Attached files
| file | filename |
|---|---|
| 8-K - 8-K - FACET BIOTECH CORP | a10-3358_18k.htm |
Exhibit 99.1
|
|
BIO CEO and Investor Conference February 9, 2010 |
|
|
Facet Biotech Corporation Forward-looking Statements This presentation contains forward-looking statements involving risks and uncertainties and Facet’s actual results could differ materially from those, express or implied, in this presentation. The forward-looking statements include, without limitation, that we expect: (i) to initiate the DECIDE phase 3 trial for daclizumab in 1H 2010, (ii) to receive milestone payments related to the development of daclizumab, (iii) to fund our current operations through the end of 2012, (iv) MS market dynamics to shift towards higher efficacy therapeutics, and (v) daclizumab would compete with these higher efficacy therapeutics. Various factors may cause differences between our expectations and actual results including: the development and commercial potential of daclizumab and elotuzumab could be adversely impacted by changes in our plans or timelines, including because of unexpected safety or efficacy data observed during clinical trials, enrollment rates in clinical trials, changes in expected competition and changes in regulatory support for our development path; changes in clinical development program expectations, including regarding the advancement, slowing or termination of clinical development programs, unexpected litigation or other disputes and other unexpected events could adversely impact our cash utilization and costs and expenses. Other risk factors we face are discussed in the “Risk Factors” sections of our SEC filings, which may be obtained at the “Investors” section of our website at www.facetbiotech.com. We expressly disclaim any obligation or undertaking to update or revise any forward-looking statements to reflect any change in expectations, even as new information becomes available or other events occur in the future. All forward-looking statements in this presentation are qualified in their entirety by this cautionary statement. Copyright Notice All of the content in this presentation is copyrighted and may not be reproduced, distributed or revised in any manner without the prior written consent of Facet Biotech. Any use without consent may violate copyright law and other laws or regulations. © Copyright 2010, Facet Biotech Corporation. All Rights Reserved. |
|
|
Investment highlights Robust pipeline of five clinical-stage programs Compelling data seen to-date; significant value-driving inflection points through 2011 Promising proprietary protein engineering platform Opportunities in global “biobetter” and “biosimilar” markets Financial strength to fund company to achieve key milestones through 2012 $316.1 million in cash and related at November 30, 2009 Strategic process continues; discussions remain ongoing |
|
|
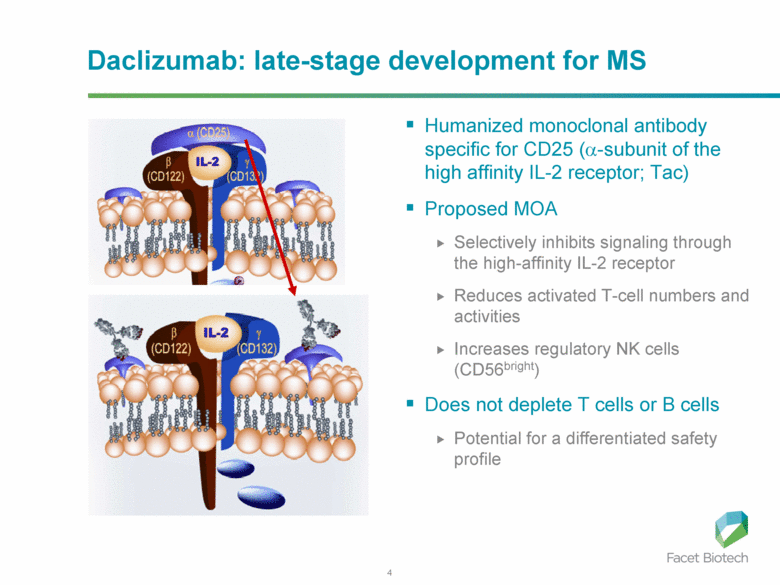
Daclizumab: late-stage development for MS Humanized monoclonal antibody specific for CD25 (α-subunit of the high affinity IL-2 receptor; Tac) Proposed MOA Selectively inhibits signaling through the high-affinity IL-2 receptor Reduces activated T-cell numbers and activities Increases regulatory NK cells (CD56bright) Does not deplete T cells or B cells Potential for a differentiated safety profile |
|
|
Strong rationale for daclizumab in MS 1. Rose et. al. Neurology 2007 69:785-789; 2. Rose et. al. Ann Neurol 2004 56:864-869; 3. Bielekova et.al. PNAS USA 2004 101:8705-8708; 4. Bielekova et. al. Arch Neur Vol. 66 (No. 4) Apr. 2009. Open-label, P1/2 investigator-sponsored trials P2 CHOICE trial: daclizumab + IFN P2 SELECT trial: daclizumab monotherapy Daclizumab and CD56bright NK cells P1/2 monotherapy data suggest activity in excess of 80% reduction in Gd-enhancing lesions and 50% reduction in ARR1-4 P2 CHOICE data demonstrate high activity and an acceptable safety profile for daclizumab + IFN SELECT interim futility analysis passed and data assessment completed Potential drug-biomarker combination in MS |
|
|
SELECT: first registration-enabling trial 6 Year 1 Year 2 (Extension) Randomization ~ 600 RRMS Subjects Daclizumab 300mg SC every 4 wks (n = 200) Daclizumab 150mg SC every 4 wks (n = 200) Placebo (n = 200) Daclizumab 300 mg Daclizumab 150 mg (+/- washout) 1 year Superiority (ARR) 2 year Safety Daclizumab 300 mg (+/- washout) Daclizumab 150 mg Screening 600-patient study assessing 150 mg and 300 mg doses SC every 4 weeks Interim futility analysis and data assessment performed July 2009 on 150 patients who had been on therapy at least 6 months Trial enrollment projected to be completed by end of 2010; primary endpoint data readout by end of 2011 |
|
|
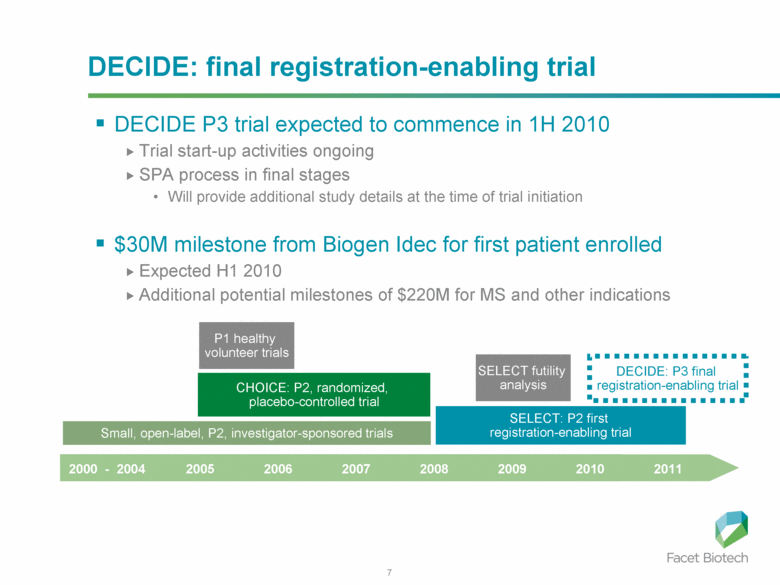
7 DECIDE: final registration-enabling trial DECIDE P3 trial expected to commence in 1H 2010 Trial start-up activities ongoing SPA process in final stages Will provide additional study details at the time of trial initiation $30M milestone from Biogen Idec for first patient enrolled Expected H1 2010 Additional potential milestones of $220M for MS and other indications 2000 - 2004 2005 2006 2007 2008 2009 2010 2011 Small, open-label, P2, investigator-sponsored trials P1 healthy volunteer trials CHOICE: P2, randomized, placebo-controlled trial SELECT: P2 first registration-enabling trial DECIDE: P3 final registration-enabling trial SELECT futility analysis |
|
|
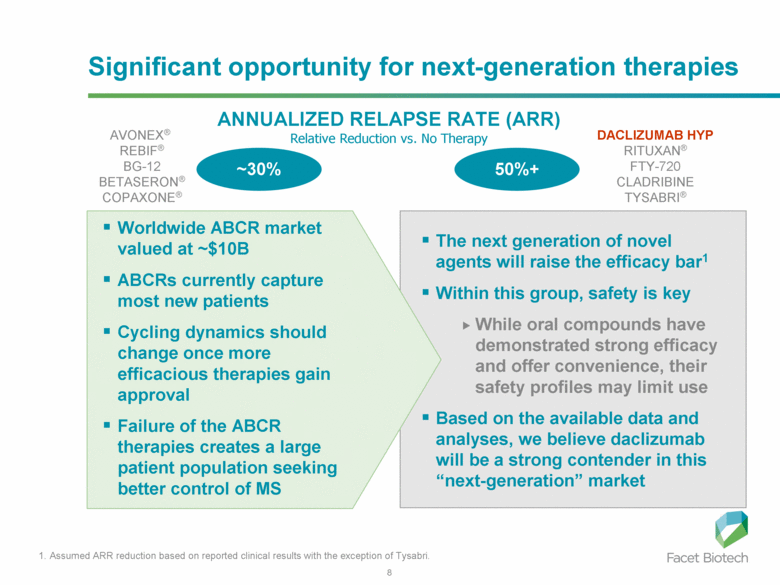
Significant opportunity for next-generation therapies ANNUALIZED RELAPSE RATE (ARR) Relative Reduction vs. No Therapy AVONEX® REBIF® BG-12 BETASERON® COPAXONE® DACLIZUMAB HYP RITUXAN® FTY-720 CLADRIBINE TYSABRI® ~30% Worldwide ABCR market valued at ~$10B ABCRs currently capture most new patients Cycling dynamics should change once more efficacious therapies gain approval Failure of the ABCR therapies creates a large patient population seeking better control of MS 1. Assumed ARR reduction based on reported clinical results with the exception of Tysabri. 50%+ The next generation of novel agents will raise the efficacy bar1 Within this group, safety is key While oral compounds have demonstrated strong efficacy and offer convenience, their safety profiles may limit use Based on the available data and analyses, we believe daclizumab will be a strong contender in this “next-generation” market |
|
|
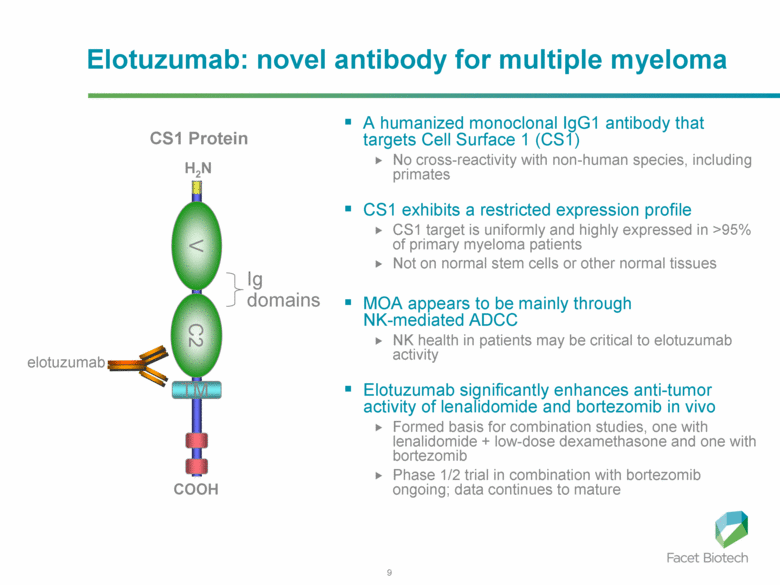
Elotuzumab: novel antibody for multiple myeloma A humanized monoclonal IgG1 antibody that targets Cell Surface 1 (CS1) No cross-reactivity with non-human species, including primates CS1 exhibits a restricted expression profile CS1 target is uniformly and highly expressed in >95% of primary myeloma patients Not on normal stem cells or other normal tissues MOA appears to be mainly through NK-mediated ADCC NK health in patients may be critical to elotuzumab activity Elotuzumab significantly enhances anti-tumor activity of lenalidomide and bortezomib in vivo Formed basis for combination studies, one with lenalidomide + low-dose dexamethasone and one with bortezomib Phase 1/2 trial in combination with bortezomib ongoing; data continues to mature H2N COOH V C2 TM Ig domains CS1 Protein elotuzumab |
|
|
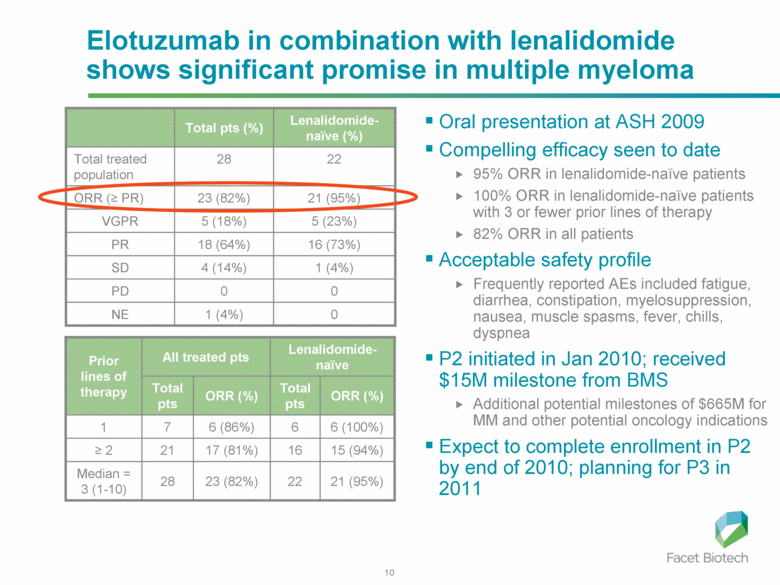
Elotuzumab in combination with lenalidomide shows significant promise in multiple myeloma 0 0 PD 1 (4%) 4 (14%) SD 0 1 (4%) NE 16 (73%) 5 (23%) 21 (95%) 22 Lenalidomide-naïve (%) 18 (64%) 5 (18%) 23 (82%) 28 Total pts (%) VGPR ORR (≥ PR) Total treated population PR Oral presentation at ASH 2009 Compelling efficacy seen to date 95% ORR in lenalidomide-naïve patients 100% ORR in lenalidomide-naïve patients with 3 or fewer prior lines of therapy 82% ORR in all patients Acceptable safety profile Frequently reported AEs included fatigue, diarrhea, constipation, myelosuppression, nausea, muscle spasms, fever, chills, dyspnea P2 initiated in Jan 2010; received $15M milestone from BMS Additional potential milestones of $665M for MM and other potential oncology indications Expect to complete enrollment in P2 by end of 2010; planning for P3 in 2011 Lenalidomide-naïve All treated pts 23 (82%) 17 (81%) 6 (86%) ORR (%) 22 16 6 Total pts 21 (95%) 15 (94%) 6 (100%) ORR (%) 28 21 7 Total pts Median = 3 (1-10) ≥ 2 1 Prior lines of therapy |
|
|
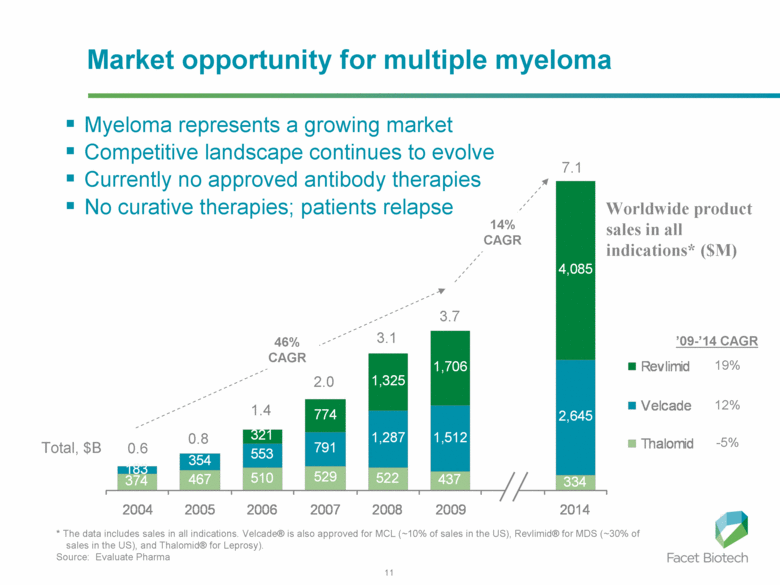
Worldwide product sales in all indications* ($M) Total, $B 0.6 0.8 1.4 3.7 7.1 14% CAGR 46% CAGR ’09-’14 CAGR 19% 12% -5% 2.0 3.1 Market opportunity for multiple myeloma Myeloma represents a growing market Competitive landscape continues to evolve Currently no approved antibody therapies No curative therapies; patients relapse * The data includes sales in all indications. Velcade® is also approved for MCL (~10% of sales in the US), Revlimid® for MDS (~30% of sales in the US), and Thalomid® for Leprosy). Source: Evaluate Pharma 374 467 510 529 522 437 334 183 354 553 791 1,287 1,512 2,645 321 774 1,325 1,706 4,085 2004 2005 2006 2007 2008 2009 2014 Revlimid Velcade Thalomid |
|
|
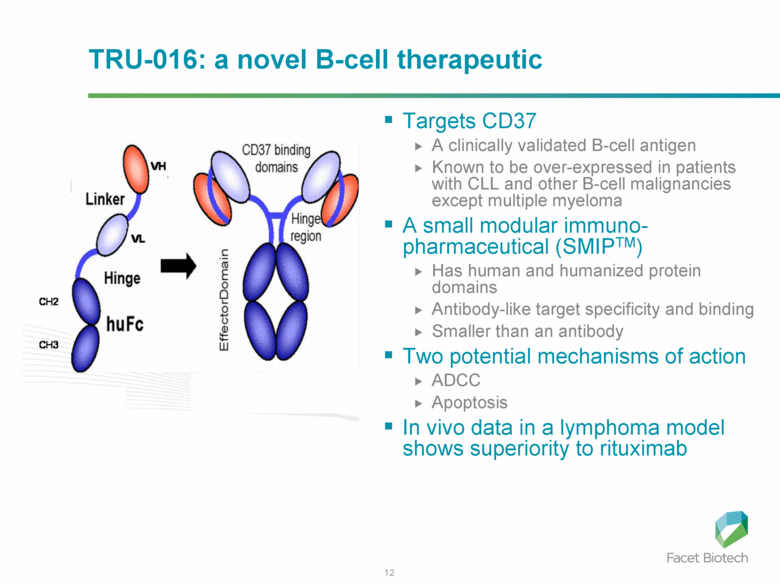
TRU-016: a novel B-cell therapeutic Targets CD37 A clinically validated B-cell antigen Known to be over-expressed in patients with CLL and other B-cell malignancies except multiple myeloma A small modular immuno-pharmaceutical (SMIPTM) Has human and humanized protein domains Antibody-like target specificity and binding Smaller than an antibody Two potential mechanisms of action ADCC Apoptosis In vivo data in a lymphoma model shows superiority to rituximab |
|
|
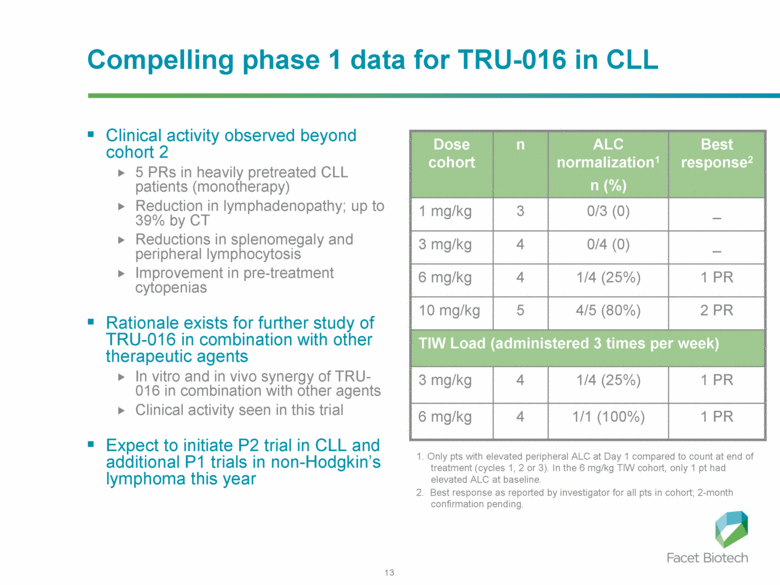
Compelling phase 1 data for TRU-016 in CLL Clinical activity observed beyond cohort 2 5 PRs in heavily pretreated CLL patients (monotherapy) Reduction in lymphadenopathy; up to 39% by CT Reductions in splenomegaly and peripheral lymphocytosis Improvement in pre-treatment cytopenias Rationale exists for further study of TRU-016 in combination with other therapeutic agents In vitro and in vivo synergy of TRU-016 in combination with other agents Clinical activity seen in this trial Expect to initiate P2 trial in CLL and additional P1 trials in non-Hodgkin’s lymphoma this year TIW Load (administered 3 times per week) 1/1 (100%) 1/4 (25%) 4/5 (80%) 1/4 (25%) 0/4 (0) 0/3 (0) ALC normalization1 n (%) 1 PR 4 6 mg/kg 1 PR 4 3 mg/kg 2 PR 5 10 mg/kg 1 PR 4 6 mg/kg - 4 3 mg/kg - 3 1 mg/kg Best response2 n Dose cohort 1. Only pts with elevated peripheral ALC at Day 1 compared to count at end of treatment (cycles 1, 2 or 3). In the 6 mg/kg TIW cohort, only 1 pt had elevated ALC at baseline. 2. Best response as reported by investigator for all pts in cohort; 2-month confirmation pending. |
|
|
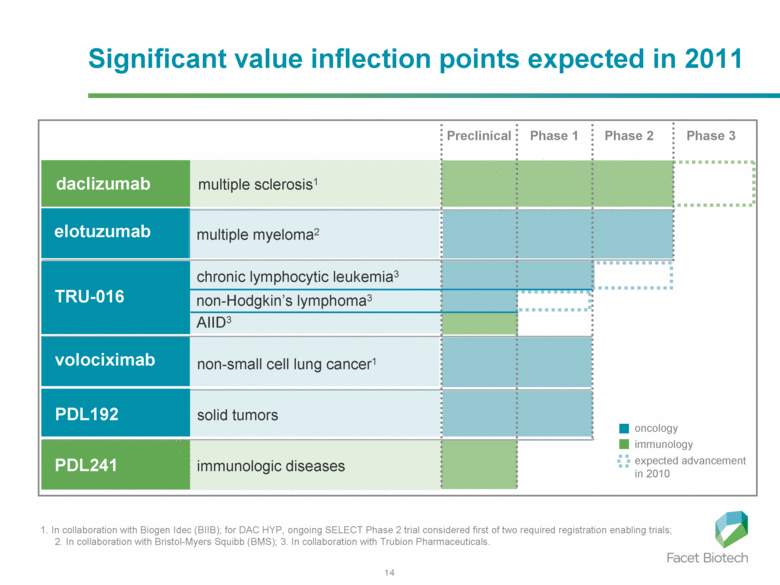
1. In collaboration with Biogen Idec (BIIB); for DAC HYP, ongoing SELECT Phase 2 trial considered first of two required registration enabling trials; 2. In collaboration with Bristol-Myers Squibb (BMS); 3. In collaboration with Trubion Pharmaceuticals. oncology immunology expected advancement in 2010 daclizumab Preclinical Phase 1 Phase 2 Phase 3 multiple sclerosis1 non-small cell lung cancer1 volociximab solid tumors PDL192 immunologic diseases PDL241 multiple myeloma2 elotuzumab Significant value inflection points expected in 2011 chronic lymphocytic leukemia3 TRU-016 non-Hodgkin’s lymphoma3 AIID3 |
|
|
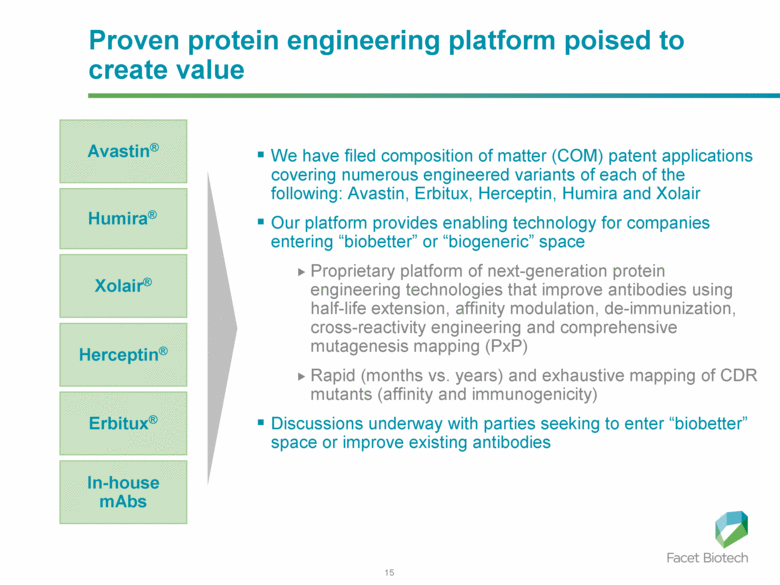
Proven protein engineering platform poised to create value We have filed composition of matter (COM) patent applications covering numerous engineered variants of each of the following: Avastin, Erbitux, Herceptin, Humira and Xolair Our platform provides enabling technology for companies entering “biobetter” or “biogeneric” space Proprietary platform of next-generation protein engineering technologies that improve antibodies using half-life extension, affinity modulation, de-immunization, cross-reactivity engineering and comprehensive mutagenesis mapping (PxP) Rapid (months vs. years) and exhaustive mapping of CDR mutants (affinity and immunogenicity) Discussions underway with parties seeking to enter “biobetter” space or improve existing antibodies Avastin® Xolair® Humira® Herceptin® Erbitux® In-house mAbs |
|
|
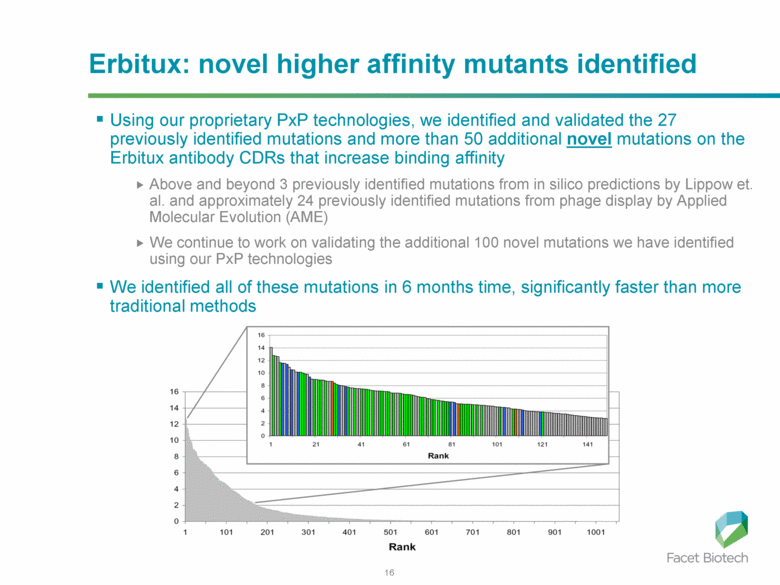
Erbitux: novel higher affinity mutants identified Using our proprietary PxP technologies, we identified and validated the 27 previously identified mutations and more than 50 additional novel mutations on the Erbitux antibody CDRs that increase binding affinity Above and beyond 3 previously identified mutations from in silico predictions by Lippow et. al. and approximately 24 previously identified mutations from phage display by Applied Molecular Evolution (AME) We continue to work on validating the additional 100 novel mutations we have identified using our PxP technologies We identified all of these mutations in 6 months time, significantly faster than more traditional methods |
|
|
Financial strength to achieve key milestones 1. As of November 30, 2009 and includes equity investment in Trubion; 2. Based on 25.07 million shares outstanding as of 12/04/09. $316.1M in cash, marketable and investment securities and restricted cash1 $12.61 per share based on shares outstanding2 Up to $45M in milestone payments achievable by end of first half of 2010 $1.79 per share based on shares outstanding2 $30M from Biogen Idec for initiation of daclizumab P3 trial $15M from BMS for initiation of elotuzumab P2 trial (earned and received since 11/30) |
|
|
Facet has favorable collaboration terms 50/50 cost sharing Significant milestone opportunities Commercialization and co-promotion rights 50/50 profit split in US, Canada and EU; royalty ROW Biogen Idec 20/80 Facet/BMS cost sharing Significant milestone opportunities 30/70 Facet/BMS U.S. profit split; royalty ex-US Bristol-Myers Squibb 50/50 cost sharing Risk-mitigated outbound milestone payments given late-stage nature Commercialization rights worldwide 50/50 profit split worldwide Flexibility to assign or terminate Trubion Terms |
|
|
Investment highlights Robust pipeline of five clinical-stage programs Compelling data seen to-date; significant value-driving inflection points through 2011 Promising proprietary protein engineering platform Opportunities in global “biobetter” and “biosimilar” markets Financial strength to fund company to achieve key milestones through 2012 $316.1 million in cash and related at November 30, 2009 Strategic process continues; discussions remain ongoing |