Attached files
| file | filename |
|---|---|
| EX-10 - INTEGRATED BIOPHARMA INC | exhibit10_1.htm |
| EX-32 - INTEGRATED BIOPHARMA INC | exhibit32_2.htm |
| EX-31 - INTEGRATED BIOPHARMA INC | exhibit31_1.htm |
| EX-32 - INTEGRATED BIOPHARMA INC | exhibit32_1.htm |
| EX-31 - INTEGRATED BIOPHARMA INC | exhibit31_2.htm |
UNITED
STATES SECURITIES AND EXCHANGE COMMISSION
Washington
D.C. 20549
____________
FORM
10-Q
x Quarterly Report
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of
1934
For the
quarterly period ended December 31, 2009
OR
o
Transition Report Pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934
For the
transition period
from to
Commission
File Number 001-31668
INTEGRATED BIOPHARMA,
INC.
(Exact
name of registrant, as specified in its charter)
| Delaware | 22-2407475 |
| (State or other jurisdiction of | (I.R.S. Employer |
| incorporaton or organization | Identification No.) |
|
|
|
225 Long Ave.,
Hillside, New Jersey
|
07205
|
| (Address of principal executive offices) |
(Zip
Code)
|
(888)
319-6962
(Registrant's telephone number,
including Area Code)
Not
Applicable
(Former
name, former address and former fiscal year, if changed since last
report)
Indicate
by check mark whether the Registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during
the preceding 12 months (or for such shorter period that the Registrant was
required to file such reports), and (2) has been subject to such filing
requirements for the past 90 days.
Yes x No o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
the definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act. (Check
one):
|
Large
accelerated filer
|
Accelerated
filer
|
Non-accelerated
filer
|
Smaller
reporting company þ
|
Indicate by check whether the
registrant is a shell company (as defined in Rule 12b-2 of the Exchange
Act).
Yes o No x
Applicable
only to Corporate Issuers:
The
number of shares outstanding of each of the issuer’s class of common stock, as
of the latest practicable date:
| Class |
Outstanding at February 12,
2010
|
| Common Stock, $0.002 par value | 20,469,342 Shares |
|
|
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
FORM
10-Q QUARTERLY REPORT
For
the Six Months Ended December 31, 2009
INDEX
|
Page
|
||
|
Part
I. Financial Information
|
||
|
Item
1.
|
Condensed
Consolidated Statements of Operations for the Three and Six Months Ended
December 31, 2009 and 2008 (unaudited)
|
2
|
|
Condensed
Consolidated Balance Sheets as of December 31, 2009 (unaudited) and June
30, 2009
|
3
|
|
|
Condensed
Consolidated Statements of Changes in Stockholders’ Equity for the Six
Months Ended December 31, 2009 (unaudited)
|
4
|
|
|
Condensed
Consolidated Statements of Cash Flows for the Six Months Ended December
31, 2009 and 2008 (unaudited)
|
5
|
|
|
Notes
to Condensed Consolidated Statements
|
6
|
|
|
Item
2.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
22
|
|
Item
3.
|
Quantitative
and Qualitative Disclosures about Market Risk
|
30
|
|
Item
4T.
|
Controls
and Procedures
|
30
|
|
Part
II. Other Information
|
||
|
Item
1.
|
Legal
Proceedings
|
31
|
|
Item
1A.
|
Risk
Factors
|
31
|
|
Item
2.
|
Unregistered
Sales of Equity Securities and Use of Proceeds
|
32
|
|
Item
3.
|
Defaults
Upon Senior Securities
|
32
|
|
Item
4.
|
Submission
of Matters to a Vote of Security Holders
|
32
|
|
Item
5.
|
Other
Information
|
32
|
|
Item
6.
|
Exhibits
|
33
|
|
Other
|
||
|
Signatures
|
34
|
|
Disclosure Regarding Forward-Looking
Statements Disclosure Regarding Forward-Looking Statements Certain
statements in the Quarterly Report on Form 10-Q may constitute “forward-looking”
statements as defined in Section 27A of the Securities Act of 1933, as amended
(the “Securities Act”), Section 21E of the Securities Act of 1934, as amended
(the “Exchange Act”), the Private Securities Litigation Reform Act of 1995 (the
“PSLRA”) or in releases made by the Securities and Exchange Commission, all as
may be amended from time to time. Such forward-looking statements involve known
and unknown risks, uncertainties and other factors which may cause the actual
results, performance or achievements of Integrated BioPharma, Inc. (the
“Company”) or industry results, to be materially different from any future
results, performance or achievements expressed or implied by such
forward-looking statements. Statements that are not historical fact are
forward-looking statements. Forward-looking statements can be identified by,
among other things, the use of forward-looking language, such as the words,
“plan”, “believe”, “expect”, “anticipate”, “intend”, “estimate”, “project”,
“may”, “will”, “would”, “could”, “should”, “seeks”, or “scheduled to”, or other
similar words, or the negative of these terms or other variations of these terms
or comparable language, or by discussion of strategy or intentions. These
cautionary statements are being made pursuant to the Securities Act, the
Exchange Act and the PSLRA with the intention of obtaining the benefits of the
“safe harbor” provisions of such laws. The Company cautions investors that any
forward-looking statements made by the Company are not guarantees or indicative
of future performance. Important assumptions and other important factors that
could cause actual results to differ materially from those forward-looking
statements with respect to the Company, include, but are not limited to, the
risks and uncertainties affecting its businesses described in Items 1 and 1A of
the Company’s Annual Report filed on Form 10-K for the year ended June 30, 2009
and in registration statements and other securities filings by the Company.
Although the Company believes that its plans, intentions and expectations
reflected in or suggested by such forward-looking statements are reasonable,
actual results could differ materially from a projection or assumption in any of
the forward-looking statements and are subject to change due inherent risks and
uncertainties. The forward-looking statements contained in this Quarterly Report
on Form 10-Q are made only as of the date hereof and the Company does not have
or undertake any obligation to update or revise any forward-looking statements
whether as a result of new information, subsequent events or otherwise, unless
otherwise required by law.
1
ITEM 1. FINANCIAL
STATEMENTS
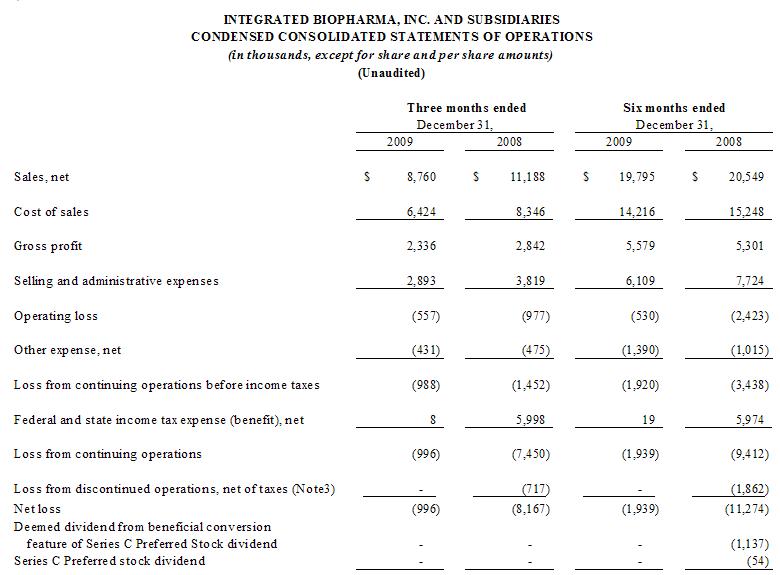
2


3

4
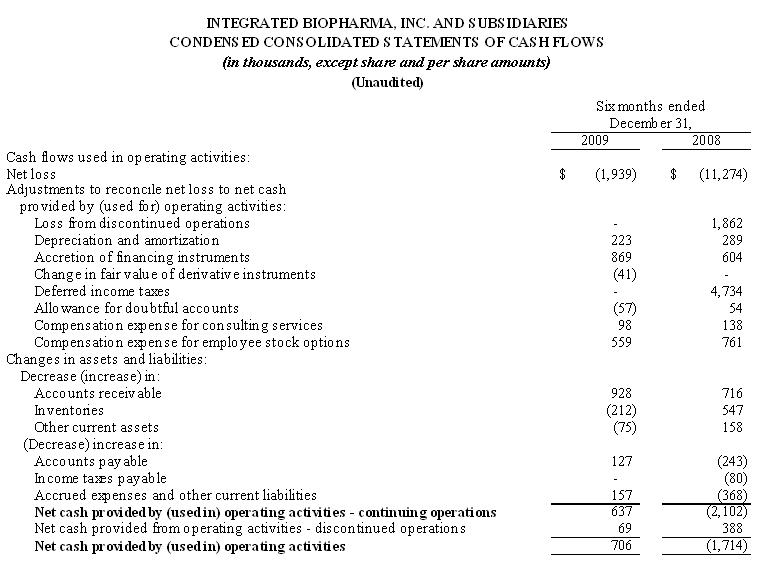

5
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Note
1. Principles of Consolidation and Basis of Presentation
The
accompanying condensed financial statements for the interim periods are
unaudited and include the accounts of the Company. The interim condensed
consolidated financial statements have been prepared in conformity with Rule
10-01 of Regulation S-X of the Securities and Exchange Commission (“SEC”) and
therefore do not include information or footnotes necessary for a complete
presentation of financial position, results of operations and cash flows in
conformity with accounting principles generally accepted in the United States of
America. However, all adjustments (consisting only of normal recurring
adjustments) which are, in the opinion of management, necessary for a fair
presentation of the financial position and operating results for the periods
presented have been included. These condensed financial statements should be
read in conjunction with the financial statements and notes thereto, together
with Management’s Discussion and Analysis of Financial Condition and Results of
Operations, contained in the Company’s Annual Report on Form 10-K for the fiscal
year ended June 30, 2009 (“10-K”), as filed with the SEC. The June 30, 2009
balance sheet was derived from audited financial statements, but does not
include all disclosures required by accounting principles generally accepted in
the United States of America. The results of operations for the three and six
months ended December 31, 2009, are not necessarily indicative of the results
for the full fiscal year ending June 30, 2010 or for any other
period.
Integrated
BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the
“Company”), is engaged primarily in manufacturing, distributing, marketing and
sales of vitamins, nutritional supplements and herbal products. The
Company’s customers are located primarily in the United States. The Company was
previously known as Integrated Health Technologies, Inc. and, prior to that, as
Chem International, Inc. The Company was reincorporated in its current form in
Delaware in 1995. As of September 22, 2009, the Company’s common stock trades on
the OTC Bulletin Board under the symbol INBP.OB. From February 27,
2009 through September 22, 2009, the Company’s common stock traded on the Pink
Sheets under the symbol INBP.PK. Immediately prior to February 27,
2009, the Company’s common stock traded on the NASDAQ Global Market under the
symbol “INBP.” The Company continues to do business with certain of
its customers and vendors as Chem International, Inc.
The
Company, subsequent to the spin-off of its Biotechnologies segment and the sale
of the Pharmaceutical segment, which occurred in Fiscal 2009, see Note 3. –
Discontinued Operations, has one remaining reportable segment for its operation,
the Nutraceutical segment.
The
Nutraceutical segment, our one remaining business operation, includes:
InB:Manhattan Drug Company, Inc. (“Manhattan Drug”), which manufactures vitamins
and nutritional supplements for sale to distributors, multilevel marketers and
specialized health-care providers; AgroLabs, Inc. (“AgroLabs”), which
distributes for sale through major mass market, grocery, drug and vitamin
retailers, healthful nutritional products under the following brands: Naturally
Noni, Naturally Pomegranate, Naturally Aloe, Aloe Pure, Naturally Thai
Mangosteen, Peaceful Sleep, Green Envy, 1st
Choice Multi-Vitamin, ACAI Extra, ACAI Immune, ACAI Cleanse, and other products
which are being introduced into the market, these are referred to as our branded
proprietary Nutraceutical business and/or products; and The Vitamin Factory,
which sells private label Manhattan Drug products, as well as our AgroLabs
products, through mail order catalogs and the Internet.
The
Company also distributes fine natural chemicals through its wholly-owned
subsidiary IHT Health Products, Inc. and is a distributor of certain raw
materials for DSM Nutritional Products, Inc.
These
condensed consolidated financial statements, reflect the spin-off of iBio, Inc.
("iBio"), the sale of InB: Hauser Pharmaceuticals, Inc. (“Hauser”), the
discontinued operations of The Organic Beverage Company (“TOBC”), and related
transactions (see Note 3. – Discontinued Operations).
6
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Reclassifications.
Certain reclassifications have
been made to the prior year data to conform with the current year
presentation.
Principles of
Consolidation. The accompanying condensed consolidated financial
statements include the accounts of the Company and its wholly-owned
subsidiaries. Intercompany transactions and accounts are eliminated in
consolidation.
Estimates.
The preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect the reported amounts of assets and liabilities and disclosure of
contingent assets and liabilities at the date of the financial statements and
the reported amounts of revenues and expenses during the reporting period.
Management bases its estimates on historical experience and on various other
assumptions that are believed to be reasonable under the circumstances, the
results of which form the basis for making judgments about the carrying values
of assets and liabilities that are not readily apparent from other sources. The
most significant estimates include:
|
·
|
sales
returns and allowances;
|
|
·
|
trade
marketing and merchandising;
|
|
·
|
allowance
for doubtful accounts;
|
|
·
|
inventory
valuation;
|
|
·
|
valuation
and recoverability of long-lived and intangible assets, including the
values assigned to acquired intangible
assets;
|
|
·
|
income
taxes and valuation allowance on deferred income taxes;
and,
|
|
·
|
accruals
for, and the probability of, the outcome of current
litigation.
|
On a
continual basis, management reviews its estimates utilizing currently available
information, changes in facts and circumstances, historical experience and
reasonable assumptions. After such reviews, and if deemed appropriate, those
estimates are adjusted accordingly. Actual results could differ from those
estimates. Nothing has come to our attention which would cause a change in these
estimates.
Subsequent
Events. For purposes of preparing the accompanying condensed
consolidated financial statements and the following notes to these condensed
financial statements, the Company evaluated subsequent events through the date
the condensed financial statements were issued.
Investment in
iBio, Inc. The Company accounts for its investment in iBio on the cost
basis as it retained approximately 6% of its interest in iBio at the time of the
spin-off of this subsidiary (see Note 3. Discontinued
Operations). The Company reviews its investment in iBio for
impairment and records a loss when there is deemed to be an impairment of the
investment.
Revenue
Recognition. For product sales, the Company recognizes revenue when the
product’s title and risk of loss transfers to the customer. The Company believes
this revenue recognition practice is appropriate because the Company’s sales
policies meet the four criteria of Staff Accounting Bulletin 104 which are: (i)
persuasive evidence that an arrangement exists, (ii) delivery has occurred,
(iii) the seller’s price to the buyer is fixed and determinable and (iv)
collectability is reasonably assured. The Company’s sales policy is to require
customers to provide purchase orders establishing selling prices and shipping
terms. The Company evaluates the credit risk of each customer and establishes an
allowance of doubtful accounts for any credit risk. Sales returns and allowances
are estimated upon shipment.
7
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Shipping and
Handling Costs. Shipping and handling costs are included in cost of
sales.
Trade Marketing
and Merchandising. In order to support the Company’s proprietary
Nutraceutical product lines, various promotional activities are conducted
through the retail trade, distributors or directly with consumers, including
in-store display and product placement programs, feature price discounts,
coupons, and other similar activities. The Company regularly reviews and
revises, when it deems necessary, estimates of costs to the Company for these
promotional programs based on estimates of what will be redeemed by the retail
trade, distributors, or consumers. These estimates are made using various
techniques, including historical data on performance of similar promotional
programs. Differences between estimated expense and actual performance are
generally not material and are recognized as a change in management’s estimate
in a subsequent period.
Supplemental
Statement of Cash Flows

Earnings Per
Share. Basic earnings per common share amounts are based on weighted
average number of common shares outstanding. Diluted earnings per share amounts
are based on the weighted average number of common shares outstanding, plus the
incremental shares that would have been outstanding upon the assumed exercise of
all potentially dilutive stock options, warrants and convertible preferred
stock, subject to anti-dilution limitations using the treasury stock
method.
For both
the three and six months periods ended December 31, 2009 and 2008, total options
and warrants of 3,007,888 and 3,073,017, respectively, to purchase shares of
common stock, were outstanding but were not included in the computation of
diluted earnings per share as they were anti-dilutive as a result of net losses
applicable to common shareholders during the periods.
8
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
For both
the three and six month periods ended December 31, 2009 and 2008, options to
purchase shares of common stock with exercise prices below the market price,
respectively, were outstanding but were not included in the computation of
diluted earnings per share as they are anti-dilutive as a result of net losses
during the period.
For both
the three and six month periods ended December 31, 2009 and 2008, common share
equivalents of 2,250,000 and 1,779,755, respectively, related to the Convertible
Note Payable were not included in the computation of diluted earnings per share
as they were anti-dilutive as a result of net losses applicable to common
shareholders.
Recent Accounting
Pronouncements.
The
Financial Accounting Standards Board released an update to guidance for fair
value measurements and disclosures. This update requires new disclosures for
significant transfers in and out of Level 1 and Level 2 fair value measurements
and separate presentation of certain information in the reconciliation for fair
value measurements using significant unobservable inputs. In addition, the
updated guidance clarifies the requirements of existing disclosures. The update
is effective for interim and annual reporting periods beginning after December
15, 2009, which is the third quarter of fiscal 2010 for the Company. The Company
does not expect this update to have a material impact on its financial
statements.
Note 2.
Liquidity. The Company’s condensed consolidated financial statements have
been prepared assuming that it will continue as a going concern. The Company has
incurred recurring operating losses and negative operating cash flows for the
three consecutive fiscal years ended in June 30, 2009, including a net loss
attributable to common stockholders of $19,367 and negative operating cash flows
of $2,752 for the year ended June 30, 2009. For the six months ended December
31, 2009, the Company had a net operating loss of $530 and a net loss of
$1,939. At December 31, 2009, the Company had cash and cash
equivalents of $1,518, a working capital deficit of $3,693, primarily
attributable to the amended Notes Payable (See Note 7. (c)) in the amount of
$7,805, which were due on November 15, 2009, and an accumulated deficit of
$46,304.
As of
February 12, 2010, the Company has not repaid its amended Notes Payable in the
amount of $7,805, nor has it completed the negotiations of modified terms,
including an extension or refinancing thereof. The Company also has
not received any demand of payment from the amended Notes Payable holders nor
have they exercised their rights to foreclose on substantially all of the assets
of the Company, which were pledged as collateral for the Company’s obligation
under the amended Notes Payable.
Assuming
the Company is able to raise additional capital and/or refinance its Notes
Payable, and it is not adversely affected by the current economic conditions,
the Company believes that its available capital as of December 31, 2009 will
enable it to continue as a going concern through the third quarter of the fiscal
year ending June 30, 2011. There can be no assurance that the Company will be
able to raise additional capital or successfully refinance the amended Notes
Payable of at least $7,805, upon acceptable terms, nor that the current economic
conditions will not negatively impact it. If the Company is unable to raise
additional capital or successfully refinance its amended Notes Payable of at
least $7,805 upon acceptable terms, it would have a material adverse effect on
the Company. The accompanying condensed consolidated financial statements do not
include any adjustments that might result from this uncertainty.
9
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Note
3. Discontinued Operations
In the
fiscal year ended June 30, 2009, the Company classified the spin-off of iBio in
August 2008, the sale of Hauser in March 2009 and the discontinued operations of
TOBC in June 2009 as discontinued operations for the current and prior periods
and the associated results of operations, financial position and cash flows are
separately reported for all periods presented. The remaining net assets of TOBC
are classified as assets and liabilities related to discontinued operations in
the Company’s condensed consolidated balance sheet as of December 31, 2009 and
June 30, 2009.
The net
assets from discontinued operations were comprised of the
following:
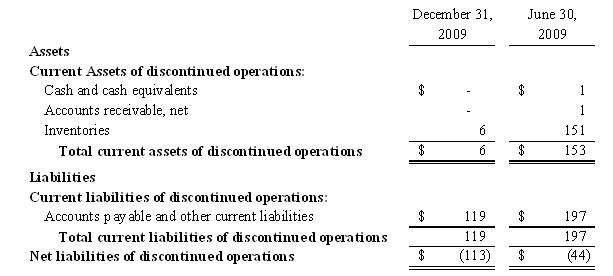
|
(a)
|
Spin-off of
iBio – In August 2008, the Company completed the spin-off of iBio.
As a result, the Company recognized an after-tax loss of $105 during the
first quarter of the fiscal year ended June 30, 2009. iBio revenues from
discontinued operations were $169 for the three and six months ended
December 31, 2008. The Company’s loss from the discontinued
operations of iBio, net of taxes, was $105 for the three and six months
ended December 31, 2008.
|
|
(b)
|
Sale of
Hauser – In March 2009, the Company entered into a stock purchase
agreement and consummated the sale of all of the issued and outstanding
shares of common stock of its wholly owned subsidiary Hauser to Cedarburg
Pharmaceuticals, Inc. (“Cedarburg”). Prior to the sale of Hauser, the
Company sold substantially all the assets of INB: Paxis Pharmaceuticals,
Inc. (“Paxis”) and transferred outstanding payables owed by Paxis (the
“Net Assets of Paxis”) to Hauser in consideration for the outstanding
intercompany debt between these two subsidiaries of the
Company. The Net Assets of Paxis transferred under this
transaction were owned by Hauser at the time of the sale of Hauser’s
common stock to Cedarburg and are no longer assets and liabilities of the
Company. The Company continues to own certain assets of Paxis through its
ownership of common stock of Paxis. The purchase price received by the
Company in connection with the sale of Hauser consisted of $1,160 in cash
and a promissory note in favor of the Company in the principal amount of
$340. The promissory note matures on March 17, 2010 and bears interest at
a rate of 12% per annum, payable quarterly. On April 7, 2009, this
promissory note was sold to CD Financial, LLC, a related party and the
holder of the Company’s Convertible Note Payable (see Note 7(a)), for the
full principal amount of $340 and accrued interest of
$2.
|
As a
result of the sale of Hauser, the Company recognized a loss of $629 during the
fiscal year ended June 30, 2009. The Hauser revenues from discontinued
operations were $1,043 and $2,183 for the three and six months ended December
31, 2008, respectively. The Company’s net loss from the Hauser discontinued
operations, net of taxes were $598 and $1,385 for the three and six months ended
December 31, 2008, respectively.
10
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
|
(c)
|
Discontinued
operations of TOBC – During the six months ended September 30,
2008, the Company curtailed its operations of TOBC and combined the sales
efforts for the Syzmo™ product line with the AgroLabs
products. In June 2009, the Company determined that the Syzmo™
product line was to be discontinued as the Company does not have the
financial resources to pursue the further development of the Syzmo™
product in the very competitive energy drink market
place.
|
Revenues
from TOBC’s discontinued operations were $(5) and $151 for the three and six
months ended December 31, 2008. The Company’s net losses from TOBC’s
discontinued operations were $119 and $372 for the three and six months ended
December 31, 2008.
The
Company’s revenue and loss from discontinued operations, net of taxes, from
these events were $1,038 and $717 for the three months ended December 31, 2008,
respectively, as follows:
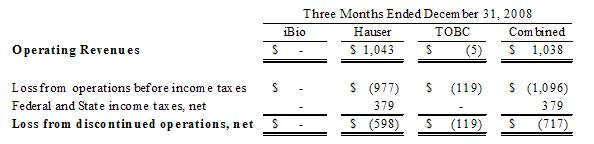
The
Company’s revenue and loss from discontinued operations, net of taxes, from
these events were $2,503 and $1,862 for the six months ended December 31, 2008,
respectively, as follows:
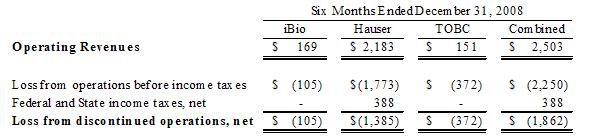
11
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Note 4.
Intangible Assets, net
Intangible
assets consist of trade names, license fees, and unpatented technology. The
carrying amount of other intangible assets, net is as follows as
of:
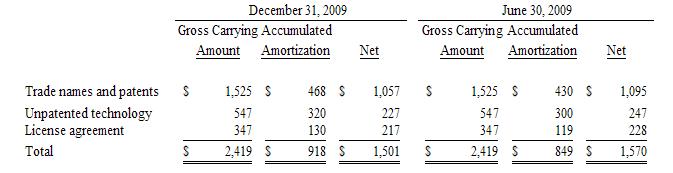
Amortization
expense from continuing operations recorded on the intangible assets for each of
the three and six months ended December 31, 2009 and 2008 was $34 and $69,
respectively. Amortization expense is recorded on the straight-line method over
periods ranging from 2 years to 20 years based on contractual or estimated lives
and is included in selling and administrative expenses. Included in the
Company’s loss from discontinued operations is amortization expense of $25 and
$83 for the three and six months ended December 31, 2008,
respectively.
The
estimated annual amortization expense for intangible assets for the five
succeeding fiscal years is as follows:
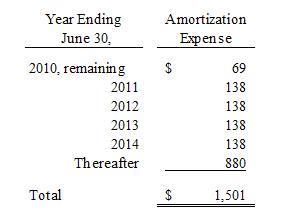
Note
5. Inventories
Inventories
are stated at the lower of cost or market using the first-in, first-out method
and consist of the following as of:
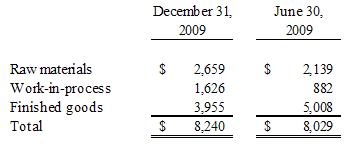
12
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Note
6. Property and Equipment, net
Property
and equipment consists of the following as of:
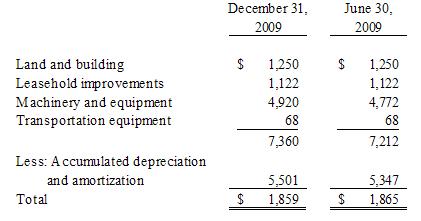
Depreciation
and amortization expense was $79 and $104 for the three months ended December
31, 2009 and 2008, respectively and $154 and $220 for the six months ended
December 31, 2009 and 2008, respectively.
Note
7. Notes Payable, Convertible Note Payable – CD Financial, LLC and Series C
Redeemable Convertible Preferred Stock
On
February 21, 2008, the Company entered into two Securities Purchase Agreements
(the "SPAs") relating to a private placement of securities with two investors,
one of whom is an affiliate of Carl DeSantis, a director of the Company, which
resulted in gross proceeds, in the aggregate, of $17,337 to the Company. The
private placement involved the sale of (i) 6,000 shares of newly designated
Series C Redeemable Convertible Preferred Stock (the “Series C Preferred Stock”)
with a stated value of $1,000 per share (see Note 11(d) Series C Redeemable
Convertible Preferred Stock), (ii) $4,500 in principal amount of 9.5%
Convertible Note Payable (the “Convertible Note Payable”), (iii) $7,000 in
principal amount of 8.0% Notes Payable (the “Notes Payable”) and (iv) 200,000
shares of the Company’s common stock. The Company also has recorded $218 of
deferred financing costs associated with the SPAs and $130 of such deferred
financing costs were netted against the gross proceeds received. These costs
were allocated to each of the components of the transaction, based on the
relative fair values and are amortized based on the terms of the component of
the transaction to which the costs were allocated. As of December 31 and June
30, 2009, the Company had $29 and $41 of deferred financing costs remaining,
respectively, of which is to be amortized to interest expense over two to
eighteen months. The Notes Payable and the Convertible Note Payable are secured
by a pledge of substantially all of the Company’s assets. Concurrently with
entry into the SPAs, the Company terminated its outstanding credit facilities
with Amalgamated Bank in the amount of $16,333 with the repayment of
$16,006.
(a) CD
Financial, LLC (“CD Financial”), a related party, provided gross proceeds of
$7,500, exclusive of a $163 discount to be repaid by the Company at a future
date, in exchange for 3,000 shares of Series C Preferred Stock, with a stated
value of $1,000 per share, and $4,500 in principal amount of Convertible Note
Payable. The Company allocated the proceeds and the discount based on the
relative fair value of the Convertible Note Payable and the Series C Preferred
Stock. The Company is amortizing to interest expense the discount applied to the
Convertible Note Payable over the term of the note, and charged to Additional
Paid in Capital the discount applied to the Series C Preferred Stock. The
Company recorded a beneficial conversion feature of $715 on the Convertible Note
Payable that was being accreted over the three-year period until maturity or the
redemption of the Convertible Note Payable. The Company also recorded a
beneficial conversion feature on the Series C Preferred Stock of $608 which was
accreted over the five-year maturity period until the redemption of the Series C
Preferred Stock in August 2008. As of December 31 and June 30, 2009, the unpaid
discount on the Series C Preferred Stock and Convertible Note Payable in the
amount of $163 is included in accrued expenses in the accompanying Condensed
Consolidated Balance Sheet.
13
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
The
beneficial conversion features were accreted using the effective interest rate
method until July 1, 2009 when the Company adopted “Accounting For Derivative
Financial Instruments Indexed to, and Potentially Settled in, a Company's Own
Stock”. As of July 1, 2009, the Company recorded an accumulated
adjustment to account for the embedded derivative liability of the conversion
feature of the Convertible Note Payable, resulting in a decrease to additional
paid in capital of $715, a decrease in accumulated deficit of $2,097, a decrease
in the carry value of the Convertible Note Payable of $1,426 and the recognition
of a derivative liability of $44. As of December 31, 2009, the
related derivative liability to the Convertible Note Payable decreased to $7 and
is included in accrued expenses and other current liabilities in the
accompanying condensed balance sheets. The embedded derivative
liability created in connection with the Convertible Note Payable was valued at
a fair value using the Black-Scholes option pricing model as of the issuance
date and subsequently as of July 1, 2009 and December 31, 2009.
The
Company used the following assumptions to calculate the fair value of the
derivative liability:
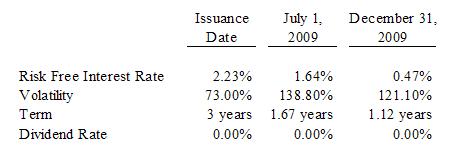
The
Convertible Note Payable bears interest at an annual rate of 9.5% and matures on
or before February 21, 2011. It may be converted, at any time and at the
holder’s option, into shares of our common stock based on a conversion price as
set out in the Convertible Note Payable. The conversion price is a formula that
bases the conversion price on the greater of (i) 90% of the average Volume
Weighted Average Price (the "VWAP") market price of our common stock for 20
trading days immediately preceding the conversion date and (ii) $2.00, subject
to adjustment in the event of a stock dividend, stock split or combination,
reclassification or similar event and upon certain issuances below the
conversion price. We have the option to prepay the Convertible Note
Payable.
Included
in Other Expense, net in the accompanying Condensed Consolidated Statement of
Operations, is $8 and $16 related to the accretion of the discount and $240 and
$461 related to the accretion of the embedded derivative liability related to
the Convertible Note Payable, for the three and six months ended December 31,
2009, respectively, and $8 and $16 related to the accretion of the discount and
$57 and $114 accretion of the beneficial conversion feature on the Convertible
Note Payable, respectively, for the three and six months ended December 31,
2008. As of December 31, 2009, the Company had interest in arrears of
$182 on the Convertible Note Payable. In March 2009, the Company and
CD Financial entered into an oral agreement to suspend the cash interest
payments on the Convertible Note Payable until the Company returned to positive
cash flows in its operations. In this oral agreement, CD Financial
agreed not to give any default notices or increase interest rates due to such
default (the default interest rate as defined in the Convertible Note Payable is
18%).
Also, in
accordance with the Convertible Note Payable, the Company will issue and deliver
to CD Financial, for no additional consideration, 50,000 shares of common stock,
on a quarterly basis in arrears, commencing with the three-month anniversary of
the issuance date, until the Convertible Note Payable has been repaid in full,
after which the Company's obligations to issue shares of common stock will no
longer be applicable.
14
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
(b) On
November 24, 2009, MDC, a wholly owned subsidiary of the Company, entered into a
$300 promissory note (the “CD Note”) with CD Financial (see Note
7(a)). The CD Note matures on November 24, 2010 and bears interest at
the rate of 5%. Interest is accrued monthly and is payable upon
maturity.
(c)
Imperium, provided gross proceeds of $9,837, including a discount of $163, in
exchange for 3,000 shares of Series C Preferred Stock, with a stated value of
$1,000 per share, $7,000 in principal amount of 8.0% Notes Payable and 200,000
shares of the Company’s common stock. The Notes Payable originally matured on
February 21, 2009. The Company allocated the proceeds and the discount based on
the relative fair value of the Notes Payable, the Series C Preferred Stock and
the Company’s common stock. The Company amortized, to interest expense, the
discount applied to the Notes Payable over the term of the notes and charged to
Additional Paid in Capital the discounts applied to the Series C Preferred Stock
and the common stock. The Company recorded a beneficial conversion feature on
the Series C Preferred Stock of $608. The beneficial conversion feature was
accreted over the five-year maturity period until the redemption of the Series C
Preferred Stock in August 2008. The beneficial conversion features were accreted
using the effective interest rate method. For the three and six months ended
December 31, 2008, included in Other Expense, net in the accompanying Condensed
Consolidated Statement of Operations, is $198 and $322, respectively, related to
the accretion of the discount on the Notes Payable.
On
October 14, 2008, the Company and the Notes Payable holders amended their SPA to
extend the maturity from February 21, 2009 to November 15, 2009 (See Note 2.
Liquidity). In consideration for extending the maturity of the Notes Payable,
the Notes Payable holders will forgo the 200,000 shares of common stock as
additional interest and the Company (i) granted a 11.5% premium on
the principal, thus aggregating a principal balance due of $7,805 and certain
other amounts payable under the Notes Payable, if any, (ii) certain new
covenants are applicable to the Company effective October 14, 2008, (iii) the
Company issued warrants to purchase 500,000 shares of the Company’s Common
Stock, with a five year term at an exercise price of $0.80 per share, and (iv)
the registration of the resale of the shares of the Company’s Common Stock for
which the warrants are exercisable. Since the October 14, 2008 amendment
significantly modified the terms of the original Notes Payable, the Company
accounted for the amendment as an extinguishment of the original Notes Payable
and issuance of new Notes Payable in accordance with “Debtor’s Accounting for a
Modification or Exchange of Debt Instruments”. As a result of the extinguishment
of the original Notes Payable, the Company accelerated the amortization of the
then remaining discount of $178 and prepaid financing costs of $32 applied to
the original Notes Payable to interest expense. Furthermore, the Company
reversed the accrual of additional consideration of $208 related to the 200,000
shares of the Company’s common stock.
The
amended Notes Payable in the amount of $7,000 bear an interest rate of 8.0% and
matured on November 15, 2009. The Company accreted the premium of $805 over the
term of the amendment, using the effective interest method, which resulted in
additional interest expense for the three and six months ended December 31, 2009
of $96 and $288. As of July 1, 2009, the warrants issued were deemed to have a
derivative liability resulting in an accumulated adjustment to account for the
embedded derivative liability of the strike price resulting in a decrease to
additional paid in capital of $169, a decrease in accumulated deficit of $140, a
decrease in the carry value of the amended Notes Payable of $6 and the
recognition of a derivative liability of $34. As of December 31,
2009, the related derivative liability to the amended Notes Payable decreased to
$30 and is included in accrued expenses and other current liabilities in the
accompanying condensed balance sheets. The embedded derivative liability created
in connection with the warrants issued was valued at a fair value using the
Black-Scholes option pricing model as of the issuance date and subsequently as
of July 1, 2009 and December 31, 2009.
15
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
The
Company used the following assumptions to calculate the fair value of the
derivative liability:
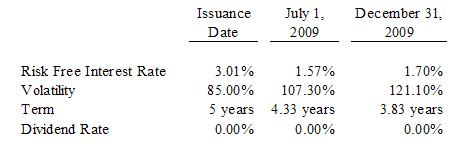
The
discount to the amended Notes Payable for the warrants which is being accreted
using the effective interest method resulted in interest expense for the three
and six months ended December 31, 2009 of $22 and $65, respectively and $33 of
interest expense for each of the three and six months ended December 31, 2008.
The Company also recorded an additional $10 of deferred financing costs as a
result of the issuance of the amended Notes Payable. The Company is
amortizing to interest expense the deferred financing costs using the effective
interest method. The amount amortized to interest expense relating to the
amended Notes Payable for the three and six months ended December 31, 2009 was
less than $1 and $3, respectively and $2 for each of the three and six months
ended December 31, 2008.
The
following table presents the stated value/principal of each of the securities
issued in connection with the debt outstanding as of December 31,
2009:
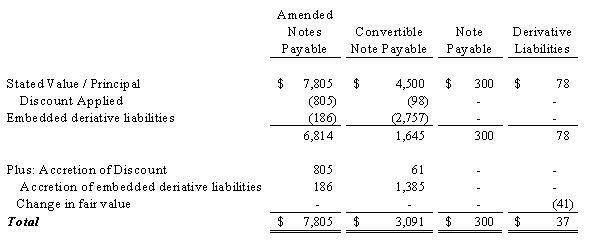
The
Company accreted $54 and $1,137, in the three and six months ended December 31,
2008, for the Series C Preferred Stock dividend and for the acceleration of the
deemed dividend from the beneficial conversion feature of the Series C Preferred
Stock, respectively (See Note 11(d) Series C Redeemable Convertible Preferred
Stock). Such amounts are included in the accompanying Condensed
Consolidated Statement of Operations.
The
weighted average interest rate paid was 8.59% in each of the three and six
months ended December 31, 2009 and 2008. As of December 31, 2009 and
June 30, 2009, the Company had accrued and unpaid interest of approximately $267
and $192, respectively, for the Notes Payable and Convertible Note
Payable. (See Note 7(a) above).
As of
December 31, 2009, the Company is in technical default of the financial
covenants of the amended Notes Payable relating to the Company's tangible net
worth requirements and minimum net capital requirements and has not repaid the
amended Notes Payable in the amount of $7,805. As of February 12,
2010, the Company continues to work with the note holders to work out the
refinancing of the amounts due under the amended Notes Payable, however at this
time the Company and the note holders have not yet come to an agreement and the
note holders have not exercised their rights, with respect to the amended Notes
Payable, based on the Company's non payment of the amended Notes
Payable. Upon the occurrence of an event of default, the note holders
have the right, to give the Company an Acceleration Notice, which would (i)
accelerate the payment of all unpaid principal and accrued and unpaid interest
(including default interest (if any)) on the Notes Payable, and (ii) require the
Company to pay an amount equal to the sum of all of the amounts described in the
preceding clause (i) in same day funds on the payment date specified in the
notice, provided such date must be at least two (2) business days following the
date on which the notice is delivered to the
Company.
16
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Note
8. Significant Risks and Uncertainties
(a)
Concentrations of Credit Risk-Cash. The Company maintains balances at
several financial institutions. Deposits at each institution are insured by the
Federal Deposit Insurance Corporation up to $250 through December 31, 2013. The
FDIC is temporarily insuring deposits up to $250 at financial institutions
through December 31, 2013. As of January 1, 2010, the Company had $870 of
uninsured deposits at these financial institutions.
(b)
Concentrations of Credit Risk-Receivables. The Company routinely assesses
the financial strength of its customers and, based upon factors surrounding the
credit risk of its customers, establishes an allowance for uncollectible
accounts and, as a consequence, believes that its accounts receivable credit
risk exposure beyond such allowances is limited. The Company does not require
collateral in relation to its trade accounts receivable credit risk. The amount
of the allowance for uncollectible accounts and other allowances was $241 and
$298 at December 31, 2009 and June 30, 2009, respectively.
(c) Major
Customers. For the three and six months ended December 31, 2009,
approximately 76.6 % of revenues was derived from two customers. For
the three and six months ended December 31, 2008 approximately 79.2% and 79.0%
of revenues were derived from three customers. Accounts receivable as
of December 31, 2009 from the two major customers represented approximately 37%
of total accounts receivable. The loss of these customers would have an adverse
affect on the Company’s operations. Major customers are those customers who
account for more than 10% of net sales.
(d) Other
Business Risks. The Company insures it business and assets against
insurable risks, to the extent that it deems appropriate, based upon an analysis
of the relative risks and costs. The Company believes that the risk of loss from
non-insurable events would not have a material adverse effect on the Company’s
operations as a whole.
The raw
materials used by the Company are primarily commodities and agricultural-based
products. Raw materials used by the Company in the manufacture of its
Nutraceutical products are purchased from independent suppliers. Raw materials
are available from numerous sources and the Company believes that it will
continue to obtain adequate supplies.
Approximately
50% the Company’s employees, located in its New Jersey facility, are covered by
a union contract. The contract was renewed in August 2006 and will expire in
August 2010.
Note
9. Commitments and Contingencies
(a)
Leases
Related Party
Leases. Warehouse and office facilities are leased from Vitamin Realty
Associates, L.L.C., a limited liability company, which is 90% owned by the
Chairman of the Company’s Board of Directors, a director and majority
shareholder and certain of his family members and 10% owned by an employee of
the Company. The lease provides for minimum annual rental payment of $324
through May 31, 2015 plus increases in real estate taxes and building operating
expenses. On July 1, 2004, the Company leased an additional 24,810 square feet
of warehouse space on a month-to month basis. Rent expense for the three and six
months ended December 31, 2009 and 2008 on these leases were $177 and $183 and
$405 and $393, respectively, and are included in both cost of sales and selling
and administrative expenses in the accompanying Condensed Consolidated
Statements of Operations. At December 31, 2009 and June 30, 2009, the Company
had an outstanding obligation of $544 and $443, respectively, included in
accounts payable in the accompanying Condensed Consolidated Balance
Sheet.
17
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Other Lease
Commitments. The Company has entered into certain non-cancelable
operating lease agreements expiring up through May 31, 2015, related to office
and warehouse space, equipment and vehicles. Total rent expense, including real
estate taxes and maintenance charges, was approximately $240 and $265 for the
three months ended December 31, 2009 and 2008 and $532 and $536 for the six
months ended December 31, 2009 and 2008, respectively. Rent expense is stated
net of sublease income of approximately $9 for each of the three months ended
December 31, 2009 and 2008 and $18 for each of the six month periods ended
December 31, 2009 and 2008. This is included in both cost of sales
and selling and administrative expenses in the accompanying Condensed
Consolidated Statements of Operations.
The
minimum rental commitment for long-term non-cancelable leases is as
follows:
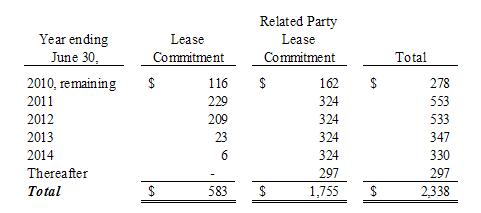
(b)
Consulting Agreements.
Effective
July 1, 2008, the Company entered into a three year consulting agreement with
Jeffrey Leach (an employee of the Company as of the date of the agreement and
its former President and Chief Executive Officer). Pursuant to the consulting
agreement, Mr. Leach is to provide consulting and specialized services to the
Company in the area of finance, acquisition of product lines, refinancing of
existing debt and capital raising under the direction of the Company, including
for any company in which the Company has an ownership interest. In connection
with the consulting agreement, the Company issued 250,000 shares of the
Company’s common stock to Mr. Leach.
(c)
Legal Proceedings.
On June
16, 2008, the State of Texas filed an Original Petition for injunctive relief
and civil penalties in the 101st Judicial District, Dallas, Texas (the "Court"),
against AgroLabs Inc., the Company, Kurt Cahill and Gerald Kay (collectively the
"Defendants"). The State alleged that the Defendants sold or
distributed juices and dietary supplements marketed with inappropriate disease
and nutritional claims. AgroLabs has appeared in the lawsuit and
filed an answer denying all claims. Additionally, AgroLabs filed a
counterclaim against the State for declaratory relief, in which AgroLabs sought
a declaratory judgment from the Court that the State's causes of action were
preempted under federal law because the product benefit claims at issue are
fully compliant with applicable federal law.
18
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
The
Company and Mr. Kay filed motions to dismiss the lawsuit for lack of personal
jurisdiction. In November 2009, the State of Texas agreed to dismiss
the Company and Mr. Kay from the lawsuit. The parties have now
resolved all of the remaining issues in this lawsuit. Neither party
has admitted any liability. Under the settlement agreement, the
Company will make a payment to the State of Texas in the amount of $130,000 to
be allocated to the State of Texas' judicial fund for programs approved by the
Texas Supreme Court that provide basic civil legal services to the indigent;
attorneys' fees and investigation costs incurred by the Office of the Attorney
General; and investigative costs incurred by the Texas Department of State
Health Services. The Company recognized this payment obligation in
its results of operations for the three and six months ended December 31,
2009.
On April
23, 2009, Braker Five & Eight Investors, L.P., (the “Landlord”) filed an
Original Petition relief and damages pursuant to a Lease Agreement for the
premises located in Austin, Texas in the 126th Judicial District, Travis County,
Texas, against BevSpec, Inc., Bioscience Technologies, Inc. dba The Organic
Beverage Company, and Integrated BioPharma, Inc., as Guarantor (collectively,
the “Defendants”). The Landlord has sued for sums due under the Lease
under breach of contract and guaranty theories. The Company believes
it has several meritorious defenses which would relieve it of all liability to
the Landlord and has filed an answer in which it generally denies liability to
the Landlord and asserts several affirmative defenses. The parties
are presently engaged in the discovery process; no trial date has been
set. The Company is unable, at this time, to make a determination as
to the likelihood of an unfavorable outcome or to estimate the amount or range
or possible loss or gain and the impact, if any, this claim will have on the
Company and its operations.
On or
about August 10, 2009, AgroLabs, Inc. commenced an action in the Superior Court
of New Jersey, Law Division, against defendants Kurt E. Cahill, Cheryl A.
Cahill, Joseph E. Cahill, Jr. and Monty C. Lloyd (all of whom were previously
employed by AgroLabs, Inc.) for, among other things, breach of contract, breach
of fiduciary duty, negligent performance of duties and other and related
relief. On or about September 1, 2009, the defendants removed the action
to the United States District Court for the District of New Jersey. On or
about September 15, 2009, the defendants filed an answer and affirmative
defenses. The defendants, however, asserted no counterclaims. The
parties have exchanged initial disclosures and other information, and
the parties have also engaged in settlement discussions. In the absence of
a settlement, the Company is unable to make a determination as to the likelihood
of a favorable outcome or to estimate the amount or range or possible loss or
gain and the impact, if any, of this claim will have on the Company and its
operations.
Note
10. Related Party Transactions
The
Company has a verbal consulting agreement with Eugene Kay, a former employee of
the Company and a brother of E. Gerald Kay, the Chairman of the Company’s Board
of Directors, a director and majority shareholder. This agreement is on a
month-to-month basis for $1 per month. The total consulting expense recorded per
this agreement for each of the three and six months ended December 31, 2009 and
2008 was $3 and $6 in each period. The Company had another consulting agreement
with EVJ, LLC, a limited liability company controlled by Robert Kay, a director
of the Company, the Chairman of its subsidiary, Paxis, and a brother of E.
Gerald Kay and Eugene Kay. This agreement was assumed by and became a liability
of the Company as a part of the Company's acquisition of Paxis in the fiscal
year ended June 30, 2004. The total consulting expense under this agreement was
$15 for the six month period ended December 31, 2008 and is included in
discontinued operations in the accompanying condensed consolidated statement of
operations. The agreement was terminated in August 2008.
Carl
DeSantis, a director of the Company and a member of CD Financial (see Note 7(a))
and CD Financial have guaranteed certain liabilities of the
Company. On April 7, 2009, CD Financial entered into a Guaranty
Agreement with Creative Flavors, Inc. (“CFC”), a major supplier of the Company,
guaranteeing up to $500 of the Company’s outstanding obligation with
CFC. The guaranty is continuing and remains in effect until
terminated by written notice to CFC. As of December 31, 2009, the
Company had an outstanding obligation to CFC in the amount of $907, which amount
is included in accounts payable in the Company’s Condensed Consolidated Balance
Sheet.
19
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
On July
1, 2009, the Company entered into a credit and payment agreement (the “Payment
Agreement”) with a major supplier, Triarco, Inc. (“Triarco”). Under
the terms of the Payment Agreement, the Company is obligated to pay its past due
balance in eight equal installments of $50 beginning August 1, 2009 and Mr.
DeSantis agreed to separately guaranty (the “Personal Guaranty”) the Company’s
obligations to Triarco. In exchange, Triarco agreed to extend additional credit
of $400 (the “Additional Amount Outstanding”) on net thirty day terms beginning
with trade payables dated June 24, 2009. The Personal Guaranty is
limited to the lesser of the aggregate amount owed to Triarco, or
$800. As of December 31, 2009, the Company owes Triarco $135, $32
under the Additional Amount Outstanding and $104 was past due, these amounts are
included in accounts payable in the Company’s Condensed Consolidated Balance
Sheet.
CD
Financial and Mr. DeSantis did not receive any compensation from the Company in
connection with these guarantees.
In August
2008, the Company ceased allocating corporate overhead to iBio and entered into
a Transitional Services Agreement (the “TS Agreement”) with iBio. The
transitional services agreement permitted the Company to continue to provide
certain corporate services to iBio in exchange for a management charge. The
scope of these services was limited to legal, strategic financial planning, SEC
reporting, and tax services by certain corporate employees of the Company. The
TS Agreement provided for a per annum fee of $100. In the six months
ended December 31, 2009 and 2008, the Company billed iBio approximately $8 and
$37, respectively under the TS Agreement. The TS Agreement was
terminated in August 2009.
See Note
7(a) and (b) – Notes Payable and Convertible Note Payable – CD Financial, LLC
for related party securities transactions.
See Note
9(a) - Leases for related party lease transactions.
Note
11. Equity Transactions
(a) Stock Option
Plan and Warrants. There were no stock options or warrants issued in the
three and six months ended December 31, 2009. During the three and
six months ended December 31, 2008, there were stock options authorized by the
Board of Directors and issued in January 2009 to Company employees and Directors
to purchase 1,000,000 shares of common stock and warrants to purchase 500,000
shares of common stock at $0.80 in connection with the amended Notes Payable
agreement (See Note 7(b) Notes Payable). During the three and six months ended
December 31, 2009 and 2008, the Company has incurred stock compensation expense
of $281 and $462 and $559 and $761, respectively. Included in
discontinued operations is stock compensation of $5 and $21 for the three and
six months ended December 31, 2008. In the six month period ended
December 31, 2008, certain key executives and a significant shareholder of the
Company exercised stock options for shares of common stock of 2,095,852 which
provided cash proceeds to the Company of approximately $1,341.
(b) Restricted
Stock Award. There were no
restricted stock awards issued in the three and six months ended December 31,
2009 and 2008, respectively.
(c) Consulting
Agreements. Effective July 1, 2008, the Company entered into two
three-year consulting agreements which resulted in the issuance of 350,000
shares of the Company’s common stock. On the effective date of these
consulting agreements, the Company recognized prepaid consulting expenses of
$830 with a corresponding increase in common stock and additional paid in
capital. During the three and six months ended December 31, 2009 and 2008, the
Company amortized $49 and $69, and $98 and $138, respectively, to selling and
administrative expenses in the Company’s Condensed Consolidated Statement of
Operations. The consulting expenses will continue to be amortized into selling
and administrative expenses over the three year terms, of the consulting
agreements.
20
INTEGRATED
BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO
THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF
DECEMBER 31, 2009 AND JUNE 30, 2009 AND FOR THE
THREE AND
SIX MONTHS ENDED DECEMBER 31, 2009 AND 2008
(in
thousands, except share and per share amounts)
Of the
common stock issued in connection with the consulting agreements, 100,000 shares
of common stock have not been registered under the Securities Act of 1933, as
amended (the "Securities Act"), and were issued and sold in reliance upon the
exemption from registration contained in Section 4(2) of the Securities Act and
Regulation D promulgated thereunder. These shares of common stock may not be
offered or sold in the United States in the absence of an effective registration
statement or exemption from the registration requirements under the Securities
Act. In March 2009, these 100,000 shares were cancelled as the
related consulting agreement was rescinded by both parties.
(d) Series C
Preferred Stock. On February 21, 2008, the Company raised
$5,788 in net proceeds from the sale of 6,000 shares of the Company’s Series C
Preferred Stock, par value $1,000 per share, at a purchase price of $1,000 per
share. Upon issuance of the Series C Preferred Stock, the Company recorded the
beneficial conversion feature of $1,216 and such amounts were being accreted
over the five year period until the mandatory redemption date of the Series C
Preferred Stock, the fifth anniversary of closing.
During
July and August 2008, all 6,269 Series C Preferred Stock (inclusive of
cumulative dividends of 269 shares of Series C Preferred Stock) were converted
into 2,639,204 shares of the Company’s common stock. The conversion resulted in
an increase to common stock of $5 and additional paid in capital of $6,264. Also
during the six months ended December 31, 2008, the Company incurred a deemed
dividend from beneficial conversion feature of the Series C Preferred Stock of
$1,137 as a result of accelerating the accretion of the beneficial conversion
feature and the discount, respectively.
Dividends
of the Series C Preferred Stock were 10% per annum, payable on an annual basis,
by the Company in shares of the Company's Series C Preferred Stock. Accordingly,
the Company had accrued approximately $216 at June 30, 2008, and incurred $54
during the six months ended December 31, 2008, which were paid in Series C
Preferred Stock and cash for the fractional shares during the period ended
December 31, 2008. The redemption of the shares of Series C Preferred Stock
accelerated a payment of a dividend on the Series C Preferred
Stock.
Note
12. Income Taxes
The
Company recognizes deferred tax assets, net of applicable valuation allowances,
related to net operating loss carry-forwards and certain temporary differences
and deferred tax liabilities related to certain temporary differences. The
Company recognizes a future tax benefit to the extent that realization of such
benefit is considered to be more likely than not. This determination is based on
projected taxable income and tax planning strategies. Otherwise, a valuation
allowance is applied.
In the
fiscal year ended June 30, 2009, the Company’s deferred tax asset as well as
projected taxable income was reviewed for expected utilization using the “more
likely than not” approach by assessing the available positive and negative
evidence surrounding its recoverability. A full valuation allowance of $5,955
was recorded in the fiscal year ended June 30, 2009, against the Company’s
deferred tax asset, as it was determined based upon past losses, the Company’s
liquidity concerns and the current economic environment, that it was “more
likely than not” that the Company’s deferred tax assets may not be realized. In
future years, if the deferred tax assets are determined by management to be more
likely than not to be realized, the recognized tax benefits relating to the
reversal of the valuation allowance will be recorded.
21
Item
2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANICAL CONDITION AND RESULTS OF
OPERATION
|
|
Certain
statements set forth under this caption constitute “forward-looking statements.”
See “Disclosure Regarding Forward-Looking Statements” on page 1 of this Report
for additional factors relating to such statements. The following discussion
should also be read in conjunction with the condensed consolidated financial
statements of the Company and Notes thereto included elsewhere herein and the
Company’s Annual Report on Form 10-K.
The
Company is engaged primarily in the manufacturing, distributing, marketing and
sales of vitamins, nutritional supplements and herbal products. The Company’s
customers are located primarily throughout North America.
Business
Outlook
Our
future results of operations and the other forward-looking statements contained
in this Form 10-Q, including this MD&A, involve a number of risks and
uncertainties—in particular, the statements regarding our goals and strategies,
new product introductions, plans to cultivate new businesses, pending
divestitures, future economic conditions, revenue, pricing, gross margin and
costs, the tax rate, and pending legal proceedings. We are focusing on efforts
to improve operational efficiency and reduce spending that may have an impact on
expense levels and gross margin. In addition to the various important factors
discussed above, a number of other important factors could cause actual results
to differ significantly from our expectations. See the risks described in “Risk
Factors” in Part II, Item 1A of this Form 10-Q.
Our
financial results are substantially dependent on net sales of our Nutraceutical
product lines. Net sales is partly a function of the mix of branded
proprietary Nutraceutical products, contract manufactured products and other
Nutraceutical, all of which are difficult to forecast. The varied
sales pricing among our products and promotional support in the form of consumer
coupons or other sales price allowances, along with the mix of products sold
affects the average selling price that we will realize and has a large impact on
our revenue and gross margins. Net sales is affected by the timing of new
product introductions and the demand for and market acceptance of our products;
actions taken by our competitors, including new product offerings and
introductions, marketing programs and pricing pressures, and our response to
such actions; our ability to respond quickly to consumer tastes and needs; and
the availability of sufficient raw materials and production lead-time from
suppliers to meet demand. Factors that could cause demand to be different from
our expectations include customer acceptance of our products and our competitors
products; changes in customer order patterns, including order returns; changes
in the level of inventory at customers; and changes in business and economic
conditions, including conditions in the credit market that could affect consumer
confidence and result in lower than expected demand for our
products.
We
believe that we have the product offerings and introductions, facilities,
personnel, and competitive and financial resources in place for business
success; however, future revenue, costs, gross margins, and profits are all
influenced by a number of factors, including those discussed above, all of which
are inherently difficult to forecast.
Critical
Accounting Policies and Estimates
There
have been no changes to our critical accounting policies in the six months ended
December 31, 2009. Critical accounting policies and the significant estimates
made in accordance with them are regularly discussed with our Audit Committee.
Those policies are discussed under “Critical Accounting Policies” in our
“Management’s Discussion and Analysis of Financial Condition and Results of
Operations” included in Item 7 of our Annual Report on Form 10-K for the year
ended June 30, 2009.
22
Results
of Operations
Our
results from operations in the following table, which for the three and six
months ended December 31, 2008, have been reclassified as a result of
discontinued operations, sets forth the income statement data of our results as
a percentage of net sales for the periods indicated:
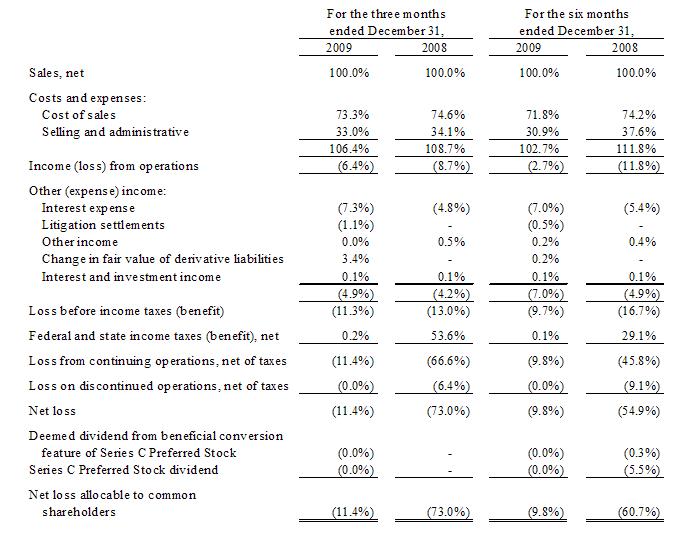
For
the six months ended December 31, 2009 compared to the six months ended December
31, 2008
Sales, net. Sales, net, for the six months ended
December 31, 2009 and 2008 were $19.8 million and $20.5 million, respectively, a
decrease of $754 or 3.7%. The decrease is comprised of the
following:
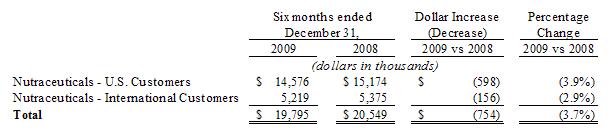
For the
six months ended December 31, 2009, approximately 77% of total net sales were
derived from two customers as compared to 79% of total net sales from three
customers for the six months ended December 31, 2008. The loss of any
of these customers would have an adverse affect on our operations. We
continue to expand our customer base by expanding from selling our proprietary
branded Nutraceutical products primarily to “club” stores to the retail sales
segment and expanding our sales in the international market and e-commerce
markets.
23
The
decrease in our net sales is the result of a decrease our branded proprietary
Nutraceutical product line of approximately $1.3 million due to declining sales
of our original three products introduced to the market four years
ago. Management believes that these products may have reached their
marketing life cycle and has focused its efforts on introducing new products
into the market place, including packaging, product size and new
formulations. Our contract manufacturing product sales increased by
approximately $0.7 million primarily from increased sales from its major
customer, Herbalife. The remaining Nutraceutical product lines had
net sales decreases of approximately $0.2 million compared to the prior
period.
Cost of
sales. Cost of sales decreased by $1.0 million to $14.2
million for the six months ended December 31, 2009 as compared to $15.2 million
for the six months ended December 31, 2008. Cost of sales decreased as a
percentage of sales to 71.8% for the six months ended December 31, 2009 as
compared to 74.2% for the six months ended December 31, 2008. The decrease in
cost of goods sold amount was primarily the result of decreased
sales. The lower cost of goods sold as a percentage of sales is the
result of increased sales in the contract manufacturing business which has fixed
manufacturing costs regardless of the dollars sold and better cost controls in
our branded nutraceutical business line.
Selling and
Administrative Expenses. Selling and administrative expenses
were $6.1 million for the six months ended December 31, 2009, as compared to
$7.7 million for the six months ended December 31, 2008, a decrease of $1.6
million or 20.9%. As a percentage of sales, net, selling and administrative
expenses were 30.9% for the six months ended December 31, 2009 and 37.6% for the
prior comparable period.
The net
decrease in selling and administrative expenses of $1.6 million is mainly due to
decreases aggregating approximately $1.6 million in:
|
·
|
advertising
and marketing ($0.6 million)
|
|
·
|
compensation
and employee benefits ($0.3
million)
|
|
·
|
stock
compensation expenses ($0.2
million),
|
|
·
|
travel
and entertainment ($0.2 million)
|
|
·
|
professional
fess ($0.1 million) and
|
|
·
|
other
expenses ($0.2 million);
|
Our
advertising and marketing costs decreased by approximately $0.6 million in the
six months ended December 31, 2009 compared to the six months ended December 31,
2008 primarily as a result of a decrease in in-store demonstrations (“demos”) of
our products at one of our customers store locations. Our management
has temporally suspended demos as a form of advertising with this customer as it
was determined that it was not producing a significant enough increase in the
number of units being sold through to the consumer.
Our
compensation and employee benefits decreased by $0.3 million as a result of
decreasing our corporate staff by two employees, the suspension of the company
match of employee’s retirement savings deferrals in the profit sharing plan and
the decreased cost resulting from switching professional employment
organizations (reduced administrative costs with outsourcing our human resources
functions and employee benefits). Our stock compensation expense
decreased by $0.2 million primarily due to the significant decrease in the
market value of our common stock from year to year at the measurement date of
the stock option grants (the market value of our common stock is one of several
factors used in determining the fair value of the stock compensation at the time
of the award and ultimate expense to our consolidated financial
statements).
Travel
and entertainment decreased by $0.2 million as a result of the decreased head
count in the corporate staff and decreased spending on meals and
entertainment. Professional fees decreased by $0.1 million due to
decreased costs incurred in the six months ended December 31, 2009 for the spin
off of iBio compared to the 2008 period. Other expenses decreased by
$0.2 million as a result of decreased spending primarily on product liability
insurance, office expenses, investor relations and regulatory
costs.
24
Other expense,
net. Other
expense, net was approximately $1.4 million for the six months ended December
31, 2009 compared to $1.0 million for the six months ended December 31, 2008, an
increase of $0.4 million. Interest expense represents approximately
$1.4 million of other expense in the six months ended December 31, 2009 compared
to $1.1 million in the six months ended December 31, 2008, an increase of $0.26
million. The increase in interest expense is attributable to the
adoption of the accounting for derivative liabilities in connection with
embedded derivatives in our Convertible Note Payable and Warrants issued in
connection with our amended Notes Payable. The balance of the
increase, or approximately $0.14 million, is primarily a result of settling with
the State of Texas for $0.10 million.
Federal and state income tax,
net. For the six months ended December 31, 2009 and December 31, 2008, we
had a small amount of state tax expenses. We continue to provide a full reserve
on our deferred tax assets as it has been determined that based upon past
losses, the Company’s liquidity concerns and the current economic environment,
that it is “more likely than not” that the Company’s deferred tax assets may not
be realized.
Loss from discontinued operations.
On August 18, 2008, we completed our distribution of our Biotechnologies
segment. The net loss from our Biotechnologies segment, included in our results
for the six months ended December 31, 2008, was $0.1 million.
On March
17, 2009, we entered into a stock purchase agreement and consummated the sale of
all of the issued and outstanding shares of common stock of our wholly owned
subsidiary Hauser to Cedarburg. On January 31, 2009, the Company sold
substantially all the assets of Paxis net of its outstanding payables, to Hauser
in consideration for the outstanding intercompany debt between these two
subsidiaries of the Company. The net loss from the Pharmaceuticals
segment, included in our results for the six months ended December 31, 2008, was
$1.4 million.
In June
2009, we discontinued the operations of our subsidiary TOBC, as we do not have
the financial resources to pursue the further development of the Syzmo™ product
in the very competitive energy drink market place. The net loss from
this discontinued product line was $0.4 million for the six months ended
December 31, 2008.
Net loss applicable to common
shareholders. Our net loss applicable to common shareholders for the six
months ended December 31, 2009 was $1.9 million as compared to $12.5 million for
the six months ended December 31, 2008. This decrease in net loss applicable to
common shareholders of approximately $10.6 million is primarily the result of
decreases in: (i) operating losses from continuing operations of $1.9 million,
(ii) income tax expense of $6.0 million, (iii) net losses from discontinued
operations of $1.9 million; and (iv) Series C Preferred Stock dividend and
deemed dividend from beneficial conversion of the preferred stock of $1.2
million, which was a result of the holders of the Series C Preferred Stock
converting their respective shares of Series C Preferred Stock into shares of
our common stock in the six months ended December 31, 2008. This
conversion resulted in permanent equity for us, as the Series C Preferred Stock
replaced $6,000 of our current and long-term obligations. These decreases were
offset by an increase in other expenses, net of $0.4 million.
25
For
the three months ended December 31, 2009 compared to the three months ended
December 31, 2008
Sales, net. Sales, net, for the three months ended
December 31, 2009 and 2008 were $8.8 million and $11.2 million, respectively, a
decrease of $2.4 million or 21.7%. The decrease is comprised of the
following:
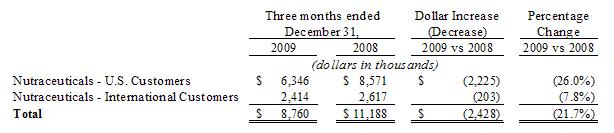
For the
three months ended December 31, 2009, approximately 76% of total net sales were
derived from two customers as compared to 79% of total net sales from three
customers for the three months ended December 31, 2008. The loss of
any of these customers would have an adverse affect on our
operations. We continue to expand our customer base by expanding from
selling our proprietary branded Nutraceutical products primarily to “club”
stores to the retail sales segment and expanding our sales in the international
market and e-commerce markets.
The
decrease in our net sales is the result of decreased sales in our branded
proprietary Nutraceutical product line of approximately $2.5 million in part due
to decreased sales to the club stores that the Company has been dependent on for
sales for its products. In the three months ended December 31, 2009,
the Company experienced increased competition in the market place for product
placement in the club level stores. The remaining Nutraceutical product lines
had net sales increases of approximately $0.1 million compared to the prior
period.
Cost of
sales. Cost of sales decreased by $1.9 million to $6.4 million
for the three months ended December 31, 2009 as compared to $8.3 million for the
three months ended December 31, 2008. Cost of sales decreased as a percentage of
sales to 73.3% for the three months ended December 31, 2009 as compared to 74.6%
for the three months ended December 31, 2008. The decrease in costs of sales as
a percentage of net sales is primarily the result of incurring $0.2 million less
in mark downs in our inventory in the 2009 period compared to the 2008
period. We also experienced a decrease in the cost of freight of
approximately $0.3 million in the three months ended December 31, 2009 compared
to the three months ended December 31, 2008, as a result of changing our
products over from glass to plastic, significantly decreasing the weight factor
in our shipping costs.
Selling and
Administrative Expenses. Selling and administrative expenses
were $2.9 million for the three months ended December 31, 2009, as compared to
$3.8 million for the three months ended December 31, 2008, a decrease of $0.9
million or 24.3%. As a percentage of sales, net, selling and administrative
expenses were 33.0% for the three months ended December 31, 2009 and 34.1% for
the prior comparable period.
The net
decrease in selling and administrative expenses of $0.9 million is mainly due to
decreases in:
|
·
|
advertising
and marketing ($0.3 million)
|
|
·
|
other
expenses ($0.2 million)
|
|
·
|
travel
and entertainment ($0.1 million)
|
|
·
|
professional
fees ($0.1 million), and
|
|
·
|
compensation
and employee benefits ($0.1
million).
|
Our
advertising and marketing costs decreased by approximately $0.3 million in the
three months ended December 31, 2009 compared to the three months ended December
31, 2008 primarily as a result of a net decrease of $0.1 million in in-store
demonstrations (“demos”) of our products at two of our customers stores’
locations in the aggregate amount of $0.2 million offset by increased spending
in our major customers stores of $0.1 million. The remaining decrease
of $0.2 million is from decreases in advertising for customer accounts that we
did not sell product to in the three months ended December 31, 2009 compared to
the three months ended December 31, 2008. Other expenses decreased by
$0.2 million as a result of decreased spending primarily on product liability
insurance, office expenses, investor relations and regulatory
costs. Travel and entertainment decreased by $0.1 million as a result
of the decreased head count in the corporate staff and decreased spending on
meals and entertainment. Professional fees decreased by $0.1 million
due to decreased costs incurred in the three months ended December 31, 2009 on
the State of Texas litigation compared to the three months ended December 31,
2008. Lastly, our compensation and employee benefits decreased by
$0.1 million from the three months ended December 31, 2009 from the three months
ended December 31, 2008 due a reduction of work force, the suspension of the
company match of employee’s retirement savings deferrals in the profit sharing
plan and the decreased cost resulting from switching professional employment
organizations (reduced administrative costs with outsourcing our human resources
functions and employee benefits).
26
Other expense,
net. Other
expense, net was approximately $0.43 million for the three months ended December
31, 2009 compared to $0.47 million for the three months ended December 31, 2008,
a net decrease of $0.03 million. Interest expense represents
approximately $0.64 million of other expense in the three months ended December
31, 2009 compared to $0.53 million in the three months ended December 31, 2008,
a decrease of $0.11 million. The decrease in interest expense is
attributable to the maturity of the amended Notes Payable on November 15, 2009,
offset by an increase in interest as a result of the accounting policy adoption
for derivative liabilities in connection with embedded derivatives in our
Convertible Note Payable and Warrants issued in connection with our amended
Notes Payable. Another decrease in other expense, net, in the
approximate amount of $0.3 million, is also a result of the adoption of the
accounting for derivative liabilities and relates to the increased fair value of
the derivative liabilities from the September 30, 2009 valuation to December 31,
2009, resulting in a gain of $0.3 million for the three months ended December
31, 2009. Another component of other expenses is a litigation
settlement amount of $0.1 million relating to the State of Texas in the three
months ended December 31, 2009 with no such expenditure in the three months
ended December 31, 2008.
Federal and state income tax,
net. For the three months ended December 31, 2009 and December 31, 2008,
we had a small amount of state tax expenses. We continue to provide a full
reserve on our deferred tax assets as it has been determined that based upon
past losses, the Company’s liquidity concerns and the current economic
environment, that it is “more likely than not” that the Company’s deferred tax
assets may not be realized.
Loss from discontinued operations.
On March 17, 2009, we entered into a stock purchase agreement and
consummated the sale of all of the issued and outstanding shares of common stock
of our wholly owned subsidiary Hauser to Cedarburg. On January 31, 2009, the
Company sold substantially all the assets of Paxis net of its outstanding
payables, to Hauser in consideration for the outstanding intercompany debt
between these two subsidiaries of the Company. The net loss from the
Pharmaceuticals segment, included in our results for the three months ended
December 31, 2008, was $0.6 million.
In the
June 2009, we discontinued the operations of our subsidiary TOBC, as we do not
have the financial resources to pursue the further development of the Syzmo™
product in the very competitive energy drink market place. The net
loss from this discontinued product line was $0.1 million for the three months
ended December 31, 2008.
Net loss applicable to common
shareholders. Our net loss applicable to common shareholders for the
three months ended December 31, 2009 was $1.0 million as compared to $8.2
million for the three months ended December 31, 2008. This decrease in net loss
applicable to common shareholders of approximately $7.2 million is primarily the
result of decreases in: (i) operating losses from continuing operations of $0.4
million, (ii) income tax expense of $6.0 million; and (iii) net losses from
discontinued operations of $0.7 million.
Seasonality
The
Nutraceutical business segment tends to be seasonal. We have found that in our
first fiscal quarter ending on September 30th of
each year, orders for our branded proprietary Nutraceutical products usually
slow (absent the addition of new customers or a new product launch with a
significant first time order), as buyers in various markets may have purchased
sufficient inventory to carry them through the summer months. Conversely, in our
second fiscal quarter, ending on December 31st of
each year, orders for our products increase as the demand for our branded
Nutraceutical products seems to increase in late December to early January as
consumers become health conscious as they enter the new year.
27
The
Company believes that there are other non-seasonal factors that also may also
influence the variability of quarterly results including, but not limited to,
general economic and industry conditions that affect consumer spending, changing
consumer demands and current news on nutritional supplements. In addition, our
recent growth has caused additional variability in our quarterly results.
Accordingly, a comparison of the Company’s results of operations from
consecutive periods is not necessarily meaningful, and the Company’s results of
operations for any period are not necessarily indicative of future
periods.
Liquidity
and Capital Resources
The
following table sets forth, for the periods indicated, the Company’s net cash
flows used in operating, investing and financing activities, its period end cash
and cash equivalents and other operating measures:
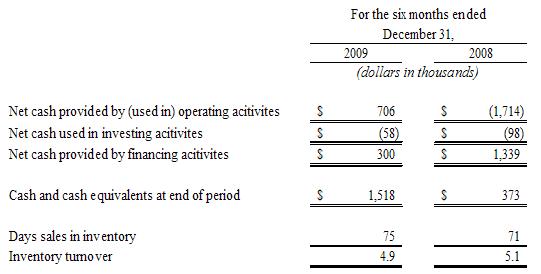
At
December 31, 2009, our working capital deficit was approximately $3.7 million,
an increase of approximately $0.7 million from the working capital deficit of
approximately $2.9 million at June 30, 2009. Our current assets increased by
$0.3 million and our current liabilities increased by $1.1 million.
Net cash
provided by operating activities of $0.7 million in the six months ended
December 31, 2009, includes a net loss of $1.9 million. After excluding the
effects of non-cash expenses, including, depreciation and amortization,
compensation expense for employee stock options and consultants and changes in
the fair value of derivative liabilities, the adjusted cash used in operations
before the effect of the changes in working capital components was $0.3 million.
Cash was provided by continuing operations from our working capital assets and
liabilities in the amount of approximately $0.9 million, was the result of a
decrease in accounts receivable of $0.9 million, offset by an increase in
inventory of $0.2 million and prepaid expenses and other assets of $0.1 million,
and decreases in accounts payable, accrued expenses and other liabilities of
$0.3 million. Less than approximately $0.07 million of cash was
provided by operating activities from our discontinued operations.
Net cash
used in operating activities of $1.7 million for the six months ended December
31, 2008 included a net loss of $11.3 million. After excluding the effects of
non-cash expenses, including the net loss from discontinued operations, deferred
taxes, deferred income taxes, impairment charges, depreciation and amortization
and compensation expense for employee stock options, the adjusted cash used
before the effect of the changes in working capital components was approximately
$2.8 million. Cash provided by continuing operations from working capital
components in the amount of approximately $0.7 million was the result of a
decrease in accounts receivable of $0.7 million, inventory of $0.5 million and
prepaid expenses and other expenses of $0.1 million, offset by a net decrease in
accounts payable and accrued expenses and other liabilities of approximately
$0.7 million. Approximately $0.4 million of cash was provided by
operating activities of our discontinued operations.
28
Cash used
in investing activities was used for the purchase of fixed assets and was less
than $0.1 million in each of the periods ended December 31, 2009 and 2008,
respectively. Cash used in our discontinued operations for investing
activities for the six months ended December 31, 2008 was also less than $0.1
million.
Cash
provided by financing activities was $0.3 million in the six months ended
December 31, 2009 and was provided by financing under a short term promissory
note entered into with CD Financial, a related party and the holder of our
Convertible Note Payable. Cash provided by financing activities was
approximately $1.3 million for the six months ended December 31, 2008 and was
the result of proceeds from employees exercising stock options during the
period.
Our
condensed consolidated financial statements have been prepared assuming that the
Company will continue as a going concern. We have incurred recurring operating
losses and negative operating cash flows for our past three fiscal years ended
June 30, 2009, including a net loss attributable to common stockholders of $19.4
million and negative operating cash flows of $2.8 million for the fiscal year
ended June 30, 2009. For the six months ended December 31, 2009, we had net
operating losses of approximately $0.5 million and a net loss of approximately
$1.9 million. As of December 31, 2009, we had cash and cash
equivalents of approximately $1.5 million, a working capital deficit of
approximately $3.7 million, primarily attributable to the matured amended Notes
Payable in the amount of $7.8 million which were due on November 15, 2009 and an
accumulated deficit of $46.3 million.
As of the
date of this filing, February 12, 2010, we have not received a Default Notice,
nor have we finalized any extensions or refinancing of the amended Notes Payable
in the amount of $7.8 million which were due on November 15,
2009. We have historically raised capital in private
placements; however, we continue to sustain losses and we only recently had
positive cash flows from our operations; this may hinder our efforts in raising
new capital or refinancing our matured debt. Additionally, current economic
conditions may cause a decline in business and consumer spending which could
adversely affect our business and financial performance. These factors raise
substantial doubt as to our ability to continue as a going concern. Assuming we
are able to raise additional capital and/or refinance at least $7.8 million of
the amended Notes Payable, and we are not adversely affected by the current
economic conditions, we believe that our available capital as of December 31,
2009, plus the additional $7.8 million of additional capital and/or refinancing
of our amended Notes Payable, will enable us to continue as a going concern
through the second quarter of our fiscal year ending June 30, 2011. There are no
assurances that we will be able to raise additional capital as needed or
refinance our current amended Notes Payable upon acceptable terms, nor that the
current economic conditions will not negatively impact us. If the current
economic conditions negatively impact us or our operations, or we are unable to
raise additional capital as needed upon acceptable terms, it would have a
material adverse effect on the Company.
Our total
annual commitments at December 30, 2009 for long term non-cancelable leases of
approximately $553 consists of obligations under operating leases for facilities
and lease agreements for the rental of warehouse equipment, office equipment and
automobiles.
Capital
Expenditures
The
Company's capital expenditures for the six months ended December 31, 2009 and
2008 were approximately $0.1 million and less than $0.1 million, respectively.
The Company has budgeted approximately $0.3 million for capital expenditures for
fiscal 2010. The total amount is expected to be funded from cash provided from
its operations and from lease financing.
Off-Balance
Sheet Arrangements
The
Company has no off-balance sheet arrangements.
29
Recent
Accounting Pronouncement
Please
refer to Note 1 to our condensed financial statements which can be found at page
6, herein.
Impact of Inflation
The
Company does not believe that inflation has significantly affected its results
of operations.
Item
3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not
applicable.
Item
4T. CONTROLS AND PROCEDURES
Disclosure
Controls and Procedures
Disclosure
controls and procedures are controls and other procedures that are designed to
ensure that information required to be disclosed by the Company in the
reports it files or submits under the Securities Exchange Act of 1934,
as amended (the “Exchange Act”) is recorded, processed, summarized, and reported
within the time periods specified by the Commission’s rules and forms.
Disclosure controls and procedures include, without limitation, controls and
procedures designed to provide reasonable assurance that information required to
be disclosed by the Company in the reports it files or
submits under the Exchange Act is accumulated and communicated to management,
including the Chief Executive Officer and Chief Financial Officer, as
appropriate, to allow timely decisions regarding required
disclosure.
Under the
supervision and with the participation of management, including the Chief
Executive Officer and Chief Financial Officer, the Company has evaluated the
effectiveness of its disclosure controls and procedures (as such term is defined
in Rule 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31,
2009, and, based upon this evaluation, the Chief Executive Officer and Chief
Financial Officer have concluded that these controls and procedures are
effective in providing reasonable assurance of compliance.
Changes
in Internal Control over Financial Reporting
No change
in our internal control over financial reporting occurred during the three and
six months ended December 31, 2009 that has materially affected, or is
reasonably likely to materially affect, our internal control over financial
reporting.
30
PART
II – OTHER INFORMATION
Item
1. LEGAL PROCEEDINGS
On June
16, 2008, the State of Texas filed an Original Petition for injunctive relief
and civil penalties in the 101st Judicial District, Dallas, Texas (the "Court"),
against AgroLabs Inc., the Company, Kurt Cahill and Gerald Kay (collectively the
"Defendants"). The State alleged that the Defendants sold or
distributed juices and dietary supplements marketed with inappropriate disease
and nutritional claims. AgroLabs has appeared in the lawsuit and
filed an answer denying all claims. Additionally, AgroLabs filed a
counterclaim against the State for declaratory relief, in which AgroLabs sought
a declaratory judgment from the Court that the State's causes of action were
preempted under federal law because the product benefit claims at issue are
fully compliant with applicable federal law.
The
Company and Mr. Kay filed motions to dismiss the lawsuit for lack of personal
jurisdiction. In November 2009, the State of Texas agreed to dismiss
the Company and Mr. Kay from the lawsuit. The parties have now
resolved all of the remaining issues in this lawsuit. Neither party
has admitted any liability. Under the settlement agreement, the
Company will make a payment to the State of Texas in the amount of $130,000 to
be allocated to the State of Texas' judicial fund for programs approved by the
Texas Supreme Court that provide basic civil legal services to the indigent;
attorneys' fees and investigation costs incurred by the Office of the Attorney
General; and investigative costs incurred by the Texas Department of State
Health Services. The Company recognized this payment obligation in
its results of operations for the three and six months ended December 31,
2009.
On April
23, 2009, Braker Five & Eight Investors, L.P., (the “Landlord”) filed an
Original Petition relief and damages pursuant to a Lease Agreement for the
premises located in Austin, Texas in the 126th Judicial District, Travis County,
Texas, against BevSpec, Inc., Bioscience Technologies, Inc. dba The Organic
Beverage Company, and Integrated BioPharma, Inc., as Guarantor (collectively,
the “Defendants”). The Landlord has sued for sums due under the Lease
under breach of contract and guaranty theories. The Company believes
it has several meritorious defenses which would relieve it of all liability to
the Landlord and has filed an answer in which it generally denies liability to
the Landlord and asserts several affirmative defenses. The parties
are presently engaged in the discovery process; no trial date has been
set. The Company is unable, at this time, to make a determination as
to the likelihood of an unfavorable outcome or to estimate the amount or range
or possible loss or gain and the impact, if any, this claim will have on the
Company and its operations.
On or
about August 10, 2009, AgroLabs, Inc. commenced an action in the Superior Court
of New Jersey, Law Division, against defendants Kurt E. Cahill, Cheryl A.
Cahill, Joseph E. Cahill, Jr. and Monty C. Lloyd (all of whom were previously
employed by AgroLabs, Inc.) for, among other things, breach of contract, breach
of fiduciary duty, negligent performance of duties and other and related
relief. On or about September 1, 2009, the defendants removed the action
to the United States District Court for the District of New Jersey. On or
about September 15, 2009, the defendants filed an answer and affirmative
defenses. The defendants, however, asserted no counterclaims. The
parties have exchanged initial disclosures and other information, and
the parties have also engaged in settlement discussions. In the absence of
a settlement, the Company is unable to make a determination as to the likelihood
of a favorable outcome or to estimate the amount or range or possible loss or
gain and the impact, if any, of this claim will have on the Company and its
operations.
Item
1A. Risk Factors
The risks
described in Item 1A, Risk Factors, in our Annual Report on Form 10-K for the
year ended June 30, 2009, could materially and adversely affect our business,
financial condition and results of operations. The risk factors discussed in
that Form 10-K do not identify all risks that we face because our business
operations could also be affected by additional factors that are not presently
known to us or that we currently consider to be immaterial to our operations.
There have been no material changes to our risk factors from those disclosed in
our Form 10-K for the year ended June 30, 2009.
31
Item
2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
Item
3. DEFAULTS UPON SENIOR SECURITIES
None.
Item
4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
Two
matters were submitted for a vote at the Company’s Annual Meeting of
Stockholders held on November 23, 2009 by holders of record of the Company’s
common stock, par value of $0.002 per share at the close of business on October
23, 2009 (the “Record Date”), which was determined to be 20,249,442 shares of
common stock. The holders of 13,223,600 shares of common stock, a majority, were
present in person or represented by proxy at the meeting. The three matters and
the results of the voting at the Annual Meeting are as follows:
|
1.
|
To
elect three Class I directors for a three year term to serve until 2012
Annual Meeting of Stockholders. The number of votes cast at the meeting
for the election of the three (3) directors for a three year term to the
2012 Annual Meeting of Stockholders were as follows:
 |
|
2.
|
To
ratify increasing the number of shares under the Company’s Stock Option
Plan from 11,000,000 to 13,000,000 were as follows:
 |
|
3.
|
To
ratify the appointment of Friedman LLP as the Company’s independent
auditors. The number of votes cast at the meeting for the proposal to
ratify Friedman LLP as the Company’s independent auditors were as
follows:
 |
Item
5. OTHER INFORMATION
On
November 24, 2009, MDC, a wholly owned subsidiary of the Company, entered into a
$300 promissory note (the “CD Note”) with CD Financial, LLC (“CD
Financial”). The CD Note matures on November 24, 2010 and bears
interest at the rate of 5%. Interest is accrued monthly and is
payable upon maturity. In the
event of default or change of control occurs; CD Financial has the right to
accelerate the payment of all unpaid principal and accrued and unpaid interest
(including default interest (if any)) on this promissory note. For a more
detailed description of the promissory note from CD Financial please see copy of
the CD Note attached as Exhibit 10.1 to this Quarterly Report on Form
10-Q.
32
Item
6. EXHIBITS
(a) Exhibits
Exhibit
Number
|
10.1
|
Promissory
Note, dated November 24, 2009
|
|
31.1
|
Certification
of pursuant to Section 302 of Section 302 of the Sarbanes-Oxley Act of
2002 by Chief Executive Officer.
|
|
31.2
|
Certification
of pursuant to Section 302 of Section 302 of the Sarbanes-Oxley Act of
2002 by Chief Financial Officer.
|
|
32.1
|
Certification
of periodic financial report pursuant to Section 906 of the Sarbanes-Oxley
Act of 2002 by Chief Executive Officer.
|
|
32.2
|
Certification
of periodic financial report pursuant to Section 906 of the Sarbanes-Oxley
Act of 2002 by Chief Financial Officer.
|
33
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the Company has duly caused this report to be signed on its behalf by the
undersigned thereunto duly authorized.
INTEGRATED BIOPHARMA, INC.
| Date: February 12, 2010 | By: | /s/ E. Gerald Kay | |
| E. Gerald Kay | |||
| President and Chief Executive Officer |
| Date: February 12, 2010 | By: | /s/ Dina L. Masi | |
| Dina L. Masi | |||
| Chief Financial Officer & Senior Vice President |
34
