Attached files
| file | filename |
|---|---|
| EX-21 - INTEGRATED BIOPHARMA INC | exhibit21.htm |
| EX-23 - INTEGRATED BIOPHARMA INC | exhibit23.htm |
| EX-31 - INTEGRATED BIOPHARMA INC | exhibit31_2.htm |
| EX-32 - INTEGRATED BIOPHARMA INC | exhibit32_2.htm |
| EX-31 - INTEGRATED BIOPHARMA INC | exhibit31_1.htm |
| EX-32 - INTEGRATED BIOPHARMA INC | exhibit32_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
____________
FORM 10-K
Annual Report Under Section 13 or 15(d)
of the Securities Exchange Act of 1934
|
For the fiscal year ended June 30, 2009 |
Commission File Number 001-31668 |
INTEGRATED BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
22-2407475 |
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.) |
|
225 Long Ave., Hillside, New Jersey |
07205 |
|
(Address of principal executive offices) |
(Zip code) |
Registrant’s telephone number: (888) 319-6962
Securities registered under Section 12(b) of the Exchange Act:
|
Title of Each Class |
Name of Each Exchange on Which Registered |
|
Common Stock, $.002 par value per share |
None |
Securities registered under Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
|
Yes | | |
No | X | |
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
|
Yes | | |
No | X | |
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
|
Yes | X | |
No | | |
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
|
----- |
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “accelerated filer,” “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated Filer | | |
|
Accelerated Filer | | |
|
Non-accelerated Filer | | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
|
Yes | | |
No | X | |
The aggregate market value of the voting stock held by non-affiliates of the Registrant based on the trading price of the Registrant’s Common Stock on December 31, 2008 was 1,499,639
The number of shares outstanding of each of the Registrant’s classes of common equity, as of the latest practicable date:
|
Class |
Outstanding at October 9, 2009 |
|
Common Stock, $.002 par value |
20,249,442 Shares |
DOCUMENTS INCORPORATED BY REFERENCE
The information required by part III will be incorporated by reference from certain portions of a definitive Proxy Statement which is expected to be filed by the Registrant within 120 days after the close of its fiscal year.
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
FORM 10-K ANNUAL REPORT
INDEX
|
Part I |
Page |
|
|
Item 1. |
Description of Business |
4 |
|
Item 1A. |
Risk Factors |
9 |
|
Item 1B. |
Unresolved Staff Comments |
12 |
|
Item 2. |
Properties |
13 |
|
Item 3. |
Legal Proceedings |
13 |
|
Item 4. |
Submission of Matters to a Vote of Security Holders |
14 |
|
Part II |
||
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Registrant Purchases of Equity Securities |
15 |
|
Item 6. |
Selected Financial Data |
16 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
16 |
|
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
28 |
|
Item 8. |
Financial Statements |
28 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and |
|
|
Financial Disclosure |
28 |
|
|
Item 9A. |
Controls and Procedures |
28 |
|
Item 9B. |
Other Information |
29 |
|
Part III |
||
|
Item 10. |
Directors and Executive Officers of the Registrant |
30 |
|
Item 11. |
Executive Compensation |
30 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management |
|
|
and Related Stockholder Matters |
30 |
|
|
Item 13. |
Certain Relationships and Related Transactions |
30 |
|
Item 14. |
Principal Accountant Fees and Services |
30 |
|
Part IV |
||
|
Item 15. |
Exhibits and Financial Statement Schedules |
31 |
|
Signatures |
68 |
|
|
|
||
2
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this Annual Report on Form 10-K may constitute forward-looking statements as defined in Section 27A of the Securities Act of 1933 (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”), the Private Securities Litigation Reform Act of 1995 (the “PSLRA”) or in releases made by the Securities and Exchange Commission (“SEC”), all as may be amended from time to time. Such forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause the actual results, performance or achievements of Integrated BioPharma, Inc. and its subsidiaries (“INBP”) or industry results, to differ materially from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors including, among others, changes in general economic and business conditions; loss of market share through competition; introduction of competing products by other companies; the timing of regulatory approval and the introduction of new products by INBP; changes in industry capacity; pressure on prices from competition or from purchasers of INBP's products; regulatory changes in the Pharmaceutical manufacturing industry and Nutraceutical industry; regulatory obstacles to the introduction of new technologies or products that are important to INBP; availability of qualified personnel; the loss of any significant customers or suppliers; and other factors both referenced and not referenced in this Report. Statements that are not historical fact are forward-looking statements. Forward looking-statements can be identified, by among other things, the use of forward-looking language, such as the words “plan”, “believe”, “expect”, “anticipate”, “intend”, “estimate”, “project”, “may”, “will”, “would”, “could”, “should”, “seeks”, or “scheduled to”, or other similar words, or the negative of these terms or other variations of these terms or comparable language, or by discussion of strategy or intentions. These cautionary statements are being made pursuant to the Securities Act, the Exchange Act and the PSLRA with the intention of obtaining the benefits of the “safe harbor” provisions of such laws. INBP cautions investors that any forward-looking statements made by INBP are not guarantees or indicative of future performance. Important assumptions and other important factors that could cause actual results to differ materially from those forward-looking statements with respect to INBP include, but are not limited to, the risks and uncertainties affecting their businesses described in Item 1A of this Annual Report on Form 10-K and in other securities filings by INBP.
Although INBP believes that its plans, intentions and expectations reflected in or suggested by such forward-looking statements are reasonable, actual results could differ materially from a projection or assumption in any of its forward-looking statements. INBP’s future financial condition and results of operations, as well as any forward-looking statements, are subject to change and inherent risks and uncertainties. The forward-looking statements contained in this Annual Report on Form 10-K are made only as of the date hereof and INBP does not have or undertake any obligation to update or revise any forward-looking statements whether as a result of new information, subsequent events or otherwise, unless otherwise required by law.
3
Item 1. Description of Business
General
Integrated BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the “Company” or “INBP”), is engaged primarily in manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily in the United States. The Company was previously known as
Integrated Health Technologies, Inc. and, prior to that, as Chem International, Inc. The Company was reincorporated in its current form in Delaware in 1995. As of September 22, 2009, the Company’s common stock trades on the OTC Bulletin Board under the symbol INBP.OB. From February 27, 2009 through September 22, 2009, the Company’s common stock traded on the Pink Sheets under the symbol INBP.PK. Immediately prior to February 27, 2009, the Company’s common stock traded
on the NASDAQ Global Market under the symbol “INBP.” The Company continues to do business as Chem International, Inc. with certain its customers and certain vendors.
The Company, subsequent to the spin-off of its Biotechnologies segment and the sale of the Pharmaceutical segment, as discussed in more detail below, has one remaining reportable segment for its operation, the Nutraceuticals segment.
The Nutraceutical segment, our one remaining business operation includes: InB:Manhattan Drug Company, Inc. (“Manhattan Drug”), which manufactures vitamins and nutritional supplements for sale to distributors, multilevel marketers and specialized health-care providers; The Vitamin Factory, which sells private label Manhattan Drug products, as well as our AgroLabs products, through mail order catalogs and the Internet.
AgroLabs, Inc. (“AgroLabs”), which oversees the manufacture of and distributes for sales through major mass market, grocery, drug and vitamin retailers, healthful nutritional products under the following brands: Naturally Noni, Naturally Pomegranate, Naturally Aloe, Aloe Pure, Naturally Thai Mangosteen, Peaceful Sleep, Green Envy, 1st Choice Multi-Vitamin, ACAI Extra, ACAI Immune, ACAI Cleanse, and other products which are being introduced into the market, these are referred to as our branded proprietary Nutraceutical business and/or products.
The Company also distributes fine natural chemicals through its wholly-owned subsidiary IHT Health Products, Inc. and is a distributor of certain raw materials for DSM Nutritional Products, Inc.
During the fiscal year ended June 30, 2007, The Organic Beverage Company (TOBC), formerly Bioscience Technologies, Inc., completed the acquisition from BevSpec, Inc. (“BevSpec”) of the Syzmo™ product, which is a USDA organic energy drink. During the first quarter of the fiscal year ended June 30, 2009, we curtailed operations of our TOBC subsidiary and combined the sales efforts for this product line with the AgroLabs products. In June 2009, we further determined that this product line was to be discontinued as we do not have the financial resources to pursue the further development of the Syzmo™ product in the very competitive energy drink market place.
The Pharmaceutical segment included InB:Paxis Pharmaceuticals, Inc. (“Paxis”) and InB:Hauser Pharmaceutical Services, Inc. (“Hauser”). Paxis manufactured and distributed Paclitaxel, and Hauser provided research, development and manufacturing testing services to the specialty chemical, pharmaceutical and natural products industries. On March 17, 2009, we consummated the sale of all of the issued and outstanding shares of the common stock of Hauser to Cedarburg Pharmaceuticals, Inc. Prior to the sale of Hauser, we sold substantially all of the assets of Paxis to Hauser and transferred the outstanding payables owed by Paxis to Hauser in consideration for the outstanding intercompany debt between Hauser and Paxis. The assets and liabilities transferred under this transaction were owned by Hauser at the time of the sale of Hauser’s common stock to Cedarburg and are no longer our assets or our liabilities. We continue to own certain assets of Paxis through our common stock ownership of Paxis. Our selling price received in connection with the sale of Hauser consisted of approximately $1.2 million in cash and a promissory note in our favor in the principal amount of $340,000, which note matures in twelve months on March 17, 2010 and bears interest at a rate of 12% per annum, payable quarterly. On April 7, 2009, we sold this promissory note to CD Financial, LLC, a related party and the holder of our Convertible Note Payable in the amount of $4.5 million, for the full principal amount of $340,000 and accrued interest of approximately $2,000.
4
The Biotechnologies segment included iBio, Inc. (formerly, iBioPharma, Inc.) (“iBio”), which was focused on the discovery, development and commercialization of proprietary products from plants. On August 18, 2008, our distribution (the “Distribution”) of the Biotechnologies segment was completed and each of our shareholders received one share of iBio’s common stock for each share they owned of our common stock as of August 12, 2008, the (the “Record Date”). The Distribution qualified as a tax-free reorganization under Section 355 of the Internal Revenue Code of 1986, as amended. The Separation and Distribution Agreement prohibits iBio from issuing any additional shares of its common stock in excess of the shares issued with respect to the Distribution for the two years immediately following the effective date of the Distribution.
On August 19, 2008, we entered into a Conversion Agreement with iBio, where approximately $5.2 million of the intercompany debt with iBio was contributed to additional paid in capital and $2.7 million of the intercompany debt purchased approximately 1.3 million shares of iBio, representing 6% of the then outstanding shares of iBio.
Additionally, on August 19, 2008, iBio closed on its $5.0 million capital raise in connection with its private placement of approximately ten percent (10%) of iBio. Such funds were released to iBio from an escrow account and it issued approximately 2.3 million shares of iBio’s par value $0.001 common stock, at an estimated purchase price of approximately $2.13 per share. This private placement reduced
our ownership in iBio to 5.4%.
The financial statements contained herein, reflect the spin-off, sale and discontinued operations and related transactions of iBio, Hauser and TOBC, respectively.
Significant Revenues from Major Customers
A significant portion of our net sales are concentrated among three customers, Herbalife International of America, Inc., Costco Wholesale, Inc. and Sam’s Club. For the years ended June 30, 2009 and 2008, these customers represented approximately 81% and 79% of total net sales, respectively. The loss of any of these customers could have a significant adverse impact on our financial condition and results of operations.
Raw Materials
The principal raw materials used in the manufacturing process in the Company’s Nutraceutical segment are natural and synthetic vitamins, minerals, herbs, related nutritional supplements, gelatin capsules, coating materials, organic and natural fruit extracts, fruit juices and the necessary components for packaging the finished products. The raw materials are available from numerous
sources within the United States and abroad. The gelatin capsules, coating materials and packaging materials are similarly widely available. The Company generally purchases its raw materials, on a purchase order basis, without long-term commitments.
Our principal suppliers are Creative Flavor Concepts, Inc., Triarco Industries, Inc., and DSM Nutritional Products, Inc.
Development and Supply Agreement
On March 13, 1998, the Company signed a development and supply agreement with Herbalife International of America, Inc. (“Herbalife”) whereby the Company develops, manufactures and supplies certain nutritional products to Herbalife. This agreement is effective through December 31, 2009. This agreement does not, however, obligate the Company to supply any particular amount of goods to Herbalife, nor does it obligate Herbalife to commit to a minimum order, if any. In December 2008, the Company and Herbalife entered into an amendment to their development and supply agreement whereby, Herbalife committed to order an estimated $13.0 million of products in the calendar year ended December 31, 2009 and an additional $7.0 million in calendar year 2010. The amendment, however; does not provide for any penalties if such commitment is not met.
5
Seasonality
The Nutraceutical business segment tends to be seasonal. We have found that in our first fiscal quarter ending on September 30th of each year, orders for our branded proprietary Nutraceutical products usually slow (absent the addition of new customers or a new product launch with a significant first time order), as buyers in various markets may have purchased
sufficient inventory to carry them through the summer months. Conversely, in our second fiscal quarter, ending on December 31st of each year, orders for our products increase as the demand for our branded Nutraceutical products seems to increase in late December to early January as consumers become health conscious as they enter the new year.
We believe that there are other non-seasonal factors that also may influence the variability of quarterly results including, but not limited to, general economic and industry conditions that affect consumer spending, changing consumer demands and current news on nutritional supplements. Accordingly, a comparison of our results of operations from consecutive periods is not
necessarily meaningful, and our results of operations for any period are not necessarily indicative of future periods.
Variability of Quarterly Results and Impact of Advertising
In connection with our program to expand our branded Nutraceutical business, advertising and promotional expenses, including those classified as a reduction in sales, were $7.4 million or 18.8% of net sales, in the fiscal year ended June 30, 2009, as compared to $7.2 million or 16.4% of net sales, in the fiscal year ended June 30, 2008. As we continue this program we may continue to incur increased advertising and promotional expenses. Such expenses include promotional activities conducted through the retail trade, distributors or directly with consumers, including in-store displays, product placement programs, coupons, radio and print advertising, and other similar activities. Since such expenses may occur in fiscal quarters before increases, if any, in revenues occur, as a result of the advertising and promotion, the program may increase variability of our quarterly results. Other factors that also may influence the variability of quarterly results include general economic and industry conditions that affect consumer spending, changing consumer demands and current news on nutritional supplements. Accordingly, a comparison of our results of operations from consecutive periods is not necessarily meaningful, and our results of operations for any period are not necessarily indicative of future periods.
Government Regulations
The manufacturing, processing, formulation, packaging, labeling and advertising of our products are subject to regulation by a number of federal agencies, including the Food and Drug Administration (“FDA”), the Federal Trade Commission (“FTC”), the United States Postal Service, the Consumer Product Safety Commission and the United States Department
of Agriculture. Our activities are also regulated by various state and local agencies in which our products are sold. The FDA is primarily responsible for the regulation of the manufacturing, labeling and sale of our products. The operation of our vitamin manufacturing facility is subject to regulation by the FDA as a food manufacturing facility. The United States Postal Service and the FTC regulate advertising claims with respect to the Company’s products. In addition, we
manufacture and market certain of our products in compliance with the guidelines promulgated by the United States Pharmacopoeia Convention, Inc. (“USP”) and other voluntary standard organizations.
In May 2007, we obtained from Quality Assurance International, certification that our records and facilities for the Syzmo™ beverage are in accordance with The Organic Foods Production Act of 1990, 7 CFR Part 205 and with general guidelines established by the USDA’s National Organic Program. Since we have discontinued the operations of the Syzmo™ beverage product line, we did not renew this certification.
The Dietary Supplement Health and Education Act of 1994 (“DSHEA”) was enacted on October 25, 1994. The Dietary Supplement Act amends the Federal Food, Drug and Cosmetic Act (“FFD&CA”) by defining dietary supplements, which include vitamins, minerals, nutritional supplements and herbs, and by providing a regulatory framework to ensure safe, quality dietary supplements and the dissemination of accurate information about such products. Dietary supplements are regulated as foods under the DSHEA. Accordingly, the FDA is generally prohibited from regulating the active ingredients in dietary supplements as food additives, or as drugs unless product claims trigger drug status. The DSHEA requires the FDA to regulate dietary
6
supplements so as to guarantee consumer access to beneficial dietary supplements, allowing only truthful and proven claims. Generally, dietary ingredients that were on the market before October 15, 1994 may be sold without FDA pre-approval and without notifying the FDA. However, new dietary ingredients (those not used in dietary supplements marketed before October 15, 1994) require
pre-market submission to the FDA of evidence of a history of their safe use, or other evidence establishing that they are reasonably expected to be safe. There can be no assurance that the FDA will accept the evidence of safety for any new dietary ingredient we may decide to use. The FDA’s refusal to accept such evidence could result in regulation of such dietary ingredients as food additives, requiring the FDA pre-approval based on newly conducted, costly safety testing.
DSHEA provides for specific nutritional labeling requirements for dietary supplements effective January 1, 1997. The Dietary Supplement Act permits substantiated, truthful and non-misleading statements of nutritional support to be made in labeling, such as statements describing general well-being from consumption of a dietary ingredient or the role of a nutrient or dietary ingredient in affecting or maintaining the structure or function of the body. The FDA requires the Company to notify the FDA of such statements. There can be no assurance that the FDA will not consider particular labeling statements used by us to be drug claims rather than acceptable statements of nutritional support, necessitating approval of a costly new drug application, or re-labeling to delete such statements. It is also possible that the FDA could allege false statements were submitted to it if structure/function claim notifications were either non-existent or so lacking in scientific support as to be plainly false.
As authorized by DSHEA, the FDA adopted Good Manufacturing Practices (“GMP”) specifically for dietary supplements. These new GMP regulations, which became effective in June 2008, are more detailed than the GMPs that previously applied to dietary supplements and require, among other things, dietary supplements to be prepared, packaged and held in compliance with specific rules, and require quality controls similar to those required by GMP regulations for drugs. We believe our manufacturing and distribution practices comply with the new rules.
Dietary supplements are also subject to the Nutrition, Labeling and Education Act (“NLEA”), which regulates health claims, ingredient labeling and nutrient content claims characterizing the level of a nutrient in a product. NLEA prohibits the use of any health claim for dietary supplements unless the health claim is supported by significant agreement within the scientific community and is pre-approved by the FDA.
In certain markets, including the United States, claims made with respect to dietary supplements may change the regulatory status of our products. For example, in the United States, the FDA could possibly take the position that claims made for some of our products classify those products as new drugs requiring pre-approval by the FDA. The FDA could also place those products within the scope of its over-the-counter (“OTC”) drug regulations and require us to comply with a
published FDA OTC monograph. OTC monographs dictate permissible ingredients, appropriate labeling language and require the marketer or supplier of the products to register and file annual drug listing information with the FDA. We do not, at present, sell OTC drug products. If the FDA were to assert that our product claims cause them to be considered new drugs or to fall within the scope of OTC regulations, we would be required to either, file a new drug application, comply with the
applicable monographs, or change the claims made in connection with those products.
The FTC regulates the marketing practices and advertising of all our products. In recent years, the FTC instituted enforcement actions against several dietary supplement companies for false and misleading marketing practices and advertising of certain products. These enforcement actions have resulted in consent decrees and monetary payments by the companies involved. Under FTC standards,
the dissemination of any false advertising constitutes an unfair or deceptive act or practice actionable under Section 45 of the Fair Trade Commission Act and a false advertisement actionable under Section 52 of that Act. A false advertisement is one that is “misleading in a material respect.” In determining whether an advertisement or labeling information is misleading in a material respect, the FTC determines not only whether overt and implied representations are false but
also whether the advertisement fails to reveal material facts. Under the FTC’s standards, any health benefit representation made in advertising must be backed by “competent and reliable scientific evidence” by which the FTC means:
tests, analyses, research studies, or other evidence based upon the expertise of professionals in the relevant area, that have been conducted and evaluated in an objective manner by persons qualified to do so, using procedures generally accepted by the profession to yield accurate and reliable results.
7
The FTC has increased its review of the use of the type of testimonials that may be used to market our products. The FTC requires competent and reliable evidence substantiating claims and testimonials at the time that such claims of health benefit are first made. The failure to have this evidence when product claims are first made violates the Federal Trade Commission Act. Although the FTC has never threatened an enforcement action against the Company for the advertising of its products, there can be no assurance that the FTC will not question the advertising for our products in the future.
We believe we are currently in compliance with all applicable government regulations. We cannot predict what new legislation or regulations governing our operations will be enacted by legislative bodies or promulgated by agencies that regulate its activities. We recognize our industry has come under increased scrutiny, principally due to the FDA’s investigation of the use of ephedrine alkaloids (ephedra). The FDA is expected to increase its enforcement activity against dietary supplements that it considers to be in violation of FFD&CA. In particular, the FDA is increasing its enforcement of DSHEA provisions. Those activities will be enhanced by the appropriation for increased FDA budgets for dietary supplement regulation enforcement.
We believe we may become subject to additional laws or regulations administered by the FDA or other federal, state, or foreign regulatory authorities. We also believe the laws or regulations which are considered favorable may be repealed, or more stringent interpretations of current laws or regulations may be implemented. Any or all of such requirements could be a burden to us. Future regulations could require us to:
|
· |
change the way it conducts business; |
|
· |
use expanded or different labeling; |
|
· |
recall, reformulate or discontinue certain products; |
|
· |
keep additional records; |
|
· |
increase the available documentation of the properties of its products; and/or |
|
· |
increase the scientific proof of product ingredients, safety, and/or usefulness. |
Competition
The business of manufacturing, distributing and marketing vitamins and nutritional supplements is highly competitive. Many of our competitors are substantially larger and have greater financial resources with which to manufacture and market their products. In particular, the retail segment is highly competitive. Many direct marketers not only focus on selling their own branded products, but offer national brands at discounts as well. Many competitors have established brand names recognizable to consumers. In addition, major pharmaceutical companies offer nationally advertised multivitamin products.
Many of our competitors in the retailing segment have the financial resources to advertise freely, to promote sales and to produce sophisticated catalogs. In many cases, such competitors are able to offer price incentives for retail purchasers and to offer participation in frequent buyers programs. Some retail competitors also manufacture their own products whereby they have the ability and financial incentive to sell their own product.
We intend to compete by stressing the quality of our manufactured product, providing prompt service, competitive pricing of products in our marketing segment and by focusing on niche products in international retail markets. We have also increased our advertising spending dollars to continue to promote our proprietary branded Nutraceutical product line and have expanded our advertising medias, to include radio and print.
Research and Development Activities
We do not conduct any significant research and development activities.
Environmental Compliance
We are subject to regulation under Federal, state and local environmental laws. While we believe we are in material compliance with applicable environmental laws, continued compliance may require substantial capital expenditures. We have not incurred any major costs for any environmental compliance during the years ended June 30, 2009 and 2008.
8
Employees
As of September 18, 2009, we had approximately 115 full time employees of whom 57 belong to the local unit of the Teamsters Union and are covered by a collective bargaining agreement, which expires August 31, 2010. Approximately 32 employees are administrative and professional personnel, 12 are laboratory personnel and 14 employees are production and shipping personnel. We
consider our relations with our employees to be good.
In January 2009, we entered into an agreement with a Professional Employer Organization (“PEO”) which established a three-way relationship between our non union employees, the PEO and us. We and the PEO are co-employers of our non-union employees. The PEO has taken responsibility for our Human Resources administration and compliance, which allows us to continue to exercise control over our business while accessing quality employee benefits. We have been using PEOs since January 2007.
Available Information
We file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission (the “SEC”). These filings are available to the public via the Internet at the SEC's website located at http://www.sec.gov. You may also read and copy any document we file with the SEC at the SEC's public reference room located at
450 Fifth Street, N.W., Washington, D.C. 20549. For more information, please call the SEC at 1-800-SEC-0330.
Our website is located at www.integratedbiopharma.com. You may request a copy of our filings with the SEC (excluding exhibits) at no cost by writing or telephoning us at the following address or telephone number:
Integrated BioPharma, Inc.
225 Long Avenue
Hillside, New Jersey 07205
Tel: 888-319-6962
Attn: Investor Relations
Item 1A. Risk Factors
Please carefully consider the following risk factors which could materially adversely affect our business, financial condition, operating results and cash flows. The risk factors described below are not the only ones we face. Risks and uncertainties not known to us currently, or that we currently deem immaterial, also may materially adversely affect our business, financial condition, operating results and cash flows.
Our inability to repay or refinance our Notes Payable, with a stated principal balance of $7.8 million, upon any default notice or at maturity could adversely affect our liquidity, business, financial performance and going concern.
As of June 30, 2009, the Company is in technical default of certain financial covenants relating to the Company’s tangible net worth requirements and minimum net capital requirements, under that certain Securities Purchase Agreement, dated as of February 21, 2009, pursuant to which the Company issued notes payable (the “Notes Payable”), the current stated principal balance of which is $7.8 million. The Company remains in technical default with respect to the Notes Payable and, although the Company continues to work with the note holders to obtain a formal waiver for the default of the covenants, at this time the Company has not obtained such waiver. Upon the occurrence of an event of default, the note holders have the right to give the Company a written notice of such event of default (an “Acceleration Notice”), which would (i) accelerate the payment of all unpaid principal and accrued and unpaid interest (including default interest (if any)) on the Notes Payable, and (ii) require the Company to pay an amount equal to the sum of all of the amounts described in the preceding clause (i) in same day funds on the payment date specified in the notice, provided such date must be at least two (2) business days following the date on which the notice is delivered to the Company. If such an Acceleration Notice was sent to the Company, our inability to repay or refinance the Notes Payable would adversely affect our liquidity, business and financial performance and our ability to continue as a going concern is at risk.
9
In addition, if we are unsuccessful in our refinancing efforts with respect to the Notes Payable with our current lenders and/or replacing our current lenders, our ability to continue as a going concern is at risk.
Our revenue would decline significantly if we lose one or more of our most significant customers, which could have a significant adverse impact on us.
A significant portion of our revenues are concentrated among three customers, Herbalife International of America, Inc., Costco Wholesale, Inc. and Sam’s Club. For the years ended June 30, 2009 and 2008, these customers represented approximately 81% and 79% of total revenue, respectively. The loss of any of these customers could have a significant adverse impact on our financial condition and results of operations.
Complying with new and existing government regulation, both in the U.S. and abroad, could increase our costs significantly and adversely affect our financial results.
The processing, formulation, manufacturing, packaging, labeling, advertising, distribution and sale of our products are subject to regulation by several U.S. federal agencies, including the FDA, the FTC, the Consumer Product Safety Commission, the Department of Agriculture and the EPA, as well as various state,
local and international laws and agencies of the localities in which our products are sold. Government regulations may prevent or delay the introduction, or require the reformulation, of our products. Some agencies, such as the FDA or state agencies, could require us to remove a particular product from the market, delay or prevent the import of raw
materials for the manufacture of our products, or otherwise disrupt the marketing of our products. Any such government actions would result in additional costs to us, including lost revenues from any additional products that we are required to remove from the market, which additional costs could be material. Any such government actions also could lead to liability, substantial costs and reduced growth prospects. Moreover, there can be no assurance that new laws or regulations imposing
more stringent regulatory requirements on the dietary supplement industry will not be enacted or issued. In addition, complying with adverse event reporting requirements imposes additional costs on us, which costs could become significant in the event more demanding reporting requirements are put into place.
Additional or more stringent regulations of dietary supplements and other products have been considered from time to time. These developments could require reformulation of certain products to meet new standards, recalls or discontinuance of certain products that cannot be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, adverse event reporting or other new requirements. These developments also could increase our costs significantly. For example, the FDA issued rules which became effective in 2008 that imposed substantial new regulatory requirements for dietary supplements, including GMPs. Congress also passed legislation requiring adverse event reporting and related record keeping which imposed additional costs on us. See Item 1. "Business—Government Regulation" for additional information.
We may be exposed to legal proceedings initiated by regulators either in the United States or abroad which could increase our costs and adversely affect our reputation, revenues and operating income.
In the United States and abroad, non-compliance with relevant legislation can result in regulators bringing administrative or, in some cases, criminal proceedings. As manufacturers of nutraceutical products, our products are regulated by various governments and it is common for regulators to prosecute retailers and manufacturers for non-compliance with legislation governing foodstuffs and medicines. Failures by us or our subsidiaries to comply with applicable legislation could occur from time to time and prosecution for any such violations could have a material adverse effect on our business, results of operations, financial condition and cash flows. See Item 1. "Business—Government Regulation," for additional information and Item 3. Legal Proceedings.
10
We depend on our senior management, the loss of whom would have an adverse effect on us.
We presently are dependent upon the executive abilities of our Chairman of the Board, President and Chief Executive Officer, E. Gerald Kay, and our other executive officers. Our business and operations to date chiefly have been implemented under the direction of these individuals, who presently are, and in the future will be, responsible for the implementation of our anticipated plans and programs. The loss or unavailability of the services of one or more of our principal executives would have an adverse effect on us. We may encounter difficulty in our ability to recruit and ultimately hire any replacement or additional executive officers having similar background, experience and qualifications as those of our current executive officers.
There is no assurance that we will remain listed on an active trading market.
Our common stock is currently trading on the OTC Bulletin Board. From February 27, 2009 through September 22, 2009, our common stock was trading in the Pink Sheets. As of February 27, 2009, our common stock is no longer quoted on the NASDAQ Global Market, and there can be no assurance that we will, in the future, be able to meet all the requirements for reinstatement on that exchange. In such event, the liquidity and stock price in the secondary market may be adversely affected.
We have entered into several transactions with entities controlled by some of our officers and directors, which could pose a conflict of interest.
We have entered into several agreements and arrangements described in our previous SEC public filings and to be fully described in our proxy statement for our 2009 annual meeting of stockholders, including the lease of real property from Vitamin Realty Associates, L.L.C., the sale of our financial debt securities, and issuance of our common stock, which involved transactions with entities significantly owned by members of the Kay family and other of our significant shareholders and/or executive officers, who collectively own a majority of our shares of common stock. Although we believe that these transactions were advantageous to us and were on terms no less favorable to us than could have been obtained from unaffiliated third parties, transactions with related parties can potentially pose a conflict of interest.
Our Executive Officers and Directors have majority voting power and may take actions that may not be in the best interest of other stockholders, but in their own interest.
Our Executive Officers and Directors beneficially own approximately 68% of our outstanding shares. If these stockholders act together, they may be able to exert significant control over our management and affairs since significant corporate transactions require stockholder approval. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of all our stockholders.
We have a staggered Board of Directors, which could impede an attempt to acquire the Company or remove our management.
Our Board of Directors is divided into three classes, each of which serves for a staggered term of three years. This division of our Board of Directors could have the effect of impeding an attempt to take over our company or change or remove management, since only one class will be elected annually. Thus, only approximately one-third of the existing Board of Directors could be replaced at any election of directors.
Our product liability insurance may be insufficient to cover possible claims against us.
Our company, like other manufacturers, wholesalers and distributors of vitamin and nutritional supplement products, faces an inherent risk of exposure to product liability claims if, among other things, the use or ingestion of our products, result in sickness or injury. We currently maintain a product liability insurance policy that provides a total of $5.0 million of coverage per occurrence and $5.0 million of coverage in the aggregate. However, there can be no assurance that existing or future insurance coverage will be sufficient to cover any possible product liability risks or that such insurance will continue to be available to us on economically feasible terms.
11
Our Nutraceutical products are manufactured using various raw materials consisting of vitamins, minerals, herbs, fruit extracts and other ingredients that we regard as safe when taken as recommended by us and that various scientific studies have suggested may provide health benefits. We could be adversely affected if any our products or any similar products distributed by other companies should prove or be asserted to be harmful to consumers or should scientific studies provide unfavorable findings regarding the effectiveness of our products.
We may not be able to obtain raw materials used in certain of our manufactured products.
The principal raw materials used in the manufacturing process in the Company’s Nutraceutical segment are natural and synthetic vitamins, minerals, herbs, related nutritional supplements, gelatin capsules, coating materials, fruit extracts, fruit juices and the necessary components for packaging the finished products. The raw materials are available from numerous sources within the
United States and abroad. The gelatin capsules, coating materials and packaging materials are similarly widely available. We generally purchase our raw materials, on a purchase order basis, without long-term commitments.
Our principal suppliers are Creative Flavor Concepts, Inc., Triarco Industries, Inc., and DSM Nutritional Products, Inc.
If we are unable to maintain our relationships with our major suppliers, we may not be able to find alternate sourcing of our raw materials or at the same pricing that we receive from our current suppliers and/or quickly enough to make timely shipments to our customers. These factors could decrease our sales and/or increase our cost of sales.
Current economic conditions may cause a decline in business and consumer spending which could adversely affect our business and financial performance.
Our operating results are impacted by the health of the North American economies. Our business and financial performance, including collection of our accounts receivable, recoverability of assets including investments, may be adversely affected by current and future economic conditions, such as a reduction in the availability of credit, financial market volatility, recession, etc. Additionally, we may experience difficulties in scaling our operations to react to economic pressures in the U.S.
We incur significant accounting and other control costs that impact our financial condition.
As a publicly traded corporation, we incur certain costs to comply with regulatory requirements. If regulatory requirements were to become more stringent or if controls thought to be effective later fail, we may be forced to make additional expenditures, the amounts of which could be material. Some of our competitors are privately owned so their accounting and control costs can be a competitive disadvantage for us. Should our sales decline or if we are unsuccessful at increasing prices to cover higher expenditures for internal controls and audits, our costs associated with regulatory compliance will rise as a percentage of sales.
Other issues and uncertainties may include:
|
· |
New accounting pronouncements or changes in accounting policies; and |
|
· |
Legislation or other governmental action that detrimentally impacts our expenses or reduces sales by adversely affecting our customers. |
Item 1B. Unresolved Staff Comments
Not applicable.
12
Item 2. Properties
On January 10, 1997, we entered into a lease agreement for approximately 75,000 square feet of factory, warehouse and office facilities in Hillside, New Jersey. The facilities are leased from Vitamin Realty Associates, L.L.C., a limited liability company, which is 90% owned by our Chairman of the Board, President, Chief Executive Officer and principal stockholder and
certain family members and 10% owned by an employee of the Company. The lease expires May 2015 and provides for a base annual rental of $0.3 million plus increases in real estate taxes and building expenses. At our option, we have the right to renew the lease for an additional five-year period.
We also own a 40,000 square foot manufacturing facility in Hillside, New Jersey. The space is utilized for Manhattan Drug’s tablet manufacturing operations.
On May 16, 2007, AgroLabs, Inc. entered into a five-year lease agreement for approximately 39,000 square feet of warehouse space in Coppell, Texas. We moved to this facility in the quarter ended June 30, 2007. The facility is used for the storage and distribution of inventory for our liquid Nutraceutical products, with approximately 4,500 square feet used for office space. This lease expires in May 2012 and provides for a base annual rent of $0.2 million plus increases in real estate
taxes and building expenses. This replaced the lease that expired in May 2007 for the warehouse space in Grapevine, Texas.
In connection with the acquisition of BevSpec, Inc., The Organic Beverage Company, assumed the five-year lease agreement entered into by BevSpec, Inc., in February 2005, for approximately 12,000 square feet of warehouse space in Austin, Texas. The facility was used for the storage and distribution of inventory for Syzmo™ products. The lease was to expire in February 2010 and provided for a base annual rent of $0.1 million plus increases in real estate taxes and building expenses.
We consolidated warehouse space with AgroLabs and abandoned this facility in September 2008; however, the landlord has asserted that we remained responsible for the lease through March 2009 (See Item 3. Legal Proceedings).
Item 3. Legal Proceedings
On June 16, 2008, the State of Texas filed an Original Petition for injunctive relief and civil penalties in the 101st Judicial District, Dallas, Texas, against AgroLabs Inc., the Company, Kurt Cahill and Gerald Kay (collectively, the “Defendants”). The State alleges that the Defendants sold or distributed juices and dietary supplements marketed with inappropriate disease and nutritional claims. Agrolabs has appeared in the lawsuit and brought a counterclaim against the State for declaratory relief. The Company and Mr. Kay have filed motions to dismiss the lawsuit for lack of personal jurisdiction. The State of Texas is seeking that a permanent injunction be issued, restraining the defendants from, among other things, disseminating false or misleading advertising on product labels as well as civil penalties and attorneys fees and costs. AgroLabs, the Company and Mr. Kay vigorously contest the allegations set forth in the Petition. The Company is unable at this time to make a determination as to the likelihood of an unfavorable outcome or to estimate the amount or range or possible loss or gain and the impact, if any, of such claims will have on the Company and its operations.
On April 23, 2009, Braker Five & Eight Investors, L.P., (the “Landlord”) filed an Original Petition relief and damages pursuant to a Lease Agreement for the premises located in Austin, Texas in the 126th Judicial District, Travis County, Texas, against BevSpec, Inc., Bioscience Technologies, Inc. dba The Organic Beverage Company, and Integrated BioPharma, Inc., as Guarantor (collectively, the “Defendants”). The Landlord is seeking damages for rental fees and charges, repairs, and other sums due under the Lease, including interest. The Company has retained local counsel and is unable to make a determination as to the likelihood of an unfavorable outcome or to estimate the amount or range or possible loss or gain and the impact, if any, of this claim will have on the Company and its operations.
On or about August 10, 2009, AgroLabs, Inc. commenced an action in the Superior Court of New Jersey, Law Division, against defendants Kurt E. Cahill, Cheryl A. Cahill, Joseph E. Cahill, Jr. and Monty C. Lloyd (all of whom were previously employed by AgroLabs, Inc.) for, among other things, breach of contract, breach of fiduciary duty, negligent performance of duties and other and related relief. On or about September 1, 2009, the defendants removed the action to the United States District Court for the District of New Jersey. On or about September 15, 2009, the defendants filed an answer and affirmative defenses. The defendants, however, asserted no counterclaims. The parties are required to exchange initial disclosures and other information, and are scheduled to appear for an initial conference with the Court
13
on November 18, 2009. The Company is unable to make a determination as to the likelihood of a favorable outcome or to estimate the amount or range or possible loss or gain and the impact, if any, of this claim will have on the Company and its operations.
Item 4. Submission of Matters to a Vote of Security Holders
No matters were submitted to a vote of security holders during the fourth quarter of the fiscal year ended June 30, 2009.
14
PART II
Item 5. Market for Common Equity, Related Stockholder Matters and Registrant Purchases of Equity Securities
Market Information
As of September 22, 2009, our common stock began trading on the NASDAQ Bulletin Board under the symbol INBP.OB. From February 27, 2009 to September 22, 2009, our common stock traded in the Pink Sheets under the symbol “INBP.PK”. Prior to February 27, 2009 and commencing on February 6, 2007, our common stock trading on the NASDAQ Global Market under the symbol
“INBP” and previously traded under the symbol INB on the American Stock Exchange under the symbol INB.
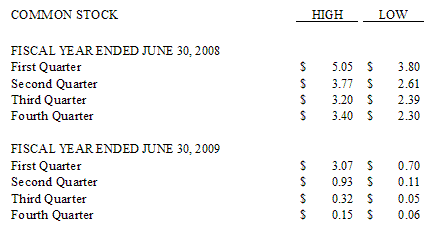
Set forth below are the high and low closing prices of the Common Stock as reported on the NASDAQ Global Market and as listed in the Pink Sheets, as applicable:
Holders
As of June 30, 2009 there were approximately 1,100 holders of record of the Company’s common stock.
Dividends
We have not declared or paid a dividend with respect to our common stock during the fiscal years ended June 30, 2009 and 2008, nor do we anticipate paying dividends in the foreseeable future.
We have paid dividends of approximately $54,000 and $216,000 with respect to our Series C Convertible Preferred Stock during fiscal years 2009 and 2008, respectively. All of our Series C Convertible Preferred Stock outstanding as of June 30, 2008 was converted to common stock in the first quarter of fiscal year 2009.
15
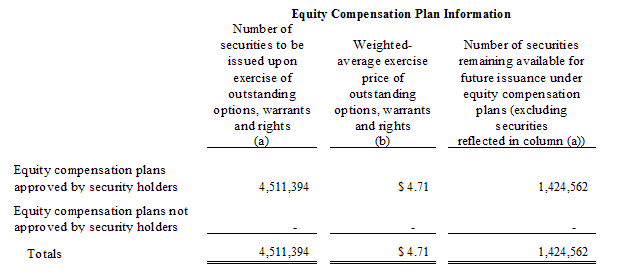
Equity Compensation Plans
The following table provides information, as of June 30, 2009, about the Company's equity compensation plans:
Recent Sales of Unregistered Securities
On January 23, 2009, the Board of Directors of the Company authorized the issuance of 1,000,000 stock options to the Company’s employees, officers and independent directors under the Company’s stock option plan. 900,000 stock options were issued with an exercise price of $0.14, a three year vesting period and with a ten year term and 100,000 stock options were issued with an exercise price of $0.15, a three year vesting period and with a five year term.
The stock options were not registered under the Securities Act of 1933 because such grants either did not involve an offer or sale for purposes of Section 2(a)(3) of the Securities Act of 1933, in reliance on the fact that the stock options were granted for no consideration, or were offered and sold in transactions not involving a public offering, exempt from registration under the Securities Act of 1933 pursuant to Section 4(2).
Item 6. Selected Financial Data
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Certain statements set forth under this caption constitute “forward-looking statements.” See “Disclosure Regarding Forward-Looking Statements” on page 1 of this Report for additional factors relating to such statements.
The Company is engaged primarily in the manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily throughout the United States.
For the fiscal year ended June 30, 2009, our net sales decreased by $4.5 million or 10.3% to $39.4 million from $43.8 million for the fiscal year ended June 30, 2008. The fourth quarter net sales for the current fiscal year were $10.5 million as compared to the fourth quarter of the prior fiscal year of approximately $10.9 million, which is a decrease of approximately 2.9%. While we experienced a slight decrease in our fourth quarter sales, our gross profit on those sales increased by
approximately 9.2% and we continued to cut our selling and administrative costs. We continue remain optimistic about the long-term performance of our Nutraceutical businesses as we continue to launch and test new products in various markets, have a dedicated sales and marketing team and are reaching out to new customers.
16
Critical Accounting Policies and Estimates
Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting
period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
|
· |
sales returns and allowances; |
|
· |
trade marketing and merchandising; |
|
· |
allowance for doubtful accounts; |
|
· |
inventory valuation; |
|
· |
valuation and recoverability of long-lived and intangible assets and goodwill, including the values assigned to acquired intangible assets; |
|
· |
income taxes and valuation allowances on deferred income taxes; and |
|
· |
accruals for, and the probability of, the outcome of current litigation. |
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Allowances for Doubtful Accounts and Sales Returns
Our management makes judgments as to its ability to collect outstanding receivables and provides allowances for the portion of receivables for which collection becomes doubtful. Provisions are made based upon a specific review of all significant outstanding amounts. We continuously monitor payments from our customers and maintain allowances for estimated losses for doubtful accounts in the period they become known.
If the historical data we use to calculate the allowance provided for doubtful accounts does not reflect the future ability to collect outstanding receivables, additional provisions for doubtful accounts may be needed and the future results of operations could be materially affected. In recording any additional allowances, a respective charge against income is reflected in the general and administrative expenses, and would reduce the operating results in the period in which the increase is recorded.
Our return policy in our contract manufacturing business is to only accept returns for defective products. If defective products are returned, our agreement with our customers is to cure the defect and re-ship the product. Based on this policy, when the product is shipped we make an estimate of any potential returns or allowances. With respect to our branded proprietary Nutraceutical products, our return policy is also to accept returns for defective products and re-ship replacement items for the damaged product. In most instances, the damaged goods are a small portion of the overall order and we instruct our customer to dispose of the damaged product and we issue them a credit for the dollar amount of the damaged goods plus any cost of disposal. We also estimate and make allowances at the time of shipment.
In the event we have an item that is discontinued in our customers retail stores, we work with our buyer and broker on the sell through and/or return such discontinued item. We make estimates of this event at both the time of shipment and at the time of the notice from our customer that our item has been discontinued, compare this to our recorded sales allowances and record any adjustments based upon the updated knowledge of a known return.
17
If the historical data we use to calculate the sales allowance for sales returns and other allowances does not reflect the amounts previously recorded, additional provisions for sales allowance may be needed and the future results of operations could be materially affected. In recording any additional sales allowances, a respective charge against income is reflected in net sales, and would reduce the profit margins and operating results in the period in which the increase is recorded.
The Company performed a sensitivity analysis to determine the impact of fluctuations in our estimates for our allowance for doubtful accounts. As of June 30, 2009, the allowance for doubtful accounts was $0.3 million. If this amount were in error by plus or minus one percent of the account receivable balance, the impact would be an additional $29,000 of income or expense.
Trade Marketing and Merchandising. In order to support the Company’s propriety Nutraceutical product lines, various promotional activities are conducted through the retail trade, distributors or directly with consumers, including in-store display and product placement programs, feature price discounts, coupons, and other similar activities. The Company regularly reviews
and revises, when it deems necessary, estimates of costs to the Company for these promotional programs based on estimates of what will be redeemed by the retail trade, distributors, or consumers. These estimates are made using various techniques, including historical data on performance of similar promotional programs. Differences between estimated expense and actual performance are generally not material and are recognized as a change in management’s estimate in a subsequent
period. Our total promotional expenditures, including amounts classified as a reduction of net sales, represent approximately 18% of net sales for the fiscal year ended June 30, 2009, the likelihood exists of materially different reported results if factors such as the level and success of the promotional programs or other conditions differ from expectations.
Inventory Valuation
Inventories are stated at the lower of cost or market (“LCM”), which reflects management’s estimates of net realizable value. Cost is determined using the first-in, first-out method. As a result of our inventory being manufactured primarily on a purchase order basis, the quantity of both raw materials and finished goods inventory provides for minimal risk of potential overstock or obsolescence.
Mail order inventory is expiration date sensitive. Accordingly, we review this inventory, consider sales levels (by SKU), term to expiration date, potential for retesting to extend expiration date, and evaluate potential for obsolescence or overstock.
We performed a sensitivity analysis to determine the impact of fluctuations in our estimates for inventory allowances. If our estimates used to value inventory were off by one percent of the total inventory balance, the impact would be an additional $0.1 million of income or expense.
Long Lived Assets
Purchased intangibles consisting of patents and unpatented technological expertise, license fees and trade names purchased as part of business acquisitions are presented net of related accumulated amortization and are being amortized on a straight-line basis over the remaining useful lives of such intangibles.
We record impairment losses on other intangible assets when events and circumstances indicate that such assets might be impaired and the estimated fair value of any such asset is less than its recorded amount in accordance with Statement of Financial Accounting Standards No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”. The Company reviews the value of its long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Conditions that would necessitate an impairment assessment include material adverse changes in operations, significant adverse differences in actual results in comparison with initial valuation forecasts prepared at the time of acquisition, a decision to abandon certain acquired products, services, or marketplaces, or other significant adverse changes that would indicate the carrying amount of the recorded asset might not be recoverable. No impairment losses were identified or recorded in the fiscal years ended June 30, 2009 and 2008.
18
Other Intangible Assets
The Financial Accounting Standards Board (“FASB”) has issued Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (“SFAS 142”). SFAS 142 requires that goodwill and intangible assets with indefinite lives no longer be amortized against earnings, but instead tested for impairment at least annually based on a
fair-value approach as described in SFAS 142.
Intangible assets with finite lives are amortized over their estimated useful lives. The useful life of an intangible asset is the period over which the asset is expected to contribute directly or indirectly to future cash flows. The carrying value of intangible assets with finite lives is evaluated whenever events or circumstances indicate that the carrying value may not be recoverable. The carrying value is not recoverable when the projected undiscounted future cash flows are less
than the carrying value. Tests for impairment or recoverability require significant management judgment, and future events affecting cash flows and market conditions could result in impairment losses. As a result of the Company’s testing, it recorded impairment charges of $32 (relating to trademarks and patents) in the fiscal year ended June 30, 2009 and $813 in the fiscal year ended June 30, 2008, of which $447 related to intellectual property, $335 related to trade names and $31
to patents. These impairment charges are included in discontinued operations in the Consolidated Statement of Operations.
Deferred Taxes
The Company accounts for income taxes pursuant to SFAS No. 109, “Accounting for Income Taxes” (SFAS 109”). SFAS 109 is an asset-and-liability approach that requires the recognition of deferred tax assets and liabilities for the expected tax consequences and events that have been recognized in the Company’s financial statements or tax returns. In the fiscal year
ended June 30, 2009, the Company recognized an income tax expense, of approximately $4.8 million and approximately $1.2 million in discontinued operations. The income tax expense in both continuing and discontinuing operations, were primarily the result of the valuation allowance recorded against our deferred tax assets. Our management, based on current factors relating to our business environment resulting, in part, from the current downward economic trends, does not have sufficient
information to determine if we will have future federal taxable income which would allow us to realize our net deferred tax assets in the future.
General Litigation
From time to time, the Company is a defendant or plaintiff in various legal actions which arise in the normal course of business. As such the Company is required to assess the likelihood of any adverse outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of the provision required for these commitments and contingencies, if any, which would be charged to earnings, is made after careful analysis of each matter. The provision may change in the future due to new developments or changes in circumstances. Changes in the provision could increase or decrease the Company’s earnings in the period the changes are made. In the opinion of management, after consultation with legal counsel, the ultimate resolution of these matters cannot be determined at this time as to the whether there could be material adverse effect on our financial condition or results of operations.
General
The Company recognizes revenue in accordance with the Securities and Exchange Commission’s Staff Accounting Bulletin 104. The Company recognizes product sales revenue, the prices of which are fixed and determinable, when title and risk of loss have transferred to the customer, when estimated provisions for product returns, rebates, charge-backs and other sales allowances are reasonably determinable, and when collectability is reasonably assured. Accruals for these items are
presented in the consolidated financial statements as reductions to sales. The Company’s net sales represent gross sales invoiced to customers, less certain related charges for discounts, returns, rebates, charge-backs and other allowances. Cost of sales includes the cost of raw materials and all labor and overhead associated with the manufacturing and packaging of the products. Gross margins are affected by, among other things, changes in the relative sales mix among our products
and valuation and/or charge off of slow moving, expired or obsolete inventories.
19
Operating results in all periods presented reflect the impact of acquisitions and discontinued operations. The timing of those acquisitions and the changing mix of businesses as acquired companies are integrated into the Company may affect the comparability of results from one period to another.
Recent Accounting Pronouncements
In April 2008, the FASB issued FASB Staff Position (FSP) SFAS No. 142-3, “Determination of the Useful Life of Intangible Assets” (“FSP FAS No. 142-3”). FSP FAS No. 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets” and was effective for fiscal years beginning after December 15, 2008. The adoption of this pronouncement by the Company, for the fiscal year ending June 30, 2010, will not have a material impact on the its consolidated financial statements.
In April 2009, the FASB issued FSP FAS No. 107-1 and Accounting Principles Board Opinion 28-1, “Interim Disclosures about Fair Value of Financial Instruments: (“APB 28-1”). APB 28-1 amends SFAS No. 107, “Fair Value of Financial Instruments” to require disclosures about fair value of financial instruments for interim reporting periods in
addition to the required disclosures in annual financial statements. APB 28-1 also amends APB Opinion 28, “Interim Financial Reporting”, to require those disclosures in summarized financial information at interim reporting periods. FSP FAS 107-1 and APB 28-1 are effective for interim reporting periods ending after June 15, 2009. The Company has adopted the provisions of this FSP effective for its interim quarter ended September 30, 2009 and it will not have a material impact
on its consolidated financial statements.
In May 2009, the FASB issued SFAS No. 165, Subsequent Events (“SFAS 165”). SFAS 165 establishes general standards for accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or available to be issued and was effective for interim and annual periods ending after June 15, 2009. The
adoption of SFAS No. 165 did not have an impact on the Company’s results of operations or financial condition. The Company evaluated all subsequent events that occurred from July 1, 2009 through October 12, 2009, inclusive, and determined there were no material subsequent events that required disclosure in its consolidated financial statements.
In June 2009, the FASB issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles” (“SFAS No. 168”). SFAS No. 168 will become the single source of authoritative nongovernmental U.S. generally accepted accounting principles (“GAAP”), superseding existing FASB, American Institute of Certified Public Accountants, Emerging Issues Task Force (“EITF”), and related accounting literature. SFAS No. 168 reorganizes the thousands of GAAP pronouncements into roughly 90 accounting topics and displays them using a consistent structure. Also included is relevant Securities and Exchange Commission guidance organized using the same topical structure in separate sections. SFAS No. 168 will be effective for financial statements issued for reporting periods that end after September 15, 2009. The adoption of SFAS No. 168 is not expected to have a material impact on the Company’s consolidated results of operations and financial condition.
In October 2008, the Financial Accounting Standards Board (“FASB”) issued FSP No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active” (“FSP 157-3”). FSP 157-3 clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in
determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 became effective for us on September 30, 2008 for all financial assets and liabilities recognized or disclosed at fair value in our Consolidated Financial Statements on a recurring basis (at least annually).
In May 2008, the FASB issued SFAS No. 162, “The Hierarchy of Generally Accepted Accounting Principles.” The statement is intended to improve financial reporting by identifying a consistent hierarchy for selecting accounting principles to be used in preparing financial statements that are presented in conformity with GAAP. Prior to the issuance of SFAS No. 162, GAAP hierarchy was defined in the American Institute of Certified Public Accountants (“AICPA”) Statement
on Auditing Standards (SAS) No. 69, The Meaning of Present Fairly in Conformity With Generally Accepted Accounting Principles. Unlike SAS No. 69, SFAS No. 162 is directed to the entity rather than the auditor. Statement No. 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board Auditing
20
amendments to AU Section 411, “The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles”. SFAS No. 162 is not expected to have any material impact on the Company’s results of operations, financial condition or liquidity.
In May 2008, the FASB issued FASB Staff Position (“FSP”) No. APB 14-1, “Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (including Partial Cash Settlement),” or (“FSP APB 14-1”), which requires separate accounting for the debt and equity components of convertible debt issuances. The requirements for separate accounting must be applied retrospectively to previously issued cash-settleable convertible instruments
as well as prospectively to newly issued instruments, negatively affecting both net income and earnings per share for issuers of the instruments. The FSB APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008. The Company is currently evaluating the impact of FSP No. APB 14-1 on the Company’s results of operations, financial condition or liquidity as of June 30, 2009.
In June 2008, the FASB issued EITF 07-5, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity's Own Stock. EITF 07-5” provides guidance in assessing whether an equity-linked financial instrument (or embedded feature) is indexed to an entity's own stock for purposes of determining whether the appropriate accounting treatment falls under the scope of SFAS 133, "Accounting For Derivative Instruments and Hedging Activities" and/or EITF 00-19,
"Accounting For Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company's Own Stock". EITF 07-5 is effective for financial statements issued for fiscal years beginning after December 15, 2008 and early application is not permitted. The Company is currently evaluating the impact of EITF 07-5 on the Company’s results of operations, financial condition or liquidity as of June 30, 2009.
21
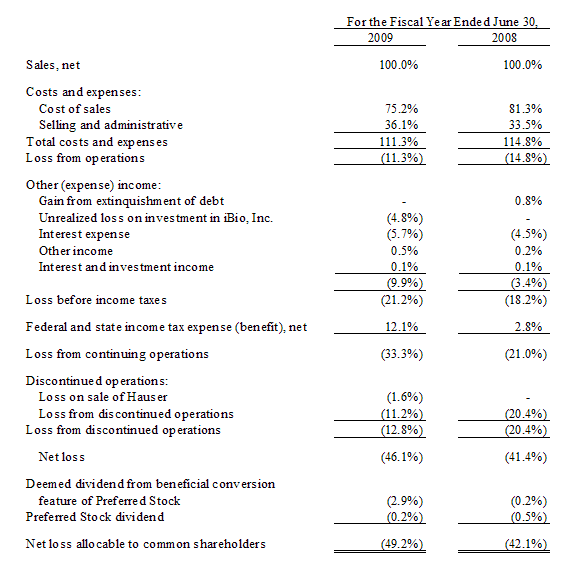
Results of Operations (in thousands, expect share and per share amount)
The following table sets forth the income statement data of the Company as a percentage of net sales for the periods indicated:
Year ended June 30, 2009 Compared to the Year ended June 30, 2008
Sales, net. Net Sales for the fiscal year ended June 30, 2009 and 2008 were $39,367 and $43,868, respectively, a decrease of $4,501 or 10.3%. The decrease is comprised of the following:
22
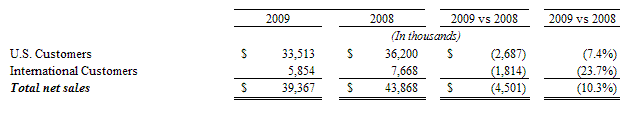
For the fiscal year ended June 30, 2009, approximately 81% of total net sales were derived from three customers as compared to 79% of total net sales for the fiscal year ended June 30, 2008. The loss of any of these customers would have an adverse affect on our operations. We continue to expand our customer base by expanding from selling our propriety branded Nutraceutical products primarily to “club” stores to the retail sales segment grocery sales segment and expanding our sales in the international market.
The decrease is, in part, the result of a decrease in sales in our contract manufacturing products sales which decreased by approximately $4.0 million, primarily due to lower volume and selling prices to one of our major customers of approximately $4.5 million, offset in part by an increase in sales to other customers of approximately $0.5 million. Additionally, sales of our branded proprietary Nutraceutical product line decreased by approximately $0.2 million in part due to increased promotional programs offering discounts to our customers which resulted in a lower selling price point on our products at our club stores as compared to the fiscal year ended June 30, 2008. The remaining Nutraceutical product lines had net sales decreases of approximately $0.3 million compared to the prior period. Management believes that the decrease in the sales in the other product lines is a result of the general depressed economic environment in the United States, the main geographic area of our customers. During the fiscal year ended June 30, 2009, we launched approximately nine new products in test markets. A few of the products launched are receiving positive indicators; however, it is too early to project future impact.
Cost of sales. Cost of sales decreased to $29.6 million for the fiscal year ended June 30, 2009, as compared to $35.7 million for the fiscal year ended June 30, 2008, which is a decrease of $6.1 million. Cost of sales decreased as a percentage of net sales to 75.2% for the fiscal year ended June 30, 2009, as compared to 81.4% for the fiscal year ended June 30, 2008.
As a result of the decrease in sales, approximately 50% of the decrease of $6.1 million or $3.5 million relates to the decrease sales volume, offset in part by an increase of 10% in the cost of goods sold in our contract manufacturing which has certain fixed overhead costs that we will incur regardless of our sales volumes. The decrease of 6.2% in cost of sales as a percentage of net sales or approximately $2.4 million, is a result of (i) a decrease in write-offs for inventory items
that either became obsolete during the respective fiscal years as a result in the changes in our customer base and product mix or the inventory items being classified as slow moving and (ii) a decrease in freight costs of $0.9 million.
Selling and Administrative Expenses. Selling and administrative expenses were $14.2 million for the fiscal year ended June 30, 2009, as compared to $14.7 million for the fiscal year ended June 30, 2008, a decrease of $0.5 million or 3.1%. As a percentage of sales, net, selling and administrative expenses were 36.1% for the fiscal year ended June 30, 2009 and 33.5%
for the prior comparable period.
The decrease in selling and administrative expenses of $0.5 million is mainly due to decreases aggregating approximately $1.3 million in:
|
· |
Stock compensation expenses ($0.5 million), |
|
· |
Professional fees ($0.4 million), |
|
· |
Commissions ($0.2 million), and |
|
· |
Travel and entertainment ($0.2 million); |
Offset, in part by increases aggregating approximately $1.0 million in:
|
· |
Advertising and marketing costs ($0.3 million), such as in store demos, |
|
· |
Warehouse expense ($0.3 million), |
|
· |
bad debt expense ($0.3 million) as a result of the current downturn in the economy, and |
23
|
· |
Investor and public relation costs ($0.1 million). |
Our stock compensation expense decreased by $0.5 million primarily due to the significant decrease in the market value of our common stock from year to year at the measurement date of the stock option grants (the market value of our common stock is one of several factors used in determining the fair value of the stock compensation at the time of the award and ultimate expense to our consolidated financial statements). Professional fees decreased by $0.4 million in fiscal year ended June 30, 2009 compared to fiscal year ended June 30, 2008 as we incurred more than normal professional fees as a result of the spin-off of iBio in the fiscal year ended June 30, 2008 as we were preparing and filing the necessary legal and regulatory documents in connection with the spin-off. This decrease was offset by an increase in legal fees in connection with new legal proceedings arising in the fiscal year ended June 30, 2009. Our commission expense decreased by $0.2 million primarily as a result of a decrease in drug store chain sales and international sales resulting in decreased commissions of approximately $0.1 million coupled with a decrease in the net cash collected (including offsets for advertising dollars spent) on which commissions are calculated also in the approximate amount of $0.1 million. Our travel and entertainment expenses decreased by $0.2 million primarily as a result in a change in compensation of certain executives whereby they receive a percentage of sales collected to spend on travel and entertainment in lieu of seeking reimbursement of employee business travel expenses from the Company and due to a cost cutting initiatives followed by other officers and key employees of the Company.
Other expense, net. Other expense, net increased by approximately $2.4 million for the fiscal year ended June 30, 2009 as compared to the fiscal year ended June 30, 2008. This is primarily due to an increase in: (i) interest expense due to the increased average total of outstanding obligations and higher interest rates on the outstanding obligations for the period ended June 30, 2009 compared to June 30, 2008, resulting in an increase in interest expense of $0.3 million; (ii) an unrealized loss on investment in our iBio common stock of $1.9 million recorded in the fourth quarter of the fiscal year ended June 30, 2009 with no such loss in the fiscal year ended June 30, 2008; and (iii) the recognition of a gain on the extinguishment of debt of $0.3 million in the fiscal year ended June 30, 2008 with no comparable gain in the fiscal year ended June 30, 2009.
Federal and state income tax, net. For the fiscal year ended June 30, 2009, we had a net tax expense of $4.8 million as compared to a net tax expense of $1.2 million for the fiscal year ended June 30, 2008. The tax expense increased $3.5 million as a result of fully reserving our deferred tax asset as it was determined based upon past losses, the Company’s
liquidity concerns and the current economic environment, that it was “more likely than not” that the Company’s deferred tax assets would not be realized.
Loss from discontinued operations. On August 18, 2008, we completed our distribution of our Biotechnologies segment. The net loss from our Biotechnologies segment, included in our results for the fiscal year ended June 30, 2009, was $0.1 million as compared to $1,555 for the fiscal year ended June 30, 2008. This decrease of approximately $1,400 is primarily the
result of a full fiscal year of revenues and expenses in the period ended June 30, 2008 versus revenues and expenses of approximately one and a half months during the period ended June 30, 2009.
On March 17, 2009, we entered into a stock purchase agreement and consummated the sale of all of the issued and outstanding shares of common stock of our wholly owned subsidiary Hauser to Cedarburg Pharmaceuticals, Inc. (“Cedarburg”). On January 31, 2009, the Company sold substantially all the assets of Paxis net of its outstanding payables, to Hauser in consideration for the outstanding intercompany debt between these two subsidiaries of the Company. The net loss from the
Pharmaceuticals segment, included in our results for the fiscal year ended June 30, 2009, was $3,081 million as compared to $3,306 for the fiscal year ended June 30, 2008. The decrease of 225 from the 2008 period to the comparable 2009 period is primarily due to the decrease of the loss from operations of $1,369 from 2008 to 2009 due to the sale of the Pharmaceuticals segment in the eighth month of the fiscal year ended June 30, 2009 period as compared to a full fiscal year in the 2008
comparable period, offset by a recognition of a loss on the sale of Hauser of $629 in the period ended June 30, 2009 with no such loss in the comparable 2008 period.
In the June 2009, we discontinued the operations of our subsidiary TOBC, as we do not have the financial resources to pursue the further development of the Syzmo™ product in the very competitive energy drink market place. The net loss from this discontinued product line was $1,232 and $4,093 for the fiscal years ended in June 30, 2009 and 2008, respectively. The decrease in the net loss of $2,861 was the result of curtailing the operations and marketing efforts of TOBC in the first quarter of our fiscal year ended June 30, 2009.
24
Net loss applicable to common shareholders. Our net loss applicable to common shareholders for the fiscal year ended June 30, 2009 was $19,367 as compared to $18,462 for the fiscal year ended June 30, 2008. This increase in net loss applicable to common shareholders of approximately $905 is primarily the result of decreases in: (i) net losses from discontinued operations of $4,535, and (ii) operating losses from continuing operations of $2,058; offset by, an increase in our net income taxes of $3,544 resulting primarily from fully reserving our deferred tax asset of $4,733; as it was determined based upon past losses, the Company’s liquidity concerns and the current economic environment, that it was “more likely than not” that the Company’s deferred tax assets would not be realized. In addition to the increase in our tax expense for the current year, our other expense increased by approximately $2,429, which is mainly attributable to an increase in our unrealized loss on investment in iBio of $1,877 and secondarily by an increase in our interest expense of $273 on our outstanding obligations in the current fiscal year. Also impact the increase in the net loss applicable to common shareholders is a net increase of $896 in Series C preferred stock dividend (decrease of $162) and deemed dividend from beneficial conversion of the preferred stock (increase of $1,058) which was a result of the Series C preferred stockholders converting their respective Series C shares into shares of our common stock in the first quarter of our fiscal year ended June 30, 2009. This conversion resulted in permanent equity for us, as the Series C preferred stock replaced $6,000 of our current and long-term obligations.
Seasonality
The Nutraceutical business segment tends to be seasonal. We have found that in our first fiscal quarter ending on September 30th of each year, orders for our branded proprietary Nutraceutical products usually slow (absent the addition of new customers or a new product launch with a significant first time order), as buyers in various markets may have purchased sufficient inventory to carry them through the summer months. Conversely, in our second fiscal quarter, ending on December 31st of each year, orders for our products increase as the demand for our branded Nutraceutical products seems to increase in late December to early January as consumers become health conscious as they enter the new year.
The Company believes that there are other non-seasonal factors that also may also influence the variability of quarterly results including, but not limited to, general economic and industry conditions that affect consumer spending, changing consumer demands and current news on nutritional supplements. In addition, our recent growth has caused additional variability in our quarterly results. Accordingly, a comparison of the Company’s results of operations from consecutive periods is not necessarily meaningful, and the Company’s results of operations for any period are not necessarily indicative of future periods.
Liquidity, Going Concern and Capital Resources
The following table sets forth, for the periods indicated, the Company’s net cash flows used in operating, investing and financing activities:
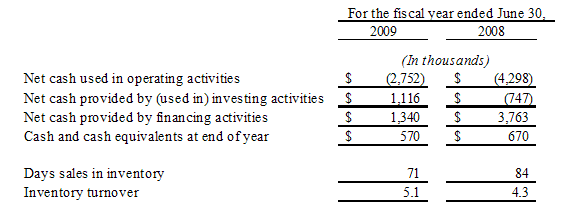
25
At June 30, 2009, the Company’s working capital deficit was approximately $2,916, a decrease of $10,220 from working capital at June 30, 2008 of $7,304. The decrease in our working capital is primarily a result of the our discounted notes payable coming due within the next twelve months ($7,458) (principal stated amount of $7,805) and recording a full valuation allowance against our deferred tax assets in the second quarter of fiscal year 2009 ($3,827) as a result of past losses, the Company’s liquidity concerns and the current economic environment. Cash and cash equivalents were $570 at June 30, 2009 and $670 at June 30, 2008.
Net cash used by operating activities of $2,752 for the fiscal year ended June 30, 2009 included a net loss of $18,176. After excluding the effects of non-cash expenses, including the net loss from discontinued operations, unrealized loss on investment in iBio, deferred taxes, impairment charges, depreciation and amortization and compensation expense for employee stock
options, the adjusted cash used before the effect of the changes in working capital components was $3,494. Cash was provided by continuing operations in the amount of approximately $1,655 and was the result of a decrease in inventory of $610, accounts receivable of $438 and other current assets of $200 and a net increase in accounts payable, accrued expenses and other liabilities of $487, these increases to cash were partially offset by a decrease in income taxes payable of $80.
Approximately $913 of cash used was for operating activities from our discontinued operations.
Net cash used in operating activities of $4,298 for the fiscal year ended June 30, 2008 included a net loss of $18,167. After excluding the effects of non-cash expenses, including the net loss from discontinued operations, deferred taxes, impairment charges, depreciation and amortization and compensation expense for employee stock options, the adjusted cash used before the effect of the changes in working capital components was $4,861. Cash provided by continuing operations for working
capital components the amount of approximately $5,603 and was the result of an increase in inventory of $4,423 and other current and non-current assets of $561, and a net decrease in accounts payable and accrued expenses and other liabilities of approximately $1,663, offset by reductions of cash from an increase in accounts receivable of $430 and decrease in income taxes payable of $614. Approximately $5,040 of cash used was for operating activities of our discontinued operations.
Cash in the amount $1,228 was provided from continuing operations in investing activities for the fiscal year ended June 30, 2009 and as compared to cash used of $265 in investing activities in the fiscal year ended June 30, 2008. In the fiscal year ended June 30, 2009, $1,290 of cash was provided from the sale of Hauser and we used $62 to purchase property and equipment, compared to the purchase of property and equipment of $255 and $10 of intangible assets in the fiscal year ended June 30, 2008. Cash used in our discontinued operations for investing activities during the fiscal years ended June 30, 2009 and 2008, was $112 and $482, respectively.
Cash provided by financing activities was $1,340 for the fiscal year ended June 30, 2009 and was the result of proceeds from employees exercising stock options during the period. Additionally, in the fiscal year ended June 30, 2009, the Company had proceeds of $750 from the issuance of notes payable, which were repaid in the same period in the amount of $750.
Cash provided by financing activities was $3,763 for the fiscal year ended June 30, 2008. Cash provided during the year ended June 30, 2008 was the result of proceeds aggregating $20,936. The components of the proceeds are from the issuance of: Series C Preferred Stock of $5,788, convertible note payable of $4,500, notes payable of $7,049, the release of restricted cash under the revolving credit facility of $2,000, proceeds from the revolving credit facility of $1,500 and the exercise of stock options of in the amount of $99. The proceeds were offset, in part, by repayments of the revolving credit facility and the term loan of $7,350 and $9,823, respectively.
Our consolidated financial statements have been prepared assuming that the Company will continue as a going concern. We have incurred recurring operating losses and negative operating cash flows for three consecutive years including a net loss attributable to common stockholders of $19,367 and negative operating cash flows of $2,752 for the fiscal year ended June 30, 2009. At June 30, 2009, we had cash and cash equivalents of $570, a working capital deficit of $2,916, primarily attributable to the discounted Notes Payable in the amount of $7,458, with a stated principal amount of $7,805, which are due on the earlier of the date stated in an Acceleration Notice (which must be at least two (2) business days following the date on which the notice is delivered), if any, or November 15, 2009, and an accumulated deficit of $46,603. We have historically raised capital in private placements, however, we continue to sustain losses and negative operating cash flows. Additionally, current economic conditions may cause a decline in business and consumer spending which could adversely affect our business and financial
26
performance. These factors raise substantial doubt as to our ability to continue as a going concern. Assuming we are able to raise additional capital and/or refinance at least $7,800 of the discounted Notes Payable, and we are not adversely affected by the current economic conditions, we believe that our available capital as of June 30, 2009, plus the additional $7,800 of additional capital and/or refinancing of our discounted Notes Payable, will enable us to continue as a going concern through the first quarter of our fiscal year ending June 30, 2011. There are no assurances that we will be able to raise additional capital as needed upon acceptable terms, nor that the current economic conditions will not negatively impact us. If the current economic conditions negatively impact us or our operations, or we are unable to raise additional capital as needed upon acceptable terms, it would have a material adverse effect on the Company.
Our total annual commitments at June 30, 2009 for long term non-cancelable leases of approximately $556 consists of obligations under operating leases for facilities and lease agreements for the rental of warehouse equipment, office equipment and automobiles.
The following table sets forth the Company’s future commitments as of June 30, 2009:
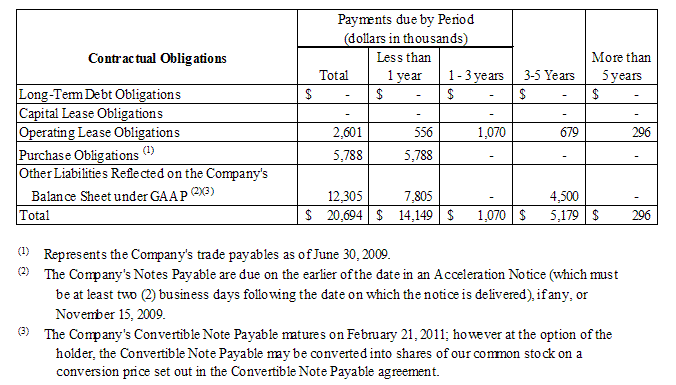
Capital Expenditures
The Company's capital expenditures for the fiscal ended June 30, 2009 and 2008 were approximately $62 and $255, respectively. The Company has budgeted approximately $0.5 million for capital expenditures for fiscal 2010. The total amount is expected to be funded from cash provided from its operations.
27
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Impact of Inflation
The Company does not believe that inflation has significantly affected its results of operations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not Applicable
Item 8. Financial Statements
For a list of financial statements filed as part of this report, see the index to financial statements at page 31.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by the Company in the reports it files or submits under the Securities Exchange Act of 1934 (the “Exchange Act”) is recorded, processed, summarized, and reported within the time periods specified by the Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to provide reasonable assurance that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of management, including the Chief Executive Officer and Chief Financial Officer, the Company has evaluated the effectiveness of its disclosure controls and procedures (as such term is defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act) as of June 30, 2009, and, based upon this evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that these controls and procedures are effective in providing reasonable assurance of compliance.
Changes in Internal Control over Financial Reporting
Under the supervision and with the participation of management, including the Chief Executive Officer and Chief Financial Officer, the Company has evaluated changes in internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the fiscal quarter ended June 30, 2009 and have concluded that no change has materially affected, or is reasonably likely to materially affect, internal control over financial reporting.
Management’s Annual Report On Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining an adequate system of internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control system was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes, in accordance with generally accepted accounting principles. Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deteriorate.
28
The Company’s management, including the Chief Executive Officer and Chief Financial Officer, has conducted an evaluation of the effectiveness of its internal control over financial reporting as of June 30, 2009 based on the framework in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, management concluded that our internal control over financial reporting was effective as of June 30, 2009.
The information set forth in this Item 9A shall not be considered filed under the Exchange Act. This annual report does not include an attestation report of Amper, Politziner & Mattia, LLP, Integrated BioPharma’s independent registered public accounting firm, regarding internal control over financial reporting. Management’s report was not subject to attestation by Amper, Politziner & Mattia, LLP pursuant to temporary rules of the SEC that permit the Company to provide only management’s report in this Form 10-K.
Item 9B. Other Information
None.
29
PART III
Item 10. Directors and Executive Officers of the Registrant.
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2009.
Item 11. Executive Compensation
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2009.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2009.
Item 13. Certain Relationships and Related Transactions
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2009.
Item 14. Principal Accountant Fees and Services
Incorporated by reference from the Company’s Proxy Statement for Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year ended June 30, 2009.
30
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) Exhibits and Index
|
(1) |
A list of the financial statements filed as part of this report is set forth in the index to financial statements at Page 31 and is incorporated herein by reference. |
(2) An index of exhibits incorporated by reference or filed with this Report is provided below.
|
Number |
Description |
|
3.1 |
Certificate of Incorporation of Integrated BioPharma, Inc., as amended (15) |
|
3.2 |
By-Laws of Registrant (13) |
|
4.1 |
Certificate of Designation of Series and Determination of Rights and Preferences of Series A Convertible Preferred Stock of Integrated BioPharma, Inc. dated June 25, 2003 (1). |
|
4.2 |
Certificate of Designation of Series C and Determination of Rights and Preferences of Series C Convertible Preferred Stock of Integrated BioPharma, Inc. dated February 21, 2008 (14) |
|
10.1 |
Lease Agreement, dated August 3, 1994, between the Company and Hillside 22 Realty Associates, L.L.C. (4) |
|
10.2 |
Lease Agreement between the Company and Vitamin Realty Associates, dated January 10, 1997 (5) |
|
10.3 |
Manufacturing Agreement between Chem International, Inc. and Herbalife International of America, Inc. dated April 9, 1998 (6) |
|
10.4 |
Integrated Health Technologies, Inc. 2001 Stock Option Plan (7) |
|
10.5 |
Loan Agreement, dated September 1, 2006, between Integrated BioPharma, Inc. and Amalgamated Bank (9) |
|
10.6 |
Asset Purchase Agreement, dated February 28, 2007, between Integrated BioPharma, Inc., Bioscience Technologies, Inc., BevSpec, Inc. (10) |
|
10.7 |
Loan Agreement, dated April 3, 2007, between Integrated BioPharma, Inc. and Amalgamated Bank (11) |
|
10.8 |
Amendment One To Revolving (Grid) Promissory Note And Loan Agreement dated April 3, 2007, between Integrated BioPharma, Inc. and Amalgamated Bank (11) |
|
10.9 |
Amendment Two To Revolving (Grid) Promissory Note And Loan Agreement dated September 27, 2007, between Integrated BioPharma, Inc. and Amalgamated Bank (12) |
|
10.10 |
Separation and Distribution Agreement dated November 14, 2007, with our subsidiary INB:Biotechnologies (12) |
|
10.11 |
Securities Purchase Agreement dated February 21, 2008, by and between Integrated BioPharma, Inc. and Imperium Master Fund, Ltd. 8% Senior Secured Note (14) |
|
10.12 |
Securities Purchase Agreement dated February 21, 2008, by and between Integrated BioPharma, Inc. and CD Financial, LLC 9.5% Convertible Senior Secured Note (14) |
|
10.13 |
Warrant Agreement by and between Integrated BioPharma, Inc. and the note holders of the Amended and Restated First Amendment to Amended and Restated Securities Purchase Agreement and 8% Senior Secured Notes dated October 14, 2008 (16) |
|
10.14 |
Registration Rights Agreement by and between Integrated BioPharma, Inc. and the each of the named Investors in such agreement dated October 14, 2008 (16) |
|
10.15 |
Amended And Restated First Amendment To Amended And Restated Securities Purchase Agreement And 8% Senior Secured Notes dated October 20, 2008 (16) |
|
10.16 |
9.5% Promissory Note by and between Integrated BioPharma, Inc. and CD Financial, LLC. dated February 5, 2009 (17) |
|
10.17 |
9.5% Promissory Note by and between Integrated BioPharma, Inc. and CD Financial, LLC. dated February 17, 2009 (17) |
|
14 |
Code of Ethics (8) |
|
21 |
Subsidiaries of the Registrant (18) |
|
23 |
Consent of Independent Registered Public Accounting Firm (18) |
31
|
31.1 |
Certification of Periodic Report by Chief Executive Officer Pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (18) |
|
31.2 |
Certification of Periodic Report by Chief Financial Officer Pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (18) |
|
32.1 |
Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18) |
|
32.2 |
Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18) |
__________________________
|
(1) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 2003, filed with the SEC on September 29, 2003. |
|
(2) |
Incorporated herein by reference to the Company’s Registration Statement on Form SB-2, Registration No. 333-5240-NY. |
|
(3) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on April 21, 2004. |
|
(4) |
Incorporated herein by reference to Amendment No. 1 to the Company’s Registration Statement on Form SB-2, Registration No. 333-5240-NY. |
|
(5) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 1997, filed with the SEC on September 29, 1997. |
|
(6) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 1998, filed with the SEC on September 24, 1998. |
|
(7) |
Incorporated herein by reference to the Company’s Registration Statement on Form S-8, filed with the SEC on May 1, 2002. |
|
(8) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-KSB for the fiscal year ended June 30, 2004, filed with the SEC on September 28, 2004, as amended on November 10, 2004. |
|
(9) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on September 7, 2006. |
|
(10) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on March 9, 2007. |
|
(11) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on April 9, 2007. |
|
(12) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on November 14, 2007. |
|
(13) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on February 12, 2008. |
|
(14) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on February 21, 2008. |
|
(15) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on May 9, 2008. |
|
(16) |
Incorporated herein by reference to the Company's Current Report on Form 8-K filed with the SEC on October 20, 2008. |
|
(17) |
Incorporated herein by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2008 filed with the SEC on February 20, 2008. |
|
(18) |
Filed herewith.
|
|
|
32
Item 8: Financial Statements
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm 34
Consolidated Statements of Operations for the fiscal years
ended June 30, 2009 and 2008 35
Consolidated Balance Sheets as of June 30, 2009 and 2008 36
Consolidated Statements of Stockholders’ (Deficiency) Equity for the fiscal years
ended June 30, 2009 and 2008 37
Consolidated Statements of Cash Flows for the fiscal years ended
ended June 30, 2009 and 2008 38
Notes to Consolidated Financial Statements 39 - 67
. . . . . . . .
33
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
Integrated BioPharma, Inc.
We have audited the accompanying balance sheets of Integrated BioPharma, Inc. and Subsidiaries (the “Company”) as of June 30, 2009 and 2008, and the related statements of operations, stockholders’ (deficiency) equity, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Integrated BioPharma, Inc. and Subsidiaries as of June 30, 2009 and 2008, and the results of their operations and their cash flows for each of the years then ended in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming Integrated BioPharma, Inc. and Subsidiaries will continue as a going concern. As more fully described in Note 2, the Company has a working capital deficiency and recurring net losses, and is the process of seeking additional capital. The Company has not yet secured sufficient capital to fund its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that may result from the outcome of this uncertainty.
/s/ Amper, Politziner & Mattia, LLP
October 12, 2009
Edison, NJ
34
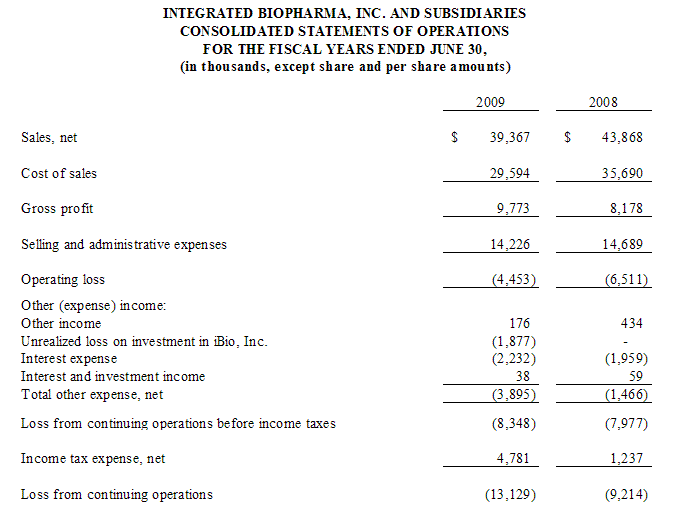
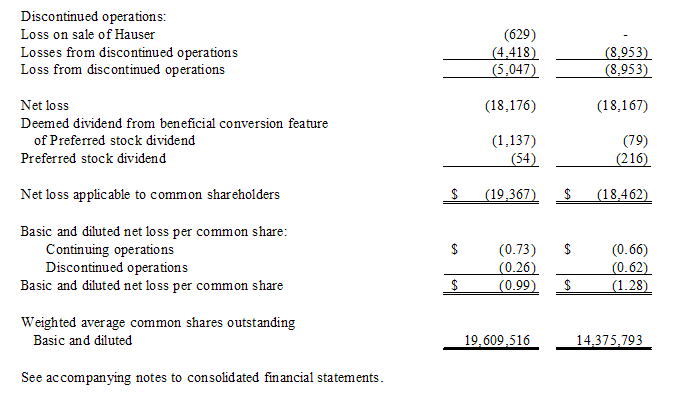
35
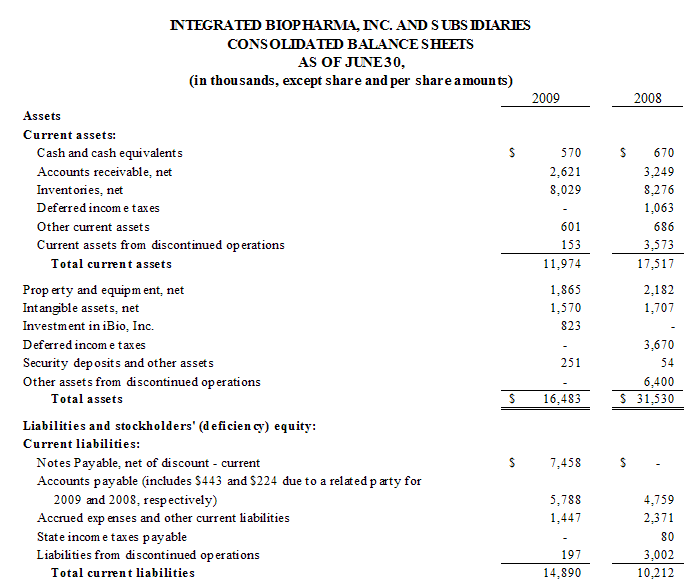

36
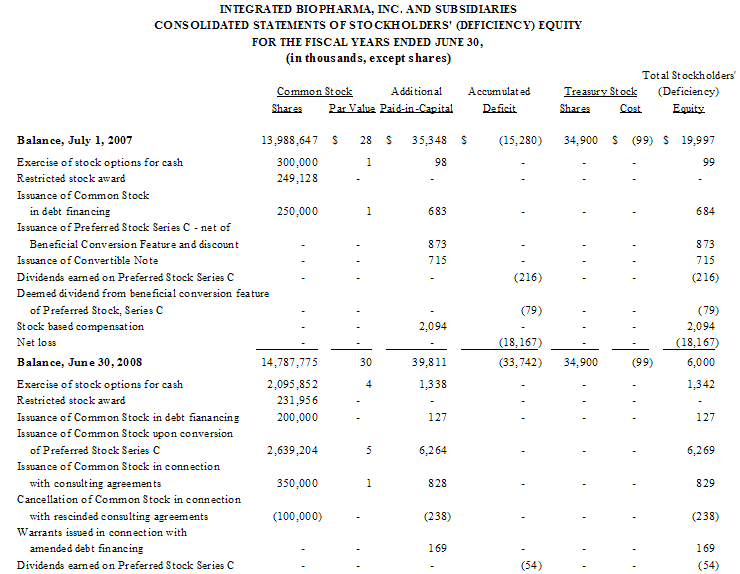
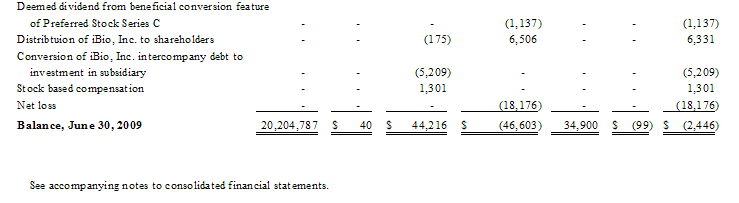
37

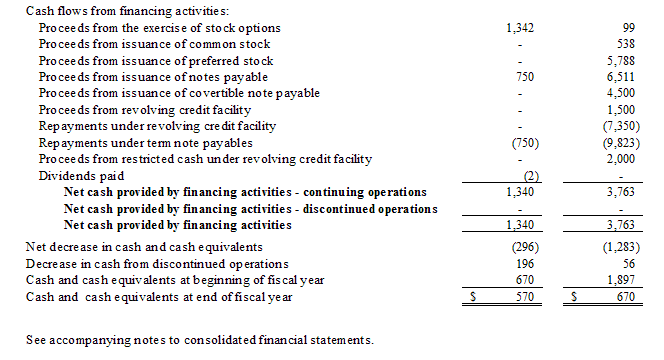
38
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Note 1. Business
Integrated BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the “Company” or “INBP”), is engaged primarily in manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily in the United States. The Company was previously known as Integrated Health
Technologies, Inc. and, prior to that, as Chem International, Inc. The Company was reincorporated in its current form in Delaware in 1995. As of September 22, 2009, the Company’s common stock trades on the OTC Bulletin Board under the symbol INBP.OB. From February 27, 2009 through September 22, 2009, the Company’s common stock traded on the Pink Sheets under the symbol INBP.PK. Immediately prior to February 27, 2009, the Company’s common stock traded on the NASDAQ
Global Market under the symbol “INBP.” The Company continues to do business as Chem International, Inc. with certain its customers and certain vendors.
The Company, subsequent to the spin-off of its Biotechnologies segment and the sale of the Pharmaceutical segment, as discussed in more detail below, has one remaining reportable segment for its operation, the Nutraceuticals segment.
The Nutraceutical segment, our one remaining business operation includes: InB:Manhattan Drug Company, Inc. (“Manhattan Drug”), which manufactures vitamins and nutritional supplements for sale to distributors, multilevel marketers and specialized health-care providers; The Vitamin Factory, which sells private label Manhattan Drug products, as well as our AgroLabs products, through mail order catalogs and the Internet.
AgroLabs, Inc. (“AgroLabs”), which oversees the manufacture of and distributes for sales through major mass market, grocery, drug and vitamin retailers, healthful nutritional products under the following brands: Naturally Noni, Naturally Pomegranate, Naturally Aloe, Aloe Pure, Naturally Thai Mangosteen, Peaceful Sleep, Green Envy, 1st Choice Multi-Vitamin, ACAI Extra, ACAI Immune, ACAI Cleanse, and other products which are being introduced into the market, these are referred to as our branded proprietary Nutraceutical business and/or products.
The Company also distributes fine natural chemicals through its wholly-owned subsidiary IHT Health Products, Inc. and is a distributor of certain raw materials for DSM Nutritional Products, Inc.
During the fiscal year ended June 30, 2007, The Organic Beverage Company (TOBC), formerly Bioscience Technologies, Inc., completed the acquisition from BevSpec, Inc. (“BevSpec”) of the Syzmo™ product, which is a USDA organic energy drink. During the first quarter of the fiscal year ended June 30, 2009, we curtailed operations of our TOBC subsidiary and combined the sales efforts for this product line with the AgroLabs products. In June 2009, we further determined that
this product line was to be discontinued as we do not have the financial resources to pursue the further development of the Syzmo™ product in the very competitive energy drink market place.
The Pharmaceutical segment included InB:Paxis Pharmaceuticals, Inc. (“Paxis”) and InB:Hauser Pharmaceutical Services, Inc. (“Hauser”). Paxis manufactured and distributed Paclitaxel, and Hauser provided research, development and manufacturing testing services to the specialty chemical, pharmaceutical and natural products industries. On March 17, 2009, we consummated the sale of all of the issued and outstanding shares of the common stock of Hauser to Cedarburg Pharmaceuticals, Inc. Prior to the sale of Hauser, we sold substantially all of the assets of Paxis to Hauser and transferred the outstanding payables owed by Paxis to Hauser in consideration for the outstanding intercompany debt between Hauser and Paxis. The assets and liabilities transferred under this transaction were owned by Hauser at the time of the sale of Hauser’s common stock to Cedarburg and are no longer our assets or our liabilities. We continue to own certain assets of Paxis through our common stock ownership of Paxis. Our selling price received in connection with the sale of Hauser consisted of $1,160 in cash and a promissory note in our favor in the principal amount of $340, which note matures in twelve months on March 17, 2010 and bears interest at a rate of 12% per annum, payable quarterly. On April 7, 2009, we sold this promissory note to CD Financial, LLC, a related party and the holder of our Convertible Note Payable in the amount of $4,500, for the full principal amount of $340 and accrued interest of approximately $2.
39
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
The Biotechnologies segment included iBio, Inc. (formerly, iBioPharma, Inc.) (“iBio”), which was focused on the discovery, development and commercialization of proprietary products from plants. On August 18, 2008, our distribution (the “Distribution”) of the Biotechnologies segment was completed and each of our shareholders received one share of iBio’s common stock for each share they owned of our common stock as of August 12, 2008, the (the “Record Date”). The Distribution qualified as a tax-free reorganization under Section 355 of the Internal Revenue Code of 1986, as amended. The Separation and Distribution Agreement prohibits iBio from issuing any additional shares of its common stock in excess of the shares issued with respect to the Distribution for the two years immediately following the effective date of the Distribution.
On August 19, 2008, we entered into a Conversion Agreement with iBio, where approximately $5,209 of the intercompany debt with iBio was contributed to additional paid in capital and $2,700 of the intercompany debt purchased approximately 1.3 million shares of iBio, representing 6% of the then outstanding shares of iBio.
Additionally, on August 19, 2008, iBio closed on its $5,000 capital raise in connection with its private placement of approximately ten percent (10%) of iBio. Such funds were released to iBio from an escrow account and it issued approximately 2.3 million shares of iBio’s par value $0.001 common stock, at an estimated purchase price of approximately $2.13 per share. This private placement reduced our ownership in iBio to 5.4%.
These consolidated financial statements, reflect the spin-off, sale of and discontinued operations and related transactions of iBio, Hauser and TOBC, respectively.
Note 2. Liquidity and Going Concern.
The Company’s consolidated financial statements have been prepared assuming that it will continue as a going concern. The Company has incurred recurring operating losses and negative operating cash flows for three consecutive years including a net loss attributable to common stockholders of $19,367 and
negative operating cash flows of $2,752 for the year ended June 30, 2009. At June 30, 2009, the Company had cash and cash equivalents of $570, a working capital deficit of $2,916, primarily attributable to the discounted Notes Payable in the amount of $7,458, with a stated principal
amount of $7,805, and which are due on the earlier of the date stated in an Acceleration Notice (which must be at least two (2) business days following the date on which the notice is delivered), if any, or November 15, 2009, and an accumulated deficit of $46,603. The Company has
historically raised capital in private placements, but continues to sustain losses and negative operating cash flows. Additionally, current economic conditions may cause a decline in business and consumer spending which could adversely affect the Company’s business and financial performance. These factors raise substantial doubt as to the Company’s ability to continue as a going concern. Assuming the Company is able to raise additional capital and/or refinance at least
$7,800 of the discounted Notes Payable, and it is not adversely affected by the current economic conditions, the Company believes that its available capital as of June 30, 2009 will enable it to continue as a going concern through the first quarter of the fiscal year ending June 30, 2011. There are no assurances that the Company will be able to raise additional capital or successfully refinance its discounted Notes Payable of at least $7,800,
upon acceptable terms, nor that the current economic conditions will not negatively impact it. If the current economic conditions negatively impact the Company, or it is unable to raise additional capital or successfully refinance its discounted Notes Payable of at least $7,800 upon acceptable terms, it would have a material adverse effect on the Company.
The accompanying consolidated financial statements do not include any adjustments that might result from this uncertainty.
Note 3. Summary of Significant Accounting Policies
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Intercompany transactions and accounts have been eliminated in consolidation.
Reclassifications. Certain reclassifications have been made to the prior year data to conform with the current year presentation.
40
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues
and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
|
· |
sales returns and allowances; |
|
· |
trade marketing and merchandising; |
|
· |
allowance for doubtful accounts; |
|
· |
inventory valuation; |
|
· |
valuation and recoverability of long-lived and intangible assets and goodwill, including the values assigned to acquired intangible assets; |
|
· |
income taxes and valuation allowance on deferred income taxes, and; |
|
· |
accruals for, and the probability of, the outcome of current litigation. |
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Revenue Recognition. For product sales, the Company recognizes revenue when the product’s title and risk of loss transfers to the customer. The Company believes this revenue recognizing practice is appropriate because the Company’s sales policies meet the four criteria of SAB 104 which are: (i) persuasive evidence that an arrangement exists, (ii) delivery has occurred, (iii) the seller’s price to the buyer is fixed and determinable and (iv) collectability is reasonably assured. The Company’s sales policy is to require customers to provide purchase orders establishing selling prices and shipping terms. The Company evaluates the credit risk of each customer and establishes an allowance of doubtful accounts for any credit risk. Sales returns and allowances are estimated upon shipment.
The Company realized fee income from managing warehouse and marketing consulting for unrelated entities of $89 and $107 in the fiscal years ended June 30, 2009 and 2008, respectively. Additionally, in the fiscal year ended Juine 30, 2009, the Company earned $87 in transitional services fee income from iBio. These amounts are included in “Other income,” in the accompanying
Consolidated Statements of Operations.
Shipping and Handling Costs. Shipping and handling costs were approximately $1,833 and $2,730 for the fiscal years ended June 30, 2009 and 2008, respectively, and are included in cost of sales in the accompanying Consolidated Statements of Operations.
Trade Marketing and Merchandising. In order to support the Company’s propriety Nutraceutical product lines, various promotional activities are conducted through the retail trade, distributors or directly with consumers, including in-store display and product placement programs, feature price discounts, coupons, and other similar activities. The Company regularly reviews and revises, when it deems necessary, estimates of costs to the Company for these promotional programs based on estimates of what will be redeemed by the retail trade, distributors, or consumers. These estimates are made using various techniques, including historical data on performance of similar promotional programs. Differences between estimated expense and actual performance are generally not material and are recognized as a change in management’s estimate in a subsequent period. The Company’s total promotional expenditures, including amounts classified as a reduction of net sales, represent approximately 18.8% and 16.4% of net sales for in the fiscal years ended June 30, 2009 and 2008, respectively.
41
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Advertising. Advertising costs are expensed as incurred. Advertising expense was approximately $4,343 and $3,445 for the fiscal years ended June 30, 2009 and 2008, respectively. These costs are included in selling and administrative expenses in the accompanying Consolidated Statements of Operations.
Stock-Based Compensation. As of June 30, 2009 and 2008, the Company has two stock-based compensation plans that have outstanding options issued in accordance with such plans. We periodically grant stock options to employees and directors in accordance with the provisions of our stock option plans, with the exercise price of the stock options being set at the closing market
price of the common stock on the date of grant. Effective as of July 1, 2005, the Company adopted Statement of Financial Standards No. 123R, “Share-Based Payment” (“SFAS No. 123R”) which requires that compensation cost relating to share-based payment transactions be recognized as an expense in the consolidated financial statements and that measurement of that cost be based on the estimated fair value of the equity or liability instrument issued.
The intrinsic value of options outstanding and exercisable at June 30, 2009 and 2008 was $2 and $1,624, respectively. There were 2,095,852 and 300,000 options exercised during the fiscal year ended June 30, 2009 and 2008, respectively.
The remaining unrecognized stock-based compensation expense at June 30, 2009 was $1,212 and will be amortized over a weighted average life of 1.5 years.
The fair value for these options was estimated at the date of each grant using a Black-Scholes option pricing model with the following weighted-average assumptions for the fiscal years ending June 30:
|
2009 |
2008 |
|||
|
Risk-free interest rate |
1.59% |
3.3% |
||
|
Expected volatility |
100% |
57% |
||
|
Dividend yield |
-- |
-- |
||
|
Expected life |
7 to 10 years |
7 to 10 years |
||
|
Forfeiture rate |
0% to 20% |
0% to 20% |
The Company calculates expected volatility for a stock-based grant based on historic daily stock price observations of our common stock during the period immediately preceding the grant that is equal in length to the expected term of the grant. The expected term of the options is estimated based on the Company’s historical exercise rate and forfeiture rates are
estimated based on employment termination experience. The risk free interest rate is based on U.S. Treasury yields for securities in effect at the time of grants with terms approximating the term of the grants. The assumptions used in the Black-Scholes option valuation model are highly subjective, and can materially affect the resulting valuations.
Income Taxes. The Company accounts for income taxes using the liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates
expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will
not be realized.
Earnings Per Share. In accordance with SFAS No. 128, “Earnings Per Share,” basic earnings per common share are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible preferred stock, subject to anti-dilution limitations.
42
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
For the fiscal years ended June 30, 2009 and 2008, options and warrants to purchase 25,000 and 5,108,002 shares of common stock with exercise prices below the market price, respectively, were outstanding but were not included in the computation of diluted earnings per share as they are anti-dilutive as a result of net losses during the period. Options and warrants to purchase 3,036,785 and 2,103,150 shares of common stock were outstanding as of June 30, 2009 and 2008, respectively, but were not included in the computation of diluted earnings per share as their exercise prices were greater than the market price of the common shares.
For the fiscal year ended June 30, 2009, Convertible Note Payable common share equivalents of 2,250,000 were not included in the computation of diluted earnings per share as they were anti-dilutive as a result of net losses applicable to common shareholders. For the fiscal year ended June 30, 2008, Convertible Series C Preferred Stock and Convertible Note Payable in the amount of 2,462,778 and 1,779,755 common share equivalents, respectively, were not included in the computation of diluted earnings per share as they were anti-dilutive as a result of net losses applicable to common shareholders.
Fair Value of Financial Instruments. Generally accepted accounting principles require disclosing the fair value of financial instruments to the extent practicable for financial instruments which are recognized or unrecognized in the balance sheet. The fair value of the financial instruments disclosed herein is not necessarily representative of the amount that
could be realized or settled, nor does the fair value amount consider the tax consequences of realization or settlement.
In assessing the fair value of financial instruments, the Company uses a variety of methods and assumptions, which are based on estimates of market conditions and risks existing at the time. For certain instruments, including cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments. All debt is based on current rates at which the Company
could borrow funds with similar remaining maturities and approximates fair value.
Cash and Cash Equivalents. Cash equivalents are comprised of certain highly liquid investments with a maturity of three months or less when purchased.
Accounts Receivable and Allowance for Doubtful Accounts. In the normal course of business, the Company extends credit to customers. Accounts receivable, less the allowance for doubtful accounts, reflect the net realizable value of receivables, and approximate fair value. The Company believes there is no concentration of credit risk with any single customer whose failure or nonperformance would materially affect the Company’s results other than as discussed in Note 13(c) – Significant Risks and Uncertainties – Major Customers. On a regular basis, the Company evaluates its accounts receivables and establishes an allowance for doubtful accounts based on a combination of specific customer circumstances, credit conditions, and historical write-offs and collections. The allowance for doubtful accounts as of June 30, 2009 and 2008 was $298 and $108, respectively. Accounts receivable are charged off against the allowance after management determines that the potential for recovery is remote.
Inventories. Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out method. Allowances for obsolete and overstock inventories are estimated based on “expiration dating” of inventory and projection of sales.
Property and Equipment. Property and equipment are recorded at cost and are depreciated over the following estimated useful lives:
Building 15 Years
Leasehold Improvements Shorter of estimated useful life or term of lease
Machinery and Equipment 7 Years
Machinery and Equipment Under Capital Leases 7 Years
Transportation Equipment 5 Years
43
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Building, machinery and equipment and transportation equipment are depreciated using straight-line methods while leasehold improvements are amortized on a straight-line basis over various periods not to exceed its useful life or the lease terms whichever is shorter.
Impairment of Long-Lived Assets. In accordance with Statement of Financial Accounting Standards No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, long-lived assets, except goodwill and indefinite-lived intangible assets, are reviewed for impairment when circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the future net cash flows estimated by the Company to be generated by such assets. If such assets are considered to be impaired, the impairment to be recognized is the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of by sale are recorded as held for sale at the lower of carrying value or estimated net realizable value. No impairment losses were identified or recorded in the fiscal years ended June 30, 2009 and 2008.
Other Intangible Assets. In accordance with Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets”, goodwill and indefinite-lived intangible assets are not amortized against earnings, but are reviewed at least annually for impairment. The Company does not have any goodwill or other intangible assets with indefinite lives.
Intangible assets with finite lives are amortized over their estimated useful lives. The useful life of an intangible asset is the period over which the asset is expected to contribute directly or indirectly to future cash flows. The carrying value of intangible assets with finite lives is evaluated whenever events or circumstances indicate that the carrying value may not be recoverable. The carrying value is not recoverable when the projected undiscounted future cash flows are less than the carrying value. Tests for impairment or recoverability require significant management judgment, and future events affecting cash flows and market conditions could result in impairment losses. As a result of its testing, the Company recorded impairment charges of $32 (relating to trademarks and patents) in the fiscal year ended June 30, 2009 and $813 in the fiscal year ended June 30, 2008, of which $447 related to intellectual property, $335 related to trade names and $31 to patents. These impairment charges are included in discontinued operations in the Consolidated Statement of Operations. Amortization expense is recorded on the straight-line method over periods ranging from 2 years to 20 years and is included in selling and administrative expenses.
Other intangible assets consist of trademarks, license fees, and unpatented technology. Amortization is being recorded on the straight-line basis over periods ranging from 2 years to 20 years based on contractual or estimated lives.
Recent Accounting Pronouncements. In April 2008, the FASB issued FASB Staff Position (FSP) SFAS No. 142-3, “Determination of the Useful Life of Intangible Assets” (“FSP SFAS No. 142-3”). FSP SFAS No. 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a
recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets” and was effective for fiscal years beginning after December 15, 2008. The adoption of this pronouncement by the Company, for the fiscal year ending June 30, 2010, will not have a material impact on the its consolidated financial statements.
In April 2009, the FASB issued FSP FAS No. 107-1 (“SFAS No. 107-1”) and Accounting Principles Board Opinion 28-1, “Interim Disclosures about Fair Value of Financial Instruments” (“APB 28-1”). APB 28-1 amends SFAS No. 107, “Fair Value of Financial Instruments” to require disclosures about fair value of financial instruments for interim reporting periods in addition to the required disclosures in annual financial statements. APB 28-1 also
amends APB Opinion 28, “Interim Financial Reporting”, to require those disclosures in summarized financial information at interim reporting periods. FSP SFAS 107-1 and APB 28-1 are effective for interim reporting periods ending after June 15, 2009. The Company has adopted the provisions of this FSP effective for its interim quarter ended September 30, 2009 and it will not have a material impact on its consolidated financial statements.
44
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events” (“SFAS 165”). SFAS 165 establishes general standards for accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or available to be issued and was effective for interim and annual periods ending after June 15, 2009. The adoption of SFAS No. 165 did not have an impact on the Company’s results of operations or financial
condition. The Company evaluated all subsequent events that occurred from July 1, 2009 through October 2, 2009, inclusive, and determined there were no material subsequent events that required disclosure in its consolidated financial statements.
In June 2009, the FASB issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles” (“SFAS No. 168”). SFAS No. 168 will become the single source of authoritative nongovernmental U.S. generally accepted accounting principles (“GAAP”), superseding existing FASB, American Institute of Certified Public Accountants, Emerging Issues Task Force (“EITF”), and related accounting literature. SFAS No. 168 reorganizes the thousands of GAAP pronouncements into roughly 90 accounting topics and displays them using a consistent structure. Also included is relevant Securities and Exchange Commission guidance organized using the same topical structure in separate sections. SFAS No. 168 will be effective for financial statements issued for reporting periods that end after September 15, 2009. The adoption of SFAS No. 168 is not expected to have a material impact on the Company’s consolidated results of operations and financial condition.
In October 2008, the Financial Accounting Standards Board (“FASB”) issued FSP No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active” (“FSP 157-3”). FSP 157-3 clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in
determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 became effective for us on September 30, 2008 for all financial assets and liabilities recognized or disclosed at fair value in our Consolidated Financial Statements on a recurring basis (at least annually).
In May 2008, the FASB issued SFAS No. 162, “The Hierarchy of Generally Accepted Accounting Principles” (“SFAS No. 162”). SFAS No. 162 is intended to improve financial reporting by identifying a consistent hierarchy for selecting accounting principles to be used in preparing financial statements that are presented in conformity with GAAP. Prior to the issuance of SFAS No. 162, GAAP hierarchy was defined in the American Institute of Certified Public Accountants
(“AICPA”) Statement on Auditing Standards (SAS) No. 69, “The Meaning of Present Fairly in Conformity With Generally Accepted Accounting Principles”. Unlike SAS No. 69, SFAS No. 162 is directed to the entity rather than the auditor. Statement No. 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board Auditing amendments to AU Section 411, “The Meaning of Present Fairly in Conformity with Generally
Accepted Accounting Principles”. SFAS No. 162 is not expected to have any material impact on the Company’s results of operations, financial condition or liquidity.
In May 2008, the FASB issued FSP No. APB 14-1, “Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (including Partial Cash Settlement)” (“FSP APB 14-1”), which requires separate accounting for the debt and equity components of convertible debt issuances. The requirements for separate accounting must be applied
retrospectively to previously issued cash-settleable convertible instruments as well as prospectively to newly issued instruments, negatively affecting both net income and earnings per share for issuers of the instruments. The FSB APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008. The Company is currently evaluating the impact of FSP No. APB 14-1 on the Company’s results of operations, financial condition or liquidity as of
June 30, 2009.
In June 2008, the FASB issued EITF 07-5, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity's Own Stock” (“EITF07-5”). EITF07-5 provides guidance in assessing whether an equity-linked financial instrument (or embedded feature) is indexed to an entity's own stock for purposes of determining whether the appropriate accounting treatment falls under the scope of SFAS 133, "Accounting For Derivative Instruments and Hedging Activities" and/or EITF 00-19, "Accounting For Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company's Own Stock". EITF 07-5 is effective for financial statements issued for fiscal years beginning after December 15, 2008 and early
45
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
application is not permitted. The Company is currently evaluating the impact of EITF 07-5 on the Company’s results of operations, financial condition or liquidity as of June 30, 2009.
Note 4. Discontinued Operations
During the Company’s 2009 fiscal year, the Company has classified the spin-off of iBio in August 2008, the sale of Hauser in March 2009 and the discontinued operations of TOBC as discontinued operations for the current and prior periods and the associated results of operations, financial position and cash flows are separately reported for all periods presented. The net assets of iBio, Hauser and TOBC are classified as assets and liabilities related to discontinued operations in the Company’s consolidated balance sheet as of June 30, 2008 and for TOBC also as of June 30, 2009. The net assets from discontinued operations as of June 30, 2008 were comprised of the following:
46
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
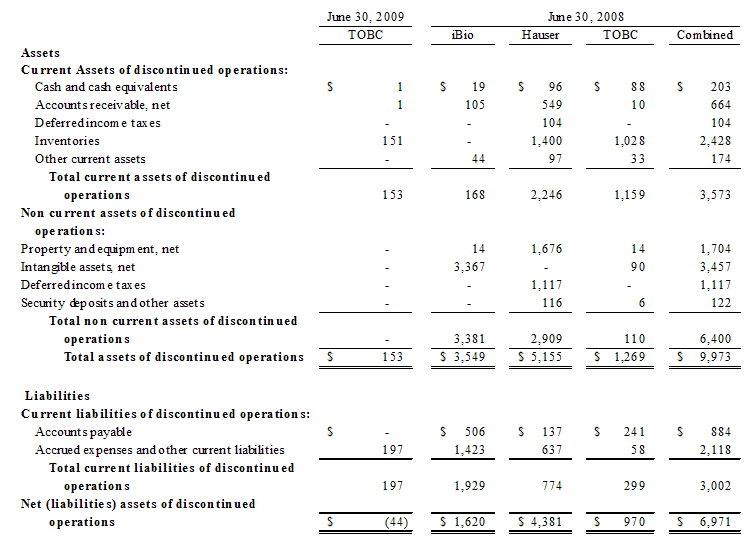
(a) Spin-off of iBio – In August 2008, the Company completed the spin-off of iBio. As a result, the Company recognized an after-tax loss of $105 during the first quarter of the fiscal year ended June 30, 2009. iBio revenues from discontinued operations were $169 and $987 for the fiscal years ended June 30, 2009 and 2008, respectively. The Company’s loss from discontinued operations, net of taxes, was $105 and $1,555 for the fiscal years ended June 30, 2009 and 2008, respectively.
47
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
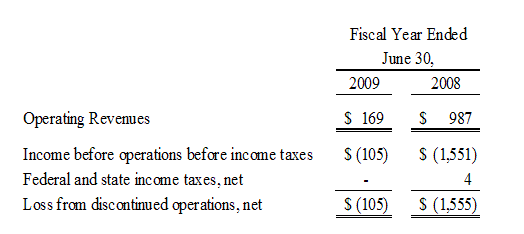
The Distribution was completed on August 18, 2008 and each shareholder of the Company received one share of iBio for each share the shareholder owned as of August 12, 2008. The Distribution qualified as a tax-free reorganization under Section 355 of the Internal Revenue Code of 1986, as amended. The Separation and Distribution Agreement prohibits iBio from issuing more than 19,845,061 of additional shares of its
common stock (representing the number of shares issued in connection with the Distribution) for the two years immediately following the effective date of the Distribution.
On August 19, 2008, the Company entered into a Conversion Agreement with iBio, whereby the Company caused approximately $5,209 of the intercompany debt to be contributed to additional paid in capital and used $2,700 of the intercompany debt to purchase approximately 1,266,706 common shares of iBio, representing 6% of the then outstanding common shares of iBio. The Company is recorded its investment in iBio on the cost basis, in the fourth quarter of its fiscal year ended June 30, 2009,
the Company recorded an unrealized (impairment) loss of $1,877 on its investment in iBio. The Company owns 5.4% of the shares outstanding of iBio as of June 30, 2009. In September 2009, iBio completed a private placement of 4,615,385 shares of its common stock for approximately $3,000 or $0.65 per share of common stock, reducing the Company’s ownership to approximately 4.5%.
In August 2008, the Company ceased allocating corporate overhead to iBio (formerly Biotechnologies Segment) and entered into a Transitional Services Agreement (the “TS Agreement”) with iBio. The transitional services agreement permits the Company to continue to provide certain corporate services to iBio in exchange for a management charge. The scope of these services is
limited to legal, strategic financial planning and SEC reporting, and tax services by certain corporate employees of the Company. The TS Agreement provides for a per annum fee of $100. In the fiscal year ended June 30, 2009, the Company billed iBio approximately $87 under the TS Agreement.
(b) Sale of Hauser – In March 2009, the Company entered into a stock purchase agreement and consummated the sale of all of the issued and outstanding shares of common stock of its wholly owned subsidiary Hauser to Cedarburg Pharmaceuticals, Inc. (“Cedarburg”). Prior to the sale of Hauser, the Company sold substantially all the assets of Paxis and transferred outstanding payables owed by Paxis (the “Net Assets of Paxis”) to Hauser in consideration for the outstanding intercompany debt between these two subsidiaries of the Company. The Net Assets of Paxis transferred under this transaction were owned by Hauser at the time of the sale of Hauser’s common stock to Cedarburg and are no longer assets and liabilities of the Company. The Company continues to own certain assets of Paxis through its common stock ownership of Paxis. The purchase price received by the Company in connection with the sale consisted of $1,160 in cash and a promissory note in favor of the Company in the principal amount of $340. The promissory note matures in twelve months, on March 17, 2010, and bears
48
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
interest at a rate of 12% per annum, payable quarterly. On April 7, 2009, this promissory note was sold to CD Financial, LLC, a related party and the holder of the Company’s Convertible Debt (see Note 6(a)), for the full principal amount of $340 and accrued interest of $2.
As a result of the sale of Hauser, the Company recognized a loss of $629 during the fiscal year ended June 30, 2009. The Hauser revenues from discontinued operations were $2,743 and $4,843 for the fiscal years ended June 30, 2009 and 2008, respectively. The Company’s net loss from discontinued operations, net of taxes was $3,081 and $3,306 for the fiscal years ended June 30, 2009 and 2008, respectively.
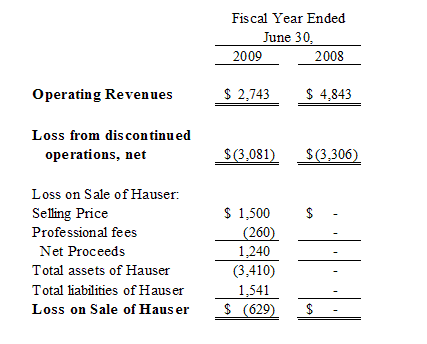
(c) Discontinued operations of TOBC – During the first quarter of the fiscal year ending 2009, the Company curtailed its operations of its TOBC subsidiary and combined the sales efforts for the Syzmo™ product line with the AgroLabs products. In June 2009, the Company determined that this product line was to be discontinued as it does not have the financial resources to pursue the further development of the Syzmo™ product in the very competitive energy drink market place.
Revenues from TOBC’s discontinued operations were $164, and $736 for the fiscal years ended June 30, 2009 and 2008, respectively. The Company’s net loss from TOBC’s discontinued operations was $1,232 and $4,093 for the fiscal years ended June 30, 2009 and 2008, respectively.
49
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)

Note 5. Supplemental Cash Flow Information
50
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
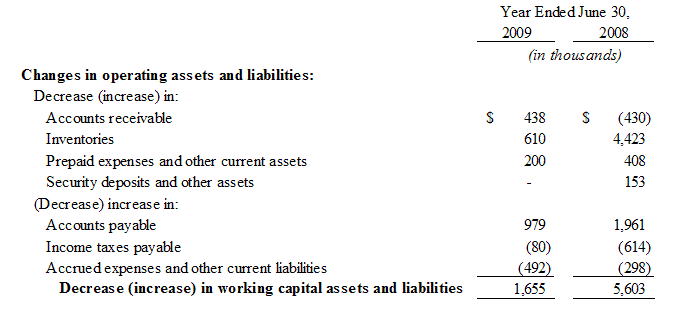
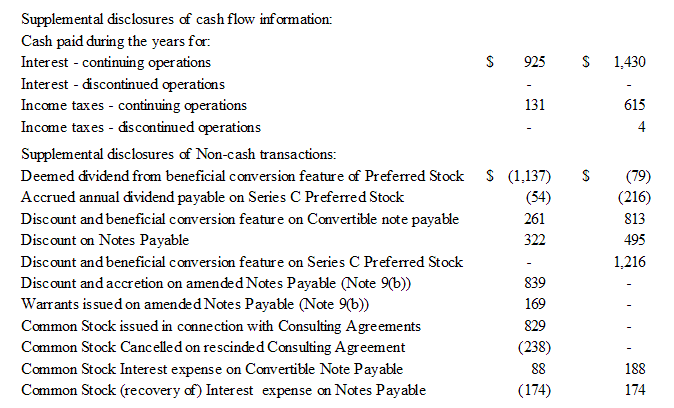
51
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Note 6. Other Intangible Assets
Other intangible assets are tested for impairment at the reporting unit level (operating segment or one level below an operating segment) on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value.
The carrying amount of acquired other intangible assets is as follows:
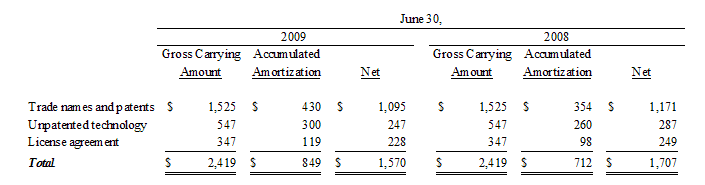
Amortization expense recorded on other intangible assets for each of the fiscal years ended June 30, 2009 and 2008 was $137. The Company recorded impairment charges of $32 (relating to trademarks and patents) in the fiscal year ended June 30, 2009 and $813 in the fiscal year ended June 30, 2008, of which $447 related to intellectual property, $335 related to trade names and $31 to patents. These impairment charges are included in discontinued operations in the Consolidated Statement of Operations. Amortization expense is recorded on the straight-line method over periods ranging from 2 years to 20 years and is included in selling and administrative expenses.
The estimated annual amortization expense for intangible assets for the five succeeding fiscal years is as follows:
52
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
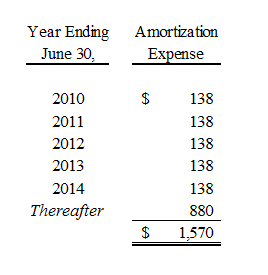
53
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Note 7. Inventories
Inventories are stated at the lower of cost or market using the first-in, first-out method and consist of the following:
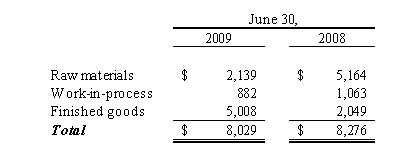
Note 8. Property and Equipment
Property and equipment consists of the following:
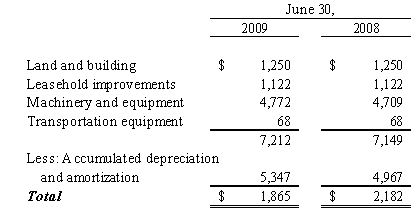
Depreciation and amortization expense was $380 and $448 for the fiscal years ended June 30, 2009 and 2008, respectively.
Note 9. Notes Payable, Convertible Note Payable – CD Financial, LLC and Series C Redeemable Convertible Preferred Stock
On February 19, 2008, the Company entered into two Securities Purchase Agreements (the "SPA") relating to a private placement of securities with two investors, one of whom is an affiliate of Carl DeSantis, a director of the Company, which resulted in gross proceeds, in the aggregate, of $17,337 to the Company. The private placement involves the sale of (i) 6,000 shares of newly designated redeemable Series C Convertible Preferred Stock (the “Series C Preferred”) with a stated value of $1,000 per share (see Note 17(d) Series C Redeemable Convertible Preferred Stock.), (ii) $4,500 in principal amount of 9.5% Convertible Note Payable (the “Convertible Note Payable ”), (iii) $7,000 in principal amount of 8.0% Notes Payable (the “Notes Payable”) and (iv) 200,000 shares of the Company’s common stock. The Company also has recorded $218 of deferred financing costs associated with the two SPA's and $130 of such deferred financing costs were netted against the gross proceeds received. These costs were allocated to the each of the components of the transaction, based on the relative fair values and are amortized based on the terms of the component of the transaction for which the costs were allocated to respectively. As of June 30, 2009 and 2008, the Company had $41 and $113, remaining respectively, of which is to be amortized to interest expense over one to three years. The Notes Payable and the Convertible Note Payable are secured by a pledge of substantially all of our assets. Concurrently with the SPA’s, the Company terminated its outstanding credit facilities with Amalgamated Bank in the amount of $16,333 with the repayment of $16,006. Consequently, upon the extinguishment of the credit facilities, the Company recognized a gain in
54
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
the amount of $327 in the third quarter and fiscal year ended June 30, 2008. Such amount is included in other income in the accompanying Consolidated Statements of Operations.
(a) CD Financial, LLC (“CD Financial”), a related party, provided gross proceeds of $7,500, exclusive of a $163 discount to be repaid by the Company at a future date, in exchange for 3,000 shares of Series C Preferred Stock, with a stated value of $1,000 per share, and $4,500 in principal amount of 9.5% Convertible Note Payable. The Company allocated the proceeds and the discount based the relative fair value of the Convertible Note Payable and the Series C Preferred Stock in connection with this transaction. The Company is amortizing to interest expense the discount applied to the Convertible Note Payable over the term of the note, and charged to Additional Paid in Capital the discount applied to the Series C Preferred Stock. The Company recorded a beneficial conversion feature, in accordance with EITF 00-27, on the Convertible Note Payable of $715 to be accreted over the three-year period until maturity or the redemption of the convertible note payable. The Company also recorded a beneficial conversion feature on the Series C Preferred Stock of $608 to be accreted over the five-year maturity period or the redemption of the Series C Preferred Stock. As of June 30, 2008, the unpaid discount on the Series C Preferred Stock and Convertible Note Payable in the amount of $163 is included in accrued expenses in the accompanying Consolidated Balance Sheet. The beneficial conversion features will be accreted using the effective interest rate method. The Convertible Notes bear interest at an annual rate of 9.5% and mature on or before February 21, 2011. They may be converted, at any time and at the holder’s option, into shares of our common stock based on a conversion price as set out in the Convertible Notes. The conversion price is a formula that bases the conversion price on the greater of (i) 90% of the average Volume Weighted Average Price (the "VWAP") market price of our common stock for 20 trading days immediately preceding the conversion date and (ii) $2.00, subject to adjustment in the event of a stock dividend, stock split or combination, reclassification or similar event and upon certain issuances below the conversion price. We have the option to prepay the Convertible Notes. For the fiscal year ended June 30, 2009 and 2008, included in Interest Expense in the accompanying Consolidated Statement of Operations, is $32 and $229 and $12 and $79, respectively, related to the accretion of the discount and accretion of the beneficial conversion feature on the Convertible Notes, respectively. As of June 30, 2009, the Company had interest in arrears of $145 on the Convertible Notes. In March 2009, the Company and CD Financial entered into an oral agreement to suspend the cash interest payments on the Convertible Notes until the Company returned to positive cash flows in its operations. In this agreement, CD Financial agreed not to give any default notices or increase interest rates due to such default (the default interest rate as defined in the Convertible Note Payable is 18%). In September 2009, the Company made an interest payment of $37, representing the payment of one month’s interest arrearage.
Each holder has the right to require the Company to redeem all or any portion of the shares held by such Holder (a "Mandatory Redemption") in cash upon the occurrence of certain events. The amount payable upon a Mandatory Redemption shall be equal to the greater of (i) the aggregate liquidation preference for the Series C Preferred Shares being redeemed as of the Mandatory Redemption Date
and (ii) the aggregate liquidation preference for such Series C Preferred Shares divided by the Conversion Price, as defined, multiplied by the Market Price, as defined, in effect on the Mandatory Redemption Date. Also, in accordance with the Convertible Note, the Company will issue and deliver to CD Financial LLC, for no additional consideration, 50,000 shares of Common Stock, on a quarterly basis in arrears, commencing with the three-month anniversary of the issuance date, until the
Note has been repaid in full, after which the Company's obligations to issue shares of Common Stock will no longer be applicable.
(b) Imperium, provided proceeds of $9,837, which includes a discount of $163, in exchange for 3,000 shares of Series C Preferred Stock, with a stated value of $1,000 per share, $7,000 in principal amount of 8.0% Notes Payable and 200,000 shares of the Company’s common stock. The Notes Payable originally matured on February 21, 2009. The Company allocated the proceeds and the discount based the relative fair value of the Notes Payable, the Series C Preferred Stock and the Company’s common stock in connection with this transaction. The Company amortized, to interest expense, the discount applied to the Notes Payable over the term of the note and charged to Additional Paid in Capital the discounts applied to the Series C Preferred Stock and the Common Stock. The Company recorded a beneficial conversion feature, in accordance with EITF 00-27, on the Series C Preferred Stock of $608. The beneficial conversion feature is to be accreted over the five-year maturity period or the redemption of the Series C Preferred Stock. The beneficial conversion features will be accreted using the effective interest rate method. For the fiscal year ended June 30, 2009 and 2008, included in Interest Expense in the accompanying Consolidates Statement of Operations, is $322 and $173, respectively, related to the accretion of the discount on the Notes Payable.
55
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
On October 14, 2008, the Company and the Notes Payable holders amended the SPA to extend the maturity from February 21, 2009 to November 15, 2009. In consideration for extending the maturity of the Notes Payable, the Notes Payable holders will forgo the 200,000 shares of common stock as additional interest and the Company will (i) grant a 11.5% premium
on the principal, thus aggregating a principal balance due of $7,805 and certain other amounts payable under the Notes Payable, if any, (ii) certain new covenants will be applicable to the Company effective October 14, 2008, (iii) the Company shall issue warrants to purchase 500,000 shares of the Company’s Common Stock, with a five year term at an exercise price of $0.80 per share, and (iv) the registration of the resale of the shares of the Company’s Common Stock for which
the warrants are exercisable. Since the October 14, 2008 amendment significantly modified the terms of the original Notes Payable, the Company has accounted for the amendment as an extinguishment of the original Notes Payable and issuance of new Notes Payable, per EITF Issue No. 96-19, “Debtor’s Accounting for a Modification or Exchange of Debt Instruments”. As a result, the Company accelerated the
amortization of the then remaining discount of $178 and prepaid financing costs of $32 applied to the original Notes Payable to interest expense as a result of the extinguishment. Furthermore, the Company reversed the accrual of additional consideration of $208 related to the 200,000 shares of the Company’s common stock.
The amended Notes Payable in the amount of $7,000 bear an interest rate of 8.0% and will mature on November 15, 2009. The Company is accreting the premium of $805 over the term of the amendment, using the effective interest method, which has resulted in additional interest expense for the fiscal year ended June 30, 2009 of $517. The warrants issued with the amended Notes Payable were valued at a fair value of $169 using the Black-Scholes option pricing model as of the issuance date. The
Company used the following assumptions to calculate the fair value of the warrants: risk free interest rate of 3.0%, expected volatility of 75.8%, a term of 5 years and a dividend yield of zero. The discount to the amended Notes Payable for the warrants which are being accreted using the effective interest method, has resulted in additional interest expense for the fiscal year ended June 30, 2009 of $110. The Company also recorded an additional $10 of deferred financing costs as a
result of the issuance of the amended Notes Payable. The amended Notes Payable agreement requires the Company to register for resale the shares of Common Stock for which the warrants are exercisable prior to 90 days after the closing date of October 14, 2008. The Company had not registered the warrants as of June 30, 2009. The failure to register the warrants results in a default payment of the greater of $8 or 2.0% of the market price as of the date on which the registration default
occurred multiplied by the number of unregistered warrants. As of the date of the filing, the Company has not registered the securities. The Company will incur a de-minimis penalty amount when it completes the registration of the warrants. The Company is amortizing to interest expense the deferred financing costs using the effective interest method. The amount amortized related to the amended Notes Payable for the fiscal year ended June 30, 2009 is $7.
56
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)

The Company has accreted $54 and $1,137, in the fiscal year ended June 30, 2009, for the Series C Preferred Stock Dividend and for the acceleration of the deemed dividend from the beneficial conversion feature of the Series C Preferred Stock, respectively (See Note 18(d) Series C Redeemable Convertible Preferred Stock). For the fiscal year ended June 30, 2008, the Company accreted $79 for the beneficial conversion feature of the Series C Preferred Stock and $216 for the Series C Preferred Stock Dividend. Such amounts are included in the accompanying Condensed Consolidated Statement of Operations.
The weighted average interest rate paid was 8.59% in each fiscal year ended June 30, 2009 and 2008. The Company had accrued and unpaid interest of approximately $192 and $82, respectively, for the Notes Payable and Convertible Note Payable. (See Note 9(a) above).
As of June 30, 2009, the Company is in technical default of the financial covenants of the Notes Payable relating to the Company’s tangible net worth requirements and minimum net capital requirements. The Company continues to work with the note holders to obtain a formal waiver for the default of the covenants, however at this time the Company has not obtained such waiver and the note holders have not exercised their rights, with respect to the Notes Payable, based on the Company’s technical default. Upon the occurrence of an event of default, the note holders have the right, to give the Company a written notice of such default (an “Acceleration Notice”), which would (i) accelerate the payment of all unpaid principal and accrued and unpaid interest (including default interest (if any)) on the Notes Payable, and (ii) require the Company to pay an amount equal to the sum of all of the amounts described in the preceding clause (i) in same day funds on the payment date specified in the notice, provided such date must be at least two (2) business days following the date on which the notice is delivered to the Company.
Note 10. Revolving Credit Facility and Restricted Cash
On February 21, 2008, as discussed in Note 9, the Company used the majority of the proceeds of $17,337 to repay Amalgamated Bank (the “Bank”) to extinguish the outstanding balance of $7,500 and $8,833 for the Revolving Credit Facility and the Term Credit Facility, respectively. The Company was released from its obligations and the
restricted cash balance was released upon repayment of $16,006 for the outstanding balance, which resulted in a gain from the extinguishment of the Credit Facilities of $327. In addition the Company paid the outstanding interest and commitment fees of $106 plus professional fees of $64.
57
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
In April, 2007, the Company entered into a $15,000 revolving credit facility (“Revolving Credit Facility”) with the Bank and $10,000 five-year term note (“Term Note”), (collectively “Credit Facilities”) with the Bank. On September 27, 2007, the Company and the Bank amended the Revolving Credit Facility, to extend the maturity from October 31, 2007 to March 31, 2008, to amend the quarterly interest rates under the Credit Facilities to equal LIBOR plus a spread that varies depending on the Company’s covenant ratio of non-GAAP financial information, as defined in the agreement, and to cap the amount available under the Revolving Credit Facility to $7,500. For the period from July 1, 2007 until compliance with the December 31, 2007 amended debt covenants, the interest rate was LIBOR plus 3.0%.
Note 11. Term Credit Facility.
On April 3, 2007, we entered into a loan agreement with Amalgamated Bank. The loan agreement provided for a five-year secured term credit facility in the amount of $10,000. Borrowings under the facility were used to refinance $5,000 under our existing $15,000 revolving credit facility with Amalgamated Bank and the balance for working capital purposes. The initial interest
rate on borrowings under the term facility was equal to 3.00% plus the applicable LIBOR rate. Interest was payable monthly, quarterly or semi-annually, at the Company’s election, in arrears not later than the end of each period. The credit facility required that all principal be repaid in $1,000 semi-annual payments beginning October 4, 2007. The facility was secured by a first priority lien on our accounts receivable, equipment, inventory and deposit accounts. The obligations
under the term credit facility were also guaranteed by each of our current and future subsidiaries.
The Company repaid this obligation, on February 19, 2008, with the gross proceeds of $17,337 from the two SPA’s as discussed in Note 9. Notes Payable, Convertible Note Payable – CD Financial, LLC and Series C Redeemable Convertible Preferred Stock.
Note 12. Interest Expense
The components of interest expense for the fiscal years ended June 30, 2009 and 2008 are presented below:

58
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Note 13. Income Taxes
Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial accounting purposes and the amounts used for income tax reporting. Significant components of the Company’s deferred tax assets are as follows:

Net operating losses (“NOL”) of approximately $29,279 will expire beginning in 2024 for federal purposes. State NOL’s of approximately $46,308 will expire beginning in 2010 through 2028 depending on the state in which the NOL’s were generated. These carryforwards could be subject to certain limitations in the event there is a change in control of the Company.
Realization of the NOL carryforwards and other deferred tax temporary differences is contingent on future taxable earnings. The Company’s deferred tax asset was reviewed for expected utilization using a “more likely than not” approach by assessing the available positive and negative evidence surrounding its recoverability. Accordingly, a valuation allowance has been recorded against the Company’s deferred tax asset, as it was determined based upon past and present losses that it was “more likely than not” that the Company’s deferred tax assets would not be realized. The valuation allowance was increased to the full carrying amount of the Company’s deferred tax assets in the fiscal year ended June 30, 2009, which is an increase of $9,242 from the fiscal year ended June 30, 2008. The valuation allowance increase is composed of $4,733 of the net carrying value of the deferred tax assets as of June 30, 2008, and the current year’s increase in deferred tax assets of $4,507. In future years, if the deferred tax assets are determined by management to be “more likely than not” to be realized, the recognized tax benefits relating to the reversal of the valuation allowance as of June 30, 2009 will be recorded. The Company will continue to assess and evaluate strategies that will enable the deferred tax asset, or portion thereof, to be utilized, and will reduce the valuation allowance appropriately as such time when it is determined that the “more likely than not” criteria is satisfied.
59
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
The components of the provision for income taxes consists of the following:

A reconciliation of the statutory tax rate to the effective tax rate is as follows:
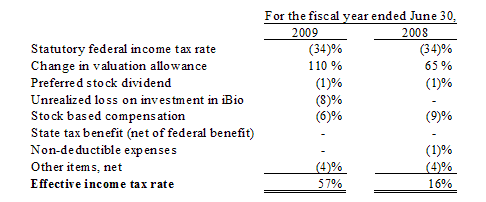
Effective July 1, 2007, the Company adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes” (“FIN No. 48”), which clarifies the accounting for uncertainty in income taxes recognized in the financial statement in accordance with FASB Statement No. 109 Accounting for Income Taxes . This interpretation
prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken, or expected to be taken, in a tax return. There were no significant matters determined to be unrecognized tax benefits taken or expected to be taken in a tax return that have been recorded on the Company’s consolidated financial statements for the year ended June 30, 2009.
Additionally, FIN No. 48 provides guidance on the recognition of interest and penalties related to income taxes. As of June 30, 2008, the Company has included in state income taxes payable $80 for interest or penalties related to income taxes that have been accrued or recognized for the years ended June 30, 2008 and 2007. There were no such interests or penalties outstanding as of or for the fiscal year ended June 30, 2009.
The federal and state tax returns for the years ending June 30, 2006, 2007 and 2008 are currently open and the tax returns for the year ended June 30, 2009 will be filed by March 15, 2010.
60
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
Note 14. Profit-Sharing Plan
The Company maintains a profit-sharing plan, which qualifies under Section 401(k) of the Internal Revenue Code, covering all nonunion employees meeting age and service requirements. Contributions are determined by matching a percentage of employee contributions. The total related expense for the fiscal years ended June 30, 2009 and 2008 was $75 and $175, respectively. As of January 1, 2009, the Company curtailed the Company’s matching percentage of employee contributions into the profit-sharing plan for the benefit of the employees. As of June 30, 2009, the Company has not determined when or if it will reinstate the discretionary employer match in the profit-sharing plan for the benefit of the employees.
Note 15. Significant Risks and Uncertainties
(a) Concentrations of Credit Risk-Cash. The Company maintains balances at several financial institutions. Deposits at each institution are insured by the Federal Deposit Insurance Corporation up to $250 through December 31, 2013. The FDIC is temporarily insuring deposits up to $250 at financial institutions through December 31, 2013. Additionally, JP Morgan Chase is participating in the FDIC’s Transaction Account Guarantee Program, whereby all non-interest bearing checking accounts (including accounts with interest rates less than 0.50%) are fully guaranteed by the FDIC for the entire amount through December 31, 2009. As of June 30, 2009, all the Company’s cash on deposit with JP Morgan Chase was insured pursuant to these two programs with the FDIC.
(b) Concentrations of Credit Risk-Receivables. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk of its customers, establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowances is limited. The Company does not require collateral in relation to its trade accounts receivable credit risk. The amount of the allowance for uncollectible accounts and other allowances as of June 30, 2009 and 2008 was $298 and $108, respectively. The Company’s bad debt expense for the years ended June 30, 2009 was $160 and the Company had a net recovery of bad debts in the amount of $99 for the fiscal year ended June 30, 2008.
(c) Major Customers. For the fiscal years ended June 30, 2009 and 2008 approximately 81% and 79%, respectively, of revenues were derived from three customers. Accounts receivable from these customers represented approximately 95% of total net accounts receivable as of June 30, 2009. The loss of any of these customers would have an adverse affect on the Company’s operations. Major customers are those customers who account for more than 10% of net sales.
(d) Business Risks. The Company insures it business and assets against insurable risks, to the extent that it deems appropriate, based upon an analysis of the relative risks and costs. The Company believes that the risk of loss from non-insurable events would not have a material adverse effect on the Company’s operations as a whole.
The raw materials used by the Company are primarily commodities and agricultural-based products. Raw materials used by the Company in the manufacture of its Nutraceutical products are purchased from independent suppliers. Raw materials are available from numerous sources and the Company believes that it will continue to obtain adequate supplies.
Approximately 50% the Company’s employees, located in its New Jersey facility, are covered by a union contract. The contract was renewed in August 2006 and will expire in August 2010.
Note 16. Commitments and Contingencies
(a) Leases
Related Party Leases. Warehouse and office facilities are leased from Vitamin Realty Associates, L.L.C. (“Vitamin Realty”), a limited liability company, which is 90% owned by the Company’s chairman, president and principal stockholder and certain family members and 10% owned by an employee of the Company. The lease provides for minimum annual rental payment of $324 through May 31, 2015 plus increases in real estate taxes and building operating
61
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
expenses. On July 1, 2004, the Company leased an additional 24,810 square feet of warehouse space on a month-to month basis. Rent expense for the fiscal years ended June 30, 2009 and 2008 on these leases were $815 and $781, respectively, and are included in both cost of sales and selling and administrative expenses in the accompanying Consolidated Statements of Operations. For the fiscal years ended June 30, 2009 and 2008, the Company had an outstanding obligation to Vitamin Realty of $443 and $224, respectively, included in accounts payable in the accompanying Consolidated Balance Sheet.
Other Lease Commitments. The Company has entered into certain non-cancelable operating lease agreements expiring up through May 31, 2015, related to office and warehouse space, equipment and vehicles. Total rent expense, including real estate taxes and maintenance charges, was approximately $1,062 and $1,082 for the fiscal years ended June 30, 2009 and 2008, respectively. Rent expense is stated net of sublease income of approximately $34 and $46 for the fiscal years ended June 30, 2009 and 2008, respectively. Rent expense is included in both cost of sales and selling and administrative expenses in the accompanying Consolidated Statements of Operations and rental income is included in other income (expense) in the accompanying Consolidated Statements of Operations.
(b) Restricted Stock Awards.
Effective July 1, 2008, the Company entered into a consulting agreement, pursuant to which the consultant is to provide consulting and specialized services to the Company in the area of finance, acquisition of product lines, refinancing of existing debt and capital raising under the direction of the Company, including for any company in which the Company has an ownership interest, for a period of
three years. In connection with the agreement, the Company issued 100,000 shares of the Company’s common stock associated with (See Note 18. Equity Transactions). In March 2009, this consulting agreement was rescinded and the shares of the Company’s common stock issued in connection with this agreement were cancelled.
Effective July 1, 2008, the Company entered into a consulting agreement with Jeffrey R. Leach, an employee of the Company, and its former President and Chief Executive Officer. Pursuant to this agreement, Mr. Leach is to provide consulting and specialized services to the Company in the area of finance, acquisition of product lines, refinancing of existing debt and capital raising under the direction of the Company, including for any company in which the Company has an ownership interest
for a period of three years. In connection with the agreement, the Company issued 250,000 shares of the Company’s common stock (See Note 18. Equity Transactions).
(c) Legal Proceedings.
On June 16, 2008, the State of Texas filed an Original Petition for injunctive relief and civil penalties in the 101st Judicial District, Dallas, Texas, against AgroLabs Inc., the Company, Kurt Cahill and Gerald Kay (collectively the “Defendants”). The State alleges that the Defendants sold or distributed juices and dietary supplements marketed with inappropriate disease and nutritional claims. Agrolabs has appeared in the lawsuit and brought a counterclaim against the State for declaratory relief. The Company and Mr. Kay have filed motions to dismiss the lawsuit for lack of personal jurisdiction. The State of Texas is seeking that a permanent injunction be issued, restraining the defendants from, among other things, disseminating false or misleading advertising on product labels as well as civil penalties and attorneys fees and costs. AgroLabs, the Company and Mr. Kay vigorously contest the allegations set forth in the Petition. The Company is unable to make a determination as to the likelihood of an unfavorable outcome or to estimate the amount or range or possible loss or gain and the impact, if any, of such claims will have on the Company and its operations.
On April 23, 2009, Braker Five & Eight Investors, L.P., (the “Landlord”) filed an Original Petition relief and damages pursuant to a Lease Agreement for the premises located in Austin, Texas in the 126th Judicial District, Travis County, Texas, against BevSpec, Inc., Bioscience Technologies, Inc. dba The Organic Beverage Company, and Integrated BioPharma, Inc., as Guarantor (collectively, the “Defendants”). The Landlord is seeking damages for rental fees and charges, repairs, and other sums due under the Lease, including interest. The Company has retained local counsel and is unable to make a determination as to the likelihood of an unfavorable outcome or to estimate the amount or range or possible loss or gain and the impact, if any, of this claim will have on the Company and its operations.
62
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
On or about August 10, 2009, AgroLabs, Inc. commenced an action in the Superior Court of New Jersey, Law Division, against defendants Kurt E. Cahill, Cheryl A. Cahill, Joseph E. Cahill, Jr. and Monty C. Lloyd (all of whom were previously employed by AgroLabs, Inc.) for, among other things, breach of contract, breach of fiduciary duty, negligent performance of duties and other and related relief. On or about September 1, 2009, the defendants removed the action to the United States District Court for the District of New Jersey. On or about September 15, 2009, the defendants filed an answer and affirmative defenses. The defendants, however, asserted no counterclaims. The parties are required to exchange initial disclosures and other information, and are scheduled to appear for an initial conference with the Court on November 18, 2009. The Company is unable to make a determination as to the likelihood of a favorable outcome or to estimate the amount or range or possible loss or gain and the impact, if any, of this claim will have on the Company and its operations.
The minimum rental commitment for long-term non-cancelable leases is as follows:
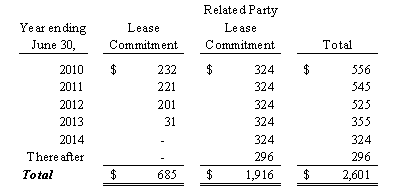
Note 17. Related Party Transactions
The Company has a consulting agreement with Eugene Kay, a former employee of the Company and a brother of E. Gerald Kay, the Company’s Chief Executive Officer, Chairman of the Board, President, Chief Executive Officer and majority shareholder. This agreement is on a month-to-month basis for $1 per month. The total consulting expense recorded per this verbal agreement for the fiscal years ended June 30, 2009 and 2008 was $13 in each year. The Company had another consulting agreement with EVJ, LLC, a limited liability company controlled by Robert Kay, a director of the Company, the Chairman of its subsidiary, InB: Paxis, and a brother of E. Gerald Kay and Eugene Kay. This agreement was assumed by and became a liability of the Company as a part of the Company's acquisition of Paxis Pharmaceuticals Inc. in the fiscal year ended June 30, 2004. The total consulting expense under this agreement was $15 and $120 for each of the fiscal years ended June 30, 2009 and 2008, respectively and is included in discontinued operations in the accompanying Statement of Operations.
Carl DeSantis, a director of the Company and a member of CD Financial (see Note 9(a)) and CD Financial have guaranteed certain liabilities of the Company. On April 7, 2009, CD Financial entered into a Guaranty Agreement with Creative Flavors, Inc. (“CFC”), a major supplier of the Company, guaranteeing up to $500 of the Company’s outstanding obligation with CFC. The guaranty is continuing and
remains in effect until terminated by written notice to CFC. As of June 30, 2009, the Company had an outstanding obligation to CFC in the amount of $1,053, which amount is included in accounts payable in the Company’s Consolidated Balance Sheet. CD Financial and Mr. DeSantis did not receive any compensation from the Company in connection with these guarantees.
On July 1, 2009, the Company entered into a credit and payment agreement (the “Payment Agreement”) with a separate major supplier, Triarco, Inc. (“Triarco”). Under the terms of the Payment Agreement, the Company is obligated to pay its past due balance in eight equal installments of $50 beginning August 1, 2009 and Mr. DeSantis agreed to separately guaranty (the “Personal Guaranty”) the Company’s obligations to Triarco. In exchange, Triarco agreed to extend additional credit of $400 (the “Additional Amount Outstanding”) on net thirty day terms beginning with trade payables dated June 24, 2009. The Personal Guaranty is limited to the lesser of the aggregate amount owed to Triarco, or $800. As of June 30, 2009, the Company owes Triarco $454, $41 under the Additional Amount Outstanding and $413 was past due, these amounts are included in accounts payable in the Company’s Consolidated Balance Sheet.
63
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
See Note 9(a) - Notes Payable, Convertible Note Payable – CD Financial, LLC and Series C Redeemable Convertible Preferred Stock for related party debt.
See Note 16(a) - Leases for Related Party Leases.
Note 18. Equity Transactions
(a) Stock Option Plan and Warrants. The Company has adopted a stock option plan for the granting of options or restricted shares to employees, officers, directors and consultants of the Company that originally provided for the purchase of up to 7,000,000 shares of common stock, at the discretion of the Board of Directors. During fiscal year 2004, the Board of Directors and stockholders approved an additional 2,000,000 common stock shares to be available for grant, for a total of 9,000,000 shares of common stock available for grant and during the fiscal year ended June 30, 2006, the Board of Directors and stockholders approved a further increase in the number of shares of common stock reserved for issuance under the Company’s Stock Option Plan to 11,000,000. Stock option grants may not be priced less than the fair market value of the Company’s common stock at the date of grant. Options granted are generally for ten-year periods, except that incentive stock options granted to a 10% stockholder (as defined) are limited to five-year terms.
During the fiscal year ended June 30, 2009, there were 1,000,000 stock options authorized by the Board of Directors and issued to Company officers, employees and independent directors with an exercise price ranging from $0.14 to $0.15, vesting over three years, with terms of either five or ten years. Warrants to purchase 500,000 shares of common stock at $0.80 in connection with the amended Notes Payable agreement (See Note 9(b) Notes Payable) were also issued in the fiscal year ended June 30, 2009. During the fiscal year ended June 30, 2008, the Company granted 648,650 incentive stock options and 148,150 non-statutory stock options, respectively for periods of five or ten years, vesting over three years, at an exercise price equal to the market price ranging from $2.96 to $3.36. There were no warrants issued in the fiscal year ended June 30, 2008. During the fiscal year ended June 30, 2009 and 2008, the Company has incurred stock compensation expense of $1,301 and $2,094, respectively. Stock compensation is also included in discontinued operations in the amount of $22 and $75 for the fiscal years ended June 30, 2009 and 2008, respectively. During the first quarter of fiscal year 2009, certain key executives and a significant shareholder of the Company exercised stock options to purchase 2,095,852 shares of common stock of the Company; which provided cash proceeds to the Company of approximately $1,342.
Additionally, in the fiscal year ended June 30, 2008, the Company granted Restricted Stock Unit Awards (“RSU”) representing 735,000 shares of the Company’s common stock. The RSUs vest equally over a three-year period on their vesting anniversary date and are subject to forfeiture and were valued using the then market price of $3.05 each. As of June 30, 2009, net shares of 98,750 were forfeited as a result of the employee’s separation of employment with the Company and another 174,221 were cancelled as a result of compensation negotiations.
64
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
A summary of the Company’s stock option activity, and related information for the years ended June 30, follows:
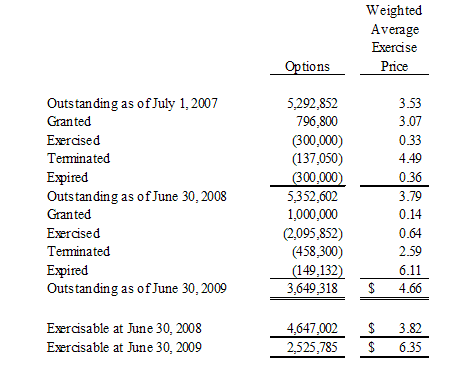
The following table summarizes the range of exercise prices and weighted-average exercise prices for stock options outstanding and exercisable as of June 30, 2009 under the Company’s stock option plans:
65
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
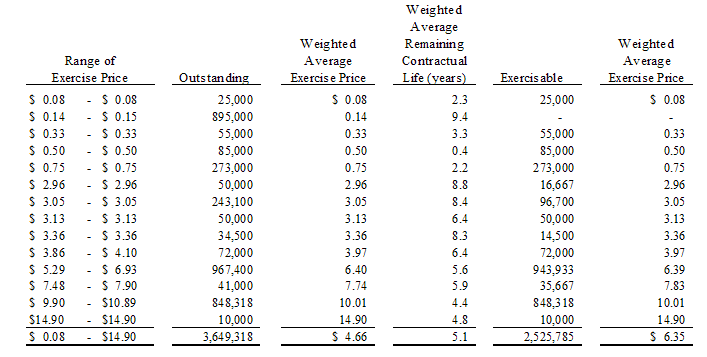
As of both June 30, 2009 and 2008, the Company has 536,000 and 461,000 warrants outstanding, respectively, to purchase shares of common stock at prices ranging from $0.80 to $14.00 and $12 to $14.00, respectively. All outstanding warrants are currently exercisable.
(b) Consulting Agreements. Effective July 1, 2008, the Company entered into two consulting agreements which resulted in the issuance of 350,000 shares of the Company’s common stock associated with these three year consulting agreements. As such, on the effective date of these agreements, the Company recognized prepaid consulting expenses of $830 with a corresponding increase in common stock and additional paid in capital. In March 2009, 100,000 shares of the Company’s common stock associated with one of the consulting agreements were cancelled as the related consulting agreement was rescinded by both parties. See Note 16(b). During the fiscal year ended June 30, 2009, the Company has amortized $197 to selling and administrative expenses in the Company’s Consolidated Statement of Operations. The consulting expense will continue to be amortized into selling and administrative expenses over the three year term of the remaining consulting agreement.
(c) Series C Redeemable Convertible Preferred Stock. On February 21, 2008, the Company raised $5,788 in net proceeds from the sale of 6,000 shares of the Company’s Series C Redeemable Convertible Preferred Stock, par value
$1,000 per share (‘the “Series C Preferred Shares’), at a purchase price of $1,000 per share, in connection with the extinguishment of the Revolving Credit Facility and the Term Credit Facility. (See Note 10. Revolving Credit Facility and Restricted Cash.)
Dividends of the Series C Preferred Shares are 10% per annum, payable on a annual basis, by the Company in shares of the Company's Common Stock, par value $0.002 per share (the "Common Stock"). Accordingly, the Company has accrued approximately $54 and $216 in the fiscal year ended June 30, 2009 and 2008, respectively.
66
INTEGRATED BIOPHARMA, INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND 2008 AND
FOR THE FISCAL YEARS ENDED JUNE 30, 2009 AND 2008
(in thousands, except share and per share amounts)
The Series C Preferred Shares are convertible at any time at the option of the holder into shares of our common stock based on a conversion price, subject to adjustment in the event of a stock dividend, stock split or combination, reclassification or similar event, and upon certain below-market issuances of our common stock. Upon the election to convert, each holder of shares of Series C Preferred Shares will receive such number of fully-paid and nonassessable shares of our common stock
as determined by dividing the aggregate liquidation preference of the shares of Series C Preferred Shares to be converted by the conversion price then in effect on the conversion date. Prior to and including August 21, 2008, the conversion price of each share of Series C Preferred Shares is a formula that bases the conversion price on the lesser of (i) the greater of (x) 90% of the average market price of our common stock for 10 trading days immediately preceding the conversion date and
(y) $2.00 and (ii) $2.94. After August 21, 2008, the conversion price of each share of Series C Preferred Shares is a formula that bases the conversion price on the greater of (i) 90% of the average market price of our common stock for 10 trading days immediately preceding the conversion date and (ii) $2.00. The liquidation preference is equal to $1,000 per share of Series C Preferred Stock held by the holder plus any accrued but unpaid dividends on such shares. The Series C Preferred
may be redeemed under certain circumstances stated in the Certificate of Designations.
Each holder has the right to require the Company to redeem all or any portion of the shares held by such Holder (a "Mandatory Redemption") in cash upon the occurrence of certain events. The amount payable upon a Mandatory Redemption shall be equal to the greater of (i) the aggregate liquidation preference for the Series C Preferred Shares being redeemed as of the Mandatory
Redemption Date and (ii) the aggregate liquidation preference for such Series C Preferred Shares divided by the Conversion Price, as defined, multiplied by the Market Price, as defined, in effect on the Mandatory Redemption Date.
The Company shall pay in cash the Mandatory Redemption Price to the holder exercising its right to redemption on or prior to the fifth (5th) Business Day following the date on which such holder delivers written notice to the Company demanding the redemption of such holder's Series C Preferred Shares specifying the number of Series C Preferred Shares to be redeemed. If the Company fails to pay the Mandatory Redemption Price to a holder on or before the Mandatory Redemption Date, such
Holder is entitled to interest until the Mandatory Redemption Price has been paid in full, at an annual rate equal to the Default Interest Rate.
If any Series C preferred shares remain outstanding on the maturity date (February 1, 2013), the Company will either (i) convert such preferred shares at a conversion rate determined by dividing 115% of the conversion amount being converted by the applicable conversion price as of the maturity date for such preferred shares or (ii) redeem such preferred shares for an amount in cash
per preferred share equal to the conversion amount. The Company is required to give sixty (60) days written notice to each holder of Series C shares, which shall state its election. The Company can redeem at maturity all or a portion of the Series C shares.
The Company recorded the beneficial conversion feature of $1,216 in accordance with EITF 00-27 and such amounts are being accreted over the five year period until the mandatory redemption date of the Series C Preferred Stock, the fifth anniversary of closing. The Company recorded accretion of $1,137 and $79 for the fiscal years ended June 30, 2009 and 2008, respectively to deemed dividend from beneficial conversion feature of Preferred stock dividend in the accompanying Consolidated
Statements of Operations.
In May 2008, the Company registered the Common Stock underlying the Series C Preferred Shares, for resale under the Securities Act of 1933 and applicable state securities laws.
During July and August 2008, all 6,269 Series C Preferred Shares (inclusive of cumulative dividends of 269 shares of Series C Preferred Stock) were converted into 2,639,204 shares of the Company’s common stock. The conversion resulted in an increase to common stock of $5 and additional paid in capital of $6,264. Also during the fiscal year ended June 30, 2009, the Company incurred a deemed dividend from beneficial conversion feature of the Series C Preferred Shares of $1,137 as a result of accelerating the accretion of the beneficial conversion feature and the discount, respectively.
67
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Company has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
INTEGRATED BIOPHARMA, INC.
Date: October 13, 2009 By: /s/ E. Gerald Kay
E. Gerald Kay
Chief Executive Officer
Date: October 13, 2009 By: /s/ Dina L. Masi
Dina L. Masi
Chief Financial Officer
68
