Attached files
| file | filename |
|---|---|
| EX-32.1 - SPAN AMERICA MEDICAL SYSTEMS INC | v169922_ex32-1.htm |
| EX-23.1 - SPAN AMERICA MEDICAL SYSTEMS INC | v169922_ex23-1.htm |
| EX-31.1 - SPAN AMERICA MEDICAL SYSTEMS INC | v169922_ex31-1.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Form
10-K
|
x
|
Annual
report pursuant to section 13 or 15(d) of the Securities Exchange Act of
1934
|
For the fiscal year ended October 3, 2009
or
|
¨
|
Transition
report pursuant to section 13 or 15(d) of the Securities Exchange Act of
1934
|
For the transition period from _______
to _______.
Commission
file number 0-11392
SPAN-AMERICA
MEDICAL SYSTEMS, INC.
(Exact
name of registrant as specified in its charter)
|
South
Carolina
|
57-0525804
|
|
(State
or other jurisdiction of incorporation or organization)
|
(I.R.S.
Employer Identification
No.)
|
|
70
Commerce Center, Greenville, South Carolina
|
29615
|
|
(Address
of principal executive offices)
|
(Zip
Code)
|
|
Registrant’s
telephone number, including area code
|
(864)
288-8877
|
Securities
registered pursuant to section 12(b) of the Act:
|
Title
of each class
|
Name
of each exchange on which registered
|
|
None
|
None
|
Securities
registered pursuant to section 12(g) of the Act:
Common
stock, no par value
(Title of
class)
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes ¨ No
x
Indicate by check mark if the
registrant is not required to file reports pursuant to Section 13 or Section
15(d) of the Act. Yes ¨ No x
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes x No
¨
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Website, if any, every Interactive Data file required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes x No
¨
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§229.405 of this chapter) is not contained herein, and will not
be contained, to the best of the registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. ¨
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting
company. See the definitions of “large accelerated filer,”
“accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act. (Check one):
Large
Accelerated Filer ¨ Accelerated
Filer ¨
Non-Accelerated
Filer x Smaller
Reporting Company ¨
Indicate by check mark whether the
registrant is a shell company (as defined in Rule 12b-2 of the
Act). Yes ¨ No x
The
aggregate market value of the voting and non-voting common equity held by
non-affiliates computed by reference to the price at which the common equity was
last sold as of the last business day of the registrant’s most recently
completed second fiscal quarter was $19,749,935.
The
number of shares of the registrant’s common stock, no par value, outstanding as
of December 22, 2009 was 2,715,101.
Documents
Incorporated By Reference
Portions
of the Company’s Definitive Proxy Statement for the annual shareholders’ meeting
to be held February 12, 2010 are incorporated by reference into Part
III.
PART
I
Item
1. Business
Forward-Looking
Statements
This annual report on Form 10-K
includes forward-looking statements that describe anticipated results for
Span-America Medical Systems, Inc. (the “Company” or
“Span-America”). These statements are estimates or forecasts about
Span-America and its markets based on our beliefs, assumptions and
expectations. These forward-looking statements therefore involve
numerous risks and uncertainties. We wish to caution the reader that
these forward-looking statements, such as, but not limited to, our expectations
for future sales or future expenses, are only predictions. Actual
events or results may differ materially as a result of risks and uncertainties
in our business. Such risks include, but are not limited to, the
“Risk Factors” described in Item 1A below and other risks referenced from time
to time in our other Securities and Exchange Commission (“SEC”)
filings. We disclaim any obligation to update any forward-looking
statement, whether as a result of new information, future events or
otherwise.
Background
Span-America Medical Systems, Inc. was
incorporated under the laws of the state of South Carolina on September 21,
1970. We manufacture and distribute a variety of therapeutic support
surfaces and related products utilizing polyurethane and other foam products for
the medical, consumer and industrial markets.
We began operations in 1975 as a
manufacturer of polyurethane foam patient positioners and later expanded our
product lines to include foam mattress overlays for the wound care market
primarily in acute care hospitals. Wound care products aid in the
treatment or prevention of pressure ulcers and diabetic ulcers commonly known as
bedsores. In the late 1970s, we also began producing foam products
for industrial applications. In 1985, we introduced the patented
Geo-Mattâ therapeutic
mattress overlay in the health care market, which became one of our leading
products. During the same time period, we began selling convoluted
foam mattress overlay products to consumer bedding retailers throughout the
United States.
We entered the replacement mattress
segment of the medical market in 1992 by acquiring certain assets of Healthflex,
Inc., including its PressureGuard® II therapeutic support surface. We
have since significantly expanded the PressureGuard product line and have added
the Geo-Mattress® product line to provide a broad line of therapeutic support
surfaces that we sell directly and through distributors to hospitals, long-term
care facilities, and home health care dealers throughout the Unites States and
Canada.
In July 2002, we acquired certain
assets of Vadus, Inc., which included patents and equipment related to the
design and production of the Secure I.V.® line of short peripheral intravenous
catheters. The product line was in the development stage at the time
of the acquisition, and we completed the development of the product and
initially launched Secure I.V. in 2004. However, we were unable to
generate sufficient sales volume in the safety catheter segment to make it a
viable business. Consequently, in October 2007, we decided to exit
the safety catheter business and try to sell the related assets. As
of September 29, 2007, we recorded an impairment charge to eliminate the book
value of our safety catheter assets. We are currently engaged in
efforts to sell this part of our business. However, our efforts to
date have been unsuccessful.
1
Our primary long-term strategy is to
become a leading health care manufacturer and marketer specializing in wound
management products used in the prevention and treatment of pressure
ulcers. We are actively seeking to develop or acquire new products in
this market segment. We also seek to further develop consumer and
industrial applications of our medical products.
Our products are distributed in the
United States and, to a lesser degree, in several foreign
countries. Total export sales during fiscal 2009 were approximately
$2.4 million or 4.3% of total net sales. The majority of our export
sales occurred in Canada. See Note 18 – Operations and Industry
Segments in the Notes to Financial Statements included in Item 8 of this
report.
We maintain a website at http://www.spanamerica.com. Our
reports and other filings made with the SEC are available free of charge on our
website, which includes a link to the Company’s filings in the SEC’s EDGAR
filing database.
Industry
Segment Data
Please see Note 18 – Operations and
Industry Segments in the Notes to Financial Statements included in Item 8 of
this report for additional information on industry segment data and revenues
from foreign sales.
Medical
Span-America’s
principal medical products consist of polyurethane foam mattress overlays,
therapeutic support surfaces (which consist of non-powered and powered
therapeutic support surfaces), patient positioners, seating products and Selan®
skin care products. We sell these products primarily in North America
to customers in the major segments of the health care market, including acute
care hospitals, long-term care facilities and home health care
providers. Sales of medical products represented 68% of
total net sales in fiscal 2009, 72% of total net sales in fiscal 2008 and 71% of
total net sales in fiscal 2007.
Mattress
Overlays. Span-America produces a variety of foam mattress
overlays, including convoluted foam pads and its patented Geo-Matt®
overlay. Span-America's overlay products are mattress pads rather
than complete mattresses and are marketed as less expensive alternatives to more
complex therapeutic support surfaces. Our mattress overlays disperse
body heat, increase air circulation beneath the patient and reduce moisture
accumulation to aid in the prevention and treatment of pressure
ulcers. Their convoluted or geometrically contoured construction also
reduces shear forces and more evenly distributes the patient's body weight,
thereby reducing the localized pressure that can cause ulcers. The
Geo-Matt design includes numerous individual foam cells that are cut to exacting
tolerances on computer-controlled equipment to create a clinically effective
mattress surface. Mattress overlays comprised approximately 7% of total net
sales in fiscal 2009. These products are designed to provide patients
with greater comfort and to assist in treating patients who have developed or
are susceptible to developing pressure ulcers. The mattress overlays
are designed for single patient use.
2
Therapeutic Support
Surfaces. Span-America’s non-powered therapeutic support
surfaces fall into two main product categories: the Geo-Mattress® all-foam
products and the non-powered portion of the PressureGuard® product
line. Geo-Mattress® products are single-density or multi-layered foam
mattresses topped with the same patented Geo-Matt surface used in our
overlays. These mattresses are sold as alternatives to standard
innerspring and all-foam mattresses often found in acute and long-term care
settings.
In 1997,
we introduced the Geo-Mattress Max, Plus, and Pro models of foam therapeutic
support surfaces. In early 1999, we extended the product line with
the release of the Geo-Mattress with Wings®, which has been a significant
contributor to overall Geo-Mattress sales. The Wings support surfaces
feature raised perimeter bolsters designed to reduce the chances of patients
rolling out of bed or becoming entrapped. We added a second line
extension, the Geo-Mattress Atlas®, in December 2000 to address the needs of
heavier patients.
Span-America’s
more complex non-powered support surfaces consist of products from the
PressureGuard® series. We acquired the PressureGuard design through
the acquisition of Healthflex, Inc. in February 1992. The original
design combined a polyurethane foam shell and static air cylinders to form a
support surface that incorporated the comfort and pressure relieving features of
both mattress overlays and more sophisticated dynamic
mattresses. This original design, which we later used as the basis
for powered versions (see below), was further refined through a complete
technical upgrade of all PressureGuard components in November 1997.
In
addition to the non-powered, static PressureGuard Renew®, we offer the
PressureGuard CFT®. This model incorporates patented design
principles of constant force technology. The PressureGuard CFT is
unique in that it is a dynamic support surface that rivals more expensive
powered surfaces in effectiveness, yet it requires no power source.
Span-America’s
powered therapeutic support surfaces constitute the remaining models in the
PressureGuard Series. In November 1993, we received Food and Drug
Administration (“FDA”) 510(k) marketing approval for the PressureGuard IV
therapeutic support surface. Building on the comfort and support of
the original PressureGuard design, PressureGuard IV was designed as a
sophisticated, powered system for providing pressure reduction and patient
comfort, with the added ability to turn the patient. The system was
designed to automatically sense the patient’s weight and position, and to
continually adjust the pressures appropriately while slowly and quietly
repositioning the patient at angles up to 30 degrees in cycles of up to two
hours. The upgraded version, renamed the PressureGuard Turn Select®,
incorporates all of these capabilities, as well as several additional
features. Of particular note is a pendant-operated,
microprocessor-controlled motion system, which is built into the support surface
rather than being suspended from the bed frame as a separate
unit.
3
Another
powered system in the PressureGuard line is the PressureGuard APM®, a simpler
but effective alternating pressure mattress. The APM is targeted
primarily at the long-term care and home care markets. In 2000, we
added a more feature-rich version of this mattress called the PressureGuard
APM2. In
2003, we further upgraded the APM2 products
with new features such as the addition of the Deluxe control
unit. The APM2 gives
caregivers the flexibility to offer either alternating pressure or a basic
lateral rotation modality by activating a toggle switch on the control
panel. In fiscal 2009, the APM2 was our
highest selling medical product line.
In late 2001, Span-America introduced
the PressureGuard Easy Air®, our first offering in the category of low-air-loss
mattresses. The Easy Air incorporates several patented design
innovations, which we believe allow it to overcome common performance
compromises inherent in competitive low-air-loss
products. Additionally, the Easy Air was independently documented to
outperform all leading competitors at that time in controlling excess skin
moisture, a key performance advantage in the competitive support surfaces
marketplace (see Ostomy/Wound
Management, January 2003, Volume 49, Issue 1, pp. 32-42).
We sell
all of the powered products in the PressureGuard Series to long-term care
facilities, usually through our distributors, and to home health care equipment
dealers for daily rental in the home care market. We also sell the
PressureGuard products in the acute care market, but in much smaller quantities
than in the long-term care and home care markets.
In fiscal
2004, we began working with Hill-Rom Company, Inc. (now Hill-Rom Holdings, Inc.
(NYSE:HRC)) to develop a private-label version of our PressureGuard CFT
therapeutic support surface to be sold under the Hill-Rom name primarily to
acute care hospitals. Hill-Rom, formerly a division of Hillenbrand
Industries, is a major supplier of hospital beds and various other types of
patient care equipment. Since the initial work in 2004, we expanded
the private label CFT line to include several other therapeutic support surfaces
with additional features designed and manufactured to meet Hill-Rom’s
specifications. As a result of the private label sales to Hill-Rom,
the PressureGuard CFT support surfaces became our fastest growing product line
in fiscal years 2006 and 2007. Our original supply agreement with
Hill-Rom expired in May 2008, and Hill-Rom decided to add competing support
surfaces to broaden their product line. We signed a new, short-term
agreement in July 2008, primarily to provide a structure for an orderly wind
down of our supply relationship with Hill-Rom. The new agreement was
cancelled by Hill-Rom in April 2009 as their transition neared
completion. Our sales to Hill-Rom in fiscal 2009 were $2.0
million. We expect sales to Hill-Rom in fiscal 2010 to be
minimal. We continue to offer private label versions of selected
medical products to other customers, depending on market conditions and customer
interest.
Therapeutic
support surfaces and related products made up approximately 46% of total Company
net sales in fiscal 2009, 54% in fiscal 2008, and 54% in fiscal
2007.
4
Patient
Positioners. We sell our specialty line of patient positioners
primarily under the trademark Span-Aids®. Span-Aids accounted for
approximately 7% of our total net sales in fiscal 2009. This is our
original product line and consists of over 300 different foam items that aid in
relieving the basic patient positioning problems of elevation, immobilization,
muscle contracture, foot drop, and foot or leg rotation. Span-Aids
patient positioners hold a patient's body in prescribed positions, provide
greater patient comfort, and generally are used to aid long-term comatose
patients or those in a weakened or immobilized condition. The
positioners also help in the prevention of pressure ulcers by promoting more
effective dispersion of pressure, heat and moisture. Span-Aids are
intended for single-patient use throughout a patient's entire treatment
program. Among the Span-Aids products that we presently market are
abduction pillows, body aligners, head supports, limb elevators and various foot
and wrist positioners. We sell patient positioners primarily to
hospitals and long-term care facilities through several national medical
products distributors.
Seating Products. Another
product category in our medical segment consists of seat cushions and related
seating products for wheelchairs, Geri-chairs (typically used in long-term care
facilities) and other health care seating needs. Our offerings in
this category can be subdivided into three main groups:
|
|
·
|
wound
healing aids,
|
|
|
·
|
patient
positioning and general pressure management products,
and
|
|
|
·
|
pressure
management products without patient positioning
features.
|
Seating
products made specifically as an aid to wound healing include the Isch-Dish® and
Sacral Dish® pressure relief cushions. Seating products made for
patient positioning and general pressure management include the Isch-Dish Thin,
the Geo-Matt® Contour® cushion, the Equalizer®, and the EZ-Dish®. The
Equalizer contoured positioning cushion has a multi-component design that
includes a viscoelastic foam top, proprietary soft polymer inserts, and a
contoured base. Like the Isch-Dish, the Equalizer is covered for
reimbursement by the Medicare system. This makes it an attractive
option for durable medical equipment suppliers and rehab seating
specialists. The EZ-Dish pressure relief cushion, which uses some of
the features of the original Isch-Dish design, offers a simpler, more affordable
solution to the seating problems of nursing home patients. The
Geo-Wave® Cushion assists with positioning and pressure reduction for patients
using specialty recliners and Geri-chairs. The Short-Wave® seat and back
cushion reduces shear and assists with patient positioning in standard
wheelchairs.
Seating
products designed to address pressure management without additional positioning
benefits include the Gel-T® cushion and the Geo-Matt and Geo-Matt PRT®
wheelchair cushions. The Gel-T is a gel/foam combination cushion that
is especially popular with elderly patients. The Geo-Matt and
Geo-Matt PRT cushions incorporate our proprietary Geo-Matt anti-shearing
surface.
Seating products accounted for
approximately 4% of total net sales in fiscal 2009.
Skin Care
Products. We also market the Selan® line of skin care creams
and lotions under a license agreement with PJ Noyes Company. The
products, which are manufactured by PJ Noyes, are used for cleaning,
moisturizing and protecting patients’ skin and are sold primarily in long-term
care and acute care settings. The license agreement with PJ Noyes
will expire on December 14, 2010. We anticipate renewing the license
agreement under similar terms and conditions prior to the 2010 expiration
date. Sales of skin care products accounted for approximately 2% of
our total net sales in fiscal 2009.
5
Fall Protection
Products. In December 2008, we introduced the Risk Manager®
bedside safety mat, which is a new product category for
Span-America. The Risk Manager is manufactured using an elastomeric
gel compound and is designed to cushion the force of impact and reduce the
chance of injury to a patient who falls at the bedside. Sales of the
Risk Manager made up 1% of our total net sales in fiscal 2009.
Distributor and Private-Label
Manufacturing Relationships. We sell our medical products to many
customers of varying sizes. We also sell our branded medical products
to several medical products distributors which resell our products to acute care
hospitals and long-term care facilities throughout North
America. Sales to our four largest medical distributors made up
approximately 45% of net sales in the medical segment during fiscal
2009. We believe our relationships with these distributors are
good. However, the loss of any one of these customers could have a
material adverse effect on our business. See Item 1A. “Risk Factors”
below for more information on our relationships with large
customers.
Custom
Products
Span-America's custom products segment
includes two major product lines: consumer bedding products and various
engineered industrial products. Our consumer product line consists
primarily of convoluted and contour-cut mattress overlays and specially designed
pillows for the consumer bedding market. The consumer products are
marketed to retailers through Louisville Bedding Company, a leading manufacturer
and distributor of bedding products in North America. Louisville
Bedding is the exclusive distributor of our consumer foam products pursuant to a
distribution agreement between us, which expires in December
2012. The agreement automatically renews for successive three-year
terms unless either party provides notice of its intent not to renew at least 60
days prior to the expiration date.
Our industrial product line consists of
specially engineered foam products used in a variety of markets, including the
water sports equipment, automotive, photographic film, durable goods and
electronics industries. Our largest industrial customers manufacture
kayaks, cars and specialty packaging products. The majority of our
industrial products are made to order according to customer
specifications. We currently have one full-time sales representative
selling our industrial foam fabrication capabilities primarily in the
southeastern United States.
The custom products segment represented
approximately 32% of our total net sales in fiscal 2009, compared with 28% in
fiscal 2008 and 29% in fiscal 2007. In fiscal 2009, approximately 79%
of our total custom products sales were distributed through Louisville Bedding
Company. The loss of this relationship would have a material adverse
effect on our business. Sales of consumer bedding products within the
custom products segment represented 27% of total Company net sales in fiscal
2009, 22% in fiscal 2008 and 23% in fiscal 2007.
6
Safety
Catheters
In July
2002, we acquired assets related to the Secure I.V.® protected short peripheral
intravenous catheter from Vadus, Inc., a privately owned designer and
manufacturer of catheters. The acquired assets consisted primarily of
patents and equipment related to the design, production and sale of the Secure
I.V. catheter. The product line was in the development stage at the
time of the acquisition, and we completed the development of the product and
initially launched Secure I.V. in 2004. However, we were unable to
generate sufficient sales volume in the safety catheter segment to make it a
viable business. Consequently, in October 2007, we decided to exit
the safety catheter business and try to sell the related assets. As
of September 29, 2007, we recorded an impairment charge to eliminate the book
value of our safety catheter assets. The losses, net of income taxes,
for the discontinued safety catheter segment were $21,000 in 2009, $50,000 in
2008, and $2.6 million in 2007 (including an after-tax impairment charge of $1.9
million).
We have
attempted to sell the assets related to the safety catheter business, but our
efforts so far have not been successful. We have ceased the use of
the safety catheter assets and are committed to a plan of sale or
abandonment. However, we have no offers pending and can give no
assurance that the assets will eventually be sold. If the assets are
not eventually sold, they will be abandoned and disposed of.
Research
and Development
Span-America’s expenditures for
research and development for the last three fiscal years are set forth in the
following table:
|
Research
and Development Expense
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
Medical
|
$ | 822,000 | $ | 657,000 | $ | 724,000 | ||||||
|
Custom
products
|
44,000 | - | - | |||||||||
|
Total
R& D - Operating
|
866,000 | 657,000 | 724,000 | |||||||||
|
Discontinued
Operations - Safety catheters
|
- | - | 83,000 | |||||||||
|
Total
R&D expense
|
$ | 866,000 | $ | 657,000 | $ | 807,000 | ||||||
We expect
research and development costs in fiscal 2010 to be similar to those of fiscal
2009.
Competition
Medical. In the
medical market segment, we face significant competition for sales of our foam
mattress overlays. Competition within the overlay market is primarily
based on price and delivery for convoluted foam overlays. For
therapeutic overlays, including Geo-Matt, competition is based on price, product
performance, quality and delivery. However, the largest single source
of competition for our mattress overlay products is from full-function
therapeutic support surfaces designed and manufactured either by us or our
competitors. Sales of overlays have generally declined since the
1990s as customers began to replace single-patient-use overlays with full
function mattresses that incorporated the pressure relieving features of
overlays. Competition with respect to our Span-Aids patient
positioners is primarily based on price. However, a secondary source
of competition for patient positioners results from alternative methods, such as
the use of pillows and other devices to position patients.
7
We believe that Span-America is one of
the largest nationwide suppliers of therapeutic mattress overlays and patient
positioners to the U.S. health care market. Our primary competitors
in the overlay and positioner markets are Sunrise Medical, Inc. and Covidien
(formerly Tyco Healthcare).
Competition in the therapeutic support
surface market is based on product performance, price and
durability. Customers typically select a product based on these
criteria after conducting a formal clinical evaluation of sample mattresses for
periods of one to six months. A secondary source of competition
results from alternative products such as mattress overlays, which are
significantly less expensive than support surfaces.
The market for therapeutic support
surfaces developed principally during the 1990s. Competitors include
The Encompass Group, Gaymar Industries, Inc., Hill-Rom Holdings, Inc., Kinetic
Concepts, Inc., Invacare Corporation, Sunrise Medical, Inc., Anodyne Medical
Device, Inc., Direct Supply, Inc. and Medline Industries, Inc. These
competitors use combinations of their own sales representatives and
manufacturer’s representatives to sell nationwide directly to hospitals,
distributors, long-term care facilities and original equipment
manufacturers.
Many of our competitors in the health
care segment are larger and have greater resources than
Span-America. We believe our competitive advantages in the medical
segment include innovative and patented product designs, product quality,
manufacturing capabilities, distribution relationships and responsiveness to
customer requirements.
Custom
Products. In the custom products segment, we have encountered
significant competition for our mattress pad and pillow products. The
competition is principally based on price, which is largely determined by foam
density and thickness. However, competition also exists due to
variations in product design and packaging. There are presently a
number of companies with the manufacturing capability to produce similar bedding
products. Our primary competitors in this market are Sleep
Innovations, Inc., E.R. Carpenter Company and Zinus, Inc., most of which are
larger and have greater resources than we do. We also have a number
of competitors in the market for our industrial products,
including CelloFoam North America, Inc., UFP Technologies,
Inc. (NASDAQ-CM:UFPT) and Foam-Tech. These competitors are larger and
have greater resources than we do. The competition for industrial
foam products is largely based on price. In many instances, however,
design, product quality and delivery capabilities are also
important. We believe that our competitive advantages in the custom
products segment include our distribution relationship with Louisville Bedding
Company, innovative product designs, manufacturing and foam fabrication
capabilities, low cost manufacturing processes and responsiveness to customer
requirements.
8
Within the last few years, we have
encountered increasing competition in the consumer bedding market from visco
foam products manufactured both in the United States and China. Visco
foam, also known as visco-elastic foam or memory foam, has greater density and
different properties than traditional polyurethane foam products. It
responds to body temperature, conforms to the shape of the body, and generally
has slower recovery time compared with traditional polyurethane
foam. Visco foam is also significantly more expensive than
traditional foam and is more difficult to handle and
fabricate. Because visco foam is more difficult to cut and shape than
traditional foam, it is more difficult for us to differentiate our products from
those of our competitors. Consequently, the visco mattress pads
currently on the market tend to be somewhat undifferentiated without unique
surface designs. In addition, since visco foam is significantly more
expensive and more dense than traditional foam, it is more cost effective for
overseas competitors (from China for example) to ship the products into the U.S.
market. This is generally because retail prices of visco foam
products are significantly higher than comparable traditional foam products,
which generates much higher revenue per square foot of retail shelf space and
lowers shipping costs as a percent of sales value.
Major
Customers
We have an agreement with Louisville
Bedding Company to distribute our consumer foam products. Sales to
Louisville Bedding during fiscal 2009 made up approximately 26% of our total net
sales and approximately 79% of sales in the custom products
segment.
In the medical segment, sales to an
additional customer with whom we have a business relationship to re-sell certain
of our medical products exceeded 10% of our total net sales. Sales to
this distributor, McKesson Medical-Surgical, during fiscal 2009 made up
approximately 12% of total net sales and approximately 18% of net sales in the
medical products segment.
See “Industry Segment Data – Medical –
Distributor and Private-Label Manufacturing Relationships” above and Note 17 –
Major Customers and Note 18 – Operations and Industry Segments in the Notes to
Financial Statements below for more information on major
customers. The loss of any of these major customers would have a
material adverse effect on our business.
9
Seasonal
Trends
Some seasonality can be identified in
certain of our medical and consumer foam products. However, the
fluctuations have minimal effect on our operations because of offsetting trends
among these product lines. We have not experienced significant
seasonal fluctuations in our industrial product line.
Patents
and Trademarks
We hold 34 United States patents and 8
foreign patents relating to various components of our patient positioners,
mattress overlays, and therapeutic support surfaces for the medical
segment. We have also filed additional patent
applications. We believe that these patents are important to our
business. However, while we have a number of products covered by
patents, there are competitive alternatives available, which are not covered by
these patents. Therefore, we do not rely solely on our patents to
maintain our competitive position in our various markets.
Our principal patents include the
patents on Geo-Matt, Geo-Mattress, PressureGuard, and Span-Aids
products. The Geo-Matt and Geo-Mattress patents have remaining lives
ranging from 1 to 4 years with additional patents pending. The
PressureGuard patents have remaining lives ranging from 5 to 9 years with
additional patents pending. The Span-Aids patents have remaining
lives ranging from less than a year to 6 years.
As previously noted, in July 2002, we
acquired assets related to the Secure I.V.® catheter product line of Vadus,
Inc., a privately owned designer and manufacturer of peripheral intravenous
catheters. The Secure I.V. has FDA 510(k) approval and is protected
by 11 U. S. patents and 9 foreign patents, all of which are owned by
Span-America. The Secure I.V. patents have remaining terms ranging
from 3 to 12 years. The mark “Secure I.V.” is also Span-America’s
registered trademark. We have also filed additional patent
applications. If we are successful in our efforts to sell the Secure
I.V. business, we expect that the purchaser will acquire the related patents and
trademarks.
We hold 37 federally registered
trademarks and 18 foreign trademark registrations, including Span-America, Span-Aids, Geo-Matt,
Geo-Mattress, PressureGuard, and Isch Dish in the medical and
consumer segments. Other federal
registration applications are presently pending. We believe that
these trademarks are readily identifiable in their respective markets and add
value to our product lines.
Raw
Materials and Backlog
Polyurethane foam and nylon/vinyl
mattress covers and tubes account for approximately 80% of our raw
materials. In addition, we use corrugated shipping containers,
polyethylene plastic packaging material and hook-and-loop
fasteners. We believe that our basic raw materials are in adequate
supply and are available from many suppliers at competitive
prices.
10
See Item 1A. “Risk Factors” and Item 7.
“Management’s Discussion and Analysis of Financial Condition and Results of
Operations” for more information on price increases for polyurethane
foam.
As of October 3, 2009, we had unshipped
orders of approximately $400,000 which represents a 67% decrease compared to our
backlog of $1.2 million at fiscal year-end 2008. We believe the
decline in our fiscal year-end level of unshipped orders from 2008 to 2009 was
an aberration caused by an unusual fluctuation in the timing of orders
received. We do not believe that the decline in unshipped orders is
indicative of expected sales trends in fiscal 2010. It is common for
our level of unshipped orders to fluctuate significantly on a daily
basis. Order volumes and shipments after October 3, 2009 have been
similar to historical levels and have generally met our
expectations. We expect to fill all orders in the current backlog in
the 2010 fiscal year.
Employees
We had 212 full-time employees as of
October 3, 2009. Of these employees, eight were officers, 14 were
management personnel, 22 were administrative and clerical personnel, 27 were
sales personnel, and 141 were manufacturing employees. We are not a
party to any collective bargaining agreement and have never experienced an
interruption or curtailment of operations due to labor
controversy. We believe that our relations with our employees are
good.
Supervision
and Regulation
The Federal Food, Drug and Cosmetic
Act, and regulations issued or proposed thereunder, provide for regulation by
the FDA of the marketing, manufacture, labeling, packaging and distribution of
medical devices, including our products. These regulations require,
among other things, that medical device manufacturers register with the FDA,
list devices manufactured by them, and file various reports. In
addition, our manufacturing facilities are subject to periodic inspections by
regulatory authorities and must comply with “good manufacturing practices” as
required by the FDA and state regulatory authorities. We believe that
we are in substantial compliance with applicable regulations and do not
anticipate having to make any material expenditures as a result of FDA or other
regulatory requirements.
We are certified as an ISO 9001 and ISO
13485 supplier for our PressureGuard mattress products from our Greenville,
South Carolina plant. These standards are prepared by the American
Society for Quality Control Standards Committee to correspond to the
International Standard ISO 9001:2000. ISO (the International
Organization for Standardization) is a worldwide federation of national
standards bodies dealing with quality-system requirements that can be used by a
supplier to demonstrate its capability and for the assessment of the capability
of a supplier by external parties. Compliance with ISO standard 13485
is required by Health Canada for all Class II medical devices sold
there. All of our powered therapeutic support surfaces for the health
care market are considered Class II medical devices. The
certification is subject to reassessment at six-month intervals. We
have maintained our certification based on the results of ISO audits conducted
during fiscal year 2009.
11
Environmental
Matters
Our manufacturing operations are
subject to various government regulations pertaining to the discharge of
materials into the environment. We believe that we are in substantial
compliance with applicable regulations. We do not anticipate that
continued compliance will have a material effect on our capital expenditures,
earnings or competitive position.
Item 1A. Risk
Factors
The loss of a key distributor or
customer in the Company’s medical or custom products segments could cause a
rapid and significant sales decline, which would likely result in a decline in
earnings. Many of our medical products are sold through large
national distributors in the United States and Canada. We do not
maintain long-term distribution agreements with most of these
distributors. Instead, we supply them based on purchase orders that
are issued by the customers on a daily or weekly basis. These
supplier-customer relationships can generally be ended by either party with
minimal notice. Consequently, if a large customer or distributor
decided to discontinue purchasing our products, our sales and earnings could
quickly decline. Our largest customer in the medical segment is
McKesson Medical-Surgical which accounted for 18% of sales in the medical
segment in fiscal 2009. In addition, all of our consumer foam
products are sold through our exclusive distributor, Louisville Bedding Company,
under a marketing and distribution agreement that expires in December
2012. The agreement automatically renews for successive three-year
terms unless either party provides notice of its intent not to renew at least 60
days prior to the expiration date.
For more information on major customers
and information on our business segments, see the discussions under Item 1.
“Business – Major Customers,” Note 17 – Major Customers and Note 18 – Operations
and Industry Segments in the Notes to Financial Statements, Item 1. “Business –
Industry Segment Data – Medical – Distributor and Private-Label Manufacturing
Relationships” and Item 1. “Business – Industry Segment Data – Custom
Products.”
The current weakness in the U.S.
economy and associated problems in the credit markets could cause our sales to
decline, which in turn could have a negative effect on our
earnings. Our fastest growing products during the last five
years have been our lines of therapeutic support surfaces, which consist of our
PressureGuard and Geo-Mattress products as well as our private-label support
surfaces. Sales of these support surfaces represented 46% of our
total net sales in fiscal 2009. These products are generally
considered by us and our customers to be capital purchase items instead of
consumable supplies. We believe that purchases of these capital goods
are more easily postponed during business downturns than purchases of
consumables. Consequently, sales of our support surfaces are likely
to be more sensitive to general economic weakness than other medical product
lines in our business. Also, tight conditions in credit markets could
make it more difficult for our customers to obtain financing for capital
expenditures, which could slow sales particularly within our support surface
product lines. Therapeutic support surface sales decreased 20% during fiscal
2009 compared with fiscal 2008. Sales of our therapeutic support
surfaces may continue to decline if the economy remains weak.
12
In addition, our industrial products
are sold primarily to the water sports, automotive and packaging industries, as
well as various other manufacturers. Our industrial business has
historically been more affected by general economic trends than other
Span-America product lines. Therefore an economic downturn is likely
to have a greater effect on sales of industrial products than on other product
lines in our business. Industrial sales decreased 26% during fiscal
2009 compared with fiscal 2008. Sales of industrial products could
continue to decline if the economy remains weak.
Since many of our operating costs are
fixed within a reasonable range of sales and production activity, sales declines
could result in proportionally greater declines in earnings
performance. We would attempt to reduce expenses in response to lower
sales levels, but we cannot give assurance that we would be able to fully offset
the effect of a rapid decline in sales volume.
Our medical business could lose sales
volume or could have a lower sales growth rate as a result of government
reimbursement changes in the medical market. A number of our
medical products are eligible for reimbursement by Medicare. We
receive no direct reimbursements from Medicare, but our customers often submit
reimbursement requests to Medicare. For example, we sell therapeutic
support surfaces to home health care dealers who in turn rent these products to
patients. Medicare reimburses the dealers for some or all of the
patient’s rental cost. If Medicare reimbursement rates are reduced,
the demand for our medical products that are covered by Medicare could also be
reduced, depending on the size of the rate reduction.
Our earnings could be negatively
affected by raw material cost increases that we are unable to recover through
sales price increases. The cost of polyurethane foam
represented approximately 41% of our total cost of goods sold in fiscal
2009. An increase in foam raw material costs that we are not able to
pass through to our customers by increasing prices could have a significant
negative effect on our profitability. Besides polyurethane foam, our
other major raw material categories include mattress covers made of various
water proof fabrics, vinyl bags, vinyl air cylinders, electronic components for
mattresses and corrugated boxes. Raw materials are our single largest
cost category, representing approximately 73% of our total cost of goods sold in
fiscal 2009. Cost increases in these raw materials could have a
significant adverse effect on earnings if we are unable to recover the higher
costs through sales price increases or operating expense
reductions.
Our sales volume could decline as a
result of competition from low-cost foreign imports. During
the last two years, we have experienced increased competition in our medical and
custom products segments from low-cost foreign imports. In the
medical segment, the number of low-cost, imported mattress products has
increased in the last two years, but it has not yet had a significant impact on
our medical business. We believe that we have potentially greater
exposure to low-cost imports in our consumer bedding product lines because those
products have more commodity-like characteristics than our medical
products. Also, our customers, which are generally national
retailers, are more likely to change suppliers to buy lower-cost
products. Therefore, we could lose significant sales volume in our
consumer bedding business and smaller parts of our medical sales volume if we
are unable to compete effectively with low-cost imports.
13
Certain of our medical products are
classified as medical devices and are regulated by the
FDA. These regulations require, among other things, that
medical device manufacturers register with the FDA, list devices manufactured by
them, and file various reports. In addition, our manufacturing
facilities are subject to periodic inspections by regulatory authorities and
must comply with “good manufacturing practices” as required by the FDA and state
regulatory authorities. Although we believe that we are in
substantial compliance with applicable regulations, the existence of the
regulations creates the risk of a product recall and related expenses as well as
the risk of additional expenses required to meet the regulatory
requirements.
Item
1B. Unresolved Staff Comments
None
Item
2. Properties
We own our principal office and
manufacturing facility, which is located in Greenville, South
Carolina. This facility contains approximately 188,000 square feet
used by the medical and custom products segments and is located on a 13-acre
site. We believe that our current manufacturing and storage space is
adequate to support our operations during the next several years, depending on
sales growth rates.
We also lease 15,000 square feet of
warehouse space in Salt Lake City, Utah for use as a distribution center for our
medical products. We lease this facility on a month-by-month basis at
a rate of $6,750 per month.
We consider the South Carolina and Utah
facilities to be suitable and adequate for their intended purposes.
Item 3. Legal
Proceedings
From time to time we are a party to
various legal actions arising in the normal course of business. We
believe that as a result of legal defenses and insurance arrangements with
parties believed to be financially capable, there are no proceedings threatened
or pending against us that, if determined adversely, would have a material
adverse effect on our financial position or results of operations.
Item
4. Submission of Matters to a Vote of Security
Holders
No
matters were submitted to a vote of security holders during the fourth quarter
of our 2009 fiscal year.
14
PART
II
Item 5. Market
for the Registrant's Common Equity, Related Stockholder Matters and Issuer
Repurchases of Equity Securities
The common stock of Span-America
Medical Systems, Inc. trades on The NASDAQ Global Market® under the symbol
SPAN. As of December 16, 2009, there were 2,715,118 common shares
outstanding, 180 shareholders of record and approximately 1,100 beneficial
shareholders. The closing price of Span-America's stock on December
16, 2009 was $15.90 per share.
The high and low sales prices for the
Company’s Common Stock in each of the last eight fiscal quarters is shown on the
following table.
|
Quarterly
Stock Price Data
|
||||||||||||||||||||
|
First
|
Second
|
Third
|
Fourth
|
Year
|
||||||||||||||||
|
For
Fiscal 2009
|
||||||||||||||||||||
|
High
|
$ | 12.94 | $ | 10.75 | $ | 11.94 | $ | 13.53 | $ | 13.53 | ||||||||||
|
Low
|
8.03 | 7.76 | 8.05 | 10.54 | 7.76 | |||||||||||||||
|
For
Fiscal 2008
|
||||||||||||||||||||
|
High
|
$ | 18.75 | $ | 13.39 | $ | 13.52 | $ | 13.50 | $ | 18.75 | ||||||||||
|
Low
|
9.88 | 10.17 | 10.17 | 10.60 | 9.88 | |||||||||||||||
The Company has paid a regular
quarterly cash dividend since January 1990. In April 2008, the Board
increased the quarterly dividend to $0.09 per share from $0.08 per
share. In November 2009, the Board increased the quarterly dividend
to $0.10 per share from $0.09 per share payable on December 4,
2009. We expect the Company to continue to pay quarterly dividends
for the foreseeable future, though the Board may discontinue paying dividends at
any time. Future dividend payments will depend upon the Company’s
earnings and liquidity position. See the discussion of our revolving
bank credit facility in Note 9 – Borrowings in the Notes to Financial Statements
for a description of restrictions on our ability to pay dividends and repurchase
our stock, which description is incorporated herein by reference.
The information regarding equity
compensation plans set forth under Item 12 below is incorporated herein by
reference.
15
ISSUER
PURCHASES OF EQUITY SECURITIES
|
Period
|
(a)
Total Number
of
Shares
Purchased
|
(b)
Average
Price
Paid per
Share
|
(c)
Total Number
of
Shares
Purchased
as Part
of
Publicly
Announced
Plans
or
Programs
|
(d)
Maximum
Number
of
Shares
that May
Yet
Be
Purchased
Under
the
Plans
|
||||||||||||
|
June
28, 2009-Aug.
1, 2009
|
1,000 | 11.28 | 1,000 | 126,991 | ||||||||||||
|
Aug.
2, 2009 – Aug. 29, 2009
|
3,662 | 11.52 | 3,662 | 123,329 | ||||||||||||
|
Aug.
30, 2009 – Oct. 3, 2009
|
5,350 | 12.45 | 5,350 | 117,979 | ||||||||||||
|
Total
|
10,012 | 12.00 | 10,012 | 117,979 | ||||||||||||
The
Company announced on November 28, 2007 that the Board of Directors authorized
the Company to repurchase up to 138,772 shares of its common
stock. On February 11, 2009, the Board expanded the
repurchase program by 100,000 shares, bringing the total number of authorized
shares to 238,772. The program may be suspended or discontinued at
any time.
16
PERFORMANCE
GRAPH
Notwithstanding
any statement in any of the Company’s previous or future filings under the
Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as
amended, incorporating future or past filings, including this Annual Report on
Form 10-K, in whole or in part, the following Performance Graph shall not be
incorporated by reference into any such filing unless the incorporation
specifically lists the following Performance Graph.
The following graph sets forth the
performance of the Company’s Common Stock for the five-year period from October
2, 2004 through October 3, 2009, compared to the Russell MicroCap Index and a
peer group index. The peer group index was prepared by an
unaffiliated third party and is comprised of all exchange-listed companies that
had the standard industry classification code 3842 (which relates to medical
products and supplies) as of October 3, 2009. The companies included
in the peer group index are shown below. All stock prices reflect the
reinvestment of cash dividends.
COMPARISON
OF CUMULATIVE TOTAL RETURN AMONG
SPAN-AMERICA
MEDICAL SYSTEMS, INC.,
THE
RUSSELL MICROCAP INDEX
AND
A PEER GROUP
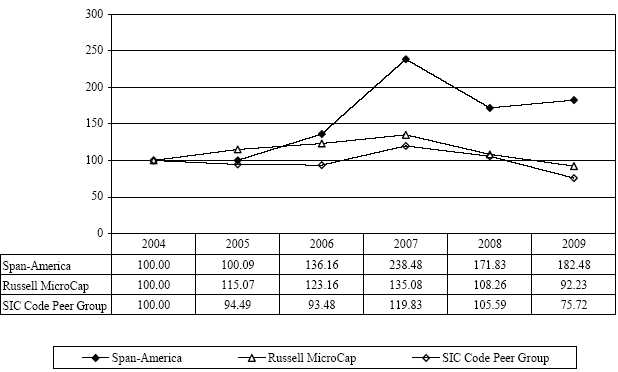
Assumes
$100 invested on October 2, 2004.
Assumes
dividends reinvested. Fiscal year ended October 3,
2009.
17
COMPANIES
INCLUDED IN PEER GROUP INDEX
Standard
Industry Classification Code 3842
at
October 3, 2009
|
American
Surgical Holdings, Inc.
|
Antares
Pharma, Inc.
|
Applied
Nanoscience Inc.
|
||
|
ATS
Medical, Inc.
|
Biocoral,
Inc.
|
Cardo
Medical, Inc.
|
||
|
Chad
Therapeutics, Inc.
|
Cytori
Therapeutics, Inc.
|
Exactech,
Inc.
|
||
|
Hansen
Medical, Inc.
|
Heritage
Worldwide, Inc.
|
Hill-Rom
Holdings, Inc.
|
||
|
Ibrands
Corp.
|
Insulet
Corp.
|
Integra
Lifesciences Holdings
|
||
|
Invacare
Corp.
|
Lakeland
Industries, Inc.
|
MB
Software Corp.
|
||
|
Medical
Action Industries Inc.
|
Medical
Solutions Management
|
Mine
Safety Appliances Co.
|
||
|
Miracor
Diagnostics, Inc.
|
Nano
Mask Inc.
|
Orthologic
Corp.
|
||
|
Otix
Global, Inc.
|
PC
Group Incorporated
|
Point
Blank Solutions, Inc.
|
||
|
Pride
Business Development Holdings
|
Quantum
MRI, Inc.
|
RTI
Biologics, Inc.
|
||
|
Sector
10, Inc.
|
Sharps
Compliance Corp.
|
Stryker
Corp.
|
||
|
Symmetry
Medical, Inc.
|
Synovis
Life Technologies
|
Theragenics
Corp.
|
||
|
Wright
Medical Group, Inc.
|
Zimmer
Holdings, Inc.
|
18
Item 6. Selected
Financial Data
Selected Financial Data for the
Company’s last five fiscal years is shown in the table below.
Five-Year
Financial Summary
(Amounts
in thousands, except per share and employee data)
|
2009
|
2008
|
2007
|
2006
|
2005
|
||||||||||||||||
|
(1)
|
(1)
|
(1)
|
||||||||||||||||||
| For the year: | ||||||||||||||||||||
|
Net
sales
|
$ | 55,867 | $ | 59,265 | $ | 60,544 | $ | 51,436 | $ | 48,433 | ||||||||||
|
Gross
profit
|
20,208 | 20,395 | 20,951 | 16,438 | 15,304 | |||||||||||||||
|
Operating
income
|
6,868 | 7,518 | 8,128 | 5,093 | 4,633 | |||||||||||||||
|
Income
from continuing operations
|
4,705 | 4,919 | 5,505 | 3,779 | 3,408 | |||||||||||||||
|
Net
income
|
4,684 | 4,869 | 2,874 | 3,055 | 2,439 | |||||||||||||||
|
Cash
flow from operations
|
6,806 | 5,250 | 6,294 | 2,497 | 2,589 | |||||||||||||||
|
Capital
expenditures for continuing operations
|
355 | 692 | 1,009 | 1,071 | 2,375 | |||||||||||||||
|
Per
share:
|
||||||||||||||||||||
|
Income
from continuing operations:
|
||||||||||||||||||||
|
Basic
|
$ | 1.72 | $ | 1.77 | $ | 2.02 | $ | 1.43 | $ | 1.31 | ||||||||||
|
Diluted
|
1.68 | 1.71 | 1.92 | 1.36 | 1.24 | |||||||||||||||
|
Net
income:
|
||||||||||||||||||||
|
Basic
|
$ | 1.72 | $ | 1.76 | $ | 1.06 | $ | 1.15 | $ | 0.94 | ||||||||||
|
Diluted
|
1.67 | 1.70 | 1.00 | 1.10 | 0.89 | |||||||||||||||
|
Cash
dividends declared (2)
|
0.36 | 0.34 | 5.30 | 0.195 | 0.565 | |||||||||||||||
|
(1)
|
As
restated to show the safety catheter segment as a discontinued operation.
See Note 12 in Notes to Financial
Statements.
|
|
(2)
|
Cash
dividends declared include special dividends of $5.00 per share in 2007
and $0.40 per share in 2005.
|
19
Five-Year
Financial Summary
(Amounts
in thousands, except per share and employee data)
|
2009
|
2008
|
2007
|
2006
|
2005
|
||||||||||||||||
|
(1)
|
(1)
|
(1)
|
||||||||||||||||||
|
At
end of year:
|
||||||||||||||||||||
|
Working
capital
|
10,858 | 8,048 | 7,447 | 13,338 | 10,638 | |||||||||||||||
|
Property
and equipment - net
|
6,159 | 6,569 | 6,537 | 6,137 | 5,812 | |||||||||||||||
|
Total
assets
|
26,835 | 24,113 | 23,838 | 31,012 | 28,666 | |||||||||||||||
|
Long
term debt
|
700 | 3,700 | ||||||||||||||||||
|
Shareholders'
equity
|
20,573 | 17,332 | 13,788 | 24,517 | 21,561 | |||||||||||||||
|
Book
value per share
|
7.59 | 6.28 | 4.97 | 9.22 | 8.26 | |||||||||||||||
|
Number
of employees from continuing operations
|
212 | 253 | 317 | 287 | 291 | |||||||||||||||
|
Key
ratios:
|
||||||||||||||||||||
|
Return
on net sales (2)
|
8.4 | % | 8.2 | % | 4.7 | % | 5.9 | % | 5.0 | % | ||||||||||
|
Return
on average shareholders' equity (2)
|
24.7 | % | 31.3 | % | 15.0 | % | 13.3 | % | 11.6 | % | ||||||||||
|
Return
on average total assets (2)
|
18.4 | % | 20.3 | % | 10.5 | % | 10.2 | % | 8.7 | % | ||||||||||
|
Current
ratio
|
3.0 | 2.5 | 2.3 | 3.8 | 3.0 | |||||||||||||||
|
(1)
|
As restated to show the safety
catheter segment as a discontinued operation. See Note 12 in Notes to Financial
Statements.
|
|
(2)
|
These
"Return" ratios are calculated using net income as shown above, which
includes losses from discontinued
operations.
|
20
Item
7. Management's Discussion and Analysis of Financial Condition and
Results of Operations
OVERVIEW
Span-America’s operations are divided
into two primary business units or segments: medical and custom
products. Our revenues, profits and cash flows are derived from the
development, manufacture and sale of products for these two market
segments. In the medical segment, we manufacture and market a
comprehensive selection of pressure management products, including Geo-Matt®,
PressureGuard®, Geo-Mattress®, Span-Aids® and Isch-Dish® products. We
license and market, but do not manufacture, Selan® skin care
products. In the custom products segment, we manufacture consumer
mattress pads and pillows for the retail bedding market and various engineered
foam products for the industrial market. Our consumer mattress pads
and pillows are marketed by our exclusive distributor, Louisville Bedding
Company. We sell the industrial product line directly to our
customers instead of using distributors. Prior to fiscal year 2008,
we had a third business unit engaged in the development, manufacture and sale of
safety catheters. We decided to exit the safety catheter business in
October 2007. Revenues and expenses related to the safety catheter
business in fiscal years 2009 and 2008 are shown in our financial statements as
a discontinued operation. Results of operations for fiscal year 2007
were restated to show the safety catheter segment as a discontinued
operation.
RESULTS
OF OPERATIONS FISCAL 2009 VS. 2008
Summary
Total sales in fiscal 2009 declined 6%
to $55.9 million compared with $59.3 million in fiscal 2008 because of lower
sales volume in our medical segment, which was partially offset by increases in
sales volume in our custom products segment. Medical sales were down
11% to $37.8 million due mainly to a decline in sales of private label
therapeutic support surfaces. Custom products sales for fiscal 2009
increased 7% to $18.1 million due to higher sales of consumer bedding products
compared with fiscal year 2008.
Income from continuing operations
declined 4% in fiscal 2009 to $4.7 million, or $1.68 per diluted share, because
of lower sales levels. Net income, which includes results from the
discontinued safety catheter segment, also fell 4% in fiscal 2009 to $4.7
million, or $1.67 per diluted share.
Sales
Total sales in our core medical
business declined 11% to $37.8 million in fiscal 2009 compared with $42.5
million in fiscal 2008. The decline in medical sales was primarily
related to the expiration of a private label supply contract with Hill-Rom that
accounted for $7.0 million in fiscal 2008 sales compared with $2.0 million in
fiscal 2009 sales. Consequently, sales of therapeutic support
surfaces, including private label products, declined by 20% to $25.5 million
during fiscal 2009 compared with $31.8 million in fiscal
2008. Therapeutic support surfaces, or mattresses, are our largest
medical product line, making up 67% of our medical segment sales in fiscal 2009
and 75% in fiscal 2008. We sell these specialty mattresses to
hospitals, long-term care facilities and home care dealers throughout the United
States and Canada. Sales of therapeutic support surfaces have
declined compared to previous fiscal years due primarily to the expiration of
the private label contract mentioned above and secondarily due to reduced
capital spending by many of our customers. Excluding sales to
Hill-Rom in both years, sales of therapeutic support surfaces would have
declined by 5% in fiscal 2009 compared with fiscal 2008.
21
Sales of the new Risk Manager™ bedside
safety mat, introduced at the beginning of our second fiscal quarter in 2009,
were $801,000 in fiscal 2009. Sales of our Span-Aids patient
positioners and mattress overlays both increased by 7% during fiscal 2009
compared with fiscal 2008. Selan skin care sales fell 4%, and sales
of seating products increased 10% during fiscal 2009. The growth in
sales of positioners, overlays and seating products in fiscal 2009 was mainly
due to sales price increases during the year. Medical sales accounted
for 68% of total net sales in fiscal year 2009 compared with 72% in fiscal
2008. We expect medical sales for 2010 to be higher than those of
2009.
Our custom products segment consists of
consumer bedding products and specialty foam products for the industrial
market. Sales in the custom products segment increased 7% during
fiscal 2009 to $18.1 million from $16.8 million in fiscal 2008. The
primary reason for the increase occurred in the consumer part of the custom
products segment, where sales increased 17% to $15.3 million compared with $13.0
million in fiscal 2008. This increase was caused by higher volumes of
consumer mattress overlays sold to existing customers and the addition of
several new customers during the year. All of our consumer products
are sold through our marketing and distribution partner, Louisville Bedding
Company.
Early in 2009, Span-America was
selected as one of three companies to supply consumer mattress pads to Sam’s
Club. We began shipping the new products in April, and our sales to
Sam’s Club in fiscal 2009 were $2.0 million. We learned in August
that Sam’s Club had selected an offshore company as its sole supplier of
mattress pads and that Span-America would not be a continuing supplier to Sam’s
Club. Our ongoing business with Wal-Mart is unaffected by the Sam’s
Club decision. We plan to sell the remaining Sam’s Club-related
inventory, and we do not expect significant future write-offs or charges related
to the loss of the Sam’s Club business. We expect consumer sales in
fiscal 2010 to be lower than they were in fiscal 2009 primarily because of the
loss of the Sam’s Club business described above.
In the other part of the custom
products segment, industrial sales decreased 26% in fiscal 2009 to $2.8 million
compared with $3.8 million in fiscal 2008. This portion of our
business has been more impacted by the weak economy as our largest customers
serve the water sports, automotive and packaging markets that have been
adversely affected by the recession. We believe that industrial sales
in fiscal 2010 will be higher than those of fiscal 2009 because we expect
increased demand in our key industrial markets as the U. S. economy
improves. There can be no assurance, however, that economic
conditions will improve or that industrial sales will increase.
22
Gross
Profit
Our gross profit decreased by 1% during
fiscal 2009 to $20.2 million compared with $20.4 million in fiscal
2008. Gross margin increased to 36.2% for fiscal 2009 from 34.4% in
fiscal 2008. The decrease in gross profit was caused mostly by lower
sales volume in the medical segment. The increase in gross margin was
the result of lower raw material costs during fiscal 2009 and the ongoing
implementation of lean manufacturing techniques during the year. As a
result of the lean manufacturing initiatives, we have improved our overall
manufacturing efficiency, and specifically we have reduced scrap rates, improved
product yields and reduced labor costs. We expect our gross profit in
fiscal 2010 to be higher than in 2009, but we believe our 2010 gross margin will
be slightly lower than 2009 because of anticipated increases in raw material
costs. Our 2010 gross profit and margin performance will depend
heavily on sales volume, product mix and raw material costs.
Selling,
Research & Development and Administrative Expenses
Selling and marketing expenses
increased 1%, or $49,000, to $9.0 million and 16.2% of net sales in fiscal 2009
compared with 15.2% of net sales in fiscal 2008. The increase
occurred in the medical segment and was primarily the result of higher incentive
compensation, which was partially offset by declines in evaluation samples
expense and shipping costs. We believe that total selling and
marketing expenses for fiscal 2010 will increase over 2009 levels.
Total research and development expenses
increased 32% to $866,000 in fiscal 2009 compared with $657,000 in fiscal
2008. Almost all of our research and development expenses are
incurred in the medical segment and are related to the development of new
products, new features of existing products and design
improvements. The increase in expense during fiscal 2009 was caused
by a greater number of new product development projects and higher incentive
compensation for R&D employees. R&D expenses will likely
fluctuate from quarter to quarter and from year to year, depending on the nature
of the development projects being pursued. We expect total R&D
expenses in fiscal 2010 to be similar to those of fiscal 2009.
General and administrative expenses
increased 6% to $3.4 million in fiscal 2009 from $3.2 million in fiscal
2008. The expense increase during fiscal 2009 was caused by higher
incentive compensation expense and an increase in depreciation expenses
associated with our new enterprise resource planning system. These
expense increases were partially offset by decreases in property and casualty
insurance and bad debt expenses. Incentive compensation is determined by
comparing actual operating earnings to planned operating earnings for the
current fiscal year. Incentive compensation expense increased in 2009 because we
exceeded our operating earnings goal by a wider margin in 2009 than in
2008. We expect administrative expenses for fiscal 2010 to be slightly
lower than they were in fiscal 2009.
Operating
Income
In the
medical segment, operating income for fiscal 2009 declined by 27% to $5.6
million compared with $7.6 million in fiscal 2008. The decrease was
caused by lower medical sales volume and higher R&D expenses compared with
last fiscal year.
Operating
income in the custom products segment increased 166% to $2.1 million in fiscal
2009 compared with $801,000 in fiscal 2008. The increase in
profitability of the custom products segment was caused by higher sales volume
in our consumer product lines, improved manufacturing efficiencies as discussed
above and lower raw material costs. The profit improvement for our
consumer product lines was partially offset by a profit decline in our
industrial product lines caused by a sharp decrease in industrial sales
volume.
23
Operating income for the total Company
declined 9% in fiscal 2009 to $6.9 million compared with $7.5 million in fiscal
2008. As discussed above, the decline in medical operating income,
caused by lower medical sales volume, was greater than the improvement in
operating income for the custom products segment.
Non-Operating
Income
Investment and other income declined by
43% to $29,000 in fiscal 2009 compared with $51,000 in fiscal
2008. The decline was caused by lower interest rates on overnight
investments in fiscal 2009 compared with 2008. We expect investment
income for fiscal 2010 to be higher than for fiscal 2009.
Interest
Expense
Interest expense in fiscal 2009
decreased 96% to $4,000 compared with $108,000 in fiscal 2008. The
decrease was caused by a lower average balance of long-term debt in fiscal 2009
compared with 2008 as we paid off the remaining balance of $700,000 on our
revolving note in the first quarter of fiscal 2009. See “Liquidity
and Capital Resources” below for further discussion about our revolving credit
facility.
Net
Income and Dividends
Net income, which includes results from
the discontinued safety catheter segment, decreased 4% in fiscal 2009 to $4.7
million, or $1.67 per diluted share, compared with $4.9 million, or $1.70 per
diluted share, in fiscal 2008. The decrease in net income was
primarily the result of lower medical sales volume.
During fiscal 2009, we paid dividends
of $983,000, or 21% of net income for the year. This amount consisted
of four quarterly dividends of $0.09 per share. During fiscal 2008,
we paid dividends of $943,000, or 19% of net income for the
year. This amount consisted of two quarterly dividends of $0.08 per
share and two quarterly dividends of $0.09 per share.
RESULTS
OF OPERATIONS FISCAL 2008 VS. 2007
Summary
Total sales in fiscal 2008 declined 2%
to $59.3 million compared with $60.5 million in fiscal 2007 because of lower
sales volume in both our medical and custom products
segments. Medical sales were down 2% to $42.5 million due mainly to a
decline in sales of private label therapeutic support
surfaces. Custom products sales for fiscal 2008 decreased 3% to $16.8
million due to lower sales of consumer bedding products compared with fiscal
year 2007.
Income from continuing operations
declined 11% in fiscal 2008 to $4.9 million, or $1.71 per diluted share, because
of lower sales levels, higher raw material costs in the medical segment, a
slight increase in selling expenses and a decline in non-operating
income.
24
Net income, which includes results from
the discontinued safety catheter segment, was up 69% in fiscal 2008 to $4.9
million, or $1.70 per diluted share. The increase in fiscal 2008 net
income was caused by our exit from the safety catheter segment in early fiscal
2008 and the resulting decrease in losses from the discontinued
operation.
Sales
Total sales in our core medical
business declined 2% to $42.5 million in fiscal 2008 compared with $43.2 million
in fiscal 2007. The decline in medical sales was caused mostly by
lower volume of private-label therapeutic support surfaces manufactured for
Hill-Rom. Sales to Hill-Rom declined by $2.7 million in fiscal 2008.
Sales of therapeutic support surfaces, including private label and branded
products, declined by 2% during fiscal 2008. Therapeutic support
surfaces, or mattresses, are our largest medical product line, making up 75% of
our medical segment sales in both fiscal 2008 and fiscal
2007. Excluding sales to Hill-Rom, sales of our therapeutic support
surfaces were up 8% in fiscal 2008, reflecting solid growth in our branded
mattress products. Growth leaders among our branded support surfaces
included the PressureGuard APM2
alternating pressure mattress and the Geo-Mattress line of all-foam support
surfaces. We had mixed results in sales of our other medical product
lines during fiscal 2008. Sales of our Span-Aids patient positioners
declined by 4% due mostly to lower export business. Sales of mattress
overlays increased 3%, Selan skin care sales rose 4% and sales of seating
products declined 1% during fiscal 2008. Medical sales accounted for
72% of total net sales in fiscal year 2008 compared with 71% in fiscal
2007.
Our custom products segment consists of
consumer bedding products and specialty foam products for the industrial
market. Sales in the custom products segment declined 3% during
fiscal 2008 to $16.8 million from $17.3 million in fiscal 2007. The
entire decline occurred in the consumer part of the custom products segment,
where sales were down 5% to $13.0 million compared with $13.7 million in fiscal
2007. The consumer sales decrease was caused mostly by the loss of
one customer due to bankruptcy and loss of another due to an acquisition by a
competitor. This lost business was partially offset by an increase in
sales of consumer mattress overlays and pillows to Wal-Mart, our largest
customer in the custom products segment.
In the other part of the custom
products segment, industrial sales increased 5% in fiscal 2008 to $3.8 million
compared with $3.6 million in fiscal 2007. The growth in industrial
sales came from a combination of new and existing customers primarily in the
automotive and water sports markets.
Gross
Profit
Our gross profit decreased by 3% during
fiscal 2008 to $20.4 million compared with $21.0 million in fiscal
2007. Gross margin declined slightly to 34.4% for fiscal 2008 from
34.6% in fiscal 2007. The decreases in gross profit and gross margin
were caused by lower sales volume in the medical and custom products segments
and higher raw material costs in the medical segment. The increase in
medical material cost was affected by higher foam costs and an increase in
warranty expense related to performance problems with two electronic components
within our therapeutic support surface product lines. The problems
were resolved in the fourth quarter of fiscal 2008. See Note 8 in the
Notes to Financial Statements for more information on warranty
expense.
25
We implemented several lean
manufacturing techniques during fiscal 2008, which improved our labor usage and
efficiency, particularly in the custom products segment. As a result,
our number of employees declined and labor costs declined both in absolute
dollars and as a percentage of sales. The decline in labor costs
partially offset higher raw material costs during the
year. Manufacturing overhead expenses remained level in fiscal years
2008 and 2007.
Selling,
Research & Development and Administrative Expenses
Selling and marketing expenses
increased 1% to $9.0 million and 15.2% of net sales in fiscal 2008 compared with
$8.9 million and 14.7% of net sales in fiscal 2007. The increase
occurred in the medical segment and was mostly in the categories of sales
commissions, evaluation samples and shipping costs. Those expenses
increased even though sales declined because we replaced most of our lost
private-label sales, which have no commission and shipping costs, with branded
product sales, which carry standard commission and shipping costs.
Total research and development expenses
declined 9% to $657,000 in fiscal 2008 compared with $724,000 in fiscal
2007. The decline was caused by the completion of product development
projects in fiscal 2008 that were ongoing in fiscal 2007 and by lower incentive
compensation expense.
General and administrative expenses
increased 1% to $3.23 million in fiscal 2008 from $3.21 million in fiscal
2007. The expense increase was caused mainly by a decline in the cash
value of corporate-owned life insurance. A decrease in the cash value
of life insurance is shown as an administrative expense, while an increase is
shown as a reduction in administrative expenses. Please see “Item 7A.
Quantitative and Qualitative Disclosures about Market Risk” below and Notes 6
and 10 in the Notes to Financial Statements for additional information on the
cash value of life insurance. The increase in administrative expenses
also reflected higher costs for property and casualty
insurance. These expense increases were mostly offset by a decline in
incentive compensation expense in fiscal 2008.
Operating
Income
In the
medical segment, operating income for fiscal 2008 declined by 17% to $7.6
million compared with $9.1 million in fiscal 2007. The decrease was
caused by lower medical sales volume, higher raw material costs and higher
medical selling expenses in fiscal 2008 compared with last fiscal
year.
The
custom products segment showed a significant improvement in operating income,
which rose to $801,000 in fiscal 2008 compared with an operating loss of
$267,000 in fiscal 2007. The increase in custom products operating
income was caused by across-the-board expense reductions in that segment as well
as an increase in industrial sales volume. Within the custom products
segment, we achieved cost reductions during the year in the categories of raw
materials, labor and manufacturing overhead as a result of ongoing efforts to
improve material usage and yields and to increase manufacturing
efficiencies. We also adopted lean manufacturing techniques to reduce
the number of employees required to meet production
requirements.
26
Operating income for the total Company
declined 7% in fiscal 2008 to $7.5 million compared with $8.1 million in fiscal
2007. As discussed above, the decline in medical operating income was
greater than the improvement in custom products operating income.
Non-Operating
Income
Investment income declined by 82% to
$51,000 in fiscal 2008 compared with $277,000 in fiscal
2007. The decrease was caused by a significant reduction in the
level of short-term investments, which in turn was related to our $5.00 per
share special dividend paid in June 2007. The $13.9 million special
dividend was funded by liquidation of our short-term investments and the
addition of $5.7 million in long-term debt during fiscal 2007.
Interest
Expense
Interest expense in fiscal 2008
increased 5% to $108,000 compared with $103,000 in fiscal 2007. The
increase was caused by a higher average balance of long-term debt in fiscal 2008
compared with 2007. Since the addition of long-term debt took place
in June 2007 as described above, the debt was outstanding for only four months
during fiscal year 2007. Although we steadily reduced the debt level
during fiscal 2008, the average balance was higher in fiscal 2008 than in
2007. See “Liquidity and Capital Resources” below for further
discussion about our revolving credit facility.
Income
from Continuing Operations, Net Income and Dividends
Income from continuing operations in
fiscal 2008 declined 11% to $4.9 million, or $1.71 per diluted share, compared
with $5.5 million, or $1.92 per diluted share, in fiscal 2007. The
decrease was caused by lower medical and custom products sales volume, higher
raw material costs in the medical segment, higher medical selling expenses and
lower investment income in fiscal 2008 compared with fiscal 2007.
Net income, which includes results from
the discontinued safety catheter segment, increased 69% in fiscal 2008 to $4.9
million, or $1.70 per diluted share, compared with $2.9 million, or $1.00 per
diluted share, in fiscal 2007. The increase in net income was the
result of discontinuing the safety catheter segment, which reduced our after-tax
loss from discontinued operations to $50,000 in fiscal 2008 from $2.6 million in
fiscal 2007.
During fiscal 2008, we paid dividends
of $943,000, or 19% of net income for the year. This amount consisted
of two quarterly dividends of $0.08 per share and two quarterly dividends of
$0.09 per share. In April 2008, the board of directors increased our
quarterly dividend by 12.5%, from $0.08 to $0.09 per share.
LIQUIDITY
AND CAPITAL RESOURCES
We generated cash from operations of
$6.8 million during fiscal 2009, which represented a 30% increase compared with
cash flow of $5.3 million in fiscal 2008. The major positive factor
affecting cash flow from operations in fiscal 2009 was a decrease in accounts
receivable during the year compared with an increase in that line item in the
previous year. The main negative factor affecting cash flow during
fiscal 2009 was an increase in prepaid expenses and other assets compared with a
decrease in that category during fiscal 2008. The balance sheet
components of these changes are described below.
27
The main uses of cash provided by
operations in fiscal 2009 were purchases of marketable securities of $3.7
million, payment of dividends of $983,000, repayment of $700,000 of long-term
debt, stock repurchases of $604,000 and equipment purchases of
$355,000.
Working capital increased by $2.8
million, or 35%, to $10.9 million during fiscal 2009. The increase in
working capital was primarily caused by a higher balance in securities available
for sale. In addition, our current ratio increased to 3.0 at fiscal
year-end 2009 from 2.5 at fiscal year-end 2008.
Accounts receivable, net of allowances,
decreased 19% to $6.3 million at the end of fiscal 2009 compared with $7.8
million at the end of fiscal 2008. The decrease was partly the result
of lower sales during fiscal 2009 compared with fiscal 2008 and partly due to
the timing of customer payments received. Our accounts receivable
balance was higher than usual at fiscal year-end 2008 and lower than usual at
fiscal year-end 2009, creating a slightly exaggerated decline in accounts
receivable in fiscal 2009. The days sales outstanding (or average
collection time), calculated using a 12-month average for trade accounts
receivable balances, decreased slightly to 43.1 days in 2009 compared with 43.5
days in 2008. The shorter collection time is primarily due to normal
month-to-month fluctuations in the timing of payments received and a higher
percentage of custom products sales. Medical sales typically have
longer collection times than custom products sales. All of our
accounts receivable are unsecured.
Inventory, net of reserves, decreased
by $82,000 to $3.9 million at fiscal year-end 2009 compared with $4.0 million at
fiscal year-end 2008. The slight change was the result of decreases
in raw material inventory for medical and industrial products, partially offset
by increases in medical and industrial finished goods
inventory. Inventory turns from continuing operations decreased
slightly to 9.0 times in fiscal 2009 compared with 9.7 times in fiscal 2008
because our cost of sales decreased at a faster rate than our total inventory
levels. We expect inventory levels in fiscal 2010 to be similar to
2009 fiscal year-end levels.
Our deferred income tax asset increased
by 46% during fiscal 2009 to $997,000 from $683,000 due mostly to an increase in
accrued incentive compensation. The increase in accrued incentive
compensation was caused by an increase in management bonuses in fiscal 2009
compared with fiscal 2008.
Prepaid expenses increased 96% to
$102,000 during fiscal 2009 from $52,000 at the end of fiscal
2008. The increase was the result of normal monthly fluctuations in
prepaid accounts.
Net property and equipment decreased by
$410,000, or 6%, during fiscal 2009. The change resulted from the
combination of $764,000 in depreciation expense offset by capital expenditures
of $355,000. We expect capital expenditure levels in fiscal 2010 to
be similar to those of fiscal 2009.
28
Other assets increased by 8% to $2.5
million during fiscal 2009 compared with $2.3 million at fiscal year-end 2008
due to increases in deposits placed with raw material suppliers.
Our accounts payable decreased by 34%
in fiscal 2009 to $1.7 million due mostly to a decrease in raw material
purchases in September 2009. Accrued and sundry liabilities increased
by 36% during the year to $3.7 million compared with $2.8 million last
year. The increase was caused by higher accruals for incentive
compensation and income taxes payable.
In the
first quarter of fiscal 2009, we repaid the remaining balance of $700,000 on our
revolving line of credit, which was outstanding at fiscal year-end
2008. As of October 3, 2009, we had no borrowings under the revolving
line of credit. The maximum principal amount we can borrow at any one
time under the agreement is $10 million. The maturity date is June 5,
2012. The agreement is unsecured and accrues interest at a variable
rate equal to 30-day LIBOR plus a margin ranging from 85 to 165 basis points
depending on our leverage ratio (as defined in the agreement). The
margin in effect during fiscal 2009 was 85 basis points. The interest
rate, including the margin, at November 20, 2008, the last date at which
interest expense was incurred, was 3.70%. Interest-only payments are
required monthly. There are no unused line fees associated with the
revolver. The agreement includes financial covenants relating to
tangible net worth and leverage ratios. The agreement restricts
dividends, stock repurchases, mergers and acquisitions, asset sales,
indebtedness, liens, and capital expenditures (see below). Violation
of loan covenants could result in acceleration of the term of the
agreement. We have pledged to grant the bank a security interest in
our accounts, instruments, and chattel paper upon its request in the event of a
default as defined in the agreement. We believe that we were in
compliance with the loan covenants as of October 3, 2009.
The
credit facility restricts dividends and stock repurchases during any fiscal year
to an aggregate amount of no more than 50% of the sum of (i) our income from
continuing operations for that fiscal year plus (ii) the absolute value of any
aggregate after-tax, non-cash and extraordinary losses for that fiscal
year. As an exception to the restriction above, we may pay a regular
quarterly dividend in an amount no greater than the previous quarter’s regular
dividend so long as we remain in compliance with the financial covenants after
giving effect to the payment of the dividend.
We believe that funds on hand, funds
generated from operations and funds available under our revolving credit
facility are adequate to finance our operations and expected capital
requirements during fiscal 2010 and for the foreseeable future.
OFF-BALANCE-SHEET
ARRANGEMENTS
We have no off-balance-sheet
arrangements.
CONTRACTUAL
OBLIGATIONS
The following table summarizes our
significant contractual obligations and commercial commitments at October 3,
2009 and the future periods in which such obligations are expected to be settled
in cash. For additional information regarding these obligations, see
the referenced footnotes in the Notes to Financial Statements under Item 8
below.
29
| Payments Due by Period | ||||||||||||||||||||
|
Contractual
Obligations
|
Less
Than
|
More
Than
|
||||||||||||||||||
|
(dollars
in thousands)
|
Total
|
1
Year
|
1-3
Years
|
3-5
Years
|
5
Years
|
|||||||||||||||
|
Operating
lease obligations - Note 19
|
$ | 37 | $ | 11 | $ | 16 | $ | 10 | ||||||||||||
|
Purchase
obligations - Note 20
|
625 | 625 | ||||||||||||||||||
|
Deferred
compensation - Note 10
|
1,278 | 114 | 227 | 227 | $ | 710 | ||||||||||||||
|
Total
contractual obligations
|
$ | 1,940 | $ | 750 | $ | 243 | $ | 237 | $ | 710 | ||||||||||
IMPACT
OF INFLATION AND COST OF RAW MATERIALS
Based on current conditions in the
markets for our primary raw materials, we expect inflation to be a moderate
factor for our operations in fiscal 2010. We experienced increases in
the cost of polyurethane foam, our primary raw material, during the first
quarter of 2009, followed by cost decreases on selected raw materials later in
fiscal 2009. We have been able to offset previous raw material cost
increases through a combination of sales price increases, efficiency
improvements and other expense reduction efforts. However, we can
give no assurance that we will be able to offset future cost increases, which
could negatively affect our profitability.
The cost of polyurethane foam is
indirectly influenced by oil prices. However, other market factors
also affect foam prices, including supply availability of component chemicals,
demand for related products from domestic and international manufacturers,
competition among domestic suppliers, our purchase volumes and regulatory
requirements. Consequently, it is difficult for us to accurately
predict the impact that inflation might have on our operations.
CRITICAL
ACCOUNTING POLICIES
This discussion and analysis of
financial condition and results of operations is based on our financial
statements that we prepare in conformity with accounting principles generally
accepted in the United States of America. The preparation of our
financial statements requires us to make estimates and assumptions that affect
the reported amounts of assets, liabilities, revenues and
expenses. These estimates and assumptions are based on historical
experience and on various other factors that we believe are reasonable under the
circumstances, the results of which form the basis for making judgments about
the carrying values of assets and liabilities that are not readily apparent from
other sources. These estimates and assumptions also require the
application of certain accounting policies, many of which require us to make
forecasts about future events and their estimated impact on amounts reported in
our financial statements and related notes. We periodically review
our accounting policies and estimates and make adjustments when facts and
circumstances dictate.
Actual results may differ from these
estimates under different assumptions or conditions. Any differences
may have a material impact on our financial condition and results of
operations.
30
In addition to the accounting policies
which are more fully described in the Notes to Financial Statements included in
this report, we have identified the following critical accounting policies used
in the preparation of our financial statements.
Allowance
for Doubtful Accounts
Credit evaluations are undertaken to
set credit limits for all customers. We regularly evaluate past due
accounts included in our accounts receivable and provide what we estimate to be
adequate reserves for doubtful accounts. Customer financial
conditions may change and increase the risk of non-collectibility and may
require additional provisions, which would negatively impact our operating
results. As of October 3, 2009, our provision for doubtful accounts
represented approximately 2.4% of total accounts receivable, or
$155,000. This compares with $235,000, or 2.9% of total accounts
receivable at fiscal year-end 2008.
Inventories
We regularly review inventory
quantities on hand and adjust for excess and obsolete inventory based primarily
on historical usage rates and our estimates of future product demand and
production. Actual demand may differ from our estimate, in which case
we may have understated or overstated the provision required for obsolete and
excess inventory, which would have an impact on our operating
results. As of October 3, 2009, our provision for excess and obsolete
inventory represented approximately 8.0% of total inventories, or
$340,000. This compares with $221,000, or 5.2% of total inventories
at fiscal year-end 2008.
Warranty
Obligations
We warrant certain of our products for
specific periods of time against manufacturing or performance
defects. We provide for the estimated future cost of warranty
obligations in cost of goods sold when the related revenue is
recognized. The accrued warranty cost represents our best estimate at
the time of sale of the total cost that we will incur to repair or replace
covered products or parts. The amount of accrued estimated warranty
cost is primarily based on historical experience as well as current information
on repair costs. Actual warranty cost could differ from the estimated
amounts. On a quarterly basis, we review the accrued balances and
update the historical warranty cost trends. If we were required to
accrue additional warranty cost in the future, it would negatively affect
operating results. Our actual warranty expense was approximately
$392,000 in fiscal 2009, $438,000 in fiscal 2008, and $222,000 in fiscal
2007. Our accrued warranty costs at October 3, 2009, were
$462,000. See Note 8 in the Notes to Financial Statements for more
information on product warranties.
Impairment
of Goodwill
We evaluate goodwill in our medical
business unit for impairment at least annually or more frequently if events
occur or circumstances change that could reduce the fair value of our medical
business unit. For fiscal year-end 2009, we determined that the fair
value of the medical business unit exceeded its carrying value and thus no
impairment charge was required. In assessing the value of goodwill,
we must make assumptions regarding estimated future cash flows and other factors
to determine the fair value of the medical business unit. If these
estimates or their related assumptions change in the future, we may be required
to record impairment charges, which would negatively impact operating
results. As of October 3, 2009, the carrying value of goodwill was
$1.9 million.
31
Impairment
of Long-Lived Assets
“Impairment” is the condition that
exists when the carrying amount of a long-lived asset or asset group is greater
than its fair value. We evaluate long-lived assets for potential
impairment whenever events occur or circumstances indicate that the carrying
amount of the assets may not be recoverable. The carrying amount of a
long-lived asset is not recoverable if it exceeds the sum of the undiscounted
cash flows expected to result from the use and eventual disposition of the
asset. If the carrying amount of a long-lived asset is not
recoverable and is greater than its fair value, the asset is impaired and an
impairment loss must be recognized. Accordingly, we recorded an
impairment charge of $2.9 million in September of 2007, which eliminated 100% of
the book value of the safety catheter assets. We have attempted to
sell the assets related to the safety catheter business, but our efforts so far
have been unsuccessful. Proceeds from a future sale of these assets,
if any, will be recorded as a gain on the disposition of assets from
discontinued operations. (See Notes 12 and 18 in the Notes to
Financial Statements.)
Present
Value of Deferred Compensation
We are obligated under the terms of a
retirement agreement to make fixed payments for the remaining lives of
Span-America’s founder and his ex-wife as discussed in Note 10 “Deferred
Compensation” in the Notes to Financial Statements. This obligation
can be funded from internally generated cash or from the cash value of
Company-owned life insurance policies, which had a value of $1.8 million at
October 3, 2009. See Item 7A below and Notes 6 and 10 in the Notes to
Financial Statements for more information on deferred compensation and the cash
value of life insurance. We have fully accrued the present value of
the expected payments due over the combined estimated life expectancy of our
founder and his ex-wife. In calculating this present value we used a
discount rate of 8%, which is an estimate of the effective long-term rate of
return on the portfolio. If the actual rate of return was
significantly lower than our estimate and we were required to accrue additional
deferred compensation costs in the future, it would negatively affect operating
results. As of October 3, 2009, we had recorded a deferred
compensation liability of approximately $822,000, including current and
long-term portions. If we reduced the discount rate by 1%, the
deferred compensation liability would be increased by approximately $42,000 and
pre-tax income would be reduced by the same amount.
Item 7A. Quantitative and
Qualitative Disclosures About Market Risk
We are exposed to financial market risk
in three areas: our short-term investments, cash value of life insurance, and
our credit facility. As of October 3, 2009, we had short-term
investments of $3.7 million which were classified as available for
sale. These short-term investments are high quality, highly liquid
corporate commercial paper and bonds known as “variable rate demand notes” or
“low floaters.” The bonds are issued by municipalities or companies
and are backed by letters of credit from federally insured banks. The
bonds carry the credit rating of the underlying bank and are therefore highly
rated. The interest rate on the bonds is a floating rate which is
reset weekly or monthly, depending on the issue, by the re-marketing agent based
on market rates for comparable securities or through an auction
process. We can liquidate the bonds at any time with a settlement
date of seven to 35 days after the trade date. Using the level of
securities available for sale at fiscal year-end 2009, a 100 basis point
increase or decrease in interest rates for one year would increase or decrease
pre-tax earnings by approximately $37,000. The effect of a 100 basis
point increase or decrease in interest rates will vary from period to period
with the dollar amount invested in our low floater portfolio.
32
We are exposed to financial market risk
because our other assets at October 3, 2009 included $1.8 million in cash value
of life insurance, which is subject to market risk related to equity pricing and
interest rate changes. The cash value is generated from life
insurance policies that are being used as the funding vehicle for a retirement
program for Span-America’s founder and former chairman. See “Present
Value of Deferred Compensation” above. The cash value is invested in
a combination of fixed income life insurance contracts and a portfolio of mutual
funds managed by an insurance company. The fixed income contracts are
similar to fixed income bond funds and are therefore subject to interest rate
and company risk. The mutual fund portfolios invest in common stocks
and bonds in accordance with their individual investment
objectives. These portfolios are exposed to stock market and interest
rate risk similar to comparable mutual funds. We believe that
substantial fluctuations in equity markets and interest rates and the resulting
changes in cash value of life insurance would not have a material adverse effect
on our financial position. During the fiscal year ended October 3,
2009, cash value of life insurance decreased by 2%, creating after-tax expense
of approximately $27,000.
Our credit facility accrues interest at
a variable rate equal to 30-day LIBOR plus a margin ranging from 85 to 165 basis
points depending on our then-applicable leverage ratio (as defined in the credit
facility). The current margin is 85 basis points. Interest
is payable monthly. There is no unused commitment fee associated with
the line of credit. An increase in interest rates would have a
negative impact on our financial condition and earnings to the extent that we
had outstanding borrowings under the facility. The degree of impact
would vary depending on the level of the borrowings. We repaid the
outstanding balance of our long-term debt during the first quarter of fiscal
year 2009. Unless we borrow under the facility or otherwise incur
significant debt with a variable interest rate, a change in interest rates would
have no material effect on our interest expense.
33
Item 8. Financial
Statements and Supplementary Data
Span-America
Medical Systems, Inc.
Financial
Statements
October
3, 2009
Contents
|
35
|
|
|
Financial
Statements
|
|
|
Statements
of Income
|
36
|
|
Balance
Sheets
|
37
|
|
Statements
of Changes in Shareholders’ Equity and Comprehensive
Income
|
38
|
|
39
|
|
|
Notes
to Financial Statements
|
40
|
34
Report
of Independent Registered Public Accounting Firm
Shareholders
and Board of Directors
Span-America
Medical Systems, Inc.
Greenville,
South Carolina
We have
audited the accompanying balance sheets of Span-America Medical Systems, Inc. as
of October 3, 2009 and September 27, 2008 and the related statements of income,
changes in shareholders’ equity and comprehensive income, and cash flows for
each of the years in the three year period ended October 3,
2009. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on
these financial statements based on our audits.
We
conducted our audits in accordance with the Standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audits to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis
for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Span-America Medical Systems, Inc.
as of October 3, 2009 and September 27, 2008 and the results of its operations
and its cash flows for each of the years in the three year period ended October
3, 2009 in conformity with accounting principles generally accepted in the
United States of America.
We were
not engaged to examine management’s assertion about the effectiveness of
Span-America Medical Systems, Inc.’s internal control over financial reporting
as of October 3, 2009 and, accordingly, we do not express an opinion
thereon.
/s/ ELLIOTT DAVIS,
LLC
Greenville,
South Carolina
December
22, 2009
35
Statements
of Income
|
Years Ended
|
||||||||||||
|
October
3,
|
September
27,
|
September
29,
|
||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
(Restated)
|
||||||||||||
|
Net
sales
|
$ | 55,867,104 | $ | 59,265,265 | $ | 60,543,829 | ||||||
|
Cost
of goods sold
|
35,658,795 | 38,869,911 | 39,592,675 | |||||||||
|
Gross
profit
|
20,208,309 | 20,395,354 | 20,951,154 | |||||||||
|
Selling
and marketing expenses
|
9,038,123 | 8,988,657 | 8,893,608 | |||||||||
|
Research
and development expenses
|
866,179 | 657,369 | 724,331 | |||||||||
|
General
and administrative expenses
|
3,436,114 | 3,230,854 | 3,205,273 | |||||||||
| 13,340,416 | 12,876,880 | 12,823,212 | ||||||||||
|
Operating
income
|
6,867,893 | 7,518,474 | 8,127,942 | |||||||||
|
Non-operating
income and expense:
|
||||||||||||
|
Investment
income and other
|
28,934 | 50,755 | 276,852 | |||||||||
|
Interest
expense
|
4,174 | 108,465 | 103,152 | |||||||||
|
Net
non-operating income (expense)
|
24,760 | (57,710 | ) | 173,700 | ||||||||
|
Income
from continuing operations before income taxes
|
6,892,653 | 7,460,764 | 8,301,642 | |||||||||
|
Income
taxes on continuing operations - Note 13
|
2,188,000 | 2,542,000 | 2,797,000 | |||||||||
|
Income
from continuing operations
|
4,704,653 | 4,918,764 | 5,504,642 | |||||||||
|
(Loss)
from discontinued operations, net of income tax benefit of $11,000 (2009),
$25,000 (2008) and $1,337,000 (2007) - Note 12
|
(20,622 | ) | (49,915 | ) | (2,630,703 | ) | ||||||
|
Net
income
|
$ | 4,684,031 | $ | 4,868,849 | $ | 2,873,939 | ||||||
|
Earnings
per share of common stock - Note 14
|
||||||||||||
|
Income
from continuing operations:
|
||||||||||||
|
Basic
|
$ | 1.72 | $ | 1.77 | $ | 2.02 | ||||||
|
Diluted
|
$ | 1.68 | $ | 1.71 | $ | 1.92 | ||||||
|
(Loss)
from discontinued operations:
|
||||||||||||
|
Basic
|
$ | (0.01 | ) | $ | (0.02 | ) | $ | (0.97 | ) | |||
|
Diluted
|
n/a | n/a | n/a | |||||||||
|
Net
income:
|
||||||||||||
|
Basic
|
$ | 1.72 | $ | 1.76 | $ | 1.06 | ||||||
|
Diluted
|
$ | 1.67 | $ | 1.70 | $ | 1.00 | ||||||
|
Dividends
per share of common stock
|
$ | 0.36 | $ | 0.34 | $ | 5.30 | ||||||
|
Weighted
average shares outstanding:
|
||||||||||||
|
Basic
|
2,730,426 | 2,771,754 | 2,723,942 | |||||||||
|
Diluted
|
2,803,625 | 2,868,494 | 2,864,820 | |||||||||
The accompanying notes are an integral
part of these financial statements.
36
Balance Sheets
|
October
3,
|
September
27,
|
|||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets:
|
||||||||
|
Cash
and cash equivalents
|
$ | 1,263,944 | $ | 833,714 | ||||
|
Securities
available for sale - Note 2
|
3,703,839 | |||||||
|
Accounts
receivable, net of allowances of $155,000 (2009) and $235,000
(2008)
|
6,305,430 | 7,771,366 | ||||||
|
Inventories
- Note 3
|
3,909,318 | 3,990,999 | ||||||
|
Deferred
income taxes - Note 13
|
997,000 | 683,000 | ||||||
|
Prepaid
expenses
|
101,835 | 51,964 | ||||||
|
Total
current assets
|
16,281,366 | 13,331,043 | ||||||
|
Property
and equipment, net - Note 4
|
6,158,977 | 6,569,091 | ||||||
|
Goodwill
- Note 5
|
1,924,131 | 1,924,131 | ||||||
|
Other
assets - Note 6
|
2,470,077 | 2,288,589 | ||||||
| $ | 26,834,551 | $ | 24,112,854 | |||||
|
LIABILITIES
AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current
liabilities:
|
||||||||
|
Accounts
payable
|
$ | 1,679,822 | $ | 2,528,934 | ||||
|
Accrued
and sundry liabilities - Note 7
|
3,743,968 | 2,753,771 | ||||||
|
Total
current liabilities
|
5,423,790 | 5,282,705 | ||||||
|
Long-term
debt - Note 9
|
700,000 | |||||||
|
Deferred
income taxes - Note 13
|
129,000 | 45,000 | ||||||
|
Deferred
compensation - Note 10
|
708,421 | 752,684 | ||||||
|
Total
long-term liabilities
|
837,421 | 1,497,684 | ||||||
|
Total
liabilities
|
6,261,211 | 6,780,389 | ||||||
|
Commitments
and contingencies - Notes 19 and 20
|
||||||||
|
Shareholders'
equity - Notes 11 and 14
|
||||||||
|
Common
stock, no par value, 20,000,000 shares authorized; issued and outstanding
shares 2,712,310 (2009) and 2,759,077 (2008)
|
792,466 | 1,308,847 | ||||||
|
Additional
paid-in capital
|
619,460 | 563,304 | ||||||
|
Retained
earnings
|
19,161,414 | 15,460,314 | ||||||
|
Total
shareholders' equity
|
20,573,340 | 17,332,465 | ||||||
| $ | 26,834,551 | $ | 24,112,854 | |||||
The
accompanying notes are an integral part of these financial
statements.
37
Statements
of Changes in Shareholders' Equity and Comprehensive Income
|
Accumulated
|
||||||||||||||||||||||||
|
Additional
|
Other
|
|||||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Retained
|
Comprehensive
|
|||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Earnings
|
Loss
|
Total
|
|||||||||||||||||||
|
Balance
at September 30, 2006
|
2,660,345 | $ | 1,032,118 | $ | 136,614 | $ | 23,352,221 | $ | (3,495 | ) | $ | 24,517,458 | ||||||||||||
|
Net
income for the 2007 fiscal year
|
2,873,939 | 2,873,939 | ||||||||||||||||||||||
|
Reclassification
adjustment from sale of securities
|
3,495 | 3,495 | ||||||||||||||||||||||
|
Comprehensive
income
|
2,877,434 | |||||||||||||||||||||||
|
Common
stock issued to Directors
|
8,500 | 132,090 | 132,090 | |||||||||||||||||||||
|
Common
stock issued on exercise of stock options
|
109,109 | 595,917 | 595,917 | |||||||||||||||||||||
|
Tax
benefits for stock options exercised
|
314,371 | 314,371 | ||||||||||||||||||||||
|
Stock
repurchase
|
(2,510 | ) | (35,900 | ) | (35,900 | ) | ||||||||||||||||||
|
Stock
option compensation expense
|
77,960 | 77,960 | ||||||||||||||||||||||
|
Cash
dividends paid or declared ($5.30 per share)
|
(14,691,663 | ) | (14,691,663 | ) | ||||||||||||||||||||
|
Balance
at September 29, 2007
|
2,775,444 | 1,724,225 | 528,945 | 11,534,497 | - | 13,787,667 | ||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Net
income for the 2008 fiscal year
|
4,868,849 | 4,868,849 | ||||||||||||||||||||||
|
Common
stock issued to Directors
|
8,500 | 111,265 | 111,265 | |||||||||||||||||||||
|
Common
stock issued on exercise of stock options
|
39,394 | 220,901 | 220,901 | |||||||||||||||||||||
|
Stock
repurchase
|
(64,261 | ) | (747,544 | ) | (747,544 | ) | ||||||||||||||||||
|
Stock
option compensation expense
|
34,359 | 34,359 | ||||||||||||||||||||||
|
Cash
dividends paid or declared ($0.34 per share)
|
(943,032 | ) | (943,032 | ) | ||||||||||||||||||||
|
Balance
at September 27, 2008
|
2,759,077 | 1,308,847 | 563,304 | 15,460,314 | - | 17,332,465 | ||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Net
income for the 2009 fiscal year
|
4,684,031 | 4,684,031 | ||||||||||||||||||||||
|
Common
stock issued to Directors
|
8,500 | 73,907 | 73,907 | |||||||||||||||||||||
|
Common
stock issued on exercise of stock options
|
1,265 | 13,491 | 13,491 | |||||||||||||||||||||
|
Stock
repurchase
|
(56,532 | ) | (603,779 | ) | (603,779 | ) | ||||||||||||||||||
|
Stock
option compensation expense
|
56,156 | 56,156 | ||||||||||||||||||||||
|
Cash
dividends paid or declared ($0.36 per share)
|
(982,931 | ) | (982,931 | ) | ||||||||||||||||||||
|
Balance
at October 3, 2009
|
2,712,310 | $ | 792,466 | $ | 619,460 | $ | 19,161,414 | $ | - | $ | 20,573,340 | |||||||||||||
The
accompanying notes are an integral part of these financial
statements.
38
Statements
of Cash Flows
|
Years Ended
|
||||||||||||
|
October
3,
|
September
27,
|
September
29,
|
||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
OPERATING
ACTIVITIES:
|
||||||||||||
|
Net
income
|
$ | 4,684,031 | $ | 4,868,849 | $ | 2,873,939 | ||||||
|
Adjustments
to reconcile net income to net cash provided by operating
activities:
|
||||||||||||
|
Impairment
charge
|
2,878,803 | |||||||||||
|
Depreciation
|
764,194 | 656,075 | 891,872 | |||||||||
|
Amortization
|
85,801 | 73,282 | 137,841 | |||||||||
|
Provision
for losses on accounts receivable
|
(54,202 | ) | 75,500 | 99,841 | ||||||||
|
Provision
for deferred income taxes
|
(230,000 | ) | 321,000 | (962,629 | ) | |||||||
|
Realized
loss on securities available for sale
|
3,595 | |||||||||||
|
Gain
on sale and disposal of property and equipment
|
(11,367 | ) | (13,781 | ) | (8,644 | ) | ||||||
|
Decrease
(Increase) in cash value of life insurance
|
17,395 | 82,987 | (138,199 | ) | ||||||||
|
Deferred
compensation
|
(44,263 | ) | (40,983 | ) | (37,947 | ) | ||||||
|
Stock
compensation expense
|
56,156 | 34,359 | 77,960 | |||||||||
|
Changes
in operating assets and liabilities:
|
||||||||||||
|
Accounts
receivable
|
1,516,299 | (782,428 | ) | (267,767 | ) | |||||||
|
Inventories
|
81,681 | 6,586 | (435,896 | ) | ||||||||
|
Prepaid
expenses and other assets
|
(208,265 | ) | 197,243 | 345,596 | ||||||||
|
Accounts
payable and accrued and sundry liabilities
|
148,339 | (228,205 | ) | 835,530 | ||||||||
|
Net
cash provided by operating activities
|
6,805,799 | 5,250,484 | 6,293,895 | |||||||||
|
INVESTING
ACTIVITIES:
|
||||||||||||
|
Purchases
of securities available for sale
|
(3,700,000 | ) | (5,095,000 | ) | ||||||||
|
Proceeds
from sales of marketable securities
|
10,187,175 | |||||||||||
|
Purchases
of property and equipment
|
(355,144 | ) | (692,043 | ) | (1,009,175 | ) | ||||||
|
Proceeds
from sale of property and equipment
|
12,431 | 17,500 | 9,137 | |||||||||
|
Payments
for other assets
|
(52,383 | ) | (73,602 | ) | (86,685 | ) | ||||||
|
Net
cash (used for) provided by investing activities
|
(4,095,096 | ) | (748,145 | ) | 4,005,452 | |||||||
|
FINANCING
ACTIVITIES:
|
||||||||||||
|
Dividends
paid
|
(982,931 | ) | (943,032 | ) | (14,691,663 | ) | ||||||
|
Proceeds
of long-term debt
|
5,700,000 | |||||||||||
|
Repayment
of long-term debt
|
(700,000 | ) | (3,000,000 | ) | (2,000,000 | ) | ||||||
|
Purchase
and retirement of common stock
|
(603,779 | ) | (747,544 | ) | (35,900 | ) | ||||||
|
Proceeds
from exercise of options for common stock
|
6,237 | 213,087 | 561,555 | |||||||||
|
Net
cash used for financing activities
|
(2,280,473 | ) | (4,477,489 | ) | (10,466,008 | ) | ||||||
|
Increase
(Decrease) in cash and cash equivalents
|
430,230 | 24,850 | (166,661 | ) | ||||||||
|
Cash
and cash equivalents at beginning of year
|
833,714 | 808,864 | 975,525 | |||||||||
|
Cash
and cash equivalents at end of year
|
$ | 1,263,944 | $ | 833,714 | $ | 808,864 | ||||||
The
accompanying notes are an integral part of these financial
statements.
39
NOTES
TO FINANCIAL STATEMENTS
October
3, 2009
1. SIGNIFICANT
ACCOUNTING POLICIES
Description
of Business
We
manufacture and distribute replacement mattresses, mattress overlays, patient
positioners, seating cushions, and skin care products for the medical market and
pillows, mattress pads and various foam products for the custom products market
throughout the United States and Canada.
Cash
and Cash Equivalents
We
consider all cash equivalents to be highly liquid investments with a maturity
when purchased of three months or less. Depending on market
conditions, we may maintain a centralized cash management program whereby our
excess cash balances are invested in commercial paper and are considered cash
equivalents. Cash balances in our accounts usually exceed federally
insured limits.
Accounts
Receivable
We
provide credit in the normal course of business and perform ongoing credit
evaluations on certain of our customers, but generally we do not require
collateral to support these receivables. We also establish an
allowance for doubtful accounts based upon factors surrounding the credit risk
of specific customers, historical trends and other
information. Account balances are charged against the allowance after
all collection efforts have been exhausted and the potential for recovery is
considered remote.
Inventories
Our
inventories are valued at the lower of cost (first-in, first-out method) or
market.
Property
and Equipment
Property
and equipment is stated at cost. Maintenance, repairs, and minor
replacements that do not improve or extend the useful lives of assets are
expensed when incurred. Depreciation is computed using the
straight-line method. Estimated useful lives for buildings and land
improvements range from 15 to 35 years. The estimated useful lives of
all other property and equipment range from 3 years to 15 years. For
income tax purposes, substantially all depreciation is computed using
accelerated methods.
Intangibles
Intangible
assets are amortized using the straight-line method. Costs of patents
are amortized over periods ranging from 10 to 17 years, and trademarks are
amortized over periods of 5 or 10 years. Goodwill, or costs in excess of the
fair value of net assets, was acquired from two separate
acquisitions. See Note 5. Accumulated amortization of
intangible assets at October 3, 2009 and September 27, 2008 was approximately
$2,539,000 and $2,463,000, respectively. We annually review the
recoverability of the carrying value of these assets. We also review
long-lived assets and the related intangible assets for impairment whenever
events or changes in circumstances indicate the carrying amount of such assets
may not be recoverable. See Note 12 – Impairment of Safety Catheter
Assets.
40
Revenue
Recognition
We
recognize revenue when goods are shipped and title passes to the
customer. There are no customer acceptance provisions, and the right
to return exists only in cases of damaged product, non-compliance with customer
specifications or warranty claims.
We have
applied the accounting and disclosure requirements of Securities and Exchange
Commission Staff Accounting Bulletin (SAB) No. 104.
Advertising
Costs
Advertising
costs are expensed as incurred.
Shipping
and Handling Costs
Shipping
and handling costs that are not reimbursed by customers are charged to selling
and marketing expenses and were approximately $1,715,000 in 2009, $1,837,000 in
2008, and $1,801,000 in 2007.
Vendor
Rebates
We offer
rebates to certain of our distributors based on predetermined sales
targets. These rebates vary by the type of product sold and by
distributor and are based on a percentage of the applicable sales
target. The rebate expense is charged as a reduction of gross
sales. Rebate expense and the associated liability are calculated and
recorded as the rebate-related revenue is recognized.
Earnings
Per Share of Common Stock
Earnings
per share of common stock are computed based on the weighted average number of
shares outstanding during each period. See Note 14 – Earnings per
Share of Common Stock.
Stock-Based
Compensation
We
measure and recognize compensation expense for all stock-based payments at fair
value. Stock-based payments include stock option
grants. We grant options to purchase common stock to some of our
employees under various plans at prices equal to the market value of the stock
on the dates the options were granted.
The fair
value of each option grant is estimated on the date of grant using the
Black-Scholes option pricing model with the following weighted average
assumptions for grants made in 2009: risk-free interest rates between 1.99% and
2.72%; dividend yield of 3.2%; volatility factor of the expected market price of
our common stock of between 43.8% and 44.4%; and a weighted average expected
life of the option of 8.2 years. No options were granted during
fiscal years 2008 or 2007.
Fiscal
Year
Our
fiscal year ends on the Saturday nearest to September 30. Fiscal year
2009 was a 53-week year. Fiscal years 2008 and 2007 were 52-week
years. Fiscal year 2010 will be a 52-week year.
41
Income
Taxes
The
liability method is used in accounting for income taxes. Under this
method, deferred tax assets and liabilities are determined based on differences
between financial reporting and tax bases of assets and liabilities and are
measured using the enacted tax rates and laws that are projected to be in effect
when the differences are expected to reverse.
Use
of Estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires us to make estimates
and assumptions that affect the amounts reported in the financial statements and
accompanying notes and the disclosure of contingent assets and
liabilities. Although these estimates are based on our knowledge of
current events and actions planned for the future, the estimates may ultimately
differ from actual results.
Recently
Issued Accounting Standards
Accounting
standards that have been issued or proposed by FASB or other standards-setting
bodies that do not require adoption until a future date are not expected to have
a material impact on our financial statements upon adoption.
Reclassifications
Results
of operations for fiscal year 2007 were restated to show the safety catheter
segment as a discontinued operation. See Note 12 – Impairment of
Safety Catheter Assets.
2. FAIR
VALUE OF FINANCIAL INSTRUMENTS
In the
first quarter of fiscal 2009, the Company adopted the new accounting
pronouncement related to fair value measurements for financial assets and
liabilities measured on a recurring basis. The accounting pronouncement applies
to all financial assets and financial liabilities that are being measured and
reported on a fair value basis. There was no impact from the adoption
of this pronouncement on the financial statements. The new guidance
establishes a framework for measuring fair value and expands disclosure about
fair value measurements. That framework provides a fair value
hierarchy that prioritizes the inputs to valuation techniques used to measure
fair value. The hierarchy gives the highest priority to unadjusted
quoted prices in active markets for identical assets or liabilities (Level 1
measurements) and the lowest priority to unobservable inputs (Level 3
measurements).
The
following is a brief description of these three levels:
Level 1 –
Unadjusted quoted prices in active markets that are accessible at the
measurement date for identical, unrestricted assets or liabilities;
Level 2 –
Quoted prices in markets that are not active, or inputs which are observable,
either directly or indirectly, for substantially the full term of the asset or
liability;
Level 3 –
Prices or valuation techniques that require inputs that are both significant to
the fair value measurement and unobservable (i.e. supported by little or no
market activity).
42
The
asset’s or liability’s fair value measurement level within the fair value
hierarchy is based on the lowest level (with “3” being the lowest) of any input
that is significant to the fair value measurement. Valuation
techniques used need to maximize the use of observable inputs and minimize the
use of unobservable inputs.
Following
is a description of the valuation methodologies used for assets measured at fair
value.
Cash
value of life insurance policy: Valued at the cash surrender value of
the life insurance policy, as determined by the issuer of the insurance policy,
which approximates fair value.
Marketable
equity securities: Valued at the closing prices reported on active
markets on which the individual securities are traded.
The
methods described above may produce a fair value calculation that may not be
indicative of net realizable value or reflective of future fair
values. Furthermore, although the Company believes its valuation
methods are appropriate and consistent with methods used by other market
participants, the use of different methodologies or assumptions to determine
fair value of certain financial instruments could result in a different fair
value measurement at the reporting date.
The
following table summarizes information on the fair value measurement of the
Company’s assets as of October 3, 2009 grouped by the categories described
above:
|
Total
|
Quoted
prices in
active
markets
(Level 1)
|
Significant
other
observable
inputs
(Level 2)
|
Significant
unobservable
inputs
(Level 3)
|
|||||||||||||
|
Cash
value of life insurance policy
|
$ | 1,820,474 | $ | 1,820,474 | ||||||||||||
|
Securities
available for sale
|
$ | 3,703,839 | $ | 3,703,839 | ||||||||||||
Securities
available for sale at October 3, 2009 were variable rate demand
notes. We had no significant unrealized holding gains or losses
during fiscal years 2009, 2008 or 2007.
3. INVENTORIES
|
2009
|
2008
|
|||||||
|
Raw
materials
|
$ | 2,478,316 | $ | 2,825,817 | ||||
|
Finished
goods
|
1,431,002 | 1,165,182 | ||||||
| $ | 3,909,318 | $ | 3,990,999 | |||||
43
4. PROPERTY
AND EQUIPMENT
|
2009
|
2008
|
|||||||
|
Land
|
$ | 469,718 | $ | 469,718 | ||||
|
Land
improvements
|
486,698 | 486,698 | ||||||
|
Buildings
|
6,793,205 | 6,783,714 | ||||||
|
Machinery
and equipment
|
6,909,820 | 6,613,918 | ||||||
|
Furniture
and fixtures
|
487,775 | 487,775 | ||||||
|
Automobiles
|
9,520 | 9,520 | ||||||
| 15,156,736 | 14,851,343 | |||||||
|
Less
accumulated depreciation
|
8,997,759 | 8,282,252 | ||||||
| $ | 6,158,977 | $ | 6,569,091 | |||||
5. GOODWILL
AND OTHER INTANGIBLES
As of
October 3, 2009 and September 27, 2008, we had goodwill of
$1,924,131. In addition, we had patents and trademarks (net of
accumulated amortization) of $293,018 as of October 3, 2009 and $316,435 as of
September 27, 2008. The goodwill is associated with the medical
segment, and the patents and trademarks are associated with the medical and
custom products segments. Goodwill has an indefinite useful
life. The useful lives of individual patents and trademarks have been
reviewed, and no material changes were required.
Amortization
expense for patents and trademarks from continuing operations during fiscal
years 2009, 2008, and 2007 was $75,801, $63,282 and $59,165,
respectively. Estimated amortization expense for the next five fiscal
years based on existing patents and trademarks is as follows:
|
Estimated
|
||||
|
Amortization
|
||||
|
Fiscal years
|
Expense
|
|||
|
2010
|
$ | 54,217 | ||
|
2011
|
35,811 | |||
|
2012
|
31,612 | |||
|
2013
|
28,536 | |||
|
2014
|
25,671 | |||
6. OTHER
ASSETS
|
2009
|
2008
|
|||||||
|
Patents
and trademarks, net of accumulated amortization of $1,510,892
(2009) and $1,435,091 (2008)
|
$ | 293,018 | $ | 316,435 | ||||
|
Cash
value of life insurance policies - Note 2
|
1,820,474 | 1,837,869 | ||||||
|
Other
|
356,585 | 134,285 | ||||||
| $ | 2,470,077 | $ | 2,288,589 | |||||
44
7. ACCRUED
AND SUNDRY LIABILITIES
|
|
2009
|
2008
|
||||||
|
Salaries
and other compensation
|
$ | 1,910,035 | $ | 1,271,859 | ||||
|
Federal
and state income taxes and sales taxes
|
455,134 | 101,359 | ||||||
|
Payroll
taxes accrued and withheld
|
143,392 | 129,992 | ||||||
|
Property
taxes
|
169,420 | 181,800 | ||||||
|
Medical
insurance
|
249,689 | 276,071 | ||||||
|
Warranty
reserve
|
461,721 | 365,721 | ||||||
|
Vendor
rebates
|
339,111 | 410,990 | ||||||
|
Other
|
15,466 | 15,979 | ||||||
| $ | 3,743,968 | $ | 2,753,771 | |||||
8. PRODUCT
WARRANTIES
We offer
warranties of various lengths to our customers, depending on the specific
product sold. The warranties require us to repair or replace
non-performing products during the warranty period at no cost to the
customer. At the time revenue is recognized for covered products, we
record a liability for estimated costs that may be incurred under our
warranties. The costs are estimated based on historical experience
and any specific warranty problems that have been
identified. Although historical warranty costs have been within our
expectations, there can be no assurance that future warranty costs will not
exceed historical amounts. We regularly evaluate the adequacy of the
warranty liability and adjust the balance as necessary.
Changes
in our product warranty liability for the years ended October 3, 2009 and
September 27, 2008 are as follows:
|
2009
|
2008
|
|||||||
|
Accrued
liability at beginning of year
|
$ | 365,721 | $ | 332,881 | ||||
|
Increases
in reserve
|
487,909 | 470,440 | ||||||
|
Expenses
|
(391,909 | ) | (437,600 | ) | ||||
|
Accrued
liability at end of year
|
$ | 461,721 | $ | 365,721 | ||||
9. BORROWINGS
Long-term
debt consists of a revolving credit facility from a bank. The maximum
principal amount that we can borrow at any one time under the agreement is $10
million. The maturity date is June 5, 2012. The agreement
is unsecured and accrues interest at a variable rate equal to 30-day LIBOR plus
a margin ranging from 85 to 165 basis points depending on our leverage ratio (as
defined in the agreement). The margin in effect for fiscal 2009 was
85 basis points. The interest rate at November 20, 2008, the last
date interest expense was incurred, was 3.7%. Interest-only payments
are required monthly. The agreement includes financial covenants
relating to tangible net worth and leverage ratios. The agreement
restricts dividends, stock repurchases, mergers and acquisitions, asset sales,
indebtedness, liens and capital expenditures. Violation of loan
covenants could result in acceleration of the term of the
agreement. We have pledged to grant the bank a security interest in
our accounts, instruments, and chattel paper upon its request in the event of a
default as defined in the agreement.
45
The
credit facility restricts dividends and stock repurchases during any fiscal year
to an aggregate amount of no more than 50% of the sum of (i) our income from
continuing operations for that fiscal year plus (ii) the absolute value of our
aggregate after-tax, non-cash and extraordinary losses for that fiscal year, if
any. As an exception to the restriction noted above, we may pay a
regular quarterly dividend in an amount no greater than the previous quarter’s
regular dividend so long as we remain in compliance with the financial covenants
after giving effect to the payment of the dividend.
We paid
interest expense of approximately $4,000 in 2009, $108,000 in 2008 and $103,000
in 2007.
10. DEFERRED
COMPENSATION
We are
obligated to make fixed payments of approximately $114,000 per year to our
founder and former chief executive officer pursuant to a retirement
agreement. The payments will be made for the longer of the
executive’s remaining life or his ex-wife's remaining life, if she survives
him. We have fully accrued the present value of the expected payments
due over the combined life expectancy of the executive and his
ex-wife. We recognized expenses of approximately $69,000 in 2009,
$73,000 in 2008, and $76,000 in 2007 related to this agreement. An 8%
discount rate was used in measuring the present value of our deferred
compensation obligation.
11. EQUITY
COMPENSATION
In
January 2007, the Board adopted the 2007 Equity Incentive Plan (“2007 Plan”),
which was approved by shareholders in February 2007. The 2007 Plan
authorized the Board to grant stock-based compensation awards to our officers,
directors and key employees for up to 250,000 shares of Company common
stock. Awards may be in the form of restricted stock, non-restricted
stock, restricted stock units, options or stock appreciation rights
(SARs). Total awards under the 2007 Plan may not exceed 250,000
shares, of which no more than 75,000 shares may be in the form of restricted
stock, non-restricted stock or restricted stock units. The per share
exercise prices of options or SARs granted under the 2007 Plan must be no less
than the fair market value of a share on the grant date. The terms
and conditions of each award may be set by the Board or a committee of the
Board. The 2007 Plan will expire on December 31, 2016 unless
terminated earlier in accordance with the plan.
In March
1997, the Board adopted the 1997 Stock Option Plan (“1997 Plan”). The
1997 Plan authorized the Board to grant options to our key officers and
employees for up to 200,000 shares of our common stock. Options
granted under the 1997 Plan are generally granted at the fair market value on
the date of grant. These options become exercisable and vest at the
greater of 1,000 shares per year or 20% of the grant. Options expire
10 years from the date of grant for continuing employees, or three months after
termination of employment for employees who leave the Company. The
1997 Plan was terminated on October 20, 2007. The termination does
not affect options outstanding under the plan, but no further options can be
granted under the 1997 Plan.
46
In
November 1991, the Board adopted the 1991 Stock Option Plan (“1991
Plan”). The 1991 Plan authorized the Board to grant options for up to
200,000 shares of Company common stock to our officers and key employees and
50,000 shares to directors who are neither officers nor employees of the
Company. All other terms and conditions of the 1991 Plan are similar
to the 1997 Plan. The 1991 Plan was terminated on November 7,
2001. The termination does not affect options outstanding under the
plan, but no further options can be granted under the 1991 Plan.
Shown
below is a summary of activity under the Company’s three stock option
plans.
|
Outstanding
|
Exercisable
|
|||||||||||||||||||
|
Weighted
|
Weighted
|
|||||||||||||||||||
|
Average
|
Average
|
|||||||||||||||||||
|
Shares
|
Number of
|
Ex. Price
|
Number of
|
Ex. Price
|
||||||||||||||||
|
Available
|
Shares
|
Per Share
|
Shares
|
Per Share
|
||||||||||||||||
|
Balance
at 9/30/06
|
23,200 | 340,352 | $ | 6.03 | 307,432 | $ | 5.66 | |||||||||||||
|
Fiscal Year 2007
|
||||||||||||||||||||
|
Granted
|
||||||||||||||||||||
|
Exercised
|
(108,089 | ) | 5.20 | |||||||||||||||||
|
Forfeited
|
2,750 | (2,750 | ) | 9.64 | ||||||||||||||||
|
New
option plan
|
250,000 | |||||||||||||||||||
|
Balance
at 9/29/07
|
275,950 | 229,513 | 6.39 | 218,860 | 6.23 | |||||||||||||||
|
Fiscal Year 2008
|
||||||||||||||||||||
|
Granted
|
||||||||||||||||||||
|
Exercised
|
(38,743 | ) | 5.50 | |||||||||||||||||
|
Forfeited
|
||||||||||||||||||||
|
1997
Plan expired
|
(25,950 | ) | ||||||||||||||||||
|
Balance
at 9/27/08
|
250,000 | 190,770 | 6.57 | 189,802 | 6.55 | |||||||||||||||
|
Fiscal Year 2009
|
||||||||||||||||||||
|
Granted
|
(22,500 | ) | 22,500 | 9.34 | ||||||||||||||||
|
Exercised
|
(645 | ) | 9.67 | |||||||||||||||||
|
Forfeited
|
(1,289 | ) | 9.88 | |||||||||||||||||
|
Balance
at 10/3/09
|
227,500 | 211,336 | $ | 6.83 | 200,336 | $ | 6.69 | |||||||||||||
47
Shown
below is a summary of stock options outstanding and exercisable at fiscal
year-end 2009.
|
Outstanding
|
Exercisable
|
|||||||||||||||||||
|
Weighted
|
||||||||||||||||||||
|
Weighted
|
Average
|
Weighted
|
||||||||||||||||||
|
Average
|
Remaining
|
Average
|
||||||||||||||||||
|
Ranges of Exercise
|
Number of
|
Ex. Price
|
Contract
|
Number of
|
Ex. Price
|
|||||||||||||||
|
Prices
|
Shares
|
Per Share
|
Life (yrs)
|
Shares
|
Per Share
|
|||||||||||||||
|
$
2.75 - $ 4.30
|
86,843 | $ | 3.87 | 1.4 | 86,843 | $ | 3.87 | |||||||||||||
|
6.18
- 9.18
|
51,324 | 7.56 | 4.3 | 51,324 | 7.56 | |||||||||||||||
|
9.34
- 10.52
|
73,169 | 9.84 | 6.8 | 62,169 | 9.93 | |||||||||||||||
|
$
2.75 - $10.52
|
211,336 | $ | 6.83 | 4.0 | 200,336 | $ | 6.69 | |||||||||||||
The Board
of Directors adopted a stock purchase incentive plan in February
2000. The 2000 Restricted Stock Plan was created to encourage our
management employees to purchase and hold Span-America common
stock. Plan benefits are paid in shares of Company common
stock. Benefits earned and accrued under the plan were $3,749 in
2009, $7,254 in 2008 and $7,814 in 2007. We issued stock valued at
$7,254 in 2009, leaving a vested balance of $3,749 at October 3,
2009.
12. IMPAIRMENT
OF SAFETY CATHETER ASSETS
In
October 2007, we decided to exit the safety catheter business and sell the
related assets because we had been unable to generate sufficient sales volume to
make it a viable business. As of September 29, 2007, we recorded an
impairment charge of approximately $2,879,000, which reduced the book value of
our safety catheter assets to zero. As a result of the degree of
uncertainty associated with any potential sale of these assets, we concluded
that we could not reasonably estimate a net realizable value for the
assets. This charge included an impairment charge for equipment of
approximately $1,712,000, impairment of patents and trademarks of approximately
$373,000 and reduction in net realizable value of inventories of approximately
$794,000. Accordingly, revenues and expenses related to the safety
catheter business in fiscal year 2009 and 2008 are shown as a discontinued
operation. Results of operations for the fiscal year 2007 were
restated to show the safety catheter segment as a discontinued
operation.
We have
ceased the use of the safety catheter assets and are committed to a plan of sale
or abandonment. We are still engaged in efforts to sell these assets
in order to maximize any value that might currently remain. However,
we have no offers pending and can give no assurance that the assets will
eventually be sold. If the assets are not eventually sold, they will
be abandoned and disposed of.
13. INCOME
TAXES
Deferred
income taxes reflect the net tax effects of temporary differences between the
carrying amounts of assets and liabilities for financial reporting purposes and
the amounts used for income tax purposes. Significant components of
our deferred tax liabilities and assets as of October 3, 2009 and September 27,
2008 are as follows:
48
|
2009
|
2008
|
|||||||
|
Deferred
tax liabilities:
|
||||||||
|
Depreciation
|
$ | (433,000 | ) | $ | (417,000 | ) | ||
|
Deferred
tax assets:
|
||||||||
|
Deferred
compensation
|
255,000 | 316,000 | ||||||
|
Accrued
expenses
|
632,000 | 271,000 | ||||||
|
Inventory
|
354,000 | 357,000 | ||||||
|
Amortization
|
49,000 | 101,000 | ||||||
|
Other
|
11,000 | 10,000 | ||||||
|
Total
deferred tax assets
|
1,301,000 | 1,055,000 | ||||||
|
Net
deferred tax assets
|
$ | 868,000 | $ | 638,000 | ||||
We made
cash income tax payments, net of refunds, of approximately $2,039,000,
$2,302,000, and $2,114,000, in fiscal years 2009, 2008 and 2007,
respectively.
Federal
and state income tax provisions consist of the following:
|
2009
|
2008
|
2007
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$ | 2,267,000 | $ | 2,048,000 | $ | 2,502,000 | ||||||
|
State
|
140,000 | 160,000 | 222,000 | |||||||||
| 2,407,000 | 2,208,000 | 2,724,000 | ||||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
(218,000 | ) | 288,000 | (1,165,000 | ) | |||||||
|
State
|
(12,000 | ) | 21,000 | (99,000 | ) | |||||||
| (230,000 | ) | 309,000 | (1,264,000 | ) | ||||||||
|
Income
tax expense
|
$ | 2,177,000 | $ | 2,517,000 | $ | 1,460,000 | ||||||
Income
tax expense differs from the amounts computed by applying the federal tax rate
to income before income taxes as follows:
|
2009
|
2008
|
2007
|
||||||||||
|
Computed
tax at the statutory rate
|
$ | 2,333,000 | $ | 2,511,000 | $ | 1,474,000 | ||||||
|
Increases
(decreases):
|
||||||||||||
|
State
income taxes, net of federal tax benefit
|
84,000 | 120,000 | 81,000 | |||||||||
|
Tax-exempt
investment income
|
(51,000 | ) | ||||||||||
|
Officer's
life insurance
|
9,000 | 31,000 | (44,000 | ) | ||||||||
|
Domestic
production deduction
|
(151,000 | ) | (133,000 | ) | (77,000 | ) | ||||||
|
Other,
net
|
(98,000 | ) | (12,000 | ) | 77,000 | |||||||
|
Income
tax expense
|
$ | 2,177,000 | $ | 2,517,000 | $ | 1,460,000 | ||||||
49
14. EARNINGS
PER SHARE OF COMMON STOCK
The
following table sets forth the computation of basic and diluted earnings per
share of common stock.
|
2009
|
2008
|
2007
|
||||||||||
|
Numerator
for basic and diluted earnings per share:
|
||||||||||||
|
Income
from continuing operations
|
$ | 4,704,653 | $ | 4,918,764 | $ | 5,504,642 | ||||||
|
(Loss)
from discontinued operations, net of income taxes
|
(20,622 | ) | (49,915 | ) | (2,630,703 | ) | ||||||
|
Net
income
|
$ | 4,684,031 | $ | 4,868,849 | $ | 2,873,939 | ||||||
|
Denominator:
|
||||||||||||
|
Denominator
for basic earnings per share:
|
||||||||||||
|
Weighted
average shares
|
2,730,426 | 2,771,754 | 2,723,942 | |||||||||
|
Effect
of dilutive securities:
|
||||||||||||
|
Employee
and board stock options
|
73,199 | 96,740 | 140,878 | |||||||||
|
Denominator
for diluted earnings per share:
|
||||||||||||
|
Adjusted
weighted average shares and assumed conversions
|
2,803,625 | 2,868,494 | 2,864,820 | |||||||||
|
Income
from continuing operations per share:
|
||||||||||||
|
Basic
|
$ | 1.72 | $ | 1.77 | $ | 2.02 | ||||||
|
Diluted
|
$ | 1.68 | $ | 1.71 | $ | 1.92 | ||||||
|
(Loss)
from discontinued operations per share:
|
||||||||||||
|
Basic
|
$ | (0.01 | ) | $ | (0.02 | ) | $ | (0.97 | ) | |||
|
Diluted
|
n/a | n/a | n/a | |||||||||
|
Net
income per share:
|
||||||||||||
|
Basic
|
$ | 1.72 | $ | 1.76 | $ | 1.06 | ||||||
|
Diluted
|
$ | 1.67 | $ | 1.70 | $ | 1.00 | ||||||
15. EMPLOYEE
BENEFITS AND INCENTIVE PLANS
We have a
401(k) plan available to employees meeting eligibility
requirements. We match a percentage of employee contributions, with
certain limitations. Our 401(k) matching contributions amounted to
approximately $172,000, $212,000 and $183,000, for the 2009, 2008 and 2007
fiscal years, respectively.
16. RELATED-PARTY
TRANSACTIONS
We had no
related-party transactions during any year in the three-year period ended
October 3, 2009.
50
17. MAJOR
CUSTOMERS
The
largest of our medical customers are distributors who sell our products to acute
care hospitals and other treatment facilities throughout the United
States. Sales generated by the largest of these distributors amounted
to approximately 12 % of net sales in 2009 and 11% of net sales in 2008 and
2007.
We have a
business relationship with another customer to distribute certain of our
consumer products. Sales to this customer amounted to 26% of net
sales in 2009, 20% of net sales in 2008 and 21% of net sales in
2007.
See Note
18 for further information about sales to major customers.
18. OPERATIONS
AND INDUSTRY SEGMENTS
For
management and reporting purposes, we divide our business into two
segments: medical and custom products. This industry
segment information corresponds to the markets in the United States and Canada
for which we manufacture and distribute our various products. See
also Note 12 – Impairment of Safety Catheter Assets.
The
following table summarizes certain information on industry
segments:
|
2009
|
2008
|
2007
|
||||||||||
|
Net
sales:
|
||||||||||||
|
Medical
|
$ | 37,813,756 | $ | 42,456,763 | $ | 43,248,331 | ||||||
|
Custom
products
|
18,053,348 | 16,808,502 | 17,295,498 | |||||||||
|
Total
|
$ | 55,867,104 | $ | 59,265,265 | $ | 60,543,829 | ||||||
|
Operating
profit (loss):
|
||||||||||||
|
Medical
|
$ | 5,586,679 | $ | 7,615,385 | $ | 9,129,693 | ||||||
|
Custom
products
|
2,129,488 | 800,849 | (266,994 | ) | ||||||||
|
Total
|
7,716,167 | 8,416,234 | 8,862,699 | |||||||||
|
Corporate
expense
|
(848,274 | ) | (897,760 | ) | (734,757 | ) | ||||||
|
Other
income (expense)
|
24,760 | (57,710 | ) | 173,700 | ||||||||
|
Income
from continuing operations before income taxes
|
$ | 6,892,653 | $ | 7,460,764 | $ | 8,301,642 | ||||||
51
|
2009
|
2008
|
2007
|
||||||||||
|
Identifiable
assets:
|
||||||||||||
|
Medical
|
$ | 14,153,908 | $ | 15,852,599 | $ | 15,869,258 | ||||||
|
Custom
products
|
5,887,425 | 5,583,170 | 5,234,675 | |||||||||
|
Corporate
|
6,793,218 | 2,677,085 | 2,734,125 | |||||||||
| $ | 26,834,551 | $ | 24,112,854 | $ | 23,838,058 | |||||||
|
Depreciation
and amortization expenses:
|
||||||||||||
|
Operating:
|
||||||||||||
|
Medical
|
$ | 564,184 | $ | 512,415 | $ | 444,751 | ||||||
|
Custom
products
|
285,271 | 216,440 | 232,522 | |||||||||
|
Corporate
|
540 | 502 | 429 | |||||||||
| 849,995 | 729,357 | 677,702 | ||||||||||
|
Other
- discontinued operations
|
352,011 | |||||||||||
| $ | 849,995 | $ | 729,357 | $ | 1,029,713 | |||||||
|
Capital
expenditures:
|
||||||||||||
|
Medical
|
$ | 240,771 | $ | 492,088 | $ | 884,655 | ||||||
|
Custom
products
|
114,373 | 199,955 | 124,520 | |||||||||
| $ | 355,144 | $ | 692,043 | $ | 1,009,175 | |||||||
Total
sales by industry segment include sales from unaffiliated customers as reported
in our statements of income. In calculating operating profit,
non-allocable general corporate expenses, interest expense, other income and
income taxes are not included, but certain corporate operating expenses incurred
for the benefit of all segments are included on an allocated basis.
Identifiable
assets are those assets that are used in the operations of each segment on an
allocated basis. Amounts shown for corporate assets consist primarily
of cash, marketable securities and cash surrender value of life
insurance.
We have
several customers whose sales represent significant portions of sales in their
respective business segments. In the medical segment, sales to the
top four distributors represented 45% of net medical sales in 2009, 52% in 2008
and 57% in 2007. In the custom products segment, sales to one
customer accounted for 79% of net custom products sales in 2009, 71% in 2008 and
72% in 2007.
Export
sales (net), primarily to Canada, were approximately $2,420,000 in 2009,
$3,287,000 in 2008 and $3,569,000 in 2007. We have no physical assets
in Canada or any other foreign country.
52
19. OPERATING
LEASES
We lease
truck equipment in South Carolina. In addition, we lease a 15,000
square foot distribution facility in Utah for $6,750 a month. The
Utah facility lease is cancellable by either party with 60 days
notice. Both leases require us to pay certain insurance and
maintenance costs.
Rental
expense for all operating leases was $102,000 in 2009, $104,000 in 2008 and
$107,000 in 2007.
20. COMMITMENTS
AND CONTINGENCIES
We are
committed to minimum purchases of $625,000 of Selan® skin care products per
calendar year through 2010. For the fiscal years ended 2009, 2008 and
2007, purchases under this commitment were $745,000, $748,000 and
$782,000.
From time
to time we are defendants in legal actions involving claims arising in the
normal course of business. We believe that, as a result of legal
defenses and insurance arrangements, none of these actions should have a
material adverse effect on our operations or financial condition.
21. QUARTERLY
FINANCIAL DATA (Unaudited)
Selected
quarterly financial data are shown in the following table.
Quarterly
Financial Data (Unaudited)
(Amounts
in thousands, except per share data)
|
First
|
Second
|
Third
|
Fourth
|
Year
|
||||||||||||||||
|
For
Fiscal 2009
|
||||||||||||||||||||
|
Net
sales
|
$ | 13,392 | $ | 13,376 | $ | 14,327 | $ | 14,771 | $ | 55,867 | ||||||||||
|
Gross
profit
|
4,690 | 4,774 | 5,004 | 5,740 | 20,208 | |||||||||||||||
|
Operating
income
|
1,346 | 1,598 | 1,748 | 2,176 | 6,868 | |||||||||||||||
|
Income
from continuing operations
|
888 | 1,056 | 1,156 | 1,605 | 4,705 | |||||||||||||||
|
Loss
from discontinued operations net of income
taxes
|
(1 | ) | - | (19 | ) | - | (21 | ) | ||||||||||||
|
Net
income
|
887 | 1,056 | 1,137 | 1,605 | 4,684 | |||||||||||||||
|
Earnings
per share
|
||||||||||||||||||||
|
Basic
|
0.32 | 0.39 | 0.42 | 0.59 | 1.72 | |||||||||||||||
|
Diluted
|
0.32 | 0.38 | 0.41 | 0.57 | 1.67 | |||||||||||||||
53
Quarterly
Financial Data (Unaudited)
(Amounts
in thousands, except per share data)
|
First
|
Second
|
Third
|
Fourth
|
Year
|
||||||||||||||||
|
For
Fiscal 2008
|
||||||||||||||||||||
|
Net
sales
|
$ | 13,664 | $ | 15,770 | $ | 14,852 | $ | 14,979 | $ | 59,265 | ||||||||||
|
Gross
profit
|
4,647 | 5,544 | 4,955 | 5,249 | 20,395 | |||||||||||||||
|
Operating
income
|
1,638 | 2,134 | 1,771 | 1,975 | 7,518 | |||||||||||||||
|
Income
from continuing operations
|
1,062 | 1,404 | 1,162 | 1,291 | 4,919 | |||||||||||||||
|
Loss
from discontinued operations net of income taxes
|
(17 | ) | (27 | ) | (4 | ) | (2 | ) | (50 | ) | ||||||||||
|
Net
income
|
1,045 | 1,377 | 1,158 | 1,289 | 4,869 | |||||||||||||||
|
Earnings
per share
|
||||||||||||||||||||
|
Basic
|
0.38 | 0.50 | 0.42 | 0.47 | 1.76 | |||||||||||||||
|
Diluted
|
0.36 | 0.48 | 0.41 | 0.45 | 1.70 | |||||||||||||||
Item 9. Changes in and
Disagreements With Accountants on Accounting and Financial
Disclosure
None
Item
9A(T). Controls and Procedures
Evaluation
of Disclosure Controls and Procedures
Our management has evaluated, with the
participation of our Chief Executive Officer and Chief Financial Officer, the
effectiveness of our disclosure controls and procedures as of the end of the
period covered by this annual report (the “Evaluation Date”), and, based on this
evaluation, our Chief Executive Officer and Chief Financial Officer have
concluded that these controls and procedures were effective at the Evaluation
Date. There were no changes in our internal controls over financial
reporting during the last quarter of fiscal 2009 that have materially affected
or are reasonably likely to materially affect our internal control over
financial reporting.
Disclosure controls and procedures
are controls and other procedures that are designed to ensure that
information required to be disclosed by us in the reports that we file or submit
under the Exchange Act is recorded, processed, summarized and reported, within
the time periods specified in the Securities and Exchange Commission’s rules and
forms. Disclosure controls and procedures include, without
limitation, controls and procedures designed to ensure that information required
to be disclosed by the Company in the reports that it files or submits under the
Exchange Act is accumulated and communicated to our management, including our
Chief Executive Officer and Chief Financial Officer, as appropriate to allow for
timely decisions regarding required disclosure.
54
Management’s
Report on Internal Control over Financial Reporting
The
management of Span-America Medical Systems, Inc. is responsible for establishing
and maintaining adequate internal control over financial reporting, as such term
is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Span-America’s
internal control system was designed to provide reasonable assurance regarding
the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted
accounting principles and includes those policies and procedures
that:
|
|
1.
|
pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of the assets of
Span-America;
|
|
|
2.
|
provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of Span-America
are being made only in accordance with authorizations of management and
directors of Span-America; and
|
|
|
3.
|
provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of Span-America’s assets that
could have a material effect on the financial
statements.
|
All
internal control systems, no matter how well designed, have inherent
limitations. Therefore, even those systems determined to be effective
can provide only reasonable assurance with respect to financial statement
preparation and presentation.
We have
assessed the effectiveness of our internal control over financial reporting as
of October 3, 2009. In making this assessment, we used the framework set
forth by the Committee of Sponsoring Organizations of the Treadway Commission
(COSO) in Internal Control-Integrated Framework. Based on that assessment,
we believe that as of October 3, 2009, our internal control over financial
reporting is effective.
This
annual report does not include an attestation report of our independent
registered public accounting firm regarding internal control over financial
reporting. Our report on internal control was not subject to
attestation by our independent registered public accounting firm pursuant to
temporary rules of the Securities and Exchange Commission that permit us to
provide only management’s report in this annual report.
Item 9B. Other
Information
None
PART
III
Item
10. Directors, Executive Officers and Corporate
Governance
Information required under Item 10 of
Part III is incorporated herein by reference to portions of the definitive Proxy
Statement filed or to be filed with the Securities and Exchange Commission on or
prior to 120 days following the end of our 2009 fiscal year under the headings
“Proposals to be Voted Upon – Election of Directors,” “Corporate Governance,”
“Executive Officers” and “Section 16(a) Beneficial Ownership Reporting
Compliance.”
55
Item
11. Executive Compensation
Information required under Item 11 of
Part III is incorporated herein by reference to portions of the definitive Proxy
Statement filed or to be filed with the Securities and Exchange Commission on or
prior to 120 days following the end of our 2009 fiscal year under the headings
“Compensation of Executive Officers,” “Corporate Governance – Director
Compensation” and “Corporate Governance – Compensation Committee Interlocks and
Insider Participation.”
Item 12. Security
Ownership of Certain Beneficial Owners and Management and Related Stockholder
Matters
The information under the heading
“Security Ownership of Certain Beneficial Owners and Management” set forth in
our definitive Proxy Statement filed or to be filed with the Securities and
Exchange Commission on or prior to 120 days following the end of our 2009 fiscal
year is incorporated herein by reference.
The
following table summarizes information regarding our equity compensation plans
as of October 3, 2009:
|
Plan category
|
Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
Number of securities
remaining available
for future issuance
under equity
compensation plans
(excluding securities
reflected in column
(a))
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity
compensation plans approved by security holders
|
211,336 | $ | 6.83 | 331,381 | ||||||||
|
Equity
compensation plans not approved by security holders
|
0 | 0 | 0 | |||||||||
|
Total
|
211,336 | $ | 6.83 | 331,381 | ||||||||
For additional information on our stock
option plans, see Note 11 in the Notes to Financial Statements for the year
ended October 3, 2009.
Item 13. Certain
Relationships and Related Transactions and Director
Independence
The information required under Item 13
of Part III is incorporated herein by reference to portions of our definitive
Proxy Statement filed or to be filed with the Securities and Exchange Commission
on or prior to 120 days following the end of our 2009 fiscal year under the
headings “Certain Relationships and Related Transactions” and “Corporate
Governance – Director Independence.”
56
Item
14. Principal Accountant Fees and Services
The information required under Item 14
of Part III is incorporated herein by reference to portions of our definitive
Proxy Statement filed or to be filed with the Securities and Exchange Commission
on or prior to 120 days following the end of our 2009 fiscal year under the
heading “Principal Accountant Fees and Services.”
PART
IV
Item 15. Exhibits
and Financial Statement Schedules
|
(a)
(1)
|
Financial Statements
|
|
|
The
response to this portion of Item 15 is submitted under Item 8, Financial
Statements and Supplementary Data, beginning on page
34.
|
||
|
(2)
|
Financial Statement
Schedules
|
|
|
The
response to this portion of Item 15 is submitted below under Item
15(c).
|
||
|
(3)
|
Listing of Exhibits
|
|
|
3.1
|
Restated
Articles of Incorporation: Incorporated by reference to Exhibit
3(a) to the Company’s Registration Statement on Form S-18, Commission File
No. 0-11392.
|
|
|
3.1.1
|
Articles
of Amendment filed with the South Carolina Secretary of State on February
6, 1989: Incorporated by reference to Exhibit 3.1.1 to the Company’s
Annual Report on Form 10-K for the fiscal year ended September 28, 1991
(the “1991 10-K”), Commission File No. 0-11392.
|
|
|
3.1.2
|
Articles
of Amendment filed with the South Carolina Secretary of State on March 5,
1992: Incorporated by reference to Exhibit 4.4 to the Company’s
Registration Statement on Form S-2 dated May 11, 1992, Commission File No.
33-47670.
|
|
|
3.1.3
|
Articles
of Amendment filed with the South Carolina Secretary of State on April 22,
1993: Incorporated by reference to Exhibit 4.1 to the Company’s Quarterly
Report on Form 10-Q for the quarter ended April 3,
1993.
|
|
|
3.2
|
Amended
and Restated By-Laws dated February 4, 1997: Incorporated by reference to
Exhibit 3.0 to the Company’s Quarterly Report on Form 10-Q for the quarter
ended March 29,
1997.
|
57
|
3.2.1
|
Amendment
to the Company's By-laws dated March 13, 2003: Incorporated by reference
to Exhibit 3.2 to the Company's report on Form 8-K dated March 13, 2003,
Commission File No. 000-11392.
|
|
|
3.2.2
|
Amendment
to the Company's By-laws dated November 7, 2003: Incorporated by reference
to Exhibit 3.2.2 to the Company’s Annual Report on Form 10-K for the
fiscal year ended September 27, 2003 (the “2003 10-K”), Commission File
No. 0-11392.
|
|
|
4.1
|
Specimen
of Common Stock certificate: Incorporated by reference to Exhibit 1 to the
Form S-8 filed on January 8, 1990, Commission File No.
33-32896.
|
|
|
4.2
|
Amended
and Restated Shareholder Rights Agreement dated March 24, 2003, between
Span-America Medical Systems, Inc. and American Stock Transfer & Trust
Company as Rights Agent: Incorporated by reference to Exhibit 4.1 to the
Company's report on Form 8-K dated March 24, 2003.
|
|
|
4.2.1
|
Amendment
No. 1 to the Amended and Restated Shareholder Rights Agreement dated
November 19, 2003: Incorporated by reference to Exhibit 4.1 to
the Company's report on Form 8-K dated December 2,
2003.
|
|
|
4.3
|
Agreement
among Span-America Medical Systems, Inc., Jerry Zucker, and Robert B.
Johnston, dated December 17, 2003, regarding nomination of Mr. Johnston to
the Span-America Board of Directors: Incorporated by reference to Exhibit
4.4 to the 2003 10-K.
|
|
|
10.1
|
Patent
Assignment and Royalty Agreement between Donald C. Spann and the Company,
with letter amendment thereto: Incorporated by reference to Exhibit 10(c)
to the Form S-18 filed on June 2, 1983, Commission File No.
2-832-74-A.
|
|
|
10.2*
|
Retirement
Agreement dated February 6, 1991 between the Company and Donald C.
Spann: Incorporated by reference to Exhibit 10.7 to the 1991
10-K.
|
|
|
10.3*
|
Voluntary
Resignation Agreement dated July 30, 1993 between the Company and Donald
C. Spann: Incorporated by reference to Exhibit 10.1 to the Company's
Quarterly Report on Form 10-Q for the quarter ended July 3, 1993,
Commission File No. 0-11392.
|
|
|
10.4*
|
1991
Stock Option Plan: Incorporated by reference to Exhibit 10.6 to
the 1991 10-K.
|
|
|
10.4.1*
|
Amendment
No. 1 to the 1991 Stock Option Plan: Incorporated by reference to Exhibit
10.4.2 to the Company’s Annual Report on Form 10-K for the fiscal year
ended October 3, 1998 (the “1998 10-K”), Commission File No.
0-11392.
|
58
|
10.5*
|
1997
Stock Option Plan: Incorporated by reference to Exhibit 10.14 to the
Company’s Annual Report on Form 10-K for the fiscal year ended September
27, 1997 (the “1997 10-K”), Commission File No.
0-11392.
|
|
|
10.5.1*
|
Amendment
No. 1 to the 1997 Stock Option Plan: Incorporated by reference to Exhibit
10.14.2 to the 1998 10-K.
|
|
|
10.6*
|
1997
Long Term Incentive Stock Option Plan: Incorporated by reference to
Exhibit 10.15 to the 1997 10-K.
|
|
|
10.7*
|
Span-America
Medical Systems, Inc. 2000 Restricted Stock Plan: Incorporated by
reference to Exhibit B to the Company’s Definitive Proxy Statement for its
2001 Annual Meeting of Shareholders filed with the Commission on January
11, 2001.
|
|
|
10.8*
|
Span-America
Medical Systems, Inc. 2005 Non-Employee Director Stock Plan: Incorporated
by reference to Appendix B to the Company’s Definitive Proxy Statement for
its 2005 Annual Meeting of Shareholders filed with the Commission on
January 10, 2005.
|
|
|
10.9*
|
Span-America
Medical Systems, Inc. 2007 Equity Incentive Plan: Incorporated by
reference to Appendix A to the Company’s Definitive Proxy Statement for
its 2007 Annual Meeting of Shareholders filed with the Commission on
January 8, 2007.
|
|
|
10.10*
|
Severance
Protection Agreement between the Company and James D. Ferguson dated July
25, 2002: Incorporated by reference to Exhibit 10.20 of the Company’s
Annual Report on Form 10-K for the fiscal year ended September 28, 2002
(the “2002 10-K”), Commission File No. 000-11392.
|
|
|
10.11*
|
Severance
Protection Agreement between the Company and Robert E. Ackley dated July
25, 2002: Incorporated by reference to Exhibit 10.21 of the 2002
10-K.
|
|
|
10.12*
|
Severance
Protection Agreement between the Company and Richard C. Coggins dated July
25, 2002: Incorporated by reference to Exhibit 10.22 of the 2002
10-K.
|
|
|
10.13*
|
Severance
Protection Agreement between the Company and James R. O’Reagan dated July
25, 2002: Incorporated by reference to Exhibit 10.23 of the 2002
10-K.
|
|
|
10.14*
|
Severance
Protection Agreement between the Company and Clyde A. Shew dated July 25,
2002: Incorporated by reference to Exhibit 10.24 of the 2002
10-K.
|
|
|
10.15*
|
Severance
Protection Agreement between the Company and Wanda J. Totton dated
February 11, 2004: Incorporated by reference to Exhibit 10.1 of the
Company’s Quarterly Report on Form 10-Q for the quarter ended April 3,
2004.
|
59
|
10.16*
|
Severance
Protection Agreement between the Company and Erick C. Herlong dated
December 1, 2008.
|
|
|
10.17*
|
Severance
Protection Agreement between the Company and Marie Sitter dated December
1, 2008.
|
|
|
10.18
|
Distribution
Agreement dated March 1, 1999 between the Company and Louisville Bedding
Corporation: Incorporated by reference to Exhibit 10.16 to the
Company’s Annual Report on Form 10-K for the fiscal year ended September
30, 2000, Commission File No. 000-11392.
|
|
|
10.18.1
|
Addendum
to Distribution Agreement between Louisville Bedding Company and
Span-America Medical Systems, Inc. dated January 1,
2002: Incorporated by reference to Exhibit 10.17 to the
Company’s Quarterly Report on Form 10-Q for the quarter ended March 30,
2002.
|
|
|
10.19
|
Loan
Agreement dated June 5, 2007 by and between Carolina First Bank, as
lender, and the Company, as borrower: Incorporated by reference
to the Company’s Current Report on Form 8-K dated June 5, 2007 and filed
with the commission on June 11, 2007.
|
|
|
10.19.1
|
Revolving
Note dated June 5, 2007 by the Company to Carolina First
Bank: Incorporated by reference to the Company’s Current Report
on Form 8-K dated June 5, 2007 and filed with the commission on June 11,
2007.
|
|
|
10.19.2
|
Negative
Pledge Agreement dated June 5, 2007 by the Company to Carolina First
Bank: Incorporated by reference to the Company’s Current Report
on Form 8-K dated June 5, 2007 and filed with the commission on June 11,
2007.
|
|
|
23.1
|
Consent
of Elliott Davis, LLC.
|
|
|
31.1
|
Officer
Certifications Pursuant to Section 302.
|
|
|
32.1
|
Officer
Certifications Pursuant to Section
906.
|
|
*
|
Management
contract or compensatory plan or
arrangement.
|
(b) Exhibits
The
exhibits required by this section of Item 15 are attached hereto or incorporated
by reference
(c)
Financial Statement
Schedules
60
Schedule
VIII Valuation and Qualifying Accounts
|
COL.
A
|
COL.
B
|
COL
C.
|
COL.
D
|
COL.
E
|
||||||||||||
|
ADDITIONS
|
||||||||||||||||
|
(1)
|
||||||||||||||||
|
Balance
at
|
Charged
to
|
Balance
at
|
||||||||||||||
|
Beginning
of
|
Costs
and
|
Deductions-
|
End
of
|
|||||||||||||
|
Description
|
Period
|
Expenses
|
Describe
|
Period
|
||||||||||||
|
Year
Ended October 3, 2009
|
||||||||||||||||
|
Deducted
from asset accounts:
|
||||||||||||||||
|
Reserve
for uncollectible accounts
|
$ | 235,000 | $ | (54,200 | ) | $ | 25,800 |
(a)
|
$ | 155,000 | ||||||
|
Year
Ended September 27, 2008
|
||||||||||||||||
|
Deducted
from asset accounts:
|
||||||||||||||||
|
Reserve
for uncollectible accounts
|
$ | 189,000 | $ | 75,500 | $ | 29,500 |
(a)
|
$ | 235,000 | |||||||
|
Year
Ended September 29, 2007
|
||||||||||||||||
|
Deducted
from asset accounts:
|
||||||||||||||||
|
Reserve
for uncollectible accounts
|
$ | 117,000 | $ | 99,841 | $ | 27,841 |
(a)
|
$ | 189,000 | |||||||
|
(a) Uncollectible
accounts written off.
|
||||||||||||||||
All other schedules for which provision
is made in the applicable accounting regulations of the Securities and Exchange
Commission are not required under the related instructions or are inapplicable,
and therefore have been omitted.
61
SIGNATURES
Pursuant to the requirements of Section
13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly
caused this report to be signed on its behalf by the undersigned, thereunto duly
authorized.
SPAN-AMERICA
MEDICAL SYSTEMS, INC.
|
By: /s/ Thomas D.
Henrion
|
December
22,
2009
|
|
Thomas
D. Henrion
|
|
|
Chairman
of the Board
|
Pursuant to the requirements of the
Securities Exchange Act of 1934, this report has been signed below by the
following persons on behalf of the registrant and in the capacities on the date
indicated.
|
/s/ James D. Ferguson
|
President,
Chief Executive Officer and Director
|
|
|
James
D. Ferguson
|
(Principal
Executive Officer)
|
|
|
/s/ Richard C.
Coggins
|
Chief
Financial Officer and Director
|
|
|
Richard
C. Coggins
|
(Principal
Financial Officer)
|
|
|
/s/ Gwendolyn L.
Randolph
|
Controller
|
|
|
Gwendolyn
L. Randolph
|
||
|
/s/ Robert H. Dick
|
Director
|
|
|
Robert
H. Dick
|
||
|
/s/ Thomas F. Grady,
Jr.
|
Director
|
|
|
Thomas
F. Grady, Jr.
|
||
|
/s/ Guy R. Guarch
|
Director
|
|
|
Guy
R. Guarch
|
||
|
/s/ Thomas D. Henrion
|
Director
|
|
|
Thomas
D. Henrion
|
||
|
/s/ Robert B. Johnston
|
Director
|
|
|
Robert
B. Johnston
|
||
|
/s/ Dan R. Lee
|
Director
|
|
|
Dan
R. Lee
|
||
|
/s/ Linda D. Norman
|
Director
|
|
|
Linda
D. Norman
|
||
|
December
22,
2009
|
62
