Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a09-33735_18k.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a09-33735_1ex99d1.htm |
Exhibit 99.2
|
|
Avanafil REVIVE (TA-301) Results November 18, 2009 |
|
|
Today’s Speakers Leland F. Wilson, Chief Executive Officer Dr. Charles H. Bowden, Senior Director Clinical Development Dr. Wesley W. Day, Vice President Clinical Development Dr. Leroy Jones, Clinical Associate Professor, Urology, University of Texas Health Science Center, San Antonio |
|
|
VIVUS cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests,“ "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the Company's current beliefs and expectations. These forward-looking statements include statements regarding the efficacy and safety of avanafil, the potential for, and timing of, an NDA submission for avanafil, the commercial and therapeutic potential of avanafil, and the potential to obtain regulatory approval for, and effectively treat erectile dysfunction with, avanafil. The inclusion of forward looking statements should not be regarded as a representation by VIVUS that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to the risk and uncertainties inherent in the VIVUS business, including, without limitation: additional analyses of data from the avanafil Phase 3 trials and any other clinical trials of avanafil may produce negative or inconclusive results, or may be inconsistent with previously announced results or previously conducted clinical trials; the FDA may not agree with the Company’s interpretation of efficacy and safety results; earlier clinical trials may not be predictive of future results; Avanafil may not receive regulatory approval on a timely basis or at all, and the FDA may require VIVUS to complete additional clinical, non-clinical or other requirements prior to the submission or the approval of NDAs for either product candidate; the potential for adverse safety findings relating to avanafil to delay or prevent regulatory approval or commercialization, or result in product liability claims, including serious adverse events that are not characterized by clinical investigators as possibly related to avanafil; the third parties on whom VIVUS relies to assist with the development programs for avanafil, including clinical investigators, contract laboratories, clinical research organizations and manufacturing organizations, may not successfully carry out their contractual duties or obligations or meet expected deadlines, and the quality or accuracy of the data or materials generated by such third parties may be of insufficient quality to include in the Company’s regulatory submissions; the ability of VIVUS and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its product candidates; VIVUS may be unable to enter a collaboration or partnership relating to avanafil for promotion to broader markets on attractive terms or at all; and other risks described in the Company's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and VIVUS undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. Information included herein is based on the Company’s review and evaluation of the clinical data. All conclusions and determinations contained herein are subject to the Company’s further analysis of the clinical data. The ultimate determination of the safety and efficacy of avanafil will be made by the FDA and other relevant regulatory authorities Forward-Looking Statements |
|
|
|
|
|
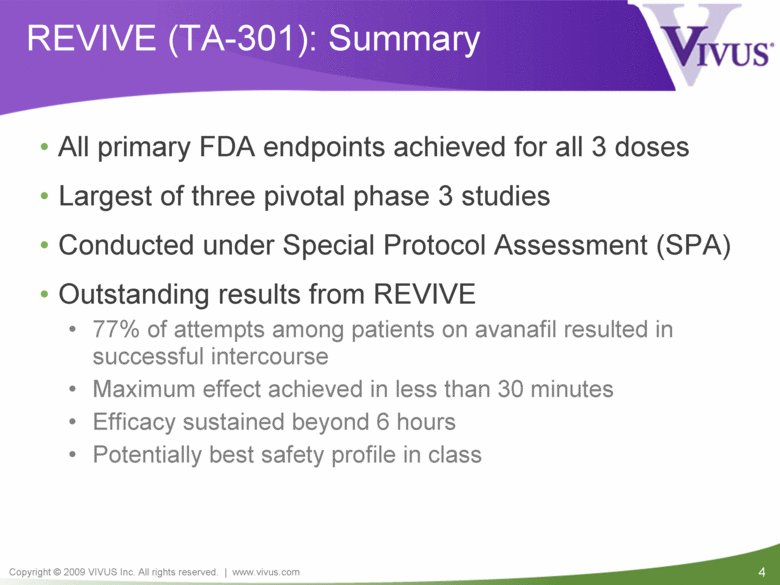
4 Copyright © 2009 VIVUS Inc. All rights reserved. | www.vivus.com REVIVE (TA-301): Summary • All primary FDA endpoints achieved for all 3 doses • Largest of three pivotal phase 3 studies • Conducted under Special Protocol Assessment (SPA) • Outstanding results from REVIVE • 77% of attempts among patients on avanafil resulted in successful intercourse • Maximum effect achieved in less than 30 minutes • Efficacy sustained beyond 6 hours • Potentially best safety profile in class |
|
|
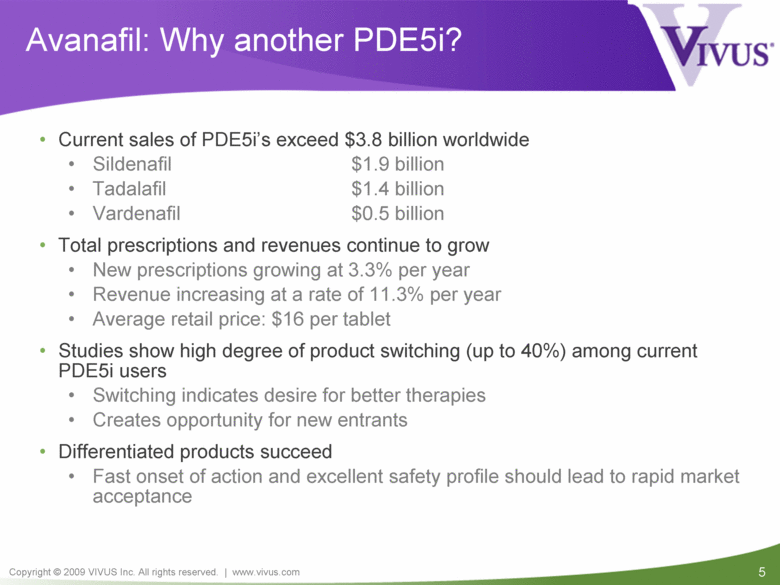
Avanafil: Why another PDE5i? Current sales of PDE5i’s exceed $3.8 billion worldwide Sildenafil $1.9 billion Tadalafil $1.4 billion Vardenafil $0.5 billion Total prescriptions and revenues continue to grow New prescriptions growing at 3.3% per year Revenue increasing at a rate of 11.3% per year Average retail price: $16 per tablet Studies show high degree of product switching (up to 40%) among current PDE5i users Switching indicates desire for better therapies Creates opportunity for new entrants Differentiated products succeed Fast onset of action and excellent safety profile should lead to rapid market acceptance |
|
|
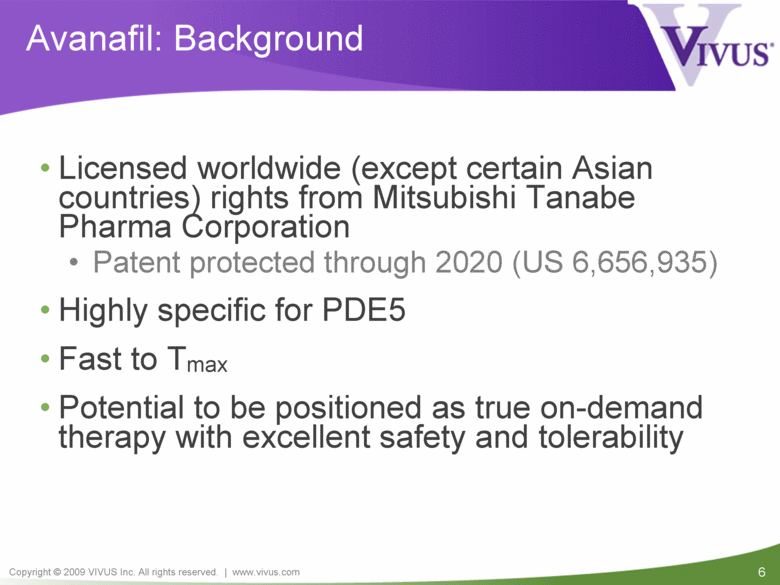
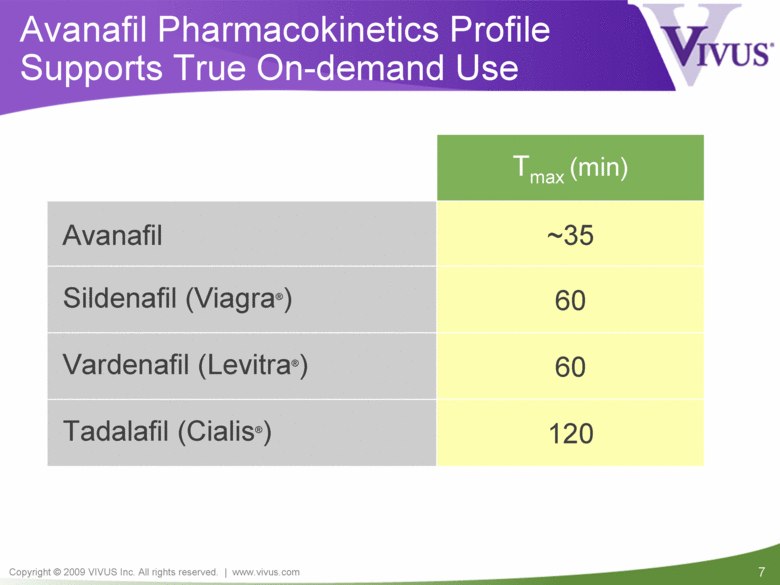
Avanafil: Background Licensed worldwide (except certain Asian countries) rights from Mitsubishi Tanabe Pharma Corporation Patent protected through 2020 (US 6,656,935) Highly specific for PDE5 Fast to Tmax Potential to be positioned as true on-demand therapy with excellent safety and tolerability |
|
|
Avanafil Pharmacokinetics Profile Supports True On-demand Use 120 Tadalafil (Cialis®) 60 Vardenafil (Levitra®) 60 Sildenafil (Viagra®) ~35 Avanafil Tmax (min) |
|
|
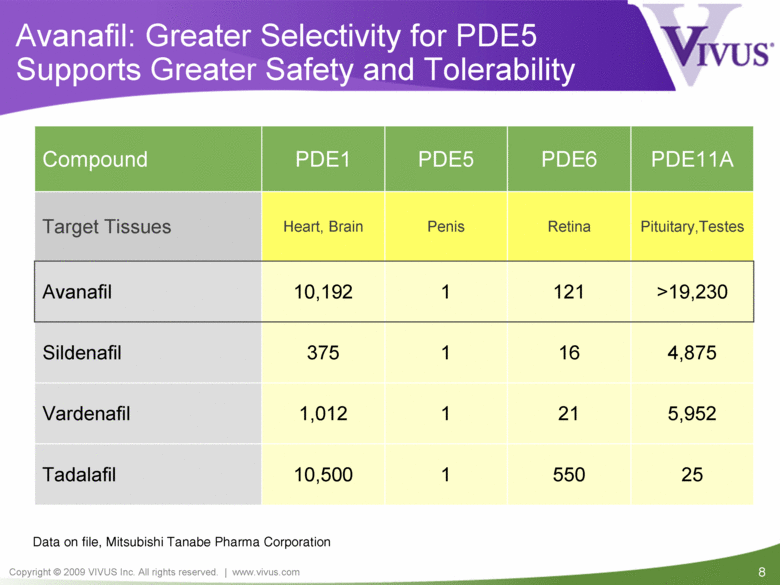
Avanafil: Greater Selectivity for PDE5 Supports Greater Safety and Tolerability Pituitary,Testes Retina Penis Heart, Brain Target Tissues 25 550 1 10,500 Tadalafil >19,230 121 1 10,192 Avanafil 1 1 PDE5 1,012 375 PDE1 Compound PDE6 PDE11A Sildenafil 16 4,875 Vardenafil 21 5,952 Data on file, Mitsubishi Tanabe Pharma Corporation |
|
|
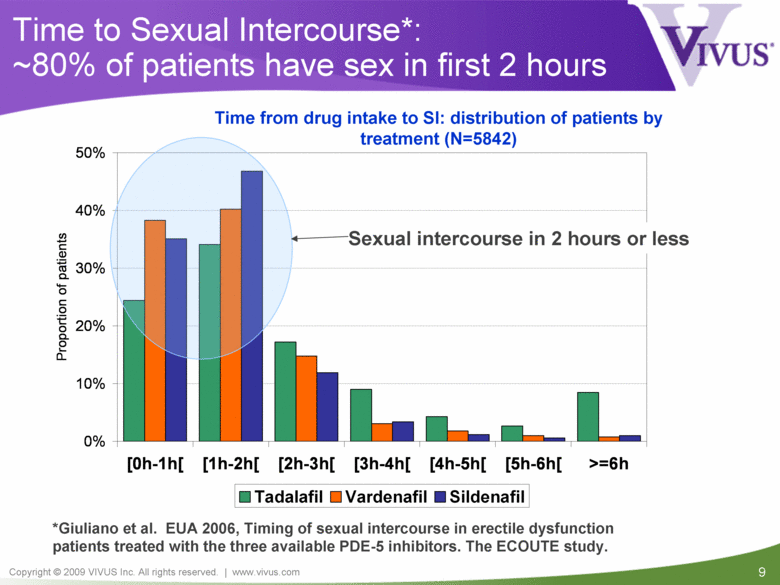
Time to Sexual Intercourse*: ~80% of patients have sex in first 2 hours Sexual intercourse in 2 hours or less *Giuliano et al. EUA 2006, Timing of sexual intercourse in erectile dysfunction patients treated with the three available PDE-5 inhibitors. The ECOUTE study. Time from drug intake to SI: distribution of patients by treatment (N=5842) 0% 10% 20% 30% 40% 50% [0h-1h[ [1h-2h[ [2h-3h[ [3h-4h[ [4h-5h[ [5h-6h[ >=6h Proportion of patients Tadalafil Vardenafil Sildenafil |
|
|
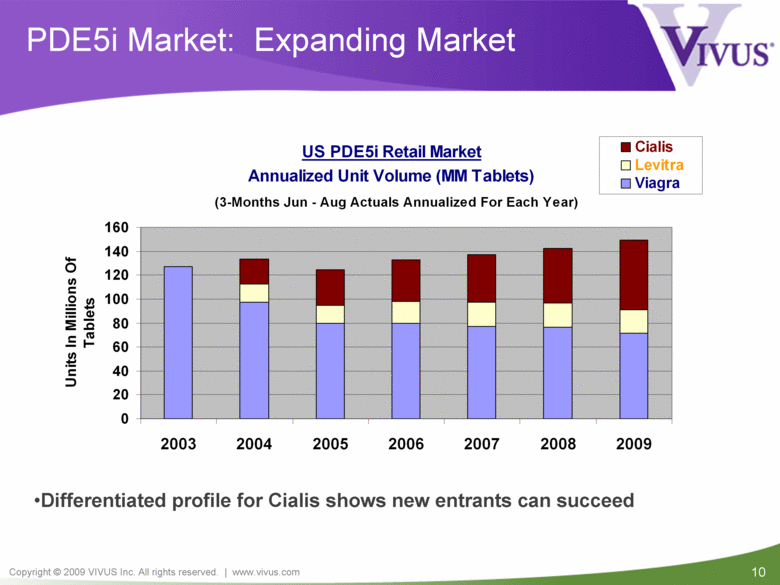
PDE5i Market: Expanding Market Differentiated profile for Cialis shows new entrants can succeed US PDE5i Retail Market Annualized Unit Volume (MM Tablets) (3-Months Jun - Aug Actuals Annualized For Each Year) 0 20 40 60 80 100 120 140 160 2003 2004 2005 2006 2007 2008 2009 Units In Millions Of Tablets Cialis Levitra Viagra |
|
|
Market Need for New PDE5 Therapy Large and growing market Patients less than satisfied, lots of switching New entrants with differentiated position able to take market share Patients looking for on-demand therapy with lower frequency of side effects |
|
|
REVIVE (TA-301): Phase 3 Results Charles H. Bowden, M.D. |
|
|
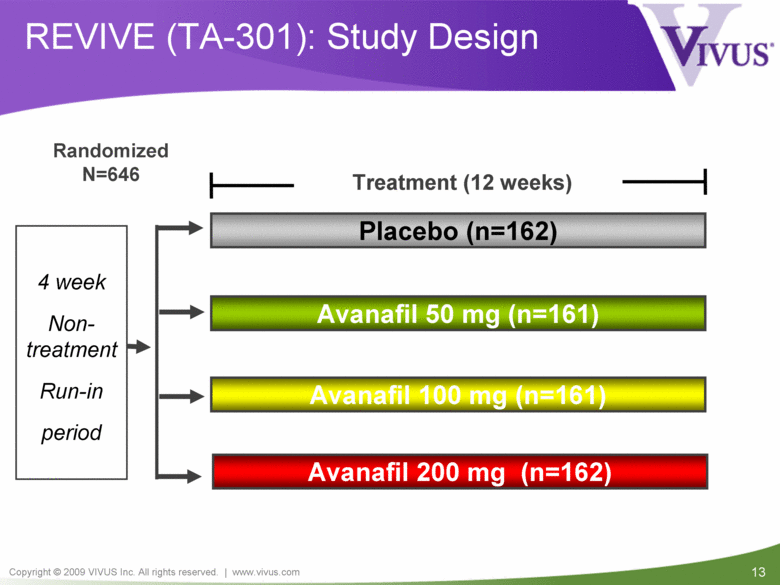
REVIVE (TA-301): Study Design Randomized N=646 Treatment (12 weeks) Avanafil 100 mg (n=161) Avanafil 50 mg (n=161) Placebo (n=162) 4 week Non-treatment Run-in period Avanafil 200 mg (n=162) |
|
|
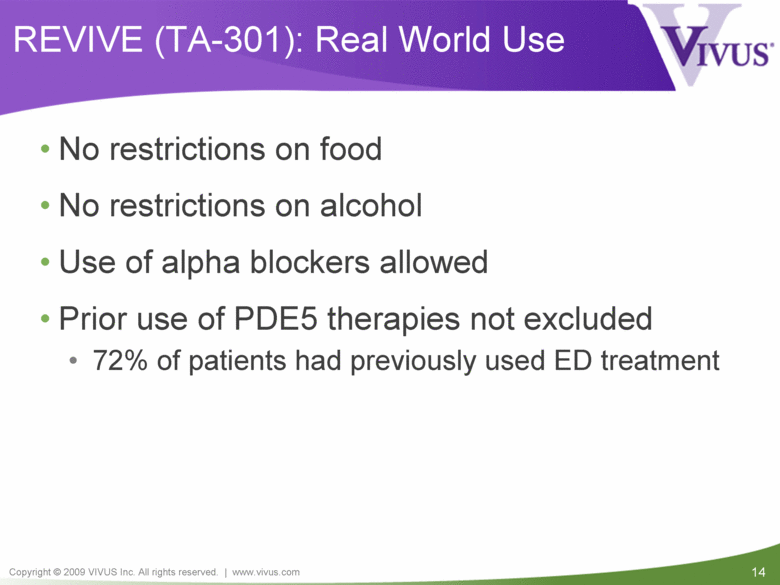
REVIVE (TA-301): Real World Use No restrictions on food No restrictions on alcohol Use of alpha blockers allowed Prior use of PDE5 therapies not excluded 72% of patients had previously used ED treatment |
|
|
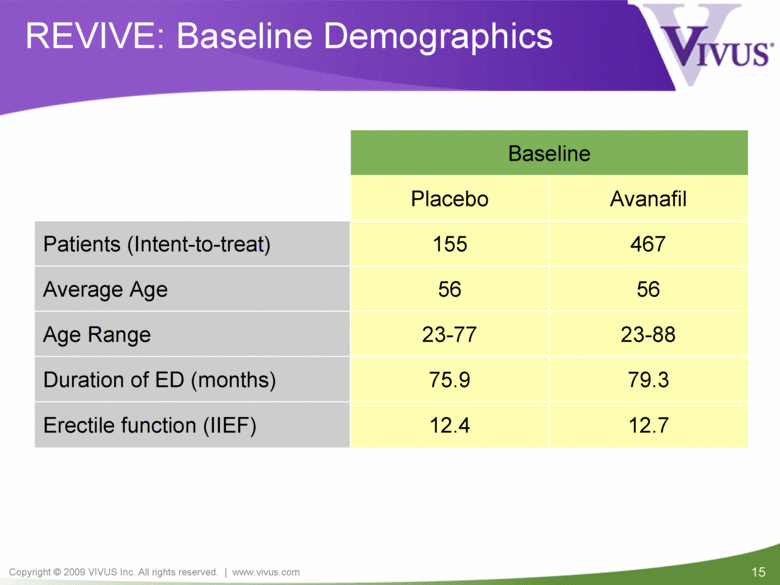
REVIVE: Baseline Demographics Baseline 467 155 Patients (Intent-to-treat) Avanafil Placebo 12.4 75.9 23-77 56 Average Age 56 Age Range 23-88 Duration of ED (months) 79.3 Erectile function (IIEF) 12.7 |
|
|
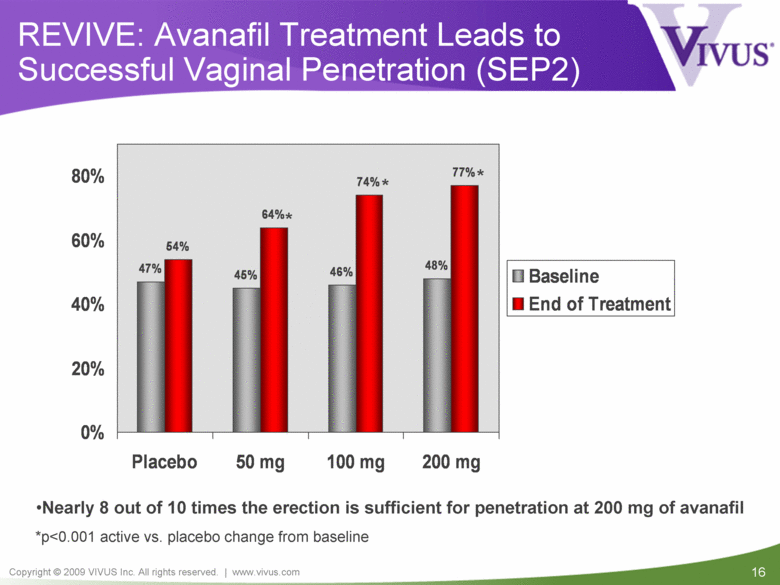
REVIVE: Avanafil Treatment Leads to Successful Vaginal Penetration (SEP2) *p<0.001 active vs. placebo change from baseline Nearly 8 out of 10 times the erection is sufficient for penetration at 200 mg of avanafil * * * 47% 45% 46% 48% 54% 64% 74% 77% 0% 20% 40% 60% 80% Placebo 50 mg 100 mg 200 mg Baseline End of Treatment |
|
|
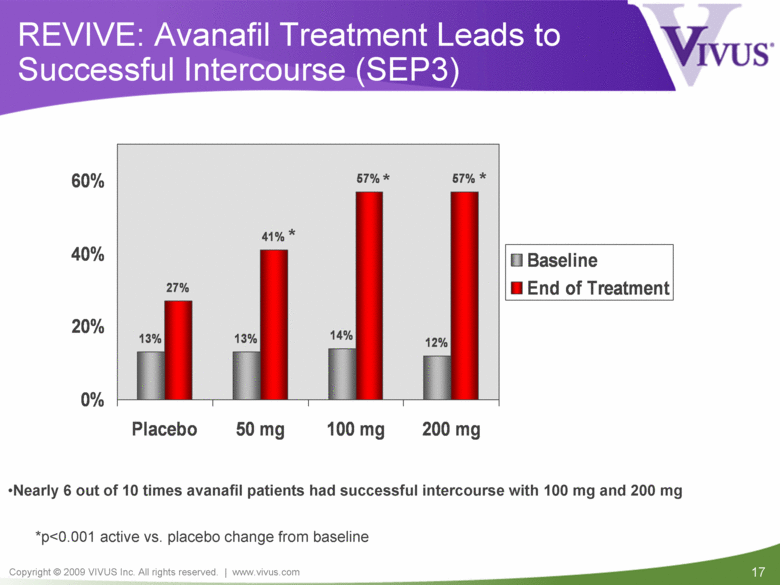
REVIVE: Avanafil Treatment Leads to Successful Intercourse (SEP3) Nearly 6 out of 10 times avanafil patients had successful intercourse with 100 mg and 200 mg *p<0.001 active vs. placebo change from baseline * * * 13% 13% 14% 12% 27% 41% 57% 57% 0% 20% 40% 60% Placebo 50 mg 100 mg 200 mg Baseline End of Treatment |
|
|
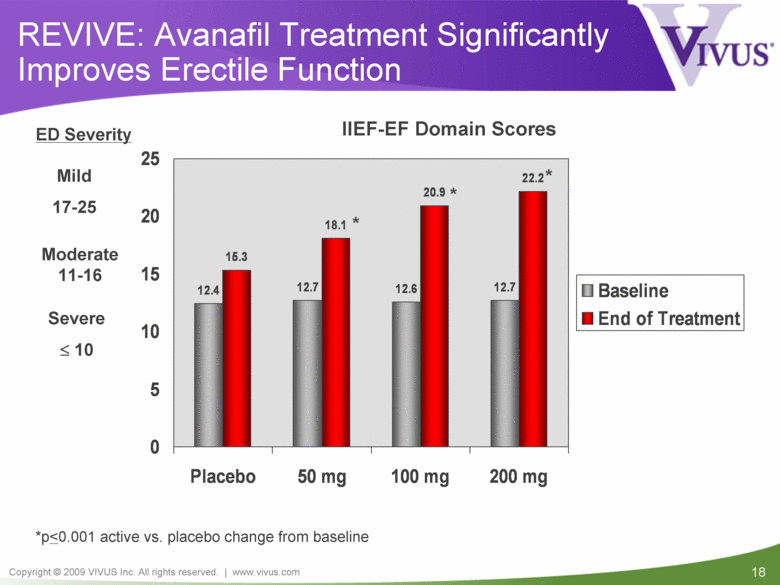
REVIVE: Avanafil Treatment Significantly Improves Erectile Function Mild 17-25 Moderate 11-16 ED Severity Severe < 10 IIEF-EF Domain Scores *p < 0.001 active vs. placebo change from baseline * * * 12.4 12.7 12.6 12.7 15.3 18.1 20.9 22.2 0 5 10 15 20 25 Placebo 50 mg 100 mg 200 mg Baseline End of Treatment |
|
|
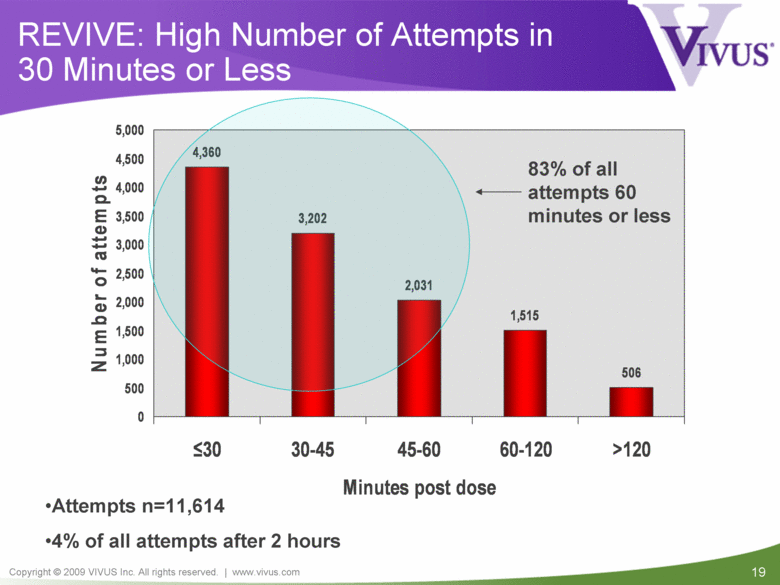
REVIVE: High Number of Attempts in 30 Minutes or Less 83% of all attempts 60 minutes or less Attempts n=11,614 4% of all attempts after 2 hours 4,360 3,202 2,031 1,515 506 0 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 4,500 5,000 < 30 30-45 45-60 60-120 >120 Minutes post dose Number of attempts |
|
|
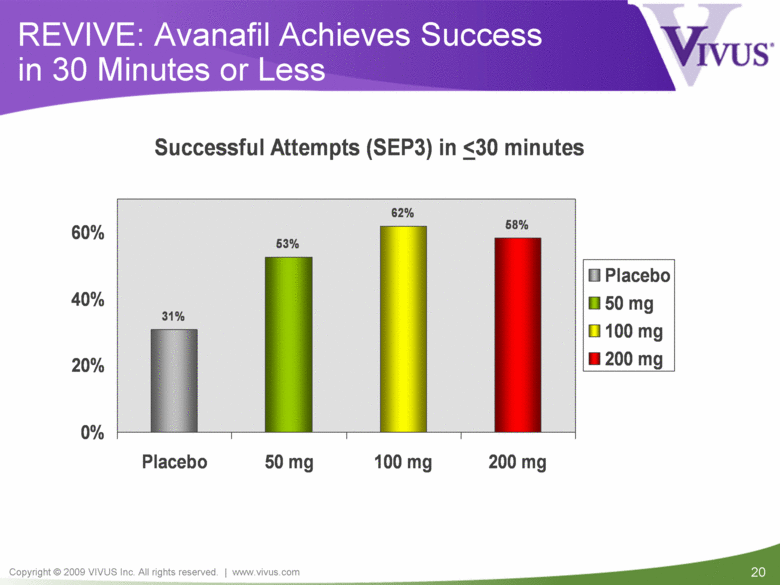
REVIVE: Avanafil Achieves Success in 30 Minutes or Less Successful Attempts (SEP3) in < 30 minutes 31% 53% 62% 58% 0% 20% 40% 60% Placebo 50 mg 100 mg 200 mg Placebo 50 mg 100 mg 200 mg |
|
|
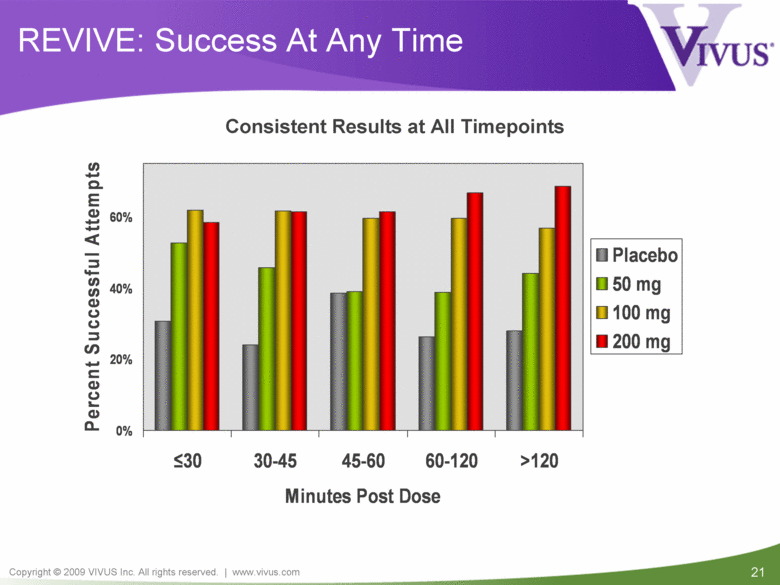
REVIVE: Success At Any Time Consistent Results at All Timepoints 0% 20% 40% 60% < 30 30-45 45-60 60-120 >120 Minutes Post Dose Percent Successful Attempts Placebo 50 mg 100 mg 200 mg |
|
|
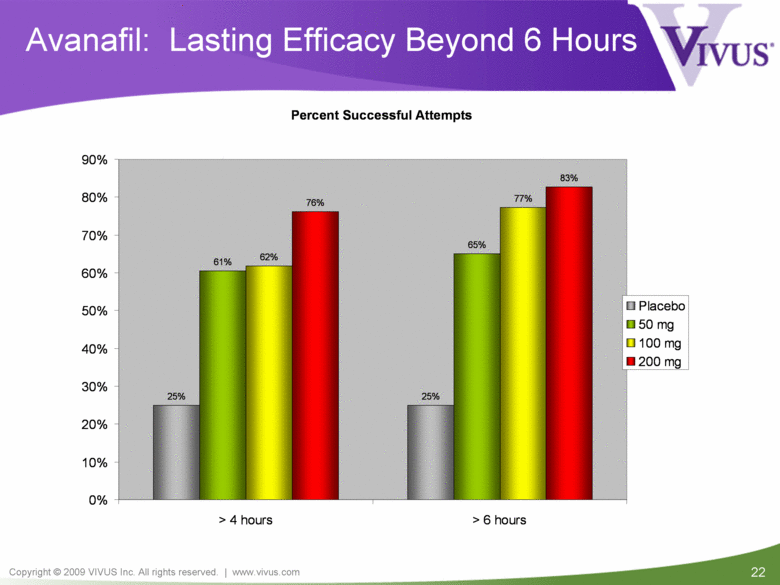
Avanafil: Lasting Efficacy Beyond 6 Hours Percent Successful Attempts 25% 25% 61% 65% 62% 77% 76% 83% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% > 4 hours > 6 hours Placebo 50 mg 100 mg 200 mg |
|
|
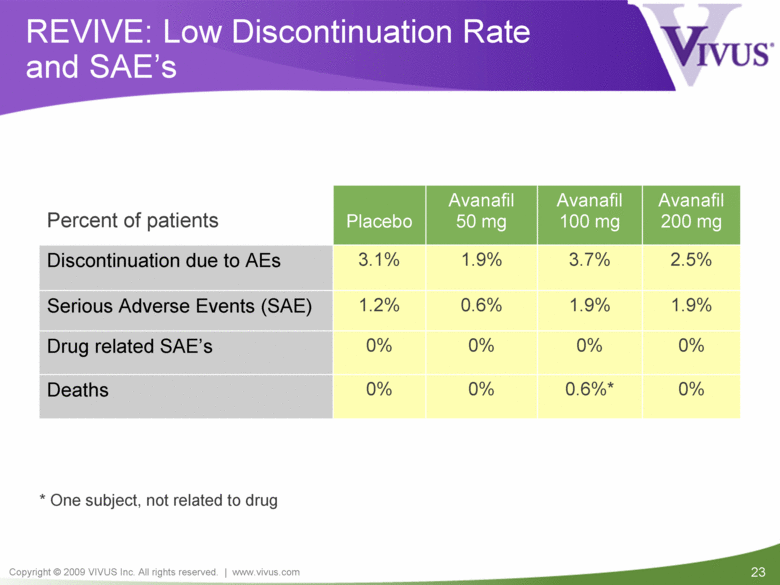
REVIVE: Low Discontinuation Rate and SAE’s 0% 0% 0% 0% Drug related SAE’s 0% 0.6%* 0% 0% Deaths Percent of patients Placebo Avanafil 50 mg Avanafil 100 mg Avanafil 200 mg Discontinuation due to AEs 3.1% 1.9% 3.7% 2.5% Serious Adverse Events (SAE) 1.2% 0.6% 1.9% 1.9% * One subject, not related to drug |
|
|
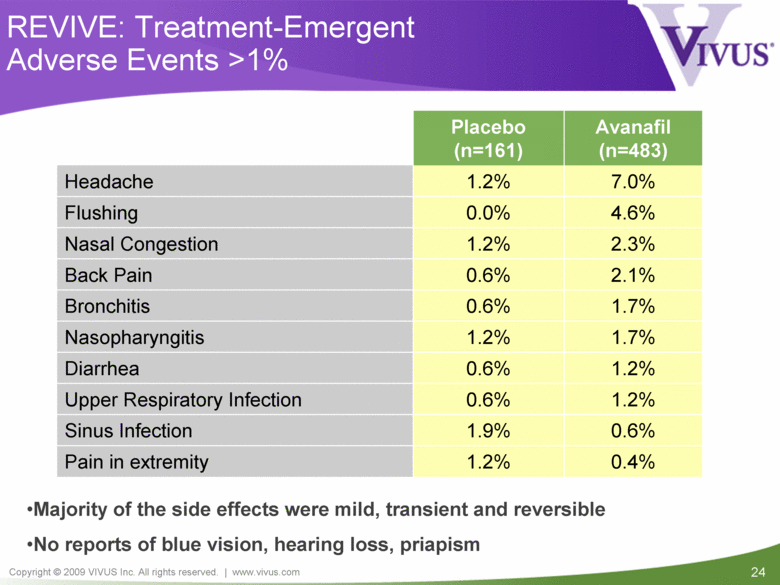
REVIVE: Treatment-Emergent Adverse Events >1% 1.7% 0.6% Bronchitis Placebo (n=161) Avanafil (n=483) Headache 1.2% 7.0% Flushing 0.0% 4.6% Nasal Congestion 1.2% 2.3% Back Pain 0.6% 2.1% Nasopharyngitis 1.2% 1.7% Diarrhea 0.6% 1.2% Upper Respiratory Infection 0.6% 1.2% Sinus Infection 1.9% 0.6% Pain in extremity 1.2% 0.4% Majority of the side effects were mild, transient and reversible No reports of blue vision, hearing loss, priapism |
|
|
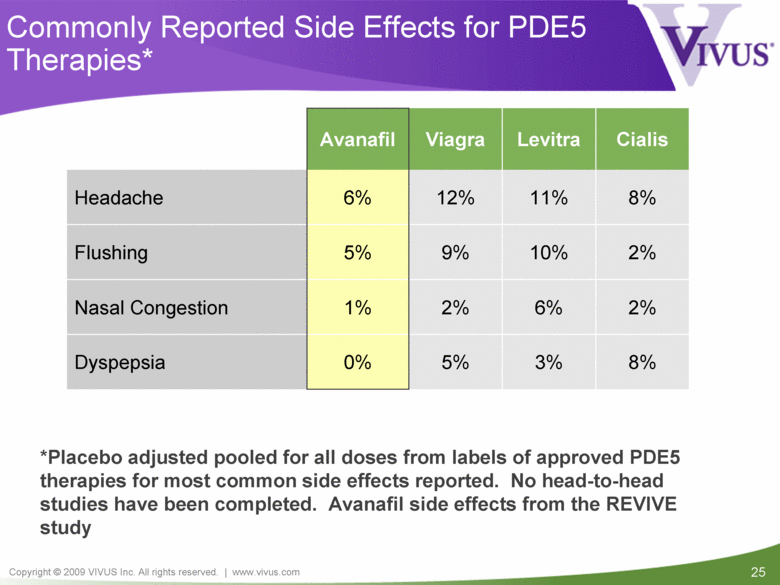
Commonly Reported Side Effects for PDE5 Therapies* 3% 6% 10% 11% Levitra 8% 5% 0% Dyspepsia 2% 9% 12% Viagra Avanafil Cialis Headache 6% 8% Flushing 5% 2% Nasal Congestion 1% 2% *Placebo adjusted pooled for all doses from labels of approved PDE5 therapies for most common side effects reported. No head-to-head studies have been completed. Avanafil side effects from the REVIVE study |
|
|
Avanafil Development Program Wesley W. Day, PhD Vice President Clinical Development |
|
|
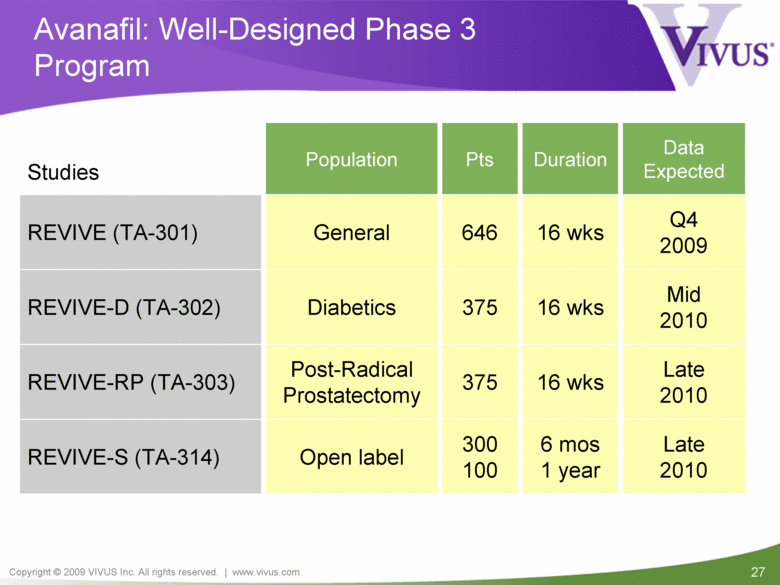
Late 2010 16 wks 375 Post-Radical Prostatectomy REVIVE-RP (TA-303) Q4 2009 16 wks 646 General REVIVE (TA-301) Mid 2010 16 wks 375 Diabetics REVIVE-D (TA-302) Late 2010 6 mos 1 year 300 100 Open label REVIVE-S (TA-314) Duration Studies Population Pts Data Expected Avanafil: Well-Designed Phase 3 Program |
|
|
Avanafil Development Program On Track All nonclinical and clinical studies necessary to support NDA are completed, in progress or planned Clinical Pharmacology OB-140 TQT study completed - no findings of concern Nitrate study complete – avanafil less interaction than sildenafil Clinical Pharmacology studies on track to complete 2010 Non-Clinical All studies on track or complete - no findings of concern Development plan on track for NDA submission in late 2010 or early 2011 |
|
|
Dr. Leroy Jones Clinical Associate Professor, Urology, University of Texas Health Science Center, San Antonio |
|
|
30 Copyright © 2009 VIVUS Inc. All rights reserved. | www.vivus.com REVIVE (TA-301): Summary • All primary FDA endpoints achieved for all 3 doses • Largest of three pivotal phase 3 studies • Conducted under Special Protocol Assessment (SPA) • Outstanding results from REVIVE • 77% of attempts among patients on avanafil resulted in successful intercourse • Maximum effect achieved in less than 30 minutes • Efficacy sustained beyond 6 hours • Potentially best safety profile in class |