Attached files
Exhibit 10.6
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Asterisks denote omissions.
Securities and Exchange Commission. Asterisks denote omissions.
Amendment 2
OEM DEVELOPMENT AND PURCHASE AGREEMENT
OEM DEVELOPMENT AND PURCHASE AGREEMENT
This Amendment 2 to the OEM Development and Purchase Agreement dated the 24th day of
July, 2003, as amended by Addendum 1 (with a date of June 5, 2006 in the footer of such document)
(the “Agreement”), by and between Aspect Medical Systems, Inc. (“Aspect”) and Mindray DS USA, Inc.
(“Datascope”) (formerly Datascope Corp.) is made as of this 3rd day of October, 2009
(the “Amendment Effective Date”).
Terms used, but not defined, in this Amendment 2, have the meaning ascribed to them in the
Agreement.
| 1. | Section 2 of the Agreement shall be replaced by the following: |
| 2 | Definitions | ||
| 2.1 | “Affiliate” shall mean, with respect to either Aspect or Datascope, any other business entity which, directly or indirectly, controls, is controlled by, or is under common control with Aspect or Datascope, respectively. The parties agree and acknowledge that Mindray and its affiliates are Affiliates of Datascope. | ||
| 2.2 | “Aspect BIS Sensor” means a single use disposable sensor manufactured by Aspect for use with the Aspect BISx Kit in the OR, ICU, and other sedation locations that is required to generate Aspect’s Bispectral Index. These sensors include the BIS Quatro Sensor, the BIS Bilateral Sensor and the BIS Pediatric Sensor. | ||
| 2.3 | “Aspect’s Bispectral Index” or “BIS” is Aspect’s proprietary processed EEG parameter that may be used as an aid in monitoring the effects of certain anesthetic agents. | ||
| 2.4 | “Aspect BISx Kit” is the Aspect components of the Datascope BISx System that are developed and manufactured by or for Aspect and licensed/sold to Datascope under this Agreement including the BISx or BISx4, and the Patient Interface Cable (“PIC”). | ||
| 2.5 | “Aspect Products” means Aspect BISx Kit and any other product that can be ordered by Datascope as listed in Exhibit A (Aspect Products and Purchase Prices). | ||
| 2.6 | “BISx” is an Aspect device that acquires up to two channels of EEG and computes BIS and other EEG parameters. | ||
| 2.7 | “BISx4” is an Aspect device that acquires up to four channels of EEG and computes BIS and other EEG parameters | ||
| 2.8 | “Documentation” means the BISx Serial Interface Specification. | ||
| 2.9 | “Monitor Cable” is a cable that connects the BISx or BISx4 to the Datascope Patient Monitor. | ||
| 2.10 | “Host Cable Connector” is the connector that is or will be (as applicable) integrated into the Monitor Cable for connection to the Datascope Patient Monitors. |
1/15
| 2.11 | “Datascope Patient Monitors” includes but not limited to multiparameter patient monitoring systems and anesthesia machines manufactured and/or sold by Datascope or its Affiliates under the Datascope brands designed for monitoring patient vital signs in anesthetizing and sedating locations. | ||
| 2.12 | “Datascope BISx System” is the combined Aspect BISx Kit and Monitor Cable. | ||
| 2.13 | “Party” or “Parties” shall mean Aspect and Datascope each individually or jointly. | ||
| 2.14 | “Software” means Aspect software programs in binary code form that are designed for use with the Aspect BISx Kit. | ||
| 2.15 | “Mindray” means Shenzen Mindray Bio-Medical Electronics Co., Ltd. |
| 2. | Section 4.3 of the Agreement shall be replaced by the following: | ||
| Distribution of Aspect BIS Sensors |
| 4.3.1 | Sensor Distribution | ||
| Aspect will provide Datascope one (1) starter kit of demonstration sensors (five (5) sensors per kit as set forth in Exhibit A (Aspect Products and Purchase prices)), at no charge with each Datascope BISx System purchased. Datascope will provide this starter kit of five (5) Aspect BIS Sensors at no charge with each Datascope BISx System sold to the customer purchasing the corresponding Datascope BISx System. | |||
| Datascope may [**] of Aspect BIS Sensors (twenty-five (25) sensors per box, except for Bilateral which is ten (10) sensors per box, as set forth in Exhibit A) solely in connection with the new sale of each Datascope BISx System and solely to the customer purchasing the corresponding Datascope BISx System. Sensors are to be delivered at time of Datascope BISx System installation. | |||
| 4.3.2 | Prices | ||
| Prices for Aspect BIS Sensors purchased by Datascope hereunder shall be as set forth in Exhibit A (Aspect Products and Purchase Prices). |
2/15
| 4.3.3 | Aspect will ensure that Datascope customers have an outlet (which may include Aspect or its Affiliates or a third party distributor) from which to purchase BIS Sensors. In the event Aspect does not provide such an alternative BIS Sensor outlet in a country or region, Aspect (or its local Affiliate) will grant Datascope or the applicable Datascope local Affiliate non-exclusive distribution rights for BIS Sensors to Datascope customers in such country or region with terms, conditions and pricing consistent with those of similar distributors in other countries or regions offering the same level of marketing, customer, and technical support as the local Datascope Affiliate is prepared to offer. Aspect shall have the right to terminate any such agreement upon three (3) months notice once Aspect has established an alternative BIS Sensor outlet for such country or region. Aspect will provide regular updates to Datascope regarding the progress toward the establishment of alternative BIS Sensor outlets. | ||
| 4.3.4 | If any sales by Datascope or its Affiliates, distributors or sub-distributors of BIS Sensors are made to any customer in contravention of Section 4.3.1, Datascope will cease such sales and Datascope will retroactively pay Aspect an [**] for every BIS Sensor previously sold to customers in contravention of Section 4.3.1. In addition, Datascope shall reimburse Aspect for the cost of any audit that confirmed the existence of any such sales. |
| 3. | Section 6.1 “Commissions on Sales of Aspect BIS Sensors” is deleted | ||
| 4. | Section 7.6 “Competitive Products and Volume Price Discounts “ is added: | ||
| Competitive Products and Volume Price Discounts | |||
| If Datascope or its Affiliates offer a directly competitive product to the Aspect BISx Kit (any parameter that utilizes EEG to measure the hypnotic effect of anesthesia) the volume discounts shown in Exhibit A [**]. | |||
| 5. | The following is added to the end of Section 10 of the Agreement: | ||
| In the event that a new Datascope BISx System (not repaired or refurbished product) is rejected by Datascope, and Aspect replaces the product as remedy, the replacement product shall be a new Datascope BISx System, not refurbished. | |||
| In the event that Datascope rejects a service exchange Datascope BISx System, and Datascope provides written notice to Aspect of the rejection within [**] days of the date of receipt, Aspect shall in good faith, promptly upon receipt of written notification, and in lieu of return receipt of the rejected service exchange Datascope BISx System, send to Datascope a replacement service exchange Datascope BISx System. Should Aspect’s subsequent examination and testing of the rejected service exchange Datascope BISx System disclose that there was no defect covered by the warranty set forth in Section 10, Aspect shall so advise Datascope and Aspect may dispose of or otherwise handle the returned service |
3/15
| exchange Datascope BISx System at Aspect’s discretion, and Datascope shall reimburse Aspect for transportation and insurance costs. |
4/15
| 6. | Section 11.1 of the Agreement shall be replaced by the following: | ||
| General | |||
| Aspect warrants solely to Datascope that Aspect Products (including Software) delivered hereunder shall perform substantially in accordance with the specifications in Exhibit B (Datascope’s BISx System) and shall be free from defects in materials and workmanship, when given normal, proper and intended usage, for [**] months from the date of shipment by Aspect to Datascope. This warranty shall not apply to expendable components and supply items, such as, but not limited to, cables (except for failures occurring within [**] days of receipt of shipment by Datascope), or disposable items such as an Aspect BIS Sensor after the expiration date marked on the Aspect BIS Sensor packaging. Aspect shall not have any obligation under this Agreement to make repairs or replacements which are required by normal wear and tear, or which result, in whole or in part, from catastrophe, fault or negligence of Datascope or Affiliate, or anyone claiming through or on behalf of Datascope, or from improper or unauthorized use of Aspect Products, or use of Aspect Products in a manner for which they were not designed, or by causes external to Aspect Products such as, but not limited to, power or air conditioning failure. | |||
| 7. | Section 11.2 of the Agreement shall be replaced by the following: | ||
| Warranty Procedures | |||
| Datascope shall notify Aspect of any Aspect products which it believes to be defective during the applicable warranty period and which are covered by the warranties set forth in Section 11.1. At Aspect’s option, such Aspect Products shall be returned by Datascope to Aspect’s designated facility for examination and testing, or may be repaired on site by Aspect. Aspect shall either repair or replace, within [**] of receipt by Aspect, any such Aspect Product found to be defective and return these Aspect Products to Datascope. Transportation and insurance costs shall be borne by Aspect. Should, Aspect’s examination and testing disclose that there was no defect covered by the foregoing warranty, Aspect shall so advise Datascope and dispose of or return the Aspect Product in accordance with Datascope’s instructions and at Datascope’s expense, and Datascope shall reimburse Aspect for transportation and insurance costs. | |||
| 8. | Section 12.2 of the Agreement shall be replaced by the following: | ||
| Service Training | |||
| Aspect agrees to provide initial service training, [**] to Datascope, to a mutually agreed upon number of Datascope service representatives prior to the market release of the BISx Systems | |||
| If there are Aspect Product changes that materially affect form, fit, or function, as described in Section 15, and based on the mutual understanding of the necessity of the training, Aspect shall provide [**] training to a mutually agreed upon number of Datascope personnel, at a mutually agreed upon schedule, [**] to Datascope. |
5/15
| 9. | Section 14.7 of the Agreement shall be replaced by the following | ||
| Recall | |||
| In the event that a corrective action (including notifications or recalls) is initiated with respect to any Aspect Product, and such action (i) is required to comply with applicable laws or regulations, (ii) is initiated by Aspect in its discretion for a reasonable business purpose or for safety reasons or (iii) is the result of the failure of Aspect Products to conform in all material respects to the applicable standards, Aspect shall be responsible for the repair or replacement of the Aspect Products without cost to Datascope. Aspect agrees to consult with Datascope to establish a reasonable process for managing the corrective action and Aspect shall be responsible for all reasonable out-of-pocket expenditures incurred by Datascope if Aspect fails to take corrective action. In the event that Aspect or Datascope is required to take such action to comply with applicable laws or regulations or such action is reasonably necessary for safety and efficacy reasons or for the failure of the Aspects Products to comply with specifications (other than minor deviations from specifications) and fails to do so within 10 days of being notified of the need for such action, Datascope shall have the right to take such action and Aspect shall reimburse Datascope for all reasonable out-of-pocket expenditures incurred in connection with such action by Datascope. If it is determined by an arbitrator that such action was not necessary for safety reasons or for the failure of Aspect Products to comply with specifications (other than a minor deviation from such specifications), Datascope will return to Aspect any amounts previously paid to Datascope by Aspect in connection with such action. | |||
| Any corrective action will be at Datascope’s expense if clearly caused by the Datascope Patient Monitor. Datascope will reimburse Aspect for Aspect’s documented cost directly resulting from such corrective actions within sixty (60) days of completion of the corrective action. | |||
| Datascope and Aspect will share the cost if both Parties’ products contribute to the cause, proportionate to the extent of each Party’s respective degree of cause as mutually agreed. Aspect and Datascope will negotiate in good faith a reasonable settlement of the issue. If after twenty (20) business days, the Parties in good faith fail to agree the issue will be escalated for resolution in accordance with Section 22. | |||
| 10. | Section 22.1 of the Agreement shall be replaced by the following | ||
| Term and Renewal | |||
| The initial term of this Agreement shall commence on the date first set forth above and shall continue for an initial period until October 25, 2009. The term of this Agreement shall thereafter be renewed automatically for successive twelve (12) month periods, unless either Party provides written notice of termination to the other Party at least ninety (90) days prior to expiration of the Agreement. | |||
| 11. | Exhibits of the Agreement shall be replaced as follows: |
| a) | “Exhibit A: Aspect Products and Purchase Prices” is replaced by the attached “Exhibit A: Aspect Products and Purchase Prices”. |
6/15
| b) | “Exhibit B: Specifications: Datascope BISx System” is replaced by the attached “Exhibit B: Specification: Datascope BISx System”. | ||
| c) | “Exhibit C: Contact Persons / Addresses” is replaced by the attached “Exhibit C: Contact Persons / Addresses”. | ||
| d) | “Exhibit D: Aspect and Datascope Trademarks” is replaced by the attached “Exhibit D: Aspect and Datascope Trademarks”. |
All terms and conditions of the Agreement not modified herein shall remain unchanged, in full force
and effect. After the Amendment Effective Date, every reference in the Agreement to the
“Agreement” shall mean the Agreement as amended by this Amendment 2.
Acceptance of this Amendment 2 is indicated by signatures below.
| Aspect Medical Systems, Inc. | Mindray DS USA, Inc. | |||||||
By:
|
/s/ J. Neal Armstrong | By: | /s/ Tim Fitzpatrick | |||||
Date:
|
11-5-09 | Date: | 2 November 2009 | |||||
Name:
|
Neal Armstrong | Name: | Tim Fitzpatrick | |||||
Title:
|
Chief Financial Officer | Title: | Corporate Secretary | |||||
7/15
EXHIBIT A
ASPECT PRODUCTS AND PURCHASE PRICES
A) Datascope BISx System:
| Aspect | Datascope | Unit of | ||||||||||||||
| Part Number | Part Number | Product Description | Measure | List Price | ||||||||||||
186-0195-DS |
0992-00-0236-01 | BISx Kit (Datascope) | 1 each | [**] | ||||||||||||
186-0224-DS
(†) |
TBD | BISx4 Kit (Datascope) | 1 each | [**] | ||||||||||||
| (†) | Estimated price, assumes a standard Datascope monitor cable and host cable connector. Customization of monitor cable or host cable connector may increase costs. BISx4 Kit development costs will be determined by the terms and conditions of Section 7 the Agreement. |
B) Volume Discounts
| Total Price for | Total Price for | |||||||||||||||||||
| Cumulative Annual Total | Price for | Datascope BISx | Datascope BISx4 | |||||||||||||||||
| Volume (in units) ‡ | Price for | Price for | Monitor | System | System | |||||||||||||||
| (Calendar Year) | BISx Kit | BISx4 Kit | Cable | 186-0195-DS | 186-0224-DS(†) | |||||||||||||||
[**] |
[**] | [**] | [**] | [**] | [**] | |||||||||||||||
[**] |
[**] | [**] | [**] | [**] | [**] | |||||||||||||||
[**] |
[**] | [**] | [**] | [**] | [**] | |||||||||||||||
| (†) | Estimated price, assumes a standard Datascope monitor cable and host cable connector. Customization of monitor cable or host cable connector may increase costs. BISx4 Kit development costs will be determined by the terms and conditions of Section 7 the Agreement. | |
| ‡ | Total calculation includes all Datascope BISx System types purchased by Datascope under this Agreement (186-0195-DS and BISx4 Kits), as well as [**]. Cumulative means the combination of volume of Mindray and [**]. Pricing from the Amendment Effective Date through [**] will be at the [**] level. Pricing for subsequent years will be as defined in section E of this Exhibit A. |
C) BIS DISPOSABLE SENSORS
| Aspect | Datascope | Unit of | Datascope | |||||||||||||
| Part Number | Part Number | Product Description | Measure | Price | ||||||||||||
186-0106* |
0600-00-0132-01 | BIS Quatro Sensor | Box of 25 | [**] | ||||||||||||
186-0200* |
0600-00-0132-03 | BIS Pediatric Sensor | Box of 25 | [**] | ||||||||||||
186-0160* |
0600-00-0132-02 | BIS Extend Sensor | Box of 25 | [**] | ||||||||||||
186-0212* |
TBD | BIS Bilateral Sensor | Box of 10 | [**] | ||||||||||||
| * | Datascope may distribute [**] of sensors (1 box = 25 sensors, except for Bilateral where 1 box = 10 sensors) bundled with the sale of the Datascope BISx System, as set forth in Section 4.3. |
ii) Demonstration sensors:
| Aspect | Datascope | Unit of | Datascope | |||||
| Part Number | Part Number | Product Description | Measure | Price | ||||
186-0150-DS**
|
0020-00-0491-01 | BIS Quatro Starter Kit | Box of 5 | [**] | ||||
186-0206-DS**
|
0020-00-0491-03 | BIS Pediatric Starter Kit | Box of 5 | [**] |
| ** | Demonstration sensors cannot be resold |
8/15
D) SPARE PARTS/ACCESSORY PRICES
| Datascope | Orderable | Unit of | Aspect | Datascope | ||||||||
| Aspect Part # | Part Number | Parts/Products | Measure | List Price | Price | |||||||
186-0142-DS
|
0012-00-1666-01 | DS PIC+ Cable (replacement) |
1 each | $ | 125.00 | [**] | ||||||
186-0201-DS
|
0012-00-1665-01 | Datascope Host Cable (Replacement) |
1 each | [**] | ||||||||
186-1018-DS
|
TBD | DS PIC4 Cable (replacement) |
1 each | $ | 200.00 | [**] | ||||||
186-0143-DS
|
0454-00-0060 | DS 2ch & 4ch Sensor Simulator |
1 each | $ | 30.00 | [**] | ||||||
| Aspect Part | Datascope | Orderable | Unit of | Aspect | Datascope | |||||||
| Number | Part Number | Parts/Products | Measure | List Price | Price | |||||||
186-0199-DS (••)
|
0992-UC-0236-01 | Datascope BISx Kit (service exchange) |
1 each | $ | 1,500.00 | [**] | ||||||
186-1030-DS (†) (••)
|
TBD | Datascope BISx4 Kit (service exchange) |
1 each | $ | 2,300.00 | [**] | ||||||
| (†) | Estimated price, assumes a standard Datascope monitor cable and host cable connector. Customization of monitor cable or host cable connector may increase costs. BISx4 Kit development costs will be determined by the terms and conditions of Section 7 the Agreement. | |
| (••) | Service exchange items may be refurbished product. Service exchange items can only be ordered in qty equal to Return Material Authorization Numbers (RMA#s) issued by Aspect. See Section 11.2 of the Agreement for details on Warranty procedures. Units exchanged under warranty may be replaced with a comparable refurbished product and carry a Repair Warranty as outlined in Section 11.3 of the Agreement. |
E) CALCULATION OF VOLUME DISCOUNT:
For the purpose of calculating the volume discount for a given calendar year, all BISx/BISx4
Kits purchased by [**], excluding Aspect Products that are shipped free of charge, will be
included in the total volume discount calculation.
The initial pricing for a given calendar year is based on the total volume of BISx/BISx4 Kits
purchased in the prior calendar year. For example, if [**] BISx Kits were purchased in year 1,
the initial volume pricing level for year 2 will be the [**] unit price level. The Parties
agree that, for the period beginning on the Amendment Effective Date and continuing through
[**], pricing will start at the [**] unit price level.
If a higher volume level of BISx/BISx4 Kits is achieved during a given calendar year, the price
on only those BISx/BISX4 Kits purchased after achieving the higher volume level will reflect
the price associated with the appropriate volume level achieved. All price adjustments will
apply on a going forward basis only. For example, if midway through year 2, [**] BISx Kits are
purchased, the price on the [**] BISx Kit will reflect the next volume break.
F) CURRENCY
United States Dollars
9/15
EXHIBIT B
SPECIFICATIONS: DATASCOPE BISx SYSTEM
SPECIFICATIONS: DATASCOPE BISx SYSTEM
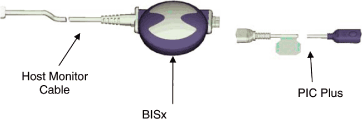
PRODUCT DESCRIPTION
The BISx Kit includes a PIC+, BISx Device, and a Monitor Cable. The BISx kit will here
forth be referred to as BISx. The BISx4 Kit includes a 4 channel PIC, BISx4 Device, and a
Monitor Cable. The BISx4 kit will here forth be referred to as BISx4.
BISx is a device that acquires up to two channels of EEG and computes BIS and other EEG
parameters. BISx4 is a device that acquires up to four channels of EEG and computes BIS
and other EEG parameters. Both the BISx and BISx4 are designed to mate with Aspect’s XP
platform 1 or 2 channel sensors. The BISx4 is designed to additionally interface with
Aspect’s Bilateral sensor, which is required for acquisition of 4 channels of EEG. BISx
and BISx4 have no display or user interface. They plug into a host monitor system for
display of EEG and processed parameters. BISx and BISx4 are designed for use wherever
sedative drugs are administered, including but not limited to the following environments:
Operating rooms, Intensive Care Units, Procedural Sedation, and Clinical Research areas.
BISx and BISx4 interface to one or more of the following interfaces: standard RS-232
asynchronous interface, RS-232 type asynchronous interface but with TTL 3.3V signal levels,
RS-485, or Universal Serial Bus (USB) interface. All interface versions also support USB
interfacing for software upgrade and download purposes. BISx and BISx4 can be connected
and disconnected to an already powered up host monitor. The host monitor should
automatically detect its presence and configure it accordingly.
10/15
The BISx connects to a sensor via the Aspect Patient Interface Cable (here forth referred
to as PIC+). The PIC+ is nominally 4.5 ft. long. The PIC+ connection is integral to the
enclosure (no pigtail), and can be detached from the BISx device for service or replacement
without the use of tools. The enclosure is sealed against liquid ingress even when the
PIC+ is detached.
The BISx4 connects to a sensor via the Aspect 4 channel Patient Interface Cable (here forth
referred to as CM PIC). The CM PIC is nominally 54 inches long. The CM PIC connection is
integral to the enclosure (no pigtail), and can be detached from the BISx4 device for
service or replacement without the use of tools. The enclosure is sealed against liquid
ingress even when the PIC+ is detached.
The BISx or BISx4 is attached to the host monitoring system via a nominal 9-foot monitor
cable. The host connector of the monitor cable is host system dependent and is the source
of future development with Datascope. The wire is narrow and highly flexible
multi-conductor cable used to supply power from the host monitor to the BISx/BISx4 as well
as allow communication between the devices. Additionally, the inability of the host
monitor to provide the BISx specified power requirements or communication requirements may
be addressed through the inclusion of a circuit which is integral to the monitor cable.
The monitor cable connection to the BISx/BISx4 device is integral to the enclosure (no
pigtail), and may require the use of tools for detachment from the BISx/BISx4 device
enclosure for the purposes of service or replacement. The enclosure is sealed against
liquid ingress only when the monitor cable is attached. There are no adjustable parts
inside the BISx. The cables may be replaced without opening the enclosure.
The BISx and BISx4 include software which is stored in reprogrammable FLASH memory.
Software upgrades can be accomplished via the serial / USB interface. Each BISx and BISx4
is given a unique serial identifier, allowing for electronic identification and tracking of
every BISx/BISx4.
PRELIMINARY TECHNICAL SPECIFICATIONS
(Unless otherwise noted specifications apply to both the BISx and BISx4)
Physical Specifications
BISx Kit
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
Weight: approx 8.0 oz without cables
approx 10.0 oz including PIC+
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
Weight: approx 8.0 oz without cables
approx 10.0 oz including PIC+
BISx Integral Cables
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (PIC+): 1.4m (4.5 ft)
11/15
BISx4 Kit
Size: 95.3 mm (3.75”) diameter x 63.5 mm (2.5”) height
Weight: 227g (8.0 oz) without cables
Weight: 227g (8.0 oz) without cables
BISx4 Integral Cables
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (CM PIC): 1.4m (4.5 ft)
Host Monitor Cable: 2.74m (9ft)
Patient Interface Cable (CM PIC): 1.4m (4.5 ft)
Safety Specifications
| • | The BISx and BISx4 comply with the essential requirements of the Medical Device Directive 93/42/EEC, as well as IEC 60601-1 and IEC 60601-2-26. | ||
| • | They are both Type BF applied parts. Both the BISx and BISx4 have internal optical coupling and an isolation transformer for patient isolation. | ||
| • | Both are protected against damage from defibrillation as long as the sensor is not located between the defibrillator pads and is resistant to artifact from electrosurgery. | ||
| • | United States federal law restricts these devices to sale by or on the order of a physician. | ||
| • | BISx, BISx4, and cables are latex free. |
Environmental Specifications
Liquid Ingress: IEC 529 IPX4
Temperature
Temperature
0 to 40 degrees C operating
-40 to 70 degrees C storage
Humidity
-40 to 70 degrees C storage
Humidity
10% to 95% non-condensing operating
10% to 95% non-condensing storage
10% to 95% non-condensing storage
Pressure
480 to 1067 hPa operating (Approx -1250 to 20,000 feet)
116 to 1067 hPa storage (Approx -1250 to 50,000 feet)
116 to 1067 hPa storage (Approx -1250 to 50,000 feet)
Performance Specifications
Measurement Ranges
Bispectral Index (BIS): 0 to 100 unitless scale
Electromyographic Strength (EMG): 25 to 100 dB where 1 µV2=40dB
Signal Quality Index (SQI): 0 to 100%
Suppression Ratio (SR): 0 to 100%
Spectral Edge Frequency (SEF): 0.5 to 30.0 Hz
Total Power: 40 to 100 dB where 1 µV RMS=40dB
Burst Count: 0 to 30
Bispectral Index (BIS): 0 to 100 unitless scale
Electromyographic Strength (EMG): 25 to 100 dB where 1 µV2=40dB
Signal Quality Index (SQI): 0 to 100%
Suppression Ratio (SR): 0 to 100%
Spectral Edge Frequency (SEF): 0.5 to 30.0 Hz
Total Power: 40 to 100 dB where 1 µV RMS=40dB
Burst Count: 0 to 30
Noise (EEG Waveform) < 0.3 µVRMS (2.0 µV peak-to-peak)
BIS Numeric Update Frequency Once per second
EEG Bandwidth 0.25 to 100 Hz (- 3dB)
Patient Leakage <10uA
BIS Numeric Update Frequency Once per second
EEG Bandwidth 0.25 to 100 Hz (- 3dB)
Patient Leakage <10uA
12/15
EXHIBIT C
CONTACT PERSONS/ADDRESSES
CONTACT PERSONS/ADDRESSES
Contact Persons and Responsibilities at Aspect:
| Person | Title | Responsibility | ||||
Joan Rubin
|
Sr. Director, | Contract | jrubin@aspectms.com | |||
| Business | ||||||
| Development | ||||||
Steve Mesrobian
|
Director, OEM | Project Manager | smesrobian@aspectms.com | |||
| Engineering | ||||||
Christine Vozella
|
Senior Director, | Quality and | cvozella@aspectms.com | |||
| RA/QA | Regulatory Matters | |||||
James Charest
|
OEM Engineer Manager | Technical Design | jcharest@aspectms.com | |||
| Issues |
Mailing Address:
|
Aspect Medical Systems, Inc. | |
| One Upland Road | ||
| Norwood, MA 02062 |
Contact Persons and Responsibilities at Datascope:
| Person | Title | Responsibility | Contact Information | |||
Richard Cipolli
|
Engineering Manager | Project Manager, | E-mail: | |||
| Spectrum O.R. | richard_cipolli@datascope.com | |||||
| Tel: 1-201-995-8024 | ||||||
| Fax: 1-201-995-8654 | ||||||
Bonita Lisnay
|
Purchasing | Purchasing | E-mail: | |||
| Supervisor | bonita_lisnay@datascope.com | |||||
| Tel: 1-201-995-8374 | ||||||
| Fax: 1-201-995-8001 | ||||||
Chuck Webster
|
Director of | Marketing | E-mail: | |||
| Marketing & Sales | chuck_webster@datascope.com | |||||
| Solutions | Tel: 1-201-995-8207 | |||||
| Fax: 1-201-995-8612 | ||||||
Kathleen Kramer
|
Supervisor, | Regulatory | E-mail: | |||
| Clinical and | kathleen_kramer@datascope.com | |||||
| Regulatory Affairs | Tel: 1-201-995-8169 Fax: 1-201-995-8605 |
Address: |
Mindray DS USA, Inc. | |||
| 800 MacArthur Blvd | ||||
| Mahwah, NJ 07430 | ||||
13/15
EXHIBIT D
ASPECT AND DATASCOPE TRADEMARKS
ASPECT AND DATASCOPE TRADEMARKS
| Aspect Trademarks | Reference | |
Aspect ®
|
Aspect is a registered trademark of Aspect Medical Systems, Inc | |
A-2000 TM
|
A-2000 is a trademark of Aspect Medical Systems, Inc. | |
Bispectral Index ®
|
Bispectral is a registered trademark of Aspect Medical Systems, Inc. | |
BIS ®
|
BIS is a registered trademark of Aspect Medical Systems, Inc. | |

|
BIS logo is a registered trademark of Aspect Medical Systems, Inc. | |
BISx ™
|
BISx is a trademark of Aspect Medical Systems, Inc. | |
BIS Ready™
|
BIS Ready is a trademark of Aspect Medical Systems, Inc. | |
BIS VISTA ™
|
BIS VISTA is a trademark of Aspect Medical Systems, Inc. | |
|
BISx logo is a trademark of Aspect Medical Systems, Inc. | |

|
BIS Ready logo is a registered trademark of Aspect Medical Systems, Inc. |
14/15
| Datascope Trademarks | Reference | |

|
Datascope logo is a U.S. registered trademark of Datascope Corp. | |
Datascope®
|
Datascope® is a U.S. registered trademark of Datascope Corp. | |
Navigator™
|
Navigator™ is a U.S. trademark of Datascope Corp. | |
Panorama™
|
Panorama™ is a U.S. trademark of Datascope Corp. | |
Spectrum™
|
Spectrum™ is a U.S. trademark of Datascope Corp. | |
View 12™
|
View 12™ is a U.S. trademark of Datascope Corp. | |
Passport 2®
|
Passport 2® is a U.S. registered trademark of Datascope Corp. | |
Auto-Set™
|
Auto-Set™ is a U.S. trademark of Datascope Corp. | |
Rescale Waves™
|
Rescale Waves™ is a U.S. trademark of Datascope Corp. | |
SmartScreen™
|
SmartScreen™ is a U.S. trademark of Datascope Corp. |
Further Datascope Trademark information to be provided by Datascope within 30 days of signing
amendment.
15/15
