Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | a54806e8vk.htm |
Exhibit 99.1

| Company Update January 11, 2010 |

| Safe Harbor Statement Statements contained in this presentation regarding matters that are not historical facts are "forward- looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Ardea's goals, its preclinical and clinical trial plans, timelines and milestones, its expectations about the size of its markets and commercial potential of its compounds, expected results of future clinical trials, expected properties of compounds under development, financial position, cash usage, licensing and partnering opportunities, liquidity and anticipated milestones. Risks that contribute to the uncertain nature of the forward-looking statements include: risks related to the outcomes of preclinical and clinical trials, risks related to regulatory approvals, delays in commencement of preclinical and clinical tests, costs associated with internal development, and the outcome of our business development activities, including collaboration or licensing agreements. These and other risks and uncertainties are described more fully in Ardea's most recently filed SEC documents, including its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, under the headings "Risk Factors." All forward-looking statements contained in this presentation speak only as of the date of this presentation, and Ardea undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof or otherwise. 2 |

| Status of Development Programs RDEA427 RDEA806 900 Series Discovery Preclinical Phase 0/1 Phase 2 A B Phase 3 NNRTI for HIV NNRTI for HIV MEKI for cancer 3 Gout RDEA594 RDEA684 MEKI + sorafenib for cancer RDEA119 Gout Partnered with Bayer RDEA119 3rd Gen NNRTIs |

| Gout/Hyperuricemia 4 |

| Refractory Disease Has Not Disappeared 5 |

| Hyperuricemia/Gout - Unmet Medical Need Gout is caused by abnormally elevated levels of uric acid (>6.8 mg/dL) Painful and debilitating disease Attacks of severe pain/Inflammation Disfiguring nodules (tophi) Kidney damage (nephropathy) Increasing incidence and severity in U.S. (> 5,000,000 potential patients) 288% increase in gout related hospitalizations from 1988-20051 $11.2 billion hospitalization cost in 20051 Only one new drug available for hyperuricemia in last 40 years Hyperuricemia linked to elevated C-Reactive Protein, hypertension, increased mortality in Chronic Kidney Disease4 and possibly other cardiovascular risk factors2 ~90% of patients are "under-excretors" of uric acid Defect in urate transporter recently found to be genetically linked to gout3 1Ann Rheum Dis 2008;67(Suppl II):96; 2 JAMA. 2008;300(8):924-932 3Nature Genetics 40, 437 - 442 (2008) 4Am J Kidney Dis 2009;53:796-803 6 |

| Hare, J. M. et al. Circulation 2003;107:1951-1953 Uric Acid Production and Elimination Allopurinol Febuxostat RDEA594 Probenecid Losartan (Cozaar(r)) Benzbromarone* 7 *Benzbromarone is a URAT1 inhibitor approved outside of the US and later withdrawn due to liver toxicity. Pegloticase URAT1 |

| >99% Reabsorption Excretion ~100% of uric acid is initially filtered through glomerular filtration URAT1 Inhibitors Increase the Urinary Excretion of Uric Acid URAT1 Enomoto; Urat1 identification in Nature May2002 D Levinson & L Sorensen; Renal Handling of Uric Acid Proximal Tubule Benzbromarone RDEA594 8 |

| 9 Selective URAT1 Transporter Inhibition: An Important Contribution to the Treatment of Hyperuricemia/Gout Inhibition of the URAT1 transporter is a well-validated target for gout Benzbromarone has over 10 million patient-years of treatment experience, with very few side effects, except liver toxicity due to structural defects of that molecule Approximately 170 million tablets of benzbromarone are currently sold per year in Japan (where it is still promoted), even with warnings about severe liver toxicity 90% of gout patients do not excrete normal amounts of uric acid; URAT1 inhibitors can normalize uric acid excretion in these patients, producing sustained reductions in the level of serum urate (sUA) The combination of benzbromarone + allopurinol produces significant reductions in tophi and has been sold as a combined pill; we are currently developing a RDEA594 + allopurinol combination pill Perez-Ruiz F, Arthritis Rheum 2002;47:356-360 Velocity of Tophi Reduction |

| Diet & Metabolism 600-800 mg/day Joint Tophus 10 mg/dL 6 mg/dL serum level Xanthine Oxidase Inhibitor URAT1 Inhibitor <5 mg/dL Combining RDEA594 with XO Inhibitor Should Accelerate Clearance of Urate from Body Pools urine urine 300 mg/day 200 mg/day 600 mg/day 10 |

| Uric Acid-Lowering Was Noticed With RDEA806 Up to 45% reductions in serum urate (sUA) were observed in healthy volunteers in the 10-day multiple ascending dose study of RDEA806 Uric acid reductions were also observed in all HIV patients in the Phase 2a monotherapy study with RDEA806 A 28-day proof-of-concept study with RDEA806 in gout patients confirmed its activity and tolerability in the target patient population with sUA reductions averaging 36%, and individual reductions up to 60% Approximately 250 subjects have received RDEA806 (and exposed to RDEA594) with: No SAEs, deaths or discontinuations due to adverse events No clinically significant changes in physical exam findings or vital signs No clinically significant changes in laboratory parameters except sUA (no evidence of renal toxicity) 11 |
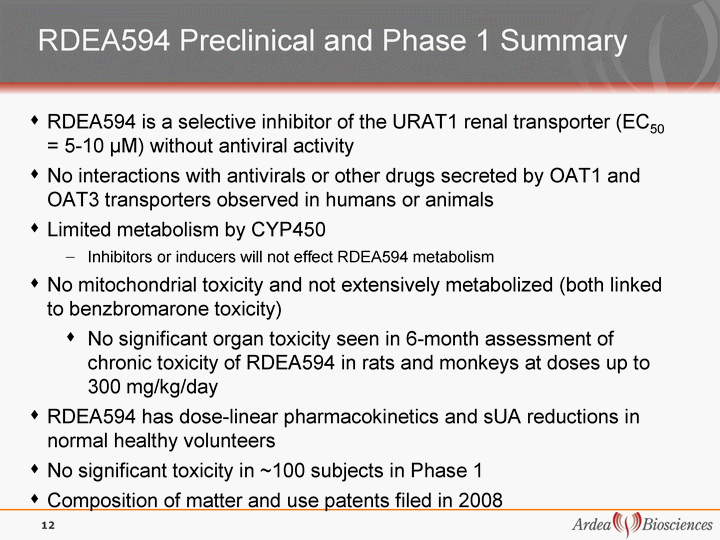
| RDEA594 Preclinical and Phase 1 Summary RDEA594 is a selective inhibitor of the URAT1 renal transporter (EC50 = 5-10 ^M) without antiviral activity No interactions with antivirals or other drugs secreted by OAT1 and OAT3 transporters observed in humans or animals Limited metabolism by CYP450 Inhibitors or inducers will not effect RDEA594 metabolism No mitochondrial toxicity and not extensively metabolized (both linked to benzbromarone toxicity) No significant organ toxicity seen in 6-month assessment of chronic toxicity of RDEA594 in rats and monkeys at doses up to 300 mg/kg/day RDEA594 has dose-linear pharmacokinetics and sUA reductions in normal healthy volunteers No significant toxicity in ~100 subjects in Phase 1 Composition of matter and use patents filed in 2008 12 |

| Arm 1:RDEA594 200 mg QD Screenin g Period Colchicine Treatment Randomize if no gout flare during 2 weeks of colchicine Off drug Off drug Arm 2: Placebo 400 mg QD Off drug Arm 3: Allopurinol 300 mg 5 pts 5 pts 11 pts 7-21 days 1 week 2 weeks total with dose escalation of RDEA594 after Week 1 1 week Population: gout patients with hyperuricemia (serum uric acid ^ 8 mg/dL; ^ 9 mg/dL for cohort 2) Cohort 2: total of 6 patients: 5 on RDEA594 + 1 placebo Duration: 4 wks: 1-wk run-in, 2-wk treatment, 1-wk follow-up Endpoint: Proportion of subjects with sUA level < 6.0 mg/dL at Day 14 RDEA594 Phase 2a Pilot Pharmacodynamic and Safety Study in Gout Patients - Completed Allopurinol 300 mg Allo+ RDEA594 400 mg QD Allo+ RDEA594 200 mg QD Off drug Cohort 2: 6 pts 13 Allopurinol 300 mg Off drug Allopurinol + Placebo Cohort 1: 21 pts |
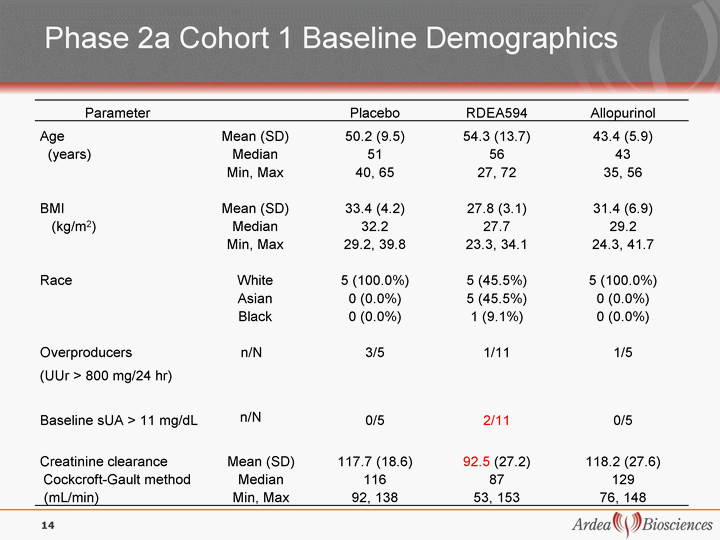
| Phase 2a Cohort 1 Baseline Demographics Parameter Placebo RDEA594 Allopurinol Age Mean (SD) Mean (SD) 50.2 (9.5) 54.3 (13.7) 43.4 (5.9) (years) Median Median 51 56 43 Min, Max Min, Max 40, 65 27, 72 35, 56 BMI Mean (SD) Mean (SD) 33.4 (4.2) 27.8 (3.1) 31.4 (6.9) (kg/m2) Median Median 32.2 27.7 29.2 Min, Max Min, Max 29.2, 39.8 23.3, 34.1 24.3, 41.7 Race White White 5 (100.0%) 5 (45.5%) 5 (100.0%) Asian Asian 0 (0.0%) 5 (45.5%) 0 (0.0%) Black Black 0 (0.0%) 1 (9.1%) 0 (0.0%) Overproducers n/N Overproducers n/N Overproducers n/N 3/5 1/11 1/5 (UUr > 800 mg/24 hr) (UUr > 800 mg/24 hr) Baseline sUA > 11 mg/dL Baseline sUA > 11 mg/dL n/N 0/5 2/11 0/5 Creatinine clearance Creatinine clearance Mean (SD) 117.7 (18.6) 92.5 (27.2) 118.2 (27.6) Cockcroft-Gault method Cockcroft-Gault method Median 116 87 129 (mL/min) (mL/min) Min, Max 92, 138 53, 153 76, 148 14 |

| Randomized Drug Washout Period All Randomized Subjects Excluding Overproducers All Randomized Subjects Excluding Overproducers All Randomized Subjects Excluding Overproducers All Randomized Subjects Excluding Overproducers Response defined as < 6.0 mg/dL Placebo % (n/N) RDEA594 % (n/N) Allopurinol % (n/N) Response Week 1 0 (0/2) 40 (4/10) 75 (3/4) Response Week 2 0 (0/2) 60 (6/10) 100 (4/4) Mean (+-SEM) Change in sUA (%) at nadir -2.6+-3.0% -46.7+-2.6% -44.7+-4.7% Both Active Arms Produced Significant Reductions in sUA 15 |
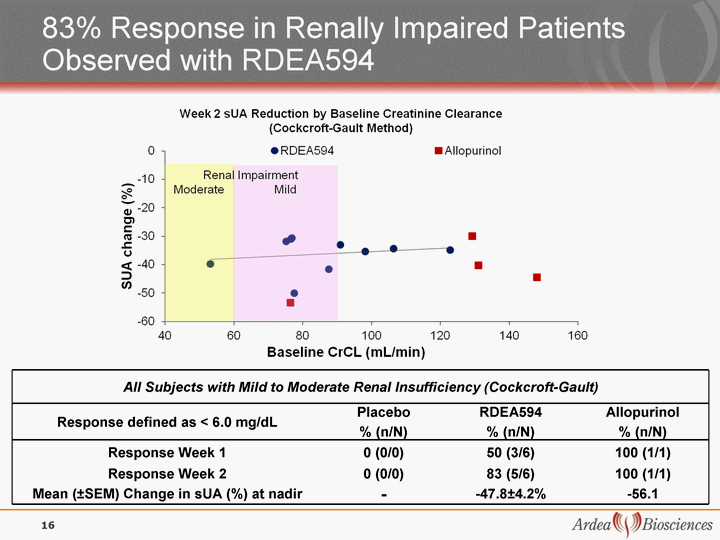
| 83% Response in Renally Impaired Patients Observed with RDEA594 All Subjects with Mild to Moderate Renal Insufficiency (Cockcroft-Gault) All Subjects with Mild to Moderate Renal Insufficiency (Cockcroft-Gault) All Subjects with Mild to Moderate Renal Insufficiency (Cockcroft-Gault) All Subjects with Mild to Moderate Renal Insufficiency (Cockcroft-Gault) Response defined as < 6.0 mg/dL Placebo % (n/N) RDEA594 % (n/N) Allopurinol % (n/N) Response Week 1 0 (0/0) 50 (3/6) 100 (1/1) Response Week 2 0 (0/0) 83 (5/6) 100 (1/1) Mean (+-SEM) Change in sUA (%) at nadir - -47.8+-4.2% -56.1 16 |

| ** ** * ** ** * *P<0.10; **P<0.05 for Allo vs combo LOCF used for allopurinol due to incomplete data 54% Reduction in SUA and 100% Response with RDEA594 Combined with Allopurinol RDEA594 400 mg + Allopurinol produced a reduction of sUA from a mean of 10.2 mg/dL to 4.7 mg/dL, with 100% of patients < 6 mg/dL and 80% below 5 mg/dL. Allopurinol 300 mg BID* *Reinders et al. Ann Rheum Dis 2009;68;892-897 17 |

| RDEA594 and Allopurinol Combination Product Under Development Allopurinol 300 mg (Zyloprim) RDEA594 400 mg RDEA594 400 mg and Allopurinol 300 mg Reference for Size, Tylenol(r) GelCap 18 |
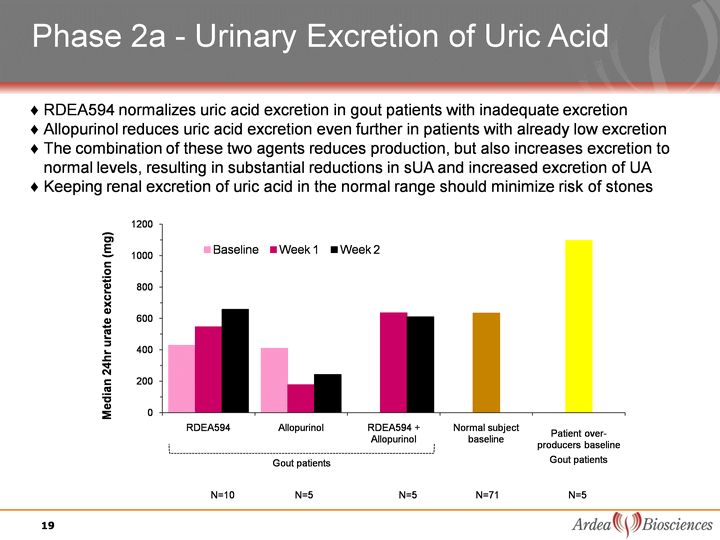
| Phase 2a - Urinary Excretion of Uric Acid Gout patients RDEA594 normalizes uric acid excretion in gout patients with inadequate excretion Allopurinol reduces uric acid excretion even further in patients with already low excretion The combination of these two agents reduces production, but also increases excretion to normal levels, resulting in substantial reductions in sUA and increased excretion of UA Keeping renal excretion of uric acid in the normal range should minimize risk of kidney stones 19 |

| Treatment Group Mild Moderate Severe Serious Adverse Event Treatment Group (grade 1) (grade 2) (grade 3) Serious Adverse Event Treatment Group N Number of Adverse Events (Number of patients, n/N) Number of Adverse Events (Number of patients, n/N) Number of Adverse Events (Number of patients, n/N) Number of Adverse Events (Number of patients, n/N) RDEA594 11 11 (6/11) 1 (1/11)* 0 0 Placebo 5 3 (2/5) 1 (1/5) 0 0 Allopurinol 5 3 (2/5) 4 (3/5) 1** (1/5) 1** (1/5) * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and a serious adverse event due to hospitalization * Grade 2 prostatitis not considered related to RDEA594 **Acute coronary syndrome was both severe and a serious adverse event due to hospitalization Treatment Group N Mild Moderate Severe Treatment Group N (grade 1) (grade 2) (grade 3/4) Treatment Group N Laboratory abnormality with increase in CTC grade Laboratory abnormality with increase in CTC grade Laboratory abnormality with increase in CTC grade RDEA594 11 AST Creatinine (2) - Placebo 5 - Glucose, ALT, Triglycerides - Allopurinol 5 ALT - Aldosterone Only Mild to Moderate Adverse Events and Grade 1 Laboratory Abnormalities with RDEA594 Severity Grade of Adverse Events (Cohort 1) Severity Grade of Laboratory Abnormalities (Cohort 1 ) No significant toxicities on RDEA594 in this study Excluding the two grade 1 increases, no apparent trend in serum creatinine, and no changes in creatinine in Cohort 2 Safety profile supports anticipated positive Phase 2b results 20 |

| Undissociated Urinary Uric Acid (UUua) is the Most Important Risk Factor for Nephrolithiasis* 21 Patients on uricosurics with UUua<20 mg/dL had same rate of lithiasis as patients on allopurinol * 2009 ACR Philadelphia, Presentation 1499 Risk Factors for Renal Lithiasis During the Treatment with Uricosuric Drugs Mean UUua on RDEA594 400 mg was 14 mg/dL |

| RDEA594-105: Phase 1 Febuxostat Drug-Drug Interaction and PD Study - Completed Population: 36 normal healthy volunteers with sUA ^ 6 mg/dL; 18 per dose panel Males and postmenopausal or sterile women 18 - 65 years of age, inclusive 2 panels with escalating doses of RDEA594 or placebo - 200 mg and 400 mg Assessments: pharmacokinetic drug interactions impact on sUA safety and tolerability Design: Each panel is randomized to one of two sequences with data pooled for analysis Sequences for each panel: Febuxostat 40 mg qd RDEA594 or Placebo qd Sequence Days 1-7 Days 8-14 Days 15-21 RDEA594 or Placebo qd Febuxostat 40 mg qd 1 2 22 |

| No Pharmacokinetic Interactions Observed Between RDEA594 and Febuxostat at Steady-State 23 Febuxostat RDEA594 Pharmacokinetic results for the 400 mg dose group are pending |

| Combination of RDEA594 and Febuxostat Produced 70% - 80% Reductions in sUA Febuxostat 160mg in healthy subjects* *Khosravan, et al. Clin Pharmacokinet 2006; 45 (8): 821-841 24 The combination arms are significantly different from febuxostat alone at all points from Days 9-15: p < 0.001 60 - 70% mean reduction with RDEA594 200 mg + Febuxostat 40 mg 70 - 80% mean reduction with RDEA594 400 mg + Febuxostat 40 mg |

| 25 Pegloticase FDA Committee Slides Approximately 50% mean reduction with q4 week regimen (9.8 to 5.2 mg/dL) Approximately 65% mean reduction with q2 week regimen (9.8 to 3.5 mg/dL) 25 |

| 26 RDEA594 Results Superimposed on Pegloticase Data RDEA594 + Allopurinol from Study 201 (projected out in time) RDEA594 + Febuxostat (projected data from NHV study) 26 |

| 27 27 The sUA lowering with RDEA594 + febuxostat is projected to be similar to what was achieved with the q2 week regimen of pegloticase; however, to be conservative we are using a 20% response rate from the q4 week regimen for powering our proposed study |

| Overall Summary of Safety for RDEA594 Through Phase 2a Patients exposed to RDEA594 (either administration of RDEA594 or RDEA806): Healthy subjects and HIV pts ~360 for up to 14 days Gout patients ~ 40 for up to 28 days Control subjects ~ 88 28 Arm Discontinuations Due to AEs N (%) Serious Adverse Events N (%) Grade 2 or Greater Laboratory Abnormalities* N (%) RDEA594 1** (0.25%) 1** (0.25%) 1***(0.28%) Control 2 (2.3%) 2 (2.3%) 7 (8%) *Increase in grade from 0 or 1 to grade 2 or greater. **Hemorrhoids considered not likely drug related *** One subject receiving RDEA806 400 mg once daily had increase in lipase from Grade 2 at baseline to Grade 3 at Day 28; however, baseline was elevated as compared to screening and was not considered drug related. |

| RDEA594 Phase 2 Program Focuses on Important Commercial Opportunities Phase 2b monotherapy dose-response, safety and efficacy study (n=140) Supports use in patients who are intolerant to allopurinol, inadequate responders to allopurinol, and first-line treatment Phase 2b allopurinol add-on study in gout patients stable on allopurinol 300 mg QD with sUA > 6 mg/dL (n=72) Preliminary results from ongoing Phase 2a - Cohort 2 supports the dose selection in the Phase 2b allopurinol combination study Targets use in 60% of allopurinol patients that do not achieve adequate reduction in uric acid with standard dose, and provides data for fixed combination product Renal impairment and pharmacodynamic study (n=12) Safety, tolerability, pharmacokinetics and impact on sUA in subjects with moderate renal insufficiency with or without allopurinol Febuxostat drug-drug interaction and pharmacodynamic study (n=36) ^ Initial results strongly support use of combination in patients with high baseline levels and tophaceous gout patients 29 |

| RDEA594 Phase 2b Monotherapy Dose- Response, Safety and Efficacy Study - Enrolling 21 days 2 weeks 4 weeks 2 weeks Population: gout patients with hyperuricemia (serum uric acid ^8 mg/dL) total of 140 patients in 4 treatment arms Duration: 8 wks: 2-wk run-in, 4-wk treatment, 2-wk follow-up: dose will be titrated up weekly to reduce possible gout flares and nephrolithiasis Endpoints: proportion of subjects with sUA level < 6.0 mg/dL at Day 28 safety and tolerability of the combination versus placebo Arm 1: RDEA594 200 mg - 35 pts Screenin g Period Washout of urate lowering therapy Colchicine Treatment Randomize if no gout flare during 2 weeks of colchicine Off drug Arm 2: RDEA594 400 mg - 35 pts Off drug Arm 4: RDEA594 Placebo - 35 pts Off drug Arm 3: RDEA594 600 mg - 35 pts Off drug 30 |

| RDEA594 Phase 2b Allopurinol Add-On Combination Safety and Efficacy Study - Enrolling Dose Escalation Based on sUA Lowering with 200 mg RDEA594: 18 patients initially, including 12 patients randomized to RDEA594 and 6 patients randomized to placebo 31 RDEA594 100 mg or placebo plus allopurinol 300 mg (n=36) RDEA594 200 mg or placebo plus allopurinol 300 mg RDEA594 200 mg or placebo plus allopurinol 300 mg (n=36 total) or Dose Escalation/Reduction Cohorts: 36 patients per dose, including 24 patients randomized to RDEA594 and 12 patients randomized to placebo per cohort and treated for 28 days Dose Reduction Cohorts: if >75% of patients have sUA < 5 mg/dL, then go to100 mg QD and full 36 patients on 200 mg QD Dose Escalation Cohorts: if <75% meet criteria, then go to full 36 patients on 200 mg and 400 mg QD RDEA594 400 mg or placebo plus allopurinol 300 mg (n=36) RDEA594 200 mg or placebo plus allopurinol 300 mg (n=36 total) Based on Phase 2a results, this trial has been modified |

| RDEA594-204: Renal Impairment in Patients with Gout and Hyperuricemia - Enrolling Cohort 1A: (6 subjects) 200 mg RDEA594 Subjects must have moderate renal impairment defined as creatinine clearance from (CrCL) ^ 30 mL/min to < 60 mL/min. Subjects will receive RDEA594 once daily for 5 days. Cohorts 1A and 2A of Segments I and II will enroll simultaneously: Segment I - patients with hyperuricemia (sUA ^ 7.0 mg/dL) not receiving urate lowering therapy; Optional Cohort 1B: (6 subjects) 100 mg to 400 mg RDEA594 , dependent on the data from Cohort 1A Cohort 2A: (6 subjects) 200 mg RDEA594* + allopurinol Segment II - patients poorly controlled on allopurinol 100 to 200 mg once daily (sUA ^ 6.0 mg/dL) Optional Cohort 2B: (6 subjects) 100 mg to 400 mg RDEA594, dependent on the data from Cohort 2A, + allopurinol *Dose can be lowered to 100 mg per day if Cohort 1A data suggests a lower dose is advised. 32 |

| 33 There is a large unmet need for new treatments for gout RDEA594, a selective URAT1 transport inhibitor, corrects the physiologic cause of 90% of hyperuricemia: uric acid under-excretion Clinical proof-of-concept has been achieved for both monotherapy, and in combination with xanthine oxidase inhibitors allopurinol and febuxostat RDEA594 has been well tolerated in almost 200 subjects at doses up to 600 mg and approx. 250 subjects dosed with its prodrug, RDEA806 Safety profile supports positive Phase 2b outcome With about 100 patients under treatment in Phase 2b, there have been no serious adverse events, no discontinuations due to laboratory abnormalities, and only one premature discontinuation due to an adverse event. Phase 2 development program for RDEA594 is designed to demonstrate its broad clinical utility and to provide multiple Phase 3 options for further development Gout/Hyperuricemia Program Summary |
| RDEA684 - Development Candidate Selection RDEA684 was selected for further development based on the following criteria: Potency improved by up to 200-fold compared to RDEA594 Clean in initial toxicology screen, including 7-day rat, genetox, mitochondrial tox, and ligand screening (MDS) Comparable microsome stability to RDEA594 in multiple species including human Good urinary excretion (retains urinary elimination pathway) Favorable PK in rat, monkey and dog Projected clinical dose is 20 mg/day Plan to complete preclinical development activities in 2H10 34 |

| Cancer 35 MEK program licensed to Bayer Healthcare on April 28, 2009 $35 million upfront license fee Payments could total $407 million, excluding royalties Low double-digit royalties on worldwide sales Ardea responsible for completing two dose-finding studies (expected completion 1H10) |

| The Age of Targeted Cancer Treatments Nexavar MEK Ras Raf c-Myc Herceptin, Erbitux Proliferation Angiogenesis Differentiation Apoptosis cPLA2 PDE4 MNK1/2 Elk-1 MAPKAPK1/3 ARRY-142886 (Ph 2) XL518 (Ph 1) RDEA119 (Ph 1/2) RTK Bcl-2 Mcl-1 c-Jun Tarceva GF ERK2 ERK2 36 |
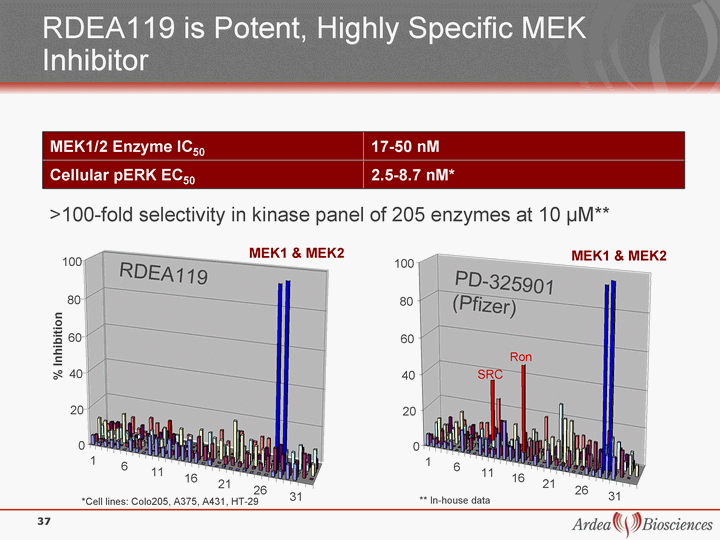
| RDEA119 is Potent, Highly Specific MEK Inhibitor MEK1/2 Enzyme IC50 17-50 nM Cellular pERK EC50 2.5-8.7 nM* >100-fold selectivity in kinase panel of 205 enzymes at 10 µM** *Cell lines: Colo205, A375, A431, HT-29 ** In-house data 1 6 11 16 21 26 31 0 20 40 60 80 100 1 6 11 16 21 26 31 MEK1 & MEK2 MEK1 & MEK2 0 20 40 60 80 100 Ron SRC RDEA119 PD-325901 (Pfizer) % Inhibition 37 |

| Synergy between RDEA119 and Sorafenib in Hepatoma Cancer Lines 38 |

| Financial Position Summary Statement of Operations (In thousands, except per share data) Nine Months Ended Sept. 30, 2009 Revenue Operating expenses Other expense $ 14,681 38,527 (691) NET LOSS $(24,537) NET LOSS PER SHARE $(1.36) 18.5 million common shares outstanding Expect 2009 year-end cash balance $50 - $55 million Multiple partnering opportunities Condensed Balance Sheet Data (In thousands) Sept. 30, 2009 Dec. 31, 2008 Cash, equivalents & ST invest Receivables Total assets Total stockholders' equity $59,589 $1,420 $64,121 $29,037 $57,743 $384 $61,475 $45,958 39 |

| Near-Term Anticipated Milestones Drug Candidate Event Date Date Gout Gout Gout Gout RDEA594 Phase 2b Start in 7/09 Phase 2b Start in 7/09 ? RDEA594 Phase 2a - Cohort 2 Allopurinol Combo Results Phase 2a - Cohort 2 Allopurinol Combo Results ? RDEA594 Febuxostat Combination Study Results Febuxostat Combination Study Results ? RDEA594 Phase 2b Monotherapy Study Results Phase 2b Monotherapy Study Results 1Q10 RDEA594 Phase 2b Allopurinol Add-On Study Results Phase 2b Allopurinol Add-On Study Results 2Q10 RDEA594 Renal Impairment Study Results Renal Impairment Study Results 1Q10 RDEA684 Phase 1 Start Phase 1 Start 2H10 HIV HIV HIV HIV RDEA427 Phase 1 start Phase 1 start post-partnering 900 series Clinical Candidate Selection Clinical Candidate Selection post-partnering Cancer - Inflammation Cancer - Inflammation Cancer - Inflammation Cancer - Inflammation RDEA119 Phase 1 advanced cancer results Phase 1 advanced cancer results 1H10 RDEA119 Phase 1/2 sorafenib combination results Phase 1/2 sorafenib combination results 1H10 40 |
