Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CURIS INC | d8k.htm |
 Corporate Overview January 7, 2010 NASDAQ: CRIS Exhibit 99.1 |
 2 Forward Looking Statements This presentation contains statements about Curis’ future expectations, plans and prospects that constitute forward-looking statements for purposes of the safe harbor provisions
of the Private Securities Litigation Reform Act of 1995. Actual results may differ materially
from those indicated by these forward-looking statements as a result of
various important factors, including risks relating to: both our and our
collaborators’ ability to successfully research, obtain regulatory
approvals for, develop and commercialize products based upon our
technologies; our ability to obtain and maintain proprietary protection for
our technologies and product candidates, including our multi- target inhibitors; competitive pressures; our ability to maintain strategic
collaborations, including with Genentech and Debiopharm; our ability to
successfully execute on, and receive favorable results from, our proprietary
drug development efforts; our ability to raise additional funds to finance our operations; and those factors described in our Quarterly Report on Form 10-Q for
the quarter ended September 30, 2009, and other reports that we file with
SEC. The forward-looking statements included in this presentation
represent our views as of the date of this presentation. We anticipate that
subsequent events and developments will cause our views to change. While we
may elect to update these forward-looking statements in the future, we specifically disclaim any obligation to do so. These forward-looking statements
should not be relied upon as representing our views as of any date
subsequent to the date of this presentation. |
 3 Curis – Innovative Cancer Therapies Developed With Balanced Business Model • Oncology company leveraging innovative signaling pathway drug technologies to create next generation targeted cancer therapies • Pivotal-stage development program with Roche/Genentech for GDC-0449 in solid tumors - First-in-class small molecule Hedgehog pathway inhibitor - Genentech overseeing 3 ongoing Phase II clinical trials in advanced basal cell carcinoma, metastatic colorectal cancer and advanced ovarian cancer • Regulatory submissions in advanced basal cell carcinoma anticipated in 2011 • Curis eligible for certain clinical development and regulatory approval milestones
and royalties • Targeted cancer “network” drug development programs with leading IP portfolio - Lead program candidate CUDC-101 designed to inhibit HDAC, EGFR, and Her2 • Phase I clinical trial ongoing; expect to expand in specific tumor types including
non-small cell lung, breast, colon and pancreatic cancers - Broad proprietary pipeline of preclinical drug candidates under development • Capital to fund planned operations into 2H 2011 |
 4 Curis Pipeline Discovery Products Early preclinical Mid preclinical Late preclinical IND- I II III NDA CUDC-101 (HDAC/EGFR/Her2 inhibitor) Multiple cancers Phase I Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) with Avastin and Chemotherapy First-line metastatic colorectal cancer Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) Advanced ovarian cancer Phase II Phase I expansion cohort Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) Advanced basal cell carcinoma Pivotal Phase II (1) Debio 0932 (Hsp90 inhibitor) Network Targeted Inhibitor Pipeline Multiple cancers Preclinical (1) –Pivotal trial is designed so that its data, if positive, may serve as the basis for NDA submission by Genentech. Multiple cancers CTA Filed |
 5 Curis Pipeline Discovery Products Early preclinical Mid preclinical Late preclinical IND- I II III NDA CUDC-101 (HDAC/EGFR/Her2 inhibitor) Multiple cancers Phase I First-line metastatic colorectal cancer Phase II Advanced ovarian cancer Phase II Advanced basal cell carcinoma Pivotal Phase II (1) Debio 0932 (Hsp90 inhibitor) Multiple cancers CTA Filed Network Targeted Inhibitor Pipeline Multiple cancers Preclinical (1) –Pivotal trial is designed so that
its data, if positive, may serve as the basis for NDA submission by Genentech. GDC-0449/RG3616 (Hedgehog pathway inhibitor) GDC-0449/RG3616 (Hedgehog pathway inhibitor) with Avastin and Chemotherapy GDC-0449/RG3616 (Hedgehog pathway inhibitor) |
 6 GDC-0449 (RG3616): First-in-Class Hedgehog Pathway Inhibitor |
 7 Hedgehog and Development • Effects of Hh during development can be grouped into two mechanistic categories - Stimulating entry of progenitor cells into cell cycle by activating cyclins and cyclin-dependent kinases - Regulating survival, growth, and differentiation by controlling the production of growth factors, neurotrophins and angiogenic factors |
 8 Hedgehog (Hh) pathway and Cancer • Mutation Driven Mechanism: - Basal cell carcinoma (BCC) / Medublastoma • Tumor in which Hh pathway is activated by mutation • Ligand Driven Mechanism: - Colon cancer (pancreatic, ovarian cancers, etc.) • Tumors in which Hh pathway regulates production of growth and angiogenic factors |
 9 Hedgehog-Associated Cancers Ligand Driven Colorectal Cancer Ovarian Cancer Small Cell Lung Cancer Pancreatic Carcinoma Stomach Cancer Esophageal Cancer Prostate Cancer Breast Cancer Liver Cancer Mutation Driven Basal Cell Carcinoma Medulloblastoma Rhabdomyosarcoma |
 10
Responses confirmed by Independent Review Facility; measured using RECIST
criteria Genentech and Roche are responsible for the clinical development and commercialization of GDC-0449 * 1 of 8 partial responses confirmed by imaging was also confirmed by clinical
examination. GDC-0449 Demonstrated Clinical Benefit in BCC Patients in Phase I Clinical Trial Von Hoff, et al., New England Journal of Medicine, September 2009 8.8+ months Duration of Response (median) 9.8+ months Duration on Study (median) 10 Clinical Examination 8* Imaging 4 Disease Progression 11 Stable Disease 16 (48.5%) Partial Response 2 (6.1%) Complete Response Patients, N=33 Best Response |
 11
Phase I Study of GDC-0449 in Locally Advanced, Multifocal or Metastatic BCC Baseline After 5 Months 60 year old with basal cell nevus syndrome with lesions of the posterior scalp Von Hoff, et al., New England Journal of Medicine, September 2009 |
 12
Phase I Study of GDC-0449 in Locally Advanced, Multifocal or Metastatic BCC 67 year old with BCC Metastatic to Lung, Liver and Bone Von Hoff, et al., Presented at American Association for Cancer Research Annual Meeting
2008 Baseline At 8 months (confirmed PR) |
 13
Phase I Study of GDC-0449 in Locally Advanced, Multifocal or Metastatic BCC Baseline After 2 Months 83 year old with BCC Lesion Invasive in Ear and Parotid Gland Von Hoff, et al., Presented at American Association for Cancer Research Annual Meeting
2008 |
 14
BCC Patients Phase I Safety Summary • No grade 5 toxicities or dose-limiting toxicities observed • Single grade 4 adverse event not determined to be related to the study drug • Grade 3 adverse events included - fatigue (n=4), hyponatremia (n=2), weight loss (n=2), dyspnea (n=2) - 1 patient each muscles spasms, atrial fibrillation, aspiration, back pain, corneal abrasian, dehydration, keratitis, lymphopenia, pneumonia, urinary tract infection, a prolonged QT interval, increased serum alkaline phosphatase and increased serum potassium • Grade 1 and 2 observed adverse events included muscle spasms, dysguesia (altered taste sensation), anorexia, weight decrease, hypocalcemia and dyspepsia • Safety data is similar to that observed in broader Phase I clinical trial Von Hoff, et al., New England Journal of Medicine, September 2009 |
 15
Hedgehog-Associated Cancers Ligand Driven Colorectal Cancer Ovarian Cancer Small Cell Lung Cancer Pancreatic Carcinoma Stomach Cancer Esophageal Cancer Prostate Cancer Breast Cancer Liver Cancer Mutation Driven Basal Cell Carcinoma Medulloblastoma Rhabdomyosarcoma |
 16
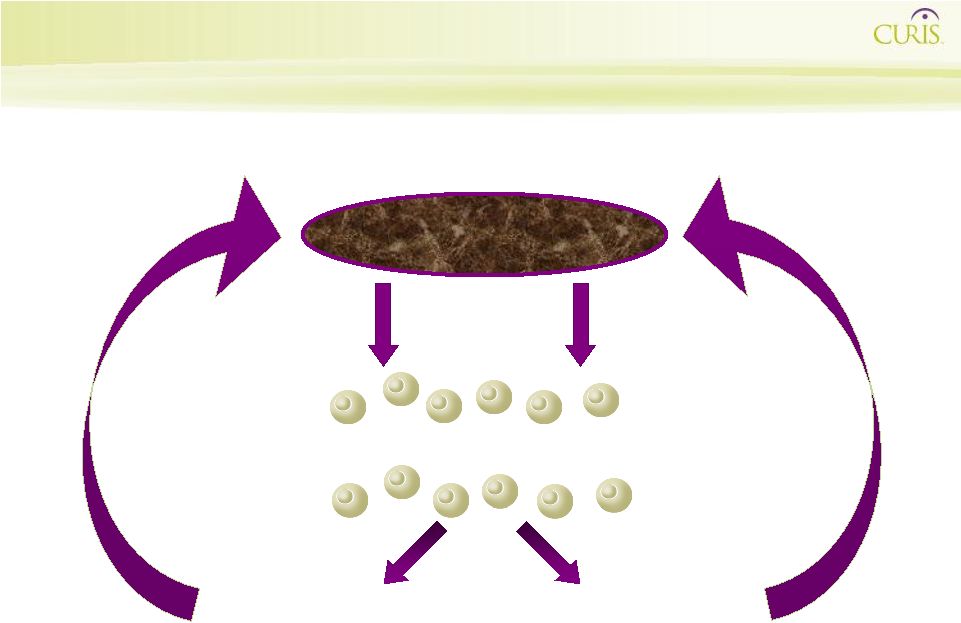
Abnormal Hedgehog Expression Supports Growth of Certain Cancers Certain Cancers Hedgehog Protein Adjacent Stromal Cells Angiogenesis Factors VEGF, Ang-1, Ang-2 Growth Factors IGF-I, BDNF, NGF |
 17
Shh Induces Angiogenesis Shh Day 0 Shh Day 6 day 0 day 6 pellet containing Shh |
 18
Abnormal Hedgehog Expression Supports Growth of Certain Cancers: Network Disruption Adjacent Stromal Cells Angiogenesis Factors VEGF, Ang-1, Ang-2 Growth Factors IGF-I, BDNF, NGF GDC-0449 |
 19
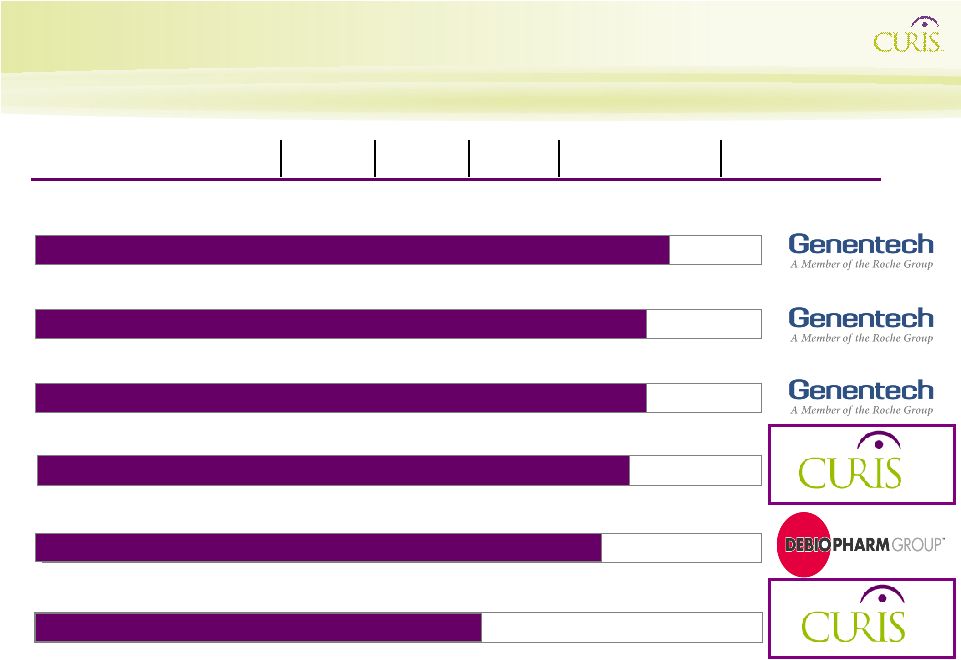
Phase II Clinical Trial Status Patient Population Advanced Basal Cell Carcinoma (Mutation-Driven) First-Line Metastatic Colorectal Cancer (Ligand-Driven) Advanced Ovarian Cancer Maintenance Therapy (Ligand-Driven) Phase Pivotal Phase II Phase II Phase II # Patients N = 100 N = 190 N = 100 Design Single agent in metastatic and inoperable locally advanced BCC FOLFOX or FOLFIRI chemotherapy with Avastin +/- GDC-0449 Single agent in ovarian cancer patients in 2 nd or 3 rd complete remission – GDC-0449 vs. placebo Status FPI Q1 2009 Enrollment completed Q2 2009 FPI Q4 Enrollment completed Q4 2009 Advanced basal cell carcinoma represents a fast-to-market opportunity that
enables first market entry for this class Positive ‘proof of concept’ data in ligand-driven cancers could result in potential rapid expansion with investment in additional indications - Small cell lung cancer - Pancreatic - Upper GI - Prostate - Multiple myeloma - Non-small cell lung cancer - Glioma - Endometrial - Melanoma |
 20
Curis Pipeline Discovery Products Early preclinical Mid preclinical Late preclinical IND- I II III NDA CUDC-101 (HDAC/EGFR/Her2 inhibitor) Multiple cancers Phase I GDC-0449/RG3616 (Hedgehog pathway inhibitor) with Avastin and
Chemotherapy First-line metastatic colorectal cancer Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) Advanced ovarian cancer Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) Advanced basal cell carcinoma Pivotal Phase II (1) Phase II* Debio 0932 (Hsp90 inhibitor) Multiple cancers CTA Filed Network Targeted Inhibitor Pipeline Multiple cancers (1) –Pivotal trial is designed so that its data, if positive, may serve as the basis
for NDA submission by Genentech. Preclinical |
 21
CUDC-101 Drug Design and Mechanism of Action: Enhance Efficacy and Overcome Resistance to EGFR/Her2 Inhibitors |
 22
Curis Multi-Targeted Inhibitor Cancer Platform: Designed for Network Disruption EGFR inhibitor HDAC Her2 inhibitor inhibitor CUDC-101 EGFR Inhibition HDAC Inhibition Her2 Inhibition Rational Design Single Small Molecule M.W. < 500 |
 23
EGFR and HER2 Tyrosine Kinases (RTK) As Cancer Targets: Clinical Benefit and Challenges • Aberrant EGFR/HER2 signaling can lead to tumor growth via enhanced cellular proliferation, survival, and metastasis • Several EGFR, HER2 and EGFR/Her2 inhibitors approved are generally less toxic than chemotherapy • Limitations observed in treating certain cancers with these tyrosine kinase inhibitors - Poor overall response rates • Low percentage of patients responsive • Poor survival benefit - Rapid emergence of drug resistance • A compelling unmet medical need in the treatment of major cancers remains for novel drugs, which are more efficacious |
 24
Limitation of Single-Target Drugs, such as Erlotinib and other RTK inhibitors Resistance via
mutation Response Resistance via adaptation Poor Response Rate and Drug Resistance HER3 MET MAPK Signaling Akt Signaling Transcription Regulation Transcription Regulation Transcription Regulation |
 25
Rationale for Multi-Targeted Inhibitors of HDAC and Receptor Tyrosine Kinases • Dysregulation of histone deacetylase (HDAC) activity plays a role in tumor onset and tumor progression
• HDAC inhibitors suppress tumor cell growth/survival via: -
Histone acetylation to reverse aberrant epigenetic changes - Non-histone oncoprotein acetylation, such as HSP90 and HIF-1 • A growing number of published studies demonstrate epigenetic effects of HDAC inhibition are synergistic with protein kinase inhibitors and other agents • Simultaneous blockade of HDAC and RTK pathways may overcome limitations observed in treating certain cancers with RTK inhibitors |
 26
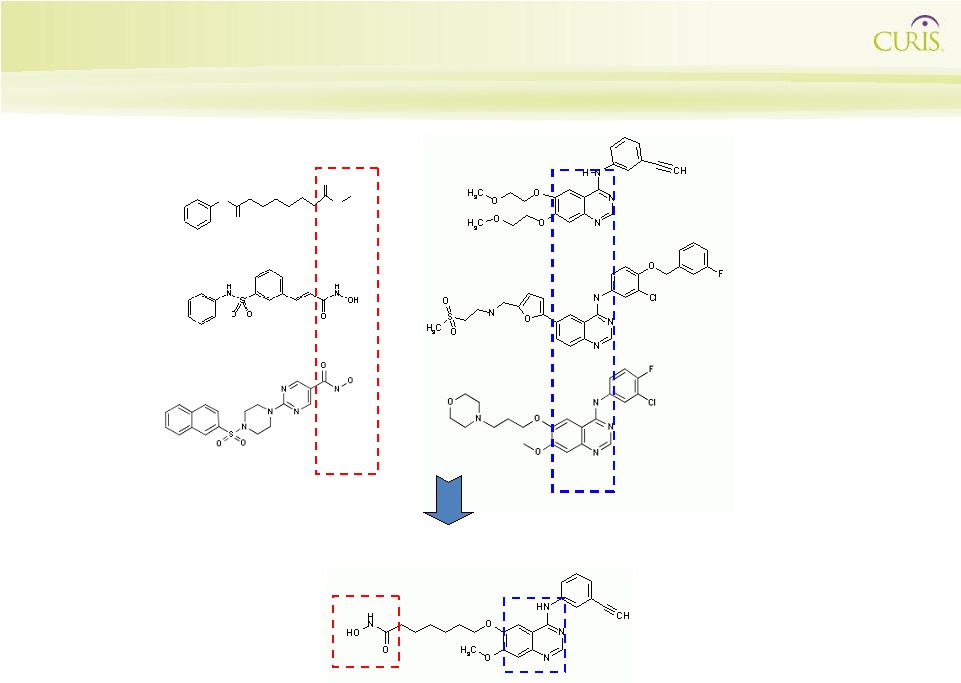
CUDC-101 MW 434.5 erlotinib MW 393.5 lapatinib MW 581.1 CUDC-101 Structure Design: integrating a hydroxamic acid HDAC
- inhibiting moiety into quinazoline RTK
inhibition pharmacophore N O N O O SAHA MW 264.3 Belinostat MW 318.35 JNJ-16241199 MW 413.45 gefitinib MW 446.9 HDAC Inhibitors EGF/Her2 Inhibitors HDAC/ EGF/Her2 Inhibitors |
 27
CUDC-101 is a Very Potent and Selective HDAC, EGFR and Her2 Inhibitor Compound IC 50 (nM) in Enzyme Assays HDAC EGFR HER2 SAHA 40.0 NA NA erlotinib NA 48.0 134.5 lapatinib NA 11.2 10.2 CUDC-101 4.4 2.4 15.7 Potency • HDAC inhibition: 5-10 fold more potent than SAHA • EGFR inhibition: 10-20 fold more potent than erlotinib • Her2 inhibition: 5-10 fold more potent than erlotinib and similar to lapatinib
Selectivity • A total of 72 kinases have been assayed • CUDC-101 appears to be a weak inhibitor of VEGFR2, Lck, Lyn, Abl-1, FGFR2,
Flt3 and Ret kinases (IC 50 values are within the range of 1-5 M) • At 10 M concentration, the inhibition of other kinases is less than 50%
|
 28
CUDC-101 Forms Additional Hydrogen Bonds with EGFR vs. Erlotinib • Compared to erlotinib, CUDC-101 has 20x higher binding affinity for EGFR (2.4 nM versus 48 nM IC 50 ); This may be explained by the additional hydrogen bond interaction of CUDC-101 with D831 in the activation loop (A-loop) CUDC-101 in EGFR Binding Domain Overlay of CUDC-101 and Erlotinib in EGFR Binding Domain erlotinib CUDC-101 |
 29
CUDC-101 Is A Pan Class I & II HDAC Inhibitor - No inhibitory activity against class III HDACs (SIRT 1-3) (IC50 in nM) HDAC1 HDAC2 HDAC3 HDAC8 HDAC4 HDAC5 HDAC6 HDAC7 HDAC9 HDAC10 4.5 12.6 9.1 79.8 13.2 11.4 5.1 373 67.2 26.1 Class I Class II Nucleus • Histones • Transcription factors Cytoplasm • Proteins (e.g., HSP90) |
 30
CUDC-101, Advantages Over Single-Target Drugs: Disruption of Signaling Network Poor Response Rate Drug Resistance Improved Response Rate Escape Drug Resistance EGFR/Her2 HER3 MET Class II HDAC Class I HDAC EGFR/HER2 CUDC-101 EGFR/HER2 Erlotinib Lapatinib EGFR/Her2 HER3 MET Transcription Regulation Transcription Regulation MAPK Signaling Akt Signaling MAPK Signaling Akt Signaling |
 31
CUDC-101 Synergistically Suppresses HER2 Signaling Through Multiple Points of Intervention HSP90 Class II HDAC Class I HDAC EGFR/HER2 Her2 GAPDH M 0 1
5 10 1 5 10 1 10 -RT ctrl CUDC-101 SAHA lapatinib HER2 p-Akt GAPDH Control CUDC-101 p-HER2 HER2 |
 32
Current CUDC-101 Clinical Trial Summary Progress Favorable PK Biomarkers High Exposure Completed Well Tolerated 75 mg/ m² 150 mg/ m² Longer T1/2 than SAHA Dose Proportionality HER2 Protein Partial Response - 22 patients enrolled to date - PK dose proportional - A patient evaluated at 275 mg/m confirmed partial response (14 weeks, 7 cycles; approximately 55% reduction in target lesion) - One patient in 150 mg/m cohort exhibited a mixed response with a reduction in the size of one target lesion - One patient in 150 mg/m cohort exhibited stable disease (received 6 cycles -12wks) Accrual Clinical Response 2 2 2 |
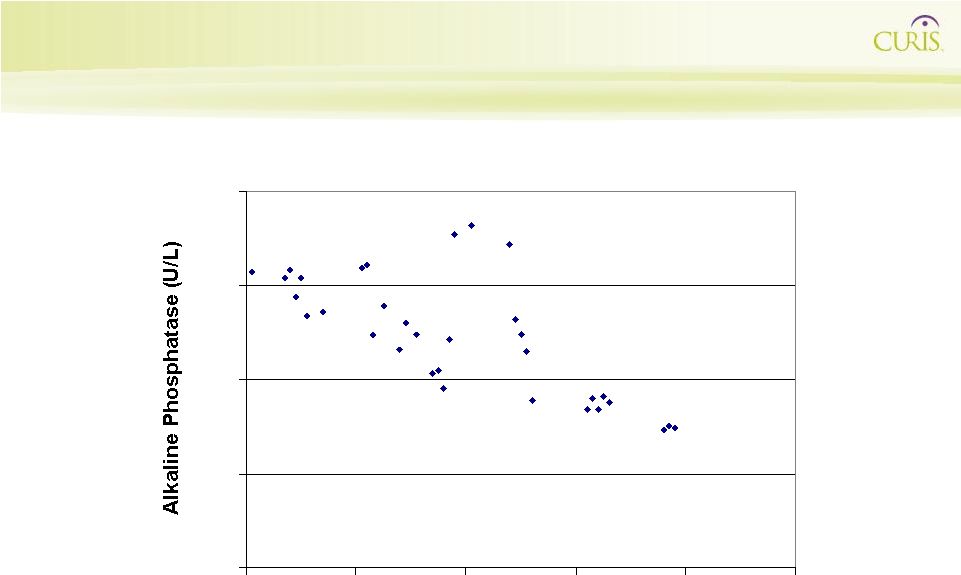 33
Reduction in Serum Alkaline Phosphatase in a Patient with Advanced Gastric Cancer (275 mg/m 2 ) 100 150 200 250 300 0 20 40 60 80 100 Time On Study (Days) |
 34
Preliminary Antitumor Activity: Metastatic Salivary Cancer (Head & Neck) Mixed Response with reduction in mediastinal mass 150 mg/m 2 Baseline Post 4 th Cycle |
 35
Current CUDC-101 Clinical Trial Summary Progress Favorable PK Biomarkers High Exposure Completed Well Tolerated 75 mg/ m² 150 mg/ m² Longer T1/2 than SAHA Dose Proportionality HER2 Protein Partial Response - Dose-limiting toxicity of transient grade 2 elevated Creatinine levels observed at 300 mg/m2 - 75 mg/m2,150 mg/m2, 225 mg/m2, and 275mg/m2 appear well- tolerated • Most frequent adverse events: rash (275 mg/m2 only) dry skin, nausea, fatigue, vomiting, pyrexia (fever), constipation, dyspnea, decreased hemoglobin, hyperglycemia (Grade 1-2) - Dry skin indicative of EGFR inhibition at dose levels below 275 mg/m2 - Grade 1-2 rash reported in patients dosed at 275 mg/m2 - Hemoglobin changes and hyperglycemia indicative of HDAC inhibition - Trend for reduced EGFR phosphorylation in skin biopsies - One patient with decrease in Her2 protein in tumor biopsy (75 mg/m2) Safety Biomarker |
 36
Curis Pipeline Discovery Products Early preclinical Mid preclinical Late preclinical IND- I II III NDA CUDC-101 (HDAC/EGFR/Her2 inhibitor) Multiple cancers Phase I GDC-0449/RG3616 (Hedgehog pathway inhibitor) with Avastin and
Chemotherapy First-line metastatic colorectal cancer Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) Advanced ovarian cancer Phase II Phase II GDC-0449/RG3616 (Hedgehog pathway inhibitor) Pivotal Phase II (1) Phase II Debio 0932 (Hsp90 inhibitor) Multiple cancers CTA Filed Phase II Phase II* Network Targeted Inhibitor Pipeline Multiple cancers Preclinical (1) –Pivotal trial is designed so that its data, if positive, may serve as the basis
for NDA submission by Genentech. Advanced basal cell carcinoma
|
 37
Debio 0932 (formerly CUDC-305): Potential Best-in-Class HSP90 inhibitor |
 38
Debio 0932 Summary Exclusive worldwide license with Debiopharm Group in August 2009 - Monetized asset for runway extension and to further balance model - Upfront, early and subsequent clinical development milestones Total potential payments of $90 million assuming the successful achievement of clinical development and regulatory objectives Upfront and early payments provide Curis with adequate capital to fund operations into 2H 2011 - Eligible for royalties on net sales by Debiopharm or its sublicensees - Debiopharm assumes all development costs and oversight Clinical trial application filing by Debiopharm completed in December 2009 - Cash payment for CTA acceptance by European regulatory authorities - Additional payment for 5 Patient treated Phase I clinical trial th |
 39
Corporate |
 40
GDC-0449 Seven Indications Phase I – Phase II Corporate Development Pipeline: Primary Value Drivers and Capital Access CUDC-101 Multiple Cancers Phase I Phase Ia Completed Initiate Phase I(b)/II 2012E 2010E 2011E IND/CTA Acceptance ($XM) CUDC-305 IND 5th Patient Phase I ($XM) Phase III Initiation NDA Submission Phase III Initiation NDA Approval Pancreatic Phase I Completed 12/09 (combination with Tarceva, gemcitabine) Phase II Data Phase II Data Phase II Data Hedgehog Pathway Inhibitor Hsp90 Inhibitor HDAC / EGFR / Her2 GDC-0449 Colorectal Phase II GDC-0449 BCC Pivotal Phase II GDC-0449 Ovarian Phase II 4Q 2009 |
 41
Financial Data Cash, cash equivalents, investments $ 27,200 Debt $
- Shareholders’ equity $ 34,400 Basic shares outstanding 66,500 Fully diluted shares outstanding 80,700 2009 net loss $ 7,100 Remaining warrants under August 2007 financing have been or are expected to be exercised under the terms of the warrant agreement, resulting in proceeds to Curis of approximately $400,000 in December 2009 and $1.8 million expected in January/February 2010. September 30, 2009 (000’s) |
 42
Appendix |
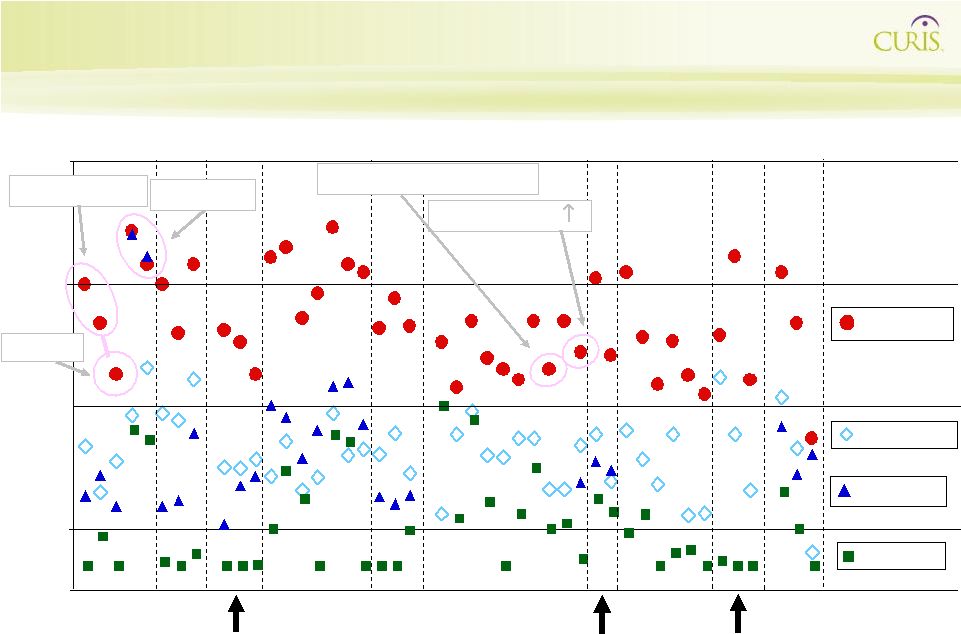 43
CUDC-101 Is More Potent Than Erlotinib, Lapatinib, or Vorinostat Against a Wide Range of Human Cancer Cell Lines Prostate (3) Sarcoma (3) Liver (3) NSCLC (10) Ovarian (2) Pancreatic (6) Breast (5) Colon (3) Glioblastoma (3) H&N (7) IC50 (anti-proliferation assay) 10 nM 100 nM 1 uM 10 uM Vorinostat CUDC-101 Erlotinib Lapatinib 45 cell lines H1993 (c-Met ) H1975(L585R/T790M) Her2 neg Her2 pos “Triple Neg” |
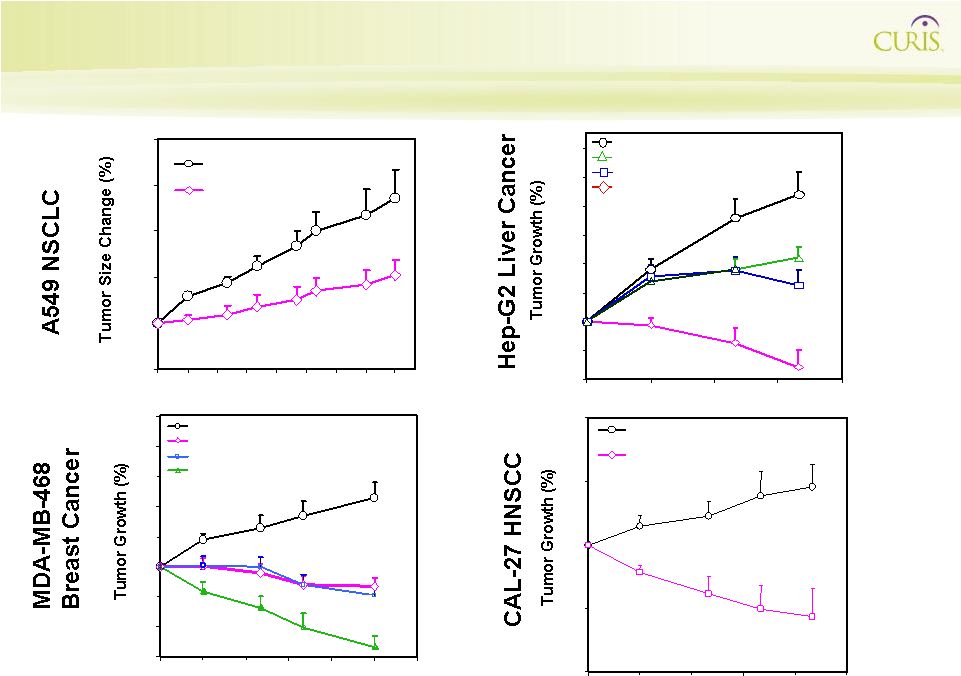 44
CUDC-101 Disrupts Signaling Network to Improve the Treatment of Drug-Resistant Tumors 0 50 100 150 200 0 3 6 9 12 15 Days Vehicle CUDC-101 120 mg/kg P<0.0001 Days 40 60 80 100 120 140 160 180 200 0 3 6 9 12 P<0.0001 P<0.0001 P<0.0001 15 18 Vehicle (Captisol®) Vehicle (Captisol®) CUDC-101 (120 mg/kg) CUDC-101 (120 mg/kg) CUDC-101 & Paclitaxel CUDC-101 & Paclitaxel Paclitaxel (12.5 mg/Kg, 2x weekly) Paclitaxel (12.5 mg/Kg, 2x weekly) 50 100 150 200 250 300 0 3 6 9 12 15 18 21 24 Days P<0.001 Vehicle CUDC-101 120 mg/kg 60 80 100 120 140 160 180 200 220 0 3 6 Days P<0.05 P<0.0001 9 12 P<0.05 Vehicle ( Vorinostat (72 mg kg) Erlotinib (25 mg/kg) Vehicle ( Captisol®) CUDC-101 (120 mg/kg ) Vorinostat (72 mg kg) Erlotinib (25 mg/kg) |
 CUDC-101 Disrupts Signaling Network to Improve the Treatment of Heterogeneous and Drug-Resistance Tumors EGFR HER2
HER3 MET p-Akt p53 ER HIF-1 CUDC-101 SAHA erlotinib ER- HIF-1 Tubulin Hr 0
2 7 24 2 7 24 24 Ctrl CUDC-101 erlotinib
SAHA p-Akt Akt Tubulin Ac-p53 p53 P-EGFR p-HER2 HER2 P-HER3 Tubulin Ctrl CUDC-101 erlotinib SAHA
Hr 0 7 18 24 Hr 0
2 7 24 2 7 24 Ctrl CUDC-101 erlotinib p-MET MET Tubulin Hr 0
2 7 24 2 7 24 24 Hr 0
3 8 24 30 erlotinib SAHA CUDC-101 p-Akt Akt Improved Response Rate Escape Drug Resistance EGFR/Her2 HER3 MET Transcription Regulation MAPK Signaling Akt Signaling |
 Corporate Overview January 7, 2010 NASDAQ: CRIS |
