Attached files
| file | filename |
|---|---|
| EX-31.1 - CERTIFICATION OF CEO PURSUANT TO SECTION 302 - VASCULAR SOLUTIONS INC | vascular100390_ex31-1.htm |
| EX-31.2 - CERTIFICATION OF CFO PURSUANT TO SECTION 302 - VASCULAR SOLUTIONS INC | vascular100390_ex31-2.htm |
| EX-32.1 - CERTIFICATION OF CEO PURSUANT TO SECTION 906 - VASCULAR SOLUTIONS INC | vascular100390_ex32-1.htm |
| EX-32.2 - CERTIFICATION OF CFO PURSUANT TO SECTION 906 - VASCULAR SOLUTIONS INC | vascular100390_ex32-2.htm |
| EX-10.13 - SECURITY AGREEMENT - VASCULAR SOLUTIONS INC | vascular100390_ex10-13.htm |
| EX-10.12 - CREDIT AGREEMENT - VASCULAR SOLUTIONS INC | vascular100390_ex10-12.htm |
| EX-10.14 - PROMISSORY NOTE - VASCULAR SOLUTIONS INC | vascular100390_ex10-14.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
|
|
|
|
(Mark One) |
|
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
For the fiscal year ended December 31, 2009 |
|
|
OR |
|
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 0-27605
VASCULAR SOLUTIONS, INC.
(Exact
name of registrant as specified in its charter)
|
|
|
|
Minnesota |
41-1859679 |
|
(State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) |
6464 Sycamore Court
Minneapolis, Minnesota 55369
(Address
of principal executive offices, including zip code)
(763) 656-4300
(Registrant’s
telephone number, including area code)
|
|
|
|
Title of each class: |
Name of each exchange on which registered: |
|
Common Stock, par value $.01 per share |
The NASDAQ Global Market |
|
Securities
registered pursuant to Section 12(g) of the Act: None
|
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or ofr such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (section 229.406 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
|
|
|
|
|
|
Large accelerated filer o |
Accelerated filer x |
Non-accelerated filer o |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold on June 30, 2009 was $122,687,487.
As of January 29, 2010, the number of shares outstanding of the registrant’s common stock was 16,732,610.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for its 2010 Annual Meeting of Shareholders to be held on April 22, 2010 are incorporated by reference in Part III of this Annual Report on Form 10-K.
1
PART I
ITEM 1. BUSINESS
Overview
Vascular Solutions, Inc. (we, us or Vascular) is a medical device company focused on bringing clinically advanced solutions to interventional cardiologists and interventional radiologists worldwide. We were incorporated in the state of Minnesota in December 1996, and began operations in February 1997. Our main product lines consist of the following:
|
|
|
|
|
|
• |
Hemostat (blood clotting) products, principally consisting of the D-Stat® Dry hemostat, a topical thrombin-based pad with a bandage used to control surface bleeding, and the D-Stat Flowable, a thick yet flowable thrombin-based mixture for preventing bleeding in subcutaneous pockets, |
|
|
|
|
|
|
• |
Extraction catheters, principally consisting of the Pronto® V3 and Pronto LP extraction catheters, mechanical systems for the removal of soft thrombus from arteries, |
|
|
|
|
|
|
• |
Vein products, principally consisting of the Vari-Lase® endovenous laser, a laser and procedure kit used for the treatment of varicose veins, |
|
|
|
|
|
|
• |
Access products, principally consisting of micro-introducer kits, MICRO Elite™ and EXPRO Elite™ snares, the Guardian® hemostasis valve, and guidewires used in connection with percutaneous access to the vasculature, and |
|
|
|
|
|
|
• |
Specialty catheters, consisting of a variety of catheters for clinical niches including the Langston® dual lumen catheters, Twin-Pass® dual access catheters, GuideLiner™ catheter, Minnie™ support catheter, and Gopher™ catheter. |
In 2000 we received FDA clearance for our first product, the Duett™ sealing device, which is used to seal the puncture site following catheterization procedures. In 2001, due to competitive developments in the sealing device market, we made the strategic decision to develop additional products and de-emphasize the promotion of our Duett sealing device. We have grown from net revenue of $6.2 million in 2000 solely from the Duett device to net revenue of $68.4 million in 2009, with 99% of our 2009 net revenue coming from products other than the Duett device. This increase in revenue represents a compound annual growth rate of 31% and was driven by our commitment to the research and development of multiple new devices to diagnose and treat existing and new vascular conditions.
As a vertically-integrated medical device company, we generate ideas, create new interventional medical devices and then deliver these devices directly to the physician through our direct domestic sales force and our international distribution network. We are currently developing several additional products that leverage our existing infrastructure to bring additional solutions to the interventional cardiologist and interventional radiologist.
Interventional Cardiology and Interventional Radiology Industry Background
An estimated 80 million Americans have one or more types of cardiovascular disease—diseases of the heart and blood vessels. Cardiovascular disease is the number one cause of death in the United States and is replacing infectious disease as the world’s pre-eminent health risk. Advances in medicine have enabled physicians to perform an increasing number of diagnostic and therapeutic treatments of cardiovascular disease using minimally invasive methods, such as catheters placed inside the arteries, instead of highly invasive open surgery. Cardiologists and radiologists use diagnostic procedures, such as angiography, to confirm, and interventional procedures, such as angioplasty and stenting, to treat diseases of the coronary and peripheral arteries. Based on industry statistics, we estimate that cardiologists and radiologists performed over nine million diagnostic and interventional catheterization procedures worldwide in 2009. The number of catheterization procedures performed is expected to continue to grow as the incidence of cardiovascular disease continues to increase and the diagnosis and treatment of cardiovascular disease expands worldwide. The worldwide market for interventional medical devices in 2009 exceeded $5 billion.
2
Each angiographic procedure using a catheter requires a puncture in an artery, usually the femoral artery in the groin area and sometimes the radial artery in the wrist of the patient to gain access for the catheter. The catheter then is deployed through an introducer sheath and into the vessel to be diagnosed or treated. Upon completion of the procedure and removal of the catheter, the physician must seal this puncture in the artery and the tissue tract that leads from the skin surface to the artery to stop bleeding.
For information about our revenue, profits or losses and total assets, see our Consolidated Financial Statements included in Item 8 of Part II of this 10-K.
Hemostat Products
Our hemostat products utilize thrombin, a powerful bovine-derived blood clotting protein, to deliver a rapid seal of bleeding with a variety of shelf-stable product configurations. Through internal development we developed a proprietary manufacturing process to terminally sterilize our thrombin-based hemostats, which has resulted in our ability to create unique advantages in storage, shipping, preparation and application of our hemostat products.
Our most popular hemostat product is the D-Stat Dry hemostat bandage. In September 2003 we received regulatory clearance and commenced sales of our D-Stat Dry hemostat bandage in the United States and international markets. The D-Stat Dry hemostat bandage is a version of our proprietary blood clotting substance that is lyophilized (freeze-dried) into a gauze pad and combined with an adhesive bandage for application. The D-Stat Dry is used as an adjunct to manual compression for managing bleeding after catheterization procedures.
The traditional method for sealing the puncture site after catheterization procedures has been a manual process whereby a healthcare professional applies direct pressure to the puncture site, sometimes using a sand bag or a large C-clamp, for 20 minutes to an hour in order to form a blood clot. The healthcare professional then monitors the patient, who must remain immobile in order to prevent dislodging of the clot, for an additional two to 24 hours. Patients subjected to manual compression generally experience significant pain and discomfort during compression of the puncture site and during the period in which they are required to be immobile. Many patients report that this pain is the most uncomfortable aspect of the catheterization procedure. In addition, patients can develop a substantial coagulated mass of blood, or hematoma, around the puncture site. Finally, the need for healthcare personnel to provide compression and the use of hospital beds during the recovery period results in substantial costs to the institution, which, under virtually all current healthcare payment systems, are not separately reimbursed.
Until 1996, manual compression was used following virtually all catheterization procedures. In late 1995, the first vascular sealing device which did not rely on compression was introduced in the United States. In addition to invasive (below the skin surface) sealing devices, starting in 2000, non-invasive “patches” began to be used as an assist to manual compression following catheterizations. Non-invasive patches are used by physicians who (principally due to cost, complexity or risk of complications) do not wish to use invasive sealing devices, and for those patients who are contra-indicated for an invasive sealing device. Based on the number of catheterization procedures performed annually by cardiologists and radiologists, industry sources report that the total market opportunity for vascular sealing devices (invasive and non-invasive) is more than $1 billion annually.
3
We completed a 376-patient, five center randomized clinical study that demonstrated a 50% reduction in the median time-to-hemostasis when using the D-Stat Dry bandage compared to simple manual compression. In the third quarter of 2006 we received Food and Drug Administration (FDA) clearance of our claim that the D-Stat Dry reduces the time-to-hemostasis in diagnostic catheterizations. In the first quarter of 2008 we received FDA clearance and began selling two new versions of the original D-Stat Dry bandage. The first new version, the D-Stat® Dry Clear hemostatic bandage, is packaged with a clear bandage which allows for better visibility of the site while the bandage is in place. The second new version, Thrombix®, uses a lower cost manufacturing process which offers price flexibility within the product line. In the first quarter of 2009 we received FDA clearance and began selling a new version of the original D-Stat Dry bandage. This new product, the D-Stat® Dry Wrap hemostatic bandage, contains a pre-cut specifically designed for the control of bleeding around indwelling lines. We believe that the market for hemostat pads following catheterization procedures has grown substantially since the first competitive patch was introduced in 2000, with a market size greater than $50 million in 2009.
We have developed additional configurations of our hemostat technology for specialized medical procedures. Our D-Stat Radial hemostat band is a version of the D-Stat Dry that includes a compression band to allow it to be applied over the radial artery in the wrist. In approximately 5% of all catheterizations in the United States, the radial artery in the wrist instead of the femoral artery in the groin is used to gain arterial access. In these cases using the radial artery, a variety of compression splints and tapes have been used for controlling bleeding following the procedure. The D-Stat Radial is the first device that contains an active blood clotting agent together with a compression collar for this purpose. We received regulatory clearance for the D-Stat Radial hemostat band in September 2003, and made manufacturing improvements to the product before launching it in the United States in early 2004. In December 2009, we made further manufacturing improvements and launched a new version called the D-Stat Rad-Band™ in the United States.
Our D-Stat Flowable hemostat, which we began selling worldwide in February 2002, is a thick yet flowable mixture of collagen, thrombin and diluent that can be delivered topically and into voids and open spaces to control active bleeding. The D-Stat Flowable hemostat can be used in a wide variety of procedures as an adjunct to hemostasis. In December 2006 we received FDA approval of our premarket approval (PMA) supplement for the use of D-Stat Flowable in the prepectoral pockets created in pacemaker and implantable cardioverter defibrillator (ICD) implants. Our PMA supplement was supported by the results of our 269-patient “Pocket Protector” clinical study that demonstrated a 48% reduction in the incidence of clinically relevant hematomas through the use of D-Stat Flowable compared to the standard of care. We estimate that the U.S. market opportunity for this prepectoral pocket indication is greater than 100,000 procedures or $10 million annually.
Our original Duett sealing device is designed to provide a complete seal of the puncture site following catheterization procedures such as angiography, angioplasty and stenting. The Duett sealing device combines an easy-to-use balloon catheter delivery mechanism with a biological procoagulant mixture, which we believe offers advantages over both manual compression and competitive vascular sealing devices. We began selling our Duett sealing device in Europe in February 1998 and in the United States in June 2000. In the fourth quarter of 2001 we introduced the Diagnostic Duett version of the Duett sealing device, which utilizes a lower dose of procoagulant for the less-challenging diagnostic subset of catheterization procedures.
At the end of the first quarter of 2004 we received regulatory clearance in the United States for the Thrombi-Pad® trauma bandage. The Thrombi-Pad trauma bandage is a larger-sized version of our D-Stat Dry designed for use in trauma indications, does not require mixing or special storage requirements and can be quickly applied to even severely bleeding wounds. During the second quarter of 2005 we received regulatory clearance in the United States for the Thrombi-Gel® hemostatic foam. The Thrombi-Gel hemostatic product contains a gelatin foam pad (instead of the non-resorbable gauze pad in the D-Stat Dry) to provide a unique, premixed, sterile, gelatin/thrombin hemostat. An additional version of the Thrombi-Gel in development is the Thrombi-Paste™, which adds a liquid to make a thick thrombin-based gel. Because the Thrombi-Pad, Thrombi-Gel and Thrombi-Paste products are expected to be utilized outside of our core market of interventional procedures, we have licensed the distribution of these products to King Pharmaceuticals, Inc., as described under “Agreements with King Pharmaceuticals, Inc.” below.
4
Extraction Catheters
Our Pronto products consist of a catheter with a proprietary distal tip and large extraction lumen that can be delivered into arteries to mechanically remove blood clots using simple vacuum suction. The Pronto extraction catheter was initially developed by Dr. Pedro Silva of Milan, Italy, who exclusively licensed the design to us in 2002. We received CE mark approval and commenced international sales of the Pronto in August 2003, and received FDA clearance in December 2003 and commenced sales in the United States in early 2004. In the fourth quarter of 2005 we launched the third generation design of the Pronto, named the Pronto V3. The V3 version of the Pronto resulted in a substantial increase in Pronto sales in 2006. The FDA cleared the Pronto V3 catheter for specific use within the coronary system in December 2006. We believe that the market size for the removal of soft thrombus is greater than $100 million per year worldwide.
We have developed four additional versions of extraction catheters -- the Pronto-Short, Pronto .035”, Pronto LP and QXT®. The Pronto-Short is a shorter and larger version designed for use in clotted dialysis grafts that was launched in August 2005. The Pronto .035” is a much larger version designed for use in large vessel peripheral indications that was launched in August 2007. The Pronto LP is a low profile version that is designed for use in smaller vessels and was launched in January 2008. The QXT is a low-cost version that is designed to be sold in certain international markets and was launched in March 2008.
Vein Products
Our Vari-Lase endovenous laser products consist of a laser console, procedure kits and accessories used in the treatment of reflux of the great saphenous vein, commonly referred to as varicose veins. More than one million people in the United States seek treatment each year for varicose veins. Left untreated, varicose veins can result in serious clinical consequences, including limited mobility and venous stasis ulcers. Historically, an invasive surgical procedure known as vein stripping was the only treatment for severe varicose veins. While vein stripping is still performed, since 2002 a non-surgical procedure using endovenous laser energy to treat and close the diseased vein has become a preferred alternative. Recent clinical data on endovenous laser therapy has demonstrated excellent clinical results and outstanding patient satisfaction. During the fourth quarter of 2004 the Center for Medicare and Medicaid Services (CMS) published the Medicare Physicians Fee Schedule which established favorable reimbursement rates for the endovenous laser procedure starting January 1, 2005. Private insurance companies also have issued reimbursement coverage decisions resulting in more physicians adding endovenous laser therapy to their practice. We believe the current market size for treating varicose veins using endovenous therapy is greater than $140 million per year.
The first product we launched in our vein product line was our Vari-Lase® procedure kit in July 2003 in the United States. Our Vari-Lase procedure kit is custom-designed for the endovenous procedure, with features supporting ease-of-use and safety, and is compatible with many of the competitive laser consoles used in this procedure. In December 2003, we received FDA clearance for our Vari-Lase laser console, which is manufactured to our specifications by MedArt, a leading Denmark-based medical laser manufacturer. Since 2004 we have continued our expansion by adding several accessory items to our vein product line. In April 2007 we launched the Vari-Lase Bright Tip™ fiber which utilizes a ceramic sleeve to the distal tip of the laser fiber to provide improved ultrasound visibility and prevent contact between the energy-transmitting fiber tip and the vein wall during the application of laser energy.
Specialty Catheters
Specialty catheters consist of a variety of catheters designed to perform unique functions within clinical niches in interventional medicine. At the end of the third quarter of 2004 we received regulatory clearance in the United States for the Langston dual lumen pigtail catheter. The Langston catheter is used for the precise measurement of intravascular pressure gradients, primarily measured to diagnose aortic valve stenosis. We believe our Langston catheter is the only dual lumen pigtail catheter on the U.S. market that can be used for this intended indication. We believe the U.S. market opportunity for the Langston catheter product line is $10 million annually.
5
During 2006 we launched the Twin-Pass dual access catheter. The Twin-Pass is a two lumen catheter designed to be used in conjunction with steerable guidewires to access discrete regions of the coronary and peripheral arterial vasculature and for use during procedures utilizing two guidewires. We believe the Twin-Pass addresses a market opportunity of between $1 million and $5 million annually within interventional cardiology.
In July of 2007 we launched the Gopher support catheter. The Gopher catheter is designed to assist in the passage of interventional devices through arterial lesions by utilizing a unique rotational force. In August 2009 we launched our new version of this catheter, referred to as the Gopher™ Gold catheter. The Gopher Gold is designed for use when treating coronary and peripheral stenoses over an existing in-place 0.014” guidewire and contains a highly radiopaque gold-plated distal tip. We believe the market opportunity of the Gopher is in excess of $2 million per year.
In January 2009 we launched the Minnie support catheter. The Minnie support catheter is designed to provide superior guidewire support and exchange for complex interventions. We believe the current market size for support catheters is greater than $15 million per year.
In November 2009 we launched the GuideLiner catheter. The GuideLiner catheter is a unique coaxial “mother and child” guide extension with rapid exchange convenience that enables deep seating, guide back-up support and selective intubation in challenging coronary interventions. We believe the market opportunity of the GuideLiner is in excess of $15 million per year.
Access Products
Access products are used to gain percutaneous access to the vasculature for a wide variety of arterial and venous procedures. We started selling access products in July 2003. Our access products include a full line of micro-introducer kits and a variety of specialty guidewires.
During 2007 we entered into an agreement with Zerusa Limited, whereby we agreed to act as the exclusive U.S. distributor of Zerusa’s Guardian® hemostatic valve. The Guardian hemostatic valve is a valve used in catheterization procedures to allow the placement of multiple devices simultaneously in the artery with a unique push-button operation that is designed to minimize blood loss. In November 2009 we launched a second generation version called the Guardian II hemostatic valve. The Guardian II has a shorter overall length and clear rotating components.
During 2008 we entered into an agreement with Radius Medical to distribute their Micro Elite™ and Expro Elite™ Snares within the United States. The Elite snares feature a highly torqueable shaft design for control and maneuverability when accessing distal targets. We believe the market opportunity for the Micro Elite Snare is in excess of $2 million per year and the market opportunity for the Expro Snares is in excess of $5 million per year.
During 2009 we entered into an agreement with Perouse Medical to distribute their Flamingo inflation device within the United States. The Flamingo is designed for use during cardiovascular procedures to create, maintain and monitor pressures.
In March 2009 we launched the Grebset™ micro-introducer kit and in November 2009 we launched the PiggyBack™ wire converter. The Grebset is a unique micro-introducer kit with an angled tip that facilitates steerability. The PiggyBack converts the 0.014” guidewire into a 0.035” delivery device, preserving the position of the 0.014” guidewire and eliminating the need to switch-out or exchange guidewires.
6
Other Products
We have developed and offer several additional clinical niche products, and additional products in international markets which are not yet approved in the United States. During the second quarter of 2002 we acquired the Acolysis® ultrasound thrombolysis system. The Acolysis system uses ultrasound energy generated by the Acolysis controller that is delivered by the disposable Acolysis probe to lyse blood clots and plaque within the artery. The Acolysis controller and probes are sold only in international markets, where it has been sold principally for the treatment of peripheral vascular disease.
The amount of total revenue contributed by each of our product lines and by geographic areas for the last three fiscal years is set forth in Notes 2 and 11 to our Consolidated Financial Statements in Item 8 of Part II of this Form 10-K.
Agreements with King Pharmaceuticals, Inc.
In January 2007, we entered into three agreements with King Pharmaceuticals, Inc., consisting of a License Agreement, a Device Supply Agreement and a Thrombin-JMI® Supply Agreement.
The effect of these three agreements was to forge a new relationship between us and King having essentially three components. First, King is selling through its direct sales force, and we are manufacturing and supplying to King, our Thrombi-Pad trauma bandage and Thrombi-Gel hemostat products (and in the future our Thrombi-Paste hemostat product). Second, we are working with King to develop additional hemostat products to be sold by King outside of our direct sales force’s call point of cardiac, peripheral and electrophysiology catheterization laboratories. Third, King is selling Thrombin-JMI® to us for use in the manufacture of our hemostatic products under a 10-year, fixed price arrangement.
Under the terms of the License Agreement, we granted to King an exclusive, royalty-free, fully-paid up, perpetual, worldwide right and license to all of our patents and know-how relating to the development, manufacture, use, sale, importation or other exploitation of our Thrombi-Pad trauma bandage, Thrombi-Gel 10, 40 and 100 hemostats, Thrombi-Paste hemostat (collectively, the “Products”) and all future medical devices having application in the Field (as defined below) and intended to produce hemostasis by accelerating the clotting process of blood (a “hemostat device”). The “Field” is defined as all applications of hemostat devices in all areas other than catheterization laboratories (cardiac and peripheral), electrophysiology laboratories and holding and recovery rooms for such laboratories. Upon execution of the License Agreement, King paid us a one-time payment of $6.0 million. No other payments are due from King to us under the License Agreement. The term of the License Agreement commenced on January 9, 2007 and continues until the later of the expiration of each licensed patent or King’s relinquishment of its license rights under the licensed know-how.
Under the terms of the Device Supply Agreement, we agreed to manufacture and supply the Products to King and King agreed to purchase the Products from us for King’s exclusive commercialization, distribution, sale and use of the Products in the Field. King does not have any minimum purchase obligations under the Device Supply Agreement. The Device Supply Agreement does not limit our ability to manufacture the Products for our own commercialization, distribution, sale and use outside of the Field. The transfer prices are fixed for each Product under the Device Supply Agreement and are adjusted for cost and inflation increases according to a market index. Upon the first commercial sale by King of a Thrombi-Gel hemostat (which occurred in May 2007), King made a one-time, non-refundable milestone payment to us of $1.0 million. Upon the first commercial sale by King of a Thrombi-Paste hemostat product, King will be required to make another one-time, non-refundable milestone payment to us of $1.0 million. As directed by King, we will continue to perform the regulatory work necessary to obtain surgical approvals for the Thrombi-Gel products. King has directed us to suspend the regulatory work on the Thrombi-Paste products until further notice. King has agreed to reimburse us for our expenses in obtaining these approvals. If, after undertaking and completing the development and regulatory plans with respect to the Thrombi-Gel and Thrombi-Paste products, such development and regulatory efforts have not resulted in regulatory approval for surgical use, we have agreed to make a one-time, non-creditable, non-refundable payment of $2.5 million to King for both the Thrombi-Gel products and Thrombi-Paste products if they are not approved by the FDA for surgical use. We believe the probability of making these one-time payments to King is remote. Under the Device Supply Agreement, King also has certain rights of first refusal with respect to any hemostatic devices for use in the Field that we may develop on our own or at the request of King. The Device Supply Agreement has an initial term of 10 years, followed by successive automatic one-year extensions, subject to termination by the parties under certain circumstances, including termination by King without cause anytime after the third anniversary of its execution upon two years prior written notice to us.
7
Under the terms of the Thrombin-JMI® Supply Agreement, King agreed to manufacture and supply thrombin to us on a non-exclusive basis. King agreed to supply us with such quantity of thrombin as we may order for use in devices not intended for sale by King in the Field at a fixed price throughout the term of the Thrombin-JMI® Supply Agreement as adjusted for inflation, variations in potency and other factors. King also agreed to provide thrombin to us under the Thrombin-JMI® Supply Agreement at no cost for incorporation into Products and hemostat devices intended for sale in the Field by King. The Thrombin-JMI® Supply Agreement has an initial term of 10 years, followed by successive automatic one-year extensions, subject to termination by the parties under certain circumstances, including (1) termination by King without cause anytime after the fifth anniversary of its execution upon five years prior written notice to us and (2) termination by us without cause anytime after the fifth anniversary of its execution upon five years prior written notice to King provided that the Device Supply Agreement has expired on its terms or the parties have agreed to terminate it.
Business Strategy
Our primary objective is to establish ourselves as a leading supplier of clinically superior medical devices for substantial, unique opportunities within interventional medicine. The key steps in achieving our primary objective are the following:
|
|
|
|
|
|
• |
Maintain and Improve our Clinically-Oriented Direct Sales Force in the United States. During 2000 we commenced sales of our products in the United States through a direct sales force of clinically trained account managers who sell and train interventional cardiologists, radiologists and catheterization laboratory personnel on the use of our products. As our product lines have increased, we have increased the size of our sales force to 92 at the end of 2009, which provides substantially complete geographic coverage of the United States. |
|
|
|
|
|
|
• |
Expand our Existing Products to Our Existing Market. Starting in 2003 we have launched multiple new products in the United States through our direct sales force to our existing markets. Pursuing this multiple product strategy has generated material sales growth, and we believe that each of our current product lines has the potential to generate continued sales growth during 2010 and beyond. |
|
|
|
|
|
|
• |
Develop New Devices to be Sold Through our Direct Sales Force to our Existing Customers. We intend to continue to leverage our direct sales force by bringing additional products to the interventional physician. |
|
|
|
|
|
|
• |
Explore Corporate Partnerships for the Distribution of Products Through our Direct Sales Force to our Existing Customers. We intend to continue to leverage our direct sales force by bringing additional products to the interventional physician through distribution agreements. Over the past three years we have partnered with Radius Medical, Zerusa Limited and Perouse Medical to distribute their products in the United States. We are in discussions with additional potential distribution partners and expect to distribute additional products as they become available. |
|
|
|
|
|
|
• |
Explore Corporate Relationships to Augment our Direct Sales Force. In markets for our products beyond the interventional physician (such as occurred with our Thrombi-Gel, Thrombi-Paste and Thrombi-Pad products) and in other situations where synergistic sales can result, we intend to enter into corporate relationships to broaden our products’ reach and increase our revenues without distracting our direct sales force. |
8
Sales, Marketing and Distribution
In 2000 we commenced sales of our Duett sealing device in the United States through our direct sales organization. As of December 31, 2009, our worldwide sales force consisted of approximately 92 employees who sell our entire line of interventional products. We believe that the majority of interventional catheterization procedures in the United States are performed in high volume catheterization laboratories, and that these institutions can be served by our focused direct sales force.
As part of our sales strategy, our sales force is clinically trained and is able to train physicians and other healthcare personnel on the use of our products. We believe that effective training is a key factor in encouraging physicians to use interventional medical devices. We have created, and will continue to work to improve, an in-the-field training program for the use of all of our products. We also develop and maintain close working relationships with our customers to continue to receive input concerning our product development plans.
We are focused on building market awareness and acceptance of our products. Our marketing department provides a wide range of programs, materials and events that support our sales force. These include product training, conference and trade show appearances and sales literature and promotional materials.
Our international sales and marketing strategy has been to sell to interventional cardiologists and interventional radiologists through established independent distributors in major international markets, subject to required regulatory approvals. In Germany, we created a wholly-owned subsidiary to sell directly to customers in the German market beginning in the fourth quarter of 2000. In the first quarter of 2008 we transitioned our sales in Germany to an independent distributor and closed our German subsidiary. We have entered into multi-year written distribution agreements with each of our independent distributors, and we ship our products to these distributors upon receipt of purchase orders. Each of our independent distributors has the exclusive right to sell our products within a defined territory. These distributors also market other medical products, although they have agreed not to sell directly competitive products. Our independent distributors purchase our products from us at a discount from list price and resell the device to hospitals and clinics. Sales to international distributors are denominated in United States dollars, with the exception of the Germany distributor where sales are denominated in Euros. The end-user price is determined by the distributor and varies from country to country.
New Product Development
Our research and development staff is currently focused on developing new products to sell to our existing customer base through our direct sales force and on developing next generation versions of our existing products. We incurred expenses of $7,847,000 in 2009, $6,333,000 in 2008, and $5,418,000 in 2007 for research and development activities. To further leverage our efficiencies, our research and development group continues to develop in-house capabilities to manufacture some of the components currently produced by outside vendors.
We expect our research and development activities to continue to expand to include evaluation of new concepts and products for the interventional cardiology and interventional radiology field. We believe that there are many potential new interventional products that would fit within the development, clinical, manufacturing and distribution network we have created for our existing products.
9
Manufacturing
We manufacture our products in our facilities located in the suburbs of Minneapolis, Minnesota. The catheter manufacturing and packaging processes occur under a controlled clean room environment. Our quality system, manufacturing facilities and processes have been certified to be compliant with the applicable European standards EN46001 and the succeeding EN13485 since July 1998. Our quality system was most recently audited by the FDA in February 2009 with no deficiencies noted.
We purchase components from various suppliers and rely on single sources for several parts of our products. We purchase our United States product requirements for thrombin (a component in the Duett and in all of the D-Stat products) under the Thrombin-JMI® Supply Agreement with King Pharmaceuticals, Inc. We purchase our International product requirements for thrombin under a supply agreement with Sigma-Aldrich Fine Chemicals, an operating division of Sigma-Aldrich, Inc. To date, we have not experienced any significant adverse effects resulting from shortages of components.
The manufacture and sale of our products entails significant risk of product liability claims. Although we have product liability insurance coverage in an amount which we consider reasonable, it may not be adequate to cover potential claims. Any product liability claims asserted against us could result in costly litigation, reduced sales and significant liabilities and divert the attention of our technical and management personnel away from the development and marketing of our products for significant periods of time.
Competition
Competition in the interventional medical device industry is intense and dominated by very large and experienced companies such as Medtronic Inc., Abbott Laboratories, Johnson & Johnson and Boston Scientific Corporation. We compete on the basis of our clinically differentiated products and focused opportunities within this interventional medical device market.
Our D-Stat Dry hemostatic bandage competes in the noninvasive topical patch market segment of sealing devices. These patches are applied directly over the puncture site and held in place with adjunctive manual compression for a period of 10-20 minutes. Competitive patches include the SyvekPatch®, which is manufactured and marketed by Marine Polymer Technologies, Inc., and the HemCon® Patch, which is distributed by Cardinal Health, Inc. and manufactured by Hemcon Medical Technologies, Inc.
The Pronto extraction catheter competes in the market segment for removal of thrombus from the arterial system. There are many companies that are selling or have developed products in this segment, including MedRad, Inc., Medtronic, Inc. and Spectranetics Corporation.
We are aware of many companies that sell a product for the endovenous laser treatment of varicose veins, including AngioDynamics Inc., biolitec, Dornier MedTech, and CoolTouch. Each of the competitive products contains similar types of components for performing endovenous laser therapy but differ in procedural training, laser wavelength, custom-designed features and customer support. In addition, Covidien sells an alternative endovenous procedure that utilizes radiofrequency as opposed to laser energy for the treatment of varicose veins.
There are many companies that are selling or have developed hemostats which compete generally with our D-Stat Flowable hemostat. Virtually all of these devices, however, are positioned as hemostats for the surgical market and are not designed specifically for use in electrophysiology procedures.
We have several competitors in the specialty catheter and access product lines, including Cook® Medical.
10
In each of our product areas, we believe that several other companies are developing new devices. The medical device industry is characterized by rapid and significant technological changes as well as the frequent emergence of new technologies. There are likely to be research and development projects related to these market areas of which we are currently unaware. A new technology or product may emerge that results in a reduced need for our products or results in a product that renders our product noncompetitive.
Regulatory Requirements
United States
Our products are regulated in the United States as medical devices by the FDA under the Federal Food, Drug and Cosmetic Act. The FDA classifies medical devices into one of three classes based upon controls the FDA considers necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls such as labeling, adherence to good manufacturing practices and maintenance of product complaint records, but are usually exempt from premarket notification requirements. Class II devices are subject to the same general controls and also are subject to special controls such as performance standards, and FDA guidelines, and may also require clinical testing prior to approval. Class III devices are subject to the highest level of controls because they are used in life-sustaining or life-supporting implantable devices. Class III devices require rigorous clinical testing prior to their approval and generally require a premarket approval (PMA) or supplement application prior to their sale.
If a medical device manufacturer can establish that a device is “substantially equivalent” to a legally marketed Class I or Class II device, or to an unclassified device, or to a Class III device for which the FDA has not called for PMAs, the manufacturer may seek clearance from the FDA to market the device by filing a 510(k) premarket notification. The 510(k) notification must be supported by appropriate data establishing the claim of substantial equivalence to the satisfaction of the FDA. Following submission of the 510(k) notification, the manufacturer may not place the device into commercial distribution in the United States until an order is issued by the FDA.
Manufacturers must file an investigated device exemption (IDE) application if human clinical studies of a device are required and if the FDA considers experimental use of the device to represent significant risk to the patient. The IDE application must be supported by data, typically including the results of animal and mechanical testing of the device. If the IDE application is approved by the FDA, human clinical studies may begin at a specific number of investigational sites with a maximum number of patients, as approved by the FDA. The clinical studies must be conducted under the review of an independent institutional review board to ensure the protection of the patients’ rights.
Generally, upon completion of these human clinical studies, a manufacturer seeks approval of a Class III medical device from the FDA by submitting a PMA application. A PMA application must be supported by extensive data, including the results of the clinical studies, as well as literature to establish the safety and effectiveness of the device.
Our Duett sealing device is classified as a Class III device and is subject to the PMA requirements. Our D-Stat Flowable is dually classified as both a Class III and Class II device based on the three distinct indications for use that have been assigned to this product.
Our D-Stat Dry, Pronto, Vari-Lase, specialty catheters and access products product lines generally are classified as Class II products and therefore require clearance of a 510(k) notification by the FDA prior to being sold in the United States. Each of the devices within these product lines was subject to a 510(k) notification which was determined to be “substantially equivalent” to a legally marketed predicate device by the FDA, thereby allowing commercial marketing in the United States.
11
Our Thrombi-Gel and Thrombi-Paste product lines are indicated for use as topical hemostats and, as such, are classified as Class II products. Approval for expanded use as surgical hemostats will place these products into the Class III designation subject to the PMA requirements.
We also are subject to FDA regulations concerning manufacturing processes and reporting obligations. These regulations require that manufacturing steps be performed according to FDA standards and in accordance with documentation, control and testing standards. We also are subject to inspection by the FDA on an on-going basis. We are required to provide information to the FDA on adverse incidents as well as maintain a documentation and record keeping system in accordance with FDA guidelines. The advertising of our products also is subject to both FDA and Federal Trade Commission jurisdiction. If the FDA believes that we are not in compliance with any aspect of the law, it can institute proceedings to detain or seize products, issue a recall, stop future violations and assess civil and criminal penalties against us, our officers and our employees.
International
The European Union has adopted rules which require that medical products receive the right to affix the CE mark, an international symbol of adherence to quality assurance standards and compliance with applicable European medical device directives. As part of the CE mark compliance, manufacturers are required to comply with the European quality systems standards. We received the CE mark approval for our Duett sealing device and certification of our quality system in July 1998, and we have subsequently received the CE mark approval for other products within our product lines.
Our hemostatic products contain bovine-derived thrombin and are subject to additional regulatory review within the European Union to minimize the risk of exposure to viral and BSE pathogens. The regulations in this area continue to evolve and our products may be subject to additional regulatory scrutiny in the future.
International sales of our products are subject to the regulatory requirements of each country in which we sell. These requirements vary from country to country but generally are less stringent than those in the United States. We have obtained regulatory approvals where required for us to sell our products in those countries.
Third Party Reimbursement
In the United States, healthcare providers that purchase medical devices generally rely on third-party payors, principally the Centers for Medicare and Medicaid Services or CMS (formerly the Health Care Financing Administration, or HCFA) and private health insurance plans, to reimburse all or part of the cost of catherization procedures. We believe that in the current United States reimbursement system, the cost of vascular sealing devices is incorporated into the overall cost of the catheter procedure. Our other products are subject to reimbursement rules depending on the specific medical procedure in which they are utilized.
CMS and the AMA Current Procedure Terminology (CPT) panel finalized the implementation of reimbursement codes for the endovenous laser ablation procedure beginning in January 2005. This action cleared the way for a consistent means of billing the Medicare program for medically necessary vein treatments using laser technologies and resulted in a favorable reimbursement rate. Reimbursement for these procedures is now well-established but adjusted annually in accordance with the normal adjustment procedures of CMS.
Market acceptance of our products in international markets is dependent in part upon the availability of reimbursement from healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country. The main types of healthcare payment systems in international markets are government-sponsored healthcare and private insurance. Countries with government-sponsored healthcare, such as the United Kingdom, have a centralized, nationalized healthcare system. New devices are brought into the system through negotiations between departments at individual hospitals at the time of budgeting. In most foreign countries, there are also private insurance systems that may offer payments for alternative therapies.
12
Patents and Intellectual Property
We file patent applications to protect technology, inventions and improvements that are significant to the development of our business, and use trade secrets and trademarks to protect other areas of our business. We currently have 10 U.S. patents issued and 12 additional patents pending concerning our Duett sealing device, Pronto catheter, Langston dual lumen pigtail catheter, D-Stat Dry, the Vari-Lase product line, and other products. We also have pursued international patent applications.
The interventional medical device market in general and the endovenous laser therapy field in particular, are characterized by frequent and substantial intellectual property litigation. Currently, we are involved in litigation with AngioDynamics, Inc., who has filed a lawsuit against us alleging that we have infringed U.S. Patent No. 7,273,478 and U.S. Patent No. 7,559,329. (See “Legal Proceedings” in Item 3 of Part I of this Form 10-K.) The interpretation of patents involves complex and evolving legal and factual questions. Intellectual property litigation in recent years has proven to be complex and expensive, and the outcome of such litigation is difficult to predict.
We may become the subject of additional intellectual property claims in the future related to our products. Our defense of any intellectual property claims, regardless of the merits of the complaint, could divert the attention of our technical and management personnel away from the development and marketing of our products for significant periods of time. The costs incurred to defend these claims could be substantial and adversely affect us, even if we are ultimately successful.
We also rely on trade secret protection for certain aspects of our technology. We typically require our employees, consultants and vendors for major components to execute confidentiality agreements upon their commencing services with us or before we disclose confidential information to them. These agreements generally provide that all confidential information developed or made known to the other party during the course of that party’s relationship with us is to be kept confidential and not disclosed to third parties, except in special circumstances. The agreements with our employees also provide that all inventions conceived or developed in the course of providing services to us shall be our exclusive property.
We also register the trademarks and trade names through which we conduct our business. To date, we have registered and use the trademarks “Acolysis®,” “Acolysis System®,” “Acolysis System Therapeutic Ultrasound Thrombolysis®,” “Auto-Fill®,” “D-Stat®,” “Gandras®,” “Langston®,” “Pronto®,” “QXT Extraction Catheter®,” “Skyway®,” “Thrombix®,” “Thrombi-Gel®,” “Twin-Pass®,” and “Vari-Lase®,” and we use the following trademarks “Axis™,” “Bright Tip™,” “Gator™,” “Gopher™,” “GrebSet™,” “GuideLiner™,” “InnerChange™,” “Jiffy™,” “Max-Support™,” “Minnie™,” “PiggyBack™,” “Rad-Band™,” “Trespass™,” and the Duett stylized logo. We acquired the registered trademark “Acolysis” in connection with our acquisition of the Acolysis therapeutic ultrasound business in 2002. U.S. trademark registrations are generally for a term of 10 years, renewable every 10 years as long as the trademark is used in the regular course of trade.
Employees
As of December 31, 2009, we had 266 full-time employees. Of these employees, 77 were in manufacturing activities, 119 were in sales and marketing activities, 28 were in research and development activities, 23 were in regulatory, quality assurance and clinical research activities and 19 were in general and administrative functions. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements. We believe our employee relations are good. We are an Equal Opportunity Employer.
13
Executive Officers of the Registrant
Our executive officers as of January 31, 2010 are as follows:
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position
|
|
Howard Root |
|
49 |
|
Chief Executive Officer and Director |
|
James Hennen |
|
37 |
|
Chief Financial Officer, Vice President of Finance and Corporate Secretary |
|
Jonathan Hammond |
|
42 |
|
Vice President of Manufacturing |
|
Brett Demchuk |
|
46 |
|
Vice President of Quality |
|
William Rutstein |
|
57 |
|
Vice President of International Sales |
|
Susan Christian |
|
41 |
|
Vice President of Sales Operations |
|
Carrie Powers |
|
35 |
|
Vice President of Marketing |
Howard Root has served as Chief Executive Officer and a member of our Board of Directors since he co-founded Vascular Solutions in February 1997. From 1990 to 1995, Mr. Root was employed by ATS Medical, Inc., a mechanical heart valve company, most recently as Vice President and General Counsel. Prior to joining ATS Medical, Mr. Root practiced corporate law, specializing in representing emerging growth companies, at the law firm of Dorsey & Whitney LLP for over five years. Mr. Root is also a member of the Board of Directors of the Medical Device Manufacturers Association (MDMA).
James Hennen has served as our Chief Financial Officer since January 2004. Mr. Hennen served as our Controller & Director of Finance from February 2002 through December 2003. Prior to joining us, Mr. Hennen served in various accounting positions, most recently as International Controller with WAM!NET, Inc., a globally networked information technology company for media transfer, where he worked from December 1997 through February 2002. From October 1995 through December 1997, Mr. Hennen was a Senior Auditor for Ernst & Young, LLP. Mr. Hennen is a Certified Public Accountant (inactive).
Jonathan Hammond has served as our Vice President of Manufacturing since January 2010. Mr. Hammond previously served as our Director of Process Development from January 2008 to December 2009, our Process Development Manager from January 2007 to December 2007, and our Senior Process Development Engineer from the time he joined us in July of 2005 until December 2006. Prior to joining us, Mr. Hammond served as Senior Manufacturing Engineer with Enpath Medical, a leading supplier of venous vessel introducers, where he worked from November 2002 through June of 2005. From March 1993 through October 2002, Mr. Hammond served in various engineering and technical product management roles for MICROVENA Corporation (ev3).
Brett Demchuk has served as our Vice President of Quality since July 2007. Prior to joining us, Mr. Demchuk worked at ATS Medical, Inc. where he was Senior Director of Operations from 1998 to July 2007 and Quality Manager from 1992 to 1998. Prior to this time, he held quality assurance engineering positions at Orthomet and GV Medical.
Bill Rutstein has served as our Vice President of International Sales since October 2008. Mr. Rutstein served as our Senior Director of International Sales from January 2008 to September 2008 and was our Director of International Sales since joining Vascular Solutions in August 1999. Prior to joining us, Mr. Rutstein was the Business Unit Director for the cardiosurgery division of Minntech Corporation, a medical device company, from April 1997 to July 1999. From November 1988 to March 1997, Mr. Rutstein worked for Daig Corporation (a St. Jude Medical Company), a medical device company specializing in cardiology and electrophysiology catheters, where he served as Regional Sales Manager, National Sales Manager, OEM Sales Manager and International Sales Manager.
Susan Christian has served as our Vice President of Sales Operations since October 2008. Ms. Christian previously served as our Senior Director of Sales Operations and Director of Sales Administration since she joined the company in September 2006. Prior to joining us, Ms. Christian served as the Senior Vice President of Finance & Operations of Tad Ware & Company, Inc., a marketing communications agency, where she worked from April 1992 to September 2006. From August 1990 through March 1992, Ms. Christian was a Tax Accountant for Arthur Anderson & Co. Ms. Christian is a Certified Public Accountant (inactive).
14
Carrie Powers has served as our Vice President of Marketing since July 2009. Ms. Powers previously served as our Senior Director of Product Management and Training from July 2008 to June 2009, Director of Training from March 2007 to July 2008, Product Manager for the Hemostasis Product Line from July 2006 to March 2007 and began her employment with us as an Associate Product Manager for the Hemostasis Product Line from January 2006 to July 2006. Prior to joining us, Ms. Powers was employed by St. Mary’s Hospital in Madison, WI from 2002 to 2006, most recently as a Registered Nurse in an Interventional Cardiac Catheterization Lab. Prior to this time, Ms. Powers worked in the areas of home health, cardiac rehabilitation and community health education.
There are no family relationships among any of our executive officers.
Available Information
We make available free of charge on or through our internet website at www.vascularsolutions.com our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to these reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (the Exchange Act) as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
ITEM 1A. RISK FACTORS
The risks and uncertainties described below are not the only ones facing our company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of the following risks occur, our business, financial condition or results of operations could be seriously harmed.
We will not be successful if the interventional medical device community does not adopt our new products.
We have launched over 50 new products since 2003. Our success will depend on the continued launch of new products and the medical community’s acceptance of our new products. We cannot predict how quickly, if at all, the medical community will accept our new products, or, if accepted, the continuation or extent of their use. Our potential customers must:
|
|
|
|
|
|
• |
believe that our products offer benefits compared to the methodologies and/or devices that they are currently using; |
|
|
|
|
|
|
• |
use our products and obtain acceptable clinical outcomes; |
|
|
|
|
|
|
• |
believe that our products are worth the price that they will be asked to pay; and |
|
|
|
|
|
|
• |
be willing to commit the time and resources required to change their current methodology. |
Because we are often selling a new technology, we have limited ability to predict the level of growth or timing in sales of these products. If we encounter difficulties in growing our sales of our new medical devices in the United States, our business will be seriously harmed.
We have a limited history of profitability and may not be profitable in the future.
From 1997 to 2007 we incurred net losses primarily from costs relating to the development and commercialization of our new products. At December 31, 2009, we had an accumulated deficit of $48.3 million. We believe that we have achieved a level of consistent profitability from our continuing operations; however, there is no assurance that this will continue, and we cannot be certain that we can sustain or increase profitability on a quarterly or annual basis.
15
We are involved in and may face additional litigation claims which could prevent us from manufacturing and selling our products or result in our incurring substantial costs and liabilities.
The interventional medical device industry is characterized by numerous patent filings and frequent and substantial intellectual property litigation. Companies in the interventional medical device industry have employed intellectual property litigation in an attempt to gain a competitive advantage. Intellectual property litigation has proven to be very complex, and the outcome of such litigation is difficult to predict. While we do not believe that any of our products infringe any existing patent, it is highly likely that we will continue to become subject to intellectual property claims with respect to our new or existing products. Currently, we are involved in litigation with AngioDynamics, Inc., who has filed a lawsuit against us alleging that we have infringed U.S. Patent No. 7,273,478 and U.S. Patent No. 7,559,329. (See “Legal Proceedings” in Item 3 of Part I of this Form 10-K.)
An adverse determination in any intellectual property litigation or interference proceedings could prohibit us from selling a product, subject us to significant immediate payments to third parties and require us to seek licenses from third parties. The costs associated with these license arrangements may be substantial and could include substantial up-front payments and ongoing royalties. Furthermore, the necessary licenses may not be available to us on satisfactory terms, if at all. Adverse determinations in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling a product.
Our defense of intellectual property claims whether ongoing or filed in the future, regardless of the merits of the complaint, could divert the attention of our technical and management personnel away from the development and marketing of our products for significant periods of time. The costs incurred to defend these claims could be substantial and seriously harm us, even if our defense is ultimately successful.
Our future operating results are difficult to predict and may vary significantly from quarter to quarter, which may adversely affect the price of our common stock.
The ongoing introduction of new products that affect the overall product mix make the prediction of future operating results difficult. You should not rely on our past revenue growth as any indication of future growth rates or operating results. The price of our common stock will likely fall in the event that our operating results do not meet the expectations of analysts and investors. Comparisons of our quarterly operating results are an unreliable indication of our future performance because they are likely to vary significantly based on many factors, including:
|
|
|
|
|
|
• |
the level of sales of our products in the United States market; |
|
|
|
|
|
|
• |
our ability to introduce new products and enhancements in a timely manner; |
|
|
|
|
|
|
• |
the demand for and acceptance of our products; |
|
|
|
|
|
|
• |
the success of our competition and the introduction of alternative products; |
|
|
|
|
|
|
• |
our ability to command favorable pricing for our products; |
|
|
|
|
|
|
• |
the growth of the market for our devices; |
|
|
|
|
|
|
• |
the expansion and rate of success of our direct sales force in the United States and our independent distributors internationally; |
16
|
|
|
|
|
|
• |
actions relating to ongoing FDA compliance; |
|
|
|
|
|
|
• |
the effect of intellectual property disputes; |
|
|
|
|
|
|
• |
the size and timing of orders from independent distributors or customers; |
|
|
|
|
|
|
• |
the attraction and retention of key personnel, particularly in sales and marketing, regulatory, manufacturing and research and development; |
|
|
|
|
|
|
• |
unanticipated delays or an inability to control costs; |
|
|
|
|
|
|
• |
general economic conditions as well as those specific to our customers and markets; and |
|
|
|
|
|
|
• |
seasonal fluctuations in revenue due to the elective nature of some procedures. |
We may face product liability claims that could result in costly litigation and significant liabilities.
The manufacture and sale of medical products entail significant risk of product liability claims. Any product liability claims, with or without merit, could result in costly litigation, reduced sales, cause us to incur significant liabilities and divert our management’s time, attention and resources. We cannot be sure that our product liability insurance coverage is adequate or that it will continue to be available to us on acceptable terms, if at all.
The market for interventional medical devices is highly competitive and will likely become more competitive, and our competitors may be able to respond more quickly to new or emerging technologies and changes in customer requirements that may render our products obsolete.
The existing market for interventional medical devices is intensely competitive. We expect competition to increase further as companies develop new products and/or modify their existing products to compete directly with ours. Each of our products encounters competition from several medical device companies, including Medtronic Inc., Boston Scientific Corporation, Covidien plc and St. Jude Medical Inc. Each of these companies has:
|
|
|
|
|
|
• |
better name recognition; |
|
|
|
|
|
|
• |
broader product lines; |
|
|
|
|
|
|
• |
greater sales, marketing and distribution capabilities; |
|
|
|
|
|
|
• |
significantly greater financial resources; |
|
|
|
|
|
|
• |
larger research and development staffs and facilities; and |
|
|
|
|
|
|
• |
existing relationships with some of our potential customers. |
We may not be able to effectively compete with these companies. In addition, broad product lines may allow our competitors to negotiate exclusive, long-term supply contracts and offer comprehensive pricing for their products. Broader product lines may also provide our competitors with a significant advantage in marketing competing products to group purchasing organizations and other managed care organizations that are increasingly seeking to reduce costs through centralized purchasing. Greater financial resources and product development capabilities may allow our competitors to respond more quickly to new or emerging technologies and changes in customer requirements that may render our products obsolete.
17
Our international sales are subject to a number of risks that could seriously harm our ability to successfully commercialize our products in any international market.
Our international sales are subject to several risks, including:
|
|
|
|
|
|
|
|
• |
the ability of our independent distributors to sell our products; |
|
|
|
|
|
|
|
|
• |
the impact of recessions in economies outside the United States; |
|
|
|
|
|
|
|
|
• |
greater difficulty in collecting accounts receivable and longer collection periods; |
|
|
|
|
|
|
|
|
• |
unexpected changes in regulatory requirements, tariffs or other trade barriers; |
|
|
|
|
|
|
|
|
• |
weaker intellectual property rights protection in some countries; |
|
|
|
|
|
|
|
|
• |
potentially adverse tax consequences; and |
|
|
|
|
|
|
|
|
• |
political and economic instability. |
The occurrence of any of these events could seriously harm our future international sales and our ability to successfully commercialize our products in any international market.
Our business and results of operations may be seriously harmed by changes in third-party reimbursement policies.
We could be seriously harmed by changes in reimbursement policies of governmental or private healthcare payors, particularly to the extent any changes affect reimbursement for catheterization procedures in which our products are used. Failure by physicians, hospitals and other users of our products to obtain sufficient reimbursement from healthcare payors for procedures in which our products are used or adverse changes in governmental and private third-party payors’ policies toward reimbursement for such procedures would seriously harm our business.
In the United States, healthcare providers, including hospitals and clinics that purchase medical devices such as our products, generally rely on third-party payors, principally federal Medicare, state Medicaid and private health insurance plans, to reimburse all or part of the cost of catheterization procedures. Any changes in this reimbursement system could seriously harm our business.
In international markets, acceptance of our products is dependent in part upon the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country. Our failure to receive international reimbursement approvals could have a negative impact on market acceptance of our products in the markets in which these approvals are sought.
Our products and our manufacturing activities are subject to extensive governmental regulation that could prevent us from selling our products in the United States or introducing new and improved products.
Our products and our manufacturing activities are subject to extensive regulation by a number of governmental agencies, including the FDA and comparable international agencies. We are required to:
|
|
|
|
|
|
|
|
• |
obtain the clearance of the FDA and international agencies before we can market and sell our products; |
|
|
|
|
|
|
|
|
• |
satisfy these agencies’ content requirements for all of our labeling, sales and promotional materials; and |
|
|
|
|
|
|
|
|
• |
undergo rigorous inspections by these agencies. |
18
Compliance with the regulations of these agencies may delay or prevent us from introducing any new model of our existing products or other new products. Furthermore, we may be subject to sanctions, including temporary or permanent suspension of operations, product recalls and marketing restrictions if we fail to comply with the laws and regulations pertaining to our business.
We are also required to demonstrate compliance with the FDA’s quality system regulations. The FDA enforces its quality system regulations through pre-approval and periodic post-approval inspections. These regulations relate to product testing, vendor qualification, design control and quality assurance, as well as the maintenance of records and documentation. If we are unable to conform to these regulations, the FDA may take actions which could seriously harm our business. In addition, government regulation may be established that could prevent, delay, modify or rescind regulatory clearance or approval of our products.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our offices are located in two buildings totaling approximately 93,000 square feet of leased space in two suburbs of Minneapolis, Minnesota (Maple Grove and Plymouth). These facilities include approximately 37,000 square feet used for manufacturing activities and approximately 7,000 square feet used for research and laboratory activities, with the remainder used for warehouse and general office space. On November 12, 2007, we amended both lease agreements to extend the terms of the leases through September 30, 2015, with additional renewal options.
ITEM 3. LEGAL PROCEEDINGS
On May 11, 2005 we initiated a lawsuit for product disparagement and false advertising against Marine Polymer Technologies, Inc., a Delaware corporation (Marine Polymer). In the lawsuit, we alleged that Marine Polymer made defamatory and disparaging statements concerning our D-Stat Dry hemostatic bandage. We sought relief in the form of an injunction to enjoin Marine Polymer from continuing to defame and disparage our products, damages as a result of such statements, and other costs, disbursements and attorneys’ fees. Marine Polymer brought a counter-claim against us including, among other claims, business defamation and product disparagement for statements allegedly made by us concerning Marine Polymer’s SyvekPatch®. Marine Polymer sought relief in the form of monetary damages, costs, disbursements and attorneys’ fees. The trial commenced on March 24, 2008 in the U.S. District Court for the District of Massachusetts. At the conclusion of the trial on April 7, 2008 the jury returned a verdict in our favor and against Marine Polymer for product disparagement concerning statements made regarding the safety of our D-Stat Dry hemostat product. In its verdict, the jury found that Marine Polymer’s statements were false and disparaged the D-Stat Dry product and awarded us $4,500,000 in monetary damages. The jury rejected Marine Polymer’s counterclaims in their entirety. Following post trial motions, on June 30, 2008, the Court upheld the jury verdict, granted our request for a permanent injunction against Marine Polymer for the statements that the jury found were false, and added prejudgment interest on the jury verdict award in the amount of $592,124.
On July 14, 2008, Marine Polymer filed a Notice of Appeal with the U.S. First Circuit Court of Appeals seeking to overturn the monetary damages and injunction issued against them. Oral arguments on Marine Polymer’s appeal were held on February 4, 2009. On December 23, 2009, the U.S. First Circuit Court of Appeals affirmed the judgment against Marine Polymer for product disparagement. As a result, the permanent injunction issued at the conclusion of the trial will remain in effect, prohibiting Marine Polymer and its representatives from making, publishing or disseminating certain disparaging statements concerning the safety of our D-Stat products. Addressing the jury’s award of $4.5 million in damages, the Court determined that, due to differences in opinion among the judges, we could either accept a $2.7 million award of damages, plus interest, or insist upon a new trial limited to the issue of determining the reasonable amount of damages. We accepted the $2.7 million award of damages plus interest, and received $3.56 million from Marine Polymer on January 22, 2010. We will record a litigation gain of $3.56 million in the first quarter of 2010.
19
On July 29, 2009 AngioDynamics, Inc. (AngioDynamics) filed a lawsuit against us in the U.S. District Court for the District of Delaware, alleging that we have infringed U.S. Patent No. 7,273,478 and U.S. Patent No. 7,559,329. Specifically, AngioDynamics alleges that doctors using our Bright Tip fibers and procedure kits are using the methods claimed in those patents, and accuses us of inducing and contributing to infringement. AngioDynamics has requested injunctive relief and compensatory damages. On December 1, 2009 we filed our answer, a counterclaim, and a separate motion to transfer the litigation to the U.S. District Court for the District of Minnesota.
We have also filed an inter partes request for reexamination of both the ‘478 and ‘329 patents with the United States Patent Office. The Patent Office granted our inter partes requests for reexamination for both the ‘478 and ‘329 patents. We expect the reexamination process for each patent will take at least three years to conclude. On January 27, 2010, we filed a motion with the U.S. District Court for the District of Delaware to stay all proceedings in the litigation pending the outcome of the inter partes reexaminations.
From time to time we are involved in additional legal proceedings arising in the normal course of our business. As of the date of this report we are not a party to any legal proceedings not described in this section in which an adverse outcome would reasonably be expected to have a material adverse effect on our results of operations or financial condition.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
20
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is traded on the NASDAQ Global Market under the symbol “VASC”. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported by the NASDAQ Global Market.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
High |
|
Low |
|
||
|
|
|
|
|
|
|
|
||
|
2009 |
|
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
9.55 |
|
$ |
5.04 |
|
|
|
Second Quarter |
|
|
8.70 |
|
|
5.70 |
|
|
|
Third Quarter |
|
|
8.80 |
|
|
7.10 |
|
|
|
Fourth Quarter |
|
|
9.80 |
|
|
7.60 |
|
|
|
|
|
|
|
|
|
|
|
|
2008 |
|
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
6.80 |
|
$ |
5.63 |
|
|
|
Second Quarter |
|
|
7.66 |
|
|
6.19 |
|
|
|
Third Quarter |
|
|
9.06 |
|
|
6.04 |
|
|
|
Fourth Quarter |
|
|
9.75 |
|
|
6.78 |
|
During the quarter ended December 31, 2009, we repurchased shares of common stock from our employees to satisfy income tax withholding obligations upon vesting of outstanding restricted share awards for those employees. Shares purchase activity for the fourth quarter of fiscal 2009 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Date |
|
Total Number |
|
Average |
|
Total Number of Shares |
|
Approximate Dollar |
|
|
October 1 - 31, 2009 |
|
1,614 |
|
$9.03 |
|
— |
|
— |
|
|
November 1 - 30, 2009 |
|
— |
|
— |
|
— |
|
— |
|
|
December 1 - 31, 2009 |
|
— |
|
— |
|
— |
|
— |
|
|
|
|
|
|
|
(1) |
At the request of our employees and pursuant to the terms of their Restricted Stock Awards, we purchased 1,614 shares of common stock at the fair market value of the common stock on the day their awards vested to satisfy income tax withholding obligations for those employees. |
Holders
As of December 31, 2009, we had 140 shareholders of record. Such number of record holders does not reflect shareholders who beneficially own common stock in nominee or street name.
Dividends
We have paid no cash dividends on our common stock, and do not intend to pay cash dividends on our common stock in the future.
21
The following graph shows a comparison of cumulative total returns for our common stock, the NASDAQ Stock Market Index (U.S.) and the NASDAQ Medical Industry Index (Medical Devices, Instruments and Supplies), assuming the investment of $100 in our common stock and each index on December 31, 2004 and the reinvestment of dividends, if any.
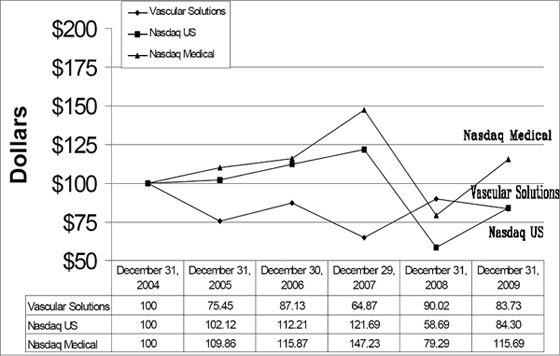
ITEM 6. SELECTED FINANCIAL DATA
The following selected financial data as of December 31, 2009 and 2008 and for the three years ended December 31, 2009, 2008 and 2007 are derived from, and should be read together with, our consolidated financial statements included elsewhere in this Form 10-K. The following selected financial data as of December 31, 2007, 2006 and 2005 and for the fiscal years ended December 31, 2006 and 2005 are derived from consolidated financial statements not included herein. The information set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” the Consolidated Financial Statements and Notes thereto and other financial information included elsewhere in this Form 10-K.
22
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
2006 |
|
2005 |
|
|||||
|
|
|
(in thousands, except per share amounts) |
|
|||||||||||||
|
Statements of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenue |
|
$ |
66,726 |
|
$ |
59,757 |
|
$ |
51,414 |
|
$ |
43,310 |
|
$ |
32,786 |
|
|
License and collaboration revenue |
|
|
1,701 |
|
|
1,464 |
|
|
1,450 |
|
|
— |
|
|
— |
|
|
Total revenue |
|
|
68,427 |
|
|
61,221 |
|
|
52,864 |
|
|
43,310 |
|
|
32,786 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
|
22,917 |
|
|
20,690 |
|
|
17,002 |
|
|
14,231 |
|
|
9,386 |
|
|
Cost of sales related to thrombin inventory |
|
|
— |
|
|
670 |
|
|
— |
|
|
— |
|
|
— |
|
|
Collaboration expenses |
|
|
850 |
|
|
632 |
|
|
685 |
|
|
— |
|
|
— |
|
|
Research and development |
|
|
7,847 |
|
|
6,333 |
|
|
5,481 |
|
|
4,578 |
|
|
3,789 |
|
|
Clinical and regulatory |
|
|
2,886 |
|
|
3,220 |
|
|
3,168 |
|
|
2,493 |
|
|
2,006 |
|
|
Sales and marketing |
|
|
21,206 |
|
|
20,482 |
|
|
19,603 |
|
|
17,097 |
|
|
13,681 |
|
|
General and administrative |
|
|
4,555 |
|
|
4,695 |
|
|
5,304 |
|
|
3,716 |
|
|
2,810 |
|
|
Thrombin qualification |
|
|
— |
|
|
— |
|
|
147 |
|
|
2,802 |
|
|
1,620 |
|
|
Litigation |
|
|
— |
|
|
1,484 |
|
|
5,800 |
|
|
— |
|
|
— |
|
|
Amortization of purchased technology |
|
|
— |
|
|
— |
|
|
— |
|
|
72 |
|
|
218 |
|
|
Total product costs and operating expenses |
|
|
60,261 |
|
|
58,206 |
|
|
57,190 |
|
|
44,989 |
|
|
33,510 |
|
|
|
||||||||||||||||
|
Operating income (loss) |
|
|
8,166 |
|
|
3,015 |
|
|
(4,326 |
) |
|
(1,679 |
) |
|
(724 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expenses): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
48 |
|
|
203 |
|
|
444 |
|
|
99 |
|
|
163 |
|
|
Interest expense |
|
|
(38 |
) |
|
(62 |
) |
|
(148 |
) |
|
(206 |
) |
|
— |
|
|
Foreign exchange loss |
|
|
(10 |
) |
|
(28 |
) |
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
8,166 |
|
|
3,128 |
|
|
(4,030 |
) |
|
(1,786 |
) |
|
(561 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit (expense) |
|
|
(2,788 |
) |
|
13,045 |
* |
|
(276 |
) |
|
— |
|
|
— |
|
|
Net income (loss) |
|
$ |
5,378 |
|
$ |
16,173 |
|
$ |
(4,306 |
) |
$ |
(1,786 |
) |
$ |
(561 |
) |
|
Net income (loss) per common share – Basic |
|
$ |
0.34 |
|
$ |
1.04 |
|
$ |
(0.28 |
) |
$ |
(0.12 |
) |
$ |
(0.04 |
) |
|
Net income (loss) per common share – Diluted |
|
$ |
0.33 |
|
$ |
1.01 |
|
$ |
(0.28 |
) |
$ |
(0.12 |
) |
$ |
(0.04 |
) |
|
Weighted average number of common shares outstanding |
|
|
16,047 |
|
|
15,588 |
|
|
15,238 |
|
|
14,910 |
|
|
14,515 |
|
* A complete discussion of the facts and circumstances surrounding this amount can be found on page 27 of this 10-K.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, |
|
|||||||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
2006 |
|
2005 |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and available-for-sale securities (includes restricted cash) |
|
$ |
17,794 |
|
$ |
7,209 |
|
$ |
10,759 |
|
$ |
2,557 |
|
$ |
4,282 |
|
|
Working capital (includes restricted cash) |
|
|
35,145 |
|
|
22,677 |
|
|
14,530 |
|
|
11,472 |
|
|
10,887 |
|
|
Total assets |
|
|
51,755 |
|
|
44,180 |
|
|
31,278 |
|
|
20,967 |
|
|
19,896 |
|
|
Total shareholders’ equity |
|
|
40,399 |
|
|
31,826 |
|
|
12,825 |
|
|
14,467 |
|
|
14,107 |
|
23
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our Consolidated Financial Statements and Notes thereto, and the other financial information included elsewhere in this Form 10-K. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains descriptions of our expectations regarding future trends affecting our business. These forward-looking statements and other forward-looking statements made elsewhere in this document that are not strictly historical fact are made in reliance upon safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based on management’s current expectations as of the date of this report but involve risks, uncertainties and other factors which may cause actual results to differ materially from those contemplated by such forward looking statements. Item 1A of Part I of this Form 10-K sets forth certain factors we believe could cause actual results to differ materially from those contemplated by the forward-looking statements. We do not intend to update any of these forward-looking statements after the date of this Form 10-K to conform them to actual results.
Overview
We are a medical device company focused on bringing solutions to interventional cardiologists and interventional radiologists. As a vertically-integrated medical device company, we generate ideas and create new interventional medical devices, and then deliver those products directly to the physician through our direct domestic sales force and international distribution network. We continue to develop new products and new applications for our existing products.
Results of Operations
The following table sets forth, for the periods indicated, certain items from our statements of operations expressed as a percentage of net sales:
24
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
Product revenue |
|
|
98 |
% |
|
98 |
% |
|
97 |
% |
|
License and collaboration revenue |
|
|
2 |
% |
|
2 |
% |
|
3 |
% |
|
Total revenue |
|
|
100 |
% |
|
100 |
% |
|
100 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
|
34 |
% |
|
34 |
% |
|
32 |
% |
|
Cost of sales related to thrombin inventory |
|
|
— |
|
|
1 |
% |
|
— |
|
|
Collaboration expenses |
|
|
1 |
% |
|
1 |
% |
|
1 |
% |
|
Research and development |
|
|
11 |
% |
|
10 |
% |
|
11 |
% |
|
Clinical and regulatory |
|
|
4 |
% |
|
5 |
% |
|
6 |
% |
|
Sales and marketing |
|
|
31 |
% |
|
34 |
% |
|
37 |
% |
|
General and administrative |
|
|
7 |
% |
|
8 |
% |
|
10 |
% |
|
Thrombin qualification |
|
|
— |
|
|
— |
|
|
— |
|
|
Litigation |
|
|
— |
|
|
2 |
% |
|
11 |
% |
|
Total product costs and operating expenses |
|
|
88 |
% |
|
95 |
% |
|
108 |
% |
|
|
||||||||||
|
Operating income (loss) |
|
|
12 |
% |
|
5 |
% |
|
(8 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income/expense and foreign exchange loss, net |
|
|
— |
|
|
— |
|
|
1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
12 |
% |
|
5 |
% |
|
(7 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit (expense) |
|
|
(4 |
%) |
|
21 |
% |
|
(1 |
%) |
|
Net Income (loss) |
|
|
8 |
% |
|
26 |
% |
|
(8 |
%) |
Our primary products are categorized into five product lines. The following table sets forth, for the periods indicated, net revenue by product line along with the change from the previous year:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For Years Ended December 31, |
|
||||||||||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
||||||||||||
|
|
|
|
|
Percent |
|
|
|
Percent |
|
|
|
Percent |
|
||||||
|
|
|
Net Revenue |
|
|
Net Revenue |
|
|
Net Revenue |
|
|
|||||||||
|
Hemostat products |
|
$ |
24,428,000 |
|
|
4 |
% |
$ |
23,475,000 |
|
|
(5% |
) |
$ |
24,712,000 |
|
|
14 |
% |
|
Extraction catheters |
|
|
16,897,000 |
|
|
13 |
% |
|
14,992,000 |
|
|
36 |
% |
|
11,016,000 |
|
|
22 |
% |
|
Vein products |
|
|
11,225,000 |
|
|
12 |
% |
|
10,035,000 |
|
|
16 |
% |
|
8,629,000 |
|
|
22 |
% |
|
Access products |
|
|
7,298,000 |
|
|
31 |
% |
|
5,561,000 |
|
|
99 |
% |
|
2,790,000 |
|
|
74 |
% |
|
Specialty catheters |
|
|
5,905,000 |
|
|
29 |
% |
|
4,563,000 |
|
|
36 |
% |
|
3,363,000 |
|
|
8 |
% |
|
Other products |
|
|
973,000 |
|
|
(14 |
%) |
|
1,131,000 |
|
|
25 |
% |
|
904,000 |
|
|
18 |
% |
|
License & Collaboration |
|
|
1,701,000 |
|
|
16 |
% |
|
1,464,000 |
|
|
1 |
% |
|
1,450,000 |
|
|
N/A |
|
|
|
|||||||||||||||||||
|
Total Net Revenue |
|
$ |
68,427,000 |
|
|
12 |
% |
$ |
61,221,000 |
|
|
16 |
% |
$ |
52,864,000 |
|
|
22 |
% |
Year ended December 31, 2009 compared to the years ended December 31, 2008 and December 31, 2007.
Net revenue increased 12% to $68,427,000 for the year ended December 31, 2009 from $61,221,000 for the year ended December 31, 2008. The increase in net revenue was primarily the result of an increased market penetration in all five of our product categories as well as the introduction of new products. Approximately 87% of our net revenue was earned in the United States and 13% of our net revenue was earned in international markets for both years ended December 31, 2009 and December 31, 2008. Net revenue increased 16% to $61,221,000 for the year ended December 31, 2008 from $52,864,000 for the year ended December 31, 2007.
25
We recognized $850,000 of licensing revenue during the year ended December 31, 2009 as the result of our License Agreement and Device Supply Agreement with King and our distribution agreement with Nicolai in Germany. We also recognized $851,000 of collaboration revenue during the year ended December 31, 2009 as a result of performing clinical and development work for King under the Device Supply Agreement. We expect to recognize approximately $850,000 of license revenue in 2010. We expect collaboration revenue will continue in 2010 but at a lower level due to us completing the enrollment of one of the clinical projects for King.
Gross margin across all product lines increased to 66% for the year ended December 31, 2009 compared to 65% for the year ended December 31, 2008. We expect product gross margins to be in the range of 65% to 67% in 2010, subject to changes in our selling mix between our lower margin products such as the Vari-Lase products and our higher margin products such as the D-Stat Dry. Gross margin across all product lines declined to 65% for the year ended December 31, 2008 compared to 67% for the year ended December 31, 2007.
Cost of sales related to thrombin inventory expenses were $-0- for the year ended December 31, 2009, compared to $670,000 for the year ended December 31, 2008. Cost of sales related to thrombin inventory expenses relate to a reserve we have recorded for the amount of thrombin we anticipate expiring prior to being used in the manufacturing of our international hemostat products. We do not anticipate incurring additional charges related to thrombin inventory during 2010.
Collaboration expenses were $850,000 for the year ended December 31, 2009, compared to $632,000 for the year ended December 31, 2008 and $685,000 for the year ended December 31, 2007. Collaboration expenses primarily are the result of our collaboration revenue related to the pre-clinical and clinical projects we are performing for King. We expect collaboration expenses to be approximately 2% of revenue during 2010, dependent upon the timing of the collaboration revenue.
Research and development expense for the year ended December 31, 2009 totaled $7,847,000, or 11% of revenue, compared to $6,333,000, or 10% of revenue for the year ended December 31, 2008 and $5,481,000, or 11% of revenue for the year ended December 31, 2007. The increase in research and development expenses resulted from additional new products moving through our development system in 2009. We expect our continuing research and development expenses to be approximately 10% to 12% of revenue in 2010 as we continue to pursue additional new products and to move our longer term development projects forward.
Clinical and regulatory expense for the year ended December 31, 2009 totaled $2,886,000, or 4% of revenue, compared to $3,220,000, or 5% of revenue for the year ended December 31, 2008 and $3,168,000, or 6% of revenue for the year ended December 31, 2007. Clinical and regulatory expenses fluctuate due to the timing of clinical studies and the number of new products coming through the regulatory system. We expect clinical and regulatory expenses to be approximately 4% to 5% of revenue in 2010.
Sales and marketing expense for the year ended December 31, 2009 totaled $21,206,000, or 31% of revenue, compared to $20,482,000, or 34% of revenue for the year ended December 31, 2008 and $19,603,000, or 37% of revenue for the year ended December 31, 2007. The primary reason for the increase in sales and marketing expenses was a $467,000, or 13% increase in our commissions paid to our direct sales force due to increased sales in 2009 over 2008. We expect to maintain the same relative size of our direct sales force during 2010 at between 85 and 90 full-time sales employees. As a result, we expect our sales and marketing expenses to continue to decrease as a percent of revenue from approximately 31% to 32% of revenue at the beginning of 2010 to between 27% and 29% of revenue by the end of 2010.
26
General and administrative expense for the year ended December 31, 2009 totaled $4,555,000, or 7% of revenue, compared to $4,695,000, or 8% of revenue for the year ended December 31, 2008 and $5,304,000, or 10% of revenue for the year ended December 31, 2007. The decrease was primarily the result of lower legal fees of approximately $380,000 as we settled the Diomed and Covidien litigation matters in the second half of 2008 (see Note 14 to the Consolidated Financial Statements included in Item 8 of Part II of this Form 10-K). We expect general and administrative expenses to be approximately 6% to 7% of revenue during 2010.
Litigation expenses were $-0- for the year ended December 31, 2009, compared to $1,484,000 for the year ended December 31, 2008 and $5,800,000 for the year ended December 31, 2007. For the year ended December 31, 2009 we did not recognize any gain resulting from the Marine Polymer jury verdict. We received $3.56 million from Marine Polymer on January 22, 2010 and the gain will be recognized in the first quarter of 2010 (see Note 16 to the Consolidated Financial Statements included in Item 8 of Part II of this Form 10-K). In the second quarter of 2008 we recorded a gain of $1,659,000 due to the settlement of our litigation with Diomed (reflecting a reduction from the $5,245,000 litigation expense incurred in 2007 due to the Diomed jury verdict) and a litigation expense of $3,116,000 upon the settlement of the litigation with Covidien (see Note 14 to the Consolidated Financial Statements included in Item 8 of Part II of this Form 10-K).
Interest income decreased to $48,000 for the year ended December 31, 2009, compared to $203,000 for the year ended December 31, 2008 and $444,000 for the year ended December 31, 2007. The decrease in interest income was primarily the result of lower interest rates being paid on our deposit funds held at the bank.
Interest expense decreased to $38,000 for the year ended December 31, 2009, compared to $62,000 for the year ended December 31, 2008 and $148,000 for the year ended December 31, 2007. The decrease in interest expense was as a result of repaying our equipment line of credit during the year ended December 31, 2008.
Foreign exchange loss decreased to $10,000 for the year ended December 31, 2009, compared to $28,000 for the year ended December 31, 2008 and $-0- for the year ended December 31, 2007.
Income tax expense increased to $2,788,000 on income before taxes of $8,166,000 resulting in an income tax rate of 34% for the year ended December 31, 2009, compared to an income tax benefit of $13,045,000 for the year ended December 31, 2008 and income tax expense of $276,000 for the year ended December 31, 2007.
We assess the likelihood that our deferred tax assets will be recovered from future taxable income during the fourth quarter of each year. We consider projected future taxable income and ongoing tax planning strategies in assessing the amount of the valuation allowance necessary to offset our deferred tax assets that will not be recoverable. Based upon management’s assessment of all available evidence, including our cumulative pretax net income for fiscal years 2009 and 2008, estimates of future profitability and the overall prospects of our business, we determined that our current deferred tax assets are adequate at December 31, 2009. In the fourth quarter of 2008 we determined it was more likely than not that we would be able to realize a portion of our deferred tax assets in the future, and as a result recorded a $13.2 million income tax benefit. To determine the amount of the reduction in the valuation allowance, we projected our income over the next five years, which approximates the ten-year life of our three most significant products at this time. The amount of the valuation allowance reduction was based on our discounted projected taxable income. We will continue to assess the potential realization of our deferred tax assets on an annual basis or on an interim basis if circumstances warrant. If our actual results and updated projections vary significantly from our prior estimates, we expect to increase or decrease our valuation allowance against our gross deferred tax assets. Any adjustment to our earnings for the deferred tax would occur in the period we make the determination. With the exception of 2009 and 2008, we have not generated any significant pre-tax income in any year and therefore have not paid any federal income taxes since our inception in December 1996.
27
As of December 31, 2009, we had approximately $40.3 million and $4.5 million of federal and state net operating loss carryforwards available to offset future taxable income which begin to expire in the year 2020. As of December 31, 2009, we also had federal and state research and development tax credit carryforwards of approximately $4.0 million which begin to expire in the year 2012. As of December 31, 2009, we also had a foreign tax loss carryforward of approximately $357,000, which does not expire. Under the United States Tax Reform Act of 1986, the amounts of and benefits from net operating loss carryforwards may be impaired or limited in certain circumstances, including significant changes in ownership interests. Future use of our existing net operating loss carryforwards may be restricted due to changes in ownership or from future tax legislation.
Liquidity and Capital Resources
Our cash and cash equivalents totaled $17,794,000 at December 31, 2009 compared to $7,209,000 in cash and cash equivalents at December 31, 2008, an increase of $10,585,000. Our cash equivalents are invested in a money market fund invested in all types of high quality, short-term money market instruments denominated in U.S. dollars such as debt instruments guaranteed by the governments of the United States, Western Europe, Australia, Japan and Canada, high quality corporate issuers and bank obligations. The money market fund’s assets are rated in the highest short-term category by nationally recognized rating agencies, such as Moody’s or Standard & Poor’s.
Cash provided by operations. We generated $10,374,000 of cash from operations during the year ended December 31, 2009. The cash generated during 2009 primarily resulted from our income before taxes of $8,166,000 since essentially all of our income taxes are offset by our deferred tax assets.
Cash used for investing activities. We used $1,325,000 of cash in investing activities during the year ended December 31, 2009. We incurred capital expenditures of $1,325,000 relating primarily to additional manufacturing equipment, leasehold improvements as part of our facility expansion and additional research and development equipment.
Cash provided by financing activities. We generated $1,536,000 of cash in financing activities during the year ended December 31, 2009. We used $354,000 of cash to repurchase shares that vested under outstanding restricted stock awards for income tax withholding purposes. This was offset by our receipt of $1,890,000 of cash we received under our Employee Stock Purchase Plan and upon the exercise of outstanding stock options.
We have a $10 million revolving line of credit with US Bank, which has a 12-month term, bears interest at the rate of LIBOR plus 1.60% and is secured by a first security interest on all of our assets. The credit facility includes one covenant that we cannot have a maximum cash flow leverage ratio greater than 2.5 to 1. The calculation of this covenant is determined by multiplying our annual lease expenses times six and adding any loans, then dividing this amount by the sum of our earnings before interest, taxes, depreciation, amortization and our annual operating lease payments. We were in compliance with this covenant on December 31, 2009. As of December 31, 2009, we had no outstanding balance on the $10 million revolving line of credit with an availability of $10 million.
The following table summarizes our contractual cash commitments as of December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period |
|
|||||||||||||
|
|
|
|
|
|
Less than |
|
|
|
|
|
|
|
More than
|
|
||
|
Contractual Obligation |
|
Total |
|
|
1 - 3 years |
|
3 - 5 years |
|
|
|||||||
|
Facility operating leases |
|
$ |
4,679,000 |
|
$ |
761,000 |
|
$ |
1,605,000 |
|
$ |
1,682,000 |
|
$ |
631,000 |
|
We do not have any other significant cash commitments related to supply agreements, nor do we have any significant commitments for capital expenditures.
28
We currently anticipate that we will experience positive cash flow from our normal operating activities for the foreseeable future. We currently believe that our working capital of $35.1 million at December 31, 2009 will be sufficient to meet all of our operating and capital requirements for the foreseeable future. However, our actual liquidity and capital requirements will depend upon numerous unpredictable factors, including the amount of revenues from sales of our existing and new products; the cost of maintaining, enforcing and defending patents and other intellectual property rights; competing technological and market developments; developments related to regulatory and third party reimbursement matters; and other factors.
Critical Accounting Policies
Management’s Discussion and Analysis of Financial Condition and Results of Operations addresses our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our consolidated financial statements requires that we make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an on-going basis, we evaluate these estimates and judgments. We base our estimates and judgments on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our accounting policies are described in Note 2 to the consolidated financial statements. We set forth below those material accounting policies that we believe are the most critical to an investor’s understanding of our financial results and condition and that require complex management judgment.
Inventory
We state our inventory at the lower of cost (first-in, first-out method) or market. The estimated value of excess, obsolete and slow-moving inventory as well as inventory with a carrying value in excess of its net realizable value is established by us on a quarterly basis through review of inventory on hand and assessment of future demand, anticipated release of new products into the market, historical experience and product expiration. Our stated value of inventory could be materially different if demand for our products decreased because of competitive conditions or market acceptance, or if products become obsolete because of advancements in the industry. We have approximately $1.3 million of Sigma thrombin in inventory at December 31, 2009, which we expect to use in our hemostat products sold in international markets. We received regulatory approval in February 2008 allowing us to use the Sigma thrombin in our international hemostat products. In the fourth quarter of 2008 we wrote off $670,000 of our Sigma thrombin which we expect will expire before we are able to use it. We will continue to review our Sigma thrombin needs and we will write off any amounts we anticipate will not be used.
Revenue Recognition
We recognize revenue in accordance with generally accepted accounting principles as outlined in the Securities and Exchange Commission’s Staff Accounting Bulletin No. 104 Revenue Recognition, [ASC 605-10-S99], which requires that four basic criteria be met before revenue can be recognized: (i) persuasive evidence of an arrangement exists; (ii) the price is fixed or determinable; (iii) collectability is reasonably assured; and (iv) product delivery has occurred or services have been rendered. We recognize revenue as products are shipped based on FOB shipping point terms when title passes to customers. We negotiate credit terms on a customer-by-customer basis and products are shipped at an agreed upon price. All product returns must be pre-approved and, if approved, customers are subject to a 20% restocking charge.
We also generate revenues from license agreements and research collaborations and recognizes these revenues when earned. In accordance with Emerging Issues Task Force (EITF) Issue No. 00-21, Revenue Arrangements with Multiple Deliverables, [ASC 605-25], for deliverables which contain multiple deliverables, the Company separates the deliverables into separate accounting units if they meet the following criteria: (i) the delivered items have a stand-alone value to the customer; (ii) the fair value of any undelivered items can be reliably determined; and (iii) if the arrangement includes a general right of return, delivery of the undelivered items is probable and substantially controlled by the seller. Deliverables that do not meet these criteria are combined with one or more other deliverables into one accounting unit. Revenue from each accounting unit is recognized based on the applicable accounting literature, primarily Staff Accounting Bulletin (SAB) 104, Revenue Recognition, [ASC 605-10-S99].
29
Effective April 1, 2008 we entered into a five-year distribution agreement with Nicolai, GmbH. As a result of entering into this distribution agreement, we no longer maintain a direct sales force in Germany. In connection with this distribution agreement, we received 500,000 Euros from Nicolai, GmbH in 2008. The payment was deferred and is being recognized ratably over the five-year term of the distribution agreement. The distribution agreement also includes provisions requiring us to pay Nicolai, GmbH specific amounts if we terminate the distribution agreement prior to the end of the five-year term. We do not intend to terminate the distribution agreement and, as such, have not recorded a liability relating to these potential future payments to Nicolai, GmbH.
On January 9, 2007, we entered into three separate agreements with King, consisting of a License Agreement, a Device Supply Agreement and a Thrombin-JMI® Supply Agreement. We licensed the exclusive rights to our products Thrombi-Pad, Thrombi-Gel and Thrombi-Paste to King for a one-time payment of $6 million. We continue to manufacture the licensed products for sale to King under the Device Supply Agreement. The Device Supply Agreement requires King to pay us a $1 million milestone payment upon the first commercial sale of Thrombi-Gel and again upon the first commercial sale of Thrombi-Paste. On May 30, 2007 we received the first $1 million payment related to King’s first commercial sale of Thrombi-Pad. In 2009 King decided to suspend indefinitely the clinical development of the Thrombi-Paste product. We are amortizing the $6 million license fee received on January 9, 2007 and the $1 million milestone payment received on May 30, 2007 on a straight-line basis over the remaining 10 years. We will amortize the second $1 million milestone payment over the remaining 10-year license period from the date it is received.
As part of the Device Supply Agreement, we agreed to conduct clinical studies for Thrombi-Gel and Thrombi-Paste, with the expected costs related to the clinical studies to be paid by King. Additionally, on May 18, 2007, we entered into a Product Development & Supply Agreement with a third party company by which we agreed to develop, manufacture and sell to this company a specialty version of our Twin-Pass dual access catheter, with the costs related to the development paid by this company. We have recognized collaboration revenue on these development agreements as it was earned under the agreements with King and the third party company. No revenue was recognized under the Product Development & Supply Agreement with the third party company for the year ended December 31, 2009 and none is expected in 2010.
In addition, we have reviewed the provisions of EITF Issue No. 07-01, Accounting for Collaborative Arrangements, [ASC 808-10], and believe the adoption of this EITF will have no impact on the amounts recorded under these agreements.
We analyze the rate of historical returns when evaluating the adequacy of the allowance for sales returns, which is included with the allowance for doubtful accounts on our balance sheet. At December 31, 2009, this reserve was $45,000 compared to $25,000 at December 31, 2008. If the historical data we use to calculate these estimates does not properly reflect future returns, revenue could be overstated.
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. This allowance is regularly evaluated by us for adequacy by taking into consideration factors such as past experience, credit quality of the customer base, age of the receivable balances, both individually and in the aggregate, and current economic conditions that may affect a customer’s ability to pay. At December 31, 2009, this reserve was $105,000 compared to $95,000 at December 31, 2008. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
30
Warranty Costs
We provide a warranty for certain products against defects in material and workmanship for periods of up to 24 months. We record a liability for warranty claims at the time of sale. The amount of the liability is based on the amount we are charged by our original equipment manufacturer to cover the warranty period. The original equipment manufacturer includes a one year warranty with each product sold to us. We record a liability for the uncovered warranty period offered to a customer, provided the warranty period offered exceeds the initial one year warranty period covered by the original equipment manufacturer. At December 31, 2009, this warranty provision was $73,000 compared to $49,000 at December 31, 2008. If the assumptions used in calculating the provision were to materially change, resulting in more defects than anticipated, an additional provision may be required.
Income Taxes
The carrying value of our net deferred tax assets assumes that we will be able to generate sufficient taxable income in the United States based on estimates and assumptions. We record a valuation allowance to reduce the carrying value of our net deferred tax asset to the amount that is more likely than not to be realized. For the year ended December 31, 2009, we recorded a $14.4 million valuation allowance and a $727,000 reserve related to our net deferred tax assets of $25.5 million as a result of our adoption of Financial Accounting Standards Board Interpretation 48 (FIN 48), Accounting for Uncertainty in Income Taxes — an Interpretation of FASB Statement No. 109, [ASC 740-10]. At December 31, 2009, we have accrued $-0- for the payment of tax related interest and there was no tax interest or penalties recognized in the statements of operations. In the fourth quarter of 2008, based upon management’s assessment of all available evidence, including our cumulative adjusted pretax net income for fiscal years 2008 and 2007 adjusted for certain non-recurring items allowed by FAS 109, [ASC 740-10], estimates of future profitability and the overall prospects of our business, we determined that it is more likely than not that we will be able to realize a portion of our deferred tax assets in the future, and as a result recorded a $13.2 million income tax benefit. To determine the amount of the reduction in the valuation allowance, we projected our income over the next five years, which approximates the ten-year life of our three most significant products at this time. The amount of the valuation allowance reduction was based on our discounted projected taxable income. We continue to assess the potential realization of our deferred tax assets on an annual basis, or on an interim basis if circumstances warrant. If our actual results and updated projections vary significantly from our projections, we would need to increase or decrease our valuation allowance against our gross deferred tax assets. We would adjust our earnings for the deferred tax in the period we make the determination. No additional income tax benefits have been recorded for the year ended December 31, 2009.
New Accounting Pronouncement
In June 2009, the FASB issued Statement of Financial Accounting Standards No. 168, The FASB Accounting Standards Codification™ and the Hierarchy of Generally Accepted Accounting Principles a Replacement of FASB Statement No. 162 (“FAS 168”), [ASC 105-10]. This Standard establishes the FASB Accounting Standards Codification™ (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. The Codification does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all the authoritative literature related to a particular topic in one place. The Codification was effective for interim and annual periods ending after September 15, 2009, and as of the effective date, all existing accounting standard documents have been superseded. The Codification was effective for us in the third quarter of 2009, and accordingly, our Quarterly Report on Form 10-Q for the quarter ending September 30, 2009 and all subsequent public filings will reference the Codification as the sole source of authoritative literature.
31
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivables. We maintain our accounts for cash and cash equivalents principally at one major bank and one investment firm in the United States. We have a formal written investment policy that restricts the placement of investments to issuers evaluated as creditworthy. We have not experienced any losses on our deposits of our cash and cash equivalents.
With respect to accounts receivable, we perform credit evaluations of our customers and do not require collateral. There have been no material losses on accounts receivables.
In the United States we sell our products directly to hospitals and clinics. In international markets, we sell our products to independent distributors who, in turn, sell to medical clinics. We sell our product in these countries through independent distributors denominated in United States dollars, with the exception of Germany, where sales are denominated in Euros.
We do not believe our operations are currently subject to significant market risks for interest rates, foreign currency exchange rates, commodity prices or other relevant market price risks of a material nature. A change of 0.1 in the Euro exchange would result in an increase or decrease of approximately $21,000 in the amount of United States dollars we receive in payment on accounts receivable from Nicolai, GmbH. Under our current policies, we do not use foreign currency derivative instruments to manage exposure to fluctuations in the Euro exchange rate.
We currently have no indebtedness, but if we were to borrow amounts from our revolving credit line, we would be exposed to changes in interest rates. Advances under our revolving credit line bear interest at an annual rate indexed to LIBOR. We will thus be exposed to interest rate risk with respect to amounts outstanding under the line of credit to the extent that interest rates rise. As we had no amounts outstanding on the line of credit at December 31, 2009, we have no exposure to interest rate changes on this credit facility. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. Additionally, we will be exposed to declines in the interest rates paid on deposited funds. A 1% decline in the current market interest rates paid on deposits would result in interest income being reduced by approximately $178,000 on an annual basis.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Consolidated Financial Statements and Notes thereto required pursuant to this Item begin on page 40 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were effective.
32
Changes in Internal Controls.
During the fiscal quarter ended December 31, 2009, there has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2009.
Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm, has issued an attestation report on our internal control over financial reporting as of December 31, 2009.
Attestation Report of Independent Registered Public Accounting Firm.
Baker Tilly Virchow Krause, LLP, an independent registered public accounting firm, has issued an attestation report on our internal control over financial reporting as of December 31, 2009. The attestation report of Baker Tilly Virchow Krause, LLP, on our internal control over financial reporting as of December 31, 2009 is included on page 40 and incorporated by reference herein.
ITEM 9B. OTHER INFORMATION
None.
33
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPRATE GOVERNANCE
Incorporated herein by reference to the Sections under the headings “Proposal 1: Election of Directors,” “Committees of the Board of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2009.
See the section under the heading “Executive Officers of the Registrant” in Item 1 of Part I herein for information regarding our executive officers.
Code of Ethics
We have adopted a code of ethics that applies to all of our directors, officers (including our chief executive officer, chief financial officer, chief accounting officer, and any person performing similar functions) and employees. We have posted our Code of Ethics in the “Corporate Governance” section of our website, http://www.vascularsolutions.com.
ITEM 11. EXECUTIVE COMPENSATION
Incorporated herein by reference to the Sections under the headings “Director Compensation” and “Executive Compensation” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2009.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Incorporated herein by reference to the Section under the heading “Security Ownership of Certain Beneficial Owners and Management” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2009.
34
Equity Compensation Plans
The following table sets forth the securities authorized to be issued under our current equity compensation plans as of December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan category |
|
Number of |
|
Weighted-average |
|
Number of securities |
|
|||
|
Equity compensation plans approved by security holders |
|
|
1,030,000 |
|
$ |
6.06 |
|
|
3,651,000 |
(1)(2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity compensation plans not approved by security holders |
|
|
None |
|
|
None |
|
|
None |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
1,030,000 |
|
$ |
6.06 |
|
|
3,651,000 |
|
|
|
|
|
|
|
(1) |
Includes 2,800,000 shares reserved and available for issuance under our Stock Option and Stock Award Plan. The shares available for issuance under our Stock Option and Stock Award Plan automatically increases on an annual basis through 2016, by the lesser of: |
||
|
|
|
||
|
|
|
• |
500,000 shares; |
|
|
|
|
|
|
|
|
• |
5% of the common-equivalent shares outstanding at the end of our prior fiscal year; or |
|
|
|
|
|
|
|
|
• |
a smaller amount determined by our Board of Directors or the committee administering the plan. |
|
|
|
|
|
|
(2) |
Includes 851,000 shares reserved and available for issuance under our Employee Stock Purchase Plan. The shares available for issuance under our Employee Stock Purchase Plan automatically increases on an annual basis through 2010, by the lesser of: |
||
|
|
|
||
|
|
|
• |
200,000 shares; |
|
|
|
|
|
|
|
|
• |
2% of the common-equivalent shares outstanding at the end of our prior fiscal year; or |
|
|
|
|
|
|
|
|
• |
a smaller amount determined by our Board of Directors or the committee administering the plan. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Incorporated herein by reference to the Sections under the headings “Related Person Transaction Policy” and “Proposal 1: Election of Directors” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2009.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Incorporated herein by reference to the Section under the heading “Additional Information about our Independent Registered Public Accounting Firm” contained in the Proxy Statement for our Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission within 120 days of the close of the year ended December 31, 2009.
35
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Documents filed as part of this Report.
|
|
|
|
|
(1) |
The following financial statements are filed herewith in Item 8 in Part II. |
|
|
|
|
|
|
|
(i) |
Reports of Independent Registered Public Accounting Firm |
|
|
|
|
|
|
(ii) |
Consolidated Balance Sheets |
|
|
|
|
|
|
(iii) |
Consolidated Statements of Operations |
|
|
|
|
|
|
(iv) |
Consolidated Statements of Changes in Shareholders’ Equity |
|
|
|
|
|
|
(v) |
Consolidated Statements of Cash Flows |
|
|
|
|
|
|
(vi) |
Notes to Consolidated Financial Statements |
|
|
|
|
|
(2) |
Financial Statement Schedules |
|
Schedule II – Valuation and Qualifying Accounts. Such schedule should be read in conjunction with the consolidated financial statements. All other supplemental schedules are omitted because of the absence of conditions under which they are required.
|
|
|
|
(3) |
Exhibits |
|
|
|
|
|
|
Exhibit |
|
Description |
|
|
3.1 |
|
Amended and Restated Articles of Incorporation of Vascular Solutions, Inc. (incorporated by reference to Exhibit 3.1 to Vascular Solutions’ Form 10-Q for the quarter ended September 30, 2000). |
|
|
3.2 |
|
Amended and Restated Bylaws of Vascular Solutions, Inc. (incorporated by reference to Exhibit 3.1 of Vascular Solutions’ Form 8-K dated October 19, 2007). |
|
|
4.1 |
|
Specimen of Common Stock certificate (incorporated by reference to Exhibit 4.1 of Vascular Solutions’ Registration Statement on Form S-1 (File No. 333-84089)). |
|
|
10.1 |
|
Lease Agreement dated December 28, 2006 by and between IRET - Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 10.4 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006). |
|
|
10.2 |
|
Amendment to Lease Agreement, dated November 12, 2007, by and between IRET - Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 99.1 of Vascular Solutions’ Form 8-K dated November 14, 2007). |
|
|
10.3 |
|
Amendment to Lease Agreement, dated November 12, 2007, by and between IRET – Plymouth, LLC as Landlord and Vascular Solutions, Inc. as Tenant (incorporated by reference to Exhibit 99.2 of Vascular Solutions’ Form 8-K dated November 14, 2007). |
|
|
10.4 |
|
Mutual and General Release dated November 9, 1998 by and between Vascular Solutions, Inc., Dr. Gary Gershony and B. Braun Medical, Inc. (incorporated by reference to Exhibit 10.5 of Vascular Solutions’ Registration Statement on Form S-1 (File No. 333-84089)). |
|
|
10.5 |
|
Purchase and Sale Agreement dated September 17, 1998 by and between Vascular Solutions, Inc. and Davol Inc. (incorporated by reference to Exhibit 10.8 of Vascular Solutions’ Registration Statement on Form S-1 (File No. 333-84089)). |
|
36
|
|
|
|
|
|
Exhibit |
|
Description |
|
|
10.7** |
|
Form of Employment Agreement by and between Vascular Solutions, Inc. and each of its executive officers (incorporated by reference to Exhibit 10.5 of Vascular Solutions’ Form 10-Q for the quarter ended March 31, 2004). |
|
|
10.8 |
|
Form of Distribution Agreement (incorporated by reference to Exhibit 10.12 of Vascular Solutions’ Registration Statement on Form S-1 (File No. 333-84089)). |
|
|
10.9** |
|
Vascular Solutions, Inc. Employee Stock Purchase Plan, as amended (incorporated by reference to Exhibit 10.14 to Vascular Solutions’ Form 10-K for the year ended December 31, 2000). |
|
|
10.10*** |
|
Supply Agreement dated October 18, 2004 by and between Vascular Solutions and Sigma-Aldrich Fine Chemicals, an operating division of Sigma-Aldrich, Inc. (incorporated by reference to Exhibit 10.12 to Vascular Solutions’ Form 10-K for the year ended December 31, 2004). |
|
|
10.11*** |
|
Amendment to Supply Agreement dated December 15, 2006 by and between Vascular Solutions and Sigma-Aldrich Fine Chemicals, an operating division of Sigma-Aldrich, Inc. (incorporated by reference to Exhibit 10.12 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006). |
|
|
10.12* |
|
Credit Agreement, dated December 21, 2009, between U.S. Bank Association and Vascular Solutions, Inc. |
|
|
10.13* |
|
Security Agreement, dated December 21, 2009, between U.S. Bank Association and Vascular Solutions, Inc. |
|
|
10.14* |
|
Promissory Note, dated December 21, 2009, between U. S. Bank Association and Vascular Solutions, Inc. |
|
|
10.15** |
|
Form of Incentive Stock Option Agreement (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated September 22, 2004). |
|
|
10.17** |
|
Form of Nonqualified Stock Option Agreement (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 8-K dated September 22, 2004). |
|
|
10.18** |
|
Form of Board of Directors Stock Option Agreement, as amended December 9, 2005 (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 8-K dated December 9, 2005). |
|
|
10.19** |
|
Form of Restricted Stock Award Agreement (incorporated by reference to Exhibit 10.3 of Vascular Solutions’ Form 8-K dated December 9, 2005). |
|
|
10.20 |
|
License agreement dated January 9, 2007 by and between Vascular Solutions and King Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.22 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006). |
|
|
10.21*** |
|
Device Supply agreement dated January 9, 2007 by and between Vascular Solutions and King Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.23 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006). |
|
|
10.22*** |
|
Thrombin-JMI® Supply Agreement dated January 9, 2007 by and between Vascular Solutions and King Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.24 of Vascular Solutions’ Form 10-K for the year ended December 31, 2006). |
|
|
10.23** |
|
Vascular Solutions, Inc. Stock Option and Stock Award Plan, as amended January 25, 2006, effective April 18, 2006 (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 10-Q for the quarter ended March 31, 2006). |
|
|
10.24 |
|
Settlement Agreement dated April 8, 2008 between Vascular Solutions, Inc. and Diomed, Inc. (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated April 10, 2008). |
|
|
10.25*** |
|
Settlement Agreement dated June 2, 2008 among VNUS Medical Technologies, Inc. (acquired by Covidien), AngioDynamics, Inc. and Vascular Solutions, Inc. (incorporated by reference to Exhibit 10.2 of Vascular Solutions’ Form 10-Q for the quarter ended June 30, 2008). |
|
|
10.26** |
|
Separation Agreement and General Release dated December 31, 2009 between Vascular Solutions, Inc. and James H. Quackenbush (incorporated by reference to Exhibit 10.1 of Vascular Solutions’ Form 8-K dated December 31, 2009). |
|
|
23.1* |
|
Consent of Baker Tilly Virchow Krause, LLP. |
|
|
24.1 |
|
Power of Attorney (included on signature page). |
|
37
|
|
|
|
|
|
Exhibit |
|
Description |
|
|
31.1* |
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
31.2* |
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
32.1* |
|
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
32.2* |
|
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
|
|
|
* Filed herewith. |
|
**Management contract or compensatory plan or arrangement required to be filed as an Exhibit to this Form 10-K. |
|
*** Pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, confidential portions of these exhibits have been deleted and filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment. |
38
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on the 2nd day of February 2010.
|
|
|
|
|
|
VASCULAR SOLUTIONS, INC. |
|
|
|
||
|
|
By: |
/s/ Howard Root |
|
|
|
Howard Root |
|
|
|
Chief Executive Officer and Director |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Howard Root and James Hennen (with full power to act alone), as his true and lawful attorneys-in-fact and agents, with full powers of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to the Annual Report on Form 10-K of Vascular Solutions, Inc. for the year ended December 31, 2009, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes, lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed on the 2nd day of February 2010, by the following persons in the capacities indicated.
|
|
|
|
|
|
|
|
|
|
Signature |
|
|
|
Title |
|
|
|
|
|
||||
|
/s/ Howard Root |
|
Chief Executive Officer and Director |
||||
|
Howard Root |
|
(principal executive officer) |
||||
|
|
|
|
||||
|
/s/ James Hennen |
|
Vice President, Finance and Chief Financial Officer and Secretary |
||||
|
James Hennen |
|
(principal financial officer) |
||||
|
|
|
|
||||
|
/s/ Timothy Slayton |
|
Controller |
||||
|
Timothy Slayton |
|
(principal accounting officer) |
||||
|
|
|
|
||||
|
/s/ Richard Nigon |
|
Director |
||||
|
Richard Nigon |
|
|
||||
|
|
|
|
||||
|
|
|
Director |
||||
|
Michael Kopp |
|
|
||||
|
|
|
|
||||
|
/s/ Paul O’Connell |
|
Director |
||||
|
Paul O’Connell |
|
|
||||
|
|
|
|
||||
|
/s/ John Erb |
|
Director |
||||
|
John Erb |
|
|
||||
|
|
|
|
||||
|
/s/ Jorge Saucedo |
|
Director |
||||
|
Jorge Saucedo |
|
|
||||
|
|
|
|
||||
|
/s/ Charmaine Sutton |
|
Director |
||||
|
Charmaine Sutton |
|
|
||||
39
Report of Independent Registered Public Accounting Firm
The Board of
Directors and Shareholders
Vascular Solutions, Inc.
Under date of February 2, 2010, we reported on the consolidated balance sheets of Vascular Solutions, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations, changes in shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2009 as contained in the annual report on Form 10-K for the year ended December 31, 2009. In connection with our audits of the aforementioned consolidated financial statements, we have also audited the related financial statement schedule as listed in the accompanying index. This financial statement schedule is the responsibility of the Company’s management. Our responsibility is to express an opinion on this financial statement schedule based on our audits.
In our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
|
|
|
|
|
/s/ Baker Tilly Virchow Krause, LLP |
|
|
|
|
Minneapolis, Minnesota |
|
|
February 2, 2010 |
|
40
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Description |
|
Balance at |
|
Additions |
|
Less |
|
Balance at |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, 2009: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales return allowance |
|
$ |
25,000 |
|
$ |
20,000 |
|
$ |
— |
|
$ |
45,000 |
|
|
Allowance for doubtful accounts |
|
|
95,000 |
|
|
62,000 |
|
|
(52,000 |
) |
|
105,000 |
|
|
Total |
|
$ |
120,000 |
|
$ |
82,000 |
|
$ |
(52,000 |
) |
$ |
150,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, 2008: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales return allowance |
|
$ |
40,000 |
|
$ |
— |
|
$ |
(15,000 |
) |
$ |
25,000 |
|
|
Allowance for doubtful accounts |
|
|
90,000 |
|
|
58,000 |
|
|
(53,000 |
) |
|
95,000 |
|
|
Total |
|
$ |
130,000 |
|
$ |
58,000 |
|
$ |
(68,000 |
) |
$ |
120,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, 2007: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales return allowance |
|
$ |
45,000 |
|
$ |
— |
|
$ |
(5,000 |
) |
$ |
40,000 |
|
|
Allowance for doubtful accounts |
|
|
65,000 |
|
|
37,000 |
|
|
(12,000 |
) |
|
90,000 |
|
|
Total |
|
$ |
110,000 |
|
$ |
37,000 |
|
$ |
(17,000 |
) |
$ |
130,000 |
|
41
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders, Audit
Committee and Board of Directors
Vascular Solutions, Inc.
Minneapolis, MN
We have audited the accompanying consolidated balance sheets of Vascular Solutions, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations, shareholders’ equity and cash flows for each of the years in the three-year period ended December 31, 2009. We also have audited Vascular Solutions, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Vascular Solutions, Inc.’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting included in Item 9A Controls and Procedures. Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the company’s internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Vascular Solutions, Inc. as of December 31, 2009 and 2008, and the consolidated results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, Vascular Solutions, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
|
|
|
/s/ Baker Tilly Virchow Krause, LLP |
|
|
|
Minneapolis, Minnesota |
|
February 2, 2010 |
42
Vascular Solutions, Inc.
Consolidated Balance Sheets
|
|
|
|
|
|
|
|
|
|
|
|
December 31 |
|
||||
|
|
|
2009 |
|
2008 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
17,794,000 |
|
$ |
7,209,000 |
|
|
Accounts receivable, net of reserves of $150,000 and $120,000 at December 31, 2009 and 2008, respectively |
|
|
9,143,000 |
|
|
8,706,000 |
|
|
Inventories |
|
|
8,977,000 |
|
|
9,974,000 |
|
|
Prepaid expenses |
|
|
1,520,000 |
|
|
1,045,000 |
|
|
Current portion of deferred tax assets |
|
|
4,500,000 |
|
|
2,680,000 |
|
|
Total current assets |
|
|
41,934,000 |
|
|
29,614,000 |
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
3,793,000 |
|
|
3,887,000 |
|
|
Intangible assets, net |
|
|
193,000 |
|
|
193,000 |
|
|
Deferred tax assets, net of current portion and liabilities |
|
|
5,835,000 |
|
|
10,486,000 |
|
|
Total assets |
|
$ |
51,755,000 |
|
$ |
44,180,000 |
|
|
|
|
|
|
|
|
|
|
|
Liabilities and shareholders’ equity |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,396,000 |
|
$ |
2,022,000 |
|
|
Accrued compensation |
|
|
2,978,000 |
|
|
2,584,000 |
|
|
Accrued expenses |
|
|
865,000 |
|
|
848,000 |
|
|
Accrued royalties |
|
|
621,000 |
|
|
576,000 |
|
|
Current portion of deferred revenue |
|
|
929,000 |
|
|
907,000 |
|
|
Total current liabilities |
|
|
6,789,000 |
|
|
6,937,000 |
|
|
|
|
|
|
|
|
|
|
|
Long-term deferred revenue, net of current portion |
|
|
4,567,000 |
|
|
5,417,000 |
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shareholders’ equity: |
|
|
|
|
|
|
|
|
Common stock, $0.01 par value: |
|
|
|
|
|
|
|
|
Authorized shares – 40,000,000 |
|
|
|
|
|
|
|
|
Issued and outstanding shares – 16,557,669 – 2009; 16,027,519 – 2008 |
|
|
166,000 |
|
|
160,000 |
|
|
Additional paid-in capital |
|
|
88,481,000 |
|
|
85,292,000 |
|
|
Other |
|
|
84,000 |
|
|
84,000 |
|
|
Accumulated deficit |
|
|
(48,332,000 |
) |
|
(53,710,000 |
) |
|
Total shareholders’ equity |
|
|
40,399,000 |
|
|
31,826,000 |
|
|
Total liabilities and shareholders’ equity |
|
$ |
51,755,000 |
|
$ |
44,180,000 |
|
See accompanying notes.
43
Vascular Solutions, Inc.
Consolidated Statements of Operations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31 |
|
|||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
Product revenue |
|
$ |
66,726,000 |
|
$ |
59,757,000 |
|
$ |
51,414,000 |
|
|
License and collaboration revenue |
|
|
1,701,000 |
|
|
1,464,000 |
|
|
1,450,000 |
|
|
Total revenue |
|
|
68,427,000 |
|
|
61,221,000 |
|
|
52,864,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold |
|
|
22,917,000 |
|
|
20,690,000 |
|
|
17,002,000 |
|
|
Cost of goods sold related to thrombin inventory |
|
|
— |
|
|
670,000 |
|
|
— |
|
|
Collaboration expenses |
|
|
850,000 |
|
|
632,000 |
|
|
685,000 |
|
|
Research and development |
|
|
7,847,000 |
|
|
6,333,000 |
|
|
5,481,000 |
|
|
Clinical and regulatory |
|
|
2,886,000 |
|
|
3,220,000 |
|
|
3,168,000 |
|
|
Sales and marketing |
|
|
21,206,000 |
|
|
20,482,000 |
|
|
19,603,000 |
|
|
General and administrative |
|
|
4,555,000 |
|
|
4,695,000 |
|
|
5,304,000 |
|
|
Litigation |
|
|
— |
|
|
1,484,000 |
|
|
5,800,000 |
|
|
Thrombin qualification |
|
|
— |
|
|
— |
|
|
147,000 |
|
|
Total product costs and operating expenses |
|
|
60,261,000 |
|
|
58,206,000 |
|
|
57,190,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
8,166,000 |
|
|
3,015,000 |
|
|
(4,326,000 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expenses): |
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
48,000 |
|
|
203,000 |
|
|
444,000 |
|
|
Interest expense |
|
|
(38,000 |
) |
|
(62,000 |
) |
|
(148,000 |
) |
|
Foreign exchange loss |
|
|
(10,000 |
) |
|
(28,000 |
) |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes |
|
|
8,166,000 |
|
|
3,128,000 |
|
|
(4,030,000 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit (expense) |
|
|
(2,788,000 |
) |
|
13,045,000 |
|
|
(276,000 |
) |
|
Net income (loss) |
|
$ |
5,378,000 |
|
$ |
16,173,000 |
|
$ |
(4,306,000 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per common share |
|
$ |
0.34 |
|
$ |
1.04 |
|
$ |
(0.28 |
) |
|
Diluted net income (loss) per common share |
|
$ |
0.33 |
|
$ |
1.01 |
|
$ |
(0.28 |
) |
|
Shares used in computing basic net income (loss) per common share |
|
|
16,046,534 |
|
|
15,588,135 |
|
|
15,237,836 |
|
|
Shares used in computing diluted net income (loss) per common share |
|
|
16,474,708 |
|
|
15,954,631 |
|
|
15,237,836 |
|
See accompanying notes.
44
Vascular Solutions, Inc.
Consolidated Statements of Changes in Shareholders’ Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
|
|
|
Accumulated |
|
|
|
|
|||||||
|
|
|
Shares |
|
Amount |
|
|
Other |
|
|
Total |
|
||||||||
|
Balance at December 31, 2006 |
|
|
15,141,181 |
|
|
151,000 |
|
|
79,841,000 |
|
|
52,000 |
|
|
(65,577,000 |
) |
|
14,467,000 |
|
|
Exercise of stock options |
|
|
208,781 |
|
|
2,000 |
|
|
473,000 |
|
|
— |
|
|
— |
|
|
475,000 |
|
|
Issuance of common stock under the Employee Stock Purchase Plan |
|
|
102,194 |
|
|
1,000 |
|
|
666,000 |
|
|
— |
|
|
— |
|
|
667,000 |
|
|
Stock option compensation |
|
|
154,500 |
|
|
2,000 |
|
|
1,455,000 |
|
|
— |
|
|
— |
|
|
1,457,000 |
|
|
Deferred compensation related to option grants |
|
|
— |
|
|
— |
|
|
21,000 |
|
|
(21,000 |
) |
|
— |
|
|
— |
|
|
Amortization of deferred compensation |
|
|
— |
|
|
— |
|
|
— |
|
|
26,000 |
|
|
— |
|
|
26,000 |
|
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(4,306,000 |
) |
|
(4,306,000 |
) |
|
Translation adjustment |
|
|
— |
|
|
— |
|
|
— |
|
|
39,000 |
|
|
— |
|
|
39,000 |
|
|
Total comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,267,000 |
) |
|
Balance at December 31, 2007 |
|
|
15,606,656 |
|
|
156,000 |
|
|
82,456,000 |
|
|
96,000 |
|
|
(69,883,000 |
) |
|
12,825,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock options |
|
|
184,860 |
|
|
2,000 |
|
|
617,000 |
|
|
— |
|
|
— |
|
|
619,000 |
|
|
Issuance of common stock under the Employee Stock Purchase Plan |
|
|
133,274 |
|
|
1,000 |
|
|
701,000 |
|
|
— |
|
|
— |
|
|
702,000 |
|
|
Stock option compensation |
|
|
130,000 |
|
|
1,000 |
|
|
1,676,000 |
|
|
— |
|
|
— |
|
|
1,677,000 |
|
|
Cancellation of common stock upon the vesting of restricted shares |
|
|
(27,271 |
) |
|
— |
|
|
(158,000 |
) |
|
— |
|
|
— |
|
|
(158,000 |
) |
|
Amortization of deferred compensation |
|
|
— |
|
|
— |
|
|
— |
|
|
9,000 |
|
|
— |
|
|
9,000 |
|
|
Comprehensive income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
16,173,000 |
|
|
16,173,000 |
|
|
Translation adjustment |
|
|
— |
|
|
— |
|
|
— |
|
|
(21,000 |
) |
|
— |
|
|
(21,000 |
) |
|
Total comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16,152,000 |
|
|
Balance at December 31, 2008 |
|
|
16,027,519 |
|
$ |
160,000 |
|
$ |
85,292,000 |
|
$ |
84,000 |
|
$ |
(53,710,000 |
) |
$ |
31,826,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock options |
|
|
247,990 |
|
|
3,000 |
|
|
1,140,000 |
|
|
— |
|
|
— |
|
|
1,143,000 |
|
|
Issuance of common stock under the Employee Stock Purchase Plan |
|
|
140,790 |
|
|
1,000 |
|
|
746,000 |
|
|
— |
|
|
— |
|
|
747,000 |
|
|
Stock option compensation |
|
|
180,500 |
|
|
2,000 |
|
|
1,657,000 |
|
|
— |
|
|
— |
|
|
1,659,000 |
|
|
Repurchase and cancellation of common stock upon the vesting of restricted shares |
|
|
(39,130 |
) |
|
— |
|
|
(354,000 |
) |
|
— |
|
|
— |
|
|
(354,000 |
) |
|
Amortization of deferred compensation |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Comprehensive income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
5,378,000 |
|
|
5,378,000 |
|
|
Translation adjustment |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Total comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,378,000 |
|
|
Balance at December 31, 2009 |
|
|
16,557,669 |
|
$ |
166,000 |
|
$ |
88,481,000 |
|
$ |
84,000 |
|
$ |
(48,332,000 |
) |
$ |
40,399,000 |
|
See accompanying notes.
45
Vascular Solutions, Inc.
Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31 |
|
|||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
5,378,000 |
|
$ |
16,173,000 |
|
$ |
(4,306,000 |
) |
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
1,418,000 |
|
|
1,469,000 |
|
|
1,376,000 |
|
|
Stock-based compensation |
|
|
1,659,000 |
|
|
1,677,000 |
|
|
1,457,000 |
|
|
Deferred compensation expense |
|
|
— |
|
|
9,000 |
|
|
26,000 |
|
|
Deferred taxes, net |
|
|
2,832,000 |
|
|
(13,194,000 |
) |
|
28,000 |
|
|
Loss on disposal of fixed assets |
|
|
— |
|
|
26,000 |
|
|
— |
|
|
Change in accounts receivable allowance |
|
|
30,000 |
|
|
(10,000 |
) |
|
20,000 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(467,000 |
) |
|
(1,330,000 |
) |
|
(848,000 |
) |
|
Inventories |
|
|
996,000 |
|
|
(1,667,000 |
) |
|
(1,080,000 |
) |
|
Prepaid expenses |
|
|
(474,000 |
) |
|
(235,000 |
) |
|
(14,000 |
) |
|
Accounts payable |
|
|
(626,000 |
) |
|
1,000 |
|
|
758,000 |
|
|
Accrued compensation and expenses |
|
|
456,000 |
|
|
(5,091,000 |
) |
|
5,524,000 |
|
|
Deferred license fees received |
|
|
— |
|
|
731,000 |
|
|
7,000,000 |
|
|
Amortization of deferred license fees and other deferred revenue |
|
|
(828,000 |
) |
|
(845,000 |
) |
|
(562,000 |
) |
|
Net cash provided by (used in) operating activities |
|
|
10,374,000 |
|
|
(2,286,000 |
) |
|
9,379,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment, net |
|
|
(1,325,000 |
) |
|
(1,536,000 |
) |
|
(1,545,000 |
) |
|
Cash deposits transferred from (to) restricted cash |
|
|
— |
|
|
5,473,000 |
|
|
(5,473,000 |
) |
|
Net cash provided by (used in) investing activities |
|
|
(1,325,000 |
) |
|
3,937,000 |
|
|
(7,018,000 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from the exercise of stock options and stock warrants |
|
|
1,143,000 |
|
|
619,000 |
|
|
475,000 |
|
|
Net proceeds from the sale of common stock, employee stock purchase plan |
|
|
747,000 |
|
|
702,000 |
|
|
667,000 |
|
|
Payments on long-term debt borrowings |
|
|
— |
|
|
(867,000 |
) |
|
(800,000 |
) |
|
Repurchase and cancellation of common shares |
|
|
(354,000 |
) |
|
(158,000 |
) |
|
— |
|
|
Net cash provided by financing activities |
|
|
1,536,000 |
|
|
296,000 |
|
|
342,000 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
|
— |
|
|
(24,000 |
) |
|
26,000 |
|
|
Increase in cash and cash equivalents |
|
|
10,585,000 |
|
|
1,923,000 |
|
|
2,729,000 |
|
|
Cash and cash equivalents at beginning of year |
|
|
7,209,000 |
|
|
5,286,000 |
|
|
2,557,000 |
|
|
Cash and cash equivalents at end of year |
|
$ |
17,794,000 |
|
$ |
7,209,000 |
|
$ |
5,286,000 |
|
|
|
||||||||||
|
Supplemental disclosure of cash flow |
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest |
|
$ |
39,000 |
|
$ |
68,000 |
|
$ |
155,000 |
|
|
Cash paid for taxes |
|
$ |
191,000 |
|
$ |
252,000 |
|
$ |
149,000 |
|
See accompanying notes.
46
1. Description of Business
Vascular Solutions, Inc. (the Company) is a medical device company focused on bringing clinically advanced solutions to interventional cardiologists and interventional radiologists. The Company’s main product lines consist of the following:
|
|
|
|
|
|
• |
Hemostat (blood clotting) products, principally consisting of the D-Stat® Dry hemostat, a topical thrombin-based pad with a bandage used to control surface bleeding, and the D-Stat Flowable, a thick yet flowable thrombin-based mixture for preventing bleeding in subcutaneous pockets, |
|
|
|
|
|
|
• |
Extraction catheters, principally consisting of the Pronto® V3 and Pronto LP extraction catheters, mechanical systems for the removal of soft thrombus from arteries, |
|
|
|
|
|
|
• |
Vein products, principally consisting of the Vari-Lase® endovenous laser, a laser and procedure kit used for the treatment of varicose veins, |
|
|
|
|
|
|
• |
Access products, principally consisting of micro-introducer kits, MICRO Elite™ and EXPRO Elite™ snares, the Guardian® hemostasis valve, and guidewires used in connection with percutaneous access to the vasculature, and |
|
|
|
|
|
|
• |
Specialty catheters, consisting of a variety of catheters for clinical niches including the Langston® dual lumen catheters, Twin-Pass® dual access catheters, GuideLiner™ catheter, Minnie™ support catheter, and Gopher™ catheter. |
As a vertically-integrated medical device company, the Company generates ideas and creates new interventional medical devices and then delivers the products directly to the physician through a direct domestic sales force and an international distribution network. The Company was incorporated in the state of Minnesota in December 1996 and began operations in February 1997.
2. Summary of Significant Accounting Policies
Basis of Consolidation
The consolidated financial statements include the accounts of Vascular Solutions, Inc. and its wholly owned subsidiary, Vascular Solutions GmbH, after elimination of intercompany accounts and transactions.
Segment Reporting
A business segment is a distinguishable component of an enterprise that is engaged in providing an individual product or service or a group of related products or services and that is subject to risks and returns that are different from those of other business segments. The Company’s segments have similar economic characteristics and are similar in the nature of the products sold, type of customers, methods used to distribute the Company’s products and regulatory environment. Management believes that the Company meets the criteria for aggregating its operating segments into a single reporting segment.
47
2. Summary of Significant Accounting Policies (Continued)
Our primary products are categorized into five product lines. The following table sets forth, for the periods indicated, net revenue by product line along with the percent change from the previous year:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For Years Ended December 31, |
|
||||||||||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
||||||||||||
|
|
|
Net Revenue |
|
Percent |
|
Net Revenue |
|
Percent |
|
Net Revenue |
|
Percent |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hemostat products |
|
$ |
24,428,000 |
|
|
4 |
% |
$ |
23,475,000 |
|
|
(5 |
%) |
$ |
24,712,000 |
|
|
14 |
% |
|
Extraction catheters |
|
|
16,897,000 |
|
|
13 |
% |
|
14,992,000 |
|
|
36 |
% |
|
11,016,000 |
|
|
22 |
% |
|
Vein products |
|
|
11,225,000 |
|
|
12 |
% |
|
10,035,000 |
|
|
16 |
% |
|
8,629,000 |
|
|
22 |
% |
|
Access products |
|
|
7,298,000 |
|
|
31 |
% |
|
5,561,000 |
|
|
99 |
% |
|
2,790,000 |
|
|
74 |
% |
|
Specialty catheters |
|
|
5,905,000 |
|
|
29 |
% |
|
4,563,000 |
|
|
36 |
% |
|
3,363,000 |
|
|
8 |
% |
|
Other products |
|
|
973,000 |
|
|
(14 |
%) |
|
1,131,000 |
|
|
25 |
% |
|
904,000 |
|
|
18 |
% |
|
License & Collaboration |
|
|
1,701,000 |
|
|
16 |
% |
|
1,464,000 |
|
|
1 |
% |
|
1,450,000 |
|
|
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Net Revenue |
|
$ |
68,427,000 |
|
|
12 |
% |
$ |
61,221,000 |
|
|
16 |
% |
$ |
52,864,000 |
|
|
22 |
% |
Foreign Currency Translation and Transactions
The Company’s German subsidiary Vascular Solutions, GmbH accounted for its transactions in its functional currency, the Euro. Foreign assets and liabilities are translated into United States dollars using the year-end exchange rates. Equity is translated at average historical exchange rates. Results of operations are translated using the average exchange rates throughout the year. Translation gains or losses are accumulated as a separate component of shareholders’ equity.
Effective April 1, 2008 the Company began to sell products to a new international distributor in Germany at prices denominated in Euros. The Company also purchases a small number of inventory items at prices denominated in Euros. As a result, the Company is exposed to foreign exchange movements during the time between the shipment of the product and payment. The Company currently has terms of net 60 days with this distributor and net 30 days with vendors under the agreements providing for payments in Euros.
Comprehensive Loss
The components of comprehensive income (loss) are net income (loss) and the effects of foreign currency translation adjustments. The accumulated other comprehensive income (loss) for the foreign currency translation adjustment at December 31, 2009 and 2008 was $84,000 and $84,000, respectively.
Fair Value of Financial Instruments
The carrying amount for cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses approximates fair value due to the immediate or short-term maturity of these financial instruments. The fair value of long-term debt approximated their carrying value because the terms are equivalent to borrowing rates currently available to the Company for debt with similar terms and maturities.
48
2. Summary of Significant Accounting Policies (Continued)
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of deferred tax assets and liabilities, as well as other amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company classifies all highly liquid investments with initial maturities of three months at the date of purchase or less as cash equivalents. Cash equivalents consist of cash and money market funds and are stated at cost, which approximates market value. The Company deposits its cash in high quality financial institutions. The balances, at times, may exceed federally insured limits.
Credit Risk and Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments. This allowance is regularly evaluated by the Company for adequacy by taking into consideration factors such as past experience, credit quality of the customer base, age of the receivable balances, both individually and in the aggregate, and current economic conditions that may affect a customer’s ability to pay. Accounts receivable over 60 days past due are considered past due. The Company does not accrue interest on past due accounts receivable. Receivables are written off only after all collection attempts have failed and are based on individual credit evaluation and the specific circumstances of the customer. At December 31, 2009 and 2008, the allowance for doubtful accounts was $105,000 and $95,000, respectively.
All product returns must be pre-approved and, if approved, customers are subject to a 20% restocking charge. The Company analyzes the rate of historical returns when evaluating the adequacy of the allowance for sales returns, which is included with the allowance for doubtful accounts on its balance sheet. At December 31, 2009 and 2008, the sales and return allowance was $45,000 and $25,000, respectively.
Accounts receivable are shown net of the combined total of the allowance for doubtful accounts and allowance for sales returns of $150,000 and $120,000 at December 31, 2009 and 2008, respectively.
Inventories
Inventories are stated at the lower of cost (first-in, first-out method) or market. Appropriate consideration is given to deterioration, obsolescence and other factors in evaluating net realizable value. Inventories are comprised of the following at December 31:
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
||
|
Raw materials |
|
$ |
4,382,000 |
|
$ |
4,943,000 |
|
|
Work-in-process |
|
|
858,000 |
|
|
871,000 |
|
|
Finished goods |
|
|
3,737,000 |
|
|
4,160,000 |
|
|
|
|
$ |
8,977,000 |
|
$ |
9,974,000 |
|
49
2. Summary of Significant Accounting Policies (Continued)
Cost of sales related to thrombin inventory expenses were $670,000 for the year ended December 31, 2008. Cost of sales related to thrombin inventory expenses relate to a reserve the Company has recorded for the amount of thrombin the Company anticipates will expire prior to being used in the manufacturing of international hemostat products. The Company has not incurred additional charges related to thrombin inventory during 2009 and does not anticipate incurring additional charges related to thrombin inventory during 2010.
Property and Equipment
Property and equipment are stated at cost. Depreciation is provided on a straight-line basis over the estimated useful lives of the assets as follows:
|
|
|
|
Manufacturing equipment |
1 to 8 years |
|
Office and computer equipment |
1 to 5 years |
|
Furniture and fixtures |
3 to 8 years |
|
Leasehold improvements |
Shorter of useful life or |
|
Research and development equipment |
3 to 7 years |
Impairment of Long-Lived Assets
The Company will record impairment losses on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. The amount of impairment loss recorded will be measured as the amount by which the carrying value of the assets exceeds the fair value of the assets. To date, the Company has determined that no impairment of long-lived assets exists.
Revenue Recognition
In the United States the Company sells its products directly to hospitals and clinics. Revenue is recognized in accordance with generally accepted accounting principles as outlined in the Securities and Exchange Commission’s Staff Accounting Bulletin No. 104, Revenue Recognition (SAB 104), [Accounting Standards Codification (“ASC”) 605-10-S99], which requires that four basic criteria be met before revenue can be recognized: (i) persuasive evidence of an arrangement exists; (ii) the price is fixed or determinable; (iii) collectibility is reasonably assured; and (iv) product delivery has occurred or services have been rendered. The Company recognizes revenue as products are shipped based on FOB shipping point terms when title passes to customers. The Company negotiates credit terms on a customer-by-customer basis and products are shipped at an agreed-upon price. All product returns must be pre-approved and, if approved, customers are subject to a 20% restocking charge.
In all international markets, the Company sells its products to international distributors which subsequently resell the products to hospitals and clinics. The Company has agreements with each of its distributors which provide that title and risk of loss pass to the distributor upon shipment of the products to the distributor. The Company warrants that its products are free from manufacturing defects at the time of shipment to the distributor. Revenue is recognized upon shipment of products to distributors following the receipt and acceptance of a distributor’s purchase order. Allowances are provided for estimated returns and warranty costs at the time of shipment. Sales and use taxes are reported on a net basis, excluding them from revenue.
50
2. Summary of Significant Accounting Policies (Continued)
The Company’s revenues from license agreements and research collaborations are recognized when earned (see Note 14). In accordance with Emerging Issues Task Force (EITF) Issue No. 00-21, Revenue Arrangements with Multiple Deliverables, [ASC 605-25], for deliverables which contain multiple deliverables, the Company separates the deliverables into separate accounting units if they meet the following criteria: (i) the delivered items have a stand-alone value to the customer; (ii) the fair value of any undelivered items can be reliably determined; and (iii) if the arrangement includes a general right of return, delivery of the undelivered items is probable and substantially controlled by the seller. Deliverables that do not meet these criteria are combined with one or more other deliverables into one accounting unit. Revenue from each accounting unit is recognized based on the applicable accounting literature, primarily Staff Accounting Bulletin (SAB) 104, Revenue Recognition, [ASC 605-10-S99].
The Company currently has a license agreement with King Pharmaceuticals, Inc. (King) under which the Company licensed the exclusive rights of Thrombi-PadTM, Thrombi-Gel® and Thrombi-PasteTM products to King in exchange for a license fee. The Company is amortizing the license fees on a straight-line basis over the projected 10 year economic life of the products. The Company determines the economic life of the products under its license agreements by evaluating similar products the Company has launched or other similar products in the medical industry. In addition, the Company has a five-year license agreement with Nicolai, GmbH in which the Company is amortizing the license fee on a straight-line basis over the five-year life of the agreement.
As part of the agreements with King, the Company agreed to conduct clinical studies for the Thrombi-Gel and Thrombi-Paste products, with the costs related to the clinical studies paid by King. Additionally, on May 18, 2007, the Company entered into a Product Development & Supply Agreement with a third party company pursuant to which the Company agreed to develop, manufacture and sell to this company a specialty version of its Twin-Pass dual access catheter, with the costs related to the development paid by this company. The Company is recognizing the collaboration revenue on these development agreements as it is earned in accordance with Emerging Issues Task Force 01-14, Income Statement Characterizations of Reimbursements Received for “Out-of-Pocket” Expenses Incurred, [ASC 605-45-45], and SAB104, [ASC 605-10-S99]. In 2009 King decided to suspend indefinitely further development of the Thrombi-Paste products.
In addition, the Company has reviewed the provisions of EITF Issue No. 07-01, Accounting for Collaborative Arrangements, [ASC 808-10], and believes the adoption of this EITF will have no impact on the amounts recorded under these agreements.
Shipping and Handling Costs
In accordance with the Emerging Issues Task Force (EITF) issue 00-10, Accounting for Shipping and Handling Fees and Costs, [ASC 605-45-45], the Company includes shipping and handling revenues in net sales and shipping and handling costs in cost of goods sold.
Research and Development Costs
All research and development costs are charged to operations as incurred.
Warranty Costs
Certain of the Company’s products are covered by warranties against defects in material and workmanship for periods of up to 24 months. The Company records a liability for warranty claims at the time of sale. The amount of the liability is based on the amount the Company is charged from its original equipment manufacturer to cover the warranty period. The original equipment manufacturer includes a one year warranty with each product sold to the Company. The Company records a liability for the uncovered warranty period offered to a customer, provided the warranty period offered exceeds the initial one year warranty period covered by the original equipment manufacturer.
51
2. Summary of Significant Accounting Policies (Continued)
Warranty provisions and claims for the years ended December 31, 2009, 2008 and 2007, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Beginning balance |
|
$ |
49,000 |
|
$ |
34,000 |
|
$ |
46,000 |
|
|
Warranty provisions |
|
|
80,000 |
|
|
66,000 |
|
|
28,000 |
|
|
Warranty claims |
|
|
(56,000 |
) |
|
(51,000 |
) |
|
(40,000 |
) |
|
Ending balance |
|
$ |
73,000 |
|
$ |
49,000 |
|
$ |
34,000 |
|
Advertising Costs
The Company follows the policy of charging production costs of advertising to expense as incurred. Advertising expense was $116,000, $110,000, and $77,000 for the years ended December 31, 2009, 2008 and 2007, respectively.
Stock-Based Compensation
The Company has various types of stock-based compensation plans. These plans are administered by the compensation committee of the Board of Directors, which selects persons to receive awards and determines the number of shares subject to each award and the terms, conditions, performance measures and other provisions of the award. Refer to Notes 8, 9 and 10 for additional information related to these stock-based compensation plans.
Effective January 1, 2006, the Company adopted Statement No. 123R, Share-Based Payment (SFAS 123R), [ASC 718], which requires companies to measure and recognize compensation expense for all stock-based payments at fair value. SFAS 123R, [ASC 718], is being applied on the modified prospective basis. Prior to the adoption of SFAS 123R, [ASC 718], the Company accounted for its stock-based compensation plans under the recognition and measurement principles of Accounting Principles Board (APB) Opinion No. 25, Accounting for Stock Issued to Employees, and related interpretations, and accordingly, recognized no compensation expense related to the stock-based plans.
Under the modified prospective approach, SFAS 123R, [ASC 718], applies to new awards and to awards that were outstanding on January 1, 2006 that are subsequently modified, repurchased, cancelled or vest. Under the modified prospective approach, compensation cost recognized in 2006 includes compensation cost for all share-based payments granted prior to, but not yet vested on, January 1, 2006, based on the grant-date fair value estimated in accordance with the provisions of SFAS 123R, [ASC 718], and compensation cost for all shared-based payments granted subsequent to January 1, 2006, based on the grant-date fair value estimated in accordance with the provisions of SFAS 123R, [ASC 718]. Prior periods were not restated to reflect the impact of adopting the new standard.
52
2. Summary of Significant Accounting Policies (Continued)
The following amounts have been recognized as stock-based compensation expense in the Consolidated Statements of Operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Stock-based compensation included in: |
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold |
|
$ |
199,000 |
|
$ |
207,000 |
|
$ |
154,000 |
|
|
Research and development |
|
|
234,000 |
|
|
170,000 |
|
|
164,000 |
|
|
Clinical and regulatory |
|
|
38,000 |
|
|
132,000 |
|
|
102,000 |
|
|
Sales and marketing |
|
|
608,000 |
|
|
601,000 |
|
|
422,000 |
|
|
General and administrative |
|
|
580,000 |
|
|
567,000 |
|
|
615,000 |
|
|
|
|
$ |
1,659,000 |
|
$ |
1,677,000 |
|
$ |
1,457,000 |
|
The Company uses the Black-Scholes option-pricing model to estimate fair value of stock-based awards with the following weighted average assumptions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Stock Options and Awards: |
|
|
|
|
|
|
|
|
|
|
|
Expected life (years) |
|
|
5.50 |
|
|
5.50 |
|
|
5.50 |
|
|
Expected volatility |
|
|
52% |
|
|
50% |
|
|
51% |
|
|
Dividend yield |
|
|
0% |
|
|
0% |
|
|
0% |
|
|
Risk-free interest rate |
|
|
1.8% |
|
|
2.75% |
|
|
4.64% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee Stock Purchase Plan: |
|
|
|
|
|
|
|
|
|
|
|
Expected life (years) |
|
|
2.0 |
|
|
2.0 |
|
|
2.0 |
|
|
Expected volatility |
|
|
52% |
|
|
42% |
|
|
36% |
|
|
Dividend yield |
|
|
0% |
|
|
0% |
|
|
0% |
|
|
Risk-free interest rate |
|
|
1.03% |
|
|
2.04% |
|
|
3.48% |
|
Restricted stock awards fair value is calculated as the market price on the date of grant for the years ended December 31, 2009 and 2008 and the fair value is amortized on a straight line basis over the requisite service period of four years for the award. The weighted average fair value of restricted stock awards granted during 2009, 2008 and 2007 was $9.14, $6.09 and $10.37, respectively.
The weighted average fair value of stock options granted with an exercise price equal to the deemed stock price on the date of grant during 2009, 2008 and 2007 was $2.65, $2.82 and $4.51, respectively.
The Company calculates expected volatility for stock options and awards using historical volatility. The starting point for the historical period used is based on a material change in the Company’s operations that occurred in the third quarter of 2003. The Company uses a 10% forfeiture rate for key employees and a 15% forfeiture rate for non-key employees for stock options and awards. The Company calculates expected volatility for employee stock purchase plan shares using historical volatility over a two-year period. A two-year period is used to coincide with the maximum two-year offering period under the employee stock purchase plan. The risk-free rates for the expected terms of the stock options and awards and the employee stock purchase plan is based on the U.S. Treasury yield curve in effect at the time of grant.
53
2. Summary of Significant Accounting Policies (Continued)
Income Taxes
Income taxes are accounted for under the liability method. Deferred income taxes are provided for temporary differences between the financial reporting and the tax bases of assets and liabilities. Deferred tax assets are reduced by a valuation allowance to the extent that realization of the related deferred tax asset is not assured. If the Company determines in the future that it is more likely than not that the Company will realize all or a portion of the deferred tax assets, the Company will adjust the valuation allowance in the period the determination is made (Note 7).
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance sheet along with any associated interest and penalties that would be payable to the taxing authorities upon examination.
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation 48 (FIN 48), Accounting for Uncertainty in Income Taxes — an Interpretation of FASB Statement No. 109, [ASC 740-10], which clarifies the accounting for uncertain income tax positions. This interpretation prescribes a financial statement recognition threshold and measurement attribute for any tax position taken or expected to be taken in a tax return. The interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Effective January 1, 2007, the Company adopted FIN 48, [ASC 740-10]. Upon adoption, the Company had $425,000 of unrecognized income tax benefits and the adoption of FIN 48, [ASC 740-10], had no effect on shareholders’ equity. The Company has recorded FIN 48, [ASC 740-10], reserves of $727,000 and $597,000 at December 31, 2009 and 2008. The impact of tax related interest and penalties is recorded as a component of income tax expense. At December 31, 2009, the Company has recorded $-0- for the payment of tax related interest and there were no tax penalties or interest recognized in the statements of operations
Net Income (Loss) Per Common Share
In accordance with SFAS No. 128, Earnings Per Share, [ASC 260-10], basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average common shares outstanding during the periods presented. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average common and potential dilutive common shares outstanding computed in accordance with the treasury stock method.
54
2. Summary of Significant Accounting Policies (Continued)
The number of shares used in earnings per share computations is as follows for the years ended December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Weighted average common shares outstanding—basic |
|
|
16,046,534 |
|
|
15,588,135 |
|
|
15,237,836 |
|
|
Dilutive effect of stock options and warrants |
|
|
428,174 |
|
|
366,496 |
|
|
— |
|
|
Weighted average common shares outstanding—diluted |
|
|
16,474,708 |
|
|
15,954,631 |
|
|
15,237,836 |
|
The dilutive effect of stock options and warrants in the above table excludes 396,000, 448,500, and 441,500 of options and warrants for which the exercise price was higher than the average market price for the years ended December 31, 2009, 2008 and 2007, respectively. In addition, dilutive potential common shares of 1,017,400 were excluded from diluted weighted average common shares outstanding for the year ended December 31, 2007, as they would be anti-dilutive due to the Company’s net loss for that year.
Goodwill and Other Intangible Assets
In fiscal 2002, the Company adopted SFAS No. 142, Goodwill and Other Intangible Assets, [ASC 350-20 and 350-30]. Goodwill is tested for impairment annually in the fourth quarter or more frequently if changes in circumstances or the occurrence of events suggest impairment exists. The Company has concluded that no impairment of goodwill existed as of December 31, 2009.
Other intangible assets consist of purchased technology. Purchased technology was amortized using the straight-line method over its estimated useful life of four years. The Company reviewed intangible assets for impairment as changes in circumstances or the occurrence of events suggested the remaining value was not recoverable.
Leases and Deferred Rent
The Company leases all office space. Leases are accounted for under the provisions of SFAS No. 13, Accounting for Leases, as amended, [ASC 840-10 and 840-20], which requires that leases be evaluated and classified as operating or capital leases for financial reporting purposes. As of December 31, 2009, all of the Company’s leases were accounted for as operating leases. For leases that contain rent escalations, the Company records the total rent payable during the lease term on a straight-line basis over the term of the lease and records the difference between the rents paid and the straight-line rent as a deferred rent. For any lease incentives the Company receives for items such as leasehold improvements, the Company records a deferred credit for the amount of the lease incentive and amortizes it over the lease term, which may or may not equal the amortization period of the leasehold improvements in accordance with FASB Technical Bulletin 88-1 “Issues Relating to Accounting for Leases,” [ASC 840-20].
New Accounting Pronouncement
In June 2009, the FASB issued Statement of Financial Accounting Standards No. 168, The FASB Accounting Standards Codification™ and the Hierarchy of Generally Accepted Accounting Principles a Replacement of FASB Statement No. 162 (“FAS 168”), [ASC 105-10]. This Standard establishes the FASB Accounting Standards Codification™ (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. The Codification does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all the authoritative literature related to a particular topic in one place. The Codification was effective for interim and annual periods ending after September 15, 2009, and as of the effective date, all existing accounting standard documents will be superseded. The Codification was effective for the Company in the third quarter of 2009, and accordingly, the Quarterly Report on Form 10-Q for the quarter ending September 30, 2009 and all subsequent public filings will reference the Codification as the sole source of authoritative literature.
55
3. Goodwill and Other Intangible Assets
The Company has adopted SFAS No. 141, Business Combinations, [ASC 805-10 and 805-20]. The Company acquired developed technology from Angiosonics, Inc. in April 2002 and subsequently amortized the amount over its useful life of four years. The goodwill of $193,000 acquired as part of this transaction is not being amortized.
4. Property and Equipment
Property and equipment consists of the following at December 31:
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
||
|
Property and equipment: |
|
|
|
|
|
|
|
|
Manufacturing equipment |
|
$ |
5,908,000 |
|
$ |
5,067,000 |
|
|
Office and computer equipment |
|
|
1,888,000 |
|
|
1,815,000 |
|
|
Furniture and fixtures |
|
|
424,000 |
|
|
463,000 |
|
|
Leasehold improvements |
|
|
1,495,000 |
|
|
1,275,000 |
|
|
Research and development equipment |
|
|
504,000 |
|
|
436,000 |
|
|
Construction-in-process |
|
|
61,000 |
|
|
213,000 |
|
|
|
|
|
10,280,000 |
|
|
9,269,000 |
|
|
Less accumulated depreciation and amortization |
|
|
(6,487,000 |
) |
|
(5,382,000 |
) |
|
Net property and equipment |
|
$ |
3,793,000 |
|
$ |
3,887,000 |
|
5. Lines of Credit
On December 21, 2009, the Company entered into a secured asset-based revolving credit agreement with U.S. Bank National Association. The revolving credit agreement is a one-year, $10,000,000 facility with availability based primarily on eligible customer receivables, inventory and property and equipment. The revolving credit agreement bears interest equal to the one-month LIBOR rate plus 1.60% and is secured by a first security interest on all of the Company’s assets. The revolving credit agreement requires a quarterly payment based on an annual fee of 0.125% of the average unused portion of the committed revolving line as determined by the bank and reviewed by management.
The revolving credit agreement includes one covenant that the Company cannot have a maximum cash flow leverage ratio greater than 2.5 to 1. The calculation of this covenant is determined by multiplying annual lease expense times six and adding any loans, then dividing this amount by the sum of earnings before interest, taxes, depreciation, amortization and annual operating lease payments. The covenant is computed quarterly based on a rolling 12-month period. The Company was in compliance with all of the covenants as of December 31, 2009.
As of December 31, 2009, the Company had no outstanding balance against the revolving credit agreement. Based on the Company’s eligible customer receivables, inventory, property and equipment and cash balances, $10,000,000 was available for borrowing as of December 31, 2009.
56
6. Leases
The Company leases two buildings totaling approximately 93,000 square-feet under separate operating leases. On November 12, 2007, both leases were amended to extend the terms until September 2015, with options to renew both leases. Rent expense related to the operating leases was approximately $1,133,000, $755,000 and $596,000 for the years ended December 31, 2009, 2008, and 2007, respectively.
Future minimum lease commitments under these operating leases as of December 31, 2009 were as follows:
|
|
|
|
|
|
|
2010 |
|
$ |
761,000 |
|
|
2011 |
|
|
797,000 |
|
|
2012 |
|
|
808,000 |
|
|
2013 |
|
|
841,000 |
|
|
2014 |
|
|
841,000 |
|
|
Thereafter |
|
|
631,000 |
|
|
|
|
$ |
4,679,000 |
|
7. Income Taxes
At December 31, 2009, the Company had net operating loss carryforwards of approximately $40,276,000 and $4,521,000 for federal and state income tax purposes that are available to offset future taxable income and begin to expire in the year 2019. Included in the U.S. amount are approximately $4.1 million of deductions resulting from disqualifying dispositions of stock options. When these deductions from disqualifying dispositions are realized for financial statement purposes they will not result in a reduction in income tax expense, rather the benefit will be recorded as additional paid-in-capital. At December 31, 2009, the Company also had federal research and development tax credit carryforwards of approximately $3,272,000 and Minnesota research and development tax credit carryforwards of approximately $760,000, which begin to expire in the year 2012. At December 31, 2009, the Company has foreign tax loss carryforwards of approximately $357,000 that do not expire. The adoption of FIN 48, Accounting for Uncertainty in Income Taxes, [ASC 740-10], has had no impact on the reported carryforwards at December 31, 2009.
The Company adopted the provisions of FASB Interpretation No. 48 (FIN 48), Accounting for Uncertainty in Income Taxes - an Interpretation of SFAS No. 109, [ASC 740-10], on January 1, 2007. This interpretation prescribes a financial statement recognition threshold and measurement attribute for any tax position taken or expected to be taken in a tax return. The interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Upon adoption, there was $425,000 of unrecognized income tax benefits and the adoption of FIN 48, [ASC 740-10], had no effect on shareholders’ equity. The impact of tax related interest and penalties will be recorded as a component of income tax expense. At December 31, 2009, the Company has accrued zero for the payment of tax related interest and there were no tax interest or penalties recognized in the statements of operations.
The Company is subject to income tax in numerous jurisdictions and at various rates and the use of estimates is required in determining the provision for income taxes. For the year ended December 31, 2009, the Company recorded a tax provision of $2,788,000 on income before tax of $8,166,000 resulting in an effective income tax rate of 34%.
The Company is subject to income tax examinations in the U.S. Federal jurisdiction, as well as in the Germany and various state jurisdictions. At December 31, 2009, the Company was not under examination by any of these taxing authorities and the open tax years are 2007 to 2009.
57
7. Income Taxes (Continued)
A reconciliation of the beginning and ending amount of unrecognized tax benefit is as follows:
|
|
|
|
|
|
|
Balance at December 31, 2007 |
|
$ |
512,000 |
|
|
Increases as a result of tax positions taken during a prior period |
|
|
5,000 |
|
|
Increases as a result of tax positions taken during the current period |
|
|
80,000 |
|
|
Reductions as a result of lapse of the applicable statute of limitations |
|
|
— |
|
|
Decreases relating to settlements with taxing authorities |
|
|
— |
|
|
Balance at December 31, 2008 |
|
|
597,000 |
|
|
Increases as a result of tax positions taken during a prior period |
|
|
2,000 |
|
|
Increases as a result of tax positions taken during the current period |
|
|
128,000 |
|
|
Reductions as a result of lapse of the applicable statute of limitations |
|
|
— |
|
|
Decreases relating to settlements with taxing authorities |
|
|
— |
|
|
Balance at December 31, 2009 |
|
$ |
727,000 |
|
The components of the Company’s deferred tax assets and liabilities as of December 31, 2009 and 2008 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
$ |
16,942,000 |
|
$ |
19,538,000 |
|
|
Tax credit carryforwards |
|
|
4,759,000 |
|
|
4,274,000 |
|
|
Deferred revenue |
|
|
2,049,000 |
|
|
2,353,000 |
|
|
Depreciation and amortization |
|
|
171,000 |
|
|
206,000 |
|
|
Accrued compensation |
|
|
335,000 |
|
|
304,000 |
|
|
Stock-based compensation |
|
|
609,000 |
|
|
530,000 |
|
|
Federal and state AMT credits |
|
|
143,000 |
|
|
195,000 |
|
|
Inventory reserve |
|
|
325,000 |
|
|
334,000 |
|
|
Other |
|
|
155,000 |
|
|
138,000 |
|
|
Gross deferred tax assets |
|
|
25,488,000 |
|
|
27,872,000 |
|
|
Deferred tax liability |
|
|
(38,000 |
) |
|
(34,000 |
) |
|
Net deferred taxes assets before FIN48 Reserve and valuation allowances |
|
|
25,450,000 |
|
|
27,838,000 |
|
|
FIN48 Reserve |
|
|
(727,000 |
) |
|
(597,000 |
) |
|
Less valuation allowances |
|
|
(14,388,000 |
) |
|
(14,075,000 |
) |
|
Net deferred tax asset / (liability) |
|
$ |
10,335,000 |
|
$ |
13,166,000 |
|
|
|
|
|
|
|
|
|
|
|
Deferred taxes recorded on the balance sheet: |
|
|
|
|
|
|
|
|
Net deferred tax assets / (liabilities) – current |
|
$ |
4,500,000 |
|
$ |
2,680,000 |
|
|
Net deferred tax assets / (liabilities) – long-term |
|
|
5,835,000 |
|
|
10,486,000 |
|
|
Net deferred tax assets |
|
$ |
10,335,000 |
|
$ |
13,166,000 |
|
58
7. Income Taxes (Continued)
The Company regularly assesses the likelihood that the deferred tax assets will be recovered from future taxable income. The Company considers projected future taxable income and ongoing tax planning strategies, then records a valuation allowance to reduce the carrying value of the net deferred taxes to an amount that is more likely than not to be realized. For the year ended December 31, 2008, based upon the Company’s assessment of all available evidence, including the previous three year cumulative income before unusual and infrequent expenses (litigation and thrombin qualification expenses), estimates of future profitability, and the Company’s overall prospects of future business, the Company determined that it was more likely than not that the Company would be able to realize a portion of the deferred tax assets in the future, and as a result recorded a $13,200,000 income tax benefit. To determine the amount of the reduction in the valuation allowance, the Company used a discounted projection of its revenue and income for the years ending December 31, 2009 through 2013, which would approximate the ten-year life of the Company’s three most significant products at this time. The amount of the valuation allowance reduction at December 31, 2008, was based on the Company’s projected discounted taxable income. The Company continues to assess the potential realization of deferred tax assets on an annual basis, or an interim basis if circumstances warrant. If the Company’s actual results and updated projections vary significantly from the projections used as a basis for this determination, the Company may need to increase or decrease the valuation allowance against the gross deferred tax assets. The Company would adjust earnings for the deferred tax in the period the determination was made. At December 31, 2009 and 2008, the valuation allowance was $14,388,000 and $14,075,000, respectively. Of these amounts, $530,000 as of December 31, 2008 was attributable to increases in the net operating loss carry forwards resulting from the exercise of stock options. This amount will be recorded as an increase to additional paid-in-capital if it is determined in the future that this portion of the valuation allowance is no longer required. The increase (decrease) in the valuation allowance was $313,000, ($14,997,000) and $1,360,000 for the years ended December 31, 2009, 2008 and 2007, respectively. For the year ended December 31, 2009 the Company recorded a stock option tax deduction of $1,062,000, which will be recorded against “additional paid-in capital” at the time at which it reduces taxes payable.
Reconciliation of the statutory federal income tax rate to the Company’s effective tax rate is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax at statutory rate |
|
|
34.0 |
% |
|
34.0 |
% |
|
(34.0 |
)% |
|
Permanent differences |
|
|
(1.4 |
) |
|
9.6 |
|
|
13.5 |
|
|
State income taxes, net of federal benefit |
|
|
4.5 |
|
|
3.7 |
|
|
(3.7 |
) |
|
Change in valuation reserve |
|
|
6.2 |
|
|
(476.4 |
) |
|
33.6 |
|
|
R&D credits generated |
|
|
(6.1 |
) |
|
(18.1 |
) |
|
(24.8 |
) |
|
FIN48 reserve |
|
|
1.6 |
|
|
2.8 |
|
|
12.7 |
|
|
Change in effective deferred tax rate |
|
|
(2.1 |
) |
|
25.4 |
|
|
4.0 |
|
|
Other adjustments |
|
|
(2.6 |
) |
|
2.0 |
|
|
5.5 |
|
|
Effective income tax rate |
|
|
34.1 |
% |
|
(417.0 |
)% |
|
6.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Current taxes |
|
|
(0.1 |
)% |
|
3.7 |
% |
|
(3.7 |
)% |
|
Deferred taxes |
|
|
34.2 |
|
|
1.3 |
|
|
10.5 |
|
|
Benefit from release of valuation reserve |
|
|
— |
|
|
(422.0 |
) |
|
— |
|
|
Effective income tax rate |
|
|
34.1 |
% |
|
(417.0 |
)% |
|
6.8 |
% |
59
8. Stock Options and Restricted Shares
Stock Option and Stock Award Plan
The Company has a stock option and stock award plan (the Stock Option Plan) which provides for the granting of stock options, restricted shares and stock appreciation rights to employees, directors, and consultants. Incentive stock options may be granted only to employees of the Company. Options which do not qualify as incentive stock options and awards of restricted shares may be granted to both employees and to non-employee directors and consultants. As of December 31, 2009, the Company had reserved 5,900,000 shares of common stock under the Stock Option Plan. Under the Stock Option Plan, stock options must be granted at an exercise price not less than the fair market value of the Company’s common stock on the grant date. Vesting requirements of all awards under this plan are time based and vary by individual grant. The options expire on the date determined by the Board of Directors but may not extend more than 10 years from the grant date. The incentive stock options generally become exercisable over a four-year period and the nonqualified stock options generally become exercisable over a two-year period. Vested and unexercised options are canceled three-months after termination, and unvested awards are canceled on the date of termination of employment and become available under the Stock Option Plan for future grants.
During 2007 to 2009, the Company granted stock options to its directors under the Stock Option Plan. The ten-year options issued to the Company’s directors vest over a one-year period based on the continuation of service as a director of the Company. The Company uses a 0% forfeiture rate for all director options granted.
Option activity is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares |
|
Plan
Options |
|
Exercise |
|
Weighted |
|
Aggregate |
|
|||||
|
|
||||||||||||||||
|
Balance at December 31, 2006 |
|
|
1,991,000 |
|
|
1,512,000 |
|
$ |
0.78–$12.00 |
|
$ |
5.24 |
|
|
|
|
|
Shares reserved |
|
|
500,000 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
Granted |
|
|
(75,000 |
) |
|
75,000 |
|
|
9.41– 9.58 |
|
|
9.44 |
|
|
|
|
|
Exercised |
|
|
— |
|
|
(116,000 |
) |
|
0.78– 9.46 |
|
|
2.01 |
|
|
|
|
|
Forfeited |
|
|
3,000 |
|
|
(3,000 |
) |
|
0.84– 9.46 |
|
|
8.27 |
|
|
|
|
|
Expired |
|
|
9,000 |
|
|
(9,000 |
) |
|
0.78– 9.46 |
|
|
4.24 |
|
|
|
|
|
Balance at December 31, 2007 |
|
|
2,428,000 |
|
|
1,459,000 |
|
$ |
0.78–$12.00 |
|
$ |
5.74 |
|
|
|
|
|
Shares reserved |
|
|
500,000 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
Granted |
|
|
(60,000 |
) |
|
60,000 |
|
|
6.36 |
|
|
6.36 |
|
|
|
|
|
Exercised |
|
|
— |
|
|
(185,000 |
) |
|
0.84– 6.74 |
|
|
3.35 |
|
|
|
|
|
Forfeited |
|
|
5,000 |
|
|
(5,000 |
) |
|
6.74– 9.46 |
|
|
9.45 |
|
|
|
|
|
Expired |
|
|
83,000 |
|
|
(83,000 |
) |
|
6.74– 12.00 |
|
|
9.52 |
|
|
|
|
|
Balance at December 31, 2008 |
|
|
2,956,000 |
|
|
1,246,000 |
|
$ |
0.78–$11.62 |
|
$ |
5.84 |
|
|
|
|
|
Shares reserved |
|
|
500,000 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
Granted |
|
|
(60,000 |
) |
|
60,000 |
|
|
6.39 |
|
|
6.39 |
|
|
|
|
|
Exercised |
|
|
— |
|
|
(248,000 |
) |
|
0.81– 6.74 |
|
|
4.61 |
|
|
|
|
|
Forfeited |
|
|
2,000 |
|
|
(2,000 |
) |
|
6.00– 11.62 |
|
|
10.22 |
|
|
|
|
|
Expired |
|
|
26,000 |
|
|
(26,000 |
) |
|
9.46– 11.62 |
|
|
11.55 |
|
|
|
|
|
Balance at December 31, 2009 |
|
|
3,424,000 |
|
1,030,000 |
|
$ |
0.78–$10.89 |
$ |
6.06 |
$ |
2,849,000 |
||||
|
Exercisable at December 31, 2009 |
|
|
|
|
|
1,010,000 |
|
|
|
$ |
6.05 |
$ |
2,809,000 |
|||
The weighted average remaining contractual term of options exercisable at December 31, 2009, was 4.3 years. The total intrinsic value of options exercised during fiscal 2009, 2008 and 2007, was $879,000, $909,000 and $903,000, respectively.
60
8. Stock Options and Restricted Shares (Continued)
The following table summarizes information about stock options outstanding at December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding |
|
Options Exercisable |
|
|||||||||||
|
Range of |
|
Outstanding |
|
Weighted |
|
Weighted |
|
Exercisable |
|
Weighted |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.78–$0.84 |
|
|
|
243,000 |
|
2.4 |
|
$ |
0.84 |
|
|
243,000 |
|
$ |
0.84 |
|
|
0.85– 2.51 |
|
|
|
76,000 |
|
1.9 |
|
|
2.39 |
|
|
76,000 |
|
|
2.39 |
|
|
2.52– 6.50 |
|
|
|
128,000 |
|
8.4 |
|
|
6.34 |
|
|
108,000 |
|
|
6.33 |
|
|
6.51– 7.48 |
|
|
|
187,000 |
|
2.9 |
|
|
6.93 |
|
|
187,000 |
|
|
6.93 |
|
|
7.49– 9.27 |
|
|
|
73,000 |
|
5.9 |
|
|
8.23 |
|
|
73,000 |
|
|
8.23 |
|
|
9.28– 10.89 |
|
|
|
323,000 |
|
5.2 |
|
|
9.72 |
|
|
323,000 |
|
|
9.72 |
|
|
|
|
|
|
1,030,000 |
|
4.3 |
|
$ |
6.06 |
|
|
1,010,000 |
$ |
6.05 |
|
|
As of December 31, 2009, there was $12,000 of total unrecognized compensation costs related to the outstanding stock options, which is expected to be recognized over a weighted average period of 0.18 years.
The holder of a restricted share award is generally entitled at all times on and after the date of issuance of the restricted shares to exercise the rights of a shareholder of the Company, including the right to vote the shares and the right to receive dividends on the shares. These shareholders do not have the ability to sell, transfer or otherwise encumber the restricted share awards until they fully vest. During 2009, 2008 and 2007 the Company granted restricted shares to employees under the Stock Option Plan. The restricted shares vest over a four-year period based on the continuation of employment.
Restricted share activity is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Shares |
|
Weighted |
|
||
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2006 |
|
|
159,000 |
|
$ |
5.42 |
|
|
Granted |
|
|
174,000 |
|
|
10.37 |
|
|
Vested |
|
|
— |
|
|
— |
|
|
Forfeited |
|
|
(20,000 |
) |
|
8.03 |
|
|
Expired |
|
|
— |
|
|
— |
|
|
Balance at December 31, 2007 |
|
|
313,000 |
|
|
8.01 |
|
|
Granted |
|
|
174,000 |
|
|
6.09 |
|
|
Vested |
|
|
(73,000 |
) |
|
5.50 |
|
|
Forfeited |
|
|
(44,000 |
) |
|
7.55 |
|
|
Expired |
|
|
— |
|
|
— |
|
|
Balance at December 31, 2008 |
|
|
370,000 |
|
|
7.68 |
|
|
Granted |
|
|
211,000 |
|
|
9.14 |
|
|
Vested |
|
|
(106,000 |
) |
|
8.86 |
|
|
Forfeited |
|
|
(30,000 |
) |
|
8.15 |
|
|
Expired |
|
|
— |
|
|
— |
|
|
Balance at December 31, 2009 |
|
|
445,000 |
|
$ |
8.07 |
|
61
8. Stock Options and Restricted Shares (Continued)
As of December 31, 2009, there was $1,112,000 of total unrecognized compensation costs related to the outstanding restricted shares, which is expected to be recognized over a weighted average period of 1.22 years. The Company estimates the forfeiture rate for restricted stock using 10% for key employees and 15% for non-key employees.
The net remaining shares available for grant under the Stock Option and Stock Award Plan is 2,800,000 shares.
Deferred Compensation
In 2009, 2008, and 2007, the Company recorded $-0-, $-0- and $21,000, respectively, of deferred compensation in connection with certain nonqualified stock options granted to medical advisory board members. The weighted average fair value of these options was $4.23. The deferred compensation recorded was amortized ratably over the period that the options vest and was adjusted for options which have been canceled. Vesting requirements for nonqualified stock options under this plan will vary by individual grant. Deferred compensation expense was $-0-, $9,000 and $26,000 for the years ended December 31, 2009, 2008, and 2007, respectively.
9. Employee Stock Purchase Plan
The Company has an Employee Stock Purchase Plan (the Purchase Plan) under which 2,100,000 shares of common stock have been reserved for issuance. Eligible employees may contribute 1% to 10% of their compensation to purchase shares of the Company’s common stock at a discount of 15% of the market value at certain plan-defined dates up to a maximum of 2,000 shares per purchasing period. The Purchase Plan terminates in July 2010. In fiscal 2009, 2008 and 2007, 140,800 shares, 133,300 shares, and 102,200 shares, respectively, were issued under the Purchase Plan. At December 31, 2009, 851,000 shares were available for issuance under the Purchase Plan.
As of December 31, 2009, there was $98,000 of total unrecognized compensation costs related to the Purchase Plan, which is expected to be recognized over a weighted average period of 0.24 years.
10. Employee Retirement Savings Plan
The Company has an employee 401(k) retirement savings plan (the Plan). The Plan provides eligible employees with an opportunity to make tax-deferred contributions into a long-term investment and savings program. All employees over the age of 21 are eligible to participate in the Plan beginning with the first quarterly open enrollment date following start of employment. The Plan allows eligible employees to contribute up to 50% of their annual compensation, subject to a maximum limit determined by the Internal Revenue Service, with the Company contributing an amount equal to 25% of the first 5% contributed to the Plan. The Company recorded an expense of $157,000, $117,000 and $144,000 for contributions to the Plan for the years ended December 31, 2009, 2008, and 2007, respectively.
11. Concentrations of Credit and Other Risks
In the United States the Company sells its products directly to hospitals and clinics. In all international markets, the Company sells its products to distributors who, in turn, sell to medical clinics. Loss, termination, or ineffectiveness of distributors to effectively promote the Company’s product could have a material adverse effect on the Company’s financial condition and results of operations.
No customer represented more than 10% of total revenue for any year ended December 31, 2009, 2008 and 2007.
62
11. Concentrations of Credit and Other Risks (Continued)
The Company performs credit evaluations of its customers and does not require collateral to establish an account receivable. No customer represented more than 10% of gross accounts receivable at December 31, 2009 and 2008. There have been no material losses on customer receivables.
Product revenue by geographic destination as a percentage of total product revenues were as follows for the years ended December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Domestic |
|
87 |
% |
|
87 |
% |
|
87 |
% |
|
|
Foreign |
|
13 |
|
|
13 |
|
|
13 |
|
|
12. Related Party Activity
During the years ended December 31, 2009, 2008 and 2007, the Company sold $458,000, $495,000 and $489,000, respectively, of product to a company of which a board member of the Company is an officer. As of December 31, 2009 and 2008, the Company had an accounts receivable balance due of $103,000 and $47,000, respectively, from this related party. In addition, the Company purchases product from this related party and during the years ended December 31, 2009, 2008 and 2007 the Company purchased $15,000, $16,000 and $24,000, respectively, of product from this related party. As of December 31, 2009 and 2008, the Company had an accounts payable balance due of $-0- to this related party.
In July 2008 the Company began utilizing the consulting services from a company owned by a board member. During the years ended December 31, 2009 and 2008, the Company utilized services in the amount of $354,000 and $170,000, respectively, from this vendor. At December 31, 2009 and 2008, the Company had an accounts payable balance due of $25,000 and $68,000 to this related party.
In July 2009 the Company began utilizing development consulting services from a company owned by the spouse of a board member. During the year ended December 31, 2009 the Company utilized services in the amount of $55,000 from this vendor. At December 31, 2009 the Company had an accounts payable balance due of $4,000 to this related party.
13. Dependence on Key Suppliers
King Pharmaceuticals
The Company purchases certain key components from single-source suppliers. Any significant component delay or interruption could require the Company to qualify new sources of supply, if available, and could have a material adverse effect on the Company’s financial condition and results of operations. The Company purchases its requirements for thrombin (a component in the Hemostat products) under a Thrombin-JMI Supply Agreement entered into with King Pharmaceuticals, Inc. (King) on January 9, 2007. Under the terms of the Thrombin-JMI Supply Agreement, King agrees to manufacture and supply thrombin to the Company on a non-exclusive basis. The Thrombin-JMI Supply Agreement does not contain any minimum purchase requirements. King agrees to supply the Company with such quantity of thrombin as the Company may order at a fixed price throughout the term of the Thrombin-JMI Supply Agreement as adjusted for inflation, variations in potency and other factors. The Thrombin-JMI Supply Agreement has an initial term of 10 years, followed by successive automatic one-year extensions, subject to termination by the parties under certain circumstances, including: (i) termination by King without cause any time after the fifth anniversary of the date of the Thrombin-JMI Supply Agreement upon five years prior written notice to the Company, and (ii) termination by the Company without cause any time after the fifth anniversary of the date of the Thrombin-JMI Supply Agreement upon five years prior written notice to King provided that the Device Supply Agreement, which the Company also entered into with King on January 9, 2007, has expired on its terms or the parties have agreed to terminate it.
63
14. Commitments and Contingencies
All legal cost related to litigation are charged to operations as incurred, except settlements which are expensed when a claim is probable and estimatable.
Diomed Litigation
On March 4, 2004, the Company was named as the defendant in an intellectual property lawsuit brought by Diomed Inc. (Diomed) in the United States District Court for the District of Massachusetts (the “Court”). The complaint requested a judgment that sales of the Company’s Vari-Lase® procedure kit and Vari-Lase laser console infringe on a single method patent (No. 6,398,777) held by Diomed and asked for relief in the form of an injunction that would prevent the Company from selling the Company’s Vari-Lase products, compensatory and treble damages caused by the manufacture and sale of the Company’s products, and other costs, disbursements and attorneys’ fees. The trial commenced on March 12, 2007, and concluded on March 28, 2007, when the jury reached a verdict that the Company contributed to and induced infringement of Diomed’s patent and awarded monetary damages in the amount of $4,100,000, plus pre-judgment interest. The jury concluded there was no willful infringement by the Company and therefore the award was not subject to treble damages or attorneys’ fees. To settle Diomed’s claims for pre-judgment interest and for additional damages for sales not considered by the jury, the Company agreed to amend the judgment amount to $4,975,000 and accrued this amount together with additional costs and attorney’s fees as of June 30, 2007 in the aggregate amount of $5,690,000. On June 20, 2007 the Company posted a supersedeas bond and appealed the jury verdict to the U.S. Court of Appeals for the Federal Circuit in Washington, D.C. On April 8, 2008, the Company announced that it entered into a settlement agreement with Diomed. Pursuant to the settlement agreement, (i) on April 29, 2008, the Company made a one-time payment of $3,586,000 to Diomed, (ii) the Company and Diomed jointly dismissed the appeal with the United States Court of Appeals for the Federal Circuit, and (iii) Diomed provided to the Company a satisfaction of judgment, releasing the Company from the monetary obligations of the judgment imposed by the Court in its entirety.
Marine Polymer Technologies, Inc.
On May 11, 2005 the Company initiated a lawsuit for product disparagement and false advertising against Marine Polymer Technologies, Inc., a Delaware corporation (Marine Polymer). In the lawsuit, the Company alleged that Marine Polymer made defamatory and disparaging statements concerning the Company’s D-Stat® Dry hemostatic bandage. The Company sought relief in the form of an injunction to enjoin Marine Polymer from continuing to defame and disparage the Company’s products, damages as a result of such statements, and other costs, disbursements and attorneys’ fees. Marine Polymer brought a counter-claim against the Company including, among other claims, business defamation and product disparagement for statements allegedly made by the Company concerning Marine Polymer’s SyvekPatch®. Marine Polymer sought relief in the form of monetary damages, costs, disbursements and attorneys’ fees. The trial commenced on March 24, 2008 in the United States District Court for the District of Massachusetts. At the conclusion of the trial on April 7, 2008 the jury returned a verdict in favor of the Company and against Marine Polymer for product disparagement concerning statements made regarding the safety of the Company’s D-Stat Dry hemostat product. In its verdict, the jury found that Marine Polymer’s statements were false and disparaged the D-Stat Dry product and awarded the Company $4,500,000 in monetary damages. The jury rejected Marine Polymer’s counter-claims in their entirety. Following post trial motions, on June 30, 2008, the Court upheld the jury verdict, granted the Company’s request for a permanent injunction against Marine Polymer for the statements that the jury found were false, and added prejudgment interest on the jury verdict award in the amount of $592,124.
64
14. Commitments and Contingencies (Continued)
On July 14, 2008, Marine Polymer filed a Notice of Appeal with the U.S. First Circuit Court of Appeals seeking to overturn the monetary damages and injunction issued against them. Marine Polymer did not appeal the Court’s rejection of its counter-claims against the Company. On February 4, 2009, oral arguments by both parties on Marine Polymer’s appeal were heard by the U.S. First Circuit Court of Appeals. On December 23, 2009, the U.S. First Circuit Court of Appeals affirmed the judgment against Marine Polymer for product disparagement. As a result, the permanent injunction issued at the conclusion of the trial will remain in effect, prohibiting Marine Polymer and its representatives from making, publishing or disseminating certain disparaging statements concerning the safety of our D-Stat products. Addressing the jury’s award of $4.5 million in damages, the Court determined that, due to differences in opinion among the judges, the Company could either accept a $2.7 million award of damages (plus interest) or insist upon a new trial limited to the issue of determining the reasonable amount of damages. The Company has accepted the $2.7 million award of damages plus interest, received $3.56 million from Marine Polymer on January 22, 2010, and will record the amount as litigation gain during the first quarter of 2010 (See Note 16).
VNUS® Medical Technologies (acquired by Covidien) Litigation
On October 13, 2005, the Company was named as one of three defendants in an intellectual property lawsuit brought by VNUS® Medical Technologies, Inc. (VNUS) in the United States District Court for the Northern District of California. The complaint requested a judgment that the Company’s Vari-Lase® procedure kit and Vari-Lase® laser console infringe on four patents held by VNUS and asked for relief in the form of an injunction that would prevent the Company from selling the Company’s Vari-Lase® products, compensatory and treble damages caused by the manufacture and sale of these products, and other costs, disbursements and attorneys’ fees. VNUS subsequently indicated that it was not pursuing its allegation of infringement concerning one of the four patents. On June 2, 2008, the Company entered into a settlement agreement with VNUS for the purpose of resolving the lawsuit. Under the terms of the settlement agreement, (i) on June 4, 2008, the Company paid VNUS a royalty payment in the aggregate amount of $3,116,000 related to all Vari-Lase products shipped within the United States through the end of the first quarter of 2008, (ii) the Company agreed to pay a quarterly royalty on all on-going U.S. shipments of Vari-Lase laser and kit products payable quarterly during the remaining life of the applicable patents, (iii) VNUS granted the Company a non-exclusive and non-sublicensable license to the applicable patents for use in endovenous laser therapy, and (iv) all litigation between the parties was dismissed.
AngioDynamics, Inc. Litigation
On July 29, 2009 AngioDynamics, Inc. (AngioDynamics) filed a lawsuit against the Company in the U.S. District Court for the District of Delaware, alleging that the Company has infringed U.S. Patent No. 7,273,478 and U.S. Patent No. 7,559,329. Specifically, AngioDynamics alleges that doctors using the Company’s Bright Tip fibers and procedure kits are using the methods claimed in those patents, and accuses the Company of inducing and contributing to infringement. AngioDynamics has requested injunctive relief and compensatory damages. On December 1, 2009 the Company filed its answer, a counterclaim, and a motion to transfer the case to the U.S. District Court for the District of Minnesota.
The Company has also filed an inter partes request for reexamination of both the ‘478 and ‘329 patents with the United States Patent Office. The Patent Office granted the inter partes requests for reexamination for both the ‘478 and ‘329 patents. The Company expects the reexamination process for each patent will take at least three years to conclude. On January 27, 2010, the Company filed a motion with the U.S. District Court for the District of Delaware to stay all proceedings in the litigation pending the outcome of the inter partes reexaminations.
65
14. Commitments and Contingencies (Continued)
From time to time, the Company is involved in additional legal proceedings arising in the normal course of business. As of the date of this report the Company is not a party to any legal proceeding not described in this section in which an adverse outcome would reasonably be expected to have a material adverse effect on the Company’s results of operations or financial condition.
King Agreements
On January 9, 2007, the Company entered into three separate agreements with King: a License Agreement, a Device Supply Agreement and a Thrombin-JMI® Supply Agreement. Under the License Agreement, the Company licensed the exclusive rights to the Company’s products Thrombi-Pad, Thrombi-Gel and Thrombi-Paste to King in exchange for a one-time license fee of $6,000,000. Under the Device Supply Agreement, the Company agreed to manufacture the licensed products for sale to King in exchange for two separate $1,000,000 milestone payments; one upon the first commercial sale of Thrombi-Gel (which was received on May 31, 2007), and one upon the first commercial sale of Thrombi-Paste. The Company is amortizing the $6,000,000 license fee on a straight-line basis over 10 years. The Company is amortizing the $1,000,000 milestone payment that was received on May 31, 2007 over the remaining 10-year license period and will amortize the additional $1,000,000 milestone payment over the remaining 10-year license period when it is received. In 2009 King suspended further development of all Thrombi-Paste products. The unamortized license fee was $4,945,000, $5,649,000 and $6,353,000 at December 31, 2009, 2008 and 2007, respectively. The amortization of license fee was $704,000, $704,000 and $647,000 for the years ended December 31, 2009, 2008 and 2007, respectively.
Under the Device Supply Agreement the Company agreed to pursue a surgical indication for the use of the Thrombi-Gel and Thrombi-Paste products from the FDA. The Device Supply Agreement requires the Company to make a one-time payment of $2,500,000 to King if the FDA does not approve the surgical indication of Thrombi-Gel and a one-time payment of $2,500,000 to King if the FDA does not approve the surgical indication of Thrombi-Paste after performing a clinical study and submitting the application. The Company believes the probability of paying these one-time payments to King is remote, and therefore has not recorded any provision for these payments.
Nicolai, GmbH Agreement
Effective April 1, 2008 the Company entered into a five-year distribution agreement with Nicolai, GmbH. As a result of entering into this distribution agreement, the Company no longer maintains a direct sales force in Germany. In connection with this distribution agreement, the Company received 500,000 Euros from Nicolai, GmbH. which was deferred and is being recognized ratably over the five-year term of the distribution agreement.
The agreement also includes provisions requiring the Company to pay Nicolai, GmbH specific amounts if the Company terminates the distribution agreement prior to the end of the five-year term. The Company does not intend to terminate the distribution agreement and, as such, has not recorded a liability relating to these potential future payments to Nicolai, GmbH. The unamortized license fee was $472,000 and $617,000 at December 31, 2009 and 2008, respectively. The amortization of license fee was $145,000 and $114,000 for the year ended December 31, 2009 and 2008, respectively.
66
15. Quarterly Financial Data (Unaudited, in Thousands, Except per Share Data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
Fourth Quarter |
|
Third |
|
Second Quarter |
|
First |
|
||||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product |
|
$ |
17,712 |
|
$ |
16,817 |
|
$ |
16,781 |
|
$ |
15,416 |
|
|
License and collaboration |
|
|
510 |
|
|
411 |
|
|
385 |
|
|
395 |
|
|
Total revenue |
|
|
18,222 |
|
|
17,228 |
|
|
17,166 |
|
|
15,811 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selected costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product |
|
|
6,219 |
|
|
5,718 |
|
|
5,765 |
|
|
5,215 |
|
|
Collaboration |
|
|
295 |
|
|
199 |
|
|
173 |
|
|
183 |
|
|
Total selected costs and expenses: |
|
|
6,514 |
|
|
5,917 |
|
|
5,938 |
|
|
5,398 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
2,228 |
|
|
2,178 |
|
|
2,247 |
|
|
1,513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
|
1,623 |
|
|
1,431 |
|
|
1,386 |
|
|
938 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per share |
|
$ |
0.10 |
|
$ |
0.09 |
|
$ |
0.09 |
|
$ |
0.06 |
|
|
Diluted net income (loss) per share |
|
$ |
0.10 |
|
$ |
0.09 |
|
$ |
0.09 |
|
$ |
0.06 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product |
|
$ |
16,059 |
|
$ |
15,089 |
|
$ |
14,872 |
|
$ |
13,737 |
|
|
License and collaboration |
|
|
345 |
|
|
376 |
|
|
366 |
|
|
377 |
|
|
Total revenue |
|
|
16,404 |
|
|
15,465 |
|
|
15,238 |
|
|
14,114 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selected costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product |
|
|
5,619 |
|
|
5,130 |
|
|
5,360 |
|
|
4,581 |
|
|
Collaboration |
|
|
126 |
|
|
138 |
|
|
187 |
|
|
181 |
|
|
Total selected costs and expenses: |
|
|
5,745 |
|
|
5,268 |
|
|
5,547 |
|
|
4,762 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
1,457 |
|
|
1,748 |
|
|
(444 |
) |
|
254 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
|
14,684 |
|
|
1,722 |
|
|
(468 |
) |
|
235 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per share |
|
$ |
0.93 |
|
$ |
0.11 |
|
$ |
(0.03 |
) |
$ |
0.02 |
|
|
Diluted net income (loss) per share |
|
$ |
0.90 |
|
$ |
0.11 |
|
$ |
(0.03 |
) |
$ |
0.01 |
|
16. Subsequent Event
On January 22, 2010 the Company received $3.56 million dollars as payment in full for the judgment against Marine Polymer in the lawsuit initiated by the Company for product disparagement and false advertising which was affirmed by the U.S. First Circuit Court of Appeals on December 23, 2009. The Company will record the $3.56 million as a litigation gain in the first quarter of 2010.
67
