Attached files
| file | filename |
|---|---|
| EX-23.01 - EXHIBIT 23.01 - MARTEK BIOSCIENCES CORP | a2195928zex-23_01.htm |
| EX-31.01 - EXHIBIT 31.01 - MARTEK BIOSCIENCES CORP | a2195928zex-31_01.htm |
| EX-32.01 - EXHIBIT 32.01 - MARTEK BIOSCIENCES CORP | a2195928zex-32_01.htm |
| EX-31.02 - EXHIBIT 31.02 - MARTEK BIOSCIENCES CORP | a2195928zex-31_02.htm |
| EX-21.01 - EXHIBIT 21.01 - MARTEK BIOSCIENCES CORP | a2195928zex-21_01.htm |
| EX-32.02 - EXHIBIT 32.02 - MARTEK BIOSCIENCES CORP | a2195928zex-32_02.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
FOR THE FISCAL YEAR ENDED OCTOBER 31, 2009 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
COMMISSION FILE NUMBER: 0-22354
MARTEK BIOSCIENCES CORPORATION
(EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
| DELAWARE (STATE OR OTHER JURISDICTION OF INCORPORATION OR ORGANIZATION) |
52-1399362 (I.R.S. EMPLOYER IDENTIFICATION NUMBER) |
6480 DOBBIN ROAD, COLUMBIA, MARYLAND 21045
(ADDRESS OF PRINCIPAL EXECUTIVE OFFICES)
REGISTRANT'S TELEPHONE NUMBER, INCLUDING AREA CODE: (410) 740-0081
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| TITLE OF EACH CLASS: | NAME OF EACH EXCHANGE ON WHICH REGISTERED: | |
|---|---|---|
| Common Stock, $.10 Par Value | The NASDAQ Stock Market |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ý NO o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES o NO ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ý Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). o Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). o Yes ý No
The aggregate market value of Common Stock held by non-affiliates of the registrant as of April 30, 2009 was $595,521,109, based on the closing price of the Common Stock on The NASDAQ Global Select Market on April 30, 2009.
The number of shares of Common Stock outstanding as of December 17, 2009 was 33,270,252.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of the Registrant's Definitive Proxy Statement for its 2010 Annual Meeting of Stockholders (which will be filed with the Commission within 120 days after the end of the Registrant's 2009 fiscal year) are incorporated by reference into Part III of this Report.
MARTEK BIOSCIENCES CORPORATION
FORM 10-K
For The Fiscal Year Ended October 31, 2009
The information in this Form 10-K for Martek Biosciences Corporation ("Martek", "we", or the "Company") contains certain forward-looking statements, including statements related to markets for the Company's products and trends in its business that involve risks and uncertainties. The Company's actual results may differ materially from the results discussed in the forward-looking statements. Factors that might cause such a difference include those discussed in Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operations" and Item 1. "Business" as well as those discussed elsewhere in this Form 10-K, including in Item 1A. "Risk Factors."
Martek Biosciences Corporation is a leader in the innovation and development of high value nutritional products from microbial sources, including algae, fungi and other microbes, that promote health and wellness. The Company's innovation and development is based on its core expertise, broad experience and proprietary technology in areas such as microbial biology, algal genomics, fermentation and oil processing. We refer to this set of complementary abilities as Martek's "research engine." Through this research engine, the Company has developed and commercialized life'sDHA™, a vegetarian source of the omega-3 fatty acid DHA (docosahexaenoic acid), for use in infant formula, pregnancy and nursing products, foods and beverages, dietary supplements and animal feed. Martek also produces life'sARA™, a vegetarian source of the omega-6 fatty acid ARA (arachidonic acid), for use in infant formula.
NUTRITIONAL PRODUCTS
We have developed production methods and intellectual property for two important fatty acids, DHA and ARA. We sell oils containing these fatty acids as life'sDHA™, DHASCO®, Neuromins®, ARASCO® and life'sARA™. We derive DHA from microalgae and ARA from fungi, using proprietary processes. Cell membranes throughout the body contain these fatty acids, and they are particularly concentrated in the brain, central nervous system, retina and heart. Research has shown that DHA and ARA may enhance mental and visual development in infants. In addition, research has shown that DHA may play a pivotal role in brain function throughout life and may reduce the risk of cardiovascular disease. Low levels of DHA in adults have been linked to a variety of health risks, including Alzheimer's disease, dementia, cardiovascular problems and various other neurological and visual disorders. Further research is underway to assess the role of supplementation with our DHA on mitigating a variety of health risks.
Adults may obtain DHA via a limited number of foods such as fish, eggs or organ meats. ARA may be obtained from foods such as red meats, fish and eggs. Pregnant women transfer DHA and ARA through the placenta to the fetus and lactating mothers pass DHA and ARA to infants through breast milk. While there are currently no universally recognized guidelines for daily consumption of DHA, several international scientific and health agencies have made recommendations for DHA and ARA consumption for infants and for DHA intake for pregnant and nursing women. A workshop in 1999 sponsored by the International Society for the Study of Fatty Acids and Lipids as well as others recommended that adults consume at least 220 mg of DHA daily. U.S. Department of Health and Human Services data indicate that dietary consumption of DHA is well below this level. We believe that greater recognition of this possible dietary deficiency will result in an increase in demand for DHA-supplemented products. Recommendations for ARA consumption by adults have not been put forth and may not be necessary as adequate amounts of ARA are likely consumed in the typical adult diet.
1
Investigators at a number of research institutions, including the National Institutes of Health ("NIH"), have observed a relationship between low levels of DHA and a variety of health risks, including Alzheimer's disease, dementia, cardiovascular problems and various other neurological and visual disorders. We sponsor and participate with others in research to determine the benefit of DHA supplementation in addressing these health concerns. Additionally, Martek continues to support and provide oils for multiple ongoing clinical trials designed to further explore the benefits of DHA supplementation during pregnancy and nursing to assess the outcomes on both mother and child.
In May 2001, the Food and Drug Administration ("FDA") completed a favorable review of our generally recognized as safe ("GRAS") notification for the use of our DHASCO® and ARASCO® oil blend in specified ratios in infant formulas. Since the first United States infant formula product introduction in February 2002, infant formulas supplemented with Martek's DHA and ARA manufactured by six of our customers have been sold in the United States: Mead Johnson Nutritionals under the Enfamil®LIPIL® brand; Abbott Nutrition under its Similac® ADVANCE® brand; Nestle under its Good Start® Supreme DHA & ARA brand; PBM Products Inc. under the brands Bright Beginnings™, Vermont Organics™ and under private label brands, including Wal-Mart Parent's Choice™; Hain Celestial under the brand Earth's Best®; and Nutricia North America under the brand Neocate®. These supplemented infant formulas include term, preterm, soy-based, specialty and toddler products. As of October 31, 2009, we estimate that formula supplemented with our oils had penetrated almost all of the U.S. infant formula market.
We are supplying over 35 infant formula customers with our nutritional oils. These companies collectively represent approximately 75% of the estimated $15 billion worldwide retail market for infant formula and nearly 100% of the estimated $4.5 billion U.S. retail market for infant formula, including the retail value of Women, Infants and Children program ("WIC") rebates. WIC is a federal grant program administered by the states for the benefit of low-income, nutritionally at-risk women, infants and children. Our customers include infant formula market leaders Mead Johnson Nutritionals, Nestle, Abbott Nutrition, Pfizer (formerly Wyeth) and Danone (formerly Numico), each of whom is selling infant formula supplemented with our nutritional oils. Our customers are now selling infant formula products containing our oils collectively in over 75 countries. In addition, certain infant formula customers are selling products that contain our nutritional oils and target the markets for children ages nine months to seven years of age and older.
In addition to the DHA we sell for use in infant formula, Martek has developed certain separate and distinct DHA technology, which we refer to as DHA-S, that is derived from a different algal strain than our DHA authorized for addition to infant formula. We have received a favorable review by the FDA of our GRAS notification for the use of DHA-S in food and beverage applications in the U.S. and have received similar approvals in Canada. We have also received authorization from the Ministry of Health in China (subject to certain conditions) and the Australia New Zealand Food Authority for the use of DHA-S oil in all foods and authorization from the European Commission and Israel for the use of our DHA-S oil as a novel food ingredient. This novel food designation authorizes the use of our DHA-S as an ingredient in certain foods such as certain dairy products, including cheese and yogurt, spreads and dressings, breakfast cereals, food supplements and dietary foods for special medical purposes in the European Community and Israel. In July 2009, the Standing Committee of the European Commission and the 27 Member States approved the expanded use of our DHA-S algal oil in beverages, bakery products and nutrition bars. DHA algal oil is also regarded as a traditional food in Japan and, as such, is acceptable for use in foods and beverages and dietary supplements.
We are currently selling DHA-S products into the dietary supplement, food and beverage, pregnancy and nursing and animal feed markets domestically and internationally. To date, over 200 domestic and international companies have launched non-infant formula products that contain DHA-S, most of which remain on the market. Our DHA-S and our infant formula DHA are each marketed under the brand life'sDHA™.
2
COLLABORATIONS
In addition to selling microbial-based nutritional products, we also perform work in connection with technical collaborations with corporate partners. This work utilizes our core expertise in microbial biology, algal genomics, fermentation and oil processing to collaborate with these corporate partners in the development of new products and technologies. It is our intent to enter into such collaborations only when, in addition to potential research payments, economic benefits can be derived through such means as future royalty arrangements, milestone payments or commercialization rights. One of Martek's initial partners for these collaborations is a subsidiary of BP p.l.c. ("BP"), for whom we are performing work on microbial oils for the development of biofuels. All intellectual property developed by Martek will be owned by BP, with an exclusive license to Martek for commercialization in nutrition, cosmetic and pharmaceutical applications. Additionally, each party is entitled to certain commercial payments from the counterparty for commercialization of the technology in the other party's fields of use.
CONTRACT MANUFACTURING
We have been providing certain contract manufacturing services at our Kingstree, South Carolina facility. We began offering these services following our September 2003 acquisition of FermPro Manufacturing, LP, which had been providing third-party manufacturing services since the mid-1960's. The facility's large fermentation capacity and numerous types of recovery equipment allow us to customize production processes for our customers and produce at significant volumes. Our facilities are particularly well-suited for the production of enzymes, specialty chemicals, vitamins and agricultural specialty products. During fiscal 2009, while revenues remained at approximately the same level as fiscal 2008, we reduced the scope of our contract manufacturing activities, and we anticipate further limiting our contract manufacturing activities over the course of fiscal 2010 in order to focus resources on our higher margin nutritional oils business. Other than the reductions to revenue and associated gross margin, we do not expect that the cessation of contract manufacturing activities will have any material impact on our results of operations.
NUTRITIONAL OILS—INFANT FORMULA APPLICATIONS
Certain microalgae and fungi produce large quantities of oils and fats containing long-chain polyunsaturated fatty acids, known as LCPUFAs, that are important to human nutrition and health. We have identified strains of microalgae that produce oils rich in DHA and have developed the means to grow them by fermentation. In addition, we have isolated and cultured a strain of fungus that produces large amounts of ARA.
DHA is the predominant omega-3 fatty acid in the brain and retina of the eye and is a key component of heart tissue in humans and other mammals. Both DHA and ARA are important for infant brain and eye development, which occurs primarily in the last trimester in utero, and continues throughout the first few years of life. During pregnancy, DHA and ARA are actively transported from the mother to the fetus via the placenta. Following birth, the infant receives these fatty acids from either breast milk (which always contains DHA and ARA) or infant formula supplemented with DHA and ARA. Studies have shown that with DHA-supplemented infant formula, formula-fed infants have blood and tissue levels of DHA that are similar to those of breastfed infants. DHA and ARA supplementation is especially important for premature infants who failed to complete the last trimester of pregnancy in utero. DHA and ARA were added to U.S. infant formulas beginning in 2002, and Martek's DHA and ARA continue to be the only DHA and ARA included in infant formula in the U.S.
3
Fish oils can also be used for DHA supplementation in infant formula. However, we believe that for a number of reasons our DHA oil is more desirable for infant formula applications than fish oil or other sources of DHA. Our oils are derived from a vegetarian source and are grown under tightly controlled conditions. As a result, Martek oils do not contain contaminants such as methylmercury, polychlorinated biphenyls ("PCBs") and dioxins that may be found in certain fish oils. Our oils do not contain significant quantities of eicosapentaenoic acid ("EPA"), an omega-3 fatty acid found in fish oil which certain studies indicate is not appropriate for consumption by infants in high levels. Both algal and fish DHA oils contain DHA that is in the form of easily digestible triglycerides similar to the major form found in breast milk. Martek oils have the further benefit, however, of higher oxidative stability and longer shelf life than certain fish oils. A study testing the benefits of DHA supplemented infant formula in premature infants conducted by Dr. M. T. Clandinin and others published in April 2005 in The Journal of Pediatrics directly compared infant formula supplemented with DHA from Martek oils to a formula supplemented with DHA from fish oil as well as unsupplemented formula. Both supplemented formulas also contained Martek ARA. The study demonstrated that infants fed algal DHA supplemented formula for twelve months "achieved body weights and lengths comparable to breastfed infants by 18 months after term, whereas infants fed unsupplemented formulas or formulas with DHA from fish oil did not."
Although not all experts agree on the essentiality, benefits or importance of DHA and ARA for infants, the following studies show the benefits of including DHA and ARA in the infant diet:
- •
- A study by Dr. J. Drover and others published in September/October 2009 in Child
Development reported new data on the effects of DHA and ARA supplementation on cognition in 9-month old infants. Participants included 229 infants enrolled in three
separate randomized clinical trials. In the trials, infants received infant formula supplemented with DHA and ARA or non-supplemented infant formula beginning at days 1-5 of
life or following 6 weeks or 4 to 6 months of breastfeeding. Results showed that infants fed supplemented formula beginning at day 1-5, or after 6 weeks of
breastfeeding, scored significantly higher in the cognitive evaluation than infants fed non-supplemented infant formula. The authors state that "these results suggest that LCPUFA
supplementation improves means-ends problem solving." Martek's oils were used in all of the supplemented formulas.
- •
- A report by Dr. R. Pivik and others published in June 2009 in Developmental
Neuropsychology evaluated resting heart rate during the first six months of life in healthy, full-term infants. The newborns were either breastfed or given cow's
milk-based or soy-based commercial infant formula containing different levels of DHA and ARA throughout the study period. Results showed that infants fed higher levels of DHA
and ARA from either breast milk or infant formula had lower heart rates and higher values for heart rate variability measures compared to infants fed lower levels of DHA and ARA. According to the
authors, these results indicate that adequate DHA is important for parasympathetic tone during this important period in development of cardiovascular regulation.
- •
- A study reported by Dr. M. Makrides and others published in January 2009 in the Journal of the American Medical Association evaluated the effects of providing a higher than usual level of DHA to 657 very premature infants (33 weeks or less) during the early weeks of life in order to achieve the DHA levels equal to those of a term infant. The results of a preplanned analysis by gender found that premature girls on the high DHA diet scored five points higher on the Bayley Mental Development Index than did the girls receiving the standard DHA diet. This resulted in a 55% reduction in mild mental delay and an 80% reduction in significant mental delay. These were statistically significant improvements versus the control. The benefits were not significant in males. Martek's oils as well as DHA derived from fish oil were used in the study.
4
- •
- An independent study reported by Dr. E. Birch and others published in May 2007 in Early
Human Development reported the results of a comparison between children who were breastfed as infants to both children who as infants used DHA and ARA supplemented formula and
children who as infants used formula not supplemented with DHA and ARA. The results indicated that children who received the supplemented formula had visual and verbal IQ scores that did not differ
significantly from children who had been breastfed and such scores were better than children who received unsupplemented formula. Martek's oils were used in the study. This was a follow up to a study
by Dr. E. Birch and others published in 2000 in Developmental Medicine & Child Neurology, which noted the results of an NIH-sponsored
study that showed a significant improvement in mental development in term infants given a commercially available infant formula supplemented with Martek DHA™ and ARA compared to infants
fed the same formula, but without DHA and ARA. In the double-blind study, infants fed the diet supplemented with our oils showed, at 18 months of age, a mean increase of 7 points on the Mental
Development Index ("MDI") of the Bayley Scales of Infant Development II. Researchers reported that "these data support a long-term cognitive advantage of infant dietary DHA supply during
the first 4 months of life. The significant correlations...support the hypothesis that early dietary supply of DHA was a significant determinant of improved performance on the MDI."
- •
- A study reported by Dr. N. Pastor and others published in November 2006 in Clinical
Pediatrics found that infants fed a formula containing 17 mg DHA and 34 mg ARA per 100 Kcal had fewer episodes of bronchiolitis and bronchitis at ages 5, 7, and 9 months
compared to infants receiving control formula not containing these added LCPUFAs. This study used Martek's oils.
- •
- A study reported by Dr. E. Birch and others published in April 2005 in the American Journal
of Clinical Nutrition found that DHA and ARA supplementation of term infant formula during the first year of life resulted in improved visual function in 12-month
old infants compared to those without supplementation. This study used Martek's oils.
- •
- A study reported by Dr. D. Hoffman and others published in the June 2003 issue of The
Journal of Pediatrics reported that infants who were breastfed from birth to between four and six months of age and then weaned onto formula supplemented with DHA and ARA
experienced significantly improved visual development at one year of age compared to infants who were breastfed and then weaned onto formula without DHA and ARA. This study used Martek's oils.
- •
- A study reported by Dr. E. Birch and others published in March 2002 in the American Journal of Clinical Nutrition found that infants who were breastfed for six weeks and then weaned to DHA and ARA supplemented infant formula had significantly better visual acuity at 17, 26 and 52 weeks of age and significantly better stereoacuity at 17 weeks of age than infants who were weaned to non-supplemented formula. This study used Martek's oils.
DHA and ARA have been recognized as important in the infant diet and recommended for inclusion in infant formula by several expert panels, including: the United Nations Food and Agricultural Organization and the World Health Organization ("FAO/WHO"); International Society for the Study of Fatty Acids and Lipids ("ISSFAL") sponsored workshop panel; an expert panel sponsored by the Child Health Foundation; and the British Nutrition Foundation ("BNF"). Other expert groups making recommendations regarding the addition of DHA and ARA to infant formula include:
- •
- Enteral Nutrient Supply for Preterm Infants: A Comment of the ESPGHAN [European Society for Paediatric Gastroenterology, Hepatology and Nutrition] Committee on Nutrition, authored by Dr. C. Agostoni and others published in Journal of Pediatric Gastroenterology and Nutrition online in October 2009, in which the ESPGHAN Committee on Nutrition updated their 1987 guidelines regarding the nutrient requirements of preterm infants. These new guidelines now include significant information regarding the importance of both DHA and ARA for the preterm infant.
5
- •
- Feeding Preterm Infants After Hospital Discharge: A Commentary by the ESPGHAN Committee on
Nutrition, authored by Dr. P. Aggett and others published in Journal of Pediatric Gastroenterology and Nutrition in May
2006, in which the ESPGHAN Committee on Nutrition reviewed available evidence and established recommendations for feeding preterm infants after hospital discharge. The Committee recommended that
formula-fed infants born prematurely should receive infant formula that provides LCPUFAs for optimal growth and development. Martek DHA and ARA are LCPUFAs added to preterm and term infant
formula in the U.S. and worldwide.
- •
- Global Standard for the Composition of Infant Formula: Recommendations of an ESPGHAN Coordinated International Expert Group, authored by Dr. B. Koletzko and others published in Journal of Pediatric Gastroenterology and Nutrition in November 2005, in which the International Expert Group, in view of beneficial effects, supported the optional addition of LCPUFAs to infant formula. Their guidelines specify that the optional addition of DHA should not exceed 0.5% of total fat; ARA contents should be at least the same concentration as DHA; and the EPA content should not exceed that of DHA.
The Committee recommended that along with the other nutrients required for optimal development, DHA and ARA should be included in preterm infant formula to provide 11-27 mg/100 Kcal DHA and 16-39 mg/100 Kcal ARA. Any EPA present should not exceed 30% of DHA supply. These recommended inclusion levels are equal to or greater than the inclusion levels generally found in preterm infant formulas currently marketed in the United States.
Our sales of nutritional oils for infant formula were approximately $285.7 million, $300.7 million and $265.6 million in fiscal 2009, 2008 and 2007, respectively, which represents 87%, 89% and 91% of total product sales, respectively. Mead Johnson Nutritionals accounted for approximately 30%, 36% and 42% of our total product sales in fiscal 2009, 2008 and 2007, respectively. Abbott Nutrition accounted for approximately 20%, 19% and 18% of our total product sales in fiscal 2009, 2008 and 2007, respectively. Nestle accounted for approximately 12%, 12% and 11% of our total product sales in fiscal 2009, 2008 and 2007, respectively. Pfizer (formerly Wyeth) accounted for approximately 8%, 9% and 9% of our total product sales in fiscal 2009, 2008 and 2007, respectively. Danone (formerly Numico) accounted for approximately 7%, 5% and 4% of our total product sales in fiscal 2009, 2008 and 2007, respectively.
NUTRITIONAL OILS—NON-INFANT FORMULA APPLICATIONS
Applications for Pregnant and Nursing Women
DHA is transferred from the mother to the fetus during pregnancy and particularly during the last trimester. Following birth, the mother transfers DHA to her newborn through breast milk. Therefore, an adequate intake of DHA during pregnancy and nursing is thought to be important and many public health agencies such as the World Health Organization ("WHO") and ISSFAL have made recommendations for DHA intake during the perinatal period. In addition, other experts have specified certain minimum levels of LCPUFA intake during pregnancy as follows:
- •
- A consensus report by Dr. B. Koletzko and others published in January 2008 in the Journal
of Perinatal Medicine recorded the recommendations of international experts in maternal and pediatric nutrition regarding the importance of LCPUFAs. The committee recommended
that "pregnant and lactating women should aim to achieve an average daily intake of at least 200 mg DHA." When breastfeeding is not possible for a term infant, the committee recommended use of "an
infant formula providing DHA at levels between 0.2 and 0.5 weight percent of total fat, and with the minimum amount of ARA equivalent to the contents of DHA."
- •
- The September 2007 issue of the Journal of the American Dietetic Association included a position statement from the American Dietetic Association and Dietitians of Canada regarding dietary
6
- •
- During the PeriLip meeting, a European Union supported Consensus Conference on "Dietary Fat Intake During the Perinatal Period" (September 2005, Germany), the following recommendation was made regarding DHA consumption: "Pregnant and lactating women should aim to achieve a dietary intake of n-3 LCPUFA [omega-3 long-chain polyunsaturated fatty acid] that supplies a DHA intake of at least 200 mg/day." These recommendations and overall results of the PeriLip project were published in the British Journal of Nutrition (November 2007).
fatty acids. The groups emphasized the importance of DHA during pregnancy, lactation, and infancy and its particular importance for neural development and function. The groups also reviewed the importance of long-chain omega-3 fatty acids for health and recommend that adults consume 500 mg of long-chain omega-3 fatty acids daily.
Supplementation of breastfeeding mothers with DHA has shown to increase the level of DHA found in breast milk. Studies have shown a benefit for breastfed infants of DHA-supplemented mothers as indicated below:
- •
- A study reported by Dr. C. Henriksen and others published in June 2008 in the journal Pediatrics evaluated the effect of enriching human milk
with DHA and ARA to be fed to premature infants. The results showed that infants receiving the
DHA/ARA enriched human milk demonstrated better recognition memory and had higher problem solving scores at six months than the non-enriched milk group. Martek's oils were used in this
study.
- •
- A study reported by Dr. S. Innis and Dr. R. Friesen published in March 2008 in the American Journal of Clinical Nutrition examined visual acuity in infants whose mothers received DHA supplementation during pregnancy. The results of the
study showed that infants from mothers who received DHA supplementation exhibited a better visual response than those from mothers who received placebo. Martek's oils were used in this study.
- •
- A study published by Dr. J. Cohen and others in November 2005 in the American Journal of
Preventive Medicine provided a statistical analysis of many studies which had examined maternal fatty acid intake and effect on infant development. The results of the analysis
showed that increases in maternal DHA intake are associated with modest improvements in child IQ.
- •
- A study reported by Dr. S. Hart and others published in August 2005 in the Journal of
Pediatric Psychology revealed a positive correlation between DHA levels in breast milk and newborn neurobehavioral function. The study analyzed the DHA content of breast milk
collected from 20 breastfeeding mothers nine days after delivery. At the same time, their infants were tested for neurobehavioral functioning using the Brazelton Neonatal Behavioral Assessment
Scale ("NBAS"), a commonly used behavioral test. An analysis of this test revealed a positive correlation between DHA levels in the mother's breast milk and the child's NBAS score.
- •
- A study reported by Dr. C. Jensen and others published in July 2005 in the American Journal of Clinical Nutrition noted that infants of mothers who supplemented with life'sDHA™ while breastfeeding had improved psychomotor skills at 21/2 years of age. The study revealed that children of DHA-supplemented mothers scored significantly higher on the Bayley Psychomotor Development Index (PDI), when compared to the children of the non-supplemented breastfeeding mothers. The study also confirmed that DHA supplementation while breastfeeding effectively increases DHA levels in the mother's milk as it noted that the mothers supplemented with DHA had 75% more DHA in their breast milk than the control group and their infants had 35% higher DHA blood levels than the control group infants. This study was partially funded by Martek.
Additional research is underway to further evaluate DHA supplementation during pregnancy and nursing. We are currently providing DHA oils to researchers who are evaluating potential benefits of
7
maternal DHA supplementation during pregnancy and nursing on pregnancy outcomes and infant development.
Other Human Applications
Investigators at universities around the world and at other research centers, such as NIH, have observed a relationship between low levels of DHA and a variety of health risks, including Alzheimer's disease, dementia, cardiovascular problems and various other neurological and visual disorders. We are currently trying to establish what contribution, if any, supplementation with our oils will make in addressing a number of these problems. We, as well as others, are supporting studies to further investigate the potential benefit of DHA supplementation on cardiovascular health, and we, as well as others, are conducting research regarding the impact of DHA supplementation on certain visual and neurological disorders.
Cognitive Function
DHA and cognitive function in adults—Discussed below are the findings of several published studies and papers that highlight potential benefits of DHA on neurological health as well as on the risk of Alzheimer's disease and age-related dementia.
- •
- A pre-clinical study reported by Dr. Q. Ma and others published in December 2007 in the Journal of Neuroscience reported that DHA was found to
decrease an important risk factor for late-onset Alzheimer's disease. Using a mouse
model, a diabetic rat model and cultured human cells, the study found that DHA increases the production of LR11, a protein vital to clearing the brain of the enzymes that make the beta amyloid plaques
that are thought to cause Alzheimer's disease. The investigators used Martek's oils for a portion of the research.
- •
- An independent study reported by Dr. R. McNamara and others published in July 2007 in the Society of Biological Psychiatry
found a deficit in the total fatty acid composition of the orbitofrontal cortex and found a selective deficit in the
level of DHA compared with controls in brain examinations of postmortem patients who had been diagnosed with major depression compared with controls. These findings show a correlation between low
tissue levels of DHA and neuropsychiatric diseases such as depression.
- •
- A study published by Dr. K. Green and others in April 2007 in The Journal of
Neuroscience used a transgenic mouse model of Alzheimer's disease to show that supplementation with DHA reduced the accumulation of both amyloid-beta and tau, two
indicators of the disease in humans. This study used Martek's DHA oils and funding for the study was provided by Martek.
- •
- A study published by Dr. E. Schaefer and others in November 2006 in the Archives of
Neurology investigated the relationship of blood DHA levels and the development of dementia in a prospective follow-up study of the participants in the Framingham
Heart Study. The results of the study noted that subjects with the highest levels of plasma DHA (top 20%) had a significant reduction in the risk of developing dementia from all causes. The study was
partially funded by Martek.
- •
- The results of an in vitro study conducted by Dr. S. Florent and others published in November 2005 in the Journal of Neurochemistry notes that DHA enrichment likely induces changes in neuronal membrane properties that may assist in the prevention of
Alzheimer's disease and other neurodegenerative diseases.
- •
- The results of an in vitro study conducted by Dr. W. Lukiw and others published in October 2005 in the Journal of Clinical Investigation suggest that DHA intake could benefit people with Alzheimer's disease by lowering the accumulation of amyloid-B peptides, which are associated with brain aging and Alzheimer's.
8
- •
- In vitro research conducted by Dr. N. Bazan and published in August 2005 in Molecular
Neurobiology reported on a metabolite of DHA that appears to have a protective role in neural cell survival and Alzheimer's disease.
- •
- A scientific review on DHA performed by Dr. J. Marszalek and Dr. H. Lodish published in June 2005 in Annual Review of Cell and Developmental
Biology suggests the significant role that DHA plays in the maintenance of normal neurological function.
- •
- The Agency for Healthcare Research and Quality ("AHRQ") of the United States Department of Health and Human Services
issued in February 2005 a report on the effects of omega-3 fatty acids on cognitive function with respect to persons experiencing aging, dementia and neurological diseases. They stated:
"Total omega3 FA [omega-3 fatty acid] consumption and consumption of DHA (but not ALA [alpha-linolenic acid] or EPA) were associated with a
significant reduction in the incidence of Alzheimer's."
- •
- In September 2004, Dr. F. Calon and others at the UCLA School of Medicine published the results of an animal study in the journal Neuron noting the effects of Martek's DHA on the advancement of Alzheimer's disease. The study found that a diet rich in DHA significantly lessened the memory loss and cell damage associated with Alzheimer's disease in laboratory mice. In 2005, this laboratory extended their findings using DHA in the European Journal of Neuroscience. The authors reported how dietary essential fatty acids may influence cognition and Alzheimer's disease risk.
Additional research is needed to evaluate the role, if any, of DHA supplementation in reducing the risk of developing these diseases.
DHA and cognitive function in children—Discussed below are the findings of a published study that highlights the benefits of DHA on cognitive development in children.
- •
- A study reported by Dr. A. Ryan and Dr. E. Nelson, both employees of Martek, was published in the May 2008 issue of Clinical Pediatrics and showed that while primary endpoints were not achieved, a preplanned regression analysis yielded a statistically significant positive association between a higher DHA level in the blood and higher scores on the Peabody Picture Vocabulary Test, a cognitive test designed to measure listening comprehension and vocabulary skills. Performance on the test is an indicator of school readiness. The study was funded by Martek and used Martek's oils.
In addition, during the November 2008 annual meeting of the Society for Neuroscience, Dr. R. McNamara and others reported findings from functional magnetic resonance imaging in healthy eight to ten year old boys. In this study, 33 subjects were randomly assigned to receive placebo, DHA (400 mg/day), or DHA (1,200 mg/day) for eight weeks. These data demonstrated that higher dietary DHA intake increased erythrocyte membrane DHA content and enhanced functional cortical activity in a region of the brain which is implicated in sustained attention and executive function. Martek's oils were used and Martek funded portions of this study.
9
Cardiovascular Health
Discussed below are recommendations from expert groups and the findings of several published studies and papers that highlight the benefits of long-chain omega-3 fatty acids, including DHA, on cardiovascular health. In some cases, the authors caution people of the potential risks associated with the intake of certain fish.
- •
- A study reported by Dr. J Virtanen and others in an electronic publication ahead of print in December 2009 in Circulation reported a study in
which, from 1984 to 1989, the serum fatty acids of 2,174 healthy men, age 42 to 60 years, were measured. At the
average follow-up period of 17.7 years, information regarding any previous diagnosis of atrial fibrillation, a common cardiac arrhythmia, was compared to baseline fatty acid status.
Results showed that DHA, and only DHA, and not EPA or ALA, was associated with a reduced risk for developing atrial fibrillation. Compared to the reference group, subjects in the highest DHA quartile
had a 38% lower risk for developing atrial fibrillation.
- •
- A study reported by Dr. A. Ryan, an employee of Martek, and others published in March-April 2009 in the American Journal of
Therapeutics reported the combined data from 16 published clinical trials examining the effects of DHA triglyceride ("TG") oil
derived from algae on serum TG levels and other related parameters. The study populations included subjects with both normal and elevated TG levels including those with persistent hypertriglyceridemia
receiving statin therapy. At doses of 1-2 g/d, algal-DHA with or without statins significantly lowered plasma TG levels up to 26%. Regression analysis showed a linear
relationship between baseline TG and magnitude of TG reduction. All of the studies in the review used Martek's oils.
- •
- A study reported by Dr. D. Kelley and others published in January 2008 in The Journal of
Nutrition demonstrated the effects of DHA supplementation on remnant-like particle-cholesterol ("RLP-C") and red blood cell omega-3 content
in plasma, two potential risk factors for cardiovascular disease. Results showed that the study participants (hypertriglyceridemic men) experienced a decrease in atherogenic RLP-C and an
increase in the cardio-protective omega-3 index, both of which are associated with improved cardiovascular health. The study used Martek's oils.
- •
- A study published by Dr. D. Kelley and others in August 2007 in the American Journal of
Clinical Nutrition found that DHA is effective in reducing the level of triglycerides in male hypertriglyceridemic patients. In this study, DHA alone was effective without EPA,
the other omega-3 commonly found in fish oil, in reducing triglycerides. Hypertriglyceridemia (high triglyceride levels) in men is associated with an increased risk of cardiovascular
disease and metabolic syndrome. Martek contributed the DHA supplements and funding for this study.
- •
- An independent study reported by Dr. H. Theobald and others published in April 2007 in The
Journal of Nutrition assessed the effects of low-dose DHA on blood pressure in healthy men and women in the United Kingdom. Compared with placebo, supplementation
with Martek's oils significantly reduced diastolic blood pressure by 3.3 mm Hg.
- •
- The results of a study reported by Dr. L. Keilson and others, which were presented in March 2007 at the American College of Cardiology 56th Annual Scientific Session, showed that supplementation with life'sDHA™ reduced triglycerides by nearly 20%, reduced pulse rate by 5 beats per minute and reduced diastolic blood pressure by 4 mm Hg. The DHA-supplemented group also showed an 8% reduction in total cholesterol and a 5% increase in HDL (good) cholesterol level. The study was conducted in men and women with hypertriglyceridemia who were already taking statin medications for the reduction of cholesterol. This study used Martek oils and funding for this study was provided by Martek.
10
- •
- An independent study reported by Dr. L. Schwellenbach and others published in December 2006 in the Journal of the American College of Nutrition
compared Martek's oils (algal oil) to DHA plus EPA (fish oil) for their ability to lower triglycerides in
subjects with coronary artery disease and elevated triglycerides, most of whom were undergoing statin therapy. Both supplements significantly lowered triglycerides, but only life'sDHA™ increased
high density ("good") cholesterol. The authors concluded that there was no added benefit provided by EPA for lowering
triglycerides at this level. The authors of the study also indicated that life'sDHA™ was better tolerated by the study participants than
fish oil, noting that a greater proportion of subjects in the fish oil group reported fishy taste as a problem with their treatment.
- •
- A study published by Dr. A. Erkkilä and others in September 2006 in the Journal of Lipid Research noted an important
relationship between plasma DHA levels and the reduced progression of cardiac disease. Specifically, women
whose DHA levels were above the median at enrollment had slower progression of coronary artery stenosis over a three-year period. This effect was not seen with the other
omega-3 fatty acids, ALA or EPA.
- •
- The results of a study conducted by Dr. K. Maki and others and published in the Journal of
the American College of Nutrition in June 2005 demonstrated that life'sDHA™ supplementation lowered triglycerides by
approximately 21%. This study was sponsored by Martek.
- •
- In May 2002, in the publication Circulation, the American Heart Association ("AHA") issued a Scientific Statement entitled "Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease." The Scientific Statement outlines the findings of a comprehensive report that examined the cardiovascular health benefit of omega-3 fatty acids, specifically DHA and EPA, from fish sources. The report concluded that consumption of such omega-3 fatty acids, either through diet or supplements, reduces the incidence of cardiovascular disease. The statement refers to studies that have indicated the following to be associated with the intake of omega-3 fatty acids: decreased risk of sudden death and arrhythmia; decreased thrombosis (blood clot); decreased triglyceride levels; decreased growth of atherosclerotic plaque; improved arterial health; and lower blood pressure.
The Scientific Statement concluded that omega-3 fatty acids have been shown in epidemiological and clinical trials to reduce the incidence of heart disease and recommends that healthy individuals eat a variety of fish (preferably oily) at least twice a week. The statement cautioned, however, that fish intake "must be balanced with concerns about environmental pollutants" because some species of fish may contain significant levels of methylmercury, polychlorinated biphenyls ("PCBs"), dioxins, and other contaminants. Both the FDA and the Environmental Protection Agency have advised children, pregnant women, women who may become pregnant and nursing mothers to limit their intake of certain fish. In consideration of the health risks posed by such contaminants, the authors of the statement conclude by stating, "The availability of high-quality omega-3 fatty acid supplements, free of contaminants, is an important prerequisite to their extensive use." Martek's DHA oil is derived from a vegetarian source and is free of contaminants that may be found in certain fish oils.
A Scientific Statement entitled "Diet and Lifestyle Recommendations Revision 2006" published by Dr. A. Lichtenstein and other members of the American Heart Association Nutrition Committee published in July 2006 in Circulation included a recommendation that people with documented heart disease consume approximately one gram of DHA plus EPA per day. They affirm that with appropriate medical advice, use of supplements may be substituted for fish.
11
In September 2004, the FDA announced that it would allow conventional foods and beverages and dietary supplements containing DHA and EPA to make a qualified health claim for reduced risk of coronary heart disease on their product packaging. A qualified health claim must be supported by credible scientific evidence. Upon review of this scientific evidence, the FDA concluded that supportive but not conclusive research shows that consumption of DHA and EPA may reduce the risk of coronary heart disease. This qualified health claim supports the benefit of Martek's DHA-S oil, as it contains both DHA and small amounts of EPA.
While there is not yet a scientific consensus on the subject, a number of clinical studies, including several listed above, have indicated that DHA provides the main cardioprotective benefits traditionally ascribed to fish consumption or to the combination of DHA plus EPA. Such research has indicated that DHA, in the absence of EPA, may have the following effects on cardiovascular risk factors:
- •
- reduces triglycerides and raises the HDL or "good" cholesterol;
- •
- reduces blood pressure;
- •
- reduces heart rate; and
- •
- increases LDL and HDL cholesterol particle size.
Other
Discussed below are the findings of some studies that highlight the benefits of DHA on other human health conditions.
- •
- The American Journal of Clinical Nutrition (December 2009) reported
12-year follow-up data from over 1,800 people who had participated in the Age-Related Eye Disease Study evaluating the development of Age Related Macular
Degeneration ("AMD"). At the end of the 12-year period, information was graded to determine each individual's AMD status and comparisons were made with the intake of DHA, EPA, and the
combination of DHA and EPA. Results showed that individuals who consumed the highest amounts of DHA alone or in combination with EPA had the lowest level of progression to AMD. EPA alone did not show
a benefit.
- •
- A study by Dr. C. Chiu and others published in September 2009 in the British Journal of
Ophthalmology reported findings from a multi-year study of the effects of diet on the progression of age-related macular degeneration. This large
clinical trial supplemented over 2,000 people with 5, 6, and 18 times the Recommended Dietary Allowance levels of vitamins C, E and/or zinc and other minerals. In this report, the investigators
quantified the dietary intake of DHA and EPA in the study groups as well. Results showed that consuming a diet rich in DHA slowed the progression of early age-related macular degeneration
and that the DHA benefit occurred independently from EPA and the usual study supplements.
- •
- A study reported by Dr. D. Kelley and others published in March 2009 in The Journal of
Nutrition evaluated the effect of DHA on several indicators of inflammation in 34 hypertriglyceridemic men. As a result of DHA supplementation, several indicators of
inflammation were improved, including: the number of circulating neutrophils; the concentrations of C-reactive protein ("CRP"); interleukin-6; and granulocyte monocyte-colony
stimulating factor. There was a corresponding increase in the concentration of anti-inflammatory matrix metalloproteinase. The authors noted that the reduction of CRP was similar to that
seen with statins. Overall, the authors concluded that supplemental DHA may lessen the inflammatory response. Martek's oils were used in this study.
- •
- An independent observational study by Dr. J.M. Norris and others published in September 2007 in the Journal of the American Medical Association was designed to determine whether the intake of omega-3 and omega-6 fatty acids is associated with the development of Type 1 diabetes in
12
- •
- A study by Dr. K. Connor and others published in July 2007 in Nature Medicine reported that increasing consumption of long-chain omega-3 fatty acids, including DHA, reduces destructive vascularization in the retina. In this animal study of retinopathy associated with prematurity, the authors summarize a series of experiments demonstrating that long-chain omega-3 fatty acids, and selected metabolites, are effective in reducing retinal vascular disease, which is a leading cause of blindness. A portion of these studies included Martek oils as the source of long-chain fatty acids.
children. This study was conducted with children known to be at genetic risk for developing Type 1 diabetes. Results showed that the omega-3 fatty acid levels were inversely correlated with the risk for developing diabetes in this group of at-risk children. These investigators are further investigating this relationship in an ongoing clinical trial to determine whether DHA, specifically, has a role in Type 1 diabetes prevention.
Life'sDHA™ is sold by Martek as an ingredient to food and beverage and supplement manufacturers and is also the brand name of Martek's consumer supplement product. Martek's Neuromins® brand, which contains life'sDHA™, is distributed by the Company and sold under license by several supplement manufacturers and can be found nationwide. In addition, life'sDHA™ can now be found in multiple supplement products, including private label products sold in Wal-Mart, CVS/pharmacy and Walgreen's.
We are continuing to aggressively pursue further penetration of our DHA oils, both domestically and internationally, in the food and beverage, pregnancy and nursing, nutritional supplement and animal feed (collectively, "non-infant formula") markets. We are in discussions with many companies to sell products containing our DHA oils for cognitive function, cardiovascular health and other applications. To date, over 200 domestic and international companies have launched non-infant formula products that contain life'sDHA™, most of which remain on the market and are believed to be meeting customer expectations (see "Sales and Marketing" below). Certain of our DHA license and supply agreements with major consumer food products companies establish Martek, subject to certain exceptions, as their exclusive supplier of DHA for minimum periods of time. We, along with our customers and certain third parties, are developing other DHA delivery methods, including powders and emulsions, to facilitate further entry into the non-infant formula markets. Management believes that over the next few years, the non-infant formula markets will continue to expand and could ultimately represent a larger opportunity than infant formula.
Our sales of nutritional oils for products outside of infant formula uses were $39.3 million, $31.3 million and $22.9 million in fiscal 2009, 2008 and 2007, respectively.
Martek is a leading innovator in the development of microbial-based nutritional products that promote health and wellness. We leverage our knowledge of microalgae and other microorganisms and expertise in fermentation sciences and natural product isolation to develop commercially attractive, proprietary and environmentally sustainable sources of nutrients which have proven or emerging health benefits. These processes and use of the products derived from these processes form the basis of our intellectual property estate. Product development involves four major activities: discovery, process development and product formulation, product safety and efficacy evaluation, and scale-up and commercial production.
Physiology/ Discovery—Having established an appropriate nutritional product target, Martek screens its large database of live and preserved, genetically diverse microalgal species to identify candidate microalgal producers. Martek's culture collection consists of microalgal strains that have been isolated from nature by Martek's scientists and those that have been obtained from both public and private culture collections. Martek's culture collection also includes non-microalgal microbial species, which we
13
believe may be increasingly important in the development of future products. Martek's microorganisms have a range of physiological and biochemical characteristics which naturally produce many different lipids, carbohydrates and proteins. Promising candidates are further developed and screened for their ability to meet desired product requirements within the desired cost structure.
Process Development and Product Formulation—Commercial processes for production of candidate products are developed by Martek's scientists and engineers. Martek's processes consist of several basic steps including microbial culture inoculum germination and expansion, fermentation, and product isolation and purification. Martek's scientists utilize a broad range of technical skills and equipment of progressively larger scale to develop reproducible and economical processes. We apply standard industrial microbiological techniques to microorganisms, including classical strain development and culture condition (growth medium composition, temperature, pH) manipulation to optimize product yield and productivity. Martek's expertise in oil processing is broadly applicable to a number of nutrients which are lipid soluble. Finally, Martek develops suitable liquid and dry powder product forms to enable our customers to utilize our products in a broad range of desired consumer products. Martek has invested in extensive lab- and large pilot-scale fermentation and product recovery equipment to enable efficient and cost-effective product development and to support on-going product cost reduction efforts.
While we do not utilize genetically-engineered microorganisms in the production of current commercial products, in the future we may use genetic engineering technology for the production of products at lower-cost or with improved functionality. For example, Martek successfully isolated the genes responsible for producing DHA in one commercial strain of microalgae, and is developing, with a corporate collaborator, the use of these genes to produce low-cost seed oil DHA and LCPUFA products in transgenic terrestrial oilseed crops.
Product Safety and Efficacy Evaluation—In the course of product development, products undergo thorough safety testing and evaluation to assure our ability to reproducibly produce products that are safe and compliant with worldwide regulatory requirements. All commercial products are produced utilizing good manufacturing practices ("GMP") conditions appropriate for the intended food and beverage, supplement, or pharmaceutical market. The health benefits and efficacy of our products are tested and demonstrated utilizing appropriate preclinical animal models and human clinical studies. These studies are conducted by Martek, academic researchers and/or corporate partners affiliated with Martek and currently focus on the areas of brain development, cognitive function, cardiovascular health, immune system health, inflammation, eye development and eye health. Results from these studies are used to establish and support product claims for market development.
Scale-up and Commercial Production—Successful exploitation of the unique characteristics of microalgae and certain other microorganisms is in large measure dependent upon the availability of large-scale culturing technology. We have successfully scaled-up several strains of microalgae capable of producing large amounts of DHA heterotrophically using common organic nutrients and salts. Heterotrophic culturing of these DHA-producing microalgae at commercially viable levels enables significantly lower production costs to be achieved, which were not possible prior to our achievements.
Aspects of our technology are the subject of many U.S. and international patents and patent applications. Martek employs a systematic process to identify, develop, prosecute and defend commercially-valuable intellectual property.
14
LICENSE AND SUPPLY AGREEMENTS AND COLLABORATIONS
Customer Agreements
We are supplying over 35 infant formula customers with our nutritional oils through license and supply agreements. These companies collectively represent approximately 75% of the estimated $15 billion worldwide retail market for infant formula and nearly 100% of the estimated $4.5 billion U.S. retail market for infant formula, including the retail value of Women, Infants and Children program ("WIC") rebates. WIC is a federal grant program administered by the states for the benefit of low-income, nutritionally at-risk women, infants and children. Our customers include infant formula market leaders Mead Johnson Nutritionals, Nestle, Abbott Nutrition, Pfizer (formerly Wyeth) and Danone (formerly Numico), each of whom is selling infant formula supplemented with our nutritional oils. Our pricing for these customers generally follows two models. For the large majority of these agreements, we receive a transfer price per kilogram upon the sale of our oils to our customers. For a limited number of such agreements, we receive a lower transfer price on sales of our oils to our customers plus ongoing royalties based on our customers' sales of infant formula products containing our oils. The most significant license agreements have remaining terms ranging from approximately eight to 19 years, contain no future purchase commitments from our customers, and, generally, may be terminated by our customers upon proper notification, which, in certain cases, only requires short notice periods. In many agreements, our customers have the right to buy other sources of DHA and/or ARA oils. However, in these cases, customers would typically pay us greater amounts for the DHA or ARA that they purchase from us.
Since 2006, we have entered into long-term supply agreements with four of our significant infant formula customers- Mead Johnson Nutritionals, Abbott Nutrition, Danone (formerly Numico) and Pfizer (formerly Wyeth). In general, these supply agreements establish, with limited exceptions, Martek as the exclusive supplier of all DHA and ARA for the respective customer's infant formula products. These agreements have terms of either ten or 15 years with certain rights for either party to terminate the arrangement as of January 1, 2012 provided that the requisite prior written notice is given. With the exception of Danone, whose agreement established a new license arrangement with Martek with a 15-year term, the original license agreements remain in effect and have been incorporated into the new supply agreements. Along with other exclusive DHA and/or ARA supply agreements entered into over the last three years with other infant formula customers, Martek now has sole source commitments, subject to limited exceptions, through at least December 31, 2011 with customers that comprise nearly 80% of Martek's current infant formula revenues.
Under the terms of the infant formula license and supply agreements, our customers are responsible for obtaining all necessary regulatory approvals with respect to the use of these nutritional oils in infant formula products and our customers are generally obligated to indemnify us against product liability claims relating to our nutritional oils unless our nutritional oils do not meet agreed-upon specifications. Under the terms of several of our agreements, we are prohibited from supplying DHA and/or ARA to any party for the inclusion into infant formula with commercial terms that are more favorable to such customer than those provided in our agreements with our current customers without either the prior written consent of the current customers or prospectively offering such new favorable terms to these customers.
Since fiscal 2005, the Company has entered into several license and supply or distribution agreements permitting the direct sale or use of life'sDHA™ in various non-infant formula applications. Certain of these agreements establish the customer as the exclusive customer of life'sDHA™ in a particular food or beverage category or categories or geographic region. Certain of the agreements are for terms of 15 years and establish Martek, subject to certain exceptions, as the licensees' exclusive provider of DHA for minimum periods of time. With limited exceptions, there are no minimum purchase requirements or other financial commitments to Martek under these agreements. Other customer agreements are non-exclusive license and supply agreements that enable the customer to
15
purchase life'sDHA™ on an "as needed" basis, subject to certain limitations. These non-exclusive arrangements generally include product pricing to the customer that is higher than the pricing to our customers who have agreed to use Martek's life'sDHA™ on an exclusive basis.
Collaborations and Third-Party Technology and In-Licensing Agreements
In August 2009, we entered into a collaboration agreement with BP for the joint development of biofuels from microbial oils. Under the terms of the agreement, which is expected to continue through at least 2011, Martek and BP will work together to establish proof of concept for large-scale, cost-effective microbial biodiesel production through fermentation. In connection with this agreement, BP has agreed to contribute up to $10 million to the initial phases of the collaboration, which utilizes Martek's significant expertise in microbial oil production and BP's production and commercialization experience in biofuels as the platform for the joint development effort. Martek will perform the biotechnology research and development associated with the initial phases and receive fees from BP for such efforts. All intellectual property owned prior to the execution of the collaboration agreement will be retained by each respective company, and all intellectual property developed during the collaboration period will be owned by BP, with an exclusive license to Martek for commercialization in nutrition, cosmetic and pharmaceutical applications. Additionally, each party is entitled to certain commercial payments from the counterparty for commercialization of the technology in the other party's fields of use.
In July 2009, we entered into the First Amended and Restated ARA Alliance, Purchase, and Production Agreement (the "Restated Agreement") with DSM Food Specialties B.V. ("DSM"). The Restated Agreement amends and restates our agreement entered into with DSM in 2004 (the "2004 DSM Agreement"). The 2004 DSM Agreement extended the existing relationship between the two companies involving the production and supply of ARA, one of our nutritional oils that we sell to our infant formula customers. Among other things, the 2004 DSM Agreement provided for the grant to Martek by DSM of a license related to certain technologies (including patents) associated with the manufacture of ARA. This grant involved a license fee totaling $10 million, which is being amortized over the 15-year term of the agreement using the straight-line method. The 2004 DSM Agreement, as amended, also provided for the granting to DSM by us of an exclusive license under certain of our patents and intellectual property rights for the production by DSM of products containing ARA for non-human applications, including animal feed products as well as for certain limited human applications. In addition, we and DSM have agreed to contribute our complementary resources to cooperative marketing and joint research and development efforts to expand the applications and fields of use for ARA, with both parties sharing any economic benefits of such efforts. The Restated Agreement, which extends the original supply term through December 31, 2023, amended, consolidated and restated all existing agreements between the two companies governing the cross-licensing, purchase, supply and production of ARA. The licenses and collaborations between the parties noted above remained in effect after the execution of the Restated Agreement. The Restated Agreement is discussed in further detail below in "Production" and in Note 4 of our Consolidated Financial Statements included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K.
In February 2009, we entered into a license agreement with an international food company for certain patented technology expected to be used in the production of Martek's life'sDHA™ for certain applications. Under the agreement, Martek was granted a perpetual and generally exclusive license to the technology. As consideration for this license, we paid an upfront license fee of $1.0 million and will pay, subject to the successful completion by the licensor of certain development goals related to the licensed technology, additional fees of up to approximately $5.0 million. In addition, Martek will be required to pay royalties of up to 4.5% of sales of products produced using the licensed technology, including certain minimum royalty payments of approximately $2.0 million, the timing of which is also subject to the successful completion of the development goals.
16
In December 2003, we entered into a collaboration agreement with a Canadian biotechnology company to co-develop DHA products from plants. In January 2007, an amendment to this agreement was executed, whereby we acquired exclusive license rights to the plant-based DHA technology developed by the co-collaborator for a period of at least 16 years. As consideration for this exclusive license, we made a license payment of $750,000, and the license is subject to minimum royalties of 1.5% of gross margin, as defined, on future sales by us of such plant-based DHA. During the term of the license, we may be required to pay additional royalties of up to approximately 1.0% of gross margin, as defined, on sales of products in the future that utilize certain licensed technologies. To further our plant-based DHA initiatives, in May 2008, we entered into a collaboration agreement with a global biotechnology company to jointly develop and commercialize a canola seed that produces DHA. We and our co-collaborator anticipate a multi-year effort to produce this oil. Our financial commitments associated with this development initiative are subject to the successful completion of identified milestones. As of October 31, 2009, our financial commitment, primarily through internal research efforts, to the first projected milestone date totals approximately $700,000. Commitments thereafter, also primarily through internal research efforts, assuming successful achievement of all identified milestones, total approximately $5.6 million.
We have also entered into various additional collaborative research and license agreements. Under these agreements, we are required to fund research or to collaborate on the development of potential products. As of October 31, 2009, we had no material commitments to fund any future development activities under these arrangements.
We manufacture oils rich in DHA at our production facilities located in Winchester, Kentucky, and Kingstree, South Carolina. The oils that we produce in these facilities are certified Kosher by the Orthodox Union and are certified Halal by the Islamic Food and Nutrition Council of America. Both manufacturing facilities have received favorable ratings by the American Institute of Baking, an independent auditor of food manufacturing facilities, and have obtained the ISO 14001 Environmental Management System ("EMS") International Standard, the most recognized EMS standard in the world. Also, the National Oceanic and Atmospheric Administration has granted Martek's Winchester plant, the Company's primary shipping location, a health certificate, which is required for import of products into many countries, including China and neighboring countries in the Pacific Rim.
Over 90% of our ARA oils are purchased from DSM. Because DSM is a third-party manufacturer, we have only limited control over the timing and level of its production volumes. The balance of our ARA requirements is produced at our Kingstree facility. In July 2009, we entered into the First Amended and Restated ARA Alliance, Purchase, and Production Agreement (the "Restated Agreement") with DSM. The Restated Agreement, which extends the original supply term through December 31, 2023, amended, consolidated and restated all existing agreements between the two parties governing the cross-licensing, purchase, supply and production of ARA. Among other things, the Restated Agreement established ARA pricing to Martek for calendar years 2009 through 2014 with the potential for only limited changes related to post-2011 volumes and provides that ARA pricing subsequent to calendar 2014 will be negotiated in the future by the parties. For the establishment of the 2009 through 2014 ARA pricing and related contractual provisions, we paid DSM approximately $11.0 million. While, subject to the limited exceptions noted below, Martek is committed to purchasing all of its ARA requirements from DSM through the term of the Restated Agreement, the Restated Agreement also sets minimum ARA purchase quantities for Martek in calendar years 2009, 2010 and 2011. As of October 31, 2009, the value of the remaining calendar year 2009 and full calendar years 2010 and 2011 minimum purchase requirements are approximately $17.2 million, $93.9 million and $87.1 million, respectively. These minimum purchase quantities approximate the amounts expected to be consumed by us in the normal course of business.
17
We have attempted to reduce the risk inherent in having a single supplier through certain elements of our supply agreement with DSM. In connection with this agreement, we have the ability to produce, either directly or through an approved third party, an unlimited amount of ARA. The sale of such self-produced ARA is limited annually, however, to the greater of (i) 100 tons of ARA oil or (ii) any amounts ordered by us that DSM is unable to fulfill. Although we currently produce ARA ourselves, our existing manufacturing capacity would not permit us to produce ARA quantities sufficient to meet current demand without impacting our production of DHA. To further improve our overall ARA supply chain, we have directly engaged a U.S.-based provider of certain post-fermentation ARA manufacturing services. Along with our ARA downstream processing capabilities at Kingstree, this third-party facility provides us with multiple U.S. sites for the full downstream processing of ARA.
When combining our current DHA and ARA production capabilities in Kingstree and Winchester with DSM's current ARA production capabilities, we have production capacity for DHA and ARA products in excess of $500 million in annualized sales, collectively, to the infant formula and non-infant formula markets. As such, our production capabilities exceed current demand; however, we have the ability to manage production levels and, to a certain extent, control our manufacturing costs. Nonetheless, when experiencing excess capacity, we may be unable to produce the required quantities of oil cost-effectively due to the existence of significant levels of fixed production costs at our plants and the plants of our suppliers.
The commercial success of our nutritional oils will depend, in part, on our ability to manufacture these oils or have them manufactured at large scale on a routine basis and at a commercially acceptable cost. Our success will also be somewhat dependent on our ability to align our production with customer demand, which is inherently uncertain. There can also be no assurance that we will be able to continue to comply with applicable regulatory requirements, including the Food and Drug Administration's "good manufacturing practice" ("GMP") requirements. Under the terms of several of our agreements with infant formula customers, those customers may elect to manufacture these oils themselves. While our customers are not required to disclose to us that they have begun the process, we are currently unaware of any of our customers producing our oils or preparing to produce our oils, and estimate that it would take a customer a minimum of one year to implement a process for making our oils.
Our raw material suppliers for production of DHA oil include major chemical companies and food and beverage ingredient suppliers. We have identified and validated multiple sources for most of our major ingredients and do not anticipate that the lack of availability of raw materials will cause future production shortages.
Our research and development focus areas include: (i) broadening the scientific evidence supporting our products; (ii) improving manufacturing processes; (iii) developing food and beverage applications; and (iv) developing new products and technologies to expand our market offerings. We perform research and development at our Columbia, Maryland, Boulder, Colorado and Winchester, Kentucky facilities. Our research and development expenditures in fiscal 2009 included, among other things, development activity directed toward (i) improving the quality, sensory properties and stability of our nutritional oils; (ii) developing new ingredient forms and applications technology for DHA-enriched food and beverage products; (iii) optimizing production characteristics of microalgal strains; (iv) investigating the clinical health benefits of DHA and ARA fatty acids; (v) increasing our DHA production yields; (vi) improving our ability to produce ARA; (vii) reducing waste and continuing to improve the overall quality of our oils; (viii) developing feasible approaches to the expression of nutritional fatty acids, especially DHA, in plant oilseeds; (ix) developing biofuels in conjunction with
18
BP; and (x) investigating the feasibility of utilizing our expertise in molecular biology to produce other bioactive compounds with applications in the health and wellness fields. We incurred total research and development expense of approximately $27.4 million, $26.2 million and $24.9 million in fiscal 2009, 2008 and 2007, respectively.
Our nutritional oils are marketed and sold primarily to the infant formula, pregnancy and nursing, food and beverage, dietary supplement and animal feed markets. Currently, we are supplying over 35 infant formula customers with our nutritional oils. These companies collectively represent approximately 75% of the estimated $15 billion worldwide retail market for infant formula and nearly 100% of the estimated $4.5 billion U.S. retail market for infant formula, including the retail value of Women, Infants & Children program ("WIC") rebates. WIC is a federal grant program administered by the states for the benefit of low-income, nutritionally at-risk women, infants and children. Our customers include infant formula market leaders Mead Johnson Nutritionals, Nestle, Abbott Nutrition, Pfizer (formerly Wyeth) and Danone (formerly Numico), each of whom is selling infant formula supplemented with our nutritional oils. Our customers are now selling infant formula products containing our oils collectively in over 75 countries. Supplemented infant formulas from Mead Johnson Nutritionals, Abbott Nutrition, PBM Products, Nestle, Hain Celestial and Nutricia North America are currently being sold in the United States. In addition, certain infant formula customers are selling products in the United States and abroad that contain our nutritional oils and target the markets for children ages nine months to seven years of age and older. One infant formula customer, Mead Johnson Nutritionals, as well as non-infant formula customers including Wal-Mart, CVS/pharmacy, Walgreen's, Everett Laboratories and Mission Pharmacal are selling products containing life'sDHA™ targeted to pregnant and nursing women.
Life'sDHA™ is sold as an ingredient to food and beverage and supplement manufacturers and is also a supplement brand sold directly by Martek. Neuromins® is a Martek supplement brand that is distributed and sold nationwide under license by several supplement manufacturers.
We are currently marketing and selling life'sDHA™, either directly or through distributors, for food and beverage, supplement and animal feed applications to both U.S. and international companies. To date, over 200 domestic and international companies, including Dean Foods, Coca-Cola/Minute Maid, JM Smucker/Crisco, Danone, Starbuck's, Parmalat Australia and Ragasa, have launched non-infant formula products that contain life'sDHA™, most of which remain on the market and are believed to be meeting customer expectations. Generally, these products are also co-branded with the life'sDHA™ logo.
Certain of our DHA license and supply agreements with major consumer food products companies establish Martek, subject to certain exceptions, as their exclusive supplier of DHA for minimum periods of time. Certain of these agreements establish the customer as the exclusive customer of life'sDHA™ in a particular food or beverage category or categories or geographic region. We, along with our customers and certain third parties, are developing other DHA delivery methods, including powders and emulsions, to facilitate further entry into the non-infant formula markets. Management believes that over the next few years, the non-infant formula markets will continue to expand and could ultimately represent a larger opportunity than infant formula.
Consumer marketing efforts are performed primarily by our customers although we play a supportive role. Our infant formula customers market their DHA and ARA supplemented formulas directly to consumers and healthcare professionals. Our dietary supplement, food and beverage and animal feed customers also create and implement their own advertising campaigns. We support these efforts through trade show participation and targeted direct mail campaigns as well as limited advertising and public relations campaigns.
19
Our DHA product is branded as life'sDHA™ and includes the tagline "Healthy brain, eyes, heart" which is designed to be consumer-friendly and to communicate the importance of DHA for health throughout life. Our ARA is branded as life'sARA™. The purpose of this branding initiative is to raise the awareness and understanding of the life'sDHA™ and life'sARA™ brands. Management believes that as consumers learn more about these trusted brands via Martek's, and certain of our customers', marketing and public relations efforts, they will be more likely to purchase products containing the life'sDHA™ and life'sARA™ logos. This, in turn, provides support to Martek's customers by further accentuating the benefits of Martek's products over other sources.
The healthcare and biological sciences industries are characterized by rapidly evolving technology and intense competition. Our competitors include major pharmaceutical, chemical, specialized biotechnology and food and beverage ingredient companies, many of whom have financial, technical and marketing resources significantly greater than ours. In addition, many specialized biotechnology companies have formed collaborations with large, established companies to support research, development and commercialization of products and technologies that may be competitive with our products and technologies. Academic institutions, governmental agencies and other public and private research organizations are also conducting research and development activities that may be competitive with our products. These organizations are seeking patent protection and may commercialize products and technologies on their own or through joint ventures that are competitive with our products and technologies. The existence of products and technologies of which we are not aware, or those that may be developed in the future, may adversely affect the marketability of the products and technologies that we have developed.
Fish oil-based products currently dominate the adult DHA supplement market and certain foods containing fish oils are on the market in various parts of the world. DHA-containing fish oil for infant formula applications provides an alternative to our DHA nutritional oil and is used by certain of our customers and other infant formula manufacturers outside the United States. In addition, in April 2006, the FDA notified Abbott Nutrition (then known as the Ross Products Division of Abbott Laboratories) that it had no questions at that time regarding Ross' conclusion that DHA-rich oil from tuna and ARA-rich oil from Mortierella alpina are safe as sources of DHA and ARA in term and post-discharge preterm infant formulas. While Abbott Nutrition has the regulatory permission to introduce fish oils into infant formula in the U.S., under the terms of the agreement executed by Abbott with us in October 2007, Abbott has agreed to purchase its total needs for DHA and ARA from Martek through at least 2011. Furthermore, we are not aware of any plans by any of our other customers to incorporate this alternative DHA and ARA blend into their infant formulas. The GRAS notification, however, removes a significant regulatory hurdle to the introduction of competitive products in the U.S.
Fish oil is generally less costly than our DHA oil, and therefore presents a substantial competitive threat to our DHA product line. However, certain fish oils have odor, stability and taste characteristics that may limit their usefulness in food and beverage products. In addition, fish oils may be negatively perceived by certain customers. Several companies, including BASF AG, DSM and Ocean Nutrition, and a number of other companies, manufacture microencapsulated fish oil products. Although microencapsulation of the oil resolves many of the odor, stability and taste issues found with fish oil, a microencapsulated product currently is more costly than regular fish oil. Because fish oil is generally less costly than our DHA oil, even when microencapsulated, and continues to improve in quality and gain general market acceptance, fish oil presents a substantial competitive threat.
We continue to work to reduce the costs of our products and to improve the sensory and stability characteristics to make it easier for our customers to incorporate our products into their products. We have also continued to refine our manufacturing processes in order to produce higher levels of DHA and thereby reduce our DHA unit costs. These improvements and changes make our DHA more cost
20
competitive with certain microencapsulated fish oils, on a price per DHA unit basis, but not on a total omega-3 basis due to the presence of large quantities of EPA and other non-DHA omega-3 fatty acids in fish oil.
Published reports have cited certain fish oils as containing chemical toxins not present in our oils. In addition, we believe the combination of low-EPA fish oil with a microbial source of ARA for use in infant formula would likely infringe upon our patent position in several countries.
We believe that our nutritional oils have the following advantages over fish oil and other currently available sources of DHA and ARA for use in infant formula, as food and beverage ingredients, or as supplements:
- •
- our oils, in general, are easier to formulate than certain fish oils in food and beverage products;
- •
- our oils can be blended in a variety of mixtures in precise ratios for specific applications, whereas the composition of
fish oils may vary;
- •
- each of our oils used in infant formula is comprised of a fatty acid blend that does not contain certain other fatty
acids, such as EPA, in significant quantities, which may not be appropriate for consumption by infants at high levels.
- •
- our oils do not contain substances found in certain fish and fish oils such as methylmercury, polychlorinated biphenyls
("PCBs"), dioxins and other toxic contaminants;
- •
- our oils have a higher oxidative stability and longer shelf life than certain fish oils and are, therefore, more amenable
to the spray drying process required for powdered formula;
- •
- our oils are not produced from animal sources and, therefore, should be more desirable for use in food and beverage
products, as the available market includes consumers who require a vegetable-sourced DHA, unlike fish oil;
- •
- our oils are produced from renewable, sustainable natural resources, unlike fish oil;
- •
- our DHA and ARA-enriched oils are in an easily digestible triglyceride form similar to that found in fish oils
and breast milk, but different from the phospholipid form found in egg yolk lipids; and
- •
- our oils can be produced in large quantities under controlled conditions satisfying strict regulatory scrutiny.
At this time, our oils are the only DHA and ARA oils used in infant formula in the U.S.
Suntory Limited, Nippon Suisan, Cargill Inc. (through a joint venture with a company in China) and other independent Chinese manufacturers are producing and distributing a fungal source of ARA. Monsanto/Solae are expecting to commercialize an inexpensive, sustainable, vegetarian omega-3 for foods and beverages in the next one to three years. This product is a soybean oil containing stearidonic acid ("SDA"). Monsanto/Solae are positioning SDA as a precursor to EPA, in addition to DHA, the other omega-3 found in fish oil. In addition, we are aware that there may be manufacturers in China and India producing or attempting to produce an algal source of DHA, but we are uncertain of the overall status and commercial potential of these development efforts. In addition, other companies, several with greater financial resources than ours, are developing plant-based DHA, other plant-based oils or, in the case of DuPont, an EPA product derived from yeast, that may compete with us, and other companies may be developing chemically synthesized DHA.
Small amounts of DHA and ARA can be derived from egg yolk lipids, but DHA and ARA of this type are not in the same molecular form as that predominantly found in breast milk (i.e., phospholipid vs. triglyceride). DHA and ARA derived from egg yolks are currently being added to some brands of infant formula marketed by several small companies. We believe that the processes to produce DHA
21
and ARA from egg lipids are more costly than the processes that we use for producing DHA and ARA from microbial sources. Furthermore, the addition of DHA and ARA from egg yolks at levels equivalent to those found in human breast milk may result in dietary levels of lecithin and cholesterol in excess of those found in human breast milk.
In December 2005, Lonza Group LTD, a Swiss chemical and biotechnology group, acquired from Nutrinova Nutrition Specialties & Food Ingredients GmbH, a wholly-owned subsidiary of Celanese Corporation, Nutrinova's business having as its product a DHA-rich microalgal oil. Since the acquisition, Lonza has actively marketed its DHA oil to the food and beverage and dietary supplement markets in Europe and China, and was actively marketing in the United States. In addition, Lonza may be actively marketing its DHA oil for infant formula applications in certain markets. Nutrinova and Lonza are defendants in patent infringement actions involving our DHA patents that we have brought in both the United States and Germany, and Lonza is a defendant in a patent infringement action in France. One of Nutrinova's customers is also a defendant in these actions in Germany. In connection with these patent lawsuits, the Company has incurred and capitalized significant external legal costs. As of October 31, 2009, the patents being defended in the Lonza matter had a net book value of approximately $4.4 million, including capitalized legal costs, which is being amortized over a weighted average remaining period of approximately five years. These lawsuits are further described in Item 3. "Legal Proceedings" of Part I of this Form 10-K.
GlaxoSmithKline is currently selling LOVAZA™, a prescription DHA/ EPA ethyl ester for treatment of hyperlipidemia. LOVAZA™ is a lipid-regulating agent which includes both EPA and DHA from fish oil. GlaxoSmithKline has filed an application with the FDA for an indication that will expand the use of LOVAZA™ and is actively evaluating new indications. Other pharmaceutical companies offering other applications using omega-3 fatty acids may be expected.
There may be other competitive sources of DHA and ARA of which we are not aware. The fact that many of the companies mentioned above are larger, more experienced and better capitalized than Martek raises the significant risk that these companies may be able to use their resources to develop less costly sources of DHA and ARA than our current technology permits.
Our competitive position will also depend on our ability to attract and retain qualified scientific and other personnel, develop effective proprietary products, implement production and marketing plans, obtain and maintain patent protection and secure adequate capital resources.
PATENTS, LICENSES AND PROPRIETARY TECHNOLOGY
We have received numerous patents protecting our nutritional products technology, including the fermentation methods of producing our DHA and ARA oils, as well as the blending and use of certain DHA and/or ARA oils in infant formula. In 1994, we received a U.S. patent covering certain blends of a microbial oil enriched with DHA and a microbial oil enriched with ARA, as well as the use of such blends in infant formulas. In 1995, we received a U.S. patent covering a process for making an edible oil containing DHA under certain specified conditions and the edible oil made by such process as well as a U.S. patent covering an infant formula comprising an edible DHA-containing oil with certain specified characteristics. In 1996, we received two additional U.S. patents covering our nutritional oils technology. The first patent protects pharmaceutical compositions and dietary supplements comprising a single cell oil in concentrations of at least 20% DHA in a triglyceride form made using our method of producing DHA oil. The second patent clarifies that our patent coverage includes the blending, in infant formula and dietary supplements, of microbially derived ARA oil with low EPA fish oils. Fish oil is a potential competitive source of DHA to Martek's algal-derived DHA oil. This patent makes it more difficult for low EPA fish oils to be combined with microbial sources of ARA oils in the U.S. without violating our patents. A U.S. patent was granted in 1997, which protects the production, use and sale of certain microbial oils rich in ARA. In 1998, a U.S. patent was issued protecting our
22
DHA-rich algal biomass. DHA-rich algal biomass is the raw product of the DHA fermentation process and represents an inexpensive source of DHA that may potentially be a low cost product itself. We also have been awarded a number of foreign patents covering various aspects of our nutritional oils, including European patents covering our DHA and ARA-rich oils.
We also own patents and applications that cover certain algal fermentation processes, lipid extraction/purification, genomic-based approaches to lipid production, arachidonic acid production and use, animal feeding protocols, and food and beverage applications for LCPUFAs. From 1992 to 2006, eight U.S. patents owned by us were issued covering the use of certain algae in the production of omega-3 LCPUFAs (e.g. DHA-S), and the use of such LCPUFAs in such products as human foods and beverages, animal feed, aquaculture and the resulting supplemented meat, seafood, milk and eggs. Four of these patents have now expired. From 1994 to 2008, twelve U.S. patents were issued covering, among other things, the fermentation of microorganisms in low chloride fermentation medium and their use in aquaculture applications. Seven of these patents have now expired. Additional patent applications covering this technology are still pending. Other U.S. patents have been issued and a number of patents are pending worldwide.
Our success is largely dependent on our ability to obtain and maintain patent protection for our products, maintain trade secret protection and operate without infringing the proprietary rights of others. Our policy is to aggressively protect our proprietary technology through patents, where appropriate, and in other cases, through trade secrets. Additionally, in certain cases, we rely on the licenses of patents and technology of third parties. We hold approximately 80 U.S. patents, covering various aspects of our technology, which will expire on various dates between 2009 and 2026. Our core infant formula-related U.S. patents expire between 2011 and 2014. We also license from DSM certain patents that provide additional protection for our nutritional oil products and expire between 2017 and 2024, and certain patent applications that could, if granted, provide added protection for our nutritional oil products. Martek has been granted U.S. patents covering certain processes for producing DHA-containing oil that may be used in foods and beverages which expire between 2010 and 2024. In addition, Martek has several pending patents related to DHA, including products and processes, which could offer long-term protection. We have filed, and intend to file, applications for additional patents covering both our products and processes as appropriate. While we have sole source supply agreements (in most cases through 2011) with customers comprising nearly 80% of our current infant formula revenues, it is uncertain how much protection that our patents or those in-licensed from DSM expiring after 2011 will provide should these customers choose ingredients from our competitors. Furthermore, even if our customers continue to use our oils, direct competition could force us to reduce the price of our products, which could materially affect future revenues and product margins.
Currently, we have over 350 issued patents and over 500 pending patent applications worldwide. There can be no assurance that:
- •
- any patent applications filed by, assigned to or licensed to us will be granted;
- •
- we will develop additional products that are patentable;
- •
- any patents issued to or licensed by us will provide us with any competitive advantages or adequate protection for
inventions;
- •
- any patents issued to or licensed by us will not be challenged, invalidated or circumvented by others;
- •
- issued patents, or patents that may be issued, will provide protection against competitive products or otherwise be
commercially valuable; or
- •
- we will be successful in securing arrangements with our customers after patent expirations that will provide economic protection similar to patents.
23
Patent law relating to the scope of claims in the fields of healthcare and biosciences is still evolving, and our patent rights are subject to this uncertainty. European, United States and Asian patent authorities have not adopted a consistent policy regarding the breadth of claims allowed for health and bioscience patents. Our products might infringe the patent rights of others, whether existing now or in the future. Similarly, the products of others could infringe our patent rights. The defense and prosecution of patent claims are both costly and time consuming, even if the outcome is ultimately in our favor. An adverse outcome in infringement actions by Martek against third parties could reduce or eliminate any competitive advantage provided by the affected Martek patent rights. An adverse outcome in infringement actions by third parties against Martek could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties and/or require us to cease selling the affected products.
In certain competitive geographic markets, we do not have patent protection and may be unable to obtain it. In other competitive markets, we may be unable to maintain (through patent expiration or otherwise) the patent protection for our nutritional oils currently afforded to us. A lack of patent protection would have a material adverse effect on our ability to gain a competitive advantage for our oils and may have a material adverse effect on our results of operations, especially on future sales of our nutritional oils. In particular, a lack of patent protection would permit our competitors to manufacture products that would be directly competitive with our nutritional oils using similar or identical processes, and it is possible that our current infant formula, supplement, or food and beverage customers, or those that may be under license in the future, may choose ingredients from our competitors if they choose to include the ingredients at all.
It is our corporate policy to vigorously protect our substantial investment in the research and development of our products and to continue to enforce our patent and other intellectual property rights against third parties who engage in the unauthorized manufacture, sale, or use of our technology. We currently have several challenges to our European patents covering our DHA and ARA oils and an anonymous party has filed Requests for Reexamination with respect to two of Martek's U.S. blended oils patents and one U.S. ARA patent. In addition, Lonza has filed Requests for Reexamination with respect to nine of Martek's DHA patents (four of which have now expired). These challenges, as well as our lawsuit against others for infringement of our patents, are described in Item 3. "Legal Proceedings" of Part I of this Form 10-K. Total patent litigation expenditures were approximately $4.3 million, $1.6 million and $2.3 million in fiscal 2009, 2008 and 2007, respectively.
We expect that, in the future, as our nutritional oils continue to be commercialized, opposition to our patents and other intellectual property by our competitors will continue and most likely increase. We may incur substantial costs in the future protecting and defending our patents and other intellectual property rights and cannot be sure that we will be able to successfully defend our patents or that our competitors will not be able to "design around" our intellectual property.
We also rely on trade secrets and proprietary know-how, which we seek to protect in part by confidentiality agreements with our collaborators, employees and consultants. There can be no assurance that these agreements will not be breached, that we will have adequate remedies for any such breach or that our trade secrets will not otherwise become known or be independently developed by competitors.
24
GOVERNMENT REGULATION AND PRODUCT TESTING
Our products and our manufacturing and research activities are subject to varying degrees of regulation by state and federal regulatory authorities in the United States, including the FDA pursuant to the Federal Food, Drug and Cosmetic Act. The products developed by us are subject to regulation by the FDA as food and beverage ingredients, dietary supplements, animal feed, drugs and/or medical devices. The regulatory status of any product is largely determined by its intended use.
Drugs and medical devices generally may not be marketed without first obtaining FDA authorization to do so. New infant formulas also are subject to premarket notification requirements. Although there are no premarket authorization requirements for whole foods per se, there are premarket approval requirements for food and beverage additives. Specifically exempt from the food additive definition and, therefore, the premarket approval requirements, are generally recognized as safe food, beverage and animal feed ingredients. FDA's Center for Food Safety and Applied Nutrition is responsible for the regulation of human food products. The Center for Veterinary Medicine ("CVM") is responsible for the regulation of animal food (feed) products. Dietary supplements for the most part are not subject to premarket authorization requirements, although there is a premarket notification requirement for certain new dietary ingredients that were not marketed as dietary supplements prior to October 1994. The FDA has established detailed GMP, labeling and other requirements for drugs, medical devices, infant formulas, foods and beverages and dietary supplements. The requirements for drugs, medical devices and infant formulas generally are much more stringent than the requirements for dietary supplements and foods and beverages.
Our infant formula customers are responsible for obtaining the requisite regulatory clearances to market their products containing our oils. Sales of our products outside the United States are subject to foreign regulatory requirements that may vary widely from country to country.
In May 2001, the FDA completed a favorable review of our generally recognized as safe ("GRAS") notification for the use of our DHASCO® and ARASCO® oil blend in specified ratios in infant formulas. Since the first product introduction in February 2002, supplemented infant formulas manufactured by six of our customers, Mead Johnson Nutritionals, Abbott Nutrition, PBM Products, Nestle, Hain Celestial and Nutricia North America, have been sold in the United States.
The FDA regulates the use and marketing of dietary supplements under the provisions of the Dietary Supplement Health and Education Act of 1994 ("DSHEA"). We are currently selling several lines of DHA dietary supplements. In addition, we are researching and developing new applications for our DHA and ARA oils. We believe that our DHA and ARA are not new dietary ingredients and, as such, are not subject to premarket notification requirements when marketed for use as dietary supplements. There can be no assurance that the FDA would agree that a premarket notification is not required or that we will be able to comply with the requirements of DSHEA or any regulations that the FDA may promulgate thereunder.
In June 2002, the Australia New Zealand Food Authority authorized the use of DHA-S oil for use as a novel food ingredient in Australia and New Zealand. In June 2003, the European Commission authorized the use of our DHA-S oil as a novel food ingredient in certain foods in the European Community. This novel food designation authorizes the use of our DHA-S as an ingredient in certain foods such as certain dairy products, including cheese and yogurt, spreads and dressings, breakfast cereals, food supplements and dietary foods for special medical purposes in the European Community. In July 2009, the Standing Committee of the European Commission and the 27 Member States approved the expanded use of our DHA-S algal oil in beverages, bakery products and nutrition bars. Official approval for the new uses for DHA-rich oil in food throughout the European Union was achieved and recognized via publication in the Official Journal of the European Union in October 2009. In February 2004, the FDA completed a favorable review of our GRAS notification for the use of DHA-S in food and beverage applications. In October 2006, Health Canada approved per serving levels
25
of Martek's DHA-S of not less than eight mg and not more than 100 mg of DHA when used as a food ingredient. In June 2007, we received approval for the use of our DHA-S oil in food and beverages in Mexico. In August 2007, the Ministry of Health in China authorized the use of our life'sDHA™ as a novel food ingredient. This designation permits the use of life'sDHA™ in foods, beverages and supplements in China for persons older than three years. This initial approval by the Ministry of Health is part of the regulatory process applicable to Chinese novel foods. In December 2007, China issued a new Novel Food Regulation requiring that a new application be filed. We have therefore prepared a new application for submission in order to obtain approval under this new regulation. In March 2008, the Israeli Ministry of Health approved DHA-rich oil from Schizochytrium (DHA-S) patterned after the 2003 approval in Europe. In 2008, the Ministry of Health Labor and Welfare in Japan confirmed that DHA algal oil is also regarded as a traditional food and, as such, is acceptable for use in foods and beverages and dietary supplements.
The regulatory status of an animal feed product is determined by CVM on a case-by-case basis. FDA cooperates with the Association of American Feed Control Officials ("AAFCO") and state authorities for the implementation of uniform policies for regulating the use of animal feed products. This includes the establishment of uniform feed ingredient definitions and proper labeling to assure the safe use of feeds. We believe that our animal feed ingredient is acceptable for use by classification under an existing AAFCO feed ingredient definition; however, there can be no assurance that the FDA would agree with this determination. Other products derived from microalgae and other organisms may be subject to potential regulation by the FDA as either medical devices or as a combination medical device/drug product to the extent that they are used in the diagnosis, mitigation, treatment, cure or prevention of diseases. Such classification would subject the products to premarket clearances and/or regulatory approvals. There can be no assurances that we, our customers or collaborators would be able to develop the extensive safety and efficacy data needed to support such FDA premarket authorizations or that the FDA ultimately would authorize the marketing of such products on a timely basis, if at all.
For potential pharmaceutical uses of products derived from microalgae and other organisms, there can be no assurance that required clinical testing will be completed successfully within any specified time period, if at all, with respect to our products. Additionally, there is no assurance that we, our customers or collaborators will be able to develop the extensive data needed to establish the safety and efficacy of these products for approval for drug uses, or that such drug products will not be subject to regulation as biological products or as controlled substances, which would affect marketing and other requirements.
Some of our products are in research or development phases. We cannot predict all of the regulatory requirements or issues that may apply to or arise in connection with our products. Changes in existing laws, regulations or policies and the adoption of new laws, regulations or policies could prevent us, our customers or collaborators from complying with such requirements.
Due to the cost and time commitment associated with the FDA regulatory process, we will decide on a product-by-product basis whether to handle relevant clearance and other requirements independently or to assign such responsibilities to our customers or future collaborative partners. There can be no assurance that we, our customers or collaborators will be able to obtain such regulatory clearances, if required, on a timely basis or at all. Delays in receipt of, or failure to receive, such clearances, the loss of previously received approvals or clearances, or failure to comply with existing or future regulatory requirements would have a material adverse effect on our business, financial condition and results of operations.
In connection with the manufacture of most of our products, we are required to adhere to applicable current "good manufacturing practice" ("GMP") regulations as required by the FDA. GMP regulations specify production and process controls, component and product testing standards, quality control and quality assurance requirements, and records and other documentation controls. The GMP
26
requirements for foods and beverages, infant formulas, drugs and medical devices vary widely. As a manufacturer of DHA and ARA that are marketed as dietary supplements and used as ingredients in infant formulas sold in the United States and in foods and beverages, we are subject to GMP and various other requirements applicable to such products. There can be no assurance that we will be able to continue to manufacture our nutritional oils in accordance with relevant food and beverage, dietary supplement and infant formula requirements for commercial use. Ongoing compliance with GMP and other applicable regulatory requirements is monitored through periodic inspections by state and federal agencies, including the FDA and comparable agencies in other countries. A determination that we are in violation of such GMP and other regulations could lead to an interruption of our production output and the imposition of civil penalties, including fines, product recalls or product seizures, and, in the most egregious cases, criminal sanctions.
As large-scale manufacturing facilities, our plants in Winchester, Kentucky and Kingstree, South Carolina are required to abide by applicable federal and state environmental and safety laws, including regulations established by the Environmental Protection Agency and the Occupational Safety and Health Administration and similar state agencies. In addition, our solvent extraction processes include the use of hexane, which is extremely flammable and subject to emission requirements. If we fail to abide by these laws we could receive fines, or if the violations were serious enough, our operations could be shut down or restricted until the problems are fixed. Such penalties could have a material adverse effect on our ability to manufacture our nutritional oils, and our financial results could be negatively impacted. While the costs of our compliance with environmental laws and regulations cannot be predicted with certainty, such costs are not expected to have a material adverse effect on our earnings or financial or competitive position.
The Federal Trade Commission ("FTC") regulates certain aspects of the advertising and marketing of our products. Under the Federal Trade Commission Act, a company must be able to substantiate both the express and implied claims that are conveyed by an advertisement. It is not uncommon for the FTC to conduct an investigation of the claims that are made about products in new and emerging areas of science that involve a potentially vulnerable population such as infants, or that relate to conditions impacting significant portions of the population. An adverse action by the FTC could have a negative impact on our results of operations and financial condition.
As of October 31, 2009, we had 587 full-time employees, 42 of whom have Ph.D.s. Approximately 139 employees are engaged in research and development activities, 283 are engaged in production or production development related activities and 165 are in administrative, business development and sales and marketing positions. We consider relations with our employees to be good. None of our employees is covered by a collective bargaining agreement.
27
EXECUTIVE OFFICERS OF THE REGISTRANT
Our executive officers are as follows:
Name
|
Age | Position | |||
|---|---|---|---|---|---|
Steve Dubin |
56 | Chief Executive Officer and Director | |||
David M. Abramson |
56 | President | |||
Peter L. Buzy |
50 | Chief Financial Officer, Treasurer and Executive Vice President for Finance and Administration | |||
David M. Feitel |
46 | Executive Vice President and General Counsel | |||
Peter A. Nitze |
51 | Chief Operating Officer and Executive Vice President | |||
Barney B. Easterling |
64 | Senior Vice President, Manufacturing | |||
Mr. Dubin became Chief Executive Officer of Martek in June 2006 after serving since September 2003 as President of Martek. Mr. Dubin joined Martek in 1992 and has served in various management positions, including CFO, Treasurer, Secretary, General Counsel and Senior Vice President of Business Development. In 2000, he moved to a part-time position of Senior Advisor—Business Development, a role he filled until his election to President of Martek in September 2003. He also spent time during 2000 through 2003 co-founding and co-managing a Maryland-based, angel-investing club that funds early-stage, high-potential businesses. He was also "Of Counsel" to the law firm Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. during part of 2001 and 2002. Prior to 1992, Mr. Dubin worked in the financing and management of early-stage businesses and, over a period of 12 years, served in various positions at Suburban Bank, now part of Bank of America, including Vice President and Treasurer of their venture capital subsidiary, Suburban Capital Corporation. Mr. Dubin received a B.S. in accounting from the University of Maryland and a Juris Doctor degree from the National Law Center at George Washington University. Mr. Dubin is a Certified Public Accountant and a member of the Maryland Bar. Mr. Dubin has been a director of Martek since July 2006. His term expires in 2009.
Mr. Abramson joined Martek in 2003 as head of Corporate Development and was elected President in September 2006. Prior to joining Martek, he was the Executive Vice President and General Counsel for U.S. Foodservice from 1996 to 2003. In this position, Mr. Abramson oversaw the legal and regulatory affairs of U.S. Foodservice, a large foodservice distributor in the United States, and advised on business development opportunities for this company. U.S. Foodservice became a subsidiary of Royal Ahold in 2000. In addition, Mr. Abramson was also the Executive Vice President for Legal Affairs at Ahold, U.S.A. from 2000 to 2003. Mr. Abramson also served on the Board of Directors of U.S. Foodservice from 1994 to 2003. Prior to joining U.S. Foodservice, from 1983 until 1996, Mr. Abramson was a partner at Levan, Schimel, Belman & Abramson, P.A., now a part of Miles & Stockbridge P.C. Mr. Abramson graduated from George Washington University in 1975, where he obtained a Bachelor of Business Administration in accounting. He received his Juris Doctor degree, with honors, from the University of Maryland School of Law in 1978. Mr. Abramson is a member of the Maryland Bar.
Mr. Buzy joined Martek in 1998 as Chief Financial Officer. Prior to joining Martek, Mr. Buzy spent 13 years with the accounting firm of Ernst & Young LLP, most recently as an audit partner in the Northern Virginia High Technology/Life Sciences Practice. Mr. Buzy is a Certified Public Accountant and a member of the American Institute of Certified Public Accountants. He received his B.S. in accounting from Salisbury University.
Mr. Feitel joined Martek in 2004 as Associate General Counsel and was elected to the position of Senior Vice President and General Counsel in December 2006 and later to Executive Vice President and General Counsel in November 2008. From 2003 until joining Martek, he practiced law at
28
Miles & Stockbridge P.C., where he had started his legal career in 1988. From 2000 to 2003, Mr. Feitel was the Vice President and General Counsel of BCE Emergis, an eCommerce service provider and a subsidiary of Bell Canada. Prior to BCE Emergis, Mr. Feitel worked for the Discovery Group, a Columbus, Ohio-based venture capital company, from 1997 through 2000. Mr. Feitel received his undergraduate degree from Duke University and his Juris Doctor from the Duke University School of Law in 1988.
Mr. Nitze joined Martek in 2005 as Chief Operating Officer. Prior to joining Martek, Mr. Nitze served as Vice President of Operations at DRS Technologies from April 2004 to July 2005, with responsibility for the alignment and deployment of the company's manufacturing and supply chain resources. Before joining DRS Technologies, Mr. Nitze served as the Chief Operating Officer of Regulatory DataCorp, a New York City firm that provides risk management services to financial services institutions, from July 2002 to April 2004. Prior to joining Regulatory DataCorp, Mr. Nitze was the business leader of the Optoelectronics venture at Honeywell International from February 2000 to November 2001, where he had previously served as the head of global operations for the Amorphous Metals division. Mr. Nitze began his career at General Electric Co. in finance and subsequently held a variety of positions in engineering, marketing, supply chain and operations management. Mr. Nitze has over 20 years of operations and general management experience with small, medium and large companies. He holds two M.S. degrees in engineering from Stanford University and a B.A. degree from Harvard University.
Mr. Easterling joined Martek in 2003 in connection with Martek's acquisition of FermPro Manufacturing, LP ("FermPro"). With the acquisition, he was named Vice President of Manufacturing of Martek, and in March 2004, he was elected to the position of Senior Vice President of Manufacturing. From 1994 to 2003, Mr. Easterling served as President and CEO of FermPro, a provider of contract fermentation services. From 1980 to 1994, Mr. Easterling served in various management capacities for Gist-Brocades. He received a B.S. in premedicine from Clemson University.
Martek was incorporated in Delaware in 1985. Martek's principal executive offices are located at 6480 Dobbin Road, Columbia, Maryland 21045. Our telephone number is (410) 740-0081 and our website address is http://www.martek.com. We make our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to these reports available on our website free of charge as soon as practicable after we file with the SEC.
Financial information prepared in accordance with U.S. generally accepted accounting principles, including information about revenues from customers, measures of profit and loss, total assets, financial information regarding geographic areas and export sales, can be found in our Consolidated Financial Statements included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K.
29
Investing in our securities involves a high degree of risk. Before making an investment decision, you should carefully consider the risk factors set forth herein, as well as other information we include in this report and the additional information in the other reports we file with the Securities and Exchange Commission (the "SEC" or the "Commission"). If any of the following risks actually occur, our business could be harmed. In such case, the trading price of our securities could decline and you could lose all or part of your investment.
Current economic and market conditions could adversely affect our revenue and profitability.
The United States economy and the global economy are recovering from a severe recession. Due to factors such as uncertainties in consumer spending, a sustained regional and/or global economic downturn or slow recovery may continue to reduce the demand for our nutritional oils. If demand for our oils continues to decline, our revenue and profitability will be adversely affected. Furthermore, challenging economic conditions also may impair the ability of our customers to pay for products they have purchased and may impair the ability of our vendors to supply us with items critical to the operation of our business. Any of these negative occurrences could adversely impact our operating results, financial condition or business, in general.
A substantial portion of our nutritional oil products sales is made to five of our existing customers under agreements with no minimum purchase requirements. If demand by these customers for our nutritional oil products decreases, our revenues may materially decline.
We rely on a substantial portion of our product sales to five of our existing customers. Approximately 77% of our product sales revenue during the year ended October 31, 2009 was generated by sales of DHA and ARA to five customers: Mead Johnson Nutritionals, Abbott Nutrition, Nestle, Pfizer (formerly Wyeth) and Danone (formerly Numico). We cannot guarantee that these customers will continue to demand our nutritional products at current or predictable levels. None of our license agreements requires our licensees to purchase any minimum amount of products from us now or in the future, and certain of our license agreements can be terminated within short periods and also allow our customers to manufacture our products themselves or purchase nutritional oils from other sources. We have limited visibility into our customers' future actual level of demand, notwithstanding our view of consumer demand. We have sole source supply agreements (in most cases through 2011) with customers comprising nearly 80% of our current infant formula revenues. If demand by any of our significant customers for our nutritional products decreases, either prior to or subsequent to the expiration of such supply agreements, we may experience a material decline in our revenues. Furthermore, if purchasing patterns by our significant customers continue to be uneven or inconsistent, we will likely experience fluctuations in our quarter-to-quarter revenues and cash flows. In addition, if these customers attempt to utilize their purchasing power in order to receive price reductions on our products, we may be unable to maintain prices of our oils at current levels, which could materially affect future revenues and product margins.
Our major customers are part of either the pharmaceutical or food and beverage industries. Mergers and acquisitions are prevalent in both industries. If one of our major customers or divisions thereof are acquired, as there are no minimum purchase requirements in our license agreements with them, there is no guarantee that the acquirer will continue purchasing our oils at current levels or continue selling infant formula at all. An acquisition of one of our major customers could have a material effect on future revenues.
Our major customers also employ differing strategies with respect to the timing of their inventory and raw material purchases. To the extent that these strategies change (i.e., further advancements to a "just-in-time" procurement process), our revenues in the quarter of such change could be materially
30
affected by this modification in customer ordering patterns. In addition, our major customers use varying inclusion levels of DHA and ARA in their infant formulas. If significant changes in their market shares occur, or, in general, our customers reduce such inclusion levels, we could experience material changes in our infant formula revenues.
If we are unable to obtain or maintain patent protection or if our patents do not provide protection or we are unable to obtain comparable protection after patent expiration against competitive products, our results of operations may be adversely affected.
Our success is largely dependent on our ability to obtain and maintain patent protection for our products, maintain trade secret protection and operate without infringing the proprietary rights of others. Our policy is to aggressively protect our proprietary technology through patents, where appropriate, and in other cases, through trade secrets. Additionally, in certain cases, we rely on the licenses of patents and technology of third parties. We hold approximately 80 U.S. patents, covering various aspects of our technology, which will expire on various dates between 2009 and 2026. Our core infant formula-related patents expire between 2011 and 2014. We also license from DSM certain patents that provide additional protection for our nutritional oil products and expire between 2017 and 2024, and certain patent applications that could, if granted, provide added protection for our nutritional oil products. We have been granted U.S. patents covering certain processes for producing DHA-containing oil that may be used in foods and beverages which expire between 2010 and 2024. We have filed, and intend to file, applications for additional patents covering both our products and processes as appropriate. While we have sole source supply agreements (in most cases through 2011) with customers comprising nearly 80% of our current infant formula revenues, it is uncertain how much protection that our patents or those in-licensed from DSM expiring after 2011 will provide should these customers choose ingredients from our competitors. Specifically, as discussed more fully in Item 3. "Legal Proceedings" of Part I of this Form 10-K, our U.S. ARA patent expiring in 2014 is under reexamination challenge and will likely emerge from this process with narrower claims. It is probable that the broader product claims will not survive. Rather, the patent will likely contain narrower product claims and process claims that, among other things, would provide patent protection to a production process that we believe: i) results in higher quality oil and higher ARA potency and ii) is cost efficient compared to other processes for producing ARA. When combined with our in-licensed patents from our ARA production partner DSM, we believe that it will be technologically difficult and less cost efficient for competitors to design around these claims. Nonetheless, it is uncertain how much protection they will provide. Furthermore, even if our customers continue to use our oils, direct competition could force us to reduce the price of our products, which could materially affect future revenues and product margins.
Currently, we have over 350 issued patents and over 500 pending patent applications worldwide. There can be no assurance that (i) any patent applications filed by, assigned to or licensed to us will be granted; (ii) we will develop additional products that are patentable; (iii) any patents issued to or licensed by us will provide us with any competitive advantages or adequate protection for inventions; (iv) any patents issued to or licensed by us will not be challenged, invalidated or circumvented by others; (v) issued patents, or patents that may be issued, will provide protection against competitive products or otherwise be commercially valuable; or (vi) we will be successful in securing arrangements with our customers after patent expirations that will provide economic protection similar to patents. Furthermore, patent law relating to the scope of claims in the fields of healthcare and biosciences is still evolving, and our patent rights are subject to this uncertainty. European, United States and Asian patent authorities have not adopted a consistent policy regarding the breadth of claims allowed for health and bioscience patents. Our products might infringe the patent rights of others, whether existing now or in the future. Similarly, the products of others could infringe our patent rights. The defense and prosecution of patent claims are both costly and time consuming, even if the outcome is ultimately in our favor. An adverse outcome in infringement actions by Martek against third parties could reduce or
31
eliminate any competitive advantage provided by the affected Martek patent rights. An adverse outcome in infringement actions by third parties against Martek could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties and/or require us to cease selling the affected products.
In certain competitive geographic markets, we do not have patent protection and may be unable to obtain it. In other competitive markets, we may be unable to maintain (through patent expiration or otherwise) the patent protection for our nutritional oils currently afforded to us. A lack of patent protection would have a material adverse effect on our ability to gain a competitive advantage for our oils and may have a material adverse effect on our results of operations, especially on future sales of our nutritional oils. In particular, a lack of patent protection would permit our competitors to manufacture products that would be directly competitive with our nutritional oils using similar or identical processes, and it is possible that our current infant formula, supplement, or food and beverage customers, or those that may be under license in the future, may choose ingredients from our competitors if they choose to include the ingredients at all.
There are a number of intellectual property proceedings pending against Martek or that Martek has pending against third parties. See Item 3. "Legal Proceedings" of Part I of this Form 10-K for further information. If any of these challenges or any other challenges to our patents that we do not currently consider material or that may arise in the future are successful and we are unable to secure customer arrangements upon patent revocation or expiration that would ensure continuity of our customers' purchases from us or obtain comparable protection after patent expiration through other patents, we may experience decreases in the future sales of our nutritional oils or we may be forced to reduce the price of our products due to our competitors' ability to produce similar products, both of which could cause decreases in future revenues as well as product margins. Specifically, the revocation of our European DHA patent or ARA patent could result in a decrease in revenues under our license agreements. Furthermore, it is our accounting policy to capitalize legal and related costs incurred in connection with patent applications and the defense of our patents. As of October 31, 2009, the net book value of our patent assets totaled $16.7 million, which includes approximately $4.4 million of costs related to our patent defenses in the Nutrinova/ Lonza matters discussed above, which is being amortized over a weighted average remaining period of approximately five years. If, in the future, it is determined to be unlikely that our patents will be successfully defended in connection with the challenges described above or if it is concluded that certain of our patents will no longer provide an economic benefit to the Company, a write-off of the costs ascribed to the particular patent or patents would be required. The effect of such write-off could be material to our results of operations.
We expect that in the future, as our nutritional oils continue to be commercialized, opposition to our patents and other intellectual property by our competitors will continue and most likely increase. We may incur substantial costs in the future protecting and defending our patents and other intellectual property rights and cannot be sure that we will be able to successfully defend our patents or that our competitors will not be able to "design around" our intellectual property. We also expect that in the future, third parties may allege that Martek infringes their intellectual property rights, which could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties and/or require us to cease selling the affected products.
We are aware of several products that are currently available, and products under development, that may present a serious competitive threat to our products. If we are unable to maintain a competitive differentiation from these products, our revenues may be adversely affected.
Our continued success and growth depends upon achieving and maintaining a superior competitive position in the infant formula, supplement and food and beverage product markets. Potential competitors include companies such as BASF AG, DSM, Cargill Inc., Nippon Suisan, Suntory Limited, Archer Daniels Midland Company, Lonza Group LTD, Nagase & Co. Ltd., Ocean Nutrition ("ONC")
32
and Monsanto. Many of these companies have substantially greater research and development capabilities, marketing, financial and managerial resources and experience in the industry. Some of our competitors are currently offering, at prices less than ours, competing sources of DHA and/or ARA for use in the food and beverage and dietary supplement markets and for use in infant formula. If our competitors' products gain widespread acceptance due to pricing or other advantages, our patents expire, or we lose our patents, the sales of our products may be materially adversely affected and our technologies rendered obsolete.
We are aware that other sources of DHA and ARA are or may be available, any of which could represent a competitive threat that could seriously harm our product sales. Specifically:
- •
- Abbott Nutrition (then known as the Ross Products Division of Abbott Laboratories), a significant Martek customer, filed a
generally recognized as safe notification on January 2, 2002 seeking Food and Drug Administration ("FDA") concurrence that its tuna oil source of DHA and its fungal source of ARA, as
manufactured by Suntory Limited, are generally recognized as safe when used as ingredients in infant formula. In April 2006, the FDA notified Ross Products that it had no questions at that time
regarding Ross' conclusion that DHA-rich oil from tuna and ARA-rich oil from Mortierella alpina are safe as sources of DHA and
ARA in term and post-discharge preterm infant formulas. While Abbott Nutrition has not announced any introduction of such oils into infant formula in the U.S. and has agreed to purchase
its total needs for DHA and ARA from us through at least 2011, and while we are not aware of any plans by our other customers to do so, the GRAS notification removes a significant regulatory hurdle to
the introduction of competitive products in the U.S.
- •
- Suntory Limited, Nippon Suisan and Cargill Inc. (through a joint venture with a company in China) and other
independent Chinese manufacturers are producing and distributing a fungal source of ARA. In addition, we are aware that there may be manufacturers in China and India attempting to produce an algal
source of DHA, but we are uncertain of the overall status and commercial potential of these efforts. ONC has also indicated their desire to enter the microbial oil market.
- •
- Some infant formulas now on the market outside the United States, including those marketed by certain of Martek's
customers, use DHA derived from other sources, such as fish oil or eggs.
- •
- In December 2005, Lonza Group LTD, a Swiss chemical and biotechnology group, acquired from Nutrinova Nutrition
Specialties & Food Ingredients GmbH, a wholly-owned subsidiary of Celanese Corporation, Nutrinova's business having as its product a DHA-rich microalgal oil. Since the
acquisition, Lonza has actively marketed its DHA oil to the food and beverage and dietary supplement markets around the world. In addition, Lonza may be actively marketing its DHA oil for infant
formula applications in certain markets. Nutrinova and Lonza are defendants in patent infringement actions involving our DHA patents that we have brought in the United States, Germany and France. One
of Nutrinova's customers is also a defendant in these actions in Germany. These lawsuits are further described below in the risk factor regarding patent protection and in Item 3. "Legal
Proceedings" of Part I of this Form 10-K.
- •
- Monsanto/Solae are expecting to commercialize an inexpensive, sustainable, vegetarian omega-3 for foods and beverages in the next one to three years. This product is a soybean oil containing stearidonic acid ("SDA"). Monsanto/Solae are positioning SDA as a precursor to EPA, in addition to DHA, the other omega-3 found in fish oil. While a large amount of SDA soybean oil must be included in a food or beverage to achieve the intake level Monsanto has recommended for SDA, the oil should be inexpensive, claims to have stability and formulation advantages and may be able to make similar cardiovascular structure-function claims to fish oil. As another omega-3 on the market, SDA soy oil could be a threat to Martek's food and beverage business.
33
- •
- GlaxoSmithKline is currently selling LOVAZA™, a prescription DHA/ EPA ethyl ester for treatment of
hyperlipidemia. LOVAZA™ is a lipid-regulating agent which includes both EPA and DHA from fish oil. GlaxoSmithKline has filed an application with the FDA for an indication that will expand
the use of LOVAZA™ and is actively evaluating new indications. Other pharmaceutical companies offering other applications using omega-3 fatty acids may be expected.
- •
- Other companies, several with greater financial resources than ours, are developing plant-based DHA, other plant-based oils or, in the case of DuPont, an EPA product derived from yeast, that may compete with us, and other companies may be developing chemically synthesized DHA.
Several large companies, including BASF AG, DSM, ONC and a number of smaller companies, manufacture microencapsulated fish oil products. Although microencapsulation of the oil resolves many of the odor, stability and taste issues found with fish oil, a microencapsulated product currently is more costly than regular fish oil. Fish oil-based products (i) are used as a DHA source by some infant formula companies, (ii) currently dominate the adult DHA supplement market and (iii) are included in certain foods on the market in various parts of the world. Because fish oil is generally less costly than our DHA oil, even when microencapsulated, and continues to improve in quality and gain general market acceptance, fish oil presents a substantial competitive threat.
Experts differ in their opinions on the importance of DHA and/or ARA in infant formula and the levels of DHA and/or ARA required to achieve health benefits for babies. Some experts feel that they are not necessary ingredients for infant development. If clinical trials do not continue to yield positive results, certain favorable regulatory guidelines are not enacted or current favorable regulatory guidelines are amended, our future revenues in the infant formula market may be limited.
Our continued success in the infant formula industry depends on sustained acceptance of our nutritional oils as necessary or beneficial ingredients in infant formulas. Notwithstanding existing clinical results that have demonstrated the beneficial effects of adding our nutritional oils to infant formula, some experts in the field of infant nutrition do not believe that our nutritional oils are necessary or that they provide any long-term beneficial effects. There have also been clinical studies where no beneficial effects have been found, possibly due to dose, duration or other factors. Experts generally recommend that mothers breastfeed rather than use infant formulas whether or not they contain our nutritional oils. Furthermore, breastfeeding advocacy groups challenge the benefits of infant formulas whether or not they contain our nutritional oils. Some experts believe that infant formulas without our oils or with greatly reduced levels are sufficient as infants can convert precursor fats into DHA and ARA as needed. In addition, some physicians are unimpressed by studies showing that infant formulas supplemented with our oils improve infants' cognitive ability at early ages, suggesting that these results may not carry over to improved results later in life. Due to these differences in opinion, if clinical studies do not continue to yield positive results, our future revenues in the infant formula market may be limited.
We are aware that in Asia as well as other international markets, certain infant formula manufacturers incorporate very low levels or, at times, no ARA in their supplemented infant formulas. Furthermore, in most countries, there are significant disparities amongst infant formula manufacturers as to the levels of DHA and ARA in their respective products. A failure by one or more regulatory authorities to enact guidelines for minimum levels of DHA and/or ARA for supplementation of infant formula products or the issuance of regulatory guidelines that establish targeted levels of DHA and/or ARA in infant formula that are lower than levels currently being used could result in lower-potency formula products in specific affected countries, which could reduce the market opportunity for DHA and ARA ingredients. Any regulatory guidelines for infant formula that permit inclusion of DHA and ARA ingredients containing higher levels of EPA than covered in Martek's patents could also reduce the market opportunity for Martek's DHA and ARA ingredients in affected countries. While the Codex
34
Alimentarius Infant Formula Standard and the European Union and Australia/ New Zealand regulations all allow the optional addition of DHA and ARA in infant formula, there are no existing regulations in any country requiring the addition of DHA and ARA.
Our opportunity in the U.S. infant formula market may be limited by the renewal rate of supplemented formulas into the Women, Infants and Children program or if the eligibility requirements for participating in the program are made more restrictive or if the amount of infant formula offered to participants is reduced.
We estimate that of the total current annual U.S. market opportunity for sales of supplemented infant formula, approximately half represents Women, Infants and Children ("WIC")-funded sales. WIC is a federal grant program that is state-administered for the benefit of low-income nutritionally at-risk women, infants and children. Most WIC state agencies provide only one brand of infant formula to its participants, depending on which company has the rebate contract in a particular state. Currently, WIC programs in 50 states and the District of Columbia offer term and certain specialty infant formula products supplemented with our oils. If supplemented formulas are removed from WIC programs that previously adopted them, eligibility requirements for participating in WIC become more restrictive and/or participation decreases, or if any of our customers fail to renew, in a timely fashion, their contract awards from WIC agencies for the adoption of a supplemented infant formula, then our future revenues from supplemented infant formula sales in the U.S. would be limited. Further, in December 2007, the USDA, the federal agency which governs WIC, issued an interim final rule which included a reduction in the amount of infant formula to be offered through WIC. State WIC agencies had until October 2009 to implement this change and the USDA is accepting comments on this interim final rule through February 2010. If there is a permanent reduction in the amount of infant formula offered through WIC, then our future infant formula revenues could be materially adversely affected.
If our non-infant formula customers do not introduce products containing our nutritional oils on a broad scale into the marketplace and consumers do not purchase such products, our sales to these markets will be limited.
We are continuing to aggressively pursue further penetration of our DHA oils in the food and beverage, pregnancy and nursing, nutritional supplement and animal feed markets. To this end, we have signed license and supply agreements with several consumer products companies. Our success in penetrating this market, however, is dependent upon these customers introducing products that contain our nutritional oils into the marketplace and is further dependent upon the end consumer purchasing such products. Although some of our customers have launched products containing our oils, we cannot control whether our existing customers or potential new customers will continue to do so in the future, nor can we control whether our current or future customers will follow through with their planned launches of products containing our oils. Furthermore, as a broad scale product launch by our customers is likely dependent on actual or perceived consumer demand, which is inherently uncertain, we cannot control whether our customers will broadly distribute such DHA-enriched products or offer them beyond niche products or line extensions. To date, our non-infant formula customers have launched over 300 products containing our oils. Of the products launched, some have failed, but most remain on the market. Despite this fact, industry averages, particularly food and beverage, would continue to suggest that many of these products will not ultimately be successful in the marketplace. As such, the introduction of a product by our customers does not guarantee that the product will remain on the market. If our non-infant formula customers do not introduce products containing our nutritional oils on a broad scale into the marketplace and end consumers do not purchase such products, our sales to the food and beverage, pregnancy and nursing, nutritional supplement and animal feed markets would be limited.
35
Our oils are very sensitive to oxidation and may not be very compatible with many liquid or dry foods that are currently on the market. If economical methods are not developed to successfully incorporate our oils into various food and beverage applications, we may never be able to gain large-scale entry into the food and beverage and nutritional supplements markets.
Due to the sensitivity of our oils to oxidation, it is possible that our oils may change over time in such a way as to negatively affect the taste, smell and/or shelf stability of food and beverage applications. While we believe that the food and beverage market could be a large market for DHA supplementation with our DHA-S oil, the potential in this market would be limited if methods are not developed that allow incorporation of the oil into various foods and beverages with, among other things, acceptable flavor, odor and texture for the duration of the shelf life of the food and beverage products. Furthermore, while DHA-enriched food and beverage products with acceptable flavor and stability have been developed, risks exist for other finished food and beverage products, including cereals, shelf-stable beverages and certain types of nutritional bars for which DHA supplementation has not yet been successfully established. Even if we can successfully incorporate our oils into foods and beverages, manufacturers of these products will have to develop methods to demonstrate feasibility in their production and distribution processes, including the packaging, storage and handling of such products. To facilitate this, our customers may require us to meet certain enhanced specifications and standards, our compliance with which cannot be assured. The timing and extent of our sales into the food and beverage market, therefore, are dependent not only on market demand, but also on customer formulation, production and distribution issues over which we have little or no control.
If clinical trials do not continue to yield positive results on the benefits of DHA on cognitive function, cardiovascular health or other health applications, our future revenues may be limited in the food and beverage and the dietary supplement markets.
Investigators at universities and at other research centers, such as NIH, have observed a relationship between low levels of DHA and a variety of health risks, including increased cardiovascular problems, Alzheimer's disease and dementia and various other neurological and visual disorders. We are currently trying to establish what contribution, if any, supplementation with our oils will make in addressing a number of these problems. Although clinical data is not required to market food and beverage ingredients or dietary supplements outside of the infant formula market, we believe that further clinical studies may be needed to validate the benefits of DHA supplementation in order to gain widespread entry into these markets. If clinical trials do not continue to yield positive results on the benefits of DHA or if these benefits are not considered significant by our targeted consumers, our future revenues in these markets may be limited.
If our oils are unable to be used in organic food and beverage products, the opportunity for sales of our oils into the food and beverage market will be limited to non-organic products.
The Organic Foods Production Act of 1990 required the U.S. Department of Agriculture ("USDA") to develop national standards for organically produced agricultural products to assure consumers that agricultural products marketed as organic meet consistent, uniform standards. Accordingly, the USDA has put in place a set of national standards (the "National Organic Program" or "NOP") that food labeled "organic" must meet, whether it is grown in the United States or imported from other countries. Under the NOP regulations, only a USDA-accredited certifying agent may make the determination that a food product may be labeled as organic. Martek is not a USDA-accredited certifying agent.
36
Some of our customers have obtained organic certification from USDA-accredited certifying agents and have received authorization to use the USDA's organic seal on certain products that contain our oils. In some instances, such products have been further reviewed and the authorization to use Martek's oils has been explicitly ratified by the USDA. Certain advocacy groups, however, have challenged these authorizations and ratifications. In addition, recent media attention directed to NOP regulations has increased the level of scrutiny being placed on these regulations. Because the NOP regulations are subject to change and interpretation, there can be no guarantee that our oils will be acceptable for use in all organic products. Organic food sales accounted for approximately 3.5% of the total U.S. food sales in 2008; however, we believe that interest from food manufacturers in producing and selling organic products is expanding. If our oils are ineligible for inclusion in products that bear the USDA organic seal, our sales opportunity in the food and beverage market may be adversely impacted.
Because food and beverage pricing is very competitive, the premium that our oils adds to the cost of a food or beverage may never allow it to be priced at levels that will allow acceptance by consumers.
Food and beverage pricing is very competitive and the market is very sensitive to product price changes. Moreover, these consumer sensitivities are further heightened during periods of economic uncertainty and downturn. Because the inclusion of our oils may add to the retail cost of these products, there is the risk that our potential customers in this market may not be able to sell supplemented products at prices that will allow them to gain market acceptance while, at the same time, remaining profitable. This may lead to our customers delaying or suspending product launches, or, at a minimum, may lead to price pressure on us. If our food and beverage customers delay product launches or we have to reduce our prices, our sales and/or profitability relative to the food and beverage market may be adversely impacted.
If we are unable to gain or maintain broad regulatory approvals for the incorporation of our oils into foods and beverages worldwide, our future revenues in the food and beverage market may be limited.
To date, we have received limited approval in several countries for the incorporation of our DHA-S oil into foods and beverages. Our DHA-S oil has received regulatory approval or is accepted for inclusion in foods and beverages, with certain country-specific limitations, in Argentina, Australia, Canada, Chile, China, the European Community, Israel, Japan, Korea, Malaysia, Mexico, New Zealand, the Philippines, Singapore, South Africa, Taiwan and the United States. With respect to approvals in Europe and Israel, the use of our DHA-S is authorized as an ingredient in certain foods such as certain dairy products, including cheese and yogurt, spreads and dressings, breakfast cereals, food supplements and dietary foods for special medical purposes. In July 2009 the Standing Committee of the European Commission and the 27 Member States approved the expanded use of our DHA-S algal oil in beverages, bakery products and nutrition bars. In parts of the world not mentioned above, laws and regulations with respect to the addition of our oils into foods and beverages are diverse and our ability to gain or maintain the necessary regulatory approvals is unclear. If we are unable to gain or maintain broad approvals for the incorporation of our oils into foods and beverages worldwide, our future revenues in the food and beverage market may be limited.
If it is determined that large amounts of eicosapentaenoic acid ("EPA") must accompany DHA in order to achieve optimal health benefits, we may never be able to gain large-scale entry into the food and beverage and dietary supplements markets.
The rationale for supplementing foods and beverages with DHA is to, in part, improve overall cardiovascular system and/or central nervous system development and health. In September 2004, the FDA authorized a qualified health claim that may be utilized for food and beverage products containing both DHA and EPA relating to the reduction of risk of coronary heart disease. No minimum amounts for either DHA or EPA were established as prerequisites for the claim. Our DHA-S
37
oil includes limited amounts of EPA and therefore products containing the DHA-S oil are eligible for use of the qualified health claim. Studies have been completed in the past to investigate the independent effects of DHA and EPA on health and additional studies may be ongoing or conducted in the future. If consensus of results from these studies establishes that relatively large amounts of EPA are required to be supplemented with DHA in order to achieve the optimal cardiovascular benefits, then our penetration of the food and beverage and dietary supplements markets may be limited.
If the FDA finalizes its proposed rule to prohibit nutrient content claims for DHA and EPA, our penetration of the food and beverage and dietary supplements markets may be limited.
In November 2007, the FDA issued a proposed rule that would prohibit the nutrient content claims for DHA and EPA that had been authorized in three previously submitted Food and Drug Administration Modernization Act notifications. The FDA is proposing to prohibit the DHA and EPA nutrient content claims because the agency does not believe they are based on an authoritative statement. The FDA specifically acknowledged that it did not conduct an independent review of the scientific evidence when evaluating these nutrient content claims. In addition to other stakeholders, we have submitted comments in opposition to this proposed rule. It is unclear when the FDA will issue a final rule. In the event the proposed rule becomes final, the potential health benefits of consuming DHA and EPA still may be communicated through the use of a qualified health claim and/or structure/function claims that are made consistent with applicable FDA requirements and/or quantitative claims. Nonetheless, our penetration of the food and beverage and dietary supplements markets may be limited if this proposed rule is implemented.
If the FDA narrowly interprets certain sections of the Food and Drug Administration Amendments Act of 2007, such an interpretation could have a material adverse effect on our business and financial condition.
In July 2008, the FDA issued a notice and request for comments on section 912 of the Food and Drug Administration Amendments Act of 2007, titled "Prohibition Against Food to Which Drugs or Biological Products Have Been Added." This section of the Act prohibits the use in food of certain authorized drugs and biological substances, including certain such substances that have been the subject of substantial clinical investigations that have been made public. The prohibition applies unless companies first marketed the substance in food prior to the FDA authorization and before the substantial clinical investigations had been instituted. We have submitted comments to the FDA in which we assert that the implementation of section 912 should be based on the intended purpose of the clinical investigation rather than on the mere existence of such an investigation. A more restrictive interpretation of the implementation of section 912 could not only discourage companies from sponsoring clinical research for new food ingredients, but could also bar the continued marketing of some food ingredients and dietary supplements already on the market. Such an interpretation by the FDA could have a material adverse effect on our results of operations and financial condition.
If we or our customers fail to secure authorized nutrition and health claims within the European Union, future revenues from sales within the European Union could be adversely affected.
In December 2006, the European Parliament and Council issued regulation EC No 1924/2006 on "nutrition and health claims made on foods". Implementation of this regulation is currently scheduled for January 2010, although the review of claim applications under the regulation is currently underway. Until the implementation date, which now appears will be postponed, claims that have been in use in Member States prior to January 2006 may continue to be used. After the implementation date, only claims listed in the regulation and those that have received European Union ("EU") authorization shall be permitted on foods and dietary supplements within the EU. Our failure, and that of our customers, to secure authorized claims for our DHA and ARA could adversely impact future market penetration of products containing our DHA and ARA in the EU. Martek's efforts to secure such claims under the
38
current regulation have so far been unsuccessful. In addition, market penetration could be limited for a period of 5 years if claim approvals are given proprietary status for a particular customer's or competitor's product.
We have a single third-party supplier of our ARA with whom we have a contractual relationship. If this supplier of our ARA is unable to supply us with our required amounts of ARA, our results of operations may be adversely affected.
In July 2009, we entered into the First Amended and Restated ARA Alliance, Purchase, and Production Agreement (the "Restated Agreement") with DSM. The Restated Agreement, which extends the original supply term through December 31, 2023, amended, consolidated and restated all existing agreements between the two parties governing the cross-licensing, purchase, supply and production of ARA, one of the Company's nutritional oils that it sells to its infant formula customers. Because DSM is a third-party manufacturer, we have only limited control over the timing and level of its production volumes. If DSM fails to supply us with required amounts of ARA under our agreement, we would not be able to meet our customers' demands unless we were able to utilize alternative sources of supply. In this regard, we would have to either manufacture the ARA at one or both of our plants, which may be more costly and would also reduce our DHA oil production capacity, or enter into other third-party manufacturer supply agreements, none of which may be achievable in a timely manner. An inability of DSM to supply us with our required amounts of ARA may have a material adverse effect on our results of operations.
We hold inventory and are committed to significant future inventory purchases. If our inventory on-hand or committed amounts cannot be sold, our results of operations and/or financial position may be adversely affected.
As of October 31, 2009, we had total inventory on-hand of $116.2 million. Such inventory equates to approximately seven months of future sales. In addition, the Restated Agreement with DSM establishes minimum ARA purchase quantities for calendar years 2009 through 2011. As of October 31, 2009, the value of the remaining calendar year 2009 and full calendar years 2010 and 2011 minimum purchase requirements are approximately $17.2 million, $93.9 million and $87.1 million, respectively. Furthermore, our ARA pricing from DSM from 2012 through 2014 is to an extent volume dependent. If our customers' demand for our ARA and DHA declines, we may have excess inventory on-hand; we may be forced to purchase excessive amounts of ARA in periods through 2011; or we could experience an increase to ARA unit costs after 2011. All such occurrences, through inventory write-downs or other, may have a material adverse effect on our results of operations and/or financial position.
Market fluctuations in the availability and cost of raw materials and energy are beyond our control and may adversely impact our business.
Raw material and energy costs are a significant operating expense of our production facilities and we are dependent on outside suppliers for nearly all of our key raw material and energy needs. The prices and availability of raw materials and energy can be volatile and are susceptible to rapid and substantial changes due to factors beyond our control such as changing economic conditions, weather conditions and supply and demand considerations. A substantial decrease in the availability of raw materials and/or energy from our suppliers, the loss of a key supplier or a substantial increase in the cost of our raw materials and/or energy could adversely impact our results of operations.
39
If customer demand for our nutritional oils requires us or our major suppliers to increase production beyond current levels, we may experience certain risks associated with the ramp-up of commercial manufacturing that could have a material adverse effect on our business, financial condition, and/or results of operations.
When combining our current DHA production capabilities in Kingstree and Winchester with DSM's current ARA production capabilities, we currently have production capacity for all DHA and ARA products in excess of $500 million in annualized sales, collectively, to the infant formula market and pregnancy and nursing, food and beverage, dietary supplement and animal feed markets. Our and DSM's ability to maintain commercial production at these higher levels has not been successfully tested.
If we or our major suppliers increase our production, we each may encounter many risks associated with our commercial manufacturing such as:
- •
- we may experience problems processing, handling and shipping the higher quantities of oil produced from suppliers' or our
expanded facilities;
- •
- the costs of expanding, operating and maintaining our production facilities may exceed our expectations;
- •
- product defects may result;
- •
- lower than anticipated fermentation success rates may result;
- •
- lower downstream processing yields may result;
- •
- environmental and safety problems may result from our production process; and
- •
- regulatory issues relating to the scale-up and operations regarding our production processes may arise.
If we were to experience any one or more of these problems, there could be a material adverse effect on our business, financial condition, and/or results of operations.
We have significantly increased our manufacturing capacity and have incurred substantial costs in doing so. If we are unable to increase our revenues from our nutritional oils produced at these facilities, we may continue to experience excess production capacity and we may be unable to recover these plant expansion costs, which could result in a write-down of certain production assets.
In connection with our efforts to alleviate supply constraints with our infant formula customers and to prepare for other applications of our products, we expanded our internal production capacity and incurred significant expansion costs in doing so. As of October 31, 2009, the Company had $34.4 million of production assets that are currently idle. Our ability to recover the costs of these and certain other assets may depend on increased volumes and revenue from the products manufactured at our facilities. There are no assurances that we will be able to achieve this goal. If it is estimated that we will not be able to ultimately recover the carrying amounts of the production assets, we would be required to record an asset impairment write-down. The effect of such write-down could be material. In addition, when experiencing excess capacity, we may be unable to produce the required quantities of oil cost-effectively, which could have a material adverse effect on our product margins and overall profitability.
Our business would be harmed if we fail to comply with applicable good manufacturing practices as required by the FDA.
In connection with the manufacture of most of our products, we are required to adhere to applicable current "good manufacturing practice" ("GMP") regulations as required by the FDA and our contractual obligations. GMP regulations specify production and process controls, component and product testing standards, quality control and quality assurance requirements, and records and other
40
documentation controls. As a manufacturer of DHA and ARA that are marketed as dietary supplements and used as ingredients in infant formulas and foods and beverages sold in the United States, we are subject to GMP and various other requirements applicable to such products. There can be no assurance that we will be able to continue to manufacture our nutritional oils in accordance with relevant food and beverage, dietary supplement and infant formula requirements for commercial use. Ongoing compliance with GMP and other applicable regulatory requirements is monitored through periodic inspections by state and federal agencies, including the FDA and comparable agencies in other countries. A determination that we are in violation of such GMP and other regulations could lead to an interruption of our production output and the imposition of civil penalties, including fines, contractual damages, product recalls or product seizures, and, in the most egregious cases, criminal sanctions.
Our manufacturing process involves the handling of hazardous materials and emission of regulated pollutants. If we fail to properly handle these hazardous materials and/or emissions, substantial costs and harm to our business could result.
In connection with our research and development and manufacturing activities, we utilize some hazardous materials and emit regulated pollutants. We are subject to federal, state and local laws and regulations governing the use, storage, handling, discharge, management and disposal of hazardous materials and the emission of regulated pollutants. The cost of compliance with these laws and regulations could be significant, and our ability to comply with certain emission requirements is somewhat dependent upon raw materials produced by others, over whom we have little or no control. Moreover, we could be subject to loss of our permits, government fines or penalties and/or other adverse governmental or private party action if our hazardous materials or waste products are used, stored, handled, emitted or otherwise managed in violation of law or any permit. In addition, we could be subject to liability if hazardous materials or waste are released into the environment. A substantial fine, penalty or judgment, the payment of significant environmental remediation costs or natural resource damages or property or personal injury damages, or the loss of a permit or other authorization to operate or engage in our ordinary course of business could result in material, unanticipated expenses and the possible inability to satisfy customer demand for our nutritional oils.
Our corporate compliance program cannot guarantee that we are in compliance with all potentially applicable federal and state regulations.
Our business is subject to extensive federal and state regulation. Current products and products in development cannot be sold if we or our customers do not obtain or maintain regulatory approvals. While we have developed and instituted a corporate compliance program, we cannot assure you that we or our employees are or will be in compliance with all potentially applicable federal and state regulations. If we fail to comply with any of these regulations, a range of actions could result, materially affecting our business and financial condition, including, but not limited to, the failure to approve a product candidate, restrictions on our products or manufacturing processes, including withdrawal of our products from the market, significant fines, or other sanctions.
Our business exposes us to liabilities related to allegedly unsubstantiated product efficacy claims.
The Federal Trade Commission regulates certain aspects of the advertising and marketing of our products and the products of our customers that incorporate our oils. Under the Federal Trade Commission Act, a company must be able to substantiate both the express and implied claims that are conveyed by an advertisement. The food and supplements industries are under increasing scrutiny by regulators, attorneys general, consumer groups, the class action bar, and others for claims appearing on product labels, websites, advertisements, and other product promotions. These actions are based on consumer protection laws and typically allege that a company lacks data that can substantiate the claims made in advertising and marketing efforts. Actions against certain food, beverage and
41
supplement companies are occurring, and product claims being made by certain food, beverage and supplement companies, some of which may relate to the benefits attributable to our ingredients, are being examined more closely. In the future, we may be subject to regulatory actions and/or litigation relating to these claims, which could adversely affect our business.
Our business exposes us to potential product liability claims and recalls, which could adversely impact our financial condition and performance.
Our development, manufacture and marketing of products involve an inherent risk of exposure to product liability claims, product recalls, product seizures and related adverse publicity. In addition, as only a small amount of our oils resides in our customers' end product, a recall of our oils could impact a much larger recall of our customers' end products. With respect to infant formula, customer recall could result from such things as contamination, spoilage, product misbranding or product tampering, whether real or perceived. Although we currently maintain product liability and recall insurance for our products in the amounts we believe to be commercially reasonable, we cannot be certain that the coverage limits of our insurance policies or those of our strategic partners will be adequate. Insurance coverage for such risks is expensive and difficult to obtain, and we may be unable to obtain coverage in the future on acceptable terms, if at all. If we are unable to obtain sufficient insurance at an acceptable cost, a product liability claim or recall could adversely impact our financial condition. Furthermore, if a product liability claim is made against us or if there is a product recall, whether fully covered by insurance or not, our future sales could be adversely impacted due to, among other things, negative publicity and the resulting inability to effectively market our products.
Concerns with the safety and quality of our nutritional oils could cause customers to avoid our products.
Recent adverse publicity about the safety of certain foods due to the actual or potential existence of certain compounds therein has heightened the sensitivities of many consumers. These safety and quality issues, whether real or perceived, may discourage customers from buying products containing or perceived to contain the compounds which give rise to such concerns. We could be adversely affected if our customers or the ultimate consumers of our products lose confidence in the safety and quality of our nutritional oils. Any negative change in customer perceptions about the safety and quality of our products could adversely affect our business and financial condition.
We may need additional capital in the future to continue our research and development efforts, to conduct product testing, including preclinical and clinical trials, and to market our products. We may also need additional capital to expand our production capacity if market demand for our products continues to grow.
As of October 31, 2009, we had approximately $141.1 million in cash and cash equivalents as well as $135 million of our revolving credit facility available to meet future capital requirements. We may require additional capital to fund, among other things, our research and development, product testing, and marketing activities. Our ability to meet future demand may require even further expansion of our production capability for our nutritional oils, which would also require additional capital. The timing and extent of our additional cash needs will primarily depend on: (a) the timing and extent of future launches of infant formula products containing our oils by our customers; (b) the timing and extent of introductions of DHA into foods and beverages and/or dietary supplements for children and adults; and (c) our ability to generate profits from the sales of our nutritional products.
To continue to fund our growth, we may pursue various sources of funding, which may include debt financing, equity issuances, asset-based borrowing, lease financing, and collaborative arrangements with partners. In September 2005, we amended and expanded our secured revolving credit facility to $135 million and extended the term until September 2010. This debt financing arrangement requires us to comply with financial covenants, which we may not be able to meet if demand for our products was to significantly decline, if there was a significant change in our financial position or if our cash needs are greater than we currently anticipate. Additionally, funding from other sources may not be available,
42
if needed, due to financial covenant compliance or credit facility expiration, or may not be available on terms that would be commercially acceptable or permit us to continue the planned commercialization of our products or expansion of our production capacity. Furthermore, future equity issuances may be dilutive to our existing shareholders. If we obtain funds through collaborative or strategic partners, these partners may require us to give them technology or product rights, including patent rights, that could ultimately diminish our value. If we cannot secure adequate funding, we may need to scale back our research, development, manufacturing, and commercialization programs, which may have a material adverse effect on our future business.
The market price of our common stock may experience a high level of volatility due to factors such as the volatility in the market for biotechnology stocks generally, and the short-term effect of a number of possible events.
We are a public biosciences company. As frequently occurs among these companies, the market price for our common stock may experience a high level of volatility. During the fifty-two week period ending October 31, 2009, our common stock traded between $31.60 and $15.36 per share. During the fifty-two week period ending October 31, 2008, our common stock traded between $39.60 and $22.47 per share. The following are examples of items that may significantly impact the market price for our common stock:
- •
- announcements of technical innovations, new commercial products and product launches by us or our competitors;
- •
- announcements of use of or intention to use competitors' DHA and/or ARA products by our customers;
- •
- arrangements or strategic partnerships by us or our competitors;
- •
- announcements of license agreements, acquisitions or strategic alliances by us or our competitors;
- •
- announcements of sales by us or our competitors;
- •
- announcements of results of clinical trials by us, our competitors or others;
- •
- patent or other intellectual property achievements or adverse developments;
- •
- quarterly fluctuations in our revenues and results of operations;
- •
- failure to enter into favorable third-party manufacturing agreements;
- •
- regulatory decisions (approvals or disapprovals) or changes concerning our products and our competitors' products;
- •
- events related to threatened, new or existing litigation, or the results thereof;
- •
- changes in our estimates of financial performance or changes in recommendations by securities analysts; and
- •
- general market conditions for bioscience companies.
Because we may experience a high level of volatility in our common stock, you should not invest in our stock unless you are prepared to absorb a significant loss of your capital. At any given time, you may not be able to sell your shares at a price that you think is acceptable.
The market liquidity for our stock is relatively low. As of October 31, 2009, we had 33,269,686 shares of common stock outstanding. The average daily trading volume in our common stock during the fifty-two week period ending October 31, 2009 was approximately 400,000 shares. Although a more active trading market may develop in the future, the limited market liquidity for our stock may affect your ability to sell at a price that is satisfactory to you.
43
As a single reporting unit, we evaluate the realizability of our goodwill using the market approach. Under this approach, market value is determined utilizing our market capitalization plus a control premium. A control premium is representative of premiums paid in the marketplace to acquire a controlling interest in a company. As such, the realizability of our goodwill is somewhat dependent on the market value of Martek's stock. Should the market value of our stock decline significantly, we may need to record a goodwill impairment write-down and the effect of such write-down could be material.
If significant shares eligible for future sales are sold, the result may depress our stock price by increasing the supply of our shares in the market at a time when demand may be limited.
As of October 31, 2009, we had 33,269,686 shares of common stock and approximately 600,000 unvested restricted stock units outstanding, as well as stock options outstanding to purchase an aggregate of approximately 2.4 million shares of common stock. Of the stock options, substantially all were exercisable at December 17, 2009, and approximately 300,000 had exercise prices that were below the market price on this date. The restricted stock units will vest and common stock will issue at various dates through 2014. To the extent that these options for our common stock are exercised, restricted stock units vest or we issue additional shares to raise capital, the increase in the number of our outstanding shares of common stock may adversely affect the price for our common stock. This could hurt our ability to raise capital through the future sale of equity securities. If we require additional outside sources of capital to finance, among other things, our research and development, product testing and the manufacturing and marketing of our products, we may need to raise additional capital through the sale of equity securities.
If we pursue and ultimately consummate a business combination, we may not realize all of the anticipated benefits of the merger.
We have an ongoing process of evaluating potential merger and acquisition candidates that will support our future growth and expand our product offerings. To be successful after any consummated merger, we would need to combine and integrate the separate organizations and operations of the two companies. The combination of two independent companies is a complex, costly, and time-consuming process. As a result, we would need to devote significant management attention and resources to integrating the diverse business practices and operations of the two companies. We may encounter difficulties that could harm the combined businesses, adversely affect our financial condition, and cause our stock price to decline. Furthermore, even if the operations of the two organizations are integrated successfully, the combined company may not realize the expected benefits of the transaction, including the synergies, cost savings, or sales or growth opportunities.
Changes in foreign currency exchange rates or interest rates could reduce profitability.
In July 2009, we entered into the Restated Agreement with DSM. As part of the agreement, it was established that 25% of the ARA we buy from DSM will be denominated in Euros. As such, consistent with our payment arrangements with DSM prior to the execution of the Restated Agreement, we are exposed to risks related to changes in exchange rates between the U.S. dollar and the Euro. Fluctuations in the Euro-U.S. dollar exchange rate can adversely impact our cost of ARA oil and our gross margins. To reduce the risk of unpredictable changes in these costs, we may, from time to time, enter into forward foreign exchange contracts. However, due to the variability of timing and amount of payments under these contracts, the forward foreign exchange contracts may not mitigate the potential adverse impact on our financial results and, in fact, may themselves cause financial harm. Furthermore, these contracts have inherent levels of counterparty risk over which we have no control. While we had no foreign currency forward contracts outstanding at October 31, 2009, we expect to enter into new forward contracts in the future. We estimate that a 5% change in the Euro-U.S. dollar exchange rate would impact gross margins of our infant formula products by less than 0.5%.
44
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
We lease an aggregate of approximately 79,000 square feet of laboratory, technical and administrative space in Columbia, Maryland. The lease for approximately 13,000 square feet expires in January 2010 and the remainder expires in January 2011. This facility acts as Martek's corporate headquarters.
We also lease an aggregate of approximately 19,000 square feet of laboratory, technical and administrative space in Boulder, Colorado. The lease expires in May 2011.
We own a fermentation and oil processing facility in Winchester, Kentucky where we can produce our nutritional oils. The facility is located on 30 acres with buildings occupying approximately 114,000 square feet holding multiple fermentation vessels totaling 1.2 million liters of capacity.
We also own a fermentation and oil processing facility in Kingstree, South Carolina where we produce our nutritional oils and provide contract manufacturing services. The facility is located on more than 500 acres with buildings occupying approximately 419,000 square feet and holding multiple fermentation vessels totaling 2.7 million liters of capacity. The large majority of the fermentation capacity is intended for the production of DHA and ARA.
Aventis S.A. and Nagase & Co. Ltd. are challenging our European patent covering our DHA-containing oils, which expires in 2011. At a hearing in October 2000, the Opposition Division of the European Patent Office ("EPO") revoked our patent on the grounds that it was not novel. We immediately appealed this ruling, and in July 2002 we received a positive ruling from an Appeal Board of the EPO, setting aside the prior decision to revoke this patent. The patent was returned to the Opposition Division for a determination as to whether it has met the legal requirement of "inventive step". A hearing in August 2005 resulted in a ruling by the Opposition Division that this requirement had been met and the validity of the patent was upheld. Aventis appealed the decision to the Appeal Board of the EPO. Martek filed its answer to Aventis' grounds for appeal in July 2006. The appeal hearing was scheduled for March 2009. Martek submitted new evidence that the appeal was inadmissible because Aventis was not the proper party. Because the Appeal Board found that there are questions relating to the admissibility of Aventis' appeal, it postponed the hearing and directed the parties to submit additional briefs on this issue. Aventis submitted its brief in May 2009 and Martek submitted its brief in August 2009. Aventis has filed a further brief, and Martek intends to respond. The filing and consideration of these briefs will likely delay the appeal hearing for about one to two years from the original hearing date. Martek's patent will remain in full force and effect during the pendency of these proceedings. Claim 1 of this patent is the basis of the patent infringement suit against Nutrinova and Lonza in Germany and against Lonza in France, discussed below. In the event Martek were to lose this appeal, this DHA patent would be revoked. The revocation of this patent would result in the dismissal of the patent infringement suit against Nutrinova and Lonza in Germany and against Lonza in France, discussed below, and patent protection for Martek's DHA-containing oils for use in infant formula would be compromised in Europe. Currently, annual sales of Martek's DHA for use in infant formula in Europe to companies other than those with whom Martek has or expects to have an exclusive supply agreement are less than $1 million. Such exclusive agreements generally run through 2011. An adverse decision would not impact Martek's ARA patent position in Europe. Moreover, the outcome of this appeal will not affect the patent infringement lawsuit against Lonza in the U.S., as the U.S. lawsuit is based on a different family of patents.
45
In April 2007, the EPO granted another patent to Martek for ARA oil made from Martek's microbial source for use in infant formula. This divisional ARA patent strengthens Martek's intellectual property position by providing commercially significant protection through the expiration date of the original patent in January 2012. Suntory, N.V. Nutricia and Cargill filed oppositions against this divisional ARA patent. N.V. Nutricia has withdrawn from the opposition. At a hearing held in June 2009 before the Opposition Division of the EPO in The Hague, Netherlands, the Opposition Division upheld the patent with narrower claims. The claims were narrowed by adding a specified ratio of ARA to eicosapentaenoic acid, or EPA, in infant formula applications. The narrower claims cover the use of Martek's blended microbial oil products in infant formula, but do not cover microbial ARA when blended with certain fish oils in infant formula. Martek appealed the decision to the Board of Appeals of the EPO in December 2009. During the pendency of the appeal, which will likely take between one and two years from the date the appeal was filed, the original claims of the patent will remain in full force and effect. Martek does not believe this decision will have a material adverse impact on its infant formula revenues.
Prior to our purchase of OmegaTech in fiscal 2002, Aventis Research and Technologies GmbH & Co. KG, and Nagase Limited challenged OmegaTech's European patent covering its DHA-containing oils. At a hearing in December 2000, the Opposition Division of the EPO upheld some of the claims and revoked other claims. OmegaTech immediately appealed this ruling, as did Aventis. At an appeal hearing in May 2005, we received a favorable decision from the Appeal Board of the EPO, which overturned the decision of the Opposition Division and returned the case to the Opposition Division for review on the merits of the patent claims. In a November 2007 hearing, the Opposition Division upheld claims that are narrower than the claims originally granted but broader than the claims that were previously upheld in the December 2000 Opposition Division hearing. Martek and Aventis have appealed. The patent, which expires in late 2010, will remain in full force and effect during the appeal process. This appeal suffers from the same admissibility problems that Aventis has with the appeal of the DHA patent discussed above.
In October 2007, the EPO granted a patent to Martek for fermentation processes for producing microbial lipids (e.g., DHA oil) under low dissolved oxygen conditions. Lonza AG filed an opposition against this process patent in July 2008, and Martek filed a written response in April 2009. A hearing before the Opposition Division of the EPO in Munich, Germany is scheduled for April 2010.
In September 2003, we filed a patent infringement lawsuit in the U.S. District Court in Delaware against Nutrinova Inc., Nutrinova Nutrition Specialties & Food Ingredients GmbH, Celanese Ventures GmbH, and Celanese AG. Celanese Ventures GmbH and Celanese AG were dropped from the lawsuit. Lonza Ltd. was added to the lawsuit. In October 2006, after an almost two week trial in Wilmington, Delaware, the jury returned a favorable verdict to Martek, deciding that all three of the asserted Martek DHA patents were valid and infringed, and that one was willfully infringed. In October 2007, the judge upheld the October 2006 jury verdict that the defendants infringed all of the asserted claims of U.S. Patent Nos. 5,340,594 and 6,410,281 and that these patents were not invalid. The judge has granted a permanent injunction against the defendants with respect to those two patents. The judge also upheld the jury verdict that the defendants had acted willfully in their infringement of U.S. Patent No. 6,410,281. Regarding the third patent involved in the case, U.S. Patent No. 6,451,567, the judge reversed the jury verdict and found that the asserted claims of this patent were invalid. Martek's request to the judge to reconsider his ruling on the third patent was denied. Martek and the defendants appealed aspects of the judge's final decision and a hearing was held before the U.S. Court of Appeals for the Federal Circuit in April 2009. In September 2009, the Court of Appeals ruled in Martek's favor on all of the patents that were the subject of the appeal, which included U.S. Patent Nos. 5,340,594, 6,410,281, 6,451,567 noted above and 5,698,244, which was included in Martek's appeal as a result of the trial court's decision at a pre-trial hearing on the meaning and scope of the patent claims in dispute. With respect to U.S. Patent No. 5,698,244, the Court of Appeals reversed the trial court's interpretation of certain claim language and remanded this patent to the trial court for further
46
proceedings. Lonza subsequently filed a request with the U.S. Patent and Trademark Office seeking reexamination of this patent. U.S. Patent Nos. 5,340,594 and 6,454,567 have expired and U.S. Patent Nos. 6,410,281 and 5,698,244 are scheduled to expire in August 2011 and December 2014, respectively. The defendants requested a rehearing with the Court of Appeals on the decision, but their request was denied. The defendants may seek an appeal to the U.S. Supreme Court with regard to certain of the patents.
We also filed a patent infringement suit involving Nutrinova Nutrition Specialties & Food Ingredients GmbH and Celanese Ventures GmbH in Germany in January 2004. Lonza Ltd. and a customer of Nutrinova have also been added to this lawsuit. The complaint alleges infringement of our European patent relating to DHA-containing oils. A hearing was held in a district court in Dusseldorf in September 2007 and the court issued its decision in October 2007, ruling that Martek's patent was infringed by the defendants. The defendants have appealed, and an appeal hearing was scheduled for February 2009. Martek and the defendants requested that the appeal hearing be delayed until the Appeal Board of the EPO decides whether to uphold Martek's European patent covering our DHA-containing oils. This EPO Appeal Board hearing was scheduled for March 2009 and has been postponed. In December 2008, Martek requested that the court expand the appeal to include Lonza's production of DHA in Germany, based on evidence discovered in October 2008.
We filed a patent infringement suit involving Lonza Ltd AG and Capsugel France in France in November 2008. The complaint alleges infringement of our European patent relating to DHA-containing oils. We agreed to dismiss our claims against Capsugel France in November 2009.
Martek is opposing two of Suntory's low sterol ARA oil patents in Europe and one in Australia. The patents are generally directed to processes for producing microbial ARA oil having a low ratio of certain sterols, the resulting oil and its use in infant formula. Martek believes that the patents are invalid for a number of reasons, including prior art that anticipates the claims relevant to Martek. An Opposition Division hearing on the first European patent was held in April 2008, and the Opposition Division revoked the Suntory patent. Suntory has appealed, during which time the patent will remain in full force and effect. A hearing on the other European patent took place in December 2009, resulting in the revocation of the Suntory patent. Suntory will likely appeal. A hearing on the Australian patent is expected in 2010.
Third parties have filed Requests for Reexamination ("Requests") of twelve of Martek's U.S. patents. Nine of these Requests were filed by Lonza with respect to nine of Martek's DHA patents which, with the exception of U.S. Patent No. 5,698,244 described below, are generally not relevant to Martek's infant formula business. Four of these patents have now expired. Additionally, an anonymous party filed the other three Requests with respect to two of Martek's blended oils patents and one ARA patent. The U.S. Patent and Trademark Office has granted eleven of the Requests to initiate a Reexamination process. As a result of these Reexaminations, the claims of the subject patents may be upheld in their current form, narrowed, abandoned, or revoked, or the term of a patent may be shortened. Not all of the claims of the patents are subject to Reexamination. With respect to the ARA patent, which is scheduled to expire in 2014, we received a second adverse office action in July 2009 and conducted interviews with the examiners. In October 2009, we received a positive advisory action from the examiners regarding 80 claims pending in the reexamination of the ARA patent. While the examiners have indicated that the broader composition claims in the patent likely will not be upheld, we believe that certain process claims that have been deemed allowable will provide a competitive advantage. We also received positive advisory actions from the examiners regarding claims pending in the reexaminations of two DHA patents that have not expired and the two blended oils patents. Notices of Intent to Issue a Reexamination Certificate have been received for the two DHA patents and one of the blended oils patents. We expect the fourth will issue in December 2009. The reexaminations are continuing for the remaining two unexpired DHA patents, and we have been conducting further proceedings with the examiners on these two DHA patents. If we are unable to
47
obtain commercially meaningful claim coverage, we plan to appeal within the U.S Patent and Trademark Office, and in the event of a negative outcome, we will have an opportunity to further appeal to the federal courts. These patents will remain in full force and effect during the appeal process. However, if the appeals are not successful or are not pursued, some or all of the claims of these patents could be revoked. In November 2009, Lonza filed a request for reexamination of Martek's U.S. Patent No. 5,698,244, which is the patent that the Court of Appeals for the Federal Circuit remanded to the District Court for trial, as discussed above. The U.S. Patent and Trademark Office determined that the request did not comply with Office requirements, and Lonza has submitted a replacement request.
With respect to our infant formula business, as stated previously, our blended oils patents expire in December 2011, which generally coincides with the expiration of our customers' sole source purchase obligations. As for the ARA patent discussed immediately above, it is likely that the patent will emerge from the reexamination process with narrower claims. Specifically, it is probable that the broader product claims will not survive. Rather, the patent will likely contain narrower product claims and process claims that, among other things, would provide patent protection to a production process that we believe: i) results in higher quality oil and higher ARA potency and ii) is cost efficient compared to other processes for producing ARA. When combined with our in-licensed patents from our ARA production partner DSM, we believe that it will be technologically difficult and less cost efficient for competitors to design around these claims. Nonetheless, it is uncertain how much protection they will provide.
There are additional intellectual property proceedings pending against Martek or that Martek has pending against third parties that are not considered material.
In addition, from time to time, Martek is a party to additional litigation or administrative proceedings relating to claims arising from its operations in the normal course of business or other matters. Management believes that the ultimate resolution of any such additional litigation or administrative proceedings currently pending against Martek is unlikely, either individually or in the aggregate, to have a material adverse effect on Martek's results of operations or financial condition.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
No matter was submitted to a vote of security holders during the fourth quarter of the fiscal year covered by this report.
48
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
The Company's common stock is traded on The NASDAQ Global Select Market under the symbol MATK. As of December 17, 2009, there were approximately 265 holders of record of the Company's common stock. The price of the Company's common stock was $17.79 on December 17, 2009. No cash dividends have been paid on the common stock and the Company does not anticipate paying any cash dividend in the foreseeable future. Dividend payments are restricted under the Company's Amended and Restated Loan and Security Agreement dated September 30, 2005. The following table sets forth, for the calendar periods indicated, the range of high and low sales prices for the Company's common stock as reported by NASDAQ:
Sales Price Range of Common Stock
Fiscal 2008
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
November 1, 2007 - January 31, 2008 |
$ | 34.70 | $ | 24.18 | |||
February 1, 2008 - April 30, 2008 |
$ | 36.80 | $ | 27.52 | |||
May 1, 2008 - July 31, 2008 |
$ | 38.55 | $ | 32.00 | |||
August 1, 2008 - October 31, 2008 |
$ | 39.60 | $ | 22.47 | |||
Fiscal 2009
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
November 1, 2008 - January 31, 2009 |
$ | 31.60 | $ | 24.80 | |||
February 1, 2009 - April 30, 2009 |
$ | 26.79 | $ | 15.36 | |||
May 1, 2009 - July 31, 2009 |
$ | 24.14 | $ | 17.54 | |||
August 1, 2009 - October 31, 2009 |
$ | 25.43 | $ | 17.95 | |||
No repurchases of common stock took place during fiscal 2009.
49
Performance Graph
The following graph sets forth the Company's total cumulative stockholder return as compared to the NASDAQ Stock Market Composite Index and the NASDAQ Biotechnology Index, for the period beginning October 31, 2004 and ending October 31, 2009. Total stockholder return assumes $100 invested at the beginning of the period in the common stock of the Company, the stocks represented in the NASDAQ Composite Index and the NASDAQ Biotechnology Index, respectively. Total return assumes reinvestment of dividends; the Company has paid no dividends on its common stock. Historical price performance should not be relied upon as indicative of future stock performance.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Martek Biosciences Corporation, The NASDAQ Composite Index
And The NASDAQ Biotechnology Index
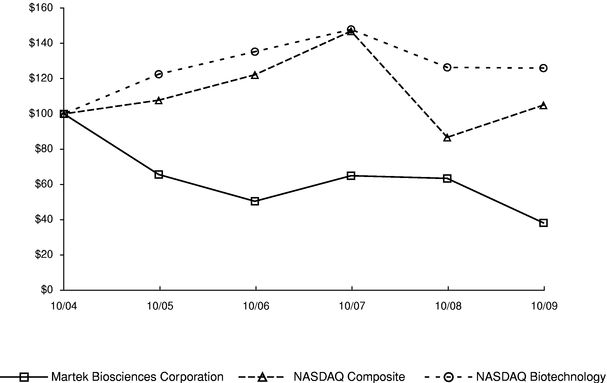
- *
- $100 invested on 10/31/04 in stock or index, including reinvestment of dividends. Fiscal year ending October 31.
50
ITEM 6. SELECTED FINANCIAL DATA.
The selected financial data set forth below with respect to the Company's consolidated statements of income for each of the years in the three-year period ended October 31, 2009 and with respect to the consolidated balance sheets as of October 31, 2009 and 2008 are derived from the audited consolidated financial statements included elsewhere in this Form 10-K. The statements of operations data for each of the years in the two-year period ended October 31, 2006 and the balance sheet data at October 31, 2007, 2006 and 2005 are derived from audited financial statements not included in this Form 10-K.
The following selected financial data should be read in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the consolidated financial statements and notes contained in this Form 10-K.
| |
Year ended October 31, | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
In thousands, except per share data
|
2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||
Consolidated Statements of Operations Data |
|||||||||||||||||
Revenues: |
|||||||||||||||||
Product sales |
$ | 329,135 | $ | 336,609 | $ | 292,549 | $ | 255,838 | $ | 203,765 | |||||||
Contract manufacturing and services |
16,062 | 15,753 | 14,264 | 14,816 | 14,087 | ||||||||||||
Total revenues |
345,197 |
352,362 |
306,813 |
270,654 |
217,852 |
||||||||||||
Cost of revenues: |
|||||||||||||||||
Cost of product sales |
182,385 | 192,848 | 179,367 | 158,600 | 120,865 | ||||||||||||
Cost of contract manufacturing and services |
14,252 | 14,000 | 13,952 | 14,676 | 12,516 | ||||||||||||
Total cost of revenues |
196,637 |
206,848 |
193,319 |
173,276 |
133,381 |
||||||||||||
Gross margin |
148,560 |
145,514 |
113,494 |
97,378 |
84,471 |
||||||||||||
Operating expenses: |
|||||||||||||||||
Research and development |
27,448 | 26,223 | 24,853 | 24,044 | 19,415 | ||||||||||||
Selling, general and administrative |
50,027 | 54,181 | 44,855 | 39,597 | 31,968 | ||||||||||||
Amortization of intangible assets |
6,389 | 7,422 | 6,558 | 2,796 | 2,489 | ||||||||||||
Restructuring charge |
— | — | 853 | 4,729 | — | ||||||||||||
Other operating expenses |
1,080 | 1,504 | 1,614 | 1,158 | 7,654 | ||||||||||||
Total operating expenses |
84,944 |
89,330 |
78,733 |
72,324 |
61,526 |
||||||||||||
Income from operations |
63,616 |
56,184 |
34,761 |
25,054 |
22,945 |
||||||||||||
Interest and other income (expense), net |
428 |
1,137 |
(1,089 |
) |
(1,528 |
) |
1,125 |
||||||||||
Income before income tax provision |
64,044 |
57,321 |
33,672 |
23,526 |
24,070 |
||||||||||||
Income tax provision |
23,454 | 19,654 | 1,659 | 8,588 | 8,786 | ||||||||||||
Net income |
$ |
40,590 |
$ |
37,667 |
$ |
32,013 |
$ |
14,938 |
$ |
15,284 |
|||||||
Net income per share, basic |
$ |
1.22 |
$ |
1.14 |
$ |
0.99 |
$ |
0.47 |
$ |
0.49 |
|||||||
Net income per share, diluted |
$ | 1.22 | $ | 1.13 | $ | 0.98 | $ | 0.46 | $ | 0.48 | |||||||
Weighted average common shares outstanding, basic |
33,207 |
32,951 |
32,336 |
32,113 |
31,164 |
||||||||||||
Weighted average common shares outstanding, diluted |
33,363 | 33,284 | 32,593 | 32,343 | 32,032 | ||||||||||||
51
| |
October 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||
Consolidated Balance Sheets and Other Data |
||||||||||||||||
Cash, cash equivalents, short-term investments and marketable securities |
$ | 148,364 | $ | 102,495 | $ | 21,648 | $ | 26,828 | $ | 33,347 | ||||||
Working capital |
303,634 | 224,831 | 149,345 | 128,488 | 124,208 | |||||||||||
Total assets |
689,817 | 645,981 | 596,695 | 597,973 | 578,485 | |||||||||||
Long-term debt, notes payable and other long-term obligations |
400 | 793 | 9,310 | 46,277 | 66,115 | |||||||||||
Long-term portion of deferred revenue |
8,426 | 9,263 | 9,517 | 9,335 | 8,959 | |||||||||||
Retained earnings (accumulated deficit) |
75,972 | 35,382 | (2,285 | ) | (34,298 | ) | (49,236 | ) | ||||||||
Total stockholders' equity |
636,144 | 588,583 | 531,727 | 492,575 | 469,205 | |||||||||||
Cash dividends declared—common stock |
— | — | — | — | — | |||||||||||
52
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
This Management's Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements concerning our business and operations, including, among other things, statements concerning the following:
- •
- expectations regarding future revenue, revenue growth, gross margin, operating cash flow and
overall profitability;
- •
- expectations regarding product introductions and growth in nutritional product
sales;
- •
- expectations regarding potential collaborations and
acquisitions;
- •
- expectations regarding demand for products with our nutritional
oils;
- •
- expectations regarding sales to and by our infant formula customers and supplemented infant
formula market penetration levels;
- •
- expectations regarding marketing of our oils by our infant formula
customers;
- •
- expectations regarding future agreements with, and revenues from, companies in the food and
beverage, pregnancy and nursing, nutritional supplement and animal feed markets;
- •
- expectations regarding future revenues from contract manufacturing
customers;
- •
- expectations regarding future revenues from
collaborations;
- •
- expectations regarding growing consumer recognition of the key health benefits of DHA and
ARA;
- •
- expectations regarding competitive products;
- •
- expectations regarding future efficiencies and improvements in manufacturing processes and the
cost of production of our nutritional oils;
- •
- expectations regarding future purchase volumes and costs of third-party manufactured
oils;
- •
- expectations regarding the amount of production capacity and our ability to meet future demands
for our nutritional oils;
- •
- expectations regarding the amount of inventory held by us or our
customers;
- •
- expectations regarding production capacity utilization and the effects of excess production
capacity;
- •
- expectations regarding future selling, general and administrative and research and development
costs;
- •
- expectations regarding future capital
expenditures;
- •
- expectations regarding levels of consumption through governmental programs of infant formula
products containing our nutritional oils; and
- •
- expectations regarding our ability to maintain and protect our intellectual property.
Forward-looking statements include those statements containing words such as the following:
- •
- "will,"
- •
- "should,"
- •
- "could,"
- •
- "anticipate,"
- •
- "believe,"
53
- •
- "plan,"
- •
- "estimate,"
- •
- "expect,"
- •
- "intend," and other similar expressions.
All of these forward-looking statements involve risks and uncertainties. They and other forward-looking statements in this Form 10-K are all made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. We wish to caution you that our actual results may differ significantly from the results we discuss in our forward-looking statements. We discuss some of the risks that could cause such differences in Part I, Item 1A."Risk Factors" in this report on Form 10-K and in our various other filings with the Securities and Exchange Commission. Our forward-looking statements speak only as of the date of this document, and we do not intend to update these statements to reflect events or circumstances that occur after that date.
Martek was founded in 1985. We are a leader in the innovation and development of high value nutritional products from microbial sources, including algae, fungi and other microbes, that promote health and wellness. Our innovation and development is based on our core expertise, broad experience and proprietary technology in areas such as microbial biology, algal genomics, fermentation and oil processing. We refer to this set of complementary abilities as Martek's "research engine." Through this research engine, we have developed and commercialized life'sDHA™, a vegetarian source of the omega-3 fatty acid DHA (docosahexaenoic acid), for use in infant formula, pregnancy and nursing products, foods and beverages, dietary supplements and animal feed. We also produce life'sARA™, a vegetarian source of the omega-6 fatty acid ARA (arachidonic acid), for use in infant formula.
We sell oils containing these fatty acids as life'sDHA™, DHASCO®, Neuromins®, ARASCO® and life'sARA™. We derive DHA from microalgae and ARA from fungi, using proprietary processes. Cell membranes throughout the body contain these fatty acids, and they are particularly concentrated in the brain, central nervous system, retina and heart. Research has shown that DHA and ARA may enhance mental and visual development in infants. In addition, research has shown that DHA may play a pivotal role in brain function throughout life and may reduce the risk of cardiovascular disease. Low levels of DHA in adults have been linked to a variety of health risks, including Alzheimer's disease, dementia, cardiovascular problems and various other neurological and visual disorders. Further research is underway to assess the role of supplementation with our DHA on mitigating a variety of health risks.
In 1992, we realized our first revenues from license fees related to our nutritional oils containing DHA and ARA and sales of sample quantities of these oils. In 1995, we recognized our first product and royalty revenues from sales of infant formula containing these oils, and in 1996 we began to realize revenues from the sale of Neuromins®, a DHA dietary supplement. In 2001, the FDA completed a favorable review of our generally recognized as safe notification for the use of our DHA and ARA oil blend in specified ratios in infant formula. We are supplying over 35 infant formula customers with our nutritional oils. These companies collectively represent approximately 75% of the estimated $15 billion worldwide retail market for infant formula and nearly 100% of the estimated $4.5 billion U.S. retail market for infant formula, including the retail value of Women, Infants and Children program ("WIC") rebates. WIC is a federal grant program administered by the states for the benefit of low-income, nutritionally at-risk women, infants and children. Our customers include infant formula market leaders Mead Johnson Nutritionals, Nestle, Abbott Nutrition, Pfizer (formerly Wyeth) and Danone (formerly Numico), each of whom is selling infant formula supplemented with our nutritional oils. Our customers are now selling infant formula products containing our oils collectively in over 75 countries. Supplemented infant formulas from Mead Johnson Nutritionals, Abbott Nutrition, PBM Products, Nestle, Hain Celestial and Nutricia North America are currently being sold in the United States. In
54
addition, certain infant formula customers are selling products in the United States and abroad that contain our nutritional oils and target the markets for children ages nine months to seven years of age and older.
We are currently marketing and selling life'sDHA™, either directly or through distributors, for food and beverage, supplement and animal feed applications to both U.S. and international companies. To date, over 200 domestic and international companies have launched non-infant formula products that contain life'sDHA™, most of which remain on the market and are believed to be meeting customer expectations. Certain of our DHA license and supply agreements with major consumer food products companies establish Martek, subject to certain exceptions, as their exclusive supplier of DHA for minimum periods of time. Certain of these agreements establish the customer as the exclusive customer of life'sDHA™ in a particular food or beverage category or categories or geographic region. We, along with our customers and certain third parties, are developing other DHA delivery methods, including powders and emulsions, to facilitate further entry into the non-infant formula markets. Management believes that over the next few years, the non-infant formula markets will continue to expand and could ultimately represent a larger opportunity than infant formula.
We have received a favorable review by the FDA of our GRAS notification for the use of DHA-S in food and beverage applications in the U.S. and have received similar approvals in Canada. We have also received authorization from the Ministry of Health in China (subject to certain conditions) and the Australia New Zealand Food Authority for the use of DHA-S oil in all foods and authorization from the European Commission and Israel for the use of our DHA-S oil as a novel food ingredient. This novel food designation authorizes the use of our DHA-S as an ingredient in certain foods such as certain dairy products, including cheese and yogurt, spreads and dressings, breakfast cereals, food supplements and dietary foods for special medical purposes in the European Community and Israel. In addition, in July 2009 the Standing Committee of the European Commission and the 27 Member States approved the expanded use of our DHA-S algal oil in beverages, bakery products and nutrition bars. DHA algal oil is also regarded as a traditional food in Japan and, as such, is acceptable for use in foods and beverages and dietary supplements.
During the past several years, many new products were launched that contained life'sDHA™. Several of Martek's customers such as Dean Foods, Coca-Cola/Minute Maid, JM Smucker/Crisco, Danone, Starbuck's, Parmalat Australia, Ragasa and others have launched food and beverage products containing life'sDHA™ in the U.S. and other countries. Some of our customers are currently selling products containing life'sDHA™ targeted to pregnant and nursing women, including Mead Johnson Nutritionals, Wal-Mart, CVS/pharmacy, Walgreen's, Everett Laboratories and Mission Pharmacal. Generally, these products are also co-branded with the life'sDHA™ logo.
Although we anticipate future growth in annual sales of our nutritional oils, we are likely to continue to experience quarter-to-quarter and year-to-year fluctuations in our future operating results, some of which may be significant. The timing and extent of future oils-related revenues are largely dependent upon the following factors:
- •
- the timing of international infant formula market introductions by our customers;
- •
- the timing of our customers' ordering patterns;
- •
- the timing and extent of stocking and de-stocking of inventory by our customers;
- •
- the timing and extent of our customers' production campaigns and plant maintenance shutdowns;
- •
- the timing and extent of introductions of DHA into various child and/or adult applications and the marketplace success of
such applications;
- •
- the levels of inclusion of our oils in infant formula;
55
- •
- the continued acceptance, and extent thereof, of products containing our oils under WIC and other regulatory programs in
the U.S.;
- •
- the continued acceptance of these products by consumers and continued demand by our customers;
- •
- the ability of our customers to incorporate our oils into various foods and beverages;
- •
- our ability to protect against competitive products through our patents;
- •
- competition from alternative sources of DHA and ARA; and
- •
- agreements with other future third-party collaborators to market our products or develop new products.
As such, the likelihood, timing and extent of future profitability are largely dependent on factors such as those mentioned above, as well as others, over which we have limited or no control.
We manufacture oils rich in DHA at our production facilities located in Winchester, Kentucky, and Kingstree, South Carolina. The oils that we produce in these facilities are certified Kosher by the Orthodox Union and are certified Halal by the Islamic Food and Nutrition Council of America. Both manufacturing facilities have received favorable ratings by the American Institute of Baking, an independent auditor of food manufacturing facilities, and have obtained the ISO 14001 Environmental Management System ("EMS") International Standard, the most recognized EMS standard in the world. Also, the National Oceanic and Atmospheric Administration has granted Martek's Winchester plant, the Company's primary shipping location, a health certificate, which is required for import of products into many countries, including China and neighboring countries in the Pacific Rim.
Over 90% of our ARA oils are purchased from DSM. Because DSM is a third-party manufacturer, we have only limited control over the timing and level of its production volumes. The balance of our ARA requirements is produced at our Kingstree facility. In July 2009, we entered into the First Amended and Restated ARA Alliance, Purchase, and Production Agreement (the "Restated Agreement") with DSM. The Restated Agreement, which extends the original supply term through December 31, 2023, amended, consolidated and restated all existing agreements between the two parties governing the cross-licensing, purchase, supply and production of ARA. Among other things, the Restated Agreement established ARA pricing to Martek for calendar years 2009 through 2014 with the potential for only limited changes related to post-2011 volumes and provides that ARA pricing subsequent to calendar 2014 will be negotiated in the future by the parties. For the establishment of the 2009 through 2014 ARA pricing and related contractual provisions, we paid DSM approximately $11.0 million. While, subject to the limited exceptions noted below, Martek is committed to purchasing all of its ARA requirements from DSM through the term of the Restated Agreement, the Restated Agreement also sets minimum ARA purchase quantities for Martek in calendar years 2009, 2010 and 2011. As of October 31, 2009, the value of the remaining calendar year 2009 and full calendar years 2010 and 2011 minimum purchase requirements are approximately $17.2 million, $93.9 million and $87.1 million, respectively. These minimum purchase quantities approximate the amounts expected to be consumed by us in the normal course of business.
We have attempted to reduce the risk inherent in having a single supplier through certain elements of our supply agreement with DSM. In connection with this agreement, we have the ability to produce, either directly or through an approved third party, an unlimited amount of ARA. The sale of such self-produced ARA is limited annually, however, to the greater of (i) 100 tons of ARA oil or (ii) any amounts ordered by us that DSM is unable to fulfill. Although we currently produce ARA ourselves, our existing manufacturing capacity would not permit us to produce ARA quantities sufficient to meet current demand without impacting our production of DHA. To further improve our overall ARA
56
supply chain, we have directly engaged a U.S.-based provider of certain post-fermentation ARA manufacturing services. Along with our ARA downstream processing capabilities at Kingstree, this third-party facility provides us with multiple U.S. sites for the full downstream processing of ARA.
When combining our current DHA and ARA production capabilities in Kingstree and Winchester with DSM's current ARA production capabilities, we have production capacity for DHA and ARA products in excess of $500 million in annualized sales, collectively, to the infant formula and non-infant formula markets. As such, our production capabilities exceed current demand; however, we have the ability to manage production levels and, to a certain extent, control our manufacturing costs. Nonetheless, when experiencing excess capacity, we may be unable to produce the required quantities of oil cost-effectively due to the existence of significant levels of fixed production costs at our plants and the plants of our suppliers.
The commercial success of our nutritional oils will depend, in part, on our ability to manufacture these oils or have them manufactured at large scale on a routine basis and at a commercially acceptable cost. Our success will also be somewhat dependent on our ability to align our production with customer demand, which is inherently uncertain. There can also be no assurance that we will be able to continue to comply with applicable regulatory requirements, including the Food and Drug Administration's "good manufacturing practice" ("GMP") requirements. Under the terms of several of our agreements with infant formula customers, those customers may elect to manufacture these oils themselves. While our customers are not required to disclose to us that they have begun the process, we are currently unaware of any of our customers producing our oils or preparing to produce our oils, and estimate that it would take a customer a minimum of one year to implement a process for making our oils.
CRITICAL ACCOUNTING POLICIES AND
THE USE OF ESTIMATES
The preparation of our consolidated financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. On an ongoing basis, we evaluate our estimates and judgments, which are based on historical and anticipated results and trends and on various other assumptions that we believe are reasonable under the circumstances, including assumptions as to future events. By their nature, estimates are subject to an inherent degree of uncertainty and, as such, actual results may differ from our estimates. We believe that the following significant accounting policies and assumptions involve a higher degree of judgment and complexity than others.
Valuation of Long-lived Assets We review our long-lived assets, including fixed assets and certain identifiable intangibles, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. As of October 31, 2009, these long-lived assets had a total net book value of $294.9 million. Included in these long-lived assets is approximately $34.4 million of production equipment whose use is not currently required based on present customer demand. Undiscounted cash flow analyses are used to assess impairment. The estimates of future cash flows involve considerable management judgment and are based on many assumptions for each target market. Such assumptions include market size, penetration levels and future product margins. While management believes that its projections are reasonable and that no impairment of these assets exists, different assumptions could affect these evaluations and result in material impairment charges against the carrying value of these assets.
Impairment of Goodwill We assess the possible impairment of goodwill annually, on August 1, or more frequently when events occur or circumstances change that would more likely than not reduce the fair value of the asset below its carrying amount. Events or circumstances that may require an interim impairment assessment include experiencing disruptions to business, unexpected significant declines in
57
operating results, divestiture of a significant component of the business or a sustained decline in market capitalization. The first step of the goodwill impairment test, used to identify potential impairment, compares the fair value of a reporting unit with its carrying amount, including goodwill. During fiscal 2009, as in prior years, we operated as one reporting unit and therefore compared the carrying amount of our goodwill to our market value. Market value is determined utilizing our market capitalization plus a control premium. A control premium is representative of premiums paid in the marketplace to acquire a controlling interest in a company. If our market value exceeds our carrying amount, goodwill is considered not impaired, thus the second step of the impairment test is not necessary. If our carrying amount exceeds our market value, we would then need to determine the implied fair value of the reporting unit's goodwill via a hypothetical allocation of purchase price. To the extent that the implied fair value of goodwill is less than carrying value, an impairment loss is recognized for such difference.
As a result of its annual impairment test, management concluded that there was no impairment of goodwill as of August 1, 2009. Subsequent to our 2009 annual impairment test, we experienced a decline in our market capitalization to an amount below book value. As a result, we reconsidered our analysis and we noted that applying a control premium at October 31, 2009 similar to the one applied for our annual impairment test resulted in a market value in excess of carrying value. Therefore, we concluded that it was not more likely than not that the goodwill was impaired and we did not perform an interim impairment test as of October 31, 2009.
We will continue to monitor our market capitalization as a potential impairment indicator considering overall market conditions and other industry events. Any impairment charges that may result will be recorded in the period in which the impairment is identified. While we believe we have made reasonable estimates and assumptions to calculate the fair values of our reporting unit, it is possible a material change could occur. That is, if our market capitalization declines, our goodwill could become impaired and a charge to our results of operations may be required.
Revenue Recognition We derive revenue principally from three sources: product sales, contract manufacturing and collaborations. We recognize product sales revenue when persuasive evidence of an arrangement exists, the fee is fixed or determinable, collectibility is probable, the product is shipped and title and risk of loss are transferred. A number of our infant formula license contracts include an upfront license fee, a prepayment of product sales and established pricing on future product sales, which also may include discounts based on the achievement of certain volume purchases. The consideration from these contracts is allocated based on the relative fair values of the separate elements. Revenue is recognized on product sales when goods are shipped and all other conditions for revenue recognition are met. If volume pricing discounts are deemed to be a separate element, revenue on related product shipments is recognized using the estimated average price to the customer over the term of the discount period, which requires an estimation of total production shipments over that time frame. Amounts billed in excess of the estimated average price are recorded as deferred revenue. Conversely, a receivable is recorded for the excess of the estimated average price over amounts billed. Estimates of average prices are reviewed and, if necessary, adjusted periodically based on updated estimates of product shipments during each contract year. Our historical estimates of product shipments have approximated actual results. Amounts recorded as either deferred revenue or a receivable are settled at the end of each contract year, which generally is December 31. Cash received as a prepayment on future product purchases is deferred and recognized as revenue when product is shipped. Revenue from product licenses is deferred and recognized on a straight-line basis over the term of the agreement. Our terms of sale allow for limited rights of return with such rights based solely on compliance with agreed-upon customer product specifications. We test the product for compliance with customer product specifications prior to shipment. Such returns have not been material. Royalty income is recorded when earned, based on information provided by our customers.
58
Contract manufacturing revenue is recognized when goods are shipped to customers and all other conditions for revenue recognition are met. Cash received that is related to future performance under such contracts is deferred and recognized as revenue when earned.
Revenue earned from collaboration work may come from stand-alone arrangements for certain discrete development work or multiple deliverable arrangements that include such development work followed by larger-scale manufacturing efforts. Revenue is recognized based on the nature of the arrangements, with each of the multiple deliverables in a given arrangement having distinct and separate fair values. Fair values are determined via consistent pricing between stand-alone arrangements and multiple deliverable arrangements, as well as a competitive bidding process. Collaborations may be performed on a time and materials basis or fixed fee basis. For time and materials arrangements, revenue is recognized as services are performed and billed. For fixed fee arrangements, revenue is recognized upon completion and acceptance by the customer.
Deferred Income Taxes and Uncertain Tax Positions We provide for income taxes in accordance with the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on temporary differences between the financial reporting bases and the tax bases of assets and liabilities. We also recognize deferred tax assets for tax net operating loss carryforwards. These deferred tax assets and liabilities are measured using the enacted tax rates and laws expected to be in effect when such amounts are projected to reverse or be utilized. The realization of total deferred tax assets is contingent upon the generation of future taxable income in the tax jurisdictions in which the deferred tax assets are located. As of October 31, 2009, our total gross deferred tax asset was $50.1 million, which will require future taxable income of approximately $135 million for realization. When appropriate, we recognize a valuation allowance to reduce such deferred tax assets to amounts more likely than not to be ultimately realized. The calculation of deferred tax assets (including valuation allowances) and liabilities requires management to apply significant judgment related to such factors as the application of complex tax laws and the changes in such laws. We have also considered our future operating results, which require assumptions such as future market penetration levels, forecasted revenues and the mix of earnings in the jurisdictions in which we operate, in determining the need for a valuation allowance. We review our deferred tax assets on a quarterly basis to determine if a change to our valuation allowance is required based upon these factors. Our deferred tax asset valuation allowance of $2.9 million as of October 31, 2009 relates to certain state net operating loss and credit carryforwards whose realizability is uncertain. Changes in our assessment of the need for a valuation allowance could give rise to a change in such allowance, potentially resulting in material amounts of additional tax expense or benefit in the period of change.
Effective November 1, 2007, we began recognizing the benefits of tax positions in the financial statements if such positions are more likely than not to be sustained upon examination by the taxing authority and satisfy the appropriate measurement criteria. If the recognition threshold is met, the tax benefit is generally measured and recognized as the tax benefit having the highest likelihood, in our judgment, of being realized upon ultimate settlement with the taxing authority, assuming full knowledge of the position and all relevant facts. At October 31, 2009, we had unrecognized tax benefits totaling approximately $3.3 million. The determination of these unrecognized amounts requires significant judgments and interpretation of complex tax laws. Different judgments or interpretations could result in material changes to the amount of unrecognized tax benefits.
Inventory We carry our inventory at the lower of cost or market and include appropriate elements of material, labor and indirect costs. Inventories on-hand total $116.2 million as of October 31 2009 and are valued using a weighted average approach that approximates the first-in, first-out method. In addition, the Restated Agreement with DSM also sets minimum ARA purchase quantities for Martek in calendar years 2009, 2010 and 2011. As of October 31, 2009, the value of the remaining calendar year 2009 and full calendar years 2010 and 2011 minimum purchase requirements are approximately $17.2 million, $93.9 million and $87.1 million, respectively. We regularly review inventory quantities
59
on-hand and our future inventory purchase commitments and record a reserve for excess, obsolete and "off-spec" inventory based primarily on an estimated forecast of product demand and the likelihood of consumption in the normal course of manufacturing operations. Those reserves are based on significant estimates. Our estimates of future product demand or assessments of future consumption may prove to be inaccurate, in which case we may have understated or overstated the provision required. Although we make every effort to ensure the accuracy of our forecasts and assessments, any significant unanticipated changes, particularly in demand or competition levels, could have a significant impact on the values of our inventory and our reported operating results. In addition, abnormal amounts of inventory costs related to, among other things, idle facilities, freight handling and waste material expenses are recognized as period charges and expensed as incurred. The determination of such period costs requires the use of judgment in establishing the level of production that the Company considers normal. A different conclusion as to what constitutes normal production levels could result in material changes to idle capacity expenses recognized.
Patent Cost Capitalization We incur certain external legal and related costs in connection with patent applications. If a future economic benefit is anticipated from the resulting patent or an alternate future use is available to the Company, such costs are capitalized and amortized over the expected life of the patent. We also capitalize external legal costs incurred in the defense of our patents when it is believed that the future economic benefit of the patent will be maintained or increased and a successful defense is probable. Capitalized patent defense costs are amortized over the remaining life of the related patent. Our assessment of future economic benefit and/or a successful defense of our patents involves considerable management judgment. A different conclusion could result in material write-offs of the carrying value of these assets.
Fair Value Measurements As of November 1, 2008, the Company adopted the provisions of guidance codified as Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") 820, "Fair Value Measurements and Disclosures" ("ASC 820") for financial instruments. ASC 820 defines fair value, establishes a fair value hierarchy for assets and liabilities measured at fair value and requires expanded disclosures about fair value measurements. The ASC 820 hierarchy ranks the quality and reliability of inputs, or assumptions, used in the determination of fair value and requires assets and liabilities carried at fair value to be classified and disclosed as either a Level 1, 2 or 3 fair value instrument. Different from Levels 1 and 2, Level 3 instruments are valued using unobservable inputs that are not corroborated by market data. At October 31, 2009, we had Level 3 assets of $11.8 million, which include both auction rate securities and an auction rate securities rights agreement (the "Put Agreement"). Of this amount, fair value changes in assets totaling $7.3 million have been recorded through earnings and changes in the remainder have been recorded through other comprehensive income. Some of the unobservable inputs into the discounted cash flow models on which we base our Level 3 valuations include, but are not limited to, periodic coupon rates, market required rates of return and the expected term of each security. Changes to the inputs used as of October 31, 2009 would cause fluctuations to the fair value of the affected instruments and such fair value changes could be material.
60
Revenues
The following table presents revenues by category (in thousands):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Product sales |
$ | 329,135 | $ | 336,609 | $ | 292,549 | |||||
Contract manufacturing and services |
16,062 | 15,753 | 14,264 | ||||||||
Total revenues |
$ | 345,197 | $ | 352,362 | $ | 306,813 | |||||
Product sales decreased by $7.5 million or 2% in fiscal 2009 as compared to fiscal 2008 and increased by $44.1 million or 15% in fiscal 2008 as compared to fiscal 2007. Product sales were comprised of the following (in thousands):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Infant formula market |
$ | 285,664 | $ | 300,742 | $ | 265,563 | |||||
Food and beverage market |
10,692 | 10,431 | 5,483 | ||||||||
Pregnancy and nursing, nutritional supplements and animal feeds |
28,615 | 20,835 | 17,439 | ||||||||
Non-nutritional products |
4,164 | 4,601 | 4,064 | ||||||||
Total product sales |
$ | 329,135 | $ | 336,609 | $ | 292,549 | |||||
Sales to the infant formula market decreased in fiscal 2009 compared to fiscal 2008 due primarily to de-stocking of inventory by certain of our major infant formula customers and the impact of declining U.S. births. These declines offset the growth experienced during fiscal 2009 in our international infant formula markets. Sales to the infant formula market increased in fiscal 2008 over 2007 due to the growth in demand in both the U.S. and international infant formula markets during that period. Launches of new products and the growth of existing products containing life'sDHA™ resulted in slightly higher sales to the food and beverage market in fiscal 2009 compared to the prior fiscal year. In addition, sales into the pregnancy and nursing, nutritional supplements and animal feeds markets increased significantly in fiscal 2009 due to an overall expansion of Martek's customer base in these markets, particularly pregnancy and nursing. Customer base expansion was also experienced in fiscal 2008 in the food and beverage, pregnancy and nursing and nutritional supplements markets which led to sales growth in these areas from fiscal 2007 to fiscal 2008.
Approximately 77%, 80% and 85% of our product sales in fiscal 2009, 2008 and 2007, respectively, were generated by sales to Mead Johnson Nutritionals, Abbott Nutrition, Nestle, Pfizer (formerly Wyeth) and Danone (formerly Numico). Although we are not given precise information by our customers as to the countries in which infant formula containing our oils is ultimately sold, we estimate that approximately 50%, 52% and 59% of our sales to infant formula customers for fiscal 2009, 2008 and 2007, respectively, relate to sales in the U.S. As of October 31, 2009, we estimate that formula supplemented with our oils had penetrated almost all of the U.S. infant formula market.
Although we anticipate that annual product sales will continue to grow, our future sales growth is subject to quarter-to-quarter fluctuations and is dependent to a significant degree upon the following factors: (i) the expansions of current products containing our nutritional oils by our customers in new and existing markets; (ii) the launches of new products containing our nutritional oils by current or future customers and the success in the marketplace of such launches; (iii) the timing and extent of stocking and de-stocking of inventory by our customers; (iv) the levels of inclusion of our oils in infant formula; (v) the timing and extent of our customers' production campaigns and plant maintenance shutdowns; and (vi) the availability and use by our customers and others of competitive products.
61
Contract manufacturing and services revenues, totaling approximately $16.1 million, $15.8 million and $14.3 million in fiscal 2009, 2008 and 2007, respectively, relate mainly to fermentation work performed for various third parties at our Kingstree, South Carolina facility. The slight increase in fiscal 2009 over fiscal 2008 was due to the commencement of revenues associated with Martek's joint development agreement with BP for work on microbial oils for use as biofuels. The increase in fiscal 2008 was primarily due to additional orders from one existing contract manufacturing customer. During fiscal 2009, while revenues remained at approximately the same level as fiscal 2008, we reduced the scope of our contract manufacturing activities, and we anticipate further limiting our contract manufacturing activities over the course of fiscal 2010 in order to focus resources on our higher margin nutritional oils business. Other than the reductions to revenue and associated gross margin, we do not expect that the cessation of contract manufacturing activities will have any material impact on our results of operations. Our collaborative work with BP is expected to continue through at least 2011.
As a result of the above, total revenues decreased by $7.2 million or 2% in fiscal 2009 as compared to fiscal 2008 and increased by $45.5 million or 15% in fiscal 2008 as compared to fiscal 2007.
Cost of Revenues
The following table presents our cost of revenues (in thousands):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Cost of product sales |
$ | 182,385 | $ | 192,848 | $ | 179,367 | |||||
Cost of contract manufacturing and services |
14,252 | 14,000 | 13,952 | ||||||||
Total cost of revenues |
$ | 196,637 | $ | 206,848 | $ | 193,319 | |||||
Cost of product sales as a percentage of product sales has improved in each of the last two years with decreases to 55% in fiscal 2009 from 57% in fiscal 2008 and 61% in fiscal 2007. The decreases were due mainly to DHA productivity improvements (1% improvement in both comparative periods) and decreases in our overall cost of ARA (1% in fiscal 2009 from fiscal 2008 and 3% in fiscal 2008 from fiscal 2007).
Cost of contract manufacturing and services was $14.3 million, $14.0 million and $14.0 million in fiscal 2009, 2008 and 2007. Our contract manufacturing and services margins vary between periods primarily due to contract mix and volume.
See "Management Outlook" for discussion of expected revenue and gross profit margin growth for fiscal 2010.
Operating Expenses
The following table presents our operating expenses (in thousands):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Research and development |
$ | 27,448 | $ | 26,223 | $ | 24,853 | |||||
Selling, general and administrative |
50,027 | 54,181 | 44,855 | ||||||||
Amortization of intangible assets |
6,389 | 7,422 | 6,558 | ||||||||
Restructuring charge |
— | — | 853 | ||||||||
Other operating expenses |
1,080 | 1,504 | 1,614 | ||||||||
Total operating expenses |
$ | 84,944 | $ | 89,330 | $ | 78,733 | |||||
62
Research and Development Our research and development costs increased by $1.2 million or 5% in fiscal 2009 as compared to fiscal 2008 due primarily to continued focus on clinical research and product development. Our research and development costs increased by $1.4 million or 6% in fiscal 2008 as compared to fiscal 2007 due primarily to additional resources dedicated to, among other things, the development of new food and beverage applications for life'sDHA™. Our research and development efforts continue to focus on: (i) broadening the scientific evidence supporting our products; (ii) improving manufacturing processes; (iii) developing food and beverage applications; and (iv) developing new products and technologies to expand our market offerings. We expect to continue to experience quarter-to-quarter fluctuations in research and development expenses primarily due to the timing of outside services, including third-party clinical trial services.
Selling, General and Administrative Our selling, general and administrative costs decreased by $4.2 million or 8% in fiscal 2009 as compared to fiscal 2008. As a percentage of revenue, our selling, general and administrative expenses decreased to 14.5% in fiscal 2009 from 15.4% in fiscal 2008. The decrease is due, in part, to close management of spending levels, which led to reductions in areas such as personnel, travel and marketing costs. The fiscal 2009 decrease also resulted from reductions in annual incentive compensation payouts due to the attainment of only a limited number of the pre-established operational and financial goals for the year.
Our selling, general and administrative costs increased by $9.3 million or 21% in fiscal 2008 as compared to fiscal 2007. As a percentage of revenue, our selling, general and administrative expenses increased to 15.4% in fiscal 2008 from 14.6% in fiscal 2007. This increase resulted primarily from higher personnel costs driven by continued investment in personnel needed to support our growth as well as an increase over fiscal 2007 associated with the variable component of Company-wide compensation resulting from our improved overall financial performance.
Amortization of Intangible Assets We capitalize patent application and patent defense costs in addition to certain other external costs related to our intellectual property portfolio to the extent that we anticipate a future economic benefit or an alternate future use is available to us from such expenditures. We amortize these costs over the expected life of the respective assets. We recorded amortization expense related to our intangible assets of $6.4 million, $7.4 million and $6.6 million during fiscal 2009, 2008 and 2007, respectively. The decrease in fiscal 2009 from fiscal 2008 resulted primarily from the expiration of certain patents and licenses in fiscal 2009. The increase in fiscal 2008 from fiscal 2007 resulted primarily from the amortization associated with a new license arrangement entered into during fiscal 2008. See Item 3. "Legal Proceedings" of Part I of this Form 10-K for further discussion of certain patent matters.
Other Operating Expenses We incurred other operating expenses of $1.1 million, $1.5 million and $1.6 million in fiscal 2009, 2008 and 2007, respectively. These costs in fiscal 2009, 2008 and 2007 primarily include production trials and start-up costs incurred in the expanded utilization of our internal manufacturing facilities.
Interest and Other Income, Net
Interest and other income, net, decreased by $800,000 in fiscal 2009 as compared to fiscal 2008 due primarily to lower interest earned on cash balances. Interest and other income, net, increased by $500,000 in fiscal 2008 as compared to fiscal 2007, due primarily to varying levels of cash, cash equivalents and investment balances and variable interest rates earned.
63
Interest Expense
Interest expense decreased by $100,000 in fiscal 2009 as compared to fiscal 2008 and decreased by $1.7 million in fiscal 2008 as compared to fiscal 2007. The decrease in fiscal 2008 from fiscal 2007 is due to reduced levels of debt outstanding under our revolving credit facility and the repayment in full during 2008 of our former term debt prior to its scheduled maturity. Since October 31, 2007, there have been no amounts outstanding under our revolving credit facility. See "Liquidity and Capital Resources" for further discussion.
Income Tax Provision
The provision for income taxes totaled $23.5 million, $19.7 million and $1.7 million in fiscal 2009, 2008 and 2007, respectively, which results in an effective tax rate of approximately 36.6%, 34.3% and 4.9% in fiscal 2009, 2008 and 2007, respectively. During fiscal 2008, the Company recorded an income tax benefit of approximately $1.6 million of previously unrecognized research and development tax credits, which served to decrease our effective tax rate in that year by 2.8%. During fiscal 2007, it was determined that certain net operating loss carryforwards, whose related deferred tax asset had previously been fully reserved, were more likely than not to be realized. This determination was made based upon the Company's projection of future taxable income which, it was concluded, had less uncertainty due to longer histories and certain recently executed contractual arrangements with its customer base. This valuation allowance reversal resulted in an income tax benefit of approximately $10.8 million related to net operating loss carryforwards generated from operations and a decrease to goodwill of approximately $7.4 million related to net operating loss carryforwards acquired from OmegaTech in 2002.
Realization of deferred tax assets is contingent upon the generation of future taxable income in the tax jurisdictions in which the deferred tax assets are located. As of October 31, 2009, the total gross value of our deferred tax asset was $50.1 million, which will require future taxable income of approximately $135 million for realization. Our deferred tax asset valuation allowance of $2.9 million as of October 31, 2009 relates to certain state net operating loss and credit carryforwards whose realizability is uncertain. Should realization of these deferred tax assets become more likely than not, the resulting valuation allowance reversal would be reflected as additional benefit in the period of change.
As of October 31, 2009, we had net operating loss carryforwards for Federal income tax purposes of approximately $82 million, which expire at various dates between 2021 and 2025. The timing and manner in which U.S. net operating loss carryforwards may be utilized may be limited if we incur a change in ownership as defined under Section 382 of the Internal Revenue Code. Although we have net operating losses available to offset future taxable income, we may be subject to Federal alternative minimum taxes.
Net Income
As a result of the foregoing, net income was $40.6 million in fiscal 2009 as compared to net income of $37.7 million in fiscal 2008 and net income of $32.0 million in fiscal 2007.
64
RECENTLY ISSUED
ACCOUNTING PRONOUNCEMENTS
In June 2009, the Financial Accounting Standards Board ("FASB") established the FASB Accounting Standards Codification ("ASC"), or Codification, which on July 1, 2009 became the single official source of authoritative, nongovernmental GAAP, superseding existing FASB, AICPA, EITF and related literature. Prospectively, only one level of authoritative GAAP will exist, excluding the guidance issued by the SEC. All other literature will be non-authoritative. The Codification does not change GAAP but instead reorganizes the U.S. GAAP pronouncements into accounting topics, and displays all topics using a consistent structure. As the Codification does not change GAAP, it did not have a material impact on the Company's financial statements. Previous references to applicable literature via our disclosures have been updated with references to the new Codification section.
In December 2007, the FASB issued Statement No. 141(R), "Business Combinations", which has principally been codified in ASC 805, "Business Combinations" ("ASC 805"). ASC 805 establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree and the goodwill acquired. ASC 805 also establishes disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. ASC 805 is effective for financial statements issued for fiscal years beginning after December 15, 2008 and will be adopted by us in the first quarter of fiscal 2010. We are currently evaluating the overall impact of the adoption of ASC 805 on our consolidated financial position and results of operations, which impact will be largely dependent on the size and nature of any business combination completed after the adoption of this standard. However, as ASC 805 requires the immediate expensing of acquisition-related costs, to the extent that we incur such costs in connection with our ongoing process of evaluating merger and acquisition candidates, these costs will have an impact on our results of operations in the period incurred.
In April 2009, the FASB issued guidance that has been codified as the following: (i) ASC 820-10-65-4, "Transition Related to FASB Staff Position FAS 157-4, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly", (ii) ASC 320-10-65-1, "Transition Related to FASB Staff Position FAS 115-2 and FAS 124-2, Recognition and Presentation of Other-Than-Temporary Impairments", and (iii) ASC 825-10-65-1, "Transition Related to FASB Staff Position FAS 107-1 and APB 28-1, Interim Disclosures about Fair Value of Financial Instruments", each of which was effective for the Company beginning with the third quarter of fiscal 2009. ASC 820-10-65-4 provides guidance on how to determine the fair value of assets and liabilities in the current economic environment and reemphasizes that the objective of a fair value measurement remains the determination of an exit price. ASC 320-10-65-1 modifies the requirements for recognizing other-than-temporarily impaired debt securities and revises the existing impairment model for such securities by modifying the current intent and ability indicator in determining whether a debt security is other-than-temporarily impaired. ASC 825-10-65-1 enhances the disclosure of instruments for both interim and annual periods. The adoption of these pronouncements did not have a material effect on the Company's consolidated financial position, results of operations or cash flows.
LIQUIDITY AND CAPITAL RESOURCES
Since fiscal 2006, we have financed our operations primarily from the following sources:
- •
- cash generated from operations;
- •
- cash received from the exercise of stock options; and
- •
- debt financing.
65
Our cash flows for the years ended October 31, 2009, 2008 and 2007 were as follows (in thousands):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Net cash provided by operating activities |
$ | 65,822 | $ | 106,548 | $ | 45,860 | |||||
Net cash used in investing activities |
(27,267 | ) | (22,130 | ) | (10,724 | ) | |||||
Net cash provided by (used in) financing activities |
13 | 1,104 | (33,741 | ) | |||||||
Total cash inflows |
$ | 38,568 | $ | 85,522 | $ | 1,395 | |||||
At October 31, 2009, our primary sources of liquidity were our cash and cash equivalents totaling $141.1 million as well as the $135 million available under our revolving credit facility. Cash and cash equivalents increased $38.6 million since October 31, 2008, largely due to our net income during that period which contributed to the generation of $65.8 million of cash from operating activities. These operating cash flows reflect a reduction of $40.7 million from fiscal 2008 which was caused primarily by decreases in our current liabilities versus the prior year as well as the inventory increases caused by the timing and extent of Martek's fiscal 2009 ARA purchases from DSM. Consistent with prior years, the timing of Martek's purchases of ARA are largely dependent upon DSM's scheduled production runs. Operating cash flows increased from fiscal 2007 to fiscal 2008 by $60.7 million primarily due to earnings levels between periods as well as inventory and current liability fluctuations.
Our fiscal 2009 investing cash flows reflect certain cash outflows associated with the Restated Agreement with DSM as payments totaling approximately $11.0 million were made in fiscal 2009 related to the establishment of ARA pricing from 2009 through 2014 and related contractual provisions (see Note 4 to the Consolidated Financial Statements included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K). We anticipate recovering this cash within 36 months of the outlay through the future ARA pricing reductions included in the Restated Agreement.
Capital expenditures totaled $8.9 million in fiscal 2009, a level consistent with fiscal 2008 and 2007. In general, we believe that our current production infrastructure can accommodate our short- and medium-term growth objectives in all material respects. We expect that capital expenditures over the next twelve months will not exceed $20 million.
Our financing activities have been relatively insignificant since our full repayment of outstanding draws on our credit facility in fiscal 2007. See below for further discussion of this credit facility.
At October 31, 2009, our investments had a fair value of $11.8 million and consisted primarily of auction rate securities ("ARS") whose underlying assets are student loans originated under the Federal Family Education Loan Program ("FFELP"). FFELP student loans are guaranteed by state guarantors who have reinsurance agreements with the U.S. Department of Education. These ARS are intended to provide liquidity via an auction process that resets the applicable interest rate approximately every 30 days and allows investors to either roll over their holdings or gain immediate liquidity by selling such investments at par. The underlying maturities of these investments range from 17 to 38 years. Since February 2008, as a result of negative conditions in the global credit markets, the large majority of the auctions for our investment in these securities have failed to settle, resulting in Martek continuing to hold such securities. Consequently, the investments are not currently liquid and we will not be able to access these funds, except as noted below, until a future auction of these investments is successful, a buyer is found outside of the auction process or the investments reach their contractual maturity date. To this end, in November 2008, we executed an auction rate securities rights agreement (the "Put Agreement") with a financial institution that provides us the ability to sell certain of our ARS to the financial institution and allows the financial institution, at its sole discretion, to purchase
66
such ARS at par during the period June 30, 2010 through July 2, 2012. Our ARS holdings to which this relates have a cost basis of approximately $7.4 million and a fair value at October 31, 2009 of approximately $6.1 million. The Put Agreement, which is deemed a discrete short-term investment, has a recorded fair value at October 31, 2009 of approximately $1.2 million. The time period until the initial date on which the exercise of the Put Agreement is permitted is currently less than 12 months; therefore, the investment values of the Put Agreement and the ARS to which the Put Agreement relates are classified as short-term investments in the accompanying consolidated balance sheet as of October 31, 2009. We based our valuation of these ARS and the Put Agreement on discounted cash flow models that include various unobservable inputs. Changes to the inputs used as of October 31, 2009 would cause fluctuations to the fair value of the affected instruments and such fair value changes could be material.
The following table sets forth our future minimum payments under contractual obligations at October 31, 2009:
In thousands
|
Total | Less than 1 year |
1-3 years |
3-5 years |
More than 5 years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Notes payable(1) |
$ | 593 | $ | 119 | $ | 77 | $ | 77 | $ | 320 | ||||||
Operating lease obligations |
2,061 | 1,221 | 528 | 91 | 221 | |||||||||||
Unconditional purchase obligations(2),(3) |
198,204 | 95,437 | 102,767 | — | — | |||||||||||
Total contractual cash obligations |
$ | 200,858 | $ | 96,777 | $ | 103,372 | $ | 168 | $ | 541 | ||||||
- (1)
- Minimum
payments above include interest and principal due under these notes.
- (2)
- Comprised
of future inventory purchases from DSM pursuant to minimum purchase commitment. Excludes any additional future inventory purchases from DSM
pursuant to the Restated Agreement or the potential payment by us associated with our rights to terminate the Restated Agreement after 2012. A termination payment by us as of January 1, 2013
would currently range from $15 million to $20 million and a termination payment as of January 1, 2016 would currently range from less than $1 million to $7 million
(see Note 4 to the Consolidated Financial Statements included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on
Form 10-K).
- (3)
- Excludes
$5.6 million of financial commitments associated with a research collaboration that are contingent upon the successful completion of
identified milestones. Also excludes $7.0 million of financial commitments associated with a license agreement and subject to successful completion by the licensor of certain development goals
(see Notes 9 and 12 to the Consolidated Financial Statements included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on
Form 10-K).
- (4)
- The table above excludes uncertain tax payments of $3.3 million, the timing of which is uncertain.
We have a $135 million secured revolving credit facility that is collateralized by accounts receivable, inventory and all capital stock of our subsidiaries and expires in September 2010. There were no amounts outstanding under the revolving credit facility during fiscal 2009 or 2008. The weighted average interest rate on amounts outstanding under the credit facility was approximately 7.1% for the year ended October 31, 2007. The weighted average annual commitment fee rate on unused amounts was approximately 0.1% in each of the years ended October 31, 2009, 2008 and 2007. Both the interest and commitment fee rates are based on LIBOR and our current leverage ratio. Among other things, the credit facility agreement contains restrictions on future debt, the payment of dividends and the further encumbrance of assets. In addition, the credit facility requires that we comply with specified financial ratios and tests, including minimum coverage ratios and maximum leverage ratios.
67
We do not believe that these covenants restrict our ability to carry out our current business plan. As of October 31, 2009, we were in compliance with all of these debt covenants.
As noted above, our current revolving credit facility expires in September 2010. We have begun discussion with our current creditors and expect to have a new credit line available at market rates prior to expiration of the existing facility. These discussions notwithstanding, we believe that a credit facility will not be necessary to fund the future operations of our current business and that our cash and cash equivalents of $141.1 million on-hand at October 31, 2009, and anticipated operating cash flows thereafter, will provide us with adequate capital to meet our obligations for at least the next 12 to 18 months. The ultimate amount of capital that we may require will depend, among other things, on one or more of the following factors:
- •
- our ability to operate profitably and generate positive cash flow;
- •
- growth in our infant formula, food and beverage and other nutritional product sales;
- •
- the extent and progress of our research and development programs;
- •
- the cost and progress of pre-clinical and clinical studies;
- •
- the time and costs of obtaining and maintaining regulatory clearances for our products that are subject to such
clearances;
- •
- the costs involved in filing, protecting and enforcing patent claims;
- •
- competing technological and market developments;
- •
- the development or acquisition of new products;
- •
- the cost of acquiring additional and/or operating and expanding existing manufacturing facilities for our various products
and potential products (depending on which products we decide to manufacture and continue to manufacture ourselves);
- •
- the costs associated with our internal build-up of inventory levels;
- •
- the costs associated with litigation to which we are a party;
- •
- the costs of, and any capital requirements related to, merger and acquisition activity; and
- •
- the costs of marketing and commercializing our products.
We can offer no assurance that, if needed, any of our financing alternatives will be available to us on terms that would be acceptable, if at all.
In fiscal 2010, we are anticipating modest growth in revenues from sales of our oils for infant formula over fiscal 2009 as penetration in the overseas markets is expected to outpace any decreases domestically due to declining birth rates or any additional de-stocking of inventory by our customers. We expect that most of this growth will take place in the second half of the fiscal year.
We continue to see expansions in our non-infant formula markets reflecting the quality of Martek's products and the desire of consumers to improve their health and well-being, even in times of economic uncertainty. We project meaningful growth in the non-infant formula category during fiscal 2010 with a number of new products from current customers set to launch during the first half of fiscal 2010 and with important new customers also entering the global marketplace with products during that period.
Our gross margin continued to improve during fiscal 2009 as compared to fiscal 2008, and we expect this trend to continue in fiscal 2010 through further manufacturing efficiencies coupled with
68
additional decreases in our ARA costs. In addition, consistent with fiscal 2009, we will be closely managing our operating expenses.
On an overall basis, for fiscal 2010, we expect growth in both revenues and profitability over fiscal 2009 with profitability growing at a higher rate than revenues primarily due to improvements in gross profit margins.
Although we expect the annual growth noted above, we are likely to experience quarter-to-quarter fluctuations in both infant formula and non-infant formula nutritional revenues due primarily to variability in customer ordering patterns and the timing of product launches.
OFF-BALANCE SHEET ARRANGEMENTS
We have entered into lease agreements, primarily for certain laboratory and administrative space, with rental payments aggregating $2.1 million over the remaining lease terms, which expire primarily through 2014.
We do not engage in any other off-balance sheet financing arrangements. In particular, we do not have any interest in entities referred to as variable interest entities, which include special purpose entities and structured finance entities.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We are subject to market risk associated with changes in foreign currency exchange rates and interest rates.
In July 2009, we entered into the First Amended and Restated ARA Alliance, Purchase, and Production Agreement (the "Restated Agreement") with DSM. As part of the agreement, it was established that 25% of the ARA we buy from DSM will be denominated in Euros. As such, consistent with our payment arrangements with DSM prior to the execution of the Restated Agreement, we are exposed to risks related to changes in exchange rates between the U.S. dollar and the Euro. We enter into foreign currency cash flow hedges to reduce the related market risk on our payment obligations. We do not enter into foreign currency cash flow hedges for speculative purposes. While we had no foreign currency forward contracts outstanding at October 31, 2009, we expect to enter into new forward contracts in the future. We estimate that a 5% change in the Euro-U.S. dollar exchange rate would impact gross margins of our infant formula products by less than 0.5%.
We are subject to risk from adverse changes in interest rates, primarily relating to variable-rate borrowings used to maintain liquidity; however, at October 31, 2009, there was no variable-rate debt outstanding.
We have investments at October 31, 2009 with a fair value of $11.8 million, which consist primarily of auction rate securities ("ARS"). These ARS are intended to provide liquidity via an auction process that resets the applicable interest rate approximately every 30 days and allows investors to either roll over their holdings or gain immediate liquidity by selling such investments at par. Since February 2008, as a result of negative conditions in the global credit markets, the large majority of the auctions for our investment in these securities have failed to settle, resulting in our continuing to hold such securities. Based on the estimated fair value of the ARS, during fiscal 2008 and fiscal 2009, we recorded net unrealized losses on these securities totaling approximately $2.4 million ($1.5 million, net of income tax benefit), reflecting the decline in the estimated fair value of these securities. We continue to monitor the market for auction rate securities and consider its impact, if any, on the fair value of these investments. If current market conditions deteriorate further, the Company may be required to record additional write-downs. In November 2008, we executed an auction rate securities rights agreement (the "Put Agreement") with a financial institution that provides us the ability to sell certain of our ARS to the financial institution and allows the financial institution, at its sole discretion, to purchase such ARS
69
at par during the period June 30, 2010 through July 2, 2012. Our ARS holdings to which this relates have a cost basis of approximately $7.4 million and a fair value at October 31, 2009 of approximately $6.1 million. The Put Agreement, which is deemed a discrete short-term investment, has a recorded fair value of $1.2 million. The benefits of the Put Agreement are subject to the continued expected performance by the financial institution of its obligations under the agreement. We based our valuation of these ARS and the Put Agreement on discounted cash flow models that include various unobservable inputs. Changes to the inputs used as of October 31, 2009 would cause fluctuations to the fair value of the affected instruments and such fair value changes could be material.
70
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
71
MANAGEMENT'S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of Martek Biosciences Corporation ("Martek") is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Securities Exchange Act Rule 13a-15(f). Martek's internal control system is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Martek's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect Martek's transactions and dispositions of assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that Martek's receipts and expenditures are being made only in accordance with authorizations of Martek's management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of Martek's assets that could have a material effect on the financial statements.
There are inherent limitations in the effectiveness of any internal control over financial reporting, including the possibility of human error and the circumvention or overriding of controls. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance with respect to financial statement preparation.
Martek's management, including the principal executive officer and principal financial officer, conducted an evaluation of the effectiveness of Martek's internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the evaluation under the framework in Internal Control—Integrated Framework, management concluded that Martek's internal control over financial reporting was effective as of October 31, 2009 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles.
Management discussed its assessment with the Audit Committee of the Board of Directors. The effectiveness of Martek's internal control over financial reporting as of October 31, 2009 has been audited by Ernst & Young LLP, independent registered public accounting firm, as stated in their report which is included herein.
| /s/ STEVE DUBIN Steve Dubin Chief Executive Officer and Director |
/s/ PETER L. BUZY Peter L. Buzy Chief Financial Officer, Treasurer and Executive Vice President for Finance and Administration |
|
December 29, 2009 |
December 29, 2009 |
72
REPORT OF ERNST & YOUNG LLP, INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM, ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The
Board of Directors and Stockholders
Martek Biosciences Corporation
We have audited Martek Biosciences Corporation's internal control over financial reporting as of October 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Martek Biosciences Corporation's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Martek Biosciences Corporation maintained, in all material respects, effective internal control over financial reporting as of October 31, 2009, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the accompanying consolidated balance sheets of Martek Biosciences Corporation as of October 31, 2009 and 2008, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the three years in the period ended October 31, 2009 of Martek Biosciences Corporation, and our report dated December 29, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Baltimore,
Maryland
December 29, 2009
73
REPORT OF ERNST & YOUNG LLP, INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The
Board of Directors and Stockholders
Martek Biosciences Corporation
We have audited the accompanying consolidated balance sheets of Martek Biosciences Corporation as of October 31, 2009 and 2008, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the three years in the period ended October 31, 2009. Our audits also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Martek Biosciences Corporation at October 31, 2009 and 2008, and the consolidated results of its operations and its cash flows for each of the three years in the period ended October 31, 2009, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule referred to above, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Martek Biosciences Corporation's internal control over financial reporting as of October 31, 2009, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated December 29, 2009 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Baltimore,
Maryland
December 29, 2009
74
MARTEK BIOSCIENCES CORPORATION
CONSOLIDATED BALANCE SHEETS
| |
October 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|
In thousands, except share and per share data
|
2009 | 2008 | |||||||
Assets |
|||||||||
Current assets |
|||||||||
Cash and cash equivalents |
$ | 141,063 | $ | 102,495 | |||||
Short-term investments |
7,301 | — | |||||||
Accounts receivable, net |
44,304 | 40,438 | |||||||
Inventories, net |
116,179 | 99,553 | |||||||
Deferred tax asset |
24,303 | 24,821 | |||||||
Other current assets |
5,240 | 4,866 | |||||||
Total current assets |
338,390 | 272,173 | |||||||
Property, plant and equipment, net |
252,279 |
265,900 |
|||||||
Deferred tax asset |
— | 13,535 | |||||||
Long-term investments |
4,495 | 11,336 | |||||||
Goodwill |
51,592 | 51,592 | |||||||
Other intangible assets, net |
42,631 | 30,997 | |||||||
Other assets, net |
430 | 448 | |||||||
Total assets |
$ | 689,817 | $ | 645,981 | |||||
Liabilities and stockholders' equity |
|||||||||
Current liabilities |
|||||||||
Accounts payable |
$ | 13,122 | $ | 17,105 | |||||
Accrued liabilities |
18,243 | 28,819 | |||||||
Current portion of notes payable and other long-term obligations |
410 | 374 | |||||||
Current portion of deferred revenue |
2,981 | 1,044 | |||||||
Total current liabilities |
34,756 | 47,342 | |||||||
Notes payable and other long-term obligations |
400 |
793 |
|||||||
Long-term portion of deferred revenue |
8,426 | 9,263 | |||||||
Deferred tax liability |
10,091 | — | |||||||
Total liabilities |
53,673 | 57,398 | |||||||
Commitments |
|||||||||
Stockholders' equity |
|||||||||
Preferred stock, $.01 par value, 4,700,000 shares authorized; none issued or outstanding |
— | — | |||||||
Common stock, $.10 par value; 100,000,000 shares authorized; 33,269,686 and 33,147,360 shares issued and outstanding, respectively |
3,327 | 3,315 | |||||||
Additional paid-in capital |
557,519 | 553,871 | |||||||
Accumulated other comprehensive loss |
(674 | ) | (3,985 | ) | |||||
Retained earnings |
75,972 | 35,382 | |||||||
Total stockholders' equity |
636,144 | 588,583 | |||||||
Total liabilities and stockholders' equity |
$ | 689,817 | $ | 645,981 | |||||
See accompanying notes.
75
MARTEK BIOSCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
In thousands, except per share data
|
2009 | 2008 | 2007 | ||||||||
Revenues: |
|||||||||||
Product sales |
$ | 329,135 | $ | 336,609 | $ | 292,549 | |||||
Contract manufacturing and services |
16,062 | 15,753 | 14,264 | ||||||||
Total revenues |
345,197 | 352,362 | 306,813 | ||||||||
Cost of revenues: |
|||||||||||
Cost of product sales |
182,385 | 192,848 | 179,367 | ||||||||
Cost of contract manufacturing and services |
14,252 | 14,000 | 13,952 | ||||||||
Total cost of revenues |
196,637 | 206,848 | 193,319 | ||||||||
Gross margin |
148,560 | 145,514 | 113,494 | ||||||||
Operating expenses: |
|||||||||||
Research and development |
27,448 | 26,223 | 24,853 | ||||||||
Selling, general and administrative |
50,027 | 54,181 | 44,855 | ||||||||
Amortization of intangible assets |
6,389 | 7,422 | 6,558 | ||||||||
Restructuring charge |
— | — | 853 | ||||||||
Other operating expenses |
1,080 | 1,504 | 1,614 | ||||||||
Total operating expenses |
84,944 | 89,330 | 78,733 | ||||||||
Income from operations |
63,616 | 56,184 | 34,761 | ||||||||
Interest and other income, net |
806 |
1,647 |
1,144 |
||||||||
Interest expense |
(378 | ) | (510 | ) | (2,233 | ) | |||||
Income before income tax provision |
64,044 |
57,321 |
33,672 |
||||||||
Income tax provision |
23,454 | 19,654 | 1,659 | ||||||||
Net income |
$ | 40,590 | $ | 37,667 | $ | 32,013 | |||||
Net income per share |
|||||||||||
Basic |
$ | 1.22 | $ | 1.14 | $ | 0.99 | |||||
Diluted |
$ | 1.22 | $ | 1.13 | $ | 0.98 | |||||
Weighted average common shares outstanding |
|||||||||||
Basic |
33,207 | 32,951 | 32,336 | ||||||||
Diluted |
33,363 | 33,284 | 32,593 | ||||||||
See accompanying notes.
76
MARTEK BIOSCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
| |
Common Stock | |
Accumulated Other Comprehensive Income (Loss) |
Retained Earnings (Accumulated Deficit) |
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-in Capital |
|
||||||||||||||||||||
In thousands, except share data
|
Shares | Amount | Total | |||||||||||||||||||
Balance at October 31, 2006 |
32,156,162 | $ | 3,216 | $ | 523,486 | $ | 171 | $ | (34,298 | ) | $ | 492,575 | ||||||||||
Issuance of common stock under employee stock plans |
179,460 | 18 | 2,747 | — | — | 2,765 | ||||||||||||||||
Equity-based compensation |
— | — | 2,710 | — | — | 2,710 | ||||||||||||||||
Tax impact of exercise of non-qualified stock options |
— | — | 1,632 | — | — | 1,632 | ||||||||||||||||
Net income |
— | — | — | — | 32,013 | 32,013 | ||||||||||||||||
Other comprehensive income (loss): |
||||||||||||||||||||||
Realized gain on exchange rate forward contracts, net of tax of $(102) |
— | — | — | (171 | ) | — | (171 | ) | ||||||||||||||
Unrealized gain on exchange rate forward contracts, net of tax of $116 |
— | — | — | 203 | — | 203 | ||||||||||||||||
Comprehensive income |
32,045 | |||||||||||||||||||||
Balance at October 31, 2007 |
32,335,622 | 3,234 | 530,575 | 203 | (2,285 | ) | 531,727 | |||||||||||||||
Issuance of shares related to OmegaTech contingent purchase price |
340,946 | 34 | 10,130 | — | — | 10,164 | ||||||||||||||||
Issuance of common stock under employee stock plans |
470,792 | 47 | 8,817 | — | — | 8,864 | ||||||||||||||||
Shares withheld for tax payments upon vesting of restricted stock units |
— | — | (626 | ) | — | — | (626 | ) | ||||||||||||||
Equity-based compensation |
— | — | 3,192 | — | — | 3,192 | ||||||||||||||||
Tax impact of exercise of non-qualified stock options and vesting of restricted stock units, net |
— | — | 1,783 | — | — | 1,783 | ||||||||||||||||
Net income |
— | — | — | — | 37,667 | 37,667 | ||||||||||||||||
Other comprehensive income (loss): |
||||||||||||||||||||||
Unrealized loss on available-for-sale securities, net of tax of $(669) |
— | — | — | (1,120 | ) | — | (1,120 | ) | ||||||||||||||
Realized gain on exchange rate forward contracts, net of tax of $(163) |
— | — | — | (286 | ) | — | (286 | ) | ||||||||||||||
Unrealized loss on exchange rate forward contracts, net of tax of $(1,666) |
— | — | — | (2,782 | ) | — | (2,782 | ) | ||||||||||||||
Comprehensive income |
33,479 | |||||||||||||||||||||
Balance at October 31, 2008 |
33,147,360 | 3,315 | 553,871 | (3,985 | ) | 35,382 | 588,583 | |||||||||||||||
Issuance of common stock under employee stock plans |
122,326 | 12 | 664 | — | — | 676 | ||||||||||||||||
Shares withheld for tax payments upon vesting of restricted stock units |
— | — | (545 | ) | — | — | (545 | ) | ||||||||||||||
Equity-based compensation |
— | — | 3,993 | — | — | 3,993 | ||||||||||||||||
Tax impact of exercise of non-qualified stock options and vesting of restricted stock units, net |
— | — | (464 | ) | — | — | (464 | ) | ||||||||||||||
Net income |
— | — | — | — | 40,590 | 40,590 | ||||||||||||||||
Other comprehensive income (loss): |
||||||||||||||||||||||
Reclassification of available-for-sale securities, net of tax of $383 |
— | — | — | 646 | — | 646 | ||||||||||||||||
Unrealized loss on available-for-sale securities, net of tax of $(119) |
— | — | — | (201 | ) | — | (201 | ) | ||||||||||||||
Realized loss on exchange rate forward contracts, net of tax of $799 |
— | — | — | 1,362 | — | 1,362 | ||||||||||||||||
Unrealized gain on exchange rate forward contracts, net of tax of $912 |
— | — | — | 1,504 | — | 1,504 | ||||||||||||||||
Comprehensive income |
43,901 | |||||||||||||||||||||
Balance at October 31, 2009 |
33,269,686 | $ | 3,327 | $ | 557,519 | $ | (674 | ) | $ | 75,972 | $ | 636,144 | ||||||||||
See accompanying notes.
77
MARTEK BIOSCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
Year ended October 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
In thousands
|
2009 | 2008 | 2007 | ||||||||||
Operating activities |
|||||||||||||
Net income |
$ |
40,590 |
$ |
37,667 |
$ |
32,013 |
|||||||
Adjustments to reconcile net income to net cash provided by operating activities: |
|||||||||||||
Depreciation and amortization |
28,948 | 28,633 | 26,466 | ||||||||||
Provision for inventory obsolescence |
3,300 | 2,200 | 900 | ||||||||||
Deferred tax provision |
21,567 | 16,636 | 479 | ||||||||||
Equity-based compensation expense |
3,947 | 3,200 | 2,744 | ||||||||||
Incremental tax benefit from exercise of non-qualified stock options and vesting of restricted stock units |
— | (1,783 | ) | (507 | ) | ||||||||
Loss on asset disposal and other, net |
775 | 958 | 1,104 | ||||||||||
Changes in operating assets and liabilities: |
|||||||||||||
Accounts receivable |
(3,867 | ) | 1,205 | (9,129 | ) | ||||||||
Inventories |
(19,880 | ) | 7,648 | 1,976 | |||||||||
Other assets |
(355 | ) | 2,892 | 1,968 | |||||||||
Accounts payable |
(4,207 | ) | (1,382 | ) | (3,545 | ) | |||||||
Accrued liabilities |
(5,856 | ) | 9,980 | (7,642 | ) | ||||||||
Deferred revenue and other liabilities |
860 | (1,306 | ) | (967 | ) | ||||||||
Net cash provided by operating activities |
65,822 | 106,548 | 45,860 | ||||||||||
Investing activities |
|||||||||||||
Sales and maturities of investments |
200 |
8,475 |
6,850 |
||||||||||
Purchases of investments |
— | (16,925 | ) | (275 | ) | ||||||||
Expenditures for property, plant and equipment |
(8,932 | ) | (9,785 | ) | (8,279 | ) | |||||||
Repurchase from sale-leaseback transaction and other |
— | — | (3,010 | ) | |||||||||
Capitalization of intangible and other assets |
(18,535 | ) | (3,895 | ) | (6,010 | ) | |||||||
Net cash used in investing activities |
(27,267 | ) | (22,130 | ) | (10,724 | ) | |||||||
Financing activities |
|||||||||||||
Repayments of notes payable and other long-term obligations |
(118 |
) |
(8,917 |
) |
(1,013 |
) |
|||||||
Repayments under revolving credit facility, net |
— | — | (36,000 | ) | |||||||||
Issuance of common stock under employee stock plans |
676 | 8,864 | 2,765 | ||||||||||
Tax payments from shares withheld upon vesting of restricted stock units |
(545 | ) | (626 | ) | — | ||||||||
Incremental tax benefit from exercise of non-qualified stock options and vesting of restricted stock units |
— | 1,783 | 507 | ||||||||||
Net cash provided by (used in) financing activities |
13 | 1,104 | (33,741 | ) | |||||||||
Net increase in cash and cash equivalents |
38,568 | 85,522 | 1,395 | ||||||||||
Cash and cash equivalents, beginning of year |
102,495 | 16,973 | 15,578 | ||||||||||
Cash and cash equivalents, end of year |
$ | 141,063 | $ | 102,495 | $ | 16,973 | |||||||
Supplemental cash flow disclosures: |
|||||||||||||
Interest paid |
$ | 199 | $ | 464 | $ | 2,478 | |||||||
Income taxes paid |
$ | 1,367 | $ | 2,902 | $ | 530 | |||||||
Issuance of shares related to OmegaTech contingent purchase price |
$ | — | $ | 10,164 | $ | — | |||||||
Accrual of contingent purchase price to be settled in shares of Martek common stock |
$ | — | $ | — | $ | 10,164 | |||||||
See accompanying notes.
78
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND DESCRIPTION OF BUSINESS
Martek Biosciences Corporation (the "Company" or "Martek"), a Delaware corporation, was founded in 1985. The Company is a leader in the innovation and development of high value nutritional products from microbial sources, including algae, fungi and other microbes, that promote health and wellness. The Company's innovation and development is based on its core expertise, broad experience and proprietary technology in areas such as microbial biology, algal genomics, fermentation and oil processing. The Company refers to this set of complementary abilities as Martek's "research engine." Through this research engine, Martek has developed and commercialized life'sDHA™, a vegetarian source of the omega-3 fatty acid DHA (docosahexaenoic acid), for use in infant formula, pregnancy and nursing products, foods and beverages, dietary supplements and animal feed. Martek also produces life'sARA™, a vegetarian source of the omega-6 fatty acid ARA (arachidonic acid), for use in infant formula.
Research has shown that DHA and ARA may enhance mental and visual development in infants. In addition, research has shown that DHA may play a pivotal role in brain function throughout life and may reduce the risk of cardiovascular disease. Low levels of DHA in adults have also been linked to a variety of health risks, including Alzheimer's disease, dementia, cardiovascular problems and various other neurological and visual disorders. Further research is underway to assess the role of supplementation with the Company's DHA on mitigating a variety of health risks.
Martek also provides contract manufacturing and performs technical collaboration work with corporate partners. The contract manufacturing services are for both large and small companies and relate primarily to the production of enzymes, specialty chemicals, vitamins, and agricultural specialty products. Collaboration work utilizes the Company's core expertise in microbial biology, algal genomics, fermentation and oil processing to collaborate with these corporate partners in the development of new products and technologies.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation The consolidated financial statements include the accounts of Martek and its wholly-owned subsidiaries, Martek Biosciences Boulder Corporation and Martek Biosciences Kingstree Corporation, after elimination of all significant intercompany balances and transactions. In the opinion of management, all adjustments (consisting of normal recurring adjustments) considered necessary for a fair presentation have been included.
Subsequent Events Management has evaluated subsequent events through December 29, 2009, which is the date the Company's financial statements were issued. No material subsequent events have occurred during the period October 31, 2009 to December 29, 2009 that would require recognition or additional disclosure in these financial statements.
Use of Estimates The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the Company's consolidated financial statements and accompanying notes. On an ongoing basis, the Company evaluates its estimates and judgments, which are based on historical and anticipated results and trends and on various other assumptions that the Company believes to be reasonable under the circumstances. By their nature, estimates are subject to an inherent degree of uncertainty and, as such, actual results may differ from the Company's estimates.
79
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Segment Information The Company currently operates in one material business segment, the development and commercialization of high value nutritional products from microbial sources, including algae, fungi and other microbes. The Company is managed and operated as one business. The entire business is comprehensively managed by a single management team that reports to the Chief Executive Officer. The Company does not operate any material separate lines of business or separate business entities with respect to its products or product candidates. Accordingly, the Company does not have separately reportable segments.
Revenue Recognition The Company derives revenue from three sources: product sales, contract manufacturing and collaborations. The Company recognizes product sales revenue when persuasive evidence of an arrangement exists, the fee is fixed or determinable, collectibility is probable, the product is shipped and title and risk of loss are transferred. A number of infant formula license contracts include an upfront license fee, a prepayment of product sales and established pricing on future product sales, which also may include discounts based on the achievement of certain volume purchases. The consideration from these contracts is allocated based on the relative fair values of the separate elements. Revenue is recognized on product sales when goods are shipped and all other conditions for revenue recognition are met. If volume pricing discounts are deemed to be a separate element, revenue on related product shipments is recognized using the estimated average price to the customer over the term of the discount period, which requires an estimation of total production shipments over that time frame. Amounts billed in excess of the estimated average price are recorded as deferred revenue. Conversely, a receivable is recorded for the excess of the estimated average price over amounts billed. Estimates of average prices are reviewed and, if necessary, adjusted periodically based on updated estimates of product shipments during each contract year. The Company's historical estimates of product shipments have approximated actual results. Amounts recorded as either deferred revenue or a receivable are settled at the end of each contract year, which generally is December 31. Cash received as a prepayment on future product purchases is deferred and recognized as revenue when product is shipped. Revenue from product licenses is deferred and recognized on a straight-line basis over the term of the agreement. The Company's terms of sale allow for limited rights of return with such rights based solely on compliance with agreed-upon customer product specifications. The Company tests the product for compliance with customer product specifications prior to shipment. Such returns have not been material.
Royalty income is recorded when earned, based on information provided by the Company's customers. Royalty income was approximately $1.3 million, $2.1 million and $2.4 million in fiscal 2009, 2008 and 2007, respectively, and is included in product sales revenue in the consolidated statements of income.
Contract manufacturing revenue is recognized when goods are shipped to customers and all other conditions for revenue recognition are met. Cash received that is related to future performance under such contracts is deferred and recognized as revenue when earned.
Revenue earned from collaboration work may come from stand-alone arrangements for certain discrete development work or multiple deliverable arrangements that include such development work followed by larger-scale manufacturing efforts. Revenue is recognized based on the nature of the arrangements, with each of the multiple deliverables in a given arrangement having distinct and separate fair values. Fair values are determined via consistent pricing between stand-alone arrangements and multiple deliverable arrangements, as well as a competitive bidding process. Collaborations may be performed on a time and materials basis or fixed fee basis. For time and
80
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
materials arrangements, revenue is recognized as services are performed and billed. For fixed fee arrangements, revenue is recognized upon completion and acceptance by the customer.
Shipping Income and Costs Shipping costs charged to customers are recorded as revenue in the period that the related product sale revenue is recorded, and associated costs of shipping are included in cost of revenues. Shipping and handling costs were approximately $2.3 million, $2.7 million and $1.6 million in fiscal 2009, 2008 and 2007, respectively.
Income Taxes Deferred tax assets and liabilities are determined based on temporary differences between the financial reporting bases and the tax bases of assets and liabilities. Deferred tax assets are also recognized for tax net operating loss carryforwards. These deferred tax assets and liabilities are measured using the enacted tax rates and laws expected to be in effect when such amounts are projected to reverse or be utilized. The realization of total deferred tax assets is contingent upon the generation of future taxable income in the tax jurisdictions in which the deferred tax assets are located. Valuation allowances are provided to reduce such deferred tax assets to amounts more likely than not to be ultimately realized.
The Company recognizes the benefits of tax positions in the financial statements if such positions are more likely than not to be sustained upon examination by the taxing authority and satisfy the appropriate measurement criteria. If the recognition threshold is met, the tax benefit is generally measured and recognized as the tax benefit having the highest likelihood, in management's judgment, of being realized upon ultimate settlement with the taxing authority, assuming full knowledge of the position and all relevant facts. The Company also recognizes interest and penalties accrued related to unrecognized tax benefits in the provision for income taxes. The Company believes appropriate provisions for all outstanding issues have been made for all jurisdictions and all open tax years.
Foreign Currency Transactions and Hedging Activities Foreign currency transactions are translated into U.S. dollars at prevailing rates. Gains or losses resulting from foreign currency transactions are included in current period income or loss as incurred. All material transactions of the Company are denominated in U.S. dollars with the exception of a portion of purchases of ARA from DSM Food Specialties B.V. ("DSM"), which are denominated in Euros.
The Company periodically enters into foreign currency forward contracts to reduce its transactional foreign currency exposures associated with the purchases of ARA from DSM. The Company does not use derivative financial instruments for speculative purposes. These forward contracts are highly effective cash flow hedges and qualify for hedge accounting. Consequently, the resulting unrealized gains and losses are recorded as a component of other comprehensive income until the related product is sold. As of October 31, 2009, there were no outstanding forward contracts. Amounts recorded due to hedge ineffectiveness have not been material.
Research and Development Research and development costs are charged to operations as incurred. These costs include internal labor, materials and overhead expenses associated with the Company's ongoing research and development activity as well as third-party costs for contracted work and clinical trials.
Advertising Advertising costs are expensed as incurred. Advertising costs, including print, television and internet-based advertising, were approximately $1.9 million in fiscal 2009 and $2.7 million in each of fiscal 2008 and fiscal 2007.
81
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Other Operating Expenses Other operating expenses relate primarily to internal production and contract manufacturing start-up costs, including materials, training and other such costs, incurred in connection with the expansion of the Company's internal manufacturing operations and costs incurred in connection with production trials and the qualification of certain third-party manufacturers. All such costs are expensed as incurred.
Equity-Based Compensation The Company records compensation cost for all equity-based payments based on the grant date fair value. The Company utilizes the "straight-line" method for allocating compensation cost by period.
Net Income Per Share Basic net income per share is computed using the weighted average number of shares of common stock outstanding during the period. Diluted net income per share is computed using the weighted average number of shares of common stock outstanding, giving effect to stock options and restricted stock units using the treasury stock method.
Comprehensive Income Comprehensive income is comprised of net earnings and other comprehensive income, which includes certain changes in equity that are excluded from net income. The Company includes unrealized holding gains and losses on available-for-sale securities as well as changes in the market value of exchange rate forward contracts designated as cash flow hedges in other comprehensive income in the Consolidated Statements of Stockholders' Equity.
Cash and Cash Equivalents Cash equivalents consist of highly liquid investments with an original maturity from date of purchase of three months or less.
Investments and Marketable Securities The Company has classified investments and marketable securities at October 31, 2009 as either trading or available-for-sale and at October 31, 2008 as available-for-sale. Unrealized gains and losses on available-for-sale securities are reported as accumulated other comprehensive income, which is a separate component of stockholders' equity. Unrealized gains and losses on trading securities and realized gains and losses on both types of securities are included in other income as incurred based on the specific identification method.
The Company periodically evaluates whether any declines in the fair value of its available-for-sale investments are other than temporary. This evaluation consists of a review of several factors, including, but not limited to: length of time and extent that a security has been in an unrealized loss position; the existence of an event that would impair the issuer's future earnings potential; the near term prospects for recovery of the market value of a security; the intent of the Company to sell the impaired security; and whether the Company will be required to sell the security prior to the anticipated recovery in market value. Declines in value below cost for debt securities where it is considered probable that all contractual terms of the security will be satisfied, where the decline is due primarily to changes in interest rates (and not because of increased credit risk), and where the Company does not intend to sell the investment prior to a period of time sufficient to allow a market recovery, are assumed to be temporary. If management determines that an other-than-temporary impairment exists, the carrying value of the investment will be reduced to the current fair value of the investment and if such impairment results from credit-related matters, the Company will recognize a charge in the consolidated statements of income equal to the amount of the carrying value reduction. Other-than-temporary impairment write-downs resulting from non-credit-related matters are recognized in other comprehensive income.
82
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The fair value option for financial assets and liabilities permits an entity to elect to measure eligible items at fair value ("fair value option"), including many financial instruments. The decision to elect the fair value option is made individually for each instrument and is irrevocable once made. Changes in fair value for the selected instruments are recorded in earnings. The Company has elected the fair value option for the auction rate securities rights agreement (the "Put Agreement"), which is recorded within short-term investments at October 31, 2009. The Put Agreement is the only instrument of its nature or type held by the Company and for which the Company has elected the fair value option. See Note 5 for further discussion.
The Company classifies its investments as either current or long-term based upon the investments' contractual maturities and the Company's ability and intent to convert such instruments to cash within one year.
Fair Value of Financial Instruments The Company considers the carrying cost of its financial assets and liabilities, which consist primarily of cash and cash equivalents, investments and marketable securities, accounts receivable, accounts payable, notes payable and long-term debt, to approximate the fair value of the respective assets and liabilities at October 31, 2009 and 2008. See Note 6 for further discussion of the Company's fair value measurements.
Trade Receivables Trade receivables are reported in the consolidated balance sheets at outstanding principal less any allowance for doubtful accounts. The Company writes off uncollectible receivables against the allowance for doubtful accounts when the likelihood of collection is remote. The Company considers receivables past due if not paid by their respective due date. The Company performs ongoing credit evaluations of its customers and, in general, extends credit without requiring collateral. The Company maintains an allowance for doubtful accounts, which is determined based on historical experience, existing economic conditions and management's expectations of losses. The Company analyzes historical bad debts, customer concentrations, customer creditworthiness and current economic trends when evaluating the adequacy of the allowance for doubtful accounts. Losses have historically been within management's expectations. The allowance for doubtful accounts was approximately $100,000 and $20,000 as of October 31, 2009 and 2008, respectively.
Concentration of Credit Risk and Significant Customers Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of accounts receivable. Concentrations of credit risk with respect to accounts receivable are present due to the relatively small number of customers comprising the Company's customer base. However, the credit risk is reduced through the Company's ongoing efforts to monitor its exposure for credit losses. Five customers accounted for approximately 77%, 80% and 85% of the Company's product sales in fiscal 2009, 2008 and 2007, respectively. At October 31, 2009 and 2008, five customers accounted for approximately 60% and 74%, respectively, of the Company's outstanding accounts receivable balance. Included in these amounts is one of the Company's customers that accounted for approximately 30%, 36%, and 42% of total product sales in fiscal 2009, 2008 and 2007, respectively, and represented 26% and 34% of the Company's outstanding accounts receivable balance at October 31, 2009 and 2008, respectively. Approximately 53%, 55% and 60% of the Company's sales were to domestic customers in each of fiscal 2009, 2008 and 2007, respectively.
Inventories Inventories are stated at the lower of cost or market and include appropriate elements of material, labor and indirect costs. Inventories are valued using a weighted average approach that approximates the first-in, first-out method. The Company analyzes both historical and projected sales
83
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
volumes and, when needed, reserves for inventory that is either obsolete, slow moving or impaired. Abnormal amounts of inventory costs related to, among other things, idle facilities, freight handling and waste material expenses are recognized as period charges and expensed as incurred.
Property, Plant and Equipment Property, plant and equipment, including leasehold improvements, is stated at cost and depreciated or amortized when available for commercial use by applying the straight-line method, based on useful lives as follows:
Asset Description
|
Useful Life (years) | |||
|---|---|---|---|---|
Building |
15 - 30 | |||
Fermentation equipment |
10 - 20 | |||
Oil processing equipment |
10 - 20 | |||
Other machinery and equipment |
5 - 10 | |||
Furniture and fixtures |
5 - 7 | |||
Computer hardware and software |
3 - 7 | |||
Leasehold improvements are amortized over the shorter of the useful life of the asset or the lease term, including renewals when probable. Costs for capital assets not yet available for commercial use have been capitalized as construction in progress. Costs for repairs and maintenance are expensed as incurred.
Patent Costs The Company has filed a number of patent applications in the U.S. and in foreign countries. Certain external legal and related costs are incurred in connection with patent applications. If a future economic benefit is anticipated from the resulting patent or an alternate future use is available to the Company, such costs are capitalized and amortized over the expected life of the patent. The Company also capitalizes external legal costs incurred in the defense of its patents when it is believed that the future economic benefit of the patent will be maintained or increased and a successful defense is probable. Capitalized patent defense costs are amortized over the remaining life of the related patent.
Goodwill and Other Intangible Assets Goodwill is tested for impairment annually, on August 1, or more frequently when events occur or circumstances change that would more likely than not reduce the fair value of the asset below its carrying amount. The Company operates as one reporting unit and determines fair value of the reporting unit using the market approach.
Purchased intangible assets other than goodwill are amortized over their useful lives unless these lives are determined to be indefinite. The Company's intangible assets are carried at cost less accumulated amortization. Amortization is computed over the estimated useful lives of the respective assets, generally ten to seventeen years.
Impairment of Long-Lived Assets The Company reviews long-lived assets and certain identifiable intangibles for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. Assets are grouped and evaluated for impairment at the lowest level for which there is identified cash flows. The Company deems an asset to be impaired if a forecast of undiscounted cash flows is less than its carrying amount. The impairment to be recognized is measured by the amount by which the carrying amount of assets exceeds the fair value of the assets. The
84
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Company generally measures fair value by discounting projected future cash flows. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Recently Issued Accounting Pronouncements
In June 2009, the Financial Accounting Standards Board ("FASB") established the FASB Accounting Standards Codification ("ASC"), or Codification, which on July 1, 2009 became the single official source of authoritative, nongovernmental GAAP, superseding existing FASB, AICPA, EITF and related literature. Prospectively, only one level of authoritative GAAP will exist, excluding the guidance issued by the SEC. All other literature will be non-authoritative. The Codification does not change GAAP but instead reorganizes the U.S. GAAP pronouncements into accounting topics, and displays all topics using a consistent structure. As the Codification does not change GAAP, it did not have a material impact on the Company's financial statements. Previous references to applicable literature via the Company's disclosures have been updated with references to the new Codification section.
In December 2007, the FASB issued Statement No. 141(R), "Business Combinations", which has principally been codified in ASC 805, "Business Combinations" ("ASC 805"). ASC 805 establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, any noncontrolling interest in the acquiree and the goodwill acquired. ASC 805 also establishes disclosure requirements to enable the evaluation of the nature and financial effects of the business combination. ASC 805 is effective for financial statements issued for fiscal years beginning after December 15, 2008 and will be adopted by the Company in the first quarter of fiscal 2010. The Company is currently evaluating the overall impact of the adoption of ASC 805 on its consolidated financial position and results of operations, which impact will be largely dependent on the size and nature of any business combination completed after the adoption of this standard. However, as ASC 805 requires the immediate expensing of acquisition-related costs, to the extent that the Company incurs such costs in connection with its ongoing process of evaluating merger and acquisition candidates, these costs will have an impact on the Company's results of operations in the period incurred.
In April 2009, the FASB issued guidance that has been codified as the following: (i) ASC 820-10-65-4, "Transition Related to FASB Staff Position FAS 157-4, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly", (ii) ASC 320-10-65-1, "Transition Related to FASB Staff Position FAS 115-2 and FAS 124-2, Recognition and Presentation of Other-Than-Temporary Impairments", and (iii) ASC 825-10-65-1, "Transition Related to FASB Staff Position FAS 107-1 and APB 28-1, Interim Disclosures about Fair Value of Financial Instruments", each of which was effective for the Company beginning with the third quarter of fiscal 2009. ASC 820-10-65-4 provides guidance on how to determine the fair value of assets and liabilities in the current economic environment and reemphasizes that the objective of a fair value measurement remains the determination of an exit price. ASC 320-10-65-1 modifies the requirements for recognizing other-than-temporarily impaired debt securities and revises the existing impairment model for such securities by modifying the current intent and ability indicator in determining whether a debt security is other-than-temporarily impaired. ASC 825-10-65-1 enhances the disclosure of instruments for both interim and annual periods. The adoption of these pronouncements did not have a material effect on the Company's consolidated financial position, results of operations or cash flows.
85
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. EQUITY-BASED COMPENSATION
As of October 31, 2009, the Company had stock options and restricted stock units outstanding that were previously granted under the Company's 1997 Stock Option Plan, the 2001 Stock Option Plan, the 2002 Stock Incentive Plan, the 2003 New Employee Stock Option Plan and the 2004 Stock Incentive Plan, collectively referred to as the "Equity-Based Compensation Plans." Option awards under the Equity-Based Compensation Plans are granted at prices as determined by the Compensation Committee, but are not less than the fair market value of the Company's common stock on the date of grant. Stock options granted and outstanding include only non-qualified options and vest over a period of up to five years and have a maximum term of ten years from the date of grant. Restricted stock units granted generally vest over periods of up to 62 months from the date of grant. At October 31, 2009, approximately 1.1 million shares of common stock were available for future grants under the Equity-Based Compensation Plans.
Stock Options
The Company did not grant any stock options during the years ended October 31, 2009 and 2007, and the Company granted 18,094 stock options during the year ended October 31, 2008. The Company has utilized the Black-Scholes-Merton valuation model for estimating the fair value of the stock options granted. Based upon the Black-Scholes-Merton valuation model, the stock options granted in fiscal 2008 had a total fair value of $150,000. As follows are the weighted average assumptions used in valuing the stock options granted during the year ended October 31, 2008 and a discussion of the Company's methodology for developing each of the assumptions used:
| |
Year ended October 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||
Expected volatility |
n/a | 48.5 | % | n/a | |||
Risk-free interest rate |
n/a | 3.2 | % | n/a | |||
Expected life of options |
n/a | 2 years | n/a | ||||
Expected dividend yield |
n/a | 0 | % | n/a | |||
Forfeiture rate |
n/a | 0 | % | n/a | |||
Expected Volatility—Volatility is a measure of the amount by which a financial variable such as a share price has fluctuated (historical volatility) or is expected to fluctuate (expected volatility) during a period. The Company uses the historical volatility over the preceding five-year period to estimate expected volatility. From fiscal 2005 through fiscal 2009, the Company's annual volatility ranged from 48.5% to 62.9% with an average of 58.0%.
Risk-Free Interest Rate—This is the average U.S. Treasury rate (having a term that most closely resembles the expected life of the option) for the quarter in which the option was granted.
Expected Life of Options—This is the period of time that the options granted are expected to remain outstanding. This estimate is based primarily on historical exercise data. Options granted during the year ended October 31, 2008 have a maximum term of ten years.
Expected Dividend Yield—The Company has never declared or paid dividends on its common stock and does not anticipate paying any dividends in the foreseeable future.
Forfeiture Rate—This is the estimated percentage of options granted that are expected to be forfeited or cancelled on an annual basis before becoming fully vested. The Company estimates the
86
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. EQUITY-BASED COMPENSATION (Continued)
forfeiture rate based on past turnover data with further consideration given to the level of the employees to whom the options were granted.
The weighted average fair market value of the options at the date of grant for options granted during the year ended October 31, 2008 was $8.29. A summary of the Company's options as of October 31, 2009 is presented below:
| |
Number of Shares (in thousands) |
Weighted Average Price per Share |
Aggregate Intrinsic Value (in thousands) |
Weighted Average Remaining Contractual Term (years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Options outstanding at November 1, 2008 |
2,587 | $ | 41.04 | ||||||||||
Granted |
— | $ | — | ||||||||||
Exercised |
(51 | ) | $ | 13.68 | |||||||||
Cancelled and forfeited |
(88 | ) | $ | 46.67 | |||||||||
Options outstanding at October 31, 2009 |
2,448 | $ | 41.41 | $ | 929 | 3.8 | |||||||
Options exercisable at October 31, 2009 |
2,444 | $ | 41.43 | $ | 929 | 3.8 | |||||||
Vested and expected to vest at October 31, 2009 |
2,448 | $ | 41.41 | $ | 929 | 3.8 | |||||||
The total intrinsic value of stock options exercised during the years ended October 31, 2009, 2008 and 2007 was approximately $500,000, $4.5 million and $1.6 million, respectively. The total fair value of options vested during the years ended October 31, 2009, 2008 and 2007 was approximately $200,000, $1.0 million and $2.8 million, respectively. Unrecognized compensation cost related to unvested stock options as of October 31, 2009 is not material.
Restricted Stock Units
Restricted stock units represent rights to receive common shares at a future date. There is no exercise price and no monetary payment is required for receipt of restricted stock units or the shares issued in settlement of the award. The Company granted 302,801, 223,143 and 281,395 restricted stock units during years ended October 31, 2009, 2008 and 2007, respectively, which generally vest over periods of up to 62 months from the date of grant. The total fair value of the restricted stock units granted during the years ended October 31, 2009, 2008 and 2007 of $7.9 million, $7.3 million and $6.4 million, respectively, was based on the fair market value of the Company's common stock on the date of grant.
The fair value at the time of the grant is amortized to expense on a straight-line basis over the period of vesting. Pre-vesting forfeitures were estimated to be approximately 5%. The weighted average grant date fair value of restricted stock units granted during the years ended October 31, 2009, 2008 and 2007 was $26.03, $32.88 and $22.69, respectively. The total fair value of restricted stock units vested during the years ended October 31, 2009 and 2008 was approximately $2.9 million ($28.25 weighted average fair value per share) and $1.8 million ($23.06 weighted average fair value per share),
87
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. EQUITY-BASED COMPENSATION (Continued)
respectively. A summary of the Company's restricted stock units as of October 31, 2009 is presented below:
| |
Number of Shares (in thousands) |
Aggregate Intrinsic Value (in thousands) |
Weighted Average Remaining Contractual Term (years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Restricted stock units outstanding at November 1, 2008 |
386 | |||||||||
Granted |
303 | |||||||||
Vested |
(102 | ) | ||||||||
Cancelled and forfeited |
(15 | ) | ||||||||
Restricted stock units outstanding at October 31, 2009 |
572 | $ | 10,263 | 7.4 | ||||||
Vested and expected to vest at October 31, 2009 |
538 | $ | 9,654 | 7.4 | ||||||
As of October 31, 2009, there was approximately $12.7 million remaining in unrecognized compensation cost related to restricted stock units. The cost is expected to be recognized through fiscal 2014 with a weighted average recognition period of approximately two years.
The Company recognized $4.0 million, $3.2 million and $2.7 million in the years ended October 31, 2009, 2008 and 2007, respectively, in compensation cost related to equity-based compensation arrangements. Such costs were recorded approximately 75%, 15% and 10% as selling, general and administrative expenses, research and development expenses and cost of revenues, respectively, in all periods. The total income tax benefit recognized in the income statement for equity-based compensation arrangements was approximately $1.5 million, $1.2 million and $1.0 million in the years ended October 31, 2009, 2008 and 2007, respectively. Equity-based compensation cost capitalized as part of inventory during the years ended October 31, 2009, 2008 and 2007 was approximately $400,000, $300,000 and $300,000, respectively.
4. DSM SUPPLY AND LICENSE AGREEMENT
In July 2009, the Company entered into the First Amended and Restated ARA Alliance, Purchase, and Production Agreement (the "Restated Agreement") with DSM. The Restated Agreement, which extends the original supply term through December 31, 2023, amended, consolidated and restated all existing agreements between the two parties governing the cross-licensing, purchase, supply and production of ARA. Among other things, the Restated Agreement established ARA pricing to Martek for calendar years 2009 through 2014 with the potential for only limited changes related to post-2011 volumes and provides that ARA pricing subsequent to calendar 2014 will be negotiated in the future by the parties. For the establishment of the 2009 through 2014 ARA pricing and related contractual provisions, Martek paid DSM approximately $11.0 million. Such payment represents an acquired contract right and thus has been recorded within intangible assets in the accompanying consolidated balance sheet and is being amortized through cost of goods sold through 2014. While, subject to certain limited exceptions, Martek is committed to purchasing all of its ARA requirements from DSM through the term of the Restated Agreement, the Restated Agreement also sets minimum ARA purchase quantities for Martek in calendar years 2009, 2010 and 2011. As of October 31, 2009, the value of the remaining calendar year 2009 and full calendar years 2010 and 2011 minimum purchase requirements
88
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. DSM SUPPLY AND LICENSE AGREEMENT (Continued)
are approximately $17.2 million, $93.9 million and $87.1 million, respectively. These minimum purchase quantities approximate the amounts expected to be consumed by Martek in the normal course of business.
Under certain circumstances, either Martek or DSM may terminate the Restated Agreement after 2012. Upon early termination by Martek, Martek would be required to make a payment to DSM with the value of such payment decreasing over the remaining term of the Restated Agreement and being dependent upon DSM's physical infrastructure at the early termination date. A termination payment by Martek as of January 1, 2013 would currently range from $15 million to $20 million and a termination payment as of January 1, 2016 would currently range from less than $1 million to $7 million.
See Note 9 for discussion of the license granted by DSM to the Company in connection with the April 2004 agreement between the parties.
5. INVESTMENTS
At October 31, 2009 and October 31, 2008, the Company had investments consisting of auction rate securities ("ARS") whose underlying assets are student loans originated under the Federal Family Education Loan Program ("FFELP"). FFELP student loans are guaranteed by state guarantors who have reinsurance agreements with the U.S. Department of Education. These ARS are intended to provide liquidity via an auction process that resets the applicable interest rate approximately every 30 days and allows the Company to either roll over its holdings or gain immediate liquidity by selling such investments at par. The underlying maturities of these investments range from 17 to 38 years. Since February 2008, as a result of negative conditions in the global credit markets, the large majority of the auctions for the Company's investment in these securities have failed to settle, resulting in Martek continuing to hold such securities. Consequently, the investments are not currently liquid and the Company will not be able to access these funds, except as noted below, until a future auction of these investments is successful, a buyer is found outside of the auction process or the investments reach their contractual maturity date.
While Martek continues to receive interest payments on these investments involved in failed auctions, the Company believes that the estimated fair value of these ARS no longer approximates par value. Such fair value was estimated by the Company and considers, among other items, the creditworthiness of the issuer, the collateralization underlying the securities and the timing of expected future cash flows. Based upon these fair value estimates, as of October 31, 2009, Martek has recorded temporary impairments of $1.1 million and other-than-temporary impairments of $1.3 million on such ARS whose original cost basis totals $12.9 million (see below for further discussion of the accounting for such impairments).
As of October 31, 2008, the Company's ARS were classified as "available-for-sale" and totaled $11.3 million. As of October 31, 2008, the Company had recorded an unrealized loss on these ARS of approximately $1.8 million in other comprehensive income. In November 2008, the Company executed a Put Agreement with a financial institution that provides Martek the ability to sell certain of its ARS to the financial institution and allows the financial institution, at its sole discretion, to purchase such ARS at par during the period June 30, 2010 through July 2, 2012. The Company's ARS holdings to which this relates have a cost basis of approximately $7.4 million and a fair value at October 31, 2009 of approximately $6.1 million. Upon execution of the Put Agreement, the Company no longer had the intent or unilateral ability to hold the ARS covered by the Put Agreement to maturity. Therefore, the Company has reclassified such investments from "available-for-sale" to "trading" and has recognized a
89
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. INVESTMENTS (Continued)
loss of approximately $1.3 million during the year ended October 31, 2009. This $1.3 million loss, which was recorded in other income in the accompanying consolidated statements of income, includes approximately $1.0 million of other-than-temporary impairments reclassified from other comprehensive income and approximately $300,000 of net unrealized trading losses occurring after such reclassification.
During the year ended October 31, 2009, the Company recorded the Put Agreement at its fair value, which as of October 31, 2009 is approximately $1.2 million, and recognized a net gain equal to this amount in other income within the accompanying consolidated statements of income. The Company elected to adopt the fair value option for the Put Agreement so that future changes in the fair value of this asset will largely offset the fair value movements of the related ARS. The Company believes that the accounting for the Put Agreement will then match its purpose as an economic hedge to the changes in the fair value of the related ARS. The ability of the Put Agreement to act as an economic hedge is subject to the continued expected performance by the financial institution of its obligations under the agreement. The fair value of the Put Agreement considers, among other things, the creditworthiness of the issuer and the liquidity of the financial instrument. Due to the Company's intent to sell its ARS covered by the Put Agreement to the financial institution on June 30, 2010, the fair value of such ARS along with the fair value of the Put Agreement are classified as short-term investments in the accompanying consolidated balance sheet as of October 31, 2009.
As of October 31, 2009, the Company's ARS holdings not covered by the Put Agreement have a cost basis of approximately $5.6 million and a fair value of approximately $4.5 million. The total decline in fair value of $1.1 million has been recorded as a net reduction to other comprehensive income with approximately $300,000 of such total recorded during the year ended October 31, 2009. The Company believes that the unrealized losses on these ARS are temporary. In making this determination, Martek primarily considered the financial condition of the issuers, collateralization underlying the securities, the intent of the Company to sell the impaired security, and whether the Company will be required to sell the security prior to the anticipated recovery in market value. The Company continues to monitor the market for ARS and consider its impact, if any, on the fair value of these investments. If the Company determines that any valuation adjustment is other than temporary, the Company would record an impairment charge to earnings. Due to the underlying maturities of these investments and the Company's belief that the market for the ARS not covered by the Put Agreement may take in excess of twelve months to fully recover, the fair value of such ARS is classified as long-term investments in the accompanying consolidated balance sheet as of October 31, 2009.
6. FAIR VALUE MEASUREMENTS
As of November 1, 2008, the Company adopted the provisions of guidance codified as ASC 820, "Fair Value Measurements and Disclosures" ("ASC 820") for financial instruments. ASC 820 defines fair value, establishes a fair value hierarchy for assets and liabilities measured at fair value and requires expanded disclosures about fair value measurements. The adoption of ASC 820 did not have a material impact on the Company's financial condition, results of operations, or cash flows; however, the additional disclosures required by ASC 820 are presented below. The ASC 820 hierarchy ranks the quality and reliability of inputs, or assumptions, used in the determination of fair value and requires
90
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. FAIR VALUE MEASUREMENTS (Continued)
assets and liabilities carried at fair value to be classified and disclosed in one of the following three categories:
| Level 1 — | quoted prices in active markets for identical assets and liabilities; | |
Level 2 — |
inputs other than Level 1 quoted prices that are directly or indirectly observable; and |
|
Level 3 — |
unobservable inputs that are not corroborated by market data. |
In October 2008, the FASB issued Staff Position No. 157-3, "Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active" ("FSP 157-3"). FSP 157-3 clarifies the application of ASC 820 to financial assets for which an active market does not exist. Specifically, FSP 157-3 addresses the following application issues: (1) how a reporting entity's own assumptions should be considered in measuring fair value when observable inputs do not exist; (2) how observable inputs in inactive markets should be considered when measuring fair value; and (3) how the use of market quotes should be considered when assessing the relevance of inputs available to measure fair value. FSP 157-3 applies to financial assets within the scope of accounting pronouncements that require or permit fair value measurements in accordance with ASC 820 and was effective upon issuance. Adoption of FSP 157-3 did not materially affect the Company's methodology for determining Level 3 pricing as discussed below.
The Company evaluates financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level at which to classify them for each reporting period. This determination requires highly subjective judgments as to the significance of inputs used in determining fair value and where such inputs lie within the ASC 820 hierarchy.
As of October 31, 2009, the Company held certain assets that are required to be measured at fair value on a recurring basis. These financial assets were as follows (in thousands):
| |
As of October 31, 2009 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level 1 | Level 2 | Level 3 | Balance | |||||||||
Available-for-sale securities(1) |
$ | — | $ | — | $ | 4,495 | $ | 4,495 | |||||
Trading securities(2) |
— | — | 6,080 | 6,080 | |||||||||
Put Agreement(2) |
— | — | 1,221 | 1,221 | |||||||||
Investments in money market funds(3) |
99,007 | — | — | 99,007 | |||||||||
Total |
$ | 99,007 | $ | — | $ | 11,796 | $ | 110,803 | |||||
- (1)
- Included
in long-term investments in the accompanying consolidated balance sheets.
- (2)
- Included
in short-term investments in the accompanying consolidated balance sheets.
- (3)
- Included in cash and cash equivalents in the accompanying consolidated balance sheets.
91
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. FAIR VALUE MEASUREMENTS (Continued)
The table below provides a reconciliation of the beginning and ending balances of the Company's investments measured at fair value using significant unobservable inputs (Level 3) for the year ended October 31, 2009 (in thousands):
| |
Auction Rate Securities |
Put Agreement | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance on November 1, 2008 |
$ | 11,336 | $ | — | $ | 11,336 | ||||||
Transfers to/(from) Level 3 |
— |
— |
— |
|||||||||
Total gains (losses) (realized or unrealized):(1) |
||||||||||||
Included in earnings |
(1,270 | ) | 1,221 | (49 | ) | |||||||
Included in other comprehensive income |
709 | — | 709 | |||||||||
Purchases, sales, issuances and settlements, net |
(200 | ) | — | (200 | ) | |||||||
Balance on October 31, 2009 |
$ | 10,575 | $ | 1,221 | $ | 11,796 | ||||||
- (1)
- See Note 5 for discussion of Auction Rate Securities and related Put Agreement.
Some of the inputs into the discounted cash flow models from which the Company bases its Level 3 valuations for the ARS and Put Agreement are unobservable in the market and have a significant effect on valuation. The assumptions used in preparing the models include, but are not limited to, periodic coupon rates, market required rates of return and the expected term of each security. The coupon rate was estimated using implied forward rate data on interest rate swaps and U.S. Treasuries, and limited where necessary by any contractual maximum rate paid under a scenario of continuing auction failures. Assumptions regarding required rates of return were based on risk-free interest rates and credit spreads for investments of similar credit quality. The expected term for the ARS was based on a weighted probability-based estimate of the time the principal will become available to the Company. The expected term for the Put Agreement was based on the earliest date on which the Company can exercise the Put Agreement rights.
7. INVENTORIES
Inventories consist of the following (in thousands):
| |
October 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||
Finished goods |
$ | 56,203 | $ | 45,871 | |||
Work in process |
56,501 | 49,042 | |||||
Raw materials |
3,475 | 4,640 | |||||
Inventories, net |
$ | 116,179 | $ | 99,553 | |||
Inventory levels are evaluated by management based upon anticipated product demand, shelf-life, future marketing plans and other factors. Reserves for obsolete and slow-moving inventories are recorded for amounts that may not be realizable.
92
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consists of the following (in thousands):
| |
October 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||
Land |
$ | 2,320 | $ | 2,320 | |||
Building and improvements |
67,094 | 64,731 | |||||
Machinery and equipment |
266,257 | 262,831 | |||||
Furniture and fixtures |
3,094 | 2,963 | |||||
Computer hardware and software |
17,220 | 16,119 | |||||
|
355,985 | 348,964 | |||||
Less: accumulated depreciation and amortization |
(113,437 | ) | (91,268 | ) | |||
|
242,548 | 257,696 | |||||
Construction in progress |
9,731 | 8,204 | |||||
Property, plant and equipment, net |
$ | 252,279 | $ | 265,900 | |||
Depreciation and amortization expense on property, plant and equipment totaled approximately $22.5 million, $21.5 million and $20.6 million for the years ended October 31, 2009, 2008 and 2007, respectively.
Assets available for commercial use that were not in productive service had a net book value of $34.4 million and $38.2 million at October 31, 2009 and 2008, respectively. Depreciation as well as certain other fixed costs related to such idle assets is recorded as cost of product sales in the accompanying consolidated statements of income.
9. GOODWILL AND OTHER INTANGIBLE ASSETS
Intangible assets and related accumulated amortization consist of the following (in thousands):
| |
October 31, 2009 | October 31, 2008 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Intangible Asset
|
Gross | Accumulated Amortization |
Net | Gross | Accumulated Amortization |
Net | |||||||||||||
Trademarks |
$ | 2,202 | $ | (1,004 | ) | $ | 1,198 | $ | 2,153 | $ | (850 | ) | $ | 1,303 | |||||
Patents |
25,732 | (9,046 | ) | 16,686 | 22,533 | (7,795 | ) | 14,738 | |||||||||||
Core technology |
1,708 | (797 | ) | 911 | 1,708 | (683 | ) | 1,025 | |||||||||||
Current products |
10,676 | (5,363 | ) | 5,313 | 10,676 | (4,652 | ) | 6,024 | |||||||||||
Licenses and other |
23,191 | (4,668 | ) | 18,523 | 11,968 | (4,061 | ) | 7,907 | |||||||||||
|
63,509 | (20,878 | ) | 42,631 | 49,038 | (18,041 | ) | 30,997 | |||||||||||
Goodwill |
51,592 | — | 51,592 | 51,592 | — | 51,592 | |||||||||||||
|
$ | 115,101 | $ | (20,878 | ) | $ | 94,223 | $ | 100,630 | $ | (18,041 | ) | $ | 82,589 | |||||
In April 2004, the Company was granted a license by DSM related to certain technologies associated with the manufacture of ARA. This grant involved a license fee totaling $10 million, which is being amortized over 15 years using the straight-line method. In addition, as part of the July 2009 Restated Agreement, certain other intangible assets were acquired from DSM by Martek for approximately $11.0 million. See Note 4 for further discussion of these intangible assets.
93
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. GOODWILL AND OTHER INTANGIBLE ASSETS (Continued)
In February 2009, Martek entered into a license agreement with an international food company for certain patented technology expected to be used in the production of Martek's life'sDHA™ for certain applications. Under the agreement, Martek was granted a perpetual and generally exclusive license to the technology. As consideration for this license, Martek paid an upfront license fee of $1.0 million and will pay, subject to the successful completion by the licensor of certain development goals related to the licensed technology, additional fees of up to approximately $5.0 million. In addition, Martek will be required to pay royalties of up to 4.5% of sales of products produced using the licensed technology, including certain minimum royalty payments of approximately $2.0 million, the timing of which is also subject to the successful completion of the development goals. The license fees paid in connection with this arrangement are being amortized over 10 years.
Core technology and current products relate to the value assigned to the assets acquired as part of the OmegaTech acquisition in fiscal 2002.
Most of the amortization associated with the Company's intangible assets is reflected in amortization of intangible assets in the accompanying consolidated statements of income. Included in amortization of intangible assets is approximately $5.6 million, $6.6 million and $5.3 million in the years ended October 31, 2009, 2008 and 2007, respectively, related to assets supporting the Company's commercial products and approximately $800,000, $800,000 and $1.2 million, respectively, related to assets supporting the Company's research and development initiatives. Based on the current amount of intangible assets subject to amortization, the estimated total amortization expense for each year in fiscal 2010 through fiscal 2014 will be approximately $7.6 million, $6.9 million, $5.7 million, $5.5 million and $5.3 million, respectively. As of October 31, 2009, the weighted average remaining useful lives of the Company's trademarks, patents, core technology, current products and licenses are 8 years, 8 years, 8 years, 8 years and 7 years, respectively.
The Company recorded patent amortization expense of approximately $4.0 million, $4.7 million and $4.2 million in the years ended October 31, 2009, 2008 and 2007, respectively.
10. ACCRUED LIABILITIES
Accrued liabilities consist of the following (in thousands):
| |
October 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||
Compensation and employee benefits |
$ | 9,677 | $ | 14,789 | |||
Income and business tax liabilities |
4,091 | 2,535 | |||||
Customer-related |
1,490 | 3,422 | |||||
Forward contracts liabilities |
— | 4,577 | |||||
Other |
2,985 | 3,496 | |||||
|
$ | 18,243 | $ | 28,819 | |||
Customer- related accrued liabilities primarily relate to deferred customer credits and estimated discounts on volume purchases.
94
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. NOTES PAYABLE AND LONG-TERM DEBT
The Company has a $135 million secured revolving credit facility that is collateralized by accounts receivable, inventory and all capital stock of the Company's subsidiaries and expires in September 2010. There were no amounts outstanding under the credit facility in fiscal 2009 or 2008. The weighted average interest rate on amounts outstanding under the revolving credit facility was approximately 7.1% for the year ended October 31, 2007. The weighted average annual commitment fee rate on unused amounts was approximately 0.1% in each of the years ended October 31, 2009, 2008 and 2007. Both the interest and commitment fee rates are based on LIBOR and the Company's current leverage ratio. Among other things, the credit facility agreement contains restrictions on future debt, the payment of dividends and the further encumbrance of assets. In addition, the credit facility requires that the Company comply with specified financial ratios and tests, including minimum coverage ratios and maximum leverage ratios. As of October 31, 2009, the Company was in compliance with all of these debt covenants.
In connection with the purchase of certain assets and the assumption of certain liabilities of FermPro in fiscal 2003, the Company assumed a $10 million secured note. The note was amended in January 2004 and was an unsecured obligation of the Company. The note had a stated interest rate of 5% and principal was amortized utilizing a 20-year period with the outstanding principal due at the maturity date of December 31, 2008. During fiscal 2008, the Company repaid prior to scheduled maturity the full $7.8 million due under this note. The Company also repaid in full during fiscal 2008 $600,000 of principal due under notes payable with originally scheduled maturities of April 2015.
The annual maturities of the Company's notes payable and long-term debt at October 31, 2009 are summarized as follows (in thousands):
Fiscal Year
|
|
|||
|---|---|---|---|---|
2010 |
$ | 95 | ||
2011 |
17 | |||
2012 |
18 | |||
2013 |
19 | |||
2014 |
21 | |||
Thereafter |
242 | |||
|
$ | 412 | ||
During the years ended October 31, 2009, 2008 and 2007, the Company incurred interest on borrowings of approximately $200,000, $500,000 and $2.4 million, respectively, and recorded amortization of related deferred financing fees of approximately $200,000 in each year. Interest costs have been capitalized to the extent that the related borrowings were used to cover the balance of projects under construction. Accordingly, during the years ended October 31, 2008 and 2007, approximately $100,000 and $300,000 of interest, respectively, was capitalized. No interest was capitalized in the year ended October 31, 2009.
The carrying amounts of notes payable at October 31, 2009 and 2008 approximate their fair values based on instruments of similar terms available to the Company.
95
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. COMMITMENTS AND CONTINGENCIES
Leases The Company leases its Columbia, Maryland premises under an operating lease. In fiscal 2009 and 2007, the Company expanded its Columbia leased space by an additional 30%. The leases expire through January 2011 and include annual rent escalations of 2.5% to 3.0%.
The Company also leases its premises in Boulder, Colorado under an operating lease that expires in May 2011. The terms of the lease include annual rent escalations of 3.5%. Additionally, the Company leases certain equipment under operating leases at its Winchester, Kentucky and Kingstree, South Carolina manufacturing facilities and its Boulder offices.
Rent expense was approximately $2.1 million, $2.0 million and $1.9 million for the years ended October 31, 2009, 2008 and 2007, respectively.
Future minimum lease payments under operating leases at October 31, 2009 are as follows (in thousands):
Fiscal Year
|
|
|||
|---|---|---|---|---|
2010 |
$ | 1,221 | ||
2011 |
445 | |||
2012 |
83 | |||
2013 |
50 | |||
2014 |
41 | |||
Thereafter |
221 | |||
|
$ | 2,061 | ||
Scientific Research Collaborations The Company has entered into various collaborative research and license agreements for its algal technology. Under these agreements, the Company is required to fund research or to collaborate on the development of potential products. Certain of these agreements also commit the Company to pay royalties upon the sale of certain products resulting from such collaborations. Royalty payments in fiscal years 2009, 2008 and 2007 associated with these arrangements were not material.
In December 2003, the Company entered into a collaboration agreement with a Canadian biotechnology company to co-develop DHA products from plants. In January 2007, an amendment to this agreement was executed, whereby the Company acquired exclusive license rights to the plant-based DHA technology developed by the co-collaborator for a period of at least 16 years. As consideration for this exclusive license, the Company made a license payment of $750,000, and the license is subject to minimum royalties of 1.5% of gross margin, as defined, on future sales by Martek of such plant-based DHA. During the term of the license, the Company may be required to pay additional royalties of up to approximately 1.0% of gross margin, as defined, on sales of products in the future that utilize certain licensed technologies.
In May 2008, the Company entered into a collaboration agreement with a global biotechnology company to jointly develop and commercialize a canola seed that produces DHA. Martek and its co-collaborator anticipate a multi-year effort to produce this oil. The Company's financial commitments associated with this development initiative are subject to the successful completion of identified milestones. As of October 31, 2009, the Company's financial commitment, primarily through internal research efforts, to the first projected milestone date totals approximately $700,000. Commitments
96
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. COMMITMENTS AND CONTINGENCIES (Continued)
thereafter, also primarily through internal research efforts, assuming successful achievement of all identified milestones, total approximately $5.6 million.
In August 2009, the Company entered into a collaboration agreement with BP for the joint development of biofuels from microbial oils. Under the terms of the agreement, which is expected to continue through at least 2011, Martek and BP will work together to establish proof of concept for large-scale, cost-effective microbial biodiesel production through fermentation. In connection with this agreement, BP has agreed to contribute up to $10 million to the initial phases of the collaboration, which utilizes Martek's significant expertise in microbial oil production and BP's production and commercialization experience in biofuels as the platform for the joint development effort. Martek will perform the biotechnology research and development associated with the initial phases and receive fees from BP for such efforts. All intellectual property owned prior to the execution of the collaboration agreement will be retained by each respective company, and all intellectual property developed during the collaboration period will be owned by BP, with an exclusive license to Martek for commercialization in nutrition, cosmetic and pharmaceutical applications. Additionally, each party is entitled to certain commercial payments from the counterparty for commercialization of the technology in the other party's fields of use.
Purchase Commitments See Note 4 for discussion of purchase commitments to DSM.
OmegaTech Contingent Purchase Price In April 2002, the Company completed its acquisition of OmegaTech, a DHA producer located in Boulder, Colorado. In connection with the purchase, the Company issued 1,765,728 shares of the Company's common stock in exchange for all of the outstanding capital stock of OmegaTech. The aggregate purchase price for OmegaTech was approximately $54.1 million. The purchase agreement also provided for additional stock consideration of up to $40 million, subject to certain pricing adjustments, if four milestones were met. Two of these milestones related to operating results and two related to regulatory and labeling approvals in the U.S. and Europe. In June 2003, the conditions of one of the regulatory milestones were met, and accordingly, approximately 358,566 shares of Martek common stock, valued at approximately $14.2 million, were issued. The payment of this additional consideration was recorded as goodwill.
Beginning in 2004, disputes existed and litigation was ongoing between the Company and the representative of the former OmegaTech stockholders related to whether certain of the other milestones had been achieved. In October 2007, the Company entered into a settlement with the former OmegaTech stockholders regarding the disputed contingent consideration associated with these milestones. In connection with the settlement, the Company issued 340,946 shares of its common stock to the former OmegaTech stockholders in December 2007. The shares issued to the former OmegaTech stockholders resulted in the recognition of approximately $10.2 million of additional purchase price consideration which has been recorded as goodwill by the Company. The settlement eliminates the potential for any additional shares to be issued to the former OmegaTech stockholders.
Patent Infringement Litigation In September 2003, the Company filed a patent infringement lawsuit in the U.S. District Court in Delaware against Nutrinova Nutrition Specialties & Food Ingredients GmbH ("Nutrinova") and others alleging infringement of certain of our U.S. patents. In December 2005, Nutrinova's DHA business was sold to Lonza Group LTD, a Swiss chemical and biotechnology group, and the parties agreed to add Lonza to the U.S. lawsuit. In October 2006, the infringement action in the United States was tried, and a verdict favorable to Martek was returned. The jury found that the defendants infringed all the asserted claims of three Martek patents and that these
97
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. COMMITMENTS AND CONTINGENCIES (Continued)
patents were valid. It also found that the defendants willfully infringed one of these patents. In October 2007, the judge upheld the October 2006 jury verdict that the defendants infringed all of the asserted claims of U.S. Patent Nos. 5,340,594 and 6,410,281 and that these patents were not invalid. The judge has granted a permanent injunction against the defendants with respect to those two patents. The judge also upheld the jury verdict that the defendants had acted willfully in their infringement of U.S. Patent No. 6,410,281. Regarding the third patent involved in the case, U.S. Patent No. 6,451,567, the judge reversed the jury verdict and found that the asserted claims of this patent were invalid. Martek's request to the judge to reconsider his ruling on the third patent was denied. Martek and the defendants appealed aspects of the judge's final decision and a hearing was held before the U.S. Court of Appeals in April 2009. In September 2009, the Court of Appeals ruled in Martek's favor on all of the patents that were the subject of the appeal, which included U.S. Patent Nos. 5,340,594, 6,410,281, 6,451,567 noted above and 5,698,244, which was included in Martek's appeal as a result of the trial court's decision at a pre-trial hearing on the meaning and scope of the patent claims in dispute. With respect to U.S. Patent No. 5,698,244, the Court of Appeals reversed the trial court's interpretation of certain claim language and remanded this patent to the trial court for further proceedings. U.S. Patent Nos. 5,340,594 and 6,454,567 have expired and U.S. Patent Nos. 6,410,281 and 5,698,244 are scheduled to expire in August 2011 and December 2014, respectively. The defendants requested a rehearing with the Court of Appeals on the decision, but their request was denied. The defendants may seek an appeal to the U.S. Supreme Court with regard to certain of the patents. The Company does not believe that the results of any potential appeal, even if unfavorable, will have a material impact on its results of operations. Additionally, the defendants have requested reexamination of patent 5,698,244 in the U.S. Patent and Trademark Office.
In January 2004, the Company filed a patent infringement lawsuit in Germany against Nutrinova and Celanese Ventures GmbH. Lonza Ltd. and a customer of Nutrinova have also been added to this lawsuit. The complaint alleges infringement of Martek's European patent relating to DHA-containing oils. A hearing was held in a district court in Dusseldorf in September 2007 and the court issued its decision in October 2007, ruling that Martek's patent was infringed by the defendants. The defendants have appealed, and the appeal is expected to be heard in 2010.
In connection with these patent lawsuits, the Company has incurred and capitalized significant external legal costs. As of October 31, 2009, the patents being defended in the Lonza matter had a net book value of approximately $4.4 million, including capitalized legal costs, which is being amortized over a weighted average remaining period of approximately five years.
These lawsuits are further described in Item 3. "Legal Proceedings" of Part I of this Form 10-K.
Other The Company is involved in various other legal actions. Management believes that these actions, either individually or in the aggregate, will not have a material adverse effect on the Company's results of operations or financial condition.
98
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. NET INCOME PER SHARE
Basic net income per share is computed using the weighted average number of common shares outstanding. Diluted net income per share is computed using the weighted average number of common shares outstanding, giving effect to stock options and restricted stock units using the treasury stock method.
The following table presents the calculation of basic and diluted net income per share (in thousands, except per share amounts):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Net income |
$ | 40,590 | $ | 37,667 | $ | 32,013 | |||||
Weighted average shares outstanding, basic |
33,207 |
32,951 |
32,336 |
||||||||
Effect of dilutive potential common shares: |
|||||||||||
Stock options |
92 | 256 | 223 | ||||||||
Restricted stock units |
64 | 77 | 34 | ||||||||
Total dilutive potential common shares |
156 | 333 | 257 | ||||||||
Weighted average shares outstanding, diluted |
33,363 | 33,284 | 32,593 | ||||||||
Net income per share, basic |
$ | 1.22 | $ | 1.14 | $ | 0.99 | |||||
Net income per share, diluted |
$ | 1.22 | $ | 1.13 | $ | 0.98 | |||||
Stock options to purchase approximately 2.1 million, 1.5 million and 2.3 million shares were outstanding but were not included in the computation of diluted net income per share for the years ended October 31, 2009, 2008 and 2007, respectively, because the effects would have been antidilutive.
14. INCOME TAXES
The income tax provision consisted of the following (in thousands):
| |
Year ended October 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
Current provision: |
|||||||||||
Federal |
$ | 531 | $ | 768 | $ | 530 | |||||
State |
1,356 | 2,250 | 43 | ||||||||
Deferred provision: |
|||||||||||
Federal |
20,736 | 15,520 | 850 | ||||||||
State |
831 | 1,116 | 236 | ||||||||
Income tax provision |
$ | 23,454 | $ | 19,654 | $ | 1,659 | |||||
99
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. INCOME TAXES (Continued)
The difference between the tax provision and the amount that would be computed by applying the statutory Federal income tax rate to income before taxes is attributable to the following (in thousands):
| |
Year ended October 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | |||||||
Federal income tax expense at 35% |
$ | 22,415 | $ | 20,062 | $ | 11,785 | ||||
State taxes, net of Federal benefit |
1,422 |
1,981 |
351 |
|||||||
Previously unrecognized research and development tax credits |
(186 | ) | (1,608 | ) | — | |||||
Change in valuation allowance |
385 | (66 | ) | (10,841 | ) | |||||
Other |
(582 | ) | (715 | ) | 364 | |||||
Income tax provision |
$ | 23,454 | $ | 19,654 | $ | 1,659 | ||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes as well as net operating loss carryforwards. Significant components of the Company's net deferred income taxes are as follows (in thousands):
| |
October 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | ||||||
Deferred tax assets: |
||||||||
Accruals and reserves |
$ | 1,994 | $ | 4,006 | ||||
Net operating loss carryforwards and credits |
40,132 | 51,639 | ||||||
Forward contracts and investments |
521 | 2,496 | ||||||
Deferred revenue |
3,745 | 3,702 | ||||||
Equity-based compensation |
2,607 | 2,457 | ||||||
Other |
1,068 | 508 | ||||||
Total assets |
50,067 | 64,808 | ||||||
Deferred tax liabilities: |
||||||||
Property, plant and equipment |
(26,544 | ) | (19,467 | ) | ||||
Patents and trademarks |
(2,342 | ) | (1,417 | ) | ||||
Acquired intangibles |
(2,343 | ) | (2,647 | ) | ||||
Goodwill |
(1,711 | ) | (1,437 | ) | ||||
Total liabilities |
(32,940 | ) | (24,968 | ) | ||||
Total deferred tax asset |
17,127 |
39,840 |
||||||
Valuation allowance |
(2,915 | ) | (1,484 | ) | ||||
Deferred tax asset, net of valuation allowance |
14,212 | 38,356 | ||||||
Less: current deferred tax asset |
(24,303 | ) | (24,821 | ) | ||||
Long-term deferred tax (liability) asset |
$ | (10,091 | ) | $ | 13,535 | |||
Realization of total deferred tax assets is contingent upon the generation of future taxable income in the tax jurisdictions in which the deferred tax assets are located. During fiscal 2007, it was
100
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. INCOME TAXES (Continued)
determined that certain net operating loss carryforwards, whose related deferred tax asset had previously been fully reserved, were more likely than not to be realized. This determination was made based upon the Company's projection of future taxable income which, it was concluded, had less uncertainty due to longer histories and certain recently executed contractual arrangements with its customer base. This valuation allowance reversal resulted in an income tax benefit of approximately $10.8 million related to net operating loss carryforwards generated from operations and a decrease to goodwill of approximately $7.4 million related to net operating loss carryforwards acquired from OmegaTech in 2002. The deferred tax asset valuation allowance of $2.9 million and $1.5 million as of October 31, 2009 and 2008, respectively, relates to certain state net operating loss and credit carryforwards whose realizability is uncertain. Should realization of these deferred tax assets become more likely than not, the resulting valuation allowance reversal would be reflected in income.
The Company has adopted the tax law method for determining the order in which deductions, carryforwards and credits are realized by the Company. In fiscal 2008 and 2007, the Company recorded increases to additional paid-in capital of approximately $1.4 million and $1.6 million in fiscal 2008 and 2007, respectively, which related to the realization of the excess tax deductions associated with exercises of non-qualified stock options. Deferred tax assets associated with these deductions have only been recognized to the extent that they reduce current taxes payable. In fiscal 2009, due to declines in the Company's stock price since the granting of certain restricted stock units that vested during the year, Martek recorded a reduction to additional paid-in capital of approximately $500,000.
As of October 31, 2009, the Company had net operating loss carryforwards for Federal income tax purposes of approximately $82 million, which expire at various dates between 2021 and 2025. The timing and manner in which U.S. net operating loss carryforwards may be utilized may be limited if the Company incurs a change in ownership as defined under Section 382 of the Internal Revenue Code. Although the Company has net operating losses available to offset future taxable income, the Company may be subject to Federal alternative minimum taxes.
The Company adopted the provisions of FIN 48 (which is principally codified in ASC 740, "Income Taxes"), effective November 1, 2007. The adoption did not have a material effect on the Company's consolidated financial position or results of operations.
For the years ended October 31, 2009 and 2008, interest and penalties recorded were not material.
101
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. INCOME TAXES (Continued)
A reconciliation of the beginning and ending balance of the total amounts of unrecognized tax benefits for the year is as follows (in thousands):
Unrecognized tax benefits at November 1, 2007 |
$ | 711 | |||
Gross increases—tax positions in prior period |
1,390 | ||||
Gross decreases—tax positions in prior period |
— | ||||
Gross increases—tax positions in the current period |
— | ||||
Settlements with taxing authorities |
(352 | ) | |||
Lapse of statute of limitations |
— | ||||
Unrecognized tax benefits at October 31, 2008 |
1,749 | ||||
Gross increases—tax positions in prior period |
328 | ||||
Gross decreases—tax positions in prior period |
— | ||||
Gross increases—tax positions in the current period |
1,229 | ||||
Settlements with taxing authorities |
(36 | ) | |||
Lapse of statute of limitations |
— | ||||
Unrecognized tax benefits at October 31, 2009 |
$ | 3,270 | |||
As of October 31, 2009, approximately $1.9 million of unrecognized tax benefits were included in accrued liabilities with the remainder reducing deferred tax assets. The entire $3.3 million of unrecognized tax benefits at October 31, 2009 would affect the effective tax rate if recognized. It is reasonably possible that the total amount of unrecognized tax benefits will change within the next 12 months as various uncertainties are resolved. The Company cannot reasonably estimate the range of potential outcomes.
The Company is subject to U.S. federal income tax as well as state and local tax in various jurisdictions. Because the Company's 2006 U.S. federal income tax return used net operating loss carryforwards dating, in part, back to fiscal 1991, some elements of the income tax returns back to fiscal 1991 are subject to examination. The Company believes that appropriate provisions for all outstanding items have been made for all jurisdictions and for all open years.
15. EMPLOYEE 401(K) PLAN
The Company maintains an employee 401(k) Plan (the "Plan"). The Plan, which covers all employees 21 years of age or older, stipulates that participating employees may elect an amount up to 100% of their total compensation to contribute to the Plan, not to exceed the maximum allowable by Internal Revenue Service regulations. The Company may make "matching contributions" equal to a discretionary percentage up to 3% of a participant's salary, based on deductions of up to 6% of a participant's salary. All amounts deferred by a participant under the 401(k) Plan's salary reduction feature vest immediately in the participant's account while contributions the Company may make would vest over a five-year period in the participant's account. The Company contribution was approximately $1.1 million, $1.0 million and $900,000 for the years ended October 31, 2009, 2008 and 2007, respectively.
102
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
16. QUARTERLY FINANCIAL INFORMATION (unaudited)
Quarterly financial information for fiscal 2008 and 2007 is presented in the following table (in thousands, except per share data):
| |
1st Quarter |
2nd Quarter |
3rd Quarter |
4th Quarter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2009 |
|||||||||||||
Total revenues |
$ | 87,363 | $ | 92,411 | $ | 77,834 | $ | 87,589 | |||||
Cost of revenues |
50,318 | 53,316 | 43,804 | 49,199 | |||||||||
Income from operations |
15,265 | 16,899 | 14,219 | 17,233 | |||||||||
Net income |
9,606 | 11,017 | 8,928 | 11,039 | |||||||||
Net income per share, basic |
0.29 | 0.33 | 0.27 | 0.33 | |||||||||
Net income per share, diluted |
0.29 | 0.33 | 0.27 | 0.33 | |||||||||
2008 |
|||||||||||||
Total revenues |
$ | 82,881 | $ | 90,726 | $ | 88,403 | $ | 90,352 | |||||
Cost of revenues |
48,785 | 53,343 | 51,708 | 53,012 | |||||||||
Income from operations |
13,302 | 14,357 | 14,603 | 13,922 | |||||||||
Net income |
8,669 | 9,202 | 9,332 | 10,464 | (1) | ||||||||
Net income per share, basic |
0.26 | 0.28 | 0.28 | 0.32 | (1) | ||||||||
Net income per share, diluted |
0.26 | 0.28 | 0.28 | 0.31 | (1) | ||||||||
- (1)
- In the fourth quarter of fiscal 2008, Martek recognized an income tax benefit of $1.6 million related primarily to certain previously unrecognized research and development tax credits.
103
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
- a)
- Evaluation of Disclosure Controls and Procedures. As of the end of the period covered by this report, we,
under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, have evaluated the effectiveness of our disclosure controls and
procedures pursuant to Exchange Act rules 13a—15(e) and 15d—15(e). Based on this evaluation, our Chief Executive Officer and Chief Financial Officer have
concluded that, as of the end of the period covered by this report, the disclosure controls and procedures were effective.
- b)
- Internal Control Over Financial Reporting. The report called for by Item 308(a) of Regulation S-K is incorporated herein by reference to Management's Report on Internal Control Over Financial Reporting, included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K. The attestation report called for by Item 308(b) of Regulation S-K is incorporated herein by reference to the attestation report of Ernst & Young LLP, our independent registered public accounting firm, on management's assessment of internal control over financial reporting, included in Part II, Item 8. "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K. There was no change in our internal control over financial reporting during our last fiscal quarter that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Not applicable.
104
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Information relating to our Executive Officers is set forth in Part I of this report under the caption Item 1. "Business"—"Executive Officers of the Registrant." Information on our directors and the other information required by this item will be contained in the following sections of our 2010 Definitive Proxy Statement, which sections are hereby incorporated by reference:
Election of Directors
Board Committees
Section 16(a) Beneficial Ownership Reporting Compliance
As part of our system of corporate governance, our Board of Directors has adopted a code of ethics for senior financial officers that is specifically applicable to our chief executive officer, president, chief financial officer and controller. This code of ethics is available on the corporate governance page of the investor information section of our website at http://www.martek.com. We intend to satisfy any disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of this code of ethics by posting such information on our website at the address above.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by this item will be contained in the following sections of our 2010 Definitive Proxy Statement, which sections are hereby incorporated by reference:
Directors Compensation
Executive Compensation
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by this item will be contained in the following sections of our 2010 Definitive Proxy Statement, which sections are hereby incorporated by reference:
Beneficial Ownership of Common Stock
Equity Compensation Plan Information
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required by this item will be contained in the following sections of our 2010 Definitive Proxy Statement, which section is hereby incorporated by reference:
Transactions with Related Persons
Director Independence
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The information required by this item will be contained in the following sections of our 2010 Definitive Proxy Statement, which section is hereby incorporated by reference:
Independent Auditors
105
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a)(1) Index to Consolidated Financial Statements
The Financial Statements listed in the Index to Consolidated Financial Statements are filed as part of this Annual Report on Form 10-K. See Part II, Item 8. "Financial Statements and Supplementary Data."
(a)(2) Financial Statement Schedules
Schedule II Valuation and Qualifying Accounts—Years Ended October 31, 2009 and 2008 |
107 |
Other financial statement schedules for the years ended October 31, 2009 and 2008 and financial statement schedules for the year ended October 31, 2007 have been omitted since they are either not required, not applicable, or the information is otherwise included in the consolidated financial statements or the notes to consolidated financial statements.
(a)(3) Exhibits
The Exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this report.
106
MARTEK BIOSCIENCES CORPORATION
VALUATION AND QUALIFYING ACCOUNTS
YEARS ENDED OCTOBER 31, 2009 AND 2008
In thousands Description |
Balance at Beginning of Year |
Additions | Deductions | Balance at End of Year |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year ended October 31, 2009: |
||||||||||||||
Deferred tax valuation allowance |
$ | 1,484 | $ | 1,766 | $ | (335 | ) | $ | 2,915 | |||||
Reserve for inventory obsolescence |
$ | 2,600 | $ | 3,300 | $ | (4,500 | ) | $ | 1,400 | |||||
Year ended October 31, 2008: |
||||||||||||||
Deferred tax valuation allowance |
$ | 1,550 | $ | — | $ | (66 | ) | $ | 1,484 | |||||
Reserve for inventory obsolescence |
$ | 1,300 | $ | 2,200 | $ | (900 | ) | $ | 2,600 | |||||
107
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized on December 29, 2009.
| MARTEK BIOSCIENCES CORPORATION | ||||
By |
/s/ STEVE DUBIN Steve Dubin Chief Executive Officer and Director |
|||
Pursuant to the requirement of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated.
Signatures
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ STEVE DUBIN Steve Dubin |
Chief Executive Officer and Director (Principal Executive Officer) | December 29, 2009 | ||
/s/ PETER L. BUZY Peter L. Buzy |
Chief Financial Officer, Treasurer and Executive Vice President for Finance and Administration (Principal Financial Officer and Accounting Officer) |
December 29, 2009 |
||
/s/ JAMES R. BEERY James R. Beery |
Director |
December 29, 2009 |
||
/s/ HARRY J. D'ANDREA Harry J. D'Andrea |
Director |
December 29, 2009 |
||
/s/ MICHAEL G. DEVINE Michael G. Devine |
Director |
December 29, 2009 |
||
/s/ ROBERT J. FLANAGAN Robert J. Flanagan |
Director |
December 29, 2009 |
||
/s/ POLLY B. KAWALEK Polly B. Kawalek |
Director |
December 29, 2009 |
108
Signatures
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ JEROME C. KELLER Jerome C. Keller |
Director | December 29, 2009 | ||
/s/ DOUGLAS J. MACMASTER, JR. Douglas J. MacMaster, Jr. |
Director |
December 29, 2009 |
||
/s/ ROBERT H. MAYER Robert H. Mayer |
Director |
December 29, 2009 |
||
/s/ DAVID M. PERNOCK David M. Pernock |
Director |
December 29, 2009 |
||
/s/ EUGENE H. ROTBERG Eugene H. Rotberg |
Director |
December 29, 2009 |
109
| EXHIBIT NUMBER# |
DESCRIPTION | ||
|---|---|---|---|
| 3.01 | Revised Restated Certificate of Incorporation. | ||
3.02 |
Certificate of Amendment to the Restated Certificate of Incorporation (filed as Exhibit 3.1 to the Company's Registration Statement on Form S-3, File No. 33-89760, filed March 15, 1995, and incorporated by reference herein). |
||
3.03 |
Certificate of Elimination of Series A Junior Participating Preferred Stock (filed as Exhibit 3.2 to the Company's current report on Form 8-K, File No. 0-22354, filed on February 8, 2006, and incorporated by reference herein). |
||
3.04 |
Certificate of Amendment to the Restated Certificate of Incorporation of the Company (filed as exhibit 3.07 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended January 31, 2002, and incorporated by reference herein). |
||
3.05 |
Amended By-Laws of Registrant (filed as exhibit 3.1 to the Company's current report on Form 8-K, File No. 0-22354, filed on September 22, 2008, and incorporated by reference herein). |
||
3.06 |
Certificate of Designation, Preferences and Rights of Series B Junior Participating Preferred Stock (filed as Exhibit 3.1 to the Company's current report on Form 8-K, File No. 0-22354, filed on February 8, 2006, and incorporated by reference herein). |
||
3.07 |
Certificate of Elimination of Series B Junior Participating Preferred Stock (filed as Exhibit 3.07 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2008, and incorporated by reference herein). |
||
4.01 |
Specimen Stock Certificate for Common Stock. |
||
10.01 |
Form of Indemnification Agreement for directors and officers (filed as Exhibit 10.01 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended April 30, 2009, and incorporated by reference herein).+ |
||
10.02 |
License Agreement, dated October 28, 1992, between the Company and Mead Johnson & Company (filed as Exhibit 10.02 to the Company's current report on Form 8-K, File No. 0-22354, dated December 15, 2006 and incorporated by reference herein).* |
||
10.02A |
Exhibits to October 28, 1992 License Agreement between the Company and Mead Johnson & Company.* |
||
10.03 |
Supply Agreement with Mead Johnson & Company dated May 17, 2006 (filed as Exhibit 10.01 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended April 30, 2006, and incorporated by reference herein).* |
||
10.04 |
License Agreement, dated January 28, 1993 between the Company and American Home Products Corporation represented by the Wyeth-Ayerst Division (Domestic Version) and American Home Products Corporation represented by its agent Wyeth-Ayerst International (International Version) (filed as Exhibit 10.03 to the Company's current report on Form 8-K, File No. 0-22354, dated December 15, 2006 and incorporated by reference herein).* |
||
10.04A |
Exhibits to January 28, 1993 License Agreements between the Company and American Home Products Corporation represented by the Wyeth-Ayerst Division (Domestic Version) and American Home Products Corporation represented by its agent Wyeth-Ayerst International (International Version).* |
||
110
| EXHIBIT NUMBER# |
DESCRIPTION | ||
|---|---|---|---|
| 10.05 | Supply Agreement, executed on October 31, 2008 but made effective as of November 21, 2008, by and between AHP Manufacturing B.V. trading as Wyeth Nutritionals Ireland and the Company (filed as Exhibit 10.07 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2008, and incorporated by reference herein).* | ||
10.06 |
License Agreement, dated March 31, 2000 between the Company and Abbott Laboratories (filed as Exhibit 10.30 to the Company's quarterly report on Form 10-Q for the quarter ended April 30, 2000, and incorporated by reference herein).* |
||
10.07 |
Supply Agreement, executed October 5, 2007 and effective January 1, 2007, by and between Abbott Nutrition, a division of Abbott Laboratories, and the Company (filed as Exhibit 10.49 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2007, and incorporated by reference herein).^ |
||
10.08 |
License and Supply Agreement, dated June 13, 2003 between the Company and Nestec Ltd. (filed as exhibit 10.50 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended April 30, 2003, and incorporated by reference herein).* |
||
10.09 |
License and Supply Agreement, executed February 13, 2008 and effective January 1, 2008, by and between Numico Trading B.V. and the Company (filed as Exhibit 10.01 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended January 31, 2008, and incorporated by reference herein).* |
||
10.10 |
First Amended and Restated ARA Alliance, Purchase and Production Agreement, dated as of July 13, 2009, by and between Martek Biosciences Corporation and DSM Food Specialties B.V. (filed as Exhibit 10.01 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended July 31, 2009, and incorporated by reference herein).* |
||
10.11 |
Amended and Restated Loan and Security Agreement by and among the Company, as Borrower, and the Lenders party thereto and Manufacturers and Traders Trust Company, as Administrative Agent and Sole Book Runner, and Bank of America, NA, as Syndication Agent, and SunTrust Bank, as Documentation Agent, dated September 30, 2005 (filed as Exhibit 10.1 to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). |
||
10.11A |
Form of Revolving Loan Promissory Note (filed as Exhibit 10.1A to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). |
||
10.11B |
Form of Guaranty Agreement (filed as Exhibit 10.1B to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). |
||
10.11C |
Form of Security Agreement from Guarantors (filed as Exhibit 10.1C to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). |
||
10.11D |
Form of Stock Pledge Agreement (filed as Exhibit 10.1D to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). |
||
10.11E |
Form of Lender Assignment and Acceptance Agreement (filed as Exhibit 10.1E to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). |
||
111
| EXHIBIT NUMBER# |
DESCRIPTION | ||
|---|---|---|---|
| 10.12 | Second Modification Agreement effective as of September 30, 2005 by and between Manufacturers and Traders Trust Company, as Administrative Agent, the lenders named therein, Martek Biosciences Corporation, Martek Biosciences Boulder Corporation, and Martek Biosciences Kingstree Corporation (filed as Exhibit 10.2 to the Company's current report on Form 8-K, File No. 0-22354, filed on October 6, 2005 and incorporated by reference herein). | ||
10.13 |
Settlement Agreement and Release, dated October 15, 2007, by and between the Company, Robert Zuccaro, on his own behalf and as Stockholders' Representative of certain former interest holders of OmegaTech, Inc. (filed as Exhibit 10.50 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2007, and incorporated by reference herein). |
||
10.14 |
Martek Biosciences Corporation 1997 Stock Option Plan (filed as Exhibit 4.1(e) to the Company's Registration Statement on Form S-8, File No. 333-27671, filed May 22, 1997, and incorporated by reference herein).+ |
||
10.15 |
Martek Biosciences Corporation 2001 Stock Option Plan (filed as Exhibit 10.01 to the Company's Registration Statement on Form S-8, File No. 333-74092, filed on November 28, 2001, and incorporated by reference herein).+ |
||
10.16 |
Martek Biosciences Corporation Amended And Restated Management Cash Bonus Incentive Plan effective April 25, 2002 (filed as Exhibit 10.36 to the Company's quarterly report on Form 10-Q, File No.0-22354, for the quarter ended April 30, 2002, and incorporated by reference herein).+ |
||
10.17 |
OmegaTech, Inc. 1996 Stock Option Plan, as amended on April 26, 2001 (filed as Exhibit 10.37 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended April 30, 2002, and incorporated by reference herein).+ |
||
10.18 |
Employment Agreement dated April 25, 2002 between the Company and James Flatt (filed as Exhibit 10.39 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended April 30, 2002, and incorporated by reference herein).+ |
||
10.19 |
Martek Biosciences Corporation 2002 Stock Incentive Plan (filed as Exhibit 1 to the Company's Definitive Proxy, Schedule 14A, File No. 0-22354, filed on February 8, 2002 and incorporated by reference herein).+ |
||
10.20 |
Martek Biosciences Corporation 2003 New Employee Stock Option Plan (filed as Exhibit 10.55 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended July 31, 2003, and incorporated by reference herein).+ |
||
10.21 |
Form of Nonqualified Stock Option Award Agreement under Martek Biosciences Corporation 2003 New Employee Stock Option Plan (filed as Exhibit 10.46 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2004, and incorporated by reference herein).+ |
||
10.22 |
Form of Nonqualified Stock Option Award Agreement under Martek Biosciences Corporation 2004 Stock Incentive Plan (filed as Exhibit 10.47 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2004, and incorporated by reference herein).+ |
||
10.23 |
Martek Biosciences Corporation Amended and Restated 2004 Stock Incentive Plan (filed as Appendix A to the Company's Definitive Proxy, Schedule 14A, File No. 0-22354, filed on February 8, 2005 and incorporated by reference herein).+ |
||
112
| EXHIBIT NUMBER# |
DESCRIPTION | ||
|---|---|---|---|
| 10.24 | Form of Nonqualified Stock Option Agreement under Martek Biosciences Corporation Amended and Restated 2004 Stock Incentive Plan (filed as Exhibit 10.03 to the Company's Registration Statement on Form S-8, File No. 333-125802, filed June 14, 2005, and incorporated by reference herein).+ | ||
10.25 |
Form of Employment Agreement between the Company and Peter L. Buzy dated November 10, 2006 (filed as Exhibit 10.42 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2006, and incorporated by reference herein).+ |
||
10.26 |
Form of Employment Agreement between the Company and Steve Dubin dated December 21, 2006. (filed as Exhibit 10.43 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2006, and incorporated by reference herein).+ |
||
10.27 |
Form of Employment Agreement between the Company and Peter A. Nitze dated November 10, 2006 (filed as Exhibit 10.44 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2006, and incorporated by reference herein).+ |
||
10.28 |
Form of Employment Agreement between the Company and David Abramson dated November 10, 2006 (filed as Exhibit 10.45 to the Company's annual report on Form 10-K, File No. 0-22354, for the year ended October 31, 2006, and incorporated by reference herein).+ |
||
10.29 |
Form of Restricted Stock Unit Agreement for executives under the Martek Bioscience Corporation Amended and Restated 2004 Stock Incentive Plan (filed as Exhibit 10.01 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended January 31, 2007, and incorporated by reference herein).+ |
||
10.30 |
Form of Restricted Stock Unit Agreement for directors under the Martek Bioscience Corporation Amended and Restated 2004 Stock Incentive Plan (filed as Exhibit 10.02 to the Company's quarterly report on Form 10-Q, File No. 0-22354, for the quarter ended January 31, 2007, and incorporated by reference herein).+ |
||
21.01 |
Subsidiaries of the Registrant.** |
||
23.01 |
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm.** |
||
31.01 |
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a).** |
||
31.02 |
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a).** |
||
32.01 |
Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.** |
||
32.02 |
Certification of Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.** |
||
- *
- Confidential
treatment was granted by the Securities and Exchange Commission for certain portions of these agreements. The confidential portions were filed
separately with the Commission.
- **
- Filed
herewith.
- ^
- Confidential treatment was requested for certain portions of this agreement. The confidential portions were filed separately with the Commission.
113
- #
- Unless
otherwise noted, all Exhibits are incorporated by reference as an Exhibit to the Registrant's Registration Statement on Form S-1
(No. 33-68522). The registrant will furnish a copy of any exhibit upon receipt of a written request and the payment of a specified reasonable fee which fee shall be limited to the
registrant's reasonable expenses in furnishing such exhibit.
- +
- Denotes management contract or compensatory arrangement required to be filed as an exhibit to this form.
114
