Attached files
| file | filename |
|---|---|
| EX-31.1 - CardioGenics Holdings Inc. | v173825_ex31-1.htm |
| EX-31.2 - CardioGenics Holdings Inc. | v173825_ex31-2.htm |
| EX-23.2 - CardioGenics Holdings Inc. | v173825_ex23-2.htm |
| EX-21.1 - CardioGenics Holdings Inc. | v173825_ex21-1.htm |
| EX-23.1 - CardioGenics Holdings Inc. | v173825_ex23-1.htm |
| EX-10.29 - CardioGenics Holdings Inc. | v173825_ex10-29.htm |
| EX-10.28 - CardioGenics Holdings Inc. | v173825_ex10-28.htm |
| EX-10.30 - CardioGenics Holdings Inc. | v173825_ex10-30.htm |
| EX-10.31 - CardioGenics Holdings Inc. | v173825_ex10-31.htm |
| EX-32.1 - CardioGenics Holdings Inc. | v173825_ex32-1.htm |
UNITED STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
|
|
þ
|
Annual report pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934 for the fiscal
year ended October 31, 2009
|
OR
|
|
¨
|
Transition report pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934 for the
transition period from __________ to
__________.
|
Commission
file number: 000-28761
CARDIOGENICS
HOLDINGS INC.
(Exact
name of registrant as specified in its charter)
|
Nevada
|
88-0380546
|
|
(State
or other jurisdiction of
|
(I.R.S.
Employer
|
|
incorporation
or organization)
|
Identification
Number)
|
|
6295
Northam Drive, Unit 8, Mississauga, Ontario L4V 1W8
(Address
of principal executive offices) (Zip code)
(905)
673-8501
(Registrant’s
telephone number, including area code)
Securities
registered pursuant to Section 12(b) of the
Act: None
|
|
Securities
registered pursuant to Section 12(g) of the Act:
Common
Stock—$0.00001 par value
Series
2 Class B Common Stock—$0.00001 par value
Series
3 Class B Common Stock—$0.00001 par
value
|
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes ¨ No þ
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes ¨ No þ.
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes þ No ¨.
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes ¨ No þ.
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. þ
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, non-accelerated filer or a small. See definition
of “large accelerated filer, accelerated filer and smaller reporting company” in
Rule 12b-2 of the Exchange Act. (Check one):
|
Large
accelerated filer ¨
|
Accelerated
filer ¨
|
Non-accelerated
filer ¨ (Do
not check if smaller reporting company)
|
Smaller
reporting company
þ
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Act). Yes ¨ No þ.
The
aggregate market value of the registrant’s voting and non-voting common stock
held by non-affiliates on February 10, 2010 (based on the closing stock price on
the OTC Bulletin Board) on such date was approximately $32,268,221.
As of
February 10, 2010 the Registrant had the following number of shares of its
capital stock outstanding: 218,006,202 shares of Common Stock, 1 share of Series
1 Preferred Voting Stock, par value $0.0001, representing 16 exchangeable shares
of the Registrant’s subsidiary, CardioGenics ExchangeCo Inc., which are
exchangeable into 276,655,415 shares of the Registrant’s Common Stock, 380,931
shares of Series 2 Class B Common Stock and 21,500 shares of Series 3 Class B
Common Stock.
CARDIOGENICS
HOLDINGS INC.
ANNUAL
REPORT ON FORM 10-K
FOR
THE FISCAL YEAR ENDED OCTOBER 31, 2009
TABLE
OF CONTENTS
|
Page
|
|
|
Part
I
|
1
|
|
Item
1. Business
|
1
|
|
Item
1A. Risk Factors
|
15
|
|
Item
1B. Unresolved Staff Comments
|
26
|
|
Item
2. Properties
|
26
|
|
Item
3. Legal Proceedings
|
27
|
|
Item
4. Submission of Matters to a Vote of Security Holders
|
27
|
|
Part
II
|
28
|
|
Item
5. Market for Registrant’s Common Equity, Related Stockholder Matters and
Issuer Purchases of Securities
|
28
|
|
Item
6. Selected Financial Data
|
30
|
|
Item
7. Management’s Discussion and Analysis or Plan of
Operation
|
30
|
|
Item7A.
Quantitive and Qualitative Disclosure about Market Risk
|
36
|
|
Item
8. Financial Statements and Supplementary Data
|
36
|
|
Item
9. Changes in and Disagreements with Accountants on Accounting and
Financial Disclosure
|
37
|
|
Item
9A(T). Controls and Procedures
|
37
|
|
Item
9B. Other Information
|
38
|
|
Part
III
|
38
|
|
Item
10. Directors, Executive Officers and Corporate Governance
|
38
|
|
Item
11. Executive Compensation
|
40
|
|
Item
12. Security Ownership of Certain Beneficial Owners and Related
Stockholder Matters
|
43
|
|
Item
13. Certain Relationships and Related Transactions, and Director
Independence
|
44
|
|
Item
14. Principal Accounting Fees and Services
|
45
|
|
Part
IV
|
46
|
|
Item
15. Exhibits, Financial Statement Schedules
|
46
|
i
PART
I
ITEM
1. BUSINESS
This
Annual Report on Form 10-K contains forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section
21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Such
statements are based upon current expectations that involve risks and
uncertainties. Any statements contained herein that are not statements of
historical fact may be deemed to be forward-looking statements. Words such as
“may,” “will,” “should,” “estimates,” “predicts,” “potential,” “continue,”
“strategy,” “believes,” “anticipates,” “plans,” “expects,” “intends” and similar
expressions are intended to identify forward-looking statements. Our discussions
relating to our liquidity and capital resources, our business strategy, our
competition, and the future of our market segment, our acquisition of
CardioGenics Inc., an Ontario Canada corporation (“CardioGenics”), among
others, contain such statements. Our actual results and the timing of certain
events may differ significantly from the results discussed in the
forward-looking statements.
Our
forward-looking statements in this Annual Report on Form 10-K are based on
management’s current views and assumptions regarding future events and speak
only as of their dates. We undertake no obligation to publicly update or revise
any forward-looking statements, whether as a result of new information, future
events or otherwise, except as required by the federal securities laws. Unless
the context requires otherwise, the terms “we,” “us” and “our” refer to
CardioGenics Holdings Inc., our predecessors and subsidiaries. Our acquisition
of CardioGenics as discussed in this Annual Report on Form 10-K is sometimes
referred to as the “CardioGenics Acquisition.”
OVERVIEW
Prior to
the CardioGenics Acquisition, our primary business was providing financial and
investment information to the investment community which we have been doing
since 1989. In May 1999, we began offering our services on a subscription fee
basis to the general public for the first time through our website at
jagnotes.com. Through our website and our traditional fax-based service, we
offer timely financial data, reports and commentary.
Our
online services currently consist of a subscription-based service that offers
two specific products, the JAGNotes (Upgrade/Downgrade) Report and the Rumor
Room, providing timely market reports, including breaking news and potentially
market moving information. We currently derive revenues primarily from the sale
of subscriptions.
In July
2009, we consummated the CardioGenics Acquisition and the main focus of our
business switched from offering our customers fee-based financial information to
the development of products targeting the immunoassay segment of the
point-of-care In Vitro Diagnostic (“IVD”) testing market. See “—Our
Strategy—Acquisition of CardioGenics.” In order to better reflect the new focus
of our business, we changed our name to CardioGenics Holdings Inc. in October
2009.
We are a
Nevada corporation. Our address is 6295 Northam Drive, Unit 8, Mississauga,
Ontario, Canada L4V 1W8, and our telephone number is 905-673-8501.
COMPANY
BACKGROUND
JagNotes,
Inc.
We have
been providing financial information to the investment community since 1989. In
May 1999, we began offering our services on a subscription fee basis to the
general public for the first time through our website at jagnotes.com. Through
our website and our traditional fax-based service, we offer timely financial
data, reports and commentary.
Our
online services currently consist of a subscription-based service that offers
two specific products, the JAGNotes (Upgrade/Downgrade) Report and the Rumor
Room, providing timely market reports, including breaking news and potentially
market moving information. We currently derive revenues primarily from the sale
of subscriptions.
From 1989
to 1992, we operated as an unincorporated business entity. In 1992, we
incorporated in the State of New Jersey as New Jag, Inc. On December 14, 1993,
JagNotes, Inc. merged with and into New Jag Inc., and we changed our name to
JagNotes, Inc. We operated as JagNotes, Inc. until March 1999 when we were
acquired by Professional Perceptions, Inc., a Nevada corporation, which
subsequently changed our name to JagNotes.com Inc.
Until
1999, we targeted only a limited audience of financial professionals and did not
engage in organized sales and marketing efforts. In 1999, we decided to change
focus by expanding onto the Internet and targeting retail subscribers with the
hope of expanding our subscriber base and business.
1
We
undertook a corporate reorganization in January 2002 in order to distinguish and
better manage separate areas of business. On January 4, 2002, we formed JAG
Media LLC, a Delaware limited liability company and wholly-owned subsidiary. The
assets and liabilities of our current fax and Internet subscription business
were transferred to JAG Media LLC. In order to better reflect the overall
business in which we expected to engage and the corporate structure we intended
to use to conduct that business, we changed our name from JagNotes.com Inc. to
JAG Media Holdings, Inc. effective April 8, 2002.
On
November 24, 2004, through one of our subsidiaries, Pixaya (UK) Limited (“Pixaya”), we purchased certain
development stage software products and related assets in the United Kingdom
from TComm Limited, a company organized in the United Kingdom. We subsequently
changed the name of our subsidiary, JAG Media LLC, to Pixaya LLC in order to
better reflect its role as owner of Pixaya and primary provider of support for
our Pixaya products in the United States. Due to cash constraints, we ceased
financing development and marketing by Pixaya of our SurvayaCam product, a
mobile surveillance system which streams live video in real time from the point
of use back to a control center and, if desired, to other locations. To date, we
have only made minimal sales of SurvayaCam as part of our prior marketing and
distribution efforts.
In light
of the difficulties we encountered in growing our JAG Notes subscription
business and Pixaya business, we began seeking merger and acquisition
candidates, in related and unrelated lines of businesses, to augment our current
business. On July 31, 2009, we completed the acquisition of CardioGenics, a
developer of products targeting the immunoassay segment of the Point-of-Care IVD
testing market, based in Ontario, Canada. See “—Our Acquisition of
CardioGenics.”
CardioGenics
Inc.
CardioGenics
was founded in Toronto, Canada in 1997 by Dr. Yahia Gawad to develop technology
and products targeting the immunoassay segment of the IVD testing market. These
include:
|
|
§
|
The
QL Care Analyzer (the “QLCA”), a
state-of-the-art proprietary Point-of-Care (“POC”) immunoassay
analyzer;
|
|
|
§
|
A
series of immunoassay tests to detect cardiac markers (the “Cardiovascular Tests”);
and,
|
|
|
§
|
Paramagnetic
beads developed through our proprietary method, which improves their light
collection (the “Beads”).
|
OUR
INDUSTRY
CardioGenics IVD POC Testing
Markets
IVD
Market
In
vitro diagnostics (IVD) refers to testing that aims for the identification of
disease states outside the body, using samples such as body fluids (blood,
urine) and tissues (biopsies and tissue sections). The IVD is a well established
market, offering essential products (tests, components and machinery) used by
physicians and clinical chemistry personnel to assess disease conditions. The
world market for IVD is estimated at $42 billion in 2007 and is expected to grow
6% annually to $56.3 billion by 20121. North America, Europe,
Japan and Western Europe currently make up 81% of the total IVD market, and this
is expected to decrease to 76% by 2012 as China and India become more
significant players in the IVD market. Sales of IVD products in emerging
economies in Latin America and Eastern Europe are expected to grow from 4% of
the market in 2007 to 5% in 2012. Overall, sales growth of IVD products in
emerging markets will account for 10-20% annual growth in the IVD market, while
the developed world will see annual growth of 3-6%.2
1 This
includes all laboratory, hospital-based products and OTC products, according to
Kalorama Information, The Worldwide Market for In Vitro Diagnostics Tests,
6th
Edition, June 2008
2 Kalorama
Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th
Edition, June 2008, p3
2
The
following table summarizes the market size and projections of the IVD market and
the sub-sectors where our products will compete:
|
Product
|
2007
|
2008
|
2009
|
2010
|
2011
|
|||||||||||||||
|
IVD (billions)
|
42.1 | 44.5 | 47.1 | 49.1 | 52.9 | |||||||||||||||
|
Immunoassay
Testing (billions)
|
4.185 | 4.435 | 4.695 | 4.975 | 5.260 | |||||||||||||||
|
POC
Testing (billions)
|
1.625 | 1.715 | 1.815 | 1.910 | 2.02 | |||||||||||||||
|
Cardiac
Marker Tests (millions)
|
425 | 471.75 | 523.64 | 581.24 | 645.17 | |||||||||||||||
In 2007,
16 top tier IVD companies occupied 78% of the global market ($32
billion). Since 2005, there has been a trend toward consolidation at
all levels of the IVD market. In 2007, three top tier companies, DPC,
Dade Behring and Bayer Diagnostics, merged to become Siemens Medical
Diagnostics.
Immunoassay
Market
The 2007
world market for all immunoassays, excluding infectious diseases, is estimated
at $4,185 million3,
and by 2012 the market is projected to grow by 6% annually to reach $5,605
million worldwide. Immunoassays sales for cardiac markers were 785 million in
2007, or 12% of market, and this is expected to increase to 1,050 million (12%)
by 20124. The
following table illustrates the relationships between the top IVD companies and
sales of IVD products.
Revenue History of Leading
Immunoassay Vendors, $ million 2005-20075
|
2007
|
2006
|
2005
|
||||||||||
|
Abbott
Diagnostics
|
2,100 | 1,900 | 1,800 | |||||||||
|
Siemans/Dade
Behring
|
825 | 785 | 750 | |||||||||
|
Siemens/Bayer
|
750 | 714 | 680 | |||||||||
|
Beckman
Coulter
|
596 | 484 | 402 | |||||||||
|
Siemens/DPC
|
595 | 517 | 473 | |||||||||
|
Roche
|
575 | 509 | 450 | |||||||||
|
bioMérieux
|
363 | 362 | 353 | |||||||||
|
Fujirebio
|
299 | 277 | 279 | |||||||||
|
Ortho
|
200 | 190 | 160 | |||||||||
|
TOTAL
|
6,303 | 5,738 | 5,347 | |||||||||
Immunoassay
testing segment of the IVD market is characterized by:
|
·
|
Expanding
opportunities after completion of the human genome
project.
|
|
·
|
Demand
for automated and sensitive POC immunoassay
analyzers.
|
|
·
|
Search
for an ideal POC platform.
|
4 Kalorama
Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th
Edition, June 2008, p401
5
Estimated. Kalorama Information, The Worldwide Market for In Vitro Diagnostics
Tests, 6th
Edition, June 2008, p402
3
|
·
|
Increased
mergers and acquisition among top tier IVD companies to achieve more
complete product lines
|
|
·
|
Greater
cooperation between test developers and top tier IVD
companies.
|
Over the
next 5-10 years, the immunoassay business will see:
|
|
·
|
The
continued automation of routine immunoassays – thyroid,
anemia, fertility, therapeutic drug monitoring and drugs of
abuse; and
|
|
|
·
|
More
new assays and test categories for disease risk evaluation.6
|
Point-Of-Care
(POC) Testing Market
Point Of
Care (POC) testing refers to a laboratory assay that can be performed outside of
a centralized facility, with results available within minutes. POC testing is
divided into personal use tests, such as pregnancy tests, and professional use
tests, that are administered in a physician‘s office or hospital emergency ward.
Our tests will compete in the professional use testing market
sector.
The
market for professional7 POC immunoassays is
estimated at $1,625 million in 2007 and with the 14% projected growth, this
market will reach $2,770 million in 2012. It is anticipated that most of the
growth will come from increased use of cardiac markers and new assays for cancer
markers and diabetes/cardiac disease markers. The market for professional POC
tests for cardiac markers is estimated at $425 million in 2007 (11%) and this is
expected to increase to $850 million (15%) by 2012.7
There is
a wide perception that POC tests are more expensive than lab-based tests and
that patient test results are lost to the historical record. There is also the
perception that once the patient leaves the acute care area, the baseline POC
tests done in that unit are of little value because the POC testing results do
not correlate with lab-based systems.
Two
critical characteristics are necessary for potential POC test products to become
more prevalent; POC testing results must correlate with lab results and the POC
devices must be more consistent and robust in delivering those
results.
The
impact of POC testing on improving patients’ care is clear and has been well
documented. Further, the impact of POC testing on saving healthcare resources
was also demonstrated by numerous agencies and institutions.
Cardiovascular
Disease Testing Market
Cardiac
markers are proteins released from heart muscle when it is damaged as a result
of a heart attack (myocardial infarction), when the blood supply to part of the
heart is interrupted. Physicians use cardiac markers in two ways – to diagnose a
cardiac event in a hospital emergency room or within the hospital or to evaluate
a risk of a cardiovascular event occurring. The routine markers of myocardial
infarction – CK-MB, troponin and myoglobin and recently BNP are used in the
acute care and tests such as cholesterol are used to evaluate risk.
The world
market for cardiac markers is estimated at $740 million in 2007, and with
projected annual growth of 5%, will reach $1,050 million in 2012.
Until
recently, Troponin and CK-MB were the lead cardiac markers. Brain Natriuetic
Pepetide (BNP) was recently introduced to differentiate between a myocardial
infarction and heart failure. A number of companies are focused on developing
new cardiac markers.
4
Magnetic
Particles Market
Magnetic
particles, or beads, are widely used as the solid phase for binding tests for
automating and simplifying the methods for isolation and detection of
biomolecules in both research and routine clinical laboratories. Eight of the
top 10 IVD companies employ magnetic particles in their fully automated
analyzers.
An
independent 2006 market research report, prepared for CardioGenics by Adventus
Research Inc. (the “Adventus Report”) and sponsored by the National Research
Council of Canada (NRC), estimated the market for magnetic beads for
immunoassays and molecular diagnostics to be approximately $900 million (between
$833 million and $1.3 billion). The report of market size did not include
magnetic beads produced in-house by some of the IVD test manufacturers or beads
produced for research applications. The Adventus Report was conducted using
several methods, including interviews with leading particle-manufacturers and
the end-users, published industry reports and data from leading IVD
manufacturers.
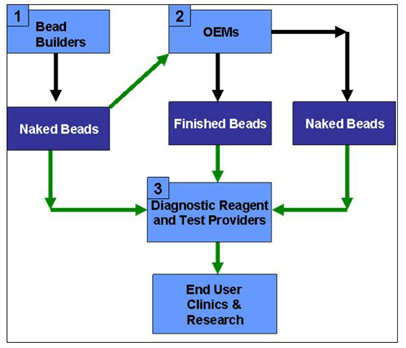
As stated
in the Adventus Report, according to Dynal, a leading magnetic beads
manufacturer, the largest part of its Molecular Systems’ business is OEM sales
of magnetic beads to IVD companies. Dynal stated that “the IVD market is very
large, and still growing. However, the magnetic bead-based part of this market
is growing at an even higher rate per year”.8 According to Dynal,
immunoassays make up more than USD 4 billion of the IVD market, and magnetic
beads are now the gold standard for immunoassay testing, as opposed to older
technologies such as microtitre plate based tests. Nucleic acid testing makes up
a smaller portion of the IVD market, USD 2 billion, but is fast growing.
Magnetic beads are also the most common solid phase employed in this
market.
Furthermore,
according to Dynal, as stated in the Adventus Report, end-user business rather
than OEM business (referred to as functionalized and naked beads markets
respectively) goes to research and routine laboratories within Genomics,
Expression Profiling and Proteomics. The market size for Genomics, including DNA
and RNA extraction and purification products was USD 300 million in 2001 while
the market size of Pharmacogenomics was estimated to be USD 2.3 billion in
2001.
5
As stated
in the Adventus Report, according to Gen-Probe, which is a leading DNA clinical
testing company, other markets that are employing magnetic beads as a solid
phase are growing also. Further, magnetic particles are used for Separation of
Microorganisms in Food and Water Testing and also for HLA testing for organ
transplantation.
Source:
Gen-Probe presentation- May 2006
JAG Notes & Pixaya
Markets
Internet-Based
Financial Information Market
The
growth of the Internet has changed the way investors seek information and manage
their portfolios. Individual investors are increasingly seeking
access to information that was formerly available only to financial
professionals. Professional investors who have traditionally relied
on print and other media for information are demanding faster information and
greater accessibility.
According
to tracking surveys conducted by Pew Internet & American Life Project as of
September 2007, approximately 36% use the Internet to obtain financial
information and approximately 11% use the Internet to buy or sell stocks, bonds
or mutual funds. (http://www.pewinternet.org/trends/Internet_Activities_7.22.08.htm).
Although this represents a decrease from the 44% and 13%, respectively for these
activities, reported by Pew in 2005, the Internet population base has steadily
risen during this period as has broadband usage.
Mobile
& Wireless Market
With
respect to our Pixaya business unit, the mobile and wireless industries are
experiencing significant growth and changing the way businesses operate and
people communicate. Both industries are also giving rise to new forms
of mobile entertainment, communication and information not available just a few
short years ago. As of June 2008 there were more than 262 million
wireless subscribers in the U.S., reported wireless minutes of use exceeded 2.2
trillion and wireless penetration reached 84% of the total U.S. population
(http://www.ctia.org/advocacy/research/index.cfm/AID/10323).
In
addition, wireless carriers are updating their networks and are in the process
of deploying their next-generation high-speed broadband networks, commonly
referred to as 3G. Such 3G networks will significantly improve uplink and
downlink speeds, thereby providing an enhanced user experience when viewing
video, accessing the Internet or working with any large data
files.
6
OUR
PRODUCTS
The CardioGenics
Products
QL
Care Analyzer (QLCA)

The QLCA
represents a shift in the design of POC analyzers. The QLCA is a small,
portable, stand-alone and completely automated point-of-care immunoassay
analyzer. The QLCA has successfully miniaturized lab test technology, and
combined it with a simplified mechanical design and proprietary triggering
mechanism.
The QLCA
uses a proprietary self-metering cartridge to perform immunoassay tests at the
POC. Each cartridge is pre-loaded with our beads, which have been coated with
specific bind reagents and bioluminescent proteins linked to the target marker.
A drop of whole blood added to the Cartridge creates the chemiluminescent
reaction needed to deliver sensitive and accurate test results. Operation of the
QLCA does not require specialized training and testing can be completed in 15
minutes.
POC
immunoassay analyzers are not new; however, none of the commercial analyzers can
replicate the sensitivity and accuracy of a test done in a medical lab. The QLCA
delivers the required laboratory sensitivity and accuracy. The QLCA employs
chemical light generation or “chemiluminescence“ (“CL“), the same technology
used in the medical labs. The QLCA uses a patented automated electronic process
to trigger CL, which enhances light collection, speeds up marker binding and
increases sensitivity.
We have
rigorously tested the QLCA protocols and have compared our test results against
medical laboratory test data. Based on these internal test results, we have
consistently met or exceeded the sensitivity standards of medical laboratory
immunoassay equipment.
Cardiovascular
Tests
To
support the use of the QLCA, we have developed four immunoassay tests designed
to identify cardiac markers in the blood at the time of a heart
attack.
|
Test
|
Description
|
||
|
Troponin
I (TnI)
|
§
|
TnI
testing is the current routine testing for a heart
attack.
|
|
|
§
|
TnI
is a heart muscle protein, released in the bloodstream shortly after a
heart attack (myocardial infarction or MI).
|
||
|
§
|
Current
laboratory analyzers cannot detect TnI before 4-6 hours after the onset of
symptoms, when TnI concentration in the blood reaches its detection
threshold.
|
||
|
§
|
Our
test will take only 15 minutes to deliver quantitative results, allowing
physicians to obtain much more rapid results and therefore accelerate
patient triage.
|
||
|
Plasminogen
Activator Inhibitor Type-1 (PAI-1)
|
§
|
This
test will help to optimize the performance of a heart drug (“tPA” or tissue
Plasminogen Activator), a clot buster used as the first line of therapy
for MI patients.
|
|
|
§
|
This
proprietary whole blood test will quantify PAI-1 levels within 15
minutes.
|
||
|
§
|
Forty
percent of patients do not respond to tPA, a fact recognized only after
the “golden hour” (the time period in which permanent heart damage can be
prevented) has passed.
|
||
|
Heart
Failure Risk Stratification (HFRS)
|
§
|
We
have discovered a family of related proteins that are released into the
bloodstream during heart
failure.
|
|
7
|
Test
|
Description
|
||
|
§
|
We
are developing a proprietary test, the Heart Failure Risk Stratification
or HFRS test to stratify the risk of death in patients with heart failure,
thus permitting the initiation of appropriate therapy at an early
stage.
|
||
|
Heart
Failure Genomics Risk (HFGR)
|
§
|
We
are developing a proprietary HFGR test that predicts the response of heart
failure patients to routinely administered drugs.
|
|
|
§
|
The
need to measure the precise response to these drugs in a timely manner
would minimize the trial and error methods now used by doctors to optimize
drugs best suited to each
patient.
|
||
These
tests are designed to be administered in the diagnostic and management process
of patients with heart disease. The full scope of our core technology, as well
as the know-how we have developed respecting aspects of chemical entrapment in
bioassays, are covered under our patent applications.
Upon
receipt of FDA approval, we intend to market the QLCA and the Cardiovascular
Tests through a major IVD distributor. We have initiated discussions with a
number of the Tier 1 IVD companies, and we anticipate that we will select a
partner before we receive FDA approval. In
accordance with industry practice, we intend to enter into a license agreement
with our distribution partner for the manufacture and distribution of our
products.
Paramagnetic
Beads
Medical
laboratories widely use paramagnetic particles as a solid surface in
heterogeneous immunoassay tests utilizing the process of phase separation done
by magnetic field. Such tests involve the measurement of light
generated on the surface area of paramagnetic beads coated with bio-organic
material.

Our Beads
represent a significant product advance. Most paramagnetic beads are made of
iron oxide, and all are traditionally black or brown. We have developed a
proprietary process that coats the beads with a layer of silver, making them
white, and more sensitive to light. Our production process is also significantly
less expensive than those used by our competitors. We have internally tested our
Beads against all commercially available beads, and have found our silver-coated
Beads to be five times more sensitive than traditional black or brown magnetic
particles.
Since the
CardioGenics business described above is now our primary business, we intend to
examine the possibility of divesting our Pixaya and JAG Notes subscription
businesses so we can focus our attention and resources exclusively on our
primary CardioGenics business. Consistent with this objective, we have engaged
in preliminary discussions with several parties that have expressed an interest
in acquiring one or more of these businesses.
The JAG Notes and Pixaya
Products
JAG
Notes Subscription Products
We have
been providing financial and investment information to the investment community
since 1989. In 1999, we began offering our services on a subscription
fee basis to the general public for the first time through our
website.
Our jagnotes.com website
consists solely of a subscription-based service. We offer our subscribers two
targeted products: The JAGNotes (Upgrade/Downgrade) Report, targeted primarily
at institutional subscribers, and the Rumor Room, targeted primarily at retail
individual customers. These two products are accessible only to paid
subscribers. Subscriptions are offered to individuals at the rate of $9.95 per
month, or $99.95 per year. We have offered free trial subscriptions at times in
the past and may do so in the future.
8
JAGNotes
(Upgrade/Downgrade) Report
The
JAGNotes Report is a daily consolidated investment report that summarizes newly
issued research, analyst opinions, upgrades, downgrades and analyst coverage
changes from various investment banks and brokerage houses. Each morning we
gather this information, then compile and release it in a concise, easy to read
format before the markets open. We believe that this report gives early,
convenient access to its subscribers of potentially market moving information
that was traditionally not available in a convenient format when the market
opens. We update this report from time to time during the trading
day.
Our
current strategy involves phasing out retail subscribers for the JAGNotes Report
and refocusing on institutional customers and professional traders. We have
always maintained our original JAGNotes fax-based service for a limited number
of mostly institutional subscribers. Through this service, we provide our
subscribers faxed copies of our daily JAGNotes Report, which is provided through
installments as information is received every weekday morning before the stock
market opens. We also allow our subscribers access to our U.S. website by
providing them with a specified number of access codes. The price for this
combined service is approximately $1,500 to $2,150 per year. The website
contains substantially all of the information provided in the faxed reports,
updates of such information and access to the Rumor Room.
We intend
to continue providing our combined fax/internet service primarily to
institutional subscribers. We believe that some financial
institutions are willing to pay a higher price for this combined service because
they consider a faxed report to be a more user friendly means of receiving the
information even if their employees have direct internet access.
While we
intend to focus efforts exclusively on offering the JAGNotes Report to
institutional customers, we recognize that it remains of interest to some retail
customers as well. However, to avoid the cost of accessing such
individuals we expect to do so almost exclusively through strategic affiliations
such as our current arrangement with Track Data.
Rumor
Room
Because
rumors can move equities, we have established the Rumor Room where we post
rumors that have been heard on the street about various stocks. When
we hear rumors, we post the information in the Rumor Room and indicate the date
and time of the rumor. While we realize that rumors are inherently
unreliable as indicated by a cautionary note introducing this portion of our
website (jagnotes.com), we believe that every trader and investor, both large
and small, should have access to this information to determine its
usefulness. The Rumor Room is available to our subscribers and
updated whenever we receive relevant information.
Although
targeted primarily at individual subscribers, the Rumor Room is also made
available through our website to institutional investors as described
above. As described below, if we can obtain funding and we can
complete development of our Pixaya Mobile software product, which delivers
on-demand video/audio clips and text messages to mobile phones, we intend to try
to distribute the Rumor Room through mobile phones as well. We
believe such a distribution channel might better access our target
audience. There can be no assurance, however, that such development
will be successfully completed or, if completed, that customers will embrace
this new mode of distribution. For the time being, we have stopped
further development of our Pixaya Mobile software product in light of our lack
of liquidity.
Advertising
Revenue
While we
expect the primary source of our revenue for our JAGNotes business to continue
to be from subscriptions for our JAGNotes Report and the Rumor Room, we may
supplement this with advertising revenue. Such revenues have,
however, not been substantial to date. We do not expect such
advertising revenues to become material until (i) there is a major upturn from
current levels in internet banner advertising generally or we are able to offer
advertisers various media advertising alternatives to banners, and, (ii) we are
successful in increasing the number of unique visitors to our
website
Pixaya
Products
SurvayaCam
In
November 2004, we purchased certain development stage software products and
related assets from TComm Limited, a company organized in the United
Kingdom. At the time of acquisition, TComm Limited was in various
stages of development of four software products. One product that we
continued to develop is SurvayaCam (previously CCMTV), a mobile surveillance
system that consists of software programs and related hardware intended to
permit field personnel to send encrypted real-time video streams from the field
to a central location (or multiple locations) where they can be viewed. The
system can be used as a fixed-in-place or body worn surveillance system. We have
only made minimal sales of such product as part of our prior marketing and
distribution efforts. In light of our cash constraints prior to the CardioGenics
Acquisition and the revised focus of our business as a result of that
acquisition, we previously suspended marketing and further development of
SurvayaCam and do not anticipate re-engaging in any further development and
marketing efforts in connection with this product.
9
Pixaya
Mobile and Other Pixaya Products
Due to
our lack of funding, we have for the time being discontinued developing Pixaya
Mobile (previously known as TComm TV), which delivers on-demand video/audio
clips and text messages to various Java-based and Symbian-based mobile phones.
We have also discontinued our support of the other products we acquired through
our Pixaya acquisition. In addition, after the acquisition we first
supported and then had to suspend development of a new mobile phone product
named “SOS Guides,” which are mobile travel guides deliverable to users through
their mobile phones.
OUR
STRATEGY
The
success of our business depends on our ability to obtain the requisite financing
and be able to:
|
|
●
|
complete
the development of our QLCA and our cardiovascular
tests;
|
|
|
●
|
obtain
FDA approval of our QLCA and the cardiovascular
tests;
|
|
|
●
|
develop
further tests that can be run on our
QLCA;
|
|
|
●
|
commercialize
our Beads.
|
We will
require additional funds in order to implement our full business strategy.
Accordingly, we will need to raise additional funds through public or private
financing, strategic relationships or other arrangements. We do not anticipate
generating any significant revenue until after our first cardiovascular test has
been approved by the FDA and our Beads are commercialized by Merck Chimie
pursuant to our agreement with them.
Since our
strength is product development and innovation, our strategy is focused on
exploiting this strength. In terms of product development and innovation, we
employ our internal resources to develop our products through the various phases
of development. We also rely on external service providers to supplement our
internal talents in product development.
We will
outsource product manufacturing. In terms of the QLCA, both the cartridge
assembly as well as the analyzer assembly will be contracted out to different
OEM providers with the facilities and expertise to deliver quality products. We
will maintain a quality control process to ensure that the products meet the
predetermined specifications.
Product
marketing and distribution will by achieved through partnerships with global
companies with wide reach. As we have done with our magnetic beads, the QLCA
will be marketed by a third party through licensing and distribution
agreements.
We are
also focusing on protecting our intellectual property and know how through
maintaining a patent filing process on a global basis as well as
maintaining confidentiality agreements with our staff, employees and service
providers under contractual agreements.
While we
have continued to operate our original JAGNotes subscription business since the
CardioGenics Acquisition we have also, during that time, evaluated whether it is
in the best interests of the Company and its stockholders to continue to devote
resources to operating this business unit. Given our new focus on the
CardioGenics business as a result of the CardioGenics Acquisition, we concluded
that the Company and its stockholders would be best served by devoting all of
our efforts and resources to our core CardioGenics business.
Accordingly,
we began considering offers for the purchase of our Pixaya business unit
(including our JAGNotes subscription business). On February 10, 2010 we entered
into an agreement to sell our 100% membership interest in our Pixaya LLC
subsidiary and the transaction closed on February 11, 2010.
Although
we believe in these strategies, goals and targets, we cannot guarantee that we
will be successful in implementing them or that, even if implemented, they will
be effective in creating a profitable business. In addition, we are dependent on
having sufficient cash to carry out our strategies
Regulation
CardioGenics
Products
Our QL
Care Analyzer, Cartridge and Tests are classified as medical devices. Our
beads are reagents of medical testing equipment. Accordingly, they are subject
to a number of regulations in the jurisdictions where our products will be
sold.
10
United
States
The
testing, production and sale of IVD products are subject to regulation by
numerous state and federal government authorities, principally the
FDA.
Pursuant
to the U.S. Federal Food, Drug
and Cosmetic Act (“FD&C Act”), the FDA regulates the preclinical and
clinical testing, manufacture, labeling, distribution and promotion of medical
devices.
Medical
devices are classified into three categories, Class I, Class II or Class III.
The classification of a device is based on the level of control necessary to
assure the safety and effectiveness of the device. Generally, the complexity of
the submission and the approval times are based on the regulatory class of the
device. Device classification depends on the intended use and also the
indications for use of the device. Classification is also based on the risk the
device poses to the patient and/or the user. Class I devices include devices
with the lowest risk, and Class III devices are those with the greatest risk.
Class I devices are subject to general control, Class II devices are subject to
general controls and special controls, and Class III devices are subject to
general controls and must receive a Premarket Assessment or PMA by the
FDA.
Before
some Class I and most Class II devices can be introduced in the market, either
the manufacturer or distributor of the device is required to follow the
pre-market notification process described in section 510(k) of the FD&C Act.
A 510(k) is a pre-marketing submission made to the FDA to demonstrate that the
device to be marketed is as safe and effective, and is substantially equivalent
to a legally marketed device. Applicants must compare their 510(k) device to one
or more similar devices currently on the US market and support their claims for
substantial equivalency. The FDA requires a rigorous demonstration of
substantial equivalency. It generally takes three to six months from submission
to obtain 510(k) clearance. If any device cleared through 510(k) is modified or
enhanced, or if there is a change of use of the device, a new amended 510(k)
application must be submitted. According to FDA regulations and our management
team’s prior experiences with submissions of similar products, our QLCA and
launch product (TnI) will be classified as a Class II device and will be
subjected to the 510(K) process. Further, a second test product of ours (HFRS)
will also be subjected to the same 510(K) process. As for both tests, predicate
devices are commercially available. For other test products,
depending on the claims and with a prior agreement with the FDA, the submissions
would be either a PMA or 510(K). We have not yet approached the FDA for that
purpose.
Canada
Health
Canada sets out the requirements governing the sale, importation and
advertisement of medical devices. These regulations are intended to ensure that
medical devices distributed in Canada are both safe and effective. We are also
required to comply with certain procedures for the disposal of waste products
under the Canadian Code of Practice for the Management of Biological Waste (the
“Code”). We believe we are currently in compliance with all required Code
provisions.
Europe
Our
products will be subject to registration under the EU Medical Device Directives
for in-vitro diagnostic products.
Other
countries
Our
products will be subject to the regulations of any country where they are sold,
and we will make the necessary applications for approval on a country-by-country
basis.
JAGNotes and Pixaya
Products
The
securities industry is subject to extensive regulation under federal and state
laws in the United States, and companies that provide financial advice to
investors are generally required to register as investment advisers at either
the federal or state level. We believe that our JAGNotes business consists of a
publishing activity for which investment adviser registration and regulation do
not apply under applicable federal or state law, and thus we are not registered
as an investment adviser with either the Securities and Exchange Commission (the
“SEC”) or any of the
various states. The regulatory environment in which it operates is
subject to change, however, and we could be required to register as an
investment adviser with an appropriate regulatory agency at some point in the
future.
In
addition, we operate in an environment of uncertainty about potential government
regulation of the Internet and internet-based service providers. We
believe that our business is not currently subject to direct regulation other
than regulations applicable to businesses generally. However, the
Internet is evolving rapidly, and governmental agencies have not yet been able
to adapt all existing regulations to the Internet environment. The
United States Congress has passed legislation that regulates certain aspects of
the Internet, including on-line content, copyright infringement, user privacy,
liability for third-party activities and jurisdiction. Specifically,
with respect to one aspect of copyright law, on October 28, 1998, the United
States Congress passed the Digital Millennium Copyright Act (“DMCA”). The DMCA
includes statutory licenses for the performance of sound recordings and for the
making of recordings to facilitate transmissions. Under these
statutory licenses, depending on our future business activities, we and our
customers may be required to pay licensing fees in connection with digital sound
recordings which we may deliver to our customers. Additionally,
federal, state, local and foreign governmental organizations from time to time
consider other legislative and regulatory proposals that would regulate the
Internet. The distribution of content to mobile phones is also
regulated.
11
We cannot
predict what new laws will be enacted or how courts will interpret both existing
and new laws. As a result, we are uncertain as to how new laws or the
application of existing laws may affect our business. For example,
while we are not aware of any pending laws or regulations that would restrict
our ability to disseminate market-based rumors and other information of
unsubstantiated reliability, it is possible that such laws or regulations may be
passed in the future. Increased regulation in this area could
decrease the demand for our JAGNotes subscription services, increase our cost of
operating such business or otherwise have a material adverse effect on that
business, its results of operations and financial condition. In
addition, the ways in which Internet companies deal with copyrighted content
which appears on their sites are in flux.
Competition
CardioGenics
Competitors
Numerous
companies provide Point Of Care (POC) products, many with cardiovascular test
products. However, in terms of quantitative POC
products, few companies operate in this space with marketed devices. These
include:
|
|
●
|
Biosite
Diagnostics Incorporated;
|
|
|
●
|
Response
Biomedicals Corp.;
|
|
|
●
|
Roche
POC division; and
|
|
|
●
|
i-Stat
division of Abbott Diagnostics.
|
The first
3 companies employ fluorescence measurements in their platforms, while i-Stat
employs electrochemical testing. We believe that our technology and products in
development will offer superior products to the POC market. None of the above
companies offer chemiluminescence in its platform, a technology that is
well-recognized for its superiority as evidenced by its dominance in the
laboratory testing market. We believe that harnessing chemiluminescence in our
QLCA will fulfill the clinical demands for fast and accurate quantitative
results at patient bedsides.
JAG
Notes Competitors
Providing
financial information and analysis over the internet is an intensely competitive
business. A large number of web-based financial information providers
are competing for subscribers, customers, advertisers, content providers,
analysts, commentators and staff. In addition, cable television is an
increasingly important source of financial news and therefore
competition.
Our
business competes to a different degree with the following information sources,
many of which provide their information without charge:
|
|
●
|
Online
financial news and information providers, such as Yahoo Finance,
Marketwatch, TheStreet.com, Forbes.com, Briefing.com, America Online
Personal Finance, Reuters and
MotleyFool.com;
|
|
|
●
|
Internet
portals and search engines such as Google, AOL, MSN and
Yahoo;
|
|
|
●
|
Traditional
media sources such as The Wall Street Journal, Investor’s Business Daily,
The Financial Times, Barrons, CNN/Money, and MSN Money/CNBC, all of which
also have an Internet presence;
|
|
|
●
|
Terminal-based
financial news providers including Bloomberg, Reuters and Dow Jones;
and,
|
|
|
●
|
Online
brokerage firms such as TD Ameritrade, E*Trade Financial, Charles Schwab
and Fidelity;
|
Because
there is not a readily defined market in which we compete, we cannot predict
which information source or sources will be our primary competition in the
future. However, we expect competition from each of the above
information sources to intensify and increase in the future. Most of
our current and potential competitors have greater name recognition, larger
financial, technical and marketing resources, and more extensive customer bases
than we do, all of which could be leveraged to gain market share to our
detriment. Such advantages would also permit our competitors to enter
new sectors such as distribution through mobile phones, more easily than we
would be able to do.
12
It is not
difficult for new competitors to enter the market. Many blogs now
provide financial information at no cost. Much of the information we
provide is publicly available and it does not have any patented or otherwise
protected technologies that would preclude or inhibit competitors from entering
our markets. Our current and future competitors may develop or offer
services that have significant price, content, creative or other advantages over
the services we provide.
In order
for us to successfully compete, we will need to reliably provide valuable
services to a greater number of institutional and other subscribers who are
willing to pay fees sufficient to support such services. We believe
that over time, if we can obtain sufficient funding, a successful implementation
of our business strategy will allow us to compete successfully as a focused
provider of timely investment information to institutional and retail
customers.
Intellectual Property
In
connection with our CardioGenics business, we have filed seven (7) full patent
applications relating to the QLCA and its core technology, the self-metering
cartridge, our battery of cardiovascular tests and the Beads.
With our
intellectual properties, we file an initial application with the Patent
Cooperation Treaty (PCT) which is responsible for intellectual property
protection globally. Thereafter we file patent applications in individual
jurisdictions, once the patent is granted by the PCT.
We have
filed 3 patent applications covering the core technology, an application
covering one of its test products, 2 applications covering the detection
technology and a patent application covering the development of a self-metering
cartridge for the QLCA. We also choose to maintain some of our intellectual
property as proprietary “know-how“, rather than disclosing such proprietary
information to the public domain through patent applications.
With
respect to our JAGNotes business, we are the owner of the trademarks “JAG NOTES
— AHEAD OF THE MONEY,” “STREETSIDE” and “STREETSIDE WITH DAN
DORFMAN.” Each of the foregoing trademarks was registered in 2002 and
has a duration of ten years, at which time each of the trademarks must be
renewed or they will expire. We do not consider these trademarks to
be material to our business.
Research
and Development
Our
efforts are focused on the development and commercialization of our products
under development.
The
Company has spent approximately $1,597,000 over the last two (2) fiscal years
developing its QLCA and cardiovascular tests to be used therein and its
paramagnetic beads
Website
Technical Information
Our
CardioGenics website (www.cardiogenics.com) is maintained by us internally and
is hosted by DreamHost, which has hosting facilities located in Brea,
California.
With
respect to our JAGNotes business, we lease one web server, which is the computer
system on which all of the content for our jagnotes.com website
is maintained and through which we operate our jagnotes.com
website. Our U.S. server is maintained by Woodbourne Solutions and is
located at their facility in Germantown, Maryland.
Our
Pixaya website (www.pixaya.com) is hosted by InnoTech. InnoTech has
offices located in southern California and Raleigh, North Carolina. Their
Internet data center is located in Orange County, California. The
Pixaya website is maintained by us internally.
Employees
As of
October 31, 2009, we had ten (10) employees of whom
seven (7) were full time and three (3) were part-time. Of those
employees, only Yahia Gawad, our Chief Executive Officer, has an employment
agreement with the Company.
Acquisition
of CardioGenics
On July
31, 2009 we completed the acquisition of CardioGenics by CardioGenics ExchangeCo
Inc. (“ExchangeCo”), our
Ontario, Canada subsidiary, pursuant to the terms of a Share Purchase Agreement
dated May 22, 2009 among ExchangeCo, JAG Media Holdings, Inc., CardioGenics and
CardioGenics’ principal stockholder, Yahia Gawad (the “Share Purchase Agreement”).
CardioGenics is considered the acquirer in the transaction for accounting and
financial reporting purposes.
13
In
connection with the acquisition, ExchangeCo acquired all of the outstanding
common shares of CardioGenics (the “CardioGenics Common Shares”),
excluding 173,869 CardioGenics Common Shares in the aggregate owned by two (2)
minority stockholders of CardioGenics (the “Dissenting Stockholders”), in
consideration for the issuance of 422,183,610 shares of our common stock to the
CardioGenics stockholders at the closing, as further described below (the “Share Consideration”). In
consideration for the surrender of their CardioGenics Common Shares, the
CardioGenics stockholders had the option to receive at the closing their
pro-rata allocation of the Share Consideration in the form of (a) our common
shares or (b) “Exchangeable Shares“ of ExchangeCo, which are exchangeable into
our common shares in accordance with the terms of a Voting and Exchange Trust
Agreement dated July 6, 2009 among JAG Media, ExchangeCo, and WeirFoulds LLP, as
trustee and the rights and preferences of the Exchangeable Shares. Those
CardioGenics stockholders who elected to receive directly our common shares were
issued, in the aggregate, 145,528,195 common shares at the closing and those
CardioGenics stockholders who elected to receive Exchangeable Shares were issued
16 Exchangeable Shares at the closing, which are exchangeable at any time into
276,655,415 of our common shares, in the aggregate. The Share Consideration
issued at the closing provided the CardioGenics stockholders with direct and
indirect ownership of approximately 85% of our outstanding common stock, on a
fully diluted basis.
Immediately
prior to the closing, all CardioGenics debenture holders converted their
debentures into CardioGenics Common Shares in accordance with the terms of their
respective debentures, as required by the terms of the Share Purchase Agreement.
Accordingly, such former debenture holders became CardioGenics stockholders for
purposes of the acquisition and received their pro-rata allotment of the Share
Consideration in the form of JAG Common Shares and/or Exchangeable Shares at the
closing in consideration for the surrender of the CardioGenics Common Shares
they received upon conversion of their debentures.
Also
prior to the closing, CardioGenics closed on an equity investment round of
financing totaling $2,715,000. These equity investors in CardioGenics became
CardioGenics stockholders for purposes of the acquisition and received their
pro-rata allotment of the Share Consideration in the form of our common
shares.
All of
our common shares received by CardioGenics stockholders in exchange for their
CardioGenics Common Shares may not be registered for resale and, therefore,
shall remain subject to the rights and restrictions of Rule 144. All
Exchangeable Shares received by CardioGenics stockholders in exchange for their
CardioGenics Common Shares (and any of our common shares into which such
Exchangeable Shares may be exchanged) also may not be registered for resale
prior to six (6) months following the closing and, therefore shall remain
subject to the rights and restrictions of Rule 144 prior to any such
registration.
Also at
the closing, all holders of CardioGenics warrants entitling the holders to
purchase CardioGenics Common Shares at various prices exchanged their
CardioGenics warrants for warrants to purchase, in the aggregate, 36,148,896 of
our common shares at exercise prices of $0.047 per share, in accordance with the
terms of the Share Purchase Agreement and the respective warrants. The terms of
these newly issued warrants did not include any registration rights for the
warrant holders. CardioGenics had no options to acquire CardioGenics Common
Shares outstanding as of the closing.
At the
closing, our then current directors resigned as directors of JAG Media and its
subsidiaries after appointing their successors and our then current officers
also resigned as officers and executives of JAG Media and its subsidiaries.
After their resignation and the closing, our former directors entered into
consulting agreements with the Company pursuant to which they are rendering
various services to assist us in connection with certain transition
matters. Each
consulting agreement is for a term of 18 months, with each party receiving
500,000 shares of the Company’s common stock, issued pursuant to our 1999
Long-Term Incentive Plan, as compensation for their services under the
consulting agreements.
Following
the closing, a majority of our stockholders approved, by written consent, an
amendment to our articles of incorporation, which provided for (a) a change in
our corporate name from “JAG Media Holdings, Inc.“ to “CardioGenics Holdings
Inc.” and (b) an increase in the number of our authorized JAG Common Shares from
500,000,000 to 650,000,000.
Financing
Arrangements
Equity
Line of Credit with YA Global
In
connection with the CardioGenics Acquisition, on March 12, 2009 we entered into
a Standby Equity Distribution Agreement with YA Global Master SPV Ltd. (“YA Ltd
”) (the “ SEDA ”) pursuant to which YA Ltd agreed to purchase up to $5,000,000
of our common stock (the “ Commitment Amount ”) over the course of the
thirty-six (36) months following the date the registration statement for the
shares to be issued pursuant to the SEDA is first declared effective (the “
Commitment Period ”). We will have the right, but not the obligation, to sell
common stock to YA Ltd during the Commitment Period. Concurrent with the
execution of the SEDA, we also entered into a Registration Rights Agreement with
YA Ltd pursuant to which we agreed to register the shares of our common stock to
be issued in connection with the SEDA. We have not yet filed such registration
statement, in accordance with the terms of the Registration Rights Agreement,
and accordingly the SEDA is not currently effective.
14
Increase
in Authorized Shares
In
October 2009 a majority of our stockholders approved, by written consent, an
amendment to our articles of incorporation, which provided for, among other
matters, an increase in the number of our authorized shares of common stock from 500,000,000 to
650,000,000.
Facilities
See “Item
2.—Properties.”
Legal
Proceedings
See “Item
3.—Legal Proceedings.”
Where
You Can Find More Information About Us
We are
required to file annual, quarterly and current reports, proxy statements and
other information with the SEC. You can read and copy any of this
information at the SEC’s Public Reference Room at 100 F Street, N.E.,
Washington, D.C. 20549 on official business days during the hours of 10:00 a.m.
to 3:00 p.m. You may obtain information on the operation of the
Public Reference Room by calling the SEC at 1-800-SEC-0330. This
information is also available from the SEC’s website at http://www.sec.gov. We
will also gladly send any filing to you upon your written request to Dr. Yahia
Gawad, our Chief Executive Officer, at 6295 Northam Drive, Unit 8, Mississauga,
Ontario L4V 1W8.
ITEM
1A. RISK FACTORS
Risks Related to Our
CardioGenics Business and Industry
The
global financial crisis has had, and may continue to have, an impact on our
business and financial condition.
The
ongoing global financial crisis may limit our ability to access the capital
markets at a time when we would like, or need, to raise capital, which could
have an impact on our ability to react to changing economic and business
conditions. Accordingly, if the global financial crisis and current economic
downturn continue or worsen, our business, results of operations and financial
condition could be materially and adversely affected.
The
requirements of being a public company may strain our resources and distract our
management
As a
public company, we are subject to the reporting requirements of the Securities
Exchange Act of 1934, as amended and the Sarbanes-Oxley Act of 2002 (the
“Sarbanes-Oxley Act”). These requirements place a strain on our systems and
resources. The Exchange Act requires that we file annual, quarterly
and current reports with respect to our business and financial condition. The
Sarbanes-Oxley Act requires that we maintain effective disclosure controls and
procedures and internal controls for financial reporting. We are
required to document and test our internal control procedures in order to
satisfy the requirements of Section 404 of the Sarbanes-Oxley Act, which
requires annual management assessments of the effectiveness of our internal
controls over financial reporting and in the future will require a report by our
independent registered public accountants addressing these assessments via an
auditor’s attestation. The auditor’s attestation requirement would be included
in our fiscal year end 2010 Form 10-K in the event that the Securities and
Exchange Commission does not extend the June 15, 2010 implementation date.
During the course of our testing, we may identify deficiencies which we may not
be able to remediate in time to meet the deadlines imposed by the Sarbanes-Oxley
Act. If we fail to achieve and maintain the adequacy of our internal
controls, as such standards are modified, supplemented or amended
from time to time, we may not be able to ensure that we can conclude on an
ongoing basis that we have effective internal controls over financial reporting
in accordance with the Sarbanes-Oxley Act.
15
In order
to maintain and improve the effectiveness of our disclosure controls and
procedures and internal control over financial reporting, significant resources
and management oversight will be required. This may divert management’s
attention from other business concerns, which could have a material adverse
effect on our business, financial condition, results of operations and cash
flows. In addition, we may need to hire additional accounting and financial
staff with appropriate public company experience and technical accounting
knowledge, and we cannot assure you that we will be able to do so in a timely
fashion.
We
have not earned any revenues in our CardioGenics business unit since its
incorporation and only have a limited operating history in its current business,
which raise doubt about our ability to continue as a going concern.
Our
CardioGenics business unit has a limited operating history in its current
business and must be considered in the development stage. It has not generated
any revenues since its inception and we will, in all likelihood, continue to
incur operating expenses without significant revenues until we complete
development of our Cardiovascular Tests and commercialize our QLCA and the
Cardiovascular Tests. The primary source of funds for our CardioGenics business
unit has been the sale of common stock. We cannot assure that we will be able to
generate any significant revenues or income. These circumstances make us
dependent on additional financial support until profitability is achieved. There
is no assurance that we will ever be profitable and we have not yet achieved
profitable operations. These factors raise substantial doubt that we will be
able to continue as a going concern.
We
need to raise additional financing to support the research and development of
our CardioGenics business but we cannot be sure that we will be able to obtain
additional financing on terms favorable to us when needed. If we are unable to
obtain additional financing to meet our needs, our operations may be adversely
affected or terminated.
Our
ability to develop new test products for our QLCA is dependent upon our ability
to raise significant additional financing when needed. If we are unable to
obtain such financing, we will not be able to fully develop and commercialize
our platform and technology. Our future capital requirements will depend upon
many factors, including:
|
|
•
|
continued
scientific progress in our research and development
programs;
|
|
|
•
|
costs
and timing of conducting clinical trials and seeking regulatory approvals
and patent prosecutions;
|
|
.
|
•
|
competing
technological and market
developments;
|
|
.
|
•
|
our
ability to establish additional collaborative relationships;
and
|
|
|
•
|
the
effect of commercialization activities and facility expansions if and as
required.
|
We have
limited financial resources and to date, no cash flow from the operations of our
CardioGenics business unit and we are dependent for funds on our ability to sell
our common stock, primarily on a private placement basis. There can be no
assurance that we will be able to obtain financing on that basis in light of
factors such as the market demand for our securities, the state of financial
markets generally and other relevant factors. Any sale of our common stock in
the future will result in dilution to existing stockholders. Furthermore, there
is no assurance that we will not incur debt in the future, that we will have
sufficient funds to repay any future indebtedness or that we will not default on
our future debts, jeopardizing our business viability. Finally, we may not be
able to borrow or raise additional capital in the future to meet our needs or to
otherwise provide the capital necessary to continue the development of our
technology, which might result in the loss of some or all of your investment in
our common stock.
We
may acquire other businesses, license rights to technologies or products, form
alliances, or dispose of or spin-off businesses, which could cause us to incur
significant expenses and could negatively affect profitability.
We
may pursue acquisitions, technology licensing arrangements, and strategic
alliances, or dispose of or spin-off some of our businesses, as part of our
business strategy. We may not complete these transactions in a timely manner, on
a cost-effective basis, or at all, and may not realize the expected benefits. If
we are successful in making an acquisition, the products and technologies that
are acquired may not be successful or may require significantly greater
resources and investments than originally anticipated. We may not be able to
integrate acquisitions successfully into our existing business and could incur
or assume significant debt and unknown or contingent liabilities. We could also
experience negative effects on our reported results of operations from
acquisition or disposition-related charges, amortization of expenses related to
intangibles and charges for impairment of long-term assets.
16
The
expiration or loss of patent protection and licenses may affect our future
revenues and operating income.
Much of
our business relies on patent and trademark and other intellectual property
protection. Although most of the challenges to our intellectual property would
likely come from other businesses, governments may also challenge intellectual
property protections. To the extent our intellectual property is successfully
challenged, invalidated, or circumvented or to the extent it does not allow us
to compete effectively, our business will suffer. To the extent that countries
do not enforce our intellectual property rights or to the extent that countries
require compulsory licensing of our intellectual property, our future revenues
and operating income will be reduced. Our principal patents and trademarks are
described in greater detail in the sections captioned, "Patents, Trademarks, and
Licenses."
Competitors'
intellectual property may prevent us from selling our products or have a
material adverse effect on our future profitability and financial
condition.
Competitors
may claim that one or more of our products infringe upon their intellectual
property. Resolving an intellectual property infringement claim can be costly
and time consuming and may require us to enter into license agreements. We
cannot guarantee that we would be able to obtain license agreements on
commercially reasonable terms. A successful claim of patent or other
intellectual property infringement could subject us to significant damages or an
injunction preventing the manufacture, sale or use of our affected products. Any
of these events could have a material adverse effect on our profitability and
financial condition.
We
may not be able to adequately protect our intellectual property
We
believe the patents, trade secrets and other intellectual property we use are
important to our business, and any unauthorized use of such intellectual
property by third parties may adversely affect our business and reputation. We
rely on the intellectual property laws and contractual arrangements with our
employees, business partners and others to protect such intellectual property
rights. Filing, prosecuting, defending and enforcing patents on all of our
technologies and products throughout the world would be prohibitively expensive.
Competitors may, without our authorization, use our intellectual property to
develop their own competing technologies and products in jurisdictions where we
have not obtained patent protection. These technologies and products may not be
covered by any of our patent claims or other intellectual property rights.
Furthermore, the validity, enforceability and scope of protection of
intellectual property in some countries where we may conduct business is
uncertain and still evolving, and these laws may not protect intellectual
property rights to the same extent as the laws of the United
States.
Many
companies have encountered significant problems in protecting and defending
their intellectual property rights in foreign jurisdictions. Many countries,
including certain countries in Europe, have compulsory licensing laws under
which a patent owner may be compelled to grant licenses to third parties (for
example, the patent owner has failed to “work” the invention in that country or
the third party has patented improvements). In addition, many countries limit
the enforceability of patents against government agencies or government
contractors. In these countries, the patent owner may have limited remedies,
which could materially diminish the value of the patent. Moreover, litigation
involving patent or other intellectual property matters in the United States or
in foreign countries may be necessary in the future to enforce our intellectual
property rights, which could result in substantial costs and diversion of our
resources, and have a material adverse effect on our business, financial
condition and results of operations.
We
are subject to numerous governmental regulations and it can be costly to comply
with these regulations and to develop compliant products and
processes.
Our
products are subject to regulation by the U.S. Food and Drug Administration
(“FDA”), and numerous international, federal, and state authorities. The process
of obtaining regulatory approvals to market a medical device can be costly and
time-consuming, and approvals might not be granted for future products, or
additional uses of existing products, on a timely basis, if at all. Delays in
the receipt of, or failure to obtain approvals for, future products, or
additional uses of existing products, could result in delayed realization of
product revenues, reduction in revenues, and in substantial additional costs. In
particular, in the United States our products are regulated under the 1976
Medical Device Amendments to the Food, Drug and Cosmetic Act, which is
administered by the FDA. We believe that the FDA will classify our products as
“Class II” devices, thus requiring us to submit to the FDA a pre-market
notification form or 510(k). The FDA uses the 510(k) to substantiate product
claims that are made by medical device manufacturers prior to marketing. In our
510(k) notification, we must, among other things, establish that the product we
plan to market is “substantially equivalent” to (1) a product that was on
the market prior to the adoption of the 1976 Medical Device Amendment or
(2) a product that the FDA has previously cleared.
17
The FDA
review process of a 510(k) notification can last anywhere from three to six
months, and the FDA must issue a written order finding “substantial equivalence”
before a company can market a medical device. We are currently developing a
group of cardiovascular tests that we will have to clear with the FDA through
the 510(k) notification procedures. These test products are crucial for our
success and if we do not receive 510(k) clearance for a particular product, we
will not be able to market these products in the United States, which will have
a material adverse effect on our revenues, profitability and financial
condition.
In
addition, no assurance can be given that we will remain in compliance with
applicable FDA and other regulatory requirements once clearance or approval has
been obtained for a product. We must incur expense and spend time and effort to
ensure compliance with these complex regulations. Possible regulatory actions
could include warning letters, fines, damages, injunctions, civil penalties,
recalls, seizures of our products and criminal prosecution. These actions could
result in, among other things: substantial modifications to our business
practices and operations; refunds, recalls, or seizures of our products; a total
or partial shutdown of production while we or our suppliers remedy the alleged
violation; the inability to obtain future pre-market clearances or approvals;
and, withdrawals or suspensions of current products from the market. Any of
these events could disrupt our business and have a material adverse effect on
our revenues, profitability and financial condition.
Changes
in third-party payor reimbursement regulations can negatively affect our
business.
By
regulating the maximum amount of reimbursement they will provide for blood
testing services, third-party payors, such as HMOs, pay-per-service insurance
plans, Medicare and Medicaid, can indirectly affect the pricing or the relative
attractiveness of our diagnostic products. For example, the Centers for Medicare
and Medicaid Services set the level of reimbursement of fees for blood testing
services for Medicare beneficiaries. If third-party payors decrease the
reimbursement amounts for blood testing services, it may decrease the amount
that physicians and hospitals are able to charge patients for such services.
Consequently, we would either need to charge less for our products or incur a
reduction in our profit margins. If the government and third-party payors do not
provide for adequate coverage and reimbursement levels to allow health care
providers to use our products, the demand for our products will
decrease.
Laws
and regulations affecting government benefit programs could impose new
obligations on us, require us to change our business practices, and restrict our
operations in the future.
Our
industry is also subject to various federal, state, and international laws and
regulations pertaining to government benefit program reimbursement, price
reporting and regulation, and health care fraud and abuse, including
anti-kickback and false claims laws, the Medicaid Rebate Statute, the Veterans
Health Care Act, and individual state laws relating to pricing and sales and
marketing practices. Violations of these laws may be punishable by criminal
and/or civil sanctions, including, in some instances, substantial fines,
imprisonment, and exclusion from participation in federal and state health care
programs, including Medicare, Medicaid, and Veterans Administration health
programs. These laws and regulations are broad in scope and they are subject to
evolving interpretations, which could require us to incur substantial costs
associated with compliance or to alter one or more of our sales or marketing
practices. In addition, violations of these laws, or allegations of such
violations, could disrupt our business and result in a material adverse effect
on our revenues, profitability, and financial condition.
Our
research and development efforts may not succeed in developing commercially
successful products and technologies, which may cause our revenue and
profitability to decline.
To remain
competitive, we must continue to launch new products and technologies. To
accomplish this, we must commit substantial efforts, funds, and other resources
to research and development. A high rate of failure is inherent in the research
and development of new products and technologies. We must make ongoing
substantial expenditures without any assurance that its efforts will be
commercially successful. Failure can occur at any point in the process,
including after significant funds have been invested.
Promising
new product candidates may fail to reach the market or may only have limited
commercial success because of efficacy or safety concerns, failure to achieve
positive clinical outcomes, inability to obtain necessary regulatory approvals,
limited scope of approved uses, excessive costs to manufacture, the failure to
establish or maintain intellectual property rights, or infringement of the
intellectual property rights of others. Even if we successfully develop new
products or enhancements or new generations of our existing products, they may
be quickly rendered obsolete by changing customer preferences, changing industry
standards, or competitors' innovations. Innovations may not be accepted quickly
in the marketplace because of, among other things, entrenched patterns of
clinical practice or uncertainty over third-party reimbursement. We cannot state
with certainty when or whether any of our products under development will be
launched or whether any products will be commercially successful. Failure to
launch successful new products or new uses for existing products may cause our
products to become obsolete, causing our revenues and operating results to
suffer.
18
New
products and technological advances by our competitors may negatively affect our
results of operations.
Our
products face intense competition from our competitors' products. Competitors'
products may be safer, more effective, more effectively marketed or sold, or
have lower prices or superior performance features than our products. We cannot
predict with certainty the timing or impact of the introduction of competitors'
products.
We
depend on key members of our management and scientific staff and, if we fail to
retain and recruit qualified individuals, our ability to execute our business
strategy and generate sales would be harmed.
We are
highly dependent on the principal members of our management and scientific
staff. The loss of any of these key personnel, including in particular Dr. Yahia
Gawad, our Chief Executive Officer, might impede the achievement of our business
objectives. We may not be able to continue to attract and retain skilled and
experienced scientific, marketing and manufacturing personnel on acceptable
terms in the future because numerous medical products and other high technology
companies compete for the services of these qualified individuals. We currently
do not maintain key man life insurance on any of our employees.
The
manufacture of many of our products is a highly exacting and complex process,
and if we or one of our suppliers encounter problems manufacturing products, our
business could suffer.
The
manufacture of many of our products is a highly exacting and complex process,
due in part to strict regulatory requirements. Problems may arise during
manufacturing for a variety of reasons, including equipment malfunction, failure
to follow specific protocols and procedures, problems with raw materials,
natural disasters, and environmental factors. In addition, we may use single
suppliers for certain products and materials. If problems arise during the
production of a batch of product, that batch of product may have to be
discarded. This could, among other things, lead to increased costs, lost
revenue, damage to customer relations, time and expense spent investigating the
cause and, depending on the cause, similar losses with respect to other batches
or products. If problems are not discovered before the product is released to
the market, recall and product liability costs may also be incurred. To the
extent we or one of our suppliers experience significant manufacturing problems,
this could have a material adverse effect on our revenues and
profitability.
Significant
safety issues could arise for our products, which could have a material adverse
effect on our revenues and financial condition.
All
medical devices receive regulatory approval based on data obtained in controlled
testing environments of limited duration. Following regulatory approval, these
products will be used over longer periods of time with many patients. If new
safety issues arise, we may be required to change the conditions of use for a
product. For example, we may be required to provide additional warnings on a
product's label or narrow its approved use, either of which could reduce the
product's market acceptance. If serious safety issues with one of our products
arise, sales of the product could be halted by us or by regulatory authorities.
Safety issues affecting suppliers' or competitors' products also may reduce the
market acceptance of our products.
In
addition, in the ordinary course of business, we may be the subject of product
liability claims and lawsuits alleging that our products or the products of
other companies that we promote, or may be incorporated in our products, have
resulted or could result in an unsafe condition for or injury to patients.
Product liability claims and lawsuits and safety alerts or product recalls,
regardless of their ultimate outcome, may have a material adverse effect on our
business, reputation and financial condition, as well as on our ability to
attract and retain customers. Product liability losses are
self-insured.
The
international nature of our business subjects us to additional business risks
that may cause our revenue and profitability to decline.
Since we
intend to market our products internationally, our business will be subject to
risks associated with doing business internationally. The risks associated with
any such operations outside the United States include:
19
|
•
|
changes
in foreign medical reimbursement policies and
programs;
|
|
•
|
multiple
foreign regulatory requirements that are subject to change and that could
restrict our ability to manufacture, market, and sell our
products;
|
|
•
|
differing
local product preferences and product
requirements;
|
|
•
|
trade
protection measures and import or export licensing
requirements;
|
|
•
|
difficulty
in establishing, staffing, and managing foreign
operations;
|
|
•
|
differing
labor regulations;
|
|
•
|
potentially
negative consequences from changes in or interpretations of tax
laws;
|
|
•
|
political
and economic instability;
|
|
•
|
inflation,
recession and fluctuations in foreign currency exchange and interest
rates; and,
|
|
•
|
compulsory
licensing or diminished protection of intellectual
property.
|
These
risks may, individually or in the aggregate, have a material adverse effect on
our revenues and profitability.
Other
factors can have a material adverse effect on our future profitability and
financial condition.
Many
other factors can affect our profitability and financial condition,
including:
|
•
|
Changes
in or interpretations of laws and regulations including changes in
accounting standards, taxation requirements and environmental laws in
domestic or foreign jurisdictions.
|
|
•
|
Changes
in the rate of inflation (including the cost of raw materials,
commodities, and supplies), interest rates and the performance of
investments held by us.
|
|
•
|
Changes
in the creditworthiness of counterparties that transact business with or
provide services to our distributors or
us.
|
|
•
|
Changes
in business, economic, and political conditions, including: war, political
instability, terrorist attacks in the U.S. and other parts of the world,
the threat of future terrorist activity in the U.S. and other parts of the
world and related military action; natural disasters; the cost and
availability of insurance due to any of the foregoing events; labor
disputes, strikes, slow-downs, or other forms of labor or union activity;
and, pressure from third-party interest
groups.
|
|
•
|
Changes
in our business units and investments and changes in the relative and
absolute contribution of each to earnings and cash flow resulting from
evolving business strategies, changing product mix, changes in tax rates
both in the U.S. and abroad and opportunities existing now or in the
future.
|
|
•
|
Changes
in the buying patterns of a major distributor, retailer, or wholesale
customer resulting from buyer purchasing decisions, pricing, seasonality,
or other factors, or other problems with licensors, suppliers,
distributors, and business
partners.
|
|
•
|
Difficulties
related to our information technology systems, any of which could
adversely affect business operations, including any significant breakdown,
invasion, destruction, or interruption of these
systems.
|
|
•
|
Changes
in credit markets impacting our ability to obtain financing for our
business operations.
|
|
•
|
Legal
difficulties, any of which could preclude or delay commercialization of
products or adversely affect profitability, including claims asserting
statutory or regulatory violations, adverse litigation decisions, and
issues regarding compliance with any governmental consent
decree.
|
Risks related to our Pixaya
Business and Industry
We will be managed by a new
management team with no experience in our Pixaya business
sector.
As a
result of our acquisition of CardioGenics, our new management may not be able to
effectively manage the newly combined business or our Pixaya business. If
current or new management is unable to operate the new combined businesses at a
profit, it could materially and adversely affect our business, results of
operation and financial condition and could cause our stockholders to lose part
or all of their investment in our common stock.
20
We may not be able to stop
contraction of our subscriber revenues and attract sufficient institutional
customers.
Our
subscriber base has been shrinking and we have determined that we cannot expand
our retail subscriber base for our traditional product, the JAGNotes Report. We
believe that we must refocus our subscriber base on institutional customers to
be successful, but do not have the funding to do so. Our subscription
revenues have leveled off at a level which cannot support our operating
costs. During the year ended July 31, 2008, revenues from our Pixaya
business unit were approximately $177,065, and consisted entirely of revenues
from subscriptions.
Our
efforts to refocus our key subscriber base have been ineffective and
historically Internet users have only been attracted to subscription websites in
limited areas. Our competitors may be more successful than us in attracting
customers, or the number of institutional and other professional users seeking
or willing to pay for financial information of the kind we provide may not
increase or may even decrease. Any of these would adversely affect
the revenues from our Pixaya business unit. Because there is
currently limited potential for Internet banner advertising revenues, if we
cannot reverse the current shrinkage of our subscriber base or refocus such
base, we will have little, if any, financial success.
We have been forced to discontinue
our commentators and the free portion of our website, which may cause us to lose
subscribers.
In order
to attempt to reduce costs, we have been forced to discontinue all of our
commentators as well as the entire free portion of our
website. Accordingly, we run the risk that existing and potential
subscribers may not find our website valuable and our revenues may
decline. Moreover, many of our competitors offer financial
information for free and are likely to continue to do so, perhaps at an
increasing rate. Our current and potential subscribers may be
unwilling to pay for our service if they feel they can receive comparable
information for free.
We may not be successful in our
attempt to refocus our business strategy of targeting institutional investors
for our JAGNotes Report.
Our
efforts to include individual retail subscribers as part of our strategy to
increase sales of our flagship product, the JAGNotes Report, have been
unsuccessful, and we have therefore decided to refocus our strategy on offering
subscriptions solely to institutional investors and professional
traders. Due to the uncertain nature of this undertaking and our lack
of funding, this shift in business strategy may not be executed, or if executed,
may not be successful, and we may not realize any benefit from it.
We may not be successful at building
brand awareness or building strategic relationships.
Our
growth and success depends in part on our ability to build awareness of the
JAGNotes and Pixaya names. The JAGNotes and Pixaya names have only limited
recognition within the financial community and little if any recognition among
the general public. We do not currently allocate any of our working
capital to marketing and advertising the JAGNotes and Pixaya names but rather
rely solely upon strategic alliances to increase our name
recognition. Our ability to refocus our subscriber base, offer new
services or otherwise expand the business will be limited if we cannot increase
our name recognition.
We may experience difficulties in
developing new and enhanced services and products.
We
believe that our website will be more attractive to subscribers if we introduce
additional or enhanced services in the future in order to retain our current
users and attract new users. Our first attempt to introduce streaming
audio and video was not financially successful and the business was sold. While
we may consider various new enhanced services for our website, as well as new
products for our Pixaya business unit, adequate financing is not currently
available and the new focus of our business in light of the CardioGenics
acquisition will likely require that any surplus cash be used to further develop
our CardioGenics business unit.
21
In
addition, we may experience other difficulties that could delay or prevent us
from introducing such enhanced services. We may encounter
technological problems in enhancing our websites and developing new products or
enhancements to current products in our Pixaya business unit. We may
need to modify significantly the design of these services on our websites and
modify significantly (or discontinue, as we have already had to do) certain
products and services being offered through our Pixaya business
unit. Our business could be adversely affected if we experience
difficulties in introducing or maintaining new services and products, if these
new services and products are not accepted by users or if their cost exceeds the
revenue they generate.
If we
introduce enhanced service on our website that is not favorably received, our
current users may not continue using our service as frequently. New
users could also choose a competitive service over ours.
Our failure to respond to rapid
changes in technology and its applications and intense competition in the mobile
services industry could make our services obsolete.
If and
when funds become available, our Pixaya business unit hopes to again develop
software for the mobile phone and wireless environment. The mobile
and wireless services industries are subject to rapid and substantial
technological development and product innovations. To be successful,
we must respond to new developments in technology and find new applications of
existing technology in our Pixaya business unit for which we currently have no
available funds. In addition, our response may be hindered if we
require, but cannot secure, rights to essential third party intellectual
property. We compete against numerous companies offering alternative
products and services to ours, most of which have much greater financial,
marketing and technical resources to utilize in pursuing technological
development.
We may not successfully attract or
manage our strategic alliances.
We
currently intend to evaluate strategic alliances, partnerships or joint
ventures, as a means of acquiring additional distribution. Pursuing
such transactions will entail a number of risks and difficulties, including a
continuing lack of available funds and personnel. We compete with a wide variety
of information providers and there is substantial competition for distribution
channels. We can offer no guarantee that we will be able to locate suitable
candidates for alliances or risk sharing partners. If we are able to do so, we
will require a high level of managerial skill to successfully evaluate and
implement these transactions. While we have limited experience in
evaluating and implementing transactions of this type, we cannot guarantee that
we will be able to successfully pursue this strategy.
We may have to defend against
intellectual property infringement claims and libel and defamation claims, which
may cause significant operations expenditures.
Third
parties may assert claims against us that our Pixaya business unit has violated
a patent or infringed a copyright, trademark or other proprietary right
belonging to them. Parties could also bring libel, defamation or similar claims
based on the content published on our websites. Any such claims,
whether meritorious or not, could result in the expenditure of significant
financial and managerial resources on our part, which could materially adversely
affect our business, results of operations and financial condition.
Failure to maintain our reputation as
a trustworthy provider of financial news may reduce the number of our users,
which would harm our business.
It is
very important that we maintain our reputation as a trustworthy provider of
financial news. The occurrence of events, including our misreporting
a news story, could harm our reputation for trustworthiness. These events could
result in a significant reduction in the number of our subscribers, which could
materially adversely affect our business, results of operations and financial
condition.
We depend on key people in management
and operations.
Our
JAGNotes and Rumor Room products depend on our former key employees’ contacts
within the professional financial community for certain information that we
provide to our subscribers. Although we have retained Mr. Thomas J.
Mazzarisi, our former Chairman, Chief Executive Officer and General Counsel, and
Mr. Stephen J. Schoepfer, our former President, Chief Operating Officer,
Chief Financial Officer and Secretary as consultants to assist us with the
ongoing operation of our Pixaya business unit during a limited post-closing
transition period, we may also need to attract and retain additional qualified
managers, software developers and other key personnel in the future in order to
successfully manage our Pixaya business unit. We may not be able to
attract or retain the requisite personnel or have the requisite funding to hire
them.
22
We face difficulties concerning
availability of our sources of information for our products.
Our
JAGNotes Report and Rumor Room products rely on information from independent
third party sources. We do not maintain written agreements with these sources to
provide this information, so we cannot guarantee that any of these sources will
continue to provide the information necessary to maintain our
products. If information from these sources is altered, curtailed or
discontinued this could adversely affect the quality or even the viability of
these products, which could decrease the demand for our JAGNotes website and
adversely impact our revenues.
We may become party to legal
proceedings relating to the dissemination of rumors and other information of
questionable reliability.
Information
posted in the Rumor Room consists of rumors and other information received from
third party sources that may have no reasonable factual basis. We realize that
rumors are inherently unreliable, and provide a cautionary note on this portion
of our site reminding subscribers that cyberfraud is prevalent and that rumors
should not be relied upon when making investment decisions. There can be no
assurance that we will be able to prevent the unlawful posting of misleading,
defamatory, fraudulent or intentionally erroneous information or material that
infringes on the intellectual property rights of others, and the law relating to
its potential liability relating to such activity is currently unsettled. The
potential imposition of liability for unlawful activities of subscribers to our
site could require us to implement measures to reduce our exposure to such
liability, which may require us, among other things, to spend substantial
resources and/or to discontinue certain service offerings. In addition, it is
possible that we could become subject to various legal proceedings alleging,
among other things, that we have intentionally disseminated or have aided and
abetted others in intentionally disseminating false or defamatory information or
material that infringes on the intellectual property rights of
others. These claims, even without merit, could cause us to expend
significant financial and managerial resources, which could adversely affect our
business operations.
Future government regulation of the
Internet may add to our operating costs.
Like many
businesses engaging in Internet-related activities, we may face unanticipated
operating costs because of the current uncertainty surrounding potential laws
and government regulation applicable to the Internet and
e-commerce. Laws and regulations may be introduced and court
decisions reached that affect the Internet or other online services, covering
issues such as user pricing, user privacy, freedom of expression, defamation,
libel, access charges, content and quality of products and services,
advertising, intellectual property rights and information
security. For example, if the government determines that our website
and the types of activities engaged in by visitors and/or subscribers to our
website should be subject to new or existing rules or regulations, our business
model may be adversely affected and our operating costs may
increase. In addition, as an Internet company it is unclear in which
jurisdictions we are actually conducting business. Our failure to
qualify to do business in a jurisdiction that requires us to do so could subject
us to fines or penalties and could result in our inability to enforce contracts
in that jurisdiction. Even if we were able to ascertain correctly in
which jurisdictions we conduct business, many of these jurisdictions have yet to
determine the application of their existing laws to Internet-related activities
or develop laws that apply to such activities.
We could be deemed to be an
investment advisor subject to federal or state regulatory
oversight.
Companies
and individuals that provide financial advice to investors in the United States
are generally required to register as an investment adviser at either the
federal or state level, and are subject to extensive regulation. We believe that
our business consists of a publishing activity for which investment adviser
registration and regulation do not apply under applicable federal or state law,
and do not believe that we are required to register as an investment adviser
with either the SEC or any of the various states. The regulatory environment in
which we operate is subject to change, however, and we could be required to
register as an investment adviser with an appropriate regulatory agency at some
point in the future. Such registration could adversely affect our
method of operation and revenues. For example, if we were ever deemed to be in
non-compliance with applicable investment adviser regulations, we could be
subject to various penalties, including administrative or judicial proceedings
that might result in censure, fine, civil penalties (including treble damages in
the case of insider trading violations), the issuance of cease-and-desist orders
or other adverse consequences.
Our business is currently dependant
on the continued public interest in investing in the stock
market.
The
volatility of the stock market in the 1990s generated unprecedented public
interest in the stock market and trading. Our success depends upon the continued
maintenance or growth of this interest. The subsequent downturn in
the stock market may have been in part responsible for an overall decrease in
subscription revenues since the end of the second fiscal quarter of 2001. Even
after the market had recovered to some extent, our revenues generally continued
to decline. A number of factors that are out of our control, such as the recent
turmoil in global stock markets, that could lead to a stagnant or depressed
stock market that would likely decrease the public’s interest in stock trading
and financial information. If this were to happen, it is likely that we would
lose a significant percentage of our then current and potential subscriber
base.
23
Most of our current and potential
competitors have greater name recognition, financial, technical and marketing
resources, as well as more extensive customer bases and industry relationships
than we do, all of which could be leveraged to gain market share to our
detriment.
Our JAG
Notes website’s primary current competitors provide financial news, commentary
and analysis on the Internet such as Yahoo Finance, Marketwatch, TheStreet.com,
Briefing.com, America Online Personal Finance, Reuters and MotleyFool.com.
Providing financial information and analysis over the Internet is an intensely
competitive business. An increasing number of web-based financial information
providers are competing for subscribers, customers, advertisers, content
providers, analysts, commentators and staff, and we continue to face competition
from traditional news and information sources including television and print. We
expect competition from both sources to intensify and increase in the future.
Many of our competitors have substantially greater financial and other resources
than we do.
We are an intensely competitive
business with low barriers to entry.
The
barriers to entry into our JAGNotes business are relatively low (i.e., it is not
difficult for new competitors to enter the market). Many blogs now
provide financial information at no cost. Much of the information we
provide to subscribers is available and we do not have any patented or otherwise
protected technologies that would preclude or inhibit competitors from entering
our markets. Our current and future competitors may develop or offer services
that have significant price, substantive, creative or other advantages over the
services we provide. If they do so and we are unable to respond
satisfactorily, our business and financial condition will likely be adversely
affected.
We may not be able to adequately
protect ourselves against security risks.
All
Internet businesses are subject to electronic and computer security risks. We
have taken steps to protect ourselves from unauthorized access to our systems
and use of our site, but we cannot guarantee that these measures will be
effective. If our security measures are ineffective, unauthorized parties could
alter, misappropriate, or otherwise disrupt our service or
information. If such unauthorized parties were able to access certain
proprietary information, of ours or our customers’; including subscribers’
credit card numbers and personal information, we would face significant
unexpected costs and a risk of material loss, either of which could adversely
affect our business.
Risks Related to Our Capital
Structure
Our shareholders may experience
significant dilution from the exercise of warrants to purchase shares of our
common stock.
In June
2006, we issued warrants to purchase 12,000,000 shares of our common stock to YA
Global Investments L.P. (“YA Global”). To date, we have issued 11,325,000 shares
of our common stock upon the exercise of these warrants by YA Global. There are
no further warrants available for exercise by YA Global since one warrant
exercise made by YA Global was done on a “cashless basis“ resulting in a
reduction of 675,000 warrant shares. In addition, as a result of our
acquisition of CardioGenics, former CardioGenics warrant holders exchanged their
warrants to purchase CardioGenics Common Shares for warrants to purchase our
Common Shares. Currently, the warrants held by such former CardioGenics warrant
holders entitle them to purchase up to 36,148,896 of our Common Shares at prices
of $0.047 per share.
Accordingly,
you may experience substantial dilution upon exercise of these warrants. In
addition, you may experience substantial dilution if the price of our Common
Shares increases to a level greater than the exercise price of these
warrants.
The resale by YA Global of its shares
of our common stock received from us in connection with the exercise of their
warrants may lower the market price of our common stock.
The
resale by YA Global of shares of our common stock that it receives from us in
exercise of their warrants will increase the number of publicly traded shares of
our stock, which could lower the market price of our common stock. Moreover, the
shares that we issue to YA Global, or other warrant holders will be available
for immediate resale, subject to the resale restrictions of Rule 144 of the
Securities Act. There are no contractual restrictions on the ability of YA
Global to offer shares issued to it pursuant to our warrants, other than the
limitation that YA Global cannot beneficially own more than 9.99% of our then
outstanding shares of common stock. If YA Global continues to resell such
shares, the market price for our shares could decrease significantly. In
addition, the mere prospect of such transactions could lower the market price
for our common stock.
24
There
are substantial risks associated with the Standby Equity Distribution Agreement
with YA Global Master SPV Ltd., which could contribute to the decline of our
stock price and have a dilutive impact on our existing stockholders
In order
to obtain needed capital, we entered into a Standby Equity Distribution
Agreement with YA Global Master SPV Ltd. (“YA Ltd.”) dated as of March 12, 2009.
The sale of shares of our common stock pursuant to the SEDA will have a dilutive
impact on our stockholders. We believe YA Ltd. intends to promptly re-sell the
shares we issue to them under the SEDA and that such re-sales could cause the
market price of our common stock to decline significantly with advances under
the SEDA. To the extent of any such decline, any subsequent advances would
require us to issue a greater number of shares of common stock to YA Ltd. in
exchange for each dollar of the advance. Under these circumstances
our existing stockholders would experience greater dilution. The sale of our
common stock under the SEDA could encourage short sales by third parties, which
could contribute to the further decline of our stock price.
Future
Issuance of Our Common Stock Could Dilute Current Stockholder or Adversely
Affect the Market.
Future
issuances of our common stock could be at values substantially below the price
paid by the current holders of our common stock. In addition, common stock could
be issued to fend off unwanted tender offers or hostile takeovers without
further stockholder approval. Sales of substantial amounts of our common stock
in the public market, or even just the prospect of such sales, could depress the
prevailing market price of our common stock and our ability to raise equity
capital in the future.
The market for our common stock is
limited.
Our
common stock is traded on the OTC Bulletin Board. Trading activity in our stock
has fluctuated and at times been limited. We cannot guarantee that a
consistently active trading market for our stock will continue, especially while
we remain on the OTC Bulletin Board.
Because our common stock currently
trades below $5.00 per share and is quoted on the OTCBB, our common stock is
considered by the SEC to be a “penny stock,” which adversely affects our
liquidity.
Our
common stock does not currently qualify for listing on any national securities
exchange, and we do not anticipate that it will qualify for such a listing in
the short-term future. If our common stock continues to be quoted on the OTC
Bulletin Board or is traded on the Pink Sheets, and if the trading price of our
common stock remains less than $5.00 per share, our common stock is considered a
“penny stock,” and trading in our common stock is subject to the requirements of
Rule 15g-9 under the Exchange Act. Under this rule, brokers or dealers who
recommend low-priced securities to persons other than established customers and
accredited investors must satisfy special sales practice requirements. The
broker or dealer must make an individualized written suitability determination
for the purchaser and receive the purchaser’s written consent prior to the
transaction. SEC regulations also require additional disclosure in connection
with any trades involving a penny stock, including the delivery, prior to any
penny stock transaction, of a disclosure schedule explaining the penny stock
market and its associated risks. These requirements could severely limit the
liquidity of such securities in the secondary market because few brokers or
dealers are likely to undertake these compliance activities. In addition to the
applicability of the penny stock rules, another risk associated with trading in
penny stocks may be large price fluctuations.
Our amended charter contains
provisions that may discourage an unaffiliated party to take us
over.
Without
further stockholder action, our Board of Directors could authorize the issuance
of additional shares of our common stock as well as preferred stock with special
voting rights by class or with more than one vote per share, to a “white knight”
in order to deter a potential buyer. This might have the effect of
preventing or discouraging an attempt by a party unable to obtain the approval
of our Board of Directors to take over or otherwise gain control of
us.
Terms of subsequent financings may
adversely impact your investment.
We may
have to raise equity, debt or preferred stock financing in the future. Your
rights and the value of your investment in our Common Shares could be reduced.
For example, if we issue secured debt securities, the holders of the debt would
have a claim against our assets that would be prior to the rights of
stockholders until the debt is paid. Interest on these debt securities would
increase costs and negatively impact operating results.
Preferred
stock could be issued in series from time to time with such designations,
rights, preferences, and limitations as needed to raise capital. The terms of
preferred stock could be more advantageous to those investors than to the
holders of our Common Shares.
25
Our
articles of incorporation do not provide stockholders the pre-emptive right to
buy shares from the company. As a result, you will not have the automatic
ability to avoid dilution in your percentage ownership of the
company.
Control
of our stock is now held by the former CardioGenics shareholders.
The prior
shareholders of CardioGenics own, directly or indirectly, approximately 85% of
our outstanding common stock. While their percentage would decline if and to the
extent new shares of our common stock are issued, you should expect these
persons to exert continuing influence over all matters requiring shareholder
approval, including the election of directors. You may have little to no
practical control over such matters.
It
is not likely that we will pay dividends on the common stock or any other class
of stock
We intend
to retain any future earnings for the operation and expansion of our business.
We do not anticipate paying cash dividends on our common stock, or any other
class of stock, in the foreseeable future. Stockholders should look solely to
appreciation in the market price of our Common Shares to obtain a return on
investment.
Our stockholders ownership of our
common stock may be in doubt due to possible naked short selling of our common
stock.
We
believe, but cannot confirm, that speculators may have engaged in a practice
commonly known as a “naked short” sale of our common stock, which means that
certain brokers may be permitting their short selling customers to sell shares
of our common stock that their customers do not own and may have failed to
borrow and therefore deliver the shares sold to the purchaser of the shares. We
have from time to time been included by Nasdaq on the Regulation SHO Threshold
Security List, which is indicative of a significant amount of naked shorting in
the stock. Because naked shorting may result in an artificial depression of our
stock price, our stockholders could lose all or part of their investment in our
common stock. As a result of this naked short selling, there may be a
substantial number of purchasers who believe they are our stockholders, but who
in fact would not be stockholders since their brokers may never have received
any shares of our common stock for their account. In addition, investors who
believe they are our stockholders may not have received a stock dividend to
which they are entitled or may have been deprived of the right to vote some or
all of their shares.
ITEM
1B. UNRESOLVED STAFF COMMENTS
Not
Applicable.
ITEM
2. PROPERTIES
Our
executive and administrative headquarters are currently located at 6295 Northam
Drive, Units 7 & 8, Mississauga, Ontario L4V 1W8 Canada. We rent this space
at a cost of CDN$6,432 per month.
The
administrative offices for our Pixaya subsidiary are currently located at 6865
SW 18th Street, Suite B-13, in Boca Raton, Florida 33433. We rent
this space at a cost of US$1,450 per month. The lease for this space
expired in June 2009 and is currently on a month-to-month basis.
The
servers for our websites are housed at separate locations as described
above. See “Item 1.—Business—Website Technical
Information.” We believe that our facilities are adequate for our
current needs and that, if our lease is not renewed on commercially reasonable
terms, we will be able to locate suitable new office space and obtain a suitable
replacement for our executive and administrative headquarters.
26
ITEM
3. LEGAL PROCEEDINGS
On April
22, 2009, CardioGenics was served with a statement of claim from a prior
contractor claiming compensation for wrongful dismissal and ancillary causes of
action including payment of monies in realization of his investment in
CardioGenics, with an aggregate claim of $514,000. The Company considers
all the claims to be without any merit, has already delivered a statement
of defence and intends to vigorously defend the action. If the matter
eventually proceeds to trial, the Company does not expect to be found liable on
any ground or for any cause of action. The statement of claim was filed in
Ontario Superior Court of Justice (Court File No. CN09-1728-00).
On June
22, 2009 CardioGenics received a letter from Flow Capital Advisors, Inc.
regarding a Non-Circumvention Agreement dated July 16, 2004 and
a Finder's Fee Agreement dated December 13, 2004 between Flow Capital
and CardioGenics. The letter states that CardioGenics
has breached these agreements insofar as the transaction
between CardioGenics and JAG Media is concerned and advising that Flow
Capital is entitled to payment of 8% of the transaction value in accordance with
the terms of the Finder's Fee Agreement. CardioGenics' lawyers subsequently
wrote to Flow Capital denying any contractual breach and explaining why
Flow Capital’s claims are without merit.
On
January 15, 2010, Flow Capital Advisors, Inc. (“Flow Capital”) filed a lawsuit
against CardioGenics, Inc, and another defendant in the United States District
Court for the Southern District of Florida, Fort Lauderdale Division (Case No.
10-CV-60066-Martinez-Brown) (“Flow Lawsuit”). The Flow Lawsuit alleges that
CardioGenics (i) breached a Finder’s Fee Agreement in connection with the
CardioGenics Acquisition; and (ii) breached a non-circumvention
agreement. Flow Capital is claiming that it is entitled to its
finder’s fee equal to eight percent (8%) of the JAG Media Holdings shares
received by CardioGenics, or the equivalent monetary value of the stock. The
Company and its counsel are currently reviewing the Flow Lawsuit and anticipate
responding to the Flow Lawsuit in the near future.
On
January 14, 2010, Flow Capital filed a lawsuit against JAG Media Holdings Inc.
in the Circuit Court of the 17th
Judicial Circuit In and For Broward County Florida (Case No.
10001713). Pursuant to this lawsuit, Flow Capital alleges that JAG
Media Holdings breached a Non-Circumvention Agreement it had entered into with
Flow Capital, dated January 1, 2004. The Company and its counsel are currently
reviewing this lawsuit and anticipate responding to it in the near
future.
On June
20, 2002, we, along with our then President and Chief Executive Officer, Gary
Valinoti, filed a complaint in the 165th District Court of Harris County, Texas
against over 150 brokerage firms, alleging, among other things, a conspiracy
among the defendants to short sell our stock. The original lawsuit
was subsequently amended on June 24, 2002 and was removed to the United States
District Court for the Southern District of Texas. The plaintiffs
subsequently filed a motion in the United States District Court for the Southern
District of Texas to have the action remanded back to the state court where it
was originally commenced. That motion was denied and the action
proceeded in the federal district court. On October 1, 2003, the
Court denied various motions to dismiss made on behalf of the
defendants. However, in its ruling, the Court indicated that all
motions to dismiss could have been granted in light of the defective pleadings
made by plaintiffs and allowed plaintiffs 20 days to file an amended complaint
to comply with certain pleading requirements of the Court. Plaintiffs
filed an amended complaint within the required period. Discovery was
stayed while the motions to dismiss were pending.
After
plaintiffs filed their third amended complaint, 78 out of the total of
approximately 150 defendants again filed a motion to dismiss the
lawsuit. On September 6, 2004, the Court entered an order granting
the moving defendants’ motion to dismiss the lawsuit, again citing various
deficiencies in the pleadings. The Court did not grant the plaintiffs
leave to replead.
The
plaintiffs and the moving defendants have since stipulated to the entry of a
final judgment dismissing the third amended complaint against the moving
defendants with prejudice. Under this stipulation, the parties agreed
on entry of final judgment to (a) waive their right to attorneys’ fees or seek
sanctions and bear their own costs and (b) not appeal the judgment.
On
December 3, 2004, we announced that our original counsel had assigned our legal
retainer agreement in connection with the lawsuit to a legal consortium
consisting of various law firms and other consultants throughout the country,
which includes our original counsel. We have met with our new
attorneys and continue to evaluate our options for recommencing an action
against certain defendants, and possibly other parties, in light of the court’s
order and/or pursuing other strategies.
ITEM
4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
In
October 2009, following the closing of the CardioGenics Acquisition, our board
of directors approved the following amendments to our articles of incorporation,
subject to the approval of such amendments by the holders of a majority of our
common shares: (a) a change of our corporate name from “JAG Media Holdings, Inc.” to
“CardioGenics Holdings
Inc.” so as to better reflect the nature of our business following the
CardioGenics Acquisition and (b) an increase in the number of our authorized
common shares from 500,000,000 to 650,000,000. Subsequent to the approval of
such proposed amendments by our board of directors, the holders of a majority of
our common shares approved by written consent the amendments to our articles of
incorporation approved by our board of directors.
27
Under
Nevada law, the written consent of the holders of a majority of our common
shares, without convening a shareholder meeting to vote on the proposals, was
sufficient to make the above-referenced changes to our articles of
incorporation. The applicable stockholders were informed of the details of the
approved amendments by an Information Statement filed with the SEC and
distributed to the applicable stockholders subsequent to the written consent of
the holders of a majority of our common shares, but prior to such amendments
taking effect. The approved amendments to the articles of incorporation were
filed with the Secretary of State of Nevada approximately ten (10) days after
the mailing of the Information Statement to the applicable
stockholders.
PART
II
ITEM
5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS
AND ISSUER PURCHASES OF SECURITIES
For the
period covered below, our common stock (other than our class B common stock) is
traded on the OTC Bulletin Board under the symbol CGNH. In October 2009, our
symbol was changed from JAGH to CGNH as a result of the CardioGenics
Acquisition. The following table based on Bloomberg L.P. reflects quarterly high
and low bid prices of our common stock from October 31, 2007 through October 31,
2009. Such prices are inter-dealer quotations without retail
mark-ups, mark-downs or commissions, and may not represent actual
transactions.
|
Fiscal Year Ending October 31,
2009
|
||||||||
|
Quarter
Ended
|
High
$
|
Low
$
|
||||||
|
October
31, 2009
|
0.78
|
0.20
|
||||||
|
July
31, 2009
|
0.38
|
0.37
|
||||||
|
April
30, 2009
|
0.17
|
0.14
|
||||||
|
January
31, 2009
|
0.23
|
0.18
|
||||||
|
October
31, 2008
|
0.41
|
0.38
|
||||||
|
Fiscal Year Ending July 31,
2008
|
||||||||
|
Quarter
Ended
|
High
$
|
Low
$
|
||||||
|
July
31, 2008
|
0.79 | 0.17 | ||||||
|
April
30, 2008
|
0.98 | 0.68 | ||||||
|
January
31, 2008
|
1.07 | 0.66 | ||||||
|
October
31, 2007
|
1.10 | 0.59 | ||||||
On
February 10, 2010, the closing bid price for our common stock was
$0.117. A public trading market for our Series 2 and Series 3 Class B
common stock has never developed.
As of
February 10, 2010, there were 218,006,202 shares of our common stock
outstanding, 380,931 shares of our Series 2 Class B common stock
outstanding and 21,500 shares of our Series 3 Class B common stock outstanding
and 3,797 stockholders of record with respect to such
shares. There was also outstanding 1 share of Series 1 Preferred
Voting Stock, par value $0.0001, representing 16 Exchangeable Shares, which are
exchangeable into 276,655,415 shares of our common stock.
In
addition, there are 1,325 additional stockholders who did not turn in their
shares of prior classes of our common stock in connection with our
recapitalizations in 2002 and 2004. These stockholders, upon
presentation of their shares, are entitled to receive shares of our common stock
in exchange. As of February 10, 2010 174,099 Series 1 Class B common
shares, 1,076,207 Class A common shares and 125,277 original
JagNotes.com Inc. common shares remained unconverted.
Dividend
Policy
We have
never paid any cash dividends on our common stock and anticipate that, for the
foreseeable future, no cash dividends will be paid on our common
stock.
28
Equity
Compensation Plans Information
See the
information provided under “Item 12.—Security Ownership of Certain Beneficial
Owners and Related Stockholder Matters—Equity Compensation Plan
Information.”
Recent
Sales of Unregistered Securities
On May
25, 2006 JAG Media issued 1,250,000 shares of its common stock to
YA Global as repayment for $250,000 of the outstanding principal amount of the
Promissory Note and a $1,900,000 10% secured convertible debenture with a
maturity of three years in consideration of the remaining $1,750,000 of the
outstanding principal amount of the Promissory Note and $150,000 in accrued and
unpaid interest. This issuance was exempt from registration under the
Securities Act, pursuant to Section 3(a)(9) thereof.
In May of
2006, JAG Media issued two secured convertible debentures to YA Global
in an aggregate principal amount of $1,250,000, as follows: (i) a $1,250,000 10%
secured convertible debenture and (ii) a $1,000,000 10% secured convertible
debenture, each with a maturity of three years. This issuance was
exempt from registration under the Securities Act, pursuant to Section 4 (2)
thereof, as YA Global is an accredited investor.
Pursuant
to the terms of the secured convertible debentures, YA Global has converted the
entire principal and accrued interest on the debentures into an aggregate of
14,651,265 shares of JAG Media’s
common stock.
On May
25, 2006, as partial consideration for YA Global’s purchase of the secured
convertible debentures, JAG Media issued five warrants to purchase an
aggregate of 12,000,000 shares of JAG Media’s common stock, as
follows. For a description of such warrants, see “Item
1.—Business—Financing Arrangements—Warrants.” This issuance was
exempt from registration under the Securities Act, pursuant to Section 4(2)
thereof, as YA Global is an accredited investor.
Pursuant
to the terms of the warrants, as amended, YA Global has exercised all of the
shares under the warrants at exercise prices ranging from $0.40-$0.05. JAG
Media received $2,370,000 in gross proceeds from the exercise of these
warrants.
29
The
Company claims an exemption from the registration requirements of the Securities
Act of 1933, as amended, for the issuance of shares to as provided above
pursuant to Section 4(2) of the Act and/or Rule 506 of Regulation D promulgated
thereunder since, among other things, the transaction does not involve a public
offering, the purchaser is an “accredited investor” and/or qualified
institutional buyers, the purchaser has access to information about the Company
and its purchase, the purchaser will take the securities for investment and not
resale, and the Company is taking appropriate measures to restrict the transfer
of the securities.
Purchases
of Equity Securities
There
were no repurchases made for any class or series of securities in a month within
the fourth quarter of the fiscal year ended October 31, 2009.
ITEM
6. SELECTED FINANCIAL DATA
We are a
smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are
not required to provide information under this item.
ITEM
7. MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF
OPERATION
This
annual report contains forward-looking statements relating to future events or
our future financial performance. In some cases, you can identify
forward-looking statements by terminology such as "may", "should", "intends",
"expects", "plans", "anticipates", "believes", "estimates", "predicts",
"potential", or "continue" or the negative of these terms or other comparable
terminology. These statements are only predictions and involve known
and unknown risks, uncertainties and other factors which may cause our or our
industry's actual results, levels of activity or performance to be materially
different from any future results, levels of activity or performance expressed
or implied by these forward-looking statements.
Although
we believe that the expectations reflected in the forward-looking statements are
reasonable, we cannot guarantee future results, levels of activity or
performance. You should not place undue reliance on these statements,
which speak only as of the date that they were made. These cautionary
statements should be considered with any written or oral forward-looking
statements that we may issue in the future. Except as required by
applicable law, including the securities laws of the United States, we do not
intend to update any of the forward-looking statements to conform these
statements to actual results, later events or circumstances or to reflect the
occurrence of unanticipated events.
In
this annual report unless otherwise specified, all dollar amounts are expressed
in United States dollars and all references to “common shares” refer to the
common shares of our capital stock.
The
management’s discussion and analysis of our financial condition and results of
operations are based upon our financial statements, which have been prepared in
accordance with accounting principles generally accepted in the United States of
America ("GAAP").
The
financial statements contained herein include the results of CardioGenics,
Inc. and its subsidiaries and JAG Media Holdings, Inc and its subsidiaries (“JAG
Media Holdings, Inc.”) (from July 31, 2009, date of acquisition) which are
collectively referred to as the “Company.”
30
JAG Media
Holdings, Inc. is a provider of Internet-based equities research and financial
information that offers its subscribers a variety of stock market research, news
and analysis, including "JAGNotes", JAG Media Holdings, Inc.'s flagship early
morning consolidated research product.
On July
31, 2009, JAG Media Holdings, Inc. completed a reverse acquisition of privately
held CardioGenics Inc. (“CardioGenics”), an Ontario, Canada Corporation. The
acquisition was effected pursuant to a Share Purchase Agreement dated May 22,
2009 by and among JAG Media Holdings, Inc., CardioGenics Inc. and CardioGenics
ExchangeCo Inc., the Company’s wholly owned subsidiary (“ExchangeCo”). In
accordance with the terms of the Share Purchase Agreement, 99% of the holders of
common shares of CardioGenics Inc. (two (2) minority shareholders of
CardioGenics holding in aggregate 173,869 common shares of CardioGenics
Inc. did not participate) surrendered their CardioGenics Common Shares to
ExchangeCo. ExchangeCo caused JAG Media Holdings, Inc. to issue to the
CardioGenics shareholders 422,183,610 shares of the Company’s common stock, par
value $0.00001 per share (the “Share Consideration”). The Share Consideration
provides the former CardioGenics shareholders with direct and/or indirect
ownership of approximately 85% of JAG Media Holdings, Inc.’s outstanding common
stock (on a fully diluted basis) as of July 31, 2009.
On
October 27, 2009 the name of the Company was changed from JAG Media Holdings,
Inc. to CardioGenics Holdings, Inc.
CardioGenics
develops technology and products targeting the immunoassay segment of the In-Vitro Diagnostic testing
market. CardioGenics has developed the QL Care Analyzer, a proprietary Point Of
Care immuno-analyzer, which will run a number of diagnostic tests
under development by CardioGenics, the first of which will be a
series of cardiovascular diagnostic tests. As part of its core proprietary
technology, CardioGenics has also developed a proprietary method for silver
coating paramagnetic microspheres (a fundamental platform component of
immunoassay equipment), which improve instrument sensitivity to light.
CardioGenics’ principal offices are located in Mississauga, Ontario,
Canada.
With the
acquisition of CardioGenics, the Company’s business will now be refocused on
developing technologies and products for the point-of-care In Vitro Diagnostics
market.
Results
of Operations for the Years Ended October 31, 2009 and October 31,
2008
The
following table sets forth the Company’s results of operations for the years
ended October 31, 2009 and October 31, 2008 and includes the results of
operations of JAG Media Holdings, Inc. from July 31, 2009 (date of acquisition)
to October 31, 2009:
|
Year Ended October 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Revenues
|
$ | 21,539 | $ | — | ||||
|
Cost
of revenues
|
136,946 | — | ||||||
|
Gross
(loss)
|
(115,407 | ) | — | |||||
|
Operating
Expenses:
|
||||||||
|
Amortization
of property and equipment
|
26,157 | 32,248 | ||||||
|
Amortization
of patent application costs
|
4,181 | — | ||||||
|
Write-off
of patent application costs
|
23,803 | 29,928 | ||||||
|
General
and administrative expenses
|
1,597,010 | 177,169 | ||||||
|
Write-off
of goodwill
|
12,780,214 | — | ||||||
|
Research
and product development, net of investment tax credits
|
1,572,337 | 24,531 | ||||||
|
Total
|
16,003,702 | 263,876 | ||||||
|
Loss
from operations
|
(16,119,109 | ) | (263,876 | ) | ||||
|
Other
(income) expense:
|
||||||||
|
Interest
expense and bank charges, net
|
571,840 | 432,005 | ||||||
|
Loss
on change in value of derivative liability
|
12,421,023 | — | ||||||
|
Loss
(gain) on foreign exchange transactions
|
(184,389 | ) | 657,340 | |||||
|
Net
loss
|
$ | (28,927,583 | ) | $ | (1,353,221 | ) | ||
31
Revenues
Cardiogenics
is a development stage company and as such has no revenues from operations other
than that derived from the ongoing operations of the acquired company, JAG Media
Holdings, Inc.
Since
July 31, 2009 (date of acquisition of JAG Media Holdings, Inc.), revenues
consist of sales of annual, semi-annual, quarterly and monthly subscriptions
relating to our JAGNotes Report product. JAGNotes Report is a daily consolidated
investment report that summarizes newly issued research, analyst opinions,
upgrades, downgrades and analyst coverage changes from various investment banks
and brokerage houses.
Cost
of Revenues
Cost of
revenues includes the cost to transmit the JAGNotes product over telephone and
fax lines, on-line service charges for our website, costs in connection with the
development and maintenance of the website and consultants costs in assisting to
produce the JAGNotes product.
Operating
expenses
General
and administrative expenses
General
and administrative expenses consist primarily of compensation to officers and
directors, occupancy costs, professional fees and other office expenses. The
increase in general and administrative expenses is attributable primarily to
common share based compensation to officers and directors of approximately
$969,000 (2008 - $0), a common share based consulting fee paid to former
officers of JAG Media Holdings, Inc. of $380,000 (2008 - $0), a financial
consulting fee of $32,000 (2008 - $0) and administrative expenses of the
acquired company of $77,000 for the last quarter.
Write-off
of goodwill
On July
31, 2009, JAG Media Holdings, Inc. completed a reverse acquisition of privately
held CardioGenics Inc. (“CardioGenics”), an Ontario, Canada Corporation. The
acquisition was effected pursuant to a Share Purchase Agreement dated May 22,
2009 by and among JAG Media Holdings, Inc., CardioGenics Inc. and CardioGenics
ExchangeCo Inc., the Company’s wholly owned subsidiary (“ExchangeCo”). The
purchase price, arrived at based on the average price of JAG Media Holdings,
Inc.’s common shares, was allocated primarily to goodwill of $12,780,214.
Subsequent to completing the purchase management has analyzed the operations of
the acquired company and determined that considerable resources would have to be
allocated to those operations to make them significantly viable. Since the
Company intends to concentrate its activities on developing technologies and
products for the point-of-care In Vitro Diagnostics market, in November 2009,
management decided to sell its Pixaya subsidiary, along with its JAGNotes and
Pixaya UK businesses. Offers were received in the range of $40,000 to $100,000.
Based on these offers, management determined that the value of the goodwill
associated therewith was impaired. A $12.8 million goodwill impairment charge
was recorded in the fourth quarter of 2009.
Research
and product development costs, net of investment tax credits
Research
and development expenses consist primarily of salaries and wages paid to
officers and employees engaged in those activities and supplies consumed
therefor. Salaries and wages increased by common share based compensation paid
to those officers and employees of approximately $1,280,000 (2008 - $0).
Scientific consulting fees amounted to $36,000 as compared to $1,500 in
2008. Investment tax credits were $160,000 in 2009 and $392,000 in
2008. The 2009 credit is lower in that the credit applies only up to the point
at which the acquisition occurred.
32
Other
expenses (income)
Interest
expense and bank charges, net
Interest
expense in 2009 includes beneficial conversion charges on the conversion of
debentures and director’s loan of $335,000 and $117,000 respectively (2008 - $0
for both). Interest expense in 2008 included a loss on extinguishment of debt of
$231,580 (2009 - $0). Interest on debentures and director’s loan was $138,000 in
2009 and $240,000 in 2008, lower in the later year due to the loans having been
repaid during the year.
Loss
on change in value of derivative liability
The loss
on change in value of the derivative liability for the year ended October 31,
2009 arises out of the conversion of warrants of CardioGenics Inc. to warrants
of the Company on completion of the reverse acquisition on July 31, 2009 and the
issuance of options and warrants to an agent for assisting in the conversion of
debentures to common shares in concert with the acquisition. The Company
determined that, based on the guidance in “Accounting for Derivative Financial
Instruments Indexed to, and Potentially Settled in a Company’s Own Stock”, the
Company was prohibited from concluding that it would have a sufficient number of
authorized and unissued shares to net-share settle any of those warrants and
options or any other warrants or options previously issued or granted to
non-employees because the conversion of our secured convertible debentures and
the related warrants could have resulted in the issuance of an indeterminable
number of common shares as they were convertible at a discount from the market
price. The Company therefore had to record the warrants and options at fair
value of $12,325,833 and $95,190 respectively.
On
September 30, 2009, the Company’s articles of incorporation were amended to
increase the total number of common shares authorized for issuance from
500,000,000 shares to 650,000,000 shares of common stock, par value $0.00001 per
share. As a result, the total number of shares of all classes of capital stock
authorized for issuance by the Company increased from 550,440,000 shares to
700,440,000 shares with a par value of $.00001 per share, of which 50,000,000
shares are authorized for issuance as preferred stock, 500,000,000 shares are
authorized for issuance as common stock, 400,000 shares are authorized for
issuance as Series 2 Class B common stock and 40,000 shares are authorized for
issuance as Series 3 Class B common stock. The increase in authorized capital
allowed management to conclude that it does have a sufficient number of
authorized and unissued shares to net-share settle any of those warrants or any
other warrants or options previously issued or granted to employees or
non-employees. As a result of this increase, the fair value of this derivative
liability was determined to be zero and therefore the re-valued amount of
$13,501,360 was credited to Additional Paid-In Capital.
Loss
(gain) on foreign exchange transactions
The
Company conducts the majority of its transactions in Canadian dollars. The
foreign exchange loss (gain) (2009-($184,389), 2008-$657,340) results from
currency movements on transactions settled during the year.
Liquidity
and Capital Resources
For the
year ended October 31, 2009 the Company generated subscription revenues of only
$21,539 (since July 31, 2009) and does not anticipate significant increases in
revenues from this source in the foreseeable future. The Company incurred a net
loss of approximately $28,900,000 and a cash flow deficiency from operating
activities of approximately $735,000 for the year ended October 31, 2009. The
Company has not yet established an ongoing source of revenues sufficient to
cover our operating costs and allow us to continue as a going
concern. The Company has funded its activities to date almost
exclusively from debt and equity financings. These matters raise
substantial doubt about the Company’s ability to continue as a going concern and
our independent auditors included an explanatory paragraph to emphasize such
doubt in their report on the audit of our financial statements.
The
Company will continue to require substantial funds to continue research and
development, including preclinical studies and clinical trials of our products,
and to commence sales and marketing efforts. The Company’s plans
include financing activities such as private placements of its common stock and
issuances of convertible debt instruments. The Company is also
actively pursuing industry collaboration activities including product licensing
and specific project financing.
The
Company believes that it will be successful in obtaining the necessary financing
to fund its operations, meet revenue projections and manage costs; however,
there are no assurances that such additional funding will be achieved and that
the Company will succeed in its future operations.
33
Off-Balance
Sheet Arrangements
The
Company is not a party to any off balance sheet arrangements.
Seasonality
The
Company does not believe that its business is materially affected by seasonal
trends or inflation. On an ongoing basis, the Company will attempt to minimize
any effect of inflation on its operating results by controlling operating costs
and whenever possible, seeking to insure that subscription rates, and other
revenues when and if they are realized reflect increases in costs due to
inflation.
Summary
of Critical Accounting Policies and Estimates
The
discussion and analysis of the Company’s financial condition and results of its
operations are based upon its consolidated financial statements, which have been
prepared in accordance with accounting principles generally accepted in the
United States of America for financial statements filed with the
SEC.
(a)
Convertible
Debentures
In
accordance with guidance in accounting for convertible securities with
beneficial conversion features or contingently adjustable conversion ratios, the
Company recognized an imbedded beneficial conversion feature present in the
convertible debentures. The Company allocated a portion of the proceeds equal to
the intrinsic value of that feature to additional paid-in
capital. The debt discount attributed to the beneficial conversion
feature is amortized over the convertible debenture's maturity period as
interest expense using the effective yield method.
In
addition, the Company recognized the value attributable to the warrants to
additional paid-in capital and a discount against the convertible debentures.
The Company valued the warrants using the Black-Scholes pricing
model. The debt discount attributed to the value of the warrants
issued is amortized over the convertible debenture’s maturity period as interest
expense using the effective yield method.
(b)
Research
and Development Costs
Expenditures
for research and development are expensed as incurred and include, among other
costs, those related to the production of prototype products, including payroll
costs. Amounts expected to be received from governments under Scientific
Research Tax Credit arrangements are offset against current
expenses. The Company recognizes revenue from restricted grants in
the period in which the Company has incurred the expenditures in compliance with
the specific restrictions.
(c)
Income
Taxes
The
Company utilizes the liability method of accounting for income taxes as set
forth in the authoritative guidance. Under the liability method, deferred taxes
are determined based on the temporary differences between the financial
statement and tax basis of assets and liabilities using tax rates expected to be
in effect during the years in which the basis differences reverse. A valuation
allowance is recorded when it is more likely than not that some of the deferred
tax assets will not be realized. As there is no certainty that the Company will
generate taxable income in the foreseeable future to utilize tax losses
accumulated to date, no provision for ultimate tax reduction has been made in
these financial statements.
On
November 1, 2007, the Company adopted the guidance issued for accounting for
uncertainty in income taxes which provides detailed guidance for the financial
statement recognition, measurement and disclosure of uncertain tax positions
recognized in an enterprise’s financial statements. Income tax positions must
meet a more-likely-than-non recognition threshold at the effective date to be
recognized upon the adoption of the guidance and in subsequent periods. The
Company recognizes potential accrued interest and penalties related to
unrecognized tax benefits within operations as income tax expense. Upon
adoption, there were no adjustments required.
34
(d) Stock-Based
Compensation
The
Company follows the authoritative guidance for stock-based compensation which
requires that new, modified and unvested share-based payment transactions with
employees, such as grants of stock options and restricted stock, be recognized
in the financial statements based on their fair value at the grant date and
recognized as compensation expense over their vesting periods. The Company has
also considered the related guidance of the Security and Exchange Commission
(“SEC”). The Company estimates the fair value of stock options and shares issued
as compensation to employees and directors as of the date of grant using the
Black-Scholes pricing model and restricted stock based on the per share
value. The Company also follows the guidance for equity instruments
that are issued to other than employees for acquiring, or in conjunction with
selling, goods or services for equity instruments issued to consultants
which provides guidance on transactions in which (1) the fair value
of the equity instruments is more reliably measurable than the fair value of the
goods or services received and (2) the counterparty receives shares of stock,
stock options, or other equity instruments in settlement of the entire
transaction or, if the transaction is part cash and part equity instruments, in
settlement of the portion of the transaction for which the equity instruments
constitute the consideration. Options issued with a nominal exercise
price in exchange for services rendered were measured at the fair value of the
underlying services rendered on the date of grant. The expense was recorded to
the statement of operations with a corresponding increase in share capital with
no additional increase in the number of shares as they were legally not yet
exercised.
(e)
Foreign
Currency Translation
The
Company maintains its accounting records for its Canadian operations in Canadian
dollars. Transactions in United States dollars (“USD”) are translated into
Canadian dollars at rates in effect at the date of the transaction and gains or
losses on such transactions are recorded at the time of settlement in the
statement of operations.
The
Company’s reporting currency is the United States Dollar. Foreign
denominated assets and liabilities of the Company are translated into USD at the
prevailing exchange rates in effect at the end of the reporting period, the
historical rate for shareholders’ equity and a weighted average of exchange rate
in effect during the period for expenses, gains and
losses. Adjustments that arise from translation into the reporting
currency are recorded in the accumulated other comprehensive income (loss)
component of stockholders’ equity (deficit).
(f)
Goodwill
Goodwill
and other intangible assets with indefinite lives are tested for impairment
annually, as required by pronouncement, “Goodwill and Other Intangible
Assets”. First, the fair value of the reporting unit is compared to
its carrying value. If the fair value is less than the carrying value, a second
step is performed. In the second step, an implied goodwill value is determined
by deducting the fair value of all tangible and intangible net assets of the
reporting unit from the fair value of the reporting unit. If the implied fair
value of the goodwill as calculated is less than the carrying amount of the
goodwill, an impairment charge is recorded for the difference.
Recent
Accounting Pronouncements
In
September 2006, “Fair Value Measurements” pronouncement was issued which defines
fair value, establishes a framework for measuring fair value in accordance with
accounting principles generally accepted in the United States, and expands
disclosures about fair value measurements. This pronouncement is effective for
financial statements issued for the Company’s fiscal year beginning November 1,
2008, with earlier application encouraged. Any amounts recognized upon adoption
as cumulative effect adjustments will be recorded to the opening balance of
retained earnings in the year of adoption. On February 12, 2008, the effective
date for non-financial assets and liabilities was delayed to fiscal years
beginning on November 15, 2008; however, the effective date for financial assets
remains intact. The Company has adopted the fair value measurements
pronouncement for current assets and liabilities in these financial statements
which has not had a material effect on its consolidated financial
statements.
In
December 2007, “Business Combinations” pronouncement was issued, which
establishes principles and requirements
for how an acquirer recognizes and measures in its financial statements the
identifiable assets acquired, the liabilities assumed, and any non-controlling
interest in an acquiree, including the recognition and measurement of goodwill
acquired in a business combination. This Pronouncement is effective
as of the beginning of the first fiscal year beginning on or after December 15,
2008. Earlier adoption is prohibited. The Company does not
expect this pronouncement to have a material effect on its consolidated
financial statements.
35
In
December 2007, “Non-controlling Interest in Consolidated Financial Statements”,
was pronounced, which will change the accounting and reporting for minority
interests, which will be re-characterized as non-controlling interests and
classified as a component of equity within the consolidated balance
sheets. This pronouncement is effective as of the beginning of the
first fiscal year beginning on or after December 15, 2008. Earlier
adoption is prohibited. The Company believes that the adoption will
have minimal impact on its consolidated financial position, results of
operations or cash flows.
In May
2008, “Accounting for Convertible Debt Instruments That May Be Settled in Cash
upon Conversion (Including Partial Cash Settlements)” was pronounced. This
requires a portion of this type of convertible debt to be recorded as equity and
to record interest expense on the debt portion at a rate that would have been
charged on nonconvertible debt with the same terms. This pronouncement takes
effect in the first quarter of fiscal years beginning after December 15, 2008
and will be applied retrospectively for all periods presented. It will be
effective for the Company on November 1, 2009. The Company does not expect this
pronouncement to have a material effect on its consolidated financial
statements.
In June
2008, “Determining Whether Instruments Granted in Share-Based Payment
Transactions Are Participating Securities” was pronounced. Securities
participating in
dividends with common stock according to a formula are participating securities.
This pronouncement determined that unvested shares of restricted stock and stock
units with no forfeitable rights to dividends are participating securities.
Participating securities require the “two-class” method to be used to calculate
basic earnings per share. This method lowers basic earnings per common share.
This pronouncement takes effect in the first quarter of fiscal years beginning
after December 15, 2008 and will be applied retrospectively for all periods
presented. It will be effective for the Company on November 1, 2009. The Company
does not expect this pronouncement to have a material effect on its consolidated
financial statements.
In June
2009, the Financial Accounting Standards Board (“FASB”) issued guidance which
stipulates the FASB Accounting Standards Codification is the source of
authoritative U.S. Generally Accepted Accounting Principles (“GAAP”) recognized
by the FASB to be applied by non-governmental entities, and supersedes all
existing non-SEC standards. This guidance is effective for the Company’s fiscal
year beginning November 1, 2009. The Company does not expect this pronouncement
to have a material effect on its consolidated financial statements.
In
October 2009, the FASB issued guidance related to revenue recognition for
arrangements with multiple deliverables. This guidance eliminates the residual
method of allocation and requires the relative selling price method when
allocating deliverables of a multiple deliverable revenue arrangement. The
determination of the selling price for each deliverable requires the use of a
hierarchy designed to maximize the use of available objective evidence
including, vendor specific objective evidence, third party evidence of selling
price, or estimated selling price. The guidance is effective prospectively for
revenue arrangements entered into or materially modified in fiscal years
beginning on or after June 15, 2010, and must be adopted in the same period
using the same transition method. If adoption is elected in a period other than
the beginning of a fiscal year, the amendments in these standards must be
applied retrospectively to the beginning of the fiscal year. Full retrospective
application of these amendments to prior fiscal years is optional. Early
adoption of these standards may be elected. The Company is currently evaluating
the impact of this new accounting standard on the consolidated financial
statements.
ITEM
7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
We are a
smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are
not required to provide information under this item.
ITEM
8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The
financial statements and supplementary data required in this item are set forth
beginning on Page F-1 of this Annual Report on Form 10-K.
36
ITEM
9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
On July
31, 2009, JAG Media Holdings, Inc. completed a reverse acquisition of privately
held CardioGenics Inc. (“CardioGenics”), an Ontario, Canada Corporation. The
auditors of CardioGenics were BDO Dunwoody LLP. The auditors of JAG Media
Holdings, Inc. were J.H. Cohn LLP.
Given the
long history of J.H. Cohn LLP as auditors of JAG Media Holdings, Inc., the
Company elected to appoint J.H. Cohn LLP as auditors of the successor company,
herein referred to as the Company and BDO Dunwoody LLP was terminated as
auditors.
The
reports of BDO Dunwoody LLP on CardioGenics’ financial statements for the fiscal
years ended October 31, 2008 and 2007 did not contain an adverse opinion or a
disclaimer of opinion and were not qualified or modified as to uncertainty,
audit scope, or accounting principles.
During
our fiscal years ended October 31, 2008 and 2007 and the subsequent interim
period through August 31, 2009, the date on which the directors approved the
engagement of J.H. Cohn LLP and BDO Dunwoody LLP ceased being our auditors,
there were no disagreements between us and BDO Dunwoody LLP on any matter of
accounting principles or practices, financial statement disclosure, or auditing
scope or procedure, which disagreements, if not resolved to the satisfaction of
BDO Dunwoody LLP, would have caused BDO Dunwoody LLP to make reference to the
subject matter of the disagreements in connection with its audit reports on our
financial statements. During our past fiscal year ended October 31, 2009 BDO
Dunwoody LLP did not advise us of any of the matters specified in Item
304(a)(1)(v) of Regulation S-K.
ITEM
9A(T). CONTROLS AND PROCEDURES
Evaluation
of Disclosure Controls and Procedures
Our
management, consisting of our Chief Executive Officer and Chief Financial
Officer, has evaluated the effectiveness of our disclosure controls and
procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the
Exchange Act, in connection with the preparation of this Annual Report on Form
10-K, as of October 31, 2009.
Based on
the review described above, our Chief Executive Officer and Chief Financial
Officer determined that our disclosure controls and procedures were not
effective as of the end of the period covered by this report.
Management’s
Report on Internal Control Over Financial Reporting
Management
is responsible for establishing and maintaining adequate internal control over
financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the
Exchange Act) to provide reasonable assurance regarding the reliability of our
financial reporting and preparation of financial statement for external purposes
in accordance with U.S. generally accepted accounting principles. A control
system, no matter how well designed and operated, can provide only reasonable
assurance of achieving the desired control objectives. Because of the inherent
limitations in all control systems, internal controls over financial reporting
may not prevent or detect misstatements. The design and operation of a control
system must also reflect that there are resource constraints and management is
necessarily required to apply its judgment in evaluating the cost-benefit
relationship of possible controls.
Our
management concluded that during the period covered by this report our internal
controls over financial reporting were not effective. Management has identified
the following material weaknesses in our internal controls over financial
reporting:
|
|
•
|
lack
of documented policies and
procedures;
|
|
|
•
|
lack
of resources to account for complex and unusual transactions;
and
|
|
|
•
|
there
is no effective separation of duties, which includes monitoring controls,
between the members of management.
|
Management
is currently evaluating what steps can be taken in order to address these
material weaknesses.
37
This
Annual Report on Form 10-K does not include an attestation report of our
registered public accounting firm regarding internal controls over financial
reporting. Management’s report was not subject to attestation by our registered
public accounting firm pursuant to temporary rules of the SEC that permit us to
provide only a management’s report.
Changes
in Internal Controls Over Financial Reporting
There
were no significant changes (including corrective actions with regard to
significant deficiencies or material weaknesses) in our internal controls over
financial reporting that occurred during the quarter ended October 31, 2009,
that have materially affected, or are reasonably likely to materially affect,
our internal control over financial reporting.
ITEM
9B. OTHER INFORMATION
There are
no items that required disclosure in a Form 8-K during the fourth quarter of the
year covered by this Form 10-K that were not reported by the
Company.
PART
III
ITEM
10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE
The
following table sets forth the name, age and position of each of the members of
our board of directors, executive officers, and certain significant employees as
of the fiscal year ending October 31, 2009.
Board
of Directors and Executive Officers
|
Name
|
Age
|
Position
|
||
|
Yahia
Gawad
|
51
|
Director
& Chief Executive Officer
|
||
|
Chandra
Panchal
|
60
|
Director
|
||
|
Alexander
D.G. Reid
|
71
|
Director
|
||
|
J
Neil Tabatznik
|
59
|
Director/Acting
Chairman
|
||
|
Linda
J. Sterling
|
48
|
Director
& Secretary
|
||
|
James
Essex
|
61
|
Chief
Financial Officer
|
There are
no family relationships among the directors and executive officers. All
directors are elected to hold office until the next annual meeting of
stockholders following election and until their successors are duly elected and
qualified. Executive officers are appointed by the Board of Directors and serve
at the discretion of the Board.
We know
of no pending proceedings to which any director, member of senior management, or
affiliate is either a party adverse to us, or our subsidiaries, or has a
material interest adverse to us or our subsidiaries.
None of
our executive officers or directors have been involved in any bankruptcy
proceedings within the last five years, been convicted in or has pending any
criminal proceedings, been subject to any order, judgment or decree enjoining,
barring, suspending or otherwise limiting involvement in any type of business,
securities or banking activity or been found to have violated any federal, state
or provincial securities or commodities law.
38
Yahia Gawad, MB,
Ch.B., MD, MSc, (age 51, Director & Chief Executive Officer). Dr. Gawad is a
Physician/Scientist with primary training in Cardiology, Biochemistry and
Immunology. He received his medical education and post-graduate
training at the University of Alexandria and the University of Toronto. Dr.
Gawad's academic and commercial experience and expertise include many years of
designing and managing cardiovascular disease research and product
development.
Dr. Gawad
was a co-founder of a division of Nanogen (NGEN) (formerly Syn X and Skye
Pharmatech) where he held the position of Vice-President, Medical Affairs. Prior
to that, he was Director of Clinical Research and Development at Spectral
Diagnostics Inc. (now Nanogen).
For the
past 16 years, he has been working extensively on cardiac diagnostic test
products. He has prepared, submitted and obtained FDA regulatory
approvals for several cardiac test products currently being marketed (including
Cardiac Status Troponin I®, Myoglobin® and Myoglobin/CK-MB®, registered
trademarks of Spectral Diagnostics Inc.). Through his expertise and
contributions to an international committee, a new cardiac test, Troponin I, is
now in routine clinical use.
In
addition, Dr. Gawad has researched, developed and published several other tests.
Dr. Gawad has received several awards and scholarships and was a member of both
the Clinical Committee of the American Heart Association and the POC division of
the American Association for Clinical Chemistry. He has served as a reviewer for
the editorial board of the American Journal of Cardiology (1999-2003). Dr. Gawad
published extensively and presented his research and clinical findings at
national and international symposia.
Neil Tabatznik
(age 59, Director). Mr. Tabatznik is the
Chairman, CEO of Arrow Pharmaceuticals Inc. Arrow Pharmaceuticals is part of a
global generic drug company established in 2000, and has seen rapid growth from
$0 to $700 million in 8 years. The Arrow Group has sales operations in 5
continents and employs more than 1000 people worldwide. Prior to Arrow
Pharmaceuticals, Mr. Tabatznik was the Chairman, CEO of Genpharm Inc.
(1993-2000), which was acquired by MerckKGaA in 1994 and is now a part of Mylan
Inc. the world's third largest generic and specialty pharmaceutical company. He
was a Barrister-at-Law in London and was called to the Bar of England and Wales
in 1978. He has extensive expertise in pharmaceutical manufacturing and
negotiations of agreements with multinational companies.
Dr. Chandra
Panchal, (age 60, Director). Dr. Panchal is the co-founder of Ambrilia
Biopharma Inc. and was a Senior Executive of that company since inception, until
February 2008. Ambrilia Biopharma is a biopharmaceutical company specializing in
the research, discovery and development of cancer and infectious disease
treatments and diagnostics. Dr. Panchal holds a PhD in Biochemical
Engineering and has been managing the scientific affairs of Ambrilia and its
predecessor, Procyon Biopharma Inc., since inception in 1986. Under
his tenure, Ambrilia has evolved into a TSX listed biotechnology company with
several products in development and alliance agreements with multinational drug
companies. He also sits on the Board of Chemaphor (TSX.V: CFR), Canadian
Oil Remediation and Recovery Enterprises (TSX: CORRE), Axcelon Biopolymers
Corp., Rodocanachi and MaRS Innovation.
Alexander D.G.
Reid (age 71, Director). Mr. Reid has been in the
financial community with experience in public and private companies for over 30
years. He has held numerous positions and board memberships in various financial
and non-financial corporations. For many years, Mr. Reid was the author of the
market business column in the Financial Post. Through his writing, various
business models have been analysed and critiqued. He has been
involved with the Company as a shareholder since 1999;
Linda J.
Sterling, F.Inst.L.C.O. (age
48, Director & Secretary). Ms. Sterling has been in
the legal community in the capacity as a Law Clerk with both Stikeman Elliott
LLP and Davies Ward Phillips & Vineberg LLP since 1999. She developed
expertise with both public and private company legal compliance and has been
responsible for CardioGenics' compliance and maintenance of corporate governance
since 2001. She is currently licensed as a Legal Executive (F.Inst.L.C.O.), with
the Institute of Law Clerks of Ontario, of which she is a member. She has held
the position of CEO and director of Sterling Studios since 1989.
James A. Essex,
CA,
MBA (age 61, Chief Financial Officer) Mr. Essex has been with
CardioGenics since 1999. He founded J. Hunter & Associates Inc. in 1990, a
private financial consulting firm. Previously, he was a co-owner, President and
COO of Calais Investigations, Inc., a private company (from 1993 to 1998), a
Vice President of Confederation Trust (1989) and a Vice President of Chemical
Bank of Canada (now JP Morgan Chase Bank of Canada) from 1977 through
1987.
39
Board
Committees
Our Board
of Directors does not have standing audit, nominating or compensation
committees. Instead, the functions that might be delegated to such committees
are carried out by our entire Board of Directors, to the extent required.
Our Board of Directors anticipates forming one or more of such committees during
our 2010 fiscal year.
Nomination
of Directors
There
have been no material changes to the procedures by which our security holders
may recommend nominees to our Board of Directors.
Section
16(a) Beneficial Ownership Reporting Compliance
Under the
securities laws of the United States, our directors, executive officers and any
person holding more than 10% of our common stock are required to file initial
forms of ownership of our common stock and reports of changes in that ownership
at the SEC. Specific due dates for these forms have been established,
and we are required to disclose in this report any failure to file by these
dates.
Based
solely on our review of the copies of such forms received by it with respect to
fiscal year 2008, or written representations from certain reporting persons, to
the best of our knowledge, all reports were filed on a timely
basis.
Code
of Ethics
We have
adopted a Code of Ethics (our “Code of Ethics”) that applies
to our Chief Executive Officer and Chief Financial Officer. We will
provide to any person without charge, upon request, a copy of our Code of Ethics
by sending such request to the attention: Yahia Gawad, Chief
Executive Officer, CardioGenics Holdings Inc., 6295 Northam Drive, Unit 8,
Mississauga, Ontario L4V 1W8. The Company will promptly disclose any
amendments or waivers to our Code of Ethics on Form 8-K.
ITEM
11. EXECUTIVE COMPENSATION
As a
“smaller reporting company,” CardioGenics has elected to follow scaled
disclosure requirements for smaller reporting companies with respect to Part III, Item 11 – Executive
Compensation. Under the scaled disclosure obligations, CardioGenics is
not required to provide CompensationDiscussion and Analysis
and certain other tabular and narrative disclosures relating to executive
compensation. Nor is CardioGenics required to quantify payments due to the named
executives upon termination of employment. Management believes that the scaled
disclosure for the Company’s executive compensation policy and practices is
appropriate because CardioGenics is small for a publicly-traded company, has
only three named executives and has a relatively simple compensation policy and
structure that has not changed in the last fiscal year.
Summary
Compensation Table
The
following table provides information concerning compensation of CardioGenics’
named executives for CardioGenics’ last two completed fiscal years ending
October 31, 2008 and 2009.
|
Name and
|
Salary
|
Stock Awards
|
Total
|
||||||||||
|
Principal Position
|
Year
|
($)
|
($)
|
($)
|
|||||||||
|
Dr.
Yahia Gawad
|
2009
|
85,426 | (1) | 927,235 | (2) | 1,012,661 | |||||||
|
Chief
Executive Officer
|
2008
|
116,891 | (1) | 116,891 | |||||||||
|
James
A. Essex
|
2009
|
162,936 | (2) | 162,936 | |||||||||
|
Chief
Financial Officer
|
2008
|
15,585 | (1) | 15,585 | |||||||||
|
Linda
J. Sterling
|
2009
|
294,008 | (2) | 294,008 | |||||||||
| Corporate Secretary | |||||||||||||
|
(1)
|
Cash
compensation is stated in the table in U.S. dollars. To the extent any
cash compensation was paid in Canadian dollars, it has been converted into
U.S. dollars based on the average Canadian/U.S. dollar exchange rate for
the years ended October 31, 2009 and October 31, 2008.
|
40
|
(2)
|
This
amount represents the dollar amount recognized for financial statement
reporting purposes with respect to the fiscal year ended October 31, 2009
for stock awards granted in May and July 2009, a portion of which was in
respect of fiscal years 2001 through 2008 and was immediately vested. The
fair value is calculated using the price at which CardioGenics was selling
stock through private placements around the date of grant. This amount
reflects our accounting expense for these awards, and does not correspond
to the actual value that will be recognized by the named
executives.
|
Other
Benefit Plans
The
Company has no defined benefit or actuarial pension plans.
Employment
Agreements
We
currently do not have written employment agreements with any of our current
officers or executive personnel, except for Dr. Yahia Gawad who has a 3 year
employment agreement with CardioGenics Holdings Inc. with an annual salary of
$150,000, heath and dental insurance coverage on terms not less favorable than
the health insurance coverage to be offered by the Company to its employees,
performance bonuses in the form of cash and stock options to be proposed to the
Board of Directors on an annual basis, non-compete agreement for 24 months after
effective takeover and 18 months full salary severance pay and benefit for
firing without cause. Further, for each calendar year of the Term he will be
entitled to five (5) weeks paid vacation. Also, he will be eligible for Stock
Option incentives to the executives as approved by the Board of
Directors.
With
respect to our former directors and executives, Messrs. Thomas J. Mazzarisi and
Stephen J. Schoepfer, they each received an annual base salary of $150,000
pursuant to their amended and restated employment agreements.
Pursuant
to these employment agreements, Messrs. Mazzarisi and Schoepfer were also
entitled to the same medical and other benefits, including health and life
insurance coverage, as are provided to our other employees. The
agreements also provided that in the event the employment of Messrs. Mazzarisi
and Schoepfer are terminated without cause or such executive resigns
for good reason as defined in the employment agreements, they shall be entitled
to receive (i) continued medical and life insurance coverage for a period equal
to the greater of one year or the number of years and fractions thereof between
the date of such termination and the end of the term (the “Severance Period”), (ii) a
lump sum cash payment equal to the executive’s highest rate of annual salary in
effect during the term multiplied by the Severance Period, (iii) a lump sum cash
payment equal to the number of accrued and unused vacation days calculated at
the executive’s then current salary rate and (iv) accelerated vesting of all of
the executive’s outstanding stock options. Such cash payments are
required to be made within ten days of termination of employment, and shall not
be subject to offset for amounts earned by the executive in respect of any
subsequent employment, nor is the executive required to seek any such subsequent
employment.
The
employment agreements further provide that, immediately prior to a “change in
control” (as defined in our 1999 Long-Term Incentive Plan), Messrs. Mazzarisi
and Schoepfer will each be granted an option to acquire 1,000,000 shares of our
common stock (subject to equitable adjustments for stock splits, etc.) at an
exercise price equal to the fair market value of the average closing bid price
for shares of our common stock for the 30 days prior to such change in control,
which option shall be fully vested and immediately exercisable in full and
expire on a date which is the earlier of ten years from such change in control
and three years after termination of employment. Generally, under our
1999 Long-Term Incentive Plan a “change in control” shall be deemed to have
occurred (i) if there is an acquisition of 30% or more of our then outstanding
shares of common stock, (ii) Messrs. Mazzarisi and Schoepfer cease for any
reason to constitute at least a majority of the members of our Board or
Directors, or (iii) a merger, consolidation, recapitalization, reorganization,
sale or disposition of all or a substantial portion of our assets, or similar
transaction shall have occurred. However, a change in control shall
not be deemed to have occurred if consummation of such a transaction would
result in at least 70% of the total voting power represented by our voting
securities outstanding immediately after such transaction being beneficially
owned by at least 75% of the holders of our outstanding voting securities
immediately prior to the transaction, with the voting power of each such
continuing holder relative to other such continuing holders not substantially
altered in the transaction.
As the
CardioGenics Acquisition resulted in a “change in control” as described above,
Messers. Mazzarisi and Schoepfer were each granted upon their resignation at the
closing an option to acquire 1,000,000 shares of our common stock at an exercise
price of $0.34 per share, in accordance with the terms of their respective
employment agreements.
41
Option
Grants in Fiscal Year 2008
Pursuant
to our 1999 Long-Term Incentive Plan, we granted options to purchase 2,000,000
to non-employees, with a weighted average exercise price of $0.34.
The
following table sets forth information regarding options to acquire shares of
our common stock outstanding under our 1999 Long-Term Incentive Plan held by our
named executive officers as of October 31, 2009
Outstanding
Equity Awards at October 31, 2009 Fiscal Year End
|
Name
|
Number of
securities
underlying
unexercised
options
exercisable
|
Number of
securities
underlying
unexercised
options
unexercisable
|
Option exercise or
base
price per
($/Share)
|
Option
expiration
date
|
|||||||||
|
Thomas
J. Mazzarisi
|
500,000 | 0 | 0.02 |
August
31, 2011
|
|||||||||
| 1,000,000 | 0 | 0.34 |
August
1, 2019
|
||||||||||
|
Stephen
J. Schoepfer
|
250,000 | 0 | 0.02 |
August
31, 2011
|
|||||||||
| 1,000,000 | 0 | 0.34 |
August
1, 2019
|
||||||||||
Director
Compensation
Non-Employee
Directors' Compensation
In fiscal
2009 our policy for compensation of non-employee directors was as
follows:
|
|
1.
|
Non-employee
directors do not receive an annual cash base retainer.
|
|
|
2.
|
At
the discretion of the full Board of Directors, nonemployee directors may
receive shares of the Company’s common stock. The number and terms of such
shares is within the discretion of the full Board of
Directors.
|
|
|
3.
|
Directors
who are officers or employees of CardioGenics do not receive separate
consideration for their service on the Board of
Directors.
|
Fiscal
Year 2009 Director Compensation Table
|
Stock Award
|
Stock Award
|
|||||||||||
|
As Director
|
(Other) (1)
|
Total (2)
|
||||||||||
|
Name
|
$
|
$
|
$
|
|||||||||
|
Neil
Tabatznik
|
22,500 | 179,830 | 202,330 | |||||||||
|
Dr.
Chandra Panchal
|
22,500 | 22,500 | ||||||||||
|
Alexander
D. G. Reid
|
22,500 | 22,500 | ||||||||||
|
(1)
|
Issued
pursuant to debenture financing of January
2009.
|
|
(2)
|
As
of October 31, 2009, the aggregate number of shares underlying stock
awards granted to each non-employee director was as follows: Mr. Tabatznik
(5,616,477) Dr. Panchal (523,925) and Mr. Reid
(523,925).
|
42
Indemnification
of Officers and Directors
Our
amended and restated Articles of Incorporation provide that we shall indemnify
our officers, directors, employees and agents to the full extent permitted by
Nevada law. Our Bylaws include provisions to indemnify our officers
and directors and other persons against expenses (including judgments, fines and
amounts paid for settlement) incurred in connection with actions or proceedings
brought against them by reason of their serving or having served as officers,
directors or in other capacities. We do not, however, indemnify them
in actions in which it is determined that they have not acted in good faith or
have acted unlawfully or not in our best interest. In the case of an
action brought by or in the right of us, we shall indemnify them only to the
extent of expenses actually and reasonably incurred by them in connection with
the defense or settlement of these actions and we shall not indemnify them in
connection with any matter as to which they have been found to be liable to us,
unless the deciding court determines that, notwithstanding such liability, that
person is fairly entitled to indemnity in light of all the relevant
circumstances.
We do not
currently maintain director’s and officer’s liability insurance but we may do so
in the future.
Insofar
as indemnification for liabilities arising under the Securities Act may be
permitted to our directors and officers pursuant to the foregoing provisions, or
otherwise, we have been advised that in the opinion of the SEC such
indemnification is against public policy as expressed in the Securities Act and
is, therefore, unenforceable.
ITEM
12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND RELATED STOCKHOLDER
MATTERS
The
following table sets forth information regarding the beneficial ownership of our
common stock as of January 22, 2009 (except as otherwise indicated) by (i) each
person known by us to be the beneficial owner of more than 5% of our common
stock, (ii) each director and nominee to be a director, (iii) each named
executive officer and (iv) all directors and executive officers as a
group. Except as otherwise indicated below, each of the persons named
in the table has sole voting and investment power with respect to the shares set
forth opposite such person’s name. Unless otherwise indicated the
address of each person listed in this table is c/o CardioGenics Holdings Inc,
6295 Northam Drive, Unit 8, Mississauga, Ontario, Canada L4V 1W8.
|
Name & Address of Beneficial Owner
|
Number of Shares
Beneficially Owned
|
Percentage of
Class** |
||||||
|
Yahia
Gawad
|
181,446,523 | 36.78 | % | |||||
|
Chandra
Panchal
|
1,257,420 | * | ||||||
|
Alexander
D.G. Reid
|
5,231,956 | 1.06 | % | |||||
|
J.
Neil Tabatznik
|
18,825,337 | (1) | 3.80 | % | ||||
|
Linda
J. Sterling
|
15,016,172 | 3.02 | % | |||||
|
James
Essex
|
3,981,830 | * | ||||||
|
Thomas
J. Mazzarisi
|
1,743,498
|
(2) | * | |||||
|
Stephen
J. Schoepfer
|
1,530,500
|
(3) | * | |||||
|
All
executive officers and directors as a group (8 persons)
|
229,063,370
|
46.00 | % | |||||
* Less
than one percent
** Based
on 493,289,034 shares of common stock issued and
outstanding
43
(1) Includes
1,571,775 shares of common stock issuable upon exercise of a
warrant.
(2) Includes
1,500,000 shares of common stock issuable upon the exercise of stock
options.
(3) Includes
1,250,000 shares of common stock issuable upon the exercise of stock
options.
Equity
Compensation Plan Information
The
following table summarizes the shares of our common stock authorized for
issuance under our equity compensation plans as of October 31,
2009.
|
Number of
securities
to be issued upon
exercise of
outstanding options,
warrants and rights
|
Weighted average
exercise price of
outstanding
options, warrants
and rights
|
Number of
securities remaining
available for future
issuance under
equity compensation
plans (excluding
securities reflected
in column (a))
|
||||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity
compensation plans approved by security holders
|
Not applicable
|
Not applicable
|
Not applicable
|
|||||||||
|
Equity
Compensation Plans not approved by security holders
|
2,750,000 | $ | _____ | 3,250,000 | (1) | |||||||
|
TOTAL
|
2,750,000 | — | 3,250,000 | |||||||||
|
(1)
|
The
maximum number of shares that may be subject to outstanding awards under
our 1999 Long-Term Incentive Plan is 6,000,000 shares of Common
Stock. Because this limitation applies only to outstanding
awards under the plan, as the outstanding options included in column (a)
are either exercised, forfeited or expire pursuant to their terms, the
number of shares remaining available for future issuance in column (c)
shall be increased by the number of shares subject to such option so
exercised, forfeited or expired.
|
Our 1999
Long-Term Incentive Plan provides our directors, officers, employees and
consultants with the opportunity to participate in our ownership. Our
Board of Directors acts as the committee under the plan which administers the
plan, addressing participation, the awards offered and any applicable conditions
of exercise. In making these determinations, our Board of Directors
will generally consider the participant’s position and record of service to
us. The Board of Directors may issue options, stock appreciation
rights, restricted stock, deferred stock, bonus stock, awards in lieu of cash
obligations, dividend equivalents and other stock based awards, all subject to
terms and conditions to be set by the Board of Directors. The plan
also contains standard provisions dealing with matters such as adjustment of the
number of shares subject to options and covered by the plan in addition to
amendment and termination of the plan.
ITEM
13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
As a
smaller reporting company, we are required to follow the scaled disclosure
requirements with respect to this Part III, Item 13 – Certain
Relationships and Related Transactions, and Director Independence . The
disclosures related to review of related person transactions are not applicable
to smaller reporting companies.
Certain
Relationships and Related Transactions
During
the years ended October 31, 2009 and October 31, 2008, the Company utilized
advances from Dr. Yahia
Gawad totaling
approximately $885,000 bearing interest at 10% per annum. On July 31, 2009, the
advances plus interest of $108,613 were converted to common shares at a price of
$.037 per share for the advances and $.05 for the interest.
Director
Independence
The Board
of Directors currently consists of five members, three of whom are “independent”
as defined under applicable rules of the SEC and The NASDAQ Stock Market LLC.
The three independent members of the Board of Directors are Neil Tabatznik, Dr.
Chandra Panchal and Alexander D. G. Reid.
For a
director to be considered independent, the Board must determine that the
director has no relationship which, in the opinion of the Board, would interfere
with the exercise of independent judgment in carrying out the responsibilities
of a director.
44
We do not
have a standing audit, nominating or compensation committee made up of
independent directors. In light of our current limited revenues and
operations, the Board of Directors does not believe it would be cost effective
at this time to establish such committees, although it does intend to re-address
these possibilities during our current fiscal year.
ITEM
14. PRINCIPAL ACCOUNTING FEES AND SERVICES
J.H. Cohn
LLP has served as our independent auditors since August 31, 2009. The
appointment of J.H. Cohn LLP as our independent public accountants was
unanimously approved by the Board of Directors. J.H. Cohn LLP is the successor
to our former independent auditors, BDO Dunwoody LLP (“BDO”).
BDO
served as our independent auditors from December 1, 2007 until August 31,
2009.
The
following table sets forth the aggregate fees paid by CardioGenics for the
fiscal years ended October 31, 2009 and 2008 to our independent
auditors:
|
Fiscal Year
|
Fiscal Year
|
|||||||
|
Ended
|
Ended
|
|||||||
|
October
|
October
|
|||||||
|
2009
|
2008
|
|||||||
|
Audit
fees
|
$ | 60,000 | (1) | $ | 169,568 |
(2)
|
||
|
Audit
related fees
|
$ | 36,616 |
(3)
|
$ | 0 | |||
|
Tax
fees (4)
|
$ | 0 | $ | 0 | ||||
|
All
other fees
|
$ | 0 | $ | 0 | ||||
|
(1)
|
Represents
estimated audit fees for the fiscal year ended October 31,
2009.
|
|
(2)
|
Represents
charges of BDO Dunwoody LLP, CardioGenics’ auditors for fiscal year ended
October 31, 2008.
|
|
(3)
|
Represents
charges of J.H. Cohn LLP, CardioGenics’ auditor in fiscal year ended
October 31, 2009 for review of interim financial
statements.
|
|
(4)
|
Both
BDO Dunwoody LLP and J.H. Cohn LLP did not provide and did not bill for
any tax services.
|
All
Other Fees
There
were no other fees billed by J.H. Cohn LLP or BDO Dunwoody LLP in the years
ended October 31, 2009 or October 31, 2008.
Pre-Approval
Policies and Procedures
The Board
of Directors is required to pre-approve the rendering by our independent auditor
of audit or permitted non-audit services. The Board of Directors pre-approved
all of the services rendered by J.H. Cohn LLP and BDO Dunwoody LLP for the audit
of the consolidated financial statements included in our Annual Reports on Form
10-K and reviews of consolidated financial statements included in our Quarterly
Reports on Form 10-Q.
The
services provided for 2009 were 63% audit services and 37% audit related
fees. The services provided above for 2008 were 100% audit
services.
45
PART
IV
ITEM
15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
Financial
Statements and Financial Statement Schedule
|
Report
of Independent Registered Public Accounting Firms
|
F2-3
|
|
Consolidated
Balance Sheets
|
F-4
|
|
Consolidated
Statements of Operations Years Ended
|
F-5
|
|
Consolidated
Statement of Changes in Stockholders’ Deficiency
|
F-6
|
|
Consolidated
Statements of Cash Flows
|
F-13
|
|
Notes
to Consolidated Financial Statements
|
F-14
|
Exhibits
The
following Exhibits are filed as part of this Annual Report on Form 10-K or
incorporated by reference.
|
Exhibit No.
|
Description
|
|
|
3.1
|
Amended
and Restated Articles of Incorporation of
Registrant. Incorporated by reference to the Registrant’s Form
10-QSB filed with the SEC on June 19, 2006.
|
|
|
3.2
|
Bylaws
of Registrant. Incorporated by reference to the Registrant’s
Form SB-2 filed with the SEC on September 30, 1999.
|
|
|
3.3
|
Certificate
of Designation of Series 1 Preferred Stock of Registrant. Incorporated by
reference to the Registrant’s Form 8-K filed with the SEC on July 24,
2009.
|
|
|
3.4
|
Articles
of Amendment of CardioGenics ExchangeCo Inc. effective July 14 2009 and
Articles of Incorporation of CardioGenics ExchangeCo Inc. Effective May
22, 2009
|
|
|
3.5
|
Certificate
of Amendment to Articles of Incorporation of Registrant. Incorporated by
reference to the Registrant’s Form DEF 14C filed with the SEC on September
9, 2009.
|
|
|
4.1
|
Form
of Common Stock Certificate. Incorporated by reference to the
Registrant’s Form 10-KSB filed with the SEC on November 8,
2005.
|
|
|
4.2
|
Form
of Series 2 Class B Stock Certificate. Incorporated by reference to the
Registrant’s Form 10-KSB filed with the SEC on November 8,
2005.
|
|
|
4.3
|
Securities
Purchase Agreement, effective May 25, 2006, with YA Global. Incorporated
by reference to the Registrant’s Form 8-K filed with the SEC on June 1,
2006.
|
|
|
4.4
|
Letter
Agreement, dated January 31, 2008, relating to the conversion of the
remaining principal balance of the convertible secured
debentures. Incorporated by reference to the Registrant’s Form
8-K filed with the SEC on February 6, 2008.
|
|
|
4.5
|
Warrant
No. CCP-1 for 2,000,000 shares of common stock issued to YA Global,
effective May 25, 2006. Incorporated by reference to the Registrant’s Form
8-K filed with the SEC on June 1, 2006.
|
|
|
4.6
|
Warrant
No. CCP-2 for 2,000,000 shares of common stock issued to YA Global,
effective May 25, 2006. Incorporated by reference to the
Registrant’s Form 8-K filed with the SEC on June 1,
2006.
|
46
|
Exhibit No.
|
Description
|
|
|
4.7
|
Warrant
No. CCP-3 for 2,000,000 shares of common stock issued to YA Global,
effective May 25, 2006. Incorporated by reference to the
Registrant’s Form 8-K filed with the SEC on June 1,
2006.
|
|
|
4.8
|
Warrant
No. CCP-4 for 3,000,000 shares of common stock issued to YA Global,
effective May 25, 2006. Incorporated by reference to the
Registrant’s Form 8-K filed with the SEC on June 1,
2006.
|
|
|
4.9
|
Warrant
No. CCP-5 for 3,000,000 shares of common stock issued to YA Global,
effective May 25, 2006. Incorporated by reference to the
Registrant’s Form 8-K filed with the SEC on June 1,
2006.
|
|
|
4.10
|
Letter
Agreement, amending Warrant No. CCP-4. Incorporated by reference to the
Registrant’s Form 8-K filed with the SEC on October 3,
2008.
|
|
|
4.11
|
Investor
Registration Rights Agreement, effective May 25, 2006, with YA Global.
Incorporated by reference to the Registrant’s Form 8-K filed with the SEC
on June 1, 2006.
|
|
|
10.1
|
Non-Binding
Letter of Intent, dated October 1, 2008, by and among the Registrant,
BlueCreek, e2 Business and YA Global. Incorporated by reference
to the Registrant’s Form 8-K filed with the SEC on October 3,
2008.
|
|
|
10.2
|
1999
Long-Term Incentive Plan, as amended. Incorporated by reference
to Exhibit 10.1 to the Registrant’s Form S-8 filed with the SEC on
May 1, 2002.
|
|
|
10.3
|
Amended
and Restated Employment Agreement, dated August 31, 2001, between Thomas
J. Mazzarisi and Registrant. Incorporated by reference to
Exhibit 10.21 in Amendment No. 1 to the Registrant’s Form SB-2 filed with
the SEC on September 26, 2001.
|
|
|
10.4
|
Amended
and Restated Employment Agreement, dated August 31, 2001, between Stephen
J. Schoepfer and Registrant. Incorporated by reference to
Exhibit 10.20 in Amendment No. 1 to the Registrant’s Form SB-2 filed with
the SEC on September 26, 2001.
|
|
|
10.5
|
Amendment
to Amended and Restated Employment Agreement, dated as of November 3,
2005, between Registrant and Thomas J. Mazzarisi. Incorporated
by reference to the Registrant’s Form 10-KSB filed with the SEC on
November 8, 2005.
|
|
|
10.6
|
Amendment
to Amended and Restated Employment Agreement, dated as of November 3,
2005, between Registrant and Stephen J. Schoepfer. Incorporated
by reference to the Registrant’s Form 10-KSB filed with the SEC on
November 8, 2005.
|
|
|
10.7
|
Amendment
to Amended and Restated Employment Agreement, dated as of November 12,
2007, by and between Registrant and Thomas J.
Mazzarisi. Incorporated by reference to Exhibit 10.6 of
Registrant’s Form 10-K filed with the SEC on November 13,
2008.
|
|
|
10.8
|
Amendment
to Amended and Restated Employment Agreement, dated as of November 12,
2007, by and between Registrant and Stephen J. Schoepfer. Incorporated by
reference to Exhibit 10.7 of the Registrant’s Form 10-K filed with the SEC
on November 13, 2008
|
|
|
10.9
|
Extension
of Amended and Restated Employment Agreement dated as of November 12, 2008
between registrant and Thomas J. Mazzarisi. Incorporated by reference to
Exhibit 10.9 of the Registrant’s Form 10-K filed with the SEC on November
13, 2008.
|
|
|
10.10
|
Extension
of Amended and Restated Employment Agreement dated as of November 12, 2008
between registrant and Stephen J. Schoepfer. Incorporated by reference to
Exhibit 10.10 of the Registrant’s Form 10-K filed with the SEC on November
13, 2008.
|
|
|
10.11
|
Consulting
Agreement, dated November 12, 2007, between the Registrant and Walsh
Organization, Inc.
Incorporated
by reference to Exhibit 10.31 of the Registrant’s Annual Report on Form
10-KSB filed November 13,
2003.
|
47
|
Exhibit No.
|
Description
|
|
|
10.12
|
Power
of Attorney and Contingent Fee Contract, dated June 14, 2002, among the
Registrant, Gary Valinoti and the Law Firm of O’Quinn, Laminack &
Pirtle. Incorporated by reference to Exhibit 10.32 of the Registrant’s
Annual Report on Form 10-KSB filed November 13, 2003.
|
|
|
10.13
|
Subscription
Agreement, dated December 10, 2002, between the Registrant and Bay Point
Investment Partners LLC. Incorporated by reference to the Registrant’s
Registration Statement on Form SB-2 filed on January 9,
2003.
|
|
|
10.14
|
Placement
Agent Agreement, dated December 10, 2002, between the Registrant and RMC 1
Capital Markets, Inc. Incorporated by reference to the Registrant’s
Registration Statement on Form SB-2 filed on January 9,
2003.
|
|
|
10.15
|
Placement
Agent Agreement, dated as of June 19, 2003, between the Registrant and RMC
1 Capital Markets, Inc., as amended on August 12, 2003. Incorporated by
reference to the Registrant’s Current Report on Form 8-K filed on August
13, 2003.
|
|
|
10.16
|
Subscription
Agreement, dated as of June 19, 2003, between the Registrant and Bay Point
Investment Partners LLC, as amended on August 12, 2003. Incorporated by
reference to the Registrant’s Current Report on Form 8-K filed on August
13, 2003.
|
|
|
10.17
|
Subscription
Agreement, dated as of September 25, 2003, between the Registrant and
Kuekenhof Equity Fund L.P. Incorporated by reference to Exhibit 10.39 of
the Registrant’s Form 10-KSB filed with the SEC on November 13,
2003.
|
|
|
10.18
|
Non-Circumvention/Non-Disclosure
Agreement, dated as of January 1, 2004 between Flow Capital Advisors Inc.
and the Registrant. Incorporated by reference to the Registrant’s Form 8-K
filed with the SEC on July 25, 2007.
|
|
|
10.19
|
Finder’s
Fee Agreement, dated as of January 5, 2004, between the Registrant and
Flow Capital Advisors, Inc. Incorporated by reference to the Registrant’s
Form 8-K filed with the SEC on January 20, 2004.
|
|
|
10.20
|
Finder’s
Fee Agreement, dated as of March 14, 2005, by and between the Registrant
and Flow Capital Advisors, Inc. Incorporated by reference to the
Registrant’s Form 8-K filed with the SEC on July 25,
2007.
|
|
|
10.21
|
Irrevocable
Transfer Agent Instructions, effective May 25, 2006, between the
Registrant and YA Global. Incorporated by reference to the Registrant’s
Form 8-K filed with the SEC on June 1, 2006.
|
|
|
10.22
|
Letter,
dated as of June 17, 2008, from Cryptometrics regarding termination of the
agreement and plan of merger between the Registrant and Cryptometrics.
Incorporated by reference to the Registrant’s Form 8-K filed with the SEC
on June 18, 2008.
|
|
|
10.23
|
Stand-By
Equity Distribution Agreement dated March 12, 2009 between Registrant and
YA Global Master SPV Ltd. Incorporated by reference to the Registrant’s
Form 8-K filed with the SEC on March 13, 2009.
|
|
|
10.24
|
Registration
Rights Agreement dated March 12, 2009 between Registrant and YA Global
Master SPV Ltd. Incorporated by reference to the Registrant’s Form 8-K
filed with the SEC on March 13, 2009.
|
|
|
10.23
|
Share
Purchase Agreement dated May 22, 2009 between Registrant, CardioGenics
ExchnageCo Inc., CardioGenics Inc. And Yahia Gawad, Principal Shareholder
of CardioGenics Inc.
|
|
|
10.24
|
Voting
and Exchange Trust Agreement dated July 6, 2009 among Registrant,
CardioGenics ExchangeCo Inc. and Weirfoulds LLP. Incorporated by reference
to the Registrant’s Form 8-K filed with the SEC on July 6,
2009.
|
|
|
10.25
|
Support
Agreement dated July 6, 2009 between Registrant and CardioGenics
ExchangeCo Inc. Incorporated by reference to the Registrant’s Form 8-K
filed with the SEC on July 6,
2009.
|
48
|
Exhibit No.
|
Description
|
|
|
10.26
|
Agreement
dated September 10, 2009 between Registrant and The Investor’s Relations
Group, Inc. Incorporated by reference to the Registrant’s Form 8-K filed
with the SEC on September 11, 2009.
|
|
|
10.27
|
Agreement
dated September 28, 2009 between Registrant and Gilford
Securities. Incorporated by reference to the Registrant’s Form 8-K
filed with the SEC on October 2, 2009.
|
|
|
10.28
|
Retainer
Agreement dated as of January 20, 2010 between Registrant and Wolfe,
Axelrod & Weinberger Associates LLC*
|
|
|
10.29
|
Letter
of Agreement dated January 18, 2010 between Registrant and The Investor
Relations Group, Inc.*
|
|
|
10.30
|
Employment
Agreement dated July 31, 2009 between Registrant and Dr. Yahia
Gawad.*
|
|
|
10.31
|
LLC
Membership Interest Purchase Agreement dated February 10, 2010 between
Registrant and Rothcove Partners LLC.*
|
|
|
14.1
|
Code
of Ethics. Incorporated by reference to the Registrant’s Form
10-KSB filed with the SEC on November 13, 2003.
|
|
|
21.1
|
Subsidiaries
of Registrant.*
|
|
|
23.1
|
Consent of J.H. Cohn LLP | |
|
23.2
|
Consent of BDO Dunwoody LLP | |
|
31.1
|
Section
302 Certification of Chief Executive Officer*
|
|
|
31.2
|
Section
302 Certification of Chief Financial Officer*
|
|
|
32.1
|
Section
906 Certification of Chief Executive Officer and Chief Financial
Officer*
|
|
|
99.1
|
Letter of Intent dated March 12, 2009 among JAG Media Holdings Inc., CardioGenics Inc. and Yahia Gawad. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on March 13, 2009. |
*Filed
herewith.
49
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Table
of Contents
October
31, 2009 and 2008
|
Consolidated
Financial Statements
|
|
|
Report
of Independent Registered Public Accounting Firms
|
F2-3
|
|
Consolidated
Balance Sheets
|
F-4
|
|
Consolidated
Statements of Operations
|
F-5
|
|
Consolidated
Statements of Stockholders’ Equity (Deficit)
|
F-6-12
|
|
Consolidated
Statements of Cash Flows
|
F-13
|
|
Notes
to Consolidated Financial Statements
|
F-14-37
|
F-1
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Report
of Independent Registered Public Accounting Firm
To the
Board of Directors and Stockholders
CardioGenics
Holdings, Inc.
We have
audited the accompanying consolidated balance sheet of CardioGenics Holdings
Inc. (a development stage company) and Subsidiaries as of October 31, 2009, and
the related consolidated statements of operations, changes in stockholders'
equity (deficit) and cash flows for the year ended October 31, 2009 and for the
period from November 20, 1997 (date of inception) to October 31, 2009. These
consolidated financial statements are the responsibility of the Company's
management. Our responsibility is to express an opinion on these consolidated
financial statements based on our audit. The financial statements of
CardioGenics, Inc for the period from November 20, 1997 to October 31, 2008 were
audited by other auditors whose report dated July 29, 2009 , expressed
unqualified opinion on those statements with explanatory paragraphs relating to
the Company’s ability to continue as a going concern.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the
consolidated financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by management, as well
as evaluating the overall financial statement presentation. We believe that our
audit provides a reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of CardioGenics Holdings, Inc.
and Subsidiaries as of October 31, 2009 and their results of operations and cash
flows for the year ended October 31, 2009 and for the period from November 20,
1997 (date of inception) to October 31, 2009 in conformity with
accounting principles generally accepted in the United States of
America.
The
consolidated financial statements referred to above have been prepared assuming
that the Company will continue as a going concern. As further discussed in Note
2 to the consolidated financial statements, the Company's operations have
generated recurring losses and negative cash flows from operating activities.
Such matters raise substantial doubt about the Company's ability to continue as
a going concern. Management's plans concerning these matters are also described
in Note 2. The accompanying consolidated financial statements do not include any
adjustments that might result from the outcome of these
uncertainties.
|
/s/
J.H. Cohn LLP
|
|
Roseland,
New Jersey
|
|
February
12, 2010
|
F-2
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Directors and Shareholders of
CardioGenics
Holdings Inc. (formerly CardioGenics Inc.)
(A
Development Stage Company)
We have
audited the accompanying consolidated balance sheet of CardioGenics Holdings
Inc. (formerly CardioGenics Inc.) (a development stage company) as at October
31, 2008 and the related consolidated statements of operations, stockholders’
equity (deficit) and cash flows for the year ended October 31,
2008. These consolidated financial statements are the responsibility
of the Company’s management. Our responsibility is to express an
opinion on these consolidated financial statements based on our
audit.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the consolidated financial statements are free of material
misstatement. The Company is not required to have, nor were we
engaged to perform, an audit of its internal control over financial
reporting. Our audit included consideration of internal control over
financial reporting as a basis for designing audit procedures that are
appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company’s internal control over financial
reporting. Accordingly we express no such opinion. An
audit also includes examining, on a test basis, evidence supporting the amounts
and disclosures in the consolidated financial statements and assessing the
accounting principles used and significant estimates made by management, as well
as evaluating the overall financial statement presentation. We
believe that our audit provides a reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of CardioGenics Holdings Inc.
(formerly CardioGenics Inc.) (a development stage company) as at
October 31, 2008 and the results of its operations and its cash flows for
the year ended October 31, 2008 in conformity with accounting principles
generally accepted in the United States of America.
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in Note 2
to the financial statements, the Company has suffered recurring net losses and
negative cash flows from operations. These matters raise substantial
doubt about the Company’s ability to continue as a going
concern. Management’s plans regarding these matters are also
described in Note 2. The accompanying consolidated financial
statements do not include any adjustments that might result from the outcome of
this uncertainty.
(Signed)
“BDO Canada
LLP”
Chartered
Accountants, Licensed Public Accountants
Toronto,
Ontario
July 29,
2009
F-3
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Balance Sheets
|
October 31,
|
October 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Assets
|
||||||||
|
Current
|
||||||||
|
Cash
and Cash Equivalents
|
$ | 2,388,516 | $ | 253,872 | ||||
|
Deposits
and Prepaid Expenses
|
11,996 | 8,309 | ||||||
|
Refundable
Taxes Receivable
|
14,878 | 9,091 | ||||||
|
Government
Grants and Investment Tax Credits Receivable
|
175,554 | 211,024 | ||||||
| 2,590,944 | 482,296 | |||||||
|
Property
and Equipment
|
54,338 | 67,218 | ||||||
|
Patents
|
241,980 | 234,716 | ||||||
| 296,318 | 301,934 | |||||||
| $ | 2,887,262 | $ | 784,230 | |||||
|
Liabilities
and Stockholders' Equity (Deficit)
|
||||||||
|
Current
Liabilities
|
||||||||
|
Accounts
Payable and Accrued Expenses
|
$ | 751,037 | $ | 408,870 | ||||
|
Due
to Director
|
147,102 | 872,435 | ||||||
|
Accrued
Interest Payable
|
—
|
565,931 | ||||||
|
Debentures
Payable
|
25,000 | 1,006,972 | ||||||
| 923,139 | 2,854,208 | |||||||
|
Mandatorily
redeemable Class B common stock; par value $.00001 per
share:
|
||||||||
|
400,000
shares designated as series 2; 381,749 shares issued and
outstanding
|
4 |
—
|
||||||
|
40,000
shares designated as series 3; 21,500 shares issued and
outstanding
|
—
|
—
|
||||||
| 4 |
—
|
|||||||
|
Commitments
and contingencies
|
||||||||
|
Stockholders'
Equity (Deficit)
|
||||||||
|
Preferred
stock; par value $.0001 per share, 50,000,000 shares authorized, none
issued
|
—
|
—
|
||||||
|
Common
stock; par value $.00001 per share;
650,000,000 (2008-500,000,000) shares authorized, 217,671,011 and 222,410,228 common shares and 276,655,415 and 0 exchangeable shares issued and outstanding as at October 31, 2009 and 2008 respectively |
4,943 | 2,224 | ||||||
|
Additional
paid-in capital
|
35,539,274 | 2,051,017 | ||||||
|
Deficit
accumulated during development stage
|
(33,260,283 | ) | (4,332,700 | ) | ||||
|
Accumulated
other comprehensive income (loss)
|
(319,815 | ) | 209,481 | |||||
|
Total
stockholders' equity (deficit)
|
1,964,119 | (2,069,978 | ) | |||||
|
Total
liabilities and stockholders' equity (deficit)
|
$ | 2,887,262 | $ | 784,230 | ||||
See notes to consolidated
financial statements.
F-4
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Operations
For
the Years Ended October 31, 2009 and 2008 and
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Cumulative
|
||||||||||||
|
From
|
||||||||||||
|
November 20,
|
||||||||||||
|
1997
|
||||||||||||
|
(Date of
|
||||||||||||
|
For the Years Ended
|
Inception) to
|
|||||||||||
|
October 31,
|
October 31,
|
|||||||||||
|
2009
|
2008
|
2009
|
||||||||||
|
Revenues
|
||||||||||||
|
Subscription
Fees
|
$ | 21,539 |
$
|
—
|
$ | 21,539 | ||||||
|
Cost
of Revenues
|
136,946 |
—
|
136,946 | |||||||||
|
Gross
Loss
|
(115,407 | ) |
—
|
(115,407 | ) | |||||||
|
Operating
Expenses
|
||||||||||||
|
Amortization
of Property and Equipment
|
26,157 | 32,248 | 160,108 | |||||||||
|
Amortization
of Patent Application Costs
|
4,181 |
—
|
4,181 | |||||||||
|
Write-off
of Patent Application Costs
|
23,803 | 29,928 | 53,731 | |||||||||
|
General
and Administrative
|
1,597,010 | 177,169 | 3,029,222 | |||||||||
|
Write-off
of Goodwill
|
12,780,214 |
—
|
12,780,214 | |||||||||
|
Research
and Product Development, Net of Investment Tax Credits
|
1,572,337 | 24,531 | 2,595,716 | |||||||||
|
Total
operating expenses
|
16,003,702 | 263,876 | 18,623,172 | |||||||||
|
Total
operating expenses and operating loss
|
(16,119,109 | ) | (263,876 | ) | (18,738,579 | ) | ||||||
|
Other
Expenses (Income)
|
||||||||||||
|
Interest
Expense and Bank Charges (Net)
|
571,840 | 432,005 | 2,086,335 | |||||||||
|
Loss
on Change in Value of Derivative Liability
|
12,421,023 |
—
|
12,421,023 | |||||||||
|
Loss
(Gain) on Foreign Exchange Transactions
|
(184,389 | ) | 657,340 | 14,346 | ||||||||
|
Total
other expenses (income)
|
12,808,474 | 1,089,345 | 14,521,704 | |||||||||
|
Net
Loss
|
$ | (28,927,583 | ) | $ | (1,353,221 | ) | $ | (33,260,283 | ) | |||
|
Basic
and Fully Diluted Net Loss per Common Share
|
$ | (.10 | ) | $ | (.01 | ) | ||||||
|
Weighted-average
shares of Common Stock outstanding
|
303,850,580 | 223,019,571 | ||||||||||
See notes
to consolidated financial statements.
F-5
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Issuance
of common shares for cash November 1998
|
15,927,320 | $ | 159 | $ | (158 | ) | $ | 1 | ||||||||||||||||
|
Issuance
of common shares for cash December 1998, $.00
|
7,963,660 | 80 | 34,956 | 35,036 | ||||||||||||||||||||
|
Issuance
of common shares for cash March 1998, $.00
|
5,516,113 | 55 | 24,393 | 24,448 | ||||||||||||||||||||
|
Issuance
of common shares for cash April 1998, $.00
|
129,866,107 | 1,299 | 4,404 | 5,703 | ||||||||||||||||||||
|
Issuance
of common shares for cash May 1998, $.01
|
2,102,490 | 21 | 17,278 | 17,299 | ||||||||||||||||||||
|
Issuance
of common shares for cash August 1998, $.00
|
27,872,810 | 279 | (51 | ) | 228 | |||||||||||||||||||
|
Issuance
of common shares for cash September 1998, $.01
|
841,004 | 8 | 6,563 | 6,571 | ||||||||||||||||||||
|
Issuance
of common shares for cash October 1998, $..01
|
319,489 | 3 | 2,497 | 2,500 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
$ | (81,208 | ) | (81,208 | ) | |||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
$ | (2,096 | ) | (2,096 | ) | |||||||||||||||||||
|
Total
Comprehensive income (Loss)
|
(81,208 | ) | (2,096 | ) | (83,304 | ) | ||||||||||||||||||
|
Balance
at October 31, 1998
|
190,408,993 | $ | 1,904 | $ | 89,882 | $ | (81,208 | ) | $ | (2,096 | ) | $ | 8,482 | |||||||||||
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance November 1, 1998
|
190,408,993 | $ | 1,904 | $ | 89,882 | $ | (81,208 | ) | $ | (2,096 | ) | $ | 8,482 | |||||||||||
|
Issuance
of common shares for cash November 1998, $.01
|
320,663 | 3 | 2,497 | 2,500 | ||||||||||||||||||||
|
Issuance
of common shares for cash February 1999, $.01
|
1,592,732 | 16 | 14,273 | 14,289 | ||||||||||||||||||||
|
Commission
paid on issuance of common stock for cash February 1999
|
(935 | ) | (935 | ) | ||||||||||||||||||||
|
Issuance
of common shares for cash March 1999, $.01
|
2,787,281 | 28 | 24,682 | 24,710 | ||||||||||||||||||||
|
Commission
paid on issuance of common stock for cash March 1999
|
(1,647 | ) | (1,647 | ) | ||||||||||||||||||||
|
Issuance
of common shares for cash to minority shareholders April 1999,
$.01
|
— | — | 10,707 | 10,707 | ||||||||||||||||||||
|
Commission
paid on issuance of common stock for cash April 1999
|
(627 | ) | (627 | ) | ||||||||||||||||||||
|
Issuance
of common shares for cash April 1999, $.01
|
398,183 | 4 | 3,810 | 3,814 | ||||||||||||||||||||
|
Commission
paid on issuance of common stock for cash April 1999
|
(314 | ) | (314 | ) | ||||||||||||||||||||
|
Issuance
of common shares for cash July 1999, $.01
|
1,194,549 | 12 | 10,062 | 10,074 | ||||||||||||||||||||
|
Issuance
of common shares for cash August 1999, $.01
|
1,194,549 | 12 | 10,034 | 10,046 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(100,745 | ) | (100,745 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(3,489 | ) | (3,489 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(100,745 | ) | (3,489 | ) | (104,234 | ) | ||||||||||||||||||
|
Balance
at October 31, 1999
|
197,896,950 | $ | 1,979 | $ | 162,424 | $ | (181,953 | ) | $ | (5,585 | ) | $ | (23,135 | ) | ||||||||||
See notes
to consolidated financial statements.
F-6
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 1999
|
197,896,950 | $ | 1,979 | $ | 162,424 | $ | (181,953 | ) | $ | (5,585 | ) | $ | (23,135 | ) | ||||||||||
|
Issuance
of common shares for cash November 1999, $.03
|
3,185,464 | 32 | 99,968 | 100,000 | ||||||||||||||||||||
|
Issuance
of common shares for minority shareholders as employee compensation
December 1999, $.03
|
— | — | 3,396 | 3,396 | ||||||||||||||||||||
|
Issuance
of common shares for cash March 2000, $.03
|
1,672,369 | 17 | 43,109 | 43,126 | ||||||||||||||||||||
|
Issuance
of common shares for minority shareholders for cash March, 2000,
$.03
|
— | — | 25,330 | 25,330 | ||||||||||||||||||||
|
Issuance
of common shares for cash April 2000, $.03
|
238,910 | 2 | 6,126 | 6,128 | ||||||||||||||||||||
|
Loan
Payable plus interest exchanged for shares July 2000, $.03
|
3,567,720 | 36 | 111,964 | 112,000 | ||||||||||||||||||||
|
Issuance
of common shares for minority shareholders as employee compensation
October 2000, $.03
|
— | — | 6,611 | 6,611 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2000
|
— | 11,570 | 11,570 | |||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(154,365 | ) | (154,365 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
921 | 921 | ||||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(154,365 | ) | 921 | (153,444 | ) | |||||||||||||||||||
|
Balance
at October 31, 2000
|
206,561,413 | $ | 2,066 | $ | 470,498 | $ | (336,318 | ) | $ | (4,664 | ) | $ | 131,582 | |||||||||||
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2000
|
206,561,413 | $ | 2,066 | $ | 470,498 | $ | (336,318 | ) | $ | (4,664 | ) | $ | 131,582 | |||||||||||
|
Issuance
of common shares as employee compensation October 2001,
$.03
|
24,100 | — | 925 | 925 | ||||||||||||||||||||
|
Issuance
of common share for minority shareholders as employee compensation October
2001, $.03
|
— | — | 6,169 | 6,169 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2001
|
— | — | 22,269 | 22,269 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(116,261 | ) | (116,261 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(10,528 | ) | (10,528 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(116,261 | ) | (10,528 | ) | (126,789 | ) | ||||||||||||||||||
|
Balance
at October 31, 2001
|
206,585,513 | $ | 2,066 | $ | 499,861 | $ | (452,579 | ) | $ | (15,192 | ) | $ | 34,156 | |||||||||||
See notes
to consolidated financial statements.
F-7
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2001
|
206,585,513 | $ | 2,066 | $ | 499,861 | $ | (452,579 | ) | $ | (15,192 | ) | $ | 34,156 | |||||||||||
|
Issuance
of common shares for cash June 2002, $.03
|
10,512,109 | 105 | 318,917 | 319,022 | ||||||||||||||||||||
|
Issuance
of common shares to minority shareholders for cash July 2002,
$.03
|
— | — | 3,235 | 3,235 | ||||||||||||||||||||
|
Issuance
of common shares for cash September 2002, $.03
|
209,570 | 2 | 6,343 | 6,345 | ||||||||||||||||||||
|
Issuance
of common shares for minority shareholders as employee compensation
October 2002, $.03
|
— | — | 9,505 | 9,505 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2002
|
— | — | 70,518 | 70,518 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(158,457 | ) | (158,457 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(11,506 | ) | (11,506 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(158,457 | ) | (11,506 | ) | (169,963 | ) | ||||||||||||||||||
|
Balance
at October 31, 2002
|
217,307,192 | $ | 2,173 | $ | 908,379 | $ | (611,036 | ) | $ | (26,698 | ) | $ | 272,818 | |||||||||||
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2002
|
217,307,192 | $ | 2,173 | $ | 908,379 | $ | (611,036 | ) | $ | (26,698 | ) | $ | 272,818 | |||||||||||
|
Issuance
of common shares for cash May 2003, $.03
|
282,920 | 3 | 9,868 | 9,871 | ||||||||||||||||||||
|
Issuance
of common shares for minority shareholders for cash May 2003
$.03
|
— | — | 10,967 | 10,967 | ||||||||||||||||||||
|
Issuance
of warrants in conjunction with convertible debentures September
2003
|
358,406 | 358,406 | ||||||||||||||||||||||
|
Issuance
of common shares as employee compensation October 2003,
$.04
|
565,839 | 6 | 20,416 | 20,422 | ||||||||||||||||||||
|
Issuance
of common shares for minority shareholders as employee compensation
October 2003, $.04
|
— | — | 7,564 | 7,564 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2003
|
— | — | 23,580 | 23,580 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(232,818 | ) | (232,818 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
42,957 | 42,957 | ||||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(232,818 | ) | 42,957 | (189,861 | ) | |||||||||||||||||||
|
Balance
at October 31, 2003
|
218,155,951 | $ | 2,182 | $ | 1,339,180 | $ | (843,854 | ) | $ | 16,259 | $ | 513,767 | ||||||||||||
See notes
to consolidated financial statements.
F-8
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2003
|
218,155,951 | $ | 2,182 | $ | 1,339,180 | $ | (843,854 | ) | $ | 16,259 | $ | 513,767 | ||||||||||||
|
Issuance
of warrants in conjunction with convertible debentures September
2004
|
152,628 | 152,628 | ||||||||||||||||||||||
|
Issuance
of common shares as employee compensation October 2004,
$.04
|
1,236,463 | 12 | 47,305 | 47,317 | ||||||||||||||||||||
|
Issuance
of common shares as directors' compensation October 2004,
$.04
|
1,571,775 | 16 | 60,133 | 60,149 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2004
|
— | — | 27,669 | 27,669 | ||||||||||||||||||||
|
Issuance
of options to directors and committee chairmen for services rendered in
October 2004
|
54,582 | 54,582 | ||||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(602,480 | ) | (602,480 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(6,136 | ) | (6,136 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(602,480 | ) | (6,136 | ) | (608,616 | ) | ||||||||||||||||||
| Balance at October 31, 2004 | 220,964,189 | $ | 2,210 | $ | 1,681,497 | $ | (1,446,334 | ) | $ | 10,123 | $ | 247,496 | ||||||||||||
See notes
to consolidated financial statements.
F-9
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2004
|
220,964,189 | $ | 2,210 | $ | 1,681,497 | $ | (1,446,334 | ) | $ | 10,123 | $ | 247,496 | ||||||||||||
|
Issuance
of common shares as employee compensation November 2004,
$.04
|
94,307 | 1 | 3,759 | 3,760 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation December 2004,
$.04
|
94,307 | 1 | 3,691 | 3,692 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation January 2005,
$.04
|
94,307 | 1 | 3,673 | 3,674 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation February 2005,
$.04
|
94,307 | 1 | 3,628 | 3,629 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation March 2005, $.04
|
94,307 | 1 | 3,700 | 3,701 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation April 2005, $.04
|
94,307 | 1 | 3,640 | 3,641 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation May 2005, $.04
|
94,307 | 1 | 3,583 | 3,584 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation June 2005, $.04
|
94,307 | 1 | 3,627 | 3,628 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation July 2005, $.04
|
94,307 | 1 | 3,679 | 3,680 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation August 2005,
$.04
|
94,307 | 1 | 3,736 | 3,737 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation September 2005,
$.04
|
94,307 | 1 | 3,820 | 3,821 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation October 2005,
$.04
|
94,307 | — | 3,822 | 3,822 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2005
|
— | — | 33,973 | 33,973 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(693,603 | ) | (693,603 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(13,288 | ) | (13,288 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(693,603 | ) | (13,288 | ) | (706,891 | ) | ||||||||||||||||||
|
Balance
at October 31, 2005
|
222,095,873 | $ | 2,221 | $ | 1,759,828 | $ | (2,139,937 | ) | $ | (3,165 | ) | $ | (381,053 | ) | ||||||||||
See notes
to consolidated financial statements.
F-10
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2005
|
222,095,873 | $ | 2,221 | $ | 1,759,828 | $ | (2,139,937 | ) | $ | (3,165 | ) | $ | (381,053 | ) | ||||||||||
|
Issuance
of common shares as employee compensation November 2005,
$.04
|
104,785 | 1 | 4,231 | 4,232 | ||||||||||||||||||||
|
Issuance
of common shares in exchange for services rendered December 2005,
$.04
|
104,785 | 1 | 4,304 | 4,305 | ||||||||||||||||||||
|
Issuance
of common shares in exchange for services rendered January 2006,
$.04
|
104,785 | 1 | 4,320 | 4,321 | ||||||||||||||||||||
|
Issuance
of stock options in exchange for services rendered October
2006
|
— | — | 2,658 | 2,658 | ||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(531,093 | ) | (531,093 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(25,688 | ) | (25,688 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(531,093 | ) | (25,688 | ) | (556,781 | ) | ||||||||||||||||||
|
Balance
at October 31, 2006
|
222,410,228 | $ | 2,224 | $ | 1,775,341 | $ | (2,671,030 | ) | $ | (28,853 | ) | $ | (922,318 | ) | ||||||||||
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2006
|
222,410,228 | $ | 2,224 | $ | 1,775,341 | $ | (2,671,030 | ) | $ | (28,853 | ) | $ | (922,318 | ) | ||||||||||
|
Incremental
increase in fair value of warrants in conjunction with re-structuring of
debentures, April 2007
|
44,096 | 44,096 | ||||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(308,449 | ) | (308,449 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(184,432 | ) | (184,432 | ) | ||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(308,449 | ) | (184,432 | ) | (492,881 | ) | ||||||||||||||||||
|
Balance
at October 31, 2007
|
222,410,228 | $ | 2,224 | $ | 1,819,437 | $ | (2,979,479 | ) | $ | (213,285 | ) | $ | (1,371,103 | ) | ||||||||||
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2007
|
222,410,228 | $ | 2,224 | $ | 1,819,437 | $ | (2,979,479 | ) | $ | (213,285 | ) | $ | (1,371,103 | ) | ||||||||||
|
Issuance
of warrants in conjunction with re-structuring of debentures October
2008
|
231,580 | 231,580 | ||||||||||||||||||||||
|
Comprehensive
Income (Loss)
|
||||||||||||||||||||||||
|
Net
Loss
|
(1,353,221 | ) | (1,353,221 | ) | ||||||||||||||||||||
|
Other
Comprehensive Income (Loss)
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
422,766 | 422,766 | ||||||||||||||||||||||
|
Total
Comprehensive Income (Loss)
|
(1,353,221 | ) | 422,766 | (930,455 | ) | |||||||||||||||||||
|
Balance
at October 31, 2008
|
222,410,228 | $ | 2,224 | $ | 2,051,017 | $ | (4,332,700 | ) | $ | 209,481 | $ | (2,069,978 | ) | |||||||||||
See notes
to consolidated financial statements.
F-11
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Stockholders' Equity (Deficit)
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Deficit
|
||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||
|
During
|
Accumulated
|
Total
|
||||||||||||||||||||||
|
Additional
|
the
|
Other
|
Stockholders'
|
|||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Development
|
Comprehensive
|
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Stage
|
Income (Loss)
|
(Deficit)
|
|||||||||||||||||||
|
Balance
November 1, 2008
|
222,410,228 | $ | 2,224 | $ | 2,051,017 | $ | (4,332,700 | ) | $ | 209,481 | $ | (2,069,978 | ) | |||||||||||
|
Issuance
of common shares as payment of debenture interest, January 2009, $0.05 per
share
|
4,950,945 | 49 | 236,194 | 236,243 | ||||||||||||||||||||
|
Issuance
of common shares on exercise of options, April 2009
|
5,709,798 | 57 | (29 | ) | 28 | |||||||||||||||||||
|
Issuance
of common shares as employee compensation, May 2009, $0.04 per
share
|
31,538,776 | 316 | 1,298,469 | 1,298,785 | ||||||||||||||||||||
|
Issuance
of common shares to directors, pursuant to debenture financing of January
2009, May 2009, $0.04 per share
|
9,283,952 | 93 | 382,437 | 382,530 | ||||||||||||||||||||
|
Issuance
of common shares in exchange for services rendered, June 2009, $0.04 per
share
|
50,234 | - | 2,062 | 2,062 | ||||||||||||||||||||
|
Issuance
of common shares for cash June 2009, $0.04 per share
|
240,902 | 2 | 8,600 | 8,602 | ||||||||||||||||||||
|
Issuance
of common shares in exchange for services rendered, July 2009, $0.04 per
share
|
471,533 | 5 | 20,245 | 20,250 | ||||||||||||||||||||
|
Issuance
of common shares as payment of director compensation, July 2009, $0.04 per
share
|
2,410,055 | 24 | 103,476 | 103,500 | ||||||||||||||||||||
|
Issuance
of common shares as employee compensation pursuant to reverse merger
transaction, July 2009, $0.04 per share
|
11,735,920 | 117 | 503,883 | 504,000 | ||||||||||||||||||||
|
Issuance
of common shares to retire debentures, July 2009, $0.03 per
share
|
33,460,282 | 335 | 997,237 | 997,572 | ||||||||||||||||||||
|
Issuance
of common shares as payment of debenture interest, January 2009, July
2009, $0.05 per share
|
8,557,120 | 86 | 418,582 | 418,668 | ||||||||||||||||||||
|
Issuance
of common shares to retire director's loan, July 2009 $0.04 per
share
|
23,778,126 | 238 | 884,762 | 885,000 | ||||||||||||||||||||
|
Issuance
of common shares as payment of interest on director's loan, July 2009,
$0.04 per share
|
2,185,564 | 22 | 108,613 | 108,635 | ||||||||||||||||||||
|
Issuance
of common shares for cash, July 2009, $0.04 per share
|
65,400,175 | 654 | 2,714,346 | 2,715,000 | ||||||||||||||||||||
|
Issuance
of common shares as compensation for consulting contract, July 2009, $0.38
per share
|
1,000,000 | 10 | 379,990 | 380,000 | ||||||||||||||||||||
|
Issuance
of common shares on exercise of warrants by YA Global for cash August
2009
|
250,000 | 2 | 44,998 | 45,000 | ||||||||||||||||||||
|
Beneficial
conversion charge on 3rd debenture
|
—
|
335,000 | 335,000 | |||||||||||||||||||||
|
Beneficial
conversion charge on director's loan
|
—
|
117,109 | 117,109 | |||||||||||||||||||||
|
Reclassification
of warrants to derivative liability
|
(786,710 | ) | (786,710 | ) | ||||||||||||||||||||
|
Assumption
of options in reverse merger
|
644,806 | 644,806 | ||||||||||||||||||||||
|
Reclassification
of derivative liability on increase of authorized
shares
|
13,501,360 | 13,501,360 | ||||||||||||||||||||||
|
Effect
of Reverse Merger
|
70,892,816 | 709 | 11,572,827 |
—
|
—
|
11,573,536 | ||||||||||||||||||
|
Comprehensive
Loss
|
||||||||||||||||||||||||
|
Net
Loss
|
(28,927,583 | ) | (28,927,583 | ) | ||||||||||||||||||||
|
Other
Comprehensive Loss
|
||||||||||||||||||||||||
|
Currency
Translation Adjustment
|
(529,296 | ) | (529,296 | ) | ||||||||||||||||||||
|
Total
comprehensive loss
|
(28,927,583 | ) | (529,296 | ) | (29,456,879 | ) | ||||||||||||||||||
|
Balance
October 31, 2009
|
494,326,426 | $ | 4,943 | $ | 35,539,274 | $ | (33,260,283 | ) | $ | (319,815 | ) | $ | 1,964,119 | |||||||||||
See notes
to consolidated financial statements.
F-12
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Consolidated
Statements of Cash Flows
Years
Ended October 31, 2009 and 2008 and
Cumulative
from November 20, 1997 (Date of Inception) to October 31, 2009
|
Cumulative from
|
||||||||||||
|
November 20, 1997
|
||||||||||||
|
Years Ended
|
(Date of Inception)
|
|||||||||||
|
October 31
|
To October 31,
|
|||||||||||
|
2009
|
2008
|
2009
|
||||||||||
|
Cash flows from operations activities
|
||||||||||||
|
Net
Loss for the Period
|
$ | (28,927,583 | ) | $ | (1,353,221 | ) | $ | (33,260,283 | ) | |||
|
Adjustments
to reconcile net loss for the period to net cash provided (used) by
operating activities
|
||||||||||||
|
Amortization
of Property and Equipment
|
26,157 | 32,248 | 160,108 | |||||||||
|
Amortization
of Patent Application Costs
|
4,181 |
—
|
4,181 | |||||||||
|
Write-off
of Patent Application Costs
|
23,803 | 29,928 | 53,731 | |||||||||
|
Write-off
of Goodwill
|
12,780,214 |
—
|
12,780,214 | |||||||||
|
Amortization
of Deferred Debt Issuance Costs
|
—
|
—
|
511,035 | |||||||||
|
Loss
on Extinguishment of Debt
|
—
|
231,580 | 275,676 | |||||||||
|
Loss
on Change in Value of Derivative Liability
|
12,421,023 |
—
|
12,421,023 | |||||||||
|
Interest
Accrued and Foreign Exchange Loss (Gain) on Debt
|
356,608 | 187,182 | 922,539 | |||||||||
|
Unrealized
Foreign Currency Exchange Gains (Losses)
|
(184,389 | ) | 422,766 | 25,092 | ||||||||
|
Beneficial
Conversion Charge included in Interest Expense
|
452,109 |
—
|
452,109 | |||||||||
|
Common
Stock Issued as Employee or Officer/Director Compensation
|
2,288,815 |
—
|
2,508,282 | |||||||||
|
Common
Stock Issued for Services Rendered
|
402,312 |
—
|
402.312 | |||||||||
|
Stock
Options Issued for Services Rendered
|
—
|
—
|
192,238 | |||||||||
|
Stock
Options Issued to Directors and Committee Chairman
|
—
|
—
|
54,582 | |||||||||
|
Changes
in Operating Assets and Liabilities,
|
||||||||||||
|
Net
of Acquisition
|
||||||||||||
|
Deposits
and Prepaid Expenses
|
(2,898 | ) | 13,444 | (11,207 | ) | |||||||
|
Refundable
Taxes Receivable
|
(4,923 | ) | 4,860 | (14,014 | ) | |||||||
|
Investment
Tax Credits Receivable
|
55,522 | 347,756 | (155,502 | ) | ||||||||
|
Accounts
Payable and Accrued Expenses
|
(425,731 | ) | 212,654 | (16,861 | ) | |||||||
|
Advances
|
—
|
—
|
131 | |||||||||
|
Net
cash provided (used) by operating activities
|
(734,780 | ) | 129,197 | (2,694,614 | ) | |||||||
|
Cash
flows from investing activities
|
||||||||||||
|
Cash
Acquired from Acquisition
|
195,885 |
—
|
195,885 | |||||||||
|
Purchase
of Property and Equipment
|
(8,950 | ) | (1,012 | ) | (193,366 | ) | ||||||
|
Patent
Application Costs
|
(15,164 | ) | (33,824 | ) | (259,715 | ) | ||||||
|
Net
cash provided (used) by investing activities
|
171,771 | (34,836 | ) | (257,196 | ) | |||||||
|
Cash
flows from financing activities
|
||||||||||||
|
Due
to Director
|
—
|
67,546 | 872,432 | |||||||||
|
Issue
of Debentures
|
371,333 |
—
|
1,378,305 | |||||||||
|
Issue
of Common Shares on Exercise of Stock options
|
31 |
—
|
31 | |||||||||
|
Issue
of Common Shares for Cash
|
2,768,602 |
—
|
3,568,847 | |||||||||
|
Redemption
of 10% Senior Convertible Debentures
|
(369,972 | ) |
—
|
(369,972 | ) | |||||||
|
Net
cash provided by financing activities
|
2,769,994 | 67,546 | 5,449,643 | |||||||||
|
Effect
of foreign exchange on cash and cash equivalents
|
(72,341 | ) | 66,224 | (109,317 | ) | |||||||
|
Cash
and Cash Equivalents
|
||||||||||||
|
Increase
in cash and cash equivalents during the period
|
2,134,644 | 228,131 | 2,388,516 | |||||||||
|
Beginning
of Period
|
253,872 | 25,741 |
—
|
|||||||||
|
End
of Period
|
$ | 2,388,516 | $ | 253,872 | $ | 2,388,516 | ||||||
See notes
to consolidated financial statements.
F-13
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
1.
|
Nature
of Business
|
The
accompanying audited consolidated financial statements have been prepared in
accordance with the requirements of Form 10-K and Article 8 of Regulation S-X of
the Securities and Exchange Commission (the “Commission”) and include the
results of CardioGenics, Inc. and JAG Media Holdings, Inc and its subsidiaries
(”JAG Media”) (from July 31, 2009, date of acquisition) which are
collectively referred to as the “Company.”
CardioGenics
Inc. (“CardioGenics”) was incorporated on November 20, 1997 in the Province of
Ontario, Canada, and carries on the business of development and
commercialization of diagnostic test products to the In Vitro Diagnostics
testing market. CardioGenics has several test products that are in various
stages of development.
CardioGenics’
business is that of a development-stage company, with a limited history of
operations and whose revenues, to date, have been primarily comprised of grant
revenue and Scientific Research Tax Credits from government agencies. There can
be no assurance that the Company will be successful in obtaining regulatory
approval for marketing of any of the existing or future products that the
Company will succeed in developing.
On
July 31, 2009, CardioGenics acquired the business of JAG Media Holdings, Inc.
(“JAG Media” see Note 4) The business acquired is
that of gathering and compiling financial and investment information from
various financial institutions and other Wall Street professionals. Revenues of
the acquired business of JAG media are generated by releasing such financial
information to subscribers in a consolidated format on a timely basis through
facsimile transmissions and a web site. Further, software focused on streaming
video solutions was acquired through the acquisition of JAG Media by
CardioGenics. Historically, further development of this software has been
limited as a result of JAG Media’s lack of financial resources.
References
herein to CardioGenics common shares has been retrospectively adjusted to
reflect the exchange ratio of 20.957 established in the Share Purchase
Agreement.
On
October 27, 2009 the name of the Company was changed from JAG Media Holdings
Inc. to CardioGenics Holdings Inc.
|
2.
|
Basis
of Presentation
|
The
accompanying consolidated financial statements have been prepared using the
accounting principles generally accepted in the United States of America
applicable to a going concern, which contemplates the realization of assets and
the satisfaction of liabilities and commitments in the normal course of
business.
The
Company has incurred operating losses and has experienced negative cash flows
from operations since inception. The Company has an accumulated
deficit at October 31, 2009 of approximately $33.2 million. The Company has not
yet established an ongoing source of revenues sufficient to cover its operating
costs and to allow it to continue as a going concern. The Company has
funded its activities to date almost exclusively from debt and equity
financings. These conditions raise substantial doubt about the Company’s ability
to continue as a going concern.
F-14
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
The
Company will continue to require substantial funds to continue research and
development, including preclinical studies and clinical trials of its products,
and to commence sales and marketing efforts, if the FDA and other regulatory
approvals are obtained. In order to meet its operating cash flow requirements
Management’s plans include financing activities such as private placements of
its common stock and issuances of convertible debt instruments. Management is
also actively pursuing industry collaboration activities including product
licensing and specific project financing.
While the
Company believes it will be successful in obtaining the necessary financing to
fund its operations, meet revenue projections and manage costs, there are no
assurances that such additional funding will be achieved and that it will
succeed in its future operations. The financial statements do not include any
adjustments relating to the recoverability and classification of recorded asset
amounts or amounts of liabilities that might be necessary should the Company be
unable to continue in existence.
|
3.
|
Summary
of Significant Accounting Policies
|
|
|
(a)
|
Principles
of Consolidation
|
The
consolidated financial statements include the accounts of the Company and its
100% owned subsidiaries. All significant intercompany transactions and balances
have been eliminated.
|
|
(b)
|
Development
Stage Company
|
The
accompanying financial statements have been prepared in accordance with the
provisions of the guidance for development stage enterprises.
|
|
(c)
|
Cash
and Cash Equivalents
|
The
Company considers all highly liquid investments purchased with an
original maturity of three months or less to be cash equivalents.
|
|
(d)
|
Government
Grants and Investment Tax Credits
Receivable
|
The
Company’s accounts include claims for investment tax credits relating to
scientific research activities of the Company. The qualification and recording
of this activity for investment tax credit purposes is established by Canadian
Income Tax authorities when the income tax returns for the period are assessed.
The credit is recognized in the statement of operations in the year in which the
expenses were incurred.
|
|
(e)
|
Property
and Equipment
|
Property
and equipment are recorded at cost and depreciated using methods and rates as
follows:
|
Furniture
and Fixtures
|
20%
declining balance
|
|
Lab
Equipment
|
20%
declining balance
|
|
Computer
Equipment – Hardware
|
30%
declining balance
|
|
Computer
Equipment – Software
|
50%
declining balance
|
|
Leasehold
Improvements
|
Straight-line
over the lesser of the life of the asset or the life of the
lease
|
F-15
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
|
(f)
|
Patents
|
Capitalized
patent costs represent legal costs incurred to establish patents. Capitalized
patent costs are amortized on a straight line method over the related patent
term. As patents are abandoned, the net book value of the patent is written
off.
|
|
(g)
|
Impairment
or Disposal of Long-Lived
Assets
|
The
Company assesses the impairment of long-lived assets under the guidance of
standards for the impairment or disposal of long-lived assets whenever events or
changes in circumstances indicate that the carrying value may not be
recoverable. For long-lived assets to be held and used, the Company
recognizes an impairment loss only if its carrying amount is not recoverable and
exceeds its fair value. The carrying amount of the long-lived asset
is not recoverable if it exceeds the sum of the undiscounted cash flows expected
to result from the use and eventual disposal of the asset.
|
|
(h)
|
Convertible
Debentures
|
In
accordance with guidance in accounting for convertible securities with
beneficial conversion features or contingently adjustable conversion ratios, the
Company recognized an imbedded beneficial conversion feature present in the
convertible debentures. The Company allocated a portion of the proceeds equal to
the intrinsic value of that feature to additional paid-in
capital. The debt discount attributed to the beneficial conversion
feature is amortized over the convertible debenture's maturity period as
interest expense using the effective yield method.
In
addition, the Company recognized the value attributable to the warrants to
additional paid-in capital and a discount against the convertible debentures.
The Company valued the warrants using the Black-Scholes pricing
model. The debt discount attributed to the value of the warrants
issued is amortized over the convertible debenture’s maturity period as interest
expense using the effective yield method.
|
|
(i)
|
Research
and Development Costs
|
Expenditures
for research and development are expensed as incurred and include, among other
costs, those related to the production of prototype products, including payroll
costs. Amounts expected to be received from governments under Scientific
Research Tax Credit arrangements are offset against current
expenses. The Company recognizes revenue from restricted grants in
the period in which the Company has incurred the expenditures in compliance with
the specific restrictions.
|
|
(j)
|
Income
Taxes
|
The
Company utilizes the liability method of accounting for income taxes as set
forth in the authoritative guidance. Under the liability method, deferred taxes
are determined based on the temporary differences between the financial
statement and tax basis of assets and liabilities using tax rates expected to be
in effect during the years in which the basis differences reverse. A valuation
allowance is recorded when it is more likely than not that some of the deferred
tax assets will not be realized. As there is no certainty that the Company will
generate taxable income in the foreseeable future to utilize tax losses
accumulated to date, no provision for ultimate tax reduction has been made in
these financial statements.
F-16
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
On
November 1, 2007, the Company adopted the guidance issued for accounting for
uncertainty in income taxes which provides detailed guidance for the financial
statement recognition, measurement and disclosure of uncertain tax positions
recognized in an enterprise’s financial statements. Income tax positions must
meet a more-likely-than-non recognition threshold at the effective date to be
recognized upon the adoption of the guidance and in subsequent periods. The
Company recognizes potential accrued interest and penalties related to
unrecognized tax benefits within operations as income tax expense. Upon
adoption, there were no adjustments required.
|
|
(k)
|
Stock-Based
Compensation
|
The
Company follows the authoritative guidance for stock-based compensation which
requires that new, modified and unvested share-based payment transactions with
employees, such as grants of stock options and restricted stock, be recognized
in the financial statements based on their fair value at the grant date and
recognized as compensation expense over their vesting periods. The Company has
also considered the related guidance of the Security and Exchange Commission
(“SEC”). The Company estimates the fair value of stock options and shares issued
as compensation to employees and directors as of the date of grant using the
Black-Scholes pricing model and restricted stock based on the per share
value. The Company also follows the guidance for equity instruments
that are issued to other than employees for acquiring, or in conjunction with
selling, goods or services for equity instruments issued to consultants
which provides guidance on transactions in which (1) the fair value
of the equity instruments is more reliably measurable than the fair value of the
goods or services received and (2) the counterparty receives shares of stock,
stock options, or other equity instruments in settlement of the entire
transaction or, if the transaction is part cash and part equity instruments, in
settlement of the portion of the transaction for which the equity instruments
constitute the consideration. Options issued with a nominal exercise
price in exchange for services rendered were measured at the fair value of the
underlying services rendered on the date of grant. The expense was recorded to
the statement of operations with a corresponding increase in share capital with
no additional increase in the number of shares as they were legally not yet
exercised.
|
|
(l)
|
Net
Loss Per Common Share
|
Basic
loss per share is computed by dividing loss available to common stockholders by
the weighted average number of common shares outstanding during the period.
Diluted earnings (loss) per share gives effect to all dilutive potential common
shares outstanding during the period. The computation of diluted earnings (loss)
per share does not assume conversion, exercise or contingent exercise of
securities that would have an anti-dilutive effect on earnings (loss) per
share.
|
|
(m)
|
Comprehensive
Loss
|
Other
comprehensive loss, which includes only foreign currency translation
adjustments, is shown in the Statement of Changes in Stockholders’ Equity
(Deficit).
|
|
(n)
|
Concentration
of Credit Risk
|
The Company maintains cash balances, at times, with
financial institutions in excess of amounts insured by the Canada Deposit
Insurance Corporation and the Federal Deposit Insurance
Corporation. Management monitors the soundness of these institutions
and has not experienced any collection losses with these financial
institutions.
F-17
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
|
(o)
|
Use
of Estimates
|
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the dates of
the financial statements and the reported amounts of revenues and expenses
during the reporting periods. Actual results could differ from those
estimates. By their nature, these estimates are subject to
uncertainty and the effect on the consolidated financial statements of changes
in such estimates in future periods could be material.
|
|
(p)
|
Foreign
Currency Translation
|
The
Company maintains its accounting records for its Canadian operations in Canadian
dollars. Transactions in United States dollars (“USD”) are translated into
Canadian dollars at rates in effect at the date of the transaction and gains or
losses on such transactions are recorded at the time of settlement in the
statement of operations.
The
Company’s reporting currency is the United States Dollar. Foreign
denominated assets and liabilities of the Company are translated into USD at the
prevailing exchange rates in effect at the end of the reporting period, the
historical rate for stockholders’ equity and a weighted average of exchange rate
in effect during the period for expenses, gains and
losses. Adjustments that arise from translation into the reporting
currency are recorded in the accumulated other comprehensive income (loss)
component of stockholders’ equity (deficit).
|
|
(q)
|
Financial
Instruments
|
The
carrying values of cash and cash equivalents, other current assets, accounts
payable and accrued expenses approximate their fair values due to their
short-term nature. Long-term debt and convertible debentures
approximate their fair value based upon the borrowing rates available for the
nature of the underlying debt.
|
|
(r)
|
Revenue
Recognition
|
Fees for
subscriptions are generally billed in advance on a monthly, quarterly,
semi-annual or annual basis. Revenues from subscriptions are
recognized ratably over the subscription period. Subscription fees
collected that relate to periods subsequent to the date of the consolidated
balance sheets are included in deferred revenues.
|
|
(s)
|
Effects
of Recent Accounting
Pronouncements
|
In
September 2006, “Fair Value Measurements” pronouncement was issued which defines
fair value, establishes a framework for measuring fair value in accordance with
accounting principles generally accepted in the United States, and expands
disclosures about fair value measurements. This pronouncement is effective for
financial statements issued for the Company’s fiscal year beginning November 1,
2008, with earlier application encouraged. Any amounts recognized upon adoption
as cumulative effect adjustments will be recorded to the opening balance of
retained earnings in the year of adoption. On February 12, 2008, the effective
date for non-financial assets and liabilities was delayed to fiscal years
beginning after November 15, 2008; however, the effective date for financial
assets remains intact. The Company has adopted the fair value measurements
pronouncement for current assets and liabilities which has not had a material
effect on its consolidated financial statements.
In
December 2007, “Business Combinations” pronouncement was issued, which
establishes principles and requirements for how an acquirer recognizes and
measures in its financial statements the identifiable assets acquired, the
liabilities assumed, and any non-controlling interest in an acquiree, including
the recognition and measurement of goodwill acquired in a business
combination. This Pronouncement is effective as of the beginning of
the first fiscal year beginning on or after December 15,
2008. Earlier adoption is prohibited. The Company does not
expect this pronouncement to have a material effect on its consolidated
financial statements.
F-18
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
In
December 2007, “Non-controlling Interest in Consolidated Financial
Statements” was pronounced, which will change the accounting and reporting for
minority interests, which will be re-characterized as non-controlling interests
and classified as a component of equity within the consolidated balance
sheets. This pronouncement is effective as of the beginning of the
first fiscal year beginning on or after December 15, 2008. Earlier
adoption is prohibited. The Company estimates that the adoption will
have minimal impact on its consolidated financial position, results of
operations or cash flows.
In May
2008, “Accounting for Convertible Debt Instruments That May Be Settled in Cash
upon Conversion (Including Partial Cash Settlements)” was pronounced. This
requires a portion of this type of convertible debt to be recorded as equity and
to record interest expense on the debt portion at a rate that would have been
charged on nonconvertible debt with the same terms. This pronouncement takes
effect in the first quarter of fiscal years beginning after December 15, 2008
and will be applied retrospectively for all periods presented. It will be
effective for the Company on November 1, 2009. The Company does not expect this
pronouncement to have a material effect on its consolidated financial
statements.
In June
2008, “Determining Whether Instruments Granted in Share-Based Payment
Transactions Are Participating Securities” was pronounced. Securities
participating in
dividends with common stock according to a formula are participating securities.
This pronouncement determined that unvested shares of restricted stock and stock
units with no forfeitable rights to dividends are participating securities.
Participating securities require the “two-class” method to be used to calculate
basic earnings per share. This method lowers basic earnings per common share.
This pronouncement takes effect in the first quarter of fiscal years beginning
after December 15, 2008 and will be applied retrospectively for all periods
presented. It will be effective for the Company on November 1, 2009. The Company
does not expect this pronouncement to have a material effect on its consolidated
financial statements.
In June
2009, the Financial Accounting Standards Board (“FASB”) issued guidance which
stipulates the FASB Accounting Standards Codification is the source of
authoritative U.S. Generally Accepted Accounting Principles (“GAAP”) recognized
by the FASB to be applied by non-governmental entities, and supersedes all
existing non-SEC standards. This guidance is effective for the Company’s fiscal
year beginning November 1, 2009. The Company does not expect this pronouncement
to have a material effect on its consolidated financial statements.
In
October 2009, the FASB issued guidance related to revenue recognition for
arrangements with multiple deliverables. This guidance eliminates the residual
method of allocation and requires the relative selling price method when
allocating deliverables of a multiple deliverable revenue arrangement. The
determination of the selling price for each deliverable requires the use of a
hierarchy designed to maximize the use of available objective evidence
including, vendor specific objective evidence, third party evidence of selling
price, or estimated selling price. The guidance is effective prospectively for
revenue arrangements entered into or materially modified in fiscal years
beginning on or after June 15, 2010, and must be adopted in the same period
using the same transition method. If adoption is elected in a period other than
the beginning of a fiscal year, the amendments in these standards must be
applied retrospectively to the beginning of the fiscal year. Full retrospective
application of these amendments to prior fiscal years is optional. Early
adoption of these standards may be elected. The Company is currently evaluating
the impact of this new accounting standard on the consolidated financial
statements.
F-19
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
4.
|
Acquisition
|
On July
31, 2009, the Company completed a reverse acquisition of privately held
CardioGenics Inc. (“CardioGenics”), an Ontario, Canada Corporation. The
acquisition was effected pursuant to a Share Purchase Agreement dated May 22,
2009 by and among the Company, CardioGenics Inc. and CardioGenics ExchangeCo
Inc., the Company’s wholly owned subsidiary (“ExchangeCo”). In accordance with
the terms of the Share Purchase Agreement, 99% of the holders of common shares
of CardioGenics Inc. (two (2) minority shareholders of CardioGenics Inc. holding
in aggregate 173,869
common shares of CardioGenics Inc. did not participate) surrendered their
CardioGenics Common Shares. CardioGenics Inc. caused the Company to issue to
ExchangeCo or CardioGenics’ shareholders 422,183,610 shares of the Company’s
common stock, par value $0.00001 (the “Share Consideration”). The CardioGenics
Inc.’s shareholders had the option to receive their pro-rata allocation of the
Share Consideration in the form of (a) the Company’s common stock (the “JAG
Consideration Shares”) or (b) exchangeable shares of ExchangeCo. Inc., which
shares shall be exchangeable at any time after July 31, 2009 into a number of
shares of the Company’s common stock equal to such shareholders’ pro rata
allocation of the Share Consideration (the “Exchangeable Shares”). The
Exchangeable Shares have the same voting rights, dividend entitlements and other
attributes as JAG Media common stock. Exchangeable Shares will automatically be
exchanged for JAG Media common stock five years from July 31, 2009, and in
certain other events. The Share Consideration provides the former CardioGenics
shareholders with direct and/or indirect ownership of approximately 85% of JAG
Media’s outstanding common stock (on a fully diluted basis) as of July 31,
2009.
On July
31, 2009, 145,528,195 common shares of JAG Media were issued to former
shareholders of CardioGenics and 276,655,415 common shares of JAG Media were
issued to ExchangeCo. These shares are not registered for resale and, therefore,
shall remain subject to the rights and restrictions of Rule 144. All
Exchangeable Shares received by the CardioGenics shareholders in exchange for
their CardioGenics Common Shares (and any JAG Media common stock into which such
Exchangeable Shares may be exchanged) shall not be registered for resale prior
to six (6) months following July 31, 2009 and, therefore, shall remain subject
to the rights and restrictions of Rule 144 prior to any such
registration.
The Share
Consideration provided the CardioGenics Inc.’s shareholders with direct and/or
indirect ownership of approximately 85% of the Company’s outstanding common
stock (on a fully diluted basis) as of July 31, 2009. Based on the five-day
average price of the Company’s common stock of $0.16 per share, the purchase
price approximated was $11,573,536, plus approximately $342,880 of acquisition
costs plus the fair value of options and warrants assumed of
$644,806.
A summary
of the purchase price allocation is as follows:
|
Common
stock issued
|
$ | 11,573,536 | ||
|
Acquisition
costs incurred
|
342,880 | |||
|
Fair
value of options and warrants assumed
|
644,806 | |||
|
Total
purchase price
|
$ | 12,561,222 |
F-20
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
The
purchase price has been allocated as follows based on the fair values of the
assets and liabilities acquired:
|
Cash
|
$ | 195,885 | ||
|
Accounts
Payable
|
(386,177 | ) | ||
|
Derivative
liability for warrants assumed
|
(28,700 | ) | ||
|
Goodwill
|
12,780,214 | |||
|
Total
|
$ | 12,561,222 |
The
following pro forma consolidated financial information presents the combined
results of operations of JAG Media Holdings, Inc. and CardioGenics, Inc. as if
the acquisition had occurred as of November 1, 2008 and 2007, after giving
effect to certain adjustments, including the issuance of JAG Media Holdings,
Inc. common stock as part of the purchase price. For the purpose of this pro
forma presentation, both JAG Media Holdings, Inc.’s and CardioGenics, Inc.’s
financial information is presented for the years ended October 31, 2009 and
2008, respectively. The pro forma condensed consolidated financial information
does not necessarily reflect the results of operations that would have occurred
had JAG Media Holdings, Inc. and CardioGenics, Inc. been a single entity during
such periods.
|
Years ended
October
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Revenues
|
$ | 130,826 | $ | 183,914 | ||||
|
Net
Loss
|
$ | 29,859,758 | $ | 5,825,607 | ||||
|
Weighted-average
shares of Common
stock outstanding: Basic
and diluted
|
354,277,422 | 282,353,881 | ||||||
|
Basic
and diluted net loss per common share
|
$ | 0.08 | $ | 0.02 | ||||
|
5.
|
Property
and Equipment
|
The costs
and accumulated depreciation and amortization of property and equipment are
summarized as follows:
|
October 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Furniture
and Fixtures
|
$ | 7,983 | $ | 7,380 | ||||
|
Lab
Equipment
|
99,340 | 83,591 | ||||||
|
Computer
Hardware
|
19,490 | 18,019 | ||||||
|
Computer
Software
|
8,433 | 7,798 | ||||||
|
Leasehold
Improvements
|
91,269 | 84,381 | ||||||
|
Total
Property and Equipment
|
226,515 | 201,169 | ||||||
|
Less
Accumulated Depreciation and Amortization
|
172,177 | 133,951 | ||||||
|
Property
and Equipment, Net
|
$ | 54,338 | $ | 67,218 | ||||
F-21
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
Depreciation
and amortization expense amounted to $26,159 and $32,248 for the years ended
October 31, 2009 and 2008 respectively.
|
6.
|
Patents
|
The costs
and accumulated amortization of patents are summarized as follows:
|
October 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Patents
|
$ | 246,161 | $ | 234,716 | ||||
|
Less:
Accumulated Amortization
|
4,181 | 0 | ||||||
|
Patents,
Net
|
$ | 241,980 | $ | 234,716 | ||||
|
Weighted
Average Life
|
17 Years
|
17 Years
|
||||||
|
|
Amortization
expense amounted to $4,181 and $0 for the years ended October 31, 2009 and
2008 respectively. Amortization expense is expected to be
approximately $4,000 per year for the years ended October 31, 2010 through
2014. During the years ended October 31, 2009 and 2008, the
Company wrote off approximately $23,803 and $29,928 of net book value of
patents, respectively for abandoned
patents.
|
|
7.
|
Goodwill
|
Goodwill
and other intangible assets with indefinite lives are tested for impairment
annually, as required by pronouncement, “Goodwill and Other Intangible
Assets”. First, the fair value of the reporting unit is compared to
its carrying value. If the fair value is less than the carrying value, a second
step is performed. In the second step, an implied goodwill value is determined
by deducting the fair value of all tangible and intangible net assets of the
reporting unit from the fair value of the reporting unit. If the implied fair
value of the goodwill as calculated is less than the carrying amount of the
goodwill, an impairment charge is recorded for the difference.
As
indicated above, the goodwill impairment assessment is a two step
process.
A $12.8
million goodwill impairment charge was recorded in the fourth quarter of
2009. This impairment charge was determined by
management soliciting offers for sale of the JAG Notes division and
receiving therefor offers in the range of $40,000 to $60,000. In
November 2009, the company made a decision to sell the Jag Notes.
On February 10, 2010 we entered into an LLC Membership Interest
Purchase Agreement with Rothcove Partners LLC pursuant to which we would sell
our 100% membership interest in our Pixaya LLC subsidiary to Rothcove. In
consideration for its acquisition of the Pixaya LLC membership interest,
Rothcove assumed $100,000 in accounts payable of Pixaya LLC and its subsidiary
Pixaya (UK) Limited. The transaction closed on February 11, 2010.
A summary
of the change in the Company’s goodwill for the years ended October 31,
2009 is as follows:
|
Goodwill
related to purchase (see Note 4)
|
$
|
12,780,214
|
||
|
Goodwill
impairment charge- JAG
Notes
|
(12,780,214)
|
|||
|
Goodwill,
October 31, 2009
|
$
|
0
|
F-22
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
8.
|
Due
to Director
|
The
amount due to a director is due on demand and carries interest at 10% per
annum. On July 31, 2009, $885,000 due to a director was converted to
23,778,126 common shares of JAG Media. A beneficial conversion charge of
$117,109 was credited against Additional Paid-in Capital. Accrued interest on
the director’s loan of $108,635 was converted to 2,185,522 common shares of JAG
Media at the same time.
On
January 28, 2009, the Company issued to a director and an officer of the Company
a new series of Debentures in the amount of $371,333. The Debentures
were for a term of two years and carry interest at 10% per annum. At
July 31, 2009, the holders of the debentures exchanged the debentures, plus
accrued interest, for 17,017,084 common shares of JAG Media common stock. A
beneficial conversion charge of $335,000 was credited against Additional Paid-in
Capital.
|
9.
|
Income
Taxes
|
The
Company adopted the provisions of the guidance for uncertainty in income taxes
on August 1, 2007. The guidance clarifies the accounting for uncertainty in
income taxes recognized in an enterprise’s financial statement, and prescribes a
recognition threshold and measurement process for financial statement
recognition and measurement of a tax position taken or expected to be taken in a
tax return. It also provides guidance on derecognition classification, interest
and penalties accounting in interim periods disclosure and
transition.
Based on
the Company’s evaluation, management has concluded that there are no significant
tax positions requiring recognition in the consolidated financial
statements.
The
Company has incurred losses in Canada since inception, which have generated net
operating loss carryforwards for income tax purposes. The net
operating loss carryforwards arising from Canadian sources as of October 31,
2009, approximated $6,429,964 (2008 - $2,897,106) which will expire from 2014
through 2029:
All
fiscal years except 2009 have been assessed; however, claims relating to
research and development credits are open for review for the fiscal years ended
October 2009, 2008 and 2007.
As of
October 31, 2009, the Company had net operating loss carryforwards from US
sources of approximately $40,154,000 available to reduce future Federal taxable
income which will expire from 2019 through 2029.
For the
years ended October 31, 2009 and 2008, the Company’s effective tax rate differs
from the statutory rate principally due to the net operating losses for which no
benefit was recorded.
As of
October 31, 2009 and 2008, the Company’s deferred tax assets consisted of the
effects of temporary differences attributable to the following:
F-23
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
October 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Deferred
Revenues
|
$ | — | $ | — | ||||
|
Unearned
Compensation
|
—
|
—
|
||||||
|
Property
and Equipment
|
(15,232 | ) | (7,646 | ) | ||||
|
Net
operating loss carryforwards
|
16,712,575 | 813,792 | ||||||
|
Unrealized
foreign exchange
|
15,951 | 69,424 | ||||||
|
Investment
tax credits
|
(50,903 | ) | (36,840 | ) | ||||
|
Total
Deferred Tax Assets
|
16,662,391 | 838,730 | ||||||
|
Valuation
Allowance
|
(16,662,391 | ) | (838,730 | ) | ||||
|
Net
Deferred Income Taxes
|
$ | — | $ | — | ||||
A
reconciliation of the Canadian combined statutory rate to the Company’s
effective tax rate for the years ended October 31, 2009 and 2008 is as
follows:
|
October 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Statutory
rate
|
34 | % | 34 | % | ||||
|
Decrease
in income tax rate resulting from:
|
||||||||
|
Changes
in tax rate
|
— |
4.1
|
% | |||||
|
Permanent
differences
|
(33.3 | )% | (14.8 | )% | ||||
|
Other
items
|
— |
0.1
|
% | |||||
|
Change
in valuation allowance
|
(0.7 | )% | (23.4 | )% | ||||
|
Effective
tax rate
|
0.0 | % | 0.0 | % | ||||
|
10.
|
Accounts
Payable and Accrued Expenses
|
|
October 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Accounts
Payable
|
$ | 199,868 | $ | 17,512 | ||||
|
Accrued
Interest Payable
|
—
|
204,479 | ||||||
|
Research
and Development
|
31,864 | 26,384 | ||||||
|
Executive
Compensation
|
110,916 | 101,174 | ||||||
|
Patent
Application Costs
|
40,194 | 59,321 | ||||||
|
Legal
Fees
|
231,062 |
—
|
||||||
|
Accounting
Fees
|
137,133 |
—
|
||||||
|
Total
|
$ | 751,037 | $ | 408,870 | ||||
F-24
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
11.
|
Debentures
Payable
|
10%
Senior Convertible Debentures
10%
Senior Convertible Debentures due September 30, 2006 and extended to September
30, 2009. The debentures are senior in right of payment to all other
indebtedness of the Company. Interest is payable at maturity, conversion or
redemption of the debentures. Interest on interest is calculated at 10%
annually, compounded monthly, payable on redemption or conversion of the
debentures. Interest may, at the option of the Company, be paid in cash or
common shares, the latter at the rate of 20.957 common shares per $1 of
interest. The debentures may be converted in multiples of $25,000 by the holder
at any time in exchange for the lesser of (i) 20.957 shares for each $1 face
value of debentures being converted or (ii) at a price equal to 90% of the most
recent issue price of common
shares to an arm's length investor. The Company may redeem the debentures at any
time after giving to the holders of seven (7) days notice, during which time the
holders must notify The Company whether the redemption is to occur in cash or
common shares, the latter in exchange for the
lesser of (i) 20.957 common shares for each $1 face value of debentures being
converted, or (ii) at a price equal to 90% of the most recent issue price of
common shares. Interest may be converted at the option of The Company into
20.957 common shares for each $1 payable. Debentures totaling $234,972 were
payable to former officers of The Company.
On
November 12, 2008, a group of holders of Senior Convertible Debentures, holding
in total $369,972 principal amount of Senior Convertible Debentures plus accrued
interest of $235,243, issued a notice of default under the debentures,
demanding, within sixty (60) days of the notice, payment of principal, or
conversion to common shares of The Company at the rate of 83.828 common shares
for each $1 face value of Debentures outstanding, plus interest on conversion to
common shares at the rate of 20.957 common shares for each $1 of interest
payable. The debentures were settled as follows: the principal amount of
$369,972 was repaid in cash January 27, 2009 and the interest payable on that
date in the amount of $236,243 was satisfied by the issuance of 4,950,945 common
shares of The Company.
At July
31, 2009, $280,000 Senior Convertible Debentures were converted to common shares
of the Company at the rate of 20.957 common shares of The Company for each $.78
face value of the debentures. Interest was converted at the rate of 20.957
common shares of the Company for each $1 payable.
10%
Subordinated Convertible Debentures
10%
Subordinated Convertible Debentures due September 30, 2006 and extended to
September 30, 2009. The debentures are senior in right of payment to all other
indebtedness of the Company except for the senior convertible debentures
described above. Interest is payable at maturity, conversion or redemption of
the debentures. Interest on interest is calculated at 10% annually, compounded
monthly, payable on redemption or conversion of the debentures. Interest may, at
the option of the Company, be paid in cash or common shares of the Company, the
latter at the rate of 20.957 common shares of the Company per $1 of
interest. The debentures may be converted in multiples of $25,000, or
the entire outstanding principal sum if less than $25,000, by the holder at any
time in exchange for the lesser of (i) 20.957 shares for each $1 face value of
debentures being converted, or (ii) at a price equal to 90% of the most recent
issue price of common shares to an arm's length investor. The Company may redeem
the debentures at any time after the giving to the holders of seven (7) days
notice, during which time the holders must notify the Company whether the
redemption is to occur in cash or common shares, the latter in exchange for the
lesser of (i) 20.957 common shares of the Company for each $1 face value of
debentures being converted, or (ii) at a price equal to 90% of the most recent
issue price of common shares of the Company. Interest may be converted at the
option of The Company into 20.957 common shares of the Company for each $1
payable.
F-25
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
At July
31, 2009, all of the 10% subordinated convertible debentures were converted to
common shares of the Company at the rate of 20.957 common shares of the Company
for each $.78 face value of the debentures. Interest was converted at rate of
20.957 common shares of CardioGenics Inc. for each $1 payable.
Current
convertible debentures consist of:
|
October 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
10%
Senior Convertible Debentures
|
$ | 25,000 | $ | 674,972 | ||||
|
10%
Subordinated Convertible Debentures
|
- | 332,000 | ||||||
| $ | 25,000 | $ | 1,006,972 | |||||
The
convertible debentures are accounted for in accordance with the “Accounting for
Convertible Securities with Beneficial Conversion Features or Contingently
Adjustable Conversion Ratios” policy. The following summarizes the significant
terms and accounting for each convertible debenture entered into by the
Company:
F-26
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
$674,972
|
$332,000
|
|||||||
|
Debenture
|
Debenture
|
|||||||
|
Date
issued
|
9/30/2003
|
9/21/2004
|
||||||
|
Debenture
Amount
|
$ | 674,972 | $ | 332,000 | ||||
|
#
of Debentures
|
16 | 9 | ||||||
|
Gross
Proceeds
|
$ | 674,972 | $ | 332,000 | ||||
|
Net
Proceeds
|
$ | 674,972 | $ | 332,000 | ||||
|
Warrants
Issued to Investors
|
14,145,388 | 6,957724 | ||||||
|
Warrant
Exercise Price
|
$ | 0.047 | $ | 0.047 | ||||
|
Warrant
Fair Value (WFV)
|
$ | 0.02514 | $ | 0.0225 | ||||
|
Warrant
Relative Fair Value (WRFV)
|
$ | 232,926 | $ | 106,557 | ||||
|
Beneficial
Conversion Feature (BCF)
|
$ | 84,432 | $ | 33,517 | ||||
|
Black-Scholes
Model Assumptions
|
||||||||
|
Fair
value of common stock
|
$ | 0.037 | $ | 0.037 | ||||
|
Risk
Free Interest Rate (%)
|
4.73 | 4.73 | ||||||
|
Expected
Volatility
|
.7248 | .6866 | ||||||
|
Life
of Warrants (years)
|
7 | 6 | ||||||
|
Costs
associated with issuance classified as deferred debt issuance
costs
|
$ | 41,048 | $ | 12,554 | ||||
|
Amortization
of WFV and BCF as Non-cash Interest Expense
|
$ | 317,358 | $ | 140,074 | ||||
|
Principal
and Interest Converted
|
— | — | ||||||
|
Shares
Issued Upon Conversion
|
— | — | ||||||
|
Principal
and Interest Repayments in Shares Of Common Stock
|
— | — | ||||||
|
Shares
Issued for Principal and Interest Repayments
|
— | — | ||||||
|
Principal
and Interest Repayments in Cash
|
— | — | ||||||
The
accrued interest payable on the debentures was $0 and $565,931 as of October 31,
2009 and 2008, respectively.
On May 6,
2007, the maturity date of the debentures payable was extended to September 30,
2007. The term of the existing warrants associated with the original debentures
was extended from September 30, 2010 to September 30, 2011. The
conversion price was amended to the lesser of 1) a discount of 10% of whatever
forms an arm’s length financing, existing or in the future, comes in at or 2)
$0.047. At the date of the extension, The Company recorded in its
statement of operations a Loss on Extinguishment of Debt of
$44,096. The debentures were fully accreted to face value at the time
of the extension.
On
October 2, 2008 the maturity date of the debentures payable was extended to
September 30, 2009. The existing warrants associated with the original
debentures were cancelled and replaced by warrants entitling the holder to
purchase 31.44 common shares in the Company for each $1 principal amount of the
debenture at a price of $0.047 per share up to and including July 31,
2012.
On
November 12, 2008, a group of holders of Senior Convertible Debentures, holding
in total $369,972 principal amount of Senior Convertible Debentures plus accrued
interest of $236,243, issued a notice of default under the debentures,
demanding, within sixty (60) days of the notice, payment of principal, or
conversion to common shares at the rate of 83.828 common shares of the Company
for each $1 face value of Debentures outstanding, plus interest on conversion to
common shares of the Company at the rate of 20.957 common shares of the Company
for each $1 of interest payable. The debentures were settled as follows: the
principal amount of $369,972 was repaid in cash January 27, 2009 and the
interest payable on that date in the amount of $236,243 was satisfied by the
issuance of 4,950,945 common shares.
F-27
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
At the
date of the extension, the Company recorded in its statement of operations a
Loss on Extinguishment of Debt of $231,580. The debentures were fully
accreted to face value at the time of the extension.
10%
Convertible Debentures
On
January 28, 2009, the Company issued to a director and an officer of the Company
a new series of Debentures in the amount of $371,333.The Debentures were for a
term of two years and carried interest at 10% per annum, interest payable at
maturity.
At July
31, 2009 the debenture holders exchanged the debentures, plus accrued interest,
for 17,017,084 common shares of the Company’s common stock. A beneficial
conversion charge of $335,000 was credited to Additional Paid-in
Capital.
|
12.
|
Stock
Based Compensation
|
The
Company follows the guidance for stock-based compensation. Stock-based employee
compensation related to stock options for each of the years ended October 31,
2009 and 2008 amounted to $-0-.
The fair
value of each option granted is estimated on grant date using the Black-Scholes
option pricing model which takes into account as of the grant date the exercise
price and expected life of the option, the current price of the underlying stock
and its expected volatility, expected dividends on the stock and the risk-free
interest rate for the term of the option. The Company granted to a consultant
300,000
stock options during the year ended October 31, 2009 and no stock options during
the year ended October 31, 2008.
The
following is a summary of the common stock options granted, forfeited or expired
and exercised under the Plan:
|
Weighted
|
||||||||
|
Average
|
||||||||
|
Exercise
|
||||||||
|
Options
|
Price
|
|||||||
|
Outstanding
– October 31, 2007
|
9,901,198 | $ | 0.02 | |||||
|
Forfeited/expired
|
— | — | ||||||
|
Exercised
|
— | — | ||||||
|
Outstanding
– October 31, 2008
|
9,901,198 | — | ||||||
|
Granted
|
— | — | ||||||
|
Forfeited/expired
|
(4,191,400 | ) | $ | 0.06 | ||||
|
Exercised
|
(5,709,798 | ) | — | |||||
|
Assumed
upon JAG Media acquisition
|
2,750,000 | $ | 0.25 | |||||
|
Granted
|
300,000 | $ | 0.09 | |||||
|
Outstanding
– October 31, 2009
|
3,050,000 | $ | 0.23 | |||||
|
Exercisable
|
3,050,000 | $ | 0.23 | |||||
Options
typically vest immediately at the date of grant. As such, the Company
does not have any unvested options or unrecognized compensation expense at
October 31, 2009 and 2008.
F-28
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
The fair
value of each option granted and assumed is estimated on grant date using
the Black-Scholes option pricing model which takes into account as of the grant
date the exercise price and expected life of the option, the current price of
the underlying stock and its expected volatility, expected dividends on the
stock and the risk-free interest rate for the term of the option. The
following is the average of the data used to calculate the fair
value:
|
Risk
Free Interest Rate
|
1.1 – 3.4 | % | ||
|
Expected
Life (Years)
|
1.83 – 9.75 | % | ||
|
Expected
Volatility
|
50.11 – 62.14 | % | ||
|
Expected
Dividends
|
$ | — |
The
following table summarizes information on stock options outstanding at October
31, 2009:
|
Options Outstanding and
Exercisable
|
||||||||||||||||||
|
Weighted
|
||||||||||||||||||
|
Number
|
Weighted
|
Average
|
||||||||||||||||
|
Outstanding
|
Average
|
Remaining
|
Aggregate
|
|||||||||||||||
|
Range
of
|
at
|
Exercise
|
Life
|
Intrinsic
|
||||||||||||||
|
Exercise Price
|
October 31,2009
|
Price
|
(Years)
|
Value
|
||||||||||||||
| $ | 0.02 | 750,000 | $ | 0.02 | 1.83 | |||||||||||||
| $ | 0.09 | 300,000 | 0.09 | 9.75 | ||||||||||||||
| $ | 0.34 | 2,000,000 | 0.34 | 9.75 | ||||||||||||||
| 3,050,000 | 7.80 | $ | 467,500 | |||||||||||||||
|
For the Year Ended October
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Weighted
Average Fair Value of Options Granted
|
$ | 0.35 | $ | — | ||||
|
Cash
Received for Exercise of Stock Options
|
$ | 28 | $ | — | ||||
The
intrinsic value is calculated as the difference between the market value as of
October 31, 2009. and the exercise price of the
shares. The market value as of October 31, 2009. was $0.39 as reported by the
NASDAQ Stock Market.
|
13.
|
Stockholders’
Equity (Deficit)
|
Comprehensive
Loss
Comprehensive
loss, which includes net loss from the change in the foreign currency
translation account, for the years ended October 31, 2009 and 2008 respectively
is as follows:
F-29
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
2009
|
2008
|
|||||||
|
Net
(Loss)
|
$ | (28,927,583 | ) | $ | (1,353,221 | ) | ||
|
Currency
translation adjustment
|
(529,296 | ) | 422,766 | |||||
|
Comprehensive
(Loss)
|
$ | (29,456,879 | ) | $ | (930,455 | ) | ||
Equity
Instruments Issued for Services Rendered
During
the years ended October 31, 2000 through 2006 CardioGenics Inc. issued stock
options with a nominal exercise price in exchange for services rendered to
CardioGenics Inc. The fair value of each stock option was measured at the fair
value of the underlying services on the date of grant. The fair value
of each grant was charged to the related expense in the statement of
operations.
The
Company assumed options outstanding at JAG Media entitling the employees to
purchase 750,000 common shares of the Company’s stock at a price of $0.02 per
share to August 31, 2011. The Company issued options to employees entitling the
employees to purchase 2,000,000 common shares of the Company’s stock at a price
of $0.34 per share to July 31, 2019, based upon change of control provisions in
their employment agreements. All these options were immediately
vested. The fair value of the 2,750,000 options is included in the
purchase price.
At August
1, 2009, the Company issued options to a consultant entitling the consultant to
purchase 300,000 common shares of the Company’s stock at a price of $0.09 per
share to July 31, 2019. These options were immediately
vested.
F-30
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
October
31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Warrants
|
||||||||
|
Issued
to subscribers to the debenture financing of 2003 and its related
extension entitling the holder to purchase 1 common share of the Company
at an exercise price of $0.047 per common share up to and including July
31, 2012
|
21,218,082 | 21,218,082 | ||||||
|
Issued
to subscribers to the debenture financing of 2004 and its related
extension entitling the holder to purchase 1 common share in the Company
at an exercise price of $0.047 per common share up to and including July
31, 2012
|
10,436,586 | 10,436,586 | ||||||
|
Issued
to agents for the debenture financings of 2003 and 2004 entitling the
holder to purchase 1 common share in the Company at an exercise
price of $0.047 per common share up to and including July 31,
2012
|
2,084,174 | 2,084,174 | ||||||
|
Issued
to former employee entitling the holder to purchase 1 common share in the
company at an exercise price of $0.047 per common share up to and
including July 31, 2012
|
1,362,205 | 1,362,205 | ||||||
|
Issued
to Consultants July 31, 2009, entitling the holder to purchase 1 common
share of the company at an exercise price of $0.09 per share up to and
including July 31, 2012
|
1,047,850 | — | ||||||
|
Issued
to consultant August 1, 2009, entitling the holder to purchase 1 common
share in the company at an exercise price of $0.09 per common share up to
and including July 31, 2017
|
2,870,848 | — | ||||||
|
Total
Warrants outstanding
|
39,019,745 | 35,101,047 | ||||||
F-31
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
As of
July 31, 2009, the conversion of the warrants would result in the issuance of
common shares in excess of the number of common shares authorized, the Company
determined that based on the guidance on derivative financial instruments
indexed to, and potentially settled in a Company’s own stock, the Company would be prohibited
from concluding that it would have a sufficient number of authorized and
unissued shares to net-share settle any of those warrants or any other warrants
or options previously issued or granted to non-employees. Therefore, as of the
date of the reverse acquisition, the Company recorded the related fair value of
all warrants issued with prior debentures previously issued to non-employees as
a liability. Subsequent changes in the fair value of such options and warrants
at the end of each reporting period were to be recorded as charges or credits to
the Company’s results of operations. On September 30, 2009, the
Company’s authorized share capital was amended to increase the number of
authorized common shares to 650,000,000. At that time the outstanding
options and warrants were re-valued with the subsequent valuation of $13,501,360
re-classified to Additional Paid-In Capital.
At July
31, 2009, the Company assumed the remainder of warrants dated May 24, 2006
entitling YA Global to purchase 250,000 shares of the Company’s common stock for
$0.40 per share. These warrants were exercised August 5,
2009.
|
14.
|
Standby
Equity Distribution Agreement
|
On March
12, 2009, the Company and YA Global Master SPV Ltd. (“YA Ltd”) entered into a
Standby Equity Distribution Agreement (The “SEDA”) pursuant to which YA Ltd
agreed to purchase up to $5,000,000 of the Company’s common stock (the
“Commitment Amount”) over the course of the thirty-six (36) months following the
date the registration statement for the shares to be issued pursuant to the SEDA
is first declared effective (the “Commitment Period”). The Company shall have
the right, but not the obligation, to sell common stock to YA Ltd during the
Commitment Period. Each right to sell common stock to YA Ltd is an “Advance”
under the SEDA.
In order
to request an Advance under the SEDA, the Company must submit a written notice
to YA Ltd specifying the amount of the Advance (an “Advance Notice”). An Advance
Notice may be delivered to YA Ltd every five (5) trading days. The common stock
issued to YA Ltd in connection with each Advance Notice shall be issued at a
purchase price equal to 95% of the lowest Volumes Weighted Average Price
(“VWAP”) during the five trading days immediately following the date of the
Advance Notice, as reported by Bloomberg, L.P. In addition, (i) each Advance may
not exceed $250,000; (ii) the aggregate amount of the Advances pursuant to the
SEDA shall not exceed the Commitment Amount; and, (iii) in no event shall the
number of shares of common stock issuable to YA Ltd pursuant to an Advance cause
the aggregate number of shares of common stock beneficially owned by YA Ltd and
its affiliates to exceed 9.99% of the then outstanding common stock of the
Company. Further, the Company’s common stock being authorized for quotation on a
“Principal Market,” which is defined as the NASDAQ Global Select Market, the
NASDAQ Global Market, the NASDAQ Capital Market, the NYSE Euronext or the OTC
Bulletin Board of the New York Stock Exchange, shall be a condition to any
Advance. Each Advance shall also be subject to such additional terms and
conditions as are set forth in the SEDA. On the 11th trading
day following the completion of the Commencement Date, as defined in the
Registration Rights Agreement (the “Commencement Date”), the Company shall issue
to YA Ltd, as a commitment fee, shares of the Company’s common stock in an
amount equal to $250,000 divided by the average of the VWAP for each of the ten
(10) trading days following the effective date of the Acquisition (the
“Commitment Fee Shares”). The Commitment Fee Shares shall be included on any
registration statement filed by the Company after the date of the SEDA, unless
such shares may be resold without any limitation pursuant to Rule
144.
F-32
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
On March
12, 2009, concurrent with the execution of the SEDA, the Company and YA Ltd also
entered into a Registration Rights Agreement (the “Registration Rights
Agreement”) pursuant to which the Company agreed to register the shares of the
Company’s common stock to be issued in connection with the SEDA (the
“Registrable Securities”). The Company may not file the registration statement
for the Registrable Securities (the “Registration Statement”) prior to the tenth
(10th)
trading day following the Commencement Date and the Company shall not have the
ability to make any Advances under the SEDA until the Registration Statement is
declared effective. The Company shall cause the Registration Statement that has
been declared effective to remain effective at all times until all Registrable
Securities under the Registration Statement cease to be Registrable Securities.
Once issued, Registrable Securities cease to be Registrable Securities when (i)
such Registrable Securities have been disposed of pursuant to the Registration
Statement; (ii) such Registrable Securities have been sold under circumstances
under which all of the applicable conditions of Rule 144 ( or any similar
provision there in force) are met; or, (iii) in the opinion of counsel to the
Company such Registrable Securities may permanently be sold without registration
and without any time, volume or manner limitations pursuant to Rule
144.
15. Authorized
Share Capital
On
September 30, 2009, the Company’s articles of incorporation were amended to
increase the total number of common shares authorized for issuance from
500,000,000 shares to 650,000,000 shares of common stock, par value $0.00001 per
share. As a result, the total number of shares of all classes of
capital stock authorized for issuance by the Company increased from 550,440,000
shares to 700,440,000 shares with a par value of $.00001 per share, of which
50,000,000 shares are authorized for issuance as preferred stock, 650,000,000
shares are authorized for issuance as common stock, 400,000 shares are
authorized for issuance as Series 2 Class B common stock and 40,000 shares are
authorized for issuance as Series 3 Class B common stock.
The
shares of Series 2 and Series 3 Class B common stock will be non-voting, have
dividend and liquidation rights equal to Class A common stock and be
redeemable. Redemption by the Company shall be mandatory within six
months (or as soon thereafter as permitted by law) following the final
resolution of any successor lawsuit brought by the Company relating to the
subject matter of the Company’s now dismissed lawsuit against certain brokerage
firms (Jag Media Holdings, Inc. v. A.G. Edwards & Sons, et al.) in U.S.
District Court for the Southern District of Texas, which date shall be
determined by board of directors. The redemption price per share of
the Series 2 Class B common stock will be the greater of (i) the par value of
each share or (ii) the amount obtained by dividing (a) 90% of the net proceeds
to the Company of such lawsuit after payment of fees and expenses incurred in
connection with such lawsuit and all taxes on net income accrued or paid with
respect to such amount by (b) the total number of shares of Series 2 Class B
common outstanding. The redemption price per share of the Series 3
Class B common stock will be the greater of (i) par value of each share or (ii)
.0025% of 10% of the net proceeds to the Company of such lawsuit after payment
fees and expenses incurred in connection with such lawsuit and all taxes on net
income accrued or paid with respect to such amount.
Since the
value of the Series 2 and Series 3 Class B common stock is contingent upon the
outcome of a pending or successor litigation, the Company recorded the shares of
Series 2 and Series 3 Class B common stock that were originally issuable during
the year ended July 31, 2003 at their total par value of $4.20. Since
the Company will be required to distribute substantially all the proceeds of the
pending litigation to holders of Series 2 and Series 3 Class B common stock, the
Company had classified the shares as the equivalent of mandatorily redeemable
preferred stock and excluded their carrying value from stockholders’ equity
(deficit) in the accompanying October 31, 2009 consolidated balance sheets
pursuant to the rules and regulations of the Securities and Exchange Commission
and “Accounting for Certain Financial Instruments with Characteristics of Both
Liabilities and Equity.”
F-33
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
16.
|
Net
Loss per Share
|
The
following table sets forth the computation of weighted-average shares
outstanding for calculating basic and diluted earnings per share
(EPS):
|
Years Ended
October 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Weighted-average
shares - basic
|
303,850,580 | 223,019,571 | ||||||
|
Effect
of dilutive securities
|
— | — | ||||||
|
Weighted-average
shares - diluted
|
303,850,580 | 223,019,571 | ||||||
Basic
earnings per share “EPS” and diluted EPS for the years ended October 31, 2009
and 2008 have been computed by dividing the net loss available to common
stockholders for each respective period by the weighted average shares
outstanding during that period. All outstanding options, warrants and shares to
be issued upon the exercise of the outstanding options and warrants representing
42,069,745 and 45,002,245 incremental shares respectively have been excluded
from the years ended October 31, 2009 and 2008 computation of diluted EPS as
they are antidilutive given the net losses generated.
|
17.
|
Commitments
and Contingent Liabilities
|
Leases
The
Company has entered into an operating lease agreement for the use of operating
space.
Aggregate
minimum annual lease commitments of the Company under the non-cancelable
operating lease as of October31, 2009 are as follows:
|
Year
|
Amount
|
|||
|
2010
|
$ | 43,245 | ||
|
2011
|
36,408 | |||
|
Thereafter
|
— | |||
|
Total
Minimum Lease Payments
|
$ | 79,653 | ||
Lease
expense amounted to $60,883 and $71,785 for the years ended October 31, 2009 and
2008, respectively.
The
preceding data reflects existing leases and does not include replacements upon
their expiration. In the normal course of business, operating leases
are generally renewed or replaced by other leases.
F-34
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
Lawsuits
|
|
a)
|
On
April 22, 2009, the Company was served with a statement of claim from a
former employee claiming compensation for wrongful dismissal and ancillary
causes of action including payment of monies in realization of his
investment in the Company, with an aggregate claim of
$514,000. The Company considers all the claims to be without
any merit, has already delivered a statement of defense and intends to
vigorously defend the action. If the matter eventually proceeds
to trial, the Company does not expect to be found liable on any ground or
for any cause of action.
|
|
|
b)
|
On
June 22, 2009, the Company received a letter from a former advisor with
regards to a Non-Circumvention Agreement dated July 16, 2004 and a
Finder’s Fee Agreement dated December 13, 2004 with said former advisor.
The letter states that the Company has breached said agreements insofar as
the transaction between CardioGenics Inc. and the Company is concerned and
advising that said former advisor is entitled to payment of 8% of the
transaction value in accordance with the terms of the Finder’s Fee
Agreement. The Company’s lawyers have written to said former advisor
denying any contractual breach and explaining why said former advisor’s
claims are without merit.
|
On
January 15, 2010, Flow Capital Advisors, Inc. (“Flow Capital”) filed a lawsuit
against CardioGenics, Inc, and another defendant in the United States District
Court for the Southern District of Florida, Fort Lauderdale Division (Case No.
10-CV-60066-Martinez-Brown) (“Flow Lawsuit”). The Flow Lawsuit alleges that
CardioGenics (i) breached a Finder’s Fee Agreement in connection with the
CardioGenics Acquisition; and (ii) breached a non-circumvention
agreement. Flow Capital is claiming that it is entitled to its
finder’s fee equal to eight percent (8%) of the JAG Media Holdings shares
received by CardioGenics, or the equivalent monetary value of the stock. The
Company and its counsel are currently reviewing the Flow Lawsuit and anticipate
responding to the Flow Lawsuit in the near future.
On
January 14, 2010, Flow Capital filed a lawsuit against JAG Media Holdings Inc.
in the Circuit Court of the 17th
Judicial Circuit In and For Broward County Florida (Case No.
10001713). Pursuant to this lawsuit, Flow Capital alleges that JAG
Media Holdings breached a Non-Circumvention Agreement it had entered into with
Flow Capital, dated January 1, 2004. The Company and its counsel are currently
reviewing this lawsuit and anticipate responding to it in the near
future.
|
c)
|
On
June 20, 2002, we, along with our then President and Chief Executive
Officer, field a complaint in the 165th District Court of Harris County,
Texas against over 150 brokerage firms, alleging, among other things, a
conspiracy among the defendants to short sell our stock. The original
lawsuit was subsequently amended on June 24, 2002 and was removed to the
United States District Court for the Southern District of Texas. The
plaintiffs subsequently filed a motion in the United States District Court
for the Southern District of Texas to have the action remanded back to the
state court where it was originally commenced. That motion was denied and
the action proceeded in the federal district court. On October 1, 2003,
the Court denied various motions to dismiss made on behalf of the
defendants. However, in its ruling, the Court indicated that all motions
to dismiss could have been granted in light of the defective pleading made
by plaintiffs and allowed plaintiffs 20 days to file an amended complaint
to comply with pleading requirements of the Court. Plaintiffs filed an
amended complaint within the required period. Discovery was stayed while
the motions to dismiss were
pending.
|
After
plaintiffs filed their third amended complaint, 78 out of the total of
approximately 150 defendants again filed a motion to dismiss the lawsuit.
On September 6, 2004, the Court entered an order granting the moving defendants’
motion to dismiss the lawsuit, again citing various deficiencies in the
pleadings. The Court did not grant the plaintiffs leave to replead.
The
plaintiffs and the moving defendants have since stipulated to the entry of a
final judgment dismissing the third amended complaint against the moving
defendants with prejudice. Under this stipulation, the parties agreed on entry
of final judgment to (a) waive their right to attorney’s fees or sanctions and
bear their own costs and (b) not appeal the judgment.
On
December 3, 2004, we announced that our original counsel had assigned our legal
retainer agreement in connection with the lawsuit to a legal consortium
consisting of various law firms and other consultants throughout the country,
which includes our original counsel. We have met with our now attorneys and
continue to evaluate our options for recommencing an action against certain
defendants, and possibly other parties, in light of the court’s order and/or
pursing other strategies.
|
18.
|
Supplemental
Disclosure of Cash Flow Information
|
|
Years Ended
|
||||||||
|
October 31
|
||||||||
|
2009
|
2008
|
|||||||
|
Cash
paid during the year for:
|
||||||||
|
Interest
|
$ | 4,464 | $ | 2,643 | ||||
|
Income
taxes
|
$ | — | $ | — | ||||
|
19.
|
Subsequent
Events
|
In May
2009, guidance for subsequent events was issued. The guidance
established general accounting standards and disclosure for subsequent events.
The company adopted this pronouncement during the quarter ended July 31, 2009.
Accordingly the Company has evaluated subsequent events through the date and
time the consolidated financial statements were issued on February 12,
2010. Except for the mattrers disclosed in this financial
statement, no other material subsequent events have occurred since October
31, 2009 that would require recognition or disclosure in these consolidated
financial statements.
On
February 10, 2010 the Company entered into an LLC Membership Interest
Purchase Agreement with Rothcove Partners LLC pursuant to which the
Company would sell its 100% membership interest in its Pixaya LLC
subsidiary to Rothcove. In consideration for its acquisition of the Pixaya LLC
membership interest, Rothcove assumed $100,000 in accounts payable of Pixaya LLC
and its subsidiary Pixaya (UK) Limited. The transaction closed on
February 11, 2010.
|
20.
|
Segment
Reporting
|
The
Company’s operating segments are regularly reviewed by the chief operating
decision maker for purposes of allocating resources and assessing
performance.
The
Company’s financial reporting is organized into two business segments: In Vitro
Diagnostics and Jag Notes.
F-35
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
The
information presented below includes certain expense allocations between the
corporate office and the operating business segments. The information is
presented after all intercompany and intersegment eliminations and is therefore
not necessarily indicative of the results that would be achieved had the
business segments been stand-alone businesses.
The
results of operations and other data for the two operating segments for the
years ending October 31, 2009 and 2008 are as follows:
|
In Vitro
Diagnostics
|
Jag Notes
|
Consolidated
|
||||||||||
|
2009
|
||||||||||||
|
Revenue
|
$ | — | $ | 21,539 | $ | 21,539 | ||||||
|
Cost
of goods sold
|
— | 136,946 | 136,946 | |||||||||
|
General
and administrative
|
1,520,295 | 76,715 | 1,597,010 | |||||||||
|
Research
and Development
|
1,572,337 | — | 1,572,337 | |||||||||
|
Goodwill
impairment charges
|
— | 12,780,214 | 12,780,214 | |||||||||
|
Depreciation
expense and amortization of intangibles
|
54,141 | — | 54,141 | |||||||||
|
Loss
from operations
|
$ | (3,146,773 | ) | $ | (12,972,336 | ) | $ | (16,119,109 | ) | |||
|
Interest
expense
|
$ | 570,474 | $ | 1,366 | $ | 571,840 | ||||||
|
Capital
expenditures — property and equipment
|
8,950 | — | 8,950 | |||||||||
|
Capital
expenditures — Patent application
|
15,164 | — | 15,164 | |||||||||
|
Total
assets
|
2,839,865 | 47,397 | 2,887,262 | |||||||||
F-36
CardioGenics
Holdings Inc.
(A
Development Stage Company)
Notes
to Consolidated Financial Statements
October
31, 2009 and 2008
|
In Vitro Diagnostics
|
Jag Notes
|
Consolidated
|
||||||||||
|
2008
|
||||||||||||
|
Revenue
|
$ | — | $ | — | $ | — | ||||||
|
Cost
of good sold
|
— | — | — | |||||||||
|
General
and administrative
|
177,169 | — | 177,169 | |||||||||
|
Research
and Development
|
24,531 | — | 24,531 | |||||||||
|
Depreciation
expense and amortization of intangibles
|
62,176 | — | 62,176 | |||||||||
|
Loss
from operations
|
$ | (263,876 | ) | $ | — | $ | (263,876 | ) | ||||
|
Interest
expense
|
$ | 432,005 | $ | — | $ | 432,005 | ||||||
|
Capital
expenditures — property and equipment
|
1,012 | — | 1,012 | |||||||||
|
Capital
expenditures — Patent application
|
33,824 | — | 33,824 | |||||||||
|
Total
assets
|
784,230 | — | 784,230 | |||||||||
F-37
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
|
CARDIOGENICS
HOLDINGS INC.
|
|
|
By:
|
/s/ Yahia Gawad
|
|
Yahia
Gawad
|
|
|
Chief
Executive Officer
|
|
Dated:
February __, 2010
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the Registrant and in the
capacities and on the date indicated.
|
SIGNATURE
|
TITLE
|
DATE
|
||
|
/s/ Yahia
Gawad
|
Chief
Executive Officer
|
February
16, 2010
|
||
|
Yahia Gawad
|
||||
|
/s/ James
Essex
|
Chief
Financial Officer
|
February
16, 2010
|
||
|
James Essex
|
EXHIBIT
INDEX
|
31.1
|
Section
302 Certification of Chief Executive Officer
|
|
|
31.2
|
Section
302 Certification of Chief Financial Officer
|
|
|
32.1
|
Section
906 Certification of Chief Executive Officer and Chief Financial
Officer
|
|
|
21.1
|
Subsidiaries
of Registrant.
|
|
|
10.28
|
Retainer
Agreement dated January 20, 2010 between Registrant and Wolfe, Axelrod
& Weinberger Associates LLC
|
|
|
10.29
|
Letter
Agreement dated January 18, 2010 between Registrant and The Investor
Relations Group, Inc.
|
|
|
10.30
|
Employment
Agreement dated July 31, 2009 between Registrant and Dr. Yahia
Gawad.
|
|
|
10.31
|
LLC
Membership Interest Purchase Agreement dated February 10, 2010 between
Registrant and Rothcove Partners LLC.
|
|
| 23.1 | Consent of J.H. Cohn LLP | |
| 23.2 | Consent of BDO Donwoody LLP |
