureau of Statistics of China, the change in the consumer price index in China was 0.46%, (0.77%), and 1.16% in 2001, 2002 and 2003, respectively. However in connection with a 3.9% increase in 2004, the Chinese government announced measures to restrict lending and investment in China in order to reduce inflationary pressures in China’s economy. Following the government’s actions, the consumer price index decreased to 1.8% in 2005 and to 1.5% in 2006. In 2007, the consumer price index increased to 4.8%. In response, China’s central bank, the People’s Bank of China, announced that the bank reserve ratio would rise half a percentage point to 15.5% in an effort to reduce inflation pressures. China’s consumer price index growth rate reached 8.7% year over year in 2008.
Taxation
Pursuant to the EIT Law and the supplementary regulations, only high-tech companies that have been re-certified as such under the new criteria are granted the preferential enterprise income tax rate of 15%. Due to the PRC’s standardization of tax rates for all companies in China effective January 1, 2008 and promulgation of new standards for high technology companies, BDL paid the 25% tax rate in 2008. While the certification remained available in 2008, the standards were revised, requiring previously certified entities to apply for high technology certification again. BDL subsequently applied for and obtained such high technology certification approval online and expects to receive the confirmation certificate in the near future. As a result, we have calculated our 2009 estimated income tax expenses at the preferential tax rate of 15%.
54
Table of Contents
Corporate History
Our founder, Mr. Ping Chen, founded BTL on July 5, 2001 to develop and distribute medical devices. He currently owns approximately 86% of BTL, and his wife and employees of Dehaier own the remaining 14% of BTL. BTL leases some of our property to us and provides certain transportation and repair services to medical devices for which we are not obligated to perform warranty services, either because the warranty is expired or because the product was sold by another company. We do not receive any payment for such services from BTL. BTL served as the domestic partner to our joint venture pursuant to which we, a British Virgin Islands company, own the majority BDL, a PRC company in the medical device business. At the time of the formation of the joint venture, foreign enterprises were not permitted to own such companies without PRC partners.
In 2003, in order to continue to grow our business, BTL engaged in a corporate restructuring and Series A venture capital financing. As a result of those actions, Dehaier, BDL and DHK were established and a venture capital investor, De-haier Investment Holdings, Ltd., a British Virgin Islands company, became a Series A preferred shareholder in Dehaier. At the same time, Mr. Chen’s wholly-owned company, Chen Ping Ltd., became a shareholder in Dehaier, and we created the holding company structure that remains in place.
Dehaier was incorporated as an international business company in the British Virgin Islands in 2003 under the name “De-Haier Medical Systems Limited.” We changed this name to “Dehaier Medical Systems Limited” in 2005.
In 2003, we also established BDL in the PRC as a joint venture between a Chinese entity, BTL, and a foreign invested enterprise, Dehaier, in order to allow foreign investments to be used to grow our business. Because BDL is engaged in an encouraged industry under the Foreign Investment Industrial Guidance Catalogue, it was allowed to have foreign investments and to be established as a Chinese-foreign equity joint-venture. This structure allowed BDL access to foreign capital that would not have been available outside this structure. In addition to its engagement in an encouraged industry, the use of this joint venture structure allowed BDL to take advantage of favorable tax rates available prior to January 1, 2008. Before January 1, 2008, equity joint-ventures such as BDL could enjoy a preferential enterprise income tax rate of 24%. In addition to the lower standard rate, such equity joint-ventures were also allowed a two-year income tax exemption from the date they first became profitable and a 50% income tax reduction for the following three years after that time. After January 1, 2008, the income tax rates were unified at 25% under the PRC Enterprise Income Tax Law; however, BDL’s ability to pay lower income taxes prior to this date left it with more net income with which to grow its business. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Tax Matters Applicable to Our Company — PRC Enterprise Income Taxes.”
In 2003, we also founded DHK and created a holding company structure by which we are the parent company of BDL and DHK.
BDL has been focused on the development and distribution of medical devices since its inception and began developing its respiratory and oxygen homecare business in 2006.
Effective as of January 5, 2007, we completed a Series B venture capital financing with Crystal East Group Limited, a British Virgin Islands company. Crystal East Group Limited subsequently completed the transfer of its Series B preferred shares to its individual shareholder, Yijen Chen.
Effective as of October 31, 2009, we converted all of the Series A and Series B preferred shares into common shares on a 1-for-1 basis and completed a 3.66140766-for-1 share split of all common shares. At the conclusion of these actions, we have 3,000,000 common shares issued and outstanding prior to commencement of this initial public offering and no preferred shares issued and outstanding.
Relationship among Dehaier, BTL and BDL
BTL is a PRC company established on July 5, 2001. Dehaier is a BVI company established on July 22, 2003. Dehaier and BTL jointly established BDL on September 24, 2003 as a Chinese-foreign equity joint-venture under the PRC laws. Dehaier has been and is BDL’s foreign shareholder, and BTL has been and is BDL’s domestic shareholder since BDL was established. Currently, Dehaier owns 96.37% of the contributions of BDL, and BTL owns 3.63% of the contributions of BDL. Under PRC laws, the shareholders of the equity joint-venture share the profits, risks and losses in proportion to their respective contributions of the equity joint-venture.
55
Table of Contents
As described above, the business of Dehaier was historically performed by BTL before we completed our Series A venture capital financing in 2003. At the time of the Series A venture capital financing, BTL’s founder, Mr. Ping Chen, sought capital to grow the business, and a foreign (non-Chinese) investor wanted to invest in BTL. Chinese law at the time required such foreign investments in companies like BTL to be in the form of a joint venture between a foreign company and a Chinese company. For this reason, our company was restructured to create a joint venture, with BTL as the Chinese party to the joint venture and Dehaier as the foreign party to the joint venture. Dehaier was considered the foreign party to the joint venture both because it is a British Virgin Islands company and because its shareholders at the time were also British Virgin Islands companies. In connection with the joint venture, BDL was formed as the operating entity. As a result of this restructuring, BDL conducted the business previously conducted by BTL.
Dehaier presently operates as a holding company, of which DHK is its wholly owned subsidiary and BDL is its 96.37%-owned subsidiary. BTL exists primarily as the Chinese joint venture party for the reasons described above. In addition, BTL performs certain limited functions, including leasing some of our property to us and providing certain transportation and repair services to medical devices for which we are not obligated to perform warranty services, either because the warranty is expired or because the product was sold by another company. BDL is our operating entity through which we sell all of our medical products and provide all of our warranty services. We develop, assemble and market our branded products through BDL and market our distributed products through BDL.
Financings and Restructurings
2003 Series A Financing
On October 23, 2003, Dehaier agreed to issue up to $2,400,000 in Series A Preferred Shares to De-haier Investment Holdings Ltd., a British Virgin Islands company. The investor received one-half of these shares in 2003 for $1,200,000 and had an option to acquire the other half of the shares for the remainder of the purchase price. The investor ultimately elected in 2006 not to purchase the other one-half of the Series A Preferred Shares. In connection with the transaction, BDL was formed as a Sino-foreign joint venture with Dehaier holding a majority of the registered capital and BTL holding a minority of the registered capital. In connection with closing, BTL’s assets were transferred to BDL. In connection with the Series A Financing, the parties entered into a Shareholder Agreement that granted certain rights to the Series A Preferred shareholders that terminated upon conversion of the Series A Preferred Shares to common shares.
The Series A financing was exempt from registration under U.S. Securities laws by virtue of exemptions provided by Regulation S, Section 4(2) of the Securities Act of 1933 and by falling outside the an offering covered by Section 5 of the Securities Act of 1933.
2007 Series B Financing
Effective January 5, 2007, Dehaier agreed to issue up to $2,000,000 in Series B Preferred Shares to Crystal East Group Limited, a British Virgin Islands company. In connection with the Series B Financing, the parties entered into a Shareholder Agreement that granted certain rights to the Series B Preferred shareholders that terminated upon conversion of the Series B Preferred Shares to common shares.
The Series B financing was exempt from registration under U.S. Securities laws by virtue of exemptions provided by Regulation S, Section 4(2) of the Securities Act of 1933 and by falling outside the an offering covered by Section 5 of the Securities Act of 1933.
2009 Restructuring
In preparation for the commencement of this initial public offering, our company completed a restructuring to accomplish two goals. First, we sought to convert all Series A and Series B Preferred Shares into common shares. Second, we completed a shares split so that we would have, in the aggregate, 3,000,000 common shares outstanding prior to commencement of the offering. As to the first goal, our Series A and Series B Preferred shareholders agreed to convert their respective Series A and Series B Preferred Shares into an equal number of our common shares. This 1:1 conversion was completed without consideration. As to the second goal, we completed a 3.66140766-for-1 share split of common shares completed of October 31, 2009. As a result of the conversions and share split, we have 3,000,000 common shares issued and outstanding prior to commencement of this offering and no issued and outstanding preferred shares.
56
Table of Contents
Recent Corporate Developments
We believe our business has developed significantly in the last three years. In 2006, we took a major step toward becoming the company we are today by beginning to offer respiratory and oxygen homecare products to supplement the medical devices we sold at that time. As part of this development, we developed our patented “Oxi-chip” technology, which produces highly-concentrated, therapeutic-level oxygen from air at normal temperatures. We also launched our sales of our DHR family of oxygen concentrators. In the course of developing our branded products, we also received our EN ISO 13485 certification, which is a certification by the International Organization for Standardization that recognizes a comprehensive management system for the design and manufacture of medical devices. ISO 13485 certification requires an organization to demonstrate the implementation and maintenance of systems to ensure product safety and quality, and compliance with ISO 13485 is often seen as a first step in achieving compliance with European regulatory requirements. Finally, we negotiated for our second round of venture capital financing in order to continue to develop our business.
This Series B financing closed in early 2007. Upon closing this round of fundraising, we were able to begin a new round of research and development into developing our next generation of oxygen concentrators. Our ventilator product offerings continued to grow, as we began to distribute homecare MAP and S6 ventilators made by ResMed.
In 2008, we focused on growing our sales and support offerings. To that end, we opened an online sales platform for our respiratory and oxygen homecare products to make those products more easily available throughout China. We also established our first Customer Experience Center, or CEC, in Beijing. We have used this CEC to increase our marketing efforts, with the goal of increasing our market penetration in China’s large cities. These CECs are complementary to our current marketing and distribution model because the CECs give our potential customers an opportunity to experience our products first-hand in an environment that is similar to the environment in which they will use the products, whether that is a home or healthcare facility.
In 2009, we developed several CPAP, sleep diagnostic and screening products. We have begun the registration process for these products. We have also developed several technical service products, which are not themselves medical devices but which are used in connection with medical devices. For example, our ventilator trolleys and air compressors are not medical devices but enhance the utility of our ventilators. Finally, in preparation to enter the international market with our branded products, we have established an international business department within our company and have applied for and received CE certificates for our branded oxygen concentrators and air compressors. CE certificates are European conformance marks that designate a product as meeting certain safety, health or environmental requirements in European Union member countries. These CE marks are required before a product may be marketed in European Union member countries.
Since September 30, 2009 we have not developed any new products, received any new patents or entered into any new contracts with new providers. We have not had any material business or corporate developments since September 30, 2009. During this time, we have continued to market and sell our branded and distributed products. We have recently benefitted from additional spending in our industry, as government initiatives and public interest in health care have grown.
China is trending toward greater investment in our industry. The Chinese government implemented large-scale healthcare reform in April 2009. As part of China’s stimulus package, the State Council allocated $123 billion as part of its New Medical Reform Plan. Allocation of this stimulus money has resulted in increased spending in our industry. Additionally, the 2003 outbreak of SARS, the 2006 avian flu and the 2009 swine flu pandemic have heightened public awareness of safe filtering, resulting in increased spending in our industry and increased interest in our products, including, in particular, our products related to ventilators and respiratory care. See “Our Industry,” “Risk Factors” and “Our Market.”
Our Market
China’s Economic Development
China’s population of approximately 1.3 billion people is expected to grow by roughly 9 million people per year. The country’s gross national product has grown at a rate of approximately 9% for more than 25 years, the fastest growth rate for a major economy. In the same 25 year period, China has moved more than 300 million people out of poverty and quadrupled the average Chinese person’s income. The potential of this market is noted by the fact that 400 of the world’s largest 500 companies have invested in China.
In 2010, the Chinese government is posed with the difficult task of maintaining China’s economy in the midst of the global economic financial crisis. China’s economy experienced overall growth of only 9.6% in 2008, its lowest growth rate in seven years, down from a growth rate of 13% in 2007. The global financial crisis has significantly decreased demand for Chinese exports, resulting in factory closures and losses of millions of jobs. The falling demand for Chinese goods has caused the World Bank to predict economic growth of 7.2% for China in 2009, down 2.4% from the seven-year-low of 9.6% seen in 2008. This prediction is also 0.8% lower than the 8% rate the Chinese government had predicted and said would be necessary to avoid significant further job losses. While predictions for China’s growth rate have fallen, China’s growth rate remains well above the World Bank’s forecast for decline of 1.5% for the world economy in 2009. See “Risk Factors – Our operations could be adversely affected by changes in the political and economic conditions in the PRC.”
57
Table of Contents
China’s Healthcare Industry – Generally
From 1940 through the 1950s, China’s government developed a healthcare system that would address the main health considerations of the day – improved sanitation, improved diet and disease prevention. The healthcare system provided universal access to all Chinese citizens. In China’s rural areas local governments generally financed the healthcare system. The results of the government’s focus upon healthcare resulted in a dramatic long-term improvement of the health of China’s citizens. For example, in 1949, the life expectancy of a Chinese citizen was 35 years; by 2009, the life expectancy of a Chinese citizen had risen to over 73 years, more than six years greater than the worldwide average life expectancy.
In the 1980s, the Chinese government, led by Deng Xiaoping, introduced socialist market reforms designed to increase China’s presence in the international community while increasing the standard of living in China. However, these reforms also pushed an ever-increasing portion of healthcare costs upon individual citizens. Consequently, an ever-widening gap arose between the provision of healthcare services to citizens in wealthy, urban areas and those in poor, rural areas. In 1975, over 85% of the rural population was covered by government-provided healthcare; in 1997, that proportion had decreased to 10% of the rural population.
In April 2009, the Chinese government implemented large-scale healthcare reform. The State Council allocated $123 billion as part of its New Medical Reform Plan. The plan, which is part of China’s stimulus package aimed at correcting the recent global economic down-turn, contemplates the development of a universal healthcare system that will cover 90% of China’s population by 2010. In addition, the plan includes significant improvements to health care facilities and expansion of China’s health related infrastructure.
Specifically, within three years, the Chinese government aims to improve the urban healthcare system by rebuilding and restructuring approximately 3,700 existing urban community health centers and 11,000 community health clinics. The plan will also accommodate the development of approximately 2,400 new urban health centers. In effect, the plan de-emphasizes the prevalence of large, magnet facilities in favor of smaller, more accessible clinics.
In addition, the plan is designed to dramatically improve medical services available for the 800 million rural poor in China. Through the plan, the Chinese government contemplates the development of clinics in every village and a hospital in every county in China by the end of 2011. If successfully implemented, the plan would result in at least 2,000 new county hospitals and 29,000 village clinics.
China’s Healthcare Industry – Medical Device Market Background
China’s medical device market is projected to grow faster than the global medical device market. Reasons for this faster growth in China include:
A fast growing domestic economy. China’s gross domestic product is expected to grow by 7.2% in 2009 while many leading world economies are expected to contract.
Increasing desire for and utilization of more advanced technologies in Chinese hospitals and clinics. The market penetration of common medical equipment in Chinese hospitals is low compared to hospitals in more developed countries. However, we believe hospitals in China are purchasing more advanced technology as they attempt to compete for patients and generate additional profits.
Increasing availability of healthcare insurance. The increasing availability of healthcare insurance generally provides coverage for more advanced and extensive healthcare services than were previously available.
Increasing autonomy at the hospital level. Although governmental entities own and control substantially all of the hospitals in China, recent healthcare system reforms have resulted in a trend of greater operating autonomy at local levels. For example, hospitals in China today rely less and less on governmental funding and are generally expected to earn enough revenues on their own to cover 70% to 90% of their operating expenses. This has led to a greater focus on achieving efficiencies and improving services by regional hospital administrators, who now typically have the authority to make decisions regarding equipment purchases.
58
Table of Contents
Increasing government focus on improving quality of care. The outbreaks of SARS in 2003, avian flu in 2006 and swine flu in the last two years have heightened the government’s awareness of the need to improve the country’s healthcare infrastructure, and healthcare has become a priority for the PRC government.
Chinese Healthcare Institutions. According to the PRC Ministry of Health, there were approximately 19,847 hospitals and 270,000 healthcare clinics in China in 2007. The hospitals, which on average had approximately 135 beds, can be further divided into approximately 1,182 large-sized hospitals, 6,608 medium-sized hospitals and 12,057 small-sized hospitals, commonly referred to as Tier III, Tier II and Tier I and other hospitals, respectively, in China.
Chinese Medical Device Manufacturers. According to China Medical Device Manufacturer magazine, as of 2008 there were approximately 13,000 medical device manufacturers in China, but most are small- and medium-sized companies producing basic medical supplies, such as bandages, patient aids and medical or surgical instruments. However, only 60 companies in China accounted for RMB100 million in sales per year as of 2008. However, more advanced medical products are expected to be produced in China in the next few years. Those China-based companies that are able to develop and manufacture more advanced products at lower costs than their international competitors should be able to capitalize on the growing desire for better quality of care in China and emerge as leaders in domestic medical device manufacturing.
Medical Device Marketing and Distribution in China. Hospitals in China purchase a majority of their medical devices and supplies through distributors. Medical device distribution is highly specialized and localized in China. Most medical device distributors operate within relatively small territories. Few distributors are willing or able to cover the entire country. Most distributors focus on China’s eastern coastal cities, where purchasing power is concentrated, while western China tends to have very limited coverage. In addition, different provinces in China often have their own medical and insurance practices, purchasing policies and regulatory requirements which further increases the complexity of medical device distribution. As a result, most manufacturers need to appoint multiple distributors to effectively cover all of the geographic areas in China. The ability to leverage local contacts and knowledge is vital in creating an effective distribution network in China, creating a significant barrier to entry for both smaller local companies and larger international competitors that lack a meaningful local presence.
Our Strengths
We believe we have the following principal competitive strengths, enabling us to attain a leading position in China’s medical device and respiratory and oxygen homecare industries:
| • | Our Brand and Leading Market Position. We believe that we have established ourselves as one of the most recognized brands in the medical device and respiratory and oxygen homecare markets in China. We believe we have one of the most experienced management teams in China’s medical device and respiratory and oxygen homecare industries. Each of our executive officers has over 10 years of experience in the medical device and respiratory and oxygen homecare industries. Over time they have been able to develop a comprehensive understanding of local markets and customer needs within those markets. Through our experienced leadership, strong brand and market position we have developed a broad customer base throughout China, allowing for more rapid acceptance of our branded products. |
| • | Distribution, Sales and Service Network. Through our extensive distribution, sales and service network for medical devices and respiratory and oxygen homecare products in China, we have established a strong platform of business contacts and local knowledge which enables us to develop products and provide services tailored to our customers’ local needs. We have contractual distribution relationships with over 2,000 independent distributors, and we employ 70 direct sales and sales support personnel. We do not own, employ or control these independent distributors. |
| • | Research and Development Capabilities. Our research and development team has 20 engineers. We increased our annual investment in research and development activities in each of the last several years. We developed eight new home respiratory therapy products in the last four years, including three new products in 2008. We also have plans to develop six new home respiratory therapy products in 2009. In addition to developing new products, our research and development efforts have helped us to improve our assembly processes, reduce our costs, and make the development process quicker and more efficient. |
| • | Vertically Integrated Operating Model. We employ a vertically integrated operating model that enables us to efficiently develop, assemble and market quality products at competitive prices. We believe this integrated model allows us to lower our costs for materials and components, reduce our capital expenditures, create a more efficient workflow, and improve our quality control. This model also provides for a more effective upstream and downstream channel of business cooperation. |
59
Table of Contents
| • | Relationships with Foreign Medical Device Manufacturers. We distribute a wide range of medical devices in China for several foreign medical device manufacturers, including IMD, Timesco, JMS and ResMed. |
| • | Customer Support and Service Team. We have customer support teams located in our Beijing headquarters and each of our 26 offices throughout China. This extensive customer support network allows us to provide domestic training, technical support, and warranty, maintenance and repair services to end-users of our products, as well as support and service to our distributors. We also maintain a 24-hour customer service center in Beijing, staffed with senior technical support engineers prepared to provide preliminary support and on-site guidance and repair if necessary. |
Our Products – Overview
When we began to sell our products in China, substantially all of the products we sold were distributed products from foreign suppliers. When we completed our Series A financing in 2003, we used some of the proceeds from that financing to establish a research and development department in order to develop products of our own to address market needs.
At that time, our management concluded that it could develop its own products for sale through its existing network of independent distributors, who were already selling medical devices for our company to our desired customer base. We made this decision based in large part on our conclusion that we would be able to meet the needs of different markets with our branded products than we already met with our distributed products. Because our distribution team was already in place, we only needed to form research and development and assembly teams to be able to offer its branded products. As noted above, the Series A financing proceeds allowed us to form these teams. Based on feedback from our distribution team, our management has been able to provide guidance to our research and development team about what needs to address with new products and what improvements to make to existing products. This feedback has grown sales of our branded products.
Prior to 2009, our branded products have accounted for approximately one-third of our revenues, and our distributed products have accounted for approximately two-thirds of our revenues. In the nine months ended September 30, 2009, our branded products and distributed products have each accounted for approximately half of our revenues.
Our Products
Our branded and distributed products fall into three general categories: (i) medical devices; (ii) technical service products and (iii) respiratory and oxygen homecare products. Our medical devices and technical service branded and distributed products are mainly used in hospitals and clinics, while our respiratory and oxygen homecare branded and distributed products are mainly for at-home use by individual customers. Our technical service branded products consist of components for use in ventilators. Prior to 2009, our branded products have accounted for approximately one-third of our revenues, and our distributed products have accounted for approximately two-thirds of our revenues. In the nine months ended September 30, 2009, our branded products and distributed products have each accounted for approximately half of our revenues.
| For the years ended December 31, |
For the nine months ended September 30, |
|||||||||||||||||||
| 2008 | 2007 | 2009 | 2008 | |||||||||||||||||
| US$ | US$ | US$ | US$ | |||||||||||||||||
| Branded products |
3,462,559 | 35.70 | % | 2,193,764 | 33.24 | % | 4,731,057 | 50.21 | % | 2,084,210 | 32.91 | % | ||||||||
| Distributed products |
6,235,501 | 64.30 | % | 4,405,748 | 66.767 | % | 4,691,403 | 49.79 | % | 4,447,644 | 68.09 | % | ||||||||
| Total |
9,698,060 | 6,599,512 | 9,422,460 | 6,531,854 | ||||||||||||||||
60
Table of Contents
Our Branded Products
Our management believes that our branded products, which are generally less expensive than products from foreign companies, tend to be more attractive to smaller city and rural hospitals and healthcare facilities and other end-users for whom price is a significant factor in deciding whether to purchase our products. Our branded products include medical devices, technical service products and respiratory and oxygen homecare products.
Medical Devices
| • | Mobile Medical X-Ray Image Devices. We provide four types of DHR Explorer Series mobile and C-armed X-ray machines. X-ray is used for visualizing bone structures and other dense tissues such as tumors. These mobile and C-armed X-ray machines provide added convenience for use in hospitals and clinics. Our C-arm series of X-ray systems are suitable for ortho reduction and fixation procedures, intervertebral disc imaging and treatment, spinal operation, uterine and oviduct imaging, bladder and ureter imaging and gastric imaging. |
| • | Anesthesia Machines. We provide two types of DHR ORSA Series anesthesia machines. These machines are used by anesthesiologists to support the administration of anesthesia. These machines administer a precise and continuous supply of anesthetic gases and vapors to the patient at accurate and safe levels of pressure and flow. These machines maintain a continuous, closed-loop control over the pressure of gas within a patient’s mouth or respiratory according to the selected pressure input. In addition, these machines feature a modular design for mobility and ease of maintenance, cleaning and disinfection. |
| • | Patient Monitoring Devices. We provide two types of DHR 930 patient monitoring devices which monitor patients’ physiological parameters, such as heart rate, blood pressure, respiration and temperature. We also provide polysomnograph devices, which are used to monitor and record patients’ sleep data through multiple parameters. These machines are suitable for adult, pediatric and neonatal care and principally used by hospitals in intensive care units, operating rooms and emergency rooms. Our monitors have a number of optional features, such as local area network connections, optional printer features, different screen sizes, and the ability to use in monitoring up to 32 patients at a time. |
Technical Service Products
| • | Ventilator Air Compressor. We provide two types of air compressors to support medical ventilators in surgery by supplying continuous airflow for the ventilator. Where a facility lacks a central pressured air supply system, our C250 and C280 air compressors provide a portable source of such pressured air. Our air compressors feature oil-less motors, large locking castors, high flow capacity, and spill-proof switches. We have designed our air compressors to be adaptable for use with any ventilator. |
| • | Trolleys for Ventilators. We provide three types of trolleys to hold ventilators and their accessories for mobility. These trolleys can be fit with a monitor to further enhance the portability and utility. |
| • | Sterilizers for Ventilators. We provide one type of sterilizer to treat the air from patients in order to control cross-contamination and infection in a facility in general and for subsequent ventilator patients in particular. |
Respiratory and Oxygen Homecare Products
| • | Oxygen Concentrating Products. We provide two types of oxygen concentrator products, including, the DHR-3L/5L Oxi-Fairy and the DHR-3L/5L Oxi-Pioneer. These products use our patented advanced Pressure Swing Absorbing (“PSA”) technology to produce highly-concentrated, therapeutic-level oxygen (approximately 90% oxygen concentration) from air at normal temperatures. These products are used by patients with cardiovascular disease, respiratory diseases, such as chronic obstructive pulmonary disease, and geriatric patients. |
| • | Sleep Apnea Treatment Products. We have designed and expect to provide several products designed for obstructive sleep apnea (“OSA”) therapy. These products include our DHR CPAP C5, DHR Auto CPAP A8, and DHR Auto S-CPAP A9. Our DHR CPAP C5, Auto CPAP A8 and DHR S-CPAP A9 are in the process of obtaining SFDA approval and will not be available for sale until we receive such approval. While we expect to receive this approval within the first half of 2010, we cannot guarantee that we will obtain such SFDA approval in this timeframe or all. These products are all non-invasive therapy products that treat symptoms of sleep apnea. Our CPAP devices do not cure apnea but instead use air pressure to |
61
Table of Contents
| open customers’ airways to reduce snoring and apnea disturbances during sleep. Our automatic CPAP products provide air pressure at a customized, adjustable level, while our traditional CPAP products provide a constant level of air pressure. |
| • | Diagnostic Products. We have designed and expect to provide two types of screening and diagnosis products are portable sleep respiratory recording devices that can be used in a healthcare facility or in a patient’s home to assist physicians in determining whether the patient has obstructive sleep apnea requiring use of a CPAP device. We have applied to SFDA for approval of our DHR 998 and DHR 999 diagnostic products, and they will not be available for sale until we receive such approval. While we expect to receive this approval within the first half of 2010, we cannot guarantee that we will obtain such SFDA approval in this timeframe or all. |
| • | Thermotherapy Products. We have designed and expect to provide our thermotherapy device for patients with rhinitis and delivers atomized clean water at a therapeutic temperature to the affected site. We have not yet begun to market this device and cannot project when we will begin to market this device. |
Research and Development of Our Branded Products
Our success to date has in part resulted from our strong research and development capabilities, which allow us to regularly introduce new and more advanced products at more competitive prices within a shorter period of time. We increased our annual investment in research and development activities as a percentage of net revenues every year since 2003. Research and development costs were $87,660, $47,228, $50,671 and $36,989 for the years ended December 31, 2008 and 2007, and for the nine months ended September 2009 and 2008, respectively. Our research and development team consists of 20 engineers, representing more than one-tenth of our employees nationwide.
Our project selection goals focus on projects that we believe are commercially feasible, can generate significant revenue and can be introduced into the market in the near-term. While our research and development department may conduct research into areas that are likely to lead to short-, medium- and long-range business opportunities for our company, we focus our development of products on those solutions we believe are most likely to generate significant near-term revenues. Thus, we would generally devote more resources to a solution expected to have an immediate financial return (for example, a ventilator) than to a project with a potentially greater overall payoff that is more distant and tenuous (for example, an artificial lung).
Our management seeks feedback from our distribution network to learn about needs for future products and improvements to existing products that our research and development department can seek to address. Once we identify a product opportunity, our sales and service, research and development, and assembly teams work closely together to determine potential market demand for a product and how it fits with our current design and assembly capabilities. We organize regular meetings in which our sales and service, research and development and assembly teams review progress and, if necessary, adjust the emphasis of our research and development projects.
If we deem a new product to be commercially feasible, our research and development team will work closely with our assembly team to move assembly forward. This integrated approach allows us to identify potential difficulties in commercializing our branded product or product improvement. Furthermore, it enables us to make adjustments as necessary and develop cost-efficient assembly processes prior to distribution. We believe these abilities can significantly shorten the time it takes to launch a commercialized product. In the last three years, we have developed and brought to market 5 new products, which appeal to a wide range of end-users.
We maintain a 5,400 square foot research and development center in our facility in Beijing, which allows us to compete for skilled research and development technicians and managers. In addition, we are enhancing our research and development ability by cooperating with the research institutes of two top ranking Universities in China: Beijing University of Aeronautics & Astronautics and Beijing University of Technology and Science.
62
Table of Contents
Principal Suppliers – Our Branded Products
We use the following principal suppliers to manufacture the components in the products we develop and assemble:
| • | Friend of Health (Chuzhou) Medical Technology Co., Ltd. |
| • | IMI Morgren Trading (Shanghai) Co., Ltd |
| • | Yuyao Best Medical Device Parts Factory |
| • | Contec Medical Systems Co., Ltd. |
| • | Tianjin HSD Metal Product Co., Ltd. |
We believe the components provided by our suppliers are widely available and do not anticipate that we will be unable to obtain these components from other suppliers in the event our principal suppliers are unable or unwilling to supply us. Notwithstanding this fact, we currently purchase approximately 13.5% of our supplies from Friend of Health (Chuzhou) Medical Technology Co., Ltd., and this company may be considered a major supplier or our company.
We provide the technical specifications and files needed for our suppliers to manufacture components. We purchase the same components from a wide variety of suppliers; for example, we purchase a component of our oxygen concentrator, the oxi-chip, from several suppliers, including Yueqing Dingji Pneumatic Machinery Factory, Shanghai Showpeak Molecular Sieves Co., Ltd., Yuyao Best Medical Device Parts Factory and Beijing Xishenglong Electric Equipment Co., Ltd. We only provide separately outsourced core parts to Friend of Health to incorporate into components.
We outsource the production of some of our oxygen concentrators and air compressors to Friend of Health under production agreements. We provide the technical specifications and files needed for Friend of Health to manufacture these branded products. We provide separately outsourced core parts to Friend of Health to incorporate into the components they assemble and distribute for us. We test and approve each part produced by Friend of Health before they begin mass production. We require Friend of Health to maintain minimum supplies of our branded products and components for use in our branded products, and we permit them to sell our branded products only in Chuzhou. Friend of Health delivers products to us on credit, and we are required to make payment within 45 days after the date of delivery. Friend of Health is the only supplier that also assembles and distributes finished branded products for us.
Assembly of Our Branded Products
After our research and development team designs the technical specifications and computer models for our branded products, we typically work with an independent contractor to fabricate working prototypes before we commence with the production run of a product. We test prototypes to confirm that they operate as expected and with the quality we require. During the prototyping process, we apply for SFDA approval as necessary. Once both of these processes are completed, we commission a production run of components for assembly into our branded products.
We depend on component and product manufacturing and logistical services provided by third parties. All of our branded products are manufactured in whole or in part by a variety of third-party manufacturers. While these arrangements may lower operating costs, they also reduce our direct control over production. It is uncertain what effect such diminished control will have on the quality or quantity of products or services, or on our flexibility to respond to changing conditions.
We maintain a 32,000 square foot product center in Changping Science Park in Beijing. This product center contains our research and development area and our assembly facilities. Final assembly of our products is currently performed in this facility by our 40 employees in assembly and by some of our external vendors, such as Friend of Health. Currently, the supply and manufacture of many critical components is performed by sole-sourced third-party vendors in China.
Proprietary Rights for Our Branded Products
We are developing a portfolio of intellectual property rights in China to protect the technologies, inventions and improvements that we believe are significant to our business in China. We have two patents issued in China for an oxygen concentrator. We have applied for five additional patents related to our CPAP devices (2), portable sleep screening (2) and diagnostic services (1). Moreover, we possess proprietary technology and know-how in assembly processes, design and engineering. We have not filed for any patent protection outside of China. To protect our brand name recognition, we have registered the brand name “Dehaier” for trademark protection in China.
Our success in the medical equipment industry depends in substantial part on effective management of both intellectual property assets and infringement risks. In particular, we must be able to protect our own intellectual property as well as minimize the risk that any of our branded products may infringe upon the intellectual property rights of others.
63
Table of Contents
We enter into agreements with all our employees involved in research and development, under which all intellectual property generated during their employment belongs to us, and they waive all relevant rights or claims to such intellectual property. All our employees involved in research and development are also bound by a confidentiality obligation and have agreed to disclose and assign to us all inventions conceived by them during their term of employment.
We believe that we have successfully established our brand in China. We have registered trademarks in China for the Dehaier name and logo used on our own-brand products. As part of our overall strategy to protect and enhance the value of our brand, we actively enforce our registered trademarks against any unauthorized use by a third party.
Our Distributed Products
Our management believes that our distributed products, which are generally more expensive than products from Chinese companies, tend to be more attractive to larger city hospitals and more affluent healthcare facilities and other end-users for whom perceived quality is a significant factor in deciding which products to purchase. While we believe that the quality of our branded products is also strong, we understand that some consumers in China associate more well-known international brands with higher quality than they associate with domestically produced brands.
We serve as a significant distributor in China for several foreign producers of medical devices and respiratory and oxygen homecare products, including IMD, Timesco, JMS and ResMed. We believe this extensive platform allows us to be responsive to local market demand. We distribute medical devices and respiratory and oxygen homecare products for these companies.
Medical Devices
| • | Mobile Medical X-Ray Image Devices. We provide two types of X-ray machines developed by other companies: the IMD Radius Series mobile and C-armed X-ray machine and the IMD Compact Series mobile-X-ray machine. |
| • | Medical Ventilator. We provide three types of ventilators developed by other companies: the ResMed VS Serena, Ultra and Integra Ventilators. The VS Series of medical ventilators mechanically move breathable air to and from the lungs to support breathing support for patients who are physically unable to breathe or who are breathing insufficiently. |
| • | Laryngoscopes. We provide four types of laryngoscopes developed by other companies: the Optima, Optima XL and Eclipse lines of laryngoscopes from Timesco. Laryngoscopes are flexible lighted tubes that are used to look at the inside of the larynx. Anesthesiologists make use of laryngoscopes to assist with intubation in surgery. |
Respiratory and Oxygen Homecare Products
| • | Sleep Apnea Treatment Products. We provide two types of products developed by other companies designed for obstructive sleep apnea (“OSA”) therapy. These products include the ResMed S6 and MAP MiniPAP. |
Our Relationships with Suppliers of Our Distributed Products
While we develop, assemble, market and sell our branded products, we also serve as the distributor for a number of international companies looking to sell their brands of products in China. We are a distribution agent for some or all products marketed in China by IMD (Italy), Timesco (UK), ResMed (Australia) and JMS (Japan). In this capacity, we are responsible for sales, marketing and after-sale services of these products.
We sign agency agreements with these international suppliers annually with the aim of settling marketing promotion modes, costs, product training and resolution of customer service issues. The agency agreements cover purchasing price, purchasing intervals, order quantity, transportation and type of payment, spare part supply and after-sale service terms. We negotiate renewal of these agency agreements as they expire to confirm ongoing distributor expectations.
64
Table of Contents
We seek to enlarge the scope of products we are able to sell as agent for these companies and constantly try to identify competitive suppliers and products on the international market to assist them with marketing and selling their products in China.
Agreements with Current Suppliers of Our Distributed Products
We currently have supply agreements with suppliers of our distributed products. The key terms of those agreements are set forth in this section.
IMD China Co., Ltd. Our current Sales Agency Agreement with IMD is dated December 21, 2009. Under this agreement, we have been appointed to act as IMD’s exclusive agent in the PRC to sell the IMD Radius S9 and Radius R9 Mobile C-arm X-Ray Unit; provided, however, that third parties may also deal directly with IMD if they choose. We have undertaken to sell not less than $2 million of these units between January 1, 2010 and January 1, 2011. This agreement may be extended with mutual consent and may be terminated if either party fails to perform under the agreement.
Timesco of London Ltd. Our Agency Agreement with Timesco is effective September 8, 2009 for a period of one year but subject to review on performance every six months. We have been appointed to act as Timesco’s exclusive agent in the PRC, excluding Hong Kong, Taiwan and Macao, to sell its Optima, Optima XL and Eclipse laryngoscopes. Timesco may fulfill any contracts, orders or quotations prior to the effective date of the agreement but has agreed to refer all inquiries after such date to our company. We are not permitted to sell any competitors’ laryngoscopes that compete with Timesco’s Optima, Optima XL and Eclipse lines of laryngoscopes. Our annual sales target under this agreement is $200,000, and we are expected to place a minimum order for $50,000 every three months. We must pay half of the cost for such orders before shipment and the remainder within 90 days.
Dalian Wande Electronics Co., Ltd. (JMS). Our Sales Agency Agreement with JMS is effective November 1, 2009 for a period of one year. Under this agreement, we have been appointed by JMS’ General Agent, Dalian Wande, to serve as JMS’ product distributor in the PRC. We distribute JMS syringe pumps and infusion pumps under this agreement. Payment must be made prior to delivery of such pumps. We may not sell competing pumps during the term of the agreement.
ResMed Asia Pacific Limited. Our Distribution Agreement with ResMed is effective July 1, 2009 for a period of twelve months. This agreement is subject to extension for a subsequent twelve month period if we are in compliance and provide notice to ResMed of our intention to renew sixty days prior to July 1, 2010. The agreement may be further renewed upon agreement with ResMed. This Distribution Agreement appoints us as the non-exclusive distributor of ResMed products in Sichuan, Hubei, Chongqing, Guizhou, Guangxi, Yunnan, Anhui, Shanxi (except for homecare), Henan, Guangdong (except for homecare), Hunan and Hebei provinces. In our first year under this agreement, we must purchase at least $600,000 of ResMed products; if the agreement is renewed, we must purchase at least $800,000 of ResMed products during the second year. ResMed has the ability to discontinue products or increase prices of products with 60 days’ prior notice. ResMed may terminate the agreement in the event we provide non-ResMed equipment for the diagnosis or treatment of sleep disordered breathing. We are required to provide monthly forecasts of our requirements for ResMed Products for the next three months, and the first month’s projection is considered a firm commitment to purchase such products from ResMed, subject to its acceptance of such offer. In addition, we must keep sufficient ResMed stock to meet one month’s projected requirement. We are required to provide the original purchaser of ResMed Products a warranty which is to be fulfilled by ResMed. We are obligated to meet certain minimum levels of sales and marketing activity with respect to ResMed products and training.
65
Table of Contents
Principal Suppliers – Our Distributed Products
In addition to the products we design, we distribute products designed and manufactured by the following companies:
| • | IMD (Italy) |
| • | Timesco (UK) |
| • | ResMed (Australia) |
| • | JMS (Japan) and |
The exact products from these suppliers are available only from such suppliers; however, we do not anticipate that we will be unable to obtain similar products from other suppliers in the event our principal suppliers are unable or unwilling to supply us. In addition, we have designed similar products to those supplied by IMD and ResMed. See “We sell products for some of our competitors, some of which compete with our branded products.”
Service
We maintain a 24-hour customer service center in Beijing for technical support and repair. We staff our customer service center with senior technical support engineers who provide preliminary support. Our engineers attempt to quickly diagnose and assist in repairing problems over the phone, or determine whether a service visit to the customer’s premises is necessary. In some instances, our engineers will provide on-site operating guidance and repair service. We periodically review customer calls to ensure that any issues raised by our customers are resolved to their satisfaction.
Our Strategies
Our objective is to strengthen our position as one of China’s leaders in developing, distributing and marketing medical devices and home respiratory therapy products and to become an industry leader in certain select foreign markets. We intend to achieve our objective by implementing the following strategies:
| • | Increase Market Share. We have plans to develop and introduce more technologically advanced home respiratory products while maintaining steady rates of growth in the sales of our existing medical devices. We plan to establish 32 new Customer Experience Centers (“CEC”) over the next three years after completion of this offering, add more direct sales personnel, and increase our marketing activities to further penetrate China’s market. These CECs are complementary to our current marketing and distribution model because the CECs give our potential customers an opportunity to experience our products first-hand in an environment that is similar to the environment in which they will use the products, whether that is a home or healthcare facility. We plan to target distributors, hospitals, clinics and individuals with the CECs. CECs can provide marketing, training and after-sales support to distributors, hospitals and clinics and can provide a experience to individuals, whether they come in on their own or referred by a hospital doctor. We plan to establish CECs in each of China’s 32 provincial capital cities. We expect to open these CECs in order based on considerations of existing sales of the products we sell, estimated potential market size and other strategic considerations, such as geographic diversity and expected profitability. We have not yet determined the order in which we plan to establish these CECs or the cost of such CECs, as the order and exact timing will depend substantially on whether and when this offering is completed and the expense of establishing a CEC will depend on market research that we will conduct prior to beginning to establish a given CEC. We expect that the order may change over time as the foregoing variables change (for example, if a potential market grows dramatically or if one of the products we sell becomes more popular in one province than another). At present, we expect to place our highest priority on building CECs in Beijing, Shanghai and Guangzhou. The cost of building and maintaining a CEC in a large city like Beijing is approximately RMB 422,000 (approximately $61,820) in the first year. We also plan to capitalize on the anticipated market growth by leveraging our significant local industry expertise, strong brand recognition, broad customer base and established distribution network. Additionally we plan to increase our exposure through increased participation at industry exhibitions and technology forums. |
66
Table of Contents
| • | Expand into Select Foreign Markets. Assuming successful completion of this offering and the receipt of approval to sell our branded products in the European Union, we currently plan to expand initially into Europe. We have not yet qualified or applied to sell our branded products in the European Union, and we have no guarantee that we will be successful with any such application for approval. We have received CE certificates for two of our branded products (our oxygen concentrators and air compressors). We are applying for CE certificates for other branded products. CE certificates are European conformance marks that designate a product as meeting certain safety, health or environmental requirements in European Union member countries. |
| • | Increase Research and Development. We intend to broaden our market reach by introducing more advanced branded products and new branded product lines that address different end-user segments. Some of the segments we intend to direct future focus to include: oxygen therapy and health care, sleep and respiratory diagnosis and therapy, and homecare data collection and transportation. |
| • | Enhance Market Position and Brand Recognition. We plan to enhance our position in the market and bolster our brand recognition through the above mentioned 32 CECs which will be established in each of China’s 32 capital cities. The CECs will provide purchasers of our branded products with extensive pre-sale education in the use of the branded product as well as post-sale service of the branded product. We also plan to grow our international business though the establishment of a more extensive distribution network and technical service products relationships with international manufacturers and distributors. |
| • | Maintaining Our Disciplined Cost Focus. We plan to maintain our disciplined cost focus while further improving our cost structure. Our research and development team will work with our assembly team to optimize our design in order to improve our margins and competitive cost advantages. As our sales volume increases, so too will the size of our purchases of components. We intend to take advantage of this situation by leveraging our purchasing power to reduce our purchasing costs. As we build economies of scale, we anticipate further increases in our operational efficiencies. |
| • | Maintain Distribution Network. We plan to maintain our existing distribution network through active management of our distributors. We will periodically review the performance of our distributors and assess areas that need improvement; agreements with distributors who fail to perform to our standards will be terminated. |
| • | Pursue Strategic Acquisitions and Alliances. We expect to evaluate potential acquisitions of assets and companies as a part of our efforts to expand our presence in the medical device market. We expect to focus on acquisitions that enhance our ability to serve our existing customer base, enter new market segments in China or grow into new international markets. We have not yet identified any potential targets, and we do not have any understandings, commitments or agreements in place with regard to such acquisitions. We cannot guarantee that we will identify favorable opportunities or that we will be able to negotiate acceptable acquisition terms if we do locate such opportunities. |
67
Table of Contents
Customers
We have three categories of customers: distributors, hospitals and government agencies and individual consumers to whom we sell directly. Our customer base is widely dispersed on both a geographic and revenues basis.
Our distributors. Sales to our distributors make up the substantial majority of our revenues as over 90% of our sales are to distributors. Based on the expected use of products sold to distributors, we estimate that they sell approximately 60% of our products to hospitals, 20% to clinics and 10% to individuals. As a result, we estimate that approximately 67% of our products (on a revenue basis, rather than unit basis) are sold to hospitals, approximately 21% to clinics and approximately 12% to individuals. We have contractual distribution relationships with over 2,000 independent distributors. We do not own, employ or control these independent distributors.
Hospital and governmental agency customers. Our hospital and governmental agency customers account for approximately 7% of our direct sales. Our hospital and governmental agency customers primarily include hospitals as well as provincial level public health bureaus and population and family planning bureaus. These customers typically place large volume orders that are awarded based on bids submitted by competing medical equipment companies through a state-owned bidding agent.
Individual consumers. We sell our home respiratory therapy products directly to consumers through our CEC centers and through the E-commerce website: www.cpap-oxygen.com. The information on this website is not incorporated in this prospectus. Such sales account for approximately 2% of our direct sales.
Dependence on Major Customers. For the years ended December 31, 2008 and 2007, and for the nine months ended September 2009 and 2008, approximately 11%, 17%, 8% and 7%, respectively, of our sales were from one customer, Poverty Aid Office.
Our business would be harmed if we lost or reduced our relationships with Poverty Aid Office. See “Risk Factors – Our revenues are highly dependent on a limited number of customers involved in China’s healthcare device industry.”
Concentration of Receivables. At December 31, 2008 and 2007, receivables from four customers were approximately 17%, 13%, 11%, 10% and 20%, 12%, 11%, 10%, respectively. At September 2009 and 2008, receivables from four customers were approximately 9%, 9%, 8%, 7% and 16%, 14%, 9%, 8%, respectively. With the exception of Poverty Aid Office, no other customer accounted for more than ten percent of our revenues, notwithstanding having receivables at various balance sheet dates in excess of ten percent of total receivables.
The table below shows the four highest receivable percentages as of December 31, 2007 and 2008 and September 30, 2008 and 2009. The four customers vary at each period and only our major customer, Poverty Aid Office, has been identified. The loss of any other customer would not materially affect our business.
| December 31, 2007 |
December 31, 2008 |
September 30, 2008 |
September 30, 2009 | |||||||||||
| A | 20% | B (Poverty Aid Office) | 17% | B (Poverty Aid Office) | 16% | H | 9% | |||||||
| B (Poverty Aid Office) | 12% | E | 13% | A | 14% | I | 9% | |||||||
| C | 11% | F | 11% | F | 9% | J | 8% | |||||||
| D | 10% | G | 10% | E | 8% | K | 7% | |||||||
Competition – Generally
The medical device industry is characterized by rapid product development, technological advances, intense competition and a strong emphasis on proprietary information. Across all product lines and product tiers, we face direct competition from both domestic and international competitors. We compete based on factors such as price, value, customer support, brand recognition, reputation, and product functionality, reliability and compatibility. Each of our branded products competes against functionally similar products from domestic and international companies.
Our competitors include publicly traded and privately held multinational companies, such as Respironics, Inc., ResMed Inc., and Covidien, as well as domestic Chinese companies such as Beijing Aoji, Beijing Ya’ao, Jiangsu Yuyue and Zhejiang Longfei. We believe that we can continue to compete successfully in China because our established domestic distribution network and customer support and service network allows us significantly better access to China’s small and medium-sized hospitals. In addition, our strong investment in research and development, coupled with our low-cost operating model, allows us to compete effectively for sales to large hospitals.
68
Table of Contents
We believe our competitive position in China varies depending on the product in question. While we are a much smaller company overall than, for example, General Electric, Siemens or Philips and are unable to offer the range or depth of products each of those companies offers, we believe our market position is favorable in several segments. The following charts provide our marketing department’s estimations of our primary competitors by product, both as to our branded products and as to our distributed products:
| Branded Product |
Primary Competitors in China |
Dehaier’s Estimated Competitive Position* | ||
| DHR Explorer Series C-armed X-ray machine | Nanjing Pulang, Beijing Wantong, Beijing Smart, Shenzhen Nanyun | Average | ||
| DHR ORSA Series anesthesia machines | Drugg, GE, Spacelab | Average | ||
| DHR 930 patient monitoring devices | Shenzhen Mindray, Shenzhen Jinkewei, Zhuhai Baolaite | Average | ||
| C250 and C280 air compressors | Beijing Yi’an, Guangdong Puling | Greater than average | ||
| Trolleys | An OEM business model, N/A | N/A | ||
| Sterilizers | An OEM business model, N/A | N/A | ||
| DHR oxygen concentrator | Beijing Aoji, Beijing Ya’ao, Jiangsu Yuyue and Zhejiang Longfei | Smaller than average | ||
| DHR CPAP C5,* DHR Auto CPAP A8,* DHR Auto S-CPAP A9 | Foreign companies such as Respironics, ResMed, and Covidien | Not in market yet | ||
| DHR 998* and DHR 999* screening and diagnosis products | Foreign companies such as Respironics, ResMed, and Covidien | Not in market yet | ||
| DHR thermotherapy device | none | Not in market yet | ||
| Distributed Product |
Primary Competitors in China |
Dehaier’s Estimated Competitive Position | ||
| IMD C-armed X-ray machine | Philips, GE, Siemens | Smaller than average | ||
| ResMed Ventilators | Drugg (GER), GE, TYCO (USA), Newport (USA) | Smaller than average | ||
| ResMed S6 and MAP MiniPAP | Respironics (USA), Covidien (USA), GE | Smaller than average | ||
| JMS pump | B Braun (GER), Atom (JPN), Smiths (UK) | Smaller than average | ||
| Timesco laryngoscope | Kirchner & Wilhelm (GER), WelchAllyn (USA) | Greater than average | ||
*A “greater than average” position indicates Dehaier estimates its competitive position in the top third of all competitors. “Average” indicates Dehaier estimates its competitive position in the middle third of all competitors and “Smaller than average” indicates Dehaier estimates its competitive position in the bottom third of all competitors.
As we expand into international markets, our competitors will include publicly traded and privately held multinational companies such as Respironics, Inc., ResMed Inc. and Covidien. These companies typically focus on the premium segments of the market. We believe we can successfully penetrate certain international markets by offering products of comparable quality at lower prices. We will also face competition in international sales from companies that have local operations in the markets in which we sell our branded products. We believe that we can compete successfully with these companies by offering high quality branded products at comparable prices.
Methods of Competition
China’s medical device market currently features a significant number of small distributors. We seek to distinguish our company from our competitors by being able to offer branded and distributed products that address the device needs of customers that may have very different needs.
For example, China is currently investing heavily in health care nationwide; however, money for healthcare is currently unevenly distributed. There are a number of large hospitals that have significant resources and a number of rural clinics that have extremely limited budgets. We are able to provide distributed products that reach the more affluent customers, as these customers frequently tend to ascribe more perceived value to products made by well-known foreign companies, such as Timesco and ResMed. We are also able to supply our branded products to less wealthy customers, who tend to care less about perceived value and more about functionality. One of our strategies in competing in this market is to make our products as mobile as possible. For this reason, our air compressors, mobile X-ray devices, trolleys and the like are all portable. This portability addresses the budgetary limitations of, for instance, a rural clinic that can only afford to purchase a single air compressor.
69
Table of Contents
We currently compete on three levels. First, we have a network of contractual distribution relationships with over 2,000 independent distributors, and we employ 70 direct sales and sales support personnel. Second, in 2008 we opened an online sales platform for our respiratory and oxygen homecare products to make those products more easily available throughout China. Third, we established our first Customer Experience Center, or CEC, in Beijing. We have used this CEC to increase our marketing efforts, with the goal of increasing our market penetration in China’s large cities. These CECs are complementary to our current marketing and distribution model because the CECs give our potential customers an opportunity to experience our products first-hand in an environment that is similar to the environment in which they will use the products, whether that is a home or healthcare facility.
Employees
As of February 16, 2010, we had 155 employees, including 148 full-time employees and 7 part-time employees. Out of that total, 40 were employed in assembly, 20 were in research and development, 15 were in general administration, 70 were in marketing, sales, and customer support and service, and 10 were in procurement and supply management. As required by PRC regulations, we participate in various employee benefit plans that are organized by municipal and provincial governments, including pension, work-related injury benefits, maternity insurance, and medical and unemployment benefit plans. We are required under PRC law to make contributions to the employee benefit plans at specified percentages of the salaries, bonuses, housing funds and certain allowances of our employees, up to a maximum amount specified by the local government from time to time. We make contributions to the employee benefit 10% of employee salaries.
Generally, we enter into a three-year standard employment contract with all of our officers, managers and other key employees and a one-year standard employment contract with all other employees. According to these employment contracts, all of our employees are prohibited from engaging in any activities that compete with our business during the period of their employment with us.
Under Chinese law, we may only terminate employment agreements without cause and without penalty by providing notice of non-renewal one month prior to the date on which the employment agreement is scheduled to expire. If we fail to provide this notice or if we wish to terminate an employment agreement in the absence of cause, then we are obligated to pay the employee one month’s salary for each year we have employed the employee. We are, however, permitted to terminate an employee for cause without penalty to our company, where the employee has committed a crime or the employee’s actions or inactions have resulted in a material adverse effect to us.
We are headquartered and our principal executive offices are located at the Epoch Center in Beijing. We assemble and test all our branded products at our 32,000 square foot product facility at the Changping Science Park in Beijing.
| Office |
Address |
Rental Term Expiration |
Space | |||
| Principal Executive Office | 1223 Epoch Center, 31 Zi Zhu Yuan Road, Haidian District, Beijing, 100089 | December 31, 2009 (to be extended to December 31, 2011 upon expiration of current term) | 2,800 square feet | |||
| Product Center | 45 Yong An Road, Science Park, Changping District, Beijing, 102200 | September 24, 2010 | 32,000 square feet | |||
70
Table of Contents
Our products are medical devices and are subject to regulatory controls governing medical devices. As a distributor of medical equipment and supplies we are subject to regulation and oversight by different levels of the food and drug administration in China, in particular the SFDA. We are also subject to other PRC government laws and regulations. SFDA requirements include obtaining certifications, permits, compliance with clinical testing standards, assembly practices, quality standards, applicable industry standards and adverse event reporting, and advertising and packaging standards.
China’s Regulation of Medical Devices
Classification of Medical Devices
In China, medical devices are classified by the SFDA into three different categories, Class I, Class II and Class III, depending on the degree of risk associated with each medical device and the extent of control needed to ensure safety and effectiveness. Classification of a medical device is important because the class to which a medical device is assigned determines, among other things, whether a company needs to obtain a permit and the level of regulatory authority involved in obtaining such permit. Classification of a device also determines the types of registration required and the level of regulatory authority involved in effecting the product registration.
Class I devices require product certification and are those with low risk to the human body and are subject to “general controls.” Class I devices are regulated by the city level food and drug administration where the company is located. Class II devices are those with medium risk to the human body and are subject to “special controls.” Class II devices require product certification, usually through a quality system assessment, and are regulated by the provincial level food and drug administration where the company is located. Class III devices are those with high risk to the human body, such as life-sustaining, life-supporting or implantable devices. Class III devices also require product certification and are regulated by the SFDA under the strictest regulatory control.
The majority of our products are classified as Class II or Class III devices. Our anesthesia machines and ventilators are classified as Class III medical devices, while the remainder of our products are either classified as Class II or, in the case of our ventilator trolleys and sterilizers, not categorized devices.
Assembly Permit
A company must obtain a permit from the provincial level food and drug administration before commencing the assembly of Class II and Class III medical devices. No assembly permit is required for Class I devices, but the company must notify the provincial level food and drug administration where the company is located and file for record with it. An assembly permit, once obtained, is valid for five years and is renewable upon expiration.
We have a single assembly permit, which covers all products we assemble and is scheduled to expire on September 15, 2013. To renew an assembly permit, a company needs to submit to the provincial level food and drug administration an application to renew the permit, along with required information nine months before the expiration date of the permit.
Distribution License
A manufacturer or distributor must obtain a distribution license in order to engage in sales and distribution of Class II and Class III medical devices in China. A distribution license is valid for five years and is renewable upon expiration. Our distribution license will expire on May 16, 2010.
Registration Requirement
Before a medical device can be manufactured for commercial distribution, a company must effect medical device registration by proving the safety and effectiveness of the medical device to the satisfaction of respective levels of the food and drug administration. In order to conduct a clinical trial on a Class II or Class III medical device, the SFDA requires companies to apply for and obtain in advance a favorable inspection result for the device from an inspection center jointly recognized by the SFDA and the Administration of Quality Supervision, Inspection and Quarantine. The application to the inspection center must be supported by appropriate data, such as animal and
71
Table of Contents
laboratory testing results. If the inspection center approves the application for clinical trial, and the respective levels of the food and drug administration approve the institutions which will conduct the clinical trials, the company may begin the clinical trial. A registration application for a Class II or Class III device must provide required pre-clinical and clinical trial data and information about the device and its components regarding, among other things, device design, production and labeling. The provincial level food and drug administration, within 60 days of receiving an application for the registration of a Class II device, and the SFDA, within 90 days of receiving an application for the registration of a Class III device, will notify the applicant whether the application for registration is approved. If approved, a registration certificate will be issued within ten days of written approval. If the food and drug administration requires supplemental information, the approval process may take much longer. The registration is valid for four years.
The SFDA may change its policies, adopt additional regulations, revise existing regulations or tighten enforcement, each of which could block or delay the approval process for a medical device.
The following table discloses the current registration expiration dates for the products we sell. It is the obligation of that produces the product to seek registration and any renewals. We are responsible for registering our branded products but must rely on the suppliers of other products to seek registration for those products. We will either cease to sell such product or seek comparable products from other suppliers in the event the registration is not renewed on expiration.
Medical Devices
| Product Type |
Product Model |
Registration Expiration | ||
| Mobile Medical X-Ray Image Devices. | Explorer Series mobile and C-armed X-ray machine | November 2010 | ||
| IMD Compact Series mobile-X-ray machine | December 2012 | |||
| Anesthesia Machines. | DHR ORSA Series anesthesia machine | September 2013 | ||
| Patient Monitoring Devices. | DHR 930 patient monitor | December 2011 | ||
| Medical Ventilator. | Resmed Ventilator | November 2013 |
Technical Service Products
| Product |
Registration Expiration | |
| Ventilator Air Compressor. |
November 2011 | |
| Trolleys for Ventilators. |
Not a medical device | |
| Sterilizers for Ventilators. |
Not a medical device |
Respiratory and Oxygen Homecare Products
| Product Type |
Product Model |
Registration Expiration | ||
| Oxygen Concentrating Products. | Oxygen concentrator | November 2010 | ||
| Sleep Apnea Treatment Products. | CPAP C5 Auto CPAP A8 Auto S-CPAP A9 |
Application stage Application stage Application stage | ||
| Diagnostic Products. | DHR 998 DHR 999 |
Application stage Application stage | ||
| Thermotherapy Products. | Thermotherapy device | Application stage |
Continuing SFDA Regulation
We are subject to continuing regulation by the SFDA. In the event of significant modification to an approved medical device, its labeling or its assembly process, a new premarket approval or premarket approval supplement may be required. Our products are subject to, among others, the following regulations:
| • | SFDA’s quality system regulations which require companies to create, implement and follow certain design, testing, control, documentation and other quality assurance procedures; |
| • | medical device reporting regulations, which require that companies report to the SFDA certain types of adverse reaction and other events involving their products; and |
| • | SFDA’s general prohibition against promoting products for unapproved uses. |
Class II and III devices may also be subject to special controls applicable to them, such as supply purchase information, performance standards, quality inspection procedures and product testing devices which may not be
72
Table of Contents
required for Class I devices. We believe we are in compliance with the applicable SFDA guidelines, but we could be required to change our compliance activities or be subject to other special controls if the SFDA changes or modifies its existing regulations or adopts new requirements.
We are also subject to inspection and market surveillance by the SFDA to determine compliance with regulatory requirements. If the SFDA decides to enforce its regulations and rules, the agency can institute a wide variety of enforcement actions such as:
| • | fines, injunctions and civil penalties; |
| • | recall or seizure of our products; |
| • | the imposition of operating restrictions, partial suspension or complete shutdown of assembly; and |
| • | criminal prosecution. |
China Compulsory Certification Requirements
China Compulsory Certification, or CCC, inclusive of a certificate and a mark, serves as evidence that the covered products can be imported, marketed or used in China. The CCC mark is administered by the China National Certification and Accreditation Administration, which designates the China Quality Certification Center to process CCC mark applications. Some medical devices are required to have a CCC mark. We have received a certificate and a mark for each of our branded products for which a CCC mark is required.
Other National and Provincial Level Laws and Regulations in China
Beyond those laws and regulations we consider material to our business, we are subject to evolving regulations under many other laws and regulations administered by governmental authorities at the national, provincial and city levels, some of which are, or may be, applicable to our business. Our hospital customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
Laws regulating the conduct of business in our industry cover a broad array of subjects. We must comply with numerous additional state and local laws relating to matters such as safe working conditions, environmental protection and fire hazard control, which affect all companies doing business in China. We believe we are currently in compliance with these laws and regulations in all material respects. We may be required to incur significant costs to comply with these laws and regulations in the future. Unanticipated changes in existing regulatory requirements or adoption of new requirements could have a material adverse effect on our business, financial condition and results of operations.
Restriction on Foreign Ownership
The principal regulation governing foreign ownership of medical device businesses in the PRC is the Foreign Investment Industrial Guidance Catalogue, effective as of December 11, 2007 (the “Catalogue”). The Catalogue classifies the various industries into four categories: encouraged, permitted, restricted and prohibited. As confirmed by the government authorities, BDL is engaged in an encouraged industry. Such a designation offers businesses distinct advantages. For example, businesses engaged in encouraged industries:
| • | are not subject to restrictions on foreign investment, and, as such, foreign can own a majority in Sino-foreign joint ventures or establish wholly-owned foreign enterprises in the PRC; |
| • | provided such company has total investment of less than $100 million, the company is subject to regional (not central) government examination and approval which are generally more efficient and less time-consuming; and |
| • | may import certain equipment while enjoying a tariff and import-stage value-added tax exemption. |
The National Development and Reform Commission and the Ministry of Commerce periodically jointly revise the Foreign Investment Industrial Guidance Catalogue. As such, there is a possibility that our company’s business may fall outside the scope of the definition of an encouraged industry in the future. Should this occur, we would no longer benefit from such designation.
73
Table of Contents
Regulation of Foreign Currency Exchange
The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations (1996), as amended, and the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996). Under these regulations, Renminbi are freely convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions, but not for most capital account items, such as direct investment, loan, repatriation of investment and investment in securities outside China, unless the prior approval of SAFE or its local counterparts is obtained. In addition, any loans to an operating subsidiary in China that is a foreign invested enterprise, cannot, in the aggregate, exceed the difference between its respective approved total investment amount and its respective approved registered capital amount. Furthermore, any foreign loan must be registered with SAFE or its local counterparts for the loan to be effective. Any increase in the amount of the total investment and registered capital must be approved by the PRC Ministry of Commerce or its local counterpart. We may not be able to obtain these government approvals or registrations on a timely basis, if at all, which could result in a delay in the process of making these loans.
The dividends paid by the subsidiary to its shareholder are deemed shareholder income and are taxable in China. Pursuant to the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), foreign-invested enterprises in China may purchase or remit foreign exchange, subject to a cap approved by SAFE, for settlement of current account transactions without the approval of SAFE. Foreign exchange transactions under the capital account are still subject to limitations and require approvals from, or registration with, SAFE and other relevant PRC governmental authorities.
Regulation of Dividend Distribution
The principal regulations governing the distribution of dividends by foreign holding companies include the Foreign Investment Enterprise Law (1986), as amended, and the Administrative Rules under the Foreign Investment Enterprise Law (2001).
Under these regulations, foreign investment enterprises in China may pay dividends only out of their retained profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, foreign investment enterprises in China are required to allocate at least 10% of their respective retained profits each year, if any, to fund certain reserve funds unless these reserves have reached 50% of the registered capital of the enterprises. These reserves are not distributable as cash dividends.
Notice 75
On October 21, 2005, SAFE issued Notice 75, which became effective as of November 1, 2005. According to Notice 75, prior registration with the local SAFE branch is required for PRC residents to establish or to control an offshore company for the purposes of financing that offshore company with assets or equity interests in an onshore enterprise located in the PRC. An amendment to registration or filing with the local SAFE branch by such PRC resident is also required for the injection of equity interests or assets of an onshore enterprise in the offshore company or overseas funds raised by such offshore company, or any other material change involving a change in the capital of the offshore company.
Moreover, Notice 75 applies retroactively. As a result, PRC residents who have established or acquired control of offshore companies that have made onshore investments in the PRC in the past are required to complete the relevant registration procedures with the local SAFE branch. Under the relevant rules, failure to comply with the registration procedures set forth in Notice 75 may result in restrictions being imposed on the foreign exchange activities of the relevant onshore company, including the increase of its registered capital, the payment of dividends and other distributions to its offshore parent or affiliate and capital inflow from the offshore entity, and may also subject relevant PRC residents to penalties under PRC foreign exchange administration regulations.
PRC residents who control our company are required to register with SAFE in connection with their investments in us. Such individuals completed this registration in 2007, and 2008, as amended. If we use our equity interest to purchase the assets or equity interest of a PRC company owned by PRC residents in the future, such PRC residents will be subject to the registration procedures described in Notice 75.
74
Table of Contents
Trademark Rights
The PRC Trademark Law, adopted in 1982 and revised in 2001, with its implementation rules adopted in 2002, protects registered trademarks. The Trademark Office of the State Administration of Industry and Commerce (“SAIC”), handles trademark registrations and grants trademark registrations for a term of ten years.
Regulations on Offshore Parent Holding Companies’
Direct Investment in and Loans to Their PRC Subsidiaries
An offshore company may invest equity in a PRC company, which will become the PRC subsidiary of the offshore holding company after investment. Such equity investment is subject to a series of laws and regulations generally applicable to any foreign-invested enterprise in China, which include the Wholly Foreign Owned Enterprise Law, the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Contractual Joint Venture Enterprise Law, all as amended from time to time, and their respective implementing rules; the Tentative Provisions on the Foreign Exchange Registration Administration of Foreign-Invested Enterprise; and the Notice on Certain Matters Relating to the Change of Registered Capital of Foreign-Invested Enterprises.
Under the aforesaid laws and regulations, the increase of the registered capital of a foreign-invested enterprise is subject to the prior approval by the original approval authority of its establishment. In addition, the increase of registered capital and total investment amount shall both be registered with SAIC and SAFE.
Shareholder loans made by offshore parent holding companies to their PRC subsidiaries are regarded as foreign debts in China for regulatory purpose, which is subject to a number of PRC laws and regulations, including the PRC Foreign Exchange Administration Regulations, the Interim Measures on Administration on Foreign Debts, the Tentative Provisions on the Statistics Monitoring of Foreign Debts and its implementation rules, and the Administration Rules on the Settlement, Sale and Payment of Foreign Exchange.
Under these regulations, the shareholder loans made by offshore parent holding companies to their PRC subsidiaries shall be registered with SAFE. Furthermore, the total amount of foreign debts that can be borrowed by such PRC subsidiaries, including any shareholder loans, shall not exceed the difference between the total investment amount and the registered capital amount of the PRC subsidiaries, both of which are subject to the governmental approval.
75
Table of Contents
Executive Officers and Directors
The following table sets forth our executive officers and directors, their ages and the positions held by them:
| Name |
Age | Position |
Appointed | |||
| Ping Chen(1)(2) |
46 | Chief Executive Officer and Chairman and Director | 2003 | |||
| Zheng (Rita) Liu(1)(3) |
37 | Chief Financial Officer and Director | 2003 | |||
| Weibing Yang(1) |
43 | Vice President of Sales and Marketing | 2003 | |||
| Yong Wang(1) |
38 | Vice President of Research and Development | 2003 | |||
| Yunxiang (Phil) Fan(1)(3)(4)(5)(6) |
43 | Independent Director | 2009 | |||
| Jimin (Peter) Zhuo(1)(4)(5)(6)(7) |
37 | Independent Director | 2009 | |||
| Bin Qiu(1)(4)(5)(6)(7) |
40 | Independent Director | 2009 |
| (1) | The individual’s business address is c/o BDL, 1223 Epoch Center, No. 31 Zi Zhu Yuan Road, Haidian District, Beijing 100089 People’s Republic of China. |
| (2) | Class III director whose term expires in 2012. |
| (3) | Class II director whose term expires in 2011. |
| (4) | Member of audit committee. |
| (5) | Member of compensation committee. |
| (6) | Member of nominating committee. |
| (7) | Class I director whose term expires in 2010. |
Ping Chen. Mr. Chen is our Chief Executive Officer. Prior to his service as our Chief Executive Officer, from 1993-2000, Mr. Chen served as the CEO of Beijing Chengcheng Medical Electronic Equipment Co. Prior to 1993, Mr. Chen served as an engineer at the No. 2 Academy, Ministry of Aeronautics and Astronautics from 1987 to 1991 and moved up to the Head of the Civilian Products Division there from 1991-1993. Mr. Chen founded BTL in 2001 and has served as CEO since that time. Mr. Chen received his bachelor’s degree in 1984 from the National University of Defense Technology and his master’s degree in 1987 from the Ministry of Aeronautics and Astronautics.
Zheng (Rita) Liu. Ms. Liu is our Chief Financial Officer. She has served in this role since 2003. She is a Professional National Accountant of National Institute Accountants, Member of Chartered Institute of Management Accountant and Institute Financial Accountants. In the past, Ms. Liu served as the Finance Manager for Lehman Brown Investment Consulting (China) Co. and Bro-rad Laboratory Instruments (China) Co., Ltd. Ms Liu received her bachelor’s degree from the Renmin University of China in 1992 and her Master’s degree in Business Administration from Peking University in 2001.
Weibing Yang. Dr. Yang is our Vice President of Sales and Marketing. Prior to becoming our VP of Sales and Marketing in 2003, Dr. Yang served us as a Sales Director in 2003. Prior to joining our company as a Sales Director, Dr. Yang served as a Product Manager for AMTRONIX Inc. from 1996-1997. From 1993-1996, Dr. Yang served as a sales manager for Planmeca Medical Equipment Co. Dr. Yang also served as a doctor in the Beijing Ship Hospital from 1989-1992. Dr. Yang graduated from the Medical School at SooChow University in 1989.
Yong Wang. Mr. Wang is our Vice President of Research and Development. Mr. Wang served as a Control Engineer at the Engineering Institution of Beijing from 1994-1997. Mr. Wang then served as the Vice General Manager and Chief Engineer for the Beijing Sinoeverlife Medical Technology Co., Ltd., from 1997-2003. From 2003 through present, Mr. Wang has been our Vice President of Research and Development. Mr. Wang received his bachelor’s degree in 1994 from Tianjin University.
Yunxiang (Phil) Fan. Mr. Fan is a director of our company. In 2003, Mr. Fan co-founded Tri-Tech Holding Inc., a company operating in the water pollution remediation, software and engineering industry in China (“Tri-Tech”). He currently serves as the President and a director of Tri-Tech. Prior to founding Tri-Tech, Mr. Fan provided technical, engineering and management services in several U.S. engineering firms, including Black and Beatch, Parsons
76
Table of Contents
Brinckerhoff, Inc., and Chastain-Skillman, Inc. From 2003 through 2005, Mr. Fan was the Asia Regional Sales Manager for Met-Pro Corporation. Mr. Fan earned his bachelor’s and master’s degrees in environmental engineering from Hunan University and a master’s degree in civil engineering from Louisiana State University. Mr. Fan has been a registered professional engineer in the United States since 2001.
Ji Min (Peter) Zhuo. Mr. Zhuo has over 15 years of financial and accounting work experience. From June 2007 through present, Mr. Zhuo has served as vice president and chief accounting officer of Vanceinfo Technology Limited, a China-based IT outsourcing company listed on the New York Stock Exchange (NYSE Arca: VIT). From 2005 to 2006, he was chief financial officer of Ebis Company Limited, a China based IT service company. From 2004 to 2005, Mr. Zhuo worked as a controller at Morgan Stanley Properties (China) Co. Ltd. From 1994 to 2004, Mr. Zhuo was an auditor with Arthur Andersen Beijing and Sydney office, and PriceWaterhouseCoopers Beijing office. He is a China Certified Public Accountant and also passed China Bar examination and U.S CPA examination. He obtained bachelor degree with major in international accounting from Central University of Finance & Economic in China, and Master of Law (L.L.M) from University of Southern California, Gould School of Law.
Bin Qiu. Mr. Qiu has more than 17 years of experience in medicine and homecare medical devices. From May 2002 through present, he has served as a director and CEO of Beijing Dawn Aerospace Bio-Tech Co., Ltd., a Bio-Pharmacy and healthcare food R&D, manufacturing and distribution company. Before this, he was CEO of Shandong Fushan Dongsheng Industry Co., Ltd from May 2001 to April 2002. From 1998 to 2001, he was VP of Shanxi Fushan Medicine Co., Ltd. From 1995 to 1998, he was a department manager of Shandong SanZhu Industry Co., Ltd, a healthcare medicine and food company in China. Mr. Qiu earned his bachelor’s degree from Shandong Chinese Medicine University.
Executive Compensation
Summary Compensation Table
The following table shows the annual compensation paid by us for the years ended December 31, 2007 and 2008 to Ping Chen, our principal executive officer. His current employment agreement commenced on January 1, 2009 and is scheduled to expire on December 31, 2009. Mr. Chen and our company have entered into an extension of this agreement, effective December 21, 2009, through December 31, 2012. No officer had a salary during either of the previous two years of more than $100,000.
| Name and principal position |
Year | Salary | Bonus | All Other Compensation |
Total Paid | |||||||||
| Ping Chen |
2008 | $ | 27,000 | $ | 0 | $ | 0 | $ | 27,000 | |||||
| Chief Executive Officer |
2007 | $ | 27,000 | $ | 0 | $ | 0 | $ | 27,000 | |||||
Employment Agreements
Under Chinese law, we may only terminate employment agreements without cause and without penalty by providing notice of non-renewal one month prior to the date on which the employment agreement is scheduled to expire. If we fail to provide this notice or if we wish to terminate an employment agreement in the absence of cause, then we are obligated to pay the employee one month’s salary for each year we have employed the employee. We are, however, permitted to terminate an employee for cause without penalty to our company, where the employee has committed a crime or the employee’s actions or inactions have resulted in a material adverse effect to us.
Our employment agreements with our executive officers generally provide for a term of three (3) years and a salary to be paid monthly. The agreements also provide that executive officers are to work an average of forty hours per week and are entitled to all legal holidays as well as other paid leave in accordance with PRC laws and regulations and our internal work policies. Under such agreements, our executive officers can be terminated for cause without further compensation. The employment agreements also provide that we will pay for all mandatory social security programs for our executive officers in accordance with PRC regulations. During the agreement and for one (1) year afterward, our executive officers are subject to keep trade secrets confidential.
77
Table of Contents
Share Option Pool
We intend to establish a pool for share options for our employees following the completion of this offering. This pool will contain options to purchase our common shares equal to ten percent (10%) of the number of common shares outstanding at the conclusion of this offering, not including any shares underlying placement agent’s warrants. This pool will contain options to purchase up to 450,000 of our common shares subject to outstanding share options or reserved for issuance under our share incentive plan, assuming a maximum offering. The options will vest at a rate of 20% per year for five years and have a per share exercise price equal to the fair market value of one of our common shares on the date of grant. Other than those granted under this pool, we will not grant any shares or options to our employees prior to the second anniversary of the closing of this offering. We expect to grant options under this pool to certain employees as of the closing of this offering. Any options granted as of the closing of this offering will have an exercise price per common share equal to the offering price. We have not yet determined the recipients of any such grants.
Board of Directors and Board Committees
Composition of Board
Our board of directors currently consists of 5 directors. We expect that all current directors will continue to serve after this offering. There are no family relationships between any of our executive officers and directors.
The directors will be divided into three classes, as nearly equal in number as the then total number of directors permits. Class I directors shall face re-election at our annual general meeting of shareholders in 2010 and every three years thereafter. Class II directors shall face re-election at our annual general meeting of shareholders in 2011 and every three years thereafter. Class III directors shall face re-election at our annual general meeting of shareholders in 2012 and every three years thereafter.
If the number of directors changes, any increase or decrease will be apportioned among the classes so as to maintain the number of directors in each class as nearly as possible. Any additional directors of a class elected to fill a vacancy resulting from an increase in such class will hold office for a term that coincides with the remaining term of that class. Decreases in the number of directors will not shorten the term of any incumbent director. These board provisions could make it more difficult for third parties to gain control of our company by making it difficult to replace members of the Board of Directors.
A director may vote in respect of any contract or transaction in which he is interested, provided, however that the nature of the interest of any director in any such contract or transaction shall be disclosed by him at or prior to its consideration and any vote on that matter. A general notice or disclosure to the directors or otherwise contained in the minutes of a meeting or a written resolution of the directors or any committee thereof of the nature of a director’s interest shall be sufficient disclosure and after such general notice it shall not be necessary to give special notice relating to any particular transaction. A director may be counted for a quorum upon a motion in respect of any contract or arrangement which he shall make with our company, or in which he is so interested and may vote on such motion.
There are no membership qualifications for directors. Further, there are no share ownership qualifications for directors unless so fixed by us in a general meeting.
The Board of Directors maintains a majority of independent directors who are deemed to be independent under the definition of independence provided by NASDAQ Stock Market Rule 4200(a)(15). Messers. Fan, Zhuo and Qiu are our independent directors.
There are no other arrangements or understandings pursuant to which our directors are selected or nominated.
Board Committees
Currently, three committees have been established under the board: the audit committee, the compensation committee and the nominating committee. The audit committee is responsible for overseeing the accounting and financial reporting processes of our company and audits of the financial statements of our company, including the appointment, compensation and oversight of the work of our independent auditors. The compensation committee of the board of directors reviews and makes recommendations to the board regarding our compensation policies for our
78
Table of Contents
officers and all forms of compensation, and also administers our incentive compensation plans and equity-based plans (but our board retains the authority to interpret those plans). The nominating committee of the board of directors is responsible for the assessment of the performance of the board, considering and making recommendations to the board with respect to the nominations or elections of directors and other governance issues.
Board Observers
In connection with this offering, we have agreed to allow our placement agent to designate two non-voting observers to our Board of Directors until the earlier of the date that:
| • | the investors that purchase common shares in this offering beneficially own less than ten percent (10%) of our outstanding common shares; or |
| • | the average closing price per common share equals or exceeds three (3) times the offering price for a period of 15 consecutive trading days. |
Although our placement agent’s observers will not be able to vote, they may nevertheless significantly influence the outcome of matters submitted to the Board of Directors for approval. We have agreed to reimburse the observers for their expenses for attending our Board meetings, subject to a maximum reimbursement of $6,000 per meeting and $12,000 annually, which amount is not more than the reimbursement payable to our directors. The observers will be required to certify that such travel expenses are not reimbursed by any other party. We will also pay observers the same amount as our independent directors receive. As of the date of this prospectus, Mr. L. McCarthy Downs III and Mr. Ming Zhu are serving as our placement agent’s observers to our Board of Directors.
Duties of Directors
Under British Virgin Islands law, our directors have a duty to act honestly, in good faith and with a view to our best interests. Our directors also have a duty to exercise the care, diligence and skills that a reasonably prudent person would exercise in comparable circumstances. See “Description of Share Capital—Differences in Corporate Law” for additional information on our directors’ fiduciary duties under British Virgin Islands law. In fulfilling their duty of care to us, our directors must ensure compliance with our fourth amended and restated memorandum and articles of association. We have the right to seek damages if a duty owed by our directors is breached.
The functions and powers of our board of directors include, among others:
| • | appointing officers and determining the term of office of the officers; |
| • | authorizing the payment of donations to religious, charitable, public or other bodies, clubs, funds or associations as deemed advisable; |
| • | exercising the borrowing powers of the company and mortgaging the property of the company; |
| • | executing cheques, promissory notes and other negotiable instruments on behalf of the company; and |
| • | maintaining or registering a register of mortgages, charges or other encumbrances of the company. |
Interested Transactions
A director may vote, attend a board meeting or sign a document on our behalf with respect to any contract or transaction in which he or she is interested. A director must promptly disclose the interest to all other directors after becoming aware of the fact that he or she is interested in a transaction we have entered into or are to enter into. A general notice or disclosure to the board or otherwise contained in the minutes of a meeting or a written resolution of the board or any committee of the board that a director is a shareholder, director, officer or trustee of any specified firm or company and is to be regarded as interested in any transaction with such firm or company will be sufficient disclosure, and, after such general notice, it will not be necessary to give special notice relating to any particular transaction.
Remuneration and Borrowing
The directors may receive such remuneration as our board of directors may determine from time to time. Each director is entitled to be repaid or prepaid all traveling, hotel and incidental expenses reasonably incurred or expected to be incurred in attending meetings of our board of directors or committees of our board of directors or shareholder meetings or otherwise in connection with the discharge of his or her duties as a director. The compensation committee will assist the directors in reviewing and approving the compensation structure for the directors.
79
Table of Contents
Our board of directors may exercise all the powers of the company to borrow money and to mortgage or charge our undertakings and property or any part thereof, to issue debentures, debenture stock and other securities whenever money is borrowed or as security for any debt, liability or obligation of the company or of any third party.
Qualification
A director is not required to hold shares as a qualification to office.
Limitation on Liability and Other Indemnification Matters
British Virgin Islands law does not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime.
Under our memorandum and articles of association, we may indemnify our directors, officers and liquidators against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with civil, criminal, administrative or investigative proceedings to which they are party or are threatened to be made a party by reason of their acting as our director, officer or liquidator. To be entitled to indemnification, these persons must have acted honestly and in good faith with a view to the best interest of the company and, in the case of criminal proceedings, they must have had no reasonable cause to believe their conduct was unlawful.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for our directors or officers under the foregoing provisions, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable as a matter of United States law.
Director Compensation
All directors hold office until the next annual meeting of shareholders at which their respective class of directors is re-elected and until their successors have been duly elected and qualified. There are no family relationships among our directors or executive officers. Officers are elected by and serve at the discretion of the Board of Directors. Employee directors do not receive any compensation for their services. Non-employee directors are entitled to receive $2,000 per meeting for serving as directors and may receive option grants from our company. In addition, non-employee directors are entitled to receive compensation for their actual travel expenses for each Board of Directors meeting attended, up to a maximum of $6,000 per meeting and $12,000 per year.
Summary Director Compensation Table FY 2008
| Name |
Director fees earned or paid in cash |
Total(1) | ||||
| Ping Chen(2) |
$ | 0 | $ | 0 | ||
| Zheng (Rita) Liu(2) |
$ | 0 | $ | 0 | ||
| Yunxiang (Phil) Fan(3) |
$ | 0 | $ | 0 | ||
| Jimin (Peter) Zhuo(3) |
$ | 0 | $ | 0 | ||
| Bin Qiu(3) |
$ | 0 | $ | 0 | ||
| (1) | None of the directors received any common share awards, option awards, nonqualified deferred compensation earnings or non-equity incentive plan compensation in fiscal year 2008. |
| (2) | Mr. Chen and Ms. Liu received payment in their capacity as officers of our company and/or subsidiaries/affiliates but did not receive any compensation for serving as directors of our company. |
| (3) | Messers. Fan, Zhuo and Qiu did not become directors until 2009 and did not receive any payment in 2008. |
80
Table of Contents
Limitation of Director and Officer Liability
Under British Virgin Islands law, each of our directors and officers, in performing his or her functions, is required to act honestly and in good faith with a view to our best interests and exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances. Our memorandum and articles of association provide that, to the fullest extent permitted by British Virgin Islands law or any other applicable laws, our directors will not be personally liable to us or our shareholders for any acts or omissions in the performance of their duties. Such limitation of liability does not affect the availability of equitable remedies such as injunctive relief or rescission. These provisions will not limit the liability of directors under United States federal securities laws.
We may indemnify any of our directors or anyone serving at our request as a director of another entity against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with legal, administrative or investigative proceedings. We may only indemnify a director if he or she acted honestly and in good faith with the view to our best interests and, in the case of criminal proceedings, the director had no reasonable cause to believe that his or her conduct was unlawful. The decision of our board of directors as to whether the director acted honestly and in good faith with a view to our best interests and as to whether the director had no reasonable cause to believe that his or her conduct was unlawful, is in the absence of fraud sufficient for the purposes of indemnification, unless a question of law is involved. The termination of any proceedings by any judgment, order, settlement, conviction or the entry of no plea does not, by itself, create a presumption that a director did not act honestly and in good faith and with a view to our best interests or that the director had reasonable cause to believe that his or her conduct was unlawful. If a director to be indemnified has been successful in defense of any proceedings referred to above, the director is entitled to be indemnified against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred by the director or officer in connection with the proceedings.
We may purchase and maintain insurance in relation to any of our directors or officers against any liability asserted against the directors or officers and incurred by the directors or officers in that capacity, whether or not we have or would have had the power to indemnify the directors or officers against the liability as provided in our fourth amended and restated memorandum and articles of association.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for our directors or officers under the foregoing provisions, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable as a matter of United States law.
81
Table of Contents
Prior Related Party Transactions
Our chief executive officer and his spouse rent our principal executive office to our company. The current rental term will expire on December 31, 2009. Mr. Chen and his wife, who hold the property pursuant to a 70 year lease entered on January 4, 2009, have entered an agreement to rent the office to BDL for the next 2 years after expiration for a monthly rental rate of approximately $2,500 (¥17,000). We believe this rental rate is the market price based on our experience in the area; however, these terms cannot be considered negotiated at arms-length as we did not have independent directors at the time we entered into this relationship.
BTL, which holds the property pursuant to a 50 year lease entered on September 10, 2003, rents our product center to our company without charge. The current rental term will expire on September 24, 2010. BTL’s building is also pledged as collateral for BDL’s bank loans. In exchange, BDL loans money to BTL to finance BTL’s operations.
BDL will pay all utility fees for the above two properties. As noted above, both properties are held pursuant to long-term leases. Under Chinese law, land belongs to the PRC and use of such property is granted to individuals and entities pursuant to long-term leases. These leases are typically between 50 and 70 years, as is the case for these properties, and may be renewed upon expiration on terms to be negotiated at the time of renewal.
BTL has pledged its property at 45 Yong An Road, Science Park, Changping District, Beijing, 102200, as collateral for BDL’s loan contract, executed on May 5, 2009 with ICBC in Beijing. In exchange, BDL advances money to BTL to finance its operations. The advances from BDL to BTL are non-interest bearing. There is no formal agreement between BDL and BTL governing these advances. These are advances made to BTL as needed with no repayment schedule. Repayments are made when BTL has the available funds. Additionally, Mr. Chen has personally guaranteed BDL’s bank loan. BTL, in turn, has agreed to indemnify Mr. Chen and guarantee his performance should he ever be called on to perform under BDL’s bank loan.
Make-Good Shares Subject to Redemption
As described in more detail in the section entitled “Placement – Market and Pricing Consideration” our company had been valued on a forward-looking basis for purposes of this offering. We and our placement agent agreed to value our company at a multiple of approximately 6.6 times our targeted 2010 audited net after-tax income. Based on the valuation of our company at approximately $24,000,000 using this methodology, our earnings per share would be approximately $0.80.
Valuing a company on a forward-looking basis is subject to a number of risks, including the possibility that the company will not achieve the targeted income levels and that world markets may not maintain the same valuation for companies in general in the future. In order to mitigate some of this risk, certain key members of the management of our company have agreed to place, on a prorated basis, that number of common shares into escrow that is equal to 40% of the maximum number of shares to be sold in this offering. These shares will be placed into escrow prior to the time we request effectiveness of the registration statement. Upon closing of this offering, the escrow agent will return any shares in excess of 40% of the actual number of shares sold in the offering. Such escrowed shares are referred to as the “Make-Good Shares”). The Make-Good Shares will remain in escrow with SunTrust Bank or another bank acceptable to our placement agent pending the filing of our company’s Form 10-K for the year ending December 31, 2010.
To the extent our audited after-tax earnings per share for the year ending December 31, 2010 are less than the target of $0.80, excluding any expenses associated with releasing the Make-Good Shares back to the original owners, our company will redeem and cancel, pro rata, the Make-Good Shares without any consideration to the extent necessary to cause our audited after-tax earnings to be equal to the target of $0.80. We cannot guarantee that we will be able to redeem a sufficient number of Make-Good Shares to increase audited after-tax earnings per share to the target of $0.80 if our company either has low net income or any net losses in 2010. Any remaining Make-Good Shares will be released from escrow to Ping Chen, Zheng (Rita) Liu, Weibing Yang, Jian Sun and Yong Wang upon the earlier of (i) one (1) business day after the termination of this offering without closing or (ii) thirty (30) calendar days after the filing of the Form 10-K for the year ending December 31, 2010 after redeeming any Make-Good Shares. Additionally, notwithstanding any other terms of the Make-Good Escrow, if our shares trade at or above 2.5 times the price of this offering for a period of five trading days within a ten day trading period, the Make-Good Escrow will terminate and the Make-Good Shares will be released to the initial shareholders. Any delay in redeeming the Make-Good Shares will delay the release of such remaining Make-Good Shares from escrow. See “Risk Factors – A redemption of Make-Good Shares may be insufficient to cause our company to achieve targeted earnings and may reduce our management’s involvement and stake in our company.”
Because the Make-Good Shares have been escrowed as a condition of completing the initial public offering and will be released to the holders thereof without regard to such holders’ continued employment if Dehaier meets the foregoing criteria, we have determined that no compensatory arrangement exists. Accordingly, we account for the Make-Good Shares as an element of the overall transaction and we do not recognize any compensation expense upon the return of such Make-Good Shares to the holders. If our company does not meet the criteria for releasing the Make-Good Shares, then we will redeem the Make-Good Shares without payment, resulting in the reduction of Dehaier common shares outstanding.
82
Table of Contents
The following table sets forth information with respect to beneficial ownership of our common shares as of February 16, 2010 by:
| • | Each person who is known by us to beneficially own more than 5% of our outstanding common shares; |
| • | Each of our directors and named executive officers; and |
| • | All directors and named executive officers as a group. |
In addition, we have included the share ownership of each of the individuals subject to the Make-Good Share Escrow described above. See “Related Party Transactions – Make-Good Shares Subject to Redemption.” The below table assumes no redemption of any shares subject to such Make-Good Share Escrow.
The number and percentage of common shares beneficially owned before the offering are based on 3,000,000 common shares outstanding as of February 16, 2010. Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common shares. Beneficial ownership is determined in accordance with the rules of the SEC and generally requires that such person have voting or investment power with respect to securities. In computing the number of common shares beneficially owned by a person listed below and the percentage ownership of such person, common shares underlying options, warrants or convertible securities held by each such person that are exercisable or convertible within 60 days of February 16, 2010 are deemed outstanding, but are not deemed outstanding for computing the percentage ownership of any other person. Except as otherwise indicated in the footnotes to this table, or as required by applicable community property laws, all persons listed have sole voting and investment power for all common shares shown as beneficially owned by them. Unless otherwise indicated in the footnotes, the address for each principal shareholder is in the care of BDL, 1223 Epoch Center, No. 31 Zi Zhu Yuan Road, Haidian District, Beijing 100089, People’s Republic of China. As of the date of the Prospectus, we had nine (9) shareholders of record.
| Named Executive Officers and Directors |
Amount of Beneficial Ownership(1) |
Pre-Offering Percentage Ownership(2) |
Post- Minimum Offering Percentage Ownership |
Post- Maximum Offering Percentage Ownership |
||||||||
| Ping Chen, Chief Executive Officer, Director |
1,104,742 | (3)(4) | 36.82 | % | 25.99 | % | 24.55 | % | ||||
| Zheng (Rita) Liu, Chief Financial Officer, Director |
96,452 | (4) | 3.22 | % | 2.27 | % | 2.14 | % | ||||
| Yunxiang (Phil) Fan, Director |
0 | * | * | * | ||||||||
| Jimin (Peter) Zhuo, Director |
0 | * | * | * | ||||||||
| Bin Qiu, Director |
0 | * | * | * | ||||||||
| Weibing Yang, Vice President of Sales and Marketing |
263,471 | (4) | 8.78 | % | 6.20 | % | 5.85 | % | ||||
| Yong Wang, Vice President of Research and Development |
40,455 | (4) | 1.35 | % | * | * | ||||||
| All officers and directors as a group (7 persons) |
1,505,120 | 50.17 | % | 35.41 | % | 33.45 | % | |||||
| Jian Sun |
253,076 | (4) | 8.44 | % | 5.95 | % | 5.62 | % | ||||
| Chen Ping Ltd. |
1,104,742 | (3)(4) | 36.82 | % | 25.99 | % | 24.55 | % | ||||
| De-haier Investment Holdings Ltd. |
527,693 | (5) | 17.59 | % | 12.42 | % | 11.73 | % | ||||
| Yijen Chen |
624,113 | 20.80 | % | 14.69 | % | 13.87 | % |
| * | Less than 1%. |
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the common shares. |
83
Table of Contents
| (2) | The number of our common shares outstanding used in calculating the percentage for each listed person excludes the common shares underlying options held by such person. In addition, the percentage ownership assumes the return to the shareholder of all shares subject to the Make-Good Escrow. |
| (3) | Ping Chen has the sole power to direct the voting and disposition of the 1,104,742 shares held by Chen Ping Ltd. |
| (4) | These shares are subject to the Make-Good Escrow. |
| (5) | Ms. Yunli Lou and Mr. Liping Qiu share power to direct the voting and disposition of the 527,693 shares held by De-haier Investment Holdings Ltd. |
We were incorporated as an international business company under the International Business Companies Act, 1984, in the British Virgin Islands on July 22, 2003 under the name “De-Haier Medical Systems Limited.” We changed our name to “Dehaier Medical Systems Limited” on June 3, 2005. As of the date of this prospectus, we have authorized 18,307,038 common shares, of $0.002731 par value.
The following are summaries of the material provisions of our amended and restated memorandum and articles of association that will be in force at the time of the closing of this offering and the BVI Act, insofar as they relate to the material terms of our common shares. The forms of our amended and restated memorandum and articles of association are filed as exhibits to the registration statement of which this prospectus is a part.
Common Shares
General
All of our issued common shares are fully paid and non-assessable. Certificates representing the common shares are issued in registered form. Our shareholders who are non-residents of the British Virgin Islands may freely hold and vote their common shares.
Distributions
The holders of our common shares are entitled to such dividends as may be declared by our board of directors subject to the BVI Act.
Voting rights
Any action required or permitted to be taken by the shareholders must be effected at a duly called annual or special meeting of the shareholders entitled to vote on such action and may not be effected by a resolution in writing. At each general meeting, each shareholder who is present in person or by proxy (or, in the case of a shareholder being a corporation, by its duly authorized representative) will have one vote for each common share which such shareholder holds.
Election of directors
Delaware law permits cumulative voting for the election of directors only if expressly authorized in the certificate of incorporation. The laws of the British Virgin Islands, however, do not specifically prohibit or restrict the creation of cumulative voting rights for the election of our directors. Cumulative voting is not a concept that is accepted as a common practice in the British Virgin Islands, and we have made no provisions in our memorandum and articles of association to allow cumulative voting for elections of directors.
Meetings
We must provide written notice of all meetings of shareholders, stating the time, place and, in the case of a special meeting of shareholders, the purpose or purposes thereof, at least 10 days before the date of the proposed meeting to those persons whose names appear as shareholders in the register of members on the date of the notice and are entitled to vote at the meeting. Our board of directors shall call a special meeting upon the written request of shareholders holding at least 30% of our outstanding voting shares. In addition, our board of directors may call a special meeting of shareholders on its own motion. A meeting of shareholders may be called on short notice: (a) if it
84
Table of Contents
is so agreed by shareholders holding not less than 90% of the common shares entitled to vote on the matters to be considered at the meeting, or 90% of the common shares of each class or series entitled to vote together as a class or series, together with not less than 90% of the remaining votes; or (b) if all shareholders holding common shares entitled to vote on the matters to be considered at the meeting have waived notice of the meeting, and presence at the meeting shall be deemed to constitute waiver for this purpose.
At any meeting of shareholders, a quorum will be present if there are shareholders present in person or by proxy representing not less than 50% of the issued common shares entitled to vote on the resolutions to be considered at the meeting. Such quorum may be represented by only a single shareholder or proxy. If no quorum is present within two hours of the start time of the meeting, the meeting shall be dissolved if it was requested by shareholders. In any other case, the meeting shall be adjourned to the next business day, and if shareholders representing not less than one-third of the votes of the common shares or each class of shares entitled to vote on the matters to be considered at the meeting are present within one hour of the start time of the adjourned meeting, a quorum will be present. If not, the meeting will be dissolved. No business may be transacted at any general meeting unless a quorum is present at the commencement of business. If present, the chairman of our board of directors shall be the chairman presiding at any meeting of the shareholders.
A corporation that is a shareholder shall be deemed for the purpose of our memorandum and articles of association to be present in person if represented by its duly authorized representative. This duly authorized representative shall be entitled to exercise the same powers on behalf of the corporation which he represents as that corporation could exercise if it were our individual shareholder.
Protection of minority shareholders
We would normally expect British Virgin Islands courts to follow English case law precedents, which permit a minority shareholder to commence a representative action, or derivative actions in our name, to challenge (1) an act which is ultra vires or illegal, (2) an act which constitutes a fraud against the minority by parties in control of us, (3) the act complained of constitutes an infringement of individual rights of shareholders, such as the right to vote and pre-emptive rights and (4) an irregularity in the passing of a resolution which requires a special or extraordinary majority of the shareholders.
Pre-emptive rights
There are no pre-emptive rights applicable to the issue by us of new common shares under either British Virgin Islands law or our memorandum and articles of association.
Transfer of common shares
Subject to the restrictions in our memorandum and articles of association, the lock-up agreements with our placement agent described in “Shares Eligible for Future Sale— Lock-Up Agreements” and applicable securities laws, any of our shareholders may transfer all or any of his or her common shares by written instrument of transfer signed by the transferor and containing the name and address of the transferee. Our board of directors may resolve by resolution to refuse or delay the registration of the transfer of any common share. If our board of directors resolves to refuse or delay any transfer, it shall specify the reasons for such refusal in the resolution. Our directors may not resolve or refuse or delay the transfer of a common share unless: (a) the person transferring the shares has failed to pay any amount due in respect of any of those shares; or (b) such refusal or delay is deemed necessary or advisable in our view or that of our legal counsel in order to avoid violation of, or in order to ensure compliance with, any applicable, corporate, securities and other laws and regulations.
Liquidation
If we are wound up and the assets available for distribution among our shareholders are more than sufficient to repay all amounts paid to us on account of the issue of shares immediately prior to the winding up, the excess shall be distributable pari passu among those shareholders in proportion to the amount paid up immediately prior to the winding up on the shares held by them, respectively. If we are wound up and the assets available for distribution among the shareholders as such are insufficient to repay the whole of the amounts paid to us on account of the issue of shares, those assets shall be distributed so that, to the greatest extent possible, the losses shall be borne by the shareholders in proportion to the amounts paid up immediately prior to the winding up on the shares held by them, respectively.
85
Table of Contents
If we are wound up, the liquidator appointed by us may, in accordance with the BVI Act, divide among our shareholders in specie or kind the whole or any part of our assets (whether they shall consist of property of the same kind or not) and may, for such purpose, set such value as the liquidator deems fair upon any property to be divided and may determine how such division shall be carried out as between the shareholders or different classes of shareholders.
Calls on common shares and forfeiture of common shares
Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their common shares in a notice served to such shareholders at least 14 days prior to the specified time of payment. The common shares that have been called upon and remain unpaid are subject to forfeiture.
Redemption of common shares
Subject to the provisions of the BVI Act, we may issue shares on terms that are subject to redemption, at our option or at the option of the holders, on such terms and in such manner as may be determined by our memorandum and articles of association and subject to any applicable requirements imposed from time to time by, the BVI Act, the SEC, the NASDAQ Capital Market, or by any recognized stock exchange on which our securities are listed.
Modifications of rights
All or any of the special rights attached to any class of shares may, subject to the provisions of the BVI Act, be amended only pursuant to a resolution passed at a meeting by a majority of the votes cast by those entitled to vote at a meeting of the holders of the shares of that class.
Changes in the number of shares we are authorized to issue and those in issue
We may from time to time by resolution of our board of directors:
| • | amend our memorandum of association to increase or decrease the maximum number of shares we are authorized to issue; |
| • | subject to our memorandum, divide our authorized and issued shares into a larger number of shares; and |
| • | subject to our memorandum, combine our authorized and issued shares into a smaller number of shares. |
Untraceable shareholders
We are entitled to sell any shares of a shareholder who is untraceable, provided that:
| • | all checks or warrants in respect of dividends of these shares, not being less than three in number, for any sums payable in cash to the holder of such shares have remained uncashed for a period of twelve years prior to the publication of the notice and during the three months referred to in the third bullet point below; |
| • | we have not during that time received any indication of the whereabouts or existence of the shareholder or person entitled to these shares by death, bankruptcy or operation of law; and |
| • | we have caused a notice to be published in newspapers in the manner stipulated by our memorandum and articles of association, giving notice of our intention to sell these shares, and a period of three months has elapsed since such notice. |
The net proceeds of any such sale shall belong to us, and when we receive these net proceeds we shall become indebted to the former shareholder for an amount equal to the net proceeds.
Inspection of books and records
Under British Virgin Islands Law, holders of our common shares are entitled, upon giving written notice to us, to inspect (i) our memorandum and articles of association, (ii) the register of members, (iii) the register of directors and (iv) minutes of meetings and resolutions of members, and to make copies and take extracts from the documents and records. However, our directors can refuse access if they are satisfied that to allow such access would be contrary to our interests. See “Where You Can Find Additional Information.”
86
Table of Contents
Rights of non-resident or foreign shareholders
There are no limitations imposed by our memorandum and articles of association on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in our memorandum and articles of association governing the ownership threshold above which shareholder ownership must be disclosed.
Issuance of additional common shares
Our memorandum and articles of association authorizes our board of directors to issue additional common shares from authorized but unissued shares, to the extent available, from time to time as our board of directors shall determine.
Differences in Corporate Law
The BVI Act and the laws of the British Virgin Islands affecting British Virgin Islands companies like us and our shareholders differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the laws of the British Virgin Islands applicable to us and the laws applicable to companies incorporated in the United States and their shareholders.
Mergers and similar arrangements
Under the laws of the British Virgin Islands, two or more companies may merge or consolidate in accordance with Section 170 of the BVI Act. A merger means the merging of two or more constituent companies into one of the constituent companies and a consolidation means the uniting of two or more constituent companies into a new company. In order to merge or consolidate, the directors of each constituent company must approve a written plan of merger or consolidation, which must be authorized by a resolution of shareholders.
While a director may vote on the plan of merger or consolidation even if he has a financial interest in the plan, the interested director must disclose the interest to all other directors of the company promptly upon becoming aware of the fact that he is interested in a transaction entered into or to be entered into by the company.
A transaction entered into by our company in respect of which a director is interested (including a merger or consolidation) is voidable by us unless the director’s interest was (a) disclosed to the board prior to the transaction or (b) the transaction is (i) between the director and the company and (ii) the transaction is in the ordinary course of the company’s business and on usual terms and conditions.
Notwithstanding the above, a transaction entered into by the company is not voidable if the material facts of the interest are known to the shareholders and they approve or ratify it or the company received fair value for the transaction.
Shareholders not otherwise entitled to vote on the merger or consolidation may still acquire the right to vote if the plan of merger or consolidation contains any provision which, if proposed as an amendment to the memorandum or articles of association, would entitle them to vote as a class or series on the proposed amendment. In any event, all shareholders must be given a copy of the plan of merger or consolidation irrespective of whether they are entitled to vote at the meeting to approve the plan of merger or consolidation.
The shareholders of the constituent companies are not required to receive shares of the surviving or consolidated company but may receive debt obligations or other securities of the surviving or consolidated company, other assets, or a combination thereof. Further, some or all of the shares of a class or series may be converted into a kind of asset while the other shares of the same class or series may receive a different kind of asset. As such, not all the shares of a class or series must receive the same kind of consideration.
After the plan of merger or consolidation has been approved by the directors and authorized by a resolution of the shareholders, articles of merger or consolidation are executed by each company and filed with the Registrar of Corporate Affairs in the British Virgin Islands.
A shareholder may dissent from a mandatory redemption of his shares, an arrangement (if permitted by the court), a merger (unless the shareholder was a shareholder of the surviving company prior to the merger and continues to hold the same or similar shares after the merger) or a consolidation. A shareholder properly exercising his dissent rights is entitled to a cash payment equal to the fair value of his shares.
87
Table of Contents
A shareholder dissenting from a merger or consolidation must object in writing to the merger or consolidation before the vote by the shareholders on the merger or consolidation, unless notice of the meeting was not given to the shareholder. If the merger or consolidation is approved by the shareholders, the company must give notice of this fact to each shareholder within 20 days who gave written objection. These shareholders then have 20 days to give to the company their written election in the form specified by the BVI Act to dissent from the merger or consolidation, provided that in the case of a merger, the 20 days starts when the plan of merger is delivered to the shareholder.
Upon giving notice of his election to dissent, a shareholder ceases to have any shareholder rights except the right to be paid the fair value of his shares. As such, the merger or consolidation may proceed in the ordinary course notwithstanding his dissent.
Within seven days of the later of the delivery of the notice of election to dissent and the effective date of the merger or consolidation, the company must make a written offer to each dissenting shareholder to purchase his shares at a specified price per share that the company determines to be the fair value of the shares. The company and the shareholder then have 30 days to agree upon the price. If the company and a shareholder fail to agree on the price within the 30 days, then the company and the shareholder shall, within 20 days immediately following the expiration of the 30-day period, each designate an appraiser and these two appraisers shall designate a third appraiser. These three appraisers shall fix the fair value of the shares as of the close of business on the day prior to the shareholders’ approval of the transaction without taking into account any change in value as a result of the transaction.
Shareholders’ suits
There are both statutory and common law remedies available to our shareholders as a matter of British Virgin Islands law. These are summarized below:
Prejudiced members
A shareholder who considers that the affairs of the company have been, are being, or are likely to be, conducted in a manner that is, or any act or acts of the company have been, or are, likely to be oppressive, unfairly discriminatory or unfairly prejudicial to him in that capacity, can apply to the court under Section 184I of the BVI Act, inter alia, for an order that his shares be acquired, that he be provided compensation, that the Court regulate the future conduct of the company, or that any decision of the company which contravenes the BVI Act or our memorandum and articles of association be set aside.
Derivative actions
Section 184C of the BVI Act provides that a shareholder of a company may, with the leave of the Court, bring an action in the name of the company to redress any wrong done to it.
Just and equitable winding up
In addition to the statutory remedies outlined above, shareholders can also petition for the winding up of a company on the grounds that it is just and equitable for the court to so order. Save in exceptional circumstances, this remedy is only available where the company has been operated as a quasi partnership and trust and confidence between the partners has broken down.
Indemnification of directors and executive officers and limitation of liability
British Virgin Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any provision providing indemnification may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime.
Under our memorandum and articles of association, we indemnify against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with legal, administrative or investigative proceedings for any person who:
| • | is or was a party or is threatened to be made a party to any threatened, pending or completed proceedings, whether civil, criminal, administrative or investigative, by reason of the fact that the person is or was our director; or |
88
Table of Contents
| • | is or was, at our request, serving as a director or officer of, or in any other capacity is or was acting for, another body corporate or a partnership, joint venture, trust or other enterprise. |
These indemnities only apply if the person acted honestly and in good faith with a view to our best interests and, in the case of criminal proceedings, the person had no reasonable cause to believe that his conduct was unlawful.
This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Anti-takeover provisions in our memorandum and articles of association
Some provisions of our memorandum and articles of association may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable, including provisions that provide for a staggered board of directors and prevent shareholders from taking an action by written consent in lieu of a meeting. However, under British Virgin Islands law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association, as amended and restated from time to time, as they believe in good faith to be in the best interests of our company.
Directors’ fiduciary duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction and that the transaction was of fair value to the corporation.
Under British Virgin Islands law, our directors owe the company certain statutory and fiduciary duties including, among others, a duty to act honestly, in good faith, for a proper purpose and with a view to what the directors believe to be in the best interests of the company. Our directors are also required, when exercising powers or performing duties as a director, to exercise the care, diligence and skill that a reasonable director would exercise in comparable circumstances, taking into account without limitation, the nature of the company, the nature of the decision and the position of the director and the nature of the responsibilities undertaken. In the exercise of their powers, our directors must ensure neither they nor the company acts in a manner which contravenes the BVI Act or our memorandum and articles of association, as amended and re-stated from time to time. A shareholder has the right to seek damages for breaches of duties owed to us by our directors.
Shareholder action by written consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. British Virgin Islands law provides that shareholders may approve corporate matters by way of a written resolution without a meeting signed by or on behalf of shareholders sufficient to constitute the requisite majority of shareholders who would have been entitled to vote on such matter at a general meeting; provided that if the consent is less than unanimous, notice must be given to all non-consenting shareholders. However, our memorandum and articles of association do not permit shareholders to act by written consent.
89
Table of Contents
Shareholder proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings. British Virgin Islands law and our memorandum and articles of association allow our shareholders holding not less than 30% of the votes of the outstanding voting shares to requisition a shareholders’ meeting. We are not obliged by law to call shareholders’ annual general meetings, but our memorandum and articles of association do permit the directors to call such a meeting. The location of any shareholders’ meeting can be determined by the board of directors and can be held anywhere in the world.
Cumulative voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. As permitted under British Virgin Islands law, our memorandum and articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our memorandum and articles of association, directors can be removed from office, with cause, by a resolution of shareholders or by a resolution of directors passed at a meeting of directors called for the purpose of removing the director or for purposes including the removal of the director.
Transactions with interested shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or group who or which owns or owned 15% or more of the target’s outstanding voting shares within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware public corporation to negotiate the terms of any acquisition transaction with the target’s board of directors. British Virgin Islands law has no comparable statute. However, our memorandum and articles of association expressly provide for the same protection afforded by the Delaware business combination statute.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board. Under the BVI Act and our memorandum and articles of association, we may appoint a voluntary liquidator by a resolution of the shareholders or by resolution of directors.
90
Table of Contents
Variation of rights of shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. Under our memorandum and articles of association, if at any time our shares are divided into different classes of shares, the rights attached to any class may only be varied, whether or not our company is in liquidation, by a resolution passed at a meeting by a majority of the votes cast by those entitled to vote at a meeting of the holders of the issued shares in that class.
Amendment of governing documents
Under the Delaware General Corporation Law, a corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. As permitted by British Virgin Islands law, our memorandum and articles of association may be amended by a resolution of shareholders and, subject to certain exceptions, by a resolution of directors. Any amendment is effective from the date it is registered at the Registry of Corporate Affairs in the British Virgin Islands.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our common shares, and a liquid trading market for our common shares may not develop or be sustained after this offering. Future sales of substantial amounts of common shares, including common shares issued upon exercise of outstanding options and exercise of the warrants offered in this prospectus in the public market after this offering or the anticipation of those sales could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of our equity securities.
Upon the completion of the offering, we will have outstanding 4,500,000 common shares, assuming no exercise of outstanding options and the closing of the maximum offering. Of these common shares, the common shares sold in this offering will be freely tradable without restriction under the Securities Act. The remaining approximately 3,000,000 common shares outstanding will be restricted shares held by existing shareholders that could be sold pursuant to Rule 144. We have not agreed to register these restricted shares. We have not issued any warrants to purchase our common shares or other securities convertible into our common shares.
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person (or persons whose shares are aggregated) who is deemed to be an affiliate of our company at the time of sale, or at any time during the preceding three months, and who has beneficially owned restricted shares for at least six months, would be entitled to sell within any three-month period a number of our common shares that does not exceed the greater of 1% of the then outstanding common shares or the average weekly trading volume of common shares during the four calendar weeks preceding such sale. Sales under Rule 144 are subject to certain manner of sale provisions, notice requirements and the availability of current public information about our company. In addition, sales by our affiliates may be subject to the terms of lock-up agreements and Make-Good Escrow agreements. See “Shares Eligible for Future Sale – Lock-Up Agreements” and “Related Party Transactions – Make-Good Shares Subject to Redemption.”
A person who has not been our affiliate at any time during the three months preceding a sale, and who has beneficially owned his or her common shares for at least six months, would be entitled under Rule 144 to sell such shares without regard to any manner of sale, notice provisions or volume limitations described above. Any such sales must comply with the public information provision of Rule 144 until our common shares have been held for one year.
Rule 701
Securities issued in reliance on Rule 701 are also restricted and may be sold by shareholders other than affiliates of our company subject only to manner of sale provisions of Rule 144 and by affiliates under Rule 144 without compliance with its six-month holding period requirement.
91
Table of Contents
Registration on Form S-8
We intend to file a registration statement on Form S-8 under the Securities Act as soon as practicable after the closing of this offering to register up to 450,000 of our common shares subject to outstanding share options or reserved for issuance under our share incentive plan, such amount being equal to ten percent (10%) of the number of common shares issued and outstanding immediately after the closing of the offering, assuming a maximum offering. This registration will permit the resale of these common shares by nonaffiliates in the public market without restriction under the Securities Act, upon the completion of the lock-up period described below. Common shares registered pursuant to the Form S-8 held by affiliates will be subject to Rule 144 volume limitations. As of the date of this Prospectus, we have not issued any options to purchase our common shares.
Lock-Up Agreements
Each of our executive officers, directors and individuals who on the effective date of the registration statement of which this prospectus is a part are the beneficial owners of more than 5% of our common shares, has agreed not to register, offer, sell, contract to sell or grant any of our common shares or any securities convertible into or exercisable or exchangeable for our common shares or any warrants to purchase our common shares (including, without limitation, securities of our company which may be deemed to be beneficially owned by such individuals in accordance with the rules and regulations of the Securities and Exchange Commission and securities which may be issued upon the exercise of a stock option or warrant) for a period of (a) as to one-half ( 1/2) of the common shares now or in the future beneficially owned by such individual, ninety (90) days after the date of effectiveness or commencement of sales of this public offering and (b) as to the other one-half of such common shares now or in the future beneficially owned by such individual, one hundred ninety (190) days after the date of effectiveness or commencement of sales of this public offering. Upon the expiration of these lock-up agreements, additional common shares will be available for sale in the public market.
Summary of Shares Available for Future Sale
The following table summarizes the total shares potentially available for future sale. To the extent we sell a number of common shares between the minimum and maximum offering, the below tables will be adjusted proportionately as to numbers of shares available for sale (as to option pool and placement agent shares) and dates on which such shares may be sold (as to currently outstanding shares).
Minimum Offering
| Shares |
Date Available for Sale | |
| Currently Outstanding Common Shares: 3,000,000 | ||
| 1,205,001 |
After 90 days from the date of effectiveness or commencement of sales of the public offering | |
| 1,294,999 |
After 190 days from the date of effectiveness or commencement of sales of the public offering | |
| 500,000 |
After 30 days after filing of Form 10-K for the year ending December 31, 2010, assuming no redemption of Make-Good Shares; any delay in redemption will also delay the release of these shares | |
| Common Shares in Option Pool: 425,000 | From vesting dates through expiration of grants | |
| Common Shares Underlying Placement Agent’s Warrants: 125,000 |
After 180 days from the date of effectiveness or commencement of sales of the public offering | |
92
Table of Contents
Maximum Offering
| Shares |
Date Available for Sale | |
| Currently Outstanding Common Shares: 3,000,000 | ||
| 1,155,001 |
After 90 days from the date of effectiveness or commencement of sales of the public offering | |
| 1,244,999 |
After 190 days from the date of effectiveness or commencement of sales of the public offering | |
| 600,000 |
After 30 days after filing of Form 10-K for the year ending December 31, 2010, assuming no redemption of Make-Good Shares; any delay in redemption will also delay the release of these shares | |
| Common Shares in Option Pool: 450,000 | From vesting dates through expiration of grants | |
| Common Shares Underlying Placement Agent’s Warrants: 150,000 | After 180 days from the date of effectiveness or commencement of sales of the public offering | |
93
Table of Contents
TAX MATTERS APPLICABLE TO U.S. HOLDERS OF OUR COMMON SHARES
The following sets forth the material British Virgin Islands, Chinese and U.S. federal income tax consequences of an investment in our common shares. It is directed to U.S. Holders (as defined below) of our common shares and is based upon laws and relevant interpretations thereof in effect as of the date of this prospectus, all of which are subject to change. This description does not deal with all possible tax consequences relating to an investment in our common shares, such as the tax consequences under state, local and other tax laws. To the extent that the description relates to matters of British Virgin Islands tax law, it represents the opinion of Kaufman & Canoles, P.C., our British Virgin Islands counsel. To the extent that the description relates to matters of United States tax law, it represents the opinion of Kaufman & Canoles, P.C., our United States counsel. To the extent that the description relates to matters of Chinese tax law, it represents the opinion of Beijing Kang Da Law Firm, our Chinese counsel. All such tax opinions of the above counsel are set forth in full in this section. All such tax opinions rely on the factual statements made in this registration statement and are limited to the matters discussed herein. Any variance in facts from those stated in the registration statement may affect the conclusions stated below. All such opinions are as of the date of this registration statement, and each respective counsel undertakes no obligation to update its respective opinion. Each opinion is based on statutes, regulations and agency and judicial interpretations thereof, all of which may be subject to change either prospectively or retrospectively.
People’s Republic of China Enterprise Taxation
The following description of Chinese enterprise laws is designed to highlight the enterprise-level taxation on our earnings, which will affect the amount of dividends, if any, we are ultimately able to pay to our shareholders. See “Dividend Policy.”
In 2007, the PRC National People’s Congress enacted the PRC Enterprise Income Tax Law and related implementation rules, or the EIT Law, which became effective on January 1, 2008. The EIT Law imposes a single uniform income tax rate of 25% on all Chinese enterprises, including foreign-invested enterprises, and levies a withholding tax rate of 10% on dividends payable by Chinese subsidiaries to their foreign shareholders unless any such foreign shareholders’ jurisdiction of incorporation has a tax treaty with China that provides for a different withholding agreement. Under the EIT Law, enterprises established outside China but deemed to have a “de facto management body” within the country may be considered “resident enterprises” for Chinese tax purposes and, therefore, may be subject to an enterprise income tax rate of 25% on their worldwide income. Pursuant to the implementation rules of the EIT Law, “de facto management bodies” have material and overall management control over the business, personnel, accounts and properties of the enterprise. At present, the Chinese tax authority has not issued any guidance on the application of the EIT Law and its implementing rules on non-Chinese enterprise or group enterprise controlled entities whose structures are like ours. As a result, it is unclear what factors will be used by the Chinese tax authorities to determine whether we are a “de facto management organization” in China. However, as substantially all members of our management team are located in China, and may remain there in the future, we may be deemed a resident enterprise subject to an enterprise income tax rate of 25% on our worldwide income, with the possible exclusion of dividends received directly from another Chinese tax resident.
British Virgin Islands Taxation
Under the BVI Act as currently in effect, a holder of common shares who is not a resident of the British Virgin Islands is exempt from British Virgin Islands income tax on dividends paid with respect to the common shares and all holders of common shares are not liable to the British Virgin Islands for income tax on gains realized during that year on sale or disposal of such shares. The British Virgin Islands does not impose a withholding tax on dividends paid by a company incorporated or re-registered under the BVI Act.
There are no capital gains, gift or inheritance taxes levied by the British Virgin Islands on companies incorporated or re-registered under the BVI Act. In addition, shares of companies incorporated or re-registered under the BVI Act are not subject to transfer taxes, stamp duties or similar charges.
There is no income tax treaty or convention currently in effect between the United States and the British Virgin Islands or between China and the British Virgin Islands.
94
Table of Contents
United States Federal Income Taxation
The following describes the material U.S. federal income tax consequences to U.S. Holders (as defined below) under present law of an investment in our common shares. This description applies only to U.S. Holders that hold common shares as capital assets and that have the U.S. dollar as their functional currency. This description is based on the tax laws of the United States in effect as of the date of this prospectus and on U.S. Treasury regulations in effect or, in some cases, proposed, as of the date of this prospectus, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing authorities are subject to change, which change could apply retroactively and could affect the tax consequences described below.
WE URGE POTENTIAL PURCHASERS OF OUR SHARES TO CONSULT THEIR OWN TAX
ADVISORS CONCERNING THE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX
CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR SHARES.
The following does not address the tax consequences to any particular investor or to persons in special tax situations such as:
| • | banks; |
| • | financial institutions; |
| • | insurance companies; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | broker-dealers; |
| • | traders that elect to mark-to-market; |
| • | U.S. expatriates; |
| • | tax-exempt entities; |
| • | persons liable for alternative minimum tax; |
| • | persons holding our common shares as part of a straddle, hedging, conversion or integrated transaction; |
| • | persons that actually or constructively own 10% or more of our voting shares; |
| • | persons who acquired our common shares pursuant to the exercise of any employee share option or otherwise as consideration; or |
| • | persons holding our common shares through partnerships or other pass-through entities. |
Prospective purchasers are urged to consult their tax advisors about the application of the U.S. Federal tax rules to their particular circumstances as well as the state, local, foreign and other tax consequences to them of the purchase, ownership and disposition of our common shares.
The description below of the U.S. federal income tax consequences to “U.S. Holders” will apply to you if you are a beneficial owner of shares and you are, for U.S. federal income tax purposes,
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
Taxation of dividends and other distributions on our common shares
Subject to the passive foreign investment company rules discussed below, the gross amount of distributions made by us to you with respect to the common shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). The dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, for taxable years beginning before January 1, 2011, dividends will be taxed at the lower capital gains rate applicable to qualified dividend
95
Table of Contents
income, provided that (1) the common shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a passive foreign investment company (as discussed below) for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Under U.S. Internal Revenue Service authority, common shares are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on the NASDAQ Capital Market. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our common shares, including the effects of any change in law after the date of this prospectus.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our common shares will constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.”
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your common shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
Taxation of Dispositions of Common Shares
Subject to the passive foreign investment company rules discussed below, you will recognize taxable gain or loss on any sale, exchange or other taxable disposition of a share equal to the difference between the amount realized (in U.S. dollars) for the share and your tax basis (in U.S. dollars) in the common shares. The gain or loss will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the common shares for more than one year, you will be eligible for reduced tax rates of 0% (for individuals in the 10% or 15% tax brackets) or 15% for all other individuals, assuming the renewal of current capital gains rates prior to their scheduled expiration at the end of 2010. If capital gains preferential rates are not renewed, such gains would be taxable at the personal income rates then in place. The deductibility of capital losses is subject to limitations. Any such gain or loss that you recognize will generally be treated as United States source income or loss for foreign tax credit limitation purposes.
Passive Foreign Investment Company
Based on our current and anticipated operations and the composition of our assets, we do not expect to be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for our current taxable year ending December 31, 2008. Our actual PFIC status for the current taxable year ending December 31, 2008 will not be determinable until the close of such taxable year and, accordingly, there is no guarantee that we will not be a PFIC for the current taxable year. Because PFIC status is a factual determination for each taxable year which cannot be made until the close of the taxable year. A non-U.S. corporation is considered a PFIC for any taxable year if either:
| • | at least 75% of its gross income is passive income; or |
| • | at least 50% of the value of its assets (based on an average of the quarterly values of the assets during a taxable year) is attributable to assets that produce or are held for the production of passive income (the “asset test”). |
We will be treated as owning our proportionate share of the assets and earning our proportionate share of the income of any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock.
We must make a separate determination each year as to whether we are a PFIC. As a result, our PFIC status may change. In particular, because the value of our assets for purposes of the asset test will generally be determined
96
Table of Contents
based on the market price of our common shares, our PFIC status will depend in large part on the market price of our common shares. Accordingly, fluctuations in the market price of the common shares may cause us to become a PFIC. In addition, the application of the PFIC rules is subject to uncertainty in several respects and the composition of our income and assets will be affected by how, and how quickly, we spend the cash we raise in this offering. If we are a PFIC for any year during which you hold common shares, we will continue to be treated as a PFIC for all succeeding years during which you hold common shares. However, if we cease to be a PFIC, you may avoid some of the adverse effects of the PFIC regime by making a “deemed sale” election with respect to the common shares.
If we are a PFIC for any taxable year during which you hold common shares, you will be subject to special tax rules with respect to any “excess distribution” that you receive and any gain you realize from a sale or other disposition (including a pledge) of the common shares, unless you make a “mark-to-market” election as discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the common shares will be treated as an excess distribution. Under these special tax rules:
| • | the excess distribution or gain will be allocated ratably over your holding period for the common shares; |
| • | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we were a PFIC, will be treated as ordinary income, and |
| • | the amount allocated to each other year will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to years prior to the year of disposition or “excess distribution” cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the sale of the common shares cannot be treated as capital, even if you hold the common shares as capital assets.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election for such stock to elect out of the tax treatment discussed above. If you make a mark-to-market election for the common shares, you will include in income each year an amount equal to the excess, if any, of the fair market value of the common shares as of the close of your taxable year over your adjusted basis in such common shares. You are allowed a deduction for the excess, if any, of the adjusted basis of the common shares over their fair market value as of the close of the taxable year. However, deductions are allowable only to the extent of any net mark-to-market gains on the common shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as gain on the actual sale or other disposition of the common shares, are treated as ordinary income. Ordinary loss treatment also applies to the deductible portion of any mark-to-market loss on the common shares, as well as to any loss realized on the actual sale or disposition of the common shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such common shares. Your basis in the common shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, the tax rules that apply to distributions by corporations which are not PFICs would apply to distributions by us, except that the lower applicable capital gains rate for qualified dividend income discussed above under “—Taxation of Dividends and Other Distributions on our Common shares” generally would not apply.
The mark-to-market election is available only for “marketable stock”, which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a qualified exchange or other market (as defined in applicable U.S. Treasury regulations), including the NASDAQ Capital Market. If the common shares are regularly traded on the NASDAQ Capital Market and if you are a holder of common shares, the mark-to-market election would be available to you were we to be or become a PFIC.
Alternatively, a U.S. Holder of stock in a PFIC may make a “qualified electing fund” election with respect to such PFIC to elect out of the tax treatment discussed above. A U.S. Holder who makes a valid qualified electing fund election with respect to a PFIC will generally include in gross income for a taxable year such holder’s pro rata share of the corporation’s earnings and profits for the taxable year. However, the qualified electing fund election is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. We do not currently intend to prepare or provide the information that would enable you to make a qualified electing fund election.
97
Table of Contents
If you hold common shares in any year in which we are a PFIC, you will be required to file U.S. Internal Revenue Service Form 8621 regarding distributions received on the common shares and any gain realized on the disposition of the common shares.
You are urged to consult your tax advisors regarding the application of the PFIC rules to your investment in our common shares and the elections discussed above.
Information Reporting and Backup Withholding
Dividend payments with respect to our common shares and proceeds from the sale, exchange or redemption of our common shares may be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding at a current rate of 28%. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or who is otherwise exempt from backup withholding. U.S. Holders who are required to establish their exempt status generally must provide such certification on U.S. Internal Revenue Service Form W-9. U.S. Holders are urged to consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information.
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the British Virgin Islands with limited liability. We are incorporated in the British Virgin Islands because of certain benefits associated with being a British Virgin Islands corporation, such as political and economic stability, an effective judicial system, a favorable tax system, the absence of exchange control or currency restrictions and the availability of professional and support services. However, the British Virgin Islands has a less developed body of securities laws as compared to the United States and provides protections for investors to a significantly lesser extent. In addition, British Virgin Islands companies may not have standing to sue before the federal courts of the United States.
Substantially all of our assets are located outside the United States. In addition, a majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or such persons or to enforce against them or against us, judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof.
We have appointed CT Corporation System as our agent to receive service of process with respect to any action brought against us in the United States District Court for the Southern District of New York under the federal securities laws of the United States or of any State of the United States or any action brought against us in the Supreme Court of the State of New York in the County of New York under the securities laws of the State of New York.
Beijing Kang Da Law Firm, our counsel as to Chinese law, has advised us that there is uncertainty as to whether the courts of China would (1) recognize or enforce judgments of United States courts obtained against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or (2) be competent to hear original actions brought in each respective jurisdiction, against us or such persons predicated upon the securities laws of the United States or any state thereof.
Beijing Kang Da Law Firm has advised us that the recognition and enforcement of foreign judgments are provided for under the Chinese Civil Procedure Law. Chinese courts may recognize and enforce foreign judgments in accordance with the requirements of the Chinese Civil Procedure Law based either on treaties between China and the country where the judgment is made or in reciprocity between jurisdictions. China does not have any treaties or other agreements with the British Virgin Islands or the United States that provide for the reciprocal recognition and enforcement of foreign judgments. As a result, it is uncertain whether a Chinese court would enforce a judgment rendered by a court in either of these two jurisdictions.
98
Table of Contents
We have been advised by Kaufman & Canoles, our counsel as to British Virgin Islands law, that the United States and the British Virgin Islands do not have a treaty providing for reciprocal recognition and enforcement of judgments of courts of the United States in civil and commercial matters and that a final judgment for the payment of money rendered by any general or state court in the United States based on civil liability, whether or not predicated solely upon the U.S. federal securities laws, is unlikely to be enforceable in the British Virgin Islands. We have also been advised by Kaufman & Canoles that a final and conclusive judgment obtained in U.S. federal or state courts under which a sum of money is payable as compensatory damages (i.e., not being a sum claimed by a revenue authority for taxes or other charges of a similar nature by a governmental authority, or in respect of a fine or penalty or multiple or punitive damages) may be the subject of an action on a debt in the court of the British Virgin Islands under the common law doctrine of obligation.
We have engaged Anderson & Strudwick, Incorporated to conduct this offering on a “best efforts, minimum/maximum” basis. The offering is being made without a firm commitment by the placement agent, which has no obligation or commitment to purchase any of our shares. None of our officers, directors or affiliates may purchase shares in this offering.
Unless sooner withdrawn or canceled by either us or the placement agent, the offering will continue until the earlier of (i) a date mutually acceptable to us and our placement agent after which the minimum offering is sold or (ii) May 31, 2010 (the “Offering Termination Date”). The Placement Agent has agreed in accordance with the provisions of SEC Rule 15c2-4 to cause all funds received by the Placement Agent for the sale of the common shares to be promptly deposited in an escrow account maintained by SunTrust Bank, N.A. (the “Escrow Agent”) as escrow agent for the investors in the offering. The Escrow Agent will exercise signature control on the escrow account and will act based on joint instructions from our company and the placement agent. On the closing date for the offering, net proceeds in the escrow account maintained by the Escrow Agent will be delivered to our company. We will not be able to use such proceeds in China, however, until we complete certain remittance procedures in China. If we do not complete this offering before the Offering Termination Date, all amounts will be promptly returned as described below. If we complete this offering, then on the closing date, we will issue shares to investors and placement agent warrants to our placement agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of common shares sold in This offering. In the event of any dispute between our company and the placement, including about whether the minimum offering has been sold and whether and how funds are to be reimbursed, the escrow agent is entitled to petition a court of competent jurisdiction to resolve any such dispute.
Investors must pay in full for all common shares at the time of investment. Payment for the common shares may be made (i) by check, bank draft or money order made payable to “SunTrust Bank” and delivered to the Placement Agent no less than four business days before the date of closing, or (ii) by authorization of withdrawal from securities accounts maintained with the Placement Agent. If payment is made by authorization of withdrawal from securities accounts, the funds authorized to be withdrawn from a securities account will continue to accrue interest, if any interest is to accrue on such amounts, at the contractual rates until closing or termination of the offering, but a hold will be placed on such funds, thereby making them unavailable to the purchaser until closing or termination of the offering. If a purchaser authorizes the Placement Agent to withdraw the amount of the purchase price from a securities account, such Placement Agent will do so as of the date of closing. The Placement Agents will inform prospective purchasers of the anticipated date of closing. If payment is made by check, investors should make all checks payable to the Escrow Agent.
Proceeds deposited in escrow with the Escrow Agent may not be withdrawn by investors prior to the earlier of the closing of the offering or the Offering Termination Date. If the offering is withdrawn or canceled or if the 1,250,000 share minimum offering is not reached and proceeds therefrom are not received by us on or prior to the Offering Termination Date, all proceeds will be promptly returned by the Escrow Agent without interest or deduction to the persons from which they are received (within one business day) in accordance with applicable securities laws. All such proceeds will be placed in a non-interest bearing account pending such time.
Pursuant to that certain placement agreement by and between the placement agent and us, the obligations of the placement agent to solicit offers to purchase the shares and of investors solicited by the placement agent to purchase
99
Table of Contents
our common shares are subject to approval of certain legal matters by counsel to the placement agent. The placement agent’s ability to complete this “best efforts minimum/maximum” transaction is dependent upon the existence of stable U.S. trading markets. As such, the placement agent’s obligations under the placement agreement are also subject to various conditions which are customary in transactions of this type, including that, as of the closing of the offering, there shall not have occurred (i) a suspension or material limitation in trading in securities or the publication of quotations on the NASDAQ Stock Market (National Market System or Capital Market); (ii) a general moratorium on commercial banking activities in the State of New York or China; (iii) the engagement by the United States or China in hostilities which have resulted in the declaration of a national emergency or war if any such event would have a material adverse effect, in the placement agent’s reasonable judgment, as to make it impracticable or inadvisable to proceed with the solicitation of offers to consummate the offering with respect to investors solicited by the placement agent on the terms and conditions contemplated herein.
We have agreed to indemnify the placement agent against certain liabilities, including liabilities under the Securities Act of 1933, or to contribute to payments the placement agent may be required to make in respect of those liabilities.
The placement agent is offering the common shares, subject to prior sale, when, as and if issued to and accepted by it, subject to conditions contained in the placement agreement, such as the receipt by the placement agent of officers’ certificates and legal opinions. The placement agent reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part in the event (i) our representations or warranties are incorrect or misleading or we fail to fulfill our agreements with the placement agent; (ii) a material adverse change occurs affecting our business, management, property, assets, results of operations, condition or prospects; (iii) trading is suspended on any national securities exchange; (iv) war is declared; (v) a banking moratorium is declared in Virginia, New York or the U.S.; or (vi) any laws, regulations, court or administrative order or other governmental or agency act causes the placement agent to believe that our business or the U.S. securities markets will be materially adversely affected. The placement agent’s discretion in this regard is broad.
The placement agent intends to offer our common shares to its retail customers only in states in which we are permitted to offer our common shares. We have relied on an exemption to the blue sky registration requirements afforded to “covered securities”. Securities listed on the NASDAQ Capital Market are “covered securities.” If we were unable to meet the NASDAQ Capital Market’s listing standards, then we would be unable to rely on the covered securities exemption to blue sky registration requirements and we would need to register the offering in each state in which we planned to sell shares. Consequently, we will not complete this offering unless we meet the NASDAQ Capital Market’s listing requirements.
In connection with this offering, the placement agent or certain of the securities dealers may distribute prospectuses electronically. No forms of prospectus other than printed prospectuses and electronically distributed prospectuses that are printable in Adobe PDF format will be used in connection with this offering.
Foreign Regulatory Restrictions on Purchase of our Shares
We have not taken any action to permit a public offering of our shares outside the United States or to permit the possession or distribution of this prospectus outside the United States. People outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering of our shares and the distribution of this prospectus outside the United States.
Commissions and Discounts
The placement agent has advised us that it proposes to offer the common shares to the public at the initial public offering price on the cover page of this prospectus. The following table shows the public offering price, placement agent fee to be paid by us to the placement agent and the proceeds, before expenses, to us.
| Per Common share | Minimum Offering | Maximum Offering | |||||||
| Assumed public offering price |
$ | 8.00 | $ | 10,000,000 | $ | 12,000,000 | |||
| Placement discount |
$ | 0.56 | $ | 700,000 | $ | 840,000 | |||
| Proceeds to us, before expenses |
$ | 7.44 | $ | 9,300,000 | $ | 11,160,000 | |||
100
Table of Contents
We expect our total cash expenses for this offering to be approximately $500,000, exclusive of the above commissions. In addition, we will pay the placement agent a non-accountable expense allowance of 1% of the amount of the offering, or up to $120,000 (maximum offering, exclusive of shares registered under Rule 462(b)) or $100,000 (minimum offering). The placement agent must sell the minimum number of securities offered (1,250,000 common shares) if any are sold. The placement agent is required to use only its best efforts to sell the securities offered. The offering will terminate upon the earlier of: (i) a date mutually acceptable to us and our placement agent after which the minimum offering is sold or (ii) May 31, 2010. Until we sell at least 1,250,000 shares, all investor funds will be held in an escrow account at SunTrust Bank, Richmond, Virginia. If we do not sell at least 1,250,000 shares by May 31, 2010, all funds will be promptly returned to investors (within one business day) without interest or deduction. If we complete this offering, then on the closing date, we will issue shares to investors and placement agent warrants to our placement agent.
Placement Agent’s Warrants
We have agreed to sell to the placement agent, on the closing date of this offering, at a price of $0.001 per warrant, placement agent’s warrants exercisable at a rate of one warrant per share to purchase 10% of the number of common shares issued by us in connection with the offering. We will issue between 125,000 and 150,000 placement agent’s warrants in connection with this offering, depending on the number of common shares sold in this offering. Each placement agent’s warrant will be exercisable to purchase one common share. The placement agent’s warrants will be exercisable at 125% the offering price per common share for a period of five years after issuance on the closing date of this offering. The placement agent’s warrants may not be exercised, sold, transferred, pledged, assigned or hypothecated for a period of 180 days after the date of effectiveness or commencement of sales of the public offering, except to officers or partners and shareholders of the placement agent. This restriction is imposed pursuant to the requirements of FINRA Rule 5110(g)(1). If we do not complete this offering by selling at least the minimum number of common shares, we will not issue any placement agent’s warrants to our placement agent exercisable at a rate of one warrant per share to purchase up to 10% of the aggregate number of common shares sold in this offering.
For the life of the placement agent’s warrants, the holders thereof are given, at nominal costs, the opportunity to profit from a rise in the market price of our common shares with a resulting dilution in the interest of other shareholders. Further, the holders may be expected to exercise the placement agent’s warrant at a time when we would, in all likelihood, be able to obtain equity capital on terms more favorable than those provided in the placement agent’s warrants.
We are required for the life of the placement agent’s warrants to reserve sufficient common shares to deliver upon exercise of the warrants and to take all necessary actions to ensure that we may validly and legally issue fully paid and non-assessable shares on exercise of the warrants.
The placement agent has a right to demand registration of the common shares in the event registered common shares are not available at the time of exercise and an exemption from such registration is not otherwise available. If this happens, we will be required to file the registration statement within ninety (90) days after demand and to pay the costs associated with the registration other than the placement agent’s counsel fees and any underwriting or selling commissions. We are required to seek the listing of the shares on the same exchange on which our common shares trade, if any.
To the extent we are unable to register the shares, the placement agent may exercise the warrant with a cashless exercise, which is designed to give the placement agent the economic benefit of exercising the placement agent’s warrants. A cashless exercise, however, would not result in the payment of any exercise price to us.
The placement agent’s warrants also contain anti-dilution provisions, consistent with applicable FINRA rules, to adjust the terms of the placement agent’s warrants as necessary to protect against dilution in the event we reorganize, consolidate, merge or subdivide our shares.
Market and Pricing Considerations
There is not an established market for our common shares. We negotiated with our placement agent to determine the offering price of our common shares in this offering using a multiple of approximately 6.6 times our targeted after-tax net income for the fiscal year ending December 31, 2010. Noting past offerings completed by our placement agent, we believe that this multiple approximates the valuation multiples utilized in similar offerings for similarly-sized companies.
101
Table of Contents
In addition to prevailing market conditions, the factors considered in determining the applicable multiples were:
| • | The history of, and the prospects for, our company and the industry in which we compete; |
| • | An assessment of our management, its past and present operation, and the prospects for, and timing of, our future revenues; |
| • | The present state of our development; and |
| • | The factors listed above in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
If we are unable to achieve the targeted after-tax earnings upon which our valuation is based, then there is a risk that our company would be considered overvalued based on this multiple. In order to mitigate some of this risk, each of Ping Chen, Zheng (Rita) Liu, Weibing Yang, Jian Sun and Yong Wang has agreed to place, on a prorated basis, that number of beneficially owned common shares into escrow that is equal to 40% of the maximum number of shares to be sold in this offering. See “Related Party Transactions - Make-Good Shares Subject to Redemption.”
An active trading market for our common shares may not develop. It is possible that after this offering the common shares will not trade in the public market at or above the initial offering price.
The exercise price for the placement agent’s warrants issued to our placement agent in connection with, and conditional on the closing of, this offering has been negotiated between our company and the placement agent. The exercise price (125% of the offering price of common shares in this offering), along with the length of time the placement agent must wait before exercise (at least 180 days after the closing of this offering) are influenced by the valuation attributed by FINRA in its calculation of the acceptability of aggregate placement consideration.
Discretionary Shares
The placement agent will not sell any shares in this offering to accounts over which it exercises discretionary authority, without first receiving written consent from those accounts.
Application for Listing on the NASDAQ Capital Market
We have applied to list our common shares on the NASDAQ Capital Market under the symbol “DHRM.” As this offering is a best-efforts offering, the NASDAQ Capital Market has indicated that it is unable to admit our common shares for listing until the completion of the offering and, consequently, the satisfaction of NASDAQ Capital Market listing standards. If so admitted, we expect our common shares to begin trading on the NASDAQ Capital Market on the day following the closing of this offering. If our common shares are eventually listed on the NASDAQ Capital Market, we will be subject to continued listing requirements and corporate governance standards. We expect these new rules and regulations to significantly increase our legal, accounting and financial compliance costs. We and our placement agent agree that we will not close this offering unless we satisfy the NASDAQ Capital Market’s listing standards and will be able to trade on the NASDAQ Capital Market; however, we have not yet received such admission and have no assurance that we will receive such admission.
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of the common shares, the placement agent may engage in transactions that stabilize, maintain or otherwise affect the price of the common shares. In order to facilitate the offering, the placement agent may, but is not required to, bid for, and purchase, common shares in the open market to stabilize the price of the common shares. These activities may raise or maintain the market price of the common shares above independent market levels or prevent or retard a decline in the market price of the common shares. The placement agent is not required to engage in these activities, and may end any of these activities at any time. We and the placement agent have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
Certain matters as to Virginia law and U.S. federal law in connection with this offering will be passed upon for us and for the placement agent by Kaufman & Canoles, P.C. The validity of the shares and certain legal matters relating to the offering as to British Virgin Islands law will be passed upon for us by Kaufman & Canoles, P.C.
102
Table of Contents
Certain legal matters relating to the offering as to Chinese law will be passed upon for us by Beijing Kang Da Law Firm, People’s Republic of China. Kaufman & Canoles, P.C. may rely upon Beijing Kang Da Law Firm with respect to matters governed by PRC law.
Financial statements as of December 31, 2008 and 2007, and for the years then ended appearing in this prospectus, have been included herein and in the registration statement in reliance upon the report of Friedman LLP, an independent registered public accounting firm, appearing elsewhere herein, and upon the authority of that firm as experts in accounting and auditing.
INTERESTS OF EXPERTS AND COUNSEL
Attorneys with Kaufman & Canoles, P.C., representing our company with respect to this offering beneficially own 44,999 common shares of our company as of the date of this prospectus.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933 with respect to our common shares offered by this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information regarding us and our common shares offered hereby, please refer to the registration statement and the exhibits filed as part of the registration statement.
This registration statement, including exhibits thereto, and any periodic reports we may in the future be required to file may be inspected without charge at the Public Reference Room maintained by the SEC at 100 F Street, NE, Washington, D.C. 20549. You may obtain copies of the registration statement, including the exhibits thereto, and all of our periodic reports after payment of the fees prescribed by the SEC. For additional information regarding the operation of the Public Reference Room, you may call the SEC at 1-800-SEC-0330. The SEC also maintains a website which provides on-line access to reports and other information regarding registrants that file electronically with the SEC at the address: http://www.sec.gov.
EXPENSES RELATED TO THIS OFFERING
The estimated expenses payable by us in connection with this offering (other than the placement discounts and commissions) will be as follows. With the exception of the filing fees for the U.S. Securities Exchange Commission, FINRA and NASDAQ, all amounts are estimates.
| U.S. Securities Exchange Commission registration fee |
$ | 845 | |
| FINRA filing fee |
$ | 2,013 | |
| NASDAQ listing fee |
$ | 50,000 | |
| Legal fees and expenses for Chinese counsel |
$ | 95,000 | |
| Legal fees and expenses for British Virgin Islands counsel |
$ | 12,000 | |
| Legal fees and expenses for U.S. counsel |
$ | 160,000 | |
| Accounting fees and expenses |
$ | 150,000 | |
| Printing fees |
$ | 25,000 | |
| Miscellaneous |
$ | 5,142 | |
| Total |
$ | 500,000 |
103
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
F-1
Table of Contents

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
Dehaier Medical Systems Limited
We have audited the accompanying consolidated balance sheets of Dehaier Medical Systems Limited and Affiliate as of December 31, 2008 and 2007, and the consolidated related statements of operations, cash flows and shareholders’ equity for the years then ended. Dehaier Medical Systems Limited and Affiliate management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Dehaier Medical Systems Limited and Affiliate as of December 31, 2008 and 2007, and the consolidated results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ Friedman LLP
New York, New York
February 15, 2010

F- 2
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
CONSOLIDATED BALANCE SHEETS
| December 31, | September 30, | |||||
| 2008 | 2007 | 2009 | ||||
| US$ | US$ | US$ | ||||
| (Unaudited) | ||||||
| ASSETS |
||||||
| CURRENT ASSETS: |
||||||
| Cash and cash equivalents |
282,603 | 1,143,352 | 937,970 | |||
| Accounts receivable, less allowance for doubtful accounts of $141,926, $101,830 and $148,570 |
5,416,702 | 3,170,103 | 6,059,480 | |||
| Other receivables |
1,187,360 | 538,886 | 1,461,635 | |||
| Prepaid expenses and other current assets |
1,798,887 | 726,073 | 1,300,742 | |||
| Inventory |
1,575,228 | 2,277,110 | 2,625,682 | |||
| Due from shareholders |
— | 9,280 | — | |||
| Due from officer |
— | 27,418 | — | |||
| Total current assets | 10,260,780 | 7,892,222 | 12,385,509 | |||
| Property and equipment, net |
2,196,127 | 1,609,521 | 2,927,592 | |||
| Intangible assets, net |
36,525 | 73,049 | 9,131 | |||
| Tax receivable |
552,968 | 354,071 | 1,163,479 | |||
| Total assets | 13,046,400 | 9,928,863 | 16,485,711 | |||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||
| CURRENT LIABILITIES: |
||||||
| Short-term borrowings |
1,465,740 | 1,233,792 | 1,464,920 | |||
| Accounts payable |
74,320 | 48,894 | 79,855 | |||
| Advances from customers |
230,394 | 159,540 | 59,695 | |||
| Accrued expenses and other current liabilities |
250,839 | 224,308 | 297,448 | |||
| Taxes payable |
3,052,137 | 1,785,007 | 4,461,781 | |||
| Warranty obligation |
158,065 | 98,964 | 199,727 | |||
| Due to officer |
969 | — | 2,947 | |||
| Total current liabilities | 5,232,464 | 3,550,505 | 6,566,373 | |||
| Commitments and contingency |
||||||
| Shareholders’ equity | ||||||
| Preferred Stock Series A, $0.01 par value, 120,000 shares authorized, 120,000 shares issued and outstanding ( liquidation value of $1,200,000 ) |
1,200 | 1,200 | 1,200 | |||
| Preferred Stock Series B, $0.01 par value, 380,000 shares authorized, 182,635 shares issued and outstanding ( liquidation value of $2,000,000 ) |
1,826 | 1,826 | 1,826 | |||
| Common stock, $0.002731 par value, 16,476,300 shares authorized, 1,891,930 shares issued and outstanding * |
5,167 | 5,167 | 5,167 | |||
| Additional paid-in capital |
3,196,974 | 3,196,974 | 3,196,974 | |||
| Retained earnings |
2,624,771 | 1,706,751 | 4,696,547 | |||
| Accumulated other comprehensive income |
778,766 | 398,581 | 774,531 | |||
| Total Dehaier Medical Systems Limited shareholders’ equity | 6,608,704 | 5,310,499 | 8,676,245 | |||
| Non-controlling interest |
1,205,232 | 1,067,859 | 1,243,093 | |||
| Total shareholders’ equity | 7,813,936 | 6,378,358 | 9,919,338 | |||
| Total liabilities and shareholders’ equity | 13,046,400 | 9,928,863 | 16,485,711 | |||
| * | The change in par value of common shares pursuant to the stock split was retroactively adjusted. |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the years ended December 31, |
For the nine months ended September 30, |
|||||||||||
| 2008 | 2007 | 2009 | 2008 | |||||||||
| US$ | US$ | US$ | US$ | |||||||||
| (Unaudited) | (Unaudited) | |||||||||||
| Revenue |
9,414,430 | 6,599,512 | 9,422,460 | 6,531,854 | ||||||||
| Cost of revenues |
(5,931,087 | ) | (4,279,571 | ) | (5,688,352 | ) | (4,110,704 | ) | ||||
| Gross profit | 3,483,343 | 2,319,941 | 3,734,108 | 2,421,150 | ||||||||
| Service income |
418,483 | 369,001 | 298,036 | 301,622 | ||||||||
| Service expense |
(105,436 | ) | (79,815 | ) | (113,066 | ) | (115,674 | ) | ||||
| General and administrative expense |
(1,326,406 | ) | (757,642 | ) | (787,784 | ) | (917,943 | ) | ||||
| Selling expense |
(973,898 | ) | (880,863 | ) | (544,470 | ) | (785,132 | ) | ||||
| Operating income | 1,496,086 | 970,622 | 2,586,824 | 904,023 | ||||||||
| Financial expense ( including interest expense of $97,122 and $45,221, $67,704 and $65,022) |
(104,474 | ) | (43,953 | ) | (66,384 | ) | (71,029 | ) | ||||
| Other income (expense) |
4,071 | (4,600 | ) | — | 3,306 | |||||||
| Income before provision for income taxes and non-controlling interest |
1,395,683 | 922,069 | 2,520,440 | 836,300 | ||||||||
| Provision for income taxes |
(415,332 | ) | (73,648 | ) | (410,162 | ) | (270,300 | ) | ||||
| Net income | 980,351 | 848,421 | 2,110,278 | 566,000 | ||||||||
| Non-controlling interest in income |
(62,331 | ) | (53,214 | ) | (38,502 | ) | (44,437 | ) | ||||
| Net income attributable to Dehaier Medical Systems Limited |
918,020 | 795,207 | 2,071,776 | 521,563 | ||||||||
| Earnings per share | ||||||||||||
| -Basic |
0.48 | 0.42 | 1.10 | 0.27 | ||||||||
| -Diluted |
0.31 | 0.32 | 0.69 | 0.17 | ||||||||
| Weighted average number of common shares used in computation |
||||||||||||
| -Basic |
1,891,930 | 1,891,930 | 1,891,930 | 1,891,930 | ||||||||
| -Diluted |
3,000,000 | 2,451,624 | 3,000,000 | 3,000,000 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
| Dehaier Medical Systems Limited | ||||||||||||||||||||||||||||
| Common stock Shares US$ |
Preferred A Shares US$ |
Preferred B Shares US$ |
Additional paid-in capital US$ |
Retained earnings US$ |
Accumulated other comprehensive income US$ |
Comprehensive income US$ |
Non-controlling interest US$ |
Total US$ |
||||||||||||||||||||
| Balance as of January 1, 2007 |
1,891,930 | 5,167 | 120,000 | 1,200 | 1,198,800 | 911,544 | 102,714 | 946,293 | 3,165,718 | |||||||||||||||||||
| Shares issued |
182,635 | 1,826 | 1,998,174 | 2,000,000 | ||||||||||||||||||||||||
| Foreign currency translation |
295,867 | 295,867 | 68,352 | 364,219 | ||||||||||||||||||||||||
| Net income |
795,207 | 795,207 | 53,214 | 848,421 | ||||||||||||||||||||||||
| Comprehensive income |
1,091,074 | |||||||||||||||||||||||||||
| Balance as of December 31, 2007 |
1,891,930 | 5,167 | 120,000 | 1,200 | 182,635 | 1,826 | 3,196,974 | 1,706,751 | 398,581 | 1,067,859 | 6,378,358 | |||||||||||||||||
| Foreign currency translation |
380,185 | 380,185 | 75,042 | 455,227 | ||||||||||||||||||||||||
| Net income |
918,020 | 918,020 | 62,331 | 980,351 | ||||||||||||||||||||||||
| Comprehensive income |
1,298,205 | |||||||||||||||||||||||||||
| Balance as of December 31, 2008 |
1,891,930 | 5,167 | 120,000 | 1,200 | 182,635 | 1,826 | 3,196,974 | 2,624,771 | 778,766 | 1,205,232 | 7,813,936 | |||||||||||||||||
| Foreign currency translation |
(4,235 | ) | (4,235 | ) | (641 | ) | (4,876 | ) | ||||||||||||||||||||
| Net income |
2,071,776 | 2,071,776 | 38,502 | 2,110,278 | ||||||||||||||||||||||||
| Comprehensive income |
2,067,541 | |||||||||||||||||||||||||||
| Balance as of September 30, 2009 (unaudited) |
1,891,930 | 5,167 | 120,000 | 1,200 | 182,635 | 1,826 | 3,196,974 | 4,696,547 | 774,531 | 1,243,093 | 9,919,338 | |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the years ended December 31, |
For the nine months ended September 30, |
|||||||||||
| 2008 | 2007 | 2009 | 2008 | |||||||||
| US$ | US$ | US$ | US$ | |||||||||
| (Unaudited) | (Unaudited) | |||||||||||
| Operating Activities | ||||||||||||
| Net income |
980,351 | 848,421 | 2,110,278 | 566,000 | ||||||||
| Adjustments to reconcile net income to net cash provided by (used in) operating activities |
||||||||||||
| Depreciation and amortization |
235,867 | 147,801 | 210,231 | 155,423 | ||||||||
| Provision for (recovery of ) doubtful accounts |
23,297 | 112,358 | (18,765 | ) | ||||||||
| Provision for (recovery of ) warranty |
59,102 | 14,111 | 41,662 | (3,907 | ) | |||||||
| Gain on sale of equipment |
(3,324 | ) | — | — | (3,306 | ) | ||||||
| Changes in assets and liabilities: |
||||||||||||
| Increase in accounts receivable |
(2,269,896 | ) | (344,628 | ) | (642,778 | ) | (1,371,519 | ) | ||||
| Decrease (increase) in prepayments and other current assets |
(1,072,813 | ) | (496,610 | ) | 498,145 | (117,116 | ) | |||||
| Increase in other receivables |
(648,474 | ) | (119,052 | ) | (274,275 | ) | (691,451 | ) | ||||
| Decrease (increase) in inventory |
701,882 | (1,280,919 | ) | (1,050,453 | ) | (304,441 | ) | |||||
| Increase in tax receivable |
(198,896 | ) | (352,981 | ) | (610,511 | ) | (205,295 | ) | ||||
| (Decrease) increase in accounts payable |
25,425 | (308,326 | ) | 5,536 | 101,926 | |||||||
| (Decrease) increase in advances from customers |
70,854 | (484,448 | ) | (170,699 | ) | 55,054 | ||||||
| (Decrease) increase in accrued expenses and other current liabilities |
26,531 | 81,730 | 46,609 | (18,830 | ) | |||||||
| Increase in tax payable |
1,267,130 | 525,598 | 1,409,645 | 878,075 | ||||||||
| Net cash provided by (used in )operating activities |
(802,964 | ) | (1,656,945 | ) | 1,573,390 | (978,152 | ) | |||||
| Investing Activities |
||||||||||||
| Proceeds from sale of equipment |
33,102 | — | — | 32,929 | ||||||||
| Capital expenditures and other additions |
(708,580 | ) | (510,871 | ) | (915,693 | ) | (668,027 | ) | ||||
| Proceeds from (advances to) related parties |
37,667 | (31,530 | ) | 1,976 | 37,622 | |||||||
| Net cash used in investing activities |
(637,811 | ) | (542,401 | ) | (913,717 | ) | (597,476 | ) | ||||
| Financing Activities |
||||||||||||
| Proceeds from bank loans |
231,948 | 849,378 | — | 238,984 | ||||||||
| Proceeds from issuance of Series B preferred shares |
— | 2,000,000 | — | — | ||||||||
| Net cash provided by financing activities |
231,948 | 2,849,378 | — | 238,984 | ||||||||
| Effect of exchange rate fluctuations on cash and cash equivalents |
348,078 | 288,549 | (4,306 | ) | 394,095 | |||||||
| Net increase (decrease) in cash and cash equivalents |
(860,749 | ) | 938,581 | 655,367 | (942,549 | ) | ||||||
| Cash and cash equivalents at beginning of period |
1,143,352 | 204,771 | 282,603 | 1,143,352 | ||||||||
| Cash and cash equivalents at end of period |
282,603 | 1,143,352 | 937,970 | 200,803 | ||||||||
| Supplemental cash flow information |
||||||||||||
| Income tax paid |
3,765 | 130 | 12,824 | 3,745 | ||||||||
| Interest paid |
97,122 | 45,221 | 67,704 | 65,022 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
Dehaier Medical Systems Limited (“Dehaier”) was incorporated in the British Virgin Islands in 2003 as a limited liability company. Dehaier distributes and provides after-sale services for medical equipment in China mainly through its majority-owned subsidiary Beijing Dehaier Medical Technology Co. Limited (“BDL”) and its affiliate Beijing Dehaier Technology Limited (“BTL”). On October 23, 2003, Dehaier established a wholly-owned subsidiary in Hong Kong, De-haier Medical System (Hong Kong) Limited (“DHK”), (collectively, the “Company”). Both BDL and BTL were incorporated in the People’s Republic of China (“PRC”). The Company distributes branded, proprietary medical equipment, such as sleep apnea machines, patient monitors, air compressors, and oxygen generators; moreover, standard product registration, product certification and quality management system have been established in the Company. ISO13485 industry standard has also already been passed. It also has the distribution rights for a number of international medical equipment suppliers for products including anesthesia equipment, patient monitors, mobile C-arm X-ray machines and other medical equipment accessories.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements of the Company are prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
The accompanying consolidated interim financial statements include all adjustments that in the opinion of management, are necessary in order to make the consolidated interim financial statements not misleading.
Basis of Consolidation
The consolidated financial statements include the accounts of Dehaier, BDL, its majority-owned subsidiary, and its wholly-owned subsidiary, DHK as well as BTL. All significant inter-company transactions and balances are eliminated in consolidation.
A group of shareholders, including the Chief Executive officer, holds more than 50% of the voting ownership interest of Dehaier, BDL and BTL. BTL is a variable interest entity, and BDL is the primary beneficiary. BTL owns a building which is pledged as collateral for BDL’s bank loans. In exchange, BDL loans money to BTL to finance its operations. BTL’s primary operation is to provide repairs and transportation services to BDL’s customers. Because of these arrangements, BDL is the primary beneficiary of BTL, which is the entity that is most closely associated with BTL. Dehaier has included BTL in its consolidated financial statements through the consolidation with BDL since December 31, 2006. Dehaier, BDL and BTL were under common control until October 31, 2009, when each share of preferred stock was converted into a common share (see note 13). Because the chief executive officer held more than 50% of the voting ownership interest of each of Dehaier, BDL, BTL and DHK at December 31, 2006, the Company initially measured the assets, liabilities and noncontrolling interest of the variable interest entity at the amounts at which they were carried in the accounts of the reporting entity that controls the variables interest entity pursuant to FASB ASC 810-10.
F-7
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
The carrying amount and classification of BTL’s assets and liabilities included in the consolidated balance sheets are as follows:
| December 31, | September 30, | |||||
| 2008 | 2007 | 2009 | ||||
| US$ | US$ | US$ | ||||
| Total current assets |
392,431 | 770,598 | 268,407 | |||
| Total assets |
1,573,073 | 1,988,906 | 1,380,680 | |||
| Total current liabilities |
367,842 | 921,407 | 137,587 | |||
| Total liabilities |
367,842 | 921,407 | 137,587 | |||
The accounts of BTL are consolidated in the accompanying financial statements pursuant to Accounting Standards Codification (“ASC”) 810-10, “Consolidation”. As a VIE, BTL’s revenues are included in the Company’s service income, and its income from operations is consolidated with the Company’s. Because of the arrangements, the Company had a pecuniary interest in BTL that requires consolidation of the Company’s and BTL’s financial statements.
On January 1, 2009, the Company adopted the transition rules within ASC 810-10-65, regarding accounting and reporting standards for the non-controlling interest in a subsidiary.
Use of Estimates
The preparation of the consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting periods. Estimates are adjusted to reflect actual experience when necessary. Significant accounting estimates reflected in the Company’s consolidated financial statements include revenue recognition, allowance for doubtful accounts, useful lives of property and equipment and intangible assets. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash on hand and highly liquid investments which are unrestricted as to withdrawal or use, and which have maturities of three months or less when purchased. The Company maintains uninsured cash and cash equivalents with various financial institutions mainly in the PRC and Hong Kong.
Accounts Receivable
Accounts receivable are recorded at net realizable value. Accounts receivable terms typically are net 60-180 days from the end of the month in which the services were provided. The company generally does not require collateral or other security to support accounts receivable. An allowance, if required, is based on a combination of historical experience, aging analysis, and an evaluation of the collectibility of specific accounts. Receivables are considered past due after 3 years and written off.
F-8
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Advances to Suppliers and Advances from Customers
The Company, as is the common practice in the PRC, will often pay advance payments to suppliers for unassembled parts, or receive advance payments from customers. Advances to suppliers were $1,798,887, $675,108 and $1,300,742 as of December 31, 2008 and 2007 and September 30, 2009, respectively. Advances from customers were $230,394, $159,540 and $59,695 as of December 31, 2008 and 2007 and September 30, 2009, respectively.
Fair Value of Financial Instruments
The carrying amounts reported in the consolidated financial statements for current assets and current liabilities approximate fair value due to the short-term nature of these financial instruments.
In 2008, the Company adopted ASC 820-10, “Fair Value Measurements and Disclosures”, which establishes a single authoritative definition of fair value and a framework for measuring fair value and expands disclosure of fair value measurements for both financial and nonfinancial assets and liabilities. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flows) and the cost approach (cost to replace the service capacity of an asset or replacement cost). For purposes of ASC 820-10-15, nonfinancial assets and nonfinancial liabilities would include all assets and liabilities other than those meeting the definition of a financial asset or financial liability as defined in ASC 820-10-15-15-1A. Management elected the deferral option available for one year for nonfinancial assets and liabilities as permitted by ASC-820-10.
The Company decided not to elect the fair value option prescribed by Financial Accounting Standards Board (“FASB”) ASC-825-10 for its financial assets and liabilities.
Inventory
Inventory is stated at the lower of cost or market and consists of assembled and unassembled parts relating to medical devices. The Company reviews its inventory annually for possible obsolete goods and to determine if any reserves are necessary for potential obsolescence. At December 31, 2008 and 2007 and September 30, 2009, no reserve for obsolescence is considered necessary.
Property and Equipment
Property and equipment are recorded at cost less accumulated depreciation and amortization. Depreciation and amortization are calculated on a straight-line basis over the following estimated useful lives:
| Leasehold improvements |
Shorter of the useful lives or the lease term | |
| Building and land use rights |
20-40 years | |
| Machinery and equipment |
10-15 years | |
| Furniture and office equipment |
5 years | |
| Motor vehicles |
5 years |
F-9
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Intangible Assets
Intangible assets consist primarily of purchased technology rights with finite lives and are stated at cost less accumulated amortization. Amortization is calculated on a straight-line basis over the estimated useful lives of these assets of 6 to 10 years and recognized in cost of revenues.
The Company reviews intangible assets for impairment in accordance with the provisions of ASC 360-10, “Impairment or Disposal of Long-Lived Assets.”
There was no impairment of intangible assets for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008. Although management believes the assumptions used in testing for impairment are reasonable, changes in any one of the assumptions could produce a significantly different result.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may no longer be recoverable. When these events occur, the Company measures impairment by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to result from the use of the asset and eventual disposition. If the sum of the expected future cash flows is less than the carrying amount of the asset, an impairment loss, equal to the excess of the carrying amount over the fair market value of the asset, is recognized. Management has determined no impairment exists at the balance sheet dates.
Revenue Recognition
The Company recognizes revenues when all the followings conditions have been satisfied:
* Persuasive evidence of an arrangement exists;
* Delivery and/or installation has occurred (e.g., risks and rewards of ownership has passed);
* The sales price is fixed or determinable; and,
* Collectibility is reasonably assured.
All revenues are based on firm customer orders with fixed terms and conditions. Because the products are assembled to the customers’ specification, there is no right of return. The Company does not provide its customers with price protection or cash rebates. For products which include software, the software is an off-the-shelf package and an integral part of the products being delivered. The Company does not provide any significant post-sale customer support services and does not provide customers with upgrades. The software is incidental to the product as a whole. For products that do not require installation, revenues are recognized when the products are delivered. For products that require installation, revenues are recognized when the installation is completed.
For all service income, the Company recognizes the revenue upon the completion of the repairs when the equipment has been returned to and accepted by the customers.
In the PRC, value added tax (VAT) of 17% of the invoice amount is collected in respect of the sales of goods on behalf of tax authorities. The VAT collected is not revenue of the Company; instead, the amount is recorded as a liability on the balance sheet until such VAT is paid to the authorities.
Cost of Revenues
Cost of revenues primarily includes wages to assemble parts and the costs of unassembled parts, handling charges, and other expenses associated with the assembly and distribution of product.
F-10
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Service income and expense
Service income and expense represents activities related to repair and transportation service provided for the customers by BTL.
Foreign Currency Translation
The accounts of Dehaier, BDL, BTL and DHK are measured using the currency of the primary economic environment in which the entity operates (the “functional currency”). Dehaier’s functional currency is US dollars (“$”) while BDL and BTL maintain their accounts in Renminbi (“RMB”) and DHK maintains its currency in Hong Kong dollars (“HKD”). The accompanying consolidated financial statements are presented in US dollars. Foreign currency transactions are translated into US dollars using fixed exchange rates in effect at the time of the transaction. Generally foreign exchange gains and losses resulting from the settlement of such transactions are recognized in the consolidated statements of operations. The foreign currency accounts of DHK, BDL and BTL are translated in accordance with ASC 830-10, “Foreign Currency Matters”. Assets and liabilities are translated at current exchange rates quoted by the People’s Bank of China at the balance sheet dates and revenues and expenses are translated at average exchange rates in effect during the periods. Resulting translation adjustments are recorded as other comprehensive income (loss) and accumulated as a separate component of equity in non-controlling interest.
Warranty Costs
The Company provides for the estimated cost of product warranties at the time revenue is recognized. Warranty costs are included in general and administrative expenses. The Company’s warranty obligation is affected by product failure rates and material usage and service delivery costs incurred in correcting product failure. Should actual product failure rates, material usage or service delivery costs differ from the Company’s estimates, the Company may be required to revise its estimated product warranty liability. The term of the product warranty is generally twelve months. Provision for warranty costs was $158,065, $98,964 and $199,727 at December 31, 2008 and 2007 and September 30, 2009, respectively. Warranty expense for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008 was $150,382, $60,298, $83,588 and $72,801, respectively.
Research and Development Costs
Research and development costs relating to the development of new products and processes, including significant improvements and refinements to existing products, are expensed as incurred. Research and development costs were $87,660, $47,228, $50,671 and $36,989 for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008, respectively.
Shipping and Handling Expenses
Shipping and handling expenses of $68,135, $67,308, $49,591 and $36,711 for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008 were included in operating expenses in the consolidated statements of operations, respectively.
Advertising Costs
Advertising costs are expensed as incurred. Advertising costs were $52,677, $18,591, $42,758 and $51,130 for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008, respectively.
F-11
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Earnings Per Share
The Company follows the provisions of ASC 260-10, “Earnings Per Share”. Basic earnings per share is computed by dividing net income attributable to holders of common shares by the weighted average number of common shares outstanding during the years. Diluted earnings per share reflect the potential dilution that could occur if securities or other contracts to issue common shares were exercised or converted into common shares. Convertible preferred shares are included in the computation of diluted earnings per share on an “if converted” basis, when the impact is dilutive.
The following table sets forth computation of basic and diluted weighted average share information:
| Year Ended | Nine Months Ended | |||||||
| December 31, | September 30, | |||||||
| 2008 | 2007 | 2009 | 2008 | |||||
| Weighted average number of common shares outstanding |
1,891,930 | 1,891,930 | 1,891,930 | 1,891,930 | ||||
| Dilutive effect of convertible preferred shares |
1,108,070 | 559,694 | 1,108,070 | 1,108,070 | ||||
| Weighted average number of common shares outstanding, assuming dilution |
3,000,000 | 2,451,624 | 3,000,000 | 3,000,000 | ||||
Value Added Tax
The Company reports revenues net of PRC’s value added tax for all the periods presented in the consolidated statements of operations.
Stock-Based Compensation
The Company follows the provisions of ASC 718-10, “Share-Based Payment Arrangement.” On December 7, 2009, the Company’s Board of Directors and shareholders have approved the creation of a share incentive plan to be implemented after the Company has completed its public offering. This plan will authorize the issuance of up to 10% of the number of shares outstanding after the Company has completed its public offering. Pursuant to the anticipated plan, the Company may issue options to purchase its common shares to employees and directors of the Company and its affiliates. The Company will fair value share-based awards to be granted under the new plan. Accordingly, compensation will be measured on the grant date using appropriate valuation models.
F-12
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC 740-10, “Accounting for Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year; and, (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if, based on the weight of available positive and negative evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. As of December 31, 2008 and 2007, and as of September 30, 2009, the Company did not record any deferred tax assets or liabilities.
ASC 740-10 prescribes a recognition threshold and measurement attribute for the financial statement recognition of a tax position taken or expected to be taken on a tax return. Under ASC 740-10, a tax benefit from an uncertain tax position taken or expected to be taken may be recognized only if it is “more likely than not” that the position is sustainable upon examination, based on its technical merits. The tax benefit of a qualifying position under ASC 740-10 would equal the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all the relevant information. A liability (including interest and penalties, if applicable) is established in the financial statements to the extent a current benefit has been recognized on a tax return for matters that are considered contingent upon the outcome of an uncertain tax position. Related interest and penalties, if any, are included as components of income tax expense and income taxes payable. The Company is awaiting resolution of certain complex tax issues and has not yet filed its 2007 and 2008 Value Added Tax (“VAT”) tax returns for some of its customers. However, all the potential VAT liabilities on these VAT returns were accrued and included in the accompanying consolidated financial statements.
The implementation of ASC 740-10 resulted in no material liability for unrecognized tax benefits and no material change to the beginning retained earnings of the Company. As of December 31, 2008 and 2007, and September 30, 2009 the Company did not have a liability for any unrecognized tax benefits. The Company recognizes interest and penalties, if any, related to unrecognized tax benefits as income tax expense in the statement of operations. During the years ended December 31, 2008 and 2007, and the nine months ended September 30, 2009 and 2008, the Company did not incur any interest or penalties.
Income tax returns for the years prior to 2004 are no longer subject to examination by tax authorities.
Subsequent Events
The accompanying consolidated financial statements were approved by management and the board of directors and were issued on February 15, 2010. Management has evaluated subsequent events through this date.
F-13
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Recently Issued Accounting Standards
In June 2009, the FASB issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles – a Replacement of FASB Statement No. 162”. The objective of this statement is to replace SFAS No. 162 and to establish the FASB Accounting Standards Codification as the source of the authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative GAAP for SEC registrants. This statement shall be effective for financial statements issued for interim and annual periods ending after September 15, 2009. On the effective date of this statement, all then existing non-SEC accounting and reporting standards are superseded.
In June 2009, the FASB issued SFAS No. 167, an amendment to FASB Interpretation 46(R), “Consolidation of Variable Interest Entities.” The statement requires an entity to perform an analysis to determine whether the entity’s variable interest give it a controlling financial interest in a variable interest entity by rationalizing characteristics that would give it power to direct the activities of a variable interest entity and the obligation to absorb losses or the right to receive benefits from the entity that could potentially be significant to the variable interest entity. The statement is effective for years beginning after November 15, 2009 and is not expected to have a material effect on the Company’s consolidated financial statements.
In June 2009, the FASB issued SFAS No. 166, “Accounting for Transfers of Financial Assets”, an amendment to SFAS NO. 140, “Accounting for Transfers and Servicing of Financial Assets and Extinguishment of Liabilities.” The statement defines the term “participating interest” to establish specific conditions for reporting a transfer of financial assets as a sale and improves financial reporting by eliminating (a) the exception for qualifying special-purpose entities from consolidation guidance and (b) the exception that permitted sale accounting for certain mortgage securitizations when a transferor has not surrendered control over the transferred financial assets. The statement is effective for annual reports for years beginning after November 15, 2009 and is not expected to have a material effect on the Company’s consolidated financial statements.
F-14
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES-continued
Recently Issued Accounting Standards-continued
On October 7, 2009, the FASB issued Accounting Standards Update (ASU) No. 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements—a Consensus of the FASB Emerging Issues Task Force. The amendments to FASB ASC 605-25, Revenue Recognition: Multiple-Element Arrangements, permit vendors to account for products and services separately rather than as a combined unit. Any vendor who enters into multiple-deliverable arrangements with customers that are covered by Subtopic 605-25 will be affected, the FASB said. As a result of the changes, multiple-deliverable arrangements will be separated in more circumstances than under existing guidance. With the changes to Subtopic 605-25, the FASB is eliminating the residual method of allocation and instead requiring entities to allocate the arrangement consolidation at the inception of the arrangement to all deliverables using the relative selling price method. Vendors will be required to determine their best estimate of the selling price consistently with the method they use to determine the selling price when the good or service is sold separately. The changes in ASU No. 2009-13 will be effective for revenue arrangements that begin or are changed in fiscal years that start June 15, 2010, or later. Entities that adopt the changes before then will have to apply them to their results from the beginning of their fiscal years. The adoption of this accounting standard is not expected to have a material effect on the Company’s consolidated financial statements.
On August 26, 2009, the FASB issued Accounting Standard Update (ASU) 2009-05, Measuring Liabilities at Fair Value, to clarify how entities should estimate the fair value of liabilities under the FASB ASC Topic 820, Fair Value Measurements and Disclosures. The amendments in ASU 2009-05 reduce potential ambiguity in financial reporting when measuring the fair value of liabilities. Therefore, preparers, investors, and other users of financial statements will have a better understanding of how the fair value of liabilities was measured, helping to improve consistency in the application of Topic 820. The FASB issued ASU 2009-05 as a result of expressed concern that there may be a lack of observable market information to measure the fair value of a liability. For example, in the hypothetical transfer of an asset subject to a restriction there will be no observable data available to measure the liability because it is restricted from being transferred. This guidance is effective for the first reporting period (including interim periods) beginning after issuance. The adoption of this accounting standard is not expected to have a material effect on the Company’s consolidated financial statements.
F-15
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
3. OTHER RECEIVABLES
Other receivables consist of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 | ||||
| US$ | US$ | US$ | ||||
| Due from suppliers |
251,697 | 220,547 | 351,581 | |||
| Deposits for ongoing contracts |
874,260 | 215,694 | 1,029,114 | |||
| Staff advance |
61,403 | 102,645 | 80,940 | |||
| 1,187,360 | 538,886 | 1,461,635 | ||||
4. PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consist of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 | ||||
| US$ | US$ | US$ | ||||
| Advances to suppliers | 505,140 | 285,918 | 139,815 | |||
| Prepayment for equipment purchase | 1,293,747 | 389,190 | 1,160,927 | |||
| Prepaid expenses |
— | 50,965 | — | |||
| 1,798,887 | 726,073 | 1,300,742 | ||||
F-16
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
5. PROPERTY AND EQUIPMENT, NET
Property and equipment consist of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 |
|||||||
| US$ | US$ | US$ | |||||||
| Building |
1,227,641 | 1,144,019 | 1,215,473 | ||||||
| Land use rights |
284,940 | 266,500 | 284,780 | ||||||
| Machinery and equipment |
1,040,771 | 365,642 | 1,947,659 | ||||||
| Automobiles |
56,428 | 90,805 | 56,148 | ||||||
| Office and computer equipment |
267,125 | 201,228 | 277,396 | ||||||
| 2,876,905 | 2,068,194 | 3,781,456 | |||||||
| Less: Accumulated depreciation and amortization |
(680,778 | ) | (458,673 | ) | (853,864 | ) | |||
| Property and equipment, net |
2,196,127 | 1,609,521 | 2,927,592 | ||||||
At December 31, 2008 and 2007 and September 30, 2009, BTL’s building was pledged to a bank as collateral for short-term borrowings of RMB10,000,000 (US$1,465,740), RMB9,000,000((US$1,233,792) and RMB 10,000,000 (US$1,464,920), respectively (see Note 8).
Depreciation and amortization expense was $235,867, $147,801, $210,231 and $182,816, for the years ended December 31, 2008 and 2007, and for the nine months ended September 2009 and 2008, respectively.
Land Use Rights
There is no private ownership of land in China. Land is owned by the government and the government grants land use rights for specified terms. The Company’s land use rights are reported at the purchase price (RMB1,944,000 in 2002).
F-17
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
6. INTANGIBLE ASSETS, NET
Intangible assets consist of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 |
|||||||
| US$ | US$ | US$ | |||||||
| Technology rights |
182,623 | 182,623 | 182,623 | ||||||
| Less: accumulated amortization |
(146,098 |
) |
(109,574 |
) |
(173,492 |
) | |||
| Intangible assets, net |
36,525 | 73,049 | 9,131 | ||||||
Intangible assets represent technology rights relating to the anesthesia machines under the brand of Kontron. Amortization expense for each of the years ended December 31, 2008 and 2007 was $36,525, and for each of the nine months ended September 2009 and 2008 was $27,394.
7. TAX RECEIVABLE
Tax receivable consists of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 | ||||
| US$ | US$ | US$ | ||||
| Value added tax receivable |
552,968 | 354,071 | 1,163,479 | |||
Enterprises or individuals who sell commodities, engage in repair and maintenance or import and export goods in the PRC are subject to a value added tax in accordance with Chinese laws. The value added tax standard rate is 17% of the gross sales price. A credit is available whereby VAT paid on the purchases of unassembled medical components of the Company’s product used in contract and production can be used to offset the VAT due on sales of the product.
The tax receivable as of December 31, 2008 and 2007, and September 30, 2009 was $552,968, $354,071 and $1,163,479, respectively, which represents VAT credit on the purchased products. These amounts can be used to offset the VAT due on sales of the finished product.
8. SHORT-TERM BORROWINGS
The Company has a line of credit for RMB10,000,000 with a commercial bank in China to finance its working capital. The credit line bears interest at a variable rate and is renewed annually on May 18th. Average interest rates for the years ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 were 7.84%, 6.86% and 7.66%, respectively. Pursuant to the terms of the agreement, the line of credit is secured by BTL’s building (see note 5) and guaranteed by BDL and an officer of the Company.
On June 2, 2009, the bank renewed the Company’s credit line with payments due on November 20, 2009, March 3, 2010 and May 20, 2010 for RMB2,000,000, RMB3,000,000 and RMB5,000,000, respectively.
9. ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other payables consist of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 | ||||
| US$ | US$ | US$ | ||||
| Accrued salaries and social welfare |
153,952 | 113,972 | 172,325 | |||
| Accrued expenses |
19,054 | 17,822 | 19,044 | |||
| Other payables, non-trade vendors |
77,833 | 92,514 | 106,079 | |||
| 250,839 | 224,308 | 297,448 | ||||
F-18
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
10. TAXES PAYABLE
Taxes payable consists of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 | ||||
| US$ | US$ | US$ | ||||
| Value added tax |
2,542,227 | 1,699,153 | 3,555,625 | |||
| Enterprise income tax |
504,445 | 79,767 | 901,853 | |||
| Employee withholding taxes |
3,777 | 4,112 | 3,221 | |||
| Business tax |
1,174 | 487 | 673 | |||
| City construction tax |
514 | 1,488 | 409 | |||
| 3,052,137 | 1,785,007 | 4,461,781 | ||||
11. NON-CONTROLLING INTEREST
Non-controlling interest consists of the following:
| December 31, 2008 |
December 31, 2007 |
September 30, 2009 | ||||
| US$ | US$ | US$ | ||||
| Paid-in capital |
384,211 | 384,211 | 384,211 | |||
| Retained earnings |
642,940 | 580,609 | 681,442 | |||
| Accumulated other comprehensive income |
178,081 | 103,039 | 177,440 | |||
| 1,205,232 | 1,067,859 | 1,243,093 | ||||
F-19
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
12. COMMITMENTS AND CONTINGENCY
Leases
The lease commitments are for office premises and a warehouse facility, all of which are classified as operating leases. These non-cancelable leases have lease terms between one and two years. Future minimum lease payments under these leases at December 31, 2008, are as follows:
| Year Ending December 31, |
US$ | |
| 2009 |
18,328 | |
| 2010 |
1,527 | |
| Total minimum lease payments |
19,855 | |
Rent expense for the year ended December 31, 2008 and 2007, and for the nine months ended September 30, 2009 and 2008 was $ 32,011, $ 28,399, $ 36,174, and $ 25,507 respectively.
Rent expense paid to the spouse of the chief executive officer for the years ended December 31, 2008 and 2007 and for the nine months ended September 30, 2009 and 2008 was $ 0, $ 0, $ 17,418 and $ 0, respectively.
On December 15, 2009, BTL agreed to lease its building to BDL free of any charges through September 24, 2010.
Employment Contracts
Under the PRC labor law, all employees have signed employment contracts with the Company. Management employees have employment contracts with terms up to three years and non-management employees have a one year employment contract renewable on an annual basis.
Contingency
The Labor Contract Law of the People’s Republic of China, effective as of January 1, 2008, requires employers to assure the liability of the severance payments if employees are terminated and have been working for the employers for at least two years prior to January 1, 2008. The Company has estimated its possible severance payments of approximately $88,000 and $154,000 as of December 31, 2008 and September 30, 2009, respectively, which have not been reflected in its consolidated financial statements.
F-20
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
13. SHAREHOLDERS’ EQUITY
Common Shares
At December 31, 2008 and 2007, and September 30, 2009, the Company’s Memorandum and Articles of Association authorized share capital of US $50,000 was comprised of 4,500,000 common shares and 500,000 Preferred Shares (120,000 shares of Series A Preferred Shares and 380,000 shares of Series B Preferred Shares) of US$0.01 par value.
On October 31, 2009, the board of directors approved a 3.6614-for-1 stock split of the Company’s common shares. Accordingly, all common share and per share information in the accompanying consolidated financial statements give retroactive effect to the stock split. Prior to the stock split, there were 516,722 common shares issued and outstanding at September 30, 2009, December 31, 2008 and 2007. The par value of the common stock was $.01 per share prior to the stock split.
The founders of the Company agreed to place, on a pro rata basis, that number of common shares of the Company into escrow that is equal to 40% of the maximum number of shares to be sold in an initial public offering (“IPO”) until such time as the Company files its Form 10-K with the Securities and Exchange Commission for the year ending December 31, 2010, or the termination of the IPO without closing, if earlier. The shares in escrow (Make-Good Shares) will be accounted for as an element in the IPO and the Company will not recognize any compensation expense upon the return of such Make-Good Shares to the holders. To the extent the Company’s earnings per share for the year ending December 31, 2010 are less than $.80, the Company shall redeem, pro rata, such shares. These shares are included as part of the calculation of the basic and diluted earning per share for all the periods presented in the accompanying consolidated financial statements.
F-21
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
13. SHAREHOLDERS’ EQUITY- continued
Convertible Preferred Shares
In October 2003, the Company issued 120,000 Series A convertible preferred shares (“Series A Preferred Shares”) for total proceeds of $1,200,000.
During 2007, the Company issued in aggregate 182,635 Series B convertible preferred shares (“Series B Preferred Shares”) for total proceeds of $2,000,000.
Series A Preferred Shares and Series B Preferred Shares are collectively referred to as the “Preferred Shares”.
Preferred Shares have priority over the common stock “Ordinary Shares”. Certain rights, preferences and privileges of the Preferred Shares are listed below:
The holders of Preferred Shares are entitled to receive noncumulative dividends, when and if declared by the board of directors. Dividends are not mandatory and shall not accrue.
Preferred Shares Series A and B have a liquidation preference of $10 and $10.95074 per share, respectively.
The holders of Preferred Shares are entitled to one vote for each share of common stock the Preferred Shares could be converted to.
Preferred Shares are non-redeemable.
On October 31, 2009, each share of preferred stock was converted into common shares on a 1-to-1 basis. In addition, all preferred shares in authorized capital on October 31, 2009, were re-designated as common shares.
Following is a pro-forma earnings per share calculation for the year ended December 31, 2008 and the nine months ended September 30, 2009 giving effect to the conversion of all the outstanding preferred shares and the effect of the stock split:
| December 31, 2008 |
September 30, 2009 | |||
| US$ | US$ | |||
| Basic earnings per share |
0.31 | 0.69 |
F-22
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
14. INCOME TAXES
British Virgin Islands
Dehaier is a tax-exempt company incorporated in the British Virgin Islands. BDL and BTL were incorporated in the PRC and are governed by the PRC laws.
Hong Kong
DHK is subject to Hong Kong profits tax at a rate of 17.5% on its assessable profits. No Hong Kong profits tax has been provided as the Company did not have any taxable profit that was earned in or derived from Hong Kong during the years presented.
PRC
BDL is entitled to a preferential tax rate of 15% as a “high technology” company, and is entitled to a three-year exemption from income taxes commencing in 2004, followed by a 50% reduction in tax rates for the succeeding three years.
In May 2008, PRC issued new standards for “high technology” companies and required the Company to update their certification. Dehaier did not get the updated certification in 2008. On May 18, 2009, the State Tax of the Beijing Changping District issued “the Notice to Tax Affairs” (Tax Notice [2009]7013) to cancel “Approved to relief the Enterprise Income Tax” act. The tax rate for BDL is 25% in 2008.
PRC government grants a preferential income tax rate of 15% to government-certified high technology companies, and under the new standard the period of validity for the certification of high technology companies is three years. In 2009, BDL is engaged in updating its certification for “high technology” company and should get the government approval online at the end of November 2009 and certification later. Therefore, BDL used a 15% income tax rate to calculate the income tax expense for nine months ended September 30, 2009.
BTL is entitled to a preferential tax rate of 15% as a “high technology” company, and is entitled to a three-year exemption from income taxes commencing in 2002, followed by a 50% reduction in tax rates for the succeeding three years.
A reconciliation of income tax expense and the amount computed by applying the statutory income tax rate to the income before income tax provision is as follows:
| Year Ended December 31, |
Nine Months Ended September 30, | |||||||
| 2008 | 2007 | 2009 | 2008 | |||||
| US$ | US$ | US$ | US$ | |||||
| Tax computed at statutory rate |
415,332 | 73,648 | 410,162 | 270,300 | ||||
| Increase in income taxes resulting from temporary differences |
— | — | — | — | ||||
| Permanent differences |
— | — | — | — | ||||
| 415,332 | 73,648 | 410,162 | 270,300 | |||||
F-23
Table of Contents
DEHAIER MEDICAL SYSTEMS LIMITED AND AFFILIATE
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—Continued
15. RELATED PARTY TRANSACTIONS
At December 31, 2007, amounts due from shareholders and an officer were $9,280 and $27,418, respectively, for temporary advances. These amounts were repaid in 2008.
At December 31 2008, the amount due to an officer was $969 representing expenses paid by an officer of the Company. This amount was repaid in the first six months of 2009.
At September 30, 2009, the amount due to an officer was $2,947 representing expenses paid by an officer of the Company.
16. CONCENTRATIONS
Major Customers
For the years ended December 31, 2008 and 2007, and for the nine months ended September 2009 and 2008, approximately 11%, 17%, 8% and 7%, respectively, of the Company’s revenues were to one customer. At December 31, 2008 and 2007, receivables from four customers were approximately 17%, 13%, 11%, 10% and 20%, 12%, 11%, 10%, respectively. At September 2009 and 2008, receivables from four customers were approximately 9%, 9%, 8%, 7% and 16%, 14%, 9%, 8%, respectively.
Revenues
For the years ended December 31, 2008 and 2007, and for the nine months ended September 2009 and 2008, the Company’s three top selling products accounted, in the aggregate, for approximately 73%, 65%, 61% and 57%, respectively, of its total net revenues.
The following represents the revenues by products, all derived from China:
| For the years ended December 31, |
For the nine months ended September 30, | |||||||
| Products |
2008 | 2007 | 2009 | 2008 | ||||
| US$ | US$ | US$ | US$ | |||||
| Medical Devices |
8,814,682 | 6,281,865 | 7,292,406 | 6,057,131 | ||||
| Technical Service |
484,799 | 264,414 | 1,231,993 | 381,093 | ||||
| Respiratory and Oxygen Homecare |
114,949 | 53,233 | 898,061 | 93,630 | ||||
| 9,414,430 | 6,599,512 | 9,422,460 | 6,531,854 | |||||
F-24
Table of Contents

Table of Contents
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution. |
The estimated expenses payable by us in connection with the offering described in this registration statement (other than the placement discounts and commissions) will be as follows. With the exception of the filing fees for the U.S. Securities Exchange Commission, FINRA and NASDAQ, all amounts are estimates.
| U.S. Securities Exchange Commission registration fee |
$ | 845 | |
| FINRA filing fee |
$ | 2,013 | |
| NASDAQ listing fee |
$ | 50,000 | |
| Legal fees and expenses for Chinese counsel |
$ | 95,000 | |
| Legal fees and expenses for British Virgin Islands counsel |
$ | 12,000 | |
| Legal fees and expenses for U.S. counsel |
$ | 160,000 | |
| Accounting fees and expenses |
$ | 150,000 | |
| Printing fees |
$ | 25,000 | |
| Miscellaneous |
$ | 5,142 | |
| Total |
$ | 500,000 |
All fees and expenses other than the fees for the Securities and Exchange Commission, FINRA and NASDAQ are estimated.
| Item 14. | Indemnification of Directors and Officers |
British Virgin Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Under the memorandum and articles of association of CHJA, or the Registrant, the Registrant may indemnify its directors, officers and liquidators against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with civil, criminal, administrative or investigative proceedings to which they are party or are threatened to be made a party by reason of their acting as our director, officer or liquidator. To be entitled to indemnification, these persons must have acted honestly and in good faith with a view to the best interest of the Registrant and, in the case of criminal proceedings, they must have had no reasonable cause to believe their conduct was unlawful.
The Placement Agreement, the form of which is filed as Exhibit 1.1 to this registration statement, will also provide for indemnification of the Registrant and its officers and directors.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
| Item 15. | Recent Sales of Unregistered Securities |
2007 Series B Convertible Preferred Share Issuance.
Prior to the reorganization of our company, effective as of January 5, 2007, we issued, in the aggregate, 182,635 Series B convertible preferred shares for total proceeds of $2,000,000 to Crystal East Group Limited, a British Virgin Islands company. This issuance was deemed to be exempt under the Securities Act by virtue of Section 4(2) thereof as a transaction not involving any public offering. In addition, this issuance was deemed not to fall within Section 5 under the Securities Act and to be further exempt under Rule 901 and 903(b)(1) of Regulation S by virtue of being offers and sales of securities by non-U.S. companies to non-U.S. citizens or residents, conducted outside the United States and not using any element of interstate commerce. These Series B convertible preferred shares were converted into the same number of common shares prior to the reorganization described below in this Item 15. Crystal East Group Limited subsequently completed the transfer of its shares to its individual shareholder, Yijen Chen.
II-1
Table of Contents
2009 Reorganization of Dehaier.
We issued 3,000,000 shares in the aggregate to nine (9) shareholders upon the reorganization of our company as of October 31, 2009. These issuances were not required to be registered under the Securities Act of 1933. The nine shareholders are as follows: Chen Ping Limited; De-haier Investment Holdings Limited; Broadview-Richfield Holdings, LLC; RMCC Investments, LLC; Yijen Chen; Weibing Yang; Jian Sun; Yong Wang; and Zheng (Rita) Liu.
These issuances consisted of (i) the conversion of previously issued Series A preferred shares held by De-haier Investment Holdings Limited into common shares at a rate of 1 Series A preferred share to 1 common share; (ii) the conversion of previously issued Series B preferred shares held by Crystal East Group Limited into common shares at a rate of 1 Series A preferred share to 1 common share and transfer of such shares to Crystal East Group Limited’s shareholder, Yijen Chen; and, finally, (iii) the subdivision of all common shares, including the converted Series A and Series B preferred shares into an aggregate of 3,000,000 common shares, a rate of 3.66140766-for-1. Of these 3,000,000 shares, up to 600,000, assuming a maximum offering, will be held in escrow pursuant to the Make-Good Escrow described in the section of the prospectus titled “Related Party Transactions - Make-Good Shares Subject to Redemption.”
All issuances of common shares to these shareholders were deemed to be exempt under the Securities Act by virtue of Section 4(2) thereof as a transaction not involving any public offering. In addition, the issuance of shares to Chen Ping Limited, Weibing Yang, Jian Sun, Yong Wang, Zheng (Rita) Liu, De-haier Investment Holding Ltd. and Yijen Chen were deemed not to fall within Section 5 under the Securities Act and to be further exempt under Rule 901 and 903(b)(1) of Regulation S by virtue of being offers and sales of securities by non-U.S. companies to non-U.S. citizens or residents, conducted outside the United States and not using any element of interstate commerce.
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits
The following exhibits are filed herewith or incorporated by reference in this prospectus:
| Exhibit Number |
Document | |
| 1.1 | Form of Placement Agreement(1) | |
| 3(i).1 | Articles of Association of the Registrant(1) | |
| 3(ii).1 | Memorandum of Association of the Registrant(1) | |
| 4.1 | Specimen Share Certificate(1) | |
| 4.2 | Form of Placement Agent’s Warrant (included in Ex. 10.1)(1) | |
| 5.1 | Form of Opinion of Kaufman & Canoles, P.C., Virginia counsel(2) | |
| 5.2 | Form of Opinion of Kaufman & Canoles, P.C., British Virgin Islands counsel(2) | |
| 8.1 | Opinion of Kaufman & Canoles, P.C., Virginia counsel, as to certain tax matters(2) | |
| 8.2 | Opinion of Kaufman & Canoles, P.C., British Virgin Islands counsel, as to certain tax matters(2) | |
| 8.3 | Opinion of Beijing Kang Da Law Firm, Chinese counsel, as to certain tax matters(2) | |
| 10.1 | Form of Placement Agent’s Warrant Agreement(1) | |
| 10.2 | Translation of Form of Registrant’s Executive Officer Employment Agreement(1) | |
| 10.3 | Form of Lock-Up Agreement(1) | |
| 10.4 | Form of Share Option Plan(1) | |
| 10.5 | Form of Make Good Escrow Agreement(1) | |
| 10.6 | Translation of lease agreement for Product Center dated September 23, 2008(1) | |
| 10.7 | Translation of lease agreement for Principal Executive Office dated December 21, 2009, effective January 1, 2010(1) | |
| 10.8 | Distribution agreement with IMD(1) | |
II-2
Table of Contents
| 10.9 | Distribution agreement with Timesco(1) | |
| 10.10 | Translation of distribution agreement with JMS(1) | |
| 10.11 | Distribution agreement with ResMed(1) | |
| 10.12 | Translation of form of independent distributor agreement(1) | |
| 10.13 | Translation of letter of credit agreement with ICBC(1) | |
| 10.14 | Form of Initial Public Offering Escrow Agreement between Dehaier, Placement Agent and SunTrust Bank(1) | |
| 10.15 | Translation of Executive Officer Employment Agreement for Ping Chen(1) | |
| 10.16 | Translation of Executive Officer Employment Agreement for Weibing Yang(1) | |
| 10.17 | Translation of Executive Officer Employment Agreement for Zheng Liu(1) | |
| 10.18 | Translation of Executive Officer Employment Agreement for Yong Wang(1) | |
| 10.19 | Translation of Form of Purchase Agreement with Poverty Aid Office(1) | |
| 10.20 | Translation of Production Agreement with Friend of Health (Chuzhou) Medical Technology Co., Ltd.(1) | |
| 10.21 | Translation of Guarantee Contract between Ping Chen and ICBC(2) | |
| 10.22 | Mortgage Contract between ICBC and BTL(2) | |
| 10.23 | Indemnification and Guarantee Contract between Ping Chen and BTL(2) | |
| 10.24 | Description of oral loan contract between BTL and BDL(2) | |
| 21.1 | Subsidiaries of the Registrant(1) | |
| 23.1 | Consent of Friedman LLP(2) | |
| 23.2 | Form of Consent of Kaufman & Canoles (included in Exhibit 5.1)(2) | |
| 23.3 | Form of Consent of Kaufman & Canoles (included in Exhibit 5.2)(2) | |
| 24.1 | Power of Attorney (included on page II-5 of the Registration Statement)(1) | |
| 99.1 | Code of Business Conduct and Ethics(1) | |
| 99.2 | Opinion of Beijing Kang Da Law Firm(1) | |
| (1) | Previously filed. |
| (2) | Filed herewith. |
| (3) | To be filed by amendment. |
(b) Financial Statement Schedules
None.
| Item 17. | Undertakings |
The Registrant hereby undertakes:
| (a) | to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to: |
| (i) | include any prospectus required by section 10(a)(3) of the Securities Act; |
| (ii) | reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (b) | that, for the purpose of determining any liability under the Securities Act, each post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (c) | to file a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering. |
| (d) | to file a post-effective amendment to include any financial statements required by Form 10-K at the start of any delayed offering or throughout a continuous offering. |
II-3
Table of Contents
| (e) | that insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant, the Registrant has been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registration of expenses incurred or paid by a director, officer or controlling person to the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| (f) | that, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (g) | that, for the purpose of determining liability of the Registrant under the Securities Act to any purchaser in the initial distribution of the securities, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | any preliminary prospectus or prospectus of the Registrant relating to the offering filed pursuant to Rule 424; |
| (ii) | any free writing prospectus relating to the offering prepared by or on behalf of the Registrant or used or referred to by the Registrant; |
| (iii) | the portion of any other free writing prospectus relating to the offering containing material information about the Registrant or its securities provided by or on behalf of the Registrant; and |
| (iv) | any other communication that is an offer in the offering made by the Registrant to the purchaser. |
| (h) | to provide to the Placement Agent at the closing specified in the placement agent agreements, certificates in such denominations and registered in such names as required by the Placement Agent to permit prompt delivery to each purchaser. |
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the People’s Republic of China, on February 16, 2010.
| Dehaier Medical Systems Limited, | ||
| By: |
/S/ PING CHEN | |
| Name: | Ping Chen | |
| Title: | Chief Executive Officer (Principal Executive Officer) | |
| Date: | February 16, 2010 | |
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| Signature |
Title |
Date | ||
| /S/ PING CHEN |
Chief Executive Officer and Director | February 16, 2010 | ||
| Ping Chen | (Principal Executive Officer) | |||
| /S/ ZHENG (RITA) LIU |
Chief Financial Officer (Principal Accounting and | February 16, 2010 | ||
| Zheng (Rita) Liu | Financial Officer) and Director | |||
| * |
Director (Authorized Representative in the United States) | February 16, 2010 | ||
| Yunxiang (Phil) Fan | ||||
|
|
Director | |||
| Jimin (Peter) Zhuo | ||||
|
|
Director | |||
| Bin Qiu | ||||
| * By: | /S/ PING CHEN | |
| Ping Chen | ||
| Attorney-in-Fact | ||
| February 16, 2010 |
II-5

