Attached files
China Pediatric Pharmaceuticals, Inc.
As filed with the United States Securities and Exchange Commission on January 28, 2010
Registration No. 000-52007
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CHINA PEDIATRIC PHARMACEUTICALS, INC.
Formerly Lid Hair Studios International, Inc.
(Name of small business issuer in its charter)
|
Nevada |
2834 |
20-271 8075 |
|
(State or other jurisdiction of
incorporation or organization) |
(primary standard industrial
classification code number) |
(I.R.S. Employer
Identification No.) |
9th Floor, No. 29 Nanxin Street,
Xi’an, Shaanxi Province
P.R.C., 710004
86 29 8727 1818
(Address and telephone number of principal executive offices and principal place of business)
__________
Copies to:
William Macdonald
MACDONALD TUSKEY
CORPORATE AND SECURITIES LAWYERS
(W.L. Macdonald Law Corporation)
1210 - 777 Hornby Street
Vancouver BC V6Z 1S4
Tel: 604.689.1022 Fax: 604.681.4760
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer |
¨ |
|
Accelerated Filer |
¨ |
|
Non-Accelerated Filer |
¨ |
Smaller Reporting Company |
þ |
If the delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. ¨
|
Title Of Each
Class of Securities
To be Registered |
Amount To
Be Registered |
Proposed
Maximum
Offering Price
Per Share(1) |
Proposed
Maximum
Aggregate
Offering Price |
Amount of
Registration Fee(3) |
||||||||||||
|
Common stock, par value $0.001 per share (2)(3) |
1,250,000 | $ | 3.00 | $ | 3,750,000 | $ | 267.38 | |||||||||
|
Common stock, par value $0.001 per share (2)(3) |
1,250,000 | $ | 5.00 | $ | 6,250,000 | $ | 445.63 | |||||||||
|
Total |
2,500,000 | $ | 10,000,000 | $ | 713.00 | |||||||||||
———————
(1) The price is estimated in accordance with Rule 457(h)(1) under the Securities Act of 1933, as amended, solely for the purpose of calculating the registration fee. Our estimate is based on the average of the high and low prices for our common stock as reported on the National Association of Securities Dealers Inc.'s
OTC Bulletin Board on January 28, 2010
(2) An indeterminate number of additional shares of common stock shall be issuable pursuant to Rule 416 to prevent dilution resulting from stock splits, stock dividends or similar transactions and in such an event the number of shares registered shall automatically be increased to cover the additional shares in accordance
with Rule 416 under the Securities Act.
(3) We are registering up to 2,500,000 shares of our common stock that we may issue upon the exercise of share purchase warrants.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities
Act of 1933, as amended, or until the registration statement shall become effective on such date as the United States Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the United States Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state
where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED January 28, 2010
PRELIMINARY PROSPECTUS
CHINA PEDIATRIC PHARMACEUTICALS, INC.
2,500,000 shares of common stock
This prospectus relates to the resale of up to 2,500,000 shares of our common stock, $0.001 par value per share, by certain of our stockholders. These persons, together with their transferees, are referred to throughout this prospectus as “selling stockholders.”
We issued all of the shares described above in private placement transactions completed prior to the filing of this registration statement.
The selling stockholders may offer to sell the shares of common stock being offered in this prospectus at fixed prices, at prevailing market prices at the time of sale, at varying prices or at negotiated prices. Our common stock is quoted on the OTC Bulletin Board under the symbol "CPDU". On January 28, 2010, the closing
bid price for one share of our common stock on the OTC Bulletin Board was $2.38.
You should consider carefully the risk factors beginning on page 4 of this prospectus.
Neither the United States Securities and Exchange Commission nor any state securities commission has approved of these securities or determined that this prospectus is accurate or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is:
You should rely only on the information contained in the prospectus. We have not authorized anyone to provide you with information or to make any representations about us, the selling stockholders, the securities or any matter discussed in this prospectus, other than that contained in the prospectus. If any other information
or representation is given or made, such information or representations may not be relied upon as having been authorized by us or any selling stockholder. The selling stockholders are offering to sell and seeking offers to buy shares of our common stock only in jurisdictions where offers and sales are permitted. This prospectus does not constitute an offer to sell, or a solicitation of an offer to buy the securities in any circumstances under which the offer or solicitation is unlawful. The information contained
in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock. Neither the delivery of this prospectus nor any distribution of securities in accordance with this prospectus shall, under any circumstances, imply that there has been no change in our affairs since the date of this prospectus. The prospectus will be updated and updated prospectuses made available for delivery to the extent required by the federal securities
laws.
When used in this prospectus, the terms:
|
|
● |
“Asia Pharm,” refers to Asia Pharm Holdings, Inc., a British Virgin Islands Company |
|
|
● |
“China Pediatric,” “China Pediatric Pharmaceuticals,” “Lid Hair Studios International, Inc.,” “CPDU,” “LHSI,” the “Company,” “we,” “our” and “us” refers to China Pediatric Pharmaceuticals, Inc., formerly Lid Hair Studios International, Inc. a Nevada corporation. |
|
|
● |
“Coova Children Pharmaceuticals Co. Ltd.” refers to our wholly owned subsidiary China Children Pharmaceuticals Co., Ltd., a limited liability company organized under the laws of the Hong Kong. |
|
|
● |
“China Children Pharmaceuticals” refers to our variable interest entity (“VIE”), Shaanxi Jial Pharmaceutical Co., Ltd. a company organized under the laws of the PRC that has entered into a series of agreements with Coova Children Pharmaceuticals Co. Ltd. that (i) give Coova Children Pharmaceuticals Co. Ltd. control over the board of directors, officers, operations and finances of China
Children Pharmaceuticals; (ii) permit China Children Pharmaceuticals to be treated as a subsidiary of Coova Children Pharmaceuticals Co. Ltd. under the laws of the People’s Republic of China; and (iii) allow us to consolidate its financial statements under GAAP. |
|
|
● |
“WOFE” refers to Coova Children Pharmaceuticals Co. Ltd. which is a “wholly owned foreign enterprise” under the laws of the People’s Republic of China. |
|
|
● |
“China Children Shareholders” refers to the shareholders of China Children Pharmaceuticals who are also some of the shareholders of China Pediatric Pharmaceuticals. |
|
|
● |
“Yuan” or “RMB” refer to the Chinese yuan (also known as the Renminbi). According to the currency website xe.com, as of December 16, 2009, $1 = 6.82990 yuan. |
|
|
● |
“PRC” or “China” refers to the People’s Republic of China. |
|
|
● |
“SFDA” refers to the PRC State Food and Drug Administration |
|
|
● |
“OTC Bulletin Board” or the “OTCBB” refers to the Over-the-Counter Bulletin Board, an electronic quotation system for equity securities overseen by the Financial Industry Regulatory Authority (formerly the National Association of Securities Dealers), which is accessible through its website at WWW.OTCBB.COM |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND OTHER INFORMATION CONTAINED IN THIS PROSPECTUS
This prospectus contains some forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties. Forward-looking statements
include statements regarding, among other things, (a) projected sales, profitability, and cash flows, (b) growth strategies, (c) anticipated trends, (d) future financing plans and (e) anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,” “should,” “anticipates,” “estimate,” “plans,” “potential,” “projects,” “continuing,” “ongoing,” “expects,”
“management believes,” “we believe,” “we intend,” or the negative of these words or other variations on these words or comparable terminology. These statements may be found under “Management’s Discussion and Analysis of Financial Condition and Plan of Operation” and “Business,” as well as in this prospectus generally. In particular, these include statements relating to future actions, prospective products or product approvals, future performance
or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results.
Any or all of the forward-looking statements in this report may turn out to be inaccurate. They can be affected by inaccurate assumptions we might make or by known or unknown risks or uncertainties. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially as a result of various factors, including,
without limitation, the risks outlined under “Risk Factors” and matters described in the prospectus generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this filing will in fact occur. You should not place undue reliance on these forward-looking statements.
This summary highlights selected information contained elsewhere in this prospectus. It is not complete and does not contain all of the information that you should consider before investing in our common stock. To understand this offering fully, you should read the entire prospectus carefully, including “Risk Factors”
and the Consolidated Financial Statements and the Related Notes.
Shaanxi Jiali was incorporated in the PRC under the name Shaanxi Jiali Technologies Co, Ltd. on July 1, 1998. In October 2000, Shaanxi Jiali Technologies changed its name to Shaanxi Jiali Pharmaceutical Co. Ltd. Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August
4, 2008, Asia-Pharm through its wholly owned subsidiary China Children Pharmaceutical, Inc. (“China Children”), became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and pharmaceutical developer, manufacturer and marketer in the PRC. China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm. Xi-an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) is a “wholly
owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly owned subsidiary of China Children. On August 4, 2008, an Entrustment Management Agreement was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China Children shall receive all net profits and assume all operational
losses of Shaanxi Jiali through Xi’an Coova.
As more fully described in Item 2.01 below, we acquired China Children Pharmaceutical, Inc. (“China Children”), a Hong Kong based pharmaceutical manufacturer and distributor company in accordance with a Share Exchange Agreement dated September 30, 2009 (“Exchange Agreement”) made by and among Lid Hair Studios International,
Inc., (“Company”) a Nevada corporation, Eric Anderson the (“Principal Company Shareholder”), and Asia-Pharm Holding (“Asia-Pharm”), Inc. a company incorporated in the British Virgin Islands, the sole shareholder of China Children.
The close of the Share Exchange transaction (the "Closing") took place on September 30, 2009 (the “Closing Date”). On the Closing Date, pursuant to the terms of the Exchange Agreement, we acquired all of the outstanding capital stock and ownership interests of China Children Pharmaceuticals Inc.(the “Interests”)
from Asia-Pharm and they transferred and contributed all of their Interests to us. In exchange, we issued to Asia-Pharm 7,000,000 shares of our common stock.
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm through its wholly owned subsidiary China Children became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and pharmaceutical developer, manufacturer and marketer
in the PRC.
China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm.
Xi-an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) was a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly owned subsidiary of China Children.
Shaanxi Jiali was incorporated in the PRC under the name Shaanxi Jiali Technologies Co, Ltd. on July 1, 1998. In October 2000, Shaanxi Jiali Technologies changed its name to Shaanxi Jiali Pharmaceutical Co. Ltd. Shaanxi Jiali is our operating company and is in the business of manufacturing, marketing and sales of
pharmaceuticals in China with pediatric medicine as its focus.
As described below, on August 4, 2008, an Entrustment Management Agreement (described in detail on page X) was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China
Children shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
Xi’an Coova acquired management control over Shaanxi Jiali, in the same manner as if it were a wholly owned subsidiary under PRC law.
China Children, Xi’an Coova, and Shaanxi Jiali shall be referred to herein collectively as “Shaanxi Jiali." Shaanxi Jiali is principally engaged in the development and distribution of medicines, active pharmaceutical ingredients and pharmaceutical intermediaries.
This transaction is discussed more fully in Section 2.01 of this Report. The information therein is hereby incorporated in this Section 1.01 by reference.
Pursuant to the share exchange agreement of September 30, 2009, Eric Anderson transferred 5,000,000 shares of common stock of Lid Hair Studios to Lid Hair Studios for cancellation and forgives the outstanding shareholder’s loan to the company in the amount of $86,173in exchange for 100% of the issued and outstanding shares of Lid Hair
Studios’ wholly-owned subsidiary, Belford Enterprises B.C. Ltd., d/b/a Lid Hair Studio. Lid Hair Studios cancelled the shares of common stock received from Eric Anderson prior to affecting the reverse stock split which reduce the amount of common stock then issued and outstanding. Eric Anderson and Lid Hair Studios entered into a binding agreement with respect to such transaction on September 30, 2009.
Our operations are headquartered in Xi’an, Shaanxi Province, China. We are a profitable, mid-sized Chinese pharmaceutical company that identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional and Traditional Chinese Medicines (“TCMs”),
for the treatment of some of the most common ailments and diseases, with pediatric medicine as its focus.
We currently have one operating company: Shaanxi Jiali identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional and Traditional Chinese Medicines (“TCMs”), for the treatment of some of the most common ailments and diseases, with pediatric medicine
as its focus.
Our operating company has its own manufacturing facility located in Baoji, Shaanxi Province. Our manufacturing facility was issued a Good Manufacturing Practices1 (“GMP”) certification by the SFDA2 in
2004. We were awarded the National High-tech Enterprise Award by the Shaanxi Technology Administration in 2006. As a result of receiving the National High-tech Award Shaanxi Jiali is entitled to the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Shaanxi Jiali may enjoy an
exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax.
We distribute our high value, branded medicines, both prescription and OTC, through exclusive territory agents who sell our products directly to local pharmacies who in turn sell them to their retail customers.
Lid Hair Studios International, Inc. was established on April 20, 2005 in the state of Nevada. The company was originally incorporated under the name Belford Enterprises, Inc. and changed its name to Lid Hair Studios International Inc. on August 15, 2005. Our operating subsidiary Belford Enterprises B.C. Ltd., doing business
as Lid Hair Studio was established June 7, 2005, in the City of Vancouver, British Columbia Canada. There are no bankruptcies, receivership, or similar proceedings against the parent or operating subsidiary.
On June 18, 2005, the company purchased hair salon equipment and the rights to an office lease from Farideh Jafari in the amount of approximately $39,150US ($45,000CDN).
___________________________
1 Good Manufacturing Practices (“GMP”) is an internationally-recognized standard for pharmaceutical plant design and construction. GMP has been defined as “that part of quality assurance which ensures that products are
consistently produced and controlled to the quality standards appropriate for their intended use and as required by the marketing authorization” (World Health Organization). GMP covers all aspects of the manufacturing process: defined manufacturing process; validated critical manufacturing steps; suitable premises, storage, transport; qualified and trained production and quality control personnel; adequate laboratory facilities; approved written procedures and instructions; records to show all steps of
defined procedures taken; full traceability of a product through batch processing records and distribution records; and systems for recall and investigation of complaints.
2 The Chinese government agency, SFDA, is analogous to the Food and Drug Administration (“FDA”) in the United States. Unlike the FDA, however, the SFDA provides intellectual property and competitive protection to certain classes
of approved drugs.
On July 4, 2005 we entered into a lease agreement by way of Assignment of Lease by Tenant with Landlord’s Consent. The lease commenced July 1, 2005 and terminates June 30, 2015.
Effective on October 9, 2009, the Company changed its name from Lid Hair Studios International Inc. to China Pediatric Pharmaceuticals, Inc. The name change was effected through a parent/subsidiary merger of our wholly-owned subsidiary, China Pediatric Pharmaceuticals, Inc., with and into the Company, with the Company as the surviving
corporation. To effectuate the merger, the Company filed its Articles of Merger with the Nevada Secretary of State and the merger became effective on October 9, 2009. A copy of the filed Articles of Merger is being filed under Exhibit 3.3. The Company’s board of directors approved the merger which resulted in the name change on October 9, 2009. In accordance with Section 92A.180 of the Nevada Revised Statutes,
shareholder approval of the merger was not required. On the effective date of the merger, the Company’s name was changed to “China Pediatric Pharmaceuticals, Inc.” and the Company’s Articles of Incorporation were amended to reflect this name change. Following the Combination, the combined Company will continue to be traded on the OTCBB, under the symbol “LHSI”, however we will request a new symbol closer in form to China Pediatric Pharmaceuticals, Inc.
Shaanxi Jiali was incorporated in the PRC under the name Shaanxi Jiali Technologies Co, Ltd. on July 1, 1998. In October 2000, Shaanxi Jiali Technologies changed its name to Shaanxi Jiali Pharmaceutical Co. Ltd. Shaanxi Jiali is our operating company and is in the business of manufacturing, marketing and sales of
pharmaceuticals in China with pediatric medicine as its focus.
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm through its wholly owned subsidiary China Children Pharmaceutical, Inc. (“China Children”), became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and
pharmaceutical developer, manufacturer and marketer in the PRC.
China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm.
Xi-an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) is a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly owned subsidiary of China Children.
As described below, on August 4, 2008, an Entrustment Management Agreement (described in detail on page X) was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China
Children shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
Xi’an Coova entered into a Management Entrustment Agreement with Shaanxi Jiali and the shareholders of Shaanxi Jiali (the “Management Entrustment Agreement”), in which Shaanxi Jiali and its shareholders agreed to transfer control, or entrust, the operations and management of its business to Xi’an Coova. Under
the agreement, Xi’an Coova manages the operations and assets of Shaanxi Jiali , controls all of the cash flows of Shaanxi Jiali through a bank account controlled by Xi’an Coova, is entitled to 100% of earnings before tax of Shaanxi Jiali, a management fee, and is obligated to pay all payables and loan payments of Shaanxi Jiali. In addition, under the terms of the Management Entrustment Agreement, Xi’an Coova has been granted certain rights which include, in part, the right to appoint and terminate
members of Shaanxi Jiali’s Board of Directors, hire management and administrative personnel and control decisions relating to entering and performing customer contracts and other instruments. We anticipate that Shaanxi Jiali will continue to be the contracting party under its customer contracts, bank loans and certain other instruments unless Xi’an Coova exercises its option. The agreement does not terminate unless the business of Shaanxi Jiali is terminated or Xi’an Coova exercises its option
to acquire all of the assets or equity of Shaanxi Jiali under the terms of the Exclusive Option Agreement as more fully described below and completes the acquisition of Shaanxi Jiali.
In exchange for causing Shaanxi Jiali to enter into the Management Entrustment Agreement, Xi’an Coova, through its parent company Asia-Pharm, issued an aggregate of 12,000,000 shares of Asia-Pharm’s common stock to the shareholders of Shaanxi Jiali which was allocated based on their respective pro rata ownership of Shaanxi Jiali.
In order to give Xi’an Coova further control over Shaanxi Jiali, the Shaanxi Jiali shareholders and Xi’an Coova, a wholly owned subsidiary of China Children in the PRC, entered into a shareholders’ Voting Proxy Agreement (the “Voting Proxy
Agreement”) whereby the Shaanxi Jiali shareholders irrevocably and exclusively appointed the members of Xi’an Coova’s board of directors, as their proxies to vote on all matters that require Shaanxi Jiali shareholder approval, including, without limitation, the right to appoint members of the board of directors of Shaanxi
Jiali. The agreement further provides that Shaanxi Jiali will appoint all of the board of directors of Xi’an Coova as its board of directors. Xi’an Coova’s board of directors changes, Shaanxi Jiali must remove and appoint new members to its board. The agreement terminates upon the exercise of the option by Xi’an Coova to purchase the shares of Shaanxi Jiali as described below and is governed by the laws of the PRC.
In order to permit Shaanxi Jiali to become an indirectly wholly owned subsidiary of China Children when permitted under PRC law, Xi’an Coova, Shaanxi Jiali and the Shaanxi Jiali shareholders entered into an exclusive option agreement (the “Exclusive Option Agreement”) whereby the Shaanxi Jiali shareholders granted Xi’an
Coova an irrevocable and exclusive purchase option (the “Option”) to acquire Shaanxi Jiali’s equity and/or remaining assets, but only to the extent that the acquisition does not violate limitations imposed by PRC law on such transactions. Current PRC law does not specifically provide for the equity of a non-PRC entity to be used as consideration for the purchase of a PRC entity’s assets or equity unless the value of the shares are equal to or greater than the value of the enterprise acquired.
In addition, there is a lengthy appraisal process which must be approved by the provincial PRC government entities. The consideration for the exercise of the Option is to be determined by the parties and memorialized in future definitive agreements setting forth the kind and value of such consideration. Accordingly, we will consider exercising the Option under such circumstances we believe will be in our best interests and our shareholders and the Exclusive Option Agreement has been drafted to give us such flexibility.
In considering whether or not we will exercise the Option we may consider such factors as (1) if the exercise price can be lower than the appraised value under current PRC law (2) availability of funds, (3) any relevant tax considerations at the time, (4) any other relevant PRC laws that may exist at the time, (5) the value of our shares that were previously paid to shareholders of Shaanxi Jiali, and (6) whether or not the exercise of the Option will provide any other additional benefits to us or our shareholders. Upon
exercise of the Option, the parties will prepare transfer documents to be submitted for governmental approval and work together to obtain all approvals and permits. The agreement may be terminated by agreement of all parties or by with 30 days notice and is governed by the laws of the PRC.
In order to further solidify China Children’s rights, benefits and control of Shaanxi Jiali through its ownership of Xi’an Coova under the Entrusted Management Agreement, Voting Proxy Agreement and Exclusive Option Agreement, Xi’an Coova and the Shaanxi Jiali shareholders entered into a share pledge agreement (the
“Share Pledge Agreement”) whereby the Shaanxi Jiali shareholders pledged all of their equity interests in Shaanxi Jiali, including the proceeds thereof, to guarantee the performance by the shareholders of all of the agreements they entered into with Xi’an Coova. Upon breach by any of the shareholders of any of the Management Entrustment Agreement, Voting Proxy Agreement, the Exclusive Option Agreement or the Share Pledge Agreement, Xi’an Coova is entitled by operation of law to become
the beneficial owner of the shares of Shaanxi Jiali. Prior to termination of the Share Pledge Agreement, the pledged equity interests of Shaanxi Jiali cannot be transferred without Xi’an Coova’s prior written consent. The provisions of the Share Pledge Agreement and the Voting Proxy Agreement work together to give the board of directors of Xi’an Coova control over transfers by the shareholders of Shaanxi Jiali . The agreement will not terminate until agreed to by all of the parties
in writing and is governed by the laws of the PRC.
On September 30, 2009, we acquired a Hong Kong based pharmaceutical manufacturer and distributor company in accordance with a Share Exchange Agreement dated September 30, 2009 (“Exchange Agreement”) made by and among Lid Hair Studios International, Inc., (“Company”) a Nevada corporation, Eric Anderson
the (“Principal Company Shareholder”), and Asia-Pharm Holding (“Asia-Pharm”), Inc. a company incorporated in the British Virgin Islands.
The close of the Share Exchange transaction (the "Closing") took place on September 30, 2009 (the “Closing Date”). On the Closing Date, pursuant to the terms of the Exchange Agreement, we acquired all of the outstanding capital stock and ownership interests of China Children Pharmaceuticals Inc.(the “Interests”)
from Asia-Pharm, and they transferred and contributed all of their Interests to us. In exchange, we issued to Asia-Pharm 7,000,000 shares of our common stock.
Shaanxi Jiali was incorporated in the PRC under the name Shaanxi Jiali Technologies Co, Ltd. on July 1, 1998. In October 2000, Shaanxi Jiali Technologies changed its name to Shaanxi Jiali Pharmaceutical Co. Ltd. Shaanxi Jiali is our operating company and is in the business of research and development, manufacturing,
marketing and sales, and distribution of pharmaceuticals in China, with pediatric medicine as its focus.
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm through its wholly owned subsidiary China Children Pharmaceutical, Inc. (“China Children”), became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and pharmaceutical
developer, manufacturer and marketer in the PRC.
China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm. Xi-an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) is a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly
owned subsidiary of China Children. On August 4, 2008, an Entrustment Management Agreement (described in detail on page 7) was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China Children shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
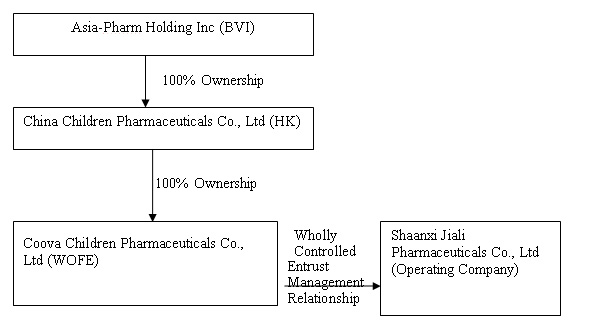
On September 30, 2009, Asia-Pharm, Eric Anderson and Lid Hair Studios, entered into a definitive Share Exchange Agreement (“Exchange Agreement”) for the combination of us and China Children Pharmaceuticals (the “Combination”). The Combination was accomplished by means of a share exchange in which Asia-Pharm exchanged
all of their stock in China Children Pharma for the new issuance of our common stock. Under the terms of the Exchange Agreement and as a result of the Combination:
|
● |
China Children became our wholly owned subsidiary; |
|
● |
In exchange for all of their shares of China Children stock, Asia-Pharm received 7,000,000 newly issued shares of our common stock. |
|
● |
Immediately following the closing of the Combination, such shares of CPPI common stock represent approximately 65.25% of our issued and outstanding shares on a fully diluted basis. |
The organization and ownership structure of the Company subsequent to the consummation of the combination as summarized in the paragraphs above is as follows:
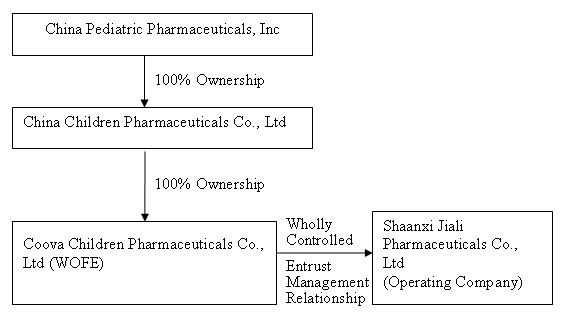
This transaction closed on September 30, 2009.
|
Common stock outstanding |
|
8,305,288 shares as of December 31, 2009. |
|
Common stock that may be offered by selling stockholders |
Up to 2,500,000 shares. | |
|
Total proceeds raised by offering |
Total Proceeds will be $10,000,000 covered from the shares of the prospectus. | |
|
Common Stock Outstanding after the offering |
10,805,288 | |
|
Risk factors |
There are significant risks involved in investing in our company. For a discussion of risk factors you should consider before buying our common stock, see “Risk Factors” beginning on page 4. These risk factors include:
·
Various risks of doing business in China since all of our revenues are derived from operations of China Children Pharmaceuticals in China including changes in political and economics in China, changes in the laws of the People’s Republic of China, exchange rate issues, difficulty in establishing management, legal and financial
controls;
·
The risk of the loss of China Children Pharmaceuticals as our operating business;
|
| Risks associated with Shaanxi Jiali’s business, which include among other, competition, ability to manage growth, discovery and development of new products, need for additional capital to fund operations, dependence on key personnel, need for regulatory approval of products, need to obtain renewal of licenses to
operate business, governmental price controls, products being replaced by newer products
·
Risk related to our common stock, which include but are not limited to the illiquidity of our stock price, need for internal controls, additional expenses due to regulatory requirements, penny stock regulations that will be applicable to our common stock. | ||
|
Use of Proceeds |
The shares of common stock offered hereby are being registered for the account of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of the common stock will go to the selling
stockholders and we will not receive any proceeds from the resale of the common stock by the selling stockholders, although we could receive proceeds
of up to $10,000,000 if all of the share purchase warrants are exercised. We will, however, incur all costs associated with this registration statement and prospectus. |
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated into this offering that are not historic facts are forward-looking
statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Business
|
● |
WE NEED TO MANAGE GROWTH IN OPERATIONS TO MAXIMIZE OUR POTENTIAL GROWTH AND ACHIEVE OUR EXPECTED REVENUES AND OUR FAILURE TO MANAGE GROWTH WILL CAUSE A DISRUPTION OF OUR OPERATIONS RESULTING IN THE FAILURE TO GENERATE REVENUE. |
In order to maximize potential growth in our current and potential markets, we believe that we must expand our manufacturing and marketing operations. This expansion will place a significant strain on our management and our operational, accounting, and information systems. We expect that we will need to continue to improve our financial controls,
operating procedures, and management information systems. We will also need to effectively train, motivate, and manage our employees. Our failure to manage our growth could disrupt our operations and ultimately prevent us from generating the revenues we expect.
|
● |
WE CANNOT ASSURE YOU THAT OUR ORGANIC GROWTH STRATEGY WILL BE SUCCESSFUL WHICH MAY RESULT IN A NEGATIVE IMPACT ON OUR GROWTH, FINANCIAL CONDITION, RESULTS OF OPERATIONS AND CASH FLOW. |
One of our strategies is to grow organically through increasing the distribution and sales of our products by penetrating existing markets in PRC and entering new geographic markets in PRC. However, many obstacles to entering such new markets exist, including, but not limited to, trade and tariff barriers, shipping and delivery costs, costs
associated with marketing efforts. We cannot, therefore, assure you that we will be able to successfully overcome such obstacles and establish our products in any additional markets. Our inability to implement this organic growth strategy successfully may have a negative impact on our growth, future financial condition, results of operations or cash flows.
|
● |
WE CANNOT ASSURE YOU THAT OUR ACQUISITION GROWTH STRATEGY WILL BE SUCCESSFUL RESULTING IN OUR FAILURE TO MEET GROWTH AND REVENUE EXPECTATIONS. |
In addition to our organic growth strategy, we also expect to grow through strategic acquisitions. We intend to pursue opportunities to acquire businesses in PRC that are complementary or related in product lines and business structure to us. We may not be able to locate suitable acquisition candidates at prices that we consider appropriate
or to finance acquisitions on terms that are satisfactory to us. If we do identify an appropriate acquisition candidate, we may not be able to negotiate successfully the terms of an acquisition, or, if the acquisition occurs, integrate the acquired business into our existing business. Acquisitions of businesses or other material operations may require debt financing or additional equity financing, resulting in leverage or dilution of ownership. Integration of acquired business operations could disrupt our business
by diverting management away from day-to-day operations. The difficulties of integration may be increased by the necessity of coordinating geographically dispersed organizations, integrating personnel with disparate business backgrounds and combining different corporate cultures. We also may not be able to maintain key employees or customers of an acquired business or realize cost efficiencies or synergies or other benefits we anticipated when selecting our acquisition candidates. In addition, we may need to
record write-downs from future impairments of intangible assets, which could reduce our future reported earnings. At times, acquisition candidates may have liabilities or adverse operating issues that we fail to discover through due diligence prior to the acquisition. In addition to the above, acquisitions in PRC, including of state owned businesses, will be required to comply with laws of the People's Republic of China ("PRC"), to the extent applicable. There can be no assurance that any given proposed acquisition
will be able to comply with PRC requirements, rules and/or regulations, or that we will successfully obtain governmental approvals which are necessary to consummate such acquisitions, to the extent required. If our acquisition strategy is unsuccessful, we will not grow our operations and revenues at the rate that we anticipate.
|
● |
IF WE ARE NOT ABLE TO IMPLEMENT OUR STRATEGIES IN ACHIEVING OUR BUSINESS OBJECTIVES, OUR BUSINESS OPERATIONS AND FINANCIAL PERFORMANCE MAY BE ADVERSELY AFFECTED. |
Our business plan is based on circumstances currently prevailing and the bases and assumptions that certain circumstances will or will not occur, as well as the inherent risks and uncertainties involved in various stages of development. However, there is no assurance that we will be successful in implementing our strategies or that our strategies,
even if implemented, will lead to the successful achievement of our objectives. If we are not able to successfully implement our strategies, our business operations and financial performance may be adversely affected.
|
● |
IF WE NEED ADDITIONAL CAPITAL TO FUND OUR GROWING OPERATIONS, WE MAY NOT BE ABLE TO OBTAIN SUFFICIENT CAPITAL AND MAY BE FORCED TO LIMIT THE SCOPE OF OUR OPERATIONS. |
If adequate additional financing is not available on reasonable terms, we may not be able to undertake plant expansion, purchase additional machinery and purchase equipment for our operations and we would have to modify our business plans accordingly. There is no assurance that additional financing will be available to us after this Offering.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competition;
(iii) the level of our investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
If we cannot obtain additional funding, we may be required to: (i) limit our investments in research and development; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures.
Such reductions could materially adversely affect our business and our ability to compete.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are acceptable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible
debt securities issued by us to obtain financing could have rights, preferences and privileges senior to our common stock. We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
|
●
|
SHAANXI JIAL IS CURRENTLY ENTITLED TO A BENEFICIAL TAX EXEMPTION FOR A FIVE YEAR PERIOD; HOWEVER, SUCH TAX EXEMPTION MAY BE INTERPRETED TO BE NOT IN COMPLIANCE WITH PRC TAX LAWS IN THE FUTURE CAUSING US TO SET ASIDE CERTAIN CONTINGENCY FUNDS FOR DEALING WITH POTENTIAL RETROSPECTIVE TAX LIABILITIES. |
Shaanxi Jiali is entitled to enjoy the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Shaanxi Jiali may enjoy
an exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax.
As to the tax treatment promised by local governments to purely domestic enterprises, i.e., Shaanxi Jiali, invested by non-local (but not foreign) investors under the so called preferential policy announced by local governments, our consultation with PRC certified public accountants and lawyers, is that the above policy is not compliant with
the PRC laws. Even though such practice exists in many areas across the country, the policy faces the risk of being ruled illegal at any time for non-compliance with relevant laws. In this event, there is a risk that we might be assessed retrospective tax liabilities.
|
● |
WE MAY HAVE DIFFICULTY DEFENDING OUR INTELLECTUAL PROPERTY RIGHTS FROM INFRINGEMENT RESULTING IN LAWSUITS REQUIRING US TO DEVOTE FINANCIAL AND MANAGEMENT RESOURCES THAT WOULD HAVE A NEGATIVE IMPACT ON OUR OPERATING RESULTS. |
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property as critical to our success. We rely on trademark, patent and trade secret law, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights. We have received trademark
and patent protection for certain of our products in the People's Republic of China. No assurance can be given that our patents and licenses will not be challenged, invalidated, infringed or circumvented, or that our intellectual property rights will provide competitive advantages to us. There can be no assurance that we will be able to obtain a license from a third-party technology that we may need to conduct our business or that such technology can be licensed at a reasonable cost.
Presently, we sell our products mainly in PRC. To date, no trademark or patent filings have been made other than in PRC. To the extent that we market our products in other countries, we may have to take additional action to protect our intellectual property. The measures we take to protect our proprietary rights may be inadequate and we cannot
give you any assurance that our competitors will not independently develop formulations and processes that are substantially equivalent or superior to our own or copy our products.
Currently, the SFDA does not automatically stay drug registration approval upon initiation of an infringement lawsuit by a third party. At present, we must wait until a copycat manufacturer has received marketing approval from SFDA before we can bring an infringement lawsuit. Furthermore, Chinese courts have been hesitant to issue preliminary
injunctions to suspend sales until a final judgment is issued in the lawsuit. Our sales could be lowered were a competitor to infringe our intellectual property rights by marketing one or more versions of SFDA-approved drugs proprietary to us, such as the Qiweiben capsule, until we can curtail such infringement through legal action. Pursuing infringement lawsuits would require us to devote financial and management resources that could impact the results of our operations.
|
● |
WE DEPEND ON THE SUPPLY OF RAW MATERIALS, AND ANY ADVERSE CHANGES IN SUCH INTENSE COMPETITION FROM EXISTING AND NEW ENTITIES MAY ADVERSELY AFFECT OUR REVENUES AND PROFITABILITY. |
We compete with other companies, many of whom are developing or can be expected to develop products similar to ours. Our markets are large with many competitors. The market for pharmaceutical raw materials manufacturing is particularly competitive. In this market, our competitors include a number of contract manufacturers and, from time to
time, demand for particular products may be greatly exceeded by production capacity. Many of our competitors are more established than we are, and have significantly greater financial, technical, marketing and other resources than us. Some of our competitors may have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive
terms to customers, and adopt more aggressive pricing policies. We intend to create greater brand awareness for our brand name so that we can successfully compete with our competitors. We cannot assure you that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
|
● |
OUR PRODUCTS AND THE PROCESSES COULD EXPOSE US TO SUBSTANTIAL PRODUCT LIABILITY CLAIMS WHICH WILL NEGATIVELY IMPACT OUR PROFITABILITY. |
We face an inherent business risk of exposure to product liability claims in the event that the use of our products is alleged to have resulted in adverse side effects. Side effects or marketing or manufacturing problems pertaining to any of our products could result in product liability claims or adverse publicity. These risks will exist
for those products in clinical development and with respect to those products that have received regulatory approval for commercial sale. To date, we have not experienced any product liability claims. However, that does not mean that we will not have any such claims with respect to our products in the future which will negatively impact our profitability.
|
● |
WE DEPEND ON OUR KEY MANAGEMENT PERSONNEL AND THE LOSS OF THEIR SERVICES COULD ADVERSELY AFFECT OUR BUSINESS. |
We place substantial reliance upon the efforts and abilities of our executive officers, Jun Xia, our Chairman and Chief Executive Officer and Minggang Xiao, our Chief Financial Officer. The loss of the services of any of our executive officers could have a material adverse effect on our business, operations, revenues or prospects. We do not
maintain key man life insurance on the lives of these individuals.
|
● |
WE MAY NEVER PAY ANY DIVIDENDS TO SHAREHOLDERS. |
We did not declare any dividends for the year ended December 31, 2008 and have not declared any dividends to date in 2009. Our board of directors does not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors, and will depend upon,
among other things, the results of our operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and, if dividends are paid, there is no assurance with respect to the amount of any such dividend.
|
● |
MANAGEMENT EXERCISES SIGNIFICANT CONTROL OVER MATTERS REQUIRING SHAREHOLDER APPROVAL WHICH MAY RESULT IN THE DELAY OR PREVENTION OF A CHANGE IN OUR CONTROL. |
Mr. Xia, our Chief Executive Officer, through his common stock ownership, currently has voting power equal to approximately 22.8% of our voting securities. As a result, management through such stock ownership exercises significant control over all matters requiring shareholder approval, including the election of directors and approval of significant
corporate transactions. This concentration of ownership in management may also have the effect of delaying or preventing a change in control of us that may be otherwise viewed as beneficial by shareholders other than management.
|
● |
WE MAY INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS. |
We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations
to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals
to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
|
● |
WE MAY NOT BE ABLE TO MEET THE ACCELERATED FILING AND INTERNAL CONTROL REPORTING REQUIREMENTS IMPOSED BY THE SECURITIES AND EXCHANGE COMMISSION RESULTING IN A POSSIBLE DECLINE IN THE PRICE OF OUR COMMON STOCK AND OUR INABILITY TO OBTAIN FUTURE FINANCING. |
As directed by Section 404 of the Sarbanes-Oxley Act, the Securities and Exchange Commission adopted rules requiring each public company to include a report of management on the company's internal controls over financial reporting in its annual reports. In addition, the independent registered public accounting firm auditing a company's financial
statements must
also attest to and report on management's assessment of the effectiveness of the company's internal controls over financial reporting as well as the operating effectiveness of the company's internal controls.
While we expect to expend significant resources in developing the necessary documentation and testing procedures required by Section 404 of the Sarbanes-Oxley Act, there is a risk that we may not be able to comply timely with all of the requirements imposed by this rule. In the event that we are unable to receive a positive attestation from
our independent registered public accounting firm with respect to our internal controls, investors and others may lose confidence in the reliability of our financial statements and our stock price and ability to obtain equity or debt financing as needed could suffer.
In addition, in the event that our independent registered public accounting firm is unable to rely on our internal controls in connection with its audit of our financial statements, and in the further event that it is unable to devise alternative procedures in order to satisfy itself as to the material accuracy of our financial statements
and related disclosures, it is possible that we would be unable to file our Annual Report on Form 10-K with the Securities and Exchange Commission, which could also adversely affect the market price of our common stock and our ability to secure additional financing as needed.
|
● |
WE MAY HAVE DIFFICULTY RAISING NECESSARY CAPITAL TO FUND OPERATIONS AS A RESULT OF MARKET PRICE VOLATILITY FOR OUR SHARES OF COMMON STOCK. |
In recent years, the securities markets in the United States have experienced a high level of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For
these reasons, our shares of common stock can also be expected to be subject to volatility resulting from purely market forces over which we will have no control. If our business development plans are successful, we may require additional financing to continue to develop and exploit existing and new technologies and to expand into new markets. The exploitation of our technologies may, therefore, be dependent upon our ability to obtain financing through debt and equity or other means.
Risks Relating to the People's Republic of China
|
● |
THE SALES PRICES OF SOME MEDICINES ARE CURRENTLY CONTROLLED BY THE CHINESE GOVERNMENT AND THAT MAY ADVERSELY AFFECT OUR BUSINESS. |
Prices paid by end consumers for many of our medicines are regulated by PRC's State Development and Reform Commission. PRC justifies its need to control the drug prices on the basis that, at present, only workers at state or private companies have health insurance. About 900 million rural Chinese people and 35 million urban unemployed Chinese
people lack insurance coverage and cannot afford expensive drugs. Our future profitability might suffer if a significant portion of our revenues were to be derived from products whose final selling prices were state-controlled and if those prices were held at levels close to or below our cost of sales.
|
● |
SALES OF OUR PRODUCTS COULD BE HARMED BY THE WIDESPREAD PRESENCE OF COUNTERFEIT MEDICATION IN PRC NEGATIVELY IMPACTING OUR PROFITABILITY. |
Chinese counterfeiting of pharmaceuticals and other products affecting public health has grown in tandem with counterfeiting and piracy of goods such as brand-name clothing, compact discs and computer software. Exact data are impossible to collect, but the FBI believes that more than half of the pharmaceuticals sold in PRC are counterfeit.
Examples of the seriousness of the problem include: six months after Viagra was introduced in 2002, state media reported that some 90 percent of little blue pills sold in Shanghai were counterfeit; and 192,000 Chinese patients were reported to have died in 2001 from fake drugs. Counterfeit products shrink markets for legitimate goods. This situation affects Shaanxi, Jiali and other major domestic and foreign drug manufacturers in PRC, especially for products marketed through the OTC rather than hospital channel.
However, we believe the Chinese authorities are becoming increasingly vigilant against counterfeiting. .
|
● |
THERE COULD BE CHANGES IN GOVERNMENT REGULATIONS TOWARDS THE PHARMACEUTICAL AND HEALTH SUPPLEMENT INDUSTRIES THAT MAY ADVERSELY AFFECT OUR GROWTH AND PROFITABILITY. |
The manufacture and sale of APIs in the PRC is heavily regulated by many state, provincial and local authorities. These regulations have significantly increased the difficulty and costs involved in obtaining and maintaining regulatory approvals for marketing new and existing products. Our future growth and profitability depend to a large extent
on our ability to obtain regulatory approvals.
The SFDA of PRC recently implemented new guidelines for licensing of APIs. All existing manufacturers with licenses were required to apply for GMP certifications and to receive approvals. However, should we fail to receive or maintain the GMP certifications under the new guidelines in the future; our businesses would be materially and adversely affected.
Moreover, the laws and regulations regarding acquisitions of the pharmaceutical industry in the PRC may also change and may significantly impact our ability to grow through acquisitions.
|
● |
CERTAIN POLITICAL AND ECONOMIC CONSIDERATIONS RELATING TO THE PRC COULD ADVERSELY AFFECT OUR COMPANY. |
The PRC is transitioning from a planned economy to a market economy. While the PRC government has pursued economic reforms since its adoption of the open-door policy in 1978, a large portion of the PRC economy is still operating under five-year plans and annual state plans. Through these plans and other economic measures, such as control on
foreign exchange, taxation and restrictions on foreign participation in the domestic market of various industries, the PRC government exerts considerable direct and indirect influence on the economy. Many of the economic reforms carried out by the PRC government are unprecedented or experimental, and are expected to be refined and improved. Other political, economic and social factors can also lead to further readjustment of such reforms. This refining and readjustment process may not necessarily have a positive
effect on our operations or future business development. Our operating results may be adversely affected by changes in the PRC's economic and social conditions as well as by changes in the policies of the PRC government, such as changes in laws and regulations (or the official interpretation thereof), measures which may be introduced to control inflation, changes in the interest rate or method of taxation, and the imposition of additional restrictions on currency conversion.
|
● |
THE RECENT NATURE AND UNCERTAIN APPLICATION OF MANY PRC LAWS APPLICABLE TO US CREATE AN UNCERTAIN ENVIRONMENT FOR BUSINESS OPERATIONS AND THEY COULD HAVE A NEGATIVE EFFECT ON US. |
The PRC legal system is a civil law system. Unlike the common law system, the civil law system is based on written statutes in which decided legal cases have little value as precedents. In 1979, the PRC began to promulgate a comprehensive system of laws and has since introduced many laws and regulations to provide general guidance on economic
and business practices in the PRC and to regulate foreign investment. Progress has been made in the promulgation of laws and regulations dealing with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. The promulgation of new laws, changes of existing laws and the abrogation of local regulations by national laws could have a negative impact on our business and business prospects. In addition, as these laws, regulations and legal requirements are relatively
recent, their interpretation and enforcement involve significant uncertainty.
|
● |
RECENT PRC REGULATIONS RELATING TO THE ESTABLISHMENT OF OFFSHORE SPECIAL PURPOSE COMPANIES BY PRC RESIDENTS MAY SUBJECT OUR PRC RESIDENT SHAREHOLDERS TO PERSONAL LIABILITY AND LIMIT OUR ABILITY TO INJECT CAPITAL INTO OUR PRC SUBSIDIARIES, LIMIT OUR PRC SUBSIDIARIES’ ABILITY TO DISTRIBUTE PROFITS TO US, OR OTHERWISE ADVERSELY AFFECT US. |
SAFE issued a public notice in October 2005, or the SAFE notice, requiring PRC residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of PRC companies, referred to in the notice as an “offshore special purpose company.”
PRC residents that are shareholders of offshore special purpose companies established before November 1, 2005 were required to register with the local SAFE branch before March 31, 2006. Our current beneficial owners who are PRC residents have registered with the local SAFE branch as required under the SAFE notice. The failure of these beneficial owners to timely amend their SAFE registrations pursuant to the SAFE notice or the failure of future beneficial owners of our company who are PRC residents
to comply with the registration procedures set forth in the SAFE notice may subject such beneficial owners to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to our company or otherwise adversely affect our business.
Other Risks
|
● |
CURRENCY CONVERSION AND EXCHANGE RATE VOLATILITY COULD ADVERSELY AFFECT OUR FINANCIAL CONDITION. |
The PRC government imposes control over the conversion of Renminbi (“RMB”) into foreign currencies. Under the current unified floating exchange rate system, the People's Bank of China publishes an exchange rate, which we refer to as the PBOC exchange rate, based on the previous day's dealings in the inter-bank foreign exchange
market. Financial institutions authorized to deal in foreign currency may enter into foreign exchange transactions at exchange rates within an authorized range above or below the PBOC exchange rate according to market conditions.
Pursuant to the Foreign Exchange Control Regulations of the PRC issued by the State Council which came into effect on April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which came into effect on July 1, 1996, regarding foreign exchange control, conversion of Renminbi into foreign
exchange by Foreign Investment Enterprises, or FIEs, for use on current account items, including the distribution of dividends and profits to foreign investors, is permissible. FIEs are permitted to convert their after-tax dividends and profits to foreign exchange and remit such foreign exchange to their foreign exchange bank accounts in the PRC. Conversion of Renminbi into foreign currencies for capital account items, including direct investment, loans, and security investment, is still under certain restrictions.
On January 14, 1997, the State Council amended the Foreign Exchange Control Regulations and added, among other things, an important provision, which provides that the PRC government shall not impose restrictions on recurring international payments and transfers under current account items.
Enterprises in the PRC (including FIEs) which require foreign exchange for transactions relating to current account items, may, without approval of the State Administration of Foreign Exchange, or SAFE, effect payment from their foreign exchange account or convert and pay at the designated foreign exchange banks by providing valid receipts
and proofs.
Convertibility of foreign exchange in respect of capital account items, such as direct investment and capital contribution, is still subject to certain restrictions, and prior approval from the SAFE or its relevant branches must be sought.
Since 1994, the exchange rate for Renminbi against the United States dollar has remained relatively stable, most of the time in the region of approximately RMB8.28 to $1.00. However, in 2005, the Chinese government announced that it would begin pegging the exchange rate of the Chinese Renminbi against a number of currencies, rather than just
the U.S. dollar and, the exchange rate for the Renminbi against the U.S. dollar became RMB8.02 to $1.00. As our operations are primarily in PRC, any significant revaluation or devaluation of the Chinese Renminbi may materially and adversely affect our cash flows, revenues and financial condition. We may not be able to hedge effectively against in any such case. For example, to the extent that we need to convert United States dollars into Chinese Renminbi for our operations, appreciation of this currency against
the United States dollar could have a material adverse effect on our business, financial condition and results of operations. Conversely, if we decide to convert Chinese Renminbi into United States dollars for other business purposes and the United States dollar appreciates against this currency, the United States dollar equivalent of the Chinese Renminbi we convert would be reduced. There can be no assurance that future movements in the exchange rate of Renminbi and other currencies will not have an adverse
effect on our financial condition. China Pediatric’s operating companies are FIEs to which the Foreign Exchange Control Regulations are applicable. There can be no assurance that we will be able to obtain sufficient foreign exchange to pay dividends or satisfy other foreign exchange requirements in the future.
|
● |
IT MAY BE DIFFICULT TO AFFECT SERVICE OF PROCESS AND ENFORCEMENT OF LEGAL JUDGMENTS UPON OUR COMPANY AND OUR OFFICERS AND DIRECTORS BECAUSE THEY RESIDE OUTSIDE THE UNITED STATES. |
As our operations are presently based in PRC and a majority of our directors and all of our officers reside in PRC, service of process on our company and such directors and officers may be difficult to effect within the United States. Also, our main assets are located in PRC and any judgment obtained in the United States against us may not
be enforceable outside the United States.
|
● |
ANY FUTURE OUTBREAK OF AVIAN INFLUENZA, OR THE ASIAN BIRD FLU, OR ANY OTHER EPIDEMIC IN PRC COULD HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS OPERATIONS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
Since mid-December 2003, a number of Asian countries have reported outbreaks of highly pathogenic avian influenza in chickens and ducks. Since all of our operations are in PRC, an outbreak of the Asian Bird Flu in PRC in the future may disrupt our business operations and have a material adverse effect on our financial condition and results
of operations. For example, a new outbreak of Asian Bird Flu, or any other epidemic, may reduce the level of economic activity in affected areas, which may lead to a reduction in our revenue if our clients cancel existing contracts or defer future expenditures. In addition, health or other government regulations may require temporary closure
of our offices, or the offices of our customers or partners, which will severely disrupt our business operations and have a material adverse effect on our financial condition and results of operations.
|
● |
OUR BUSINESS MAY BE AFFECTED BY UNEXPECTED CHANGES IN REGULATORY REQUIREMENTS IN THE JURISDICTIONS IN WHICH WE OPERATE. |
We are subject to many general regulations governing business entities and their behavior in PRC and in other jurisdictions in which we have, or plan to have, operations and market our products. In particular, we are subject to laws and regulations covering food, dietary supplements and APIs. Such regulations typically deal with licensing,
approvals and permits. Any change in product licensing may make our products more or less available on the market. Such changes may have a positive or negative impact on the sale of our products and may directly impact the associated costs in compliance and our operational and financial viability. Such regulatory environment also covers any existing or potential trade barriers in the form of import tariff and taxes that may make it difficult for us to import our products to certain countries and regions, such
as Hong Kong, which would limit our international expansion.
|
● |
SINCE MOST OF OUR ASSETS ARE LOCATED IN PRC, ANY DIVIDENDS OF PROCEEDS FROM LIQUIDATION IS SUBJECT TO THE APPROVAL OF THE RELEVANT CHINESE GOVERNMENT AGENCIES. |
Our assets are predominantly located inside PRC. Under the laws governing foreign invested enterprises in PRC, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. Any dividend payment will be subject to the decision of the board of directors and subject to foreign exchange
rules governing such repatriation. Any liquidation is subject to the relevant government agency's approval and supervision as well as the foreign exchange control. This may generate additional risk for our investors in case of dividend payment and liquidation.
Risks Associated with Our Shares of Common Stock
|
● |
OUR SHARES OF COMMON STOCK ARE VERY THINLY TRADED, AND THE PRICE MAY NOT REFLECT OUR VALUE AND THERE CAN BE NO ASSURANCE THAT THERE WILL BE AN ACTIVE MARKET FOR OUR SHARES OF COMMON STOCK EITHER NOW OR IN THE FUTURE. |
Our shares of common stock are very thinly traded, and the price if traded may not reflect our value. There can be no assurance that there will be an active market for our shares of common stock either now or in the future. The market liquidity will be dependent on the perception of our operating business and any steps that our management
might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, investors may not be able to liquidate their investment or liquidate it at a price that reflects the value of the business. If a more active market should develop, the price may be highly volatile. Because there may be a low price for our shares of common stock, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds
a broker willing to effect a transaction in the shares of our common stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price. Further, many lending institutions will not permit the use of such shares of common stock as collateral for any loans.
|
● |
WE MAY BE SUBJECT TO THE PENNY STOCK RULES WHICH WILL MAKE THE SHARES OF OUR COMMON STOCK MORE DIFFICULT TO SELL. |
We may be subject now and in the future to the SEC’s “penny stock” rules if our shares of common stock sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC
which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer's account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally
or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer's confirmation.
In addition, the penny stock rules require that prior to a transaction, the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any offerings
and reduce the trading activity for shares of our common stock. As long as our shares of common stock are subject to the penny stock rules, the holders of such shares of common stock may find it more difficult to sell their securities.
|
● |
SALES OF OUR CURRENTLY ISSUED AND OUTSTANDING STOCK MAY BECOME FREELY TRADABLE PURSUANT TO RULE 144 AND MAY DILUTE THE MARKET FOR YOUR SHARES AND HAVE A DEPRESSIVE EFFECT ON THE PRICE OF THE SHARES OF OUR COMMON STOCK. |
A substantial majority of our outstanding shares of common stock are "restricted securities" within the meaning of Rule 144 under the Securities Act. As restricted shares, these shares may be resold only pursuant to an effective registration statement or under the requirements of Rule 144 or other applicable exemptions from registration under
the Act and as required under applicable state securities laws. Rule 144 provides in essence that a person who has held restricted securities for a period of at least one year may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed the greater of 1% of a company's outstanding shares of common stock or the average weekly trading volume during the four calendar weeks prior to the sale (the four calendar week rule does not apply to companies quoted
on the OTC Bulletin Board). There is no limit on the amount of restricted securities that may be sold by a non-affiliate after the restricted securities have been held by the owner for a period of two years or more and such owner has not been an affiliate for the 90 day period prior to sale. A sale under Rule 144 or under any other exemption from the Act, if available, or pursuant to subsequent registrations of our shares of common stock, may have a depressive effect upon the price of our shares of common stock
in any active market that may develop.
|
● |
THERE CAN BE NO ASSURANCE THAT THE PRICE OF OUR SHARES OF COMMON STOCK WILL MEET OR EXCEED THE EXERCISE PRICE OF THE WARRANTS DURING THE EXERCISE PERIOD OR AT ANY TIME THEREAFTER. |
Unless the price of our shares of Common Stock equals or exceeds the exercise price of the Warrants at the time of such exercise, an Investor may not be able to exercise his Warrants profitably. There can be no assurance that the price of our shares of Common Stock will meet or exceed the exercise price of the Warrants during the exercise
period or at any time thereafter. Accordingly, should an Investor choose to exercise the Warrants, the value of our shares of Common Stock purchased upon such exercise may be less than the Warrant exercise price the Investor pays. The Warrant may be worthless and expire unexercised if the price of our shares of Common Stock does not exceed the Warrant exercise price. Please see “Description of Securities - Warrants” for additional information as to the terms of the Warrants.
|
● |
MANAGEMENT EXERCISES SIGNIFICANT CONTROL OVER MATTERS REQUIRING SHAREHOLDER APPROVAL AND INVESTORS WILL HAVE CONTROL OVER COMPANY ACTIONS. |
As a practical matter, our officers and directors will have control of us and will be able to assert significant influence over the election of directors and other matters presented for a vote of stockholders. Even after the Offering is complete, a majority of our shares of Common Stock will be owned by Mr. Xia. Through his concentration of
voting power, he could delay, deter or prevent a change in our control or other business combinations that might otherwise be beneficial to the other stockholders. In deciding how to vote on such matters, Mr. Xia may be influenced by interests that conflict with other stockholders’ interests. Investors will not have a voice in management decisions and will exercise very little control. In the event that the requisite approval of stockholders is obtained, dissenting or non-participating stockholders generally
would be bound by such vote.
|
● |
INVESTORS MAY EXPERIENCE IMMEDIATE AND SUBSTANTIAL DILUTION IN NET TANGIBLE BOOK VALUE PER SHARE OF OUR COMMON STOCK IF THEY ELECT TO EXERCISE THE WARRANTS. |
Investors may experience immediate and substantial dilution in net tangible book value per share of our common stock if they elect to exercise the Warrants. The exercise price of the Warrants may be substantially higher than the net tangible book value per share. Accordingly, if Investors exercise the Warrants, they will likely experience
immediate and substantial dilution in the net tangible book value per share and further dilution if we issue shares of our common stock in the future. As a result of this dilution, in the event of our subsequent liquidation, Investors exercising their Warrants may receive significantly less than the full exercise price that they paid for the shares of our Common Stock.
|
●
|
INVESTORS MAY EXPERIENCE IMMEDIATE AND SUBSTANTIAL DILUTION IN NET TANGIBLE BOOK VALUE PER SHARE OF OUR COMMON STOCK IN THE EVENT WE ISSUE SHARES OF OUR COMMON STOCK IN THE FUTURE. |
There are additional authorized but unissued shares of our Common Stock that may be later issued by our management for any purpose without the consent or vote of the stockholders. Investors purchasing in this Offering may be further diluted in their percentage ownership on an as-converted basis in the event additional shares are issued by
us in the future.
The shares of common stock offered hereby are being registered for the account of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of the common stock will go to the selling stockholders and we will not receive any proceeds from the resale of the common stock by the selling stockholders,
although we could receive proceeds of up to $10,000,000 if all of the share purchase warrants are exercised. We will, however, incur all costs associated with this registration statement and prospectus."
Market Information
Our common stock, having $.001 par value per share ("Common Stock"), is traded on the Over-The-Counter Bulletin Board ("OTCBB") under the symbol "CPDU".
The following table sets forth, for the periods indicated, the reported high and low closing bid quotations for CPDU’s common stock as reported on the OTCBB. The bid prices reflect inter-dealer quotations, do not include retail mark-ups, markdowns or commissions and do not necessarily reflect actual transactions.
Common Stock
|
Period Ended: |
High |
Low | |
|
October 1, 2009 to December 31, 2009 Adjusted for Share Split 7 old for 2 new |
6.12 |
.21 | |
|
June 1, 2009 to September 30, 2009 |
0.06 |
0.06 | |
|
March 1, 2009 to May 31, 2009 |
0.06 |
0.06 | |
|
December 1, 2008 to February 28, 2009 |
0.20 |
0.06 | |
|
September 1, 2008 to November 30, 2008 |
1.00 |
0.20 | |
|
June 1, 2008 to August 31, 2008 |
1.10 |
1.00 | |
|
March 1, 2008 to May 31, 2008 |
2.50 |
1.10 | |
|
December 1, 2007 to February 28, 2008 |
2.50 |
2.50 | |
|
September 1, 2007 to November 30, 2007 |
2.50 |
0.10 | |
|
March 31, 2007 to May 31, 2007 |
0.10 |
0.10 |
Holders
As of January 25, 2010, there were 8,305,288 shares of our common stock issued and outstanding, and there were approximately 418 holders of record of our common stock.
Dividends
Any future determination as to the declaration and payment of dividends on shares of our Common Stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of Common Stock. In addition, we currently
have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in
the future if at the time we are unable to obtain sufficient dividend distributions from China Children Pharmaceutical and Coova and Jiali. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
Determination of Market Price
Our shares of common stock are very thinly traded, and the price if traded may not reflect our value. The selling stockholders may offer to sell the shares of common stock being offered in this prospectus at fixed prices, at prevailing market prices at the time of sale, at varying prices or at negotiated prices. Our common stock
is quoted on the OTC Bulletin Board under the symbol "CPDU". On January 28, 2010, the closing bid price for one share of our common stock on the OTC Bulletin Board was $2.38.
The fixed exercise prices of $3.00 and $5.00 per warrant was arrived at after the Company entered into the Management Agreement and related agreements with Shaanxi Jiali Pharmaceuticals, based upon advice of investment bankers after taking into account our prospects, revenue anticipated to be generated by China Children Pharmaceuticals and
by using the overall valuation of our Company. No assurance, however, can be given that any warrants will be exercised at the $3.00 and $5.00 price. The public offering price does not necessarily bear any relation to our asset value, earnings, net financial condition or other established criteria of value applicable to us and should not be regarded as the actual value or future market price of our common stock. Such prices are subject to change as a result of market conditions and other factors and no
assurance can be given that the shares can be resold at the public offering price.
Securities Authorized for Issuance Under Equity Compensation Plans
We do not have any equity compensation plans.
The following discussion should be read in conjunction with our financial statements and the notes thereto which appear elsewhere in this report. The results shown herein are not necessarily indicative of the results to be expected in any future periods. This discussion contains forward-looking statements based on current expectations, which
involve uncertainties. In some cases, you can identify forward-looking statements by terminology such as "anticipate," "estimate," "plan," "project," "predict," "potential," "continue," "ongoing," "expect," "believe," "intend," "may," "will," "should," "could," or the negative of these terms or other comparable terminology. All forward-looking statements included in this document are based on information available to the management on the date hereof. Actual
results and the timing of events could differ materially from the forward-looking statements as a result of a number of factors. Readers should also carefully review factors set forth in other reports or documents that we file from time to time with the Securities and Exchange Commission.
Our discussion and analysis of our financial condition and results of operations are based upon our audited consolidated financial statements for the years’ ended December 31, 2008 and 2007 and our reviewed consolidated financial statement as of September 30, 2009 and September 30, 2008 which have been prepared in accordance with accounting
principles generally accepted in the United States. Through various contractual arrangements completed on September 30, 2009 Coova Children Pharmaceuticals Co. Ltd. has a controlling interest in China Children Pharmaceuticals and therefore we are required to consolidate Coova Children Pharmaceuticals Co. Ltd.’s and Shaanxi Jiali Pharmaceuticals financial statements and ultimately consolidate them with the financial statements of China Pediatric Pharmaceuticals. The preparation of financial statements
in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of any contingent liabilities at the financial statement date and reported amounts of revenue and expenses during the reporting period. On an on-going basis we review our estimates and assumptions. Our estimates are based on our historical experience and other assumptions that we believe to be reasonable under the
circumstances. Actual results are likely to differ from those estimates under different assumptions or conditions. Our critical accounting policies, the policies we believe are most important to the presentation of our financial statements and require the most difficult, subjective and complex judgments are outlined below in “Critical Accounting Policies.”
FORWARD-LOOKING STATEMENTS
Certain statements made in this report may constitute “forward-looking statements on our current expectations and projections about future events.” These forward-looking statements involve known or unknown risks, uncertainties and other
factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases you can identify forward-looking statements by terminology such
as “may,” “should,” “potential,” “continue,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” and similar expressions. These statements are based on our current beliefs, expectations, and assumptions and are subject to a number of risks and uncertainties. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot
guarantee future results, levels of activity, performance or achievements. These forward-looking statements are made as of the date of this report, and we assume no obligation to update these forward-looking statements whether as a result of new information, future events, or otherwise, other than as required by law. In light of these assumptions, risks, and uncertainties, the forward-looking events discussed in this report might not occur and actual results and events may vary significantly from those discussed
in the forward-looking statements.
AND CAPITAL RESOURCES AND RESULTS OF OPERATIONS AS OF JUNE 30, 2009 AND 2008 AND FOR THE SIX MONTHS THEN ENDED
LIQUIDITY AND CAPITAL RESOURCES AND RESULTS OF OPERATIONS AS OF SEPTEMBER 30, 2009 AND 2008 AND FOR THE NINE MONTHS THEN ENDED
LIQUIDITY AND CAPITAL RESOURCES
As of September 30, 2009, we had cash and cash equivalents of approximately $2.8 million. We believe our existing cash and cash equivalents will be sufficient to maintain our operations at present level for at least the next twelve months. We plan to review acquisition opportunities as a strategy for further growth.
Net cash generated in operating activities for the nine months ended September 30, 2009 was $1,565,534. This was primarily due to the net income of $2,583,391, adjusted by non-cash related income including reversal of allowance for doubtful accounts of $63,767 and non-cash related expenses including depreciation and amortization of $330,824
and allowance for doubtful accounts of $108,180 offset by a net decrease in working capital items of $1,393,094. The net decrease in working capital items was mainly due to increase in accounts receivable which resulted from the significant increase in revenues during the nine months ended September 30, 2009 and the longer credit term provided to customers as part of sales promotion, increase in prepayments to suppliers, and increase in inventories to prepare for the ongoing sales promotion, decrease in accounts
payable, decrease in VAT and income tax payable. The net decrease in working capital items was partially offset by the increase in accrued expenses.
Net cash generated in operating activities for the nine months ended September 30, 2008, was $753,149. This was primarily due to the net income of $1,437,256, adjusted by non-cash related expenses including depreciation and amortization of $327,324, offset by a net decrease in working capital items of $1,011,431. The net decrease in working
capital items was mainly due to increase in inventory, increase in prepayments to suppliers, a decrease in accrued expenses and decrease in VAT tax payable. The net decrease in working capital items was partially offset by increase in accounts receivable and the increase in accounts payable and income tax payable.
There was no cash used in investing activities for the nine months ended September 30, 2009. Net cash used in investing activities for the nine months ended September 30, 2008 was $10,365. This was due to addition of properties, plant and equipment of $10,365.
Net cash generated in financing activities for the nine months ended September 30, 2009 was $438,539. This was due to the inception of new bank loans of $438,526 and subscription received of $13. There was no cash used in financing activities for the nine months ended September 30, 2008.
COMPARISON OF RESULTS FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009, TO THE NINE MONTHS ENDED SEPTEMBER 30, 2008
NET SALES
|
Nine Months Ended September 30, |
% of |
|||||||||||||||||||
|
2009 |
2008 |
change |
||||||||||||||||||
|
"Xianzhi" Series |
$ | 1,460,527 | 13 | % | $ | 1,682,968 | 16 | % | (13 | ) % | ||||||||||
|
"Cooer" Series |
7,923,754 | 70 | % | 6,084,574 | 60 | % | 30 | % | ||||||||||||
|
"Qingsongling" Series |
1,938,862 | 17 | % | 2,471,047 | 24 | % | (22 | ) % | ||||||||||||
|
Total net sales |
$ | 11,323,143 | 100 | % | $ | 10,238,589 | 100 | % | 11 | % | ||||||||||
Total net sales for the nine months ended September 30, 2009 increased by $1,084,554 or approximately 11% compared to the same period of 2008. This was mainly due to increases in sales of "Cooer" Series by 30% in 2009, as a result of the intensive promotion in 2008.
COST OF SALES
|
Nine Months Ended September 30, |
% of |
|||||||||||||||||||
|
2009 |
2008 |
change |
||||||||||||||||||
|
"Xianzhi" Series |
$ | 345,422 | 8 | % | $ | 277,691 | 9 | % | 24 | % | ||||||||||
|
"Cooer" Series |
3,005,207 | 69 | % | 1,993,840 | 60 | % | 51 | % | ||||||||||||
|
"Qingsongling" Series |
1,025,912 | 23 | % | 1,027,448 | 31 | % | (1 | ) % | ||||||||||||
|
Total cost of sales |
$ | 4,376,541 | 100 | % | $ | 3,298,979 | 100 | % | 33 | % | ||||||||||
Compared to last period, cost of sales increased $1,077,562 or 33% in the nine months ended September 30, 2009. This was primarily resulting from the increase in product net sales. The increase in average unit costs was mainly caused by the general increase in costs of raw material in China in the year 2009.
GROSS PROFIT
|
Nine Months Ended September 30, |
|||||||||||||||
|
2009 |
2008 |
||||||||||||||
|
gross profit |
gross profit |
% of |
|||||||||||||
|
margin |
margin |
change |
|||||||||||||
|
"Xianzhi" Series |
$ |
1,115,105 |
76 |
% |
$ |
1,405,277 |
84 |
% |
(21) |
% | |||||
|
"Cooer" Series |
4,918,547 |
62 |
% |
4,090,734 |
67 |
% |
20 |
% | |||||||
|
"Qingsongling" Series |
912,950 |
47 |
% |
1,443,599 |
58 |
% |
(37) |
% | |||||||
|
Total |
$ |
6,946,602 |
61 |
% |
$ |
6,939,610 |
68 |
% |
0 |
% | |||||
GP slightly increased compared with the same period in 2008 due to the promotion of Cooer series was successful.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
|
Nine Months Ended September 30, |
|||||||||||||||
|
2009 |
2008 |
||||||||||||||
|
% of total |
% of total |
% of |
|||||||||||||
|
net sales |
net sales |
change |
|||||||||||||
|
Selling, general and administrative expenses |
$ |
3,930,219 |
35 |
% |
$ |
5,253,803 |
51 |
% |
(24) |
% | |||||
In 2009, decreases in selling, general and administrative expenses in absolute dollars of $1,323,584 and as a percentage of total net sales also decreased were mainly due to the decrease in promotional and advertising expenditures of around $1.6 million. Other items contributed to the decrease in selling, general and administrative
expenses for the nine months ended 2009 included decrease in operating expenses like office supply, telecommunication expenses. Under this circumstance, the selling, general and administrative expenses were decreased 50%.
PROVISION FOR INCOME TAXES
|
Nine Months Ended September 30, |
||||||||
|
2009 |
2008 |
|||||||
|
Provision for income taxes |
$ |
422,716 |
$ |
276,477 |
||||
|
Effective tax rate |
15 |
% |
15 |
% | ||||
The amount of Enterprise Income Tax payable is computed on the basis of the taxable income and by applying the tax rate of 25%. The Company enjoyed a reduced rate of 15% from the Enterprises Income Tax in 2008 and 2009 because the Company is "High Technology Business" under PRC Income Tax Laws.
In 2008, the income tax calculation was based on the Company's unaudited profit. But, the audited profit before tax was lower than the amount of tax charged based on unaudited figures so that the amount of tax overcharged was deducted to offset part of the 2009's income tax. However, the selling and administrative expenses for the nine month
end 30 September, 2009 was decreased by approximately $1.3 million, therefore the assessable income was increased significantly.
NET INCOME
As a result of the above, in the nine months ended September 30, 2009, the net income was $2,583,392 compared to a net income of $1,437,256 for the nine months ended September 30, 2008.
We believe the following critical accounting policies, among others, affect management's more significant judgments and estimates used in the preparation of the financial statements:
Revenue Recognition
The Company's revenue recognition policies are in compliance with Staff Accounting Bulletin ("SAB") 104, "Revenue Recognition." Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist
and collectibility is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue.
Allowance for Doubtful Accounts
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserves
are recorded primarily on a specific identification basis.
The following table sets out the aging of our accounts receivable for each balance sheet periods presented.
|
Accounts Receivable Aging |
Total |
1-30 days |
31-60 days |
61-90 days |
91-180 days |
181-365 days |
> 365 days |
|||||||||||||||||||||
|
As of September 30, 2009 |
$ |
3,908,552 |
$ |
616,661 |
$ |
1,658,190 |
$ |
586,624 |
$ |
464,196 |
$ |
402,208 |
$ |
180,673 |
||||||||||||||
|
As of September 30, 2008 |
$ |
2,404,947 |
$ |
- |
$ |
399,063 |
$ |
125,464 |
$ |
1,477,920 |
$ |
389,305 |
$ |
13,195 |
||||||||||||||
|
As of December 31, 2008 |
$ |
3,286,863 |
$ |
1,792,090 |
$ |
1,308,980 |
$ |
132,918 |
$ |
- |
$ |
- |
$ |
52,875 |
||||||||||||||
The following presents the days sales outstanding calculated based on sales and accounts receivables in RMB term for each income statement periods presented.
|
Nine Months Ended September 30, |
Year Ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Days sales outstanding |
93 | 63 | 79 | 72 | ||||||||||||
Inventory
Management compares the cost of inventories with the market value. We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand, future pricing and market conditions.
Property, plant and Equipment
Property, plant and equipment are stated at historical cost less accumulated depreciation and amortization. Expenditures for maintenance and repairs are charged to earnings as incurred, additions, renewals and betterments are capitalised. When property and equipment are retired or otherwise disposal of, the related cost and accumulated depreciation
are removed from the respective accounts, and any gain or loss in included in operations. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Judgment is required to determine the estimated useful lives of assets, especially for plant and equipment and transportation equipment, including determining how long existing equipment can function and when new technologies will be introduced at cost-effective price points to replace existing equipment. Changes in these
estimates and assumptions could materially impact the financial position and results of operations.
Accounting for Stock-Based Compensation
The account for stock-based compensation based on the provisions of Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees", as amended by the Financial Accounting Standards Board Interpretation No. 44, "Accounting for Certain Transactions Involving Stock Compensation." Accounting Principles Board Opinion No.
25 and Financial Accounting Standards Board Interpretation No. 44 state that no compensation expense is recorded for stock options or other stock-based awards to employees that are granted with an exercise price equal to or above the estimated fair value per share of the company’s common stock on the grant date. We adopted the disclosure requirements of Statement of Financial Accounting Standards No. 123, "Accounting for Stock-Based Compensation" which requires compensation expense to be disclosed based
on the fair value of the options granted at the date of the grant.
In December 2004, the FASB issued FASB Statement No. 123R, "Share-Based Payment, an Amendment of FASB Statement No. 123" ("FAS No. 123R"). FAS No. 123R requires companies to recognize in the statement of operations the grant date fair value of stock options and other equity-based compensation issued to employees. FAS No. 123R is effective
beginning in the first quarter of fiscal 2006.
We did not issue any stock options to employees during the year ended December 31, 2007, therefore pro forma disclosures are not required.
Accounting for Defined Benefit Pensions and Other Postretirement Plans
In September 2006, FASB issued SFAS No. 158, “Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans – an amendment of FASB Statements No. 87, 88, 106, and 132(R)” (“SFAS No. 158”). SFAS No. 158 improves financial reporting by requiring an employer to recognize the over funded or
under funded status of a defined benefit postretirement plan (other than a multiemployer plan) as an asset or liability in its statement of financial position and to recognize changes in that funded statues in the year in which the changes occur through comprehensive income of a business entity or changes in unrestricted net assets of a not-for-profit organization. This Statement also improves financial reporting by requiring an employer to measure the funded status of a plan as of the date of its
year-end statement of financial position, with limited exceptions. An employer with publicly traded equity securities is required to initially recognize the funded status of a defined benefit postretirement plan and to provide the required disclosures as of the end of the fiscal year ending after December 15, 2006. An employer without publicly traded equity securities is required to recognize the funded status of a defined benefit postretirement plan and to provide the required disclosures as of the
end of the fiscal year ending after June 15, 2007. However, an employer without publicly traded equity securities is required to disclose the following information in the notes to financial statements for a fiscal year ending after December 15, 2006, but before June 16, 2007, unless it has applied the recognition provisions of this Statement in preparing those financial statements.
|
a. |
A brief description of the provisions of this Statement |
|
b. |
The date that adoption is required |
|
c. |
The date the employer plans to adopt the recognition provisions of this Statement, if earlier. |
The requirement to measure plan assets and benefit obligations as of the date of the employer’s fiscal year-end statement of financial position is effective for fiscal years ending after December 15, 2008.
The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Valuation of Intangibles
From time to time, we acquire intangible assets that are beneficial to our product development processes. Management periodically evaluates the carrying value of intangibles, including the related amortization periods. In evaluating acquired intangible assets, management determines whether there has been impairment by comparing the anticipated
undiscounted cash flows from the operation and eventual disposition of the product line with its carrying value. If the undiscounted cash flows are less than the carrying value, the amount of the impairment, if any, will be determined by comparing the carrying value of each intangible asset with its fair value. Fair value is generally based on either a discounted cash flows analysis or market analysis. Future operating income is based on various assumptions, including regulatory approvals, patents being granted,
and the type and nature of competing products. If regulatory approvals or patents are not obtained or are substantially delayed, or other competing technologies are developed and obtain general market acceptance or market conditions otherwise change, our intangibles may have a substantially reduced value, which could be material.
In April 2008, the FASB issued FASB Staff Position (FSP) FAS 142-3, “Determination of the Useful Life of Intangible Assets,” which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, “Goodwill
and Other Intangible Assets.” This Staff Position is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The adoption of this new FSP did not have a material impact on the Company’s financial position, results of operations or cash flows.
The Fair Value Option for Financial Assets and Financial Liabilities
In February, 2007, FASB issued SFAS 159, ‘The Fair Value Option for Financial Assets and Financial Liabilities – Including an Amendment of FASB Statement No. 115.” This Statement permits entities to choose to measure many financial instruments and certain other items at fair value. This Statement is expected to
expand the use of fair value measurement, which is consistent with the Board’s long-term measurement objectives for accounting for financial instruments. This Statement is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Income Taxes
The Company utilizes SFAS No. 109, "Accounting for Income Taxes," which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future
years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), "Accounting for uncertainty in Income Taxes." FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement,
classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, "Accounting for Contingencies." FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company's financial statements.
Pursuant to the PRC Income Tax Laws, the Enterprise Income Tax ("EIT") through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high technology business and under PRC Income
Tax Laws, it is entitled to a two-year tax exemption for 2006 through 2007. Starting from January 1, 2008, the EIT is at a reduced rate of 15% to the Company.
Foreign Currency
Our functional currency is the U.S. dollar and our subsidiary and our operating company in China use their respective local currencies as their functional currencies, i.e. the Chinese Yuan Renminbi (CNY). An entity's functional currency is the currency of the primary economic environment in which the entity operates. Management must use judgment
in determining an entity's functional currency, assessing economic factors including cash flow, sales price, sales market, expense, financing and inter-company transactions and arrangements. Impact from exchange rate changes related to transactions denominated in currencies other than the functional currency is recorded as a gain and loss in the statements of operations, while impact from
exchange rate changes related to translating a foreign entity's financial statements from the functional currency to its reporting currency, the U.S. dollar, is disclosed and accumulated in a separate component under the equity section of the balance sheets. Different judgments or assumptions resulting in a change of functional currency may
materially impact our financial position and results of operations.
STATUTORY RESERVE
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax
to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of
cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of September 30, 2009 and September 30, 2008, the Company had allocated $880,133 and $605,622, respectively, to these non-distributable reserve funds.
CONTRACTUAL OBLIGATIONS
There was no contractual obligations as of September 30, 2009.
INFLATION
Inflation in recent years have affected the business results of the Company. First of all, on the global economic expansion, supply has been restricted and coupled with the fact that USA has adopted a relaxed currency policy etc., these increase inflation risks. Secondly, GNP increases in China also elevates consumption
ability and production cost, prices increase as a natural tendency. Finally, as a result of the macro economic trend, prices increase, the Company’s procurement prices are also affected resulting in increase in cost of sales.
The Company operates in China and as such, the Company’s business activities, financial position and operational results will be affected by PRC politics, economic and legal environments and also affected by the overall economic situation of China. The business of the Company may be affected by the relevant laws, regulations,
anti-inflation measures, currency conversion and overseas remittance and exchange rates issues etc that are related to China politics.
OFF-BALANCE SHEET COMMITMENTS AND ARRANGEMENTS
We do not have any financial undertaking or any other guarantee responsible for third party obligations and commitments. We have not entered into any derivative products contracts having the Company’s equity linked or that have not been reflected in the consolidated Pro Forma financial statements. Furthermore, in
passing our assets to those providing credits, clearing or providing market risks support Bodies, we have not reserved any rights or undefined rights on the assets passing. The Company does not have any benefits from Bodies providing finance, clearing or market risks or credit support or with Bodies we engage in leasing, hedging or research and development services.
Effective on October 9, 2009, the Company changed its name from Lid Hair Studios International Inc. to China Pediatric Pharmaceuticals, Inc. The name change was effected through a parent/subsidiary merger of our wholly-owned subsidiary, China Pediatric Pharmaceuticals, Inc., with and into the Company, with the Company as the surviving
corporation. To effectuate the merger, the Company filed its Articles of Merger with the Nevada Secretary of State and the merger became effective on October 9, 2009. A copy of the filed Articles of Merger is being filed herewith.
The Company’s board of directors approved the merger which resulted in the name change on October 9, 2009. In accordance with Section 92A.180 of the Nevada Revised Statutes, shareholder approval of the merger was not required. On the effective date of the merger, the Company’s name was changed to “China
Pediatric Pharmaceuticals, Inc.” and the Company’s Articles of Incorporation were amended to reflect this name change. Following the Combination, the combined Company will continue to be traded on the OTCBB, under the symbol “LHSI”, however we will request a new symbol closer in form to China Pediatric Pharmaceuticals, Inc. Please see Exhibit 3.3.
Effective on October 14, 2009, the company filed an Amendment to our Articles of Incorporation to provide for a seven old common shares for two new common shares reverse share split.
Effective on October 14, 2009, the company changed our year ending from May 31 to December 31.
Our operations are headquartered in Xi’an, Shaanxi Province, China. We are a profitable, mid-sized Chinese pharmaceutical company that identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional and Traditional Chinese Medicines (“TCMs”),
for the treatment of some of the most common ailments and diseases, with pediatric medicine as its focus.
We currently have one operating company: Shaanxi Jiali identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional and Traditional Chinese Medicines (“TCMs”), for the treatment of some of the most common ailments and diseases, with pediatric medicine
as its focus. We distribute our high value, branded medicines, both prescription and OTC, through exclusive territory agents who sell our products directly to local pharmacies who in turn sell them to their retail customers.
We currently have 26 products that are manufactured by both our in-house production facility and through OEM companies.
Shaanxi Jiali intends to further develop the series of products based on the Coors Brand and designed to target the pediatric medicine market. These products will continue be sold through exclusive territory agents who in turn distribute to local pharmacies in their assigned territories. Shaanxi Jiali intends to further expand its distribution
channels by increasing the number of exclusive territory agents. These additional exclusive territory agents will be contracted to distribute Shaanxi Jaili products to local pharmacies in several additional provinces or regions throughout China.
We will continue to evaluate and develop additional product candidates, both through our in-house research and development department and working with our research and development partners, to expand our pipeline where we perceive an unmet need and commercial potential. We
will face the risk that in developing new products we may spend substantial sums of money and the new products developed may not effectively meet the perceived need or may not be successfully commercialized.
With intense price competition among many similar or identical products in the industry, we believe that building brand equity is the primary means to generate and sustain profitable growth in the future. The company intends to focus its brand development efforts on building the Cooers brand name with the intent on it becoming a leading pediatric
medicine brand in China.
We intend to grow our internal marketing and sales function and increase our relationships with other exclusive territory distributors to expand the distribution and presence of our pharmaceutical products. In expanding market share of our products, we intend to take advantage of our large manufacturing scale and reasonable cost control mechanisms,
and our strong sales network. In addition, our goal is to establish our products as a preferred choice for children’s medicines in local pharmacies. We hope to add other pharmaceutical products into this channel over the next few years. We may face risks in obtaining adequate quantities of raw materials at reasonable prices in order to meet increased demand for our products that result from any growth. In seeking additional employees, sales representatives, and exclusive territory agents, we will compete
with many other established pharmaceutical manufacturers that may have greater resources than we do.
Our continuing success in optimizing our manufacturing processes and minimizing our production costs provides us with a competitive advantage.
China’s thousands of domestic companies account for 70 percent of the pharmaceutical market. Anticipating the effects of WTO entry and in an effort to compete with foreign firms, the Chinese government has decided to nurture its own large pharmaceutical companies, by encouraging the consolidation of its government-owned companies. To
this end, the Chinese State Economic and Trade Commission (SETC) announced plans to consolidate the industry and support the development of 10 to 15 largest pharmaceutical firms. According to government statistics China currently has about 3,500 drug companies, down from over 5,000 in 2004. The number is expected to drop further. As the industry undergoes further consolidation, Lid Hair will have the opportunity to grow by acquisition.
Description of our Business
Lid Hair Studios International, Inc. was established on April 20, 2005 in the state of Nevada. The company was originally incorporated under the name Belford Enterprises, Inc. and changed its name to Lid Hair Studios International Inc. on August 15, 2005. Our operating subsidiary Belford Enterprises B.C. Ltd., doing business
as Lid Hair Studio was established June 7, 2005, in the City of Vancouver, British Columbia Canada. There are no bankruptcies, receivership, or similar proceedings against the parent or operating subsidiary.
On June 18, 2005, the company purchased hair salon equipment and the rights to an office lease from Farideh Jafari in the amount of approximately $39,150US ($45,000CDN).
On July 4, 2005 we entered into a lease agreement by way of Assignment of Lease by Tenant with Landlord’s Consent. The lease commenced July 1, 2005 and terminates June 30, 2015.
Effective on October 9, 2009, the Company changed its name from Lid Hair Studios International Inc. to China Pediatric Pharmaceuticals, Inc. The name change was effected through a parent/subsidiary merger of our wholly-owned subsidiary, China Pediatric Pharmaceuticals, Inc., with and into the Company, with the Company as the surviving
corporation. To effectuate the merger, the Company filed its Articles of Merger with the Nevada Secretary of State and the merger became effective on October 9, 2009. A copy of the filed Articles of Merger is being filed under Exhibit 3.3. The Company’s board of directors approved the merger which resulted in the name change on October 9, 2009. In accordance with Section 92A.180 of the Nevada Revised Statutes,
shareholder approval of the merger was not required. On the effective date of the merger, the Company’s name was changed to “China Pediatric Pharmaceuticals, Inc.” and the Company’s Articles of Incorporation were amended to reflect this name change. Following the Combination, the combined Company will continue to be traded on the OTCBB, under the symbol “LHSI”, however we will request a new symbol closer in form to China Pediatric Pharmaceuticals, Inc.
Shaanxi Jiali was incorporated in the PRC under the name Shaanxi Jiali Technologies Co, Ltd. on July 1, 1998. In October 2000, Shaanxi Jiali Technologies changed its name to Shaanxi Jiali Pharmaceutical Co. Ltd. Shaanxi Jiali is our operating company and is in the business of manufacturing, marketing and sales of pharmaceuticals
in China with pediatric medicine as its focus.
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm through its wholly owned subsidiary China Children Pharmaceutical, Inc. (“China Children”), became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and pharmaceutical
developer, manufacturer and marketer in the PRC.
China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm.
Xi-an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) is a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly owned subsidiary of China Children.
As described below, on August 4, 2008, an Entrustment Management Agreement (described in detail on page X) was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China
Children shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
Xi’an Coova entered into a Management Entrustment Agreement with Shaanxi Jiali and the shareholders of Shaanxi Jiali (the “Management Entrustment Agreement”), in which Shaanxi Jiali and its shareholders agreed to transfer control, or entrust, the operations and management of its business to Xi’an Coova. Under
the agreement, Xi’an Coova manages the operations and assets of Shaanxi Jiali , controls all of the cash flows of Shaanxi Jiali through a bank account controlled by Xi’an Coova, is entitled to 100% of earnings before tax of Shaanxi Jiali, a management fee, and is obligated to pay all payables and loan payments of Shaanxi Jiali. In addition, under the terms of the Management Entrustment Agreement, Xi’an Coova has been granted certain rights which include, in part, the right to appoint and terminate
members of Shaanxi Jiali’s Board of Directors, hire management and administrative personnel and control decisions relating to entering and performing
customer contracts and other instruments. We anticipate that Shaanxi Jiali will continue to be the contracting party under its customer contracts, bank loans and certain other instruments unless Xi’an Coova exercises its option. The agreement does not terminate unless the business of Shaanxi Jiali is terminated or Xi’an Coova exercises
its option to acquire all of the assets or equity of Shaanxi Jiali under the terms of the Exclusive Option Agreement as more fully described below and completes the acquisition of Shaanxi Jiali.
In exchange for causing Shaanxi Jiali to enter into the Management Entrustment Agreement, Xi’an Coova, through its parent company Asia-Pharm, issued an aggregate of 12,000,000 shares of Asia-Pharm’s common stock to the shareholders of Shaanxi Jiali which was allocated based on their respective pro rata ownership of Shaanxi Jiali.
In order to give Xi’an Coova further control over Shaanxi Jiali, the Shaanxi Jiali shareholders and Xi’an Coova, a wholly owned subsidiary of China Children in the PRC, entered into a shareholders’ Voting Proxy Agreement (the “Voting Proxy Agreement”) whereby the Shaanxi Jiali shareholders irrevocably and
exclusively appointed the members of Xi’an Coova’s board of directors, as their proxies to vote on all matters that require Shaanxi Jiali shareholder approval, including, without limitation, the right to appoint members of the board of directors of Shaanxi Jiali. The agreement further provides that Shaanxi Jiali will appoint all of the board of directors of Xi’an Coova as its board of directors. Xi’an Coova’s board of directors changes, Shaanxi Jiali must remove and appoint
new members to its board. The agreement terminates upon the exercise of the option by Xi’an Coova to purchase the shares of Shaanxi Jiali as described below and is governed by the laws of the PRC.
In order to permit Shaanxi Jiali to become an indirectly wholly owned subsidiary of China Children when permitted under PRC law, Xi’an Coova, Shaanxi Jiali and the Shaanxi Jiali shareholders entered into an exclusive option agreement (the “Exclusive Option Agreement”) whereby the Shaanxi Jiali shareholders granted Xi’an
Coova an irrevocable and exclusive purchase option (the “Option”) to acquire Shaanxi Jiali’s equity and/or remaining assets, but only to the extent that the acquisition does not violate limitations imposed by PRC law on such transactions. Current PRC law does not specifically provide for the equity of a non-PRC entity to be used as consideration for the purchase of a PRC entity’s assets or equity unless the value of the shares are equal to or greater than the value of the enterprise acquired.
In addition, there is a lengthy appraisal process which must be approved by the provincial PRC government entities. The consideration for the exercise of the Option is to be determined by the parties and memorialized in future definitive agreements setting forth the kind and value of such consideration. Accordingly, we will consider exercising the Option under such circumstances we believe will be in our best interests and our shareholders and the Exclusive Option Agreement has been drafted to give us such flexibility.
In considering whether or not we will exercise the Option we may consider such factors as (1) if the exercise price can be lower than the appraised value under current PRC law (2) availability of funds, (3) any relevant tax considerations at the time, (4) any other relevant PRC laws that may exist at the time, (5) the value of our shares that were previously paid to shareholders of Shaanxi Jiali, and (6) whether or not the exercise of the Option will provide any other additional benefits to us or our shareholders. Upon
exercise of the Option, the parties will prepare transfer documents to be submitted for governmental approval and work together to obtain all approvals and permits. The agreement may be terminated by agreement of all parties or by with 30 days notice and is governed by the laws of the PRC.
In order to further solidify China Children’s rights, benefits and control of Shaanxi Jiali through its ownership of Xi’an Coova under the Entrusted Management Agreement, Voting Proxy Agreement and Exclusive Option Agreement, Xi’an Coova and the Shaanxi Jiali shareholders entered into a share pledge agreement (the
“Share Pledge Agreement”) whereby the Shaanxi Jiali shareholders pledged all of their equity interests in Shaanxi Jiali, including the proceeds thereof, to guarantee the performance by the shareholders of all of the agreements they entered into with Xi’an Coova. Upon breach by any of the shareholders of any of the Management Entrustment Agreement, Voting Proxy Agreement, the Exclusive Option Agreement or the Share Pledge Agreement, Xi’an Coova is entitled by operation of law to become
the beneficial owner of the shares of Shaanxi Jiali. Prior to termination of the Share Pledge Agreement, the pledged equity interests of Shaanxi Jiali cannot be transferred without Xi’an Coova’s prior written consent. The provisions of the Share Pledge Agreement and the Voting Proxy Agreement work together to give the board of directors of Xi’an Coova control over transfers by the shareholders of Shaanxi Jiali . The agreement will not terminate until agreed to by all of the parties in writing
and is governed by the laws of the PRC.
On September 30, 2009, we acquired a Hong Kong based pharmaceutical manufacturer and distributor company in accordance with a Share Exchange Agreement dated September 30, 2009 (“Exchange Agreement”) made by and among Lid Hair Studios International, Inc., (“Company”) a Nevada corporation, Eric Anderson
the (“Principal Company Shareholder”), and Asia-Pharm Holding (“Asia-Pharm”), Inc. a company incorporated in the British Virgin Islands.
The close of the Share Exchange transaction (the "Closing") took place on September 30, 2009 (the “Closing Date”). On the Closing Date, pursuant to the terms of the Exchange Agreement, we acquired all of the outstanding capital stock and
ownership interests of China Children Pharmaceuticals Inc.(the “Interests”) from Asia-Pharm, and they transferred and contributed all of their Interests to us. In exchange, we issued to Asia-Pharm 7,000,000 shares of our common stock. The material terms of the Exchange Agreement are more fully described in Exhibit 2.1
of this report.
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm through its wholly owned subsidiary China Children Pharmaceutical, Inc. (“China Children”), became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and pharmaceutical
developer, manufacturer and marketer in the PRC.
China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm. Xi’an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) is a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is
a wholly owned subsidiary of China Children. On August 4, 2008, an Entrustment Management Agreement (described in detail on page 7) was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China Children shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
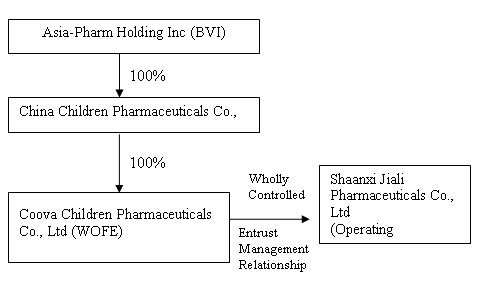
Combination
On September 30, 2009, Asia-Pharm, Eric Anderson and Lid Hair Studios, entered into a definitive Share Exchange Agreement (“Exchange Agreement”) for the combination of us and China Children Pharmaceuticals (the “Combination”). The Combination was accomplished by means of a share exchange in which Asia-Pharm exchanged
all of their stock in China Children Pharm for the new issuance of our common stock. Under the terms of the Exchange Agreement and as a result of the Combination:
|
● |
China Children became our wholly owned subsidiary; |
|
● |
In exchange for all of their shares of China Children stock, Asia-Pharm received 7,000,000 newly issued shares of our common stock. |
|
● |
Immediately following the closing of the Combination, such shares of CPPI common stock represent approximately 65.25% of our issued and outstanding shares on a fully diluted basis. |
The organization and ownership structure of the Company subsequent to the consummation of the combination as summarized in the paragraphs above is as follows:
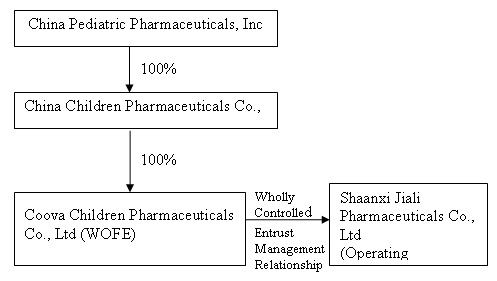
This transaction closed on September 30, 2009.
Our operations are headquartered in Xi’an, Shaanxi Province, China. We are a profitable, mid-sized Chinese pharmaceutical company that identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional and Traditional Chinese Medicines (“TCMs”),
for the treatment of some of the most common ailments and diseases, with pediatric medicine as its focus.
We currently have one operating company: Shaanxi Jiali identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional and Traditional Chinese Medicines (“TCMs”), for the treatment of some of the most common ailments and diseases, with pediatric medicine
as its focus.
Our operating company has its own manufacturing facility located in Baoji, Shaanxi Province. Our manufacturing facility was issued a Good Manufacturing Practices1 (“GMP”) certification by the SFDA2 in
2004. We were awarded the National High-tech Enterprise Award by the Shaanxi Technology Administration in 2006. As a result of receiving the National High-tech Award Shaanxi Jiali is entitled to the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Shaanxi Jiali may enjoy an
exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax.
We distribute our high value, branded medicines, both prescription and OTC, through exclusive territory agents who sell our products directly to local pharmacies who in turn sell them to their retail customers.
___________________________
1 Good Manufacturing Practices (“GMP”) is an internationally-recognized standard for pharmaceutical plant design and construction. GMP has been defined as “that part of quality assurance which ensures that products are
consistently produced and controlled to the quality standards appropriate for their intended use and as required by the marketing authorization” (World Health Organization). GMP covers all aspects of the manufacturing process: defined manufacturing process; validated critical manufacturing steps; suitable premises, storage, transport; qualified and trained production and quality control personnel; adequate laboratory facilities; approved written procedures and instructions; records to show all steps of
defined procedures taken; full traceability of a product through batch processing records and distribution records; and systems for recall and investigation of complaints.
2 The Chinese government agency, SFDA, is analogous to the Food and Drug Administration (“FDA”) in the United States. Unlike the FDA, however, the SFDA provides intellectual property and competitive protection to certain classes
of approved drugs.
In 2008, our revenues were principally derived from sales of products listed below. We have SFDA approval for all medicines and active pharmaceutical ingredients that we market. The sales of ready-made Chinese Traditional Medicines are required to obtain approval of SFDA. While sales of the raw materials of Chinese herbs are not required to
obtain SFDA approval.
Main Products
We currently have 26 products that are manufactured by both our in-house production facility and through OEM companies.
Table 1
As shown below we produce 13 of our medicines in-house at our production plant.
|
Manufacturer |
Brand Name &Product |
Type: Prescription/
OTC Conventional
OTC TCM |
Primary Function | |||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Radix lsatidis Granule (5g) |
OTC TCM |
Treatment for inflammation and pain. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Radix lsatidis Granule (10g) |
OTC TCM |
Treatment for inflammation and pain. | |||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Da shanzha Granule |
OTC TCM |
Treatment for indigestion. | |||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Paracetamol, Artificial Cow-bezoar and Chlorphenamine Maleale Granule |
OTC Conventional |
Treatment for inflammation, pain and fever. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Erythromycin Estolate Granule |
OTC Conventional |
Treatment for indigestion. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Compound Danshen Tablet |
OTC TCM |
Treatment for heart and blood conditions. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Ganmao Tuire Granule |
OTC TCM |
Treatment for inflammatory and pain. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Qingrejiedu Tablet |
OTC TCM |
Treatment for respiratory tract inflammation. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Erythromycin Ethylsuccinate Tablets (0.125 g) |
OTC Conventional |
Treatment for indigestion. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Yuanhu Pain killer |
OTC TCM |
Treatment for pain. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Vitamin C Tablets (50mg) |
OTC Conventional |
Vitamin | |||
|
Shaanxi Jiali Pharmaceuticals |
Xianzhi Series
Kechuanqing Tablet |
Prescription |
Treatment for bronchitis and asthma. | |||
|
Shaanxi Jiali Pharmaceuticals |
Qingsongling Series
Paracetamol,Caffein,Atificial Cow-bezoar and chlorphenamine Maleate Capsules |
OTC Conventional |
Treatment for common colds. |
Table 2
The table below represents our 13 of our medicines that are produced by OEM companies.
|
Manufacturer |
Brand Name &Product |
Type: Prescription/
OTC Conventional
OTC TCM |
Primary Function | |||
|
OEM: Hubei Kanger Jian Bioengineering Co. Ltd. |
Cooer Jin 21 Multivitamin Chewing Tablets
|
OTC Conventional |
Vitamin. | |||
|
OEM: Hubei Kanger Jian Bioengineering Co. Ltd. |
Cooer Lacidophilin Granules
|
OTC Conventional |
Treatment for indigestion and diarrhea. | |||
|
OEM Partner: Taiji Group Sichuan Nanchong Pharmaceutical Factory |
Cooer Xiaochaihu Granules
|
OTC TCM |
Treatment for indigestion. | |||
|
OEM Partner: Taiji Group Sichuan Nanchong Pharmaceutical Factory |
Cooer Xiaoer Expectorant Granules
|
OTC TCM |
Treatment for coughs. | |||
|
OEM: Taiji Group Sichuan Nanchong Pharmaceutical Factory |
Cooer Xiaoer Throat-cleaning Granules
|
OTC TCM |
Treatment for throat pain. | |||
|
OEM : Taiji Group Sichuan Nanchong Pharmaceutical Factory |
Cooer Xiaoer Cough-curing Syrup
|
OTC TCM |
Treatment for coughs. | |||
|
OEM: Xi’an KeLi Pharmaceuticals Co. Ltd. |
Cooer Xiaoer Xishi Granule
|
OTC TCM |
Treatment for indigestion and diarrhea. | |||
|
OEM: Xi’an KeLi Pharmaceuticals Co. Ltd. |
Cooer Xiaoer Kechuanling Granule
|
OTC TCM |
Treatment for respiratory tract infection and coughs. | |||
|
OEM: Xi’an KeLi Pharmaceuticals Co. Ltd. |
Cooer I+1 Suit
Cooer Xiaoer Paracetamol, |
OTC Conventional |
Treatment for fever. | |||
|
OEM : Fosham City Yichuang Biochemistry Science and Technology Co. Ltd. |
Cooer Fever Release Paste
|
OTC TCM |
Treatment for fever | |||
|
OEM: Jiyuan City, Taiji Medical Supplies Co. Ltd. |
Cooer Paste for Pediatric Diarrhea
|
OTC TCM |
Treatment for diarrhea. | |||
|
OEM Partner: Jiyuan City, Taiji Medical Supplies Co. Ltd. |
Cooer Paste for Pediatric Cough & Asthma
|
OTC TCM |
Treatment for asthma and coughs. | |||
|
OEM: Jiyuan City, Taiji Medical Supplies Co. Ltd. |
Cooer Paste for Pediatric Cold
|
OTC TCM |
Treatment for the common cold. |
Our Three Brands include:
Cooers Brand Products
The Cooers Brand product line is focused on the pediatric medicine market. The product line includes both prescription and over–the-counter pharmaceutical products, including both conventional and traditional Chinese medicines. The Cooers product line has 15 products that address numerous ailments including, but not limited,
to pain relief, anti-inflammatory, anti-bacteria, infection, digestion, respiratory tract infections, gangrene, diarrhea and fever, cold and cough remedies and vitamins.
Qingsongling Brand Products
The Qingsongling product line is focused on the adult market. This product line includes both prescription and over –the-counter pharmaceutical products including both conventional and traditional Chinese medicines. The Qingsongling product line has 8 products for treatment
of numerous ailments, including but not limited to, treatments for heart disease and angina, respiratory tract infection, influenza, gangrene, anti-inflammatory pain relief, cold and fever remedies and vitamins.
Zianzhi Brand Product
The Zianzhi product line is focused on the adult medicine market. Xianzhi series includes one product which is prescription medicine (TCM). The Zianzhi product line has one product for treatment for chronic bronchitis and asthma.
Shaanxi Jiali’s high value branded prescription and OTC medicines are distributed through the following distribution channels: Pharmaceutical Manufacturer (the company) - Exclusive Agents - Pharmacies – Patients.
Shaanxi Jaili currently sells its products thru 15 exclusive territory agents in 7 provinces throughout China. The exclusive territory agents sell the company’s products directly to local pharmacies in their assigned territory. This method of distribution minimizes the company’s need to maintain a large direct sales force.
Exclusive Territory Agents
Shaanxi Jiali has entered into contracts with Exclusive Territory Agents for the sale and distribution of our products to local pharmacies in the agent’s respective exclusive territory. In 2008, we signed six Exclusive Territory Agent contracts and as of September 30, 2009 we have increased our number of contracts to a total
of fifteen. As the company expands into new markets and gains more access to additional capital, we expect to increase the number of contracts with Exclusive Territory Agents. Please see Exhibit 10.2.
Special Marketing Initiatives
Coors Brand Initiative
Shaanxi Jaili intends to further develop the series of products based on the Coors Brand and designed to target the pediatric medicine market. These products will continue be sold through exclusive territory agents who in turn distribute to local pharmacies in their assigned territories.
Regional Expansion Initiative
Shaanxi Jiali intends to further expand its distribution channels by increasing the number of exclusive territory agents. These additional exclusive territory agents will be contracted to distribute Shaanxi Jaili products to local pharmacies in several additional provinces or regions throughout China.
Status of Publicly Announced New Products
We expect that the following products in our development pipeline will generate growth in our revenues in the next few years. We expect to begin mass production for products for which we recently received SFDA approval as soon as possible.
Development Status of Key Products in our pipeline as of September 30, 2009:
|
Proposed Manufacturer |
Name of Brand and Product name |
Type:
Prescription/
OTC Conventional/
OTC TCM |
Primary Function |
SFDA Status | ||||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Fuxiening Granules |
OTC TCM |
Treatment for common cold symptoms |
Application Submitted to SFDA waiting for approval | ||||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Kechuanling Granule |
OTC TCM |
Treatment for common cold symptoms |
Application Submitted to SFDA waiting for approval | ||||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Kangganjiedu Granules |
OTC TCM |
Treatment for common cold symptoms |
Application Submitted to SFDA waiting for approval | ||||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Digesting Tablet |
OTC TCM |
Treatment for common cold symptoms |
Application Submitted to SFDA waiting for approval | ||||
|
Shaanxi Jiali Pharmaceuticals |
Cooer Xiaoer Jianpibuxue Granules |
OTC TCM |
Treatment for common cold symptoms |
Application Submitted to SFDA waiting for approval |
There are certain opportunities for growth through the following growth strategies:
Rapidly growing Chinese pharmaceutical market With approximately one-fifth of the world’s population and a fast-growing gross domestic product, China represents a significant potential market for the pharmaceutical industry. We believe the significant expected
growth of the pharmaceutical market in China is due to factors such as robust economic growth and increased pharmaceutical expenditure, aging population and increased lifestyle-related diseases, government support of the pharmaceutical industry, the relatively low research and development and clinical trial costs in China as compared to developed countries, as well as the increased availability of funding for medical insurance and industry consolidation in China.
Currently China has about 3,500 drug companies, falling from more than 5,000 in 2004, according to government figures. The number is expected to drop further. The domestic companies compete in the $10 billion market without a dominant leader. Most often cited adverse factors include a lack of protection of intellectual property rights, a lack
of visibility for drug approval procedures, a lack of effective governmental incentives, poor corporate support for drug research and differences in the treatment in China accorded to local and foreign firms. The profile of the pharmaceutical industry in China remains very low. China accounts for 20% of the world’s population but only 1.5% of the global drug market. As of 2008, China is the world's eighth largest drug market.
New Product Development. We will continue to evaluate and develop additional product candidates, both through our in-house research and development department and working with our research and development partners, to expand our pipeline where we perceive an unmet
need and commercial potential. We will face the risk that in developing new products we may spend substantial sums of money and the new products developed may not effectively meet the perceived need or may not be successfully commercialized.
Focus on brand development. With intense price competition among many similar or identical products in the industry, we believe that building brand equity is the primary means to generate and sustain profitable growth in the future. The company intends to focus its
brand development efforts on building the Cooers brand name with the intent on it becoming a leading pediatric medicine brand in China.
Marketing and Sales Function. We intend to grow our internal marketing and sales function and increase our relationships with other exclusive territory distributors to expand the distribution and presence of our pharmaceutical products. In expanding market share of
our products, we intend to take advantage of our large manufacturing scale and reasonable cost control mechanisms, and our strong sales network. In addition, our goal is to establish our products as a preferred choice for children’s medicines in local pharmacies. We hope to add other pharmaceutical products into this channel over the next few years. We may face risks in obtaining adequate quantities of raw materials at reasonable prices in order to meet increased demand for our products that result from
any growth. In seeking additional employees, sales representatives, and exclusive territory agents, we will compete with many other established pharmaceutical manufacturers that may have greater resources than we do.
Low cost producer. Our continuing success in optimizing our manufacturing processes and minimizing our production costs provides us with a competitive advantage.
Government sponsored industry consolidation presents opportunities for acquisitions. China’s thousands of domestic companies account for 70 percent of the pharmaceutical market. Anticipating the effects
of WTO entry and in an effort to compete with foreign firms, the Chinese government has decided to nurture its own large pharmaceutical companies, by encouraging the consolidation of its government-owned companies. To this end, the Chinese State Economic and Trade Commission (SETC) announced plans to consolidate the industry and support the development of 10 to 15 largest pharmaceutical firms. According to government statistics China currently has about 3,500 drug companies, down from over 5,000 in 2004. The
number is expected to drop further. As the industry undergoes further consolidation, China Pediatric will have the opportunity to grow by acquisition.
The raw materials used to manufacture our products include various medicinal herbs, which we obtain from specified and qualified suppliers with national qualifications. We also enter into trading agreements for the supply of many of the materials used to manufacture and package our products. Shaanxi Jiali has written agreements with substantially
all of its suppliers.
The five largest suppliers of Shaanxi Jaili in 2008 are as follows:
|
Supplier |
% | |
|
1 |
Shaanxi Aokesen Laboratory Co., Ltd |
21 |
|
2 |
Baoji Weixin Tangye Wholesale Shop |
16 |
|
3 |
Baoji Meixian lvyuan Chinese Herbal Medicine Planting and R&D Co., Ltd |
13 |
|
4 |
Baoji Chencang Qu JieLi Carton Factory |
11 |
|
5 |
Shaanxi Daxin Suye Co.Ltd |
10 |
Shaanxi Aokesen Laboratory Co., Ltd.
Shaanxi Jiali’s largest supplier of bulk chemicals used in the production of conventional over-the-counter pharmaceuticals is Shaanxi Aokesen Laboratory Co, Ltd. In 2008, Shaanxi Jiali purchased raw materials, for the production of our conventional medicines, including gypsum and Erythromycin Ethylsuccinate powder, from this supplier
equivalent to 21% of Shaanxi Jaili’s raw materials.
Baoji Weixin Tangye Wholesale Shop
Shaanxi Jiali’s second largest supplier of bulk chemicals used in the production of over-the-counter TCM pharmaceuticals is Baoji Weixin Tangye Wholesale Shop. In 2008, Shaanxi Jiali purchased raw supporting materials including, cane sugar and Amylin, from this supplier equivalent to 16% of Shaanxi Jaili’s raw materials.
Baoji Meixian Lvyuan Chinese Herbal Medicine Planting and R&D Co, Ltd.
Shaanxi Jiali’s third largest supplier of bulk chemicals used in the production of over-the-counter TCM pharmaceuticals is Baoji Meixian lvyuan Chinese Herbal Medicine Planting and R&D Co., Ltd. In 2008, Shaanxi Jiali purchased raw materials for our TCMs including, Radix Isatidis and Fomes Japonica, from this supplier equivalent
to 13% of Shaanxi Jaili’s raw materials.
Baoji Chencang Qu JieLi Carton Factory
Shaanxi Jiali’s largest supplier of packaging material and our fourth largest overall supplier over is Baoji Chencang Qu JieLi Carton Factory. In 2008, Shaanxi Jiali purchased packaging material, including instructions and boxes, from this supplier equivalent to 11% of Shaanxi Jaili’s overall supply of raw and packaging materials.
Shaanxi Daxin Suye Co. Ltd.
Shaanxi Jiali’s fourth largest supplier of bulk chemicals and firth largest overal supplier is Shaanxi Daxin Suye Co.Ltd. In 2008, Shaanxi Jiali purchased raw materials including, Polyvinyl Chloride (PVC) and Press Through Package (PTP), from this supplier equivalent to 10% of Shaanxi Jaili’s raw materials.
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property as critical factors to our success. We rely on patent, trademark and trade secret law, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights.
Pursuant to the PRC TCM Protection Regulation, certain ready- made TCM products which have received SFDA approval have automatic protected intellectual property rights. For the Protection of the TCM in China, there is an administrative protection period for 8 years. There is transformation of protection to TCM from administrative
protection to legal protection to TCM which will cover all dosage type of this certain medicine approved which is patent protection period of 20 years. Our patents listed in below have a protection period of 20 years to 2025. Once this protection period has expired, a company may apply for patent protection.
To a large extent, we rely on such protection regulation to protect our intellectual property rights with respect to such products.
Two types of medicine-related patents exist in PRC: the medicine production technique patent and medicine invention formula patent. In PRC, illegal infringements of production technique patents are widespread. A tiny modification to a filed medicine production technique might be construed as a new one and argued as not violating the patent
laws. Invention formula patents are as easily imitated as production technique patents. However, since only one SFDA production certificate can be issued on new branded medicine based on the major pharmaceutical ingredients used, a minor modification to the formula would not warrant a new SFDA production certificate. This means that, even if another manufacturer gets to know a patented formula, it won’t be able to produce it, in PRC, without the proper SFDA production certificate.
Shaanxi Jiali currently holds two patents: ZL03 1 08011.1 for the treatment of chronic bronchitis and ZL 03 1 08012.X and for the treatment of dermatosis
|
Product Description |
Patent No. |
Public date | ||
|
A Chinese Traditional Medicine for the treatment of chronic bronchitis disease and the pharmacy |
ZL03 1 08011.1 |
Sept. 14, 2005 | ||
|
A Chinese Traditional Medicine for the treatment of dermatosis and gynecology disease and the pharmacy |
ZL 03 1 08012.X |
Sept. 14, 2005 |
Shaanxi Jiali currently holds nine registered trade names for the following medicine names: Image, Sanseqi, Cooer, Tailaishi, Qingsongling, Beishiting, Zhiben, Xianzhi, Chugan, Yunlaitongjingbao and has filed trademark applications, the approvals of which are pending, for 3 other medicine names or general trademarks.
|
Brand Name |
Certificate No. |
Type of
Products |
Validity Period | |||
|
Sanseqi |
1795539 |
5 |
2002/6/28 to 2012/06/27 | |||
|
Cooer |
3180187 |
5 |
2003/11/21to 2013/ 11/ 20 | |||
|
Tailaishi |
3180186 |
5 |
2003/11/21 to 2013/11/20 | |||
|
Qingsongling |
3180188 |
5 |
2003/11/21 to 2013/11/20 | |||
|
Beishiting |
3241933 |
5 |
2004/01/07 to 2014/ 01/06 | |||
|
Zhiben |
3389585 |
5 |
2004/07/21 to 2014/07/20 | |||
|
Xianzhi |
3898392 |
5 |
2006/06/28 to 2016/06/27 | |||
|
Chugan |
4203833 |
5 |
2009/02/28 to 2019/02/27 | |||
|
Yunlaitongjingbao |
4506025 |
5 |
2008/07/07 to 2018/07/06 |
Activities During the Prior Two Fiscal Years
Research and Development
We develop new products in-house as well as through an arrangement with the Shaanxi Research Institution of Chinese Traditional Medicines, in Xi’an. During 2008, we applied for approvals f drug production for five new drug batches. We expect to continue to develop additional new drugs under this method. During the last two fiscal years,
Shaanxi Jiali spent 1.0 million US dollars exclusively on research and development (“R&D”).
Shaanxi Jiali’s in-house Research & Development department coordinates and supervises the contract based outsourced R&D processes. Shaanxi Jiali’s Research & Development had a staff of five during the year s 2008 and 2009.
Shaanxi Jiali entered into a Medicine Research and Development Agreement with the Shaanxi Research Institute of Chinese Traditional Medicines on December 10, 2007. Under the terms of the Medicine Research and Development Agreement the Shaanxi Research Institute of Chinese Traditional Medicines was contracted for the research and
development and for the application of productional approval of five new “pediatric cold treatment medicines”: Cooer Xiaoer Fuxiening Granules;
Cooer Xiaoer Kechuanling Granule; Cooer Xiaoer Kangganjiedu Granules; Cooer Xiaoer Digesting Table , and Cooer Xiaoer Jianpibuxue Granules. Under the terms of the research and development agreement Shaanxi Jiali is committed to paying the Shaanxi Research Institute of Chinese Traditional Medicines $293,000
for each of the five new products contracted for development. Under the terms of the research and development agreement Shaanxi Jiali owns the Intellectual Property rights for the five new pharmaceutical products developed by the Shaanxi Research Institute of Chinese Traditional Medicines. Please see Exhibit 10.3 Medicine Research and Development Agreement.
The testing, approval, manufacturing, labeling, advertising and marketing, post-approval safety reporting, of our products or product candidates are extensively regulated by governmental authorities in the PRC. Our sole sales market is presently in China. We are subject to the Drug Administration Law of China, which governs the licensing,
manufacturing, marketing and distribution of pharmaceutical products in China and sets penalties for violations of the law. Additionally, we are subject to various regulations and permit systems by the Chinese government. These regulations and their impact on the business of Shaanxi Jiali Pharmaceutical are set forth in more detail below.
|
|
● |
Drug Administration Law of the PRC was promulgated by the Standing Committee of National People’s Congress on February 28, 2001 and effective as of December 1, 2001, and its implemental rules were promulgated by the State Council on August 4, 2004 and effective as of September 15, 2002. According to Drug Administration Law of the PRC
and its implemental rules, a pharmaceutical manufacturer is to obtain a Pharmaceutical Manufacturing Permit and the Drug Approval Number for each manufactured medicine from relevant SFDA’s provincial branch, which are valid for five years and are renewable upon application before expiration. Shaanxi Jiali Pharmaceuticals is required to file for these approvals for each of its medicines and renew them prior to expiration. |
|
|
● |
Administration Regulations for Drug Registration was promulgated by the SFDA on July 10, 2007, and was effective as of October 1, 2007. Administration Regulations for Drug Registration specifies the requirements and procedures of obtaining a Drug Approval Number for new drugs, including the requirements for clinical trial of new drugs, procedures of
registering imported medicines and report and approval procedures of generic medicines. The Drug Approval Number is valid for five years and can be re-registered upon expiration. Shaanxi Jiali Pharmaceuticals is required to obtain a Drug Approval Number for each of its new drugs and reapply prior to the expiration date. |
|
|
● |
Good Manufacturing Practices (GMP) for Pharmaceutical Products, as revised in 1998 was promulgated by the SFDA on June 18, 1999 and became effective as of August 1, 1999, and the Authentication Regulations for Drug GMP was promulgated by the SFDA on September 7, 2005 and became effective on
of October1, 2005. A pharmaceutical manufacturer must meet the GMP standards and obtain the GMP Certificate with a five-year validity period from SFDA. Before the GMP Certification expires, the pharmaceutical manufacturer must apply again and complete the relevant procedures, which may take about 120 working days, to obtain a new GMP Certificate. On October 24, 2007, the SFDA issued the new guideline for authentication standards of GMP, effective
as of January 1, 2008. The new guideline may result in a rise of cost for a pharmaceutical manufacturer to meet the new standards so as to maintain the GMP qualification. If a pharmaceutical manufacturer fails to obtain or maintain GMP Certification and still carry on its production, it will be fined and the Pharmaceutical Manufacturing Permit may be revoked under serious circumstances. Shaanxi Jiali Pharmaceuticals
holds one GMP certificate that expire August 3, 2011, July 1, 2012 and July 1, 2012 and is required to reapply prior to the expiration date and maintain its Pharmaceutical Manufacturing Permit. |
|
|
● |
Administration Regulations for Drug Call-back was promulgated by the SFDA on December 10, 2007 and effective on the same day. According to the Administration Regulations for Drug Call-back, the pharmaceutical manufacturer should establish a drug call-back system and collect information regarding the drug safety. If a manufacturer discovers any unreasonable
danger of drug that threatens people’s safety and health, it should immediately stop the manufacturing and sale of such drug, notify the distributors and report to the branch of the SFDA. This regulation also stipulates the procedures of drug call-back and danger valuation standards established and maintain a drug call back system in conformance the regulations. |
|
|
● |
Administration Regulations for Drug Instructions and Labels was promulgated by the SFDA on March 15, 2006 and was effective as of June 1, 2006. According to Administration Regulations for Drug Instructions and Labels, the contents of instructions and labels of each drug must be approved by the SFDA, and the smallest packing unit of |
|
|
|
drug shall be attached with instructions. Shaanxi Jiali Pharmaceuticals received approval and maintains drug labeling in conformance with the regulations for its existing products and must do so for new products. |
|
|
● |
Supervision Administration Regulations for Drug Distribution was promulgated by the SFDA on January 31, 2007 and effective as of May 1, 2007. According to Supervision Administration Regulations for Drug Distribution, a pharmaceutical manufacturer can only sell drugs produced by itself, and it shall not sell drugs produced by other manufacturers or produced
by itself but for commissioning manufacturing purpose. Shaanxi Jiali Pharmaceuticals does not resell any other pharmaceutical manufacturers drugs. |
|
|
● |
Regulations for Drug Advertisement Censoring was promulgated by the SFDA and State Administration for Industry and Commerce (the “SAIC”) on March 13, 2007 and effective as of May 1, 2007. Standards for Drug Advertisement Censoring and
Publication promulgated by the SFDA and the SAIC on March 3, 2007 and effective as of May 1, 2007. According to Regulations for Drug Advertisement Censoring, a pharmaceutical manufacturer must obtain a Drug Advertisement Approval Number from the provincial branch of the SFDA which is valid period of one year if the drug advertisement describes the functions or benefits of a drug. However, if an over the counter drug advertisement in
any media, or a prescription drug advertisement in professional medical magazine, only refers to the name of the drug, including the general name and commercial name, without any other addition promotional information, the advertisement does not need to be censored or approved. Shaanxi Jiali Pharmaceuticals obtained a Drug Advertisement Approval Number and reviews all of its over the counter and prescription drug advertisements so that it is in conformance with the regulations relating to advertising its products.
The application and approval procedure in China for a newly developed drug product has numerous steps. New drug applicants prepare the documentation of pharmacological study, toxicity study and pharmacokinetics and drug metabolism (PKDM) study and new drug samples. Documentation and samples are then submitted to provincial food and drug administration
(“provincial FDA”). The provincial FDA sends its officials to the applicant to check the applicant’s research and development facilities and to arrange new drug examination committee meeting for approval deliberations. This process usually takes three months. After the documentation and samples are approved by the provincial FDA, the provincial FDA will submit the approved documentation and samples to SFDA. SFDA examines the documentation and tests the samples and arranges new drug examination
committee meeting for approval deliberations. If the application is approved by SFDA, SFDA will issue a clinical trial license to the applicant for clinical trials. The clinical trial license approval typically takes one year. The applicant completes the clinical trial process and prepares documentation and files that are submitted to SFDA for new drug approval. The clinical trial process usually takes one year or two depending on the category and class of the new drug. SFDA examines the documentation and gives
final approval for the new drug and issues the new drug license to the applicant. This process usually takes eight months. The whole process for new drug approval usually takes three to four years.
The SFDA and China Traditional Medicine Administration Bureau regulate the process for new drug approval and licensing in China, which can involve many layers of authority, lacks transparency, and presents one of the greatest obstacles for companies in introducing new drugs into the market. One of the preliminary aspects of the application
process involves a review of the Chinese market’s need for a particular drug. If the SFDA determines that the market niche for a particular drug is saturated, the drug will not receive further consideration and the licensing application will be denied. According to industry analysts, eighty-five percent of the applications for new drugs licensing are determined by SFDA to be in saturated markets and thus are not considered for approval. Only fifteen percent of new-to-market drug applications are
considered for approval by the SFDA.
Shaanxi Jiali Pharmaceuticals’ receipt of the GMP certificate and approval by the SFDA of its prescription and OTC drugs represents a significant competitive advantage as these approvals present a significant barrier to entry by new companies. Any new products may not pass the clinical review and testing process which can negatively
affect cash flow and income. |
We comply with the Environmental Protection Law of PRC as well as applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in
the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
We comply with the Environmental Protection Law of PRC as well as applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in
the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
China Pediatric Pharmaceuticals’ operation and facilities are subject to environmental laws and regulations stipulated by the national and the local environment protection bureaus in China. Relevant laws and regulations include provisions governing air emissions, water discharges and the management and disposal of hazardous substances
and wastes. The PRC regulatory authorities require pharmaceutical companies to carry out environmental impact studies before engaging in new construction projects to ensure that their production processes meet the required environmental standards.
China Pediatric Pharmaceuticals maintains controls at its production facilities to facilitate compliance with environmental rules and regulations. China Pediatric Pharmaceuticals is not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has it been subject to any action
made by any environmental administration authorities of the PRC. To management’s knowledge, China Pediatric Pharmaceuticals’ operation meets or exceeds the existing requirements of the PRC.
Advertisement Law of the People’s Republic of China and Rules of Medicine Advertisements Management from State Admission for Industry and Commerce, Regulations on Control of Advertisements (tentative) from State Council provide guidelines for advertising prescription and OTC drugs and nutrients made by Shaanxi Jiali Pharmaceuticals.
The rules limit where advertisements may be place and govern the claims that may be made by the manufacturer.
The prices of approximately 1,500 pharmaceuticals are presently set by the Chinese government. These constitute approximately 10% of all distributing drugs. The prices for the other 90%, or 12,000 pharmaceuticals, are established by the market (by the companies themselves). Corporations typically establish these prices based on operating costs,
and market supply and demand. The Supervision Department of the Chinese government will intervene only if there is a significant fluctuation in prices or a monopoly develops in a particular drug. We have not had any products subject to specific pricing control and production and trading of none of our pharmaceutical products constitutes a monopoly.
We have approximately 141 full-time employees. Our employee breakdown includes twenty-one in management and administration, fifty-nine in sales, marketing and distribution, fifty-six in production and quality control, and five in research and development. Our employees are not unionised nor represented by a labor group for purposes of collective
bargaining. We have good employee relations with little turnover in staff.
Production Facilities and Equipment
Locations
Our properties are located in Baoji and Xi'an, which is the capital of the Shaanxi province in the People's Republic of China and a sub-provincial city. As one of the oldest cities in Chinese history, Xi'an is one of the Four Great Ancient Capitals of China because it has been the capital of some of the most important dynasties in Chinese
history, including the Zhou, Qin, Han, the Sui, and Tang dynasties. Xi'an is the eastern terminus of the Silk Road and known as the site of the Terracotta Army, made during the Qin Dynasty. The city has more than 3,100 years of history, and was known as Chang'an before the Ming Dynasty. Since the 1990s, as part of the economic revival of interior China especially for the central and northwest regions, the city of Xi'an has re-emerged as an important cultural, industrial and educational center of the central-northwest
region, with facilities for research and development, national security and China's space exploration program.
Headquarters and Administration Offices
Shaanxi Jiali leases approximately 2,200 sq feet of premium office space at the 9th Floor of the China Construction Bank Building, No. 29 Nanxin Street, Xi’an, Shaanxi, PRC, for the purposes of accommodating the company’s executive and administration
staff. The annual lease expense is RMB 156,240.which equals approximately USD22, 900. Please refer to the Exhibit 10.4 Lease Agreement.
Production Plant
Shaanxi Jiali owns the land use rights to approximately 54,000 square feet of land in Shaanxi province. The location of our manufacturing facility, and 28,500 square feet building, is located at No. 48 Xibao Road, Weibin District, City of Baoji , Shaanxi Province, P.R.C. We have a Certificate of House Ownership and Appendix Permit
Certificate of State-owned Land Usufruct.
In connection with the Exchange Agreement, we appointed five new directors to our board and hired six new officers. Furthermore, concurrent with the closing of the Exchange Agreement, Mr. Eric Anderson, our former President, Treasurer, Secretary, and sole Director, resigned from these positions.
The following table sets forth the names, ages, and positions of our new executive officers and directors as of the Closing Date. Executive officers are elected annually by our Board of Directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. Directors are elected
annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
|
NAME |
AGE |
POSITION | ||
|
Jun Xia |
37 |
Chairman, Chief Executive Officer, and President | ||
|
Minggang Xiao |
28 |
Director, Chief Financial Officer | ||
|
Wanxiang Li |
68 |
Independent Director | ||
|
Nanjing Lin |
71 |
Independent Director | ||
|
Xaioying Zhang |
30 |
Independent Director |
Jun Xia, Chairman, Chief Executive Officer and President became our Chairman, Chief Executive Officer and President upon the closing of the Share Exchange transaction on September 30, 2009. Mr. Xia founded Shaanxi Jiali Pharmaceuticals on July 1, 1998. Prior to founding Shaanxi Jiali Pharmaceuticals, Mr.
Xia founded Shaanxi Jiali Broadcasting Co., Ltd in the year 1996 which provided advertising marketing promotion and brand management strategies to clients that included Volkswagon AG and major drink manufacture Wahaha. Mr. Xia is the Standing Director of Shaanxi Pharmaceuticals Association and Vice Chairman of Biomedical Branch of Shaanxi Scientific and Research Association of Chinese Traditional Medicines. In 2001, Mr. Xia was named “Youngest Entrepreneur in Shaanxi Province” by Xi’an
National Hi-Tech Industrial Development Zone. Mr. Jun Xia, graduated from Economy Management School of Northwest University, in 1995. Mr. Xia also attended the Fudan University in Shaanhai
Minggang Xiao, Director & Chief Financial Officer became our director and Chief Financial Officer upon the closing of the Share Exchange transaction on September 30, 2009. Mr. Xiao has been with Shaanxi Jiali since 2006. Mr. Xiao manages the day-to-day operations of the Shaanxi Jiali’s Finance Department. Prior
to joining Shaanxi Jaili Mr. Xiao was the Finance Manager at Shaanxi Xinchuang Management Co. Ltd. in Xi’an from 2005 to 2006. From 2004 to 2005, Mr. Xiao was an Accountant with Financial Bureau of Baoji City, Shaanxi. Mr. Xiao holds the professional designation of Accountant. Mr. Xiao graduated from Xi’an Industrial University with his Bachelor of Accounting Degree in 2004.
Wanxiang Li, Independent Director, became an Independent Director upon the closing of the Share Exchange transaction on September 30, 2009. Ms. Li has been a Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University in Xi;an since 1984.. From 1969 to 1984, Ms. Li was
an Accountant in the Finance Department of the Science and Technology Management Bureau of Government Shaanxi in Xi’an. Ms. Li holds the professional designation of Senior Accountant. Ms Li graduated from the Economy Management School of China Agricultural University in Xi’an with her Master’s Degree in Accounting in 1964.
Nanjing Lin, Independent Director, became an Independent Director upon the closing of the Share Exchange transaction on September 30, 2009. Mr. Lin has been Committee Chief of Government Consulting & Policies Management in Shaanxi province in Xi’an since 1993, after joining in 1986. From 1964 to 1986, Mr.
Lin was Department Head, Finance, at Shayuan Farm of Agr-cultivation Bureau of Shaanxi province in Xi’an. Mr. Lin holds the professional designation of Senior Accountant. Mr. Lin graduated from the Economy Management School of Shaanxi Finance and Economy University where he earned his Bachelor’s degree of Accounting in the year 1964.
Xiaoying Zhang, Independent Director became an Independent Director upon the closing of the Share Exchange transaction on September 30, 2009. Ms. Zhang has been Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd. in Xi’an since 2007. Prior
to her employment with Shaanxia Deli Energy Ms. Zhang was employed as an Accountant with the Department of Finance of Xi’an Instrument Group Ltd. Co. in Xi’an from 2003 until 2007. Ms. Zhang holds the professional designation of Accountant. Ms. Zhang graduated from the Xi’an International University, in Xi’an in 2003 and earned her Bachelor’s Degree of Accounting.
Committees of the Board of Directors
We have appointed the follow committees of the Board of Directors with membership as follows:
Audit Committee
|
● |
Nanjing Lin, Chair, Committee Chief of Government Consulting & Policies Management in Shaanxi province |
|
● |
Wanxiang Li, Member Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University |
| ● | Xiaoying Zhang, Member, Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd. |
Our audit committee consists of our independent directors Mr. Lin, Ms. Li and Ms. Zhang and is chaired by Mr. Lin. The Committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
|
● |
selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors; |
|
● |
reviewing with our independent auditors any audit problems or difficulties and management’s response; |
|
● |
reviewing and approving all proposed related-party transactions, as defined in Item 404 of Regulation S-K under the Securities Act; |
|
● |
discussing the annual audited financial statements with management and our independent auditors; |
|
● |
reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies; |
|
● |
annually reviewing and reassessing the adequacy of our audit committee charter; |
|
● |
such other matters that are specifically delegated to our audit committee by our board of directors from time to time; |
|
● |
meeting separately and periodically with management and our internal and independent auditors; and |
|
● |
reporting regularly to the full board of directors. |
Compensation Committee
|
● |
Wanxiang Li, Chair, Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University |
|
|
● |
Nanjing Lin, Member, Committee Chief of Government Consulting & Policies Management in Shaanxi province | |
| ● | Xiaoying Zhang, Member, Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd. | |
Our compensation committee consists of our independent directors Ms. Li, Mr. Lin and Ms. Zhang and is the Chaired by Ms. Li.
Our compensation committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Members of the compensation committee are not prohibited from direct involvement in determining their
own compensation. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
|
|
● |
approving and overseeing the compensation package for our executive officers; |
|
|
● |
reviewing and making recommendations to the board with respect to the compensation of our directors; |
|
|
● |
reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, evaluating the performance of our chief executive officer in light of those goals and objectives, and setting the compensation level of our chief executive officer based on this evaluation; and |
|
|
● |
reviewing periodically and making recommendations to the board regarding any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans. |
Corporate Governance and Nominating Committee
|
● |
Wanxiang Li, Chair, Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University |
|
● |
Nanjing Lin, Member, Committee Chief of Government Consulting & Policies Management in Shaanxi province |
| ● | Xiaoying Zhang, Member, Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd. |
Our corporate governance and nominating committee consists of our independent directors Ms. Lin, Mr. Li and Ms Zhang. The corporate governance and nominating committee assists the board of directors in identifying individuals qualified to
become our directors and in determining the composition of the board and its committees. The corporate governance and nominating committee is responsible for, among other things:
|
|
● |
identifying and recommending to the board nominees for election or re-election to the board, or for appointment to fill any vacancy; |
|
|
● |
reviewing annually with the board the current composition of the board in light of the characteristics of independence, age, skills, experience and availability of service to us; |
|
|
● |
identifying and recommending to the board the directors to serve as members of the board’s committees; |
|
|
● |
advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any corrective action to be taken; and |
|
|
● |
monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
To the best of our knowledge, none of the following ever occurred to any of our directors and officers.
(1) Any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
(2) Any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
(3) Being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or
(4) Being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated.
We currently have a code of ethics that applies to our officers, employees and directors, including our Chief Executive Officer and senior executives.
Certain potential conflicts of interest are inherent in the relationships between our officers and directors, and us.
From time to time, one or more of our affiliates may form or hold an ownership interest in and/or manage other businesses both related and unrelated to the type of business that we own and operate. These persons expect to continue to form, hold an ownership interest in and/or manage additional other businesses which may compete with ours with
respect to operations, including financing and marketing, management time and services and potential customers. These activities may give rise to conflicts between or among the interests of us and other businesses with which our affiliates are associated. Our affiliates are in no way prohibited from undertaking such activities, and neither we nor our shareholders will have any right to require participation in such other activities.
Further, because we intend to transact business with some of our officers, directors and affiliates, as well as with firms in which some of our officers, directors or affiliates have a material interest, potential conflicts may arise between the respective interests of us and these related persons or entities. We believe that such transactions
will be effected on terms at least as favorable to us as those available from unrelated third parties.
With respect to transactions involving real or apparent conflicts of interest, we have adopted policies and procedures which require that: (i) the fact of the relationship or interest giving rise to the potential conflict be disclosed or known to the directors who authorize or approve the transaction prior to such authorization or approval,
(ii) the transaction be approved by
a majority of our disinterested outside directors, and (iii) the transaction be fair and reasonable to us at the time it is authorized or approved by our directors.
Neither we, nor any of our controlled affiliates, are involved in any lawsuit outside the ordinary course of business, the disposition of which would have a material effect upon either our results of operations, financial position, or cash flows.
Our board of directors has determined that Ms. Wanxiang Li, Xiaoying Zhang and Mr. Nanjing Lin are “independent” in accordance with rule 4200(a) (15) of the NASDAQ Stock Market Rules. Messrs. Jun Xia and Minggang Xiao are not “independent” because they are officers of ours. There are no family relationships among our
directors or officers and none of our directors have any arrangement or understanding with any other person pursuant to which they were selected as a director.
Share Exchange Related Transaction
Pursuant to the share exchange agreement of September 30, 2009, Eric Anderson transferred 5,000,000 shares of common stock of Lid Hair Studios to Lid Hair Studios and forgive the outstanding shareholder’s loan to the company in the amount of $86,173in exchange for 100% of the issued and outstanding shares of Lid Hair Studios’ wholly-owned
subsidiary, Belford Enterprises B.C. Ltd., d/b/a Lid Hair Studio. Lid Hair Studios cancelled the shares of common stock received from Eric Anderson prior to affecting the reverse stock split which reduce the amount of common stock then issued and outstanding. Eric Anderson and Lid Hair Studios entered into a binding agreement with respect to such transaction on September 30, 2009.
Shaanxi Jiali Reorganization Related Transactions
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm through its wholly owned subsidiary China Children Pharmaceutical, Inc. (“China Children”), became the indirect holding company for Shaanxi Jiali Pharmaceutical Co. Ltd. ("Shaanxi Jiali"), a medical and pharmaceutical
developer, manufacturer and marketer in the PRC.
China Children was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm.
Xi-an Coova Children Pharmaceuticals Co., Ltd, (“Xi-an Coova”) is a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly owned subsidiary of China Children.
On August 4, 2008, an Entrustment Management Agreement (described in detail on page X) was entered into between Xi-an Coova and Shaanxi Jiali, to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China Children shall receive
all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
The organization and ownership structure of the Company subsequent to the consummation of the reorganization as summarized in the paragraphs above is as follows:
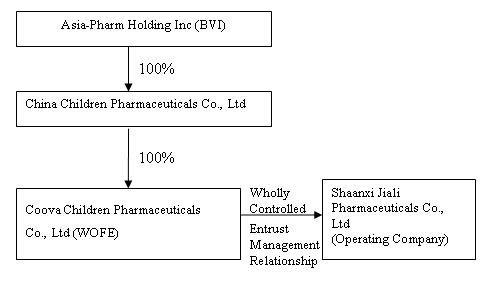
CPPI EXECUTIVE COMPENSATION SUMMARY
The following Executive Compensation Chart highlights the compensation for our executive officers. No other executive officers received salary and bonus in excess of $100,000 for the prior three fiscal years.
Compensation of Directors and Executive Members
|
Long Term Compensation |
||||||||||||||||
|
Annual Compensation |
Awards |
Payouts |
||||||||||||||
|
Name
and
Principal
Position |
Year |
Salary
($) |
Bonus ($) |
Other
Annual Compensation ($) |
Restricted
Stock
Award(s)
($) |
Securities
Underlying Options/
SARs (#) (#) |
LTIP
Payouts ($) |
All Other
Compensation
($) | ||||||||
|
Eric Anderson (former CEO and President) (1) |
2009
2008
2007 |
$1,173
$0
$0 |
$0
$0
$0 |
$0
$0
$0 |
N/A
N/A
N/A |
N/A
N/A
N/A |
N/A
N/A
N/A |
N/A
N/A
N/A | ||||||||
|
Jun Xia (CEO)(2) |
2008 |
$10,553 |
$0 |
$0 |
N/A |
N/A |
N/A |
$0 | ||||||||
|
Minggang Xiao (CFO)(2) |
2008 |
$7,036 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Jing Fu, Senior Vice President |
2008 |
$8,794 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Qina Sun, Vice President Sales & Marketing |
2008 |
$6,156 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Yanxin Li, Vice President Operations |
2008 |
$5,277 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Lijuan Feng, Vice President Administration |
2008 |
$5,277 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Juan Wei, Vice President Corporate Affairs |
$5,277 |
|||||||||||||||
|
(1) |
Eric Anderson resigned as our Chairman, Chief Executive Officer, President and Chief Financial Officer on September 30, 2009. |
|
(2) |
As of the Closing of the Share Exchange Agreement we executed employment agreements with our CEO and CFO. The employment agreement for Jun Xia is for a term of three years. The employment agreement for Minggang Xiao is for one year. The employment agreements will provide for annual salaries and annual bonuses in amounts not less
than the amounts set forth in the table above. |
SHAANXI JIALI EXECUTIVE COMPENSATION SUMMARY
Summary Compensation Table
The following table sets forth all cash compensation paid by Shaanix Jiali, for last fiscal year, specifically, for the year ending December 31, 2008. The table below sets for the positions for each person at Shaanix Jiali. All amounts are in USD.
|
Name/Principal Position |
Year |
Salary (Cash & Non-Cash) |
Bonus |
|||||||
|
Jun Xia, Chairman of the Board of Directors and Chief Executive Officer |
2008 |
$ | 10,553 | $ | 0 | |||||
|
Minggang Xiao, Director & Chief Financial Officer |
2008 |
$ | 7,036 | $ | 0 | |||||
|
Jing Fu, Senior Vice President |
2008 |
$ | 8,794 | $ | 0 | |||||
|
Qina Sun, Vice President, Sales & Marketing |
2008 |
$ | 6,156 | $ | 0 | |||||
|
Yanxin Li, Vice President of Operations |
2008 |
$ | 5,277 | $ | 0 | |||||
|
Lijuan Feng, Vice President Administration |
2008 |
$ | 5,277 | $ | 0 | |||||
|
Juan Wei, Vice President Corporate Affairs |
2008 |
$ | 5,277 | $ | 0 | |||||
Option Grants
We do not maintain any equity incentive or stock option plan. Accordingly, we did not grant options to purchase any equity interests to any employees or officers, and no stock options are issued or outstanding to any officers.
Employment Contracts
As of the Closing, we executed employment agreements with each of our executive officers, specifically, Jun Xia, our Chief Executive Officer; and, our Chief Financial Officer. The employment agreement for our CEO has a term of three years. The employment agreement for our CFO is for a term of one year. The employment agreements
provide for annual salaries and annual bonuses in amounts as set forth in the table above entitled “Shaanxi Jiali” Please see Exhibit 10.5 and 10.6 Employment Contracts.
CPPI EXECUTIVE COMPENSATION SUMMARY
The following Executive Compensation Chart highlights the compensation for our executive officers. No other executive officers received salary and bonus in excess of $100,000 for the prior three fiscal years.
Compensation of Directors and Executive Members
|
Long Term Compensation |
||||||||||||||||
|
Annual Compensation |
Awards |
Payouts |
||||||||||||||
|
Name
and
Principal
Position |
Year |
Salary
($) |
Bonus ($) |
Other
Annual Compensation ($) |
Restricted
Stock
Award(s)
($) |
Securities
Underlying Options/
SARs (#) (#) |
LTIP
Payouts ($) |
All Other
Compensation
($) | ||||||||
|
Eric Anderson (former CEO and President) (1) |
2009
2008
2007 |
$1,173
$0
$0 |
$0
$0
$0 |
$0
$0
$0 |
N/A
N/A
N/A |
N/A
N/A
N/A |
N/A
N/A
N/A |
N/A
N/A
N/A | ||||||||
|
Jun Xia (CEO)(2) |
2008 |
$10,553 |
$0 |
$0 |
N/A |
N/A |
N/A |
$0 | ||||||||
|
Minggang Xiao (CFO)(2) |
2008 |
$7,036 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Jing Fu, Senior Vice President |
2008 |
$8,794 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Qina Sun, Vice President Sales & Marketing |
2008 |
$6,156 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Yanxin Li, Vice President Operations |
2008 |
$5,277 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Lijuan Feng, Vice President Administration |
2008 |
$5,277 |
$0 |
$0 |
N/A |
N/A |
N/A |
N/A | ||||||||
|
Juan Wei, Vice President Corporate Affairs |
$5,277 |
|||||||||||||||
|
(1) |
Eric Anderson resigned as our Chairman, Chief Executive Officer, President and Chief Financial Officer on September 30, 2009. |
|
(2) |
As of the Closing of the Share Exchange Agreement we executed employment agreements with our CEO and CFO. The employment agreement for Jun Xia is for a term of three years. The employment agreement for Minggang Xiao is for one year. The employment agreements will provide for annual salaries and annual bonuses in amounts not less
than the amounts set forth in the table above. |
SHAANXI JIALI EXECUTIVE COMPENSATION SUMMARY
Summary Compensation Table
The following table sets forth all cash compensation paid by Shaanix Jiali, for last fiscal year, specifically, for the year ending December 31, 2008. The table below sets for the positions for each person at Shaanix Jiali. All amounts are in USD.
|
Name/Principal Position |
Year |
Salary (Cash & Non-Cash) |
Bonus |
|||||||
|
Jun Xia, Chairman of the Board of Directors and Chief Executive Officer |
2008 |
$ | 10,553 | $ | 0 | |||||
|
Minggang Xiao, Director & Chief Financial Officer |
2008 |
$ | 7,036 | $ | 0 | |||||
|
Jing Fu, Senior Vice President |
2008 |
$ | 8,794 | $ | 0 | |||||
|
Qina Sun, Vice President, Sales & Marketing |
2008 |
$ | 6,156 | $ | 0 | |||||
|
Yanxin Li, Vice President of Operations |
2008 |
$ | 5,277 | $ | 0 | |||||
|
Lijuan Feng, Vice President Administration |
2008 |
$ | 5,277 | $ | 0 | |||||
|
Juan Wei, Vice President Corporate Affairs |
2008 |
$ | 5,277 | $ | 0 | |||||
Option Grants
We do not maintain any equity incentive or stock option plan. Accordingly, we did not grant options to purchase any equity interests to any employees or officers, and no stock options are issued or outstanding to any officers.
Employment Contracts
As of the Closing, we executed employment agreements with each of our executive officers, specifically, Jun Xia, our Chief Executive Officer; and, our Chief Financial Officer. The employment agreement for our CEO has a term of three years. The employment agreement for our CFO is for a term of one year. The employment agreements
provide for annual salaries and annual bonuses in amounts as set forth in the table above entitled “Shaanxi Jiali” Please see Exhibit 10.5 and 10.6 Employment Contracts.
The following table sets forth certain information regarding common stock beneficially owned on September 30, 2009, after the closing, for (i) each stockholder known to be the beneficial owner of 5% or more of our outstanding common stock, (ii) each executive officer and director, and (iii) all executive officers and directors as a group,
to reflect the closing of the Exchange and the exercise of the Warrants.
Assuming the exercise of the Warrants, as of September 30, 2009, we have 10,728,571 shares of common stock outstanding.
|
Name of Beneficial Owner |
Amount of Beneficial Ownership |
Percent of Beneficial Ownership (4) |
||||||
|
Jun Xia, Director & CEO |
2,450,000 | 28.8 | % | |||||
|
Yangling Bodisen Biotech Development, Inc.(1) |
2,018,590 | 18.8 | % | |||||
|
IFG Investments Services, Inc. |
1,200,000 | 11.2 | % | |||||
|
China National Information Network Trust |
800,000 | 7.5 | % | |||||
|
Minggang Xiao, Director & CFO |
0 | 0 | % | |||||
|
Wanxiang Li, Director |
0 | 0 | % | |||||
|
Nanjing Lin, Director |
0 | 0 | % | |||||
|
Xiaoying Zhang, Director |
0 | 0 | % | |||||
|
All Executive Officers and Directors as a group (1 persons) |
2,450,000 | 28.8 | % | |||||
|
(1) |
Chen Bo is the beneficial owner of Yahling Bodisen Biotech Developement, Inc. |
|
(2) |
Daniel MacMullin is the beneficial owner of IFG Investments Services, Inc. |
|
(3) |
Clare Donahue is the beneficial owner of China National Information Network, Trust |
|
(4) |
Based on 10,728,571 shares issued and outstanding as of the Closing of the Exchange Agreement. |
The table below sets forth the name of each person who is offering for resale shares of common stock covered by this prospectus, the number of shares of common stock beneficially owned by each person, the number of shares of common stock that may be sold in this offering, and the number of shares of common stock each person will own after
the offering, assuming they sell all of the shares offered. To the extent that any successor(s) to the named selling stockholders wishes to sell under this prospectus, we will file a prospectus supplement identifying such successor(s) as selling stockholders. None of the selling stockholders are affiliates of broker-dealers.
The shares of common stock being offered in this prospectus (including shares issuable upon the conversion of convertible promissory notes) were issued in private placement transactions by us, each of which was exempt from the registration requirements of the Securities Act of 1933, as amended, pursuant to Section 4(2) of the Securities
Act.
Because the selling stockholders may offer all, some, or none of their shares of our common stock, we cannot provide a definitive estimate of the number of shares that the selling stockholders will hold after this offering.
None of the selling stockholders has at any time during the past three years acted as one of our employees, officers, or directors or otherwise had a material relationship with us.
For purposes of the following table, beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission. Each selling stockholder’s percentage of ownership in the following table is based on 10,805,288 shares of common stock outstanding as of December 31, 2009.
|
Selling Stockholder |
Shares beneficially
owned prior to the
offering |
Number of
common shares
registered in
this prospectus |
Shares beneficially
owned after the
offering | |||||||
|
Number |
Percent |
Number |
Percent | |||||||
|
IFG Investments Service, Inc. (1) |
|
1,200,000 |
|
11.2 |
|
1,200,000 |
|
0 |
|
0% |
|
China National Information Network Trust (2) |
800,000 |
7.5 |
800,000 |
0 |
0% | |||||
|
Kang Xiulan |
500,000 |
4.7 |
500,000 |
0 |
0% | |||||
|
———————
| |
|
(1) |
Daniel MacMullin is the beneficial owner of IFG Investments Services, Inc. |
| (2) | Clare Donahue is the beneficial owner of China National Information Network, Trust |
The selling stockholders and any of their respective pledgees, donees, assignees, and other successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions.
We have agreed, subject to certain limits, to bear all costs, expenses, and fees of registration of the shares of our common stock offered by the selling stockholders for resale. However, any brokerage commissions, discounts, concessions, or other fees, if any, payable to broker-dealers in connection with any sale of shares of common stock
will be borne by the selling stockholders selling those shares or by the purchasers of those shares.
On our being notified by a selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of shares through a block trade, special offering, exchange distribution, or secondary distribution, or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to
Rule 424(b) under the Securities Act, disclosing the following:
| ● | the name of each such selling stockholder and of any participating broker-dealer |
| ● | the number of securities involved |
| ● | the price at which such securities were sold |
| ● | the commissions paid or discounts or concessions allowed to any broker-dealer, where applicable that any broker-dealer did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus |
| ● | other facts material to the transaction. |
| ● | the price at which such securities were sold |
| ● | the commissions paid or discounts or concessions allowed to any broker-dealer, where applicable that any broker-dealer did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus |
| ● | other facts material to the transaction. |
The selling stockholders may use any one or more of the following methods when selling shares:
| ● | directly as principals |
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers |
| ● | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account |
| ● | an exchange distribution in accordance with the rules of the applicable exchange |
| ● | privately negotiated transactions |
| ● | short sales that are in compliance with the applicable laws and regulations of any state or the United States |
| ● | broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share |
| ● | a combination of any such methods of sale |
| ● | any other method permitted pursuant to applicable law |
The selling stockholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Any sales of the shares may be effected through the OTC Bulletin Board, in private transactions or otherwise, and the shares may be sold at market prices prevailing at the time of sale, at prices related to prevailing market prices.
The selling stockholders may also engage in short sales against the box, puts and calls, and other transactions in our securities or derivatives of our securities and may sell or deliver shares in connection with these trades. The selling stockholders may pledge their shares to their brokers under the margin provisions of customer agreements.
If a selling stockholder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares. We believe that the selling stockholders have not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding sale of their shares other than ordinary course brokerage arrangements, nor is there an underwriter or coordinating broker acting in connection with the proposed sale of shares by the selling stockholders.
Broker-dealers engaged by the selling stockholders may arrange for other brokers-dealers to participate in sales. If the selling stockholders effect sales through underwriters, brokers, dealers or agents, such firms may receive compensation in the form of discounts, concessions or commissions from the selling stockholders or the purchasers
of the shares for whom they may act as agent, principal or both in amounts to be negotiated. Those persons who act as broker-dealers or underwriters in connection with the sale of the shares may be selected by the selling stockholders and may have other business relationships with, and perform services for, us. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
Any selling stockholder or broker-dealer who participates in the sale of the shares may be deemed to be an “underwriter” within the meaning of section 2(11) of the Securities Act. Any commissions received by any underwriter or broker-dealer and any profit on any sale of the shares as principal may be deemed to be underwriting discounts
and commissions under the Securities Act.
The anti-manipulation provisions of Rules 101 through 104 of Regulation M promulgated under the Exchange Act may apply to purchases and sales of shares of common stock by the selling stockholders. In addition, there are restrictions on market-making activities by persons engaged in the distribution of the common stock.
Under the securities laws of certain states, the shares may be sold in those states only through registered or licensed brokers or dealers. In addition, in certain states the shares may not be able to be sold unless our common stock has been registered or qualified for sale in that state or an exemption from registration or qualification is
available and is complied with.
We are required to pay expenses incident to the registration, offering, and sale of the shares under this offering. We estimate that our expenses will total approximately $50,000. We have agreed to indemnify certain selling stockholders and certain
other persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments to which those selling stockholders or their respective pledgees, donees, transferees or other successors in interest may be required to make in respect thereof. Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers and controlling persons, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
As of September 30, 2009, our authorized capital stock consists of 75,000,000 shares of common stock, par value $0.001 per share. As of December 15, 2009, an aggregate of 8,305,288 shares of Common Stock were outstanding, including shares issued pursuant to the Closing.
There are no shares of preferred stock outstanding.
Common Stock
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of Common Stock are entitled to receive dividends out of assets legally available therefore at times and in amounts as our board of directors may determine. Each stockholder is entitled to one vote for each share of
Common Stock held on all matters submitted to a vote of the stockholders. Cumulative voting is not provided for in our amended articles of incorporation, which means that the majority of the shares voted can elect all of the directors then standing for election. The Common Stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon the occurrence of a liquidation, dissolution or winding-up, the holders of shares of Common Stock are entitled to share rateably in all assets
remaining after payment of liabilities and satisfaction of preferential rights of any outstanding preferred stock. There are no sinking fund provisions applicable to the Common Stock. The outstanding shares of Common Stock are, and the shares of Common Stock to be issued upon conversion of the Warrants will be, fully paid and non-assessable.
Voting Rights
Holders of our common stock have the right to cast one vote for each share of stock in their name on the books of our company, whether represented in person or by proxy, on all matters submitted to a vote of holders of common stock, including election of directors. There is no right to cumulative voting in the election of directors. Except
where a greater requirement is provided by statute or by the articles of incorporation, or in the by-laws, the presence, in person or by proxy duly authorized, of the one or more holders of a majority of the outstanding shares of our common stock constitutes a quorum for the transaction of business. The vote by the holders of a majority of outstanding shares is required to effect certain fundamental corporate changes such as liquidation, merger, or amendment of our articles of incorporation.
Dividends
Any future determination as to the declaration and payment of dividends on shares of our Common Stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of Common Stock. In addition, we currently
have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from China Children Pharmaceutical and Coova and Jiali. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable
future. See “Risk Factors.”
Preemptive Rights
Holders of our common stock are not entitled to preemptive rights, and no redemption or sinking fund provisions are applicable to our common stock. All outstanding shares of our common stock are, and the shares of common stock sold in the offering will when issued be fully paid and non-assessable.
TranShare Corporation, 5105 DTC Parkway, Suite 325, Greenwood Village, CO 80111-2608 Tel: 303-662-1112.
The Company approved and authorized the engagement of Acquavella, Chiarelli, Shuster, Berkower & Co., LLP Certified Public Accountants and Advisors, 518 Route One, Suite 4103, Iselin, NJ 08830 as the principal independent accountant for the Company. In addition, effective October 14, 2009, the Company selected Acquavella, Chiarelli,
Shuster, Berkower & Co. LLP, Certified Public Accountants and Advisors as the independent public accountants for the Company for the fiscal year ending December 31, 2009.
No expert or counsel named in this registration statement as having prepared or certified any part of this statement or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or
will receive, in connection with the offering, a substantial interest, direct or indirect, in us. Nor was any such person connected with us as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
Neither we, nor any of our controlled affiliates, are involved in any lawsuit outside the ordinary course of business, the disposition of which would have a material effect upon either our results of operations, financial position, or cash flows.
The General Corporation Law of Nevada provides that directors, officers, employees or agents of Nevada corporations are entitled, under certain circumstances, to be indemnified against expenses (including attorneys’ fees) and other liabilities actually and reasonably incurred by them in connection with any suit brought against
them in their capacity as a director, officer, employee or agent, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. This statute provides that directors, officers, employees and agents may also be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by them in
connection with a derivative suit brought against them in their capacity as a director, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification may be made without court approval if such person was adjudged liable to the corporation.
Our by-laws provide that we shall indemnify our officers and directors in any action, suit or proceeding unless such officer or director shall be adjudged to be derelict in his or her duties.
Our Certificate of Incorporation and By-Laws provide that we will indemnify our directors and officers from all liabilities incurred by them in connection with any action, suit or proceeding in which they are involved by reason of their acting as our directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore,
unenforceable.
None.
In accordance with the Securities Act of 1933, we filed with the SEC a registration statement on Form S-1 covering the securities in this offering. As permitted by rules and regulations of the SEC, this prospectus does not contain all of the information in the registration statement. For further information regarding both our company
and the securities in this offering, we refer you to the registration statement, including all exhibits and schedules, which you may inspect without charge at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549 on official business days during the hours of 10:00 a.m. to 3:00 p.m. You may obtain information on the operation of the Public
Reference room by calling the Commission at 1-800-SEC-0330. The Commission maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Commission. The address of this Internet site is (http://www.sec.gov).
We expect to be subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, and in accordance with the Securities Exchange Act of 1934, we expect to file annual, quarterly and special reports, and other information with the SEC. These periodic reports and other information will be available for inspection and copying at the public reference facilities and website of the SEC referred to above. You also may request a copy of the registration statement and these filings
by writing or calling us at 9th Floor, No. 29 Nanxin Street, Xi’an, Shaanxi Province P.R.C., 710004, telephone: 86 29 8727 1818.
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC.)
CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
TABLE OF CONTENTS
ACSBAcquavella, Chiarelli, Shuster, Berkower & Co., LLP
|
517 Route one |
1 Penn Plaza |
|
Iselin, New Jersey, 08830 |
36the Floor |
|
732.855.9600 |
New York, NY 10119 |
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
China Children Pharmaceuticals Co., Ltd.
We have reviewed the accompanying consolidated balance sheets of China Children Pharmaceuticals Co., Ltd., a wholly owned subsidiary of Asia-Pharm Holdings, Inc. as of September 30, 2009 and 2008 and the consolidated statements of operations for the nine months ended
September 30, 2009 and 2008 and consolidated statements of cash flows for the nine months then ended. These financial statements are the responsibility of the Company's management.
We conducted our review in accordance with standards of the Public Company Accounting Oversight Board (United States). A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an
audit conducted in accordance with standards of the Public Company Accounting Oversight Board (United States), the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our reviews, we are not aware of any material modifications that should be made to the accompanying interim financial statements referred to above for them to be in conformity with accounting principles generally accepted in the United States of America.
We have previously audited, in accordance with auditing standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of China Children Pharmaceuticals Co., Ltd. as of December 31, 2008 and the related consolidated statements of income, retained earnings and comprehensive income, and consolidated
statement of cash flows for the year then ended; and in our report dated September 14, 2009 we expressed an unqualified opinion on those financial statements. In our opinion, the information set forth in the accompanying consolidated balance sheets as of December 31, 2008, is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
Certified Public Accountants
New York, N.Y.
November 23, 2009
F-1
|
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC.)
CONSOLIDATED BALANCE SHEETS
AS OF SEPTEMBER, 2009 AND DECEMBER 31, 2008 |
||||||||
|
ASSETS |
9/30/2009 |
12/31/2008 |
||||||
|
Current Assets |
||||||||
|
Cash and cash equivalents |
$ |
2,821,187 |
$ |
813,252 |
||||
|
Accounts receivable, net |
3,908,552 |
3,286,863 |
||||||
|
Other receivable |
8,649 |
41,578 |
||||||
|
Due from related parites |
6,684 |
- |
||||||
|
Inventory |
1,711,196 |
543,879 |
||||||
|
Prepaid expenses |
3,336,038 |
2,935,404 |
||||||
|
Total Current Assets |
11,792,306 |
7,620,976 |
||||||
|
Property & equipment, net |
819,466 |
900,857 |
||||||
|
Goodwill |
612,745 |
612,745 |
||||||
|
Intangible Assets |
1,939,942 |
2,189,375 |
||||||
|
Total Assets |
$ |
15,164,459 |
$ |
11,323,953 |
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
||||||||
|
Current Liabilities |
||||||||
|
Accounts payable |
$ |
431,134 |
$ |
451,182 |
||||
|
Accrued expenses and other payables |
2,381,301 |
1,490,671 |
||||||
|
Due to related party |
121,686 |
- |
||||||
|
Trade deposit received |
6,308 |
5,936 |
||||||
|
Short-term bank loan |
438,750 |
|||||||
|
VAT tax payable |
16,592 |
147,612 |
||||||
|
Income tax payable |
155,021 |
213,228 |
||||||
|
Total Current Liabilities |
3,550,792 |
2,308,629 |
||||||
|
Stockholders' Equity |
||||||||
|
Capital stock |
$ |
13 |
$ |
13 |
||||
|
Subscription receivables |
- |
(13 |
) | |||||
|
Additional paid in capital |
1,486,363 |
1,486,363 |
||||||
|
Statutory reserve |
880,133 |
721,553 |
||||||
|
Other comprehensive income |
328,368 |
313,429 |
||||||
|
Retained earnings |
8,918,790 |
6,493,979 |
||||||
|
Total Stockholders' Equity |
11,613,667 |
9,015,324 |
||||||
|
Total Liabilities and Stockholders' Equity |
$ |
15,164,459 |
$ |
11,323,953 |
||||
The accompanying notes are an integral part of these financial statements.
F-2
|
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC.) |
||||||||||||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS |
||||||||||||||||
|
(UNAUDITED) |
||||||||||||||||
|
Three Months Ended |
Nine Months Ended |
|||||||||||||||
|
September 30, |
September 30, |
|||||||||||||||
|
2009 |
2008 |
2009 |
2008 |
|||||||||||||
|
Sales, net |
$ |
4,180,368 |
$ |
3,654,066 |
$ |
11,323,143 |
$ |
10,238,589 |
||||||||
|
Cost of sales |
1,589,853 |
1,165,029 |
4,376,541 |
3,298,979 |
||||||||||||
|
Gross profit |
2,590,515 |
2,489,037 |
6,946,602 |
6,939,610 |
||||||||||||
|
Selling, general and administrative expenses |
1,427,655 |
2,209,522 |
3,930,219 |
5,253,803 |
||||||||||||
|
Income from operations |
1,162,860 |
279,515 |
3,016,383 |
1,685,807 |
||||||||||||
|
Other Income (Expense) |
||||||||||||||||
|
Interest income |
61 |
12,532 |
61 |
27,962 |
||||||||||||
|
Other income |
- |
6 |
287 |
109 |
||||||||||||
|
Interest expense |
(10,525 |
) |
(10,525 |
) |
- |
|||||||||||
|
Other expense |
(69 |
) |
(92 |
) |
(99 |
) |
(145 |
) | ||||||||
|
Total other Income (Expense) |
(10,533 |
) |
12,446 |
(10,276 |
) |
27,926 |
||||||||||
|
Income before income taxes |
1,152,327 |
291,961 |
3,006,107 |
1,713,733 |
||||||||||||
|
Provision for income taxes |
154,734 |
5,315 |
422,716 |
276,477 |
||||||||||||
|
Net income |
$ |
997,593 |
$ |
286,646 |
$ |
2,583,391 |
$ |
1,437,256 |
||||||||
|
Consolidated Statement of Comprehensive Income |
||||||||||||||||
|
Net income |
$ |
997,593 |
$ |
286,646 |
$ |
2,583,391 |
$ |
1,437,256 |
||||||||
|
Foreign currency translation adjustment |
8,057 |
8,730 |
14,939 |
185,169 |
||||||||||||
|
Comprehensive income |
$ |
1,005,650 |
$ |
301,598 |
$ |
2,598,330 |
$ |
1,622,425 |
||||||||
The accompanying notes are an integral part of these financial statements.
F-3
|
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC.)
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008 |
||||||||
|
2009 |
2008 |
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES |
||||||||
|
Net Income |
$ |
2,583,391 |
$ |
1,437,256 |
||||
|
Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||
|
Depreciation |
81,391 |
83,393 |
||||||
|
Amortization of intangible assets |
249,433 |
243,931 |
||||||
|
Bad debt expense |
16,771 |
- |
||||||
|
Provision for doubt other receivable |
27,642 |
|||||||
|
(Increase) / decrease in assets: |
||||||||
|
Accounts receivables |
(658,121 |
) |
248,167 |
|||||
|
Inventory |
(1,165,437 |
) |
(400,075 |
) | ||||
|
Prepaid expense |
(393,383 |
) |
(564,055 |
) | ||||
|
Other receivable |
33,019 |
(25,404 |
) | |||||
|
Due from (to) shareholders |
114,947 |
- |
||||||
|
Increase / (decrease) in current liabilities: |
||||||||
|
Accounts payable |
(21,062 |
) |
102,929 |
|||||
|
Accrued expenses and other payables |
886,604 |
(222,303 |
) | |||||
|
Trade deposit received |
357 |
- |
||||||
|
VAT payable |
(131,311 |
) |
(177,395 |
) | ||||
|
Income taxes payable |
(58,707 |
) |
2,228 |
|||||
|
Total Adjustments |
(1,017,857 |
) |
(684,107 |
) | ||||
|
Net cash provided/(used) by operating activities |
1,565,534 |
753,149 |
||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES |
||||||||
|
Acquisitions of property, plant and equipment |
- |
(10,365 |
) | |||||
|
Net cash used by investing activities |
- |
(10,365 |
) | |||||
|
CASH FLOWS FROM FINANCING ACTIVITIES |
||||||||
|
Proceeds from new bank loans |
438,526 |
- |
||||||
|
Subscription received |
13 |
|||||||
|
Net cash used by financing activities |
438,539 |
- |
||||||
|
Effect of exchange rate changes on cash and cash equivalents |
3,862 |
69,304 |
||||||
|
Net change in cash and cash equivalents |
2,007,935 |
812,088 |
||||||
|
Cash and cash equivalents, beginning balance |
813,252 |
542,150 |
||||||
|
Cash and cash equivalents, ending balance |
$ |
2,821,187 |
$ |
1,354,238 |
||||
|
SUPPLEMENTAL DISCLOSURES: |
||||||||
|
Cash paid during the year for: |
||||||||
|
Income tax payments |
$ |
213,429 |
$ |
- |
||||
|
Interest payments |
$ |
10,525 |
$ |
- |
||||
The accompanying notes are an integral part of these financial statements.
F-4
|
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC.)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 |
|
Additional |
Other |
Total |
||||||||||||||||||||||||||
|
Capital Stock |
Subscription |
Paid-in |
Comprehensive |
Statutory |
Retained |
Stockholders |
||||||||||||||||||||||
|
Amount |
Receivables |
Capital |
Income |
Reserves |
Earnings |
Equity |
||||||||||||||||||||||
|
Balance December 31, 2008 |
$ |
13 |
$ |
(13 |
) |
$ |
1,486,363 |
$ |
313,429 |
$ |
721,553 |
$ |
6,493,979 |
$ |
9,015,324 |
|||||||||||||
|
Foreign currency translation adjustments |
14,939 |
14,939 |
||||||||||||||||||||||||||
|
Subscription received |
13 |
13 |
||||||||||||||||||||||||||
|
Transferred to Statutory reserve |
158,580 |
(158,580 |
) |
- |
||||||||||||||||||||||||
|
Income for the nine months ended September 30, 2009 |
2,583,391 |
2,583,391 |
||||||||||||||||||||||||||
|
Balance September 30, 2009 |
$ |
13 |
$ |
- |
1,486,363 |
$ |
328,368 |
$ |
880,133 |
$ |
8,918,790 |
$ |
11,613,667 |
|||||||||||||||
The accompanying notes are an integral part of these financial statements
F-5
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 1 – ORGANIZATION
China Children Pharmaceuticals Co., Ltd., a wholly owned subsidiary of Asia-Pharm Holding Inc., (the “Company”) was formed on June 27, 2008 under the laws of Hong Kong. The Company formed Coova Children Pharmaceuticals Co., Ltd,)”Coova” or the “WOFE”) was formed on July 25, 2008. Coova is a wholly
owned subsidiary of China Children Pharmaceuticals Co., Ltd.
On August 4, 2008, Coova Children Pharmaceuticals Co., Ltd, entered into a Management Entrustment Agreement, with Shaanxi Jiali Pharmaceuticals Co., Ltd. (Jiali") and its shareholders (the “Transaction”). Shaanxi Jiali Pharmaceuticals Co., Ltd. is a corporation formed under the laws of the PRC. According to this Agreement, Coova
acquired management control of Jiali whereby Coova is entitled to all of the net profits of Jiali, as a management fee, and is obligated to fund Jiali operations and pay all of the debts.
The contractual arrangements completed on August 4, 2008 provide that Coova has controlling interest in Jiali as defined by FASB Interpretation No. 46R, “Consolidation of Variable Interest Entities” (“FIN 46R”), an Interpretation of Accounting Research Bulletin No. 51, which requires Coova to consolidate the
financial statements of Jilai and ultimately consolidate with its parent company, China Children Pharmaceuticals Co., Ltd.
The Company, through its subsidiary, and exclusive contractual arrangement with Shaanxi Jiali Pharmaceuticals Co., Ltd., is engaged in the business of manufacturing and marketing over-the-counter (“OTC”) and prescription pharmaceutical products for the Chinese marketplace as treatment for a variety of diseases and conditions.
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The Company's functional currency is the Chinese Renminbi, however the accompanying consolidated financial statements have been translated and presented in United States Dollars.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiary and variable interest entity (“VIE”) for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted the Consolidation Topic
of the FASB Accounting Standards Codification (“ASC 810”) which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
In determining Shaanxi Jiali Pharmaceutical Co. Ltd. is the VIE of Coova Children Pharmaceuticals Co., Ltd., the Company considered the following indicators, among others:
|
● |
Coova has the full right to control and administrate the financial affairs and daily operation of Jiali and has the right to manage and control all assets of Jiali. The equity holders of Jiali as a group have no right to make any decision about Jiali’s activities without the consent of Coova. |
F-6
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
● |
Coova was assigned all voting rights of Jiali and has the right to appoint all directors and senior management personnel of Jiali. The equity holders of Jiali possess no substantive voting rights. |
|
● |
Coova will provide financial support if Jiali requires additional funds to maintain its operations and to repay its debts. |
|
● |
Coova should be paid a management fee equal to Jiali’s earnings before taxes. If there are no earnings before taxes and other cash expenses, then no fee shall be paid. If Jiali sustains losses, they will be carried over to the next period and deducted from the next management fee. Coova should assume
all operation risks of Jiali and bear all losses of Jiali. Therefore, Coova is the primary beneficiary of Jiali. |
Translation Adjustment
As of September 30, 2009 and December 31, 2008, the accounts of the Company were maintained, and its financial statements were expressed, in Chinese Yuan Renminbi (“CNY”). Such financial statements were translated into U.S. Dollars (“USD”) in accordance with the Foreign Currency Matters Topic of the FASB
Accounting Standards Codification (“ASC 830”), with the CNY as the functional currency. According to ASC 830, all assets and liabilities were translated at the current exchange rate, stockholders’ equity are translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with the Comprehensive Income Topic Of the FASB Accounting
Standards Codification (“ASC 220”), as a component of shareholders’ equity. Transaction gains and losses are reflected in the income statement.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues
and expenses during the reporting period. Actual results could differ from those estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”). Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
F-7
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Risks and Uncertainties
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC’s economy. The Company’s business may be influenced
by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company is subject to substantial risks from, among other things, intense competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history, foreign currency exchange rates and the volatility of public markets.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise
of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably
possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed.
Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of
these reserves. Reserves are recorded primarily on a specific identification basis. Allowances for doubtful accounts as of September 30, 2009 and December 31, 2008 were $16,767 and $ 0, respectively.
F-8
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Inventories
Inventories are valued at the lower of cost (determined on a weighted average basis) or market. The Management compares the cost of inventories with the market value and allowance is made for writing down their inventories to market value, if lower. As of September 30, 2009 and December 31, 2008, inventory consist of the following:
|
2009 |
2008 |
|||||||
|
Raw materials |
$ |
919,569 |
$ |
406,666 |
||||
|
Finished goods |
791,627 |
301,199 |
||||||
|
Provision for obsolescence |
- |
(163,986 |
) | |||||
|
Total |
$ |
1,711,196 |
$ |
543,879 |
||||
Property, Plant & Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain
or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Buildings |
30 years |
|
Plant and equipment |
5-14 years |
|
Transportation equipment |
5-10 years |
|
Office equipment |
5-10 years |
As of September 30, 2009 and December 31, 2008, Property, Plant & Equipment consist of the following:
|
2009 |
2008 |
|||||||
|
Buildings |
$ |
445,845 |
$ |
445,845 |
||||
|
Plant and equipment |
859,665 |
859,665 |
||||||
|
Transportation equipment |
5,609 |
5,609 |
||||||
|
Office equipment |
40,354 |
40,354 |
||||||
|
Total |
1,351,473 |
1,351,473 |
||||||
|
Accumulated depreciation |
(532,007 |
) |
(450,616 |
) | ||||
|
$ |
819,466 |
$ |
900,857 |
|||||
Depreciation expense for the nine months ended September 30, 2009 and 2008 were $81,390 and $83,393, respectively.
F-9
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Goodwill
Goodwill represents the excess cost of a business acquisition over the fair value of the net assets acquired. In accordance with the Intangibles, Goodwill and other topic of the FASB Accounting Standard Codification (“ASC 350”), indefinite-life identifiable intangible assets and goodwill are not amortized. Under
the provisions of ASC 350, we are required to perform an annual impairment test of our goodwill. Goodwill impairment is determined using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of a reporting unit, which we define as our business segments, with its net book value or carrying amount including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered
not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test compares the implied fair value of the reporting unit's goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit's goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The implied fair value of
goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. The fair value of the reporting unit is allocated to all of the assets and liabilities of that unit including any unrecognized intangible assets as if the reporting unit had been acquired in a business combination and the fair value of the reporting unit was the purchase price paid to acquire the reporting unit. Goodwill as of September 30, 2009 and December 31, 2008 are $ 612,745 and
$ 612,745.
Intangible Assets
Intangible assets are amortized using the straight-line method over their estimated period of benefit, ranging from ten to fifty years. Management evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists.
No impairments of intangible assets have been identified during any of the periods presented. The land use rights will expire in 2056 and 2058. The proprietary technologies were contributed by four shareholders of the Company and relate to the production of the Company's five state approved drugs. All of the Company’s intangible assets are subject to amortization with estimated lives of:
|
Land use right |
50 years |
|
Proprietary technologies |
10 years |
The components of finite-lived intangible assets are as follows:
|
September 30, |
December 31, |
|||||||
|
2009 |
2008 |
|||||||
|
Land use right |
$ |
264,625 |
$ |
264,625 |
||||
|
Proprietary technologies |
2,966,355 |
2,966,355 |
||||||
|
3,230,980 |
3,230,980 |
|||||||
|
Less: Accumulated amortization |
(1,291,038 |
) |
(1,041,605 |
) | ||||
|
$ |
1,939,942 |
$ |
2,189,375 |
|||||
Amortization expense for the nine months ended September 30, 2009 and 2008 were $249,433 and $243,931, respectively.
F-10
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
The estimated future amortization expenses related to intangible asset as of September 30, 2009 are as follows:
|
2010 |
332,648 |
|||
|
2011 |
332,648 |
|||
|
2012 |
332,648 |
|||
|
2013 |
332,648 |
|||
|
2014 |
332,648 |
|||
|
Thereafter |
$ |
276,702 |
Long-Lived Assets
Effective January 1, 2002, the Company adopted the Property, Plant and Equipment Topic of the FASB Accounting Standard Codification (“ASC 360”), which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, “Accounting for the Impairment of Long-Lived Assets
and for Long-Lived Assets to be Disposed Of,” and the accounting and reporting provisions of Accounting Principles Board Opinion No. 30, “Reporting the Results of Operations for a Disposal of a Segment of a Business.” The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with ASC 360, which requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash
flows estimated to be generated by those assets are less than the assets carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. Based on its review, the Company believes that, as of September 30, 2009, there were no significant impairments of its long-lived assets.
Fair Value of Financial Instruments
The Financial Instrument Topic of the FASB Accounting Standards Codification (“ASC 825”) requires that the Company disclose estimated fair values of financial instruments. The carrying amounts reported in the statements of financial position for current assets and current liabilities qualifying as financial instruments
are a reasonable estimate of fair value.
Revenue Recognition
The Company’s revenue recognition policies are in compliance with the Revenue Recognition Topic of the FASB Accounting Standards Codification (“ASC 605”). Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other
significant obligations of the Company exist and collectibility is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue.
F-11
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Advertising
Advertising expenses consist primarily of costs of promotion for corporate image and product marketing and costs of direct advertising. The Company expenses all advertising costs as incurred.
Income Taxes
The Company utilizes SFAS No. 109, “Accounting for Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences
in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), “Accounting for uncertainty in Income Taxes.” FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition,
measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, “Accounting for Contingencies.” FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company’s financial statements. SFAS No. 109 and FIN 48 were superseded by the Income Taxes Topic of the
FASB Accounting Standards Codification (“ ASC 740”) in September 2009.
Statement of Cash Flows
In accordance with the Statement of Cash Flows Topic of the FASB Accounting Standards Codification (“ASC 230”), cash flows from the Company’s operations are based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes
in the corresponding balances on the balance sheet.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, accounts receivable and other receivables arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base, most of which are
in China. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited.
F-12
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Recent Accounting Pronouncements
In April 2009, the Financial Accounting Standards Board (“FASB”) issued the following new accounting standards:
|
● |
FASB Staff Position FAS No. 157-4, Determining Whether a Market Is Not Active and a Transaction Is Not Distressed, (“FSP FAS No. 157-4”) provides guidelines for making fair value measurements more consistent with the principles presented in SFAS No. 157. FSP FAS
No. 157-4 provides additional authoritative guidance in determining whether a market is active or inactive and whether a transaction is distressed. It is applicable to all assets and liabilities (i.e., financial and non-financial) and will require enhanced disclosures. FSP FAS No. 157-4 was superseded by the Fair Value Measurements and Disclosures Topic of the FASB Accounting Standards Codification (“ASC 820”). |
|
● |
FASB Staff Positions FAS No. 115-2, FAS 124-2, and EITF No. 99-20-2, Recognition and Presentation of Other-Than-Temporary Impairments, (“FSP FAS No. 115-2, FAS No. 124-2, and EITF No. 99-20-2”) provides additional guidance to provide greater clarity about the credit and noncredit
component of an other-than-temporary impairment event and to more effectively communicate when an other-than-temporary impairment event has occurred. This FSP applies to debt securities. FSP FAS No. 115-2 and FAS No. 124-2 were superseded by the Investments-Debt and Equity Securities Topic of the FASB Accounting Standards Codification (“ASC 320”). |
|
● |
FASB Staff Position FAS No. 107-1 and APB No. 28-1, Interim Disclosures about Fair Value of Financial Instruments, (“FSP FAS No. 107-1 and APB No. 28-1”) amends FASB Statement No. 107, Disclosures about Fair Value of Financial
Instruments, to require disclosures about fair value of financial instruments in interim as well as in annual financial statements. This FSP also amends APB Opinion No. 28, Interim Financial Reporting, to require those disclosures in all interim financial statements. FSP FAS No. 107-1 and APB No. 28-1were superseded by the Financial Instruments Topic of the FASB Accounting Standards Codification (“ASC 825”). |
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent
events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB
Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded
all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The
F-13
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair Value, which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used.
However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or
quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In September 2009, the FASB issued ASU No. 2009-12, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent), that amends ASC 820 to provide guidance on measuring the fair value of certain alternative investments such as hedge funds, private
equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the
scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued ASU No. 2009-13, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force, that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these
amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence
is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based
on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. ASU No. 2009-13 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard
on its consolidated results of operations and financial condition.
In October 2009, the FASB issued ASU No. 2009-14, Certain Revenue Arrangements That Include Software Elements—a consensus of the FASB Emerging Issues Task Force, that reduces the types of
F-14
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
transactions that fall within the current scope of software revenue recognition guidance. Existing software revenue recognition guidance requires that its provisions be applied to an entire arrangement when the sale of any products or services containing or utilizing software when the software is considered more than incidental to the product
or service. As a result of the amendments included in ASU No. 2009-14, many tangible products and services that rely on software will be accounted for under the multiple-element arrangements revenue recognition guidance rather than under the software revenue recognition guidance. Under the ASU, the following components would be excluded from the scope of software revenue recognition guidance: the tangible element of the product, software products bundled with tangible products where the software components
and non-software components function together to deliver the product’s essential functionality, and undelivered components that relate to software that is essential to the tangible product’s functionality. The ASU also provides guidance on how to allocate transaction consideration when an arrangement contains both deliverables within the scope of software revenue guidance (software deliverables) and deliverables not within the scope of that guidance (non-software deliverables). ASU No. 2009-14
is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
Note 3 – OTHER RECEIVABLES
Other receivables mainly consist of cash advances to employees. As of September 30, 2009 and December 31, 2008, the other receivables were $8,649 and $41,578, respectively.
Note 4 – DUE FROM/TO RELATED PARTY
The Company has receivables due from a related party. As of September 30, 2009 and December 31, 2008, due from related party were $ 6,684 and $ 0, respectively. The Company also has payables due to a related party. As of September 30, 2009 and December 31, 2008, due to related party were $ 121,686 and $ 0, respectively.
Note 5 – COMPENSATED ABSENCES
Regulation 45 of the local labor law of the People’s Republic of China (“PRC”) entitles employees to annual vacation leave after 1 year of service. In general, all leave must be utilized annually, with proper notification. Any unutilized leave is cancelled.
F-15
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC. )
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 6 – INCOME TAXES
Pursuant to the PRC Income Tax Laws, the Enterprise Income Tax (“EIT”) through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high tech enterprise and under
PRC Income Tax Laws, it is entitled to a two-year exemption for 2006 through 2007. Starting from January 1, 2008, the EIT is at a discounted rate of 15%.
The following is a reconciliation of income tax expense:
|
9/30/2009 |
International |
Total |
||||||
|
Current |
$ |
422,716 |
$ |
422,716 |
||||
|
Deferred |
- |
- |
||||||
|
Total |
$ |
422,716 |
$ |
422,716 |
||||
|
9/30/2008 |
International |
Total |
||||||
|
Current |
$ |
276,477 |
$ |
276,477 |
||||
|
Deferred |
- |
|||||||
|
Total |
$ |
276,477 |
$ |
276,477 |
||||
Note 7 – COMMITMENTS & CONTINGENCIES
The Company leases facilities under operating leases, extending through July 2009, that it pays for on an annual basis and accrues for throughout the year. For the nine months ended September 30, 2009 and 2008, rent expense was $2,420 and $0, respectively.
Note 8 – STATUTORY RESERVE
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax
to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of
cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of September 30, 2009 and December 31, 2008, the Company had allocated $880,133 and $721,553, respectively, to these non-distributable reserve funds.
F-16
CHINA CHILDREN PHARMACEUTICALS CO. LTD.
(A WHOLLY OWNED SUBSIDIARY OF ASIA-PHARM HOLDING, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2009
Note 9 – OTHER COMPREHENSIVE INCOME
Balances of related after-tax components comprising accumulated other comprehensive income, included in stockholders’ equity, at of September 30, 2009, are as follows:
|
Foreign Currency Translation Adjustment |
Accumulated Other Comprehensive Income |
|||||||
|
Balance at December 31, 2008 |
$ | 313,429 | $ | 313,429 | ||||
|
Change for 2009 |
14,939 | 14,939 | ||||||
|
Balance at September 30, 2009 |
$ | 328,368 | $ | 328,368 | ||||
Note 10 – CURRENT VULNERABILITY DUE TO CERTAIN RISK FACTORS
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC's economy. The Company’s business may be influenced by changes in governmental
policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Note 11 – MAJOR CUSTOMERS AND CREDIT RISK
Four customers accounted for more than 10% of accounts receivable at September 30, 2009, totaling 47%; at September 30, 2008, five customers were greater than 10% of accounts receivable, totaling 90%. Four vendors accounted for more than 10% of accounts payable at September 30, 2009, totaling 82%; at September 30, 2008, four vendors accounted
for greater than 10%, totaling 58%.
Six customers accounted for 73% of sales for the nine months ended September 30, 2009 and six customers accounted for 100% of sales for the nine months ended September 30, 2008. Four vendors supplied 80% of purchases for the nine months ended September 30, 2009. Three vendors supplied 51% of purchases for the nine months ended September 30,
2008.
F-17
PART II
Information Required Not Part of the Prospectus
Item 13. Other Expense of Issuance and Distribution
We will pay all expenses in connection with the registration and sale of the common stock by the selling stockholders. The estimated expenses of issuance and distribution are set forth below:
|
Registration fees |
$ | 713.00 | ||
|
Legal fees |
$ | 5,000.00 | ||
|
Accounting fees |
$ | 20,000.00 | ||
|
Miscellaneous |
$ | 5,000.00 | ||
|
Total estimated costs of offering |
$ | 30,713.00 | ||
Item 14. Indemnification of Directors and Officers
The General Corporation Law of Nevada provides that directors, officers, employees or agents of Nevada corporations are entitled, under certain circumstances, to be indemnified against expenses (including attorneys’ fees) and other liabilities actually and reasonably incurred by them in connection with any suit brought against
them in their capacity as a director, officer, employee or agent, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. This statute provides that directors, officers, employees and agents may also be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by them in
connection with a derivative suit brought against them in their capacity as a director, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification may be made without court approval if such person was adjudged liable to the corporation.
Our by-laws provide that we shall indemnify our officers and directors in any action, suit or proceeding unless such officer or director shall be adjudged to be derelict in his or her duties.
Item 15. Recent Sale of Unregistered Securities
Pursuant to the Exchange Agreement, on September 30, 2009, we issued 7,000,000 shares of our Common Stock to Asia-Pharm in exchange for 100% of the outstanding shares of China Children Holdings Limited. Such securities were not registered under the Securities Act of 1933. The issuance of these shares was exempt from registration, in part pursuant
to Regulation S and Regulation D under the Securities Act of 1933 and in part pursuant to Section 4(2) of the Securities Act of 1933. We made this determination based on the representations of China Children which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders
were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that Asia-Pharm understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Concurrent with the Share Exchange Agreement on September 30, 2009, we issued to the consultants two year warrants to purchase 1,250,000 shares of our common stock at an exercise price of $3.00 per share with three year piggyback warrants to purchase 1,250,000 shares of our common stock at an exercise price of $5.00 per share. Such securities
were not registered under the Securities Act of 1933. The issuance of these securities was exempt from registration under Section 4(2) of the Securities Act. We made this determination based on the representations of the Placement Agent, which included, in pertinent part, that such Placement Agent was an "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act and that the Placement Agent was acquiring our common stock for investment purposes for its own
respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Placement Agent understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Warrants
Each Warrant and piggy-back warrant entitles the holder to purchase one share of our Common Stock. The Warrants will be exercisable, in whole or in part, at an exercise price equal to $3.00 per share (“Exercise Price”). The Piggyback Warrants will be exercisable, in whole or in part, at an exercise price equal to $5.00 per share
(“Exercise Price”). The Warrants may be exercised at any time upon the election of the Investor, beginning on the date of issuance and ending of the second anniversary of the closing of the Share Exchange Agreement. The Piggy-back Warrants may be exercised at any time upon the election of the Investor, beginning on the date of issuance and ending of the third anniversary of the closing of the Share Exchange Agreement.
The Warrants will be detachable and separately transferable only during the Warrant exercise period; upon the expiration of the Warrant exercise period, the Warrants will expire and become void.
In order to exercise the Warrants, the Warrant must be surrendered at the office of the Warrant Agent prior to the expiration of the Warrant exercise period, with the form of exercise appearing with the Warrant completed and executed as indicated, accompanied by payment of the full exercise price for the number of Warrants being exercised.
Payment shall be by certified funds or cashier's check payable to “China Pediatric Pharmaceuticals, Inc.” In the case of partial exercise, the Warrant Agent will issue a new warrant to the exercising warrant holder, or assigns, evidencing the Warrants which remain unexercised. In our discretion, the Warrant Agent may designate a location other than our office for surrender of Warrants in the case of transfer or exercise.
The exercise price and number of shares of Common Stock to be received upon the exercise of Warrants are subject to adjustment upon the occurrence of certain events, such as stock splits, stock dividends or our recapitalization. In the event of our liquidation, dissolution or winding up, the holders of Warrants will not be entitled to participate
in the distribution of our assets.
If we shall make any dividend or other distribution of assets to holders of its common stock (a “Distribution”), then the Exercise Price shall be reduced by multiplying such Exercise Price by a fraction of which (i) the numerator shall be the closing bid price of the shares of common stock on the trading day immediately preceding
such record date minus the value of the Distribution applicable to one share of common stock, and (ii) the denominator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date. Additionally, the number of shares underlying the Warrant shall be increased to a number of shares of common stock obtainable immediately prior to the close of business on the record date fixed for determining holders of shares entitled to receive the Distribution multiplied
by the reciprocal of the fraction set forth above. Holders of Warrants will have no voting, pre-emptive, subscription or other rights of shareholders in respect of the Warrants, nor shall the Holders be entitled to receive dividends.
Registration Rights with Respect to Common Stock Underlying the Warrants
We have agreed to register an amount of stock equal to 100% of the shares of Common Stock issuable in connection with the Warrants (“Underlying Common Stock”), on a registration statement to be filed by us. We shall pay the usual costs of such registration.
No holder of any of our currently outstanding securities has any registration rights with respect to the securities held by them. We shall not file any other registration statement for any of our securities (other than the shares of Common Stock underlying the warrants) until such time as the Registration Statement has been filed
and declared effective; provided, however, we may, subject to stockholder approval, establish an equity performance or stock option plan for the benefit of our employees and directors for up to 5% of the outstanding shares of our Common Stock and file a registration statement to register such shares on Form S-8 or a comparable form for such purpose.
On October 2, 2009, we issued 76,600 shares of our Common Stock to employees of the Company as part of their 2009 compensation packages. Such securities were not registered under the Securities Act of 1933. The issuance of these shares was exempt from registration, in part pursuant to Regulation S and Regulation D under the Securities Act
of 1933 and in part pursuant to Section 4(2) of the Securities Act of 1933. We made this determination based on the representations of employees which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Act, and that such shareholders were acquiring our common stock, for investment purposes for their
own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the employees understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Item 16. Exhibits
|
Exhibit Number |
Description | |
|
2.1* |
Share Exchange Agreement by and among Lid Hair Studios International, Inc., Eric Anderson, and Asia-Pharm Holdings, Inc. dated September 30, 2009 * | |
|
2.2* |
Share Exchange Agreement between Lid Hair Studios International, Inc. and Eric Anderson dated September 30, 2009 | |
|
3.1* |
Charter and Articles of Incorporation of Lid Hair Studios International, Inc., a Nevada corporation. (April 2005) | |
|
3.2* |
Amended Articles of Incorporation of Lid Hair Studios International, Inc., a Nevada Corporation (August 2005) | |
|
3.3* |
Amended Articles – Name Change to China Pediatric Pharmaceuticals, Inc. a Nevada Corporation (October 2009) and Plan of Merger Agreement | |
|
4.2* |
Specimen Stock Certificate for Shares of Common Stock of the Company | |
|
10.1* |
Agreement with Exclusive Territory Agents - Sample Agreement Previously Filed | |
|
10.2* |
Medicine Research and Development Agreement – Sample Agreement Previously Filed | |
|
10.3* |
Form of Common Stock Purchase Warrant Previously Filed | |
|
10.4* |
Lease Agreement Previously Filed | |
|
10.5* |
Employment Agreement with Jun Xia, President and CEO Previously Filed | |
|
10.6* |
Employment Agreement with Minggang Xiao, CFO Previously Filed | |
|
10.7* |
Warrant Agreement – IFG Investments Services, Inc. | |
|
10.8* |
Warrant Agreement – China National Information Network Trust Amended October 8, 2009 | |
|
10.9* |
Warrant Agreement – Kang Xiulan Previously Filed | |
|
10.10* |
Warrant Terms and Conditions General Previously Filed | |
|
10.11* |
Consulting Agreement IFG Investment Services, Inc. Previously Filed | |
|
10.12* |
Investor Relations Agreement Amended October 8, 2009 | |
|
10.13* |
Referral Agreement Kang Xiulan Previously Filed | |
|
99.2* |
Patents Previously Filed | |
|
99.3* |
Trademarks Previously Filed |
*Previously Filed on Form 8-K and 8-K/A as Exhibits and can be found on the SEC’s website at www.sec.gov
Item 17. Undertakings
The undersigned registrant hereby undertakes that it will:
|
1. |
File, during any period in which it offers or sells securities, a post- effective amendment to this registration statement to: |
|
|
i. |
Include any prospectus required by section 10(a)(3) of the Securities Act; |
|
|
ii. |
Reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) (§ 230.424(b) of this chapter) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the ‘‘Calculation of Registration Fee’’ table in the effective registration statement; and |
|
|
iii. |
Include any additional or changed material information on the plan of distribution. |
2. For determining liability under the Securities Act, treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
3. File a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 (the ‘‘Act’’) may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Signatures
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements of filing on Form S-1 and authorized this registration statement to be signed on its behalf by the undersigned, in Xi’an City, People’s Republic of China
on January 28, 2010.
|
CHINA PEDIATRIC PHARMACEUTICALS, INC. |
|||||
|
By: |
/s/ Jun Xia |
||||
|
Jun Xia |
|||||
|
Chairman |
|||||
|
(principal executive officer) |
|||||
|
By: |
/s/ Minggang Xiao |
||||
|
Minggang Xiao |
|||||
|
Chief Financial Officer |
|||||
|
(principal financial officer and principal accounting officer) |
|||||
In accordance with the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Date: |
January 28, 2010 |
By: |
/s/ Jun Xia |
||
|
Jun Xia |
|||||
|
Chairman |
|||||
|
(principal executive officer) |
|||||
|
Date: |
January 28, 2010 |
|
By: |
/s/ Minggang Xiao |
|
|
Minggang Xiao |
|||||
|
Chief Financial Officer |
|||||
|
(principal financial officer and principal accounting officer ) |
|||||
|
Date: |
January 28, 2010 |
By: |
/s/ Wanxiang Li |
||
|
Wanxiang Li |
|||||
|
Director |
|||||
|
Date: |
January 28, 2010 |
By: |
/s/ Nanjing Lin |
||
|
Nanjing Lin |
|||||
|
Director |
|||||
|
Date: |
January 28, 2010 |
By: |
/s/ Xiaoying Zhang |
||
|
Xiaoying Zhang |
|||||
|
Director |
Page | 75
